- 1College of Animal Science and Technology, Tarim,University, Alar, China
- 2Animal Husbandy and Veterinany Station of the Third Division, Tumushuke, China
- 3Key Laboratory of Tarim Animal Husbandry Science and Technology, Xinjiang Production and Construction Corps, Tarim University, Alar, China
- 4Key Laboratory of Livestock and Forage Resources Utilization around Tarim, Ministry of Agriculture and Rural Affairs, Tarim University, Alar, China
Introduction: Licorice aerial parts are widely cultivated in China but often underutilized due to nutrient loss during haymaking. Ensiling with whole-plant corn may enhance their nutritional value and utilization in ruminant diets.
Methods: This study evaluated the effects of mixed silages containing aerial parts of licorice and whole-plant corn at inclusion levels of 0, 22, 28, and 34% on nutrient digestibility, rumen fermentation, and gastrointestinal microbiota in Simmental cattle. Forty-eight male Simmental cattle were randomly assigned to four groups and fed the experimental diets for 75 days. Apparent nutrient digestibility, rumen fermentation parameters, and microbial profiles in rumen fluid and feces were analyzed.
Results: The 22% and 28% silage groups showed significantly higher digestibility of neutral detergent fiber (NDf) and acid detergent filber (ADF), along with increased concentrations of rumen acetate and total volatile fatty acids (TVFA), compared to the control and 34% groups. Rumen pH was significantly lower in these groups. Microbial diversity (Chao1 index increased inthe 34% group, while the 22% group exhibited a higher relative abundance of beneficial rumen bacteria such as Oscillospiraceae and NK4A214 group, with Oscillospiraceae negatively correlated with rumen pH. in feces, Firmicutes was enriched in the 28%group and identified as a key biomarker, Other beneficial taxa, including Christensenellaceae, Monoglobaceae and Ruminococcus, also increased with silage supplementation.
Discussion/conclusion: These findings suggest that incorporating 28% licorice-corn mixed silage into the diet optimizes nutrient digestibility, enhances rumen fermentation, and improves gut microbial composition, thereby boosting feeding efficiency in fattening Simmental cattle.
Introduction
The shortage of high-quality roughage supplies is a major constraint to the development of the cattle industry in many developing countries. Southern Xinjiang, China’s largest area of saline soil and desertification, is characterized by low rainfall and poor soil fertility. Due to these harsh environmental conditions, the availability of high-quality roughage in southern Xinjiang is extremely limited. A promising solution is the promotion of saline-tolerant forage crops. Licorice (Glycyrrhiza uralensis Fisch), a perennial plant belonging to the genus Glycyrrhiza in the legume family, is one of the most valuable plant resources adapted to arid and semi-arid conditions (1). It is primarily distributed in the dry regions of northeastern, northern, and northwestern China, as well as Mongolia, Central Asia, and Russia, with Xinjiang being one of the main production areas (2, 3). The aerial parts of licorice are a promising roughage resource, containing over 16% crude protein (CP) and rich in bioactive compounds such as flavonoids and glycosides, which exhibit antiviral, antioxidant, and immunomodulatory activities (4–6). These nutritional and functional properties have supported their successful incorporation into feed for sheep (7, 8), poultry (9), and fish (10), where they have significantly enhanced growth performance. However, their use as a roughage is limited by practical challenges: during haymaking, they are prone to substantial nutrient losses, and their low water-soluble carbohydrate (WSC) content hinders effective ensiling. Corn silage, known as the “king of feed” for ruminants due to its low crude fiber, high palatability, and rich soluble sugars, is typically used in whole-plant form. Co-ensiling licorice aerial parts with whole-plant corn or sweet sorghum has been shown to improve silage quality and compensate for the limitations of licorice alone (11), offering a viable strategy to enhance their utilization as a high-quality roughage source.
In vitro studies have shown that increasing the proportion of licorice aerial parts beyond 4.5% of dietary dry matter impairs fermentation characteristics and gas production, suggesting threshold effects for optimal digestibility (4). In vivo trials in sheep supplemented with licorice extract up to 4.5% of DM reported enhanced antioxidant status and immune function without adverse effects on growth or intake, while higher levels reduced rumen fermentation efficiency (5). Furthermore, glycyrrhizic acid, the primary active compound in licorice, has been associated with dose-dependent toxicity, including hypertension and electrolyte imbalance, at excessive intake levels (6). Based on these findings, the inclusion levels of 22, 28, and 34% were selected to represent moderate, effective, and upper-limit doses to evaluate both beneficial and potentially adverse effects. We hypothesized that appropriate inclusion levels of mixed silage containing licorice aerial parts and whole-plant corn would not only improve silage fermentation quality, but also enhance nutrient digestibility, optimize rumen fermentation parameters, and beneficially modulate the gastrointestinal microbiota in Simmental cattle. Therefore, mixed silage composed of licorice aerial parts and whole-plant corn was selected for investigation. Simmental cattle were used as experimental animals, and different inclusion levels of the mixed silage were during the late fattening stage to evaluate the effects on apparent nutrient digestibility, rumen fermentation parameters, and gastrointestinal microbiota. The findings from this study would provide valuable insights for the practical application of mixed silage in ruminant nutrition.
Materials and methods
Mixed silages production
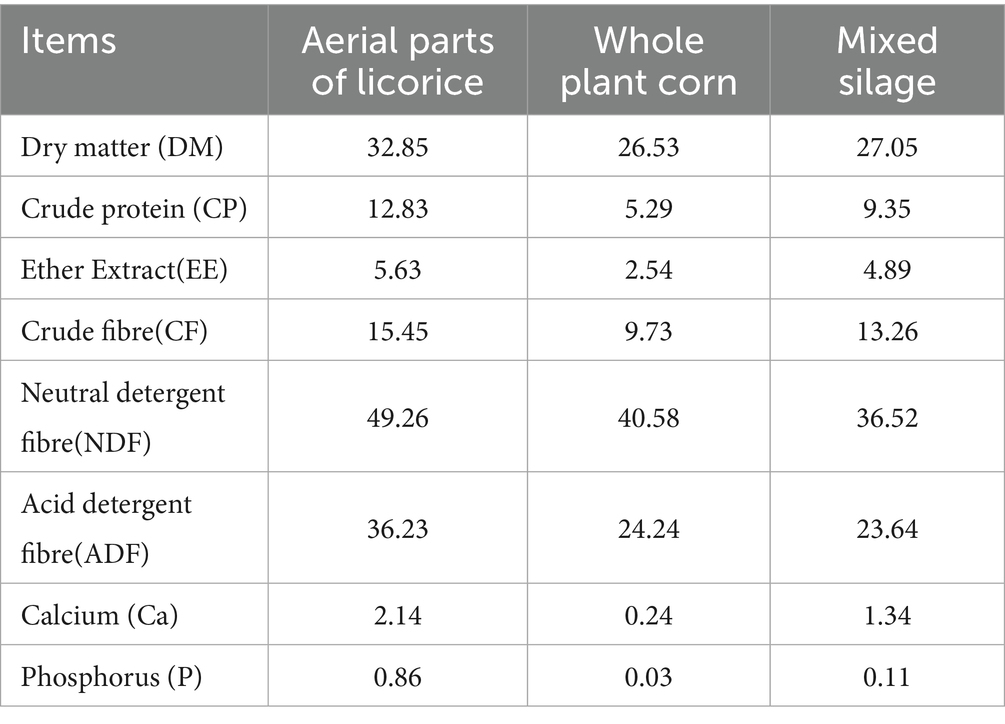
Aerial parts of licorice and whole-plant corn were sourced from Tumshuk City, Xinjiang Production and Construction Corps, China. Samples were collected on September 21, 2023. The sampling location is situated between 78°07′–78°21′E, and 39°22′–39°30’N, at an elevation ranging from 1,100 m to 2,063 m, and features a temperate continental arid climate.
Both the licorice aerial parts and whole-plant corn were harvested using a silage harvester and chopped to a length of approximately 1–2 cm. The moisture content was adjusted to 65–70%, and the two components were mixed at a mass ratio of 1:1. The mixture was thoroughly blended, compacted into a silo, tightly sealed with a double-layer plastic film. The white outer layer reflected sunlight to reduce heat accumulation, while the black inner layer blocked light to inhibit the growth of aerobic bacteria. The edges of the film were secured using sandbags, tires, or soil to prevent air leakage and maintain anaerobic conditions. The silage was fermented at room temperature for 90 days before use. The main nutritional composition of the mixed silage is presented in Table 1.
Experimental design
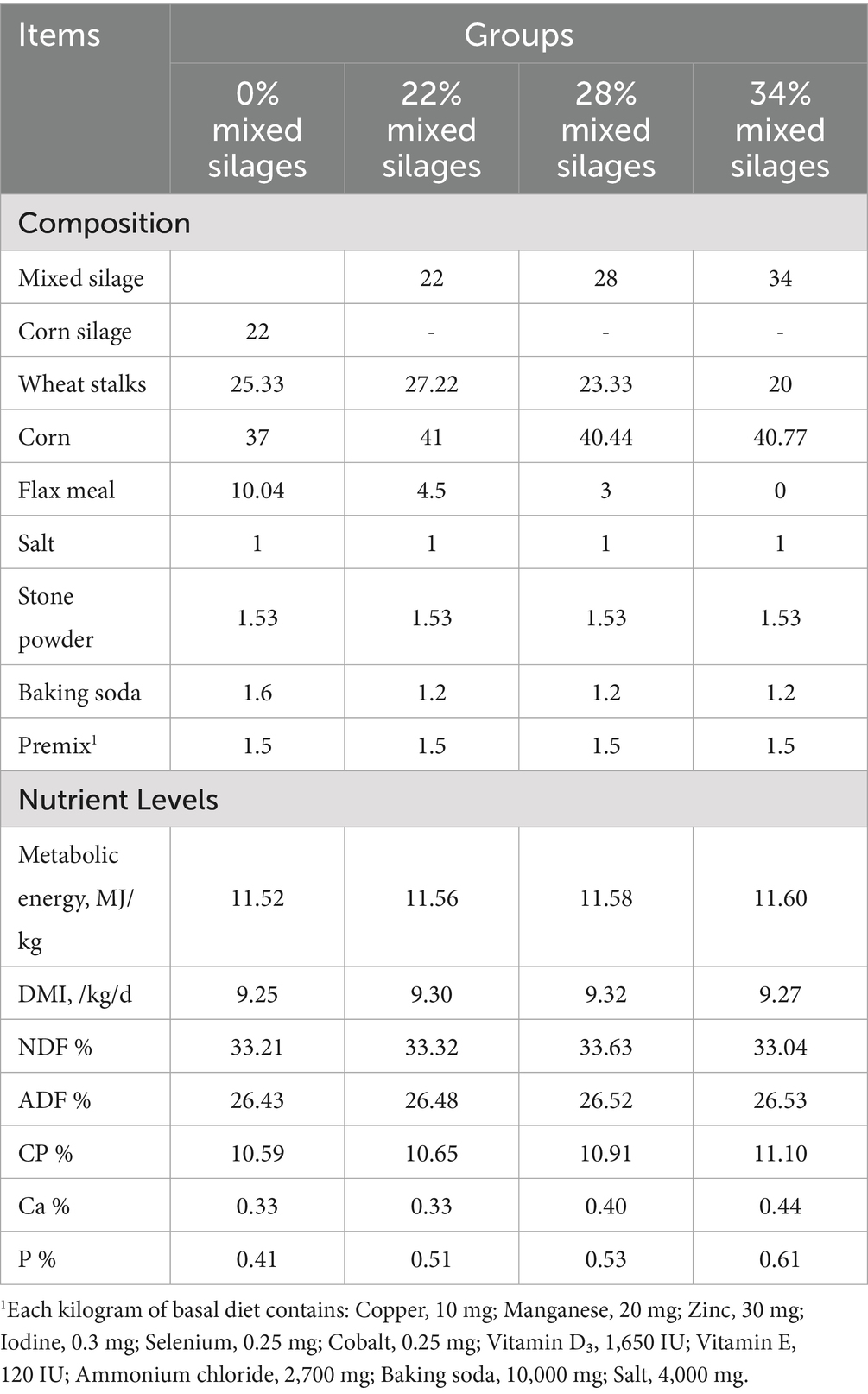
A completely randomized design was employed for this study. Forty-eight male Simmental cattle, approximately 18–20 months old, each weighing 550 ± 10 kg and with a body condition score (BCS) of 6, were randomly assigned to four groups (n = 12 per group). The groups received total mixed rations (TMR) supplemented with 0% (control), 22, 28%, or 34% mixed silage, respectively. The TMRs were formulated based on nutritional requirements outlined in the Chinese Feeding Standards for Beef Cattle (NY/ T815-2004) (7). The ingredient composition and nutritional values of each treatment group are provided in Table 2. Nutrient concentrations of crude protein (CP), calcium (Ca), and phosphorus (P) in the feed supplied was determined according to standard AOAC methods (8). CP was measured using the Kjeldahl method, Ca was determined viapotassium permanganate titration, and P content was measured by absorbance photometry (Table 2).
