- 1Department of Veterinary Medicine, College of Animal Science and Technology, Jilin Agricultural University, Changchun, China
- 2Institute of Animal Science, Ningxia Academy of Agriculture and Forestry, Yinchuan, China
- 3Institute of Agricultural Mechanization, Xinjiang Academy of Agricultural Sciences, Wulumuqi, China
Introduction: Fine particulate matter (PM2.5) is an important factor in the induction of a variety of respiratory diseases and associated cellular damage. The composition of PM2.5 in the animal farm environments is complex, which poses a significant threat to the respiratory health of both workers and livestock, but the causative mechanisms are unclear.
Methods: In order to investigate targeted treatment options, this study focused on the role of microbial components in cowshed PM2.5-induced respiratory damage. Utilizing the common pathogenic bacteria (Pasteurella multocida) in cowshed PM2.5 as a perspective, the intrinsic connection and interaction mechanism between PM2.5 particles and bacterial components were explored through in vivo and in vitro experiments. Bacterial components can interact with PM2.5 and are important factors in the respiratory toxicity of PM2.5 in farm animal environments by scanning electron microscopy (SEM), Fourier infrared spectroscopy (FTIR) and Zeta potential measurements.
Results: We demonstrate that Bacteria adhered to PM2.5 particles and modified the original surface functional groups characteristics, significantly enhanced toxic effects of PM2.5 on cells (including oxidative stress levels, release of inflammatory factors, etc.). Furthermore, PM2.5 particles significantly enhanced bacterial intracellular invasion, initiated the guanylate-binding protein 2 (GBP2)-mediated intracellular bacterial defense mechanism, further triggered the non-canonical NLRP3 pathway, and ultimately induced a cascade of inflammatory responses and pyroptosis. To explore therapeutic strategies, siRNA silencing of GBP2 and inhibition of NLRP3 were done; GBP2 silencing initially delayed cytotoxicity, but eventually increased the inflammatory response. However, inhibition of NLRP3 expression maintained cell viability and delayed pyroptosis, with potential as an effective solution for treatment of PM2.5-induced lung injury in farm-animal environments.
Conclusion: In conclusion, the results of this study demonstrated the interaction between particulate matter and bacteria during cowshed PM2.5-induced respiratory injury and clarified the signaling mechanisms among intracellular bacteria, GBP2, NLRP3, and pyroptosis. These findings provide a theoretical basis for developing therapeutic strategies against PM2.5-related respiratory diseases in farm-animal environments.
1 Introduction
Fine particulate matter (PM2.5) is a global pollution in the air that affects human health (1). There is a clear association between PM2.5 and lung disease. According to the relevant statistics, the 3.7 million people in the same area, for every 10 μg/m3 increase in ambient PM2.5 concentration, physician visits for suspected pneumonia and number of respiratory infections increased by 6.32 and 4.72%, respectively (2). In addition to pneumonia, direct and indirect exposure to PM2.5 also a contributes to chronic obstructive pulmonary disease (COPD) (3), asthma (4), pulmonary fibrosis (5) and even lung cancer (6). Therefore, elucidating mechanisms of PM2.5-induced damage to the respiratory system and digging up effective strategies for treating PM2.5-associated respiratory diseases based on this mechanism are crucial.
Composition, microscopic characteristics, and biological toxicity of PM2.5 vary among environments. Research has indicated that industrial activities, including vehicle exhaust and factory emissions, contribute to an increase in the presence of heavy metals and organic compounds in atmospheric PM2.5. These components have been identified as the primary contributors to respiratory diseases caused by atmospheric PM2.5 (7). However, the composition of PM2.5 that induces respiratory diseases is also different in some specific environments. In the farm-animal environments, elevated stocking densities, poor ventilation, the equipment operation, and the frequent animal activity result in PM2.5 concentrations that are often maintained at high levels (6), whereas animal feed, feces, feathers, and bedding also affect PM2.5, resulting in its higher microbial abundance (8). In addition, due to the smaller aerodynamic diameter of PM2.5, it may transport disease-causing microorganisms to the end of the bronchi, thereby facilitating the transmission and induction of animal diseases. This is a major threat to staff health and animal health and production (9). At present, most studies focus on microbiological composition of PM2.5 in farm-animal environments. Some studies have found that poultry house PM2.5 contains pathogenic genera such as Staphylococcus and Corynebacterium, and harmful fungi such as Aspergillus and Bombyx mori (10). Bacterial aerosols in hog house also identified pathogenic bacterial genera such as Streptococcus, Fusobacterium, and Pseudomonas (11). In addition, Bacteria such as Staphylococcus and Streptococcus were also frequently detected in cowshed PM2.5 (12). The present studies have focused on the microbial composition of farmed environmental PM2.5. However, the biotoxicity profile at higher PM2.5 microbial levels in inducing respiratory damage remains to be elucidated. Moreover, in the molecular mechanisms of respiratory damage caused by PM2.5 in animal farm environments, the intrinsic link between microbial components and PM2.5 particles are largely unknown.
In our previous study of the microbial diversity of ambient cowshed PM2.5 samples, Pasteurella multocida (P. multocida) was detected in all samples (13). P. multocida is a conditionally pathogenic bacterium, airborne transmission is an important means of its transmission, and it is a common causative agent of causing upper respiratory tract diseases (14). P. multocida has a wide range of hosts and can cause respiratory infections in a variety of domestic and wild animals, and it is the causative agent of bovine haemorrhagic septicaemia (15), porcine pneumonic disease (16), avian cholera (17), and rabbit pasteurellosis (18), as well as bacteraemia, meningitis, etc., in humans (19, 20). However, there are doubts regarding the role of P. multocida in biological toxicity of PM2.5, as well as interactions between P. multocida and PM2.5 and the specific mechanism of respiratory injury under the synergy between P. multocida and PM2.5.
Organisms have complex self-defense mechanisms when faced with microbial invasion and infection. Among them, guanylate-binding proteins (GBPs), a conserved family of interferon-induced GTPases, which play a pivotal role in the immune system’s defense against bacterial, viral and protozoan pathogens that infect the host (21). In our previous transcriptomics study by the research group, GBP2 was aberrantly expressed in alveolar macrophages by cowshed PM2.5 stimulation. GBP2, a member of the GBPs family, participate in host defense against intracellular pathogens (22). Studies have shown that GBP2 lyses vesicular membranes harboring pathogens, thereby facilitating host cell recognition and subsequent immune responses, exhibiting potent antimicrobial activity in both in vivo and in vitro models (23). Recent studies have suggested that GBP2 is closely linked to the activation of the NLRP3 inflammasome (24), but the mechanism is unclear. NLRP3 inflammasome, a member of the NOD-like receptor family, is widely distributed among various types of immune cells (25). As a key component of the innate immune system, NLRP3 can promote the generation of active Caspase-1, which drives maturation and secretion of inflammatory cytokines IL-1β and IL-18 (26). NLRP3 involves regulation and synergy of complex molecular signaling in cellular metabolic state, oxidative stress, pyroptosis and autophagy (27, 28). However, it is unknown whether GBP2 and NLRP3 have roles in cowshed PM2.5-induced respiratory damage. In addition, mechanisms of activation of GBP2 and NLRP3 by cowshed PM2.5 and mechanisms of the transmission mechanism among the three are also unknown.
Given the many unknowns about the respiratory damage caused by cowshed PM2.5 as mentioned above, we hypothesized that microbial components are the primary reason of the observed cytotoxicity. Therefore, this study established an in vivo model of respiratory exposure to cowshed PM2.5 to investigate the specific mechanisms underlying the respiratory injury inflicted by cowshed PM2.5 on model animals (rats). In vitro, a model of synergistic infection of alveolar macrophages by P. multocida and PM2.5 was established, in order to investigate the intrinsic connections and interaction mechanisms between PM2.5 and microbial components. Furthermore, studies on the intrinsic association of GBP2 and NLPR3 were conducted, to determine mechanisms of respiratory damage and cellular defense in the presence of cowshed PM2.5. These studies will inform treatment options for PM2.5-induced respiratory system diseases in farm-animal environments.
2 Materials and methods
2.1 PM2.5 sample preparation
Based on previous studies by our research group (29), PM2.5 was collected using a multi-level flow particulate sampler on cattle farms in Changchun, Jilin Province. These PM2.5 samples were analyzed for chemical and microbiological constituents (13). The PM2.5 standard (ERM-CZ110) was purchased from the JRC Science Hub and its main components are shown (Supplementary Table S1).
2.2 Animal model establishment and ethical statements
Experimental animals were selected 6-week-old specific pathogen free (SPF) grade Sprague Dawley (SD) rats (180–220 g) from Liaoning Changsheng Biotechnology Co, with ad libitum access to water and rat chow. After 1 week to acclimatize, exposure was done in an exposure box, as described in the group’s previous studies (30, 31). During the experiment, rats were allocated into a Control group (exposed to clean air) and a PM2.5 exposure group (exposure to 4 times the cowshed environment PM2.5 concentration), 6 rats per group. The rats of the PM2.5 exposure group were exposed to PM2.5 for 6 h a day for 30 days, to be able to simulate the daily exposure patterns of animals in the cowshed. At the end of the experiment, to be able to simulate the daily exposure patterns of animals in the cowshed. Rats were anaesthetized by intraperitoneal injection of 3% sodium pentobarbital (40 mg/kg).
An ethical review of animal welfare was conducted by the Animal Experimentation Ethics Committee at Haihua Biotechnology Group Co., Ltd., who adhered to the ARRIVE guidelines and the Chinese National Standard Laboratory Animal Guidelines. The protocol was approved (Animal Experimentation Ethics Number: AUP-20231117-001).
2.3 Histopathological examination
Portions of lung were fixed in 4% paraformaldehyde, embedded in paraffin, cut into 5 μm sections, stained with hematoxylin and eosin (H&E), and observed with made a microscope (Phenix, China). Three samples were randomly selected from each group for histopathological assessment.
2.4 Lung wet/dry ratio
Immediately after death, a portion of the lung tissue of each mouse was excised and wet and dry, weights (after drying in an oven at 65°C for 24 h) were recorded and used to detect pulmonary edema (based on the W/D ratio of the lungs).
2.5 Cell model establishment and grouping
Rat alveolar macrophage cells (NR8383) were purchased from Shanghai Cell Bank and cultured in DMEM medium containing 10% fetal bovine serum and 1% penicillin–streptomycin (C0222). Cells were grown at 37°C in a 5% CO2 environment and seeded into 6-well plates (106 cells/well). Cells were cultured to 80% density and then required substances were added.
To determine effects of cowshed PM2.5 on NR8383 cells, we chose the concentration of cowshed PM2.5 based on the relevant literature (32, 33). These cells were divided into four groups (Control and low-, medium- and high-concentrations of PM2.5 [0, 60, 120, and 240 μg/mL, respectively]), and stimulated for 12 h. To avoid other factors influencing the results, cells were infected with a mixture of P. multocida and PM2.5 standard to mimic the effects of microorganisms during cowshed PM2.5 infection. For this, cells were divided into Control group, PM2.5 standard group (120 μg/mL), P. multocida group (1 × 107 CFU/mL) and Mixture group (PM2.5 standard + P. multocida, for intuitive comparison, half doses were used for both the PM2.5 standard and P. multocida), and stimulated for 12 h. In addition. In addition, referring to the relevant literature (34), an NLRP3 inhibitor (MCC950, HY-12815A, MCE, China) was used to validate the role of NLRP3 in cowshed PM2.5-induced cellular damage. Cells were divided into the Control group, MCC950 group, PM2.5 group, and PM2.5 + MCC950 group, and stimulated for 12 h.
