- 1Southern Branch, Taiwan Livestock Research Institute, Ministry of Agriculture, Pingtung, Taiwan
- 2Graduate Institute of Bioresources, National Pingtung University of Science and Technology, Pingtung, Taiwan
- 3Department of Animal Science, National Pingtung University of Science and Technology, Neipu, Pingtung, Taiwan
Artificial insemination (AI) is a critical tool for genetic improvement and fertility management in goats. This study examined the effects of semen deposition site (uterine body, cervix, vagina) and vaginal mucus type (cloudy, turbid, clear) on pregnancy rate, kidding rate, and average litter size in 300 CIDR-synchronized Alpine does in southern Taiwan. Semen deposited in the uterine body combined with cloudy mucus yielded the highest pregnancy rate (55.9%), while vaginal deposition with clear mucus resulted in the lowest (30.7%). Two-way ANOVA showed significant main effects and interactions for pregnancy rate and average litter size (p < 0.05), but no significant effect on kidding rate. Pregnant does exhibited lower vaginal mucus electrical conductivity, higher pH, and elevated temperature compared to non-pregnant does, suggesting these parameters as potential biomarkers for estrus detection. Findings highlight the importance of precise semen placement and optimal mucus condition for improving AI protocols in goats.
1 Introduction
Artificial insemination (AI) is one of the most significant reproductive biotechnologies in modern goat production. It plays a critical role in genetic improvement, enhancing productivity, and enabling more effective management of breeding programs (1–3). Through AI, farmers can access superior genetic resources from elite bucks, resulting in offspring with higher milk yield, faster growth, and greater disease resistance. Moreover, AI reduces the risk of disease transmission and allows strategic planning of breeding seasons, thereby supporting the sustainable development of livestock systems (4–6).
Despite its advantages, the success of AI is influenced by multiple biological and environmental factors, among which semen deposition site and vaginal mucus characteristics are especially important (7). Previous studies have consistently shown that deeper semen deposition within the female reproductive tract is associated with higher pregnancy rates. Laparoscopic intrauterine insemination (LAI), which directly deposits semen into the uterine horns, typically achieves the highest success rates (8–10), followed by transcervical insemination, while vaginal insemination yields the lowest (11–13). This occurs because closer semen deposition to the fertilization site reduces the distance sperm must travel and the physiological barriers they encounter, consistent with current models of sperm-tract interactions (14–16).
Beyond deposition site, vaginal and cervical mucus characteristics including appearance, consistency, pH, and electrical conductivity vary throughout the estrous cycle and directly influence sperm survival and transport (17, 18). Cervical mucus is generally clear and fluid during estrus, facilitating sperm passage, but becomes increasingly viscous after estrus, which restricts sperm motility (18–20). Vaginal electrical resistance (VER) typically declines around estrus, making it a useful adjunct for AI timing. Recent work has provided reference ranges for goats and supports the use of electrical measures as objective biomarkers (21, 22). In addition, external factors such as ambient temperature and meteorological conditions have been shown further to modulate fertility following AI (23–25).
Although these parameters are well documented as indicators for estrus detection and ovulation timing, their potential as predictive biomarkers at the actual moment of insemination remains underexplored. Most prior research has focused on determining whether a doe is in estrus and estimating ovulation rather than systematically documenting mucus appearance and physicochemical properties such as conductivity, pH, and temperature synchronously with AI procedures. As a result, there is a lack of systematic field data directly linking these real-time measurements to reproductive outcomes, including pregnancy rate, kidding rate, and litter size. This gap limits the development of practical, science-based tools that could enhance AI efficiency under commercial farm conditions.
This study aimed to evaluate how semen deposition site and vaginal mucus characteristics influence AI outcomes in Alpine goats and determine whether mucus physicochemical properties (pH, conductivity, temperature) can serve as practical biomarkers for optimal insemination timing. By integrating anatomical precision with real-time physiological assessment, we sought to establish evidence-based guidelines for improving reproductive management in commercial goat production systems.
2 Materials and methods
2.1 Animals
2.1.1 Does
Field data from 300 Alpine does inseminated was collected in this study. The animals were sourced from three private farms in southern Taiwan, namely Kaixiang dairy goat farm in Zhongpu, Chiayi, Songjun livestock farm, and Jiata farm. The data collection period focused on the breeding season from October to November of 2024. All does were between 2 and 3 years of age and had given birth 1 to 2 times. The average daily milk yield ranged from 2.3 to 2.7 kg.
The 300 does were distributed across nine treatment combinations (3 deposition sites × 3 mucus types) as follows: uterine body deposition (n = 84: cloudy n = 28, turbid n = 37, clear n = 19), cervical deposition (n = 155: cloudy n = 42, turbid n = 67, clear n = 46), and vaginal deposition (n = 61: cloudy n = 15, turbid n = 23, clear n = 23). The uneven distribution reflects the natural occurrence of different mucus types and the technical challenges associated with achieving deeper semen deposition in field conditions.
Importantly, no grouping of animals was conducted at the time of data collection. Vaginal mucus observations, including electrical conductivity, pH, and vaginal temperature, were measured and recorded on-site at the time of insemination. Subsequently, the dataset was classified retrospectively according to pregnancy rate, kidding rate, and average litter size.
2.1.2 Bucks and semen source of frozen semen and artificial insemination
The frozen semen for artificial insemination (AI) was purchased from the Southern Branch of Livestock Research Institute, Council of Agriculture Taiwan and one buck was the origin of semen. Each straw contained 0.5 mL of semen with a concentration of 150 × 10⁶ sperm/0.5 mL. Only seminal samples with a post-thaw sperm motility >50% along with a progressive motility >40% were selected. The buck was 4 years 10 months old and had sired 2,200 doses of frozen semen.
2.1.3 Preparation of semen and composition of the extender
Semen straws were thawed in a 37 °C water bath for 30 s before use. Semen quality at post-thaw was analyzed using a computer-assisted sperm analyzer (Sperm Class Analyzer® CASA System, MICROPTIC, Barcelona, Spain). Semen with ≥50% total motility and ≥50% progressive motility were only included. The semen extender was formulated per 100 mL as follows: 2.42 g Tris (hydroxymethyl aminomethane; T1503), 1.48 g citric acid (C0759), 1.00 g glucose (G8270), 1 mL penicillin–streptomycin solution (P4333), 6% low-density lipoprotein (LDL) on a dry matter basis. The concentration of glycerol (G5516) was set to 7% (v/v). All chemicals were obtained from Sigma-Aldrich.