Animal feeding and management
The experimental cattle were housed in tie stalls for a total of 75 days, which included a 15-day adaptation period and a 60-day experimental feeding period. All cattle were dewormed and ear-tagged prior to the start of the trial. Animals were fed twice daily, at 10 am and 6 pm, with feed offered ad libitum and free access to clean drinking water provided throughout the trial. To ensure proper feed intake, feed bunks were checked daily before the morning feeding, and refusals were maintained between 0 and 5% of the total feed offered.
Sample collection: 5 days before the end of the experiment, feed and residual materials were collected, thoroughly mixed, and quartered. Subsamples were oven-dried at 65 °C to a constant weight, ground, and stored in self-sealing bags for later nutrient analysis. On day 60, rumen fluid was collected from each anima using a rumen fluid collector. The first aliquot was discarded to avoid contamination, and subsequent fluid was collected. Rumen contents were filtered through four layers of sterile gauze. The pH of the filtrate was measured immediately and the remainder was stored in 5 mL cryovials at −80 °C for analysis of rumen fermentation parameters and microbial composition via 16S rDNA sequencing. Between days 56 and 60, 400 g of fresh feces were collected daily from the rectum of each animal. A portion of each sample was treated with 10% sulfuric acid to preserve nitrogen content. Fecal samples from each animal were pooled over the 5-day period, dried at 65 °C, ground, and stored for determination of apparent nutrient digestibility. Additional fecal samples were snap-frozen in liquid nitrogen and stored at −80 °C for subsequent microbial analysis via 16S rDNA sequencing.
Analysis of nutritional content and apparent digestibility of feed
Feed and fecal samples were ground and passed through a 40-mesh sieve prior to analysis. Dry matter (DM) was determined by drying at 105 °C in a forced-air oven for 4 h. Nitrogen (N) content in feed, feces, urine and microbial sampleswere measured using the Kjeldahl method, and crude protein (CP) was calculated as N × 6.25 (8). Ash content was determined by complete combustion in a muffle furnace at 600 °C for 6 h (8). Neutral detergent fiber (NDF) and acid detergent fiber (ADF) were according to the Van Soest method (9). Acid-insoluble ash (AIA) in feces and feed served as an internal markerfor calculating the apparent digestibility of each nutrient.
Apparent digestibility (%) was calculated using the following equation; 100–100 x (AIA content in the diet/AIA in feces) × (nutrient in feces/ The amount of this nutrient in the diet) (10).
Analysis of rumen fermentation
The pH of the rumen fluid was measured immediately after collection using a portable pH meter (FE28, Mettler Toledo, China). Ammonia nitrogen (NH₃-N) concentrations were measured using Broderick’s alkaline sodium hypochlorite-phenol spectrophotometry method (11). Volatile fatty acids (VFAs), including acetate, propionate, and butyrate, were quantified using gas chromatography (SP7800, Beijing Jingke Ruida, China) following the method of Yang et al. (12). The chromatographic conditions were as follows: capillary column GP-sil88 (30 m × 0.25 mm × 0.25 μm); flame ionization detector (FID) at 260 °C; inlet temperature 230 °C; injection volume 1 μL. Carrier gas pressures were: nitrogen 0.04 MPa, hydrogen 0.05 MPa, air 0.05 MPa, and tail blow (make-up gas) 0.05 MPa.
Rumen liquid and fecal samples 16S rDNA gene analysis
DNA extraction PCR amplification, and product purification
Genomic DNA was extracted using the PowerSoil® DNA Isolation Kit (Qiagen, United States) according to the manufacturer’s instructions. The purity and concentration of the extracted DNA were assessed by 1% agarose gel electrophoresis. The 16S rDNA gene was amplified using the universal bacterial primers 27F (AGRGTTTGATYNTGGCTCAG) and 1492R (TASGGHTACCTTGTTASGACTT). PCR reactions were performed in a 20 μL reaction system, containing 1 μL of each forward and reverse primers, 20 μL of Solexa PCR mix, and 5–50 ng of template DNA. PCR was performed with an initial denaturation at 95 °C for 5 min, followed by 30 cycles of 95 °C for 30 s, 50 °C for 30 s, and 72 °C for 1 min/kb, with a final extension at 72 °C for 7 min. PCR products were quantified, and samples were pooled according to the required sequencing depth and fragment size for each sample. The pooled PCR products were purified using 0.8 × volume of magnetic beads to remove impurities and primer dimers.
Library preparation and sequencing
Sequencing libraries were generated using the NEB Next® Ultra™ II FS DNA PCR-free Library Prep Kit (New England Biolabs, United States, Catalog #: E7430L) following manufacturer’s recommendations, and unique indexes were added to each sample. The libraries were quantified usinga Qubit fluorometer and real-time PCR, and the fragment size distribution was assessed using a Bioanalyzer. Quantified libraries were pooled based on effective concentration and required data output, and sequencing was performed on the PacBio Sequel II platform.
Paired-end reads assembly and quality control
Raw subreads were processed to obtain circular consensus sequencing (CCS) reads using SMRT Link software (version 8.0). Demultiplexing of CCS reads was performed with the Lima tool (version 1.7.0) based on barcode sequences to separate samples. Chimeric sequences were identified and removed using UCHIME (version 8.1) (13), resulting in a set of high-quality, non-chimeric CCS reads for downstream analysis.
Operational taxonomic units denoise and taxonomic annotation
Sequences were clustered into operational taxonomic units (OTUs) at a 97% similarity threshold using USEARCH (version 10.0) (14), with a minimum abundance filter set at 0.005% of the total sequence count to remove low-frequency noise (15). Taxonomic annotation of representative OTU sequences was performed using the RDP Classifier (version 2.2)1 with a confidence threshold of 0.8. Classification was conducted against the SILVA 16S rRNA database2 using Clustal W/X (version 2.0) for alignment.
Alpha diversity, beta diversity and LEfSe analysis
Alpha diversity indices, including the Shannon and Chao1 indices, were calculated using Mothur software (version 1.3).3 Beta diversity was assessed using non-metric multidimensional scaling (NMDS) based on Bray-Curtis and unweighted UniFrac distances, visualized with the ggplot2 and ade4 packages in R (version 3.5.3). LEfSe analysis4 was conducted to identify significant bacterial biomarkers differentiating between groups.
Statistical analysis
Differences in apparent nutrient digestibility and rumen fermentation parameters were analyzed by one-way ANOVA, followed by Duncan’s multiple range test for post-hoc comparisons. Microbial community analysis, including statistical comparisons of relative bacterial abundance, was performed by Tsingke Biotechnology Co., Ltd. (Beijing, China). The company conducted normality testing to determine the suitability of parametric methods and applied the Benjamini–Hochberg false discovery rate (FDR) correction to adjust p-values for multiple comparisons. Results were expressed as means ± SEM, and p < 0.05 was considered statistically significant. Graphs were generated using OriginPro 2021 (version 9.8.0.200).
Results
Effect of mixed silage of aerial parts of licorice with whole plant corn on apparent nutrient digestibility in Simmental cattle
The apparent nutrient digestibility of Simmental cattle fed diets containing different proportions of mixed silage composed of aerial parts of licorice and whole plant corn is presented in Table 3. As the proportion of mixed silage increased, the apparent digestibility of nutrients initially increased and then declined. Notably, the crude protein (CP) digestibility in the 34% mixed silage group was significantly lower compared to the 22 and 28% mixed silage groups (p = 0.017). The neutral detergent fiber (NDF) and acid detergent fiber (ADF) digestibility in the 22 and 28% groups were significantly higher than those in the control group (0%) and the 34% group (p = 0.001 for NDF; p = 0.009 for ADF) No significant differences were observed in the ether extract (EE) digestibility among all treatment groups (p > 0.05; Table 3).
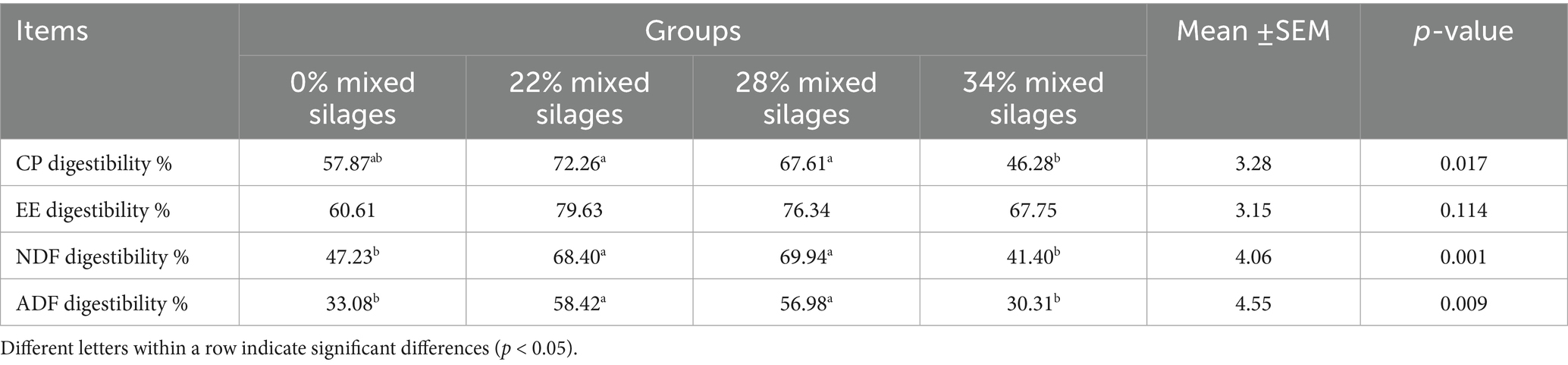
Table 3. Effect of mixed silages of aerial parts of licorice with whole plant corn on apparent digestibility of nutrients in Simmental cattle.
