- 1Department of Neurosurgery, Zhumadian Central Hospital, Affiliated Central Hospital of Huanghuai University, Zhumadian, China
- 2College of Medicine, Huanghuai University, Zhumadian, China
- 3College of Life Science and Technology, Beijing University of Chemical Technology, Beijing, China
Aeromonas hydrophila (A. hydrophila) is a common pathogen in aquaculture that also causes opportunistic infections and sporadic food- and water-borne illness in humans. Phage therapy is increasingly considered a promising complementary medicine for antibiotic therapy. In this study, we isolated a novel A. hydrophila phage (designated BUCT551) using A. hydrophila strain Ah18 as an indicator. The one-step growth curve demonstrated that BUCT551 had a latent period of 20 min and a burst size of 32 PFU/cell at its optimal multiplicity of infection (0.1). BUCT551 had a survival pH range from 5 to 10 and could tolerant temperatures from 4 °C to 50 °C. Host range analysis showed that the phage was able to lyse not only A. hydrophila, but also Aeromonas veronii. Whole-genome sequencing of BUCT551 revealed a linear DNA genome of 61,382 bp. Bioinformatics analysis demonstrated that the genome of phage BUCT551 contains 74 predicted open reading frames (ORFs), of which 27 were annotated as functional proteins with assigned biological roles. Notably, no lysogeny-associated genes, antimicrobial resistance determinants, virulence factors, or tRNA genes were identified in this phage genome. A comparative genomic analysis showed that phage BUCT551 is the closest relative to Aeromonas phage LAh_7 and shares the same new branch in the phylogenetic tree. Characterization of the phage BUCT551 enriches our knowledge about the diversity of A. hydrophila phages.
1 Introduction
Aeromonas hydrophila (A. hydrophila) is a Gram-negative, non-spore-forming, rod-shaped, facultative anaerobic organism belonging to the Aeromonadaceae family (1). It is ubiquitous in the natural environment because of its wide range of survival and reproductive temperatures (from 4 °C to 40 °C) (2). A. hydrophila is usually found in aquatic environments, such as fresh river water, and is recognized as an opportunistic pathogenic of poikilothermic and homoeothermic animals including humans (3, 4). A. hydrophila is considered the causative agent of multiple diseases in many fish species, including ulcerative disease, hemorrhagic disease, red sore disease, and septicemia, leading to severe economic losses to the aquaculture industry (5–7). Furthermore, A. hydrophila is pathogenic to humans, especially immunocompromised individuals, as it colonizes the human intestinal tract and has been linked to gastroenteritis, skin diseases, and septicemia (5, 8–10). A. hydrophila produces virulence factors, including aerolysin, hemolysin, adhesion factor, enterotoxin, and extracellular protease, that act individually or in synergy, resulting in tissue damage and contributing to pathogenicity (8). Aeromonas infections have been reported in recent decades, and data from Japan indicated that Aeromonas species were isolated from 5% of travelers (23,215 persons in total) who returned from developing countries (10). In 2018–2019, Hilt et al. isolated several multidrug-resistant A. hydrophila isolates from two solid organ transplant patients (11). In another recent case, Hasan et al. reported A. hydrophila infection in an immunocompromised 69-year-old woman, with surgical site sepsis/infection and several comorbidities (12).
The increasing levels of antibiotic resistance among bacteria present a great challenge to conventional medicine and pose a real threat to human life (13, 14). Antibiotic resistance among Aeromonas spp. has attracted increasing attention in recent years. Elbehiry et al. isolated A. hydrophila from 150 meat and water samples to screen their antibiotic resistance, and found that some A. hydrophila strains were resistant to ampicillin, cefotaxime, pefloxacin, ceftazidime, and ciprofloxacin (15). In another study, A. hydrophila was found to have acquired a gene encoding the carbapenemase GES-24, conferring carbapenem resistance (16). Given that A. hydrophila exhibits pathogenicity in both humans and animals, and outbreaks lead to economic losses in agriculture/aquaculture or public health incidents, the emergence of antibiotic resistance in this pathogen should be a focus of research.
As a result of increasing antibiotic drug resistance, phage therapy has gained attention, with the potential for use either alone or in combination with antibiotics (17–19). Phages are exclusively virulent against prokaryotic microbes and do not lyse animal and human cells. They can be classified into lytic phage and temperate phage depending on their life cycle. Lytic phages lyse host bacteria immediately after undergoing replication and assembly in the host, leading to the elimination of host bacteria. The characteristics of lytic phages endow therapeutic potential against bacterial infection (20). Some phages demonstrate high specificity to a single species or even a certain strain of bacteria (21). To enhance the efficacy when using phages as antibacterial agents, a strategy has been suggested that involves mixing a cocktail of different phages into one formulation (22). However, only a few A. hydrophila phages have been isolated and characterized (23). In this study, we isolated and characterized a novel phage specific to drug-resistant A. hydrophila. Additionally, we evaluated the application potential of this phage by characterizing its physiological properties, lytic activity, host range, and genomic features. This systematic assessment provides a comprehensive basis for determining its suitability in therapeutic scenarios.
2 Materials and methods
2.1 Bacterial strain isolation and identification
The host strain Ah18 was isolated from an aquaculture farm (Nanjing, China). Molecular identification of strain Ah18 was conducted based on 16S rRNA gene sequencing. The gene was amplified from genomic DNA using the universal primers 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-GGCTACCTTGTTACGACTT-3′). The resulting amplicons were sequenced by RuiBiotech (Beijing, China). The obtained sequence was subjected to BLASTn analysis via the NCBI database, which revealed 100% identity with the 16S rRNA sequence of A. hydrophila strain GSH8-2 (accession no. AP019193.1). Based on these results, we confirmed that Ah18 belonged to species A. hydrophila. Strain Ah18 was cultured at 28–30 °C with shaking at 220 × g in Tryptic Soy Broth (TSB) medium (BD Difco).
2.2 Drug sensitivity test of A. hydrophila strain Ah18
The antimicrobial susceptibility profile of strain Ah18 was determined using the broth microdilution method as previously described (24). The antibiotics used in the test were purchased from Fosun Diagnostics (Shanghai, China). The bacterial isolate Ah18 was cultured on Mueller–Hinton (MH) agar and incubated at 28 °C for 24 h. A bacterial suspension adjusted to 0.5 McFarland standard was prepared in sterile saline, diluted with MH broth to approximately 1 × 106 CFU/mL, and 50 μL of the diluted suspension was aliquoted into each well of a microplate. Two wells were designated as controls: one containing only bacterial suspension (positive control) and another containing only sterile MH broth (negative control). The microplate was incubated at 28 °C for 24 h. Escherichia coli ATCC 25922 and Staphylococcus aureus ATCC 29213 were used as quality control strains and incubated at 37 °C. The minimum inhibitory concentration (MIC) was defined as the lowest antimicrobial concentration that completely inhibited visible bacterial growth. MIC values were interpreted as sensitive, intermediate, or resistant according to the Clinical and Laboratory Standards Institute (CLSI) guidelines (CLSI VET03/VET04-S2, 2014).
2.3 Phage isolation and purification
The sewage sample collected from Nanjing aquatic market was centrifuged at 12,000 × g for 10 min, then the supernatant was filtered through a 0.22-μm membrane. The phage filtrate was pipetted into a culture solution containing A. hydrophila strain Ah18 at logarithmic phase. The mixture was incubated overnight at 28 °C in a shaker to allow the phages to proliferate. The phage stock was obtained by centrifugation at 12,000 × g for 10 min at 4 °C. To verify the phage stock, a sample was applied onto double-layer agar plates, with the upper agar mixing with Ah18, and the plates were incubated until the appearance of plaques. After verification, purification of the phage isolate was performed as previously described (25). The diluent at optimum concentration was mixed with host bacteria to generate double-layer agar plates. After incubation, single plaques were selected and cultured in TSB medium using Ah18 as an indicator. Phages were re-harvested by centrifugation and filtration. The purification processes were conducted three times.
2.4 Transmission electron microscopy
Transmission electron microscopy (TEM) was performed to determine the morphological characteristics of phages after concentration using cesium chloride (CsCl) gradient centrifugation (26). A phage suspension was pipetted onto carbon-coated support membrane copper mesh, left for 20 min, then negatively stained with 2% phosphotungstic acid solution for a 1-min retention time. After further drying, phage particles were observed under an electron microscope (JEM-1200EX, Japan) at an accelerating voltage of 100 kV.
2.5 Determination of multiplicity of infection
The multiplicity of infection (MOI) signifies the ratio of the number of phage in a system to the total number of bacteria and it can be interpreted as the number of phage added to each cell during infection. A single colony of strain Ah18 was inoculated into 5 mL of fresh TSB medium and cultured with shaking at 28 °C until reaching the early-exponential phase (approximately 1 × 107 CFU/mL). Subsequently, different concentrations of phage particles, diluted with sterile TSB, were separately introduced into parallel bacterial cultures to achieve MOI of 0.01, 0.1, 1, 10, 100. The mixtures were then incubated with shaking at 28 °C for 12 h. Finally, the phage titers under each MOI condition were determined using the double layer agar method (27).
