- 1Reproductive Biotechnology Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
- 2Sina Fanavaran Mandegar Company, Alborz Science and Technology Park, Kamalshahr, Iran
- 3Department of Molecular Biotechnology, National Institute of Genetic Engineering and Biotechnology (NIGEB), Tehran, Iran
- 4Avicenna Fertility Centre, Avicenna Research Institute, ACECR, Tehran, Iran
- 5Department of Animal Science, College of Agriculture, Urmia University, Urmia, Iran
- 6Department of Internal Medicine, Reproduction and Population Medicine, Faculty of Veterinary Medicine, Ghent University, Merelbeke, Belgium
- 7Department of Animal Science, University of Tennessee Institute of Agriculture and AgResearch, Knoxville, TN, United States
Nano-sized extracellular vesicles (EVs) possess a lipid bilayer and are secreted from cells into their surrounding environment. The transport of multiple biomolecules, including DNA together with RNA, microRNAs (miRNAs), lipids, proteins, and metabolites, happens through biofluids via EVs for intercellular communication. Extracellular vesicles play crucial roles during the in vitro embryo production (IVEP) process. Specifically, the maturing oocyte benefits from EVs that facilitate cell-to-cell communication and transfer important biomolecules, which improve oocyte development potential. Moreover, EVs help establish important molecular control needed for oocytes to advance into the metaphase II phase, which enables proper fertilization events. In fact, the fertilization process depends heavily on EVs because seminal plasma-derived EVs play an essential role during fertilization, and they improve sperm motility as well as capacitation and the acrosome reaction, which are required for successful fertilization. EVs transport proteins together with RNAs, which enhance sperm capacity to fertilize. Embryos benefit from the optimal growth environment, which is maintained by oviduct and uterus-derived extracellular vesicles (EVs), as they support proper gene expression regulation. EVs produced in the oviduct enable embryo development, and those released by the uterus serve as communication channels for embryo-maternal environment integration required during implantation. These vesicles contain bioactive molecules such as miR-21, miR-26a, and HSP70, which are involved in key reproductive functions including granulosa cell (GC) signaling, oocyte maturation, and sperm function regulation. Overall, the reproductive system relies heavily on EVs because these vesicles manage oocyte development as well as the process of fertilization and embryonic development. The communication features of EVs using regulatory molecules indicate their potential role in assisted reproductive technologies (ARTs). Advancing our knowledge regarding EVs' mechanisms will support the development of novel strategies to enhance IVEP outcomes. This review provides an overview of the current understanding of the roles of EVs in oocyte maturation, fertilization, and embryo development.
1 Introduction
Population growth triggers the increasing worldwide need for animal-based food products, including dairy items and meat products (1–3). Consequently, the reproductive performance of farm animals has been improved through the widespread application of assisted reproductive technologies (ARTs), particularly in vitro embryo production (IVEP) (4). According to the International Embryo Technology Society (IETS), more than one million bovine embryos are produced in vitro annually worldwide, highlighting the scale and impact of these technologies (5). Over the past two decades, the animal breeding field has realized the usefulness of IVEP to enhance genetic advancement and reproductive efficiency (6). In this context, assisted reproductive techniques (ARTs) such as in vitro fertilization (IVF), intracytoplasmic sperm injection, somatic cell nuclear transfer, and in vitro maturation (IVM) are used for breeding cattle, buffalo, goats, as well as sheep and camels (7, 8). Similarly, IVF serves as an essential ART for human fertility, as it offers a solution for individuals affected by infertility (9, 10). Thus, IVF plays a dual role since it improves livestock breeding methodologies and facilitates human reproductive medicine (11).
Furthermore, researchers working in the field of IVEP have dedicated substantial efforts to developing improved strategies for assessing embryo quality under laboratory conditions (10, 12–14). Research studies have identified EVs as essential components responsible for cell-to-cell communications as well as embryonic developmental processes. EVs function primarily as carriers of bioactive molecules-such as proteins, mRNAs, and miRNAs-facilitating intercellular communication within reproductive tissues (Figure 1) (15, 16). Therefore, embryo development, together with implantation, depends on this vital molecular transfer mechanism that enables cell-to-cell communication (17, 18).
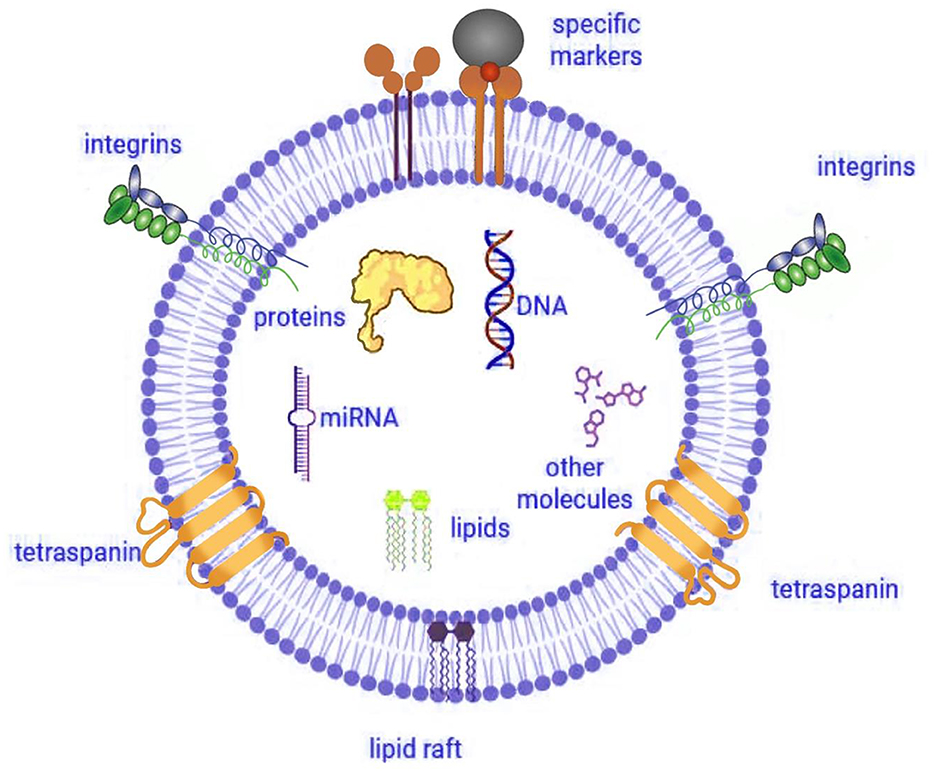
Figure 1. Structure and cargo of extracellular vesicle. Illustration created using Digital Paint and Adobe Photoshop 2023 and was inspired by Truby et al. (319).
To explore these functions, researchers have applied EVs for IVEP investigation in cattle (19), horses (20), dogs (21), mice (22), pigs (23, 24), and humans (25). In addition, EVs in reproductive biology offer researchers critical cellular information and demonstrate potential usage as embryo viability predictors. Notably, functional characteristics of EVs mainly result from their protein cargo. These vesicles contain multiple tetraspanins, including CD9, CD63, and CD81, that assist cells with signaling activities and membrane processes (26). Moreover, the protein content of EVs includes essential proteins ALIX (27) as well as TSG101 (28) and MHC I (16) and HSP90 (29) together with integrins (α2, α6, β1, and β4) which facilitate critical embryo-maternal signaling processes (30).
This review discusses the essential functions of EVs throughout the IVEP process. Specifically, it begins with an explanation of EV types and their creation processes before discussing isolation and analytical methods. Finally, the review will investigate how these EVs affect IVF stages, starting from the expansion of cumulus cells up to embryo hatching, while demonstrating their ability to regulate embryonic developmental processes.
2 The classification of EVs
EVs are lipid bilayer-enclosed particles that are naturally released by most cell types into the extracellular space. They function as central mediators of intercellular communication by transporting a wide range of biomolecules, including RNAs, proteins, and lipids (31–33). According to MISEV2023 guidelines published by the International Society for Extracellular Vesicles (ISEV), EVs should be classified using operational terms based on measurable characteristics such as size, density, and biochemical composition. Commonly accepted categories include small EVs (sEVs; < 200 nm), medium/large EVs (m/lEVs; >200 nm), and apoptotic bodies (Table 1). The use of terms such as “exosomes” or “microvesicles” is not recommended unless their specific biogenesis pathways are experimentally confirmed (34).
Small EVs, typically ranging from 30 to 150 nanometers in diameter, are often associated with endosomal origin. They form as intraluminal vesicles within multivesicular bodies (MVBs), which then fuse with the plasma membrane to release their contents into the extracellular environment. Although commonly referred to as “exosomes” in earlier literature, MISEV2023 recommends using the term only when the endosomal origin has been experimentally verified (35–37).
Ectocytosis allows the release of m/lEVs from 50 to 1,000 nanometers in size through direct outward budding from plasma membranes. The formation process of plasma membrane-derived exosomes sets them apart from other exosomes that develop from endosomes (38, 39). The formation of m/lEVs proceeds differently from sEVs because it takes place from the plasma membrane surface instead of intracellular membranes or the endosomal system (40). On the other hand, apoptotic bodies take a wider size range from 1 to 5 μm in diameter during programmed cell death (41). These various types of EVs play significant roles in different biological processes, including cell motility (42–44), differentiation (45–47), proliferation (48), apoptosis (49), reprogramming (49–52), and immunity (53). The involvement of EVs in these processes highlights their potential for clinical applications (54).
Although the current ISEV guidelines recommend using the terms “exosomes,” “microvesicles,” and “apoptotic bodies” with caution-unless supported by highly specific isolation and characterization techniques-these categories are still widely used in the literature due to their distinct biogenetic origins. Exosomes (30–150 nm) are formed within multivesicular bodies (MVBs) and released when MVBs fuse with the plasma membrane. Microvesicles or ectosomes (100–1,000 nm) are generated through direct outward budding of the plasma membrane. Apoptotic bodies (500–2,000 nm), in contrast, are produced during programmed cell death and often contain fragmented nuclear material, organelles, and cytoplasmic content (55).
Intercellular communication through cellular exchanges regulates numerous physiological and pathological operations by sEVs and m/lEVs (56). Cargo of these EVs is transferred to nearby cells transfer to nearby cells (57, 58). The illustration in Figure 2 shows EVs are classified into sEVs, m/lEVs, and apoptotic bodies based on their size, biogenesis, and molecular characteristics, under MISEV2023 guidelines (Figure 2).
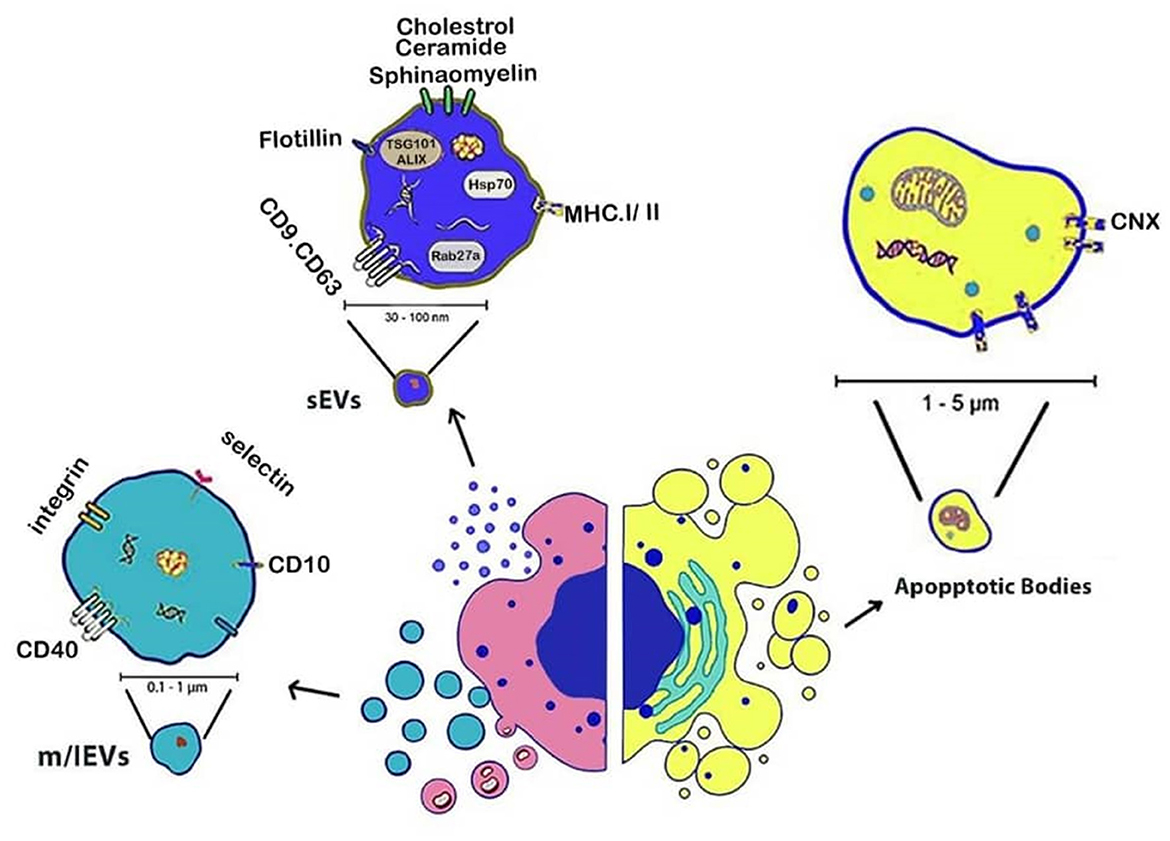
Figure 2. Schematic representation of EVs subtypes based on their biogenesis. small EVs (<200 nm) originate from the endosomal system, medium/large EVs (>200 nm) bud directly from the plasma membrane, and apoptotic bodies (>500 nm) are released during programmed cell death. Illustration created using Digital Paint and Adobe Photoshop 2023.
3 Cellular and biofluid sources of EVs in in-vitro embryo production
Extracellular vesicles originate from diverse cells and reproductive biofluids to function as vital items within natural communication paths between cells. During IVEP research, EVs from reproductive fluids and cells strengthen the maturation of oocytes and help with fertilization and contribute to embryo development (59, 60).
3.1 Reproductive fluids (follicular, oviductal, and uterine fluid)
Reproductive biofluids, including follicular fluid (FF), uterine fluid (UF), and oviductal fluid (OF), contain significant concentrations of EVs that enable essential communication processes between different reproductive cells. The FF-derived EVs employ different bioactive molecules that amplify oocyte competence as they accelerate both maturation stages of the cytoplasm and nucleus (61, 62).
The release of OF-derived EVs leads to sperm capacitation while promoting fertilization success by establishing maternal-embryo dialogue (63, 64). Many extracellular vesicles (EVs) present in follicular fluid are derived from granulosa and cumulus cells and contribute to oocyte development by transferring regulatory biomolecules. These EVs carry key microRNAs (miRNAs) such as miR-21, miR-26a, and miR-375, which modulate gene expression in follicular cells and enhance oocyte competence. Proteins such as HSP70, Annexins, and OVGP1 have also been identified in follicular EVs and are linked to improved fertilization and embryo development outcomes. Studies have shown that the addition of follicular fluid-derived EVs to IVM media can improve oocyte maturation and subsequent embryo development (65, 66).
The delivery of EVs within UF plays an essential role in embryo development and creates endometrial receptivity, which results in better implantation rates in assisted reproduction procedures, as presented in (67).
3.2 Granulosa and cumulus cell-derived EVs
Oocytes receive regulation through EVs produced by granulosa cells (GCs) and cumulus cells that contain miRNAs, proteins, and growth factors, which influence both oocyte quality and embryonic development competency. These EVs are enriched with molecules that influence mitochondrial function, epigenetic modifications, and cumulus expansion, all of which are critical for proper oocyte maturation. For example, miR-375 has been reported to improve mitochondrial membrane potential in oocytes, and CD63 and TSG101 have been used as markers for exosome-mediated signaling in cumulus–oocyte communication. Cumulus-derived EVs have also been associated with enhanced tolerance to in vitro stress conditions, contributing to better developmental potential post-fertilization (68). Multiple studies demonstrate that these EVs affect mitochondrial functions and epigenetic characteristics as well as in vitro production (IVP) embryo stress tolerance capabilities (69, 70).
3.3 Embryo-derived EVs
The early embryonic cells produce specialized signals that lead both to cell self-preservation and the regulation of neighboring cells during developmental processes. Recent studies have demonstrated that EVs function as essential communicative mechanisms that transport signals from cell to cell for vital embryo-maternal (71). It has been shown that the vital importance of embryo-derived EVs as cellular signaling mediators in both preimplantation embryonic development and the maternal environment is because their molecular components affect the embryonic development cycle and implantation, which leads to pregnancy establishment (72). A study showed that adding embryo-derived EVs from outgrowth embryos to culture medium enhanced both preimplantation embryonic development as well as implantation capability (73). The embryo-derived EVs contain gene-modifying molecules, which include mRNAs, miRNAs, and DNA fragments that reform gene expression and modify the cellular behavior of recipient cells. For example, blastocyst formation as well as implantation processes are influenced by specific EVs-delivered miRNAs (72). Embryo-derived EVs have been shown to carry common exosomal markers such as CD9, CD63, CD81, ALIX, TSG101, and HSP70, which contribute to embryo-maternal communication (74).
Also, new evidence shows that the embryo starts secreting EVs during early pregnancy, coming from the trophectoderm and inner cell mass. Likewise, the blastocoel fluid of preimplantation human embryos contains exosomes, which represent a specific subtype of EVs according to research (75). In addition, research suggests that human embryo-derived EVs have a connection to embryonic quality and can control maternal pregnancy recognition through modifications of endometrial epithelial cell transcript expression. Additionally, specific microRNAs, including miR-30c and miR-378, have been implicated in promoting trophoblast adhesion and regulating gene expression during implantation. Furthermore, research shows that maternal responses occurred only upon exposure to EVs derived from high-quality embryos, yet no detectable reactions were observed from EVs produced by degenerated embryos (76–78).
Embryo origin (in vivo or in vitro) together with culture conditions affect both embryonic EVs and maternal EVs concentration, size, and molecular profile, enabling changes in embryo-maternal signaling (71, 79, 80). Current research shows that EVs released into culture medium possess the potential to act as biomarkers that help evaluate embryonic quality and developmental competence. Embryos with poor developmental quality tend to produce increased concentrations of EVs, likely due to stress (81, 82).
Moreover, research indicates that EVs' dimensions present in human embryo culture medium serve as a non-invasive indicator for developmental competency in embryos (83). Research findings present inconsistent outcomes about the relation of EV size to embryo viability because some evidence links bigger EVs with healthy embryos, yet other studies show non-viable embryos generate larger quantities of large EVs that indicate stress symptoms. Research on bovine embryos produces contradictory results regarding the link between embryonic vesicle size and embryonic quality assessment (84, 85).
Further research reveals that human embryos release higher concentrations of EVs during later developmental stages, potentially reflecting increased cellular activity and structural complexity associated with processes such as blastulation (72, 86).
4 Methodological overview
Comprehensive information about the physicochemical properties of EVs-including size, shape, density, surface charge, and porosity, is essential for understanding their biological functions and interactions (87, 88). Several widely used characterization techniques assist in this analysis, including.
4.1 Nanoparticle tracking analysis (NTA)
Nanoparticle tracking analysis (NTA) is a widely used technique for the biophysical characterization of EVs. It operates based on the principle of light scattering and enables the tracking of the Brownian motion of individual nanoparticles in a liquid suspension (89). In the context of EV analysis, NTA tracks the movement of each particle through image analysis, measuring their velocity (90), which is correlated with their size (91). This technique is capable of examining the concentration and size distribution of EVs within a size range of ~50–1,000 nm (92). Moreover, NTA can also analyze the zeta potential, which reflects the surface charge of EVs. This measurement provides valuable information about vesicle stability, aggregation tendency, and interactions with biological membranes. NTA requires minimal sample preparation, and the analysis process is relatively quick (93, 94). Additionally, NTA offers a fluorescence mode that allows for the probing of EV surface antigens using labeled antibodies (95). The success of NTA heavily depends on proper sample preparation and the correct dilution factor (96).
4.2 Dynamic light scattering (DLS)
Dynamic light scattering (DLS) represents a widespread analytical method for nanoparticle size distribution measurements, with EVs represented as part of this group of nanoparticles (97). DLS measures scattering light intensity fluctuations produced by particles performing Brownian motion in suspension in order to calculate their hydrodynamic diameter. However, it is important to note that DLS does not provide information on particle concentration, which limits its utility when quantitative analysis of EVs is required (93, 98).
There are some advantages of using DLS including: (1) non-invasive and rapid: DLS is a non-destructive method that requires relatively small sample volumes, making it suitable for analyzing precious biological samples such as EVs (99). (2) Broad size range: the technique is capable of measuring particles ranging from ~1 nm to several micrometers, encompassing the typical size range of EVs (93). (3) High throughput: DLS can quickly provide size distribution data, facilitating rapid screening and analysis of multiple samples (100). The analysis of vesicular structures through DLS shows two major limitations due to its excessive detection of bigger particles and its incapability to locate small vesicles among larger ones. The technique fails to accurately measure diverse particle combinations found in heterogeneous mixtures because it cannot account for non-spherical vesicle shapes (101). Additionally it does not provide data regarding biochemical characteristics, origin or functional properties of EVs (93). Despite its limitations, DLS has been effectively employed in various studies involving EVs: (1) size distribution analysis: DLS has been used to assess the size distribution of EVs derived from different cell types, such as red blood cells and ovarian cancer cells, aiding in understanding their biophysical properties (93). (2) Quality control: the technique serves as a quality control measure to detect aggregation or changes in EV size distribution during isolation and storage processes (99). (3) Comparative studies: DLS has been utilized alongside other characterization methods, such as NTA and electron microscopy, to provide complementary data on EV populations (92, 101).
The detection of smaller particles remains challenging for this technique because larger particles within a mixture can prevent the successful detection of the smaller ones (101, 102). DLS demonstrates remarkable capability for measuring vesicles of various origins, such as red blood cells and ovarian cancer cells, regardless of its size measurement limitation (103). The diameter measurements from DLS are straightforward, but this technique lacks the capability to identify the origin or constituents of EVs (104). EVs' size analysis is possible with this tool yet researchers need supplementary equipment to uncover EVs' complete biological properties fully (105, 106).
Dynamic light scattering functions as a significant analytical technique for studying EVs by enabling fast and non-destructive distribution measurements of their sizes (107). The assessment of EVs demands awareness of DLS's two main constraints, which include the size measurement effect on bigger particles and insufficient biochemical data (108). Getting a full understanding of EVs requires the use of DLS alongside other analytical methods which reveal their molecular structure along with functional characteristics.
