- 1Taiwan Livestock Research Institute, Ministry of Agriculture, Pingtung, Taiwan
- 2Graduate Institute of Bioresources, National Pingtung University of Science and Technology, Pingtung, Taiwan
- 3Department of Animal Science, National Pingtung University of Science and Technology, Neipu, Pingtung, Taiwan
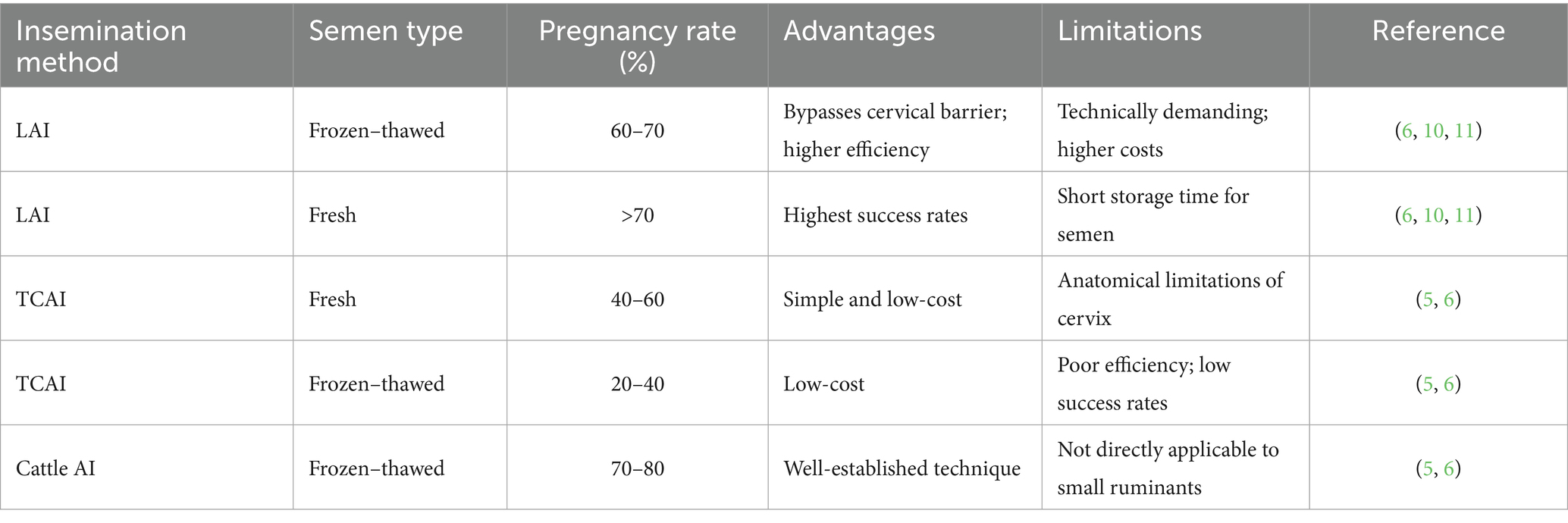
Laparoscopic artificial insemination (LAI) has emerged as a cornerstone technology for genetic improvement in small ruminants. By directly depositing semen into the uterine horns through minimally invasive surgery, LAI effectively bypasses the cervical anatomical barrier that hinders transcervical insemination. This approach has elevated pregnancy rates with frozen–thawed semen from 20 to 40% using conventional methods to 60–70%, establishing LAI as the “gold standard” in small ruminant reproduction. This review develops an integrated framework centered on LAI, systematically highlighting how emerging technologies directly enhance its precision and automation. Artificial intelligence–driven multivariate prediction models now enable pregnancy rate forecasting (AUC = 0.86), while computer vision technologies provide highly accurate estrus detection (98.56% accuracy), optimizing insemination timing. Robotic-assisted systems further refine surgical precision, and the integration of Internet of Things (IoT) and digital twin platforms enables end-to-end intelligent reproductive management. Economic evaluations indicate that LAI delivers significant returns on investment when the genetic value of disseminated germplasm is sufficiently high. Although technical complexity and equipment costs remain challenges, the integration of LAI with emerging technologies is driving a paradigm shift toward precision and intelligent management in the small ruminant industry, offering critical support for global food security and sustainable development.
1 Introduction
The global small ruminant industry faces the dual challenge of enhancing both production efficiency and genetic quality. With the increasing demand for animal protein driven by population growth and the ongoing threats of climate change, livestock systems must achieve higher productivity under limited resources (1, 2). Genetic improvement remains the most fundamental pathway to enhancing herd performance, and efficient assisted reproductive technologies are critical tools for accelerating the dissemination of superior genes (3). Among these technologies, artificial insemination [Artificial intelligence (AI)] is widely adopted for maximizing the genetic potential of elite sires. However, conventional transcervical artificial insemination [Transcervical artificial insemination (TCAI)] is constrained by the unique anatomical complexity of the sheep and goat cervix, characterized by tortuous spiral folds that create a natural barrier to sperm transport (4, 5). While these structures may have evolved to provide reproductive protection, they represent a major bottleneck in AI. Pregnancy rates achieved with TCAI average only 40–60% when fresh semen is used, and drop further to 20–40% with frozen–thawed semen considerably lower than the 70–80% success rates reported in cattle (5, 6). Laparoscopic artificial insemination (LAI), first introduced in the early 1980s, addressed this limitation by directly depositing semen into the uterine horns through minimally invasive surgery (7–9). Since then, LAI has become the “gold standard” for small ruminant reproduction, reliably achieving pregnancy rates of 60–70% even with frozen–thawed semen (6, 10, 11). Beyond improving semen utilization efficiency, LAI has opened new opportunities for global germplasm exchange and conservation (3, 8). Nevertheless, most current studies have focused on isolated technical aspects, lacking a systems-level perspective that positions LAI at the core of reproductive management. With the rapid advancement of artificial intelligence, robotics, and the Internet of Things, LAI is now poised for transformative innovation (12, 13).
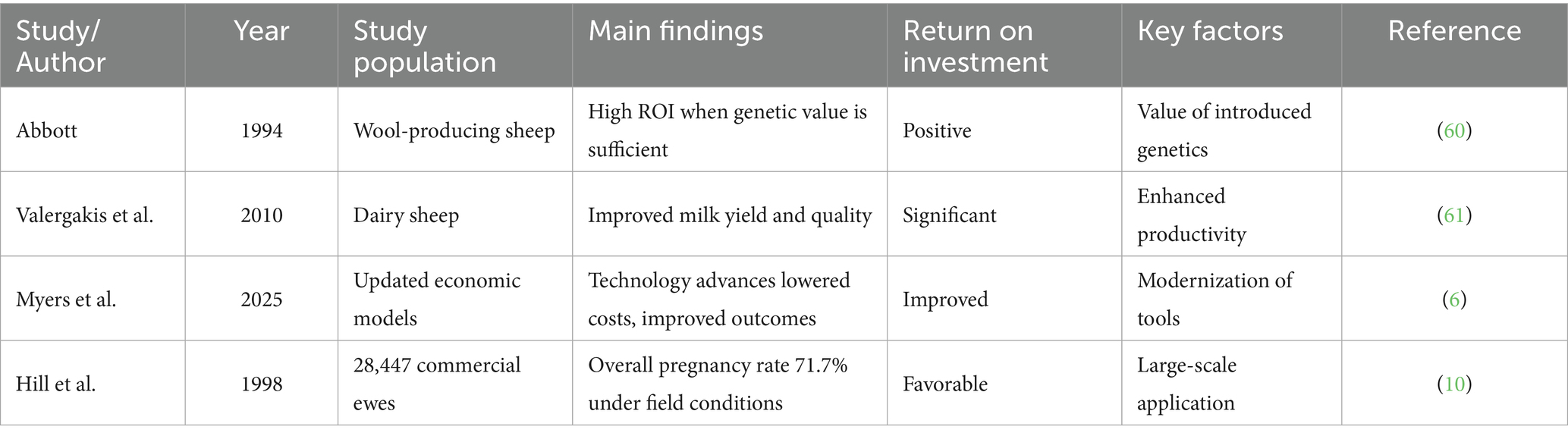
This review establishes a LAI-centered integrative framework to demonstrate how emerging technologies support critical decision points in LAI, while also assessing its economic feasibility and long-term prospects for the small ruminant industry.