2.2 Estrus synchronization application to does
All does were synchronized using a Controlled Internal Drug Release device (CIDR®; EAZI–breed, Rydalmere, Australia) in combination with pregnant mare serum gonadotropin (PMSG, Prospec-Tany, Israel) and a prostaglandin F2α analog (cloprostenol, Estrumate®, Merck Animal Health, United States). CIDR® was administered intravaginally on Day 0. An intramuscular injection of PGF2α (5.3 mg/0.5 mL) and PMSG (500 IU) was performed on Day 9. CIDR® was withdrawn at Day 11 and AI was performed 40 h after CIDR withdrawal.
2.3 Measurement and assessment criteria of all parameters
2.3.1 Pregnancy rate (%)
Pregnancy was determined 45 days after laparoscopic AI by transrectal ultrasonography with an ultrasound scanner (Aloka SSD-500, Japan) and a transrectal linear probe (3.5 MHz). Pregnancy was diagnosed by recognition of uterine horn shapes and images of fetuses.
2.3.2 Kidding rate (%)
The proportion of does conceived around 150 ± 7 days post-laparoscopic artificial insemination.
2.3.3 Average litter size-kid
Number of kids born/Number of does that kidded.
2.4 Artificial insemination process
2.4.1 Semen deposition site
The inseminator categorized semen deposition sites into 3 distinct types according to the depth of sheathization and tactile sensation (Figure 1):
• Vaginal: An insemination sheath was inserted through the external cervical, but the tip was not inserted into the cervix.
• Cervical: The artificial insemination sheath was inserted approximately 2 cm into the cervix, the tip of the sheath partially through the screw circle the cervix, but not into the uterine body, when injection, no reflux of the semen back to the vagina.
• Body of the uterus: The sheath entered the cervix, and ejaculate was placed directly into the body of the uterus.
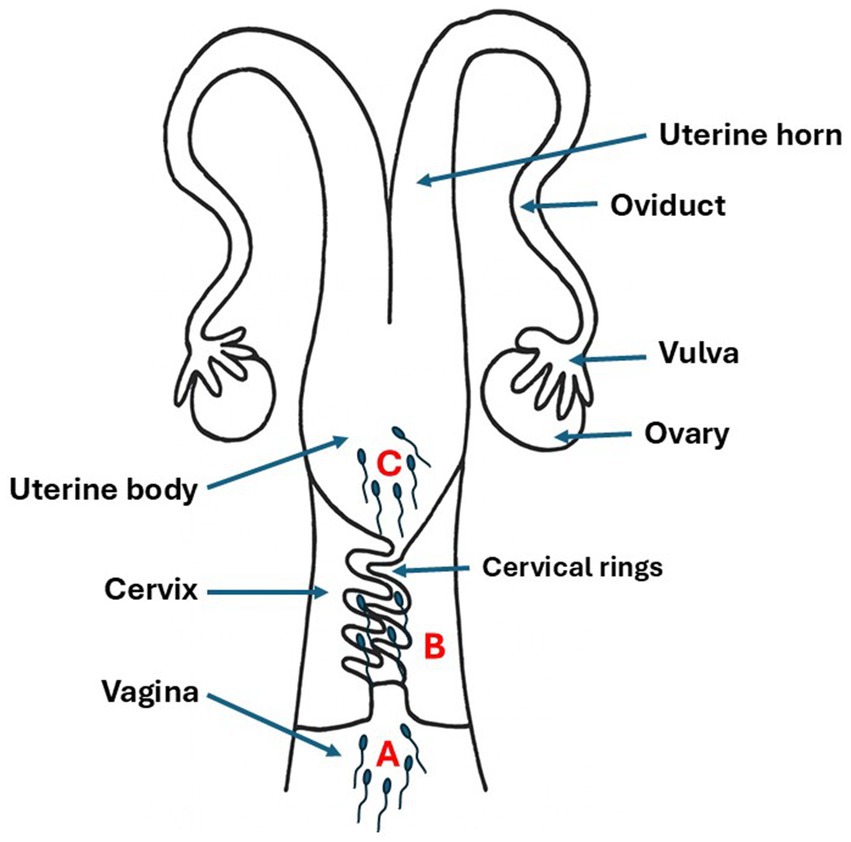
Figure 1. Schematic representation of semen deposition sites during artificial insemination in goats. (A) Vaginal deposition: semen placed in vaginal cavity without cervical penetration. (B) Cervical deposition: semen deposited 2 cm into cervix through cervical rings. (C) Uterine body deposition: semen placed directly into uterine body through complete cervical passage. Arrows indicate semen placement locations.
