- 1College of Animal Science and Technology, Henan University of Animal Husbandry and Economy, Zhengzhou, China
- 2Department of Molecular Microbiology, Washington University School of Medicine, St. Louis, MO, United States
Rotavirus (RV) remains a leading cause of severe gastroenteritis in infants and young animals worldwide, contributing to significant morbidity and mortality despite the availability of vaccines. The gastrointestinal tract’s health, shaped by complex interactions between nutrition, the gut microbiota, and the host immune system, plays a crucial role in RV pathogenesis and outcomes. Emerging evidence suggests that dietary components not only influence the composition and function of the gut microbiota but also modulate immune responses essential for protection against RV. This review integrates findings from human and animal models to elucidate the interplay between nutrition, microbiota, and immunity in the context of RV infection. We aim to shed light on the mechanisms by which dietary factors and microbial communities influence RV susceptibility and severity, and how this knowledge could guide the development of more effective preventive and therapeutic strategies.
1 Introduction
Rotavirus (RV) remains one of the leading causes of severe gastroenteritis, particularly among infants and young children and animals, causing substantial global morbidity and mortality. Despite advances in vaccination, RV infections continue to present significant public health challenges, especially in resource-limited regions (1). A growing body of research has underscored the pivotal role of gut health in modulating the immune response to viral infections. The gut microbiota, a complex community of microorganisms residing in the intestines, plays a crucial role in both maintaining intestinal homeostasis and modulating immune functions. Dysbiosis, or imbalance in the microbiota, has been implicated in exacerbating disease outcomes during RV infections (2). The gut microbiota plays a central role in shaping immune function, and nutrition—an important determinant of immune responses—further modulates these interactions, influencing susceptibility to RV infection. Dietary components can alter the composition and function of the gut microbiota, thereby impacting the host’s immune response to infections (3). Recent studies leveraging human and animal models have highlighted potential therapeutic avenues through nutritional and microbiota-based interventions, offering potential avenues for therapeutic strategies (4). This review aims to explore the intricate interplay between nutrition, gut microbiota, and immunity in the context of RV infection.
2 RV biology and immune response
2.1 Host immune responses to RV
RV is a non-enveloped, double-stranded RNA virus belonging to the Reoviridae family. It is characterized by an 11-segmented genome encapsulated within a triple-layered protein capsid, which is essential for its stability and infectivity (5). RV infection triggers innate immunity at the intestinal epithelium. Enterocytes detect viral dsRNA via RIG-I, MDA5, and TLR3, which activate IRF3 and NF-κB, driving type I (IFN-α/β) and type III (IFN-λ) interferon production (6). These interferons establish an antiviral state by inducing interferon-stimulated genes (ISGs) that limit viral replication. Additionally, RV modulates host responses through viral proteins such as NSP1, which antagonizes IFN signaling by targeting IRF3 and other regulatory factors for proteasomal degradation (6, 7). In the adaptive phase, mucosal immunity is critical for protection. The infection stimulates gut-associated lymphoid tissue (GALT), particularly Peyer’s patches and mesenteric lymph nodes, where antigen-presenting cells such as dendritic cells activate naïve CD4+ T cells, leading to B cell class switching and the generation of IgA-producing plasma cells (8). These plasma cells home to the intestinal lamina propria via CCR9 and α4β7 integrins and secrete virus-specific secretory IgA (sIgA) that neutralizes virus particles in the lumen and on the epithelial surface, preventing reinfection. CD8+ cytotoxic T lymphocytes also play a role in clearing infected cells, especially in the early stages of infection (8). Protective immunity is largely non-sterilizing but is enhanced by repeated exposure, which boosts both the magnitude and breadth of sIgA and systemic IgG responses (5). In vaccine contexts, the generation of intestinal sIgA and memory B cells has been correlated with long-term protection, though no absolute immunologic correlate of protection has been universally accepted (9).
2.2 Limitations of RV vaccines
Recent data further underscore the geographic disparities in rotavirus vaccine performance. According to WHO surveillance and Gavi reports, vaccine effectiveness exceeds 80–90% in many high-income countries but drops significantly in low- and middle-income countries (LMICs), often ranging from 50 to 60% (10). The limitations of RV vaccines in providing effective immune protection are multifaceted, involving both host and viral factors that compromise efficacy, particularly in LMICs. A major constraint is the incomplete understanding of immune correlates of protection; while serum IgA and neutralizing antibodies have been associated with protection, they are not fully reliable predictors of vaccine efficacy, complicating the assessment and improvement of vaccines (11). The mucosal immunity required for robust protection is often inadequately induced, particularly in settings where environmental factors like malnutrition, high pathogen exposure, and environmental enteric dysfunction impair immune responses (12, 13). Maternal antibodies transferred via the placenta or breast milk can neutralize vaccine virus replication in the infant gut, dampening the immune response (12). Additionally, the gut microbiome composition, altered by geography and nutrition, influences vaccine take and efficacy, with evidence suggesting that dysbiosis can reduce immunogenicity (13).
The genetic diversity of RV also presents a significant hurdle; although current vaccines provide some cross-protection, they are primarily based on specific strains, and the continual emergence of novel genotypes may limit broad protective coverage (1, 14). Moreover, vaccine-induced immunity may wane after the first 2 years of life, particularly in LMICs, leaving children susceptible to later infections (13). There are operational challenges as well, including the need for cold chain logistics and multiple doses, which can limit vaccine coverage and timely administration (14). These combined factors highlight the need for next-generation vaccines that can induce stronger and more durable mucosal immunity, broaden strain coverage, and overcome host and environmental barriers to ensure equitable global protection against RV (11, 14).
3 Gut microbiota and RV infection
3.1 Influence on susceptibility and severity
The gut microbiota profoundly influences host susceptibility, immune responses, and vaccine efficacy against RV through modulation of intestinal barrier function, immune signaling, and viral replication dynamics. Heyman et al. (15) found that germ-free mice infected with RV exhibited increased intestinal permeability and abnormal absorption of macromolecules like bovine serum albumin, suggesting that microbiota are critical in maintaining tight junction integrity and limiting paracellular leakage during infection. Hulst et al. (16) provided molecular evidence from germ-free piglets, where RV infection triggered an exaggerated transcriptional response in the jejunum, particularly upregulation of genes related to innate immunity, such as interferon-stimulated genes (ISGs), acute phase reactants, and inflammatory cytokines, indicating that microbiota normally calibrate the epithelial and mucosal immune tone to prevent hyperactivation. Uchiyama et al. (17) further demonstrated that broad-spectrum antibiotic treatment, which depletes gut microbiota, significantly reduced RV replication in mice and simultaneously enhanced virus-specific humoral immunity, including higher titers of IgA and IgG. This suggests that certain bacterial species may provide metabolites or immune cues that favor viral replication while dampening adaptive immune responses. Ngo et al. (18) extended these findings by identifying microbial taxa, particularly some Clostridia and Bacteroidetes members, that impair RV vaccine efficacy by suppressing type I interferon pathways and antigen-presenting cell functions, thereby reducing the priming of protective immunity. Collectively, these studies underscore that the microbiota shapes the epithelial barrier, regulates innate immune thresholds, modulates viral replication niches, and influences both infection outcomes and vaccine responses to RV.
3.2 Microbial metabolites and immunomodulation
The gut microbiota produces a diverse array of metabolites that profoundly influence the host’s immune system, antiviral activity, and barrier function (19–21). Several microbial metabolites and compounds modulate RV infection through diverse cellular pathways and antiviral mechanisms (Table 1). Butyrate, a short-chain fatty acid produced by gut bacteria, exerts protective effects against RV-induced damage by attenuating endoplasmic reticulum (ER) stress-mediated apoptosis via suppression of the PERK-eIF2α signaling pathway, thereby reducing epithelial cell death (22). Additionally, sodium butyrate preserves intestinal barrier integrity by activating the AMPK-Nrf2 signaling pathway, leading to enhanced antioxidant defenses and tight junction protein expression in IPEC-J2 cells (23–25).

Table 1. Bioactive compounds and host-derived factors modulating RV infection: mechanisms and outcomes across models.
Beyond butyrate, bile acids and synthetic farnesoid X receptor (FXR) agonists inhibit RV replication by disrupting viral transcription and replication processes, suggesting bile acid receptors play a regulatory role in antiviral defenses (26, 27). Bacterial-derived sialidases impede porcine RV replication by cleaving host cell surface sialic acids, interfering with viral attachment and entry during early infection stages (28).
Additionally, the interaction between RV and the host’s serotonergic system influences diarrheal outcomes, with RV triggering serotonin release that modulates gut motility and secretion, exacerbating diarrhea (29). Metabolomic studies further highlight that host–microbe interactions shape the metabolic landscape influencing RV pathogenesis, though specific metabolic mediators require further elucidation (30). Lastly, carbohydrate malabsorption syndrome contributes to the pathogenesis of RV diarrhea by promoting osmotic imbalances and altering the gut microbiota composition, thereby aggravating disease severity (31). Together, these findings emphasize the therapeutic potential of microbial metabolites like butyrate, bile acids, and bacterial enzymes in mitigating RV infection and its associated intestinal damage.
3.3 Microbiota and vaccine responses
The gut microbiota plays a significant role in shaping both mucosal and systemic immune responses to vaccines, including those against RV (32). Multiple bacterial species modulate RV (RV) infection through distinct mechanisms involving direct viral inhibition, microbiota modulation, immune priming, and glycan interactions (Table 2). Lactobacillus rhamnosus GG and Lactobacillus acidophilus reduce RV diarrhea severity by enhancing mucosal barrier integrity, producing antiviral metabolites, and modulating immune responses such as increasing sIgA levels (33–35). Bifidobacterium longum inhibits RV replication in vitro by competitive exclusion and modulation of host cell antiviral pathways, such as upregulating interferon-stimulated genes (36).

