- Department of Anatomy, College of Medicine, Taif University, Taif, Saudi Arabia
Background: Glyphosate, the most widely used herbicide globally, accumulates in renal tissue causing kidney damage through incompletely understood mechanisms. This study evaluated quercetin’s nephroprotective effect against glyphosate-induced kidney injury in rats.
Methods: Five groups of male Wistar rats (n = 10 each) received daily treatments for 21 days: control, glyphosate (25 mg/kg), quercetin (50 mg/kg), and quercetin+glyphosate at low (25 mg/kg) or high (50 mg/kg) doses. All treatments were administered by oral gavage for 21 days. Renal parameters, oxidative stress markers, inflammatory mediators, and apoptotic indicators were assessed using spectrophotometric assays, ELISA, qRT-PCR, and histology.
Results: Glyphosate impaired renal function, increased kidney weight, and elevated kidney injury molecule-1 (KIM-1) levels. It suppressed antioxidant enzymes (CAT, SOD, GPX) and downregulated their mRNA expression (Cat, Sod2, and Gpx-1, respectively), while depleting GSH and increasing oxidative markers (MDA, NO). Notably, glyphosate reduced Nrf2 protein and Nfe2l2 gene expression, disrupting this master regulator of antioxidant responses, with concurrent Hmox-1 downregulation. Glyphosate upregulated pro-inflammatory cytokines (TNF-α, IL-1β, IL-6), increased TLR-4 and NOS2 expression, activated mitochondrial apoptosis by increasing pro-apoptotic proteins (BAX, CYTOCHROME C, and CASPASE-3) while decreasing anti-apoptotic BCL-2 protein levels, with corresponding changes in gene expression. Consistent with protein findings, Bcl-2 gene expression was significantly downregulated, further confirming the shift toward pro-apoptotic signaling. Quercetin dose-dependently attenuated these alterations, with high-dose providing superior protection compared to low-dose by restoring gene expression and enzyme activities. Histopathological examination confirmed quercetin mitigated glyphosate-induced tubular degeneration and glomerular atrophy.
Conclusion: Quercetin protects against glyphosate nephrotoxicity through antioxidative, anti-inflammatory, and anti-apoptotic mechanisms, suggesting therapeutic potential against herbicide-induced kidney injury.
1 Introduction
Nephrotoxicity represents a significant global health concern characterized by deteriorating renal function, resulting in metabolic waste accumulation, electrolyte imbalances, and increased risk of renal failure (1). Multiple factors contribute to kidney injury, including ischemia–reperfusion injury, nephrotoxic medications, sepsis, and environmental toxicants (2). Among environmental xenobiotics, glyphosate [N-(phosphonomethyl) glycine], the world’s most widely used herbicide, has emerged as a concerning potential nephrotoxic agent with increasing clinical relevance (3).
Glyphosate is extensively used in agriculture for weed control in crops like soybean, corn, and cotton, as well as in non-agricultural settings for vegetation management (4). In many ecosystems, substances like glyphosate accumulate through the food chain, ultimately affecting human health through chronic low-dose exposure (5).
Given its preferential accumulation in the kidneys more so than in the spleen, liver, or neural tissue (5), glyphosate raises particular concern for nephrotoxicity, especially during sub-acute (6) and sub-chronic exposure (7) scenarios.
The precise mechanisms of glyphosate-induced nephrotoxicity remain incompletely understood. However, emerging evidence suggests oxidative stress plays a pivotal role in the pathogenesis of glyphosate-induced renal injury (8). Prolonged exposure suppresses endogenous antioxidant defense systems [catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPX)] while enhancing reactive oxygen species (ROS) production. This persistent oxidative imbalance leads to lipid peroxidation, protein oxidation, and DNA damage, culminating in renal cellular dysfunction and death (9).
Additionally, glyphosate exposure activates inflammatory pathways, notably through Toll-like receptor 4 (TLR-4) signaling, resulting in pro-inflammatory cytokine production [tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6)] (10). This inflammatory cascade exacerbates renal injury through progressive leukocyte infiltration and tubular damage [15]. The mitochondrial apoptotic pathway, characterized by altered Bcl-2-associated X protein (BAX)/ B-cell lymphoma 2 (BCL-2) ratio, CYTOCHROME C release, and CASPASE-3 activation, has been implicated in glyphosate-induced renal cell death during continuous exposure (11).
Given widespread glyphosate exposure and limited treatments for chemical-induced nephrotoxicity, effective interventions were urgently needed. Natural antioxidants have gained significant attention for mitigating nephrotoxicity by modulating oxidative stress, inflammation, and apoptotic signaling (12). Quercetin (3,3′,4′,5,7-pentahydroxyflavone), a plant-derived flavonoid, demonstrates potent antioxidant, anti-inflammatory, and anti-apoptotic properties (13).
Quercetin’s protective effects stem from free radical scavenging and enhancement of endogenous antioxidant defenses, while also regulating inflammatory mediators and inhibiting inflammatory pathways (14). Previous studies have shown quercetin has protective effects against liver injury (15), kidney injury (16), and other model diseases such arteriosclerosis (14), but its potential against glyphosate-induced nephrotoxicity and underlying molecular mechanisms remain largely unexplored.
Recent evidence suggests the interaction between oxidative stress and inflammatory pathways represents a critical axis in xenobiotic-induced kidney injury (17), creating a self-amplifying cycle leading to mitochondrial dysfunction, apoptosis, and renal impairment (18). This study aimed to investigate quercetin’s nephroprotective effects against glyphosate-induced kidney injury in rats exposed for 3 weeks, hypothesizing that quercetin would attenuate renal damage by modulating oxidative stress, inflammatory responses, and apoptotic pathways during sub-acute exposure.
2 Materials and methods
2.1 Experimental animals
Fifty male Wistar rats (150–200 g) were purchased from King Fahd Medical Research Center (KFMRC), King Abdulaziz University, Saudi Arabia. Before the start of the experiment, animals were acclimatized for 1 week under standard conditions. Rats were housed in plastic cages at room temperature (22–25°C) with a 12-h light/dark cycle, and had free access to standard laboratory diet and water.
2.2 Chemicals
Glyphosate (CAS No. 1071-83-6; Cat: 337757) and Quercetin (CAS No. 117–39-5; Cat: Q4951) were purchased from Sigma-Aldrich Co. LLC., St. Louis, MO, USA and freshly prepared in distilled water prior to administration.
2.3 Animal grouping and treatment
A total of 50 male Wistar rats were randomly divided into five groups (n = 10 per group). All treatments were administered once daily via oral gavage for 21 consecutive days. The groupings were as follows:
Group 1 (Control group, Control): Rats received 0.9% physiological saline intraperitoneally as a vehicle control.
Group 2 (Glyphosate group, Glyphosate): Rats received glyphosate at a dose of 25 mg/kg/day (19) to induce sub-acute nephrotoxicity.
Group 3 (Quercetin group, Quercetin): Rats received quercetin alone (50 mg/kg/day) (20) to assess its impact on normal renal function and exclude any intrinsic toxicity of the high dose.
Group 4 (Quercetin low-dose + Glyphosate group, Que-25 + Glyp): Rats received quercetin (25 mg/kg/day) (21), administered 1 h prior to glyphosate injection to evaluate the protective effect of a low dose.
Group 5 (Quercetin high-dose + Glyphosate group, Que-50 + Glyp): Rats received quercetin (50 mg/kg/day), administered 1 h prior to glyphosate injection to assess the dose-dependent protective effects against glyphosate-induced renal damage.
Sample size was determined based on previous similar toxicological studies evaluating nephroprotective agents in rats, with consideration of anticipated effect size, variability, and statistical power. A group size of 10 animals per group was selected to ensure sufficient power (80%) to detect significant differences with a confidence level of 95% and considering potential data loss.
2.4 Anesthesia and sample collection
On day 22, animals were anesthetized with thiopental sodium (100 mg/kg, intraperitoneally). Adequate anesthesia was confirmed by the absence of the pedal withdrawal reflex. Blood samples were collected immediately via cardiac puncture using sterile 5 mL syringes with 21-gage needles under aseptic conditions (as it enables sufficient terminal blood collection for biochemical and molecular analyses). The animals were then euthanized by cervical decapitation. Kidneys were excised, rinsed with ice-cold saline, and processed for histological, biochemical, and molecular analyses. Blood samples were allowed to clot at room temperature for 30 min and centrifuged at 3000 rpm for 10 min. The obtained serum was separated and stored at −80°C until biochemical analysis.
2.5 Serum renal function tests
Renal function was assessed by measuring serum creatinine, urea, and uric acid, using commercially available diagnostic kit (Randox Laboratories Ltd., Crumlin, County Antrim, UK). All assays were performed according to the manufacturers’ protocols.
2.6 Determination of kidney weight
The relative kidney weight was calculated according to the method described by Almeer et al. (22). To ensure consistency across all samples, the left kidney was selected for weight measurement. The right kidney was preserved for histopathological and molecular analyses requiring intact tissue integrity.
2.7 Relative kidney weight
Relative kidney weight was calculated using the formula: (left kidney weight / body weight) × 100.
