- 1International Office, Veterinary Medicines Directorate, Department for the Environment, Food and Rural Affairs, Surrey, United Kingdom
- 2Safe Medicines for Animals-Regulatory Training, London, United Kingdom
The regulation of veterinary medicines is important for animal health and welfare, for human health, for sustainable food production, and for minimising impacts on the environment. The capability to regulate these medicines is therefore also important to provide confidence to stakeholders, particularly the public. Although there is a Global Benchmarking Tool to assess the capability of regulatory bodies for human medicines, developed by the World Health Organisation (WHO), the veterinary regulatory sector lacks a similar global, comprehensive scheme and associated guidance. A review of schemes that address veterinary medicines regulatory bodies was undertaken and compared to the WHO scheme to develop a proposed scheme for regulators of veterinary medicines. This new tool will provide a comprehensive and systematic approach to strengthening regulatory systems, fostering harmonisation, and ensuring the quality, safety, and efficacy of veterinary medicinal products.
1 Introduction
Veterinary medicines play a critical role in preventing and treating animal diseases. Consequently, they are essential for supporting livestock production, an industry that has an estimated 1.3–1.7 billion people reliant on the sector for their livelihoods, of which approximately 930 million are specified as low-income Africans and South Asians (1, 2).
Effective regulation of veterinary medicines is essential to ensure their quality, efficacy, and safety. Broadly, these regulations cover animal field trials; dossier review for efficacy, safety, quality; product labelling as part of considering licensing of the product; assessment of adverse events post-marketing; compliance with good manufacturing and good distribution practices; batch release testing certification for vaccines; and monitoring of veterinary medicines residues in food products of animal origin intended for human consumption. These services are provided by national regulatory agencies, and in some cases by a regional medicine regulator, e.g., the European Medicines Agency in the European Union.
The maturity of national veterinary medicine regulatory systems varies significantly between countries, leading to inconsistent stakeholder confidence in the regulatory process, especially by the pharmaceutical industry, which discourages manufacturers from bringing products to markets with an inefficient regulatory system. Pharmaceutical manufacturers are more likely to invest and bring products to markets with efficient and mature regulatory systems.
It has long been recognised for human medicines regulation that a means of assessing the capability of human medicine regulatory authorities is required because of the important gateway assurance function they perform. Consequently, the World Health Organisation established in 1997 a Global Benchmarking Tool for a uniform and standardised way to assess capability (3).
The value of benchmarking to good regulation is that it informs and prioritises where development is needed, provides a way of measuring the success of that development, supports medicines pre-qualification programmes, helps identify the best-suited regional leads for mutual recognition of medicines activities, and supports opportunities for mutual and unilateral reliance on the licencing approvals of other regulators.
There is no global benchmarking scheme for regulatory authorities for veterinary medicines. This has been identified as a weakness (4).
This study aimed to identify and develop a suitable self-assessment/benchmarking tool for veterinary medicines National Regulatory Agencies by reviewing several existing tools used by different institutions in both the human and veterinary sectors, thereby providing countries with a systematic method for strengthening their regulatory systems, fostering regulatory reliance and harmonisation, further assuring animal and public health, and increasing timely access to quality-assured veterinary medicinal products.
2 Materials and methods
Existing assessment and benchmarking tools were analysed and compared to assess their breadth and depth of assessment and applicability to veterinary medicines regulatory functions. These were the World Health Organisation (WHO) Global Benchmarking Tool (GBT) for regulators of human medicines (5), the World Organisation for Animal Health (WOAH) Performance of Veterinary Services (PVS) Pathway tool (6), the European Union Benchmarking of European Medicines Agencies (BEMA) scheme (7), and outcomes from the World Bank’s Enabling the Business of Agriculture (EBA) reports (8). Each tool was assessed based on its framework, regulatory functions covered, the indicators and sub-indicators used to measure capability, and their level of scope and granularity. The authors possessed extensive experience of the BEMA scheme both as a body being assessed and as an assessor. Consultations were held with the Regulatory Systems Strengthening team at the WHO to better understand all aspects of the GBT scheme, and where necessary, specific discussions were held with WOAH to confirm the veterinary medicines regulation components of the PVS scheme and methods of inspection and evaluation.
Appetite and need for such a veterinary medicines regulator-specific assessment/benchmarking tool for National Regulatory Agencies (NRAs) was determined from broad-based discussions with 27 country NRAs in sub-Saharan Africa, two mature NRAs from Europe, WOAH, the Food and Agriculture Organisation (FAO), and the pharmaceutical industry (HealthforAnimals; HfA) as key stakeholders.
Based on the analysis and review of the different tools, the one most suited to serve as a template for veterinary medicines regulators was identified (the WHO GBT; see Results and Discussion sections), and veterinary medicine-specific prototypes were developed with a modified list of functions, indicators, and sub-indicators. These were shared with the FAO, WOAH, HfA, ANSES (the French veterinary medicines regulator and a WOAH-designated Collaborating Centre for Veterinary Medicinal Products), and 15 sub-Saharan African NRAs for review and feedback. All feedback related to the sub-indicators and software was considered, and changes were made, resulting in the development of a revised prototype software tool (ElSebaie & Co., based on WHO-developed software) (3, 5).
This revised prototype was piloted in two developed countries (the UK and Australia) and two developing countries (Botswana and Rwanda). Training was provided on how to populate the tool, either online or through workshop-style hands-on training. The assessors completing the tool were also provided with a feedback document where software and sub-indicator-specific observations, comments, or suggestions could be made. This exercise resulted in further refinement of the tool.
3 Results
Two of the four schemes, the WHO GBT for human medicines and medical devices, and the EU BEMA for human or veterinary medicines, were comprehensive and detailed for medicines regulation, whereas the WOAH PVS scheme covered veterinary medicines regulation as part of an overall evaluation of veterinary services provision, and the World Bank EBT was restricted to aspects of veterinary medicine regulation that were business customer specific.
