- Division of Animal Science and Institute of Agricultural and Life Science, Gyeongsang National University, Jinju, Republic of Korea
Introduction: The prohibition of antibiotics in animal feed has increased interest in alternatives, such as phytogenic compounds, pro- and prebiotics, organic acids, and exogenous enzymes. Among these, Bacillus-based biotics and enzyme cocktail are the most commonly used feed additives. However, their effects on growth performance, immunity, and gut health in nursery pigs, as well as their interactions with pathogens under commercial conditions, remain unclear. This study investigated the impact of these additives on growth performance, immunity, and pathogenic microorganisms in the gut under commercial conditions.
Material and methods: Two hundred nursery pigs were assigned to one of five dietary treatments: (1) CON: a basal corn-soybean meal diet, (2) A: basal diet with 0.05% probiotics, (3) B: basal diet with 0.1% synbiotics containing one strain, (4) C: basal diet with 0.1% synbiotics containing two strains, and (5) D: basal diet with 0.1% enzyme cocktail.
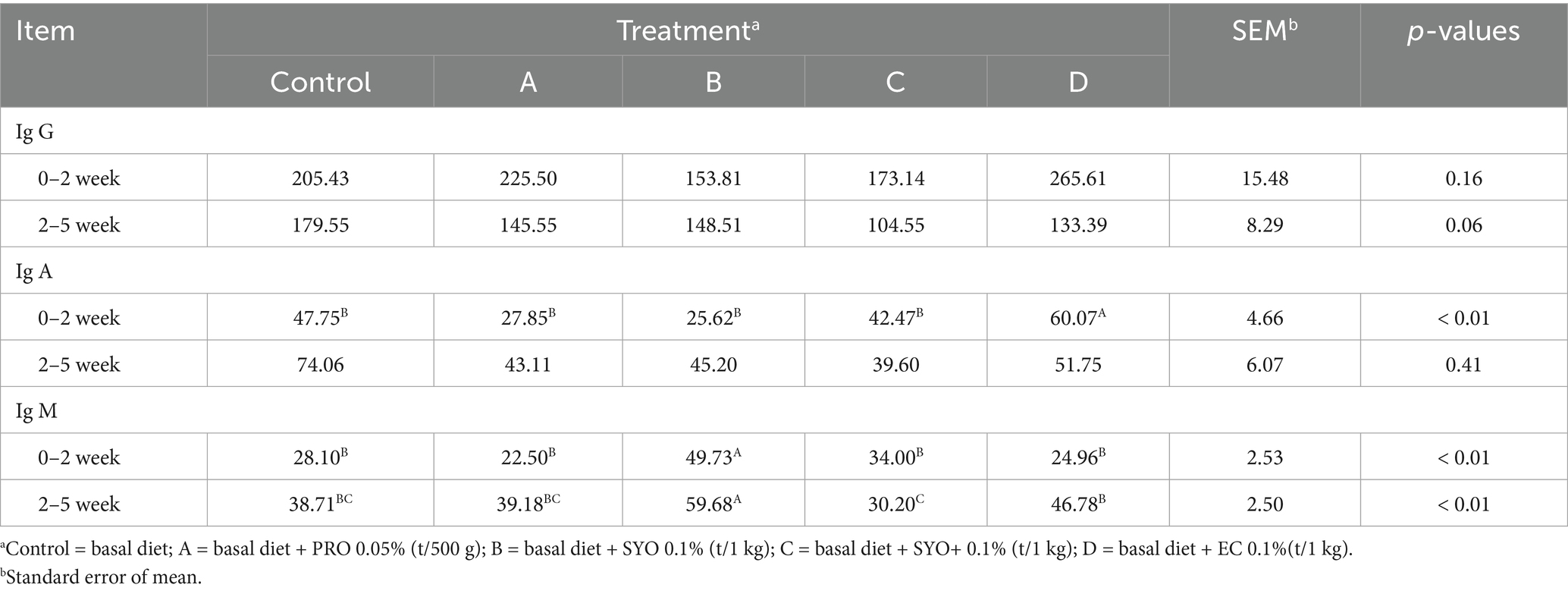
Results: The growth performance did not show significant differences according to the feed additives. In terms of immunity, B treatment increased immunoglobulin M levels, while D treatment increased immunoglobulin A levels during weeks 0–2 (p < 0.01). Additionally, both B and D treatments decreased Mycoplasma spp. in the gut, as indicated by log fold change (LFC) values of −1.571 and −1.529, respectively.
Conclusion and implications: Therefore, this study highlights the potential of Bacillus-based biotics and enzyme cocktail as practical alternatives for reducing pathogenic microorganisms such as Mycoplasma spp. and improving immunity in nursery pigs under commercial conditions.
1 Introduction
The weaning period is one of the most stressful phases for nursery pigs, characterized by various stressors such as environmental, social, and dietary changes, which can lead to negative effects such as reduced feed intake and weight loss (1, 2). These changes can adversely affect the gastrointestinal tract and can lead to diseases such as diarrhea in vulnerable nursery pigs. However, the prohibition of antibiotics and some minerals due to issues such as antibiotics resistance and residues in the body has prompted research into alternative feed additives (3, 4). Among these additives, probiotics, synbiotics, and enzyme cocktail have been commonly used in swine diet.
Probiotics are defined as live microorganisms that when given in adequate amounts, and they help establish a balanced gut microbial community in the intestine (5, 6). The major effects of orally administered probiotics on the gut include: (1) regulation of intestinal microbial communities; (2) inhibition of pathogenic bacteria; (3) immune regulation; (4) enhancement of epithelial cell proliferation and differentiation and strengthening of the intestinal barrier (7). These key effects enhance the responsiveness of intestinal epithelial and immune cells to the gut microbiota, improve the overall metabolic function of the gut microbial community, and suppress pathogens, thereby shifting the gut microbiota towards a more beneficial state (8). Synbiotics, which combine probiotics and prebiotics, offer many of the same benefits as probiotics alone. For instance, Girard et al., (9) showed that synbiotics supplementation promotes the growth of beneficial gut bacteria while reducing harmful metabolites. Notably, many probiotics and synbiotics formulations utilize Bacillus spp. as their primary bacterial component, with Bacillus subtilis and Bacillus licheniformis being the most commonly used species (10). Bacillus subtilis is known for secreting numerous antimicrobial peptides, including bacteriocins and lipopeptides, which collectively inhibit a broad range of gut pathogens (11), and Bacillus licheniformis produces lichenysin, a lipopeptide antibiotic that disrupts pathogen cell membranes and biofilms and provides strong antibacterial effects in the intestinal environment (12). Both species help maintain gut health and are often used alone or in combination to achieve complementary benefits (13).
Enzyme cocktails are a mixture of multiple enzymes designed to maximize efficiency by utilizing the synergy between each enzyme. These blended enzymes hydrolyze cell-wall polysaccharides and phytic acids to maximize the utilization of phosphorus, proteins, and minerals, thereby improving the digestibility of energy and amino acids (14). Concurrently, enzyme cocktails modulate the gut microbiota by generating fermentable oligosaccharides through exogenous carbohydrases (15). These oligosaccharides promote the growth of beneficial bacteria, increase microbial diversity, and improve fermentation profiles, thus supporting intestinal health and function (16). Several studies have also shown that supplementing pig diets with enzyme cocktails improves growth performance, enhances nutrient digestibility, and beneficially modulates the gut bacterial community (17, 18).
Pig farms are complex environments with diverse microorganisms, some of which can impact the productivity and health of pigs. Notably, porcine reproductive and respiratory syndrome virus (PRRSV), porcine circovirus-2 (PCV-2), Mycoplasma hyopneumonia, (MH), Pasteurella multicide A (PMA), Haemophilus parasuis (HP), Actinobacillus pleuropneumonia type 2, (APP2), and Actinobacillus pleuropneumonia type 5 (APP5) are major pathogens in the porcine respiratory disease complex (PRDC), responsible for enzootic pneumonia (19, 20). Among these pathogens, MH is the primary causative agent of enzootic pneumonia in swine, leading to chronic respiratory disease and economic losses (21). In response, attempts have been made to regulate the pathogenic gut microbiota using probiotics and prebiotics as alternative approaches (22, 23). For instance, Pahumunto et al. (24) evaluated potential probiotics strains for their effects on porcine pathogens and immune modulation. However, the mechanisms of interaction between probiotics, synbiotics, enzyme cocktail, and the gut microbiota are not yet clearly understood, and the effects of these feed additives in nursery diets have shown inconsistent results.
Therefore, this study is notable for being conducted directly under commercial farm conditions, with the aim of evaluating the effects of Bacillus-based biotics and enzyme cocktail supplementation in nursery pig diets on growth performance, immune status, gut health, and the reduction of pathogenic microorganisms. By conducting the research under commercial farm conditions, this study provides practical insights into how these additives perform in real-world settings, offering applicable nutritional strategies beyond controlled laboratory conditions.
2 Materials and methods
All experimental protocols involving animals in the present study were approved by the Institutional Animal Care and Use Committee (IACUC) of the Gyeongsang National University (GNU-231030-P0204). This experiment was conducted in a commercial farm to investigate the antibody titers of PRRSV, PCV-2, MH, APP2, APP5, PMA, and HP, which are considered major causative agents of porcine respiratory disease complex, using a serum ELISA test. In this experimental farm, seropositive reactions were observed for PRRSV, PCV-2, and MH.
2.1 Obtained feed additives
Feed additive used in all experiments were composed as follows: Probiotics (PRO) Bacillus subtilis 1.0 × 108 cfu/g, Bacillus coagulans 1.0 × 108 cfu/g, and Clostridium butyricum 1.0 × 106 cfu/g; synbiotics (SYO) Bacillus licheniformis 1.0 × 108 cfu/g and more than 2% fructooligosaccharides; synbiotics + (SYO+) Bacillus licheniformis 1.0 × 108 cfu/g, Bacillus subtilis 1.0 × 108 cfu/g, and more than 2% fructooligosaccharides; enzyme cocktail (EC) At least 500,000 unit/kg of phytase, at least 1,500,000 unit/kg of α-amylase, at least 40,000 unit/kg of cellulase, at least 50,000 unit/kg of xylanase, at least 300,000 unit/kg of neutral protease, at least 500 unit/kg of lipase, and at least 1,500,000 unit/kg of α-amylase. All feed additives were provided by Woogene B&G (WOOGENE B&G CO. LTD, Seoul, Republic of Korea).
