- 1Key Laboratory of Clinical Diagnosis and Treatment Techniques for Animal Disease, Ministry of Agriculture, Inner Mongolia Agricultural University, Hohhot, China
- 2Laboratory of Veterinary Clinical Pharmacology, College of Veterinary Medicine, Inner Mongolia Agricultural University, Hohhot, China
- 3Inner Mongolia Bayannaoer City Municipal Center for Disease Control and Prevention, Bayannur, China
- 4Inner Mongolia Academy of Agricultural & Animal Husbandry Sciences, Hohhot, China
Escherichia coli (E. coli) is the primary causative agent of bovine mastitis. Currently, antibiotic therapy remains the cornerstone of mastitis treatment; necessitating the identification of alternative therapeutic options. This study employed in vitro cultured bovine bone marrow-derived macrophages (BMDMs) to systematically assess the potential of microsomal prostaglandin e synthase-1 (mPGES-1) inhibitors (MF63, MK886) and EP4 receptor inhibitor (Grapiprant) in modulating inflammatory responses and reducing tissue damage. Cells were pre-treated with mPGES-1 inhibitors and an EP4 receptor inhibitor before infection with E. coli. Following infection, extracellular bacteria were removed, and assays—including ELISA, Western blot, and qRT-PCR—were conducted to analyze inflammatory mediators, protein expression, and gene expression. E. coli infection significantly induced PGE₂ synthesis in BMDMs, which exacerbated the inflammatory response and tissue damage via NF-κB and MAPK signaling pathways, elevating TNF-α, IL-1β, IL-6, and IL-8. Treatment with MF63, MK886 and Grapiprant effectively reduced PGE₂ levels, inhibited NF-κB and MAPK signaling pathways, decreased inflammatory mediators, and enhanced macrophage bactericidal activity, thereby demonstrating potent anti-inflammatory and immunomodulatory effects. Moreover, inhibition of the mPGES-PGE₂-EP4 signaling pathway was found to reduce the expression of damage-associated molecular patterns (HMGB-1 and HABP-2), suggesting alleviation of E. coli-induced tissue damage. Based on the role of PGE₂ in mediating immune and inflammatory responses via the EP4 receptor, inhibiting the mPGES-1-PGE₂-EP4 signaling axis to reduce inflammation and tissue damage will facilitate further investigation into the regulatory mechanisms of the PGE₂ signaling axis in the pathogenesis of mastitis. This approach provides a theoretical foundation and experimental basis for the development of alternative anti-inflammatory therapies to replace antibiotics.
1 Introduction
Milk is recognized as one of the most significant animal-derived foods worldwide, characterized by its rich composition of high-quality proteins, fats, vitamins, and minerals, and its irreplaceable role in human nutrition (1). The escalating demand for dairy products has prompted modern dairy farming to prioritize increasing milk yield and improving milk quality (2). However, mastitis, a major disease affecting udder health, significantly reduces milk yield, compromises milk quality, and results in substantial economic losses for the dairy industry (3). Given the complex pathogenesis of mastitis, current treatment strategies predominantly rely on antibiotics (4). Yet, prolonged antibiotic use may lead to bacterial resistance and drug residues, posing a threat to food safety (5, 6). Therefore, understanding the pathogenesis of mastitis and exploring safer, more effective alternative therapies are essential for the health of dairy cows and the sustainable development of the dairy industry.
Escherichia coli (E. coli) is a major pathogen responsible for mastitis among dairy cows (7), utilizing various virulence factors to inflict damage on host cells, induce inflammation, and evade the immune system. These effects compromise the integrity of mammary tissue and exacerbate mastitis progression (8–10). Mastitis does not only reduce milk quality but also facilitates the entry of pathogens and their virulence factors into milk, thereby affecting food safety (11). Therefore, understanding the pathogenic mechanisms of E. coli, along with its interaction with the host immune responses, is crucial for preventing mastitis and ensuring dairy product safety.
Macrophages residing in mammary tissue, primarily originating from the bone marrow, are key immune cells in pathogen defense (12). The M1 macrophage phenotype is associated with inflammatory and antitumor functions. Monocytes and macrophages in the bone marrow and peripheral blood are primarily derived from hematopoietic stem cells in the bone marrow; therefore, we used bovine bone marrow-derived macrophages (BMDMs) in the present study (13). Their roles encompass pathogen recognition, phagocytosis, and elimination, while also maintaining tissue homeostasis by regulating inflammatory responses (14, 15). However, under pathological conditions, damage to mammary tissue may result in the disruption of the blood-milk barrier, leading to the excessive release of pro-inflammatory cytokines and exacerbating tissue damage, thereby creating a vicious cycle of inflammatory imbalance (16).
Mastitis is an inflammatory process of the mammary gland that leads to the production of prostaglandins, resulting in increased body and mammary surface temperature (17). Studies have shown that prostaglandins, particularly prostaglandin E2 (PGE₂), play a significant role in the pathophysiology and severity of E. coli-induced mastitis, especially during the early lactation phase in dairy cows (18, 19). PGE2, synthesized during arachidonic acid (AA) metabolism by cyclooxygenase (COX-1/COX-2) and prostaglandin E synthase (PGES), is a key bioactive lipid molecule involved in regulating inflammation, immune responses, and tissue repair (20). It exerts its biological effects through four G protein-coupled receptors (EP1–EP4) (21), with EP4 being particularly critical in immune regulation and tissue damage repair during mastitis (22).
In the pathological process of bovine mastitis, E. coli infection of mammary tissue activates the TLR4/NF-κB signaling pathway (23), which subsequently leads to the upregulation of COX-2 expression, promoting the synthesis of PGE₂ and activating the EP4 receptor (24, 25). The PGE₂-EP4 signaling pathway not only influences inflammation regulation and tissue repair, but may also affect the efficacy of antibiotic treatment for inflammation and the development of resistance (26). The study suggests that PGE₂ can upregulate the expression of drug resistance-associated genes through the EP4 receptor, thereby reducing the therapeutic efficacy of antibiotics (27). Therefore, targeting the PGE₂-EP4 signaling pathway may represent a novel strategy for the treatment of mastitis, helping to alleviate inflammatory damage while potentially replacing the use of antibiotics and reducing the risk of resistance.
This study utilized mPGES-1 inhibitors (MF63, MK886) and EP4 receptor inhibitor (Grapiprant) to block the PGE₂-EP4 signaling pathway. MF63—compared to earlier mPGES-1 inhibitors—demonstrates stronger potency and higher selectivity in cellular assays, making it a more promising candidate for targeting PGE₂ inhibition (28). Preclinical studies have shown that MF63 effectively inhibits the synthesis of inflammatory PGE₂ while preserving other prostaglandin biosynthetic pathways, thus minimizing off-target effects and enhancing its therapeutic potential in inflammatory conditions (29). MK-886 also serves as an effective mPGES-1 inhibitor thatobstructs the intracellular biosynthesis of PGE₂ (30). Widely used in experimental models of inflammation, allergy, cancer, and cardiovascular diseases, MK-886 represents an established tool for investigating PGE₂-related pathways (31). Grapiprant, a highly selective EP4 receptor antagonist, inhibits the PGE₂-EP4 signaling pathway and downstream inflammatory responses (32). Unlike traditional nonsteroidal anti-inflammatory drugs (NSAID) that broadly inhibit COX activity, Grapiprant specifically targets the EP4 receptor. Approved for the treatment of osteoarthritis-related pain and inflammation in dogs, Grapiprant has demonstrated efficacy and safety in preclinical and clinical studies, alleviating arthritis inflammation, relieving pain, and improving mobility by blocking EP4-mediated pro-inflammatory signaling (33). Accordingly, it can be speculated that the PGE₂-EP4 signaling pathway may play a crucial role in the treatment of mastitis in dairy cows.
Although there is an abundance of literature on mastitis in dairy cows, data regarding its underlying mechanisms remain insufficient. Given the detrimental effects associated with antibiotic use for the treatment of bovine mastitis in humans, the potential of PGE₂-EP4 inhibitors for managing mastitis has garnered renewed interest. However, the role of PGE₂-EP4 inhibitors in treating mastitis in dairy cows, particularly concerning the underlying molecular mechanisms, has largely been overlooked.
This study aims to investigate the anti-inflammatory effects of PGE₂-EP4 inhibitors in an E. coli-induced macrophage model, their impact on phagocytic killing ability against E. coli, and the underlying molecular mechanisms.
2 Materials and methods
2.1 Ethical statement
Bovine ribs were obtained postmortem from adult dairy cows that were slaughtered for commercial food production at Beiya Halal Slaughterhouse (Hohhot, Inner Mongolia, China). No animals were euthanized for the purposes of this study, and no live animal interventions were conducted. As a result, ethical approval was not necessary.
2.2 Bacterial strains
A 1 mL suspension of E. coli O157 (34, 35) strain (at a concentration of 1 × 107 CFU) preserved in the laboratory was inoculated into 100 mL of Luria-Bertani (LB) broth (Oxoid, Basingstoke, LTD, UK). Incubate the culture at 37°C with shaking at 200 rpm for 12 h, or until the OD600 of the culture reaches 0.9. The bacterial suspension was serially diluted and plated onto LB agar. After incubation at 37°C for 18 h, colonies were counted, and the concentration was quantified as CFU/mL.
2.3 Culture of bovine BMDMs
The bovine rib marrow cavity was washed with phosphate-buffered saline (PBS), and the cell suspension was filtered through a cell strainer into a 50 mL centrifuge tube (Hyclone, UT, United States). The suspension was centrifuged at 2900 g for 8 min, and the supernatant was discarded. Red blood cells were lysed using red blood cell lysis buffer for 5 min, followed by centrifugation at 1300 g for 8 min. The bone marrow cells were then collected and cultured in Roswell Park Memorial Institute (RPMI) 1,640 medium with 20% fetal bovine serum (Hyclone, UT, United States) and 20 ng/mL macrophage colony-stimulating factor (Kingfisher, MN, United States) at 37°C in a 5% CO2 incubator. After 7 days of induction, non-adherent cells were removed, and adherent cells were treated with 1 μg/mL lipopolysaccharide (LPS, PeproTech, NJ, United States) for 24 h to differentiate M0 macrophages into M1 macrophages for subsequent experiments.
2.4 Experimental infection and treatment in vitro
Cells were treated with mPGES-1 inhibitors (MF63, MK886; Cayman Chemical Company, Ann Arbor, MI, United States) for 24 h and EP4 receptor inhibitor (Grapiprant; MedChemExpress, Shanghai, China) for 4 h, followed by E. coli infection. At 1-h post-infection, cells were washed with fresh medium containing 100 μg/mL tobramycin to remove extracellular bacteria (36).
2.5 Enzyme-linked immunosorbent assay
After pretreatment with mPGES-1 inhibitors (MF63, MK886) and EP4 receptor inhibitor (Grapiprant), cell supernatants were collected 6 h after E. coli infection. The secretion of PGE2, TNF-α, IL-1β, IL-6, IL-8, and IL-10 in the BMDM supernatant was measured using ELISA kits for bovine PGE2 (Cayman Chemical Company, MI, United States), TNF-α, IL-6 (R&D Systems, MN, United States), and IL-1β, IL-8, and IL-10 (Kingfisher Biotech, MN, United States).