2.6 Bacterial strains and culture conditions
Pasteurella multocida strains were isolated from the cowshed PM2.5 samples. The strain was cultured and identified by streaking onto BHI agar plates and incubating at 37°C. Then, single colonies were picked and inoculated into the BHI liquid medium and incubated at 37°C with shaking. In the experiments, sterile and pyrogen-free BHI liquid medium was used to dilute the bacterial solution.
2.7 CCK-8
Cell viability was measured using the CCK-8 kit (C0037). For this, NR8383 cells were added to 96-well plates at a density of 5 × 103 cells per well. After 12 h of stimulation under various conditions, 10 μL of CCK-8 was added to each well and incubated for 1 h. The OD values were read at 450 nm on an enzyme labeler. Cell viability was determined in accordance with the manufacturer’s guidelines.
2.8 Oxidative stress indices
Kits were purchased from Nanjing Jiancheng Bioengineering Institute. Reactive oxygen species (ROS), malonic dialdehyde (MDA) and superoxide dismutase (SOD) were determined according to the manufacturer’s instructions.
2.9 PM2.5 surface characterization
To investigate effects of P. multocida on surface properties of PM2.5. Samples were divided into PM2.5 standard group, P. multocida group and PM2.5 standard + P. multocida group. Samples were fixed with 2% glutaraldehyde, dehydrated with anhydrous ethanol, and subsequently transferred to Shanghai Jiao Tong University for scanning electron microscopy acquisition, Zeta potential determination, and Fourier infrared spectroscopy.
2.10 Intracellular bacterial levels
Cells were seeded in 12-well plates at a density of 3 × 105 cells/well using antibiotic-free medium and assigned to four groups: Control, PM2.5 standard, P. multocida, and PM2.5 standard + P. multocida. Following 8 h of incubation at 37°C in 5% CO₂, the medium was replaced with DMEM containing 50 μg/mL gentamicin to eliminate extracellular bacteria. After 1 h, cells were lysed with 300 μL of 1% Triton X-100. Lysates were serially diluted 10-fold and plated on BHI agar for CFU enumeration.
2.11 siRNA transfection
GBP2 siRNA was transfected into NR8383 cells cultured in 6-well plates for 24 h using Lipofectamine 3000 (L3000015) following the manufacturer’s protocol. Three individual siRNAs targeting GBP2 were used. After 12 h, the transfection medium was replaced with complete medium. Western blotting was performed 48 h post-transfection to validate GBP2 silencing efficiency. For experimental treatments, cells were divided into five groups: Control, NC-siRNA, GBP2-siRNA, PM2.5, and PM2.5 + GBP2-siRNA.
2.12 ELISA
The levels of the inflammatory cytokine IL-1β were measured using ELISA kit (ml037361) according to the manufacturer’s instructions.
2.13 LDH
The levels of the inflammatory cytokine LDH release were measured LDH cytotoxicity assay kit (C0016) according to the manufacturer’s instructions.
2.14 Immunofluorescence
Immunofluorescence staining was performed to assess the expression of GSDMD-N in cells after receiving various stimuli. Cells were fixed with 4% paraformaldehyde for 20 min and permeabilized with 0.3% Triton-X 100 for 10 min. After blocking with BSA for 1 h at room temperature, cells were incubated with GSDMD-N antibody (1:100, ab215203) overnight at 4°C. CY3-coupled secondary antibody (1:100, SA00009-2) was incubated for 1 h at room temperature. Following thorough washing, DAPI was used for nuclear staining, and images were captured under a fluorescence microscope (Olympus).
2.15 RT-qPCR
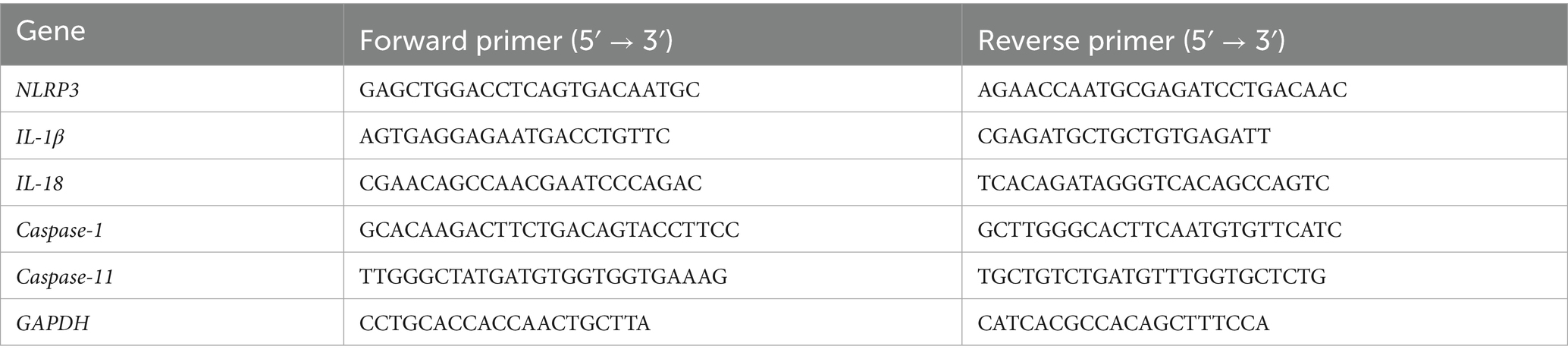
Total RNA was extracted from the cells using the Total RNA Extraction Kit (B511311) and reverse transcribed to cDNA using the PrimeScript™ RT Kit (RR047A). RT-qPCR was performed according to the instructions of the RT-qPCR kit. qPCR reactions were performed using TB Green Mix (RR820A). The target gene expression’s CT value was then contrasted with that of the Control group. GAPDH was employed as a housekeeping control. The 2−ΔΔCT method was used to conduct a relative quantitative analysis. The following primer sequences were used (Table 1).
2.16 Western blotting
RIPA lysate containing 1% PMSF (C500005) was used to lyse cultured cells to extract total protein. Sample protein concentrations were determined using a BCA protein quantification kit (P0010S), denatured and subjected to SDS-PAGE gel electrophoresis. Proteins were transferred to PVDF membranes (GVWP04700) and blocked with 5% skimmed milk powder for 2 h. Then, the membrane was incubated overnight with the corresponding primary antibody, and secondary antibodies were incubated for 2 h at room temperature. After sufficient washing using TBST solution, an ECL luminescence assay (PW30601S) was performed to assess protein expression. Protein bands were detected using an Amersham Imager 680. Image analysis was performed using ImageJ (Version: 1.54f). The details of the antibodies used are provided in Supplementary Table S2.
2.17 Statistical analysis
Each experiment was repeated at least three times and values expressed as the mean ± SD of the measurements, with plots using GraphPad Prism version 8.0 software (GraphPad Software, San Diego, CA, USA). Statistical comparisons were made using unpaired Student’s t-tests. A one-way analysis of variance (ANOVA) was used to compare data between multiple groups, and the Tukey–Kramer post-test was used to locate differences. Statistical significance was defined as p < 0.05.
3 Results
3.1 Cowshed PM2.5 induced rat lung and in vitro alveolar macrophage damage
To assess the effects of cowshed PM2.5 on the respiratory system, changes of in vivo rat pulmonary assessments and in vitro alveolar macrophages were independently evaluated after exposure to cowshed PM2.5. In vivo, in comparison with the Control group, lung tissue obvious pathological damage, including alveolar hemorrhage, alveolar wall thickening, inflammatory cell infiltration, and alveolar interstitial exudates (Figure 1A). Moreover, comparison to the Control group, exposure of rats to cowshed PM2.5 significantly increased the lung W/D ratio and concentrations IL-1β and IL-18 (Figures 1B–E).
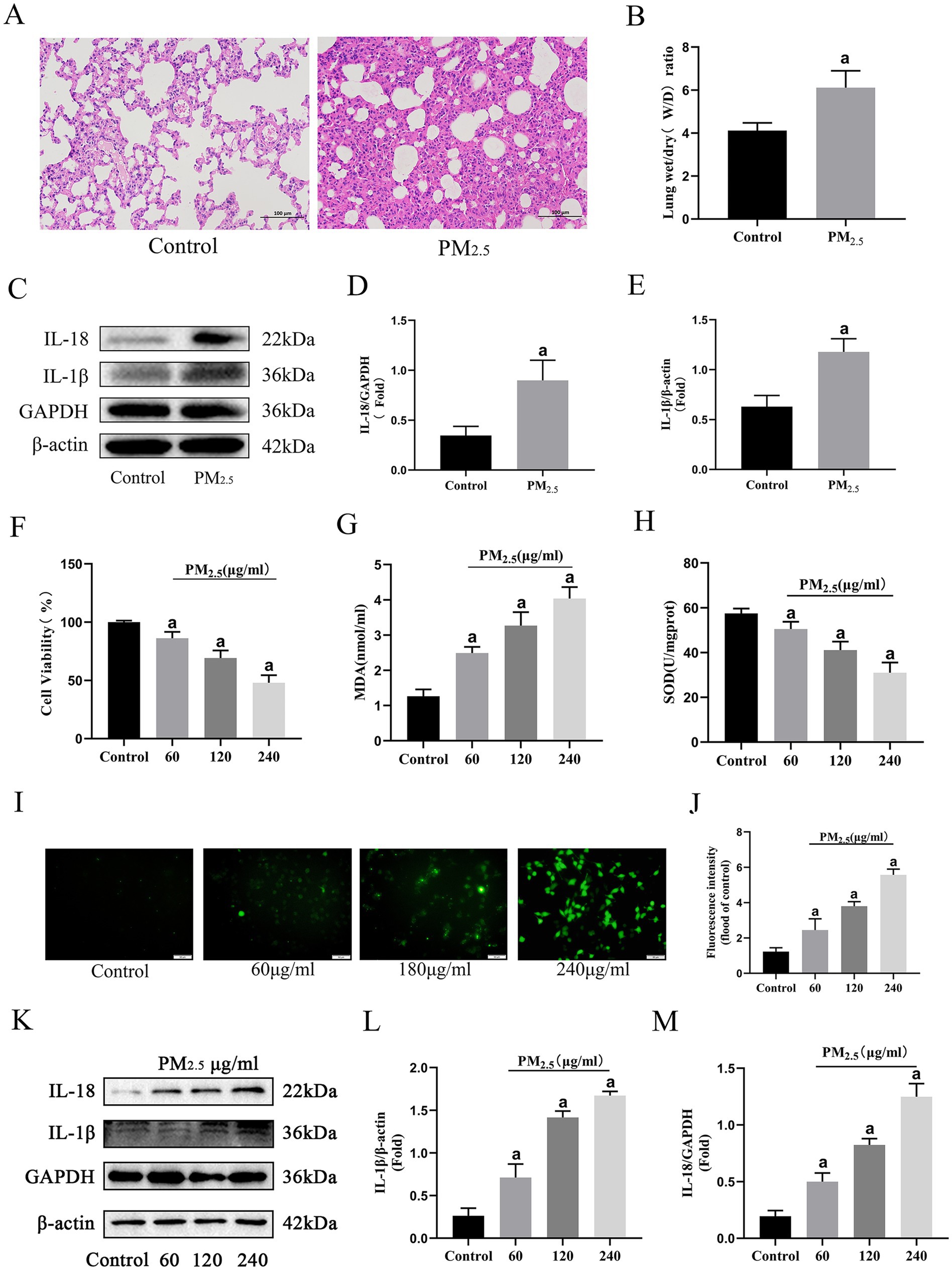
Figure 1. Cowshed PM2.5 induced rat lung and in vitro alveolar macrophage damage. (A) H&E stained sections of rat lungs after cowshed PM2.5 exposure treatment. Scale bar = 100 μm. (B) W/D ratio in rat lung. (C–E) Expression levels of inflammatory factors in rat lungs. (F) Cell viability levels of NR8383 cells upon exposure to cowshed PM2.5. (G–J) Oxidative stress in NR8383 cells during exposure to cowshed PM2.5. (K–M) Inflammatory factor levels in NR8383 cells during exposure to cowshed PM2.5. Results are expressed as mean ± SD deviation of three determinations. ap < 0.05, compared to the Control group.
