- 1Department of Parasitology, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt
- 2Department of Pathology, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt
- 3Department of Aquatic Animal Medicine and Management, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt
- 4Department of Biology, College of Science, Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia
- 5Department of Biology, College of Science, King Khalid University, Abha, Saudi Arabia
- 6Department of Biology, College of Science, Jazan University, Jazan, Saudi Arabia
- 7Department of Agricultural Microbiology, Faculty of Agriculture, Zagazig University, Zagazig, Egypt
- 8Department of Biology, College of Science, United Arab Emirates University, Al Ain, United Arab Emirates
- 9Department of Poultry Diseases, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt
- 10Department of Diseases of Birds, Rabbits, Fish and their Care and Wildlife, School of Veterinary Medicine, Badr University in Cairo (BUC), Cairo, Egypt
Introduction: The helmeted guineafowl is a ground-dwelling bird native to Africa, easily recognized by its bald, bluish-gray head and the distinctive horn-like casque (helmet) on top of its head. Parasitic coinfection with Ascaridia worms and Eimeria in chickens poses a significant health challenge, as both parasites damage the intestinal tract and impair nutrient absorption. Ascaridia galli competes for nutrients and causes mechanical irritation, while Eimeria tenella induces mucosal injury and inflammation. Their combined effect leads to severe enteritis, reduced growth performance, poor feed conversion, and increased susceptibility to secondary infections. This synergistic impact exacerbates economic losses in poultry production and highlights the importance of integrated parasite control strategies.
Methods: This study investigated the cause of mortality in helmeted guineafowl on a private farm. Clinical examination, necropsy, parasitological analysis, molecular characterization, and histopathological examination were conducted.
Results: Preliminary findings indicated a mixed gastrointestinal parasitic infection, with A. galli and E. tenella identified as the causative agents of co-infection. Molecular analysis targeting the ITS rDNA and COX1 regions of A. galli and the ITS and 18S rDNA regions of E. tenella confirmed their identities and revealed genetic diversity among the isolates. Phylogenetic analysis clustered the isolates within well-supported clades of their respective species. Clinical signs included depression and sporadic hemorrhagic droppings, while postmortem lesions varied, featuring enteritis, hemorrhagic typhlitis, splenic necrosis, and hepatic lesions. Histopathological examination revealed severe intestinal damage, including hemorrhage, epithelial desquamation, and the presence of multiple parasite developmental stages. The co-infection led to a 10% mortality rate.
Discussion: The current study offers insights into the impact of A. galli and E. tenella co-infection in helmeted guineafowl, underscoring the importance of molecular surveillance in monitoring poultry parasite populations. Additional research is recommended to establish routine parasitological monitoring, implement targeted deworming initiatives, enhance sanitation, and enforce biosecurity protocols to reduce parasite load and prevent epidemics.
Introduction
Helmeted guineafowl (Numida meleagris) are birds native to sub-Saharan Africa but introduced in various regions around the globe for domestic rearing and/or production. These birds are deemed more resistant to parasitic infections (1). Poultry production faces significant challenges from parasitic infections, with more than 30 helminths identified in hens (2). Among these, Ascaridia galli stands out as the most prevalent, often occurring in mixed infections with the caecal nematode Heterakis gallinarum (3–5).
While A. galli has been observed in chickens across various housing systems globally, its prevalence was lower in laying hens housed in traditional battery cages (6, 7). The persistence of A. galli in poultry environments is facilitated by its resilient eggs, which can survive for extended periods. Although most ascarid eggs are destroyed within months, a small percentage (up to 3%) can remain viable for up to 2 years (8). This longevity, coupled with the parasite’s high reproductive rate and the host’s compromised immune response, leads to an escalating intensity of infection over time (9). As the largest intestinal helminth in chickens, A. galli significantly impacts egg and meat production, thereby affecting both economic outcomes and animal protein availability (10–12).
The parasite’s life cycle is direct, involving a single host. The adult worms in the small intestine produce eggs that are expelled into the environment (13). These eggs are protected by three layers: an outer thin albuminous layer, a thick, resistant covering, and an inner permeable vitelline membrane (2, 13). The eggs develop internally to the infectious third larval stage (L3) without hatching in the environment (14). Under typical conditions in chicken barns, A. galli eggs rapidly develop into infectious larvae, a process known as embryonation in ovo (14, 15). Laboratory studies have demonstrated that under optimal conditions, nearly 88% of A. galli eggs complete development within 1–2 weeks (16, 17).
The pathogenesis of A. galli infection primarily stems from the larval stages, although both adult and immature parasites contribute to intestinal health deterioration (18). Transmission of A. galli occurs through ingestion of contaminated food or water or through ingestion of earthworms containing larvae (15). Earthworms can serve as paratenic hosts, ingesting the infectious stage and subsequently transmitting the infection to definitive hosts (17). Infection via earthworm consumption is more efficient than direct egg ingestion from the environment, provided the earthworm is consumed within 96 h of ingesting the eggs (17).
In addition to helminth infections, the poultry industry faces significant challenges from protozoan diseases, particularly coccidiosis. This disease accounts for 6–10% of all broiler mortalities and causes substantial economic losses worldwide (19). Coccidiosis is caused by apicomplexan protozoa of the genus Eimeria, affecting both domestic and wild chickens (20). The severity of coccidiosis can be classified into three levels: coccidiosis (a mild infection with no adverse effects), subclinical coccidiosis, and clinical coccidiosis (21–24).
Subclinical infections can reduce bird growth performance through various mechanisms, including reduced voluntary feed intake, decreased nutrient digestibility, and diversion of nutrients for tissue repair and immune responses (25–27). The impact of Eimeria infections on bird growth performance is influenced by factors related to the pathogen, host, and environment (28). Host factors such as age, sex, genetic line, and pathogen factors like Eimeria species can modulate the impact of the infection. The disease progression, including incubation, acute, and recovery phases, is crucial in determining the overall effect on performance (29).
Multiple Eimeria species affect chickens, each with its specific predilection site in the gut, oocyst morphometrics, and immunogenicity (30). Reid et al. (31) classified Eimeria species based on their effects on the host: hemorrhagic (E. tenella, E. necatrix, and E. brunetti), malabsorptive (E. maxima and E. acervulina), and lesser degree infections (E. mitis and E. praecox). Four species are of particular concern due to their global disease and economic impact: E. acervulina, E. maxima, E. tenella, and E. necatrix. The life cycle of Eimeria spp., involves both exogenous and endogenous stages, with well-documented durations that vary slightly between species (31). For instance, the prepatent (incubation) phase ranges from 4 days for E. acervulina to 5–6 days for E. maxima and 6–7 days for E. tenella (32).
The endogenous phases (schizogony and gametogony) disrupt host intestinal cells to form parasite “zoites.” Coccidiosis typically manifests as an acute invasion and destruction of intestinal mucosa by the protozoa Eimeria or Isospora. Clinical symptoms include diarrhea, fever, decreased appetite, weight loss, and emaciation, with severe cases potentially leading to death (33). The disease compromises the mucous membranes throughout the intestinal tract, resulting in absorption imbalances that cause diarrhea and, in extreme cases, mortality (34). However, many infections remain subclinical, posing challenges for detection and management (34).
Molecular sequencing techniques have revolutionized the identification and characterization of parasites in birds, offering unprecedented accuracy and reliability in diagnosing infections such as those caused by A. galli and E. tenella (35–37). These advanced methods enable precise species identification and provide valuable insights into the genetic diversity of parasites, contributing significantly to our understanding of host–parasite dynamics and the epidemiology of avian diseases in natural ecosystems (37).
Interestingly, A. galli and Eimeria spp., often occur as concurrent infections in poultry with prominent pathological lesions potentially exacerbating the negative impacts on host health and productivity (38–40). This co-occurrence can lead to complex interactions between the parasites and the host immune system, further complicating diagnosis and treatment strategies. Furthermore, co-infections’ timing can significantly influence the severity of the disease and the induced pathological lesions, with A. galli infection preceding other etiological agents, resulting in more severe outcomes (40, 41).
Given the significant impact of parasitic infections on poultry health and production, there is a critical need for further investigation into the interactions between A. galli and Eimeria spp. in concurrent infections. Thus, the current study aimed to investigate the causative agents of mortality in black-colored chickens (N. meleagris) on a private farm, with a particular focus on identifying and characterizing the parasitic infections present. The study also aims to molecularly characterize both parasites using COX1, ITS, and 18S rRNA sequencing and to assess the impact of concurrent infections through histopathological examination.
Materials and methods
Ethical approval
The work was carried out in accordance with the IACUC guidelines and was approved by the Ethical Committee of the Faculty of Veterinary Medicine, Cairo University, Cairo, Egypt, with the code “Vet CU 8032022511.”
Study design and sample collection
The current study was conducted in response to an unusual mortality pattern observed among helmeted guineafowl on a private farm. The farm reported a significant increase in chicken mortalities over a two-week period, reaching approximately 30%. This mortality rate initially indicated the overall expected losses among the entire flock on the farm over the 2 weeks preceding our examination, as conveyed by the farm owner, which prompted an inquiry into suspected causal agents, notably parasitic diseases.
Following the initial report, a series of farm visits were conducted between October to November 2024. During these visits, comprehensive farm inspections were carried out, focusing on housing conditions, feed quality, and general management practices that might contribute to disease susceptibility.
A total of 60 chickens were included in the study, and this number of birds was the total number of the remaining birds after mortality and they were clinically diseased birds and available for monitoring and intervention during our visits. The mortality during the farm investigation has reached 10% (6 birds out of 60); the age of dead birds ranges from 18 to 30 months. Birds were selected based on clinical signs suggestive of parasitic infection, including diarrhea, weight loss, and reduced egg production.
Sampling included freshly dead birds as well as droppings samples from the surrounding environment. The samples were then transported to the laboratory of the Department of Parasitology, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt, for comprehensive post-mortem examination and tissue sampling under sterile conditions. Fecal samples were collected in sterile containers with 2.5% potassium dichromate solution for oocyst preservation.
Clinical examination and necropsy
Diseased birds were subjected to thorough clinical examination, recording all observable symptoms. Birds that succumbed to the disease were promptly necropsied following standard veterinary protocols recommended by Berhe et al. (42). During necropsy, all visceral organs were systematically examined for gross pathological changes.
Particular attention was paid to the gastrointestinal tract, with the mucosa of the duodenum, jejunum, ileum, and ceca being carefully inspected for the presence of parasites, especially Eimeria and Ascaridia spp., following the method described by Razmi and Kalideri (43).
Parasitological examination
Microscopic examination
Wet smears of mucosal scrapings from intestinal and caecal tissues were prepared and examined using Olympus BH-2 (Olympus Optical Co. Ltd., Tokyo, Japan) light microscope equipped with a digital camera and software (Jenoptik ProgRes Camera, C12plus, Frankfurt, Germany). Eimeria spp. were identified based on the site of infection, oocyst morphology (including size, color, presence or absence of micropyle and micropyle cap), and sporulation time, according to the criteria established by Long and Rose (44).
