- 1Key Laboratory of Animal Disease Clinical Diagnosis and Treatment Technology, College of Veterinary Medicine, Inner Mongolia Agricultural University, Hohhot, China
- 2National Key Laboratory of Agricultural Microbiology, Huazhong Agricultural University, Wuhan, China
- 3Department of Husbandry and Veterinary, Ulanqab Vocational College, Ulanqab, China
- 4Inner Mongolia Key Laboratory of Tick-Borne Zoonotic Infectious Disease, Department of Medicine, Hetao College, Bavan Nur, China
- 5Key Laboratory of Animal Disease Clinical Diagnosis and Treatment Technology, College of Life Science, Inner Mongolia Agricultural University, Hohhot, China
Internal parasitic infections are a persistent challenge for horse owners, in the absence of effective vaccines and the growing challenge of drug resistance, leading many researchers to view current control strategies as unsustainable. Despite slow progress over the past two decades, effective parasitic diagnosis remains crucial for controlling infections and preventing the growing issue of drug resistance. This review examines the research progress in serological and molecular biological diagnostic methods for major equine parasites. Currently, most diagnostic techniques are based on genes such as ITS1, ITS2, COI, and IGS, which have been applied to equine strongylids, including Strongylus spp., Cylicocyclus spp., and Cylicostephanus spp. These methods are particularly suitable for large-scale epidemiological studies and rapid species identification. Although many diagnostic methods have been developed, most remain confined to laboratory research and have seldom been used for real-time field diagnostics. Future research should prioritize precise diagnostic methods and clinically applicable alternatives. Additionally, whole genome sequencing has been widely used in eukaryotes for population genetics and the development of diagnostic markers. However, comprehensive genomic data on parasitic species infecting equines is still limited. With the decrease in sequencing costs in the post-genomic era, a growing number of genome assemblies are expected to be released soon. These genome maps will offer comprehensive genomic data to identify specific genetic markers and variations associated with parasitic infections, enabling more accurate and reliable diagnostic techniques. High-throughput sequencing technologies will significantly accelerate progress in equine parasitology research and the development of diagnostic tools like enzyme-linked immunosorbent assay (ELISA) and TaqMan quantitative PCR (qPCR). At the same time, this paper also provides some insights into the research direction of sustainable control programs and equine parasite diagnostic methods.
1 Introduction
It is estimated that approximately 116 million equines (donkeys, horses, and mules) are distributed globally. According to FAO data, the number of equines has remained relatively stable over the past 50 years (1961–2019). World Mapper estimates that the global horse population will reach about 60 million by 2024. Despite millions of years of biological evolution, Equus remains highly susceptible to multiple internal parasites due to its unique living habits and feeding conditions. More than 60 common parasites, including protozoa, trematodes, cestodes, and nematodes, are known to cause parasitic infections (1). In most cases, equines exhibit symptoms similar to those of other livestock when infected with internal parasites, such as abdominal swelling, nutritional deficiencies, and weight loss. These symptoms can be assessed through body condition scoring, and are often accompanied by digestive disorders, poor appetite, and stunted growth, especially in immunocompromised foals (2). During gastrointestinal parasitic infections, equines may experience disruptions in their intestinal microbiota and impaired nutrient absorption, in addition to clinical signs. This results in increased feed conversion ratios and a higher demand for proteins and amino acids. Incorrect feeding of concentrates and changes in diet formulation may also result in the occurrence of “Friday disease” (3, 4).
For a long time, parasitic detection methods have traditionally relied on microscopic examination and necropsy of collected parasite tissues (5, 6), fecal samples (7, 8), and blood smears (9–12), thus enabling the diagnosis of internal and external parasitic infections in livestock. Some equine parasites, such as Parascaris equorum (P. equorum), Anoplocephala perfoliata (A. perfoliate), and Oxyuris equi, expel their eggs with feces, while others, such as the horse stomach bot fly, are expelled only when the larvae mature and the infection load becomes substantial. Furthermore, parasitic examinations of horses require trained personnel to collect feces and perform microscopic examination, necessitating the individual restraint and examination of each horse, which makes it impractical for analyzing large populations. Due to the lack of simple and effective internal parasite detection methods, deworming in equines is typically performed 2–4 times per year, depending on their use. Since the discovery of anthelmintics, animal husbandry has largely relied on the frequent use of chemical drugs, such as Benzimidazoles (BZDs), macrolides (MLs), and tetrahydropyrimidines, for year-round parasitic control (13, 14). Most competitive sport horses undergo deworming four or more times a year to enhance performance, which has contributed to the growing issue of resistance to deworming drugs. Anthelmintic Resistance (AR) refers to the inability of previously effective drugs to kill parasite populations when exposed to therapeutic doses (15). Over time, sensitive parasites transmit drug resistance to their offspring through genetic mechanisms, leading to the loss of sensitivity in previously susceptible populations (16). As the livestock industry has rapidly developed, the prevalence of drug resistance has become particularly severe, especially in cattle (17, 18), sheep, and goats (19, 20), which are economic animals. In recent years, an increasing number of clinical and epidemiological reports have described the occurrence of AR in various parasites of equines (21–23). Global equine parasitic epidemiological surveys report resistance to these four classes of anthelmintics/dewormers in over 70 species of parasites, including nematodes, Gasterophilus spp., and P. equorum (24–26), further highlighting the need to reassess parasite control strategies.
While there are differing opinions on the mechanisms behind the development of drug resistance, most researchers agree on the importance of early monitoring of parasitic infections. Early detection and control of parasitic infections and AR are critical. Many proposed deworming programs recommend regular population-wide testing using fecal samples; however, this poses a challenge for equine-specific parasites that are not detectable through fecal egg analysis. The rapid evolution of AR necessitates the urgent development of efficient and standardized diagnostic methods for internal parasites (27). Early and accurate identification of parasitic infections in individuals, along with an understanding of their development mechanisms, will help implement sustained and effective deworming programs and may assist in slowing the development of resistance. Additionally, the development of in vitro diagnostic methods is mostly based on the development of host-parasite immune-related protective antigens, which will also contribute to the development of new anthelmintic drugs and vaccines. In the foreseeable future, parasites in the body will still need to be controlled using chemical drugs for a long time. Although many researchers are exploring some alternative strategies to combat parasitic infections, such as breeding resistant varieties, increasing protein supplementation, and implementing “low-parasite” pasture strategies, most methods to counter resistance focus on boosting the host's immunity to enhance parasite resistance. Research has shown that adding certain bioactive substances to the diet can play a significant role in reducing parasitic infections and preventing AR development (28). However, while this approach can mitigate some of the impact parasites have on livestock traits, it may also lead to increased feed consumption. Currently, many in vitro diagnostic methods are used in parasitic research on ruminants such as cattle and sheep, and these methods can also detect host AR. However, related studies have few examples of parasites in horses, such as P. equorum, Strongylus vulgaris (S. vulgaris) and Gastrophilus spp., which can also allow us to obtain a lot of reference from the study of ruminants in the diagnostic test of horse parasites and the development mechanism of AR. However, it is worth thinking that the delay of related research also further illustrates that the development of related detection methods, with many areas in need of improvement. The purpose of this review is to systematically describe the diagnostic methods of common parasites in horses, focusing on in vitro detection techniques based on serology and molecular biology, and to explain their application and scientific strategies in diagnosing horse parasites, aiming to support the prevention and control of AR in horses. Additionally, this review presents opinions on the development of the horse industry and the sustainable control of parasites in equine animals in the future.
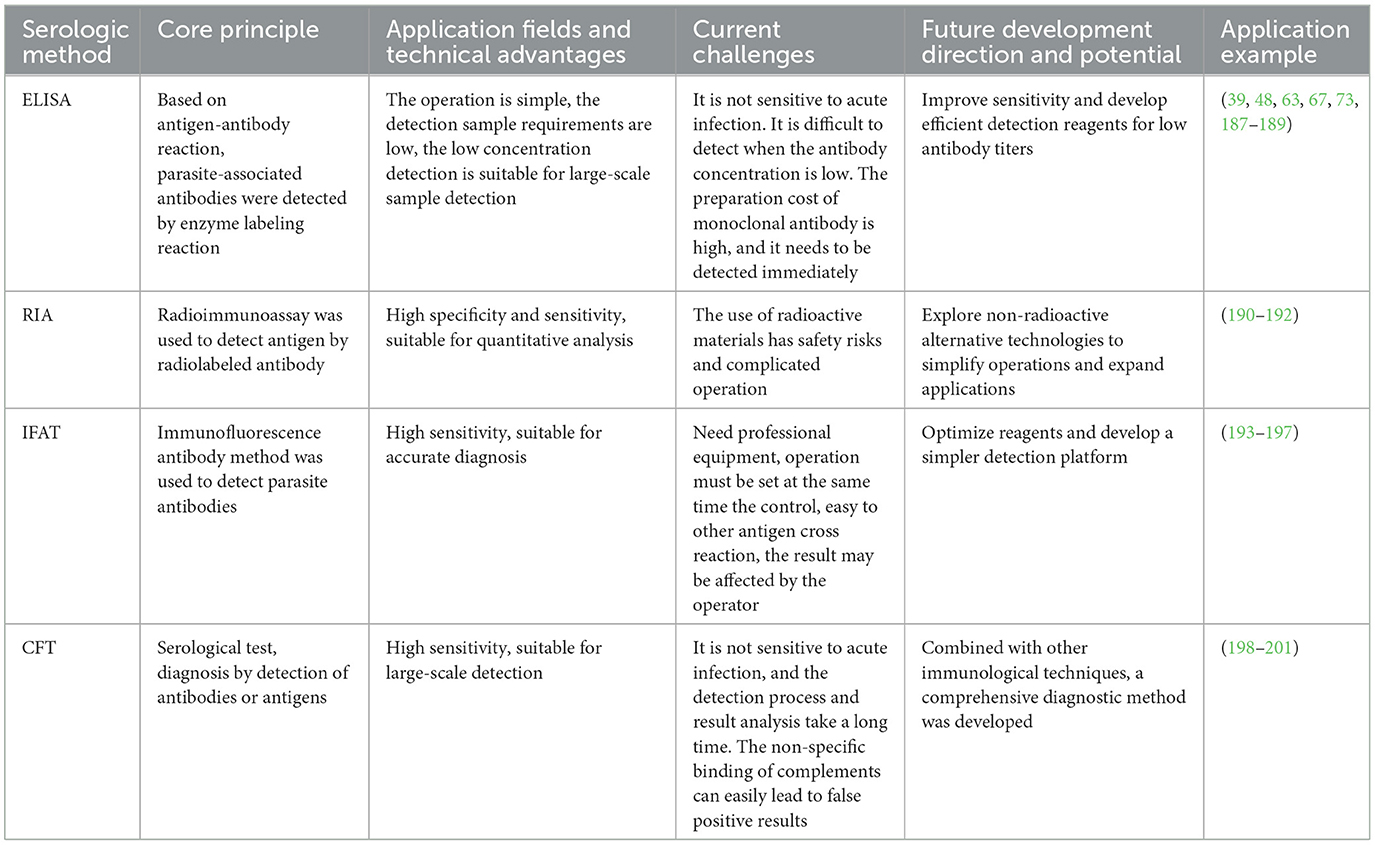
2 Serological detection
2.1 Overview of serological detection and its role in the diagnosis
Serology mainly achieves the purpose of detection and diagnosis by detecting antibodies in serum, plasma, saliva, semen, or cerebrospinal fluid of the host (29, 30). In some clinical settings, serological testing remains the gold standard for diagnosing parasitic infections when biological samples cannot be directly obtained. Although serological analysis is similar to microscopic examination in terms of operational convenience and test turnaround time, for parasites such as Babesia and Theileria, which are difficult to accurately identify on blood smears, or some horses infected with Trypanosoma cruzi, these individuals may be mild or asymptomatic, serological analysis can show higher sensitivity and specificity, which is crucial for the diagnostic process (31). The parasitic stage in the host triggers both nonspecific and specific immune responses, which are influenced by the location of the parasite within the host, the host's health condition, and the characteristics of the parasite antigens. Therefore, a deep understanding of the host's immune response is critical in the development of serological diagnostic methods (32). Equine parasites are typically obligate parasites that remain in the host for extended periods. Their life cycles primarily occur within the host, with eggs or adult parasites expelled in the feces. Among the current diagnostic methods, serological diagnostics serve as an effective alternative to parasitological examinations and autopsy diagnoses. During the larvae are in the migration stage in the host or fail to find signs of parasitic infection on the body surface, serological detection is a simple and effective method for rapid diagnosis of parasitic infection. As large animals, horses typically do not experience significant pain or noticeable damage to the host's body from internal parasitic infections, making it difficult for horse owners to determine whether their horses are infected with parasites through daily observation. However, during the early stages of parasitic infection (especially during the larval development stage), the establishment of early diagnostic methods could provide strong support for timely drug treatment and intervention, preventing or mitigating the development of resistance.
2.2 Host immune mechanisms in parasitic infections
At present, studies on ruminants infected with liver flukes have shown that during the parasitic infection of the host, both humoral and cell-mediated immune responses occur, and there is a complex interaction between the parasite and the host's immune system after infection (33). Common equine parasitic nematodes such as P. equorum, Strongyloides westeri, and Gastrophilus spp. regulate the immune system by releasing soluble mediators. These mediators interact with the host's immune cells and molecules, leading to the expression of secretory proteins and carbohydrates. In nematode studies, it has been clarified that cysteine and serine protease inhibitors (cystatins and serpins) can block T-cell responses, regulate macrophage function, and alter the activity of eosinophils and neutrophils (34). The regulation of the host immune system is both rapid and sustained, allowing parasites to persist for extended periods within the host. During the process of maintaining immune system homeostasis, both parasites and host-derived damage-associated molecular patterns are crucial for protective immune responses and tissue repair mechanisms. In studies involving fly larvae parasites, the life cycle and parasitic stages of Hypoderma bovis (H. bovis) share many similarities with certain obligate equine parasites, such as the horse stomach bot fly. The larvae of H. bovis can penetrate the host's skin after hatching, triggering an immune response. Currently, immunological techniques detecting excretory-secretory antigens (ESA) specific to parasites can identify anti-parasitic antibodies within 3–5 weeks post-infection (35, 36). Although natural ESA are immunogenic, their accuracy and sensitivity are limited, especially in low-intensity infections, where they are particularly ineffective, especially in large animals like cattle and horses, where such methods provide only coarse diagnostic results. In contrast, recombinant antigens exhibit greater sensitivity and specificity than natural crude antigens. Their standardized production not only improves yield but also has significant cost-control implications (37). However, despite its diagnostic value, serological testing faces limitations in large-scale epidemiological surveys. For instance, after horses undergo systematic deworming treatment, antibody titers remain elevated for an extended period, which can significantly affect serological test results and lead to a considerable number of “false positives” (38).
