- 1Department of Animal Nutrition, College of Veterinary and Animal Science University, Rani Lakshmi Bai Central Agricultural University, Jhansi, India
- 2Animal Nutrition Division, ICAR-Indian Veterinary Research Institute, Izatnagar, India
- 3ILRI, South East Asia Office, New Delhi, India
Introduction: The present study aimed to understand the shift in the rumen microbiome of buffaloes fed diets with and without phyto-additives. The rationale was based on the hypothesis that plant-based additives can modulate the microbial population in the rumen, potentially reducing methane production and enhancing fiber degradation. Given the possibility that prolonged use of the same additives may lead to microbial adaptation and diminished efficacy, the study also investigated the effects of periodically switching additives.
Methods: Three male buffalo calves were fed a control diet, while another three received additive-supplemented diets. Two additive formulations were used: FAI (a blend of garlic Allium sativum, ajwain Trachyspermum ammi, harad Terminalia chebula, and soapnut Sapindus mukorossi) and FAII (ajwain oil). The additives were alternated every 15 days to prevent microbial adaptation. After 21 days of feeding, rumen liquor samples were collected 2 hours post-feeding for metagenomic analysis. The study included both in vivo and in vitro assessments of rumen fermentation.
Results: Metagenomic analysis revealed that dominant bacterial phyla included Prevotella, Bacteroides, Succiniclasticum, Fibrobacter, Clostridium, Alistipes, Ruminococcus, and Butyrivibrio, with over 50 bacterial species consistently present across all animals. The main archaeal phylum was Euryarchaeota (>85%), along with notable presence of Candidatus_Bathyarchaeota and Thaumarchaeota. At the genus level, Methanomicrobium and Methanobrevibacter each accounted for approximately 30% of the archaeal community, followed by Methanosphaera, Methanosarcina, and Methanomassiliicoccus. While total abundances of Archaea and Bacteroidota were not significantly different among groups, specific taxa within these phyla showed marked changes.
Discussion: The inclusion of phyto-additives in the buffalo diet influenced the rumen microbiome composition by reducing methanogen populations, particularly Methanobrevibacter, and enhancing fiber-degrading microbial communities. These microbial shifts were associated with improved fiber utilization and decreased methane emissions. Rotating the additives every 15 days appeared to sustain their efficacy over time, potentially by preventing microbial adaptation. This approach may offer a sustainable strategy to optimize rumen function and reduce enteric methane emissions in ruminants.
Introduction
Metagenomics is the analysis of the entire genetic material of microbiota in a living environment and is an important way to understand the taxonomic and functional characterization of intestinal microbiota. Animal gut microbiota, often termed the second genome, exhibit a strong association with host genetics. The bovine gastrointestinal system harbors a rich and diverse microbial community, where microbes from the rumen and gut play a critical role in host metabolism and overall health. These microbes facilitate energy production by fermenting undigested feed, and even slight alterations in their composition can profoundly influence nutrient absorption and livestock productivity. Numerous studies have reported that microbiota present in the gastrointestinal tract is closely related to cattle lactation, fat deposition, feed efficiency, and disease (1, 2). Although metagenomics has been used in various animals, such as humans, pigs, and mice, few studies using it to investigate cattle with different RFI phenotypes and correlated microbiota abundance with host gene expression have been reported.
The unique fermentation capability of the rumen is bestowed on microbes residing in their gut. These microbes produce an array of enzymes that utilize the forage and feed and, in turn, produce metabolites such as volatile fatty acids (VFAs), ammonia, lactic acid, CO2, methane, and hydrogen. These metabolites are the precursors for animals’ maintenance and their products. In recent years, plant secondary metabolites (PSMs) have long been claimed to favorably modulate rumen microbiome and its fermentation. Samal et al. (3) reported reduced methane and better feed efficiency among buffaloes fed with garlic, ajwain, harad, and soapnut. Wadhwa and Bakshi (4) and Chaturvedi et al. (5) found similar benefits with ajwain oil and herbal blends. A 2025 goat study showed a 22–33% lower methane with ajwain oil and bahera (6). However, the effect of these PSMs has been highly variable due to differences in the active principles, making it difficult to make concrete (7). In such a scenario, it becomes imperative to assess the PSMs under in vivo conditions to see how the PSM changes the rumen microbiome, fermentation pattern, and what degree of interdependence or correlations exist between these factors. In the present study, a blend of phyto feed additives using Allium sativum (garlic) bulb, Trachyspermum ammi (ajwain) seeds, essential oil, Terminalia chebula (harad) pulp, and Sapindus mukorossi (soapnut) nuts was used to study the above-mentioned objectives. Allium sativum is known for its organosulfur compounds such as allicin, diallyl sulfide, and ajoene, which possess antimicrobial, antioxidant, and immunomodulatory effects (8, 9). Trachyspermum ammi seeds and essential oil are rich in thymol, γ-terpinene, and p-cymene, offering strong antimicrobial, antifungal, and digestive properties (10, 11). Terminalia chebula contains tannins, gallic acid, chebulagic acid, and ellagic acid, which exhibit potent antioxidant, antimicrobial, and gut-toning effects (12, 13). Sapindus mukorossi is primarily composed of saponins and triterpenoids, known for their surfactant, anti-protozoal, and microbial population-modulating properties (14, 15). Together, these phytogenic additives offer a wide range of secondary metabolites that can potentially alter the microbial ecosystem of the rumen, suppress methanogenesis, enhance fermentation efficiency, and improve nutrient utilization.
Materials and methods
Experimental animals
Six male buffalo calves, 12–15 months of age with an average body weight of 165 ± 4 kg, were divided into two groups based on body weight in a completely randomized design. Animals were housed in the Animal Nutrition shed of the Indian Veterinary Research Institute, Izatnagar. Prior to the initiation of the experiment, animals were treated against ecto- and endo-parasites according to the standard protocol. The feeding trial was continued for 6 months.
Feeding management and treatment
Animals were kept individually in a well-ventilated shed with a cement floor and were fed a concentrate mixture and wheat straw in a 50:50 ratio, targeting a growth rate of 500 g/d as per ICAR (2013). The concentrate mixture of 20% protein comprised 35% maize, 24% soybean meal, 38% wheat bran, 2% mineral mixture, and 1% salt on a DM basis. The wheat straw was offered after the concentrate mixture was consumed. To meet the vitamin requirement, 5 kg of chopped green maize per animal was provided once a week. Fresh and clean drinking water was made accessible ad libitum twice a day, at 9 AM and 5 PM.
The additives used in the present study were FAI, a blend of garlic (Allium sativum), ajwain (Trachyspermum ammi), harad (Terminalia chebula), and soapnut (Sapindus mukorossi) in equal proportion at the rate of 1% of DMI and FAII, ajwain oil, at a rate of 1 mL/kg DMI alternatively every 15 days for 6 months. The FAs were mixed well with the concentrate mixture. The two treatments were Con- Control and Treat-Treatment, which were fed alternatively every 15 days for 6 months. The additive PFAI was a powder of Allium sativum bulb (garlic), Trachyspermum ammi seeds (ajwain), Terminalia chebula pulp (harad), and Sapindus mukorossi nut (soapnut), mixed in equal proportion, and PFAII was Trachyspermum ammi essential oil. The four plant parts used in PFAI were dried, powdered, and then mixed in equal proportions. The PFAs were mixed well with the concentrate mixture daily for the entire experimental period and ensured the complete consumption of conc. mix. Chopped wheat straw was offered after the concentrate mixture was completely consumed.
Sampling of rumen liquor
The rumen liquor was collected from three buffaloes of each group by using a stomach tube at the end of the feeding trial to assess the diversity of the rumen microbiome and its related functions. The rumen liquor was collected 2 h after feeding and then mixed with an equal volume of RNA later and then stored at - 80°C till further use. After that, next-generation sequencing for all the samples was performed on the Illumina Platform with a chemistry of 2 × 150 base pair (bp). The master denovo assembly to prepare contigs was carried out for all six samples using CLC Genomics Workbench v6.1, and FASTA sequences were generated. The statistical components of the assemblies were determined using custom Perl scripts developed in-house.
