- College of Animal Science and Veterinary Medicine, HeiLongJiang BaYi Agricultural University, Daqing, China
Introduction: Escherichia coli infection causes severe diarrhea, decreases growth performance, and increases mortality of poultry, which imposes a significant economic burden on the poultry industry and severely limits its growth.
Methods: Here, to investigate the effects of Lactobacillus on the intestinal health, immune response, and growth performance of E. coli-infected goslings, we established a geese model infected with an Stx2f gene-carrying E. coli strain and analyzed the probiotic characteristics of three Lactobacillus isolates obtained from the cecum of healthy geese. In an in vivo study, Zi geese were administered daily gavage of L. johnsonii MC006, L. salivarius MC013, or L. fermentum MC018 (109 CFU/mL) from 1 d of age for 21 d, followed by treatment with E. coli XH197291 gavage (109 CFU/mL) on day 8.
Results: The results showed that E. coli XH197291-infected geese exhibited depression, intestinal damage, reduced average daily gain, increased feed conversion ratio, and 100% diarrhea incidence within 48 h post-infection. Remarkably, among the three Lactobacillus isolates, L. fermentum MC018 showed the potential to function as a probiotic because of its ability to resist acid and bile degradation, antibacterial effect, and adhesion property. Notably, oral supplementation containing L. fermentum MC018 alleviated diarrhea and intestinal histological lesions, reduced E. coli counts in both ileum and rectum, increased the population of lactic acid bacteria, and improved the growth performance of E. coli-infected geese. Geese treated with L. fermentum MC018 gavage had higher serum diamine oxidase (p < 0.01) and IgM (p < 0.05) levels than those in the model group. L. fermentum MC018 reduced the levels of IL-1β, IL-6, and TNF-α in intestinal tissues following E. coli infection. Compared to L. salivarius MC013, L. fermentum MC018 increased the levels of ZO-1 in the duodenum and Claudin-1 in the ileum.
Discussion: These findings suggest that L. fermentum MC018 is a promising probiotic strain for use as a potential alternative to antibiotics for controlling avian colibacillosis.
1 Introduction
Avian pathogenic Escherichia coli (APEC) infection can cause avian colibacillosis, leading to severe diarrhea, decreased feed intake, reduced growth performance and egg production, and increased mortality of poultry (1). Avian colibacillosis is a key infectious disease in poultry farms with a broad global distribution and has resulted in major economic losses to the poultry industry (2). Infected poultry and contaminated poultry meat or eggs as pathogen carriers pose a serious threat to human health and food safety (3). Because of the intense pressure on the livestock and poultry feed industry to limit or ban antibiotic usage (4, 5), it is very critical to explore new strategies and alternatives for antibiotics to prevent and control APEC infection in poultry for improving their intestinal health and growth performance (6).
Lactobacillus, as a live microbial feed supplement, could serve as a potential alternative strategy for antibiotic use in poultry feed (7, 8). Lactobacillus can also improve intestinal barrier function (9), modulate immune response (10, 11), and enhance growth performance (12). Several Lactobacillus species, for example, L. acidophilus, L. plantarum, L. fermentum, L. salivarius, and L. johnsonii, are known for their remarkable probiotic qualities (13–16). Previous studies have shown that L. acidophilus CGMCC14437 and L. plantarum B1 improved intestinal barrier function and growth performance and suppressed Escherichia coli infection in broiler chickens (3, 17). However, the role of Lactobacillus in attenuating E. coli infection in geese remains unclear.
Hence, in the present study, we established a geese model of E. coli infection by referring to previous studies (3, 17). Next, we isolated and identified three Lactobacillus strains from the cecal content of healthy Zi geese, evaluated their biological characteristics, and investigated the effects of oral supplementation of these Lactobacillus strains on the intestinal health, immune response, and growth performance of the E. coli-infected geese model. The findings suggest that L. fermentum MC018 could serve as a promising alternative for antibiotics in controlling avian colibacillosis.
2 Materials and methods
2.1 Ethics statement
The study protocol was approved by the Institutional Animal Care and Use Committee of HeiLongJiang BaYi Agricultural University (Approval No. DWKJXY2024231). The study was conducted in accordance with the local legislation and institutional requirements.
2.2 Isolation and identification of Lactobacillus strains from geese cecal content
By using labeled sterile tubes, cecal content samples were collected from three healthy Zi geese slaughtered at the age of 3 months in a local farm of Daqing in Heilongjiang Province, China. The Zi goose, an excellent local breed in Northeast China, boasts advantages including cold resistance, tolerance for rough forage, high-quality meat, and high egg production (18). The samples were serially diluted in physiological saline (0.85% NaCl, w/v) and cultured on De Man-Rogosa-Sharpe (MRS, Aobox, China) agar plates under anaerobic conditions at 37 °C for 24–48 h for bacterial isolation. The isolated colonies were randomly selected based on their morphology and analyzed by Gram staining and optical microscopy. The isolated strains were identified by PCR and phylogenetic analysis. The following universal primers were used for amplifying 16S rRNA sequences of the strains based on a previous study (19): forward (5′-AGA GTT TGA TCC TGG CTC AG − 3′) and reverse (5′-GGT TAC CTT GTT ACG ACT T-3′).
PCR was performed in a 25-μL reaction mixture containing 1 μL of DNA, 12.5 μL of 2 × Taq Master Mix (Qiagen, Germany), 0.5 μL of each primer (10 μM), and 10.5 μL of nuclease-free water. For each PCR reaction, a negative control containing sterile double distilled water was used. The mixture was amplified under the following conditions: initial denaturation at 95 °C for 7 min, followed by 35 cycles of denaturation at 94 °C for 60 s, annealing at 56 °C for 60 s, and elongation at 72 °C for 90 s and the final elongation step at 72 °C for 10 min. The amplified PCR products were analyzed by 1.5% agarose gel electrophoresis, and positive bands were detected using an ultraviolet trans-illuminator. All PCR products were sent to Harbin Ruibiotech Biotechnology Co., Ltd. for DNA sequencing. The 16S rRNA sequences of the isolated strains were subjected to NCBI BLAST search, and the phylogenetic tree was constructed using MEGA7.0 software.
2.3 Characterization of the isolated Lactobacillus strains
Growth and pH curves were used to evaluate the growth and acid production ability of the isolated Lactobacillus strains, respectively. A single colony was selected and inoculated into 5 mL MRS broth, and the broth was incubated at 37 °C for 24 h to obtain purified cultures. The purified strains were inoculated into MRS broth at 1:100 dilution for 36 h, and the OD600 value and pH of the culture medium were measured every 2 h. The growth and pH curves were plotted based on the measured data.
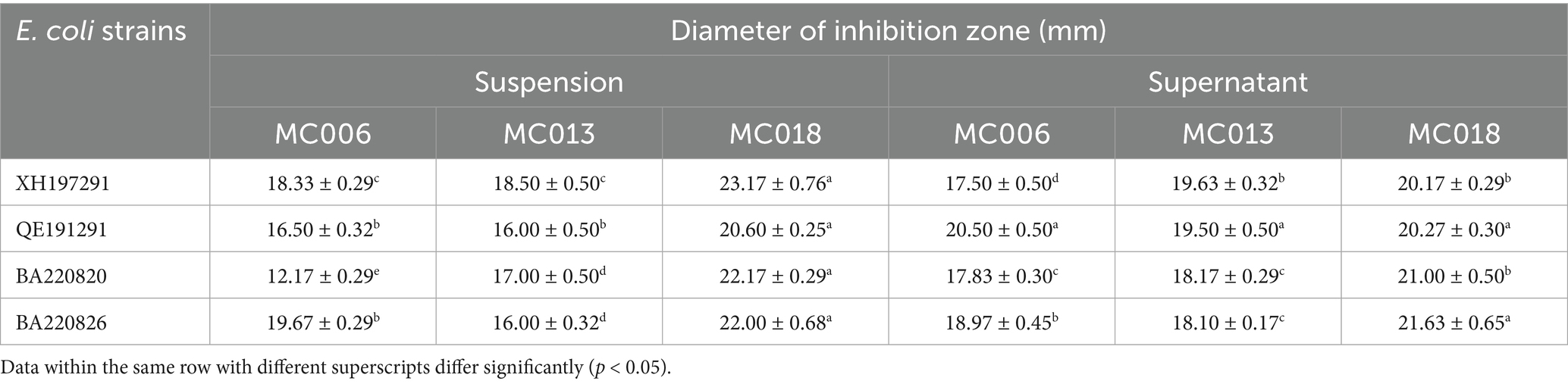
The antibacterial ability of the isolated strains was measured using their suspension and culture supernatant by the Oxford cup method. The purified cultures were transferred to a 5 mL MRS broth with 1% inoculation and grown for 24 h. The suspension was centrifuged at 10,000 rpm for 5 min. The supernatant was collected and used for the subsequent bactericidal test. Four goose-derived pathogenic E. coli strains (XH197291, GenBank: PV426822.1; QE191291, GenBank: PV535338.1; BA220820, GenBank: PV535342.1; BA220826, GenBank: PV535340.1) preserved in our laboratory were used to determine the antibacterial activity of the isolated Lactobacillus strains. These E. coli strains were evenly spread on nutrient agar plates at the cell density of 1 × 106 CFU/plate. Approximately 200 μL of the supernatant was added to each Oxford cup (Tuopu, China), and the plates were incubated at 37 °C for 12 h. The size of the inhibition zone was then measured.
The ability of the isolated strains to resist degradation by acid and bile salt was evaluated by a survival assay with MRS broth at pH 2.0 and 3.0 or containing bile salt at concentrations of 0.2 and 0.3%, as reported previously (20). Cells of the purified strains were collected by centrifugation, washed twice with phosphate-buffered saline (PBS), and resuspended in MRS broth with pH 2.0 and 3.0 or containing bile salt at concentrations of 0.2 and 0.3%. The cell suspensions (2 × 108 CFU/mL) were incubated at 37 °C for 3 h. The survival rate was defined as the ratio of the OD600 value after treatment to the initial OD600 value. Data are presented as mean ± SD of triplicates from 3 independent experiments.
