- 1Veterinary Drugs and Biologics Division, Animal and Plant Quarantine Agency, Gimcheon-si, Gyeongsangbuk-do, Republic of Korea
- 2College of Veterinary Medicine and Institute for Veterinary Biomedical Science, Kyungpook National University, Daegu, Republic of Korea
- 3Bacterial Disease Division, Animal and Plant Quarantine Agency, Gimcheon-si, Gyeongsangbuk-do, Republic of Korea
Ensuring the safety and efficacy of veterinary vaccines requires reliable methods for detecting microbial contamination, particularly from Mycoplasma species, which pose a significant risk in cell-culture-derived vaccines. In the Republic of Korea, polymerase chain reaction (PCR) is predominantly used for Mycoplasma testing due to its faster turnaround compared to culture-based methods. However, in combination with vaccines containing Erysipelothrix rhusiopathiae and classical swine fever virus, PCR is rendered ineffective because of cross-reactivity between Mycoplasma universal primers and E. rhusiopathiae, resulting in non-specific amplification. This limitation necessitates reliance on the labor-intensive culture method, underscoring the need for more accurate and efficient alternatives. This study aimed to develop and validate next-generation sequencing (NGS)-based methods for detecting Mycoplasma contamination in veterinary vaccines and to compare their performance with that of PCR. Five species, including Acholeplasma laidlawii (genus Acholeplasma) and four Mycoplasma species—Mycoplasma fermentans, Mycoplasma orale, Mycoplasma hyorhinis, and Mycoplasma synoviae–were spiked into samples containing E. rhusiopathiae, a common vaccine component. Two NGS-based approaches were evaluated: (1) a reference-mapping method incorporating two-step alignment and de novo assembly, and (2) a 16S rRNA-based metabarcoding analysis using DADA2 and Qiime2. The reference-mapping method effectively filtered non-specific reads and accurately reconstructed Mycoplasma-derived contigs, whereas the metabarcoding approach enabled taxonomic profiling with quantitative resolution. The detection limits of NGS-based methods were substantially lower than those of PCR, demonstrating improvements of up to 100-fold depending on the species. Notably, omission of the initial mapping step resulted in excessive non-specific contig formation, highlighting the importance of the dual-step reference-mapping strategy. Although metabarcoding provided valuable abundance data, it was more prone to non-specific hits due to limited read overlap. In conclusion, the reference-mapping method demonstrated superior sensitivity, specificity, and quantification compared to both conventional PCR and metabarcoding, supporting its use as a robust tool for vaccine quality control. Implementing NGS-based detection methods could significantly enhance the safety and effectiveness of veterinary vaccines, ultimately enhancing vaccine quality control.
1 Introduction
Before veterinary vaccines can be marketed, they must undergo testing to ensure compliance with quality standards, safety regulations, and efficacy requirements established by regulatory authorities (1). These tests are conducted both in the countries where the products are approved for sale and internally by manufacturers. Commercial vaccines are vulnerable to contamination by various adventitious agents during the manufacturing process, including bacteria, viruses, fungi, and Mycoplasma species. Among these contaminants, Mycoplasma has emerged as a particularly concerning issue due to its potential impact on animal health and vaccine effectiveness (2).
Mycoplasma species are a type of bacteria lacking cell walls and are capable of infecting a range of animal hosts, resulting in substantial economic losses in the agricultural sector (3). Their lack of a cell wall makes them inherently resistant to antibiotics that target cell wall synthesis, complicating treatment efforts (4). Mycoplasma can also contaminate cell cultures and biological products without causing visible signs of infection, making detection difficult. Conventional methods for identifying Mycoplasma contamination include culture-based techniques and polymerase chain reaction (PCR) assays, both of which are used by the Animal and Plant Quarantine Agency (APQA) in the Republic of Korea for testing veterinary biologicals. Culture-based methods are widely recognized for their ability to detect viable organisms, but they are time-consuming, often requiring up to 28 days for results (1, 5). This delay presents challenges for timely quality control during vaccine production. At APQA, a broad-range PCR assay targeting multiple Mycoplasma species is employed as the initial screening method. PCR-positive samples are subsequently subjected to culture, and the results of culture are used to make the final determination of Mycoplasma contamination.
During routine national lot release testing of combination vaccines containing Erysipelothrix rhusiopathiae (E. rhusiopathiae) and classical swine fever virus (CSFV) as antigens, non-specific bands in Mycoplasma PCR assays were consistently observed. Analysis of cumulative results from the national lot release tests conducted by APQA in Korea further confirmed that these non-specific findings occurred repeatedly. Basic Local Alignment Search Tool (BLAST) analysis identified these bands as sequences derived from E. rhusiopathiae. Alignment of E. rhusiopathiae sequences with Mycoplasma universal primers revealed near-complete complementarity, with only a few nucleotide mismatches (Figure 1). This finding suggests that genetic similarities between E. rhusiopathiae and Mycoplasma species can interfere with PCR specificity, resulting in non-specific amplification and potentially compromising the accuracy of Mycoplasma testing (6, 7).