Oocyst isolation and quantification
Eimeria spp. oocysts were isolated from caecal and lower intestinal mucosa using the saturated sodium chloride flotation technique as described by Permin and Hansen (45). Oocyst quantification was performed using a modified McMaster’s oocyst-counting technique (46, 47). For sporulation, intestinal contents were collected from freshly dead birds and incubated in a 2.5% aqueous solution of potassium dichromate at 27 °C for 48–72 h with regular aeration.
Helminth collection and preservation
Helminths were carefully extracted from the intestinal tract and washed repeatedly with phosphate-buffered saline (PBS, pH 7.2). Specimens were preserved in 70% glycerine alcohol for morphological studies and mounted using glycerol jelly, following the method of Salem and Attia (48).
Molecular identification of Ascaridia sp.
Individual worm specimens underwent thorough cleansing with five sterile distilled water rinses before genomic DNA extraction using a DNeasy tissue kit (Qiagen, Hilden, Germany) following manufacturer protocols. DNA quality and concentration were assessed using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific Inc., Waltham, Massachusetts, United States).
Two complementary genetic regions were targeted to ensure robust species identification and phylogenetic resolution. These were the internal transcribed spacer (ITS) region of ribosomal DNA and the cytochrome c oxidase subunit 1 (COX1) gene. The ITS region was amplified using universal primers BD1 (5′-GTCGTAACAAGGTTTCCGTA-3′) and BD2 (5′-TATGCTTAAATTCAGCGGGT-3′) (49), while COX1 amplification employed nematode-specific primers Ag Cox1F (5′-ATTATTACTGCTCATGCTATTTTGATG-3′) and Ag Cox1R (5′-CAAAACAAATGTTGATAAATCAAAGG-3′) (37).
PCR reactions were optimized in 50 μL volumes containing 5 μL of 10 × buffer, 5 μL of each dNTP at 10 mM, 10 μL of each primer at 1 pmol/μl, 0.3 μL of Taq polymerase (5 U/mL), 2.5 μL magnesium chloride (50 mM), and 2 μL of extracted gDNA. Amplification products were visualized on 1.5% agarose gels in 1X TAE buffer and purified using QIAquick PCR Purification Kit (Qiagen) prior to bi-directional Sanger sequencing (Macrogen Inc., Seoul, South Korea).
Phylogenetic analysis
Consensus sequences were aligned with representative GenBank sequences spanning Ascaridia spp., Heterakis spp., Ascaris spp., and related ascaridoid taxa using ClustalW for multiple sequence alignment in MEGA 11 (50). Outgroup selection was based on phylogenetic distance and data availability with Mastophorus muris (MK829006) for ITS analysis and Neoentomelas asatoi (LC632117) for COX1, representing closely related but distinct nematode lineages.
Maximum Likelihood phylogenetic reconstruction employed the GTR + G + I model, selected for its ability to accommodate variable substitution rates and site-specific evolutionary constraints common in ribosomal and mitochondrial sequences. Bootstrap support (1,000 replicates) provided statistical validation of tree topology.
Molecular identification of Eimeria sp.
Purified oocysts underwent four freeze–thaw cycles to disrupt oocyst walls before single-oocyst DNA extraction using DNeasy Tissue Kit (Qiagen). The complementary marker approach targeted both 18S rDNA and ITS regions to maximize phylogenetic resolution and enable comparison with existing databases. The 18S rDNA was amplified using primers ERIB1 (5′-ACCTGGTTGATCCTGCCAG-3′) and ERIB10 (5′-CTTCCGCAGGTTCACCTACGG-3′), while the ITS1-ITS2 regions were targeted using primers ITS-1 (5′-GGATGCAAAAGTCGTAACACGG-3′) and ITS-2 (5′-TCCTCCGCTTAATAATATGC-3′) (35). Both primer sets were selected for their proven efficacy in identifying apicomplexan parasites.
Phylogenetic analysis followed identical protocols, using appropriate outgroups: Coccidia sp. (MH590232) for ITS analysis and Isospora belli (AF106935) for 18S rDNA analysis. These outgroups were chosen to represent related coccidian parasites while maintaining sufficient evolutionary distance for tree rooting.
Histopathological examination
The histopathological examination involved tissue samples from the duodenum, ileum, and cecum. The samples were preserved in 10% neutral buffered formalin for 24 h, then routinely processed, embedded in paraffin, sectioned at 3–5 μm thickness, and finally stained with hematoxylin and eosin using standard protocols (51).
Histopathological lesions and the presence or absence of Eimeria spp. and Ascaridias were recorded. Tissue sections were examined, and the lesions were photographed using an Olympus light microscope.
Results
Clinical examination and postmortem findings
The investigated birds exhibited a range of clinical signs indicative of severe parasitic infection. As seen in Figure 1, the most common signs observed were mortalities, ruffled feathers, lack of appetite, drowsiness, planes in com and wattles, inability to walk, some birds revealed submandibular oedema, many birds were seen huddling together, and a significant proportion displayed bloody diarrhea. Out of the 60 birds examined, 10% succumbed to the infection during the study period.

Figure 1. Postmortem examination of freshly dead helmeted guineafowl showing (A) high mortality during the farm investigation; (B) submandibular edema (yellow arrow); (C) planes in comb and wattles.
As seen in Figure 2, freshly dead birds’ postmortem examinations showed consistent, recognizable lesions as the caeca’s appeared filled with blood; sausage-like appearance and the mesenteric blood vessels’ engorgement were the most common findings. The ceca had bloody contents when it was cut, and in a few cases, cecal cores were visible. The appearance of the infected hens’ spleens varied; some were pale, while others had surface petechial hemorrhages with necrosis. Areas of mucosal thickening and hyperemia were frequently observed in the small intestine, especially the ileum. The liver showed multiple pale areas with subcapsular hemorrhages.
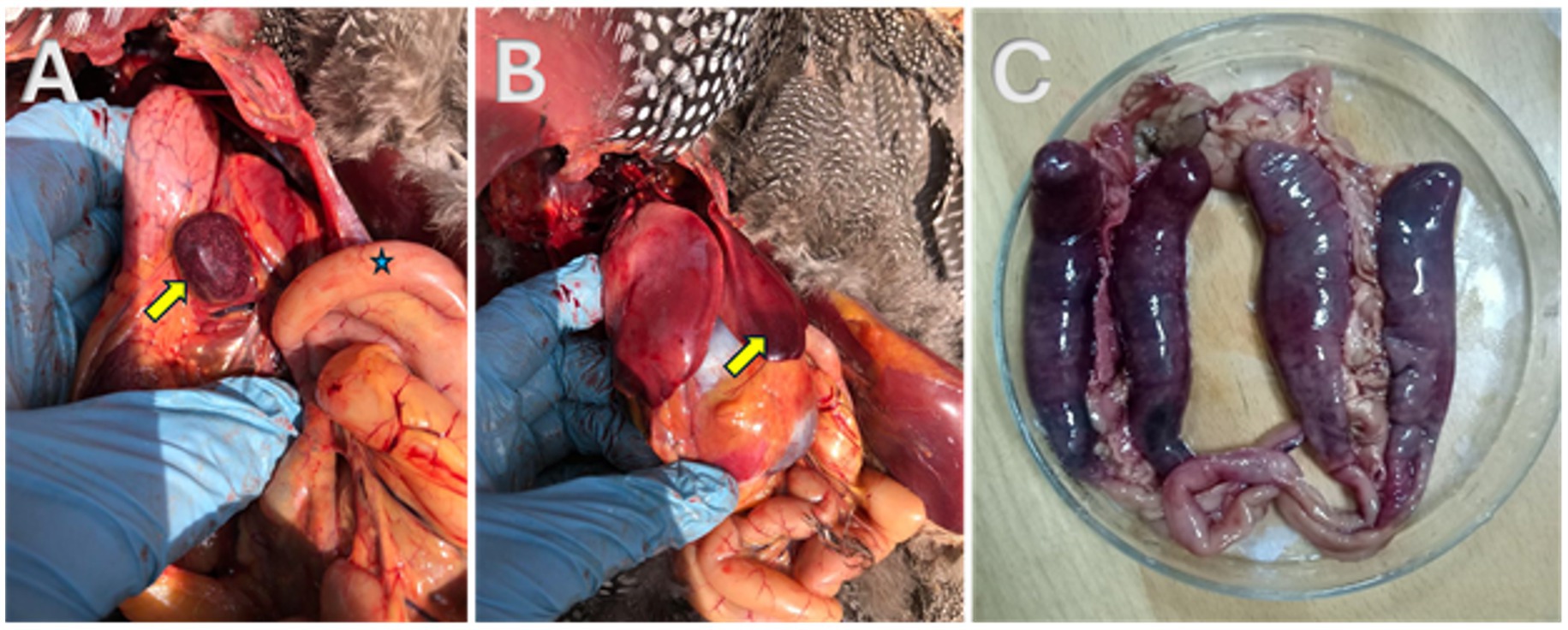
Figure 2. Postmortem examination of freshly dead helmeted guineafowl showing (A) necrosis in the spleen (yellow arrow); (B) subcapsular hemorrhage in the liver (yellow arrow); (C) blood sausage appearance of the two blind ceci.
Morphology and parasitological examination
The parasites identified in this study were A. galli and E. tenella. Fecal flotation tests revealed the presence of Eimeria oocysts in 90% of examined samples, with an average oocyst count of 8,000–12,000 ± 100 oocysts per gram of feces. A. galli eggs were detected in 60% of fecal samples, with an average egg count of 600–1,200 ± 25 eggs per gram of feces.
A. galli specimens exhibited distinct sexual dimorphism. Female worms, ranging from 75 to 115 mm in length, were notably larger than males. The body was cylindrical, creamy-white, and semi-transparent, with a thick, proteinaceous cuticle displaying transverse striations. The anterior end was characterized by three large, trilobed lips encircling a large mouth. Male A. galli, measuring between 50 and 76 mm in length, had distinctly pointed and curled tails with 10 pairs of caudal papillae (Figure 3).