Given the crucial role of alveolar macrophages in defending against PM2.5 pulmonary invasion (35), rat alveolar macrophages (NR8383) was selected for in vitro experiments. There was a notable decline in cell viability with as PM2.5 concentration increased (Figure 1F). Moreover, cell viability approached 50% at the exposure concentration of 240 μg/mL. Thus, 120 μg/mL was chosen for subsequent experiments. Regarding oxidative stress, there was a significant decrease in SOD and significant increases in MDA and ROS compared to the Control group, (Figures 1G–J). Furthermore, consistent with in vivo findings, exposure to cowshed PM2.5 significantly elevated IL-1β and IL-18 (Figures 1K–M). In conclusion, the in vivo and in vitro fundamental data demonstrate that cowshed PM2.5 is respiratory toxic and poses a significant threat to animal respiratory health.
3.2 Microbiological components are important reason for cowshed PM2.5-induced respiratory damage, and bacteria strongly amplified toxic effects of PM2.5 particles on cells
To determine the role of microbial components while minimizing alterations to cowshed PM2.5 composition, heat inactivation treatment was done and cellular toxicity assessed. Cell viability exhibited partial restoration (Figure 2A), concomitant with the restoration of ROS, MDA, and SOD contents in the cowshed PM2.5 inactivation group (Figures 2B,C,E,F), in comparison to the cowshed PM2.5 group. Furthermore, restoration of inflammatory factors IL-1β and IL-18 concentrations was most prominent (Figures 2I–K). The results provide strong evidence that the presence of microorganisms is a significant cause of cowshed PM2.5-induced respiratory damage.
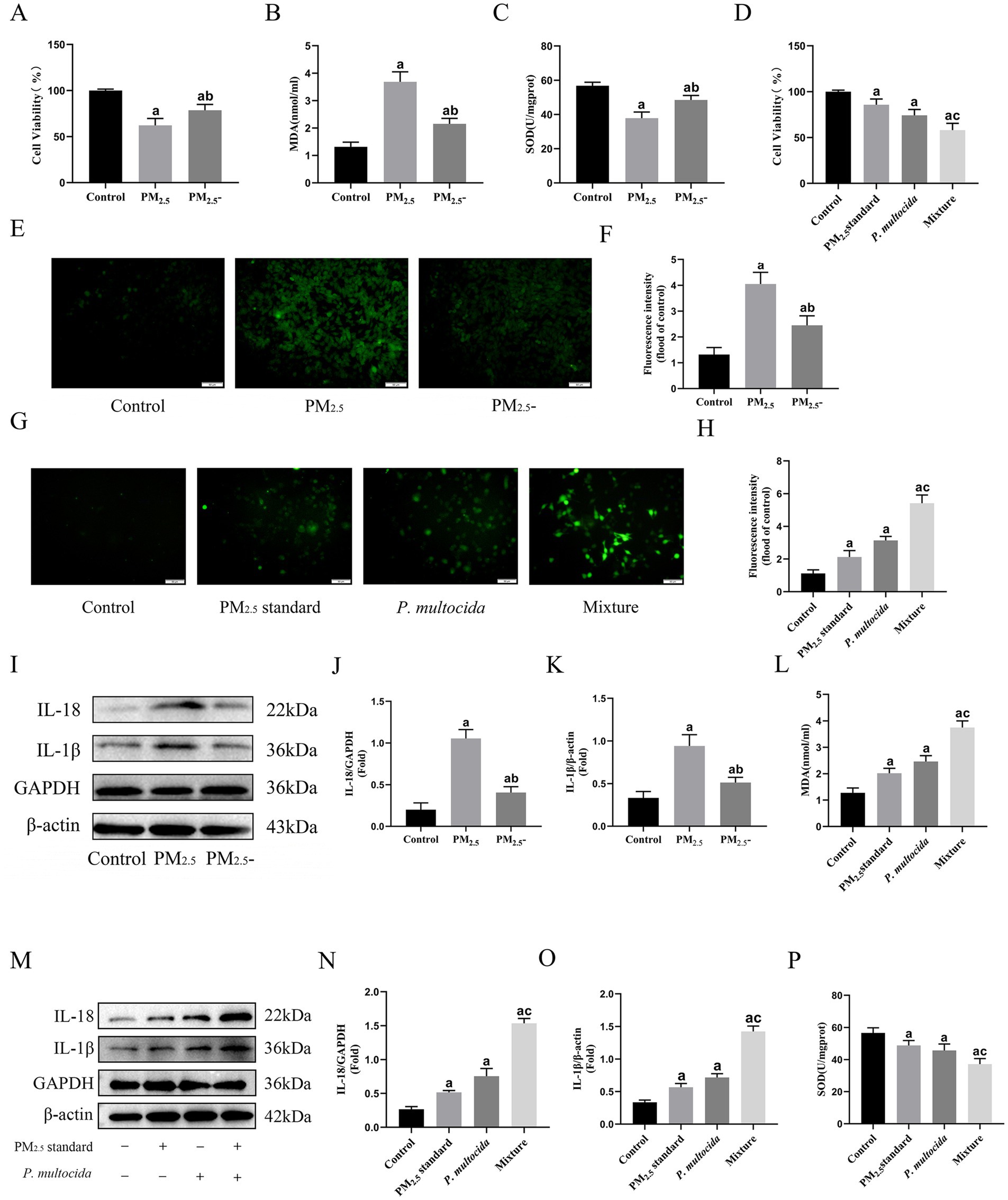
Figure 2. Microbiological components are important reason for cowshed PM2.5-induced respiratory damage, and bacteria strongly amplified toxic effects of PM2.5 particles on cells. (A) Viability of NR8383 cells after inactivation of cowshed PM2.5. (B,C,E,F) Oxidative stress on NR8383 cells after inactivation of cowshed PM2.5. (D) Cell viability under the synergistic effect of PM2.5 standard and P. multocida. (G,H,L,P) Oxidative stress in NR8383 in response to the synergistic effect of PM2.5 standard and P. multocida. (I–K) Inflammatory factors in NR8383 cells after inactivation of cowshed PM2.5. (M–O) Inflammatory factors in NR8383 cells under the synergistic effects of PM2.5 standard and P. multocida. Results are expressed as mean ± SD deviation of three determinations. ap < 0.05, compared to the Control group. bp < 0.05, the PM2.5-group compared to the PM2.5 group, cp < 0.05, the Mixture group compared to the PM2.5 standard group.
The microbial species in cowshed PM2.5 are diverse, in order to explore the mechanisms and intrinsic links between microbial components in their induction of cytotoxicity, it is first necessary to clear components and control variables. In this regard, this study used PM2.5 standard with well-defined compositions, as well as P. multocida, a common pathogenic bacteria in cowshed environments to simulate cowshed PM2.5 and reevaluated cellular toxicity. The results showed that although both the PM2.5 standard group and the P. multocida group showed some cytotoxicity. However, the Mixture group at half the dose showed more significant cytotoxicity. Compared to the PM2.5 standard group, the Mixture group had significant decreases in cell viability and the antioxidant factor SOD (Figures 2D,P), significant increases in the oxidant factors MDA and ROS, and a more pronounced increase in the inflammatory factors IL-1β and IL-18 (Figures 2G–H,L-O). These results indicate that the bacterial component appears to strongly amplify the cytotoxic effect of PM2.5 during PM2.5 stimulation of cells, although at a much lower dose.
3.3 Interactions between bacteria and PM2.5 particles enhanced intracellular invasion and surface group modification
To clarify the mechanism of cowshed PM2.5-induced respiratory damage, this study focused on the interaction between PM2.5 particles and bacteria. Firstly, with regard to effects of PM2.5 on bacteria, compared to the P. multocida single group, the PM2.5 standard + P. multocida group showed a significant increase in intracellular bacterial count (Figure 3A), indicating that PM2.5 enhances bacterial entry into the cytosol. The elevated viable bacteria in the cytosol may be a key factor for enhanced toxicity. Scanning electron microscopy further revealed that PM2.5 particles could adhere to and encapsulate bacteria (Figure 3B), directly explaining the increased intracellular bacterial load in the PM2.5 standard + P. multocida group.
