- Department of Animal and Food Sciences, University of Kentucky, Lexington, KY, United States
Globally, heat stress (HS) is a major concern in poultry farming, adversely impacting bird productivity, health, welfare, and economic returns. As climate change intensifies, the occurrence and severity of HS are anticipated to rise, posing greater risks to the poultry industry and the increasing demand for food. Birds respond to HS by exhibiting different mechanisms, including behavioral and physiological changes, to regulate their body temperature. In poultry, HS has been associated with reduced feed consumption, growth, feed efficiency, quantity and quality of eggs produced, meat quality, reproductive performance, impaired gut health, and increased mortality. Also, HS induces acid–base imbalance, causing both respiratory alkalosis and metabolic acidosis. During HS, birds pant to cool down and exhale excessive carbon dioxide, leading to a decrease in blood pH. Nutritional interventions have emerged as a viable strategy to mitigate HS effects, with various dietary supplements demonstrating efficacy in improving poultry resilience. Vitamins (A, C, D, and E), minerals (selenium, zinc, chromium, sodium, potassium, and chloride), fat, amino acids, electrolytes, and in ovo feeding have been revealed to boost thermotolerance, support growth, and improve feed efficiency of birds under HS conditions. This review integrates current literature on the impact of HS on poultry production and examines how nutritional supplements can help alleviate the effects of this environmental stressor in the avian species.
1 Introduction
1.1 Significance of global poultry production
The contribution of poultry to the global economic and food system cannot be overstated. Poultry meat and eggs are widely consumed globally, providing a readily available and affordable source of high-quality protein, essential vitamins (A, B2, and B12), and important minerals (calcium, zinc, and iron), thereby proving to be essential for healthy human nutrition (1). Also, poultry products (meat and eggs) have no cultural or religious taboos (2), which contribute to their widespread global acceptance and consumption (3). Globally, 144.02 million tons of meat is generated from poultry (Figure 1), accounting for over one-third of total meat produced (4). In 2024, the United States generated $70.2 billion from the sales of poultry products, including eggs from laying hens and meat from broilers and turkey breeders (5). Also, the generally low cost of poultry products makes them an accessible source of nutrition for individuals in both developing and developed countries, ultimately supporting food security and improving livelihoods (6). This highlights the vital role of poultry products in the global economy, supporting food security, rural development, and poverty alleviation, especially in developing nations (7).
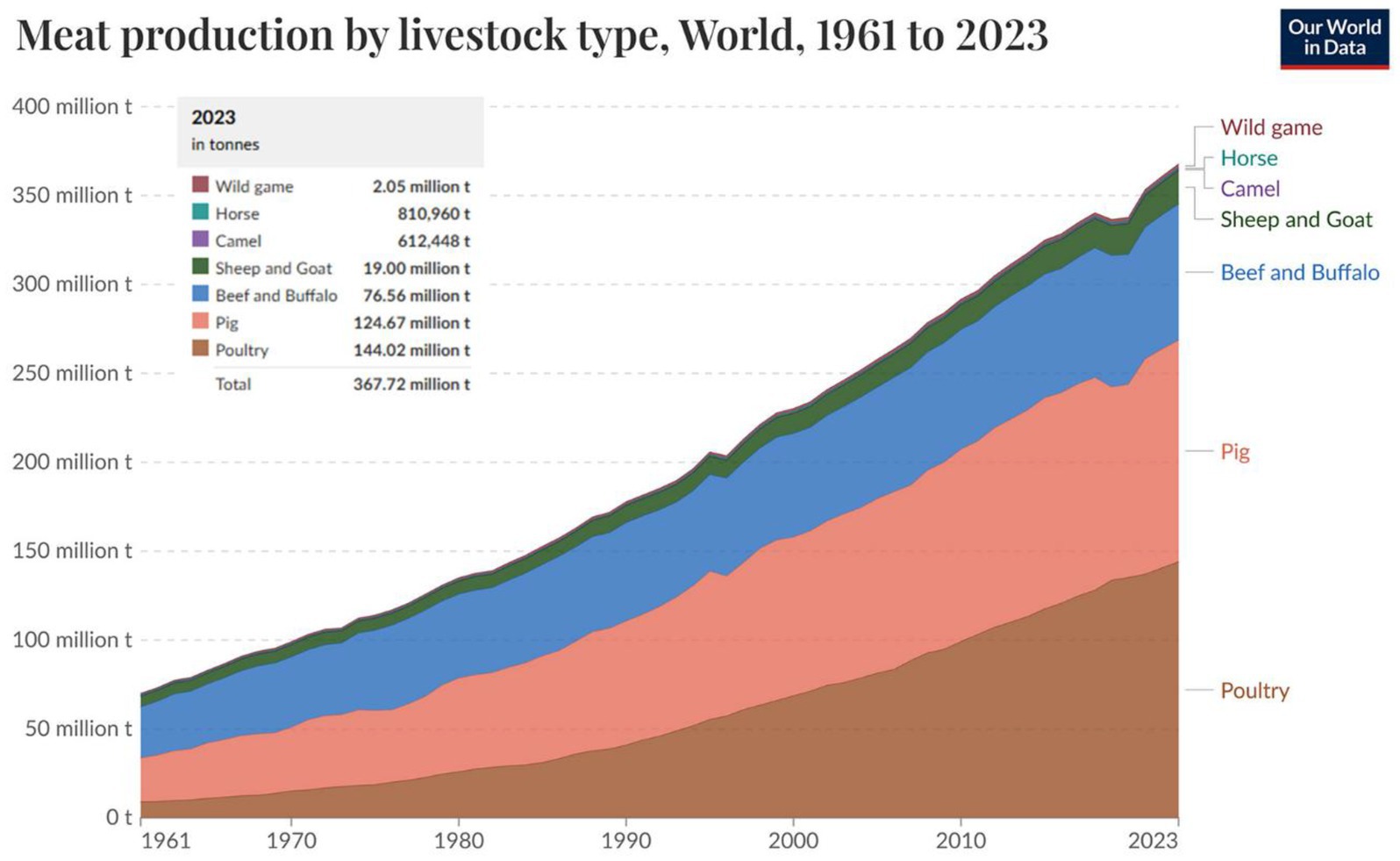
Figure 1. Livestock meat production from commercial and farm slaughter based on dressed carcass weights and slaughter fats. Source: Adapted from FAO (4).
