- 1Department of Animal Science, Cornell University, Ithaca, NY, United States
- 2Department of Clinical Sciences, College of Veterinary Medicine, Cornell University, Ithaca, NY, United States
- 3Toxicology Research Laboratory, Department of Pharmacology, University of Illinois at Chicago, Chicago, IL, United States
- 4Department of Molecular Medicine, Cornell University, Ithaca, NY, United States
Introduction: The rapidly expanding market for therapeutic cannabinoid products has intensified research on their safety and efficacy in pets. Full-spectrum hemp extracts contain compounds such as terpenoids and flavonoids that may act synergistically via the “entourage effect,” yet their application in companion animals remains underexplored. This study assessed the pharmacokinetics and safety of isolated and full-spectrum cannabinoids in dogs.
Methods: Eight healthy adult Beagle dogs (four males and four females) were randomly assigned to a 4 × 4 Latin square design (two dogs per kennel, same sex), consisting of four experimental periods and four treatments: CBD isolate (1 mg/kg), CBDA isolate (1 mg/kg), CBDA full spectrum (FS) (1 mg/kg), and a combined CBD/CBDA FS (1 mg/kg). Treatments were administered twice daily (every 12 h). In the morning, dogs received their assigned treatment following their daily ration of dry kibble and were immediately offered 122 grams of wet food. Each experimental period lasted 1 week and was followed by a three-week washout period.
Results: No adverse events were associated with any treatment. CBDA showed higher Cmax and AUC than CBD in both isolate and FS forms (p < 0.001). CBDA in CBD/CBDA FS had a shorter Tmax compared to CBD (p = 0.019). Mean residence time and elimination half-life did not differ among treatments.
Conclusion: CBDA demonstrated superior absorption compared to CBD. No evidence supported enhanced absorption from full-spectrum products, suggesting the “entourage effect” may involve receptor-level interactions rather than absorption. All treatments were well tolerated, with normal CBC and chemistry results, indicating that administering CBD or CBDA, either as isolates or in full-spectrum extracts, at 1 mg/kg every 12 h for 1 week is safe in healthy adult dogs. This is the first comprehensive comparison of full-spectrum, isolate, and acidic cannabinoid forms in dogs.
1 Introduction
In companion animal medicine, cannabinoids have gained significant interest over the past decade. In 2018, Epidiolex, a hemp-based CBD isolate drug for epilepsy in young children, received FDA approval and was successfully launched on the market, demonstrating safety for long-term use (1, 2). Both cannabinoid isolates and full-spectrum CBD-rich products have also been evaluated in animals for safety and pharmaceutical applications (3, 4). In dogs diagnosed with idiopathic epilepsy, CBD- and CBDA-rich hemp products have been associated with reduced seizure frequency (5). According to a recent published review article (3), five human studies have shown that CBD can alleviate pain, thereby improving quality of life and allowing for reduced dosage of other medications as part of a multimodal pain management approach.
Additionally, seven other studies have reported beneficial effects of CBD/CBDA-based nutraceuticals in treating canine osteoarthritis (3). However, data on the absorption, retention, and clinical efficacy of various cannabinoid form in dogs remain limited. To date, only three studies (6–8) have investigated the safety and tolerability of acidic cannabinoids such as CBDA in dogs. While some publications have addressed the efficacy of full hemp extract, comprehensive data on full-spectrum cannabinoids are still lacking. Preclinical studies in rodents and humans suggest that differences may exist in the absorption kinetics of CBD and CBDA when delivered as isolates versus full-spectrum hemp products containing other minor cannabinoids (9, 10). A thorough understanding of the pharmacokinetics and safety profiles of CBD and CBDA in dogs is essential before developing evidence-based guidelines for their use in veterinary medicine.
The objectives of this research were twofold: first, to determine the pharmacokinetics of full-spectrum cannabinoid products versus isolates (CBD and CBDA) and limited metabolite production in adult beagle dogs; and second, to assess the safety and tolerability of both cannabinoid forms, the isolates and the full-spectrum formulations (CBD isolate oil, CBDA isolate oil, full-spectrum CBDA oil, and a full-spectrum CBD/CBDA mixture) in healthy adult beagles. We hypothesized that, compared to CBD, acidic cannabinoids (CBDA) and full-spectrum (FS) cannabinoids would exhibit greater absorption in adult dogs.
2 Materials and methods
2.1 Animals and study design
All experimental procedures were approved by the Cornell University Animal Care and Use Committee (protocol #2022–0211).
Eight healthy adult Beagles (four males and four females), all 2 years old and weighing between 7.5 and 11.3 kg, were used in this study. The dogs were owned by the Cornell University College of Veterinary Medicine teaching dog colony. Prior to the study, same-sex dogs were housed in groups of four in separate temperature-controlled rooms maintained at 26°C. For the study, dogs were re-paired and housed in pairs within 2 × 5 m kennels, with two kennels per room. Dogs were acclimated to the new housing arrangement for 2 days before the experimental procedures began.
Kennels were randomly assigned to a 4 × 4 Latin square design with four experimental periods and four treatments: CBD isolate (1 mg/kg), CBDA isolate (1 mg/kg), CBDA FS (1 mg/kg), and CBD/CBDA FS (1 mg/kg). Treatments were administered twice daily (every 12 hours). In the morning, dogs received their assigned treatment following their daily ration of dry kibble and were immediately offered 122 grams of wet food. Each experimental period lasted 1 week and was followed by a three-week washout period. At the beginning of each experimental period, dogs were weighed to calculate the appropriate dosage of cannabinoids per treatment. The initial body weight (mean kg ± standard deviation) across treatment groups were 9.27 ± 1.33 (CBD isolate), 9.26 ± 1.35 (CBDA isolate), 9.40 ± 1.48 (CBDA FS) and 9.44 ± 1.44 (CBD/CBDA FS).
All dogs were fed dry food (LabDiet 5 L18, PMI Nutrition International, Brentwood, MO, USA) once a day in the morning (06.00). During each experimental period, treatments were administered directly into the dogs’ mouth using a 1-mL Luer-slip type syringe. Following dosing, each dog received an additional portion of their daily dietary allocation consisting of 122 grams of wet food (Purina Pro Plan Savory Chicken and Rice Formula, Nestle Purina, St. Louis, MO) in the morning (07.00) and the evening (19.00), immediately after treatments throughout the study.