2.6 Thermal stability and pH sensitivity
To investigate the vigor of phage BUCT551 under multiple environmental conditions, we measured the phage titers across diverse temperatures and pH values using previously described methods (25). In the thermal stability assay, phages (approximately 109 PFU/mL) were incubated in a water bath at different temperatures (4 °C, 30 °C, 40 °C, 50 °C, 60 °C, 70 °C, 80 °C) for 1 h, then the phage titers were determined by the double layer agar method. For pH stability assessment, 100 μL of phage suspension (2 × 1010 PFU/mL) was mixed with 900 μL of TSB pre-adjusted to pH 3–12 using NaOH or HCl. Following incubation at 28 °C for 1 h, then the phage titers were calculated by the double layer agar method. All experiments were performed in triplicate and the results are presented as the mean value.
2.7 One-step growth curve assay
The one-step growth curve assay was performed to determine the eclipse period and burst size of phage BUCT551. Phage BUCT551 was mixed with A. hydrophila strain Ah18 at exponential phase (approximately 8 × 107 CFU/mL) and optimal MOI, then 30 min absorption at 28 °C was performed. The mixture was centrifuged at 12,000 × g for 1 min to separate free phage particles within host bacteria immediately after the absorption procedure. The harvested cells were washed twice with sterile TSB and resuspended in 50 mL of TSB medium. Subsequently, the mixture was cultured with shaking at 28 °C for 205 min and samples were collected at 0, 10, 20, 30, 40, 55, 70, 85, 100, 115, 130, 145, 160, 175, 190, and 205 min during incubation. The phage titers were determined by the double-layer agar method as described above. According to the one-step growth curve, the latent and lysis periods for the phage were obtained. The burst size of phage BUCT551 was calculated using the following formula: Burst size (PFU/cell) = (Final phage titer at plateau phase) / (Initial number of infected host bacteria) (28).
2.8 Host range analysis
The bacterial susceptibility level was inspected using spotting method as previously described (26). Eight strains of A. hydrophila (Ah 154, ATCC 419140, AS1. 18031, Ah 7, Ah 9, Ah 10, Ah 17, Ah 18, No. 201308071) and eight strains of A. veronii (Av 4, Av 6, Av 15, Av 26, Av 25, Av 13, Av 21, Av 3) that were stored at the Biosafety Technology Research Center, Beijing University of Chemical Technology, were selected for host range determination. In brief, 500 μL of the A. hydrophila culture was transferred to 5 mL of TSB medium containing 0.75% agar, which was promptly poured onto a tryptic soy agar (TSA) plate. After solidification, 2.5 μL of phage BUCT551 was pipetted onto the double-layer agar, then the plates were cultured overnight to observe plaque formation. Additionally, the susceptibility of the tested bacterial strain to phage BUCT551 was determined using the efficiency of plaquing (EOP) assay (29). EOP values were calculated by normalizing plaque formation efficiency on the target strain relative to that observed on the reference strain Ah18.
2.9 Genome extraction, sequencing and analysis
The genome of phage BUCT551 was extracted using the proteinase K/SDS method as described by Yang et al. (30). Subsequently, the DNA library was constructed using NEBNext Ultra II FS DNA Library Prep Kit (NEB) as manufacturer’s instructions. The concentration of nucleic acid was determined using Qubit 2.0 (Invitrogen, United States). Whole-genome sequencing of phage BUCT551 was performed using the MiSeq platform (Illumina, United States). Newbler v3.13.0 software (Roche 454) and CLC software (CLC Bio) were used for genomic sequence assembly (31). Low-quality sequences were filtered out using the Trimmomatic (v0.32) program (32). The Phred score cut-off was set at Q ≥ 30, with a scanning window size of 20 bp, and a minimum read length cut-off of 50 bp.
Possible protein-coding genes were initially predicted using the Rapid Annotation using Subsystem Technology server.1 The predicted open reading frames (ORFs) were confirmed with the ORF Finder,2 and their putative functions were annotated using BLASTp.3 The genome function map was generated by the laboratory’s self-built script and modified using Inkscape 0.92.3.0. The genomic sequences of phage BUCT551 and phage LAh_7 were aligned using the Easyfig 2.2.3 (33). The tRNAs in the phage BUCT551 genome were predicted using the software tRNAscan-SE v.2.0 (34). The possible virulence and pathogen genes carried by phage genome were predicted with the VFDB (35) and PathogenFinder (36), respectively.
A phylogenetic tree was constructed based on the amino acid sequence of the conserved major capsid and terminase large subunit to analyze the relationship between phage BUCT551 and other phages of the same subfamily recorded in the International Committee on Taxonomy of Viruses database (ICTV). MEGA version 7.0 was employed to construct the phylogenetic tree (37).
2.10 In vitro antibacterial curve determination
The antibacterial activity of phage BUCT551 was assessed according to a previously established method (38). Briefly, exponential-phase A. hydrophila Ah18 cultures (1 × 108 CFU/mL) were co-incubated with an equal volume of phage suspensions at different MOIs of 0.01, 0.1, 1, and 10, respectively. The mixtures were incubated with shaking at 28 °C for 12 h. Untreated A. hydrophila cultures (supplemented with an equivalent volume of TSB broth) served as controls. Bacterial growth was monitored by measuring optical density at 600 nm (OD₆₀₀) at each time point. All experiments were performed in triplicate.
2.11 Statistical analysis
GraphPad Prism 8.0.1 (GrapPad Software, San Diego, CA, United States) was used to plot the data. One-way analysis of variance (ANOVA) was used to evaluate the difference between the experimental and control groups. The p-value < 0.05 was considered statistically significant.
3 Results
3.1 Drug sensitivity analysis of A. hydrophila Ah18
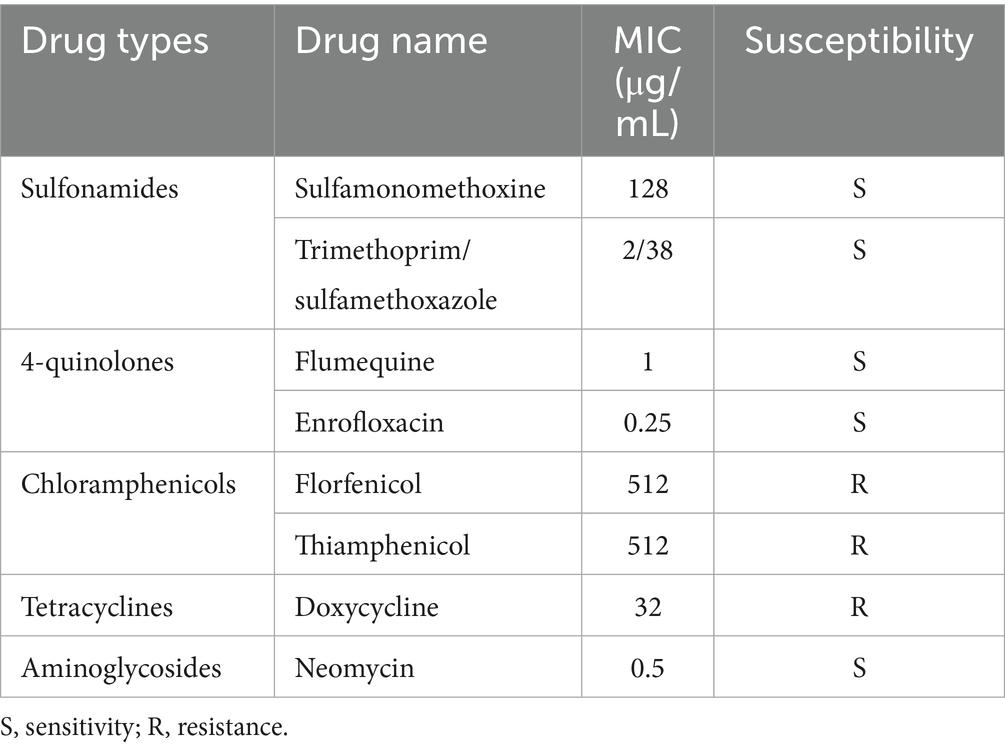
Antimicrobial susceptibility testing of A. hydrophila isolate Ah18 was conducted using antibiotics commonly employed in aquaculture. The results revealed distinct resistance in Ah18, specifically to florfenicol, thiamphenicol, and doxycycline (Table 1). This finding underscores that prolonged antibiotic usage in aquaculture can drive bacterial resistance development, ultimately limiting the efficacy of antimicrobial therapies.
3.2 Phage morphology
An A. hydrophila phage was isolated from aquacultural sewage and was designated BUCT551. Phage BUCT551 formed clear plaques of 0.5–1 mm in diameter after 8 h incubation on the lawn of A. hydrophila strain Ah18 (Figure 1a). TEM revealed that phage BUCT551 had a polyhedral capsid (50 ± 3 nm in diameter) and a long tail (180 ± 5 nm) (Figure 1b).