Gene prediction
The master assembled transcripts were subjected to coding gene prediction using Prodigal (v2.6.3), considering the metagenome gene prediction method. To determine the genes expressed in each sample, reads of each sample were mapped to these predicted genes using bwa v0.7.12. The genes having reads mapped were considered expressed for that particular sample. The identified genes for each sample were then subjected to further taxonomic and functional analysis.
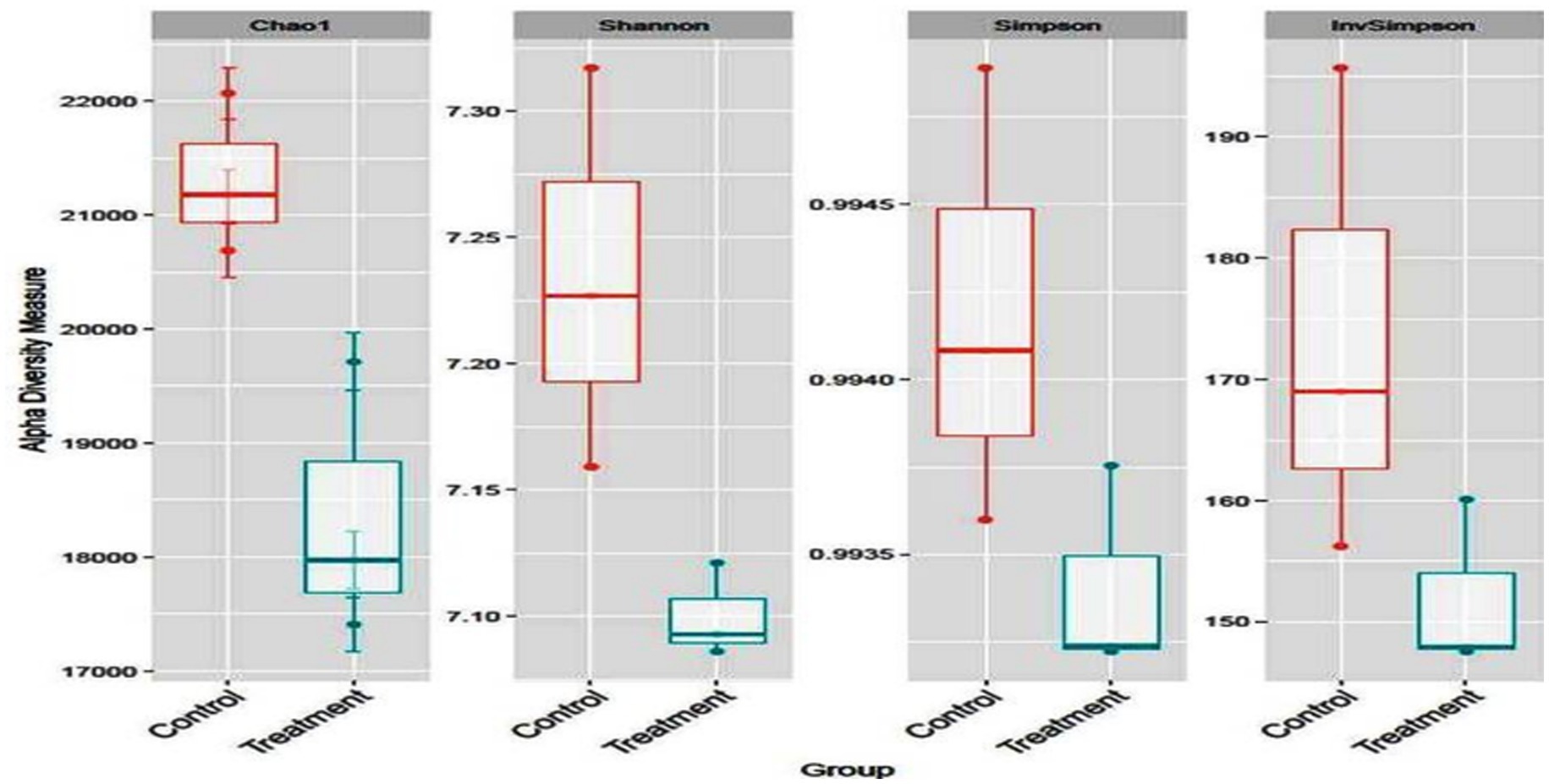
Taxonomic annotation of CDS
Kaiju is an efficient and standalone metagenome classifier that uses the Burrows–Wheeler transform algorithm to detect the highest exact protein-level matches. It classifies individual metagenomic reads by referencing a database of annotated protein-coding genes from various microbial genomes, which are primarily sourced from NCBI RefSeq. The final gene sequences obtained from prodigal were taken as input in Standalone Kaiju for assessing the taxa of the rumen microbial sequences under study with Database-Nr, Run mode-Greedy, Minimum match score-75, and Allowed mismatches-5. The output file obtained from KAIJU, having taxonomy assigned information, was converted to OTU and taxa using R. Eventually, these data files in control and treatment were converted into a phyloseq object to derive alpha diversity using “estimate_richness” of the phyloseq package. Alpha diversity was obtained in terms of Chao1, Shannon, Simpson, and Inv Simpson, and was expressed in the form of a boxplot using the “plot_richness” function. Data filtration and normalization were performed, and the OTUs were divided into the Bacteria and Archaea domains. Other taxonomy analyses for the phylum, genus, and species were carried out using MicrobiomeAnalyst, using the OTU table, taxa table, and metadata files. Feature filtration was performed for bacteria as well as archaea using a low-count filter with a minimum count of 2 and a prevalence of 10% of the samples. A low variance filter was applied to remove features having an inter-quantile range (IQR) of 5%. Core microbiome analysis was carried out using MicrobiomeAnalyst using the following criteria: sample prevalence (%): 20% relative abundance (%): 0.01. The core microbiome was identified at the genus level for the bacterial domain.
Network analysis for microbial genus and functional parameters
Correlation Network was constructed at the phylum and class level for the bacterial domain using the Spearman’s rank correlation algorithm at a p-value threshold of 0.001 and a correlation threshold of 0.5. For archaea, a correlation network was constructed at the phylum and class levels for the archaeal domain using the Spearman’s rank correlation algorithm at a default p-value threshold of 0.05 and a correlation threshold of 0.3. Here, each node represents a taxon with its color based on the mean abundance and its size based on the number of connections to that taxon. Two taxa are connected by an edge if the correlation between the two taxa meets the p-value and correlation threshold. The edge size also reflects the magnitude of the correlation. Green color represents the control group, whereas orange color represents the treatment group.
CAZyme analysis
The CAZyme database was downloaded from dbCAN2. The protein sequences obtained from the prodigal were searched for similarity using BlastP against CAZyDB with an E-value of 1e-06. To estimate CAZyme profiles, reads were mapped to CDS having CAZy information using bwa v0.7.12 and quantified using Samtools v1.4.1. Thus, the abundance of all CAZymes was calculated. A matrix having 604 CAZyme families with read count for all six samples was provided as the shotgun data profiling (SDP) option of MicrobiomeAnalyst to calculate PCA, LEfSe, and differential analysis. The features for CAZymes were filtered at default parameters, i.e., a low count filter of minimum count 4, i.e., a low count filter of minimum count 4, and prevalence in 20% samples was applied, i.e., for any feature to be retained, at least 20% of its values should contain at least 4 counts. By default, a low variance filter was applied to remove features having an inter-quantile range (IQR) of 10%. Some features were removed based on low abundance and prevalence. Finally, these filtered features were normalized using the default option of cumulative sum scaling (CSS) and used for analysis.
Diversity at the phylum, order, family, genus, and species levels for bacteria
Bacterial domain
Due to the presence of a large number of classifications present for the bacterial domain, the groupwise top 50 phylum, class, order, family, genus, and species were plotted for ease of representation. Furthermore, linear discriminant analysis (LDA) effect size (LEfSe) was estimated at phylum and genus levels for the bacterial domain using the LEfSe option of MicrobiomeAnalyst with a p-value of 0.05 and log LDA score of 2. The top 15 significant differential features at the phylum and genus levels were plotted.
