- 1Facultad de Medicina Veterinaria y Zootecnia No. 1, Universidad Autónoma de Guerrero, Chilpancingo, Mexico
- 2Forestry and Wood Technology Department, Faculty of Agriculture (El-Shatby), Alexandria University, Alexandria, Egypt
- 3Facultad de Medicina Veterinaria y Zootecnia, Universidad Autónoma del Estado de México, Toluca, Mexico
- 4Department of Pesticide Chemistry and Technology, Faculty of Desert and Environmental Agriculture, Matrouh University, Mersa Matruh, Egypt
- 5Department of Industrial Engineering, University of Applied Sciences Technikum Wien, Vienna, Austria
- 6Dipartimento di Scienze del Suolo, Della Pianta e Degli Alimenti, Università Degli Studi di Bari, Bari, Italy
Caesalpinia coriaria (Jacq.) Willd [syn.: Libidibia coriaria (Jacq.) Schltdl.], a member of the Fabaceae family and the Caesalpinioideae subfamily, is commonly known in Mexican vernacular as “cascalote“. Various botanical parts of this tree, such as leaves, pods, flowers, seeds, branches, and bark, have been studied due to their bioactivity and their astringent, antiparasitic, antiseptic, and anti-inflammatory properties. Extracts obtained from C. coriaria contain a wide range of bioactive compounds, including tannins, terpenoids, phenols, coumarins, quinones, flavonoids, saponins, carbohydrates, proteins, glycosides, cardiac glycosides, anthraquinones, steroids, and polyphenols. During the fattening phase in ruminants, these plant extracts may be used to reduce gastrointestinal parasitism, promote growth, and decrease drug residues in animal-derived products. This review aims to highlight the importance of the bioactivities of C. coriaria extracts and their active compounds. In vitro studies have demonstrated that the phenolic and flavonoid compounds present in this species inhibit bacterial growth by disrupting membrane integrity and enzymatic activity, often outperforming conventional antibiotics. In livestock production systems, the presence of pathogenic bacteria leads to significant economic losses; in this context, the use of polyphenolic compounds derived from C. coriaria may have a positive effect on animal productivity. Moreover, the extracts from this tree represent a promising source of bioactive compounds for various industrial applications.
Introduction
In recent years, the use of plant-derived extracts, particularly from legumes and aromatic species, has generated growing interest as a natural and ecological alternative to synthetic antibiotics and anthelmintics in animal production systems (1). This trend reflects the need to improve animal health and productivity while mitigating the risks associated with antimicrobial resistance, pharmaceutical residues in animal products, and the environmental impact of livestock farming (1, 2). In this context, Caesalpinia coriaria (Jack) Willd. (syn.: Libidibia coriaria (Jacq.) Schltdl.), commonly known in Mexico as “cascalote,” is a species native to tropical and subtropical regions that belongs to the Fabaceae family (3); however, despite being a legume, it does not have the capacity to fix atmospheric nitrogen and therefore does not contribute to enriching the soil with this element (4). Its presence predominates on the Pacific coasts in the states of Oaxaca, Michoacán, Jalisco, and Sinaloa (4, 5) and is known to be a species commonly used by traditional medicine for the treatment of various ailments.
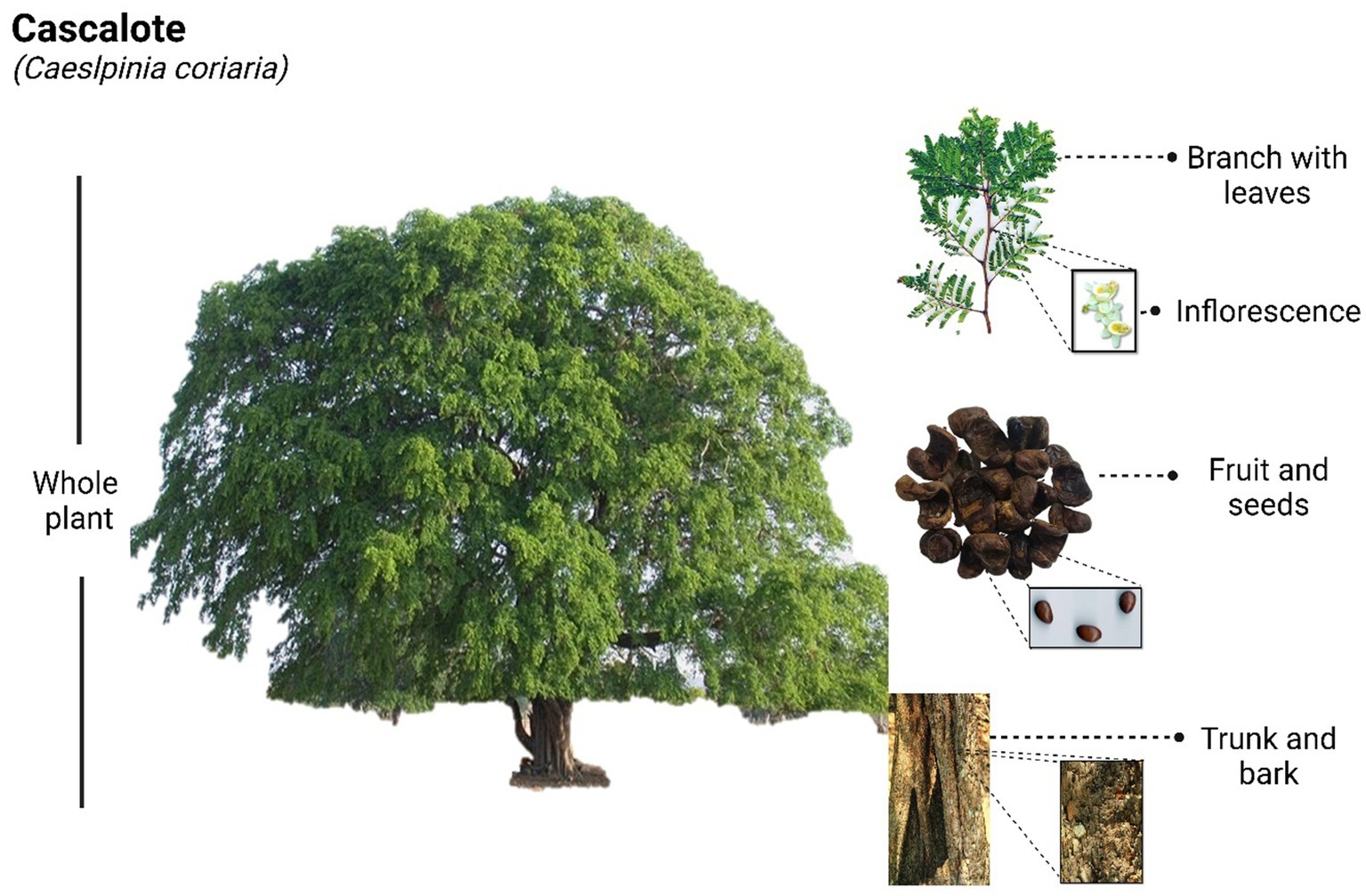
Caesalpinia coriaria, which can grow up to 6 meters tall, bears individual thorns along its branches and produces orange or yellow flowers with red stripes, measuring up to 1.5 cm in diameter. Its green leaves, with pale undersides, are composed of three to five pairs of oval leaflets (2 to 6 cm long), and its leguminous fruits are small, reddish, cylindrical pods that typically contain about five seeds each (Figure 1). All parts of the plant are used to treat various ailments, including the bark and leaves as astringents, the flowers for heart disease and digestive issues, the roots for their antiseptic properties in ulcer treatment, and nut-based infusions for relieving tonsillitis (6). The applications of this species extend beyond human medicine, as several studies have demonstrated its potential as ruminant fodder with promising results, and its use in artisanal leather tanning processes is also well docum (4). Incorporating C. coriaria fruit waste into ruminant diets reduces methane and carbon dioxide emissions while improving ruminal fermentation, offering an eco-friendly approach to livestock management (7, 8). The combined use of C. coriaria fruit and fungal agents provides an effective, sustainable alternative for controlling gastrointestinal nematodes in sheep (9). Its fruit, which resembles a twisted pod, is called nacascolotl in Náhuatl, meaning “twisted ear,” and is the most valued part of the plant due to its astringent, antiseptic, and anti-inflammatory properties (10).
