- 1Seibozaka Animal Hospital, Tokyo, Japan
- 2Vet Surg Tokyo, Tokyo, Japan
Introduction: This prospective, randomized clinical study with a sequential design aimed to evaluate whether a novel quadratus lumborum block (QLB) technique applied in sternal recumbency could reduce isoflurane requirements and enhance procedural safety in cats undergoing ovariohysterectomy.
Methods: Thirty-five American Society of Anesthesiologists physical status (ASA-PS) I female cats, either client-owned or sheltered, undergoing ovariohysterectomy. Cats were randomly assigned to different groups to undergo either conventional QLB technique in lateral recumbency (CQLB group, n = 17) or novel QLB technique in sternal recumbency (NQLB group, n = 18). Ultrasound-guided injections were performed bilaterally, targeting the fascial plane between the quadratus lumborum and psoas minor muscles, with 0.4 mL kg−1 bupivacaine administered per side. Cats were premedicated with dexmedetomidine, anesthetized with propofol and isoflurane. The end-tidal isoflurane concentration (ETiso) was adjusted based on heart rate (HR), respiratory rate, and mean arterial pressure (MAP). Outcomes included total mean ETiso, phase-specific ETiso, total mean HR and MAP, rate of needle proximity to abdominal organs, rates of bradycardia, hypotension, and the need for postoperative analgesics, assessed using both the Short-form UNESP-Botucatu Multidimensional Composite Pain Scale and the Feline Grimace Scale.
Results: Cats in the NQLB group demonstrated slightly lower total mean ETiso (p = 0.046) and significantly reduced ETiso during right ovariectomy (p = 0.022) and hysterectomy (p = 0.007) compared with cats in the CQLB group. Total mean HR and MAP did not differ between groups. Needle proximity to abdominal organs was observed in all CQLB cats but not in any NQLB cats. No bradycardia or hypotension was observed. There was no significant difference in the requirement for postoperative rescue analgesic between the groups.
Discussion: The novel QLB technique demonstrated a superior isoflurane-sparing effect and safety compared with that of conventional QLB in cats. This approach may benefit cats undergoing ovariohysterectomy.
1 Introduction
Regional anesthesia is increasingly recognized as an important component of nociceptive management in both human and veterinary medicine, with growing evidence supporting its efficiency (1). In addition to providing analgesia, regional anesthesia is known to reduce the requirement for inhalational anesthesia in both dogs and cats (2). Given the cardiovascular and pulmonary depressive effects of isoflurane in cats (3, 4), minimizing its use can provide significant perioperative benefits. Although sevoflurane has been suggested in some studies and clinical contexts to offer cardiopulmonary advantages over isoflurane (5), isoflurane remains more widely used in veterinary anesthesia due to its lower cost and established clinical use (6).
Various regional anesthesia techniques, including epidural anesthesia (7, 8), transversus abdominis plane (TAP) block (9), and rectus sheath block (10), are commonly used for celiotomy in cats. The quadratus lumborum block (QLB), a fascial plane block involving the injection of local anesthesia around the quadratus lumborum muscle, was developed as a modification of the TAP block used in human medicine (11). Unlike TAP blocks, which primarily provide somatosensory blockade, QLB also modulates visceral nociception by spreading around visceral afferent fibers that course alongside the sympathetic trunk (12). In human medicine, QLB is widely used for abdominal procedures, including hysterectomy (13–15), cesarean section (16, 17), and nephrectomy (18). Despite its increasing use in human medicine, only a few clinical studies have investigated QLB in cats (19–21).
Existing QLB techniques used in cats, which vary according to the injection site around the quadratus lumborum muscle, are typically performed in lateral recumbency (22–24). However, this approach requires needle advancement in a ventrolateral-to-dorsomedial direction, raising concerns about the risk of injuring abdominal organs such as the kidney and spleen (25). A cadaveric study demonstrated that this technique resulted in injectate staining the retroperitoneal cavity (22). To mitigate these risks, a dorsolateral-to-ventromedial needle trajectory was proposed, but this modification exhibited poor ultrasound visibility (23). In the present study, we investigated a novel QLB approach performed in sternal recumbency. This technique employs dorsolateral-to-ventromedial needle insertion, aiming to improve ultrasound visibility while avoiding needle trajectory proximity to abdominal organs. This study aimed to evaluate the isoflurane-sparing effect, safety, and feasibility of this novel QLB technique in cats undergoing ovariohysterectomy. Preliminary clinical impressions suggested that this approach might reduce intraoperative anesthetic requirements compared to the conventional technique. Therefore, we hypothesized that the novel technique would be associated with lower end-tidal isoflurane concentrations and a reduced risk of needle proximity to abdominal organs. The primary outcome was the intraoperative end-tidal isoflurane concentration (ETiso) required to maintain anesthesia, used as a practical surrogate for anesthetic depth and clinical anesthetic requirement. Secondary outcomes included the proximity of abdominal organs to the needle trajectory, the incidence of cardiovascular complications, QLB-related complications, and postoperative pain scores.
2 Materials and methods
2.1 Animals and study design
This study followed a sequential design and was approved by the Institutional Animal Care and Use Committee of Kimura Animal Hospital (KAH2023-005). Informed consent was obtained from the owners or caregivers of all enrolled cats.
A total of 38 healthy female cats scheduled for elective ovariohysterectomy were initially enrolled. Cats were randomly assigned to either the CQLB group, in which QLB was performed via the conventional lateral recumbency approach as described by dos-Santos et al. (22), or the NQLB group, in which the novel QLB was performed in sternal recumbency. Randomization was performed by one investigator (FT) using an online list generator,1 which assigned numbers 1–38 to either group. Cats were then allocated to these numbers according to the order of their scheduled procedures.
Of the 38 cats enrolled, three were excluded: one due to an ASA-PS classification of II associated with respiratory disease, and two due to withdrawal of owner consent. Consequently, data from 35 cats were included in the final analysis (CQLB group: n = 17; NQLB group: n = 18).
Preanesthetic examinations, including physical examination, complete blood count, blood biochemistry, and thoracic radiography, were performed within 1 week before surgery. On the day of surgery, the cats underwent body weight measurement, Body Condition Score (BCS) assessment using a 9-point scale (26), and baseline pain assessments using the Short-form UNESP-Botucatu Multidimensional Composite Pain Scale (MCPS-SF) for Cats (27) and the Feline Grimace Scale (FGS) (28).
Cats with an American Society of Anesthesiologists physical status (ASA-PS) classification of I, scheduled for ovariohysterectomy, and either client-owned or sheltered, were enrolled in the study. Cats were excluded from the study if they met any of the following criteria: ASA-PS classification of II or greater; BCS lower than 4 or higher than 7; age younger than 4 months or older than 3 years; skin infection or any other lesion at the QLB site; pregnancy; painful conditions (MCPS-SF score ≥ 4); or prior analgesic treatment.
