- Anhui Agricultural University, Hefei, China
Background: Strawberry vines are nutrient-rich but contain high levels of tannins and have a moisture content of up to 70%, making ensiling a crucial strategy for preserving their nutritional value.
Methods: Two lactic acid bacteria (LAB) strains, designated Z and R, were isolated from strawberry vines. Fresh strawberry vines were ensiled in three groups: two experimental groups inoculated with strains Z and R (1.0 × 106 CFU/g fresh weight) and a control group (CK) treated with physiological saline. Samples were collected on days 1, 3, 5, 7, 15, 30, and 45 of fermentation for analysis. The LAB-treated silage was subsequently incorporated into Hu sheep diets to evaluate its effects on growth performance.
Results: The identification results indicated that strain Z was Lactiplantibacillus plantarum and strain R was Lactococcus lactis. Both treatment groups exhibited significantly higher crude protein (CP) content (p < 0.05) and markedly lower NH3-N content (p < 0.05) compared to the CK group. Notably, supplementation with L. plantarum significantly reduced tannin content in strawberry vines (p < 0.05) compared to other two groups. By day 3, the pH values in the treatment groups were significantly lower than those in the CK group (p < 0.05), with L. plantarum treatment group showing significantly lower pH than L. lactis group throughout days 3 to 15 (p < 0.05), indicating more rapid silage stabilization. In terms of fermentation quality, lactic acid (LA) and acetic acid (AA) contents in the treatment groups were significantly higher than in the CK group (p < 0.05). Microbial community analysis demonstrated that both treatment groups effectively suppressed the growth of harmful microorganisms at both the phylum and genus levels, with Lactiplantibacillus genus abundance reaching 54.18% in L. plantarum group compared to only 0.98% in CK by day 3. Furthermore, when used as roughage for Hu sheep, LAB-fermented strawberry vine silage significantly improved average daily gain (ADG) (p < 0.05) and enhanced the apparent digestibility of dry matter (DM), neutral detergent fiber (NDF), and CP (p < 0.05). Inclusion of strawberry vine silage fermented by L. plantarum markedly reduced the relative abundances of the predominant ruminal genera Clostridium and Ercella.
Conclusion: LAB supplementation significantly improved the silage quality of strawberry vines by effectively inhibiting putrefactive processes during the late fermentation stage of high-moisture silage. Moreover, the use of strawberry vine silage as feed markedly enhanced the growth performance in Hu sheep.
Highlights
• This study represents the first attempt to use strawberry vines as feed.
• Anaerobic fermentation using LAB reduces the tannin content in strawberry vines.
• The addition of two LAB strains significantly increased organic acid content.
• The two LAB strains effectively preserved the nutrients of strawberry vines.
• Strawberry vines silage effectively improves the growth performance in Hu sheep.
1 Introduction
A sufficient supply of high-quality roughage is essential for the sustainable development of herbivorous livestock industries. However, China currently faces a shortage of nearly 50 million tons of premium roughage, highlighting the need to explore alternative resources (1). The efficient utilization of crop straw offers a promising solution for producing high-quality roughage. Although China generates approximately 800 million tons of crop straw annually, only about 20% is utilized in ruminant farming owing to its low nutritional value and poor palatability (2).
Strawberry (Fragaria genus, Rosaceae family), a perennial herbaceous plant, is rich in bioactive and nutritional compounds, such as anthocyanins, flavonols, ellagitannins, folic acid, and various vitamins. These constituents contribute to antioxidant activity, immune system enhancement, anti-aging effects, and cholesterol reduction. Strawberry vines, which contain CP, ether extract (EE), vitamin C, and trace minerals, exhibit good palatability and represent a source of high-quality forage. To date, relatively few studies have investigated strawberry vines, and their potential has not been effectively utilized. Following strawberry production, the residual by-product—the strawberry vines—remains unprocessed, resulting in environmental damage. Harvested during the rainy season in April and May in southern China, fresh strawberry vines typically exhibit a moisture content of approximately 70%, rendering natural drying impractical for preserving their nutritional quality. Ensiling offers an effective solution for nutrient preservation and the production of high-quality silage. This approach not only meets the demand for premium forage among herbivores but also helps reduce agricultural waste pollution, thereby supporting sustainable agro-pastoral development. Utilizing strawberry vine feed can mitigate environmental impacts, support the sustainable and circular development of the strawberry industry, and provide a substantial source of roughage for ruminant production.
Silage fermentation depends on LAB naturally present on fresh crops. These bacteria utilize water-soluble carbohydrates (WSC) under anaerobic conditions to produce LA, thereby creating an acidic environment that inhibits the growth of spoilage microorganisms (3). In the initial phase of ensiling, LAB dominates by metabolizing WSC into organic acids, which suppress the proliferation of harmful microbes (4–6). However, the competitive dynamics between LAB and other microorganisms are influenced by factors such as moisture content, airtightness, and the physiological characteristics of the LAB strains involved. The ability of LAB to rapidly adapt to the ensiling environment and reduce pH is crucial for achieving high-quality silage.
High-moisture silage (moisture content >70%) is susceptible to clostridial fermentation, which leads to the production of butyric acid and a consequent decline in silage quality (7). Excessive moisture dilutes WSC and LAB, hindering a rapid decline in pH (8). This environment further promotes clostridial activity, resulting in increased DM losses, protein hydrolysis, and butyrate accumulation. These factors collectively reduce feed digestibility, nitrogen utilization, and intake (9). Field wilting is a commonly used strategy to reduce moisture content; however, it carries risks such as physical losses, respiratory consumption of WSC, dependence on weather conditions, and mold contamination. As an alternative, additives can modulate microbial fermentation, suppress harmful microbes, and improve high-moisture silage quality. Different silage materials contain varying levels of anti-nutritional factors, such as tannins, which can affect the activity of LAB during ensiling and, consequently, influence silage quality (10). The tannin content of strawberry vines exceeds 2%, classifying them as a high-tannin silage material. Therefore, exploring indigenous LAB from strawberry vines could be beneficial for improving their silage quality. To address the challenges associated with the high moisture content and tannin-rich composition of strawberry vines, this study isolated LAB strains from strawberry vines and employed them for ensiling. The fermented silage was subsequently fed to Hu sheep to evaluate the effects of native LAB on the fermentation quality of high-moisture strawberry vines silage and its effectiveness in ruminant production.
2 Materials and methods
2.1 Experimental materials
Fresh strawberry vines were harvested in Changfeng County (117.23°N,32.12°E), Hefei City, Anhui Province, China. The strawberry vines used in the experiments were picked at the end of April.
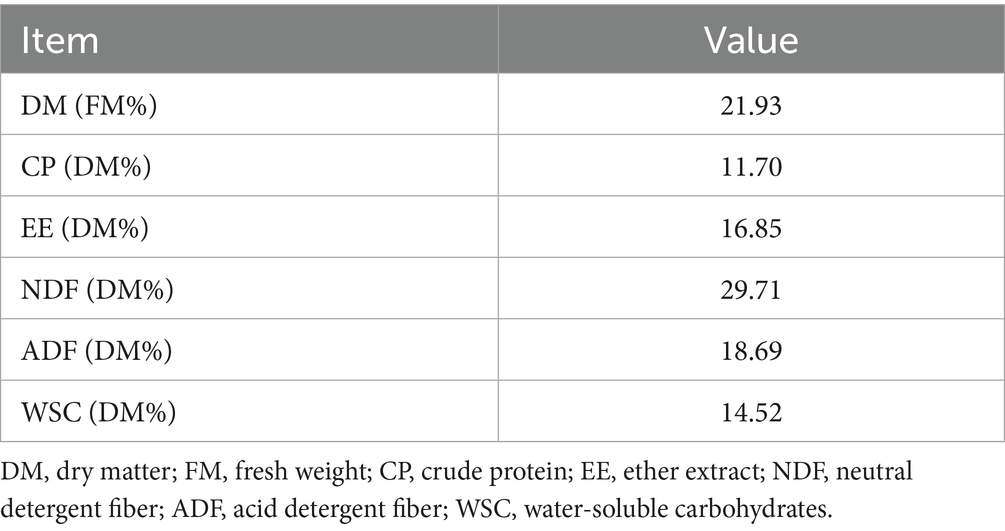
The nutritional composition of the raw strawberry vines material is presented in the Table 1. The DM content of strawberry vines raw materials was 21.93%. On a DM basis, the composition included 11.70% CP, 16.85% EE, 29.71% NDF, 18.69% acid detergent fiber (ADF), and 14.52% WSC.
2.2 Experimental design and sampling
2.2.1 Isolation of LAB from strawberry vines
The isolation and identification of LAB shall refer to the Chinese national standard GB 4789.35–2023 (11). A 10 g sample of fresh strawberry vines was placed in a conical flask containing 90 mL of sterile saline solution for extraction. The resulting extract was serially diluted to concentrations of 10−1, 10−2, 10−3, and 10−4 under aseptic conditions. Aliquots from each dilution were spread onto De Man, Rogosa and Sharpe (MRS) agar plates using a sterile spreader and incubated at 37 °C for 24 h. Colonies exhibiting differences in morphology, size, and color were selected and repeatedly streaked onto fresh MRS agar plates for purification. Pure strains were preserved in 20% (v/v) glycerol at −80 °C.
2.2.2 Silage preparation
Bacterial suspensions (strains Z and R) in the logarithmic growth phase were centrifuged at 4 °C for 3 min. The resulting pellets were washed three times with phosphate-buffered saline (PBS) to prepare the inoculants. Fresh strawberry vines were cut into 2–3 cm segments and mixed with the LAB inoculants under vacuum sealing. Three treatments were established as follows:
• CK group: Fresh strawberry vines + physiological saline (in a volume equivalent to that of the LAB inoculants).
• Treatment I group: Fresh strawberry vines + L. plantarum (strain Z, 1.0 × 106 CFU/g fresh weight).
• Treatment II group: Fresh strawberry vines + L. lactis (strain R, 1.0 × 106 CFU/g fresh weight).