2.2 Experimental procedure and diet
A total of 200 crossbred ([Landrace × Yorkshire]) × Duroc) nursery pigs (initial body weight of 5.60 ± 0.05 kg) were used in this study. The pigs were allocated to five dietary treatments based on sex and initial body weight (BW), using a randomized complete block design (RCBD), with four replicates of 10 pigs per pen. The experimental treatments included: (1) CON: a corn-soybean meal-basal diet (basal diet), (2) Treatment A: basal diet supplemented with 0.05% probiotics (PRO), (3) Treatment B: basal diet with 0.1% synbiotics (SYO) containing one strain, (4) Treatment C: basal diet with 0.1% SYO containing two strains, and (5) Treatment D: basal diet with 0.1% enzyme cocktail (EC). The study lasted for a total of 35 days.
The experimental diets (Table 1) were formulated according to the nutrient requirements outlined by the National Research Council (NRC) 2012 and were divided into two phases following the phase feeding program for nursery pigs: Phase 1 (P1) and Phase 2 (P2). Phase 1 lasted for 14 days, and Phase 2 for 20 days. The chemical composition of the diets included 22.2% crude protein (CP) and 3,400 kcal/kg metabolizable energy (ME) for Phase 1, and 20.1% CP with the same ME level for Phase 2.
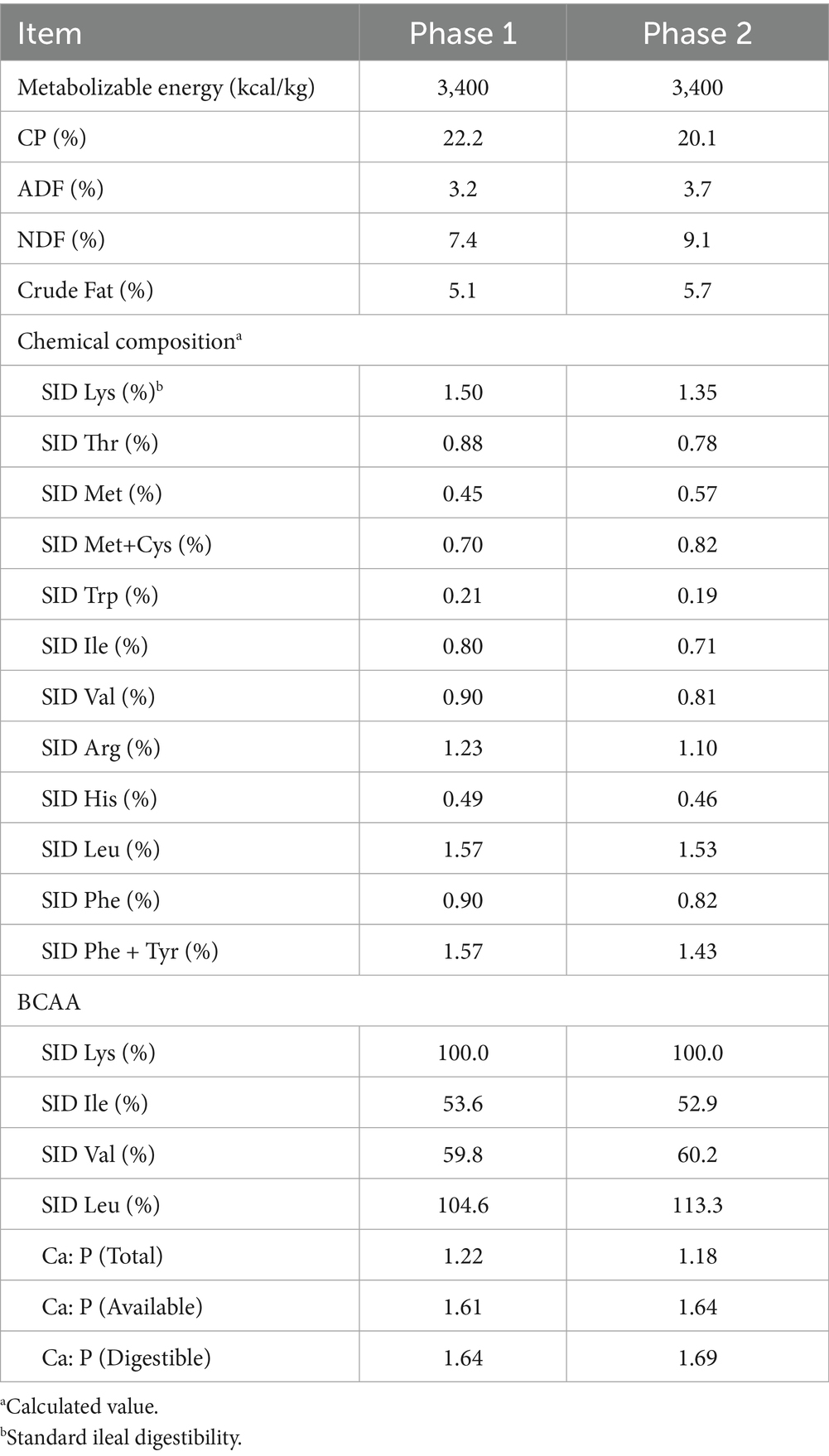
Table 1. Composition of the basal nursery pig diet (Phase 1, Phase 2)a.
2.3 Housing and sampling
Each replicate consists of 10 pigs housed in pens with a slotted floor structure (3.0 × 1.8 m2). Each pen was equipped with a feeder and an automatic waterer, allowing pigs ad libitum access to feed and water throughout the experimental period. The room’s temperature was initially maintained at 30°C and progressively lowered to 25°C until the completion of the trial with humidity between 60 and 70%. Body weight (BW) was recorded at the start of the experiment, d 14 (P1), and d 35 (P2). Feed intake was recorded daily, and residual feed was measured at d 14 and d 35 to calculate average daily gain (ADG), average daily feed disappearance (ADFD), and gain to feed ratio (G: F ratio).
Blood samples were collected from the jugular vein of two randomly selected male pigs from each pen at d 14 and d 35 to analyze the immune indicators. Additionally, fecal samples were collected from one pig per pen, selected based on proximity to the average body weight, at the end of the experiment for gut microbiota analysis.
2.4 Sample analysis
Blood samples were collected in serum separator tubes (SST) and centrifuged at 3,500 RPM for 15 min to separate the serum. The separated serum was transferred to a 1.5 mL microtubes and stored at −25°C. To assess immune responses, levels of immune globulin G (IgG), immune globulin A (IgA), and immune globulin M (IgM) were analyzed using enzyme-linked immunosorbent assay (ELISA) kits, following the manufacturer’s protocol [IgG (ab291065, Abcam, USA), IgA (ab190536, Abcam Singapore Pte Ltd., USA) IgM (ab190537, Abcam, USA)].
Fecal samples were collected in fecal tubes, and processed according to the 16S sequencing standard workflow (Illumina, San Diego, USA). The sequence data generated were analyzed using QIIME2 (ver. 2023.9, QIIME2 development team, Boulder, CO, USA). Raw reads were demultiplexed and quality-filtered using the DADA2 plugin in QIIME2, truncating reads with Phred scores below 20 and removing chimeric sequences. Taxonomic assignment was performed against the SILVA 138 reference database (25). Then, amplicon sequence variants (ASVs) were obtained through the feature-table generated by the QIIME2 pipeline. Alpha-diversity metrics, including Chao1 richness, Shannon diversity, and Simpson diversity, were analyzed to compare the control and treatment groups using QIIME2. For beta-diversity, Jaccard and Bray-Curtis indices were computed, and permutational multivariate analysis of variance (PERMANOVA) was conducted to evaluate the statistical significance of microbial distribution difference. Additionally, the log fold change (LFC) of microbial communities between the control and treatment groups was obtained at both the family and genus levels through ANCOM-BC analysis. The LFC obtained from ANCOM-BC analysis were adjusted using the false discovery rate (FDR) procedure (26).
2.5 Statistical analysis
Statistical analyses were performed using the general linear model (GLM) of SAS (SAS 9.4, SAS Institute Inc., Cary, NC, U. S) to evaluate the significance of the collected data, with dietary treatment as the fixed effect. Outliers were identified and removed by applying the adjusted quartile method to each pen and cage. For PERMANOVA analysis, distribution-free permutation techniques were employed to obtain p-values via QIIME2 (27). Statistical differences were considered highly significant at p < 0.01, significant at p < 0.05, and tendencies were noted if 0.05 < p 0.1.
3 Results
3.1 Growth performance
The effects of adding PRO, SYO, SYO+, and EC to the nursery pig diet on the growth performance of nursery pigs are shown in Table 2. BW showed no significant differences at the initial, P1, and P2, and the ADG, ADFD, and G: F ratio were also not significantly different among treatments.