2.2 Preoperative management
Food but not water was withheld for at least 6 h before anesthesia. All cats were premedicated with dexmedetomidine (Dexdomitor 0.1 mg mL−1; Orion Corporation, Espoo, Finland) at 4 μg kg-1 intramuscularly (IM). Ten minutes later, an intravenous catheter (Surflo Flash 24-gage 3/4 inch; Terumo Corporation, Tokyo, Japan) was placed in the medial saphenous vein for the administration of anesthetic agents and electrolyte fluids. Propofol (Propoflo 28; Zoetis Inc., Parsippany, NJ, United States) was then administered intravenously for anesthetic induction. Once the swallowing reflex disappeared, 0.1 mL of 2% lidocaine (Lidocaine Hydrochloride Injection 2% “Nissin”; Nissin Pharmaceutical Co., Ltd., Yamagata, Japan) was applied around the larynx. After 30–60 s, an appropriately sized cuffed endotracheal tube (3.5–4.0 mm internal diameter, Spiral Endotracheal Tube with Stylet and Cuff; Fuji Systems Corporation, Tokyo, Japan) was placed. The propofol dose required for anesthetic induction (mg kg−1) was recorded. The endotracheal tube was connected to a non-rebreathing circle system (SafeSigh Non-Rebreathing System with Manometer; Vetamac, Rossville, IN, United States) integrated with an anesthetic machine (Acoma Veterinary Anesthesia Machine FO-20A; ACOMA Medical Industry Co., Ltd., Tokyo, Japan).
2.3 Intraoperative management
Anesthesia was initially maintained using isoflurane (Isoflurane; Viatris Healthcare G. K., Tokyo, Japan) with a vaporizer setting of 2.0, 100% oxygen, and a fresh gas flow rate of 500 mL kg−1 min−1. Spontaneous ventilation was maintained, and manual ventilation was temporarily provided if end-tidal carbon dioxide (ETCO₂) exceeded 45 mmHg, to prevent hypoventilation. Lactated Ringer’s solution (Solulact Infusion 250 mL; Terumo Corporation, Tokyo, Japan) was administered at 5 mL kg−1 h−1 throughout anesthesia. Following induction, cefazolin (25 mg kg−1 IV; Cefazolin Injection “Fujita”; Fujita Pharmaceutical Co., Ltd., Tokyo, Japan) and meloxicam (0.3 mg kg−1 subcutaneously; Inflacam® 0.5% Injection; Virbac Japan Co., Ltd., Osaka, Japan) were administered. Heart rate (HR), respiratory rate (fR), oxygen saturation of hemoglobin, ETCO₂, ETiso, and esophageal temperature were continuously measured using a multiparametric monitor (ePM12M Vet; Mindray, Shenzhen, China). ETCO₂ and ETiso were measured using a side-stream type capnometer integrated into the multiparametric monitor. The ePM12M Vet gas analyzer continuously performs automatic calibration for the measurement of the concentration of anesthetic gas, including isoflurane, as per the manufacturer’s specifications. According to the manufacturer’s recommendations, annual accuracy verification using a standard calibration gas was performed to ensure measurement reliability. Non-invasive systolic, diastolic, and mean arterial pressure (SAP, DAP, and MAP, respectively) were measured at 2.5-min intervals using an oscillometric device (PetMAP Graphic II; CardioCommand, Inc., Tampa, FL, United States) with a cuff sized to approximately 40% of the circumference of the antebrachium.
2.4 QLB procedures
After instrumentation, QLB was performed according to the allocated group. In both groups, 0.125% bupivacaine (Marcaine Injection 0.125%; Nissin Pharmaceutical Co., Ltd.) was administered at 1 mg kg-1 (total 0.8 mL kg−1, 0.4 mL kg−1 per side). All QLBs were performed by a single anesthetist (FT).
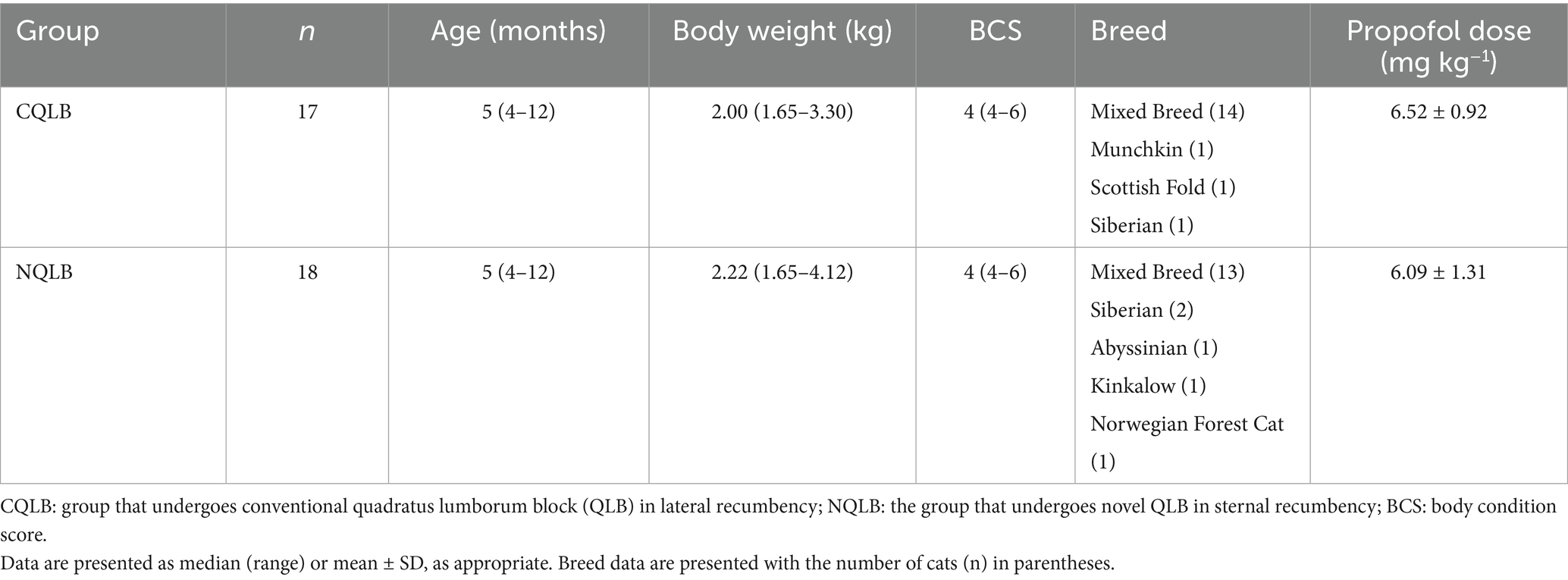
In the CQLB group, cats were positioned in left lateral recumbency. The hair around the second lumbar vertebra (L2) was clipped, and the skin was disinfected with 70% alcohol. A linear ultrasound (US) probe (11 L-D Linear Array Transducer; GE Healthcare, Chicago, IL, United States) connected to a US machine (LOGIQ S8; GE Healthcare) was placed perpendicular to the longitudinal axis of the cat to locate the L2 transverse process (Figure 1a), identified by counting from the last rib on the US image. The probe marker was oriented laterally (toward the right side of the cat), resulting in the lateral side appearing on the right side of the ultrasound image and the medial side on the left. The L2 transverse process and associated muscular structures were visualized in the transverse plane. Relevant anatomical structures for QLB, including the second lumbar vertebral body, transverse process, quadratus lumborum (QL) muscle, psoas minor (Pm) muscle, and the fascia between these muscles, were identified on the US image. A 22-gage, 50-mm echogenic needle (Sonolect Needle; Hakko Co., Ltd., Chikuma, Japan) was inserted at the ventral edge of the probe using the in-plane technique in a ventrolateral-to-dorsomedial direction. The needle tip was positioned within the fascia between the QL and Pm muscles (Figure 1b). Absence of blood on aspiration was confirmed, and a test injection containing 0.1–0.3 mL of injectate was performed to verify hydrodissection. After confirming hydrodissection, the remaining injectate was administered slowly. The procedure was then repeated on the contralateral side with the cat repositioned in right lateral recumbency.
