- 1Liaocheng Research Institute of Donkey High-Efficiency Breeding and Ecological Feeding, Liaocheng University, Liaocheng, China
- 2Department of Breeding and Genetics, Cholistan University of Veterinary and Animal Sciences, Bahawalpur, Pakistan
Intense neuroendocrine and molecular pathways with environmental sensitivity maintain reproductive efficiency in seasonal breeders, together with donkeys. The hypothalamic–pituitary-gonadal (HPG) axis functions as a primary controller through modifying gonadotropin-releasing hormone (GnRH) secretion that depends on melatonin levels, which induces photoperiodic instructions to the system. The activation of HPG axis is triggered by decreasing melatonin levels during long-day seasons, yet sustained high levels of melatonin during short-day seasons cause its suppression. The reproductive pulsatility of GnRH depends on kisspeptin-neurokinin B-dynorphin (KNDy) neurons, which are controlled by melatonin through activity regulation to produce seasonal reproductive suppression. Reproductive ability depends on metabolic signaling, which connects nutrient availability to gonadal functions to maintain fertility during optimum nutritional status. Studies have demonstrated that oxidative stress is a primary disruptor of reproductive functions as it produces gonadal cell damage while stopping steroid synthesis and increasing cell death. Endocrine-disrupting chemicals (EDCs) cause additional reproductive problems through interfering with steroidogenic enzymes, which results in hormonal imbalance and infertility. Prolactin works in association with gonadotropins and metabolic pathways to control reproductive adaptations under seasonal variation. Understanding of molecular mechanisms is essential for increasing reproductive success among donkeys and other seasonal breeders in general. The breeding programs might benefit from solutions such as photoperiod manipulation and melatonin treatments, together with nutritional supplementation and antioxidant therapies. The review focuses on seasonal reproductive processes, endocrinology, assisted reproductive technologies (ARTs), and peculiarities of anatomy and behavior. Discoveries in sperm vitrification, testicular immunology, metabolic endocrinology, and follicular dynamics give important clues to fertility manipulation in this species and suggest interventions to be pursued to enhance fertility outcomes and conservation approaches.
Introduction
Donkeys are long-lived polyestrous equids that are vital to livelihoods and biodiversity. The reproductive efficiency of farm mammals, including donkeys, is affected by seasonal variations, which subsequently impacts production traits by affecting milk yield together with meat quality and reproductive outcomes (1). Some mammals such as Cattle exhibit regular reproductive cyclicity throughout the year, yet sheep, together with goats, horses, and donkeys, demonstrate seasonal breeding cycles with peak births in late stages and early spring to maximize offspring survival (2). The reproduction cycles of these species function through neuroendocrine systems that control the frequency of ovulation along with spermatogenic activity, gamete quality, and sexual behavior (3). The regulation of seasonal reproduction depends on two main factors: natural endogenous circannual rhythms and external photoperiod signals, which the pineal gland, mediating melatonin secretion, controls (4). External signals adjust hypothalamic–pituitary-gonadal (HPG) axis functioning so the reproductive cycles undergo major neuroendocrine alterations (5). Although donkeys are distributed worldwide, the reproductive inefficiency of reproduction, especially due to seasonality and metabolic-endocrine interactions, restricts their productivity and conservation (6).
Artificial breeding programs use photoperiodic manipulation through external daylight exposure for mares, sheep, and goats, together with melatonin supplementation specifically for sheep and goats to achieve seasonal reproductive synchronization as well as seasonal reproductive control (7). The interventions alongside genetic selection programs focus on maximizing reproductive performance within controlled breeding programs (8).
Donkeys are long-day breeders as the estrous cycle is more regular and pronounced during long-day periods (9). The reproductive activity peaks in spring and summer, while it is reduced or exhibits anestrus in autumn and winter (10). Almost every reproductive parameter of donkeys varies with seasonal variability, like foaling rate, which is higher in warmer months due to increased mating success and favorable conditions, improved semen motility and concentration during spring and early summer (11).
Donkey reproductive patterns respond to various molecular systems that combine hormones with energy homeostasis and natural environmental stimuli, including light duration, weather, and diet quality (9, 11). Unlike horses, donkeys have a distinctive reproductive physiology that requires species-specific investigations and molecular treatments (12). The purpose of this review is to summarize what is known about donkey sexual biology and suggest biologically realistic ways to improve fertility.
Hypothalamic–pituitary-gonadal axis dysregulation
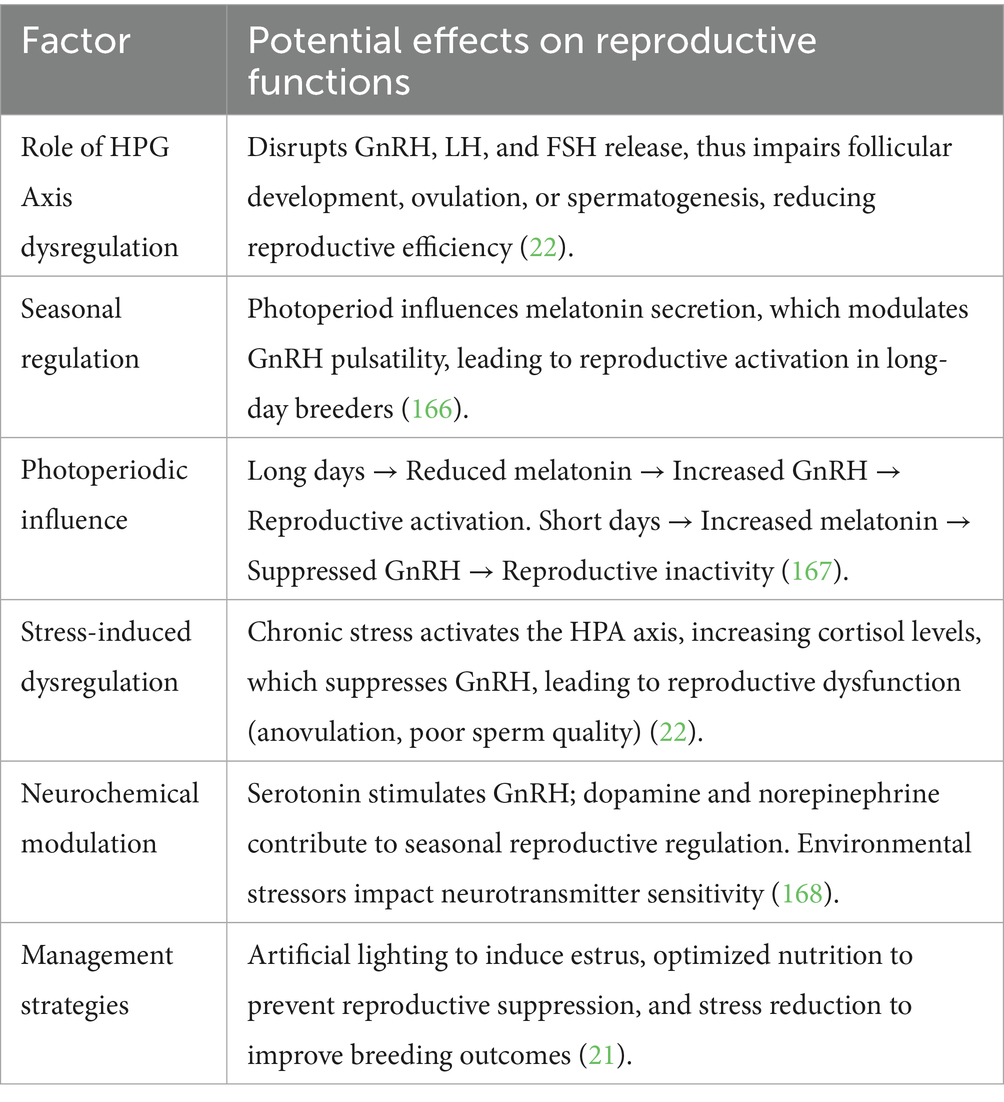
The hypothalamic–pituitary-gonadal (HPG) axis functions as the primary mechanism to regulate reproductive functions among all mammals, including the donkey, although it operates as a seasonal breeder (11). Through this axis, the hypothalamus produces gonadotropin-releasing hormone (GnRH) in pulsatile patterns that trigger the anterior pituitary to release both luteinizing hormone (LH) and follicle-stimulating hormone (FSH) (13). The gonadotropins exercise their effects on the gonads to control gametogenesis as well as hormone synthesis, where males produce testosterone and females produce estrogen and progesterone (14).
Seasonal regulation of the HPG axis
Seasonal breeders closely link their reproductive efficiency to environmental cues that mainly include photoperiod (day length), temperature, and nutritional status (15). The pineal gland produces melatonin as a response to dark conditions, which controls the seasonal pattern of GnRH secretion (16). From winter months’ short-day periods, melatonin secretion extends over time until it suppresses GnRH release, which reduces LH and FSH secretion and causes reproductive inactivity (17). The reduction of melatonin during long-day periods results in the reactivation of GnRH pulsatile action and restores reproductive capacity (18).
Relational dynamics of the HPG axis are most prominent in mares together with sheep, goats, and donkeys because their breeding patterns match photoperiod modifications of melatonin release (16). Donkeys share the reproductive pattern of horses by being long-day breeders, and their breeding season occurs during spring and summer when day length expands (11). The natural birth cycle results in foal births when environmental conditions offer the best resources.
Stress-induced dysregulation of the HPG axis
The hypothalamic–pituitary–adrenal (HPA) axis that controls stress responses creates a feedback mechanism with the HPG axis. Between chronic stress and HPA axis activation arises the production of corticotropin-releasing hormone (CRH) and adrenocorticotropic hormone (ACTH) that stimulate cortisol production from adrenal glands (19). The release of GnRH diminishes when cortisol levels increase, which subsequently decreases LH and FSH production, thus leading to reproductive system suppression.
The release of GnRH decreases when cortisol levels increase, which subsequently reduces LH and FSH production, thereby leading to suppression of the reproductive system (20). The reproductive system of female seasonal breeders shows adverse effects from chronic stress because this results in anovulation together with irregular estrous cycles and reduced estrogen production, which frequently causes ovarian dysfunction and persistent follicles or ovarian cysts (21). The prolonged exposure to stress in male individuals decreases testosterone levels along with spermatogenesis and causes sperm quality to decline while diminishing sexual desire, so fertility remains impaired in breeding periods (22).
The neurochemical agents serotonin (5-HT), along with dopamine and norepinephrine (NE), function as vital elements for controlling HPG axis responses under stress conditions (23). GnRH release receives stimulation from serotonin, although the changing sensitivity of serotonin receptors during seasonal periods may contribute to reproductive suppression caused by environmental stressors like nutritional deficiencies, changes in social standing, and climate patterns (24).
Metabolic and nutritional effects on the HPG axis
The HPG axis operates under significant control from energy balance regulation. Reproductive function regulation occurs through the AMPK-mTOR signaling pathway that detects energy levels by influencing GnRH neurons (19). AMPK activation stops GnRH secretion to cause reproductive dormancy when the body faces nutritional hardships or negative energy conditions (such as winter months). The HPG axis receives a signal from adequate energy storage to activate mTOR signaling, which then triggers GnRH release and increases reproductive capability (25).
The reproductive hormone regulation of donkeys that experience seasonality depends on their body condition changes and how much they eat between seasons (26). Studies performed on mares and sheep proved that minimal body fat, together with low leptin levels, restrict normal GnRH signal pulsing which causes breeding season delays. The HPG axis becomes fully active once the nutritional condition improves, thus breeding occurs at the most appropriate time for the environment (27). The molecular pathway Hypothalamic–Pituitary-Gonadal (HPG) Axis Dysregulation is shown in Figure 1.
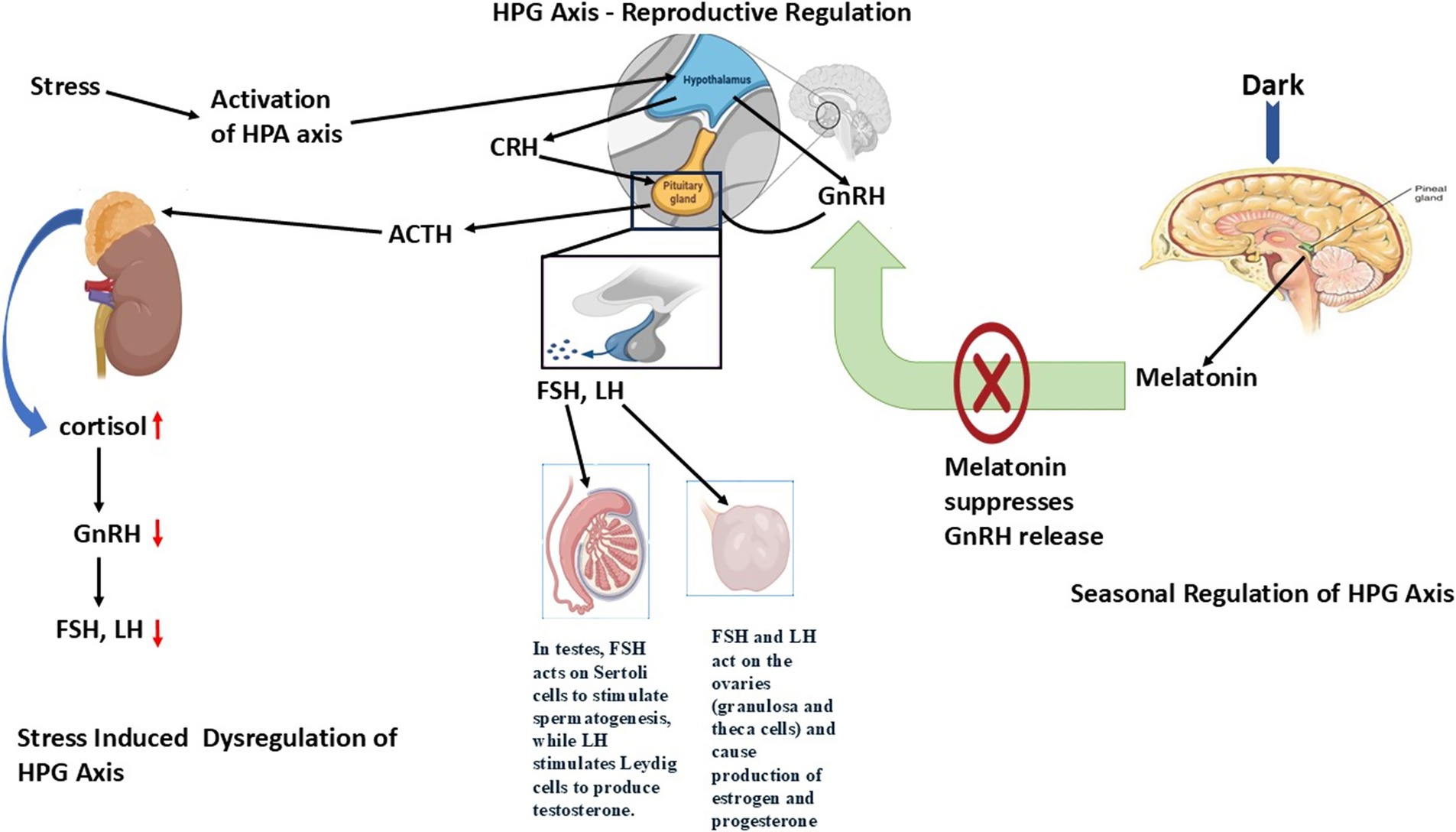
Figure 1. Role of hypothalamic–pituitary-gonadal (HPG) Axis dysregulation in regulating reproduction in seasonal breeders. The HPG axis is a hormonal system involving the hypothalamus, pituitary gland, and gonads that controls reproduction through GnRH, LH/FSH, and sex steroids. Dysregulation occurs due to stress, malnutrition, endocrine disruptors, or seasonal changes, leading to impaired hormone secretion, disrupted gametogenesis, and reduced fertility.
There is a different gonadotropin secretion pattern in donkeys. Jennies exhibit two FSH peaks during one estrous cycle and a long-lasting LH surge that frequently continues after ovulation (28). This differs from the single peak of FSH and closely timed LH surge in mares and ewes. These hormonal patterns can provide distinct follicular and luteal sustainability processes in donkeys (29).
An impressive molecular difference is seen in the ligand specificity of the FSH receptor (FSHR). Cloned FSHRs in donkeys can to bind FSH and LH/chorionic gonadotropin (CG) in a ligand promiscuous manner, which has not been observed in horses or sheep (30, 31). This is due to differences in amino acids of the extracellular domain of the receptor (~96% homology with equine FSHR). The physiological significance of such receptor flexibility on follicular development has yet to be understood (32).