The mixtures were homogenized, packed into silage bags (400 g per bag), vacuum-sealed, and stored in darkness at room temperature. Triplicate samples from each treatment were collected on days 1, 3, 5, 7, 15, 30, and 45 of ensiling for subsequent analysis.
2.2.3 Feeding trial
Fresh strawberry vines were cut into 2–3 cm segments and ensiled with L. plantarum and L. lactis at a concentration of 1.0 × 106 CFU/g. Each bag contained 50 kg of material, was vacuum-sealed, and stored for subsequent feeding to Hu sheep. Eighteen healthy male Hu sheep (initial body weight 27.15 ± 0.34 kg), with similar genetic backgrounds, were randomly assigned to three groups (n = 6 per group) as follows:
• CK group: Basal diet formulated according to the Nutrient Requirements of Meat Sheep (NY/T 816–2021).
• Treatment I group: Basal diet + 15% L. plantarum-fermented strawberry vines silage.
• Treatment II group: Basal diet + 15% L. lactis-fermented strawberry vines silage.
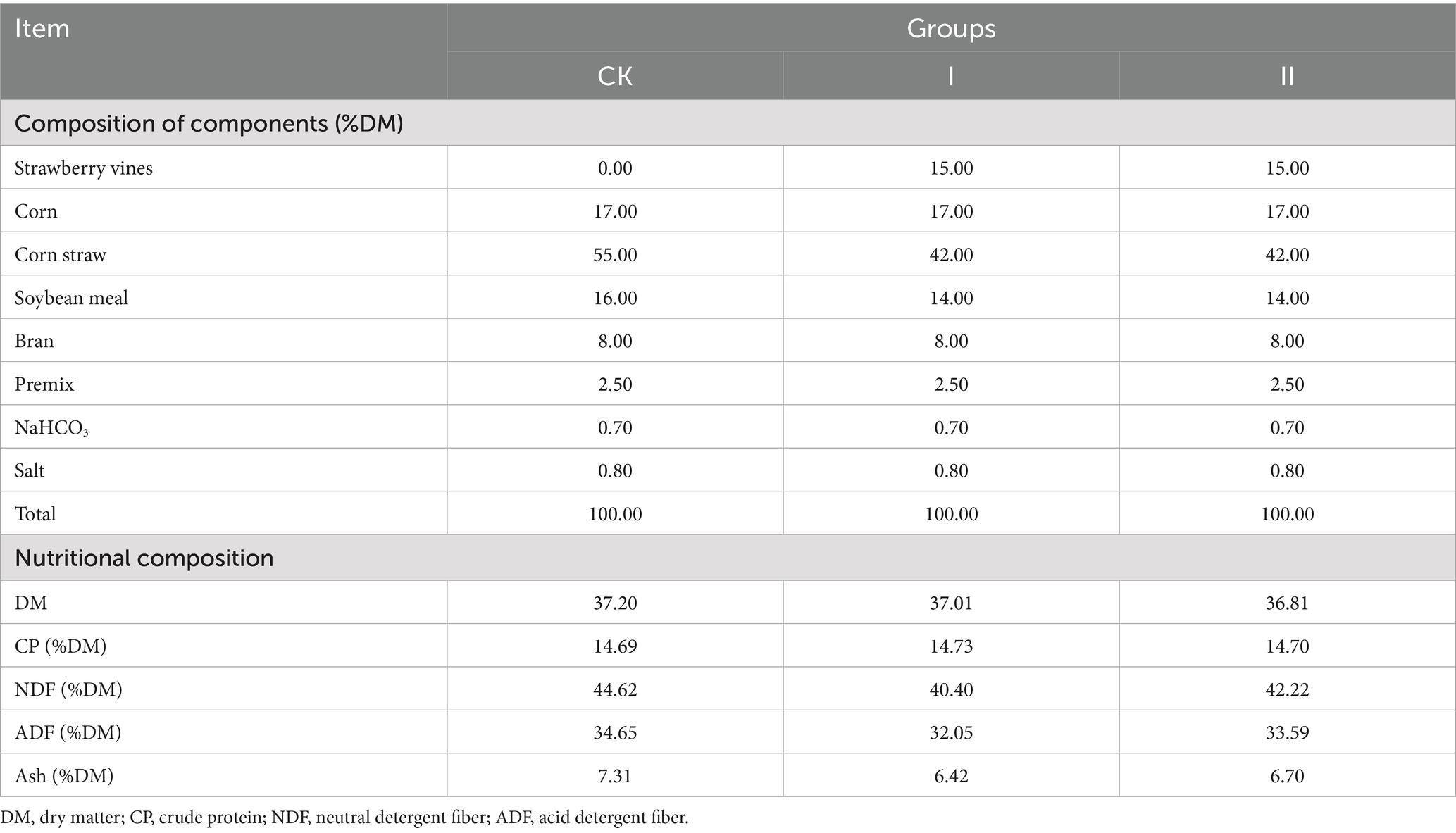
The composition and nutrients of Hu sheep feed are shown in Table 2. Sheep were housed in disinfected pens, dewormed, and acclimatized for 10 days prior to the commencement of the 60-day formal trial. A total mixed ration (TMR) was provided twice daily (at 08:00 and 16:00), with free access to water. Residual feed was collected and weighed daily to calculate dry matter intake (DMI). Body weights were recorded at both the start and end of the trial to determine the ADG and the feed-to-gain ratio (F/G). On day 60 of the trial, rumen fluid samples (5 mL) were collected following a 12-h fasting period for 16S rRNA sequencing to analyze microbial community structure. On day 60, feed and fecal samples were collected to assess apparent digestibility.
2.3 Measurements
2.3.1 LAB identification
Isolated strains were subjected to catalase testing, gas production assays from glucose fermentation, and carbohydrate fermentation assays. Genomic DNA was extracted from enriched cultures using the BIOMIGA DNA Extraction Kit following the manufacturer’s instructions. Polymerase Chain Reaction (PCR) amplification of the 16S rRNA gene was performed using universal primers (27F: 5′-AGRGTTYGATYMTGGCTCAG-3′; 1492R: 5′-RGYTACCTTGTTACGACTT-3′). The reaction mixture (50 μL) consisted of 10 μL of 5 × Fast Pfu Buffer, 2 μL of 2.5 mM dNTPs, 1 μL each of forward and reverse primers (5 μM), 0.5 μL of Fast Pfu Polymerase, 10 ng of template DNA, and ddH₂O. PCR conditions were as follows: initial denaturation at 98 °C for 2 min; 35 cycles of 98 °C for 10 s, 57 °C for 10 s, and 72 °C for 45 s; final extension at 72 °C for 5 min; followed by a hold at 4 °C. The amplified products were sequenced and analyzed via BLAST against the NCBI database (BLAST: Basic Local Alignment Search Tool) for species identification.
2.3.2 Fermentation characteristics and tannin content
For pH measurement, 10 g of silage was mixed with 90 mL of distilled water, extracted at 4 °C for 24 h, and filtered through four layers of gauze. For organic acid analysis, 20 g of silage was homogenized with 180 mL of ultrapure water, filtered through quantitative filter paper and 0.45 μm cellulose ester membranes, and analyzed for LA and AA using high-performance liquid chromatography (HPLC), following the method described by Wang et al. (6). NH₃-N was quantified according to the method of Broderick and Kang et al. (12). The DM content of both silage and apparent digestibility was determined using the oven-drying method according to AOAC standards (13). The CP content of silage and apparent digestibility was analyzed using a fully automated Kjeldahl nitrogen analyzer following AOAC standards (13). Meanwhile, the NDF and ADF contents of silage and apparent digestibility were determined using the methods described by Van Soest et al. (14). EE was determined following GB/T 6433-2006 (15); and WSC was quantified using the Abbkine Soluble Sugar Assay Kit (Abbkine Scientific Co., Ltd., KTB1320). Tannin content was assessed using the Abbkine Tannin Content Assay Kit (Abbkine Scientific Co., Ltd., KTB1541).
Apparent nutrient digestibility was calculated using acid-insoluble ash as an internal marker, according to the following formula:
Where X = apparent digestibility of the nutrient; N = acid-insoluble ash content in the feed; M = acid-insoluble ash content in the feces; K = nutrient content in the feces; L = nutrient content in the feed.
2.3.3 Microbial diversity analysis
Bacterial DNA was extracted from silage and rumen fluid samples using the MagPure Tissue DNA LQ Kit (Magen, D6321-02). DNA concentration and integrity were assessed via NanoDrop 2000 spectrophotometry (Thermo Fisher Scientific, USA) and agarose gel electrophoresis. The V3–V4 hypervariable regions of the 16S rRNA gene were amplified using primers 343F (5′-TACGGRAGGCAGCAG-3′) and 798R (5′-AGGGTATCTAATCCT-3′), with reverse primers containing sample-specific barcodes. PCR products were purified using Agencourt AMPure XP beads (Beckman Coulter, A63880), quantified with the Qubit dsDNA Assay Kit (Thermo Fisher Scientific, Q32854), and sequenced on the Illumina NovaSeq 6000 platform (250 bp paired-end reads; OE Biotech Co., Shanghai, China).
2.4 Data processing and analysis
Data were processed using Excel 2019 and analyzed by one-way Analysis of Variance (ANOVA) followed by Duncan’s multiple range test in SPSS version 20.0. Results are presented as the mean ± standard deviation. Microbial diversity metrics were analyzed using the Personalbio GeneCloud platform (Shanghai Personal Biotechnology Co., Ltd., Shanghai, China). Statistical significance was defined at p < 0.05.
