- 1School of Life and Environmental Sciences, Faculty of Science, The University of Sydney, Sydney, NSW, Australia
- 2Department of Veterinary Medicine and Animal Production, University of Naples Federico II, Naples, Italy
- 3Department of Animal Reproduction, Faculty of Veterinary Medicine and Animal Science, University of São Paulo, São Paulo, Brazil
The establishment of a pregnancy in cattle relies on crosstalk between an embryo with high developmental competence and a responsive uterus. This often fails and the pregnancy rate in cattle is around 60–70% with natural mating and 50–60% for embryo transfer, with pregnancies typically higher in beef than high performing dairy. These pregnancy rates are primarily due to the loss of embryos in the 21-day window from fertilization to the initiation of attachment of the conceptus to the uterus. Considerable research has been devoted to defining high quality embryos; however, embryonic mortality remains a major cause of pregnancy failure. The latter highlights the critical importance of uterine receptivity in establishing a pregnancy. The uterus must be responsive to signals from the developing embryo to undergo a major structural and functional transformation to prepare for attachment of the conceptus and establishment of pregnancy. The chemokine CXCL12 and its receptor CXCR4 are expressed across somatic and neural tissues and are associated with tissue remodeling including angiogenesis. These are features of the change the uterus undergoes as it develops receptivity to the conceptus. The developing embryo produces CXCL12 and CXCR4 is present in uterine tissue, and a role for the CXCL12-CXCR4 axis have been demonstrated in early pregnancy. Chemokines including CXCL12 are likely to be important in embryonic survival and pregnancy in cattle.
1 Background
Pregnancy rates in cattle following natural mating or with assisted reproductive technology have remained relatively constant at around 60–70% with natural mating and 50–60% for embryo transfer, with pregnancies typically higher in beef than high performing dairy (1–3). The primary reason why pregnancy rates have not improved as might have been expected is the failure to overcome the large embryonic loss that occurs in the period before and during the attachment of an embryo to the uterus to establish a pregnancy (2, 4–6). The period of early embryonic development involves continuous crosstalk between the embryo and uterus (7, 8). The embryo initiates this crosstalk by secreting interferon tau (IFNτ) which prevents the uterus from generating an immune response against the allogeneic embryo (9–12). Interferon tau-stimulated gene expression in blood mononuclear cells was evaluated as a biomarker of early pregnancy in cattle (13–16). The developing embryo also secretes factors that induce changes in the structure and function of the uterus, which prepares the uterus for embryonic attachment (16–20). The preparation of the uterus for attachment confers uterine receptivity (21, 22). Chemokines and their receptors have an important role in this process and an example is the embryonic chemokine ligand stromal-derived factor 1α (CXCL12) which binds to its uterine receptor CXCR4 (23). As noted below, CXCL12 and CXCR4 are expressed across somatic and neural tissues and are associated with tissue remodeling including angiogenesis. These are features of changes the uterus undergoes as it develops receptivity to the conceptus. The present review draws on information for cytokines and their receptors and in particular CXCL12-CXCR4 in female reproduction in several species to highlight the need for further research in cattle. A potential outcome of further research could be the identification of CXCL12-CXCR4 gene polymorphisms that are linked to uterine receptivity and fertility in cattle (24). This would require the collection of phenotypic information on large cohorts of cattle to achieve statistical power to identify meaningful polymorphisms. Given fertilization and formation of a zygote in cattle is typically greater than 75%, we have argued that the next step change in reproductive success in cattle will require a reduction in embryonic loss with both natural mating and assisted reproductive technology (10, 20, 25, 26).
2 Female effect on fertility in cattle
The capacity of female cattle to conceive and wean a calf on an annual basis is the primary driver of profitability in cattle enterprises (27). As noted above, fertilization rates in cattle are typically greater than 75% with both natural mating and artificial insemination (25). Fertilization per se is therefore not the major reason for reproductive failure in cattle. The main cause of reproductive failure in cattle, and indeed females of other species, is the large loss of embryos that occurs in the 21-day window from fertilization to the initiation of attachment of the embryo to the uterus (20, 25, 28, 29). Embryonic survival was identified early as arguably the most important factor in determining pregnancy outcome in cattle (30–32). In one study, a significant recipient effect was observed in pregnancy rate when Hereford x Friesian heifers received six cycles of embryo transfer (31). Heifers retrospectively classified as ‘high fertility’ had an overall pregnancy rate of 76% and heifers classified as ‘low fertility’ had a pregnancy rate of 11% (31). At day 14 after embryo transfer, more embryos had undergone elongation in ‘high fertility’ heifers (67%) compared with ‘low fertility’ heifers (14%) (31). The heifer effect was noticeable during the period of embryonic attachment and pregnancy establishment, with no apparent effect after day 60 when the determination of the effect was diminished (31, 32). In another study also involving serial embryo transfer, beef heifers classified ‘high fertile’ showed a pregnancy rate of 71% compared with a pregnancy rate of 20% for heifers classified ‘infertile’ (33). Similar with the earlier study in dairy heifers, elongating conceptuses were longer in ‘high fertile’ beef heifers compared with ‘infertile’ heifers (33). ‘High fertile’ heifers showed greater uterine expression of genes associated with conceptus-uterus crosstalk which was interpreted to indicate that ‘high fertile’ heifers had a greater capacity to support conceptus growth, attachment and pregnancy (33). Studies in Holstein cows led to the conclusion that the difference in fertility between ‘high fertile’ (Fert+) and ‘low fertile’ (Fert-) cows was related to embryonic and uterine events after day 7, which likely included the capacity of cows to support ongoing embryonic development, attachment and pregnancy (34). In the above studies, oocytes and embryos from high and low fertile females did not differ in gene expression and other functional parameters providing further evidence of the importance of the uterine response to the embryo in pregnancy (4, 33, 34). Pregnancy does, however, rely on the combination of a good quality embryo with high developmental competence and a responsive uterus (4).