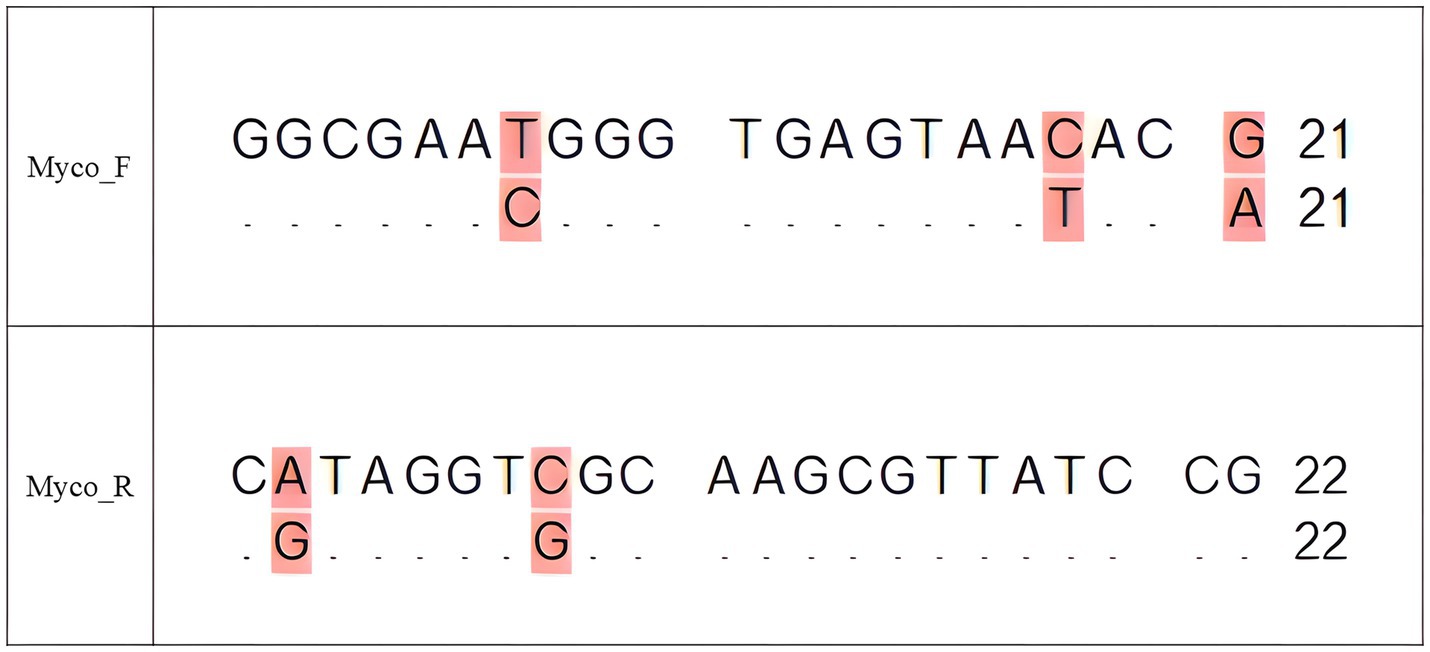
Figure 1. Sequence alignment of the universal Mycoplasma forward (Myco_F) and reverse (Myco_R) primers with the 16S rRNA sequence of E. rhusiopathiae (GenBank accession no. NR_040837). For each primer, the upper line represents the primer sequence and the lower line represents the aligned E. rhusiopathiae sequence. Identical nucleotides are indicated by dots (“.”) in the lower sequence, while mismatches are shown as their respective bases and highlighted in pink to indicate regions of divergence that may result in non-specific binding. The forward primer aligns to positions 95–115 bp, and the reverse primer aligns to positions 534–555 bp of the E. rhusiopathiae 16S rRNA gene. This alignment highlights the potential for cross-reactivity that could generate false positives in PCR assays targeting Mycoplasma spp.
Next-generation sequencing (NGS) technologies have recently proven to be highly effective for detecting and identifying microorganisms. NGS offers high sensitivity and the ability to identify a broad range of microbes without requiring prior assumptions about their presence (8). It provides results within hours to days, addressing the significant delays associated with culture-based methods, which rely on microbial isolation and can take several days or weeks. In addition, culture methods often struggle to accurately identify organisms that are slow-growing or fastidious. NGS overcomes these challenges by enabling direct, unbiased sequencing of microbial DNA, thereby delivering faster and more accurate results (9).
This study aims to address the limitations of PCR by developing a more precise and reliable NGS-based approach for detecting Mycoplasma contamination in veterinary vaccines. The goal is to resolve issues of non-specific amplification and enable clear differentiation between Mycoplasma and E. rhusiopathiae in mixed samples. By achieving accurate detection even in the presence of both pathogens, this method is expected to improve the reliability of vaccine quality control.
2 Materials and methods
2.1 Bacterial strains
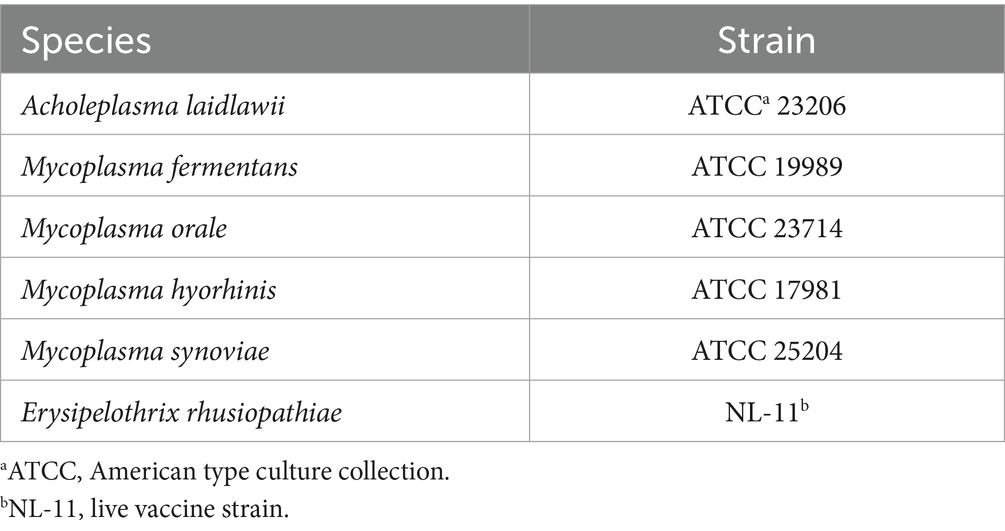
The test materials were selected in accordance with the International Cooperation on Harmonisation of Technical Requirements for Registration of Veterinary Medicinal Products guideline 34, which serves as a standard for assessing the safety, quality, and efficacy of veterinary drugs, particularly biological products (Table 1). To assess potential contamination, the guideline mandates the use of five specific organisms belonging to the class Mollicutes. Although these organisms belong to different families, they include one species of Acholeplasma (A. laidlawii) and four species of Mycoplasma (M. fermentans, M. orale, M. hyorhinis, and M. synoviae). These species were chosen based on several factors, including antibiotic susceptibility, culture requirements, potential for contamination, and pathogenicity (10). These organisms originate from a range of hosts, including mammals, birds, and humans. The five species used in this study were obtained from the American Type Culture Collection. In addition, E. rhusiopathiae was selected as a test material for the development of bioinformatics-based analytical methods due to its genetic similarity to Mycoplasma, which makes it difficult to distinguish using PCR. A commercial vaccine approved in the Republic of Korea containing CSFV and E. rhusiopathiae antigens was used in the study.
2.2 Sample preparation
Initially, both E. rhusiopathiae and Mycoplasma species were diluted using phosphate-buffered saline. E. rhusiopathiae was adjusted to achieve a concentration equivalent to two vaccine doses. Equal volumes (1 mL each) of the E. rhusiopathiae vaccine and serial dilutions of Mycoplasma species were then mixed. This ensured that the final concentration of E. rhusiopathiae in the mixed sample corresponded to a single vaccine dose, while Mycoplasma concentrations varied. The spike assay confirmed that E. rhusiopathiae remained at 1.6 × 109 CFU/mL, while the starting concentrations of the five Mycoplasma species before dilution were as follows: 1.35 × 106 CFU/mL for A. laidlawii, 1.05 × 106 CFU/mL for M. fermentans, 7.15 × 105 CFU/mL for M. orale, 7.95 × 103 CFU/mL for M. hyorhinis, and 2.5 × 106 CFU/mL for M. synoviae. In addition to the spiked samples, a separate group containing only Mycoplasma, without E. rhusiopathiae, was prepared to assess the detection threshold by PCR. All experiments, including sample preparation and downstream analysis, were performed in triplicate to ensure reliability and reproducibility.