3 Results
3.1 Isolation and identification of LAB
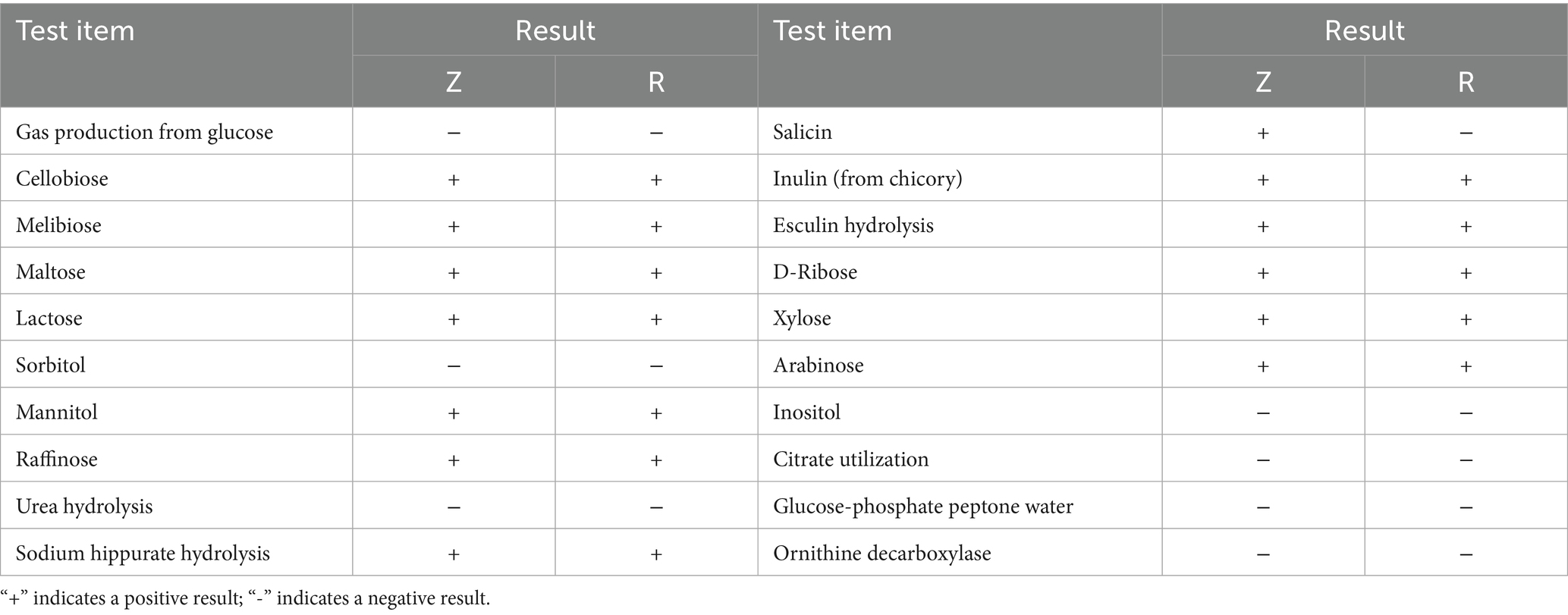
To mitigate tannin-mediated inhibition of LAB activity, we isolated two LAB strains (designated Z and R) from strawberry vines. Their morphological characteristics, Gram staining results, and cellular morphology are detailed in Table 3, while their physiological and biochemical profiles are presented in Table 4. Carbon utilization assays revealed comparable metabolic capabilities between strains Z and R across most substrates, with the notable exception of salicin metabolism, which was unique to strain Z and absent in strain R. 16S rRNA gene sequencing, conducted via PCR amplification followed by BLAST alignment, confirmed 99.39% sequence homology between strain Z and L. plantarum, while strain R exhibited 99.57% homology with L. lactis. An integrative analysis of morphological and molecular data conclusively identified strain Z as L. plantarum and strain R as L. lactis.
3.2 Effect of LAB on the nutritional composition of strawberry vines silage
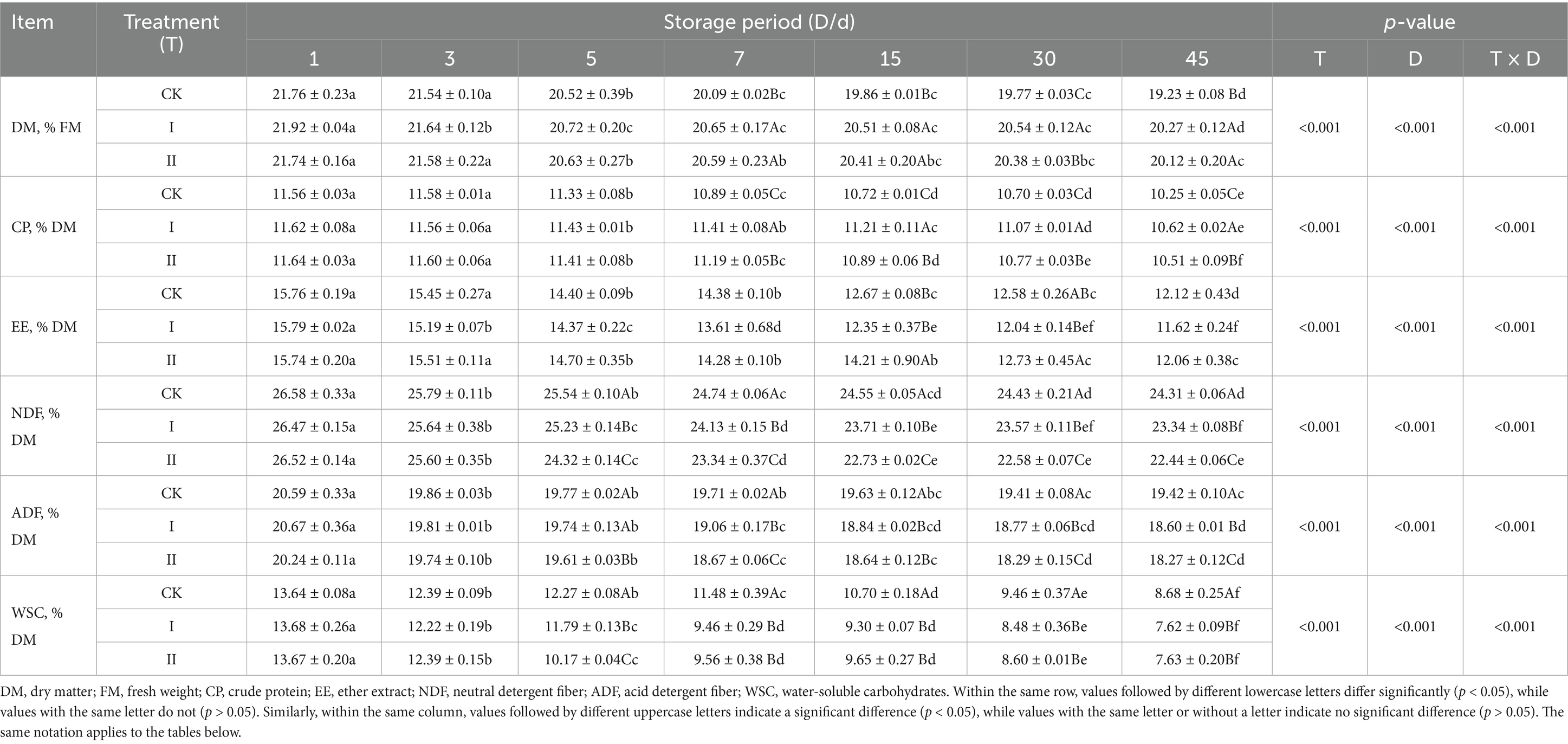
Table 5 presents the nutritional changes observed during the ensiling process of strawberry vines. The DM content in all groups decreased during the initial ensiling phase and stabilized after 15 days. At 7 days, Group I exhibited significantly higher DM content compared to both the CK group and Group II (p < 0.05). The WSC content in the experimental groups declined rapidly during the early stages of ensiling and was significantly lower than that of the CK group after 5 days (p < 0.05). Throughout the ensiling period, CP content declined across all groups; however, Group I experienced a slower rate of decrease. At 45 days, Group I maintained significantly higher CP content than both the CK group and Group II (p < 0.05). Group II exhibited a slower reduction in EE content at 15 and 30 days, maintaining significantly higher values than the other groups (p < 0.05); however, no significant differences were observed among groups at 45 days (p > 0.05). Both NDF and ADF contents decreased progressively during ensiling, with NDF showing a slower rate of decline. After 5 days, the CK group maintained significantly higher NDF and ADF contents compared to the experimental groups (p < 0.05).
3.3 Effect of LAB on the fermentation quality and tannin content of strawberry vines silage
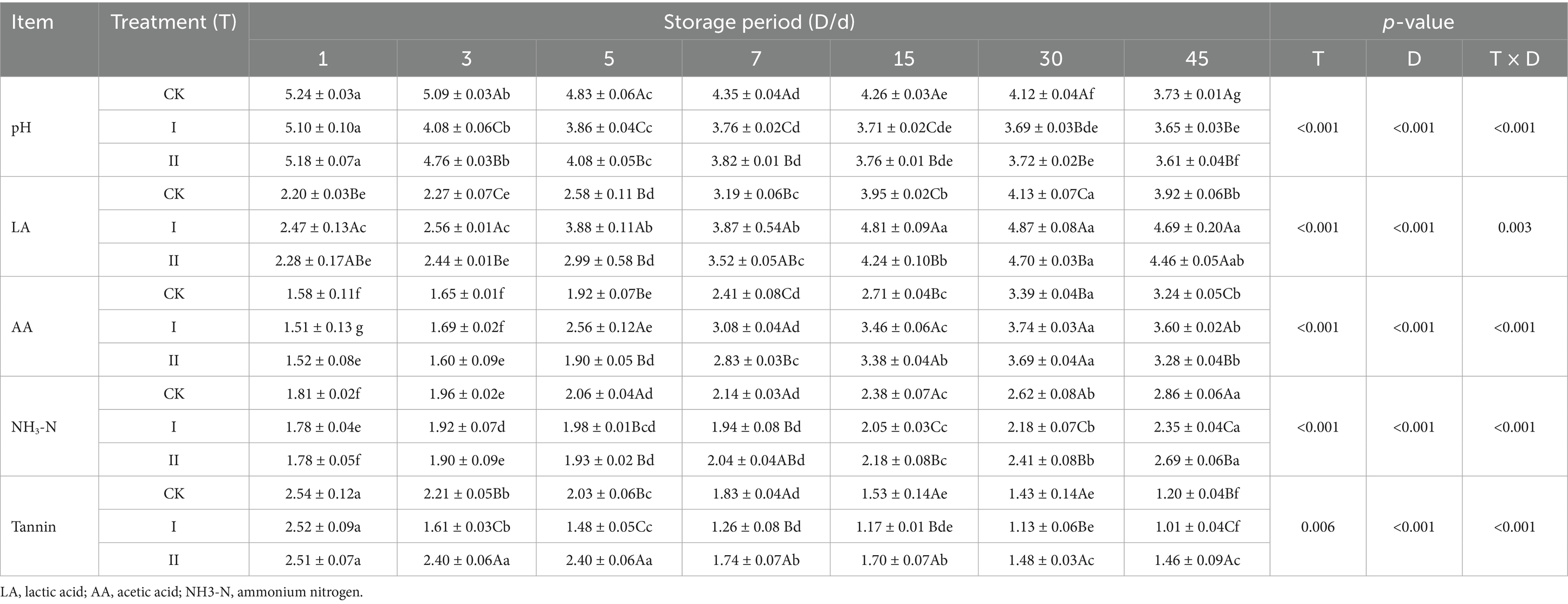
The fermentation quality and tannin content of ensiled strawberry vines are presented in Table 6. In Groups I and II, pH decreased rapidly during the early stages of ensiling, falling below 4 by days 5 and 7, respectively. From day 3 onward, both experimental groups maintained significantly lower pH values than the CK group (p < 0.05), with Group I showing significantly lower pH than Group II throughout days 3 to 15 (p < 0.05).