2.2 Cannabinoid products
Isolates of CBD and CBDA (Open Book Extracts, Roxboro, NC) were each emulsified in sesame oil to produce 30 mg/mL suspensions. A full hemp extract (CBDA FS) obtained from Cultivate Biologics (Louisville, CO) was emulsified in sesame seed oil to yield a suspension containing 25 mg/mL of CBDA, 1.1 mg/mL THCA, 1.2 mg/mL CBCA, 0.5 mg/mL CBGA, 0.1 mg/mL CBDVA, and 1.1 mg/mL CBD. Concentrations of other cannabinoids were below the limits of detection. A full-spectrum hemp extract (CBD/CBDA FS) from Cultivate Biologics (Louisville, CO) was emulsified in sesame oil to yield a suspension of 28.2 mg/mL CBD, 27 mg/mL CBDA, 0.5 mg/mL CBG, 0.4 mg/mL CBGA, 1.9 mg/mL THC, 0.6 mg/mL THCA, 1.4 mg/mL CBC, and 1.7 mg/mL CBCA, with other cannabinoids being below the limits of detection. All extracts were tested every 2 weeks and showed less than 5% variation from the original screening, indicating stability.
2.3 Data collection
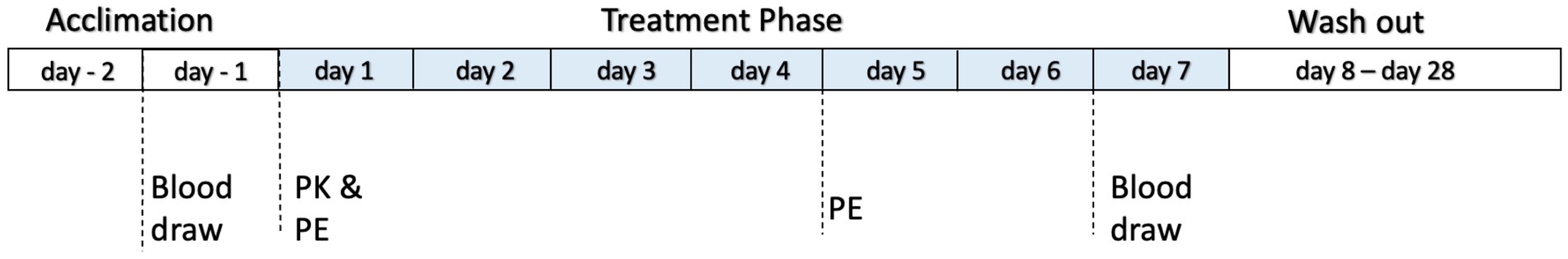
On day 1, pharmacokinetic (PK) sampling was conducted over a 12-h period, with blood samples (2 mL) collected at 0.5, 1, 2, 4, 8, and 12 h post oral dosing. From days 2 to 7, dogs received twice-daily dosing at 12-h intervals. Blood samples (6 mL) were collected 1 day prior to the start of the study and 6 h after the morning dosing on day 7 to assess hepatic chemistry and serum cannabinoid profiles. A timeline of this study is presented in Figure 1. The PK sampling was conducted over a limited 12-h time window, as the highest observed concentration (Cmax), the time point to reach maximum concentration (Tmax), and the area under the concentration–time curve (AUC), representing total systemic exposure, were the primary parameters used to assess absorption rate and extent. This design was informed by prior literature indicating that absorption occurs within 2 h in many studies involving dogs. An abbreviated PK protocol was therefore implemented, with careful consideration given to dog welfare. Physical examinations were performed on days 1 and 5 at 1 and 4 h post dosing, with a primary focus on identifying any neurological adverse events.
For blood sample collection, dogs were manually restrained. Either cephalic or jugular vein was used, and blood was transferred into serum tubes for the analysis of cannabinoids and hepatic serum chemistry. Samples were allowed to coagulate for 30–60 min, at room temperature, followed by centrifugation at 1,700 × g for 6 min. Serum was then aliquoted: one 2-mL aliquot was submitted for clinical biochemistry, and two 0.5-mL aliquots were immediately frozen at −80°C for subsequent cannabinoid profile analysis.
2.4 Physical exam
Prior to enrollment in the study (day 0), physical examinations were conducted to evaluate the dogs’ health. On days 1 and 5, dogs were examined at 1 and 4 h post dosing. Heart rate was measured and neurologic functions, including hopping proprioception response, protrusion of the nictitating membrane, and palpebral reflex, were assessed as potential adverse events in dogs (11, 12). Signs of lethargy, vomiting, and diarrhea were also closely monitored.
2.5 Sample analyses
2.5.1 Complete blood count and serum chemistry
Blood samples for complete blood counts and serum biochemistry profile were submitted to Cornell University Diagnostic Laboratory Clinical Pathology service for analyses.
2.5.2 Serum cannabinoids
Analysis of cannabinoids in dog serum was performed at the Toxicology Research Laboratory, University of Illinois at Chicago, as described previously for various species (6, 13–16), with the modification that 6-OH-CBD was added with a reference analyte, as shown in Supplementary Table 1.
2.5.3 Pharmacokinetics
The non-compartmental 12-h pharmacokinetic analysis for each hemp-derived cannabinoid (CBD, CBDA, CBG, CBGA, Δ9-THC, THCA) was performed utilizing a commercial software system (PK solutions 2.0, Summit PK, Montrose, CO). Semi-log plots were utilized to determine linearity of the elimination profiles. The results generated were time to maximal concentrations (Tmax), maximum serum concentration (Cmax), elimination half-life (T 12), area under the curve to the last time-point (AUC0–12), and mean residence time (MRT). The program predicts steady state average serum concentrations (Css Ave) based on the assumption that steady state levels are achieved after 5 half-lives with selected frequencies of administration.
2.6 Statistical analysis
Statistical analysis of PK parameters was performed using R version 4.3.2 (RStudio, PBC, Boston, MA, USA). Pharmacokinetic parameters, including Cmax, Tmax, T½ elimination, AUC and MRT, were assessed for normality using the Shapiro–Wilk test. As the data were not normally distributed (p < 0.05), all PK parameters were log-transformed prior to statistical analysis. Treatment effects were evaluated using a linear mixed-effects model, with period and treatment as fixed effects and dog and kennel as random effects to account for individual and housing variability.