2.3 Limitations and challenges of current serological methods
Currently developed serological diagnostic methods include the complement fixation test (CFT), agglutination test (AT), indirect immunofluorescence assay (IFA), Western blot, and enzyme-linked immunosorbent assay (ELISA). These methods significantly enhance diagnostic sensitivity. A highly sensitive and specific ELISA method for detecting Fasciola hepatica has been developed, based on recombinant F. hepatica-specific antigens (39). These antigens include enzymes essential for the parasite's survival within the host, such as glutathione S-transferases (40–42). Research on has shown that after initial infection with H. bovis larvae, first-stage larvae (L1) produce trypsin and chymotrypsin. These proteases regulate the immune response by inhibiting both specific and nonspecific host reactions (43, 44). IgG antibodies in cattle typically appear around 45 days after H. bovis infection, peaking at approximately 18 weeks. This is critical for implementing early serological diagnostic strategies. Although the larvae emerge from the host's back skin during the third larval stage (L3), antibodies remain at a high level for about 4 months. Diagnosing during this period is essential for formulating an effective, scientifically informed deworming strategy (45). However, the prevalence of parasitic diseases is often associated with poor pasture management or prolonged exposure to parasitic-contaminated environments. Most horses on pastures or farms are wild and free-roaming, which presents significant challenges for tasks such as restraining horses and collecting blood samples. The collection process may result in varying degrees of injury to the horses. Additionally, these diagnostic methods also have inherent limitations, such as cross-reactivity, which can lead to false-positive results.
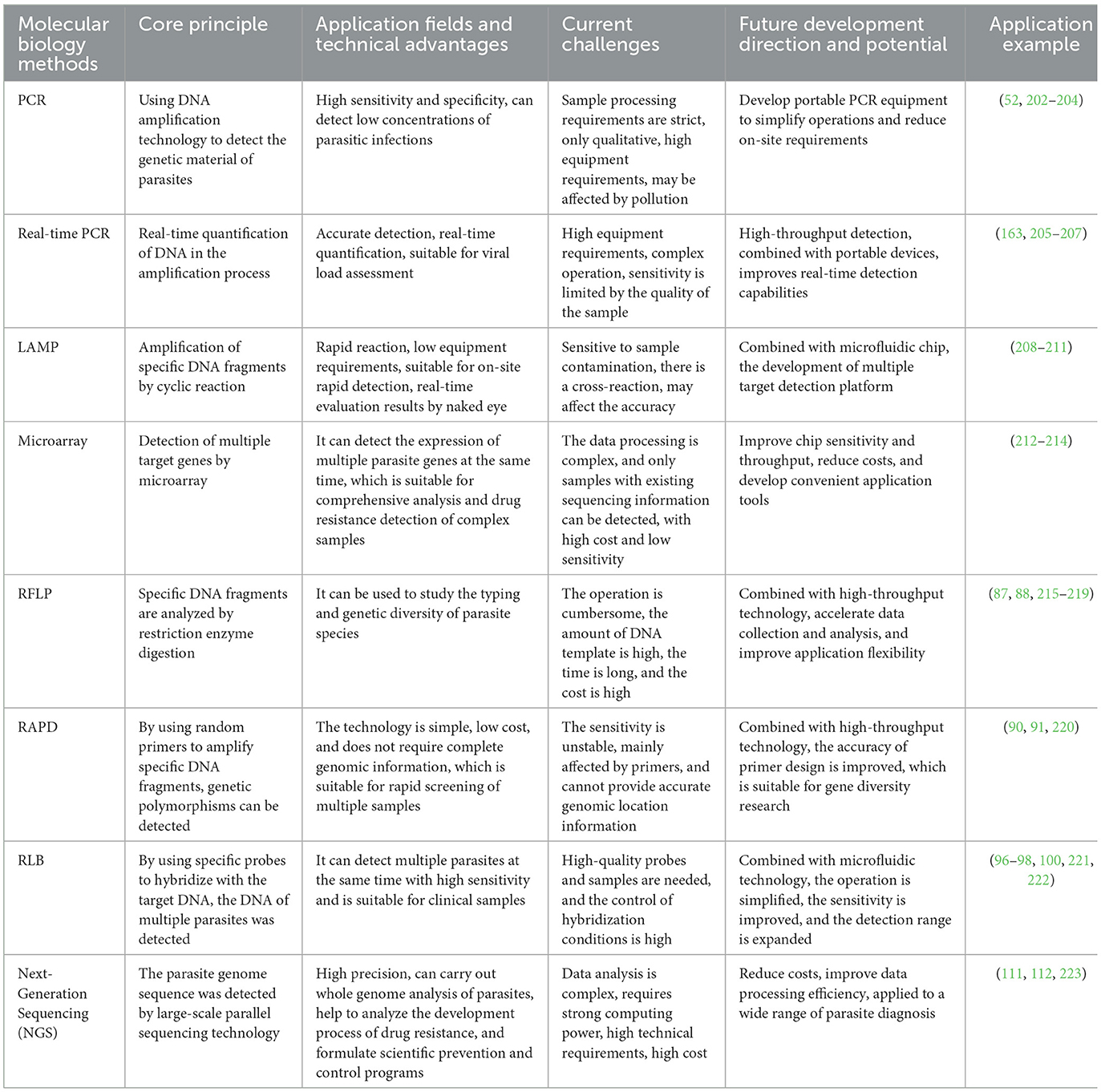
3 Molecular biology detection
3.1 Limitations of traditional diagnostic methods
Since the advent of the optical microscope, microscopic examination has been considered the most commonly used and economical gold standard for clinical parasitic diagnosis. Parasitological research originated from taxonomic studies, where researchers identified species by examining the morphological characteristics of parasites under the microscope. Although simple and cost-effective, this method has several limitations. For instance, it is labor-intensive, time-consuming, requires highly trained personnel, and makes it difficult to distinguish subtle differences between closely related species (46), During the microscopic examination of roundworms and nematodes, FEC values do not always correlate with worm burden (47), leading parasitologists to increasingly explore molecular biology techniques, such as gene amplification. As previously mentioned, immunodiagnostic tests have significant limitations. Currently, there are no commercially available or FDA-approved antibody tests for diagnosing parasitic diseases, such as cryptosporidiosis, schistosomiasis, and African trypanosomiasis. Commonly used antigen preparations include crude proteins, recombinant purified proteins, adult parasites, eggs, and non-standardized test antigens, leading to significant variability in experimental outcomes. In the development of diagnostic methods for Theileria haneyi (T. haneyi), since T. equi and T. haneyi are closely related, the current equi merozoite antigen 1 -based competitive ELISA used in the U.S. detects T. equi but not T. haneyi. Previous studies on serological diagnostic methods have found that immunoblot results show antigen cross-reactivity between species within the same genus, especially in regions where more than one parasite is prevalent, leading to no results or false-positive results (48). Furthermore, when the number of parasites in the host is low, serological tests tend to have lower sensitivity, which poses significant challenges for detection. Studies have shown that circulating antigens may persist in the host for some time after systematic deworming treatment, leading to false-positive results when compared with parasitic test outcomes. Therefore, the most critical time to apply this diagnostic method is during the acute phase of parasitic infection, ensuring the accuracy and reliability of the test results (49–51).
3.2 Emerging molecular techniques for parasitic diagnosis
With the ongoing development of molecular biology techniques, polymerase chain reaction (PCR) technology has been increasingly applied to parasite detection. In addition to traditional PCR, multiplex PCR and nested PCR techniques have also emerged. Multiplex PCR can detect multiple sequences in a single reaction, enabling the simultaneous detection of various parasites (52). Several molecular biology-based diagnostic methods, including amplified fragment length polymorphism, restriction fragment length polymorphism (RFLP), and real-time quantitative PCR (qPCR), have been utilized in parasitic detection. Loop-mediated Isothermal Amplification (LAMP) and Luminex Multi-Analyte Profiling (xMAP) technologies are emerging as novel methods for parasitic disease diagnosis. LAMP is a rapid, isothermal DNA amplification method that operates at a constant temperature (60–65 °C) using Bst DNA polymerase. It forms stem-loop structures containing repeated target DNA sequences, enabling the amplification of DNA to high copy numbers within 1 h. Nucleic acid-based molecular techniques provide superior sensitivity and specificity compared to traditional diagnostic tests. In microscopic parasitology, species identification based solely on egg observation is challenging, especially for certain parasite intermediate hosts. Equine fasciolosis is a significant re-emerging parasitic disease of horses, donkeys, and mules, caused by trematodes of the Fasciola genus, infecting animals through the ingestion of encysted metacercariae found on plants. To date, no diagnostic method is considered the gold standard for detecting equine fasciolosis (53). Molecular biology techniques can detect parasitic infections sensitively from small host samples, such as blood and saliva, even in asymptomatic hosts, making them highly valuable for early diagnosis and the detection of subclinical infections (54). qPCR uses fluorescently labeled probes or double-stranded DNA-specific fluorescent dyes to analyze the fluorescence signal of amplification products in real time, eliminating the need for complex electrophoresis steps (55). Due to its rapid speed, high specificity, excellent sensitivity, broad dynamic range, and low risk of cross-contamination, qPCR is widely used in the development of diagnostic methods for various diseases. It is particularly favored by researchers (56, 57). Additionally, RT-qPCR can be used to study parasite vector capability, host infectivity, diagnostics, and drug efficacy (22). TaqMan probe-based qPCR utilizes a non-extendable DNA probe that hybridizes to a specific region within the amplicon, ensuring high specificity and protecting quantitative precision by preventing the amplification of nonspecific molecules (58). This method enables accurate assessment of infection intensity by the same parasite in different hosts, with exceptional specificity and quantitative capability. This characteristic makes it an ideal tool for assessing host infection loads, providing a scientific basis for developing individualized deworming treatment plans. Molecular biology-based diagnostic techniques allow precise quantification of infection levels, providing important references for developing more effective and accurate deworming treatment plans, thereby playing a significant role in improving treatment efficacy and reducing drug misuse.
4 Application of serological techniques in parasite diagnosis of equids
4.1 Importance of serological techniques in field diagnosis
For most equine parasites, the humoral immune response typically precedes clinical symptoms during the initial infection. Horses often display notable behavioral changes only when they experience abdominal pain or more severe limb and hoof disorders. Parasitic infections are challenging to diagnose through routine visual inspection. During microscopic examination, parasites such as F. hepatica, Clonorchis sinensis, and Strongyloides can be detected in fecal samples. However, due to intermittent shedding or sampling limitations, eggs per gram (EPG) are typically very low when using the modified McMaster method (56). A retrospective study found that horses with EPG values below 500 had significantly fewer parasites in their intestines than those suggested by the fecal egg count (FEC). In summary, fecal egg tests have issues with missed diagnoses and instability. Serological methods may exhibit cross-reactivity between phylogenetically closely related species, and combining immunochroma-tographic tests with rapid immunoenzymatic assays (RIA) can greatly enhance the specificity and sensitivity of these tests, improving diagnostic accuracy (59, 60). Most serological methods for detecting equine parasites rely on ELISA (61).
4.2 Serological diagnosis of Cyathostomins
Cyathostomins (small strongyles) are the most prevalent and significant parasites affecting horses (62). Over the past two decades, the Jacqueline B. Matthews team at the Moredun Research Institute in the UK has made significant progress in developing in vitro diagnostic methods for Cyathostomins. Early studies identified two diagnostic antigens (20 and 25 kDa) and developed an ELISA method based on serum-specific IgG(T) responses (63–65). Although this method exhibits low cross-reactivity with S. edentatus and S. vulgaris, it remains valuable as a diagnostic antigen. Subsequent studies involved constructing a cDNA library from mixed Cyathostomins species and immunoscreening, resulting in the identification of the gut-related larval antigen Cy-GALA protein, which acts as an immune marker for the developmental larval stage. This antigen is exclusively expressed during the larval stage and is the first diagnostic method capable of detecting the latent phase of Cyathostomins (66). Further research focused on recombinantly expressing two proteins from 14 Cyathostomins species, leading to the selection of an antigen cocktail (Cocktail 3; CT3), which, based on specific recombinant antigen IgG(T), provides a standard curve to generate a “serum score” for each horse. The results demonstrated excellent detection performance (receiver operating characteristic area under the curve values >0.9), making it suitable for diagnosing horses infected with over 1,000–10,000 Cyathostomins in the mucosa and intestines, with sensitivity and specificity greater than 90 and 70%, respectively, making it commercially viable (67).
4.3 Serological diagnosis of A. perfoliate
Early studies on A. perfoliate involved serological research on scolex antigens (68), whole worm extracts, and excretory-secretory (ES) antigens (69). However, crude protein antigens, although easy to prepare, complicate the protein composition, with at least 14 different proteins identified, predominantly low molecular weight proteins in the 31–45 kDa range. Among the crude antigen mixture, two highly immunogenic proteins (12 and 13 kDa) were identified. Researchers measured the negative threshold values for anti-12/13 kDa IgG and IgG(T) ELISA systems, with critical OD values of 0.123 and 0.103, and sensitivity values of 56 and 62%, respectively. This suggests that anti-12/13 kDa IgG(T) may more accurately predict infection intensity in A. perfoliate (70). However, this method also yielded negative results in horses with low infection intensity, suggesting that diagnostic factors in parasitic diseases are not solely based on the parasite presence (especially in large animals) but also depend on the host's infection intensity (71). Among common equine parasitic infections, Anoplocephala spp. exhibit mixed infections, with A. perfoliata having significantly higher infection levels than other species. Immunological studies have yet to resolve antigen cross-reactivity between A. perfoliata and A. magna (72). No cross-reactivity has been observed between 12/13 kDa ES antigens and other parasite antigens such as A. mamillana and Gastrophilus spp. (70). Currently, commercial diagnostic kits for equine tapeworm, including the Tapeworm Blood Test and Saliva Test launched in 2014 by Austin Davis Biologics Ltd., are based on these 12/13 kDa ES antigens. The saliva test has a sensitivity of 83% and specificity of 85%, with no misdiagnoses when detecting infections with more than 20 adult tapeworms, yielding results comparable to serological tests (73).
4.4 Serological diagnosis of Gastrophilus
Jin et al. developed an indirect ELISA detection method using crude L3 antigens from Gastrophilus pecorum and salivary gland antigens. Only salivary gland antigens could be used for fecal sample detection, exhibiting high specificity (100%) and sensitivity (83.33%), while crude protein may non-specifically bind with other substances in the feces, hindering direct antigen detection (74).
4.5 Serological diagnosis of P. equorum
P. equorum, a nematode that threatens foal health, begins shedding eggs approximately 10–15 weeks after the initial infection (75). Serological diagnostic tools are crucial for assessing the likelihood of latent P. equorum infections. Currently, many parasite ES products from Ascaris species are utilized in antigen-based diagnostic development (76), including those from Ascaris. Lumbricoides (77) and Ascaris suum (78). In previous studies on P. equorum ES products, the fifth stage (L5) of P. equorum has a molecular weight range of 12–189 kDa, while L2/L3 products range from 12 to 94 kDa. Blood samples from two naturally infected foals showed antigen recognition at approximately 19, 22, 26, and 34 kDa for ES products in Silver-stained SDS-PAGE analysis (79). However, IgG(T) antibodies were not detected in foal serum prior to colostrum ingestion.
4.6 Challenges and considerations in serological testing
According to the performance assessment by Gasser et al. (80), ES antigens, when used as the capture layer in ES-ELISA, show significantly higher sensitivity than the other two antigens, although this increased sensitivity compromises antigen specificity. Acquiring a detailed treatment history is especially important for serological testing. Common deworming drugs, such as MLs, do not provide prolonged deworming effects, and IgG levels typically decrease over a period of 6 months following treatment (81, 82). It is generally recommended to conduct serological testing at least 4 months after treatment. A key issue in serology is the potential for antibody cross-reactivity. Although current serological diagnostic methods have achieved acceptable specificity, identifying and accurately expressing target antigens, as well as ensuring their high quality, are critical for their diagnostic application (83). We hope that equine parasite serology will advance to the same level of advancement as virology and bacteriology. Table 1 summarizes the advantages of various serological detection methods in parasite diagnosis and the main challenges faced by the current technology. Additionally, the current research progress and factors influencing future developments are summarized.