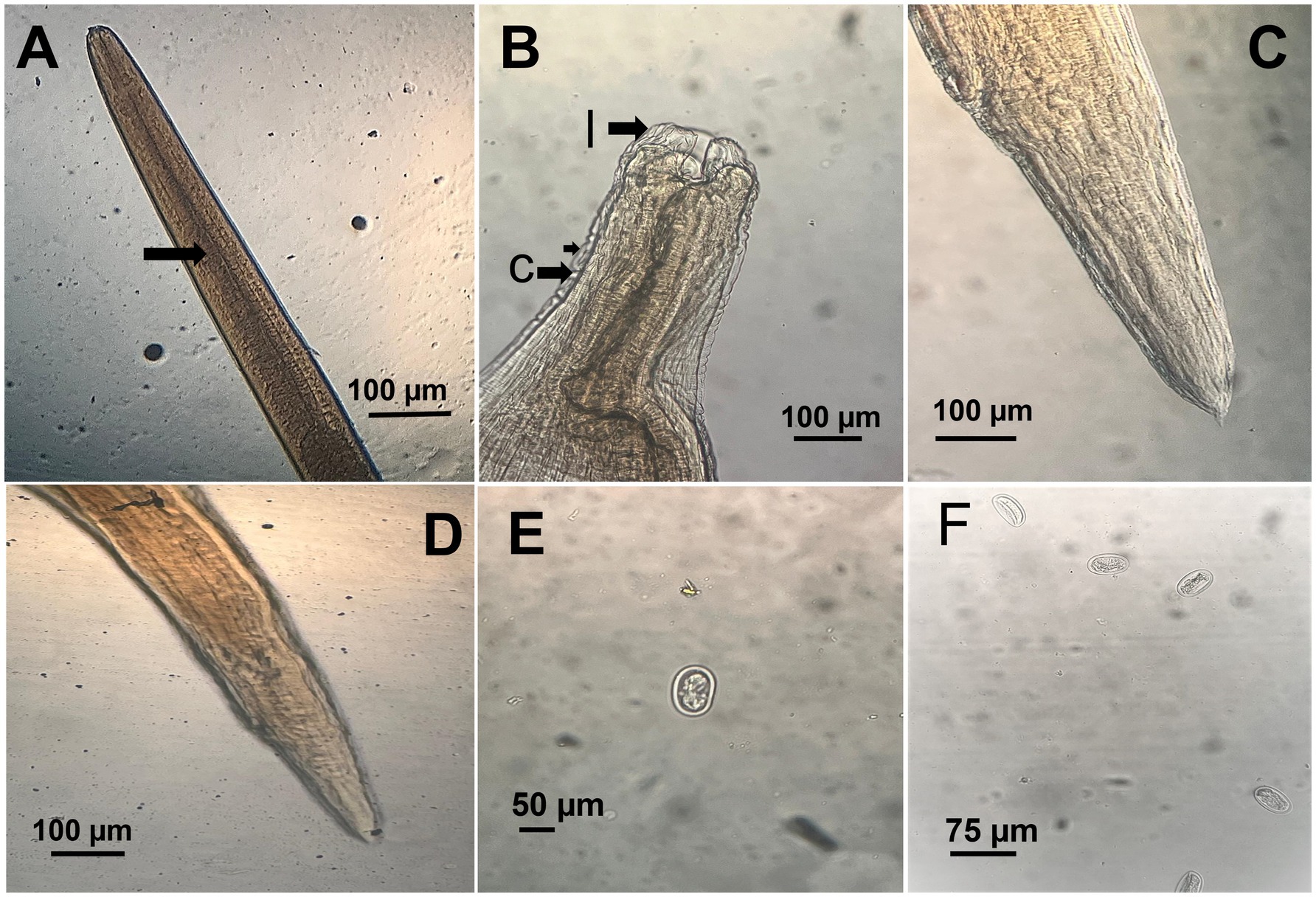
Figure 3. Microscopic morphology of Ascaridia galli. (A) Anterior part of adult worm showing the thick cuticle (arrow). (B) Close view of the cephalic end displaying trilobed lips (l) and cervical alae (c). (C) Posterior end of male with characteristic pointed tail. (D) Posterior end of female with bluntly rounded tail. (E) Unembryonated A. galli egg recovered from feces, showing smooth shell. (F) Multiple A. galli eggs in flotation preparation.
Eggs collected from infected birds’ feces were oval shaped with smooth shells, measuring 75–96 μm by 48–59 μm. Adult A. galli worms were recovered from the small intestines of half of the necropsied birds, with an average worm burden of 15–30 worms per bird. On the other hand, E. tenella oocysts were identified by their ovoid shape and double-layered oocyst wall, lacking a micropyle and micropylar cap, sporulated oocysts measured 25 (21–27) μm in length and 18 (15–29) μm in width. Direct microscopic examination of intestinal scrapings revealed various developmental stages of Eimeria, including schizonts, merozoites, and gametocytes, in the majority of examined birds (Figure 4).
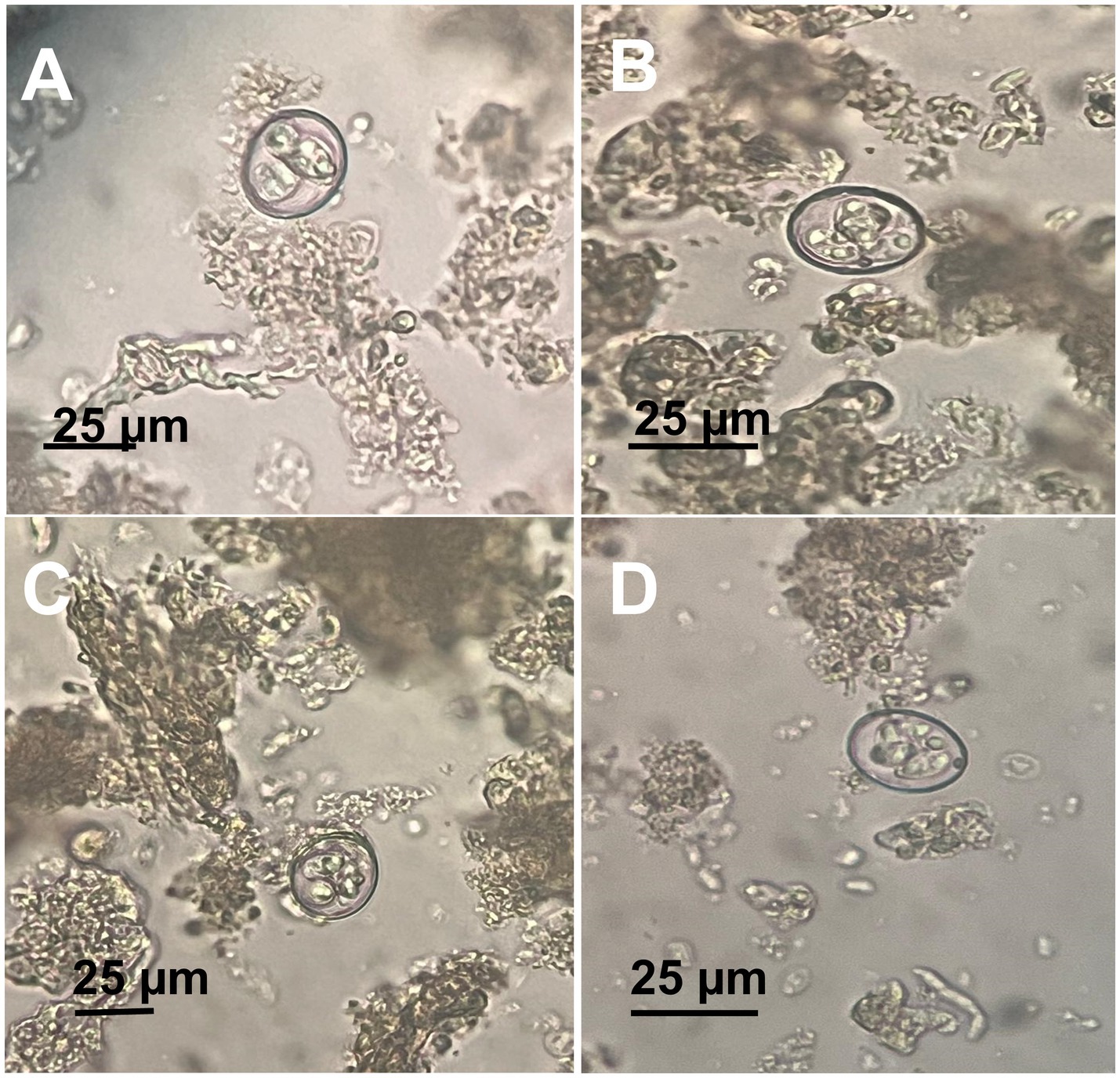
Figure 4. Microscopic appearance of Eimeria tenella sporulated oocysts. (A–D) Different views showing a fully mature sporulated oocyst.
Molecular identification of Ascaridia sp.
ITS and COX1 sequencing of Ascaridia sp.
Table 1 demonstrates that the ITS rDNA region was effectively amplified from three distinct Ascaridia specimens, producing sequences of 981, 982, and 972 base pairs, with accession numbers PQ047113, PQ047114, and PQ047115, respectively. The COX1 gene was successfully amplified from the same three specimens, producing sequences of 526, 525, and 526 base pairs, deposited as PQ106343, PQ106344, and PQ106345, respectively. Sequence analysis confirmed taxonomic identification as A. galli.
Detailed comparison revealed specific intraspecific variations: ITS sequence PQ047113 differed from PQ047114 by 9 base pair substitutions and 1 gap, while exhibiting 4 base pair differences and 9 gaps compared to PQ047115. COX1 sequence PQ106343 exhibited 7 base pair substitutions compared to PQ106344, and 4 base pair differences relative to PQ106345. Interspecific comparisons revealed lower similarities: A. columbae (95.20–96.78% for ITS, 85.52% for COX1), other Ascaridia species (76.53–87.94%), and Heterakis species (77.61–86.82%).
Phylogenetic analysis of Ascaridia sp.
Phylogenetic analysis based on ITS sequences revealed the three A. galli isolates formed a well-supported monophyletic group with 100% bootstrap support, clustering closely with other A. galli sequences (Figure 5). COX1 phylogenetic analysis showed identical patterns with the three isolates forming a monophyletic clade (100% bootstrap support) distinct from other Ascaridia and Heterakis species (Figure 6).