Effect of mixed silage of aerial parts of licorice with whole plant corn on rumen fermentation parameters in Simmental cattle
The rumen fermentation parameters of Simmental cattle fed mixed silage containing different proportions of aerial parts of licorice and whole plant corn are presented in Table 4. As the proportion of mixed silage increased the concentrations of ruminal acetate, propionate, and TVFA initially increased and then decreased. The 22 and 28% mixed silage groups showed significantly higher concentrations of acetate and TVFA compared to the control and 34% mixed silage groups (p = 0.001). Rumen pH was significantly higher in the control and 34% mixed silage groups than in the 22 and 28% mixed silage groups (p = 0.001). Propionate concentrations were also significantly higher in in the 22 and 28% groups compared to the 34% group (p = 0.042). No significant differences were observed in ruminal butyrate and NH₃-N concentrations among the groups (p > 0.05; Table 4).
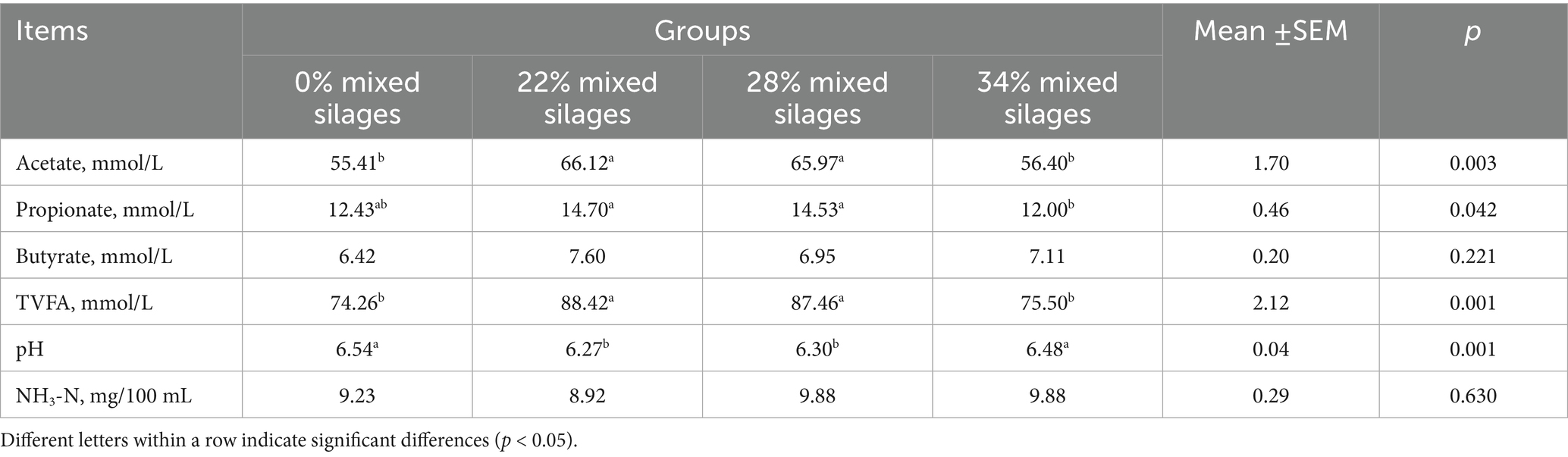
Table 4. Effect of mixed silages of aerial parts of licorice with whole plant corn on rumen fermentation parameters in Simmental cattle.
Effect of mixed silages of aerial parts of licorice with whole plant corn on gastrointestinal microbiome in Simmental cattle
Effect of mixed silages of aerial parts of licorice with whole plant corn on bacterial species richness and diversity in Simmental cattle
The rarefaction curves based on the number of Features indicate that when the original sequence number exceeds 7,500, the number of detected species plateaus, suggesting that the sequencing depth was sufficient to cover the majority of microbial diversity (Figures 1A,B). Hierarchical clustering analysis further showed a relatively homogeneous distribution of microbial species among samples (Figures 1C,D). Operational Taxonomic Units (OTUs) were clustered from rumen and fecal samples. According to the Venn diagrams, the rumen samples showed 124 unique OTUs in the control group, 52 in the 22% mixed silage group, 108 in the 28% group, and 141 in the 34% group, with 488 shared core OTUs (Figure 1E). In fecal samples, the control group had 65 unique OTUs, while the 22, 28, and 34% mixed silage groups had 130, 164, and 210 unique OTUs, respectively. The number of shared core OTUs in feces was also 488 (Figure 1F).
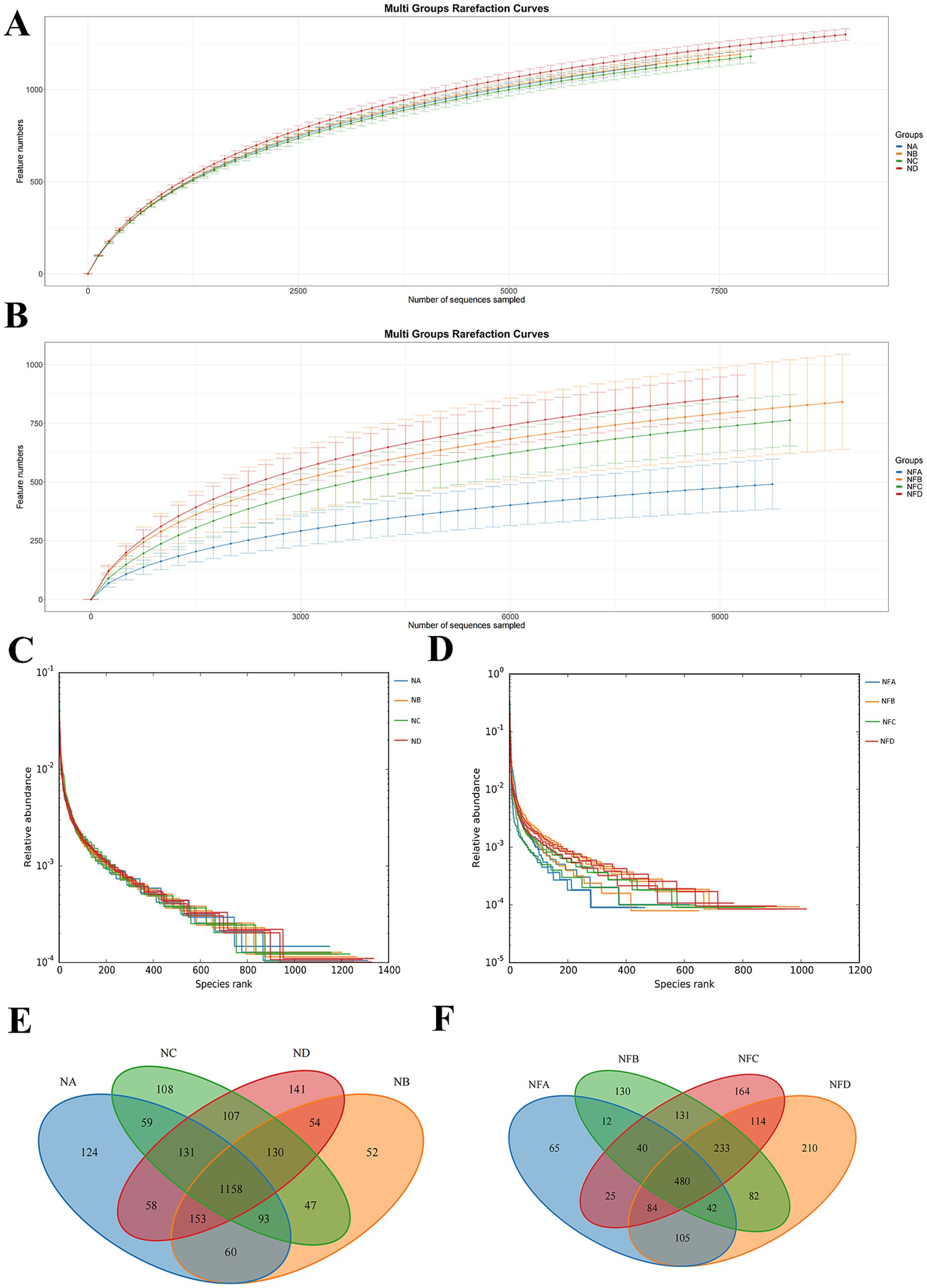
Figure 1. Microbial community diversity and structural analysis in Simmental cattle. (A,B) Rarefaction curves illustrating sequencing depth and observed amplicon sequence variant (ASV) richness for rumen (A) and fecal (B) samples. A plateau indicates sufficient sequencing depth to capture the majority of microbial diversity within a sample. (C,D) Hierarchical clustering diagrams (dendrograms) based on microbial community dissimilarity, showing relationships among rumen (C) and fecal (D) samples. Closer branches indicate more similar microbial community compositions. (E,F) Venn diagrams of shared and unique amplicon sequence variants (ASVs) among different treatment groups in rumen (E) and fecal (F) samples, illustrating community overlap and distinct ASV profiles. Sample groups are defined as: NA (0% mixed silage rumen), NB (22% mixed silage rumen), NC (28% mixed silage rumen), ND (another 22% mixed silage rumen); NFA (0% mixed silage fecal), NFB (22% mixed silage fecal), NFC (28% mixed silage fecal), NFD (another 22% mixed silage fecal).
Effect of mixed silages of aerial parts of licorice with whole plant corn on bacterial effective sequence, alpha diversity and Beta diversity in Simmental cattle
After splicing and quality filtering of 16S rDNA sequencing data from rumen and fecal samples, a total of 289,500 high-quality Circular Consensus Sequences (CCS) were obtained, averaging 12,062.5 sequences per sample. Clustering at 100% similarity produced 24,000 operational taxonomic units (OTUs). Alpha diversity analysis showed significant differences in the rumen Chao1 index between the 28 and 34% mixed silage groups (p = 0.044), as well as in the fecal Chao1 index between the control and 34% mixed silage groups (p = 0.03; Figures 2A,B). Although there were no statistically significant differences in the Shannon index for either rumen or fecal microbiota (p > 0.05), the trends in Shannon index were consistent with those of the Chao1 index (Figures 2C,D).