Caesalpinia coriaria is a leguminous tree with well-documented ethnomedical applications (11, 12), whose pharmacological relevance lies in its content of bioactive phenolic compounds with anthelmintic properties, particularly effective for controlling parasitic infections in cattle and small ruminants (13–15); this makes it a valuable resource for farmers who lack access to synthetic veterinary drugs, as the use of its extracts during the fattening phase not only contributes to reducing gastrointestinal parasitism, but also enhances growth performance and minimizes drug residues in animal-derived products (16). The therapeutic potential of this species is further supported by the long-standing use of its leaves and fruits for their anti-inflammatory (10), antioxidant (17), and antibacterial (18, 19) activities, with traditional preparations such as decoctions of dried fruits and leaves being employed to relieve gastrointestinal discomfort and stomach cramps (20). In particular, the pods are known to contain high concentrations of phenolic compounds with strong antioxidant capacity (21, 22), while both leaves and fruits have been found to be rich in saponins, tannins, flavonoids, ethyl gallate, and gallic acid (15, 18, 22), all of which contribute to their bioactivity. Among these, methyl gallate, ethyl gallate, and corilagin stand out as predominant phenolic constituents, whose multiple biological activities, including potent free radical scavenging effects, have been identified and described in several studies (3, 21, 23–25).
By modulating nitric oxide production, as well as anti-inflammatory and antioxidant pathways, methanol extracts of C. coriaria pods significantly reduced gastrointestinal lesions in rat models, showing effects comparable to conventional medications, possibly due to the presence of gallic acid derivatives (21). The hydroalcoholic extracts from fruits exhibit larvicidal and ovicidal properties against the parasitic worm (Haemonchus contortus Rudolphi, 1803) Cobb, 1898 (family Trichostrongylidae) in ruminants, indicating that they may be used as a natural anthelmintic (9, 26). To emphasize the biological benefits of C. coriaria extracts from various botanical parts and their bioactive components, this review was conducted.
Extraction techniques for phytochemical analysis
In the field of medicinal and aromatic plants, there are several methods for the extraction from different parts of plants (seeds, leaves, bark, wood, roots, flowers, and branches) including soaking or maceration, solid–liquid extraction, and others using several solvents (27–31). In this regard, C. coriaria fruits were extracted using maceration methods with two solvents: polar (ethyl acetate) and less polar (hexane) solvents (32). To generate the hydroalcoholic extract, which was then concentrated using a rotary evaporator at 50–55°C, the dried fruit material was macerated with water and methanol (70%, 1:10, w/v) or aqueous methanol at room temperature for 24 h (13, 15, 24). An organic fraction; ethyl acetate (EtOAc-F) and an aqueous fraction; water (Aq-F) were obtained by liquid–liquid extraction of the hydroalcoholic extract.
At room temperature, 600 g of the collected pods were dried, ground into a powder, and extracted using 2 L of a solvent mixture of ethanol, acetone, and water (80:10:10, v/v). The extract after 72 h was filtered and concentrated under lower pressure (3). In triplicate, 900 g of C. coriaria pods were macerated in 2 L of methanol for 24 h at room temperature. The Whatman filter paper was used to filter the resultant extract. A rotary evaporator was used to remove the solvent following filtering (21). Dried C. coriaria fruits were ground to 1 mm using a hammer mill to create the aqueous extract. In 2.5 L of distilled water, 1 kilogram of pulverized C. coriaria fruits were steeped. The contents were filtered to produce the aqueous extract after the combination was allowed to stand for 72 h (16). Additionally, the chromatographic analysis methods for identifying chemical compounds from C. coriaria were primarily employed in high-pressure liquid chromatography (HPLC) and gas chromatography–mass spectroscopy (GC–MS) analyses (32).
Phytochemical profile and bioactive compounds
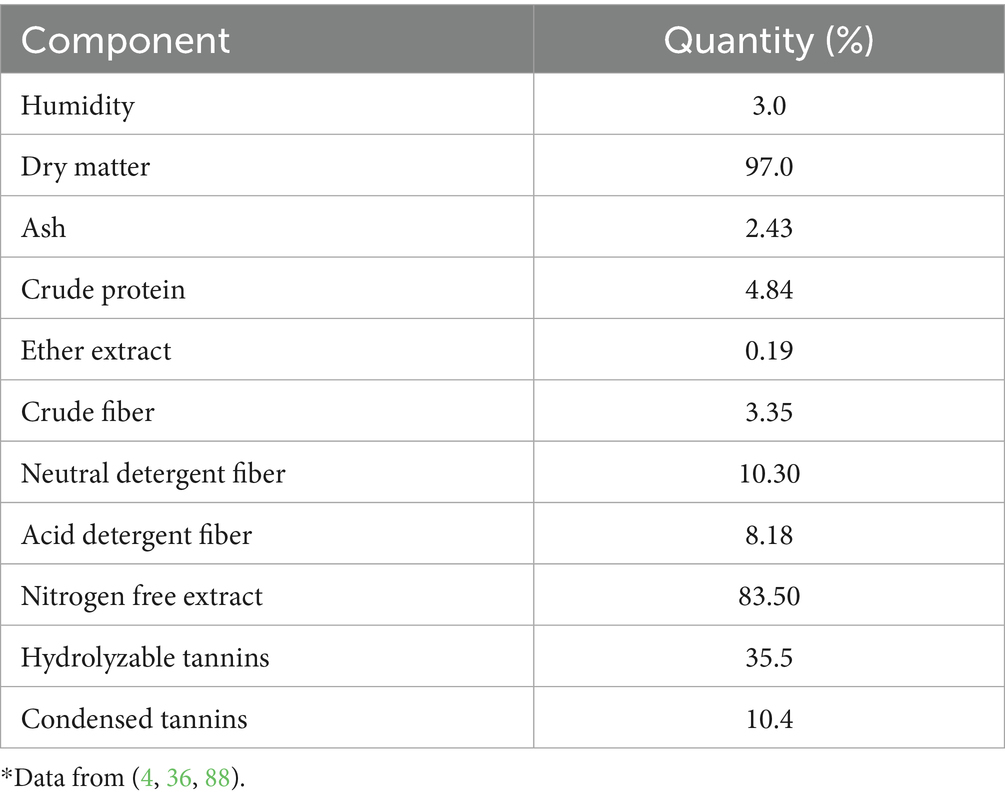
Figure 2 displays the chemical structures of the bioactive substances found in C. coriaria extracts as collected from the literature, and Table 1 shows the fruits’ proximate analysis. The extract of C. coriaria has been reported to contain phenols, tannins (including condensed tannins and proanthocyanidins), flavonoids, quinones, coumarins, and saponins (25, 33). Tannins are polyphenolic secondary metabolites derived from gallic acid, characterized by their high molecular weight, water solubility, and bitter taste (8); they are synthesized by plants during their growth and development and are distinguished by their ability to form stable, high-strength complexes with proteins (4, 34). Based on their chemical structure, tannins are broadly classified into two main types: hydrolyzable and condensed tannins. In the methanolic extract of C. coriaria pods, considerable concentrations of total polyphenolic compounds (439.08 mg/g), condensed tannins (7.72 mg/g), flavones and flavonols (149.50 mg/g), as well as total flavonoids (16.84 mg/g) have been reported (21). One of the main reasons C. coriaria has attracted significant research interest is its high tannin content, particularly concentrated in the leaves and pods (5). Furthermore, ethyl acetate and hexane extracts obtained from the fruits have shown insecticidal activity against Spodoptera frugiperda (32). HPLC analysis identified phenolic compounds, including ellagic acid, in the ethyl acetate extract, while GC–MS analysis of the hexane extract revealed hexadecanoic acid, 11-methylheptacosane, dodecanoic acid, and nonacosane as the major constituents (32).
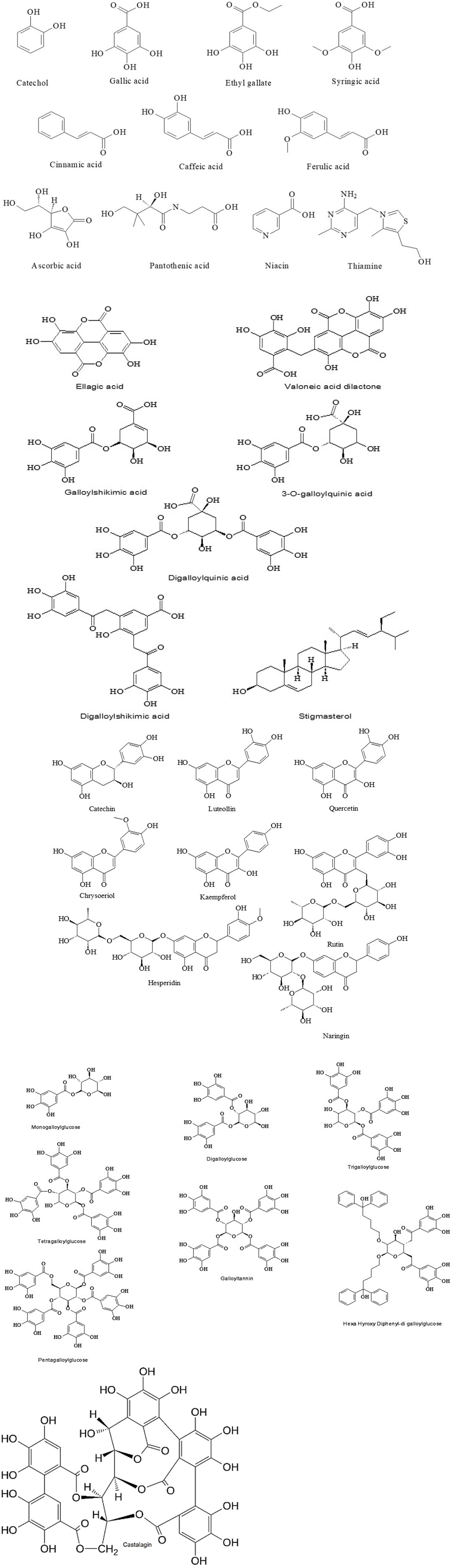
Figure 2. Chemical structures of the compounds present in Caesalpinia coriaria extracts (15, 16, 21, 24, 32).