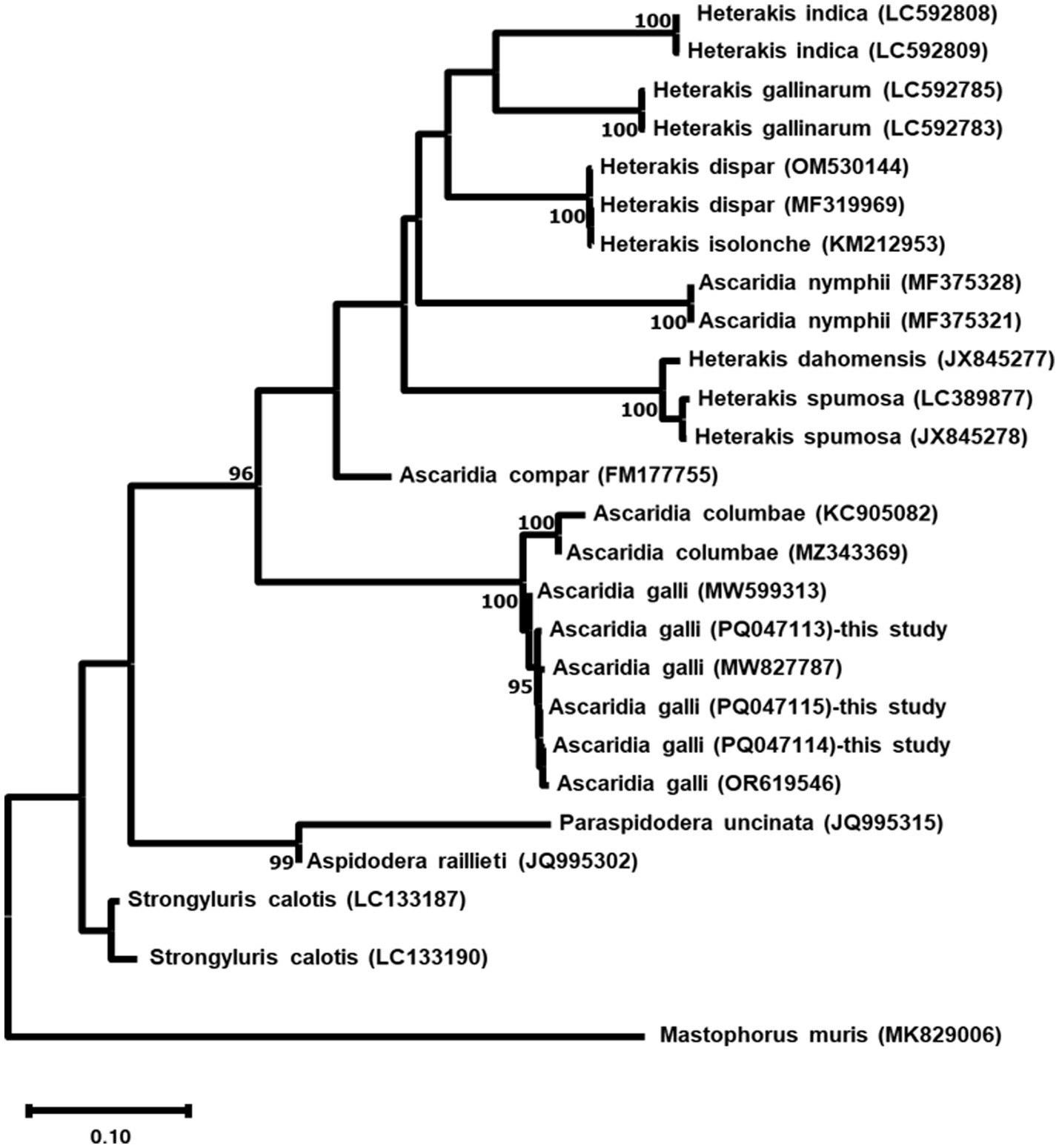
Figure 5. Maximum likelihood phylogenetic tree based on ITS sequences of three Ascaridia galli isolates and related nematode species. The tree was constructed using the GTR + G + I model. Numbers at nodes indicate bootstrap support values (%) from 1,000 replicates; only values >90% are shown. Mastophorus muris was used as an outgroup. Scale bar represents 0.10 nucleotide substitutions per site.
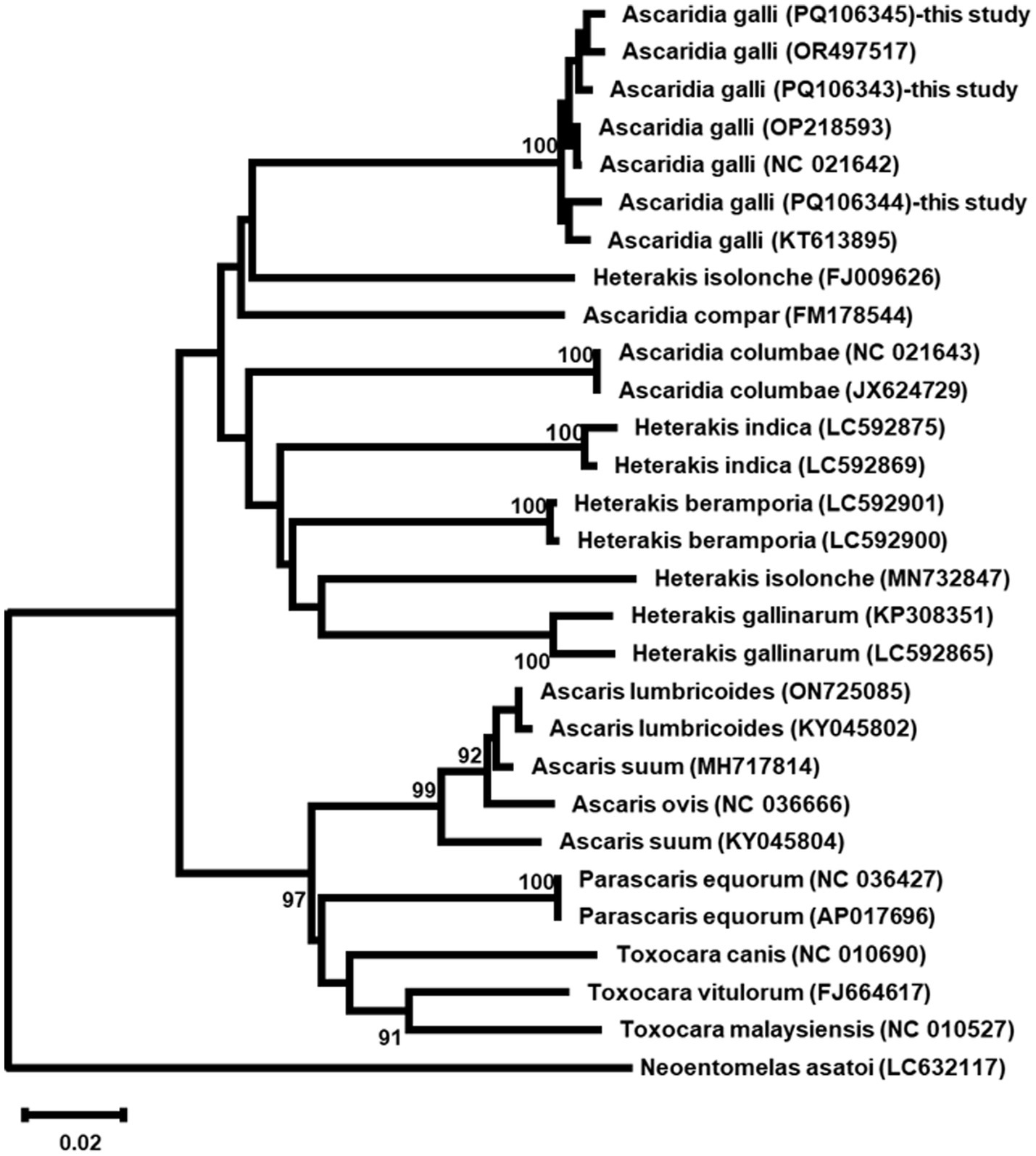
Figure 6. Phylogenetic maximum likelihood tree of COX1 sequences from three Ascaridia galli isolates and other related taxa. The tree was constructed using the GTR + G + I model. Numbers at nodes indicate bootstrap support values (%) from 1,000 replicates; only values >90% are shown. Neoentomelas asatoi was used as an outgroup. Scale bar represents 0.02 nucleotide substitutions per site.
ITS and 18S rDNA sequencing of Eimeria sp.
Table 2 illustrates that the ITS rDNA region was effectively amplified from three Eimeria sp. samples, yielding sequences of 925, 923, and 923 base pairs, with given accession numbers PQ047140, PQ047141, and PQ047142, respectively. The 18S rDNA was successfully amplified from the same three isolates, yielding sequences of 1,754, 1,753, and 1,754 base pairs, deposited under accession numbers PQ142692, PQ142693, and PQ142694, respectively. Sequence analysis confirmed taxonomic identification as E. tenella.
Specific intraspecific variations included: ITS sequence PQ047140 differed from PQ047141 by 10 base pair substitutions and 2 gaps, while exhibiting 9 base pair differences and 2 gaps compared to PQ047142. The 18S rDNA sequence PQ142692 showed 6 base pair substitutions compared to PQ142693, and 7 base pair differences relative to PQ142694. Interspecific comparisons showed lower similarities with other Eimeria species: E. gallopavonis (97.83% for 18S rDNA), and other Eimeria species (93.47–97.82%).
Phylogenetic relationships
Maximum Likelihood analysis of both ITS and 18S rDNA sequences revealed the three E. tenella isolates formed well-supported monophyletic groups (99% bootstrap support), clustering with other E. tenella sequences while maintaining clear separation from other Eimeria species (Figures 7, 8).
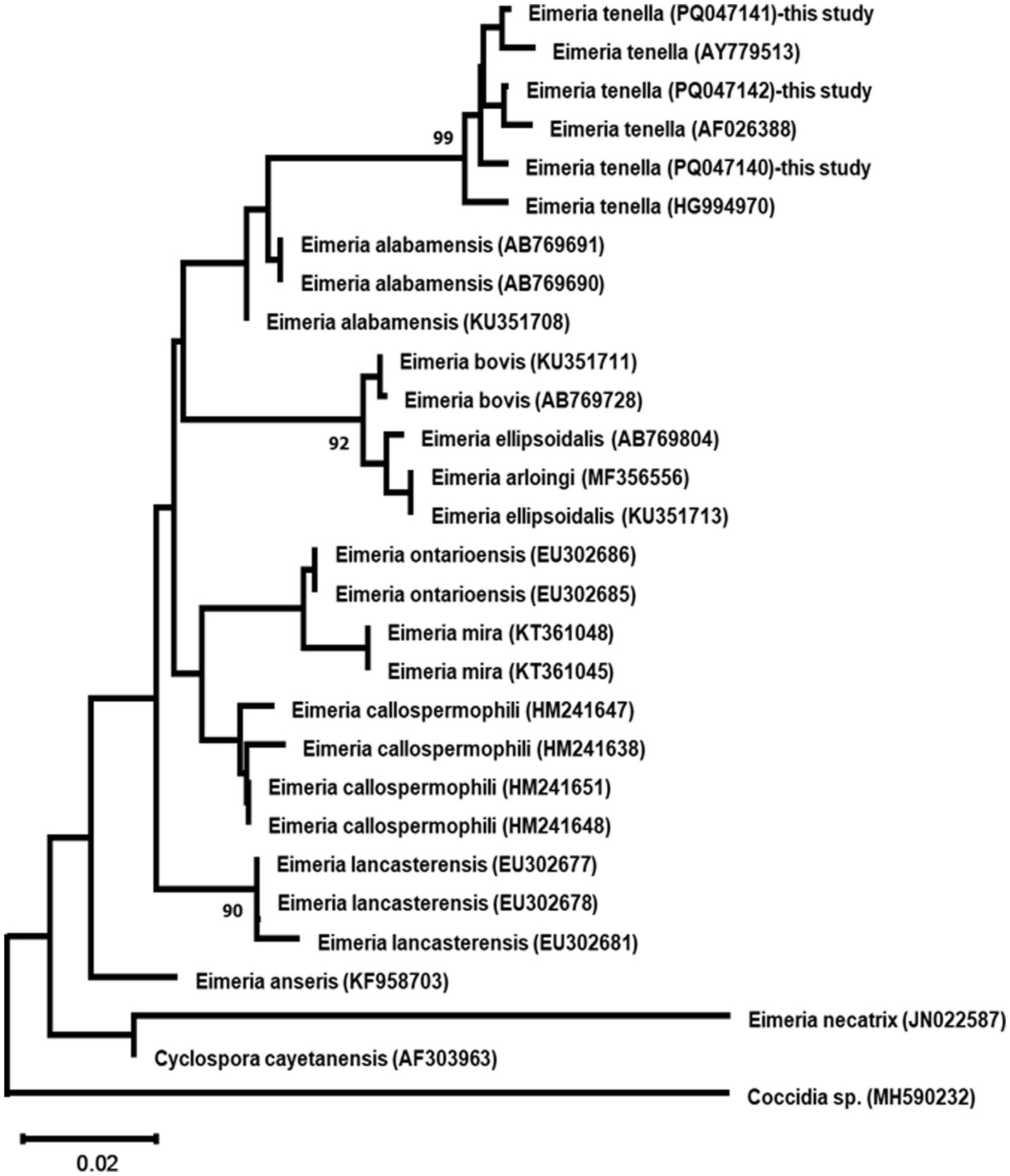
Figure 7. Maximum likelihood phylogenetic tree based on ITS region sequences from three Eimeria tenella isolates and other related taxa. The tree was constructed using the GTR + G + I model. Numbers at nodes indicate bootstrap support values (%) from 1,000 replicates; only values >90% are shown. Coccidia sp. serves as an outgroup. Scale bar represents 0.02 nucleotide substitutions per site.

