- 1Faculty of Veterinary Science, Prince of Songkla University, Songkhla, Thailand
- 2District Livestock Office, Singha Nakhon District Office, Songkhla, Thailand
- 3Public Health and Environment Division, Trang City Municipality, Trang, Thailand
Canine gestation typically lasts 63–65 days after the LH surge. Although ultrasonography is well established for monitoring fetal development in dogs, little is known about the dynamics of amniotic fluid. This study aimed to evaluate allantoamniotic fluid (AAF) using ultrasonographic examinations and correlate it with various factors of normal fetal development. Pregnant American Bulldogs underwent ultrasonographic examination (from weeks 3 to 8), and fetal parameters were collected weekly from 29 fetuses (n = 29). This study determined values for allantoamniotic fluid by cross-sectional view (AAFDC), allantoamniotic fluid by longitudinal view (AAFDL), biparietal diameter (BPD), cardio-thoracic ratio (CTR), abdominal cross-sectional area (ACA), gastric area (GA), and intercostal space (ICS). The correlation between fetal parameters and gestational age was analyzed. The AAFDC and AAFDL were first detected at week 3. Their maximum values, 34.60 ± 7.89 mm and 33.51 ± 9.59 mm, respectively, were observed at week 6 (p < 0.05). Subsequently, these values significantly and steadily declined from week 7 to the end (p < 0.05). In contrast, the BPD, ACA, GA, and ICS increased significantly from week 8 and were highly significantly correlated with gestation (p < 0.0001). Moreover, AAFDC and AAFDL showed a moderate correlation with day of gestation (DG) and day before parturition (DBP) (p < 0.0001). In conclusion, these findings propose the establishment of AAFDC and AAFDL as novel parameters for evaluating fetal wellbeing in canine practice.
1 Introduction
In humans, the routine monitoring of fetal development is standard, with practical guidelines for perinatal pregnancy. However, the clear guidelines for the monitoring of normal fetal development are not yet well-established in dogs. Previous studies in dogs have been reported primarily through radiography and ultrasonography with parturition predictors and important indicators, e.g., visible organ development, size of fetal organs, fetal heartbeats, and fetal movement (1). Assessing fetal health in puppies presents challenges and considerations by gestational age and breed-specific characteristics. There is a direct impact on the evaluation and detection of fetal maturity and development. Recently, there have been limited studies on this topic (2–4). The gestational period of a dog normally ranges from 57 to 72 days after breeding (1, 5–7). Radiography and ultrasonography are routinely used for pregnancy monitoring and parturition prediction. However, these techniques have been used in the case of (1) the first detection of fetal organs to estimate gestational day (such as gestational sac, placenta, stomach, kidney, eyes, and gastrointestinal tract) (1, 4, 8–10) and (2) the determination of parturition date by formulating the size of extra-embryonic and extra-fetal structures [such as inner chorionic cavity (ICC), placental thickness, and outer uterine diameter (OUD)] or fetal structures [such as the size of crown rump length (CRL), biparietal diameter (BPD), body diameter, deep portion of diencephalo-telencephalic vesicle (DPTV), and kidney diameter] (8, 11–15).
In human antenatal care, assessing congenital abnormalities is also critically important beyond estimating gestational age and predicting parturition date. For example, assessment of amniotic fluid (AF) appearance and volume (16, 17), which is a standard parameter, can assist in evaluating the risk of specific abnormalities, such as oligohydramnios, which can indicate urinary tract obstruction, dysfunctional kidneys, abnormal placental function, and neonatal intensive care admission (18, 19). In addition, the volume and characteristics of amniotic fluid reflect the important aspects of fetal development and maturity (16, 20, 21).
For pregnant dogs, precisely recognizing fetal maturity is also key to planning the delivery date and ensuring viable, healthy neonates (22). However, estimating fetal development in dogs is complicated by interbreed variability, which limits the universal applicability of existing parameters. Previous studies have speculated that the allantoamniotic fluid (AAF) may provide important insights into neonatal maturity in dogs, as in humans, where scientific findings have already been established (22, 23). While the veterinary literature on AAF is limited, it predominantly addresses other species (24, 25). Consequently, the potential diagnostic role of amniotic fluid in dogs remains largely unknown (26, 27). To assess the volume of AF with non-invasive techniques, AF index measurements and the single deepest vertical pocket (SDVP) have been well established for assessment during pregnancy (28, 29). However, this AF assessment protocol has not yet been studied in dogs. Therefore, this study is the first to non-invasively evaluate the patterns and interrelationships of AAF with other fetal parameters. This study proposes these findings as complementary diagnostic markers. These markers can effectively challenge the interbreed variability in canine perinatal evaluation.
To date, no study has reported on the ultrasonographic assessment of AAF during fetal development throughout pregnancy in dogs. Therefore, the primary objective of this study was to describe the developmental changes of various fetal parameters (AAFDC, AAFDL, BPD, CTR, ACA, GA, and ICS) in normal puppies using ultrasonographic measurements. A secondary objective was to determine the correlation between these parameters and day of gestation (DG) and day before parturition (DBP), as well as their interrelationships. We, therefore, hypothesized that these parameters are positively correlated with gestational age and that a strong interrelationship exists among them, reflecting a synchronized and predictable growth pattern.
2 Materials and methods
This study was ethically performed according to the Prince of Songkla University Animal Care and Use Protocol (accession no. 2563–05-041). A G-power analysis was performed to calculate the minimum number of fetuses that should be included for this study. G-power analysis showed that the minimum number of animals needed was 29, considering an r value of 0.5, an alpha value (two-tailed) of 0.05, and a power of 0.8. The formulation of the total sample size is [(Zα + Zβ)/C]2 + 3. The fetal measurements were obtained from the fetuses (n = 29) by transabdominal ultrasonography. Five female American bulldogs, aged 2–4 years old and weighing between 20 and 25 kilograms, were included in this study. The litter size ranged from 5 to 8 puppies per dam. Before breeding, all dogs were routinely examined for health status and breeding soundness examinations. All puppies included in the study were required to be healthy and free from congenital disorders, particularly those affecting the cardiovascular, digestive, and urinary systems, as well as any other disorders leading to neonatal death.
2.1 Study designs
The mating plan used for breeding in this study was adapted from the protocol described in (9, 30). First, the timing of ovulation of the study was determined by a combination of serial vaginal cytology and serial progesterone assays, a widely accepted method for accurate breeding management (30–32). Each female dog was monitored for ovulation time by measuring serum progesterone levels using Bionote’s Vcheck V200 analyzer (Bionote Inc., Hwaseong, South Korea). Artificial insemination (AI) was then carried out using fresh semen. Ultrasonographic examination was performed weekly from days 21 (week 3) to 62 (week 9) after ovulation to confirm pregnancy and collect fetal parameters (3, 4, 8, 9). The date of Cesarean section was determined by both presentation of labor signs (e.g., excitement, dropped core body temperature, or clear fluid from the vagina) and a decrease in serum progesterone level, which was not greater than 4.00 ng/mL (33).