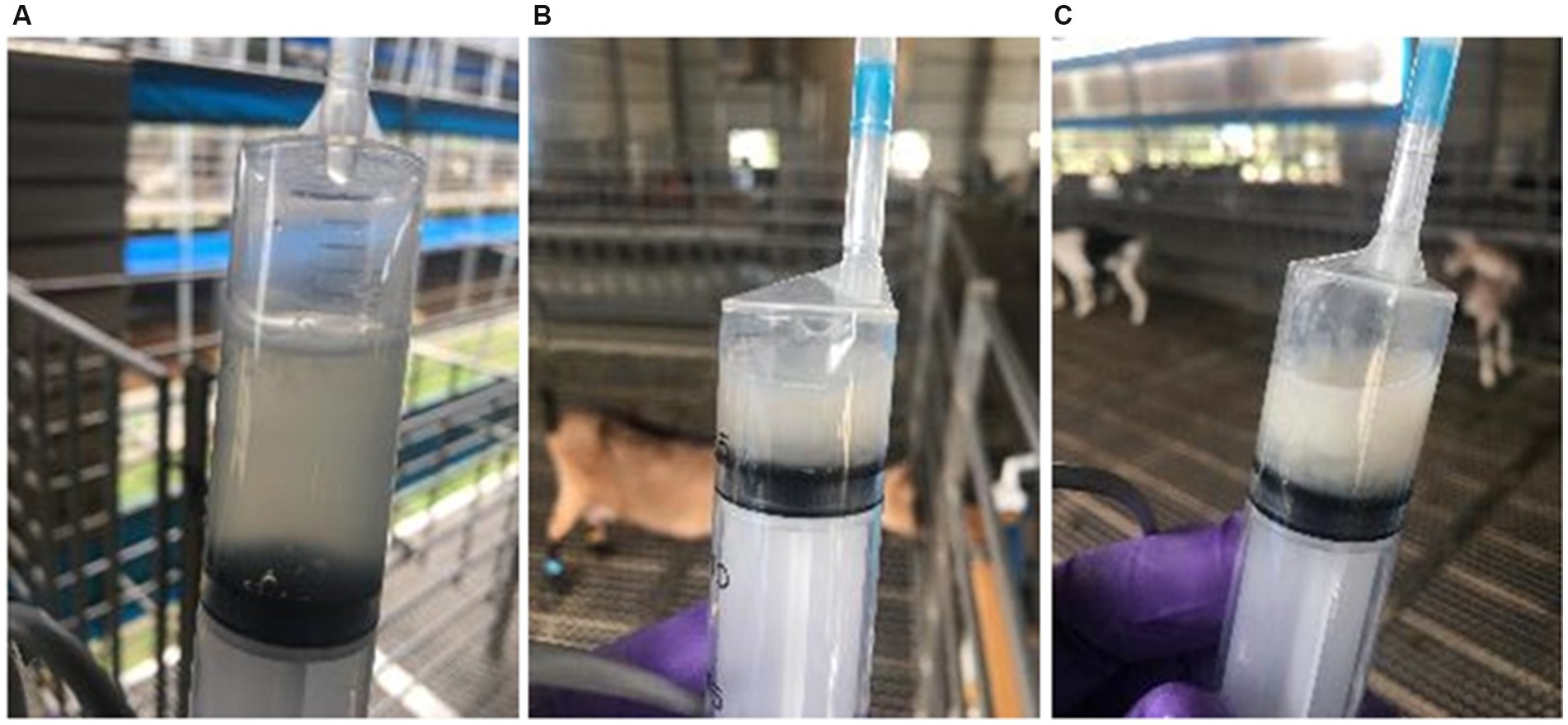
2.4.2 Vaginal mucus type
Prior to insemination, vaginal mucus was evaluated visually by the technician, with the aid of a speculum and sorted into 3 categories (Figure 2):
• Clear: Transparent with good fluidity, no noticeable odor or suspended particles.
• Turbid: Opaque with flocculent substances, moderate fluidity.
• Cloudy: Milky and viscous, poor fluidity often adheres to the vaginal wall.
The combination of the two factors (site of semen deposition × type of mucus) resulted in a 3 × 3 factorial arrangements consisting of nine observations.
2.5 Measurement of vaginal mucus conductivity, pH, and vaginal temperature and personnel
2.5.1 Personnel
All artificial insemination procedures were performed by the same operator to minimize subjective variability in assessing vaginal mucus characteristics and semen deposition sites.
2.5.2 Measurement of vaginal mucus conductivity, pH, and temperature
During artificial insemination, the physical properties of vaginal mucus were measured using a 3-in-1 detector (TES–1381) manufactured by TES Electrical Electronic Corp. The pH measurement had a resolution of 0.001 and an accuracy of ± 0.01. Conductivity resolution ranged from 0.001 μS/cm to 0.01 mS/cm, with accuracy between ± 2% and ± 5% of full scale (FS). Temperature resolution was 0.1 °C or 0.1 °F, with an accuracy of ± 0.5 °C for Celsius measurements and ± 0.9 °F for Fahrenheit.
2.6 Sperm motility assessment
Prior to use, all frozen semen samples were thawed and assessed for motion and progressive movement through Object Computer-Assisted Sperm Analysis (CASA). Only samples with post-thaw motility >50% and progressive motility >40% were considered.
2.7 Artificial insemination procedure
The frozen semen straws were obtained from storage in liquid nitrogen, and were suspended in the water bath at 37 °C and thawed for 30 s. Upon thawing, the straw was dried with tissue paper, the seal severed, and the semen transferred into the insemination gun. A lubricated speculum (K-Y jelly, Johnson and Johnson Medical, Ltd., Gargrave, Skipton, BD23 3RX, United Kingdom), was carefully placed in the vagina, its narrow end first and then it was rotated 90° to view the cervical, prior to insemination. The insemination gun was advanced through the cervix; resistance to the passage of the gun was noted, and insertion was halted, and insemination was conducted to prevent tissue damage or immunological reactions along with likely reduction in conception percentage.
2.8 Statistical analysis
All statistical analyses were performed by SAS® Enterprise Guide version 7.4 (SAS Institute Inc., Cary, NC, United States). Values are expressed as the mean ± SE. Pregnancy rates were tested among semen deposition sites and mucus types using one-way ANOVA followed by Tukey’s Honestly Significant Difference (HSD) post-hoc analysis. Means with different lowercase letters (a, b, c) within the same column are significantly different (p < 0.05).
Multiple linear regression and two-way analysis of variance (Two-way ANOVA) were conducted to evaluate the effects of cervical mucus condition and semen deposition location on pregnancy rate, kidding rate, and average litter size.
In the multiple linear regression analysis, cervical mucus condition and semen deposition location were coded as ordinal variables (mucus: 1 = clear, 2 = turbid, 3 = cloudy; location: 1 = vagina, 2 = cervix, 3 = uterine body) to explore whether a monotonic relationship exists across levels. This approach does not assume strict linearity but provides an initial assessment of potential trend-like associations (e.g., increasing mucus opacity or deeper semen deposition site may correlate with altered reproductive outcomes).
Although mucus condition and semen deposition site were coded numerically (1–3) for regression analysis, both variables remained categorical in nature. This ordinal coding was employed to test monotonic trends across treatment levels rather than to assume strict linearity. The consistency between ordinal regression and categorical ANOVA results validates the robustness of our statistical approach and confirms that observed associations reflect genuine biological relationships rather than methodological artifacts.