5 Application of molecular biology techniques in parasite diagnosis of equids
5.1 Traditional PCR-based molecular diagnostic methods
In recent years, significant progress has been made in parasitic research of great public health and veterinary importance, particularly in phylogenetic systematics and diagnostics, due to ongoing advancements in life sciences and technology. The application of molecular biology has introduced new approaches and methods for the early diagnosis and precise control of parasites. Studies aimed at differentiating A. magna and A. perfoliata have shown that both parasites, whether in crude or recombinant protein form, contain low molecular weight immunoreactive components in their antigens. These components are recognized by Anoplocephala-positive sera at the genus level, but not at the species level (84). Currently, no reliable serological assays are available to differentiate A. magna from A. perfoliata, primarily due to the absence of species-specific antigens. This diagnostic limitation highlights the critical importance of molecular-based approaches in differentiating these closely related species. Several PCR-based diagnostic methods have been developed for equine parasite detection, primarily utilizing ITS1 and ITS2 sequence regions. These two sequences, employed as phylogenetic molecular markers in molecular classification and phylogenetic diagnosis, have significantly corroborated taxonomic frameworks derived from traditional morphological and life history analyses (85). To confirm infections in equines caused by S. vulgaris, S. equinus, A. perfoliata, and Parascaris species, fecal egg counts are commonly conducted, but the identification of the parasite species causing the infection cannot be determined by egg morphology alone. Accurate diagnosis often requires culturing the collected feces for a certain period to allow development to the third larval stage (L3). DNA fingerprinting techniques, such as restriction fragment length polymorphism (RFLP), single-strand conformation polymorphism (SSCP), and random amplified polymorphic DNA (RAPD), have been widely used in the development of molecular diagnostic methods for equine parasites, due to their speed, cost-effectiveness, and the fact that they do not require complete genome information. The fundamental principle of RFLP involves the digestion of DNA fragments from target genes using specific restriction endonucleases. Genotypes are identified based on the electrophoretic patterns of the resulting digestion products, eliminating the need for further sequencing or sequence alignment analysis (86). In 1995, Campbell et al. first used PCR-RFLP technology to amplify the ITS2 region of Strongylus spp., and the results indicated that the ITS2 sequence, as a diagnostic target gene, exhibits low intraspecific variation and high interspecific variation. This study laid the foundation for the ITS2 region as the basis for molecular identification. Additionally, RFLP technology in this study also provided a means of separating amplified products using agarose gel based on fragment size, allowing for rapid identification of Strongylus spp. without sequencing or sequence analysis (87). In subsequent studies, Gasser and Monti optimized PCR-RFLP and PCR-SSCP methods to distinguish 16 species of equine strongyles (88, 89). PCR-RAPD technology has been widely used in the classification of numerous parasites (90, 91). In equine parasitic detection, this technology has been applied to define species of two Cylicocyclus species, such as C. insigne and C. elongatus, which are morphologically very similar but can be accurately distinguished, further demonstrating that this method is suitable for closely related species. Over the past 30 years, researchers have primarily developed diagnostic methods for equine parasites based on ribosomal RNA genes, particularly the ITS and IGS (Intergenic Spacer) regions. In studies of equine strongylids, by comparing the performance of two molecular barcodes (COI gene and ITS-2 rDNA gene) in different biological type samples, it was found that although the results of the two barcodes were fairly consistent, the PCR amplification efficiency of the COI gene was lower than that of ITS2, particularly in certain strongyle species (e.g., Cylicostephanus calicatus and Cylicostephanus longibursatus), where the COI barcode amplification efficiency was less than 70%. In group identification, the COI gene, due to its higher genetic diversity and larger sequence variation, can lead to PCR amplification bias, making it difficult to identify specific life cycle stages of some species and affecting the final sequencing results (92).
5.2 Emerging molecular diagnostic approaches
Hodgkinson et al. (93, 94) used Southern blot to validate the design of digoxigenin (DIG)-labeled probes targeting the IGS conserved region of four highly prevalent species (C. ashworthi, C. nassatus, C. longibursatus, C. goldi), achieving molecular identification of single eggs, L3 larvae, and L4 larvae. The research team later developed a PCR-ELISA method, which was completely consistent with the Southern blot results and could identify low-abundance species (e.g., C. catinatum, which accounted for only 1.7%). This method was used to assess the therapeutic efficacy in horses treated with BZDs. Compared to the RFLP probe method, it was more suitable for analyzing polymorphic fragments (95). The widely used Reverse Line Blot (RLB) detection method for diagnosing strongyloid species currently involves non-radioactive hybridization of PCR amplicons with different oligonucleotide probes in a single reaction, allowing for the detection and identification of more than 16 strongyloid species simultaneously (96–98). This method has also been widely applied to study the relationship between drug resistance and changes in Cyathostomin population structure, thereby exploring the potential mechanisms behind the shortened egg reappearance period after treatment (99, 100). Martins amplified the ITS2 DNA fragment of S. vulgaris by PCR and compared it with the results of fecal culture L3, demonstrating a lower consistency, further confirming that PCR diagnostic methods have become the most effective tool for diagnosing S. vulgaris (101). Nielsen developed a dual-labeled TaqMan qPCR assay specifically targeting S. vulgaris, which exhibited no cross-reactivity with other equine parasitic nematodes. This assay demonstrated a detection limit of 0.5 egg equivalents, providing a precise and efficient tool for diagnosing S. vulgaris infections. Similarly, Zhou et al. developed a novel real-time quantitative PCR assay based on the TaqMan-MGB probe, specifically for detecting T. haneyi, a pathogen causing equine piroplasmosis. The assay demonstrated excellent specificity and sensitivity, with a detection limit of 1 × 102 copies/μl, and was validated by comparison with nested PCR. This new diagnostic method promises to enhance early detection and control of T. haneyi infections, complementing existing PCR techniques for Theileria equi (T. equi), Babesia caballi (B. caballi). The LAMP-based diagnostic method for T. equi and B. caballi has been developed, providing rapid on-site results within 1 h (102). Fecal samples, the most accessible for on-site diagnosis, contain more potential inhibitors than blood samples, which can reduce the sensitivity and accuracy of the LAMP assay. Furthermore, fecal samples often require more rigorous pretreatment and ultrapure DNA extraction (103, 104). The advancement of microfluidic chip technology has enabled the successful application of isothermal amplification methods for detecting various veterinary parasites (105–107). Chen et al. (108) developed a microfluidic LAMP system that can identify five parasites. This method integrates sample pretreatment, biological separation, biochemical reactions, and signal analysis into microchips just a few square centimeters in size, achieving both miniaturization and automation in parasite detection (109, 110).
5.3 Next-generation sequencing for comprehensive parasitic diagnosis
With the ongoing development of high-throughput sequencing technology, next-generation sequencing (NGS) has become widely applied in the diagnosis and research of equine parasites. Mitchell used NGS technology to conduct qualitative and quantitative analysis of the species and egg abundance in equine fecal samples, replacing traditional larval culturing and necropsy methods. This also marks the first recorded instance of the equine “nemabiome” (111). Ghazanfar et al. combined NGS technology with the modified McMaster technique to study the prevalence and diversity of gastrointestinal nematodes in Australian racehorses. Compared to traditional microscopic examination or PCR methods, NGS proved more efficient and precise, identifying 23 strongylid species, including 18 cyathostomins and five large strongyle species. Using high-throughput data from NGS, the species abundance of various parasites in fecal samples was analyzed. The three most abundant species of small strongyles were Cylicostephanus longibursatus (28%), Cylicocyclus nassatus (23%), and Coronocyclus coronatus (23%), which together accounted for 95.8% of the entire parasite community. The study also revealed the diversity of nematode communities across different climatic zones and age groups. In foals and weanlings, the most common strongylid species was Cylicostephanus longibursatus, with relative abundances of 35.3% in foals and 34.1% in weanlings. Nematode community diversity in the Non-Seasonal Rainfall Zone and Winter Rainfall Zone was significantly higher than in the Mediterranean Rainfall Zone (112). Recently, Hamad et al. employed ITS-2 rDNA metabolic barcoding sequencing technology with the Illumina MiSeq platform to study the strongylid nematode populations on a large equine farm in Thailand. By analyzing fecal samples from 57 horses on the farm, they successfully identified 14 strongylid species, including Cylicocyclus nassatus and Cylicostephanus longibursatus. The study revealed the complexity of the strongylid nematode community and the relative abundance of species, while also validated the quantitative potential of high-throughput technology in equine parasite detection. It provides an important molecular data foundation for future drug resistance monitoring and parasite management (113, 114). Table 2 summarizes the advantages of widely used molecular biological detection methods in parasite diagnosis, along with the main challenges faced by current technologies. It also outlines the current research progress and factors influencing future developments.
6 Pasture management and sustainable control strategies for equine parasites
This review provides a clear summary of current serological and molecular biological methods for detecting equine parasites. Traditional fecal examination techniques remain a key component in the development of detection methods. Serological and molecular biology-based diagnostic tools are crucial in the early detection of parasitic infections in horses. However, these methods face several limitations regarding their development and practical field application. Table 3 presents a comparative analysis of the two diagnostic methods, highlighting key factors such as sensitivity, field applicability, and commercial viability. These factors are vital for the selection of appropriate clinical diagnostic tools.
Gastrointestinal parasites, particularly cyathostomins, are among the most common equine parasitic infections and are prevalent in global epidemiological surveys, with over 50 species identified (115). In pastures where horses are free-ranging, the infection rate can reach up to 100%, with most horses carrying 3–4 different internal parasites. Due to the misuse of deworming drugs and unscientific medication practices, numerous failures in control measures have occurred, and the failure of recommended treatment protocols has indicated widespread resistance to deworming medications. This poses new challenges in parasite control and resistance management. Effective parasite control requires planning and management at each developmental stage. The use of broad-spectrum dewormers, such as ivermectin, should be assessed according to pasture management conditions, reducing deworming frequency and drug dosages while avoiding frequent large doses. Scientific recommendations on whether horses need deworming should be provided through in vitro diagnostic methods for equine parasites. Growing resistance has created new challenges for farm and ranch owners, urgently necessitating the re-evaluation of management strategies and the formulation of scientifically sound control plans. Large-scale fecal collection and analysis should be performed, with control plans based on diagnostic results. Horses can be grouped according to parasite species and number, and high- and low-risk areas should be established. High-risk areas include high-density breeding zones and young horses (2–5 years old) raised on low-quality forage, with high FEC and initial indications of AR. Low-risk areas consist of low-density breeding or individually housed horses, fed high-quality, clean pasture, and exhibiting low FEC as confirmed by parasite testing. Horses in low-risk areas only need to maintain good feeding environments and fodder hygiene. With veterinary evaluation, the frequency of fecal egg count monitoring can be reduced in these areas. Grazing management is especially critical for herds where parasite control is failing. If rotational grazing is used, the pasture should be regularly cleaned to prevent overgrazing, which can effectively control the risk of parasitic infection.
For the past half-century, the horse industry has depended on the use of chemical drugs for deworming. In recent years, an increasing amount of research shows that uncontrolled single or frequent rotations of drugs accelerate the emergence and spread of resistant populations, rendering a limited number of drugs ineffective (116). With the rapid development of the global equine and animal husbandry industries, sustainable pasture management strategies have become a key focus. In ruminant research, many natural plants or feed additives similar to probiotics have been found to combat parasites, but research in equines is relatively limited. Beg et al. administered water-soluble herbal extracts of Nigella sativa (Kalwanji) seeds, Fumaria parviflora (Burgi Shatara) leaves, and Fumaria macrophylla (Jantaan) leaves via nasogastric tube on Day 0 and 18, with doses of 0.05, 0.10, and 0.15 g/kg, respectively, and evaluated efficacy based on the eggs per gram of feces (EPG). The results showed that all herbal extracts significantly reduced EPG after the first treatment on Day 18, with the best results at Day 28, demonstrating that N. sativa has strong anthelmintic effects against P. equorum (117). Payne et al. conducted crude extractions from 37 native Australian plants and tested their in vitro effects on the development of Cyathostomins. Seven plants, including Acacia baileyana, Acacia melanoxylon, Acacia podalyriifolia, Alectryon oleifolius, Duboisia hopwoodii, Eucalyptus gomphocephala, and Santalum spicatum, completely inhibited the development of Cyathostomins larvae, with an IC50 value of just 30.9 μg/ml to achieve 50% developmental inhibition. Other tested plant extracts displayed varying degrees of anthelmintic activity in vitro (118). Alectryon oleifolius was one of the most effective plants in inhibiting larvae. The main active component in the plant is Procyanidin A2, which, after purification, completely inhibited larval development at a low concentration of 50 μg/ml, with an IC50 value of 12.6 μg/ml. Compared to commonly used anthelmintics such as ivermectin (IC50 = 0.22 ng/ml) and levamisole (IC50 = 115 ng/ml), Procyanidin A2 exhibited excellent anthelmintic properties (119). Some studies suggest that tannins and total phenols are considered the key active components in plants for combating parasites. Tannins can bind to proline, an amino acid in the nematode's cuticle, causing larval death (120–122). Moringa oleifera (M. oleifera) leaves contain abundant vitamin E and quercetin, which protect the plant from microbial damage during growth and possess strong antioxidant activity (123–125). Mbogning-Tayo et al. (126) used M. oleifera leaf extract for egg hatching inhibition tests on Haemonchus contortus eggs, showing that at concentrations of 3.75 and 5 mg/ml of M. oleifera extract, the egg development was inhibited by 60.3% ± 8.2% and 92.8% ± 6.2%, respectively. Although research on M. oleifera components is incomplete, the maximum anti-parasitic effect likely results from the coordinated action of multiple components (127). Buono et al. extracted allicin, the active component of garlic, and conducted egg hatching inhibition assay (EHA) and larval migration inhibition tests on Cyathostominae eggs and L3 larvae, showing inhibitory activity. Allicin also exhibited growth inhibition in in vitro studies of B. caballi and Theleria equi. However, in naturally infected horses, it did not exhibit significant anti-parasitic effects (128–130). This suggests that garlic's mechanism of action may not directly kill the parasites, but rather enhance resistance by modulating the immune system. Other herbs, such as Acacia nilotica, Rumex abyssinicus, and Zingiber officinale, also exhibit strong anti-parasitic effects (131).
With growing attention to the sustainable development of grassland ecosystems and livestock, biological control-based management strategies are being increasingly implemented. Duddingtonia flagrans (D. flagrans) and Pochonia chlamydosporia have been shown to be effective and feasible alternatives to chemical dewormers. Horses consuming these fungi can survive in the gastrointestinal tract without disrupting the existing gut microbiota (132). After excretion with feces, predatory fungi form capture networks that significantly reduce larval contamination of pastures and the environment, thus potentially affecting the survival of nematode larvae (133–135). Commercial products based on D. flagrans have already been launched on the market, primarily applied through feed supplementation. Following a period of research and use, these products have significantly reduced the number of parasites in pastures (132, 136–138). Research indicates that equine gastrointestinal worm infections significantly disrupt the host's intestinal microbial (139, 140). Adding probiotic dietary supplements to the diet is undoubtedly an effective way to reduce parasitic infections in horses (141).
