- 1Clinical Unit of Internal Medicine Small Animals, Department for Companion Animals and Horses, University of Veterinary Medicine, Vienna, Austria
- 2Clinical Unit of Anesthesiology and Perioperative Intensive Care, Department for Companion Animals and Horses, University of Veterinary Medicine, Vienna, Austria
- 3Institute of Pathology, University of Veterinary Medicine, Vienna, Austria
- 4Diagnostic Imaging, Department for Companion Animals and Horses, University of Veterinary Medicine, Vienna, Austria
- 5Small Animal Surgery, Department of Companion Animals and Horses, University of Veterinary Medicine, Vienna, Austria
Alpha-1 antitrypsin deficiency (A1ATD) is a rare genetic condition in both humans and animals, caused by mutations in the SERPINA1 gene that lead to reduced or absent production of alpha-1 antitrypsin (A1AT). This case report describes a 3-year-old male dog presenting with persistent pleural effusion, chronic nonproductive cough, and respiratory distress. Despite an extensive diagnostic evaluation that included computed tomography (CT), a definitive diagnosis of A1ATD was only reached after a histopathological examination of lung tissue, which revealed acinar emphysema characterized by the destruction of alveolar walls. Serum A1AT levels were undetectable, confirming the diagnosis. The absence of liver involvement aligned with the lung-predominant phenotype described in human A1ATD. This is the first reported case of A1ATD-associated emphysema and pleural effusion in a dog, emphasizing the need for further research into its pathophysiology, diagnosis, and management in canine patients.
Introduction
Alpha-1 antitrypsin (A1AT) is a serine protease inhibitor that blocks neutrophil elastase and helps maintain protease/antiprotease balance in the lung (1). A1AT also demonstrates antioxidative and anti-inflammatory properties (2). It is primarily produced in the liver by hepatocytes, but also by alveolar monocytes and macrophages (3, 4). Genetic mutations in the SERPINA1 gene can result in reduced or no production of A1AT from birth or early life (5). Alpha-1 antitrypsin deficiency (A1ATD) is an underdiagnosed autosomal genetic disorder in humans that leads to liver disease and emphysema, often presenting with chronic obstructive pulmonary disease (COPD) and small airway dysfunction (6, 7). Reports of A1ATD in dogs are very rare, and comprehensive studies are lacking; however, the limited information available suggests that genetic, diagnostic, and potential therapeutic pathways may be similar to those in humans (8–10). To the best of our knowledge, this is the first case report describing a dog with A1ATD presenting with persistent pleural effusion and pulmonary emphysema.
Case description
A 3-year-old male intact mixed-breed dog was referred to the Small Animal Internal Medicine Unit of our university for further investigation of transudative pleural effusion, chronic non-productive cough, chronic expiratory respiratory distress, lethargy, reduced appetite, and weight loss. The dog lived in an urban area with no known exposure to smoke, toxic substances, or heavy metals. The referring veterinarian had performed routine blood work, including a CBC, serum biochemistry, and C-reactive protein (CRP) testing, all of which were within normal limits. Thoracic radiographs revealed the presence of pleural effusion. Thoracic computed tomography (CT) revealed moderate pleural effusion and multiple small, round nodules in the left lung lobes, measuring 1–4 mm in diameter (Figure 1). Differential diagnoses included inflammatory lesions, granulomas, small areas of atelectasis, and neoplasia. Effusion analysis performed by the referring veterinarian, including total protein, cytology, LDH, and cell count, classified the pleural effusion as a transudate. No etiological cause for the transudate in the pleural space or persistent cough could be identified by the referring veterinarian. Prior to referral, the dog was treated with several medications, including antibiotics (amoxicillin with clavulanic acid, enrofloxacin, and doxycycline), furosemide, meloxicam, antihistamines, and a short course of dexamethasone at an anti-inflammatory dosage, without clinical improvement. However, detailed dosage regimens were not