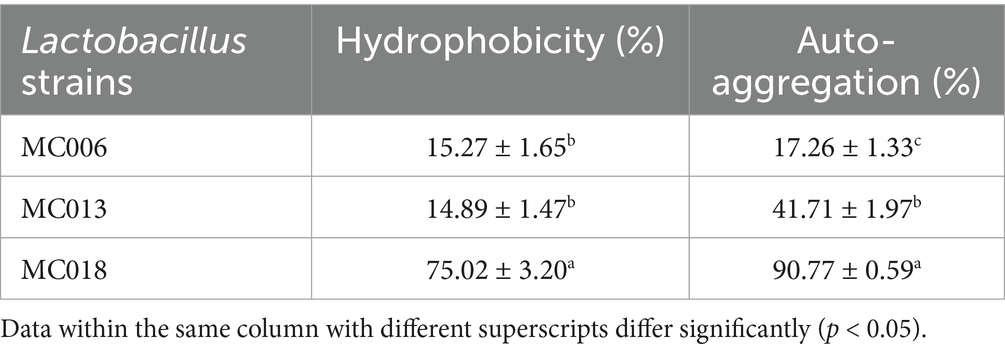
The surface hydrophobicity (H%) and auto-aggregation (Auto-A%) abilities were tested based on previous studies (21, 22). Cells of the isolated strains were collected by centrifugation, washed, and resuspended in sterile PBS to achieve an absorbance reading of 0.8 (ODinitial) at 600 nm. Next, 3 mL of the suspension was mixed with 1 mL of xylene and incubated at 37 °C for 10 min. The mixture was vortexed briefly and incubated at 37 °C for 3 h for phase separation. The aqueous phase was gently removed for measuring absorbance (ODfinal) at 600 nm. Surface hydrophobicity (H%) was calculated using the following equation: H% = (ODinitial − ODfinal)/ODinitial × 100%. Additionally, bacterial cells were collected again and resuspended in PBS to achieve an absorbance value of 0.8 (ODinitial) at 600 nm. The suspension was incubated at 37 °C for 3 h. The upper suspension was used to measure the absorbance reading (ODfinal) at 600 nm. Auto-A% was calculated using the following equation: Auto-A% = (ODinitial − ODfinal)/ODinitial × 100%.
To conduct the hemolysis test, the purified strains were streaked on agar plates containing 5% sheep blood. The plates were then incubated at 37 °C for 24 h and examined for the presence of green-hued zones (α-hemolysis), white or transparent zones (β-hemolysis), or the absence of hemolysis (γ-hemolysis) around the colonies. Staphylococcus aureus DQ13160 (GenBank: PV535337.1) was used as a positive control. The in vivo safety was also evaluated using one-day-old healthy Zi geese. Each goose in all Lactobacillus-treated groups was orally gavaged with 1 mL of the corresponding purified bacterial suspension at 109 CFU/mL cell density for 7 continuous days (d). At the end of the experiment, all geese were euthanized by intravenous sodium pentobarbital (100 mg/kg of body weight) injection as reported previously (3). Clinical signs and anatomical changes in the geese were examined and compared with the control group (PBS-fed geese).
2.4 Animals and experimental design
First, a goose model of E. coli infection was established by referring to previous reports (3, 23). A total of 648 one-day-old Zi geese were adaptively reared until 8 d of age. The geese were then randomly assigned to nine groups: E. coli XH197291-infected group (1 mL, 1 × 108 CFU/mL), E. coli XH197291-infected group (1 mL, 1 × 109 CFU/mL), E. coli QE191291-infected group (1 mL, 1 × 108 CFU/mL), E. coli QE191291-infected group (1 mL, 1 × 109 CFU/mL), E. coli BA220820-infected group (1 mL, 1 × 108 CFU/mL), E. coli BA220820-infected group (1 mL, 1 × 109 CFU/mL), E. coli BA220826-infected group (1 mL, 1 × 108 CFU/mL), E. coli BA220826-infected group (1 mL, 1 × 109 CFU/mL), and mock-infected group. Six replicates per group and 12 geese per replicate were used. During the experiment, all geese were kept in cages and fed a commercial antibiotic-free, corn-soybean meal-based gosling diet (nutrient levels of the diet are shown in Supplementary Table S1) and had free access to feed and drinking water every day. The indoor temperature was maintained at 30–31 °C in the first week and then decreased by 2–3 °C per week, and the ambient humidity was controlled at 60–65% (24, 25). Each goose in the eight infected groups was orally gavaged with 1 mL of the corresponding E. coli suspension at 108 or 109 CFU/mL cell density, and the geese in the mock-infected group were administered 1 mL of PBS. Clinical signs and anatomical changes were observed within 7 d after E. coli infection. Blood samples were collected from the heart and centrifuged to obtain the serum. All experimental geese were then euthanized by intravenous sodium pentobarbital (100 mg/kg of body weight) injection to collect intestinal tissue and anal swab samples as reported previously (3). The effects of E. coli infection were evaluated through clinical, bacteriological, and histopathological assessments. The diarrhea rate was reported as the percentage of geese with diarrhea.
Subsequently, based on the E. coli XH197291 infection model, we evaluated the effects of three goose-derived Lactobacillus strains on the growth performance, intestinal health, and immune response of Zi geese. A total of 360 one-day-old Zi geese (initial body weight: 101.6 ± 2.3 g) were randomly assigned to five groups: E. coli XH197291-infected model group, E. coli XH197291 + L. johnsonii MC006 group, E. coli XH197291 + L. salivarius MC013 group, E. coli XH197291 + L. fermentum MC018 group, and mock-infected group. Six replicates per group and 12 geese per replicate were used. During 1–7 d of age, each goose in the mock-infected and model groups was administered 1 mL of PBS, and the geese in the three Lactobacillus-supplemented groups were orally gavaged with 1 mL of the corresponding Lactobacillus suspension at 109 CFU/mL cell density (7). At 8 d of age, each goose in the model group and three Lactobacillus-supplemented groups was orally gavaged with 1 mL of E. coli XH197291 suspension at 109 CFU/mL cell density. During 8–21 d of age, each goose in the three Lactobacillus-supplemented groups was orally gavaged with 1 mL of the corresponding Lactobacillus suspension at 109 CFU/mL cell density. Clinical signs, diarrhea rate, and growth performance were recorded and analyzed during the experiment. Intestinal tissues, anal swab samples, and blood samples were collected for subsequent analyses.
2.5 Histological analysis
Segments of the duodenum and ileum were collected at 48 h post-infection (hpi) and fixed with 4% paraformaldehyde. Next, 4- to 6-mm-thick paraffin-embedded tissue sections were stained with hematoxylin and eosin. The sections were then examined under a light microscope. Five different villi and crypts per section were measured to assess villus height and crypt depth, and the ratio of villus height to crypt depth (V/C) was determined.
2.6 PCR detection for Stx2f gene
To detect the presence of Stx2f gene-carrying E. coli XH197291 used for challenge in the model group, anal swab samples from each goose in the mock-infected and model groups were tested by PCR for the Stx2f gene at 48 hpi. The following self-designed specific primers were used for amplifying the Stx2f gene (GenBank: PX099224): forward (5′-ATG ACG ACG GAC AGC AGT T-3′) and reverse (5′-CAA AGT GCT CAG CTG ACA GGG-3′). PCR cycling conditions included an initial denaturation step at 95 °C for 5 min, followed by 34 cycles at 94 °C for 60 s, 58 °C for 60 s, and 72 °C for 60 s and the final elongation step at 72 °C for 6 min. The amplified PCR products were visualized by 1.5% agarose gel electrophoresis.
2.7 E. coli and lactic acid bacteria (LAB) count
In accordance with previous studies (17, 26), 0.5 g of ileal or rectal contents were diluted with 4.5 mL of sterile PBS and then serially diluted 10-fold from 10−1 to 10−8. The contents were plated on MacConkey’s agar (02-005 K, Aobox, Beijing, China) to culture E. coli, and the agar plates were incubated at 37 °C for 24 h; the contents were also plated on MRS agar to culture LAB, and the agar plates were incubated under anaerobic conditions at 37 °C for 24 h. All bacteria were enumerated (CFU/g contents) by a visual count of colonies. The colony counts were log transformed before statistical analysis.
2.8 Quantitative real-time PCR (qRT-PCR) analysis
Total RNA was extracted from the duodenum and ileum tissue samples by using TRIzol reagent (15596026CN, Invitrogen Life Technologies, Carlsbad, CA) in accordance with the manufacturer’s instructions. The mRNA expression levels of Claudin-1, Occludin, ZO-1, IL-1β, IL-6, and TNF-α in the duodenum and ileum at 48 hpi were measured by qRT-PCR with the CFX96 Touch Real-Time PCR Detection System (Bio-Rad, Hercules, CA) using SYBR Premix Ex Taq II (RR820A, TaKaRa Biotechnology, Dalian, China). GAPDH was used as the internal control gene, with self-designed specific primers (Supplementary Table S2). The reaction cycling conditions were as follows: initial denaturation at 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s, 60 °C for 30 s, and 70 °C for 30 s. A final melting curve analysis was performed from 65 °C to 95 °C at the rate of 0.1 °C/s (continuous acquisition). Each sample was tested in triplicate, and the fold differences in gene expression were calculated using the 2−ΔΔCt method with normalization to GAPDH.
2.9 Western blot analysis
Protein levels of Claudin-1, Occludin, and ZO-1 in the duodenum and ileum at 48 hpi were measured by Western blotting. Total protein was extracted from the duodenum and ileum tissue samples with 100–150 μL of radioimmunoprecipitation assay buffer (Beyotime, Shanghai, China) containing 15 mM phenylmethylsulfonyl fluoride (Beyotime, Shanghai, China), before quantifying using a bicinchoninic acid assay kit (P0012S, Beyotime, Shanghai, China) in accordance with the manufacturer’s instructions. Approximately 30 μg of total protein was separated by electrophoresis and transferred to a polyvinylidene fluoride membrane (0.45 μm; EMD Millipore, Billerica, MA, USA), which was blocked with 5% non-fat milk in TBST (Tris-buffered saline, NaCl, and Tween 20) for 1–2 h at room temperature, and then incubated with primary antibodies including anti-Claudin-1 (GB112543, Servicebio, Wuhan, China), anti-Occludin (GB11149, Servicebio, Wuhan, China), anti-ZO-1 (GB111402, Servicebio, Wuhan, China), and anti-GAPDH (10494-1-AP, Proteintech, Rosemont, USA) overnight at 4 °C. After three rinses for 15 min with TBST, the membranes were incubated with horseradish peroxidase (HRP)-conjugated AffiniPure goat anti-rabbit IgG (H + L) (SA00001-2, Proteintech) for 1 h at room temperature. Subsequently, the membranes were rinsed three times for 15 min with TBST. Finally, the protein bands were visualized using a chemiluminescent HRP substrate (EMD Millipore), quantified using a Western blot imaging system (e-Blot Touch Imager; e-BLOT Life Science, Shanghai, China), and compared using GraphPad Prism version 8.0 (GraphPad Software, Inc.).
2.10 ELISA analysis
At 48 hpi, 400 μL of blood sample was collected from the heart and centrifuged to obtain the serum. The serum levels of IgA (kit No. YX-090701G), IgM (kit No. YX-090714G), and DAO (kit No. YX-040115G) were determined in accordance with the manufacturer’s instructions (Sinobestbio, Shanghai, China). Meanwhile, the production of IL-1β (kit No. ml061217), IL-6 (kit No. ml061135), and TNF-α (kit No. ml036890) in the duodenum and ileum was measured following the manufacturer’s instructions (MLbio, Shanghai, China).
2.11 Growth performance analysis
The body weight and feed intake of geese were measured before morning feeding at 1, 7, 8, and 21 d of age. Average daily gain (ADG), average daily feed intake (ADFI), and feed conversion ratio (feed: body weight gain, g: g, FCR) were calculated at different experimental periods (1–7 d and 8–21 d).