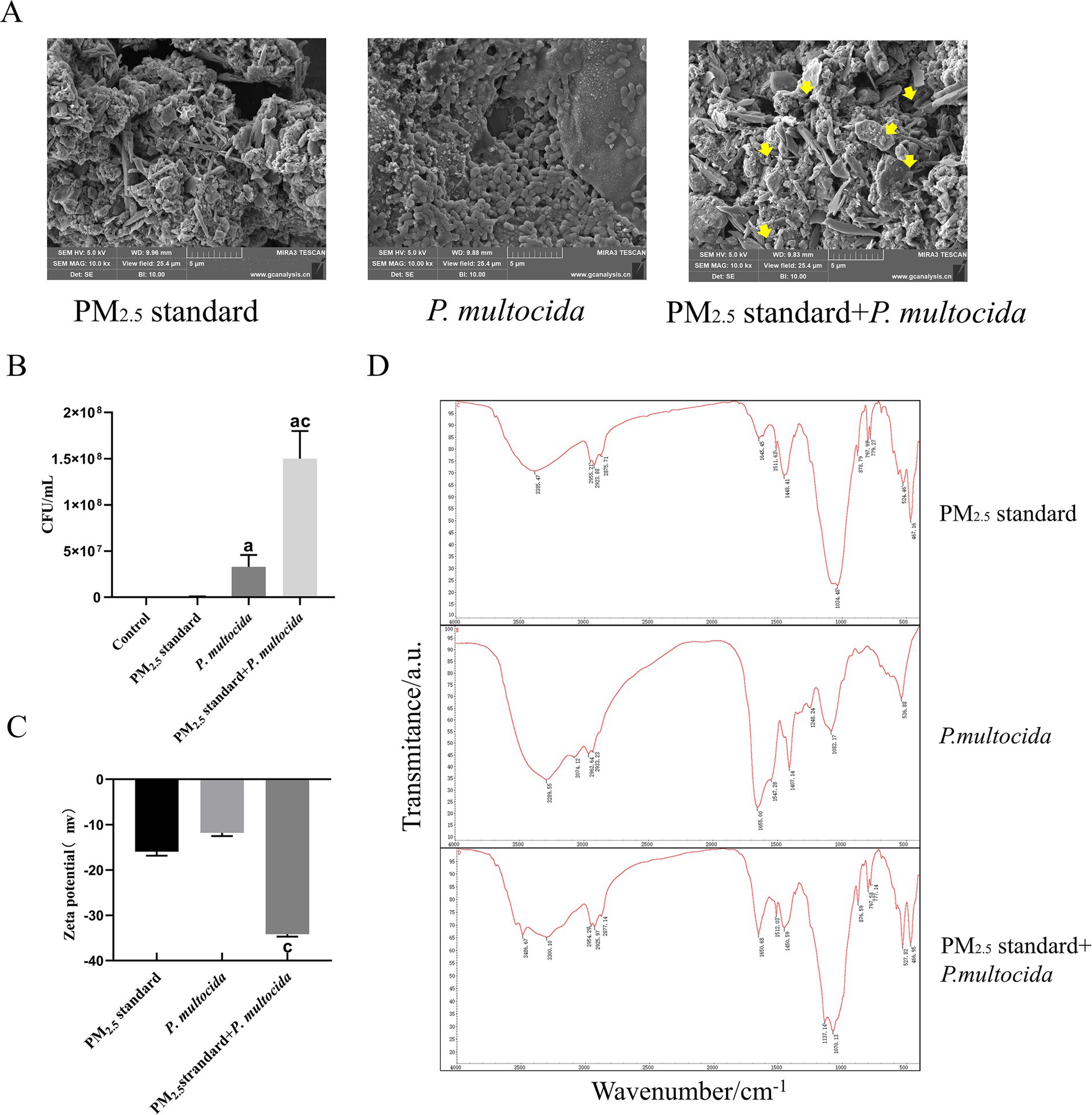
Figure 3. Interactions between bacteria and PM2.5 particles enhanced intracellular invasion and surface group modifications. (A) Scanning electron microscopy revealed P. multocida adhered to PM2.5 particles. (B) Significant increase in the number of intracellular bacteria after synergistic interaction of PM2.5 standard and P. multocida. (C) Zeta potential shifted significantly in the negative direction after synergistic interaction of PM2.5 standard and P. multocida. (D) Changes in PM2.5 surface groups after synergistic interaction of PM2.5 standard and P. multocida. Results are expressed as mean ± SD deviation of three determinations. ap < 0.05, compared to the Control group. bp < 0.05, compared to the PM2.5 standard group.
In addition, whether the bacterial component also alters the surface properties of PM2.5 particles is unclear. To evaluate bacterial effects on PM2.5 particles, we first measured Zeta potential changes. The findings indicated a notable negative shift in zeta potential in the Mixture group in comparison with the PM2.5 standard group (Figure 3C). The significant changes in zeta potential indicate greater stability within the system (36). This suggests that the bacterium enhances the anti-aggregation and anti-deposition properties of PM2.5 particles. Subsequently, every group was subjected to analysis by FTIR. The results demonstrated that the absorption peaks of the PM2.5 standard included O–H and methyl stretching vibrations, etc., whereas absorption peaks of P. multocida included antisymmetric and symmetric stretching vibrations of amino N–H, and stretching vibrations of amide C=O, amongst others. In comparison to the PM2.5 standard group, the absorption peak at 3,385 cm−1 in the PM2.5 standard + P. multocida group was red-shifted towards 3,300 cm−1. The change there suggests that bacterial cells induced elongation of O–H and N–H bonds in the PM2.5 standard + P. multocida group, leading to hydrogen bond association. The enhanced absorption peak at 1,650 cm−1 and the emergence of a new peak at 527 cm−1 suggest specific binding between bacterial organic groups and PM2.5 particles, which potentiates the biological toxicity of PM2.5 (Figure 3D). These alterations in the peaks of absorption indicated that P. multocida modified the surface groups of the PM2.5 standard. The results supported the hypothesis that interactions occurred between the bacterium and PM2.5 particles and indicated that the two were mutually promoting in terms of enhancing cytotoxicity.
3.4 Cowshed PM2.5 activates cellular GBP2 expression in response to defense against intracellular bacteria
Based on the finding that PM2.5 increases the probability of bacteria entering the cytosol as an important cause of cytotoxicity. This study focuses on digging into the signaling of alveolar macrophages to intracellular bacterial defenses to investigate the specific mechanisms of cowshed PM2.5 respiratory toxicity. Based on a bioinformatics screen for cowshed PM2.5-induced cellular differential genes, there was a significant increase in guanylate binding protein 2 (GBP2) expression in the lung under the role of cowshed PM2.5. Under the role of cowshed PM2.5, the mRNA and protein expression levels of GBP2 are shown (Figures 4A–C). However, whether the high expression of GBP2 was induced by an increase in intracellular bacteria and whether it was involved in the host cell defense process against gram-negative cytoplasmic bacteria was not clear. Firstly, still used PM2.5 standard and P. multocida to simulate cowshed PM2.5. In comparison to the Control group, neither PM2.5 standard nor P. multocida groups effectively activated GBP2 expression. However, GBP2 expression was significantly higher in the Mixture group (Figures 4D,E). This result provides substantial evidence that, under the influence of PM2.5 in the cowshed, GBP2 expression is induced by an increase in intracellular bacteria.
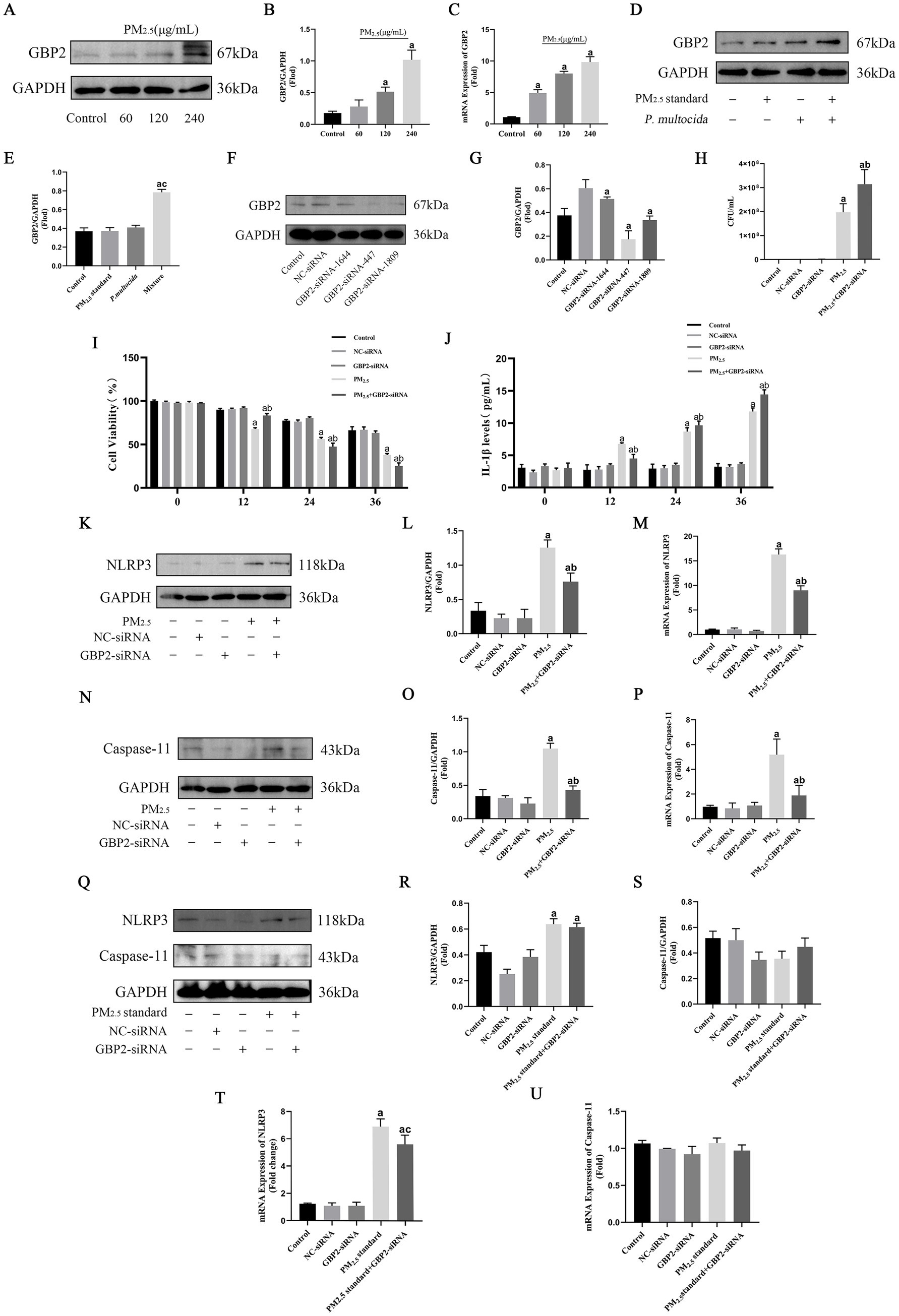
Figure 4. Cowshed PM2.5 activates cellular GBP2 expression in response to defenses against intracellular bacteria, whereas recognition of intracellular bacteria by GBP2 activates Caspase-11-mediated non-classical NLRP3. (A,B) Expression of GBP2 protein in NR8383 after cowshed PM2.5 treatment was detected by Western blotting. (C) Expression of GBP2 mRNA in NR8383 after cowshed PM2.5 treatment was detected by qRT-RCR. (D,E) Expression of GBP2 under the synergistic effect of PM2.5 standard and P. multocida. (F,G) Silencing effect was verified for three siRNAs of GBP2. (H) The number of intracellular bacteria after interference with GBP2 under the influence of cowshed PM2.5. (I) Changes in cell viability over time after interfering with GBP2 under the influence of cowshed PM2.5. (J) Changes in IL-1β over time after interfering with GBP2 under the influence of cowshed PM2.5. (K–M) Decreased expression of NLRP3 after interference with GBP2 under the influence of cowshed PM2.5. (N–P) Decreased expression of Caspase-11 after interference with GBP2 under the influence of cowshed PM2.5. (Q–U) Interference with NLRP3 and Caspase-11 expression after interference GBP2 under the influence of PM2.5 standard. Results are expressed as mean ± SD deviation of three determinations. ap < 0.05, compared to the Control group. bp < 0.05, compared to the PM2.5 group, cp < 0.05, compared to PM2.5 standard group.
In order to further validation of the above results, siRNA was used to silence GBP2 expression. First, the silencing effect was tested for three siRNAs. The results showed that all three siRNAs effectively inhibited GBP2 mRNA expression; however, as siRNA-447 had the best silencing effect (Figures 4F,G). Therefore, siRNA-447 was selected for the following studies. Then, the number of intracellular bacteria was assessed after stimulation of normal and GBP2-silenced cells with cowshed PM2.5 for 24 h each. It was found that the number of intracellular bacteria was significantly increased in the GBP2-silenced group compared to the unsilenced group (Figure 5H). This suggests that GBP2 plays an important role in the intracellular bacterial defense of host cells against cowshed PM2.5 invasion. In addition, during the above process, cell viability of the silenced group was relatively high compared to the unsilenced group for 12 h. However, after 12 h, cell viability plummeted, and after 24 h, cell viability of the unsilenced group was higher than that of the silenced group (Figure 4I). The expression level of IL-1β in the silenced group was initially lower than in the unsilenced group, but over time, expression of IL-1β in the silenced group increased abruptly and was higher than in the unsilenced group (Figure 4J). These data suggest that GBP2 is able to intervene in the cowshed PM2.5-induced cytotoxic response, but appears to have complex intrinsic mechanisms.