Diversity at the phylum, order, family, genus, and species levels for archaea
Archaeal domain
As very limited OTUs were representing the archaeal domain, group-wise all phylum, class, order, family, genus represented by the archaeal domain were plotted, except for species. At the species level, the top 100 most abundant were plotted for ease of presentation. Linear discriminant analysis (LDA) effect size (LEfSe) was estimated at the phylum, genus, and species levels for the archaeal domain using the LEfSe option of MicrobiomeAnalyst with a p-value of 0.05 and a log LDA score of 2. Significant differential features at the phylum, genus, and species levels plotted were 12, 16, and 15, respectively.
Principal coordinate analysis for bacterial genus and archaeal species
The distance method used was the Bray-Curtis index, the taxonomic level was genus for bacteria and species for archaea, and the statistical method used was permutation MANOVA (PERMANOVA).
Functional annotation
To study the functional relationship to the observed rumen microbiome and to assess the functional capacities of microbial communities, functional annotation was conducted using COGNIZER (v0.9b) at default parameters, taking the predicted genes for each sample as input. Cognizer is an all-in-one, standalone framework designed to simultaneously deliver COG, KEGG, Pfam, GO, and FIG fam annotations for individual sequences within metagenomic datasets. The results obtained from Cognizer were further analyzed using MicrobiomeAnalyst.
KEGG pathway analysis
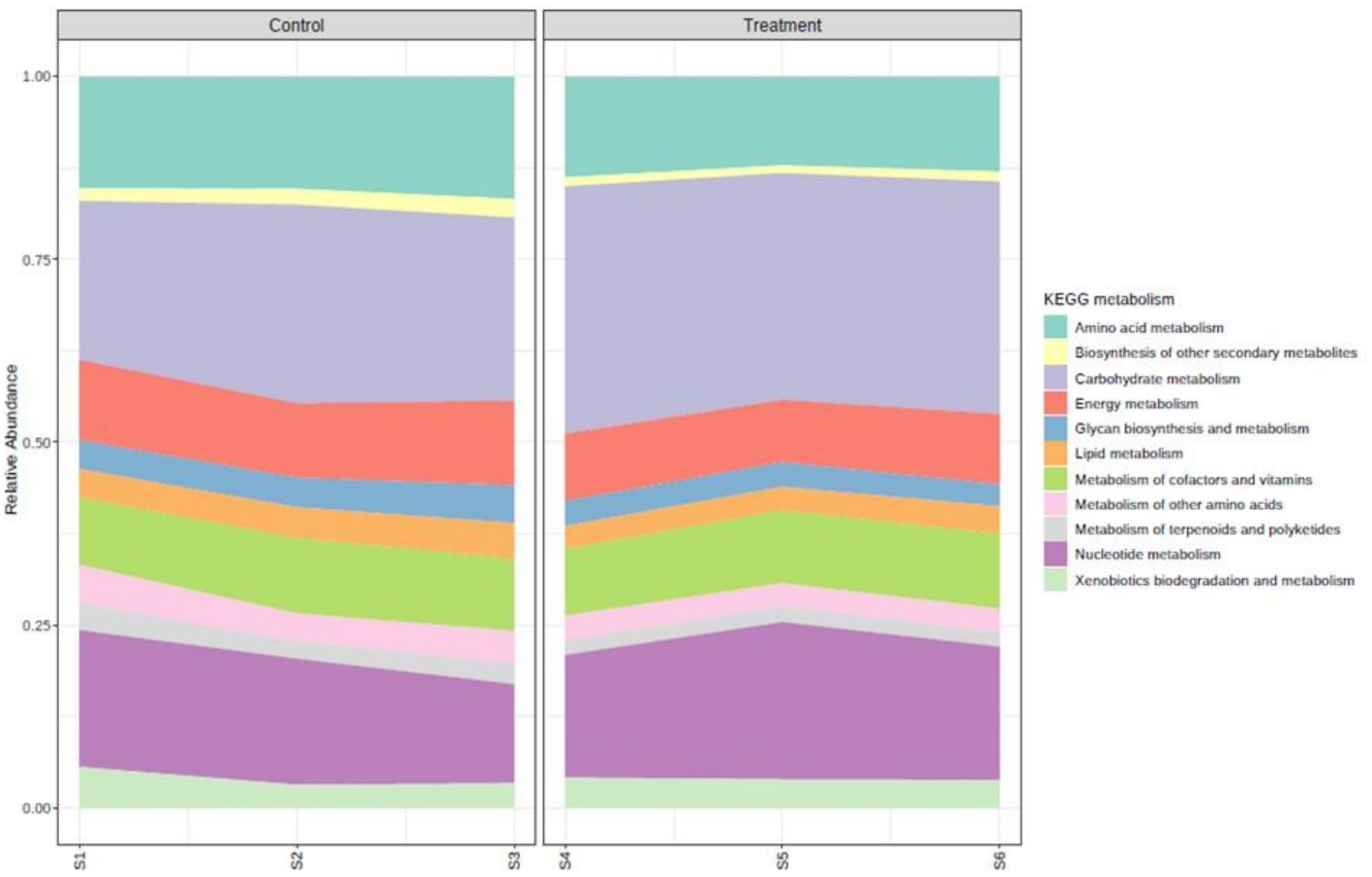
For metabolic pathway analysis, reads were mapped to CDS having KEGG information using bwa v0.7.12 and quantified using Samtools v1.4.1. Thus, the abundance of all pathways was calculated. A matrix having 3,866 KO IDs with read count for all six samples was provided as the shotgun data profiling (SDP) option of MicrobiomeAnalyst to calculate PCA, LEfSe, and differential analysis. The features were filtered using default parameters, including a low count filter (minimum count of 4 and presence in at least 20% of the samples). Additionally, a low variance filter was applied to remove features with an inter-quantile range (IQR) below 10%. Finally, these filtered features were normalized using the default option of cumulative sum scaling (CSS) and used for analysis. Functional diversity profiling was carried out for KEGG KO IDs. The category abundance was calculated based on total hits and grouped by control and treatment. Functional diversity profiling was carried out using three functional categories, namely KEGG metabolism, KEGG pathways, and COG functional categories.
Linear discriminant analysis (LDA) effect size (LEfSe)
LEfSe analysis was carried out at a p-value cutoff of 0.05 and a log LDA score of 2.0. A total of 28 significant features were found and plotted in the form of a barplot. It contained a total of 15,666 bacterial OTUs (OTUs with count > = 2 are 13,811) and 433 archaeal OTUs (OTUs with count > = 2 are 369).
Feature filtration
After filtration, the following were the stats:
(A) For bacteria a total of 3,977 low-abundance features were removed based on prevalence. A total of 492 features exhibiting low variance were discarded based on the interquartile range (IQR). The number of features remaining after the data filtration step is 9,342. Finally, these filtered features were normalized using default, total sum scaling (TSS), and used for analysis.
(B) For archaea a total of 184 low-abundance features were removed based on prevalence. A total of 10 low-variance features were removed based on IQR. The number of features remaining after the data filtration step is 175. Finally, these filtered features were normalized using default, total sum scaling (TSS), and used for analysis.
Statistical analysis
Alpha diversity was derived using “estimate_richness” of the phyloseq package. Taxonomy analysis was carried out using MicrobiomeAnalyst using OTU table, taxa table, and metadata file.
Results and discussion
Metagenomic analysis of a highly complex rumen microbiome provides a holistic approach to explore both cultivable and non-cultivable microbes. This experiment aimed at studying the changes in the microbial community structure of rumen microbiota patterns through the supplementation of additives in buffaloes.
Diversity at the phylum, order, family, genus, and species levels for bacteria
Alpha diversity was obtained in terms of Chao1, Shannon, Simpson, and Inv Simpson. It was similar across all the groups (p = 0.093) showing a trend where higher diversity was obtained for the treatment group than control (Figure 1). Many metagenomic studies on the rumen microbiome reported similarity of abundance of alpha and beta diversity of rumen microbiome and predominance of Bacteroidetes and Firmicutes (at the phylum level) and Prevotella (at genus level) in buffaloes fed on various proportions of concentrate and roughage (28) and TDN (16) and in nitrate supplemented steers at 0, 1, and 2% (29).