2.6 Cell viability assay
The MTT assay was used to assess the impact of the drugs on cell viability. BMDM (10⁴ cells per well) were seeded into a 96-well plate and cultured in 180 μL of medium under conditions of 37°C and 5% CO₂. Following treatment with MF63, MK886, and Grapiprant, the MTT assay was performed according to the manufacturer’s instructions (Solarbio, Beijing, China).
2.7 Western blot analysis
After pretreatment with mPGES-1 inhibitors (MF63, MK886) and EP4 receptor inhibitor (Grapiprant), cells were collected after 15, 30 and 60 min of E. coli infection. Total protein was extracted from treated cells using M-PER Mammalian Protein Extraction Reagent (Thermo Scientific, MA, United States) and quantified with the BCA Protein Assay Kit (Thermo Scientific, IL, United States). An aliquot of 10 μg of total protein was loaded onto each lane, and the samples were separated by 12% SDS-PAGE. Following membrane transfer, the membrane was blocked at room temperature for 1 h and then incubated overnight with primary antibodies at 4°C. The primary antibodies employed included anti-phospho-ERK, anti-ERK, anti-phospho-p38, anti-p38, anti-phospho-NF-κB p65, anti-NF-κB p65 (1:1000, Cell Signaling Technology, MA, United States), and anti-GAPDH (1:1000). The protein bands were visualized using enzyme-linked secondary antibodies and Pierce SuperSignal West Femto Chemiluminescent Substrate (Thermo Scientific, IL, United States). Image analysis was conducted using ImageJ software (version 1.48; NIH, MD, United States).
2.8 Quantitative real-time polymerase chain reaction
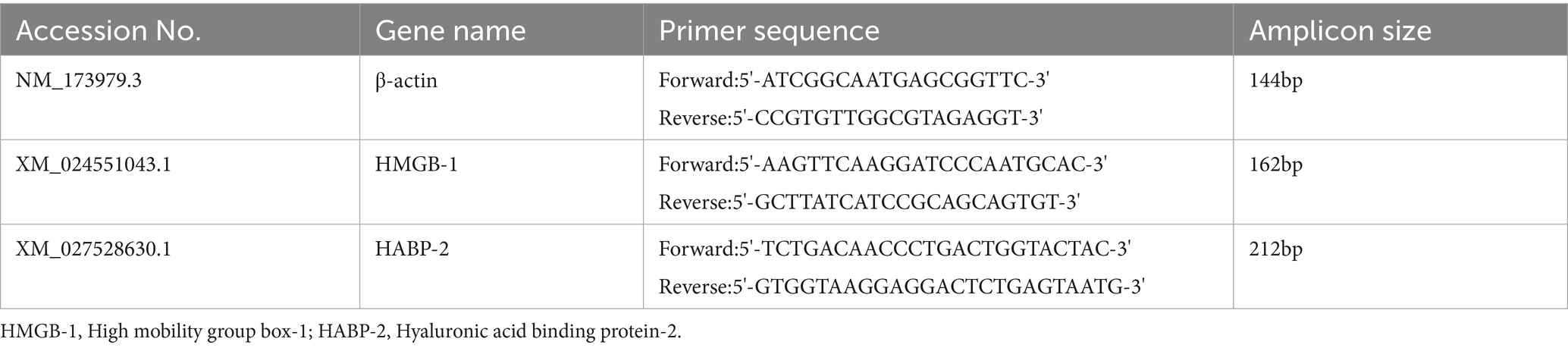
After pretreatment with mPGES-1 inhibitors (MF63, MK886) and EP4 receptor inhibitor (Grapiprant), cells were collected at 2, 4, and 6 h post-E. coli infection. Total RNA was extracted using an RNA extraction kit (Axygen Scientific, CA, United States), and reverse transcription was performed with the RevertAid First Strand cDNA Synthesis Kit (Vazyme, Nanjing, China). Quantitative real-time PCR was conducted on an ABI PCR System (Bio-Rad, Hercules, CA, United States) using FastStart SYBR Green Master (Roche Applied Science, Mannheim, Germany). PCR conditions included 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 60 s. Primers for quantitative PCR are listed in Table 1. Relative gene expression was normalized to β-actin and calculated using the 2 −ΔΔCt method.
2.9 Microscopy assay of bacterial phagocytosis and tetrazolium dye reduction for bacterial killing
To investigate the effects of mPGES-1 and EP4 treatments on phagocytosis and killing of E. coli by BMDM, cells were cultured at a density of 2 × 105 in 35 mm dishes and treated with or without MF63, MK886, and Grapiprant. The cells were co-incubated with 8 μM 1,1′-octadecyl-3,3,3′,3′-tetramethylindodicarbocyanine perchlorate (Thermo Scientific, CA, United States) for 20 min to label cell membranes, and E. coli along with Hoechst 33258 for 20 min to label the bacteria. Following this, BMDM were infected with E. coli for 0.5 or 2.5 h, fixed with 4% paraformaldehyde, and observed at 400 × magnification using a confocal microscope (LSM 800; Carl Zeiss, Oberkochen, Germany).
2.10 Data analysis
Data were analyzed using GraphPad Prism 10 software (GraphPad InStat Software, CA, United States) and expressed as mean ± standard deviation (SD). Statistical significance was determined by one-way or two-way ANOVA with appropriate post hoc tests (Tukey’s or Bonferroni). p values < 0.05 were considered significant (*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001).
3 Results
3.1 Selection of drug concentrations of mPGES-1 inhibitors in Escherichia coli infected BMDM
We conducted a series of concentration-gradient experiments to select the optimal drug concentration. The results showed that during E. coli infection of BMDM, PGE2 secretion was significantly reduced at an MF63 concentration of 1 × 10−9 M and an MK886 concentration of 1 × 10−7 M within the drug concentration range of 1 × 10−7 M to 1 × 10−10 M, indicating optimal therapeutic efficacy (Figures 1A,B, P < 0.05). Further refinement revealed the lowest secretion of PGE2 at a concentrations of 1 × 10−9 M and 4 × 10−7. Accordingly, MF63 (1 × 10−9 M) and MK886 (4 × 10−7 M) were chosen as the optimal drug concentration for use in E. coli-infected BMDM in subsequent experiments (Figures 1C,D, P < 0.05). Cell viability, measured using the MTT assay according to the manufacturer’s instructions, demonstrated no difference between the drug groups (MF63, MK886) and the control group, suggesting a lack of toxic effects on BMDM (Figures 1E,F).
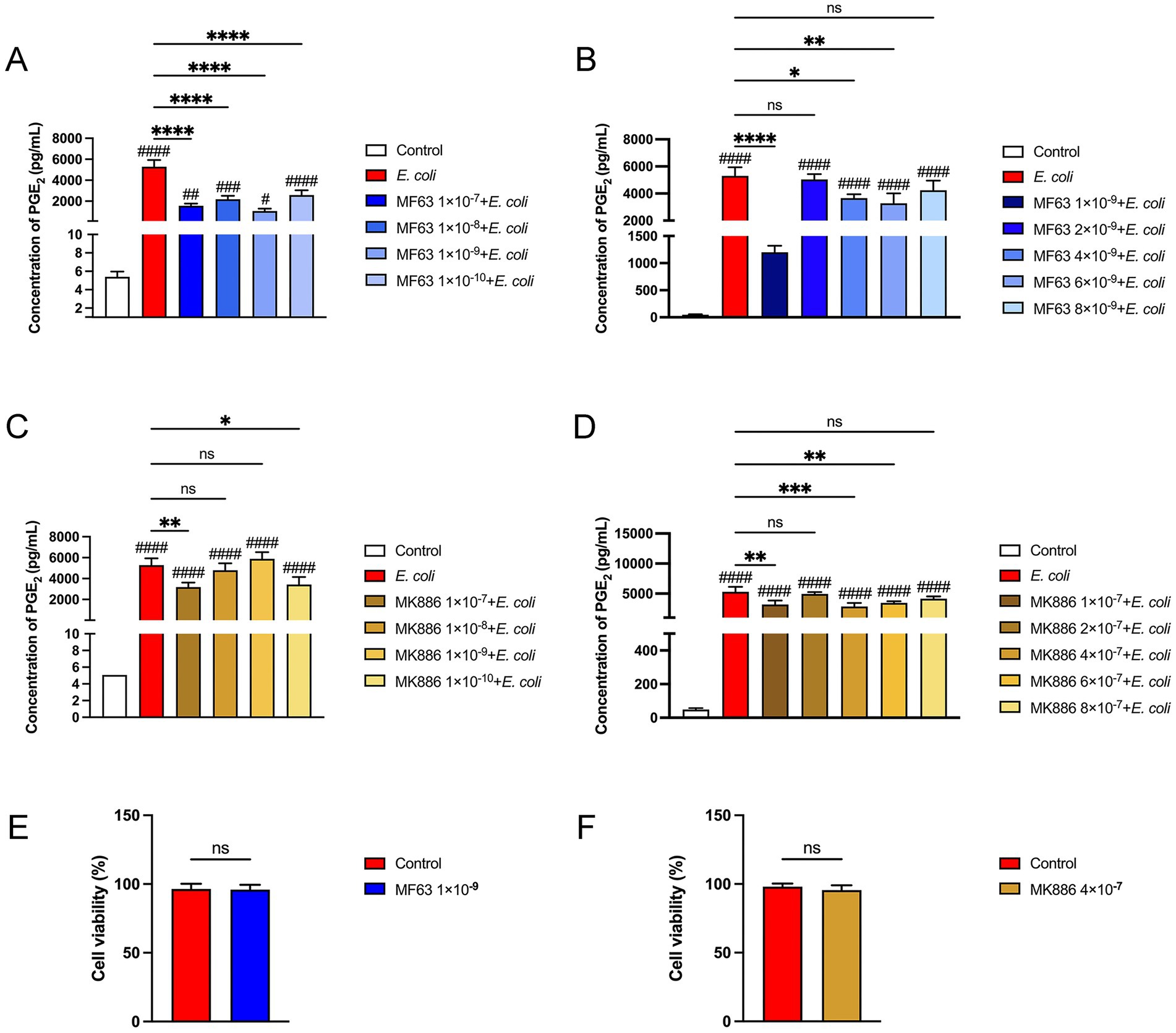
Figure 1. Results of drug selection. (A,B) Effect of varying concentrations of MF63 on PGE₂ secretion in E. coli-infected BMDMs. (C,D) Effect of varying concentrations of MF63 on PGE₂ secretion in E. coli-infected BMDMs. (E) Cell viability of MF63 (1 × 10−9 M)-treated BMDMs was assessed using the MTT assay. (F) Cell viability of MK886 (4 × 10−7 M)-treated BMDMs was assessed using the MTT assay. Data are expressed as mean ± SD. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 vs. E. coli group; # p < 0.05, ## p < 0.01 ### p < 0.001, #### p < 0.0001 vs. control group.