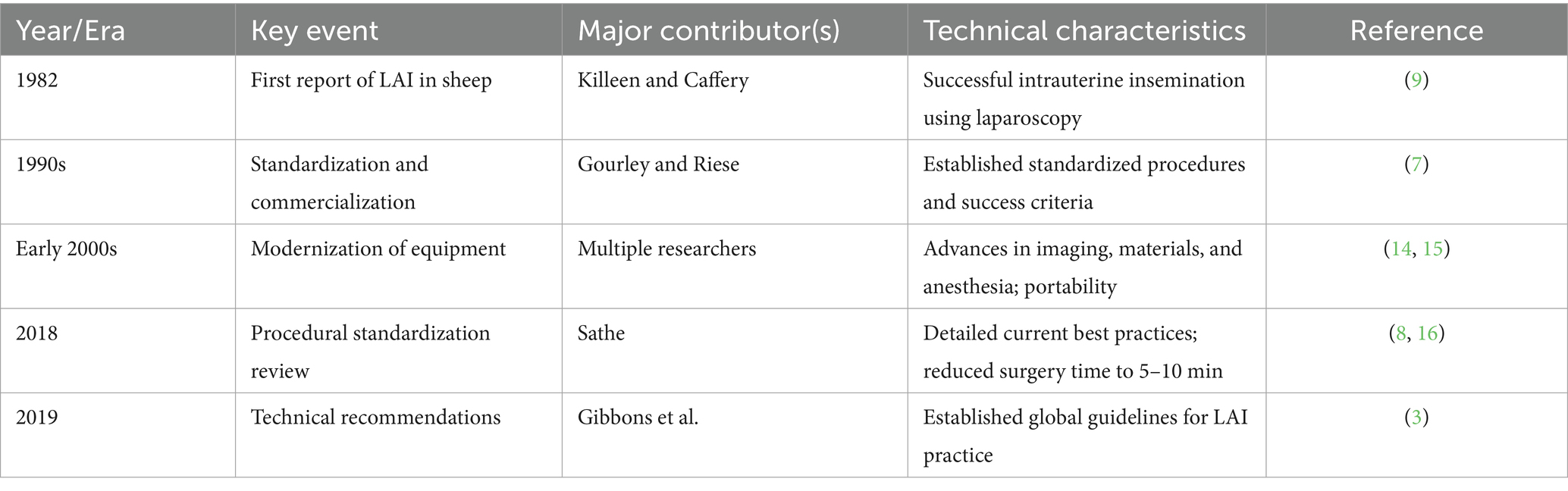
2 Evolution and standardization of LAI technology
2.1 Historical development and technical maturation
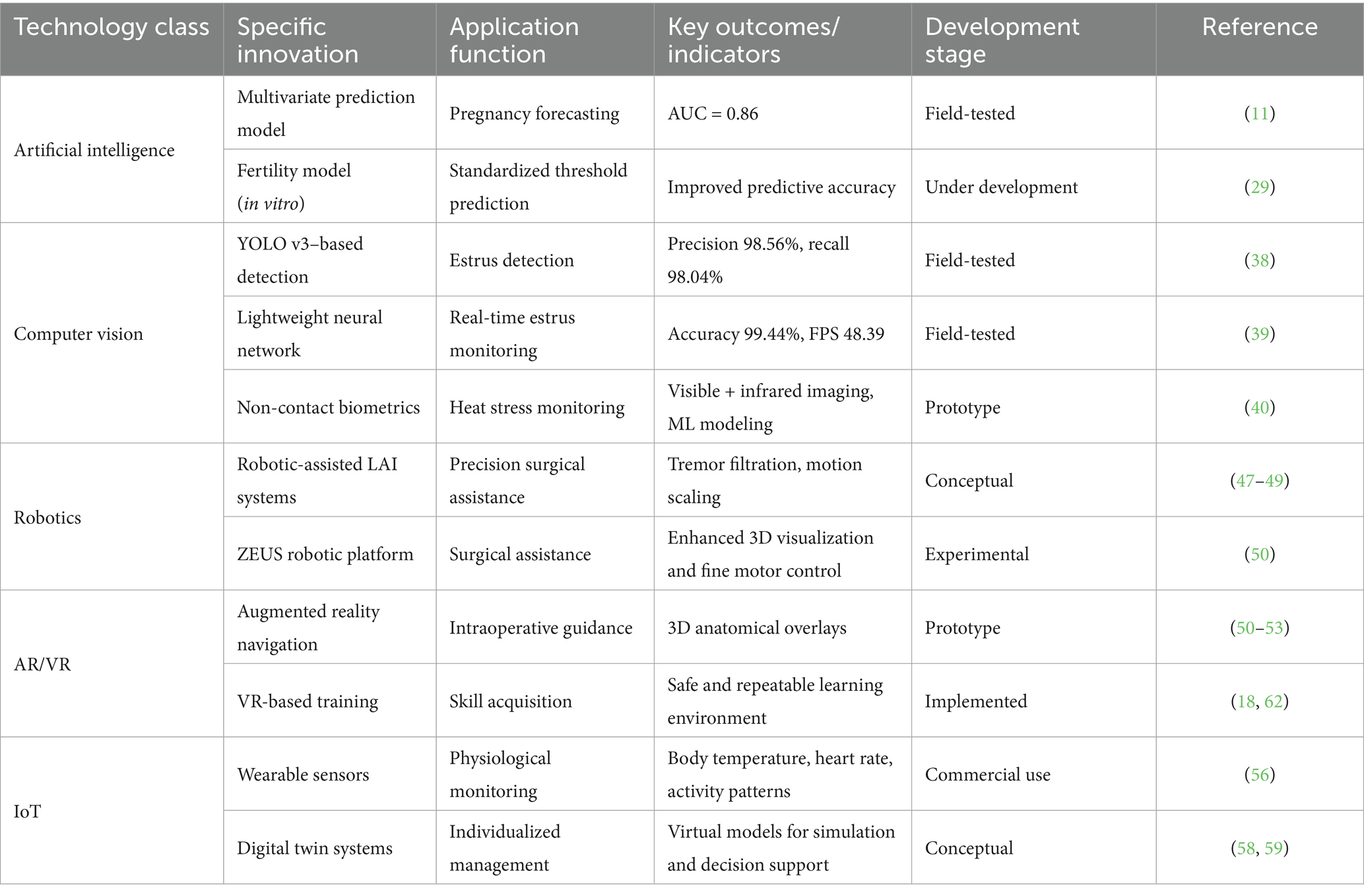
The development of laparoscopic artificial insemination (LAI) exemplifies the convergence of veterinary surgery and reproductive biology. In 1982, Killeen and Caffery first reported successful intrauterine insemination in ewes using laparoscopy, marking the inception of this technique (9). Early LAI systems were bulky and technically demanding, requiring specialized surgical teams, which limited their adoption. During the 1990s, the systematic review published by Gourley and Riese signaled the maturation of the technique and its progression into commercial use (7). Their work detailed standardized procedures and identified critical success factors, laying the foundation for subsequent refinements. With advances in optical imaging, materials science, and anesthesiology in the early 21st century, LAI equipment became more portable and durable, while the surgical procedure itself became safer and faster (14, 15). Modern LAI is now highly standardized. Sathe’s procedural review provided comprehensive best practices, covering equipment selection, animal preparation, surgical techniques, and postoperative care (8). These refinements reduced procedure time to 5–10 min, substantially improving operational efficiency and animal welfare (8, 16) (Table 1).
2.2 Standardized procedures and key technical considerations
Successful LAI begins with meticulous planning and adherence to standardized protocols. The technical recommendations published by Gibbons et al. now serve as authoritative global guidelines for LAI practice (3). The key steps include:
2.2.1 Animal selection and preparation
Selecting healthy ewes with a body condition score [Body condition score (BCS)] of 2.5–3.5/5 is essential for success. Animals should be free of reproductive disorders, aged 1–6 years, and in moderate body weight (3, 17). Preoperative evaluation typically includes general health checks, reproductive tract assessment, and basic blood biochemistry (17, 18).
2.2.2 Estrus synchronization
Hormonal synchronization is critical for ensuring LAI success. The most common protocol involves vaginal sponges containing fluorogestone acetate [Fluorogestone acetate (FGA)] or medroxyprogesterone acetate [Medroxyprogesterone acetate (MAP)] for 12–14 days, followed by Pregnant mare serum gonadotropin (PMSG) injection at withdrawal to induce ovulation (19–22). Yu et al. compared five synchronization protocols and reported that Group II (11-day FGA + PGF2α on day 9 + 330 IU PMSG at withdrawal) yielded the best balance of lambing rate, twinning rate, and cost-effectiveness (19). Tirpan et al. further confirmed that 300 IU PMSG is sufficient for effective synchronization (20).
2.2.3 Anesthesia and analgesia
Modern LAI typically combines local anesthesia with light sedation to minimize stress while ensuring smooth surgery (15, 17). Haan et al. compared CO₂ and medical air for abdominal insufflation, reporting no significant differences in respiratory parameters, though CO₂ was absorbed more quickly and improved postoperative recovery (16). Pain management has become integral to LAI, with preventive analgesia prior to surgery and extended postoperative pain control now considered best practice (15, 18, 23).
2.2.4 Surgical technique
Feed is withheld for 12–24 h before surgery to reduce gastrointestinal distension. Following CO₂ insufflation, two 5–10 mm incisions are made for the laparoscope and surgical instruments. The uterine horns are located under direct visualization, and puncture is performed at well-vascularized, high-tension sites (8, 16). The choice of trocar system and insertion technique strongly influence outcomes; Kang et al. compared different trocar systems to guide optimal device selection (24).