2.2 Sonographic measurements
Dogs underwent ultrasonographic examination in dorsal recumbency and were performed without sedation or anesthesia using transabdominal ultrasonography (Mindray DC-80 X-Insight, Shenzhen Mindray Bio-Medical Electronics Co., Ltd., Shenzhen, China) with a 4.0–10.0 MHz micro-convex probe for the diagnosis of pregnancy and fetal measurements. All dogs generally underwent sonographic evaluation with hair clipping and the use of acoustic coupling gel on the ventral abdomen. All fetal parameters were weekly performed and marked the position of the fetus, and then ultrasonographic images were measured by using SYNAPSE® software version 4.4.1 (Fujifilm Medical Systems, NC, USA). Three trained observers independently performed the ultrasonographic examinations and measurements, and the mean value of each parameter was used in this study. Fetal measurements were calculated as follows:
1. Allantoamniotic fluid volume (AAFV) was evaluated by using both allantoamniotic fluid depth by cross-sectional view (AAFDC) and longitudinal view (AAFDL) without the fetus’s presentation. AAFDC was an average of the two deepest regions at the fetal trunk, whereas the AAFDL was an average of the deepest sac (inner-to-inner edges) at the cranial and caudal regions (Figure 1). These values were adapted from single deepest pocket (SDP) and deepest vertical pocket (DVP) measurements in humans (34, 35).
2. BPD was measured from the distance between both sides of the parietal bone (outer-to-outer edge) in a symmetrically cross-sectional sonograph.
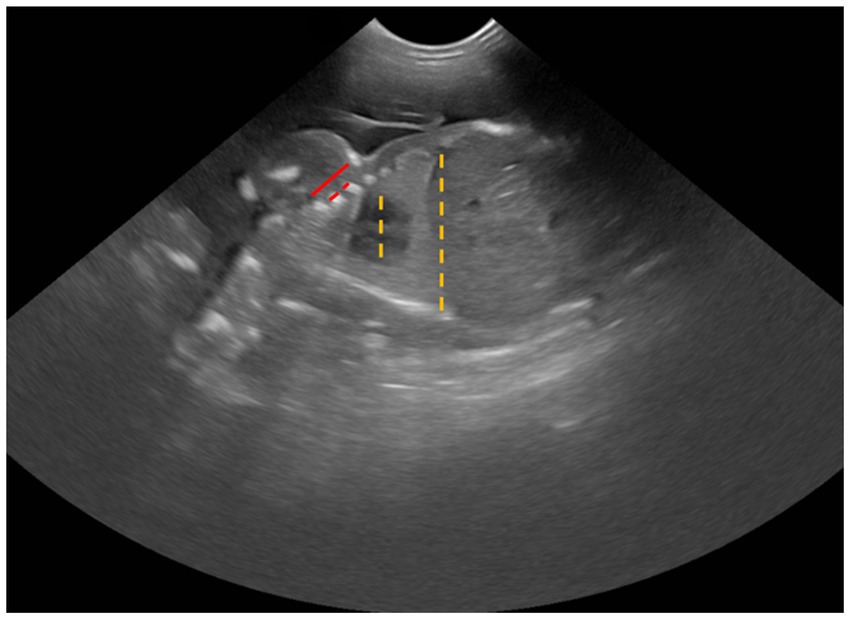
3. Cardio-thoracic ratio (CTR) was calculated by measuring the largest distance at the middle level of the fetal heart (outer-to-outer edge) and then dividing by the longest distance at the middle level of the last rib (Figure 2). Both measurements were done in a dorsal plane sonograph.
4. Abdominal cross-s.ectional area (ACA) was calculated from the two largest perpendicular truncal wall diameters (outer-to-outer edges) in a cross-sectional sonograph.
5. Gastric area (GA) was calculated from the largest cross-sectional stomach diameter in two perpendicular axes (outer-to-outer edges).
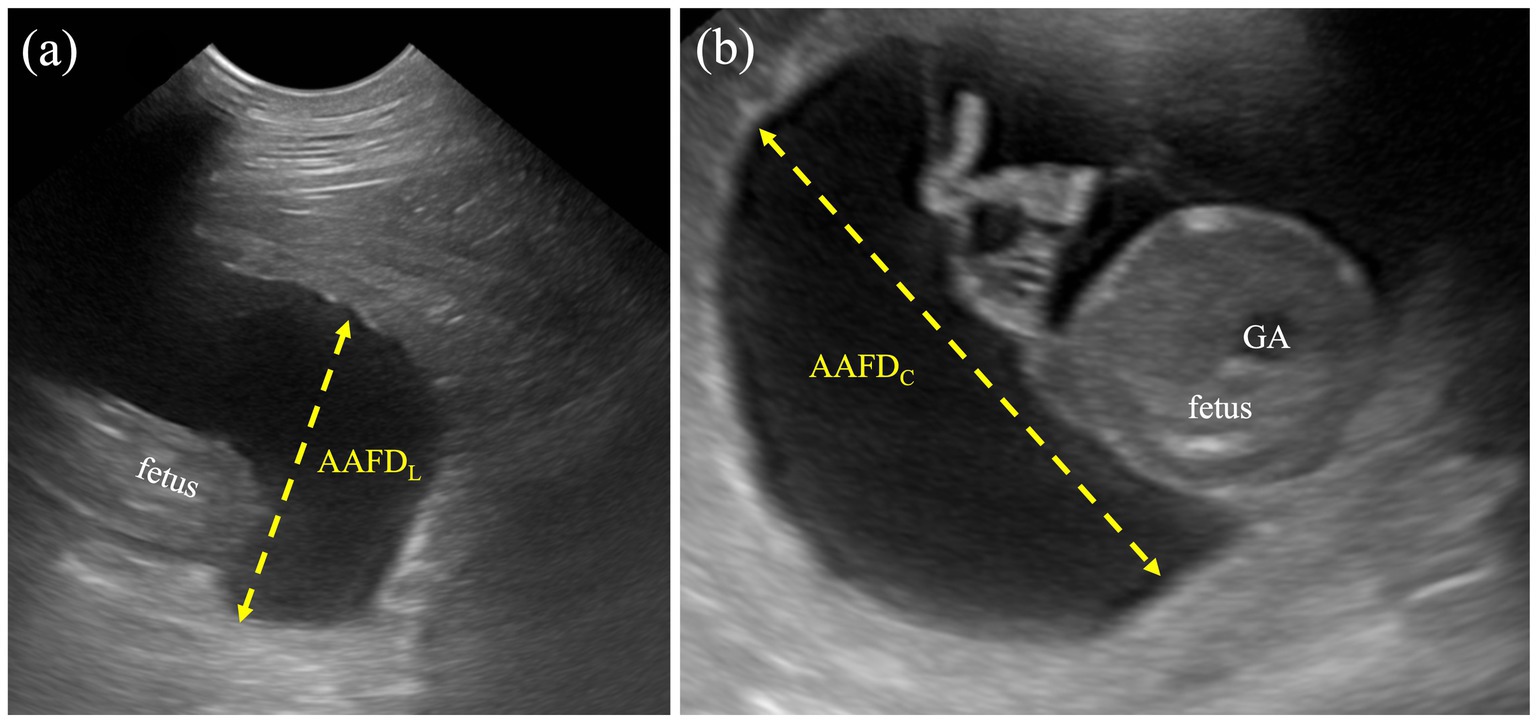
Figure 1. Measurement protocol for AAFDL and AAFDC. (a) The AAFDL was measured as the deepest sac at the caudal regions of the fetus, from inner edge to inner edge, excluding fetal structures. (b) The AAFDC was measured in the deepest region of the fetal trunk, specifically at the widest part of the cross-sectional view of the fetus.