3.2 Analysis of cytokines and signaling pathways of Escherichia coli infected BMDM by inhibiting the mPGES-1-PGE2 axis
Cytokines and chemokines function as key mediators in the inflammatory response to bacterial infections. We investigated the effects of mPGES-1 inhibitors (MF63 and MK886) on the production of pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6), the anti-inflammatory cytokine IL-10, and the chemokine IL-8 in macrophages infected with E. coli at a multiplicity of infection (MOI) of 5:1. As shown in Figures 2A–E, E. coli infection significantly increased cytokine and chemokine secretion in BMDM (p < 0.0001). However, treatment with MF63 and MK886 markedly reduced the secretion of TNF-α, IL-1β, IL-6, and IL-8, while elevating IL-10 secretion compared to the E. coli-infected group (p < 0.0001). To further elucidate the underlying mechanism, we examined the activation of the MAPK (ERK, p38) and NF-κB (p65) signaling pathways by assessing the phosphorylation status of ERK, p38, and p65 proteins. Western blot analysis revealed that E. coli infection enhanced the phosphorylation of these signaling molecules, whereas treatment with MF63 or MK886 significantly suppressed their phosphorylation levels (p < 0.01, Figures 2F–2I). These findings suggest that MF63 and MK886 may suppress the secretion of pro-inflammatory cytokines and chemokines while promoting the release of anti-inflammatory mediators during E. coli infection, potentially by inhibiting E. coli-induced activation of the MAPK and NF-κB signaling pathways in BMDM.
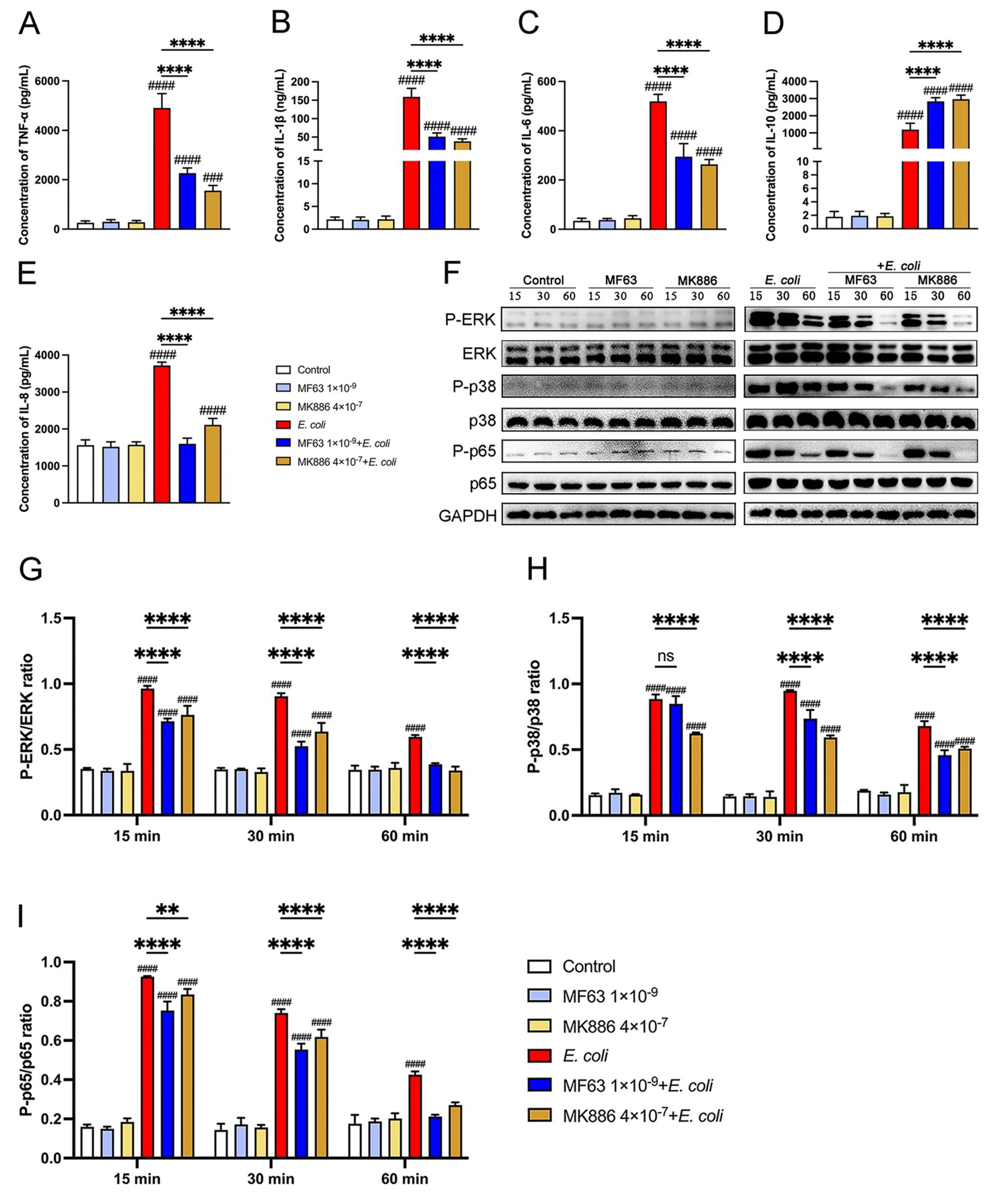
Figure 2. Impact of mPGES-1 inhibitors (MF63 and MK886) on cytokine secretion and signaling pathways in E. coli-infected BMDMs. (A–E) BMDMs were pretreated with MF63 and MK886 and subsequently infected with E. coli at a MOI of 5:1. The secretion levels of TNF-α, IL-1β, IL-6, IL-10, and IL-8 in the BMDMs culture supernatants were measured by ELISA. (F–I) Phosphorylation levels of ERK, p38, and p65 were assessed by western blotting at 15-, 30-, and 60-min post-infection, with GAPDH used as the loading control. Grayscale values were quantified using ImageJ software. Results are presented as mean ± SD from three independent experiments, analyzed by Tukey’s multiple comparisons and two-way ANOVA. Significance is denoted as *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 vs E. coli group; #p < 0.05, ##p < 0.01 ###p < 0.001, ####p < 0.0001 vs Control group.
3.3 Analysis of the effect of mPGES-1-PGE₂ axis inhibition on the phagocytic and bactericidal activity of BMDM against Escherichia coli
Next, we used DiI-labeled BMDM and Hoechst-stained E. coli to evaluate the phagocytic and intracellular killing abilities of the BMDM. At 0.5 h post-E. coli infection, pretreatment with mPGES-1 inhibitors did not affect the macrophages’ ability to phagocytose E. coli (Figures 3A,B). However, at 2.5 h post-infection, confocal laser microscopy showed a significantly reduced fluorescence intensity in the mPGES-1 inhibitor groups compared to the infection group (Figures 3C,D, P < 0.001). This indicates that inhibiting PGE2 synthesis enhances the macrophages’ ability to eradicate E. coli. Consistent with these observations, an MTT assay was used to assess the effect of mPGES-1-PGE2 axis inhibition on the survival of intracellular E. coli. Inhibition of PGE2 synthesis resulted in a reduction of E. coli survival in BMDM, further demonstrating that inhibition of PGE2 synthesis enhances the macrophages’ bactericidal ability (Figure 3E, P < 0.05).
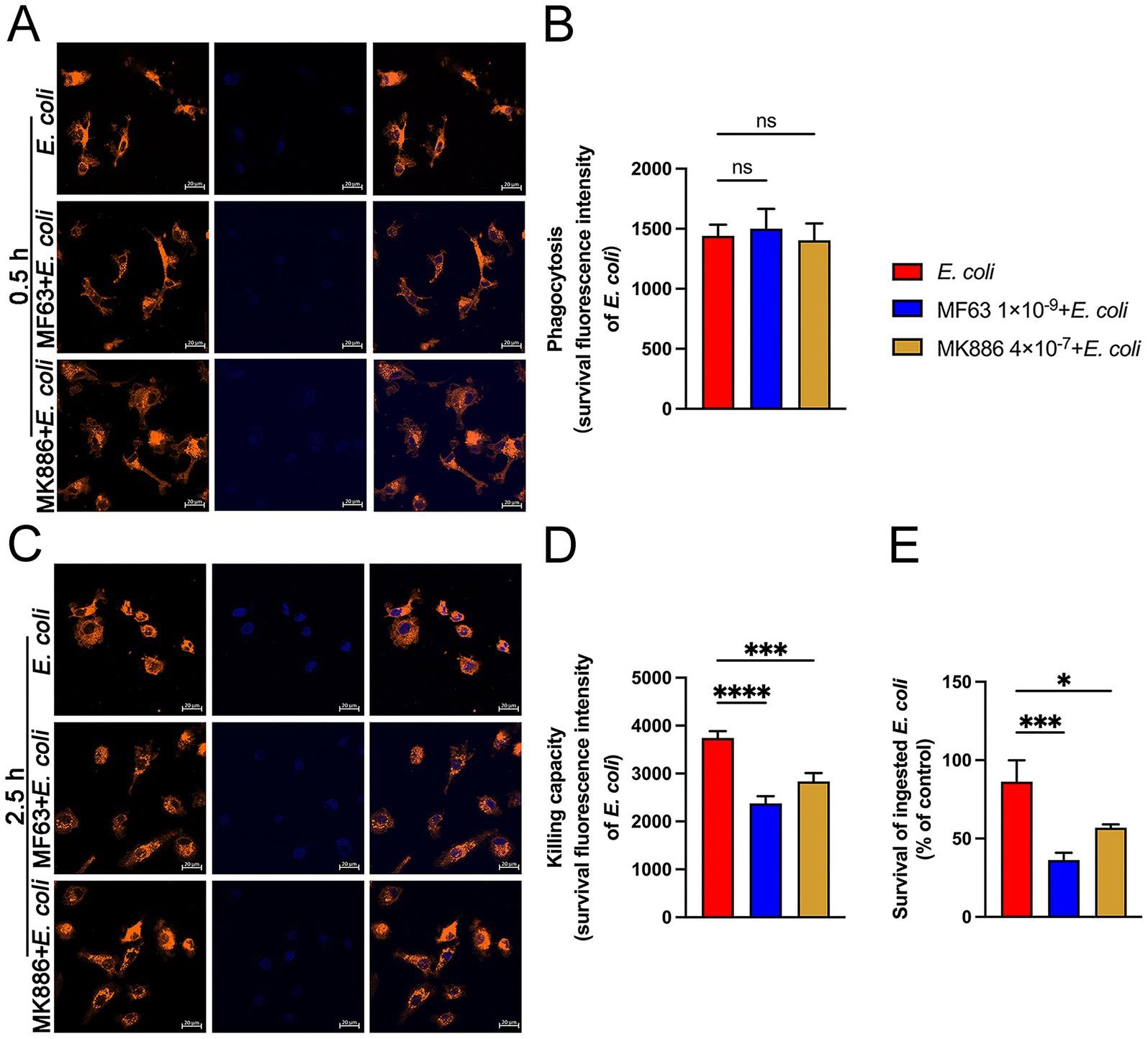
Figure 3. Effects of MF63 and MK886 on the phagocytosis and killing of E. coli by BMDMs. (A,B) BMDMs were pretreated with MF63 and MK886 for 24 h, followed by infection with E. coli (MOI 5:1) for 0.5 h. Phagocytosis of Hoechst 33258-labeled E. coli (blue) by DiI-labeled BMDMs (orange) was analyzed using microscopy at ×400 magnification (scale bar = 20 μm). (C,D) Effect of MF63 and MK886 on the bactericidal capacity of BMDMs against E. coli under an MOI of 5:1 (scale bar = 20 μm). (E) MTT assay was used to assess the effect of MF63 and MK886 on E. coli viability in BMDMs. Results are presented as mean ± SD from three independent experiments, analyzed by Tukey’s multiple comparisons and two-way ANOVA. Significance is denoted as *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 vs E. coli group; #p < 0.05, ##p < 0.01 ###p < 0.001, ####p < 0.0001 vs Control group.
