- 1Institute of Pathogens and Vectors, Yunnan Provincial Key Laboratory for Zoonosis Control and Prevention, Dali University, Dali, China
- 2Institute of Entomology, Guizhou University, Guiyang, China
Objective: Rodents and other sympatric small mammals serve as reservoir hosts for zoonotic diseases including scrub typhus and hemorrhagic fever with renal syndrome (HFRS), with their ectoparasitic mites (chiggers and gamasid mites) acting as vectors. This 12-month study investigated mite infestation, community structure, seasonal dynamics, and climatic drivers on small mammal hosts in Jingha, southern Yunnan, China–a known scrub typhus and HFRS.
Methods: We calculated infestation metrics (prevalence [PM], mean abundance [MA], mean intensity [MI], constituent ratio [Cr]) and community indices (richness [R], Shannon-Wiener diversity [H], Pielou evenness [E], Simpson dominance [D]). Generalized additive models (GAMs) analyzed spatiotemporal and climatic patterns.
Results: From 2,424 small mammal hosts (15 species), we collected 142,471 mites (158 species). Chiggers (109 species, 109,093 individuals) significantly outnumbered gamasid mites (49 species, 33,378 individuals; P < 0.001) and showed greater richness (R = 9.31 vs. 4.61), diversity (H = 2.13 vs. 1.97). Rattus andamanensis was the dominant host. Chigger infestation (PM = 86.14%, MA = 45.01, MI = 52.25) significantly exceeded gamasid mites (PM = 67.16%, MA = 13.77, MI = 20.50; P < 0.001), particularly on female and adult hosts. Four species dominated (Cr = 65.40%): chiggers Walchia micropelta, Ascoschoengastia indica, Leptotrombidium deliense, and gamasid mite Laelaps nuttalli. Primary vectors among 23 species included chiggers L. deliense, A. indica, L. scutellare, and gamasid Laelaps echidninus (Cr = 38.46%).
Conclusion: Community indices fluctuated monthly without distinct peaks, while dominant species abundances varied significantly. Climatic factors exerted species-specific effects: L. deliense peaked in July (30.0 mites/host; 95% CI: 29.2–30.8) coinciding with maximal temperatures, while A. indica peaked in August (25.1 mites/host; 95% CI: 24.5–25.8), lagging peak rainfall. Non-overlapping confidence intervals indicated temporal niche separation between species. Mite-mite networks revealed positive intragroup correlations but no significant intergroup correlations. Host-mite networks demonstrated low host specificity: individual hosts harbored multiple mite species, and individual mite species parasitized multiple hosts. High mite abundance, co-occurrence of multiple vector species, and low host specificity collectively elevate transmission risks and persistence of scrub typhus and HFRS.
1 Introduction
The small mammals in this study include rodents (Rodentia) and sympatric species such as shrews and tree shrews (1, 2). They commonly harbor numerous ectoparasites—predominantly fleas, sucking lice, chigger mites, and gamasid mites—with occasional occurrences of ticks. These mammal hosts, alongside their ectoparasitic arthropods, can serve as critical reservoir hosts for zoonotic diseases such as plague, murine typhus, scrub typhus, and hemorrhagic fever with renal syndrome (HFRS) (3–5). Chiggers (chigger mites) and gamasid mites constitute the predominant arachnid groups infesting these hosts. Besides directly inducing dermatitis through their bites, these mites can act as recognized or potential vectors and as reservoir hosts of several zoonotic pathogens. Due to their medical significance, chiggers and gamasid mites are important subjects for epidemiological research (6–9).
Taxonomically classified within subphylum Chelicerata, class Arachnida, and subclass Acari, chiggers and gamasid mites exhibit phylogenetic divergence: chiggers constitute the order Trombidiformes (infraclass Acariformes), whereas gamasid mites belong to order Mesostigmata (superorder Parasitiformes) (10, 11).
Chiggers are the larvae of trombiculid mites (Trombiculidae), which exhibit a seven-stage life cycle, with larvae (chiggers or chigger mites) being the sole ectoparasitic phase. These larvae primarily parasitize rodents and other small mammals and serve as the exclusive vectors for scrub typhus (12–14). Orientia tsutsugamushi, the etiological agent of scrub typhus, circulates among rodent reservoirs via chigger bites and may transmit to humans. Certain chigger species (e.g., Leptotrombidium scutellare) additionally function as potential vectors for HFRS, which is caused by hantavirus (12, 15–17). These zoonotic diseases pose a significant global health threat, demonstrating rapidly increasing prevalence and expanding endemicity in recent years (18–20). Examples include the recent confirmation of autochthonous scrub typhu transmissions in the United Arab Emirates and Chile, along with suspected pathogen presence in Kenya, despite the disease having been historically confined to the Asia-Pacific region (21–24). The life cycle of gamasid mites comprises eggs, larvae, protonymphs (first nymphal stage), deutonymphs (second nymphal stage), and adult males and females. All developmental stages of gamasid mites can parasitize rodents and other small mammals as ectoparasites. While gamasid mites frequently infest and bite humans, leading to dermatitis, certain species also function as vectors of rickettsial pox and are regarded as potential vectors of HFRS (8, 25–27). Further epidemiological significance associated with gamasid mites on rodents and small mammals is implicated in the transmission of more than 20 additional zoonotic diseases, including endemic typhus (murine typhus), plague, and leptospirosis (28–30).
Yunnan Province in southwestern China represents a significant natural endemic focus for scrub typhus and HFRS. The disease burden is particularly severe in southern regions, exemplified by Xishuangbanna Prefecture (7, 12). This prefecture, bordering northern Myanmar, is a high-risk area for zoonotic diseases including scrub typhus and HFRS, with 1,208 scrub typhus cases were documented between 2006 and 2017. Northern Myanmar similarly experiences outbreaks of scrub typhus and HFRS (15, 17, 31). Mites parasitizing small mammals (e.g., chiggers and gamasid mites) may disperse across borders through host migration, which may facilitate disease transmission. Host migration across international borders elevates zoonotic disease transmission risks, consequently intensifying threats to the growing frequency of international trade and tourism within this region (32, 33). Consequently, the research on small-mammal-associated mites in southern Yunnan's China-Myanmar border region holds critical public health significance.
Both chiggers and gamasid mite groups demonstrate remarkable species diversity, with >3,000 chigger species and >8,000 gamasid mite species documented globally (27, 34, 35). The species-level identification of these mites is taxonomically challenging, requiring slide-mounted specimens for detailed microscopic comparison of morphological characters. The chigger identification proves particularly demanding, necessitating high-magnification (including oil-immersion) microscopy for meticulous observation, morphometric analysis, and comparative assessment—a specialized, labor-intensive process (16, 17, 22). Owing to their diversity, complex identification requirements, and methodological constraints, prior studies have predominantly examined these mite groups separately (6, 7). Despite significant taxonomic divergence (distinct orders/superorders), they frequently co-occur on rodent hosts, forming an integrated ecological mite community. The holistic investigation of this community is therefore warranted.
We conducted monthly surveys at a fixed site in Jingha village, Jinghong County (Xishuangbanna Dai Autonomous Prefecture, southern Yunnan) from April 2016 to March 2017. Using this 12-month dataset, we conceptualize chigger and gamasid mite assemblages on small mammals as a cohesive “mite community.” We characterized its structure and seasonal dynamics while employing Generalized Additive Models (GAMs) to quantify climatic drivers of mite distribution. This integrated approach aims to: (1) advance medical acarology, (2) develop novel methodologies for studying medically significant arthropods and epizoic parasites, and (3) establish scientific foundations for monitoring and controlling vector mites and associated zoonotic diseases in the China-Myanmar bordering region.
2 Materials and methods
2.1 Field investigations
We selected Jingha village, Jinghong County (Xishuangbanna Dai Autonomous Prefecture, southern Yunnan; Figure 1) as a fixed study site. Monthly field surveys (each spanning 15–20 days) were conducted over 12 consecutive months from April 2016 to March 2017. Situated along the Lancang River (Mekong River, flowing northwest-southeast through Yunnan) at 21°50′N, 100°52′E, the study area ranges from 500 to 700 m elevation. This characteristic valley basin landscape encompassed representative habitats including rubber plantations, banana cultivations, farmlands, shrublands, and broadleaf forests (36, 37). Meteorological data were acquired from publicly accessible online records published by regional weather authorities during 2016–2017 (source: https://tianqi.911cha.com/jinghong/).
Figure 1. Location map of Jingha survey site in Jinghong County, Xishuangbanna Prefecture, southern Yunnan, China (April 2016–March 2017).
2.2 Collection of mites and host animals
Small mammal hosts (primarily rodents) were captured using baited wire cages (18 × 12 × 9 cm; Guixi Rodent Trap Factory, Guixi, Jiangxi, China). Each host individual was isolated in a separate cotton bag and transported to a field laboratory. Following anesthesia, the mammal hosts underwent examination in a large white tray where chiggers (Trombiculidae) and gamasid mites (Mesostigmata) were systematically collected using standardized parasitological protocols (38). By the help of 10× magnifying lenses, all gamasid mites on the body surface of each host were collected as completely as possible. Due to the microscopic size of chiggers (<0.2 mm), 10× magnifying lenses and surgical scalpels were used to meticulously collect chiggers and chigger-like particulates from hosts' tender skin such as pinnae, auditory canal openings, inguinal regions, perianal areas, and axillae where chiggers often attach. Adhering to a strict “one host to one container” principle, all chiggers and gamasid mites from each host were preserved in 70% ethanol within a pre-labeled centrifuge tube. The hosts were identified to species according to their comprehensive morphology such as size and shape, pelage coloration, morphometrics (body weight, body length, tail length, ear height, hindfoot length), and other morphological features (39–41). Voucher specimens of mites and representative hosts were deposited in the Institute of Pathogens and Vectors, Dali University. The use of animals (including animal euthanasia) for our research was officially approved by the Animals' Ethics Committee of Dali University, approval code: DLXY2001-1116, approval date: 16 November 2001.
2.3 Preparation and taxonomic identification of mite specimens
Chiggers, chigger-like particulates and gamasid mites were sorted out and rinsed 2–3 times in clean/distilled water under a stereomicroscope to remove non-mite impurities. Specimens were mounted on glass slides using Hoyer's medium, followed by drying and clearing. Each slide was examined under high-power magnification (100–200×) or oil immersion microscopy (400×), during which morphological structures were meticulously observed, compared, and measured.
According to the hierarchical taxonomic levels (family, subfamily, genus, species), each mite specimen was ultimately identified to species using taxonomic keys and literature (42–44). Specimens unidentifiable to species due to damage, debris obstruction, ambiguous morphology or suspected new species were recorded as species inquirendae (sp. inq.) in original datasheets. All unidentified specimens were subsequently excluded from statistical analyses. Given the technical complexity of chigger and gamasid mite taxonomy, over 3,000 working hours of dedicated effort were devoted to identification, necessitating significant workload.
2.4 Infestation indices and community structure statistics
After taxonomic identification, a verified mite dataset was compiled. Standard statistics of mite parasitism including constituent ratios (Cr), prevalence (PM), mean abundance (MA), and mean intensity (MI) were calculated. Statistical significance tests were conducted with SPSS 26.0 (α = 0.05): Chi-square test for Cr and PM, and Non-parametric test (Mann-Whitney U or Kruskal-Wallis H) for MA and MI.
In the above formulas, Ni represents the individuals of mite species i. N represents the total individuals of all mite species. M represents the total number of the hosts, Mi represents the number of infested hosts by mite i.
Mite community structure was quantified with Margalef richness index (R), Shannon-Wiener diversity index (H), Pielou's evenness index (E), and Simpson's dominance index (D). Subcommunity structures of chigger mites and gamasid mites were statistically compared.
2.5 Analysis of seasonal dynamics
The corresponding months for each season are as follows: the spring is from March to May, summer from June to August, autumn from September to November, and winter from December to February.
2.6 Analysis of climatic drivers for the seasonal dynamics
The overdispersion was corrected by using quasi-Poisson distributions (scale parameters: 1.8–3.2), with Bootstrap test confirming the stability of parameters (consistency > 97%).
2.7 Analysis of climate factors driving mite community dynamics
We analyzed climate drivers of mite community distributions on mammal hosts using Generalized Additive Models (GAMs). Models were formulated with the “mgcv” package in R v4.4.3 to fit monthly abundance data of dominant mite species against climate variables (mean temperature, relative humidity, precipitation) (45, 46). A Poisson distribution family and thin-plate regression splines characterized non-linear relationships, with basis dimensions (k) fixed at 5 for all smooth terms. Smoothing parameters were optimized via the Unbiased Risk Estimator (UBRE), and 95% confidence intervals (CIs) computed to quantify prediction uncertainty.
(1) Data preprocessing using “tidyverse” package ensure variable validity and model compliance. For each mite species, we specified the GAM as: Mite abundance ~ s(mean temperature) + s(relative humidity) + s(precipitation).
where s() denotes a smoothing function capturing non-linear climate-abundance relationships with basis dimension k = 5.