Both ACA and GA were calculated by the equation of an ellipse (π/4 *A*B; A = diameter from the outer-to-outer edge of the truncal wall or stomach, and B = diameter from the perpendicular axis).
1. Intercostal space (ICS) was determined by measuring the distance from the caudal edge of the first rib to the cranial edge of the fourth rib, subtracting the diameters of the second and third ribs, and then dividing that total by three (Figure 2).
2.3 Statistical analysis
All data in this study were expressed as mean and standard deviation, and the statistical analysis was performed using GraphPad Prism (version 9.5.0 (730) GraphPad Software, San Diego, USA). Comparisons between values of fetal parameters from different weeks were analyzed by ANOVA. Pearson’s Square was applied to analyze the coefficient of determination (r2) between fetal parameters. The level of significance was set at a p-value of <0.05. Linear regression between gestational age, which included DG and DBP, and fetal parameters, was performed and presented as y = a + bx equation form (y = gestational age, a = intercept of the coefficient, and b = first-order coefficient).
3 Results
3.1 Descriptive analysis of allantoamniotic fluid (AAF) by ultrasonographic examinations
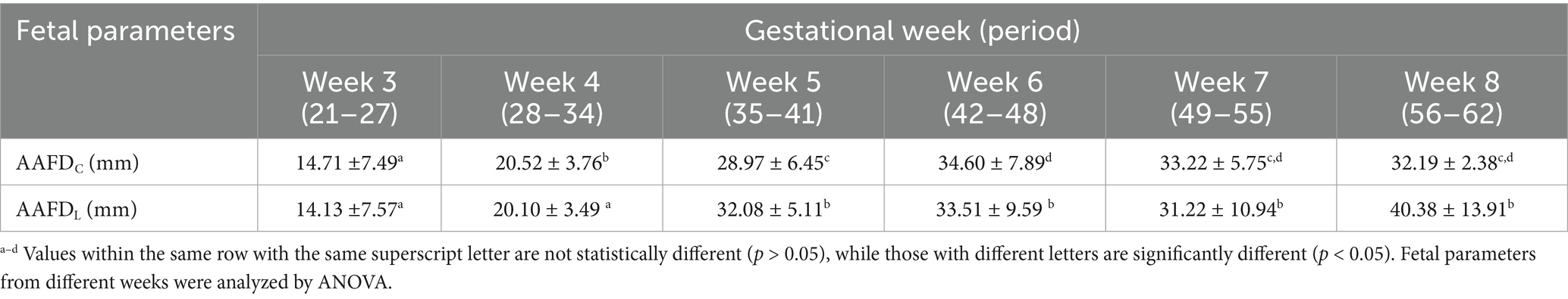
To determine the depth of AAF, the deepest region between the inner edges of the embryonic sac was measured. The AAFDC and AAFDL were first detected at week 3 with mean values of 14.71 ± 7.49 mm and 14.13 ± 7.57 mm, respectively. Maximum AAFDC and AAFDL were measured at week 6 as 34.60 ± 7.89 and 33.22 ± 5.75 mm, respectively (p < 0.05) (Figure 3; Table 1). Subsequently, both parameters significantly and steadily declined from week 7 to the end (p < 0.05).
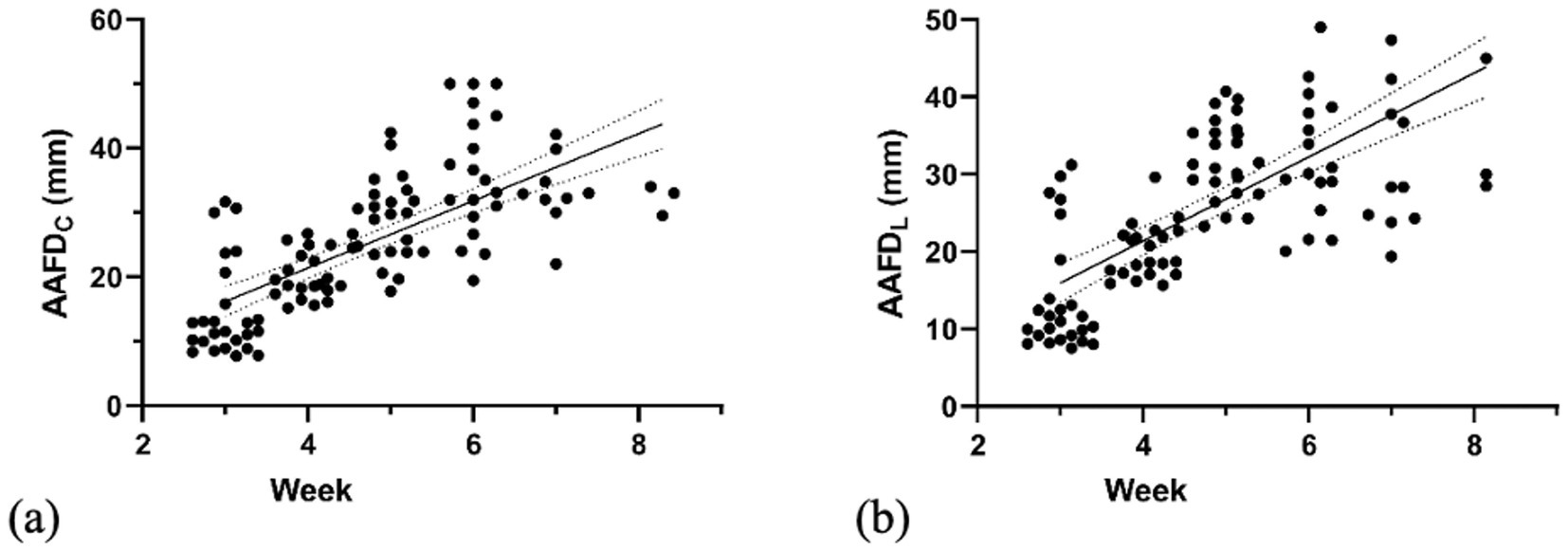
Figure 3. Linear regression graph illustrates the correlation of (a) AAFDC (weeks 3–8) and (b) AAFDL (weeks 3–8) with gestational weeks.
