- 1Key Laboratory of Animal Disease-Resistant Nutrition, Ministry of Education, Animal Nutrition Institute, Sichuan Agricultural University, Chengdu, China
- 2Departments of Traditional Chinese Medicine, Sichuan Academy of Medical Sciences, Provincial People’s Hospital, Chengdu, China
- 3Chenguang Biological Technology Group Co, Ltd., Handan, China
- 4Hebei Natural Pigment Technology Innovation Center, Handan, China
Isochlorogenic acid (ICGA), a phenolic compound with demonstrated antioxidant, antibacterial, and anti-inflammatory properties, is widely present in plants. This study investigated the effects of dietary ICGA supplementation on growth performance, diarrhea incidence, antioxidant status, immune function, and intestinal microbiota in weaned piglets. A total of 180 crossbred piglets (Duroc × Landrace × Yorkshire) with an average initial body weight of 6.77 ± 0.18 kg were randomly allocated to five dietary treatments based on gender and weight. The diets consisted of a basal formulation supplemented with 0 (CON), 100, 200, 400, or 800 mg/kg ICGA for 28 days. Each treatment comprised six replicates, with six piglets per pen. Supplementation with 200 mg/kg ICGA significantly increased the average daily gain (ADG) by 3.49% during days 15–28 compared to the CON group (p < 0.05). Furthermore, diets containing 200 and 400 mg/kg ICGA improved the apparent total tract digestibility (ATTD) of dry matter (by 1.84 and 1.54%), crude protein (by 4.48 and 4.39%), gross energy (by 3.01 and 2.99%), ether extract (by 23.18 and 17.49%), and ash (by 8.80 and 5.13%) (p < 0.01). On day 14, serum catalase (CAT) activity increased by 47.78% in the 400 mg/kg group (p < 0.05), and this increase reached 77.65% by day 28 (p < 0.05). Meanwhile, the 200 mg/kg group exhibited a 75.78% elevation in total antioxidant capacity (T-AOC) on day 28 (p < 0.05). Serum immunoglobulin levels were also enhanced; 200 and 400 mg/kg ICGA up-regulated IgA by 23.77 and 33.42%, and IgM by 18.81 and 30.86% on day 14 (p < 0.01). Microbiota analysis indicated that ICGA supplementation increased the abundance of beneficial Bacteroidota and Prevotella, while reducing pathogenic taxa such as Peptostreptococcaceae, Proteobacteria, and Staphylococcus. In conclusion, dietary ICGA at 200–400 mg/kg effectively reduced diarrhea incidence, enhanced nutrient digestibility, improved antioxidant capacity, strengthened humoral immunity, and positively modulated gut microbiota in weaned piglets. Further research is warranted to elucidate the underlying mechanisms and assess the potential for practical application in swine production.
1 Introduction
Weaning is a critical challenge in pig production, as multiple stressors compromise piglet health and growth. Abrupt separation from the sow, dietary shift from milk to solid feed, and environmental alterations frequently trigger post-weaning syndrome, manifested as reduced feed intake, intestinal inflammation, oxidative stress, and heightened susceptibility to enteric infections. These disruptions lead to growth retardation, impaired nutrient utilization, and increased morbidity and mortality, causing substantial economic losses. Although antibiotic growth promoters have been widely employed to enhance growth and prevent disease, rising concerns regarding antimicrobial resistance have driven the pursuit of sustainable plant-based alternatives that support gastrointestinal health and immune function without relying on conventional antimicrobials.
Isochlorogenic acid (ICGA), a structural analog of chlorogenic acid (CGA), is a naturally occurring polyphenolic compound synthesized via the shikimic acid pathway during aerobic respiration in plants (1). The shikimic acid pathway, a key metabolic route in plants and microorganisms, produces aromatic amino acids and secondary metabolites. It initiates with the condensation of phosphoenolpyruvate and erythrose-4-phosphate, ultimately generating precursors for numerous phenolic compounds like ICGA. ICGA is found in a variety of plant species, including Ilex hainanensis, Atractylodes macrocephala, Stevia rebaudiana, and Lonicera periclymenum (2, 3). This compound exists as three distinct isomers, ICGA A, B, and C, which differ in their molecular structures (molecular formula: C25H24O12; molecular weight: 516.45) (4). In contrast to CGA, ICGA contains one molecule of quinic acid linked to two molecules of caffeic acid, making it structurally more complex by having an additional caffeic acid group (5, 6). Research has demonstrated that ICGA exhibits a broad spectrum of biological activities, including antioxidant, antibacterial, antiviral, anti-inflammatory, hepatoprotective, and neuroprotective effects, highlighting its therapeutic potential (5, 7–10). Additionally, ICGA is included as an active ingredient in several established pharmaceutical formulations, such as Siji-kangbingdu Mixture, Shuanghuanglian Granules, and Reduning Injection, underscoring its relevance in both traditional and modern medicinal applications (5). Given its diverse biological activities, ICGA presents a promising alternative to conventional antibiotic feed additives in the livestock and poultry industries, offering a potential strategy for promoting animal health while reducing reliance on antibiotics.
CGA has garnered significant attention as a promising alternative to antibiotics in livestock production, with growing evidence supporting its beneficial effects on animal health, especially in pigs, CGA is also known to alleviate intestinal damage, ameliorate oxidative stress, and regulate mitochondrial function (11, 12). However, despite their structural similarities, the biological and pharmacological properties of ICGA may differ from those of CGA due to variations in their chemical configurations. While the antimicrobial mechanisms of CGA, including bacterial injury repair, are well documented (13), few studies have examined ICGA’s effects in pigs. Recent research on other plant-derived supplements supports the potential of phytogenic additives. For example, Litsea cubeba essential oil enhanced growth performance, immunity, antioxidant status, nutrient digestibility, and fecal microflora in pigs (14). Similarly, rumen-protected lysine supplementation improved nitrogen utilization and modified hindgut microbiota in dairy cows (15), illustrating how targeted dietary interventions can modulate gut health. To address the lack of ICGA-specific studies, we evaluated the effects of dietary ICGA supplementation on growth performance, antioxidant status, immune function, and gut microbiota in weaned piglets. This study aims to elucidate ICGA’s potential as a novel feed additive for sustainable livestock production.
2 Materials and methods
2.1 Experimental animals, diet, design and housing
The ICGA used in this experiment was sodium isochlorogenic acid (Chenguang Biotechnology Group Co., Ltd., Hebei, China, batch number: 2-0969-200613), which was derived from stevia (Stevia rebaudiana) through a standardized extraction process. The active ingredient, ICGA content in this brownish-yellow powder was 51.3%. The animal care and experimental procedures were approved by the Institutional Animal Care and Use Committee of Sichuan Agricultural University (No. 20190129).
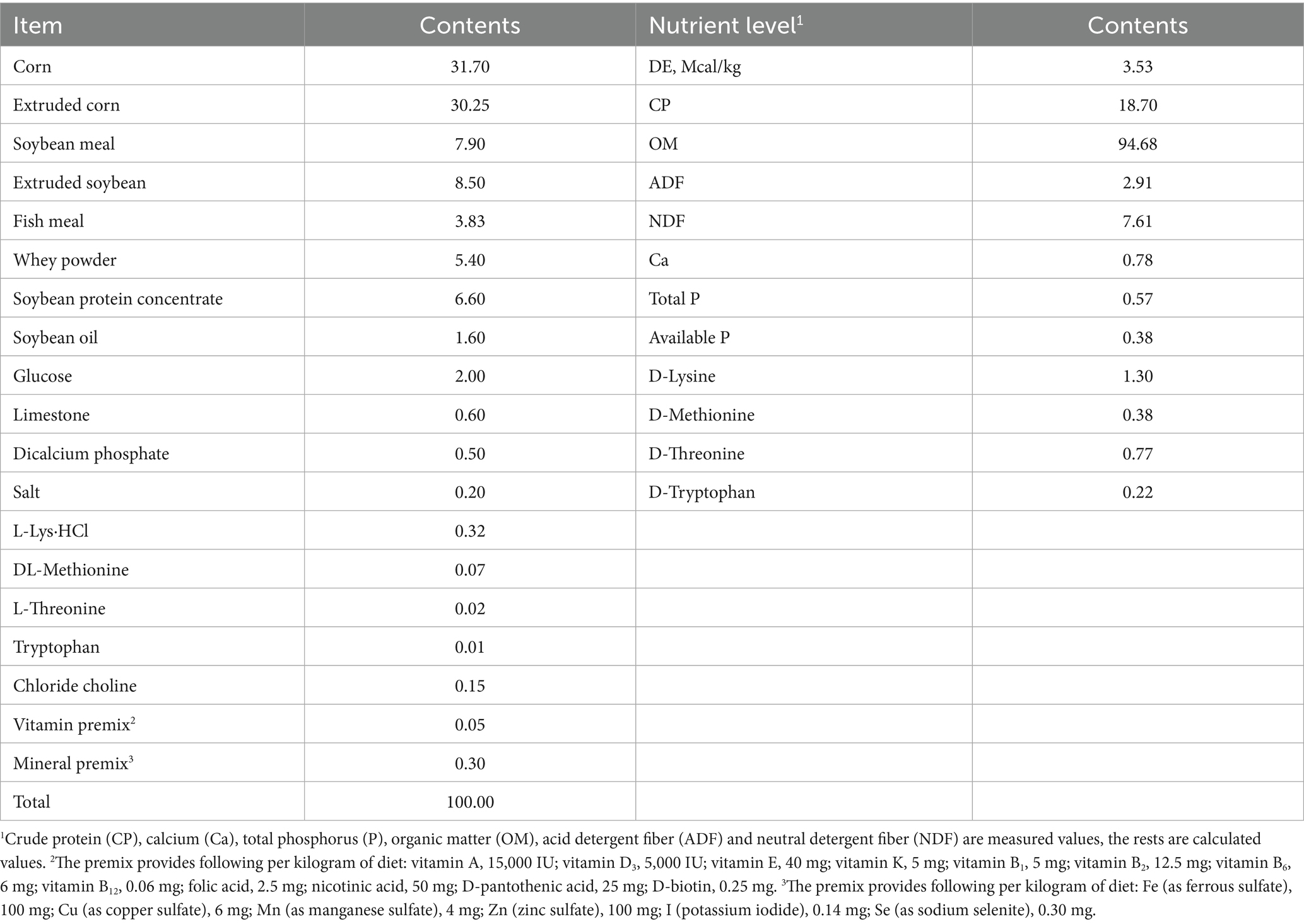
In this experiment, 180 weaned piglets (Duroc × Landrace × Yorkshire; 21 d of age; initial BW = 6.77 ± 0.18 kg) were allotted to 5 dietary treatments. Each treatment comprised 6 replicate pens with 6 piglets per pen (3 barrows and 3 gilts). The basal diet was supplemented with 0 (CON), 100, 200, 400, or 800 mg/kg of ICGA. We selected this dose range based on the efficacy of its structural analog, CGA, which improves growth performance and intestinal health in weaned pigs at 500–1000 mg/kg (16). The 100–800 mg/kg gradient was designed to identify the optimal and potentially lower effective dose of ICGA, considering its distinct bioavailability and efficacy. The 28-day experimental period encompassed the critical post-weaning recovery and growth phase. This duration allowed sufficient time for significant differences in average daily gain (ADG) and feed efficiency (G: F) to manifest and is consistent with established methodologies for assessing the medium-term effects of dietary additives in swine. The nutrient composition of the basal diet (Table 1) met or exceeded the dietary requirements for weaned piglets as outlined by the National Research Council (NRC, 2012).