In many pastures where horses are free-ranging, many horse owners engage in unscientific, irregular, and non-systematic deworming practices. The need for parasite testing and guided deworming should be emphasized through public education and government regulatory oversight. A comprehensive, year-round control strategy should be developed based on regional differences and parasite distribution. Regular cleaning of feces from horses and other livestock to maintain pasture hygiene has long been considered a fundamental method for controlling equine parasitic infections. However, this task is time-consuming and labor-intensive, making it difficult for many pasture owners to adopt. Despite the current available parasite control strategies, these actions should still be undertaken.
7 Conclusion and future directions
Over the past 30 years, numerous diagnostic kits for identifying and diagnosing equine parasites have been developed, leading to significant advancements. However, most of these methods detect the presence of infection in horses, often requiring expensive instruments and reagents. Additionally, diagnosing parasitic diseases during their latent phase is challenging, which likely explains why few findings have been widely implemented. Although current parasitic diagnostic technologies, such as ELISA and qPCR, offer highly sensitive and specific detection methods, they still face several limitations in field applications, particularly when compared to traditional fecal egg detection methods. Main obstacles include high equipment costs, the need for specialized training, and difficulties in transporting equipment and reagents to remote regions. In contrast, although FEC testing has relatively lower sensitivity, it offers significant advantages in cost, examination time, and operational convenience, making it the most widely used screening tool in resource-limited areas. Bridging the gap between laboratory-based technologies and their field applications requires the establishment of mobile diagnostic platforms that integrate serology and molecular biology (142). The widespread adoption of smartphones has led to the development of efficient smartphone-based parasitic diagnostic platforms, including those for egg identification, remote diagnosis, and colorimetric assays (143–145). Additionally, expanding veterinary training programs is crucial to ensure on-site professionals become proficient in these diagnostic techniques, thereby enhancing their effectiveness in field applications. Automated mobile diagnostic platforms can significantly reduce labor costs. Compared to manual methods for sample extraction and preparation, automated platforms use fewer reagents and exhibit smaller variability between groups (108). Point-of-care tests (POCTs) have emerged as a practical alternative and are rapidly growing in veterinary diagnostics (146). Fecal egg count reduction tests are currently considered the “gold standard” for anti-resistance detection in horses (147–149). In vitro tests, including egg hatching tests and larval development tests, are also commonly used as supplementary methods for anti-resistance testing (27, 150, 151). However, it is currently difficult to define the AR profiles of various parasites in horses, as each parasite may display resistance to different classes of anthelmintics, and the mechanisms of resistance associated with each drug may vary (152, 153). Attributing this resistance to specific gene SNP mutations is not always straightforward. Furthermore, many resistance mechanisms remain poorly understood (154). Research on resistance suggests that the development of parasitic resistance is likely influenced by multiple genes and various mechanisms of action (155–157). Current diagnostic methods face significant challenges in specifically identifying resistant populations, resulting in prevention and control strategies lacking specificity. The lack of related research is associated with horses being a special non-economic livestock species. The horse parasite genomic information available from WormBase ParaSite and NCBI parasite genome databases is still limited compared to ruminant parasite genomic information, further reflecting the significant gap between research teams and funding investment.
Genomic and transcriptomic data related to equine parasites are currently scarce. Whole-genome sequencing of different parasitic species, including both sensitive and drug-resistant strains, would provide deeper insights into the quantity and mechanisms of resistance genes (158–161). Constructing genetic maps of these species' genomes to identify unique genetic markers for species identification will aid in the development of early diagnostic methods. Additionally, research on veterinary helminths has confirmed that genetic markers of AR to BZDs, Levamisole (LEV), and Monepantel are identified using known SNP markers. Expanding the genomic maps of parasitic strains will enhance the diagnosis and detection of resistance (154, 162). Serological and molecular biological techniques currently allow for the study of parasite species, population genetic diversity, and mutations between strains in different regions. In the future, multiplex qPCR diagnostic methods can be developed for common or target parasite species on pastures (163–165). This method can detect infection status in horses and estimate infection quantities and ratios, thereby assessing the AR situation of specific parasites (166). LAMP has good potential for on-site testing and has been widely applied in research on bacteria, viruses, and parasites (167–170). However, parasitic detection samples are often feces or eggs, which are small and contain numerous contaminants, posing challenges for developing effective detection methods (171).
With the rapid advancement of artificial intelligence (AI), an increasing number of researchers are integrating parasitic diagnostic techniques with artificial intelligence (AI) technology to enhance detection efficiency and accuracy. Li et al. developed a low-cost, fully automated diagnostic system that integrates a portable robotic-assisted microscope with convolutional neural networks for the counting of parasite eggs in fecal samples. The system was tested on eggs of Eimeria, Strongyles, and Trichuris spp., with an error margin of fewer than one egg per McMaster counting chamber (172). Numerous machine learning (ML) and artificial neural network-based (ANN) models have been employed for the automated identification of parasite eggs (173–175) Januário et al. proposed an innovative approach that integrates genomic selection, machine learning, and image analysis to optimize resistance to gastrointestinal nematodes (GIN) in sheep (176). This method classifies sheep into resistant, resilient, and susceptible categories using machine learning techniques such as multinomial logistic regression, random forest (RF), and artificial neural networks (ANN), while evaluating susceptibility levels across different farms based on fecal egg count (FEC), packed cell volume, and Famacha© scores (177, 178). Additionally, the RF model was applied to segment ocular conjunctiva images of sheep, automatically classifying anemia levels according to Famacha© scores, thus improving the accuracy of parasite infection detection. For genetic analysis, Bayesian methods, including BayesA, BayesB, and Bayesian Lasso (BLASSO), were employed to estimate the breeding values (EBVs) of traits related to resistance (179–181). Datasets that are continuously enriched will substantially enhance diagnostic efficiency and accuracy, and AI will offer more efficient solutions for screening parasites and drug resistance (182–186). The development of these technologies facilitates large-scale parasite control strategies for equines, benefiting more key stakeholders. Additionally, specific immune antigens and drug targets can be identified for the development of vaccines and other biological products for major parasites, providing more effective means of safeguarding horse health.
In conclusion, while there are some ambiguities in the Swedish, Danish, Dutch, and ESCCAP guidelines concerning equine parasitism and farm management, and differences in deworming timing and diagnostic testing methods due to certain policy factors, there is a shared consensus on advocating monitoring-based control programs. Countries and organizations should proactively offer continuing education webinars on parasite control, providing horse owners and stable managers with up-to-date, evidence-based information to reduce confusion from outdated knowledge. There should also be a concerted effort to implement standardized parasite control strategies across the industry, promoting diagnostic-driven deworming over traditional blanket deworming. Given the current prevalence of drug resistance, not all categories of deworming agents are available in all countries, and strengthening regulation on deworming drug use is essential to preserving their efficacy. Policies should encourage the development of new deworming drugs and explore alternative control measures, especially through long-term studies on combination drug strategies. Furthermore, fostering collaboration among veterinary schools, pharmaceutical companies, and regulatory agencies will ensure that, in the coming years, parasite control guidelines become more unified and widely adopted, effectively curbing resistance development and safeguarding horse health.
Author contributions
TW: Conceptualization, Writing – original draft, Writing – review & editing. XC: Supervision, Writing – review & editing. XY: Writing – review & editing. YS: Writing – review & editing. WG: Writing – review & editing. CL: Supervision, Writing – review & editing. WW: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Basic scientific research operating expenses project of universities directly under the Inner Mongolia Autonomous Region (BR231406), the National Natural Science Foundation of China (32360884), the Scientific Research Special Project for First-Class Disciplines of the Department of Education of Inner Mongolia Autonomous Region (YLXKZX-NND-012).
Acknowledgments
We would like to thank the parasite research team from the College of Veterinary Medicine, Inner Mongolia Agricultural University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Dissanayake S, Rajapakse RPVJ, Rajakaruna RS. Gastrointestinal parasites of domesticated and Feral Horses (Equus caballus) in Sri Lanka. Ceylon J Sci. (2017) 46:17. doi: 10.4038/cjs.v46i1.7414
2. Owen J, Slocombe D. Pathogenesis of helminths in equines. Vet Parasitol. (1985) 18:139–53. doi: 10.1016/0304-4017(85)90063-9
3. Wolf D, Hermosilla C, Taubert A, Fox MT, Uche UE, Vaillant C, et al. Effects of Ostertagia ostertagi and omeprazole treatment on feed intake and gastrin-related responses in the calf. Vet Parasitol. (2002) 105:285–301. doi: 10.1016/S0304-4017(02)00026-2
4. Coop RL, Holmes PH. Nutrition and parasite interaction. Int J Parasitol. (1996) 26:951–62. doi: 10.1016/S0020-7519(96)80070-1
5. Sims TA, Hay J, Talbot IC. An electron microscope and immunohistochemical study of the intracellular location of Toxoplasma tissue cysts within the brains of mice with congenital toxoplasmosis. Br J Exp Pathol. (1989) 70:317–25.
6. Ferguson DJP, Hutchison WM, Pettersen E. Tissue cyst rupture in mice chronically infected with Toxoplasma gondii. Parasitol Res. (1989) 75:599–603. doi: 10.1007/BF00930955
7. Chappuis F, Rijal S, Soto A, Menten J, Boelaert M. A meta-analysis of the diagnostic performance of the direct agglutination test and rK39 dipstick for visceral leishmaniasis. BMJ. (2006) 333:723. doi: 10.1136/bmj.38917.503056.7C
8. Ozensoy S, Ozbel Y, Turgay N, Alkan MZ, Gul K, Gilman-Sachs A, et al. Serodiagnosis and epidemiology of visceral leishmaniasis in Turkey. Am J Trop Med Hyg. (1998) 59:363–9. doi: 10.4269/ajtmh.1998.59.363
9. Healy GR, Ruebush TK. Morphology of Babesia microti in human blood smears. Am J Clin Pathol. (1980) 73:107–9. doi: 10.1093/ajcp/73.1.107
10. Torres-Guerrero E, Quintanilla-Cedillo MR, Ruiz-Esmenjaud J, Arenas R. Leishmaniasis: a review. F1000Res. (2017) 6:750. doi: 10.12688/f1000research.11120.1
11. Duffy P, Fried M. Malaria: new diagnostics for an old problem. Am J Trop Med Hyg. (2005) 73:482–3. doi: 10.4269/ajtmh.2005.73.482
12. Croft AM, Kitson MM, Jackson CJ, Minton EJ, Friend HM. African Trypanosomiasis in a British Soldier. Mil Med. (2007) 172:765–9. doi: 10.7205/MILMED.172.7.765
13. Scott I, Bishop R, Pomroy W. Anthelmintic resistance in equine helminth parasites – a growing issue for horse owners and veterinarians in New Zealand? N Z Vet J. (2015) 63:188–98. doi: 10.1080/00480169.2014.987840
14. Peregrine AS, Molento MB, Kaplan RM, Nielsen MK. Anthelmintic resistance in important parasites of horses: does it really matter? Vet Parasitol. (2014) 201:1–8. doi: 10.1016/j.vetpar.2014.01.004
15. Jabbar A, Iqbal Z, Kerboeuf D, Muhammad G, Khan MN, Afaq M. Anthelmintic resistance: the state of play revisited. Life Sci. (2006) 79:2413–31. doi: 10.1016/j.lfs.2006.08.010
16. Köhler P. The biochemical basis of anthelmintic action and resistance. Int J Parasitol. (2001) 31:336–45. doi: 10.1016/S0020-7519(01)00131-X
17. Lifschitz A, Suarez VH, Sallovitz J, Cristel SL, Imperiale F, Ahoussou S, et al. Cattle nematodes resistant to macrocyclic lactones: comparative effects of P-glycoprotein modulation on the efficacy and disposition kinetics of ivermectin and moxidectin. Exp Parasitol. (2010) 125:172–8. doi: 10.1016/j.exppara.2010.01.009
18. Geurden T, Chartier C, Fanke J, di Regalbono AF, Traversa D, von Samson-Himmelstjerna G, et al. Anthelmintic resistance to ivermectin and moxidectin in gastrointestinal nematodes of cattle in Europe. Int J Parasitol Drugs Drug Resist. (2015) 5:163–71. doi: 10.1016/j.ijpddr.2015.08.001
19. Coles GC. Anthelmintic resistance – looking to the future: a UK perspective. Res Vet Sci. (2005) 78:99–108. doi: 10.1016/j.rvsc.2004.09.001
20. Domke AVM, Chartier C, Gjerde B, Höglund J, Leine N, Vatn S, et al. Prevalence of anthelmintic resistance in gastrointestinal nematodes of sheep and goats in Norway. Parasitol Res. (2012) 111:185–93. doi: 10.1007/s00436-012-2817-x
21. Geurden T, Betsch J-M, Maillard K, Vanimisetti B, D'Espois M, Besognet B. Determination of anthelmintic efficacy against equine cyathostomins and Parascaris equorum in France. Equine Vet Educ. (2013) 25:304–7. doi: 10.1111/j.2042-3292.2012.00454.x
22. Wolf D, Hermosilla C, Taubert A. Oxyuris equi: lack of efficacy in treatment with macrocyclic lactones. Vet Parasitol. (2014) 201:163–8. doi: 10.1016/j.vetpar.2013.12.009
23. Saes IL, Vera JHS, Fachiolli DF, Yamada PH, Dellaqua JVT, de Lima Saes R, et al. Time required by different anthelmintics to reach expected efficacy levels in horses infected by strongyles. Vet Parasitol. (2016) 229:90–2. doi: 10.1016/j.vetpar.2016.10.002
24. Abbas G, Ghafar A, McConnell E, Beasley A, Bauquier J, Wilkes EJA, et al. A national survey of anthelmintic resistance in ascarid and strongylid nematodes in Australian thoroughbred horses. Int J Parasitol Drugs Drug Resist. (2024) 24:100517. doi: 10.1016/j.ijpddr.2023.11.006