This study observed increased LA and AA concentrations, with Groups I and II demonstrating more rapid LA accumulation, which became significantly higher than that of the CK group by day 3 (p < 0.05). Throughout the ensiling process, Group I consistently maintained higher LA levels than the other groups, while its AA content was significantly greater than both CK and Group II from day 5 onward (p < 0.05). In the CK group, NH3-N increased rapidly during the early ensiling period, significantly exceeding levels in the experimental groups after 5 days (p < 0.05). From day 7 onward, Group I exhibited significantly lower NH3-N concentrations than Group II (p < 0.05).
After 45 days of ensiling, Group I exhibited significantly lower tannin content compared to both the CK and Group II (p < 0.05), whereas Group II did not improve tannin degradation efficiency.
3.4 Effect of LAB on the microbial community structure of strawberry vines silage
3.4.1 Analysis of microbial community composition at the phylum level
The phylum-level microbial community structure in strawberry vines silage is illustrated in Figure 1. In the present study, phylum-level community analysis revealed that Bacillota and Pseudomonadota predominated in the CK group and Group II during the early stages of ensiling, with Pseudomonadota abundance reaching 82.39% in the CK group and 56.19% in Group II. The abundance of Bacillota progressively increased with the duration of ensiling in these groups. Notably, Bacillota abundance in Group I reached 87.26% by day 3, demonstrating the superior adaptability of L. plantarum to ensiling conditions.
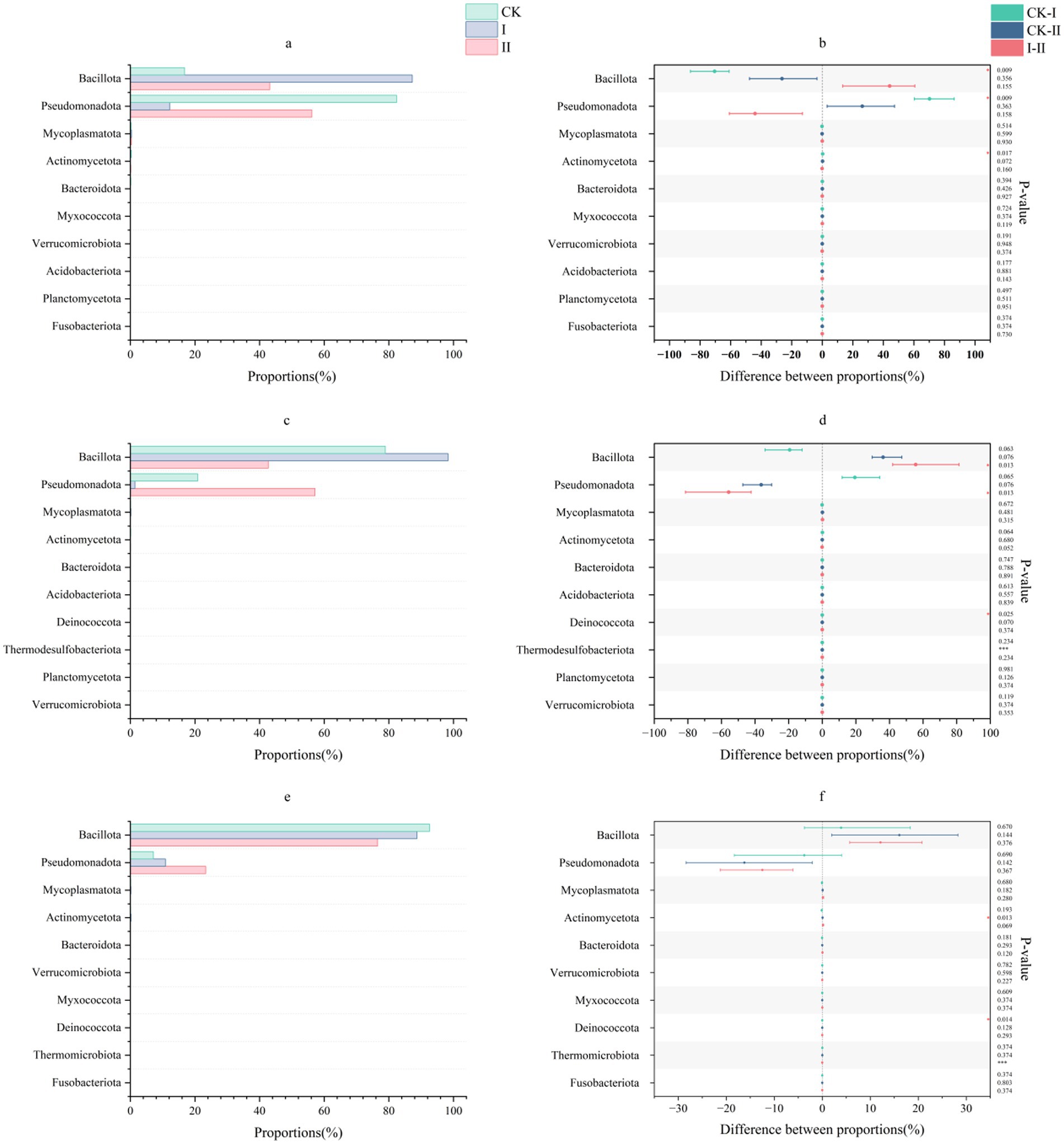
Figure 1. Effect of LAB on the microbial phylum level of strawberry vines silage over time. (a,c,e) Phylum-level microbial composition of strawberry vines silage on days 3, 7, and 45. (b,d,f) Differences in phylum-level microbial communities of strawberry vines silage on days 3, 7, and 45.
3.4.2 Analysis of microbial community composition at the genus level
The genus-level microbial community structure in strawberry vines silage is depicted in Figure 2. At the genus level, the CK group during the early stages of ensiling showed relative abundances of 14.83% for Clostridioides, 46.20% for Enterobacter, and 20.38% for Pantoea. Group II initially exhibited 42.03% Clostridioides, 25.75% Enterobacter, and 16.80% Pantoea, without significant Lactococcus presence. Notably, the abundance of Klebsiella in Group II increased from 10.71 to 34.09% by day 7. In contrast, Group I was dominated by Lactiplantibacillus (54.18%) and Clostridioides (32.38%) during the early stages of ensiling. Throughout the ensiling process, the abundance of Lactiplantibacillus progressively increased across all groups, while the populations of Clostridioides, Enterobacter, and Pantoea declined.