2.8 Renal injury biomarkers
Kidney Injury Molecule-1 (KIM-1), a highly sensitive early biomarker of proximal tubular damage, was measured in kidney tissue homogenates using ELISA kit (Elabscience Biotechnology Inc., Houston, TX, USA) according to the manufacturer’s instructions.
2.9 Redox status determination
2.9.1 Oxidative stress markers
Oxidative stress markers were assessed in kidney tissue homogenates to evaluate the extent of cellular damage induced by glyphosate exposure. Malondialdehyde (MDA) levels were determined the procedure established by Ohkawa et al. (23). Reduced glutathione (GSH) content was determined using the method outlined by Ellman (24). Nitric oxide (NO) content in renal samples was measured by Griess reagent (25).
2.9.2 Antioxidant enzyme activities
To assess the antioxidant defense system, several key enzymatic activities were measured. SOD activity was evaluated using the technique described by Nishikimi et al. (26). CAT activity was measured using the method of Lück (27). GPX activity was assessed using the technique described by Paglia and Valentine (28). The level of glutathione reductase (GR,) was assessed using the technique described by Moron et al. (29). Nuclear factor erythroid 2-related factor 2 (Nrf2) levels were quantified using ELISA kit (Elabscience Biotechnology Inc., Houston, TX, USA) according to the manufacturer’s instructions.
2.10 Inflammatory cytokines assessment
Levels of tumor necrosis factor-alpha in kidney tissue homogenates were quantified using commercial ELISA kits (TNF-α), IL-1β, and IL-6 (Elabscience Biotechnology Inc., Houston, TX, USA), according to the manufacturer’s instructions.
2.11 Apoptotic markers
Apoptotic proteins in kidney tissue were quantified using commercial ELISA kits. BCL-2 and BAX levels were measured with kits from BioVision Inc., Milpitas, CA, USA and expressed as ng mg−1 protein. CASPASE-3 activity was determined with a colorimetric assay kit from BioVision Inc., Milpitas, CA, USA, while CYTOCHROME C levels were assessed using an ELISA kit from Elabscience Biotechnology Inc., Houston, TX, USA (expressed as nmol mg−1 protein) to evaluate mitochondrial integrity. All assays were performed in accordance with the respective manufacturers’ protocols.
2.12 Gene expression analysis
Total RNA was extracted from kidney tissue samples using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. RNA purity and concentration were determined spectrophotometrically using NanoDrop (Thermo Fisher Scientific, Waltham, MA, USA) by measuring absorbance at 260/280 nm. Complementary DNA (cDNA) was synthesized from 1 μg of total RNA using RevertAid™ H Minus Reverse Transcriptase (30) following the manufacturer’s protocol. Quantitative real-time PCR (qRT-PCR) was performed using QuantiFast SYBR Green PCR kit (Qiagen, Hilden, Germany) on an Applied Biosystems 7,500 system (Thermo Fisher Scientific, CA, USA). The PCR cycling conditions included initial denaturation at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 15 s and annealing/extension at 60°C for 60s. All reactions were conducted in triplicate, and the relative gene expression levels were calculated using the 2^–ΔΔCt method with β-actin (Actb) as the internal control (30). Target genes analyzed included antioxidant response markers (Nfe2l2, Hmox-1, Sod2, Cat, GPx-1), inflammatory markers (TLR-4, TNF-α, IL-6, IL-1β), antioxidative stress markers (nitric oxide synthase NOS2), and apoptotic markers (Bax, Bcl-2, Caspase-3, Cycs). Primer sequences for all genes were listed in Table 1.
2.13 Histopathological examination
Kidney samples were fixed in 10% formalin for 24 h, dehydrated through graded ethanol (70, 80, 90, and 100% for 5 min each), cleared in xylene, embedded in paraffin wax, sectioned at 5 μm, and stained with hematoxylin and eosin (H&E) (31). The stained sections were evaluated under a light microscope (Nikon Eclipse E200) to assess pathological changes.
Histopathological evaluation: Renal tissue sections were scored semi-quantitatively in a blinded manner using the Endothelial–Glomerular–Tubular–Interstitial (EGTI) scoring system, as previously described by Toprak et al. (32). Lesions were graded on a 0–3 scale for each component (0 = none, 1 = mild, 2 = moderate, 3 = severe, 4 = very severe). Three non-overlapping fields per section were examined at 400 × magnification by a blinded pathologist. Data were presented as mean ± standard deviation (SD). Statistical significance was assessed using one-way ANOVA followed by Tukey’s post hoc test for multiple comparisons.
2.14 Statistical analysis
All data were presented as mean ± SD. Statistical comparisons among experimental groups were conducted using one-way analysis of variance (ANOVA), followed by Tukey’s post hoc test to determine pairwise differences. Statistical analyses were performed using SPSS software (version 16.0). A p-value < 0.05 was considered statistically significant. All biochemical assays were conducted in triplicates to ensure accuracy and reproducibility. Statistical notation: # indicates significant difference vs. control; $ indicates significant difference vs. glyphosate (p < 0.05).
3 Results
3.1 Kidney weight and relative kidney weight assessment
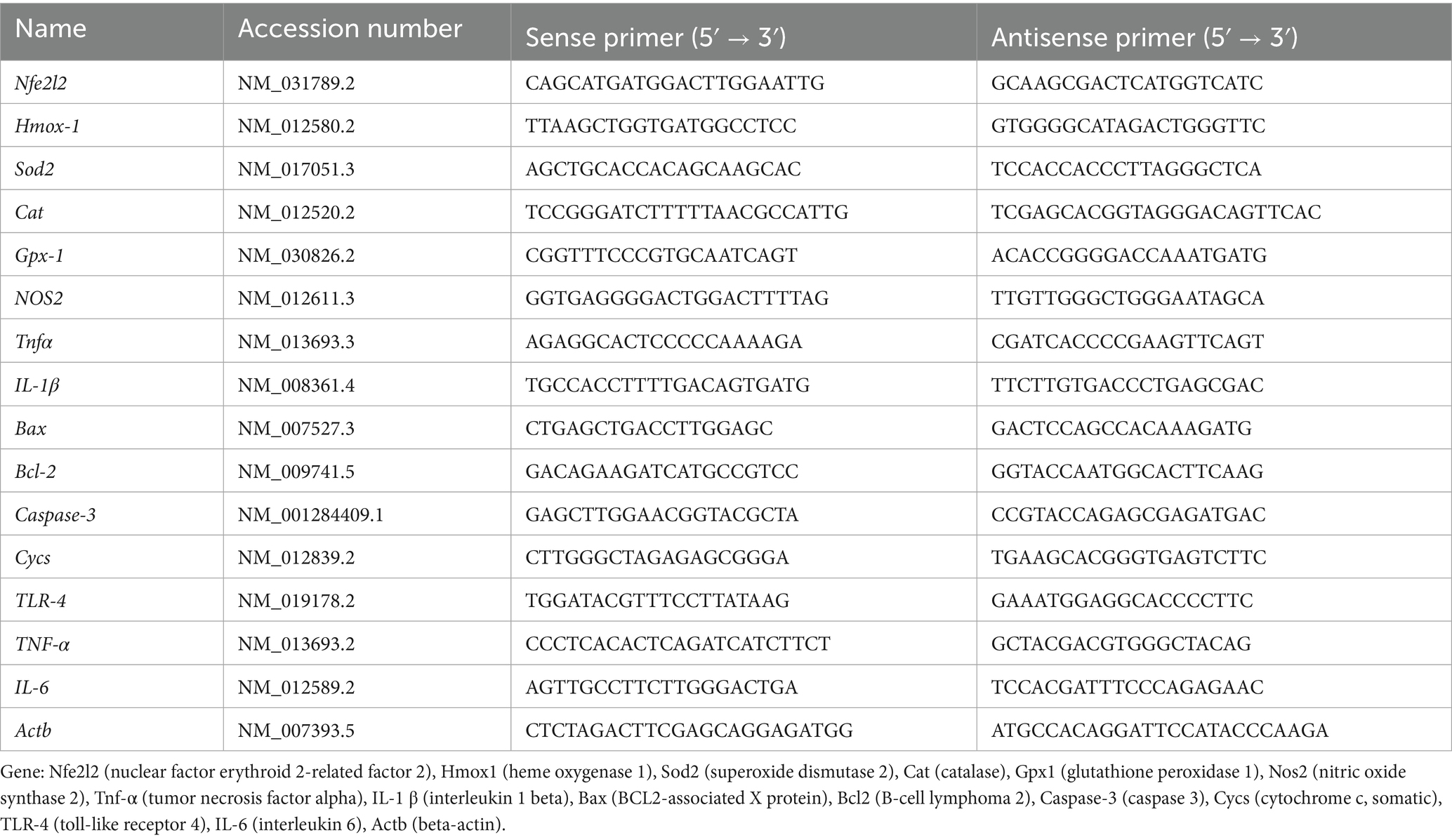
Morphometric analysis revealed significant alterations in kidney weight parameters across experimental groups. Glyphosate administration significantly increased kidney weight and relative kidney weight compared to controls (p < 0.05), indicating renal hypertrophy possibly due to inflammatory processes or cellular edema. Quercetin treatment dose-dependently attenuated these morphological alterations, with high-dose quercetin normalizing values to near-control levels, while low-dose treatment showed moderate improvement (Figure 1). This progressive normalization represents initial macroscopic evidence for quercetin’s nephroprotective properties. No statistically significant differences were observed between the initial and final body weights within each group, including the control and glyphosate-treated groups (p > 0.05, Table 2).