3.1 WHO Global Benchmarking Tool (GBT)
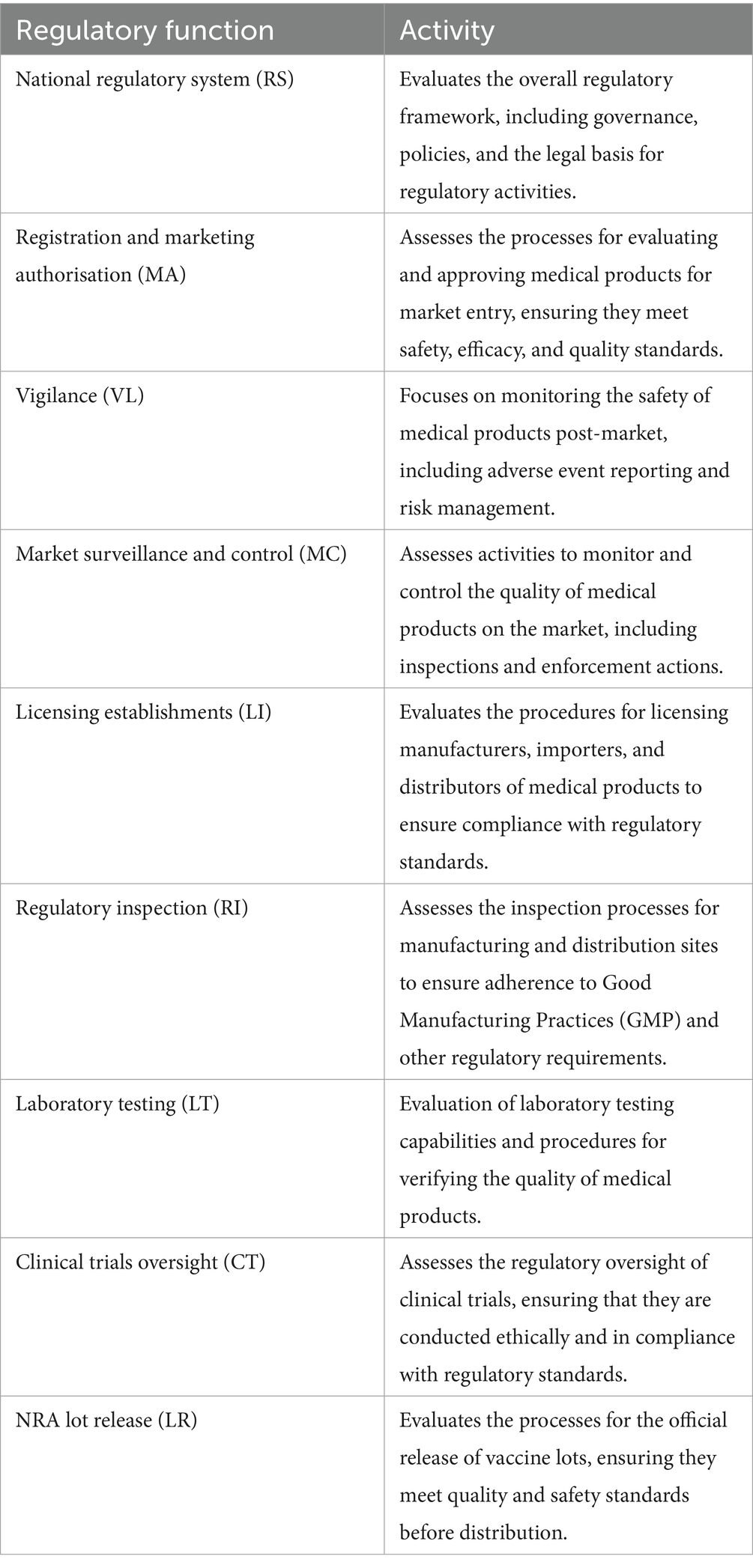
This is a comprehensive tool that provides a mechanism to benchmark the overarching framework of a country’s regulatory system and covers the nine key regulatory functions (Table 1).
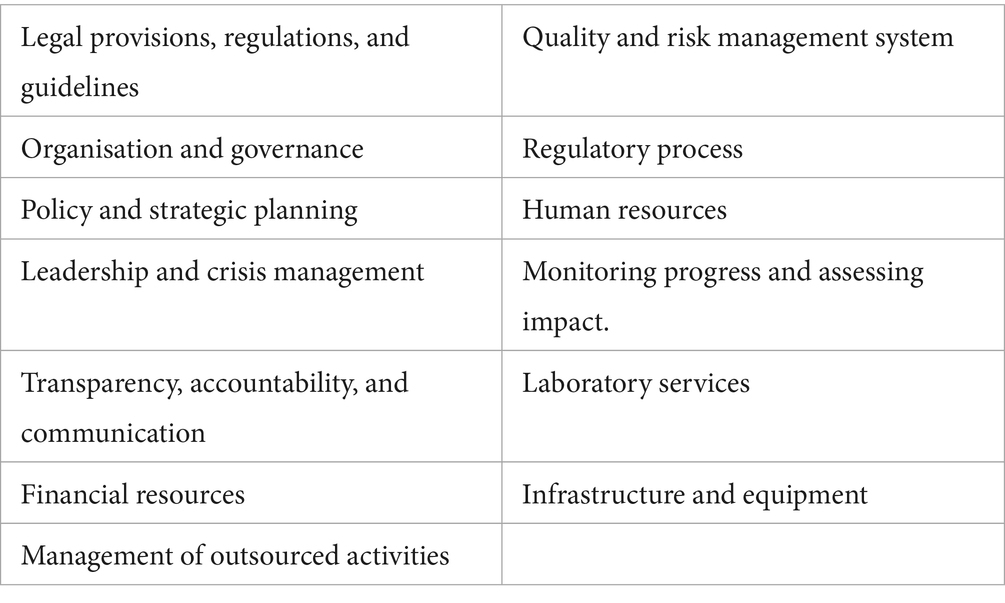
In turn, each regulatory function is composed of a maximum of 13 indicators (Table 2), each of which is subdivided into detailed sub-indicators, yielding a total of 268 sub-indicators.