4.3 Tunable resistive pulse sensing (TRPS)
TRPS stands as a significant technique for determining both the size dimensions and concentration values of EVs (109). This technology allows researchers to perform unclouded analysis of individual sample constituents along with precise characterization of their profiles. TRPS provides effective characterization results for colloidal particles along with diverse nanoparticles and biomolecules in suspension from 50 nm particles through cellular dimensions, thus enabling investigations of cellular functionality and EV uptake (109, 110). TRPS encounters technical limitations concerning system stability, together with sensitivity issues. The stability of the system will decrease when particles block the pores. The usage of coating solutions that decrease surface binding washes of non-specific molecules has demonstrated improvement in measurement accuracy according to (111). Small particles present measurement difficulties because they become challenging to detect among background signals. The intensity of light scattering occurring in DLS techniques causes larger particles to overpower smaller ones when present together. System performance can be improved when technicians optimize three key components, such as noise reduction measures and sensitivity cutoff boundaries, with precise measurement performance protocols. The technical roadblocks do not limit TRPS's effectiveness as it continues to function as a flexible and highly effective analytical approach. The technique has found applications, including studying the DNA binding process to magnetic nanoparticles along with characterizing leukemia-derived EVs between 200 and 300 nm during their interaction with the extracellular matrix (ECM) (112). Research has extensively focused on TRPS to determine the size distribution of EVs in various studies (113, 114). TRPS exists in two specialized versions that function as delivery platforms for enzymes against Alzheimer's disease while simultaneously delivering anticancer miRNAs to tumor cells (115–117).
4.4 Flow cytometry
Flow cytometry operates as a strong analytical tool to assess EVs, including exosomes, through detecting laser-exited light scattering and fluorescent emissions from liquid streams containing these particles (118). Due to the small size of EVs, flow cytometry typically requires specialized approaches such as nano-flow cytometry (nFCM) or technical modifications to conventional instruments to achieve accurate detection. Flow cytometry accomplishes comprehensive structural analysis of EVs while measuring essential morphological and parametric characteristics of thousands of particles each second (119, 120). Flow cytometry provides a specific advantage as it enables precise counting and separation, and purification of EV populations present in suspension (121).
Unlike ultracentrifugation, which is a method for EV isolation, flow cytometry is an analytical technique that additionally enables EV sorting. This feature provides a key advantage, particularly when studying EV subpopulations (111). Analysis of EVs becomes possible through this method without needing previous isolation or concentration procedures when working with limited sample volumes or requiring speedy assessment processes (122).
Standard flow cytometry equipment proves inadequate for examining exosomes between 30 and 150 nm since their dimensions approach instrument detection levels and particle light scattering matches background noise. The development of advanced flow cytometers has included features for enhanced sensitiveness in addition to improved forward scatter detection and fluorescence amplification and high-resolution imaging functions (123, 124). The innovations enhance EV sizing resolution especially for small vesicles < 200 nm diameter (71), through fast classification and antigen measurement at the single vesicle level (125).
To perform flow cytometry analysis experts must be available together with state-of-the-art laboratories. The process of precise detection of EVs usually demands labeling with fluorescent dyes or antibodies yet these requirements might sometimes affect subsequent examination procedures. Measuring and identifying EV signals through flow cytometry becomes difficult due to the inherent limitations posed by their small dimensions and low refractive properties (126, 127). The advancement of both instrumentation techniques and labeling methods intends to resolve these analytical difficulties, thereby boosting the sensitivity and specificity of EV flow cytometric analysis. While flow cytometry allows high-throughput multiparametric analysis and sorting of EVs, it still faces limitations in sensitivity and resolution when analyzing small vesicles, unless advanced setups like nFCM are used.
4.5 Transmission electron microscopy technique
Transmission electron microscopy (TEM) operates as the fundamental approach to inspect biological components, specifically EVs, through evaluating their dimensions and morphological elements, and physical features (128). With electrons instead of light as the imaging source, TEM resolves down to the nanometer range, thus allowing scientists to study EV morphology in detail (129). The exposure of a thin sample layer to an electron beam produces an image that shows a diffraction pattern through electromagnetic lens detection of electron scattering. The technique enables both optic diameter measurements of the vesicles and evaluations of their structural condition (130, 131).
Biological specimens demand special laboratory handling before TEM analysis to keep their biological structures intact. TEM imaging of EVs exhibits multiple morphological patterns that include round and cup-shaped structures which represent their naturally diverse biological sources and operational tasks (132, 133). The TEM electron beam possesses the ability to create damage to biological specimens which creates distorted results that hinder correct interpretation (134, 135). Cryo-TEM serves as an investigative method to reduce both beam-induced damage and dehydration artifacts in studies of EVs (136). Samples undergo rapid vitrification under this method which safeguards their biological composition through the maintenance of vitreous ice instead of require fixation or dehydration applications. The ultrastructural integrity of EVs remains intact through Cryo-TEM analysis because it stops both the modification of structure and the relocation of elements (137).
Cryo-TEM represents an ideal approach to study biological molecules by avoiding deformations caused by dehydration while providing clear visualization of EVs alongside their membranous components and lumens (87). The study of EV biological functions requires accurate detection of specific proteins positioned inside their cargo (31, 138). The bright fluorescent signals from labeled proteins make it hard for TEM to view the labeled EVs. Researchers typically use immunogold labeling TEM to observe EVs because this method shows antibody-probed EVs under the microscope (139, 140). The localization technique depends on gold nanoparticles, which link to specific target protein antibodies to identify particular proteins both inside and outside EV structures under TEM analysis (131, 141).
The detailed characterization of EVs strongly relies on TEM together with its variant methods, including Cryo-TEM and immunogold labeling. These investigation methods reveal important information about EV shape and chemical structure, as well as working mechanisms that advance scientific knowledge of biological processes involving EVs.
4.6 Atomic force microscopy (AFM) for EVs characterization
Numerous studies have demonstrated the effective use of AFM for studying the physicochemical characteristics of EVs obtained from different biofluids such as blood, saliva, and synovial fluid, according to (142). High-resolution topographical imaging of EVs becomes possible through AFM because the technology works under near-physiological conditions while providing vital information about EV morphology and biomechanical properties, along with composition details (143, 144), significantly enhancing our understanding of these vesicles at both the single-vesicle and sub-vesicular levels (145).
The probing-tip interference with EV surfaces allows AFM to generate 3D topographic images through atomic-scale detection of mechanical surface interactions (146). The method delivers multiple advantages compared to electron diffraction-based techniques since it offers better sample management and enables damage-free imaging functions. The analysis of EVs by AFM works without fixing EVs, so scientists can maintain their natural state while preserving their structural integrity (147). AFM technology provides high-resolution observation of single and sub-vesicular EV structures, which helps scientists measure their dimensions and surface features (148). The biomechanical properties of EVs become measurable through AFM since it assesses both the elasticity and stiffness as well as adhesion characteristics of these vesicles, which assists researchers in understanding their cellular uptake capability and signaling mechanisms. AFM analysis requires minimal sample preparation because researchers avoid using damaging procedures, which avoids artifact formation during the observation of EVs (147, 149).
Technical issues hinder the application of AFM for EVs characterization, even though it offers various benefits. Experimental control under standardization remains essential because the natural state of samples from EVs changes considerably (150). Controls must be taken to address three sources of artifacts following AFM imaging because the technique remains highly sensitive to sample preparation, along with substrate interactions and scanning speed, leading to EVs' structural topographical distortions and mechanical deformations (151, 152). Low-throughput operations are a characteristic of AFM since it demands time-intensive expertise and analysis procedures (149).
When studying EVs characterization through mechanical assessment and topographic analysis of nanoscale features both AFM reveals itself as a powerful non-specific technology for sample examination without affecting underlying structures. To achieve valid experimental results as well as reproducibility researchers must optimize their experimental settings and standardize research methods in EVs identification work. The utility of automated AFM systems combined with machine learning-driven image analysis can advance to increase high-throughput characterization of EVs in the future.
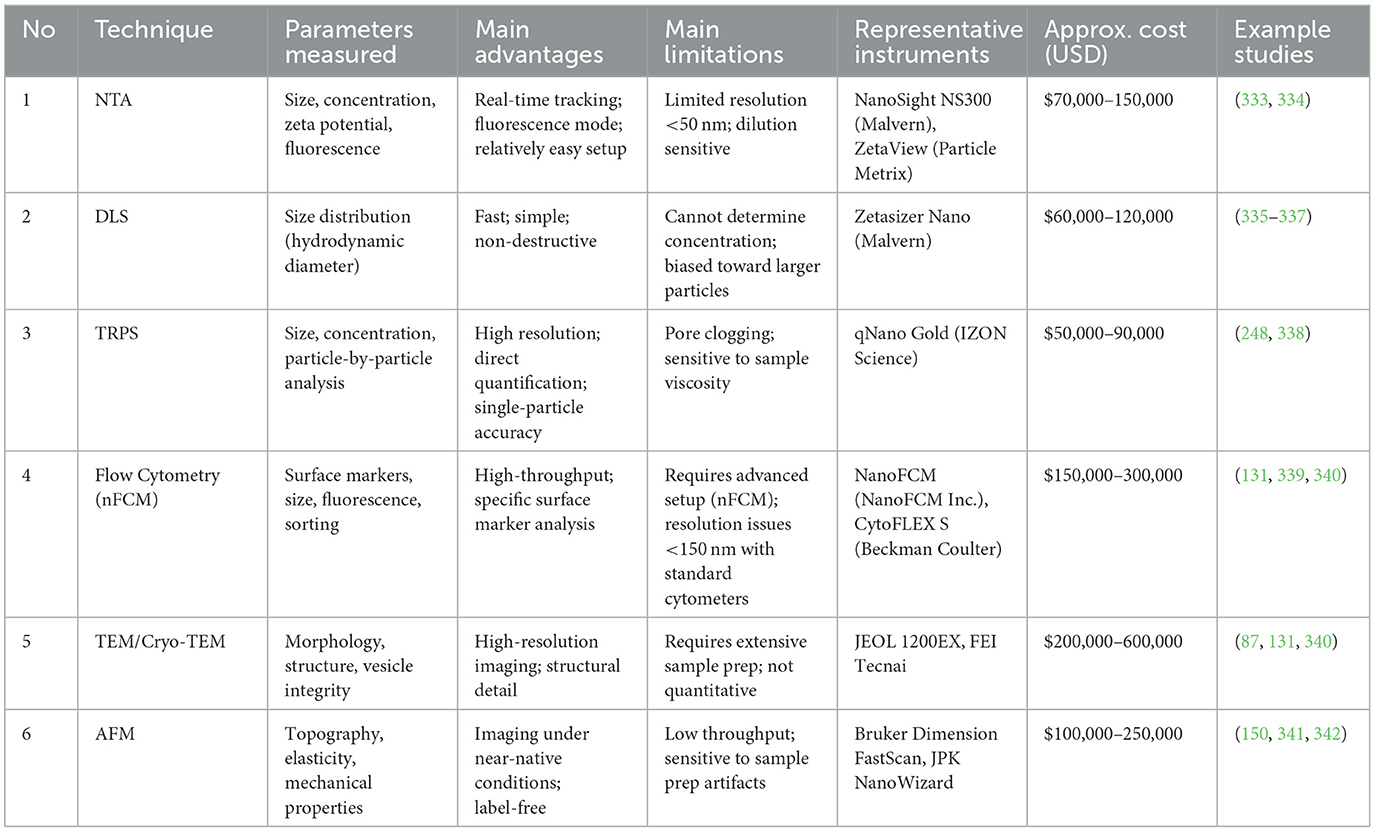
To aid researchers in selecting the most suitable EV analysis techniques, Table 2 summarizes the parameters evaluated by each method, their primary advantages and limitations, representative instruments, estimated costs, and relevant publications.
5 EVs in reproductive fluids and their role in in-vitro embryo production
Reproductive fluids such as FF, OF, endometrial fluid, amniotic fluid, and seminal fluid have been reported to contain EVs (Figure 3), which have been molecularly characterized by the presence of tetraspanins such as CD63 and CD81, and proteins like TSG101 and HSP70 (153). These EVs participate (e.g., miR-21, miR-132, miR-145) in oocyte maturation alongside fertilization and early embryonic development and implantation processes, making them critical mediators for IVP and ARTs (154).
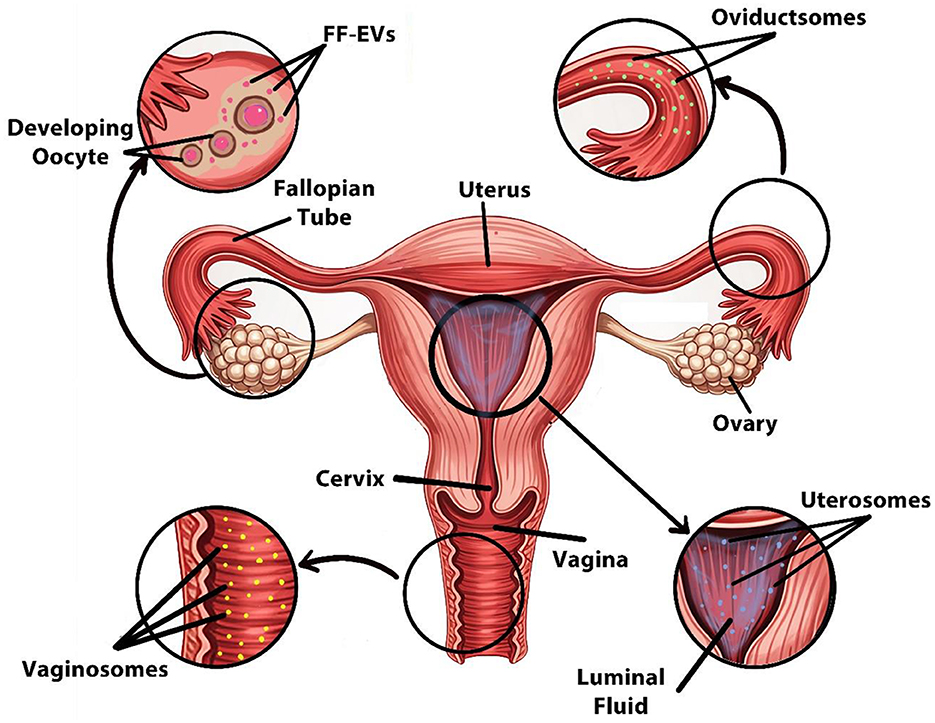
Figure 3. Sources of EVs in the female reproductive system. Designed using Digital Paint and Adobe Photoshop 2023.
Specifically, FF-EVs carry miRNA particles together with proteins and lipids, which are involved in granulosa cell signaling and oocyte maturation, thereby improving embryo development (155).
Similarly, oviductal EVs, often referred to as oviductosomes, contain specific glycoproteins, including OVGP1, Annexins, and HSP70, that serve to remodel the zona pellucida while improving sperm-oocyte binding performance. These EVs facilitate fertilization and early zygote development by transporting essential regulatory molecules. The hormonal control mechanism regulates secretion levels, which reach their maximum during the peri-ovulatory phase to enhance fertilization conditions inside the oviduct (156–158).
In addition, the embryo-maternal communication process relies on endometrial fluid–derived EVs that enhance endometrial receptivity by delivering miRNAs (such as miR-30d and miR-200c) and adhesion-related molecules (e.g., integrins αVβ3) to help activate crucial signaling pathways of early trophoblasts necessary for proper (159–161).
Moreover, the significant function of seminal EVs impacts male fertility, together with embryo quality performance. Seminal EVs influence sperm functionality and support capacitation, especially through delivery of prostasomes enriched with CD9 and enzymes such as P34H that modulate acrosome reaction and motility (162).
EVs found in amniotic fluid enriched in surfactant proteins and inflammatory mediators (e.g., IL-6, TNF-α), likewise, provide insights into maternal–fetal communication while scientists evaluate their potential as diagnostic markers for IVP embryonic health assessment (163).
Additionally, antimicrobial peptides together with defensins located in vaginal epithelial EVs known as vaginosomes help sustain proper vaginal microbiota equilibrium. The vesicles play a regulatory role in sperm selection along with early sperm survival based on hormone-controlled estrous cycle fluctuations (68, 164).
Finally, research has investigated EVs extracted from reproductive fluids because they may serve as embryonic biomarkers in ART and effective modulators to improve IVP success (165, 166).
6 Role of male reproductive tract EVs in in vitro embryo production
Extracellular vesicles secreted by the male reproductive (Figure 4) tract assist the maturation of sperm cells while improving their capability to capacitate and fertilize, which enables successful IVEP. Notably, research has mainly focused on epididymal and seminal fluid-derived EVs because they impact sperm physiological processes. Also, the testis together with the epididymis, vas deferens, and prostate actively secrete EVs (167–172).
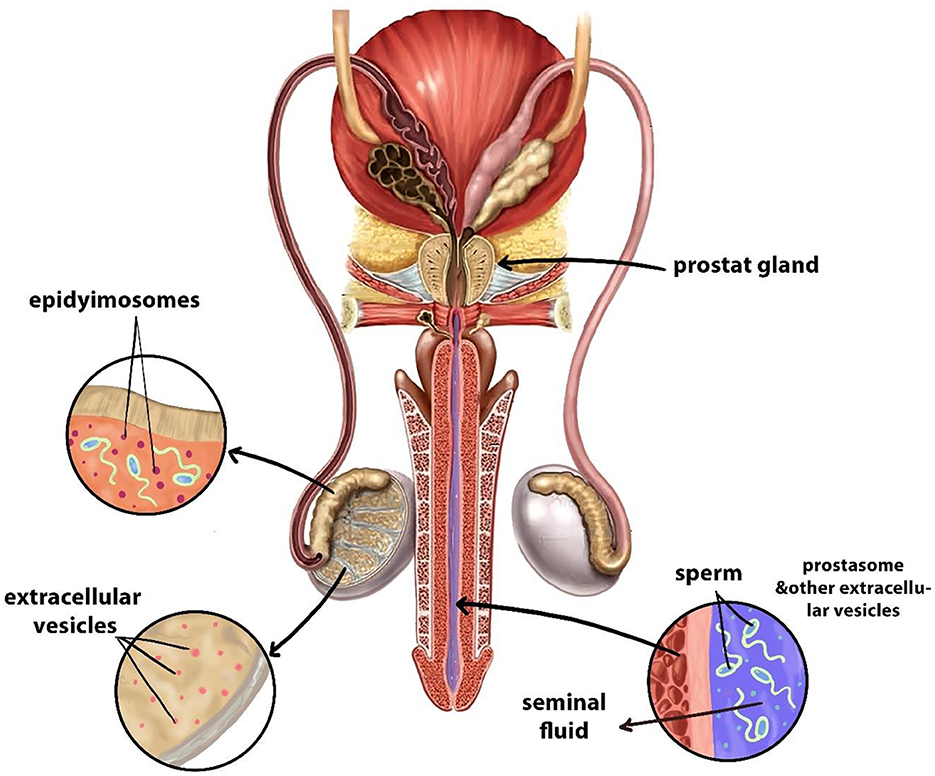
Figure 4. Sources of EVs in the male reproductive system. Designed using Digital Paint and Adobe Photoshop 2023.
Specifically, EVs released by the epididymis, epididymosomes, contain proteins like CRISP1 and miRNAs such as miR-888 and miR-891a help guide essential processes of sperm development, which are known scientifically as epididymosomal activities. These vesicles carry bioactive molecules to sperm cells to promote membrane changes and increase mobility and better fertilization ability (171, 172). Interestingly, the biochemical structure of epididymosomes changes throughout the epididymal regions, which leads to distinct effects on sperm development stages. The different compositions of vesicles among epididymal regions potentially make them suitable to serve as biomarkers for sperm selection during IVF procedures (173).
In addition, the male reproductive tract cells originating from different areas produce a variety of EVs that are now used to be classified as prostasomes (173). The essential functions of these EVs include protecting spermatozoa from oxidative stress, as well as regulating immune responses in the female reproductive tract following fertilization and sperm-oocyte interaction facilitation (174, 175). They are enriched with bioactive molecules such as tetraspanins (CD9, CD63, and CD81), enzymes like P34H and HSP70, and regulatory RNAs including miR-34c and miR-210, which play key roles in enhancing sperm motility, acrosome reaction, and fertilization efficiency (176–178).
Therefore, the male reproductive tract EVs both maintain fertility functions along with opening new opportunities for ARTs. These EVs demonstrate potential applications as supplementary agents in IVP procedures that enhance sperm capacitation capabilities, the handling process, and subsequent fertilization results. They are enriched with bioactive molecules such as tetraspanins (CD9, CD63, and CD81), enzymes like P34H and HSP70, and regulatory RNAs including miR-34c and miR-210, which play key roles in enhancing sperm motility, acrosome reaction, and fertilization efficiency. Furthermore, further study of these vesicles as well as their active substances could develop new approaches to assess sperm quality together with optimizing cell culture environments within IVP systems (174, 175).
7 Role of female reproductive tract EVs in in vitro embryo production
EVs originate from ovarian follicular cells (Figure 3) as well as oviductal epithelial cells and in vitro-fertilized embryos, and endometrial cells to mediate crucial biological functions during oocyte maturation, fertilization, and embryo-maternal interactions. Recent studies using human, bovine, and equine models reveal that EVs hold significant promise for increasing the effectiveness of IVP (179, 180).
7.1 Follicular fluid EVs and oocyte maturation
Interestingly, FF itself and FF-EVs are a rich supply of bioactive molecules essential for controlling cumulus cell activity and oocyte maturation. According to recent research, supplementation of FF during in vitro maturation can enhance cumulus growth and improve the quality of the resulting embryo in a dose-dependent manner (181). Specifically, the microenvironment of the oocyte contains FF that releases EVs by the trio of granulosa cells, cumulus cells, and theca cells (182). The bioactive contents owned by EF-derived EVs regulate oocyte growth and maturation through the delivery mechanisms of miRNAs, proteins, and lipids. Notably, several specific molecules such as miR-130b, miR-21, BMP15, and GDF9 have been identified in FF-EVs, contributing to cumulus cell expansion, inhibition of apoptosis, and promotion of oocyte meiotic competence (65). In addition, several studies have demonstrated that supplementation of FF-EVs in IVP culture media enhanced developmental competence, together with higher blastocyst formation rates (16, 183–185).