Maximum serum concentration, Tmax, and AUC were calculated using data from all eight dogs. However, in several cases, CBD or CBDA blood concentrations were insufficient to estimate T1/2 el, MRT, and Css. Values for those parameters were not available for one dog in the CBD isolate treatment, three dogs in the CBDA isolate treatment, three dogs in the CBDA FS treatment, three dogs in the CBD component of the CBD/CBDA FS treatment, and four dogs in the CBDA component of the CBD/CBDA FS treatment. To address our specific hypotheses, treatment comparisons were pre-planned prior to data collection and analysis, as follows: CBD isolate vs. CBD in CBD/CBDA FS, CBDA isolate vs. CBDA in CBD/CBDA FS, CBD isolate versus CBDA isolate, and CBDA isolate versus CBDA FS. Statistical significance was set at p < 0.05. Differences in CBD and CBDA serum concentrations on day 7 in the respective primary treatment groups were evaluated. Paired T-test test was used to determine the difference for CBD between CBD isolate and CBD/CBDA FS, and Friedman test was conducted to determine the difference in CBDA between CBDA isolate, CBDA FS and CBDA/CBD FS.
Differences in 12-h serum cannabinoid metabolite concentrations between CBD isolate and CBDA/CBD FS groups were evaluated. Following confirmation of normal data distribution with Shapiro–Wilk test, Welch’s t-tests were used to compare concentrations between CBD isolate and CBDA/CBD FS groups for each metabolite.
3 Results
3.1 Twelve-hour pharmacokinetics
Pharmacokinetic data and p-values are presented in Tables 1, 2. For Cmax, CBDA isolate had a higher peak serum concentration compared to CBD isolate (235.51 ± 65.59 vs. 69.80 ± 35.44 ng/mL, p < 0.001). CBDA in CBD/CBDA FS had a higher Cmax than CBD in the same formulation (229.30 ± 145.51 vs. 64.66 ± 37.05 ng/mL, p < 0.001). No other treatment comparisons differed for Cmax.
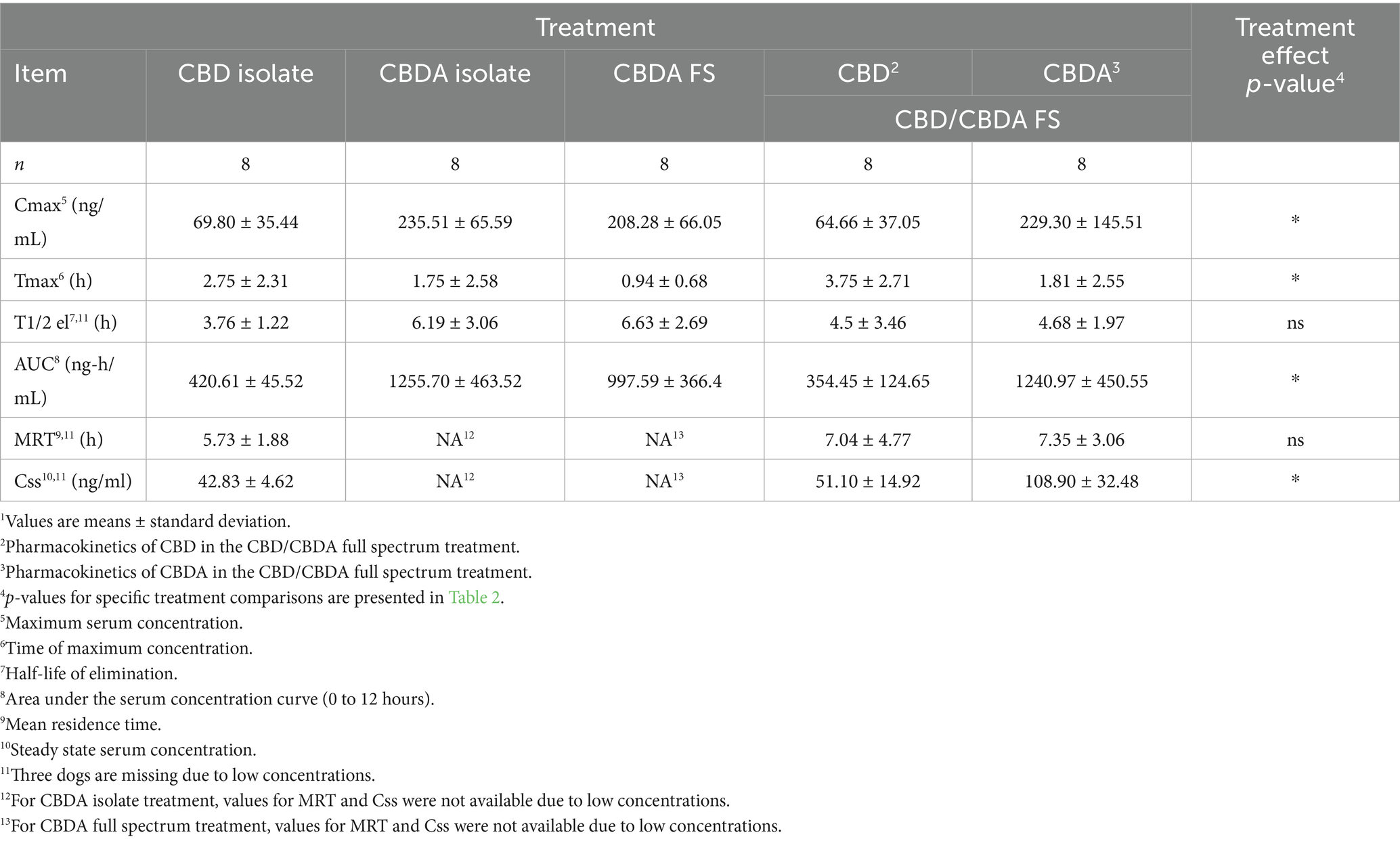
Table 1. Pharmacokinetics of CBD and CBDA in dogs administrated with CBD isolate, CBDA isolate, CBDA FS, CBD/CBDA FS oil1.