Figure 1. Effects of glyphosate and quercetin on kidney weight and relative kidney weight. Rats were administered with glyphosate and/or quercetin at low dose or high dose for 21 days. Results were presented as mean± SD (n = 10). Statistical significance: # indicates significant difference compared to the control group (p < 0.05); $ indicates significant difference compared to the glyphosate-treated group (p < 0.05).
Table 2. Mean ± SD of initial and final body weights of experimental animals in different treatment groups.
3.2 Renal function and injury biomarkers
Glyphosate exposure significantly impaired kidney function, as evidenced by pronounced elevation in serum creatinine, urea, and uric acid compared to controls (p < 0.05) (Figure 2). These results indicate a significant impairment of renal excretory function. KIM-1, a sensitive biomarker of proximal tubular damage, showed significant upregulation after glyphosate administration (p < 0.05, Figure 2), indicating that tubular epithelial injury is a primary event in glyphosate-induced nephrotoxicity. Quercetin treatment dose-dependently mitigated these impairments, with high-dose intervention (50 mg/kg/day) demonstrating superior normalization compared to low-dose treatment (25 mg/kg/day) and significantly reducing KIM-1 levels versus glyphosate-only exposure (p < 0.05), suggesting marked attenuation of tubular injury.

Figure 2. Effects of glyphosate and quercetin on serum renal function biomarkers and KIM-1. Rats were administered with glyphosate and/or quercetin at low dose or high dose for 21 days. Parameters measured include serum creatinine, urea, uric acid, and kidney tissue KIM-1 levels. Results were presented as mean± SD (n = 10). Statistical significance: # indicates significant difference compared to the control group (p < 0.05); $ indicates significant difference compared to the glyphosate-treated group (p < 0.05).
3.3 Oxidative stress markers and related gene expression
Glyphosate exposure significantly disrupted redox homeostasis, as evidenced by profound alterations in oxidative stress parameters. GSH, a critical non-enzymatic antioxidant, was markedly depleted compared to control levels (p < 0.05), indicating severe compromise of cellular antioxidant capacity (Figure 3). Concurrently, MDA levels, indicative of lipid peroxidation, were dramatically elevated versus control (p < 0.05) (Figure 3), suggesting significant membrane integrity compromise.
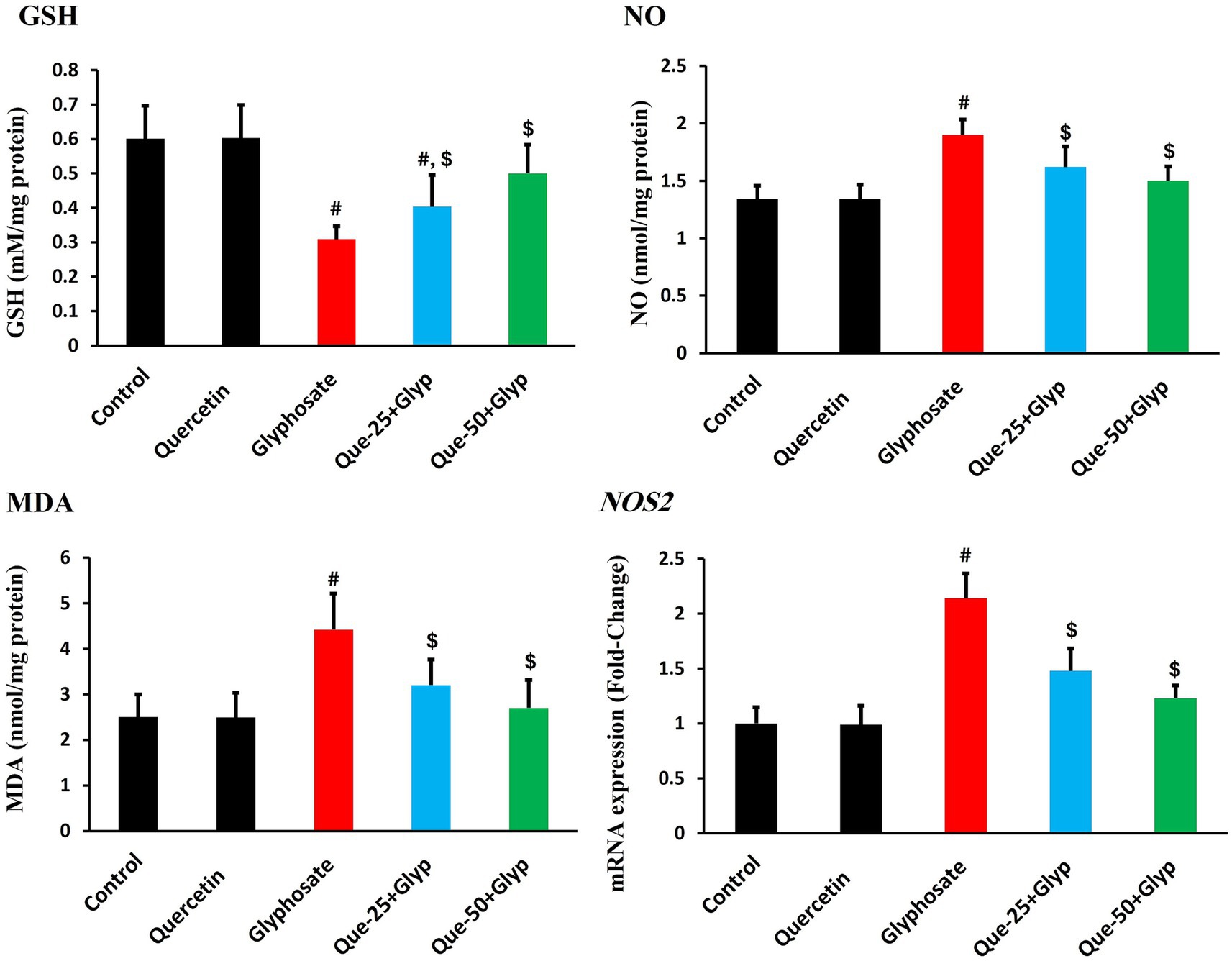
Figure 3. Effects of glyphosate and quercetin on oxidative stress parameters in renal tissue. Rats were administered with glyphosate and/or quercetin for 21 days. The figure shows levels of GSH, NO, MDA, and mRNA expression of inducible NOS2. Results were presented as mean± SD (n = 10). Statistical significance: # indicates significant difference compared to control (p < 0.05); $ indicates significant difference compared to glyphosate group (p < 0.05).
Nitrosative stress parameters showed parallel dysregulation, with NO production substantially increased compared to control (p < 0.05, Figure 3). This elevation was accompanied by pronounced upregulation of inducible NOS2 gene expression relative to control (p < 0.05), confirming enhanced nitrosative stress at both the metabolite and regulatory levels (Figure 3).
Quercetin intervention effectively attenuated these oxidative stress parameters in a dose-dependent pattern. High-dose quercetin (50 mg/kg) restored GSH content, approaching control values, while considerably reducing MDA (p < 0.05). Similarly, nitrosative stress markers were normalized, with NO levels and NOS2 expression declining, comparable to control levels (p < 0.05). Low-dose quercetin (25 mg/kg) provided moderate protection across all parameters, though less effectively than the high-dose regimen (Figure 3).
3.4 Antioxidant parameters and related gene expression
Comprehensive assessment revealed marked impairment of antioxidant enzymes following glyphosate exposure. CAT activity was profoundly reduced compared to control (p < 0.05), with concurrent downregulation of Cat gene expression relative to control (Figure 4). Similarly, GPX activity substantially decreased (p < 0.05), with corresponding reduction in Gpx-1 gene expression. SOD activity declined considerably (p < 0.05, Figure 4), with Sod2 gene expression notably decreased. GR activity was likewise dramatically diminished compared to control (p < 0.05) (Figure 4).

Figure 4. Effects of glyphosate and quercetin on antioxidant enzyme activities and their gene expression in renal tissue. Rats were administered with glyphosate and/or quercetin at low dose or high dose for 21 days. Left panels show enzyme activities of CAT, GPX, SOD, and GR. Right panels show corresponding mRNA expression levels (Cat, Gpx-1, and Sod2) presented as fold-change relative to control. Results were presented as mean± SD (n = 10). Statistical significance: # indicates significant difference compared to the control group (p < 0.05); $ indicates significant difference compared to the glyphosate-treated group (p < 0.05).
Quercetin treatment dose-dependently restored these parameters. High-dose quercetin (50 mg/kg) effectively increased CAT, GPX, SOD, and GR activities compared to the glyphosate group (p < 0.05), with moderate improvement in the low-dose group (25 mg/kg). This protective effect extended to restoration of corresponding gene expression (Cat, Gpx-1, Sod2), with transcript levels approaching control values in the high-dose group (p < 0.05). Notably, quercetin monotherapy robustly upregulated antioxidant genes above baseline control levels (p < 0.05), suggesting potential priming effects that may enhance cellular resilience against subsequent oxidative challenges (Figure 4).