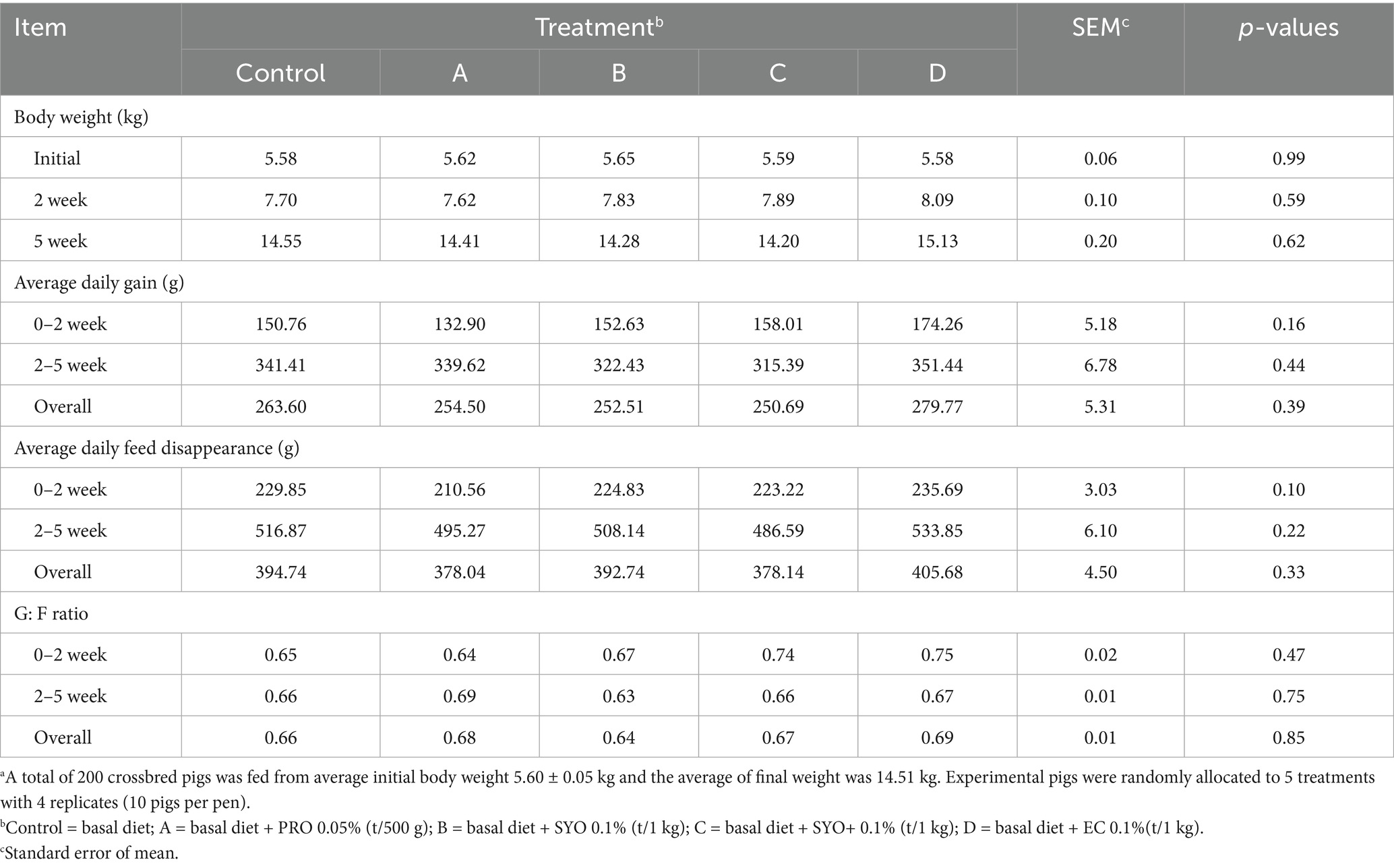
Table 2. Effects of adding PRO, SYO, SYO+, and EC on growth performance in nursery pigsa.
3.2 Immune status
Table 3 showed the immune status of nursery pigs fed diets supplemented with PRO, SYO, SYO+, and EC. IgG concentration showed no significant differences during P1 but displayed tendencies during P2 (p = 0.06). IgA concentrations were highly significant in Treatment D during P1 (p < 0.01), with no significant differences observed during P2 (p > 0.1). IgM concentrations showed highly significant differences during P1 (p < 0.01) and P2 (p < 0.01) in Treatment B.
3.3 Fecal microbiota diversity analysis
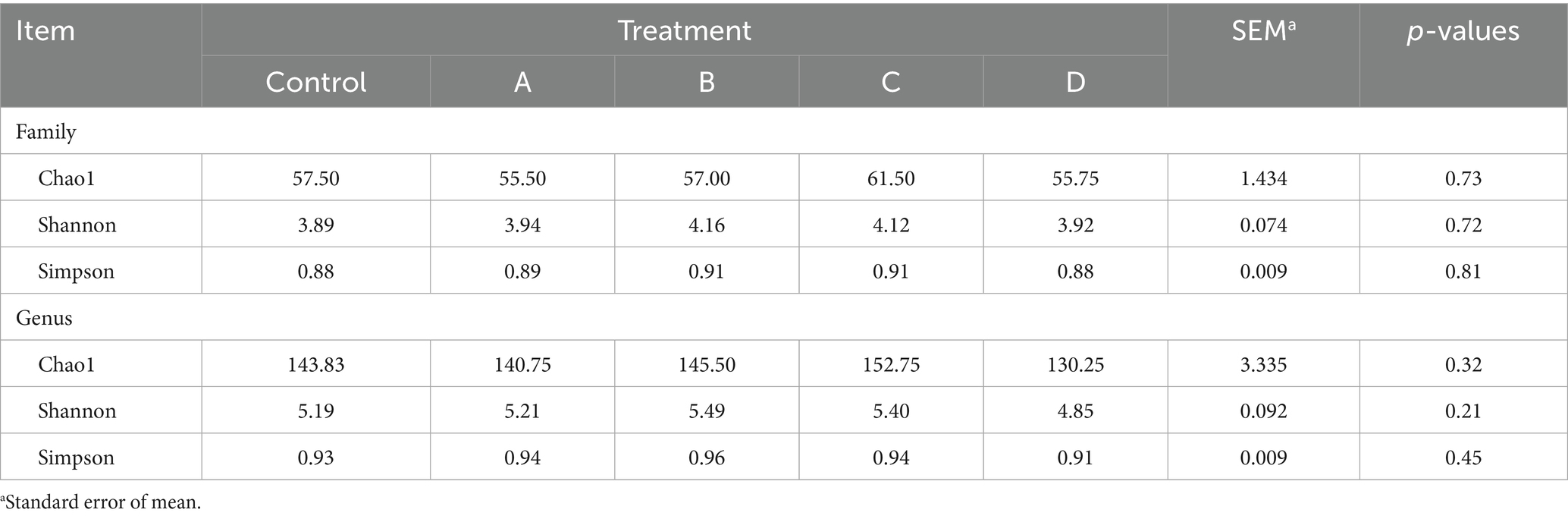
Alpha-diversity at the family and genus levels, estimated using Chao1 richness, Shannon diversity, and Simpson diversity (Table 4), was not affected by the addition of PRO, SYO, SYO+, and EC to nursery pig diets. Beta-diversity analysis using PERMANOVA indicated no significant differences between the control (CON) and other treatments, as shown by both Jaccard and Bray-Curtis indices (Table 5).
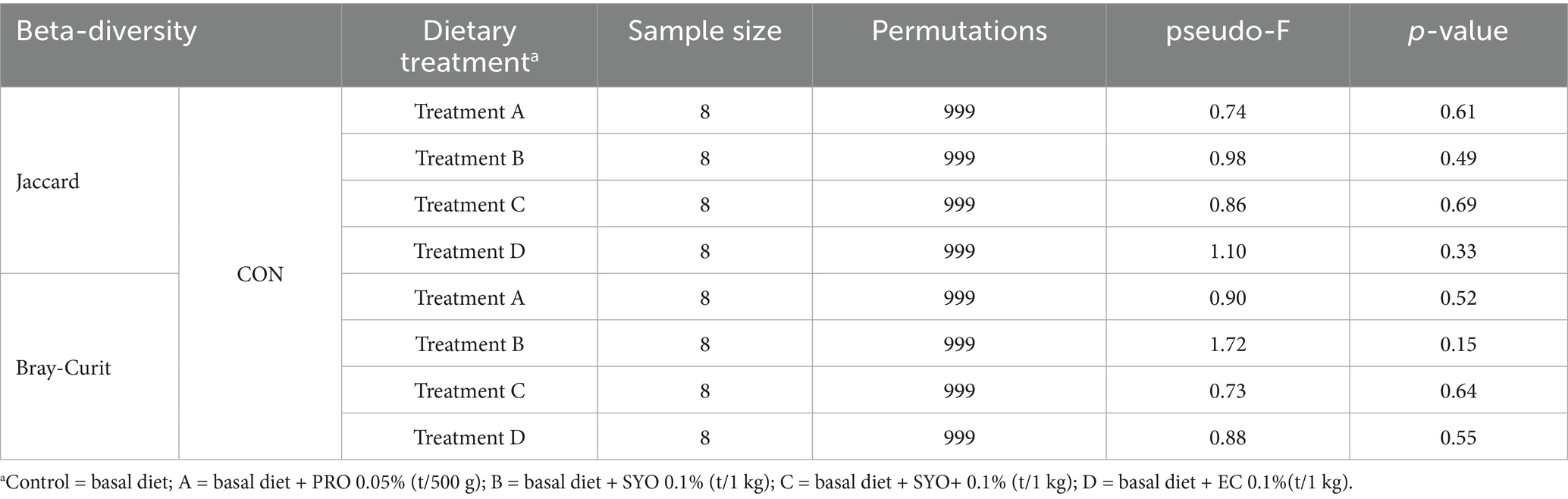
Table 5. Effects of adding PRO, SYO, SYO+, and EC on beta-diversity PERMANOVA results in nursery pigs.
3.4 Fecal microbiota LFC analysis
Figure 1 illustrates the log fold change (LFC) in microbial communities between the control and Treatment A (PRO). At the family level, Anaerovoracaceae increased (lfc = 0.178) while Deferribacteraceae decreased (lfc = −1.836). At the genus level, Pectinophilus decreased (lfc = −3.735). Figure 2 shows LFC comparing the control and Treatment B. At the family level, increases were observed in Pirellulaceae (lfc = 1.597), Corynebacteriaceae (lfc = 1.461), Christensenellaceae (lfc = 1.204), RF39 (lfc = 1.043), Muribaculaceae (lfc = 0.980), and Anaerovoracaceae (lfc = 0.707). At the genus level, Mycoplasma (lfc = −1.571) and Pectinophilus (lfc = −3.904) decreased. Figure 3 presents the LFC between the control and Treatment C. At the family level, Anaerovoracaceae decreased (lfc = −0.081). At the genus level, Eubacterium_nodatum (lfc = −2.406) and Pectinophilus (lfc = −3.904) decreased, while UCG-002 increased (lfc = 1.003). Figure 4 illustrates the LFC at the family level comparing the control and Treatment D, with increases in Pirellulaceae (lfc = 1.897) and Anaerovoracaceae (lfc = 0.752), while decreases were observed in Mycoplasmataceae (lfc = −1.700) and Selenomonadaceae (lfc = −3.374). At the genus level, Mycoplasma (lfc = −1.529) and Pectinophilus (lfc = −3.374) decreased.