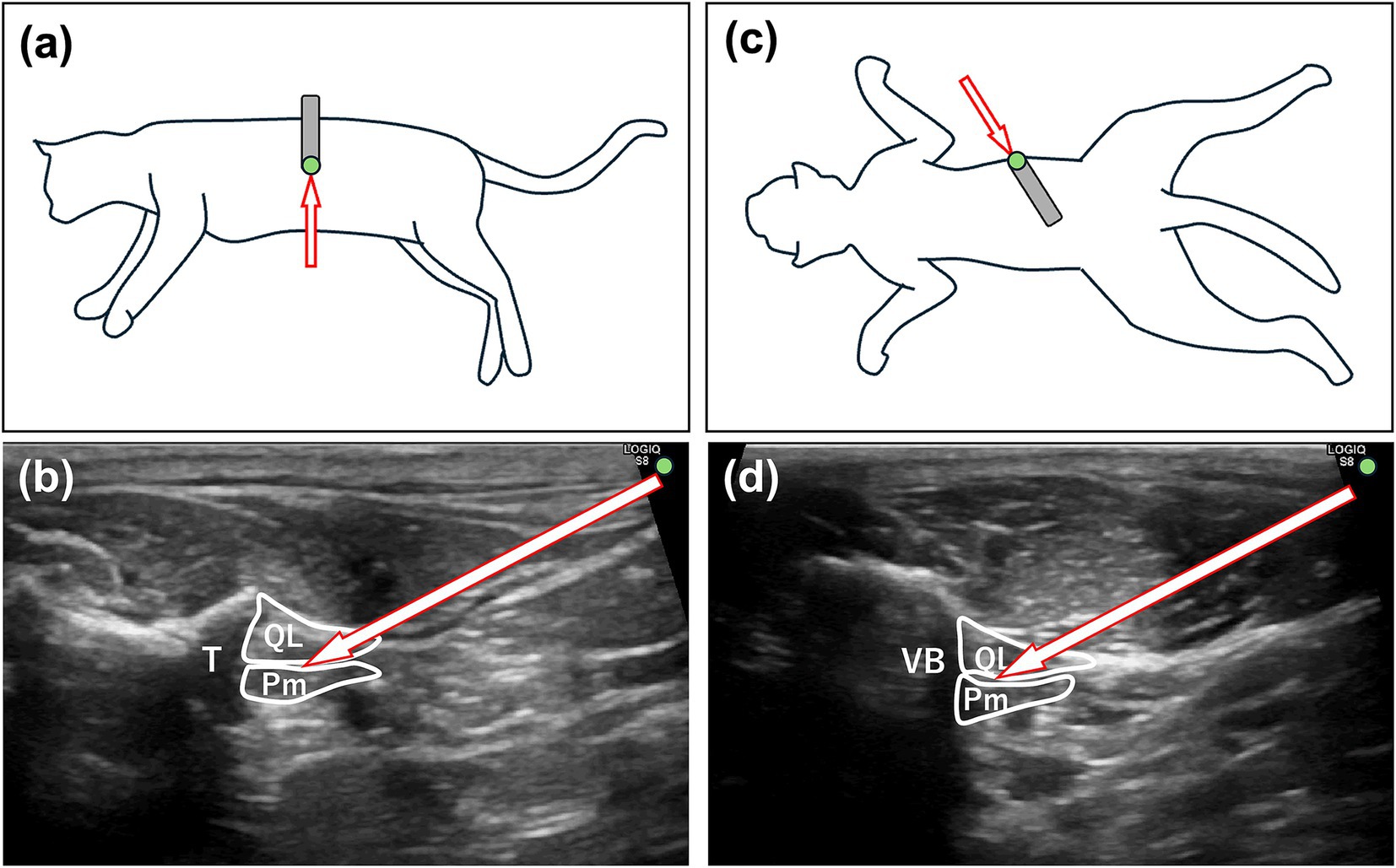
Figure 1. Ultrasound-guided quadratus lumborum block (QLB) in cats. (a) Ultrasound probe placement (gray square) and needle trajectory (arrow) in the CQLB group. A green circle indicates the position of the reference marker. (b) Corresponding ultrasound image in the CQLB group, with the arrow indicating the needle direction. A green circle indicates the direction of the reference marker. (c) Ultrasound probe placement (gray square) and needle trajectory (arrow) in the NQLB group. **A green circle indicates the position of the reference marker. (d) Corresponding ultrasound image in the NQLB group, with the arrow indicating the needle direction. A green circle indicates the direction of the reference marker. Pm, psoas minor muscle; QL, quadratus lumborum muscle; R, right; T, transverse process; VB, vertebral body; CQLB, conventional quadratus lumborum block; NQLB, novel quadratus lumborum block.
Cats in the NQLB group were positioned in sternal recumbency. The hair around the L2 region was clipped bilaterally, and the skin was disinfected with 70% alcohol. The space between the transverse processes of the second and the third lumbar vertebrae was located by counting from the last rib on the US image. The probe was placed perpendicular to the longitudinal axis of the cat at the identified location and then rotated parallel to the transverse processes to identify the QL and Pm muscles (Figure 1c). The probe marker was oriented toward the right side of the cat. Accordingly, in transverse imaging, the right side of the cat (lateral) appears on the right side of the screen, and the left side (medial) on the left. A 22-gage, 50-mm echogenic needle, similar to the one used in the CQLB group, was inserted at the lateral edge of the probe using the in-plane technique in a dorsolateral-to-ventromedial direction, with the tip positioned within the fascia between the QL and Pm muscles (Figure 1d). The injection procedure was then performed as described for the CQLB group.
During needle insertion, needle visibility and proximity to abdominal organs were evaluated subjectively by the anesthetist under real-time ultrasound guidance. Needle visibility was classified as excellent when the entire needle shaft was visualized on the ultrasound image, or poor when any part of the needle was not visible. Proximity to abdominal organs was categorized as near, if any abdominal organs (e.g., kidney or spleen) were visualized along the needle trajectory, or far if no abdominal organs were observed in the vicinity of the needle path. Any complications related to the QLB technique, including unintended needle trajectories with the potential to penetrate abdominal organs, were documented.
