- 1Department of Animal Production, School of Veterinary Medicine and Sciences, University of Ngaoundere, Ngaoundere, Cameroon
- 2Associated Laboratory for Green Chemistry (LAQV) of the Network of Chemistry and Technology (REQUIMTE), Faculty of Pharmacy, University of Porto, Porto, Portugal
- 3Department of Comparative Biomedicine and Food Science, University of Padua, Viale Legnaro, Italy
- 4Department of Comparative Biomedicine and Food Science, University of Padua, Viale Legnaro, Italy
- 5Veterinary Epidemiology Unit, Department of Internal Medicine, Reproduction and Population Medicine, Faculty of Veterinary Medicine, Ghent University, Merelbeke, Belgium
- 6Scientific Veterinary Institute Novi Sad, Novi Sad, Serbia
- 7Departament de Sanitat i Anatomia Animals, Universitat Autònoma de Barcelona (UAB), Bellaterra, Barcelona, Spain
Introduction: Modern poultry production systems inherently concentrate large numbers of birds, which also increases the risk and potential impact of disease outbreaks. Biosecurity is widely recognized as the most important tool for reducing the risk of disease introduction, establishment, and spread to, within, and from an animal population. Thus, effective biosecurity is essential for sustainable poultry production, and assessing its implementation represents a crucial step. This systematic review aimed to evaluate biosecurity implementation in poultry farms across European and neighboring countries
Methods: The Cochrane Handbook and PRISMA 2020 guidelines were followed to perform the systematic review.
Results: Of the 1,515 articles retrieved from four databases, only 44 met the inclusion criteria and 16 provided usable data for assessing biosecurity implementation. Despite relatively broad geographical coverage, including eight multi-country studies involving 36 national assessments, the distribution of studies was uneven. Moreover, most studies (77%) were pathogen- or disease-specific (e.g., Campylobacter spp., avian influenza, etc.) and focused on a single poultry species, primarily broilers (55%), while assessments involving minor poultry species were rare. There was also marked variability in the methods used to assess biosecurity, and the level of biosecurity implementation differed significantly across countries. Based on descriptive evaluations, 58% of farms implemented all the biosecurity measures assessed. According to scoring-based assessments, the overall average biosecurity score was 66.9 out of 100. The most frequently implemented measures were those related to infrastructure and control of biological vectors, disease management, and purchase of one-day-old chicks.
Discussion: The heterogeneity of results, driven by differences in study design, poultry species, production systems, and methodological approach, highlights the complexity of evaluating biosecurity across diverse national contexts. This variability may reflect differences in epidemiological conditions, research funding, and national priorities. Although this review focused solely on primary research studies, the findings underscore the need to promote cross-country collaboration to enhance knowledge sharing and data harmonization.
1 Introduction
Among the major contributors to global poultry meat and egg production, European (EU) countries play a significant role in setting high standards for quality and animal welfare, serving as both a major producer and supplier to international markets (1). Achieving high levels of production is the result of a combination of production policies, market regulations, integrated health and production management strategies. Biosecurity is widely recognized as a key tool for preventing the introduction of infectious diseases (external biosecurity) and the establishment and spread (internal biosecurity) from and within an animal production site, which in turn reduces antimicrobial usage and enhances animal production performance (2–4). Although all EU member states are subject to the same overarching legal framework for animal health (5), the practical implementation of biosecurity measures can vary significantly across countries (2, 6–8). These differences reflect the diverse needs of national poultry sectors and are expressed through tailor-made national biosecurity legislation (9) and/or country-specific quality label regulations (10, 11). The implementation of biosecurity measures also depends on various country-specific factors, such as geographical location of farms, their structural characteristics (e.g., size, production type, and category), the national epidemiological situation (e.g., presence, prevalence, or risk profile of poultry diseases), available financial resources, and the awareness and knowledge of stakeholders regarding biosecurity (12–14). A further challenge is the scarcity of available data on the actual level of biosecurity implementation in poultry farms across European countries (15–17). This data gap represents a bottleneck for the design of effective interventions aimed at improving biosecurity implementation (9, 12, 18). In response to these knowledge gaps, the COST Action CA20103 - Biosecurity Enhanced Through Training Evaluation and Raising Awareness (BETTER) - was launched in 2021. One of its aims is to gather and consolidate knowledge on biosecurity regulatory frameworks.1 To this end, an overview of biosecurity implementation as mandated by national legislation and other regulatory frameworks in intensive poultry production was performed (8). The analysis revealed a general lack of data on the actual level of implementation of biosecurity measures in most countries. Therefore, a comprehensive and systematic assessment was needed to address this important gap. The objective of this study was to assess the level of biosecurity implementation in poultry farms across key European countries contributing to the global poultry market. In addition to European COST member countries, Turkey (Full COST Member), Tunisia (Near Neighbor Country), and Israel (Cooperating Member), were also included in the analysis.
2 Methods
This systematic review was conducted in accordance with the Cochrane Handbook for Systematic Reviews (19) and is reported in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (20).
2.1 Protocol and registration
The protocol was developed according to PRISMA-P guidelines (21) and was archived in the University of Padua’s Research Archive institutional repository.2 It was also registered on the Systematic Reviews for Animals and Food (SYREAF) website.3
2.2 Information sources and search strategy
Four databases were searched: Web of Science (WOS) and PubMed via the University of Padua in Italy, and Agricola (Proquest) and CAB Abstract (Ovid) via the University of Bern in Switzerland. No publication date restrictions were applied. The search targeted primary research assessing biosecurity implementation in poultry farms across European and neighboring countries. The strategy followed the PICO framework, and employed a multi-stranded approach, using the following search terms: [Poultry] AND [Biosecurity] AND [Implementation] AND [European Countries OR Turkey OR Tunisia OR Israel]. The detailed search strategy, carried out in CAB Abstract on December 20, 2023, is reported in Supplementary material 1.
Studies published in English, Spanish, or French and reporting primary data on biosecurity implementation in intensive poultry farms (broiler, layer, duck, goose and turkey) within the selected countries were considered. In line with a protocol deviation, randomized controlled trials, cohort studies, and case–control studies were excluded. Studies involving experimentally challenged animals or model-based designs were also excluded.
2.3 Study selection
All identified records were deduplicated using Zotero (version 7.0), and screened using Rayyan.4 Six reviewers, working in pairs, independently screened titles and abstracts. Full texts of potentially eligible studies were retrieved and assessed. Each pair screened one-third of the articles, and calibration exercises were conducted prior to each step using randomly selected papers. Discrepancies between pairs of reviewers were resolved through discussion or with a third reviewer.
Eligibility was determined using the following criteria:
1. Is the publication in English, French, or Spanish? Yes [Include], No [Exclude]
2. Is the full text available? Yes [Include], No [Exclude]
3. Is the article original research? Yes [Include], No [Exclude], Unclear [Include]
4. Does it concern broilers, layers, turkeys, breeders, ducks, or geese? Yes [Include], No [Exclude]
5. Does it concern intensive poultry farming? Yes [Include], No [Exclude]
6. Does it assess biosecurity implementation at farm level? Yes [Include], No [Exclude]
7. Is the study conducted in Europe, Israel, Tunisia, or Turkey? Yes [Include], No [Exclude]
8. Is the publication a randomized controlled trial, case–control, or case-series study? No [Include], Yes [Exclude]
2.4 Data extraction
Data extraction was performed by six reviewers working in pairs, each handling one-third of the included studies. A Microsoft ExcelⓇ Spreadsheet (version 2020), developed by two authors and validated during a calibration phase on five randomly selected papers, was used. Extraction was done independently, and conflicts were solved as previously described. As a deviation from the protocol, only studies with data collection occurring from 2010 onward were included for biosecurity-related data, to ensure consistency with current legislative frameworks. Studies using models to estimate biosecurity levels were excluded, based on the assumption that non-model-based studies better reflect field conditions.
2.5 Data items
The following information was extracted from each study: year of publication; country; study time-frame; poultry category (broilers, layers, ducks, turkeys, breeders, other minor species); number of farms and number of flocks; production type (e.g., conventional, organic, antibiotic-free, outdoor, free-range, multi-species); type of analysis (e.g., scoring system, descriptive statistics, probability estimates using risk models or artificial intelligence); specific pathogen or disease (if relevant to biosecurity assessment). The full data extraction sheet is available upon request from the corresponding author.