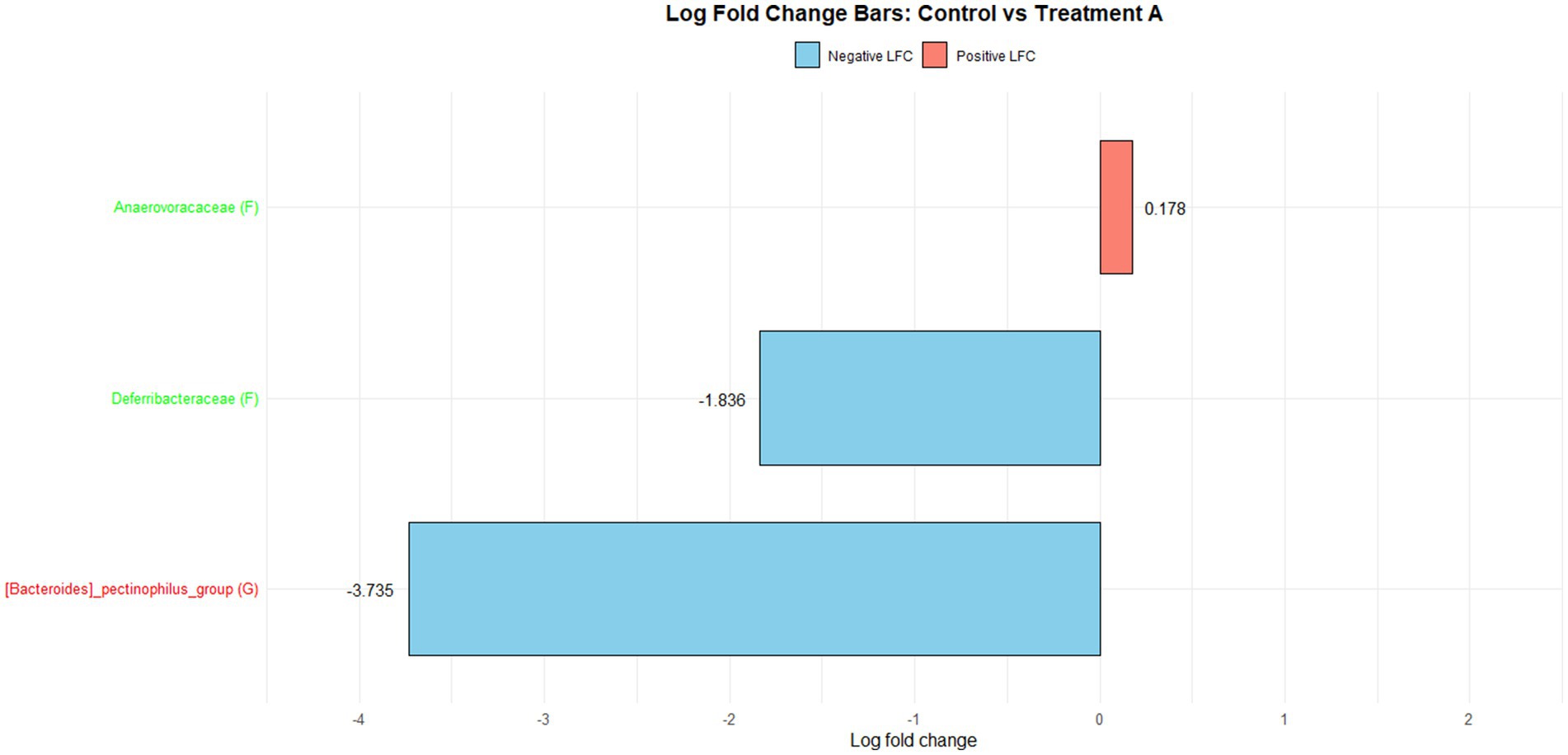
Figure 1. The log fold change (Control vs. A group/Family & Genus), where ‘f__’ represent family and ‘g__’ represent genus.
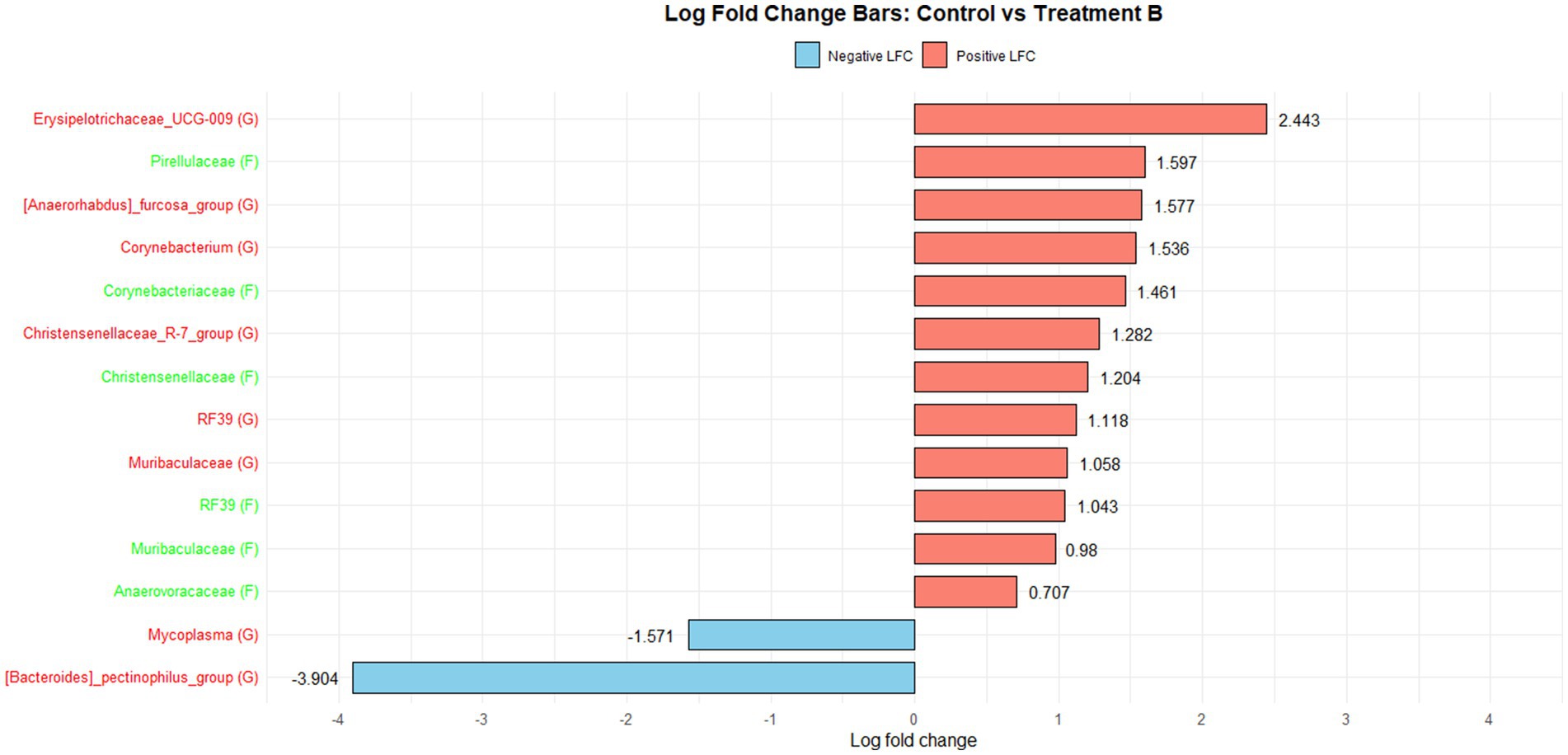
Figure 2. The log fold change (Control vs. B group/Family & Genus), where the left side represents family (f__) and the right side represent genus (g__).
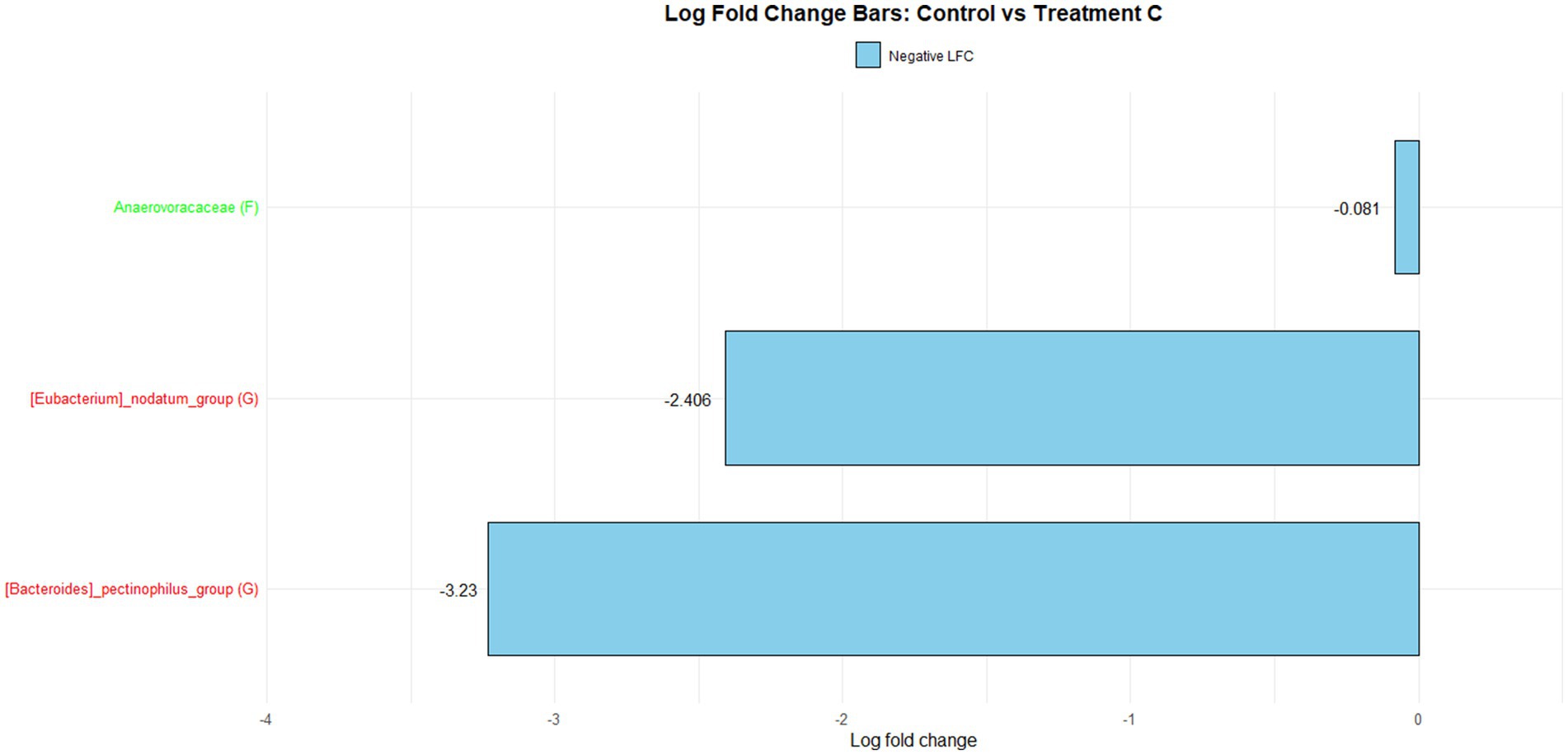
Figure 3. The log fold change (Control vs. C group/Family & Genus), where ‘f__’ represent family and ‘g__’ represent genus.