Figure 8. Maximum Likelihood phylogenetic tree based on 18S rDNA sequences from three Eimeria tenella isolates and other related taxa. The tree was constructed using the GTR + G + I model. Numbers at nodes indicate bootstrap support values (%) from 1,000 replicates; only values >90% are shown. Isospore belli serves as outgroups. Scale bar represents 0.01 nucleotide substitutions per site.
Histopathological findings
Histopathological examination of infected birds revealed severe pathological lesions in the intestine. Various stages of Eimeria parasites were observed in the epithelial cells of the intestinal mucosa and within the intestinal lumen. In some instances, parasites were aggregated in the lamina propria. Extensive infection with numerous schizonts was noted in the intestinal lumen, associated with hemorrhages and epithelial desquamation (Figure 9A). Concurrent infection with nematode parasites (A. galli) was evident, with cross-sections of nematode worms observed alongside dispersed schizonts among remnants of desquamated epithelial cells (Figures 9B,C).
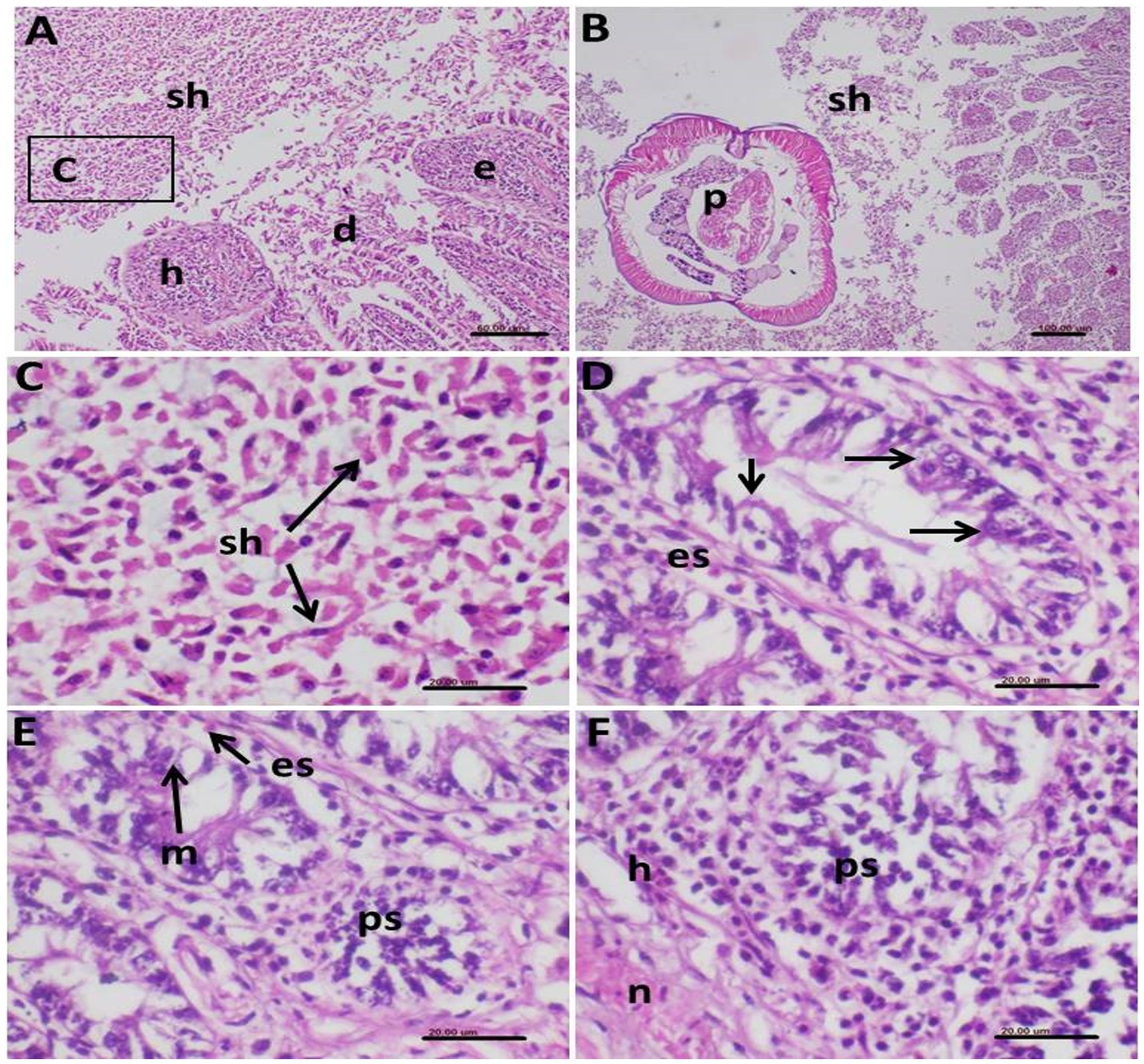
Figure 9. Photomicrograph of histopathological section of the intestine of infected birds with Eimeria tenella and stained with hematoxylin and eosin showing (A) very extensive infection with many schizonts (sh) in the intestinal lumen associated with hemorrhages (h) and desquamated (d) epithelium (e); (B) Some parts of the intestine showed concomitant infection of the shizonts (sh) and nematodes parasites (p); (C) Higher magnification of the shizonts of (A) demonstrating its characteristic elliptical shape of the schizonts dispersed in remnants of the desquamated epithelial cells; (D) Intestinal mucosal epithelium showing intracellular stages of E. tenella (arrows) with infiltration with eosinophils in the surrounding tissue; (E) Macrogametes (m) of the parasite and other parasite stages (ps) in the epithelium together with eosinophils (es) infiltration; (F) Infected mucosal cells showing stages of the E. tenella parasite (ps), hemorrhages (h) and necrosis (n).
The intestinal mucosal epithelium exhibited intracellular stages of E. tenella, accompanied by eosinophilic infiltration in the surrounding tissue (Figure 9D). Macrogametes and other parasite stages were also observed in the epithelium, along with eosinophil infiltration (Figure 9E). Hemorrhage and necrosis were consistently observed in areas of infected mucosal cells (Figure 9F).
E. tenella stages in the infected intestinal mucosa showed a positive reaction to Giemsa staining (Figures 10A–C). Some sections of the intestine displayed minor pathological changes, primarily congestion of blood vessels and oedema in the serosal layer (Figure 10D). Secondary pathological changes were noted in other internal organs of the infected birds. The spleen exhibited a depletion of lymphocytes in splenic follicles and a thickening of follicular arteriole walls (Figure 10E).
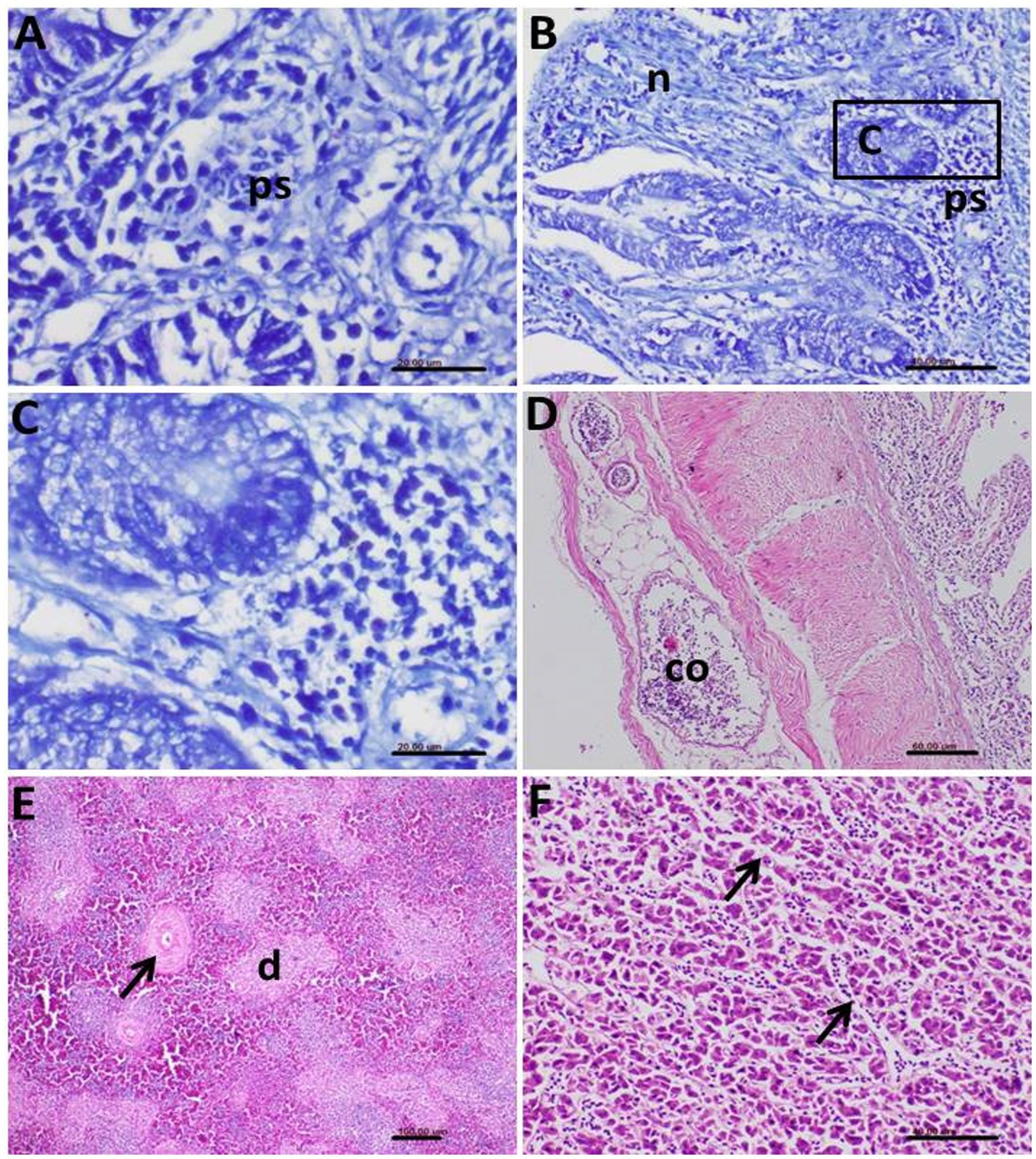
Figure 10. Photomicrograph of histopathological section of the intestine of infected birds with Eimeria tenella showing (A) Positively stained Eimeria stages (ps) by Giemsa stain; (B) Giemsa-stained parasite stages (ps) and prominent necrosis (n) of the intestinal epithelium; (C) Higher magnification of the previous photo showing many stages of the Eimeria and hyperactivity of the mucous glands; (D) Congestion (co) of the blood vessels and edema in the serosal layer of the infected intestine; (E) Spleen of the infected bird showing depletion (d) of the lymphocytes and thickening in the wall of the follicular arterioles (arrow); (F) Liver of the infected birds showing congestion of the hepatic sinusoids (arrows).