In recent years, the demand for more animal-derived protein has risen due to population growth, increasing income, and urbanization (8), leading to a rapid increase in global poultry production (7). The increase in the poultry industry’s growth is favored by its products’ affordability and short generational interval (9). Likewise, research advancements in genetics, nutrition, disease control, and housing management have contributed to this growth (6). For example, compared to other livestock, broilers have evolved to have a relatively short generational interval and high feed efficiency, making them a highly efficient source of affordable protein for the expanding global population (10). Also, laying hens have been genetically selected and improved over time for longer production cycles, thereby increasing their contribution to the supply of animal protein. Economically, the poultry industry supports millions of livelihoods (11), particularly in developing countries, where small-scale and backyard farming provide both food and income. From an ecological perspective, poultry farming generates a lower environmental footprint than other livestock, like cattle and swine (12).
1.2 Stress
Poultry is one of the fastest-growing sources of animal protein (7). Nevertheless, poultry production faces numerous challenges, including stress, high feed cost, and disease outbreaks, which threaten the performance, efficiency, sustainability, and profitability of poultry production. Selye (13) articulated the concept of stress as “the nonspecific response of the body to any demand,” while a stressor was considered as “an agent that induces stress at any given time.” Birds are vulnerable to various stressors, including environmental stressors (heat and cold stress, light, air quality, and humidity), management stressors (litter quality, stocking density, poor ventilation, beak trimming, vaccination, transportation, and handling), nutritional stressors (nutrient deficiencies, feed contamination, and mycotoxins), and biological or internal stressors such as diseases and gut microbiota imbalances (14). Poultry exposed to stress experience a disruption in their normal physiological homeostasis.
Physiologically, stress occurs when there is a shift away from ideal internal and external conditions. During stress, the hypothalamic–pituitary–adrenal (HPA) axis, the immune system, and the autonomic nervous system work together to restore homeostatic balance. Exposure to stress induces a series of regulatory mechanisms in the body, causing metabolic adjustments such as heightened energy mobilization and metabolic shifts that impair poultry performance (15). Modern poultry management practices, including optimized nutrition and environmental control, have greatly reduced the incidence of stress-induced nutritional diseases like encephalomalacia and muscular dystrophy (16). Nevertheless, stress continues to adversely affect poultry productivity and reproduction, leading to considerable economic losses.
This review identified heat stress (HS) as an environmental stressor, focusing on its impacts on poultry performance, productivity, and health. It also discusses the efficacy of nutritional supplements like vitamins, minerals, and amino acids to alleviate HS and support gut health in poultry. By linking these dietary strategies to improved gut function, resilience, and productivity, the review provides practical insights for optimizing poultry health and performance while identifying areas for future research.
2 Thermal stress in poultry
Similar to other warm-blooded animals, poultry are homeothermic and maintain a relatively constant body temperature through a complex thermoregulatory system that balances heat production and dissipation. This balance is most efficient within a thermoneutral zone of 21°C to 28°C (17), where birds are comfortable and can balance the amount of heat generated and lost by the body (Figure 2). However, the internal temperature of poultry species can vary with size, breed, and sex, making them exhibit different responses to temperature outside the thermoneutral zone. When environmental conditions (temperature and humidity) exceed the birds’ thermal comfort zone, their capacity to regulate body temperature is compromised, resulting in HS. Different variants of birds show varying levels of resistance to HS, with the fast-growing breeds exhibiting significantly reduced resistance (18). Moreover, the susceptibility of poultry, especially broilers, to HS is compounded by their inherent physiological characteristics, including a high metabolic rate that generates significant internal heat and the absence of sweat glands to effectively disperse heat through sweating, which is a primary mechanism utilized by other species like horses, donkeys, and camels (17, 19).