Along with the spiked samples, 31 negative vaccine samples—corresponding to half of the 62 vials assigned for quality control at APQA in 2024—were also analyzed. These samples consisted of commercial combination vaccines containing only CSFV and E. rhusiopathiae, and were presumed to be free of Mycoplasma contamination. The negative field samples were used to verify assay specificity and to serve as negative controls for downstream PCR and NGS-based analyses.
DNA extraction was performed using an automated nucleic acid platform (Maelstrom 4810, TANBead, Taiwan, China) and a magnetic bead-based protocol with the TANBead Nucleic Acid Extraction Kit (TANBead). Following the manufacturer’s instructions, 300 μL of sample and 10 μL of Proteinase K were used as input. The DNA was eluted in 80 μL of elution buffer.
2.3 PCR and amplicon sequencing
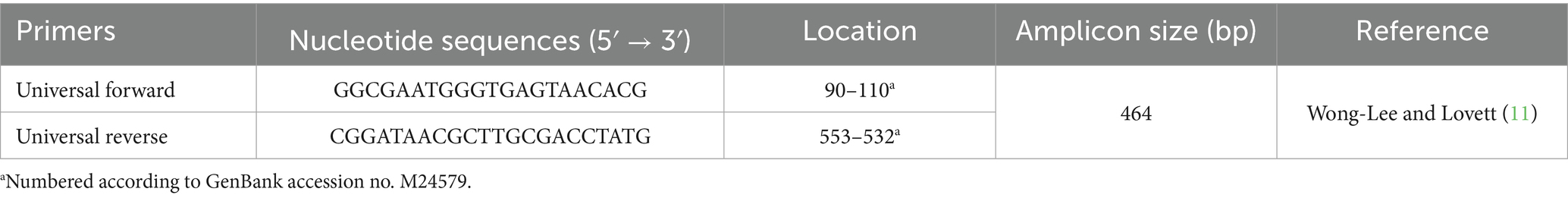
The 16S rRNA molecule, approximately 1,500 base pairs in length, is a widely conserved sequence that contains essential structural regions along with variable regions that enable differentiation between bacterial species. This makes it a commonly used tool in microbiology for bacterial identification and phylogenetic analysis (7). In this study, primers were designed to amplify the 16S rRNA region (11) with the following sequences (Table 2): universal forward primer (5′-GGC GAA TGG GTG AGT AAC ACG-3′) and universal reverse primer (5′-CGG ATA ACG CTT GCG ACC TAT C-3′). These primers follow the National Regulatory Standards for Veterinary Biologicals established by the Republic of Korea (1). PCR was carried out using the Maxime™ PCR premix (i-Taq, iNtRON Biotechnology) in a total reaction volume of 20 μL comprising 1 μL (10 pmol) of forward primer, 1 μL (10 pmol) of reverse primer, 2 μL of DNA, and 16 μL of distilled water. Thermal cycling conditions included an initial denaturation at 94 °C for 5 min; 30 cycles of denaturation (1 min at 94 °C), annealing (1 min at 60 °C), and extension (1 min 30 s at 72 °C); followed by a final extension at 72 °C for 7 min. PCR products were analyzed by electrophoresis on a 1.5% agarose gel, and the presence of a specific band at 464 base pairs (bp) was confirmed using a UV transilluminator.
The PCR products were submitted to a commercial provider (BIONICS, Daejeon, Republic of Korea) for library preparation, quality control, and sequencing using the BITseq next-generation sequencing service. Sequencing was performed with a target output of 30,000 reads, and data were delivered in FASTQ format containing 150 bp paired-end reads. These reads were used as raw data for downstream analysis.
2.4 Reference-mapping analysis
The raw sequencing data were analyzed using two distinct approaches: a reference-mapping pipeline and a metabarcoding workflow, each employing specialized software tailored to its respective analytical purpose (Figure 2). The reference-mapping method was developed to isolate specific strains from heterogeneous samples. BBMap (v39.01) was used to align sequencing reads to predefined reference genomes (12), while SPAdes (v3.13.1) served as the assembler for genome reconstruction (13, 14). Key features of this pipeline included high-accuracy alignment to reference genomes and targeted extraction of Mycoplasma-derived contigs.
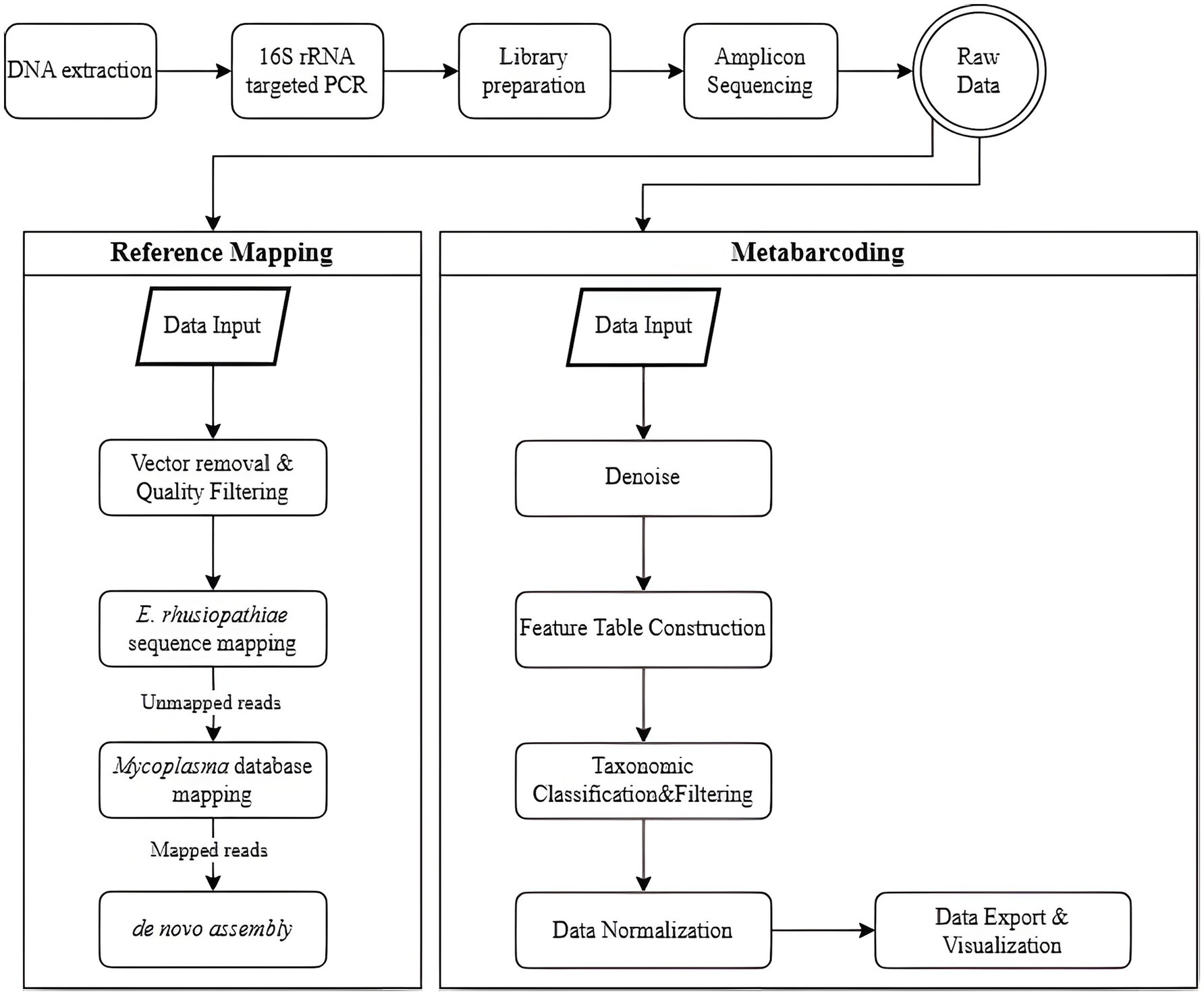
Figure 2. Workflow for preprocessing and analyzing next-generation sequencing (NGS) data, comparing two methods: reference mapping and metabarcoding. In the reference-mapping method, the BBMap and SPAdes tools are used. Raw reads undergo vector removal and quality filtering, followed by sequential mapping-first to the E. rhusiopathiae reference genome, then to a Mycoplasma spp. database-with mapped reads subjected to de novo assembly. In the metabarcoding method, Qiime2 and MicrobiomeAnalyst are employed. Denoising is performed to correct sequencing errors and identify unique amplicon sequence variants (ASVs), which are classified taxonomically. Contaminants, low-confidence ASVs, and unclassified sequences are removed. Normalization and rarefaction are applied to control for sequencing depth before relative abundance is analyzed.