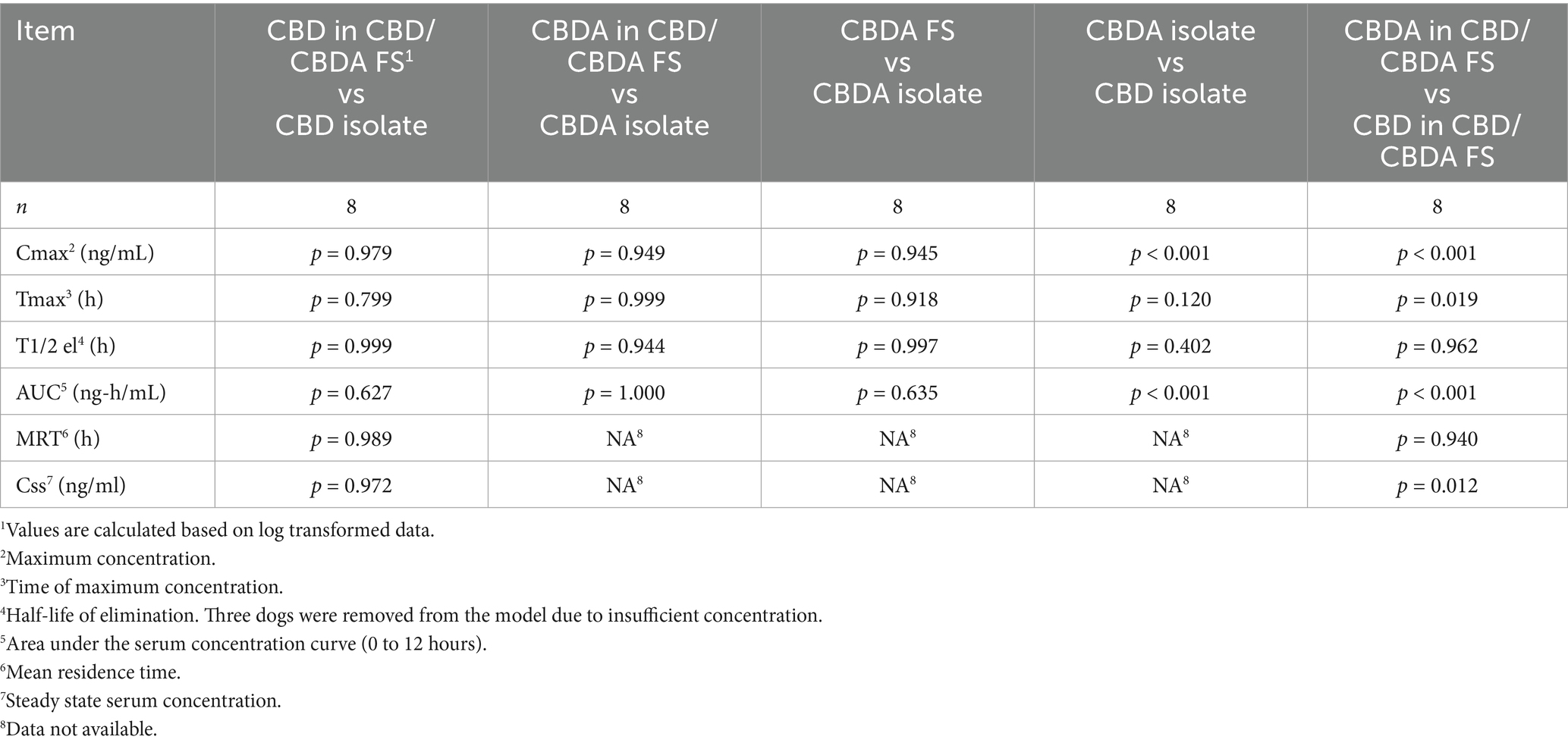
Table 2. p-value of specific treatment comparisons of the pharmacokinetics for dogs administrated with CBD isolate, CBDA isolate, CBDA FS, CBD/CBDA FS oil1.
For Tmax, CBDA in CBD/CBDA FS reached peak concentration earlier than CBD in the same formulation (1.81 ± 2.55 vs. 3.75 ± 2.71 h, p = 0.019). All other Tmax comparisons showed no differences.
The elimination half-life did not differ among any treatments. For AUC, CBDA isolate had a higher AUC than CBD isolate (1255.70 ± 463.52 vs. 420.61 ± 45.52 ng·h/mL, p < 0.001). CBDA in CBD/CBDA FS had a higher AUC than CBD in the same formulation (1240.97 ± 450.55 vs. 354.45 ± 124.65 ng·h/mL, p < 0.001). No other AUC comparisons differed.
Mean residence time and steady-state serum concentrations of CBD did not differ between CBD/CBDA FS and CBD isolate groups. Steady-state serum concentration of CBDA was higher than CBD in the CBD/CBDA FS group (108.9 ± 32.48 vs. 51.1 ± 14.92 ng/mL, p = 0.012).
On day 7, serum concentrations of CBD, CBDA, THC, and THCA were measured (Supplementary Figure 1). CBD and CBDA concentrations did not differ between treatments (p = 0.392). Trace amounts of THC and THCA were detected, primarily in the CBD/CBDA FS and CBDA FS groups.
Serum concentrations of the metabolites 6-OH-CBD and 7-COOH-CBD are presented in Supplementary Figures 2–4. No differences were observed between treatments for 6-OH-CBD or 7-COOH-CBD (p = 0.578 and p = 0.788, respectively). In the CBD/CBDA FS group, 7-COOH-CBD serum concentrations peaked at approximately 4 h post-dose. In the CBD isolate group, peak concentrations occurred between 4 and 8 h. Serum 6-OH-CBD concentrations in the CBD isolate group peaked at approximately 2 h and declined gradually. In the CBD/CBDA FS group, peak 6-OH-CBD concentrations were reached around 2 h, followed by a steady decline over 12 h.
3.2 Complete blood counts and serum chemistry
Mean and standard deviations of CBC values on days 1 and 7 are presented in Supplementary Tables 2, 3. All values were within reference ranges. Blood chemistry profiles are summarized in Supplementary Tables 4, 5. Urea nitrogen and phosphate levels were above reference ranges in all treatments. For the blood chemistry profile on days 1 and 7 (Supplementary Tables 4, 5), across all groups, urea nitrogen and phosphate levels were closer to the higher end of the reference range, while total protein and globulin were closer to the lower end. These variations were likely related to food intake timing and hydration status, and are considered clinically irrelevant, as multiple other studies have demonstrated the safety of long-term exposure. Total protein and globulin levels were below reference ranges. All other parameters of electrolyte balance, renal function, liver function, muscle function, and mineral homeostasis were within reference ranges.
3.3 Physical examination and health data
Physical examination data, including heart rate and body weight, are presented in Supplementary Table 6. Throughout the duration of the study, no signs of neurological changes such as pupil dilation, protrusion of the nictitating membrane, or proprioceptive delays were observed. Vomiting was noted in two groups of dogs (CBDA in FS, CBD/CBDA in FS) on day two of the fourth period following the evening dose. Vomiting observed in these two groups may have been due to cumulative gastrointestinal sensitivity or mild irritation associated with repeated dosing over multiple periods. The sesame seed oil formulation could also have contributed to gastric upset, especially when administered in the evening, when gastric emptying is slower. Notably, the absence of other changes in appetite or increased incidence of diarrhea suggests that this effect was transient and formulation-related rather than indicative of systemic intolerance or broader gastrointestinal dysfunction.