3 Uterine (endometrial) receptivity
The capacity of the uterus to support attachment of the conceptus, followed by the events that establish a pregnancy, relies on uterine (endometrial) receptivity irrespective of the type of placentation. The change from a non-receptive to receptive uterus occurs in response to the conceptus and involves major changes in uterine structure and function (16–18, 35, 36). The endometrium in cattle undergoes a major change in preparation for embryonic attachment and pregnancy (8, 20). The ovarian steroids oestradiol and progesterone induce initial changes in the uterine endometrium in cattle and further change is a result of ‘mutual reprogramming’ between the conceptus and uterus (21, 22). Changes in endometrial gene expression around day 15 in cattle are induced by embryonic IFNτ (16). The application of machine learning identified endometrial transcriptomic biomarkers that predicted uterine receptivity with around 95% accuracy in cattle (37, 38). The latter suggested that establishing uterine receptivity through a uterine biopsy could potentially be used as a fertility trait in cattle (38, 39). Embryos also induce changes in uterine fluid microRNAs and exosomes in cattle (40, 41). Uterine receptivity has been extensively studied in women to more precisely define the ‘implantation window’ in conjunction with efforts to increase the efficiency of IVF and embryo transfer (42–46). In Mediterranean buffaloes, the period of implantation is associated with changes in blood flow and capillary permeability of uterine caruncles (47, 48).
4 Chemokines and their receptors
Chemokines are a family of chemoattractant cytokines that have important roles in cell migration and angiogenesis (49–51). Cell differentiation and migration, and angiogenesis, are central to tumor metastasis and a large body of literature describes the role of CXCL12 in conditioning stromal cells for invasion by cancer cells (52–58). Stromal-derived factor 1α (CXCL12) is an important chemokine that is expressed in both somatic and neural tissues (52, 59–61). The receptor for CXCL12, CXCR4, is also widely distributed in somatic and neural tissues (53, 55, 62). Most studies on CXCR4 have been in cancer biology and other diseases (50, 52–55, 61, 63–66). CXCL12 can also bind to the orphan receptor CXCR7 (ACKR3) which functions as a scavenger and could have a role in the local actions of CXCL12 (56).
Both CXCL12 and CXCR4 have been characterized at the genomic and protein level. In cattle, the gene CXCL12 is identified as ENSBTAG00000005077 (primary assembly Bos taurus genome, ARS-UCD2.0) and is located at base-pair position 28:45021867–450525521. The gene has two variants each of which contains four exons. ENSEMBL identifiers for the transcripts are ENSBTAT00000015300.1 (CXCL12-201) and ENSBTAT00000031279.5 (CXCL12-202). Cattle CXCR4 is tagged ENSBTAG00000001060, is located at 2:612249996–61254590, and has three transcripts and also splice variants2. The human CXCL12 gene is located at 10q11.1 and the promoter region has binding sites for the transcription factors SP1 and CTF (52, 60, 66). CXCL12 is unique among CXC chemokines in that it has differential mRNA splicing with six splice variants which give rise to six different isoforms in humans, with three isoforms in mice (60). Both the CXCL12 gene and protein show high (90%) homology between humans and mice (60). Typical CXCL12 protein is relatively small with 68 amino acids (52). The CXCR4 gene is located at human 2q21 and the CXCR4 protein has 352 amino acids (64, 65). CXCR4 is a G protein-coupled receptor and signaling/transducing pathways include mammalian target of rapamycin (mTOR), phosphoinositol 3 kinase/protein kinase B and Janus kinase/signal transducers and activators of transcription (JAK/STAT), among other pathways (52, 64, 65).
5 CXCL12 and CXCR4 in uterine remodeling and receptivity
The uterine epithelium and stroma undergo major cellular reorganization in response to the presence of an embryo and in preparation for attachment, implantation, and the establishment of a pregnancy (7, 21, 36, 45, 46). Chemokines are now recognized as having an important role in the changes that occur in the uterine endometrium during the period before attachment of the conceptus (23, 67–69). The C-C and CXC-motif chemokines were shown to influence endometrial epithelial cell function, implantation and embryo survival in cattle (70–76). In humans, CXCL12 is produced by embryonic trophoblast cells and induces uterine stromal cells to express its receptor CXCR4 (77, 78). Both CXCL12 and CXCR4 are expressed in uterine endometrial epithelial cells and stromal cells and are considered to have an important autocrine role in remodeling of the epithelium in preparation for attachment of the conceptus (Figure 1) (23, 67–69). CXCL12-CXCR4 facilitated infiltration of the uterus by natural killer (NK) cells which is part of the immune cell remodeling of the epithelium and stroma mice (79). CXCR4 knock-out mice had reduced NK cells and increased fetal resorption and significantly reduced implantation (78). CXCL12 obtained from pre- and peri-implanting mice increased angiogenesis and embryo attachment in in vitro cultures of mouse tissues (80). Treatment with CXCL12 induced CXCR4+ Treg cells to infiltrate the uterus and create a supportive environment for attachment and pregnancy in a diabetic mouse model (81). The CXC chemokines have been implicated in the pathology of endometritis in women but this field is outside the scope of the present article (82, 83).