25. Ashrafzadeh-Shiraz M, Tavassoli M, Dalir-Naghadeh B, Sazmand A. Impaired efficacy of fenbendazole and ivermectin against intestinal nematodes in adult horses in Iran. Res Vet Sci. (2024) 166:105078. doi: 10.1016/j.rvsc.2023.105078
26. Canever RJ, Braga PRC, Boeckh A, Grycajuck M, Bier D, Molento MB. Lack of Cyathostomin sp. reduction after anthelmintic treatment in horses in Brazil. Vet Parasitol. (2013) 194:35–9. doi: 10.1016/j.vetpar.2012.12.020
27. Coles GC, Jackson F, Pomroy WE, Prichard RK, von Samson-Himmelstjerna G, Silvestre A, et al. The detection of anthelmintic resistance in nematodes of veterinary importance. Vet Parasitol. (2006) 136:167–85. doi: 10.1016/j.vetpar.2005.11.019
28. Taylor MA, Hunt KR, Goodyear KL. Anthelmintic resistance detection methods. Vet Parasitol. (2002) 103:183–94. doi: 10.1016/S0304-4017(01)00604-5
29. Vainionpää R, Waris M, Leinikki P. Diagnostic techniques: serological and molecular approaches. Ref Mod Biomed Sci. (2015) 6:B978-0-12-801238-3.02558-7. doi: 10.1016/B978-0-12-801238-3.02558-7
30. Baboo ASA, Naeem M, Behan AA, Rajput N. Serum cortisol concentration is a reliable tool to assess stress level among stereotypic and non-stereotypic thoroughbred horses. PVJ. (2024) 44:155–61. doi: 10.29261/pakvetj/2023.115
31. Apsari IAP, Swacita IBN, Dharmawan NS, Winaya IBO, Praing UYA, Agustina KK, et al. Investigation into Trypanosoma evansi infection in horses in East Sumba-Indonesia. Int J Vet Sci. (2024) 13:794–8. doi: 10.47278/journal.ijvs/2024.171
32. Verma R, Das G, Manjunathachar HV, Muwel N. Advances in diagnostics of parasitic diseases: current trends and future prospects. Int J Curr Microbiol App Sci. (2018) 7:3261–77. doi: 10.20546/ijcmas.2018.707.380
33. Dalton JP, Robinson MW, Mulcahy G, O'Neill SM, Donnelly S. Immunomodulatory molecules of Fasciola hepatica: candidates for both vaccine and immunotherapeutic development. Vet Parasitol. (2013) 195:272–85. doi: 10.1016/j.vetpar.2013.04.008
34. Johnston MJG, MacDonald JA, McKay DM. Parasitic helminths: a pharmacopeia of anti-inflammatory molecules. Parasitology. (2009) 136:125–47. doi: 10.1017/S0031182008005210
35. Mezo M, González-Warleta M, Ubeira FM. Optimized serodiagnosis of sheep fascioliasis by fast-D protein liquid chromatography fractionation of Fasciola hepatica. excretory–secretory antigens. J Parasitol. (2003) 89:843–9. doi: 10.1645/GE-74RI.1
36. Marzok M, Gattan HS, Albokhadaim I, Alruhaili MH, Salem M, Selim A. Seroprevalence and risk factors associated with Anaplasma phagocytophilum infection in horses in Egypt. Kafkas Univ Vet Fak Derg. (2024) 30:787–92 doi: 10.9775/kvfd.2024.32527
37. Kuerpick B, Schnieder T, Strube C. Evaluation of a recombinant cathepsin L1 ELISA and comparison with the Pourquier and ES ELISA for the detection of antibodies against Fasciola hepatica. Vet Parasitol. (2013) 193:206–13. doi: 10.1016/j.vetpar.2012.11.021
38. Sánchez-Andrade R, Paz-Silva A, Suárez JL, Panadero R, Pedreira J, Díez-Baños P, et al. Effect of fasciolicides on the antigenaemia in sheep naturally infected with Fasciola hepatica. Parasitol Res. (2001) 87:609–14. doi: 10.1007/s004360100425
39. Gonzales Santana B, Dalton JP, Vasquez Camargo F, Parkinson M, Ndao M. The diagnosis of human fascioliasis by enzyme-linked immunosorbent assay (ELISA) using recombinant Cathepsin L protease. PLoS Negl Trop Dis. (2013) 7:e2414. doi: 10.1371/journal.pntd.0002414
40. Kang J-M, Bahk Y-Y, Cho P-Y, Hong S-J, Kim T-S, Sohn W-M, et al. Family of cathepsin F cysteine proteases of Clonorchis sinensis is the major secreted proteins that are expressed in the intestine of the parasite. Mol Biochem Parasitol. (2010) 170:7–16. doi: 10.1016/j.molbiopara.2009.11.006
41. Norbury LJ, Beckham S, Pike RN, Grams R, Spithill TW, Fecondo JV, et al. Adult and juvenile Fasciola cathepsin L proteases: different enzymes for different roles. Biochimie. (2011) 93:604–11. doi: 10.1016/j.biochi.2010.12.004
42. Meemon K. Sobhon P. Juvenile-specific cathepsin proteases in Fasciola spp: their characteristics and vaccine efficacies. Parasitol Res. (2015) 114:2807–13. doi: 10.1007/s00436-015-4589-6
43. Boulard C, Villejoubert C. Use of pooled serum or milk samples for the epidemiological surveillance of bovine hypodermosis. Vet Parasitol. (1991) 39:171–83. doi: 10.1016/0304-4017(91)90072-4
44. Boulard C. Degradation of bovine C3 by serine proteases from parasites Hypoderma lineatum (Diptera, Oestridae). Vet Immunol Immunopathol. (1989) 20:387–98. doi: 10.1016/0165-2427(89)90083-4
45. Atelge M, Inci A, Yildirim A, Sozdutmaz I, Adler PH. First molecular characterization of hypodermin genes of Hypoderma bovis and serodiagnosis of bovine hypodermosis with recombinant hypodermin C antigen and a synthetic peptide containing its linear B-cell epitope. Vet Parasitol. (2021) 292:109394. doi: 10.1016/j.vetpar.2021.109394
46. Reyburn H, Mbatia R, Drakeley C, Carneiro I, Mwakasungula E, Mwerinde O, et al. Overdiagnosis of malaria in patients with severe febrile illness in Tanzania: a prospective study. BMJ. (2004) 329:1212. doi: 10.1136/bmj.38251.658229.55
47. Andersen UV, Howe DK, Olsen SN, Nielsen MK. Recent advances in diagnosing pathogenic equine gastrointestinal helminths: the challenge of prepatent detection. Vet Parasitol. (2013) 192:1–9. doi: 10.1016/j.vetpar.2012.11.003
48. Bastos RG, Sears KP, Dinkel KD, Kappmeyer L, Ueti MW, Knowles DP, et al. Development of an indirect ELISA to detect equine antibodies to Theileria haneyi. Pathogens. (2021) 10:270. doi: 10.3390/pathogens10030270
49. Tjitra E, Suprianto S, Dyer M, Currie BJ. Anstey NM. Field evaluation of the ICT malaria Pf/Pv immunochromatographic test for detection of Plasmodium falciparum and Plasmodium vivax in patients with a presumptive clinical diagnosis of malaria in Eastern Indonesia. J Clin Microbiol. (1999) 37:2412–7. doi: 10.1128/JCM.37.8.2412-2417.1999
50. Mayxay M, Pukrittayakamee S, Chotivanich K, Looareesuwan S, White NJ. Persistence of Plasmodium falciparum HRP-2 in successfully treated acute falciparum malaria. Trans R Soc Trop Med Hyg. (2001) 95:179–82. doi: 10.1016/S0035-9203(01)90156-7
51. Moody A. Rapid diagnostic tests for malaria parasites. Clin Microbiol Rev. (2002) 15:66–78. doi: 10.1128/CMR.15.1.66-78.2002
52. Zarlenga DS, Higgins J. PCR as a diagnostic and quantitative technique in veterinary parasitology. Vet Parasitol. (2001) 101:215–30. doi: 10.1016/S0304-4017(01)00568-4
53. Gorman T, Aballay J, Fredes F, Silva M, Aguillón JC, Alcaíno HA, et al. Fasciolosis in horses: a neglected, re-emerging disease. Equine Vet Educ. (2017) 29:202–4. doi: 10.1111/eve.12521
54. Mens P, Spieker N, Omar S, Heijnen M, Schallig H, Kager PA. Is molecular biology the best alternative for diagnosis of malaria to microscopy? A comparison between microscopy, antigen detection and molecular tests in rural Kenya and urban Tanzania. Tropical Med Int Health. (2007) 12:238–44. doi: 10.1111/j.1365-3156.2006.01779.x
55. Wang L, Lv Q, He Y, Gu R, Zhou B, Chen J, et al. Integrated qPCR and staining methods for detection and quantification of Enterocytozoon hepatopenaei in Shrimp Litopenaeus vannamei. Microorganisms. (2020) 8:1366. doi: 10.3390/microorganisms8091366
56. de Paiva-Cavalcanti M, de Morais RCS, Pessoa-E-Silva R, Trajano-Silva LAM, Gonçalves-de-Albuquerque SC, Tavares DHC, et al. Leishmaniases diagnosis: an update on the use of immunological and molecular tools. Cell Biosci. (2015) 5:31. doi: 10.1186/s13578-015-0021-2
57. Duncan R. Advancing molecular diagnostics for Trypanosomatid parasites. J Mol Diagn. (2014) 16:379–81. doi: 10.1016/j.jmoldx.2014.04.001
58. Guo S-Q, Fu Y-W, Hou T-L, Huang S-L, Zhang Q-Z. Establishment and application of TaqMan probe-based quantitative real-time PCR for rapid detection and quantification of Ichthyophthirius multifiliis in farming environments and fish tissues. Vet Parasitol. (2025) 334:110381. doi: 10.1016/j.vetpar.2024.110381
59. Souza AP, Soto M, Costa JML, Boaventura VS, de Oliveira CI, Cristal JR, et al. Towards a more precise serological diagnosis of human Tegumentary Leishmaniasis using Leishmania recombinant proteins. PLoS One. (2013) 8:e66110. doi: 10.1371/journal.pone.0066110
60. da Silveira JF, Umezawa ES, Luquetti AO. Chagas disease: recombinant Trypanosoma cruzi antigens for serological diagnosis. Trends Parasitol. (2001) 17:286–91. doi: 10.1016/S1471-4922(01)01897-9
61. Alruhaili MH, Marzok M, Gattan HS, Salem M, Abd El-Lateef HM, Selim A. Seroprevalence and assessment of risk factors associated to Borrelia burgdorferi infection in Egyptian horses. Kafkas Univ Vet Fak Derg. (2024) 30:349–54. doi: 10.9775/kvfd.2023.31264
62. Nielsen MK, Baptiste KE, Tolliver SC, Collins SS, Lyons ET. Analysis of multiyear studies in horses in Kentucky to ascertain whether counts of eggs and larvae per gram of feces are reliable indicators of numbers of strongyles and ascarids present. Vet Parasitol. (2010) 174:77–84. doi: 10.1016/j.vetpar.2010.08.007
63. Dowdall SMJ, Matthews JB, Mair T, Murphy D, Love S, Proudman CJ. Antigen-specific IgG(T) responses in natural and experimental cyathostominae infection in horses. Vet Parasitol. (2002) 106:225–42. doi: 10.1016/S0304-4017(02)00085-7
64. Dowdall SMJ, Proudman CJ, Klei TR, Mair T, Matthews JB. Characterisation of IgG(T) serum antibody responses to two larval antigen complexes in horses naturally- or experimentally-infected with cyathostomins. Int J Parasitol. (2004) 34:101–8. doi: 10.1016/j.ijpara.2003.09.008
65. Dowdall SMJ, Proudman CJ, Love S, Klei TR, Matthews JB. Purification and analyses of the specificity of two putative diagnostic antigens for larval cyathostomin infection in horses. Res Vet Sci. (2003) 75:223–9. doi: 10.1016/S0034-5288(03)00116-4
66. McWilliam HEG, Nisbet AJ, Dowdall SMJ, Hodgkinson JE, Matthews JB. Identification and characterisation of an immunodiagnostic marker for cyathostomin developing stage larvae. Int J Parasitol. (2010) 40:265–75. doi: 10.1016/j.ijpara.2009.08.004
67. Tzelos T, Geyer KK, Mitchell MC, McWilliam HEG, Kharchenko VO, Burgess STG, et al. Characterisation of serum IgG(T) responses to potential diagnostic antigens for equine cyathostominosis. Int J Parasitol. (2020) 50:289–98. doi: 10.1016/j.ijpara.2020.01.004
68. Höglund J, Ljungström B-L, Nilsson O, Uggla A. Enzyme-linked immunosorbent assay (ELISA) for the detection of antibodies to Anoplocephala perfoliata in horse sera. Vet Parasitol. (1995) 59:97–106. doi: 10.1016/0304-4017(94)00755-2
69. Proudman CJ, Trees AJ. Use of excretory/secretory antigens for the serodiagnosis of Anoplocephala perfoliata cestodosis. Vet Parasitol. (1996) 61:239–47. doi: 10.1016/0304-4017(95)00837-3
70. PROUDMAN CJ, TREES AJ. Correlation of antigen specific IgG and IgG(T) responses with Anoplocephala perfoliata infection intensity in the horse. Parasite Immunol. (1996) 18:499–506. doi: 10.1046/j.1365-3024.1996.d01-18.x
71. Anderson RM, May RM. Infectious Diseases of Humans: Dynamics and Control. Oxford, UK: Oxford University Press (1991). doi: 10.1093/oso/9780198545996.001.0001
72. Bohórquez A, Meana A, Pato NF, Luzón M. Coprologically diagnosing Anoplocephala perfoliata in the presence of A. magna. Vet Parasitol. (2014) 204:396–401. doi: 10.1016/j.vetpar.2014.04.023
73. Lightbody KL, Davis PJ, Austin CJ. Validation of a novel saliva-based ELISA test for diagnosing tapeworm burden in horses. Vet Clin Pathol. (2016) 45:335–46. doi: 10.1111/vcp.12364
74. Jin D. Indirect ELISA Procedures of Horse Gastric myiasis and Study on the Discharge Dynamics of Gasterophilus spp. Larvae after Administration of Ivermectin. Beijing Forestry University (2017).
75. Lyons ET, Drudge JH, Tolliver SC. Studies on the development and chemotherapy of larvae of Parascaris equorum (Nematoda: Ascaridoidea) in experimentally and naturally infected foals. J Parasitol. (1976) 62:453. doi: 10.2307/3279157
76. de Savigny DH, Voller A, Woodruff AW. Toxocariasis: serological diagnosis by enzyme immunoassay. J Clin Pathol. (1979) 32:284–8. doi: 10.1136/jcp.32.3.284
77. Chatterjee BP, Santra A, Karmakar PR, Mazumder DNG. Evaluation of IgG4 response in ascariasis by ELISA for serodiagnosis. Tropical Med Int Health. (1996) 1:633–9. doi: 10.1111/j.1365-3156.1996.tb00088.x
78. Lind P, Eriksen L, Nansen P, Nilsson O, Roepstorff A. Response to repeated inoculations with Ascaris suum eggs in pigs during the fattening period. Parasitol Res. (1993) 79:240–4. doi: 10.1007/BF00931899
79. Burk SV, Dangoudoubiyam S, Brewster-Barnes T, Bryant UK, Howe DK, Carter CN, et al. In vitro culture of Parascaris equorum larvae and initial investigation of parasite excretory-secretory products. Parasitol Res. (2014) 113:4217–24. doi: 10.1007/s00436-014-4097-0
80. Gasser RB, Jenkins DJ, Heath DD, Lawrence SB. Use of Echinococcus granulosus worm antigens for immunodiagnosis of E. granulosus infection in dogs. Vet Parasitol. (1992) 45:89–100. doi: 10.1016/0304-4017(92)90030-D
81. Abbott JB, Mellor DJ, Barrett EJ, Proudman CJ, Love S. Serological changes observed in horses infected with Anoplocephala perfoliata after treatment with praziquantel and natural reinfection. Vet Rec. (2008) 162:50–3. doi: 10.1136/vr.162.2.50
82. Proudman CJ, Holdstock NB. Investigation of an outbreak of tapeworm-associated colic in a training yard. Equine Vet J Suppl. (2000) 32:37–41. doi: 10.1111/j.2042-3306.2000.tb05332.x
83. Voller A, De Savigny D. Diagnostic serology of tropical parasitic diseases. J Immunol Methods. (1981) 46:1–29. doi: 10.1016/0022-1759(81)90328-8
84. Bohórquez A, Meana A, Luzón M. Differential diagnosis of equine cestodosis based on E/S and somatic Anoplocephala perfoliata and Anoplocephala magna antigens. Vet Parasitol. (2012) 190:87–94. doi: 10.1016/j.vetpar.2012.06.001
85. Rinaldi L, Krücken J, Martinez-Valladares M, Pepe P, Maurelli MP, de Queiroz C, et al. Advances in diagnosis of gastrointestinal nematodes in livestock and companion animals. Adv Parasitol. (2022) 118:85–176. doi: 10.1016/bs.apar.2022.07.002
86. Mu X, Guo J, Wang H, Li Y, Yuan K, Xu H, et al. Establishment and preliminary application of PCR-RFLP genotyping method for Giardia duodenalis in goats. BMC Vet Res. (2024) 20:527. doi: 10.1186/s12917-024-04386-0
87. Campbell AJD, Gasser RB, Chilton NB. Differences in a ribosomal DNA sequence of strongylus species allows identification of single eggs. Int J Parasitol. (1995) 25:359–65. doi: 10.1016/0020-7519(94)00116-6
88. Gasser RB, Stevenson LA, Chilton NB, Nansen P, Bucknell DG, Beveridge I. Species markers for equine strongyles detected in intergenic rDNA by PCR-RFLP. Mol Cell Probes. (1996) 10:371–8. doi: 10.1006/mcpr.1996.0050
89. Gasser RB, Monti JR. Identification of parasitic nematodes by PCR-SSCP of ITS-2 rDNA. Mol Cell Probes. (1997) 11:201–9. doi: 10.1006/mcpr.1997.0106
90. Siles-Lucas M, Felleisen R, Cuesta-Bandera C, Gottstein B, Eckert J. Comparative genetic analysis of Swiss and Spanish isolates of Echinococcus granulosus by southern hybridization and random amplified polymorphic DNA technique. Appl Parasitol. (1994) 35:107–17.