Given that the phenolic compounds present in the pods are recognized for their antioxidant properties, the concentration of these compounds in the methanolic extract of Caesalpinia coriaria pods is likely to influence the plant’s overall antioxidant capacity. According to liquid chromatography-mass spectroscopy (LC–MS) analysis, the main constituents of the extract include gallic acid, 3-O-galloylquinic acid, digalloylglucose, tetragalloylglucose, valoneic acid dilactone, pentagalloylglucose, digalloylshikimic acid, and ellagic acid (Figure 2; 21). These compounds have demonstrated not only strong antioxidant activity but also significant efficacy in reducing methane emissions when incorporated into animal diets, along with antibacterial (35) and antiparasitic properties. Estimates suggest that the tannin content in C. coriaria fruits ranges from 34 to 47% (36), with some studies reporting approximately 35% hydrolyzable tannins and around 10% condensed tannins (4), and it is further estimated that approximately 20,000 tons of C. coriaria pods are produced annually in Mexico (5), with tannin extraction from the fruit powder yielding up to 47.0% by weight in total tannins, of which 30.0% corresponds to hydrolyzable tannins (37).
Ethyl gallate and gallic acid were identified and characterized through spectroscopic data analysis and comparison with previously published literature, while stigmasterol was confirmed by direct comparison with the spectroscopic data of a reference standard (3, 5, 38). Gallic acid has been described as the main compound in a hydroalcoholic extract of C. coriaria (15, 24, 39), and its fruit extract has also been shown to contain water-soluble vitamins such as thiamine, pantothenic acid, and niacin (16). Quantitative and phytochemical analyses of the pod material showed that the tannin fraction is what gives it its antibacterial properties. According to the findings, C. coriaria may be a good choice for managing organisms with antibacterial properties (19).
Antimicrobial activity
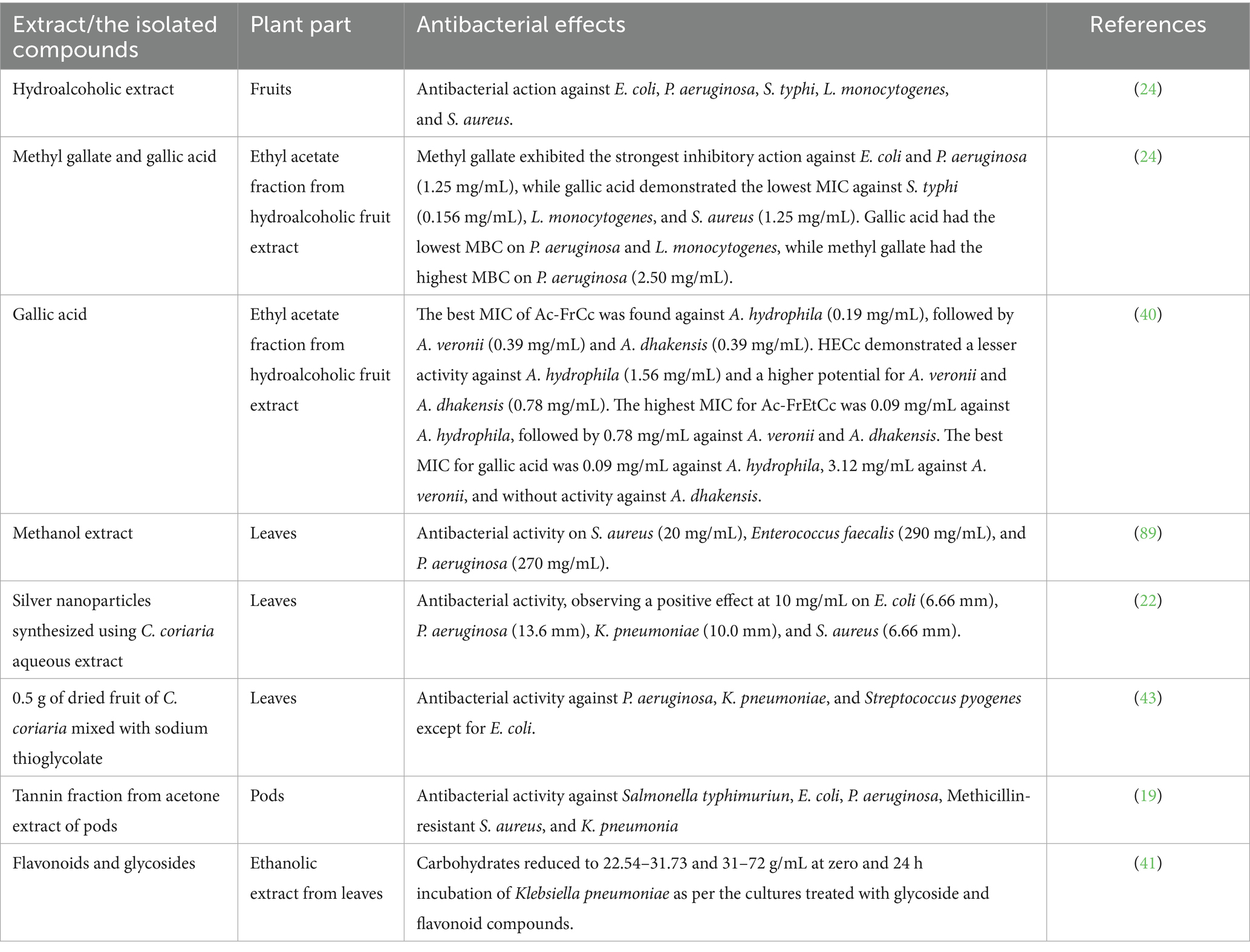
Extracts and isolated compounds from C. coriaria exhibit significant antibacterial effects against human pathogens (e.g., Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, Salmonella typhi, and Listeria monocytogenes) and aquaculture-relevant bacteria (Aeromonas spp.), often outperforming standard antibiotics in vitro (7, 24, 40). The bioactive substances flavonoids and glycosides from the ethanolic extract of C. coriaria penetrate the inner membrane and deactivate the respiratory chain dehydrogenase enzyme system of E. coli, S. aureus, and Klebsiella pneumonia, preventing cell growth and respiration (41). Table 2 summarizes the antimicrobial potential.
Treatment options for disorders caused by the genus Aeromonas may include the hydroalcoholic extract of C. coriaria and its fractions, which have antibacterial activity against Aeromonas hydrophila, Aeromonas veronii, and Aeromonas dhakensis (40). Ruminant parasitic nematodes were killed by the C. coriaria ethyl acetate fraction, with gallic acid serving as the primary chemical responsible for the observed ovicidal activity (15). In vitro, gallic acid also showed activity against bacteria of public health importance at concentrations of 5–10 mg/mL (24). Regarding the mechanism of action, the antibacterial activity of the methanolic extract of C. coriaria can be attributed to the presence of phenolic compounds, particularly phenolic acids; however, these are not the only secondary metabolites present in C. coriaria (18). It was reported that the antibacterial activity in vitro is attributed to the synergistic action of acidic and phenolic fractions, the activity of which is lost when these components are separated (42). Hexane, methanol, acetone, and water extracts by the maceration method from C. coriaria pod were tested in vitro against some pathogenic bacteria, and all of which are significantly inhibited by the acetone extract (19) as shown in Figure 3. Additionally, the plant extract was found to have an inhibitory activity of its acetone extract (10, 15, 20, 25, and 30 μg) on S. typhi, E. coli, P. aeruginosa, S. aureus and K. pneumonia (19). According to certain research, the fruit’s aqueous extract and the leaves’ alcoholic extract have antibacterial properties against P. aeruginosa, E. coli, Xanthomonas pathovars, and S. aureus (18, 43).