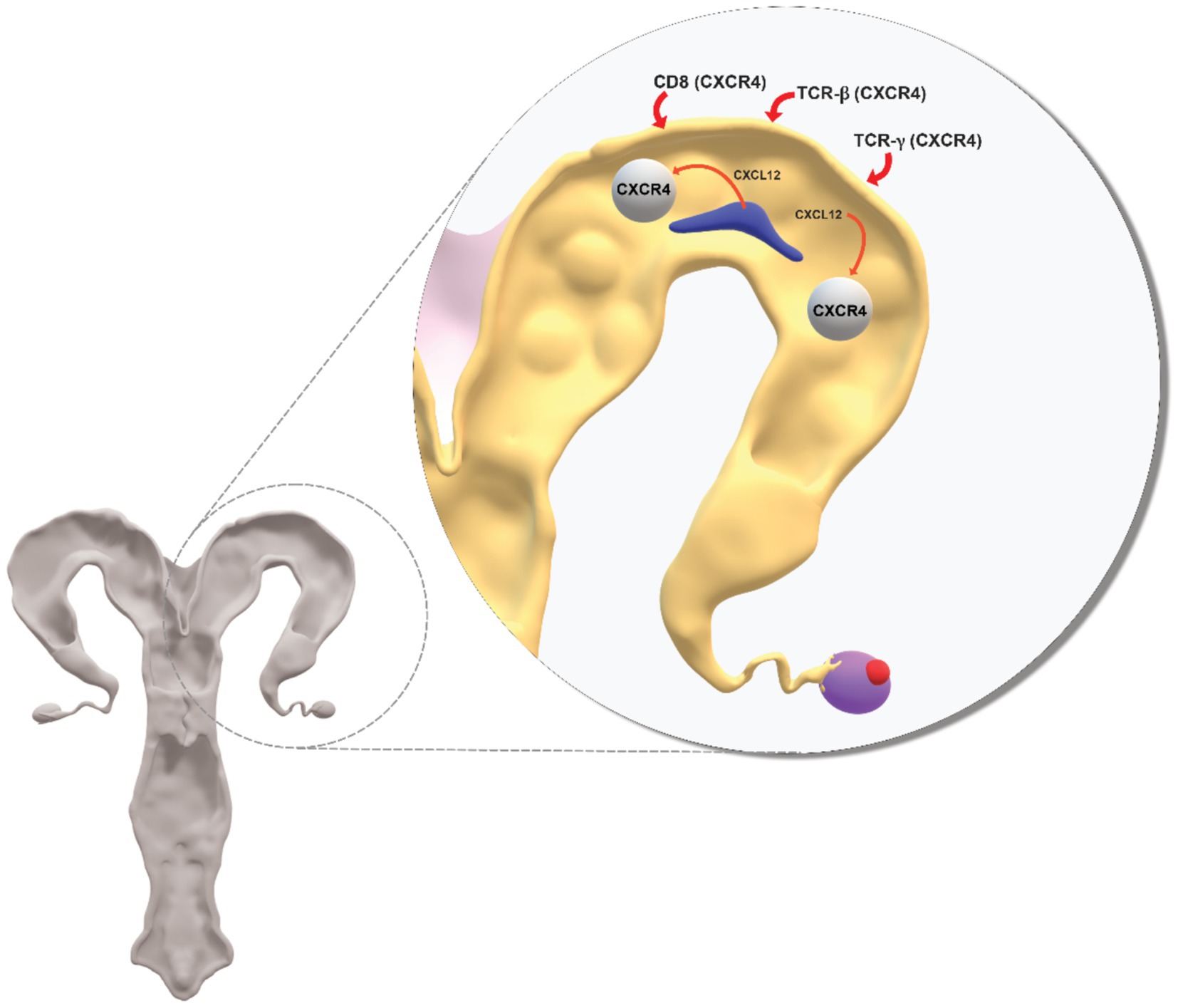
Figure 1. Conceptual diagram on role of CXCL12-CXCR4 in crosstalk between the conceptus and uterus during the period when the uterine endometrium undergoes major structural and functional change in preparation for embryo attachment to the epithelium, implantation and pregnancy. CXCL12 secreted by the conceptus acts at its CXCR4 receptor to induce changes at the uterus. CXCL12 additionally acts at CXCR4 receptors on immune cells (CD8, TCR-β, TCR-γ) recruited from blood and which are involved in inflammatory processes associated with the establishment of uterine receptivity. Support for the model of CXCL12-CXCR4 is in the cited literature.
Ewes treated with the CXCR4 antagonist AMD3100 from day 12 to day 20 after breeding had diminished uterine levels of angiogenic factors which demonstrated the role of CXCL12-CXCR4 in vascularisation of the utero-placental unit (84). In a second study of similar design in sheep, treatment with the antagonist AMD3100 from day 12 to day 35 after breeding was associated with increased autophagy induction at the fetal-placental unit (85). Also in sheep, intra-uterine treatment with antagonist AMD3100 from day 7 to day 14 after mating resulted in abnormal placental function (86). In a further study in sheep, expression of CXCL12 and CXCR4 were increased in conceptus and uterus around the time of attachment and placentation (87). CXCL12 expression in trophoblast and endometrial stroma of sheep was greater in natural mated ewes compared with ewes that received IVF embryos (88). The expression of CXCL12 in endometrial stroma was interpreted to indicate that CXCL12 can have a paracrine and/or autocrine action (88). CXCL12 and CXCR4 were reported to be associated with luminal epithelial cell remodeling in pigs (69, 89). In cattle, CXCR4 mRNA in endometrium did not change from day 14 to day 50 in pregnant cows (90). CXCR4 mRNA was, however, increased in blood on day 20 to day 32 which coincided with the period of implantation in cattle. A secondary increased in blood CXCR4 mRNA from day 30 coincided with caruncular-cotyledonary placentome development in cattle. mRNA for immune cells CD8, TCR-β and TCR-γ was increased in blood and mRNA for CD8 and TCR-β was increased in endometrium on day 19 (90). It was proposed that blood-derived immune cells that express CXCR4 populate the uterus and are involved in uterine inflammation associated with embryo attachment, vascularisation and placentome formation in cattle (90).