LH and eCG bioactivity
Equine chorionic gonadotropin (eCG) is LH-like, as well as FSH-like, in non-equines (33). Nevertheless, in donkeys, LH and CG are largely LH-active with little FSH-like activity in in vitro assays. This also emphasizes species-specific hormone-receptor interactions that may have effects on ovulatory regulation and folliculogenesis (29).
Implications of HPG axis for seasonal breeding management
The mechanism through the HPG axis functions in seasonal breeders complicates the management of reproduction and breeding operations. The following potential strategies can be used to improve reproductive efficiency in seasonal breeders (34):
1. Artificial lighting techniques that replicate long-day conditions can be employed to induce early estrus in mares and donkeys, thus increasing their reproductive effectiveness.
2. The body condition managed properly during the pre-breeding period reduces the impact of seasonal reproductive suppression.
3. The outcome of fertility improves when stress levels decrease through reducing environmental and social pressure factors.
The reproductive system of donkeys, alongside other seasonal breeders, controls the HPG axis through interactions between photoperiod signals, metabolic indicators, and stress responses (35). The reproductive efficiency of animals becomes compromised when the stress axis becomes nonfunctioning due to chronic stress combined with poor nutritional status, along with unsuitable environmental elements (36). Understanding seasonal regulatory processes better will allow scientists to create specific measures that enhance the breeding performance of seasonal species. The summary of the molecular pathway is shown in Table 1.
Melatonin signaling pathway (photoperiodic regulation)
In seasonal breeders, the information on photoperiod is encoded in the melatonin secretion of the pineal gland, which regulates the hypothalamic–pituitary-gonadal (HPG) axis (12). In sheep, melatonin can influence the pituitary pars tuberalis MT1 and MT2 receptors in the pars tuberalis to modulate TSH and downstream thyroid hormones, which then modulate kisspeptin and GnRH release (37). It is the same with horses, which express melatonin receptors in the hypothalamus, pituitary, and ovary (38).
Comparatively, there is no information on the expression and signaling of melatonin receptors in donkeys. The submissive photoperiodic reactivity of the species and the lack of clear seasonal patterns indicate that melatonin transduction may be distorted or an alternative environmental signaling may be used (39).
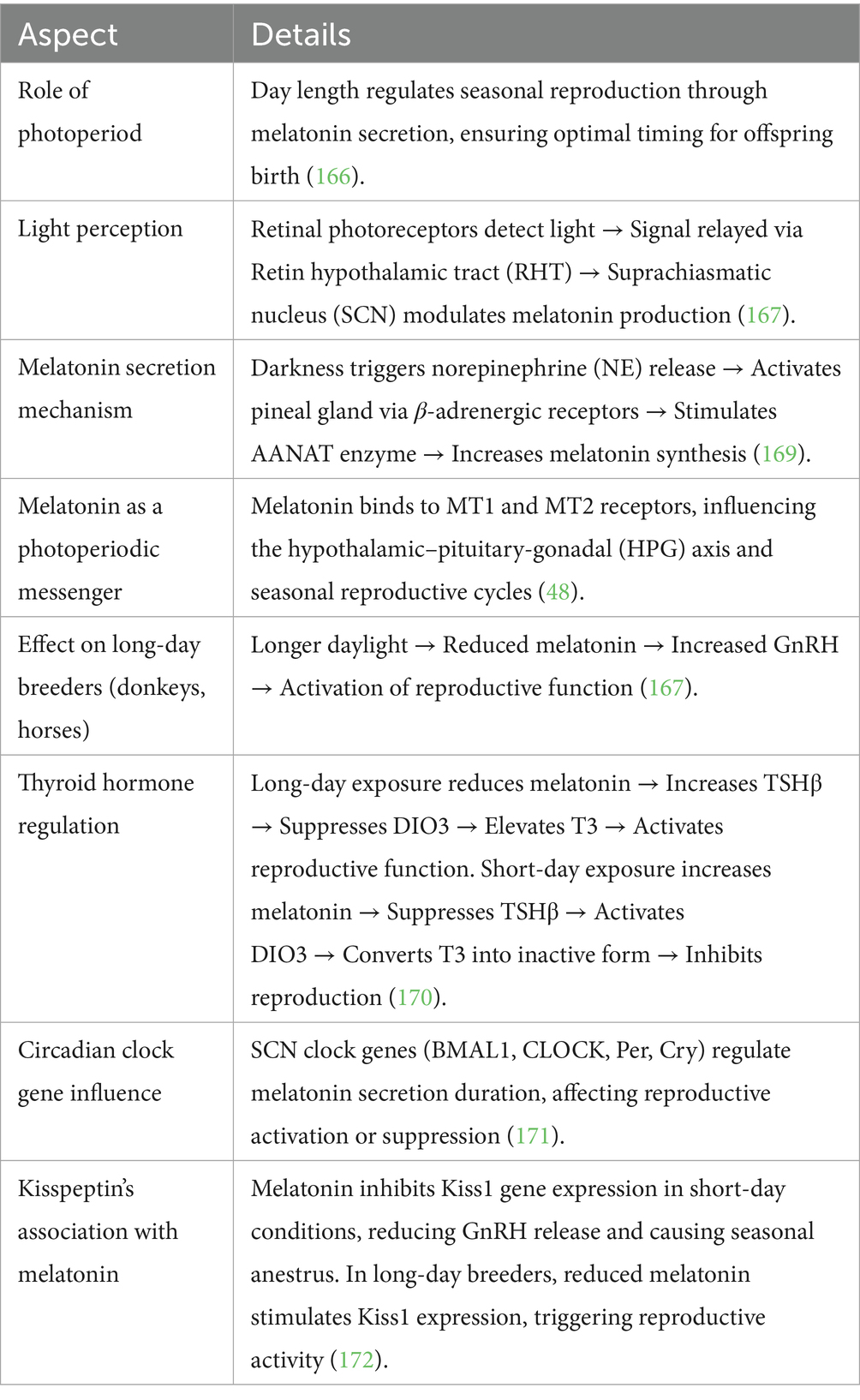
Role of photoperiod in seasonal reproduction
The reproductive patterns of seasonal breeders are controlled through environmental signals that primarily include changes in day length (photoperiod) (40). The adaptation brings about offspring births in optimal times, which usually matches the spring season when environmental factors create favorable conditions for survival. The main regulator for this process functions through melatonin, which the pineal gland produces because of daily darkness. Through its neuroendocrine role, melatonin carries photoperiodic data, which subsequently affects reproductive hormone release (41).
Horses, together with donkeys, show suppressed melatonin production when daylight stretches out, which activates their gonads (42). Short-day breeders like sheep and goats activate reproduction during periods when their nightly melatonin hormone production reaches higher levels. Knowledge of the melatonin signaling pathway stands vital for determining the seasonal mechanisms thatdonkey reproductive effectiveness (43).
Mechanism of mammalian seasonal reproduction
Light perception and transmission to the pineal gland
Relying on the retina for light detection stands as the main photoreceptor mechanism for mammals since birds use deep-brain photoreceptors (44). The ipRGCs inside the retina carry melanopsin photopigment as they detect light exposure through their intrinsic photosensitive function. The photic signals travel through the retinohypothalamic tract (RHT) until they reach the suprachiasmatic nucleus (SCN) of the hypothalamus, which acts as the central circadian pacemaker (45). Light information from the SCN passes through the PVN and IML section of the spinal cord before reaching the SCG, which makes its way to the pineal gland (46).
When light stimulation ends in darkness, the SCN exhibits reduced activity, while norepinephrine (NE) produced in the SCG activates β-adrenergic receptors in pinealocytes through these receptors. The successive neural events increase the activity of arylalkylamine N-acetyltransferase (AANAT), which results in nighttime melatonin production (47).
Melatonin as a photoperiodic messenger
The secretion of melatonin follows a daily cycle of 24 h, where the hormone remains in the body for an amount equivalent to the duration. It connects to MT1 and MT2 melatonin receptors, which exist mainly inside the pars tuberalis (PT) from the pituitary gland as well as the hypothalamus (48). The MT1 receptor functions as the primary photoperiodic information transmitter within seasonal breeders because it displays high expression levels in these animals (49).
The secretion of melatonin decreases in donkeys and horses when the photoperiod lasts longer, which activates the hypothalamic–pituitary-gonadal (HPG) axis to increase gonadotropin-releasing hormone (GnRH) release (50). The regulatory hormone melatonin creates positive effects on gonadal activity through thyroid hormone regulation of the mediobasal hypothalamus (MBH) during extended exposure durations in short-day breeders like sheep and goats (51).
Thyroid hormone regulation in seasonal breeders
The regulation of seasonal reproduction by melatonin occurs mainly through modifications in thyroid hormone metabolic patterns. The posterior pituitary (PT), a region of the pituitary gland, plays a crucial role in this process (52).
The reduction of melatonin activates thyroid-stimulating hormone (TSHβ) expression in the PT under long-day stimulus conditions. The activity of type 2 deiodinase (DIO2) is downregulated, resulting in the hypothalamus producing less triiodothyronine (T3). The stimulation of the reproductive axis occurs because of this process, and it advances both follicular development and spermatogenesis (53).
The extended exposure to melatonin stimulates TSHβ expression in the PT, which activates DIO3 to convert T3 into inactive reverse T3 through its enzymatic activity. The hormone GnRH becomes suppressed, which prevents the release of reproductive signals during seasonal anestrous periods (54).
Molecular mechanisms involved in melatonin signaling
Role of circadian clock genes
The SCN acts as a circadian oscillator that manages melatonin production by controlling the expression of BMAL1 and CLOCK together with Period (Per1, Per2) and Cryptochrome (Cry1, Cry2). All these genes create a transcription-translation feedback loop that controls the length of melatonin production based on photoperiod (55).
Long-day conditions cause changes in the phase relationships of SCN neurons, which affect clock gene expression patterns and decrease melatonin production levels. The expression patterns of clock genes under short-day conditions extend melatonin production, that results in reproductive inhibition in donkeys long-day breeders (56).
Kisspeptin, originating from the Kiss1 gene, operates as a strong activator of GnRH release. The arcuate nucleus of the hypothalamus experiences decreased Kiss1 expression because of melatonin effects, which results in reproductive inactivity (57).
The reduction of melatonin levels in donkeys with long-day breeding patterns stimulates Kiss1 gene expression to trigger the activation of GnRH along with gonadotropins that initiate reproductive functions. Sheep display seasonal anestrus by having melatonin suppress Kiss1 expression, which prevents the release of GnRH (58).
Role of RFamide-related peptides
The mammalian ortholog of gonadotropin-inhibitory hormone (GnIH) is RFamide-related peptide-3 (RFRP-3), which controls the activity of the HPG axis by suppression. The secretion of RFRP-3 shows both melatonin-regulated patterns and species-specific responses toward GnRH release (59). Sheep experience seasonal anestrus because RFRP-3 reduces GnRH secretion in their system. Short-day conditions stimulate RFRP-3 to boost GnRH secretion in the brains of hamsters, that helps the reproductive system to function normally (60). Researchers have not confirmed the function of RFRP-3 in donkeys, even though its relationship to Kisspeptin and thyroid hormone regulation might help understand seasonal reproductive patterns (61).
Species-specific photoperiodic mechanisms
In sheep and horses, the melatonin TSH thyroid kisspeptin GnRH cascade regulates photoperiodic reproduction tightly (62). Donkeys might not be fully involved in this axis. Their reproduction physiology seems to be less responsive to a change of daylight, which suggests that they depend on other stimuli like dietary conditions, temperature, or socialization (63). This hypothesis has not been molecularly confirmed because there is a lack of neuroendocrine mapping.
Applications in reproductive management of seasonal breeders
The examination of the melatonin signaling pathway enabled researchers to create methods that control reproductive patterns in donkeys along with other seasonal breeders (64).
1. Strategies involving artificial lighting can halt the production of melatonin, which leads to the acceleration of the breeding period. The technique is applied most frequently in equine breeding operations.
2. The administration of exogenous melatonin through implants provides a treatment that can halt reproduction while improving breeding seasons in animals with distinct seasonal cycle patterns.
3. Genetic Selection based on changing the photoperiodic responses has the potential to improve breeding outcomes of donkeys during unfavorable seasonal periods (Figure 2).
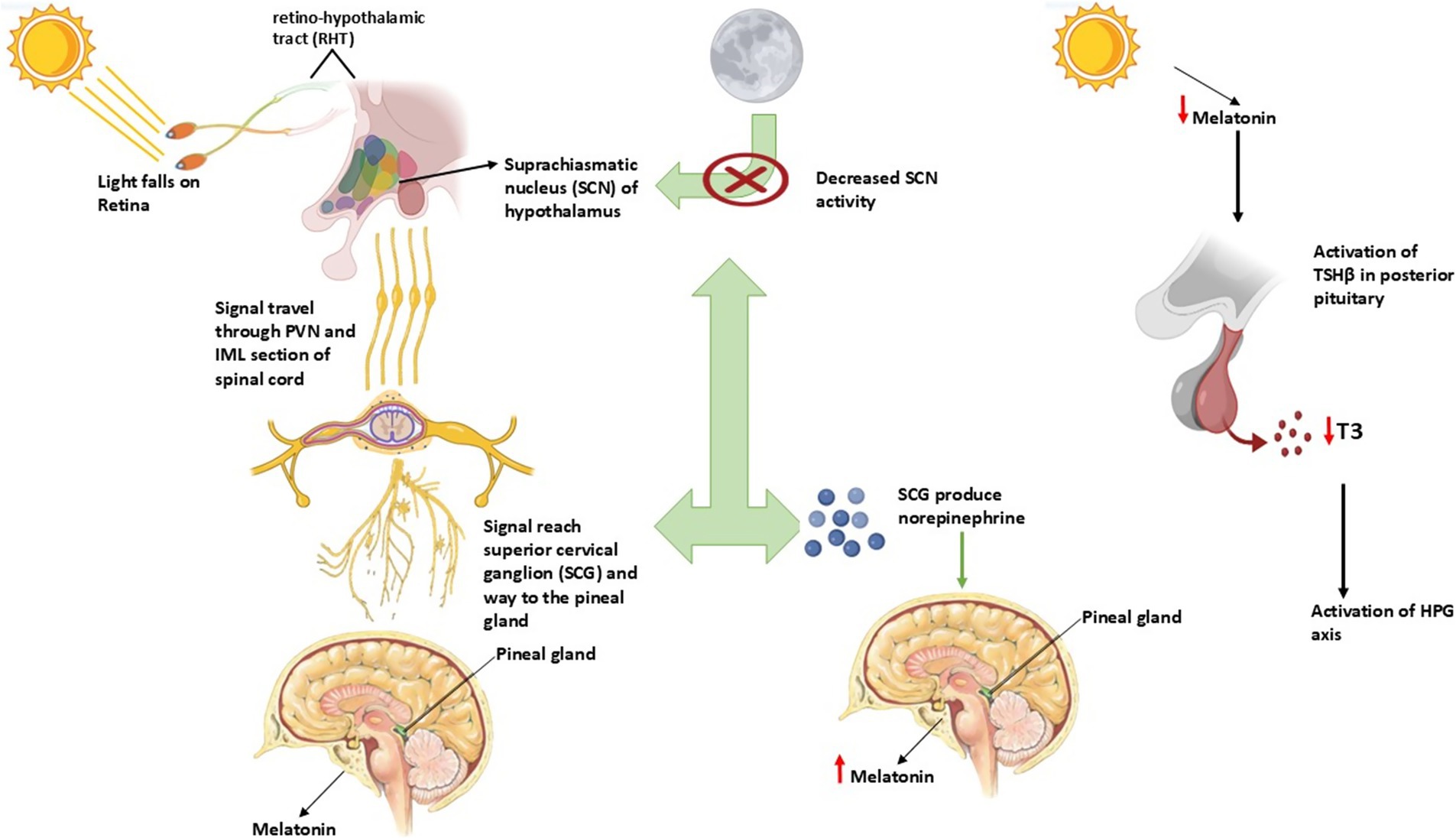
Figure 2. Role of photoperiod and its association with melatonin affecting reproduction in seasonal breeders.
The reproductive efficiency of seasonal breeders, including donkeys heavily depends on the functioning of their melatonin signaling pathway. The neuroendocrine transducer function of melatonin depends on its ability to process photoperiodic cues with the SCN while working with PT and thyroid hormones alongside Kisspeptin and RFamide-related peptides (65). Knowledge advancement regarding these mechanisms enables developers to create successful breeding management strategies that improve reproductive success for donkeys and other seasonal breeders (66). The summary of the molecular pathway is shown in Table 2.