Prior to analysis, reference sequences for E. rhusiopathiae and Mycoplasma spp. were compiled. For E. rhusiopathiae, the 16S rRNA partial sequence of strain ATCC 19414 was obtained from the NCBI reference sequence database. Concurrently, 16S rRNA gene reference sequences were obtained from the SILVA ribosomal RNA gene database (released ver. 138.2, SSURef NR99) (15). To construct a targeted database for mapping, sequences were filtered to retain only those assigned to the families Mycoplasmataceae, Acholeplasmataceae, Metamycoplasmataceae, and Mycoplasmoidaceae according to SILVA taxonomy (Supplementary Data Sheet 1). In parallel, a UniVec database containing adapter, linker, and primer sequences commonly introduced during cloning workflows was incorporated (16). Although the library preparation process did not explicitly confirm the presence of such sequences, vector screening was included as a precautionary measure to mitigate potential contamination and safeguard data integrity. Vector sequence removal and quality filtering are critical in reference mapping to prevent non-specific alignment of contaminant reads to the reference genome. This pre-processing step is essential for reducing false-positive alignments in downstream mapping.
The reference-mapping protocol consisted of four sequential stages, beginning with vector removal and quality filtering. The bbduk.sh script was used for both tasks. In the first stage, reads were aligned to the UniVec database and separated into vector-contaminated reads (outm1, outm2) and cleaned reads (outu1, outu2). The key parameters included k = 31 (k-mer length of 31) and hdist = 1 (maximum permitted Hamming distance of 1). In the second stage, adapter trimming and quality filtering were performed using the same script with the following parameters: ktrim = r (trimming from the right), k = 23 (k-mer length of 23), mink = 11 (minimum k-mer length of 11), hdist = 1 (maximum permitted Hamming distance of 1), qtrim = rl (quality trimming from both ends), trimq = 20 (quality score threshold of 20), and minlen = 50 (minimum read length of 50 bases).
The next two steps involved reference-based alignment using the bbmap.sh script. In step three, reads that did not align with the E. rhusiopathiae reference genome were retained using minid = 0.95 (minimum identity of 95%) and maxindel = 3 (maximum allowable insertion/deletion length of 3 bases). In step four, the remaining reads were aligned to the Mycoplasma reference database with stricter parameters: minid = 0.99 (minimum identity of 99%) and maxindel = 3. Genome assembly was then performed using the SPAdes assembler (spades.py) (14). The detection limit of Mycoplasma was established by evaluating colony-forming unit (CFU) counts and identifying contig formation at corresponding dilution levels.
To enable accurate comparisons across samples, sequencing depth was normalized using the Total Count (TC) method. This involved calculating the total coverage for each contig (contig length multiplied by average coverage) and dividing it by the total number of reads in the sample (17). This normalization approach minimizes technical bias introduced by varying sequencing depths and enables consistent interpretation across datasets. The formula used is as follows:
2.5 Metabarcoding analysis
The metabarcoding analysis was conducted using Qiime2 (v2024.05) to examine sequencing data from diverse microbial communities present in the samples (18). The analysis included quality filtering, sequence clustering, and taxonomic classification at the order level to identify and categorize different species. MicrobiomeAnalyst was also used for downstream data visualization and interpretation (19).
Initial sequencing data were processed in Qiime2 (v2024.05) through a series of bioinformatic procedures (15, 18). Raw sequence reads were imported into the pipeline, and quality control steps were applied. This included preprocessing with DADA2 to remove noise, correct sequencing errors, and identify amplicon sequence variants (ASVs), providing a high-resolution representation of microbial diversity. Following denoising, a feature table was constructed to record the occurrence of each ASV across all samples. Taxonomic classification of ASVs was performed by aligning sequences with the SILVA 16S rRNA reference database (15), followed by filtering to remove low-confidence assignments and potential contaminants. The data were then normalized to account for variations in sequencing depth, using methods such as rarefaction to allow comparability between samples (18, 19). Finally, the processed data were exported for statistical analysis and visualized using various tools within MicrobiomeAnalyst (19). We assessed the prevalence of Mycoplasma in each sample based on visual outputs and evaluated detection limits accordingly.
3 Results
3.1 Detection limit of conventional PCR: a benchmark for detection
The detection limits for five Mycoplasma species—A. laidlawii, M. fermentans, M. orale, M. hyorhinis, and M. synoviae—were evaluated (Table 3). Amplification for A. laidlawii was consistently observed at 3.13. log CFU/mL across all replicates, with no detection at lower concentrations. M. fermentans showed a consistent detection threshold of 3.02 log CFU/mL in all three replicates. Similarly, M. orale produced amplification at 3.85 log CFU/mL across all replicates. In the case of M. hyorhinis, the detection limit varied slightly, with amplification observed at 2.9, 1.9, and 1.9 log CFU/mL, respectively; however, 1.9 log CFU/mL was the most consistent threshold. M. synoviae demonstrated reproducible amplification at 3.4 log CFU/mL across all replicates. Collectively, these results indicate variation in PCR detection efficiency among Mycoplasma species, with amplification generally decreasing as target concentrations decline.