6 Summary
Fertilization rates in female cattle are typically greater than 75% with both natural mating and artificial insemination. The lack of fertilization per se is therefore not the major reason for reproductive failure in cattle. The main cause of reproductive failure in cattle, and indeed females of other species, is the large loss of embryos which occurs in the 21-day window from fertilization to attachment of the embryo to the uterus. As noted above, the establishment of a pregnancy relies on the combination of a good quality embryo with high developmental competence and a responsive uterus. This mini review has brought together information which highlights the important role of uterine receptivity in embryonic survival. A greater understanding of uterine receptivity is necessary for a meaningful step change in reproductive success in cattle. This could include studies involving endometrial biopsies in early stages of pregnancy for transcriptomic and proteomic profiling, linked with genotyping. This approach would however require significant resources. MicroRNAs are now known to regulate pathways associated with uterine receptivity and the interaction with CXCL12-CXCR4 is a further area of research (91). In a recent study, polymorphism in a region in proximity to the CXCR4 gene was suggested as a putative causal variant for fertility in highly fertile Brahman cattle (24). This was consistent with a role for CXCL12-CXCR4 in uterine receptivity and fertility in cattle. There is a clear need to undertake mechanistic studies to demonstrate a role for the CXCL12-CXCR4 axis in uterine receptivity in cattle. There is also a need for large phenotype-genome/proteome studies to identify additional polymorphisms in the CXCL12-CXCR4 genes and other genes associated with uterine receptivity and fertility in cattle.
Author contributions
MD’O: Conceptualization, Writing – original draft, Writing – review & editing. GC: Conceptualization, Writing – original draft, Writing – review & editing. PB: Conceptualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors thank Dr. Fabio de Moraes Francisco for producing the figure.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
1. ^https://asia.ensembl.org/Bos_taurus/Location/View?r=28%3A45021867-45052552;www.cattlegeneatlas.roslin.ed.ac.uk
2. ^https://asia.ensembl.org/Bos_taurus/Gene/Splice?db=core;g=ENSBTAG00000001060;r=2:61250084-61254502
References
1. Ealy, AD, Wooldridge, LK, and McCoski, SR. Board Invited Review: post-transfer consequences of in vitro-produced embryos in cattle. J Anim Sci. (2019) 97:2555–68. doi: 10.1093/jas/skz11
2. Hansen, PJ. The incompletely fulfilled promise of embryo transfer in cattle—why aren’t pregnancy rates greater and what can we do about it? J Anim Sci. (2020) 98:1–20. doi: 10.1093/jas/skaa288
3. Smith, BD, Poliakiwski, B, Polanco, O, Singleton, S, de Melo, GD, Muntari, M, et al. Decisive points for pregnancy losses in beef cattle. Reprod Fert Develop. (2023) 35:70–83. doi: 10.1071/RD22206
4. Peterson, AJ, and Lee, RSF. Improving successful pregnancies after embryo transfer. Theriogenology. (2003) 59:687–97. doi: 10.1016/s0093-691x(02)01248-7
5. Hue, I, Degrelle, SA, and Turenne, N. Conceptus elongation in cattle: genes, models and questions. Anim Reprod Sci. (2012) 134:19–28. doi: 10.1016/j.anireprosci.2012.08.007
6. Reese, ST, Franco, GA, Poole, RK, Hood, R, Montero, LF, Filho, RVO, et al. Pregnancy loss in cattle: a meta-analysis. Anim Reprod Sci. (2020) 212:106251. doi: 10.1016/j.anireprosci.2019.106251
7. Governini, L, Luongo, FP, Haxhiu, A, Piomboni, P, and Luddi, A. Main actors behind the endometrial receptivity and successful implantation. Tissue Cell. (2021) 73:101656. doi: 10.1016/j.tice.2021.101656
8. Tinning, H, Edge, JC, DeBem, THC, Deligianni, F, Giovanardi, G, Pensabene, V, et al. Endometrial function in pregnancy establishment in cattle. Animal. (2023) 17:100751. doi: 10.1016/j.animal.2023.100751
9. Sánchez, JM, Mathew, DJ, Passaro, C, Fair, T, and Lonergan, P. Embryonic maternal interaction in cattle and its relationship with fertility. Reprod Domest Anim. (2018) 53:20–7. doi: 10.1111/rda.13297
10. D’Occhio, MJ, Campanile, G, Zicarelli, L, Visintin, JA, and Baruselli, PS. Adhesion molecules in gamete transport, fertilization, early embryonic development, and implantation—role in establishing a pregnancy in cattle: a review. Mol Reprod Dev. (2020) 87:206–22. doi: 10.1002/mrd.23312