4 Discussion
The cannabis plant contains more than 400 compounds, including over 120 phytocannabinoids, more than 140 terpenoids, as well as various flavonoids and alkaloids. Cannabigerolic acid (CBGA) is the precursor to other major cannabinoids including Δ9-tetrahydrocannabinol (THC), cannabidiol (CBD), and cannabichromene (17). While many of these compounds have yet to be fully characterized, isolated forms such as THC and CBD have been well-studied in medicinal research. Although several studies have investigated the safety and tolerability of the decarboxylated forms of cannabinoids, particularly CBD in dogs, data on the safety and tolerability of full-spectrum and acidic forms remain limited (5, 6, 13, 17, 18).
In the cannabis plant, cannabinoids and other compounds function synergistically as secondary metabolites serving as antioxidants and contributing to the plant’s defense against predators (18). Following purification, cannabinoids can be present in various formats, including as isolates of single cannabinoids either in their decarboxylated or acidic forms, as full spectrum extracts preserving the plant’s native cannabinoid and terpene profile, or as mixtures containing two or more cannabinoids. The term “entourage effect” refers to the enhance overall impact when cannabinoids are used in combination with other minor compounds, e.g., terpenoids and flavonoids (9).
For the acidic form, pharmacokinetic studies have demonstrated its safety when administered to rabbits, horses, cats, dogs, humans and parrots (13–15, 19–21), with all of those studies suggesting superior absorption of CBDA (6, 13, 14, 17–19). Prior data from human pharmacokinetics have shown that CBD absorption appears to be enhanced when a portion of CBDA is delivered together (10). Further examination of rodents (mice) has suggested the CBDA absorption is relatively poor as an isolate, and the absorption was nearly 14 fold better while delivering in a full spectrum mixture. It was proposed that the other minor cannabinoids allowed for inhibition of enterocyte efflux enzymes allowing for better absorption as the primary mechanism (9). Therefore it is expected that there may be differing effects of full spectrum products compared to isolates which has been reported in the human literature. Full-spectrum cannabinoids are suggested to enhance symptom relief when treating mood or anxiety disorders, as well as seizures (22). Although we found little evidence to support that the major cannabinoids CBD and CBDA were enhanced by administrated as full spectrum products, there appears to be a numerical trend over 7 days on full spectrum CBDA absorption, therefore the “entourage effect” is not likely to be related to the major cannabinoid absorption, but rather to the minor components found in full spectrum products. However, our data is limited, and additional studies are needed to determine these concentrations at steady state.
Pharmacokinetic data that compares full spectrum cannabinoids to isolate cannabinoid forms was unknown in dogs prior to this study. The objectives of this study were to (1) determine the pharmacokinetics of full-spectrum hemp extract and cannabinoid isolates including quantification of CBD metabolites (6-OH-CBD, 7-COOH-CBD) in healthy adult dogs, and to (2) assess the tolerability and safety of orally dosed cannabinoid isolates (CBD, CBDA) and a hemp extract in adult beagle dogs. It was hypothesized that, compared to the isolate cannabinoid (CBD), acidic cannabinoids (CBDA) and full-spectrum cannabinoids would exhibit greater absorption in adult dogs. No significant changes in complete blood count (CBC) and serum chemistry profiles were anticipated during the one-week period of cannabinoid administration.
Earlier studies have reported that the oral bioavailability of cannabinoids in dogs is low due to extensive first-pass metabolism (23, 24). However, transdermal administration of cannabinoids has not resulted in serum concentrations comparable to those achieved with oral dosing (25). Due to the lipophilic nature of cannabinoids, and the stratum corneum, their absorption through the skin epithelium is limited (16).
In our study, the absorption of orally dosed CBD isolate at 1 mg/kg BW was low (69.8 ± 35.44 ng/mL) and comparable to that of other studies. Gamble et al. reported Cmax value of 102 ng/mL for CBD dosed at 1 mg/kg BW in a long chain triglyceride oil vehicle format to fasted dogs. Wakshlag et al. reported Cmax values of 145 ± 69 and 124 ± 62 ng/mL, respectively, for CBD administered at the same dose (1 mg/kg BW) formulated with either a mixture of medium chain triglycerides (MCT) and a sesame oil or a mixture of sunflower lecithin and sesame oil. Della Rocca et al. reported a Cmax of 206.77 ± 167.07 ng/mL for CBD administered at 1 mg/kg BW in MCT oil. In contrast, Vaughn et al. reported a significantly lower Cmax 30 ± 7 ng/mL for the same CBD dose and carrier oil. In contrast, in the studies by Deabold et al. and Wakshlag et al., when CBD was dosed at 1 mg/kg using an enriched soft chew, Cmax was greater, with values of 301 ± 63 ng/mL and 226 ± 89 ng/mL, respectively. Therefore, the type of carrier vehicle to dose CBD appears to be a driving factor in CBD absorption. In addition to carrier types, various absorption of cannabinoids were observed in other species with fasted animals or fed before dosing (13, 20). In our study, dogs were fed dry kibble before dosing, and wet food immediately after. At the same dosage (1 mg/kg), the CBD AUC observed in this study (420.61 ± 45.52 ng-h/mL) was comparable to that reported by Della Roca et al. (536.05 ng-h/mL) using MCT oil as carrier vehicle. In Doran et al., feeding dogs before dosing showed a twofold increase in AUC at 5, 10, 20 mg/kg dosage. Although additional feeding did not lead to a higher AUC in our study, feeding state and encapsulation materials used could be the other driving factors when considering cannabinoids absorption (3, 26).
In our study, Cmax, AUC, and Css for CBDA isolates were greater than those observed for the CBD isolate. These findings support previous research in dogs, cats, horses and steers, suggesting that the acidic form of cannabinoids exhibit superior absorption compared to their decarboxylated counterparts in mammalian species (7, 14, 15, 19). Similar to results reported by Gamble et al., co-administration of CBDA and CBD (each at 1 mg/kg) did not enhance the Cmax of CBD (99 ± 29.13 ng/mL). Likewise, in our study, the presence of CBDA in the CBD/CBDA FS mixture did not influence the Cmax of CBD when compared to the CBD isolate (64.66 ± 37.05 ng/mL vs. 69.8 ± 35.44 ng/mL, respectively). In contrast, Tittle et al. reported higher Cmax values for CBD in dogs dosed with a CBD-rich hemp extract formulated as either a soft gel (267.6 ± 98.9 ng/mL) or in sesame oil (184.5 ± 55.8 ng/mL), with both formulations containing CBD and CBDA at 1 mg/kg. This discrepancy may indicate that the absorption of acidic cannabinoids is less affected by feeding status compared to their decarboxylated counterparts.