7.2 Oviductal EVs and early embryo development
The oviduct serves as an essential environment where fertilization occurs, along with early embryo maturation taking place. The OF released by epithelial cells generates EVs known as OF-EVs, which engage in continuous interactions with gametes and early developing embryos. Research shows that these vesicles improve sperm capacitation while increasing both sperm viability and fertilization potential because of carrying proteins and regulatory RNAs to target cells (186). These OF-EVs have been shown to contain OVGP1, annexins, and small RNAs like miR-375, which facilitate sperm-egg interaction, enhance embryo cleavage, and improve zona pellucida remodeling (187). Moreover, it has been revealed that supplementation of OF-EVs into culture media enhanced both cleavage rates and blastocyst formation, thus confirming their role in improving IVP systems (188). Consequently, studies indicate that OF-EVs as biological substances that can enhance IVP outcomes (162).
7.3 Endometrial EVs and embryo-maternal communication
The maternal communication pathway between embryo and tissue becomes active during the post-fertilization period due to EM-EVs' role as essential mediators. These vesicles, secreted by endometrial epithelial and stromal cells, carry signaling molecules that aid trophoblast adhesion during embryo implantation (189). Recent findings highlight the presence of miR-30d, integrin αvβ3, LIF, and HSP70 in EM-EVs, which modulate immune tolerance and promote trophoblast attachment and invasion into the maternal endometrium (190–194).
Furthermore, the process of successful implantation needs maternal immune tolerance to maintain pregnancy because EM-EV signaling helps regulate this essential aspect (195). Importantly, the enhanced maternal endometrium responsiveness enables EM-EVs to prove useful for embryo transfer processes in reproductive medicine assistance (195). As a result, research now examines EM-EVs for use as implantation success biomarkers and treatment options to enhance the implantation potential of IVP-derived embryos, since implantation stands as a primary challenge for ART success rates.
8 The role of seminal plasma and oviductal EVs in IVP
Spermatogenesis depends on EVs within seminal plasma and the female reproductive tract because these vesicles regulate sperm function during capacitation as well as fertilization processes (196, 197). Notably, these EVs maintain their impact on sperm performance from before to after fertilization and create new possibilities for improving the techniques of sperm preparation, cryopreservation, and sperm-oocyte interaction in IVP.
8.1 Seminal plasma EVs and their impact on sperm function
Seminal plasma consists of various testicular and epididymal fluids alongside fluids from accessory sex glands that keep spermatozoa enveloped from ejaculation time until after ejaculation occurs (198). Importantly, EVs isolated from seminal plasma transport biological molecules that direct sperm maturation and fertilization processes (199). Given that sperm cells are largely transcriptionally inactive, the relevance of this EV-mediated regulation lies primarily in post-transcriptional mechanisms and direct cellular interactions (200). Seminal plasma EVs, including exosomes, interact with spermatozoa and other cells in both the male and female reproductive tracts (201). This interaction involves transferring regulatory cargo such as miRNAs, proteins, lipids, and various small non-coding RNAs (sncRNAs) like tRNA, Y RNA, piwi-RNA, and ribosomal RNA, which are present at high concentrations in seminal plasma EVs. While mRNA and DNA have also been found within EVs, their specific roles in intercellular communication and gene expression within the recipient sperm remain under investigation (193, 202). The pro-survival effect of these vesicles on sperm cells stems from the ability of their miRNAs to prevent apoptotic gene expression, including BAX and CASP9, and CASP3 genes (203). This protective effect also helps protect sperm against oxidative stress, which is essential for maintaining sperm quality and function (204, 205).
Furthermore, researchers have shown that low-fertility sperm function improves when incubated with high-fertility donor EVs extracted from seminal plasma during in vitro production in both bovine and equine species (206). Also, the research demonstrates how seminal plasma-derived EVs could serve as valuable tools for improving sperm selection techniques and generating better results in IVF applications (199). Also, the research demonstrates how seminal plasma-derived EVs could serve as valuable tools for improving sperm selection techniques and generating better results in IVF applications (171). These improvements are mediated by the molecular cargo of EVs, which includes proteins such as PDIA4, Gelsolin, and CRISP1—known to enhance sperm capacitation, motility, and acrosome reaction—while proteins like SNF8, aldehyde oxidase, and Mucin 15 have been linked to poor semen quality. Additionally, seminal EVs carry miRNAs (e.g., miR-21-5p, miR-222) and other small non-coding RNAs (tRNA, piRNA, and Y RNA) that post-transcriptionally regulate sperm function, despite the limited transcriptional activity of mature sperm. These molecules influence sperm performance by modulating calcium signaling (e.g., via CatSper channels), membrane fluidity, and apoptotic pathways, as well as protecting against oxidative stress. Moreover, seminal EVs interact with the female reproductive tract by promoting uterine immune tolerance and decidualization, thereby enhancing the environment for fertilization and implantation (207, 208, 208–213).
8.2 Application of EVs in sperm cryopreservation for IVP
Cryopreservation is commonly applied in ART; however, it causes such severe damage to sperm that it negatively affects their ability to move and remain viable, together with their fertility potential. Recent studies demonstrate that the addition of EVs from seminal plasma combined with the oviduct can minimize the negative impacts caused by freezing on sperm cells. The addition of EVs to freezing procedures demonstrates their ability to protect sperm membranes while boosting post-freeze motility, which results in better blastocyst formation (199). This protective effect is thought to be mediated by EVs carrying heat shock proteins (e.g., HSP70), aquaporins, and annexins, which help stabilize the sperm plasma membrane and reduce cryo-induced damage (207, 214–216).
Interestingly, the temporary reduction of sperm mobility occurred when sperm interacted with porcine oviductal EVs, yet their survival rate improved (44). However, successful IVP requires proper optimization of EV concentration because such optimization remains essential for clinical implementation. Moreover, EVs from the oviduct and seminal plasma also deliver miRNAs (such as miR-34c and miR-19b) that regulate oxidative stress responses and support mitochondrial function in sperm cells during cryopreservation (68).
8.3 Oviductal EVs and their role in sperm capacitation for IVP
The absence of complete oviductal secretions during in vitro sperm capacitation prevents a proper execution of sperm storage selection and activation processes (217). Recent discoveries show OF-EVs fulfill three essential roles by enhancing zona pellucida sperm binding (218) and enforcing sperm hyperactivation alongside capacitation changes (219), along with controlling sperm survival inside the oviduct before ovulation (220). These effects are attributed to the transfer of specific proteins such as OVGP1, PMCA4, and CatSper regulators, which induce calcium signaling and promote tyrosine phosphorylation—key steps in capacitation (221–223).
In equine species, the addition of EVs isolated from equine oviduct tissue during IVF increases the success rates because they replicate natural fertilization signals (224). Similarly, adding oviductal EVs to bovine IVP systems improved embryo quality, which highlights the promising role of EVs as bioactive supplements for this field (225) Additionally, OF-EVs contain lipid mediators such as cholesterol and sphingomyelin that modulate membrane fluidity and support the acrosome reaction, further enhancing sperm-oocyte interaction (226).
8.4 Biomarkers of sperm fertility in EVs
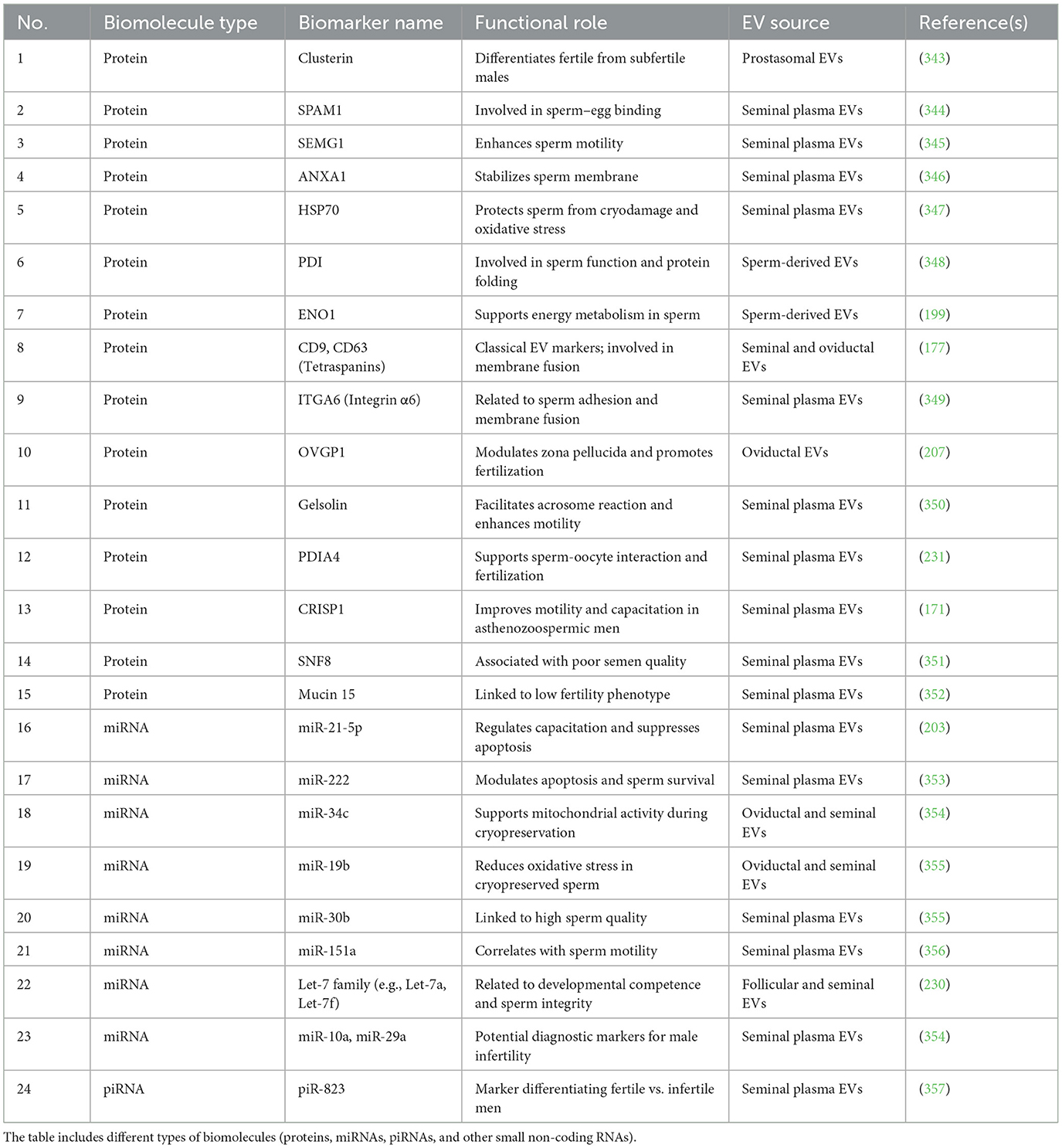
The molecular biomarkers encapsulated within EVs present information that demonstrates both the level of sperm quality and fertility potential of affected males. For example, a differentiated pattern of the diagnostic potential expressing eight miRNAs exists between EVs released from normozoospermic and oligoasthenozoospermic men. Research demonstrates that prostasome-derived EVs contain Clusterin as a protein marker, which shows the ability to differentiate between fertile and subfertile males (178, 227). Moreover, RPL investigations of spermatic EVs from affected couples' partners showed 106 proteins decreased while 71 proteins increased, indicating impaired embryo development (228). In addition to Clusterin, other EV-associated proteins such as SPAM1, SEMG1, ANXA1, and HSP70 have been linked to sperm quality, motility, and fertilization capacity. Furthermore, tetraspanins (CD9, CD63), integrins (ITGA6), and enzymes like PDI and ENO1 have been studied as potential markers in seminal EVs, reflecting sperm functional status and fertility outcomes. Several small non-coding RNAs, including miR-30b, miR-151a, and piR-823, have also been identified in EVs from high- vs. low-fertility males (177, 229–231).
Sperm evaluation through EVs offers a method for assessment that shows potential for enhancing both diagnostic performance and therapeutic outcomes at IVF clinics. A summary table (Table 3) has been added to highlight key biomarkers, their roles, and associated literature references.
9 The role of extracellular vesicles in cumulus expansion
Cumulus expansion is a critical step in oocyte maturation and subsequent fertilization. Recent research has highlighted the involvement of EVs in mediating this process through the transfer of signaling molecules and genetic regulators. This section discusses the emerging role of EVs derived from various reproductive sources in modulating cumulus cell function, matrix remodeling, and oocyte developmental competence, with particular focus on their molecular mechanisms and applications in ARTs (232–234).
9.1 EVs enhance cumulus expansion and oocyte maturation
Multiple studies have shown that EVs isolated from FF FF-EVs, plasma, seminal plasma, and oviductal fluid influence the expansion of the cumulus cells through their effects on gene expression and cell division, as well as matrix remodeling. Notably, plasma-derived EVs have been shown to enhance both cumulus cell expansion and improve the oocyte maturation rate to the MII stage during in vitro maturation (22).
Similarly, exposure to bovine FF-EVs results in increased transcription of key cumulus expansion genes-PTGS2, PTX3, and TNFAIP6- in murine cumulus cells, suggesting a conserved mechanism across species (235). Moreover, the presence of proliferative and maturation-related molecules in plasma EVs activates HAS2 and PTGS2 expression, which speeds up cumulus expansion and oocyte developmental progression (22). These findings are consistent with evidence that FF-EVs enhance both the expansion of bovine cumulus cells and the maturation status of oocytes in IVM systems (235).
In addition, the expression profiles of bovine cumulus cells' miRNAs shift based on the progesterone levels and estrous cycle phase, which is mirrored by sEVs from FF (236). Of particular interest, sEVs from low-progesterone follicles demonstrate the ability to activate genes linked to reproduction and immune response, which indicates their value as biomarkers for determining oocyte competence (237).
Furthermore, seminal plasma EVs of both small and large subtypes interact with porcine cumulus cells, which affects their gene networks that control hormone production as well as cumulus expansion (238). Conversely, different studies have presented contradictory evidence about whether FF-EVs successfully stimulate the expansion of cumulus cells. While several studies have demonstrated positive actions regarding cumulus cell enhancement (239, 240). These discrepancies likely stem from species-specific differences in EV cargo composition, as well as variations in experimental design, EV isolation methods, and culture systems.
In equine models, it has been demonstrated that FF-EVs enhanced cumulus expansion through Method II, which used two-step IVM, but they did not benefit Method I, which depended on continuous cultures (90). Moreover, studies show that FF-EVs cause different impacts on compacted and expanded COCs; for instance, they enhance viability in compacted COCs while decreasing in expanded groups (241). These observations underscore the importance of timing and cellular context in determining the responsiveness of cumulus cells to EV signals.
Finally, the development of rabbit oocytes benefits from EVs derived from testis, prostate, and epididymis that induce an increase in KISS1, MDK, NTF3, ADAM17, and VEGFA factors, which support cumulus expansion and oocyte maturation (242).
9.2 Mechanisms of EV-mediated cumulus expansion
Extracellular vesicles enable cumulus expansion by transporting bioactive substances that direct extracellular matrix (ECM) formation and key signaling activities that support the developmental competence of the oocyte. The major mechanisms include: (1) firstly, the activation of expansion-related genes constitutes the first mechanism through which EVs affect ECM development by stimulating the expression of HAS2 and PTGS2 genes along with PTX3 and TNFAIP6 factors (243). (2) Secondly, the mitogen-activated protein kinase (MAPK) pathway is regulated through EV-delivered proteins and miRNAs that activate this pathway to initiate cumulus expansion, together with cell differentiation processes (89). (3) Thirdly, the WNT signaling regulatory mechanism involves WNT signaling pathway control by EVs released from follicular and oviductal origins that facilitate follicular development and control cumulus cell functions (175). (4) Lastly, the small vesicles deliver miRNAs to target cumulus cells to modify their genetic expression, which affects their biological activities along with oocyte developmental readiness. The miRNA expression patterns in cumulus cells differ based on the maturation phase of the releasing follicle, according to research (236).
Collectively, FF-EVs demonstrate great potential for ART applications through their function as minimally invasive markers that help measure oocyte quality and developmental potential. When applied appropriately, adding appropriate doses of FF-EVs and plasma-derived EVs to IVM media enhances both cumulus cell homeostasis and the oocyte maturation process, which leads to superior blastocyst production in IVP systems, according to the literature (243, 244). However, the wide range of EVs impacts between species requires researchers to establish separate optimal EVs utilization procedures for each species that aims to use them in IVM and IVP research. Therefore, to maximize the beneficial effects of EVs and outcomes from reproductive technology, it requires a dedicated assessment of the EVs' source type together with their concentration levels, developmental stage of application, and target cell condition.
10 The role of EVs in granulosa cell proliferation
Oocytes require the specialized somatic cells known as granulosa cells (GCs), which surround them and form the follicular structure together with theca and cumulus cells. GCs are vital for oocyte growth and follicular development, and steroid hormone production. Specifically, the oocyte resides within these granulosa cells, which develop into single or multiple layers within the follicular wall that support follicular growth as well as produce estrogen and progesterone, and establish oocyte competence (245, 246).
Importantly, follicular expansion and oocyte development depend on the proliferation of granulosa cells, a process regulated by paracrine factors as well as EVs regulate (247).
Recent studies have shown that EVs function as vital mediators supporting granulosa cells proliferation and follicular function. For instance, it has been revealed that EVs separated from FF promoted granulosa cells proliferation at different rates, depending on the follicular origin and size of the antral follicles (68, 245). Moreover, the bioactive cargo of EVs function as key regulators for cellular functions necessary for follicular development. The received molecules modify cell cycle regulatory mechanisms as well as differentiation processes and metabolic functions after entering recipient cells (190, 248).
Specifically, studies have identified miRNAs (e.g., miR-21, miR-26a), proteins such as GDF9, BMP15, and metabolic enzymes like ENO1 within FF-derived EVs, which enhance GC proliferation and function (249).
miR-21, for example, has been shown to promote GC survival by suppressing pro-apoptotic genes, while GDF9 and BMP15 are known to activate SMAD signaling in granulosa cells (203).
In terms of uptake, granulosa cells internalize EVs by means of two dominant endocytic pathways known as clathrin-mediated endocytosis and caveolae-mediated endocytosis. Together, paracrine signals from the oocyte strengthen the vesicle entry process by working with membrane invaginations through the combined action of clathrin-coated pits and plasma membrane caveolae (250–252).
Additionally, other internalization mechanisms like macropinocytosis and phagocytosis contribute to EV uptake pathways which influence subsequent functions of the granulosa cells (253, 254). Once internalized, EVs cargo activates intercellular signaling pathways that drive granulosa cells proliferation and activity.
Notably, the activation of MAPK pathway by EVs frequently occurs as it significantly influences granulosa cells proliferation together with differentiation. Similarly, studies indicate that granulosa cells proliferation from EV signaling likely requires additional signaling networks like Src kinase and phosphoinositide 3-kinase (PI3K) yet scientists need to fully clarify their precise effects (253–255).
FF-EVs have been shown to influence the expression of FSH receptor and aromatase (CYP19A1) genes, thereby modulating the endocrine function of granulosa cells (256).
Furthermore, it has been shown that EVs affect granulosa cell proliferation patterns and this affects the outcomes of both IVEP and ARTs. Through their actions, microvesicles perform dual actions that first stimulate follicular development before they enhance hormonal communication which leads to better quality oocytes and higher maturation rates and embryonic development during in vitro.
Consequently, studies indicate that EVs demonstrate promising indications as fertility markers and create new possibilities to enhance IVM protocols across various species. However, it remains essential to determine the particular molecular elements inside EVs that promote granulosa cells proliferation together with how these compounds impact follicles differently between diverse animal models.
11 The role of EVs in oocyte maturation
The process of oocyte maturation involves crucial female reproductive development because it makes an immature oocyte prepared for fertilization. This maturation process occurs within the ovarian follicle, where the maturation process develops and remains under hormonal control and intercellular signals with specific molecular pathways doing the regulation (257–259). Following meiotic resumption, the oocyte moves from germinal vesicle stage to MII stage while chromosomal condenses and the first polar body extrusion while experiencing major cytoplasmic changes. Throughout this process, the oocyte competence becomes possible due to the combination of metabolic support and paracrine signaling from granulosa and cumulus cells (260–262).
During in vitro maturation the resumption of meiosis starts prior to complete cytoplasmic maturation potentially leading to diminished developmental competence. Notably, the success of fertilization and the subsequent embryo development depends on three critical aspects of cytoplasmic maturation: mRNA accumulation and organelle redistribution, and metabolic adjustments. The second messenger, cyclic adenosine monophosphate (cAMP) functions as the fundamental regulator which sustains meiotic arrest in the follicle. However, the reduction in cAMP after follicular removal leads to meiotic resumption. Therefore, to improve IVM results, it is crucial to postpone nuclear maturation while completing cytoplasmic maturation by the modification of cAMP levels (263–266).
Recent research has demonstrated that EVs act as vital messengers between follicles to transfer microenvironment signals that control oocyte development. The miRNAs together with proteins and lipids from FF, oviductal secretions, and cumulus cells act as controlling factors during oocyte maturation. Research indicates that EVs have distinctive effects on oocyte maturation across different species (267). For example, the interaction of EVs derived from seminal plasma with porcine cumulus cells during IVM shows no significant impact on oocyte maturation rates. Interestingly, it has been identified that a particular group of EVs referred to as large EVs that affect steroidogenesis-related genes which influence the functionality of cumulus cells (238). Conversely, mice plasma-derived EVs show the ability to accelerate oocyte maturation while indicating potential improvements for developmental competence (22).
In bovine studies, supplementation of FF-derived EVs during IVM has been shown to significantly increase blastocyst yield and oocyte maturation rate. Equine FF-EVs have also enhanced COC viability in compacted follicles, indicating species-specific functional responses. Furthermore, supplementation with oviductal EVs improved cytoplasmic maturation markers including mitochondrial redistribution and cortical granule alignment in bovine oocytes (180, 268).
Moreover, MAPK signaling pathway acts as a principal pathway for EV-mediated oocyte maturation and cumulus expansion (269, 270). In particular, EVs that carry particular miRNAs, including miR-21, miR-378, and miR-146a, which efficiently regulate MAPK activity, induce oocyte maturation and improved cumulus cell function. Similarly, evidence shows EVs affect the PI3K/AKT signaling cascade which enables metabolic coupling and maintains mitochondrial function between oocytes and cumulus cells (271–273).
Other candidate molecules include TGF-β1, BMP15, and GDF9-related transcripts delivered via FF-EVs, which are linked to cumulus expansion and oocyte competence.
In addition to direct contact, oocyte–granulosa cell communication is mediated by paracrine signaling and EV-mediated pathways. This dual mode of communication through gap junctions and transzonal projections enables precise control of maturation processes (274, 275). It has been shown that the molecular contents of EVs, establish the direction of developmental progression for oocytes and cumulus cells and administer their cellular functions (276, 277). Remarkably, recent investigations confirm EVs with miRNAs can enter oocytes by penetrating the zona pellucida without transfection agents so they may offer clinical value to IVM protocols (278–280).