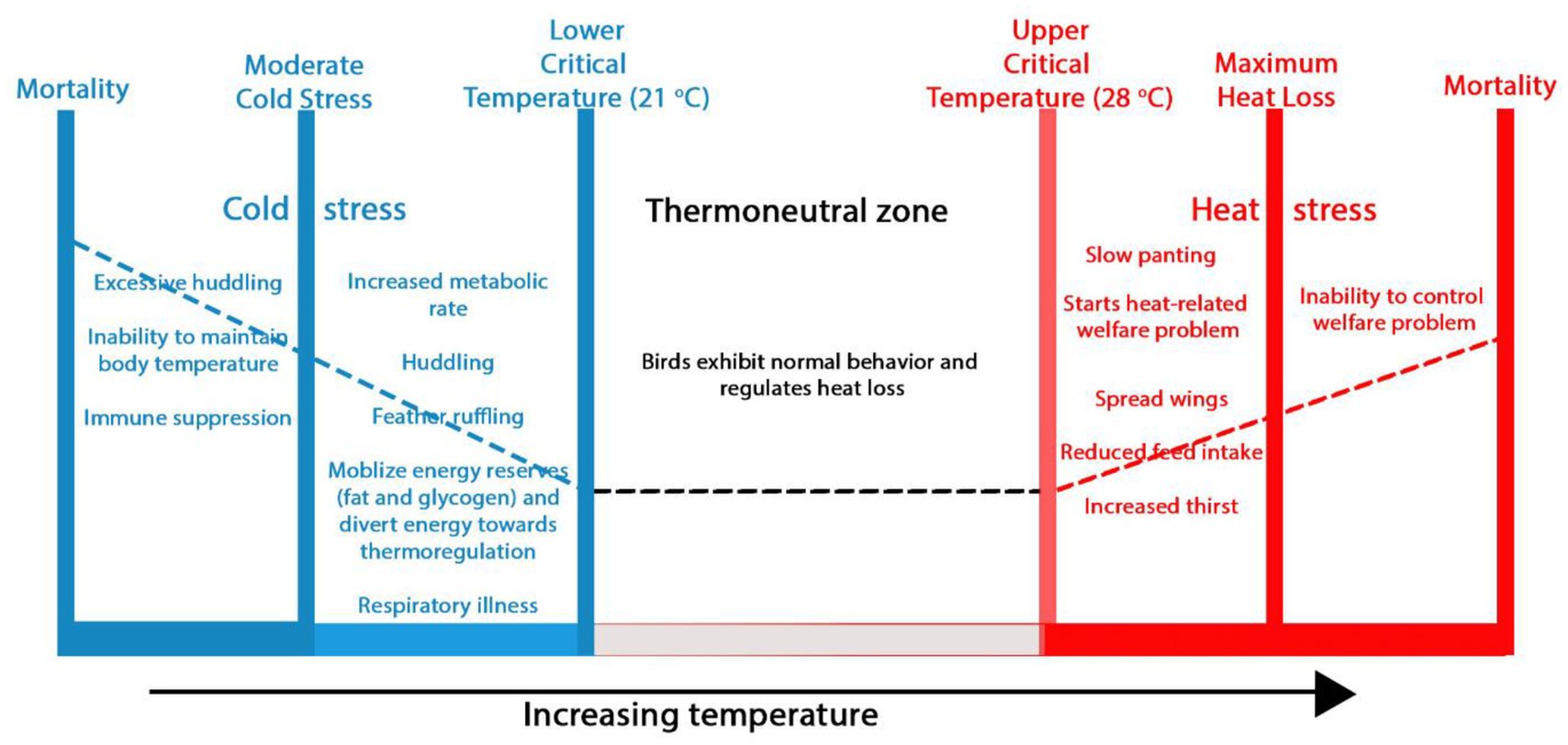
Figure 2. Impact of ambient temperature on poultry behavior and welfare. Within the thermoneutral zone, birds maintain a stable body temperature through physical heat regulation and display normal behaviors. Beyond the lower and upper critical zones, chickens experience cold and heat stress, respectively, leading to welfare issues and even death.
2.1 Mechanism of heat stress
Poultry respond differently to HS, depending on the heat intensity and duration of exposure. The neuroendocrine system is pivotal in sustaining optimal physiological functions in living organisms. Elevated ambient temperatures impact the neuroendocrine system by activating the sympathetic-adrenal medullary (SAM) and the HPA axes (Figure 3) (20), which are the primary pathways for modifying the immune response (21). This activation promotes increased glucose synthesis, which is vital for the survival of chickens under stressful conditions (22). The SAM regulates the fight against cell invasion by detecting stimuli and transferring information from the hypothalamus to the adrenal gland (23). In stressful situations, the SAM secretes catecholamines, like norepinephrine and epinephrine (adrenaline), triggering a rapid response characterized by an increased heart rate and glucose production (24).