available. Upon first clinical examination, the dog demonstrated mild expiratory respiratory distress, a body condition score of 4/9, and increased lung sounds cranially on both sides, while the rest of the clinical examination was unremarkable. Upon admission, blood tests, including CBC and serum biochemistry, were repeated. The hematocrit was at the lower limit (37%; RI 37–55%), while total protein and albumin were slightly decreased (4.24 g/dL, [RI 6.00–7.50 g/dL] and 2.29 g/dL [RI 2.58–4.73 g/dL], respectively). Blood urea nitrogen was increased (48.5 mg/dL; RI 20–40 mg/dL). CRP was within normal range (0.8 mg/dL; RI < 35 mg/dL) and remained low throughout all future rechecks. After a positive fecal occult blood test, the above findings were attributed to gastrointestinal (GI) bleeding, probably due to prior treatment with meloxicam and dexamethasone. With treatment with omeprazole (1 mg/kg IV q12h), sucralfate (30 mg/kg PO q8h), and maropitant (1 mg/kg IV q24h), the GI bleeding ceased, and clinical parameters normalized within 5 days. Liver function testing (bile acids, ammonia, and coagulation panel) and serum osmolality (302 mOsm/kg; RI: 290–310 mOsm/kg) were within normal limits. Analysis of the pleural effusion confirmed a pure transudate, with a total protein of 0.5 g/dL and no inflammatory cells. Bacterial culture of the transudate was negative. Echocardiography revealed no abnormalities. Antigen enzyme-linked immunosorbent assay (ELISA) tests for Dirofilaria immitis and Angiostrongylus vasorum were negative. Fecal flotation and larval migration (Baermann–Wetzel test) for lungworms were also negative. Coagulation testing, including D-dimer and viscoelastic analysis, ruled out a thrombotic state. Serum antinuclear antibody levels were within normal limits, and serum protein electrophoresis was unremarkable. Furthermore, serologic testing for Leishmania infantum, Ehrlichia canis, and Borrelia burgdorferi yielded negative results (all below 1:20). Urinalysis was unremarkable, and the urine protein-to-creatinine ratio (UPC) was within normal range. Abdominal ultrasound showed no abnormalities except slight signs of gastroenteritis. A follow-up thoracic CT scan (Somatom X.cite VA30, Siemens Healthcare GmbH, 91,052, Germany), performed 78 days after the first one, was followed by bronchoscopy with bronchoalveolar lavage (BAL) and ultrasound-guided fine-needle aspiration (FNA) of the lung. On CT, the previously described lung nodules (round shadows) were no longer present; however, multifocal consolidation of the right lung lobes and pleural effusion were noted (Figure 2). Flexible fiberoptic bronchoscopy, along with cytologic evaluation of both the BAL fluid and the FNA from a consolidated area of the right lung, revealed predominantly alveolar macrophages with very few neutrophils and was considered unremarkable. Bacterial cultures and susceptibility testing detected very low concentrations of Pasteurella multocida, which was sensitive to amoxicillin. Polymerase chain reaction (PCR) testing for Mycobacterium spp., Toxoplasma gondii, and mycology from both the pleural effusion and pulmonary FNA material yielded negative results. After exclusion of all the above mentioned potential causes, differential diagnoses included rare causes of interstitial lung disease, such as environmental and occupational exposures (e.g., asbestosis, silicosis, and extrinsic allergic alveolitis), pulmonary alveolar proteinosis, parasitic infections (Pneumocystis carinii and Mesocestoides), A1ATD, lipid pneumonia, systemic inflammatory diseases (e.g., lupus or polyarteritis nodosa), and, finally, idiopathic interstitial pneumonia.

Figure 1. First CT study: Sagittal reconstruction of the left lung (maximum intensity projection, 3 mm thick section) (A) and transverse CT image at the level of the right middle lung lobe (averaged intensity projection; 0,7 mm thin section; WL -600, WW 1200) (B). Round shadows in the left lung lobes (arrows) and multifocal consolidation with air bronchograms (demonstrated in the right middle lung lobe; asterisk) and pleural effusion were the major findings. Attenuation of non-consolidated lung parenchyma: -850 to -800 Hounsfield Units (HU) (reference range of normal lung tissue: -846 to -713 HU (34).