Table 2. Effects of bacterial species on RV infection and vaccine response in human and animal models.
Segmented filamentous bacteria (SFB) exhibit potent anti-RV effects by inducing a Th17-mediated immune response and enhancing retinoic acid receptor signaling, which primes intestinal epithelial cells for rapid interferon production, creating an antiviral state that blocks RV replication (37, 38). Similarly, Bacteroides thetaiotaomicron and Lactobacillus casei secrete glycan-modifying enzymes and soluble factors that alter host cell surface glycans, thereby impeding RV binding to its receptors, specifically by reducing sialic acid-containing glycan availability (39). Lactobacillus reuteri, isolated from pigs, exhibits bile tolerance and produces antimicrobial compounds like reuterin that inhibit both RV and co-infecting bacterial pathogens, indirectly reducing RV infectivity by controlling microbial dysbiosis (40). Probiotics also enhance mucus production and fortify tight junction proteins, limiting RV penetration (41). Furthermore, probiotic colonization in neonatal gnotobiotic pigs modulates dendritic cell and macrophage populations, promoting antiviral cytokine production such as IL-12 and IFN-γ (42). Lipopolysaccharide (LPS)-rich bacteria increase TLR4 activation, influencing innate immune responses that modulate live attenuated RV vaccine virus shedding (43). Glycan-mediated interactions between certain commensals and RV can competitively block viral entry by saturating glycan receptors on epithelial cells, adding an additional antiviral defense layer (44).
RV infection itself reshapes the gut microbiome by increasing glycan availability in the ileum, facilitating the overgrowth of specific bacteria that may enhance or inhibit subsequent RV infection (45). Human studies demonstrate that antibiotic-mediated microbiome depletion reduces RV vaccine immunogenicity by impairing microbial-derived adjuvant signals necessary for optimal immune priming (32). Specific microbiota profiles correlate with oral RV vaccine efficacy, particularly involving bacteria that modulate bile acid metabolism and TLR signaling (2, 18, 46). Early probiotic supplementation with Bifidobacterium bifidum and Streptococcus thermophilus reduced RV diarrhea and viral shedding, potentially via enhanced colonization resistance and modulation of gut pH to inhibit RV replication (47). Overall, these findings underscore that diverse bacterial taxa employ multi-modal mechanisms — including modulation of host immunity, competitive exclusion, glycan receptor modification, and metabolic interactions — to mitigate RV infection and improve vaccine responses.
4 Nutritional factors modulating RV infection and immunity
4.1 Macronutrients
Adequate intake of macronutrients and micronutrients is fundamental to maintaining a competent immune system and mitigating the impact of RV infection (48). Protein-energy malnutrition (PEM) profoundly disrupts intestinal integrity and immune defense against RV (RV) infection through multiple mechanisms (Table 3). In neonatal pigs and mice, PEM impairs small intestinal epithelial regeneration and mucosal repair post-RV infection by reducing villus height, crypt depth, and epithelial cell proliferation, thereby delaying recovery (49–51). Mechanistically, protein deficiency downregulates tight junction proteins and mucins, weakening the epithelial barrier and facilitating viral invasion (3). PEM also diminishes expression of pattern recognition receptors like TLR3 and RIG-I, essential for recognizing RV and initiating antiviral responses (48). Concurrently, protein malnutrition skews immune responses by reducing type I and III interferons, IL-22, and antimicrobial peptides, all critical for limiting RV replication (3). Furthermore, PEM alters gut microbiota composition—favoring pathobionts and depleting beneficial commensals—which exacerbates inflammation and diminishes the efficacy of oral RV vaccines (52, 53).
Dietary supplementation with whey protein concentrates or animal plasma enhances intestinal repair by increasing anti-inflammatory cytokines (e.g., IL-10) and sIgA, and restoring mucosal immunity, thereby reducing RV-associated diarrhea and intestinal damage (54–58). Milk fat globule membrane components specifically block RV replication by disrupting viral attachment and entry processes (59). In terms of energy and fatty acids, saturated fatty acids such as palmitic and stearic acids enhance RV infectivity by promoting membrane fluidity and facilitating viral entry and uncoating (60). In contrast, oleic acid supplementation and modulation of lipid droplet metabolism can reduce RV replication by altering host lipid metabolism, including beta-oxidation and lipid droplet formation, which RV exploits for its replication complexes (61). Ceramide metabolism is particularly critical, as ceramide-enriched membrane domains are necessary for RV assembly and budding; disruption of ceramide synthesis impairs these viral processes (61).
Carbohydrates impact RV infection both structurally and functionally. Proper glycosylation and disulfide bond formation in RV proteins, particularly VP7, are carbohydrate-dependent processes necessary for forming mature, infectious virions (62). Malabsorption of carbohydrates during RV infection leads to osmotic diarrhea, exacerbating fluid loss and disease severity (63). Intestinal glycolipids and glycoconjugates rich in sialic acid residues act as critical cell surface receptors for RV, mediating viral attachment and entry (64, 65). Inhibitory glycans and synthetic carbohydrate analogs can competitively block these binding sites, preventing infection (62). Additionally, Portulaca oleracea L. polysaccharide (POP) has been shown to inhibit porcine RV both in vitro and in vivo by reducing viral replication, enhancing mucosal barrier function, modulating the NF-κB pathway, and lowering inflammatory cytokines such as IL-6 and TNF-α (66, 67). Altogether, adequate intake of protein, energy, specific fatty acids, and digestible carbohydrates not only supports epithelial and immune defenses but also limits RV replication and pathogenesis by directly modulating viral entry, replication, and host–microbe interactions.
4.2 Micronutrients
Micronutrients are equally vital. Vitamin A and D have been extensively studied for their modulatory effects on RV (RV) infection and immunity (Table 4). Vitamin A deficiency (VAD) exacerbates RV infection through compromised gut integrity and impaired immune responses. In mice, VAD led to severe diarrhea, villous atrophy, and extensive gut involvement, reducing epithelial regeneration and delaying viral clearance (68, 69). Mechanistically, VAD diminished mucosal and systemic immunity by decreasing RV-specific intestinal IgA and systemic IgG, as well as impairing T cell proliferation (70). In gnotobiotic piglets, VAD prenatally acquired from sows resulted in reduced maturation of dendritic cells and suppressed type I interferon responses (IFN-α, IL-12), weakening innate defenses (71). Adaptive immunity was also compromised, with reduced B cell antibody responses (IgA, IgG) and impaired CD4 + and CD8 + T cell responses to both vaccination and RV challenge (72, 73).

Table 4. Effects of vitamin A and vitamin D on RV infection: summary of experimental and clinical findings across animal and human models.
Vitamin A supplementation effectively reversed these deficits. Oral vitamin A in VAD pregnant sows enhanced RV-specific IgA and IgG in colostrum and serum, strengthening maternal adaptive immunity and passive protection in piglets (74). Further, supplementation restored innate immune function by increasing natural killer (NK) cell activity and IFN-γ production, and promoted robust CD8 + T cell responses (75). Clinically, vitamin A supplementation improved outcomes in preschool children with acute RV diarrhea, shortening disease duration and severity. Mechanistically, vitamin A and its metabolites (retinoids) enhance antiviral defenses by supporting mucosal immunity, promoting epithelial repair, and modulating the Th1/Th2 cytokine balance (73).
Vitamin D supplementation similarly alleviates RV infection through distinct pathways. In pigs and IPEC-J2 cells, vitamin D3 activated autophagy by upregulating Beclin1 and LC3, promoting viral degradation and reducing replication (76). In humans, low serum vitamin D was associated with higher susceptibility to rotaviral diarrhea in children (77). Mechanistically, 25-hydroxyvitamin D3 inhibited RV replication by suppressing the RIG-I and MDA5 pathways, leading to lower IFN-β production and inflammatory signaling in infected IPEC-J2 cells (78). Vitamin D also regulates miR-155-5p. This modulation of the TBK1/IRF3 axis tempers excessive interferon responses while maintaining control of viral replication (79). Additionally, 1α,25-dihydroxyvitamin D3 protected against RV-induced ferroptosis in intestinal epithelial cells via the ATF3-SLC7A11-GPX4 pathway, enhancing cellular antioxidant defenses and survival (80). In pigs, dietary vitamin D also modulated RIG-I signaling and reduced pro-inflammatory cytokine expression during RV infection, contributing to a balanced immune response (81). Collectively, these findings underscore that vitamin A primarily bolsters gut barrier function, mucosal IgA, and T cell immunity, whereas vitamin D mitigates RV pathogenesis via autophagy activation, innate immune signaling modulation, miRNA-mediated regulation, and protection against infection-induced ferroptosis, all contributing to improved viral control and vaccine efficacy.