(2) Predictions generated via stats::predict() (with se.fit = TRUE) enabled confidence interval calculation. Species-specific smooth curves with 95% CIs were visualized using “ggplot2”, faceted by species via facet_wrap. Graphical outputs were refined with theme_publish and exported as high-resolution figures using ggplot2::ggsave().
2.8 Analysis of interspecific correlations among dominant mite species
Pearson correlation coefficients were calculated for dominant mite species with larger population sizes within the mite community to evaluate interspecific relationships with a with a significance threshold of α = 0.05. Absolute correlation coefficient (r) values were categorized as follows: 0.00–|0.19| (very weak), |0.20|–|0.39| (weak), |0.40|–|0.59| (moderate), and ≥|0.60| (strong).
Correlation matrices and heatmaps generated using Origin 2024 software visually represent relationships among dominant mite species (47).
2.9 Analysis of mite-host interactions
Parallel sets diagrams were employed to analyze interactions between mites and their hosts, focusing on dominant mite species with larger population sizes within the community. The analytical workflow comprised the following steps:
(1) The host–mite abundance matrix was converted to long format (host species × mite species). Mite species columns were transformed into attribute columns using Power Query's Unpivot Columns tool to generate a standardized data table.
(2) Parallel sets diagram parameters were configured as follows:
(2.1) Node width: dynamically scaled according to total abundance proportions of hosts and mites using:
where Wi denotes node width and Totali represents total abundance (host or mite).
(2.2) Ribbon transparency: determined by abundance values, with transparency ranging from 0.2 to 1.0 corresponding to 0–100% abundance:
3 Results
3.1 Species composition and infestations of mites
A total of 2,424 small mammal hosts captured from Jingha survey site (April 2016 to March 2017) were taxonomically classified into 15 species, 10 genera, five families, and three orders (Rodentia, Eulipotyphla, Scandentia; Table 1). Rattus andamanensis was the dominant host species, comprising 84.69% (2,053/2,424) of all the hosts. From these hosts, we recorded an amount of 148,958 mite individuals, with 142,471 of which were identified as 158 species, 24 genera, and three families [Trombiculidae (chiggers): 109,093 individuals, 109 species, 12 genera; Laelapidae and Macronyssidae (gamasid mites): 33,378 individuals, 49 species, 12 genera; Table 2]. The unidentified 6,487 mites (4.35%) were excluded from analyses. Among the 158 identified species, 23 (12 chiggers, 11 gamasid mites) were confirmed or potential vectors for scrub typhus or HFRS (Table 2).
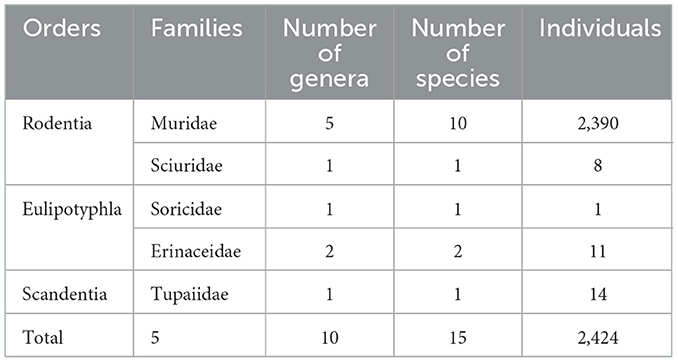
Table 1. Taxonomic identification of small mammal hosts at Jingha survey site in southern Yunnan of southwest China (April 2016–March 2017).

Table 2. Taxonomic identification of mites on small mammal hosts at Jingha survey site in southern Yunnan of southwest China (April 2016–March 2017).
Of the 158 mite species and 142,471 individuals, chiggers accounted for 68.99% (109/158) of species composition and 76.57% (109,093/142,471) of abundance, outnumbering gamasid mites with 31.01% of species (49/158) and 23.43% of abundance (33,378/142,471; Table 3).
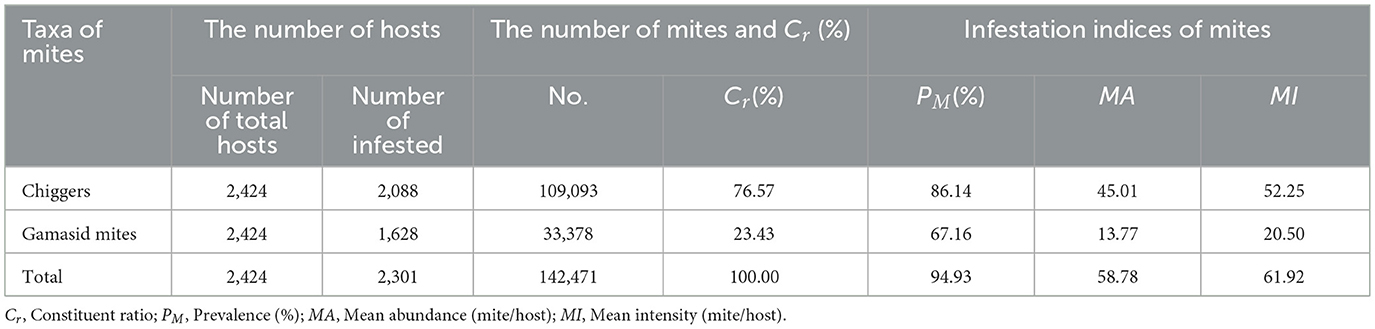
Table 3. Statistics for overall infestation indices of mites on small mammal host at Jingha survey site in southern Yunnan, China (April 2016–March 2017).
Figure 2 illustrates the taxonomic composition of chiggers and gamasid mites at the family, genus, and species levels. At the genus level (12 genera in total), 96.31% of chigger individuals (105,065/109,093) and 73.34% of chigger species (3/12) came from three genera, Walchia (Cr: 39.69% for individuals and 15.60% for species), Ascoschoengastia (Cr: 25.38% for individuals and 9.17% for species), and Leptotrombidium (Cr: 31.24% for individuals and 38.53% for species). The genus Leptotrombidium exhibited the highest species richness (42 species). At the species level (109 species in total), 75.51% of chiggers (82,374/109,093) came from three species, W. micropelta (Cr = 31.99%, 34,904/109,093), A. indica (Cr = 24.01%, 26,193/109,093), and L. deliense (Cr = 19.50%, 21,277/109,093), with W. micropelta showing the highest constituent ratio. Low Cr values ( ≤ 5%) for the remaining 106 species indicated a high proportion of rare species. Notably, Leptotrombidium deliense and L. scutellare—the primary scrub typhus vectors in China, with the latter also a potential HFRS vector—accounted for 19.50% and 3.62% of total chigger individuals, respectively.
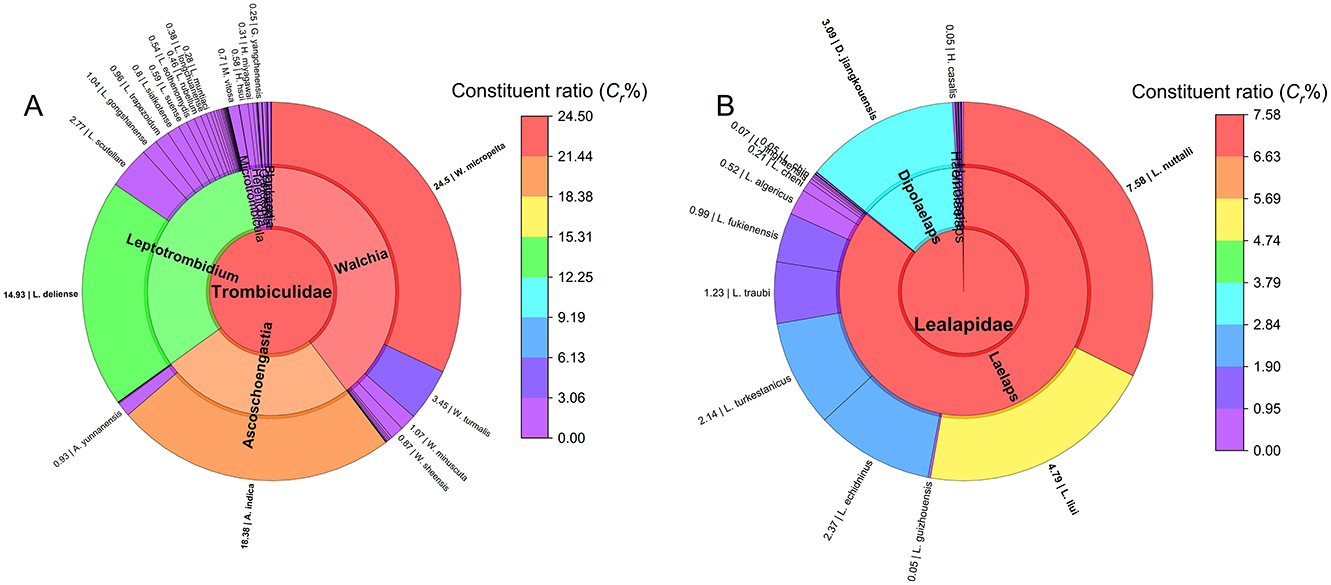
Figure 2. The composition of two mite groups at Jingha in southern Yunnan of southwest China (April 2016–March 2017). (A) The constituent ratios (Cr) of family, genus and species of chiggers (chigger mites); (B) The constituent ratios (Cr) of family, genus and species of gamasid mites.
In gamasid mite subcommunity, the family Laelapidae predominated (Cr = 99.84%, 33,323/33,378) at the family level (2 families). The genus-level analysis (12 genera in total) revealed the genus Laelaps as dominant (Cr = 85.71%, 28,607/33,378). Among 49 species of gamasid mites, Laelaps nuttalli (Cr = 32.34%, 10,795/33,378), L. liui (Cr = 20.46%, 6,830/33,378), and L. echidninus (Cr = 10.10%, 3,370/33,378) were the most abundant, collectively representing 62.90% of individuals (20,995/33,378; Figure 2).
Overall infestation indices revealed that chiggers exhibited significantly higher prevalence (PM = 86.14%), mean abundance (MA = 45.01 mites per examined host), and mean intensity (MI = 52.25 mites per infested host) than gamasid mites (PM = 67.16%, MA = 13.77, MI = 20.50). These differences were statistically significant (PM: χ2 = 243.87, P < 0.001; MA: Z = −30.05, P < 0.001; MI: Z = −27.67, P < 0.001; Table 3).
3.2 Host selection of mites
The results demonstrate rodents—particularly Muridae, Rattus, and R. andamanensis —constitute primary mite hosts. Sankey diagram visualization (Figure 3) of 142,471 mites across four host taxonomic levels (order, family, genus, species) revealed pronounced host preference. At the order level (n = 3), Rodentia harbored 99.01% of mites (Cr = 99.01%; 141,062/142,471). Among families (n = 5), Muridae hosted 98.73% of mites (Cr = 98.73%; 140,667/142,471). At the genus level (n = 10), The mites on Rattus predominated (Cr = 83.96%; 119,612/142,471), followed distantly by Berylmys (Cr = 9.58%). Species-level analysis (n = 15) showed 79.14% of mites infested Rattus andamanensis (Cr = 79.14; 112,752/142,471).
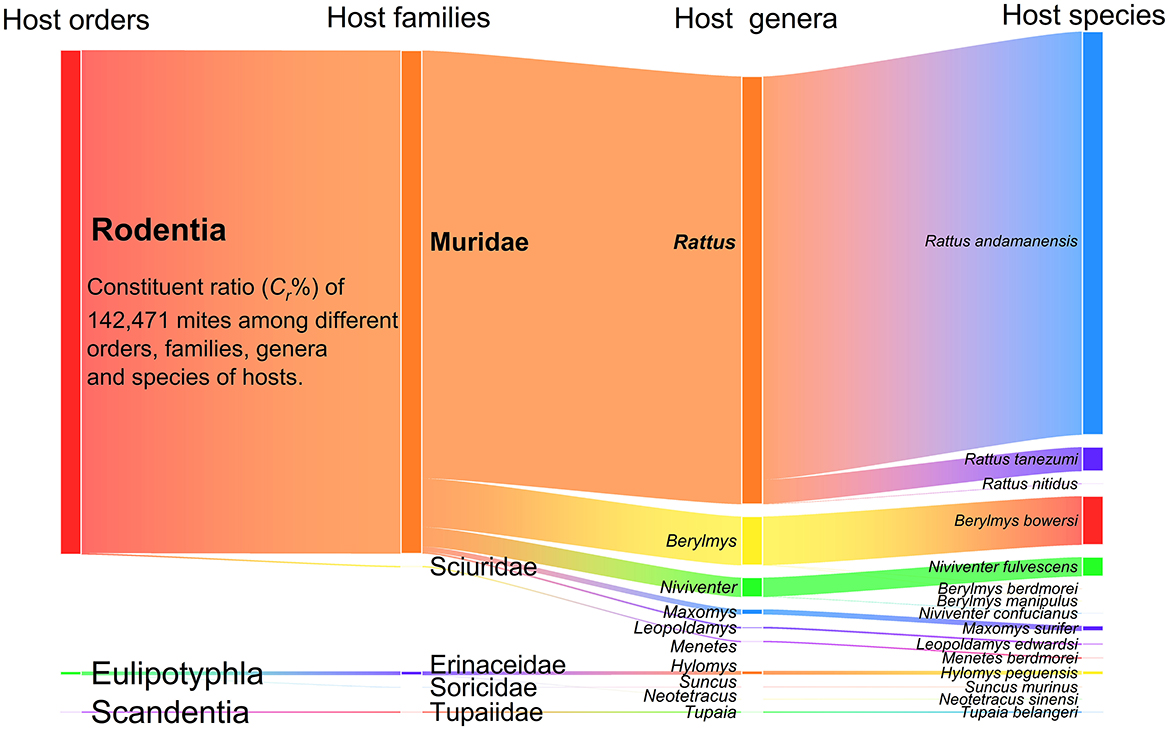
Figure 3. Visualization of mite distribution among different orders, families, genera and species of small mammal hosts at Jingha survey site in southern Yunnan of southwest China (April 2016-March 2017).