The Cmax and AUC values for CBDA in CBD/CBDA FS were greater compared to those for CBD in CBD/CBDA FS. This is the first study that compares CBD and CBDA pharmacokinetics in a full spectrum mixture. This finding aligns closely with the CBDA isolate treatment, which had greater absorption compared to CBD isolate treatment. CBDA has a low affinity for both CB1 or CB2, is one of the minor cannabinoids that functions as allosteric regulators for 5-HT1α receptors, and reported to be 1,000 time more potent than CBD in binding to these receptor sites (27). CBDA also showed better anti-hyperalgesia, anti-nausea and anti-seizure effects in rodent models (18). Those findings in the rodent studies align with the previous studies in dogs, cats and horses (7, 15, 21, 28), suggesting a better potency in the cannabinoid acidic form.
The Cmax for CBDA in the CBD/CBDA FS mixture remained unaffected with the addition of CBD, other minor cannabinoids, terpenes and flavonoids in the mixture, compared to the CBDA isolate (229.30 ± 145.51 vs. 235.51 ± 65.59) and similarly for Tmax, AUC and Css. When comparing the CBDA FS to CBDA isolate, no differences between these treatments were observed, similar to CBD results, suggesting that FS formulations play little role in the absorption and or/retention of CBD or CBDA.
The time-course serum concentrations of two primary CBD metabolites 6-OH-CBD and 7-COOH-CBD (Supplementary Figures 2–4) indicate potential treatment-dependent differences in metabolite levels. Interpretation of these data should be made with caution, as several time points were below the detection limit, and proper statistical analyses could not be conducted. In Supplementary Figure 2, dogs receiving the CBD isolate for 1 week appeared to show slightly higher 6-OH-CBD levels than those receiving the CBD/CBDA FS treatment, with peak concentration of 6-OH-CBD for both groups occurring between 2 and 4 h. Furthermore, 6-OH-CBD appeared higher than the traditional metabolite 7-COOH-CBD, as supported by previous studies in dogs (3), suggesting that dogs undergo differential metabolism of CBD when compared to humans and horses. These results are consistent with the known first-pass hepatic metabolism, where 6-OH-CBD serves as a primary oxidative metabolite that rapidly forms and either clears through renal excretion or undergoes further unidentified metabolism for biliary excretion (29). Moreover, prior pharmacokinetic studies has shown that dogs exhibit only 1–2% of the 7-COOH-CBD levels observed in humans receiving similar dosages (3, 7).
Further research is warranted to evaluate the biological activity and potency of 6-OH-CBD as a minor metabolite in dogs. In addition, CBDA metabolism has not been thoroughly investigated with only one study in extrahepatic microsomes suggesting that there is direct glucuronidation of CBDA at the acidic carboxylic acid (30). These findings reinforce that cannabinoid metabolism is sensitive to formulation, feeding status, and co-administered compounds, and that species-specific differences in CBD metabolism are likely substantial.
While metabolic differences were observed between treatments, it is important to also consider how the presence of additional cannabinoids and terpenes may influence the pharmacological effects of CBD through the proposed entourage effect. The entourage effect was first suggested in a preclinical study in 1998 when describing metabolites interactions (31). This term was later widely used in the field of medicinal cannabinoids, as the example of polypharmacy (use of multiple drugs to treat a single condition), e.g., full spectrum cannabinoids. The potential entourage effect can be variable (32). One of the common misleading concepts is that the entourage effects are often described as beneficial synergistic effects, while adverse effects, either caused by antagonism or additive effects are often ignored, leading to a public awareness of the beneficial sides only of the entourage effect (33). While many studies have emphasized the entourage effect in both humans and animals, limited research has been done to provide robust evidence. The pharmacokinetic results of this study do not support a better absorption of either CBD or CBDA when provided in a full spectrum. This may be due to the industrial purification methods, or that the specific cannabis chemovars used in this study did not contain the effective terpenoids or flavonoids or proportions of minor cannabinoids to promote enhanced absorption.
In our study, no adverse events were associated with a 7-day administration of CBD or CBDA isolates, or of these cannabinoids in various combinations and full spectrum. There was no elevated serum ALT, which has been observed in previous studies in cats and dogs (5, 25) nor the more innocuous ALP enzyme in this study. However, to thoroughly understand the mechanisms and hepatic safety behind the entourage effect, additional research is needed.
The results of this study are the first of a comprehensive comparison between full-spectrum, isolate, and acidic forms of cannabinoids. The study further supports that CBDA, the acidic form of CBD, is absorbed better in dogs. Previous research in mouse suggests that CBDA interacts with transporter breast cancer resistance protein (BCRP), and that CBG and THC inhibit BCRP-mediated transport of CBDA, thus leading to an elevated plasma concentration for CBDA (9). In our study, we found no evidence that full-spectrum cannabinoids have enhanced absorption in dogs. Therefore, there is a need for further validation with isolate CBDA and addition of CBG to test out the entourage effect in the canine model, as it relates to receptor biology rather than cannabinoids promoting synergistic absorption. Based on the physical assessment, the lack of adverse events, and CBC and chemical profiles falling within reference ranges, CBD, CBDA, and these isolates combined or presented in full spectrum are safe for dogs when provided at 1 mg/kg every 12 h during a one-week treatment protocol.
Due to the complex variations in cannabis chemovars, the findings are only applicable to the specific formulations tested in this study. Additionally, due to the limitations of the small sample size and the short duration of the trial, the generalizability remains limited. Future studies with larger sample sizes, different ages and breeds, and longer trial durations are necessary before providing comprehensive guidelines for cannabinoid usage in veterinary medicine.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by Cornell University Institutional Animal Care and Use Committee. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
TW: Data curation, Formal analysis, Methodology, Investigation, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. JW: Supervision, Writing – review & editing. AL: Writing – review & editing. AZ: Writing – review & editing. BG: Writing – review & editing. WS: Methodology, Writing – review & editing. NT: Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by ElleVet Sciences. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Acknowledgments
The authors would like to thank Stephen Parry from the Cornell Statistical Consulting Center for guidance in selecting statistical models.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2025.1639846/full#supplementary-material
SUPPLEMENTARY FIGURE 1 | Average serum concentrations of major cannabinoids on day 7 by treatment group. Values represent means ± standard error of the mean. One dog was excluded from the CBD isolate group due to unsuccessful dosing.