Zinc exerts multifaceted protective effects against RV infection through both immunological and direct antiviral mechanisms. Clinically, higher serum zinc concentrations are associated with a lower incidence of RV gastroenteritis, partly by enhancing the mucosal immune response, particularly in infants receiving delayed RV vaccination (82). Zinc supplementation reduces the duration and severity of diarrhea by promoting epithelial barrier integrity, reducing intestinal permeability, and enhancing mucosal repair (83). Immunologically, zinc upregulates immune globulins (IgA, IgG), complement proteins, and suppresses pro-inflammatory cytokines including TNF-α, IL-6, and IL-17, which are typically elevated during RV infection-induced intestinal inflammation. Moreover, zinc influences gut microbiota composition, facilitating microbial shifts that support mucosal health and recovery post-infection (84). Mechanistically, zinc ions play a critical structural role in the RV virion: they stabilize the VP6 protein, a major capsid component essential for viral assembly and maintenance of the triple-layered particle, directly impacting viral integrity and infectivity (85). Additionally, zinc oxide nanoparticles have been shown to directly inhibit RV replication in vitro, likely through the generation of reactive oxygen species (ROS) that damage viral components and disrupt replication cycles (86). The economic benefits of zinc supplementation in RV management, particularly in reducing treatment duration and healthcare costs, further emphasize its therapeutic potential. Combination therapies with zinc, selenium, and probiotics demonstrate synergistic benefits by collectively enhancing immune resilience and antiviral defenses (87).
Copper exhibits antiviral properties against RV, with in vitro evidence showing that copper oxide nanoparticles suppress RV multiplication. This effect is mechanistically linked to ROS generation, which leads to oxidative stress and denaturation of viral proteins, impairing the viral life cycle (88).
Calcium plays a dual role in the RV replication cycle. Physiologically, calcium ions facilitate the early stages of RV infection by mediating virus attachment and entry into host cells through endocytic pathways (89). However, intracellular calcium depletion alters viral protein processing: chelation of calcium activates the RV RNA-dependent RNA polymerase (RdRp), enhancing viral transcription (90). Conversely, sustained calcium depletion impairs the oligomerization of viral proteins within the endoplasmic reticulum, blocking proper virus maturation and assembly (91). Thus, calcium homeostasis is critical for balancing viral replication, assembly, and maturation processes. Collectively, these findings highlight the multifaceted roles of minerals in modulating RV infection and replication across clinical and molecular levels (Table 5).
4.3 Human milk components
Human milk contains multiple bioactive components that protect against RV (RV) infection through distinct and synergistic mechanisms (Table 6). Human milk oligosaccharides (HMOs) inhibit RV infection by acting as soluble decoy receptors that mimic host cell surface glycans, particularly histo-blood group antigens (HBGAs), which are recognized by the viral VP8* protein, thereby preventing RV attachment and entry into enterocytes (92, 93). In piglet models, HMOs not only reduce diarrhea severity and viral shedding but also enhance mucosal immunity by increasing intestinal sIgA and modulating cytokine responses, while reshaping the colonic microbiota toward protective bacterial profiles such as increased Bifidobacteria (94, 95). The milk microbiome itself, in concert with HMOs, promotes the colonization of beneficial microbes that can enhance gut barrier function and indirectly reduce RV infection susceptibility (95).

Table 6. Protective effects of human milk components against RV infection: mechanisms and experimental evidence.
Mucins (MUC1, MUC4) present in human milk exhibit sialylated and fucosylated glycans that bind RV particles, physically blocking the interaction with epithelial cell receptors necessary for viral internalization (96). Lactadherin, another glycoprotein, contains phosphatidylserine-binding domains that enable it to bind directly to RV outer capsid proteins, interfering with viral attachment and entry into enterocytes; higher lactadherin concentrations in milk are associated with reduced symptomatic RV infections in infants (97).
Extracellular vesicles (EVs) isolated from colostrum carry miRNAs, lipids, and proteins capable of modulating host cell antiviral responses, including blocking viral entry, possibly through receptor competition or modulation of intracellular signaling pathways involved in viral replication (98). Anti-RV antibodies (IgA, IgG) in milk directly neutralize RV by binding to viral capsid proteins, preventing attachment, facilitating agglutination, and promoting immune exclusion in the gut lumen (99).
Additionally, novel glycans in human milk have been identified using glycan microarrays; these glycans, particularly those with unique sialylation and fucosylation patterns, act as decoy receptors targeting specific RV strains prevalent in neonates (100). Milk proteins, such as lactoferrin, inhibit RV by binding to viral particles or blocking cell surface receptors, while also possessing iron-chelating properties that can impair viral replication (101). Lastly, studies on whole breastmilk show that certain components can inhibit the replication of live oral RV vaccines in vitro, indicating that while protective, some milk constituents may impede vaccine virus replication, potentially affecting seroconversion rates in infants (102). Collectively, these human milk factors provide both direct antiviral effects and indirect immunomodulatory actions that contribute to the protection of infants against RV infection.
4.4 Dietary fibers, prebiotics, and probiotics
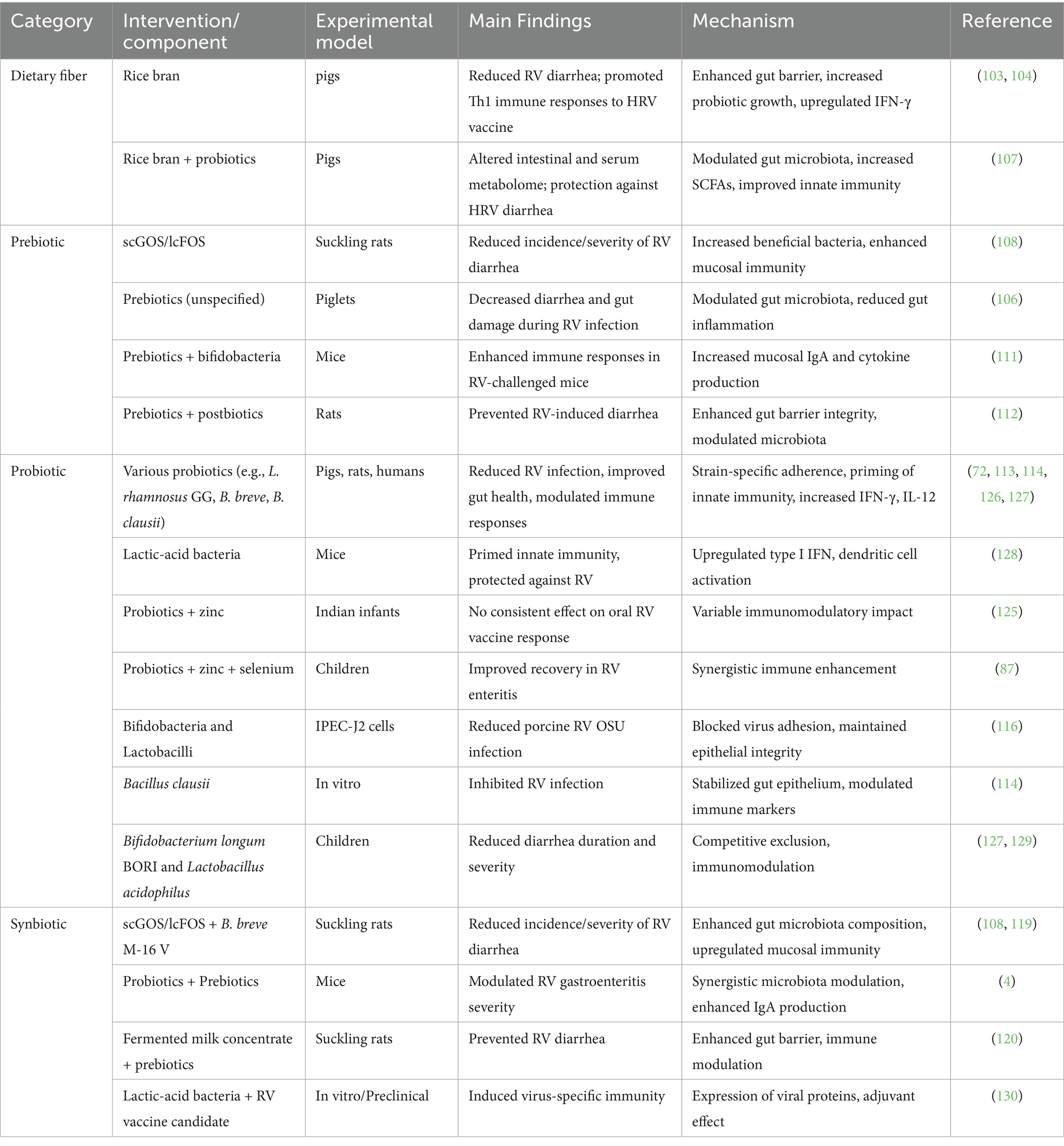
Dietary fibers, prebiotics, and probiotics represent nutritional strategies with significant potential to modulate the gut microbiota, enhance immune responses, and confer protection against RV infection (Table 7) (103–105). Dietary fiber, particularly rice bran, protects against RV (RV) infection by multiple mechanisms, including enhancing intestinal barrier integrity, modulating gut microbiota, and promoting Th1-biased immune responses. In gnotobiotic pigs, rice bran supplementation reduced RV diarrhea and elevated IFN-γ and IL-12 production, boosting the response to RV vaccination (106). Rice bran also increased the abundance of Lactobacillus and Bifidobacterium, improved mucin production, and upregulated innate immune genes such as TLRs and antimicrobial peptides, leading to stronger epithelial defenses (104, 107). Prebiotics, such as the scGOS/lcFOS mixture, decreased RV-induced diarrhea in suckling rats by enriching Bifidobacterium and Lactobacillus populations, which enhance SCFA production, mucosal IgA secretion, and the expression of tight junction proteins, maintaining gut permeability (108–110). Prebiotic supplementation, together with bifidobacteria, enhanced both systemic and mucosal immunity in mice, with increased RV-specific IgA, IgG responses, and higher levels of IL-4 and IFN-γ, indicating balanced Th1/Th2 responses (111). Similarly, a prebiotic-postbiotic mixture strengthened gut barrier function, reduced intestinal permeability, and modulated inflammatory cytokines such as IL-10 and TNF-α in a neonatal rat RV infection model (112). Probiotics, including Bifidobacterium breve, Lactobacillus rhamnosus GG, and Bacillus clausii, have shown strain-specific antiviral effects through competitive exclusion, production of antiviral metabolites, and modulation of host immunity. For instance, B. breve M-16 V prevented diarrhea by increasing dendritic cell maturation and enhancing Th1 cytokine profiles (113), while B. clausii induced epithelial stabilization, reduced oxidative stress, and upregulated type I IFN pathways, curbing RV replication in vitro (114). In pigs, probiotics modulated T cell responses, increasing CD8 + T cells and IFN-γ levels, which are critical for viral clearance (115). Probiotics also reduced RV infection in porcine IPEC-J2 epithelial cells by enhancing expression of tight junction proteins (claudin-1, occludin), reducing viral adhesion, and decreasing NF-κB mediated inflammation (116–118).