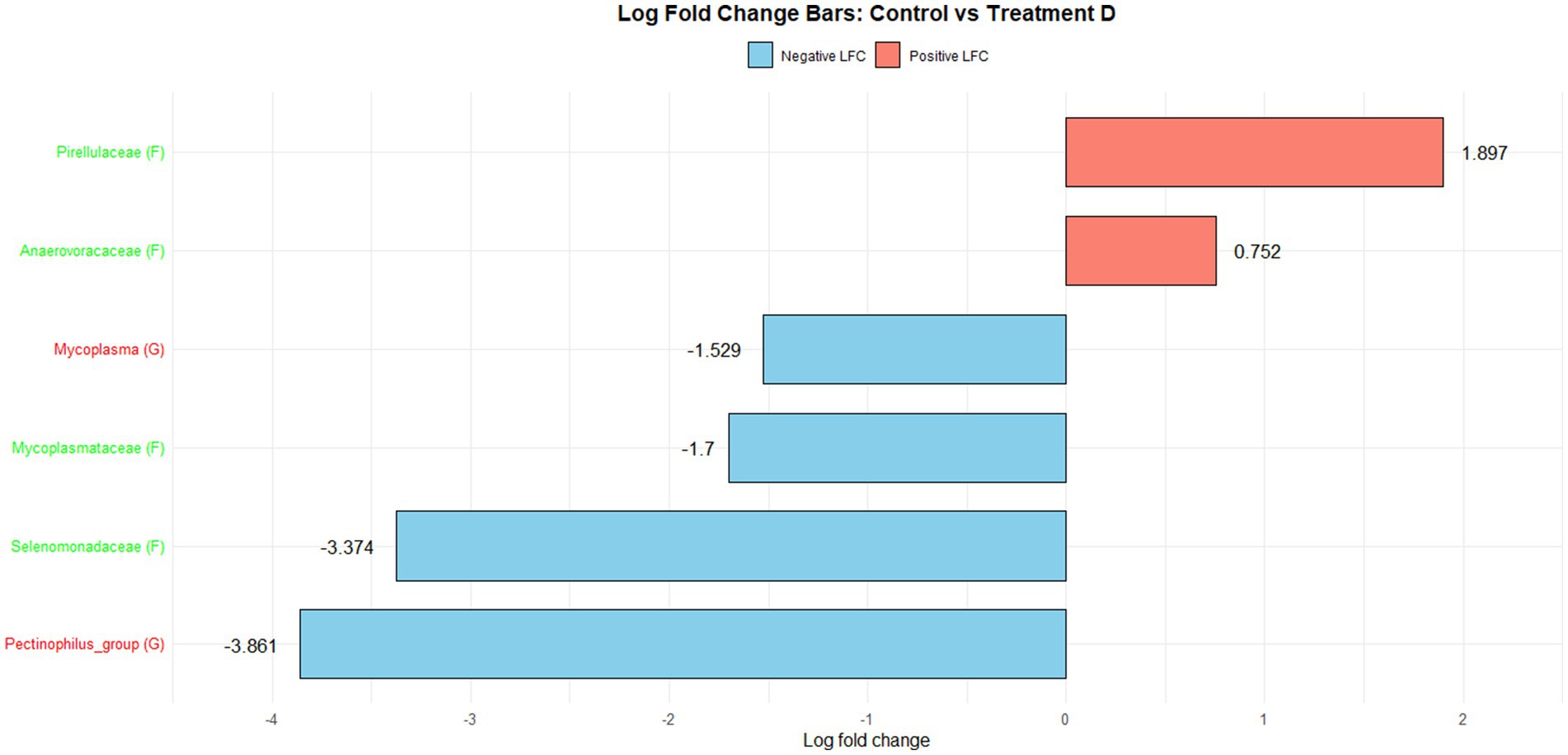
Figure 4. The log fold change (Control vs. D group/Family & Genus), where the left side represents family (f__) and the right side represent genus (g__).
4 Discussion
4.1 Effects of various feed additives on pig growth performance
Feed additives are commonly used in animal nutrition to enhance the health and productivity of livestock, with probiotics, synbiotics, and enzyme cocktails being the most frequently applied. Probiotics help maintain intestinal microbial balance and promote overall health by improving gut epithelial barrier function, activating the immune system, degrading toxin receptors, and modulating the gut microbiota (28, 29). Synbiotics complement probiotics by providing specific substrates for fermentation, enhancing the availability of beneficial bacteria and offering additional health benefits to the host (30). In this study, the PRO, SYO, and SYO + used Bacillus spp. or Clostridium spp., which are representative strains in probiotics and synbiotics known for producing various enzymes and short-chain fatty acids (SCFA) (31). SCFA, including acetate, propionate, and butyrate, interact with SCFA-activated receptors free fatty acid receptor 2 (FFA2) and free fatty acid receptor 3 (FFA3) in L-cells, influencing appetite regulation (32). L-cells secrete glucagon-like peptide-1 (GLP-1) and peptide YY (PYY), which act via the vagus-brainstem-hypothalamic pathway (33). Within the hypothalamus, the arcuate nucleus (ARC) involves pro-opiomelanocortin (POMC) and cocaine and amphetamine regulated transcript (CART), where GLP-1 and PYY interact with Y2 receptors to increase POMC activity and suppress appetite (34). This mechanism may explain the lack of significant differences in overall ADFD between the PRO, SYO, and SYO + treatment groups and the Control group. Batterham et al. (35) found that chronic peripheral administration of PYY3-36 in mice increased POMC activity, decreased appetite, and reduced body weight. However, these results were not inconsistent. According to a study by Cheng and Kim et al. (36) found that supplementing microorganisms as feedstuffs and feed additives improved the ADG by 1.83%, and the daily feed intake by 0.24% in pigs and broilers. Also, Duarte et al. (37) highlighted that adding synbiotics consisting of xylanases and Bacillus spp. to nursery pig diets improved ADG and the G: F ratio, demonstrating their potential to enhance the intestinal health of nursery pigs challenged with enterotoxigenic Escherichia coli. Therefore, the complexity of the effects of probiotics and synbiotics on nursery pig growth and health suggests that their efficacy depends on specific microbial compositions, strain types, specific condition, and physiological contexts. In addition, the limitations of the additive effects seem to be influenced by the following socio-environmental factors. First, the experiment was conducted in a commercial setting with 10 animals per pen, making it difficult to control for social factors among individuals. Second, the limited number of repetitions made it challenging to achieve statistical significance between treatment groups. Third, due to the nature of commercial farms, there were inherent limitations in controlling environmental factors such as various diseases. However, this study did not measure SCFAs, so these findings remain hypothetical. Therefore, we suggest that additional gut metabolites are needed.
Enzyme cocktail can enhance nutrient digestibility, as seen with phytase activity increasing phosphorus bioavailability (38). Additionally, unlike probiotics and synbiotics, enzyme cocktails directly degrade specific nutrients, improving their availability and potentially enhancing the growth performance of nursery pigs (39). However, in this study, EC treatment did not significantly affect nursery pig growth performance. These inconsistencies highlight the complexity of enzyme cocktail efficacy, which may be influenced by factors such as host response, and environmental conditions. Also, several studies have identified varying factors affecting enzyme cocktail performance, including enzyme activity, enzyme activity level, substrate availability, fiber composition, enzyme matrix, and experimental conditions (40–43). Nevertheless, Trindade Neto et al. (44) reported that adding enzyme cocktail on nursery pig diet improved growth performance. Zhang et al. (45) found that supplementing corn-soybean meal diets for nursery pigs with exogenous multi-enzyme has the potential to enhance gut health, improve nutrient digestion, and boost growth performance. Although similar results were not observed, the enzyme cocktail suggests potential for improving growth performance in nursery pigs. Therefore, future research should evaluate various combination and supplementation levels of enzymes to optimize enzyme cocktail efficacy for digestibility and growth performance.
4.2 Effects of various feed additives on pig immune status
The representative indicators of serum immunoglobulins, IgG, IgA, and IgM are produced by B cell, which primarily manage humoral immunity. IgM provides a rapid immune response to maintain tissue balance, IgA forms the immune system in the gastrointestinal mucosa, and IgG is the most abundant antibody and plays the largest role in defense against antigens (46, 47).