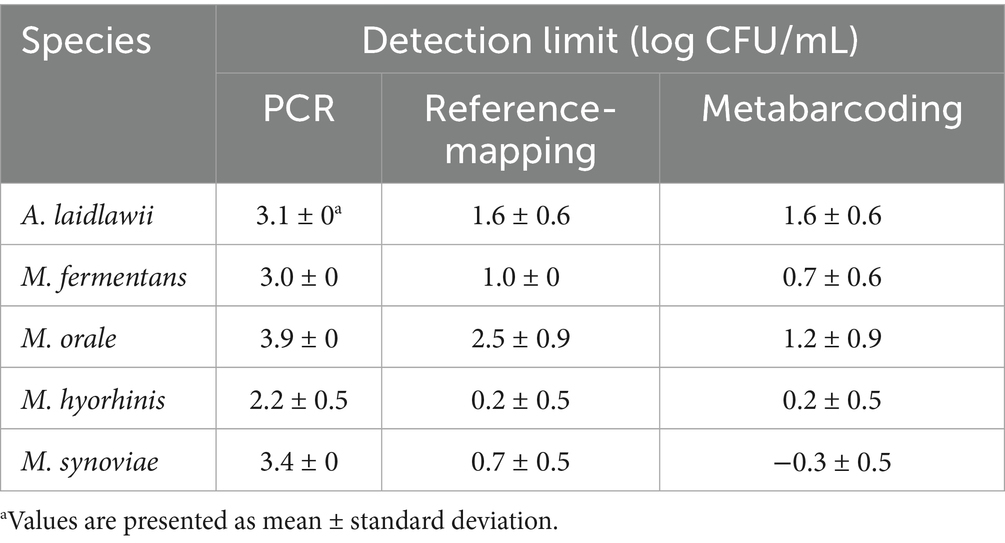
Table 3. Detection limits of Mycoplasma species using three analytical methods: conventional PCR, reference mapping, and metabarcoding.
3.2 Reference mapping analysis for high-precision detection
The reference-mapping method was applied to assess its performance in detecting multiple Mycoplasma spp. in spiked samples. The results present an overview of the read retention at each step of the reference-mapping process and demonstrate how mapped read counts vary according to bacterial concentration.
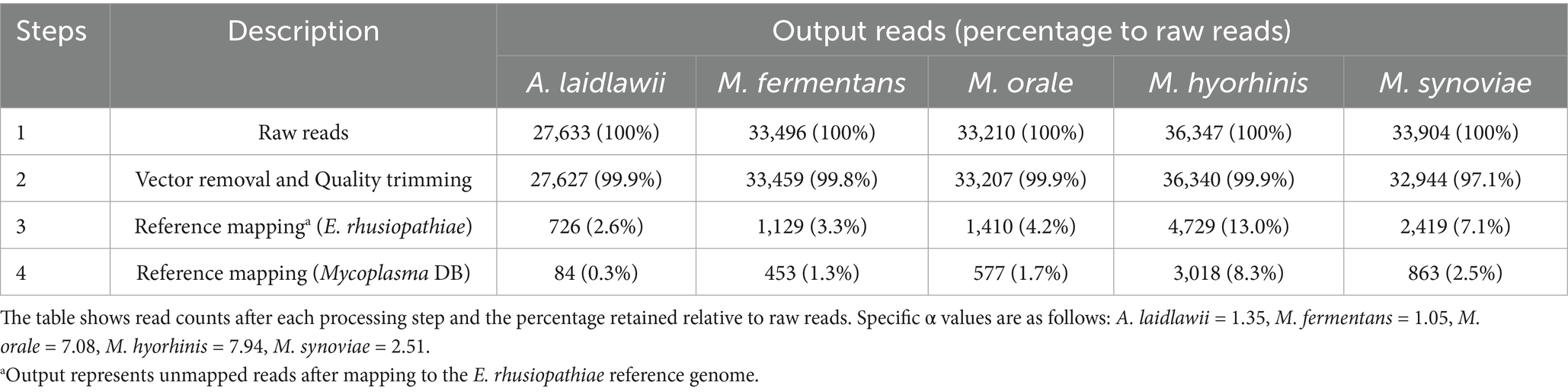
Sequencing yielded consistently high read counts across all species: 27,633 reads for A. laidlawii, 33,496 for M. fermentans, 33,210 for M. orale, 36,347 for M. hyorhinis, and 33,904 for M. synoviae, with 100% read retention at the initial stage. After vector sequence removal and quality trimming, nearly all reads were retained, indicating minimal data loss and high initial sequence quality.
Following the first step against E. rhusiopathiae, read counts decreased significantly across species: A. laidlawii retained 2.6% (726 reads), M. fermentans 3.4% (1,129 reads), M. orale 4.2% (1,410 reads), M. hyorhinis 13.0% (4,729 reads), and M. synoviae 7.1% (2,419 reads). A second mapping step against the Mycoplasma database further reduced reads, yielding 0.3% (84 reads) for A. laidlawii, 1.3% (453) for M. fermentans, 1.7% (577) for M. orale, 8.3% (3,018) for M. hyorhinis, and 2.5% (863) for M. synoviae (Table 4). These findings demonstrate the effectiveness of the two-step reference-mapping strategy in removing non-specific reads and emphasize the value of sequential mapping for accurate downstream analysis.
3.3 Metabarcoding analysis for comprehensive detection
The metabarcoding method was employed to evaluate the relative abundance of Mycoplasma spp. across various dilution levels, offering insights into sample composition and detection limits, For comparison, a table presenting both relative coverage and relative abundance is included (Table 5).