Synbiotics, like scGOS/lcFOS combined with B. breve M-16 V, provided synergistic protection by reinforcing the epithelial barrier, elevating mucosal IgA, and stimulating TLR expression, leading to heightened pathogen recognition and immune activation (108, 119). A fermented milk with prebiotics induced similar protection via upregulating mucin production and fortifying tight junctions (20, 120). Additionally, a probiotic-prebiotic combination reduced severe gastroenteritis in mice by increasing intestinal IFN-γ and IL-10 levels, balancing inflammation (4). Together, these interventions work by a multifaceted mechanism involving gut microbiota modulation, enhancement of epithelial integrity, and immune system priming, leading to effective mitigation of RV infection. Incorporating dietary fibers, prebiotics, and probiotics into nutritional strategies offers a promising approach to bolster gut health, enhance immune defenses, and reduce the impact of RV infections.
5 Gaps in knowledge and future directions
The interplay between nutrition, gut microbiota, and immunity is a cornerstone of the body’s response to RV infection, influencing both the susceptibility to infection and the severity of the disease. RV, as a leading cause of diarrheal disease, remains a major global health challenge, particularly in LMICswhere high rates of malnutrition and dysbiosis increase the vulnerability of populations to more severe outcomes (1). The recent research reviewed herein highlights the multifaceted roles that dietary factors, microbiota composition, and immune responses play in shaping the overall RV disease process. Nutrition exerts profound effects on the gut microbiota, which in turn influences immune responses critical for defending against RV infection. Macronutrients, micronutrients, probiotics, and prebiotics contribute to maintaining a balanced and diverse microbiota, which is essential for promoting efficient immune responses, strengthening gut barrier integrity, and reducing disease severity. The ability of specific dietary components to modulate immune function is a promising therapeutic avenue for improving RV outcomes, particularly when used in conjunction with vaccines (Figure 1).
Despite significant advancements in understanding the interplay between nutrition, microbiota, and immunity in RV infection, several critical knowledge gaps remain. Addressing these gaps is essential to develop more effective prevention and therapeutic strategies, particularly in vulnerable populations with high disease burdens. While numerous studies have established associations between gut microbiota composition and immune responses to RV, the specific molecular and cellular mechanisms underlying these interactions are not fully elucidated. Future research should focus on delineating how specific microbial taxa and their metabolites modulate mucosal and systemic immunity against RV. The variability in oral RV vaccine efficacy between high-income and LMICsremains poorly understood. Factors such as environmental enteric dysfunction, microbiota composition, nutritional deficiencies, and genetic diversity likely contribute to these differences. Comprehensive multi-omic studies integrating microbiome, metabolome, and host genetic data are needed to identify key determinants of vaccine performance. Although numerous nutritional interventions (e.g., probiotics, prebiotics, micronutrients) have shown promise in modulating gut health and immunity, the optimal combinations, dosages, and timing of these interventions remain to be established. More randomized controlled trials are necessary to define standardized, population-specific nutritional protocols. Personalized nutrition and microbiota modulation offer great potential but face practical challenges in implementation, particularly in resource-limited settings. Developing cost-effective, scalable methods for microbiota profiling and individualized dietary interventions is a critical area for future research. The long-term effects of early-life nutritional and microbiota-targeted interventions on RV immunity, susceptibility to other enteric infections, and overall health remain unclear. Longitudinal cohort studies are needed to assess how early interventions influence immune development and disease outcomes across the lifespan. Environmental factors such as sanitation, hygiene, and exposure to pathogens significantly influence gut microbiota and immunity. Future studies should integrate environmental and socioeconomic variables to develop holistic interventions that address the broader determinants of health affecting RV outcomes. Innovative vaccine strategies that harness or modulate the microbiota to enhance immune responses are still in nascent stages. Research into microbiota-directed vaccines and adjuvants could revolutionize vaccination strategies, especially in populations with dysbiosis or compromised gut health. Implementing personalized nutrition and microbiome-based interventions raises ethical considerations related to data privacy, equity in access, and informed consent. Recent progress in three-dimensional intestinal organoid technology has also provided robust and physiologically relevant platforms for investigating RV–host interactions. This technology complements existing human, animal, and in vitro models and holds great promise for advancing mechanistic understanding and therapeutic discovery in RV infection. Recent advances in artificial intelligence and machine learning offer promising avenues for dissecting the complex interplay between nutrition, microbiota, and immune responses in rotavirus infection. These applications in clinical environments are evolving—encompassing tasks ranging from diagnostic support to therapeutic stratification—and are poised to bridge the gap between bench findings and real-world implementation (121). In the context of our topic, these tools could be leveraged to integrate microbiome composition, nutritional status, immune profiles, and clinical outcomes, thereby refining intervention strategies. Establishing ethical frameworks and guidelines will be essential as these approaches move closer to clinical and public health applications. Future research should prioritize interdisciplinary collaborations integrating microbiology, immunology, nutrition, genomics, and public health. Investments in technological innovations, such as low-cost microbiota sequencing and machine learning algorithms for predictive modeling, will accelerate progress. Policymakers and healthcare providers must also be engaged to translate scientific insights into practical, equitable interventions that can be implemented globally. By addressing these knowledge gaps and advancing integrated strategies, the scientific and medical communities can enhance RV prevention, treatment, and vaccine efficacy, ultimately reducing the global burden of this significant enteric pathogen.
Author contributions
WH: Conceptualization, Data curation, Formal analysis, Writing – original draft. YQ: Investigation, Methodology, Writing – review & editing. EL: Methodology, Writing – review & editing. ML: Investigation, Methodology, Writing – review & editing. LC: Investigation, Methodology, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This project was supported by Henan University of Animal Husbandry and Economy Doctoral Research Start-up Funding Project (2019HNUAHEDF039).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Franco, MA, and Greenberg, HB. Challenges for rotavirus vaccines. Virology. (2001) 281:153–5. doi: 10.1006/viro.2001.0830
2. Magwira, CA, and Taylor, MB. Composition of gut microbiota and its influence on the immunogenicity of oral rotavirus vaccines. Vaccine. (2018) 36:3427–33. doi: 10.1016/j.vaccine.2018.04.091
3. Vlasova, AN, Paim, FC, Kandasamy, S, Alhamo, MA, Fischer, DD, Langel, SN, et al. Protein malnutrition modifies innate immunity and gene expression by intestinal epithelial cells and human rotavirus infection in neonatal gnotobiotic pigs. mSphere. (2017) 2:17. doi: 10.1128/mSphere.00046-17