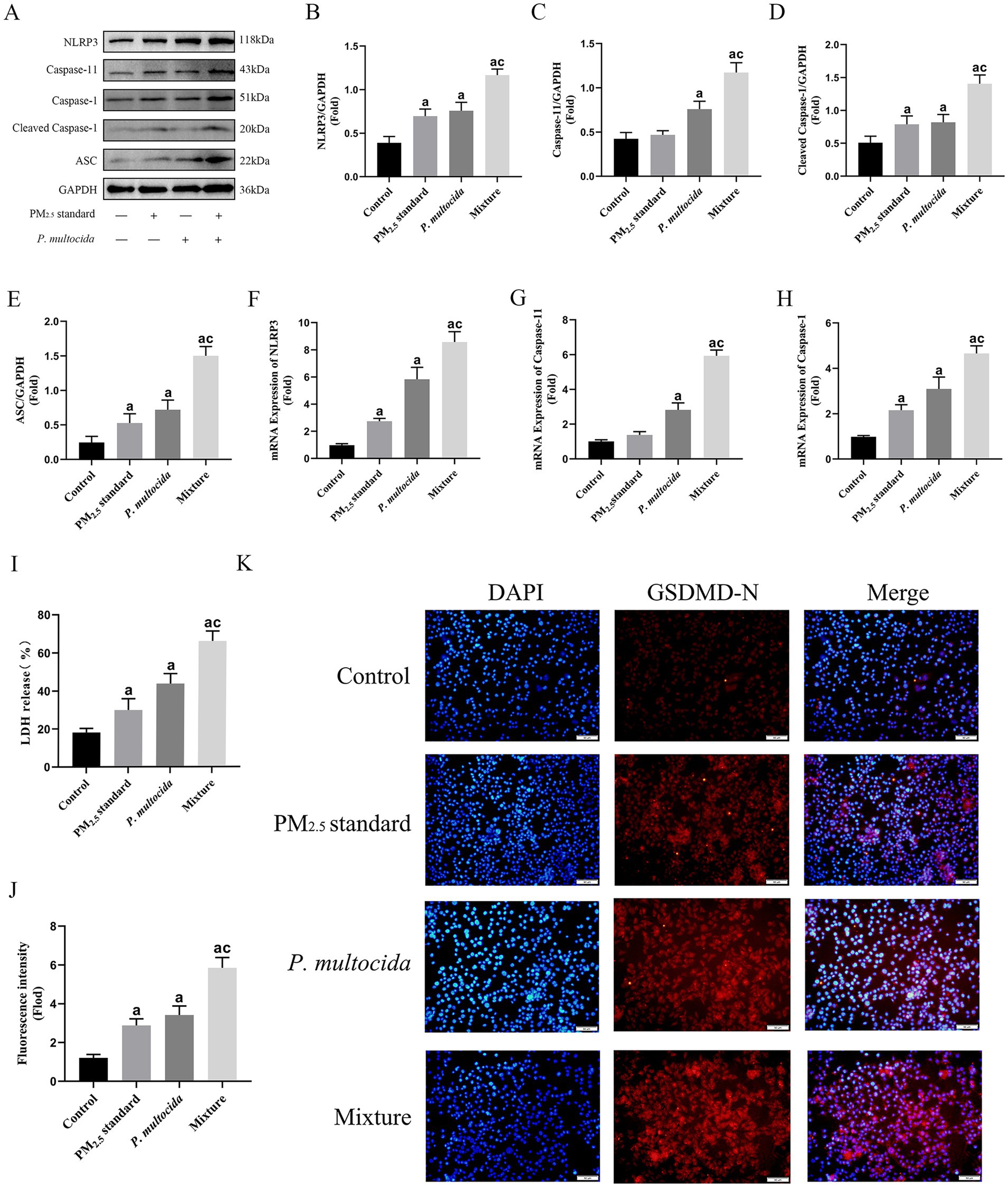
Figure 5. PM2.5 enhanced intracellular invasion of P. multocida and efficiently initiated Caspase-11-mediated non-classical NLRP3 activation and exacerbated pyroptosis. (A–E) Protein expression levels of NLRP3, Caspase-1, Caspase-11, and ASC under the synergistic effect of PM2.5 standard and P. multocida. (F–H) The mRNA expression levels of NLRP3, Caspase-1, Caspase-11 under the synergistic effect of PM2.5 standard and P. multocida. (I) LDH levels under the synergistic effect of PM2.5 standard and P. multocida. (J,K) GSDMD levels under the synergistic effect of PM2.5 standard and P. multocida. Results are expressed as mean ± SD deviation of three determinations. ap < 0.05, compared to the Control group. cp < 0.05, compared to the PM2.5 standard group.
3.5 Recognition of intracellular bacteria by GBP2 activated Caspase-11-mediated non-classical NLRP3
IL-1β is an important effector of NLRP3 expression, and in order to explain the phenomenon of the lag in changes in cell viability and IL-1β expression after GBP2 silencing in the above study. This study focused on investigating the relationship between GBP2 silencing and changes in NLRP3 expression. The results showed that NLRP3 was significantly highly expressed under the effect of cowshed PM2.5, whereas silencing GBP2 decreased expression of NLRP3 (Figures 4K–M). This suggests a link between GBP2 and NLRP3. The activation pathway of NLRP3 is relatively complex, and to further explore the relationship between GBP2 and NLRP3, this study focused on the intracellular Caspase-11-mediated non-classical NLRP3 activation pathway. The results showed a relative decrease in the expression of Caspase-11 in the silenced group compared to the unsilenced group (Figures 4N–P), suggesting that GBP2 was associated with the non-classical NLRP3 activation pathway mediated by Caspase-11. Furthermore, silencing or non-silencing of GBP2 had no significant effect on the expression of Caspase-11 and NLRP3 when cells were stimulated with PM2.5 standard (Figures 4Q–U). This strongly supports that under the effects of cowshed PM2.5, GBP2 intervenes with the hypothesis that Caspase-11-mediated nonclassical NLRP3 activation is associated with the presence of intracellular bacteria. The phenomenon of the lag in changes in cell viability and IL-1β expression after GBP2 silencing may be influenced by the failure to activate Caspase-11 in time to mediate non-classical NLRP3.
3.6 PM2.5 enhanced intracellular invasion of Pasteurella multocida, initiated Caspase-11-mediated non-classical NLRP3 activation, and exacerbates pyroptosis
To further investigate in depth the mechanisms of respiratory toxicity induced by signal transduction in alveolar macrophages in response to intracellular bacterial defenses, similarly, PM2.5 standard and P. multocida were used to simulate cowshed PM2.5. Although only half the dose was used in the Mixture group, in comparison to the PM2.5 standard group, expression levels of NLRP3, ASC, cleaved-Caspase-1, and Caspase-11 were elevated (Figures 5A–H). This suggests that during this process, the intracellular transport function of P. multocida due to PM2.5 particles plays a crucial role in Caspase-11-mediated non-classical NLRP3 activation. This data further elucidates why the Mixture group has an amplifying effect on cytotoxicity in the above results. Activation of the NLRP3 is a significant factor in the induction of pyroptosis, a major manifestation of cellular toxicity, during cellular physiological processes (37). Consequently, alterations in the level of pyroptosis were measured. The results showed that expression levels of GSDMD-N and LDH were significantly increased in the Mixture group compared to the PM2.5 standard group (Figures 5I–K). It is shown that pyroptosis is an effective form of cellular damage in the above process and is associated with enhanced intracellular invasion of P. multocida by PM2.5.
3.7 Intervention of NLRP3 expression was an effective respond to cowshed PM2.5-induced cellular damage
In this study, although GBP2 silencing initially preserved cell viability, the loss of intracellular bacterial control led to delayed cytotoxicity, indicating that targeting this Gram-negative bacterial defense protein is not a viable therapeutic strategy. Conversely, since cowshed PM2.5 triggers Caspase-11-mediated non-classical NLRP3 activation, NLRP3 inhibition was evaluated using an NLRP3 inhibitor (MCC950, MCE). As shown in Figures 6A–C, activation of NLRP3 was significantly inhibited in the PM2.5 + MCC950 group compared to the PM2.5 group. In addition, the expression levels of cleaved-Caspase-1, IL-1β and IL-18 were also significantly reduced (Figures 6E–K). In terms of pyroptosis, the expression of GSDMD-N and LDH was significantly suppressed in the PM2.5 + MCC950 group compared to the cowshed PM2.5 group, and cell viability was maintained for an interval in the PM2.5 + MCC950 group (Figures 6D,L–N). These data suggest that the use of NLRP3 inhibitors attenuates the onset of pyroptosis and maintains cell viability to some extent, which may be an effective means of treating cowshed PM2.5-induced respiratory damage.