2.6 Quality appraisal
Each included study was critically appraised using the tool developed by Downes et al. (22). This tool covers multiple aspects including study objective, methodology, results, and discussion. Appraisal was conducted independently by two reviewers, with discrepancies resolved through discussion.
2.7 Data synthesis
The selection process was summarized in the PRISMA flowchart. Descriptive statistics were used to summarize study characteristics. As described previously (23) and to avoid misclassification, for each study, biosecurity measures were grouped according to Biocheck. UGent™ poultry subcategories (purchase of one-day chicks, depopulation of broilers, feed and water, removal of manure and carcasses, visitors and farmworkers, material supply, infrastructure and biological vectors, location of the farm, disease management, cleaning and disinfection and materials and measurements between compartments) to ensure consistency across studies (24). For example, the subcategory “purchase of one-day-old chicks” included measures such as introduction of new animals, flock registration (origin, number of poultry), transport, number and health status of source herds; the “depopulation of broilers” category included measures like “all-in/all-out” poultry production on site and thinning. Studies were grouped based on the method used to assess biosecurity: (1) descriptive methods, where pooled results were expressed as the percentage of farms implementing specific categories of biosecurity measures; and (2) scoring methods, where pooled results were expressed as scores of biosecurity implementation. As described in the protocol, the intention of this review was to conduct a meta-analysis. However, due to the limited number of studies in each group and their heterogeneity, a meta-analysis, and therefore sensitivity analysis and publication bias assessment, were not conducted. For each biosecurity subcategory, the mean and interquartile range (IQR) were calculated.
3 Results
3.1 Study selection
A total of 1,515 articles were retrieved from four databases. After removing duplicates, 799 unique records were screened. Following the selection process, 44 articles were included, of which only 16 contained extractable data on biosecurity implementation. During the full text screening, the majority of articles (23 out of 64) were excluded because they did not concern the assessment of biosecurity implementation. The flow of study selection is summarized in Figure 1.
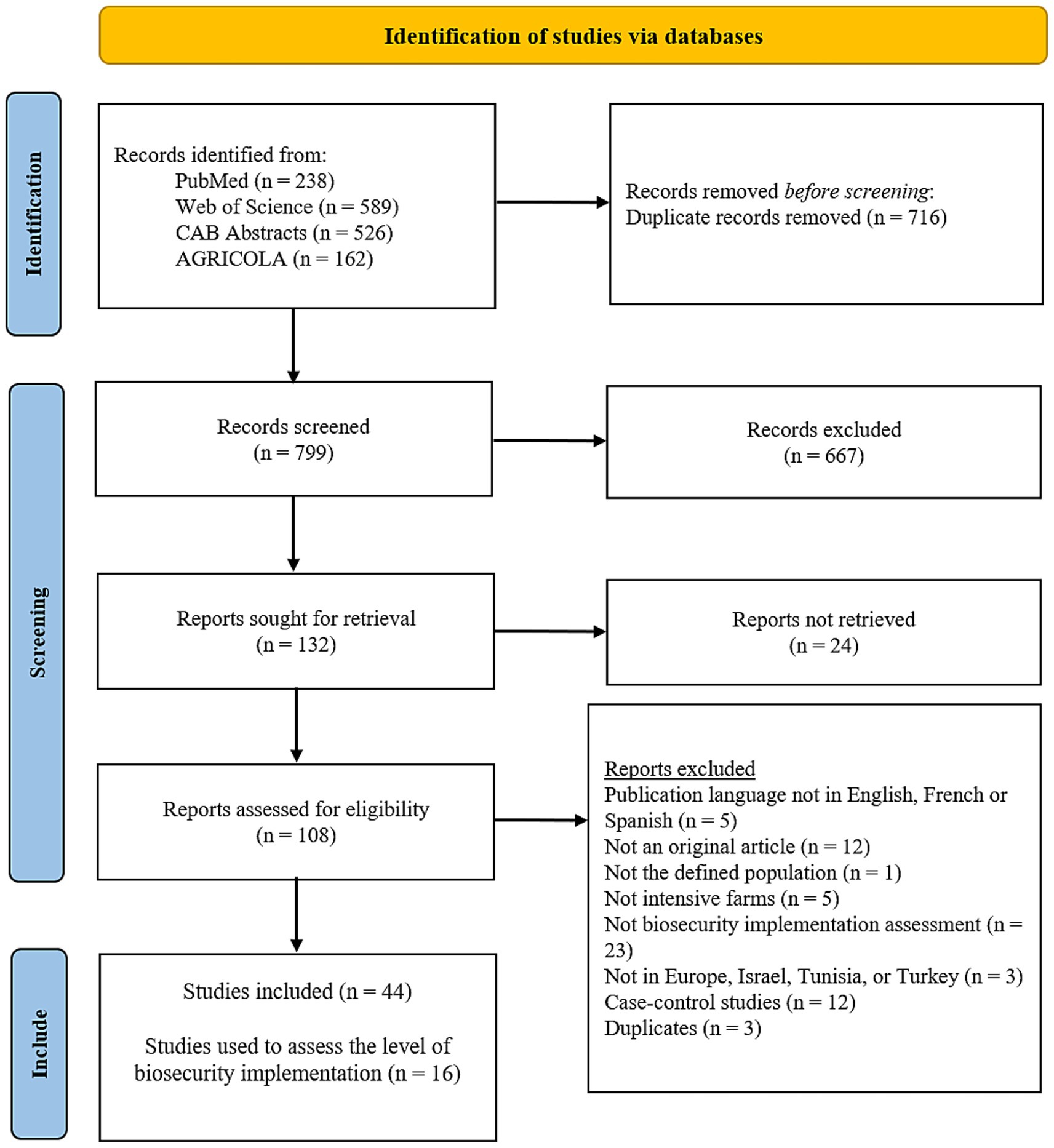
Figure 1. PRISMA flow diagram illustrating the selection process of studies included in the systematic review, from initial identification through screening and final inclusion. The process follows the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) 2020 guidelines.