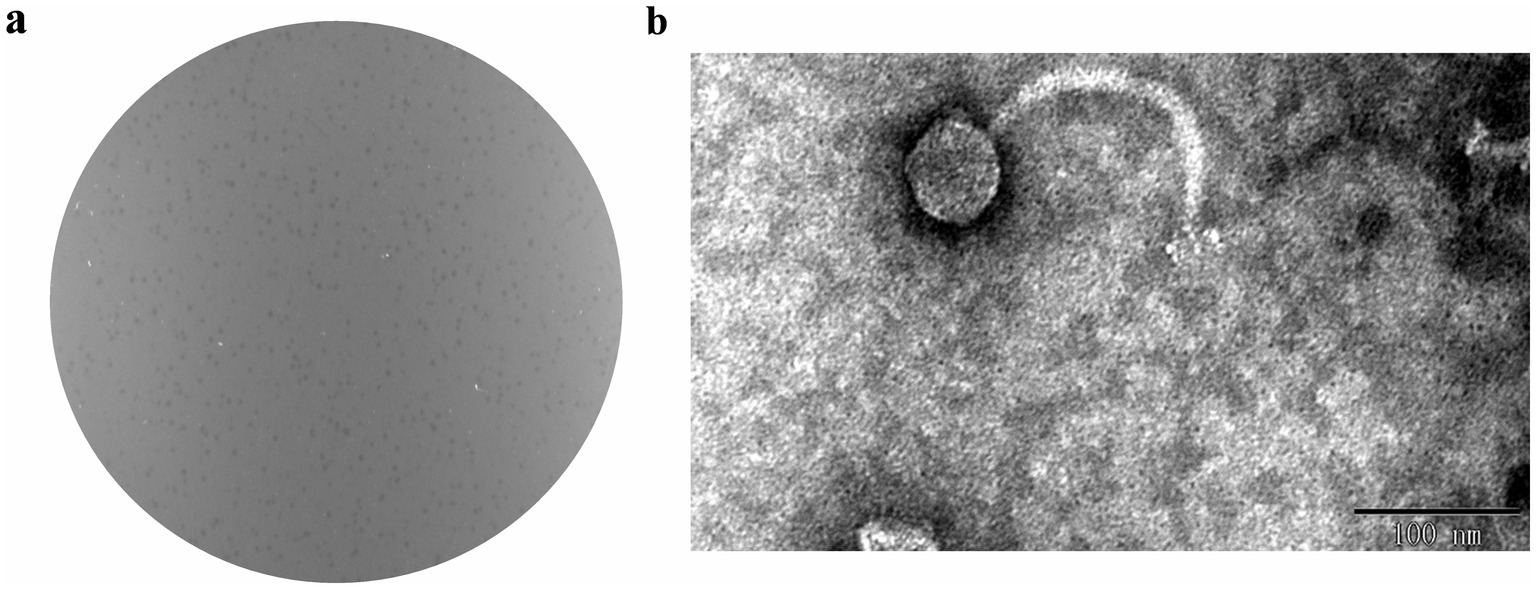
Figure 1. The morphology of phage BUCT55. (a) Plaques formed by phage BUCT551 on A. hydrophila strain Ah18 using the double agar overlay method. (b) Transmission electron micrograph of phage BUCT551. Scale bar: 100 nm.
3.3 Thermal stability and pH sensitivity
A thermal stability assay confirmed that phage BUCT551 could survive across a temperature range of 4–50 °C. The survival rate decreased significantly over 50 °C, and the phage was inactivated completely after incubating at 80 °C for 1 h (Figure 2a). The pH sensitivity assay confirmed that phage BUCT551 exhibited relative stability within the pH range of 5–10. Phage titers decreased dramatically after 1 h of incubation at pH < 5 or pH > 10, with complete inactivation observed at pH 3 and pH 12 (Figure 2b). Phage activity was significantly reduced following exposure to pH 3 and pH 12, indicating loss of infectivity under these extreme conditions.
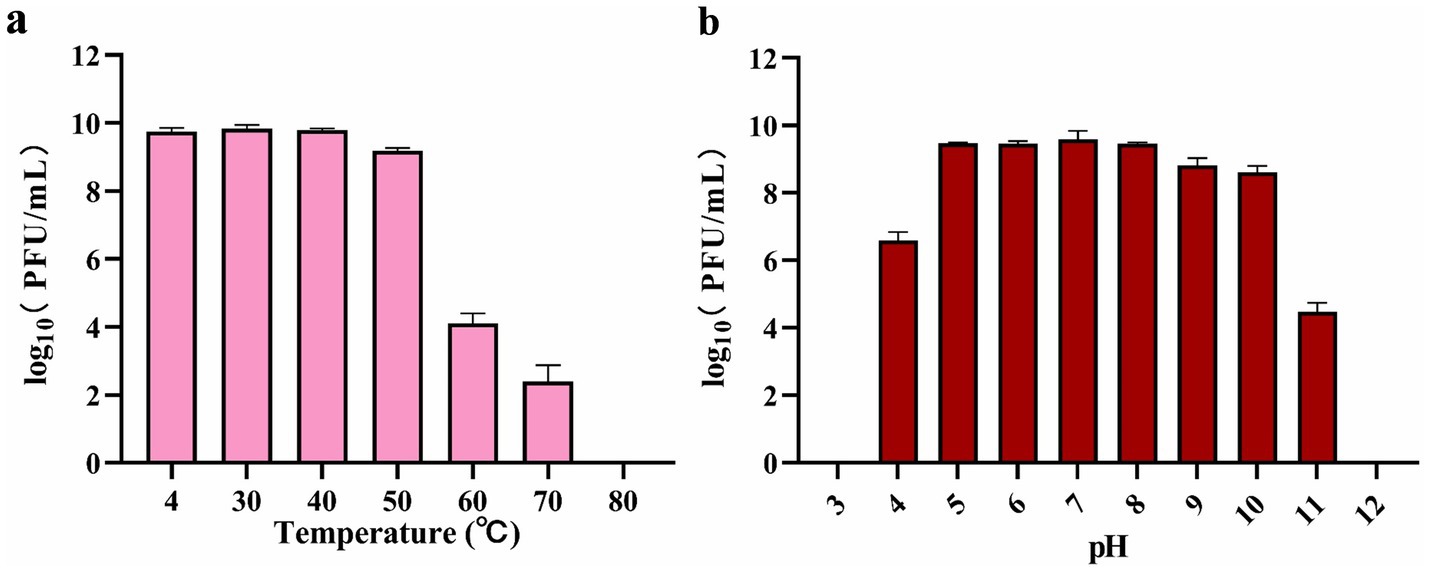
Figure 2. Stabilities of phage BUCT551 under (a) temperature 4 °C to 80 °C and (b) pH 3 to 12. Results are presented as mean values ± SD.
3.4 Optimal MOI and one-step growth curve
The titer of phage BUCT551 was tested under different MOIs infection conditions. On the basis of the maximum titer obtained after phage BUCT551 infection of A. hydrophila strain Ah18, the optimal MOI of the phage was 0.1 (Figure 3a). A one-step growth curve assay revealed that phage BUCT551 had a latent period of 20 min and a growth period of 80 min, and reached a plateau at 100 min, when the phage titer was 2.6 × 109 PFU/mL (Figure 3b). According to the formula described in the Methods section, the burst size of phage BUCT551 was calculated to be 32 PFU/cell.
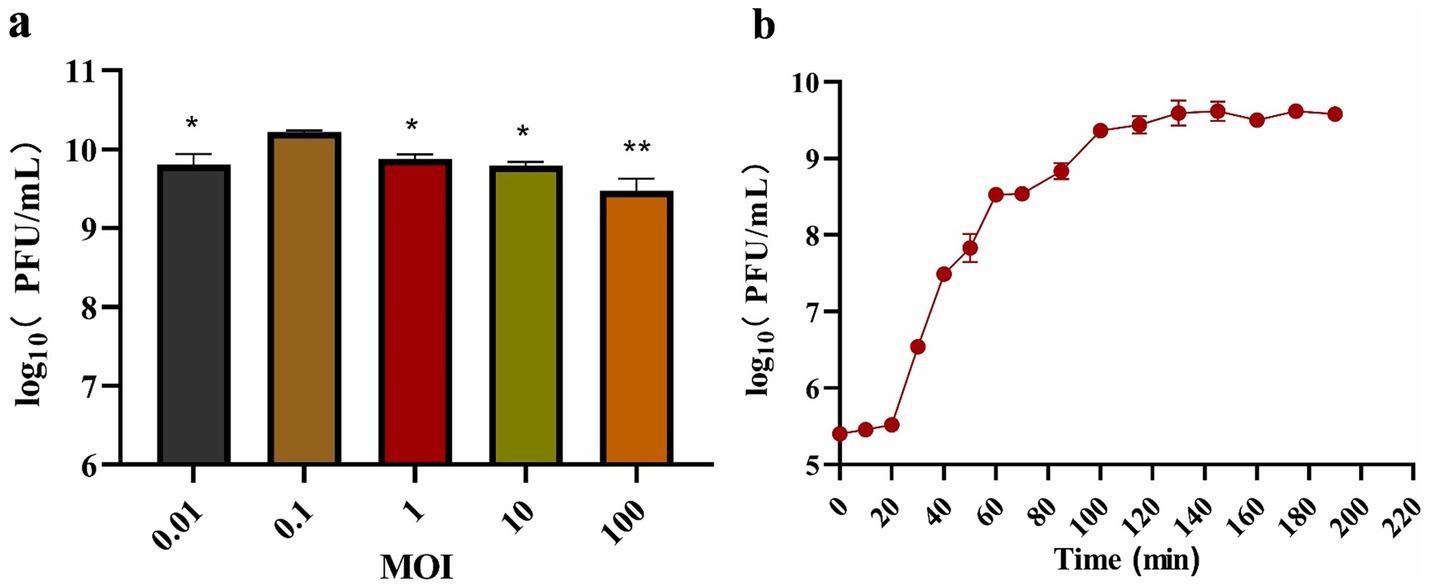
Figure 3. Growth characterization of phage BUCT551. The determination results of the (a) optimal MOI and (b) one-step growth curve. Results are presented as mean values ± SD, *p < 0.05 or **p < 0.01 indicates a significant difference compared to the MOI 0.1.