2.2.5 Semen handling and deposition
The thawing and preparation of frozen semen critically affect fertilization outcomes. Rickard and de Graaf outlined best practices, including optimal thawing temperature, extender choice, and sperm viability assessment (25). Typically, 0.1–0.2 ml of semen containing 20–50 × 106 motile spermatozoa is deposited per uterine horn (25, 26).
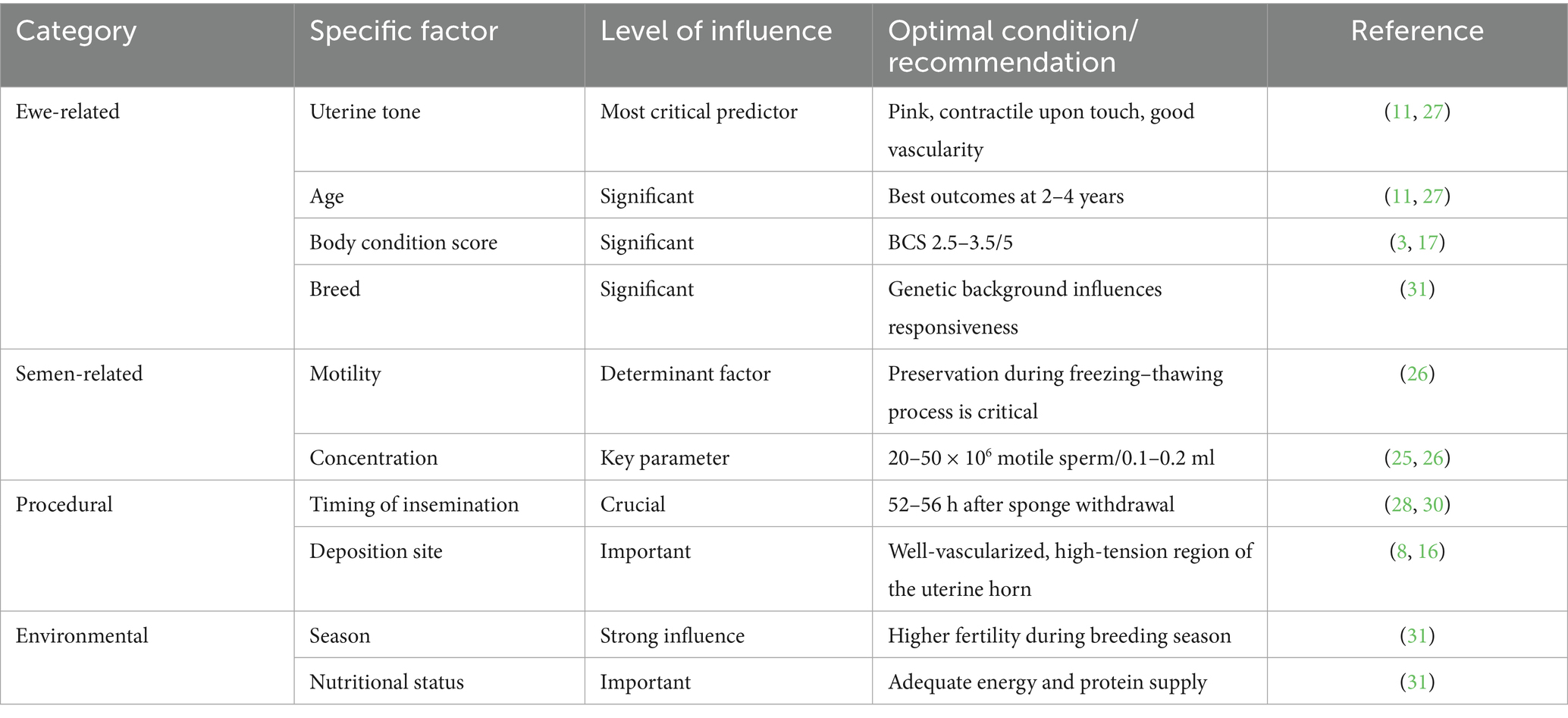
2.3 Key factors influencing success rates
The success of laparoscopic artificial insemination (LAI) is shaped by multiple interacting factors. Recent large-scale studies have provided scientific evidence to quantify their relative importance. Hill et al., analyzing 28,447 commercial LAI cases, reported an overall pregnancy rate of 71.7% and identified key determinants, including progesterone source, PMSG dosage, semen type (fresh vs. frozen–thawed), breed, and operator workload (10). More recently, Spanner et al. developed a multivariate prediction model based on 30,254 ewes, refining the analysis of predictors with modern statistical methods (11) (Table 2).
2.3.1 Ewe-related factors
Uterine tone is the single most critical predictor, reflecting the receptivity of the uterine environment under hormonal regulation. A well-toned uterus is pink in color, contracts upon palpation, and shows strong vascularization (11, 27). Age, body condition score (BCS), breed, parity, and lactational status also significantly affect pregnancy outcomes. Young ewes (2–4 years old) generally have higher fertility, though primiparous animals may exhibit slightly lower rates due to heightened stress responses (11, 27).
2.3.2 Semen-related factors
Sperm motility during the freezing–thawing process is a decisive determinant of fertility. Perkins et al. demonstrated that sperm motility and concentration directly affected pregnancy outcomes following LAI (26). Paulenz et al. further examined the role of extenders and storage conditions, providing technical support for the standardization of semen handling (28, 29).
2.3.3 Procedural factors
The timing of insemination is critical. The optimal window is 52–56 h after sponge withdrawal, coinciding with follicular maturity just before ovulation (28, 30). O’Hara et al. showed that storage temperature and duration significantly affect the viability of fresh semen, informing guidelines for transport and short-term use (30). Interestingly, unilateral deposition yields result comparable to bilateral insemination but reduces surgical time and improves animal welfare (28, 30).
2.3.4 Environmental and management factors
Seasonal effects, nutritional status, stress level, and overall flock management substantially influence pregnancy outcomes. Donovan et al. reported differences between natural and synchronized estrus conditions, while Fair et al. highlighted breed-specific responsiveness to LAI (31). Proper nutrition, stress reduction, and breeding during the natural season can significantly enhance reproductive success.
3 Integration of LAI with reproductive management
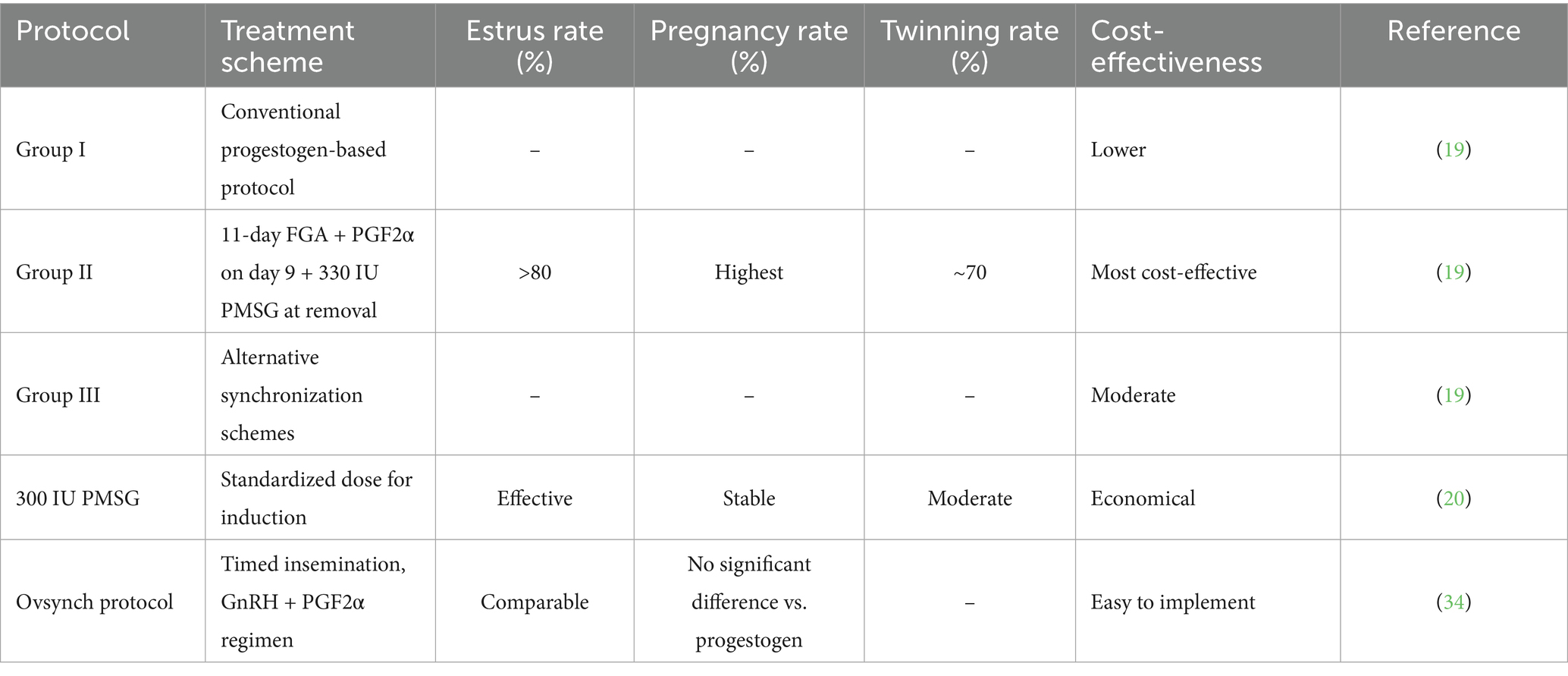
3.1 Optimization and innovation in Estrus synchronization
Estrus synchronization serves as the foundation for successful LAI, as its effectiveness directly determines insemination outcomes. Conventional protocols rely on combinations of progestogens and gonadotropins, yet new strategies continue to emerge as our understanding of small ruminant reproductive physiology deepens. Wildeus provided a seminal review outlining the theoretical framework of estrus synchronization in sheep and goats, emphasizing the pivotal role of PMSG in ovulation induction and the importance of tailoring dosages to breed, season, and individual variation (21). More recently, Habeeb highlighted the unique challenges of synchronizing seasonal breeders, further refining the theoretical basis for practical application (22). Field studies have confirmed these principles. Yu et al. compared five synchronization protocols in Hu sheep and demonstrated that Group II (11-day FGA + PGF2α on day 9 + 330 IU PMSG at withdrawal) achieved >80% estrus rate, the highest pregnancy outcomes, and up to 70% twinning rates, representing the most cost-effective solution for commercial farms (19). Tirpan et al. further showed that 300 IU PMSG was sufficient for synchronization, while higher doses increased the risk of excessive multiple births and dystocia (20). Earlier evidence by Mutiga et al. compared synchronization methods and PMSG dosages, providing historical support for dosage optimization (32). More recently, Shehabeldin et al. developed a PMSG-loaded chitosan nanoparticle system for controlled release, which enhanced synchronization efficiency and reduced the number of required injections, improving animal welfare (33). Alternative approaches have also been explored. Vallejo et al. compared Ovsynch with intravaginal progesterone devices in hair sheep and reported no significant differences in pregnancy rates, though Ovsynch was operationally simpler (34). Similarly, Deac et al. validated timed AI protocols during the non-breeding season, extending the applicability of LAI beyond seasonal constraints (35). In goats, Whitley and Jackson stressed that higher hormonal sensitivity requires species-specific dosage adjustments (36). A broader comparative review by Arya et al. summarized similarities and differences in synchronization strategies across cattle, sheep, and goats, offering insights for cross-species adaptation (37) (Table 3).