Each of these sub-indicators is categorised as expectations for achievement of a particular ‘Maturity Level’ designation (referred to as pre-designated requirements) and is supported by a ‘fact sheet’ that provides an extensive description of the scope, evidence requirements, description, and guidance on how to complete the response. The sub-indicators are assessed and scored (between nought and one) using a sliding rating scale of ‘Not implemented’ (score of 0), ‘Ongoing implementation’ (score of 0.25), ‘Partially implemented’ (score of 0.75), ‘Implemented’ (score of 1), or ‘Not applicable’. The scores for each of the sub-indicators are then used to calculate the ‘Maturity Level’ (ML) for the function.
There are four performance maturity levels, which are derived from the International Standard Organisation (ISO) 9004 for quality management (9). These levels reflect the degree to which a regulatory system has been established as stable, efficient, and cohesive. Based on the degree of implementation, an ML of 1 to 4 is ascribed to each of the functions. These can be derived from a ‘strict’ algorithm or a ‘flexible’ algorithm, and the ML achieved is distinguished by flexible/strict qualification, e.g., ML2 strict or ML2 flexible. To determine the overall ML of a regulatory body, there are several sub-indicators that are mandatory for a particular ML designation at the institute level. For the ‘strict’ algorithm, all sub-indicators mandated for a particular ML designation must be implemented. For the ‘flexible’ algorithm, a minimum of 80% of the sub-indicators for a particular ML designation must be in place, and the remaining 20% of these essential sub-indicators must be in the process of being implemented. The degree of flexibility varies for each ML. For ML2, 95% of ML1 + ML2 must be implemented, with the remaining 5% in the process of implementation (i.e., ongoing implementation or partially implemented). For ML3, 100% of ML1 + ML2 must be implemented, and 90% of ML3, with the remaining 10% in the process of implementation. For ML4, 100% of all lower ML requirements must be implemented, and 80% of ML4, with the remaining 20% in the process of implementation.
The WHO GBT also includes the formulation of an Institutional Development Plan (IDP) as an essential component. It is linked directly to any sub-indicators that have not been fully met and outlines prioritised and context-specific actions to close the gaps.
3.2 Benchmarking of European Medicines Agencies (BEMAs)
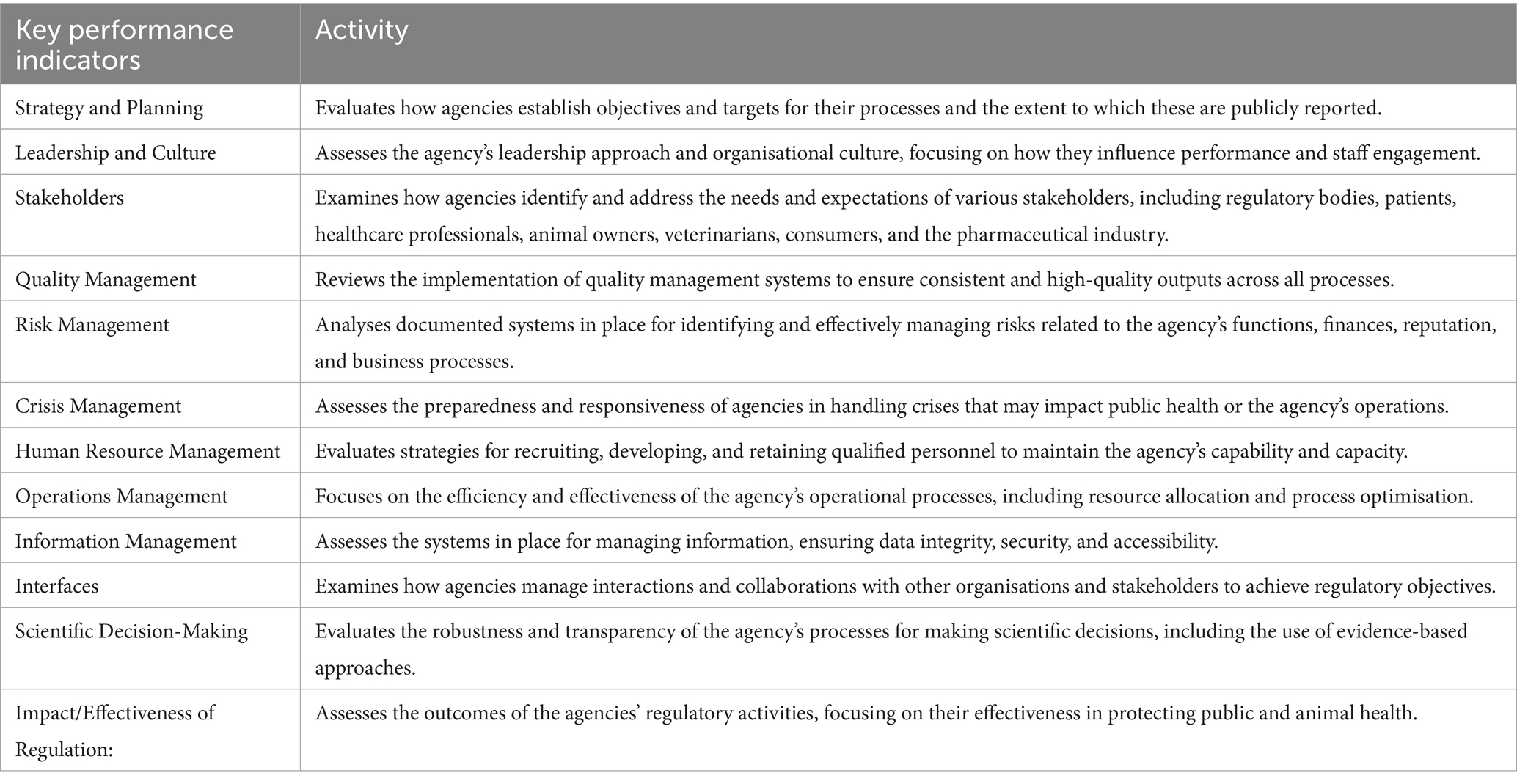
The BEMA tool (7) was developed for both human and veterinary regulators in the European Union. It includes 12 high-level Key Performance Indicators (KPIs) (Table 3), where each KPI is further divided into specific sub-indicators, assessed using a sliding rating scale from 1 to 5.
The options for scoring were assessed by the BEMA Strategy Group and the Heads of EU Medicines Agencies, and are based on ISO 9004 for Quality Management (9), which involves assigning ‘Maturity Levels’ to each process or system. There are five BEMA Maturity Levels: (1) no formal approach, (2) reactive approach, (3) stable formal system approach, (4) continuous improvement emphasised, and (5) best-in-class performance.