Bacterial domain
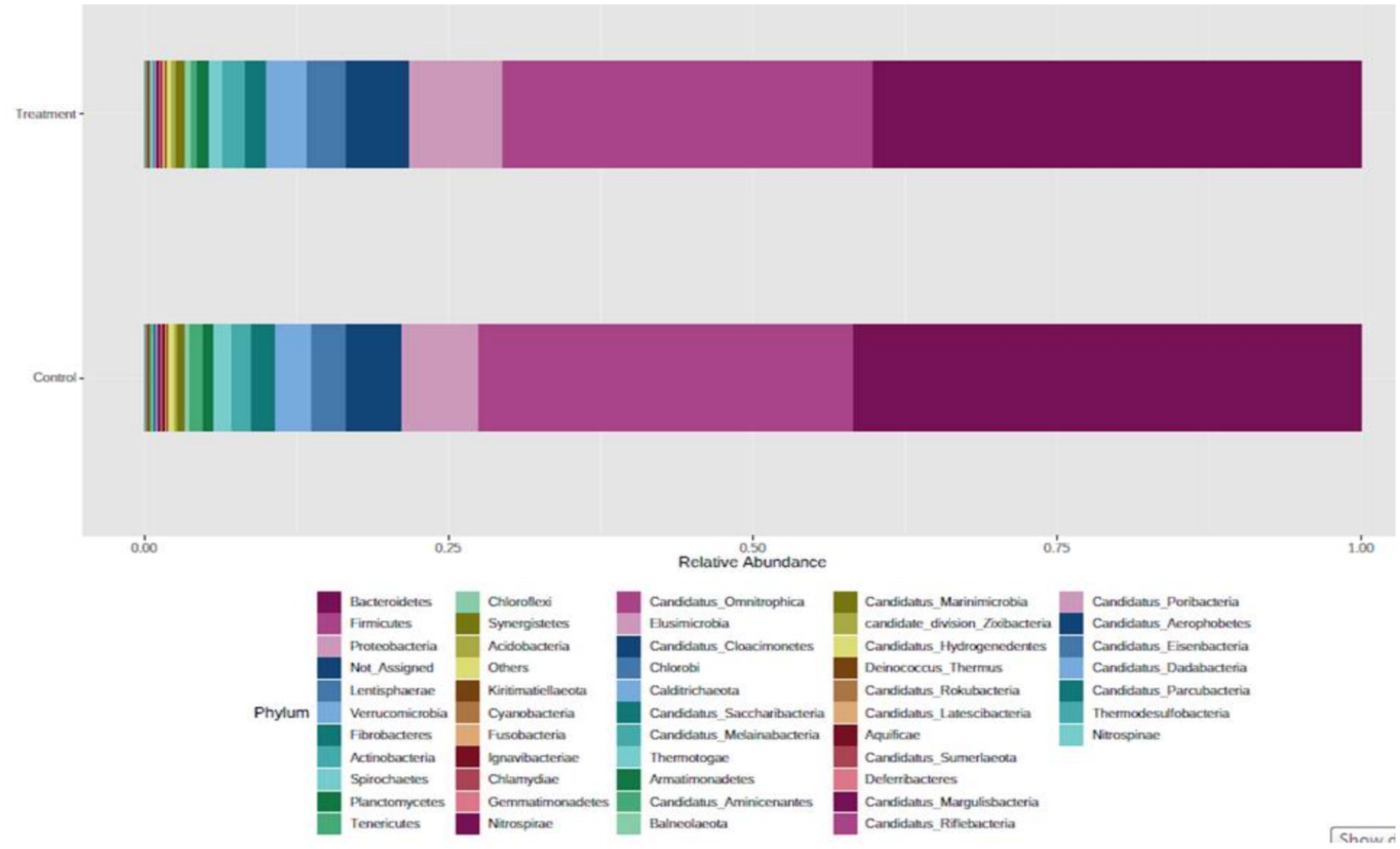
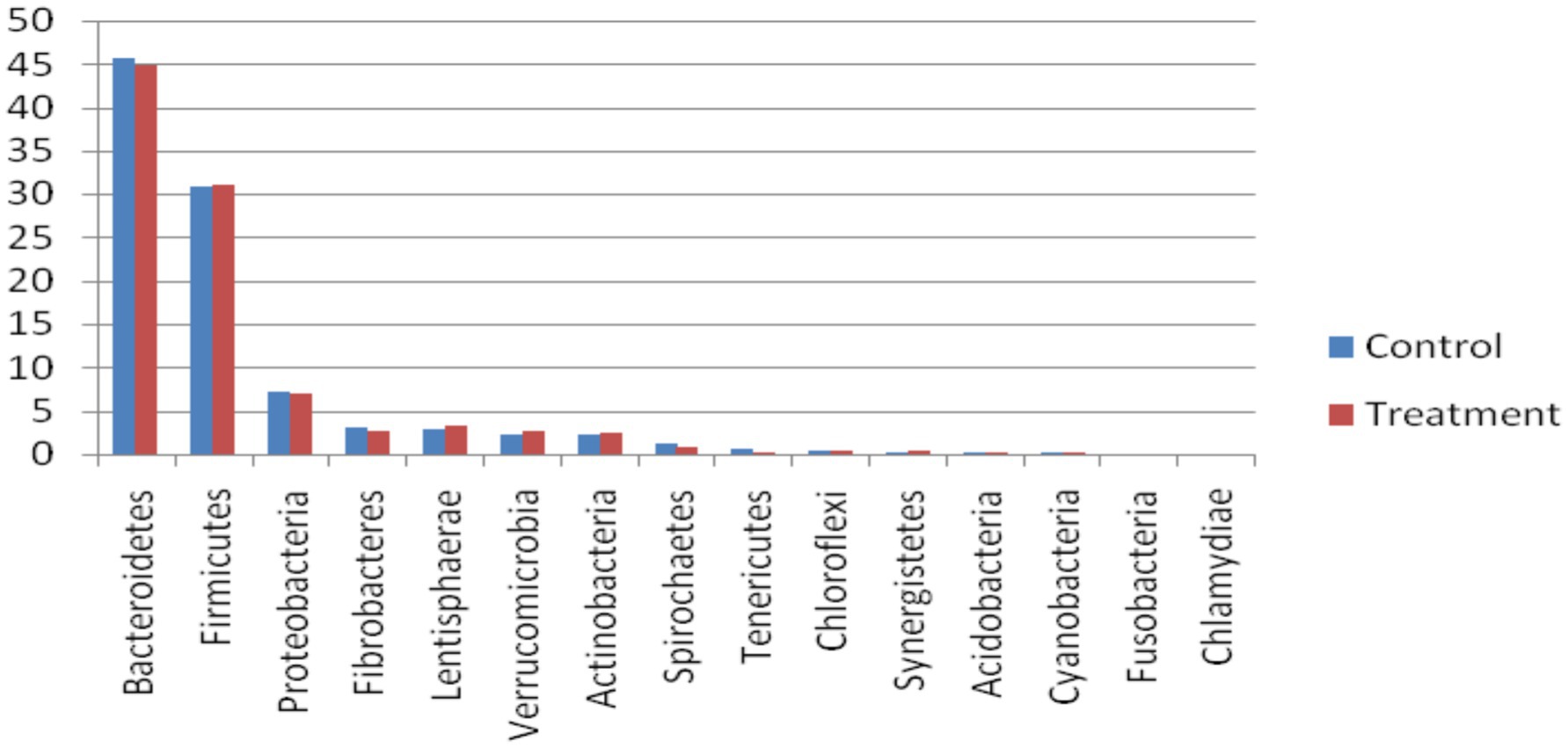
In the bacterial domain, at the phylum level, Bacteroidetes was the most abundant phylum, followed by Firmicutes, Proteobacteria, Lentisphaerae, Verrucomicrobia, Actinobacteria, and Fibrobacteres (Figure 2). At the class level, Bacteroidia was most abundant, followed by Clostridia, Gammaproteobacteria, Negativicutes, Bacilli, Flavobacteria, and Fibrobacteria. A good proportion of bacteria were placed in the not assigned category, indicating that the rumen microbes remain unknown at large. Similar to our observations, Nardi et al. (17) reported that bacterial phyla, such as Bacteroidetes, Firmicutes, Tenericutes, and Euryarchaeota, were abundant (> 1%), and Fibrobacteres were less abundant (0.1–1%) in the rumen of control, polyphenol, and organic acid supplemented heifers. The highest abundance of Bacteroidetes and Firmicutes has been reported by several researchers (16, 18–20).