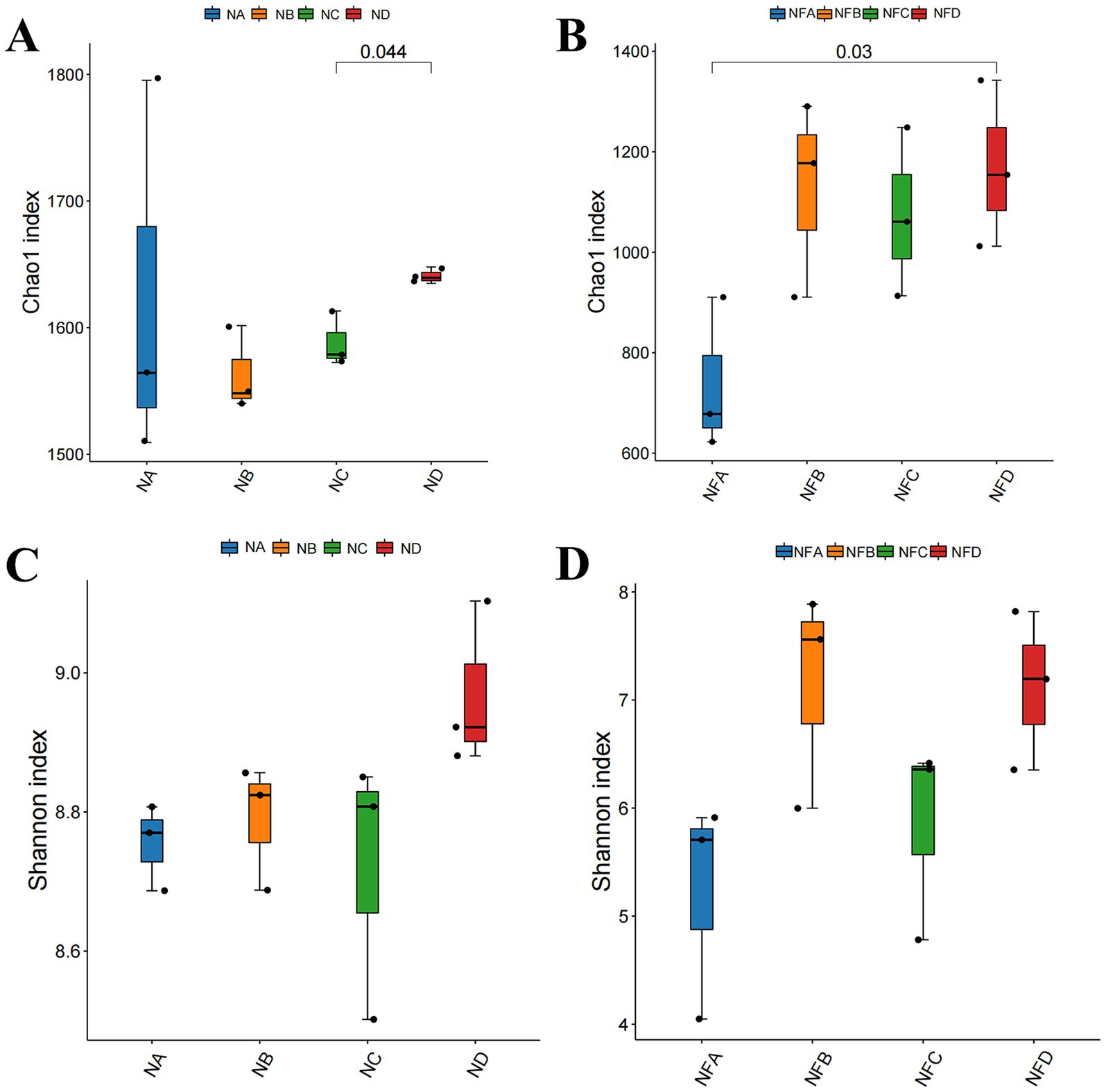
Figure 2. Alpha diversity indices of gastrointestinal microbiota in different treatment groups. Alpha diversity represents the diversity within a single sample. (A) Rumen microbial alpha diversity (e.g., Chao1 index); (B) Rumen microbial alpha diversity (e.g., Shannon index); (C) Fecal microbial alpha diversity (e.g., Chao1 index); (D) Fecal microbial alpha diversity (e.g., Shannon index). Different lowercase letters above bars indicate statistically significant differences (p < 0.05) among groups. Sample groups are defined as: NA, NB, NC, ND for rumen; NFA, NFB, NFC, NFD for fecal.
Beta diversity analysis using Bray-Curtis distance-based non-metric multidimensional scaling (NMDS) showed stress values below 0.2, indicating reliable ordination. Notably, supplementation with different levels of mixed silage resulted in distinct shifts in both rumen and fecal microbial communities (Figure 3).
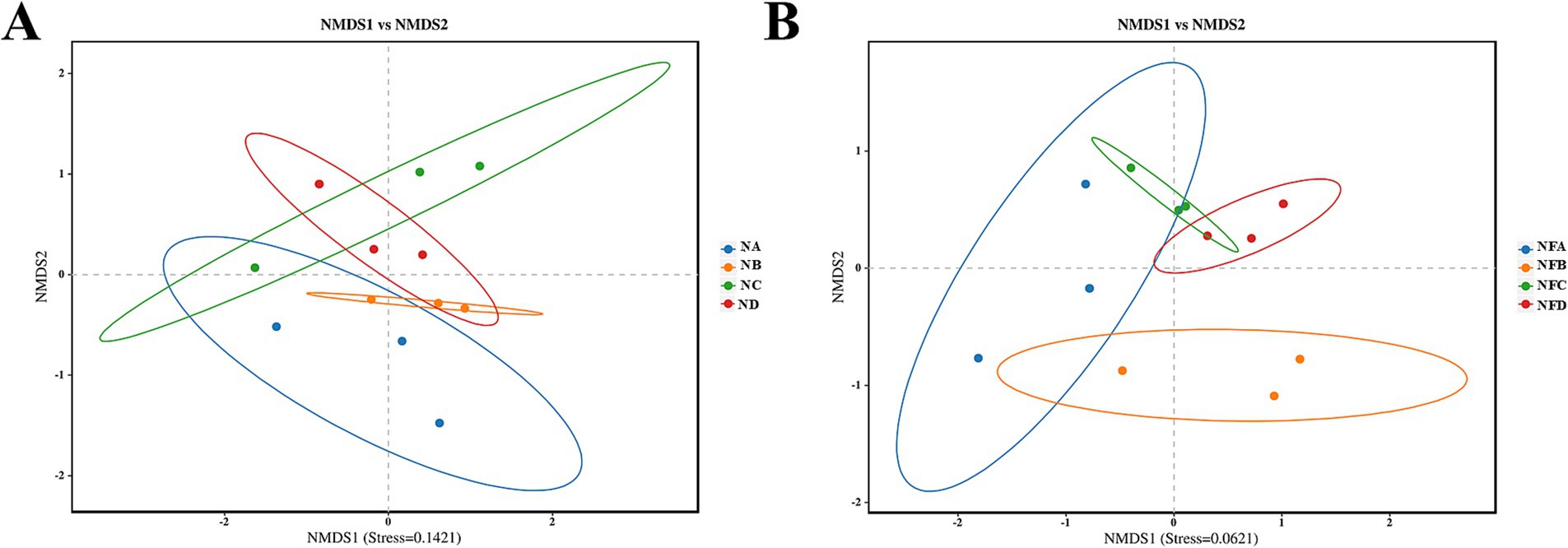
Figure 3. Non-metric Multidimensional Scaling (NMDS) plots illustrating beta diversity of gastrointestinal microbial communities in different treatment groups. Beta diversity represents the dissimilarity in microbial community composition between samples. (A) Rumen microbial beta diversity; (B) Fecal microbial beta diversity. Each point represents a sample, and the distance between points reflects the dissimilarity of their microbial communities. Sample sizes for each group are n = 3. Sample groups are defined as: NA, NB, NC, ND for rumen; NFA, NFB, NFC, NFD for fecal.
Effect of mixed silages of aerial parts of licorice with whole plant corn on bacterial species composition in Simmental cattle
Effect of mixed silages of aerial parts of licorice with whole plant corn on the composition of rumen bacteria in Simmental cattle
A total of 23 phyla, 167 families, and 329 genera were identified in the rumen samples. Bacteroidota and Firmicutes were the dominant phyla across all groups, together accounting for over 80% of the total relative abundance, with no significant differences observed among groups (p > 0.05; Figure 4A). The relative abundance of Desulfobacterota was significantly higher in the 34% mixed silage group compared to the control group (p = 0.009). Prevotellaceae and Lachnospiraceae were the dominant families in the rumen across all groups, with Lachnospiraceae having a relative abundance greater than 10%. In addition, the relative abundance of Oscillospiraceae in the 22% mixed silage group was significantly greater than that in the control group (p = 0.037; Figure 4B). Prevotella was the predominant genus in the rumen samples of all groups, with an average relative abundance exceeding 20%. The relative abundance of NK4A214_group in the 22% mixed silage group was significantly higher than in the control group (p = 0.035; Figure 4C).
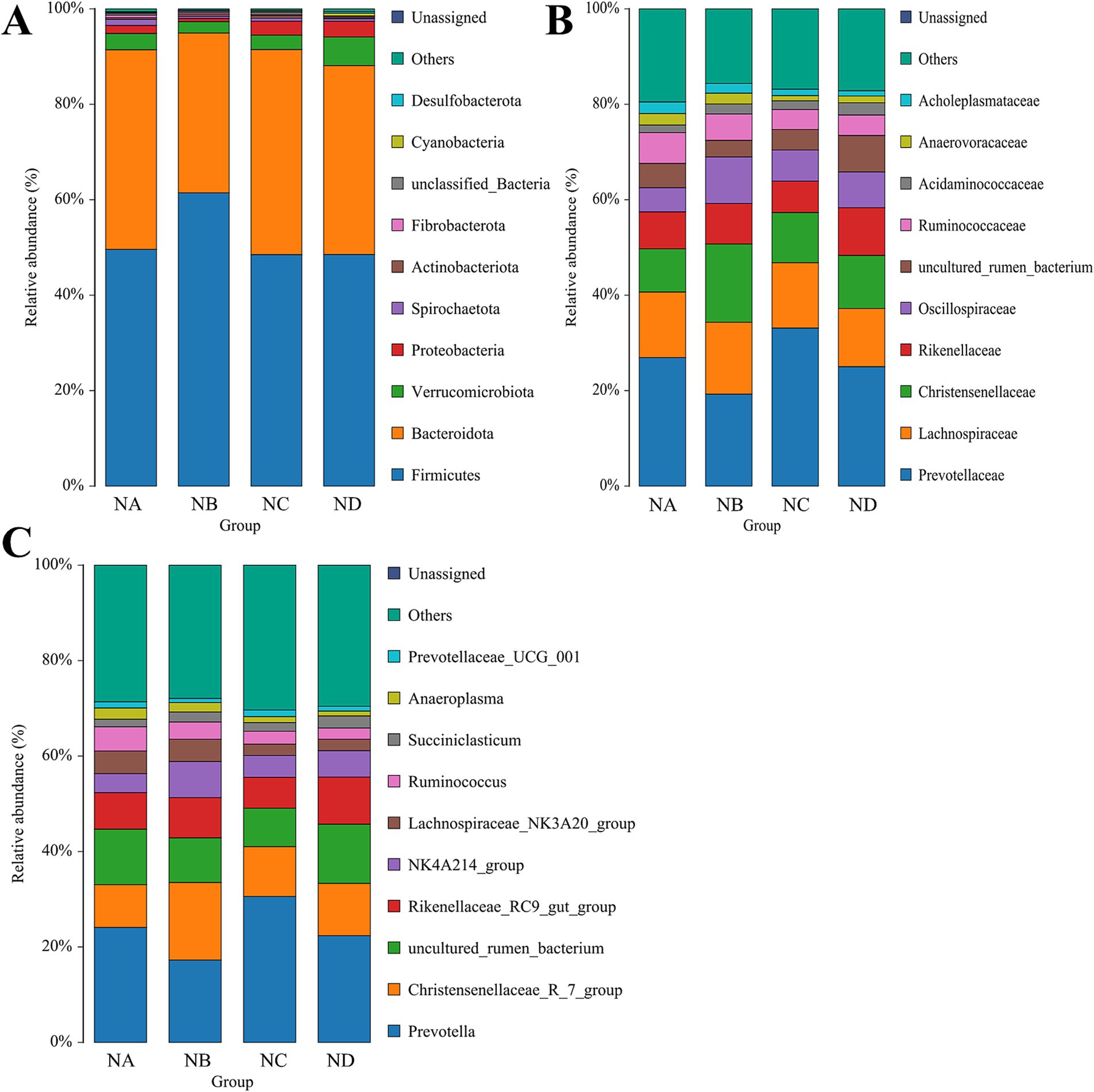
Figure 4. Bar charts showing the relative abundances of rumen bacteria in different treatment groups. (A) Phylum level; (B) Family level; (C) Genus level. Only taxa with relative abundance greater than 1% are displayed to highlight dominant microbial groups. These hierarchical levels illustrate the overall structure and key taxonomic shifts within the rumen microbial community. Sample groups are defined as: NA, NB, NC, ND.