2.12 Statistical analysis
All data are expressed as mean ± standard deviation and analyzed with Student’s unpaired t-test, one-way ANOVA, or two-way ANOVA by using GraphPad Prism version 8.0 (GraphPad Software, Inc.). A p-value of <0.05 was considered statistically significant. All samples were assayed in triplicate.
3 Results
3.1 Identification of Lactobacillus strains
We isolated 3 strains of Gram-positive bacilli, designated as strain MC006 (GenBank: PV426770.1), strain MC013 (GenBank: PV426771.1), and strain MC018 (GenBank: PV426772.1). The strains exhibited good growth in MRS medium (Figures 1A,C). The colony morphology of these strains on MRS agar and their Gram staining characteristics are shown in Figure 1A. Phylogenetic analysis (Figure 1B) revealed that strains MC006, MC013, and MC018 belonged to L. johnsonii, L. salivarius, and L. fermentum, respectively.
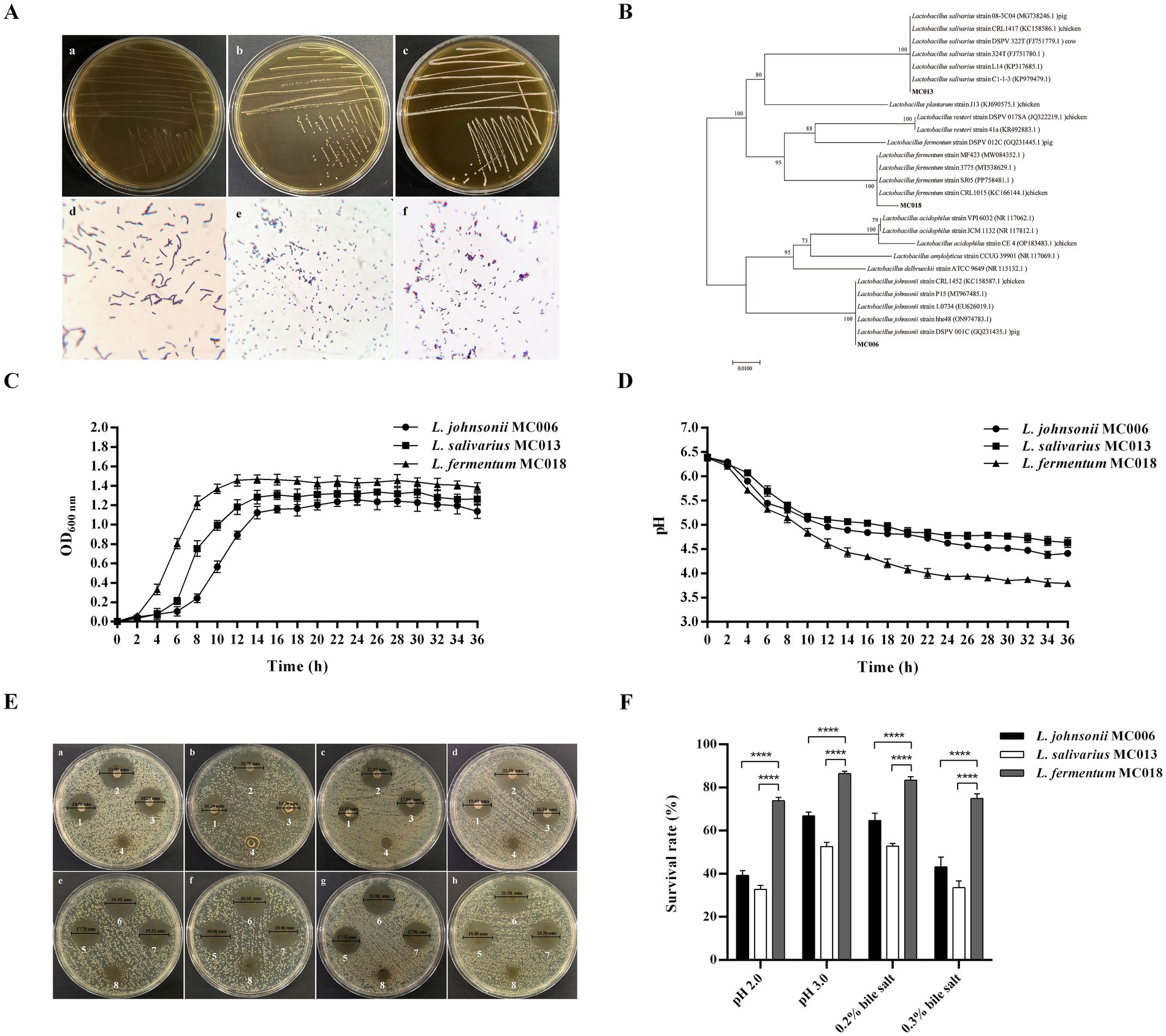
Figure 1. Identification and characterization of three goose-derived Lactobacillus strains. (A) Colony characteristics and Gram staining. Colony characteristics: (a) L. johnsonii MC006 colonies, (b) L. salivarius MC013 colonies, (c) L. fermentum MC018 colonies. Gram staining (original magnification, 1,000×): (d) L. johnsonii MC006, (e) L. salivarius MC013, (f) L. fermentum MC018; (B) Phylogenetic tree; (C) Growth curves; (D) pH curves; (E) Antibacterial ability of the isolated strains against E. coli was evaluated by the Oxford cup method. Bacterial suspensions of L. johnsonii MC006 (1), L. fermentum MC018 (2), or L. salivarius MC013 (3) were used for inhibiting E. coli XH197291 (a), E. coli QE191291 (b), E. coli BA220820 (c), or E. coli BA220826 (d). The supernatants of L. johnsonii MC006 (5), L. fermentum MC018 (6), or L. salivarius MC013 (7) were used for inhibiting E. coli, XH197291 (e), E. coli QE191291 (f), E. coli BA220820 (g), or E. coli BA220826 (h). MRS broth was used as the control (4, 8); (F) Acid and bile salt resistance characteristics, ****p < 0.0001. Data are presented as mean ± SD (n = 3 per group) and analyzed using two-way ANOVA.
3.2 Characterization of the Lactobacillus strains
As shown in Figure 1C, the three Lactobacillus strains were in the logarithmic growth phase from 4 to 14 h, stable from 14 to 30 h, and entered the cell death phase at 30 h. The pH of the culture medium of the three strains gradually decreased with bacterial growth and stabilized after 24 h (Figure 1D). At 36 h, the pH values of the culture medium of strains MC006, MC013, and MC018 were 4.41, 4.64, and 3.79, respectively.
As shown in Figure 1E, the bacterial culture solution and supernatant of the three Lactobacillus strains exhibited antibacterial activity against the four E. coli test strains. Notably, as compared to the other two Lactobacillus strains, L. fermentum MC018 exhibited higher antibacterial activity against E. coli XH197291, E. coli QE191291, E. coli BA220820, and E. coli BA220826, with mean inhibitory zone diameters of 23.17 ± 0.76 mm, 20.60 ± 0.25 mm, 22.17 ± 0.29 mm, and 22.00 ± 0.68 mm, respectively (Table 1).
The survival rates of strains MC006, MC013, and MC018 under pH 2.0 acidic conditions were 39.2% ± 2.3, 32.8% ± 1.8, and 73.8% ± 1.6%, respectively, and their survival rates under 0.3% bile salt conditions were 43.2% ± 4.5, 33.6% ± 3.1, and 75.0% ± 2.1%, respectively (Figure 1F). Additionally, the hydrophobicity level (Table 2) of strains MC006, MC013, and MC018 were 15.27% ± 1.65, 14.89% ± 1.47, and 75.02% ± 3.20%, respectively, and strain MC018 showed the highest hydrophobicity level. The auto-aggregation levels (Table 2) of strains MC006, MC013, and MC018 were 17.26% ± 1.33, 41.71% ± 1.97, and 90.77% ± 0.59%, respectively, with strain MC018 showing the maximum aggregation percentage.
In vitro biosafety of strains MC006, MC013 and MC018 was confirmed by a negative result for blood hemolytic activity (Supplementary Figure S1). Additionally, none of the geese treated with strains MC006, MC013, and MC018 exhibited clinical signs of illness.
3.3 Establishment of a goose model of E. coli infection
Geese were infected with E. coli XH197291, E. coli QE191291, E. coli BA220820, and E. coli BA220826 by administration through oral gavage. At 24 hpi, geese in each infection group had different degrees of diarrhea. Notably, the diarrhea rate of the E. coli XH197291 group (1 mL, 1 × 109 CFU/mL) was 100% within 48 hpi (Figure 2A). Compared to the other infection groups, the duration of diarrhea in geese from the E. coli XH197291 group was the longest (5 d) (Figure 2A). As shown in Figure 2B, the Stx2f gene was detected in the anal swab samples of geese from the E. coli XH197291-infected group, but not in geese from the mock-infected group.
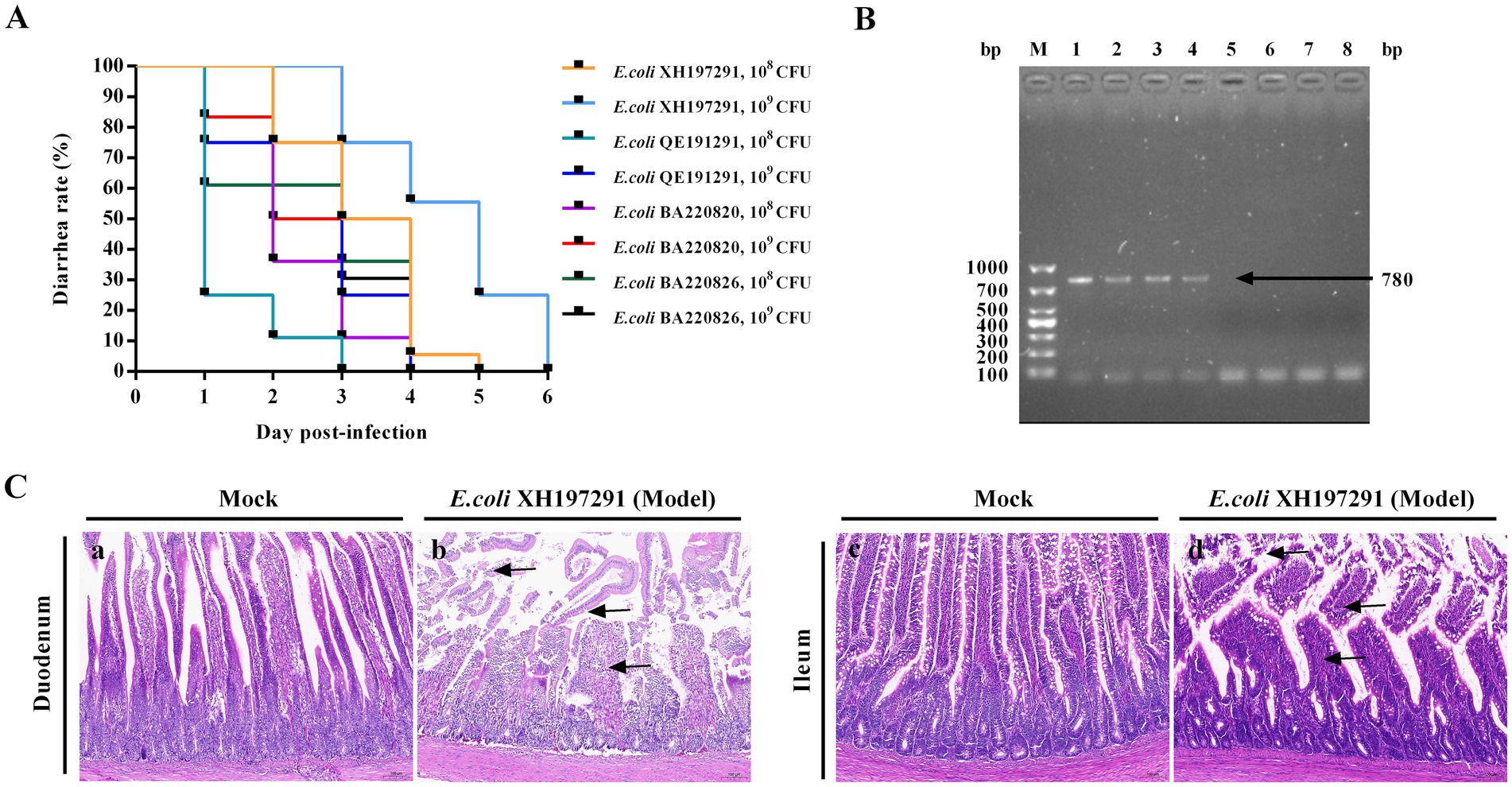
Figure 2. Establishment of a goose model of E. coli infection. (A) Diarrhea rate; (B) PCR detection of Stx2f gene. Lane M: 1000 bp DNA marker, line 1: E. coli XH197291 was used as the positive control, line 2–4: the fecal swab samples of the geese in the model group, line 5–7: the fecal swab samples of the geese in the mock-infected group, line 8: sterile distilled water was used as the negative control; (C) Histological lesions of the duodenum and ileum of the geese in the mock-infected group (a, c) and the model group (b, d).