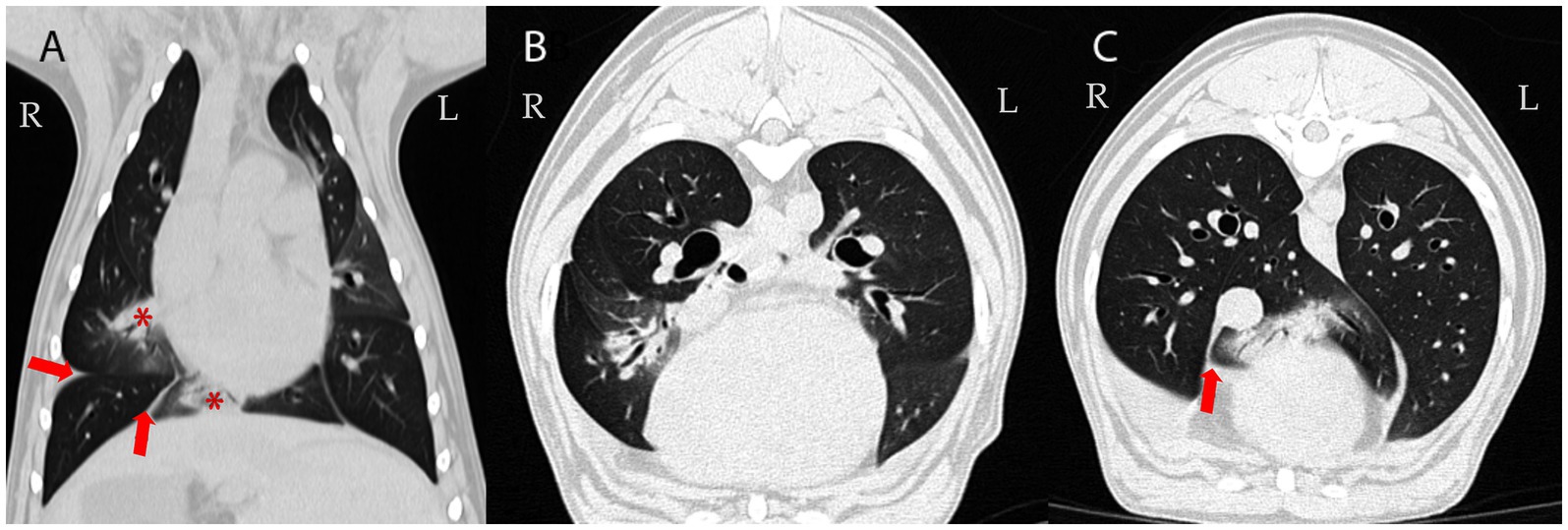
Figure 2. Follow-up CT examination 78 days later: Dorsal (A) and transverse CT images of the lung (B,C); averaged intensity projection; 0,6 mm thin section; WL -600, WW 1200. Multifocal airway-associated consolidation (demonstrated in the right middle and accessory lung lobes; asterisk in A) and pleural effusion persisted (arrows). Attenuation of non-consolidated lung parenchyma: -850 to -800 Hounsfield Units (HU) (reference range of normal lung tissue: -846 to -713 HU (34).
As no etiological diagnosis could be established and the patient continued to experience recurrent pleural effusions despite multiple thoracocenteses, surgical biopsy and histopathology of the lung and pleura were performed. After right lateral thoracotomy, a total lobectomy of the right middle lobe and a biopsy of the parietal pleura were performed. A right lateral thoracotomy was selected because imaging demonstrated the most pronounced lesions in the right hemithorax, and the right middle lobe was both severely affected and surgically the most accessible. Grossly, the affected lobe exhibited regions of overexpansion with thin, fragile parenchyma interspersed with areas of collapse, consistent with emphysematous changes and atelectasis. The remaining lung lobes, pleura, pericardium, mediastinum, and regional lymph nodes appeared unremarkable on both inspection and palpation. Complete lobectomy was performed both to obtain sufficient tissue for histopathological examination and to remove the most diseased parenchyma, following standard surgical technique. For the management of the persistent pleural effusion, a 14F pleural port (PP102K, Norfolk Vet Products Inc., 60,076 Illinois, USA) was installed in the eighth right intercostal space. For histopathological examination of the dissected lung and pleura, routine tissue sections stained with hematoxylin and eosin were produced. Regions of largely expanded alveoli with confluence of alveolar spaces due to destruction of alveolar walls/septa (emphysema) (Figure 3) were intermixed with regions of almost complete alveolar collapse. Alveolar collapse was interpreted as compression atelectasis secondary to pleural effusion. Atelectatic regions were characterized by closely arranged alveolar walls interspersed by slit-like residual alveolar spaces. No inflammation was found within the pulmonary airways, interstitial space, visceral pleura, or vessels. Special stains for iron revealed few alveolar macrophages containing hemosiderin. No fungi (such as Pneumocystis carinii) or other infectious agents were detected using the periodic acid-Schiff (PAS) reaction.