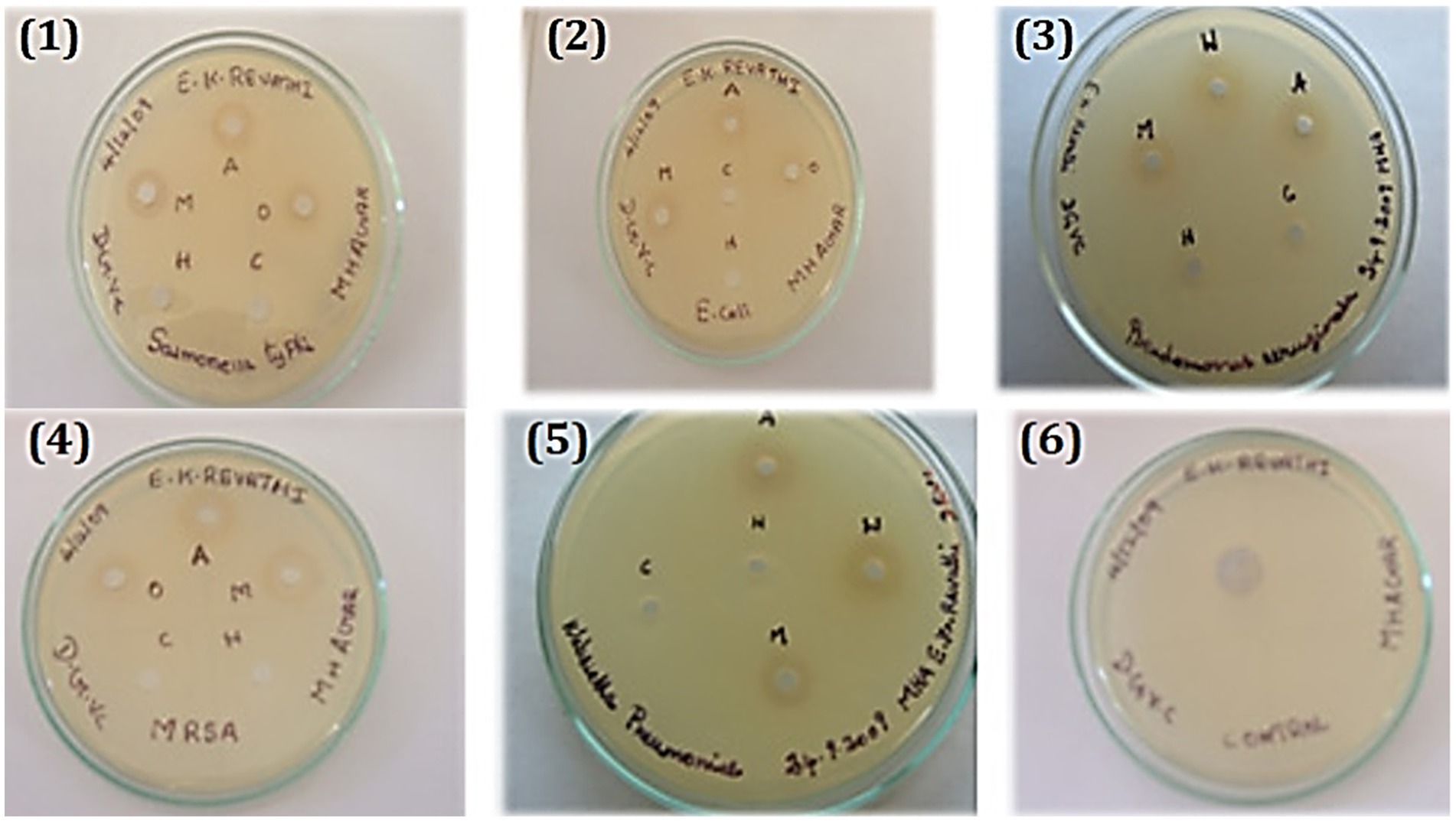
Figure 3. Antibacterial activity of bacterial strains toward Caesalpinia coriaria (19). 1. Salmonella typhi, 2. Escherichia coli, 3. Pseudomonas aeruginosa, 4. Staphylococcus aureus, 5. Klebsiella pneumonia and 6. Control. Reproduced from (19). This work is licensed under a Creative Commons Attribution 4.0 International License.
Antiparasitic activity
One of the primary issues affecting small ruminants is parasitism, and among them, gastrointestinal nematodes (GIN) are the leading cause of death for sheep and goats in Mexico’s tropical regions (44–46). With a 70% prevalence in tropical areas, Haemonchus contortus is the most significant epidemiological nematode that parasitizes the abomasum of ruminants, including cattle, sheep, and goats (47, 48). The most dangerous parasite feeds on blood, damages the abomasal epithelium, and causes inflammation, emaciation, anemia, and hypoproteinemia in addition to submandibular edema, drooping of the productive parameters (the production of wool, milk, and meat), and frequently the death of infected animals (48, 49). From a biological and economic perspective, parasites are an issue, particularly when chemical dewormers are misused, which has aided in the emergence of anthelmintic resistance (50). One of the factors contributing to the development of resistance in these microorganisms is the regular use of anthelmintics to control parasites (51).
In this regard, medicinal plants are recommended to have bioactive compounds to act as nematicidal properties of use in ruminants (47, 52). Gallic acid is the chemical that caused the anthelmintic activity, as evidenced by the in vitro data, which demonstrated the larvicidal impact of the hydroalcoholic extract of C. coriaria against the nematode H. contortus (13). The most active effective concentrations with LC50 and LC90 values were 0.01 and 5.42 mg/mL, respectively. The animals in the C. coriaria fruit group did not exhibit a decrease in eggs per gram (EPG) at the beginning of the trial, which took place on day 7 of the first week. But by the end of the study (day 42), this group’s EPG reduction was much lower than that of the ivermectin-dewormed group (78.6 vs 52.6%) (13) for a group of animals that received the fruits of C. coriaria showed no reduction in the EPG. Under grazing conditions, this plant may act as a natural anthelmintic to prevent haemonchosis in goats. The study’s findings indicate that C. coriaria fruits have a significant anthelmintic effect in both in vitro and in vivo settings. The nematocidal qualities of the phytochemical elements derived from the fruits of this arboreal legume are demonstrated by the larvicidal impact (13).
Sheep naturally infected with mixed gastrointestinal nematode species that were fed 100 g of C. coriaria fruits showed a considerable reduction in eggs per gram of feces (70%) (9). Consequently, a notable anthelmintic impact in goats was attained at a 10% level of inclusion in the overall diet (53). Condensed tannins (CT) are abundant in the fruits of this arboreal legume; each gram contains 0.367 g of CT, according to Camacho-Díaz et al. (54). Recent research on the consumption of C. coriaria fruits (10% DM or 3.67% of CT) is comparable to that of Manuel-Pablo et al. (53), they determined that the levels of CT employed (1.5, 3.0, and 4.5%) in the basal diet did not affect productive parameters after evaluating the effect of C. coriaria fruit supplementation on goat productivity. The concentration affected the extracts’ inhibitory effects; for the acetonic extract, the inhibitory activity was very comparable to the positive control doses of 1.2 mg/mL, and for the ethanolic extract, 0.78 mg/mL (55). The results demonstrate that H. contortus eggs are inhibited by extracts prepared using C. coriaria fruits in acetonic and ethanolic solvents (55).
Hydroalcoholic extracts from C. coriaria leaves and mature fruits exhibit ovicidal effect on H. contortus and H. placei (Place, 1893) (Nematoda, Trichostrongylidae, Haemonchinae; 59). Hydro-alcoholic extracts from both leaves and fruits showed a concentration-dependent ovicidal activity, with a 25.0 mg/mL concentration showing 100% efficiency against both nematode species (56). The hatching suppression of five gastrointestinal parasitic nematode eggs (Haemonchus spp., Cooperia spp., Ostertagia spp., Trichostrongylus spp., and Oesophagostomum spp.) was linked to gallic acid and various galloyl derivatives extracted from C. coriaria fruits.
Hydro-alcoholic extract (HA-E) and ethyl acetate fraction (EtOAc-F) from the fruits showed ovicidal activity at an LC50 of 0.92 and 0.16 mg/mL, respectively (15). Galloyl derivatives showed ovicidal activity against cattle gastrointestinal parasitic nematodes close to 100% at a 1 mg/mL concentration. Additionally, it was demonstrated that methyl gallate was ineffective against these parasites (15).
Possible bioactive mechanisms of the chemical compounds
Catechol, gallic acid, ethyl gallate, syringic acid, ferulic acid, caffeic acid, and cinnamic acid are members of the phenolic acid group, exhibiting diverse biological activities because of their antioxidant, anti-inflammatory, and antimicrobial properties (57). Catechol, a simple benzenediol, serves as a precursor in lignin and flavonoid biosynthesis, while gallic acid and its derivative ethyl gallate act as potent antioxidants with demonstrated antiparasitic activity (58). Hydroxycinnamic acids such as ferulic, caffeic, and cinnamic acid exhibited antibarasitoc effects on two Haemonchus contortus isolates (59). Ascorbic acid (vitamin C), pantothenic acid (vitamin B5), niacin (vitamin B3), and thiamine (vitamin B1) are essential water-soluble vitamins with critical metabolic functions (60). Ascorbic acid is a crucial antioxidant and cofactor in collagen synthesis, pantothenic acid is integral to coenzyme A synthesis and energy metabolism, niacin participates in redox reactions as NAD+/NADH, and thiamine (as thiamine pyrophosphate) is essential for carbohydrate metabolism and neurological function (61).