3.2 Descriptive analysis of other fetal parameters
This part describes the normal fetal development of the head, thorax, and abdomen sections. This study examined the developmental changes in fetal parameters (BPD, CTR, ACA, GA, and ICS) across gestational weeks (Figure 4 and Table 2). Continued growth was observed in most parameters. Specifically, BPD increased significantly from weeks 4 to 8 (p < 0.0001). Similarly, GA, ACA, and ICS also showed significant increases from weeks 6 to 8 (p < 0.0001). In contrast, the CTR showed steady values throughout the measurement period (p > 0.05).
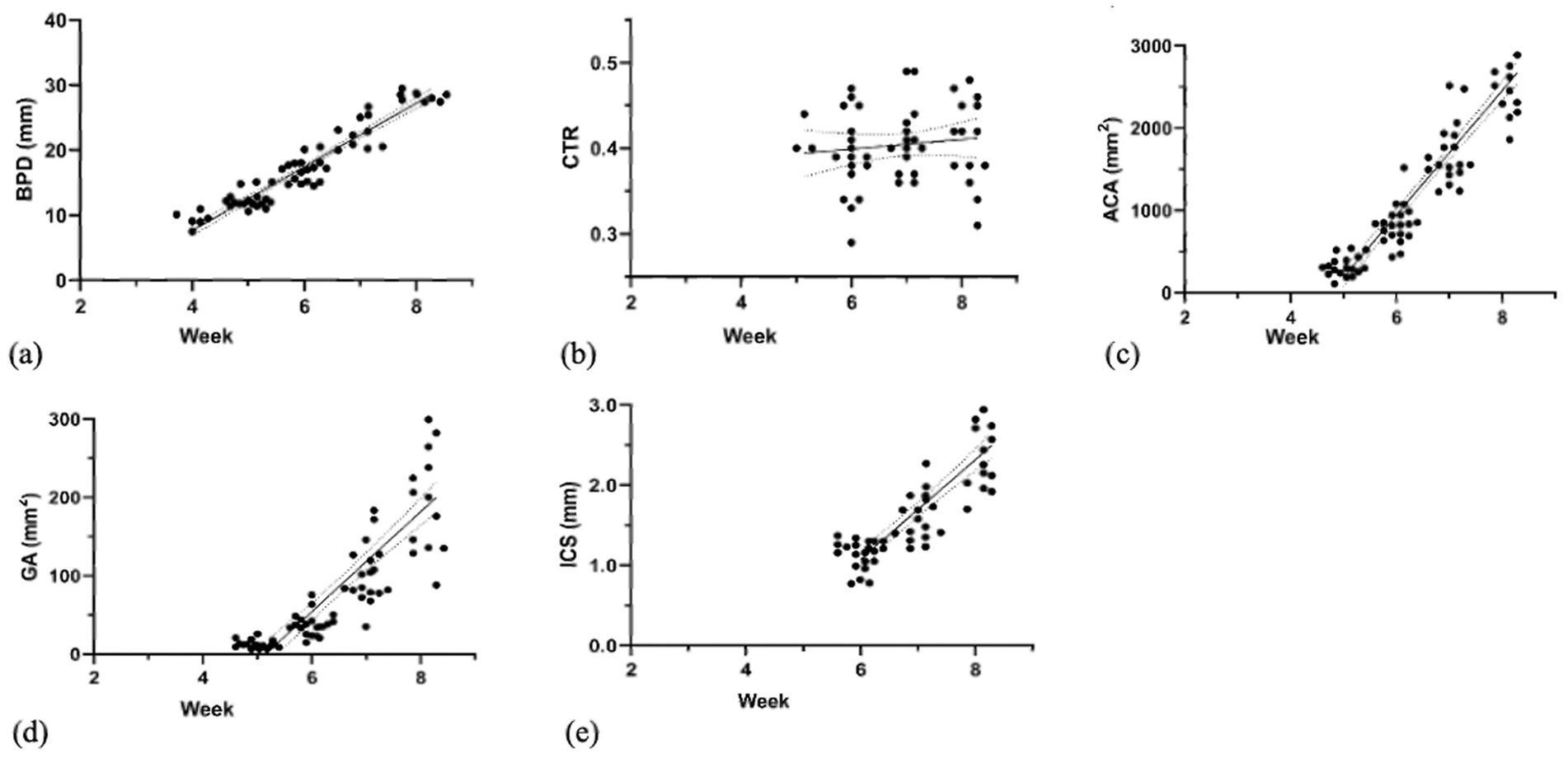
Figure 4. Linear regression graph illustrates the correlation of (a) BPD (weeks 4–8), (b) CTR (weeks 5–8), (c) ACA (weeks 5–8), (d) GA (weeks 5–8), and (e) ICS (weeks 6–8) with gestational weeks.