To address potential concerns regarding the assumption of linearity, the same factors were also analyzed as categorical variables in the two-way ANOVA model. Tukey’s post-hoc test was applied for multiple comparisons where appropriate. The consistency of results across both analytical approaches was used to validate the robustness of the findings. Statistical significance was set at p < 0.05.
Sample size calculations were based on detecting a 20% difference in pregnancy rates between treatment groups with 80% power and α = 0.05. Post-hoc power analysis confirmed adequate statistical power (>85%) for detecting the observed effect sizes in pregnancy rate and litter size outcomes.
3 Results
This study evaluated the influence of semen deposition sites (uterine body, cervical, and vaginal) and vaginal mucus characteristics (cloudy, turbid, and clear) on reproductive performance in goat, including pregnancy rate, kidding rate, and average litter size following artificial insemination.
3.1 Effects of semen deposition site and mucus characteristics on reproductive efficiency
The results showed in Table 1. Both semen deposition site and mucus type significant affected pregnancy rate and litter size (p < 0.05), whereas kidding rate remained stable across treatments (p > 0.05). Pregnancy outcomes were consistently higher when semen was deposited deeper within the reproductive tract (uterine body or cervix) and when mucus was more viscous (cloudy or turbid).
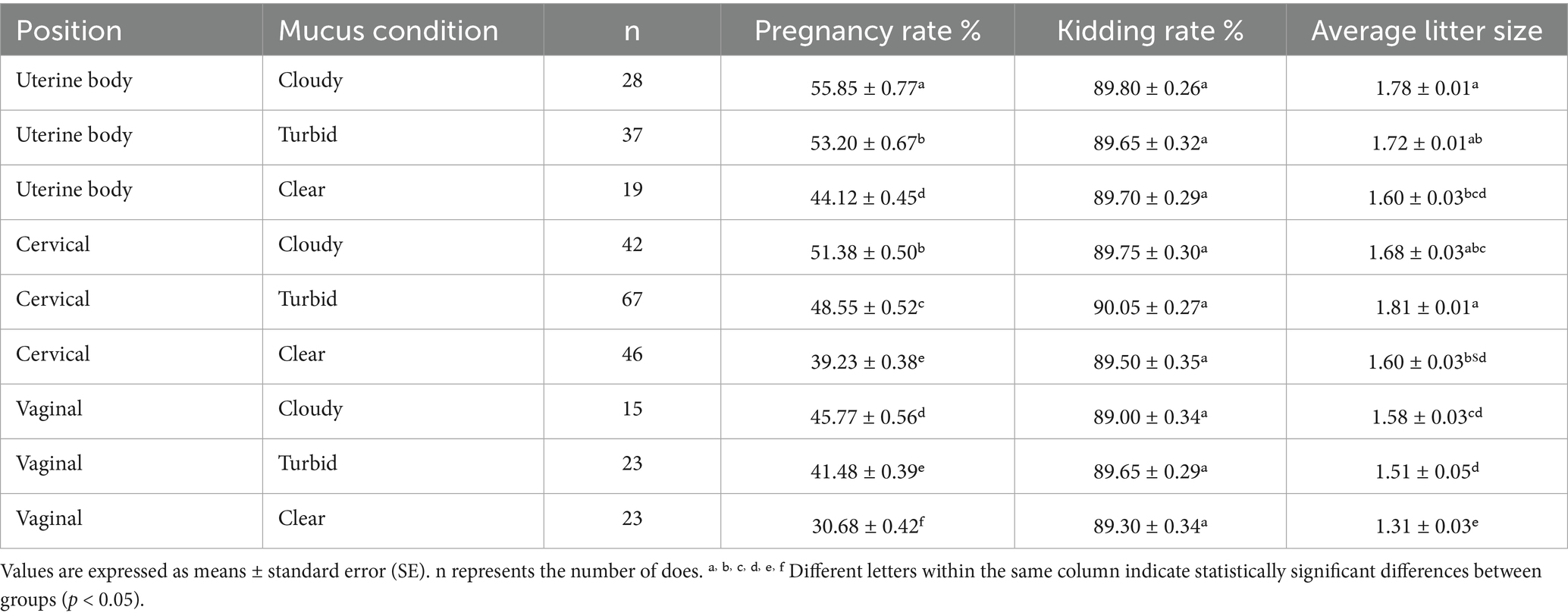
Table 1. Effects of semen deposition site and mucus characteristics on reproductive performance in goats.
The uterine body–cloudy combination achieved the highest pregnancy rate (55.9, 95% CI, 54.4–57.4), significantly outperforming most other combinations. By contrast, the lowest rate occurred in the vaginal–clear mucus group (30.68, 95% CI, 29.84–31.52).