Nrf2, the master regulator of antioxidants, showed substantial reduction in protein levels compared to control (p < 0.05), accompanied by concurrent suppression of Nfe2l2 gene expression, indicating inhibition of this master regulatory pathway at both transcriptional and post-transcriptional levels (Figure 5). Consequently, expression of direct target Hmox-1 was pronouncedly downregulated (p < 0.05, Figure 5). High-dose quercetin appreciably ameliorated this dysregulation, increasing both Nrf2 protein levels and Nfe2l2 gene expression compared to the glyphosate group (p < 0.05), with restoration of Hmox-1 expression (Figure 5).

Figure 5. Effects of glyphosate and quercetin on Nrf2 protein levels and related gene expression in renal tissue. Rats were administered with glyphosate and/or quercetin for 21 days. The figure shows Nrf2 protein levels, and mRNA expression of Nfe2l2 and Hmox-1, presented as fold-change relative to control. Results were presented as mean± SD (n = 10). Statistical significance: # indicates significant difference compared to control (p < 0.05); $ indicates significant difference compared to glyphosate group (p < 0.05).
3.5 Inflammatory markers and related gene expression
Glyphosate exposure triggered pronounced inflammatory responses in renal tissue. Protein levels of TNF-α, IL-6, IL-1β, and TLR4 were markedly increased compared to control (p < 0.05), indicating activation of pro-inflammatory cytokine networks (Figure 6). Gene expression analysis revealed even more significant upregulation of these inflammatory markers (p < 0.05), with fold-changes exceeding those observed at the protein level, suggesting activation of common transcriptional regulatory mechanisms (Figure 6).

Figure 6. Effects of glyphosate and quercetin on inflammatory markers in renal tissue. Rats were administered with glyphosate and/or quercetin for 21 days. Left panels show protein levels of TNF-α, IL-6, IL-1β, and TLR-4. Right panels show corresponding mRNA expression presented as fold-change relative to control. Results were presented as mean± SD (n = 10). Statistical significance: # indicates significant difference compared to control (p < 0.05); $ indicates significant difference compared to glyphosate group (p < 0.05).
Quercetin treatment dose-dependently normalized both protein levels and gene expression of inflammatory markers. High-dose quercetin (50 mg/kg) showed superior anti-inflammatory effects compared to low-dose intervention (25 mg/kg), with significant reductions (p < 0.05) in both protein and transcript levels. Quercetin monotherapy did not significantly alter baseline inflammatory markers, indicating its effects were most pronounced in the context of pre-existing inflammatory activation (Figure 6).
3.6 Apoptotic markers and related gene expression
Glyphosate administration significantly dysregulated apoptotic pathways. Pro-apoptotic BAX, CASPASE-3, and CYTOCHROME C levels were markedly elevated (p < 0.05), indicating activation of the intrinsic mitochondrial apoptotic pathway. Anti-apoptotic BCL-2 protein levels were significantly decreased (p < 0.05) compared to control, dropping from approximately 2.3 ng/mg to 0.7 ng/mg, further confirming the shift toward pro-apoptotic signaling (Figure 7).
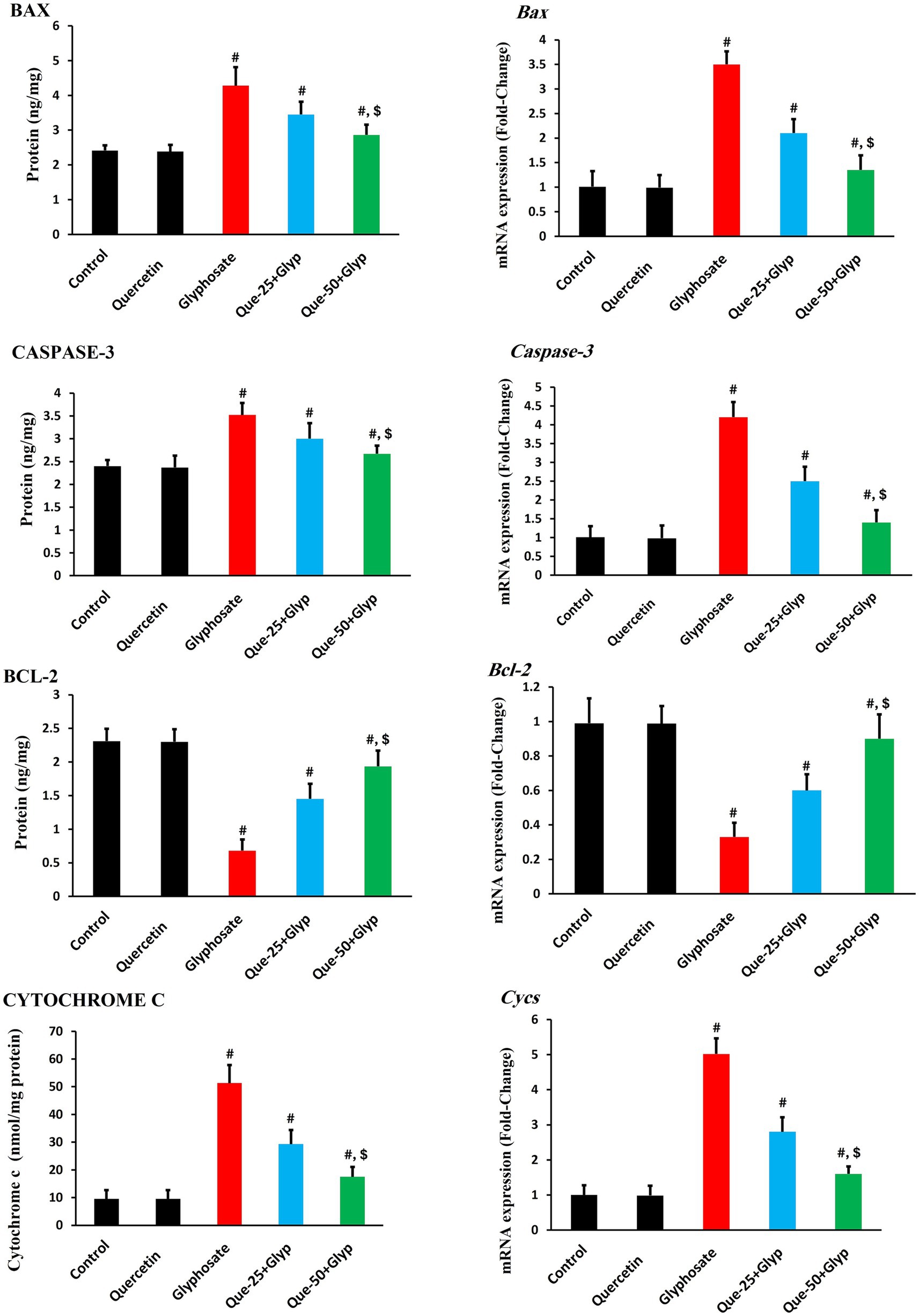
Figure 7. Effects of glyphosate and quercetin on apoptotic markers in renal tissue. Rats were administered with glyphosate and/or quercetin for 21 days. Left panels show protein levels of pro-apoptotic markers (BAX, CASPASE-3, CYTOCHROME C) and anti-apoptotic marker (BCL-2). Right panels show corresponding mRNA expression presented as fold-change relative to control. Results were presented as mean± SD (n = 10). Statistical significance: # indicates significant difference compared to control (p < 0.05); $ indicates significant difference compared to glyphosate group (p < 0.05).
Gene expression analysis revealed upregulation of Bax, caspase-3, and Cycs genes (p < 0.05), consistent with protein findings. Bcl-2 gene expression was significantly downregulated (p < 0.05) compared to control (Figure 7).
Quercetin treatment effectively normalized these apoptotic parameters dose-dependently. High-dose quercetin (50 mg/kg) demonstrated superior effects compared to low-dose treatment (25 mg/kg) (p < 0.05), restoring Bcl-2 protein levels to approximately 1.9 ng/mg and mRNA expression to 0.9-fold, while reducing pro-apoptotic markers and restoring the critical BAX/BCL-2 balance that regulates mitochondrial integrity and cellular survival (Figure 7).
3.7 Histopathological examination
Histopathological assessment revealed distinct morphological differences among experimental groups. Kidney tissues collected from both the control and quercetin-treated groups exhibited normal renal architecture, with well-preserved glomeruli and intact tubular structures. In contrast, glyphosate-treated kidneys demonstrated marked renal damage, characterized by atrophy of the glomerular tuft with severe hemorrhage and congestion, inflammatory cellular infiltration of the glomerular tuft, interstitial leukocyte infiltration, epithelial cloudy swelling, extensive cellular apoptosis, and proximal tubular dilation with hyaline cast formation (Figure 8). These histopathological alterations confirm glyphosate-induced nephrotoxicity and correlate with the observed biochemical and molecular changes.