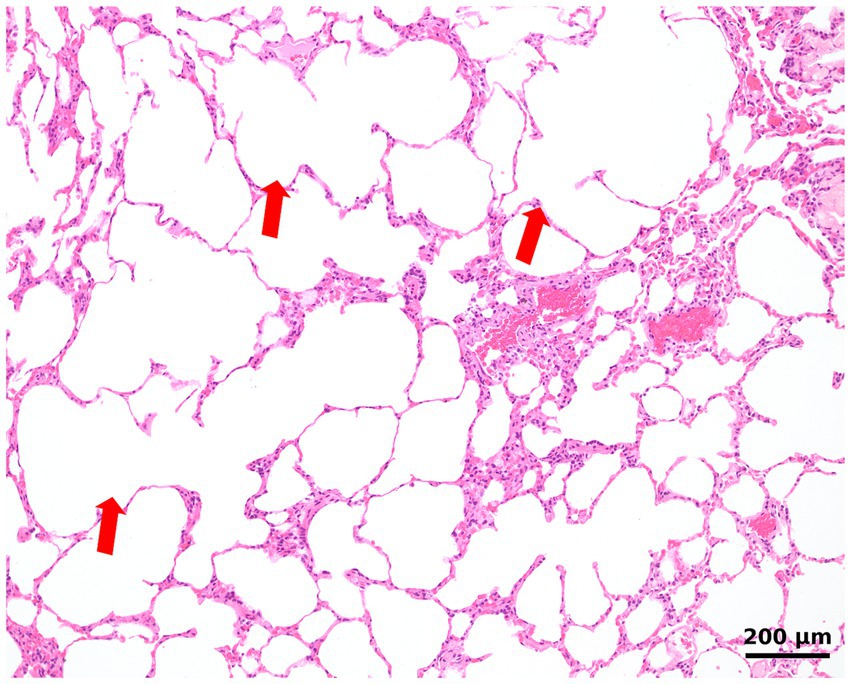
Figure 3. Histologic image of the lung shows largely expanded alveoli with the confluence of alveolar spaces, consistent with emphysema (arrows). The lung is devoid of inflammation.
In addition to histopathology, bacterial and mycological cultures of the tissues, and PCR for Mycobacterium spp., were performed and turned out to be negative. Based on histological findings, A1ATD was considered a possible differential diagnosis for the emphysema and the persistent pleural effusion. Quantitative measurement of serum A1AT was performed with a sandwich ELISA similar to what was previously described in the literature (11). In two consecutive measurements, the concentration of serum A1AT was unmeasurably low (<180 mg/L) compared to a surplus serum sample from a healthy dog, strongly suggesting a diagnosis of A1ATD. To further confirm the diagnosis, a second serum A1AT measurement was performed at a different laboratory using the immunoassay described by Heilmann et al. (12). The A1AT concentration was again low (13.6 mg/L, RI 732–1802 mg/L). The patient was discharged 3 days after surgery with supportive treatment and instructions to drain the effusion through the pleural port if persistent respiratory distress occurred, as no causal treatment for this condition in dogs has yet been described. During follow-up examinations at 2 weeks, 4 weeks, 2 months, 6 months, and 1 year, the cough had resolved, and the dog remained clinically healthy, aside from persistent pleural effusion. Drainage via the port was initially needed biweekly and later reduced to once a month.
Discussion
The first cases of A1ATD in humans were described more than 50 years ago, and since then, various phenotypic variations with differing disease severity and prognosis have been reported (13, 14). Alpha-1 antitrypsin deficiency is a rare genetic disorder that can cause emphysema in humans and is commonly mistaken for asthma (5). The pathophysiology is attributed to the lack of A1AT function, which normally protects lung tissues, largely by neutrophil elastase, from proteolytic destruction (5). Chronic deficiency of A1AT can lead to severe emphysema and liver cirrhosis due to the lack of inhibition of neutrophil elastase (15, 16). To date, the only available treatment in humans is augmentation therapy, which is considered safe, although the effectiveness and benefit are controversial (17, 18). Augmentation therapy involves the intravenous administration of A1AT derived from pooled human plasma to increase serum levels and protect lung tissue from proteolytic damage. Augmentation therapy can lead to a slower progression of emphysema in patients with severe A1ATD by reducing the decline in lung function (19). Safe conclusions have yet to be drawn, as the number of clinical studies is low due to disease rarity and its phenotypic variability (5).
There are very few studies on canine A1ATD, which suggest its connection to chronic liver disease or inflammatory diseases such as panniculitis, polyarthritis, and meningitis in dogs (10, 20–22). This condition is particularly suspected in breeds such as Cocker Spaniels and Bedlington terriers, although it remains unclear whether A1ATD is a direct cause of liver disease or a sign of hepatocellular injury or dysfunction. A case report described decreased A1AT concentrations in a dog with Bartonella spp. infection and panniculitis, polyarthritis, and meningitis (22), but another case series could not establish a connection between panniculitis and A1ATD (21). Moreover, the involvement of A1AT (or alpha-1 proteinase inhibitor) has been more thoroughly investigated in canine chronic gastrointestinal disease (23). Alpha-1 proteinase inhibitor (α1-PI) levels in fecal samples can be a helpful diagnostic tool in addition to routine diagnostics and histopathology for diagnosing and monitoring chronic inflammatory gastrointestinal disease (23). Because α1-PI is resistant to breakdown, it is an effective indicator of intestinal protein loss.