2.5 Intraoperative management and ovariohysterectomy
After completion of QLB, cats were positioned in dorsal recumbency. During surgical preparation for ovariohysterectomy, the isoflurane vaporizer was initially set to achieve an ETiso of 1.0%. ETiso, HR, fR, and MAP were recorded 10 min after completion of QLB (T0) and subsequently every 2.5 min throughout anesthesia. Throughout anesthesia, ETiso was adjusted as follows: increased by 0.2% if HR, fR, or MAP exceeded 20% of T0; increased by 0.5–1.0% if these parameters exceeded 30% of T0 or if the palpebral reflex, increased jaw tone, or purposeful movement were observed; decreased by 0.2% if MAP dropped below 70 mmHg; or the vaporizer setting was decreased by 0.5–1.0% if MAP dropped below 60 mmHg. If ETiso exceeded 2.8%, which was predetermined as approximately 1.5 times the minimum alveolar concentration (MAC) of isoflurane in cats (1.87%) based on previous studies (29), remifentanil (0.01 mg kg−1 h−1 IV; Ultiva Injection 2 mg; Janssen Pharmaceutical K. K., Tokyo, Japan) was administered as rescue analgesia, In all such cases, buprenorphine was also administered postoperatively for ongoing analgesic support. The occurrence of intraoperative rescue remifentanil administration was recorded, and data collected after its administration were excluded from further analysis. If MAP remained below 70 mmHg despite reducing ETiso to below 0.6% or until a light plane of anesthesia was observed, indicated by explicit palpebral reflex, increased jaw tone, or purposeful movement, appropriate interventions were initiated. These included a bolus administration of lactated Ringer’s solution (3 mL kg−1 over 5 min), atropine (0.04 mg kg−1 IV; Atropine Sulfate Injection 0.5 mg “Nipro”; Nipro ES Pharma Co., Ltd., Osaka, Japan), dopamine (0.005 mg kg−1 min−1 IV; Dopamine Hydrochloride Intravenous Infusion 100 mg “NP”; Nipro Corporation, Osaka, Japan), or ephedrine (0.1 mg kg−1 IV; Ephedrine “Nagai” Injection 40 mg; Nichi-Iko Pharmaceutical Co., Ltd., Toyama, Japan). Cases requiring pharmacological intervention for hypotension were excluded from data analysis. Intraoperative cardiovascular depression, defined as bradycardia (HR < 100 bpm) or hypotension (MAP < 70 mmHg requiring drug intervention), was documented.
Ovariohysterectomy was initiated 2.5–5.0 min after recording of T0 and performed by a single surgeon (YT) who was blinded to group allocation using a standardized technique (30). The surgery was divided into five periods: T1 (celiotomy), T2 (left ovariectomy), T3 (right ovariectomy), T4 (hysterectomy), and T5 (closure), with corresponding durations of 5, 5, 5, 5, and 7.5 min, respectively, as shown in Figure 2. During surgery, a forced-air warming blanket (Bair Hugger; 3 M Company, St. Paul, MN, United States) was used to maintain esophageal temperature above 36.5 °C. After surgery, isoflurane was discontinued, and the cats were extubated once the swallowing reflex was restored.

Figure 2. Timeline of surgical periods and corresponding elapsed times for ovariohysterectomy performed in this study. Solid black circles indicate the time points at which ETiso (end-tidal isoflurane concentration), HR (heart rate), and MAP (mean arterial pressure) are recorded.
2.6 Postoperative pain and QLB-related complications
Postoperative pain was assessed by the anesthetist (FT) using MCPS-SF and/or FGS at 1, 2, 4, and 8 h after extubation. If either or both pain scores reached 4 or higher, intramuscular buprenorphine (0.02 mg kg−1; Lepetan Injection 0.2 mg; Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan) was administered as rescue analgesia, and subsequent data were excluded from analysis. Pain scores were reassessed 30 min after the administration of the rescue analgesic, and additional doses were administered as needed. The number of cats in each group requiring postoperative rescue analgesia, along with the time to its first administration when applicable, was recorded.
Postoperative complications related to QLB, such as postoperative ataxia, needle-site bleeding, or signs of local anesthetic systemic toxicity, were monitored and documented if observed.
2.7 Statistical analysis
The sample size was calculated during the planning phase using G*Power version 3.1.9.7 (31), based on an expected 20% difference in mean end-tidal isoflurane concentration (ETiso), with a standard deviation (SD) of 0.34 derived from our internal pilot data. Assuming a two-sided test with 80% power and a 5% significance level, a minimum of 17 cats per group was required. To account for a potential dropout rate of approximately 10%, 19 cats were included in each group.
The total mean ETiso, HR, and MAP were calculated as the area under the curve (AUC), determined using the trapezoidal rule, and divided by the total surgery time. Similarly, the mean ETiso for each surgical period (T1–T5) was calculated as the AUC, determined using the trapezoidal rule, and divided by the respective procedure time. The Shapiro–Wilk test was used to examine the normality of data distribution. Continuous variables are expressed as mean ± SD if normally distributed and as median (range) if non-normally distributed. Rates of postoperative rescue analgesia requirement were expressed as proportions.
The total mean ETiso, HR, and MAP were compared between groups using a t-test. The mean ETiso during each procedure was compared using repeated measures analysis of variance, followed by Tukey’s post hoc test for pairwise comparisons at each time point. Postoperative incidence of rescue analgesia was compared using Kaplan–Meier survival analysis and the log-rank test. A p-value of <0.05 was considered statistically significant. All statistical analyses were performed using EZR version 1.68 (Saitama Medical Center, Jichi Medical University, Saitama, Japan).
3 Results
Demographic data, including age, body weight, BCS, breed distribution, and propofol dose for anesthetic induction, are summarized in Table 1. The groups were comparable in terms of all demographic variables. Total mean HR was 137 ± 12.3 in the CQLB group and 138 ± 7.22 in the NQLB group, with no statistically significant difference (p = 0.823; 95% CI: −7.65 to 6.13). Total mean MAP was 83 ± 6.91 mmHg in the CQLB group and 87.1 ± 9.12 mmHg in the NQLB group, also showing no significant difference (p = 0.141; 95% CI: −9.73 to 1.45).
The total ETiso was 1.39% ± 0.22 and 1.58% ± 0.31% in the NQLB and CQLB groups, respectively, with a significantly lower value observed in the NQLB group (p = 0.046; 95% CI: 0.00–0.37). The mean ETiso at each surgical period is shown in Figure 3. The mean ETiso in the NQLB group was significantly lower than that in the CQLB group at T3 (1.49% ± 0.34% vs. 1.79% ± 0.38%; p = 0.022; 95% CI: 0.04–0.54) and T4 (1.51% ± 0.30% vs. 1.90% ± 0.48%; p = 0.007; 95% CI: 0.11–0.66), whereas no significant differences were observed at other surgical periods (T0: p = 0.228; 95% CI: −0.14–0.04, T1: p = 0.109; 95% CI: −0.18–0.02, T2: p = 0.819; 95% CI: −0.14–0.17, T5: p = 0.052; 95% CI: −0.00–0.58).