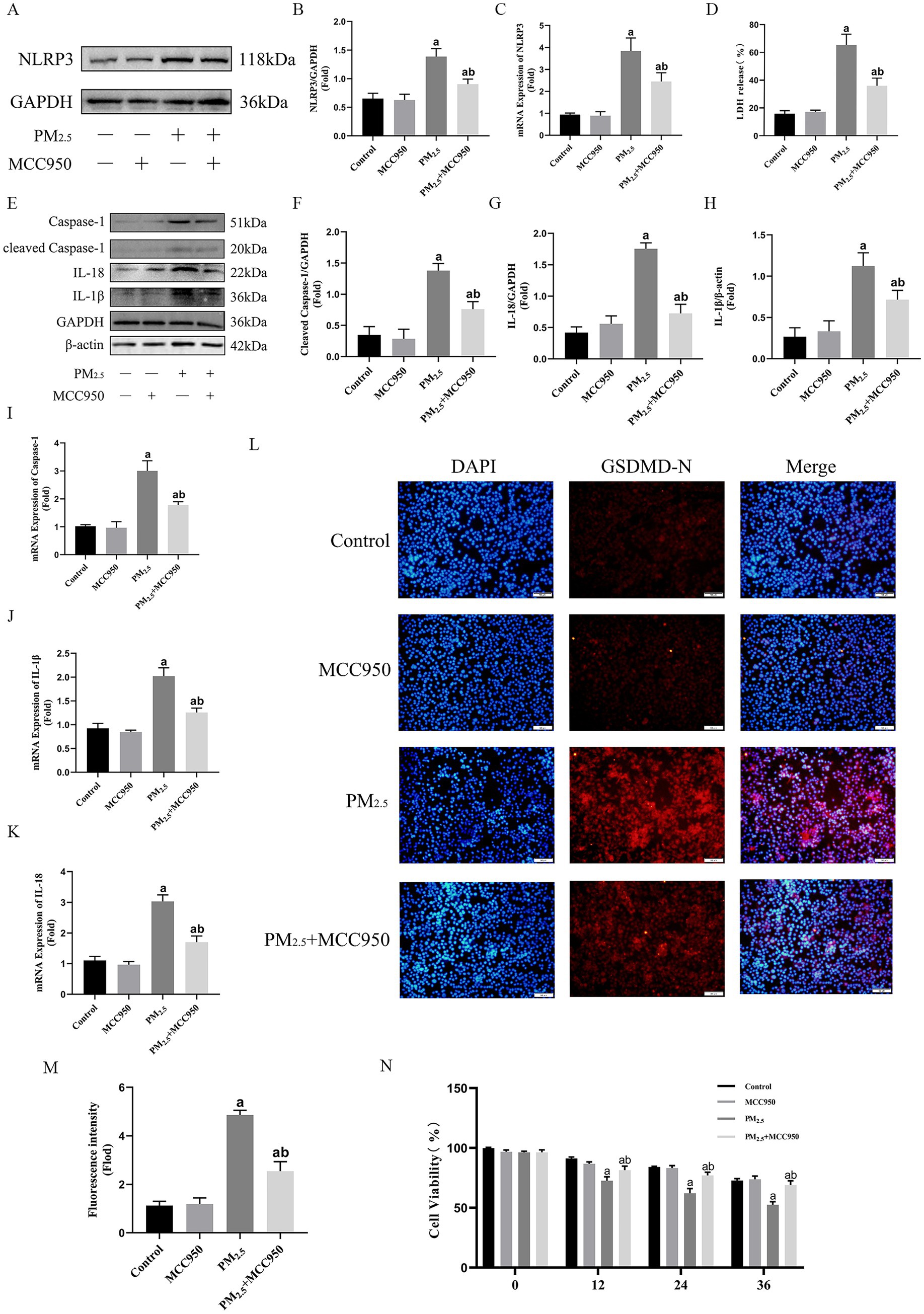
Figure 6. Intervention of NLRP3 expression was an effective respond to cowshed PM2.5-induced cellular damage. (A,B) Protein levels of NLRP3 after addition of MCC950 under the influence of cowshed PM2.5. (C) mRNA levels of NLRP3 after addition of MCC950 under the influence of cowshed PM2.5. (D) LDH levels in NR8383 after addition of MCC950 under the influence cowshed PM2.5. (E–H) Caspase-1, IL-18, and IL-1β protein expression levels after addition of MCC950 under the influence of cowshed PM2.5. (I–K) Caspase-1, IL-18, and IL-1β mRNA expression levels after addition of MCC950 under the influence of cowshed PM2.5. (L–M) GSDMD levels in NR8383 after addition of MCC950 under the influence cowshed PM2.5. (N) Changes in cell viability levels over time after addition of MCC950 under the influence of cowshed PM2.5. Results are expressed as mean ± SD deviation of three determinations. ap < 0.05, compared to the Control group. bp < 0.05, compared to the PM2.5 group.
4 Discussion
4.1 Microbial components are critical factors in cowshed PM2.5-induced respiratory toxicity
The higher feeding densities and inefficient ventilation in animal farms results in elevates PM2.5 concentrations and prolongs exposure longer, thereby increasing the risk of respiratory illness and infection for both animals and workers (38). In the present study, there was substantial pathological damage to the lungs of the model animal (rat) after exposure to cowshed PM2.5, accompanied by the release of inflammatory factors. Microorganisms, a major component of PM2.5 in the farm-animal environments, are widely present in all aspects of livestock production (39). Furthermore, physiological activities such as ruminating and flatulence are also sources of airborne microorganisms in cowsheds (40); they can utilize particulate matter as a medium for cultivation and transmission. This can result in the emergence of a range of airborne diseases, impairing animal growth, production and welfare, with potential for zoonotic diseases (41). Studies have shown that dairy cows kept for extended intervals in cowsheds with high PM2.5 concentrations are more prone to developing respiratory diseases (41). The presence of PM2.5 in piggeries has been demonstrated to induce oxidative stress and inflammatory responses in alveolar macrophages, which compromises pig immunity to a certain extent. Furthermore, this can significantly accelerate the course of disease when primary or secondary pathogenic infections are present (42).
Pathogenic bacteria, including Pseudomonas aeruginosa, Shigella escherichii, Acinetobacter, Streptococcus, and Staphylococcus have been detected in the air of cowsheds. These bacteria pose a serious risk to the organism’s health since they can result in bacteremia or respiratory infections (43). Exposure to PM2.5 in the animal farm environment increases the susceptibility of livestock to microorganisms, and this synergistic effect leads to more severe respiratory damage and inflammatory responses (44). In our previous analysis of the composition of cowshed PM2.5, bacteria accounted for 61.39% of the microbial composition of cowshed PM2.5 (13). Therefore, we speculated that microbiological components had an important role in cowshed PM2.5-induced cytotoxicity. In this study, when cytotoxicity experiments were conducted following inactivation of cowshed PM2.5, there was a significant decrease in toxicity compared to the not inactivated cowshed PM2.5, confirming the above hypothesis. However, the present study employed high temperatures to inactivate cowshed PM2.5, a method that is inherently limited in scope. On the one hand, high temperatures can only transform the microbiologically active components of cowshed PM2.5 into inactive components, and do not serve to remove the toxins. On the other hand, circulating high temperatures in localized air within the animal farmhouse is not a widespread practice. Nevertheless, UV light or microwave radiation to address recirculating airflow within the environment has been demonstrated to be an efficacious approach for curbing airborne microbial activity. This may be a viable solution for regulating the biological toxicity of PM2.5 and the dissemination of pathogens within the farm-animal environment (45).
4.2 Interactions and toxicity amplification mechanisms between PM2.5 and bacteria
In addition to this, by analyzing how the microbial component of PM2.5 from livestock facilities enhances cytotoxicity, should inform treatment options for animals infected with respiratory diseases. In order to investigate the specific mechanism of microbial components in the induction of cellular damage by cowshed PM2.5, we used P. multocida isolated from cowshed PM2.5 in synergy with PM2.5 standard of well-defined compositions to simulate cowshed PM2.5. This combination not only excludes as much as possible the unknown effects of the other components, but also shows the characteristics of the cowshed PM2.5 as much as possible. Since during PM2.5 invasion of the lungs, alveolar macrophages have an important barrier role in the intake and processing of PM2.5 particles. Consequently, it is necessary to investigate the response of alveolar macrophages (NR8383) to cowshed PM2.5. The experimental results obtained in this study demonstrate that when P. multocida is synergized with the PM2.5 standard, despite the fact that the stoichiometry is half that of the original, the cellular damage induced by the Mixture group is significantly increased. This also suggests that the microbiological component of PM2.5 may play a significant role in the effects on livestock.
In a study of measurements of biomass components in atmospheric PM2.5, fungi were found to be the predominant bioactive substances in PM2.5, due to the adsorption function of PM2.5 particles (46). Therefore, the exploration of the reasons for the strong amplification of PM2.5 cytotoxic effects by bacteria may be pivotal in elucidating the mechanism of cowshed PM2.5-induced respiratory damage. In order to verify the aforementioned speculations, an examination was conducted of the surface characteristics of particle samples of synergizing PM2.5 standard with P. multocida, and it was confirmed that P. multocida was able to adhere to PM2.5 particles. It has been shown that the selenium yeast A. brasilense of the genus Azospirilla can reduce selenite to basic selenium in the form of selenium nanospheres (47). Silver nanoparticles exhibit significant antimicrobial activity subsequent to interaction with bacterial extracellular polymers (48). This all suggests that there may be a degree of interaction between particulate matter and microorganisms. In this study, the Zeta potential shifted in a negative direction when the PM2.5 standard and P. multocida were mixed compared to the PM2.5 standard group. It has been demonstrated that negatively charged groups secreted by bacteria adsorbed onto the calcite surface induce a shift in the Zeta potential towards a negative direction (49). In the case of P. multocida, it has certain surface characteristics and charge distribution itself. After interaction with PM2.5, due to adsorption, binding, or other physicochemical processes, a change in the surface charge of PM2.5. The shift of the Zeta potential in a negative direction resulted in a reduction in bacterial aggregation and sedimentation, while concurrently enhancing the stability of the PM2.5 standard and the P. multocida system (50). In the cowshed environment, this would serve to further enhance the spread, survival and pathogenicity of P. multocida. Moreover, it would facilitate the more easily formation of bioaerosols, thereby enhancing the toxic effects of PM2.5. In the FTIR spectrum, compared to the PM2.5 standard group, the red shift of the absorption peak at 3,385 cm−1 indicates that the O–H and N–H bond lengths have risen and the bond energy has reduced in the synergistic effect of PM2.5 standard and P. multocida. This suggests that the two form a stable complex through hydrogen bonding, binding PM2.5 to the bacteria. This lays a physical foundation for the subsequent enhancement of intracellular invasion efficiency. Enhancement of the absorption peak at 1,650 cm−1 indicates an increase in C=O groups of bacterial origin within the mixture. This is direct evidence of bacterial components being adsorbed onto PM2.5 surfaces, thereby altering their chemical composition. This alteration may permit PM2.5 to carry more bacterial antigens or toxins (e.g., endotoxin LPS), which in turn enhances immune stimulation. The appearance of the 527 cm−1 absorption peak provides further evidence to support that specific chemical binding of bacteria to PM2.5 results in the introduction of new toxic groups (e.g., organic acids, cellular metabolites such as carboxyl and hydroxyl groups in proteins) and it changes the PM2.5 surface charge distribution, which enhances the retention time of the complex in the respiratory tract and the cell adhesion ability. It has also been shown that in the animal farm environment, PM2.5 particles that carry P. multocida increase the biological toxicity of the particles themselves. Concurrently, PM2.5 particles carrying P. multocida are more prone to penetrate the organism’s deeper layers, thereby elevating the probability of disease infection in animals.
4.3 Cowshed PM2.5 induces cellular pyroptosis and inflammatory responses through activation of the GBP2/Caspase-11/NLRP3 pathway
The present study investigated the effect on the level of intracellular infection of P. multocida in the presence of PM2.5 particles. It was found that PM2.5 greatly increased the probability of intracellular entry of P. multocida, which became a pivotal factor in amplifying PM2.5 cytotoxicity. However, after PM2.5 carries P. multocida into cells, the specific mechanism of causing toxicity remains to be elucidated. In this context, bioinformatics and transcriptomics screening revealed a significant up-regulation of GBP2 expression in the lungs in response to cowshed PM2.5. GBP2 plays an important role in the immune response. Studies have shown that GBP2 is capable of recognizing and binding to pathogen-associated molecular patterns, thereby initiating immune defense mechanisms (51). Therefore, we speculate that the complex microbiological composition of cowshed PM2.5 may be a significant contributing factor to the elevated GBP2 expression. An increase in the number of intracellular bacteria, an enhanced effect of cowshed PM2.5 on cell viability, and elevated expression of IL-1β were detected for a period of time following interference with GBP2 expression. This further suggests that microbial components in PM2.5 induce high GBP2 expression, and suggests that GBP2 is associated with inflammatory responses induced by microbial components. However, after interfering with GBP2 expression, there was a lag in both cell viability and inflammatory factor expression under the influence of cowshed PM2.5, which may be related to the failure of GBP2 to activate Caspase-11 in a timely manner. When GBP2 is silenced, P. multocida enters the cell. The clearance of the bacteria by the cell is diminished, and the bacteria continue to multiply intracellularly. However, they have not yet reached the threshold for triggering intense inflammation. This results in a transient increase in cell viability in the initial period (within 12 h). In the present study, it was also detected that after interfering with GBP2, there was no activation of Caspase-11 expression in the presence of cowshed PM2.5. However, it has been demonstrated that persistent intracellular bacterial reproduction can ultimately trigger the lagging activation of the non-classical NLRP3 pathway, leading to a surge of inflammatory factors and, ultimately, triggering a sudden drop in cell viability. This phenomenon reveals the pivotal function of GBP2 in maintaining a balance between antimicrobial defense and inflammation control. Furthermore, these findings suggest that the silencing of GBP2, although protective in the short term, leads to more severe damage over time.