3.4 Effect of inhibiting the mPGES-1-PGE2 axis on the expression of DAMPs during Escherichia coli infection
To investigate the effect of inhibiting the mPGES-1–PGE₂ axis on the expression of DAMPs during E. coli infection, we measured the mRNA levels of key DAMPs, such as HMGB-1 and HABP-2, in BMDM following treatment with mPGES-1 inhibitors (MF63 and MK886). E. coli infection significantly increased the expression of both HMGB-1 and HABP-2 in BMDM. However, treatment with mPGES-1 inhibitors notably reduced the mRNA expression levels of these DAMPs at 4 and 6 h post-infection, compared to the E. coli infection group (Figure 4, p < 0.0001). This suggests that inhibition of the mPGES-1–PGE₂ axis modulates the expression of DAMPs, potentially mitigating inflammation and tissue damage during E. coli infection.
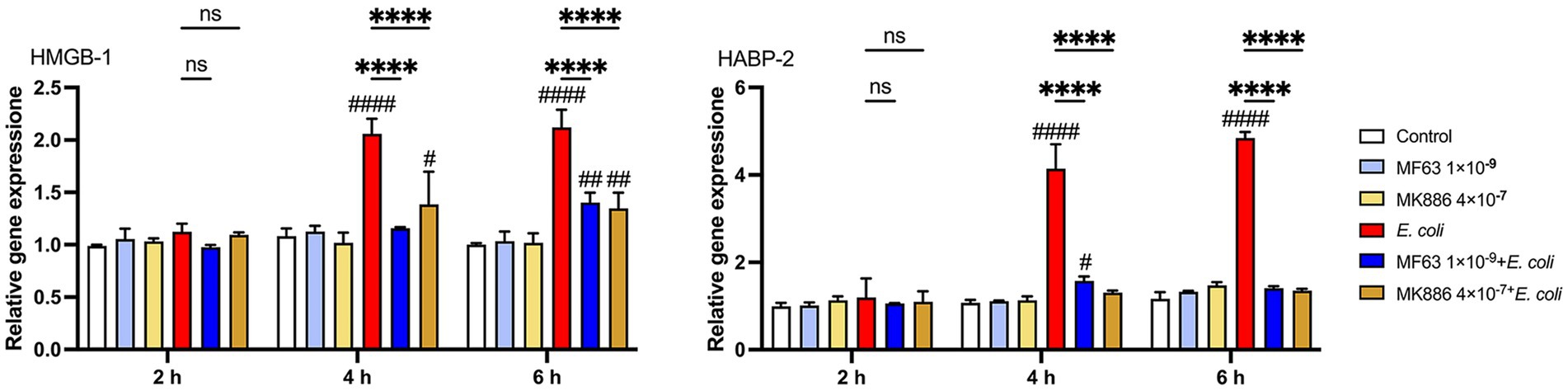
Figure 4. Effect of mPGES-1 inhibitors (MF63 and MK886) on DAMPs in E. coli-infected BMDMs. Effect of MF63 and MK886 on HMGB-1 and HABP-2 mRNA expression in E. coli-infected BMDMs. Results are presented as mean ± SD from three independent experiments, analyzed by Tukey’s multiple comparisons and two-way ANOVA. Significance is denoted as *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 vs E. coli group; #p < 0.05, ##p < 0.01 ###p < 0.001, ####p < 0.0001 vs Control group.
3.5 Selection of drug concentrations of EP4 inhibitor in Escherichia coli infected BMDM
The EP4 inhibitor (Grapiprant), tested at concentrations ranging from 1 × 10−7 M to 1 × 10−10 M, was applied to E. coli-infected BMDM. The concentration of 1 × 10−8 M was identified as the most effective in influencing PGE2 secretion (Figure 5A, P < 0.001). Further refinement revealed the lowest secretion of PGE2 at a concentration of 4 × 10−8 (Figure 5B, P < 0.01). Accordingly, Grapiprant (4 × 10−8 M) was chosen as the optimal drug concentration for treating endometritis in dairy cows in subsequent experiments. In addition, MTT results showed that Grapiprant exerted no toxic effects on BMDM (Figure 5C).
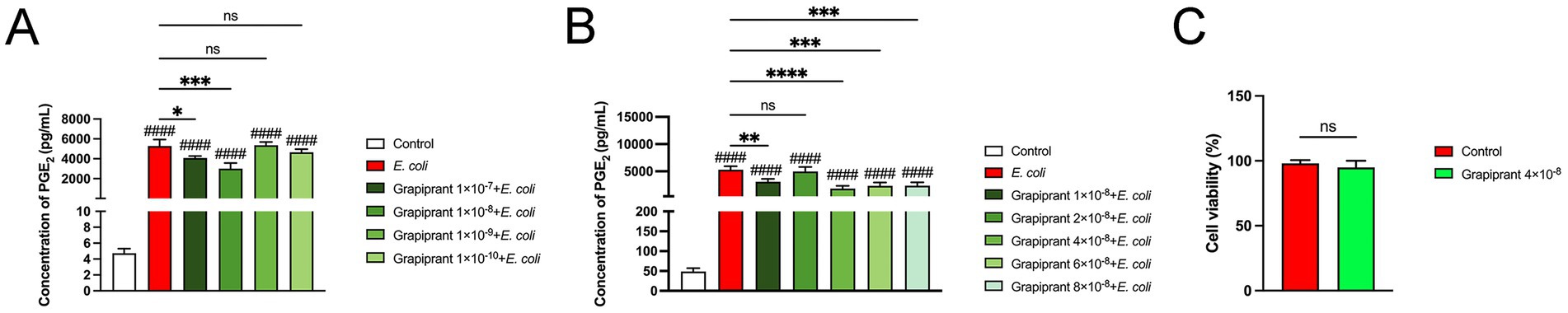
Figure 5. Grapiprant drug selection results. (A,B) Effect of varying concentrations of Grapiprant on PGE₂ secretion in E. coli-infected BMDMs. (C) Cell viability of Grapiprant (4 × 10−8 M)-treated BMDMs was assessed using the MTT assay. Results are presented as mean ± SD from three independent experiments, analyzed by Tukey’s multiple comparisons and two-way ANOVA. Significance is denoted as *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 vs E. coli group; #p < 0.05, ##p < 0.01 ###p < 0.001, ####p < 0.0001 vs Control group.
3.6 Analysis of cytokines and signaling pathways of Escherichia coli infected BMDM by inhibiting the PGE2-EP4 axis
To investigate the role of the PGE2-EP4 pathway in inflammation, we assessed the impact of the EP4 receptor inhibitor, Grapiprant, on cytokine production in E. coli-infected BMDM. E. coli infection significantly enhanced the secretion of pro-inflammatory cytokines and chemokine in BMDM. However, Grapiprant treatment markedly reduced the secretion of pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6) and the chemokine IL-8, while significantly increasing the secretion of the anti-inflammatory cytokine IL-10 in E. coli-infected BMDM (Figures 6A–E, P < 0.01). Furthermore, we examined the effect of the PGE2-EP4 receptor pathway on the activation of MAPK and NF-κB signaling in E. coli-infected BMDM, performed through Western blotting, indicated that Grapiprant treatment significantly reduced the phosphorylation of ERK, p38, and p65 compared to the E. coli infection group (Figures 6F–I, P < 0.01), indicating that the PGE2-EP4 pathway modulates these keys signaling pathways in the inflammatory response.
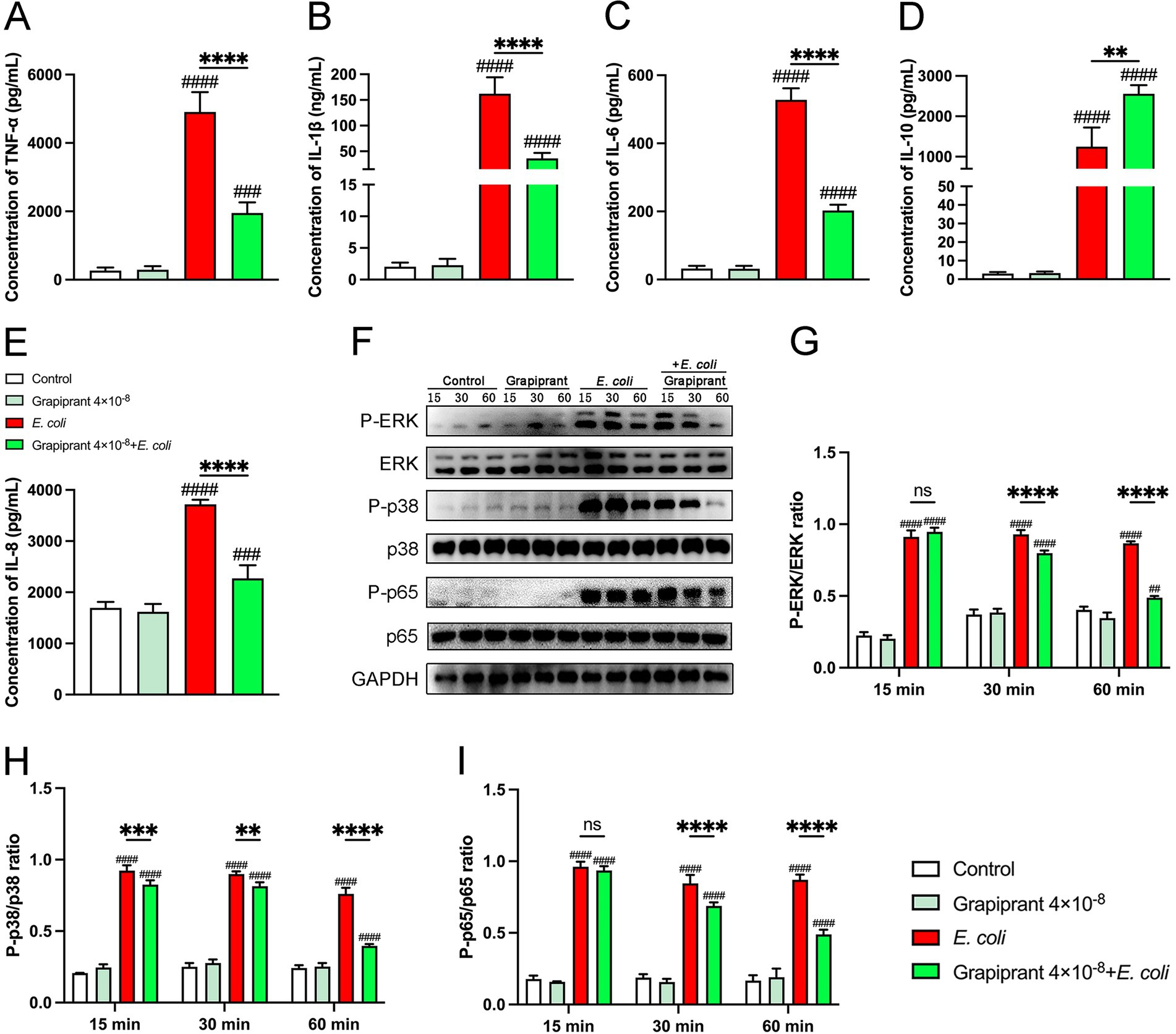
Figure 6. Impact of EP4 inhibitor (Grapiprant) on cytokine secretion and signaling pathways in E. coli-infected BMDMs. (A–E) BMDMs were pretreated with Grapiprant and subsequently infected with E. coli at a MOI of 5:1. The secretion levels of TNF-α, IL-1β, IL-6, IL-10, and IL-8 in the BMDMs culture supernatants were measured by ELISA. (F–I) Phosphorylation levels of ERK, p38, and p65 were assessed by western blotting at 15-, 30-, and 60-min post-infection, with GAPDH used as the loading control. Grayscale values were quantified using ImageJ software. Results are presented as mean ± SD from three independent experiments, analyzed by Tukey’s multiple comparisons and two-way ANOVA. Significance is denoted as *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 vs E. coli group; #p < 0.05, ##p < 0.01 ###p < 0.001, ####p < 0.0001 vs Control group.