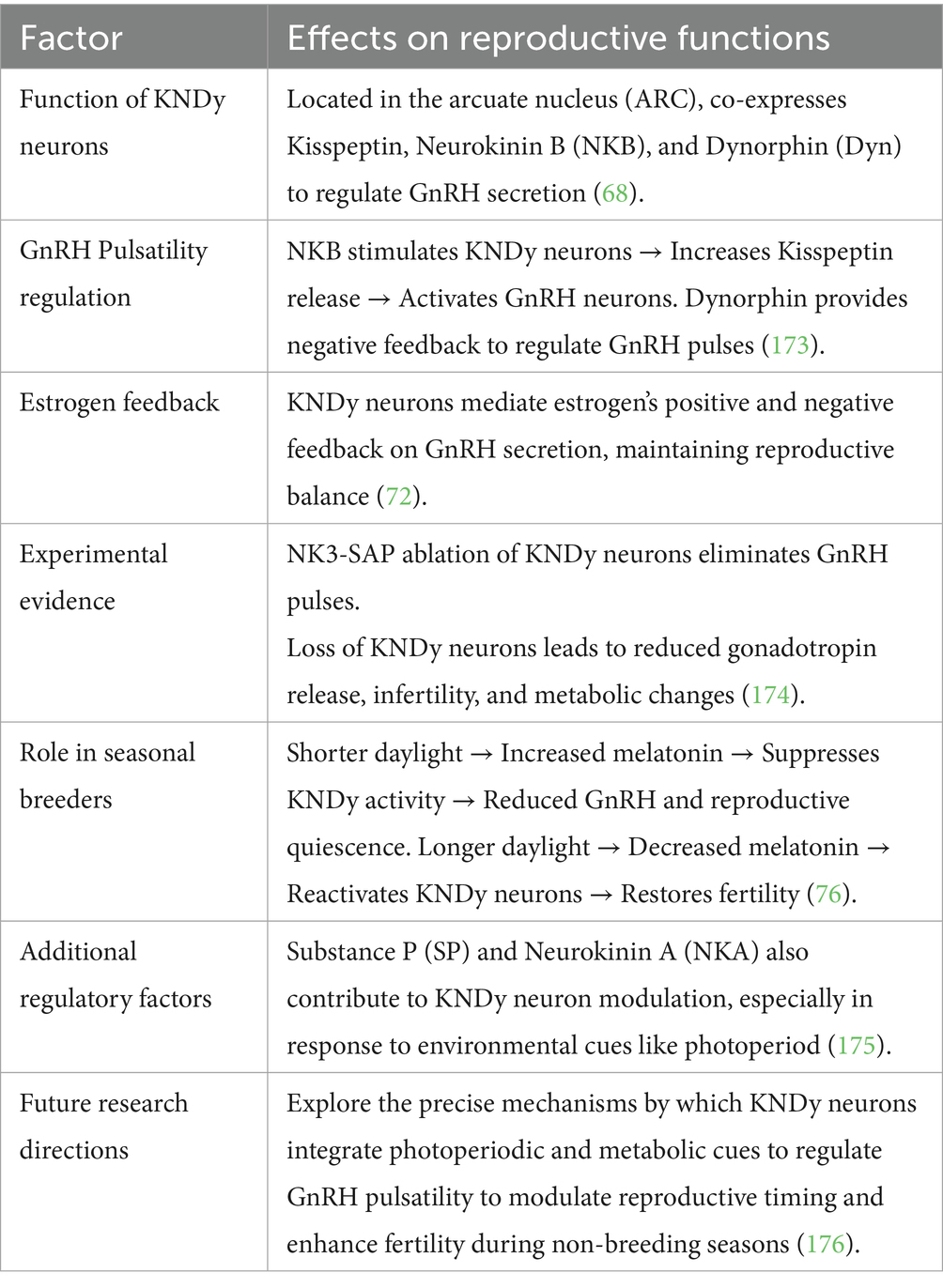
Kisspeptin-neurokinin B-dynorphin neuron regulation
A major role in gonadotropin-releasing hormone (GnRH) secretion regulation belongs to the KNDy neuron system, which resides in the hypothalamic arcuate nucleus (ARC) (67). These neurons co-express three key neuropeptides: kisspeptin, neurokinin B (NKB), and dynorphin (Dyn). Kisspeptin functions as a powerful stimulant for GnRH release through the KNDy, neurons yet NKB activates the KNDy neurons at the same time Dyn plays an inhibitory role to maintain reproductive hormone balance (68).
Kisspeptin neurons play an important role in being upstream controllers of GnRH. Their expression is photoperiod-dependent and has been mapped in sheep and horses, where they gate seasonal activation of reproductive activity (69). The axis seems to be intact in donkeys: a kisspeptin analog (C6 peptide) can trigger ovulation and LH surges. The distribution, the density, and the photoperiodic control of kisspeptin neurons are however, still unknown, and this restricts us to comprehend its complete role in the reproductive physiology of the donkey (70, 71).
Mechanism of KNDy neuron function
GnRH pulsatility regulation
The reproductive axis functions properly because GnRH secretion exists as pulsatile signals. The mechanical pulsations of neural signals depend on KNDy neurons through an auto-regulatory feedback mechanism (68). Neurokinin B (NKB) activates KNDy neurons through its stimulating effect, which produces more kisspeptin release. The direct activation of GnRH neurons by Kisspeptin results in elevated levels of GnRH hormone that is released into the bloodstream (72). The neurochemical activity of dynorphin creates negative feedback that limits KNDy neuron function for controlling GnRH release during required periods. The complex regulatory system maintains the correct timing of GnRH secretion because it functions as a crucial factor for reproductive health (73).
Experimental evidence of KNDy neuron function
• The GnRH pulse generator disappears permanently when scientists use NK3-SAP to destroy KNDy neurons, proving these cells hold the essential position for reproductive regulation (74).
• Studies involving ablating KNDy neurons establish that their destruction results in reduced gonadotropin release, infertility and body weight alterations (75).
KNDy neurons in seasonal breeders
• Donkeys belong to the seasonal breeders whose reproductive functions are controlled through seasonal photoperiod changes (11).
• The reduction of GnRH secretion and reproductive quiescence occurs because shorter daylight hours trigger melatonin secretion, which downregulates KNDy neuron activity (68).
• The reduction of melatonin secretion during breeding seasons enables KNDy neurons to become active once more, which results in fertility (76).
Additional regulatory factors
• The three main regulators that control KNDy neuron function are kisspeptin, together with NKB and Dyn, but SP (Substance P) and NKA (Neurokinin A) may also contribute to this modulation (77).
• Additional research about alternative regulatory mechanisms of GnRH secretion that bypass kisspeptin pathways should be conducted (76) (Figure 3).
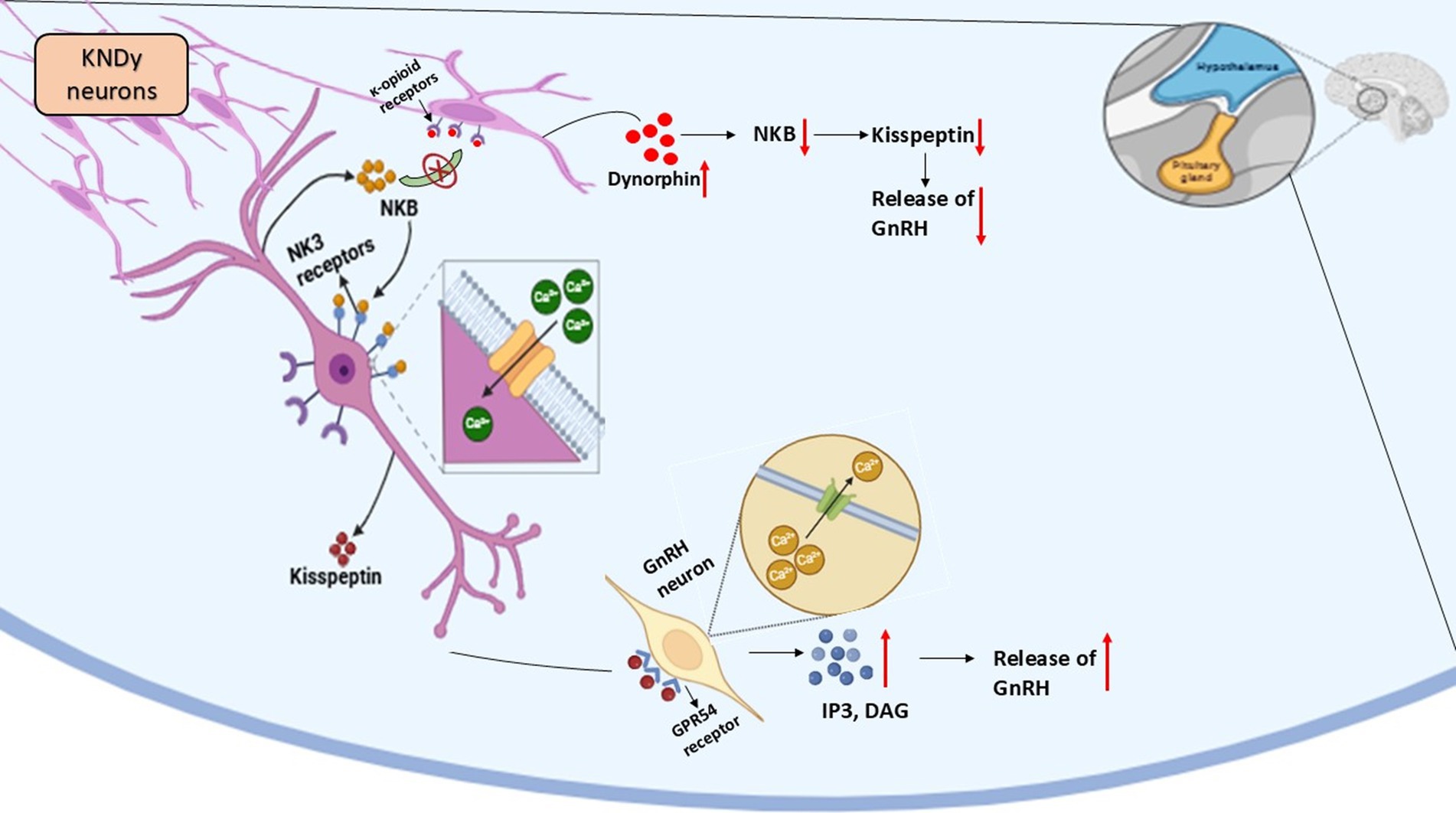
Figure 3. Kisspeptin-neurokinin B-Dynorphin (KNDy) neuronal regulation of reproduction in seasonal breeders.
The summary of the molecular pathway is shown in Table 3.
AMPK-MTOR energy sensing pathway (nutritional effect on reproduction)
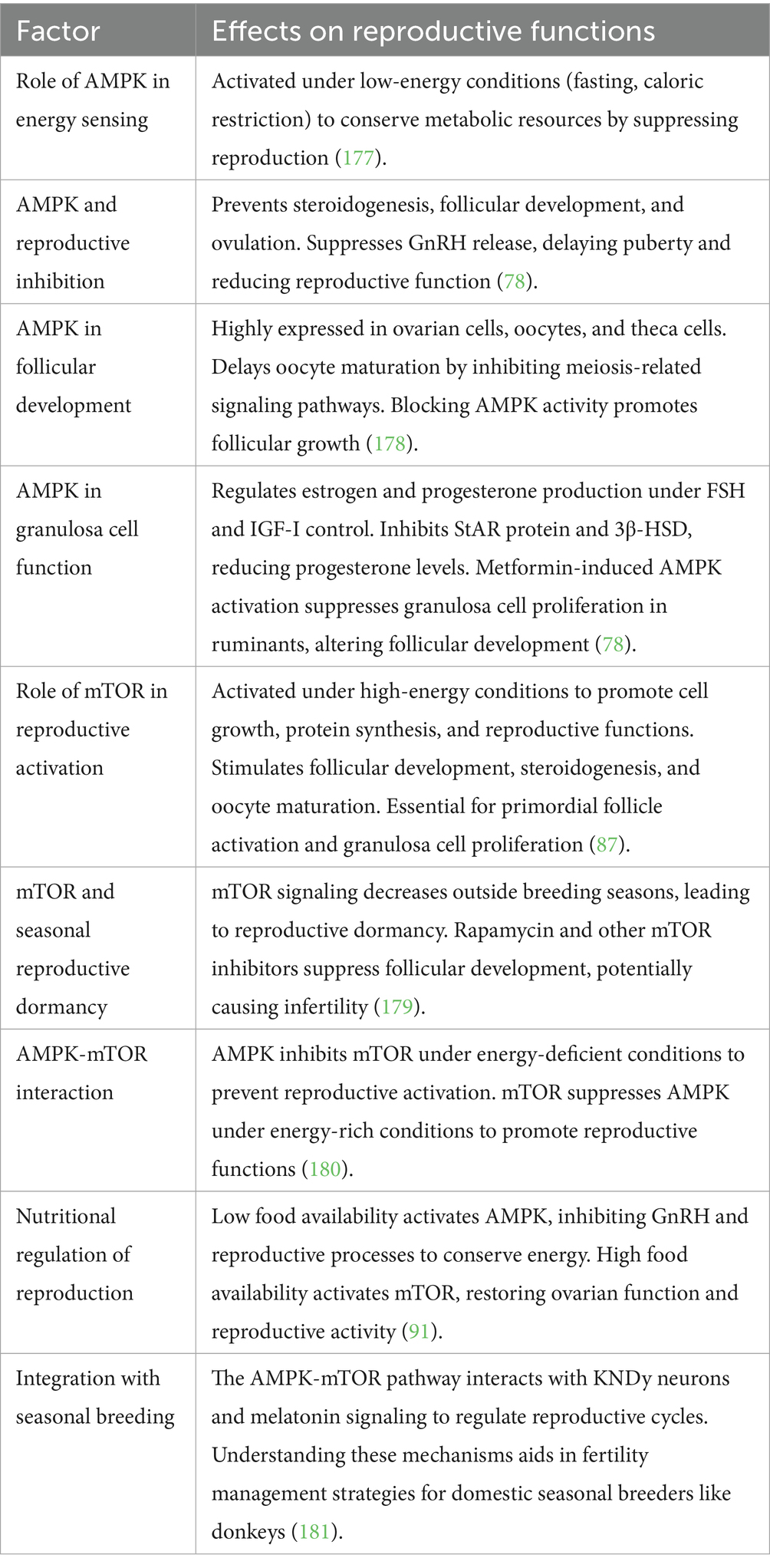
Organisms need high amounts of energy to produce offspring, and metabolic conditions control reproductive regulatory mechanisms. The AMP-activated protein kinase (AMPK), together with the mammalian target of rapamycin (mTOR), constitutes important cellular energy sensors regulate reproductive function by sensing metabolic signals (78).
AMPK: the energy sensor in reproductive regulation
Activation and function
• The enzyme AMPK starts its operation when energy supplies fall low (such as during fasting or periods of caloric restriction) to save fuel (79).
• The energy-saving process includes steroidogenesis, follicular development, and ovulation, which AMPK prevents during times of low energy (80).
• The activation of AMPK leads to the prevention of GnRH release, which results in delayed puberty and deficient reproductive function (78).
AMPK in follicular development
• Healthy Ovarian cells, together with oocytes and theca cells, express high levels of AMPK protein (81).
• The maturation of oocytes becomes delayed through AMPK activation because it blocks signaling pathways required for meiosis (82).
• Research findings demonstrate that blocking AMPK activity helps follicles grow, which implies that activated AMPK controls reproductive function as an energy deficit regulator (83).
AMPK in granulosa cell function and hormone secretion
• Under FSH and IGF-I regulation, granulosa cells create both estrogen and progesterone compounds (84).
• Through activation of AMPK, the production of progesterone decreases by preventing the function of steroidogenic acute regulatory (StAR) protein and 3β-hydroxysteroid dehydrogenase (3β-HSD) (78).
• The activation of AMPK by metformin treatment results in suppressed granulosa cell proliferation in ruminant cattle, which leads to modifications in follicular development (85).
mTOR: the energy sensor for reproductive activation
Under high-energy conditions, mTOR acts as a crucial controller of reproductive function while managing cell growth together with protein synthesis and reproductive abilities (86). The activation of mTOR leads to cellular growth, protein synthesis, and reproductive functions through stimulation of follicular development, steroid hormone production, and oocyte maturation. The ovarian system requires mTOR activation to activate primordial follicles and stimulate granulosa cell growth, together with ovulation (87). The medication rapamycin, together with other mTOR inhibitors, blocks follicular development, which can result in infertility. The research on seasonal breeders has established that mTOR signaling decreases in periods outside breeding seasons, which results in reproductive dormancy (88).