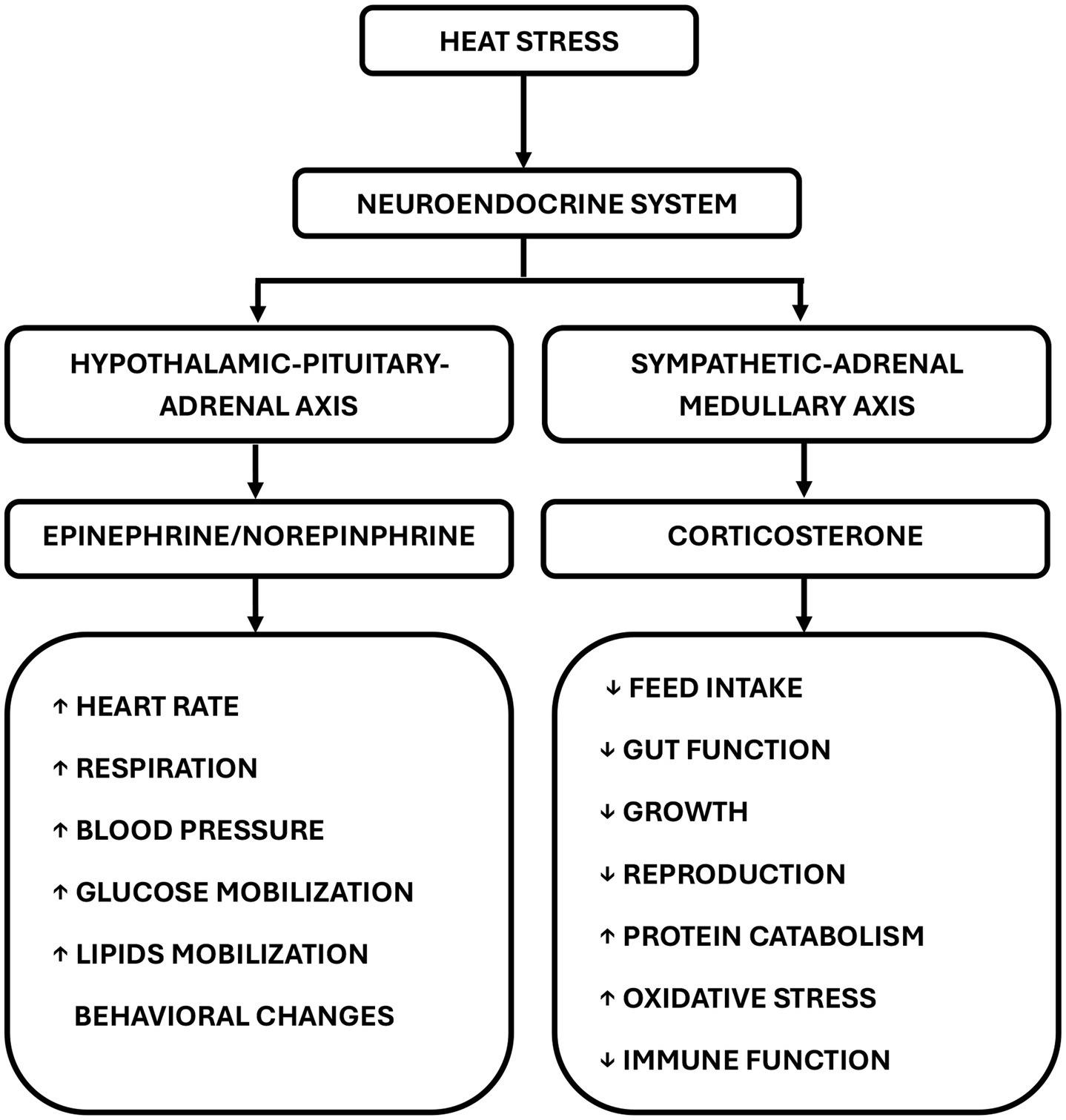
Figure 3. Mechanism of heat stress. Heat stress stimulates the hypothalamic–pituitary–adrenal and sympathetic-adrenal medullary axis to generate several physiological effects. The secretion of epinephrine or norepinephrine increases respiration, heart rate, blood pressure, glycogenolysis, and lipolysis. The release of corticosterone compromises the immune system, reduces feed intake, and impairs gut function.
The presence of primary glucocorticoids (cortisol and corticosterone) varies among species, with corticosterone predominantly found in avians and rodents, while cortisol is more commonly observed in ruminants, swine, and fish (25). Corticosterone is released from the HPA axis and the pituitary gland through the action of adrenocorticotropic hormone (ACTH) (26). Unlike adrenaline, corticosterone is secreted more slowly, leading to sustained physiological effects (25), making it a dependable stress indicator (21). In chickens, persistent corticosterone secretion is associated with cardiovascular diseases, a compromised immune system, depression, and muscle breakdown due to gluconeogenesis and reduced cognition (26).
2.2 Poultry responses to heat stress
2.2.1 Regulation of body temperature
Under HS, birds regulate their body temperature through the hypothalamus because they lack sweat glands. At first, birds disperse heat to the environment through conduction (transfer of body heat to cooler surfaces in direct contact), convection (loss of heat to surrounding air as warmer air near the body surface rises and is replaced by cooler air), and radiation, which is the emission of heat in the form of infrared energy from the body surface to cooler objects in the environment (17, 27). This thermoregulation system functions optimally within the thermoneutral range, and the size of the comb and wattle significantly contributes to this mechanism. These structures provide the body with ample bare skin to facilitate blood circulation, leading to the dissipation of heat from the body to the head and losing this heat more efficiently. Approximately 40% of the heat that the bird seeks to dissipate is lost through these mechanisms (17). As environmental temperatures hit the upper critical threshold (>26–29°C), birds change their heat loss mechanism to evaporative processes (28). The evaporative mechanism involves thermal exhaustion and intensified evaporative cooling, resulting from increased blood flow to the skin, elevated heart rate, and dilation of peripheral blood vessels. This mechanism is facilitated by the air sacs, lungs, and mucous area between the nasal openings and the tracheal base through increased gaseous exchange and air circulation on surfaces (17, 21, 28).