The piglets were housed in standard flat-bed pens, each equipped with duckbill drinkers, adjustable feeders, and perforated plastic flooring. Throughout the experiment, the piglets had ad libitum access to both feed and water. The environmental conditions within the pens were carefully controlled, with a temperature range maintained between 25 to 28 °C and relative humidity held at 60 to 70%. To uphold optimal health and hygiene standards, the pens were thoroughly cleaned and disinfected daily, ensuring a safe and sterile environment for the animals throughout the study period.
2.2 Growth performance and diarrhea
The piglets were monitored daily to assess their health status throughout the experiment. Daily feed intake for each pen was recorded, and the average daily feed intake (ADFI) was calculated. The body weight of fasted piglets was measured on days 1, 15, and 29 of the trial to determine the average daily gain (ADG) and feed-to-gain ratio (F: G). To evaluate the incidence and severity of diarrhea, fecal consistency was scored on a 0 to 3 scale: 0 = normal, firm feces; 1 = soft feces, potential slight diarrhea; 2 = unformed, moderately fluid feces; and 3 = very watery, frothy diarrhea. Diarrhea was defined as a score of 2 or greater. The diarrhea rate and diarrhea index were calculated as follows: diarrhea rate (%) = (number of piglets with diarrhea per pen × days of diarrhea) / (total number of piglets × 28 days) × 100, and diarrhea index = sum of diarrhea scores per pen/(number of piglets per pen × total days) (17).
2.3 Sampling and measurements
Fecal samples were obtained on d 25 and 28 to capture any changes in nutrient utilization once piglets had adapted to their diets. Immediately after collection, each 100 g sample of fresh manure (from pens housing 6 piglets) was treated with 10 mL of 10% H2SO4 to reduce nitrogen volatilization. At the conclusion of the trial, all fecal samples from each pen were thoroughly mixed, dried at 65 °C for 96 h, and then finely ground through a 1 mm screen to ensure uniformity.
All feed and fecal samples were analyzed for dry matter (Method 930.15), crude protein (Method 990.03), ether extract (Method 920.39), and ash (Method 942.05), according to AOAC (2005). Gross energy was quantified using an adiabatic oxygen bomb calorimeter (Parr Instrument Co., Moline, IL, USA). The organic matter (OM) content in feeds and feces was calculated by subtracting the crude ash content from the dry matter (DM). Neutral detergent fiber (NDF) and acid detergent fiber (ADF) in feeds and feces was determined using Method 973.18 with an Ankom A200i fiber analyzer (Ankom Technology, Macedon, NY, USA). The analysis employed heat-stable α-amylase and sodium sulfite, with residual ash uncorrected. Acid-insoluble ash (AIA), a robust endogenous marker for nutrient digestibility, was determined using the procedure described by China Standards Press (2009). Apparent total digestibility (ATTD) of nutrients was then calculated based on (18), using the following equation:
Blood samples (10 mL) were collected on d 14 and d 28 from the anterior cava vein of one piglet per pen. The samples were drawn into vacuum tubes lacking anticoagulant and centrifuged at 3,500 × g for 10 min at 4 °C to separate the serum. Serum aliquots were immediately transferred into clean centrifuge tubes and stored at −20 °C for subsequent laboratory analyses. To further investigate local intestinal immune status, one piglet (per replicate pen) was chosen at the end of the trial and euthanized via an intravenous injection of chlorpromazine hydrochloride (3 mg/kg body weight). After opening the abdominal cavity, the jejunum, ileum, colon, and cecum were isolated according to standard anatomical markers. The contents of the ileum, colon, and cecum were aseptically gathered in sterile cryopreservation tubes for microbiological and biochemical evaluations. Additionally, approximately 10 cm of jejunum and ileum (the same segment from each pig) were dissected longitudinally, rinsed with 0.9% ice-cold saline to remove digesta, and gently scraped with a sterile microscope slide to collect the mucosal layer. Care was taken to use a new slide for each segment to reduce cross-contamination; all procedures were carried out on ice. Mucosal samples were then placed in sterile frozen storage tubes and preserved at −80 °C.
Serum antioxidant capacity was measured by determining T-AOC (catalog No. A015-1-2), CAT activity (catalog No. A007-1-1), SOD activity (catalog No. A001-1-2), GSH-Px activity (catalog No. A005-1-2), and MDA concentration (catalog No. A003-1-2). These assays followed the instructions provided by the kit manufacturer (Nanjing Jiancheng Institute of Bioengineering, Jiangsu, China). Serum concentrations of IgA (catalog No. 8101), IgG (catalog No. 528), and IgM (catalog No. 521) were quantified using ELISA kits from Jiangsu Meimian Industrial Co., Ltd. (Jiangsu, China). In parallel, intestinal mucosal samples were analyzed for sIgA (catalog No. 9505), IL-2 (catalog No. 5010), IL-4 (catalog No. 5005), IL-10 (catalog No. 5026), and IFN-γ (catalog No. 23114) using an ELISA kit obtained from the same supplier.
2.4 Analysis for microbial community by 16S rRNA sequences
Colonic digesta samples (n = 30) were collected from pigs assigned to five dietary treatments: control, 100 mg/kg ICGA, 200 mg/kg ICGA, 400 mg/kg ICGA, and 800 mg/kg ICGA, with six animals per group. To profile the resident microbiota, the V3–V4 hypervariable region of the bacterial 16S rRNA gene was amplified and sequenced. Sample preparation, DNA extraction and validation, PCR amplification, product purification, library construction and quality assessment, as well as high-throughput sequencing using the NovaSeq platform, were performed by Beijing Novogene Biotechnology Co., Ltd.
Raw reads were preliminarily filtered and merged to remove low-quality or chimeric sequences, resulting in a clean dataset suitable for downstream analyses. DADA2 (Version 1.8) was then used to denoise these reads, with sequences falling below a minimum abundance threshold of 5 excluded according to Li et al (19). The remaining ASVs served as the basis for taxonomic classification and quantification of relative abundances. Representative ASVs were annotated with species-level identifiers, facilitating both compositional and alpha diversity assessments. To further investigate potential group-specific microbial signatures, pairwise t-tests were performed on the final dataset, and LEfSe was employed to identify differentially enriched taxa among the categorized samples.
2.5 Statistical analysis
We collated all experimental data using Microsoft Excel 2019. Prior to statistical analysis, we assessed data normality with the Shapiro–Wilk test. We then performed a one-way ANOVA using SAS 9.4 (SAS Inst. Inc., Cary, NC). When the ANOVA indicated significant differences, we applied Duncan’s multiple range test for post hoc comparisons. To evaluate dose–response relationships, we conducted linear and quadratic regression analyses on the effects of dietary ICGA supplementation. Results are expressed as mean ± standard error (SE). We considered differences statistically significant at p < 0.05 and indicative of a trend at 0.05 ≤ p < 0.10. The statistical models are as follows:
: Observed value of the j-th replicate in the i-th treatment group. : Grand mean, the theoretical average of all observations. : Fixed effect of the i-th treatment, representing its deviation from (∑=0). : Random error term, independently and identically distributed as N (0,σ2).
: Response value of the i-th observation. : Regression intercept, the predicted when =0. : Regression slope, indicating the average change in per 1-unit increase in . : ICGA dose level (continuous variable, values: 0, 100, 200, 400, 800 mg/kg). : Random error term, following N (0, σ2).
: Response value of the i-th observation. : Regression intercept, the predicted when =0. : Regression slope, indicating the average change in per 1-unit increase in . : ICGA dose level (continuous variable, values: 0, 100, 200, 400, 800 mg/kg). : Random error term, following N (0,σ2). : Quadratic term coefficient, reflecting nonlinear (curvilinear) effects. If ≠0, a quadratic relationship exists.
3 Results
3.1 Growth performance and diarrhea rate
The effects of ICGA supplementation in the diets on growth performance, diarrhea rate and diarrhea index are presented in Table 2. From days 15 to 28, dietary supplementation with 200 mg/kg ICGA significantly increased the average daily gain (ADG) by 3.49% compared with the CON group (p < 0.05). The ICGA dosage also showed a quadratic regression relationship (p < 0.05). ICGA supplementation linearly reduced diarrhea rate of weaning piglets during days 0–14 and 0–28 (p < 0.05).