3.7 Analysis of the effect of PGE₂-EP4 axis inhibition on the phagocytic and bactericidal activity of BMDM against Escherichia coli
At 0.5 h post-E. coli infection, Grapiprant did not significantly affect the phagocytic ability of macrophages compared to the E. coli infection group (Figures 7A,B). However, at 2.5 h post-infection, a notable reduction in fluorescence intensity was observed in the Grapiprant treated group (Figures 7C,D, P < 0.01). These results suggest that inhibition of the PGE2-EP4 receptor pathway enhances the bactericidal activity of macrophages against E. coli. Additionally, the MTT assay was employed to evaluate the impact of the PGE2-EP4 receptor pathway on the survival of intracellular E. coli. In line with the immunofluorescence findings, Grapiprant reduced the survival rate of E. coli in BMDM, further confirming that targeting the PGE2-EP4 receptor pathway enhances the bactericidal capacity of BMDM (Figure 7E, P < 0.05).
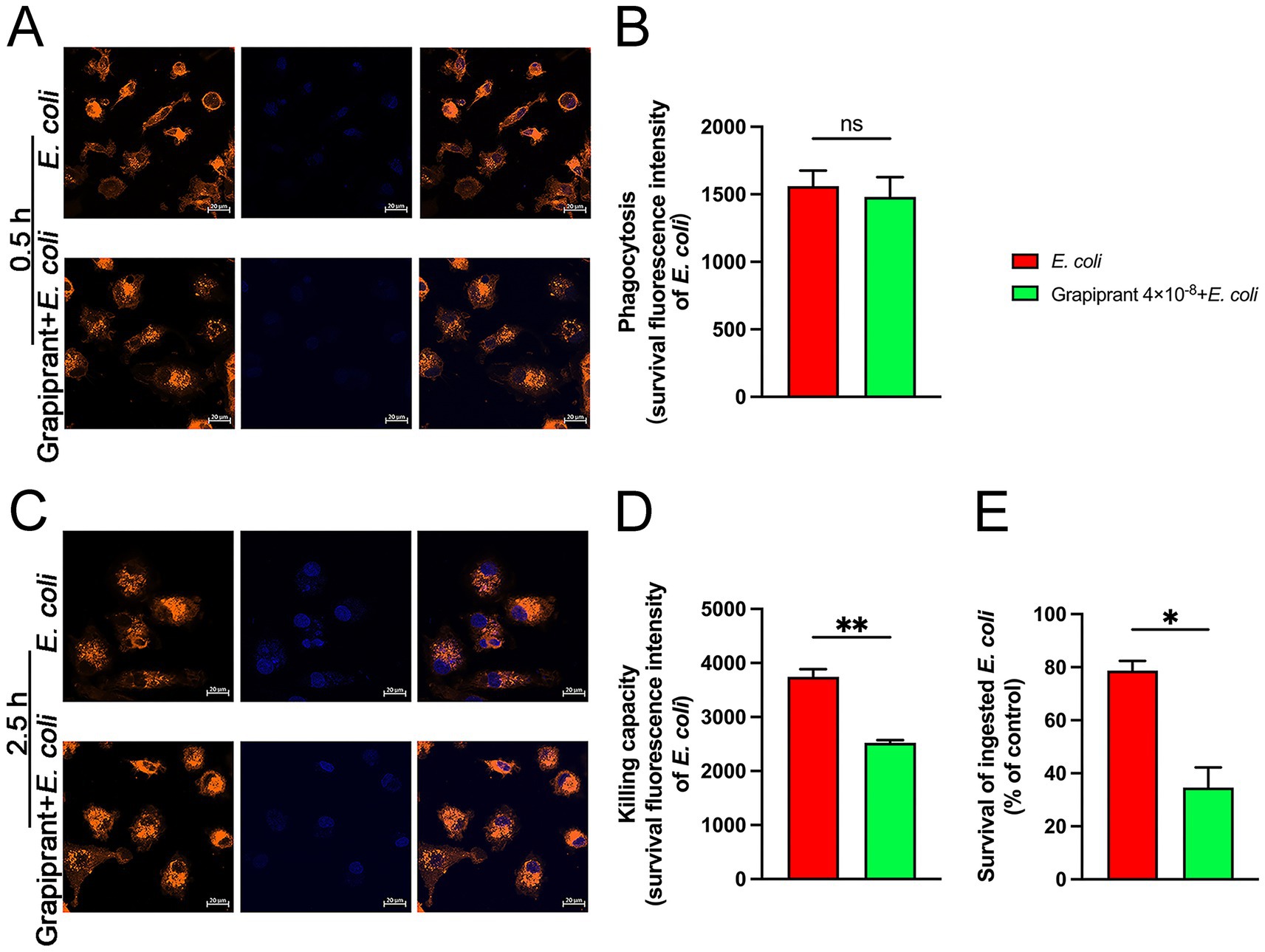
Figure 7. Effects of EP4 inhibitor (Grapiprant) on the phagocytosis and killing of E. coli by BMDMs. (A,B) BMDMs were pretreated with Grapiprant for 4 h, followed by infection with E. coli (MOI 5:1) for 0.5 h. Phagocytosis of Hoechst 33258-labeled E. coli (blue) by DiI-labeled BMDMs (orange) was analyzed using microscopy at ×400 magnification (scale bar = 20 μm). (C,D) Effect of Grapiprant on the bactericidal capacity of BMDMs against E. coli under an MOI of 5:1 (scale bar = 20 μm). (E) MTT assay was used to assess the effect of Grapiprant on E. coli viability in BMDMs. Results are presented as mean ± SD from three independent experiments, analyzed by Tukey’s multiple comparisons and two-way ANOVA. Significance is denoted as *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 vs E. coli group; #p < 0.05, ##p < 0.01 ###p < 0.001, ####p < 0.0001 vs Control group.
3.8 Analysis of the effect of PGE₂-EP4 axis inhibition on the phagocytic and bactericidal activity of BMDM against Escherichia coli
To examine the impact of inhibiting the PGE2-EP4 axis on DAMPs expression during E. coli infection, we assessed the mRNA expression of HMGB-1 and HABP-2 in BMDM. The results showed that E. coli infection significantly upregulated the expression of both HMGB-1 and HABP-2 compared to the control group. However, treatment with Grapiprant led to a notable reduction in the mRNA expression levels of HMGB-1 and HABP-2 at 4 and 6 h post-infection (Figure 8, p < 0.0001). These findings indicate that inhibition of the PGE2-EP4 pathway can modulate the release of DAMPs during the inflammatory response to E. coli infection.
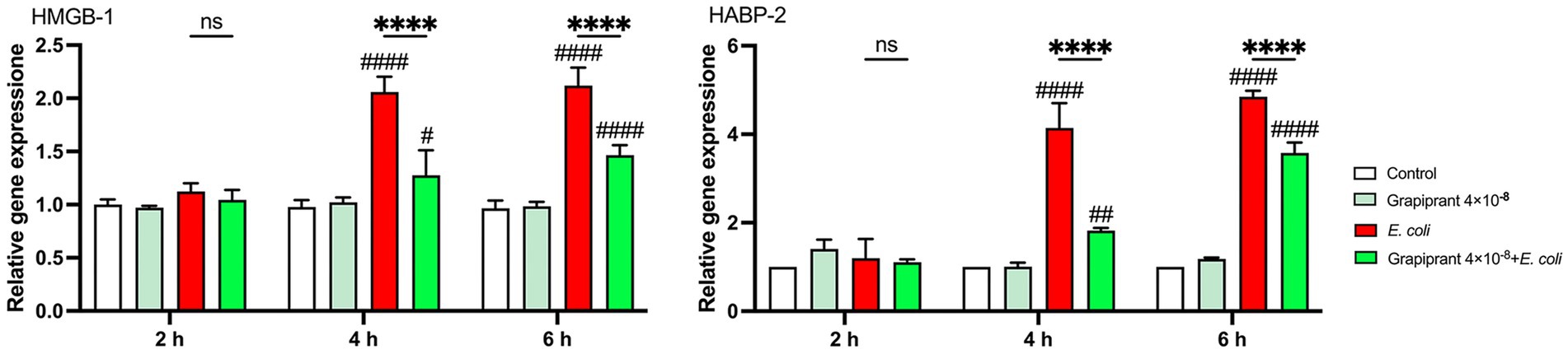
Figure 8. Effect of EP4 inhibitor (Grapiprant) on DAMPs in E. coli-infected BMDMs. Effect of Grapiprant on HMGB-1 and HABP-2 mRNA expression in E. coli-infected BMDMs. Results are presented as mean ± SD from three independent experiments, analyzed by Tukey’s multiple comparisons and two-way ANOVA. Significance is denoted as *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 vs E. coli group; #p < 0.05, ##p < 0.01 ###p < 0.001, ####p < 0.0001 vs Control group.
4 Discussion
This study was conducted to elucidate the effects of mPGES-1 inhibitors and EP4 receptor antagonists on inflammatory responses mediated by E. coli infection in BMDM. The key findings revealed that these targeted interventions significantly reduced PGE₂ levels, mitigated the activation of inflammatory signaling pathways, and enhanced macrophage bactericidal activity, thereby demonstrating potential as viable alternatives to conventional antibiotic therapy in managing mastitis.