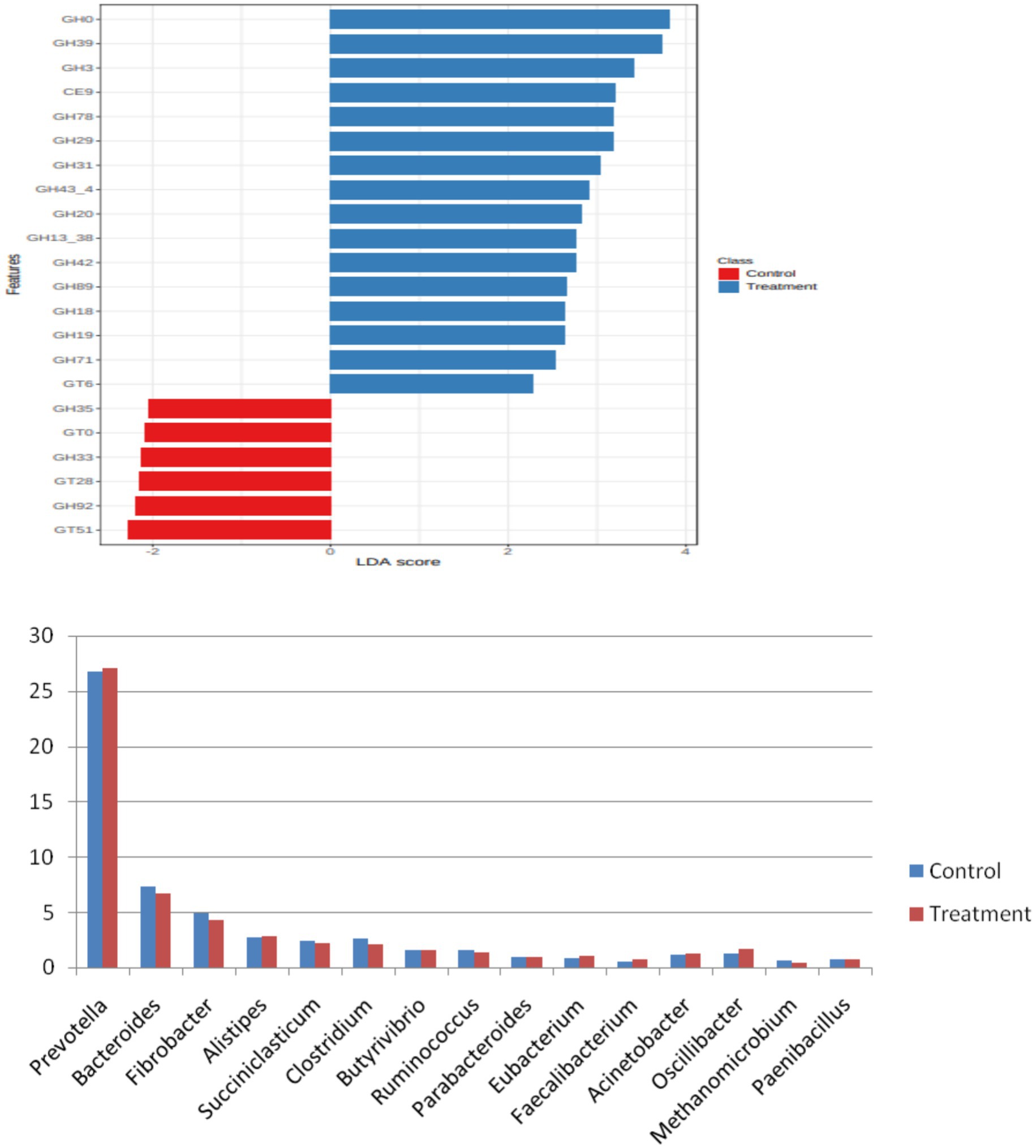
When mined at the family level, the most abundant chunk of reads was assigned to the ‘not assigned’ group, followed by families Prevotellaceae, Lachnospiraceae, Bacteroidaceae, Ruminococcaceae, and Clostridiaceae. These other top 50 family members are presented in Figure 2. At the genus level among the most abundant 50 genera, Prevotella was the major abundant genus after the ‘not assigned’ group, followed by Bacteroides, Succiniclasticum, Alistipes, Fibrobacter, Clostridium, Oscillibacter, Acinetobacter, Ruminococcus, Butyrivibrio, and Eubacterium. Fibrobacter and Ruminococcus have long been thought to be the major microbes for fiber degradation in the rumen, but their lower abundance (%) hints that there are microbes in the rumen other than these well-established fibrolytic bacteria, which play a crucial role in fiber utilization. Ruminococcus breaks down hemicellulose, pectin, and cellulose in the plant cell wall by producing a variety of cellulolytic and hemicellulolytic enzymes (21). Similarly, Stevenson and Weimer (22) found that key fibrolytic bacteria, such as R. flavefaciens, R. albus, and F. succinogenes, accounted for only approximately 2% or less of the ruminal bacterial 16S rRNA. In the present study, Bacteroides was the second most abundant bacteria in the rumen, followed by Prevotella. Naas et al. (23) have reported that Bacteroides has cellulolytic activity and the presence of polysaccharide-utilizing loci (PUL), a system of lignocellulosic feed utilization other than extracellular and cell-bound cellulosomes in their genome. This finding strengthens the hypothesis of the presence of an alternate mechanism of fibre utilization (used by other rumen microbes) in the rumen other than that of Fibrobacter and Ruminococcus species.
Furthermore, the LDA and LEfSe were estimated at the phylum and genus levels for the bacterial domain, with a p-value of 0.05 and a log LDA score of 2, identified 18 significant phyla and 274 significant species that varied between the two groups. The top 15 significant differential features included Bacteroidetes, Tenericutes, Spirochaetes, and Thermotogae in control, whereas in treatment, Proteobacteria, Lentisphaerae, Actinobacteria, Kiritimatiellaeota, and Chlamydiae were found to differ in the two groups. At the genus level, Succiniclasticum, Clostridium, Coprobacillus, Butyrivibrio, Paludibacter, Parabacteroides, Anaeroplasma in the control, and Oscillibacter, Acinetobacter, Escherichia, Enterococcus, Pseudoflavonifractor, Kangiella, and Flavonifractor in the treatment group were different. In the rumen, a higher intake of dietary concentrate caused an increase in the abundance of Proteobacteria, while the levels of Bacteroidetes and Firmicutes were reduced (24).
The core microbiome assessment of the two groups was done individually and in combination to have an idea of the core group of rumen bacteria that were essentially present in all experimental animals. It was observed that in control, the core microbiome consisted of genera such as Prevotella, Bacteroides, Succiniclasticum, Fibrobacter, Clostridium, Alistipes, Ruminococcus, and Butyrivibrio. During treatment, the core microbiome remained similar to the control with some exceptions. In treatment, Butyrivibrio was not part of the treatment core microbiome, whereas Oscillibacter and Actinobacteria were additionally found in the core microbiome of treatment but not control. Furthermore, the abundance of Fibrobacter and Ruminococcus was higher in the control group than in the treatment group. However, the largest proportion of the core microbiome remained ‘unassigned’, showing that the core microbiome in ruminants comprises many species that remain unknown in large numbers. Hence, unknown microbes and the metabolic pathways they adopt may be the reason for not finding any change in the feed digestibility of animals despite a change in the relative number of important known fibre degraders in the treatment group compared to the control.