3.2 Correlation between vaginal mucus characteristics, semen deposition site, and reproductive performance
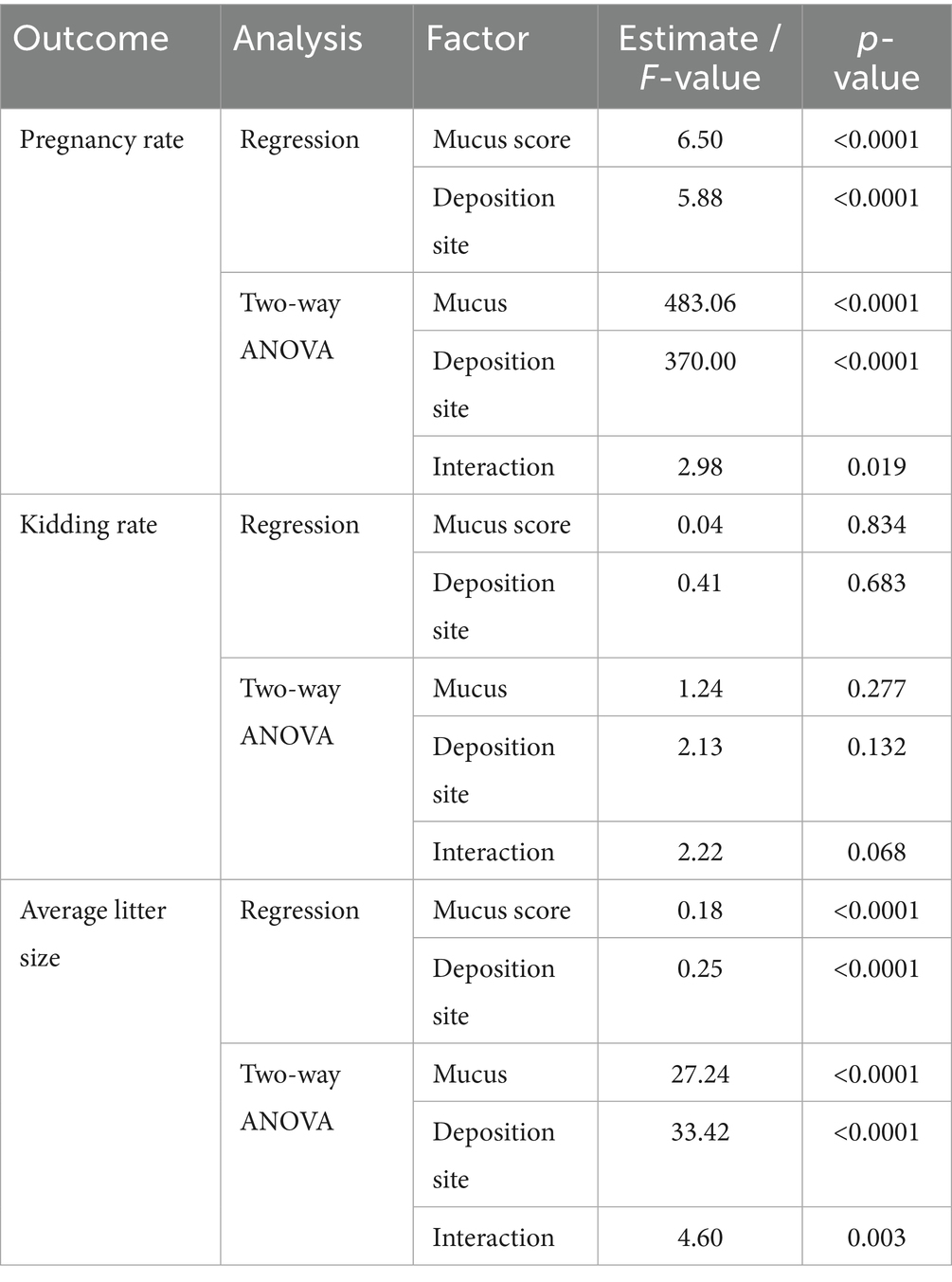
Regression and two-way ANOVA analyses (Table 2) demonstrated that pregnancy rate was the most responsive outcome to both experimental factors, followed by litter size, while kidding rate showed little variation.
3.2.1 Pregnancy rate
Mucus score (estimate = 6.50, p < 0.0001) and deposition site (estimate = 5.88, p < 0.0001) were both strongly associated with improved outcomes, with a significant interaction effect (F = 2.98, p = 0.019).
3.2.2 Kidding rate
Neither mucus quality nor deposition site had significant effects (p > 0.05), although the interaction approached significance (p = 0.068).
3.2.3 Average litter size
Both factors contributed positively to offspring number (mucus estimate = 0.18; site estimate = 0.25; both p < 0.0001), with a significant interaction effect (F = 4.60, p = 0.003).
These results emphasize that optimizing mucus quality (favoring turbid or cloudy) and semen deposition site (uterine body or cervix) produces synergistic improvements in conception probability and litter size, while exerting limited influence on kidding success.
3.3 Vaginal mucus electrical conductivity, pH, and temperature between pregnant and non-pregnant goats
Physiological properties of vaginal mucus differed significantly between pregnant and non-pregnant does (Table 3). Pregnant goats consistently exhibited lower electrical conductivity, higher pH, and slightly elevated vaginal temperatures compared with non-pregnant counterparts.
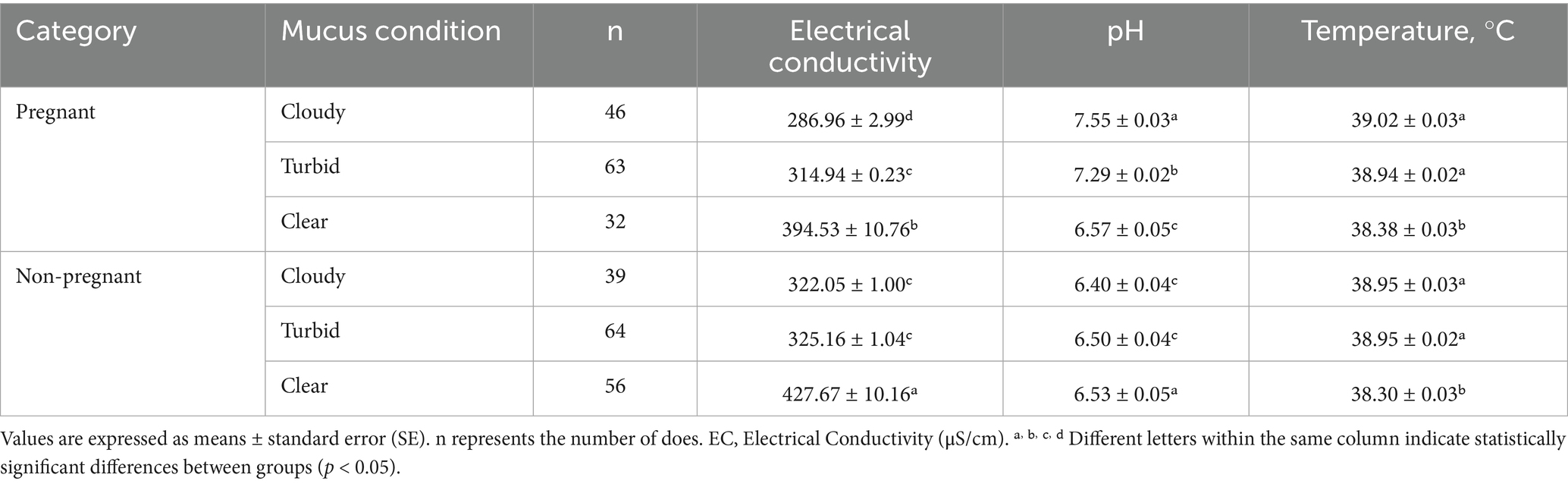
Table 3. Vaginal mucus electrical resistance, pH, and vaginal temperature in pregnant and non-pregnant goats.