To the best of our knowledge, this is the first case of a dog with acinar pulmonary emphysema and pleural effusion associated with A1ATD. The owner reported no exposure to excessive air pollutants or smoking. In our case, no indications suggesting hepatic disease were present. All liver enzymes and liver function parameters were within normal range, and no signs of hepatopathy were detected in ultrasonography or CT. In people with A1ATD, three phenotypes have been reported. In individuals with the lung-predominant phenotype, emphysema can develop independently of liver disease due to the condition’s distinct mechanisms (24). This process is particularly pronounced in individuals with severe deficiency genotypes and is often exacerbated by environmental factors such as smoking or air pollution. In contrast, liver disease in A1ATD arises from the accumulation of misfolded A1AT protein in hepatocytes, a process dependent on specific genetic mutations and protein folding abnormalities (24). While both complications can occur in the same individual, emphysema without liver disease is possible, particularly in cases where protein misfolding and hepatocyte accumulation are minimal, as observed in certain genotypes (7, 24). Spontaneous pneumothorax (SP) represents another clinically significant complication of A1ATD in human patients, typically resulting from the rupture of subpleural bullae in emphysematous lungs. SP may constitute the initial clinical manifestation of previously undiagnosed A1ATD and may recur even after surgical intervention (25). In the current case, CT and thoracotomy did not identify pulmonary bullae, and no episodes of SP occurred during the follow-up period. However, this association remains important for future investigations, as the occurrence of SP in young dogs without trauma or other predisposing factors could indicate underlying A1ATD.
Computed tomography did not reveal any signs of generalized emphysema; however, acinar emphysema was confirmed histopathologically. Similar to findings described in humans (26), round- and lobular-like homogeneous increases in pulmonary parenchymal attenuation that obscured the margins of vessels, airway walls, and air bronchograms, were present on CT. Hypothetically, these changes could correspond to microparticles, such as dust or other environmental contaminants, inducing multifocal pneumonia. Unlike most human cases or other reported dogs with emphysema of different origins (27, 28), our case did not exhibit hyperlucent lung fields, diaphragmatic flattening, or even pulmonary that are responsible for decreased alveolar surface area for gas exchange and contribute to symptoms such as labored breathing, exercise intolerance, and, over time, chronic respiratory failure. Instead, multifocal consolidation (29), rather than emphysema, was primarily observed on CT scan. Moreover, the round lesions noted during the initial CT resolved over time, indicating that a major breakdown of alveolar walls leading to emphysema was not observed during CT, which had a minimal in-plane resolution of 0.4 mm. The resolution of the pulmonary nodules observed on follow-up CT suggests that these lesions were most likely transient inflammatory or infectious foci rather than permanent emphysematous changes attributable to A1ATD. This distinction is important, as emphysema caused by A1ATD is progressive and irreversible, while the disappearance of nodular opacities indicates a separate, self-limiting process. Therefore, the resolution of these lesions does not contradict the diagnosis of A1ATD but rather highlights the concurrent presence of transient pulmonary abnormalities that complicated the diagnostic evaluation. Naturally, it is not possible to differentiate interstitial pneumonia, atelectasis, or tissue breakdown from fluid-filled spaces within consolidated areas by CT. CT consistently underestimates the extent of centriacinar and panacinar emphysema, often missing most lesions less than 0.03–0.04 cm in diameter (30), and is considered insensitive in detecting the earliest or smaller emphysematous lesions in humans (30). This limitation probably applies even more to smaller dogs. However, high-resolution CT was not performed (31). Despite CT’s limited spatial and contrast resolution, histopathology provided significantly higher sensitivity for detecting or confirming acinar emphysema. However, CT showed that the lung was not overinflated.