11. Noguchi, T, Hayashi, T, Inoue, Y, Hara, S, Shirasuna, K, and Iwata, H. Predicting of molecules mediating an interaction between bovine embryos and uterine epithelial cells. J Reprod Dev. (2022) 68:318–23. doi: 10.1262/jrd.2022-046
12. Ma, B, Cui, H, Wang, X, Feng, W, Zhang, J, Chen, N, et al. IFNT-induced IRF1 enhances bovine endometrial receptivity by transactivating LIFR. J Reprod Immunol. (2024) 163:104212. doi: 10.1016/j.jri.2024.104212
13. de Melo, GD, Mello, BP, Ferreira, CA, Filho, CASG, Rocha, CC, Silva, AG, et al. Applied use of interferon-tau stimulated genes expression in polymorphonuclear cells to detect pregnancy compared to other early predictors in beef cattle. Theriogenology. (2020) 152:94–105. doi: 10.1016/j.theriogenology.2020.04.001
14. Ferraz, PA, Filho, CASG, Rocha, CC, Neto, AL, Bruni, GA, Oshiro, TSI, et al. Feasibility and accuracy of using different methods to detect pregnancy by conceptus-stimulated genes in dairy cattle. JDS Commun. (2021) 2:153–8. doi: 10.3168/jdsc.2020-0062
15. Ferraz, PA, Poit, DAS, Pinto, LMF, Guerra, AC, Neto, AL, do Prado, FL, et al. Accuracy of early pregnancy diagnosis and determining pregnancy loss using different biomarkers and machine learning applications in dairy cattle. Theriogenology. (2024) 224:82–93. doi: 10.1016/j.theriogenology.2024.05.006
16. Johnson, GA, Bazer, FW, Burghardt, RC, Seo, H, Wu, G, Cain, JW, et al. The history of interferon-stimulated genes in pregnant cattle, sheep and pigs. Reproduction. (2024) 168:e240130. doi: 10.1530/REP-24-0130
17. Sponchiado, M, Gomes, NS, Fontes, PK, Martins, T, del Collado, M, Pastore, AA, et al. Pre-hatching embryo-dependent and -independent programming of endometrial function in cattle. PLoS One. (2017) 12:e0175954. doi: 10.1371/journal.pone.0175954
18. Sponchiado, M, Gonella-Diaza, AM, Rocha, CC, Turco, EGL, Pugliesi, G, Leroy, JLMR, et al. The pre-hatching bovine embryo transforms the uterine luminal metabolite composition in vivo. Sci Rep. (2019) 9:8354. doi: 10.1038/s41598-019-44590-9
19. Sánchez, JM, Simintiras, CA, and Lonergan, P. Aspects of embryo-maternal communication in establishment of pregnancy in cattle. Anim Reprod. (2019) 16:376–85. doi: 10.21451/1984-3143-AR2019-0075
20. D’Occhio, MJ, Campanile, G, Baruselli, PS, Porto Neto, LR, Hayes, BJ, Collins Snr, A, et al. Pleomorphic adenoma gene1 in reproduction and implication for embryonic survival in cattle: a review. J Anim Sci. (2024) 102:skae103. doi: 10.1093/jas/skae103
21. Forde, N, and Lonergan, P. Transcriptomic analysis of the bovine endometrium: what is required to establish uterine receptivity to implantation in cattle? J Reprod Develop. (2012) 58:189–95. doi: 10.1262/jrd.2011-021
22. Binelli, M, Silva, FACC, Rocha, CC, Martins, T, Sponchiado, M, Van Hoeck, V, et al. Endometrial receptivity in cattle: the mutual reprogramming paradigm. Anim Reprod. (2022) 19:e20220097. doi: 10.1590/1984-3143-AR2022-0097
23. Ao, D, Li, D-J, and Li, M-Q. CXCL12 in normal and pathological pregnancies: a review. Amer. J Reprod Immunol. (2020) 84:e13280. doi: 10.1111/aji.13280
24. Forutan, M, Engle, BN, Chamberlain, AJ, Ross, EM, Nguyen, LT, D’Occhio, MJ, et al. Genome-wide association and expression quantitative trait loci in cattle reveals common genes regulating mammalian fertility. Commun Biol. (2024) 7:724. doi: 10.1038/s42003-024-06403-2
25. D’Occhio, MJ, Campanile, G, and Baruselli, PS. Transforming growth factor-β superfamily and interferon-τ in ovarian function and embryo development in female cattle: review of biology and application. Reprod Fert Develop. (2020) 32:539–52. doi: 10.1071/RD19123
26. Campanile, G, Baruselli, PS, Limone, A, and D’Occhio, MJ. Local action of cytokines and immune cells in communication between the conceptus and uterus during the critical period of early embryo development, attachment and implantation – implications for embryo survival in cattle: a review. Theriogenology. (2021) 167:1–12. doi: 10.1016/j.theriogenology.2021.02.020
27. Kertz, NC, Banerjee, P, Dyce, PW, and Diniz, WJS. Harnessing genomics and transcriptomics approaches to improve female fertility in beef cattle—a review. Animals. (2023) 13:3284. doi: 10.3390/ani13203284
28. McMillan, WH, Peterson, AJ, Donnison, M, Pugh, PA, and Lambert, MG. 1996, Is fetal loss random during pregnancy in cattle? Proc Inter Cong Anim Reprod. (2023) 13:11–5.