3.2 Study characteristics
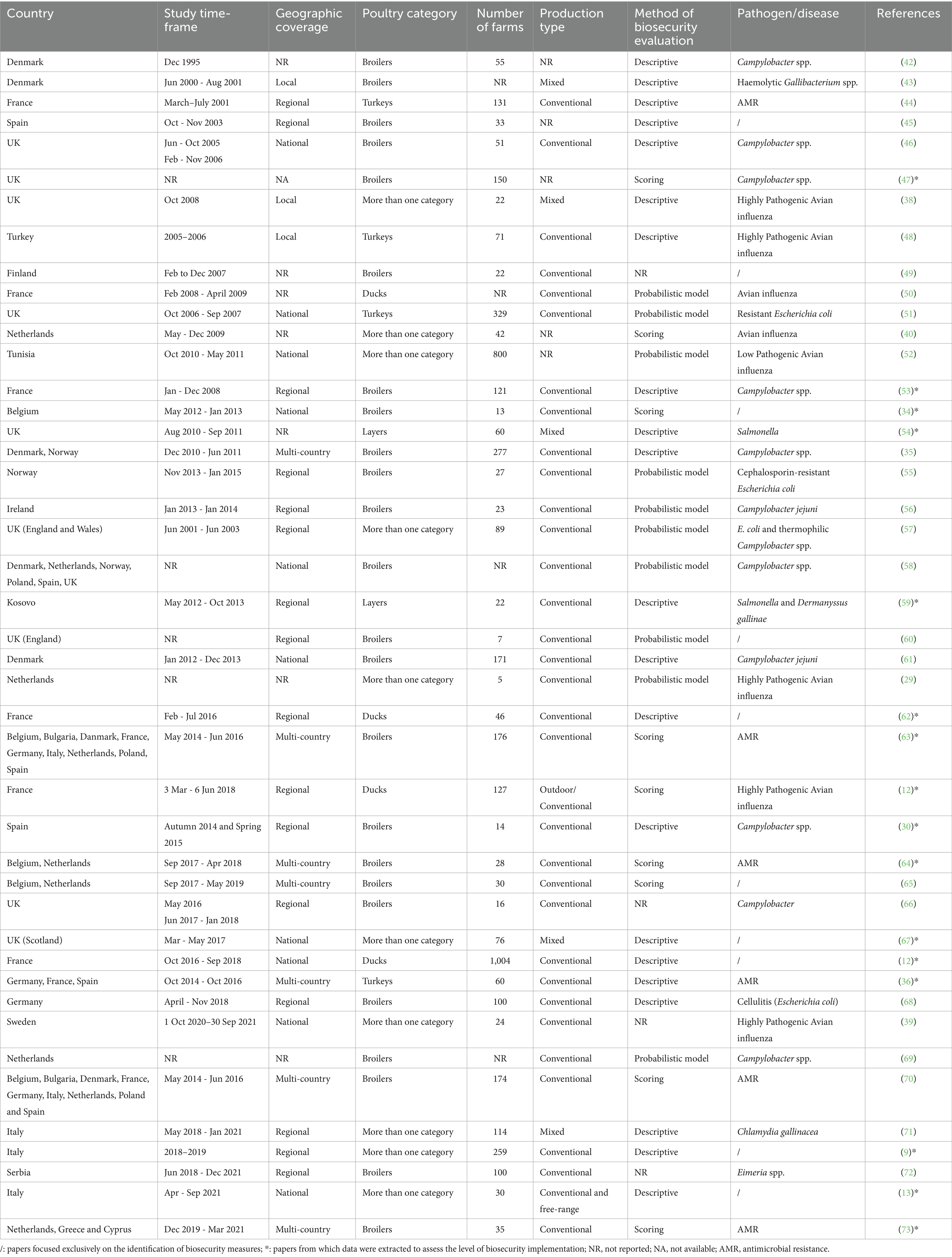
The characteristics of the included studies are reported in Table 1. Overall, there was an increasing trend in publications between 2000 and 2023. The study periods ranged from 1 to 43 months, with an average of 13 months. Among the countries represented, the United Kingdom had the highest number of studies (n = 10), followed by France and The Netherlands (n = 9 each), Denmark (n = 7), and Spain (n = 6). About 20% of countries were represented by only one study, including Cyprus, Finland, Greece, Ireland, Kosovo, Serbia, Sweden, Tunisia, and Turkey. In terms of geographical coverage, three studies focused on individual sites, 16 covered specific regions and nine provided national-level assessments. Eight studies were multi-country in scope, resulting in a total of 36 country-level analyses. Most studies focused on a single poultry species (77%), predominantly broilers (55%). The number of farms included in the studies ranged from 5 to 1,004 farms, with an average of 123. Biosecurity assessments for layers and turkeys appeared in 9% of studies, while 23% included more than one poultry category. Seventy-three percent of studies concerned conventional production systems. Only 10 out of 44 studies had biosecurity evaluation as their primary objective; the remainder (n = 6) focused on specific pathogens or diseases. The most frequently investigated topics included Campylobacter spp. (n = 12), antimicrobial resistance (n = 8), avian influenza (n = 8), Escherichia coli (n = 4), and Salmonella spp. (n = 2). Regarding methodological approaches to biosecurity assessment, 22 studies used descriptive analysis (e.g., percentage of farms implementing/having certain measures), nine applied scoring systems, and eight relied on probabilistic models. Five studies did not report any method for biosecurity assessment.
3.3 Results of individual studies
Out of the 44 articles included in the review, data extraction concerning biosecurity implementation was possible for only 16. The remaining 28 were excluded from synthesis due to the following reasons: five studies were published before 2010; 10 used probabilistic models; and 13 did not contain extractable data. As a result, all downstream analyses were performed on 16 papers. A summary of the extracted results is presented in Tables 2, 3.
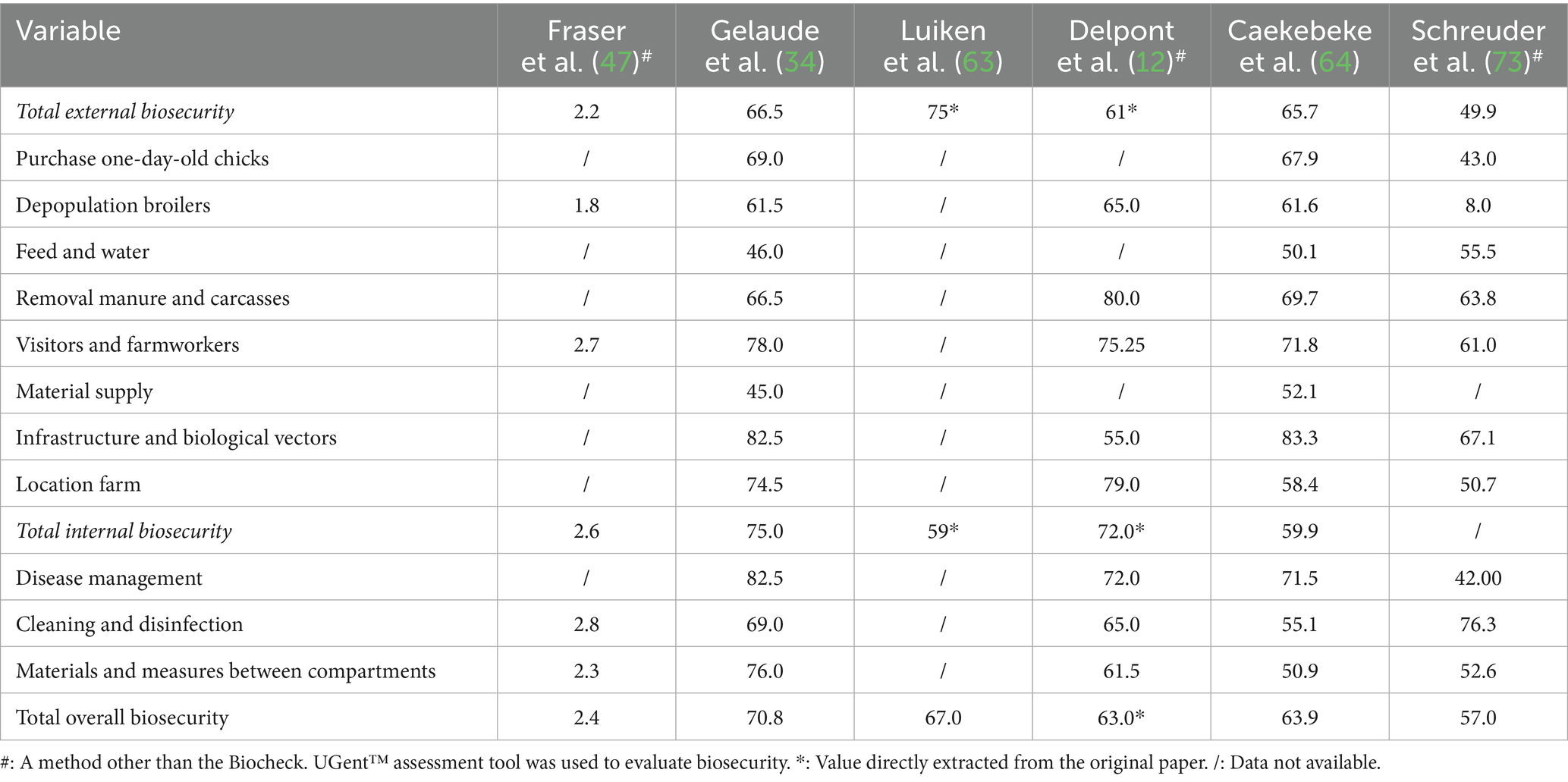
Table 2. Results of the included studies using scoring systems to evaluate biosecurity implementation.