Effect of mixed silages of aerial parts of licorice with whole plant corn on the composition of fecal bacteria in Simmental cattle
A total of 18 phyla, 153 families, and 327 genera were identified in fecal samples. Firmicutes and Bacteroidetes were the predominant fecal bacterial phyla across all groups, together accounting for more than 90% of the total relative abundance, with Firmicutes showing an absolute predominance. The relative abundance of Firmicutes was significantly higher in the 28% mixed silage group compared to the 22% mixed silage group (p = 0.038), while Bacteroidetes were more abundant in the 22% mixed silage group compared to the control and 28% mixed silage groups (p = 0.046). In addition, the relative abundance of Cyanobacteria in the 34% mixed silage group was significantly higher than in the control and 22% mixed silage groups (p = 0.007; Figure 5A).
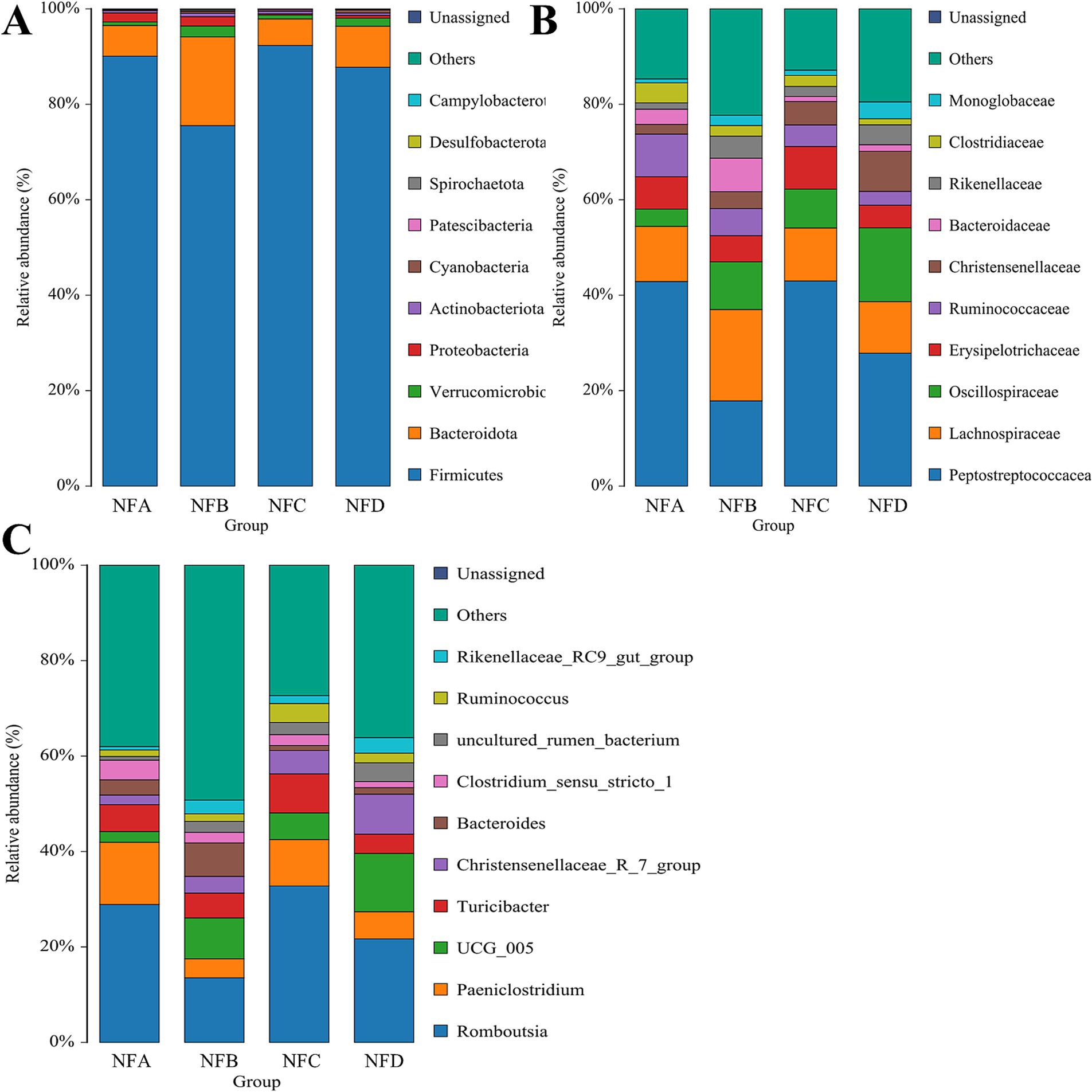
Figure 5. Bar charts showing the relative abundances of fecal bacteria in different treatment groups. (A) Phylum level; (B) Family level; (C) Genus level. Only taxa with relative abundance greater than 1% are displayed to highlight dominant microbial groups. These hierarchical levels illustrate the overall structure and key taxonomic shifts within the fecal microbial community. Sample groups are defined as: NFA, NFB, NFC, NFD.
At the family level, Peptostreptococcaceae and Lachnospiraceae were dominant in fecal samples, with Peptostreptococcaceae accounting for over 30% of the total relative abundance. Lachnospiraceae also exhibited a relative abundance exceeding 10%. The 34% mixed silage group showed a significantly higher relative abundance of Oscillospiraceae compared to the control group (p = 0.031). Christensenellaceae were significantly more abundant in all mixed silage groups (22, 28, and 34%) compared to the control group (p < 0.001). Conversely, Clostridiaceae were more abundant in the control group than in the 34% mixed silage group (p = 0.039). Additionally, Monoglobaceae were significantly more abundant in the 34% mixed silage group compared to the control and 28% mixed silage groups (Figure 5B).
At the genus level, Romboutsia was the dominant genus across all groups, with an average relative abundance above 20%. The relative abundance of UCG_005 was significantly higher in the 34% mixed silage group compared to the control group (p = 0.027). Christensenellaceae_R-7_group showed a markedly higher relative abundance in the 34% mixed silage group compared to the control, 22, and 28% mixed silage groups (p < 0.0001). In contrast, Clostridium_sensu_stricto_1 was significantly more abundant in the control group than in the 28 and 34% mixed silage groups (p = 0.042). Ruminococcus was significantly more abundant in the 28% mixed silage group compared to the control group (p = 0.030). Finally, Rikenellaceae_RC9_gut_group was significantly more abundant in the 34% mixed silage group than in the control group (p = 0.022; Figure 5C).
LEfSe analysis of Simmental cattle bacteria in mixed silages
LEfSe analysis of Simmental cattle rumen bacteria in mixed silages
To further investigate the key bacterial taxa influenced by mixed silage in the rumen of Simmental cattle, LEfSe analysis was performed to identify significant biomarkers. The analysis revealed 11, 8, 12, and 12 key biomarkers in the control group, 22% mixed silage group, 28% mixed silage group, and 34% mixed silage group, respectively (Figure 6A). These biomarkers were detected across various taxonomic levels, including phylum, family, and genus. However, some bacteria at these taxonomic levels were not identified as significant biomarkers (Figure 6B).
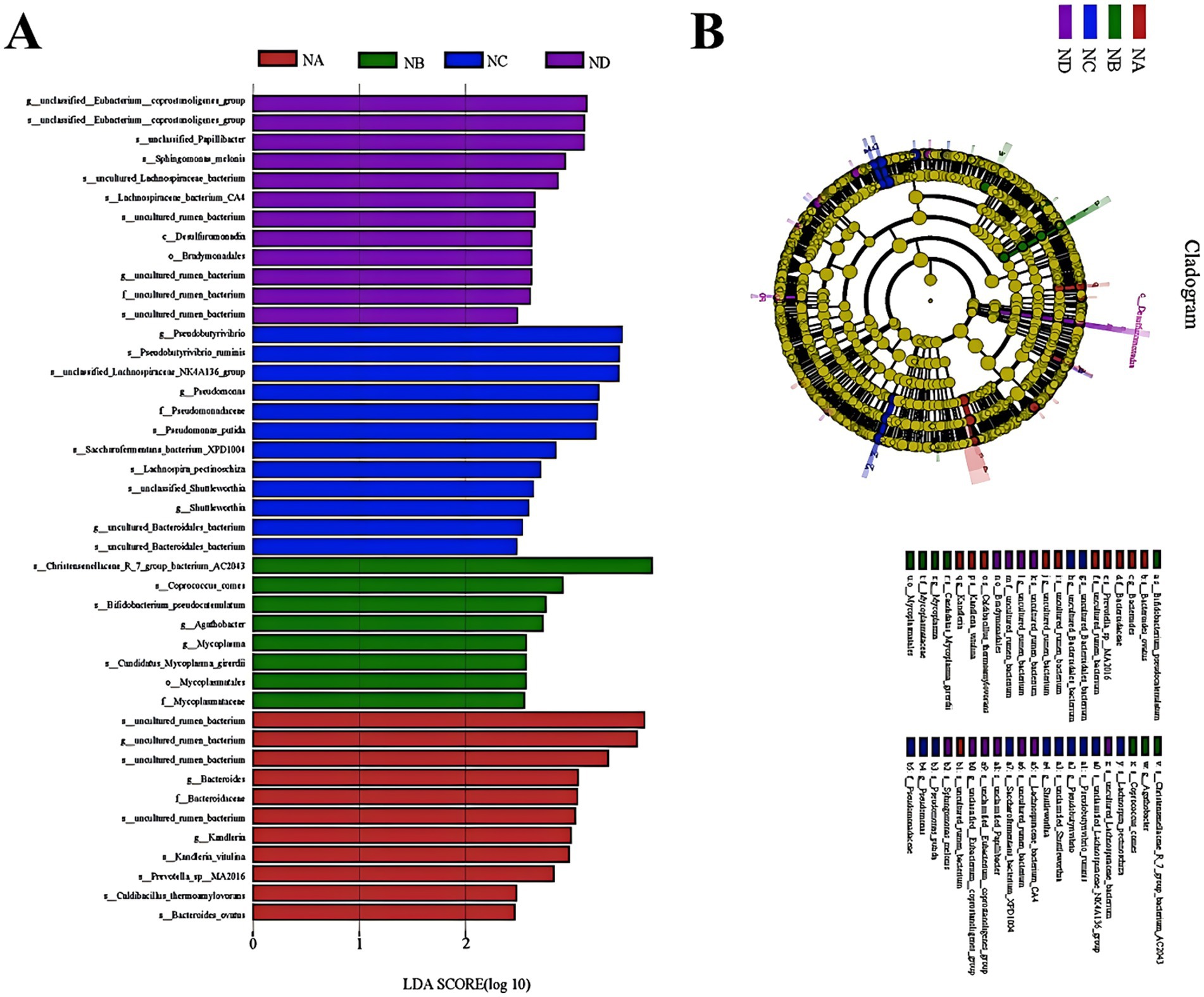
Figure 6. Linear Discriminant Analysis Effect Size (LEfSe) analysis identifying differentially abundant rumen bacterial taxa with an LDA score greater than 2.0. (A) LDA score distribution plot showing the effect size of each differentially abundant taxon. The length of the bar indicates the LDA score (log 10), representing the magnitude of the difference in abundance. Colors correspond to the group in which the taxon is enriched: NA (red), NB (green), NC (blue), ND (purple). (B) Taxonomic cladogram illustrating the phylogenetic relationships of the significantly enriched taxa identified by LEfSe analysis. The concentric circles represent taxonomic levels from the outermost to the innermost: phylum, class, order, family, genus, and species. Colored nodes and shading in the phylogenetic tree represent taxa that are significantly enriched in the corresponding group, while yellow nodes indicate taxa with no significant differences among groups. This cladogram visually represents the taxonomic hierarchy and enrichment patterns of the taxa shown in the LDA score plot (A). Sample groups are defined as: NA, NB, NC, ND.