Reproductive function requires the oppositional regulatory mechanism between mTOR and AMPK, which interact with each other (89). Under situations of energy deficiency, AMPK becomes active, thus it blocks mTOR signaling to reduce reproductive processes for metabolic energy conservation (90). The reproductive process gets activated through mTOR signaling, while energy-rich conditions lead to AMPK suppression. The AMPK, together with mTOR, works in a balanced opposition to maintain reproductive outcomes based on metabolic health status (91).
Essential for determining reproductive cycles in donkeys and other seasonal breeding species is the metabolic regulation mechanism (78). Low food availability leads to AMPK activation, which inhibits GnRH secretion along with reproductive functions, thus stopping the expenditure of energy for reproduction. An increase in food availability results in mTOR activation, which leads to ovarian function, thereby allowing reproduction to occur only in favorable metabolic situations. Seasonal breeding creatures use nutrition-dependent reproductive regulation to maximize their reproductive performance (92).
The KNDy neuron system together with the AMPK-mTOR pathway acts as an important regulatory mechanism for reproductive efficiency in seasonal breeding animals (93). GnRH pulsatility depends on signals from KNDy neurons, which receive photoperiod information through melatonin signaling along with the AMPK-mTOR pathway acting as a metabolic control mechanism for reproduction under sufficient energy conditions. Knowledge about these pathways reveals crucial information about seasonal reproductive control, thus offering possibilities to develop fertility enhancement practices for domestic animals (79). The summary of the molecular pathway is shown in Table 4 (Figure 4).
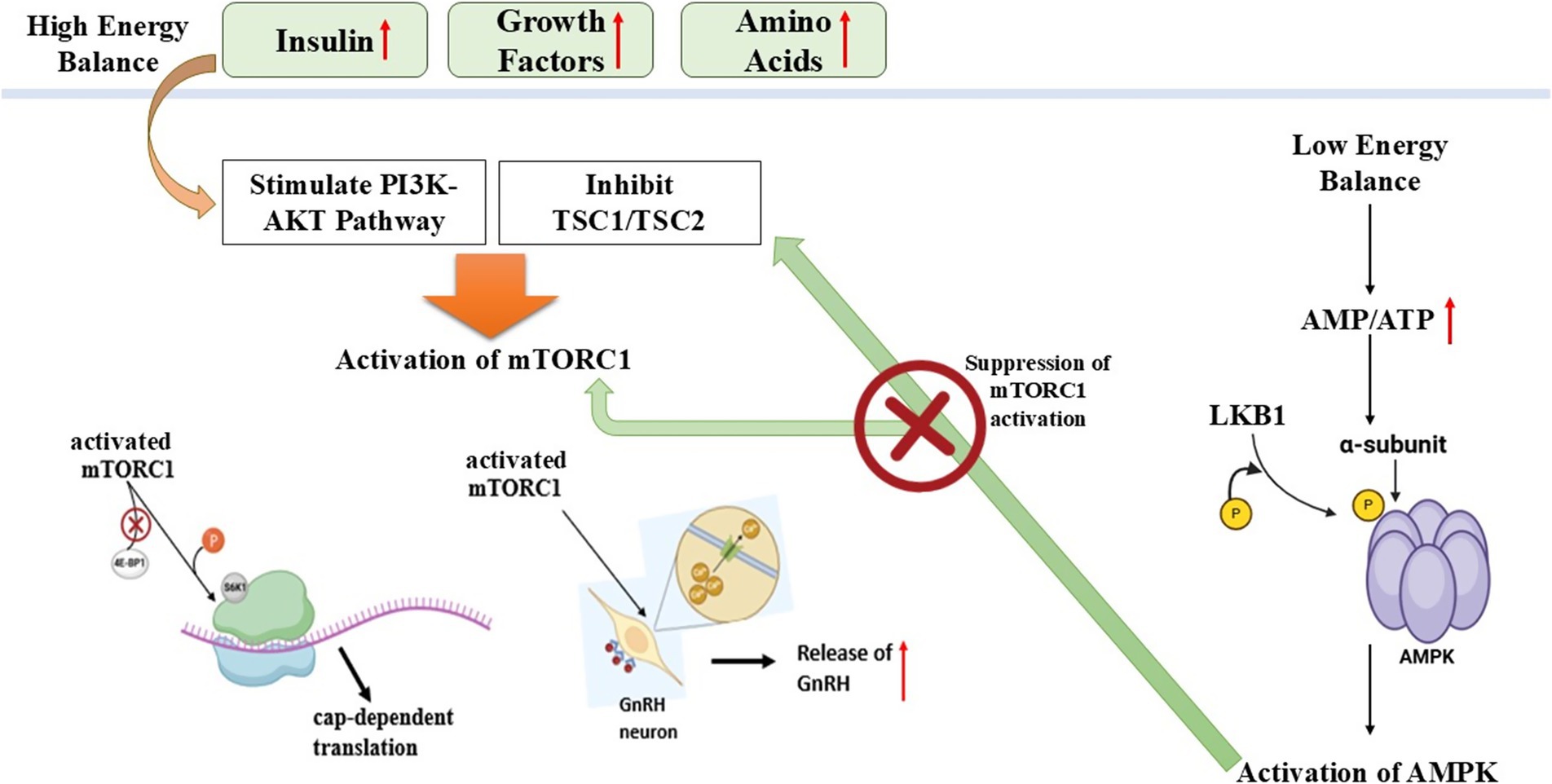
Figure 4. Schematic illustration of AMPK-MTOR energy sensing pathway regulating reproduction in seasonal breeders (Nutritional effect on reproduction).
Ovarian transcriptomic profiles of donkeys
Transcriptomic study of donkey granulosa cells has demonstrated high enrichment of PI3K-Akt and focal adhesion pathways, suggesting active participation in cell proliferation, steroidogenesis, and follicular support (32). Differential expression of genes, e.g., endomucin (EMCN) and synaptotagmin-like protein 12 (SYT12), indicates potential molecular actors that are specific to donkey follicular biology (94). Sheep follicular gene networks are well described, and horse research is also growing, but donkeys are poorly characterized at the transcriptomic level (95).
Steroidogenesis pathway disruption
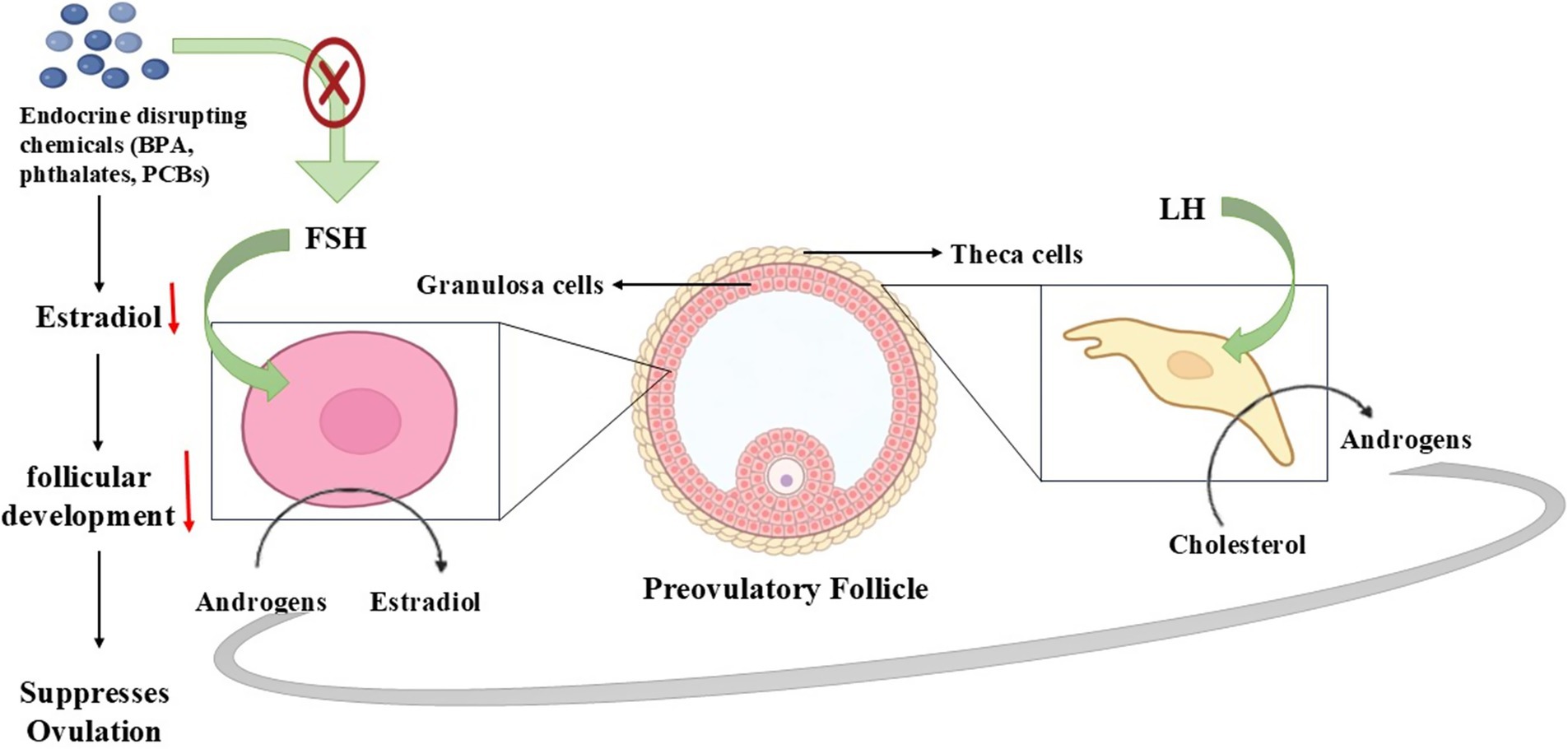
The reproductive efficiency of seasonal breeders, including donkeys, is regulated by ovarian steroidogenesis through its vital function. The reproductive cycles depend on correct sex hormone synthesis that results from this process to support follicular development and regulate ovulation (96). The pathway of ovarian steroidogenesis undergoes disruption when exposed to endocrine-disrupting chemicals (EDCs) since these chemicals create hormonal imbalances that negatively affect fertility processes (97). The function of sex hormones and hormone receptors becomes disrupted because of environmental chemicals, which are mainly present in pesticides, plastics, and industrial waste, thus resulting in reproductive complications. The evaluation of seasonal breeders requires knowledge about how EDCs modify steroidogenesis at the molecular level (98).
Ovarian steroidogenesis and its regulation
The ovary produces sex hormones through a coordinated process involving two different cell types as well as two different hormones (99). Luteinizing hormone (LH) activates cholesterol conversion to androgens in the theca cells so that these hormones move on to granulosa cells. The hormone FSH in granulosa cells turns on aromatase activity that transforms androgens into estradiol (100). The restrictive hormone control system completes the proper functioning of estrous cycles and ovulation while sustaining pregnancy in seasonal reproduction cycles. Hormone production becomes impaired through disruptions in this pathway, which occurs from environmental stressors or EDC exposure, thus causing irregular reproductive cycles along with infertility (101).
Endocrine disrupting chemicals interference in ovarian steroidogenesis
EDCs interrupt ovarian steroidogenesis either by blocking essential enzymes, duplicating natural hormones, or obstructing various receptors (97). Studies show that the chemical substances bisphenol A (BPA), phthalates, and Polychlorinated Biphenyls (PCBs) block aromatase activity, which decreases estradiol production. Pesticides together with dioxins disrupt the steroidogenic acute regulatory (StAR) protein required for cholesterol transport into mitochondria (102). These environmental toxins interfere with vital molecular pathways to change the regulation of the estrous cycle as well as the reproductive efficiency of species that align with seasonal mating patterns (103).
Impact on reproductive function in seasonal breeders
The reproduction of seasonal breeders such as donkeys strongly depends on environmental clues, including photoperiod and nutrition, because disruptions in steroidogenesis cause major reproductive effects (104). Endocrine-disrupting compounds affecting sex hormone equilibrium control the duration of the estrous cycle, delay ovulation, and decrease fertility potential (105). Juvenile animals become unable to sustain pregnancy because the corpus luteum function fails to maintain normal progesterone levels, and when estrogen production becomes disrupted, it affects follicle maturation. The dependence of these species on hormonal changes for seasonal reproduction makes exposure to EDCs a possible cause of declining fertility and reproductive problems (106).
The essential hormonal process of steroidogenesis controls reproductive efficiency, but environmental pollutants create major difficulties for seasonal breeders to maintain their reproductive functions (107). The impact of environmental pollutants on hormone biosynthesis steps results in reproductive breakdowns, which creates permanent effects on fertility (108). Research must be conducted to determine how much seasonal breeders encounter environmental pollutants while developing new approaches to lessen the reproductive health damage. The identification of environmental polluting factors will enable better decision-making regarding protection plans for species suffering from pollution exposure effects (109) (Figure 5).
The summary of the molecular pathway is shown in Table 5.
Oxidative stress pathways and apoptosis in gonads
The reproductive performance of seasonal breeders, including donkeys, depends significantly on oxidative stress (OS) because their reproductive cycles follow environmental signals closely (110). The correct relationship between reactive oxygen species (ROS) and antioxidants is necessary for proper reproductive system operation (111). When ROS production becomes excessive, it interferes with the balance, which subsequently damages cells through steroidal hormone production failure and triggers cell death in gonadal tissue (112). The reproductive functions of oocyte maturation and sperm function, together with embryonic development, undergo disturbances that affect fertility and seasonal breeding performance (113).
Oxidative stress in reproductive tissues
The metabolism of cells produces ROS byproducts mainly in mitochondria, which serve as crucial signaling agents during folliculogenesis and ovulation and corpus luteum development (114). High levels of ROS exceed the capacity of antioxidants to control them, which results in damage to lipids, proteins, and DNA, leading to gonadal cell death through apoptosis (115). The reproductive patterns of seasonal breeders respond directly to photoperiodic changes and metabolic status, thus making this research important for their breeding cycles (116). The level of oxidative stress tends to increase throughout the non-breeding cycle to maintain reproductive dormancy, yet specific ROS regulation helps execute important reproductive processes, including follicular rupture together with sperm capacitation during the breeding period (117).
Apoptosis in gonads and its regulation
Gonadal functionality depends on programmed cell death known as apoptosis since this process regulates the death of follicles while also controlling sperm formation (118). Seasonal breeders primarily depend on the intrinsic apoptotic pathway, which originates from mitochondrial dysfunction combined with oxidative damage to their cells (119). Excessive ROS activates cytochrome c release from mitochondria to activate caspases, which in turn causes the death of follicular cells and germ cells (120). The existence of a balance between pro-apoptotic proteins BAX and BAK and anti-apoptotic protein BCL-2 decides whether cells will survive. The reproductive efficiency of reproductive systems is impacted by season-dependent modifications of gonadotropin levels and melatonin signaling that control oxidative stress mechanisms and apoptosis rates in gonadal tissues (121).
Impact on reproductive efficiency in seasonal breeders
The reproductive efficiency of seasonal breeders such as donkeys is directly affected by oxidative stress and apoptosis because they harm gametes and their reproductive organs’ functionality (122). Controlled oxidative signaling supports ovulation together with sperm maturation during the breeding season. Excessive oxidative damage during times outside the breeding period quickens the process of follicular atresia while causing sperm viability to decrease (123). Reproductive success suffers from environmental stressors such as heat exposure, poor nutrition, and toxic environmental substances, which increase the rate of oxidative damage in animals (124).
Strategies to mitigate oxidative stress
The improvement of reproductive performance in seasonal breeders depends on implementing methods that reduce oxidative stress damage (125). The combination of antioxidant supplements, including vitamins C and E, and selenium, and melatonin, leads to better gonadal function and fertility results (126). The reproductive potential can benefit from nutritional measures that activate endogenous antioxidant enzymes, including superoxide dismutase, catalase, and glutathione peroxidase (127). Proper control of environmental stressors together with appropriate nutritional provision during breeding seasons will help reduce oxidative damage, which in turn leads to improved reproductive outcomes across donkeys and other seasonal breeders (128).
The regulation of reproductive efficiency in seasonal breeders depends heavily on oxidative stress together with apoptosis mechanisms (129). The body needs regulated ROS production to maintain normal reproductive functions, yet too much oxidative damage triggers problems with gamete quality along with hormonal imbalancing and infertility (130). Laboratory research on oxidative stress mechanisms interacting with seasonal reproductive signals will enable scientists to develop better treatments for enhancing donkey breeding performance alongside other seasonal breeders (131) (Figure 6).