3.3 Community structure of mites and their dominant species
The mite community comprised two subcommunities: chigger and gamasid mite subcommunities. The chigger subcommunity exhibited greater species richness (109 vs. 49), Margalef index (9.31 vs. 4.61), and Shannon-Wiener diversity (2.13 vs. 1.97) than the gamasid mite subcommunity. Conversely, the gamasid mite subcommunity showed marginally higher Simpson dominance (D = 0.81 vs. 0.80) than the chigger subcommunity (Table 4).
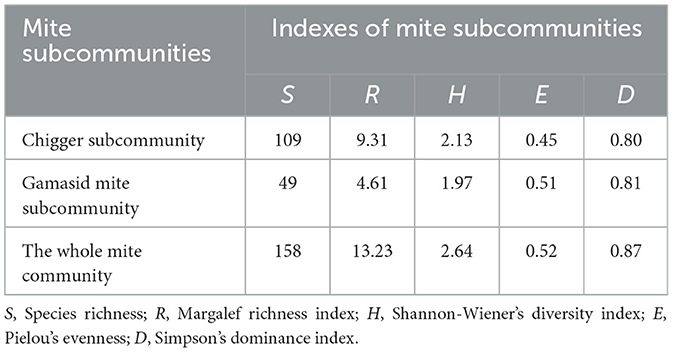
Table 4. Community indexes of two mite groups on small mammal hosts at Jingha survey site in southern Yunnan of southwest China (April 2016–March 2017).
Four species (three from chigger subcommunity and one from gamasid subcommunity) exhibit dominance (Cr = 65.40%) in the combined mite community. In the chigger subcommunity, W. micropelta (Cr = 31.99%, 34,904/109,093), A. indica (Cr = 24.01%, 26,193/109,093), and L. deliense (Cr = 19.50%, 21,277/109,093) were the three dominant species. Their prevalence (PM) was 69.06%, 61.18%, and 62.83%, respectively, with high mean abundance (MA = 14.40; 10.81; 8.78) and mean intensity (MI = 20.85; 17.66; 13.97). In the gamasid subcommunity, Laelaps nuttalli was the predominant species, comprising 32.24% of all gamasid mites (Cr = 32.24%, 10,795/33,378), with PM = of 53.42%, MA = 4.45, and MI = of 8.34. The dominating chigger species showed higher constituent ratio (Cr) and infestation indices (PM, MA, MI) than the dominating gamasid species.
3.4 Vector mite species and their infestation status
We documented four primary confirmed or putative vectors of zoonotic diseases in Jingha: three chigger species (L. deliense, A. indica, L. scutellare) and one gamasid mite species (Laelaps echidninus). These species collectively accounted for 38.46% (54,788/142,471) of all the 158 identified species (109 chigger mites + 49 gamasid mites). Among these four vector mite species, L. deliense (Cr = 14.93%, 21,277/142,471) and A. indica (Cr = 18.38%, 26,193/142,471) were the most abundant with high infestation indices (Table 5).
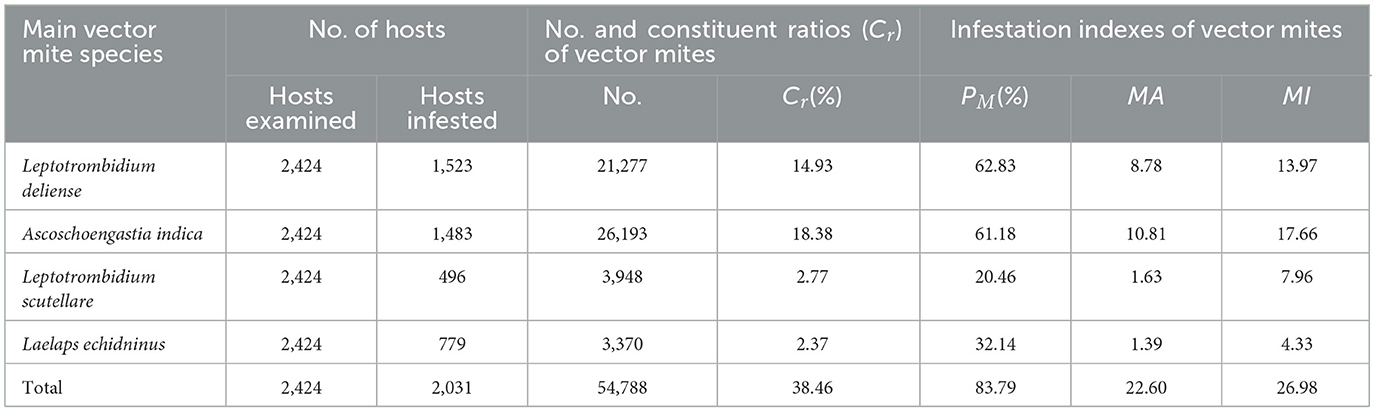
Table 5. Infestation indexes of four main vector mite species at Jingha survey site in southern Yunnan of southwest China (April 2016–March 2017).
3.5 Seasonal dynamics of mite communities
The constituent ratios (Cr) and overall infestation indices (PM, MA, MI) of mites on small mammal hosts fluctuated in different months. The variations of Cr, MA and MI in different months were of statistical significance with χ2 = 5,884.50 and P < 0.001 (Chi-square test) for Cr, H' = 59.25 and P < 0.001 for MA (Kruskal-Wallis H test), and H' = 56.66 and P < 0.001 for MI (Kruskal-Wallis H test). The difference of PM, however, was of no statistical significance with χ2 = 14.83 and P = 0.19 in Chi-square test (Table 6, Figure 4).
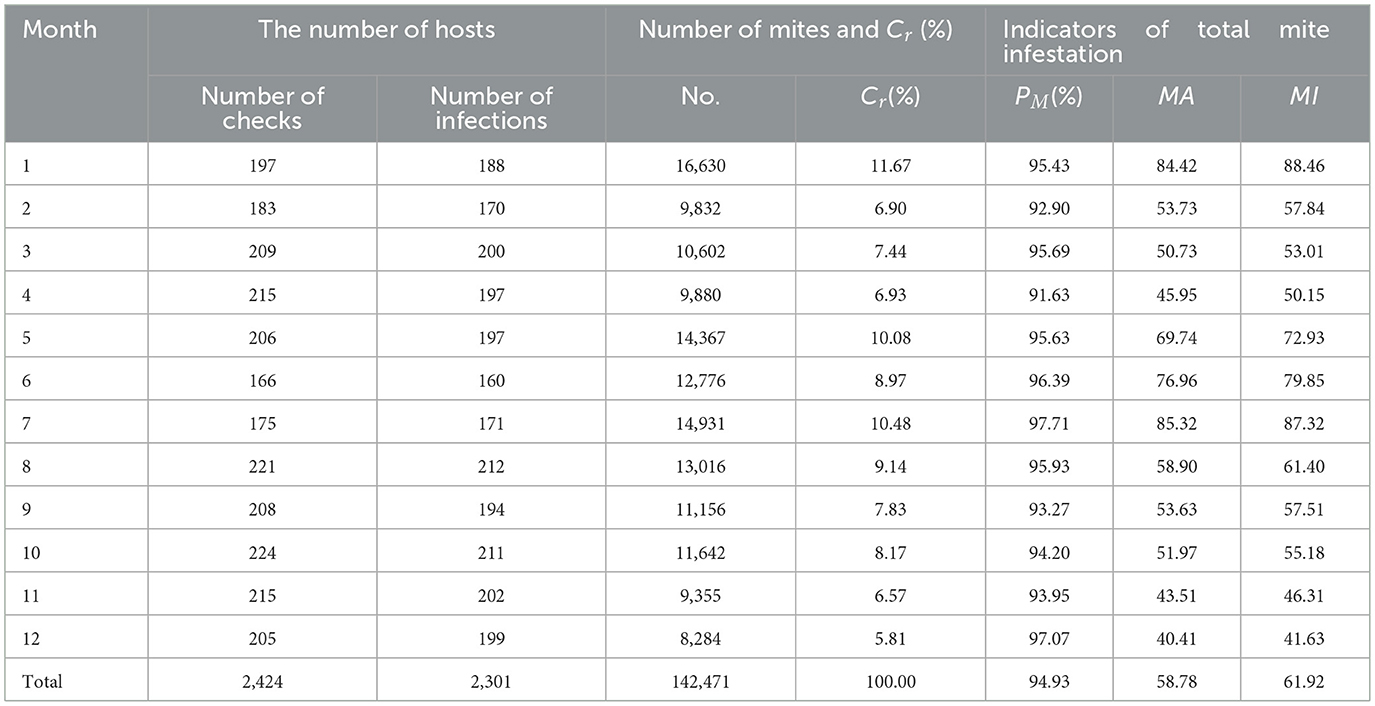
Table 6. Monthly variation of mite infestation indexes on small mammal host at Jingha survey site in southern Yunnan of southwest China (April 2016–March 2017).
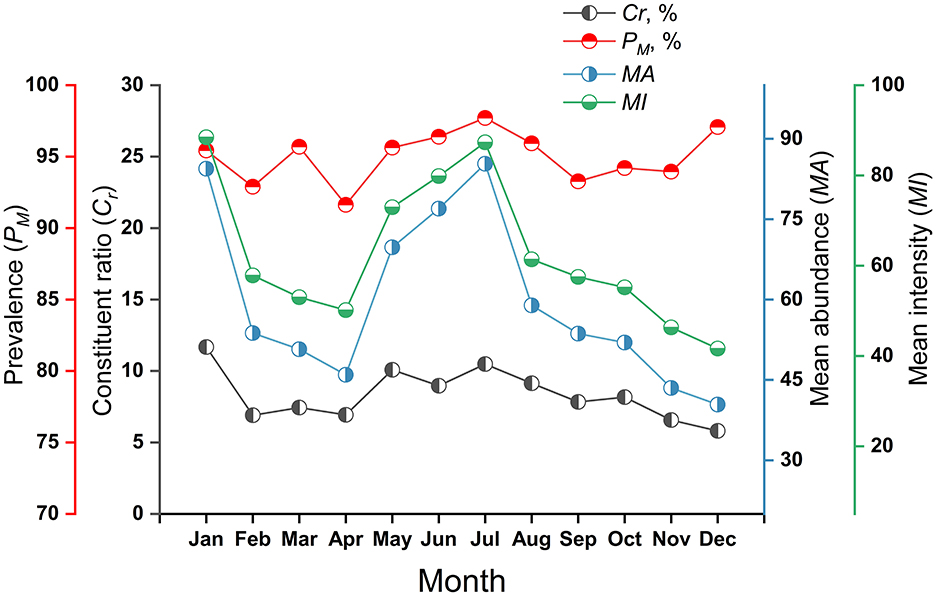
Figure 4. Monthly variations of mite constituent ratios (Cr) and mite infestation indexes (PM, MA, MI) on small mammal hosts at Jingha survey site in southern Yunnan of southwest China (April 2016–March 2017).
The monthly fluctuated values of Cr ranged from 5.81% (December) to 11.67% (January), peaking in January. The values of PM fluctuated between 91.63% (April) and 97.71% (July), with marginally elevated values in summer (July) and winter (December), but no pronounced peak. The MA varied from 40.41 (December) to 85.32 (July), exhibiting distinct bimodal peaks in July and December. Similarly, MI ranged from 41.63 (December) to 88.46 (January), with analogous bimodal peaks during July and December (Table 6, Figure 4).
The diversity indices of the mite community in each month also showed varying degrees of fluctuations. Shannon-Wiener diversity (H) and Pielou's evenness (E) showed two minor peaks in February and December, with low ebbs occurring in October. Margalef's richness (R) and Simpson's dominance (D) slight fluctuates in different months without an obvious pattern (Figure 5).