There are some limitations in our case report. First, the dog was previously treated with multiple antibiotics and glucocorticoids from the referring veterinarian, which could have a noteworthy influence on our diagnosis. Glucocorticoid administration can influence serum A1AT concentrations. As an acute-phase reactant, A1AT hepatic synthesis typically increases in response to inflammation or stress (32). Nevertheless, as the A1AT serum concentration was unmeasurable, an influence of the glucocorticoid treatment on the diagnosis seems unlikely. Furthermore, quantitative A1AT measurement was performed by ELISA and by the validated radioimmunoassay that has been established and could provide more reliable results, as it is independent of antibody specificity (12, 33). The sample yielded a value of 13.5 mg/L, markedly below the published reference interval (RI; >700 mg/L) (12). However, parallel measurement of control serum also resulted in concentrations below the reported RI, suggesting a potential issue with assay performance. To address this, three control serum samples were used for the measurement with radioimmunoassay. Diagnosis of A1AT in people relies not only on quantitative measurement of serum A1AT but also on the detection of the mutation in the SERPINA1 gene. No such analysis is currently accessible for dogs and could therefore not be performed to confirm the gene mutation. Although assay variability and methodological differences can influence results, the concordance of two independent assays, combined with the histopathological finding of emphysema in a young dog without other explanatory causes, provides strong support for a diagnosis of A1ATD. We, therefore, consider the diagnosis to be accurate, while acknowledging that genetic sequencing would represent the gold standard once available in dogs.
Here, we report the first documented case of A1ATD causing persistent pleural effusion and emphysema in a young dog. In our case, the exact pathophysiological mechanism leading to pleural effusion remains uncertain. A plausible explanation is that the lack of A1AT results in increased neutrophil elastase activity and subsequent alveolar wall destruction, which may alter pulmonary microvascular integrity and promote the leakage of low-protein transudate into the pleural space. Additionally, impaired antiprotease defense may contribute to subtle chronic inflammation and increased capillary permeability, even in the absence of overt inflammatory infiltrates. Despite extensive imaging and diagnostic workup, including CT, the emphysematous changes were confirmed only through histopathological examination, emphasizing its superior sensitivity in detecting emphysematous lesions. The absence of hematologic and radiologic evidence of liver involvement is consistent with the lung-predominant phenotype observed in certain genotypes of human A1ATD. Although no specific treatment options are available in dogs, supportive treatment and regular drainage of pleural effusion through a chest port can offer an acceptable quality of life. Further studies are warranted to better understand the clinical presentation, genetic basis, and optimal management of canine A1ATD.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the studies involving animals in accordance with the local legislation and institutional requirements, because this manuscript is a case report. Written informed consent was obtained from the owners for the participation of their animals in this study. Written informed consent was obtained from the participants for the publication of this case report.
Author contributions
PD: Conceptualization, Data curation, Investigation, Methodology, Project administration, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. CF-R: Conceptualization, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. CB: Data curation, Investigation, Validation, Writing – original draft, Writing – review & editing. OG: Data curation, Investigation, Writing – original draft, Writing – review & editing. SK: Conceptualization, Data curation, Investigation, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing. BD: Data curation, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. RH: Data curation, Investigation, Supervision, Validation, Writing – original draft, Writing – review & editing. IB: Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. NL-Z: Data curation, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Open access funding provided by University of Veterinary Medicine Vienna.
Acknowledgments
We thank the central laboratory service of the General Hospital of Vienna for performing the A1AT measurements.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Toumpanakis, D, and Usmani, OS. Small airways disease in patients with alpha-1 antitrypsin deficiency. Respir Med. (2023) 211:107222. doi: 10.1016/j.rmed.2023.107222
2. Bergin, DA, Hurley, K, McElvaney, NG, and Reeves, EP. Alpha-1 antitrypsin: a potent anti-inflammatory and potential novel therapeutic agent. Arch Immunol Ther Exp. (2012) 60:81–97. doi: 10.1007/s00005-012-0162-5
3. Kelly, E, Greene, CM, Carroll, TP, McElvaney, NG, and O'Neill, SJ. Alpha-1 antitrypsin deficiency. Respir Med. (2010) 104:763–72. doi: 10.1016/j.rmed.2010.01.016
4. Belchamber, KB, Walker, EM, Stockley, RA, and Sapey, E. Monocytes and macrophages in alpha-1 antitrypsin deficiency. Int J Chron Obstruct Pulmon Dis. (2020) 15:3183–92. doi: 10.2147/COPD.S276792
5. Miravitlles, M, Dirksen, A, Ferrarotti, I, Koblizek, V, Lange, P, Mahadeva, R, et al. European Respiratory Society statement: diagnosis and treatment of pulmonary disease in α1-antitrypsin deficiency. Eur Respir J. (2017) 50:1700610. doi: 10.1183/13993003.00610-2017
6. Tejwani, V, and Stoller, JK. The spectrum of clinical sequelae associated with alpha-1 antitrypsin deficiency. Ther Adv Chronic Dis. (2021) 12_suppl:2040622321995691. doi: 10.1177/2040622321995691
7. Stoller, JK, and Aboussouan, LS. A review of α1-antitrypsin deficiency. Am J Respir Crit Care Med. (2012) 185:246–59. doi: 10.1164/rccm.201108-1428CI
8. Ferrarotti, I, Wencker, M, and Chorostowska-Wynimko, J. Rare variants in alpha 1 antitrypsin deficiency: a systematic literature review. Orphanet J Rare Dis. (2024) 19:82. doi: 10.1186/s13023-024-03069-1
9. Mitchell, EL, and Khan, Z. Liver disease in Alpha-1 antitrypsin deficiency: current approaches and future directions. Curr Pathobiol Rep. (2017) 5:243–52. doi: 10.1007/s40139-017-0147-5
10. Sevelius, E, Andersson, M, and Jönsson, L. Hepatic accumulation of alpha-1-antitrypsin in chronic liver disease in the dog. J Comp Pathol. (1994) 111:401–12. doi: 10.1016/s0021-9975(05)80098-2
11. Melgarejo, T, Williams, DA, and Asem, EK. Enzyme-linked immunosorbent assay for canine alpha 1-protease inhibitor. Am J Vet Res. (1998) 59:127–30.