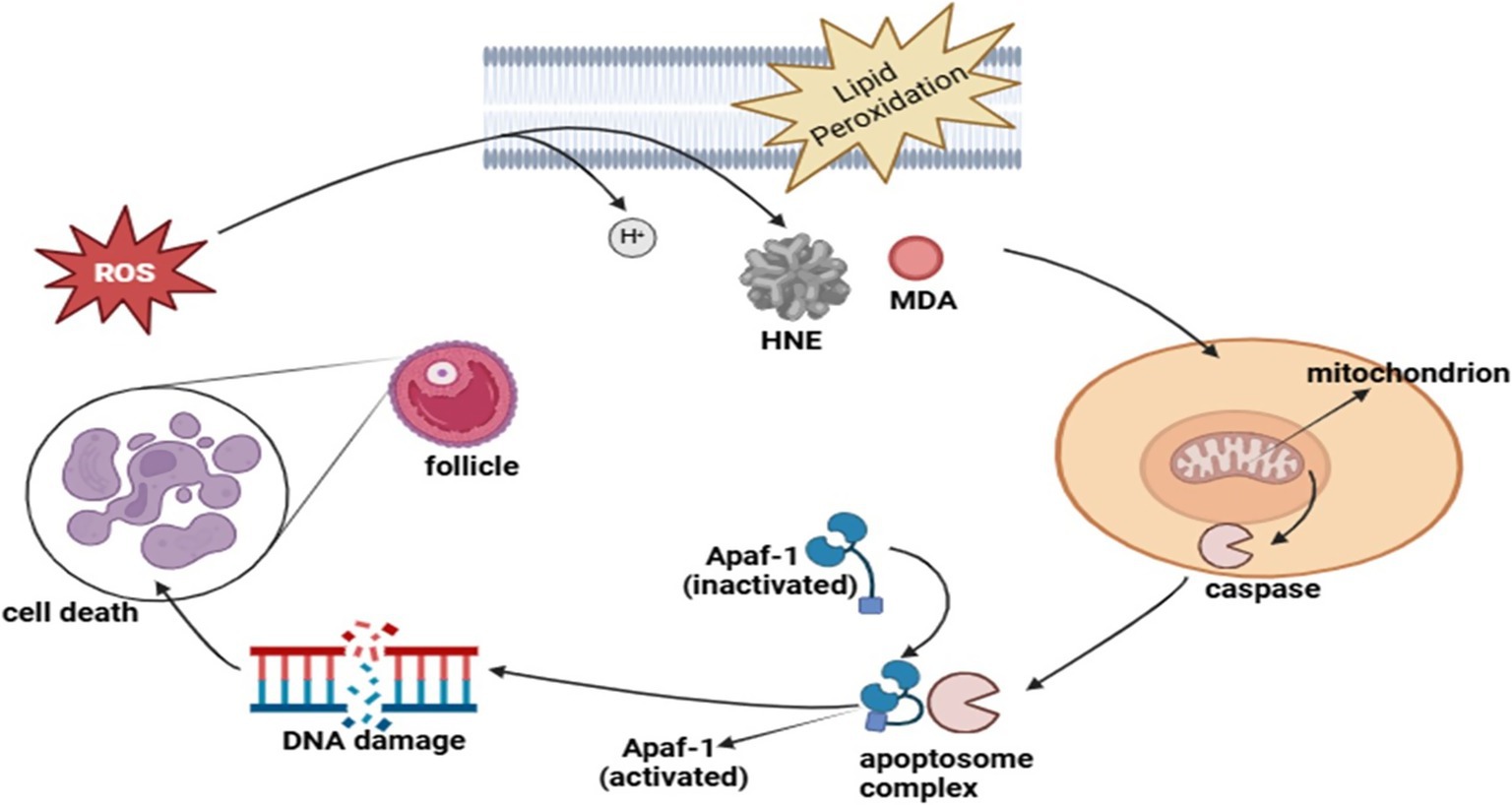
Figure 6. Role of oxidative stress pathways and apoptosis in gonads in the regulation of reproduction in seasonal breeders.
The summary of the molecular pathway is shown in Table 6.
Prolactin pathway in seasonal breeders
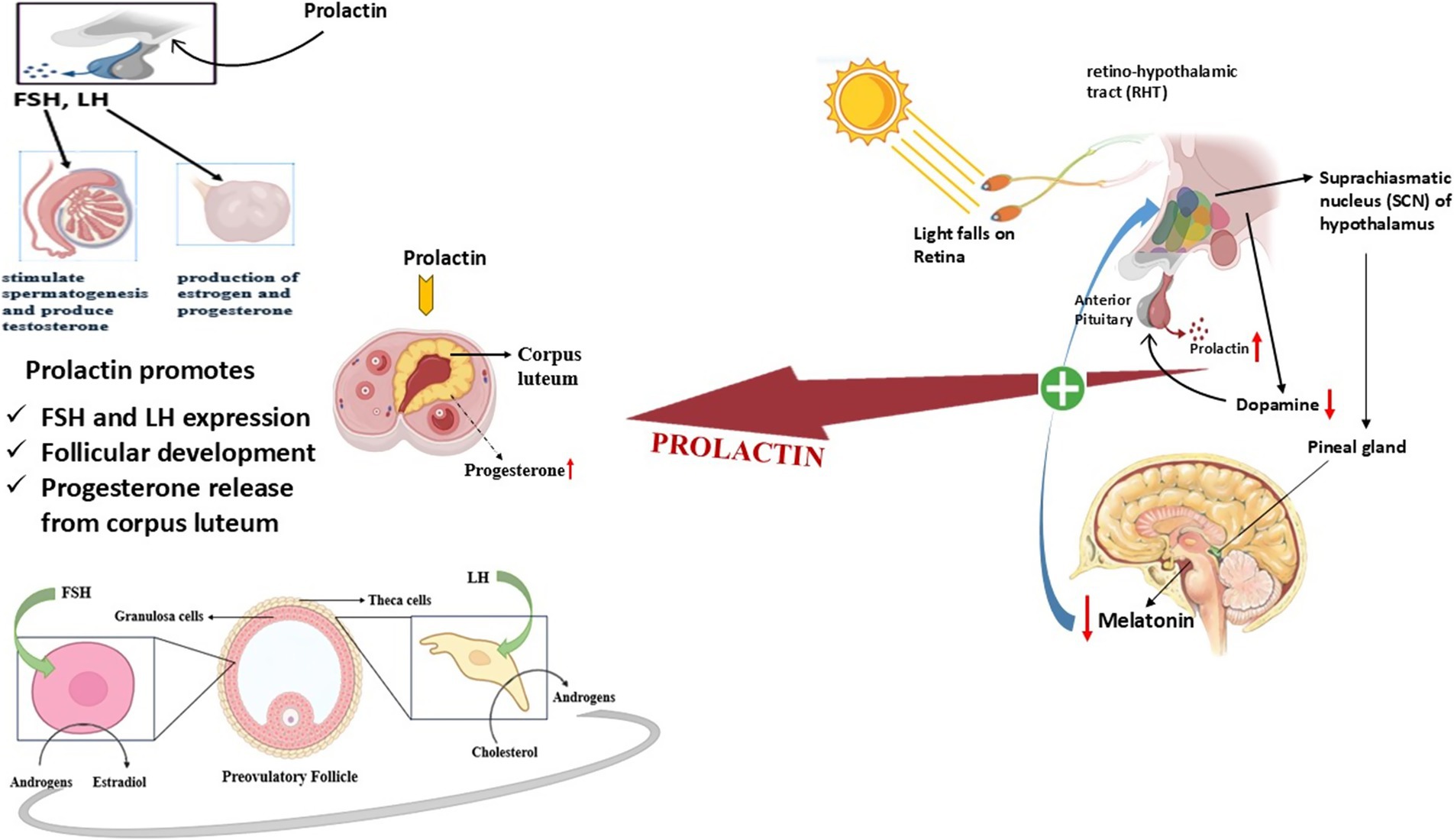
As a key hormone in seasonally breeding animals, prolactin (PRL) regulates reproductive periods and helps the body adapt to environmental shifts (132). The hormone shows seasonal patterns where the secretion rate reaches its highest point during the spring and summer months and its lowest point in the autumn and winter months (133). The hormonal regulation of prolactin depends mostly on Photoperiod which controls pineal gland melatonin production and ultimately directs prolactin output (134).
Photoperiodic regulation of prolactin
The duration of daylight throughout seasons strongly controls the production of melatonin and therefore controls the anterior pituitary’s release of prolactin (135). The hypothalamus, along with the pars tuberalis of the pituitary, contains receptors that allow melatonin to trigger seasonal endocrine responses (136). The control mechanism for both gonadotropins as well as prolactin functions through the shared regulation of luteinizing hormone (LH), follicle-stimulating hormone (FSH), and prolactin by melatonin (137).
The mechanisms that regulate seasonal prolactin changes differ from gonadotropin patterns because prolactin relies on direct neuroendocrine regulation, but gonadotropins follow feedback-based control (138). Current research does not provide enough evidence to prove that winter prolactin reduction happens only through increased dopamine inhibition (139). At this time, pituitary becomes more responsive to dopamine, which could be a factor in the decrease of prolactin, secretion throughout seasonal cycles. The intricate relationship between melatonin, prolactin and gonadotropins demonstrates how the human body readjusts reproductive and metabolic systems because of seasonal variations (132).
Prolactin’s role in seasonal reproduction
• Certain species rely on prolactin as their luteotrophic factor for corpus luteum maintenance during pregnancy (140).
• The activity of prolactin as an implantation delay factor affects the timing of embryo attachment in Bennett’s wallaby, along with the tammar wallaby (141).
• The seasonal prolactin secretion pattern seems to be a fundamental biological trait that affects reproductive cycles as well as fur shedding (molt) and bodily metabolism (142).
Prolactin and pituitary interactions in seasonal breeders
• The pituitary gland contains prolactin receptor proteins inside both the pars distalis and pars tuberalis areas indicating a paracrine regulatory internal process (132).
• Gonadotropes (LH and FSH-secreting cells) and lactotropes (PRL-secreting cells) demonstrate direct physical contact throughout the pituitary, but their structural relationships fluctuate between seasons according to research findings (134).
• The hormone dopamine functions to block prolactin release while some species demonstrate that prolactin maintains control over how gonadotropes react to GnRH which helps stop the glands from overstimulation (143).
• The photoperiod of the breeding environment determines how powerfully prolactin inhibits gonadotropin secretion through its photoperiodic dependency, where short-day breeders (sheep) demonstrate stronger inhibition but long-day breeders (horses) display a more regulatory effect (144).
Molecular and cellular mechanisms
The regulatory patterns of pituitary hormone secretion might be influenced by seasonal shifts observed in folliculostellate cells, which belong to the category of pituitary support cells (145). Research indicates that breeding_season triggers an increase in cell-adherens junctions between these cells while prolactin and gonadotropin interactions simultaneously evolve (146). The GnRH hormone canstimulate prolactin release, but its effect on this process depends on the season, the species, and the reproductive condition of the animal (147).
Many mammals use prolactin as their main seasonal regulatory agent while photoperiodic cues processed through melatonin pathways control its secretions (148). Prolactin plays an essential part in seasonal physiological adaptations since it controls molt, metabolism and energy balance beyond reproduction (149). The complex dynamic system of prolactin together with gonadotropins and hypothalamic regulatory components shows that it plays an essential role in reproductive adaptation to environmental changes while managing energy, and reproductive resource distribution annually (150) (Figure 7).
The summary of the molecular pathway is shown in Table 7.
Thus, the prolactin pathway serves as a vital neuroendocrine mechanism in seasonal breeders, regulating reproductive timing and physiological adjustments in response to the photoperiod (133). The regulation of prolactin secretion in response to melatonin affects both the reproductive and metabolic functions (132). The pituitary paracrine interactions and dopamine modulation indicate the association between prolactin and gonadotropin output (151). Thus, it indicates the dual role of prolactin in regulating internal hormonal rhythms and external seasonal changes.
The molecular pathways coordinate in response to photoperiod and nutritional availability, ensuring optimal reproduction during long days (151). The summary of these molecular pathways is shown in Table 8. It is well established that the reproductive activity in donkeys is regulated by interconnected hormonal, signaling, and metabolic pathways that respond to the environmental and seasonal variability (16). Thus, disruptions in the HPG axis, melatonin signaling, KNDy neurons, and prolactin pathway result in decreased production of GnRH and gonadocorticoids (10). Malnutrition also affects the AMPK-mTOR pathway, resulting in declined reproductive performance, and oxidative stress leads to gonadal cell damage. Understanding these mechanisms broadens our knowledge of seasonal fertility and opens the horizon for improving reproductive efficiency through modulation of molecular pathways using hormonal, nutritional, and management strategies in seasonal breeders, particularly in donkeys.
Scope of ART in donkey
Thus, donkeys have seasonally regulated reproductive patterns which are mainly regulated by photoperiod (152). Reproductive traits of males (e.g., testicular size, semen quality, hormonal changes (e.g., testosterone)) differ in breeding and non-breeding seasons (153). The results of the immunohistochemical studies of the epididymis indicate the presence of higher epithelial activity and sperm in the spring, whereas higher markers of oxidative stress and autophagy are observed in off-seasons (154). These results deny the previous hypotheses of low seasonality and support the importance of time-adjusted breeding plans. In addition, Dezhou donkeys immunized against inhibin exhibited elevated levels of FSH, LH, testosterone, and activin A, especially out of breeding season (155).
Donkeys and horses are very different in their reproductive behavior, the duration of a cycle, and anatomical characteristics (27). The estrous cycle and the gestation period of Jennies are longer, and they also respond to factors other than photoperiod (12). Jacks have bigger reproductive organs, and they take more time to ejaculate (152). Behavioral peculiarities of the donkey reproduction, like territoriality and non-harem mating patterns, as well as reduced spermatogenic efficiency, also distinguish it among other equids (27). These characteristics require specific assisted reproductive technology (ART) regimens. It has been found that duration of the follicular phase, not luteolysis, is the main factor determining variability in interovulatory interval (IOI) (156). Longer IOIs are associated with longer estrus, slower follicle development, and larger follicles at ovulation. Such findings are important to schedule insemination and forecast fertility periods (27).
Thc sperm of donkeys has some cryobiological difficulties. The traditional freezing techniques produce uneven fertility outcomes, particularly in jennies (157). Nevertheless, the latest developments in sperm vitrification show positive results. Straws with outer covers, using 0.25 mL straws, showed similar or better motility and in vivo fertility than standard frozen semen (158). Remarkably, vitrified semen caused a less severe and short-term uterine inflammatory reaction (159). Moreover, Phospholipase C Zeta (PLCzeta) localization in donkey sperm showed that it was competent in oocyte activation, particularly during intracytoplasmic sperm injection (ICSI) into horse oocytes (160). These interspecies ICSI outcomes confirm the utilization of donkey sperm in the creation of mules and imply the expansion of ARTs (161).
Endometritis is a significant limitation to fertility in donkeys. The use of equine-based histopathological grading of donkey uteri has been effective, and cytological and biopsy-based assessment would improve the level of diagnosis (162). These aids enable a superior categorization of uterine health and an even more accurate treatment regimen. Donkeys are prone to metabolic problems that affect reproduction. Metabolic disorders like insulin dysregulation, hyperlipemia, and Pituitary Pars Intermedia Dysfunction (PPID) are usually compounded by obesity (29). These conditions either directly or through systemic effects lead to impaired reproductive performance (e.g., laminitis, organ dysfunction). Most unfortunately, the majority of hormonal reference ranges and treatment protocols are based on horses, in spite of pharmacokinetic differences (163).
Breed size also plays a great role in reproductive performance. Big-bodied breeds such as the Dezhou donkey are more fertile and produce more milk than smaller breeds (e.g., Cullen donkeys) (164). Surveys conducted in Northern China indicate that formalized farm activities, especially with national/provincial institutions, are associated with improved ART adoption and reproductive success (153). The survey shows that about 73 percent of the surveyed farms are using artificial insemination, indicating the rising use of ART. Such results support the importance of breed selection, genetic advancement, and farm standardization in optimizing fertility. Donkeys are increasingly being used in protecting endangered equids. ARTs have advanced in horses, but adaptation to donkeys and wild equids is continuing (12). Donkeys can be used as fertility models as well as surrogates in conservation programs, particularly due to their reproductive strength and availability (165). Nevertheless, the molecular variability of gamete behavior and endocrine response requires specific studies. The donkey ARTs should be aligned with the principles of conservation biology in order to save genetic diversity in Equus (159).