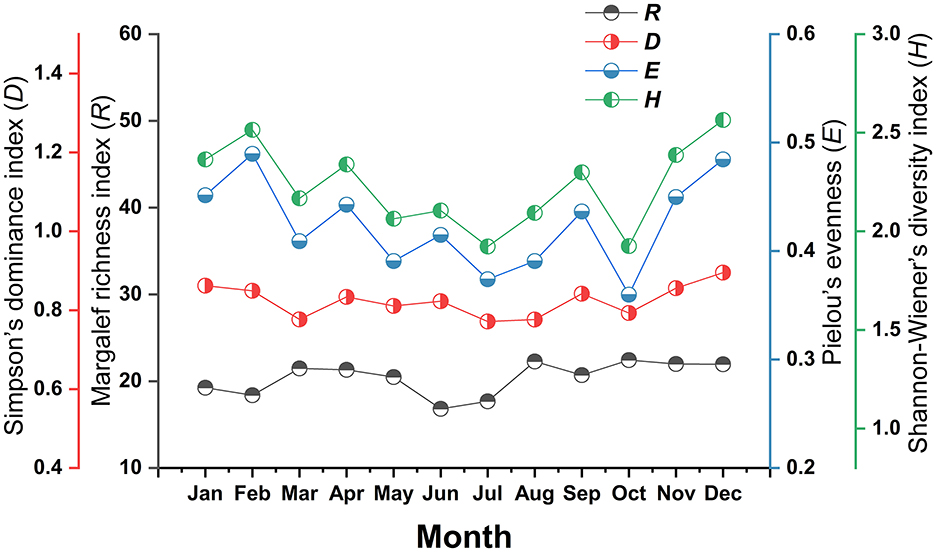
Figure 5. Monthly variation of mite community indexes on small mammal host at Jingha survey site in southern Yunnan of southwest China (April 2016–March 2017).
3.6 Seasonal fluctuations of dominant mite species
Of the dominative three chigger species (W. micropelta, A. indica, L. deliense) and one gamasid mite species (L. nuttalli), the constituent ratios (Cr) exhibited monthly fluctuations. Specifically, W. micropelta peaked in early spring (March: Cr = 43.70%, 4,633/10,602), while A. indica peaked in late summer (August: Cr = 42.69%, 5,556/13,016). Leptotrombidium deliense displayed a bimodal peak distribution, with a primary peak occurring in summer (June: Cr = 31.73%, 4,054/12,776; July: Cr = 35.12%, 5,244/14,931) and a secondary peak in autumn (October: Cr = 24.95%, 2,905/11,642; November: Cr = 25.64%, 2,399/9,355). Laelap nuttalli exhibited minor peaks in early summer (June: Cr = 12.05%, 1,539/12,776) and early winter (December: Cr = 13.19%, 1,093/8,284; Table 7, Figure 6).
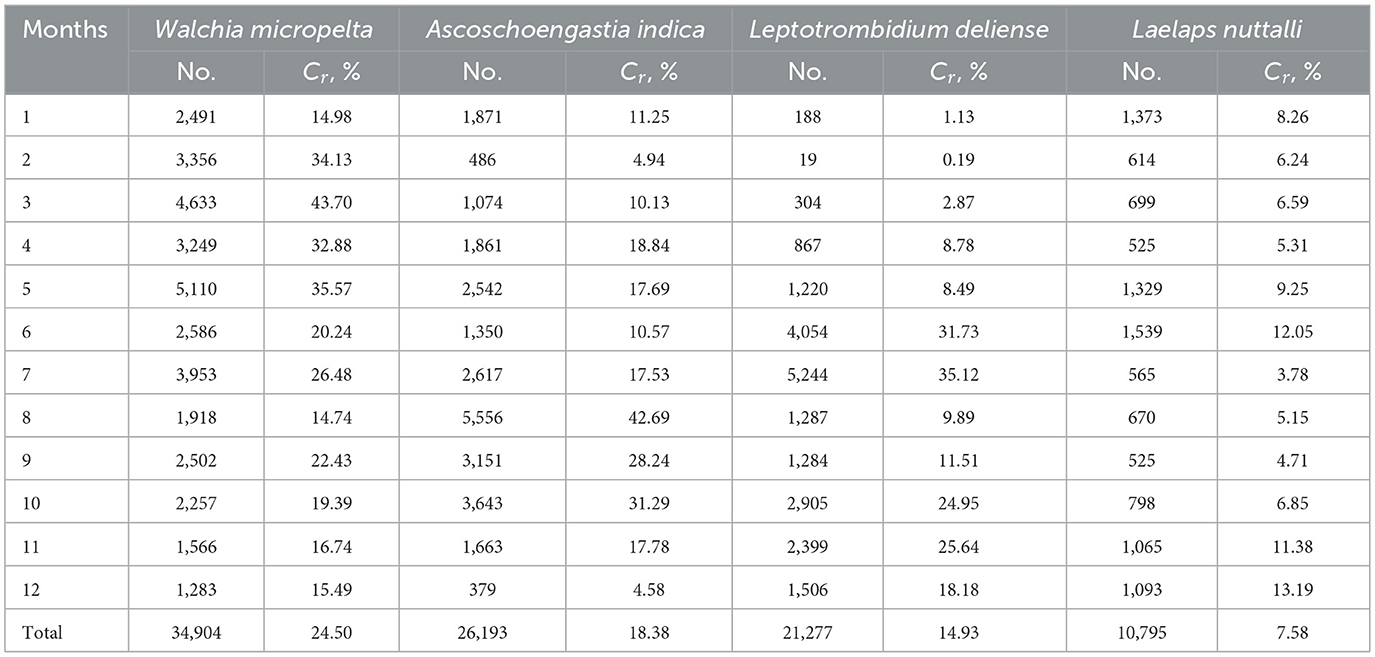
Table 7. Monthly variations of numbers and constituent ratios (Cr) of four dominant mite species at Jingha survey site in southern Yunnan of southwest China (April 2016–March 2017).
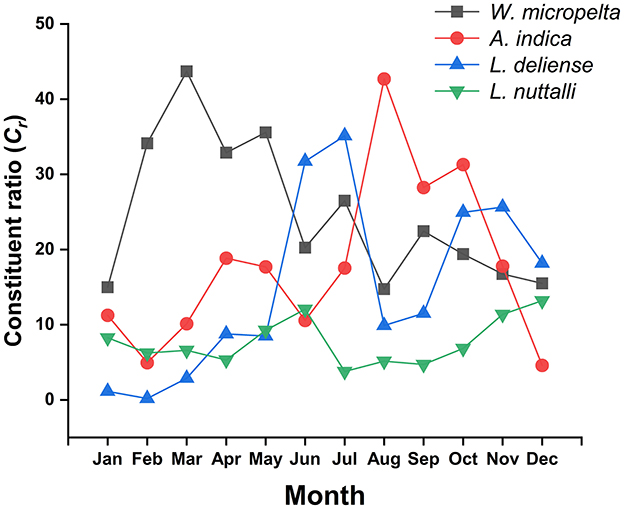
Figure 6. Monthly variations of constituent ratios (Cr) of four dominant mite species at Jingha survey site in southern Yunnan of southwest China (April 2016–March 2017).
3.7 Seasonal fluctuations of vector mite species
The three chigger (L. deliense, A. indica, L. scutellare) and one gamasid (L. echidninus) vector species varied significantly in monthly constituent ratios (Cr), with L. deliense and A. indica demonstrating the most substantial fluctuations. Leptotrombidium deliense showed bimodal peaks (summer primary peak: June-July; autumn secondary peak: October–November). Ascoschoengastia indica peaked in late summer (August: Cr = 42.69%, 5,556/13,016), while both L. scutellare and L. echidninus exhibited minor and indistinct peaks during December (Figure 7).
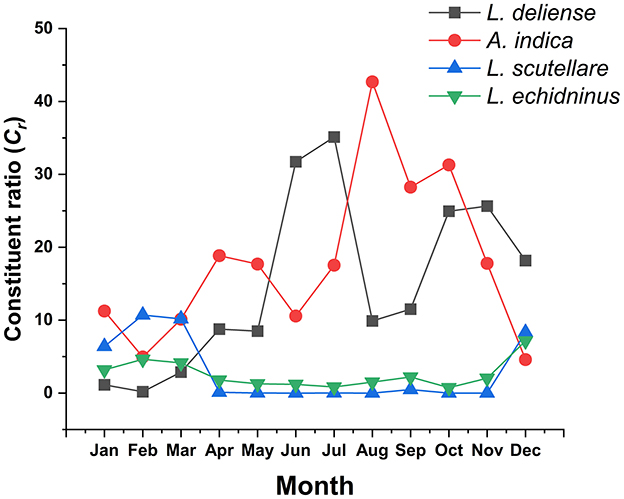
Figure 7. Monthly variations of constituent ratios (Cr) of four vector mite species at Jingha survey site in southern Yunnan of southwest China (April 2016–March 2017).
3.8 Host sex and age bias in mite infestation
Host sexes and ages significantly influenced mite infestation patterns. Of the 2,424 hosts, five lacked sex data and 95 lacked age records, which were not included in the analysis of the present study. After the exclusion, the analysis included 2,419 hosts with sex records (1,253 female; 1,166 male) and 2,329 hosts with age records (1,951 adult; 378 juvenile). Female hosts exhibited significantly higher infestation indices than males (PM: 96.01% vs. 94.08%, χ2 = 4.80, P < 0.05; MA: 63.01 vs. 54.46, Z = −4.23, P < 0.001; MI: 65.63 vs. 57.88, Z = −3.68, P < 0.001; Table 8, Figure 8).
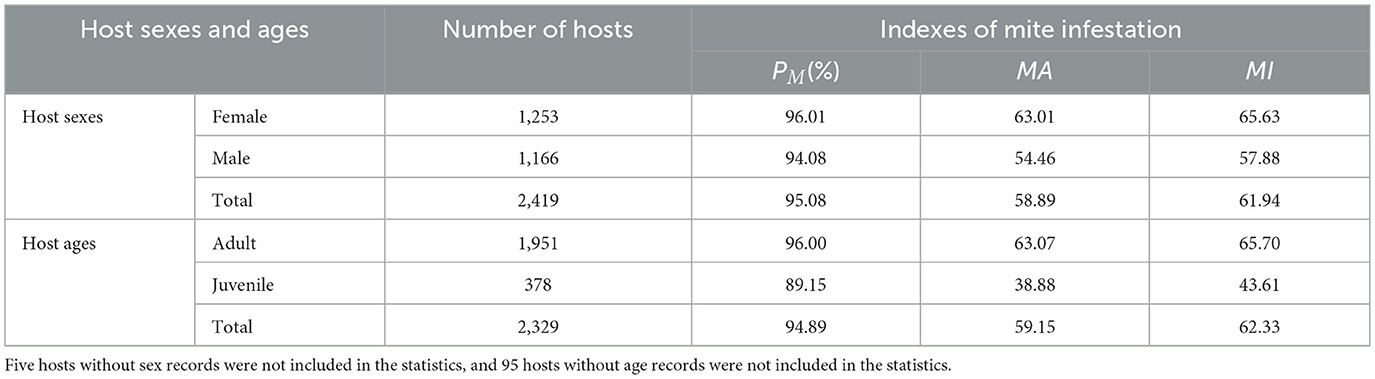
Table 8. Infestation indices of mites by sex and age of hosts at the Jingha survey site, southern Yunnan, China (April 2016–March 2017).
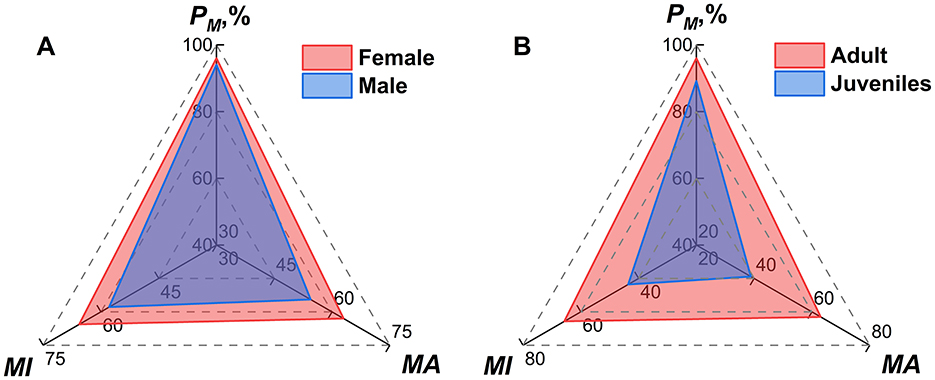
Figure 8. Radar chart visualization of mite infestation indexes on different sexes and ages of small mammal hosts at Jingha survey site in southern Yunnan of southwest China (April 2016–March 2017).
Adult hosts showed higher infestation indices of mites than juveniles (PM: 96.00% vs. 89.15%, χ2 = 30.63, P < 0.001; MA: 63.07 vs. 38.88, Z = −7.71, P < 0.001; MI: 65.70 vs. 43.61, Z = −6.21, P < 0.001). All differences were statistically significant (P < 0.05; Figure 8).