SUPPLEMENTARY FIGURE 2 | Serum 6-OH-CBD and 7-COOH-CBD metabolite concentrations on day 7 in dogs receiving CBD isolate and CBD/CBDA FS. One dog was excluded from the CBD isolate group due to unsuccessful dosing.
SUPPLEMENTARY FIGURE 3 | Serum concentration of 7-COOH-CBD over 12 hours. Values represent means ± standard error of the mean.
SUPPLEMENTARY FIGURE 4 | Serum concentration of 6-OH-CBD over 12 hours. Values represent means ± standard error of the mean.
References
1. Georgieva, D, Langley, J, Hartkopf, K, Hawk, L, Margolis, A, Struck, A, et al. Real-world, long-term evaluation of the tolerability and therapy retention of Epidiolex® (cannabidiol) in patients with refractory epilepsy. Epilepsy Behav. (2023) 141:109159. doi: 10.1016/j.yebeh.2023.109159
2. Smith, RT, and Gruber, SA. Contemplating cannabis? The complex relationship between cannabinoids and hepatic metabolism resulting in the potential for drug-drug interactions. Front Psych. (2023) 13:1055481. doi: 10.3389/fpsyt.2022.1055481
3. Di Salvo, A, Conti, MB, and della Rocca, G. Pharmacokinetics, efficacy, and safety of cannabidiol in dogs: an update of current knowledge. Front Vet Sci. (2023) 10:1204526. doi: 10.3389/fvets.2023.1204526
4. Interlandi, C, Tabbì, M, Di Pietro, S, D’Angelo, F, Costa, GL, Arfuso, F, et al. Improved quality of life and pain relief in mature horses with osteoarthritis after oral transmucosal cannabidiol oil administration as part of an analgesic regimen. Front Vet Sci. (2024) 11:1396. doi: 10.3389/fvets.2024.1341396
5. McGrath, S, Bartner, LR, Rao, S, Packer, RA, and Gustafson, DL. Randomized blinded controlled clinical trial to assess the effect of oral cannabidiol administration in addition to conventional antiepileptic treatment on seizure frequency in dogs with intractable idiopathic epilepsy. J Am Vet Med Assoc. (2019) 254:1301–8. doi: 10.2460/javma.254.11.1301
6. Gamble, LJ, Boesch, JM, Frye, CW, Schwark, WS, Mann, S, Wolfe, L, et al. Pharmacokinetics, safety, and clinical efficacy of Cannabidiol treatment in osteoarthritic dogs. Front Vet Sci. (2018) 5:165. doi: 10.3389/fvets.2018.00165
7. Wakshlag, JJ, Schwark, WS, Deabold, KA, Talsma, BN, Cital, S, Lyubimov, A, et al. Pharmacokinetics of Cannabidiol, Cannabidiolic acid, Δ9-tetrahydrocannabinol, Tetrahydrocannabinolic acid and related metabolites in canine serum after dosing with three Oral forms of hemp extract. Front Vet Sci. (2020) 7:505. doi: 10.3389/fvets.2020.00505
8. Klatzkow, S, Davis, G, Shmalberg, J, Gallastegui, A, Miscioscia, E, Tarricone, J, et al. Evaluation of the efficacy of a cannabidiol and cannabidiolic acid rich hemp extract for pain in dogs following a tibial plateau leveling osteotomy. Front Vet Sci. (2023) 9:6056. doi: 10.3389/fvets.2022.1036056
9. Anderson, LL, Etchart, MG, Bahceci, D, Golembiewski, TA, and Arnold, JC. Cannabis constituents interact at the drug efflux pump BCRP to markedly increase plasma cannabidiolic acid concentrations. Sci Rep. (2021) 11:14948. doi: 10.1038/s41598-021-94212-6
10. Eichler, M, Spinedi, L, Unfer-Grauwiler, S, Bodmer, M, Surber, C, Luedi, M, et al. Heat exposure of Cannabis sativa extracts affects the pharmacokinetic and metabolic profile in healthy male subjects. Planta Med. (2012) 78:686–91. doi: 10.1055/s-0031-1298334
11. Vaughn, D, Kulpa, J, and Paulionis, L. Preliminary investigation of the safety of escalating cannabinoid doses in healthy dogs. Front Vet Sci. (2020) 7:51. doi: 10.3389/fvets.2020.00051
12. Chicoine, A, Illing, K, Vuong, S, Pinto, KR, Alcorn, J, and Cosford, K. Pharmacokinetic and safety evaluation of various Oral doses of a novel 1:20 THC:CBD Cannabis herbal extract in dogs. Front Vet Sci. (2020) 7:583404. doi: 10.3389/fvets.2020.583404
13. Rooney, TA, Carpenter, JW, KuKanich, B, Gardhouse, SM, Magnin, GC, and Tully, TN. Feeding decreases the oral bioavailability of cannabidiol and cannabidiolic acid in hemp oil in New Zealand white rabbits (Oryctolagus cuniculus). Am J Vet Res. (2022) 83:ajvr.22.01.0006. doi: 10.2460/ajvr.22.01.0006
14. Kleinhenz, MD, Weeder, M, Montgomery, S, Martin, M, Curtis, A, Magnin, G, et al. Short term feeding of industrial hemp with a high cannabidiolic acid (CBDA) content increases lying behavior and reduces biomarkers of stress and inflammation in Holstein steers. Sci Rep. (2022) 12:3683. doi: 10.1038/s41598-022-07795-z
15. Wang, T, Zakharov, A, Gomez, B, Lyubimov, A, Trottier, NL, Schwark, WS, et al. Serum cannabinoid 24 h and 1 week steady state pharmacokinetic assessment in cats using a CBD/CBDA rich hemp paste. Front Vet Sci. (2022) 9:895368. doi: 10.3389/fvets.2022.895368
16. Mahmoudinoodezh, H, Telukutla, SR, Bhangu, SK, Bachari, A, Cavalieri, F, and Mantri, N. The transdermal delivery of therapeutic cannabinoids. Pharmaceutics. (2022) 14:438. doi: 10.3390/pharmaceutics14020438
17. Martinez, AS, Lanaridi, O, Stagel, K, Halbwirth, H, Schnürch, M, and Bica-Schröder, K. Extraction techniques for bioactive compounds of cannabis. Nat Prod Rep. (2023) 40:676–717. doi: 10.1039/D2NP00059H