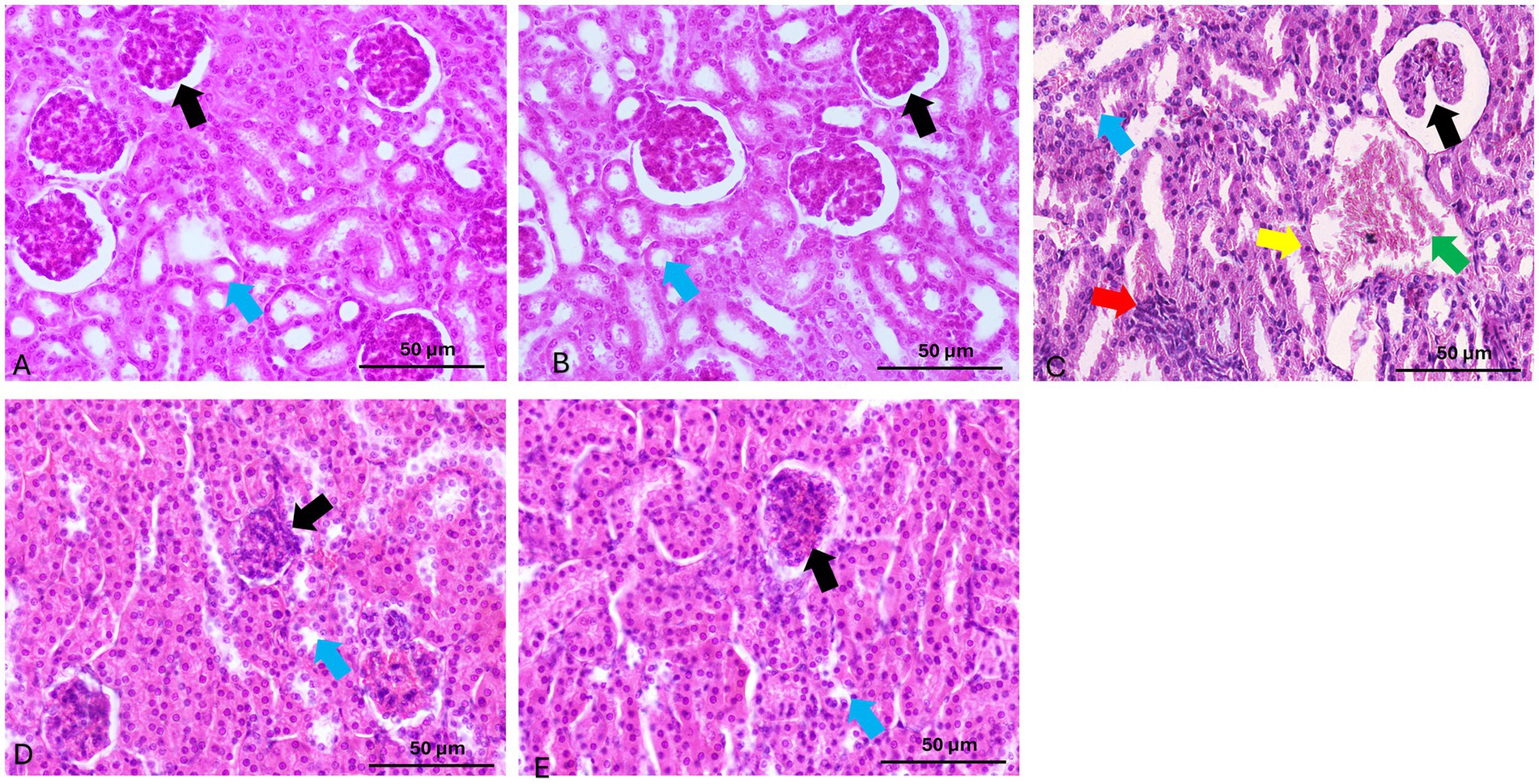
Figure 8. Representative photomicrographs of renal tissue sections stained with hematoxylin and eosin (H&E) from experimental groups. (A,B) Control and Quercetin groups show normal renal histology with intact glomeruli (black arrow) and tubules (blue arrow). (C) Glyphosate group reveals marked renal damage, including glomerular tuft atrophy with severe hemorrhage and congestion, cellular infiltration of the glomerular tuft (black arrow), leukocyte infiltration (red arrow), cloudy swelling (blue arrow), apoptosis (yellow arrow), and tubular dilation of the proximal tubule with hyaline casts (green arrow). (D) Que-25 + Glyp group shows glomeruli with reduced damage (black arrow) and preserved tubules (blue arrow). (E) Que-50 + Glyp demonstrates near-normal renal morphology with minimal histopathological changes in glomeruli (black arrow) and tubules (blue arrow). H&E stain, scale bar = 50 μm.
Quercetin treatment dose-dependently ameliorated these histopathological alterations. Low-dose quercetin (25 mg/kg) partially improved histological features, with moderate reduction in tubular degeneration and leukocytic infiltration. High-dose quercetin (50 mg/kg) showed marked improvement in renal architecture, with nearly normal glomeruli and minimal tubular damage. These morphological improvements corresponded with molecular findings, showing significant downregulation of pro-inflammatory and apoptotic genes and restoration of antioxidant genes in the high-dose quercetin group, suggesting comprehensive nephroprotection at both structural and molecular levels (Figure 8).
The semi-quantitative analysis revealed that glyphosate treatment significantly increased all renal damage parameters compared to control groups, with scores ranging from 2.0 to 3.0 across tubular dilatation, apoptotic cell abundance, glomerular atrophy, and leukocyte infiltration. Quercetin alone showed no significant histopathological changes compared to controls, confirming its safety profile. Both quercetin pretreatment groups demonstrated significant dose-dependent protective effects against glyphosate-induced nephrotoxicity, with the high-dose group (Que-50 + Glyp) showing superior protection, reducing damage scores to approximately 0.4–0.5 compared to 1.2–1.5 in the low-dose group (Que-25 + Glyp) as seen in Table 3.
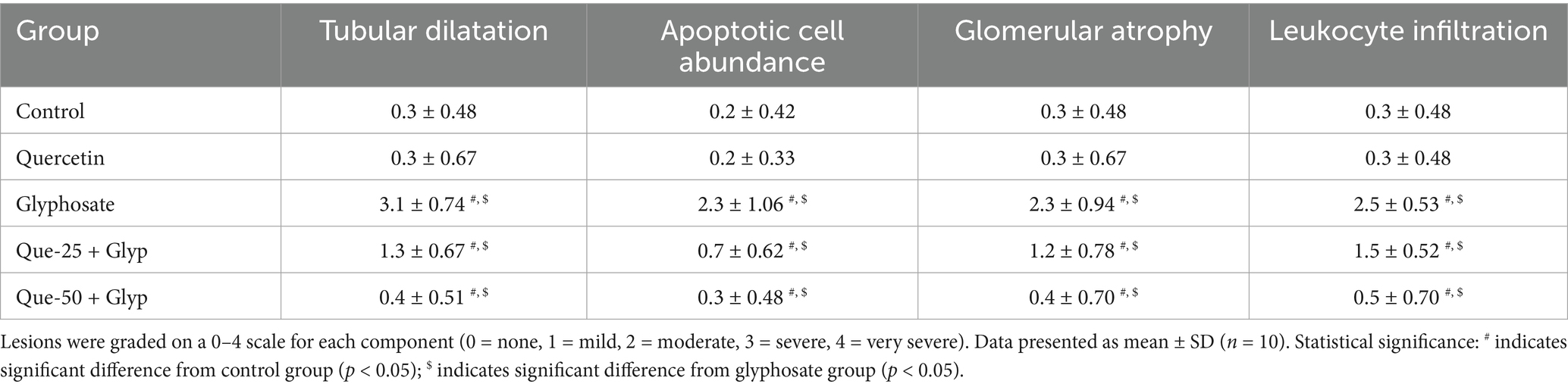
Table 3. Semi-quantitative histopathological scoring of renal damage parameters in different experimental groups.
4 Discussion
This study aimed to evaluate the nephrotoxic effects of glyphosate and investigate quercetin’s protective role against glyphosate-induced renal damage. Our findings revealed significant alterations at morphological, functional, biochemical, and molecular levels, confirming glyphosate’s nephrotoxicity and highlighting quercetin’s therapeutic potential.
The increased kidney weight after glyphosate administration represents a significant morphological manifestation of renal injury, likely reflecting inflammatory cell infiltration and cellular swelling, as reported by Karimi Jashni et al. (33). Quercetin dose-dependently normalized these parameters, consistent with its anti-inflammatory properties (34). Functional consequences of glyphosate exposure were evident through elevated renal biomarkers. Serum creatinine increased substantially, indicating impaired excretory function, with parallel rises in urea and uric acid levels, aligning with Nacano et al. (35).
Renal tubules, responsible for water and electrolyte reabsorption, were particularly vulnerable to toxic injury. Our study demonstrated that glyphosate exposure significantly damages proximal tubular epithelial cells, as evidenced by markedly elevated KIM-1 levels (p < 0.05) in exposed animals. KIM-1, a transmembrane glycoprotein specifically upregulated in injured proximal tubular cells, surpassed changes in conventional biomarkers, indicating the proximal tubular epithelium as the primary target of glyphosate toxicity. This finding aligns with Han et al. (36), who established KIM-1 as a sensitive biomarker for acute tubular damage. Our histopathological observations showing tubular epithelial degeneration provide morphological confirmation of this conclusion, suggesting that tubular injury precedes functional impairment. Reddy et al. (37) similarly reported that herbicide exposure primarily affects tubular structures before altering glomerular filtration. Importantly, quercetin treatment dose-dependently attenuated these tubular injury markers, demonstrating its specific protective effect against glyphosate-induced tubular damage.