The assessment process involves a combination of self-evaluation and peer review provided by a visiting team of experts from other EU medicines regulators, facilitating the identification of strengths, best practises, and areas for improvement within the regulatory agency being assessed. Unlike the WHO scheme, there are no algorithm choices permitting a ‘flexible’ score.
3.3 WOAH PVS pathway
The WOAH (formerly known as OIE) PVS Pathway is a tool for evaluating the Performance of Veterinary Services (PVS). It covers a wide scope presented in four chapters, including human, physical, and financial resources, technical authority and capability, stakeholder interactions, and access to markets. The PVS Tool forms the fundamental methodological basis of the WOAH multi-staged PVS Pathway cycle of Veterinary Services support.
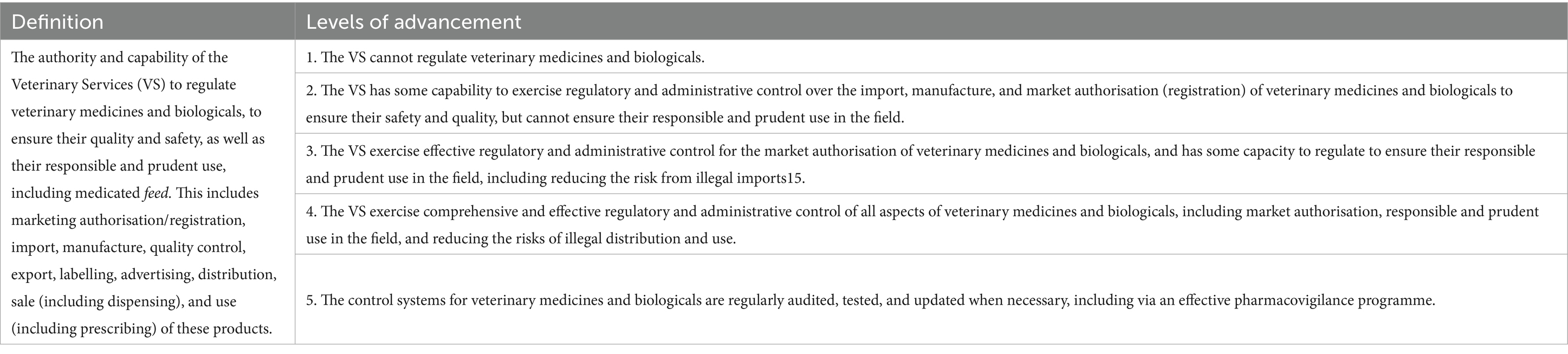
The main chapter that deals with the regulation of veterinary medicines is Chapter II – Technical Authority and Capability. There is one competency within Chapter II (II-8), Veterinary Medicines and Biologicals, that deals with veterinary medicines, and there are three other competencies that have elements dealing with veterinary medicines within them. These are Antimicrobial Resistance and Antimicrobial Use (II-9), Residue Testing, Monitoring, and Management (II-10), and Animal Feed Safety (II-11). Chapter III: Interaction with Stakeholders and Chapter IV: Access to markets also address veterinary medicines. The main competency of Chapter 11 (II-8), Veterinary Medicines and Biologicals, assesses the authority and capability of veterinary services to regulate veterinary medicines and biologicals. It covers market authorisation, import, manufacture, quality control, export, labelling, advertising, distribution, sale, and use of these products.
The PVS Pathway uses a five-level advancement system (Table 4); for example, for Chapter II (II-8), it ranges from ‘Cannot regulate veterinary medicines and biologicals (level 1)’ to ‘The control systems for veterinary medicines and biologicals are regularly audited, tested, and updated when necessary, including via an effective pharmacovigilance programme (level 5)’.
The experts conducting the evaluation use this high-level framework to derive the level of advancement of the regulatory capacity of the country, which is presented as a report (6).
3.4 World Bank Enabling the Business of Agriculture (EBA)
The EBA report focuses on laws and regulations affecting agricultural productivity, market access, and the policy environment for agriculture. It includes a questionnaire on Veterinary Medicinal Products (VMPs) that assesses the regulatory framework, implementation, and efficiency. The questionnaire examines (1) the requirement for VMPs to be registered prior to commercialisation under normal circumstances, (2) legally defined timeframes for the review of registration dossiers, (3) public availability of an official list of registered VMPs on the relevant regulatory authority’s website, (4) legal provisions allowing the registration of generic versions of existing brand-name VMPs, (5) specified proprietary periods between the registration of a brand-name VMP and its generic counterparts, and (6) requirements for registration holders to implement mechanisms for reporting adverse reactions to marketed VMPs.
By analysing these components, the EBA identifies strengths and weaknesses in countries’ regulatory frameworks related to veterinary medicines (8).
4 Description of the proposed new veterinary medicines regulatory agency self-assessment tool (VMRA-SAT)
Based on the above findings, the WHO GBT tool was adopted for the development of the VMRA-SAT, a veterinary medicine dedicated assessment/benchmarking scheme (see Discussion section for description of the rationale), adopting the functions, indicators, and sub-indicators approach. Each of these was reviewed to determine their applicability to a veterinary medicine-specific tool and to identify the changes that needed to be made (Table 5). All but one of the WHO GBT functions (Table 1) were adopted, with “Veterinary Medicines” added as a prefix to their names (see Figure 1), and two of the names of the functions were modified to reflect the commonly used language of veterinary medicines regulation, with Vigilance becoming Pharmacovigilance and Lot Release becoming Batch Release (Figure 1). The function not adopted was ‘Clinical Trials’, as this function is not as complex as in human medicine regulation. It was replaced by a ‘critical’ sub-indicator, clinical field trials (Table 5; all critical sub-indicators are also available in the Supplementary material). There are 13 indicators (Figure 1), which are comparable to the 13 indicators used in the WHO GBT.