Given these findings, EVs have a significant capacity to regulate oocyte maturity, which would increase the success rates of IVEP and ART treatments. By synchronizing the nuclear and cytoplasmic maturation, EVs promote improved development competence while enhancing fertilization results and blastocyst development. Consequently, the utility of EV-based strategies creates biologically accurate protections for in vivo follicles which enhance the performance of IVM systems across different animal species.
12 The role of EVs in embryo development and quality
In both natural conception and ARTs, embryo development and quality are critical factors that determine successful implantation and pregnancy outcomes. Recent research has emphasized EVs as important regulators of early embryonic development which they function as intercellular messengers that alter gene expression and cellular communication.
12.1 EVs in preimplantation embryo development
During early embryogenesis, embryos produce a milieu containing biochemical molecules, including EVs, which facilitate both autocrine and paracrine functions. In the porcine model, it has been shownthat developing embryos release exosomal marker CD9 along with vesicles measuring 30–120 nm in diameter, consistent with the size of exosome. These vesicles deliver mRNAs, including OCT4, SOX2, and KLF4 at different developmental stages of embryonic development. Moreover, embryonic vesicles have the capability to cross the zona pellucida before being taken up by blastomeres, supporting intercellular communication during embryonic development (76, 197, 276). Nevertheless, scientists still need to identify all the mechanisms through which embryo development depends on EVs.
Recent studies have demonstrated that supplementing embryo culture media with EVs derived from maternal reproductive tract fluids—especially oviductal and uterine EVs—can significantly enhance blastocyst formation, hatching rates, and overall embryo quality. For example, bovine oviductal EVs added during early cleavage stages improved blastocyst rates and upregulated key pluripotency markers such as OCT4 and NANOG (281). Similarly, in porcine embryos, oviductal EVs enhanced mitochondrial activity and cell proliferation, supporting superior blastocyst development. Uterine EVs collected during the peri-implantation phase have also been shown to promote trophoblast elongation and differentiation by delivering integrins, growth factors, and miRNAs. These findings highlight the importance of EV source and developmental timing when applying EVs to in vitro production (IVP) systems (187, 282).
Similarly, EVs in reproductive fluids such as FF and AOF transport miRNAs, proteins together with lipids which contribute to embryo development. A study by Asaadi et al. (16) showed that EVs obtained from both FF and AOF fluid improved blastocyst quality by enhancing TE and ICM development as well as reducing the apoptotic cell ratio. In addition, studies have shown that in the bovine model, epithelial EVs derived from the oviductal tissue improve embryo quality and increase total cell numbers, and enhance vitrification survival rates (79, 283).
Furthermore, embryonic gene expression is fundamentally modulated by the miRNA content carried by EVs. For instance, investigations have identified two particular miRNAs named miR-21 and miR-2861 which demonstrate potential in enhancing embryo quality alongside development. Conversely, research indicates that embryonic development can be negatively affected by EVs contents, such as miRNA-146b, which has been shown to impair embryo development (284–286).
In addition to maternal sources, embryo-derived EVs also appear to act in an autocrine manner to modulate their own development. For instance, EVs secreted by preimplantation embryos have been shown to influence cell lineage allocation, possibly by redistributing miRNAs and lncRNAs that regulate transcriptional activity in blastomeres. Moreover, beyond miR-21 and miR-2861, other miRNAs such as miR-320a and miR-30c have been associated with improved blastocyst viability and reduced apoptosis in both mouse and bovine models (287–289).
On the contrary, studies have found that excessive expression of miR-155 or miR-146b in EVs is associated with reduced developmental rates and impaired ICM formation. These insights suggest that EV content profiling may help screen for supportive vs. detrimental signals in embryo culture (285).
12.2 EV-mediated communication between the embryo and the oviduct
Effective communication between the embryo and the oviduct is crucial for proper development. The developing embryo requires optimal environmental conditions provided by, the oviduct, while its secretions-particularly EVs-function as essential mediators in the interaction between embryo and oviduct (77, 290). These EVs carry specific molecular cargos including proteins (e.g., oviductin), lipids, and miRNAs (e.g., miR-30c, miR-375), which modulate gene expression and support embryo development (74). Moreover, embryo physiology influenced by vesicles isolated from various region of the oviduct, such as ampulla and isthmus. For example, EVs from the ampulla region promote early cleavage, while isthmus-derived EVs enhance blastocyst formation and quality in bovine and porcine models (287). Oviductal EVs modify their miRNA miRNA composition in response to the presence of an embryo, indicating bidirectional communication between embryo and oviduct (291–293). Transcriptomic analysis has revealed altered miRNA profiles in oviductal EVs, such as increased let-7a and miR-200 family members, reflecting maternal adaptation to embryonic signals (294).
Similarly, the culture media of developing bovine preimplantation embryos contains EVs, whose size and concentration are associated with embryonic quality. Studies have shown that higher concentrations of embryo-derived EVs (30–150 nm) are correlated with improved ICM/TE ratio and mitochondrial activity (207). Therefore, it has been proposed that these EVs could be used to assess embryonic competence along with predicting implantation outcomes using these EVs (295–297).
13 The role of EVs in embryo hatching and pre-implantation development
Embryos must undergo hatching to exit their zona pellucida envelope during pre-implantation development (Figure 5). A successful hatching process not only allows embryonic development to continue but also develops implantation competence. One of the main challenges during IVEP involves establishing optimized conditions that promote embryo hatching and developmental success (358, 359).
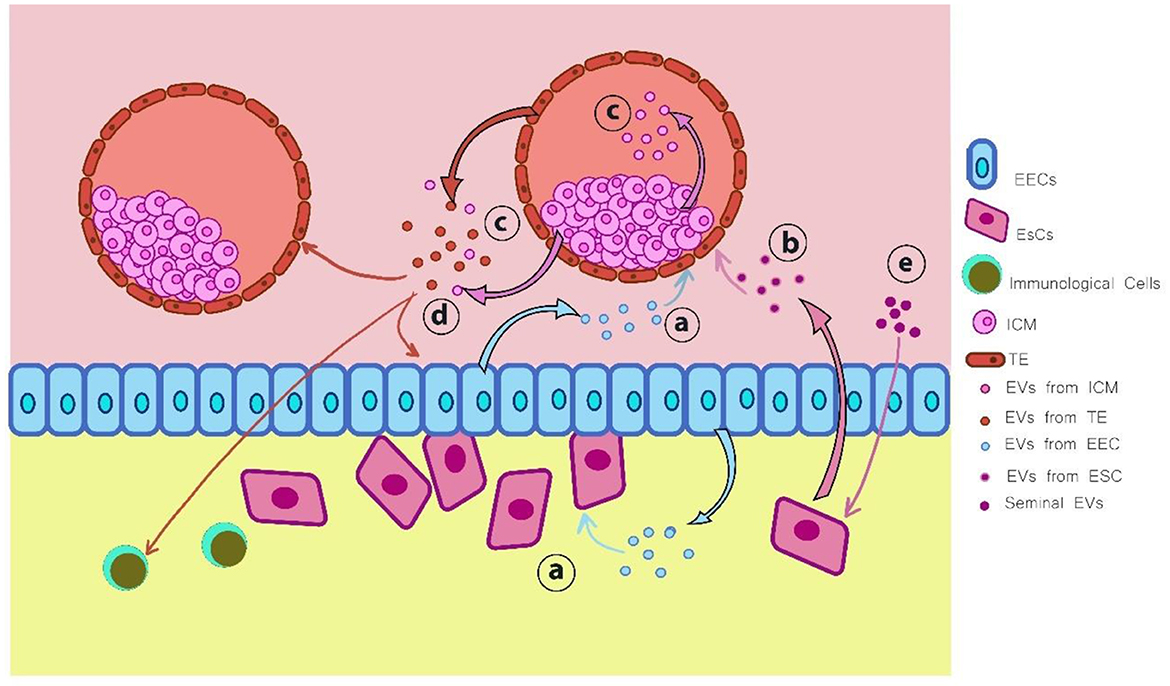
Figure 5. Extracellular vesicles (EVs) are secreted by various types of cells during the implantation process: (a) EVs derived from endometrial epithelial cells (EECs) interact with both the embryo and uterine fibroblasts; (b) EVs secreted by endometrial stromal cells (ESCs) influence embryonic development; (c) EVs originating from the inner cell mass (ICM) act on the trophectoderm (TE) and can also be transferred outside the blastocyst; (d) EVs produced by the embryo, including those from both TE and ICM, modulate the function of EECs, neighboring embryos, and immune cells; (e) Seminal EVs exert regulatory effects on ESCs. This figure was inspired by the work of Chen et al. (211). Illustration created using Digital Paint and Adobe Photoshop 2023.
Interestingly, both embryos and the maternal reproductive tract secrete EVs, which have been shown to regulate early developmental processes. Assessment data show that pre-implantation embryos actively release embryo-derived EVs into the in vitro culture medium throughout their development (360, 361). For example, Giacomini et al. (86) proved that pre-implantation human embryos produce EVs that express CD63, CD9, and ALIX markers which carry stemness-related gene transcripts and HLA-G protein. The identified embryonic EVs show potential as self-regulatory agents that may help blastocysts communicate with their neighbors before implantation occurs.
Furthermore, research using bovine IVEP models has shown that embryonic vesicles promote both blastocyst development and the hatching process. Specifically, miRNA-378a-3p, present within embryonic EVs, plays a regulatory role in bovine blastocyst hatching (298). In addition, recent studies suggest that circular RNAs also play a developmental role in embryos. For instance, circAGO2 is proposed to function as a binding molecule for RNAs involved in EVs-mediated communication, which may influence blastocyst hatching (86). Moreover, it has been reported that bovine embryo hatching rates improved following the inhibition of the small RNA tsRNA tDR-14:32-Glu-CTC-, which was also associated with altered gene expression revealed to hatching (78). Consequently, the cargo of embryonic vesicle cargoes contribute to embryonic developmental competence and holds promise for improving embryo quality IVEP systems.
EV-mediated signaling not only guides embryonic development through embryo-derived vesicles but also reflects inputs from maternal reproductive conditions. Interestingly, studies have revealed that endometrial EVs are present in uterine fluid across menstrual and estrous cycles, with their concentration peaking during the implantation window (299–303).
Proteomic analysis further show that the protein content of endometrial EVs varies according to hormonal fluctuations throughout the menstrual cycle (299). Furthermore, epithelial-origin endometrial cells have been shown to absorb EVs from recipient cells, resulting in receptor-modulating effects (304). Additionally, studies have found that EVs are enriched with proteins associated with extracellular matrix remodeling, cell adhesion, and immune modulation, all of which are essential for embryo-endometrium interactions in vivo (305–312). As a result, researchers are exploring the utilization of endometrial EVs in IVEP systems, which the aim of enhancing embryo competence and improving implantation outcomes.
In the same context, EVs isolated from ruminant reproductive tracts are of particular scientific interest due to their role in supporting pre-implantation embryos. In sheep, conceptus survival and pregnancy recognition are influenced by EVs present in uterine luminal fluid, which also regulate modulate immune responses (313). Likewise, bovine uterine EVs appear to affect trophoblast-endometrial communication by delivering conceptus-derived interferon tau, which promotesendometrial receptivity (314). Incorporating embryonic vesicles into embryo culture systems helps creates an environment that more closely mimics physiological conditions, thereby minimizing negative effects from laboratory culture on embryonic development (315).
Applications of exosomes in ARTs show promising translational potential by improving both embryo assessment and culture environments. Currently, embryo grading depends primarily on morphological criteria and invasive biopsy methods, which often fail to accurately identify embryos with high implantation potential (316, 317). Conversely, EVs profiles differ between viable and degenerate embryos, suggesting that EVs may serve as non-invasive biomarkers for assessing embryo competence (15). Moreover, the assessment of embryonic development in IVEP now incorporates EVs derived from stem cells and reproductive tract secretions (318).
Despite being in early stages, available evidence strongly supports the vital importance of EVs in embryonic development despite early stages of functional research in IVEP. Future research should focus on optimizing EV-based techniques to improve IVEP outcomes-including enhanced culture conditions, better embryo selection methods, and novel therapeutic applications. Obviously, the development of improved EVs isolation and characterization methods will open new avenues for their integration into ART.
14 Conclusion
Extracellular vesicles are increasingly recognized as critical modulators in the context of in vitro embryo production, facilitating vital intercellular communication throughout the processes of oocyte maturation, sperm functionality, fertilization, and embryonic development. By carrying bioactive molecules, including proteins, microRNAs, and lipids, EVs exert an impact on gene expression and cellular dynamics in both gametes and embryos. They serve not only as messengers but also as promising tools for diagnosis and therapy in assisted reproductive technologies.
Moreover, EVs hold promise as non-invasive biomarkers for assessing gamete and embryo quality, offering safer and more precise alternatives to current invasive methods.
Notwithstanding their considerable potential, obstacles persist concerning the standardization of EV isolation, characterization, and application across diverse species and clinical environments. Further investigation is needed to understand how EV heterogeneity across different follicular stages and species influences reproducibility and functional outcomes. Advancing analytical technologies such as single-vesicle profiling, multi-omics integration, and real-time EV tracking will be critical in unlocking their full potential.
Future studies should focus on revealing the specific mechanisms underlying EV functionality and enhancing their integration into IVEP contexts. Eventually, the incorporation of EV-based methodologies has the potential to enhance embryo quality, elevate implantation success rates, and contribute to improved reproductive outcomes in both agricultural and human fertility interventions. Ultimately, the development of EV-based supplements or engineered culture systems could significantly improve embryo viability, implantation success rates, and long-term ART outcomes.
Author contributions
MP: Conceptualization, Investigation, Visualization, Writing – original draft, Writing – review & editing. NM: Writing – review & editing. RE: Writing – review & editing. SE: Writing – review & editing. TN: Writing – review & editing. NA-D: Conceptualization, Investigation, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
MP and NM were employed by Sina Fanavaran Mandegar Company.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Pournourali M, Tarang A, Mashayekhi F. Chromosomal analysis of two buffalo breeds of Mazani and Azeri from Iran. J Vet Sci Technol. (2015) 7:22–31. doi: 10.22067/veterinary.v7i1.36731
2. Malekpour A, Shirazi A, Borjian Boroujeni S, Sarvari A, Naderi MM, Pournourali M, et al. The effect of simulated physiological oocyte maturation (SPOM) and L-carnitine on bovine oocyte developmental competence. Avicenna J Med Biotechnol. (2024) 16:260–7. doi: 10.18502/ajmb.v16i4.16742
3. Pournourali M, Tarang A, Mashayekhi F. Karyotyping studies and Chromosome morphology in Mazani and Azeri buffaloes. Res J Livest Sci. (2015) 28:13–24.
4. Sarvari A, Niasari-Naslaji A, Shirazi A, Heidari B, Boroujeni SB, Moradi MH, et al. Effect of intra-ovarian injection of mesenchymal stem cells or its conditioned media on repeated OPU-IVEP outcomes in jersey heifers and its relationship with follicular fluid inflammatory markers. Avicenna J Med Biotechnol. (2024) 16:16–28. doi: 10.18502/ajmb.v16i1.14167
5. Viana J. 2021 statistics of embryo production and transfer in domestic farm animals. Embryo Tech Newsletter. (2022) 40:22–40.
6. Guo H, Su Z, Yang X, Xu S, Pan H. Greenhouse gas emissions from beef cattle breeding based on the ecological cycle model. Int J Environ Res Public Health. (2022) 19:9481. doi: 10.3390/ijerph19159481
7. Duszewska AM, Raçpala L, Trzeciak P, Daçbrowski S, Piliszek A. Obtaining farm animal embryos in vitro. J Anim Feed Sci. (2012) 21:217–33. doi: 10.22358/jafs/66068/2012
8. Hadi S. Effect of sperm selection by ”Swim-Up“ technique on the sex ratio of in vitro produced ovine embryos. Iraqi J Vet Med. (2013) 37:175–9. doi: 10.30539/iraqijvm.v37i2.279
9. Zaninovic N, Rosenwaks Z. Artificial intelligence in human in vitro fertilization and embryology. Fertil Steril. (2020) 114:914–20. doi: 10.1016/j.fertnstert.2020.09.157
10. Amini MS, Naderi MM, Shirazi A, Aminafshar M, Boroujeni SB, Pournourali M, et al. Bioactive materials derived from menstrual blood stem cells enhance the quality of in vitro bovine embryos. Avicenna J Med Biotechnol. (2022) 14:287–93. doi: 10.18502/ajmb.v14i4.10483
11. Ferré LB, Kjelland ME, Strøbech LB, Hyttel P, Mermillod P, Ross PJ. Review: Recent advances in bovine in vitro embryo production: reproductive biotechnology history and methods. Animal. (2020) 14:991–1004. doi: 10.1017/S1751731119002775
12. Pérez-Pé R, Grasa P, Fernández-Juan M, Peleato ML, Cebrián-Pérez JÁ, Muiño-Blanco T. Seminal plasma proteins reduce protein tyrosine phosphorylation in the plasma membrane of cold-shocked ram spermatozoa. Mol Reprod Dev. (2002) 61:226–33. doi: 10.1002/mrd.1152
13. Menchaca A, dos Santos-Neto PC, Cuadro F, Souza-Neves M, Crispo M. From reproductive technologies to genome editing in small ruminants: an embryo's journey. Anim Reprod. (2018) 15:984–95. doi: 10.21451/1984-3143-AR2018-0022
14. Shirazi A, Bahiraee A, Ahmadi E, Nazari H, Heidari B, Borjian S. The effect of the duration of in vitro maturation (IVM) on parthenogenetic development of ovine oocytes. Avicenna J Med Biotechnol. (2009) 1:181–91.