29. Berg, DK, Ledgard, A, Donnison, M, McDonald, R, Henderson, HV, Meier, S, et al. The first week following insemination is the period of major pregnancy failure in pasture-grazed dairy cows. J Dairy Sci. (2022) 105:9253–70. doi: 10.3168/jds.2021-21773
30. McMillan, WH. Statistical models predicting embryo survival to term in cattle after embryo transfer. Theriogenology. (1998) 50:1053–70. doi: 10.1016/S0093-691X(98)00207-6
31. McMillan, WH, and Donnison, MJ. Understanding maternal contributions to fertility in recipient cattle: development of herds with contrasting pregnancy rates. Anim Reprod Sci. (1999) 57:127–40. doi: 10.1016/s0378-4320(99)00063-9
32. McMillan, WH. Potential survival rates to term for transferred in vitro and in vivo derived bovine embryos. Theriogenology. (1996) 45:233. doi: 10.1016/0093-691X(96)84706-6
33. Geary, TW, Burns, GW, Moraes, JGN, Moss, JI, Denicol, AC, Dobbs, KB, et al. Identification of beef heifers with superior uterine capacity for pregnancy. Biol Reprod. (2016) 95:1–12. doi: 10.1095/biolreprod.116.141390
34. Moraes, JGN, Behura, SK, Geary, TW, Hansen, PJ, Neibergs, HL, and Spencer, TE. Uterine influences on conceptus development in fertility-classified animals. Proc Natl Acad Sci USA. (2018) 115:E1749–58. doi: 10.1073/pnas.1721191115
35. Moore, SG, Cummins, SB, Mamo, S, Lonergan, P, Fair, T, and Butler, ST. Genetic merit for fertility traits in Holstein cows: VI. Oocyte developmental competence and embryo development. J Dairy Sci. (2019) 102:4651–61. doi: 10.3168/jds.2018-15813
36. Egashira, M, and Hirota, Y. Uterine receptivity and embryo–uterine interactions in embryo implantation: lessons from mice. Reprod Med Biol. (2013) 12:127–32. doi: 10.1007/s12522-013-0153-1
37. Altmäe, S, Koel, M, Võsa, U, Adler, P, Suhorutšenko, M, Laisk-Podar, T, et al. Meta-signature of human endometrial receptivity: a meta-analysis and validation study of transcriptomic biomarkers. Sci Rep. (2017) 7:10077. doi: 10.1038/s41598-017-10098-3
38. Rabaglino, MB, and Kadarmideen, HN. Machine learning approach to integrated endometrial transcriptomic datasets reveals biomarkers predicting uterine receptivity in cattle at seven days after estrous. Sci Rep. (2020) 10:16981. doi: 10.1038/s41598-020-72988-3
39. Tobolski, D, Lukasik, K, Baclawska, A, Skarzynski, DJ, Hostens, M, and Baranski, W. Prediction of calving to conception interval length using algorithmic analysis of endometrial mRNA expression in bovine. Animals. (2021) 11:236. doi: 10.3390/ani11010236
40. Kusama, K, Rashid, MB, Kowsar, R, Marey, MA, Talukder, AK, Nagaoka, K, et al. Day 7 embryos change the proteomics and exosomal micro-RNAs content of bovine uterine fluid: involvement of innate immune functions. Front Genet. (2021) 12:676791. doi: 10.3389/fgene.2021.676791
41. Mazzarella, R, Sánchez, JM, Fernandez-Fuertes, BF, Egido, SG, McDonald, M, Alvarez-Barrientos, A, et al. Embryo-induced changes in the protein profile of bovine oviductal extracellular vesicles. Mol Cell Proteomics. (2025) 24:100935. doi: 10.1016/j.mcpro.2025.100935
42. Fukui, Y, Hirota, Y, Matsuo, M, Gebril, M, Akaeda, S, Hiraoka, T, et al. Uterine receptivity, embryo attachment, and embryo invasion: multistep processes in embryo implantation. Reprod Med Biol. (2019) 18:234–40. doi: 10.1002/rmb2.12280
43. Cakmak, H, and Taylor, HS. Implantation failure: molecular mechanisms and clinical treatment. Hum Reprod Update. (2011) 17:242–53. doi: 10.1093/humupd/dmq037
44. Lessey, BA, and Young, SL. What exactly is endometrial receptivity? Fertil Steril. (2019) 111:611–7. doi: 10.1016/j.fertnstert.2019.02.009
45. Bakkensen, JB, Agarwal, R, and Shapiro, M. Recent advances and current perspectives on endometrial receptivity. Curr Obstet Gynec Reports. (2021) 10:45–52. doi: 10.1007/s13669-021-00313-4
46. Enciso, M, Aizpurua, J, Rodríguez-Estrada, B, Jurado, I, Ferrández-Rives, M, Rodríguez, E, et al. The precise determination of the window of implantation significantly improves ART outcomes. Sci Rep. (2021) 11:13420. doi: 10.1038/s41598-021-92955-w
47. Balestrieri, ML, Gasparrini, B, Neglia, G, Vecchio, D, Strazzullo, M, Giovane, A, et al. Proteomic profiles of the embryonic chorioamnion and uterine caruncles in buffaloes (Bubalus bubalis) with normal and retarded embryonic development. Biol Reprod. (2013) 88:119. doi: 10.1095/biolreprod.113.108696
48. Strazzullo, M, Gasparrini, B, Neglia, G, Balestrieri, ML, Francioso, R, Rossetti, C, et al. Global transcriptome profiles of Italian Mediterranean buffalo embryos with normal and retarded growth. PLoS One. (2014) 9:e90027. doi: 10.1371/journal.pone.0090027
49. Godessart, N, and Kunkel, SL. Chemokines in autoimmune disease. Curr Opin Immunol. (2001) 13:670–5. doi: 10.1016/S0952-7915(01)00277-1
50. Zhao, L, Liang, D, Wu, X, Li, Y, Niu, J, Zhou, C, et al. Contribution and underlying mechanisms of CXCR4 overexpression in patients with systemic lupus erythematosus. Cell Mol Immunol. (2017) 14:842–9. doi: 10.1038/cmi.2016.47