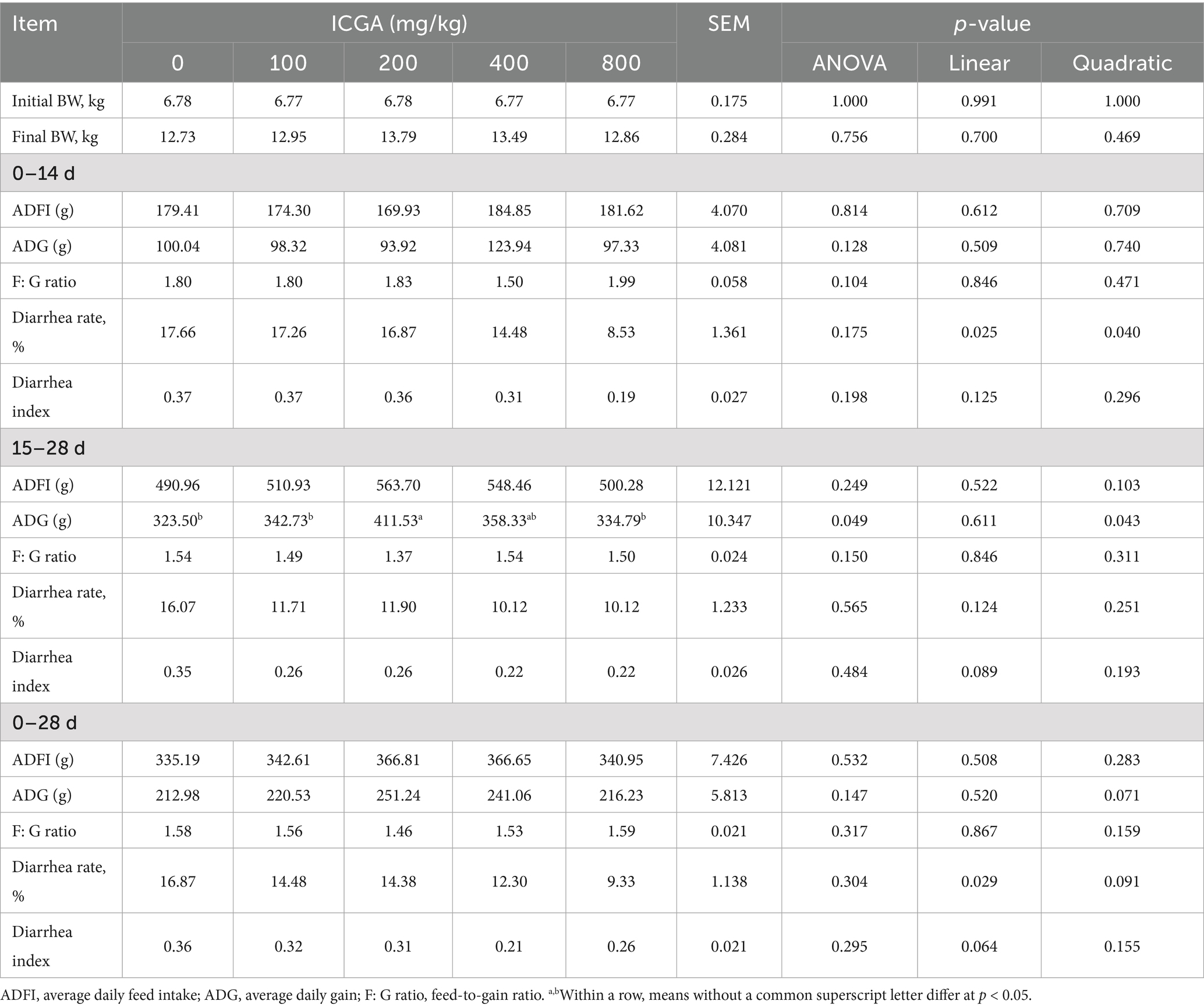
Table 2. Effects of isochlorogenic acid (ICGA) supplementation on growth performance, diarrhea rate and diarrhea index in weaned piglets.
3.2 Nutrient digestibility
As shown in Table 3, Relative to the CON group, pigs receiving 200 or 400 mg/kg ICGA exhibited significantly improved apparent total tract digestibility (ATTD; p < 0.01) of dry matter (1.84 and 1.54%, respectively), crude protein (4.48 and 4.39%), gross energy (3.01 and 2.99%), ether extract (23.18 and 17.49%), and ash (8.80 and 5.13%).
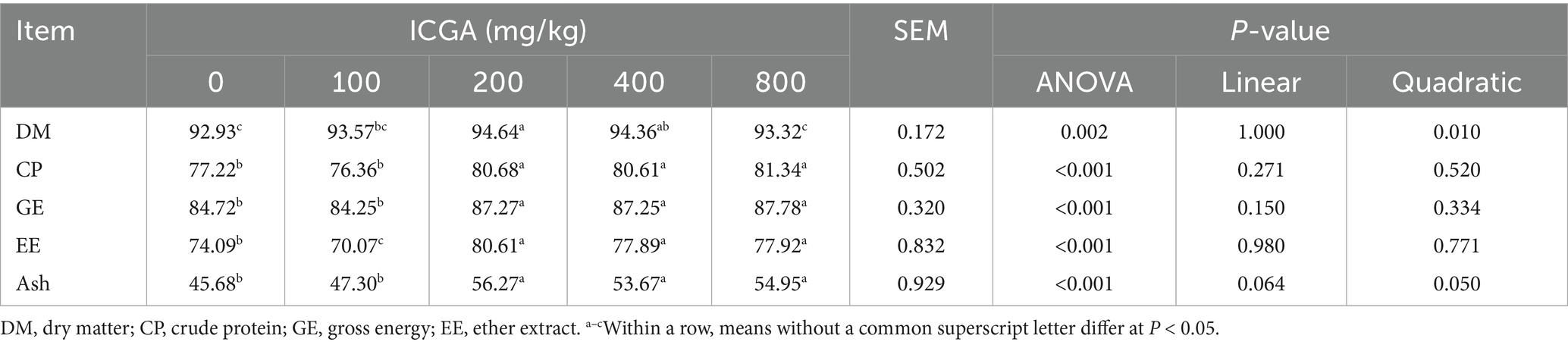
Table 3. Effects of isochlorogenic acid (ICGA) supplementation on nutrient digestibility in weaned piglets (%).
3.3 Serum anti-oxidative properties
The effects of dietary ICGA supplementation on serum anti-oxidation are shown in Table 4. On day 14, the 400 mg/kg ICGA group showed a 47.78% increase in serum catalase (CAT) activity compared with the CON group (p < 0.05), which further increased to 77.65% by day 28 (p < 0.05). Meanwhile, supplementation with 200 mg/kg ICGA elevated serum total antioxidant capacity (T-AOC) by 75.78% on day 28 (p < 0.05), demonstrating a significant quadratic dose–response relationship (p < 0.05).
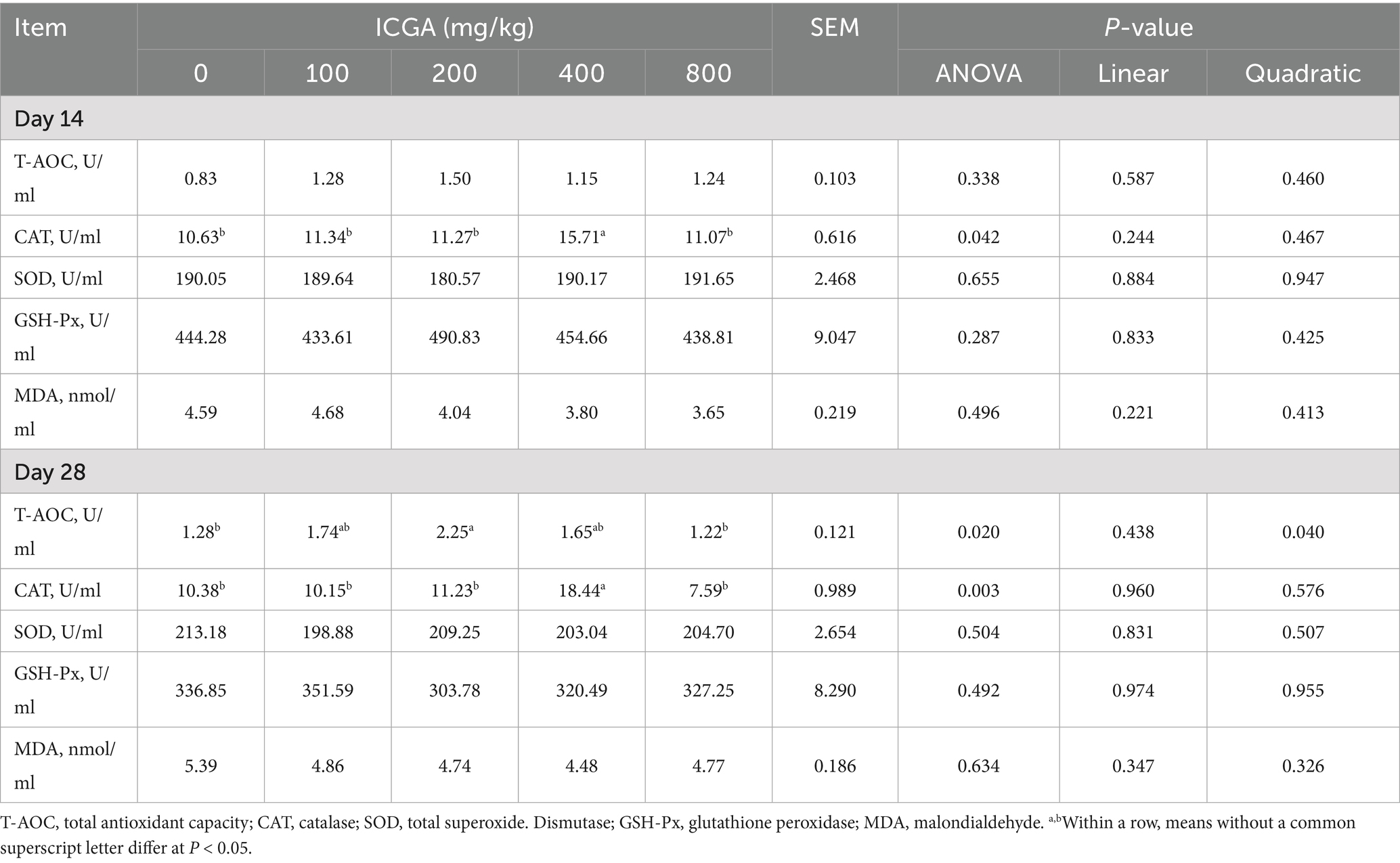
Table 4. Effects of isochlorogenic acid (ICGA) supplementation on antioxidant index in serum of piglets.
3.4 Immunoglobulins in serum, cytokines in intestinal mucosa and sIgA
The effects of dietary ICGA supplementation on immunoglobulins in serum are shown in Table 5. At day 14, serum immunoglobulin A (IgA) levels increased by 23.77 and 33.42%, and immunoglobulin M (IgM) by 18.81 and 30.86%, in the 200 and 400 mg/kg ICGA groups, respectively (p < 0.01). Immunoglobulin levels exhibited a significant quadratic relationship with ICGA dose (p < 0.01), peaking at 400 mg/kg. The effects of dietary ICGA supplementation on cytokines in intestinal mucosa are shown in Table 6. Obviously, no differences were observed for sIgA, IL-2, IL-4, IL-10, and IFN-γ in ileal and jejunal mucosa of weaned piglets among the 5 dietary treatments (p > 0.05).