3.9 Climatic drivers for the seasonal dynamics of mites
All parametric and smooth items were statistical significance (P < 0.001) in generalized additive models (GAMs) for the abundance of four dominant mite species: W. micropelta, A. indica, L. deliense, and L. nuttall. These outcomes demonstrating the substantial driving effect of climatic factors (monthly mean temperature, relative humidity, and precipitation) on mite abundance. The climatic driving effect on the seasonal dynamics of mites differed significantly depending on mite species. Leptotrombidium deliense exhibited the strongest climatic dependency with 30.6% of deviation interpretation rate (Table 9) and 73.4% of total accumulative contribution rate of climatic factors (28.1% of temperature contribution rate + 45.3% of precipitation contribution rate). Laelap nuttalli displayed minimal climatic responsiveness (6.47% of deviation interpretation rate).
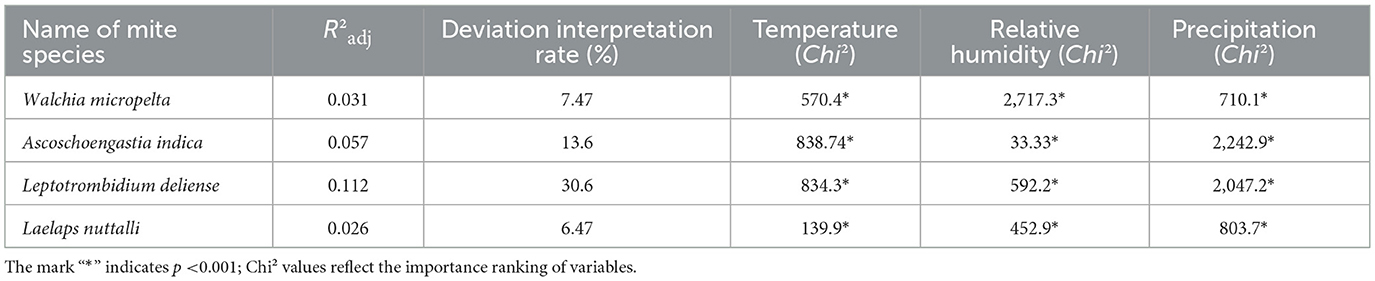
Table 9. Analysis of non-linear relationships between the abundance of four dominant mite species and climate factors by using poisson-based generalized additive models (GAMs) at Jingha survey site in southern Yunnan of southwest China (April 2016–March 2017).
Kruskal-Wallis tests confirmed the temporal niche differentiation of four dominant mite species (W. micropelta, A. indica, L. deliense, and L. nuttalli) at Jingha site (χ2 = 38.70, P < 0.001), with four types of climatic responses as follows (Figure 9).
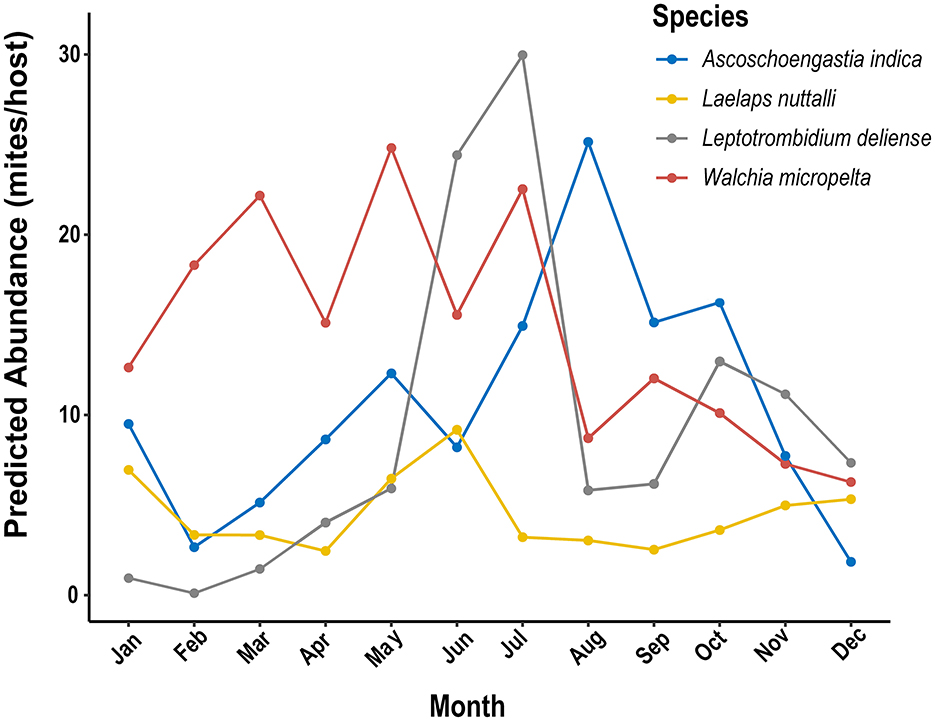
Figure 9. Monthly dynamics of predicted abundance of four dominant mite species driven by climate factors (temperature, relative humidity and precipitation) by using generalized additive models (GAMs) at Jingha survey site in southern Yunnan of southwest China (April 2016–March 2017).
(1) Multimodal temperature-response type: The abundance of W. micropelta peaked in March (22.2 mites/host; 95% CI: 21.5–22.8), May (24.8 mites/host; 95% CI: 24.1–25.5) and July (22.5 mites/host; 95% CI: 21.8–23.2).
(2) Precipitation-lagged response type: the abundance of A. indica peaked in August (25.1 mites/host; 95% CI: 24.5–25.8), which was 1 month lagged behind the maximum precipitation at Jingha site in July. The confidence interval (95% CI) for the abundance of A. indica did not overlap with that of L. nuttalli (3.0 mites/host; 95% CI: 2.8–3.3), with an obvious temporal niche differentiation (P < 0.01).
(3) High temperature strategic type: the abundance of L. deliense showed an outbreak increase in summer with high temperature and relative humidity, and its highest peak appeared in July (30.0 mites/host; 95% CI: 29.2–30.8). Leptotrombidium deliense showed a high adaptability to high temperature and relative humidity with the thermal tolerance of 12 °C−32 °C (mean edf =3.66 ± 0.12).
(4) Broad climate-adaptive type: in 12 months of a year, L. nuttalli showed an inapparent seasonal fluctuation with two minor peaks of mite abundance in January (7.0 mites/host) and June (9.2 mites/host), and the confidence intervals was broad (Levene's test: F = 7.32, P = 0.002), indicating that L. nuttalli has a broad adaptability to different types of climates throughout the year.
GAMs further revealed the response heterogeneity of different mite species to climates. The abundance of A. indica showed an exponential growth with the increase of precipitation and reached 25.1 mites/host (95% CI: 24.5–25.8) when the precipitation was >150 mm/month, which was 7.4 times that in the dry season when the precipitation was <50 mm. Leptotrombidium deliense showed a strong adaptability to the high temperature and humidity in summer with the highest peak of mite abundance in July. Laelpas nuttalli, however, had an inapparent seasonal fluctuation of mite abundance, and it showed a strong tolerance to different types of climates all the year round (Figures 9, 10).
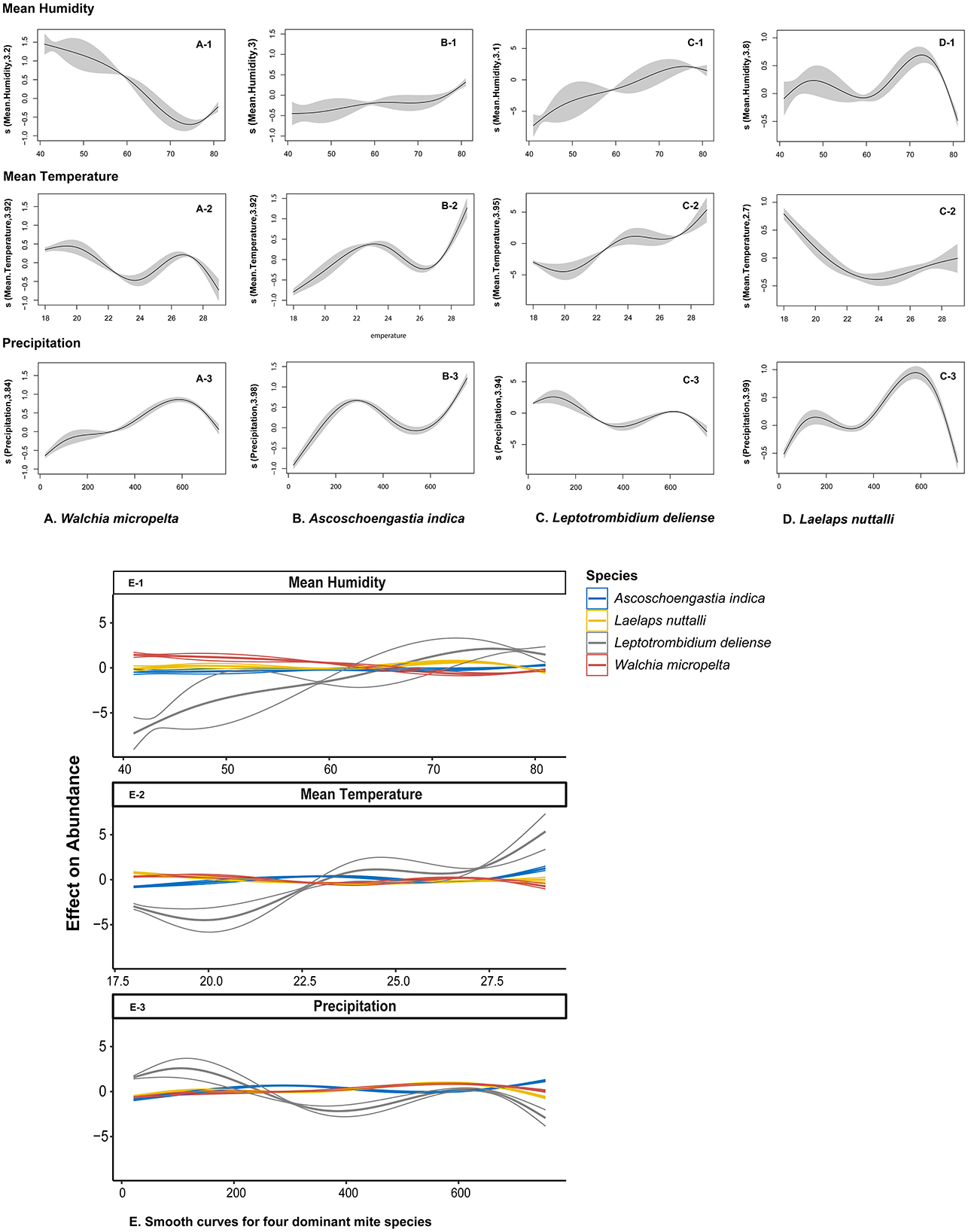
Figure 10. Non-linear effect of climate factors on mite abundance of four dominant mite species at Jingha survey site in southern Yunnan of southwest China (April 2016–March 2017). The curves were fitted with generalized additive models (GAMs), in which the shaded area represents 95% confidence intervals.
3.10 Mite-mite network analysis
The Pearson coefficient heatmap, indicating linear correlations between species pairs (mite-mite networks), comprises 18 main mite species (individuals >1,000, accounted for 72.87% of total mites, 103,821/142,471; Figure 11). Among the resulting 153 species-pairs, 32.68% of which (50/153) showed varying degrees of correlation with statistical significance (P < 0.05). Positive correlations mainly occurred within the same mite group (chigger–chigger or gamasid–gamasid mites), which accounted for 96.00% of all the correlated species-pairs (48/50). For example, a high positive correlation existed between two pairs of gamasid mites with correlation coefficients r = 0.94 for Dipolaelaps jiangkouensis vs. L. liui (P < 0.001) and r = 0.82 for L. turkestanicus vs. L. fukienensis (P < 0.001). The moderate and low positive correlations mainly occurred within chigger species (r: 0.40–0.59 for moderate correlations; r: 0.20–0.39 for low correlations) such as W. micropelta vs. W. turmalis (r = 0.57, P < 0.001), A. indica vs. A. yunnanensis (r = 0.32, P < 0.001), A. indica vs. W. turmalis (r = 0.31, P < 0.001), A. indica vs. L. gongshanense (r = 0.21, P < 0.001), and L. deliense vs. W. turmalis/W. micropelta/A. indica (r = 0.21 for all, P < 0.001). There was almost no correlation between chiggers and gamasid mites, as indicated by correlation coefficients (r) being very close to zero (0). For example, r = −0.02 (P < 0.05) for the chigger L. deliense and gamasid mite L. echidninus (Figure 11).