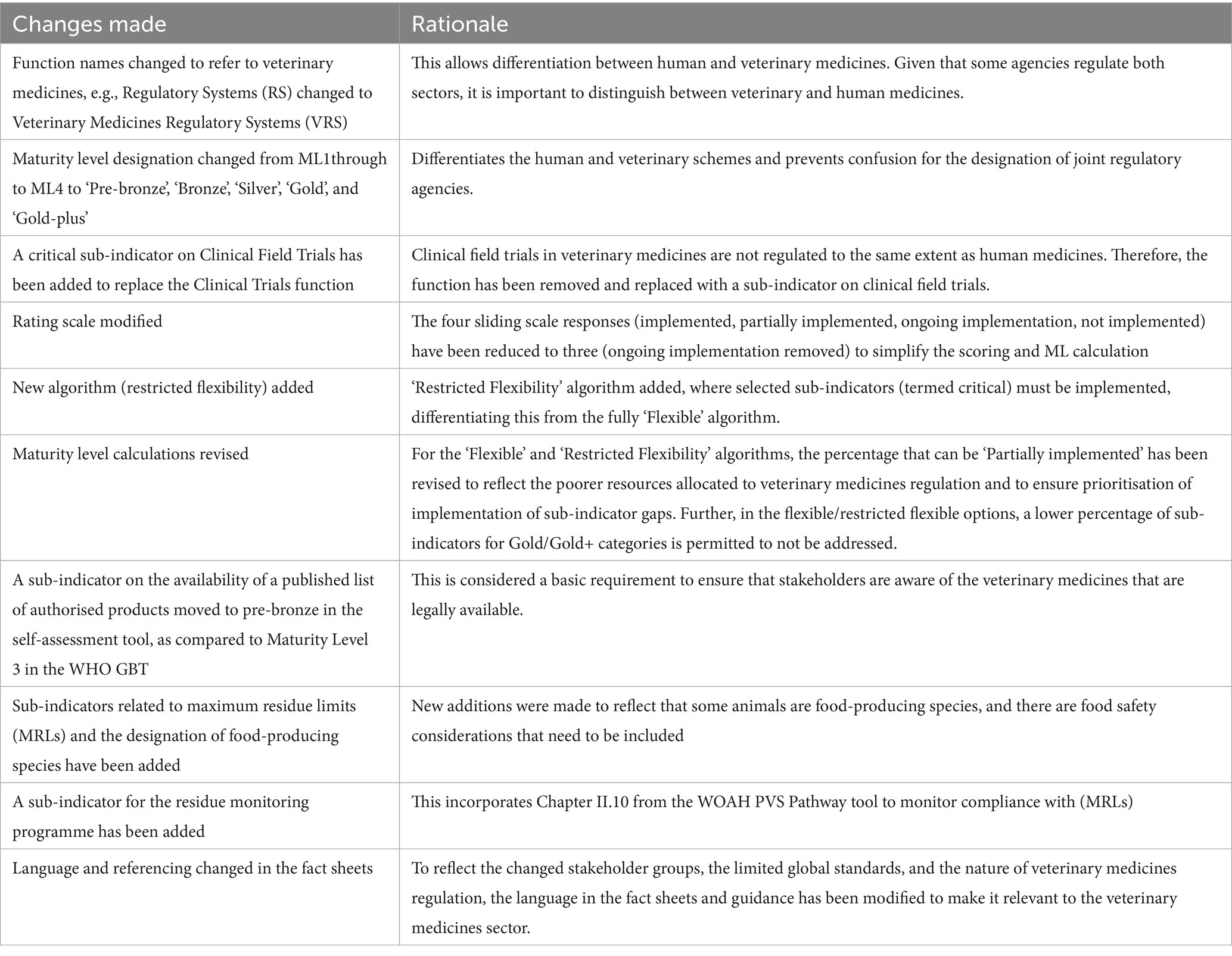
Table 5. Changes made to the WHO GBT for the development of the self-assessment/benchmarking tool for veterinary medicines regulators.
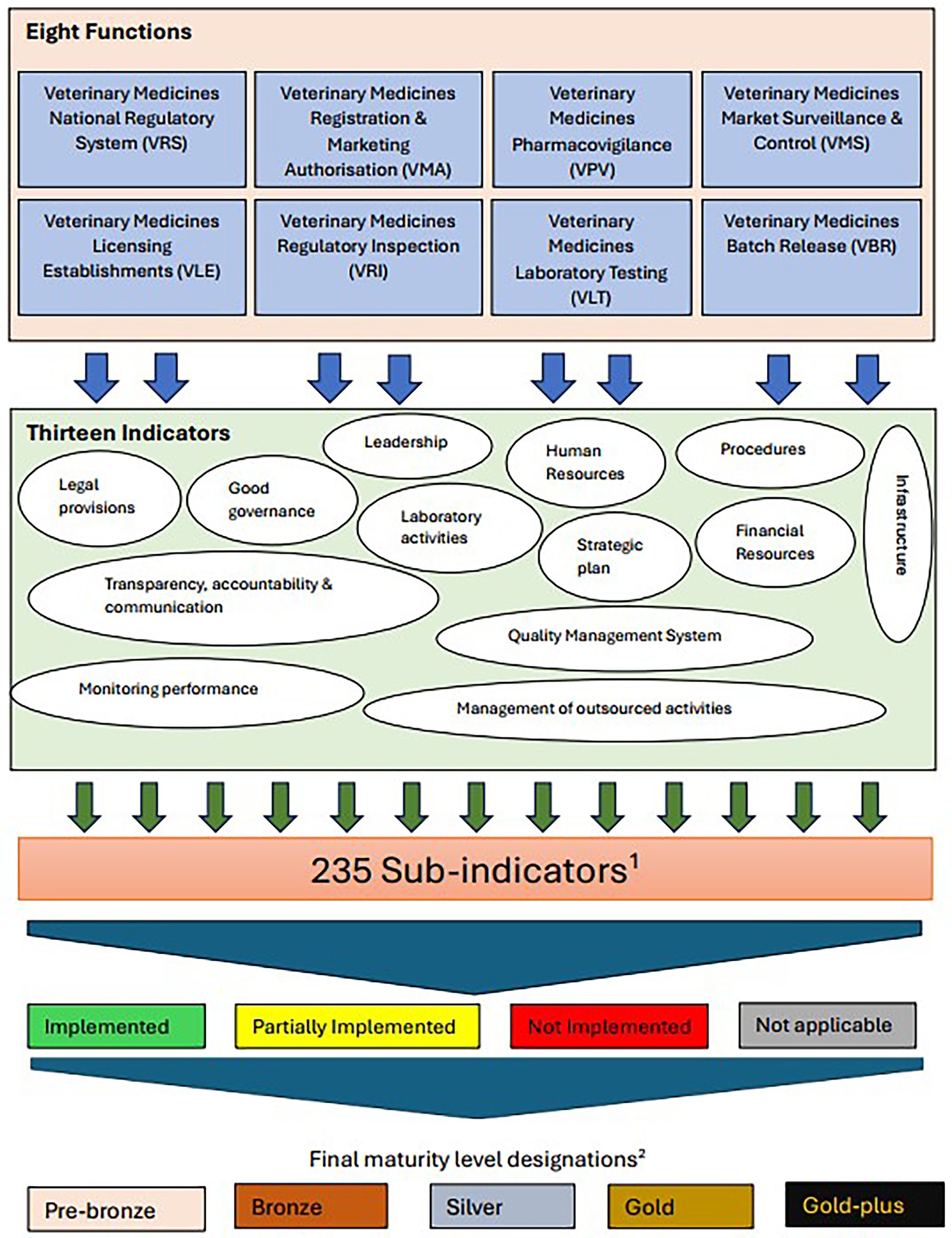
Figure 1. An outline of the different functions, indicators and sub-indicators of the self-assessment tool. 1Sub-indicators are categorised by level of implementation. 2Definition of maturity levels; number of pre-designated sub-indicators in parentheses. Pre-bronze (6): Minimum operating capability. Bronze (21): Have elements of regulatory system beyond the minimum operating capability. Silver (28): Evolving system that performs essential regulatory functions. Gold (154): Stable, well-functioning and integrated regulatory system. Gold-plus (26): Advanced level of performance.
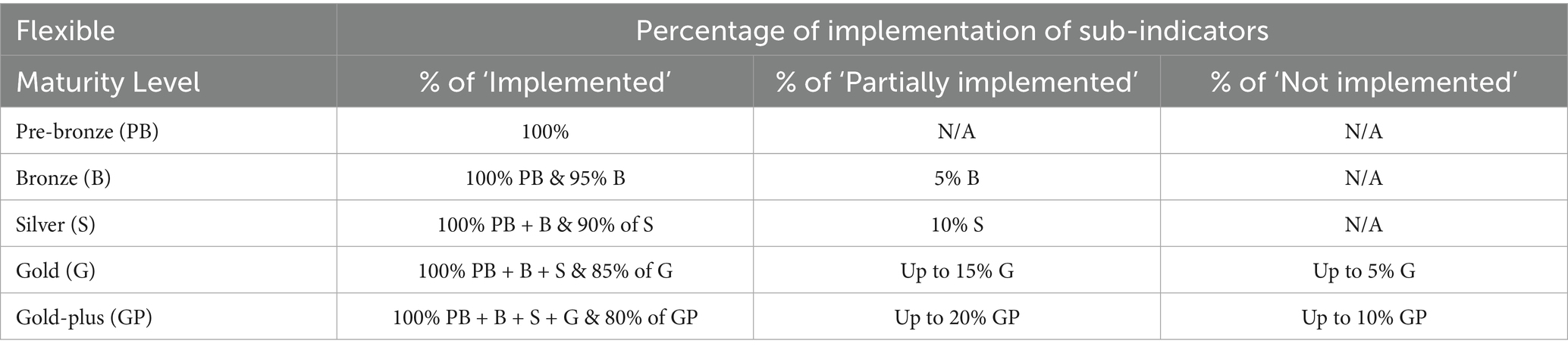
These indicators each contain several sub-indicators, yielding a total of 235 sub-indicators, which are pre-designated as requirements for each ML. The Maturity Levels for the veterinary tool have been named as Pre-bronze, Bronze, Silver, Gold, and Gold-plus (Figure 1).
The sub-indicators are assessed based on the WHO GBT sliding rating scale, the key differences being the omission of the ‘Ongoing Implementation’ category and a change in the score for the Partially Implemented category from 0.75 to 0.5. This was based on the response from consultation and pilots. The scores for each of the sub-indicators are used to calculate the ML for the function, depending on the algorithm selected. For the strict algorithm, all sub-indicator requirements for the maturity designation sought must be implemented. For the flexible algorithm, there is some flexibility for different MLs, other than for Pre-bronze (Table 6). The Pre-bronze level requires a legal foundation (three sub-indicators), an established source of funding, information on agency contacts for services, and an up-to-date list of veterinary medicines permitted on the national market. This represents minimum operating capability. The algorithms deployed for the new tool have also been modified from those of the WHO GBT by the addition of a new algorithm termed ‘restricted flexibility’. The application of both ‘strict’ and ‘flexible’ (Table 5) remains broadly the same as the WHO GBT, whereas the ‘restricted flexibility’ algorithm combined elements from both WHO GBT algorithms.