3.5 Host range analysis
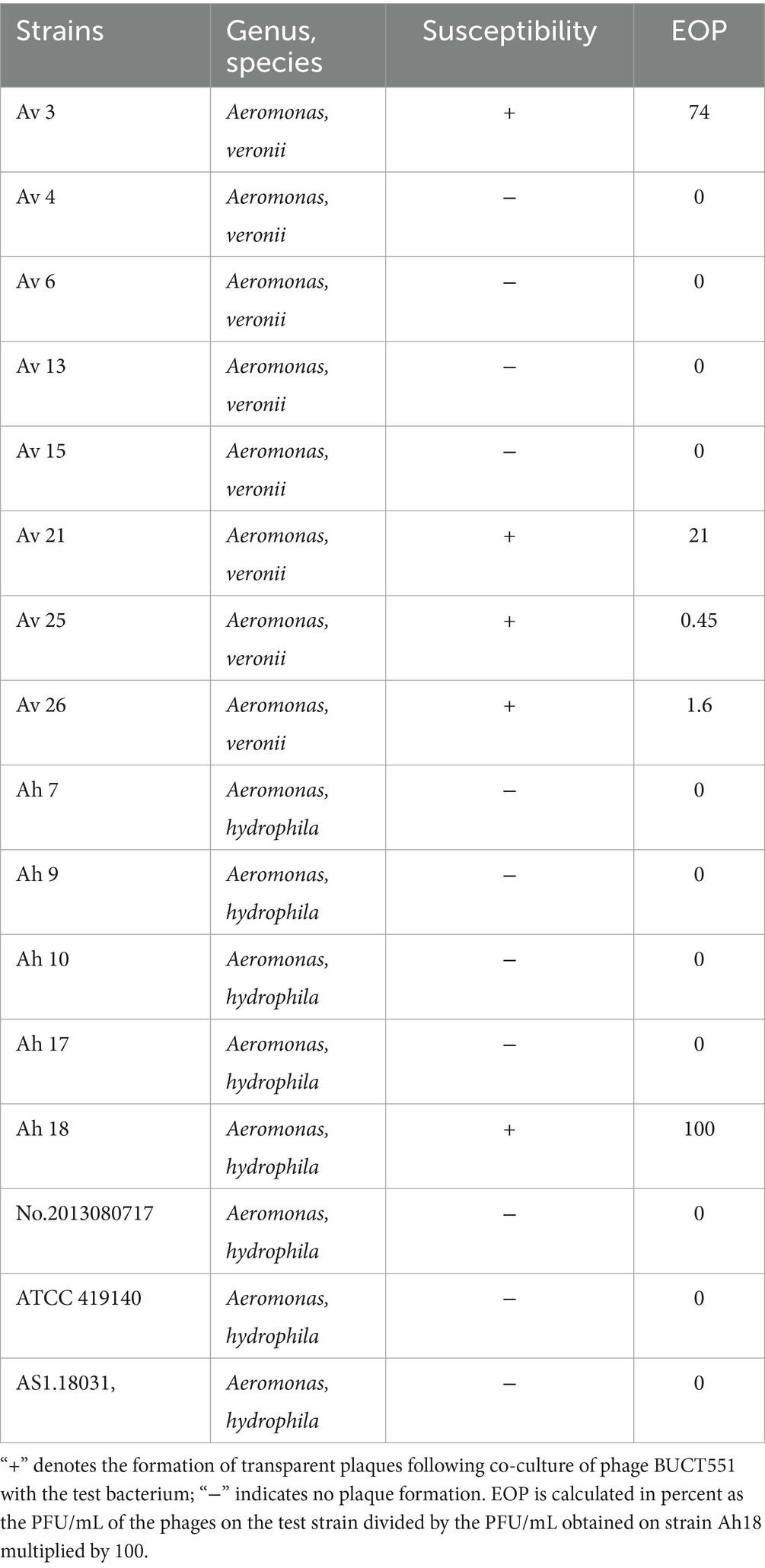
In order to understand the lysis range of phage BUCT551, all eight strains of A. hydrophila and eight strains of A. veronii in our laboratory were selected for examination. The results indicated that one strain of A. hydrophila and four strains of A. veronii were susceptible to phage BUCT551, suggesting that BUCT551 has therapeutic potential for Aeromonas infection (Table 2). Unfortunately, phage BUCT551 did not exhibit lytic activity against Vibrio, a genus closely related to Aeromonas, which reflects its narrow host specificity (Supplementary Table S1).
3.6 Whole-genome analysis
Whole-genome sequencing revealed that BUCT551 has a linear genome of 61,382 bp with a GC content of 61.77%. Restriction endonucleases specifically act on double-stranded DNA, and therefore restriction endonuclease digestion followed by electrophoresis can be employed to characterize nucleic acids. The enzyme-digested phage BUCT551 genome exhibited multiple electrophoretic bands, which preliminarily verified that BUCT551 is a double-stranded DNA phage (Supplementary Figure S1). BLASTn analysis revealed that phage BUCT551 shares 86.75% homology (87% genome coverage) with A. hydrophila phage LAh_7. Comparative analysis of the genomic sequences between bacteriophages BUCT551 and LAh_7 was conducted using deduced amino acid sequences of all ORFs. The results revealed significant similarity at the protein level (Figure 4). Notably, sequence identity in the 3′-terminal region of the genome, which encodes multiple proteins with unknown functions, was relatively lower than the genome-wide average. This observed divergence may represent adaptive mutations acquired by the bacteriophages in response to evolutionary pressures within their respective ecological niches (39).

Figure 4. The homology comparison between phage BUCT551 and phage LAh_7. The gradual red vertical bar in the lower right corner of the figure indicates the genetic homology between BUCT551 and LAh_7 in the picture.
3.7 Functional open reading frame analysis
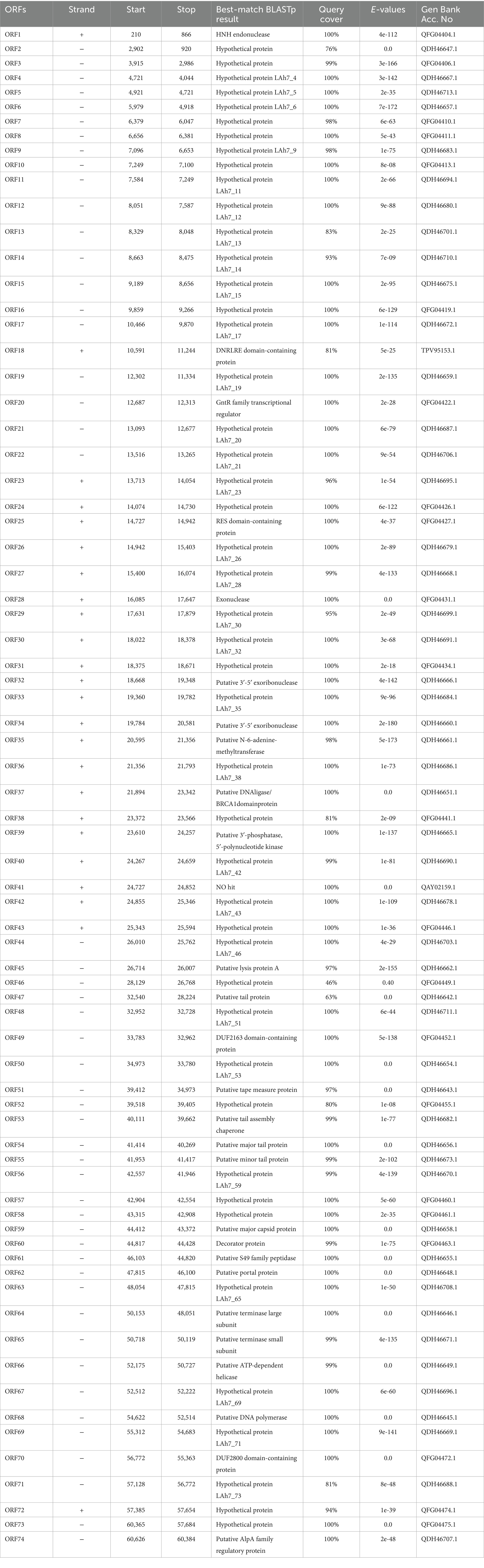
RAST gene annotation identified 74 ORFs within the phage BUCT551 genome. ATG was the initiation codon for most ORFs. Among the 74 predicted protein coding genes, 27 were identified as functional coding sequences, with the remainders annotated as proteins of unknown function or hypothetical proteins (Table 3). Further bioinformatic analysis revealed that the bacteriophage BUCT551 genome harbors no lysogeny-associated genes, antimicrobial resistance determinants, virulence factors, or tRNA genes. These findings collectively indicate that BUCT551 demonstrates genomic safety at the nucleotide level, fulfilling a critical prerequisite for its potential therapeutic application.