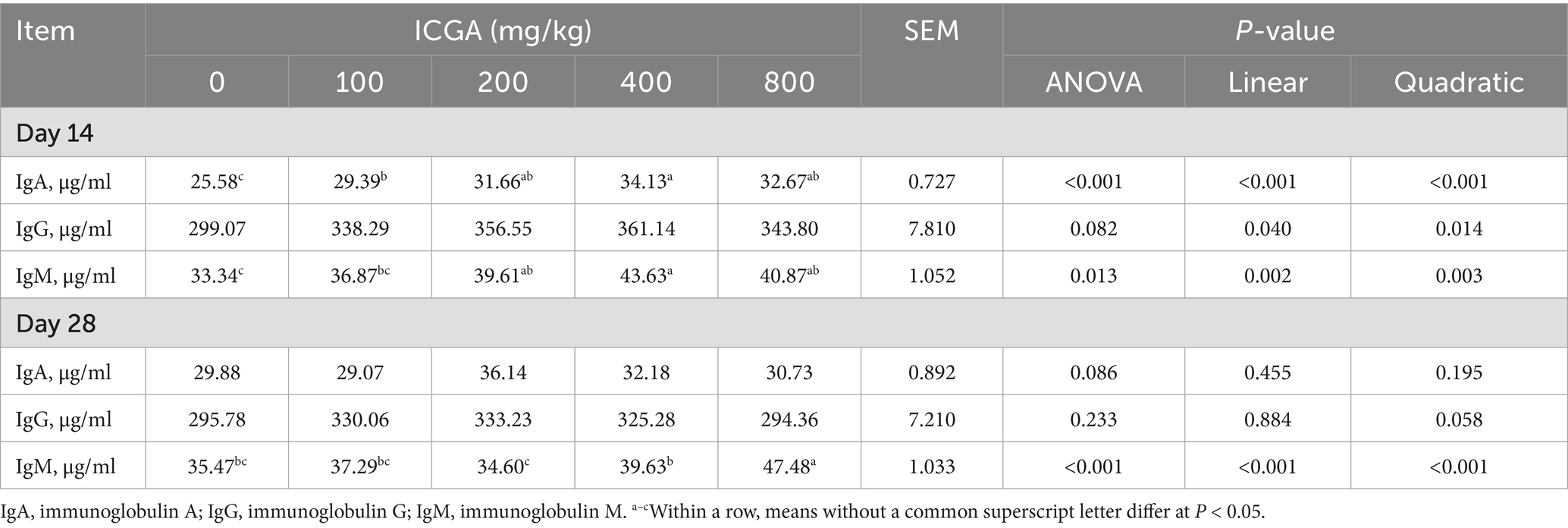
Table 5. Effects of isochlorogenic acid (ICGA) supplementation on immunoglobulins in serum of piglets.
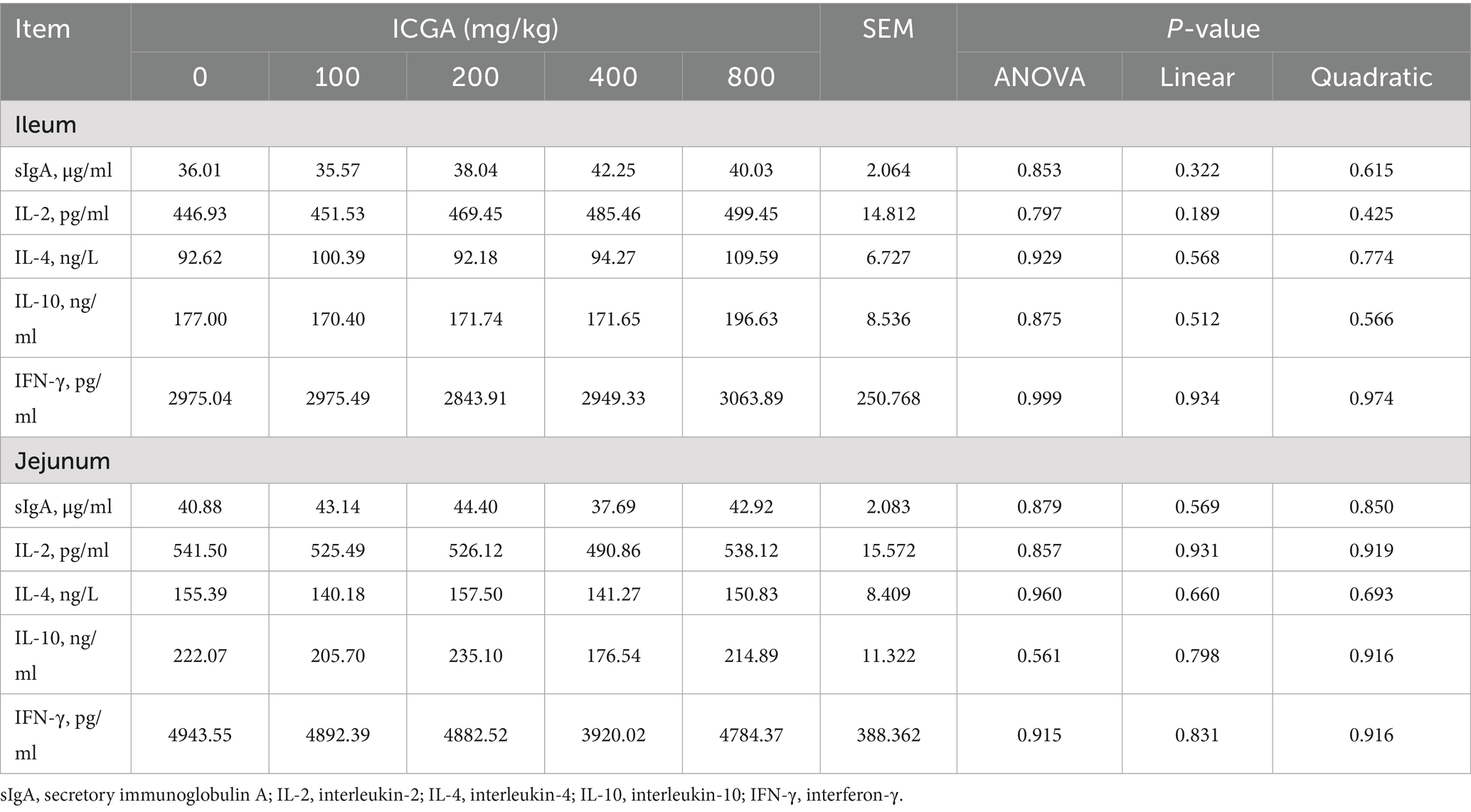
Table 6. Effects of isochlorogenic acid (ICGA) supplementation on sIgA and cytokines levels in intestinal mucosa of piglets.
3.5 Microflora community
The change in bacterial diversity was investigated using the 16 s rRNA sequencing. The alpha diversity analysis index (observed_otus, shannon, simpson, chao1, and goods_coverage) of each sample is counted. As shown in Table 7, there were no statistical differences in the alpha diversity of colonic digesta microbial communities among the 5 treatments.
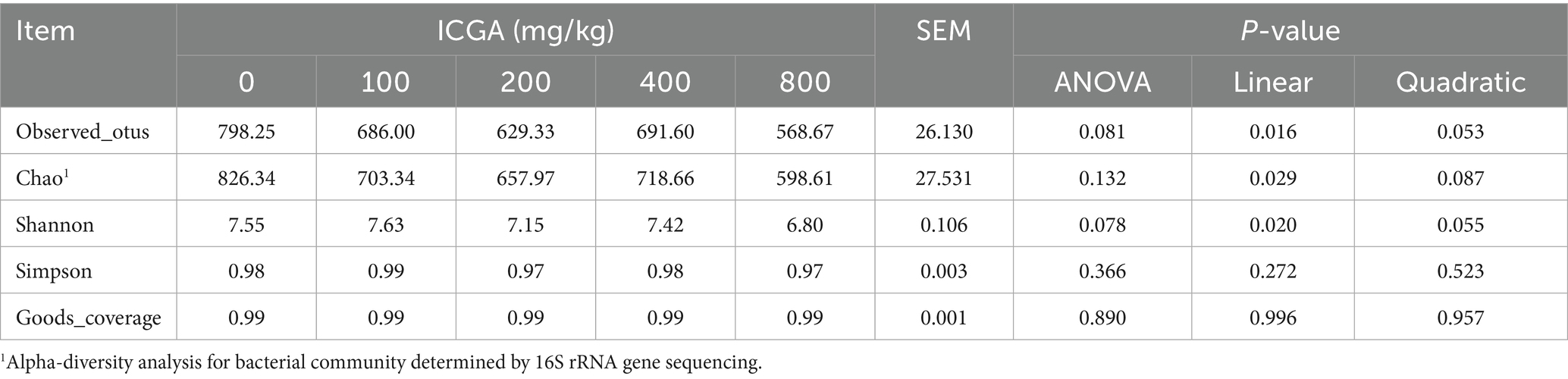
Table 7. Effects of isochlorogenic acid (ICGA) supplementation on the α-diversity1 of microbial communities of weaning piglets in colonic digesta.
The histogram of relative abundance of species can not only display the dominant species and composition of each sample, but also clearly observe the change trend of the abundance of dominant species in different species. At the phylum level, Firmicutes, Bacteroidota, Actinobacteriota and Proteobacteria were the dominant microbial divisions (Figure 1A). At the genus level, Lactobacillus, Methanobrevibacter, Prevotella, Streptococcus, Muribaculaceae, Sarcina, Clostridia_UCG-014, Clostridium_sensu_stricto_1, Terrisporobacter, Blautia, Faecalibacterium, and Eubacterium_coprostanoligenes_group were predominant (Figure 1B). The heatmap plot (according to the top 35, the most different genera) showed the relative abundance of genera in different groups (Figure 2). The color gradient and similarity degree on the heatmap plot reflected the similarity and difference of community composition among multiple samples.
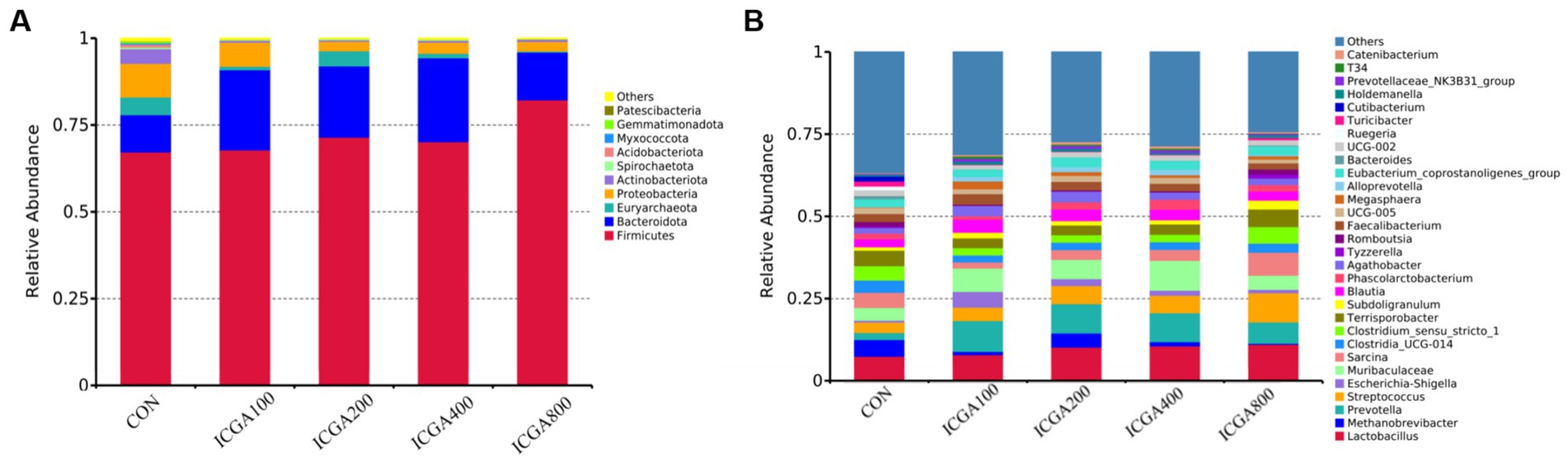
Figure 1. The relative abundance of top 10 microbial community bar plot on the phylum and genus level. Values are the means (n = 6 replicates per treatment). (A) The relative abundance of top 10 microbial community bar plot on the phylum level. (B) The relative abundance of top 30 microbial community bar plot on the genus level.