Milk, one of the most essential animal-derived food products, plays a crucial role in meeting the nutritional needs of humans (1). The performance of dairy cows, particularly regarding milk production and quality, directly impacts dairy supply and the sustainability of the food industry (37). However, mastitis, a common postpartum infection of the mammary gland, severely affects mammary health, leading to decreased milk production and quality, and causing significant economic losses in dairy farming (38). Mastitis development is influenced by various factors, with pathogenic bacterial infections being a primary contributor. Among these, E. coli is a major pathogen responsible for mastitis (7). Through the release of virulence factors, such as lipopolysaccharides, adhesins, and toxins, E. coli induces damage and inflammatory responses in mammary tissues, potentially leading to abscess formation and further loss of mammary function in severe cases (39). However, the pathogenesis of E. coli-induced mastitis remains inadequately understood. This study demonstrated that E. coli infection significantly elevated PGE₂ secretion in macrophages. As a key inflammatory mediator, PGE₂ plays a critical role in the progression of mastitis (18). Currently, antibiotic treatment is still the main means of controlling mastitis in dairy cows, but the health risks associated with its long-term use cannot be ignored. In addition, antibiotic use may adversely affect the intestinal flora of dairy cows, which in turn affects the immune system’s response and exacerbates the onset and progression of mastitis (40, 41). Consequently, exploring alternative therapeutic strategies targeting PGE₂ may provide a novel approach to treat mastitis, reduce reliance on antibiotics, and minimize their adverse effects on both health and immune function in humans.
PGE₂ is widely present in various tissues and organs in both humans and animals, where its synthesis and release typically respond to stimuli such as infection and inflammation (42). PGE₂, a biologically active lipid, is synthesized from AA by COX and different isoforms of PGES, with mPGES-1 being the most critical. mPGES-1 is co-expressed with COX-2 and plays a significant role in inflammatory processes by catalyzing the conversion of PGH₂ to PGE₂, thereby enhancing the inflammatory response (43). Given its crucial role, mPGES-1 inhibition has been explored as a strategy to modulate PGE₂ levels and attenuate inflammation. For instance, inhibitors like MF63 and MK886 reduce PGE₂ synthesis, alleviating the excessive inflammatory response (44, 45). Consistent with the findings of this study, both MF63 and MK886 significantly reduced PGE₂ secretion in E. coli-infected BMDM, indicating their effective inhibition of PGE₂'s pro-inflammatory role. However, the precise mechanisms by which these inhibitors affect the onset and progression of mastitis remain unclear. Further research is necessary elucidate the therapeutic potential of mPGES-1 inhibitors in mastitis and provide a foundation for developing novel anti-inflammatory therapies.
In conditions such as mastitis, the release of pro-inflammatory cytokines, including TNF-α, IL-1β, IL-6, and IL-8, significantly intensifies the inflammatory response, thereby exacerbating tissue damage and pathological alterations (46, 47). Additionally, when cells are exposed to pathogenic factors, the MAPK signaling pathways (e.g., ERK, p38) are activated. This activation triggers a cascade of kinases, ultimately leading to the activation of transcription factors such as NF-κB in the cell nucleus, which in turn regulates the transcription of pro-inflammatory cytokines and chemokines (48). In vitro studies have shown that sulforaphane downregulates the mRNA expression of inflammatory cytokines, inhibits the expression of inflammatory mediators such as COX-2 and inducible nitric oxide synthase, and suppresses NF-κB activation, thereby alleviating LPS-induced mastitis (49). Consistent with these findings, our study demonstrated that MF63 and MK886 significantly reduced the secretion of TNF-α, IL-1β, IL-6 and IL-8 in E. coli-infected BMDM, while significantly increasing the secretion of the anti-inflammatory cytokine IL-10. Moreover, these compounds effectively inhibited the activation of both NF-κB and MAPK signaling pathways. These results suggest that MF63 and MK886 mitigate inflammation by suppressing PGE2 synthesis, thereby inhibiting the activation of NF-κB and MAPK signaling pathways, reducing pro-inflammatory cytokine secretion, and effectively alleviating the inflammatory response. This indicates that MF63 and MK886 play a critical role in mitigating inflammation.
Macrophages are instrumental in immune responses, including pathogen and tumor cell clearance, cytokine production, and intercellular interactions (50). Phagocytosis and intracellular killing are essential steps in bacterial clearance. Phagocytosis involves the internalization of bacteria, while subsequent killing mechanisms, activated once bacteria are engulfed, work together to eliminate the pathogens (51). Previous studies have shown that PGE₂ inhibits the bactericidal activity of alveolar macrophages against Klebsiella pneumoniae (52) and suppresses H₂O₂ generation during the clearance of apoptotic cells, impairing Streptococcus pneumoniae clearance (53). In line with our findings, inhibition of PGE₂ synthesis by MF63 and MK886 did not affect the phagocytic activity of BMDM but significantly enhanced their bactericidal ability against E. coli. This suggests that PGE₂ modulates the immune response intensity by affecting macrophage bactericidal function without directly influencing phagocytosis. Similarly, decursinol enhances the bactericidal activity of macrophages without significantly affecting their phagocytic function, as shown in studies against methicillin-resistant Staphylococcus aureus, while also reducing excessive pro-inflammatory cytokine expression and the inflammatory response (54). These findings underscore the crucial role of PGE₂ inhibition in regulating inflammation and modulating the effectiveness of the immune response by influencing bacterial clearance.
Following Mycobacterium tuberculosis infection of M1 macrophages, lactate significantly reduced bacterial load and alleviated tissue damage by enhancing macrophage clearance ability (55). Similarly, polysaccharides from Codonopsis pilosula (a perennial flowering plant in the bellflower family) improve macrophage pathogen clearance, thereby reducing bacterial load and mitigating pulmonary pathology (56). These findings highlight the close relationship between macrophage killing ability and tissue damage. During inflammation, the massive release of pro-inflammatory cytokines and chemokines promotes immune cell recruitment and activation, while inducing oxidative stress, extracellular matrix degradation, and increased vascular permeability, all of which exacerbate tissue damage. High mobility group box 1 (HMGB-1) and hyaluronan-binding protein-2 (HABP-2)—two key damage-associated molecular patterns (DAMPs)—are released during cell damage or death, triggering inflammation. HMGB-1 is released from the nucleus into the extracellular space, while HABP-2 is upregulated following tissue injury, promoting tissue repair through cell adhesion and immune cell function (57, 58). In this study, the mRNA expression of HMGB-1 and HABP-2 in E. coli-infected BMDM was significantly reduced, suggesting that inhibiting PGE2 synthesis may alleviate tissue damage and improve mammary health by modulating DAMP release. Consistent with the results of this study, in vitro study has shown that mPGES-1 exacerbates neuronal injury by producing PGE2 (59). Furthermore, MF63 and MK886 alleviated endometrial damage in E. coli-infected bovine tissue by inhibiting PGE2 synthesis and blocking DAMP expression (60).
PGE₂ is an important bioactive molecule that exerts its effects through binding to four G-protein-coupled receptors (EP1, EP2, EP3, and EP4), with the EP4 receptor playing a crucial role in immune responses and inflammation (61). Grapiprant, a selective EP4 receptor antagonist, is widely used in treating PGE₂-mediated inflammatory conditions, particularly in arthritis management in dogs. In animal models, Grapiprant has shown promise in alleviating pain and improving function (62). However, its role and mechanism in E. coli-induced mastitis remain poorly understood. Peptidoglycan induces cytokine production in RAW 264.7 macrophages via the PGE₂-EP4-NF-κB and PGE₂-EP4-MAPK pathways (63), and PGE₂ inhibits lipopolysaccharide-induced cytokine production in macrophages by suppressing EP4 signaling, likely through blocking NF-κB activation (64). In this study, Grapiprant inhibited PGE₂-EP4 signaling, leading to reduced activation of NF-κB and MAPK pathways in E. coli-induced BMDM. This resulted in decreased secretion of pro-inflammatory cytokines and chemokines, while significantly increasing the secretion of the anti-inflammatory cytokine IL-10. Additionally, Grapiprant enhanced the bactericidal activity of BMDM against E. coli and reduced the expression of DAMPs. These findings highlight the significant anti-inflammatory potential of targeting PGE₂-EP4 signaling in the inhibition of E. coli-induced mastitis. A previous study has shown that PGE₂ induces T helper 1 (Th1) cell differentiation and Th17 cell expansion in vitro. Treatment with an EP4 inhibitor reduces Th1 and Th17 cell accumulation in regional lymph nodes, thereby inhibiting the progression of chronic inflammation (65). Additionally, the EP4 inhibitor suppresses pro-inflammatory cytokine IL-6, chemokine CXCL8, and inflammation-dependent bone metastasis, while alleviating immune suppression and restoring anti-tumor immunity (66). Furthermore, the EP4 antagonist significantly reduces peritoneal fibrosis and improves dysfunction by inhibiting NLRP3 inflammasome and p-p65-mediated inflammatory responses (67). These findings, consistent with our own, underscore the critical role of the PGE₂-EP4 signaling pathway in inflammation and its potential as a therapeutic target.
This study has some limitations. The experimental design primarily focused on in vitro studies, and therefore, further validation through in vivo experiments is necessary.