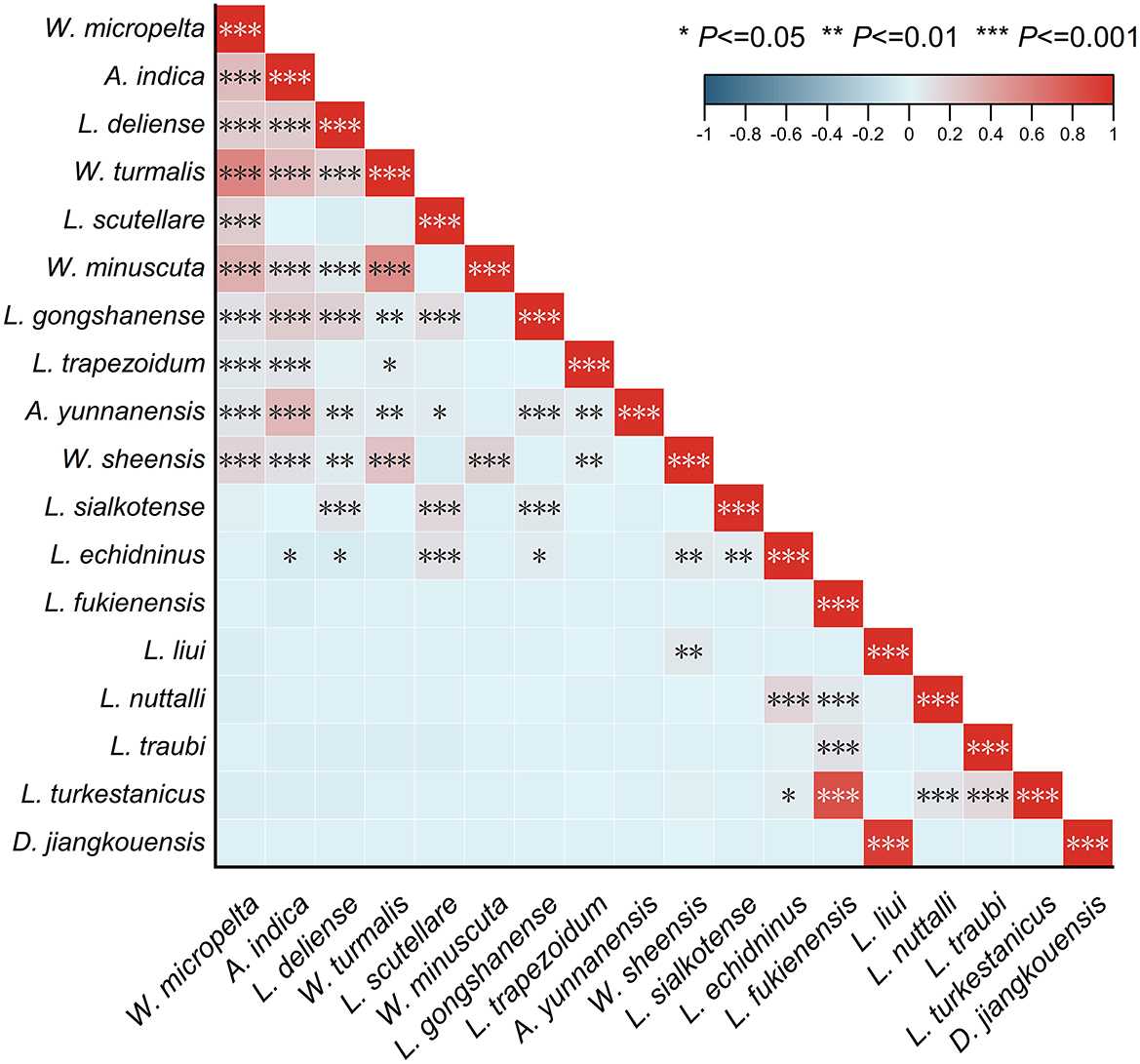
Figure 11. Heat map visualization for the relationship between any two of 18 main mite species at Jingha survey site in southern Yunnan of southwest China (April 2016–March 2017).
3.11 Host-mite network analysis
The analysis of bilateral relationships was conducted between the 18 main mite species and their corresponding small mammal hosts (host-mite networks), and the results was visualized with the parallel sets diagram (Figure 12). Of the 18 main mite species, W. micropelta (32,395), A. indica (24,490) and L. deliense (18,867) were the most abundant. The 18 main mite species parasitized 15 small mammal species, of which R. andamanensis was predominant (Cr = 84.69%, 2,053/2,424). The results showed that one host species could harbor up to 2–18 mite species and one mite species could parasitize up to 3–11 host species with low host specificity. For example, 6,669 of L. liui gamasid mites were found on 12 host species, 34,904 of W. micropelta chiggers appeared on 11 host species, and 4,375 of D. jiangkouensis gamasid mites occurred on 9 host species. The host specificity of the gamasid mite D. jiangkouensis, however, seemed relatively high, and 4,401 of D. jiangkouensis mites came from only three host species, B. bowersi, R. andamanensis, and R. tanezumi (Figure 12).
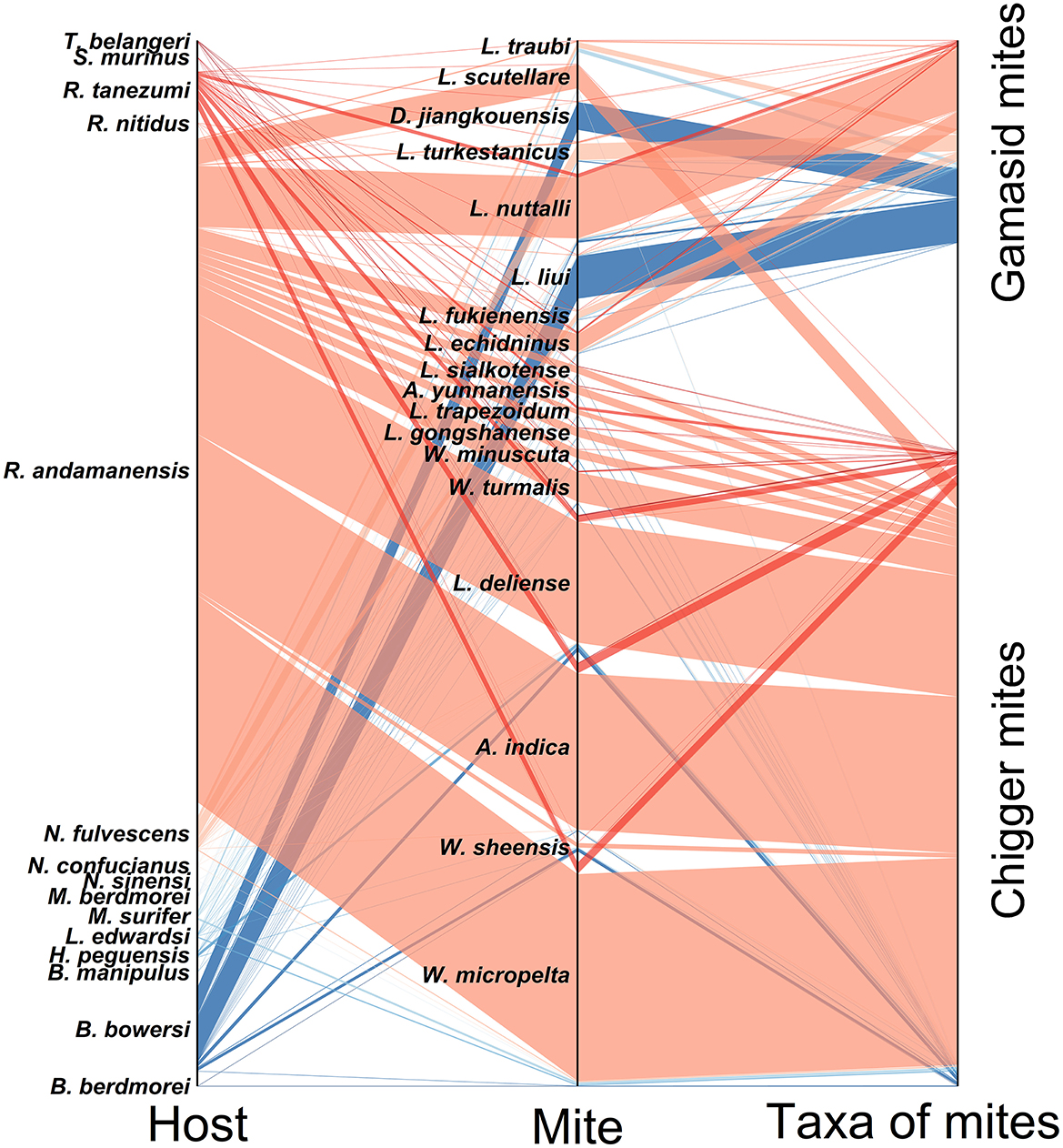
Figure 12. Parallel set map visualization for mutual relationships among main mite species and their small mammal hosts at Jingha survey site in southern Yunnan of southwest China (April 2016–March 2017).
4 Discussion
4.1 Mite infestation and characteristics of mite communities
Compares to previous reports (6, 8, 12, 13, 15–17, 22, 26–29), mite infestation in Jingha was relatively high, considering almost all small mammals were parasitized and the great numbers of mite individual and species collected. This pattern is comparative to other low-latitude and low-altitude regions in southern China, where warm and humid climates facilitate ectoparasites' survival and reproduction (48, 49). Previous studies in subtropical monsoon areas also reported high infestation level for chigger and gamasid mites (17, 22, 27). However, our results, especially for chiggers, are among the highest recorded (29, 34, 50). This may be due to both the favorable climate and the low host specificity. Compared with surveys in other parts of Yunnan, the chigger mite community in Jingha has higher species richness and yielded higher diversity metrics, while gamasid mite's diversity is comparatively lower. Rattus andamanensis is the primary host, supporting diverse mite communities and sustaining zoonotic disease cycles in similar habitats across Southeast Asia (50, 51). The dominance of chiggers over gamasid mites in both number and infestation indices implies their main role in disease transmission in this area.
4.2 Dominant and vector mite species
Several confirmed and potential vector species were found in Jingha, including L. deliense, A. indica, L. scutellare, and L. echidninus (Table 2). Importantly, A. indica and L. deliense were both dominant mites and key vectors (Tables 5, 10). Similar groups of species have been linked to persistent foci of scrub typhus and HFRS in Guangxi, Hainan, and northern Vietnam (52–55), highlighting the need for extra attention. Leptotrombidium deliense and A. indica are both prevalent and epidemiologically important. Their overlapping activity periods may increase the risk of pathogen spillover. Leptotrombidium scutellare, although less common in Jingha, is an important vector in cooler areas from central China to the north. Its presence in Jingha suggests a broader environmental tolerance than previously recognized. Laelaps echidninus is a widely distributed gamasid mite and has been linked to HFRS in several provinces, supporting the view that gamasid mites act as secondary but persistent vectors (17, 54, 55). The combination of high species diversity, low host specificity, and vector dominance in Jingha may raise the long-term risk of zoonotic disease transmission, necessitating increased surveillance efforts.
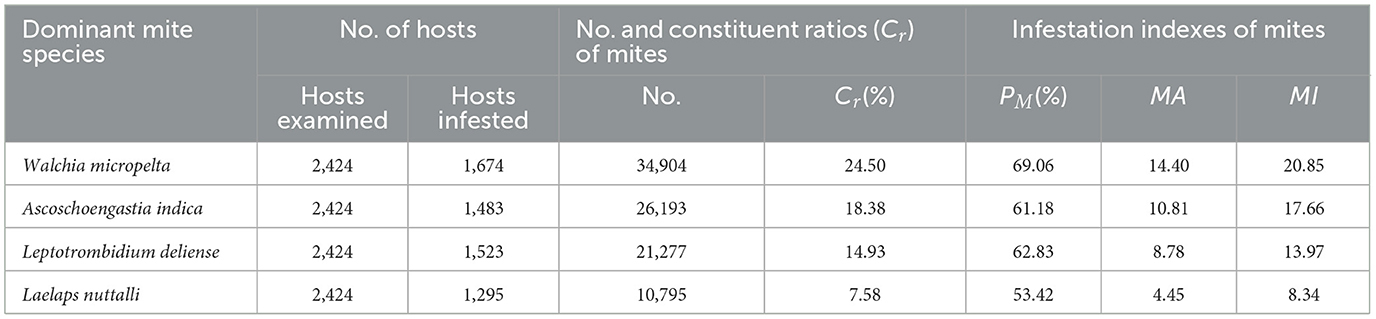
Table 10. Infestation indexes of four dominant mite species at Jingha survey site in southern Yunnan of southwest China (April 2016–March 2017).
4.3 Seasonal dynamics and driving factors
Previous studies have posited that the seasonal fluctuation patterns of chiggers and gamasid mites vary with mite species and geographical regions, i.e., different mite species exhibit different seasonal fluctuation patterns peaked in different seasons (12, 27). Moreover, the same mite species may show geographical variations in seasonal trends and peak timing (27, 35, 55). The seasonal changes of dominant mite species in Jingha show a mix of climate-specialist and climate-generalist patterns. This agrees with reports from other subtropical areas (7, 12, 22, 56).