18. Walsh, KB, McKinney, AE, and Holmes, AE. Minor cannabinoids: biosynthesis, molecular pharmacology and potential therapeutic uses. Front Pharmacol. (2021) 12:777804. doi: 10.3389/fphar.2021.777804
19. Thomson, ACS, McCarrel, TM, Zakharov, A, Gomez, B, Lyubimov, A, Schwark, WS, et al. Pharmacokinetics and tolerability of single-dose enteral cannabidiol and cannabidiolic acid rich hemp in horses (Equus caballus). Front Vet Sci. (2024) 11:6463. doi: 10.3389/fvets.2024.1356463
20. Sosa-Higareda, M, Guzman, DSM, Knych, H, Lyubimov, A, Zakharov, A, Gomez, B, et al. Twice-daily oral administration of a cannabidiol and cannabidiolic acid–rich hemp extract was well tolerated in orange-winged Amazon parrots (Amazona amazonica) and has a favorable pharmacokinetic profile. Am J Vet Res. (2023) 84:197. doi: 10.2460/ajvr.22.11.0197
21. Aragona, F, Tabbì, M, Gugliandolo, E, Giannetto, C, D’Angelo, F, Fazio, F, et al. Role of cannabidiolic acid or the combination of cannabigerol/cannabidiol in pain modulation and welfare improvement in horses with chronic osteoarthritis. Front Vet Sci. (2024) 11:1496473. doi: 10.3389/fvets.2024.1496473
22. Ferber, SG, Namdar, D, Hen-Shoval, D, Eger, G, Koltai, H, Shoval, G, et al. The “entourage effect”: terpenes coupled with cannabinoids for the treatment of mood disorders and anxiety disorders. Curr Neuropharmacol. (2020) 18:87–96. doi: 10.2174/1570159X17666190903103923
23. Samara, E, Bialer, M, and Mechoulam, R. Pharmacokinetics of cannabidiol in dogs. Drug Metab Dispos Biol Fate Chem. (1988) 16:469–72. doi: 10.1016/S0090-9556(25)06962-4
24. della Rocca, G, Paoletti, F, Conti, MB, Galarini, R, Chiaradia, E, Sforna, M, et al. Pharmacokinetics of cannabidiol following single oral and oral transmucosal administration in dogs. Front Vet Sci. (2023) 9:1104152. doi: 10.3389/fvets.2022.1104152
25. Bartner, LR, McGrath, S, Rao, S, Hyatt, LK, and Wittenburg, LA. Pharmacokinetics of cannabidiol administered by 3 delivery methods at 2 different dosages to healthy dogs. Can J Vet Res. (2018) 82:178–83.
26. Sitovs, A, Logviss, K, Lauberte, L, and Mohylyuk, V. Oral delivery of cannabidiol: revealing the formulation and absorption challenges. J Drug Deliv Sci Technol. (2024) 92:105316. doi: 10.1016/j.jddst.2023.105316
27. Bolognini, D, Rock, EM, Cluny, NL, Cascio, MG, Limebeer, CL, Duncan, M, et al. Cannabidiolic acid prevents vomiting in Suncus murinus and nausea-induced behaviour in rats by enhancing 5-HT1A receptor activation. Br J Pharmacol. (2013) 168:1456–70. doi: 10.1111/bph.12043
28. Tittle, DJ, Wakshlag, JJ, Schwark, WS, Lyubimov, A, Zakharov, A, and Gomez, B. Twenty-four hour and one-week steady state pharmacokinetics of cannabinoids in two formulations of cannabidiol and cannabidiolic acid rich hemp in dogs. Med Res Arch. (2022) 10:2907. doi: 10.18103/mra.v10i7.2907
29. Mills, T, Myers, S, Hughes, D, and Wakshlag, J. Tolerability of 2 and 4 mg/kg dosing every 12 hour of a cannabidiol- and cannabidiolic acid-rich hemp extract on mixed-breed dogs utilized for teaching in a closed colony. Animals. (2024) 14:1863. doi: 10.3390/ani14131863
30. Court, MH, Mealey, KL, Burke, NS, Jimenez, TP, Zhu, Z, and Wakshlag, JJ. Cannabidiol and cannabidiolic acid: preliminary in vitro evaluation of metabolism and drug–drug interactions involving canine cytochrome P-450, UDP-glucuronosyltransferase, and P-glycoprotein. J Vet Pharmacol Ther. (2024) 47:1–13. doi: 10.1111/jvp.13403
31. Ben-Shabat, S, Fride, E, Sheskin, T, Tamiri, T, Rhee, MH, Vogel, Z, et al. An entourage effect: inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity. Eur J Pharmacol. (1998) 353:23–31. doi: 10.1016/S0014-2999(98)00392-6
32. McPartland, JM, Guy, GW, and Di Marzo, V. Care and feeding of the endocannabinoid system: a systematic review of potential clinical interventions that upregulate the endocannabinoid system. PLoS One. (2014) 9:e89566. doi: 10.1371/journal.pone.0089566
Keywords: pharmacokinetic, CBD, CBDA, cannabinoids, canine, safety
Citation: Wang TC, Wakshlag JJ, Lyubimov A, Zakharov A, Gomez B, Schwark W and Trottier NL (2025) Limited 12-hour pharmacokinetic assessment of CBD and CBDA isolates compared to their full-spectrum extracts in healthy adult beagles. Front. Vet. Sci. 12:1639846. doi: 10.3389/fvets.2025.1639846
Edited by:
Narda Gail Robinson, CuraCore Integrative Medicine & Education Center, United StatesReviewed by:
Arun H. S. Kumar, University College Dublin, IrelandClaudia Interlandi, University of Messina, Italy
Copyright © 2025 Wang, Wakshlag, Lyubimov, Zakharov, Gomez, Schwark and Trottier. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nathalie L. Trottier, dHJvdHRpZXJAY29ybmVsbC5lZHU=
 Tongxin Charlotte Wang
Tongxin Charlotte Wang Joseph J. Wakshlag
Joseph J. Wakshlag Alex Lyubimov3
Alex Lyubimov3 Alexander Zakharov
Alexander Zakharov Wayne Schwark
Wayne Schwark