The duodenums of mock-infected geese showed no significant histological lesions (Figures 2C–a,c). E. coli XH197291-infected geese showed necrosis and shedding of mucosal epithelial cells, breakage of intestinal villi, and inflammatory cell infiltration (Figures 2C–b). Moreover, the ileums of E. coli XH197291-infected geese showed degeneration and atrophy of intestinal villi, necrosis and shedding of mucosal epithelial cells, and inflammatory cell infiltration (Figures 2C–d).
3.4 L. fermentum MC018 reduced the rate and duration of diarrhea in geese infected with E. coli XH197291
All three Lactobacillus strains reduced the diarrhea rate of E. coli XH197291-infected geese (Figure 3). Compared to geese in the other Lactobacillus treatment groups and the model group, geese in the L. fermentum MC018 group had the lowest diarrhea rate at 24, 48, and 72 hpi (39, 25, and 8%, respectively). Additionally, compared to the model group, the L. fermentum MC018 group showed a reduction in the duration of diarrhea to 3 days. None of the geese treated with strains MC006, MC013, and MC018 showed diarrhea.
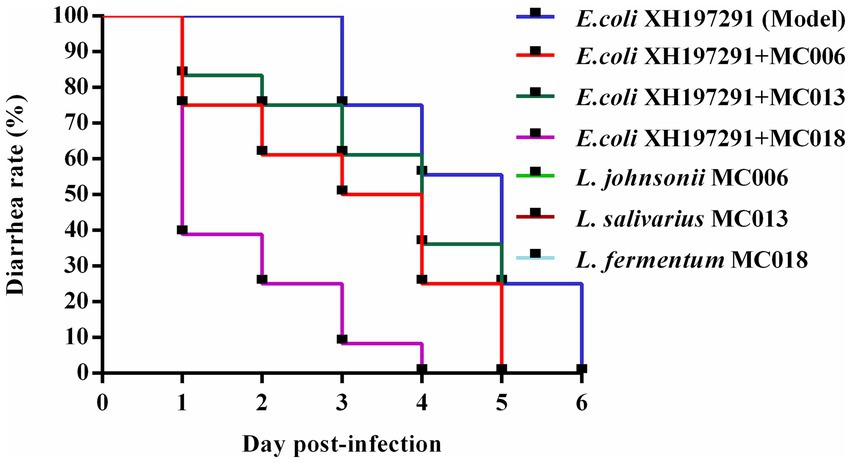
Figure 3. Effect of oral supplementation of the isolated Lactobacillus strains on the diarrhea rate of geese infected with E. coli.
3.5 L. fermentum MC018 reduced E. coli count and increased LAB count in E. coli-infected geese
Compared to the mock-infected group, the model group exhibited significantly increased E. coli counts in both ileal and rectal contents, concomitant with a marked reduction in LAB populations (Figure 4). Notably, L. fermentum MC018 treatment significantly reduced E. coli count (Figures 4A,B) and increased LAB count (Figures 4C,D) in E. coli-infected geese; however, the other two Lactobacillus strains did not significantly affect E. coli and LAB counts.
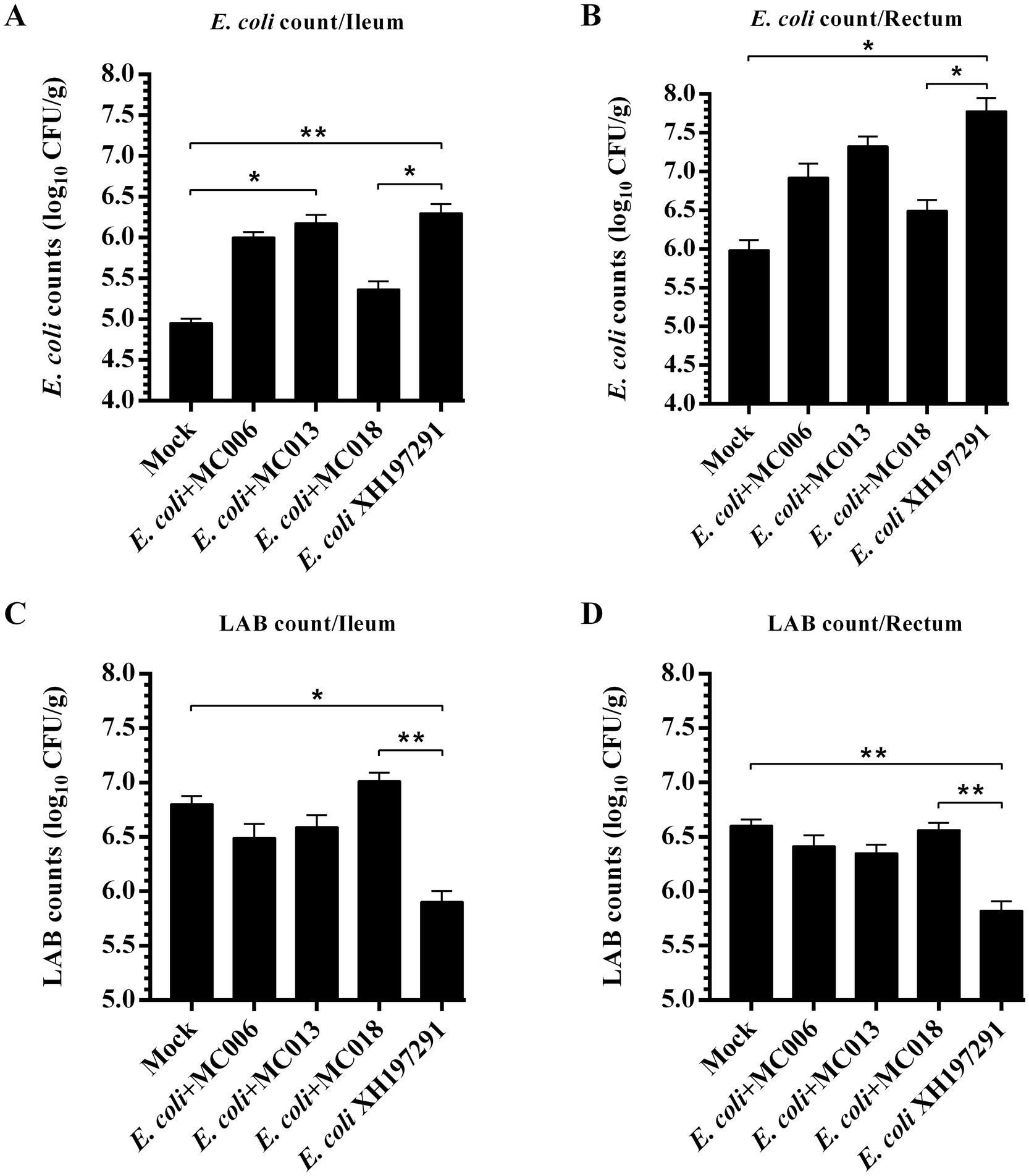
Figure 4. Effects of L. fermentum MC018 on the counts of E. coli and lactic acid bacteria (LAB) in the ileum and rectum of geese infected with E. coli XH197291. (A) E. coli count in the ileum; (B) E. coli count in the rectum; (C) LAB count in the ileum; (D) LAB count in the rectum; *p < 0.05, **p < 0.01 compared with the mock-infected group. Data are presented as mean ± SD (n = 6 per group) and analyzed using one-way ANOVA.
3.6 L. fermentum MC018 ameliorated E. coli XH197291-induced histological lesions in the duodenum and ileum
Compared to the E. coli XH197291-infected group, the geese groups with L. johnsonii MC006, L. salivarius MC013, or L. fermentum MC018 oral supplementation displayed amelioration of histological lesions in both duodenum and ileum (Figure 5). The mock-infected group showed no significant histological lesions (Figures 5A–a,E–a). E. coli XH197291 infection also significantly decreased the villus heights of the duodenum (p < 0.0001, Figure 5B) and ileum (p < 0.001, Figure 5F). Notably, compared to the E. coli XH197291-infected group, the L. fermentum MC018 group showed a significant increase in the villus heights of the duodenum (p < 0.0001, Figure 5B) and ileum (p < 0.01, Figure 5F). However, no significant differences were noted in the crypt depths of the duodenum and ileum between the groups. Moreover, oral supplementation of L. johnsonii MC006, L. salivarius MC013, or L. fermentum MC018 significantly increased the V/C ratio in the duodenum as compared to that in the E. coli-infected group (Figure 5D). However, oral supplementation of L. fermentum MC018 alone significantly increased the V/C ratio in the ileum (p < 0.05, Figure 5H).