Leptotrombidium deliense dually peaks in summer and autumn, similar to those reported in Guangxi, Guangdong, Taiwan and northern Thailand, which may attribute to that high temperature and humidity increase reproduction (57–62). Ascoschoengastia indica peaked in late summer in August, conforming to the “summer-autumn” pattern. This species displayed “rainfall-lag response” characteristics with precipitation being its primary climatic driver. Walchia micropelta peaked in early spring, which is different from the summer–autumn peaks of most other vector mites. This separation of peak activity may reduce competition between species and help stabilize populations (63, 64). The ecology and vector role of W. micropelta are not well studied, and further research is needed. Laelaps nuttalli and L. echidninus had stable populations throughout the year, reflecting broad climate tolerance. This stability allows them to act as constant pathogen reservoirs, complementing the seasonal risk from chiggers (8, 65). The observed patterns indicate that preventive practice for scrub typhus and HFRS in southern Yunnan should concentrate on summer and autumn, the peak activity periods of vectors, yet remain ongoing throughout the year due to the resilience of climate-tolerant gamasid mites.
4.4 Host sex and age biases in mite infestation
In this study, the mite infestation indices (PM, MA, MI) were significantly different on different sexes and ages of small mammal hosts, indicating the sex and age biases of hosts to mite infestation. Female hosts showed significantly higher infestation indices than males. Adult hosts also demonstrated markedly higher infestation levels than juveniles. Previous studies report that host sexes and ages commonly influence susceptibility to both internal and external parasites. Some research indicates that males exhibit higher parasite vulnerability than females, potentially due to males' high androgen levels, energy-intensive mating behaviors, and male-male competition (66–68), despite that greater female susceptibility to parasite infections were also reported (69–71). The significantly higher infestation in adults aligns with previous studies. This age bias pattern likely arises from adults' larger body surface area available for parasites and their expanded activity ranges during foraging and mating, increasing exposure opportunities (28, 33, 72, 73).
4.5 Mite-mite and host-mite relationships
Regarding host selection, different degrees of positive/negative correlations existed among 18 dominant mite species. Positive correlations predominantly occurred within gamasid or chigger subcommunities. The correlations within chigger mites were frequent but weak, while the correlations within gamasid mites were comparatively stronger (r > 0.60). This indicates coexisting tendencies within mite groups when colonizing hosts, aligning with prior reports on these taxa (28, 74–76). Among the 153 species pairs, only two showed negative correlations, while correlations between chiggers and gamasid mites were extremely weak. This suggests that chiggers and gamasid mites independently choose their hosts, showing neither mutual attraction nor repulsion.
The host-mite relationships demonstrate the low host specificity of mites. A certain host species can harbor multiple mite species, and a single mite species can parasitize diverse hosts. The broad host range enables mites to circulate zoonotic pathogens (e.g., Orientia tsutsugamushi for scrub typhus, hantavirus for HFRS) across different animal hosts. Consequently, it may increase the potential risk of persistently maintaining the endemic foci of zoonotic diseases.
5 Conclusion
The ectoparasitic mite community on small mammals in Jingha on the bordering zone of China and Myanmar comprises diverse gamasid and chigger mites with high species diversity, particularly pronounced in chigger mites. Both chiggers and gamasid mites have a wide range of small mammal hosts with low host specificity, and the rat R. andamanensis (Rodentia: Muridae) is the primary host species. Chigger mites exhibit significantly higher infestation intensity than gamasid mites. Adult hosts show greater infestation than juveniles. The dominant mite species include W. micropelta, A. indica, L. deliense, and L. nuttalli. Multiple vector mite species coexist in Jingha and the four main vector species include three chigger mites (L. deliense, A. indica, L. scutellare) and one gamasid mite (L. echidninus). Three dominant vectors (W. micropelta, A. indica, L. deliense) display markedly seasonal variations. Climatic factors (temperature, humidity, rainfall) drive the seasonal dynamics of mites. Abundance of L. deliense, one of the primary scrub typhus vectors in China, peaks in July of summer, with temperature being its main driver. Ascoschoengastia indica, a potential vector of scrub typhus, has 1-month lag in response to peak rainfall in July, and the precipitation mainly drives its seasonal dynamics. The summer-autumn seasonal pattern of main vector mites suggests that the surveillance of scrub typhus and HFRS in southern Yunnan should be prioritized in summer and autumn.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
P-WY: Data curation, Formal analysis, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. YL: Conceptualization, Formal analysis, Validation, Writing – review & editing, Writing – original draft. X-GG: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing, Writing – original draft. W-YS: Methodology, Validation, Writing – review & editing. RF: Data curation, Investigation, Writing – review & editing. C-FZ: Data curation, Investigation, Writing – review & editing. Z-WZ: Data curation, Investigation, Writing – review & editing. Y-FZ: Data curation, Investigation, Writing – review & editing. W-GD: Resources, Validation, Writing – review & editing. D-CJ: Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was supported by the National Natural Science Foundation of China (Nos. 82160400 and 81960380) to Xian-Guo Guo.
Acknowledgments
We would express our sincere thanks in the field investigation and laboratory work: Yun-Ji Zou, Sheng-Zhen Li, He Sha, Long Zhou, some colleagues and college students in Dali University. We would also express our special thanks to the above financial supports.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wei FW, Yang QS, Wu Y, Jiang XL, Liu SY, Hu YB, et al. Catalogue of mammals in China (2024). Acta Theriologica Sinica. (2025) 45:1–16. doi: 10.16829/j.slxb.151039
2. Oguge NO, Durden LA, Keirans JE, Balami HD, Schwan T. Ectoparasites (sucking lice, fleas and ticks) of small mammals in southeastern Kenya. Med Vet Entomol. (2009) 23:387–92. doi: 10.1111/j.1365-2915.2009.00820.x
3. Eslami A, Yousefi A, Dowling AP. Prevalence of ectoparasites in black rat (Rattus rattus) from mangrove forests of Qeshm Island, Iran. Comp Clin Path. (2018) 27:1583–6. doi: 10.1007/s00580-018-2777-3
4. Wei YH, Huang Y, Li XN, Ma Y, Tao X, Wu XW, et al. Climate variability, animal reservoir and transmission of scrub typhus in southern China. PLoS Negl Trop Dis. (2017) 11:e0005447. doi: 10.1371/journal.pntd.0005447
5. Meng FF, Xu Q, Chen JJ, Ji Y, Zhang WH, Fan ZW, et al. A dataset of distribution and diversity of blood-sucking mites in China. Sci Data. (2021) 8:204. doi: 10.1038/s41597-021-00994-9
6. Peng PY, Guo XG, Song WY, Hou P, Zou YJ, Fan R. Ectoparasitic chigger mites on large oriental vole (Eothenomys miletus) across southwest, China. Parasitol Res. (2016) 115:623–32. doi: 10.1007/s00436-015-4780-9
7. Lv Y, Guo XG, Jin DC. Research Progress on Leptotrombidium deliense. Korean J Parasitol. (2018) 56:313–24. doi: 10.3347/kjp.2018.56.4.313
8. Zhou JX, Guo XG, Song WY, Zhao CF, Zhang ZW, Fan R, et al. Preliminary study on species diversity and community characteristics of gamasid mites on small mammals in three parallel rivers area of China. Animals. (2022) 12:3217. doi: 10.3390/ani12223217
9. Wu DL, Shih HC, Wang JK, Teng HJ, Kuo CC. Commensal rodent habitat expansion enhances arthropod disease vectors on a tropical volcanic island. Front Vet Sci. (2021) 8:736216. doi: 10.3389/fvets.2021.736216
11. Ren TG, Guo XG, Jin DC, Wu D, Fletcher Q. A new species of chigger mite (Acari: Trombiculidae) from rodents in southwest China. Korean J Parasitol. (2014) 52:63. doi: 10.3347/kjp.2014.52.1.63
12. Lv Y, Guo XG, Jin DC, Song WY, Fan R, Zhao CF, et al. Host selection and seasonal fluctuation of Leptotrombidium deliense (Walch, 1922) (Trombidiformes: Trombiculidae) at a localized area of southern Yunnan, China. Syst Appl Acarol. (2019) 24:2253–71. doi: 10.11158/saa.24.11.15
13. Lv Y, Guo XG, Jin DC, Song WY, Fan R, Zhao CF, et al. Relative abundance of a vector of scrub typhus, Leptotrombidium sialkotense, in southern Yunnan Province, China. Korean J Parasitol. (2020) 58:153. doi: 10.3347/kjp.2020.58.2.153
14. Song WY, Lv Y, Yin PW, Yang YY, Guo XG. Potential distribution of Leptotrombidium scutellare in Yunnan and Sichuan Provinces, China, and its association with mite-borne disease transmission. Parasit Vectors. (2023) 16:164. doi: 10.1186/s13071-023-05789-y
15. Guo Y, Guo XG, Song WY, Lv Y, Yin PW, Jin DC. Comparison of chiggers (acari: trombiculidae, leeuwenhoekiidae) on two sibling mouse species, Apodemus draco and A. ilex (Rodentia: Muridae), in southwest China. Animals. (2023) 13:1480. doi: 10.3390/ani13091480
16. Peng PY, Guo XG, Jin DC, Dong WG, Qian TJ, Qin F, et al. Landscapes with different biodiversity influence distribution of small mammals and their ectoparasitic chigger mites: a comparative study from southwest China. PLoS ONE. (2018) 13:189987. doi: 10.1371/journal.pone.0189987
17. Liu QY, Fan R, Song WY, Peng PY, Zhao YF, Jin DC, et al. The distribution and host-association of the vector chigger species Leptotrombidium imphalum in southwest China. Insects. (2024) 15:504. doi: 10.3390/insects15070504
18. Mahapatra CS. Hantaviruses as Emergent Zoonoses: A Global Threat. Emerging Human Viral Diseases, Volume I. Respiratory and Haemorrhagic Fever: Springer, Singapore (2023). p. 377–400. doi: 10.1007/978-981-99-2820-0_15
19. Sehgal A, Mehta S, Sahay K, Martynova E, Rizvanov A, Baranwal M, et al. Hemorrhagic fever with renal syndrome in Asia: history, pathogenesis, diagnosis, treatment, and prevention. Viruses. (2023) 15:561. doi: 10.3390/v15020561
20. Zhou WF, Dong YH, Liu XQ, Ding S, Si HY, Yang C, et al. A bibliometric analysis of domestic and international research on hemorrhagic fever with renal syndrome over the past 2 decades. Medicine. (2024) 103:e39737. doi: 10.1097/MD.0000000000039737
21. Weitzel T, Silva-De La Fuente MC, Martínez-Valdebenito C, Stekolnikov AA, Pérez C, Pérez R, et al. Novel vector of scrub typhus in sub-Antarctic Chile: evidence from human exposure. Clin Infect Dis. (2022) 74:1862–5. doi: 10.1093/cid/ciab748
22. Lv Y, Guo XG, Jin DC, Song WY, Peng PY, Lin H, et al. Infestation and seasonal fluctuation of chigger mites on the Southeast Asian house rat (Rattus brunneusculus) in southern Yunnan Province, China. Int J Parasitol Parasites Wildl. (2021) 14:141–9. doi: 10.1016/j.ijppaw.2021.02.005
23. Costa C, Ferrari A, Binazzi R, Beltrame A, Tacconi D, Moro L, et al. Imported scrub typhus in Europe: report of three cases and a literature review. Travel Med Infect Dis. (2021) 42:102062. doi: 10.1016/j.tmaid.2021.102062
24. Lee HS, Kim SY, Lee HI. A novel strain of orientia tsutsugamushi detected from chiggers (acari: trombiculidae) on wild rodents. Pathogens. (2025) 14:29. doi: 10.3390/pathogens14010029
25. Montag A. Diseases caused by arthropods. Braun-Falco' s Dermatology. Springer: New York (2021). p. 1–45. doi: 10.1007/978-3-662-58713-3_23-1
26. Yin PW, Guo XG, Jin DC, Fan R, Zhao CF, Zhang ZW, et al. Distribution and host selection of tropical rat mite, Ornithonyssus bacoti, in Yunnan Province of Southwest China. Animals. (2021) 11:110. doi: 10.3390/ani11010110
27. Yin PW, Guo XG, Jin DC, Song WY, Zhang L, Zhao CF, et al. Infestation and seasonal fluctuation of gamasid mites (parasitiformes: gamasida) on indochinese forest rat, Rattus andamanensis (rodentia: muridae) in Southern Yunnan of China. Biology. (2021) 10:1297. doi: 10.3390/biology10121297
28. Ding F, Guo XG, Song WY, Fan R, Zhao CF, Mao KY, et al. Infestation and distribution of chigger mites on Brown rat (Rattus norvegicus) in Yunnan Province, Southwest China. Trop Biomed. (2021) 38:111–21. doi: 10.47665/tb.38.1.020
29. Guo XG, Dong WG, Men XY, Qian TJ, Wu D, Ren TG, et al. Species abundance distribution of ectoparasites on norway rats (Rattus norvegicus) from a localized area in Southwest China. J Arthropod Borne Dis. (2016) 10:192–200. Available online at: http://jad.tums.ac.ir
30. Griffiths J, Yeo HL, Yap G, Mailepessov D, Johansson P, Low HT, et al. Survey of rodent-borne pathogens in Singapore reveals the circulation of Leptospira spp, Seoul hantavirus, and Rickettsia typhi. Sci Reports. (2022) 12:2692. doi: 10.1038/s41598-021-03954-w
31. Swe MMM, Phyo AP, Cooper BS, White NJ, Smithuis F, Ashley EA, et al. A systematic review of neglected tropical diseases (NTDs) in Myanmar. PLoS Negl Trop Dis. (2023) 17:e0011706. doi: 10.1371/journal.pntd.0011706
32. Liu RJ, Guo XG, Zhao CF, Zhao YF, Peng PY, Jin DC. An ecological survey of chiggers (acariformes: trombiculidae) associated with small mammals in an epidemic focus of scrub typhus on the China–Myanmar border in Southwest China. Insects. (2024) 15:812. doi: 10.3390/insects15100812
33. Liu QY, Guo XG, Fan R, Song WY, Peng PY, Zhao YF, et al. A retrospective report on the infestation and distribution of chiggers on an endemic rodent species (Apodemus latronum) in Southwest China. Vet Sci. (2024) 11:547. doi: 10.3390/vetsci11110547
34. Liu RJ, Guo XG, Peng PY, Lv Y, Yin PW, Song WY, et al. Mite infestation on Rattus tanezum rats in southwest China concerning risk models. Front Vet Sci. (2025) 12:1519188. doi: 10.3389/fvets.2025.1519188
35. Alkathiry H, Al-Rofaai A, Ya'cob Z, Cutmore TS, Mohd-Azami SNI, Husin NA, et al. Habitat and season drive chigger mite diversity and abundance on small mammals in Peninsular Malaysia. Pathogens. (2022) 11:1087. doi: 10.3390/pathogens11101087
36. Sun K, Qiao Y, Qin B. A green pearl of the southwestern frontier of China—the topography, climate, plant species and collection of Xishuangbanna. Biol Teach. (2000) 25:37–8.