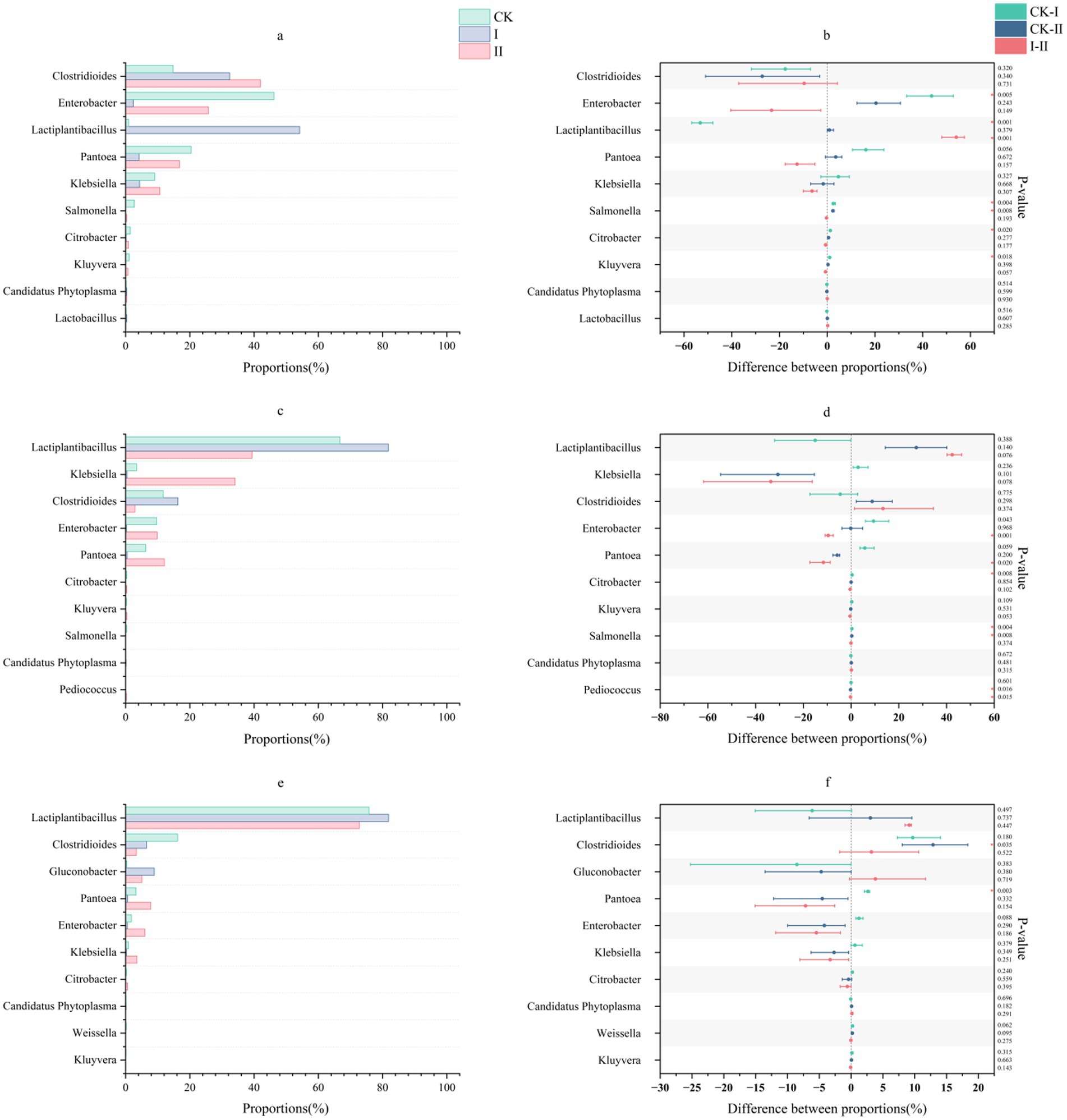
Figure 2. Effect of LAB on the microbial genus level of strawberry vines silage over time. (a,c,e) Genus-level microbial composition of strawberry vines silage on days 3, 7, and 45. (b,d,f) Differences in genus-level microbial communities of strawberry vines silage on days 3, 7, and 45.
3.5 Effect of strawberry vines silage on production performance in Hu sheep
The effects of strawberry vines silage on production performance and apparent digestibility in Hu sheep are presented in Table 7. In this study, supplementation with LAB-fermented silage resulted in a significantly higher final body weight in Group I compared to the CK group (p < 0.05). Both experimental groups demonstrated significantly improved ADG and reduced F/G relative to the CK group (p < 0.05), although no significant differences were observed in DMI.
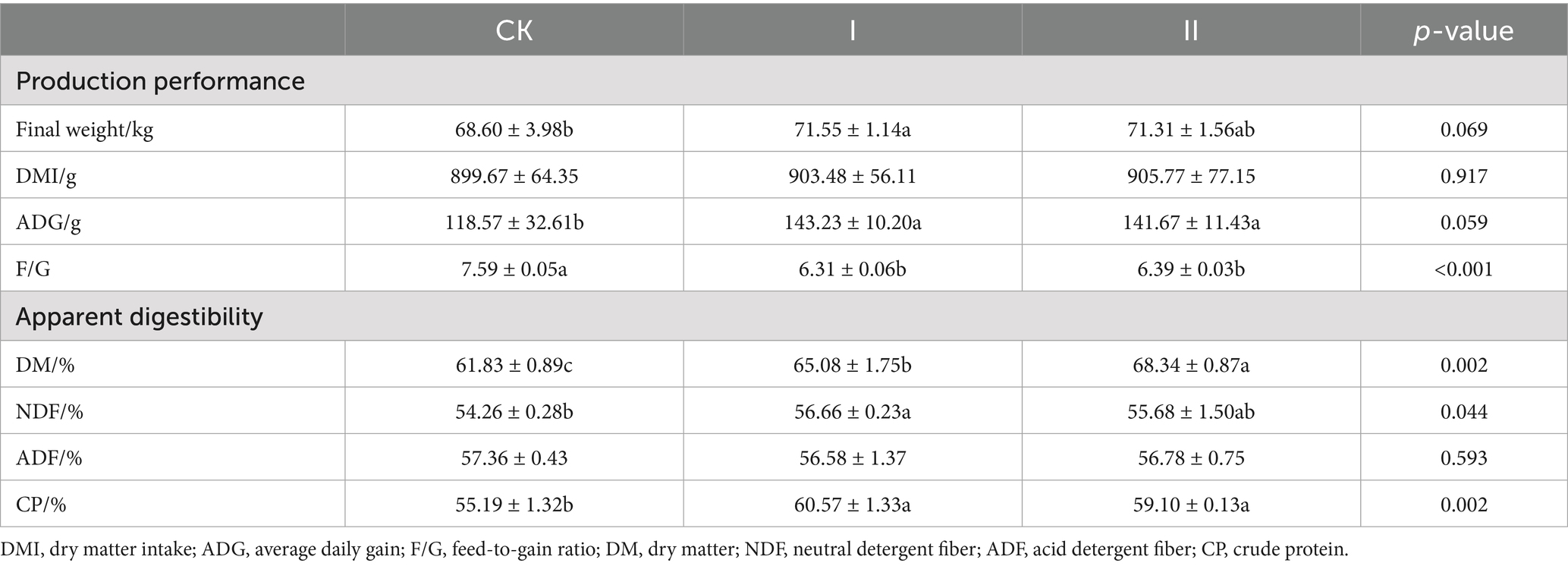
Table 7. Effect of strawberry vines silage on the production performance and apparent digestibility of Hu sheep.
In the present study, LAB-fermented silage significantly improved the apparent digestibility of DM, NDF, and CP in Hu sheep (p < 0.05), although no significant enhancement was observed in ADF digestibility.
3.6 Effect of strawberry vines silage on the rumen microbial community structure in Hu sheep
The phylum-level composition of ruminal microbiota in Hu sheep is illustrated in Figures 3a,b. In the present study, Bacteroidota and Bacillota were the dominant phyla across all groups, with no significant intergroup differences observed. Genus-level microbial profiles of ruminal fluid, presented in Figures 3c,d, reveal predominant genera including Prevotella, Bacteroides, Capnocytophaga, and Clostridium. Compared to the CK group, both experimental groups exhibited a reduction in Prevotella abundance, although no inter-experimental group differences were observed.
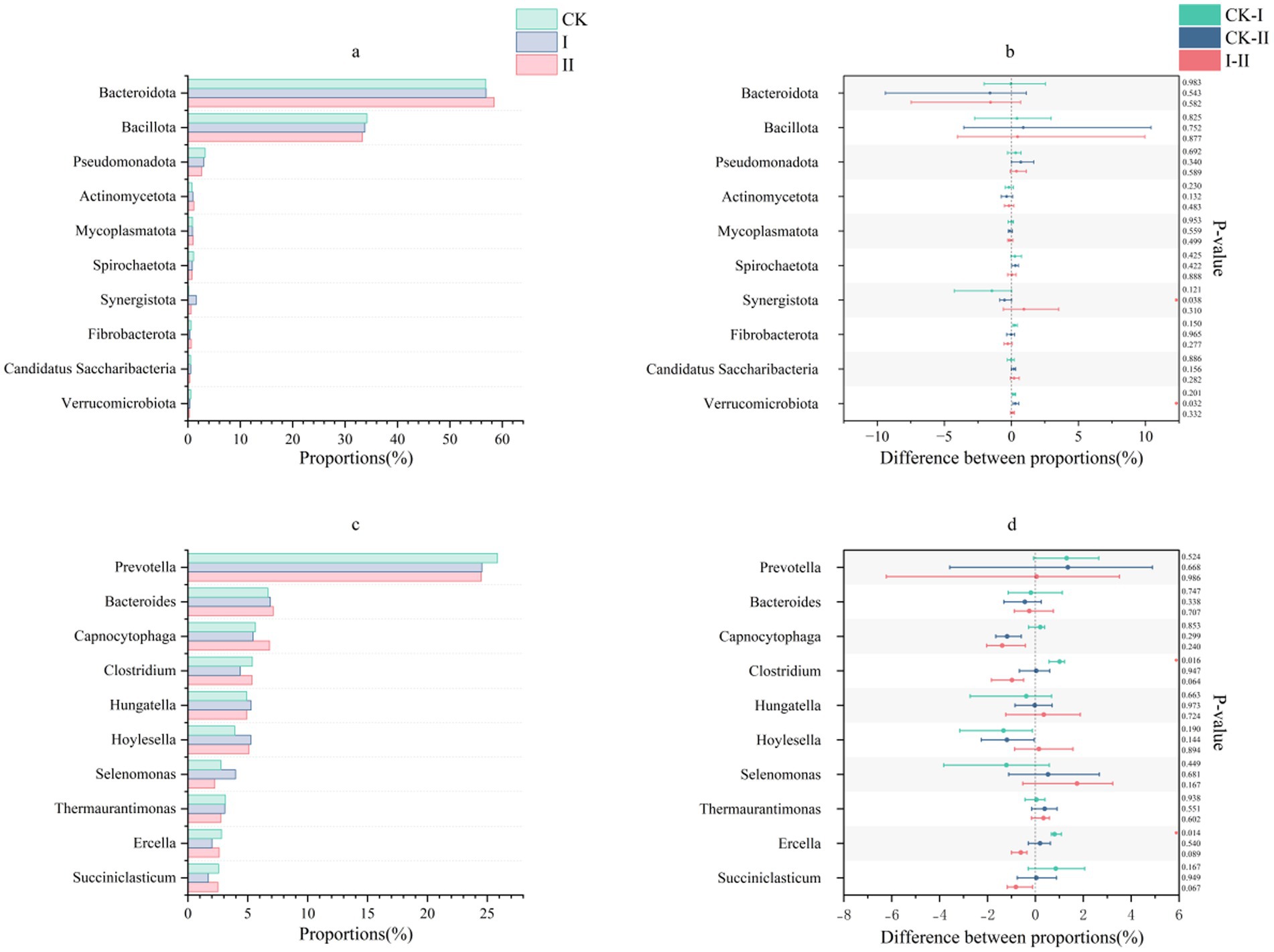
Figure 3. Effects of strawberry vines silage on the rumen microbiota composition in Hu sheep at the phylum and genus levels. (a,b) Composition and differences in the rumen fluid microbiota at the phylum level. (c,d) Composition and differences in the rumen fluid microbiota at the genus level.