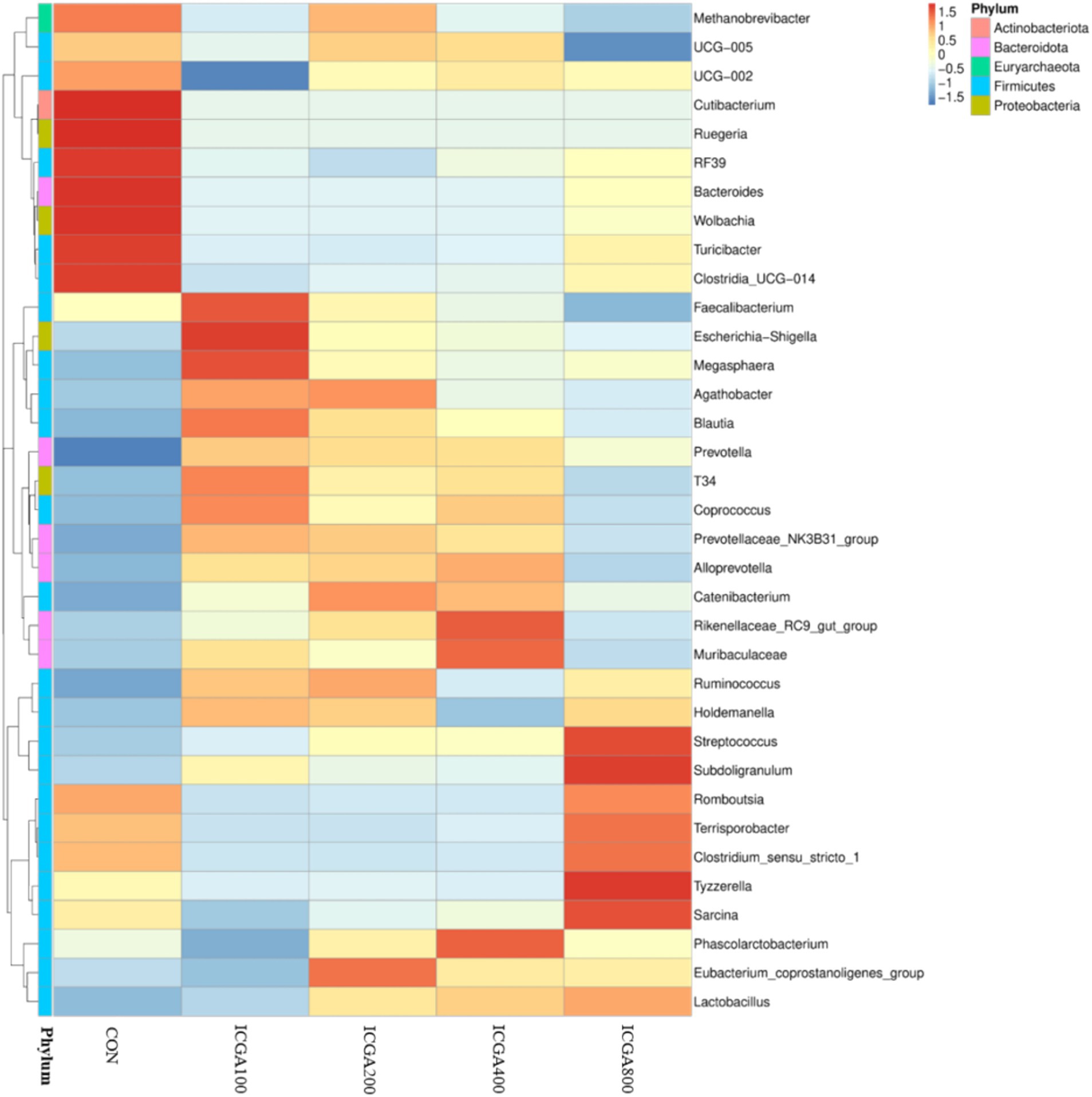
Figure 2. Heatmap of top 35 genera (relative abundances) among the groups. Values are the means (n = 6 replicates per treatment).
LEfSe analysis was used to study the influence degree of species that were significantly different (LDA score > 4.0) from phylum to genus level, and results included LDA value distribution histogram. (Figure 3). We found that Bacteroidota, Prevotella, and Muribaculaceae were the dominant species in the ICGA400 group. Peptostreptococcaceae, Actinobacteriota, and Proteobacteria were the main components of gut microbiota in the CON group.
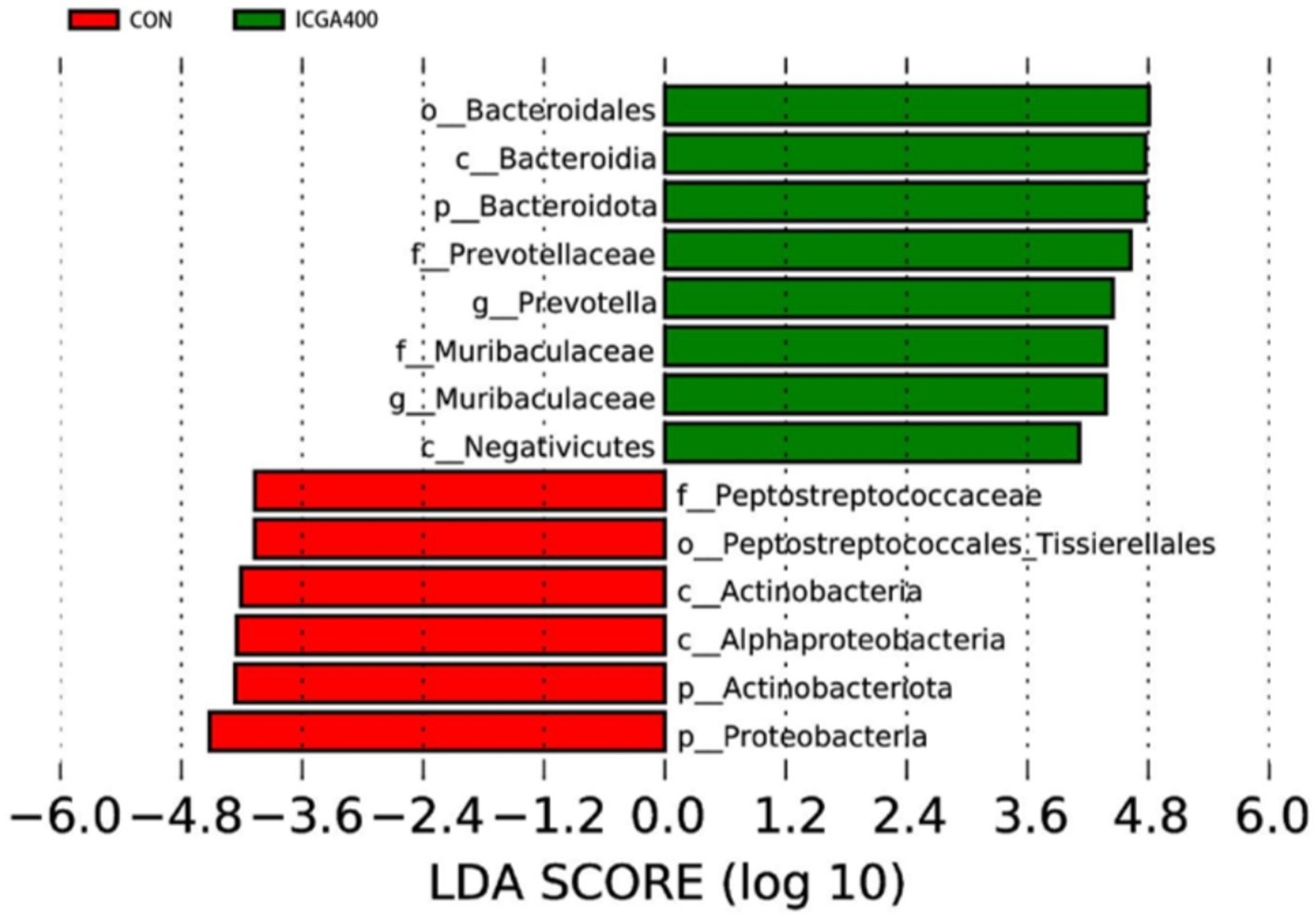
Figure 3. Linear discriminant analysis (LDA) scores (>4.0) computed for features at the ASV level. Letters represented the taxonomy of the bacteria: p, phylum, c, class; o, order; f, family; g, genus. All the values contained 6 repetitions. ICGA400 = 400 mg/kg isochlorogenic acid. Values are the means (n = 6 replicates per treatment).
T-test can be used to find species with significant differences between groups at each taxonomic level. (Figures 4A,B). ICGA400 treatments enhanced the Bacteroides (p < 0.05) in contrast to the CON group, while Proteobacteria (p < 0.01), Actinobacteriota (p < 0.05), Acidobacteriota (p < 0.05), Gemmatimonadota (p < 0.01), and Verrucomicrobiata (p < 0.05) decreased notably. Compared with the CON group, Terrisporobacter (p < 0.05), Romboutsia (p < 0.05), Ruegeria (p < 0.05), Turicibacter (p < 0.05), Cutibacterium (p < 0.05), and Staphylococcus (p < 0.05) showed a dramatic reduction in the ICGA400 group, while the Megasphaera (p < 0.01), Catenibacterium (p < 0.05), Rikenellaceae_RC9_gut_group (p < 0.05), Selenomonas (p < 0.05), Lachnospiraceae_AC2044_group (p < 0.05), and Prevotellaceae_UCG-003 (p < 0.05) increased significantly.