Archaeal domain
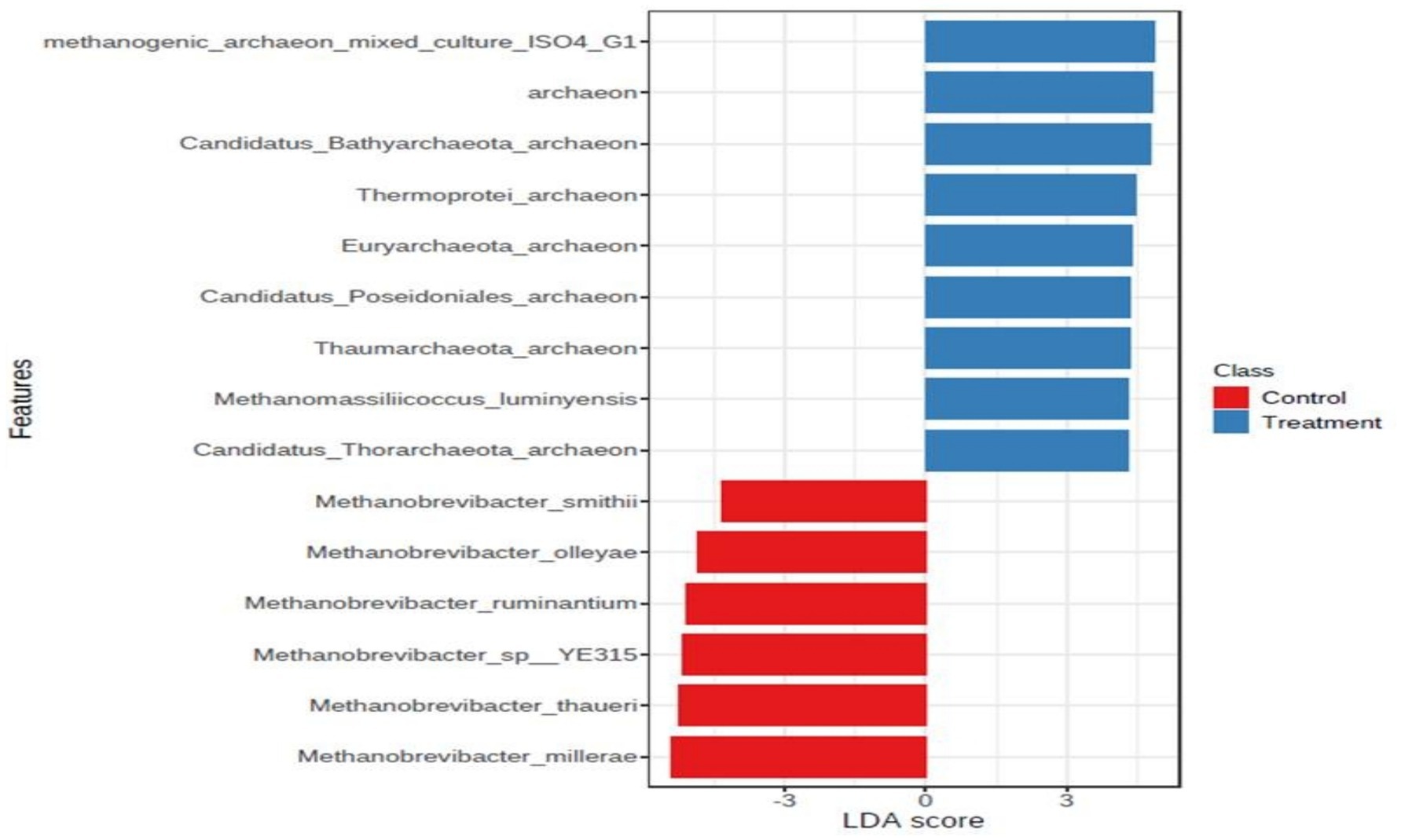
In archaea, at the phylum level, Euryarchaeota was the most abundant phylum, constituting more than 85% of the abundance. The other major phyla included Candidatus_Bathyarchaeota, Crenarchaeota, Candidatus_Lokiarchaeota, Candidatus_Woesearchaeota, and Thaumarchaeota. In the archaeal phylum, a very major share was possessed by the ‘Not Assigned’ group. At the genus level, Methanomicrobium and Methanobrevibacter were the most abundant genera, to a tune of 30% each followed by Methanosphaera, Methanosarcina, the genus level, Methanomicrobium and Methanobrevibacter were the most abundant genera, to the tune of 30% each, followed by Methanosphaera, Methanosarcina, and Methanomassiliicoccus, to name a few. At the species level, Methanomicrobium_mobile was the most abundant species with an abundance of 30%. The other major species were methanogenic_archaeon_mixed_culture_ISO4_G1, Methanomassiliicoccales archaeon Mx 02, Methanobrevibactermillerae, Methanobrevibacter_sp__YE315, Methanobrevibacter_thaueri, methanogenic_archaeon_ISO4_H5, Methanomassiliicoccales_archaeon_Mx_06, Methanobrevibacter_ruminantium, Methanobrevibacter_olleyae, Methanosphaera_sp__BMS, Methanobrevibacter_oralis, Methanosarcina_acetivorans, and Methanobrevibacter_smithii. It was noticeable that the composition of archaea within the archaeal niche was different for control and treatment. At the phylum level, Euryarchaeota was more abundant in control than the phylum level, Euryarchaea were more abundant in the control than in the treatment. The Euryarchaeota phylum contains most of the methanogenic archaea, and its decrease in control indicates that the additive affected some selective species of the archaeal community. At the genus level, the abundance of Methanobrevibacter was lower in the treatment group than in the control. Interestingly, it appeared that the niche emptied by Methanobrevibacter was occupied by other archaea such as Methanomicrobium and some unassigned sequences, as their number was higher in the treatment group than control. Methanosphaera was also higher in treatment group than the control. Methanosphaera is known to utilize hydrogen without producing methane. So, it is evident that the supplementation of the additive restructured the archaeal abundance within the niche and decreased the abundance of microbes involved in methane production and favored microbes, which decreased methane emission. At the species level also, the species belonging to Methanobrevibacter, such as Methanobrevibacter millerae, Methanobrevibacter_sp__YE315, and Methanobrevibacter_thaueri were decreased in the treatment group compared to the control, clearly shown in the LDA score (Figure 3).
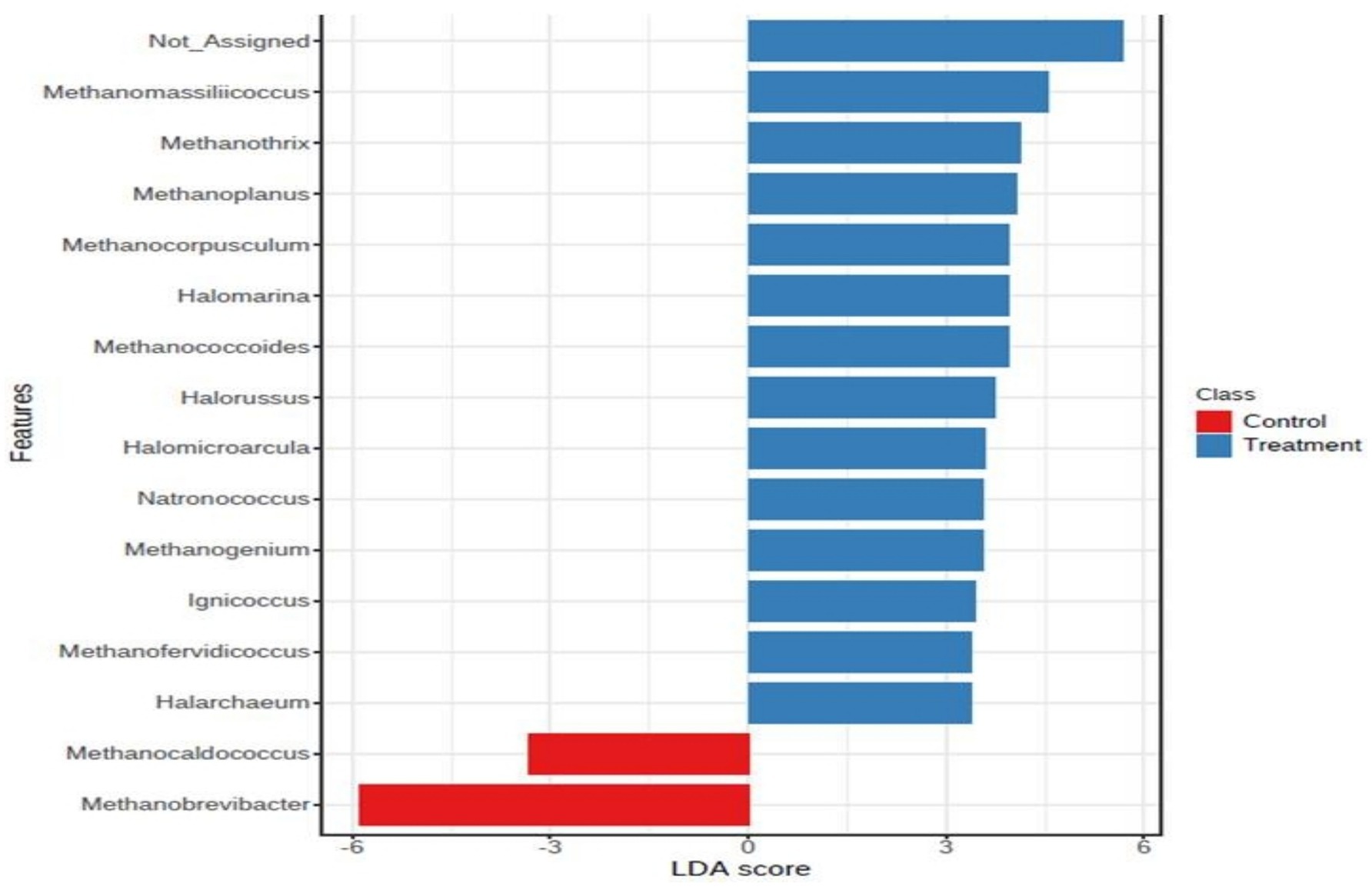
The core microbiome of archaea at species level clearly showed that the core microbiome was majorly contributed by Methanomicrobium_mobile, Methanobrevibacter millerae, Methanobrevibacter_sp__YE315, Methanobrevibacter_thaueri, Methanobrevibacter_ruminantium, and Methanobrevibacter_olleyae, whereas the treatment core microbiome consisted of Methanomicrobium_mobile, methanogenic_archaeon_mixed_culture_ISO4_G1, Methanomassiliicoccales_archaeon_Mx_02, Methanobrevibacter_millerae, Methanobrevibacter_sp__YE315, methanogenic_archaeon_ISO4_H5, Candidatus_Bathyarchaeota_archaeon, Methanobrevibacter_thaueri, and methanogenic_archaeon_ISO4_H5, archaeon (Figure 4).