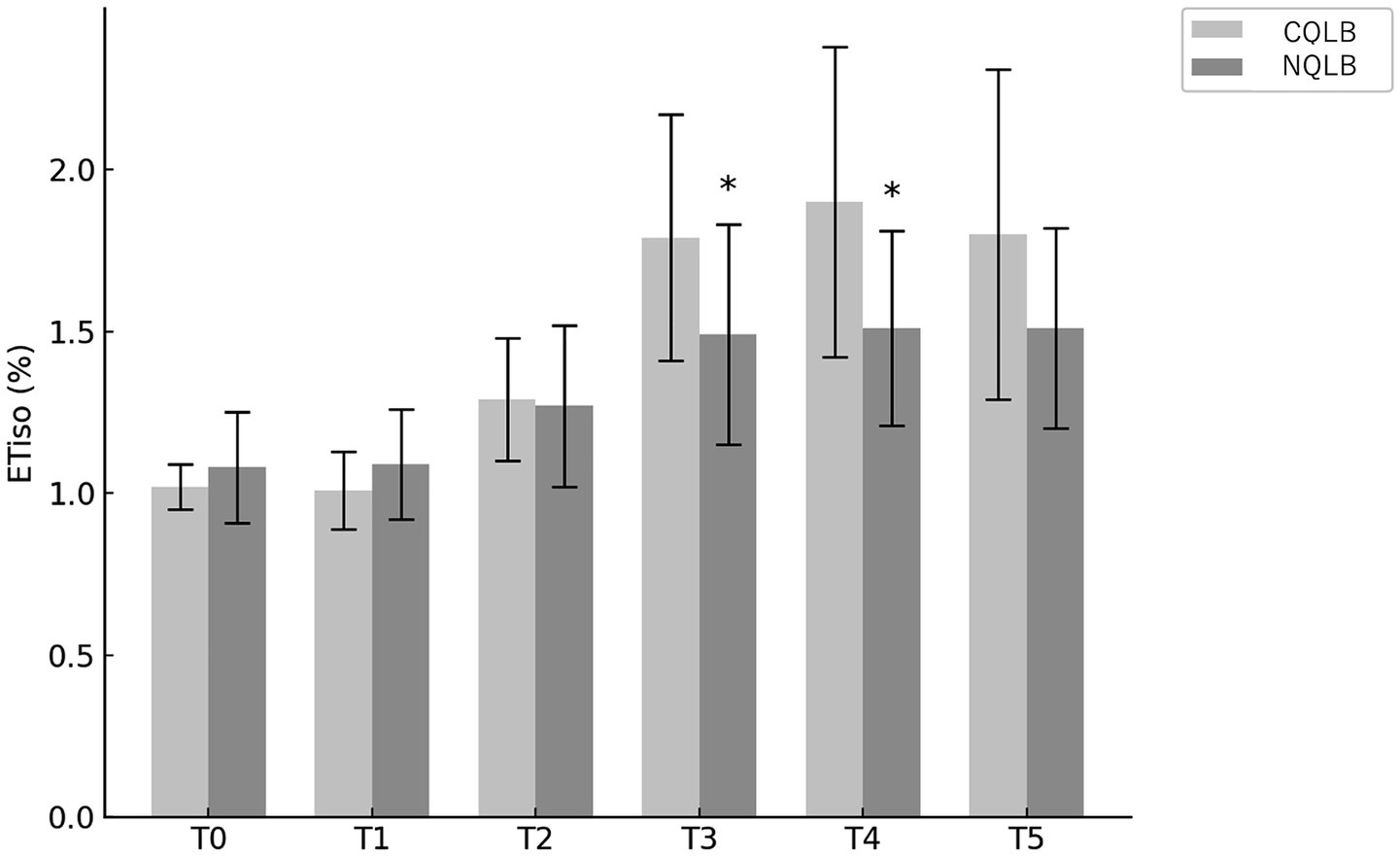
Figure 3. Mean end-tidal isoflurane concentration (ETiso) was recorded in 35 cats (CQLB group: n = 17; NQLB group: n = 18) enrolled in the study. Bars represent the mean ETiso, and whiskers indicate the standard deviation. The symbol (*) denotes statistically significant differences between groups. CQLB: refers to the conventional quadratus lumborum block (QLB) performed in lateral recumbency, and NQLB refers to the novel QLB performed in sternal recumbency. T0 represents the baseline, while T1 through T5 correspond to specific surgical stages: T1, celiotomy; T2, left ovariectomy; T3, right ovariectomy; T4, hysterectomy; and T5, closure.
In both groups, the visibility of the needle tract was rated as excellent in all cats, as the entire shaft was visualized on ultrasound images. In the CQLB group, at least one abdominal organ—such as the right kidney, spleen, or aorta—was visualized adjacent to the needle trajectory in all cats and thus categorized as “near.” In contrast, no abdominal organs were detected along the needle trajectory in the NQLB group, and all cases were classified as “far.” No intraoperative bradycardia or hypotension requiring drug treatment was observed in either group. Additionally, no complications related to the QLB technique were observed during anesthesia.
The proportion of cats requiring postoperative rescue analgesia was 9/17 (52.9%) in the CQLB group and 8/18 (44.4%) in the NQLB group. Figure 4 presents the Kaplan–Meier curve depicting the probability of not requiring rescue analgesia over time in both groups. No significant difference in the requirement for postoperative rescue analgesia was observed between the CQLB and NQLB groups (p = 0.505). Among the cats requiring rescue analgesia, administration was triggered by the FGS alone in 5/9 cats in the CQLB group and 7/8 cats in the NQLB group, and by both the FGS and the MCPS-SF in 4/9 cats in the CQLB group and 1/8 cats in the NQLB group. All cats that received rescue analgesia required only a single dose. Postoperative pain assessment was censored for 4/17 (23.5%) cats in the CQLB group and 2/18 (11.1%) cats in the NQLB group due to aggressive behavior. These censored cats were administered buprenorphine (0.02 mg kg−1 IM) regardless of their final pain scores. No clinically recognized postoperative complications related to the QLB technique were observed.
Figure 4. Kaplan–Meier curve depicting the rate and timing of postoperative rescue analgesic requirement in 35 cats (CQLB group: n = 17; NQLB group: n = 18). The x-axis (RescueTime) indicates the timing of postoperative assessments, while the y-axis represents the probability of not requiring postoperative rescue analgesia. Horizontal short lines on each curve represent censoring, with the number of lines indicating the number of censored cats. CQLB refers to the conventional quadratus lumborum block (QLB) performed in lateral recumbency, and NQLB refers to the novel QLB performed in sternal recumbency.
4 Discussion
The results of this study suggest that the novel QLB technique performed in sternal recumbency was associated with a lower isoflurane requirement compared to the conventional technique in lateral recumbency. The absence of complications, such as cardiovascular depression or abdominal organ penetration, further supports the safety of this technique.
In the present study, ovariohysterectomy was selected as a representative mid-to-lower abdominal surgery due to its involvement of a wider area of visceral innervation compared to ovariectomy, allowing for a more comprehensive evaluation of the novel QLB’s antinociceptive effects.
The isoflurane-sparing effect, observed particularly during right ovariectomy and hysterectomy performed using the novel technique, may have occurred primarily as a result of antinociceptive efficacy. Reducing the requirement for isoflurane is beneficial for cats, because this agent has been reported to induce dose-dependent cardiopulmonary depression (3). This reduction may contribute to improved hemodynamic stability during anesthesia, supporting the perioperative advantages of this novel technique.