5 Discussion
Although the schemes reviewed differed in several ways, at the highest level, the key components covered were (a) legislative/legal foundation, (b) capacity and sustainability of capacity, i.e., are there appropriate human and financial resources and infrastructure, and (c) the scope of medicine regulation performed and/or commissioned, and to what standards.
The WOAH PVS Pathway, whilst covering relevant aspects of veterinary medicines regulation, was too high-level for direct application as a benchmarking tool and would have required extensive modification. The World Bank EBA requirements were limited as they focused on those elements of regulation most relevant to market access, thereby precluding an extensive review by veterinary medicines regulators. The two most comparable schemes regarding breadth and depth were the WHO GBT and the EU BEMA schemes. The key difference in the components covered is the assessment of an appropriate legislative framework, which is not a component of BEMA. The absence of this from BEMA is because the regulation of medicines is set by the EU and adopted in all member states. A key difference in the approach between these two schemes is that the WHO GBT has a framework that starts with key regulatory functions and assesses the functions firstly at the level of 9 themes composed of 13 indicators, which cover the operational considerations required for delivery of the function, e.g., resources, strategy, and risk management, whereas the BEMA scheme starts with an equivalence of 12 indicators (referred to in the scheme as key performance indicators). Again, the difference is explained by the fact that the functions required for delivery of the regulations are set by the EU, and therefore common to all EU regulators.