Many studies have shown that PM2.5 can induce a variety of programmed cell deaths, including apoptosis, autophagy and pyroptosis, and so on (52). However, the relationship and signaling mechanisms between PM2.5 and various forms of programmed cell death are complex. Among the various forms of programmed cell death described above, pyroptosis is capable of triggering a more intense inflammatory response, a process that helps to protect the host from microbial infections (53). However, the consequences of excessive pyroptosis are also evident, with excessive cellular pyroptosis leading to a series of inflammatory storms that can cause sepsis and autoimmune diseases (54). Therefore, it is crucial to explore the mechanism of pyroptosis induced by cowshed PM2.5. Studies have shown that upon bacterial entry into cells, GBP2 cleaves pathogen-containing vesicles (PVs), thereby releasing bacteria and their associated LPS into the host cell cytoplasm and assembling a Caspase-11 activation platform on LPS-containing membranes as the first step of the inflammasome signaling, and activating the non-classical NLRP3 inflammasome (55). The mechanism of activation of the NLRP3 inflammasome is similarly intricate. Studies have shown that chemicals endocytosed into the cytoplasm by macrophages trigger lysosomal rupture and the release of histone B, leading to activation of the NLRP3 inflammasome (56). The rupture of the cytoplasmic membrane with K+ efflux caused by the PAHs component contained in PM2.5 may also be a significant reason for the activation of NLRP3 (57). In this study, the expression of NLRP3 and Caspase-11 did not change significantly after interfering with the expression of GBP2 under the role of PM2.5 standard. However, interference with GBP2 was found to have a substantial impact on the expression of NLRP3, cleaved-Caspase-1, Caspase-11, GSDMD, and LDH, under the synergistic effect of PM2.5 standard and P. multocida. These results provide validation for the upregulation of GBP2 expression upon the entry of cowshed PM2.5 into cells. Subsequently, GBP2 is able to recognize and bind to microbial components in cowshed PM2.5, further affecting the activation of the NLRP3 non-classical pathway mediated by Caspase-11.
4.4 Inhibition of NLRP3 can stably suppresses cowshed PM2.5-induced respiratory toxicity
In this study, it was determined that GBP2 is not an effective target against cowshed PM2.5-induced pyroptosis. Therefore, we focused our perspective on NLRP3, the activation of which is the central channel for pyroptosis caused by cowshed PM2.5 in the present study. In this regard, the present study attempted a therapeutic option to reduce cowshed PM2.5-induced damage by inhibiting NLRP3. The development of cellular inflammation and pyroptosis was effectively controlled by using an NLRP3 inhibitor (MCC950) to inhibit NLRP3 expression. This demonstrates the effectiveness of this regimen. This also provides a valuable therapeutic target and dosing direction for the treatment of cowshed PM2.5-induced lung injury. However, there are some limitations to this study. This study only assessed the interrelationships and mechanisms of action between PM2.5 and bacteria, and therefore does not reflect the full picture of the microbiological landscape within cowshed PM2.5. This encompasses the potential involvement of disease-causing microorganisms (such as fungi, viruses, and parasites) in the process of damage to organisms. Further research is required to elucidate this aspect, which will be the focus of our subsequent studies.
5 Conclusion
In summary, the present study demonstrated that microbiological components were non-negligible and important factors in animal farm environment PM2.5-induced lung injury. Bacterial components alter the surface characteristics of PM2.5 particles, whereas PM2.5 particles enabled bacteria to get inside the cells. The interaction between the two amplified the biological toxicity of PM2.5. Intracellularly, recognition and defense of bacteria by GBP2 activated pyroptosis induced by non-classical NLRP3. The present study elucidated the relationship among intracellular bacteria, GBP2, and NLRP3 as a key process in cowshed PM2.5-induced pyroptosis. In addition, inhibition of NLRP3 has potential for the treatment of PM2.5-induced lung injury in a farm-animal environment.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was approved by the Animal Experimentation Ethics Committee at Haihua Biotechnology Group Co. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
XD: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. ZM: Methodology, Supervision, Visualization, Writing – review & editing. YS: Methodology, Visualization, Writing – review & editing. YJ: Methodology, Writing – review & editing. XZ: Visualization, Writing – review & editing. CZ: Data curation, Writing – review & editing. XL: Visualization, Writing – review & editing. XY: Conceptualization, Formal analysis, Investigation, Resources, Validation, Writing – review & editing. YG: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Xinjiang Uygur Autonomous Region Key R&D Program (2024B02011-3), Xinjiang Uygur Autonomous Region Centralized Guided Local Science and Technology Development Fund Project (ZYYD2025QY04), Ningxia Hui Autonomous Region Agricultural Science and Technology Independent Innovation Project (NGSB-2021-12), Supported by the earmarked fund for JLARS; Special Program for the Construction of National Modern Agricultural Industrial Technology System (CARS-37).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2025.1631913/full#supplementary-material
References
1. Martins, NR, and Guilherme, CDGA. Impact of PM2.5 in indoor urban environments: a review. Sustain Cities Soc. (2018) 42:S2210670718309272. doi: 10.1016/j.scs.2018.07.011
2. Ge, E, Lai, K, Xiao, X, Luo, M, Fang, Z, Zeng, Y, et al. Differential effects of size-specific particulate matter on emergency department visits for respiratory and cardiovascular diseases in Guangzhou, China. Environ Pollut. (2018) 243:336–45. doi: 10.1016/j.envpol.2018.08.068
3. Zhang, K, Guo, L, Wei, Q, Song, Q, Liu, J, Niu, J, et al. COPD rat model is more susceptible to cold stress and PM2.5 exposure and the underlying mechanism. Environ Pollut. (2018) 241:26–34. doi: 10.1016/j.envpol.2018.05.034
4. Zhao, L, Li, B, Zhou, L, Song, C, Kang, T, Xu, Y, et al. PM2.5 exposure promotes asthma in aged Brown-Norway rats: implication of multiomics analysis. Ecotoxicol Environ Saf. (2023) 263:115393. doi: 10.1016/j.ecoenv.2023.115393
5. Xu, Z, Li, Z, Liao, Z, Gao, S, Hua, L, Ye, X, et al. PM2.5 induced pulmonary fibrosis in vivo and in vitro. Ecotoxicol Environ Saf. (2019) 171:112–21. doi: 10.1016/j.ecoenv.2018.12.061
6. Wang, TH, Huang, KY, Chen, CC, Chang, YH, Chen, HY, Hsueh, C, et al. PM2.5 promotes lung cancer progression through activation of theAhR‐TMPRSS2‐IL18 pathway. EMBO Mol Med. (2023) 15:e17014. doi: 10.15252/emmm.202217014
7. Jia, YY, Wang, Q, and Liu,. Toxicity research of PM2.5 compositions in vitro. IJERPH. (2017) 14:232. doi: 10.3390/ijerph14030232
8. Leenen, KV, Jouret, J, Demeyer, P, Vermeir, P, and Pardon, B. Particulate matter and airborne endotoxin concentration in calf barns and their association with lung consolidation, inflammation, and infection. J Dairy Sci. (2021) 104. doi: 10.3168/jds.2020-18981
9. Wang, M, Peng, S, Liu, D, Long, D, Liu, Z, and Pu, S. Characteristics and traceability analysis of microbial assemblage in fine particulate matter from a pig house. Animals. (2023) 13:5932–5947. doi: 10.3390/ani13061058
10. Dai, P, Shen, D, Tang, Q, Huang, K, and Li, C. PM2.5 from a broiler breeding production system: the characteristics and microbial community analysis. Environ Pollut. (2020) 256:113368. doi: 10.1016/j.envpol.2019.113368
11. Tang, Q, Huang, K, Liu, J, Jin, X, and Li, C. Distribution characteristics of bioaerosols inside pig houses and the respiratory tract of pigs. Ecotoxicol Environ Saf. (2021) 212:112006. doi: 10.1016/j.ecoenv.2021.112006
12. Chmielowiec-Korzeniowska, A, Trawińska, B, Tymczyna, L, Bis-Wencel, H, and Matuszewski,. Microbial contamination of the air in livestock buildings as a threat to human and animal health – a review. Ann Anim Sci. (2021) 21:417–31. doi: 10.2478/aoas-2020-0080
13. Zhang, X, Ma, Z, Hao, P, Ji, S, and Gao, Y. Characteristics and health impacts of bioaerosols in animal barns: a comprehensive study. Ecotoxicol Environ Saf. (2024) 278:116381. doi: 10.1016/j.ecoenv.2024.116381
14. Peng, Z, Wang, X, Zhou, R, Chen, H, and Wu, B. Pasteurella multocida: genotypes and genomics. Microbiol Mol Biol Rev. (2019) 83. doi: 10.1128/MMBR.00014-19
15. Prajapati, A, Yogisharadhya, R, Mohanty, NN, Mendem, SK, Nizamuddin, A, Chanda, MM, et al. Comparative genome analysis of Pasteurella multocida serogroup B: 2 strains causing haemorrhagic septicaemia (HS) in bovines. Gene. (2022) 826:146452. doi: 10.1016/j.gene.2022.146452
16. Amass, SF, Clark, LK, Van Alstine, WG, Bowersock, TL, Murphy, DA, Knox, KE, et al. Interaction of mycoplasma hyopneumoniae and Pasteurella multocida infections in swine. J Am Vet Med Assoc. (1994) 204:102–7. doi: 10.2460/javma.1994.204.01.102
17. Petruzzi, B, Rami, ALR, Evans, T, Pierson, NP, Inzana, FW, and Biofilm, TJ. Biofilm formation and avian immune response following experimental acute and chronic avian cholera due to Pasteurella multocida. Vet Microbiol. (2018) 222:114–23. doi: 10.1016/j.vetmic.2018.07.005
18. Santiani, F, Henker, LC, Schwetz, CI, Lorenzett, MP, Mendes, RE, and Casagrande, RA. Pasteurellosis outbreak due to Pasteurella multocida type A in rabbits (Oryctolagus cuniculus). Acta entiae Veterinariae. (2019). doi: 10.22456/1679-9216.93928