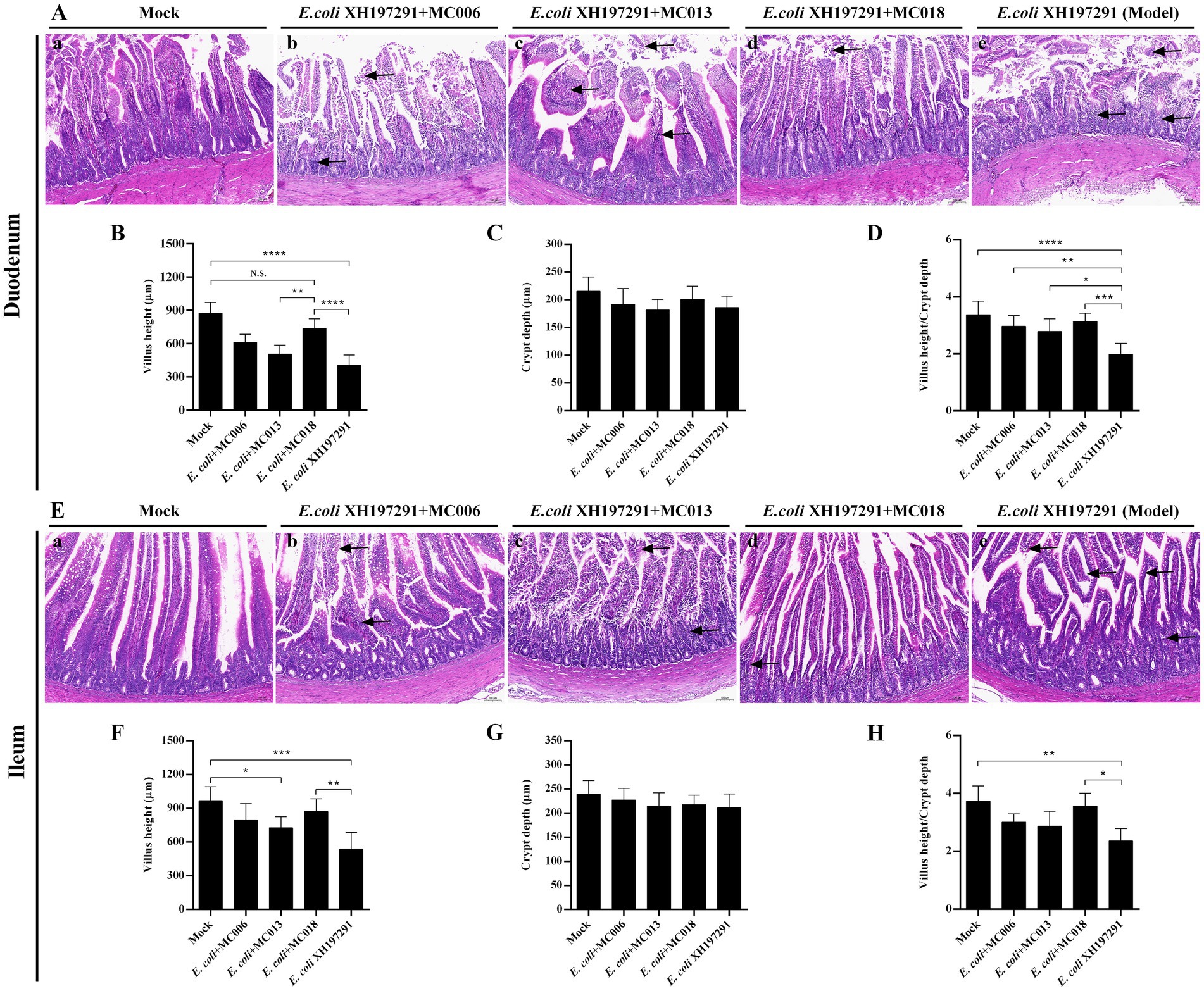
Figure 5. Effect of oral supplementation of the isolated Lactobacillus strains on intestinal histopathological changes of geese with E. coli infection. (A) Histological lesions in the duodenum; (B) Villus heights of the duodenum; (C) Crypt depths of the duodenum; (D) The ratio of villus height/crypt depth (V/C) in the duodenum; (E) Histological lesions in the ileum; (F) Villus heights of the ileum; (G) Crypt depths of the ileum; (H) The V/C ratio in the ileum; (a) The mock-infected group, (b) L. johnsonii MC006 group, (c) L. salivarius MC013 group, (d) L. fermentum MC018 group, (e) The model group (E. coli XH197291); N. S.: not significant, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 compared with the mock-infected group. Data are presented as mean ± SD (n = 6 per group) and analyzed using one-way ANOVA.
3.7 L. fermentum MC018 improved the intestinal barrier in E. coli XH197291-infected geese
The results of Western blot analysis showed that the protein levels of Claudin-1, Occludin, and ZO-1 in the duodenum and ileum were significantly decreased in the E. coli XH197291-infected group as compared to those in the mock-infected group (Figure 6). Notably, L. fermentum MC018 significantly restored the protein levels of Claudin-1, Occludin, and ZO-1 in both duodenum (Figures 6A–C) and ileum (Figures 6H–J) as compared to those in the E. coli-infected group. Compared to L. salivarius MC013, L. fermentum MC018 significantly increased the expression levels of ZO-1 in the duodenum (p < 0.05, Figure 6C) and ileum (p < 0.001, Figure 6J). Additionally, ZO-1 expression was significantly upregulated in the ileum of the L. fermentum MC018 group as compared to that in the L. johnsonii MC006 group (p < 0.001, Figure 6J). The qRT-PCR data also confirmed these findings, showing that L. fermentum MC018 supplementation notably decreased the mRNA levels of Claudin-1, Occludin, and ZO-1 in both duodenum (Figures 6E–G) and ileum (Figures 6L–N) as compared to those in the E. coli-infected group. Serum DAO levels were measured using ELISA kits. E. coli infection significantly increased the serum DAO level as compared to that in the mock-infected group (p < 0.001, Figure 7C). In contrast, L. fermentum MC018 significantly decreased the serum DAO levels as compared to that in the E. coli-infected group (p < 0.01, Figure 7C).
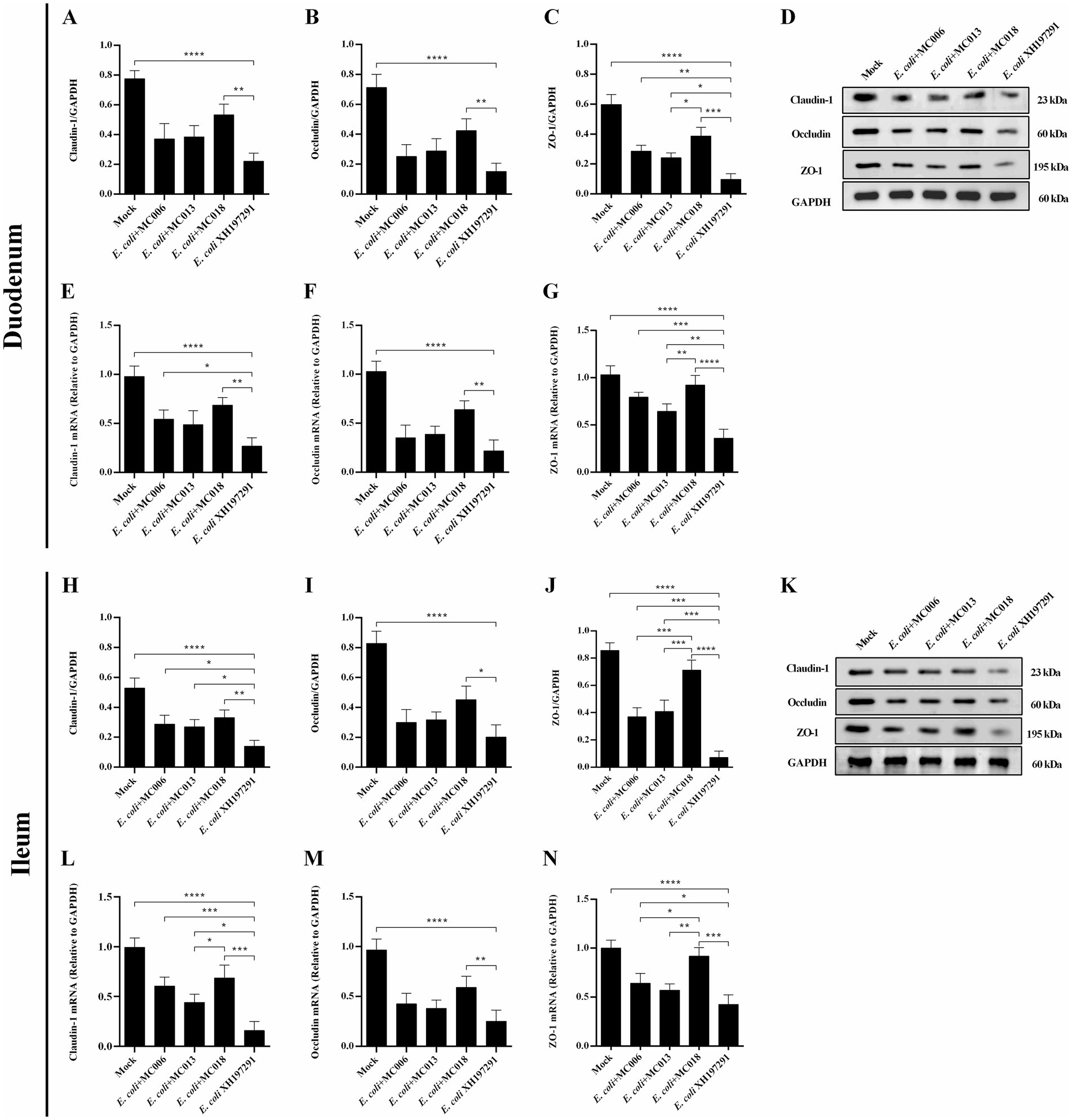
Figure 6. Effect of oral supplementation of the isolated Lactobacillus strains on the levels of Claudin-1, Occludin, and ZO-1 in the duodenum and ileum of geese with E. coli infection. The protein levels of Claudin-1 (A), Occludin (B), and ZO-1 (C) in the duodenum; The mRNA levels of Claudin-1 (E), Occludin (F), and ZO-1 (G) in the duodenum; The protein levels of Claudin-1 (H), Occludin (I), and ZO-1 (J) in the ileum; The mRNA levels of Claudin-1 (L), Occludin (M), and ZO-1 (N) in the ileum; The representative results of Western blot analysis of Claudin-1, Occludin, and ZO-1 in the duodenum (D) and ileum (K); *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 compared with the mock-infected group. Data are presented as mean ± SD (n = 6 per group) and analyzed using one-way ANOVA.