4. Gonzalez-Ochoa, G, Flores-Mendoza, LK, Icedo-Garcia, R, Gomez-Flores, R, and Tamez-Guerra, P. Modulation of rotavirus severe gastroenteritis by the combination of probiotics and prebiotics. Arch Microbiol. (2017) 199:953–61. doi: 10.1007/s00203-017-1400-3
5. Angel, J, Franco, MA, and Greenberg, HB. Rotavirus immune responses and correlates of protection. Curr Opin Virol. (2012) 2:419–25. doi: 10.1016/j.coviro.2012.05.003
6. Holloway, G, and Coulson, BS. Innate cellular responses to rotavirus infection. J Gen Virol. (2013) 94:1151–60. doi: 10.1099/vir.0.051276-0
7. Li, E, Feng, N, Zeng, Q, Sanchez-Tacuba, L, Kawagishi, T, Branham, G, et al. Rhesus rotavirus NSP1 mediates extra-intestinal infection and is a contributing factor for biliary obstruction. PLoS Pathog. (2024) 20:e1012609. doi: 10.1371/journal.ppat.1012609
8. Yuan, L, and Saif, LJ. Induction of mucosal immune responses and protection against enteric viruses: rotavirus infection of gnotobiotic pigs as a model. Vet Immunol Immunopathol. (2002) 87:147–60. doi: 10.1016/s0165-2427(02)00046-6
9. Desselberger, U, and Huppertz, HI. Immune responses to rotavirus infection and vaccination and associated correlates of protection. J Infect Dis. (2011) 203:188–95. doi: 10.1093/infdis/jiq031
10. Cohen, AL, Platts-Mills, JA, Nakamura, T, Operario, DJ, Antoni, S, Mwenda, JM, et al. Aetiology and incidence of diarrhoea requiring hospitalisation in children under 5 years of age in 28 low-income and middle-income countries: findings from the global Pediatric Diarrhea surveillance network. BMJ Glob Health. (2022) 7:548. doi: 10.1136/bmjgh-2022-009548
11. Angel, J, Steele, AD, and Franco, MA. Correlates of protection for rotavirus vaccines: possible alternative trial endpoints, opportunities, and challenges. Hum Vaccin Immunother. (2014) 10:3659–71. doi: 10.4161/hv.34361
12. Grimwood, K, and Lambert, SB. Rotavirus vaccines: opportunities and challenges. Hum Vaccin. (2009) 5:57–69. doi: 10.4161/hv.5.2.6924
13. Burnett, E, Parashar, U, and Tate, J. Rotavirus vaccines: effectiveness, safety, and future directions. Paediatr Drugs. (2018) 20:223–33. doi: 10.1007/s40272-018-0283-3
14. Carvalho, MF, and Gill, D. Rotavirus vaccine efficacy: current status and areas for improvement. Hum Vaccin Immunother. (2019) 15:1237–50. doi: 10.1080/21645515.2018.1520583
15. Heyman, M, Corthier, G, Petit, A, Meslin, JC, Moreau, C, and Desjeux, JF. Intestinal absorption of macromolecules during viral enteritis: an experimental study on rotavirus-infected conventional and germ-free mice. Pediatr Res. (1987) 22:72–8. doi: 10.1203/00006450-198707000-00017
16. Hulst, M, Kerstens, H, de Wit, A, Smits, M, van der Meulen, J, and Niewold, T. Early transcriptional response in the jejunum of germ-free piglets after oral infection with virulent rotavirus. Arch Virol. (2008) 153:1311–22. doi: 10.1007/s00705-008-0118-6
17. Uchiyama, R, Chassaing, B, Zhang, B, and Gewirtz, AT. Antibiotic treatment suppresses rotavirus infection and enhances specific humoral immunity. J Infect Dis. (2014) 210:171–82. doi: 10.1093/infdis/jiu037
18. Ngo, VL, Wang, Y, Wang, Y, Shi, Z, Britton, R, Zou, J, et al. Select Gut Microbiota Impede Rotavirus Vaccine Efficacy. Cell Mol Gastroenterol Hepatol. (2024) 18:101393. doi: 10.1016/j.jcmgh.2024.101393
19. Li, E, Li, C, Horn, N, and Ajuwon, KM. PPARgamma activation inhibits endocytosis of claudin-4 and protects against deoxynivalenol-induced intestinal barrier dysfunction in IPEC-J2 cells and weaned piglets. Toxicol Lett. (2023) 375:8–20. doi: 10.1016/j.toxlet.2022.12.015
20. Li, E, Li, C, Horn, N, and Ajuwon, KM. Quercetin attenuates deoxynivalenol-induced intestinal barrier dysfunction by activation of Nrf2 signaling pathway in IPEC-J2 cells and weaned piglets. Curr Res Toxicol. (2023) 5:100122. doi: 10.1016/j.crtox.2023.100122
21. Chanez-Paredes, SD, Abtahi, S, Zha, J, Li, E, Marsischky, G, Zuo, L, et al. Mechanisms underlying distinct subcellular localization and regulation of epithelial long myosin light-chain kinase splice variants. J Biol Chem. (2024) 300:105643. doi: 10.1016/j.jbc.2024.105643
22. Zhao, Y, Hu, N, Jiang, Q, Zhu, L, Zhang, M, Jiang, J, et al. Protective effects of sodium butyrate on rotavirus inducing endoplasmic reticulum stress-mediated apoptosis via PERK-eIF2alpha signaling pathway in IPEC-J2 cells. J Anim Sci Biotechnol. (2021) 12:69. doi: 10.1186/s40104-021-00592-0
23. Dong, X, Wang, Y, Zhu, X, Shen, L, Chen, L, Niu, L, et al. Sodium butyrate protects against rotavirus-induced intestinal epithelial barrier damage by activating AMPK-Nrf2 signaling pathway in IPEC-J2 cells. Int J Biol Macromol. (2023) 228:186–96. doi: 10.1016/j.ijbiomac.2022.12.219
24. Li, E, and Ajuwon, KM. Mechanism of endocytic regulation of intestinal tight junction remodeling during nutrient starvation in jejunal IPEC-J2 cells. FASEB J. (2021) 35:e21356. doi: 10.1096/fj.202002098R
25. Li, E, Horn, N, and Ajuwon, KM. Mechanisms of deoxynivalenol-induced endocytosis and degradation of tight junction proteins in jejunal IPEC-J2 cells involve selective activation of the MAPK pathways. Arch Toxicol. (2021) 95:2065–79. doi: 10.1007/s00204-021-03044-w
26. Kim, Y, and Chang, KO. Inhibitory effects of bile acids and synthetic farnesoid X receptor agonists on rotavirus replication. J Virol. (2011) 85:12570–7. doi: 10.1128/JVI.05839-11
27. Kong, F, Saif, LJ, and Wang, Q. Roles of bile acids in enteric virus replication. Anim Dis. (2021) 1:2. doi: 10.1186/s44149-021-00003-x
28. Huang, Y, Zhu, Q, Wang, Y, and Zhu, K. Bacterial-derived sialidases inhibit porcine rotavirus OSU replication by interfering with the early steps of infection. Microb Pathog. (2024) 190:106628. doi: 10.1016/j.micpath.2024.106628
29. Bialowas, S, Hagbom, M, Nordgren, J, Karlsson, T, Sharma, S, Magnusson, KE, et al. Rotavirus and serotonin Cross-talk in diarrhoea. PLoS One. (2016) 11:e0159660. doi: 10.1371/journal.pone.0159660
30. Mametja, PM, Motshudi, MC, Naidoo, CM, Rakau, K, Seheri, LM, and Mkolo, NM. Tapping into metabolomics for understanding host and rotavirus group a interactome. Life (Basel). (2025) 15:15. doi: 10.3390/life15050765
31. Vorobiova, N, and Usachova, E. Influence of carbohydrate malabsorption syndrome on the clinical course of rotavirus infection in children at an early age. Georgian Med News. (2021) 311:120–5.
32. Harris, VC, Haak, BW, Handley, SA, Jiang, B, Velasquez, DE, Hykes, BL, et al. Effect of antibiotic-mediated microbiome modulation on rotavirus vaccine immunogenicity: a human, randomized-control proof-of-concept trial. Cell Host Microbe. (2018) 24:197–207.e194. doi: 10.1016/j.chom.2018.07.005
33. Majamaa, H, Isolauri, E, Saxelin, M, and Vesikari, T. Lactic acid bacteria in the treatment of acute rotavirus gastroenteritis. J Pediatr Gastroenterol Nutr. (1995) 20:333–8. doi: 10.1097/00005176-199504000-00012
34. Lee, DK, Park, JE, Kim, MJ, Seo, JG, Lee, JH, Ha, NJP, et al. Acidophilus inhibit infection by rotavirus in vitro and decrease the duration of diarrhea in pediatric patients. Clin Res Hepatol Gastroenterol. (2015) 39:237–44. doi: 10.1016/j.clinre.2014.09.006
35. Pant, N, Marcotte, H, Brussow, H, Svensson, L, and Hammarstrom, L. Effective prophylaxis against rotavirus diarrhea using a combination of Lactobacillus rhamnosus GG and antibodies. BMC Microbiol. (2007) 7:86. doi: 10.1186/1471-2180-7-86
36. Kang, JY, Lee, DK, Ha, NJ, and Shin, HS. Antiviral effects of Lactobacillus ruminis SPM0211 and Bifidobacterium longum SPM1205 and SPM1206 on rotavirus-infected Caco-2 cells and a neonatal mouse model. J Microbiol. (2015) 53:796–803. doi: 10.1007/s12275-015-5302-2
37. Shi, Z, Zou, J, Zhang, Z, Zhao, X, Noriega, J, Zhang, B, et al. Segmented filamentous Bacteria prevent and cure rotavirus infection. Cell. (2019) 179:644-658 e613. doi: 10.1016/j.cell.2019.09.028
38. Ngo, VL, Shi, Z, Jiang, B, and Gewirtz, AT. Segmented filamentous bacteria impede rotavirus infection via retinoic acid receptor-mediated signaling. Gut Microbes. (2023) 15:2174407. doi: 10.1080/19490976.2023.2174407