KEGG pathway analysis
A total of 290 low-abundance features were removed based on prevalence. A total of 349 low-variance features were removed based on IQR. The number of features remaining after the data filtration step is 3,134. The KEGG pathway analysis revealed that a total of 133 pathways were observed to be expressed in the experimental animals without any effect of treatment (Figure 5). The major KEGG metabolism pathways that were highly expressed in order of abundance in both experimental groups were amino acid metabolism, biosynthesis of other secondary metabolites, carbohydrate metabolism, energy metabolism, glycan biosynthesis and metabolism, lipid metabolism, metabolism of cofactors and vitamins, metabolism of other amino acids, metabolism of terpenoids and polyketides, nucleotide metabolism, and xenobiotics biodegradation and metabolism. The expression of these pathways did not vary between the two groups.
LEfSe analysis was carried (p-value cutoff 0.05 and log LDA score of 2.0). A total of 28 significant features for KEGG metabolism pathways were found to be different in the treatment group than the control (Figure 5).
CAZyme analysis
Rumen fluid harbors complex of enzymes which work in synergistic manner to bring about fiber utilization and these enzymes are contributed by rumen microbes. The CAZyme class majorly includes glycoside hydrolases (GHs), which hydrolyze glycosidic bonds of carbohydrates, cellulose binding modules (CBMs), involved in enzyme substrate binding, carbohydrate esterases (CEs), which break the ester bonds between lignin and carbohydrates in the rumen. LEfSe analysis was carried out at a p-value cutoff of 0.05 and a log LDA score of 2.0. A total of 22 significant features was found and plotted in the form of a barplot. The differential CAZyme analysis showed 101 CAZymes to be differentially expressed in treatment and control. The top 20 are plotted in Figure 6. These differentially expressed families mainly included the GH family, which is mainly involved in the deconstruction of fibrous feed in the rumen. On the other hand, members of GT and CBM groups were also among the differentially expressed families. This indicated that the supplementation of additives modulated the expression of fibre-degrading enzymes in the rumen. The dominant phyla in the rumen of Holstein cattle include Bacteroidota, constituting about half, and Firmicutes, accounting for approximately a third of the total. Archaea are reported to constitute only a small quantity, equivalent to 0.5–1% of the total DNA in the rumen microbiome of Holstein cattle (25). Carbohydrate processing enzymes (CAZymes) are classified into families (CAZy families) based on their amino acid sequence similarity (26, 27). Archaea are notoriously known to encode few CAZymes, whereas bacterial species belonging to, e.g., Bacteroidota and Firmicutes are endowed with a strong palette of various CAZymes (Figure 7).
Conclusion
The study explored the effects of feed additives on the rumen microbiome of buffaloes, highlighting significant changes in microbial community structure and functional capabilities. In the bacterial domain, Bacteroidetes and Firmicutes were the dominant phyla, with Prevotella as the most abundant genus, while other fibrolytic microbes displayed reduced abundance. These findings suggest the presence of alternate fiber-degrading pathways apart from traditional cellulolytic microbes such as Fibrobacter and Ruminococcus. Archaeal analysis revealed a reduction in methanogenic archaea, particularly Methanobrevibacter, in the treatment group, indicating the additive’s role in reducing methane production. Core microbiome analysis identified common genera but also highlighted novel unassigned microbes playing crucial roles in rumen function. KEGG pathway analysis showed consistent metabolic pathways across all groups, while CAZyme analysis revealed enhanced fiber-degrading enzyme expression in the treatment group. Overall, the study demonstrates that phyto feed additives modulate the rumen microbiome, reduce methane production, and potentially enhance fiber utilization, contributing to sustainable livestock management practices.
Data availability statement
The data presented in the study are deposited in NCBI BioSample repository, under the accession numbers SAMN52663503 and SAMN52663504.
Ethics statement
The study was conducted at ICAR-Indian Veterinary Research Institute, Izatnagar-243122, Uttar Pradesh (India). All experimental protocols were approved (protocol number IAEC/26.07.2021/L4 dated 12.01.2022) as per the guidelines established by Institutional Animal Ethics Committee constituted (IAEC) under Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India vide letter no. V-11011 (13)/19/2021- CPCSEA-DADF dated 07.01.2022.
Author contributions
PS: Formal analysis, Investigation, Methodology, Writing – original draft. AK: Conceptualization, Data curation, Software, Validation, Writing – review & editing. PA: Data curation, Formal analysis, Methodology, Software, Writing – review & editing. RD: Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing. HR: Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing. KV: Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing. LC: Conceptualization, Methodology, Project administration, Supervision, Validation, Visualization, Data curation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was financially supported by the Indian Council of Agricultural Research (ICAR) and the International Livestock Research Institute (ILRI) under the ICAR-ILRI collaborative project (Project code: ICAR-ILRI ICA00703).