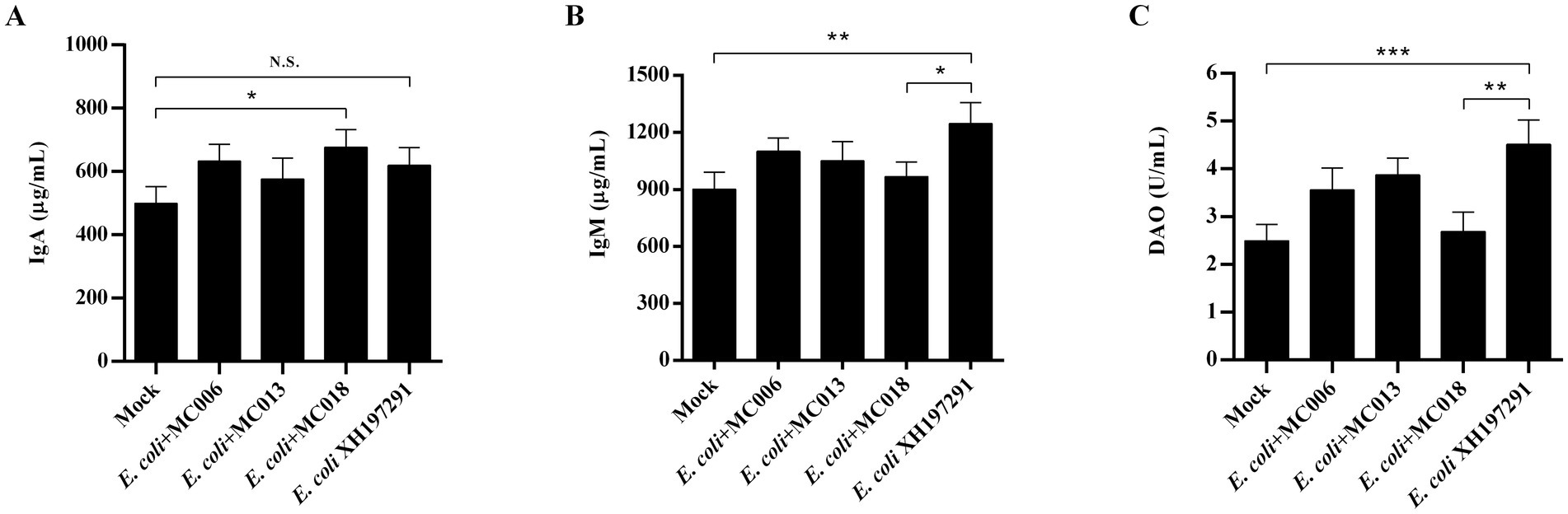
Figure 7. Effect of oral supplementation of the isolated Lactobacillus strains on the levels of IgA, IgM, and DAO in the serum of geese with E. coli infection. (A) The serum IgA level; (B) The serum IgM level; (C) The serum DAO level. N.S.: not significant, *p < 0.05, **p < 0.01, ***p < 0.001 compared with the mock-infected group. Data are presented as mean ± SD (n = 6 per group) and analyzed using one-way ANOVA.
3.8 L. fermentum MC018 decreased the levels of inflammatory cytokines in the duodenum and ileum of geese infected with E. coli XH197291
ELISA analysis showed that the protein levels of IL-1β, IL-6, and TNF-α in both duodenum and ileum were significantly elevated in the E. coli XH197291-infected group as compared to those in the mock-infected group (Figure 8). More importantly, L. fermentum MC018 supplementation remarkably reduced the protein levels of IL-1β, IL-6, and TNF-α in both duodenum and ileum as compared to those in the E. coli-infected group. Additionally, compared to L. salivarius MC013, L. fermentum MC018 significantly decreased the expression of IL-1β (p < 0.05, Figure 8A) and TNF-α (p < 0.05, Figure 8C) in the duodenum. The IL-1β expression level was significantly decreased in the duodenum of the L. fermentum MC018 group when compared with that in the duodenum of the L. johnsonii MC006 group (p < 0.05, Figure 8A). The qRT-PCR data also confirmed these findings, showing that L. fermentum MC018 supplementation notably decreased the mRNA levels of IL-1β, IL-6, and TNF-α in both duodenum (Figures 8D–F) and ileum (Figures 8J–L) as compared to those in the E. coli-infected group.
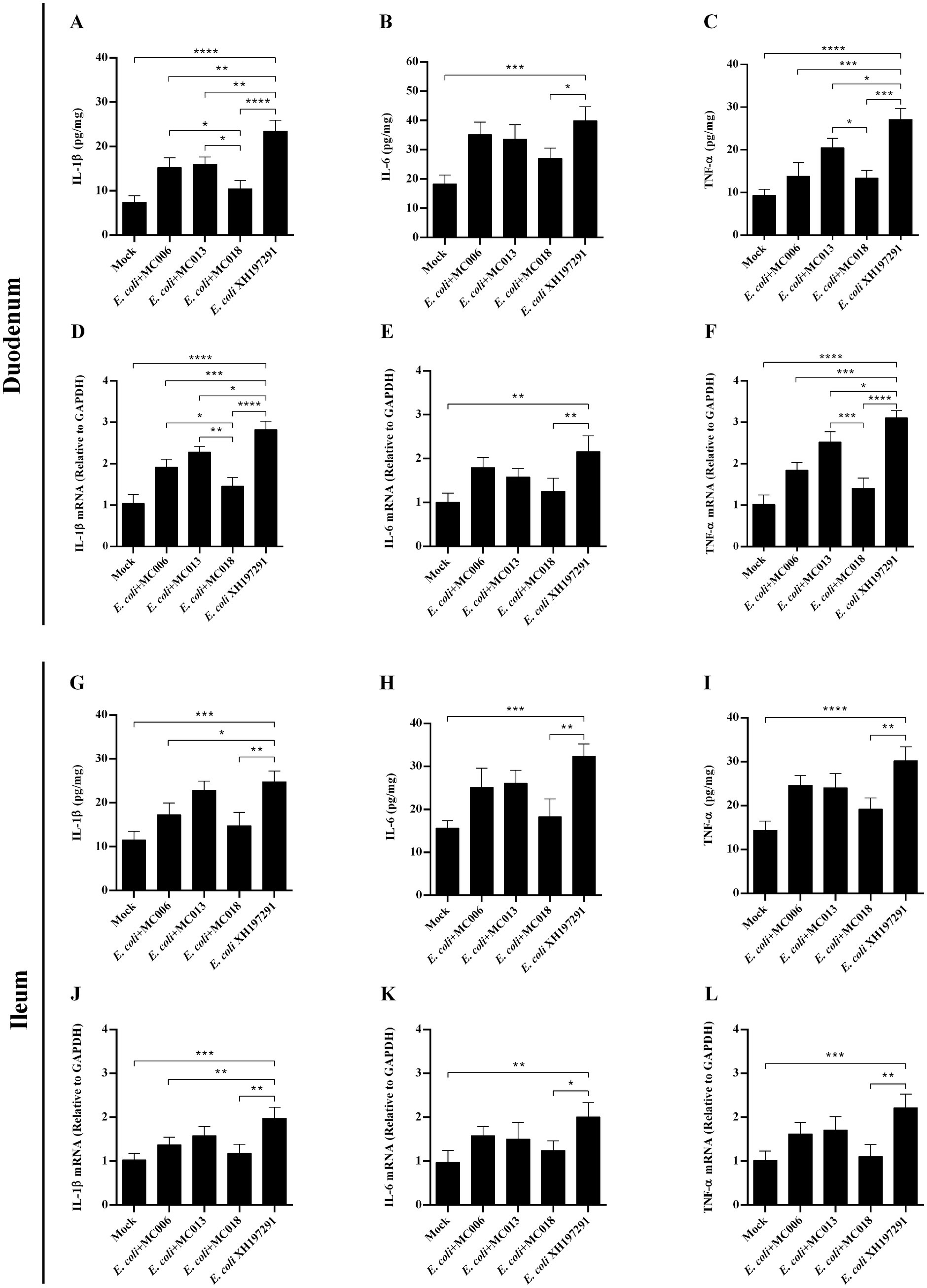
Figure 8. Effect of oral supplementation of the isolated Lactobacillus strains on the levels of IL-1β, IL-6, and TNF-α in the duodenum and ileum of geese with E. coli infection. The protein levels of IL-1β (A), IL-6 (B), and TNF-α (C) in the duodenum; The mRNA levels of IL-1β (D), IL-6 (E), and TNF-α (F) in the duodenum; The protein levels of IL-1β (G), IL-6 (H), and TNF-α (I) in the ileum; The mRNA levels of IL-1β (J), IL-6 (K), and TNF-α (L) in the ileum; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 compared with the mock-infected group. Data are presented as mean ± SD (n = 6 per group) and analyzed using one-way ANOVA.
3.9 Serum IgA and IgM levels
ELISA analysis showed that E. coli XH197291 infection significantly increased the serum IgM levels (p < 0.01) but did not significantly affect the serum IgA levels compared to mock infection (Figures 7A,B). Notably, the serum IgM levels were remarkably decreased in the L. fermentum MC018 supplementation group as compared to those in the E. coli-infected group (p < 0.05, Figure 7B). Moreover, IgA levels were significantly increased in the L. fermentum MC018 group when compared with those in the mock-infected group (p < 0.05, Figure 7A).
3.10 L. fermentum MC018 increased the ADG and reduced the FCR in E. coli XH197291-infected geese during 8–21 d of age
The L. salivarius MC013 and L. fermentum MC018 groups showed significant differences in the FCR during 1 to 7 d (p < 0.05, Table 3). E. coli XH197291 infection significantly reduced the ADG (p < 0.05) and increased the FCR (p < 0.05) compared to mock infection during 8 to 21 d. More importantly, L. fermentum MC018 significantly increased the ADG and reduced the FCR compared to the E. coli-infected group (Table 3).
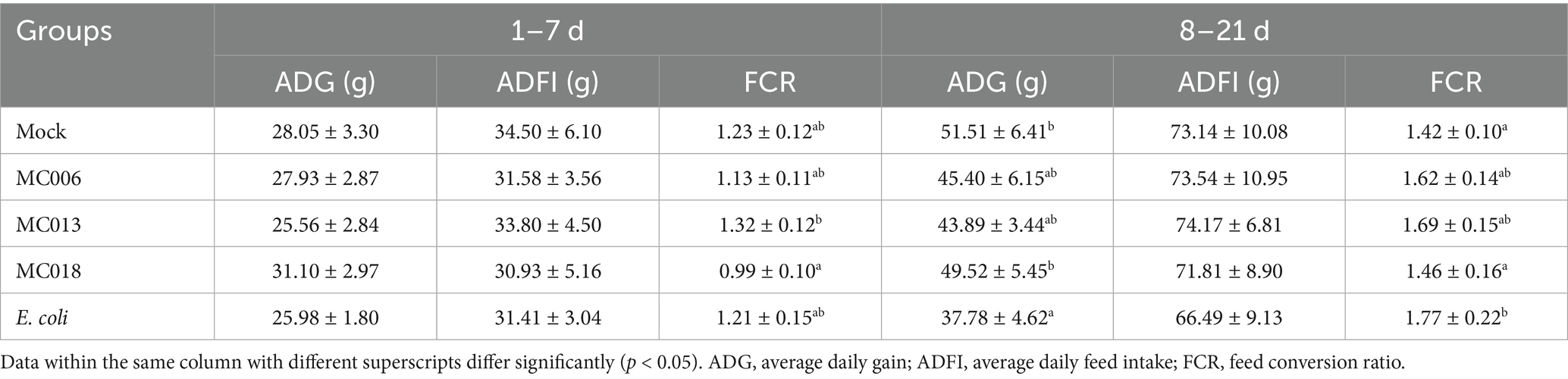
Table 3. Effect of oral supplementation of the isolated Lactobacillus strains on growth performance of geese infected with E. coli.