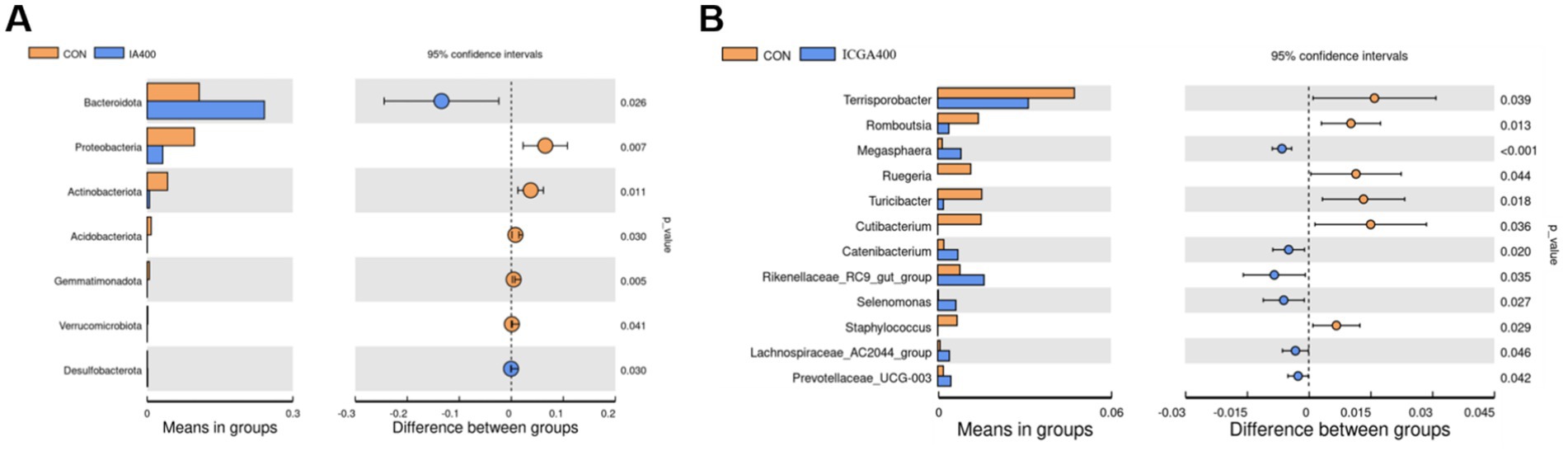
Figure 4. The t-test bar plot between groups. (A) The t-test bar plot with significantly different phylum between groups. (B) The t-test bar plot with significantly different genera between groups. Values are the means (n = 6 replicates per treatment).
4 Discussion
CGA and ICGA are natural phenolic compounds abundantly present in coffee, fruits, and vegetables. ICGA, also known as dicaffeoylquinic acids, predominantly include 3,5-dicaffeoylquinic acid (isochlorogenic acid A), 3,4-dicaffeoylquinic acid (isochlorogenic acid B), and 4,5-dicaffeoylquinic acid (isochlorogenic acid C) (20–22). Owing to their dietary prevalence and favorable biological functions, these compounds have attracted considerable interest and are extensively utilized in pharmaceuticals, food additives, and related fields (23, 24). However, research focusing on the use of ICGA in livestock and poultry production remains relatively limited compared to other disciplines, highlighting the need for further investigation into their potential applications in animal nutrition.
As natural bioactive compounds, chlorogenic acids (CGA) are known to exert antidiarrheal and growth-promoting effects when incorporated into animal diets. Numerous studies in broilers have reported that dietary CGA supplementation enhances growth performance, improves meat quality, and reduces the feed/gain ratio (F/G) (25–28). Similar benefits have been observed in monogastric animals, where CGA has been associated with improved growth and intestinal function (29–31). Notably, both CGA and isochlorogenic acids (ICGA) possess structurally similar phenolic profiles and exhibit comparable antioxidant, antibacterial, and anti-inflammatory activities. In the present study, dietary ICGA supplementation significantly increased the average daily gain (ADG) of weaned piglets, which is consistent with earlier findings on CGA. However, differences in absorption and metabolic pathways between CGA and ICGA may lead to distinct in vivo effects. We found that piglets receiving 200 or 400 mg/kg ICGA exhibited higher apparent digestibility of crude protein, gross energy, ether extract, and ash. This result aligns with previous reports that ICGAs can inhibit ɑ-glucosidase and ɑ-amylase (32, 33), suggesting a modulation of nutrient hydrolysis and absorption. In particular, the improved digestibility of ether extract (crude fat) may indicate enhanced lipid utilization, a phenomenon also reported in other models where bioactive compounds such as pectic polysaccharides inhibited intestinal lipid absorption and promoted fecal lipid excretion (34). Although the numerical reduction in diarrhea incidence did not reach statistical significance, factors including ICGA dosage, animal health, and environmental conditions may have influenced these outcomes. Further studies employing higher dosages or different delivery strategies may help clarify the antidiarrheal potential of ICGA in weaned piglets.
Weaning is widely recognized as a critical period for piglets, often leading to oxidative stress caused by an imbalance in pro-oxidant and antioxidant systems (35). Antioxidant indicators, including T-AOC, SOD, CAT, GSH-Px, and MDA, provide insights into an animal’s endogenous defenses against reactive oxygen species (ROS). Enhancing these antioxidant defenses can help alleviate oxidative stress (36). ICGA, a polyphenolic compound abundant in various food sources, has been reported to exhibit potent antioxidant effects (23, 37). Structural features, such as the multiple hydroxyl groups found in caffeoylquinic acid moieties, are thought to underlie ICGA’s capacity to scavenge ROS (38). Previous work suggests that the antioxidant activity of ICGA surpasses that of CGA, possibly due to a greater number of hydroxyl functional groups (22). Our findings indicate that dietary ICGA supplementation boosted serum T-AOC and CAT activities, consistent with earlier studies (39, 40). In the study of (20) revealed that ICGA isoforms can counter oxidative stress by scavenging ROS in Caco-2 cells treated with pro-inflammatory proteins, potentially through activation of the Nrf2-Keap1-ARE signaling pathway. While the present results underscore ICGA’s beneficial impact on antioxidant capacity in weaned piglets, knowledge remains limited regarding its exact molecular mechanisms in swine. Factors such as dosage, synergy with other dietary antioxidants, and variations in immune status may all contribute to the observed antioxidant response.
Recent research has highlighted that ICGA, a prominent dietary polyphenolic compound, exhibits diverse biological functions, notably immunomodulatory capabilities (21, 41). The immune system encompasses an intricate network of tissues, organs, specialized immune cells, and bioactive immune molecules (42). Immunoglobulins, as pivotal immune effectors, play essential roles within the humoral immune response, mediating the host’s defense against pathogenic invasion (43). In the present study, supplementation of ICGA significantly elevated serum IgA and IgM concentrations in weaned piglets at day 14 compared to the control (CON) group, reaching peak values at the dietary inclusion level of 400 mg/kg. Furthermore, on day 28, a significant enhancement in IgM concentration was observed in the serum of piglets receiving the highest dietary dose (800 mg/kg ICGA), relative to the CON group. The balance and interaction between pro-inflammatory and anti-inflammatory cytokines are crucial determinants of effective immune protection against pathogens (44). Numerous studies indicate that ICGA possesses marked anti-inflammatory, antimicrobial, and antiviral properties (5). However, in this investigation, dietary ICGA supplementation did not affect mucosal concentrations of sIgA or the cytokines IL-2, IL-4, IL-10, and IFN-γ in the jejunum and ileum of weaned piglets. It is important to note the scarcity of existing data specifically addressing the immunomodulatory effects of ICGA in piglets, and the current experimental conditions may not have presented a sufficient pathogenic or environmental challenge. Further exploration is therefore necessary to comprehensively evaluate the potential of ICGA to modulate immune function and confer protection against pathogenic threats in piglets.
The gut microbiota fundamentally regulates host metabolism, gastrointestinal function, and immune development (45), and serves as a key biomarker of intestinal health (46). Among external factors, diet exerts a dominant influence on the structure and function of the gut microbial community (47). Specific dietary components—including functional ingredients and feed additives—may improve growth performance and gastrointestinal health by modulating microbial composition and stability (48). In this study, however, ICGA supplementation did not significantly alter the α-diversity of colonic microbiota—as measured by observed_otus, Shannon, Simpson, Chao1, and Goods_coverage indices—across treatment groups. Notably, this absence of alpha-diversity modulation contrasts with reports in low-birth-weight (LBW) piglets, where a hydrolyzed protein formula significantly reduced microbial diversity indices (e.g., Chao1, ACE, Shannon, Simpson), yet enhanced barrier function and immune outcomes (49). This discrepancy may arise from differences in basal health status between normal and LBW models, or from distinct bioactivities of the supplements. Nevertheless, our results imply that ICGA may promote gastrointestinal homeostasis without disrupting microbial diversity, potentially sustaining a stable colonization niche that facilitates immune maturation—a particularly desirable effect in vulnerable populations such as LBW neonates.
The relative abundance histogram indicated that Firmicutes and Bacteroidetes were predominant bacterial phyla, aligning with prior findings (50). Bacteroidetes are recognized as highly competitive commensal organisms within the gut microbiota, significantly contributing to essential metabolic processes in the colon, particularly the fermentation of carbohydrates and metabolism of nitrogenous substrates (51). Major metabolic by-products generated by anaerobic fermentation in Bacteroidetes include acetic acid, succinic acid, and isovaleric acid, which serve as energy sources for the host (52). Additionally, these bacteria play a protective role by suppressing the colonization of pathogenic microorganisms in the gut ecosystem (53). In the present study, we observed that dietary ICGA significantly increased the abundance of the Bacteroidetes in the colonic digesta of piglets. A similar outcome was described before (54), who observed increased cecal Bacteroidetes abundance in piglets fed chlorogenic acid. The LEfSe, designed to detect biomarkers with significant differential abundance within microbial communities (55), indicated Bacteroides, Prevotella, and Muribaculaceae as dominant taxa in piglets from the ICGA400 treatment, whereas Peptostreptococcus, Actinomycetes, and Proteobacteria prevailed in the CON group. Prevotella possesses the ability to degrade plant-derived polysaccharides and host-derived mucins, generating beneficial metabolites such as short-chain fatty acids, including propionate (56). Muribaculaceae, a recently reclassified bacterial family formerly known as S24-7, belongs to Bacteroidetes and responds variably to dietary modifications and host physiological conditions, though its precise functional roles require further clarification (57). Alterations in the abundance of Muribaculaceae appear closely related to dietary interventions and host-specific physiological conditions; however, the precise functional roles and mechanisms of these bacteria remain poorly defined, necessitating further detailed investigations. Another notable observation from this study was the marked decrease in Proteobacteria abundance within cecal microbiota samples following ICGA supplementation. Proteobacteria, characterized as Gram-negative organisms possessing an outer membrane rich in lipopolysaccharides, represent a diverse and clinically significant bacterial phylum encompassing well-known pathogenic genera, such as Escherichia and Helicobacter (58). Elevated proportions of Proteobacteria are typically indicative of intestinal dysbiosis or host pathology, underscoring its potential as a microbial biomarker of compromised gut health (59). The beneficial effects of ICGA on growth performance and gut health in piglets may be attributed to its ability to modulate intestinal microbiota through probiotic-driven competitive exclusion. Probiotics potentially inhibit pathogen proliferation by competing for essential nutrients, colonization sites, and secreting antimicrobial metabolites (60). At the genus level, compared with the CON group, Terrisporobacter, Romboutsia, Ruegeria, Turicibacter, Cutibacterium, and Staphylococcus were significantly reduced in the ICGA400 group, while the Megasphaera, Catenibacterium, Rikenellaceae_RC9_gut_group, Selenomonas, Lachnospiraceae_AC2044_group, and Prevotellaceae_UCG-003 increased significantly. These beneficial microbes are recognized producers of short-chain fatty acids, known to strengthen the antioxidant defense system and activate anti-inflammatory signaling pathways, thus maintaining intestinal homeostasis and overall health (61). Furthermore, ICGA supplementation can modulate the intestinal microbial ecosystem by effectively suppressing the proliferation of potential pathogens, thus promoting a balanced gut microbiota and enhancing overall intestinal health. Maintaining this microbial equilibrium is crucial in preventing dysbiosis-associated disorders and contributes to improved physiological resilience in animals.