39. Varyukhina, S, Freitas, M, Bardin, S, Robillard, E, Tavan, E, Sapin, C, et al. Glycan-modifying bacteria-derived soluble factors from Bacteroides thetaiotaomicron and Lactobacillus casei inhibit rotavirus infection in human intestinal cells. Microbes Infect. (2012) 14:273–8. doi: 10.1016/j.micinf.2011.10.007
40. Seo, BJ, Mun, MR, J R, K, Kim, CJ, Lee, I, Chang, YH, et al. Bile tolerant Lactobacillus reuteri isolated from pig feces inhibits enteric bacterial pathogens and porcine rotavirus. Vet Res Commun. (2010) 34:323–33. doi: 10.1007/s11259-010-9357-6
41. Juntunen, M, Kirjavainen, PV, Ouwehand, AC, Salminen, SJ, and Isolauri, E. Adherence of probiotic bacteria to human intestinal mucus in healthy infants and during rotavirus infection. Clin Diagn Lab Immunol. (2001) 8:293–6. doi: 10.1128/CDLI.8.2.293-296.2001
42. Zhang, W, Wen, K, Azevedo, MS, Gonzalez, A, Saif, LJ, Li, G, et al. Lactic acid bacterial colonization and human rotavirus infection influence distribution and frequencies of monocytes/macrophages and dendritic cells in neonatal gnotobiotic pigs. Vet Immunol Immunopathol. (2008) 121:222–31. doi: 10.1016/j.vetimm.2007.10.001
43. Kgosana, LP, Seheri, ML, and Magwira, CA. Abundance of selected lipopolysaccharide-Rich Bacteria and levels of toll-like receptor 4 and interleukin 8 expression are significantly associated with live attenuated rotavirus vaccine shedding among south African infants. Vaccines (Basel). (2024) 12:273. doi: 10.3390/vaccines12030273
44. Raev, SA, Omwando, AM, Guo, Y, Raque, MS, Amimo, JO, Saif, LJ, et al. Glycan-mediated interactions between bacteria, rotavirus and the host cells provide an additional mechanism of antiviral defence. Benef Microbes. (2022) 13:383–96. doi: 10.3920/BM2022.0026
45. Engevik, MA, Banks, LD, Engevik, KA, Chang-Graham, AL, Perry, JL, Hutchinson, DS, et al. Rotavirus infection induces glycan availability to promote ileum-specific changes in the microbiome aiding rotavirus virulence. Gut Microbes. (2020) 11:1324–47. doi: 10.1080/19490976.2020.1754714
46. Parker, EPK, Praharaj, I, Zekavati, A, Lazarus, RP, Giri, S, Operario, DJ, et al. Influence of the intestinal microbiota on the immunogenicity of oral rotavirus vaccine given to infants in South India. Vaccine. (2018) 36:264–72. doi: 10.1016/j.vaccine.2017.11.031
47. Saavedra, JM, Bauman, NA, Oung, I, Perman, JA, and Yolken, RH. Feeding of Bifidobacterium bifidum and Streptococcus thermophilus to infants in hospital for prevention of diarrhoea and shedding of rotavirus. Lancet. (1994) 344:1046–9. doi: 10.1016/s0140-6736(94)91708-6
48. Fischer, DD, Kandasamy, S, Paim, FC, Langel, SN, Alhamo, MA, Shao, L, et al. Protein malnutrition alters tryptophan and angiotensin-converting enzyme 2 homeostasis and adaptive immune responses in human rotavirus-infected gnotobiotic pigs with human infant Fecal microbiota transplant. Clin Vaccine Immunol. (2017) 24:17. doi: 10.1128/CVI.00172-17
49. Zijlstra, RT, Donovan, SM, Odle, J, Gelberg, HB, Petschow, BW, and Gaskins, HR. Protein-energy malnutrition delays small-intestinal recovery in neonatal pigs infected with rotavirus. J Nutr. (1997) 127:1118–27. doi: 10.1093/jn/127.6.1118
50. Zijlstra, RT, McCracken, BA, Odle, J, Donovan, SM, Gelberg, HB, Petschow, BW, et al. Malnutrition modifies pig small intestinal inflammatory responses to rotavirus. J Nutr. (1999) 129:838–43. doi: 10.1093/jn/129.4.838
51. Oladele, P, Li, E, Lu, H, Cozannet, P, Nakatsu, C, Johnson, T, et al. Effect of a carbohydrase admixture in growing pigs fed wheat-based diets in thermoneutral and heat stress conditions. J Anim Sci. (2021) 99:254. doi: 10.1093/jas/skab254
52. Kumar, A, Vlasova, AN, Deblais, L, Huang, HC, Wijeratne, A, Kandasamy, S, et al. Impact of nutrition and rotavirus infection on the infant gut microbiota in a humanized pig model. BMC Gastroenterol. (2018) 18:93. doi: 10.1186/s12876-018-0810-2
53. Srivastava, V, Deblais, L, Huang, HC, Miyazaki, A, Kandasamy, S, Langel, SN, et al. Reduced rotavirus vaccine efficacy in protein malnourished human-faecal-microbiota-transplanted gnotobiotic pig model is in part attributed to the gut microbiota. Benef Microbes. (2020) 11:733–51. doi: 10.3920/BM2019.0139
54. Perez-Cano, FJ, Marin-Gallen, S, Castell, M, Rodriguez-Palmero, M, Rivero, M, Castellote, C, et al. Supplementing suckling rats with whey protein concentrate modulates the immune response and ameliorates rat rotavirus-induced diarrhea. J Nutr. (2008) 138:2392–8. doi: 10.3945/jn.108.093856
55. Corl, BA, Harrell, RJ, Moon, HK, Phillips, O, Weaver, EM, Campbell, JM, et al. Effect of animal plasma proteins on intestinal damage and recovery of neonatal pigs infected with rotavirus. J Nutr Biochem. (2007) 18:778–84. doi: 10.1016/j.jnutbio.2006.12.011
56. Wolber, FM, Broomfield, AM, Fray, L, Cross, ML, and Dey, D. Supplemental dietary whey protein concentrate reduces rotavirus-induced disease symptoms in suckling mice. J Nutr. (2005) 135:1470–4. doi: 10.1093/jn/135.6.1470
57. Wang, W, Chen, Y, Wang, J, Lv, Z, Li, E, Zhao, J, et al. Effects of reduced dietary protein at high temperature in summer on growth performance and carcass quality of finishing pigs. Animals (Basel). (2022) 12:599. doi: 10.3390/ani12050599
58. Fu, Y, Li, E, Casey, TM, Johnson, TA, Adeola, O, and Ajuwon, KM. Impact of maternal live yeast supplementation to sows on intestinal inflammatory cytokine expression and tight junction proteins in suckling and weanling piglets. J Anim Sci. (2024) 102:8. doi: 10.1093/jas/skae008
59. Monaco, MH, Gross, G, and Donovan, SM. Whey protein lipid concentrate high in Milk fat globule membrane components inhibit porcine and human rotavirus in vitro. Front Pediatr. (2021) 9:731005. doi: 10.3389/fped.2021.731005
60. Superti, F, Marziano, ML, Donelli, G, Marchetti, M, and Seganti, L. Enhancement of rotavirus infectivity by saturated fatty acids. Comp Immunol Microbiol Infect Dis. (1995) 18:129–35. doi: 10.1016/0147-9571(95)98854-b
61. Tao, R, Cheng, X, Gu, L, Zhou, J, Zhu, X, Zhang, X, et al. Lipidomics reveals the significance and mechanism of the cellular ceramide metabolism for rotavirus replication. J Virol. (2024) 98:e0006424. doi: 10.1128/jvi.00064-24
62. Mirazimi, A, and Svensson, L. Carbohydrates facilitate correct disulfide bond formation and folding of rotavirus VP7. J Virol. (1998) 72:3887–92. doi: 10.1128/JVI.72.5.3887-3892.1998
63. Sack, DA, Rhoads, M, Molla, A, Molla, AM, and Wahed, MA. Carbohydrate malabsorption in infants with rotavirus diarrhea. Am J Clin Nutr. (1982) 36:1112–8. doi: 10.1093/ajcn/36.6.1112
64. Isa, P, Arias, CF, and Lopez, S. Role of sialic acids in rotavirus infection. Glycoconj J. (2006) 23:27–37. doi: 10.1007/s10719-006-5435-y
65. Srnka, CA, Tiemeyer, M, Gilbert, JH, Moreland, M, Schweingruber, H, de Lappe, BW, et al. Cell surface ligands for rotavirus: mouse intestinal glycolipids and synthetic carbohydrate analogs. Virology. (1992) 190:794–805. doi: 10.1016/0042-6822(92)90917-e
66. Zhou, X, Li, Y, Li, T, Cao, J, Guan, Z, Xu, T, et al. Polysaccharide inhibits porcine rotavirus in vitro. Animals (Basel). (2023) 13:306. doi: 10.3390/ani13142306
67. Li, Y, Zhou, X, Qi, S, Jia, G, Cao, J, Guan, Z, et al. Polysaccharide on piglets infected with porcine rotavirus. Microb Pathog. (2025) 200:107355. doi: 10.1016/j.micpath.2025.107355
68. Ahmed, F, Jones, DB, and Jackson, AA. The interaction of vitamin a deficiency and rotavirus infection in the mouse. Br J Nutr. (1990) 63:363–73. doi: 10.1079/bjn19900122
69. Reifen, R, Mor, A, and Nyska, A. Vitamin a deficiency aggravates rotavirus infection in CD-1 mice through extensive involvement of the gut. Int J Vitam Nutr Res. (2004) 74:355–61. doi: 10.1024/0300-9831.74.5.355
70. Ahmed, F, Jones, DB, and Jackson, AA. Effect of vitamin a deficiency on the immune response to epizootic diarrhoea of infant mice (EDIM) rotavirus infection in mice. Br J Nutr. (1991) 65:475–85. doi: 10.1079/bjn19910106