The differences in isoflurane requirements between the groups during certain phases of the procedure in the present study may be explained by variations in local anesthetic distribution. The novel dorsolateral-to-ventromedial needle trajectory may improve local anesthetic spread to the sympathetic trunk and visceral afferents compared to the conventional approach. However, a previous cadaveric study (23) reported no significant difference in injectate spread in dorsolateral-to-ventromedial and ventrolateral-to-dorsomedial lateral recumbency. This difference in findings may be attributed to the distinct nature of cadaveric and live animal studies, as factors such as tissue compliance and perfusion can influence injectate distribution in vivo (32, 33). Additionally, the needle bevel faced the ventrolateral direction in the dorsolateral-to-ventromedial approach in the previous cadaveric study (23), while it faced medially in the present study. This difference in bevel direction may partly explain the discrepancy in results between the two studies, as seen in other fascial-plane blocks in humans (33, 34). Future studies focusing on needle direction, bevel orientation, and the spread of the injectate in QLB are warranted.
Interestingly, we failed to detect a superior isoflurane-sparing effect in the novel QLB during left ovariectomy. No previous studies have reported differences in injectate spread between the left and right sides for QLB in any species. However, local anesthetic distribution may differ between sides in vivo, as anatomical variations in the QLB target have been observed between the right and left sides in human studies (35). Additionally, the fixed 5-min duration for ovariectomy in the present study may have been too short to capture detectable changes in ETiso during left ovariectomy.
A progressive increase in ETiso was observed from T3 onward in both groups, which may reflect the waning effect of the α2-agonist used for premedication, as its peak plasma concentration (Tmax) is reached at approximately 26 min and declines thereafter (36). Although it is difficult to determine whether the changes in ETiso during each surgical phase were due to the block or other factors (e.g., nociceptive stimuli or anesthetic effects), both groups followed the same anesthetic protocol. Therefore, such systemic influences were equally present and are unlikely to explain the between-group differences observed during these phases.
A feline cadaveric study on QLB using the ventrolateral-to-dorsomedial needle insertion technique in lateral recumbency reported that the colorant stained the retroperitoneal cavity in all cadavers (22). The same authors later investigated the dorsolateral-to-ventromedial technique in lateral recumbency to reduce the risk of penetrating abdominal organs; however, poor needle visibility was reported, likely due to interference from surrounding anatomical structures (23). In contrast, the novel QLB technique used in the present study offered excellent needle visibility and a trajectory distant from abdominal organs, minimizing the perceived risk of organ penetration. These features likely contributed to the operator’s impression of enhanced procedural safety. In this study, the proximity between the needle and abdominal organs was assessed subjectively, as simple absolute distance does not necessarily reflect the true safety profile. Future investigations are warranted to objectively evaluate the safety of this novel technique using more sophisticated and standardized methods.
A feline cadaveric study showed that both QLB with dorsolateral-to-ventromedial and ventrolateral-to-dorsomedial needle insertion in lateral recumbency showed similar injectate spread patterns to the lumbar nerves innervating the abdominal wall (23). Consistent with these findings, similar isoflurane requirements during celiotomy and closure were observed for both QLB techniques in the present live animal study. Given the absence of significant differences in isoflurane requirements between groups during these phases, the total difference in isoflurane requirements appears relatively small.
In previous studies using QLB in cats, the incidence of hypotension was very low (20, 21). Consistent with these findings, both QLB techniques in the present study provided hemodynamic stability. A previous clinical study comparing the effects of QLB and sacrococcygeal epidural anesthesia (SCE) in cats undergoing ovariectomy reported a significantly lower proportion of intraoperative hypotension in the QLB group compared with that in the SCE group (19). These positive hemodynamic profiles support the selection of QLB as a beneficial option.
Postoperative pain was assessed using both the MCPS-SF and FGS to minimize the risk of incomplete assessments, given that no exclusion criteria regarding behavior were applied. However, in the study, some cats could not be assessed with both scales due to their aggressive behavior. Hence, it was necessary to censor the postoperative pain assessments.
Postoperative rescue analgesic requirements in this study were higher than those reported in previous studies (19, 20), which observed considerably lower rates of postoperative analgesic use (5.6–20.0%). This discrepancy may be attributed to differences in the concentration of local anesthetic used for QLB. In this study, 0.125% bupivacaine was used. The efficacy of this low concentration for interfascial plane blocks has not been specifically studied in veterinary medicine. However, the dose was chosen based on recommendations for cats (37, 38). Additionally, the selected dose considered the relatively high injection volume (0.4 mL kg−1 per side) required for adequate fascial plane distribution. The use of lower concentrations is to minimize the risk of systemic toxicity while still achieving effective analgesia, though it may have contributed to the higher rate of postoperative rescue analgesic use observed in this study. Additionally, previous studies (19–21) administered both methadone and dexmedetomidine as premedication, whereas only dexmedetomidine was used in the present study. Methadone provides effective postoperative analgesia in cats undergoing ovariohysterectomy (39), and this may have contributed to differences in postoperative lock analgesic requirements between studies. Furthermore, as observed in previous studies (40), the aggressive behavior of some cats in this study may have compromised the reliability of pain assessments. Further research is needed to evaluate the postoperative analgesic efficacy of novel QLB techniques, particularly in a cat population with moderate behavior, to minimize behavioral bias in pain assessment, and to explore broader clinical benefits such as postoperative well-being and recovery.
The present study had some limitations. First, the anesthetist, who also performed postoperative pain assessments, was not blinded to group allocation, potentially introducing bias. However, intraoperative ETiso adjustments were based on objective cardiopulmonary parameters, which likely minimized bias during data collection. The subjective nature of the postoperative pain scale may have affected reliability, and future studies should include blinded assessors to further reduce bias. Second, the novel QLB method used in this study has not been evaluated in cadaveric studies, leaving its sonoanatomy unverified against gross anatomy and its injectate spread unassessed using colorants or contrast agents. Similarly, while the injection site and volume of the local anesthetic were determined based on previous studies (19, 21, 22), the optimal parameters for QLB in cats remain unidentified. Further research is needed to investigate the sonoanatomy, injectate distribution, and optimal injection site and volume for this technique. Third, this study lacked a control group (no QLB), limiting the evaluation to a comparison of the efficacy of the two techniques. A negative control group was excluded due to ethical concerns, as the pilot study showed that cats without QLB required an ETiso exceeding 2.8%. While prior studies demonstrated the efficacy of QLB in lateral recumbency (19–21), the novel technique used in this study achieved an even greater reduction in isoflurane requirements, suggesting enhanced efficacy despite the absence of a negative control group. Fourth, intraoperative changes in cardiovascular parameters induced by noxious stimuli were managed by adjusting isoflurane concentration. Although, currently recommended practices suggest addressing such changes with antinociceptive interventions, such as opioid administration, the authors opted to titrate isoflurane in response to nociceptive signs due to concerns about adverse effects reported in cats receiving potent opioids—particularly fentanyl and remifentanil—including dysphoria, increased locomotor activity, tachycardia, and acid–base disturbances (41, 42). Furthermore, the 2.8% ETiso threshold used to trigger intraoperative rescue analgesia in the present study was based on a MAC of 1.86% in cats reported by Belli et al. (27). As reported, MAC values vary substantially among studies, establishing a definitive cut-off for adequate surgical anesthetic depth remains challenging. Future studies are warranted to compare opioid-based protocols with the novel QLB technique and to evaluate the potential opioid-sparing effect of this approach under protocols allowing timely systemic analgesic rescue, including investigation of its ability to reduce opioid-related adverse effects. Finally, the sample size estimation in this study was based on the total ETiso requirement as the primary outcome. Therefore, the interpretation of other analyses, including mean ETiso at each surgical period, mean total HR and MAP, and postoperative rescue analgesic requirements, is limited. Additionally, the relatively small sample size (n = 35) may limit the generalizability of these findings to broader clinical settings. Any significant differences observed in these secondary outcomes may not accurately reflect the true effect size owing to the possibility of an overestimated effect. Conversely, non-significant differences may have resulted from insufficient statistical power (type II error), potentially obscuring clinically relevant findings. Future studies with larger sample sizes specifically designed to evaluate these secondary outcomes and enhance the generalizability of the findings are warranted.