Catechin, luteolin, quercetin, chrysoeriol, kaempferol, rutin, naringin, and hesperidin are bioactive flavonoids, a diverse class of polyphenolic compounds widely found in plants and known for their significant antimicrobial properties (62). Catechin, a flavan-3-ol, shows strong antioxidant and cardioprotective effects, while luteolin and quercetin, both flavones and flavonols, respectively, exhibit potent antimicrobial and antiparasitic activities with ability to modulate signaling pathways and scavenge reactive oxygen species (63, 64). Chrysoeriol and kaempferol, also flavonols, contribute to plant defense mechanisms and display antimicrobial effects (65, 66). Naringin and hesperidin, classified as flavanone glycosides, are prevalent in citrus fruits, where they contribute to antioxidant defense, cholesterol metabolism, and anti-inflammatory responses, with hesperidin also enhancing cardiovascular health through the improvement of endothelial function (67, 68).
The natural compounds ellagic acid, valoneic acid, galloyl shikimic acid, 3-O-galloylquinic acid, digalloylquinic acid, digalloyl shikimic acid, and stigmasterol belong to distinct biochemical classes with significant biological and pharmacological importance (69, 70). Ellagic acid, a dimeric derivative of gallic acid, is a hydrolyzable tannin metabolite derived from ellagitannins and demonstrates potent antioxidant, anti-inflammatory, and anticancer properties by modulating cellular signaling pathways (71). The galloyl shikimic acid and digalloyl shikimic acid derivatives, intermediates in the shikimate pathway, are key precursors in plant polyphenol biosynthesis and exhibit antimicrobial and anti-inflammatory effects, particularly in Eucalyptus species as well as in Scots pine and Norway spruce (72, 73). Similarly, 3-O-galloylquinic acid and digalloylquinic acid, both classified as hydrolyzable tannins, are abundant in plants like Terminalia chebula and display hepatoprotective and antidiabetic activities through free radical scavenging and regulation of glucose metabolism (74). In contrast, stigmasterol, a phytosterol, is crucial for membrane structure in plants and exhibits cholesterol-lowering, anti-inflammatory, and immunomodulatory effects in humans, making it valuable in managing cardiovascular and metabolic diseases (75).
Monogalloylglucose, digalloylglucose, trigalloylglucose, tetragalloylglucose, and pentagalloylglucose are part of the class of hydrolyzable tannins, specifically gallotannins, which are esters of glucose and gallic acid with varying degrees of galloylation (76). These compounds show significant biological activities, including antioxidant, antimicrobial, and anti-inflammatory properties, because of their capacity to scavenge free radicals and interact with cellular proteins and enzymes (77). Galloyltannins, a broader subgroup, demonstrate enhanced bioactivity with increasing galloyl units, as observed in pentagalloylglucose, which is particularly noted for its potent protein-binding and enzyme-inhibitory effects (78). Hexahydroxydiphenyl-digalloylglucose, an ellagitannin precursor, further adds to the structural diversity and functional importance of tannins, contributing to potential therapeutic applications (21).
Most of the studies about the chemical compounds identified in C. coriaria showed the presence of polyphenolic compounds including gallic acid, galloyl derivatives, tannins, and flavonoids. From other plant species, the methanol extracts of Ceratonia siliqua L. (family Fabaceae) and Ziziphus spina-christi (L.) Desf. (family Rhamnaceae) leaves and branched showed the presence of several bioactive compounds including gallic acid, syringic acid, methyl gallate, catechin, coumaric acid, ellagic acid, and chlorogenic acid with potential antifungal activities (27).
The biological activity of flavonoids seems to be due to their amphipathic characteristics, which strengthen the chemical structure’s antibacterial activities, especially those of the hydrophobic substituents (prenyl groups, alkylamino, and alkyl chains, and heterocyclic fractions with N or O) (79, 80). Quercetin, rutin, vanillic acid, caffeic acid, apigenin, chlorogenic acid, ferulic acid, and cinnamic acid have been reported in polar extracts of various medicinal plants (81, 82).
In many ways, gallic acid is one of the most significant plant polyphenols with health-promoting properties. Numerous investigations have demonstrated that gallic acid suppresses bacterial growth by changing the shape of the membrane, bacterial metabolism, and the development and growth of biofilms (83). Disrupting bacterial cell membranes, preventing the formation of biofilms, and possibly interfering with bacterial DNA repair pathways are the main ways that gallic acid works against bacteria. Additionally, it can make other antibiotics more effective (84, 85). E. coli, P. aeruginosa, S. aureus, and L. monocytogenes were used to test the mechanisms of action of gallic and ferulic acids, hydroxybenzoic acid, and hydroxycinnamic acid. Through changes in hydrophobicity, a decrease in negative surface charge, and the occurrence of local rupture or pore formation in the cell membranes with the subsequent leakage of vital intracellular constituents, gallic and ferulic acids caused irreversible changes in membrane properties (charge, intra and extracellular permeability, and physicochemical properties) (86, 87).
Conclusions and future perspectives
Caesalpinia coriaria is shown as an alternative for the formulation of antimicrobial and anthelmintic drugs due to its content of bioactive compounds. C. coriaria is a promising source of bioactive molecules with various applications, including anthelmintic properties, antibacterial effects, and environmental benefits. From the future perspective, in vivo studies or concrete industrial applications should be done, in order to provide greater practical value to the extracts from several botanical parts of C. coriaria, which are rich in phenolic and tannin chemicals, have the potential to improve agriculture, medicine, and sustainable technologies.
Author contributions
MC-S: Validation, Writing – review & editing, Visualization, Writing – original draft. MS: Validation, Visualization, Writing – original draft, Writing – review & editing. ME: Validation, Visualization, Writing – original draft, Writing – review & editing. SS: Validation, Writing – review & editing. ML: Writing – original draft, Writing – review & editing. AS: Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Elghandour, MMM, Maggiolino, A, Vázquez-Mendoza, P, Alvarado-Ramírez, ER, Cedillo-Monroy, J, De Palo, P, et al. Moringa oleifera as a natural alternative for the control of gastrointestinal parasites in equines: a review. Plan Theory. (2023) 12:1921. doi: 10.3390/plants12091921
2. Martínez, AGL, Ramírez, ERA, Aguirre, DL, Pedroza, SIM, Chávez-Soto, DY, and Rivas-Jacobo, M. Effect of Syzygium aromaticum L. extract on ruminal fermentation and degradability in vitro. Ecosistemas Recurs Agropecu. (2025) 12:e4069. doi: 10.19136/era.a12n1.4069
3. Sánchez-Carranza, J, Alvarez, L, Marquina-Bahena, S, Salas-Vidal, E, Cuevas, V, Jiménez, E, et al. Phenolic compounds isolated from Caesalpinia coriaria induce S and G2/M phase cell cycle arrest differentially and trigger cell death by interfering with microtubule dynamics in Cancer cell lines. Molecules. (2017) 22:666. doi: 10.3390/molecules22040666
4. Mora-Santacruz, A, Román-Miranda, M, González-Cueva, G, and Barrientos-Ramírez, L. Chemical composition of cascalote Caesalpinia coriaria (Jacq.) Willd. And diversity of uses in the rural areas of dry tropics. Rev Investig Desarr. (2018) 4:24–18.
5. Veloz-García, RA, Marín-Martínez, R, Veloz-Rodríguez, R, Muñoz-Sánchez, CI, Guevara-Olvera, L, Miranda-López, R, et al. Antimutagenic and antioxidant activities of cascalote (Caesalpinia cacalaco) phenolics. J Sci Food Agric. (2004) 84:1632–8. doi: 10.1002/jsfa.1852
6. Hersch-Martínez, P. La flora medicinal en las comunidades indígenas. SIPIG- UNAM. (2009). Available online at: http://nacionmulticultural.unam.mx/edespig/diagnostico_y_perspectivas/RECUADROS/CAPITULO%208/1%20La%20flora%20medicinal.pdf.
7. Campos-Pérez, A, Camacho-Diaz, L, Adegbeye, M, Elghandour, M, Salem, A, Barbabosa-Pliego, A, et al. Valorization of Caesalpinia coriaria fruit waste to enhance the ruminal mitigation of greenhouse gases production. Waste Biomass Valor. (2021) 12:4991–5000. doi: 10.1007/s12649-021-01361-w
8. Manuel-Pablo, A. (2018). Efecto de los taninos del fruto del cascalote (Caesalpinia coriaria jacq. willd) sobre el comportamiento productivo, parámetros de fermentación ruminal, rendimiento y calidad de canal caprina. [Master’s thesis, Universidad Autónoma de Guerrero (México)].