4 Discussion
One of the promising strategies to prevent and treat APEC infection is diet supplementation with Lactobacillus probiotics (3, 17). To exert its health-improving effects, Lactobacillus must overcome obstacles such as the presence of acid in the stomach and bile in the intestine. In the present study, L. johnsonii MC006, L. salivarius MC013, and L. fermentum MC018 exhibited resistance to degradation by acid and bile, suggesting that these strains could possibly survive under acidic conditions in the stomach and intestine. Additionally, the ability of bacteria to auto-aggregate reflects their persistence in the gut, and this aggregation eliminates or reduces pathogen adherence (22). Surface hydrophobicity represents the capacity of Lactobacillus to adhere to and colonize the intestinal tract, with a higher hydrophobicity level indicating stronger adhesion ability. A previous study reported that the auto-aggregation level of L. acidophilus LA7 and L. plantarum Lp9 was 46.5 and 31%, respectively, which confirmed their ability to auto-aggregate (27). Lactobacillus with surface hydrophobicity of >50% is strongly hydrophobic (22). In the present study, we found that the auto-aggregation and hydrophobicity levels of L. fermentum MC018 were 90.77 and 75.02%, respectively; this finding indicated the ability of this strain to survive and colonize the gastrointestinal tract, thus confirming its probiotic properties.
To investigate the protective effects of goose-derived Lactobacillus against E. coli infection in geese, suitable animal models are essential and may serve as useful experimental tools. However, to date, few studies have established a goose model of E. coli infection. As reported previously, both intraperitoneal injection and oral administration can successfully induce E. coli infection in broilers (3, 24, 28). The infected broilers exhibited typical symptoms, including loss of appetite and diarrhea, and some broilers died due to infection. E. coli infection caused intestinal damage and inflammation and decreased the growth performance of broilers (28). In the present study, Zi geese were infected with E. coli XH197291, E. coli QE191291, E. coli BA220820, or E. coli BA220826 through oral gavage. Consistent with the results of previous studies, we found that E. coli XH197291-infected geese exhibited depression, loss of appetite, diarrhea, intestinal damage, reduced ADG, and increased FCR. More importantly, compared to the other infection groups, the E. coli XH197291 infection group showed a diarrhea rate of 100% within 48 hpi, with the longest time of diarrhea duration (up to 5 d). These findings confirmed the successful establishment of a Zi goose model of E. coli XH197291 infection and provided a basis to evaluate the protective effects of Lactobacillus supplementation against E. coli infection.
Lactobacillus supplementation in E. coli-infected poultry can reduce diarrhea rate and mortality, alleviate intestinal injury, ameliorate intestinal barrier function, and improve growth performance. According to previous studies, L. acidophilus supplementation reduced the mortality of E. coli-infected broilers and elevated serum DAO levels and mRNA expression levels of Occludin and ZO-1 in the jejunum and ileum (3). Compound feed additive containing L. acidophilus reduced the diarrhea rate in E. coli-infected broilers (24). Oral administration of L. acidophilus and Bacillus subtilis alleviated the pathological damage in the intestine of E. coli-infected broilers without reducing the diarrhea rate (29). In the present study, we found that L. fermentum MC018 supplementation not only alleviated intestinal injury of geese with E. coli infection and elevated the levels of Claudin-1, Occludin, ZO-1, and DAO, but also reduced the diarrhea rate and E. coli count and increased LAB count in both ileum and rectum. These results suggest that L. fermentum MC018 could be a candidate probiotic to prevent and alleviate diarrhea and intestinal damage and improve intestinal barrier function in E. coli-infected geese. The villus height and V/C ratio are the relevant measures of intestinal absorptive capacity and intestinal health (3). A decrease in the villus height and V/C ratio diminishes the nutrient absorption capacity of the small intestine, reduces disease resistance, and lowers the growth performance of poultry (17, 28). Similarly, in the present study, E. coli XH197291 infection decreased the villus height and V/C ratio in the duodenum and ileum of geese; moreover, this reduction was accompanied by decreased ADG and increased FCR of infected geese. More importantly, compared to the E. coli-infected group, L. fermentum MC018 significantly enhanced the V/C ratios in the duodenum and ileum and decreased FCR; this result was consistent with the findings of previous studies that reported an improvement in the V/C ratio and growth performance of broilers treated with L. acidophilus (CGMCC 14437) or L. plantarum B1 (3, 17).
IL-1β and TNF-α are two important proinflammatory cytokines that participate in the regulation of inflammatory response at the early infection period (30). E. coli infection increased IL-1β and TNF-α levels in the jejunum of broilers. (3, 31). Our results also showed that E. coli XH197291 infection elevated IL-1β and TNF-α levels in the duodenum and ileum of Zi geese. Notably, L. fermentum MC018 decreased IL-1β and TNF-α levels in the duodenum and ileum of E. coli-infected geese; this finding was consistent with the conclusions of previous studies that reported a reduction in IL-1β and TNF-α levels in broilers treated with L. acidophilus or L. plantarum (3, 17). These findings suggest that L. fermentum MC018 exerts an anti-inflammatory effect on the gut of E. coli-infected geese. IL-6 shows a critical immunomodulatory effect on B cell activation, antibody secretion, and the activation and proliferation of cytotoxic T cells (32). A previous study reported that L. acidophilus supplementation did not significantly affect IL-6 gene expression in the jejunum and spleen of E. coli-infected broilers (3). In contrast, the present study found that oral supplementation of L. fermentum MC018 substantially downregulated IL-6 mRNA expression in the duodenum and ileum of infected geese as compared to that in the E. coli-infected group. This might be due to differences in animal models, Lactobacillus species, and host status.
The immune globulins (Ig) such as IgA and IgM are produced by mature B cells in response to antigenic stimulation and play a pivotal role in various immune responses (33, 34). The present study found that E. coli challenge increased serum IgA and IgM levels at the early stage of infection; this finding is consistent with the results of Wu et al. (3). When geese are in the early stages of infection, E. coli acts as a foreign antigen that stimulates B cells to regulate the immune system and produce antibodies (3). In addition, L. acidophilus supplementation did not significantly affect the serum levels of IgA and IgM in E. coli-infected broilers. However, our findings revealed that L. fermentum MC018 remarkably decreased the serum IgM levels as compared to that in the E. coli-infected group. IgM can regulate immunity, sterilize, agglutinate, and activate the complement system in the early stages of pathogen infection (35). The decreased IgM levels observed in this study might be due to a decrease in the number of pathogens in the intestines of E. coli-infected geese fed L. fermentum MC018 during the infection period, which was insufficient to stimulate the immune system to produce more specific Ig.
The combined use of multiple probiotic strains demonstrates favorable synergistic effects. In mice, the co-administration of L. acidophilus NCFM and L. plantarum Lp-115 effectively limited Helicobacter pylori colonization on gastric mucosa while also suppressing inflammation (36). Dietary supplementation with L. acidophilus and B. subtilis enhanced intestinal barrier function and preserved immunological homeostasis in hens (9). Additionally, L. johnsonii relieved enterohaemorrhagic E. coli-induced diarrhea and altered the structure of intestinal flora in rats (37). In broilers challenged with coccidia and Clostridium perfringens, dietary L. fermentum improved intestinal health by strengthening the intestinal barrier and reducing inflammation (38). Similarly, our research discovered that oral supplementation with L. johnsonii MC006 or L. fermentum MC018 ameliorated diarrhea and intestinal histological lesions in E. coli-infected geese, as well as reduce the expression of pro-inflammatory cytokines (IL-1β and TNF-α) in the duodenum and ileum. However, the potential synergistic interaction between L. johnsonii MC006 and L. fermentum MC018 needs to be further studied and confirmed.