51. Tang, P, and Wang, JM. Chemokines: the past, the present and the future. Cell Mol Immunol. (2018) 15:295–8. doi: 10.1038/cmi.2018.9
52. Guo, F, Wang, Y, Liu, J, Mok, SC, Xue, F, and Zhang, W. CXCL12/CXCR4: a symbiotic bridge linking cancer cells and their stromal neighbors in oncogenic communication networks. Oncogene. (2016) 35:816–26. doi: 10.1038/onc.2015.139
53. Tulotta, C, Stefanescu, C, Chen, Q, Torraca, V, Meijer, AH, and Snaar-Jagalska, BE. CXCR4 signaling regulates metastatic onset by controlling neutrophil motility and response to malignant cells. Sci Rep. (2019) 9:2399. doi: 10.1038/s41598-019-38643-2
54. Britton, C, Poznansky, MC, and Reeves, P. Polyfunctionality of the CXCR4/CXCL12 axis in health and disease: implications for therapeutic interventions in cancer and immune-mediated diseases. FASEB J. (2021) 35:e21260. doi: 10.1096/fj.202001273R
55. Scala, S, D’Alterio, C, Milanesi, S, Castagna, A, Carriero, R, Farina, FM, et al. New insights on the emerging genomic landscape of CXCR4 in Cancer: a lesson from WHIM. Vaccine. (2020) 8:164. doi: 10.3390/vaccines8020164
56. Shi, Y, Riese, DJ II, and Shen, J. The role of the CXCL12/CXCR4/CXCR7 chemokine axis in cancer. Front Pharmacol. (2020) 11:574667. doi: 10.3389/fphar.2020.574667
57. Garg, P, Jallepalli, VR, and Verma, S. Unravelling the CXCL12/CXCR4 axis in breast cancer: insights into metastasis, microenvironment interactions, and therapeutic opportunities. Hum Gene. (2024) 40:201272–2. doi: 10.1016/j.humgen.2024.201272
58. Mempel, TR, Lill, JK, and Altenburger, LM. How chemokines organize the tumour environment. Nat Rev Cancer. (2024) 24:28–50. doi: 10.1038/s41568-023-00635-w
59. Yu, Y, Xiao, CH, Tan, LD, Wang, QS, Li, XQ, and Feng, YM. Cancer-associated fibroblasts induce epithelial-mesenchymal transition of breast cancer cells through paracrine TGF-ß signalling. Br J Cancer. (2014) 110:724–32. doi: 10.1038/bjc.2013.768
60. Janssens, R, Struyf, S, and Proost, P. The unique structural and functional features of CXCL12. Cell Mol Immunol. (2018) 15:299–311. doi: 10.1038/cmi.2017.107
61. Wu, X, Qian, L, Zhao, H, Lei, W, Liu, Y, Xu, X, et al. CXCL12/CXCR4: an amazing challenge and opportunity in the fight against fibrosis. Ageing Res Rev. (2023) 83:101809. doi: 10.1016/j.arr.2022.101809
62. Pawig, L, Klasen, C, Weber, C, Bernhagen, J, and Noels, H. Diversity and inter-connections in the CXCR4 chemokine receptor/ligand family: molecular perspectives. Front Immunol. (2015) 6:429. doi: 10.3389/fimmu.2015.0042
63. Teicher, BA, and Fricker, SP. CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res. (2010) 16:2927–31. doi: 10.1158/1078-0432.CCR-09-2329
64. Bianchi, ME, and Mezzapelle, R. The chemokine receptor CXCR4 in cell proliferation and tissue regeneration. Front Immunol. (2020) 11:2109. doi: 10.3389/fimmu.2020.02109
65. Mousavi, A. CXCL12/CXCR4 signal transduction in diseases and its molecular approaches in targeted-therapy. Immunol Lett. (2020) 217:91–115. doi: 10.1016/j.imlet.2019.11.007
66. Busillo, JM, and Benovic, JL. Regulation of CXCR4 signaling. Biochim Biophys Acta. (2007) 1768:952–63. doi: 10.1016/j.bbamem.2006.11.002
67. Wang, L, Li, X, Zhao, Y, Fang, C, Lian, Y, Gou, W, et al. Insights into the mechanism of CXCL12-mediated signaling in trophoblast functions and placental angiogenesis. Acta Biochim Biophys Sin. (2015) 47:663–72. doi: 10.1093/abbs/gmv064
68. Zheng, J, Wang, H, and Zhou, W. Modulatory effects of trophoblast-secreted CXCL12 on the migration and invasion of human first-trimester decidual epithelial cells are mediated by CXCR4 rather than CXCR7. Reprod Biol Endocrinol. (2018) 16:17. doi: 10.1186/s12958-018-0333-2
69. Złotkowska, A, and Andronowska, A. Chemokines as the modulators of endometrial epithelial cells remodelling. Sci Rep. (2019) 9:12968. doi: 10.1038/s41598-019-49502-5
70. Tribulo, P, Siqueira, LGB, Oliveira, LJ, Scheffler, TS, and Hansen, PJ. Identification of potential embryokines in the bovine reproductive tract. J Dairy Sci. (2017) 101:690–704. doi: 10.3168/jds.2017-13221
71. Wagener, K, Drillich, M, Aurich, C, and Gabler, C. Endometrial inflammation at the time of insemination and its effect on subsequent fertility of dairy cows. Animals. (2021) 11:1858. doi: 10.3390/ani11071858
72. Sakumoto, R. Role of chemokines in regulating luteal and uterine function in pregnant cows. J Reprod Dev. (2024) 70:145–51. doi: 10.1262/jrd.2023-100
73. Sakumoto, R, Hayashi, KG, Fuji, S, Kanahara, H, Hosoe, M, Furusawa, T, et al. Possible roles of CC- and CXC-chemokines in regulating bovine endometrial function during early pregnancy. Int J Mol Sci. (2017) 18:742. doi: 10.3390/ijms18040742