In the liver, congestion of hepatic sinusoids and multifocal areas of hepatocellular degeneration were common findings (Figure 10F). The severity and extent of these histopathological lesions correlated strongly with the clinical signs and gross pathological findings observed in the affected birds, providing clear evidence of the significant impact of the parasitic infections on the health of the helmeted guineafowl.
Discussion
The poultry industry is one of the fastest-growing sectors in global animal agriculture, playing a critical role in food security and economic development (52–54). Parasitic diseases remain a significant constraint to poultry health and production worldwide (11, 12). They contribute to substantial economic losses through reduced growth rates, poor feed conversion, decreased egg production, and increased mortality (55).
Parasitism is ubiquitous in wild birds, with individual birds typically hosting a diverse array of parasites throughout their lives (33, 56, 57). However, studying disease in free-ranging species presents unique challenges compared to research in humans or domestic birds (54, 58). These challenges include insufficient baseline data for host species, limited understanding of avian life cycle features, and difficulties in measuring disease-related variables (59). These limitations often hinder the calculation of fundamental epidemiological metrics such as prevalence, incidence, morbidity, and mortality rates, which are crucial for evaluating the impact of parasites on bird populations (57, 60). In the context of domestic poultry, parasitic co-infections pose a significant threat to the chicken industry, potentially causing substantial economic losses (41, 61).
Our study revealed that co-infection with A. galli and E. tenella resulted in a 10% mortality rate in the flock studied. This finding differs from that reported by Mousa et al. (41), who observed variable mortalities due to the parasitic coinfections in broiler and layer chickens in their study. The disparity in mortality rates could be attributed to differences in bird management practices, environmental conditions, or the genetic background of the chickens, highlighting the need for further research to elucidate factors influencing the severity of co-infections. A. galli may cause physical disruption to the intestinal wall and decrease immunity which predispose to E. tenella.
The parasites identified in our study, A. galli and E. tenella, are among the most common gastrointestinal parasites found in the avian gut, along with various cestodes and other Eimeria species (11, 12, 29, 62–64). These parasites have the capacity to modulate host immune responses; upregulating genes associated with cell-mediated immunity and stimulating humoral immune receptors. This immunomodulation can lead to the onset of mucosal immunity and various developmental stages of the parasites, which are typically observed during histopathological examination of affected tissues (65–68).
Our morphological analysis of A. galli eggs found in infected chicken excreta revealed oval, smooth-shelled eggs measuring 75–96 by 48–59 μm. These findings are consistent with those reported by Mousa et al. (41), who described un-embryonated, ellipsoidal eggs with thick, smooth outer shells measuring 65 to 85 × 49 to 55 μm. The slight variations in size ranges between studies may be due to differences in environmental conditions or host factors affecting parasite development.
The E. tenella oocysts identified in our study were characterized by their ovoid shape and double-layered wall, with sporulated oocysts measuring 25 (21–27) μm in length and 18 (15–29) μm in breadth. These dimensions are in close agreement with those reported by Mohamed et al. (69), Mares et al. (70), and Nana-Mariam et al. (71), further confirming the accuracy of our species’ identification.
The molecular characterization of A. galli and E. tenella isolates from wild birds in this study provides valuable insights into the genetic diversity and phylogenetic relationships of these parasites in natural ecosystems. The successful amplification and sequencing of both the ITS rDNA and COX1 regions for A. galli and the ITS and 18S rDNA regions for E. tenella allowed for a comprehensive genetic analysis of these important avian parasites in non-domestic settings.
For A. galli, the ITS rDNA sequences (PQ047113, PQ047114, PQ047115) and COX1 sequences (PQ106343, PQ106344, PQ106345) demonstrated high similarity to previously reported A. galli sequences, confirming their identity in wild bird populations. The observed intraspecific variations, though minor, highlight the genetic diversity within A. galli populations in natural environments. This diversity could be attributed to factors such as geographical isolation, adaptation to different wild bird species, or natural genetic drift (37, 72). The genetic variation observed in these wild isolates may differ from that seen in domestic poultry, potentially reflecting adaptations to diverse wild hosts or environmental conditions.
Phylogenetic analysis of both ITS and COX1 sequences consistently placed our A. galli isolates from wild birds within a well-supported monophyletic clade, closely related to other A. galli sequences. This robust clustering confirms the utility of these genetic markers for species identification across different host species and environments. The clear separation between Ascaridia and other genera like Heterakis in the phylogenetic trees underscores the effectiveness of these markers in resolving taxonomic relationships at both the genus and species levels, even in diverse wild bird populations.
For E. tenella, the ITS rDNA sequences (PQ047140, PQ047141, PQ047142) and 18S rDNA sequences (PQ142692, PQ142693, PQ142694) also demonstrated high similarity to known E. tenella sequences, confirming their identity in wild birds. The slight intraspecific variations observed suggest some level of genetic diversity within the E. tenella population studied. This diversity could be particularly important in wild bird populations, where parasite transmission dynamics and host–parasite interactions may differ significantly from those in domestic settings (73).
The phylogenetic analyses of both ITS and 18S rDNA sequences placed our E. tenella isolates from wild birds in well-supported clades with other E. tenella sequences. Comparison with global strains shows close relationships with domestic isolates but reveals subtle differentiation suggesting host adaptation to wild bird transmission cycles. The close phylogenetic relationships observed between our wild bird isolates and those from domestic poultry suggest that E. tenella may have a broad host range or that there might be frequent transmission between wild and domestic bird populations. This finding has important implications for the epidemiology and control of coccidiosis in both wild and domestic birds (74).
The molecular analysis revealed significant interspecific divergence between E. tenella and other Eimeria spp., demonstrating the differential discriminatory power of the two molecular markers used. For 18S rDNA, sequence similarities with other Eimeria spp. ranged from 93.47 to 97.82%, with the closest relationship observed with E. necatrix (98.80% similarity) and E. gallopavonis (97.83% similarity). In contrast, ITS rDNA showed much greater interspecific divergence, with E. necatrix similarities ranging only from 75.81 to 76.99%, highlighting the superior discriminatory power of ITS regions for species differentiation. These divergence patterns reflect the evolutionary constraints on different genomic regions, where the highly conserved 18S rDNA maintains higher similarities between species, while the more variable ITS regions better capture species-specific evolutionary signatures. The interspecific distance patterns exceed established taxonomic thresholds for Eimeria species boundaries (>2–3% for 18S rDNA, >5% for ITS), confirming robust molecular species delineation (75).
The consistent clustering of E. tenella sequences away from other species validates the complementary use of both ribosomal markers for accurate species identification and supports current taxonomic classifications (76). This is particularly important when studying parasites in diverse wild bird communities, where multiple Eimeria species may co-exist (77, 78).
Histopathological examination revealed severe pathological lesions caused by E. tenella in the intestinal mucosa, particularly in the colon. The observed lesions, including the presence of various developmental stages of the parasite in epithelial cells and the lamina propria, extensive hemorrhages, and desquamation of the epithelium, are consistent with the findings of Mohamed et al. (69) and Salem et al. (12). The concurrent presence of nematode parasites (A. galli) alongside E. tenella schizonts in some intestinal sections indicates the complexity of the co-infection and its potential for exacerbating tissue damage.
The histopathological changes observed in our study, including necrosis of intestinal mucosa, desquamated epithelial tissue in the intestinal lumen, and inflammatory reaction in the mucosa and submucosa, were closely parallel with the previous reports. These findings highlight the significant impact of parasitic co-infections on intestinal health and function, which likely contribute to the observed clinical signs and mortality. The presence of both A. galli and E. tenella in the same host raises important questions about potential interactions between these parasites. The co-occurrence of both parasites in histological sections suggests the possibility of synergistic or antagonistic effects. Future research should focus on elucidating the nature of these interactions and their implications for host health and parasite control strategies.
Conclusion
The current study provides valuable insights into the impact of A. galli and E. tenella co-infection in helmeted guineafowl. The observed mortality rate, coupled with the severe histopathological changes, underscores the significant threat posed by these parasites to poultry health and production. Future studies should focus on investigating potential interactions between A. galli and E. tenella, as well as exploring breed-specific susceptibilities to these parasites. Such research will be crucial for developing more effective strategies for preventing and controlling parasitic infections in poultry, ultimately contributing to improved bird health and economic outcomes in the poultry industry.
In addition, this study confirms the utility of ITS rDNA, COX1, and 18S rDNA as reliable genetic markers for the identification and phylogenetic analysis of A. galli and E. tenella in wild bird populations. The genetic diversity observed within these parasites underscores the importance of continued molecular surveillance in natural ecosystems to monitor for potential changes in parasite populations that could impact wild bird health or pose risks to domestic poultry. The genetic similarities between our wild bird isolates and those from domestic birds highlight the potential for cross-transmission and the need for integrated approaches to parasite management in both wild and domestic avian populations. Further studies incorporating samples from diverse wild bird species across various geographical locations could further elucidate the genetic structure, host specificity, and evolution of these important avian parasites in natural environments.
We recommend that chicken farmers and field veterinarians do regular fecal screenings and necropsy assessments to promptly identify co-infections, particularly in systems with insufficient biosecurity measures. To address dual-pathogen loads, anthelmintic and anticoccidial management strategies should be integrated rather than species-specific. This study provides valuable insights for enhancing guineafowl parasite management strategies and lays a foundation for future research on multi-parasite infections in other avian species.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The work was carried out according to the IACUC guidelines and was approved by the Ethical Committee, Faculty of Veterinary Medicine, Cairo University, Cairo, Egypt with code “Vet CU 8032022511.” The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
MMA: Writing – original draft, Writing – review & editing. MAM: Writing – review & editing, Methodology. MA: Methodology, Writing – review & editing, Writing – original draft. LA: Investigation, Writing – original draft, Resources, Writing – review & editing, Project administration. MAA: Writing – original draft, Writing – review & editing. SA: Methodology, Supervision, Writing – review & editing, Writing – original draft, Project administration. ME-S: Funding acquisition, Supervision, Writing – original draft, Software, Methodology, Formal analysis, Conceptualization, Investigation, Visualization, Data curation, Validation, Writing – review & editing, Resources, Project administration. KE-T: Writing – original draft, Writing – review & editing, Project administration, Validation, Supervision. HS: Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the UAEU Program for Advanced Research, grant number 12S169 to KE-T. Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2025R457), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. King Khalid University for funding this work through Large Research Project under grant number (R.G.P2/581/46).