LEfSe analysis of Simmental cattle fecal bacteria in mixed silages
To further investigate the key bacterial taxa influenced by mixed silage in the feces of Simmental cattle, LEfSe analysis was performed. The analysis revealed 7, 5, 3, and 9 taxa with differential abundance (LDA > 2) in the control, 22, 28, and 34% mixed silage groups, respectively (Figure 7A). While Firmicutes were enriched in the 28% mixed silage group, this reflects a broader trend in microbial composition rather than a precise biomarker. Within this phylum, genera such as Ruminococcus and families like Christensenellaceae and Lachnospiraceae, which are associated with fiber degradation and gut health, contributed substantially to the LEfSe signal. In contrast, Clostridiaceae was enriched in the control group, and Christensenellaceae, Monoglobaceae, and Christensenellaceae_R-7_group were more abundant in the 34% group (Figure 7B).
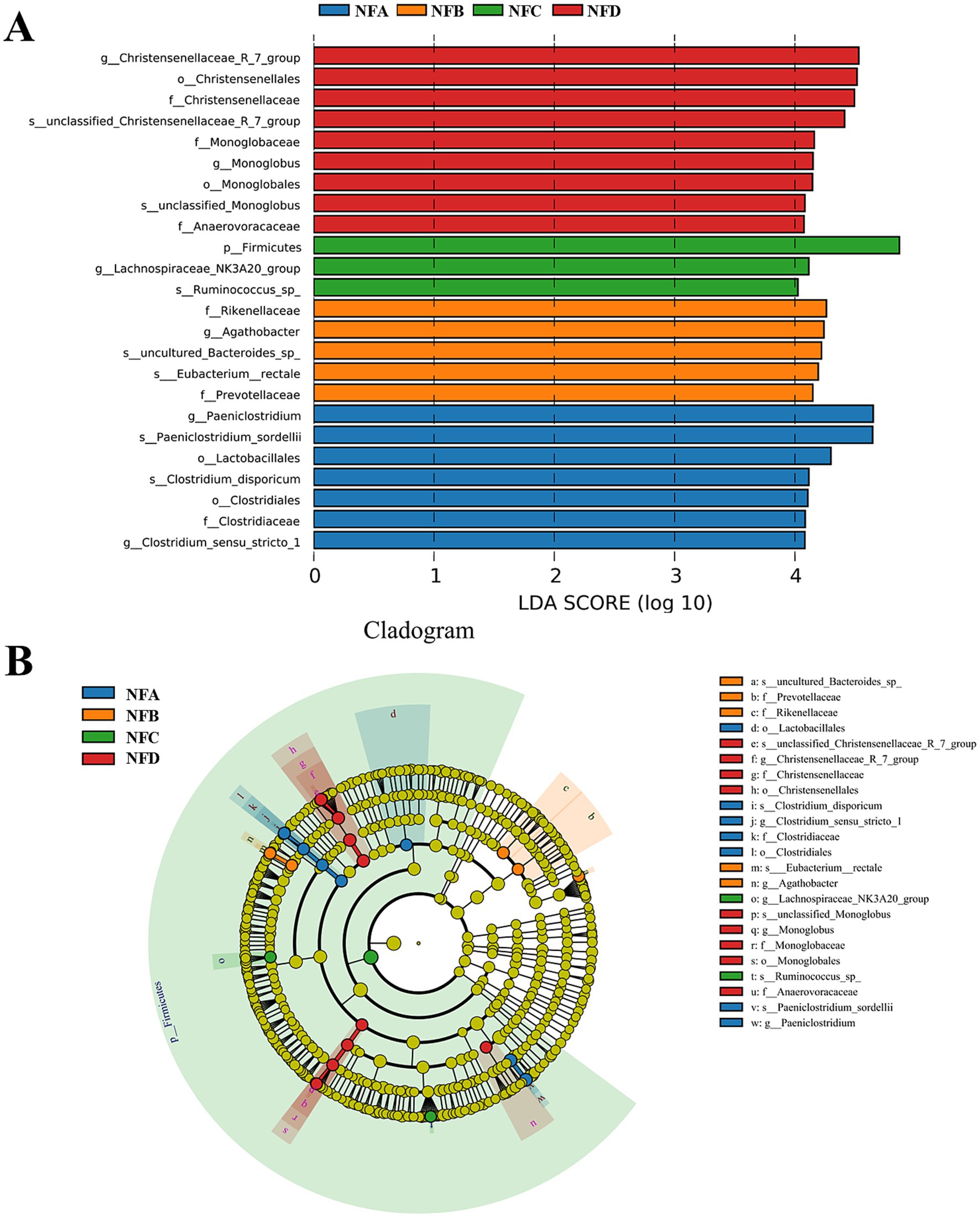
Figure 7. Linear Discriminant Analysis Effect Size (LEfSe) analysis identifying differentially abundant fecal bacterial taxa with an LDA score greater than 4.0. (A) LDA score distribution plot showing the effect size of each differentially abundant taxon. The length of the bar indicates the LDA score (log 10), representing the magnitude of the difference in abundance. Colors correspond to the group in which the taxon is enriched: NFA (red), NFB (green), NFC (blue), NFD (purple). The higher LDA score threshold (4.0) highlights more robust or highly abundant biomarkers. (B) Taxonomic cladogram illustrating the phylogenetic relationships of the significantly enriched taxa identified by LEfSe analysis. The concentric circles represent taxonomic levels from the outermost to the innermost: phylum, class, order, family, genus, and species. Colored nodes and shading in the phylogenetic tree represent bacterial taxa significantly enriched in the corresponding group, while yellow nodes indicate taxa with no significant differences among groups. This cladogram visually represents the taxonomic hierarchy and enrichment patterns of the taxa shown in the LDA score plot (A). Sample groups are defined as: NFA, NFB, NFC, NFD.
Correlation analysis between gastrointestinal bacteria, apparent digestibility of nutrients, and rumen fermentation
An analysis of the associations among rumen and fecal microbial differences, apparent nutrient digestibility, and rumen fermentation parameters was conducted. The abundance of rumen Oscillospiraceae showed a significant positive correlation with ether extract (EE) digestibility and a significant negative correlation with rumen pH (p < 0.0001). Similarly, the rumen NK4A214_group was negatively correlated with rumen pH (p < 0.0001; Figure 8).
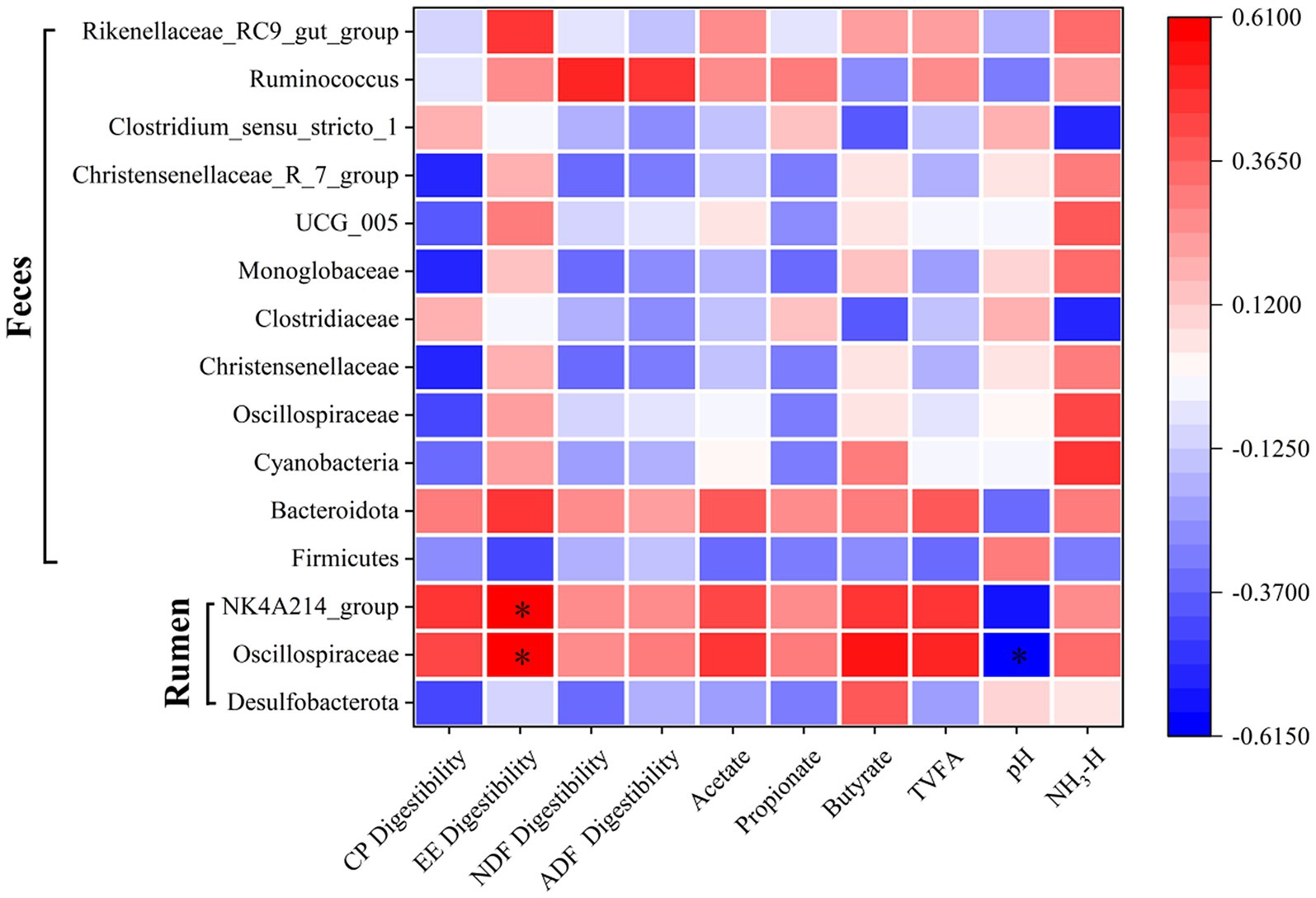
Figure 8. Spearman correlation analysis between gastrointestinal bacterial taxa, apparent nutrient digestibility parameters, and rumen fermentation parameters. The heatmap visualizes the correlation coefficients, where color intensity and direction (e.g., blue for positive, red for negative) indicate the strength and nature of the relationship. Key bacterial taxa (e.g., [List a few examples of specific taxa or taxonomic levels included]), apparent nutrient digestibility parameters (e.g., dry matter digestibility, crude protein digestibility, neutral detergent fiber digestibility), and rumen fermentation parameters (e.g., pH, total volatile fatty acids, acetate, propionate, butyrate) were included in the analysis. An asterisk (*) indicates a statistically significant correlation (p < 0.05).