We classified the 27 proteins with annotated functions into four functional divisions: replication, modification and regulation, structural and packaging, and lytic division. The detailed annotation and organization of the BUCT551 genome is illustrated in Figure 5. Both BLASTp and RAST analyses demonstrated that ORF64 and ORF65 encode the large and small subunits of the terminase, respectively, which plays a significant role in phage DNA packaging. With regard to the phage replication division, ORF68 (DNA polymerase), ORF32 (putative 3′-5′ exoribonuclease), ORF34 (putative 3′-5′ exoribonuclease), and ORF39 (putative 3′-phosphatase, 5′-polynucleotide kinase), encoded by phage BUCT551, participate in the regulation of phage DNA replication. Furthermore, the DNA helicase encoded by ORF66 and the HNH endonuclease encoded by ORF1 have been speculated to play a crucial role in DNA replication and regulation, and subsequent modification (40). ORF35 encodes N-6-adenine-methyltransferase, a regulatory enzyme that is likely to participate in phage DNA modification, whose aim was to circumvent the protection system of host bacteria (41).
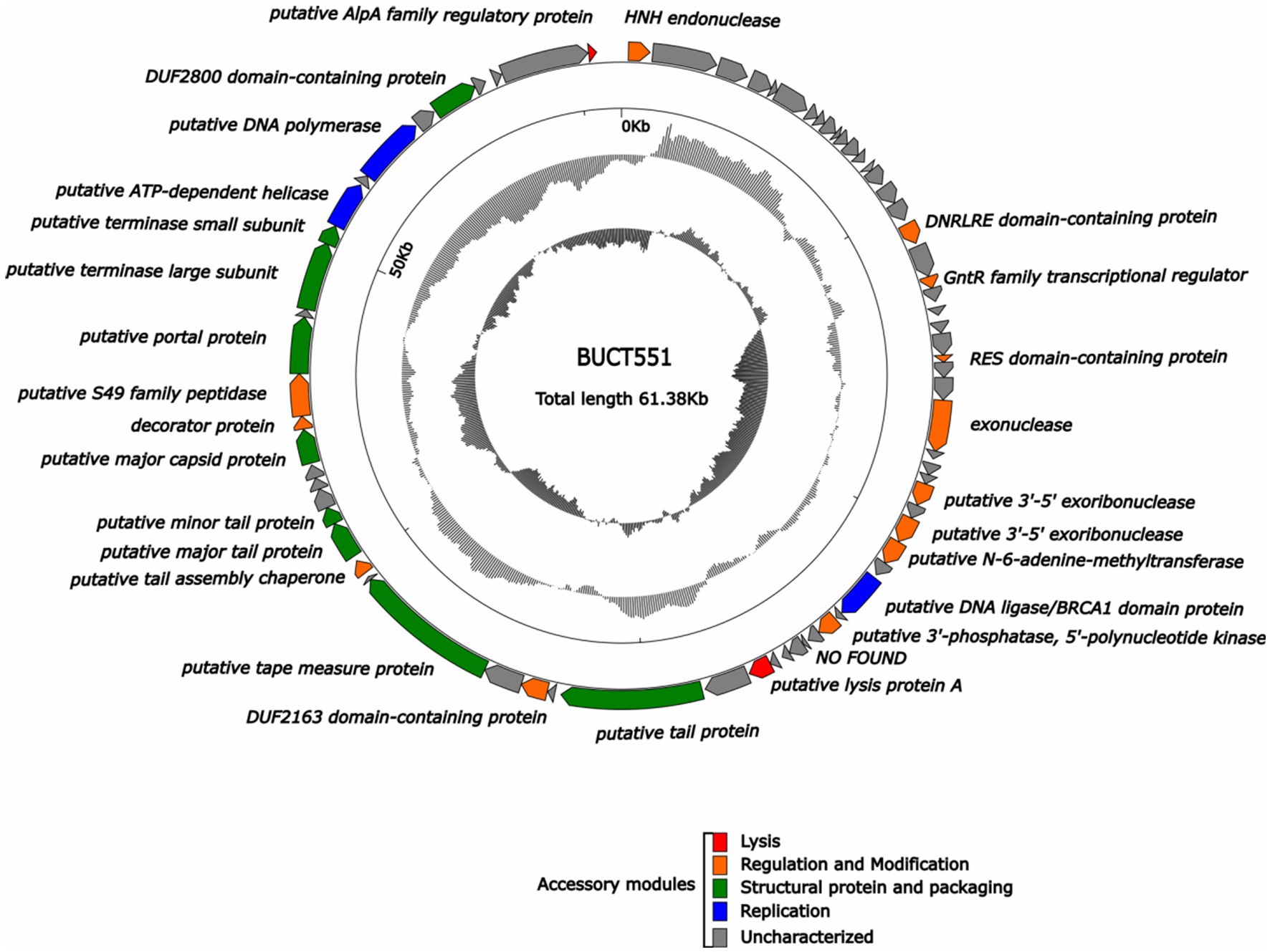
Figure 5. Whole genome map of BUCT551. The outermost layer displayed 74 open reading frames encoded in the genome, different colors represent different functions. The innermost circle represents the GC skew (G-C/G + C. Outwards indicates > 0 and inwards indicates < 0).
Phage structural proteins are encoded by multiple ORFs including ORF47 (putative tail protein), ORF51 (putative tape measure protein), ORF54 (putative major tail protein), ORF55 (putative minor tail protein), ORF53 (putative tail assembly chaperone), ORF59 (putative major capsid protein), and ORF62 (putative portal protein). Portal protein, encoded by ORF62, is considered to function in channel formation of the capsid and phage genome injection into host cells (42). The tape measure protein encoded by ORF51 plays a role in the assembly of phage capsid protein (43).
ORF18 (DNRLRE domain-containing protein), ORF20 (GntR family transcriptional regulator), ORF25 (RES domain-containing protein), ORF49 (DUF2163 domain-containing protein), ORF53 (putative tail assembly chaperone), ORF60 (decorator protein), and ORF61 (putative S49 family peptidase) encode proteins that play roles in phage modification and regulation. ORF53, encoding the tail assembly chaperone, exists widely in the genomes of Siphoviridae and Myoviridae phages, and is generally located between genes encoding the tail and tape measure proteins, thereby participating in regulation via frameshift mutations (44). ORF60 encodes a decoration protein. Decoration proteins are a class of proteins present in DNA viruses that locate on the surface of certain phage capsids, especially phages with double-stranded DNA genomes, where they protect the phage from extreme environmental changes by enhancing and promoting the robustness of the capsid (45). Many decoration proteins also possess other functions, such as facilitating target cell recognition, participating in phage assembly, and regulating host gene expression (45). Previous research has demonstrated that the GntR family of transcription factors encoded by ORF20 generally exist in prokaryotes and certain viruses and regulate various important metabolic pathways (46). The GntR family of transcription factors are also recognized to play a vital role in the growth and reproduction in bacteria (47).
ORF45 (putative lysis protein A) and ORF74 (putative AlpA family regulatory protein) belonging to the phage lytic division, probably encode lytic peptidases and enable the phage to lyse host cells effectively (48).
Notably, the majority of ORFs (47 in total) were classified as proteins of unknown function. Future research is warranted to clarify the function of these proteins to gain further insight into this phage.
3.8 Phylogenetic tree analysis for phage BUCT551
The major capsid and terminase large subunit proteins are commonly used as a marker gene to study phage evolutionary relationships (49–51). The evolutionary relationship between phage BUCT551 and other phages was determined based on the sequence of major capsid (ORF59) and terminase large subunit (ORF 64) to determine the phylogeny of BUCT551. Phylogenetic trees constructed based on the major capsid and large terminase subunit sequences both revealed that phage BUCT551 clustered within the same clade as phage LAh_7 (Figure 6), indicating a shared common ancestor. In accordance with the ICTV classification criteria, phage BUCT551 should be classified as a novel species within the genus Sharonstreetvirus.