2.2.2 Behavioral responses
As temperature rises further, birds exhibits various behavioral strategies to regulate their body temperature and mitigate the effects of HS. Under HS, birds often become exhausted, lose their appetite, spread their wings away from their bodies, pant to increase their respiratory rate and facilitate heat dissipation, and seek cooler environments (17). Heat-stressed birds modify their feeding and activity patterns with dramatic reductions in feed intake (FI) during hot periods (29). This response is a strategy aimed at lowering metabolic heat production, as feed digestion, absorption, and nutrient utilization generate additional heat; therefore, eating less helps limit their internal heat production (30). While beneficial for thermoregulation, reduced FI directly impacts growth and egg production. To compensate for water lost through panting and to maintain hydration, chickens often increase their water intake.
Additionally, heat-stressed birds become notably lethargic, spending more time resting on the ground (to maximize heat transfer to the cooler floor) and less time moving. Activities are minimized to reduce metabolic heat production from physical activities, and blood flow is redirected from internal organs to the skin to facilitate heat dissipation (31). In flocks, birds may spread out to avoid crowding (seeking space for better airflow) or seek cooler microclimates within their environment (17, 31). Behavioral signs of discomfort can include panting, drooping wings, ruffled feathers, and sometimes mild aggression (pecking in laying hens) or restlessness as they seek relief (32). Some of these behaviors consume energy; for instance, panting and wing-flapping require muscular effort, which slightly increases maintenance energy expenditure (33). Consequently, prolonged panting diverts energy from growth or egg production toward combating HS, leading to performance loss.
2.2.3 Physiological responses
The physiological response to HS in birds is mediated by the hypothalamus, pituitary gland, and adrenal gland. High humidity limits evaporative and convective cooling, thereby increasing the heat load on the bird (34). Notably, modern fast-growing broilers are prone to HS (35) due to their high metabolic heat production and relatively underdeveloped cardio-respiratory capacity (36). Fast-growing broilers with higher body weights show more severe hyperthermia and lower heat tolerance than smaller and slow-growing breeds under the same conditions (37). In both broilers and layers, prolonged heat exposure raises the internal body temperature beyond the thermoneutral range, resulting in systemic disturbances such as dehydration (due to panting-related water loss) and electrolyte imbalances.
High temperatures affect poultry’s neuroendocrine system, triggering the HPA axis to release corticotropin-releasing factor (CRF) from the hypothalamus (38). This signals the pituitary gland to release ACTH and stimulates the adrenal cortex to produce corticosteroids, which increase electrolyte and bicarbonate losses (17). Elevated corticosterone is a hallmark of acute HS response in chickens and chronically heat-stressed birds often show persistently high corticosterone levels. This hormonal shift has multiple downstream effects, including induced muscle proteolysis, suppressed protein synthesis, and increased lipogenesis (fat deposition) (33). These catabolic effects partly explain the reduction in lean muscle growth and increased abdominal fat often observed in heat-stressed broilers (33). Elevated plasma concentrations of corticosteroids have been shown to be immunosuppressive (39), modifying glucose production and mineral metabolism, which contributes to the pathogenesis of gastrointestinal lesions, cardiovascular disorders, hypercholesterolemia, and alterations in immune function in heat-stressed birds (40).
In addition to adrenal hormones, thyroid hormones are markedly affected by HS. The homeostasis of triiodothyronine (T3) and thyroxine (T4), is important for regulating body temperature and metabolism (28). Although the impact of high temperature on T4 levels varies, the concentration of T3 levels in the blood reduces under high temperature (41, 42). Literature has reported a reduction in both T3 and T4 concentrations in heat-stressed broilers (43, 44) and laying hens (45). The hypothyroid response is thought to be an adaptive mechanism to lower basal metabolic rate and decrease metabolic heat production during chronic HS (46). Reducing T3 and T4 levels may decrease internal heat production, but it leads to slower growth (47), increased carcass fat from lower lipid metabolism (48), and poorer egg production and eggshell quality in laying hens (49). Decreased thyroid activity can partially explain the growth depression and poorer eggshell calcification observed during HS. Additionally, the thyroid gland is crucial in initiating puberty and regulating reproductive functions in avian species. Therefore, a disruption to thyroid activity due to HS would adversely affect hens’ reproductive performance (42).