9. Chavarría-Joya, L, Alonso-Díaz, M, Olmedo-Juárez, A, De Von Son- Fernex, E, and Mendoza-De-Gives, P. Assessing the individual and combined use of Caesalpinia coriaria (Plantae: Fabaceae) and Duddingtonia flagrans (Fungi: Orbiliaceae) as sustainable alternatives of control of sheep parasitic nematodes. Biocontrol Sci Tech. (2022) 32:1260–74. doi: 10.1080/09583157.2021.2022600
10. Soto Núñez, JC, and Sánchez, MS. Plantas Medicinales de la Cuenca del Rio Balsas 25. Universidad Nacional Autonoma de Mexico Distrito Federal, Mexico, pp. 197–198. (1995).
12. Yukes, JE, and Balick, MJ. Dominican medicinal plants: A guide for health care providers. New York: New York Botanical Garden (2010).
13. García-Hernández, C, Rojo-Rubio, R, Gives, PM-d, González-Cortazar, M, Zamilpa, A, Mondragón-Ancelmo, J, et al. In vitro and in vivo anthelmintic properties of Caesalpinia coriaria fruits against Haemonchus contortus. Exp Parasitol. (2022) 242:108401. doi: 10.1016/j.exppara.2022.108401
14. Rojas-Morales, D, Cubides-Cárdenas, J, Montenegro, AC, Martínez, CA, Ortíz-Cuadros, R, and Rios-de, ÁL. Anthelmintic effect of four extracts obtained from Caesalpinia coriaria foliage against the eggs and larvae of Haemonchus contortus. Rev Bras Parasitol Vet. (2021) 30:e002521. doi: 10.1590/s1984-29612021057
15. García-Hernández, C, Rojo-Rubio, R, Olmedo-Juárez, A, Zamilpa, A, Mendoza de Gives, P, Antonio-Romo, IA, et al. Galloyl derivatives from Caesalpinia coriaria exhibit in vitro ovicidal activity against cattle gastrointestinal parasitic nematodes. Exp Parasitol. (2019) 200:16–23. doi: 10.1016/j.exppara.2019.03.012
16. Ruiz, P, Elghandour, M, Ponce-Covarrubias, J, Burgos, B, Adegbeye, M, Mellado, M, et al. Exploring the potential of phenolic and antioxidant compounds identified and quantified of Caesalpinia coriaria fruits and their impacts on lambs’ performance and health. EuroBiotech J. (2024) 8:74–94. doi: 10.2478/ebtj-2024-0008
17. Pájaro González, Y, Méndez Cuadro, D, Fernández Daza, E, Franco Ospina, LA, Redondo Bermúdez, C, and Díaz Castillo, F. Inhibitory activity of the protein carbonylation and hepatoprotective effect of the ethanol-soluble extract of Caesalpinia coriaria Jacq. Orient Pharm Exp Med. (2016) 16:225–32. doi: 10.1007/s13596-016-0228-8
18. Mohana, D, and Raveesha, K. Anti-bacterial activity of Caesalpinia coriaria (Jacq.) Willd. Against plant pathogenic Xanthomonas pathovars: an eco-friendly approach. J Agric Technol. (2006) 2:317–27.
19. Anandhi, D, Srinivasan, P, Revathi, K, and Revathy, E. Antibacterial activity of Caesalpinia coriaria. Biosci Biotechnol Res Asia. (2016) 8:759–64.
20. Lopez-Pazos, SA, Pitre-Ruiz, L, Galv’an-Ayala, D, Avila Mendez, KJ, and Castro-Uriana, O. In vitro antimicrobial activity of Caesalpinia coriaria (Jacq.) Willd extracts on streptococcus pyogenes and Candida albicans. Vitae. (2021) 28:381. doi: 10.17533/udea.vitae.v28n2a345381
21. Pineda-Peña, E, Capistran-Amezcua, D, Reyes-Ramírez, A, Xolalpa-Molina, S, Chávez-Piña, A, Figueroa, M, et al. Gastroprotective effect methanol extract of Caesalpinia coriaria pods against indomethacin- and ethanol-induced gastric lesions in Wistar rats. J Ethnopharmacol. (2023) 305:116057. doi: 10.1016/j.jep.2022.116057
22. Jeeva, K, Thiyagarajan, M, Elangovan, V, Geetha, N, and Venkatachalam, P. Caesalpinia coriaria leaf extracts mediated biosynthesis of metallic silver nanoparticles and their antibacterial activity against clinically isolated pathogens. Ind Crop Prod. (2014) 52:714–20. doi: 10.1016/j.indcrop.2013.11.037
23. Li, X, Deng, Y, Zheng, Z, Huang, W, Chen, L, Tong, Q, et al. Corilagin, a promising medicinal herbal agent. Biomed Pharmacother. (2018) 99:43–50. doi: 10.1016/j.biopha.2018.01.030
24. Olmedo-Juárez, A, Briones-Robles, TI, Zaragoza-Bastida, A, Zamilpa, A, Ojeda-Ramírez, D, De Gives, M, et al. Antibacterial activity of compounds isolated from Caesalpinia coriaria (Jacq) Willd against important bacteria in public health. Microb Pathog. (2019):103660. doi: 10.1016/j.micpath.2019.103660
25. Anandhi, D, and Revathi, K. In vitro anticancer activity of Caesalpinia coriaria (Jacq) Willd (pods) on SiHa cell lines. Biochem Cell Arch. (2013) 13:155–60.
26. Jesús-Martínez, X, Olmedo-Juárez, A, Hernández, S, Zamilpa, A, Gives, P, López-Arellano, M, et al. Evaluation of the hydroalcoholic extract elaborated with Caesalpinia coriaria Jacq Willd tree fruits in the control of Haemonchus contortus Rudolphi. Agrofor Syst. (2019) 94:1315–21. doi: 10.1007/s10457-019-00398-0
27. Salem, MZM, Hassan, AGA, Amer, AME, Abdullah, MFG, Ahmed, SMA, Mahmoud, MM, et al. Bio-based chemical analysis of extracts from the biomass residues of Ceratonia siliqua and Ziziphus spina-christi with their bioactivities against molecularly identified fungi. Biomass Convers Biorefinery. (2025) 2025:651. doi: 10.1007/s13399-025-06651-0
28. Salem, MZM, EL-Shanhorey, NA, Mohamed, NH, and Mohamed, AA. Phenolic and flavonoid compounds from leaves and branches of Schotia brachypetala for the development of biofungicide for wood protection. BioResour. (2025) 20:1069–87. doi: 10.15376/biores.20.1.1069-1087
29. Alara, OR, Abdurahman, NH, and Ukaegbu, CI. Extraction of phenolic compounds: a review. Curr Res Food Sci. (2021) 4:200–14. doi: 10.1016/j.crfs.2021.03.011
30. Osorio-Tobón, JF. Recent advances and comparisons of conventional and alternative extraction techniques of phenolic compounds. J Food Sci Technol. (2020) 57:4299–315. doi: 10.1007/s13197-020-04433-2
31. Salem, A-FZ, Salem, MZM, Gonzalez-Ronquillo, M, Camacho, L, and Cipriano, M. Major chemical constituents of Leucaena leucocephala and Salix babylonica leaf extracts. J Trop Agric. (2011) 49:95–8.
32. Sánchez-Alonso, I, Fonseca-González, A, Olmedo-Juárez, A, Olivares-Pérez, J, González-Cortazar, M, Monteon-Ojeda, A, et al. Insecticidal activity of two organic extracts from Libidibia coriaria (Jacq.) Schltdl. Fruits against Spodoptera frugiperda J. E. Smith. Nat Prod Res. (2024):1–7. doi: 10.1080/14786419.2024.2368274
33. Pizzani, P, Matute, I, De Martino, G, Arias, A, Godoy, S, Pereira, L, et al. Composición Fitoquímica y Nutricional de Algunos Frutos de Árboles de Interés Forrajero de Los Llanos Centrales de Venezuela. Rev Cien Fac Vet. (2006) 47:105–11.
34. Wischer, G, Boguhn, J, Steingaß, H, Schollenberger, M, and Rodehutscord, M. Effects of different tannin-rich extracts and rapeseed tannin monomers on methane formation and microbial protein synthesis in vitro. Animal. (2013) 7:1796–805. doi: 10.1017/S1751731113001481
35. Sánchez, N, Mendoza, GD, Martínez, JA, Hernández, PA, Camacho Diaz, LM, Lee-Rangel, HA, et al. Effect of Caesalpinia coriaria fruits and soybean oil on finishing lamb performance and meat characteristics. Biomed Res Int. (2018) 2018:9486258. doi: 10.1155/2018/9486258
36. Palma, J. Control of parasites and protein protection through implementation of silvopastoral systems. Rev Colomb Zootec. (2019) 5:18–25.