Research gaps and future directions
Although there has been an improvement, there are still considerable gaps in our knowledge of donkey reproductive physiology and molecular control. The principal areas in need of focus are:
• Creation of hormonal and metabolic reference values in donkeys
• Pharmacological validation of this species
• Explanation of molecular mechanisms of seasonal modulation of fertility
• Improving ART procedures such as ICSI and embryo transfer
• Reproductive studies of donkey, horse, and mule to compare to find out some unique limitations and possibilities
To resolve these problems, interdisciplinary cooperation, the use of sophisticated molecular technologies, and the dedication to the species-specific research framework will be needed.
Conclusion
The multifactorial nature of the reproductive inefficiency in donkeys has its basis in the underrecognized physiological peculiarity and the lack of specific molecular studies. This review offers convincing details of photoperiod-induced seasonal fertility, metabolic endocrine imbalances, and anatomical differences that determine reproductive fitness. New opportunities are available with recent advances in ARTs, endocrinology, and histological profiling as ways to improve fertility and conservation results. It is now necessary to strategically invest in donkey-specific research and comparative reproductive biology to deliver these insights into productive breeding innovations and long-term species sustainability. In addition, the combination of management practices with molecular insights can increase reproductive efficiency, improve animal welfare, and increase the productivity of the donkey populations. Such integration of scientific basic research and applied animal husbandry will be essential to overcoming the limitations imposed by seasonal infertility and maximizing reproductive performance in this important but often neglected species. So, to boost farm production efficiency of seasonal breeders like donkeys, upgrading reproductive efficiency by adopting cutting-edge animal biotechnological tools and breeding technologies is the future of donkey farming.
Author contributions
MA: Conceptualization, Writing – original draft, Writing – review & editing. SA: Writing – review & editing, Formal analysis. FH: Project administration, Writing – review & editing. WC: Project administration, Writing – review & editing, Supervision, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the National Key R&D Program of China (grant nos. 2022YFD1600103 and 2023YFD1302004), the Shandong Province Modern Agricultural Technology System Donkey Industrial Innovation Team (grant no. SDAIT-27), Livestock and Poultry Breeding Industry Project of the Ministry of Agriculture and Rural Affairs (grant no. 19211162), the National Natural Science Foundation of China (grant no. 31671287), The Open Project of Liaocheng University Animal Husbandry Discipline (grant no. 319312101–14), the Open Project of Shandong Collaborative Innovation Center for Donkey Industry Technology (grant no. 3193308), Doctoral research start-up fee of the Science and Technology Department (grant no. 318/318052367), Excellent foreign young scientists NSFC (grant no. 307/307272201).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Martin, G, Milton, J, Davidson, RH, Banchero, GE, Lindsay, DR, and Blache, D. Natural methods for increasing reproductive efficiency in small ruminants. Anim Reprod Sci. (2004) 82:231–45. doi: 10.1016/j.anireprosci.2004.05.014
2. Blanc, F, Martin, GB, and Bocquier, F. Modelling reproduction in farm animals: a review. Reprod Fertil Dev. (2001) 13:337–53. doi: 10.1071/RD01038
4. Hafez, E, Jainudeen, M, and Rosnina, Y. Hormones, growth factors, and reproduction. Reproduction in farm animals. Hoboken, NJ: Wiley, pp. 31–54 (2000).
5. Dobson, H, and Smith, R. Stress and reproduction in farm animals. J Reprod Fertil. (1995) 49:451–62.
6. MacKenzie, KC, Graaf, BM, Syrimis, A, Zhao, Y, Brosens, E, Mancini, GMS, et al. Goldberg–Shprintzen syndrome is determined by the absence, or reduced expression levels, of KIFBP. Hum Mutat. (2020) 41:1906–17. doi: 10.1002/humu.24097
7. Singh, AK. Advancements in management practices from far-off dry period to initial lactation period for improved production, reproduction, and health performances in dairy animals: a review. Int J Livest Res. (2021) 11:25–41. doi: 10.5455/ijlr.20200827114032
8. Da Silva, JC, Noordhuizen, JPTM, Vagneur, M, Bexiga, R, Gelfert, CC, and Baumgartner, W. Veterinary dairy herd health management in Europe constraints and perspectives. Vet Q. (2006) 28:23–32. doi: 10.1080/01652176.2006.9695203
9. Miragaya, MH, Neild, DM, and Alonso, AE. A review of reproductive biology and biotechnologies in donkeys. J Equine Vet Sci. (2018) 65:55–61. doi: 10.1016/j.jevs.2017.12.005
10. Dawson, FL. Recent advances in equine reproduction. Equine Vet J. (1977) 9:4–11. doi: 10.1111/j.2042-3306.1977.tb03960.x
11. Canisso, IF, Panzani, D, Miró, J, and Ellerbrock, RE. Key aspects of donkey and mule reproduction. Vet Clin N Am Equine Pract. (2019) 35:607–42. doi: 10.1016/j.cveq.2019.08.014
12. Wang, Y, Hua, X, Shi, X, and Wang, C. Origin, evolution, and research development of donkeys. Genes. (2022) 13:1945. doi: 10.3390/genes13111945
13. Pugh, D. Donkey reproduction. in Proceedings of the Annual American Association of Equine Practitioners. (2002).
14. Austin, CR, and Short, RV. Hormonal control of reproduction. Cambridge, UK: Cambridge University Press (1984).
15. Goodman, HM. Hormonal control of reproduction in the female: the menstrual cycle In: HM Goodman, editor. Essential medical physiology. Cape Town: Juta Limited (2004). 737.
16. Christensen, A, Bentley, G, Cabrera, R, Ortega, H, Perfito, N, Wu, T, et al. Hormonal regulation of female reproduction. Horm Metab Res. (2012) 44:587–91. doi: 10.1055/s-0032-1306301
17. Findlay, JK, Robertson, DM, Clarke, IJ, Klein, R, Doughton, BW, Xiao, S, et al. Hormonal regulation of reproduction—general concepts. Anim Reprod Sci. (1992) 28:319–28. doi: 10.1016/0378-4320(92)90118-W
18. Lamming, G, Hafs, H, and Manns, J. Hormonal control of reproduction in cattle. Proc Br Soc Anim Prod. (1972) 4:71–8.
19. Boersma, G, Salton, S, Spritzer, P, Steele, C, and Carbone, D. Models and mechanisms of metabolic regulation: genes, stress, and the HPA and HPG axes. Horm Metab Res. (2012) 44:598–606. doi: 10.1055/s-0032-1311576
20. Mbiydzenyuy, NE, and Qulu, L-A. Stress, hypothalamic-pituitary-adrenal axis, hypothalamic-pituitary-gonadal axis, and aggression. Metab Brain Dis. (2024) 39:1613–36. doi: 10.1007/s11011-024-01393-w
21. Acevedo-Rodriguez, A, Kauffman, AS, Cherrington, BD, Borges, CS, Roepke, TA, and Laconi, M. Emerging insights into hypothalamic-pituitary-gonadal axis regulation and interaction with stress signalling. J Neuroendocrinol. (2018) 30:e12590. doi: 10.1111/jne.12590
22. Toufexis, D, Rivarola, MA, Lara, H, and Viau, V. Stress and the reproductive axis. J Neuroendocrinol. (2014) 26:573–86. doi: 10.1111/jne.12179
23. Du, X, Pang, TY, Mo, C, Renoir, T, Wright, DJ, and Hannan, AJ. The influence of the HPG axis on stress response and depressive-like behaviour in a transgenic mouse model of Huntington's disease. Exp Neurol. (2015) 263:63–71. doi: 10.1016/j.expneurol.2014.09.009
24. Son, YL, Ubuka, T, and Tsutsui, K. Regulation of stress response on the hypothalamic-pituitary-gonadal axis via gonadotropin-inhibitory hormone. Front Neuroendocrinol. (2022) 64:100953. doi: 10.1016/j.yfrne.2021.100953
25. Zhang, Y, Luo, C, Huang, P, Chen, L, Ma, Y, and Ding, H. Effects of chronic exposure to a high fat diet, nutritive or non-nutritive sweeteners on hypothalamic-pituitary-adrenal (HPA) and-gonadal (HPG) axes of male Sprague-Dawley rats. Eur J Nutr. (2024) 63:2209–20. doi: 10.1007/s00394-024-03427-6
26. Lencioni, GC, Montechese, ACD, Boakari, Y, Alonso, MA, Fernandes, CB, and McLean, AK. The effect of stress on equine reproduction and welfare In: JC Gardón and KS Ambrojo, editors. Assisted reproductive Technologies in Animals Volume 1: Current trends for reproductive management. Berlin: Springer (2024). 195–217.
27. Van den Branden, E, et al. Reproduction in Equidae: a comparative study of donkeys and horses. Ghent, Belgium: Ghent University (2021).
28. Li, N, Yang, F, Yu, J, Yang, W, Wu, S, Ma, J, et al. Characteristics of follicular dynamics and reproductive hormone profiles during oestrous cycles of jennies over an entire year. Reprod Domest Anim. (2021) 56:448–58. doi: 10.1111/rda.13883
29. Mendoza, FJ, Toribio, RE, and Perez-Ecija, A. Metabolic and endocrine insights in donkeys. Animals. (2024) 14:590. doi: 10.3390/ani14040590
30. Richard, F, Martinat, N, Remy, JJ, Salesse, R, and Combarnous, Y. Cloning, sequencing and in vitro functional expression of recombinant donkey follicle-stimulating hormone receptor: a new insight into the binding specificity of gonadotrophin receptors. J Mol Endocrinol. (1997) 18:193–202. doi: 10.1677/jme.0.0180193
31. Xia, Y, Wang, Q, He, XD, Chen, Y, JiGe, MT, and Zi, XD. Cloning and expression analysis of the follicle-stimulating hormone receptor (FSHR) gene in the reproductive axis of female yaks (Bos grunniens). Domest Anim Endocrinol. (2020) 70:106383. doi: 10.1016/j.domaniend.2019.07.011
32. Wang, Y, Gao, Z, Zhang, Q, Guo, X, Xia, W, Gu, X, et al. Unraveling the transcriptomic profiles of large and small donkey follicles. Genes. (2025) 16:602. doi: 10.3390/genes16050602
33. Abdelnaby, EA, Alhaider, AK, el-Maaty, AMA, Ragab, RSA, Seida, AA, and el-Badry, DA. Ovarian and uterine arteries blood flow velocities waveform, hormones and nitric oxide in relation to ovulation in cows superstimulated with equine chorionic gonadotropin and luteolysis induction 10 and 17 days after ovulation. BMC Vet Res. (2023) 19:205. doi: 10.1186/s12917-023-03692-3
34. Whittier, JM, and Crews, D. Seasonal reproduction: patterns and control In: DO Norris and RE Jones, editors. Hormones and reproduction in fishes, amphibians, and reptiles. Berlin: Springer (1987). 385–409.
35. Batsaikhan, D, Bayar-Enkh, B, and Tuvshin, B. Interrelationship of some hormones belonging to HPG and energy axes inhorses during various reproductive states. Mongol J Agric Sci. (2015) 15:10–5. doi: 10.5564/mjas.v15i2.538
36. Busato1, EM, Abreu, AC, Bergstein-Galan, TG, Bertol, MA, and Weiss, RR. Reproductive physiology of the equine In: TG Bergstein-Galan, editor. Reproduction biotechnology in farm animals. Hyderabad: Avid Science (2017). 2.
37. Kárpáti, E, Fürlinger, D, Pleskó, A Móczáné, Gulyás, L, Gáspárdy, A, and Becskei, Z. Various approaches to influence melatonin level in sheep reproduction. Vet Glas. (2023) 77:16–34. doi: 10.2298/VETGL220308007K
38. Gáspárdy, A, Gallagher, G, Bartha, B, Cseh, S, Fekete, SG, and Somoskői, B. Plasma melatonin concentration during the early post-partum period in thoroughbred mares and their foals. Acta Vet Hung. (2023) 71:119–27. doi: 10.1556/004.2023.00883
39. Gao, Y, Zhao, S, Zhang, Y, and Zhang, Q. Melatonin receptors: a key mediator in animal reproduction. Vet Sci. (2022) 9:309. doi: 10.3390/vetsci9070309
40. Reiter, R. Photoperiod: its importance as an impeller of pineal and seasonal reproductive rhythms. Int J Biometeorol. (1980) 24:57–63. doi: 10.1007/BF02245542
41. Sumpter, JP. General concepts of seasonal reproduction In: JP Sumpter, editor. Reproductive seasonality in Teleosts. Boca Raton, FL: CRC Press (2019). 13–32.
42. Nakane, Y, and Yoshimura, T. Photoperiodic regulation of reproduction in vertebrates. Ann Rev Anim Biosci. (2019) 7:173–94. doi: 10.1146/annurev-animal-020518-115216
43. Karsch, FJ, Bittman, EL, Foster, DL, Goodman, RL, Legan, SJ, and Robinson, JE. Neuroendocrine basis of seasonal reproduction. in Proceedings of the 1983 Laurentian hormone conference. Amsterdam, Netherlands: Elsevier (1984).
44. Pitkänen, M. DMT, pineal gland, and the new view about sensory perception. Available online at: https://tgdtheory.fi/public_html/articles/dmtpineal.pdf.
45. Aleandri, V, Spina, V, and Morini, A. The pineal gland and reproduction. Hum Reprod Update. (1996) 2:225–35. doi: 10.1093/humupd/2.3.225
46. Horodincu, L, and Solcan, C. Influence of different light spectra on melatonin synthesis by the pineal gland and influence on the immune system in chickens. Animals. (2023) 13:2095. doi: 10.3390/ani13132095