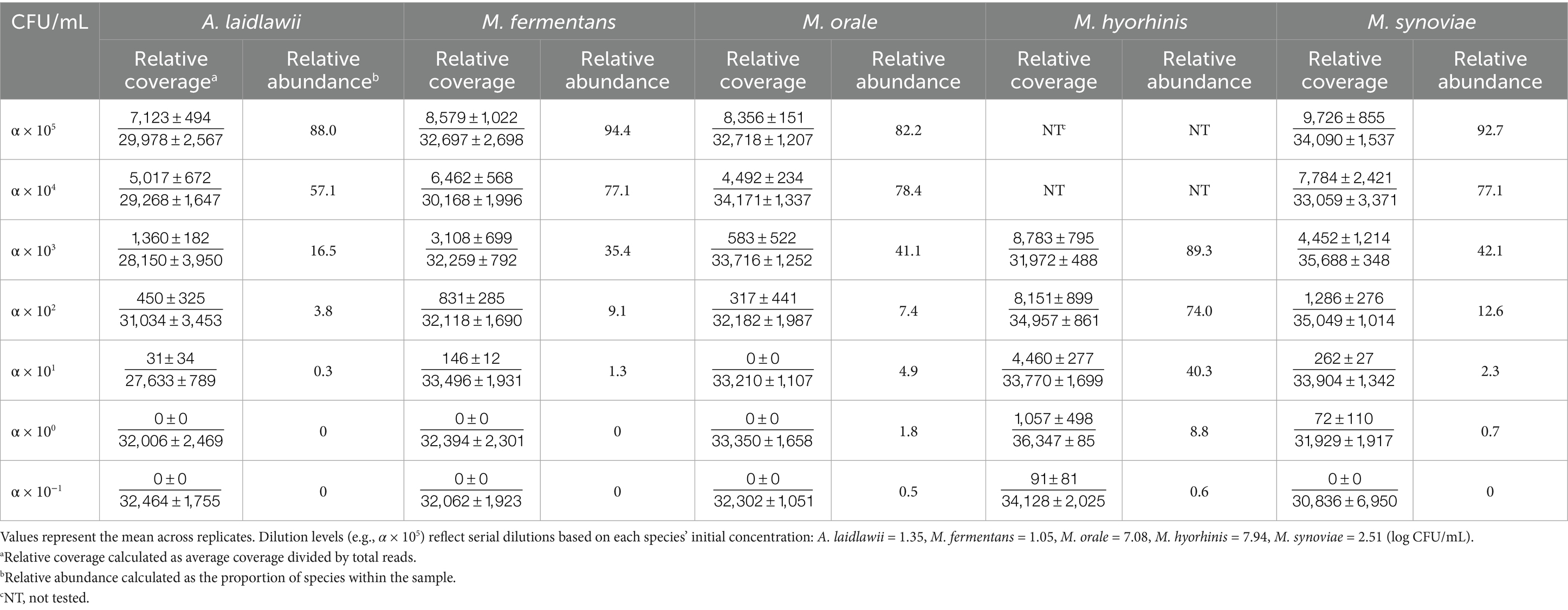
Table 5. Relative coverage and relative abundance of five Mycoplasma species across dilution levels (CFU/mL).
Relative coverage, derived from the reference-mapping method, refers to the proportion of reads mapped to a reference genome out of the total reads in a sample. It is calculated as the sum of the average coverage values across all genome regions divided by the total read count. Relative abundance, in contrast, reflects the proportion of each species within the total microbial population, providing information on species diversity and distribution. Although the relative coverage data are presented for reference, the primary focus is on abundance patterns obtained from the metabarcoding method.
For A. laidlawii, the relative abundance at the highest concentration (α × 105 CFU/mL) was 88.0%. As the dilution increased, abundance dropped sharply, reaching 0% at α × 100 and α × 10−1 CFU/mL, where no contigs were detected. M. fermentans showed a similar pattern, with 94.4% abundance at α × 105 CFU/mL, decreasing to 0% at the lowest concentrations. M. orale followed this trend, starting at 82.2% and declining to 0% with increasing dilution. M. synoviae exhibited the highest starting abundance (92.7%) at α × 105 CFU/mL, which also dropped to 0% at lower levels. In contrast, M. hyorhinis, which began testing at α × 103 CFU/mL due to limited starting material, showed 89.3% abundance at that level. Higher concentration experiments were not conducted for this species, resulting in “Not Tested” values. Despite this, the metabarcoding successfully detected M. hyorhinis at lower concentrations, demonstrating its utility in identifying less abundant species in spiked samples. Non-specific reads from genera such as Bacillus and Staphylococcus were also detected.
The detection limits of Mycoplasma spp. were compared across three methods: conventional PCR, the reference-mapping method, and the metabarcoding method (Table 3). Across all three methods, standard deviation remained below one log unit across triplicate experiments. PCR exhibited the highest detection thresholds, while both NGS-based methods yielded lower detection limits across all species.
3.4 Analysis of negative vaccine samples for Mycoplasma contamination
Initial reference-mapping method yielded mycoplasma-specific contigs in 5 of the 31 negative vaccine samples. The contigs were 447, 444, 439, 447, and 449 bp in length, respectively, and all exhibited sequencing depth below 10. BLAST analysis revealed that four contigs corresponded to M. synoviae, whereas the 449 bp contig was identified as M. fermentans. Concurrently, the sequenced data from these samples were reanalyzed using our metabarcoding method pipeline (Qiime2). This method did not detect any reads classifiable to Mycoplasma.
4 Discussion
PCR is generally considered an effective method for pathogen detection (20). In the Republic of Korea, PCR assays using Mycoplasma-specific primer sets are employed alongside bacterial culture to detect Mycoplasma contamination in veterinary vaccines. If the PCR result is negative, the vaccine is deemed acceptable (1). If the result is positive, a Mycoplasma culture test is conducted. However, PCR has notable limitations, particularly the risk of non-specific amplification under certain conditions. This occurs when primers bind to DNA sequences that are similar—but not identical—to the intended target (6). In the case of combination vaccines containing E. rhusiopathiae and viral antigens, the 16S rRNA region of E. rhusiopathiae shares sequence similarity with Mycoplasma-specific primers, leading to non-specific amplification (Figure 1). This study addresses these limitations by improving sensitivity and accuracy through NGS and bioinformatics-based methods. NGS has emerged as a transformative solution that overcomes the constraints of conventional methods, thereby improving the safety and quality of biopharmaceuticals (21). Regulatory agencies, such as the U.S. Food and Drug Administration and the Ministry of Food and Drug Safety in the Republic of Korea, have adopted NGS-based testing to detect both exogenous and endogenous viruses, thereby expanding detection capabilities and significantly reducing analysis time (22, 23).
In this study, we simulated co-infection scenarios by preparing spiked samples containing E. rhusiopathiae and various Mycoplasma spp. To distinguish between the two, we used two NGS-based analytical approaches: reference mapping and metabarcoding a widely used approach for bacterial taxonomic analysis (24), typically targeting the V3–V4 hypervariable region of the 16S rRNA gene (25). Since the PCR method used for Mycoplasma detection also amplifies the V3–V4 region, we examined whether metabarcoding could be incorporated into the testing process. Empirical results showed that relative abundance values decreased proportionally as Mycoplasma concentrations declined in the spiked samples. This pattern illustrates the method’s quantitative characteristics and reproducibility. However, its utility in our specific context was limited. The metabarcoding analysis detected low levels of non-specific sequences, such as those from Bacillus and Staphylococcus, alongside Mycoplasma. Standard 16S rRNA-based metabarcoding typically relies on paired-end sequencing of at least 250 bp to ensure sufficient read overlap for error correction. In this study, however, the V3–V4 region (464 bp) was sequenced using 150 bp paired-end reads, resulting in insufficient overlap between forward and reverse reads. As a result, denoising algorithms such as DADA2 may not have corrected errors completely, potentially leading to the identification of non-specific sequences due to limitations in chimera removal and ASV detection. Future studies should focus on optimizing overlap length to improve metabarcoding accuracy. This could be achieved by targeting a shorter region, such as the V4 alone (250 bp), or by increasing the paired-end read length to 250 or 300 bp. However, such improvements may increase sequencing costs.