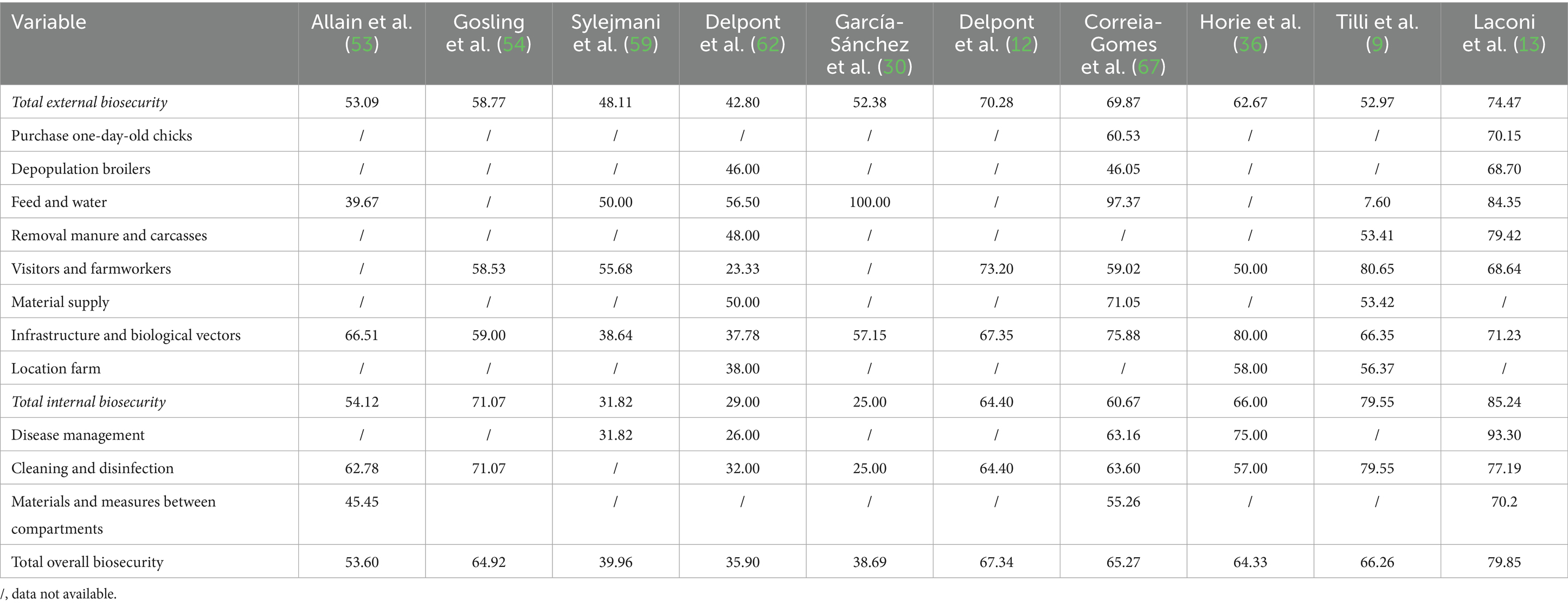
Table 3. Results of the included studies using descriptive methods to evaluate biosecurity implementation.
3.4 Quality assessment results
The quality appraisal was performed only for the 16 articles containing extractable data on biosecurity implementation evaluated through scoring or descriptive methods. The results are presented in Supplementary material 2. Overall, the methodological quality of the studies was deemed good. However, the majority of studies (87.5%) did not provide justification for the chosen sample size.
3.5 Synthesis of results
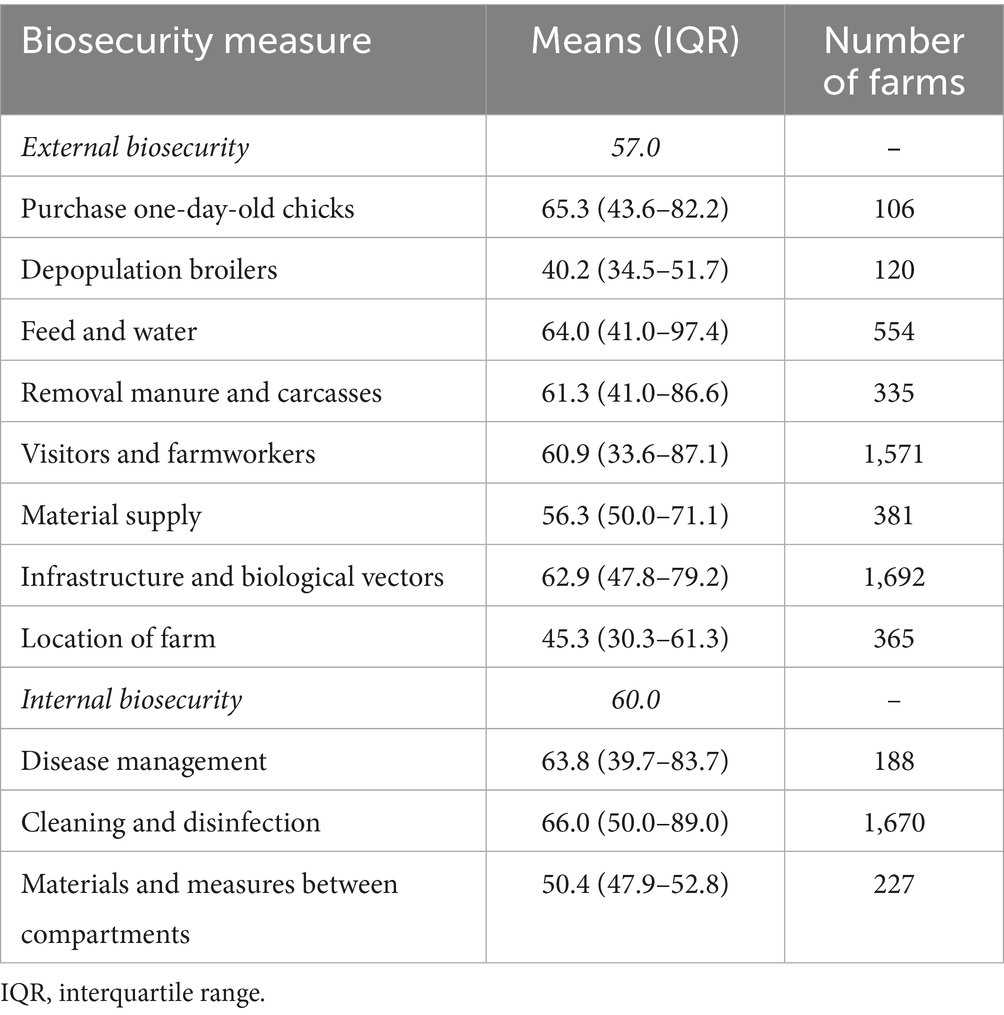
The included studies were grouped according to the type of biosecurity assessment method used, namely descriptive and scoring methods. Table 4 shows the pooled levels of biosecurity implementation (percentage of farmers implementing a biosecurity measure) based on descriptive data from 1,692 farms. In general, 58% of farms implemented all the biosecurity measures assessed in the study. External and internal biosecurity measures were implemented by 57 and 60% of farmers, respectively. Measures related to cleaning and disinfection (66.0%, IQR 50.0–89.0%) and purchase of one-day-old chicks (65.3%, IQR 43.6–82.2%) showed the highest level of implementation. Less than half of farmers implemented biosecurity measures related to depopulation (40.2%, IQR 34.5–51.7%) and farm location (45.3%, IQR 30.3–61.3%).
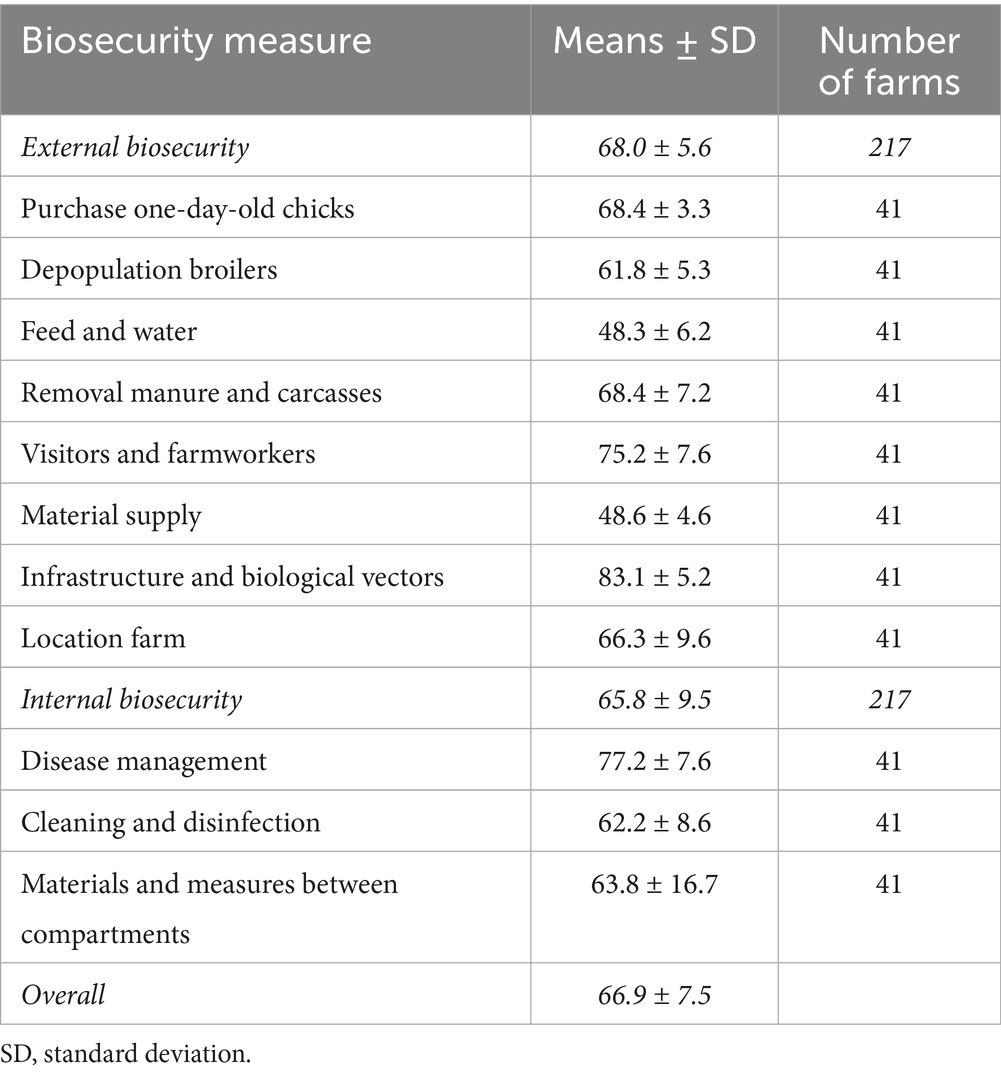
Results based on scoring assessments are presented in Table 5, covering 217 poultry farms. The overall average biosecurity score was 66.9/100. On average, the external and internal biosecurity scores were estimated at 68.0/100 and 65.8/100, respectively. The highest biosecurity scores were recorded for measures related to infrastructure and biological vectors (83.1), and disease management (77.2). Of all the biosecurity subcategories, “feed and water” (48.3) and “material supply” (48.6) received the lowest scores.