74. Lim, W, Bae, H, Bazer, F, Kim, SM, and Song, G. C—C motif chemokine ligand 2 regulates lps‐induced inflammation and ER stress to enhance proliferation of bovine endometrial epithelial cells. J Cell Physiol. (2018) 233:3141–51. doi: 10.1002/jcp.26151
75. Lim, W, Bae, H, Bazer, FW, and Song, G. C-C motif chemokine ligand 23 abolishes ER stress- and LPS-induced reduction in proliferation of bovine endometrial epithelial cells. J Cell Physiol. (2018) 233:3529–39. doi: 10.1002/jcp.26210
76. Yun, CS, Saito, Y, Rahman, ANMI, Suzuki, T, Takahashi, H, Kizaki, K, et al. C-C motif chemokine ligand 2 regulates prostaglandin synthesis and embryo attachment of the bovine endometrium during implantation. Cell Tissue Res. (2024) 396:231–43. doi: 10.1007/s00441-024-03869-8
77. Ren, L, Liu, Y-Q, Zhou, W-H, and Zhang, Y-Z. Trophoblast-derived chemokine CXCL12 promotes CXCR4 expression and invasion of human first-trimester decidual stromal cells. Hum Reprod. (2012) 27:366–74. doi: 10.1093/humrep/der395
78. Zheng, J, Qu, D, Wang, C, Ding, L, and Zhou, W. Involvement of CXCL12/CXCR4 in the motility of human first-trimester endometrial epithelial cells through an autocrine mechanism by activating PI3K/AKT signaling. BMC Pregnancy Childbirth. (2020) 20:87. doi: 10.1186/s12884-020-2788-3
79. Lyu, F, Burzynski, C, Fang, YY, Tal, A, Chen, AY, Kisa, J, et al. Maternal CXCR4 deletion results in placental defects and pregnancy loss mediated by immune dysregulation. JCI Insight. (2023) 8:e172216. doi: 10.1172/jci.insight.172216
80. Koo, HS, Yoon, MJ, Hong, SH, Ahn, J, Cha, H, Lee, D, et al. CXCL12 enhances pregnancy outcome via improvement of endometrial receptivity in mice. Sci Rep. (2021) 11:7397. doi: 10.1038/s41598-021-86956-y
81. Lin, Y, Xu, L, Jin, H, Zhong, Y, Di, J, and Lin, Q. CXCL12 enhances exogenous CD4+CD25+ T cell migration and prevents embryo loss in non-obese diabetic mice. Fertil Steril. (2009) 91:2687–96. doi: 10.1016/j.fertnstert.2008.01.109
82. Nash, DM, and Giles, JL. Uterine inflammation and lessons from large animal models of endometritis. Nat Rev Immunol. (2025) 22:2. doi: 10.1038/s41577-025-01200-2
83. Yan, X, Jiao, J, and Wang, X. The pathogenesis, diagnosis, and treatment of chronic endometritis: a comprehensive review. Front Endocrinol. (2025) 16:1603570. doi: 10.3389/fendo.2025.1603570
84. Runyan, CL, McIntosh, SZ, Maestas, MM, Quinn, KE, Boren, BP, and Ashley, RL. CXCR4 signaling at the ovine fetal–maternal interface regulates vascularization, CD34+ cell presence, and autophagy in the endometrium. Biol Reprod. (2019) 101:102–11. doi: 10.1093/biolre/ioz073
85. Ashley, RL, Runyan, CL, Maestas, MM, Trigo, E, and Silver, G. Inhibition of the C-X-C motif chemokine 12 (CXCL12) and its receptor CXCR4 reduces utero-placental expression of the VEGF system and increases utero-placental autophagy. Front Vet Sci. (2021) 8:850687. doi: 10.3389/fvets.2021.650687
86. Ashley, RL, Trigo, EM, and Ervin, JM. Placental insufficiency and heavier placentas in sheep after suppressing CXCL12/CXCR4 signaling during implantation. Biol Reprod. (2023) 109:982–93. doi: 10.1093/biolre/ioad122
87. Ashley, RL, Antoniazzi, AQ, Anthony, RV, and Hansen, TR. The chemokine receptor XCXR4 and its ligand CXCL12 are activated during implantation and placentation in sheep. Reprod Biol Endocrinol. (2011) 9:148. doi: 10.1186/1477-7827-9-148
88. Quinn, KE, Reynolds, LP, Grazul-Bilsk, AT, Borowicz, PP, and Ashley, RL. Placental development during early pregnancy: effects of embryo origin on expression of chemokine ligand twelve (CXCL12). Placenta. (2016) 43:77–80. doi: 10.1016/j.placenta.2016.05.008
89. Zlotkowska, A, and Andronowska, A. Modulatory effect of chemokines on porcine endometrial stromal and endothelial cells. Dom Anim Endocrinol. (2020) 72:106475. doi: 10.1016/j.domaniend.2020.106475
90. Ashley, RL, Smirnova, NP, and Hansen, TR. The expression profile of the chemokine receptor CXCR4 and specific T-cell markers in peripheral blood and endometrium during early pregnancy in cows. Biol Reprod. (2009) 81:603–3. doi: 10.1093/biolreprod/81.s1.603
Keywords: CXCL12, CXCR4, cow, embryo, uterus, receptivity, fertility
Citation: D’Occhio MJ, Campanile G and Baruselli PS (2025) Involvement of chemokine CXCL12 and its receptor CXCR4 in uterine receptivity and potential relationship to fertility in cattle: a mini review. Front. Vet. Sci. 12:1651593. doi: 10.3389/fvets.2025.1651593
Edited by:
Dariusz Jan Skarzynski, Wrocław University of Environmental and Live Sciences, PolandReviewed by:
Emsal Sinem Özdemir Salci, Bursa Uludag Universitesi, TürkiyeDawid Tobolski, Warsaw University of Life Sciences, Poland
Sally Ibrahim, National Research Centre, Egypt
Copyright © 2025 D’Occhio, Campanile and Baruselli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael J. D’Occhio, bWljaGFlbC5kb2NjaGlvQGJpZ3BvbmQuY29t
 Michael J. D’Occhio
Michael J. D’Occhio Giuseppe Campanile
Giuseppe Campanile Pietro S. Baruselli3
Pietro S. Baruselli3