3.2 Precision Estrus detection and optimization of insemination timing
Traditional estrus detection relies heavily on visual observation, which is labor-intensive, subjective, and prone to errors. With advances in computer vision and artificial intelligence, automated estrus detection has emerged as a practical solution. Yu et al. developed a multi-target detection neural network based on an improved YOLO v3 architecture, incorporating K-means++ clustering and additional detection layers, achieving 98.56% precision and 98.04% recall in identifying ewe mounting behavior (38). This provides an accurate “time anchor” for LAI. Later, Yu et al. introduced a lightweight network (EfficientNet-B0 with Senet modules), delivering 99.44% accuracy with a model size of only 40.6 MB and 48.39 FPS, suitable for real-time deployment (39). Complementary innovations include non-contact biometric monitoring. Fuentes et al. integrated visible and infrared thermal imaging with machine learning algorithms to assess heat stress in sheep (40). Such tools not only aid estrus detection but also expand to animal health monitoring. Additionally, Hu et al. applied an enhanced YOLOv5 model to identify grazing behaviors, while Yang et al. reviewed applications of computer vision for body condition scoring (41, 42). Integrating multiple sensing modalities—such as ultrasonography, temperature loggers, and accelerometers—offers a pathway toward comprehensive estrus monitoring. These multimodal decision-support systems are shifting insemination decisions from experience-based to data-driven, enabling a transition from group-level scheduling to precise individual-level timing (39, 40).
3.3 Integration with genetic improvement strategies
Laparoscopic artificial insemination (LAI) and genomic selection [Genomic selection (GS)] form a complementary pair of technologies that collectively accelerate genetic progress in small ruminants. Genomic selection, which leverages dense SNP chip data to estimate genomic breeding values [Genomic estimated breeding values (GEBVs)], provides unprecedented accuracy in identifying elite sires. LAI then acts as a “genetic multiplier,” enabling these elite sires to disseminate their germplasm broadly across ewe populations, unrestricted by geography or time (43, 44). Van der Werf and Banks emphasized the transformative potential of GS in sheep breeding, particularly its ability to shorten generation intervals and improve selection accuracy. Similarly, Daetwyler et al. demonstrated the value of whole-genome sequencing for dissecting complex traits, while Moghaddar et al. confirmed that GS outperforms pedigree-based approaches in young animals (43, 44). The economic implications of GS integration with LAI are substantial. Shumbusho et al. evaluated genomic selection in meat sheep breeding programs, showing that GS combined with reproductive technologies generates considerable returns on investment (45). In dairy sheep, Baloche et al. validated the accuracy of genomic predictions in the French Lacaune breed, providing technical assurance for genetic progress in milk traits (46).
An optimal integration strategy involves:
• Screening and selecting top young rams through GS.
• Collecting, freezing, and storing semen from these sires.
• Disseminating semen on a large scale via LAI.
• Feeding back progeny performance data to refine GS models.
This closed-loop “GS selection—LAI dissemination—data feedback” cycle maximizes genetic gain (45, 46).
Modern breeding programs must also align with sustainability goals. Brito et al. highlighted the importance of large-scale phenotyping to improve animal welfare and resilience (12). Likewise, González-Recio et al. incorporated methane emissions into dairy cattle breeding objectives, demonstrating the potential of genetic selection for environmental mitigation (13). Similar principles can be extended to small ruminants, where combining GS with LAI could yield breeds that are not only more productive but also climate-resilient and environmentally sustainable.
4 Emerging technologies empowering LAI
4.1 Artificial intelligence–driven prediction models and decision support
Artificial intelligence (AI) applications in LAI are transitioning from conceptual to practical. Spanner et al. developed a multivariate prediction model that integrates ewe characteristics, intraoperative uterine tone scores, and semen quality parameters to forecast pregnancy outcomes, achieving an AUC of 0.86 (11).
The model’s utility lies in several functions:
• Individualized selection—identifying ewes with extremely low predicted fertility, thus avoiding unnecessary costs and animal stress.
• Semen optimization—matching semen samples to ewes predicted to achieve the highest pregnancy rates, maximizing genetic resource efficiency.
• Dose adjustment—increasing insemination dose or applying bilateral insemination for borderline cases to improve outcomes.
Brito et al. emphasized that large-scale phenotyping is essential for training robust AI models with strong generalizability (12). More recently, Spanner et al. introduced a fertility model based on standardized in vitro semen thresholds, expanding AI-based predictive tools for field applications (29).
Ultimately, these AI systems are shifting LAI from an empirical practice to a data-driven, precision-guided intervention. The integration of genomic, environmental, and historical reproductive data will further enhance the personalization of reproductive management (12, 13) (Tables 4, 5).
4.2 Development and application of robotic-assisted surgery
Robotic-assisted surgery is already well established in human medicine and is gradually being adapted for veterinary use. These systems mitigate inherent limitations such as hand tremor and operator fatigue (47). For LAI, robotic systems offer several advantages:
• Enhanced flexibility and precision through robotic arms that exceed the range of human motion.
• Tremor filtration and motion scaling, which convert larger operator movements into precise micromovements (48).
• Improved 3D visualization, offering greater depth perception and clarity of surgical fields (47, 49).
Marescaux and Rubino described the ZEUS robotic platform in both experimental and clinical settings, highlighting its potential relevance for veterinary surgery (50). Although high costs remain a barrier, decreasing equipment prices and integration with AI and vision technologies could enable future “fully automated LAI workstations,” capable of completing insemination procedures from anesthesia to semen deposition with minimal human intervention.
4.3 Augmented reality and 3D imaging integration
Augmented reality (AR) overlays computer-generated information onto the real surgical field in real time, providing novel applications for LAI. Blixt and O’Brien systematically reviewed AR applications in veterinary surgery, reporting that AR can significantly enhance surgical accuracy and safety (51). In human medicine, Pratt et al. demonstrated that HoloLens-based AR systems can display three-dimensional (3D) vascular models intraoperatively, enabling surgeons to localize critical structures with greater precision (52). Similarly, Tepper et al. explored mixed reality integration, highlighting the combined potential of virtual and augmented reality in the operating room (53).
In LAI, AR applications could include:
• Intraoperative navigation: Overlaying virtual markers to guide precise puncture sites within the uterine horns.
• Critical structure annotation: Highlighting blood vessels and nerves to prevent iatrogenic injury.
• Remote guidance and training: Enabling experts to supervise procedures remotely in real time.