47. Axelrod, J. The pineal gland and its endocrine role, vol. 65. Berlin: Springer Science and Business Media (2013).
48. Erren, TC, and Reiter, RJ. Melatonin: a universal time messenger. Neuroendocrinol Lett. (2015) 36:187–92.
49. Vanecek, J. Cellular mechanisms of melatonin action. Physiol Rev. (1998) 78:687–721. doi: 10.1152/physrev.1998.78.3.687
50. Yasuo, S, Yoshimura, T, Ebihara, S, and Korf, HW. Melatonin transmits photoperiodic signals through the MT1 melatonin receptor. J Neurosci. (2009) 29:2885–9. doi: 10.1523/JNEUROSCI.0145-09.2009
51. Cardinali, DP, and Pévet, P. Basic aspects of melatonin action. Sleep Med Rev. (1998) 2:175–90. doi: 10.1016/S1087-0792(98)90020-X
52. Lomet, D, Cognié, J, Chesneau, D, Dubois, E, Hazlerigg, D, and Dardente, H. The impact of thyroid hormone in seasonal breeding has a restricted transcriptional signature. Cell Mol Life Sci. (2018) 75:905–19. doi: 10.1007/s00018-017-2667-x
53. Yoshimura, T. Thyroid hormone and seasonal regulation of reproduction. Front Neuroendocrinol. (2013) 34:157–66. doi: 10.1016/j.yfrne.2013.04.002
54. Shinomiya, A, Shimmura, T, Nishiwaki-Ohkawa, T, and Yoshimura, T. Regulation of seasonal reproduction by hypothalamic activation of thyroid hormone. Front Endocrinol. (2014) 5:12. doi: 10.3389/fendo.2014.00012
55. Stehle, J, Von Gall, C, and Korf, HW. Melatonin: a clock-output, a clock-input. J Neuroendocrinol. (2003) 15:383–9. doi: 10.1046/j.1365-2826.2003.01001.x
56. Peschke, E, Bähr, I, and Mühlbauer, E. Experimental and clinical aspects of melatonin and clock genes in diabetes. J Pineal Res. (2015) 59:1–23. doi: 10.1111/jpi.12240
57. Revel, FG, Ansel, L, Klosen, P, Saboureau, M, Pévet, P, Mikkelsen, JD, et al. Kisspeptin: a key link to seasonal breeding. Rev Endocr Metab Disord. (2007) 8:57–65. doi: 10.1007/s11154-007-9031-7
58. Simonneaux, V. A kiss to drive rhythms in reproduction. Eur J Neurosci. (2020) 51:509–30. doi: 10.1111/ejn.14287
59. Revel, FG, Saboureau, M, Pévet, P, Simonneaux, V, and Mikkelsen, JD. RFamide-related peptide gene is a melatonin-driven photoperiodic gene. Endocrinology. (2008) 149:902–12. doi: 10.1210/en.2007-0848
60. Anjum, S, Khattak, MNK, Tsutsui, K, and Krishna, A. RF-amide related peptide-3 (RFRP-3): a novel neuroendocrine regulator of energy homeostasis, metabolism, and reproduction. Mol Biol Rep. (2021) 48:1837–52. doi: 10.1007/s11033-021-06198-z
61. Mohapatra, SS, Mukherjee, J, Banerjee, D, Das, P, Ghosh, PR, and Das, K. RFamide peptides, the novel regulators of mammalian HPG axis: a review. Vet World. (2021) 14:1867–73. doi: 10.14202/vetworld.2021.1867-1873
62. Tkachev, A, Tkacheva, O, and Gutyj, B. The modern methods of reproduction physiology of horses. Ukr J Vet Agric Sci. (2019) 2:18–23. doi: 10.32718/ujvas2-1.04
63. Díaz-Duran, M, Zarco, L, and Boeta, AM. Ovarian dynamics and estrous cycle length in the donkey (Equus asinus). Theriogenology. (2017) 103:1–8. doi: 10.1016/j.theriogenology.2017.07.003
64. Talpur, H, Chandio, IB, Brohi, RD, Worku, T, Rehman, Z, Bhattarai, D, et al. Research progress on the role of melatonin and its receptors in animal reproduction: a comprehensive review. Reprod Domest Anim. (2018) 53:831–49. doi: 10.1111/rda.13188
65. Ake, AS, Ayo, JO, Aluwong, T, and Mohammed, A. Effects of melatonin on hematologic and biochemical changes and the effects of circadian rhythm on hematologic changes in donkeys (Equus asinus) subjected to packing during the hot-dry season. Vet Clin Pathol. (2023) 52:299–312. doi: 10.1111/vcp.13193
66. Ake, AS, Ayo, JO, Aluwong, T, Mohammed, A, and Minka, NS. Melatonin modulates rectal and body surface temperatures and their circadian rhythmicity in donkeys (Equus asinus) subjected to packing during the hot-dry season. Int J Biometeorol. (2023) 67:389–404. doi: 10.1007/s00484-022-02418-8
67. Lehman, MN, Coolen, LM, and Goodman, RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. (2010) 151:3479–89. doi: 10.1210/en.2010-0022
68. Uenoyama, Y, Nagae, M, Tsuchida, H, Inoue, N, and Tsukamura, H. Role of KNDy neurons expressing kisspeptin, neurokinin B, and dynorphin a as a GnRH pulse generator controlling mammalian reproduction. Front Endocrinol. (2021) 12:724632. doi: 10.3389/fendo.2021.724632
69. Magee, C, Bruemmer, JE, Kirkley, KS, Sylvester, LA, Runyan, B, Nett, TM, et al. Kisspeptin has an independent and direct effect on the pituitary gland in the mare. Theriogenology. (2020) 157:199–209. doi: 10.1016/j.theriogenology.2020.07.031
70. Fanelli, D, Beltramo, M, Conte, G, Cerretini, B, Lomet, D, Rota, A, et al. The Kisspeptin analogue C6 induces ovulation in jennies. Theriogenology. (2022) 189:107–12. doi: 10.1016/j.theriogenology.2022.06.014
71. Moroni, R, Fanelli, D, Tesi, M, Cerretini, B, Lomet, D, Rota, A, et al. Effect of administration of the C6 kisspeptin analogue in jennies in estrus. J Equine Vet Sci. (2022) 113:103997. doi: 10.1016/j.jevs.2022.103997
72. Mittelman-Smith, MA, Williams, H, Krajewski-Hall, SJ, Lai, J, Ciofi, P, McMullen, NT, et al. Arcuate kisspeptin/neurokinin B/dynorphin (KNDy) neurons mediate the estrogen suppression of gonadotropin secretion and body weight. Endocrinology. (2012) 153:2800–12. doi: 10.1210/en.2012-1045
73. Goodman, RL, Hileman, SM, Nestor, CC, Porter, KL, Connors, JM, Hardy, SL, et al. Kisspeptin, neurokinin B, and dynorphin act in the arcuate nucleus to control activity of the GnRH pulse generator in ewes. Endocrinology. (2013) 154:4259–69. doi: 10.1210/en.2013-1331
74. Smith, MA. The role of KNDy neurons in estrogen modulation of LH release, body weight, and thermoregulation. Arizona: The University of Arizona (2012).
75. Uenoyama, Y, and Tsukamura, H. KNDy neurones and GnRH/LH pulse generation: current understanding and future aspects. J Neuroendocrinol. (2023) 35:e13285. doi: 10.1111/jne.13285
76. Merkley, CM. The role of kisspeptin and KNDy cells in the reproductive neuroendocrine system. Ontario, Canada: The University of Western (2013).
77. Hu, G, Lin, C, He, M, and Wong, AOL. Neurokinin B and reproductive functions: “KNDy neuron” model in mammals and the emerging story in fish. Gen Comp Endocrinol. (2014) 208:94–108. doi: 10.1016/j.ygcen.2014.08.009
78. Yang, W, Wang, L, Wang, F, and Yuan, S. Roles of AMP-activated protein kinase (AMPK) in mammalian reproduction. Front Cell Dev Biol. (2020) 8:593005. doi: 10.3389/fcell.2020.593005
79. Assaf, L, Eid, AA, and Nassif, J. Role of AMPK/mTOR, mitochondria, and ROS in the pathogenesis of endometriosis. Life Sci. (2022) 306:120805. doi: 10.1016/j.lfs.2022.120805
80. Cao, N, Hu, C, Xia, B, He, Y, Huang, J, Yuan, Z, et al. The activated ampk/mtorc2 signaling pathway associated with oxidative stress in seminal plasma contributes to idiopathic asthenozoospermia. Oxidative Med Cell Longev. (2022) 2022:4240490. doi: 10.1155/2022/4240490
81. Steinberg, GR, and Hardie, DG. New insights into activation and function of the AMPK. Nat Rev Mol Cell Biol. (2023) 24:255–72. doi: 10.1038/s41580-022-00547-x
82. Bertoldo, MJ, Faure, M, Dupont, J, and Froment, P. AMPK: a master energy regulator for gonadal function. Front Neurosci. (2015) 9:235. doi: 10.3389/fnins.2015.00235
83. Mo, D, Zeng, ZH, Sui, X, Li, R, and Yang, YH. Role of glucose metabolism and signaling pathways at different stages of ovarian folliculogenesis. Reprod Dev Med. (2024) 8:111–20. doi: 10.1097/RD9.0000000000000079
84. Kayampilly, PP, and Menon, K. AMPK activation by dihydrotestosterone reduces FSH-stimulated cell proliferation in rat granulosa cells by inhibiting ERK signaling pathway. Endocrinology. (2012) 153:2831–8. doi: 10.1210/en.2011-1967
85. Wu, G, Li, C, Tao, J, Liu, Z, Li, X, Zang, Z, et al. FSH mediates estradiol synthesis in hypoxic granulosa cells by activating glycolytic metabolism through the HIF-1α–AMPK–GLUT1 signaling pathway. J Biol Chem. (2022) 298:101830. doi: 10.1016/j.jbc.2022.101830
86. Roa, J, and Tena-Sempere, M. Connecting metabolism and reproduction: roles of central energy sensors and key molecular mediators. Mol Cell Endocrinol. (2014) 397:4–14. doi: 10.1016/j.mce.2014.09.027
87. Guo, Z, and Yu, Q. Role of mTOR signaling in female reproduction. Front Endocrinol. (2019) 10:692. doi: 10.3389/fendo.2019.00692
88. Morentin, P, Martinez-Sanchez, N, Roa, J, Ferno, J, Nogueiras, R, Tena-Sempere, M, et al. Hypothalamic mTOR: the rookie energy sensor. Curr Mol Med. (2014) 14:3–21. doi: 10.2174/1566524013666131118103706
89. Correia, B, Sousa, MI, and Ramalho-Santos, J. The mTOR pathway in reproduction: from gonadal function to developmental coordination. Reproduction. (2020) 159:R173–88. doi: 10.1530/REP-19-0057
90. Moreira, BP, Oliveira, PF, and Alves, MG. Molecular mechanisms controlled by mTOR in male reproductive system. Int J Mol Sci. (2019) 20:1633. doi: 10.3390/ijms20071633
91. Dupont, J, Reverchon, M, Bertoldo, MJ, and Froment, P. Nutritional signals and reproduction. Mol Cell Endocrinol. (2014) 382:527–37. doi: 10.1016/j.mce.2013.09.028
92. Jesus, TT, Oliveira, PF, Sousa, M, Cheng, CY, and Alves, MG. Mammalian target of rapamycin (mTOR): a central regulator of male fertility? Crit Rev Biochem Mol Biol. (2017) 52:235–53. doi: 10.1080/10409238.2017.1279120
93. Ran, Z, Liu, R, Shi, H, Wang, X, Wu, Z, Zhou, S, et al. mTOR signaling mediates energy metabolic equilibrium in bovine and mouse oocytes during the ovulatory phase. Biol Reprod. (2025) 112:474–84. doi: 10.1093/biolre/ioae182
94. Tian, Y, Niu, Y, Zhang, X, Wang, T, Tian, Z, Zhang, X, et al. The transcriptomic signature of donkey ovarian tissue revealed by cross-species comparative analysis at single-cell resolution. Animals. (2025) 15:1761. doi: 10.3390/ani15121761
95. Chronowska, E. High-throughput analysis of ovarian granulosa cell transcriptome. Biomed Res Int. (2014) 2014:213570. doi: 10.1155/2014/213570
96. Craig, ZR, Wang, W, and Flaws, JA. Endocrine-disrupting chemicals in ovarian function: effects on steroidogenesis, metabolism and nuclear receptor signaling. Reproduction. (2011) 142:633–46. doi: 10.1530/REP-11-0136
97. Mlynarcikova, A, Fickova, M, and Scsukova, S. Impact of endocrine disruptors on ovarian steroidogenesis. Endocr Regul. (2014) 48:201–24. doi: 10.4149/endo_2014_04_201
98. Bornstein, S, Rutkowski, H, and Vrezas, I. Cytokines and steroidogenesis. Mol Cell Endocrinol. (2004) 215:135–41. doi: 10.1016/j.mce.2003.11.022
99. Rajakumar, A, and Senthilkumaran, B. Steroidogenesis and its regulation in teleost-a review. Fish Physiol Biochem. (2020) 46:803–18. doi: 10.1007/s10695-019-00752-0
100. Selvaraj, V, Stocco, DM, and Clark, BJ. Current knowledge on the acute regulation of steroidogenesis. Biol Reprod. (2018) 99:13–26. doi: 10.1093/biolre/ioy102
101. Hu, Z, Shen, WJ, Kraemer, FB, and Azhar, S. Regulation of adrenal and ovarian steroidogenesis by miR-132. J Mol Endocrinol. (2017) 59:269–83. doi: 10.1530/JME-17-0011
102. Patel, S, Zhou, C, Rattan, S, and Flaws, JA. Effects of endocrine-disrupting chemicals on the ovary. Biol Reprod. (2015) 93:20. doi: 10.1095/biolreprod.115.130336
103. Bloom, MS, Mok-Lin, E, and Fujimoto, VY. Bisphenol a and ovarian steroidogenesis. Fertil Steril. (2016) 106:857–63. doi: 10.1016/j.fertnstert.2016.08.021
104. Ottinger, MA, Lavoie, E, Thompson, N, Barton, A, Whitehouse, K, Barton, M, et al. Neuroendocrine and behavioral effects of embryonic exposure to endocrine disrupting chemicals in birds. Brain Res Rev. (2008) 57:376–85. doi: 10.1016/j.brainresrev.2007.08.011
105. Schantz, SL, and Widholm, JJ. Cognitive effects of endocrine-disrupting chemicals in animals. Environ Health Perspect. (2001) 109:1197–206. doi: 10.1289/ehp.011091197
106. Clotfelter, ED, Bell, AM, and Levering, KR. The role of animal behaviour in the study of endocrine-disrupting chemicals. Anim Behav. (2004) 68:665–76. doi: 10.1016/j.anbehav.2004.05.004
107. Diamanti-Kandarakis, E, Bourguignon, JP, Giudice, LC, Hauser, R, Prins, GS, Soto, AM, et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. (2009) 30:293–342. doi: 10.1210/er.2009-0002
108. Nelson, W, Wang, YX, Sakwari, G, and Ding, YB. Review of the effects of perinatal exposure to endocrine-disrupting chemicals in animals and humans. Rev Environ Contam Toxicol. (2020) 251:131–84. doi: 10.1007/398_2019_30
109. Patisaul, HB, Fenton, SE, and Aylor, D. Animal models of endocrine disruption. Best Pract Res Clin Endocrinol Metab. (2018) 32:283–97. doi: 10.1016/j.beem.2018.03.011
110. Asadi, A, Ghahremani, R, Abdolmaleki, A, and Rajaei, F. Role of sperm apoptosis and oxidative stress in male infertility: a narrative review. Int J Reprod Biomed. (2021) 19:493–504. doi: 10.18502/ijrm.v19i6.9371
111. Johnstone, J, Nash, S, Hernandez, E, and Rahman, MS. Effects of elevated temperature on gonadal functions, cellular apoptosis, and oxidative stress in Atlantic Sea urchin Arbacia punculata. Mar Environ Res. (2019) 149:40–9. doi: 10.1016/j.marenvres.2019.05.017
112. Zhou, Z, Zhou, B, Chen, H, Lu, K, and Wang, Y. Oxidative stress activates the Nrf2-mediated antioxidant response and P38 MAPK pathway: a possible apoptotic mechanism induced by BDE-47 in rainbow trout (Oncorhynchus mykiss) gonadal RTG-2 cells. Environ Pollut. (2021) 287:117341. doi: 10.1016/j.envpol.2021.117341
113. Yilmaz, BO, Yildizbayrak, N, Aydin, Y, and Erkan, M. Evidence of acrylamide-and glycidamide-induced oxidative stress and apoptosis in Leydig and Sertoli cells. Hum Exp Toxicol. (2017) 36:1225–35. doi: 10.1177/0960327116686818
114. Fujii, J, Iuchi, Y, Matsuki, S, and Ishii, T. Cooperative function of antioxidant and redox systems against oxidative stress in male reproductive tissues. Asian J Androl. (2003) 5:231–42.
115. Agarwal, A, Gupta, S, and Sharma, RK. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol. (2005) 3:1–21. doi: 10.1186/1477-7827-3-28
116. Agarwal, A, Said, TM, Bedaiwy, MA, Banerjee, J, and Alvarez, JG. Oxidative stress in an assisted reproductive techniques setting. Fertil Steril. (2006) 86:503–12. doi: 10.1016/j.fertnstert.2006.02.088
117. Wang, L, Tang, J, Wang, L, Tan, F, Song, H, Zhou, J, et al. Oxidative stress in oocyte aging and female reproduction. J Cell Physiol. (2021) 236:7966–83. doi: 10.1002/jcp.30468
118. Billig, H, Chun, SY, Eisenhauer, K, and Hsueh, AJ. Gonadal cell apoptosis: hormone-regulated cell demise. Hum Reprod Update. (1996) 2:103–17. doi: 10.1093/humupd/2.2.103
119. Vukusic Pusic, T, Janjic, T, Dujmovic, I, Poljicanin, A, Soljic, V, Saraga-Babic, M, et al. The involvement of proliferation and apoptosis in the early human gonad development. J Mol Histol. (2013) 44:55–63. doi: 10.1007/s10735-012-9455-6
120. Shaha, C, Tripathi, R, and Mishra, DP. Male germ cell apoptosis: regulation and biology. Philos Trans R Soc B Biol Sci. (2010) 365:1501–15. doi: 10.1098/rstb.2009.0124
121. Matsui, Y. Regulation of germ cell death in mammalian gonads. APMIS. (1998) 106:142–8. doi: 10.1111/j.1699-0463.1998.tb01329.x
122. Mishra, A, Reddy, I, and Mondal, S. Modern concept in optimizing reproductive efficiency of seasonal breeders. Livest Res Int. (2015) 1:51–7.