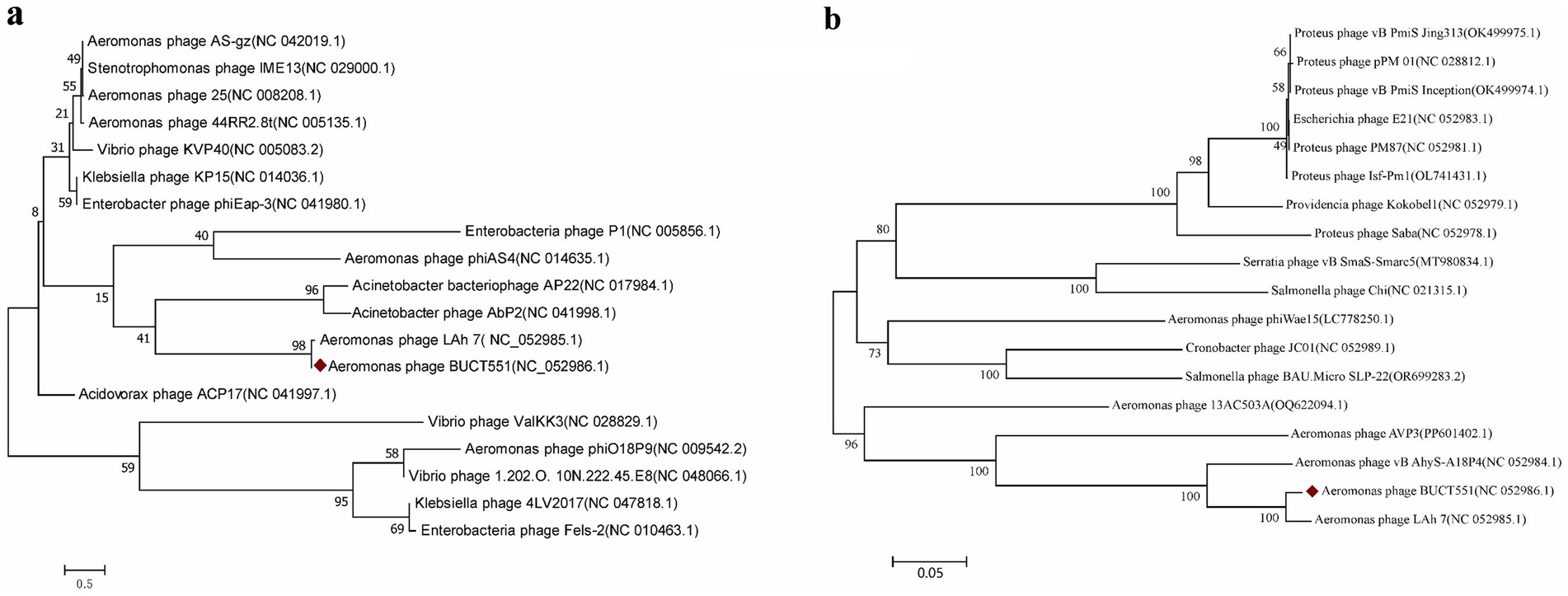
Figure 6. The phylogenetic tree was constructed using (a) capsid proteins and (b) terminase large subunit. Phylogenetic trees were constructed using the neighbor joining method of 1,000 bootstrap copies in MEGA7.
3.9 In vitro phage efficacy in Aeromonas control
To further evaluate the application potential of phage BUCT551, its ability to inhibit the growth of A. hydrophila Ah18 was assessed in vitro. As shown in Figure 7, the absorbance of Ah18 cultured without phage continued to increase over 12 h. In contrast, the phage-treated groups exhibited an initial increase in OD₆₀₀ during the first 2 h, followed by a significant decline. By 6 h post-infection, OD₆₀₀ values in all MOI groups reached minimum levels. Moreover, from 6 to 12 h, the OD₆₀₀ remained stably minimized across all MOI conditions. These results demonstrate that phage BUCT551 effectively inhibits the growth of Ah18 in vitro, indicating its promising application potential.

Figure 7. Bacteriolytic activity of phage BUCT551 at different MOIs against drug-resistance A. hydrophila strain Ah18 in liquid culture. Results are presented as mean values ± SD.
4 Discussion
Advancements in aquaculture practices have promoted the intensification of cultivation systems, resulting in significantly elevated stocking densities of cultivated fish. However, this intensification process has concurrently increased the risk of disease outbreaks in cultivated fish populations (52). A. hydrophila is a well-established pathogen that infects a large range of cultured fish, including Atlantic salmon, trout, and turbot, with the main symptoms being tail bleeding and epidermis fester (5). At present, the main methods of prevention and treatment of A. hydrophila infection still depend on disinfectants and antibiotics. However, the long-term use of antibiotics may lead to drug resistance. Novel antibacterial therapies are therefore needed. As a novel type of antibacterial agent, phages offer advantages such as high specificity, no obvious side effects, easily to acquire, and leave no toxic residue (53). Phage therapy therefore provides a new alternative to control diseases caused by A. hydrophila.
In this study, a strain of A. hydrophila (designated Ah18) was isolated from an aquaculture facility culturing the yellow catfish (Pelteobagrus fulvidraco). Antimicrobial susceptibility testing revealed multidrug resistance in this strain (Table 1), which poses significant challenges for antibiotic-based therapy. To identify alternative therapeutic approaches, we conducted a systematic screening for lytic phages using strain Ah18 as the host bacterium. Phage BUCT551 against strain Ah18 was isolated through enrichment culture techniques followed by biological characterization, with the objective of developing phage-based interventions to combat antibiotic-resistant A. hydrophila s in aquaculture systems.
When considering tailored phage therapy, thorough characterization of individual phages is essential. TEM analysis of phage BUCT551 confirmed its morphological similarity to phages belonging to the Siphoviridae family, which is the most abundant taxonomic group within the Caudoviricetes class (54). Phage BUCT551 maintained high viability (≥95% titer retention) across 4–40 °C. Furthermore, pH stability tests indicated that phage activity remained relatively stable within a pH range of 5 to 10, aligning with the ecological tolerance of aquatic pathogens in the study environment. These biological characteristics indicated that phage BUCT551 has robust and stable bacteriolytic competence, which is an important basis for phage therapy. In the host range analysis assay, we revealed that five out of 16 strains of Aeromonas were susceptible to phage BUCT551. Interestingly, four out of these five strains were identified as A. veronii, which is also pathogenic to fish (55). Generally, phages exhibit a narrow host range, typically infecting only their indicator hosts, while a broad host range is often highly advantageous for combating polymicrobial infections (56). In previous studies, several Aeromonas phages also have been reported to exhibit a broad host spectrum. Wang et al. reported a lytic phage of A. hydrophila Aph2 that infected not only A. hydrophila but also Aeromonas salmonicida (57).
Typically, to ensure the suitability of phages as antimicrobial agents, comprehensive genotypic analysis is essential in addition to phenotypic characterization. Phages harboring antibiotic resistance genes (ARGs) or bacterial virulence genes (VGs) in their genomes may facilitate horizontal transfer of these genes during large-scale production or between bacterial hosts susceptible to phage infection. Consequently, the presence of ARGs or VGs is recognized as an unsuitable trait for phage-based preparations intended for therapeutic or biocontrol applications (58). Sequencing and analysis of phage genomes enhance the understanding of phage genomics and improve the reliability and safety of phages for therapeutic applications (59). Bioinformatic analysis of putative phage-encoded proteins revealed no known antibiotic resistance genes or virulence factor genes were found in phage BUCT551 genome. This absence of pathogenic elements, coupled with strictly lytic behavior observed in prior characterization, indicates that bacteriophage BUCT551 could potentially serve as a safe and targeted therapeutic agent against drug-resistant A hydrophila strain Ah18 infections.
Phage BUCT551 possesses a double-stranded DNA genome of 61,382 bp in length, with a GC content of 61.77%. In the phage BUCT551 genome, no genes associated with viral genome integration (such as genes encoding an integrase) were detected (Table 3), implying that it is a lytic phage. Through the identified predicted proteins, we found that phage BUCT551 encodes a N-6-adenine-methyltransferase (ORF35), which plays a vital role in evading bacterial defense systems (60, 61). Previous in vitro studies have demonstrated that phage-encoded methyltransferases can protect phage DNA from cleavage by host restriction endonucleases (41, 62). N-6-adenine-methyltransferase methylates the genome of phage BUCT551, which may facilitate evasion of the restriction–modification phage defense system of the host.
Through genomic sequencing and comparative genomic analysis, combined with phylogenetic trees constructed based on the highly conserved terminase large subunit and capsid protein genes, we demonstrate that phage BUCT551 belongs to the genus Sharonstreetvirus within the class Caudoviricetes. It is noteworthy that although the NCBI database contains extensive genomic data from Aeromonas phages, the genomic sequence similarity between BUCT551 and its closest relative (Aeromonas phage Lah_7) is only 86.75%, which is well below the 95% species demarcation threshold established by the ICTV (63)—supporting its classification as a novel species. Comprehensive characterization of BUCT551 thus advances our understanding of Aeromonas phages diversity, with implications for phage-based biocontrol strategies in aquaculture.
5 Conclusion
As a result of the large-scale use of antibiotics, the application of phage to control bacterial infections of fish in aquatic environments offers a sustainable and safer alternative. In this study, the A. hydrophila phage BUCT551 was identified and characterized. Our results indicated that phage BUCT551, along with other A. hydrophila phages, has great potential for use in phage cocktail therapy, or in combination with antibiotics and other disinfection strategies. Given the lack of identified A. hydrophila phages, exploring the large number of hypothetical proteins encoded within its genome will be the priority of our future research.