Discussion
Effect of mixed silages of aerial parts of licorice with whole plant corn on apparent digestibility of nutrients in Simmental cattle
Apparent nutrient digestibility reflects the digestion and absorption efficiency of nutrients from feed and serves as a key indicator for evaluating feed nutritional value (16). Guo et al. (5) reported that supplementing with licorice extract can improve apparent nutrient digestibility in Karakul sheep. Similarly, Chen et al. (4) found that an appropriate proportion of mixed silage with forage grass (sweet sorghum) and aerial parts of licorice mixed silage significantly increased the degradationrate of CP, ADF, and NDF in the sheep rumen. In this study, as the proportion of mixed silage increased, apparent nutrient digestibility first increased and then decreased. Specifically, apparent digestibility of NDF and ADF significantly improved in the 22 and 28% mixed silage groups, with CP digestibility peaking at the 28% mixed silage group. Previous research suggested that licorice stems and leaves might inhibit fiber-degrading bacteria, and when their proportion in the diet is too high, rumen fermentation can be suppressed (17). This may explain the reduced digestibility of CP, NDF, and ADF observed in the 34% mixed silage group. This reduction at higher licorice inclusion aligns with earlier findings that excessive levels of licorice aerial parts or glycyrrhizic acid can suppress microbial fermentation or alter ruminal balance, especially when exceeding ~4.5% of dietary dry matter. Overall, these results indicate that mixed silages of aerial parts of licorice with whole plant corn can enhance the apparent digestibility of CP, ADF, and NDF in Simmental cattle, with an optimal inclusion rate not exceeding 28%. Effect of mixed silages of aerial parts of licorice with whole plant corn on rumen fermentation parameters in Simmental cattle.
Rumen pH reflects the coordinated acid–base balance maintained by the rumen microbial population and host metabolism (18), and it typically shows a negative correlation with VFA concentrations (19). In this study, as the proportion of mixed silage increased, the contents of rumen acetate and total volatile fatty acids (TVFA) showed an opposite trend to rumen pH. Specifically, the highest concentrations of acetate and TVFA were observed in the 22 and 28% mixed silage groups, while the lowest were found in the control (0%) and 34% groups. This pattern aligns with the improved fiber digestibility seen at moderate inclusion levels. A prior study has reported that licorice extract supplementation reduced TVFA and acetate levels in the rumen of Karakul sheep while increasing propionate and butyrate concentrations (5). Moreover, in vitro experiments showed no significant effect of licorice extract on VFA synthesis (20). In the present study, propionate levels aligned with these prior findings, whereas acetate and TVFA did not. Propionate is mainly produced by the fermentation of starch and soluble sugars, while acetate primarily derives from the fermentation of fiber and semi-fiber components (16). Considering the apparent nutrient digestibility data from this study, NDF and ADF digestibility significantly increased in the 22 and 28% mixed silage groups, which likely contributed to the elevated acetate and TVFA concentrations observed.
Rumen ammonia nitrogen (NH3-N) content serves as an indicator of microbial protein metabolism from dietary nitrogen (21), and there is generally a negative relationship between protein digestibility and ammonia nitrogen levels. For example, replacing legume fodder with oat hay has been shown to improve nitrogen utilization efficiency in Simmental cattle (22). In this study, rumen NH3-N content did not differ significantly across mixed silage groups, despite the increased crude protein digestibility in the 22 and 28% groups. This may indicate that the mixed silages of aerial parts of licorice with whole plant corn facilitated enhanced protein degradation and utilization. However, as NH₃-N levels remained unchanged, the interpretation of efficient nitrogen capture remains speculative in the absence of direct measures such as microbial nitrogen incorporation or blood urea concentrations. Further investigation is needed to clarify the nitrogen utilization pathways involved.
Effect of mixed silages of aerial parts of licorice with whole plant corn on bacterial alpha diversity and Beta diversity in Simmental cattle
Intestinal bacteria play a crucial role in animal digestion, nutrient absorption, and immune regulation. In ruminants, rumen microorganisms are particularly important for fiber degradation, fermentation of sugars and starch, and microbial protein synthesis (23). The structure and composition of the gut microbiota are influenced by several factors, including diet composition and feeding strategy. Alpha diversity reflects the richness and evenness of microbial communities. The Chao1 index indicates microbial abundance, while the Shannon index reflects community diversity (24). In this study, the rumen Chao1 index was significantly higher in the 34% mixed silage group compared to the 28% group. Similarly, in fecal samples, the Chao1 index of the 34% mixed silage group was significantly greater than that of the control group. Although the Shannon index followed a similar trend in both rumen and feces, the differences were not statistically significant. These findings may be partially influenced by the bioactive components of licorice stems and leaves, which are rich in flavonoids such as liquiritin, isoliquiritigenin, and glabridin (25). Some studies suggest that specific flavonoids can promote microbial richness and diversity by modulating gut microbial composition and supporting beneficial bacteria (26). However, as flavonoid effects can vary depending on structure, dosage, and microbial context, further research is needed to confirm their specific role in this setting. NMDS (non-metric multidimensional scaling) analysis based on Bray-Curtis distance further confirmed that different proportions of mixed silage led to distinct alterations in both rumen and fecal microbial communities. Overall, these results suggest that supplementing diets with mixed silages of aerial parts of licorice and whole plant corn may influence the structure and enhance the diversity of gastrointestinal microbiota in Simmental cattle; however, further studies are needed to determine whether these microbial shifts translate into improved gut function or feed efficiency. Effect of mixed silages of aerial parts of licorice with whole plant corn on bacterial species composition in Simmental cattle.
The rumen microbiome is a complex and dynamic ecosystem essential for nutrient digestion and absorption in ruminants (27). Previous studies have shown that Bacteroidetes and Firmicutes are the dominant bacterial phyla in Simmental cattle (28, 29). Consistent with these findings, Bacteroidetes and Firmicutes were the most abundant phyla in both rumen and feces in this study. Bacteroidetes are primarily involved in the fermentation of carbohydrates and polysaccharides in the plant cell wall (30), whereas Firmicutes contribute significantly to the degradation of cellulose, proteins, and other carbohydrates (31). In this study, there were no significant differences in the relative abundance of Bacteroidetes in the rumen across groups. However, in fecal samples, the 22% mixed silage group showed a higher relative abundance of Bacteroidetes, whereas Firmicutes were more abundant in the 28% group, where they were also identified as biomarkers. Considering the nutrient digestibility and volatile fatty acid (VFA) profiles, it appears that supplementation with mixed silages of aerial parts of licorice and whole plant corn may enhance the relative abundance of both Bacteroidetes and Firmicutes, thereby improving nutrient digestion and utilization. At the family level, Prevotellaceae, Lachnospiraceae, and Peptostreptococcaceae were dominant in both rumen and feces, which is consistent with the microbiota typically found in beef cattle (32). Notably, the 22% mixed silage group exhibited a significantly higher abundance of Oscillospiraceae in the rumen, while the 34% mixed silage group had increased levels of Oscillospiraceae, Christensenellaceae, and Monoglobaceae. Oscillospiraceae are associated with carbohydrate digestion and the production of short-chain fatty acids (33). Christensenellaceae are involved in protein catabolism and gut metabolite regulation (34), and are thought to support gut health and possibly alleviate inflammatory bowel conditions (35, 36). Monoglobaceae are strongly linked with host immunoinflammation (37) and have been noted as biomarker taxa in migratory yaks (38). Considering the enhancements in neutral detergent fiber (NDF), acid detergent fiber (ADF), and crude protein (CP) digestibility, along with increased total VFAs (TVFA), and the observed negative correlation between Oscillospiraceae and rumen pH, these results support the idea that dietary inclusion of licorice-based silages can beneficially modulate gastrointestinal microbiota. This modulation leads to enhanced nutrient utilization, in part due to the established inverse relationship between rumen pH and VFA concentration (19).
At the genus level, the relative abundance of NK4A214_group in the rumen was significantly increased in the mixed silage groups. Moreover, higher relative abundances of Ruminococcus, UCG_005, Christensenellaceae_R_7_group, and Rikenellaceae_RC9_gut_group were observed, while Clostridium_sensu_stricto_1 was significantly reduced. Rikenellaceae_RC9_gut_group plays a role in degrading both soluble polysaccharides and insoluble cellulose, producing succinate and propionate, and alleviating intestinal inflammation (39, 40). Ruminococcus is a key fibrolytic genus that efficiently digests hemicellulose and cellulose (41). NK4A214_group is associated with acetate production and plays a role in host energy metabolism (42). In this study, increased digestibility of NDF, ADF, CP, and higher TVFA levels were observed alongside these microbial changes. Taken together, the results indicate that mixed silages of aerial parts of licorice with whole plant corn can enhance the abundance of beneficial microbial taxa such as Rikenellaceae_RC9_gut_group, Christensenellaceae, and Ruminococcus. This improvement supports better carbohydrate metabolism, energy availability, and immune function in Simmental cattle.
Conclusion
Feeding Simmental cattle with a diet containing 28% mixed silages of aerial parts of licorice and whole plant corn significantly improved the apparent digestibility of NDF and ADF, and increased rumen acetate and total volatile fatty acid (TVFA) concentrations. This dietary treatment also enhanced gastrointestinal microbial diversity, with notable increases in the relative abundance of Oscillospiraceae and NK4A214_group in the rumen. In the feces, members of the phylum Firmicutes were enriched in the 28% mixed silage group, reflecting a broader microbial trend. More specifically, LEfSe analysis indicated that this enrichment was driven by genera such as Ruminococcus and families including Christensenellaceae and Lachnospiraceae, which are associated with fiber degradation and gut health. Additionally, the relative abundances of Oscillospiraceae, Christensenellaceae, Monoglobaceae, UCG_005, Christensenellaceae_R_7_group, Ruminococcus, and Rikenellaceae_RC9_gut_group were significantly elevated in the feces.
Based on these findings, we recommend that the inclusion of licorice aerial parts in mixed silage be limited to no more than 28% of the total dry matter, as this level provided the most favorable effects on nutrient digestibility, fermentation characteristics, antioxidant capacity, and microbial balance without adverse outcomes.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://www.ncbi.nlm.nih.gov/, 1148123.
Ethics statement
All animal procedures were approved by the Animal Ethic Committee of Tarim University (Xinjiang, China), conducted in accordance with the Guidelines for the Care and Use of Research Animals in China (GB14925-2001). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
HL: Formal analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. LT: Conceptualization, Data curation, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. XX: Writing – original draft, Writing – review & editing. QG: Writing – review & editing. YS: Writing – review & editing. WL: Writing – review & editing. DL: Writing – review & editing. HY: Writing – review & editing. TJ: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the earmarked fund for XJARS (XJARS-11) and the XPCC Key Core Technology Research Project on Agriculture, ‘Cultivation of New Strains of West China Cow’s Milk and Meat (Military Reclamation Type)’ (NYHXGG, 2023AA205). It was also supported by the presidential research fund of Tarim University for the project ‘Efficient Utilization and Demonstration of Licorice Stems and Leaves in the Production of Beef Cattle’ (TDZXZX202303).
Acknowledgments
All authors would like to thank the Science and Technology Innovation Program of the Xinjiang Production and Construction Corps, China, for financial support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
1. Yuxuan, H, Zhuoni, H, Xuemin, Z, Kaijing, Y, Zongsuo, L, and Qiuling, H. Important changes in germination, seedling tolerance, and active components content due to drought stress on three licorice (Glycyrrhiza) species[J]. Industrial Crops & Products. (2022) 175.