71. Vlasova, AN, Chattha, KS, Kandasamy, S, Siegismund, CS, and Saif, LJ. Prenatally acquired vitamin a deficiency alters innate immune responses to human rotavirus in a gnotobiotic pig model. J Immunol. (2013) 190:4742–53. doi: 10.4049/jimmunol.1203575
72. Chattha, KS, Kandasamy, S, Vlasova, AN, and Saif, LJ. Vitamin a deficiency impairs adaptive B and T cell responses to a prototype monovalent attenuated human rotavirus vaccine and virulent human rotavirus challenge in a gnotobiotic piglet model. PLoS One. (2013) 8:e82966. doi: 10.1371/journal.pone.0082966
73. Kandasamy, S, Chattha, KS, Vlasova, AN, and Saif, LJ. Prenatal vitamin A deficiency impairs adaptive immune responses to pentavalent rotavirus vaccine (RotaTeq(R)) in a neonatal gnotobiotic pig model. Vaccine. (2014) 32:816–24. doi: 10.1016/j.vaccine.2013.12.039
74. Chepngeno, J, Amimo, JO, Michael, H, Jung, K, Raev, S, Lee, MV, et al. Rotavirus a inoculation and Oral vitamin a supplementation of vitamin a deficient pregnant sows enhances maternal adaptive immunity and passive protection of piglets against virulent rotavirus a. Viruses. (2022) 14:12354. doi: 10.3390/v14112354
75. Chepngeno, J, Amimo, JO, Michael, H, Raev, SA, Jung, K, Lee, MV, et al. Vitamin a deficiency and vitamin a supplementation affect innate and T cell immune responses to rotavirus a infection in a conventional sow model. Front Immunol. (2023) 14:1188757. doi: 10.3389/fimmu.2023.1188757
76. Tian, G, Liang, X, Chen, D, Mao, X, Yu, J, Zheng, P, et al. Vitamin D3 supplementation alleviates rotavirus infection in pigs and IPEC-J2 cells via regulating the autophagy signaling pathway. J Steroid Biochem Mol Biol. (2016) 163:157–63. doi: 10.1016/j.jsbmb.2016.05.004
77. Bucak, IH, Ozturk, AB, Almis, H, Cevik, MO, Tekin, M, Konca, C, et al. Is there a relationship between low vitamin D and rotaviral diarrhea? Pediatr Int. (2016) 58:270–3. doi: 10.1111/ped.12809
78. Zhao, Y, Yu, B, Mao, X, He, J, Huang, Z, Zheng, P, et al. Effect of 25-hydroxyvitamin D3 on rotavirus replication and gene expressions of RIG-I signalling molecule in porcine rotavirus-infected IPEC-J2 cells. Arch Anim Nutr. (2015) 69:227–35. doi: 10.1080/1745039X.2015.1034522
79. Zhao, Y, Ran, Z, Jiang, Q, Hu, N, Yu, B, Zhu, L, et al. Vitamin D alleviates rotavirus infection through a Microrna-155-5p mediated regulation of the TBK1/IRF3 Signaling pathway in vivo and in vitro. Int J Mol Sci. (2019) 20:3562. doi: 10.3390/ijms20143562
80. Zhao, Y, Zhu, X, Lan, Q, Wei, Z, Shang, P, Song, L, et al. 1alpha,25-hydroxyvitamin D(3) alleviated rotavirus infection induced ferroptosis in IPEC-J2 cells by regulating the ATF3-SLC7A11-GPX4 axis. Int J Biol Macromol. (2024) 283:137484. doi: 10.1016/j.ijbiomac.2024.137484
81. Zhao, Y, Yu, B, Mao, X, He, J, Huang, Z, Zheng, P, et al. Dietary vitamin D supplementation attenuates immune responses of pigs challenged with rotavirus potentially through the retinoic acid-inducible gene I signalling pathway. Br J Nutr. (2014) 112:381–9. doi: 10.1017/S000711451400097X
82. Colgate, ER, Haque, R, Dickson, DM, Carmolli, MP, Mychaleckyj, JC, Nayak, U, et al. Delayed dosing of Oral rotavirus vaccine demonstrates decreased risk of rotavirus gastroenteritis associated with serum zinc: a randomized controlled trial. Clin Infect Dis. (2016) 63:634–41. doi: 10.1093/cid/ciw346
83. Dalgic, N, Sancar, M, Bayraktar, B, Pullu, M, and Hasim, O. Probiotic, zinc and lactose-free formula in children with rotavirus diarrhea: are they effective? Pediatr Int. (2011) 53:677–82. doi: 10.1111/j.1442-200X.2011.03325.x
84. Xu, N, Zhang, W, Huo, J, Tao, R, Jin, T, Zhang, Y, et al. Characterization of changes in the intestinal microbiome following combination therapy with zinc preparation and conventional treatment for children with rotavirus enteritis. Front Cell Infect Microbiol. (2023) 13:1153701. doi: 10.3389/fcimb.2023.1153701
85. Erk, I, Huet, JC, Duarte, M, Duquerroy, S, Rey, F, Cohen, J, et al. A zinc ion controls assembly and stability of the major capsid protein of rotavirus. J Virol. (2003) 77:3595–601. doi: 10.1128/jvi.77.6.3595-3601.2003
86. Minaeian, S, Khales, P, Hosseini-Hosseinabad, SM, Farahmand, M, Poortahmasebi, V, Habib, Z, et al. Evaluation of activity of zinc oxide nanoparticles on human rotavirus and multi-drug resistant Acinetobacter baumannii. Pharm Nanotechnol. (2023) 11:475–85. doi: 10.2174/2211738511666230504121506
87. Cai, Y, Wang, X, Li, C, Li, F, Yan, Z, Ma, N, et al. Probiotics combined with zinc and selenium preparation in the treatment of child rotavirus enteritis. Am J Transl Res. (2022) 14:1043–50.
88. Hossieni, M, Kiani, SJ, Tavakoli, A, Kachooei, A, Habib, Z, and Monavari, SH. In vitro inhibition of rotavirus multiplication by copper oxide nanoparticles. Arch Razi Inst. (2024) 79:83–91. doi: 10.32592/ARI.2024.79.1.83
89. Pando, V, Isa, P, Arias, CF, and Lopez, S. Influence of calcium on the early steps of rotavirus infection. Virology. (2002) 295:190–200. doi: 10.1006/viro.2001.1337
90. Cohen, J, Laporte, J, Charpilienne, A, and Scherrer, R. Activation of rotavirus RNA polymerase by calcium chelation. Arch Virol. (1979) 60:177–86. doi: 10.1007/BF01317489
91. Poruchynsky, MS, Maass, DR, and Atkinson, PH. Calcium depletion blocks the maturation of rotavirus by altering the oligomerization of virus-encoded proteins in the ER. J Cell Biol. (1991) 114:651–6. doi: 10.1083/jcb.114.4.651
92. Hester, SN, Chen, X, Li, M, Monaco, MH, Comstock, SS, Kuhlenschmidt, TB, et al. Human milk oligosaccharides inhibit rotavirus infectivity in vitro and in acutely infected piglets. Br J Nutr. (2013) 110:1233–42. doi: 10.1017/S0007114513000391
93. Laucirica, DR, Triantis, V, Schoemaker, R, Estes, MK, and Ramani, S. Milk oligosaccharides inhibit human rotavirus infectivity in MA104 cells. J Nutr. (2017) 147:1709–14. doi: 10.3945/jn.116.246090
94. Li, M, Monaco, MH, Wang, M, Comstock, SS, Kuhlenschmidt, TB, Fahey, GC, et al. Human milk oligosaccharides shorten rotavirus-induced diarrhea and modulate piglet mucosal immunity and colonic microbiota. ISME J. (2014) 8:1609–20. doi: 10.1038/ismej.2014.10
95. Ramani, S, Stewart, CJ, Laucirica, DR, Ajami, NJ, Robertson, B, Autran, CA, et al. Human milk oligosaccharides, milk microbiome and infant gut microbiome modulate neonatal rotavirus infection. Nat Commun. (2018) 9:5010. doi: 10.1038/s41467-018-07476-4
96. Yolken, RH, Peterson, JA, Vonderfecht, SL, Fouts, ET, Midthun, K, and Newburg, DS. Human milk mucin inhibits rotavirus replication and prevents experimental gastroenteritis. J Clin Invest. (1992) 90:1984–91. doi: 10.1172/JCI116078
97. Newburg, DS, Peterson, JA, Ruiz-Palacios, GM, Matson, DO, Morrow, AL, Shults, J, et al. Role of human-milk lactadherin in protection against symptomatic rotavirus infection. Lancet. (1998) 351:1160–4. doi: 10.1016/s0140-6736(97)10322-1
98. Civra, A, Francese, R, Donalisio, M, Tonetto, P, Coscia, A, Sottemano, S, et al. Human colostrum and derived extracellular vesicles prevent infection by human rotavirus and respiratory syncytial virus in vitro. J Hum Lact. (2021) 37:122–34. doi: 10.1177/0890334420988239
99. Asensi, MT, Martinez-Costa, C, and Buesa, J. Anti-rotavirus antibodies in human milk: quantification and neutralizing activity. J Pediatr Gastroenterol Nutr. (2006) 42:560–7. doi: 10.1097/01.mpg.0000221892.59371.b3
100. Yu, Y, Lasanajak, Y, Song, X, Hu, L, Ramani, S, Mickum, ML, et al. Human milk contains novel glycans that are potential decoy receptors for neonatal rotaviruses. Mol Cell Proteomics. (2014) 13:2944–60. doi: 10.1074/mcp.M114.039875
101. Kvistgaard, AS, Pallesen, LT, Arias, CF, Lopez, S, Petersen, TE, Heegaard, CW, et al. Inhibitory effects of human and bovine milk constituents on rotavirus infections. J Dairy Sci. (2004) 87:4088–96. doi: 10.3168/jds.S0022-0302(04)73551-1
102. Kazimbaya, KM, Chisenga, CC, Simuyandi, M, Phiri, CM, Laban, NM, Bosomprah, S, et al. In-vitro inhibitory effect of maternal breastmilk components on rotavirus vaccine replication and association with infant seroconversion to live oral rotavirus vaccine. PLoS One. (2020) 15:e0240714. doi: 10.1371/journal.pone.0240714