Advances in 3D imaging have provided a foundation for these AR applications. Fergo et al. demonstrated that 3D laparoscopy significantly improved depth perception and hand–eye coordination compared with 2D high-definition systems (54). Currò et al. further confirmed the value of 3D visualization for enhancing surgical precision (55). The combination of 3D/4 K laparoscopic imaging with AR guidance could transform LAI into a safer, more intuitive, and highly standardized procedure.
4.4 IoT and digital twin integration
The Internet of Things (IoT) leverages distributed sensor networks to provide continuous monitoring of animals and their environment. Neethirajan reviewed wearable biosensors that capture physiological parameters such as body temperature, heart rate, activity levels, and rumination behavior (56). Wolfert et al. emphasized IoT as a key enabler of smart farming, where data-driven insights underpin precision livestock management (57).
In the context of LAI, IoT systems can:
• Monitor ewe physiological indices (temperature, activity, feed intake) in real time using wearable devices.
• Track environmental parameters (temperature, humidity, air quality) with fixed sensors to ensure optimal housing conditions.
• Stream collected data to cloud-based platforms, providing AI-driven models with continuous updates (56, 57).
Digital twin technology represents the most advanced form of IoT integration. Rasheed et al. described digital twins as dynamic, data-driven virtual replicas that evolve alongside their physical counterparts (58). Pylianidis et al. further highlighted their agricultural potential, noting their ability to integrate multi-source data into predictive models (59).
For LAI, digital twins could create individualized ewe models that incorporate genomic profiles, reproductive histories, real-time physiological data, and environmental conditions. These virtual models can:
• Simulate different management strategies to predict reproductive outcomes.
• Continuously update based on real-time data, refining recommendations dynamically.
• Provide decision support to optimize insemination timing and protocols for each ewe.
This level of integration offers the potential for highly personalized reproductive management, transforming LAI from a population-level intervention into a truly individualized precision technology.
5 Economic evaluation and industrial challenges
5.1 Cost–benefit analysis and economic models
The economic evaluation of laparoscopic artificial insemination (LAI) requires a comprehensive assessment of direct costs, indirect benefits, and long-term impacts. Abbott conducted one of the earliest systematic cost–benefit studies on artificial insemination in wool sheep, concluding that LAI yields substantial returns on investment when the value of introduced germplasm is sufficiently high (60). Similarly, Valergakis et al. evaluated profitability in dairy sheep genetic improvement programs and found that LAI significantly enhanced milk yield and quality (61). More recent analyses by Myers et al. updated these models, incorporating the influence of modern technological advances on cost structures (6). Costs of LAI include equipment investment (laparoscopes, CO₂ insufflators, surgical tools), training and certification, semen procurement and storage, consumables, anesthesia and analgesics, and perioperative animal care (6, 60, 61). Benefits derive from higher pregnancy rates, improved semen utilization, rapid dissemination of elite genes, and reduced risks of sexually transmitted diseases compared with natural mating (6, 60, 61). Although single-procedure costs exceed those of transcervical insemination (TCAI), the superior pregnancy rates (60–70% vs. 20–40%) and long-term genetic and economic gains make LAI more profitable in structured breeding programs (6) (Table 6).
5.2 Major barriers to adoption and potential solutions
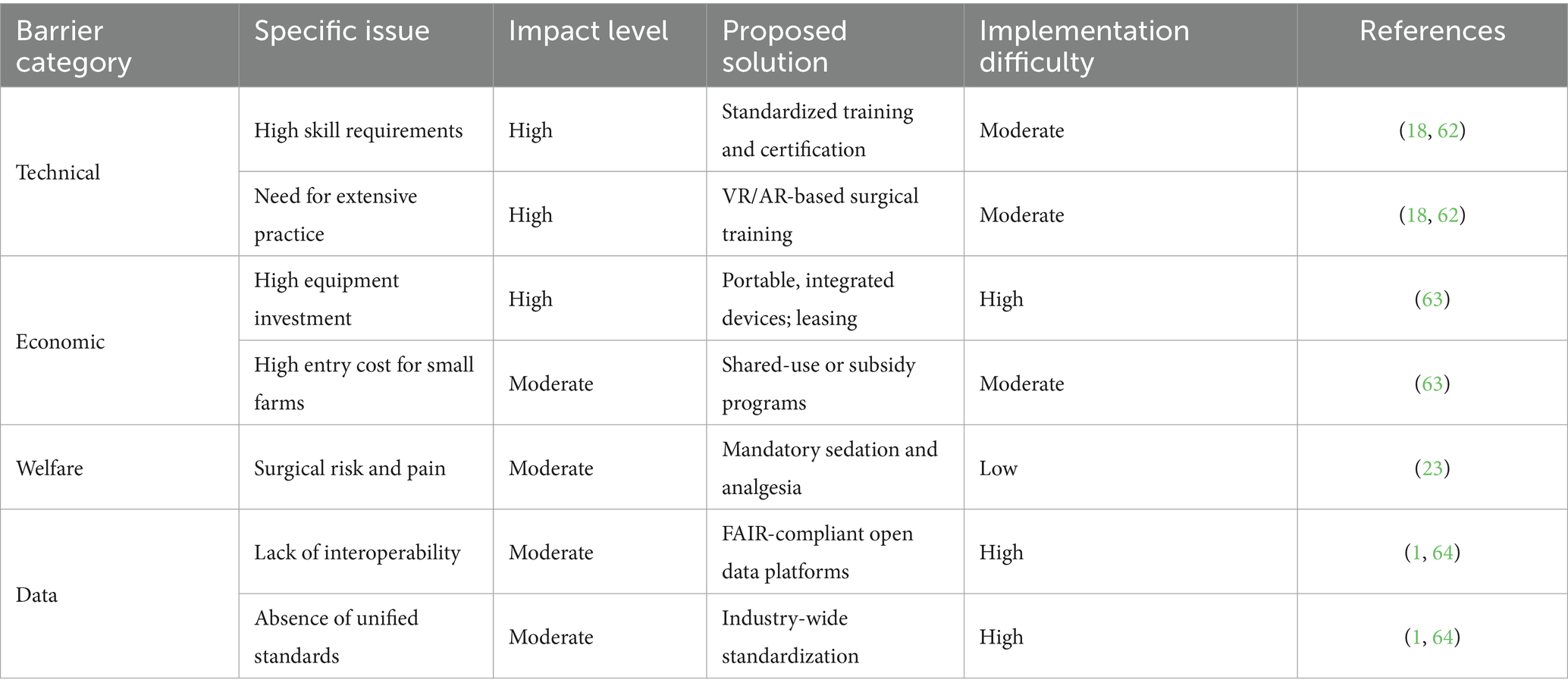
Despite its proven effectiveness, the widespread adoption of LAI is constrained by several challenges:
• High technical threshold: LAI requires specialized surgical skills and extensive practice, making operator training a major bottleneck. Solutions include standardized certification systems, competency-based evaluation, and training with VR/AR simulation tools. Mentorship programs can accelerate skill transfer across generations (18, 62).
• High equipment costs: The initial investment for LAI equipment remains a barrier for small-scale farms. Portable, integrated devices, leasing models, and government subsidies could lower adoption thresholds (63).
• Animal welfare concerns: LAI involves surgical procedures that require strict pain management protocols. Ensuring mandatory preoperative sedation, intraoperative monitoring, and postoperative analgesia is essential (15, 23).
• Lack of data standardization: Inconsistent data formats limit AI and digital system applications. Implementing FAIR principles (Findable, Accessible, Interoperable, Reusable) and establishing industry-wide data standards would facilitate large-scale integration (1, 64) (Table 7).
5.3 Industrial trends and policy support
The industrialization of LAI depends on synergistic advances in research, policy, and market forces. The USDA Animal Genome Research Blueprint (2018–2027) emphasized the importance of genomics and reproductive technologies in advancing livestock health, productivity, and welfare (2). Similar frameworks highlight the role of policy in supporting LAI’s development through research funding, workforce training, and international collaboration. On the market side, consumer demand for high-quality animal products, environmental sustainability pressures, and global competition are driving adoption. As Brito et al. observed for dairy cattle, future livestock systems must balance productivity with animal welfare and ecological sustainability (65). These principles equally apply to small ruminants, underscoring LAI’s relevance as both productivity-enhancing and sustainability-enabling technology.
6 Future perspectives and technological outlook
6.1 Technological convergence and system integration
Future developments in LAI will be characterized by high levels of integration across hardware and software. At the hardware level, the next generation of intelligent LAI workstations will likely incorporate high-definition 3D laparoscopes, robotic arms, AI decision modules, and automated semen-handling systems. These platforms could deliver fully automated workflows, from animal preparation to postoperative monitoring, reducing reliance on specialist operators (50).
On the software side, integrated platforms will unify multi-source data—IoT sensor readings, genomic profiles, historical reproductive records, and environmental metrics—into centralized decision-support systems. By leveraging advanced AI algorithms, these platforms will provide individualized reproductive recommendations, effectively building a comprehensive digital reproductive twin for each ewe (56–58).