5 Conclusion
Based on the in vitro study, goose-derived L. fermentum MC018 appears to be a promising probiotic because of its ability to resist acid and bile degradation, exert antibacterial activity on E. coli, and adhere strongly to the intestinal tract. Additionally, the in vivo study revealed that oral supplementation of E. coli-infected geese with L. fermentum MC018 alleviated diarrhea, intestinal damage, and inflammation; reduced E. coli counts in both ileum and rectum; increased intestinal LAB population, and improved intestinal barrier function and growth performance. These findings provide a scientific basis to explore Lactobacillus supplementation-based strategies to prevent E. coli infection and promote growth in geese.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by Institutional Animal Care and Use Committee of HeiLongJiang BaYi Agricultural University (Approval No. DWKJXY2024231). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
YaL: Conceptualization, Data curation, Formal analysis, Writing – original draft. ML: Data curation, Formal analysis, Conceptualization, Writing – original draft. ZL: Data curation, Software, Writing – review & editing. MD: Data curation, Software, Writing – review & editing. LH: Writing – review & editing, Methodology. PL: Writing – review & editing, Data curation, Investigation. RC: Writing – review & editing, Data curation, Software. YueL: Data curation, Writing – review & editing, Investigation. LY: Data curation, Investigation, Writing – review & editing. FL: Writing – review & editing, Methodology. YZ: Writing – review & editing, Supervision. ZZ: Supervision, Writing – review & editing. YuL: Supervision, Writing – review & editing, Funding acquisition, Project administration.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Heilongjiang Bayi Agricultural University Talent Research Start-up Fund (XYB202107).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2025.1666985/full#supplementary-material
References
1. Wu, Y, Wang, W, Kim, IH, and Yang, Y. Dietary hydrolyzed wheat gluten supplementation ameliorated intestinal barrier dysfunctions of broilers challenged with Escherichia Coli O78. Poult Sci. (2022) 101:101615. doi: 10.1016/j.psj.2021.101615
2. Ma, K, Wang, H, Lv, Z, Hu, Y, Wang, H, Shu, F, et al. The two-component system Cpxra affects antibiotic susceptibility and biofilm formation in avian pathogenic Escherichia Coli. Animals. (2023) 13:383. doi: 10.3390/ani13030383
3. Wu, Z, Yang, K, Zhang, A, Chang, W, Zheng, A, Chen, Z, et al. Effects of Lactobacillus acidophilus on the growth performance, immune response, and intestinal barrier function of broiler chickens challenged with Escherichia coli O157. Poult Sci. (2021) 100:101323. doi: 10.1016/j.psj.2021.101323
4. Ban, Y, and Guan, LL. Implication and challenges of direct-fed microbial supplementation to improve ruminant production and health. J Anim Sci Biotechnol. (2021) 12:109. doi: 10.1186/s40104-021-00630-x
5. Zhang, Y, Meng, Z, Li, S, Liu, T, Song, J, Li, J, et al. Two antimicrobial peptides derived from Bacillus and their properties. Molecules. (2023) 28:7899. doi: 10.3390/molecules28237899
6. Wang, Y, Li, J, Dai, X, Wang, Z, Ni, X, Zeng, D, et al. Effects of antimicrobial peptides Gal-13 on the growth performance, intestinal microbiota, digestive enzyme activities, intestinal morphology, Antioxidative activities, and immunity of broilers. Probiotics Antimicrob Proteins. (2023) 15:694–705. doi: 10.1007/s12602-021-09905-1
7. Yang, Y, Hong, J, Zhang, Z, Zheng, M, Zhao, J, Fang, X, et al. Oral supplementation with lactic acid Bacteria improve the intestinal epithelial barrier and gut microbiota of broiler chicks to alleviate Salmonella enteritidis infection. Poult Sci. (2024) 103:104385. doi: 10.1016/j.psj.2024.104385
8. Yin, Y, Liao, Y, Li, J, Pei, Z, Wang, L, Shi, Y, et al. Lactobacillus plantarum Gx17 benefits growth performance and improves functions of intestinal barrier/intestinal Flora among yellow-feathered broilers. Front Immunol. (2023) 14:1195382. doi: 10.3389/fimmu.2023.1195382
9. Chen, X, Chen, W, Ci, W, Zheng, Y, Han, X, Huang, J, et al. Effects of dietary supplementation with Lactobacillus acidophilus and Bacillus subtilis on mucosal immunity and intestinal barrier are associated with its modulation of gut metabolites and microbiota in late-phase laying Lens. Probiotics Antimicrob Proteins. (2023) 15:912–24. doi: 10.1007/s12602-022-09923-7
10. Neveling, DP, and Dicks, LMT. Probiotics: an antibiotic replacement strategy for healthy broilers and productive rearing. Probiotics Antimicrob Proteins. (2021) 13:1–11. doi: 10.1007/s12602-020-09640-z
11. Liu, Q, Ni, X, Wang, Q, Peng, Z, Niu, L, Wang, H, et al. Lactobacillus plantarum Bsgp201683 Isolated from Giant panda feces attenuated inflammation and improved gut microflora in mice challenged with Enterotoxigenic Escherichia coli. Front Microbiol. (2017) 8:1885. doi: 10.3389/fmicb.2017.01885
12. Asghar, S, Arif, M, Nawaz, M, Muhammad, K, Ali, MA, Ahmad, MD, et al. Selection, characterisation and evaluation of potential probiotic Lactobacillus Spp. Isolated from Poultry Droppings Benef Microbes. (2016) 7:35–44. doi: 10.3920/BM2015.0020
13. Shah, AB, Baiseitova, A, Zahoor, M, Ahmad, I, Ikram, M, Bakhsh, A, et al. Probiotic significance of Lactobacillus strains: a comprehensive review on Healthimpacts, research gaps, and future prospects. Gut Microbes. (2024) 16:2431643. doi: 10.1080/19490976.2024.2431643
14. Naghmouchi, K, Belguesmia, Y, Bendali, F, Spano, G, Seal, BS, and Drider, D. Lactobacillus fermentum: a bacterial species with potential for food preservation and biomedical applications. Crit Rev Food Sci Nutr. (2020) 60:3387–99. doi: 10.1080/10408398.2019.1688250
15. Messaoudi, S, Manai, M, Kergourlay, G, Prévost, H, Connil, N, Chobert, JM, et al. Lactobacillus salivarius: bacteriocin and probiotic activity. Food Microbiol. (2013) 36:296–304. doi: 10.1016/j.fm.2013.05.010
16. Zhang, Z, Zhao, L, Wu, J, Pan, Y, Zhao, G, Li, Z, et al. The effects of Lactobacillus johnsonii on diseases and its potential applications. Microorganisms. (2023) 11:2580. doi: 10.3390/microorganisms11102580
17. Wang, S, Peng, Q, Jia, HM, Zeng, XF, Zhu, JL, Hou, CL, et al. Prevention of Escherichia coli infection in broiler chickens with Lactobacillus plantarum B1. Poult Sci. (2017) 96:2576–86. doi: 10.3382/ps/pex061
18. Ni, H, Zhang, Y, Yang, Y, Yin, Y, Ren, J, Xiao, Q, et al. Integrated analysis of whole genome and transcriptome sequencing uncovers genetic differences between Zi goose and Xianghai flying goose. Anim Genet. (2024) 55:147–51. doi: 10.1111/age.13388
19. Sukrama, IDM, Franciska, J, and Suardana, IW. Evaluation of the Bacteriocin produced by strain 9 lactic acid Bacteria isolate for biopreservation. Vet World. (2020) 13:2012–9. doi: 10.14202/vetworld.2020.2012-2019
20. Whitehead, K, Versalovic, J, Roos, S, and Britton, RA. Genomic and genetic characterization of the bile stress response of probiotic Lactobacillus reuteri Atcc 55730. Appl Environ Microbiol. (2008) 74:1812–9. doi: 10.1128/AEM.02259-07
21. Solieri, L, Bianchi, A, Mottolese, G, Lemmetti, F, and Giudici, P. Tailoring the probiotic potential of non-starter Lactobacillus strains from ripened Parmigiano Reggiano cheese by in vitro screening and principal component analysis. Food Microbiol. (2014) 38:240–9. doi: 10.1016/j.fm.2013.10.003
22. Farid, W, Masud, T, Sohail, A, Ahmad, N, Naqvi, SMS, Khan, S, et al. Gastrointestinal transit tolerance, cell surface hydrophobicity, and functional attributes of Lactobacillus acidophilus strains isolated from indigenous Dahi. Food Sci Nutr. (2021) 9:5092–102. doi: 10.1002/fsn3.2468
23. Li, P, Zhao, S, Teng, Y, Han, S, Yang, Y, Wu, M, et al. Dietary supplementary with Ellagic acid improves the intestinal barrier function and Flora structure of broiler chicken challenged with E. coli K88. Poult Sci. (2024) 103:104429. doi: 10.1016/j.psj.2024.104429
24. Zheng, J, Liang, S, Zhang, Y, Sun, X, Li, Y, Diao, J, et al. Effects of compound Chinese herbal medicine additive on growth performance and gut microbiota diversity of Zi goose. Animals. (2022) 12:2942. doi: 10.3390/ani12212942
25. Ni, H, Zhang, Y, Yang, Y, Li, Y, Yin, Y, Sun, X, et al. Comparative analyses of production performance, meat quality, and gut microbial composition between two Chinese goose breeds. Animals. (2022) 12:1815. doi: 10.3390/ani12141815
26. Garrido Margarita, N, Skjervheim, M, Oppegaard, H, and Sørum, H. Acidified litter benefits the intestinal Flora balance of broiler chickens. Appl Environ Microbiol. (2004) 70:5208–13. doi: 10.1128/AEM.70.9.5208-5213.2004
27. Kaushik, JK, Kumar, A, Duary, RK, Mohanty, AK, Grover, S, and Batish, VK. Functional and probiotic attributes of an indigenous isolate of Lactobacillus plantarum. PLoS One. (2009) 4:e8099. doi: 10.1371/journal.pone.0008099
28. Mao, N, Yu, Y, Cui, J, He, J, Yang, Y, and Wang, D. Effect of Matrine on growth performance, gut health, and gut microbiota in chickens infected with avian pathogenic Escherichia coli. Poult Sci. (2025) 104:104520. doi: 10.1016/j.psj.2024.104520
29. Liang, W, Li, H, Zhou, H, Wang, M, Zhao, X, Sun, X, et al. Effects of Taraxacum and Astragalus extracts combined with probiotic Bacillus subtilis and Lactobacillus on Escherichia coli–infected broiler chickens. Poult Sci. (2021) 100:101007. doi: 10.1016/j.psj.2021.01.030
30. Wang, RP-H, Huang, J, Chan, KWY, Leung, WK, Goto, T, Ho, Y-S, et al. Il-1β and Tnf-Α play an important role in modulating the risk of periodontitis and Alzheimer’s disease. J Neuroinflammation. (2023) 20:71. doi: 10.1186/s12974-023-02747-4
31. Cao, GT, Zeng, XF, Chen, AG, Zhou, L, Zhang, L, Xiao, YP, et al. Effects of a probiotic, Enterococcus faecium, on growth performance, intestinal morphology, immune response, and Cecal microflora in broiler chickens challenged with Escherichia coli K88. Poult Sci. (2013) 92:2949–55. doi: 10.3382/ps.2013-03366
32. Zhou, L, Che, Z, Zhang, X, Zhou, P, Li, X, Xu, X, et al. Influence of neonatal gender on cord blood Cd34+ cell amplification and gene expression. Exp Ther Med. (2019) 18:105–18. doi: 10.3892/etm.2019.7549
33. Pan, S, Manabe, N, and Yamaguchi, Y. 3d structures of Iga, Igm, and components. Int J Mol Sci. (2021) 22:12776. doi: 10.3390/ijms222312776
34. Zhang, Y, Fear, DJ, Willis-Owen, SAG, Cookson, WO, and Moffatt, MF. Global gene regulation during activation of immunoglobulin class switching in human B cells. Sci Rep. (2016) 6:37988. doi: 10.1038/srep37988
35. Liu, J, Liu, G, Chen, Z, Zheng, A, Cai, H, Chang, W, et al. Effects of glucose oxidase on growth performance, immune function, and intestinal barrier of ducks infected with Escherichia coli O88. Poult Sci. (2020) 99:6549–58. doi: 10.1016/j.psj.2020.09.038
36. Shen, S, Ren, F, Qin, H, Bukhari, I, Yang, J, Gao, D, et al. Lactobacillus acidophilus Ncfm and Lactiplantibacillus plantarum Lp-115 inhibit Helicobacter pylori colonization and gastric inflammation in a murine model. Front Cell Infect Microbiol. (2023) 13:1196084. doi: 10.3389/fcimb.2023.1196084
37. Hu, Y, Zhao, M, Lu, Z, Lv, F, Zhao, H, and Bie, X. L. johnsonii, L. plantarum, and L. rhamnosus alleviated enterohaemorrhagic Escherichia coli-induced Diarrhoea in mice by regulating gut microbiota. Microb Pathog. (2021) 154:104856. doi: 10.1016/j.micpath.2021.104856
Keywords: geese, Lactobacillus fermentum , Escherichia coli , intestinal health, growth performance
Citation: Li Y, Liu M, Li Z, Dong M, He L, Li P, Chen R, Liang Y, Yang L, Li F, Zhou Y, Zhu Z and Liu Y (2025) Oral supplementation with Lactobacillus fermentum MC018 improves intestinal health, immune response, and growth performance of Zi geese infected with Escherichia coli XH197291. Front. Vet. Sci. 12:1666985. doi: 10.3389/fvets.2025.1666985
Edited by:
Jianzhu Liu, Shandong Agricultural University, ChinaReviewed by:
Bo Ni, China Animal Health and Epidemiology Center, ChinaFeng Pang, Guizhou University, China
Copyright © 2025 Li, Liu, Li, Dong, He, Li, Chen, Liang, Yang, Li, Zhou, Zhu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Liu, bGl1eXV5ZkAxNjMuY29t; Zhanbo Zhu, emhhbmJvemh1QGJ5YXUuZWR1LmNu
 Yang Li
Yang Li Yulong Zhou
Yulong Zhou Zhanbo Zhu
Zhanbo Zhu Yu Liu
Yu Liu