Acknowledgments
The authors gratefully acknowledge Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2025R457), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. The authors extend their appreciation to the Deanship of Research and Graduate Studies at King Khalid University for funding this work through Large Research Project under grant number (R.G.P2/581/46).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ortúzar-Ferreira, CN , Dorna-Santos, L , de Oliveira, MS , de Lima, VM , Duszynski, DW , and Berto, BP . Coccidia of Guinea fowls: validity of recorded Eimeria spp. (Apicomplexa: Eimeriidae) and first molecular identification of Eimeria grenieri Yvoré & Aycardi, 1967. Parasitol Int. (2024) 103:102937. doi: 10.1016/j.parint.2024.102937
2. Shifaw, A , Feyera, T , Elliott, T , Sharpe, B , Walkden-Brown, SW , and Ruhnke, I . Comparison of the modified McMaster and Mini-FLOTAC methods for the enumeration of nematode eggs in egg spiked and naturally infected chicken excreta. Vet Parasitol. (2021) 299:109582. doi: 10.1016/j.vetpar.2021.109582
3. Feyera, T , Sharpe, B , Elliott, T , Shifaw, AY , Ruhnke, I , and Walkden-Brown, SW . Anthelmintic efficacy evaluation against different developmental stages of Ascaridia galli following individual or group administration in artificially trickle-infected chickens. Vet Parasitol. (2022) 301:109636. doi: 10.1016/j.vetpar.2021.109636
4. Shifaw, A , Ruhnke, I , Elliott, T , Sharpe, B , Feyera, T , and Walkden-Brown, SW . Ascaridia galli eggs obtained from fresh excreta, worm uteri or worms cultured in artificial media differ in embryonation capacity and infectivity. Vet Parasitol. (2022) 310:109792. doi: 10.1016/j.vetpar.2022.109792
5. El-Saied, MA , Attia, MM , Ibrahim, MA , Elaish, M , and Mousa, MR . Morphomolecular identification of heavy parasitic typhlitis in layer flocks: tissue response and cell-mediated reaction. Acta Vet Scand. (2024) 66:27. doi: 10.1186/s13028-024-00748-8
6. Jansson, DS , Nyman, A , Vågsholm, I , Christensson, D , Göransson, M , Fossum, O, et al. Ascarid infections in laying hens kept in different housing systems. Avian Pathol. (2010) 39:525–32. doi: 10.1080/03079457.2010.527923
7. Sherwin, CM , Nasr, MA , Gale, E , Petek, M , Stafford, K , Turp, M, et al. Prevalence of nematode infection and faecal egg counts in free-range laying hens: relations to housing and husbandry. Br Poult Sci. (2013) 54:12–23. doi: 10.1080/00071668.2012.757577
8. Thapa, S , Thamsborg, SM , Meyling, NV , Dhakal, S , and Mejer, H . Survival and development of chicken ascarid eggs in temperate pastures. Parasitology. (2017) 144:1243–52. doi: 10.1017/S0031182017000555
9. Wongrak, K , Gauly, M , and Daş, G . Diurnal fluctuations in nematode egg excretion in naturally and in experimentally infected chickens. Vet Parasitol. (2015) 208:195–203. doi: 10.1016/j.vetpar.2015.01.020
10. Quiroz-Castañeda, RE , and Dantán-González, E . Control of avian coccidiosis: future and present natural alternatives. Biomed Res Int. (2015) 2015:430610. doi: 10.1155/2015/430610
11. Salem, HM , Khattab, MS , Yehia, N , Abd El-Hack, ME , El-Saadony, MT , Alhimaidi, AR, et al. Morphological and molecular characterization of Ascaridia columbae in the domestic pigeon (Columba livia domestica) and the assessment of its immunological responses. Poult Sci. (2022a) 101:101596. doi: 10.1016/j.psj.2021.101596
12. Salem, HM , Salaeh, NM , Ragni, M , Swelum, AA , Alqhtani, AH , Abd El-Hack, ME, et al. Incidence of gastrointestinal parasites in pigeons with an assessment of the nematocidal activity of chitosan nanoparticles against Ascaridia columbae. Poult Sci. (2022b) 101:101820. doi: 10.1016/j.psj.2022.101820
13. Wuthijaree, K , Lambertz, C , and Gauly, M . Prevalence of gastrointestinal helminth infections in free-range laying hens under mountain farming production conditions. Br Poult Sci. (2017) 58:649–55. doi: 10.1080/00071668.2017.1379049
14. Joyner, LP , and Long, PL . The specific characters of the Eimeria, with special reference to the coccidia of the fowl. Avian Pathol. (1974) 3:145–57. doi: 10.1080/03079457409353827
15. Tarbiat, B , Rahimian, S , Jansson, DS , Halvarsson, P , and Höglund, J . Developmental capacity of Ascaridia galli eggs is preserved after anaerobic storage in faeces. Vet Parasitol. (2018) 255:38–42. doi: 10.1016/j.vetpar.2018.03.025
16. Tarbiat, B , Jansson, DS , and Höglund, J . Environmental tolerance of free-living stages of the poultry roundworm Ascaridia galli. Vet Parasitol. (2015) 209:101–7. doi: 10.1016/j.vetpar.2015.01.024
17. Rahimian, S , Gauly, M , and Daş, G . Embryonation ability of Ascaridia galli eggs isolated from worm uteri or host faeces. Vet Parasitol. (2016) 215:29–34. doi: 10.1016/j.vetpar.2015.10.026
18. Gilbert, ER , Cox, CM , Williams, PM , McElroy, AP , Dalloul, RA , Ray, WK, et al. Eimeria species and genetic background influence the serum protein profile of broilers with coccidiosis. PLoS One. (2011) 6:e14636. doi: 10.1371/journal.pone.0014636
19. Williams, RB . Anticoccidial vaccines for broiler chickens: pathways to success. Avian Pathol. (2002) 31:317–53. doi: 10.1080/03079450220148988
20. Zhang, P , Xue, M , Gong, J , Lei, W , and Guo, D . Coccidiostat activity of Mahonia bealei (fort.) leaves extract against Eimeria tenella in chickens. Pak Vet J. (2024) 44:292–7. doi: 10.29261/pakvetj/2024.176
21. Williams, RB . Intercurrent coccidiosis and necrotic enteritis of chickens: rational, integrated disease management by maintenance of gut integrity. Avian Pathol. (2005) 34:159–80. doi: 10.1080/03079450500112195
22. El-Shall, NA , Abd El-Hack, ME , Albaqami, NM , Khafaga, AF , Taha, AE , Swelum, AA, et al. Phytochemical control of poultry coccidiosis: a review. Poult Sci. (2022) 101:101542. doi: 10.1016/j.psj.2021.101542
23. Ahmad, S , Rizwan, M , and Saeed, Z . Alternative therapeutic strategies for histomonosis: a review. Int J Agricult Biosci. (2022) 11:238–45. doi: 10.47278/journal.ijab/2022.032
24. Xu, L , Xiang, Q , Li, M , Sun, X , Lu, M , Yan, R, et al. Pathogenic effects of single or mixed infections of Eimeria mitis, Eimeria necatrix, and Eimeria tenella in chickens. Vet Sci. (2022) 9:657. doi: 10.3390/vetsci9120657
25. Sandberg, FB , Emmans, GC , and Kyriazakis, I . The effects of pathogen challenges on the performance of naïve and immune animals: the problem of prediction. Animal. (2007) 1:67–86. doi: 10.1017/S175173110765784X
26. Moraes, PO , Andretta, I , Cardinal, KM , Ceron, M , Vilella, L , Borille, R, et al. Effect of functional oils on the immune response of broilers challenged with Eimeria spp. Animal. (2019) 13:2190–8. doi: 10.1017/S1751731119000600
27. Kim, E , Létourneau-Montminy, MP , Lambert, W , Chalvon-Demersay, T , and Kiarie, EG . Centennial review: a meta-analysis of the significance of Eimeria infection on apparent ileal amino acid digestibility in broiler chickens. Poult Sci. (2022) 101:101625. doi: 10.1016/j.psj.2021.101625
28. Kipper, M , Andretta, I , Lehnen, CR , Lovatto, PA , and Monteiro, SG . Meta-analysis of the performance variation in broilers experimentally challenged by Eimeria spp. Vet Parasitol. (2013) 196:77–84. doi: 10.1016/j.vetpar.2013.01.013
29. Aljohani, ASM . Phenolics of botanical origin for the control of coccidiosis in poultry. Pak Vet J. (2024) 44:222–8. doi: 10.29261/pakvetj/2024.179
30. Chapman, HD . Milestones in avian coccidiosis research: a review. Poult Sci. (2014) 93:501–11. doi: 10.3382/ps.2013-03634
31. Reid, AJ , Blake, DP , Ansari, HR , Billington, K , Browne, HP , Bryant, J, et al. Genomic analysis of the causative agents of coccidiosis in domestic chickens. Genome Res. (2014) 24:1676–85. doi: 10.1101/gr.168955.113
32. Bangoura, B , and Daugschies, A . Eimeria In: M Florin-Christensen and L Schnittger , editors. Parasitic protozoa of farm animals and pets. Cham, Switzerland: Springer (2018). 55–102. doi: 10.1007/978-3-319-70132-5
33. Attia, MM , Mohamed, RI , and Salem, HM . Impact of Eimeria tenella experimental infection on intestinal and splenic reaction of broiler chickens. J Parasit Dis. (2023) 47:829–36. doi: 10.1007/s12639-023-01629-z
34. López-Osorio, S , Chaparro-Gutiérrez, JJ , and Gómez-Osorio, LM . Overview of poultry Eimeria life cycle and host–parasite interactions. Front Vet Sci. (2020) 7:384. doi: 10.3389/fvets.2020.00384
35. Schwarz, RS , Jenkins, MC , Klopp, S , and Miska, KB . Genomic analysis of Eimeria spp. populations in relation to performance levels of broiler chicken farms in Arkansas and North Carolina. J Parasitol. (2009) 95:871–80. doi: 10.1645/GE-1898.1
36. Schwarz, A. (2011) The influence of non-starch-polysaccharides on experimental infections with Ascaridia galli and Heterakis gallinarum in layer chicken (Gallus gallus domesticus). Ph. D. thesis, University of Veterinary Medicine Hanover, Germany.