Quercetin intervention attenuated these injury markers dose-dependently. Our findings provide substantial evidence that oxidative stress represents a central mechanism in glyphosate-induced renal injury. The disruption of the antioxidant defense system was evident through suppression of enzymatic antioxidants, with CAT, GPX, and SOD showing marked inhibition. This was accompanied by depletion of GSH content. Similar patterns have been documented by Zhou et al. (38), suggesting systematic disruption of cellular redox homeostasis. The consequences of this antioxidant defense collapse were manifested through elevation of oxidative damage markers, including MDA levels. Chang et al. (39) similarly reported that glyphosate-based formulations induce significant increases in oxidative stress biomarkers. The membrane peroxidation may compromise renal epithelial cell integrity, potentially explaining the glomerular and tubular damage observed histopathologically, as suggested by Romualdo et al. (40).
Concurrently, significant elevation in NO levels was observed following glyphosate exposure, accompanied by marked upregulation of NOS2 gene expression. This indicates coordinated dysregulation of nitrosative stress at both metabolic and genetic regulatory levels, providing an additional mechanistic link between glyphosate exposure and renal injury. Quercetin treatment dose-dependently normalized both NO production and NOS2 expression, suggesting that modulation of nitrosative stress represents an important mechanism contributing to quercetin’s nephroprotective effects.
Quercetin intervention effectively restored redox balance through multiple complementary mechanisms. The dose-dependent normalization of antioxidant enzyme activities (CAT, GPX, SOD) and GSH content suggests enhancement of endogenous antioxidant defense systems. Concurrently, the progressive reduction in oxidative damage markers (MDA and NO) indicates effective mitigation of free radical-mediated cellular injury. These findings align with reports by Alharbi et al. (41) and Zhang et al. (42), who documented quercetin’s capacity to restore antioxidant defenses and attenuate oxidative damage in various experimental models of oxidative stress-mediated tissue injury.
The molecular analysis of antioxidant gene expression revealed significant transcriptional dysregulation following glyphosate exposure, providing mechanistic insight into the observed enzymatic impairments. The coordinated downregulation of multiple antioxidant genes, including Sod2, Cat, Gpx-1, and Hmox-1, suggests suppressing a common transcriptional regulatory mechanism rather than gene-specific effects. The strong correlations between these expression patterns (r-values ranging from 0.82 to 0.88, all p < 0.05) further support their coordinated regulation through a shared pathway. Similar patterns of antioxidant gene suppression have been documented by Liu et al. (43) and Yengkokpam and Mazumder (44), who reported comparable transcriptional alterations in models of pesticide-induced oxidative stress.
The significant reduction in Nrf2 protein levels observed in our study provides mechanistic insight into the coordinated suppression of antioxidant genes. This protein reduction was accompanied by parallel downregulation of Nfe2l2 gene expression, suggesting that glyphosate disrupts this pathway at multiple regulatory levels. As the master regulator of cellular antioxidant defense, Nrf2 controls numerous cytoprotective genes through binding to antioxidant response elements in their promoter regions. This dysregulation of the Nrf2-ARE pathway appears to be a central mechanism underlying the observed comprehensive impairment of antioxidant defense systems, creating a cellular environment highly susceptible to oxidative damage (45). Quercetin’s ability to restore both Nrf2 protein levels and gene expression likely represents a key mechanism behind its nephroprotective effects, as similarly reported by Wang et al. (46) in models of oxidative stress-induced renal injury.
Quercetin intervention effectively restored antioxidant gene expression in a dose-dependent manner, with high-dose quercetin (50 mg/kg) normalizing transcription of Sod2, Cat, Gpx-1, and Hmox-1 to near-control levels. Notably, quercetin monotherapy induced substantial upregulation of these genes above baseline, with Hmox-1 showing particularly pronounced induction. This transcriptional enhancement reveals quercetin’s capacity to activate antioxidant gene expression, as documented by Zhao et al. (47) and Kenan Kinaci et al. (48) across multiple experimental models.
The histopathological findings of leukocytic infiltration in glyphosate-exposed renal tissue were corroborated by molecular evidence of inflammatory pathway activation. Significant upregulation of TLR4 suggests recognition of glyphosate-induced damage-associated molecular patterns as inflammatory triggers, activating downstream signaling pathways. Similar TLR4-mediated inflammatory signaling has been reported by Wu et al. (49) in models of nephrotoxic acute kidney injury. Elevated expression of pro-inflammatory cytokines (TNF-α, IL-6, IL-1β) further confirms activation of inflammatory networks in response to glyphosate. These cytokines mediate local inflammatory responses and contribute to renal tissue injury through direct cytotoxic effects and promotion of oxidative stress. Inflammatory markers were markedly elevated in glyphosate-treated animals, highlighting inflammation as a key contributor to nephrotoxicity. Quercetin treatment effectively suppressed this inflammatory response in a dose-dependent manner, supporting its nephroprotective potential. The simultaneous improvement in both inflammatory and oxidative stress parameters suggests that these two pathways may be closely interlinked in the pathogenesis of glyphosate-induced renal damage. Similar anti-inflammatory effects of quercetin have been documented by Chen et al. (50). Our analysis revealed that inflammatory gene expression changes consistently exceeded and preceded protein alterations, suggesting significant post-transcriptional regulation during toxic stress. Quercetin treatment normalized both processes, with gene expression responding more rapidly than protein levels, indicating primary transcriptional regulatory effects. Similar regulatory patterns were reported Gaspard et al. (51) in xenobiotic-induced inflammatory responses.
Although cleaved CASPASE-3 is the definitive marker of irreversible apoptosis, this study relied on ELISA and qRT-PCR methods to evaluate CASPASE-3expression. While these approaches provide valuable insights into apoptotic activity, they do not differentiate between pro-CASPASE-3 and its cleaved active form. Future studies employing Western blot or immunohistochemistry would provide more direct confirmation of CASPASE-3 activation. Our findings establish the mitochondrial apoptotic pathway as a significant mechanism of cell death in glyphosate-induced nephrotoxicity. The observed upregulation of pro-apoptotic BAX coupled with downregulation of anti-apoptotic BCL-2 indicates disruption of the critical BAX/ BCL-2 balance governing mitochondrial membrane integrity. This mitochondrial destabilization is evidenced by significant CYTOCHROME C release, which is associated with the activation of the CASPASE cascade, as confirmed by elevated caspase-3 expression and activity. Similar activation of the mitochondrial apoptotic pathway following glyphosate exposure has been reported by Lu et al. (52) and Gui et al. (11) in various cellular models.
The correlations between oxidative stress and apoptotic suggest that oxidative damage, particularly to cellular membranes, serves as a key contributor to the apoptotic cascade. The lipid peroxidation indicated by elevated MDA levels may compromise membrane integrity, particularly in organelles with high phospholipid content. Additionally, the impaired antioxidant defense, indicated by Sod2 downregulation, likely exacerbates oxidative damage, further promoting cellular dysfunction and apoptotic signaling. Sule et al. (53) documented the relationship between oxidative stress and apoptotic activation in pesticide-induced cytotoxicity, and reported that ROS and RNS trigger mitochondrial apoptotic pathways.
Notably, we observed increased BCL-2 protein levels despite significant downregulation of Bcl-2 gene expression, suggesting complex post-transcriptional regulatory mechanisms that may include enhanced protein stability or decreased degradation in response to toxic stress. This discordance highlights the importance of multi-level analysis for comprehensive understanding of cellular responses to toxicants.
Quercetin treatment effectively mitigated these apoptotic changes in a dose-dependent manner, normalizing the BAX/ BCL-2 ratio, reducing CYTOCHROME C release, and suppressing CASPASE-3 activation. This anti-apoptotic effect appears to result from multiple complementary mechanisms, including enhanced antioxidant protection as evidenced by restored antioxidant enzyme activities and reduced oxidative damage markers, which collectively help maintain cellular integrity and prevent progression to apoptotic cell death. These findings were consistent with reports by Chen et al. (54), who documented quercetin’s capacity to modulate apoptotic signaling in various models of toxicant-induced cell death. Specifically, Chen et al. (54) demonstrated that quercetin alleviates zearalenone-induced apoptosis and necroptosis in porcine renal epithelial cells by inhibiting the calcium-sensing receptor/calcium–calmodulin-dependent protein kinase II signaling pathway and by protecting the cells from oxidative damage caused by zearalenone exposure. Analysis of our findings suggests an integrated mechanistic model for glyphosate-induced nephrotoxicity centered on the interaction between inflammatory and oxidative pathways. Glyphosate exposure triggers inflammatory responses via TLR-4 upregulation while suppressing antioxidant gene expression, creating a self-amplifying cycle that compromises mitochondrial integrity, activates apoptotic cascades, and results in tubular epithelial damage and functional impairment. Similar integrated mechanisms have been proposed by Ferrante et al. (55) in their review of glyphosate toxicity. Quercetin’s protective effects appear mediated through multiple complementary mechanisms: enhanced antioxidant gene expression (Sod2, Cat, Gpx-1, and Hmox-1) and restored enzyme activities, alongside attenuated inflammatory signaling through reduced expression of inflammatory mediators and TLR-4. These effects interrupt the inflammation-oxidative stress cycle, preserve mitochondrial integrity, and prevent apoptotic cell death as indicated by normalized CYTOCHROME C release, restored BCL-2 protein and gene expression levels, reduced BAX levels, and decreased CASPASE-3 activity. These multifaceted protective mechanisms align with findings by Song et al. (56). The dose-dependent nature of quercetin’s protective effects, with superior efficacy at 50 mg/kg, suggests potential therapeutic applications.