5 Conclusion
This study demonstrated that the novel quadratus lumborum block (QLB) technique, performed in sternal recumbency with a dorsolateral-to-ventromedial needle trajectory, was associated with a reduced isoflurane requirement and appeared to enhance needle placement safety when compared to the conventional lateral recumbency approach in cats undergoing ovariohysterectomy. However, given the limitations of the present study—including lack of blinding, small sample size, and the sole focus on intraoperative isoflurane concentrations—these results should be interpreted with caution.
Future investigations are warranted to evaluate the postoperative analgesic efficacy of this technique, optimize the injection site and volume, and assess the spread of local anesthetic agents in both cadaveric and live feline models.
Overall, these findings may contribute to the refinement of regional anesthetic protocols in feline surgery.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal studies were approved by The Institutional Animal Care and Use Committee of Kimura Animal Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
FT: Writing – original draft, Investigation, Methodology, Formal analysis, Project administration, Conceptualization. YT: Writing – review & editing. TK: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors thank the staff at Seibozaka Animal Hospital for their technical and organizational support during the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that Gen AI was used in the creation of this manuscript. To improve language and readability using ChatGPT (OpenAI). The content was subsequently reviewed and edited by the authors to ensure accuracy and integrity.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
1. Campoy, L. Development of enhanced recovery after surgery (ERAS) protocols in veterinary medicine through a one-health approach: the role of anesthesia and locoregional techniques. J Am Vet Med Assoc. (2022) 260:1751–9. doi: 10.2460/javma.22.08.0354
2. Grubb, T, and Lobprise, H. Local and regional anaesthesia in dogs and cats: overview of concepts and drugs (part 1). Vet Med Sci. (2020) 6:209–17. doi: 10.1002/vms3.219
3. Steffey, EP, and Howland, D. Isoflurane potency in the dog and cat. Am J Vet Res. (1977) 38:1833–6. doi: 10.2460/ajvr.1977.38.11.1833
4. Hodgson, DS, Dunlop, CI, Chapman, PL, and Grandy, JL. Cardiopulmonary effects of anesthesia induced and maintained with isoflurane in cats. Am J Vet Res. (1998) 59:182–5. doi: 10.2460/ajvr.1998.59.02.182
5. Hikasa, Y, Takase, K, and Ogasawara, S. Cardiopulmonary effects of sevoflurane, isoflurane, halothane, and enflurane in cats. Am J Vet Res. (1996) 57:1259–66.
6. Elzahaby, D, Mirra, A, Levionnois, OL, and Spadavecchia, C. Inhalational anaesthetic agent consumption within a multidisciplinary veterinary teaching hospital: an environmental audit. Sci Rep. (2024) 14:17973. doi: 10.1038/s41598-024-68157-5
7. Souza, SS, Intelisano, TR, De Biaggi, CP, Moura, CA, Selmi, AL, Dias, RA, et al. Cardiopulmonary and isoflurane-sparing effects of epidural or intravenous infusion of dexmedetomidine in cats undergoing surgery with epidural lidocaine. Vet Anaesth Analg. (2010) 37:106–15. doi: 10.1111/j.1467-2995.2009.00512.x
8. Dourado, A, Gomes, A, Teixeira, P, Lobo, L, Azevedo, JT, Dias, IR, et al. Antinociceptive effect of a sacro-coccygeal epidural of morphine and lidocaine in cats undergoing ovariohysterectomy. Vet Sci. (2022) 9:623. doi: 10.3390/vetsci9110623
9. Skouropoulou, D, Lacitignola, L, Centonze, P, Simone, A, Crovace, AM, and Staffieri, F. Perioperative analgesic effects of an ultrasound-guided transversus abdominis plane block with a mixture of bupivacaine and lidocaine in cats undergoing ovariectomy. Vet Anaesth Analg. (2018) 45:374–83. doi: 10.1016/j.vaa.2018.01.005
10. Josso, M, Topie, O, Bencharif, D, Doran, DH, Desbois, C, and Touzot-Jourde, G. Ultrasound-guided rectus abdominis sheath block in cats undergoing ovariectomy: a prospective, randomized, investigator-blinded, placebo-controlled clinical trial. J Vet Sci. (2022) 7:000218. doi: 10.23880/oajvsr-16000218
11. Blanco, R. TAP block under ultrasound guidance: the description of a “non-pops technique”. Reg Anesth Pain Med. (2007) 32:130–5.