15. Pavani KC, Hendrix A, Van Den Broeck W, Couck L, Szymanska K, Lin X, et al. Isolation and characterization of functionally active extracellular vesicles from culture medium conditioned by bovine embryos in vitro. Int J Mol Sci. (2018) 20:38. doi: 10.3390/ijms20010038
16. Asaadi A, Dolatabad NA, Atashi H, Raes A, Damme P Van, Hoelker M, et al. Extracellular vesicles from follicular and ampullary fluid isolated by density gradient ultracentrifugation improve bovine embryo development and quality. Int J Mol Sci. (2021) 22:578. doi: 10.3390/ijms22020578
17. Angel-Velez D, De Coster T, Azari-Dolatabad N, Fernández-Montoro A, Benedetti C, Pavani K, et al. Embryo morphokinetics derived from fresh and vitrified bovine oocytes predict blastocyst development and nuclear abnormalities. Sci Rep. (2023) 13:4765. doi: 10.1038/s41598-023-31268-6
18. Pournourali M, Tarang A, Haghighi SF, Yousefi M, Bahadori MH. Polymorphism variant of MnSOD A16V and risk of female infertility in northern Iran. Taiwan J Obstet Gynecol. (2016) 55:801–3. doi: 10.1016/j.tjog.2016.06.018
19. Mellisho EA, Velásquez AE, Nuñez MJ, Cabezas JG, Cueto JA, Fader C, et al. Identification and characteristics of extracellular vesicles from bovine blastocysts produced in vitro. PLoS ONE. (2017) 12:e0178306. doi: 10.1371/journal.pone.0178306
20. Rodriguez-Caro H, Dragovic R, Shen M, Dombi E, Mounce G, Field K, et al. In vitro decidualisation of human endometrial stromal cells is enhanced by seminal fluid extracellular vesicles. J Extracell Vesicles. (2019) 8:1565262. doi: 10.1080/20013078.2019.1565262
21. Harris EA, Stephens KK, Winuthayanon W. Extracellular vesicles and the oviduct function. Int J Mol Sci. (2020) 21:8280. doi: 10.20944/preprints202010.0638.v1
22. Javadi M, Soleimani Rad J, Pashaiasl M, Farashah MSG, Roshangar L. The effects of plasma-derived extracellular vesicles on cumulus expansion and oocyte maturation in mice. Reprod Biol. (2022) 22:100593. doi: 10.1016/j.repbio.2021.100593
23. Fang X, Tanga BM, Bang S, Seong G, Saadeldin IM, Lee S, et al. Oviduct epithelial cells-derived extracellular vesicles improve preimplantation developmental competence of in vitro produced porcine parthenogenetic and cloned embryos. Mol Reprod Dev. (2022) 89:54–65. doi: 10.1002/mrd.23550
24. Hua R, Liu Q, Lian W, Kang T ting, Gao D, Huang C, et al. Extracellular vesicles derived from endometrial epithelial cells deliver exogenous miR-92b-3p to affect the function of embryonic trophoblast cells via targeting TSC1 and DKK3. Reprod Biol Endocrinol. (2022) 20:152. doi: 10.1186/s12958-022-01023-z
25. Vyas P, Balakier H, Librach CL. Ultrastructural identification of CD9 positive extracellular vesicles released from human embryos and transported through the zona pellucida. Syst Biol Reprod Med. (2019) 65:273–80. doi: 10.1080/19396368.2019.1619858
26. Estébanez B, Visavadiya NP, de Paz JA, Whitehurst M, Cuevas MJ, González-Gallego J, et al. Resistance training diminishes the expression of exosome cd63 protein without modification of plasma mir-146a-5p and cfDNA in the elderly. Nutrients. (2021) 13:665. doi: 10.3390/nu13020665
27. Sun R, Liu Y, Lu M, Ding Q, Wang P, Zhang H, et al. ALIX increases protein content and protective function of iPSC-derived exosomes. J Mol Med. (2019) 97:829–44. doi: 10.1007/s00109-019-01767-z
28. Yoon EJ, Choi Y, Kim TM, Choi EK, Kim YB, Park D. The neuroprotective effects of exosomes derived from TSG101-overexpressing human neural stem cells in a stroke model. Int J Mol Sci. (2022) 23:9532. doi: 10.3390/ijms23179532
29. Lawson EF, Gibb Z, de Ruijter-Villani M, Smith ND, Stout TA, Clutton-Brock, et al. Proteomic analysis of pregnant mare uterine fluid. J Equine Vet Sci. (2018) 66:171–2. doi: 10.1016/j.jevs.2018.05.064
30. Jiang K, Dong C, Yin Z, Li R, Mao J, Wang C, et al. Exosome-derived ENO1 regulates integrin α6β4 expression and promotes hepatocellular carcinoma growth and metastasis. Cell Death Dis. (2020) 11:972. doi: 10.1038/s41419-020-03179-1
31. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. (2020) 367:eaau6977. doi: 10.1126/science.aau6977
32. Van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. (2018) 19:213–28. doi: 10.1038/nrm.2017.125
33. Couch Y, Buzàs EI, Vizio D Di, Gho YS, Harrison P, Hill AF, et al. A brief history of nearly EV-erything – The rise and rise of extracellular vesicles. J Extracell Vesicles. (2021) 10:e12144:. doi: 10.1002/jev2.12144
34. Welsh JA, Goberdhan DCI, O'Driscoll L, Buzas EI, Blenkiron C, Bussolati B, et al. Minimal information for studies of extracellular vesicles (MISEV2023): from basic to advanced approaches. J Extracell Vesicles. (2024) 13:e12404. doi: 10.1002/jev2.12451
35. Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. (2014) 30:255–89. doi: 10.1146/annurev-cellbio-101512-122326
36. Gurunathan S, Kang MH, Kim JH. A comprehensive review on factors influences biogenesis, functions, therapeutic and clinical implications of exosomes. Int J Nanomedicine. (2021) 16:1281–312. doi: 10.2147/IJN.S291956
37. Donoso-Quezada J, Ayala-Mar S, González-Valdez J. The role of lipids in exosome biology and intercellular communication: function, analytics and applications. Traffic. (2021) 22:204–20. doi: 10.1111/tra.12803
38. Stahl PD, Raposo G. Extracellular vesicles: exosomes and microvesicles, integrators of homeostasis. Physiology. (2019) 34:169–77. doi: 10.1152/physiol.00045.2018
39. Muralidharan-Chari V, Clancy JW, Sedgwick A, D'Souza-Schorey C. Microvesicles: mediators of extracellular communication during cancer progression. J Cell Sci. (2010) 123:1603–11. doi: 10.1242/jcs.064386
40. Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. (2013) 200:373–83. doi: 10.1083/jcb.201211138
41. Obeng E. Brazilian Journal of Biology Apoptosis (programmed cell death) and its signals-A review. Braz J Biol. (2021) 81:1133–43. doi: 10.1590/1519-6984.228437
42. Ferraz M de AMM, Carothers A, Dahal R, Noonan MJ, Songsasen N. Oviductal extracellular vesicles interact with the spermatozoon's head and mid-piece and improves its motility and fertilizing ability in the domestic cat. Sci Rep. (2019) 9:9484. doi: 10.1038/s41598-019-45857-x
43. Silva TA, Smuczek B, Valadão IC, Dzik LM, Iglesia RP, Cruz MC, et al. AHNAK enables mammary carcinoma cells to produce extracellular vesicles that increase neighboring fibroblast cell motility. Oncotarget. (2016) 7:49998–50016. doi: 10.18632/oncotarget.10307
44. Alcântara-Neto AS, Schmaltz L, Caldas E, Blache MC, Mermillod P, Almiñana C. Porcine oviductal extracellular vesicles interact with gametes and regulate sperm motility and survival. Theriogenology. (2020) 155:240–55. doi: 10.1016/j.theriogenology.2020.05.043
45. Meningher T, Barsheshet Y, Ofir-Birin Y, Gold D, Brant B, Dekel E, et al. Schistosomal extracellular vesicle-enclosed miRNAs modulate host T helper cell differentiation. EMBO Rep. (2020) 21:e47882. doi: 10.15252/embr.201947882
46. Oh K, Kim SR, Kim DK, Seo MW, Lee C, Lee HM, et al. In vivo differentiation of therapeutic insulin-producing cells from bone marrow cells via extracellular vesicle-mimetic nanovesicles. ACS Nano. (2015) 9:11718–27. doi: 10.1021/acsnano.5b02997
47. Stronati E, Conti R, Cacci E, Cardarelli S, Biagioni S, Poiana G. Extracellular vesicle-induced differentiation of neural stem progenitor cells. Int J Mol Sci. (2019) 20:3691. doi: 10.3390/ijms20153691
48. Ozawa PMM, Alkhilaiwi F, Cavalli IJ, Malheiros D, de Souza Fonseca Ribeiro EM, Cavalli LR. Extracellular vesicles from triple-negative breast cancer cells promote proliferation and drug resistance in non-tumorigenic breast cells. Breast Cancer Res Treat. (2018) 172:713–23. doi: 10.1007/s10549-018-4925-5
49. Paone S, Baxter AA, Hulett MD, Poon IKH. Endothelial cell apoptosis and the role of endothelial cell-derived extracellular vesicles in the progression of atherosclerosis. Cell Mol Life Sci. (2019) 76:1093–106. doi: 10.1007/s00018-018-2983-9
50. Parayath NN, Padmakumar S, Amiji MM. Extracellular vesicle-mediated nucleic acid transfer and reprogramming in the tumor microenvironment. Cancer Lett. (2020) 482:33–43. doi: 10.1016/j.canlet.2020.04.009
51. Brena D, Huang MB, Bond V. Extracellular vesicle-mediated transport: reprogramming a tumor microenvironment conducive with breast cancer progression and metastasis. Transl Oncol. (2022) 15:101286. doi: 10.1016/j.tranon.2021.101286
52. Zhang DX, Vu LT, Ismail NN, Le MTN, Grimson A. Landscape of extracellular vesicles in the tumour microenvironment: interactions with stromal cells and with non-cell components, and impacts on metabolic reprogramming, horizontal transfer of neoplastic traits, and the emergence of therapeutic resistance. Semin Cancer Biol. (2021) 74:24–44. doi: 10.1016/j.semcancer.2021.01.007
53. Jiang J, Mei J, Ma Y, Jiang S, Zhang J, Yi S, et al. Tumor hijacks macrophages and microbiota through extracellular vesicles. Exploration. (2022) 2:20210144. doi: 10.1002/EXP.20210144
54. Rayyan M, Zheutlin A, Byrd JB. Clinical research using extracellular vesicles: insights from the International Society for Extracellular Vesicles 2018 Annual Meeting. J Extracell Vesicles. (2018) 7:1535744. doi: 10.1080/20013078.2018.1535744
55. Magaraggia I, Krauskopf J, Ramaekers JG, You Y, de Nijs L, Briedé JJ, et al. Harnessing brain-derived extracellular vesicles to support RDoC-based drug development. Neurosci Appl. (2025) 4:105406. doi: 10.1016/j.nsa.2024.105406
56. Petroni D, Fabbri C, Babboni S, Menichetti L, Basta G, Del Turco S. Extracellular vesicles and intercellular communication: challenges for in vivo molecular imaging and tracking. Pharmaceutics. (2023) 15:1639. doi: 10.3390/pharmaceutics15061639
57. Giunti D, Marini C, Parodi B, Usai C, Milanese M, Bonanno G, et al. Role of miRNAs shuttled by mesenchymal stem cell-derived small extracellular vesicles in modulating neuroinflammation. Sci Rep. (2021) 11:1740. doi: 10.1038/s41598-021-81039-4
58. Fernández-Messina L, Gutiérrez-Vázquez C, Rivas-García E, Sánchez-Madrid F, de la Fuente H. Immunomodulatory role of microRNAs transferred by extracellular vesicles. Biol Cell. (2015) 107:61–77. doi: 10.1111/boc.201400081
59. Stavrou A, Ortiz A. Extracellular vesicles: a novel tool in nanomedicine and cancer treatment. Cancers. (2022) 14:4450. doi: 10.3390/cancers14184450
60. Kumar MA, Baba SK, Sadida HQ, Marzooqi S Al, Jerobin J, Altemani FH, et al. Extracellular vesicles as tools and targets in therapy for diseases. Signal Transduct Target Ther. (2024) 9:27. doi: 10.1038/s41392-024-01735-1
61. Saadeldin IM, Gad A, Mermillod P. Editorial: biofluid extracellular vesicles and their involvement in animal reproductive physiology. Front Vet Sci. (2021) 8:747138. doi: 10.3389/fvets.2021.747138
62. Shekari F, Alibhai FJ, Baharvand H, Börger V, Bruno S, Davies O, et al. Cell culture-derived extracellular vesicles: considerations for reporting cell culturing parameters. J Extracell Biol. (2023) 2:e115. doi: 10.1002/jex2.115
63. Momen-Heravi F, Getting SJ, Moschos SA. Extracellular vesicles and their nucleic acids for biomarker discovery. Pharmacol Ther. (2018) 192:170–87. doi: 10.1016/j.pharmthera.2018.08.002
64. Oshchepkova A, Zenkova M, Vlassov V. Extracellular vesicles for therapeutic nucleic acid delivery: loading strategies and challenges. Int J Mol Sci. (2023) 24:7287. doi: 10.3390/ijms24087287
65. Voros C, Athanasiou D, Mavrogianni D, Varthaliti A, Bananis K, Athanasiou A, et al. Exosomal communication between cumulus–oocyte complexes and granulosa cells: a new molecular axis for oocyte competence in human-assisted reproduction. Int J Mol Sci. (2025) 26:5363. doi: 10.3390/ijms26115363
66. Pal A, Karanwal S, Habib MA, Josan F, Gaur V, Patel A, et al. Extracellular vesicles in seminal plasma of Sahiwal cattle bulls carry a differential abundance of sperm fertility-associated proteins for augmenting the functional quality of low-fertile bull spermatozoa. Sci Rep. (2025) 15:3587. doi: 10.1038/s41598-025-87998-2
67. Giacomini E, Scotti GM, Vanni VS, Lazarevic D, Makieva S, Privitera L, et al. Global transcriptomic changes occur in uterine fluid-derived extracellular vesicles during the endometrial window for embryo implantation. Hum Reprod. (2021) 36:2249–74. doi: 10.1093/humrep/deab123
68. Tesfaye D, Menjivar N, Gebremedhn S. Current knowledge and the future potential of extracellular vesicles in mammalian reproduction. Reprod Fertil Dev. (2022) 34:174–89. doi: 10.1071/RD21277
69. Wen C, Seeger RC, Fabbri M, Wang L, Wayne AS, Jong AY. Biological roles and potential applications of immune cell-derived extracellular vesicles. J Extracell Vesicles. (2017) 6:1400370. doi: 10.1080/20013078.2017.1400370
70. Ogawa R, Tanaka C, Sato M, Nagasaki H, Sugimura K, Okumura K, et al. Adipocyte-derived microvesicles contain RNA that is transported into macrophages and might be secreted into blood circulation. Biochem Biophys Res Commun. (2010) 398:723–9. doi: 10.1016/j.bbrc.2010.07.008
71. Aguilera C, Wong YS, Gutierrez-Reinoso MA, Velásquez AE, Melo-Báez B, Cabezas J, et al. Embryo-maternal communication mediated by extracellular vesicles in the early stages of embryonic development is modified by in vitro conditions. Theriogenology. (2024) 214:43–56. doi: 10.1016/j.theriogenology.2023.10.005
72. Ovčar A, Kovačič B. Biogenesis of extracellular vesicles (EVs) and the potential use of embryo-derived evs in medically assisted reproduction. Int J Mol Sci. (2025) 26:42. doi: 10.3390/ijms26010042
73. Xue Y, Zheng H, Xiong Y, Li K. Extracellular vesicles affecting embryo development in vitro: a potential culture medium supplement. Front Pharmacol. (2024) 15:1366992. doi: 10.3389/fphar.2024.1366992
74. Fazeli A, Godakumara K. The evolving roles of extracellular vesicles in embryo-maternal communication. Commun Biol. (2024) 7:754. doi: 10.1038/s42003-024-06442-9
75. Mashayekhi F, Mostafa Y, Zivar S, Pournourali M. The association of −656T > G and 1349T > G polymorphisms of ApE1 gene and the risk of female infertility. J Obstet Gynaecol. (2016) 36:544–7. doi: 10.3109/01443615.2015.1127903
76. Saadeldin IM, Oh HJ, Lee BC. Embryonic–maternal cross-talk via exosomes: potential implications. Stem Cells Cloning. (2015) 8:103–7. doi: 10.2147/SCCAA.S84991
77. Saint-Dizier M, Schoen J, Chen S, Banliat C, Mermillod P. Composing the early embryonic microenvironment: physiology and regulation of oviductal secretions. Int J Mol Sci. (2020) 21:223. doi: 10.3390/ijms21010223
78. Fan Y, Pavani KC, Smits K, Van Soom A, Peelman L. tRNAGlu-derived fragments from embryonic extracellular vesicles modulate bovine embryo hatching. J Anim Sci Biotechnol. (2024) 15:23. doi: 10.1186/s40104-024-00997-7
79. Dissanayake K, Nõmm M, Lättekivi F, Ord J, Ressaissi Y, Godakumara K, et al. Oviduct as a sensor of embryo quality: deciphering the extracellular vesicle (EV)-mediated embryo-maternal dialogue. J Mol Med. (2021) 99:685–97. doi: 10.1007/s00109-021-02042-w
80. Saadeldin IM, Pavani KC, Gnagnarelli J, Ehab S, Assiri AM, Van Soom A. Unlocking a decade of research on embryo-derived extracellular vesicles: discoveries made and paths ahead. Stem Cell Rev Rep. (2025) 21:698–708. doi: 10.1007/s12015-025-10844-5
81. Mellisho EA, Briones MA, Velásquez AE, Cabezas J, Castro FO, Rodríguez-Álvarez L. Extracellular vesicles secreted during blastulation show viability of bovine embryos. Reproduction. (2019) 158:477–92. doi: 10.1530/REP-19-0233
82. Zhao Z, Sun Y, Guo R, Liang J, Dai W, Jiang Y, et al. Extracellular vesicles: roles in oocytes and emerging therapeutic opportunities. Chin Med J. (2025) 138:1050–60. doi: 10.1097/CM9.0000000000003578
83. Veraguas D, Aguilera C, Henriquez C, Velasquez AE, Melo-Baez B, Silva-Ibañez P, et al. Evaluation of extracellular vesicles and gDNA from culture medium as a possible indicator of developmental competence in human embryos. Zygote. (2021) 29:138–49. doi: 10.1017/S0967199420000593
84. Melo-Baez B, Wong YS, Aguilera CJ, Cabezas J, Mançanares ACF, Riadi G, et al. Micrornas from extracellular vesicles secreted by bovine embryos as early biomarkers of developmental competence. Int J Mol Sci. (2020) 21:1–17. doi: 10.3390/ijms21238888
85. Nagy B, Bognár Z, Csabai TJ, Fekete N, Buzás EI, Kovács ÁF, et al. Effects of light exposure during IVF: transcriptomic analysis of murine embryos and embryo-derived EVs. Front Immunol. (2025) 16:101. doi: 10.3389/fimmu.2025.1429252
86. Giacomini E, Vago R, Sanchez AM, Podini P, Zarovni N, Murdica V, et al. Secretome of in vitro cultured human embryos contains extracellular vesicles that are uptaken by the maternal side. Sci Rep. (2017) 7:5210. doi: 10.1038/s41598-017-05549-w
87. Tiwari S, Kumar V, Randhawa S, Verma SK. Preparation and characterization of extracellular vesicles. Am J Reprod Immunol. (2021) 85:e13367. doi: 10.1111/aji.13367
88. Tang P, Song F, Chen Y, Gao C, Ran X, Li Y, et al. Preparation and characterization of extracellular vesicles and their cutting-edge applications in regenerative medicine. Appl Mater Today. (2024) 37:102084. doi: 10.1016/j.apmt.2024.102084
89. Gabryś J, Kij-Mitka B, Sawicki S, Kochan J, Nowak A, Łojko J, et al. Extracellular vesicles from follicular fluid may improve the nuclear maturation rate of in vitro matured mare oocytes. Theriogenology. (2022) 188:116–24. doi: 10.1016/j.theriogenology.2022.05.022
90. Dragovic RA, Gardiner C, Brooks AS, Tannetta DS, Ferguson DJP, Hole P, et al. Sizing and phenotyping of cellular vesicles using nanoparticle tracking analysis. Nanomedicine. (2011) 7:780–8. doi: 10.1016/j.nano.2011.04.003
91. De Necochea-Campion R, Gonda A, Kabagwira J, Mirshahidi S, Cao H, Reeves ME, et al. A practical approach to extracellular vesicle characterization among similar biological samples. Biomed Phys Eng Express. (2018) 4:1429252. doi: 10.1088/2057-1976/aad6d8
92. Zivko C, Fuhrmann K, Fuhrmann G, Luciani P. Tracking matricellular protein SPARC in extracellular vesicles as a non-destructive method to evaluate lipid-based antifibrotic treatments. Commun Biol. (2022) 5:1155. doi: 10.1038/s42003-022-04123-z
93. Szatanek R, Baj-Krzyworzeka M, Zimoch J, Lekka M, Siedlar M, Baran J. The methods of choice for extracellular vesicles (EVs) characterization. Int J Mol Sci. (2017) 18:1153. doi: 10.3390/ijms18061153
94. Vestad B, Llorente A, Neurauter A, Phuyal S, Kierulf B, Kierulf P, et al. Size and concentration analyses of extracellular vesicles by nanoparticle tracking analysis: a variation study. J Extracell Vesicles. (2017) 6:1344087. doi: 10.1080/20013078.2017.1344087
95. Nolan J, Duggan E, Van Keuren-Jensen KJ, Huentelman M, Reiman R, Arango J, et al. Vesicle flow cytometry of extracellular vesicles in cerebral spinal fluid. J Extracell Vesicles. (2015) 4:29225.