5 Conclusion
This study systematically investigated the role of mPGES-PGE₂-EP4 pathway in mastitis pathogenesis during E. coli infection and evaluated the therapeutic value of mPGES-1 inhibitors (MF63, MK886) and EP4 receptor antagonists (Grapiprant). E. coli infection significantly induced PGE₂ synthesis and release in BMDM, activating NF-κB and MAPK pathways, upregulating pro-inflammatory cytokines and chemokines, thereby exacerbating inflammation and tissue damage. Moreover, PGE₂ impairs macrophage pathogen-killing ability, reducing the host’s efficiency in pathogen clearance. Application of MF63, MK886, and Grapiprant significantly reduces PGE₂ levels, inhibits NF-κB and MAPK activation, decreases inflammatory factor secretion, and enhances macrophage bactericidal capacity, thereby demonstrating anti-inflammatory and immunoregulatory effects. Additionally, inhibiting mPGES-PGE₂-EP4 signaling pathway reduces DAMP expression, such as HMGB-1 and HABP-2, suggesting its role in alleviating E. coli-induced damage and improving udder health. These findings not only deepen our understanding of the molecular mechanisms underlying E. coli-induced mastitis but also highlight the mPGES-PGE₂-EP4 pathway as a promising therapeutic target. This work provides new insights into the development of targeted anti-inflammatory strategies, offering potential benefits for improving dairy cow health, reducing antibiotic reliance, and promoting the sustainable development of the dairy industry.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Author contributions
XY: Data curation, Writing – original draft, Writing – review & editing, Conceptualization, Methodology. XL: Investigation, Software, Visualization, Writing – review & editing. LG: Conceptualization, Data curation, Investigation, Methodology, Writing – review & editing. PG: Data curation, Formal analysis, Methodology, Writing – review & editing. YQ: Formal analysis, Investigation, Validation, Writing – review & editing. SZ: Conceptualization, Project administration, Supervision, Writing – review & editing. BL: Conceptualization, Formal analysis, Investigation, Supervision, Writing – review & editing. WG: Investigation, Software, Visualization, Writing – review & editing. HB: Conceptualization, Project administration, Supervision, Visualization, Writing – review & editing. WM: Conceptualization, Funding acquisition, Investigation, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Inner Mongolia Autonomous Region project for “Young Scientific and Technological Talents in Colleges and Universities” (NJYT22041); the Special Program to Enhance the Research Capacity of Young Faculty at Inner Mongolia Agricultural University (BR220111); the Natural Science Foundation of Inner Mongolia Autonomous Region (2022MS03048, 2023LHMS03015); and the Special Project for First-Class Discipline Research by the Inner Mongolia Autonomous Region Education Department [YLXKZX-NND-012].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hanuš, O, Samková, E, Křížová, L, Hasoňová, L, and Kala, R. Role of fatty acids in Milk fat and the influence of selected factors on their variability-a review. Molecules. (2018) 23:1636. doi: 10.3390/molecules23071636
2. Alothman, M, Hogan, SA, Hennessy, D, Dillon, P, Kilcawley, KN, O’Donovan, M, et al. The "grass-fed" Milk story: understanding the impact of pasture feeding on the composition and quality of bovine Milk. Food Secur. (2019) 8:350. doi: 10.3390/foods8080350
3. Xie, X, Cao, M, Yan, S, Gao, H, Yang, Y, Zeng, J, et al. The preventive effect of probiotic Lactobacillus plantarum X86 isolated from raw milk on Staphylococcus aureus-induced mastitis in rats. Front Vet Sci. (2025) 12:1476232. doi: 10.3389/fvets.2025.1476232
4. Tomanić, D, Božić, DD, Kladar, N, Samardžija, M, Apić, J, Baljak, J, et al. Clinical evidence on expansion of essential oil-based formulation's pharmacological activity in bovine mastitis treatment: antifungal potential as added value. Antibiotics (Basel). (2024) 13:575. doi: 10.3390/antibiotics13070575
5. Wang, Y, Chen, G, Liu, C, Liao, Y, Wei, L, Wang, H, et al. Preliminary study on the effects of dry powder and water extract of Neolamarckia cadamba on growth performance, immunity, and intestinal health of yellow-feathered broilers. BMC Vet Res. (2025) 21:233. doi: 10.1186/s12917-025-04481-w
6. Cao, L, Wang, T, Mi, X, Ji, P, Zhao, X, and Zhang, Y. Exploring the action mechanism of the active ingredient of quercetin in Ligustrum lucidum on the mouse mastitis model based on network pharmacology and molecular biology validation. Evid Based Complement Alternat Med. (2022) 2022:4236222:e01464-20. doi: 10.1155/2022/4236222
7. Jung, D, Park, S, Ruffini, J, Dufour, S, and Ronholm, J. Draft genome sequences of 113 Escherichia coli strains isolated from Intramammary infections in dairy cattle. Microbiol Resour Announc. (2021) 10:e01464-20. doi: 10.1128/MRA.01464-20
8. Zaatout, N. An overview on mastitis-associated Escherichia coli: pathogenicity, host immunity and the use of alternative therapies. Microbiol Res. (2022) 256:126960. doi: 10.1016/j.micres.2021.126960
9. Goulart, DB, and Mellata, M. Escherichia coli mastitis in dairy cattle: etiology, diagnosis, and treatment challenges. Front Microbiol. (2022) 13:928346. doi: 10.3389/fmicb.2022.928346
10. Lehtolainen, T, Pohjanvirta, T, Pyörälä, S, and Pelkonen, S. Association between virulence factors and clinical course of Escherichia coli mastitis. Acta Vet Scand. (2003) 44:203–5. doi: 10.1186/1751-0147-44-203
11. Kandeel, SA, and Megahed, AA. Editorial: infectious diseases, microbial ecology, and antimicrobial resistance dynamics in food animals. Front Vet Sci. (2023) 10:1266980. doi: 10.3389/fvets.2023.1266980
12. Barsky, SH, Mcphail, K, Wang, J, Hoffman, RM, and Ye, Y. Bone marrow origin of mammary phagocytic Intraductal macrophages (foam cells). Int J Mol Sci. (2025) 26:1699. doi: 10.3390/ijms26041699
13. De Angelis, C, Fu, X, Cataldo, ML, Nardone, A, Pereira, R, Veeraraghavan, J, et al. Activation of the IFN signaling pathway is associated with resistance to CDK4/6 inhibitors and immune checkpoint activation in ER-positive breast Cancer. Clin Cancer Res. (2021) 27:4870–82. doi: 10.1158/1078-0432.CCR-19-4191
14. Jäppinen, N, Félix, I, Lokka, E, Tyystjärvi, S, Pynttäri, A, Lahtela, T, et al. Fetal-derived macrophages dominate in adult mammary glands. Nat Commun. (2019) 10:281. doi: 10.1038/s41467-018-08065-1
15. Brady, NJ, Chuntova, P, and Schwertfeger, KL. Macrophages: regulators of the inflammatory microenvironment during mammary gland development and breast Cancer. Mediat Inflamm. (2016) 2016:4549676. doi: 10.1155/2016/4549676
16. Kobayashi, K, Oyama, S, Numata, A, Rahman, MM, and Kumura, H. Lipopolysaccharide disrupts the milk-blood barrier by modulating claudins in mammary alveolar tight junctions. PLoS One. (2013) 8:e62187. doi: 10.1371/journal.pone.0062187
17. Tommasoni, C, Fiore, E, Lisuzzo, A, and Gianesella, M. Mastitis in dairy cattle: on-farm diagnostics and future perspectives. Animals (Basel). (2023) 13:2538. doi: 10.3390/ani13152538
18. Peter, AT, Clark, PW, Van Roekel, DE, Luker, CW, Gaines, JD, and Bosu, WT. Temporal changes in metabolites of prostanoids in milk of heifers after intramammary infusion of Escherichia coli organisms. Prostaglandins. (1990) 39:451–7. doi: 10.1016/0090-6980(90)90125-f
19. Vangroenweghe, F, Duchateau, L, Boutet, P, Lekeux, P, Rainard, P, Paape, MJ, et al. Effect of carprofen treatment following experimentally induced Escherichia coli mastitis in primiparous cows. J Dairy Sci. (2005) 88:2361–76. doi: 10.3168/jds.S0022-0302(05)72914-3
20. Kaiser-Graf, D, Schulz, A, Mangelsen, E, Rothe, M, Bolbrinker, J, and Kreutz, R. Tissue lipidomic profiling supports a mechanistic role of the prostaglandin E2 pathway for albuminuria development in glomerular hyperfiltration. Front Netw Physiol. (2023) 3:1271042. doi: 10.3389/fnetp.2023.1271042
21. Tang, EH, Tang, EHC, Libby, P, Vanhoutte, PM, and Xu, A. Anti-inflammation therapy by activation of prostaglandin EP4 receptor in cardiovascular and other inflammatory diseases. J Cardiovasc Pharmacol. (2012) 59:116–23. doi: 10.1097/FJC.0b013e3182244a12
22. Hosono, K, Isonaka, R, Kawakami, T, Narumiya, S, and Majima, M. Signaling of prostaglandin E receptors, EP3 and EP4 facilitates wound healing and Lymphangiogenesis with enhanced recruitment of M2 macrophages in mice. PLoS One. (2016) 11:e0162532. doi: 10.1371/journal.pone.0162532
23. Hu, X, Tang, R, Zhao, C, Mu, R, Wang, Y, Cao, Y, et al. The prevention effect of Bacillus subtilis on Escherichia coli-induced mastitis in mice by suppressing the NF-kappaB and MAPK signaling pathways. Prob Antim Proteins. (2023) 15:74–81. doi: 10.1007/s12602-021-09854-9
24. Cadassou, O, Raza, MZ, Machon, C, Gudefin, L, Armanet, C, Chettab, K, et al. Enhanced migration of breast and lung cancer cells deficient for cN-II and CD73 via COX-2/PGE2/AKT axis regulation. Cell Oncol (Dordr). (2021) 44:151–65. doi: 10.1007/s13402-020-00558-w
25. Guimarães, JCM, Petrucci, G, Prada, J, Pires, I, and Queiroga, FL. Immunohistochemical expression and prognostic value of COX-2 and alpha-smooth muscle actin-positive Cancer-associated fibroblasts in feline mammary Cancer. In Vivo. (2024) 38:598–605. doi: 10.21873/invivo.13478
26. Ikeh, MAC, Fidel, PL Jr, and Noverr, MC. Prostaglandin E(2) receptor antagonist with antimicrobial activity against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. (2018) 62:e01920-17. doi: 10.1128/AAC.01920-17
27. Cai, JY, Hou, YN, Li, J, Ma, K, Yao, GD, Liu, WW, et al. Prostaglandin E2 attenuates synergistic bactericidal effects between COX inhibitors and antibiotics on Staphylococcus aureus. Prostaglandins Leukot Essent Fatty Acids. (2018) 133:16–22. doi: 10.1016/j.plefa.2018.04.005
28. Xu, D, Rowland, SE, Clark, P, Giroux, A, Côté, B, Guiral, S, et al. MF63 [2-(6-chloro-1H-phenanthro[9,10-d]imidazol-2-yl)-isophthalonitrile], a selective microsomal prostaglandin E synthase-1 inhibitor, relieves pyresis and pain in preclinical models of inflammation. J Pharmacol Exp Ther. (2008) 326:754–63. doi: 10.1124/jpet.108.138776