4 Discussion
LAB constitutes one of the critical determinants influencing silage quality (16). LAB can effectively improve the fermentation quality of silage by rapidly lowering the pH of the feed and inhibiting the reproduction of harmful microorganisms. Their adaptability to the silage environment is modulated by the chemical composition of the feedstock, which is particularly affected by anti-nutritional factors such as tannins that inhibit LAB activity. Boonaert et al. reported that the physiological and biochemical characteristics of LAB isolated from plant surfaces may correlate with host plant chemistry (17), suggesting that epiphytic LAB strains exhibit enhanced environmental adaptation to their native host plants. This implies that host-specific epiphytic LAB inoculants could optimize silage quality when applied to homologous plant materials. Ke et al. demonstrated that tannins suppress LAB metabolic activity and delay their proliferation during ensiling (18). Notably, the high tannin content in the strawberry vines used in the present study likely impeded the early-phase pH reduction in the silage system. To mitigate tannin-mediated inhibition of LAB activity, thereby effectively retaining the nutrients in strawberry vines and improving their palatability for use in livestock production, we isolated two LAB strains from strawberry vines, one being L. lactis and the other L. plantarum.
Zhu et al. emphasized the pivotal role of carbon source utilization capacity in LAB performance (19), noting that strains with broad metabolic versatility can rapidly acidify the silage environment (through pH reduction), suppress the growth of undesirable microorganisms, and preserve the nutritional integrity of the feedstock, thereby enhancing silage quality. L. plantarum possesses a unique ability to metabolize salicin compared to L. lactis. The phenylpropanoid pathway, mediated by phenylalanine ammonia-lyase (PAL), produces salicylic acid and tannins. Ibrahim et al. reported a synergistic relationship between salicylic acid and tannin biosynthesis (20), whereby increased salicylic acid levels enhance PAL and chalcone synthase (CHS) activities, thereby promoting the synthesis of trans-cinnamic acid derivatives, flavonoids, and tannins in plants. Given the high tannin content in strawberry vines, elevated salicylic acid concentrations were hypothesized in this system. Strain Z’s ability to metabolize salicylic acid may underlie its rapid pH-lowering effect observed during ensiling.
Borreani et al. demonstrated that aerobic respiration during ensiling results in varying degrees of DM loss in forage (9), with lower WSC content exacerbating this depletion. In the present study, the relatively high WSC content in strawberry vines provided sufficient substrate for LAB fermentation, which rapidly lowered pH and effectively preserved DM content. After 45 days of ensiling, both experimental groups showed significantly lower WSC content and higher DM content compared to the CK group, consistent with the findings of Liu et al. (21). Moreover, both experimental groups showed significantly higher CP content than the CK group at 45 days, indicating that LAB additives effectively inhibited protein degradation, in agreement with the conclusions of Khota et al. (22). Han et al. reported that post-harvest plant lipases decompose lipids into glycerol and free fatty acids, with high moisture content accelerating this process (23). In the present study, the rapid pH reduction observed in Group I may also be associated with LA production via glycerol metabolism mediated by L. plantarum. Zhang et al. suggested that LAB metabolism during ensiling degrades NDF and ADF (24). The present study observed similar degradation patterns for NDF and ADF during fermentation, with experimental groups showing significantly lower values than the CK group (p < 0.05).
The pH reduction in silage primarily results from organic acid production through LAB metabolism during fermentation. Ma et al. demonstrated that silage additives can effectively lower pH, with this reduction enhancing aerobic stability and facilitating long-term preservation (25). Experimental Groups I and II exhibited significantly LA and AA contents compared to the CK group, accompanied by markedly lower pH values. Dai et al. reported that hydrolyzable tannins in plants possess unstable structures that are susceptible to enzymatic degradation by tannase or lyase, potentially generating gallic acid (26), which may oxidize into stronger acids such as oxalic acid. Additionally, salicin present in strawberry vines may undergo microbial hydrolysis to form salicylic acid during fermentation, simultaneously serving as a carbon source for LAB. The early addition of L. plantarum in Group I accelerated tannin degradation into acidic compounds such as gallic acid, thereby further promoting pH reduction. LA and AA in silage, predominantly produced through LAB metabolism, serve as crucial indicators for evaluating fermentation quality (27). In this study, LA and AA contents were significantly higher in both experimental groups than in the CK group. These findings indicate that supplementation with L. plantarum accelerated fermentation stabilization and effectively preserved nutrients in strawberry vines silage. NH3-N in silage typically arises from protein metabolism by undesirable microorganisms (28). Elevated NH3-N levels not only reduce CP content but also decrease animal feed intake. In this study, NH3-N content was significantly higher in the CK group than in the two treatment groups. The result demonstrate that LAB inoculation effectively regulated NH3-N production, thereby improving the ensiling quality of strawberry vines.
Plants produce anti-nutritional factors to combat adverse environmental conditions or to regulate their nutritional composition. However, excessive levels of these anti-nutritional factors in feed can inhibit livestock growth and impair reproductive performance (29). Therefore, quantification of anti-nutritional factors becomes crucial. Tannins, as important secondary metabolites in plants, rank second only to lignin in abundance. Tuominen et al. demonstrated that the unique chemical structure of tannins renders them susceptible to decomposition under variations in temperature and pH (30). In this study, the tannin degradation rate showed a positive correlation with the relative abundance of L. plantarum. This correlation might be attributed either to tannin degradation by L. plantarum during its growth phase or to spontaneous tannin decomposition facilitated by sustained heat generation during ensiling. Additionally, Brutti et al. revealed that tannins can form complexes with CP, reducing its degradability and thereby increasing rumen-bypass CP content (31). Notably, Group I not only demonstrated significantly reduced tannin content but also maintained higher CP levels than both CK and Group II. This suggests that L. plantarum Z exhibits a strong capacity for protein preservation.
Long et al. reported that during the initial ensiling phase, the complex microbial communities present on silage material surfaces impede LAB from establishing dominance owing to competitive interactions with undesirable microorganisms, which may lead to an increased relative abundance of harmful species (32). As major phyla involved in silage fermentation, Bacillota and Pseudomonadota have been shown by Geddes and Rangan et al. to degrade macromolecular substances through the secretion of cellulases and hemicellulases (33, 34), with some species capable of synthesizing organic acids such as LA and AA from organic matter. The observed changes in fiber content in the present study likely correspond to the metabolic activities of these phyla. However, an excessive presence of Pseudomonadota, which are predominantly gram-negative bacteria, may contribute to silage spoilage and reduce aerobic stability. Throughout the ensiling process, the abundance of Pseudomonadota gradually declined across all groups. Bao et al. demonstrated that selected LAB strains exhibit superior environmental adaptability, enabling rapid pH reduction (35). In Group I, inoculation with L. plantarum facilitated the swift establishment of dominance and sustained high abundance during early ensiling. Bacillota abundance in Group I reached 87.26% by day 3, demonstrating the superior adaptability of L. plantarum to ensiling conditions.
Shafiee and Feng et al. demonstrated that strawberries and their vines contain endogenous salicylic acid (36, 37). As a precursor to salicylic acid, salicin serves as a carbon source for L. plantarum but not for L. lactis. Concurrently, Mukherjee et al. reported that L. lactis exhibits low tolerance to tannins, with elevated concentrations of tannic acid inhibiting its growth (38).
Group I was dominated by Lactiplantibacillus and Clostridioides during the early stages of ensiling, while Group II did not show significant Lactobacillus abundance. This divergence likely stems from differences in substrate utilization: unlike Lactiplantibacillus, L. lactis lacks the capacity to effectively metabolize salicin as a carbon source. Furthermore, the elevated tannin content in strawberry vines may have suppressed L. lactis growth, accounting for the limited abundance of this bacterium in Group II. Fang et al. reported that in high-moisture silage, low pH alone cannot completely inhibit Clostridioides proliferation (39), potentially leading to butyric acid fermentation and consequent losses of DM and energy. During the later stages of ensiling, both experimental groups maintained stable LA content and pH levels without significant fluctuations, suggesting that inoculation with L. plantarum and L. lactis enhanced silage stability and effectively controlled Clostridioides proliferation. Although initially lower in abundance, L. lactis populations remained stable over the prolonged ensiling period. Additionally, Klebsiella abundance gradually decreased over time, with Lactiplantibacillus relative abundance remaining predominant in Group II by day 45.
DMI, ADG, and F/G are key indicators for evaluating growth performance in meat sheep (40). Volatile fatty acids (VFAs) in the rumen serve as primary energy sources for ruminants and play crucial roles in rumen health and energy metabolism. In this study, supplementation with LAB-fermented silage resulted in a significantly higher ADG and lower F/G relative to the CK group, although no significant differences were observed in DMI. This phenomenon may be attributed to the increased levels of LA and AA in the silage-supplemented diets, which directly contributed to enhanced energy supply for Hu sheep. The reduction in tannin content through ensiling likely lowered it below the threshold that inhibits DMI (41), while physiological adaptations in small ruminants, including increased salivary secretion, helped mitigate tannin-induced effects. These findings align with those of Lin et al. (42), who reported that a tannin concentration of 0.2% maintained stable DMI while enhancing ADG and reducing F/G by suppressing excessive ruminal protein degradation and improving intestinal amino acid absorption efficiency. In the present study, tannin content in the strawberry vines of Group I was significantly reduced compared to Group II (p < 0.05), a change attributed to L. plantarum supplementation. This reduction in tannin attenuated its inhibitory effects on the growth performance in Hu sheep. Although Group I exhibited numerically higher ADG and lower F/G than Group II, these differences were not statistically significant (p > 0.05).