12. Akerman, M, Pejčić, N, and Veličković, I. A review of the quadratus lumborum block and ERAS. Front Med (Lausanne). (2018) 5:44. doi: 10.3389/fmed.2018.00044
13. Baran, O. Quadratus lumborum and erector spinae plane blocks are effective for analgesia in laparoscopic hysterectomy: a randomized controlled trial. Eur Rev Med Pharmacol Sci. (2023) 27:11323–33. doi: 10.26355/eurrev_202312_34571
14. Omara, AF, Elbadry, AA, and Abo Hagar, AM. Quadratus lumborum block against coupled transversus abdominis block and ilioinguinal/iliohypogastric nerve blocks for postoperative analgesia after total abdominal hysterectomy: a randomized controlled trial. Anesthesiol Pain Med. (2023) 13:e134845. doi: 10.5812/aapm-134845
15. Baran, O, Şahin, A, and Arar, C. Comparative efficacy of erector spinae plane and quadratus lumborum blocks in managing postoperative pain for total abdominal hysterectomy: a randomized controlled trial. Medicine. (2024) 103:e40313. doi: 10.1097/MD.0000000000040313
16. Zhao, Z, Xu, K, Zhang, Y, Chen, G, and Zhou, Y. Quadratus lumborum block for postoperative analgesia after cesarean section: a meta-analysis of randomized controlled trials with trial sequential analysis. Sci Rep. (2021) 11:18104. doi: 10.1038/s41598-021-96546-7
17. Yetik, F, Yilmaz, C, Karasu, D, Haliloğlu Dastan, N, Dayioğlu, M, and Baytar, Ç. Comparison of ultrasound-guided quadratus lumborum block-2 and quadratus lumborum block-3 for postoperative pain in cesarean section: a randomized clinical trial. Medicine (Baltimore). (2022) 101:e31844. doi: 10.1097/MD.0000000000031844
18. Wang, J, Chu, T, Sun, R, and Xu, A. Analgesic efficacy of quadratus lumborum block in patients undergoing nephrectomy: a systematic review and meta-analysis. Pain Med. (2023) 24:476–87. doi: 10.1093/pm/pnac166
19. dos-Santos, JD, Ginja, M, Martins, J, Cabral, P, Alves-Pimenta, S, Ribeiro, L, et al. Comparison between bilateral ultrasound-guided quadratus lumborum block and sacrococcygeal epidural in cats undergoing ovariectomy. Vet Sci. (2024) 11:25. doi: 10.3390/vetsci11010025
20. Lazzarini, E, Gioeni, D, Del Prete, G, Baio, M, and Carotenuto, AM. Ultrasound-guided quadratus lumborum block with 0.5 ml of 0.2% bupivacaine/kg is a valuable perioperative analgesic adjunct for cats undergoing ovariectomy. J Am Vet Med Assoc. (2024) 262:1491–8. doi: 10.2460/javma.24.03.0202
21. Paolini, A, Bianchi, A, Bucci, R, Parrillo, S, Di Giosia, A, Ristori, C, et al. Use of a quadratus lumborum block in queens undergoing ovariectomy: a randomised controlled trial. J Feline Med Surg. (2024) 26:1098612X241275277. doi: 10.1177/1098612X241275277
22. dos-Santos, JD, Ginja, M, Alves-Pimenta, S, Otero, PE, Ribeiro, L, and Colaço, B. A description of an ultrasound-guided technique for a quadratus lumborum block in the cat: a cadaver study. Vet Anaesth Analg. (2021) 48:804–8. doi: 10.1016/j.vaa.2021.03.017
23. dos-Santos, JD, Ginja, M, Alves-Pimenta, S, Otero, E, Ribeiro, L, and Colaço, B. Comparison of dorsoventral and ventrodorsal approaches for ultrasound-guided quadratus lumborum block in cats: a cadaver study. Vet Anaesth Analg. (2022) 49:481–9. doi: 10.1016/j.vaa.2022.05.003
24. Polo-Paredes, G, Laredo, FG, Gil, F, Soler, M, Agut, A, and Belda, E. Modified ultrasound-guided dorsal quadratus lumborum block in cat cadavers. Animals (Basel). (2023) 13:3798. doi: 10.3390/ani13243798
25. Viscasillas, J, Terrado, J, Marti-Scharfhausen, R, Castiñeiras, D, Esteve, V, Clancy, N, et al. A modified approach for the ultrasound-guided quadratus lumborum block in dogs: a cadaveric study. Animals (Basel). (2021) 11:2945. doi: 10.3390/ani11102945
26. Laflamme, DP. Development and validation of a body condition score for cats. Feline Pract. (1997) 25:13–8.
27. Belli, M, de Oliveira, AR, de Lima, MT, Trindade, PHE, Steagall, PV, and Luna, SPL. Clinical validation of the short and long Unesp-Botucatu scales for feline pain assessment. PeerJ. (2021) 9:e11225. doi: 10.7717/peerj.11225
28. Evangelista, MC, Watanabe, R, Leung, VSY, Monteiro, BP, O’Toole, E, Pang, DSJ, et al. Facial expressions of pain in cats: the development and validation of a feline grimace scale. Sci Rep. (2019) 9:19128. doi: 10.1038/s41598-019-55693-8
29. Brosnan, RJ, Pypendop, BH, and Cenani, A. Effects of trazodone and dexmedetomidine on fentanyl-mediated reduction of isoflurane minimum alveolar concentration in cats. Vet Anaesth Analg. (2024) 51:80–9. doi: 10.1016/j.vaa.2023.09.130
31. Faul, F, Erdfelder, E, Lang, A-G, and Buchner, A. G*power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. (2007) 39:175–91. doi: 10.3758/bf03193146
32. Elsharkawy, H, Pawa, A, and Mariano, ER. Interfascial plane blocks: Back to basics. Reg Anesth Pain Med. (2018) 43:341–6. doi: 10.1097/AAP.0000000000000750
33. Greenhalgh, K, Womack, J, and Marcangelo, S. Injectate spread in erector spinae plane block. Anaesthesia. (2019) 74:126–7. doi: 10.1111/anae.14523
34. Sondekoppam, RV, and Tsui, BCH. ‘Minimally invasive’ regional anesthesia and the expanding use of interfascial plane blocks: the need for more systematic evaluation. Can J Anaesth. (2019) 66:855–63. doi: 10.1007/s12630-019-01400-0
35. Diwan, S, Kulkarni, M, Kulkarni, N, and Nair, A. Journey of a quadratus lumborum plane catheter: is it important to know? Saudi J Anaesth. (2019) 13:278–9. doi: 10.4103/sja.SJA_787_18
36. Fernandes, NS, Passos, YDB, Arcoverde, KN, Mouta, AN, Paiva, TC, Oliveira, KDS, et al. Clinical effects and pharmacokinetic profile of intramuscular dexmedetomidine (10 μg/kg) in cats. Animals (Basel). (2024) 14:2274. doi: 10.3390/ani14152274
37. Lumb, WV, and Jones, EW. Veterinary anesthesia and analgesia. 5th ed. Hoboken, NJ: Wiley-Blackwell (2019). 540 p.
38. Seymour, C, and Gleed, R eds. Small animal anesthetic techniques. 2nd ed. Hoboken, NJ: Wiley-Blackwell (2019). 289 p.
39. Warne, LN, Beths, T, Holm, M, and Bauquier, SH. Comparison of perioperative analgesic efficacy between methadone and butorphanol in cats. J Am Vet Med Assoc. (2013) 243:844–50. doi: 10.2460/javma.243.6.844
40. Buisman, M, Hasiuk, MMM, Gunn, M, and Pang, DSJ. The influence of demeanor on scores from two validated feline pain assessment scales during the perioperative period. Vet Anaesth Analg. (2017) 44:646–55. doi: 10.1016/j.vaa.2016.09.001
41. Brosnan, RJ, Pypendop, BH, Siao, KT, and Stanley, SD. Effects of remifentanil on measures of anesthetic immobility and analgesia in cats. Am J Vet Res. (2010) 70:1065–71. doi: 10.2460/ajvr.70.9.1065
Keywords: feline, QLB, isoflurane-sparing effect, regional anesthesia, ultrasound-guided nerve block
Citation: Takusagawa F, Takusagawa Y and Kimura T (2025) Effect of a novel sternal recumbency approach for quadratus lumborum block on isoflurane requirements in cats undergoing ovariohysterectomy. Front. Vet. Sci. 12:1648665. doi: 10.3389/fvets.2025.1648665
Edited by:
Angela Briganti, University of Pisa, ItalyReviewed by:
Magdalena Schrank, University of Padua, ItalyVefa Tohumcu, Atatürk Üniversitesi, Türkiye
Copyright © 2025 Takusagawa, Takusagawa and Kimura. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fumihiko Takusagawa, VGFrdXNhZ2F3YUBzZWlib3pha2EtYWguY29t
 Fumihiko Takusagawa
Fumihiko Takusagawa Yoshimi Takusagawa1
Yoshimi Takusagawa1 Taro Kimura
Taro Kimura