123. Viblanc, VA, Schull, Q, Roth, JD, Rabdeau, J, Saraux, C, Uhlrich, P, et al. Maternal oxidative stress and reproduction. Funct Ecol. (2018) 32:722–35. doi: 10.1111/1365-2435.13032
124. Metcalfe, NB, and Monaghan, P. Does reproduction cause oxidative stress? An open question. Trends Ecol Evol. (2013) 28:347–50. doi: 10.1016/j.tree.2013.01.015
125. Sikiru, A. Oxidative stress and reproductive inefficiencies: the science, evidences, and solutions. Agric. Ext J. (2018) 2018:136. doi: 10.22377/aextj.v2i01.54
126. Ruder, EH, Hartman, TJ, Blumberg, J, and Goldman, MB. Oxidative stress and antioxidants: exposure and impact on female fertility. Hum Reprod Update. (2008) 14:345–57. doi: 10.1093/humupd/dmn011
127. Liew, FF, Dutta, S, and Sengupta, P. Fertility treatment-induced oxidative stress and reproductive disorders. J Integr Sci Technol. (2024) 12:756–6. doi: 10.62110/sciencein.jist.2024.v12.756
128. Banerjee, P, and Bhattacharya, J. Impact of oxidative stress on infertility, with emphasis on infertility management strategies. Glob J Fertil Res. (2019) 4:010–8. doi: 10.17352/gjfr.000012
129. Gupta, S, Sekhon, L, Kim, Y, and Agarwal, A. The role of oxidative stress and antioxidants in assisted reproduction. Curr Womens Health Rev. (2010) 6:227–38. doi: 10.2174/157340410792007046
130. O’Flaherty, C, and Scarlata, E. Oxidative stress and reproductive function: the protection of mammalian spermatozoa against oxidative stress. Reproduction. (2022) 164:F67–78. doi: 10.1530/REP-22-0200
131. Almansa-Ordonez, A, Bellido, R, Vassena, R, Barragan, M, and Zambelli, F. Oxidative stress in reproduction: a mitochondrial perspective. Biology. (2020) 9:269. doi: 10.3390/biology9090269
132. Curlewis, J. Seasonal prolactin secretion and its role in seasonal reproduction: a review. Reprod Fertil Dev. (1992) 4:1–23. doi: 10.1071/RD9920001
133. Stewart, C, and Marshall, CJ. Seasonality of prolactin in birds and mammals. J Exp Zool A Ecol Integr Physiol. (2022) 337:919–38. doi: 10.1002/jez.2634
134. Sharp, PJ, and Blache, D. A neuroendocrine model for prolactin as the key mediator of seasonal breeding in birds under long-and short-day photoperiods. Can J Physiol Pharmacol. (2003) 81:350–8. doi: 10.1139/y03-025
135. Johnston, J. Photoperiodic regulation of prolactin secretion: changes in intra-pituitary signalling and lactotroph heterogeneity. J Endocrinol. (2004) 180:351–6. doi: 10.1677/joe.0.1800351
136. Morgan, PJ. The pars tuberalis: the missing link in the photoperiodic regulation of prolactin secretion? J Neuroendocrinol. (2000) 12:287–95. doi: 10.1046/j.1365-2826.2000.00459.x
137. Lincoln, G, Andersson, H, and Hazlerigg, D. Clock genes and the long-term regulation of prolactin secretion: evidence for a photoperiod/circannual timer in the pars tuberalis. J Neuroendocrinol. (2003) 15:390–7. doi: 10.1046/j.1365-2826.2003.00990.x
138. Stirland, J, Stirland, JA, Johnston, JD, Cagampang, FR, Morgan, PJ, Castro, MG, et al. Photoperiodic regulation of prolactin gene expression in the Syrian hamster by a pars tuberalis-derived factor. J Neuroendocrinol. (2001) 13:147–57. doi: 10.1046/j.1365-2826.2001.00611.x
139. Crawford, HM, Morin, DE, Wall, EH, McFadden, TB, and Dahl, GE. Evidence for a role of prolactin in mediating effects of photoperiod during the dry period. Animals. (2015) 5:803–20. doi: 10.3390/ani5030385
140. Okkens, A, Okkens, AC, Bevers, MM, Dieleman, SJ, and Willemse, AH. Evidence for prolactin as the main luteotrophic factor in the cyclic dog. Vet Q. (1990) 12:193–201. doi: 10.1080/01652176.1990.9694266
141. Iancu, ME, Albu, AI, and Albu, DN. Prolactin relationship with fertility and in vitro fertilization outcomes—a review of the literature. Pharmaceuticals. (2023) 16:122. doi: 10.3390/ph16010122
142. Hoffmann, B, Schams, D, Bopp, R, Ender, ML, Giménez, T, and Karg, H. Luteotrophic factors in the cow: evidence for LH rather than prolactin. Reproduction. (1974) 40:77–85. doi: 10.1530/jrf.0.0400077
143. Tortonese, DJ. Intrapituitary mechanisms underlying the control of fertility: key players in seasonal breeding. Domest Anim Endocrinol. (2016) 56:S191–203. doi: 10.1016/j.domaniend.2016.01.002
144. Thiéry, J-C, Chemineau, P, Hernandez, X, Migaud, M, and Malpaux, B. Neuroendocrine interactions and seasonality. Domest Anim Endocrinol. (2002) 23:87–100. doi: 10.1016/S0739-7240(02)00148-0
145. Dobolyi, A, Oláh, S, Keller, D, Kumari, R, Fazekas, EA, Csikós, V, et al. Secretion and function of pituitary prolactin in evolutionary perspective. Front Neurosci. (2020) 14:621. doi: 10.3389/fnins.2020.00621
146. Roy, KS, and Prakash, BS. Seasonal variation and circadian rhythmicity of the prolactin profile during the summer months in repeat-breeding Murrah buffalo heifers. Reprod Fertil Dev. (2007) 19:569–75. doi: 10.1071/RD06093
147. Brooks, CL. Molecular mechanisms of prolactin and its receptor. Endocr Rev. (2012) 33:504–25. doi: 10.1210/er.2011-1040
148. Yu-Lee, L-Y. Molecular actions of prolactin in the immune system. Proc Soc Exp Biol Med. (1997) 215:35–52. doi: 10.3181/00379727-215-44111
149. Freeman, ME, Kanyicska, B, Lerant, A, and Nagy, G. Prolactin: structure, function, and regulation of secretion. Physiol Rev. (2000) 80:1523–631. doi: 10.1152/physrev.2000.80.4.1523
151. Koike, K, Miyake, A, Aono, T, Sakumoto, T, Ohmichi, M, Yamaguchi, M, et al. Effect of prolactin on the secretion of hypothalamic GnRH and pituitary gonadotropins. Horm Res. (1991) 35:5–12. doi: 10.1159/000181921
152. Aıssanou, S, Besseboua, O, and Ayad, A. Some reproductive characteristics in common donkey male (Equus asinus)-a mini review. Turk J Vet Res. (2022) 6:77–84. doi: 10.47748/tjvr.1083287
153. Zhang, Z, Huang, B, Wang, Y, Zhu, M, Liu, G, and Wang, C. A survey report on the donkey original breeding farms in China: current aspects and future prospective. Front Vet Sci. (2023) 10:1126138. doi: 10.3389/fvets.2023.1126138
154. Abdel-maksoud, FM, Zayed, AE, Abdelhafez, EA, and Hussein, MT. Seasonal variations of the epididymis in donkeys (Equus asinus) with special reference to blood epididymal barrier. Microsc Res Tech. (2024) 87:326–38. doi: 10.1002/jemt.24436
155. Akhtar, MF, Umar, M, Chai, W, Li, L, Ahmad, E, and Wang, C. Effect of inhibin immunization on reproductive hormones and testicular morphology of Dezhou donkeys during the non-breeding season. Animals. (2025) 15:813. doi: 10.3390/ani15060813
156. Segabinazzi, LG, Segabinazzi, LGTM, Gilbert, RO, Ambrosia, RL, Bergfelt, DR, Samper, JC, et al. Structural and functional dynamics of the ovary and uterus during the estrous cycle in donkeys in the eastern Caribbean. Animals. (2022) 13:74. doi: 10.3390/ani13010074
157. Díaz-Jiménez, M, Rota, A, Dorado, J, Consuegra, C, Pereira, B, Camillo, F, et al. First pregnancies in jennies with vitrified donkey semen using a new warming method. Animal. (2021) 15:100097. doi: 10.1016/j.animal.2020.100097
158. González, N, Peñalosa, A, de Blas, I, and Gil, L. Sustainable alternatives to the reduction of plastic straws used with chilled equine semen. Animals. (2024) 14:3388. doi: 10.3390/ani14233388
159. Gambini, A, Smith, JM, Gurkin, RJ, and Palacios, PD. Current and emerging advanced techniques for breeding donkeys and mules. Animals. (2025) 15:990. doi: 10.3390/ani15070990
160. BuSaleh, LS. Investigating the role played by the sperm-specific oocyte activation factor Phspholipase C zeta (PLCzeta) in embryo development and fertility treatment outcome in human patients. Saudi Arabia: Alfaisal University (2022).
161. Arroyo-Salvo, C, Cogollo Villarreal, MY, Clérico, G, Flores Bragulat, AP, Niño Vargas, A, Castañeira, C, et al. The ability of donkey sperm to induce oocyte activation and mule embryo development after ICSI. Theriogenology. (2024) 218:200–7. doi: 10.1016/j.theriogenology.2024.02.002
162. Wu, R, Yu, F, Holyoak, GR, Gao, Y, Zhu, Y, and Li, J. Use of the endometrial histopathology to improve diagnosis of donkeys with endometritis. Equine Vet Educ. (2024) 36:43–50. doi: 10.1111/eve.13815
163. Sullivan, R, and Boocock, H. Managing obesity and metabolic disease in donkeys. In Pract. (2025) 47:32–7. doi: 10.1002/inpr.504
164. Zhang, Z, Gao, X, Faheem, M, Wang, Y, Wang, T, Shi, X, et al. Comparative analysis of growth and development characteristics of two Dezhou donkey strains. Livest Sci. (2022) 263:105024. doi: 10.1016/j.livsci.2022.105024
165. Rigg, R. Are donkey’s good livestock guardians? Carnivore Damage Prevention News, No. 24. pp. 11–17. (2022).
166. Kopycińska, K, Wojtulewicz, K, Herman, AP, and Tomaszewska-Zaremba, D. The effect of photoperiodic conditions on GnRH/LH secretion in ewes. Animals. (2022) 12:283. doi: 10.3390/ani12030283
167. Masson-Pévet, M, and Gauer, F. Seasonality and melatonin receptors in the pars tuberalis in some long day breeders. Neurosignals. (2004) 3:63–70. doi: 10.1159/000109527
168. Frohlich, P, and Meston, C. Evidence that serotonin affects female sexual functioning via peripheral mechanisms. Physiol Behav. (2000) 71:383–93. doi: 10.1016/S0031-9384(00)00344-9
169. Ezzati, M, Velaei, K, and Kheirjou, R. Melatonin and its mechanism of action in the female reproductive system and related malignancies. Mol Cell Biochem. (2021) 476:3177–90. doi: 10.1007/s11010-021-04151-z
170. Thompson, D, Godke, R, and Nett, T. Effects of melatonin and thyrotropin releasing hormone on mares during the nonbreeding season. J Anim Sci. (1983) 56:668–77. doi: 10.2527/jas1983.563668x
171. Giannetto, C, Fazio, F, Alberghina, D, Giudice, E, and Piccione, G. Clock genes expression in peripheral leukocytes and plasma melatonin daily rhythm in horses. J Equine Vet Sci. (2020) 84:102856. doi: 10.1016/j.jevs.2019.102856
172. Nejad, SZ, Tehrani, FR, and Zadeh-Vakili, A. The role of kisspeptin in female reproduction. Int J Endocrinol Metab. (2017) 15:e44337. doi: 10.5812/ijem.44337
173. Moore, AM, Novak, AG, and Lehman, MN. KNDy neurons of the hypothalamus and their role in GnRH pulse generation: an update. Endocrinology. (2024) 165:bqad194. doi: 10.1210/endocr/bqad194
174. Mittelman-Smith, MA, Krajewski-Hall, SJ, McMullen, NT, and Rance, NE. Ablation of KNDy neurons results in hypogonadotropic hypogonadism and amplifies the steroid-induced LH surge in female rats. Endocrinology. (2016) 157:2015–27. doi: 10.1210/en.2015-1740
176. Fergani, C, and Navarro, VM. Expanding the role of tachykinins in the neuroendocrine control of reproduction. Reproduction. (2016) 153:R1–R14. doi: 10.1530/REP-16-0378
177. Garcia, D, and Shaw, RJ. AMPK: mechanisms of cellular energy sensing and restoration of metabolic balance. Mol Cell. (2017) 66:789–800. doi: 10.1016/j.molcel.2017.05.032
178. Dupont, J, Reverchon, M, Cloix, L, Froment, P, and Ramé, C. Involvement of adipokines, AMPK, PI3K and the PPAR signaling pathways in ovarian follicle development and cancer. Int J Dev Biol. (2012) 56:959–67. doi: 10.1387/ijdb.120134jd
179. Wu, C-W, and Storey, KB. mTOR signaling in metabolic stress adaptation. Biomolecules. (2021) 11:681. doi: 10.3390/biom11050681
180. Xu, J, Ji, J, and Yan, X-H. Cross-talk between AMPK and mTOR in regulating energy balance. Crit Rev Food Sci Nutr. (2012) 52:373–81. doi: 10.1080/10408398.2010.500245
181. Robinson, J. Nutrition and reproduction. Anim Reprod Sci. (1996) 42:25–34. doi: 10.1016/0378-4320(96)01526-6
182. Guzmán, C, Hernández-Bello, R, and Morales-Montor, J. Regulation of steroidogenesis in reproductive, adrenal and neural tissues by cytokines. Open Neuroendocrinol J. (2010) 3:161–9.
183. Fowler, PA, Bellingham, M, Sinclair, KD, Evans, NP, Pocar, P, Fischer, B, et al. Impact of endocrine-disrupting compounds (EDCs) on female reproductive health. Mol Cell Endocrinol. (2012) 355:231–9. doi: 10.1016/j.mce.2011.10.021
184. Sharma, A, Mollier, J, Brocklesby, RWK, Caves, C, Jayasena, CN, and Minhas, S. Endocrine-disrupting chemicals and male reproductive health. Reprod Med Biol. (2020) 19:243–53. doi: 10.1002/rmb2.12326
185. Zlatnik, MG. Endocrine-disrupting chemicals and reproductive health. J Midwifery Womens Health. (2016) 61:442–55. doi: 10.1111/jmwh.12500
186. Ahmad, G, et al. Overview and sources of reactive oxygen species (ROS) in the reproductive system In: G Ahmad, editor. Oxidative stress in human reproduction: Shedding light on a complicated phenomenon. Berlin: Springer (2017). 1–16.
187. Prasad, S, Tiwari, M, Pandey, AN, Shrivastav, TG, and Chaube, SK. Impact of stress on oocyte quality and reproductive outcome. J Biomed Sci. (2016) 23:1–5. doi: 10.1186/s12929-016-0253-4
188. Poljsak, B. Strategies for reducing or preventing the generation of oxidative stress. Oxidative Med Cell Longev. (2011) 2011:194586. doi: 10.1155/2011/194586
189. Moore, KE. Interactions between prolactin and dopaminergic neurons. Biol Reprod. (1987) 36:47–58. doi: 10.1095/biolreprod36.1.47
190. Hurcombe, SD. Hypothalamic-pituitary gland axis function and dysfunction in horses. Vet Clin. (2011) 27:1–17. doi: 10.1016/j.cveq.2010.12.006
191. Reiter, RJ, Tan, DX, Manchester, LC, Paredes, SD, Mayo, JC, and Sainz, RM. Melatonin and reproduction revisited. Biol Reprod. (2009) 81:445–56. doi: 10.1095/biolreprod.108.075655
192. Sukumaran, A, Choi, K, and Dasgupta, B. Insight on transcriptional regulation of the energy sensing AMPK and biosynthetic mTOR pathway genes. Front Cell Dev Biol. (2020) 8:671. doi: 10.3389/fcell.2020.00671
Keywords: seasonal breeding, donkeys, hypothalamic–pituitary-gonadal axis, melatonin, KNDy neurons, oxidative stress, steroidogenesis, reproductive efficiency
Citation: Akhtar MF, Ali S, Hassan F, Changfa W (2025) Molecular pathways affecting reproductive efficiency in seasonal breeders: prospects and implications for improving fertility in donkeys. Front. Vet. Sci. 12:1633945. doi: 10.3389/fvets.2025.1633945
Edited by:
Amal M. Aboelmaaty, National Research Centre (Egypt), EgyptReviewed by:
Ahm Musleh Uddin, University of Adelaide, AustraliaLina Maria Correa Estrad, Santo Tomás University, Chile
Copyright © 2025 Akhtar, Ali, Hassan and Changfa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Faizul Hassan, Zi5oYXNzYW5AY3V2YXMuZWR1LnBr; Muhammad Faheem Akhtar, ZmFoZWVtQGxjdS5lZHUuY24=; Wang Changfa, d2FuZ2NoYW5nZmFAbGN1LmVkdS5jbg==
 Muhammad Faheem Akhtar
Muhammad Faheem Akhtar Shahzad Ali
Shahzad Ali Faizul Hassan
Faizul Hassan Wang Changfa1*
Wang Changfa1*