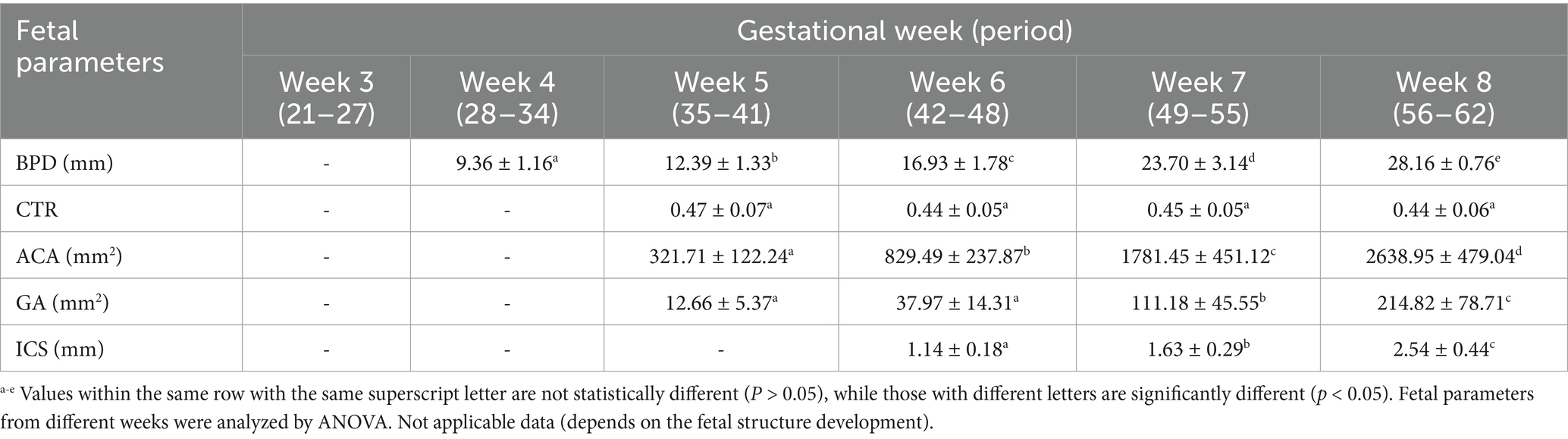
Table 2. Mean values with standard deviation (x̄±SD) of other fetal parameters (BPD, CTR, ACA, GA, and ICS) of gestational weeks.
3.3 Correlation of fetal parameters with day of gestation (DG) and day before parturition (DBP)
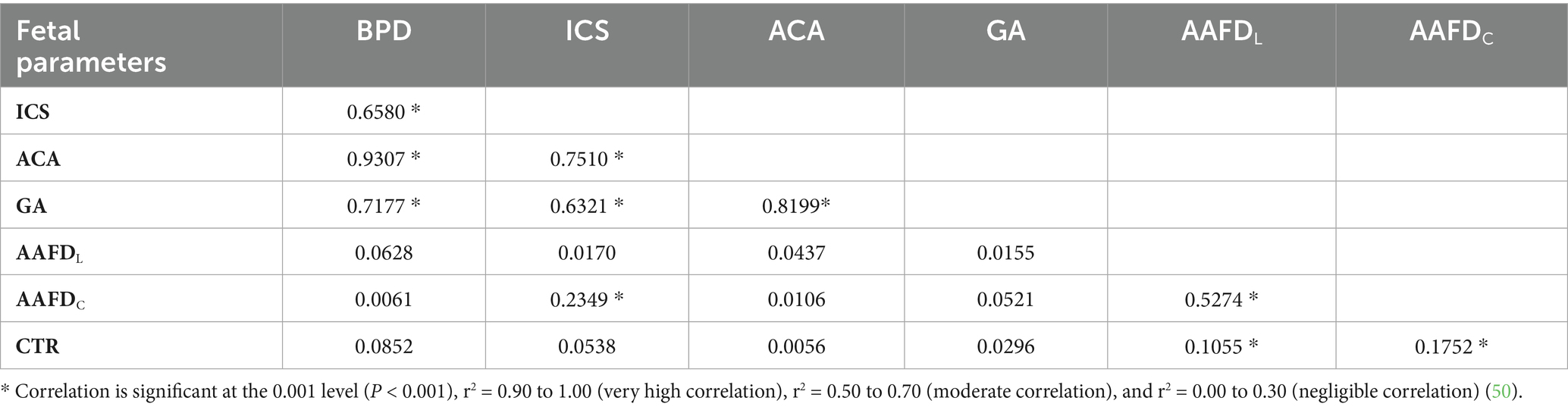
This study investigated the relationship between fetal parameters (BPD, ACA, ICS, GA, AAFDC, and AAFDL) and both the day of gestation (DG) and the day before parturition (DBP) (Table 3). Our analysis revealed that BPD, ACA, ICS, and GA were highly significantly correlated (p < 0.0001) with DG and DBP. In contrast, AAFDC and AAFDL showed a moderately significant correlation, and CTR had a negligibly significant correlation with DG and DBP. Linear model equations were then generated for all significantly correlated parameters (Table 3).
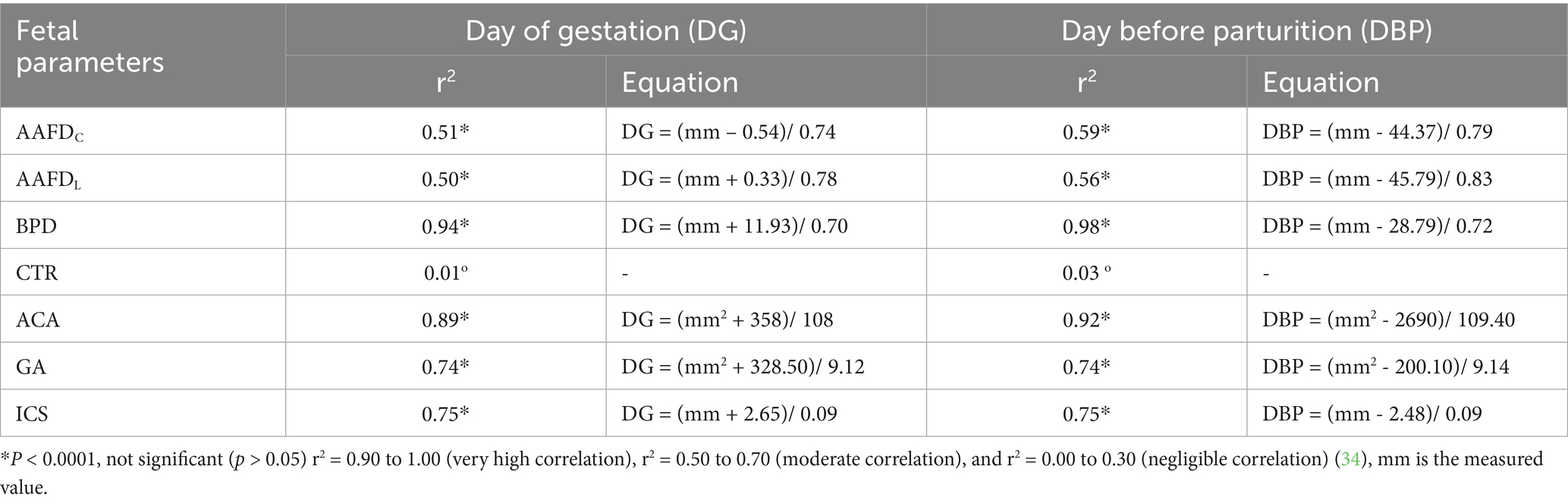
Table 3. Coefficient of determination (r2) between each fetal parameter and the day of gestation (DG) and the day before parturition (DBP) with a linear regression equation.