91. Humbert JF, Cabaret J. Use of random amplified polymorphic DNA for identification of ruminant trichostrongylid nematodes. Parasitol Res. (1995) 81:1–5. doi: 10.1007/BF00932409
92. Courtot É, Boisseau M, Dhorne-Pollet S, Serreau D, Gesbert A, Reigner F, et al. Comparison of two molecular barcodes for the study of equine strongylid communities with amplicon sequencing. PeerJ. (2023) 11:e15124. doi: 10.7717/peerj.15124
93. Hodgkinson JE, Love S, Lichtenfels JR, Palfreman S, Ramsey YH, Matthews JB. Evaluation of the specificity of five oligoprobes for identification of cyathostomin species from horses. Int J Parasitol. (2001) 31:197–204. doi: 10.1016/S0020-7519(00)00161-2
94. Hodgkinson JE, Lichtenfels JR, Mair TS, Cripps P, Freeman KL, Ramsey YH, et al. PCR–ELISA for the identification of cyathostomin fourth-stage larvae from clinical cases of larval cyathostominosis. Int J Parasitol. (2003) 33:1427–35. doi: 10.1016/S0020-7519(03)00140-1
95. Hodgkinson JE, Freeman KL, Lichtenfels JR, Palfreman S, Love S, Matthews JB. Identification of strongyle eggs from anthelmintic-treated horses using a PCR-ELISA based on intergenic DNA sequences. Parasitol Res. (2005) 95:287–92. doi: 10.1007/s00436-004-1289-z
96. Hung G-C, Gasser RB, Beveridge I, Chilton NB. Species-specific amplification by PCR of ribosomal DNA from some equine strongyles. Parasitology. (1999) 119:69–80. doi: 10.1017/S0031182099004497
97. Cwiklinski K, Kooyman FNJ, van Doorn DCK, Matthews JB, Hodgkinson JE. New insights into sequence variation in the IGS region of 21 cyathostomin species and the implication for molecular identification. Parasitology. (2012) 139:1063–73. doi: 10.1017/S0031182012000467
98. Traversa D, Milillo P, Barnes H, von Samson-Himmelstjerna G, Schurmann S, Demeler J, et al. Distribution and species-specific occurrence of cyathostomins (Nematoda, Strongylida) in naturally infected horses from Italy, United Kingdom and Germany. Vet Parasitol. (2010) 168:84–92. doi: 10.1016/j.vetpar.2009.10.006
99. van Doorn DCK, Ploeger HW, Eysker M, Geurden T, Wagenaar JA, Kooyman FNJ. Cylicocyclus species predominate during shortened egg reappearance period in horses after treatment with ivermectin and moxidectin. Vet Parasitol. (2014) 206:246–52. doi: 10.1016/j.vetpar.2014.10.004
100. Molena RA, Peachey LE, Di Cesare A, Traversa D, Cantacessi C. Cyathostomine egg reappearance period following ivermectin treatment in a cohort of UK Thoroughbreds. Parasit Vectors. (2018) 11:61. doi: 10.1186/s13071-018-2638-6
101. Martins AV, Corrêa LL, Ribeiro MS, de Lima Coelho A, Lobão LF, Palmer JPS, et al. Identification of third stage larvae of strongyles and molecular diagnosis of Strongylus vulgaris in the feces of Thoroughbred horses kept in training centers in Rio de Janeiro, Brazil. Vet Parasitol. (2024) 50:101019. doi: 10.1016/j.vprsr.2024.101019
102. Alhassan A, Thekisoe OMM, Yokoyama N, Inoue N, Motloang MY, Mbati PA, et al. Development of loop-mediated isothermal amplification (LAMP) method for diagnosis of equine piroplasmosis. Vet Parasitol. (2007) 143:155–60. doi: 10.1016/j.vetpar.2006.08.014
103. Moghaddassani H, Mirhendi H, Hosseini M, Rokni M, Mowlavi G, Kia E. Molecular diagnosis of Strongyloides stercoralis infection by PCR detection of specific DNA in human stool samples. Iran J Parasitol. (2011) 6:23–30.
104. Levenhagen MA, Costa-Cruz JM. Update on immunologic and molecular diagnosis of human strongyloidiasis. Acta Trop. (2014) 135:33–43. doi: 10.1016/j.actatropica.2014.03.015
105. Zhang H, Thekisoe OMM, Aboge GO, Kyan H, Yamagishi J, Inoue N, et al. Toxoplasma gondii: sensitive and rapid detection of infection by loop-mediated isothermal amplification (LAMP) method. Exp Parasitol. (2009) 122:47–50. doi: 10.1016/j.exppara.2009.01.012
106. Ding L, Razavi Bazaz S, Hall T, Vesey G, Ebrahimi Warkiani M. Giardia purification from fecal samples using rigid spiral inertial microfluidics. Biomicrofluidics. (2022) 16:014105. doi: 10.1063/5.0069406
107. Melville L, Kenyon F, Javed S, McElarney I, Demeler J, Skuce P. Development of a loop-mediated isothermal amplification (LAMP) assay for the sensitive detection of Haemonchus contortus eggs in ovine faecal samples. Vet Parasitol. (2014) 206:308–12. doi: 10.1016/j.vetpar.2014.10.022
108. Chen Y-X, Lou Y-R, Duan L-J, Zhou Q-J, Xu Z-J, Chen F-J, et al. Parallel detection of multiple zoonotic parasites using a real-time fluorogenic loop-mediated isothermal amplification-based quadruple-sample microfluidic chip. Front Microbiol. (2023) 14:1238376. doi: 10.3389/fmicb.2023.1238376
109. Pattanayak P, Singh SK, Gulati M, Vishwas S, Kapoor B, Chellappan DK, et al. Microfluidic chips: recent advances, critical strategies in design, applications and future perspectives. Microfluid Nanofluid. (2021) 25:99. doi: 10.1007/s10404-021-02502-2
110. Siavash Moakhar R, Mahimkar R, Khorrami Jahromi A, Mahshid SS, del Real Mata C, Lu Y, et al. Aptamer-based electrochemical microfluidic biosensor for the detection of Cryptosporidium parvum. ACS Sens. (2023) 8:2149–58. doi: 10.1021/acssensors.2c01349
111. Mitchell CJ, O'Sullivan CM, Pinloche E, Wilkinson T, Morphew RM, Mcewan NR. Using next-generation sequencing to determine diversity of horse intestinal worms: identifying the equine ‘nemabiome'. JES. (2019) 30:1–5. doi: 10.1294/jes.30.1
112. Abbas G, Ghafar A, Bauquier J, Beasley A, Ling E, Gauci CG, et al. Prevalence and diversity of ascarid and strongylid nematodes in Australian Thoroughbred horses using next-generation sequencing and bioinformatic tools. Vet Parasitol. (2023) 323:110048. doi: 10.1016/j.vetpar.2023.110048
113. Hamad MH, Islam SI, Jitsamai W, Chinkangsadarn T, Naraporn D, Ouisuwan S, et al. Metabarcoding study to reveal the structural community of strongylid nematodes in domesticated horses in Thailand. BMC Vet Res. (2024) 20:70. doi: 10.1186/s12917-024-03934-y
114. Wang D, Zeng J, Ma H, Fouad D, Su Z. Comparative analysis of the gut microbiota between two horse species. PVJ. (2024) 44:449–57. doi: 10.29261/pakvetj/2024.151
115. Синяков MП, Ятусевич AИ, Стогначева ГA. Antiparasitic drugs for the treatment and prevention of horse diseases. Vestnik APK Verhnevolzh‘ia. (2021) 1:28–32. doi: 10.35694/YARCX.2021.53.1.005
116. Panova OA, Arkhipov IA, Baranova MV, Khrustalev AV. The problem of anthelminthic resistance in horse breeding. Russ J Parasitol. (2022) 16:230–42. doi: 10.31016/1998-8435-2022-16-2-230-242
117. Beg ZA, Roohi N, Iqbal Z, Iqbal MA, Zulfiqar A. Role of herbs as anthelmintic in the control of parascariasis in equines. J Anim Plant Sci. (2023) 33:235–40. doi: 10.36899/JAPS.2023.1.0615
118. Payne SE, Kotze AC, Durmic Z, Vercoe PE. Australian plants show anthelmintic activity toward equine cyathostomins in vitro. Vet Parasitol. (2013) 196:153–60. doi: 10.1016/j.vetpar.2013.01.012
119. Payne SE, Flematti GR, Reeder A, Kotze AC, Durmic Z, Vercoe PE. Procyanidin A2 in the Australian plant Alectryon oleifolius has anthelmintic activity against equine cyathostomins in vitro. Vet Parasitol. (2018) 249:63–9. doi: 10.1016/j.vetpar.2017.11.008
120. Sneha A, Preet S. Impact of sublethal conventional and biorational larvicidal stress on fitness status in nutritionally challenged Aedes aegypti larvae. Int J Mosq Res. (2016) 3:39–46.
121. Klongsiriwet C, Quijada J, Williams AR, Mueller-Harvey I, Williamson EM, Hoste H. Synergistic inhibition of Haemonchus contortus exsheathment by flavonoid monomers and condensed tannins. Int J Parasitol Drugs Drug Resist. (2015) 5:127–34. doi: 10.1016/j.ijpddr.2015.06.001
122. Chicaiza-Tisalema E, Barros-Rodríguez M, Zurita-Vásquez H, Mera-Andrade R, Velástegui-Espín G, Muñoz-Espinoza M, et al. Efecto Antihelmíntico in vitro del Extracto de Albizia Lophantha sobre Nematodos Gastrointestinales de Caballos (Spanish). Rev Investig Vet Perú. (2016) 27:556. doi: 10.15381/rivep.v27i3.12007
123. Leone A, Spada A, Battezzati A, Schiraldi A, Aristil J, Bertoli S. Cultivation, genetic, ethnopharmacology, phytochemistry and pharmacology of Moringa oleifera leaves: an overview. Int J Mol Sci. (2015) 16:12791–835. doi: 10.3390/ijms160612791
124. Vergara-Jimenez M, Almatrafi MM, Fernandez ML. Bioactive components in Moringa oleifera leaves protect against chronic disease. Antioxidants. (2017) 6:91. doi: 10.3390/antiox6040091
125. Ferreira PMP, Farias DF, Oliveira JTA, Carvalho AFU. Moringa oleifera: bioactive compounds and nutritional potential. Rev Nutr. (2008) 21:431–7. doi: 10.1590/S1415-52732008000400007
126. Tayo GM, Poné JW, Komtangi MC, Yondo J, Ngangout AM, Mbida M. Anthelminthic activity of Moringa oleifera leaf extracts evaluated in vitro on four developmental stages of Haemonchus contortus from goats. AJPS. (2014) 05:1702–10. doi: 10.4236/ajps.2014.511185
127. Elghandour MMMY, Maggiolino A, Vázquez-Mendoza P, Alvarado-Ramírez ER, Cedillo-Monroy J, De Palo P, et al. Moringa oleifera as a natural alternative for the control of gastrointestinal parasites in equines: a review. Plants. (2023) 12:1921. doi: 10.3390/plants12091921
128. Buono F, Pacifico L, Piantedosi D, Sgroi G, Neola B, Roncoroni C, et al. Preliminary observations of the effect of garlic on egg shedding in horses naturally infected by intestinal strongyles. J Equine Vet Sci. (2019) 72:79–83. doi: 10.1016/j.jevs.2018.10.025
129. Salama AA, AbouLaila M, Terkawi MA, Mousa A, El-Sify A, Allaam M, et al. Inhibitory effect of allicin on the growth of Babesia and Theileria equi parasites. Parasitol Res. (2014) 113:275–83. doi: 10.1007/s00436-013-3654-2
130. Bhatwalkar SB, Mondal R, Krishna SBN, Adam JK, Govender P, Anupam R. Antibacterial properties of organosulfur compounds of garlic (Allium sativum). Front Microbiol. (2021) 12:613077. doi: 10.3389/fmicb.2021.613077
131. Peachey LE, Pinchbeck GL, Matthews JB, Burden FA, Mulugeta G, Scantlebury CE, et al. An evidence-based approach to the evaluation of ethnoveterinary medicines against strongyle nematodes of equids. Vet Parasitol. (2015) 210:40–52. doi: 10.1016/j.vetpar.2015.03.009
132. Alves do Carmo T, Oliveira Mena M, de Almeida Cipriano I, Mascoli de Favare G, Jabismar Guelpa G, da Costa Pinto S, et al. Biological control of gastrointestinal nematodes in horses fed with grass in association with nematophagus fungi Duddingtonia flagrans and Pochonia chlamydosporia. Biol Control. (2023) 182:105219. doi: 10.1016/j.biocontrol.2023.105219
133. Larsen M. Prospects for controlling animal parasitic nematodes by predacious micro fungi. Parasitology. (2000) 120:121–31. doi: 10.1017/S0031182099005739
134. Araújo JV, Braga FR. Mendoza-de-Gives P, Paz-Silva A, Vilela VLR. Recent advances in the control of helminths of domestic animals by helminthophagous fungi. Parasitologia. (2021) 1:168–76. doi: 10.3390/parasitologia1030018
135. Ghafar A, Abbas G, Beasley A, Bauquier J, Wilkes EJA, Jacobson C, et al. Molecular diagnostics for gastrointestinal helminths in equids: past, present and future. Vet Parasitol. (2023) 313:109851. doi: 10.1016/j.vetpar.2022.109851
136. Duddington CL. Fungi that attack microscopic animals. Bot Rev. (1955) 21:377–439. doi: 10.1007/BF02872434
137. Buzatti A, Santos CP, Fernandes MAM, Yoshitani UY, Sprenger LK, Molento MB. Duddingtonia flagrans no controle de nematoides gastrintestinais de equinos em fases de vida livre. Arq Bras Med Vet Zootec. (2017) 69:364–70. doi: 10.1590/1678-4162-9028
138. Bampidis V, Azimonti G, de Lourdes Bastos M, Christensen H, Dusemund B, Kos Durjava M, et al. Safety and efficacy of BioWorma® (Duddingtonia flagrans NCIMB 30336) as a feed additive for all grazing animals. EFS2. (2020) 18:e06208. doi: 10.2903/j.efsa.2020.6208