96. Comfort N, Bloomquist TR, Shephard AP, Petty CR, Cunningham A, Hauptman M, et al. Isolation and characterization of extracellular vesicles in saliva of children with asthma. Extracell Vesicles Circ Nucl Acids. (2021) 2:29–48. doi: 10.20517/evcna.2020.09
97. Jia Z, Li J, Gao L, Yang D, Kanaev A. Dynamic light scattering: a powerful tool for in situ nanoparticle sizing. Colloid Interface. (2023) 7:15. doi: 10.3390/colloids7010015
98. Barranco I, Catalán J, Parra A, Martínez-Díaz P, Yeste M, Roca J, et al. Phenotypic characteristics of seminal extracellular vesicles are related to sperm cryotolerance in stallions. J Equine Vet Sci. (2025) 145:105265. doi: 10.1016/j.jevs.2024.105265
99. Khan MA, Anand S, Deshmukh SK, Singh S, Singh AP. Determining the size distribution and integrity of extracellular vesicles by dynamic light scattering. Methods Mol Biol. (2022) 2413:165–75. doi: 10.1007/978-1-0716-1896-7_17
100. Kruse T, Schneider S, Reger LN, Kampmann M, Reif OW. A novel approach for enumeration of extracellular vesicles from crude and purified cell culture samples. Eng Life Sci. (2022) 22:334–43. doi: 10.1002/elsc.202100149
101. Wu S, Zhao Y, Zhang Z, Zuo C, Wu H, Liu Y. The advances and applications of characterization technique for exosomes: from dynamic light scattering to super-resolution imaging technology. Photonics. (2024) 11:101–32. doi: 10.3390/photonics11020101
102. Bryant G, Thomas JC. Improved particle size distribution measurements using multiangle dynamic light scattering. Langmuir. (1995) 11:2480–5. doi: 10.1021/la00007a028
103. Kestens V, Roebben G, Herrmann J, Jämting Å, Coleman V, Minelli C, et al. Challenges in the size analysis of a silica nanoparticle mixture as candidate certified reference material. J Nanopart Res. (2016) 18:171. doi: 10.1007/s11051-016-3474-2
104. Lawrie AS, Albanyan A, Cardigan RA, MacKie IJ, Harrison P. Microparticle sizing by dynamic light scattering in fresh-frozen plasma. Vox Sang. (2009) 96:206–12. doi: 10.1111/j.1423-0410.2008.01151.x
105. Pucci C, Martinelli C, Ciofani G. Innovative approaches for cancer treatment: Current perspectives and new challenges. Ecancermedicalscience. (2019) 13:961. doi: 10.3332/ecancer.2019.961
106. Shao H, Im H, Castro CM, Breakefield X, Weissleder R, Lee H. New technologies for analysis of extracellular vesicles. Chem Rev. (2018) 118:1917–50. doi: 10.1021/acs.chemrev.7b00534
107. Imanbekova M, Suarasan S, Lu Y, Jurchuk S, Wachsmann-Hogiu S. Recent advances in optical label-free characterization of extracellular vesicles. Nanophotonics. (2022) 11:2827–63. doi: 10.1515/nanoph-2022-0057
108. Normak K, Papp M, Ullmann M, Paganini C, Manno M, Bongiovanni A, et al. Multiparametric orthogonal characterization of extracellular vesicles by liquid chromatography combined with in-line light scattering and fluorescence detection. Anal Chem. (2023) 95:12443–51. doi: 10.26434/chemrxiv-2023-mq843
109. Jeong MH, Son T, Tae YK, Park CH, Lee HS, Chung MJ, et al. Plasmon-enhanced single extracellular vesicle analysis for cholangiocarcinoma diagnosis. Adv Sci. (2023) 10:e2205148. doi: 10.1002/advs.202205148
110. Qiu L, Liu X, Zhu L, Luo L, Sun N, Pei R. Current advances in technologies for single extracellular vesicle analysis and its clinical applications in cancer diagnosis. Biosensors. (2023) 13:129. doi: 10.3390/bios13010129
111. Buschmann D, Mussack V, Byrd JB. Separation, characterization, and standardization of extracellular vesicles for drug delivery applications. Adv Drug Deliv Rev. (2021) 174:348–68. doi: 10.1016/j.addr.2021.04.027
112. Anderson W, Lane R, Korbie D, Trau M. Observations of tunable resistive pulse sensing for exosome analysis: improving system sensitivity and stability. Langmuir. (2015) 31:6577–87. doi: 10.1021/acs.langmuir.5b01402
113. Benedetti C, Pavani KC, Gansemans Y, Azari-Dolatabad N, Pascottini OB, Peelman L, et al. From follicle to blastocyst: microRNA-34c from follicular fluid-derived extracellular vesicles modulates blastocyst quality. J Anim Sci Biotechnol. (2024) 15:104. doi: 10.1186/s40104-024-01059-8
114. Weatherall E, Willmott GR. Applications of tunable resistive pulse sensing. Analyst. (2015) 140:3541–5. doi: 10.1039/C4AN02270J
115. Eldridge J, Colby AH, Willmott GR, Yu. S, Grinstaff MW. Use of tunable pores for accurate characterization of micro- and nanoparticle systems in nanomedicine. Regen Med Artif Cells Nanomed. (2013) 219–55. doi: 10.1142/9789814472869_0010
116. Pei Y, Vogel R, Minelli C. Tunable resistive pulse sensing (TRPS). In:Hodoroaba V-D, Unger W, Shard A, , editors. Characterization of Nanoparticles: Measurement Processes for Nanoparticles. Amsterdam: Elsevier (2019) 117–36. doi: 10.1016/B978-0-12-814182-3.00009-2
117. Sivakumaran M, Platt M. Tunable resistive pulse sensing: potential applications in nanomedicine. Nanomedicine. (2016) 11:2197–214. doi: 10.2217/nnm-2016-0097
118. Farahani MS, Hosseini-Beheshti E, Moazzeni SM, Moghadam MF. Engineered extracellular vesicles expressing ICAM-1: a promising targeted delivery system for T cell modifications. Biochim Biophys Acta Gen Subj. (2024) 1868:130541. doi: 10.1016/j.bbagen.2023.130541
119. Tertel T, Görgens A, Giebel B. Analysis of individual extracellular vesicles by imaging flow cytometry. Methods Enzymol. (2020) 645:55–78. doi: 10.1016/bs.mie.2020.05.013
120. Nolan JP, Duggan E. Analysis of individual extracellular vesicles by flow cytometry. Methods Mol Biol. (2018) 1678:79–92. doi: 10.1007/978-1-4939-7346-0_5
121. Morales-Kastresana A, Jones JC. Flow cytometric analysis of extracellular vesicles. In:Hill AF, , editor. Exosomes and Microvesicles: Methods and Protocols. New York, NY: Springer New York (2017). p. 215–25. doi: 10.1007/978-1-4939-6728-5_16
122. Maia J, Batista S, Couto N, Gregório AC, Bodo C, Elzanowska J, et al. Employing flow cytometry to extracellular vesicles sample microvolume analysis and quality control. Front Cell Dev Biol. (2020) 8:593750. doi: 10.3389/fcell.2020.593750
123. Manohar SM, Shah P, Nair A. Flow cytometry: principles, applications and recent advances. Bioanalysis. (2021) 13:181–98. doi: 10.4155/bio-2020-0267
124. Yu Y, Zheng Y, Guan C, Yi M, Chen Y, Zeng Y, et al. Detection of cells by flow cytometry: Counting, imaging, and cell classification. J Innov Opt Health Sci. (2023) 16:23300057. doi: 10.1142/S1793545823300057
125. Kobayashi H, Shiba T, Yoshida T, Bolidong D, Kato K, Sato Y, et al. Precise analysis of single small extracellular vesicles using flow cytometry. Sci Rep. (2024) 14:7465. doi: 10.1038/s41598-024-57974-3
126. Dlugolecka M, Czystowska-Kuzmicz M. Factors to consider before choosing EV labeling method for fluorescence-based techniques. Front Bioeng Biotechnol. (2024) 12:1479516. doi: 10.3389/fbioe.2024.1479516
127. Tertel T, Giebel B. Amnis imagestream - analysis of individual extracellular vesicles by imaging flow cytometry. Cytotherapy. (2020) 22:S55. doi: 10.1016/j.jcyt.2020.03.075
128. Zhao Z, Wijerathne H, Godwin AK, Soper SA. Isolation and analysis methods of extracellular vesicles (EVs). Extracell Vesicles Circ Nucl Acids. (2021) 2:80–103. doi: 10.20517/evcna.2021.07
129. Brennan K, Martin K, FitzGerald SP, O'Sullivan J, Wu Y, Blanco A, et al. A comparison of methods for the isolation and separation of extracellular vesicles from protein and lipid particles in human serum. Sci Rep. (2020) 10:1039. doi: 10.1038/s41598-020-57497-7
130. Thane KE, Davis AM, Hoffman AM. Improved methods for fluorescent labeling and detection of single extracellular vesicles using nanoparticle tracking analysis. Sci Rep. (2019) 9:12295. doi: 10.1038/s41598-019-48181-6
131. Corona ML, Hurbain I, Raposo G, van Niel G. Characterization of extracellular vesicles by transmission electron microscopy and immunolabeling electron microscopy. Methods Mol Biol. (2023) 2668:33–43. doi: 10.1007/978-1-0716-3203-1_4
132. Kaur S, Nathani A, Singh M. Exosomal delivery of cannabinoids against cancer. Cancer Lett. (2023) 566:216243. doi: 10.1016/j.canlet.2023.216243
133. Song H, Chen X, Hao Y, Wang J, Xie Q, Wang X. Nanoengineering facilitating the target mission: targeted extracellular vesicles delivery systems design. J Nanobiotechnology. (2022) 20:431. doi: 10.1186/s12951-022-01638-9
134. Brisson AR. Comment on “Quality of extracellular vesicle images by transmission electron microscopy is operator and protocol dependent.” J Extracell Vesicles. (2019) 8:1555419. doi: 10.1080/20013078.2019.1648996
135. Rikkert LG, Nieuwland R, Terstappen LWMM, Coumans FAW. Quality of extracellular vesicle images by transmission electron microscopy is operator and protocol dependent. J Extracell Vesicles. (2019) 8:1555419. doi: 10.1080/20013078.2018.1555419
136. Cizmar P, Yuana Y. Detection and characterization of extracellular vesicles by transmission and Cryo-transmission electron microscopy. Methods Mol Biol. (2017) 1660:221–32. doi: 10.1007/978-1-4939-7253-1_18
137. Hayles MF, de Winter DAM. An introduction to cryo-FIB-SEM cross-sectioning of frozen, hydrated Life Science samples. J Microsc. (2021) 281:138–56. doi: 10.1111/jmi.12951
138. Hallal S, Tuzesi Á, Grau GE, Buckland ME, Alexander KL. Understanding the extracellular vesicle surface for clinical molecular biology. J Extracell Vesicles. (2022) 11:e12260. doi: 10.1002/jev2.12260
139. Tang VA, Renner TM, Fritzsche AK, Burger D, Langlois MA. Single-particle discrimination of retroviruses from extracellular vesicles by nanoscale flow cytometry. Sci Rep. (2017) 7:17769. doi: 10.1038/s41598-017-18227-8
140. Morales-Kastresana A, Telford B, Musich TA, McKinnon K, Clayborne C, Braig Z, et al. Labeling extracellular vesicles for nanoscale flow cytometry. Sci Rep. (2017) 7:1878. doi: 10.1038/s41598-017-01731-2
141. Roberts-Dalton HD, Cocks A, Falcon-Perez JM, Sayers EJ, Webber JP, Watson P, et al. Fluorescence labelling of extracellular vesicles using a novel thiol-based strategy for quantitative analysis of cellular delivery and intracellular traffic. Nanoscale. (2017) 9:13693–706. doi: 10.1039/C7NR04128D
142. Kamińska K, Godakumara K, Swiderska B, Malinowska A, Midekessa G, Sofińska K, et al. Characteristics of size-exclusion chromatography enriched porcine follicular fluid extracellular vesicles. Theriogenology. (2023) 205:79–86. doi: 10.1016/j.theriogenology.2023.04.010
143. Puthukodan S, Hofmann M, Mairhofer M, Janout H, Schurr J, Hauser F, et al. Purification analysis, intracellular tracking, and colocalization of extracellular vesicles using atomic force and 3D single-molecule localization microscopy. Anal Chem. (2023) 95:6061–70. doi: 10.1021/acs.analchem.3c00144
144. Parisse P, Rago I, Ulloa Severino L, Perissinotto F, Ambrosetti E, Paoletti P, et al. Atomic force microscopy analysis of extracellular vesicles. Eur Biophys J. (2017) 46:813–20. doi: 10.1007/s00249-017-1252-4
145. Ghosh A, Davey M, Chute IC, Griffiths SG, Lewis S, Chacko S, et al. Rapid isolation of extracellular vesicles from cell culture and biological fluids using a synthetic peptide with specific affinity for heat shock proteins. PLoS ONE. (2014) 9:e0110443. doi: 10.1371/journal.pone.0110443
146. Wang X, Zahl P, Wang H, Altman EI, Schwarz UD. How precisely can individual molecules be analyzed? A case study on locally quantifying forces and energies using scanning probe microscopy. ACS Nano. (2024) 18:4495–506. doi: 10.1021/acsnano.3c11219
147. Arthur P, Kandoi S, Sun L, Kalvala A, Kutlehria S, Bhattacharya S, et al. Biophysical, molecular and proteomic profiling of human retinal organoid-derived exosomes. Pharm Res. (2023) 40:801–16. doi: 10.1101/2022.04.25.489461
148. Priglinger E, Strasser J, Buchroithner B, Weber F, Wolbank S, Auer D, et al. Label-free characterization of an extracellular vesicle-based therapeutic. J Extracell Vesicles. (2021) 10:e12156. doi: 10.1002/jev2.12156
149. Yurtsever A, Yoshida T, Badami Behjat A, Araki Y, Hanayama R, Fukuma T. Structural and mechanical characteristics of exosomes from osteosarcoma cells explored by 3D-atomic force microscopy. Nanoscale. (2021) 13:6661–77. doi: 10.1039/D0NR09178B
150. Skliar M, Chernyshev VS. Imaging of extracellular vesicles by atomic force microscopy. J Visual Exp. (2019) 151:59254. doi: 10.3791/59254
151. Życieńska K, Pszczółkowska B, Brzozowska B, Kamiński M, Lorenc T, Olejarz W, et al. Brownian motion influence on AFM exosomes' size measurements. Int J Mol Sci. (2022) 23:10074. doi: 10.3390/ijms231710074
152. Bagci C, Sever-Bahcekapili M, Belder N, Bennett APS, Erdener SE, Dalkara T. Overview of extracellular vesicle characterization techniques and introduction to combined reflectance and fluorescence confocal microscopy to distinguish extracellular vesicle subpopulations. Neurophotonics. (2022) 9:021903. doi: 10.1117/1.NPh.9.2.021903
153. Simpson RJ, Jensen SS, Lim JWE. Proteomic profiling of exosomes: current perspectives. Proteomics. (2008) 8:4083–99. doi: 10.1002/pmic.200800109
154. De Ávila ACFCM, Da Silveira JC. Role of extracellular vesicles during oocyte maturation and early embryo development. Reprod Fertil Dev. (2019) 32:56–64. doi: 10.1071/RD19389
155. Boere J, van de Lest CHA, de Grauw JC, Plomp SGM, Libregts SFWM, Arkesteijn GJA, et al. Extracellular vesicles in synovial fluid from juvenile horses: no age-related changes in the quantitative profile. Vet J. (2019) 244:91–3. doi: 10.1016/j.tvjl.2018.12.010
156. Tang MKS, Wong AST. Exosomes: emerging biomarkers and targets for ovarian cancer. Cancer Lett. (2015) 367:26–33. doi: 10.1016/j.canlet.2015.07.014
157. Nakamura K, Sawada K, Kobayashi M, Miyamoto M, Shimizu A, Yamamoto M, et al. Role of the exosome in ovarian cancer progression and its potential as a therapeutic target. Cancers. (2019) 11:1147. doi: 10.3390/cancers11081147
158. Zhao Y, Vanderkooi S, Kan FWK. The role of oviduct-specific glycoprotein (OVGP1) in modulating biological functions of gametes and embryos. Histochem Cell Biol. (2022) 157:371–88. doi: 10.1007/s00418-021-02065-x
159. Pal A, Karanwal S, Chera JS, Batra V, Kumaresan A, Sarwalia P, et al. Circulatory extracellular vesicle derived miR-195-5p promotes cellular apoptosis and suppresses cell proliferation in the buffalo endometrial primary cell culture. Sci Rep. (2023) 13:16703. doi: 10.1038/s41598-023-43530-y
160. Evans J, Rai A, Nguyen HPT, Poh QH, Elglass K, Simpson RJ, et al. Human endometrial extracellular vesicles functionally prepare human trophectoderm model for implantation: understanding bidirectional maternal-embryo communication. Proteomics. (2019) 19:e1800423. doi: 10.1002/pmic.201800423
161. Kurian NK, Modi D. Extracellular vesicle mediated embryo-endometrial cross talk during implantation and in pregnancy. J Assist Reprod Genet. (2019) 36:189–98. doi: 10.1007/s10815-018-1343-x
162. Witek RP, Yang L, Liu R, Jung Y, Omenetti A, Syn WK, et al. Liver cell-derived microparticles activate hedgehog signaling and alter gene expression in hepatic endothelial cells. Gastroenterology. (2009) 136:320–30. doi: 10.1053/j.gastro.2008.09.066
163. Porro C, Lepore S, Trotta T, Castellani S, Ratclif L, Battaglino A, et al. Isolation and characterization of microparticles in sputum from cystic fibrosis patients. Respir Res. (2010) 11:94. doi: 10.1186/1465-9921-11-94
164. Muñoz EL, Fuentes FB, Felmer RN, Yeste M, Arias ME. Extracellular vesicles in mammalian reproduction: a review. Zygote. (2022) 30:440–63. doi: 10.1017/S0967199422000090
165. Asea A, Jean-Pierre C, Kaur P, Rao P, Linhares IM, Skupski D, et al. Heat shock protein-containing exosomes in mid-trimester amniotic fluids. J Reprod Immunol. (2008) 79:12–7. doi: 10.1016/j.jri.2008.06.001
166. Grigor'eva AE, Tamkovich SN, Eremina AV, Tupikin AE, Kabilov MR, Chernykh VV, et al. Characteristics of exosomes andmicroparticles discovered in human tears. Biomed Khim. (2016) 62:99–106. doi: 10.18097/PBMC20166201099
167. Ronquist G, Hedström M. Restoration of detergent-inactivated adenosine triphosphatase activity of human prostatic fluid with concanavalin A. Biochim Biophys Acta. (1977) 483:483–6. doi: 10.1016/0005-2744(77)90078-X
168. Yanagimachi R, Kamiguchi Y, Mikamo K, Suzuki F, Yanagimachi H. Maturation of spermatozoa in the epididymis of the Chinese hamster. Am J Anat. (1985) 172:317–30. doi: 10.1002/aja.1001720406
169. Manin M, Lecher P, Martinez A, Tournadre S, Jean C. Exportation of mouse vas deferens protein, a protein without a signal peptide, from mouse vas deferens epithelium: a model of apocrine secretion. Biol Reprod. (1995) 52:50–62. doi: 10.1095/biolreprod52.1.50
170. Sahlén G, Nilsson O, Larsson A, Carlsson L, Norlén BJ, Ronquist G. Secretions from seminal vesicles lack characteristic markers for prostasomes. Ups J Med Sci. (2010) 115:107–12. doi: 10.3109/03009730903366067
171. Tamessar CT, Trigg NA, Nixon B, Skerrett-Byrne DA, Sharkey DJ, Robertson SA, et al. Roles of male reproductive tract extracellular vesicles in reproduction. Am J Reprod Immunol. (2021) 85:e13338. doi: 10.1111/aji.13338
172. Stewart J, Hoshino A, Rosenwaks Z, Palermo G. Understanding the role of seminal fluid exosomes within the male reproductive tract. Fertil Steril. (2019) 111:7–8. doi: 10.1016/j.fertnstert.2019.02.044
173. Höög JL, Lötvall J. Diversity of extracellular vesicles in human ejaculates revealed by cryo-electron microscopy. J Extracell Vesicles. (2015) 4:28680. doi: 10.3402/jev.v4.28680
174. Ronquist KG, Ek B, Morrell J, Stavreus-Evers A, Ström Holst B, Humblot P, et al. Prostasomes from four different species are able to produce extracellular adenosine triphosphate (ATP). Biochim Biophys Acta Gen Subj. (2013) 1830:4604–10. doi: 10.1016/j.bbagen.2013.05.019
175. Gabrielsen JS, Lipshultz LI. Rapid progression in our understanding of extracellular vesicles and male infertility. Fertil Steril. (2019) 111:881–2. doi: 10.1016/j.fertnstert.2019.02.021
176. Choy KHK, Chan SY, Lam W, Jin J, Zheng T, Yu SS, et al. The repertoire of testicular extracellular vesicles cargoes and their involvement in inter-compartmental communication required for spermatogenesis. BioRxiv [Preprint]. (2021) 2021. doi: 10.1101/2021.01.08.426002
177. Jankovičová J, Michalková K, Sečová P, Horovská L, Antalíková J. The extracellular vesicle tetraspanin CD63 journey from the testis through the epididymis to mature bull sperm. Sci Rep. (2024) 14:29449. doi: 10.1038/s41598-024-81021-w
178. Zhang Y, Zhao J, Jin Q, Zhuang L. Transcriptomic analyses and experimental validation identified immune-related lncRNA–mRNA pair MIR210HG–BPIFC regulating the progression of hypertrophic cardiomyopathy. Int J Mol Sci. (2024) 25:2816. doi: 10.3390/ijms25052816
179. de ávila ACFCM, Andrade GM, Bridi A, Gimenes LU, Meirelles FV, Perecin F, et al. Extracellular vesicles and its advances in female reproduction. Anim Reprod. (2018) 16:31–8. doi: 10.21451/1984-3143-AR2018-0101
180. Lipinska P, Smits K, Van Soom A, Pavani KC, Warzych E. Follicular-fluid extracellular vesicles support energy metabolism of bovine oocytes, improving blastocyst development and quality†. Biol Reprod. (2025) 113:109–26. doi: 10.1093/biolre/ioaf096
181. Azari-Dolatabad N, Raes A, Pavani KC, Asaadi A, Angel-Velez D, Van Damme P, et al. Follicular fluid during individual oocyte maturation enhances cumulus expansion and improves embryo development and quality in a dose-specific manner. Theriogenology. (2021) 166:38–45. doi: 10.1016/j.theriogenology.2021.02.016
182. da Silveira JC, Veeramachaneni DNR, Winger QA, Carnevale EM, Bouma GJ. Cell-secreted vesicles in equine ovarian follicular fluid contain mirnas and proteins: a possible new form of cell communication within the ovarian follicle. Biol Reprod. (2012) 86:71. doi: 10.1095/biolreprod.111.093252
183. Al-Dossary AA, Strehler EE, Martin-DeLeon PA. Expression and secretion of plasma membrane Ca2+-ATPase 4a (PMCA4a) during murine estrus: association with oviductal exosomes and uptake in sperm. PLoS ONE. (2013) 8:e80181. doi: 10.1371/journal.pone.0080181
184. Azari-Dolatabad N, Eshghi Chaharborj D, Benedetti C, Fernandez Montoro A, Leroy J, Van Soom A, et al. 212 Supplementation of follicular fluid-extracellular vesicles during bovine oocyte maturation and its effect on embryo development in a serum-free group and individual culture system. Reprod Fertil Dev. (2023) 35:235. doi: 10.1071/RDv35n2Ab212
185. Makieva S, Saenz-de-Juano MD, Almiñana C, Bauersachs S, Bernal-Ulloa S, Xie M, et al. Treatment of human oocytes with extracellular vesicles from follicular fluid during rescue in vitro maturation enhances maturation rates and modulates oocyte proteome and ultrastructure. bioRxiv [preprint]. (2025). doi: 10.1101/2025.02.05.636623
186. Ruiz-González I, Xu J, Wang X, Burghardt RC, Dunlap KA, Bazer FW. Exosomes, endogenous retroviruses and toll-like receptors: pregnancy recognition in ewes. Reproduction. (2015) 149:281–91. doi: 10.1530/REP-14-0538
187. Mazzarella R, Cañón-Beltrán K, Cajas YN, Hamdi M, González EM, da Silveira JC, et al. Extracellular vesicles-coupled miRNAs from oviduct and uterus modulate signaling pathways related to lipid metabolism and bovine early embryo development. J Anim Sci Biotechnol. (2024) 15:51. doi: 10.1186/s40104-024-01008-5
188. Tannetta D, Dragovic R, Alyahyaei Z, Southcombe J. Extracellular vesicles and reproduction-promotion of successful pregnancy. Cell Mol Immunol. (2014) 11:548–63. doi: 10.1038/cmi.2014.42
189. Adam S, Elfeky O, Kinhal V, Dutta S, Lai A, Jayabalan N, et al. Review: fetal-maternal communication via extracellular vesicles – Implications for complications of pregnancies. Placenta. (2017) 54:83–8. doi: 10.1016/j.placenta.2016.12.001
190. Salmasi S, Heidar MS, Khaksary Mahabady M, Rashidi B, Mirzaei H. MicroRNAs, endometrial receptivity and molecular pathways. Reprod Biol Endocrinol. (2024) 22:139. doi: 10.1186/s12958-024-01304-9
191. Li J, Salvador AM Li G, Valkov N, Ziegler O, Yeri A, et al. Mir-30d regulates cardiac remodeling by intracellular and paracrine signaling. Circ Res. (2021) 128:e1–23. doi: 10.1161/CIRCRESAHA.120.317244
192. Juárez-Barber E, Segura-Benítez M, Carbajo-García MC, Bas-Rivas A, Faus A, Vidal C, et al. Extracellular vesicles secreted by adenomyosis endometrial organoids contain miRNAs involved in embryo implantation and pregnancy. Reprod Biomed Online. (2023) 46:470–81. doi: 10.1016/j.rbmo.2022.12.008
193. Suleiman AA, Al-Chalabi R, Shaban SA. Integrative role of small non-coding RNAs in viral immune response: a systematic review. Mol Biol Rep. (2024) 51:107. doi: 10.1007/s11033-023-09141-6
194. Nabeel MA, Nowak RA. Extracellular Vesicles in Implantation: Cross-Talk between the Embryo and Endometrium. Cham: Springer (2025). doi: 10.1007/102_2024_8
195. Rodriguez-Martinez H, Martinez EA, Calvete JJ, Peña Vega FJ, Roca J. Seminal plasma: relevant for fertility? Int J Mol Sci. (2021) 22:4368. doi: 10.3390/ijms22094368
196. Gurunathan S, Kang MH, Song H, Kim NH, Kim JH. The role of extracellular vesicles in animal reproduction and diseases. J Anim Sci Biotechnol. (2022) 13:62. doi: 10.1186/s40104-022-00715-1
197. Machtinger R, Laurent LC, Baccarelli AA. Extracellular vesicles: roles in gamete maturation, fertilization and embryo implantation. Hum Reprod Update. (2016) 22:182–93. doi: 10.1093/humupd/dmv055
198. Casao A, Peña-Delgado V, Pérez-Pe R. From spermatogenesis to fertilisation: the role of melatonin on ram spermatozoa. Domest Anim Endocrinol. (2025) 91:106916. doi: 10.1016/j.domaniend.2025.106916
199. Parra A, Padilla L, Lucas X, Rodriguez-Martinez H, Barranco I, Roca J. Seminal extracellular vesicles and their involvement in male (In)fertility: a systematic review. Int J Mol Sci. (2023) 24:4818. doi: 10.3390/ijms24054818
200. Esmaeili A, Esmaeili V, Shahverdi A, Eslaminejad MB. Engineered extracellular vesicles: a breakthrough approach to overcoming sperm cryopreservation challenges. Reprod Biol Endocrinol. (2025) 23:75. doi: 10.1186/s12958-025-01407-x
201. Huang J, Li S, Yang Y, Li C, Zuo Z, Zheng R, et al. GPX5-enriched exosomes improve sperm quality and fertilization ability. Int J Mol Sci. (2024) 25:10569. doi: 10.3390/ijms251910569
202. Naveed M, Shen Z, Bao J. Sperm-borne small non-coding RNAs: potential functions and mechanisms as epigenetic carriers. Cell Biosci. (2025) 15:5. doi: 10.1186/s13578-025-01347-4
203. Sabry R, Williams M, Werry N, LaMarre J, Favetta LA. BPA Decreases PDCD4 in bovine granulosa cells independently of miR-21 inhibition. Int J Mol Sci. (2022) 23:8276. doi: 10.3390/ijms23158276
204. Barfar M, Saffarian Z, Edalatkhah H, Peighambarzadeh SZ, Heidarnejad A, Azedi F, et al. Melatonin-induced alterations in Bax gene expression in non-obstructive azoospermic mice. Front Biomed Technol. (2025) 12:1.