4 Discussion
This systematic review provides an overview of the level of biosecurity implementation in poultry farms across Europe and neighboring regions. Despite continuous research efforts over the past two decades (2003–2023), our findings reveal considerable variability in the implementation of biosecurity practices. This heterogeneity, driven by a wide range of study designs, poultry species, and methodological approaches, highlights the complexity of evaluating biosecurity across diverse national contexts.
This review also highlights the variability in methods used to assess biosecurity, namely descriptive analyses (e.g., reporting the percentage or the number of farms implementing specific measures), scoring systems (e.g., self-scores, Biocheck. Ugent™, national scoring systems, FAO’s Zone Biosecurity model) and probabilistic/simulation models. Similar results have recently been reported by Duarte et al. (25), who identified 33 different methods used to assess biosecurity in poultry farms in Europe and beyond. Descriptive approaches were the most common among the articles analyzed in our study. While such methods provide useful baseline data, the absence of standardized lists and definitions of biosecurity measures poses a challenge for generating comparable outputs across countries (26). In contrast, 13 studies employed scoring systems. Among these, Biocheck. UGent™5 was the most commonly used tool, enabling standardized and reproducible assessments of farm biosecurity (27). Probabilistic models (used in 10 studies) also offer valuable insights into the likelihood of pathogen introduction and spread under specific farm conditions (28). However, these models often rely on simulated data rather than empirical on-farm assessments (29). The integration of scoring systems with probabilistic modeling could serve as a standardized and powerful tool for national and regional surveillance, benchmarking, and supporting farm-level decision-making. By combining both systems, a comprehensive approach to data collection and analysis could help identify emerging trends and high-risk areas, thereby reinforcing national and regional surveillance programs. At the farm level, predictive models based on real-world data can support evidence-based decisions, such as optimizing vaccination timing against diseases or intensifying biosecurity measures during high-risk periods (e.g., for avian influenza introduction from wild birds).
Based on descriptive data from 1,692 farms, 58% of farms implemented all the biosecurity measures assessed. In addition, external and internal biosecurity measures were implemented by 57 and 60% of farmers, respectively. However, these values may not fully reflect the actual level of biosecurity implementation, as many studies using descriptive methods assessed the presence of a measure rather than its correct application. For example, in García-Sánchez et al. (30), the question “Does the farm have a vehicle wheel disinfection system?” did not assess whether the system was actually used. Future studies using descriptive methods should include tools that verify whether the measure, when present, is also implemented correctly (31). From the scoring evaluation, the overall average biosecurity score was 66.9/100 with external and internal biosecurity scoring 68.0/100 and 65.8/100, respectively. The majority of these studies focused on broiler farms, highlighting the efforts of EU countries over the last decade to strengthen biosecurity in poultry production. Regulatory frameworks, including Regulation (EU) 2016/429 and related national policies (32), have further supported implementation. Additionally, this likely reflects the economic relevance and industrial standardization of broiler production (33), but also the ease of data collection linked to their shorter production cycles.
Notable differences emerged across specific biosecurity subcategories. Measures related to cleaning and disinfection and the purchase of one-day-old chicks were most commonly implemented in studies using descriptive methods. In contrast, infrastructure and biological vectors, and disease management scored highest in studies using scoring systems. This divergence may be linked to differences in evaluation principles: descriptive evaluations do not assign weights, whereas scoring systems apply risk-based weightings (34).
Most studies (34 out of 44) were pathogen- or disease-specific (e.g., Campylobacter spp., Salmonella spp., and avian influenza), while only 10 studies focused on evaluating biosecurity measures. This suggests that biosecurity is still perceived as a reactive intervention within epidemiological frameworks rather than a proactive management strategy (30, 35, 36). A shift toward a more holistic and integrative approach, capable of preventing multiple hazards, would foster more resilient poultry health systems.
A majority of studies (77%) focused on a single poultry species, primarily broilers (55%), while assessments involving layers or turkeys were far less frequent. Although this review included solely primary research studies, this imbalance reveals a gap in biosecurity assessments for other systems (e.g., extensive or organic poultry farming, turkeys, and minor poultry species), which may also pose potential zoonotic risks (37).
Some studies (23%) evaluated multiple poultry categories, offering broader insights but often without disaggregated results by production type. Almost all such studies (7 out 10) were conducted during disease outbreaks (especially avian influenza) involving multiple poultry types in a region. For example, Knight-Jones et al. (38) administered a questionnaire during an avian influenza outbreak to holdings within 10 km of infected premises, regardless of species. In Sweden, biosecurity data were collected during a major avian influenza outbreak affecting over 2.2 million birds (39). While these studies offer comprehensive overviews, they often lack production-type–specific detail. Given that avian influenza affects various poultry species, future outbreak-based studies should enable comparisons between broilers, layers, and turkeys. Ssematimba et al. (40), for example, conducted detailed interviews with 42 farmers and 18 poultry business representatives during the H7N7 epidemic in the Netherlands. Their sample was adjusted to represent the national poultry population, allowing insights into biosecurity variation across production types. Future research should therefore combine species-specific and cross-cutting approaches when evaluating biosecurity protocols.
Despite broad geographical coverage—including eight multi-country studies with 36 national assessments—the distribution of studies remains uneven. Countries such as the United Kingdom, France, and the Netherlands are well represented, while others (e.g., Cyprus, Finland, Greece, Ireland, Kosovo, Serbia, Sweden, Tunisia, and Turkey) are covered by only a single study. This imbalance may reflect differences in research funding, national priorities, or logistical barriers to conducting longitudinal studies, or differences in the impact of national poultry production at European level.
Most reviewed studies targeted conventional systems (73%), while few addressed organic or free-range production systems. Given the growing demand for products from systems perceived to offer higher welfare (41), future studies should investigate biosecurity in these contexts. Expanding research into non-conventional systems would help fill critical knowledge gaps and support policy development.
4.1 Limitations
This systematic review has some limitations. First, only published original research articles were included, limiting the pool of available studies; in some countries, biosecurity data may exist but not publicly accessible. In some cases, country-specific data were missing, and despite attempts to contact corresponding authors, no further information was obtained, leading to the exclusion of those studies. Second, methodological heterogeneity such as differences in assessment tools/methods, species, definitions, and study designs posed challenges in synthesizing and interpreting the data. These factors may limit the generalizability of the findings and underscore the need for harmonized research protocols in future biosecurity assessments.