In the study, the enzyme cocktail used contained phytase, which breaks down phytic acid [myo-inositol 1,2,3,4,5,6-hexakis (dihydrogen phosphate); InsP6], a form of phosphorus commonly found in plants. Phytic acid is classified as an anti-nutritional factor due to its inability to be digested by pigs, which limits the availability of phosphorus. The phosphates fraction for animal availability depend on the breakdown of InsP6, which can be degraded by phytase into less-phosphorylated InsP5 to InsP1, myo-inositol, and orthophosphates (48). The inositol phosphates isomers (InsPs) produced in this process are recognized by macrophages and toll-like receptor 2 (TLR2) in the intestinal mucosa, leading to the activation of dendritic cells (DCs). The activated DCs induce regulatory T cells that modulate immune responses and increase IgA levels (49, 50).
During the 0–2 week and 0–5 week periods, the addition of SYO to the diets of nursery pigs improved IgM concentration. The increase in IgM may be related to Bacillus licheniformis present in SYO. Bacillus licheniformis is known to exert antiviral and immunoregulatory effects (45). In a study by Wang et al. (51), Bacillus licheniformis was found to reduce the expression of pro-inflammatory cytokines (TNF-α and IL-1β) and increase levels of anti-inflammatory cytokines (IL-10 and IL-4). IL-10 and IL-4 are key co-factors for B cell proliferation and promote differentiation into plasmablasts, which secrete IgM or IgG (52, 53). Therefore, this is thought that Bacillus licheniformis in SYO stimulates the immune system, inducing anti-inflammatory cytokines, which in turn increase IgM. However, in the case of SYO+, the increase in IgM was not observed, which is suggested to be due to the interaction between Bacillus licheniformis and Bacillus subtilis within SYO+. These findings suggest that interaction between Bacillus licheniformis and Bacillus subtilis in SYO + may have reduced the ability of Bacillus licheniformis to raise IgM levels observed with the single-strain SYO supplement.
Consequently, the addition of SYO and enzyme cocktail in nursery pig diet can activate the immune system. These findings suggest that the inclusion of SYO + and enzyme cocktail in nursery pig diets can stimulate the immune system, highlighting the potential for improved health management in commercial pig farming. However, this study did not measure anti-inflammatory markers, and these findings remain hypothetical. Therefore, we suggest that additional immunological or metabolomic analyses are needed.
4.3 Effects of various feed additives on pig fecal microbiota
Feed additives play a significant role in modulating gut microbiota composition. Probiotics, prebiotics, and synbiotics often referred to as bioactive components, can promote gut health by inhibiting the growth of pathogen and supporting beneficial gut microbiota. Previous studies have shown that these bioactive components influence gut microbial diversity (Alpha-diversity and Beta-diversity) and alter gut microbiota composition (54, 55). We investigated the effects of various feed additives on microbial alpha and beta diversity, with beta diversity analyzed by PERMANOVA using a multivariate distance matrix (27). However, in our study, the addition of feed additives in nursery pig feed did not significantly affect alpha-diversity and beta-diversity. The gut microbiota of nursery pigs is significantly influenced by various internal and external factors, and as it stabilizes over time, the diversity of the gut microbiota may decrease (56, 57). In this study, the result of fecal analysis did not show significant differences in microbial diversity among treatments. The microbiota is a dynamic and complex structure that can be influenced by various factors such as age, dietary composition, time, and breeding environment (58–60). According to Fonseca et al. (61) study, the alpha-diversity tended to increase during the first 4 days but no significant difference was observed at over time. Also, the study by Da Sliva et al. (62) reported that beta-diversity was unchanged over time despite the addition of various probiotics to broiler diets, which in line with our result, suggesting that microbial diversity temporarily increased during the initial period of the experiment, but the effect did not remain over time. However, in this study, we did not perform longitudinal sampling, which prevented us from analyzing microbial trajectories over time. Moreover, we did not measure gut metabolites that could support the proposed mechanisms. Future studies integrating metabolomic analyses with microbial profiling would strengthen causal inferences.
Among the major findings, the significant reduction of Mycoplasma spp. by synbiotics and enzyme cocktail supplementation is noteworthy, given the pathogen’s importance in swine respiratory disease. Log Fold Change (LFC) analysis does not represent the absolute or relative abundance within a single sample but rather indicates the fold change between two samples (63). In our study, LFC analysis of feed additive supplementation showed a reduction in pathogenic Mycoplasma spp. in the SYO treatment group. Mycoplasma spp., lacking a cell wall, can evade immune system recognition, attach to cell receptors, penetrate cell membranes, and cause inflammatory responses (64). However, the antibiotic compounds produced by certain strains stimulate the immune system by promoting anti-inflammatory cytokine release and B-cell activation, thereby enhancing immunoglobulin secretion (65, 66). Consequently, the reduction of Mycoplasma spp. in the SYO group may be attributed to lichenysin, an antibiotic produced by Bacillus licheniformis. Lichenysin, a modified form of surfactin, is a lipopeptide biosurfactant used against Mycoplasma spp. (67, 68). It penetrates the cell membrane of Mycoplasma spp., inducing micelle formation, destabilizing the membrane, and leading to growth inhibition or cell death (69).
Interestingly, the reduction in Mycoplasma spp. was not observed in the PRO and SYO + groups, possibly due to interactions between Bacillus subtilis and Bacillus licheniformis. These strains primarily utilize carbon sources as nutrients (70), and competition may reduce their inhibitory effects on Mycoplasma spp. Vijayalakshmi et al. (70) also reported that Bacillus subtilis exhibits higher stability under various conditions compared to Bacillus licheniformis. Additionally, differences in antibiotics produced by these strains may explain the lack of reduction in Mycoplasma spp. The Mycoplasma spp., lacking a cell wall, have a higher exposure and content of membrane lipids (71). Surfactin, produced by Bacillus subtilis, has lower lipid affinity and surface activity than lichenysin (72), which may account for the observed results. However, these effects have not been consistent. While some studies reported that co-culturing Bacillus subtilis and Bacillus licheniformis was effectively inhibits pathogens (73, 74). Nevertheless, studies on competitive and symbiotic interactions between the two strains in pigs were limited, we cannot rule out the possibility that they vie for the same resources. Therefore, these results suggest that in vitro co-culture and in vivo validation studies are needed to clarify their compatibility and mechanisms of interaction.
In the enzyme cocktail treatment group, a reduction in Mycoplasma spp. was observed, though the mechanism remains unclear. The EC used in this study contained phytase and lipase. Phosphorus released during phytic acid degradation by phytase can stimulate SCFA production, enhance mineral absorption, and lower pH, inhibiting pathogenic bacteria growth (75, 76). Galié et al. (77) reported that various enzymes degrade biofilm structures, thereby inhibiting pathogen adhesion and survival. However, lipid-hydrolyzing enzymes like lipase can release long-chain fatty acids, potentially promoting the growth of Mycoplasma spp. (78, 79). Thus, the reduction in Mycoplasma spp. in the EC group likely results from the interaction between phytase and lipase, balancing phytase’s inhibitory effects on pathogenic bacteria with lipase’s potential growth-promoting effects on Mycoplasma spp.
5 Conclusion
The incorporation of probiotics, synbiotics, and an enzyme cocktail into the diets of nursery pigs, under commercial farming conditions, did not significantly impact growth performance directly. However, positive effects were observed in immune status and gut microbiota. Notably, nursery pigs exhibited increased feed intake, which is anticipated to contribute positively to future growth. Therefore, this study suggests that Bacillus-based biotics and enzyme cocktails have potential as alternatives to antibiotics for controlling infections caused by pathogenic microorganisms in nursery pigs under commercial conditions. However, we suggest that additional studies with increased replicates and pen numbers are necessary for application in commercial large-scale farms.
Data availability statement
The datasets presented in this study can be found in online repositories. This data can be found here: NCBI repository, accession number PRJNA1290052.