6.2 Application of precision medicine principles
The adoption of precision medicine principles in veterinary science mirrors progress already achieved in human healthcare. In LAI, this will shift management from herd-level interventions to individualized protocols. Genomic data, obtained through whole-genome sequencing or SNP chips, will form the foundation of personalized reproductive planning by identifying genetic variants linked to fertility, disease resistance, or productivity traits (43, 44, 66–70). Epigenetic research will further expand this framework by uncovering how nutrition and environment regulate reproductive gene expression (71–77). Moreover, integrative multi-omics approaches—combining genomics, transcriptomics, proteomics, and metabolomics—will provide a systems-level view of reproductive physiology. These insights will enable targeted interventions that optimize insemination success while minimizing resource inputs (78–84).
6.3 Sustainability and environmental stewardship
The future of LAI must balance productivity gains with ecological sustainability. Thornton projected that livestock production will increasingly be judged by its environmental footprint (85), while Gerber et al. highlighted livestock’s central role in climate change mitigation (86).
LAI contributes to sustainability by:
• Improving reproductive efficiency, thereby reducing the resource demand of maintaining breeding flocks.
• Accelerating genetic progress, fostering animals that are more productive and resilient to climate stressors.
• Enabling precision management, which minimizes drug use, reduces greenhouse gas emissions, and lowers waste output.
Emerging methodologies such as life-cycle assessment [Life-cycle assessment (LCA)] will be essential for evaluating LAI’s environmental impact. Indicators like carbon footprint, water usage, and land-use efficiency will provide benchmarks to ensure that technological adoption aligns with sustainability goals (85, 86).
7 Conclusion and outlook
Over the past four decades, laparoscopic artificial insemination (LAI) has evolved from a novel surgical innovation to the gold standard of reproductive management in small ruminants. This review establishes a LAI-centered framework that highlights how emerging technologies—including AI, computer vision, robotics, IoT, and digital twins—are directly enhancing the precision, automation, and scalability of the technique.
Current LAI protocols already achieve stable pregnancy rates of 60–70% with frozen–thawed semen, a substantial improvement over transcervical methods. The integration of cutting-edge tools is driving LAI into a new era:
• AI models enable predictive, individualized reproductive management.
• Computer vision systems provide automated estrus detection with high accuracy.
• Robotics improve surgical precision and reduce operator variability.
• IoT and digital twins establish continuous, individualized reproductive monitoring.
Future development will likely focus on fully automated LAI workstations, multimodal data-driven decision systems, and non- or minimally invasive insemination technologies that further enhance efficiency while minimizing animal stress. Integration with genomic and multi-omics platforms will open new opportunities for climate-resilient, welfare-conscious breeding programs.
Ultimately, the trajectory of LAI reflects the broader transformation of livestock reproduction into a field defined by precision, sustainability, and ethical responsibility. With continued innovation and policy support, LAI has the potential to shape a resilient small ruminant industry capable of meeting the dual challenges of global food security and sustainable development.
Author contributions
T-CK: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. I-LL: Data curation, Formal analysis, Supervision, Writing – review & editing. P-CS: Conceptualization, Data curation, Formal analysis, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was funded by the Taiwan Livestock Research Institute of the Ministry of Agriculture, Taiwan.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Jacobsen, A, de Miranda Azevedo, R, Juty, N, Batista, D, Coles, S, Cornet, R, et al. FAIR principles in data management and stewardship. Encyclop Bioinform Comp Biol. (2017) 1:144–56. doi: 10.1016/B978-0-12-809633-8.20327-9
2. Rexroad, C, Vallet, J, Matukumalli, LK, Reecy, J, Bickhart, D, Blackburn, H, et al. Genome to phenome: improving animal health, production, and well-being–a new USDA blueprint for animal genome research 2018–2027. Front Genet. (2019) 10:327. doi: 10.3389/fgene.2019.00327
3. Gibbons, AE, Cueto, MI, and Simões, J. Technical recommendations for artificial insemination in sheep. Anim Reprod. (2019) 16:463–71. doi: 10.21451/1984-3143-AR2019-0063
4. Abril-Parreño, L, Druart, X, Tsikis, G, Cognié, J, Pinczak, A, Dubois, A, et al. Cervical artificial insemination with frozen-thawed semen in sheep: the secret is in the cervix of Norwegian ewe breeds. Biol Reprod. (2025) 112:84–95. doi: 10.1093/biolre/ioaf084
5. Maggi, G, Stelletta, C, Gianesella, M, Morgante, M, and Fiore, E. Double artificial insemination in sheep. Animals. (2025) 22:e20240055. doi: 10.1590/1984-3143-AR2024-0055
6. Myers, A, and Kasimanickam, R. Laparoscopic artificial insemination in sheep: review and cost-benefit analysis. Clin Theriogenol. (2025) 17:11080. doi: 10.58292/CT.v17.11080
7. Gourley, DD, and Riese, RL. Laparoscopic artificial insemination in sheep. Vet Clin North Am Food Anim Pract. (1990) 6:615–33. doi: 10.1016/S0749-0720(15)30836-7
8. Sathe, SR. Laparoscopic artificial insemination technique in small ruminants—a procedure review. Front Vet Sci. (2018) 5:266. doi: 10.3389/fvets.2018.00266
9. Killeen, ID, and Caffery, GJ. Uterine insemination of ewes with the aid of a laparoscope. Aust Vet J. (1982) 59:95. doi: 10.1111/j.1751-0813.1982.tb02737.x
10. Hill, JR, Thompson, JA, and Perkins, NR. Factors affecting pregnancy rates following laparoscopic insemination of 28,447 merino ewes under commercial conditions: a survey. Theriogenology. (1998) 49:697–709. doi: 10.1016/S0093-691X(98)00019-3
11. Spanner, EA, de Graaf, SP, and Rickard, JP. A multivariate model for the prediction of pregnancy following laparoscopic artificial insemination of sheep. Sci Rep. (2024) 14:27556. doi: 10.1038/s41598-024-79253-x
12. Brito, LF, Oliveira, HR, McConn, BR, Schinckel, AP, Arrazola, A, Marchant-Forde, JN, et al. Large-scale phenotyping of livestock welfare in commercial production systems: a new frontier in animal breeding. Front Genet. (2020) 11:793. doi: 10.3389/fgene.2020.00793
13. González-Recio, O, López-Paredes, J, Ouatahar, L, Charfeddine, N, Ugarte, E, Alenda, R, et al. Mitigation of greenhouse gases in dairy cattle via genetic selection: 2. Incorporating methane emissions into the breeding goal. J Dairy Sci. (2020) 103:7210–21. doi: 10.3168/jds.2019-17598
14. Ceko, MJ, and Gebbie, J. The application of 3D and 4K imaging in laparoscopy. J Minim Access Surg. (2019) 15:269–74. doi: 10.4103/jmas.JMAS_103_18
15. Neves, JP, and Sargison, ND. Pain management in sheep. Vet Clin Food Anim Pract. (2021) 37:1–16. doi: 10.1016/j.cvfa.2020.10.001
16. Haan, JD, Hay Kraus, BL, and Sathe, SR. A comparison of the effects of carbon dioxide and medical air for abdominal insufflation on respiratory parameters in xylazine-sedated sheep undergoing laparoscopic artificial insemination. N Z Vet J. (2018) 66:167–71. doi: 10.1080/00480169.2018.1458661
17. Fisher, A, and Roadknight, N. Welfare of sheep In: R Burton editor. The practice of sheep veterinary medicine. Melbourne: CSIRO Publishing (2021) 45–78.