5 Conclusion
This study aimed to systematically review the level of biosecurity implementation in poultry farms across Europe and neighboring countries. The findings indicate that biosecurity implementation is highly variable, with notable differences in both geographical coverage and the types of poultry systems assessed. Several key areas for improvement emerged from this review, including: (i) the need for more published data on layers, turkeys, and other poultry types beyond broilers, to develop a more comprehensive understanding of biosecurity across diverse systems; (ii) the promotion of cross-country collaboration, capacity building, and targeted resource allocation to enhance research output, data harmonization, and knowledge sharing; and (iii) increased research on alternative (e.g., extensive and organic) poultry production systems, to better understand how production models influence biosecurity implementation and effectiveness.
Addressing these gaps will strengthen future efforts to implement and monitor biosecurity measures, ultimately reducing disease transmission risks and supporting a safer, more resilient poultry sector.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
RV: Data curation, Investigation, Writing – review & editing, Formal analysis, Visualization, Writing – original draft. ML: Data curation, Formal analysis, Investigation, Visualization, Writing – original draft, Writing – review & editing. GT: Data curation, Investigation, Writing – review & editing. AL: Data curation, Investigation, Writing – review & editing. QM: Data curation, Investigation, Writing – review & editing. JP-R: Writing – review & editing, Conceptualization, Project administration, Validation. AA: Conceptualization, Project administration, Validation, Writing – review & editing. IC: Conceptualization, Project administration, Writing – review & editing, Investigation, Supervision, Validation. AP: Conceptualization, Investigation, Supervision, Writing – review & editing, Data curation, Validation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research work was performed within COST Action CA20103, Biosecurity Enhanced Through Training Evaluation and Raising Awareness (BETTER), supported by COST (European Cooperation in Science and Technology). Open Access funding provided by Università degli Studi di Padova | University of Padua, Open Science Committee.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2025.1653543/full#supplementary-material
Supplementary material 1 | Search strategy performed in CAB Abstract.
Supplementary material 2 | Results of the quality appraisal of included studies.
Footnotes
1. ^https://better-biosecurity.eu/
2. ^https://hdl.handle.net/11577/3511729
References
2. Dewulf, J, and Van Immerseel, F. Biosecurity in animal production and veterinary medicine: from principles to practice. Leuven: ACCO (2018).
3. Fountain, J, Brookes, V, Kirkeby, C, Manyweathers, J, Maru, Y, and Hernandez-Jover, M. One size does not fit all: exploring the economic and non-economic outcomes of on-farm biosecurity for bovine viral diarrhea virus in Australian beef production. Prev Vet Med. (2022) 208:105758. doi: 10.1016/j.prevetmed.2022.105758
4. Dhaka, P, Chantziaras, I, Vijay, D, Bedi, JS, Makovska, I, Biebaut, E, et al. Can improved farm biosecurity reduce the need for antimicrobials in food animals? A scoping review. Antibiotics. (2023) 12:893. doi: 10.3390/antibiotics12050893
5. Regulation (EU) 2016/429 of the European Parliament and of the council, 2016, transmissible animal diseases and amending and repealing certain acts in the area of animal health (‘animal health law’). Available online at: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32016R0429.
6. Mallioris, P, Teunis, G, Lagerweij, G, Joosten, P, Dewulf, J, Wagenaar, JA, et al. Biosecurity and antimicrobial use in broiler farms across nine European countries: toward identifying farm-specific options for reducing antimicrobial usage. Epidemiol Infect. (2023) 151:e13. doi: 10.1017/S0950268822001960
7. Souillard, R, Allain, V, Dufay-Lefort, AC, Rousset, N, Amalraj, A, Spaans, A, et al. Biosecurity implementation on large-scale poultry farms in Europe: a qualitative interview study with farmers. Prev Vet Med. (2024) 224:106119. doi: 10.1016/j.prevetmed.2024.106119
8. Mahmood, Q, Tilli, G, Laconi, A, Ngom, RV, Leite, M, Prodanov-Radulović, J, et al. Implementation of biosecurity measures according to legislation in intensive poultry production: an overview across 22 EU and non-EU countries. Prev Vet Med. (2025) 242:106571. doi: 10.1016/j.prevetmed.2025.106571
9. Tilli, G, Laconi, A, Galuppo, F, Mughini-Gras, L, and Piccirillo, A. Assessing biosecurity compliance in poultry farms: a survey in a densely populated poultry area in north East Italy. Animals. (2022) 12:1409. doi: 10.3390/ani12111409
10. Santonja, GG, Georgitzikis, K, Scalet, BM, Montobbio, P, Roudier, S, and Delgado, SL. Best available techniques (BAT) reference document for the intensive rearing of poultry or pigs. EUR 28674 EN (2017). Available online at: https://eippcb.jrc.ec.europa.eu/sites/default/files/2019-11/JRC107189_IRPP_Bref_2017_published.pdf.
11. EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare)Nielsen, SS, Alvarez, J, Bicout, DJ, Calistri, P, Canali, E, et al. Welfare of broilers on farm. EFSA J. (2023) 21:7788. doi: 10.2903/j.efsa.2023.7788
12. Delpont, M, Racicot, M, Durivage, A, Fornili, L, Guerin, JL, Vaillancourt, JP, et al. Determinants of biosecurity practices in French duck farms after a H5N8 highly pathogenic avian influenza epidemic: the effect of farmer knowledge, attitudes and personality traits. Transbound Emerg Dis. (2021) 68:51–61. doi: 10.1111/tbed.13462
13. Laconi, A, Tilli, G, Galuppo, F, Grilli, G, Souillard, R, and Piccirillo, A. Stakeholders’ perceptions of biosecurity implementation in Italian poultry farms. Animals. (2023) 13:3246. doi: 10.3390/ani13203246
14. Amalraj, A, Van Meirhaeghe, H, Lefort, AC, Rousset, N, Grillet, J, Spaans, A, et al. Factors affecting poultry producers’ attitudes towards biosecurity. Animals. (2024) 14:1603. doi: 10.3390/ani14111603
15. Can, MF, Altuğ, N, and Kaygisiz, F. Biosecurity levels of livestock enterprises in Turkey and factors affecting these levels. Turk J Vet Anim Sci. (2020) 44:632–40. doi: 10.3906/vet-1911-70
16. Sari, M, and Saatci, M. Biosecurity procedures with the all aspects in goose breeding. Turk J Agric Food Sci Technol. (2020) 8:35–41. doi: 10.24925/turjaf.v8i1.35-41.2590
17. Delpont, M, Salazar, LG, Dewulf, J, Zbikowski, A, Szeleszczuk, P, Dufay-Lefort, AC, et al. Monitoring biosecurity in poultry production: an overview of databases reporting biosecurity compliance from seven European countries. Front Vet Sci. (2023) 10:1231377. doi: 10.3389/fvets.2023.1231377
18. Maletic, J, Spalević, L, Milićević, V, Glišić, D, Kureljušić, B, Kureljušić, J, et al. Assessment of biosecurity measures implemented on the broiler farms in the region of Belgrade City. Vet Glasnik. (2023) 77:125–36. doi: 10.2298/VETGL230403003M
19. Higgins, JPT, Thomas, J, Chandler, J, Cumpston, M, Li, T, Page, MJ, et al. Cochrane handbook for systematic reviews of interventions version 6.3 (2022). Available online at: http://www.training.cochrane.org/handbook.