An important distinction between the two schemes is that the WHO GBT has two available algorithms (strict and flexible), whereas the BEMA scheme does not. This enables a more nuanced assessment of the regulatory system’s capability. The flexible algorithm allows for some variability in the assessment, accommodating differences between regulatory contexts and practises. It considers the overall performance of the function, allowing for partial fulfilment of certain criteria, enabling recognition of incremental progress and improvements. This more adaptable and inclusive evaluation ensures that regulators can demonstrate their strengths even if some sub-indicators are not fully met. Conversely, the strict algorithm requires full compliance with all specified criteria for each sub-indicator within a regulatory function. The strict algorithm is designed to encourage high standards and drive continuous improvement by identifying gaps that must be addressed to achieve a higher ML designation.
The comprehensive nature and detailed indicators of the WHO GBT make it well-suited as a model for assessing the maturity and functionality of veterinary regulatory systems. An integral part of the WHO GBT is the Institutional Development Plan (IDP), which provides context-specific, actionable steps for countries to advance their regulatory capability. The IDP helps to improve the effectiveness of regulatory strengthening efforts by setting clear, specific, and actionable activities. It also allows national regulators to monitor their progress over time, and to benchmark themselves against other regulators, if the levels are made public or if shared with others in confidence. The above considerations, the more detailed guidance providing greater support, and a view from stakeholders that were joint human and veterinary medicines agencies on preference for a scheme that was already familiar to them, led to the adoption of the WHO GBT as the basis for a proposed new global self-assessment/benchmarking tool for veterinary medicines. Several changes were required to ensure veterinary medicines terminology and the addition of veterinary medicine-specific sub-indicators, such as the setting of maximum residue limits (MRLs) of animal medicines in food from livestock, aquaculture, and apiculture, and the assessment of feed for food-producing animals. Other key changes were permitting for the flexible algorithm a low level of sub-indicators to be not implemented by the agency for silver and higher levels of maturity, and the establishment of a restricted flexibility category algorithm; establishment of a pre-bronze category, for which there is no WHO GBT equivalence; and a different emphasis on clinical trials.
The Pre-bronze level of maturity represents having in place the minimum criteria to meet minimum operating capability. As such, it automatically guides those countries at the earliest stages of establishing veterinary medicines regulatory bodies on the fundamentals that must be in place, as well as encouraging the agencies, once established, to work towards improving maturity. This includes the publication of a national product list at this early maturity stage. Such a list, following the addition of extra information (for example, on the products), may mature over time into a nationally authorised product database. This will help stakeholders to be aware of which products are legally available, support those involved in controlling illegal products, as well as WOAH’s Veterinary Monitoring and Surveillance System for Substandard and Falsified Veterinary Products (VSAFE) (10). It also supports the initiative to compile the essential veterinary medicines list (11).
The feature of allowing a few sub-indicators not to be implemented for attaining Gold and Gold-plus (using the flexible algorithm) levels was built into the tool as a way of not discouraging regulatory agencies from working towards attaining them using the strict algorithm. This stepwise approach is enhanced by the availability of the restricted flexibility algorithm, which restricts some of the sub-indicators that are allowed to be not implemented, thereby enabling prioritisation on the route to the strict algorithm. Hence, based on the country’s capability, resources, and ambition, it can tailor its development journey accordingly.
Clinical trials governance in human medicines is more complex when compared to clinical field trials in the veterinary sector. The controls in place for the manufacturing, ethics, approval, reporting, and transparency requirements are more stringent in the human sector compared to the veterinary sector. Consequently, it was considered that a whole function would not be necessary for the veterinary scheme and that a sub-indicator that addresses the existence of legal provisions for veterinary clinical field trials would suffice.
A current limitation of the benchmarking schemes is that they do not assess the quality of medicines regulation activities performed. There is one direct, specific measure of regulatory performance: Good Manufacturing Practice (GMP) inspections. Membership of the Pharmaceutical Inspection Convention and Pharmaceutical Inspection Co-operation Scheme [PIC/S, (12)] requires not only an assessment of capability (e.g., training and legislative basis) but also of performance by observation of an inspection visit and consequent report. This enables mutual reliance between regulatory jurisdictions for GMP inspections. Unfortunately, there are relatively few veterinary medicine agencies that are members of PIC/S.
Finally, the capability assessment allows an inference on potential performance to be drawn as a less well-led, trained, and resourced regulatory body is less likely to perform well than one that is more mature. However, the other key limitation of the current and the proposed new veterinary schemes is that there is no obligation for the self-assessment/benchmark findings and associated scores to be made public, although the WHO may announce when an authority reaches ML3 or ML4. This reflects the sensitivities of the regulatory body and/or the country. However, a clear independent assessment of maturity and its public availability is important. It is difficult, for example, to identify with confidence a regulatory body to participate in medicines regulation pre-qualification work, and to be able to evidence to others the reason for the choice, in the absence of such transparency. The WHO has recently initiated a performance evaluation scheme (13), whereby a regulator that has been benchmarked at Maturity Level 3 can be independently assessed for performance, and if meeting performance requirements, it may be designated as a WHO Listed Authority (WLA). There would be merit in a similar performance scheme for veterinary medicines supporting the activities of WOAH and the FAO scheme once the proposed new global veterinary scheme has achieved traction. For this to happen, the scheme ideally needs to be adopted by WOAH to become a benchmarking tool as part of their broader regulatory systems strengthening work.
Although self-assessment schemes take resources from participation, the return on that investment is beneficial. It is also the case that funders of veterinary medicine regulation improvements can either use the findings of self-assessment or support self-assessment to identify current levels of competency and to prioritise supporting work. The effectiveness of the support can be assessed by improvements in maturity scores, either by further self-assessment or by independent assessment. The new VMRA-SAT is already being used for this purpose in East Africa by GALVmed for the work it supports on regional harmonisation.