3 Effects of heat stress on productivity traits in poultry
3.1 Impact of heat stress on growth and meat quality
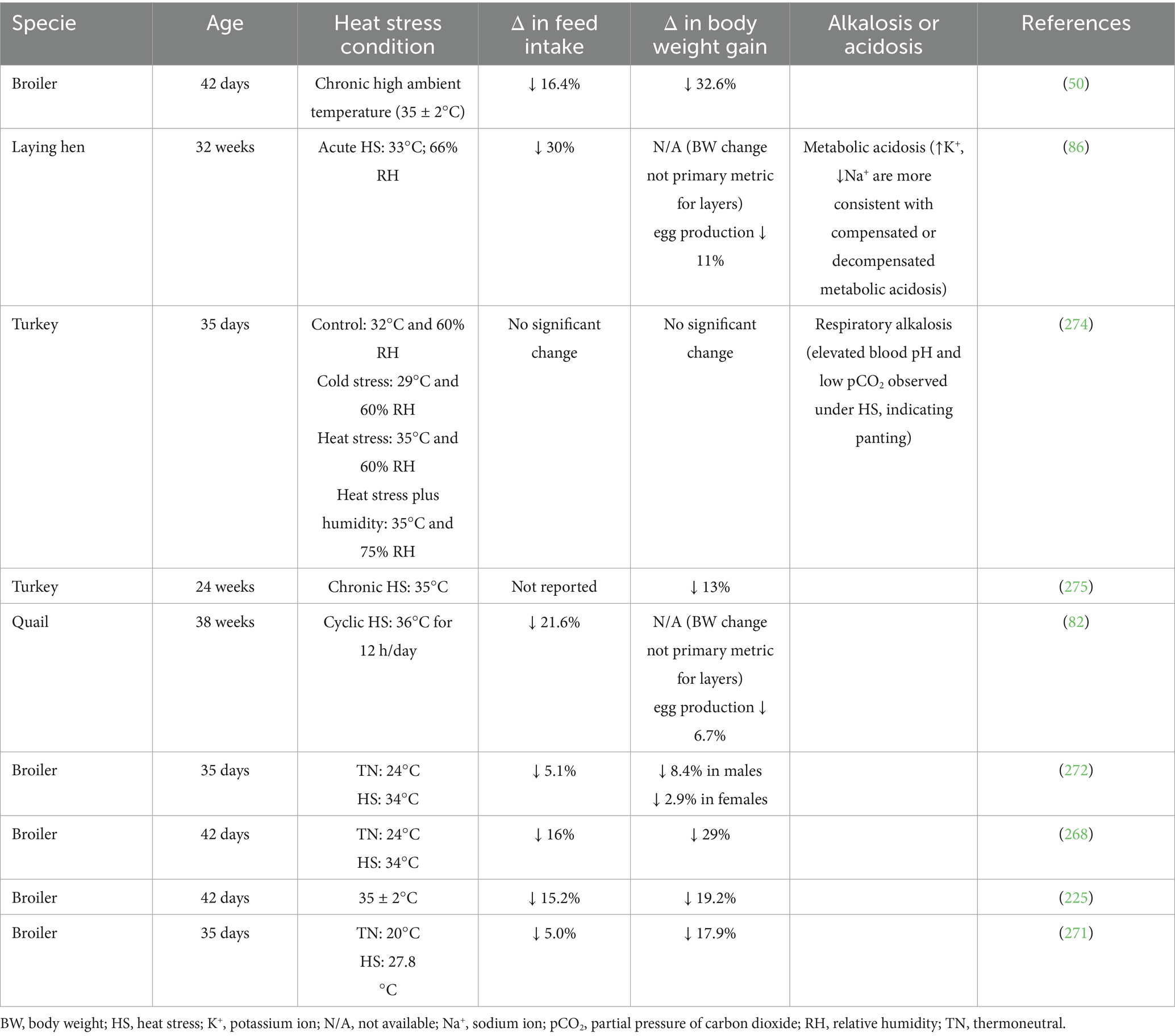
The effects of HS on bird strains and gender, as well as FI and body weight gain (BWG), are shown in Tables 1, 2, respectively. Broilers generate a large amount of heat because of the high energy density in their diets, retaining only 40% of the ingested energy and dissipating the remaining 60% (17). Exposing broilers to chronic HS resulted in 16.4, 32.6, and 25.6% reductions in FI, BWG, and feed conversion ratio (FCR), respectively (28, 50). According to Harsini et al. (51), exposing broilers to a cyclic temperature range of 23.9°C to 37°C significantly reduced BWG and FI, and increased FCR. Likewise, HS in laying hens led to reduced body weight (BW), feed efficiency, and other production metrics (52). The degree to which FI decreases varies based on different factors linked to the HS model applied to birds, complicating comparisons across studies (53). Under HS, birds reduce their FI to allocate more energy to cooling efforts, diverting energy from growth to thermoregulation. In addition to reduced FI, HS reduces the blood supply to intestinal epithelial cells, thereby reducing nutrient and oxygen supply, which in turn causes morphological changes and mucosal damage. This leads to intestinal inflammation, reduced villus height, and intestinal permeability, which impairs nutrient absorption and further contributes to poor feed efficiency and growth performance (54).
Literature shows that HS adversely impacts fat metabolism, impedes muscle growth, and detrimentally affects meat quality and chemical profile (55–58). According to Lu et al. (59), extended periods of heat stress adversely impact fat accumulation and meat quality. This was primarily attributed to electrolyte imbalance and lipid peroxidation. Dai et al. (56) and Imik et al. (60) stated that HS reduces broiler meat’s chemical composition and quality. Zhang et al. (61) reported that chronic HS reduced the breast muscle proportion and increased the thigh muscle in broilers. In the same study, birds exposed to HS had lower protein content and higher fat deposition in their meat. Broilers under HS usually exhibit lighter carcasses, reduced breast muscle, and increased abdominal and subcutaneous fat. Muscle protein accretion is hindered by heat-induced proteolysis and reduced muscle protein synthesis, while at the same time, lipogenic enzymes increase, depositing extra fat in tissues (33). This leads to poorer meat quality, characterized by paler, softer meat with lower water-holding capacity and pH and higher drip loss during storage (62). Therefore, in heat-stressed flocks, meat characteristics, including the meat color, texture, juiciness, and flavor, are often adversely affected.