20. Page, MJ, McKenzie, JE, Bossuyt, PM, and Boutron, I. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
21. Moher, D, Shamseer, L, Clarke, M, Ghersi, D, Liberati, A, Petticrew, M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. (2015) 4:1. doi: 10.1186/2046-4053-4-1
22. Downes, MJ, Brennan, ML, Williams, HC, and Dean, RS. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). BMJ Open. (2016) 6:e011458. doi: 10.1136/bmjopen-2016-011458
23. Vougat Ngom, R, Ayissi, GJ, Akoussa, AM, Laconi, A, Jajere, SM, Zangue, HA, et al. A systematic review and meta-analysis of the efficacy of biosecurity in disease prevention and control in livestock farms in Africa. Transbound Emerg Dis. (2024) 2024:8683715. doi: 10.1155/2024/8683715
24. Vougat Ngom, R, Laconi, A, Mouiche, MM, Ayissi, GJ, Akoussa, AMM, Ziebe, SD, et al. Methods and tools used for biosecurity assessment in livestock farms in Africa: a scoping review. Transbound Emerg Dis. (2024) 1:5524022. doi: 10.1155/2024/5524022
25. Duarte, F, Tamminen, LM, Kjosevski, M, Ciaravino, G, Delpont, M, Correia-Gomes, C, et al. Methods to assess on-farm biosecurity in Europe and beyond. Prev Vet Med. (2025) 239:106486. doi: 10.1016/j.prevetmed.2025.106486
26. Huber, N, Andraud, M, Sassu, EL, Prigge, C, Zoche-Golob, V, Käsbohrer, A, et al. What is a biosecurity measure? A definition proposal for animal production and linked processing operations. One Health. (2022) 15:100433. doi: 10.1016/j.onehlt.2022.100433
27. Amalraj, A, Van Meirhaeghe, H, Caekebeke, N, Creve, R, Dufay-Lefort, A-C, Rousset, N, et al. Development and use of biocheck. Ugent™ scoring system to quantify biosecurity in conventional indoor (Turkey, duck, breeder) and free-range (layer and broiler) poultry farms. Prev Vet Med. (2024) 230:106288. doi: 10.1016/j.prevetmed.2024.106288
28. Silva, G, Leotti, VB, Castro, SMJ, Silva, GS, Medeiros, AAR, Silva, APSP, et al. Assessment of biosecurity practices and development of a scoring system in swine farms using item response theory. Prev Vet Med. (2019) 167:128–36. doi: 10.1016/j.prevetmed.2019.03.020
29. Hagenaars, TJ, Boender, GJ, Bergevoet, RHM, and van Roermund, HJW. Risk of poultry compartments for transmission of highly pathogenic avian influenza. PLoS One. (2018) 13:e0207076. doi: 10.1371/journal.pone.0207076
30. García-Sánchez, L, Melero, B, Diez, AM, Jaime, I, Canepa, A, and Rovira, J. Genotyping, virulence genes and antimicrobial resistance of Campylobacter spp. isolated during two seasonal periods in Spanish poultry farms. Prev Vet Med. (2020) 176:104935. doi: 10.1016/j.prevetmed.2020.104935
31. Racicot, M, Venne, D, Durivage, A, and Vaillancourt, JP. Description of 44 biosecurity errors while entering and exiting poultry barns based on video surveillance in Quebec, Canada. Prev Vet Med. (2011) 100:193–9. doi: 10.1016/j.prevetmed.2011.04.011
32. Council of the European Union. (2019). Council conclusions on biosecurity, an overall concept with a unitary approach for protecting animal health in the EU. Available online at: https://data.consilium.europa.eu/doc/document/ST-10368–2019-REV-1/en/pdf (Accessed May 20, 2025).
33. Adaszyńska-Skwirzyńska, M, Konieczka, P, Bucław, M, Majewska, D, Pietruszka, A, Zych, S, et al. Analysis of the production and economic indicators of broiler chicken rearing in 2020-2023: a case study of a polish farm. Agriculture. (2025) 15:139. doi: 10.3390/agriculture15020139
34. Gelaude, P, Schlepers, M, Verlinden, M, Laanen, M, and Dewulf, J. Biocheck.UGent: a quantitative tool to measure biosecurity at broiler farms and the relationship with technical performances and antimicrobial use. Poult Sci. (2014) 93:2740–51. doi: 10.3382/ps.2014-04002
35. Høg, BB, Sommer, HM, Larsen, LS, Borck Høg, B, Sørensen, AIV, David, B, et al. Farm specific risk factors for Campylobacter colonisation in Danish and Norwegian broilers. Prev Vet Med. (2016) 130:137–45. doi: 10.1016/j.prevetmed.2016.04.002
36. Horie, M, Yang, D, Joosten, P, Munk, P, Wadepohl, K, Chauvin, C, et al. Risk factors for antimicrobial resistance in Turkey farms: a cross-sectional study in three European countries. Antibiotics. (2021) 10:820. doi: 10.3390/antibiotics10070820
37. Gonzales, JL, Elbers, ARW, and Beerens, N. Risk factors of primary introduction of highly pathogenic and low pathogenic avian influenza virus into European poultry holdings, considering at least material contaminated by wild birds and contact with wild birds. EFSA Support Publ. (2017) 14:1282E. doi: 10.2903/sp.efsa.2017.EN-1282
38. Knight-Jones, TJ, Gibbens, J, Wooldridge, M, and Stärk, KD. Assessment of farm-level biosecurity measures after an outbreak of avian influenza in the United Kingdom. Transbound Emerg Dis. (2011) 58:69–75. doi: 10.1111/j.1865-1682.2010.01183.x
39. Grant, M, Bröjer, C, Zohari, S, et al. Highly pathogenic avian influenza (HPAI H5Nx, clade 2.3.4.4.B) in poultry and wild birds in Sweden: synopsis of the 2020–2021 season. Vet Sci. (2022) 9:2740–51. doi: 10.3390/vetsci9070344
40. Ssematimba, A, Hagenaars, TJ, De Wit, JJ, Ruiterkamp, F, Fabri, TH, Stegeman, JA, et al. Avian influenza transmission risks: analysis of biosecurity measures and contact structure in Dutch poultry farming. Prev Vet Med. (2013) 109:106–15. doi: 10.1016/j.prevetmed.2012.09.001
41. de Jonge, J, and van Trijp, H. The impact of broiler production system practices on consumer perceptions of animal welfare. Poult Sci. (2013) 92:3080–95. doi: 10.3382/ps.2013-03334
42. Hald, B, Wedderkopp, A, and Madsen, M. Thermophilic Campylobacter spp. in Danish broiler production: a cross-sectional survey and a retrospective analysis of risk factors for occurrence in broiler flocks. Avian Pathol. (2000) 29:123–31. doi: 10.1080/03079450094153
43. Bojesen, MA, Nielsen, SS, and Bisgaard, M. Prevalence and transmission of haemolytic Gallibacterium species in chicken production systems with different biosecurity levels. Avian Pathol. (2003) 32:503–10. doi: 10.1080/0307945031000154107
44. Chauvin, C, Bouvarel, I, Belœil, PA, Orand, JP, Guillemot, D, and Sanders, P. A pharmaco-epidemiological analysis of factors associated with antimicrobial consumption level in Turkey broiler flocks. Vet Res. (2005) 36:199–211. doi: 10.1051/vetres:2004064