29. Tuure, L, Pemmari, A, Hämäläinen, M, Moilanen, T, and Moilanen, E. Regulation of gene expression by MF63, a selective inhibitor of microsomal PGE synthase 1 (mPGES1) in human osteoarthritic chondrocytes. Br J Pharmacol. (2020) 177:4134–46. doi: 10.1111/bph.15142
30. Kojima, FNH, Miyamoto, S, Beppu, M, Aoki, H, and Kawai, S. Membrane-associated prostaglandin E synthase-1 is upregulated by proinflammatory cytokines in chondrocytes from patients with osteoarthritis. Arthritis Res Ther. (2004) 6:R355–65. doi: 10.1186/ar1195
31. Koeberle, A, Siemoneit, U, Northoff, H, Hofmann, B, Schneider, G, and Werz, O. MK-886, an inhibitor of the 5-lipoxygenase-activating protein, inhibits cyclooxygenase-1 activity and suppresses platelet aggregation. Eur J Pharmacol. (2009) 608:84–90. doi: 10.1016/j.ejphar.2009.02.023
32. Kirkby Shaw, K, Rausch-Derra, LC, and Rhodes, L. Grapiprant: an EP4 prostaglandin receptor antagonist and novel therapy for pain and inflammation. Vet Med Sci. (2016) 2:3–9. doi: 10.1002/vms3.13
33. Robertson-Plouch, C, Stille, JR, Liu, P, Smith, C, Brown, D, Warner, M, et al. A randomized clinical efficacy study targeting mPGES1 or EP4 in dogs with spontaneous osteoarthritis. Sci Transl Med. (2019) 11:eaaw9993. doi: 10.1126/scitranslmed.aaw9993
34. Zhou, M, Ding, X, Ma, F, Xu, Y, Zhang, J, Zhu, G, et al. Long polar fimbriae contribute to pathogenic Escherichia coli infection to host cells. Appl Microbiol Biotechnol. (2019) 103:7317–24. doi: 10.1007/s00253-019-10014-x
35. Ahmadi, E, Mardani, K, and Amiri, A. Molecular detection and antimicrobial resistance patterns of Shiga toxigenic Escherichia coli isolated from bovine subclinical mastitis milk samples in Kurdistan, Iran. Arch Razi Inst. (2020) 75:169–77. doi: 10.22092/ari.2019.124238.1278
36. Yang, X, Yin, Y, Yan, X, Yu, Z, Liu, Y, and Cao, J. Flagellin attenuates experimental sepsis in a macrophage-dependent manner. Crit Care. (2019) 23:106. doi: 10.1186/s13054-019-2408-7
37. Mamatsios, K, Karatzia, MA, Manessis, G, Kasapidou, E, Bossis, I, and Basdagianni, Z. Effect of milking vacuum and the supplementation of vitamin E and se in milk quantity, quality, and hygiene of mammary gland in mountainous Greek sheep. Animals (Basel). (2023) 13:3400. doi: 10.3390/ani13213400
38. Wang, X, Zhang, B, Dong, W, Zhao, Y, Zhao, X, Zhang, Y, et al. SLC34A2 targets in calcium/phosphorus homeostasis of mammary gland and involvement in development of clinical mastitis in dairy cows. Animals (Basel). (2024) 14:1275. doi: 10.3390/ani14091275
39. Xiong, Y, Shen, T, Lou, P, Yang, J, Kastelic, JP, Liu, J, et al. Colostrum-derived extracellular vesicles: potential multifunctional nanomedicine for alleviating mastitis. J Nanobiotechnology. (2024) 22:627. doi: 10.1186/s12951-024-02926-2
40. Hu, X, Guo, J, Zhao, C, Jiang, P, Maimai, T, Yanyi, L, et al. The gut microbiota contributes to the development of Staphylococcus aureus-induced mastitis in mice. ISME J. (2020) 14:1897–910. doi: 10.1038/s41396-020-0651-1
41. Zhao, C, Hu, X, Bao, L, Wu, K, Feng, L, Qiu, M, et al. Aryl hydrocarbon receptor activation by Lactobacillus reuteri tryptophan metabolism alleviates Escherichia coli-induced mastitis in mice. PLoS Pathog. (2021) 17:e1009774. doi: 10.1371/journal.ppat.1009774
42. Ricciotti, E, and FitzGerald, GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. (2011) 31:986–1000. doi: 10.1161/ATVBAHA.110.207449
43. Tilley, SL, Coffman, TM, and Koller, BH. Mixed messages: modulation of inflammation and immune responses by prostaglandins and thromboxanes. J Clin Invest. (2001) 108:15–23. doi: 10.1172/JCI200113416
44. Estúa-Acosta, GA, Buentello-Volante, B, Magaña-Guerrero, FS, Flores, JE, Vivanco-Rojas, O, Castro-Salas, I, et al. Human amniotic membrane mesenchymal stem cell-synthesized PGE(2) exerts an immunomodulatory effect on neutrophil extracellular trap in a PAD-4-dependent pathway through EP2 and EP4. Cells. (2022) 11:2831. doi: 10.3390/cells11182831
45. Pein, H, Ville, A, Pace, S, Temml, V, Garscha, U, Raasch, M, et al. Endogenous metabolites of vitamin E limit inflammation by targeting 5-lipoxygenase. Nat Commun. (2018) 9:3834. doi: 10.1038/s41467-018-06158-5
46. Sánchez-Zuno, GA, González-Estevez, G, Matuz-Flores, MG, Macedo-Ojeda, G, Hernández-Bello, J, Mora-Mora, JC, et al. Vitamin D levels in COVID-19 outpatients from Western Mexico: clinical correlation and effect of its supplementation. J Clin Med. (2021) 10:2378. doi: 10.3390/jcm10112378
47. Wang, M, Zhang, D, Fu, N, Liu, M, Zhang, H, Feng, S, et al. Clinical features of cystic neutrophil granulomatous mastitis in 62 cases. Heliyon. (2025) 11:e42415. doi: 10.1016/j.heliyon.2025.e42415
48. Zubova, SV, Kosyakova, NI, Grachev, SV, and Prokhorenko, IR. Rhodobacter capsulatus PG lipopolysaccharide blocks the effects of a Lipoteichoic acid, a toll-like receptor 2 agonist. Acta Nat. (2022) 14:69–74. doi: 10.32607/actanaturae.11747
49. Shao, D, Shen, W, Miao, Y, Gao, Z, Pan, M, Wei, Q, et al. Sulforaphane prevents LPS-induced inflammation by regulating the Nrf2-mediated autophagy pathway in goat mammary epithelial cells and a mouse model of mastitis. J Anim Sci Biotechnol. (2023) 14:61. doi: 10.1186/s40104-023-00858-9
50. Liu, X, Ma, Q, Sun, X, Lu, M, Ehsan, M, Hasan, MW, et al. Effects of recombinant toxoplasma gondii citrate synthase I on the cellular functions of murine macrophages in vitro. Front Microbiol. (2017) 8:1376. doi: 10.3389/fmicb.2017.01376
51. Wu, Y, Li, D, Wang, Y, Liu, X, Zhang, Y, Qu, W, et al. Beta-Defensin 2 and 3 promote bacterial clearance of Pseudomonas aeruginosa by inhibiting macrophage autophagy through downregulation of early growth response Gene-1 and c-FOS. Front Immunol. (2018) 9:211. doi: 10.3389/fimmu.2018.00211
52. Serezani, CH, Chung, J, Ballinger, MN, Moore, BB, Aronoff, DM, and Peters-Golden, M. Prostaglandin E2 suppresses bacterial killing in alveolar macrophages by inhibiting NADPH oxidase. Am J Respir Cell Mol Biol. (2007) 37:562–70. doi: 10.1165/rcmb.2007-0153OC
53. Salina, AC, Souza, TP, Serezani, CH, and Medeiros, AI. Efferocytosis-induced prostaglandin E2 production impairs alveolar macrophage effector functions during Streptococcus pneumoniae infection. Innate Immun. (2017) 23:219–27. doi: 10.1177/1753425916684934
54. Pak, S, Thapa, B, and Lee, K. Decursinol angelate mitigates Sepsis induced by methicillin-resistant Staphylococcus aureus infection by modulating the inflammatory responses of macrophages. Int J Mol Sci. (2021) 22:10950. doi: 10.3390/ijms222010950
55. Yuk, JM, Kim, JK, Kim, IS, and Jo, EK. TNF in human tuberculosis: a double-edged sword. Immune Netw. (2024) 24:e4. doi: 10.4110/in.2024.24.e4
56. Gong, Z, Zhang, S, Gu, B, Cao, J, Mao, W, Yao, Y, et al. Codonopsis pilosula polysaccharides attenuate Escherichia coli-induced acute lung injury in mice. Food Funct. (2022) 13:7999–8011. doi: 10.1039/d2fo01221a
57. Hisaoka-Nakashima, K, Azuma, H, Ishikawa, F, Nakamura, Y, Wang, D, Liu, K, et al. Corticosterone induces HMGB1 release in primary cultured rat cortical astrocytes: involvement of Pannexin-1 and P2X7 receptor-dependent mechanisms. Cells. (2020) 9:1068. doi: 10.3390/cells9051068
58. Mambetsariev, N, Mirzapoiazova, T, Mambetsariev, B, Sammani, S, Lennon, FE, Garcia, JGN, et al. Hyaluronic acid binding protein 2 is a novel regulator of vascular integrity. Arterioscler Thromb Vasc Biol. (2010) 30:483–90. doi: 10.1161/ATVBAHA.109.200451
59. Takemiya, T, and Yamagata, K. Intercellular signaling pathway among endothelia, astrocytes and neurons in excitatory neuronal damage. Int J Mol Sci. (2013) 14:8345–57. doi: 10.3390/ijms14048345
60. Li, T, Liu, B, Mao, W, Gao, R, Wu, J, Deng, Y, et al. Prostaglandin E2 promotes nitric oxide synthase 2, platelet-activating factor receptor, and matrix metalloproteinase-2 expression in Escherichia coli-challenged ex vivo endometrial explants via the prostaglandin E2 receptor 4/protein kinase a signaling pathway. Theriogenology. (2019) 134:65–73. doi: 10.1016/j.theriogenology.2019.04.028
61. Kawahara, K, Hohjoh, H, Inazumi, T, Tsuchiya, S, and Sugimoto, Y. Prostaglandin E2-induced inflammation: relevance of prostaglandin E receptors. Biochim Biophys Acta. (2015) 1851:414–21. doi: 10.1016/j.bbalip.2014.07.008
62. Alessi, I, Banton, KL, J, S, C, ZM, CH, P, RJ, R, et al. Exploring novel non-opioid pathways and therapeutics for pain modulation. Mol Pain. (2025) 21:17448069251327840. doi: 10.1177/17448069251327840
63. Shieh, JM, Tsai, YJ, Ma, MC, Chen, CL, and Wu, WB. The cyclooxygenase-2 upregulation mediates production of PGE2 autacoid to positively regulate interleukin-6 secretion in chronic rhinosinusitis with nasal polyps and polyp-derived fibroblasts. Sci Rep. (2024) 14:7559. doi: 10.1038/s41598-024-58143-2
64. Vallerie, SN, Kramer, F, Barnhart, S, Kanter, JE, Breyer, RM, Andreasson, KI, et al. Myeloid cell prostaglandin E2 receptor EP4 modulates cytokine production but not Atherogenesis in a mouse model of type 1 diabetes. PLoS One. (2016) 11:e0158316. doi: 10.1371/journal.pone.0158316
65. Wang, D, and DuBois, RN. An inflammatory mediator, prostaglandin E2, in colorectal cancer. Cancer J. (2013) 19:502–10. doi: 10.1097/PPO.0000000000000003
66. Deng, J, Yang, H, Haak, VM, Yang, J, Kipper, FC, Barksdale, C, et al. Eicosanoid regulation of debris-stimulated metastasis. Proc Natl Acad Sci USA. (2021) 118:e2107771118. doi: 10.1073/pnas.2107771118
Keywords: Escherichia coli , mastitis, alternative treatments to antibiotics, mPGES-1 inhibitors, EP4 receptor inhibitor
Citation: Yang X, Li X, Guo L, Gong P, Qian Y, Zhang S, Liu B, Guo W, Bao H and Mao W (2025) Investigating the role of the mPGES-PGE₂-EP4 pathway in Escherichia coli-induced mastitis in dairy cows: insights for non-antibiotic therapeutic strategies. Front. Vet. Sci. 12:1628028. doi: 10.3389/fvets.2025.1628028
Edited by:
Zhigang Zhang, Northeast Agricultural University, ChinaReviewed by:
LiLi Hao, Southwest Minzu University, ChinaYaqi Zeng, Xinjiang Agricultural University, China
Copyright © 2025 Yang, Li, Guo, Gong, Qian, Zhang, Liu, Guo, Bao and Mao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Mao, bWFvd2VpMjAxNEBpbWF1LmVkdS5jbg==; Haixia Bao, aGFpeGlhYmFvQDE2My5jb20=
 Xiaolin Yang
Xiaolin Yang Xueqiang Li
Xueqiang Li Lili Guo1,2
Lili Guo1,2 Wei Mao
Wei Mao