19. Nikolaos, K, Sofia, M, Irini, D, Eleni, M, Christina, K, and Eleni, I. An unusual case of Pasteurella multocida bacteremic meningitis. J Infect Public Health. (2018) 12:95–96. doi: 10.1016/j.jiph.2018.05.012
20. Piorunek, M, Brajer-Luftmann, B, and Walkowiak, J. Pasteurella multocida infection in humans. Pathogens. (2023) 12:8. doi: 10.3390/pathogens12101210
21. Huang, S, Meng, Q, Maminska, A, and MacMicking, JD. Cell-autonomous immunity by IFN-induced GBPs in animals and plants. Curr Opin Immunol. (2019) 60:71–80. doi: 10.1016/j.coi.2019.04.017
22. Kirkby, M, Tuipulotu, ED, Feng, S, Pilato, LJ, and Man, SM. Guanylate-binding proteins: mechanisms of pattern recognition and antimicrobial functions. Trends Biochem Sci. (2023) 48:883–93. doi: 10.1016/j.tibs.2023.07.002
23. Finethy, R, Luoma, S, Orench-Rivera, N, Feeley, EM, Haldar, AK, Yamamoto, M, et al. Inflammasome activation by bacterial outer membrane vesicles requires guanylate binding proteins. MBio. (2017) 8:10.1128/mbio.01188-17. doi: 10.1128/mBio.01188-17
24. Huang, W, Zhang, Y, Zheng, B, Ling, X, Wang, G, Li, L, et al. GBP2 upregulated in LPS-stimulated macrophages-derived exosomes accelerates septic lung injury by activating epithelial cell NLRP3 signaling. Int Immunopharmacol. (2023) 124:111017. doi: 10.1016/j.intimp.2023.111017
25. Fehervari, Z. NLRP3 shapes immunity to Leishmania. Nat Immunol. (2015) 16:342. doi: 10.1038/ni.3135
26. Xu, J, and Gabriel, N. The NLRP3 inflammasome: activation and regulation. Trends Biochem Sci. (2022) 48:331–44. doi: 10.1016/j.tibs.2022.10.002
27. Jin, H, Zhu, Y, Wang, X-d, Luo, E-f, Li, Y-p, Wang, B-l, et al. BDNF corrects NLRP3 inflammasome-induced pyroptosis and glucose metabolism reprogramming through KLF2/HK1 pathway in vascular endothelial cells. Cell Signal. (2021) 78:109843. doi: 10.1016/j.cellsig.2020.109843
28. Li, P, Li, S, Wang, L, Li, H, Wang, Y, Liu, H, et al. Mitochondrial dysfunction in hearing loss: oxidative stress, autophagy and NLRP3 inflammasome. Front Cell Dev Biol. (2023) 11:9. doi: 10.3389/fcell.2023.1119773
29. Ma, Z, Du, X, Sun, Y, Sun, K, Zhang, X, Wang, L, et al. RGS2 attenuates alveolar macrophage damage by inhibiting the Gq/11-Ca2+ pathway during cowshed PM2.5 exposure, and aberrant RGS2 expression is associated with TLR2/4 activation. Toxicol Appl Pharmacol. (2024) 487:11. doi: 10.1016/j.taap.2024.116976
30. Ma, Z, Du, X, Sun, Y, Jia, Y, Liang, X, and Gao, Y. Attenuation of PM2.5-induced lung injury by 4-phenylbutyric acid: maintenance of [Ca2+]i stability between endoplasmic reticulum and mitochondria. Biomol Ther. (2024) 14:1135. doi: 10.3390/biom14091135
31. Wu, Y, Pei, C, Wang, X, Wang, Y, Huang, D, Shi, S, et al. Probiotics ameliorates pulmonary inflammation via modulating gut microbiota and rectifying Th17/Treg imbalance in a rat model of PM2.5 induced lung injury. Ecotoxicol Environ Saf. (2022) 244:114060. doi: 10.1016/j.ecoenv.2022.114060
32. Sun, Y, Sun, K, Ma, Z, Zhang, X, Du, X, Jia, Y, et al. mi R–122–5p promotes cowshed particulate matter 2.5-induced apoptosis in NR8383 by targeting COL4A1. Toxics. (2024) 12:386. doi: 10.3390/toxics12060386
33. Zeng, Y, Li, M, Zou, T, Chen, X, and Xu, H. The impact of particulate matter (PM2.5) on human retinal development in hESC-derived retinal organoids. Front Cell Dev Biol. (2021) 9:607341. doi: 10.3389/fcell.2021.607341
34. Wang, M, Zhao, M, Yu, J, Xu, Y, Zhang, J, Liu, J, et al. MCC950, a selective NLRP3 inhibitor, attenuates adverse cardiac remodeling following heart failure through improving the cardiometabolic dysfunction in obese mice. Front Cardiovasc Med. (2022) 9 2022:727474. doi: 10.3389/fcvm.2022.727474
35. Zhang, Y, Ye, F, Fu, X, Li, S, Wang, L, Chen, Y, et al. Mitochondrial regulation of macrophages in innate immunity and diverse roles of macrophages during cochlear inflammation. Neurosci Bull. (2024) 40:255. doi: 10.1007/s12264-023-01085-y
36. Quast, K. Literature review on the interaction of oleate with non-sulphide minerals using zeta potential. Miner Eng. (2016) 94:10–20. doi: 10.1016/j.mineng.2016.04.016
37. Wang, L, Qin, X, Liang, J, and Ge, P. Induction of pyroptosis: a promising strategy for cancer treatment. Front Oncol. (2021) 11:635774. doi: 10.3389/fonc.2021.635774
38. Du, L, Yang, L, Yang, C, Dominy, R, Hu, C, Du, H, et al. Investigation of bio-aerosol dispersion in a tunnel-ventilated poultry house. Comput Electron Agric. (2019) 167:105043. doi: 10.1016/j.compag.2019.105043
39. Guanliu, Y, Yao, W, Shouguo, W, Changmin, D, Liangmeng, W, Jing, G, et al. Effects of microbial aerosol in poultry house on meat ducks' immune function. Front Microbiol. (2016) 7:1245. doi: 10.3389/fmicb.2016.01245
40. Salem, E, Hägglund, S, Cassard, H, Corre, T, Näslund, K, Foret, C, et al. Pathogenesis, host innate immune response, and aerosol transmission of influenza D virus in cattle. J Virol. (2019) 93:e01853-18. doi: 10.1128/JVI.01853-18
41. Farrell, CT, Hunter, E, Wilson, PB, and White, SJ. Genomic characterisation of bioaerosols within livestock facilities: a systematic review. Sci Total Environ. (2024) 918:170722. doi: 10.1016/j.scitotenv.2024.170722
42. Tang, Q, Huang, K, Liu, J, Wu, S, and Li, C. Fine particulate matter from pig house induced immune response by activating TLR4/MAPK/NF-κB pathway and NLRP3 inflammasome in alveolar macrophages. Chemosphere. (2019) 236:124373. doi: 10.1016/j.chemosphere.2019.124373
43. Yan, X, Ma, J, Chen, X, Lei, M, Li, T, and Han, Y. Characteristics of airborne bacterial communities and antibiotic resistance genes under different air quality levels. Environ Int. (2022) 161:107127. doi: 10.1016/j.envint.2022.107127
44. Cambra-López, M, Aarnink, AJA, Zhao, Y, Calvet, S, and Torres, AG. Airborne particulate matter from livestock production systems: a review of an air pollution problem. Environ Pollut. (2010) 158:1–17. doi: 10.1016/j.envpol.2009.07.011
45. Ding, L, Zhang, Q, Wang, C, Yao, C, Shan, F, and Li, Q. A clean and health-care-focused way to reduce indoor airborne bacteria in calf house with long-wave ultraviolet. Microorganisms. (2024) 12:1472. doi: 10.3390/microorganisms12071472
46. Glikson, M, Rutherford, S, Simpson, RW, Mitchell, CA, and Yago, A. Microscopic and submicron components of atmospheric particulate matter during high asthma periods in Brisbane, Queensland, Australia. Atmos Environ. (1995) 29:549–62. doi: 10.1016/1352-2310(94)00278-S
47. Tugarova, AV, Mamchenkova, PV, Dyatlova, YA, and Kamnev, AA. FTIR and Raman spectroscopic studies of selenium nanoparticles synthesised by the bacterium Azospirillum thiophilum. Spectrochim Acta A Mol Biomol Spectrosc. (2017):458. doi: 10.1016/j.saa.2017.11.050
48. Dimkpa, CO, Calder, A, Gajjar, P, Merugu, S, Huang, W, Britt, DW, et al. Interaction of silver nanoparticles with an environmentally beneficial bacterium, Pseudomonas chlororaphis. J Hazard Mater. (2011) 188:428–35. doi: 10.1016/j.jhazmat.2011.01.118
49. Li, Q, Dong, F, Dai, Q, Zhang, C, and Yu, L. Surface properties of PM25 calcite fine particulate matter in the presence of same size bacterial cells and exocellular polymeric substances (EPS) of Bacillus mucitaginosus. Environ Sci Pollut Res Int. (2018) 25:22429–36. doi: 10.1007/s11356-017-0829-x
50. Lay, ML, Wu, HM, and Huang, CH. Study of the zeta potential of Fe (O) OH colloids. J Mater Sci. (1995) 30:5473–8. doi: 10.1007/BF00351560
51. Tretina, K, Park, ES, Maminska, A, and MacMicking, JD. Interferon-induced guanylate-binding proteins: guardians of host defense in health and disease. J Exp Med. (2019) 216:482–500. doi: 10.1084/jem.20182031
52. Wang, Y, Zhong, Y, Liao, J, and Wang, G. PM2.5-related cell death patterns. Int J Med Sci. (2021) 18:1024–9. doi: 10.7150/ijms.46421
53. Bergsbaken, T, Fink, SL, and Cookson, BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. (2009) 7:99–109. doi: 10.1038/nrmicro2070
54. Zhang, H, Du, Y, Guo, Y, Wang, Z, Li, H, Lv, Z, et al. TLR4-NLRP3-GSDMD-mediated pyroptosis plays an important role in aggravated liver injury of CD38−/− sepsis mice. J Immunol Res. (2021) 2021:1–15. doi: 10.1155/2021/6687555
55. Dickinson, MS, Kutsch, M, Sistemich, L, Hernandez, D, Piro, AS, Needham, D, et al. LPS-aggregating proteins GBP1 and GBP2 are each sufficient to enhance caspase-4 activation both in cellulo and in vitro. Proc Natl Acad Sci USA. (2023) 120:e2216028120. doi: 10.1073/pnas.2216028120
56. Ma, J, Liu, R, Wang, X, Liu, Q, Chen, Y, Valle, RP, et al. Crucial role of lateral size for graphene oxide in activating macrophages and stimulating pro-inflammatory responses in cells and animals. ACS Nano. (2015) 9:10498–515. doi: 10.1021/acsnano.5b04751
Keywords: the animal farm environment, PM2.5, Pasteurella multocida , respiratory injury, GBP2, NLRP3
Citation: Du X, Ma Z, Sun Y, Jia Y, Zhang X, Zhao C, Liang X, Yu X and Gao Y (2025) Interactions between particulate matter and bacteria during cowshed PM2.5-induced respiratory injury initiates GBP2/Caspase-11/NLRP3-mediated intracellular bacterial defense and pyroptosis. Front. Vet. Sci. 12:1631913. doi: 10.3389/fvets.2025.1631913
Edited by:
Hong Chen, Luoyang Normal University, ChinaReviewed by:
Yinbao Wu, South China Agricultural University, ChinaZhiping Zhu, Chinese Academy of Agricultural Sciences, China
Chong Wang, China Agricultural University, China
Copyright © 2025 Du, Ma, Sun, Jia, Zhang, Zhao, Liang, Yu and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunhang Gao, Z2FveXVuaGFuZ0AxNjMuY29t; Xiuzhen Yu, eXh6c2h6QDEyNi5jb20=
 Xiaohui Du1
Xiaohui Du1 Yunhang Gao
Yunhang Gao