2. Jiang, D, Li, P, Yin, Y, Ren, G, and Liu, C. Molecular cloning and functional characterization of UGTs from Glycyrrhiza uralensis flavonoid pathway. Int J Biol Macromol. (2021) 192:1108–16. doi: 10.1016/j.ijbiomac.2021.09.136
3. Kondo, K, Shiba, M, Yamaji, H, Morota, T, Zhengmin, C, Huixia, P, et al. Species identification of licorice using nrDNA and cpDNA genetic markers. Biol Pharm Bull. (2007) 30:1497–502. doi: 10.1248/bpb.30.1497
4. Chen, F, Wang, J, Zhang, S, Chaudhry, AS, and Khanaki, H. Assessing fermentation quality, aerobic stability, in vitro digestibility, and rumen degradation characteristics of silages mixed with sweet sorghum and aerial parts of licorice. Agriculture (Basel). (2024) 14:212. doi: 10.3390/agriculture14020212
5. Guo, X, Cheng, L, Liu, J, Zhang, S, Sun, X, and Al-Marashdeh, O. Effects of licorice extract supplementation on feed intake, digestion, rumen function, blood indices and live weight gain of karakul sheep. Animals-Basel. (2019) 9:279. doi: 10.3390/ani9050279
6. Steffensen, I-L, Mathisen, GH, Bruzell, E, Granum, BB, Rohloff, J, and Hetland, RB. Hazard assessment of glycyrrhizic acid from liquorice. Opinion of the panel on food additives, Flavourings, processing aids, materials in contact with food and cosmetics. Oslo, Norway: Norwegian Scientific Committee for Food and Environment (VKM) (2018).
7. Institute Of Animal Husbandry CAOA, University CA. Feeding standard of beef cattle. NY/T 815–2004. China: Industry Standard - Agriculture (2004). 30 p.
8. AOAC. Official methods of analysis. 15th ed. Arlington, VA, USA: Association of Official Analytical Chemists (1990).
9. Van Soest, PJ, Robertson, JB, and Lewis, BA. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. (1991) 74:3583–97.
10. Klobucher, KN, Stahl, TC, Islam, T, Gray, AS, Curreri, SI, and Erickson, PS. Supplementing sodium butyrate to limit-fed heifers: effects on growth, coccidiosis, urinary purine derivatives, and apparent total-tract nutrient digestibility. J Dairy Sci. (2023) 106:6894–902. doi: 10.3168/jds.2023-23275
11. Broderick, G, and Michael, CW. Metabolism of peptides and amino acids during in vitro protein degradation by mixed rumen organisms. J Dairy Sci. (1989) 72:2540–8.
12. Yang, C, Zhang, L, Cao, G, Feng, J, Yue, M, Xu, Y, et al. Effects of dietary supplementation with essential oils and organic acids on the growth performance, immune system, fecal volatile fatty acids, and microflora community in weaned piglets. J Anim Sci. (2020) 98:133–43. doi: 10.1093/jas/skz039
13. Edgar, RC, Haas, BJ, Clemente, JC, Quince, C, and Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. (2011) 27:2194–200. doi: 10.1093/bioinformatics/btr381
14. Quast, C, Pruesse, E, Yilmaz, P, Gerken, J, Schweer, T, Yarza, P, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. (2013) 41:D590–6. doi: 10.1093/nar/gks1219
15. Martı́n, MP. Towards a unified paradigm for sequence-based identification of fungi. Basel, Switzerland (2016).
17. Yongli, W, Rehemujiang, H, Chen, L, Jiangchun, W, and Yimamu, A. Effects of mixed swards meal replaced by the aerial parts of Ural licorice meal on in vitro rumenal fermentation characteristics in sheep. Chin J Anim Sci. (2015) 51:55–9. doi: 10.3390/ani9050279
18. Allen, MS. Relationship between fermentation acid production in the rumen and the requirement for physically effective Fiber. J Dairy Sci. (1997) 80:1447–62.
19. Humer, E, Aschenbach, JR, Neubauer, V, Kröger, I, Khiaosa Ard, R, Baumgartner, W, et al. Signals for identifying cows at risk of subacute ruminal acidosis in dairy veterinary practice. J Anim Physiol an N. (2018) 102:380–92. doi: 10.1111/jpn.12850
20. Liu, J. Effects of licorice extract on in vitro ruminal fermentation parameters in karakul sheep. China Anim Husb Vet Med. (2012) 9:279. doi: 10.3168/jds.S0022-0302(97)76074-0
21. Chen, T, Xiao, J, Li, T, Ma, J, Alugongo, GM, Khan, MZ, et al. Effect of the initial time of providing oat hay on performance, health, behavior and rumen fermentation in Holstein female calves. Agriculture (Basel). (2021) 11:862. doi: 10.3390/agriculture11090862
22. Du, W, Hou, F, Tsunekawa, A, Kobayashi, N, Peng, F, and Ichinohe, T. Substitution of leguminous forage for oat hay improves nitrogen utilization efficiency of crossbred Simmental calves. J Anim Physiol Anim Nutr. (2020) 104:998–1009. doi: 10.1111/jpn.13288
23. Lees, AM, Sejian, V, Lees, JC, Sullivan, ML, Lisle, AT, and Gaughan, JB. Evaluating rumen temperature as an estimate of core body temperature in Angus feedlot cattle during summer. Int J Biometeorol. (2019) 63:939–47. doi: 10.1007/s00484-019-01706-0
24. Lee, CG, Baba, Y, Asano, R, Fukuda, Y, Tada, C, and Nakai, Y. Identification of bacteria involved in the decomposition of lignocellulosic biomass treated with cow rumen fluid by metagenomic analysis. J Biosci Bioeng. (2020) 130:137–41. doi: 10.1016/j.jbiosc.2020.03.010
25. Dong, Y, Zhao, M, Zhao, T, Feng, M, Chen, H, Zhuang, M, et al. Bioactive profiles, antioxidant activities, nitrite scavenging capacities and protective effects on H2O2-injured PC12 cells of Glycyrrhiza glabra L. leaf and root extracts. Molecules. (2014) 19:9101–13. doi: 10.3390/molecules19079101
26. Xiong, H, Lin, S, Chen, L, Ouyang, K, and Wang, W. The interaction between flavonoids and intestinal microbes: a review. Foods. (2023) 12:320. doi: 10.3390/foods12020320
27. Li, F, Li, C, Chen, Y, Liu, J, Zhang, C, Irving, B, et al. Host genetics influence the rumen microbiota and heritable rumen microbial features associate with feed efficiency in cattle. Microbiome. (2019) 7:92. doi: 10.1186/s40168-019-0699-1
28. Chen, GJ, Zhang, R, Wu, JH, Shang, YS, Li, XD, Qiong, M, et al. Effects of soybean lecithin supplementation on growth performance, serum metabolites, ruminal fermentation and microbial flora of beef steers. Livest Sci. (2020) 240:104121. doi: 10.1016/j.livsci.2020.104121
29. Klein-Jobstl, D, Schornsteiner, E, Mann, E, Wagner, M, Drillich, M, and Schmitz-Esser, S. Pyrosequencing reveals diverse fecal microbiota in Simmental calves during early development. Front Microbiol. (2014) 5:622. doi: 10.3389/fmicb.2014.00622
30. Flint, HJ, and Bayer, EA. Plant cell wall breakdown by anaerobic microorganisms from the mammalian digestive tract. Ann N Y Acad Sci. (2008) 1125:280–8. doi: 10.1196/annals.1419.022
31. Barko, PC, McMichael, MA, Swanson, KS, and Williams, DA. The gastrointestinal microbiome: a review. J Vet Intern Med. (2018) 32:9–25. doi: 10.1111/jvim.14875
32. Conteville, LC, Da Silva, JV, Andrade, BGN, Cardoso, TF, Bruscadin, JJ, de Oliveira, PSN, et al. Rumen and fecal microbiomes are related to diet and production traits in Bos indicus beef cattle. Front Microbiol. (2023) 14:1282851. doi: 10.3389/fmicb.2023.1282851
33. Guilloteau, P, Martin, L, Eeckhaut, V, Ducatelle, R, Zabielski, R, and Van Immerseel, F. From the gut to the peripheral tissues: the multiple effects of butyrate. Nutr Res Rev. (2010) 23:366–84. doi: 10.1017/S0954422410000247
34. Ma, Y, Deng, X, Yang, X, Wang, J, Li, T, Hua, G, et al. Characteristics of bacterial microbiota in different intestinal segments of Aohan fine-wool sheep. Front Microbiol. (2022) 13:874536. doi: 10.3389/fmicb.2022.874536
35. Ignatyeva, O, Tolyneva, D, Kovalyov, A, Matkava, L, Terekhov, M, Kashtanova, D, et al. Christensenella minuta, a new candidate next-generation probiotic: current evidence and future trajectories. Front Microbiol. (2023) 14:1241259. doi: 10.3389/fmicb.2023.1241259
36. Liu, J, Xue, C, Sun, D, Zhu, W, and Mao, S. Impact of high-grain diet feeding on mucosa-associated bacterial community and gene expression of tight junction proteins in the small intestine of goats. Microbiol Open. (2019) 8:e745. doi: 10.1002/mbo3.745
37. Muniesa, PG, Gámez-Macías, PE, Soriano, EF, Samblas, M, Sáinz, N, and Moreno-Aliaga, MJ. Intestinal permeability, gut inflammation, and gut immune system response are linked to aging-related changes in gut microbiota composition: a study in female mice. J Gerontol: Series A. (2024) 79:1079–5006. doi: 10.1093/gerona/glae045
38. Lavrentyeva, E, Banzaraktsaeva, T, Kozyreva, L, Danilova, E, Tsyrenova, D, Dambaev, V, et al. Fecal microbiota and feeding habitats of nomadic indigenous animals (deer, yak, sheep and camel) in Baikal Siberia (Russia). Diversity. (2024) 16:52. doi: 10.3390/d16010052
39. Zhou, J, Wang, M, and Yi, X. Alteration of gut microbiota of a food-storing hibernator, Siberian chipmunk Tamias sibiricus. Microb Ecol. (2021) 84:603–12. doi: 10.1007/s00248-021-01877-7
40. Zhu, Y, Wang, Z, Hu, R, Wang, X, Li, F, Zhang, X, et al. Comparative study of the bacterial communities throughout the gastrointestinal tract in two beef cattle breeds. Appl Microbiol Biotechnol. (2020) 105:313–25. doi: 10.1007/s00253-020-11019-7
41. Thurston, B, Dawson, KA, and Strobel, HJ. Pentose utilization by the ruminal bacterium Ruminococcus albus. Appl Environ Microbiol. (1994) 60:1087–92.
Keywords: licorice–corn mixed silage, apparent nutrient digestibility, rumen fermentation parameters, gastrointestinal microbiota, Simmental cattle
Citation: Liu H, Tang L, Xu X, Gao Q, Sun Y, Li W, Lu D, Yu H and Jiang T (2025) Impact of mixed silages of licorice aerial parts and whole plant corn on nutrient digestibility, rumen fermentation, and gastrointestinal microbiota in Simmental cattle. Front. Vet. Sci. 12:1658831. doi: 10.3389/fvets.2025.1658831
Edited by:
Anum Ali Ahmad, University of Edinburgh, United KingdomReviewed by:
Mekonnen Tilahun, Chung-Ang University - Da Vinci Campus, Republic of KoreaYangyang Shen, Nanjing Agricultural University, China
Copyright © 2025 Liu, Tang, Xu, Gao, Sun, Li, Lu, Yu and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Houjun Yu, eWhqdW5zeXpAMTYzLmNvbQ==; Tao Jiang, amlhbmd0YW84ODg4ODhAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Haonan Liu1†
Haonan Liu1† Xingyu Xu
Xingyu Xu