37. Pérez-Cisneros, MA, Rengifo, R, Álvarez, A, and De Giner, GF. Proposal integral use of Divi–divi fruits (Caesalpinia coriaria) in the scope: oil well drilling, folder and social development. Banats J Biotechnol. (2019) 10:11–9. doi: 10.7904/2068-4738-X(19)-11
38. Kamatham, S, Kumar, N, and Gudipalli, P. Isolation and characterization of gallic acid and methyl gallate from the seed coats of Givotia rottleriformis Griff. And their anti-proliferative effect on human epidermoid carcinoma A431 cells. Toxicol Rep. (2015) 2:520–9. doi: 10.1016/j.toxrep.2015.03.001
39. Wyrepkowski, CC, Gomes da Costa, DLM, Sinhorin, AP, Vilegas, W, De Grandis, RA, Resende, FA, et al. Characterization and quantification of the compounds of the ethanolic extract from Caesalpinia ferrea stem bark and evaluation of their mutagenic activity. Molecules. (2014) 19:16039–57. doi: 10.3390/molecules191016039
40. Rangel-López, L, Rivero-Pérez, N, Valladares-Carranza, B, Olmedo-Juárez, A, Delgadillo-Ruiz, L, Vega-Sánchez, V, et al. Antibacterial potential of Caesalpinia coriaria (Jacq) Willd fruit against Aeromonas spp. of aquaculture importance. Animals. (2022) 12:511. doi: 10.3390/ani12040511
41. Anandhi, D, Srinivasan, PT, Kumar, GP, and Jagatheesh, S. Influence of flavonoids and glycosides from Caesalpinia coriaria (Jacq) wild as bactericidal compound. Int J Curr Microbiol Appl Sci. (2014) 3:1043–51.
42. Mohana, D, Satish, S, and Anandarao, R. Antibacterial evaluation of some plant extracts against some human pathogenic bacteria. Adv Biol Res. (2008) 2:49–55.
43. Minier, CC. Pruebas de sensibilidad y resistencia bacteriana frente a diferentes concentraciones de extracto de Caesalpinia coriaria (Guatapaná). Cienc Soc. (2007) 32:8–20. doi: 10.22206/cys.2007.v32i1.pp8-20
44. Delgado, A, Núñez, O, Aguilera-Valle, L, Palacio, D, Salas-Romero, J, Bebert, G, et al. Acción ovicida in vitro del extracto hidro-alcohólico crudo de la semilla de Pouteria sapota (mamey colorado) contra huevos de Haemonchus contortus. Primer reporte. Rev Prod Anim. (2016) 28:51–4.
45. Zapata Salas, R, Velásquez Vélez, R, Herrera Ospina, LV, Ríos Osorio, L, and Polanco Echeverry, DN. Prevalence of gastrointestinal nematodes in sheep and goat production systems under confinement, semi-confinement and grazing in municipalities of Antioquia, Colombia. Rev Investig Vet Peru. (2016) 27:344–54. doi: 10.15381/rivep.v27i2.11647
46. Canul-Ku, HL, Rodríguez-Vivas, RI, Torres-Acosta, JFJ, Aguilar-Caballero, AJ, Pérez-Cogollo, LC, and Ojeda-Chi, MM. Prevalence of cattle herds with ivermectin resistant nematodes in the hot sub-humid tropics of Mexico. Vet Parasitol. (2012) 183:292–8. doi: 10.1016/j.vetpar.2011.07.029
47. Olivares-Perez, J, Gutierrez-Segura, I, Rojas-Hernandez, S, Valencia-Almazan, MT, Mireles-Martinez, EJ, and Cordova-Izquierdo, A. Seasonal prevalence of Strongyle in creole goats of the Tierra Caliente region, state of Guerrero, Mexico. Res Opin Anim Vet Sci. (2012) 2:216–20.
48. Ehsan, M, Hu, R-S, Liang, Q-L, Hou, J-L, Song, X, Yan, R, et al. Advances in the development of anti-Haemonchus contortus vaccines: challenges, opportunities, and perspectives. Vaccine. (2020) 8:555. doi: 10.3390/vaccines8030555
49. Baltrušis, P, Komáromyová, M, Várady, M, von Samson-Himmelstjerna, G, and Höglund, J. Assessment of the F200Y mutation frequency in the β tubulin gene of Haemonchus contortus following the exposure to a discriminating concentration of thiabendazole in the egg hatch test. Exp Parasitol. (2020) 217:107957. doi: 10.1016/j.exppara.2020.107957
50. Muñiz-Lagunes, A, González-Garduño, R, López-Arellano, ME, Ramírez-Valverde, R, Ruíz-Flores, A, García-Muñiz, G, et al. Anthelmintic resistance in gastrointestinal nematodes from grazing beef cattle in Campeche state, Mexico. Trop Anim Health Prod. (2015) 47:1049–54. doi: 10.1007/s11250-015-0826-3
51. Laca Megyesi, Š, Königová, A, Babják, M, Molnár, L, Rajský, M, Szestáková, E, et al. Wild ruminants as a potential risk factor for transmission of drug resistance in the abomasal nematode Haemonchus contortus. Eur J Wildl Res. (2019) 66:9. doi: 10.1007/s10344-019-1351-x
52. León-Castro, Y, Olivares-Pérez, J, Rojas-Hernández, S, Villa-Mancera, A, Valencia-Almazán, MT, Castro, EH, et al. Chemical composition of three tree fodders and effect in control Haemonchus contortus and change of body weight in kids. Ecosist Recur Agropec. (2015) 2:193–201.
53. Manuel-Pablo, A, Elghandour, MMY, Olivares-Pérez, J, Rojas-Hernández, S, Cipriano-Salazar, M, Cruz-Lagunas, B, et al. Productive performance, rumen fermentation and carcass yield of goats supplemented with cascalote fruit (Caesalpinia coriaria J. Wild.). Agrofor Syst. (2020) 94:1381–91. doi: 10.1007/s10457-018-0312-9
54. Camacho-Díaz, L, De Jesús-Ramirez, C, Cipriano-Salazar, M, and Cruz-Lagunas, B. Taninos condensados del cascalote (Caesalpinia coriaria Jacq) y su efecto sobre el contenido de ácido linoleico conjugado (CLA) en leche de vacas doble propósito. Foro Estud Guerrero. (2015) 1:372–6.
55. Olmedo-Juárez, A, Jesús-Martínez, D, Rojas-Hernández, S, Villa-Mancera, A, Romero-Rosales, T, and Olivares-Pérez, J. Eclosion inhibition of Haemonchus contortus eggs with two extracts of Caesalpinia coriaria fruits. Rev Bio Cienc. (2022) 9:e1121. doi: 10.15741/revbio.09.e1121
56. Rojo-Rubio, R, González-Cortazar, M, Olmedo-Juárez, A, Zamilpa, A, Arece-García, J, Mendoza-Martínez, GD, et al. Caesalpinia coriaria fruits and leaves extracts possess in vitro ovicidal activity against Haemonchus contortus and Haemonchus placei. Vet Mex OA. (2019) 6:1–13. doi: 10.22201/fmvz.24486760e.2019.4.601
57. Al Mamari, H. Phenolic compounds: classification, chemistry, and updated techniques of analysis and synthesis In: FA Badria, editor. Phenolic compounds - chemistry, synthesis, diversity, non-conventional industrial, pharmaceutical and therapeutic applications. Rijeka: IntechOpen (2021). doi: 10.5772/intechopen.98958
58. Qu, S-Y, Liu, Y-H, Liu, J-T, Li, P-F, Liu, T-Q, Wang, G-X, et al. Catechol compounds as dual-targeting agents for fish protection against Ichthyophthirius multifiliis infections. Fish Shellfish Immunol. (2024) 151:109717. doi: 10.1016/j.fsi.2024.109717
59. Cortes-Morales, JA, Salinas-Sánchez, DO, de Jesús Perea-Flores, M, González-Cortazar, M, Tapia-Maruri, D, López-Arellano, ME, et al. In vitro anthelmintic activity and colocalization analysis of hydroxycinnamic acids obtained from Chamaecrista nictitans against two Haemonchus contortus isolates. Vet Parasitol. (2024) 331:110282. doi: 10.1016/j.vetpar.2024.110282
60. Pathan, AS, Jain, PG, Mahajan, AB, Kumawat, VS, Ahire, ED, Surana, KR, et al. Beneficial effects of water-soluble vitamins in nutrition and health promotion In: ED Ahire, RK Keservani, KR Surana, S Singh, and RK Kesharwani, editors. Vitamins as Nutraceuticals: Recent Advances and Applications. New York: John Wiley and Sons (2023). 235–51.