Data availability statement
All data generated for this study are included in the article/Supplementary material. NCBI accession number of BUCT551 nucleic acid sequence is NC_052986.1.
Author contributions
PH: Conceptualization, Investigation, Methodology, Writing – original draft. HQ: Formal analysis, Investigation, Methodology, Writing – original draft. CH: Software, Writing – original draft. ZD: Visualization, Writing – original draft. YY: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Key Science and Technology Project of Henan Province (Grant Nos. 252102310165 and 252102310315), Medical and Health Science and Technology Project of Shandong Province (Grant No. 202411001152), Shandong Geriatric Society Science and Technology Research Project (Grant No. LKJGG2024W023), Shandong Province Medical Staff Scientific Innovation Program (Grant No. SDYWZGKCJHLH202454).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2025.1679093/full#supplementary-material
Footnotes
References
1. Kali, A, Kalaivani, R, Charles, P, and Seetha, KS. Aeromonas hydrophila meningitis and fulminant sepsis in preterm newborn: a case report and review of literature. Indian J Med Microbiol. (2016) 34:544–7. doi: 10.4103/0255-0857.195383
2. Rasmussen-Ivey, CR, Figueras, MJ, Mcgarey, D, and Liles, MR. Virulence factors of Aeromonas hydrophila: in the wake of reclassification. Front Microbiol. (2016) 7:1337. doi: 10.3389/fmicb.2016.01337
3. Liu, D, Zhang, T, Wang, Y, Muhammad, M, Xue, W, Ju, J, et al. Knockout of alanine racemase gene attenuates the pathogenicity of Aeromonas Hydrophila. BMC Microbiol. (2019) 19:72. doi: 10.1186/S12866-019-1437-3
4. Pu, W, Guo, G, Yang, N, Li, Q, Yin, F, Wang, P, et al. Three species of Aeromonas (A. dhakensis, A. hydrophila and A. jandaei) isolated from freshwater crocodiles (Crocodylus siamensis) with pneumonia and septicemia. Lett Appl Microbiol. (2019) 68:212–8. doi: 10.1111/Lam.13112
5. El-Bahar, HM, Ali, NG, Aboyadak, IM, Khalil, S, and Ibrahim, MS. Virulence genes contributing to Aeromonas hydrophila pathogenicity in Oreochromis niloticus. Int Microbiol. (2019) 22:479–90. doi: 10.1007/s10123-019-00075-3
6. Igbinosa, IH, Igumbor, EU, Aghdasi, F, Tom, M, and Okoh, AI. Emerging Aeromonas species infections and their significance in public health. Sci World J. (2012) 2012:625023. doi: 10.1100/2012/625023
7. Citterio, B, and Francesca, B. Aeromonas hydrophila virulence. Virulence. (2015) 6:417–8. doi: 10.1080/21505594.2015.1058479
8. Stratev, D, and Odeyemi, OA. Antimicrobial resistance of Aeromonas hydrophila isolated from different food sources: a mini-review. J Infect Public Health. (2016) 9:535–44. doi: 10.1016/j.jiph.2015.10.006
9. Tu, VQ, Nguyen, TT, Tran, X, Millard, AD, Phan, HT, Le, NP, et al. Complete genome sequence of a novel lytic phage infecting Aeromonas hydrophila, an infectious agent in striped catfish (Pangasianodon hypophthalmus). Arch Virol. (2020) 165:2973–7. doi: 10.1007/S00705-020-04793-2
10. Yamada, S, Matsushita, S, Dejsirilert, S, and Kudoh, Y. Incidence and clinical symptoms of Aeromonas-associated travellers' diarrhoea in Tokyo. Epidemiol Infect. (1997) 119:121–6. doi: 10.1017/S0950268897007942
11. Anjur, N, Sabran, SF, Daud, HM, and Othman, NZ. An update on the ornamental fish industry in Malaysia: Aeromonas hydrophila-associated disease and its treatment control. Vet World. (2021) 14:1143–52. doi: 10.14202/Vetworld.2021.1143-1152
12. Hasan, O, Khan, W, Jessar, M, Pathan, AZ, and Lakdawala, RH. Bone graft donor site infection with a rare organism, Aeromonas hydrophila. A typical location, presentation and organism with 2 years follow-up. Case report. Int J Surg Case Rep. (2018) 51:154–7. doi: 10.1016/J.Ijscr.2018.08.037
13. Manyi-Loh, C, Mamphweli, S, Meyer, E, and Okoh, A. Antibiotic use in agriculture and its consequential resistance in environmental sources: potential public health implications. Molecules. (2018) 23:795. doi: 10.3390/Molecules23040795
14. Chen, J, Sun, R, Pan, C, Sun, Y, Mai, B, and Li, QX. Antibiotics and food safety in aquaculture. J Agric Food Chem. (2020) 68:11908–19. doi: 10.1021/Acs.Jafc.0c03996
15. Elbehiry, A, Marzouk, E, Abdeen, E, Al-Dubaib, M, Alsayeqh, A, Ibrahem, M, et al. Proteomic characterization and discrimination of Aeromonas species recovered from meat and water samples with a spotlight on the antimicrobial resistance of Aeromonas Hydrophila. Microbiology. (2019) 8:E782. doi: 10.1002/Mbo3.782
16. Uechi, K, Tada, T, Sawachi, Y, Hishinuma, T, Takaesu, R, Nakama, M, et al. A carbapenem-resistant clinical isolate of Aeromonas Hydrophila in Japan harbouring An acquired gene encoding Ges-24 β-lactamase. J Med Microbiol. (2018) 67:1535–7. doi: 10.1099/Jmm.0.000842
17. Pires, DP, Costa, AR, Pinto, G, Meneses, L, and Azeredo, J. Current challenges and future opportunities of phage therapy. FEMS Microbiol Rev. (2020) 44:684–700. doi: 10.1093/Femsre/Fuaa017
18. Gordillo Altamirano, FL, and Barr, JJ. Phage therapy in the postantibiotic era. Clin Microbiol Rev. (2019) 32:E00066-18. doi: 10.1128/CMR.00066-18
19. Lin, DM, Koskella, B, and Lin, HC. Phage therapy: an alternative to antibiotics in the age of multi-drug resistance. World J Gastrointest Pharmacol Ther. (2017) 8:162–73. doi: 10.4292/wjgpt.v8.i3.162
20. Kortright, KE, Chan, BK, Koff, JL, and Turner, PE. Phage therapy: a renewed approach to combat antibiotic-resistant bacteria. Cell Host Microbe. (2019) 25:219–32. doi: 10.1016/J.Chom.2019.01.014
21. Cooper, CJ, Khan Mirzaei, M, and Nilsson, AS. Adapting drug approval pathways for bacteriophage-based therapeutics. Front Microbiol. (2016) 7:1209. doi: 10.3389/fmicb.2016.01209
22. Chan, BK, Abedon, ST, and Loc-Carrillo, C. Phage cocktails and the future of phage therapy. Future Microbiol. (2013) 8:769–83. doi: 10.2217/Fmb.13.47
23. Anastasiou, E, Lorentz, KO, Stein, GJ, and Mitchell, PD. Prehistoric schistosomiasis parasite found in the Middle East. Lancet Infect Dis. (2014) 14:553–4. doi: 10.1016/S1473-3099(14)70794-7
24. Zhao, R, Wang, J, Wang, D, Wang, Y, Hu, G, and Li, S. Isolation, identification, and characterisation of a novel St2378 Aeromonas hydrophila strain from naturally diseased frogs, Rana dybowskii. Pathogens. (2024) 13:552. doi: 10.3390/Pathogens13070552
25. Han, P, Hu, Y, An, X, Song, L, Fan, H, and Tong, Y. Biochemical and genomic characterization of a novel bacteriophage Buct555 lysing Stenotrophomonas Maltophilia. Virus Res. (2021) 301:198465. doi: 10.1016/J.Virusres.2021.198465
26. Chen, Y, Sun, E, Song, J, Yang, L, and Wu, B. Complete genome sequence of a novel T7-like bacteriophage from a Pasteurella multocida capsular type a isolate. Curr Microbiol. (2018) 75:574–9. doi: 10.1007/S00284-017-1419-3
27. Han, P, Zhang, W, Pu, M, Li, Y, Song, L, An, X, et al. Characterization of the bacteriophage Buct603 and therapeutic potential evaluation against drug-resistant Stenotrophomonas Maltophilia in a mouse model. Front Microbiol. (2022) 13:906961. doi: 10.3389/Fmicb.2022.906961
28. Wang, R, Cong, Y, Mi, Z, Fan, H, Shi, T, Liu, H, et al. Characterization and complete genome sequence analysis of phage Gp4, a novel lytic Bcep22-like Podovirus. Arch Virol. (2019) 164:2339–43. doi: 10.1007/S00705-019-04309-7