Chronic heat stress induced pale, soft, exudative-like meat traits in broilers due to protein denaturation and muscle fiber shrinkage (61). Additionally, chronic heat exposure can lead to muscle fiber atrophy in broilers, reductions in muscle fiber diameter, and changes in muscle histology, which have been reported to correlate with reduced breast meat yields (61). Unlike chronic HS, acute HS has less pronounced effects on meat quality, but there is evidence that acute HS alters blood chemistry as an indication of skeletal muscle cell injury (63). Acute HS induces rapid metabolic shifts, including increased oxidative stress and increased glycogen utilization, which can temporarily impair muscle function. Under acute HS, the muscle production of lactate increases, leading to a decrease in pH and subsequently decreasing breast meat quality (64). High environmental temperatures can adversely affect ribosomal capacity by altering ribosomal gene transcription, which reduces protein synthesis and increases protein degradation (61). Increased fat accumulation may result from a reduced basal metabolism combined and physical activity (65).
Research has shown that HS negatively affects nutrient digestibility in broilers (66), laying hens (67), and quails (68). In broilers exposed to either continuous or cyclic HS, energy and nutrient digestibility were affected by HS, with dry matter and protein digestibility decreasing by 3.9 and 9.7%, respectively (66). The digestibility of essential amino acids is impacted by HS, causing a slight decrease noted for threonine, alanine, methionine, isoleucine, and leucine, and more significant reductions were observed in male birds (69). The digestibility of neutral detergent fiber was reduced in laying hens (67).
The mechanisms through which HS impairs nutrient digestibility and utilization are complex, and numerous studies have sought to clarify them. Evidence indicates that HS significantly downregulates the expression and activity of key digestive enzymes, including amylase, chymotrypsin, lipase, and maltase (70, 71). Additionally, the oxidative stress from HS caused intestinal barrier dysfunction, adversely affecting nutrient absorption efficiency (33). Heat stress alters the expression of genes coding for several macronutrient transporters. Several studies have documented that the occurrence of high temperatures over a prolonged duration results in a reduction in the expression of the glucose transporters, GLUT-2 and SGLT-1 (71–74). On the other hand, GLUT-5 expression rises, aiding in fructose transport (73). Conversely, there is an upregulation of GLUT-5, which is responsible for facilitating the transport of fructose (73).
3.2 Impact of heat stress on laying performance
Heat stress stimulates the avian HPA axis to elevate hormonal levels, including adrenocorticotropic, catecholamines, and corticosterone (75). This hormonal surge negatively impacts FI and metabolism, ultimately reducing overall performance (14). Kumar et al. (76) reported that when laying birds were exposed to HS, egg production, egg weight, BW, BWG, and daily FI were decreased by 4.99–57, 2.78–14.3, 3.74–32.6, 11–50, and 16.09–46.33%, respectively. Mack et al. (41) reported that HS reduced egg weight (13.5%) and eggshell thickness (10.5%) in White Leghorn hens. The quality of internal egg components may also be adversely affected, as producers frequently report diminished albumen quality, as evidenced by a reduction in albumen height and decreased Haugh unit scores, during periods of elevated temperatures. This decline in quality is potentially attributable to diminished FI, which can result in nutrient deficiency, as well as oxidative stress that adversely impacts yolk pigmentation and lipid stability (77).
High temperatures adversely affect FI in breeders and laying hens by decreasing FI and productive efficiency throughout the egg production cycle. While investigating the effect of HS on the ovarian function of laying hens, Rozenboim et al. (78) reported that exposing White Leghorn laying hens to heat stress (42 ± 3°C) for two days resulted in a 20% reduction in egg production. In the same study, the hens’ egg weight declined after a day of exposure to HS. Also, Buranawit et al. (79) reported that chronic HS reduces laying hens’ egg production and weight. Likewise, HS disrupts the hen’s endocrine balance, which is necessary for reproduction. Lowered estrogen levels lead to fewer mature ovarian follicles and contribute to the decline in egg production (80). Preferably, laying hens should attain sexual maturity at a weight marginally above the standard weight to induce increased food consumption, improved egg weight production, and enhanced consistency in egg production. Birds kept at 35°C have a 20–30% reduction in BW at sexual maturity when compared to hens raised at 21°C (17). Consequently, the overall egg production and quality are negatively impacted (81).
The impact of environmental temperature becomes important when laying hens attain sexual maturity. For every 1°C increase above the optimal temperature, the average feed consumed decreases by 1.6%, while the energy expenditure (metabolic energy use) increases by 2.3% (17). When temperatures exceed 30°C, both FI and egg production decrease, leading to a decline in saleable eggs (82). Notably, feed consumption was observed to decrease by 50% when temperatures increased from 21 to 38°C (17). Increasing the temperature-humidity index (THI) from 25 to 29 (29-34 °C; 34-58% relative humidity) led to a 25% reduction in total egg production (83). This decline in egg production and weight due to HS can be addressed by enhancing the nutritional content of poultry diets; however, this method does not mitigate the effects of HS on eggshell quality (17).
The THI serves as a critical environmental indicator, often used for predicting production losses that arise from livestock exposure to humid and hot climate (84). For laying hens, Zulovich and DeShazer (85) set different levels of THI: comfort (THI < 70), alert (70 < THI < 75), danger (76 < THI < 81), and emergency (THI > 81). As THI levels increase, hens experience reduced FI and lower egg production (86), and deteriorated egg quality parameters (86). Notably, higher THI levels (THI = 85) led to decreased eggshell thickness and strength, egg yolk color intensity, and Haugh unit (86).