Acknowledgments
The authors express their gratitude to the Indian Council of Agricultural Research (ICAR) and the International Livestock Research Institute (ILRI) for their financial support through the ICAR-ILRI collaborative project. They also acknowledge the fellowship granted by the University Grants Commission (UGC) to P. K. Soni and the research facilities provided by the Director of ICAR-Indian Veterinary Research Institute, Izatnagar.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Liu, L, Wu, P, Guo, A, Yang, Y, Chen, F, and Zhang, Q. Research progress on the regulation of production traits by gastrointestinal microbiota in dairy cows. Front Vet Sci. (2023) 10:1206346. doi: 10.3389/fvets.2023.1206346
2. Monteiro, HF, Zhou, Z, Gomes, MS, Peixoto, PM, Bonsaglia, EC, Canisso, IF, et al. Rumen and lower gut microbiomes relationship with feed efficiency and production traits throughout the lactation of Holstein dairy cows. Sci Rep. (2022) 12:4904. doi: 10.1038/s41598-022-08761-5
3. Samal, L, Chaudhary, LC, Agarwal, N, and Kamra, DN. Impact of phytogenic feed additives on growth performance, nutrient digestion and methanogenesis in growing buffaloes. Anim Prod Sci. (2016) 58:1056–63. doi: 10.1071/AN15610
4. Wadhwa, M, and Bakshi, MPS. Effect of supplementing total mixed ration with ajwain (Trachyspermum ammi) oil on the performance of buffalo calves. Indian J Animal Sci. (2019) 89:424–30. doi: 10.56093/ijans.v89i4.89144
5. Chaturvedi, I, Dutta, TK, Singh, PK, and Sharma, A. Effect of combined herbal feed additives on methane, total gas production and rumen fermentation. Bioinformation. (2015) 11:261. doi: 10.6026/97320630011261
6. Kumari, N, Kala, A, Agarwal, N, Chaturvedi, VB, Vijayalakshmy, K, and Chaudhary, LC. Effect of plant-derived feed additives on enteric methane emission, nutrient digestibility and immune status of adult male goats. J Anim Physiol Anim Nutr. (2025) 109:908–15. doi: 10.1111/jpn.14105
7. Ku-Vera, JC, Jiménez-Ocampo, R, Valencia-Salazar, SS, Montoya-Flores, MD, Molina-Botero, IC, Arango, J, et al. Role of secondary plant metabolites on enteric methane mitigation in ruminants. Front Vet Sci. (2020) 7:584. doi: 10.3389/fvets.2020.00584
8. Bhatwalkar, SB, Mondal, R, Krishna, SBN, Adam, JK, Govender, P, and Anupam, R. Antibacterial properties of organosulfur compounds of garlic (Allium sativum). Frontiers in Microbiology (2021). 12:613077. doi: 10.3389/fmicb.2021.613077
9. Ding, H, Ao, C, and Zhang, X. Potential use of garlic products in ruminant feeding: a review. Anim Nutr. (2023) 14:343. doi: 10.1016/j.aninu.2023.04.011
10. Kumar, K, Chaturvedi, VB, Kala, A, and Chaudhary, LC (2020). Effect of Feeding Ajwain (Trachyspermum ammi) seed as a feed additive on enteric methane production and rumen ecosystem in Murrah buffaloes. Animal Nutrition and Feed Technology, 20, 257–266. doi: 10.5958/0974-181X.2020.00023.2
11. Sengupta, S, et al. Phytochemical profile and antimicrobial potential of essential oils from Trachyspermum ammi: an updated review. Ind Crop Prod. (2022) 188:115611.
12. Sharma, A, Purena, R, and Behar, N. A review on phytochemicals & therapeutic potential of Terminalia chebula Retz. fruit. Annual Research & Review in Biology (2025) 40:106–117. doi: 10.9734/arrb/2025/v40i42226
13. Kholif, AE, Anele, UY, Patra, AK, and Varadyova, Z. Editorial: The Use of Phytogenic Feed Additives to Enhance Productivity and Health in.The Use of Phytogenic Feed Additives to Enhance Productivity and Health in Ruminants (2021) 4. doi: 10.3389/fvets.2021.685262
14. Kumar, K, Dey, A, Rose, MK, and Dahiya, SS. Impact of dietary phytogenic composite feed additives on immune response, antioxidant status, methane production, growth performance and nutrient utilization of buffalo (Bubalus bubalis) calves. Antioxidants (2022) 11:325. doi: 10.3390/antiox11020325
15. Agarwal, N, Kamra, DN, Chaudhary, LC, and Patra, AK. Effect of Sapindus mukorossi extracts on in vitro methanogenesis and fermentation characteristics in buffalo rumen liquor. Journal of Applied Animal Research (2006) 30:1–4. doi: 10.1080/09712119.2006.9706814
16. Kala, A, Kamra, DN, Agarwal, N, and Chaudhary, LC. Effect of a blend of essential oils on buffalo rumen microbial and enzyme profiles and invitro feed fermentation. Anim Nutr Feed Technol. (2017) 7:189–200. doi: 10.5958/0974-181X.2017.00020.8
17. De Nardi, R, Marchesini, G, Li, S, Khafipour, E, Plaizie, JC, Gianesella, M, et al. Metagenomic analysis of rumen microbial population in dairy heifers fed a high grain diet supplemented with dicarboxylic acids or polyphenols. BMC Vet Res. (2016) 12:29. doi: 10.1186/s12917-016-0653-4
18. Li, F, Li, ZJ, Li, SX, Ferguson, JD, Cao, YC, Yao, JH, et al. Effect of dietary physically effective fiber on ruminal fermentation and the fatty acid profile of milk in dairy goats. J Dairy Sci. (2014) 97:2281–90. doi: 10.3168/jds.2013-6895
19. Durso, LM, Harhay, GP, Smith, TP, Bono, JL, Desantis, TZ, Harhay, DM, et al. Animal-to-animal variation in fecal microbial diversity among beef cattle. Appl Environ Microbiol. (2010) 76:4858–62. doi: 10.1128/AEM.00207-10
20. Mao, S, Zhang, M, Liu, J, and Zhu, W. Characterising the bacterial microbiota across the gastrointestinal tracts of dairy cattle: membership and potential function. Sci Rep. (2015) 5:16116. doi: 10.1038/srep16116
21. Zhang, L, Chung, J, Jiang, Q, Sun, R, Zhang, J, Zhong, Y, et al. Characteristics of rumen microorganisms involved in anaerobic degradation of cellulose at various pH values. RSC Adv. (2017) 7:40303–10. doi: 10.1039/C7RA06588D
22. Stevenson, DM, and Weimer, PJ. Dominance of Prevotella and low abundance of classical ruminal bacterial species in the bovine rumen revealed by relative quantification real-time PCR. Appl Microbiol Biotechnol. (2007) 75:165–74. doi: 10.1007/s00253-006-0802-y
23. Naas, AE, Mackenzie, AK, Mravec, J, Schückel, J, Willats, WG, Eijsink, VG, et al. Do rumen Bacteroidetes utilize an alternative mechanism for cellulose degradation? MBio. (2014) 5:e01401–14. doi: 10.1128/mBio.01401-14
24. Kibegwa, FM, Bett, RC, Gachuiri, CK, Machuka, E, Stomeo, F, and Mujibi, FD. Diversity and functional analysis of rumen and fecal microbial communities associated with dietary changes in crossbreed dairy cattle. PLoS One. (2023) 18:e0274371. doi: 10.1371/journal.pone.0274371.
25. Bharanidharan, R, Lee, CH, Thirugnanasambantham, K, Ibidhi, R, Woo, YW, Lee, H, et al. Feeding systems and host breeds influence ruminal fermentation, methane production, microbial diversity and metagenomic gene abundance. Front Microbiol. (2021) 12:1–17. doi: 10.3389/fmicb.2021.701081
26. Drula, E, Garron, M, Dogan, S, Lombard, V, Henrissat, B, and Terrapon, N. The carbohydrate-active enzyme database: functions and literature. Nucleic Acids Res. (2021) 50:D571–7. doi: 10.1093/nar/gkab1045
27. Lombard, V, Golaconda Ramulu, H, Drula, E, Coutinho, PM, and Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. (2014) 42:D490–5. doi: 10.1093/nar/gkt1178
28. Singh, AK, Chaturvedi, VB, Singh, P, Verma, AK, Chaudhary, LC, and Mandal, T. Assessment of energy metabolism and methane emission in cattle and buffaloes fed TMR diets having different concentrate to roughage ratios. Indian Journal of Animal Sciences (2014) 84: 1216–1222. doi: 10.56093/ijans.v84i11.44791
Keywords: phyto-feed additives, next-generation sequencing (NGS), functional metagenomics, microbial taxonomic shift, buffalo calves
Citation: Soni PK, Kala A, Agarwal P, Deka R, Rahman H, Vijayalakshmy L and Chaudhary LC (2025) Taxonomic and functional shifts in the rumen microbiome of buffalo calves under long-term strategic supplementation of phyto-feed additives. Front. Vet. Sci. 12:1647762. doi: 10.3389/fvets.2025.1647762
Edited by:
Yanfeng Xue, Anhui Agricultural University, ChinaReviewed by:
Ahsan Naveed, University of Kentucky, United StatesSrisan Phupaboon, Khon Kaen University, Thailand
Copyright © 2025 Soni, Kala, Agarwal, Deka, Rahman, Vijayalakshmy and Chaudhary. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lal Chandra Chaudhary, bGNjaGF1ZGhhcnkxQHJlZGlmZm1haWwuY29t
 Pramod Kumar Soni
Pramod Kumar Soni Anju Kala
Anju Kala Payal Agarwal
Payal Agarwal Rampratim Deka
Rampratim Deka Habibur Rahman3
Habibur Rahman3