61. Boo, YC. Ascorbic acid (vitamin C) as a cosmeceutical to increase dermal collagen for skin antiaging purposes: emerging combination therapies. Antioxidants. (2022) 11:1663. doi: 10.3390/antiox11091663
62. Dias, MC, Pinto, DC, and Silva, AM. Plant flavonoids: chemical characteristics and biological activity. Molecules. (2021) 26:5377. doi: 10.3390/molecules26175377
63. Argüello-García, R, and Quiñonez-Bastidas, GN. Catechins as emerging and promising antiparasitic agents. Biomed J Sci Technol Res. (2020) 17:23065–71. doi: 10.26717/BJSTR.2020.30.004895
64. Mondal, C, Mandal, S, Saha, S, Ray, MS, and Lyndem, LM. Gallic acid and Catechin induce morphological alterations on the zoonotic parasite Hymenolepis diminuta. Parasitol Res. (2023) 122:2287–99. doi: 10.1007/s00436-023-07929-w
65. Chagas, MSS, Behrens, MD, Moragas-Tellis, CJ, Penedo, GXM, Silva, AR, and Gonçalves-de-Albuquerque, CF. Flavonols and flavones as potential anti-inflammatory, antioxidant, and antibacterial compounds. Oxidative Med Cell Longev. (2022) 2022:9966750. doi: 10.1155/2022/9966750
66. Jan, R, Khan, M, Asaf, S, Lubna, L, Asif, S, and Kim, K-M. Bioactivity and therapeutic potential of kaempferol and quercetin: new insights for plant and human health. Plants Theory. (2022) 11:2623. doi: 10.3390/plants11192623
67. Yi, L, Ma, S, and Ren, D. Phytochemistry and bioactivity of Citrus flavonoids: a focus on antioxidant, anti-inflammatory, anticancer and cardiovascular protection activities. Phytochem Rev. (2017) 16:479–511. doi: 10.1007/s11101-017-9497-1
68. Imperatrice, M, Cuijpers, I, Troost, FJ, and Sthijns, MM. Hesperidin functions as an ergogenic aid by increasing endothelial function and decreasing exercise-induced oxidative stress and inflammation, thereby contributing to improved exercise performance. Nutrients. (2022) 14:2955. doi: 10.3390/nu14142955
69. Vattem, DA, and Shetty, K. Biological functionality of ellagic acid: a review. J Food Biochem. (2005) 29:234–66. doi: 10.1111/j.1745-4514.2005.00031.x
70. Nawwar, MAM, Marzouk, MS, Nigge, W, and Linscheid, M. High-performance liquid chromatographic/electrospray ionization mass spectrometric screening for polyphenolic compounds of Epilobium hirsutum—the structure of the unique ellagitannin epilobamide-a. J Mass Spectrom. (1997) 32:645–54. doi: 10.1002/(SICI)1096-9888(199706)32:6<645::AID-JMS518>3.0.CO;2-P
71. Evtyugin, DD, Magina, S, and Evtuguin, DV. Recent advances in the production and applications of ellagic acid and its derivatives. A review. Molecules. (2020) 25:2745. doi: 10.3390/molecules25122745
72. Metsämuuronen, S, and Sirén, H. Bioactive phenolic compounds, metabolism and properties: a review on valuable chemical compounds in scots pine and Norway spruce. Phytochem Rev. (2019) 18:623–64. doi: 10.1007/s11101-019-09630-2
73. Moges, GW, Manahelohe, GM, and Asegie, MA. Phenolic, flavonoid contents, antioxidant, and antibacterial activity of selected Eucalyptus species. Biol Med Nat Prod Chem. (2024) 13:147–57. doi: 10.14421/biomedich.2024.131.147-157
74. Lee, DY, Kim, HW, Yang, H, and Sung, SH. Hydrolyzable tannins from the fruits of Terminalia chebula Retz and their α-glucosidase inhibitory activities. Phytochemistry. (2017) 137:109–16. doi: 10.1016/j.phytochem.2017.02.006
75. El Omari, N, Bakrim, S, Khalid, A, Abdalla, AN, Iesa, MAM, El Kadri, K, et al. Unveiling the molecular mechanisms: dietary phytosterols as guardians against cardiovascular diseases. Nat Prod Bioprospect. (2024) 14:27. doi: 10.1007/s13659-024-00451-1
76. He, H-F. Recognition of gallotannins and the physiological activities: from chemical view. Front Nutr. (2022) 9:888892. doi: 10.3389/fnut.2022.888892
77. Fraga-Corral, M, Otero, P, Echave, J, Garcia-Oliveira, P, Carpena, M, Jarboui, A, et al. By-products of Agri-food industry as tannin-rich sources: a review of tannins’ biological activities and their potential for valorization. Food Secur. (2021) 10:137. doi: 10.3390/foods10010137
78. Toda, M, Kawabata, J, and Kasai, T. Inhibitory effects of Ellagi- and Gallotannins on rat intestinal α-glucosidase complexes. Biosci Biotechnol Biochem. (2001) 65:542–7. doi: 10.1271/bbb.65.542
79. Farhadi, F, Khameneh, B, Iranshahi, M, and Iranshahy, M. Antibacterial activity of flavonoids and their structure–activity relationship: an update review. Phytother Res. (2019) 33:13–40. doi: 10.1002/ptr.6208
80. Yixi, X, Weijie, Y, Fen, T, Xiaoqing, C, and Licheng, R. Antibacterial activities of flavonoids: structure-activity relationship and mechanism. Curr Med Chem. (2015) 22:132–49. doi: 10.2174/0929867321666140916113443
81. Lojková, L, Pluháčková, H, Benešová, K, Kudláčková, B, and Cerkal, R. The highest yield, or greener solvents? Latest trends in quercetin extraction methods. TrAC Trends Anal Chem. (2023) 167:117229. doi: 10.1016/j.trac.2023.117229
82. Ali Redha, A. Review on extraction of phenolic compounds from natural sources using green deep eutectic solvents. J Agric Food Chem. (2021) 69:878–912. doi: 10.1021/acs.jafc.0c06641
83. Sang, H, Jin, H, Song, P, Xu, W, and Wang, F. Gallic acid exerts antibiofilm activity by inhibiting methicillin-resistant Staphylococcus aureus adhesion. Sci Rep. (2024) 14:17220. doi: 10.1038/s41598-024-68279-w
84. Tian, Q, Wei, S, Su, H, Zheng, S, Xu, S, Liu, M, et al. Bactericidal activity of gallic acid against multi-drug resistance Escherichia coli. Microb Pathog. (2022) 173:105824. doi: 10.1016/j.micpath.2022.105824
85. Keyvani-Ghamsari, S, Rahimi, M, and Khorsandi, K. An update on the potential mechanism of gallic acid as an antibacterial and anticancer agent. Food Sci Nutr. (2023) 11:5856–72. doi: 10.1002/fsn3.3615
86. Borges, A, Ferreira, C, Saavedra, MJ, and Simões, M. Antibacterial activity and mode of action of Ferulic and Gallic acids against pathogenic Bacteria. Microb Drug Resist. (2013) 19:256–65. doi: 10.1089/mdr.2012.0244
87. Magadla, A, Openda, YI, Mpeta, LS, and Nyokong, T. Evaluation of the antibacterial activity of gallic acid anchored phthalocyanine-doped silica nanoparticles towards Escherichia coli and Staphylococcus aureus biofilms and planktonic cells. Photodiagn Photodyn Ther. (2023) 42:103520. doi: 10.1016/j.pdpdt.2023.103520
88. Román Miranda, ML, Carvajal Hernández, S, Mora Santacruz, A, and Ochoa Ruiz, H. Forest species with diversified uses in a deciduous tropical forest in the indigenous community of Tomatlan, Jalisco, Mexico. Cienc Investig For. (2007) 11:183–92. doi: 10.52904/0718-4646.2007.85
Keywords: Caesalpinia coriaria , bioactive compounds, antimicrobial activity, antiparasitic activity, plant extracts, polyphenols
Citation: Cipriano-Salazar M, Salem MZM, Elghandour MMMY, Selim S, Lackner M and Salem AZM (2025) Phytochemical profile and biological activities of Caesalpinia coriaria extract: a review. Front. Vet. Sci. 12:1629447. doi: 10.3389/fvets.2025.1629447
Edited by:
Edwin Rafael Alvarado Ramírez, Autonomous University of Tamaulipas, MexicoReviewed by:
Agustín Olmedo-Juárez, Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP), MexicoYissel Sacnicte Valdés García, Instituto de Investigaciones en Ciencias Veterinarias, Mexicali B.C., Mexico
Nadia Alejandra Sánchez Guerra, Universidad Autónoma de Tamaulipas, Mexico
Fernando Lucio Ruiz, Instituto Nacional de Investigación Forestal, Agropecuaria (INIFAP), Mexico
Copyright © 2025 Cipriano-Salazar, Salem, Elghandour, Selim, Lackner and Salem. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maximilian Lackner, bWF4aW1pbGlhbi5sYWNrbmVyQHRlY2huaWt1bS13aWVuLmF0; Mona M. M. Y. Elghandour, bW1vaGFtZWRlQHVhZW1leC5teA==
 Moises Cipriano-Salazar1
Moises Cipriano-Salazar1 Mohamed Z. M. Salem
Mohamed Z. M. Salem Maximilian Lackner
Maximilian Lackner Abdelfattah Z. M. Salem
Abdelfattah Z. M. Salem