45. Quiles, A. Controle de Biosecurite des Gaveurs de Poulets de La Region de Murcia. Arch Zootech. (2005) 54:609–18.
46. Allen, VM, Weaver, H, Ridley, AM, Harris, JA, Sharma, M, Emery, J, et al. Sources and spread of thermophilic Campylobacter spp. during partial depopulation of broiler chicken flocks. J Food Prot. (2008) 71:264–70. doi: 10.4315/0362-028X-71.2.264
47. Fraser, RW, Williams, NT, Powell, LF, and Cook, AJ. Reducing Campylobacter and Salmonella infection: two studies of the economic Cost and attitude to adoption of on-farm biosecurity measures. Zoonoses Public Health. (2010) 57:e109–15. doi: 10.1111/j.1863-2378.2009.01295.x
48. Yalcin, C, Sipahi, C, Aral, Y, and Cevger, Y. Economic effect of the highly pathogenic avian influenza H5N1 outbreaks among Turkey producers, 2005-06, Turkey. Avian Dis. (2010) 54:390–3. doi: 10.1637/8710-031809-Reg.1
49. Siekkinen, KM, Heikkilä, J, Tammiranta, N, and Rosengren, H. Measuring the costs of biosecurity on poultry farms: a case study in broiler production in Finland. Acta Vet Scand. (2012) 54:1–8. doi: 10.1186/1751-0147-54-12
50. Duvauchelle, A, Huneau-Salaün, A, Balaine, L, Rose, N, and Michel, V. Risk factors for the introduction of avian influenza virus in breeder duck flocks during the first 24 weeks of laying. Avian Pathol. (2013) 42:447–56. doi: 10.1080/03079457.2013.823145
51. Jones, EM, Snow, LC, Carrique-Mas, JJ, Gosling, RJ, Clouting, C, and Davies, RH. Risk factors for antimicrobial resistance in Escherichia coli found in GB Turkey flocks. Vet Rec. (2013) 173:422–2. doi: 10.1136/vr.101759
52. Tombari, W, Paul, M, Bettaieb, J, Larbi, I, Nsiri, J, Elbehi, I, et al. Risk factors and characteristics of low pathogenic avian influenza virus isolated from commercial poultry in Tunisia. PLoS One. (2013) 8:e53524. doi: 10.1371/journal.pone.0053524
53. Allain, V, Chemaly, M, Laisney, MJ, et al. Prevalence of and risk factors for Campylobacter colonisation in broiler flocks at the end of the rearing period in France. Br Poult Sci. (2014) 55:452–9. doi: 10.1080/00071668.2014.941788
54. Gosling, RJ, Martelli, F, Wintrip, A, et al. Assessment of producers’ response to Salmonella biosecurity issues and uptake of advice on laying hen farms in England and Wales. Br Poult Sci. (2014) 55:559–68. doi: 10.1080/00071668.2014.949620
55. Mo, SS, Kristoffersen, AB, Sunde, M, Nødtvedt, A, and Norström, M. Risk factors for occurrence of cephalosporin-resistant Escherichia coli in Norwegian broiler flocks. Prev Vet Med. (2016) 130:112–8. doi: 10.1016/j.prevetmed.2016.06.011
56. Smith, S, Messam, LL, Meade, J, Gibbons, J, McGill, K, Bolton, D, et al. The impact of biosecurity and partial depopulation on Campylobacter prevalence in Irish broiler flocks with differing levels of hygiene and economic performance. Infect Ecol Epidemiol. (2016) 6:31454. doi: 10.3402/iee.v6.31454
57. Taylor, NM, Wales, AD, Ridley, AM, and Davies, RH. Farm level risk factors for fluoroquinolone resistance in E. Coli and thermophilic Campylobacter spp. on poultry farms. Avian Pathol. (2016) 45:559–68. doi: 10.1080/03079457.2016.1185510
58. Sommer, HM, Høg, BB, Larsen, LS, Sørensen, AIV, Williams, N, Merga, JY, et al. Analysis of farm specific risk factors for Campylobacter colonization of broilers in six European countries. Microb Risk Anal. (2016) 2-3:16–26. doi: 10.1016/j.mran.2016.06.002
59. Sylejmani, D, Musliu, A, Ramadani, N, Sparagano, O, and Hamidi, A. Associations between the level of biosecurity and occurrence of Dermanyssus gallinae and Salmonella spp. in layer farms. Avian Dis. (2016) 60:454–9. doi: 10.1637/11327-111415-Reg
60. Millman, C, Christley, R, Rigby, D, Dennis, D, O’Brien, SJ, and Williams, N. "catch 22": biosecurity awareness, interpretation and practice amongst poultry catchers. Prev Vet Med. (2017) 141:22–32. doi: 10.1016/j.prevetmed.2017.04.002
61. Sandberg, M, Dahl, J, Lindegaard, LL, and Pedersen, JR. Compliance/non-compliance with biosecurity rules specified in the Danish quality assurance system (KIK) and Campylobacter-positive broiler flocks 2012 and 2013. Poult Sci. (2017) 96:184–91. doi: 10.3382/ps/pew277
62. Delpont, M, Blondel, V, Robertet, L, Duret, H, Guerin, JL, Vaillancourt, JP, et al. Biosecurity practices on foie gras duck farms, Southwest France. Prev Vet Med. (2018) 158:78–88. doi: 10.1016/j.prevetmed.2018.07.012
63. Luiken, RE, van, L, Munk, P, Sarrazin, S, Joosten, P, Dorado-García, A, et al. Associations between antimicrobial use and the faecal resistome on broiler farms from nine European countries. J Antimicrob Chemother. (2019) 74:2596–604. doi: 10.1093/jac/dkz235
64. Caekebeke, N, Jonquiere, FJ, Ringenier, M, Tobias, TJ, Postma, M, van den Hoogen, A, et al. Comparing farm biosecurity and antimicrobial use in high-antimicrobial-consuming broiler and pig farms in the Belgian–Dutch border region. Front Vet Sci. (2020) 7:558455. doi: 10.3389/fvets.2020.558455
65. Caekebeke, N, Ringenier, M, Jonquiere, FJ, Tobias, T, Postma, M, van den Hoogen, A, et al. Coaching Belgian and Dutch broiler farmers aimed at antimicrobial stewardship and disease prevention. Antibiotics. (2021) 10:590. doi: 10.3390/antibiotics10050590
66. Royden, A, Christley, R, Prendiville, A, and Williams, NJ. The role of biosecurity in the control of Campylobacter: a qualitative study of the attitudes and perceptions of UK broiler farm workers. Front Vet Sci. (2021) 8:751699. doi: 10.3389/fvets.2021.751699
67. Correia-Gomes, C, Henry, MK, Reeves, A, and Sparks, N. Management and biosecurity practices by small to medium egg producers in Scotland. Br Poult Sci. (2021) 62:499–508. doi: 10.1080/00071668.2021.1894635
68. Bernd, KS, Kump, AWS, Freise, F, Schulze Bernd, K, Wilms-Schulze Kump, A, Reich, F, et al. Influences of biosecurity on the occurrence of cellulitis in broiler flocks. J Appl Poult Res. (2022) 31:100230. doi: 10.1016/j.japr.2021.100230
69. Horvat, A, Luning, PA, Di Gennaro, C, Rommens, E, van Daalen, E, Koene, M, et al. The impacts of biosecurity measures on Campylobacter contamination in broiler houses and slaughterhouses in the Netherlands: a simulation modelling approach. Food Control. (2022) 141:109151. doi: 10.1016/j.foodcont.2022.109151
70. Luiken, RE, Heederik, DJ, Scherpenisse, P, van Gompel, L, van Heijnsbergen, E, Greve, GD, et al. Determinants for antimicrobial resistance genes in farm dust on 333 poultry and pig farms in nine European countries. Environ Res. (2022) 208:112715. doi: 10.1016/j.envres.2022.112715
71. Marchino, M, Rizzo, F, Barzanti, P, Sparasci, OA, Bottino, P, Vicari, N, et al. Chlamydia species and related risk factors in poultry in North-Western Italy: possible bird-to-human transmission for C. Gallinacean. Int J Environ Res Public Health. (2022) 19:2174. doi: 10.3390/ijerph19042174
72. Pajić, M, Todorović, D, Knežević, S, Prunić, B, Velhner, M, Andrić, D, et al. Molecular investigation of Eimeria species in broiler farms in the province of Vojvodina, Serbia. Life. (2023) 13:1039. doi: 10.3390/life13041039
73. Schreuder, J, Simitopoulou, M, Angastiniotis, K, Ferrari, P, Wolthuis-Fillerup, M, Kefalas, G, et al. Development and implementation of a risk assessment tool for broiler farm biosecurity and a health intervention plan in the Netherlands, Greece, and Cyprus. Poult Sci. (2023) 102:102394. doi: 10.1016/j.psj.2022.102394
Keywords: biosecurity, assessment, prevention, poultry, Europe, Israel, Tunisia, Turkey
Citation: Vougat Ngom R, Leite M, Tilli G, Laconi A, Mahmood Q, Prodanov-Radulović J, Allepuz A, Chantziaras I and Piccirillo A (2025) Biosecurity implementation in poultry farms across Europe and neighboring countries: a systematic review. Front. Vet. Sci. 12:1653543. doi: 10.3389/fvets.2025.1653543
Edited by:
Ioannis Magouras, University of Bern, SwitzerlandReviewed by:
A. K. M. Dawlat Khan, Institute of Epidemiology, Disease Control and Research (IEDCR), BangladeshBehailu Assefa Wayou, Arsi University, Ethiopia
Copyright © 2025 Vougat Ngom, Leite, Tilli, Laconi, Mahmood, Prodanov-Radulović, Allepuz, Chantziaras and Piccirillo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alessandra Piccirillo, YWxlc3NhbmRyYS5waWNjaXJpbGxvQHVuaXBkLml0
§Present addresses: Marta Leite, National Institute of Agricultural and Veterinary Research (INIAV, I.P.), Vila do Conde, Portugal
Giuditta Tilli, Vetworks bvba, Poeke, Belgium
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
 Ronald Vougat Ngom
Ronald Vougat Ngom Marta Leite
Marta Leite Giuditta Tilli
Giuditta Tilli Andrea Laconi
Andrea Laconi Qamer Mahmood
Qamer Mahmood Jasna Prodanov-Radulović
Jasna Prodanov-Radulović Alberto Allepuz
Alberto Allepuz Ilias Chantziaras
Ilias Chantziaras Alessandra Piccirillo
Alessandra Piccirillo