3.4 Interrelationships among fetal parameters
This study investigated the correlations between various fetal parameters (BPD, ACA, GA, ICS, AAFDC, and AAFDL) throughout fetal development (Table 4). The relationship between BPD and ACA demonstrated the strongest correlation (r2 = 0.93, p < 0.001), indicating a close relationship in their developmental changes, whereas AAFDC showed a negligible correlation with ICS and CTR (r2 = 0.23 and 0.18, respectively, p < 0.001), indicating the effect of thoracic development.
4 Discussion
This study aimed to determine the development of AAFV and other fetal parameters in dogs by non-invasive techniques using ultrasonography, and we hypothesized that these parameters would be positively correlated during the pregnancy term, and that a strong interrelationship would exist among them, reflecting a synchronized and predictable growth pattern of fetuses. This study revealed that AAFDC (max = 34.60 ± 7.89 mm) and AAFDL (max = 33.51 ± 9.59 mm) increased during pregnancy, reached their maximum depth at week 6, and then decreased at the end of pregnancy (p < 0.05). Consistent with a previous study in dogs that measured actual volume, demonstrating a fetal fluid volume of approximately 122.28 mL (36). Like a previous study on canine and feline amniotic fluid and allantoic fluid, which found that actual amniotic volume peaked at mid-gestation before declining (36). Our study’s finding of a decrease in fetal fluid after mid-gestation, specifically AAFDC and AAFDL, is consistent with previous studies in both canine and feline species (36). This decrease is attributed to the allantoic fluid’s relationship with kidney function, urachus duct occlusion, and urethra development. Conversely, AFV increased consistently throughout gestation, although its volume never surpassed that of allantoic fluid in late pregnancy (36, 37). However, this study also presented AAFDC and AAFDL, which showed a moderate correlation throughout gestation (weeks 3 to 8, p < 0.0001).
The fluid indicated the definitive kidney, which fully developed from the metanephros in the late term of canine pregnancy (37). Moreover, the volume of human amniotic fluid at any gestational stage reflects the dynamic water balance between fetus and mother. Imbalances of these regulations result in polyhydramnios or oligohydramnios, often due to abnormal fetal or maternal disorders (38, 39). Further investigation is therefore needed to explore the practical application of this finding to perinatal pregnancy evaluation in veterinary practice, as this correlation has not yet been reported. Future research should specifically focus on monitoring intestinal and renal development to provide a more comprehensive understanding of AAFV development.
The changes in fetal parameters throughout gestation reflect continuous growth and development (p < 0.0001). Specifically, BPD indicates head growth, while ACA and GA demonstrate abdominal and internal organ expansion. ICS and CTR signify thoracic cavity enlargement, correlating with the development of intrathoracic organs (1, 40, 41). Moreover, this study reveals divergent trends for fetal BPD, ACA, GA, and ICS, which reflect fetal head, abdominal, and thoracic growth and organ dimensions. This suggests a lack of a correlation between the growth of these structures and AAFDC and AAFDL. This is further supported by the negligible correlation observed in our statistical analysis, especially concerning thoracic cavity enlargement (p < 0.001). Our results are consistent with human research showing no direct relationship between fetal fluid volume and embryonic or organ size (38, 42, 43). However, the volume of amniotic fluid can be used for assessment of lung, gastrointestinal, and urinary maturation and maldevelopment in humans (44–49) and cats (37).
5 Conclusion
This study is the first to report the development patterns of amniotic fluid volume in dogs, using a non-invasive ultrasonographic approach. It also elucidates the relationship between amniotic fluid parameters (AAFDC and AAFDL) and other relevant factors. These findings propose the establishment of AAFDC and AAFDL as novel diagnostic markers for evaluating canine fetal well-being in clinical practice. It is crucial to note that these markers are intended to be complementary tools that provide additional and supportive data to enhance the overall accuracy of perinatal evaluation.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal studies were approved by the Ethics Committee of the Prince of Songkla University Animal Care and Use Protocol (Protocol Code 2563–05-041, Ref.65/2020) for studies involving animals on 8 September 2020. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
PT: Visualization, Resources, Data curation, Methodology, Investigation, Writing – original draft. NT: Funding acquisition, Writing – review & editing, Conceptualization, Project administration, Supervision, Data curation. TI: Methodology, Conceptualization, Writing – review & editing. AD: Validation, Writing – review & editing. TA: Investigation, Formal analysis, Writing – original draft, Methodology. AU: Methodology, Writing – original draft, Investigation. PJ: Methodology, Investigation, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was financially supported by the Faculty of Veterinary Science, Prince of Songkla University.