139. Clark A, Sallé G, Ballan V, Reigner F, Meynadier A, Cortet J, et al. Strongyle infection and gut microbiota: profiling of resistant and susceptible horses over a grazing season. Front Physiol. (2018) 9:272. doi: 10.3389/fphys.2018.00272
140. Peachey LE, Molena RA, Jenkins TP, Di Cesare A, Traversa D, Hodgkinson JE, et al. The relationships between faecal egg counts and gut microbial composition in UK Thoroughbreds infected by cyathostomins. Int J Parasitol. (2018) 48:403–12. doi: 10.1016/j.ijpara.2017.11.003
141. Markowiak P, Sliżewska K. The role of probiotics, prebiotics and synbiotics in animal nutrition. Gut Pathog. (2018) 10:21. doi: 10.1186/s13099-018-0250-0
142. Gavina K, Franco LC, Khan H, Lavik J-P, Relich RF. Molecular point-of-care devices for the diagnosis of infectious diseases in resource-limited settings – a review of the current landscape, technical challenges, and clinical impact. J Clin Virol. (2023) 169:105613. doi: 10.1016/j.jcv.2023.105613
143. Escobedo P, Erenas MM, Martinez Olmos A, Carvajal MA, Tabraue Chavez M, Luque Gonzalez MA, et al. Smartphone-based diagnosis of parasitic infections with colorimetric assays in centrifuge tubes. IEEE Access. (2019) 7:185677–86. doi: 10.1109/ACCESS.2019.2961230
144. Dacal E, Bermejo-Peláez D, Lin L, Álamo E, Cuadrado D, Martínez Á, et al. Mobile microscopy and telemedicine platform assisted by deep learning for the quantification of Trichuris trichiura infection. PLoS Negl Trop Dis. (2021) 15:e0009677. doi: 10.1371/journal.pntd.0009677
145. Bucki M, Dhufaigh KN, O'Brien C, Weatherley A, Walshe N, McElligott T. Comparison of ovine faecal Strongyle egg counts from an accredited laboratory and a rapid, on-site parasite diagnostic system utilising a smartphone app and machine learning. Vet Parasitol. (2023) 320:109976. doi: 10.1016/j.vetpar.2023.109976
146. Ochwo S, Perez AM, Pérez Aguirreburualde MS. Beyond accuracy: leveraging ASSURED criteria for field evaluation of point-of-care tests for food animal diseases. Front Vet Sci. (2023) 10: doi: 10.3389/fvets.2023.1239111
147. Kaplan RM, Denwood MJ, Nielsen MK, Thamsborg SM, Torgerson PR, Gilleard JS, et al. World Association for the Advancement of Veterinary Parasitology (WAAVP) guideline for diagnosing anthelmintic resistance using the faecal egg count reduction test in ruminants, horses and swine. Vet Parasitol. (2023) 318:109936. doi: 10.1016/j.vetpar.2023.109936
148. Nielsen MK. Anthelmintic resistance in equine nematodes: current status and emerging trends. Int J Parasitol Drugs Drug Resist. (2022) 20:76–88. doi: 10.1016/j.ijpddr.2022.10.005
149. Coles GC, Bauer C, Borgsteede FHM, Geerts S, Klei TR, Taylor MA. Waller PJ. World Association for the Advancement of Veterinary Parasitology (WAAVP) methods for the detection of anthelmintic resistance in nematodes of veterinary importance. Vet Parasitol. (1992) 44:35–44. doi: 10.1016/0304-4017(92)90141-U
150. Königová A, Várady M, Corba J. Comparison of in vitro methods and faecal egg count reduction test for the detection of benzimidazole resistance in small strongyles of horses. Vet Res Commun. (2003) 27:281–8. doi: 10.1023/A:1024079907895
151. Königová A, Craven J, Bjørn H, Barnes EH, Henriksen SA, Nansen P, et al. comparison of in vitro tests and a faecal egg count reduction test in detecting anthelmintic resistance in horse strongyles. Vet Parasitol. (1999) 85:49–59. doi: 10.1016/S0304-4017(99)00113-2
152. Whittaker JH, Carlson SA, Jones DE, Brewer MT. Molecular mechanisms for anthelmintic resistance in strongyle nematode parasites of veterinary importance. Vet Pharm & Therapeutics. (2017) 40:105–15. doi: 10.1111/jvp.12330
153. Fissiha W, Kinde MZ. Anthelmintic resistance and its mechanism: a review. Infect Drug Resist. (2021) 14:5403–10. doi: 10.2147/IDR.S332378
154. Chen X, Shi Y, Wang T, Liu C, Wang W, Wang Y. Study on the effect of GCY-12 gene on albendazole sensitivity of Haemonchus contortus by RNA interference. Front Vet Sci. (2025) 12:1567869. doi: 10.3389/fvets.2025.1567869
155. von Samson-Himmelstjerna G, Thompson RA, Krücken J, Grant W, Bowman DD, Schnyder M, et al. Spread of anthelmintic resistance in intestinal helminths of dogs and cats is currently less pronounced than in ruminants and horses – Yet it is of major concern. Int J Parasitol. (2021) 17:36–45. doi: 10.1016/j.ijpddr.2021.07.003
156. Mpofu TJ, Nephawe KA, Mtileni B. Prevalence and resistance to gastrointestinal parasites in goats: a review. Vet World. (2022) 15:2442–52. doi: 10.14202/vetworld.2022.2442-2452
157. Wit J, Dilks CM, Andersen EC. Complementary approaches with free-living and parasitic nematodes to understanding anthelmintic resistance. Trends Parasitol. (2021) 37:240–50. doi: 10.1016/j.pt.2020.11.008
158. Papaiakovou M, Waeschenbach A, Ajibola O, Ajjampur SS, Anderson RM, Bailey R, et al. Global diversity of soil-transmitted helminths reveals population-biased genetic variation that impacts diagnostic targets. Nat Commun. (2025) 16:6374. doi: 10.1038/s41467-025-61687-0
159. Chen X, Wang T, Guo W, Yan X, Kou H, Yu Y, et al. Transcriptome reveals the roles and potential mechanisms of lncRNAs in the regulation of albendazole resistance in Haemonchus contortus. BMC Genomics. (2024) 25:188. doi: 10.1186/s12864-024-10096-6
160. Dabrowska J, Sroka J, Cencek T. Investigating Cryptosporidium spp. using genomic, proteomic and transcriptomic techniques: current progress and future directions. IJMS. (2023) 24:12867. doi: 10.3390/ijms241612867
161. Antonopoulos A, Gilleard JS, Charlier J. Next-generation sequencing technologies for helminth diagnostics and surveillance in ruminants: shifting diagnostic barriers. Trends Parasitol. (2024) 40:511–26. doi: 10.1016/j.pt.2024.04.013
162. Quansah E, Chen Y, Yang S, Wang J, Sun D, Zhao Y, et al. CRISPR-Cas13 in malaria parasite: diagnosis and prospective gene function identification. Front Microbiol. (2023) 14:1076947. doi: 10.3389/fmicb.2023.1076947
163. Lobanov VA, Peckle M, Massard CL, Brad Scandrett W, Gajadhar AA. Development and validation of a duplex real-time PCR assay for the diagnosis of equine piroplasmosis. Parasit Vectors. (2018) 11:125. doi: 10.1186/s13071-018-2751-6
164. Piesz J, Scro A, Corbett R, Markey Lundgren K, Smolowitz R, Gomez-Chiarri M. Development of a multiplex qPCR for the quantification of three protozoan parasites of the eastern oyster Crassostrea virginica. Dis Aquat Org. (2022) 151:111–21. doi: 10.3354/dao03694
165. Robert-Gangneux F, Duval X, Cazala C, Belaz S, Dupuis A, Guegan H, et al. Improvement of the diagnosis of intestinal protozoa using a multiplex qPCR strategy compared to classical microscopy: a prospective study on 3,500 stool samples over 3 years. J Clin Microbiol. (2025) 63:e0161024. doi: 10.1128/jcm.01610-24
166. Kotze AC, Gilleard JS, Doyle SR, Prichard RK. Challenges and opportunities for the adoption of molecular diagnostics for anthelmintic resistance. Int J Parasitol Drugs Drug Resist. (2020) 14:264–73. doi: 10.1016/j.ijpddr.2020.11.005
167. Mbong Ngwese M, Prince Manouana G, Nguema Moure PA, Ramharter M, Esen M, Adégnika AA. Diagnostic techniques of soil-transmitted helminths: impact on control measures. TropicalMed. (2020) 5:93. doi: 10.3390/tropicalmed5020093
168. Li X, Dang Z, Tang W, Zhang H, Shao J, Jiang R, et al. Detection of parasites in the field: the ever-innovating CRISPR/Cas12a. Biosensors. (2024) 14:14 5. doi: 10.3390/bios14030145
169. Feddema JJ, Fernald KDS, Keijser BJF, Kieboom J, van de Burgwal LHM. Commercial opportunity or addressing unmet needs—loop-mediated isothermal amplification (LAMP) as the future of rapid diagnostic testing? Diagnostics. (2024) 14:1845. doi: 10.3390/diagnostics14171845
170. Bari T. Al Mamun MdA, Toet H, Rathinasamy V, Larkins J-A, Beddoe T, Spithill TW, Piedrafita D, Greenhill AR. Evaluation of LAMP for Fasciola hepatica detection from faecal samples of experimentally and naturally infected cattle. Vet Parasitol. (2024) 327:110132. doi: 10.1016/j.vetpar.2024.110132
171. Doyle SR, Sankaranarayanan G, Allan F, Berger D, Jimenez Castro PD, Collins JB, et al. Evaluation of DNA extraction methods on individual helminth egg and larval stages for whole-genome sequencing. Front Genet. (2019) 10:826. doi: 10.3389/fgene.2019.00826
172. Li Y, Zheng R, Wu Y, Chu K, Xu Q, Sun M, et al. Low-cost, automated parasite diagnostic system via a portable, robotic microscope and deep learning. J Biophotonics. (2019) 12:e201800410. doi: 10.1002/jbio.201800410
173. Saha B, Tchiotsop D, Tchinda R, Wolf D, Noubom M. Automatic recognition of human parasite cysts on microscopic stools images using principal component analysis and probabilistic neural network. IJARAI. (2015) 4:26–33. doi: 10.14569/IJARAI.2015.040906
174. Widmer KW, Oshima KH, Pillai SD. Identification of Cryptosporidium parvum oocysts by an artificial neural network approach. Appl Environ Microbiol. (2002) 68:1115–21. doi: 10.1128/AEM.68.3.1115-1121.2002
175. Widmer KW, Srikumar D, Pillai SD. Use of artificial neural networks to accurately identify cryptosporidium oocyst and giardia cyst Images. Appl Environ Microbiol. (2005) 71:80–4. doi: 10.1128/AEM.71.1.80-84.2005
176. Januário LA de F. Optimization of Resistance to Gastrointestinal Nematodes in Santa Inês sheep: A Genomic Selection, Machine Learning and Image Analysis Approach. Universidade de São Paulo (2023). Available online at: https://www.teses.usp.br/teses/disponiveis/17/17135/tde-05062023-132524/ (accessed August 7, 2025).
177. Yilmaz Adkinson A, Abouhawwash M, VandeHaar MJ, Parker Gaddis KL, Burchard J, Peñagaricano F, et al. Assessing different cross-validation schemes for predicting novel traits using sensor data: an application to dry matter intake and residual feed intake using milk spectral data. J Dairy Sci. (2024) 107:8084–99. doi: 10.3168/jds.2024-24701
178. Mota LFM, Giannuzzi D, Pegolo S, Toledo-Alvarado H, Schiavon S, Gallo L, et al. Combining genetic markers, on-farm information and infrared data for the in-line prediction of blood biomarkers of metabolic disorders in Holstein cattle. J Animal Sci Biotechnol. (2024) 15:83. doi: 10.1186/s40104-024-01042-3
179. Aboshady HM, Stear MJ, Johansson A, Jonas E, Bambou JC. Immunoglobulins as biomarkers for gastrointestinal nematodes resistance in small ruminants: a systematic review. Sci Rep. (2020) 10:7765. doi: 10.1038/s41598-020-64775-x
180. Santana TEZ, Silva JCF, da Silva LOC, Alvarenga AB, Menezes GRO, Torres RAA, et al. Genome-enabled classification of stayability in Nellore cattle under a machine learning framework. Livest Sci. (2022) 260:104935. doi: 10.1016/j.livsci.2022.104935
181. Kjetså MV, Gjuvsland AB, Nordbø Ø, Grindflek E, Meuwissen T. Accuracy of genomic prediction of maternal traits in pigs using Bayesian variable selection methods. J Animal Breeding Genetics. (2022) 139:654–65. doi: 10.1111/jbg.12729
182. Quinn JA, Nakasi R, Mugagga PKB, Byanyima P, Lubega W, Andama A. Deep convolutional neural networks for microscopy-based point of care diagnostics. PMLR. (2016) 56:271–81.
183. Li S, Du Z, Meng X, Zhang Y. Multi-stage malaria parasite recognition by deep learning. GigaScience. (2021) 10:giab040. doi: 10.1093/gigascience/giab040
184. Li S, Li A, Molina Lara DA, Gómez Marín JE, Juhas M, Zhang Y. Transfer learning for Toxoplasma gondii recognition. mSystems. (2020) 5:e00445-19. doi: 10.1128/msystems.00445-19
185. Palasuwan D, Naruenatthanaset K, Kobchaisawat T, Chalidabhongse TH, Nunthanasup N, Boonpeng K, et al. (2022). “Parasitic egg detection and classification in microscopic images,” in IEEE Dataport.