In contrast, the two-step reference-mapping method proved to be a superior and highly robust solution. The reference-mapping method used in this study employed a two-step alignment procedure. First, sequencing reads were mapped to the 16S rRNA sequence of E. rhusiopathiae, and those mapped reads were removed. In the second step, the remaining reads were aligned to a Mycoplasma database, followed by de novo assembly using only the reads mapped to Mycoplasma. The data demonstrated that both mapping steps were essential for accurate identification. Our data confirmed that both steps are essential for accurate analysis. Omitting the first step makes it difficult to distinguish E. rhusiopathiae as the true source of PCR-positive results, whereas omitting the second step results in the generation of an excessive number of contigs and markedly prolonged running times. Therefore, inclusion of both steps is necessary to ensure reliable interpretation (data not shown). Results showed that sequences from E. rhusiopathiae, the vaccine’s main component, were successfully removed, enabling more precise downstream analysis. Mapping of samples spiked with Mycoplasma revealed that over 90% of total reads corresponding to A. laidlawii, M. fermentans, M. orale, and M. synoviae were removed in the first mapping step, while a greater proportion of reads remained for M. hyorhinis (Table 4). These results suggest that Mycoplasma-positive signals in conventional PCR tests of CSFV-E. rhusiopathiae vaccines may stem from non-specific amplification due to sequence similarities between E. rhusiopathiae and Mycoplasma (26–28).
The results obtained from the negative vaccine samples were inconsistent. While reference-mapping approach produced mycoplasma-specific contigs, the metabarcoding method did not detect any reads assignable to Mycoplasma. Subsequent culture test yielded no microbial growth, which could support the metabarcoding outcome. However, we cannot exclude the possibility that very small amount of Mycoplasma were present at levels insufficient for cultivation, or that laboratory contamination introduced Mycoplasma-derived reads. To further investigate this discrepancy, we reanalyzed the sequencing data using shotgun metagenomics tools, Kraken2 and Krona (29–31). In this analysis, reads classified as Mycoplasma spp. were observed in the same samples where Mycoplasma contigs had been generated by reference-mapping (data not shown).
Between the two NGS-based bioinformatics strategies evaluated, the reference-mapping method was selected as the more appropriate approach. This decision was based not only on the occurrence of non-specific signals in metabarcoding but also on the superior detection of genuine Mycoplasma-derived reads in negative vaccine samples, indicating greater analytical sensitivity. Because the method is intended as an alternative to conventional PCR, sensitivity is of paramount importance: in current QC practice, PCR-positive results require culture confirmation, and potential false positives can thus be resolved. By contrast, false negatives pose a critical risk by allowing contaminated products to pass undetected.
Despite these advantages, NGS remains limited by higher costs and the need for specialized bioinformatics expertise. Consequently, molecular methods with comparable sensitivity and specificity but lower operational burden—such as real-time PCR or RPA-CRISPR/Cas12a—may represent more practical long-term solutions for routine testing (32, 33). Nevertheless, in the present context, cross-reactivity was identified as a critical issue specifically in vaccines containing E. rhusiopathiae and CSFV. Accordingly, the reference-mapping approach was adopted to address this limitation within an otherwise well-established PCR testing system. Although in this study the NGS-based methods were primarily applied to address the specific issue of cross-reactivity with E. rhusiopathiae in conventional PCR, future study will extend beyond this case.
While NGS is an established method for adventitious agent screening in biologics like cell banks (21, 34), its application to mitigate specific PCR cross-reactivity in finished, complex vaccine products represents a significant practical advancement. Unlike raw materials, final vaccine products contain high concentrations of antigens that constitute a challenging analytical matrix. Our two-step mapping strategy is specifically tailored to overcome this matrix effect from E. rhusiopathiae, and represents a practical application of NGS to solve a persistent, real-world problem in vaccine lot release testing. This concept may also be extended to national lot release testing of viral vaccines, in which primary removal of major antigens could be followed by adventitious virus detection.
Data availability statement
The original contributions presented in the study are publicly available. This data can be found here: NCBI BioProject database under accession number PRJNA1335287.
Author contributions
S-MG: Writing – original draft, Investigation, Visualization, Formal analysis. Y-KL: Writing – original draft, Formal analysis, Methodology. J-JN: Formal analysis, Writing – review & editing, Funding acquisition. H-OG: Formal analysis, Writing – review & editing, Funding acquisition. IJ: Supervision, Methodology, Writing – review & editing, Conceptualization, Funding acquisition, Project administration. M-GS: Writing – review & editing, Supervision, Methodology.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by funds from the Animal and Plant Quarantine Agency, Republic of Korea (B-1543073-2024-26-01).
Acknowledgments
The authors would like to thank the Veterinary Drugs and Biologics Division and the Bacterial Disease Division at the Animal and Plant Quarantine Agency for providing the necessary resources and facilities for this research. We are also grateful to Jessica Hicks at the National Veterinary Services Laboratories (NVSL) for her valuable advice.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2025.1657098/full#supplementary-material
References
1. Animal and Plant Quarantine Agency. Standard manual of diagnostic tests for animals in South Korea. GPRN; (2017)
2. Fathy, M, El-Safty, MM, El-Jakee, JK, Abd-Alla, HI, and Mahmoud, H. Study the effect of Mycoplasma contamination of eggs used in virus titration and efficacy of some live attenuated poultry viral vaccines. Asian J Pharm Clin Res. (2017) 10:216–22. doi: 10.22159/ajpcr.2017.v10i1.14930
3. Citti, C, and Blanchard, A. Mycoplasmas and their host: emerging and re-emerging minimal pathogens. Trends Microbiol. (2013) 21:196–203. doi: 10.1016/j.tim.2013.01.003
4. Bébéar, C, Pereyre, S, and Peuchant, O. Mycoplasma pneumoniae: susceptibility and resistance to antibiotics. Future Microbiol. (2011) 6:423–31. doi: 10.2217/fmb.11.18
5. European Directorate for the Quality of Medicines & HealthCare (EDQM). Mycoplasma In: European Pharmacopoeia. 11th ed. Strasbourg: EDQM (2023). 210–5.
6. Stadhouders, R, Pas, SD, Anber, J, Voermans, J, Mes, TH, and Schutten, M. The effect of primer-template mismatches on the detection and quantification of nucleic acids using the 5′ nuclease assay. J Mol Diagn. (2010) 12:109–17. doi: 10.2353/jmoldx.2010.090035
7. Johansson, KE, Heldtander, MU, and Pettersson, B. Characterization of mycoplasmas by PCR and sequence analysis with universal 16S rDNA primers In: R Miles and R Nicholas, editors. Mycoplasma protocols. Totowa, NJ: Humana Press (1998). 145–65.