18. Pottle, J. Virtual reality and the transformation of medical education. Future Healthc J. (2019) 6:181–5. doi: 10.7861/fhj.2019-0036
19. Yu, X, Bai, Y, Yang, J, Zhao, X, Zhang, L, and Wang, J. Comparison of five protocols of estrous synchronization and pregnancy outcomes in Hu sheep. Animals. (2022) 12:123. doi: 10.3390/ani12010123
20. Tirpan, G, Yilmaz, O, and Sönmez, C. The effects of different doses of PMSG on estrus synchronization and pregnancy rate in Awassi ewes. J Anim Sci. (2019) 97:1234–40. doi: 10.1093/jas/skz034
21. Wildeus, S. Current concepts in synchronization of estrus: sheep and goats. J Anim Sci. (2000) 77:1–14. doi: 10.2527/jas2000.00218812007700ES0040x
22. Habeeb, AA. Recent advances in estrus synchronization in small ruminants. J Anim Sci. (2021) 99:1–12. doi: 10.1093/jas/skab012
23. Fitzpatrick, J, Scott, M, and Nolan, A. Assessment of pain and welfare in sheep. Small Rumin Res. (2006) 62:55–61. doi: 10.1016/j.smallrumres.2005.07.028
24. Kang, SS, Lee, SH, Kim, S, Park, S, and Cho, S. Comparison of different trocar systems for laparoscopic artificial insemination in sheep. J Vet Sci. (2025) 26:e1. doi: 10.4142/jvs.25047
25. Rickard, JP, and de Graaf, SP. Best practice for handling and processing sheep semen. Anim Reprod Sci. (2018) 194:3–10. doi: 10.1016/j.anireprosci.2018.03.021
26. Perkins, NR, Hill, JR, and Thompson, JA. The effect of site of insemination, sperm dose, and semen quality on the fertility of ewes inseminated laparoscopically with frozen-thawed semen. Theriogenology. (1996) 46:875–82. doi: 10.1016/0093-691X(96)00253-1
27. Spanner, EA, de Graaf, SP, and Rickard, JP. Uterine tone influences fertility of merino ewes following laparoscopic artificial insemination. Theriogenology. (2024) 219:107453. doi: 10.1016/j.theriogenology.2024.01.034
28. Paulenz, H, Söderquist, L, Pérez-Pé, R, and Berg, KA. Effect of different extenders and storage temperatures on sperm viability of liquid ram semen. Theriogenology. (2002) 57:823–36. doi: 10.1016/S0093-691X(01)00683-5
29. Spanner, EA, de Graaf, SP, and Rickard, JP. The validation and application of an ovine fertility model using standardised in vitro thresholds to predict the likelihood of pregnancy. Theriogenology. (2025) 230:45–53. doi: 10.1016/j.theriogenology.2024.11.012
30. O'Hara, L, Hanrahan, JP, Richardson, L, Donovan, A, Fair, S, Evans, AC, et al. Effect of storage duration, storage temperature, and diluent on the viability and fertility of fresh ram sperm. Theriogenology. (2010) 73:541–9. doi: 10.1016/j.theriogenology.2009.10.009
31. Paulenz, H, Söderquist, L, Adnøy, T, Fossen, OH, and Berg, KA. Effect of milk- and TRIS-based extenders on the fertility of sheep inseminated laparoscopically with liquid semen stored at 5°C. Theriogenology. (2003) 60:759–66. doi: 10.1016/S0093-691X(03)00048-7
32. Mutiga, ER, Mukasa-Mugerwa, E, Sovani, S, and Tegegne, A. Effect of the method of estrus synchronization and PMSG dosage on estrus and twinning in Ethiopian highland sheep. Theriogenology. (1992) 38:727–34. doi: 10.1016/0093-691X(92)90035-P
33. Shehabeldin, AM, Abdel-Khalek, AE, Ghobashy, H, Darwish, GM, Abdel-Hafez, MA, and El-Speiy, ME. Improving the breeding capabilities of short-term estrus synchronization protocols using PMSG and PMSG-loaded chitosan nanoparticles in Barki ewes. Animals. (2025) 15:189. doi: 10.3390/ani15020189
34. Vallejo, DA, Camacho, LE, Lemley, CO, Soto-Navarro, SA, Vonnahme, KA, and Ford, SP. Pregnancy rates in hair sheep after Ovsynch or progesterone-releasing intravaginal device treatment and artificial insemination. Animals. (2019) 9:1041. doi: 10.3390/ani9121041
35. Akter, S, Asaduzzaman, M, Saha, A, Hasan, MM, Islam, MR, and Rahman, MM. Factors affecting the pregnancy rate of Bangladeshi ewes following laparoscopic artificial insemination (LAP-AI). Vet Integr Sci. (2022) 20:371–82. doi: 10.12982/VIS.2022.024
36. Whitley, NC, and Jackson, DJ. An update on estrus synchronization in goats: a minor species. J Anim Sci. (2004) 82 E-Suppl:E270–6. doi: 10.2527/2004.8213_supplE270x
37. Arya, D, Goswami, R, and Sharma, M. Estrous synchronization in cattle, sheep and goat. Multidiscip Rev. (2023) 6:2023se0455. doi: 10.31893/multirev.2023001
38. Yu, L, Guo, J, Pu, Y, Cen, H, Li, J, Liu, S, et al. A recognition method of ewe estrus crawling behavior based on multi-target detection layer neural network. Animals. (2023) 13:413. doi: 10.3390/ani13030413
39. Yu, L, Guo, J, Pu, Y, Cen, H, Li, J, Liu, S, et al. A lightweight neural network-based method for detecting estrus behavior in ewes. Agriculture. (2022) 12:1207. doi: 10.3390/agriculture12081207
40. Fuentes, S, Gonzalez Viejo, C, Cullen, B, Tongson, E, Chauhan, SS, and Dunshea, FR. Non-invasive sheep biometrics obtained by computer vision algorithms and machine learning modeling using integrated visible/infrared thermal cameras. Sensors. (2020) 20:6334. doi: 10.3390/s20216334
41. Hu, J, Zhao, H, Li, X, Wang, Y, and Zhang, Q. Behavior recognition of grazing sheep based on improved YOLOv5. Sensors. (2023) 23:4752. doi: 10.3390/s23104752
42. Yang, C, Zhai, R, Wang, H, Liu, J, and Zhao, Y. Computer vision-based livestock body condition scoring: a systematic review. Comput Electron Agric. (2025) 218:108672. doi: 10.1016/j.compag.2024.108672
43. Daetwyler, HD, Capitan, A, Pausch, H, Stothard, P, Van Binsbergen, R, Brøndum, RF, et al. Whole-genome sequencing of 234 bulls facilitates mapping of monogenic and complex traits in cattle. Nat Genet. (2014) 46:858–65. doi: 10.1038/ng.3034
44. Ziadi, C, Demyda-Peyrás, S, Valera, M, Perdomo-González, D, Laseca, N, de los Terreros, ARS, et al. Comparative analysis of genomic and pedigree-based approaches for genetic evaluation of morphological traits in pura raza Española horses. Gene. (2025) 16:131. doi: 10.3390/genes16020131
45. Shumbusho, F, Raoul, J, Astruc, JM, Palhiere, I, and Elsen, JM. Economic evaluation of genomic selection in small ruminants: a sheep meat breeding program. Animal. (2013) 7:1573–9. doi: 10.1017/S1751731113001237
46. Baloche, G, Legarra, A, Sallé, G, Larroque, H, Astruc, JM, Robert-Granié, C, et al. Assessment of accuracy of genomic prediction for French Lacaune dairy sheep. J Dairy Sci. (2014) 97:1107–16. doi: 10.3168/jds.2013-7135
47. Rivero-Moreno, Y, Echevarria, S, Vidal-Valderrama, C, Pianetti, L, Cordova-Guilarte, J, Navarro-Gonzalez, J, et al. Robotic surgery: a comprehensive review of the literature and current trends. Cureus. (2023) 15:e42370. doi: 10.7759/cureus.42370
48. Buote, NJ. Looking to the future; veterinary robotic surgery. Vet Clin North Am Small Anim Pract. (2024) 54:735–51. doi: 10.1016/j.cvsm.2024.02.008
49. Picozzi, P, Nocco, U, Labate, C, Gambini, I, Puleo, G, Silvi, F, et al. Advances in robotic surgery: a review of new surgical platforms. Electronics. (2023) 13:4675. doi: 10.3390/electronics13234675
50. Marescaux, J, and Rubino, F. The ZEUS robotic system: experimental and clinical applications. Surg Clin North Am. (2004) 83:1305–15. doi: 10.1016/S0039-6109(03)00158-9
51. Blixt, E, and O'Brien, M. Augmented reality in veterinary surgery: a systematic review. Vet Surg. (2021) 50:947–57. doi: 10.1111/vsu.13653
52. Pratt, P, Ives, M, Lawton, G, Simmons, J, Radev, N, Spyropoulou, L, et al. Through the HoloLens™ looking glass: augmented reality for extremity reconstruction surgery using 3D vascular models with perforating vessels. Eur Radiol Exp. (2020) 4:1–7. doi: 10.1186/s41747-019-0136-8
53. Tepper, OM, Rudy, HL, Lefkowitz, A, Weimer, KA, Marks, SM, Stern, CS, et al. Mixed reality with HoloLens: where virtual reality meets augmented reality in the operating room. Plast Reconstr Surg. (2017) 140:1066–70. doi: 10.1097/PRS.0000000000003802
54. Fergo, C, Burcharth, J, Pommergaard, HC, Kildebro, N, and Rosenberg, J. Three-dimensional laparoscopy vs 2-dimensional laparoscopy with high-definition technology for abdominal surgery: a systematic review. Am J Surg. (2017) 213:159–70. doi: 10.1016/j.amjsurg.2016.07.030