12. Heilmann, RM, Ruaux, CG, Burgener, IA, Hern, JD, Suchodolski, JS, and Steiner, JM. Serum alpha1-proteinase inhibitor concentrations in healthy dogs--method validation and determination of reference interval and intra-individual variation. Vet Clin Pathol. (2013) 42:190–5. doi: 10.1111/vcp.12039
13. Eriksson, S. Pulmonary emphysema and alpha1-antitrypsin deficiency. Acta Med Scand. (1964) 175:197–205. doi: 10.1111/j.0954-6820.1964.tb00567.x
14. Papiris, SA, Veith, M, Papaioannou, AI, Apollonatou, V, Ferrarotti, I, Ottaviani, S, et al. Alpha1-antitrypsin deficiency in Greece: focus on rare variants. Pulmonology. (2024) 30:43–52. doi: 10.1016/j.pulmoe.2022.12.007
15. Sveger, T. Liver disease in alpha1-antitrypsin deficiency detected by screening of 200,000 infants. N Engl J Med. (1976) 294:1316–21. doi: 10.1056/NEJM197606102942404
16. Marek, G, Collinsworth, A, Liu, C, Brantly, M, and Clark, V. Quantitative measurement of the histological features of alpha-1 antitrypsin deficiency-associated liver disease in biopsy specimens. PLoS One. (2021) 16:e0256117. doi: 10.1371/journal.pone.0256117
17. Gøtzsche, PC, and Johansen, HK. Intravenous alpha-1 antitrypsin augmentation therapy for treating patients with alpha-1 antitrypsin deficiency and lung disease. Cochrane Database Syst Rev. (2016) 2016:CD007851. doi: 10.1002/14651858.CD007851.pub3
18. Pierce, LR. Assessing the efficacy of Alpha1-proteinase inhibitor (human) augmentation therapy for Alpha1-antitrypsin deficiency - related emphysema: challenges and opportunities. Heliyon. (2024) 10:e31183. doi: 10.1016/j.heliyon.2024.e31183
19. Stockley, RA, Parr, DG, Piitulainen, E, Stolk, J, Stoel, BC, and Dirksen, A. Therapeutic efficacy of α-1 antitrypsin augmentation therapy on the loss of lung tissue: an integrated analysis of 2 randomised clinical trials using computed tomography densitometry. Respir Res. (2010) 11:136. doi: 10.1186/1465-9921-11-136
20. Côté, E, Ettinger, SJ, and Feldman, EC. Ettinger's textbook of veterinary internal medicine. Philadelphia: Elsevier (2024).
21. Hughes, D, Goldschmidt, MH, Washabau, RJ, and Kueppers, F. Serum alpha 1-antitrypsin concentration in dogs with panniculitis. J Am Vet Med Assoc. (1996) 209:1582–4.