5 Conclusion
Taken together, the present study demonstrated that dietary ICGA supplementation could modestly reduce post-weaning diarrhea, while effectively improve nutrient digestibility, antioxidant activity and humoral immune status of weaned piglets. ICGA modulated intestinal microbiota composition by increasing the abundance of beneficial microbiota and reducing the abundance of harmful bacteria in weaned pigs.
Data availability statement
The datasets for this study can be available on request to the corresponding author. The raw sequencing data are available from NCBI repository: https://www.ncbi.nlm.nih.gov/, under accession number PRJNA1330785.
Ethics statement
The animal study was approved by the animal care and experimental procedures were approved by the Institutional Animal Care and Use Committee of Sichuan Agricultural University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
YW: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. YoL: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. HZ: Data curation, Investigation, Methodology, Software, Writing – original draft. WG: Formal analysis, Investigation, Writing – review & editing. BY: Supervision, Validation, Writing – review & editing. JH: Investigation, Resources, Writing – review & editing. WS: Formal analysis, Resources, Writing – review & editing. YuL: Methodology, Software, Writing – original draft. PZ: Investigation, Writing – original draft. XM: Visualization, Writing – review & editing. YX: Project administration, Writing – original draft. MX: Conceptualization, Investigation, Methodology, Resources, Supervision, Validation, Writing – review & editing. JY: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Precise and Intelligent Feeding with Environmental Control System of Pig Production in Sichuan (2021ZDZX0011).
Acknowledgments
Technical assistance by Huifen Wang, Quyuan Wang and Qingqing Zhu are gratefully acknowledged.
Conflict of interest
WG, WS, and MX were employed by the Chenguang Biological Technology Group Co, Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mamat, N, Dou, J, Lu, X, Eblimit, A, and Haji, AA. Isochlorogenic acid a promotes melanin synthesis in B16 cell through the β-catenin signal pathway. Acta Biochim Biophys Sin. (2017) 49:800–7. doi: 10.1093/abbs/gmx072
2. Wang, J, Wang, H, Peng, Y, Wang, GJ, and Hao, HP. Isochlorogenic acid a affects P450 and UGT enzymes in vitro and in vivo. Chin J Nat Med. (2016) 14:865–70. doi: 10.1016/S1875-5364(16)30103-0
3. Wang, X, Yang, Y, Liu, X, and Gao, X. Pharmacological properties of tanshinones, the natural products from Salvia miltiorrhiza. Advances Pharmacol. (2020) 87:43–70. doi: 10.1016/bs.apha.2019.10.001
4. Corse, J, Lundin, RE, and Waiss, AC. Identification of several components of isochlorogenic acid. Phytochemistry. (1965) 4:527–9. doi: 10.1016/S0031-9422(00)86209-3
5. Wang, HN, Shen, Z, Liu, Q, Hou, XY, Cao, Y, Liu, DH, et al. Isochlorogenic acid (ICGA): natural medicine with potentials in pharmaceutical developments. Chin J Nat Med. (2020) 18:860–71. doi: 10.1016/S1875-5364(20)60029-2
6. Fedorov, VA, Menshchikova, TK, Vargunin, AI, Nikonov, KS, Brekhovskikh, MN, and Myslitskii, OE. Processes for the preparation of high-purity arsenic and its compounds. Inorg Mater. (2021) 57:1097–108. doi: 10.1134/s0020168521110042
7. Lv, X, Feng, S, Zhang, J, Sun, S, Geng, Y, Yang, M, et al. Application of HPLC fingerprint combined with chemical pattern recognition and multi-component determination in quality evaluation of Echinacea purpurea (L.) Moench. Molecules. (2022) 27:6463. doi: 10.3390/molecules27196463
8. Yin, XL, Xu, BQ, and Zhang, YQ. Gynura divaricata rich in 3, 5−/4, 5-dicaffeoylquinic acid and chlorogenic acid reduces islet cell apoptosis and improves pancreatic function in type 2 diabetic mice. Nutr Metab. (2018) 15:73. doi: 10.1186/s12986-018-0310-y
9. Zheng, L, Lin, G, Li, R, Gan, H, Huang, X, Yao, N, et al. Isochlorogenic acid C alleviates high-fat diet-induced hyperlipemia by promoting cholesterol reverse transport. Front Pharmacol. (2022) 13:881078. doi: 10.3389/fphar.2022.881078
10. Zuo, J, Tang, W, and Xu, Y. Chapter 68- anti-hepatitis B virus activity of Chlorogenic acid and its related compounds In: VR Preedy, editor. Coffee in health and disease prevention. San Diego: Academic Press (2015). 607–13. doi: 10.1016/B978-0-12-409517-5.00068-1
11. Miao, M, and Xiang, L. Pharmacological action and potential targets of chlorogenic acid. Adv Pharmacol. (2020) 87:71–88. doi: 10.1016/bs.apha.2019.12.002
12. Naveed, M, Hejazi, V, Abbas, M, Kamboh, AA, Khan, GJ, Shumzaid, M, et al. Chlorogenic acid (CGA): a pharmacological review and call for further research. Biomed Pharmacother. (2018) 97:67–74. doi: 10.1016/j.biopha.2017.10.064
13. Zeng, M, Zou, Y, Shi, Z, Wang, J, Yang, Y, Bai, Y, et al. A broad-spectrum broth rapidly and completely repairing the sublethal injuries of Escherichia coli caused by freezing and lactic acid alone or in combination for accurate enumeration. LWT Food Sci Technol. (2024) 201:116219. doi: 10.1016/j.lwt.2024.116219
14. Chen, F, Wang, Y, Wang, K, Chen, J, Jin, K, Peng, K, et al. Effects of Litsea cubeba essential oil on growth performance, blood antioxidation, immune function, apparent digestibility of nutrients, and fecal microflora of pigs. Front Pharmacol. (2023) 14:1166022. doi: 10.3389/fphar.2023.1166022
15. Wei, X, Wu, H, Wang, Z, Zhu, J, Wang, W, Wang, J, et al. Rumen-protected lysine supplementation improved amino acid balance, nitrogen utilization and altered hindgut microbiota of dairy cows. Animal Nutrition. (2023) 15:320–31. doi: 10.1016/j.aninu.2023.08.001
16. Chen, J, Li, Y, Yu, B, Chen, D, Mao, X, Zheng, P, et al. Dietary chlorogenic acid improves growth performance of weaned pigs through maintaining antioxidant capacity and intestinal digestion and absorption function. J Anim Sci. (2018) 96:1108–18. doi: 10.1093/jas/skx078
17. Yu, J, Song, Y, Yu, B, He, J, Zheng, P, Mao, X, et al. Tannic acid prevents post-weaning diarrhea by improving intestinal barrier integrity and function in weaned piglets. J Animal Sci Biotechnol. (2020) 11:87. doi: 10.1186/s40104-020-00496-5
18. Su, W, Jiang, Z, Wang, C, Zhang, Y, Gong, T, Wang, F, et al. Co-fermented defatted rice bran alters gut microbiota and improves growth performance, antioxidant capacity, immune status and intestinal permeability of finishing pigs. Animal Nutr. (2022) 11:413–24. doi: 10.1016/j.aninu.2022.07.008
19. Li, M, Shao, D, Zhou, J, Gu, J, Qin, J, Chen, W, et al. Signatures within esophageal microbiota with progression of esophageal squamous cell carcinoma. Chinese J Cancer Research. (2020) 32:755–67. doi: 10.21147/j.issn.1000-9604.2020.06.09
20. Liang, N, and Kitts, DD. Amelioration of oxidative stress in Caco-2 cells treated with pro-inflammatory proteins by Chlorogenic acid isomers via activation of the Nrf2-Keap1-ARE-signaling pathway. J Agric Food Chem. (2018) 66:11008–17. doi: 10.1021/acs.jafc.8b03983
21. Lu, H, Tian, Z, Cui, Y, Liu, Z, and Ma, X. Chlorogenic acid: a comprehensive review of the dietary sources, processing effects, bioavailability, beneficial properties, mechanisms of action, and future directions. Compr Rev Food Sci Food Saf. (2020) 19:3130–58. doi: 10.1111/1541-4337.12620
22. Xu, JG, Hu, QP, and Liu, Y. Antioxidant and DNA-protective activities of chlorogenic acid isomers. J Agric Food Chem. (2012) 60:11625–30. doi: 10.1021/jf303771s
23. Gupta, A, Atanasov, AG, Li, Y, Kumar, N, and Bishayee, A. Chlorogenic acid for cancer prevention and therapy: current status on efficacy and mechanisms of action. Pharmacol Res. (2022) 186:106505. doi: 10.1016/j.phrs.2022.106505
24. Santana-Gálvez, J, Cisneros-Zevallos, L, and Jacobo-Velázquez, DA. Chlorogenic acid: recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules. (2017) 22:358. doi: 10.3390/molecules22030358
25. Bai, D, Liu, K, He, X, Tan, H, Liu, Y, Li, Y, et al. Effect of dietary Chlorogenic acid on growth performance, antioxidant function, and immune response of broiler breeders under immune stress and stocking density stress. Vet Sci. (2022) 9:582. doi: 10.3390/vetsci9100582
26. Zhang, X, Zhao, Q, Ci, X, Chen, S, Xie, Z, Li, H, et al. Evaluation of the efficacy of chlorogenic acid in reducing small intestine injury, oxidative stress, and inflammation in chickens challenged with Clostridium perfringens type a. Poult Sci. (2020) 99:6606–18. doi: 10.1016/j.psj.2020.09.082
27. Zhang, K, Li, X, Zhao, J, Wang, Y, Hao, X, Liu, K, et al. Protective effects of chlorogenic acid on the meat quality of oxidatively stressed broilers revealed by integrated metabolomics and antioxidant analysis. Food Funct. (2022) 13:2238–52. doi: 10.1039/D1FO03622J