While this study provides significant insights into the protective effects of quercetin against glyphosate-induced nephrotoxicity, certain limitations should be noted. Firstly, the study was limited to a sub-acute exposure model in rats, which may not fully replicate chronic exposure scenarios in humans. Secondly, only male rats were used, and sex-specific differences were not explored. Thirdly, while multiple biomarkers and gene expressions were analyzed, more advanced molecular pathways (e.g., transcriptomics or proteomics) were not assessed. Additionally, while CASPASE-3 levels were assessed using ELISA and gene expression analysis, the absence of Western blot analysis to detect cleaved CASPASE-3 is a limitation that should be addressed in future research for confirming apoptotic pathway activation at the protein level. Future studies using chronic models, both sexes, and broader mechanistic approaches would help validate and expand these findings.
5 Conclusion
The present study provides comprehensive evidence that sub-acute glyphosate exposure induces significant nephrotoxicity in rats through a complex interplay of molecular mechanisms. Our findings demonstrate that glyphosate triggers renal damage via three interconnected pathways: (1) suppression of antioxidant defense systems and induction of oxidative stress, (2) activation of TLR-4-mediated inflammatory cascades, and (3) disruption of mitochondrial integrity leading to apoptotic cell death. These pathological processes collectively culminate in tubular epithelial damage, glomerular dysfunction, and impaired renal excretory capacity. Importantly, this study establishes quercetin as a potent nephroprotective agent against glyphosate-induced renal injury. Quercetin treatment, particularly at the higher dose (50 mg/kg), effectively normalized renal morphology and function, restored antioxidant enzyme activities and gene expression, attenuated inflammatory cytokine production, and inhibited the mitochondrial apoptotic pathway. The dose-dependent protective effects observed suggest a therapeutic threshold for optimal renoprotection against xenobiotic injury.
Data availability statement
The data presented in this study were available upon reasonable request from the corresponding author.
Ethics statement
The animal study was approved by Taif University, Saudi Arabia (approval ethics number: HAPO-02-T-105). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
AA: Resources, Funding acquisition, Investigation, Writing – original draft, Software, Formal analysis, Visualization, Data curation, Project administration, Validation, Conceptualization, Writing – review & editing, Methodology, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by Taif University, Saudi Arabia, under project number TU-DSPP-2024-55.
Acknowledgments
The authors extend their appreciation to Taif University, Saudi Arabia for supporting this work through project number (TU-DSPP-2024-55).
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author declares that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kopple, JD. Pathophysiology of protein-energy wasting in chronic renal failure. J Nutr. (1999) 129:247S–51S. doi: 10.1093/jn/129.1.247S
2. Addissouky, A, Ma Ali, M, El Sayed, ET I, Wang, Y, El Baz, A, Elarabany, N, et al. Risk factors, etiology, pathology, and diagnostic methods for acute kidney injury: a review study. Res Mol Med. (2022) 10:193–204. doi: 10.32598/rmm.10.4.1265.1
3. Gunatilake, S, Seneff, S, and Orlando, L. Glyphosate’s synergistic toxicity in combination with other factors as a cause of chronic kidney disease of unknown origin. Int J Environ Res Public Health. (2019) 16:2734. doi: 10.3390/ijerph16152734
4. Green, JM. Review of glyphosate and ALS-inhibiting herbicide crop resistance and resistant weed management. Weed Technol. (2007) 21:547–58. doi: 10.1614/WT-06-004.1
5. Tizhe, EV, Ibrahim, ND-G, Fatihu, MY, Onyebuchi, II, George, BDJ, Ambali, SF, et al. Influence of zinc supplementation on histopathological changes in the stomach, liver, kidney, brain, pancreas and spleen during subchronic exposure of Wistar rats to glyphosate. Comp Clin Pathol. (2014) 23:1535–43. doi: 10.1007/s00580-013-1818-1
6. Namratha, M, Lakshman, M, Jeevanalatha, M, Kumar, B, and Rashid, S. Glyphosate induced renal toxicity and its amelioration with vitamin C in rats. Continental Vet J. (2022) 2:81–9.
7. Tizhe, EV, Ibrahim, ND-G, Fatihu, MY, Igbokwe, IO, George, B-DJ, Ambali, SF, et al. Serum biochemical assessment of hepatic and renal functions of rats during oral exposure to glyphosate with zinc. Comp Clin Pathol. (2014) 23:1043–50. doi: 10.1007/s00580-013-1740-6
8. Zheng, T, Jia, R, Cao, L, Du, J, Gu, Z, He, Q, et al. Effects of chronic glyphosate exposure on antioxdative status, metabolism and immune response in tilapia (GIFT, Oreochromis niloticus). Comp Biochem Physiol C Toxicol Pharmacol. (2021) 239:108878. doi: 10.1016/j.cbpc.2020.108878
9. Turkmen, R, Birdane, YO, Demirel, HH, Yavuz, H, Kabu, M, and Ince, S. Antioxidant and cytoprotective effects of N-acetylcysteine against subchronic oral glyphosate-based herbicide-induced oxidative stress in rats. Environ Sci Pollut Res. (2019) 26:11427–37. doi: 10.1007/s11356-019-04585-5
10. Izumi, Y, O’dell, KA, and Zorumski, CF. The herbicide glyphosate inhibits hippocampal long-term potentiation and learning through activation of pro-inflammatory signaling. Sci Rep. (2023) 13:18005. doi: 10.1038/s41598-023-44121-7
11. Gui, Y-X, Fan, X-N, Wang, H-M, Wang, G, and Chen, S-D. Glyphosate induced cell death through apoptotic and autophagic mechanisms. Neurotoxicol Teratol. (2012) 34:344–9. doi: 10.1016/j.ntt.2012.03.005
12. Fang, C-Y, Lou, D-Y, Zhou, L-Q, Wang, J-C, Yang, B, He, Q-J, et al. Natural products: potential treatments for cisplatin-induced nephrotoxicity. Acta Pharmacol Sin. (2021) 42:1951–69. doi: 10.1038/s41401-021-00620-9
13. Oo, AM, Nor, MNM, Lwin, OM, and Simbak, N. Flavonol quercetin: immunomodulatory and anticancer properties. Asian J Med Biomed. (2022) 6:17–31. doi: 10.37231/ajmb.2022.6.1.452
14. Sanders, RA, Rauscher, FM, and Watkins Iii, JB. Effects of quercetin on antioxidant defense in streptozotocin-induced diabetic rats. J Biochem Mol Toxicol. (2001) 15:143–9. doi: 10.1002/jbt.11
15. Chen, X. Protective effects of quercetin on liver injury induced by ethanol. Pharmacogn Mag. (2010) 6:135–41. doi: 10.4103/0973-1296.62900
16. Priya, SD, and Devi, CS. Protective effect of quercetin in cisplatin-induced cell injury in the rat kidney. Indian J Pharmacol. (1999) 31:422–6.
17. Rashid, H, Jali, A, Akhter, MS, and Abdi, SaH. Molecular mechanisms of oxidative stress in acute kidney injury: targeting the loci by resveratrol. Int J Mol Sci. (2023) 25:3. doi: 10.3390/ijms25010003
18. Che, R, Yuan, Y, Huang, S, and Zhang, A. Mitochondrial dysfunction in the pathophysiology of renal diseases. Am J Physiol Renal Physiol. (2014) 306:F367–78. doi: 10.1152/ajprenal.00571.2013
19. Johansson, HKL, Schwartz, CL, Nielsen, LN, Boberg, J, Vinggaard, AM, Bahl, MI, et al. Exposure to a glyphosate-based herbicide formulation, but not glyphosate alone, has only minor effects on adult rat testis. Reprod Toxicol. (2018) 82:25–31. doi: 10.1016/j.reprotox.2018.09.008
20. Behling, EB, Sendão, MC, Francescato, HD, Antunes, LM, Costa, RS, and Bianchi, MDLP. Comparative study of multiple dosage of quercetin against cisplatin-induced nephrotoxicity and oxidative stress in rat kidneys. Pharmacol Rep. (2006) 58:526–32.