22. Mellor, PJ, Fetz, K, Maggi, RG, Haugland, S, Dunning, M, Villiers, EJ, et al. Alphal-proteinase inhibitor deficiency and Bartonella infection in association with panniculitis, polyarthritis, and meningitis in a dog. J Vet Intern Med. (2006) 20:1023–8. doi: 10.1111/j.1939-1676.2006.tb01823.x
23. Heilmann, RM, Parnell, NK, Grützner, N, Mansell, J, Berghoff, N, Schellenberg, S, et al. Serum and fecal canine α1-proteinase inhibitor concentrations reflect the severity of intestinal crypt abscesses and/or lacteal dilation in dogs. Vet J. (2016) 207:131–9. doi: 10.1016/j.tvjl.2015.10.042
24. Sandhaus, RA, Turino, G, Brantly, ML, Campos, M, Cross, CE, Goodman, K, et al. The diagnosis and Management of Alpha-1 antitrypsin deficiency in the adult. Chronic Obstr Pulm Dis. (2016) 3:668–82. doi: 10.15326/jcopdf.3.3.2015.0182
25. Lepiorz, M, Großer, C, Hofmann, H-S, and Pfeifer, M. Seltene Ursache eines Spontanpneumothorax. Pneumologie. (2017) 71:590–3. doi: 10.1055/s-0043-112886
26. Mulkareddy, V, and Roman, J. Pulmonary manifestations of alpha 1 antitrypsin deficiency. Am J Med Sci. (2024) 368:1–8. doi: 10.1016/j.amjms.2024.04.002
27. Warwick, H, Guillem, J, Batchelor, D, Schwarz, T, Liuti, T, Griffin, S, et al. Imaging findings in 14 dogs and 3 cats with lobar emphysema. J Vet Intern Med. (2021) 35:1935–42. doi: 10.1111/jvim.16183
28. Huang, Y-CT, Wencker, M, and Driehuys, B. Imaging in alpha-1 antitrypsin deficiency: a window into the disease. Ther Adv Chronic Dis. (2021) 12_suppl:20406223211024523. doi: 10.1177/20406223211024523
29. Hansell, DM, Bankier, AA, MacMahon, H, McLoud, TC, Müller, NL, and Remy, J. Fleischner society: glossary of terms for thoracic imaging. Radiology. (2008) 246:697–722. doi: 10.1148/radiol.2462070712
30. Stern, EJ, and Frank, MS. CT of the lung in patients with pulmonary emphysema: diagnosis, quantification, and correlation with pathologic and physiologic findings. AJR Am J Roentgenol. (1994) 162:791–8. doi: 10.2214/ajr.162.4.8140992
31. Morandi, F, Mattoon, JS, Lakritz, J, Turk, JR, and Wisner, ER. Correlation of helical and incremental high-resolution thin-section computed tomographic imaging with histomorphometric quantitative evaluation of lungs in dogs. Am J Vet Res. (2003) 64:935–44. doi: 10.2460/ajvr.2003.64.935
32. Berger, M, Liu, M, Uknis, ME, and Koulmanda, M. Alpha-1-antitrypsin in cell and organ transplantation. Am J Transplant. (2018) 18:1589–95. doi: 10.1111/ajt.14756
33. Heilmann, RM, Paddock, CG, Ruhnke, I, Berghoff, N, Suchodolski, JS, and Steiner, JM. Development and analytical validation of a radioimmunoassay for the measurement of alpha1-proteinase inhibitor concentrations in feces from healthy puppies and adult dogs. J Vet Diagn Invest. (2011) 23:476–85. doi: 10.1177/1040638711404152
Keywords: emphysema, pulmonary, pleural effusion, canine, alpha-1 antitrypsin (AAT), histopathology
Citation: Doulidis PG, Frizzo-Ramos C, Bertram CA, Grünzweil OM, Kneissl SM, Degasperi B, Hirt RA, Burgener IA and Luckschander-Zeller N (2025) Case Report: α1-antitrypsin deficiency causing persistent pleural effusion and multilobar alveolar emphysema in a young dog. Front. Vet. Sci. 12:1678702. doi: 10.3389/fvets.2025.1678702
Edited by:
Valeria Pasciu, University of Sassari, ItalyReviewed by:
Iva Šmit, University of Zagreb, CroatiaAndrew L. Leisewitz, Auburn University, United States
Copyright © 2025 Doulidis, Frizzo-Ramos, Bertram, Grünzweil, Kneissl, Degasperi, Hirt, Burgener and Luckschander-Zeller. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pavlos G. Doulidis, UGF2bG9zLkRvdWxpZGlzQHZldG1lZHVuaS5hYy5hdA==
 Pavlos G. Doulidis
Pavlos G. Doulidis Carolina Frizzo-Ramos
Carolina Frizzo-Ramos Christof A. Bertram
Christof A. Bertram Olivia M. Grünzweil
Olivia M. Grünzweil Sibylle M. Kneissl
Sibylle M. Kneissl Brigitte Degasperi
Brigitte Degasperi Reinhard A. Hirt1
Reinhard A. Hirt1 Iwan A. Burgener
Iwan A. Burgener Nicole Luckschander-Zeller
Nicole Luckschander-Zeller