8. Quainoo, S, Coolen, JP, van Hijum, SA, Huynen, MA, Melchers, WJ, van Schaik, W, et al. Whole-genome sequencing of bacterial pathogens: the future of nosocomial outbreak analysis. Clin Microbiol Rev. (2017) 30:1015–63. doi: 10.1128/cmr.00016-17
9. Didelot, X, Bowden, R, Wilson, DJ, Peto, TEA, and Crook, DW. Transforming clinical microbiology with bacterial genome sequencing. Nat Rev Genet. (2012) 13:601–12. doi: 10.1038/nrg3226
10. International Cooperation on Harmonisation of Technical Requirements for Registration of Veterinary Medicinal Products (VICH). VICH GL34: Biologicals: Testing for the detection of Mycoplasma contamination. VICH; (2014). Available online at: https://vichsec.org/wp-content/uploads/2024/10/GL34_st7.pdf (Accessed September 25, 2025).
11. Wong-Lee, JG, and Lovett, M. Rapid and sensitive PCR method for identification of Mycoplasma species in tissue culture. In Diagnostic Molecular Microbiology: Principles and Applications. (1993):257–60.
12. Bushnell, B. BBMap: A fast, accurate, splice-aware aligner. SourceForge ; (2014). Available online at: https://sourceforge.net/projects/bbmap/ (Accessed September 26, 2024).
13. Prjibelski, A, Antipov, D, Meleshko, D, Lapidus, A, and Korobeynikov, A. Using SPAdes de novo assembler. Curr Protoc Bioinformatics. (2020) 70:e102. doi: 10.1002/cpbi.102
14. Bankevich, A, Nurk, S, Antipov, D, Gurevich, AA, Dvorkin, M, Kulikov, AS, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. (2012) 19:455–77. doi: 10.1089/cmb.2012.0021
15. Quast, C, Pruesse, E, Yilmaz, P, Gerken, J, Schweer, T, Yarza, P, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. (2013) 41:D590–6. doi: 10.1093/nar/gks1219
16. National Center for Biotechnology Information (NCBI). UniVec database [internet] (2024). Available online at: https://www.ncbi.nlm.nih.gov/tools/vecscreen/univec/ (Accessed September 26, 2024).
17. Pereira, MB, Wallroth, M, Jonsson, V, and Kristiansson, E. Comparison of normalization methods for the analysis of metagenomic gene abundance data. BMC Genomics. (2018) 19:274–17. doi: 10.1186/s12864-018-4637-6
18. Bolyen, E, Rideout, JR, Dillon, MR, Bokulich, NA, Abnet, CC, Al-Ghalith, GA, et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. (2019) 37:852–7. doi: 10.1038/s41587-019-0252-6
19. Hall, M, and Beiko, RG. 16S rRNA gene analysis with QIIME2 In: R Beiko, W Hsiao, and J Parkinson, editors. Microbiome analysis: methods and protocols. New York: Humana Press (2018). 113–29.
21. Tan, B, Ng, C, Nshimyimana, JP, Loh, LL, Gin, KYH, and Thompson, JR. Next-generation sequencing (NGS) for assessment of microbial water quality: current progress, challenges, and future opportunities. Front Microbiol. (2015) 6:1027. doi: 10.3389/fmicb.2015.01027
22. Ministry of Food and Drug Safety, Biologics Research Division. (2021). NGS-based adventitious virus testing manual for vaccine production cell lines. Cheongju: Ministry of Food and Drug Safety;
23. International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) ICH guideline Q5A(R2) on viral safety evaluation of biotechnology products derived from cell lines of human or animal origin (2022)
24. Tringe, SG, and Hugenholtz, P. A renaissance for the pioneering 16S rRNA gene. Curr Opin Microbiol. (2008) 11:442–6. doi: 10.1016/j.mib.2008.09.011
25. Claesson, MJ, Wang, Q, O'Sullivan, O, Greene-Diniz, R, Cole, JR, Ross, RP, et al. Comparison of two next-generation sequencing technologies for resolving highly complex microbiota composition using tandem variable 16S rRNA gene regions. Nucleic Acids Res. (2010) 38:e200. doi: 10.1093/nar/gkq873
26. Li, H, and Homer, N. A survey of sequence alignment algorithms for next-generation sequencing. Brief Bioinform. (2010) 11:473–83. doi: 10.1093/bib/bbq015
27. Pop, M, and Salzberg, SL. Bioinformatics challenges of new sequencing technology. Trends Genet. (2008) 24:142–9. doi: 10.1016/j.tig.2007.12.006
28. Aird, D, Ross, MG, Chen, WS, Danielsson, M, Fennell, T, Russ, C, et al. Analyzing and minimizing PCR amplification bias in Illumina sequencing libraries. Genome Biol. (2011) 12:R18–4. doi: 10.1186/gb-2011-12-2-r18
29. Wood, DE, Lu, J, and Langmead, B. Improved metagenomic analysis with kraken 2. Genome Biol. (2019) 20:257. doi: 10.1186/s13059-019-1891-0
30. Lu, J, Rincon, N, Wood, DE, Breitwieser, FP, Pockrandt, C, Langmead, B, et al. Metagenome analysis using the kraken software suite. Nat Protoc. (2022) 17:2815–39. doi: 10.1038/s41596-022-00738-y
31. Ondov, BD, Bergman, NH, and Phillippy, AM. Interactive metagenomic visualization in a web browser. BMC Bioinform. (2011) 12:385. doi: 10.1186/1471-2105-12-385
32. Templeton, KE, Scheltinga, SA, Graffelman, AW, van Schie, JM, Crielaard, JW, Sillekens, P, et al. Comparison and evaluation of real-time PCR, real-time nucleic acid sequence-based amplification, conventional PCR, and serology for diagnosis of Mycoplasma pneumoniae. J Clin Microbiol. (2003) 41:4366–71. doi: 10.1128/JCM.41.9.4366-4371.2003
33. Li, F, Xiao, J, Yang, H, Yao, Y, Li, J, Zheng, H, et al. Development of a rapid and efficient RPA-CRISPR/Cas12a assay for Mycoplasma pneumoniae detection. Front Microbiol. (2022) 13:858806. doi: 10.3389/fmicb.2022.858806
Keywords: Mycoplasma detection, next-generation sequencing, reference mapping, veterinary vaccines, vaccine quality control, PCR limitations, metabarcoding
Citation: Go S-M, Lee Y-K, Nah J-J, Gu H-O, Jang I and Seo M-G (2025) Application of next-generation sequencing for detecting Mycoplasma contamination in veterinary vaccines. Front. Vet. Sci. 12:1657098. doi: 10.3389/fvets.2025.1657098
Edited by:
Jerry William Simecka, University of North Texas Health Science Center, United StatesReviewed by:
Hugh Y. Cai, University of Guelph, CanadaHuaizu Guo, State Key Laboratory of Antibody Medicine and Targeted Therapy, China
Copyright © 2025 Go, Lee, Nah, Gu, Jang and Seo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Il Jang, bW9hMzA1aG9Aa29yZWEua3I=; Min-Goo Seo, a29yZWFzbWdAa251LmFjLmty
 Su-Min Go
Su-Min Go Yeon-Kyeong Lee3
Yeon-Kyeong Lee3 Min-Goo Seo
Min-Goo Seo