186. Hiremath PS, Bannigidad P. Identification and classification of cocci bacterial cells in digital microscopic images. IJCBDD. (2011) 4:262. doi: 10.1504/IJCBDD.2011.041414
187. El-Sayed SAE-S, Rizk MA, Baghdadi HB, Ringo AE, Sambuu G, Nugraha AB, et al. Development of a promising antigenic cocktail for the global detection of Babesia caballi in horse by ELISA. PLoS ONE. (2023) 18:e0284535. doi: 10.1371/journal.pone.0284535
188. Mitchell MC, Tzelos T, Handel I, McWilliam HEG, Hodgkinson JE, Nisbet AJ, et al. Development of a recombinant protein-based ELISA for diagnosis of larval cyathostomin infection. Parasitology. (2016) 143:1055–66. doi: 10.1017/S0031182016000627
189. Camoin M, Kocher A, Chalermwong P, Yangtarra S, Kamyingkird K, Jittapalapong S, et al. The indirect ELISA Trypanosoma evansi in equids: optimisation and application to a serological survey including racing horses, in Thailand. Biomed Res Int. (2019) 2019:1–12. doi: 10.1155/2019/2964639
190. Moore JN, Steiss J, Nicholson WE, Orth DN, Moore JN, Steiss J, et al. A case of pituitary adrenocorticotropin-dependent cushing's syndrome in the horse. Endocrinology. (1979) 104:576–82. doi: 10.1210/endo-104-3-576
191. Caporale V, Biancifiori F, Frescura F, Dimatteo A, Nannini D, Urbani G. Comparison of various tests for the serological diagnosis of Trypanosoma equiperdum infection in the horse. Comp Immunol Microbiol Infect Dis. (1981) 4:243–6. doi: 10.1016/0147-9571(81)90009-6
192. Mercer JG, Munn AE, Rees HH. Echinococcus granulosus: occurrence of ecdysteroids in protoscoleces and hydatid cyst fluid. Mol Biochem Parasitol. (1987) 24:203–14. doi: 10.1016/0166-6851(87)90107-1
193. Rüegg SR, Torgerson P, Deplazes P, Mathis A. Age-dependent dynamics of Theileria equi and Babesia caballi infections in southwest Mongolia based on IFAT and/or PCR prevalence data from domestic horses and ticks. Parasitology. (2007) 134:939–47. doi: 10.1017/S0031182007002405
194. Jongejan F, Du C, Papadopoulos E, Blanda V, Di Bella S, Cannella V, et al. Diagnostic performance of a rapid immunochromatographic test for the simultaneous detection of antibodies to Theileria equi and Babesia caballi in horses and donkeys. Parasit Vectors. (2021) 17:160. doi: 10.1186/s13071-024-06253-1
195. Borges-Silva W, de Jesus RF, Ferreira R, Gondim LFP. Reactivity of horse sera to antigens derived from Sarcocystis falcatula–Like and Sarcocystis neurona. Front Vet Sci. (2020) 7:573016. doi: 10.3389/fvets.2020.573016
196. Tirosh-Levy S, Mazuz M, Savitsky I, Pinkas D, Gottlieb Y, Steinman A. Serological and molecular prevalence of Babesia caballi in apparently healthy horses in Israel. Pathogens. (2021) 10:445. doi: 10.3390/pathogens10040445
197. Onyiche TE, Sivakumar T, Tuvshintulga B, Nugraha AB, Ahedor B, Mofokeng L, et al. Serosurvey for equine piroplasms in horses and donkeys from North-Western Nigeria using IFAT and ELISA. J Immunoassay Immunochem. (2021) 42:648–61. doi: 10.1080/15321819.2021.1935274
198. Claes F, Ilgekbayeva GD, Verloo D, Saidouldin TS, Geerts S, Buscher P, et al. Comparison of serological tests for equine trypanosomosis in naturally infected horses from Kazakhstan. Vet Parasitol. (2005) 131:221–5. doi: 10.1016/j.vetpar.2005.05.001
199. Hébert L, Froger D, Madeline A, Lecouturier F, Lemans C, Zientara S. European inter-laboratory proficiency test for dourine antibody detection using the complement fixation test. Vet Sci. (2005) 10:592. doi: 10.3390/vetsci10100592
200. Baldani CD, Machado RZ, Raso TF, Pinto AA. Serodiagnosis of Babesia equi in horses submitted to exercise stress. Pesq Vet Bras. (2007) 27:179–83. doi: 10.1590/S0100-736X2007000400009
201. Weiland G. Species-specific serodiagnosis of equine piroplasma infections by means of complement fixation test (CFT), immunofluorescence (IIF), and enzyme-linked immunosorbent assay (ELISA). Vet Parasitol. (1986) 20:43–8. doi: 10.1016/0304-4017(86)90091-9
202. Kaspar A, Pfister K, Nielsen MK, Silaghi C, Fink H, Scheuerle MC, et al. Detection of Strongylus vulgaris in equine faecal samples by real-time PCR and larval culture – method comparison and occurrence assessment. BMC Vet Res. (2016) 13:19. doi: 10.1186/s12917-016-0918-y
203. Tirosh-Levy S, Steinman A, Levy H, Katz Y, Shtilman M, Gottlieb Y. Parasite load and genotype are associated with clinical outcome of piroplasm-infected equines in Israel. Parasit Vectors. (2020) 13:267. doi: 10.1186/s13071-020-04133-y
204. Elsawy BSM, Nassar AM, Alzan HF, Bhoora RV, Ozubek S, Mahmoud MS, et al. Rapid detection of equine piroplasms using multiplex PCR and first genetic characterization of Theileria haneyi in Egypt. Pathogens. (2021) 10:1414. doi: 10.3390/pathogens10111414
205. Diekmann I, Blazejak K, Krücken J, Strube C, von Samson-Himmelstjerna G, Diekmann I, et al. Comparison of morphological and molecular Strongylus spp. identification in equine larval cultures and first report of a patent Strongylus asini infection in a horse. Equine Vet J. (2025) 57:522–9. doi: 10.1111/evj.14134
206. Stout AE, Hofmar-Glennon HG, André NM, Goodman LB, Anderson RR, Mitchell PK, et al. Infectious disease surveillance of apparently healthy horses at a multi-day show using a novel nanoscale real-time PCR panel. J VET Diagn Invest. (2021) 33:80–6. doi: 10.1177/1040638720972096
207. Chen K, Hu Z, Yang G, Guo W, Qi T, Liu D, et al. Development of a duplex real-time PCR assay for simultaneous detection and differentiation of Theileria equi and Babesia caballi. Transbound Emerg Dis. (2022) 69:e1338–49. doi: 10.1111/tbed.14464
208. Gummery L, Jallow S, Raftery AG, Bennet E, Rodgers J, Sutton DGM, et al. Comparison of loop-mediated isothermal amplification (LAMP) and PCR for the diagnosis of infection with Trypanosoma brucei ssp. in equids in The Gambia. PLoS ONE. (2020) 15:e0237187. doi: 10.1371/journal.pone.0237187
209. Ahmed ME, Eldigail MH, Elamin FM, Ali IA, Grobusch MP, Aradaib IE. Development and evaluation of real-time loop-mediated isothermal amplification assay for rapid detection of cystic echinococcosis. BMC Vet Res. (2016) 12:202. doi: 10.1186/s12917-016-0809-2
210. Salim B, Hayashida K, Mossaad E, Nakao R, Yamagishi J, Sugimoto C, et al. Development and validation of direct dry loop mediated isothermal amplification for diagnosis of Trypanosoma evansi. Vet Parasitol. (2018) 260:53–7. doi: 10.1016/j.vetpar.2018.08.009
211. Hifumi T, Akioka K, Tanaka T, Miyoshi N. Development of a loop-mediated isothermal amplification (LAMP) assay targeting the mitochondrial cytochrome b gene for the rapid detection of alveolar echinococcosis in hepatic nodules of horses. Vet Parasitol. (2021) 299:109573. doi: 10.1016/j.vetpar.2021.109573
212. Choi B, Vu HT, Vu HT, Radwanska M, Magez S, Choi B, et al. Advances in the immunology of the host–parasite interactions in African Trypanosomosis, including single-cell transcriptomics. Pathogens. (2024) 13:188. doi: 10.3390/pathogens13030188
213. Adamu L, Turaki U, BukarKolo Y, Husainy A, Dauda I, Wakil Y, et al. Current updates on diagnostic methodologies for tick-borne hemoparasitic diseases in equids: a review. J Adv Vet Anim Res. (2016) 3:84. doi: 10.5455/javar.2016.c148
214. Gavriliuc ST. Development of Non-Invasive Genomic Tools for Feral Horses. (Master's thesis, University of Calgary, Calgary, Canada) (2023). doi: 10.11575/PRISM/40699
215. Sabir N, Chaudhry ZI, Aslam A, Muhammad K, Shahid M, Hussain A, et al. A study on prevalence and molecular characterization of trypanosomal species infecting equines in Lahore region, Pakistan. J Parasit Dis. (2018) 42:96–101. doi: 10.1007/s12639-017-0972-9
216. Zuccherato LW, Furtado LF, Medeiros CS, Pinheiro CS, Rabelo ÉM. PCR-RFLP screening of polymorphisms associated with benzimidazole resistance in Necator americanus and Ascaris lumbricoides from different geographical regions in Brazil. PLoS Negl Trop Dis. (2018) 12:e0006766. doi: 10.1371/journal.pntd.0006766
217. Song J, Song R, Wang P, Zhang Y, Yan Y, Zhou J, et al. Preparation of monoclonal antibody against Ema-1 and development of rapid serological detection method for Theileria equi infection, Xinjiang, China. J Parasitol. (2020) 106:283. doi: 10.1645/19-98
218. Furtado LFV, Magalhães JGS, Rabelo ÉML, Furtado LFV, Magalhães JGS, Rabelo ÉML. Standardization and application of a modified RFLP-PCR methodology for analysis of polymorphisms linked to treatment resistance in Ancylostoma braziliense. Parasit Vectors. (2018) 11:540. doi: 10.1186/s13071-018-3125-9
219. Davaasuren B, Amgalanbaatar T, Musinguzi SP, Suganuma K, Otgonsuren D, Mossaad E, et al. The evaluation of GM6-based ELISA and ICT as diagnostic methods on a Mongolian farm with an outbreak of non-tsetse transmitted horse trypanosomosis. Vet Parasitol. (2017) 244:123–8. doi: 10.1016/j.vetpar.2017.07.036
220. Maharana BR, Tewari AK, Saravanan BC, Sudhakar NR, Maharana BR, Tewari AK, et al. Important hemoprotozoan diseases of livestock: challenges in current diagnostics and therapeutics: an update. Vet World. (2016) 9:487–95. doi: 10.14202/vetworld.2016.487-495
221. Gehlen H, Wulke N, Ertelt A, Nielsen MK, Morelli S, Traversa D, et al. Comparative analysis of intestinal helminth infections in colic and non-colic control equine patients. Animals. (2020) 10:1916. doi: 10.3390/ani10101916
222. Kooyman FNJ, van Doorn DCK, Geurden T, Wagenaar JA, Kooyman FNJ, van Doorn DCK, et al. Semi-quantitative differentiation of cyathostomin larval cultures by reverse line blot. Vet Parasitol. (2016) 216:59–65. doi: 10.1016/j.vetpar.2015.12.009
223. Johnson ACB, Biddle AS. the use of molecular profiling to track equine reinfection rates of cyathostomin species following anthelmintic administration. Animals. (2021) 11:1345. doi: 10.3390/ani11051345
224. Alzan HF, Mahmoud MS, Suarez CE. Current vaccines, experimental immunization trials, and new perspectives to control selected vector borne blood parasites of veterinary importance. Front Vet Sci. (2024) 11:1484787. doi: 10.3389/fvets.2024.1484787
225. Drescher G, dos Santos HG, Pinto MMG, Morello LG, Figueiredo FB. Diagnosis of fasciolosis antibodies in Brazilian cattle through ELISA employing both native and recombinant antigens. Microbiol Spectr. (2024) 12:e0009524. doi: 10.1128/spectrum.00095-24
226. de Albergaria IS, Elsheikha H. Diagnostic challenges and alternatives for difficult-to-detect equine gastrointestinal parasites. UK-Vet Equine. (2025) 9:138–47. doi: 10.12968/ukve.2024.0028
227. Baghdadi HBA, Abdelsalam M, Attia MM. Diagnostic innovations in equine parasitology: a nanogold-ELISA for sensitive serodiagnosis of migratory Strongylus vulgaris larvae infections. BMC Vet Res. (2024) 20:579. doi: 10.1186/s12917-024-04389-x
228. Hongsrichan N, Donthaisong P, Chaisongkram C, Eamudomkarn C, Pitaksakulrat O, Ponsrila K, et al. Epidemiological surveillance of intestinal parasites and serological analysis of Toxoplasma gondii in captive felids from Thailand zoos. Vet Med Int. (2025) 2025:1596677. doi: 10.1155/vmi/1596677
229. Attia MM, Omar HM. Gastric equine myiasis: prevalence, pathogenesis, molecular and serological implications. J Parasit Dis. (2025) 1–9. doi: 10.1007/s12639-025-01782-7
230. Gondim LFP, Mineo JR, Schares G. Importance of serological cross-reactivity among Toxoplasma gondii, Hammondia spp, Neospora spp, Sarcocystis spp and Besnoitia besnoiti. Parasitology. (2017) 144:851–68. doi: 10.1017/S0031182017000063
231. Davenport K, Liu J, Sarquis J, Beall M, Montoya A, Drexel J, et al. Performance of a point-of-care test for the detection of anti-Leishmania infantum antibodies is associated with immunofluorescent antibody titer and clinical stage of leishmaniosis in dogs from endemic regions. Vet Parasitol Reg Stud Reports. (2024) 53:101061. doi: 10.1016/j.vprsr.2024.101061
232. Toaleb NI, Shaapan RM, Elaadli H, Abdel Megeed KN, Aboelsoued D. Sarcocystis species: molecular identification and seroprevalence in water buffaloes (Bubalus bubalis). BMC Vet Res. (2025) 21:482. doi: 10.1186/s12917-025-04933-3
233. Gharban H, Sray A, Essa I. Serological prevalence of anti-Fasciola hepatica antibodies in sheep. J Vet Sci. (2024) 55:1583–90. doi: 10.21608/ejvs.2024.257463.1742
234. Hobbs EC, Colling A, Gurung RB, Allen J. The potential of diagnostic point-of-care tests (POCTs) for infectious and zoonotic animal diseases in developing countries: technical, regulatory and sociocultural considerations. Transbound Emerg Dis. (2021) 68:1835–49. doi: 10.1111/tbed.13880
235. Domrazek K, Jurka P. Application of next-generation sequencing (NGS) techniques for selected companion animals. Animals. (2024) 14:1578. doi: 10.3390/ani14111578
236. Leutenegger CM, Lozoya CE, Tereski J, Andrews J, Mitchell KD, Meeks C, et al. Comparative study of a broad qPCR panel and centrifugal flotation for detection of gastrointestinal parasites in fecal samples from dogs and cats in the United States. Parasit Vectors. (2023) 16:288. doi: 10.1186/s13071-023-05904-z
237. Baltrušis P Höglund J. Digital PCR: modern solution to parasite diagnostics and population trait genetics. Parasit Vectors. (2023) 16:143. doi: 10.1186/s13071-023-05756-7
238. Musa Abdullahi A, Mallam Hamisu T, Muhammed Chafe U, Ibrahim Daneji A. Olayinka. Alayande M. Molecular identification of Trypanosoma evansi isolated from Camels (Camelus dromedarius) in Sokoto and its environs, Nigeria. SAJOLS. (2025) 3:298–303. doi: 10.33003/sajols-2025-0302-36
239. Peña-Espinoza M, Em D, Shahi-Barogh B, Berer D, Duscher GG, van der Vloedt L, et al. Molecular pathogen screening of louse flies (Diptera: Hippoboscidae) from domestic and wild ruminants in Austria. Parasit Vectors. (2023) 16:179. doi: 10.1186/s13071-023-05810-4
Keywords: horses, parasites, serology, molecular biology, diagnosis, sustainable control
Citation: Wang T, Chen X, Yan X, Su Y, Gao W, Liu C and Wang W (2025) Progress in serology and molecular biology of equine parasite diagnosis: sustainable control strategies. Front. Vet. Sci. 12:1663577. doi: 10.3389/fvets.2025.1663577
Received: 10 July 2025; Accepted: 20 August 2025;
Published: 04 September 2025.
Edited by:
Guanghui Zhao, Northwest A&F University, ChinaReviewed by:
Qingxia Wu, Tibet Agricultural and Animal Husbandry University, ChinaAli Raza, University of Engineering and Technology, Pakistan
Copyright © 2025 Wang, Chen, Yan, Su, Gao, Liu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenlong Wang, d3dsLmltYXVAMTYzLmNvbQ==
 Tengyu Wang
Tengyu Wang Xindi Chen
Xindi Chen Xu Yan2
Xu Yan2