37. Yu Y, Meng GY, Zhang CL. Characteristics of climate change in Xishuangbanna area in the past 45 years. Meteorol Sci. (2008) 36:410–3.
38. Yin PW, Guo XG, Song WY, Dong WG, Lv Y, Jin DC. The on-site monitoring and specimen-making of ectoparasites on rodents and other small mammals. Bio-protocol. (2024) 14:e5104. doi: 10.21769/BioProtoc.5104
39. Musser G, Carleton M, Wilson D, Reeder D. Mammal Species of the World: A Taxonomic and Geographic Reference. The Johns Edited by Wilson, DE And Reeder DM Baltimore (2005). p. 894–1531.
40. Kia E, Moghddas-Sani H, Hassanpoor H, Vatandoost H, Zahabiun F, Akhavan A, et al. Ectoparasites of rodents captured in Bandar Abbas, southern Iran. Iran J Arthropod Borne Dis. (2009) 3:44.
41. Wang YX. A Complete Checklist of Mammal Species and Subspecies in China: A Taxonomic and Geographic Reference. Beijing, China: China Forestry Publishing House (2003). p. 41–46. Available online at: www.tums.ac.ir
42. Stekolnikov AA. Leptotrombidium (acari: trombiculidae) of the world. (2013) 3728:1–173. doi: 10.11646/zootaxa.3728.1.1
43. Guo XG, Speakman JR, Dong WG, Men XY, Qian TJ, Wu D, et al. Ectoparasitic insects and mites on Yunnan red-backed voles (Eothenomys miletus) from a localized area in southwest China. Parasitol Res. (2013) 112:3543–9. doi: 10.1007/s00436-013-3537-6
44. Li JC, Wang DQ, Chen XB. Trombiculid Mites of China: Studies On Vector and Pathogen of Tsutsugamushi Disease. Guangzhou: Guangdong Science and Technology Publishing (1997).
45. Wood SN. Generalized Additive Models: An Introduction with R, 2 edition. Chapman and Hall/CRC (2017). doi: 10.1201/9781315370279
46. R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria (2021). Available online at: https://www.R-project.org/
48. Yu J, Li FT, Wang Y, Lin Y, Peng ZW, Cheng K. Spatiotemporal evolution of tropical forest degradation and its impact on ecological sensitivity: a case study in Jinghong, Xishuangbanna, China. Sci Total Environ. (2020) 727:138678. doi: 10.1016/j.scitotenv.2020.138678
49. Xiong Q, Sun ZY, Cui W, Lei JZ, Fu XX, Wu L, et al. A study on sensitivities of tropical forest GPP responding to the characteristics of drought—a case study in Xishuangbanna, China. Water. (2022) 14:157. doi: 10.3390/w14020157
50. Wang T, Meng FF, Che TL, Chen JJ, Zhang HY, Ji Y, et al. Mapping the distributions of blood-sucking mites and mite-borne agents in China: a modeling study. Infect Dis Poverty. (2022) 11:41. doi: 10.1186/s40249-022-00966-0
51. Zhou Y, Duan B, Ren TG, Dong WG. Investigation of chigger mites on small mammals in Ruili, Yunnan Province, China. Trop Biomed. (2022) 39:455–61. doi: 10.47665/tb.39.3.017
52. Herrera-Mares A, Guzmán-Cornejo C, Ulloa-García A, Córdoba-Aguilar A. Silva-de la Fuente MC, Suzán G. Mites, rodents, and pathogens: a global review for a multi-species interaction in disease ecology. Acta Tropica. (2022) 232:106509. doi: 10.1016/j.actatropica.2022.106509
53. Liu CX, Guo XG, Lv Y, Yin PW, Song WY, Peng PY, et al. Abundance and infestation of mites on bower's white-toothed rat (Berylmys bowersi) in Southwest China. Vet Sci. (2025) 12:426. doi: 10.3390/vetsci12050426
54. Guo Y, Guo XG, Peng PY, Lv Y, Xiang R, Song WY, et al. Infestation and distribution of chiggers on the Anderson's white-bellied rats in southwest China. Vet Med Sci. (2023) 9:2920–6. doi: 10.1002/vms3.1275
55. Xiang R, Guo XG. Research advances of Leptotrombidium scutellare in China. Korean J Parasitol. (2021) 59:1. doi: 10.3347/kjp.2021.59.1.1
56. Chaisiri K, Gill AC, Stekolnikov AA, Hinjoy S, McGarry JW, Darby AC, et al. Ecological and microbiological diversity of chigger mites, including vectors of scrub typhus, on small mammals across stratified habitats in Thailand. Anim Microbiome. (2019) 1:1–17. doi: 10.1186/s42523-019-0019-x
57. Tsai PJ, Yeh HC. Scrub typhus islands in the Taiwan area and the association between scrub typhus disease and forest land use and farmer population density: geographically weighted regression. BMC Infect Dis. (2013) 13:1–16. doi: 10.1186/1471-2334-13-191
58. Li ZJ, Xin HL, Sun JL, Lai SJ, Zeng LJ, Zheng CJ, et al. Epidemiologic changes of scrub typhus in China, 1952–2016. Emerg Infect Dis. (2020) 26:1091. doi: 10.3201/eid2606.191168
59. Ni ZS, Li SF, Xi R, Liang KM, Song SH, Cheng CL, et al. Meteorological factors and normalized difference vegetation index drivers of scrub typhus incidence in Shandong Province based on a 16-year time-frequency analysis. BMC Public Health. (2025) 25:752. doi: 10.1186/s12889-025-21987-y
60. Han L, Sun ZB, Li ZM, Zhang YF, Tong SL, Qin T. Impacts of meteorological factors on the risk of scrub typhus in China, from 2006 to 2020: a multicenter retrospective study. Front Microbiol. (2023) 14:1118001. doi: 10.3389/fmicb.2023.1118001
61. Ma T, Hao MM, Chen S, Ding FY. The current and future risk of spread of Leptotrombidium deliense and Leptotrombidium scutellare in mainland China. Sci Total Environ. (2022) 843:156986. doi: 10.1016/j.scitotenv.2022.156986
62. Peng PY, Duan HY, Xu L, Zhang LT, Sun JQ, Zu Y, et al. Epidemiologic changes of a longitudinal surveillance study spanning 51 years of scrub typhus in mainland China. Sci Rep. (2024) 14:3138. doi: 10.1038/s41598-024-53800-y
63. Ponti R, Sannolo M. The importance of including phenology when modelling species ecological niche. Ecography. (2023) 2023:e06143. doi: 10.1111/ecog.06143
64. Brooks GC, Caruso NM, Chandler HC, Haas CA. Niche partitioning and the storage effect facilitate coexistence in an amphibian community. Ecology Evolution. (2023) 13:e10629. doi: 10.1002/ece3.10629
65. Chen YL, Guo XG, Ren TG, Zhang L, Fan R, Zhao CF, et al. A report of chigger mites on the striped field mouse, Apodemus agrarius, in Southwest China. Korean J Parasitol. (2021) 59:625. doi: 10.3347/kjp.2021.59.6.625
66. Khamesipour F, Taktaz-Hafshejani T, Tebit KE, Razavi SM, Hosseini SR. Prevalence of endo-and ecto-parasites of equines in Iran: a systematic review. Vet Med Sci. (2021) 7:25–34. doi: 10.1002/vms3.321
67. Hämäläinen A, Raharivololona B, Ravoniarimbinina P, Kraus C. Host sex and age influence endoparasite burdens in the gray mouse lemur. Front Zool. (2015) 12:1–14. doi: 10.1186/s12983-015-0118-9
68. Patterson JE, Neuhaus P, Kutz SJ, Ruckstuhl KE. Patterns of ectoparasitism in North American red squirrels (Tamiasciurus hudsonicus): sex-biases, seasonality, age, and effects on male body condition. Int J Parasitol Parasites Wildl. (2015) 4:301–6. doi: 10.1016/j.ijppaw.2015.05.002
69. Reedy AM, Cox CL, Chung AK, Evans WJ, Cox RM. Both sexes suffer increased parasitism and reduced energy storage as costs of reproduction in the brown anole, Anolis sagrei. Biol J Linn Soc. (2016) 117:516–27. doi: 10.1111/bij.12685
70. Stephenson JF. Parasite-induced plasticity in host social behaviour depends on sex and susceptibility. Biol Lett. (2019) 15:20190557. doi: 10.1098/rsbl.2019.0557
71. Habig B, Doellman MM, Woods K, Olansen J, Archie EA. Social status and parasitism in male and female vertebrates: a meta-analysis. Sci Rep. (2018) 8:3629. doi: 10.1038/s41598-018-21994-7
72. Garrido M, Adler VH, Pnini M, Abramsky Z, Krasnov BR, Gutman R, et al. Time budget, oxygen consumption and body mass responses to parasites in juvenile and adult wild rodents. Parasit Vectors. (2016) 9:1–14. doi: 10.1186/s13071-016-1407-7
73. Xiang R, Guo XG, Ren TG, Zhao CF, Fan R, Zhang ZW, et al. Infestation and distribution of mites on the Yunnan red-backed vole (Eothenomys miletus) in Yunnan Province of southwest China between 2001 and 2015. Biologia. (2022) 77:61–8. doi: 10.1007/s11756-021-00884-w
74. Li B, Guo XG, Ren TG, Peng PY, Song WY, Lv Y, et al. Analysis on infestation and related ecology of chigger mites on large Chinese voles (Eothenomys miletus) in five provincial regions of Southwest China. Int J Parasitol Parasites Wildl. (2022) 19:169–79. doi: 10.1016/j.ijppaw.2022.08.013
75. López-Pérez AM, Pesapane R, Clifford DL, Backus L, Foley P, Voll A, et al. Host species and environment drivers of ectoparasite community of rodents in a Mojave desert wetlands. PLoS ONE. (2022) 17:e0269160. doi: 10.1371/journal.pone.0269160
Keywords: chigger mites, gamasid mites, ectoparasites, community ecology, small mammals, seasonal dynamics, generalized additive model (GAM)
Citation: Yin P-W, Lv Y, Guo X-G, Song W-Y, Fan R, Zhao C-F, Zhang Z-W, Zhao Y-F, Dong W-G and Jin D-C (2025) Infestation, community structure, seasonal fluctuation and climate-driven dynamics of mites on small mammals at a focus of scrub typhus in southwest China. Front. Vet. Sci. 12:1669217. doi: 10.3389/fvets.2025.1669217
Received: 19 July 2025; Accepted: 17 September 2025;
Published: 08 October 2025.
Edited by:
Emmanuel Serrano Ferron, Autonomous University of Barcelona, SpainReviewed by:
Santiago Sanchez-Vicente, Columbia University, United StatesVictor Lizana, Universidad CEU Cardenal Herrera, Spain
Copyright © 2025 Yin, Lv, Guo, Song, Fan, Zhao, Zhang, Zhao, Dong and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xian-Guo Guo, eGdndW8yMDAyQDE2My5jb20=
†These authors have contributed equally to this work
 Peng-Wu Yin
Peng-Wu Yin Yan Lv1†
Yan Lv1† Xian-Guo Guo
Xian-Guo Guo Wen-Yu Song
Wen-Yu Song