21. Liu, C-M, Sun, Y-Z, Sun, J-M, Ma, J-Q, and Cheng, C. Protective role of quercetin against lead-induced inflammatory response in rat kidney through the ROS-mediated MAPKs and NF-κB pathway. Biochim Biophys Acta. (2012) 1820:1693–703. doi: 10.1016/j.bbagen.2012.06.011
22. Almeer, RS, Albasher, GI, Alarifi, S, Alkahtani, S, Ali, D, and Abdel Moneim, AE. Royal jelly attenuates cadmium-induced nephrotoxicity in male mice. Sci Rep. (2019) 9:5825. doi: 10.1038/s41598-019-42368-7
23. Ohkawa, H, Ohishi, N, and Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. (1979) 95:351–8. doi: 10.1016/0003-2697(79)90738-3
24. Ellman, GL. Tissue sulfhydryl groups. Arch Biochem Biophys. (1959) 82:70–7. doi: 10.1016/0003-9861(59)90090-6
25. Green, LC, Wagner, DA, Glogowski, J, Skipper, PL, Wishnok, JS, and Tannenbaum, SR. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem. (1982) 126:131–8. doi: 10.1016/0003-2697(82)90118-X
26. Nishikimi, M, Rao, NA, and Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun. (1972) 46:849–54. doi: 10.1016/S0006-291X(72)80218-3
27. Lück, H. Catalase In: HU Bergmeyer, editor. Methods of enzymatic analysis. New York: Academic Press (1965). 885–94.
28. Paglia, DE, and Valentine, WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. (1967) 70:158–69.
29. Moron, MS, Depierre, JW, and Mannervik, B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta. (1979) 582:67–78. doi: 10.1016/0304-4165(79)90289-7
30. Livak, KJ, and Schmittgen, TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. (2001) 25:402–8. doi: 10.1006/meth.2001.1262
31. Drury, R, and Wallington, E. Carleton’s histological technique. 5th ed. New York: Churchill Livingstone (1980).
32. Toprak, T, Sekerci, C, Aydın, H, Ramazanoglu, M, Arslan, F, Basok, B, et al. Protective effect of chlorogenic acid on renal ischemia/reperfusion injury in rats. Arch Ital Urol Androl. (2020) 92:153. doi: 10.4081/aiua.2020.2.153
33. Karimi Jashni, H, Novin, L, and Poorahmadi, M. Effect of the herbicide glyphosate on renal tissues in adult female rats. Pars J Med Sci. (2022) 11:9–16. doi: 10.29252/jmj.11.4.2
34. Lesjak, M, Beara, I, Simin, N, Pintać, D, Majkić, T, Bekvalac, K, et al. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J Funct Foods. (2018) 40:68–75. doi: 10.1016/j.jff.2017.10.047
35. Nacano, BRM, Convento, MB, Oliveira, ASD, Castino, R, Castino, B, Razvickas, CV, et al. Effects of glyphosate herbicide ingestion on kidney function in rats on a balanced diet. J Bras Nefrol. (2023) 46:e20230043. doi: 10.1590/2175-8239-JBN-2023-0043en
36. Han, WK, Bailly, V, Abichandani, R, Thadhani, R, and Bonventre, JV. Kidney injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. (2002) 62:237–44. doi: 10.1046/j.1523-1755.2002.00433.x
37. Reddy, PM, Jeevanalatha, M, Lakshman, M, Kalakumar, B, and Kadudhula, A. Ameliorative effect of quercetin on body weights and haematology of glyphosate induced toxicity in albino Wistar rats. Pharma Innov J. (2021) 10:782–5.
38. Zhou, J, Nie, R-C, Yin, Y-X, Cai, X-X, Xie, D, and Cai, M-Y. Protective effect of natural antioxidants on reducing cisplatin-induced nephrotoxicity. Dis Markers. (2022) 2022:1–17. doi: 10.1155/2022/1612348
39. Chang, VC, Andreotti, G, Ospina, M, Parks, CG, Liu, D, Shearer, JJ, et al. Glyphosate exposure and urinary oxidative stress biomarkers in the agricultural health study. JNCI J Natl Cancer Inst. (2023) 115:394–404. doi: 10.1093/jnci/djac242
40. Romualdo, GR, De Souza, JLH, Valente, LC, and Barbisan, LF. Assessment of the impact of glyphosate and 2, 4-D herbicides on the kidney injury and transcriptome changes in obese mice fed a Western diet. Toxicol Lett. (2023) 385:1–11. doi: 10.1016/j.toxlet.2023.08.003
41. Alharbi, HOA, Alshebremi, M, Babiker, AY, and Rahmani, AH. The role of quercetin, a flavonoid in the Management of Pathogenesis through Regulation of oxidative stress, inflammation, and biological activities. Biomol Ther. (2025) 15:151. doi: 10.3390/biom15010151
42. Zhang, Y-M, Zhang, Z-Y, and Wang, R-X. Protective mechanisms of quercetin against myocardial ischemia reperfusion injury. Front Physiol. (2020) 11:956. doi: 10.3389/fphys.2020.00956
43. Liu, L, Shi, M, Wu, Y, Hao, J, Guo, J, Li, S, et al. Protective effects of resveratrol on honeybee health: mitigating pesticide-induced oxidative stress and enhancing detoxification. Pestic Biochem Physiol. (2025) 210:106403. doi: 10.1016/j.pestbp.2025.106403
44. Yengkokpam, P, and Mazumder, PB. Antioxidant enzymatic activities and profiling of gene expression associated with organophosphate stress tolerance in Solanum melongena L. cv. Longai. 3 Biotech. (2021) 11:510. doi: 10.1007/s13205-021-03061-7
45. Dodson, M, De La Vega, MR, Cholanians, AB, Schmidlin, CJ, Chapman, E, and Zhang, DD. Modulating NRF2 in disease: timing is everything. Annu Rev Pharmacol Toxicol. (2019) 59:555–75. doi: 10.1146/annurev-pharmtox-010818-021856
46. Wang, Y, Quan, F, Cao, Q, Lin, Y, Yue, C, Bi, R, et al. Quercetin alleviates acute kidney injury by inhibiting ferroptosis. J Adv Res. (2021) 28:231–43. doi: 10.1016/j.jare.2020.07.007
47. Zhao, L, Wu, J, Yang, J, Wei, J, Gao, W, and Guo, C. Dietary quercetin supplementation increases serum antioxidant capacity and alters hepatic gene expression profile in rats. Exp Biol Med. (2011) 236:701–6. doi: 10.1258/ebm.2011.010258
48. Kinaci, K, Kinaci, M, Erkasap, N, Kucuk, A, Koken, T, and Tosun, M. Effects of quercetin on apoptosis, NF-κB and NOS gene expression in renal ischemia/reperfusion injury. Exp Ther Med. (2012) 3:249–54. doi: 10.3892/etm.2011.382
49. Wu, H, Chen, G, Wyburn, KR, Yin, J, Bertolino, P, Eris, JM, et al. TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest. (2007) 117:2847–59. doi: 10.1172/JCI31008
50. Chen, Y-Q, Chen, H-Y, Tang, Q-Q, Li, Y-F, Liu, X-S, Lu, F-H, et al. Protective effect of quercetin on kidney diseases: from chemistry to herbal medicines. Front Pharmacol. (2022) 13:968226. doi: 10.3389/fphar.2022.968226
51. Gaspard, I, Kerdine, S, Pallardy, M, and Lebrec, H. Quantitation of cytokine mRNA expression as an endpoint for prediction and diagnosis of xenobiotic-induced hypersensitivity reactions. Methods. (1999) 19:64–70. doi: 10.1006/meth.1999.0828
52. Lu, J, Zhang, C, Wang, W, Xu, W, Chen, W, Tao, L, et al. Exposure to environmental concentrations of glyphosate induces cardiotoxicity through cellular senescence and reduced cell proliferation capacity. Ecotoxicol Environ Saf. (2023) 261:115112. doi: 10.1016/j.ecoenv.2023.115112
53. Sule, RO, Condon, L, and Gomes, AV. A common feature of pesticides: oxidative stress—the role of oxidative stress in pesticide-induced toxicity. Oxidative Med Cell Longev. (2022) 2022:5563759. doi: 10.1155/2022/5563759
54. Chen, S, Xu, T, Xu, A, Chu, J, Luo, D, Shi, G, et al. Quercetin alleviates zearalenone-induced apoptosis and necroptosis of porcine renal epithelial cells by inhibiting CaSR/CaMKII signaling pathway. Food Chem Toxicol. (2023) 182:114184. doi: 10.1016/j.fct.2023.114184
55. Ferrante, M, Rapisarda, P, Grasso, A, Favara, C, and Conti, GO. Glyphosate and environmental toxicity with “one health” approach, a review. Environ Res. (2023) 235:116678. doi: 10.1016/j.envres.2023.116678
Keywords: glyphosate, quercetin, nephrotoxicity, oxidative stress, inflammation, apoptosis, antioxidant
Citation: Albrakati A (2025) Protective effects of quercetin against glyphosate-induced nephrotoxicity in rats: role of oxidative stress, inflammatory response, and apoptotic pathways. Front. Vet. Sci. 12:1624763. doi: 10.3389/fvets.2025.1624763
Edited by:
Sara Taha Elazab, Mansoura University, EgyptReviewed by:
Xiaolong Gu, Yunnan Agricultural University, ChinaAhmed M. Abdellatif, Mansoura University, Egypt
Copyright © 2025 Albrakati. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ashraf Albrakati, YS5hbGJyYWthdGlAdHUuZWR1LnNh
 Ashraf Albrakati
Ashraf Albrakati