Acknowledgments
The authors thank the staff of the Veterinary Teaching Hospital, Faculty of Veterinary Science, Prince of Songkla University, Songkhla, Thailand, and the Southland Exotic Bully Kennel for their help and support throughout the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siena, G, and Milani, C. Usefulness of maternal and fetal parameters for the prediction of parturition date in dogs. Animals. (2021) 11:878. doi: 10.3390/ani11030878
2. Milani, C, Artusi, E, Drigo, M, Mateus, L, Siena, G, Gelli, D, et al. Ultrasonographic analysis of fetal gastrointestinal motility during the peripartum period in the dog. Anim Reprod Sci. (2020) 219:106514. doi: 10.1016/j.anireprosci.2020.106514
3. Banzato, T, Zovi, G, and Milani, C. Estimation of fetal lung development using quantitative analysis of ultrasonographic images in normal canine pregnancy. Theriogenology. (2017) 96:158–63. doi: 10.1016/j.theriogenology.2017.03.011
4. Gil, EMU, Garcia, DAA, and Froes, TR. In utero development of the fetal intestine: sonographic evaluation and correlation with gestational age and fetal maturity in dogs. Theriogenology. (2015) 84:681–6. doi: 10.1016/j.theriogenology.2015.04.030
5. Concannon, PW. Reproductive cycles of the domestic bitch. Anim Reprod Sci. (2011) 124:200–10. doi: 10.1016/j.anireprosci.2010.08.028
6. Johnson, C. Pregnancy management in the bitch. Theriogenology. (2008) 70:1412–7. doi: 10.1016/j.theriogenology.2008.09.009
7. Kim, Y, Travis, AJ, and Meyers-Wallen, VN. Parturition prediction and timing of canine pregnancy. Theriogenology. (2007) 68:1177–82. doi: 10.1016/j.theriogenology.2007.08.018
8. Yeager, AE, Mohammed, HO, Meyers-Wallen, V, Vannerson, L, and Concannon, PW. Ultrasonographic appearance of the uterus, placenta, fetus, and fetal membranes throughout accurately timed pregnancy in beagles. Am J Vet Res. (1992) 53:342–51. doi: 10.2460/ajvr.1992.53.3.342
9. Kim, B-S, and Son, C-H. Time of initial detection of fetal and extra-fetal structures by ultrasonographic examination in miniature schnauzer bitches. J Vet Sci. (2007) 8:289–93. doi: 10.4142/jvs.2007.8.3.289
10. Beccaglia, M, Alonge, S, Trovo’, C, and Luvoni, G. Determination of gestational time and prediction of parturition in dogs and cats: an update. Reprod Domest Anim. (2016) 51:12–7. doi: 10.1111/rda.12782
11. Luvoni, G, and Beccaglia, M. The prediction of parturition date in canine pregnancy. Reprod Domest Anim. (2006) 41:27–32. doi: 10.1111/j.1439-0531.2006.00641.x
12. Luvoni, G, and Grioni, A. Determination of gestational age in medium and small size bitches using ultrasonographic fetal measurements. J Small Anim Pract. (2000) 41:292–4. doi: 10.1111/j.1748-5827.2000.tb03204.x
13. England, G, Allen, WE, and Porter, D. Studies on canine pregnancy using B-mode ultrasound: development of the conceptus and determination of gestational age. J Small Anim Pract. (1990) 31:324–9. doi: 10.1111/j.1748-5827.1990.tb00821.x
14. Beccaglia, M, Faustini, M, and Luvoni, G. Ultrasonographic study of deep portion of diencephalo-telencephalic vesicle for the determination of gestational age of the canine foetus. Reprod Domest Anim. (2008) 43:367–70. doi: 10.1111/j.1439-0531.2007.00916.x
15. Gil, EMU, Garcia, DAA, Giannico, AT, and Froes, TR. Early results on canine fetal kidney development: Ultrasonographic evaluation and value in prediction of delivery time. Theriogenology. (2018) 107:180–7. doi: 10.1016/j.theriogenology.2017.11.015
16. Shamsnajafabadi, H, and Soheili, ZS. Amniotic fluid characteristics and its application in stem cell therapy: a review. Int J Reprod Biomed. (2022) 20:627–43. doi: 10.18502/ijrm.v20i8.11752
17. Zaretsky, MV, McIntire, DD, Reichel, TF, and Twickler, DM. Correlation of measured amnionic fluid volume to sonographic and magnetic resonance predictions. Am J Obstet Gynecol. (2004) 191:2148–53. doi: 10.1016/j.ajog.2004.04.044
18. Dubil, EA, and Magann, EF. Amniotic fluid as a vital sign for fetal wellbeing. Austr J Ultrasound Med. (2013) 16:62–70. doi: 10.1002/j.2205-0140.2013.tb00167.x
19. Leibovitch, L, Kuint, J, Rosenfeld, E, Schushan-Eisen, I, Weissmann-Brenner, A, and Maayan-Metzger, A. Short-term outcome among term singleton infants with intrapartum oligohydramnios. Acta Paediatr. (2012) 101:727–30. doi: 10.1111/j.1651-2227.2012.02667.x
20. Bucca, S, Fogarty, U, Collins, A, and Small, V. Assessment of feto-placental well-being in the mare from mid-gestation to term: transrectal and transabdominal ultrasonographic features. Theriogenology. (2005) 64:542–57. doi: 10.1016/j.theriogenology.2005.05.011
21. Brace, RA, and Wolf, EJ. Normal amniotic fluid volume changes throughout pregnancy. Am J Obstet Gynecol. (1989) 161:382–8. doi: 10.1016/0002-9378(89)90527-9
22. Riva, F, Filipe, J, Pavlovic, R, Luciano, AM, Dall’Ara, P, Arioli, F, et al. Canine amniotic fluid at birth: from a discarded sample to a potential diagnostic of neonatal maturity. Anim Reprod Sci. (2023) 248:107184. doi: 10.1016/j.anireprosci.2022.107184
23. Leung-Pineda, V, and Gronowski, AM. Biomarker tests for fetal lung maturity. Biomark Med. (2010) 4:849–57. doi: 10.2217/bmm.10.109
24. Zaremba, W, Grunert, E, and Aurich, JE. Prophylaxis of respiratory distress syndrome in premature calves by administration of dexamethasone or a prostaglandin F2 alpha analogue to their dams before parturition. Am J Vet Res. (1997) 58:404–7. doi: 10.2460/ajvr.1997.58.04.404
25. Castagnetti, C, Mariella, J, Serrazanetti, GP, Grandis, A, Merlo, B, Fabbri, M, et al. Evaluation of lung maturity by amniotic fluid analysis in equine neonate. Theriogenology. (2007) 67:1455–62. doi: 10.1016/j.theriogenology.2007.02.013
26. Groppetti, D, Martino, PA, Ravasio, G, Bronzo, V, and Pecile, A. Prognostic potential of amniotic fluid analysis at birth on canine neonatal outcomes. Vet J. (2015) 206:423–5. doi: 10.1016/j.tvjl.2015.08.026
27. Plavec, T, Knific, T, Slapšak, A, Raspor, S, Lukanc, B, and Pipan, MZ. Canine neonatal assessment by vitality score, amniotic fluid, urine, and umbilical cord blood analysis of glucose, lactate, and cortisol: possible influence of parturition type? Animals. (2022) 12:247. doi: 10.3390/ani12101247
28. Phelan, JP, Ahn, MO, Smith, CV, Rutherford, SE, and Anderson, E. Amniotic fluid index measurements during pregnancy. J Reprod Med. (1987) 32:601–4.
29. Nabhan, AF, and Abdelmoula, YA. Amniotic fluid index versus single deepest vertical pocket as a screening test for preventing adverse pregnancy outcome. Cochrane Database Syst Rev. (2008) 2008:593. doi: 10.1002/14651858.CD006593.pub2
30. Johnston, SD, Root Kustritz, MV, and Olson, PS. Canine and feline theriogenology. 1st ed. Philadelphia: Saunders (2001).
31. Payan Carreira, R., Miranda, S., and Niżański, W. Artificial insemination in dogs. London, UK: IntechOpen Limited (2011).
32. Johnston, S., and Root, M. Serum progesterone timing of ovulation in the bitch. In Proceedings of the Proceedings of the Annual Meeting of the Society of Theriogenology: 29–30 September 1995; Nashville, Tennessee/USA, (1995); pp. 195–203.