Ethics statement
The animal study was approved by Institutional Animal Care and Use Committee (IACUC) of the Gyeongsang National University (GNU-231030-P0204). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
S-MK: Formal analysis, Investigation, Software, Visualization, Writing – original draft, Writing – review & editing. J-HL: Investigation, Writing – original draft. S-HO: Formal analysis, Software, Writing – original draft. J-CJ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by a contract research project funded by Woogene B&G. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Acknowledgments
The author(s) would like to acknowledge Woogene B&G for providing the feed additives.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2025.1627739/full#supplementary-material
References
1. Heo, JM, Opapeju, FO, Pluske, JR, Kim, JC, Hampson, DJ, and Nyachoti, CM. Gastrointestinal health and function in weaned pigs: a review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. Anim Physiol Nutr. (2013) 97:207–37. doi: 10.1111/j.1439-0396.2012.01284.x
2. Yu, SJ, Morris, A, Kayal, A, Milošević, I, Van, TTH, Bajagai, YS, et al. Pioneering gut health improvements in piglets with phytogenic feed additives. Appl Microbiol Biotechnol. (2024) 108:142. doi: 10.1007/s00253-023-12925-2
3. Chattopadhyay, MK. Use of antibiotics as feed additives: a burning question. Front Microbiol. (2014) 5:334. doi: 10.3389/fmicb.2014.00334
4. Yaqoob, MU, Wang, G, and Wang, M. An updated review on probiotics as an alternative of antibiotics in poultry — a review. Anim Biosci. (2022) 35:1109–20. doi: 10.5713/ab.21.0485
6. Yang, I, Corwin, EJ, Brennan, PA, Jordan, S, Murphy, JR, and Dunlop, A. The infant microbiome: implications for infant health and neurocognitive development. Nurs Res. (2016) 65:76–88. doi: 10.1097/NNR.0000000000000133
7. Hemarajata, P, and Versalovic, J. Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation. Ther Adv Gastroenterol. (2013) 6:39–51. doi: 10.1177/1756283X12459294
8. Bron, PA, Van Baarlen, P, and Kleerebezem, M. Emerging molecular insights into the interaction between probiotics and the host intestinal mucosa. Nat Rev Microbiol. (2012) 10:66–78. doi: 10.1038/nrmicro2690
9. Girard, M, Tretola, M, and Bee, G. A single dose of Synbiotics and vitamins at birth affects piglet microbiota before weaning and modifies post-weaning performance. Animals. (2021) 11:84. doi: 10.3390/ani11010084
10. Tang, X, Zeng, Y, Xiong, K, and Zhong, J. Bacillus spp. as potential probiotics: promoting piglet growth by improving intestinal health. Front. Vet. Sci. (2024) 11:1429233. doi: 10.3389/fvets.2024.1429233
11. Caulier, S, Nannan, C, Gillis, A, Licciardi, F, Bragard, C, and Mahillon, J. Overview of the antimicrobial compounds produced by members of the Bacillus subtilis group. Front Microbiol. (2019) 10:302. doi: 10.3389/fmicb.2019.00302
12. Zhu, J, Chen, Y, Imre, K, Arslan-Acaroz, D, Istanbullugil, FR, Fang, Y, et al. Mechanisms of probiotic Bacillus against enteric bacterial infections. One Health Adv. (2023) 1:21. doi: 10.1186/s44280-023-00020-0
13. Palkovicsné Pézsa, N, Kovács, D, Rácz, B, and Farkas, O. Effects of Bacillus licheniformis and Bacillus subtilis on gut barrier function, Proinflammatory response, ROS production and pathogen inhibition properties in IPEC-J2—Escherichia coli/Salmonella Typhimurium co-culture. Microorganisms. (2022) 10:936. doi: 10.3390/microorganisms10050936
14. Shipman, GL, Perez-Palencia, JY, Rogiewicz, A, Patterson, R, and Levesque, CL. Evaluation of multienzyme supplementation and fiber levels on nutrient and energy digestibility of diets fed to gestating sows and growing pigs. J Anim Sci. (2023) 101:skad375. doi: 10.1093/jas/skad375
15. Valente Junior, DT, Genova, JL, Kim, SW, Saraiva, A, and Rocha, GC. Carbohydrases and Phytase in poultry and pig nutrition: a review beyond the nutrients and energy matrix. Animals. (2024) 14:226. doi: 10.3390/ani14020226
16. Duarte, ME, Sparks, C, and Kim, SW. Modulation of jejunal mucosa-associated microbiota in relation to intestinal health and nutrient digestibility in pigs by supplementation of β-glucanase to corn–soybean meal-based diets with xylanase. J Anim Sci. (2021) 99:skab190. doi: 10.1093/jas/skab190
17. Aderibigbe, AS, Park, CS, Johnson, T, Velayudhan, DE, Vinyeta, E, and Adeola, O. Efficacy of a novel multi-enzyme feed additive on growth performance, nutrient digestibility and gut microbiome of weanling pigs fed corn-wheat or wheat-barley based diet. J Anim Sci. (2024) 102:64. doi: 10.1093/jas/skae064
18. Wang, JP, Hong, SM, Yan, L, Yoo, JS, Lee, JH, Jang, HD, et al. Effects of single or carbohydrases cocktail in low-nutrient-density diets on growth performance, nutrient digestibility, blood characteristics, and carcass traits in growing–finishing pigs. Livest Sci. (2009) 126:215–20. doi: 10.1016/j.livsci.2009.07.003
19. Pieters, MG, and Maes, D. Mycoplasmosis In: JJ Zimmerman, LA Karriker, A Ramirez, KJ Schwartz, GW Stevenson, and J Zhang, editors. Diseases of swine : Wiley (2019). 863–83. doi: 10.1002/9781119350927.ch56
20. Zhang, Y, Gan, Y, Wang, J, Feng, Z, Zhong, Z, Bao, H, et al. Dysbiosis of gut microbiota and intestinal barrier dysfunction in pigs with pulmonary inflammation induced by Mycoplasma hyorhinis infection. mSystems. (2022) 7:e00282-22. doi: 10.1128/msystems.00282-22
21. Zhang, Y, Zhang, Y, Liu, F, Mao, Y, Zhang, Y, Zeng, H, et al. Mechanisms and applications of probiotics in prevention and treatment of swine diseases. Porc Health Manag. (2023) 9:5. doi: 10.1186/s40813-022-00295-6
22. Huang, C-W, Liu, S-Y, Bhattarai, BP, Lee, T-Y, Chang, H-T, Lin, H-C, et al. Live multi-strain probiotics enhance growth performance by regulating intestinal morphology and microbiome population in weaning piglets. Microorganisms. (2024) 12:2334. doi: 10.3390/microorganisms12112334
23. Vega-Munguía, G, Vargas Sánchez, A, Camacho-Medina, JE, Suárez-Vélez, L, Bárcenas-Morales, G, Quintar Guerrero, D, et al. Effect of live and fragmented Saccharomyces cerevisiae in the feed of pigs challenged with Mycoplasma hyopneumoniae. Pathogens. (2024) 13:322. doi: 10.3390/pathogens13040322
24. Pahumunto, N, Dahlen, G, and Teanpaisan, R. Evaluation of potential probiotic properties of Lactobacillus and Bacillus strains derived from various sources for their potential use in swine feeding. Probiot Antimicro Prot. (2023) 15:479–90. doi: 10.1007/s12602-021-09861-w
25. Quast, C, Pruesse, E, Yilmaz, P, Gerken, J, Schweer, T, Yarza, P, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. (2012) 41:D590–6. doi: 10.1093/nar/gks1219
26. Benjamini, Y, and Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. (1995) 57:289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x
27. Anderson, MJ. A new method for non-parametric multivariate analysis of variance. Austral Ecol. (2001) 26:32–46. doi: 10.1111/j.1442-9993.2001.01070.pp.x
28. Kumar, B, and Lebeer, S. Functional mechanisms of probiotics. J Microb Biotech Food Sci. (2015) 4:321–7. doi: 10.15414/jmbfs.2015.4.4.321-327
29. Soccol, CR, Vandenberghe, LPS, Spier, MR, Medeiros, ABP, Yamaguishi, CT, Lindner, JDD, et al. The potential of probiotics: a review. Food Sci Biotechnol. (2010) 48:413–34.
30. Sarangi, NR, Babu, LK, Kumar, A, Pradhan, CR, Pati, PK, and Mishra, JP. Effect of dietary supplementation of prebiotic, probiotic, and synbiotic on growth performance and carcass characteristics of broiler chickens. Vet World. (2016) 9:313–9. doi: 10.14202/vetworld.2016.313-319
31. Kongpanna, P, Doerr, JA, Nilubol, D, and Jamikorn, U. Effect of a multi-species direct-fed microbial on growth performance, nutrient digestibility, intestinal morphology and colonic volatile fatty acids in weanling pigs. Animals. (2024) 14:1749. doi: 10.3390/ani14121749
32. Darzi, J, Frost, GS, and Robertson, MD. Do SCFA have a role in appetite regulation? Proc Nutr Soc. (2011) 70:119–28. doi: 10.1017/S0029665110004039
33. Byrne, CS, Chambers, ES, Morrison, DJ, and Frost, G. The role of short chain fatty acids in appetite regulation and energy homeostasis. Int J Obes. (2015) 39:1331–8. doi: 10.1038/ijo.2015.84
34. Silva, AD, and Bloom, SR. Gut hormones and appetite control: a focus on PYY and GLP-1 as therapeutic targets in obesity. Gut Liver. (2012) 6:10–20. doi: 10.5009/gnl.2012.6.1.10
35. Batterham, RL, Cowley, MA, Small, CJ, Herzog, H, Cohen, MA, Dakin, CL, et al. Gut hormone PYY3-36 physiologically inhibits food intake. Nature. (2002) 418:650–4. doi: 10.1038/nature00887
36. Cheng, Y-C, and Kim, SW. Use of microorganisms as nutritional and functional feedstuffs for nursery pigs and broilers. Animals. (2022) 12:3141. doi: 10.3390/ani12223141
37. Duarte, ME, Tyus, J, and Kim, SW. Synbiotic effects of enzyme and probiotics on intestinal health and growth of newly weaned pigs challenged with Enterotoxigenic F18+Escherichia coli. Front Vet Sci. (2020) 7:573. doi: 10.3389/fvets.2020.00573
38. Beaman, KR, Lilly, KGS, Gehring, CK, Turk, PJ, and Moritz, JS. Influence of pelleting on the efficacy of an exogenous enzyme cocktail using broiler performance and metabolism. J Appl Poult Res. (2012) 21:744–56. doi: 10.3382/japr.2011-00430
39. Murugesan, GR, Romero, LF, and Persia, ME. Effects of protease, phytase and a Bacillus sp. direct-fed microbial on nutrient and energy digestibility, ileal brush border digestive enzyme activity and cecal short-chain fatty acid concentration in broiler chickens. PLoS One. (2014) 9:e101888. doi: 10.1371/journal.pone.0101888
40. Adeola, O, and Cowieson, AJ. BOARD-INVITED REVIEW: opportunities and challenges in using exogenous enzymes to improve nonruminant animal production. J Anim Sci. (2011) 89:3189–218. doi: 10.2527/jas.2010-3715