103. Yang, X, Wen, K, Tin, C, Li, G, Wang, H, Kocher, J, et al. Dietary rice bran protects against rotavirus diarrhea and promotes Th1-type immune responses to human rotavirus vaccine in gnotobiotic pigs. Clin Vaccine Immunol. (2014) 21:1396–403. doi: 10.1128/CVI.00210-14
104. Yang, X, Twitchell, E, Li, G, Wen, K, Weiss, M, Kocher, J, et al. High protective efficacy of rice bran against human rotavirus diarrhea via enhancing probiotic growth, gut barrier function, and innate immunity. Sci Rep. (2015) 5:15004. doi: 10.1038/srep15004
105. Yang, C, Lu, H, Li, E, Oladele, P, and Ajuwon, KM. Inulin supplementation induces expression of hypothalamic antioxidant defence genes in weaned piglets. J Anim Physiol Anim Nutr (Berl). (2023) 107:157–64. doi: 10.1111/jpn.13698
106. Yang, H, Fan, X, Mao, X, Yu, B, He, J, Yan, H, et al. The protective role of prebiotics and probiotics on diarrhea and gut damage in the rotavirus-infected piglets. J Anim Sci Biotechnol. (2024) 15:61. doi: 10.1186/s40104-024-01018-3
107. Nealon, NJ, Yuan, L, Yang, X, and Ryan, EP. Rice bran and probiotics Alter the porcine large intestine and serum metabolomes for protection against human rotavirus Diarrhea. Front Microbiol. (2017) 8:653. doi: 10.3389/fmicb.2017.00653
108. Rigo-Adrover, M, Saldana-Ruiz, S, van Limpt, K, Knipping, K, Garssen, J, Knol, J, et al. A combination of scGOS/lcFOS with Bifidobacterium breve M-16V protects suckling rats from rotavirus gastroenteritis. Eur J Nutr. (2017) 56:1657–70. doi: 10.1007/s00394-016-1213-1
109. Wang, J, Tang, L, Wang, Y, Xing, Y, Chen, G, Jiang, Q, et al. Effects of enzymatic hydrolysate of cottonseed protein on growth performance, nutrient digestibility, blood indexes and Fecal volatile fatty acids of weaned piglets. J Anim Physiol Anim Nutr (Berl). (2025) 109:1062–71. doi: 10.1111/jpn.14121
110. Wang, Y, Li, Z, Chen, G, Xing, Y, Wang, J, Zhao, Y, et al. Dietary Galacto-oligosaccharides enhance growth performance and modulate gut microbiota in weaned piglets: a sustainable alternative to antibiotics. Animals (Basel). (2025) 15:508. doi: 10.3390/ani15111508
111. Qiao, H, Duffy, LC, Griffiths, E, Dryja, D, Leavens, A, Rossman, J, et al. Immune responses in rhesus rotavirus-challenged BALB/c mice treated with bifidobacteria and prebiotic supplements. Pediatr Res. (2002) 51:750–5. doi: 10.1203/00006450-200206000-00015
112. Morales-Ferre, C, Azagra-Boronat, I, Massot-Cladera, M, Tims, S, Knipping, K, Garssen, J, et al. Preventive effect of a postbiotic and prebiotic mixture in a rat model of early life rotavirus induced-Diarrhea. Nutrients. (2022) 14:1163. doi: 10.3390/nu14061163
113. Azagra-Boronat, I, Massot-Cladera, M, Knipping, K, Garssen, J, Ben Amor, K, Knol, J, et al. Strain-specific probiotic properties of bifidobacteria and lactobacilli for the prevention of Diarrhea caused by rotavirus in a preclinical model. Nutrients. (2020) 12:498. doi: 10.3390/nu12020498
114. Paparo, L, Tripodi, L, Bruno, C, Pisapia, L, Damiano, C, Pastore, L, et al. Protective action of Bacillus clausii probiotic strains in an in vitro model of rotavirus infection. Sci Rep. (2020) 10:12636. doi: 10.1038/s41598-020-69533-7
115. Chattha, KS, Vlasova, AN, Kandasamy, S, Rajashekara, G, and Saif, LJ. Divergent immunomodulating effects of probiotics on T cell responses to oral attenuated human rotavirus vaccine and virulent human rotavirus infection in a neonatal gnotobiotic piglet disease model. J Immunol. (2013) 191:2446–56. doi: 10.4049/jimmunol.1300678
116. Leblanc, D, Raymond, Y, Lemay, MJ, Champagne, CP, and Brassard, J. Effect of probiotic bacteria on porcine rotavirus OSU infection of porcine intestinal epithelial IPEC-J2 cells. Arch Virol. (2022) 167:1999–2010. doi: 10.1007/s00705-022-05510-x
117. Liu, F, Li, G, Wen, K, Bui, T, Cao, D, Zhang, Y, et al. Porcine small intestinal epithelial cell line (IPEC-J2) of rotavirus infection as a new model for the study of innate immune responses to rotaviruses and probiotics. Viral Immunol. (2010) 23:135–49. doi: 10.1089/vim.2009.0088
118. Li, E, Horn, N, and Ajuwon, KM. EPA and DHA inhibit endocytosis of claudin-4 and protect against deoxynivalenol-induced intestinal barrier dysfunction through PPARgamma dependent and independent pathways in jejunal IPEC-J2 cells. Food Res Int. (2022) 157:111420. doi: 10.1016/j.foodres.2022.111420
119. Rigo-Adrover, MDM, van Limpt, K, Knipping, K, Garssen, J, Knol, J, Costabile, A, et al. Preventive effect of a Synbiotic combination of Galacto- and Fructooligosaccharides mixture with Bifidobacterium breve M-16V in a model of multiple rotavirus infections. Front Immunol. (2018) 9:1318. doi: 10.3389/fimmu.2018.01318
120. Rigo-Adrover, MDM, Knipping, K, Garssen, J, van Limpt, K, Knol, J, Franch, A, et al. Prevention of rotavirus Diarrhea in suckling rats by a specific fermented Milk concentrate with prebiotic mixture. Nutrients. (2019) 11:189. doi: 10.3390/nu11010189
121. Yuan, H, Yu, K, Xie, F, Liu, M, and Sun, S. Automated machine learning with interpretation: a systematic review of methodologies and applications in healthcare. Med Adv. (2024) 2:205–37. doi: 10.1002/med4.75
122. Maier, EA, Weage, KJ, Guedes, MM, Denson, LA, McNeal, MM, Bernstein, DI, et al. Protein-energy malnutrition alters IgA responses to rotavirus vaccination and infection but does not impair vaccine efficacy in mice. Vaccine. (2013) 32:48–53. doi: 10.1016/j.vaccine.2013.10.072
123. Miyazaki, A, Kandasamy, S, Michael, H, Langel, SN, Paim, FC, Chepngeno, J, et al. Protein deficiency reduces efficacy of oral attenuated human rotavirus vaccine in a human infant fecal microbiota transplanted gnotobiotic pig model. Vaccine. (2018) 36:6270–81. doi: 10.1016/j.vaccine.2018.09.008
124. Ahmed, F, Jones, DB, and Jackson, AA. Effect of undernutrition on the immune response to rotavirus infection in mice. Ann Nutr Metab. (1990) 34:21–31. doi: 10.1159/000177566
125. Lazarus, RP, John, J, Shanmugasundaram, E, Rajan, AK, Thiagarajan, S, Giri, S, et al. The effect of probiotics and zinc supplementation on the immune response to oral rotavirus vaccine: a randomized, factorial design, placebo-controlled study among Indian infants. Vaccine. (2018) 36:273–9. doi: 10.1016/j.vaccine.2017.07.116
126. Grandy, G, Medina, M, Soria, R, Teran, CG, and Araya, M. Probiotics in the treatment of acute rotavirus diarrhoea. A randomized, double-blind, controlled trial using two different probiotic preparations in Bolivian children. BMC Infect Dis. (2010) 10:253. doi: 10.1186/1471-2334-10-253
127. Park, MS, Kwon, B, Ku, S, and Ji, GE. The efficacy of Bifidobacterium longum BORI and Lactobacillus acidophilus AD031 probiotic treatment in infants with rotavirus infection. Nutrients. (2017) 9:887. doi: 10.3390/nu9080887
128. Thompson, A, Van Moorlehem, E, and Aich, P. Probiotic-induced priming of innate immunity to protect against rotaviral infection. Probiotics Antimicrob Proteins. (2010) 2:90–7. doi: 10.1007/s12602-009-9032-9
129. Huang, YF, Liu, PY, Chen, YY, Nong, BR, Huang, IF, Hsieh, KS, et al. Three-combination probiotics therapy in children with salmonella and rotavirus gastroenteritis. J Clin Gastroenterol. (2014) 48:37–42. doi: 10.1097/MCG.0b013e31828f1c6e
Keywords: nutrition, microbiota, immunity, RV, gut health
Citation: Huo W, Qiao Y, Li E, Li M and Che L (2025) Interplay between nutrition, microbiota, and immunity in rotavirus infection: insights from human and animal models. Front. Vet. Sci. 12:1680448. doi: 10.3389/fvets.2025.1680448
Edited by:
Mengmeng Zhao, Foshan University, ChinaReviewed by:
Chaoyue Wen, Hunan Agricultural University, ChinaBangmin Liu, North Carolina State University, United States
Xuejiao Zhu, Jiangsu Academy of Agricultural Sciences (JAAS), China
Copyright © 2025 Huo, Qiao, Li, Li and Che. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenying Huo, Sm95Y2VodW9odW9AYWxpeXVuLmNvbQ==; Enkai Li, bGkuZUB3dXN0bC5lZHU=
 Wenying Huo
Wenying Huo Yingying Qiao
Yingying Qiao Enkai Li
Enkai Li Mengyun Li1
Mengyun Li1 Long Che
Long Che