205. Asadi A, Ghahremani R, Abdolmaleki A, Rajaei F. Role of sperm apoptosis and oxidative stress in male infertility: a narrative review. Int J Reprod Biomed. (2021) 19:493. doi: 10.18502/ijrm.v19i6.9371
206. Peres Campanholi S, Garcia Neto S, Basso AC, de Agostini Losano JD, Perez Siqueira AF, Nichi M, et al. Estimate of in vitro embryo production based on sperm subpopulations in Senepol bulls. Theriogenology. (2021) 161:98–107. doi: 10.1016/j.theriogenology.2020.11.019
207. Dlamini NH, Bridi A, da Silveira JC, Feugang JM. Unlocking gamete quality through extracellular vesicles: emerging perspectives. Biology. (2025) 14:198. doi: 10.3390/biology14020198
208. Wang J, Wang D, Zhang Y, Sun P, Yi L, Han A, et al. Extracellular vesicles in reproductive biology and disorders: a comprehensive review. Front Endocrinol. (2025) 16:1550068. doi: 10.3389/fendo.2025.1550068
209. Fan W, Qi Y, Wang Y, Yan H, Li X, Zhang Y. Messenger roles of extracellular vesicles during fertilization of gametes, development and implantation: recent advances. Front Cell Dev Biol. (2023) 10:1079387. doi: 10.3389/fcell.2022.1079387
210. Rasool A, Sarath T, Porteen K, Krishnakumar K, Anilkumar R. Extracellular vesicles in male and female reproduction: a comprehensive review. J Anim Res. (2024) 14:1–8. doi: 10.30954/2277-940X.01.2024.1
211. Chen K, Liang J, Qin T, Zhang Y, Chen X, Wang Z. The role of extracellular vesicles in embryo implantation. Front Endocrinol. (2022) 13:809596. doi: 10.3389/fendo.2022.809596
212. Mukherjee A, Gali J, Kar I, Datta S, Roy M, Acharya AP, et al. Candidate genes and proteins regulating bull semen quality: a review. Trop Anim Health Prod. (2023) 55:212. doi: 10.1007/s11250-023-03617-0
213. Badrhan S, Karanwal S, Pal A, Chera JS, Chauhan V, Patel A, et al. Differential protein repertoires related to sperm function identified in extracellular vesicles (EVs) in seminal plasma of distinct fertility buffalo (Bubalus bubalis) bulls. Front Cell Dev Biol. (2024) 12:1400323. doi: 10.3389/fcell.2024.1400323
214. Gusdinal H, Ramadhan R, Abimanyu AA, Ningsih WH. Impacts of cryopreservation on semen quality and sperm protein profiles of Pesisir bulls. Tropic Anim Sci J. (2025) 48:189–98. doi: 10.5398/tasj.2025.48.3.189
215. Pardede BP, Kusumawati A, Pangestu M, Purwantara B. Bovine sperm HSP-70 molecules: a potential cryo-tolerance marker associated with semen quality and fertility rate. Front Vet Sci. (2023) 10:1167594. doi: 10.3389/fvets.2023.1167594
216. Karabay AZ, Barar J, Hekmatshoar Y, Rahbar Saadat Y. Multifaceted therapeutic potential of plant-derived exosomes: immunomodulation, anticancer, anti-aging, anti-melanogenesis, detoxification, and drug delivery. Biomolecules. (2025) 15:394. doi: 10.3390/biom15030394
217. Claes A, Stout TAE. Success rate in a clinical equine in vitro embryo production program. Theriogenology. (2022) 187:215–8. doi: 10.1016/j.theriogenology.2022.04.019
218. Galli C, Colleoni S, Duchi R, Lagutina I, Lazzari G. Equine assisted reproduction and embryo technologies. Anim Reprod. (2013) 10:334–43.
219. Dell'Aquila ME, Cho YS, Minoia P, Traina V, Fusco S, Lacalandra GM, et al. Intracytoplasmic sperm injection (ICSI) versus conventional IVF on abattoir-derived and in vitro -matured equine oocytes. Theriogenology. (1997) 47:1139–56. doi: 10.1016/S0093-691X(97)82517-4
220. Suarez SS. Formation of a reservoir of sperm in the oviduct. Reprod Domest Anim. (2002) 37:140–3. doi: 10.1046/j.1439-0531.2002.00346.x
221. Chen C, Huang Z, Dong S, Ding M, Li J, Wang M, et al. Calcium signaling in oocyte quality and functionality and its application. Front Endocrinol. (2024) 15:1411000. doi: 10.3389/fendo.2024.1411000
222. Yang Y, Yang L, Han X, Wu K, Mei G, Wu B, et al. The regulation role of calcium channels in mammalian sperm function: a narrative review with a focus on humans and mice. PeerJ. (2024) 12:e18429. doi: 10.7717/peerj.18429
223. Darszon A, Ferreira JJ, López-González I, Orta G, Treviño CL, Santi CM. Voltage-dependent calcium channels (CaVs) and CatSper in spermatogenic and sperm cells. In:Zamponi GW, Weiss N, , editors. Voltage-Gated Calcium Channels. Cham: Springer (2022). p. 599–634. doi: 10.1007/978-3-031-08881-0_23
224. Lange-Consiglio A, Capra E, Giuliani D, Canesi S, Funghi F, Bosi G, et al. Endometrial and oviduct extra-cellular vescicles for in vitro equine sperm hyperactivation and oocyte fertilization. Theriogenology. (2022) 194:35–45. doi: 10.1016/j.theriogenology.2022.09.023
225. Mahé C, Lavigne R, Com E, Pineau C, Locatelli Y, Zlotkowska AM, et al. Spatiotemporal profiling of the bovine oviduct fluid proteome around the time of ovulation. Sci Rep. (2022) 12:4135. doi: 10.1038/s41598-022-07929-3
226. Zhou W, Zhang T, Lian Y, Zhang W. Roles of extracellular vesicles in human reproduction. In:Paul MK, , editor. Extracellular Vesicles-Role in Diseases, Pathogenesis and Therapy. London, UK: IntechOpen (2021). p.167–9. doi: 10.5772/intechopen.101046
227. Vickram AS, Anbarasu K, Gulothungan G, Thanigaivel S, Nanmaran R, Palanivelu J. Characterization of human prostasomes protein Clusterin (macromolecule)–a novel biomarker for male infertility diagnosis and prognosis. J Biomol Struct Dyn. (2022) 40:3979–88. doi: 10.1080/07391102.2020.1852960
228. Jena SR, Nayak J, Kumar S, Kar S, Dixit A, Samanta L. Paternal contributors in recurrent pregnancy loss: cues from comparative proteome profiling of seminal extracellular vesicles. Mol Reprod Dev. (2021) 88:96–112. doi: 10.1002/mrd.23445
229. Rana S, Lone FA, Souza-Junior JBF, Bhat GR. The potential role of seminal extracellular vesicles as biomarkers of male fertility and sperm cryotolerance in livestock species. Discover Applied Sciences. (2024) 6:619. doi: 10.1007/s42452-024-06230-4
230. Xu Z, Xie Y, Zhou C, Hu Q, Gu T, Yang J, et al. Expression pattern of seminal plasma extracellular vesicle small RNAs in boar semen. Front Vet Sci. (2020) 7:585276. doi: 10.3389/fvets.2020.585276
231. Zuo H, Pi Y, Wang Y, Zheng W, Zhang X, Zhou H, et al. Small extracellular vesicles from HO-1 modified BMMSCs alleviate steatotic liver grafts ischemia-reperfusion injury by delivering PDIA4 to promote reparative macrophage polarization. Biochim BiophysActa. (2025) 1871:167947. doi: 10.1016/j.bbadis.2025.167947
232. Turathum B, Gao E-M, Chian R-C. The function of cumulus cells in oocyte growth and maturation and in subsequent ovulation and fertilization. Cells. (2021) 10:2292. doi: 10.3390/cells10092292
233. He M, Zhang T, Yang Y, Wang C. Mechanisms of oocyte maturation and related epigenetic regulation. Front Cell Dev Biol. (2021) 9:654028. doi: 10.3389/fcell.2021.654028
234. Palma GA, Argañaraz ME, Barrera AD, Rodler D, Mutto AÁ, Sinowatz F. Biology and biotechnology of follicle development. Sci World J. (2012) 2012:938138. doi: 10.1100/2012/938138
235. Hung WT, Hong X, Christenson LK, McGinnis LK. Extracellular vesicles from bovine follicular fluid support cumulus expansion. Biol Reprod. (2015) 93:117. doi: 10.1095/biolreprod.115.132977
236. De Ávila ACFCM, Bridi A, Andrade GM, Del Collado M, Sangalli JR, Nociti RP, et al. Estrous cycle impacts microRNA content in extracellular vesicles that modulate bovine cumulus cell transcripts during in vitro maturation. Biol Reprod. (2020) 102:362–75. doi: 10.1093/biolre/ioz177
237. da Silva Rosa PM, Bridi A, de Ávila Ferronato G, Prado CM, Bastos NM, Sangalli JR, et al. Corpus luteum presence in the bovine ovary increase intrafollicular progesterone concentration: consequences in follicular cells gene expression and follicular fluid small extracellular vesicles miRNA contents. J Ovarian Res. (2024) 17:65. doi: 10.1186/s13048-024-01387-3
238. Mateo-Otero Y, Yeste M, Roca J, Llavanera M, Bucci D, Galeati G, et al. Seminal extracellular vesicles subsets modulate gene expression in cumulus cells of porcine in vitro matured oocytes. Sci Rep. (2022) 12:19096. doi: 10.1038/s41598-022-22004-7
239. Bukowska D, Kempisty B, Piotrowska H, Walczak R, Sniadek P, Dziuban J, et al. The invasive and new non-invasive methods of mammalian oocyte and embryo quality assessment: a review. Vet Med. (2012) 57:169–76. doi: 10.17221/5913-VETMED
240. Matsuno Y, Onuma A, Fujioka YA, Yasuhara K, Fujii W, Naito K, et al. Effects of exosome-like vesicles on cumulus expansion in pigs in vitro. J Reprod Dev. (2017) 63:51–8. doi: 10.1262/jrd.2016-124
241. Nevoral J, Orsák M, Klein P, Petr J, Dvoráková M, Weingartová I, et al. Cumulus cell expansion, its role in oocyte biology and perspectives of measurement: a review. Sci Agric Bohem. (2015) 45:212–25. doi: 10.1515/sab-2015-0002
242. Abumaghaid MM, Abdelazim AM, Belali TM, Alhujaily M, Saadeldin IM. Shuttle transfer of mRNA transcripts via extracellular vesicles from male reproductive tract cells to the cumulus–oocyte complex in rabbits (Oryctolagus cuniculus). Front Vet Sci. (2022) 9:816080. doi: 10.3389/fvets.2022.816080
243. Gabryś J, Gurgul A, Szmatoła T, Kij-Mitka B, Andronowska A, Karnas E, et al. Follicular fluid-derived extracellular vesicles influence on in vitro maturation of equine oocyte: impact on cumulus cell viability, expansion and transcriptome. Int J Mol Sci. (2024) 25:3262. doi: 10.3390/ijms25063262
244. Gabryś J, Kij-Mitka B, Gurgul A, Szmatoła T, Kochan J, Karnas E, et al. Extracellular vesicles from equine follicular fluid support cumulus expansion and alter cumulus cells' transcriptome and viability. J Equine Vet Sci. (2023) 125:104652. doi: 10.1016/j.jevs.2023.104652
245. Menjivar NG, Gad A, Gebremedhn S, Ghosh S, Tesfaye D. Granulosa cell-derived extracellular vesicles mitigate the detrimental impact of thermal stress on bovine oocytes and embryos. Front Cell Dev Biol. (2023) 11:1142629. doi: 10.3389/fcell.2023.1142629
246. Kimiaei Asadi K, Amiri K, Ghasemi H, Amiri I. Comparison of follicular fluid glycosaminoglycan and hydroxyproline concentration in women with polycystic ovary syndrome with healthy women. Avicenna J Med Biochem. (2023) 11:146–50. doi: 10.34172/ajmb.2444
247. Mazloomi S, Farimani MS, Tavilani H, Karimi J, Amiri I, Abbasi E, et al. Granulosa cells from immature follicles exhibit restricted glycolysis and reduced energy production: a dominant problem in polycystic ovary syndrome. J Assist Reprod Genet. (2023) 40:343–59. doi: 10.1007/s10815-022-02676-w
248. Padbury EH, Bálint Š, Carollo E, Carter DRF, Becker EBE. TRPC3 signalling contributes to the biogenesis of extracellular vesicles. J Extracell Biol. (2024) 3:e132. doi: 10.1002/jex2.132
249. Abd El Naby WS, Hagos TH, Hossain MM, Salilew-Wondim D, Gad AY, Rings F, et al. Expression analysis of regulatory microRNAs in bovine cumulus oocyte complex and preimplantation embryos. Zygote. (2013) 21:31–51. doi: 10.1017/S0967199411000566
250. Sharma DK, Brown JC, Choudhury A, Peterson TE, Holicky E, Marks DL, et al. Selective stimulation of caveolar endocytosis by glycosphingolipids and cholesterol. Mol Biol Cell. (2004) 15:3114–22. doi: 10.1091/mbc.e04-03-0189
251. Edure T, Matsuno Y, Matsushita K, Maruyama N, Fujii W, Naito K, et al. Dynamics of extracellular vesicle uptake by mural granulosa cells in mice. Mol Reprod Dev. (2024) 91:e23737. doi: 10.1002/mrd.23737
252. Hung WT, Navakanitworakul R, Khan T, Zhang P, Davis JS, McGinnis LK, et al. Stage-specific follicular extracellular vesicle uptake and regulation of bovine granulosa cell proliferation. Biol Reprod. (2017) 97:644–55. doi: 10.1093/biolre/iox106
253. Tahiri A, Puco K, Naji F, Kristensen VN, Alfsen GC, Farkas L, et al. Kinase activity profiling in renal cell carcinoma, benign renal tissue and in response to four different tyrosine kinase inhibitors. Oncotarget. (2022) 13:970–81. doi: 10.18632/oncotarget.28257
254. Gurdal H, Tuglu MM, Bostanabad SY, Dalkiliç B. Partial agonistic effect of cetuximab on epidermal growth factor receptor and Src kinase activation in triple-negative breast cancer cell lines. Int J Oncol. (2019) 54:1345–56. doi: 10.3892/ijo.2019.4697
255. Ingley E. Src family kinases: regulation of their activities, levels and identification of new pathways. Biochim Biophys Acta Proteins Proteom. (2008) 1784:56–65. doi: 10.1016/j.bbapap.2007.08.012
256. Ju W, Pan K, Zhang Q, Wang Y, Zhao S, Zhang J, et al. Differential expression of microRNA in follicular fluid-derived extracellular vesicles and mRNA in granulosa cells of patients with polycystic ovary syndrome and insulin resistance. Reprod Biomed Online. (2025) 18:105027. doi: 10.1016/j.rbmo.2025.105027
257. Fulka J, First NL, Moor RM. Nuclear and cytoplasmic determinants involved in the regulation of mammalian oocyte maturation. Mol Hum Reprod. (1998) 4:41–9. doi: 10.1093/molehr/4.1.41
258. Viveiros MM, De La Fuente R. Regulation of mammalian oocyte maturation. In: Barlow DW, Bavister BC, , editors. The Ovary. Cambridge, MA: Academic Press (2019) 165–80. doi: 10.1016/B978-0-12-813209-8.00011-X
259. Kawamura K, Kumagai J, Sudo S, Chun SY, Pisarska M, Morita H, et al. Paracrine regulation of mammalian oocyte maturation and male germ cell survival. Proc Natl Acad Sci USA. (2004) 101:7323–8. doi: 10.1073/pnas.0307061101
260. Albuz FK, Sasseville M, Lane M, Armstrong DT, Thompson JG, Gilchrist RB. Simulated physiological oocyte maturation (SPOM): a novel in vitro maturation system that substantially improves embryo yield and pregnancy outcomes. Hum Reprod. (2010) 25:2999–3011. doi: 10.1093/humrep/deq246
261. Suresh A, Shukla MK, Kumar D, Shrivastava OP, Verma N. Simulated physiological oocyte maturation (SPOM) improves developmental competence of in vitro produced goat embryos. Theriogenology. (2021) 172:193–9. doi: 10.1016/j.theriogenology.2021.06.003
262. Leal GR, Monteiro CAS, Carvalheira L de R, Souza-Fabjan JMG. The simulated physiological oocyte maturation (SPOM) system in domestic animals: a systematic review. Theriogenology. (2022) 188:90–9. doi: 10.1016/j.theriogenology.2022.05.023
263. Razza EM, Pedersen HS, Stroebech L, Fontes PK, Kadarmideen HN, Callesen H, et al. Simulated physiological oocyte maturation has side effects on bovine oocytes and embryos. J Assist Reprod Genet. (2019) 36:413–24. doi: 10.1007/s10815-018-1365-4
264. Rose RD, Gilchrist RB, Kelly JM, Thompson JG, Sutton-McDowall ML. Regulation of sheep oocyte maturation using cAMP modulators. Theriogenology. (2013) 79:142–8. doi: 10.1016/j.theriogenology.2012.09.020
265. Guimarães ALS, Pereira SA, Leme LO, Dode MAN. Evaluation of the simulated physiological oocyte maturation system for improving bovine invitro embryo production. Theriogenology. (2015) 83:52–7. doi: 10.1016/j.theriogenology.2014.07.042
266. Makutina V, Isaeva A, Krivonogova A, Deykin A. Effect of cAMP on bovine oocyte maturation in vitro. E3S Web of Conferences. (2021) 285:03013. doi: 10.1051/e3sconf/202128503013
267. Maik-Rachline G, Wexler S, Seger R. The MAPK signaling cascades. In:Bradshaw RA, Hart GW, Stahl PD, , editors. Encyclopedia of Cell Biology, Vol 4. 2nd ed. Vol 1-6. New York, NY: Elsevier (2022). p. 145–52 doi: 10.1016/B978-0-12-821618-7.00130-9
268. Gabryś J, Pietras N, Kowal-Mierzwa W, Karnas E, Andronowska A, Nowak A, et al. Investigating the impact of extracellular vesicle addition during IVM on the fertilization rate of equine oocytes following ICSI. Reprod Biol. (2024) 24:100967. doi: 10.1016/j.repbio.2024.100967
269. Rodrigues TA, Tuna KM, Alli AA, Tribulo P, Hansen PJ, Koh J, et al. Follicular fluid exosomes act on the bovine oocyte to improve oocyte competence to support development and survival to heat shock. Reprod Fertil Dev. (2019) 31:888–97. doi: 10.1071/RD18450
270. White BR, Desaulniers AT, Cederberg RA, Mills GA, Lents CA. 308 A transgenic boar model to elucidate the role of gonadotropin-releasing hormone 2 and its receptor in regulating testes and sperm function. J Anim Sci. (2017) 95:150. doi: 10.2527/asasmw.2017.308
271. Tesfaye D, Salilew-Wondim D, Gebremedhn S, Sohel MMH, Pandey HO, Hoelker M, et al. Potential role of microRNAs in mammalian female fertility. Reprod Fertil Dev. (2017) 29. doi: 10.1071/RD16266
272. Salilew-Wondim D, Gebremedhn S, Hoelker M, Tholen E, Hailay T, Tesfaye D. The role of microRNAs in mammalian fertility: From gametogenesis to embryo implantation. Int J Mol Sci. (2020) 21:585. doi: 10.3390/ijms21020585
273. Tesfaye D, Gebremedhn S, Salilew-Wondim D, Hailay T, Hoelker M, Grosse-Brinkhaus C, et al. MicroRNAs: tiny molecules with a significant role in mammalian follicular and oocyte development. Reproduction. (2018) 155:R121–35. doi: 10.1530/REP-17-0428
274. Tesfaye D, Hailay T, Salilew-Wondim D, Hoelker M, Bitseha S, Gebremedhn S. Extracellular vesicle mediated molecular signaling in ovarian follicle: implication for oocyte developmental competence. Theriogenology. (2020) 150:70–4. doi: 10.1016/j.theriogenology.2020.01.075
275. Anderson E, Albertini DF. Gap junctions between the oocyte and companion follicle cells in the mammalian ovary. J Cell Biol. (1976) 71:680–6. doi: 10.1083/jcb.71.2.680
276. da Silveira JC, Andrade GM, Collado M del, Sampaio R V., Sangalli JR, Silva LA, et al. Supplementation with small-extracellular vesicles from ovarian follicular fluid during in vitro production modulates bovine embryo development. PLoS ONE. (2017) 12:e0179451. doi: 10.1371/journal.pone.0179451
277. Xie J, Xu X, Liu S. Intercellular communication in the cumulus–oocyte complex during folliculogenesis: a review. Front Cell Dev Biol. (2023) 11:1087612. doi: 10.3389/fcell.2023.1087612
278. Bukovsky A. Ovarian stem cell niche and follicular renewal in mammals. Anat Rec. (2011) 294:1284–306. doi: 10.1002/ar.21422
279. Neyroud AS, Chiechio RM, Moulin G, Ducarre S, Heichette C, Dupont A, et al. Diversity of extracellular vesicles in human follicular fluid: morphological analysis and quantification. Int J Mol Sci. (2022) 23:11676. doi: 10.3390/ijms231911676
280. Gad A, Murin M, Bartkova A, Kinterova V, Marcollova K, Laurincik J, et al. Small-extracellular vesicles and their microRNA cargo from porcine follicular fluids: the potential association with oocyte quality. J Anim Sci Biotechnol. (2022) 13:82. doi: 10.1186/s40104-022-00723-1
281. Leal CLV, Cañón-Beltrán K, Cajas YN, Hamdi M, Yaryes A, Millán de. la Blanca MG, et al. Extracellular vesicles from oviductal and uterine fluids supplementation in sequential in vitro culture improves bovine embryo quality. J Anim Sci Biotechnol. (2022) 13:116. doi: 10.1186/s40104-022-00763-7
282. Almiñana C, Bauersachs S. Extracellular vesicles: Multi-signal messengers in the gametes/embryo-oviduct cross-talk. Theriogenology. (2020) 150:59–69. doi: 10.1016/j.theriogenology.2020.01.077