41. Agyekum, AK, Kiarie, E, Walsh, MC, and Nyachoti, CM. Postprandial portal glucose and lactate fluxes, insulin production, and portal vein-drained viscera oxygen consumption in growing pigs fed a high-fiber diet supplemented with a multi-enzyme cocktail1. J Anim Sci. (2016) 94:3760–70. doi: 10.2527/jas.2015-0076
42. Kiarie, E, Romero, LF, and Nyachoti, CM. The role of added feed enzymes in promoting gut health in swine and poultry. Nutr Res Rev. (2013) 26:71–88. doi: 10.1017/S0954422413000048
43. Nortey, TN, Patience, JF, Sands, JS, Trottier, NL, and Zijlstra, RT. Effects of xylanase supplementation on the apparent digestibility and digestible content of energy, amino acids, phosphorus, and calcium in wheat and wheat by-products from dry milling fed to grower pigs1,2. J Anim Sci. (2008) 86:3450–64. doi: 10.2527/jas.2007-0472
44. Trindade Neto, MA, Gallardo, C, Perna Junior, F, and Dadalt, JC. Apparent total and ileal digestibility of rice bran with or without multicarbohydrase and phytase in weaned piglets. Livest Sci. (2021) 245:104423. doi: 10.1016/j.livsci.2021.104423
45. Zhang, C-N, Li, X-F, Xu, W-N, Jiang, G-Z, Lu, K-L, Wang, L-N, et al. Combined effects of dietary fructooligosaccharide and Bacillus licheniformis on innate immunity, antioxidant capability and disease resistance of triangular bream (Megalobrama terminalis). Fish Shellfish Immunol. (2013) 35:1380–6. doi: 10.1016/j.fsi.2013.07.047
46. Ding, S, Yan, W, Ma, Y, and Fang, J. The impact of probiotics on gut health via alternation of immune status of monogastric animals. Anim Nutr. (2021) 7:24–30. doi: 10.1016/j.aninu.2020.11.004
47. Schroeder, HW, and Cavacini, L. Structure and function of immunoglobulins. J Allergy Clin Immunol. (2010) 125:S41–52. doi: 10.1016/j.jaci.2009.09.046
48. Zeller, E, Schollenberger, M, Witzig, M, Shastak, Y, Kühn, I, Hoelzle, LE, et al. Interactions between supplemented mineral phosphorus and phytase on phytate hydrolysis and inositol phosphates in the small intestine of broilers. Poult Sci. (2015) 94:1018–29. doi: 10.3382/ps/pev087
49. Stentz, R, Carvalho, AL, Jones, EJ, and Carding, SR. Fantastic voyage: the journey of intestinal microbiota-derived microvesicles through the body. Biochem Soc Trans. (2018) 46:1021–7. doi: 10.1042/BST20180114
50. Vucenik, I, and Shamsuddin, AM. Protection against cancer by dietary IP6 and inositol. Nutr Cancer. (2006) 55:109–25. doi: 10.1207/s15327914nc5502_1
51. Wang, Y, Du, W, Lei, K, Wang, B, Wang, Y, Zhou, Y, et al. Effects of dietary Bacillus licheniformis on gut physical barrier, immunity, and reproductive hormones of laying hens. Probiot Antim Prot. (2017) 9:292–9. doi: 10.1007/s12602-017-9252-3
52. Heine, G, Drozdenko, G, Grün, JR, Chang, H, Radbruch, A, and Worm, M. Autocrine IL-10 promotes human B-cell differentiation into IgM- or IgG-secreting plasmablasts. Eur J Immunol. (2014) 44:1615–21. doi: 10.1002/eji.201343822
53. Zong, X, Wang, TH, Lu, ZQ, Song, DG, Zhao, J, and Wang, YZ. Effects of Clostridium butyricum or in combination with Bacillus licheniformis on the growth performance, blood indexes, and intestinal barrier function of weanling piglets. Livest Sci. (2019) 220:137–42. doi: 10.1016/j.livsci.2018.12.024
54. Chen, Y-C, and Yu, Y-H. Bacillus licheniformis–fermented products improve growth performance and the fecal microbiota community in broilers. Poult Sci. (2020) 99:1432–43. doi: 10.1016/j.psj.2019.10.061
55. Lo Verso, L, Talbot, G, Morissette, B, Guay, F, Matte, JJ, Farmer, C, et al. The combination of nutraceuticals and functional feeds as additives modulates gut microbiota and blood markers associated with immune response and health in weanling piglets. J Anim Sci. (2020) 98:skaa208. doi: 10.1093/jas/skaa208
56. Bilal, M, Achard, C, Barbe, F, Chevaux, E, Ronholm, J, and Zhao, X. Bacillus pumilus and Bacillus subtilis promote early maturation of Cecal microbiota in broiler chickens. Microorganisms. (2021) 9:1899. doi: 10.3390/microorganisms9091899
57. Rodrigues, DR, Briggs, W, Duff, A, Chasser, K, Murugesan, R, Pender, C, et al. Comparative effectiveness of probiotic-based formulations on cecal microbiota modulation in broilers. PLoS One. (2020) 15:e0225871. doi: 10.1371/journal.pone.0225871
58. Bernad-Roche, M, Bellés, A, Grasa, L, Casanova-Higes, A, and Mainar-Jaime, RC. Effects of dietary supplementation with protected sodium butyrate on gut microbiota in growing-finishing pigs. Animals. (2021) 11:2137. doi: 10.3390/ani11072137
59. Kim, HB, and Isaacson, RE. The pig gut microbial diversity: understanding the pig gut microbial ecology through the next generation high throughput sequencing. Vet Microbiol. (2015) 177:242–51. doi: 10.1016/j.vetmic.2015.03.014
60. Palmer, C, Bik, EM, DiGiulio, DB, Relman, DA, and Brown, PO. Development of the human infant intestinal microbiota. PLoS Biol. (2007) 5:e177. doi: 10.1371/journal.pbio.0050177
61. Fonseca, A, Kenney, S, Van Syoc, E, Bierly, S, Dini-Andreote, F, Silverman, J, et al. Investigating antibiotic free feed additives for growth promotion in poultry: effects on performance and microbiota. Poult Sci. (2024) 103:103604. doi: 10.1016/j.psj.2024.103604
62. Da Silva, JMS, Almeida, AMDS, Borsanelli, AC, De Athayde, FRF, Nascente, EDP, Batista, JMM, et al. Intestinal microbiome profiles in broiler chickens raised with different probiotic strains. Microorganisms. (2024) 12:1639. doi: 10.3390/microorganisms12081639
63. Erhard, F. Estimating pseudocounts and fold changes for digital expression measurements. Bioinformatics. (2018) 34:4054–63. doi: 10.1093/bioinformatics/bty471
64. Razin, S, and Hayflick, L. Highlights of mycoplasma research—an historical perspective. Biologicals. (2010) 38:183–90. doi: 10.1016/j.biologicals.2009.11.008
65. Haller, D, Serrant, P, Granato, D, Schiffrin, EJ, and Blum, S. Activation of human NK cells by staphylococci and lactobacilli requires cell contact-dependent Costimulation by autologous monocytes. Clin Vaccine Immunol. (2002) 9:649–57. doi: 10.1128/CDLI.9.3.649-657.2002
66. Smits, HH, Engering, A, Van Der Kleij, D, De Jong, EC, Schipper, K, Van Capel, TMM, et al. Selective probiotic bacteria induce IL-10–producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell–specific intercellular adhesion molecule 3–grabbing nonintegrin. J Allergy Clin Immunol. (2005) 115:1260–7. doi: 10.1016/j.jaci.2005.03.036
67. Carolin C, F, Kumar, PS, and Ngueagni, PT. A review on new aspects of lipopeptide biosurfactant: types, production, properties and its application in the bioremediation process. J Hazard Mater. (2021) 407:124827. doi: 10.1016/j.jhazmat.2020.124827
68. Peypoux, F, Bonmatin, JM, and Wallach, J. Recent trends in the biochemistry of surfactin. Appl Microbiol Biotechnol. (1999) 51:553–63. doi: 10.1007/s002530051432
69. Inès, M, and Dhouha, G. Lipopeptide surfactants: production, recovery and pore forming capacity. Peptides. (2015) 71:100–12. doi: 10.1016/j.peptides.2015.07.006
70. Vijayalakshmi, S, Ranjitha, J, and Rajeswari, VD. Enzyme production ability by Bacillus subtilis and Bacillus licheniformis-a comparative study. Asian J Pharm Clin Res. (2013) 6:29–32.
71. Rottem, S. Membrane lipids of mycoplasmas. Biochim Biophys Acta Biomembr. (1980) 604:65–90. doi: 10.1016/0005-2736(80)90585-4
72. Coronel, JR, Marqués, A, Manresa, Á, Aranda, FJ, Teruel, JA, and Ortiz, A. Interaction of the Lipopeptide biosurfactant Lichenysin with phosphatidylcholine model membranes. Langmuir. (2017) 33:9997–10005. doi: 10.1021/acs.langmuir.7b01827
73. Yaderets, V, Karpova, N, Glagoleva, E, Shibaeva, A, and Dzhavakhiya, V. Bacillus subtilis RBT-7/32 and Bacillus licheniformis RBT-11/17 as new promising strains for use in probiotic feed additives. Microorganisms. (2023) 11:2729. doi: 10.3390/microorganisms11112729
74. Yang, G, Yang, D, Wang, X, and Cao, W. A novel thermostable cellulase-producing Bacillus licheniformis A5 acts synergistically with Bacillus subtilis B2 to improve degradation of Chinese distillers’ grains. Bioresour Technol. (2021) 325:124729. doi: 10.1016/j.biortech.2021.124729
75. Borda-Molina, D, Vital, M, Sommerfeld, V, Rodehutscord, M, and Camarinha-Silva, A. Insights into broilers’ gut microbiota fed with phosphorus, calcium, and Phytase supplemented diets. Front Microbiol. (2016) 7:33. doi: 10.3389/fmicb.2016.02033
76. Walugembe, M, Hsieh, JCF, Koszewski, NJ, Lamont, SJ, Persia, ME, and Rothschild, MF. Effects of dietary fiber on cecal short-chain fatty acid and cecal microbiota of broiler and laying-hen chicks. Poult Sci. (2015) 94:2351–9. doi: 10.3382/ps/pev242
77. Galié, S, García-Gutiérrez, C, Miguélez, EM, Villar, CJ, and Lombó, F. Biofilms in the food industry: health aspects and control methods. Front Microbiol. (2018) 9:898. doi: 10.3389/fmicb.2018.00898
78. Rottem, S, and Razin, S. Lipase activity of Mycoplasma. J Gen Microbiol. (1964) 37:123–34. doi: 10.1099/00221287-37-1-123
Keywords: alternative feed additives, Bacillus-based biotics, commercial farm, Mycoplasma, nursery pig
Citation: Koo S-M, Lee J-H, Oh S-H and Jang J-C (2025) Impacts of Bacillus-based biotics and an enzyme cocktail on growth performance, immunity, and gut pathogenic microorganisms of nursery pigs under commercial conditions. Front. Vet. Sci. 12:1627739. doi: 10.3389/fvets.2025.1627739
Edited by:
Elena De Felice, University of Camerino, ItalyReviewed by:
Ugonna Henry Uzoka, Michael Okpara University of Agriculture, NigeriaRuili Li, Yantai Academy of Agricultural Sciences, China
Copyright © 2025 Koo, Lee, Oh and Jang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jae-Cheol Jang, amFlamFuZzEyNzhAZ251LmFjLmty
 Seong-Min Koo
Seong-Min Koo Jae-Hyeok Lee
Jae-Hyeok Lee Jae-Cheol Jang
Jae-Cheol Jang