29. Holst Sørensen, MC, Van Alphen, LB, Fodor, C, Crowley, SM, Christensen, BB, Szymanski, CM, et al. Phase variable expression of capsular polysaccharide modifications allows Campylobacter jejuni to avoid bacteriophage infection in chickens. Front Cell Infect Microbiol. (2012) 2:11. doi: 10.3389/Fcimb.2012.00011
30. Yang, Y, Cai, L, Ma, R, Xu, Y, Tong, Y, Huang, Y, et al. A novel Roseosiphophage isolated from the oligotrophic South China Sea. Viruses. (2017) 9:109. doi: 10.3390/V9050109
31. Margulies, M, Egholm, M, Altman, WE, Attiya, S, Bader, JS, Bemben, LA, et al. Genome sequencing in microfabricated high-density picolitre reactors. Nature. (2005) 437:376–80. doi: 10.1038/Nature03959
32. Bolger, AM, Lohse, M, and Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. (2014) 30:2114–20. doi: 10.1093/Bioinformatics/Btu170
33. Sullivan, MJ, Petty, NK, and Beatson, SA. Easyfig: a genome comparison visualizer. Bioinformatics. (2011) 27:1009–10. doi: 10.1093/Bioinformatics/Btr039
34. Lowe, TM, and Chan, PP. Trnascan-se on-line: integrating search and context for analysis of transfer rna genes. Nucleic Acids Res. (2016) 44:W54–7. doi: 10.1093/Nar/Gkw413
35. Liu, B, Zheng, D, Zhou, S, Chen, L, and Yang, J. Vfdb 2022: a general classification scheme for bacterial virulence factors. Nucleic Acids Res. (2022) 50:D912–912d917. doi: 10.1093/Nar/Gkab1107
36. Cosentino, S, Voldby Larsen, M, Møller Aarestrup, F, and Lund, O. Pathogenfinder--distinguishing friend from foe using bacterial whole genome sequence data. PLoS One. (2013) 8:E77302. doi: 10.1371/Journal.Pone.0077302
37. Kumar, S, Stecher, G, and Tamura, K. Mega7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. (2016) 33:1870–4. doi: 10.1093/Molbev/Msw054
38. Liu, J, Gao, S, Dong, Y, Lu, C, and Liu, Y. Isolation and characterization of bacteriophages against virulent Aeromonas Hydrophila. BMC Microbiol. (2020) 20:141. doi: 10.1186/S12866-020-01811-W
39. Tang, C, Deng, C, Zhang, Y, Xiao, C, Wang, J, Rao, X, et al. Characterization and genomic analyses of Pseudomonas Aeruginosa Podovirus Tc6: establishment of genus Pa11virus. Front Microbiol. (2018) 9:2561. doi: 10.3389/Fmicb.2018.02561
40. Guilliam, TA, Keen, BA, Brissett, NC, and Doherty, AJ. Primase-polymerases are a functionally diverse superfamily of replication and repair enzymes. Nucleic Acids Res. (2015) 43:6651–64. doi: 10.1093/Nar/Gkv625
41. Kossykh, VG, Schlagman, SL, and Hattman, S. Phage T4 Dna [N6-adenine]methyltransferase. Overexpression, purification, and characterization. J Biol Chem. (1995) 270:14389–93. doi: 10.1074/Jbc.270.24.14389
42. Prevelige, PE Jr, and Cortines, JR. Phage assembly and the special role of the portal protein. Curr Opin Virol. (2018) 31:66–73. doi: 10.1016/j.coviro.2018.09.004
43. Geagea, H, Labrie, SJ, Subirade, M, and Moineau, S. The tape measure protein is involved in the heat stability of Lactococcus lactis phages. Appl Environ Microbiol. (2018) 84:E02082-17. doi: 10.1128/Aem.02082-17
44. Pell, LG, Cumby, N, Clark, TE, Tuite, A, Battaile, KP, Edwards, AM, et al. A conserved spiral structure for highly diverged phage tail assembly chaperones. J Mol Biol. (2013) 425:2436–49. doi: 10.1016/J.Jmb.2013.03.035
45. Dedeo, CL, Teschke, CM, and Alexandrescu, AT. Keeping it together: structures, functions, and applications of viral decoration proteins. Viruses. (2020) 12:1163. doi: 10.3390/V12101163
46. Jain, D. Allosteric control of transcription in Gntr family of transcription regulators: a structural overview. IUBMB Life. (2015) 67:556–63. doi: 10.1002/Iub.1401
47. Liu, GF, Wang, XX, Su, HZ, and Lu, GT. Progress on the Gntr family transcription regulators in bacteria. Yi Chuan. (2021) 43:66–73. doi: 10.16288/J.Yczz.20-245
48. Tsfasman, IM, Lapteva, YS, Krasovskaya, LA, Kudryakova, IV, Vasilyeva, NV, Granovsky, IE, et al. Gene expression of lytic endopeptidases Alpa and Alpb from Lysobacter sp. Xl1 in pseudomonads. J Mol Microbiol Biotechnol. (2015) 25:244–52. doi: 10.1159/000381266
49. Abrescia, NG, Bamford, DH, Grimes, JM, and Stuart, DI. Structure unifies the viral universe. Annu Rev Biochem. (2012) 81:795–822. doi: 10.1146/annurev-biochem-060910-095130
50. Nasir, A, and Caetano-Anollés, G. A phylogenomic data-driven exploration of viral origins and evolution. Sci Adv. (2015) 1:E1500527. doi: 10.1126/Sciadv.1500527
51. Liu, H, Geagea, H, Rousseau, GM, Labrie, SJ, Tremblay, DM, Liu, X, et al. Characterization of the Escherichia coli virulent myophage St32. Viruses. (2018) 10:616. doi: 10.3390/V10110616
52. Bosworth, B, Ott, B, and Torrans, L. Effects of stocking density on production traits of channel catfish×blue catfish hybrids. N Am J Aquac. (2015) 77:437–43. doi: 10.1080/15222055.2015.1024363
53. Palma, M, and Qi, B. Advancing phage therapy: a comprehensive review of the safety, efficacy, and future prospects for the targeted treatment of bacterial infections. Infect Dis Rep. (2024) 16:1127–81. doi: 10.3390/Idr16060092
54. Adriaenssens, EM, Edwards, R, Nash, J, Mahadevan, P, Seto, D, Ackermann, HW, et al. Integration of genomic and proteomic analyses in the classification of the Siphoviridae family. Virology. (2015) 477:144–54. doi: 10.1016/J.Virol.2014.10.016
55. Li, T, Raza, S, Yang, B, Sun, Y, Wang, G, Sun, W, et al. Aeromonas veronii infection in commercial freshwater fish: a potential threat to public health. Animals (Basel). (2020) 10:608. doi: 10.3390/Ani10040608
56. Chung, KM, Liau, XL, and Tang, SS. Bacteriophages and their host range in multidrug-resistant bacterial disease treatment. Pharmaceuticals (Basel). (2023) 16:1467. doi: 10.3390/Ph16101467
57. Wang, JB, Yu, MS, Tseng, TT, and Lin, LC. Molecular characterization of Ahp2, a lytic bacteriophage of Aeromonas hydrophila. Viruses. (2021) 13:477. doi: 10.3390/V13030477
58. Abedon, ST, and Thomas-Abedon, C. Phage therapy pharmacology. Curr Pharm Biotechnol. (2010) 11:28–47. doi: 10.2174/138920110790725410
59. Philipson, CW, Voegtly, LJ, Lueder, MR, Long, KA, Rice, GK, Frey, KG, et al. Characterizing phage genomes for therapeutic applications. Viruses. (2018) 10:188. doi: 10.3390/V10040188
60. Zhang, M, Gou, Z, Qu, Y, and Su, X. The indispensability of methyltransferase-like 3 in the immune system: from maintaining homeostasis to driving function. Front Immunol. (2024) 15:1456891. doi: 10.3389/Fimmu.2024.1456891
61. Muszewska, A, Steczkiewicz, K, and Ginalski, K. Dirs and Ngaro retrotransposons in Fungi. PLoS One. (2013) 8:E76319. doi: 10.1371/Journal.Pone.0076319
62. Murphy, J, Mahony, J, Ainsworth, S, Nauta, A, and Van Sinderen, D. Bacteriophage orphan DNA methyltransferases: insights from their bacterial origin, function, and occurrence. Appl Environ Microbiol. (2013) 79:7547–55. doi: 10.1128/Aem.02229-13
Keywords: Aeromonas hydrophila , Aeromonas veronii , drug resistant, phage BUCT551, genome analysis
Citation: Han P, Qin H, Hao C, Du Z and Yang Y (2025) Isolation and genomic analysis of phage BUCT551 against drug-resistant Aeromonas hydrophila. Front. Vet. Sci. 12:1679093. doi: 10.3389/fvets.2025.1679093
Edited by:
Jun Ji, Nanyang Normal University, ChinaReviewed by:
Tingting Zhang, Guizhou Medical University, ChinaSunjian Lyu, Zhejiang Academy of Agricultural Sciences, China
Copyright © 2025 Han, Qin, Hao, Du and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Yang, eWFuZ3lhbmcxOTgwMDYyN0AxNjMuY29t
†These authors have contributed equally to this work
 Pengjun Han
Pengjun Han Hongbo Qin
Hongbo Qin Chenxi Hao2
Chenxi Hao2 Zhiping Du
Zhiping Du