3.3.1 Electrical conductivity
Pregnant does had the lowest EC in the cloudy mucus group (~287 μS/cm), whereas non-pregnant does exhibited markedly higher EC in the clear mucus group (~428 μS/cm).
3.3.2 pH
Vaginal mucus in pregnant does was more alkaline, reaching pH 7.55 in cloudy mucus, compared with 6.40–6.53 in non-pregnant does.
3.3.3 Temperature
Pregnant does maintained higher vaginal temperatures (~39.0 °C) under turbid and cloudy conditions, while non-pregnant does exhibited slightly lower temperatures (~38.3–38.9 °C).
Together, these physiological markers lower EC, higher pH, and elevated temperature in viscous mucus may serve as reliable indicators of successful conception in goats.
4 Discussion
4.1 Effect of semen deposition site on artificial insemination success rate in goats
The present findings clearly demonstrate that the anatomical site of semen deposition has a decisive effect on AI success in goats. Pregnancy rates were consistently higher when semen was deposited in the uterine body compared with the cervix or vagina, particularly under favorable mucus conditions. For example, uterine body–cloudy and uterine body–turbid groups yielded conception rates exceeding 53%, whereas vaginal-clear inseminations achieved less than 31%. These observations corroborate earlier studies highlighting the advantage of deeper semen placement in small ruminants (26, 27).
Leethongdee et al. (28) further reported that semen retained in the vagina without pretreatment reduces fertility, while intrauterine insemination enhances outcomes (29) similarly showed that laparoscopic AI with frozen semen can achieve 60–80% pregnancy rates, compared with less than 40% using cervical or vaginal methods. Anatomically, the goat cervix contains four to five annular folds that impede catheter passage and present immune challenges to sperm survival (30, 31, 32). Consistent with large-scale field studies in Spanish goat breeds, accurate and deeper deposition remains central to maximizing fertility (23, 24, 33).
4.2 Influence of vaginal and cervical mucus characteristics on reproductive success
Mucus properties represent a critical physiological biomarker of estrus and directly influence insemination success. In hormonally synchronized does, clear mucus is typically observed under conditions of high estrogen dominance. This type of mucus is characterized by high water and electrolyte content and low viscosity, which facilitates sperm penetration but offers limited protection against the acidic vaginal environment (26, 27).
As ovulation approaches and the luteinizing hormone (LH) surges, mucus composition shifts toward higher concentrations of glycoproteins, particularly mucins, as well as cellular debris. These changes result in turbid or cloudy appearances with increased viscosity and stronger adhesion to the vaginal wall (27, 28). While this increased viscosity may restrict sperm motility, it also provides a more protective environment that enhances sperm survival (29).
Our findings confirmed this physiological pattern: conception rates were highest when mucus was turbid, intermediate when it was cloudy, and lowest when it was clear. These results align with earlier reports indicating that insemination timed at the transition to turbid mucus closely predicts ovulation (28, 29). Conversely, although clear mucus is strongly associated with estrogen, it may obstruct the cervix and complicate insemination procedures (30).
From a practical standpoint, artificial insemination protocols that consider mucus quality can improve reproductive outcomes. Monitoring the transition from clear to turbid/cloudy mucus provides a valuable visual biomarker of imminent ovulation for field inseminators. Moreover, adjunct strategies such as the use of hyaluronic acid to facilitate catheter passage may further enhance insemination success and fertility outcomes (28, 29).
4.3 Interaction effects of semen deposition site and mucus characteristics
Our factorial analysis revealed that semen deposition site and mucus characteristics act synergistically to determine fertility outcomes. Both pregnancy rate and litter size were significantly influenced by the combination of these two variables, while kidding rate remained unaffected.
Methodological considerations. Beyond the biological interpretation, several methodological aspects deserve clarification. In the regression models, cervical mucus condition and semen deposition site were treated as ordinal variables coded from 1 to 3. While this might raise concerns about the assumption of linearity, our intention was to test monotonic trends across levels rather than to imply strict equal-interval scaling. For example, progressive increases in mucus opacity or deeper deposition sites may reasonably be expected to produce directional changes in reproductive outcomes. To address potential concerns, we also analyzed these variables as categorical factors using two-way ANOVA. The consistency between the ordinal and categorical analyses supports the robustness of our findings and strengthens the argument that the associations observed are biologically meaningful rather than statistical artifacts.
Main effects. Cervical mucus emerged as the strongest determinant of pregnancy rate and litter size, with higher-quality mucus consistently associated with improved fertility. The effect of semen deposition site was also clear, with deeper placements leading to higher conception probabilities—an effect that parallels observations in sheep, where each additional centimeter of insemination depth enhances pregnancy likelihood (26, 27).
Interaction effects. The significant interactions revealed in our study indicate that optimal fertility requires both high-quality mucus and precise deposition. When mucus is suboptimal, accurate anatomical deposition becomes crucial, whereas favorable mucus quality can partly offset less precise semen placement.
Kidding rate. Interestingly, neither deposition site nor mucus characteristics significantly influenced kidding rate, suggesting that once conception occurs, gestation and parturition are more strongly driven by maternal health, nutrition, and environmental management factors (12, 36).
4.4 Vaginal electrical conductivity, pH, and temperature as complementary biomarkers
In addition to visual mucus assessment, vaginal electrical conductivity (EC), pH, and temperature were evaluated as complementary biomarkers of reproductive status. Physiological parameters varied consistently between pregnant and non-pregnant does under comparable mucus conditions. For instance, under turbid mucus, pregnant does showed an average pH of 7.55 compared with 6.40 in non-pregnant counterparts. Elevated pH provides a favorable environment for sperm survival, capacitation, and penetration, whereas acidic conditions impair motility (26, 28, 29).