37. Malatji, DP , van Marle-Koster, E , and Muchadeyi, FC . Gene expression profiles of the small intestine of village chickens from an Ascaridia galli infested environment. Vet Parasitol. (2019) 276:100012. doi: 10.1016/j.vpoa.2019.100012
38. Ngongeh, LA , Ugwuzor, EG , and Fakae, BB . Consequences of concurrent infections with Ascaridia galli and Eimeria in broiler chickens. Asian J Appl Sci. (2019) 7:55–62. doi: 10.24203/ajas.v7i1.5477
39. Latchumikanthan, A , Thirumavalavan, R , Ilayabarathi, D , Vengadabady, N , Vijayasarathi, MK , and Meenakshisundaram, A . Concurrent infection of intestinal coccidiosis and Ascaridia galli in native Aseel chickens (Gallus domesticus) from Villupuram district, Tamil Nadu, India. Exploratory Anim Med Res. (2021) 11:127–30. doi: 10.52635/EAMR/11.1.127-130
40. Onyeabor, AI , Uwalaka, EC , Onunkwo, DC , and Ugwuzor, E . A comparative study on the concurrent infections with Ascaridia galli and Eimeria in broiler chickens. Niger J Anim Prod. (2023) 50:152–62. doi: 10.51791/njap.v50i2.3973
41. Mousa, MR , Attia, MM , Salem, HM , Al-Hoshani, N , Thabit, H , Ibrahim, MA, et al. Coinfection of the gut with protozoal and metazoal parasites in broiler and laying chickens. Poult Sci. (2024) 103:103227. doi: 10.1016/j.psj.2023.103227
42. Berhe, M , Mekibib, B , Bsrat, A , and Atsbaha, G . Gastrointestinal helminth parasites of chicken under different management system in Mekelle town, Tigray region, Ethiopia. J Vet Med. (2019) 2019:1307582. doi: 10.1155/2019/1307582
43. Razmi, GR , and Kalideri, GA . Prevalence of subclinical coccidiosis in broiler-chicken farms in the municipality of Mashhad, Khorasan, Iran. Prev Vet Med. (2000) 44:247–53. doi: 10.1016/S0167-5877(00)00105-7
44. Long, PL , and Rose, ME . Prospects for the control of coccidiosis by immunization. World Poult Sci J. (1982) 38:85–96. doi: 10.1079/WPS19820007
45. Permin, A , and Hansen, JW . Poultry and parasites In: Epidemiology, diagnosis and control of poultry parasites. Rome: Food and Agriculture Organization of the United Nations (1998). 160 p.
46. Soulsby, EJL . Advances in immunoparasitology. Vet Parasitol. (1985) 18:303–19. doi: 10.1016/0304-4017(85)90066-4
47. Soulsby, EJL . Helminths, arthropods and Protozoa of domesticated animals. 7th ed. Reading: Baillière Tindall (1982). 809 p.
48. Salem, HM , and Attia, MM . Accidental intestinal myiasis caused by Musca domestica L. (diptera: muscidae) larvae in broiler chickens: a field study. Int J Trop Insect Sci. (2021) 41:2549–54. doi: 10.1007/s42690-021-00492-w
49. Luton, K , Walker, D , and Blair, D . Comparisons of ribosomal internal transcribed spacers from two congeneric species of flukes (Platyhelminthes: Trematoda: Digenea). Mol Biochem Parasitol. (1992) 56:323–7. doi: 10.1016/0166-6851(92)90181-I
50. Tamura, K , Stecher, G , and Kumar, S . MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol. (2021) 38:3022–7. doi: 10.1093/molbev/msab120
51. Suvarna, SK , Layton, C , and Bancroft, JD . Bancroft’s theory and practice of histological techniques. Eighth Edition, Amsterdam, Netherlands: Elsevier. (2018). 584 p.
52. Hartady, T , Syamsunarno, MRA , Priosoeryanto, BP , Jasni, S , and Balia, RL . Review of herbal medicine works in the avian species. Vet World. (2021) 14:2889–906. doi: 10.14202/vetworld.2021.2889-2906
53. Almahallawi, R , Al-Hoshani, N , Al-Nabati, E , Althubyani, SA , Negm, S , El-Ikott, AF, et al. Exploring the anticoccidial, growth-promoting, hematological, and serological potential activities of Linum usitatissimum essential oil in broiler birds. Pak Vet J. (2024) 44:117–22. doi: 10.29261/pakvetj/2024.137
54. Aruwa, CE , and Sabiu, S . Interplay of poultry–microbiome interactions–influencing factors and microbes in poultry infections and metabolic disorders. Br Poult Sci. (2024) 65:523–37. doi: 10.1080/00071668.2024.2356666
55. Kunwar, K . Gastrointestinal parasites of turkey (Meleagris gallopavo Linnaeus, 1758) in Nagarjun turkey farm, Kathmandu, Nepal. Ph.D. thesis, Tribhuvan University, Kirtipur, Nepal. (2023)
56. Abbas, A , and Alkheraije, KA . Immunomodulatory effects of Carica papaya extract against experimentally induced coccidiosis in broiler chickens. Pak Vet J. (2023) 43:628–32. doi: 10.29261/pakvetj/2023.089
57. Rebelo, CF , Ruiz, AC , Alvarado-Piqueras, A , González, FG , and Carvalho, LMD . Parasite screening in wild passerines: enhancing diagnostic approaches in wildlife rehabilitation centers. Animals. (2024) 14:3664. doi: 10.3390/ani14243664
58. Valdebenito, JO , Jones, W , and Székely, T . Evolutionary drivers of sex-specific parasite prevalence in wild birds. Proc R Soc B. (2024) 291:20241013. doi: 10.1098/rspb.2024.1013
59. Zera, AJ , and Harshman, LG . The physiology of life history trade-offs in animals. Annu Rev Ecol Syst. (2001) 32:95–126. doi: 10.1146/annurev.ecolsys.32.081501.114006
60. Ombugadu, A , Ayasi, BO , Ahmed, HO , Aliyu, AA , Aimankhu, OP , Uzoigwe, NR, et al. A post-mortem evaluation of coccidiosis and helminthiasis of poultry birds slaughtered at Lafia ultra modern market, Lafia, Nasarawa state, Nigeria. Trans Sci Technol. (2021) 8:654–66.
61. Bettridge, J. (2014) The epidemiology and ecology of infectious diseases in Ethiopian village chickens and the role of co-infection in infection risk. Ph.D. thesis, University of Liverpool, Liverpool, United Kingdom.
62. Lawal, JR , Hambali, IU , Jajere, SM , Bello, AM , Biu, AA , and Musa, G . Survey and prevalence of gastrointestinal cestodes in village chickens (Gallus gallus domesticus) slaughtered in Gombe metropolis poultry dressing slabs. Int J Livest Res. (2015) 5:21–8. doi: 10.5455/ijlr.20151217082347
63. Vermeulen, AN . Avian coccidiosis: A disturbed host-parasite relationship to be restored. Symp Soc Exp Biol. (2004) 55:211–41.
64. Rasheed, M , and Aljohani, ASM . Evaluation of anthelmintic effects of essential oil of star anise against Ascaridia galli of poultry. Pak Vet J. (2024) 44:1223–8. doi: 10.29261/pakvetj/2024.294
65. Adamu, M , Boonkaewwan, C , Gongruttananun, N , and Vongpakorn, M . Hematological, biochemical and histopathological changes caused by coccidiosis in chickens. Agricult Nat Resour. (2013) 47:238–46.
66. Hiob, L , Koethe, M , Schares, G , Goroll, T , Daugschies, A , and Bangoura, B . Experimental toxoplasma gondii and Eimeria tenella co-infection in chickens. Parasitol Res. (2017) 116:3189–203. doi: 10.1007/s00436-017-5636-2
67. Stehr, M , Grashorn, M , Dannenberger, D , Tuchscherer, A , Gauly, M , Metges, CC, et al. Resistance and tolerance to mixed nematode infections in relation to performance level in laying hens. Vet Parasitol. (2019) 275:108925. doi: 10.1016/j.vetpar.2019.108925
68. Yan, C , Zhong, Q , Jin, W , Yang, Y , Wang, Z , and Peng, D . Epidemiological study of the co-infection of immunosuppressive poultry pathogens in tissue samples of chickens in Jiangsu Province, China from 2016 to 2022. Pak Vet J. (2024) 44:330–5. doi: 10.29261/pakvetj/2024.161
69. Mohamed, SE , Dyab, AK , Mohamed, SA , and Abd-Elrahman, S . Prevalence of coccidiosis in chicken in Sohage governorate. Assiut Vet Med J. (2021) 67:1–11. doi: 10.21608/avmj.2021.205152
70. Mares, MM , Al-Quraishy, S , Abdel-Gaber, R , and Murshed, M . Morphological and molecular characterization of Eimeria spp. infecting domestic poultry Gallus gallus in Riyadh city, Saudi Arabia. Microorganisms. (2023) 11:795. doi: 10.3390/microorganisms11030795
71. Nana-Mariam, A , Suleiman, AI , Ovaino, AS , Scholastica, A , Onyaweyo, OSA , Ameh, AV, et al. Prevalence of avian coccidiosis and identification of Eimeria spp. in local broilers and chickens in Lafia modern market, Nassarawa state, Nigeria. EAS J Parasitol Infect Dis. (2023) 5:23–35. doi: 10.36349/easjpid.2023.v05i03.001
72. Teng, PY , Choi, J , Tompkins, Y , Lillehoj, H , and Kim, W . Impacts of increasing challenge with Eimeria maxima on the growth performance and gene expression of biomarkers associated with intestinal integrity and nutrient transporters. Vet Res. (2021) 52:81. doi: 10.1186/s13567-021-00949-3
73. Dolnik, OV , Palinauskas, V , and Bensch, S . Individual oocysts of Isospora (Apicomplexa: Coccidia) parasites from avian feces: from photo to sequence. J Parasitol. (2009) 95:169–74. doi: 10.1645/GE-1873.1
74. Clark, EL , Macdonald, SE , Thenmozhi, V , Kundu, K , Garg, R , Kumar, S, et al. Cryptic Eimeria genotypes are common across the southern but not northern hemisphere. Int J Parasitol. (2016) 46:537–44. doi: 10.1016/j.ijpara.2016.05.006
75. Blake, DP , Clark, EL , Macdonald, SE , Thenmozhi, V , Kundu, K , Garg, R, et al. Population, genetic, and antigenic diversity of the apicomplexan Eimeria tenella and their relevance to vaccine development. Proc Natl Acad Sci USA. (2015) 112:E5343–50. doi: 10.1073/pnas.1506468112
76. Jenkins, MC , Parker, C , O'Brien, C , Miska, K , and Fetterer, R . Differing susceptibilities of Eimeria acervulina, Eimeria maxima, and Eimeria tenella oocysts to desiccation. J Parasitol. (2013) 99:899–902. doi: 10.1645/13-192.1
77. Clark, EL , and Blake, DP . Genetic mapping and coccidial parasites: past achievements and future prospects. J Biosci. (2012) 37:879–86. doi: 10.1007/s12038-012-9251-1
Keywords: clinical examination, helmeted guineafowl, molecular surveillance, parasitological analysis, poultry parasites, phylogenetic analysis
Citation: Attia MM, Mahmoud MA, Abdelsalam M, Almutairi LA, Alqahtani MA, Areshi SM, El-Saadony MT, El-Tarabily KA and Salem HM (2025) Molecular and histopathological characterization of Ascaridia galli and Eimeria tenella co-infection in Numida meleagris. Front. Vet. Sci. 12:1622170. doi: 10.3389/fvets.2025.1622170
Edited by:
Xiaokai Song, Nanjing Agricultural University, ChinaReviewed by:
Mughees Aizaz Alvi, University of Agriculture, Faisalabad, PakistanAbdullah Inci, Erciyes University, Türkiye
Copyright © 2025 Attia, Mahmoud, Abdelsalam, Almutairi, Alqahtani, Areshi, El-Saadony, El-Tarabily and Salem. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Khaled A. El-Tarabily, a3RhcmFiaWx5QHVhZXUuYWMuYWU=
 Marwa M. Attia1
Marwa M. Attia1 Mohamed T. El-Saadony
Mohamed T. El-Saadony Khaled A. El-Tarabily
Khaled A. El-Tarabily Heba M. Salem
Heba M. Salem