28. Zhao, JS, Deng, W, and Liu, HW. Effects of chlorogenic acid-enriched extract from Eucommia ulmoides leaf on performance, meat quality, oxidative stability, and fatty acid profile of meat in heat-stressed broilers. Poult Sci. (2019) 98:3040–9. doi: 10.3382/ps/pez081
29. Chen, J, Song, Z, Ji, R, Liu, Y, Zhao, H, Liu, L, et al. Chlorogenic acid improves growth performance of weaned rabbits via modulating the intestinal epithelium functions and intestinal microbiota. Front Microbiol. (2022) 13:1027101. doi: 10.3389/fmicb.2022.1027101
30. Liu, H, Li, X, Shi, S, Zhou, Y, Zhang, K, Wang, Y, et al. Chlorogenic acid improves growth performance and intestinal health through autophagy-mediated nuclear factor erythroid 2-related factor 2 pathway in oxidatively stressed broilers induced by dexamethasone. Poult Sci. (2022) 101:102036. doi: 10.1016/j.psj.2022.102036
31. Wang, W, Li, F, Duan, Y, Guo, Q, Zhang, L, Yang, Y, et al. Effects of dietary Chlorogenic acid supplementation derived from Lonicera macranthoides hand-Mazz on growth performance, free amino acid profile, and muscle protein synthesis in a finishing pig model. Oxidative Med Cell Longev. (2022) 2022:6316611. doi: 10.1155/2022/6316611
32. Chen, Y, Geng, S, and Liu, B. Three common caffeoylquinic acids as potential hypoglycemic nutraceuticals: evaluation of α-glucosidase inhibitory activity and glucose consumption in HepG2 cells. J Food Biochem. (2020) 44:e13361. doi: 10.1111/jfbc.13361
33. Wang, S, Li, Y, Huang, D, Chen, S, Xia, Y, and Zhu, S. The inhibitory mechanism of chlorogenic acid and its acylated derivatives on α-amylase and α-glucosidase. Food Chem. (2022) 372:131334. doi: 10.1016/j.foodchem.2021.131334
34. Wang, X, Liu, Y, Xu, Y, Gao, S, Xu, Q, Gong, H, et al. Structural characterization of a pectic polysaccharide from Rubus chingii Hu. Unripe fruits and its efficacy in inhibiting intestinal lipid absorption in vivo. Carbohydr Polym. (2025) 363:123728. doi: 10.1016/j.carbpol.2025.123728
35. Zhang, L, and Piao, X. Different dietary protein sources influence growth performance, antioxidant capacity, immunity, fecal microbiota and metabolites in weaned piglets. Animal Nutr. (2022) 8:71–81. doi: 10.1016/j.aninu.2021.06.013
36. Smith, F, Clark, JE, Overman, BL, Tozel, CC, Huang, JH, Rivier, JE, et al. Early weaning stress impairs development of mucosal barrier function in the porcine intestine. Am J Physiol Gastrointest Liver Physiol. (2010) 298:G352–63. doi: 10.1152/ajpgi.00081.2009
37. Venditti, A, Maggi, F, Vittori, S, Papa, F, Serrilli, AM, Di Cecco, M, et al. Antioxidant and α-glucosidase inhibitory activities of Achillea tenorii. Pharm Biol. (2015) 53:1505–10. doi: 10.3109/13880209.2014.991833
38. Iwai, K, Kishimoto, N, Kakino, Y, Mochida, K, and Fujita, T. In vitro antioxidative effects and tyrosinase inhibitory activities of seven hydroxycinnamoyl derivatives in green coffee beans. J Agric Food Chem. (2004) 52:4893–8. doi: 10.1021/jf040048m
39. Xiong, Y, Liu, S, Xiao, H, Wu, Q, Chi, L, Zhu, L, et al. Dietary stevia residue extract supplementation improves the performance and antioxidative capacity of growing-finishing pigs. J Sci Food Agric. (2022) 102:4724–35. doi: 10.1002/jsfa.11833
40. Zhang, Y, Wang, Y, Chen, D, Yu, B, Zheng, P, Mao, X, et al. Dietary chlorogenic acid supplementation affects gut morphology, antioxidant capacity and intestinal selected bacterial populations in weaned piglets. Food Funct. (2018) 9:4968–78. doi: 10.1039/C8FO01126E
41. Cao, Z, Ding, Y, Cao, L, Ding, G, Wang, Z, and Xiao, W. Isochlorogenic acid C prevents enterovirus 71 infection via modulating redox homeostasis of glutathione. Sci Rep. (2017) 7:16278. doi: 10.1038/s41598-017-16446-7
42. Parkin, J, and Cohen, B. An overview of the immune system. Lancet. (2001) 357:1777–89. doi: 10.1016/S0140-6736(00)04904-7
43. Perez, EE, Orange, JS, Bonilla, F, Chinen, J, Chinn, IK, Dorsey, M, et al. Update on the use of immunoglobulin in human disease: a review of evidence. J Allergy Clin Immunol. (2017) 139:S1–s46. doi: 10.1016/j.jaci.2016.09.023
44. Savarin, C, and Bergmann, CC. Fine tuning the cytokine storm by IFN and IL-10 following neurotropic coronavirus encephalomyelitis. Front Immunol. (2018) 9:3022. doi: 10.3389/fimmu.2018.03022
45. Vicentini, FA, Keenan, CM, Wallace, LE, Woods, C, Cavin, JB, Flockton, AR, et al. Intestinal microbiota shapes gut physiology and regulates enteric neurons and glia. Microbiome. (2021) 9:210. doi: 10.1186/s40168-021-01165-z
46. Nathan, NN, Philpott, DJ, and Girardin, SE. The intestinal microbiota: from health to disease, and back. Microbes Infect. (2021) 23:104849. doi: 10.1016/j.micinf.2021.104849
47. Bibbò, S, Ianiro, G, Giorgio, V, Scaldaferri, F, Masucci, L, Gasbarrini, A, et al. The role of diet on gut microbiota composition. Eur Rev Med Pharmacol Sci. (2016) 20:4742–9.
48. Sánchez, B, Delgado, S, Blanco-Míguez, A, Lourenço, A, Gueimonde, M, and Margolles, A. Probiotics, gut microbiota, and their influence on host health and disease. Mol Nutr Food Res. (2017) 61:1600240. doi: 10.1002/mnfr.201600240
49. Bai, M, Liu, H, Yan, Y, Duan, S, Szeto, IM, He, J, et al. Hydrolyzed protein formula improves the nutritional tolerance by increasing intestinal development and altering cecal microbiota in low-birth-weight piglets. Front Nutr. (2024) 11:1439110. doi: 10.3389/fnut.2024.1439110
50. Zhao, J, Liu, P, Wu, Y, Guo, P, Liu, L, Ma, N, et al. Dietary Fiber increases butyrate-producing Bacteria and improves the growth performance of weaned piglets. J Agric Food Chem. (2018) 66:7995–8004. doi: 10.1021/acs.jafc.8b02545
51. Porter, NT, Luis, AS, and Martens, EC. Bacteroides thetaiotaomicron. Trends Microbiol. (2018) 26:966–7. doi: 10.1016/j.tim.2018.08.005
52. Zafar, H, and Saier, MH Jr. Gut Bacteroides species in health and disease. Gut Microbes. (2021) 13:1–20. doi: 10.1080/19490976.2020.1848158
53. Wexler, HM. Bacteroides: the good, the bad, and the nitty-gritty. Clin Microbiol Rev. (2007) 20:593–621. doi: 10.1128/CMR.00008-07
54. Chen, J, Yu, B, Chen, D, Zheng, P, Luo, Y, Huang, Z, et al. Changes of porcine gut microbiota in response to dietary chlorogenic acid supplementation. Appl Microbiol Biotechnol. (2019) 103:8157–68. doi: 10.1007/s00253-019-10025-8
55. Segata, N, Izard, J, Waldron, L, Gevers, D, Miropolsky, L, Garrett, WS, et al. Metagenomic biomarker discovery and explanation. Genome Biol. (2011) 12:R60. doi: 10.1186/gb-2011-12-6-r60
56. Kovatcheva-Datchary, P, Nilsson, A, Akrami, R, Lee, YS, De Vadder, F, Arora, T, et al. Dietary Fiber-induced improvement in glucose metabolism is associated with increased abundance of Prevotella. Cell Metab. (2015) 22:971–82. doi: 10.1016/j.cmet.2015.10.001
57. Lagkouvardos, I, Lesker, TR, Hitch, TCA, Gálvez, EJC, Smit, N, Neuhaus, K, et al. Sequence and cultivation study of Muribaculaceae reveals novel species, host preference, and functional potential of this yet undescribed family. Microbiome. (2019) 7:28. doi: 10.1186/s40168-019-0637-2
58. Rizzatti, G, Lopetuso, LR, Gibiino, G, Binda, C, and Gasbarrini, A. Proteobacteria: a common factor in human diseases. Biomed Res Int. (2017) 2017:9351507. doi: 10.1155/2017/9351507
59. Shin, NR, Whon, TW, and Bae, JW. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. (2015) 33:496–503. doi: 10.1016/j.tibtech.2015.06.011
60. Sekirov, I, Russell, SL, Antunes, LC, and Finlay, BB. Gut microbiota in health and disease. Physiol Rev. (2010) 90:859–904. doi: 10.1152/physrev.00045.2009
Keywords: isochlorogenic acid, antioxidant capacity, immune function, intestinal microbiota, weaned piglet
Citation: Wang Y, Luo Y, Zou H, Gao W, Yu B, He J, Song W, Luo Y, Zheng P, Mao X, Xuan Y, Xu M and Yu J (2025) Isochlorogenic acid derived from stevia improves antioxidant capacity, immune function and intestinal microbiota in weaned piglets. Front. Vet. Sci. 12:1672217. doi: 10.3389/fvets.2025.1672217
Edited by:
Yeni Widiawati, National Research and Innovation Agency (BRIN), IndonesiaReviewed by:
Shahid Ali Rajput, Muhammad Nawaz Shareef University of Agriculture, PakistanZhe Yang, Hunan Agricultural University, China
Copyright © 2025 Wang, Luo, Zou, Gao, Yu, He, Song, Luo, Zheng, Mao, Xuan, Xu and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meili Xu, bWVpbGl4dUB0anUuZWR1LmNu; Jie Yu, eXVqaWVAc2ljYXUuZWR1LmNu
†These authors have contributed equally to this work
 Yuxin Wang1†
Yuxin Wang1† Yong Luo
Yong Luo Wei Gao
Wei Gao Bing Yu
Bing Yu Jun He
Jun He Yuheng Luo
Yuheng Luo Xiangbing Mao
Xiangbing Mao Jie Yu
Jie Yu