Electrical measurements, including vaginal electrical resistance (VER) and EC, have been increasingly validated as reliable indicators for estrus detection in goats (30, 31). Typically, VER declines during estrus, reflecting peak estrogen activity and enhanced sperm transport conditions (29). Our findings, which demonstrated lower EC values in pregnant does—particularly under turbid and cloudy mucus—are consistent with these physiological interpretations. Although EC and VER differ technically, both capture ionic shifts in the vaginal microenvironment, and their convergence reinforces the biological relevance of these measures (29, 31).
Vaginal temperature also exhibited diagnostic potential. Pregnant does under turbid and cloudy mucus conditions maintained higher vaginal temperatures (~39.0 °C) than those under clear mucus (~38.3 °C), a pattern consistent with estrogen-induced increases in blood flow and metabolic activity (27). Furthermore, hormonal interventions such as hCG may be integrated with objective physiological markers (mucus, EC, pH, and temperature) to refine ovulation timing and improve insemination outcomes (28, 29).
In our study, electrical conductivity (EC) was measured using field-based EC meters, which directly report conductivity values (in μS/cm or mS/cm). As conductivity (σ) is the mathematical inverse of resistivity (ρ), and resistance (R) is related to resistivity via σ = 1/ρ, R = ρ·L/A, there exists a direct and well-established relationship between resistance and conductivity (21, 22).
Therefore, the use of EC in our analysis serves the same physiological and diagnostic purpose as resistance measurements—namely, characterizing the ionic and biochemical environment of vaginal mucus. The difference primarily lies in the instrumentation and unit convention, not in the biological interpretation.
From a practical perspective, the choice of EC in this study was guided by the field advantages of portable multi-parameter probes, which allow rapid and cost-effective assessment under farm conditions. Thus, combining objective biomarkers with optimized semen deposition strategies aligns with contemporary models of sperm transport, oviductal interaction, and fertilization success (30, 31).
4.5 Practical recommendations for field AI programs
Based on our findings, several practical recommendations can be made for optimizing AI protocols in commercial goat operations. First, prioritize semen deposition in the uterine body or deep cervix whenever technically feasible, as this consistently improves pregnancy rates by 15–25% compared to vaginal deposition. Second, time insemination coincides with cloudy or turbid mucus conditions, which can be easily assessed visually during routine AI procedures. Third, consider incorporating simple physiological measurements (pH, electrical conductivity, temperature) as supplementary tools for estrus detection and optimal timing, particularly in synchronized breeding programs. Finally, ensure adequate operator training to achieve consistent deposition depth and mucus assessment, as technical precision significantly influences reproductive outcomes. These evidence-based modifications to existing AI protocols could substantially improve fertility rates while maintaining cost-effectiveness in commercial goat production systems.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by Taiwan Livestock Research Institute Institutional Animal Care and Use Committee and conducted in accordance with local legislation and institutional requirements. Furthermore, the use of animals and data collection were authorized by the respective livestock farm owners.
Author contributions
T-CK: Data curation, Formal analysis, Investigation, Project administration, Writing – original draft, Writing – review & editing, Conceptualization. K-FT: Data curation, Formal analysis, Writing – review & editing. H-HL: Data curation, Formal analysis, Writing – review & editing. T-TC: Data curation, Formal analysis, Writing – review & editing. I-LL: Data curation, Formal analysis, Supervision, Writing – review & editing. P-CS: Data curation, Formal analysis, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was funded by the Taiwan Livestock Research Institute of the Ministry of Agriculture, Taiwan.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kutzler, MA. Estrus synchronization and artificial insemination in goats. Vet Clin North Am Food Anim Pract. (2007) 23:351–64. doi: 10.1016/j.cvfa.2007.03.007
2. Agossou, DJ, and Koluman, N. The effects of natural mating and artificial insemination using cryopreserved buck semen on reproductive performance in alpine goats. Arch Anim Breed. (2018) 61:459–61. doi: 10.5194/aab-61-459-2018
3. Dávila, FS, González, A, and Barragán, H. Reproduction in goats. In: oat science. INTECH. (2018) 1:1–20. doi: 10.5772/intechopen.70003
4. Luo, J, Wang, W, and Sun, S. Research advances in reproduction for dairy goats. Asian Australas J Anim Sci. (2019) 32:1284–95. doi: 10.5713/ajas.19.0486
5. Garcia-Ispierto, I, Lopez-Gatius, F, Santolaria, P, Yaniz, JL, Nogareda, C, and Lopez-Bejar, M. Factors affecting the fertility of high producing dairy herds in northeastern Spain. Theriogenology. (2007) 67:632–8. doi: 10.1016/j.theriogenology.2006.09.038
6. Menchaca, A, and Rubianes, E. New treatments associated with timed artificial insemination in small ruminants. Reprod Fertil Dev. (2004) 16:403–13. doi: 10.1071/RD04037
7. Arrebola, FA, Pardo, B, Sanchez, M, Lopez, MD, and Perez-Marin, CC. Factors influencing the success of an artificial insemination program in Florida goats. Span J Agric Res. (2012) 10:336–43. doi: 10.5424/sjar/2012102-23-11
8. Faigl, V, Vass, N, Javor, A, Kulcsar, M, Solti, L, Amiridis, G, et al. Artificial insemination of small ruminants: a review. Acta Vet Hung. (2012) 60:115–29. doi: 10.1556/AVET.2012.010