33. Nöthling, JO, Joonè, CJ, Hegarty, E, Schooley, EK, and De Cramer, KGM. Use of a point-of-care progesterone assay to predict onset of parturition in the bitch. Front Vet Sci. (2022) 9:914659. doi: 10.3389/fvets.2022.914659
34. Cho, HC, Sun, S, Min Hyun, C, Kwon, J-Y, Kim, B, Park, Y, et al. Automated ultrasound assessment of amniotic fluid index using deep learning. Med Image Anal. (2021) 69:101951. doi: 10.1016/j.media.2020.101951
35. Magann, EF, Whittington, JR, Morrison, JC, and Chauhan, SP. Amniotic fluid volume assessment: eight lessons learned. Int J Women's Health. (2021) 13:773–9. doi: 10.2147/IJWH.S316841
36. Sahraei, H, Mogheiseh, A, Nazifi, S, Divar, MR, and Iraji, F. Canine and feline foetal fluids: volume, hormonal and biochemical characterization during pregnancy. Vet Med Sci. (2024) 10:e1452. doi: 10.1002/vms3.1452
37. Bigliardi, E, Rizzi, M, Bertocchi, M, Denti, L, Bresciani, C, Vetere, A, et al. Evaluation of biochemical composition of amniotic and Allantoic fluids at different stages of pregnancy in Queens. Animals. (2022) 12:414. doi: 10.3390/ani12111414
38. Bakhsh, H, Alenizy, H, Alenazi, S, Alnasser, S, Alanazi, N, Alsowinea, M, et al. Amniotic fluid disorders and the effects on prenatal outcome: a retrospective cohort study. BMC Pregnancy Childbirth. (2021) 21:75. doi: 10.1186/s12884-021-03549-3
39. Beall, MH, van den Wijngaard, JPHM, van Gemert, MJC, and Ross, MG. Regulation of amniotic fluid volume. Placenta. (2007) 28:824–32. doi: 10.1016/j.placenta.2006.12.004
40. Pieri, N, Souza, AF, Casals, JB, Roballo, K, Ambrósio, CE, and Martins, DS. Comparative development of embryonic age by organogenesis in domestic dogs and cats. Reprod Domest Anim. (2015) 50:625–31. doi: 10.1111/rda.12539
41. Kutzler, MA, Yeager, AE, Mohammed, HO, and Meyers-Wallen, VN. Accuracy of canine parturition date prediction using fetal measurements obtained by ultrasonography. Theriogenology. (2003) 60:1309–17. doi: 10.1016/s0093-691x(03)00146-8
42. Wadnere, N, Kosta, S, and Kumar, R. Association between fetal weight and amniotic fluid index in women of Central India. Adv Biomed Res. (2014) 3:243. doi: 10.4103/2277-9175.145751
43. Jha, P, Raghu, P, Kennedy, AM, Sugi, M, Morgan, TA, Feldstein, V, et al. Assessment of amniotic fluid volume in pregnancy. Radiographics. (2023) 43:e220146. doi: 10.1148/rg.220146
44. Hooper, SB, and Harding, R. Fetal lung liquid: a major determinant of the growth and FUNCTIONAL development of the fetal lung. Clin Exp Pharmacol Physiol. (1995) 22:235–41. doi: 10.1111/j.1440-1681.1995.tb01988.x
45. Adams, FH. Functional development of the fetal lung. J Pediatr. (1966) 68:794–801. doi: 10.1016/S0022-3476(66)80456-0
46. Wu, C-S, Chen, C-M, and Chou, H-C. Pulmonary hypoplasia induced by oligohydramnios: findings from animal models and a population-based study. Pediatr Neonatol. (2017) 58:3–7. doi: 10.1016/j.pedneo.2016.04.001
47. Liu, D, Jiang, Q, Xu, Z, Li, L, and Lyu, G. Evaluating fetal lung development at various gestational weeks using two-dimensional shear wave elastography. Quant Imaging Med Surg. (2024) 14:5373–84. doi: 10.21037/qims-24-272
48. Dasgupta, S, Arya, S, Choudhary, S, and Jain, SK. Amniotic fluid: source of trophic factors for the developing intestine. World J Gastrointest Pathophysiol. (2016) 7:38–47. doi: 10.4291/wjgp.v7.i1.38
49. Rosenblum, S, Pal, A, and Reidy, K. Renal development in the fetus and premature infant. Semin Fetal Neonatal Med. (2017) 22:58–66. doi: 10.1016/j.siny.2017.01.001
50. Mukaka, MM. Statistics corner: a guide to appropriate use of correlation coefficient in medical research. Malawi Med J. (2012) 24:69–71.
Glossary
LH - Luteinizing hormone
ICC - Inner chorionic cavity
OUD - Outer uterine diameter
CRL - Crown rump length
BPD - Biparietal diameter
DPTV - Deep portion of diencephalo-telencephalic vesicle
AF - Amniotic fluid
AFV - Amniotic fluid volume
AAF - Allantoamniotic fluid
AAFV - Allantoamniotic fluid volume
AAFDC - Allantoamniotic fluid depth by cross-sectional view
AAFDL - Allantoamniotic fluid depth by longitudinal view
SDVP - Single deepest vertical pocket
AI - Artificial insemination
SDP - Single deepest pocket
DVP - Deepest vertical pocket
CTR - Cardio-thoracic ratio
ACA - Abdominal cross-sectional area
GA - Gastric area
ICS - Intercostal space
DG - Day of gestation
DBP - Day before parturition
Wk - Week
Keywords: dog, fetal parameter, ultrasonography, allantoamniotic fluid depth, pregnancy
Citation: Tantitaveewattana P, Tiptanavattana N, Ingkasri T, Dermlim A, Ampornpong T, Uppathamchat A and Jirayuwattanakun P (2025) Correlations between canine allantoamniotic fluid and fetal development throughout the pregnancy term. Front. Vet. Sci. 12:1686014. doi: 10.3389/fvets.2025.1686014
Edited by:
Regiane R. Santos, Schothorst Feed Research, NetherlandsReviewed by:
Gleice Mendes Xavier, Universidade Estadual Paulista Julio de Mesquita Filho, BrazilLubica Hornakova, University of Veterinary Medicine and Pharmacy in Košice, Slovakia
Copyright © 2025 Tantitaveewattana, Tiptanavattana, Ingkasri, Dermlim, Ampornpong, Uppathamchat and Jirayuwattanakun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Narong Tiptanavattana, bmFyb25nLnRpQHBzdS5hYy50aA==
 Phakawat Tantitaveewattana
Phakawat Tantitaveewattana Narong Tiptanavattana
Narong Tiptanavattana Thitsana Ingkasri1
Thitsana Ingkasri1 Angkhana Dermlim
Angkhana Dermlim