Emkani et al. demonstrated that LAB fermentation alters the spatial configuration of dietary proteins, thereby significantly enhancing CP apparent digestibility (43). Rumen microorganisms convert fibrous components into VFAs through fermentation, serving as vital energy sources. Hristov et al. reported that ensiling markedly improves fiber apparent digestibility (44), which is attributed to acid-induced softening of fiber structures and limited cellulase secretion by microbial activity. Besharati et al. suggested that tannin-nutrient complexes might influence digestibility, with low tannin concentrations potentially enhancing CP apparent digestibility in ruminants (45). The present experiment revealed synergistic effects between low tannin levels and silage microorganisms, including (1) promotion of DM and NDF metabolism; and (2) improved CP digestibility via the combined action of microbial activity and optimized tannin concentration. Furthermore, the reduced tannin content in Group I, mediated by L. plantarum activity, was associated with numerically higher apparent digestibility of NDF and CP compared to Group II, although these differences were not statistically significant (p > 0.05).
Wen et al. demonstrated that silage supplementation significantly improves apparent digestibility in ruminants without causing notable alterations in microbial composition at either the phylum or genus levels (46). In the present study, Bacteroidota and Bacillota showed significant differences among the three groups. Dao et al. identified Prevotella as a keystone genus involved in carbohydrate and hydrogen metabolism in ruminants (47). These cellulose-degrading genera (Prevotella, Bacteroides, and Clostridium) secrete cellulases and hemicellulases, which hydrolyze structural polysaccharides into soluble sugars and oligosaccharides, thereby enhancing feed digestibility. Additionally, Bacteroides contributes to amino acid synthesis, supplying substrates for microbial protein production. Capnocytophaga exhibits proteolytic activity, degrading dietary proteins and thereby improving CP digestibility through microbial protein synthesis. Additionally, this genus modulates host immune responses by attenuating inflammatory reactions and promoting animal health. All three genera—Bacteroides, Capnocytophaga, and Clostridium—are involved in the synthesis of short-chain fatty acids, which serve as energy sources for ruminants. Group II showed moderate increases in the abundances of Bacteroides, Capnocytophaga, and Clostridium relative to the CK group. Notably, the CK group displayed a significantly higher abundance of Clostridium than Group I.
In this study, inclusion of strawberry vine silage in Group I markedly reduced the relative abundances of the predominant ruminal genera Clostridium and Ercella. Both Ercella and Clostridium belong to the order Clostridiales, which play multiple roles in anaerobic digestion, including high rates of cellulose hydrolysis, protein catabolism, and acidogenesis, leading to the production of short-chain fatty acids, CO₂, and H₂ (48). As a dynamic and balanced system, the rumen microbiota may exhibit a reduced abundance of Ercella in Group I either because the improved pre-digestion effect lowered the rumen’s functional demand for this genus, or because the increased abundance of other genera compensated for this functional niche. Lengowski et al. reported that variations in the nutritional composition of different silages can influence the structure of the ruminal microbial community (49). Furthermore, other studies have demonstrated that microorganisms present in silage may contribute to alterations in the ruminal microbiota (50). However, the reductions in these two genera observed in Group I Hu sheep were not detected in the strawberry vine silage itself, suggesting that shifts in ruminal microbial composition were primarily attributable to differences in the nutritional structure of the feed ingredients rather than the direct introduction of exogenous microbes. Collectively, these findings indicate that supplementation with strawberry vine silage supports rumen microbial stability in Hu sheep.
5 Conclusion
Two LAB strains, L. plantarum and L. lactis, were isolated from strawberry vines. L. plantarum exhibited greater carbon source utilization and tannin-degrading capacity than L. lactis, resulting in a more pronounced pH reduction during the early stage of ensiling in the L. plantarum treatment group, which indirectly improved the silage quality of strawberry vines. When both strains were used for ensiling and the resulting silages were fed to Hu sheep, neither L. plantarum nor L. lactis was detected in the rumen microbiota via 16S rDNA analysis. However, compared with the CK group, L. plantarum treatment reduced the relative abundance of the dominant rumen genera Clostridium and Ercella. Apparent digestibility of DM, NDF, and CP was increased in the L. plantarum group, whereas improvements in DM and CP digestibility were observed in the L. lactis group. Both treatments increased ADG and decreased F/G in Hu sheep.
Data availability statement
The original contributions presented in the study are publicly available. This data can be found here: https://www.ncbi.nlm.nih.gov/ PRJNA1335296, PRJNA1335361.
Ethics statement
The animal studies were approved by Institutional Animal Care and Use Committee (IACUC), Anhui Agriculture University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
WL: Conceptualization, Formal analysis, Investigation, Visualization, Writing – original draft. ZiZ: Data curation, Formal analysis, Investigation, Writing – original draft. ZhZ: Data curation, Investigation, Writing – original draft. YL: Data curation, Investigation, Writing – original draft. ZF: Investigation, Writing – original draft. SW: Investigation, Writing – original draft. SZ: Investigation, Writing – original draft. LZ: Supervision, Writing – review & editing. YZ: Resources, Supervision, Writing – review & editing. LC: Conceptualization, Funding acquisition, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was supported by National Natural Science Foundation of China (grant no. 32172769).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Center HR. China’s strategies for overcoming the ‘neck-stuck’ dilemma in forage supply. (2024). Available online at: https://static.0471tv.org.cn/sb/pc/con/202412/27/content_77499.html.
2. Zhang, X, Wang, Z, Can, M, Bai, H, and Ta, N. Analysis of China’s crop straw yield and comprehensive utilization status 2024. Available online at: http://www.knowcat.cn/p/20241208/2101301.html.
3. Dong, L, Liu, J, Zhong, Z, Wang, S, Wang, H, Huo, Y, et al. Dietary tea tree oil supplementation improves the intestinal mucosal immunity of weanling piglets. Anim Feed Sci Technol. (2019) 255:114209. doi: 10.1016/j.anifeedsci.2019.114209
4. Eikmeyer, FG, Köfinger, P, Poschenel, A, Jünemann, S, Zakrzewski, M, Heinl, S, et al. Metagenome analyses reveal the influence of the inoculant Lactobacillus buchneri CD034 on the microbial community involved in grass ensiling. J Biotechnol. (2013) 167:334–43. doi: 10.1016/j.jbiotec.2013.07.021
5. Mogodiniyai Kasmaei, K, Dicksved, J, Spörndly, R, and Udén, P. Separating the effects of forage source and field microbiota on silage fermentation quality and aerobic stability. Grass Forage Sci. (2017) 72:281–29. doi: 10.1111/gfs.12238
6. Wang, C, He, L, Xing, Y, Zhou, W, Yang, F, Chen, X, et al. Fermentation quality and microbial community of alfalfa and stylo silage mixed with Moringa oleifera leaves. Bioresour Technol. (2019) 284:240–7. doi: 10.1016/j.biortech.2019.03.129
7. Guo, X, Undersander, D, and Combs, D. Effect of Lactobacillus inoculants and forage dry matter on the fermentation and aerobic stability of ensiled mixed-crop tall fescue and meadow fescue. J Dairy Sci. (2013) 96:1735–44. doi: 10.3168/jds.2045-5786
8. Kung, L, Stough, E, McDonell, E, Schmidt, R, Hofherr, M, Reich, L, et al. The effect of wide swathing on wilting times and nutritive value of alfalfa haylage. J Dairy Sci. (2010) 93:1770–3. doi: 10.3168/jds.2009-2451
9. Borreani, G, Tabacco, E, Schmidt, R, Holmes, B, and Muck, R. Silage review: factors affecting dry matter and quality losses in silages. J Dairy Sci. (2018) 101:3952–79. doi: 10.3168/jds.2017-13837
10. He, L, Lv, H, Xing, Y, Chen, X, and Zhang, Q. Intrinsic tannins affect ensiling characteristics and proteolysis of Neolamarckia cadamba leaf silage by largely altering bacterial community. Bioresour Technol. (2020) 311:123496. doi: 10.1016/j.biortech.2020.123496
11. China NHCotPsRo, Regulation SAfM. National Food Safety Standard – Food microbiological examination – Lactic acid Bacteria (GB 4789.35–2023). China: National Standard of the People’s Republic of China (2023).
12. Broderick, G, and Kang, J. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J Dairy Sci. (1980) 63:64–75. doi: 10.3168/jds.S0022-0302(80)82888-8
13. AOAC. Official methods of analysis. 15th ed. Arlington, VA: Association of Official Analytical Chemists (1990).
14. Van Soest, P, Robertson, JB, and Lewis, BA. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. (1991) 74:3583–97. doi: 10.3168/jds.S0022-0302(91)78551-2