55. Currò, G, La Malfa, G, Lazzara, S, Caizzone, A, and Navarra, G. Three-dimensional versus two-dimensional laparoscopic cholecystectomy: is surgeon experience relevant? J Laparoendosc Adv Surg Tech. (2015) 25:566–70. doi: 10.1089/lap.2014.0641
56. Neethirajan, S. Recent advances in wearable sensors for animal health management. Sens Bio-Sensing Res. (2017) 12:15–29. doi: 10.1016/j.sbsr.2016.11.004
57. Wolfert, S, Ge, L, Verdouw, C, and Bogaardt, MJ. Big data in smart farming–a review. Agric Syst. (2017) 153:69–80. doi: 10.1016/j.agsy.2017.01.023
58. Rasheed, A, San, O, and Kvamsdal, T. Digital twins: values, challenges and enablers from a modeling perspective. IEEE Access. (2020) 8:21980–2012. doi: 10.1109/ACCESS.2020.2970143
59. Pylianidis, C, Osinga, S, and Athanasiadis, IN. Introducing digital twins to agriculture. Comput Electron Agric. (2021) 184:105942. doi: 10.1016/j.compag.2020.105942
60. Abbott, KA. Cost-benefit evaluation of artificial insemination for genetic improvement of wool-producing sheep. Aust Vet J. (1994) 71:353–60. doi: 10.1111/j.1751-0813.1994.tb00926.x
61. Valergakis, GE, Oikonomou, G, and Arsenos, G. Profitability of a dairy sheep genetic improvement program using artificial insemination. Animal. (2010) 4:1315–21. doi: 10.1017/S1751731110000832
62. Merchant, Z, Goetz, ET, Cifuentes, L, Keeney-Kennicutt, W, and Davis, TJ. Effectiveness of virtual reality-based instruction on students' learning outcomes in K-12 and higher education: a meta-analysis. Comput Educ. (2014) 70:29–40. doi: 10.1016/j.compedu.2013.07.033
63. Mobini, S. Reproductive technologies used to make goats more productive. Prof Anim Sci. (2019) 35:231–8. doi: 10.15232/pas.2018-01820
64. Wilkinson, MD, Dumontier, M, Aalbersberg, IJ, Appleton, G, Axton, M, Baak, A, et al. The FAIR guiding principles for scientific data management and stewardship. Sci Data. (2016) 3:160018. doi: 10.1038/sdata.2016.18
65. Brito, LF, Bedere, N, Douhard, F, Oliveira, HR, Arnal, M, Peñagaricano, F, et al. Review: genetic selection of high-yielding dairy cattle toward sustainable farming systems in a rapidly changing world. Animal. (2021) 15:100292. doi: 10.1016/j.animal.2021.100292
66. Davis, GH. Major genes affecting ovulation rate in sheep. Genet Sel Evol. (2005) 37:S11–23. doi: 10.1186/1297-9686-37-S1-S11
67. Hanrahan, JP, Gregan, SM, Mulsant, P, Mullen, M, Davis, GH, Powell, R, et al. Mutations in the genes for oocyte-derived growth factors GDF9 and BMP15 are associated with both increased ovulation rate and sterility in Cambridge and Belclare sheep (Ovis aries). Biol Reprod. (2004) 70:900–9. doi: 10.1095/biolreprod.103.023093
68. Galloway, SM, McNatty, KP, Cambridge, LM, Laitinen, MP, Juengel, JL, Jokiranta, TS, et al. Mutations in an oocyte-derived growth factor gene (BMP15) cause increased ovulation rate and infertility in a dosage-sensitive manner. Nat Genet. (2000) 25:279–83. doi: 10.1038/77033
69. Juengel, JL, Hudson, NL, Heath, DA, Smith, P, Reader, KL, Lawrence, SB, et al. Growth differentiation factor 9 and bone morphogenetic protein 15 are essential for ovarian follicular development in sheep. Biol Reprod. (2002) 67:1777–89. doi: 10.1095/biolreprod.102.007146
70. Bodin, L, Di Pasquale, E, Fabre, S, Bontoux, M, Monget, P, Persani, L, et al. A novel mutation in the bone morphogenetic protein 15 gene causing defective protein secretion is associated with both increased ovulation rate and sterility in Lacaune sheep. Endocrinology. (2007) 148:393–400. doi: 10.1210/en.2006-0764
71. Knights, M, Maze, TD, Bridges, PJ, Lewis, PE, and Inskeep, EK. Short-term treatment with a controlled internal drug releasing (CIDR) device and FSH to induce fertile estrus and increase prolificacy in anestrous ewes. Theriogenology. (2001) 55:1181–91. doi: 10.1016/S0093-691X(01)00476-9
72. Ungerfeld, R, and Rubianes, E. Short term primings with different progestogen intravaginal devices (MAP, FGA and CIDR) for eCG-estrous induction in anestrus ewes. Small Rumin Res. (2002) 46:63–6. doi: 10.1016/S0921-4488(02)00105-0
73. Viñoles, C, Forsberg, M, Martin, GB, Cajarville, C, Repetto, J, and Meikle, A. Short-term nutritional supplementation of ewes in low body condition affects follicle development due to an increase in glucose and metabolic hormones. Reproduction. (2001) 122:909–17. doi: 10.1530/rep.0.1220909
74. Martin, GB, Tjondronegoro, S, and Blackberry, MA. Effects of nutrition on testicular size and the concentrations of gonadotrophins, testosterone and inhibin in plasma of mature male sheep. J Reprod Fertil. (1994) 101:121–8. doi: 10.1530/jrf.0.1010121
75. Scaramuzzi, RJ, Campbell, BK, Downing, JA, Kendall, NR, Khalid, M, Muñoz-Gutiérrez, M, et al. A review of the effects of supplementary nutrition in the ewe on the concentrations of reproductive and metabolic hormones and the mechanisms that regulate folliculogenesis and ovulation rate. Reprod Nutr Dev. (2006) 46:339–54. doi: 10.1051/rnd:2006016
76. Forcada, F, and Abecia, JA. The effect of nutrition on the seasonality of reproduction in ewes. Reprod Nutr Dev. (2006) 46:355–65. doi: 10.1051/rnd:2006017
77. Lassoued, N, Rekik, M, Mahouachi, M, and Ben Hamouda, M. The effect of nutrition prior to and during mating on ovulation rate, reproductive wastage, and lambing rate in three sheep breeds. Small Rumin Res. (2004) 52:117–25. doi: 10.1016/S0921-4488(03)00250-5
78. Nottle, MB, Hynd, PI, Seamark, RF, and Setchell, BP. Increases in ovulation rate in lupin-fed ewes are not due to lupins per se but to increased feed intake. Anim Reprod Sci. (1997) 49:27–35. doi: 10.1016/S0378-4320(97)00044-7
79. Boland, MP, Lonergan, P, and O'Callaghan, D. Effect of nutrition on endocrine parameters, ovarian physiology, and oocyte and embryo development. Theriogenology. (2001) 55:1323–40. doi: 10.1016/S0093-691X(01)00485-X
80. Viñoles, C, Meikle, A, Forsberg, M, and Rubianes, E. The effect of subluteal levels of exogenous progesterone on follicular dynamics and endocrine patterns during the early luteal phase of the ewe. Theriogenology. (1999) 51:1351–61. doi: 10.1016/S0093-691X(99)00079-5
81. Evans, AC, Duffy, P, Hynes, N, and Boland, MP. Waves of follicle development during the estrous cycle in sheep. Theriogenology. (2000) 53:699–715. doi: 10.1016/S0093-691X(99)00268-X
82. Bartlewski, PM, Beard, AP, Cook, SJ, and Rawlings, NC. Ovarian antral follicular dynamics and their relationships with endocrine variables throughout the oestrous cycle in breeds of sheep differing in prolificacy. J Reprod Fertil. (1999) 115:111–24. doi: 10.1530/jrf.0.1150111
83. Souza, CJ, Campbell, BK, McNeilly, AS, and Baird, DT. Effect of bone morphogenetic protein 2 (BMP2) on oestradiol and inhibin a production by sheep granulosa cells, and localization of BMP receptors in the ovary by immunohistochemistry. Reproduction. (2002) 123:363–9. doi: 10.1530/rep.0.1230363
84. McNatty, KP, Juengel, JL, Wilson, T, Galloway, SM, and Davis, GH. Genetic mutations influencing ovulation rate in sheep. Reprod Fertil Dev. (2001) 13:549–55. doi: 10.1071/RD01078
85. Thornton, PK. Livestock production: recent trends, future prospects. Philos Trans R Soc B. (2010) 365:2853–67. doi: 10.1098/rstb.2010.0134
Keywords: laparoscopic artificial insemination, small ruminants, precision reproductive management, artificial intelligence, computer vision, emerging technologies
Citation: Kang T-C, Lai I-L and Shen P-C (2025) Laparoscopic artificial insemination in small ruminants: technological integration, economic evaluation, and future perspectives. Front. Vet. Sci. 12:1667887. doi: 10.3389/fvets.2025.1667887
Edited by:
Charley-Lea Pollard, Department of Agriculture, Fisheries and Forestry, AustraliaReviewed by:
André Cascalho Andrade, Universidade Federal do Sul e Sudeste do Pará, BrazilMuhammad Irfan-ur-Rehman Khan, University of Veterinary and Animal Science Lahore-Pakistan, Pakistan
Copyright © 2025 Kang, Lai and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ting-Chieh Kang, Y2N1YXNrdGNAZ21haWwuY29t; Perng-Chih Shen, cGNzaGVuQG1haWwubnB1c3QuZWR1LnR3
 Ting-Chieh Kang
Ting-Chieh Kang I-Ling Lai2
I-Ling Lai2