The absence of a single global self-assessment/benchmarking tool for veterinary medicines regulators is detrimental to veterinary medicines regulation. Adoption of the proposed new VMRA-SAT would fill that gap, with the potential to have a clearer regional and/or global view on the current state of capability of veterinary medicines regulatory bodies, clearer direction to countries and funders on the improvements needed, and, over time, to build on this to establish also a measure of performance.
Author contributions
NJ: Methodology, Conceptualization, Validation, Project administration, Resources, Investigation, Writing – original draft, Software, Funding acquisition, Visualization, Formal analysis, Writing – review & editing. SB: Investigation, Validation, Funding acquisition, Writing – original draft, Writing – review & editing, Conceptualization. SE: Funding acquisition, Writing – review & editing, Project administration, Resources. OO: Writing – review & editing, Resources, Validation, Investigation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The project was funded by the Bill and Melinda Gates Foundation (BMGF), contributing directly to the Foundation’s AgDev outcomes. Investment Number – INV-045194.
Acknowledgments
The authors express their sincere gratitude to the World Health Organisation (WHO) team, with special thanks to Alireza Khadem Broojerdi for his invaluable guidance, training, and generous provision of access to the Global Benchmarking Tool (GBT), as well as to Hiiti Baran Sillo and colleagues for their support. We also extend our appreciation to the following individuals and organisations for their significant contributions to this project: colleagues at the Veterinary Medicines Directorate; Carmen Bullon (FAO); Camille Loi, Barbara Alessandrini, and David Sherman (WOAH); Mohammed ElSebaie (Developer); Jean-Pierre Orand (ANSES); Rick Clayton (HealthforAnimals); and the SSA National Regulatory Authorities (NRAs) for their constructive feedback during the tool’s development. Special thanks are also due to the Rwanda Food and Drugs Authority, the Botswana Medicines Regulatory Authority, and the Australian Pesticides and Veterinary Medicines Authority for their collaboration in piloting the tool.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2025.1648556/full#supplementary-material
References
1. FAO, Food and Agriculture Organisation of the United Nations. Decent rural employment. Livestock. (2024). Available online at: https://www.fao.org/rural/employment/agricultural-sub-sectors/livestock/en/ (Accessed May 20, 2024).
2. Bonilla-Cedrez, C, Steward, P, Rosenstock, TS, Thornton, P, Arango, J, Kropff, M, et al. Priority areas for investment in more sustainable and climate-resilient livestock systems. Nat Sustain. (2023) 6:1279–86. doi: 10.1038/s41893-023-01161-1
3. Broojerdi, AK, Sillo, HB, Dehaghi, ROA, Ward, M, Refaat, M, and Parry, J. The World Health Organization global benchmarking tool an instrument to strengthen medical products regulation and promote universal health coverage. Front Med (Lausanne). (2020) 7:457. doi: 10.3389/fmed.2020.00457
4. Pyatt, AZ, Eckford, S, Joseph, N, Borriello, SP, and Oyati, O. Veterinary medicinal product regulation in sub-Saharan Africa: identifying barriers and opportunities for enhancing VMP regulatory systems. Front Vet Sci. (2025) 12:1532098. doi: 10.3389/fvets.2025.1532098
5. WHO. Global benchmarking tool (GBT) for evaluation of national regulatory system of medical products: manual for benchmarking and formulation of institutional development plans. (2024). Available online at: https://www.who.int/publications/i/item/9789240087637 (Accessed January 17, 2025)
6. PVS Pathway. World Organisation for Animal Health (WOAH). (2025). Available online at: https://www.woah.org/en/what-we-offer/improving-veterinary-services/pvs-pathway/#ui-id-4 (Accessed February 12, 2025).
7. Pejović, G., Tošić, B., and Ruso, J., (2018). Benchmarking as the quality management tool for the excellence assessment of medicines regulatory authorities in Europe. In International Scientific Symposium SymOrg Conference (pp. 7–10).
8. Enabling the Business of Agriculture. World Bank Group. Washington, DC, USA: World Bank Publications. (2019). Available online at: https://openknowledge.worldbank.org/server/api/core/bitstreams/9cd7105f-e1c2-52ee-ad27-a63d983a2d2f/content (Accessed January 17, 2025)
9. International Standard ISO 9004:2018. Quality management — quality of an organization — guidance to achieve sustained success. (2018). Available online at: https://www.iso.org/standard/70397.html (Accessed 03 June, 2025)
10. Veterinary Monitoring and Surveillance System for Substandard and Falsified Veterinary Products (VSAFE). Workshop on substandard and falsified veterinary products for English speaking Africa. (2025). Available online at: https://rr-africa.woah.org/en/news/workshop-on-substandard-and-falsified-veterinary-products-for-english-speaking-africa/ (Accessed May 09, 2025)
11. Essential Veterinary Medicines List. Developed by the world veterinary association (WVA) and Brooke action for working horses and donkeys. (2024). Available online at: https://worldvet.org/evml/ (Accessed April 11, 2025).
12. The Pharmaceutical Inspection Co-operation Scheme (PIC/S). (2025). Available online at: https://picscheme.org/en/picscheme (Accessed June 03, 2025)
13. A Framework for Evaluating and Publicly Designating Regulatory Authorities as WHO Listed Authorities (WLA). (2025). Available online at: https://www.who.int/initiatives/who-listed-authority-reg-authorities (Accessed June 03, 2025)
Keywords: veterinary medicines, self-assessment, benchmarking, veterinary medicines regulation, National Regulatory Agency, VMPs
Citation: Joseph N, Borriello SP, Eckford S and Oyati O (2025) Development of a self-assessment/benchmarking tool for regulators of veterinary medicines. Front. Vet. Sci. 12:1648556. doi: 10.3389/fvets.2025.1648556
Edited by:
Nicola Pugliese, University of Bari Aldo Moro, ItalyReviewed by:
V. Wensley Koch, Retired, Loveland, CO, United StatesLawrence Liberti, University of Southern California, United States
Copyright © 2025 Joseph, Borriello, Eckford and Oyati. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Noel Joseph, aW50ZXJuYXRpb25hbEB2bWQuZ292LnVr
 Noel Joseph
Noel Joseph S. Peter Borriello
S. Peter Borriello Suzanne Eckford
Suzanne Eckford Osi Oyati
Osi Oyati