15. China SAotPsRo. GB/T 6433–2006: Determination of crude fat in feeds. Beijing: Standards Press of China (2006).
16. Okoye, CO, Wang, Y, Gao, L, Wu, Y, Li, X, Sun, J, et al. The performance of lactic acid bacteria in silage production: a review of modern biotechnology for silage improvement. Microbiol Res. (2023) 266:127212. doi: 10.1016/j.micres.2022.127212
17. Boonaert, CJ, and Rouxhet, PG. Surface of lactic acid bacteria: relationships between chemical composition and physicochemical properties. Appl Environ Microbiol. (2000) 66:2548–54. doi: 10.1128/AEM.66.6.2548-2554.2000
18. Ke, W, Zhang, H, Li, S, Xue, Y, Wang, Y, Dong, W, et al. Influence of condensed and hydrolysable tannins on the bacterial community, protein degradation, and fermentation quality of alfalfa silage. Animals. (2022) 12:831. doi: 10.3390/ani12070831
19. Zhu, L, Zhao, M, Yan, Y, Sun, P, Yan, X, Liu, M, et al. Characteristics of isolated lactic acid bacteria at low temperature and their effects on the silage quality. Microbiol. Spectr. (2025) 13:e0319424–4. doi: 10.1128/spectrum.03194-24
20. Ibrahim, MH, Omar, H, and Zain, NAM. Salicylic acid enhanced photosynthesis, secondary metabolites, antioxidant and lipoxygenase inhibitory activity (LOX) in Centella asiatica. Ann. Res. Rev. Biol. (2017) 17:1–14. doi: 10.9734/ARRB/2017/36153
21. Liu, Y, Du, S, Sun, L, Li, Y, Liu, M, Sun, P, et al. Volatile metabolomics and metagenomics reveal the effects of lactic acid bacteria on alfalfa silage quality, microbial communities, and volatile organic compounds. Commun. Biol. (2024) 7:1565. doi: 10.1038/s42003-024-07083-8
22. Khota, W, Pholsen, S, Higgs, D, and Cai, Y. Fermentation quality and in vitro methane production of sorghum silage prepared with cellulase and lactic acid bacteria. Asian Australas J Anim Sci. (2017) 30:1568–74. doi: 10.5713/ajas.16.0502
23. Han, L, and Zhou, H. Effects of ensiling processes and antioxidants on fatty acid concentrations and compositions in corn silages. J. Anim. Sci. Biotechnol. (2013) 4:1–7. doi: 10.1186/2049-1891-4-48
24. Zhang, J, Liu, Y, Wang, Z, Bao, J, Zhao, M, Si, Q, et al. Effects of different types of lab on dynamic fermentation quality and microbial community of native grass silage during anaerobic fermentation and aerobic exposure. Microorganisms. (2023) 11:513. doi: 10.3390/microorganisms11020513
25. Ma, J, Fan, X, Ma, Z, Huang, X, Tang, M, Yin, F, et al. Silage additives improve fermentation quality, aerobic stability and rumen degradation in mixed silage composed of amaranth and corn straw. Front Plant Sci. (2023) 14:1189747. doi: 10.3389/fpls.2023.1189747
26. Dai, X, Liu, Y, Zhuang, J, Yao, S, Liu, L, Jiang, X, et al. Discovery and characterization of tannase genes in plants: roles in hydrolysis of tannins. New Phytol. (2020) 226:1104–16. doi: 10.1111/nph.16425
27. Guan, H, Shuai, Y, Yan, Y, Ran, Q, Wang, X, Li, D, et al. Microbial community and fermentation dynamics of corn silage prepared with heat-resistant lactic acid bacteria in a hot environment. Microorganisms. (2020) 8:719. doi: 10.3390/microorganisms8050719
28. Fijałkowska, M, Pysera, B, Lipiński, K, and Strusińska, D. Changes of nitrogen compounds during ensiling of high protein herbages-a review. Ann Anim Sci. (2015) 15:289–305. doi: 10.1515/aoas-2015-0008
29. Yan, Z, Liu, Z, Zhou, C, and Tan, Z. Anti-nutritional factors of plant protein feeds for ruminants and methods for their elimination. Animals. (2025) 15:1107. doi: 10.3390/ani15081107
30. Tuominen, A, and Sundman, T. Stability and oxidation products of hydrolysable tannins in basic conditions detected by HPLC/DAD–ESI/QTOF/MS. Phytochem Anal. (2013) 24:424–35. doi: 10.1002/pca.2456
31. Brutti, D, Canozzi, M, Sartori, E, Colombatto, D, and Barcellos, J. Effects of the use of tannins on the ruminal fermentation of cattle: a meta-analysis and meta-regression. Anim Feed Sci Technol. (2023) 306:115806. doi: 10.1016/j.anifeedsci.2023.115806
32. Long, S, Li, X, Yuan, X, Su, R, Pan, J, Chang, Y, et al. The effect of early and delayed harvest on dynamics of fermentation profile, chemical composition, and bacterial Community of King Grass Silage. Front Microbiol. (2022) 13:864649. doi: 10.3389/fmicb.2022.864649
33. Rangan, KJ, Pedicord, VA, Wang, Y-C, Kim, B, Lu, Y, Shaham, S, et al. A secreted bacterial peptidoglycan hydrolase enhances tolerance to enteric pathogens. Science. (2016) 353:1434–7. doi: 10.1126/science.aaf3552
34. Geddes, EJ, Gugger, MK, Garcia, A, Chavez, MG, Lee, MR, Perlmutter, SJ, et al. Porin-independent accumulation in Pseudomonas enables antibiotic discovery. Nature. (2023) 624:145–53. doi: 10.1038/s41586-023-06760-8
35. Bao, J, Ge, G, Wang, Z, Xiao, Y, Zhao, M, Sun, L, et al. Effect of isolated lactic acid bacteria on the quality and bacterial diversity of native grass silage. Front Plant Sci. (2023) 14:1160369. doi: 10.3389/fpls.2023.1160369
36. Shafiee, M, Taghavi, T, and Babalar, M. Addition of salicylic acid to nutrient solution combined with postharvest treatments (hot water, salicylic acid, and calcium dipping) improved postharvest fruit quality of strawberry. Sci Hortic. (2010) 124:40–5. doi: 10.1016/j.scienta.2009.12.004
37. Feng, J, Zhang, M, Yang, K-N, and Zheng, C-X. Salicylic acid-primed defence response in octoploid strawberry ‘Benihoppe’leaves induces resistance against Podosphaera aphanis through enhanced accumulation of proanthocyanidins and upregulation of pathogenesis-related genes. BMC Plant Biol. (2020) 20:1–18. doi: 10.1186/s12870-020-02353-z
38. Mukherjee, A, Bhanwar, S, and Ganguli, A. Characterization of tannase production by Lactococcus lactis subsp lactis and its potential in enhancing nutritional value of a composite sourdough. Int J Genet Eng Biotechnol. (2014) 5:77–84.
39. Fang, D, Dong, Z, Wang, D, Li, B, Shi, P, Yan, J, et al. Evaluating the fermentation quality and bacterial community of high-moisture whole-plant quinoa silage ensiled with different additives. J Appl Microbiol. (2022) 132:3578–89. doi: 10.1111/jam.15506
40. Zhao, H, Hua, J, Lu, W, Yan, L, Zhang, M, Chen, C, et al. Effects of increasing levels of rubber seed cake on growth performance, nutrient digestion metabolism, serum biochemical parameters, and rumen microbiota of Hu sheep. BMC Vet Res. (2025) 21:52. doi: 10.1186/s12917-025-04503-7
41. Oliveira, LN, Pereira, MA, Oliveira, CD, Oliveira, CC, Silva, RB, Pereira, RA, et al. Effect of low dietary concentrations of Acacia mearnsii tannin extract on chewing, ruminal fermentation, digestibility, nitrogen partition, and performance of dairy cows. J Dairy Sci. (2023) 106:3203–16. doi: 10.3168/jds.2022-22521
42. Lin, L, Lu, Y, Wang, W, Luo, W, Li, T, Cao, G, et al. The influence of high-concentrate diet supplemented with tannin on growth performance, rumen fermentation, and antioxidant ability of fattening lambs. Animals. (2024) 14:2471. doi: 10.3390/ani14172471
43. Emkani, M, Oliete, B, and Saurel, R. Effect of lactic acid fermentation on legume protein properties, a review. Fermentation. (2022) 8:244. doi: 10.3390/fermentation8060244
44. Hristov, A, Harper, M, Roth, G, Canale, C, Huhtanen, P, Richard, T, et al. Effects of ensiling time on corn silage neutral detergent fiber degradability and relationship between laboratory fiber analyses and in vivo digestibility. J Dairy Sci. (2020) 103:2333–46. doi: 10.3168/jds.2019-16917
45. Besharati, M, Maggiolino, A, Palangi, V, Kaya, A, Jabbar, M, Eseceli, H, et al. Tannin in ruminant nutrition. Molecules. (2022) 27:8273. doi: 10.3390/molecules27238273
46. Wen, Z, Chen, Y, Wu, L, Tian, H, Zhu, N, Guo, Y, et al. Effects of Broussonetia papyrifera silage on rumen fermentation parameters and microbes of Holstein heifers. AMB Express. (2022) 12:62. doi: 10.1186/s13568-022-01405-x
47. Dao, T-K, Do, T-H, Le, N-G, Nguyen, H-D, Nguyen, T-Q, Le, T-T-H, et al. Understanding the role of Prevotella genus in the digestion of lignocellulose and other substrates in Vietnamese native goats’ rumen by metagenomic deep sequencing. Animals. (2021) 11:3257. doi: 10.3390/ani11113257
48. Angenent, LT, Richter, H, Buckel, W, Spirito, CM, Steinbusch, KJ, Plugge, CM, et al. Chain elongation with reactor microbiomes: open-culture biotechnology to produce biochemicals. Environ Sci Technol. (2016) 50:2796–810. doi: 10.1021/acs.est.5b04847
49. Lengowski, MB, Zuber, KH, Witzig, M, Möhring, J, Boguhn, J, and Rodehutscord, M. Changes in rumen microbial community composition during adaption to an in vitro system and the impact of different forages. PLoS One. (2016) 11:e0150115. doi: 10.1371/journal.pone.0150115
Keywords: strawberry vines, lactic acid bacteria, anti-nutritional factors, Hu sheep, growth performance
Citation: Lu W, Zhang Z, Zhu Z, Li Y, Fang Z, Wang S, Zhou S, Zhang L, Zhang Y and Chen L (2025) Tannin-rich strawberry vines fermented with lactic acid bacteria improve growth performance in Hu sheep. Front. Vet. Sci. 12:1666125. doi: 10.3389/fvets.2025.1666125
Edited by:
Arda Yıldırım, Gaziosmanpaşa University, TürkiyeReviewed by:
Yujia Tian, Tianjin Agricultural University, ChinaChangrong Wu, Guizhou University, China
Copyright © 2025 Lu, Zhang, Zhu, Li, Fang, Wang, Zhou, Zhang, Zhang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunhua Zhang, eXVuaHVhOTY4MUAxNjMuY29t; Lijuan Chen, emhhbmdjaGVubGlqdWFuQDE2My5jb20=
 Wenguang Lu
Wenguang Lu Zimo Zhang
Zimo Zhang Yunhua Zhang
Yunhua Zhang Lijuan Chen
Lijuan Chen