9. Casali, R, Pinczak, A, Cuadro, F, Guillen-Muñoz, JM, Mezzalira, A, and Menchaca, A. Semen deposition by cervical, transcervical and intrauterine route for fixed-time artificial insemination in the ewe. Theriogenology. (2017) 3:30–5. doi: 10.1016/j.theriogenology.2017.07.005
10. Foxworth, WB, Horner, S, Ho-Watson, A, Gilmore, I, Gutierrez, K, Lewis, S, et al. Comparison of transcervical and intracervical artificial insemination techniques for fixed-time artificial insemination in the goat. J Anim Sci. (2019) 97:464. doi: 10.1093/jas/skz258.914
11. Sohnrey, B, and Holtz, W. Technical note: transcervical deep cornual insemination of goats. J Anim Sci. (2005) 83:1543–8. doi: 10.2527/2005.8371543x
12. Salvador, I, Viudes-de-Castro, M, Bernacer, J, Gomez, E, and Silvestre, M. Factors affecting pregnancy rate in AI with frozen semen during non-breeding season in Murciano-Granadina goats: a field assay. Reprod omest Anim. (2005) 40:526–9. doi: 10.1111/j.1439-0531.2005.00624.x
13. Richardson, LL, Hanrahan, JP, Donovan, A, Martí, JI, Fair, S, Evans, ACO, et al. Effect of site of deposition on the fertility of sheep inseminated with frozen-thawed semen. Anim Reprod Sci. (2012) 131:160–4. doi: 10.1016/j.anireprosci.2012.03.006
14. Bhakta, HH, Refai, FH, and Avella, MA. The molecular mechanisms mediating mammalian fertilization. Development. (2019) 146:176966. doi: 10.1242/dev.176966
15. Suarez, SS, and Pacey, AA. Sperm transport in the female reproductive tract. Hum Reprod Update. (2006) 12:23–37. doi: 10.1093/humupd/dmi047
16. Wang, S, and Larina, IV. Dynamics of gametes and embryos in the oviduct: what can in vivo imaging reveal? Reproduction. (2023) 165:R25–37. doi: 10.1530/REP-22-0250
17. Murtaza, A, Khan, MIR, Abbas, M, Hameed, N, Ahmad, W, Mohsin, I, et al. Optimal timing of insemination based on standing estrus and vaginal mucus characteristics relative to ovulation in Beetal goats. Anim Reprod ci. (2020) 212:106249. doi: 10.1016/j.anireprosci.2019.106249
18. Fatet, A, Pellicer-Rubio, MT, and Leboeuf, B. Reproductive cycle of goats. Anim Reprod Sci. (2011) 124:211–9. doi: 10.1016/j.anireprosci.2010.08.029
20. Eggert-Kruse, W, Köhler, A, Rohr, G, and Runnebaum, B. The pH as an important determinant of sperm-mucus interaction. Fertil Steril. (1993) 59:617–28.
21. Glencorse, BD, Knight, TW, Death, AF, and Wyeth, TK. Factors affecting the accuracy of vaginal electrical resistance measurements for estrus detection in dairy goats. Anim Reprod Sci. (2023) 248:107134. doi: 10.1016/j.anireprosci.2022.107134
22. Kumar, A, Mehrotra, S, Singh, G, Narayanan, K, Rashid, M, Mahla, AS, et al. Estrus detection techniques in buffalo: conventional to modern. Vet World. (2025) 18:1–12. doi: 10.14202/vetworld.2025.1-12
23. Arrébola, F, Palacios, C, Gil, MJ, and Abecia, JA. Management and meteorological factors affect fertility after artificial insemination in Murciano-Granadina goats. Anim Prod Sci. (2016) 56:1028–34. doi: 10.1071/AN15176
24. Abecia, JA, Arrébola, F, Macías, A, Laviña, A, González-Casquet, O, Benítez, F, et al. Temperature and rainfall are related to fertility rate after spring artificial insemination in small ruminants. Int J Biometeorol. (2016) 60:1603–9. doi: 10.1007/s00484-016-1150-y
25. Demetrio, DGB, Santos, RM, Demetrio, CGB, and Vasconcelos, JLM. Factors affecting conception rates following artificial insemination or embryo transfer in lactating Holstein cows. J Dairy Sci. (2007) 90:5073–82. doi: 10.3168/jds.2007-0223
26. Eppleston, J, Salamon, S, Moore, N, and Evans, G. The depth of cervical insemination and site of intrauterine insemination and their relationship to the fertility of frozen-thawed ram semen. Anim Reprod Sci. (1994) 36:211–25.
27. Kumar, S, and Naqvi, SMK. Effect of the route of frozen semen deposition on fertility in Bharat merino ewes. Trop Anim Health Prod. (2014) 46:699–02. doi: 10.1007/s11250-014-0547-9
28. Leethongdee, S, Khalid, M, Bhatti, A, and Ponglowhapan, S. Cervical application of cervix with hyaluronan improves fertility in goats inseminated with frozen-thawed semen. Anim Biosci. (2020) 34:985–92. doi: 10.5713/ajas.20.0284
29. Sathe, SR. Laparoscopic artificial insemination technique in small ruminants: a procedure review. Front Vet Sci. (2018) 5:266. doi: 10.3389/fvets.2018.00266
30. Cseh, S, Faigl, V, and Amiridis, GS. Semen processing and artificial insemination in health management of small ruminants. Anim Reprod Sci. (2012) 130:187–92. doi: 10.1016/j.anireprosci.2012.01.014
31. Abril-Parreño, L, Bianchi, I, Siqueira, LC, Ortiz, G, Córdova, A, de la Fuente, M, et al. Biochemical and molecular characterization of sialylated cervical mucins in sheep. Biol Reprod. (2022) 107:419–31. doi: 10.1093/biolre/ioac077
32. Colagross-Schouten, A, Allison, D, Brent, L, and Lissner, E. Successful use of endoscopy for transcervical cannulation procedures in the goat. Reprod Domest Anim. (2014) 49:909–12. doi: 10.1111/rda.12399
Keywords: artificial insemination, biomarkers, fertility, alpine goats, semen deposition site, vaginal mucus
Citation: Kang T-C, Tseng K-F, Lin H-H, Chen T-T, Lai I-L and Shen P-C (2025) Optimizing artificial insemination in goats: semen deposition site and vaginal mucus characteristics as predictive biomarkers. Front. Vet. Sci. 12:1667834. doi: 10.3389/fvets.2025.1667834
Edited by:
Gabriele Marino, University of Messina, ItalyReviewed by:
Calogero Stelletta, University of Padua, ItalyAkhmad Dakhlan, Bandar Lampung University, Indonesia
Copyright © 2025 Kang, Tseng, Lin, Chen, Lai and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ting-Chieh Kang, Y2N1YXNrdGNAZ21haWwuY29t; Perng-Chih Shen, cGNzaGVuQG1haWwubnB1c3QuZWR1LnR3
 Ting-Chieh Kang
Ting-Chieh Kang Kai-Fei Tseng1
Kai-Fei Tseng1