283. Lopera-Vasquez R, Hamdi M, Fernandez-Fuertes B, Maillo V, Beltran-Brena P, Calle A, et al. Extracellular vesicles from boec in in vitro embryo development and quality. PLoS ONE. (2016) 11:e0148083. doi: 10.1371/journal.pone.0148083
284. Hamdi M, Sánchez JM, Fernandez-Fuertes B, Câmara DR, Bollwein H, Rizos D, et al. Oviductal extracellular vesicles miRNA cargo varies in response to embryos and their quality. BMC Genomics. (2024) 25:520. doi: 10.1186/s12864-024-10429-5
285. Pavani KC, XueFeng G, Chunduru J, Meese T, Peelman LJ, Van Nieuwerburgh F, et al. MicroRNA-146b negatively affects bovine embryo development and quality. Reproduction. (2024) 167:e230155. doi: 10.1530/REP-23-0155
286. Jenabi M, Khodarahmi P, Tafvizi F, Bostanabad SZ. Evaluation of the potential of miR-21 as a diagnostic marker for oocyte maturity and embryo quality in women undergoing ICSI. Sci Rep. (2023) 13:1440. doi: 10.1038/s41598-023-28686-x
287. Gervasi MG, Soler AJ, González-Fernández L, Alves MG, Oliveira PF, Martín-Hidalgo D. Extracellular vesicles, the road toward the improvement of ART outcomes. Animals. (2020) 10:2171. doi: 10.3390/ani10112171
288. Imakawa K, Matsuno Y, Fujiwara H. New roles for EVs, miRNA and lncRNA in bovine embryo implantation. Front Vet Sci. (2022) 9:944370. doi: 10.3389/fvets.2022.944370
289. Li Y, Zhao J, Yu S, Wang Z, He X, Su Y, et al. Extracellular vesicles long RNA sequencing reveals abundant mRNA, circRNA, and lncRNA in human blood as potential biomarkers for cancer diagnosis. Clin Chem. (2019) 65:798–808. doi: 10.1373/clinchem.2018.301291
290. Rizos D, Maillo V, Lonergan P. Role of the oviduct and oviduct-derived products in ruminant embryo development. Anim Reprod. (2016) 13:160–7. doi: 10.21451/1984-3143-AR863
291. Jeoung M, Lee S, Hawng HK, Cheon YP, Jeong YK, Gye MC, et al. Identification of a novel role for endothelins within the oviduct. Endocrinology. (2010) 151:2858–67. doi: 10.1210/en.2009-1155
292. Almiñana C, Tsikis G, Labas V, Uzbekov R, da Silveira JC, Bauersachs S, et al. Deciphering the oviductal extracellular vesicles content across the estrous cycle: Implications for the gametes-oviduct interactions and the environment of the potential embryo. BMC Genomics. (2018) 19:622. doi: 10.1186/s12864-018-4982-5
293. Mazzarella R, Bastos NM, Bridi A, del Collado M, Andrade GM, Pinzon J, et al. Changes in oviductal cells and small extracellular vesicles miRNAs in pregnant cows. Front Vet Sci. (2021) 8:639752. doi: 10.3389/fvets.2021.639752
294. Jiang N-X, Li X-L. The complicated effects of extracellular vesicles and their cargos on embryo implantation. Front Endocrinol. (2021) 12:681266. doi: 10.3389/fendo.2021.681266
295. Menjivar NG, Gad A, Thompson RE, Meyers MA, Hollinshead FK, Tesfaye D. Bovine oviductal organoids: a multi-omics approach to capture the cellular and extracellular molecular response of the oviduct to heat stress. BMC Genomics. (2023) 24:646. doi: 10.1186/s12864-023-09746-y
296. Mellisho E, Velasquez A, Nuñez MJ, Rodriguez-Alvarez L. 139 Bovine preimplantation embryos secrete extracellular vesicles, which may indicate embryo competence. Reprod Fertil Dev. (2017) 29:178. doi: 10.1071/RDv29n1Ab139
297. Dissanayake K, Nõmm M, Lättekivi F, Ressaissi Y, Godakumara K, Lavrits A, et al. Individually cultured bovine embryos produce extracellular vesicles that have the potential to be used as non-invasive embryo quality markers. Theriogenology. (2020) 149:104–16. doi: 10.1016/j.theriogenology.2020.03.008
298. Pavani KC, Meese T, Pascottini OB, Guan XF, Lin X, Peelman L, et al. Hatching is modulated by microRNA-378a-3p derived from extracellular vesicles secreted by blastocysts. Proc Natl Acad Sci USA. (2022) 119:e2122708119. doi: 10.1073/pnas.2122708119
299. Hart AR, Khan NLA, Dissanayake K, Godakumara K, Andronowska A, Eapen S, et al. The extracellular vesicles proteome of endometrial cells simulating the receptive menstrual phase differs from that of endometrial cells simulating the non-receptive menstrual phase. Biomolecules. (2023) 13:279. doi: 10.3390/biom13020279
300. Piibor J, Waldmann A, Dissanayake K, Andronowska A, Ivask M, Prasadani M, et al. Uterine fluid extracellular vesicles proteome is altered during the estrous cycle. Mol Cell Proteomics. (2023) 22:100642. doi: 10.1016/j.mcpro.2023.100642
301. Luddi A, Zarovni N, Maltinti E, Governini L, De Leo V, Cappelli V, et al. Clues to non-invasive implantation window monitoring: isolation and characterisation of endometrial exosomes. Cells. (2019) 8:811. doi: 10.3390/cells8080811
302. Burns GW, Brooks KE, O'Neil E V., Hagen DE, Behura SK, Spencer TE. Progesterone effects on extracellular vesicles in the sheep uterus. Biol Reprod. (2018) 98:612–22. doi: 10.1093/biolre/ioy011
303. O'Neil E V., Burns GW, Ferreira CR, Spencer TE. Characterization and regulation of extracellular vesicles in the lumen of the ovine uterus. Biol Reprod. (2020) 102:1020–32. doi: 10.1093/biolre/ioaa019
304. Burns GW, Brooks KE, Spencer TE. Extracellular vesicles originate from the conceptus and uterus during early pregnancy in sheep. Biol Reprod. (2016) 94:56. doi: 10.1095/biolreprod.115.134973
305. Shekibi M, Heng S, Nie G. MicroRNAs in the regulation of endometrial receptivity for embryo implantation. Int J Mol Sci. (2022) 23:6210. doi: 10.3390/ijms23116210
306. Von Grothusen C, Frisendahl C, Modhukur V, Lalitkumar PG, Peters M, Faridani OR, et al. Uterine fluid microRNAs are dysregulated in women with recurrent implantation failure. Hum Reprod. (2022) 37:734–46. doi: 10.1093/humrep/deac107.397
307. Paul ABM, Sadek ST, Mahesan AM. The role of microRNAs in human embryo implantation: a review. J Assist Reprod Genet. (2019) 36:179–87. doi: 10.1007/s10815-018-1326-y
308. Liu W, Niu Z, Li Q, Pang RTK, Chiu PCN, Yeung WSB. MicroRNA and embryo implantation. Am J Reprod Immunol. (2016) 75:263–71. doi: 10.1111/aji.12470
309. Goharitaban S, Abedelahi A, Hamdi K, Khazaei M, Esmaeilivand M, Niknafs B. Role of endometrial microRNAs in repeated implantation failure (mini-review). Front Cell Dev Biol. (2022) 10:936173. doi: 10.3389/fcell.2022.936173
310. Daneshvar M, Movahedin M, Salehi M, Noruzinia M. Alterations of miR-16, miR-let-7a and their target genes expression in human blastocysts following vitrification and re-vitrification. Reprod Biol Endocrinol. (2021) 19:155. doi: 10.1186/s12958-021-00842-w
311. Azizi E, Mofarahe ZS, Naji M. MicroRNAs, small regulatory elements with significant effects on human implantation: a review. J Assist Reprod Genet. (2023) 40:697–717. doi: 10.1007/s10815-023-02735-w
312. Nguyen MA, Karunakaran D, Geoffrion M, Cheng HS, Tandoc K, Perisic Matic L, et al. Extracellular vesicles secreted by atherogenic macrophages transfer MicroRNA to inhibit cell migration. Arterioscler Thromb Vasc Biol. (2018) 38:49–63. doi: 10.1161/ATVBAHA.117.309795
313. Burns G, Brooks K, Wildung M, Navakanitworakul R, Christenson LK, Spencer TE. Extracellular vesicles in luminal fluid of the ovine uterus. PLoS ONE. (2014) 9:e90913. doi: 10.1371/journal.pone.0090913
314. Nakamura K, Kusama K, Bai R, Sakurai T, Isuzugawa K, Godkin JD, et al. Induction of IFNT-stimulated genes by conceptus-derived exosomes during the attachment period. PLoS ONE. (2016) 11:e0158278. doi: 10.1371/journal.pone.0158278
315. Han A, Qamar AY, Bang S, Kim H, Kang H, Kim J-H, et al. Effect of extracellular vesicles derived from oviductal and uterine fluid on the development of porcine preimplantation embryos. Theriogenology. (2025) 234:216–24. doi: 10.1016/j.theriogenology.2024.12.020
316. Panzani D, Rota A, Marmorini P, Vannozzi I, Camillo F. Retrospective study of factors affecting multiple ovulations, embryo recovery, quality, and diameter in a commercial equine embryo transfer program. Theriogenology. (2014) 82:807–14. doi: 10.1016/j.theriogenology.2014.06.020
317. Alkan KK, Alkan H, Kaymaz M, Izgur IH. Multiple ovulation and embryo transfer during the breeding season in angora goats: a comparison of fresh and vitrified-thawed embryo transfer. Vet Res Forum. (2021) 12:143–8. doi: 10.30466/vrf.2020.107064.2544
318. Lange-Consiglio A, Lazzari B, Pizzi F, Idda A, Cremonesi F, Capra E. Amniotic microvesicles impact hatching and pregnancy percentages of in vitro bovine embryos and blastocyst microRNA expression versus in vivo controls. Sci Rep. (2020) 10:501. doi: 10.1038/s41598-019-57060-z
319. Truby LK, Maamari D, Saha A, Farr M, Abdulrahim J, Billia F, et al. Towards allograft longevity: leveraging omics technologies to improve heart transplant outcomes. Curr Heart Fail Rep. (2023) 20:493–503. doi: 10.1007/s11897-023-00631-z
320. Paulaitis M, Agarwal K, Nana-Sinkam P. Dynamic scaling of exosome sizes. Langmuir. (2018) 34:9387–93. doi: 10.1021/acs.langmuir.7b04080
321. Ishii N, Noguchi K, Ikemoto MJ, Yohda M, Odahara T. Optimizing exosome preparation based on size and morphology: insights from electron microscopy. Microsc Microanal. (2023) 29:2068–79. doi: 10.1093/micmic/ozad103
322. Mahmood A, Otruba Z, Weisgerber AW, Palay MD, Nguyen MT, Bills BL, et al. Exosome secretion kinetics are controlled by temperature. Biophys J. (2023) 122:1301–14. doi: 10.1101/2022.07.22.501177
323. Deng H, Sun C, Sun Y, Li H, Yang L, Wu D, et al. Lipid, protein, and MicroRNA composition within mesenchymal stem cell-derived exosomes. Cell Reprogram. (2018) 20:178–86. doi: 10.1089/cell.2017.0047
324. Dai J, Su Y, Zhong S, Cong L, Liu B, Yang J, et al. Exosomes: key players in cancer and potential therapeutic strategy. Signal Transduct Target Ther. (2020) 5:145. doi: 10.1038/s41392-020-00261-0
325. Maia J, Caja S, Strano Moraes MC, Couto N, Costa-Silva B. Exosome-based cell-cell communication in the tumor microenvironment. Front Cell Dev Biol. (2018) 6:18. doi: 10.3389/fcell.2018.00018
326. Rad F, Pourfathollah AA, Yari F, Mohammadi S, Kheirandish M. Microvesicles preparation from mesenchymal stem cells. Med J Islam Repub Iran. (2016) 30:398.
327. Zarà M, Guidetti GF, Camera M, Canobbio I, Amadio P, Torti M, et al. Biology and role of extracellular vesicles (Evs) in the pathogenesis of thrombosis. Int J Mol Sci. (2019) 20:2840. doi: 10.3390/ijms20112840
328. György B, Szabó TG, Pásztói M, Pál Z, Misják P, Aradi B, et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. (2011) 68:2667–88. doi: 10.1007/s00018-011-0689-3
329. Akers JC, Gonda D, Kim R, Carter BS, Chen CC. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol. (2013) 113:1–11. doi: 10.1007/s11060-013-1084-8
330. Battistelli M, Falcieri E. Apoptotic bodies: particular extracellular vesicles involved in intercellular communication. Adv Med Biochem Genom Physiol Pathol. (2021) 22:473–86. doi: 10.1201/9781003180449-20
331. Trotta T, Panaro MA, Cianciulli A, Mori G, Di Benedetto A, Porro C. Microglia-derived extracellular vesicles in Alzheimer's disease: a double-edged sword. Biochem Pharmacol. (2018) 148:184–92. doi: 10.1016/j.bcp.2017.12.020
332. Caruso S, Poon IKH. Apoptotic cell-derived extracellular vesicles: more than just debris. Front Immunol. (2018) 9:1486. doi: 10.3389/fimmu.2018.01486
333. Comfort N, Cai K, Bloomquist TR, Strait MD, Ferrante Jr AW, Baccarelli AA. Nanoparticle tracking analysis for the quantification and size determination of extracellular vesicles. J Vis Exp. (2021) 28:10–3791. doi: 10.3791/62447
334. Kowkabany G, Bao Y. Nanoparticle tracking analysis: an effective tool to characterize extracellular vesicles. Molecules. (2024) 29:4672. doi: 10.3390/molecules29194672
335. Aksamitiene E, Park J, Marjanovic M, Boppart SA. Defining biological variability, analytical precision and quantitative biophysiochemical characterization of human urinary extracellular vesicles. J Extracell Vesicles. (2025) 14:e70087. doi: 10.1002/jev2.70087
336. De Langhe N, Van Dorpe S, Guilbert N, Vander Cruyssen A, Roux Q, Deville S, et al. Mapping bacterial extracellular vesicle research: insights, best practices and knowledge gaps. Nat Commun. (2024) 15:9410. doi: 10.1038/s41467-024-53279-1
337. Liu X, Ma Z, Li Y, Fan T, Ge Z, Ou Z, et al. A simple modification results in a significant improvement in measuring the size of extracellular vesicles. Curr Med Sci. (2025) 45:244–52. doi: 10.1007/s11596-025-00045-z
338. Maas SLN, Broekman MLD, de Vrij J. Tunable resistive pulse sensing for the characterization of extracellular vesicles. In Hill AF, editor: Exosomes and Microvesicles: Methods and Protocols. New York, NY: Springer (2016). p. 21–33. doi: 10.1007/978-1-4939-6728-5_2
339. Liu H, Tian Y, Xue C, Niu Q, Chen C, Yan X. Analysis of extracellular vesicle DNA at the single-vesicle level by nano-flow cytometry. J Extracell Vesicles. (2022) 11:e12206. doi: 10.1002/jev2.12206
340. Nikishin I, Dulimov R, Skryabin G, Galetsky S, Tchevkina E, Bagrov D. ScanEV–A neural network-based tool for the automated detection of extracellular vesicles in TEM images. Micron. (2021) 145:103044. doi: 10.1016/j.micron.2021.103044
341. Ridolfi A, Brucale M, Montis C, Caselli L, Paolini L, Borup A, et al. AFM-based high-throughput nanomechanical screening of single extracellular vesicles. Anal Chem. (2020) 92:10274–82. doi: 10.1021/acs.analchem.9b05716
342. Gazze SA, Thomas SJ, Garcia-Parra J, James DW, Rees P, Marsh-Durban V, et al. High content, quantitative AFM analysis of the scalable biomechanical properties of extracellular vesicles. Nanoscale. (2021) 13:6129–41. doi: 10.1039/D0NR09235E
343. Jankovičová J, Sečová P, Michalková K, Antalíková J. Tetraspanins, more than markers of extracellular vesicles in reproduction. Int J Mol Sci. (2020) 21:7568. doi: 10.3390/ijms21207568
344. Zhang X, Vos HR, Tao W, Stoorvogel W. Proteomic profiling of two distinct populations of extracellular vesicles isolated from human seminal plasma. Int J Mol Sci. (2020) 21:7957. doi: 10.3390/ijms21217957
345. Governini L, Haxhiu A, Shaba E, Vantaggiato L, Mori A, Bruttini M, et al. Unraveling the multi-omic landscape of extracellular vesicles in human seminal plasma. Biomolecules. (2025) 15:836. doi: 10.3390/biom15060836
346. Huang H, Wan J, Xudong A, Qu S, Jia M, Zhao K, et al. ECM1 and ANXA1 in urinary extracellular vesicles serve as biomarkers for breast cancer. Front Oncol. (2024) 14:1408492. doi: 10.3389/fonc.2024.1408492
347. Tanrikulu MD, Cevik M, Taşli PN, Yildirim K. Cryoprotective effects of mesenchymal stem cell and seminal plasma-derived extracellular vesicles on canine sperm. Theriogenology. (2025) 244:117480. doi: 10.1016/j.theriogenology.2025.117480
348. Huang J, Su Y, Wang J, Fang Z, Zhang Y, Chen H, et al. Seminal plasma proteomics of asymptomatic COVID-19 patients reveals disruption of male reproductive function. BMC Genomics. (2025) 26:1–12. doi: 10.1186/s12864-025-11473-5
349. Liu Y, He J-X, Ji B, Wang J-F, Zhang L, Pang Z-Q, et al. Comprehensive analysis of integrin αvβ3/α6β1 in prognosis and immune escape of prostate cancer. Aging. (2023) 15:11369. doi: 10.18632/aging.205131
350. Gerber E, Asare-Werehene M, Reunov A, Burger D, Le T, Carmona E, et al. Predicting chemoresponsiveness in epithelial ovarian cancer patients using circulating small extracellular vesicle-derived plasma gelsolin. J Ovarian Res. (2023) 16:14. doi: 10.1186/s13048-022-01086-x
351. Estrada AL, Valenti ZJ, Hehn G, Amorese AJ, Williams NS, Balestrieri NP, et al. Extracellular vesicle secretion is tissue-dependent ex vivo and skeletal muscle myofiber extracellular vesicles reach the circulation in vivo. Am J Physiol Cell Physiol. (2022) 322:C246–59. doi: 10.1152/ajpcell.00580.2020
352. Kawano T, Englisch C, Hisada Y, Paul D, Archibald S, Grover S, et al. Mucin 1 and venous thrombosis in tumor-bearing mice and patients with cancer. Thromb Res. (2024) 237:23–30. doi: 10.1016/j.thromres.2024.03.022
353. Ding Y, Ding N, Zhang Y, Xie S, Huang M, Ding X, et al. MicroRNA-222 transferred from semen extracellular vesicles inhibits sperm apoptosis by targeting BCL2L11. Front Cell Dev Biol. (2021) 9:736864. doi: 10.3389/fcell.2021.736864
354. Pantos K, Grigoriadis S, Tomara P, Louka I, Maziotis E, Pantou A, et al. Investigating the role of the microRNA-34/449 family in male infertility: a critical analysis and review of the literature. Front Endocrinol. (2021) 12:709943. doi: 10.3389/fendo.2021.709943
355. Dlamini NH, Nguyen T, Gad A, Tesfaye D, Liao SF, Willard ST, et al. Characterization of extracellular vesicle-coupled miRNA profiles in seminal plasma of boars with divergent semen quality status. Int J Mol Sci. (2023) 24:3194. doi: 10.3390/ijms24043194
356. Xie Y, Peng C, He J, Wang Z, Xiang J. Seminal plasma extracellular vesicles: key mediators of intercellular communication in mammalian reproductive systems. Vet Sci. (2025) 12:585. doi: 10.3390/vetsci12060585
357. Du L, Chen W, Zhang D, Cui Y, He Z. The functions and mechanisms of piRNAs in mediating mammalian spermatogenesis and their applications in reproductive medicine. Cell Mol Life Sci. (2024) 81:379. doi: 10.1007/s00018-024-05399-6
358. Hashimoto A, Sugiura K, Hoshino A. Impact of exosome-mediated feto-maternal interactions on pregnancy maintenance and development of obstetric complications. J Biochem. (2021) 169:163–71. doi: 10.1093/jb/mvaa137
359. Abeysinghe P, Turner N, Garcia IM, Mosaad E, Peiris HN, Mitchell MD. The role of exosomal epigenetic modifiers in cell communication and fertility of dairy cows. Int J Mol Sci. (2020) 21:9106. doi: 10.3390/ijms21239106
360. Aguilera C, Velásquez AE, Gutierrez-Reinoso MA, Wong Y Sen, Melo-Baez B, Cabezas J, et al. Extracellular vesicles secreted by pre-hatching bovine embryos produced in vitro and in vivo alter the expression of IFNtau-stimulated genes in bovine endometrial cells. Int J Mol Sci. (2023) 24:7438. doi: 10.3390/ijms24087438
Keywords: extracellular vesicles, oocyte maturation, fertilization, embryo development, intercellular communication
Citation: Pournourali M, Mizban N, Ehsani R, Ebrahimian S, Nadri T and Azari-Dolatabad N (2025) Extracellular vesicles: key mediators in in vitro embryo production. Front. Vet. Sci. 12:1641966. doi: 10.3389/fvets.2025.1641966
Received: 05 June 2025; Accepted: 04 August 2025;
Published: 20 August 2025.
Edited by:
Jaime Catalán, Autonomous University of Barcelona, SpainReviewed by:
Karima Mahmoud, National Research Centre, EgyptErwin Muñoz-Acuña, University of La Frontera, Chile
Copyright © 2025 Pournourali, Mizban, Ehsani, Ebrahimian, Nadri and Azari-Dolatabad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nima Azari-Dolatabad, YXphcmluaW1hMTM2NkBnbWFpbC5jb20=
 Mostafa Pournourali
Mostafa Pournourali Nahid Mizban
Nahid Mizban Roxana Ehsani
Roxana Ehsani Somayeh Ebrahimian
Somayeh Ebrahimian Touba Nadri
Touba Nadri Nima Azari-Dolatabad
Nima Azari-Dolatabad