3.3 Impact of heat stress on reproductive function
Exposure to HS has a more pronounced impact on infertility in male breeders than on female breeders (87). Seminal characteristics, including semen production, quality, motility, and sperm metabolism, are modulated by temperature, pH, and ion concentration. When these factors are altered, they may ultimately result in infertility and the production of low-quality spermatozoa (88–90). When exposed to temperatures exceeding the thermoneutral zone, poultry species, including Japanese quail (91, 92) and broiler chicken (93), suffered from testicular abnormalities and dysfunction. These abnormalities adversely affected seminal parameters, including testicular spermatogenic cell counts, Bax (an apoptotic marker) immunopositive staining, levels of testicular Bcl-2 (an anti-apoptotic marker), the Bax/Bcl-2 ratio, and the quantity of androgen receptors, which were associated with an increase in testicular lipid peroxidation. Testicular abnormalities affect the germ cells responsible for spermatogenesis. Consequently, heat-stressed male birds exhibit reduced sperm production and impaired sperm quality, characterized by decreased motility and viability (94). Also. HS can cause testicular atrophy, shrinking the size of the testes and leading to hormonal imbalances, including reduced levels of testosterone, which further hinders reproductive function (94, 95). Ameen et al. (96) reported that HS reduced the reproductive efficiency of male cockerel breeds as semen quality and quantity were affected. Additionally, inseminating hens with semen obtained from roosters exposed to HS resulted in a higher percentage of unfertilized eggs, which is attributed to decreased sperm-egg penetration. This corroborates the report of McDaniel et al. (97), which stated that the rate of in-vivo sperm penetration into eggs was 48% lower at 27°C than at 21°C in broilers. Obidi et al. (98) reported that high temperatures promote testicular growth by increasing semen volume and concentration in the early phase of development. However, excessive heat later suppresses reproductive capacity in poultry.
Several studies have reported that exposing breeder and laying hens to excessive heat adversely affects the species’ ovulation rate, resulting in reduced fertility (89, 99), reproductive performance (98, 100, 101), and hatchability (102, 103). Heat stress lowers estrogen and progesterone levels, resulting in impaired ovarian follicular development (fewer mature ovarian follicles) and contributing to reduced egg production (80). Additionally, hens exposed to high temperatures experience infertility issues, including reduced follicular and oocyte development, and lower yolk maturation rate (104, 105). Ayo et al. (101) suggested that this effect could be due to reduced secretion of luteinizing hormone (LH), follicle-stimulating hormone, gonadotropin-releasing hormone, and alterations in antioxidant levels, fatty acid composition, and heat shock proteins (HSPs). Laying hens exposed to acute HS experienced disrupted reproductive function regulation, with reduced circulating LH levels due to reduced hypothalamic function (105). Breeder hens inseminated in the afternoon had lower fertility and hatchability rates than those inseminated in the morning (98), suggesting a potential temporal influence on reproductive success in avian species. Heat stress induces oxidative damage to the small yellow follicles, ovaries, and oviducts of ducks, laying hens, and quails, resulting in reduced relative reproductive organ weights and a decrease in the number of large follicles (76). Consequently, it leads to lower egg production, and in extreme instances, it can cause infertility (106–109).
3.4 Impact of heat stress on gut health
The gut is essential for digesting and absorbing nutrients, maintaining electrolyte balance, and supporting immune system development in living organisms (110). The gut ecosystem is inhabited by various microorganisms, including archaea, bacteria, and protozoa. Several factors, including dietary composition, environmental stressors, and temperature fluctuations, can disrupt this ecosystem (111, 112). Gastrointestinal health is adversely affected following HS, leading to reduced nutrient absorption, immune response dysfunction, and compromised gut epithelium integrity (21). In a thermoneutral environment, the gastrointestinal tract primarily absorbs nutrients through transcellular transport, a process in which molecules move across the cell membranes of intestinal epithelial cells. This mechanism is facilitated by various specific receptors and transporters located on these cells’ apical (lumen-facing) and basolateral (blood-facing) membranes. For instance, glucose and amino acids are absorbed transcellularly through sodium-dependent transporters like SGLT-1 and various amino acid transporters, while fatty acids and monoglycerides enter the enterocytes either by diffusion or through fatty acid transport proteins. While transcellular transport is dominant, a smaller portion of nutrient absorption can also occur via the paracellular pathway, which involves movement between the cells through tight junctions (TJ). This pathway is largely passive and driven by concentration gradients (113). Examples of nutrients that can be absorbed paracellularly include small ions such as sodium (Na+), chloride (Cl−), and water-soluble minerals like magnesium (Mg2+). The epithelial cells of the gut are interconnected through intercellular junction complexes to form intestinal barriers, stabilizing the epithelial barrier’s integrity (114, 115).
The junction complexes of gut epithelial cells include TJ, adherent junctions (AJ), desmosomes, and gap junctions (GJ) (116). The AJ is located beneath the TJ and contains cadherin and β-catenin as an extensive attachment to a ring of peri-junctional actin filaments and is involved in intracellular communication. Both TJ and AJ connect to the actin cytoskeleton (117, 118). Desmosomes are found beneath the AJ and are associated with keratin filaments. Desmosomes facilitate cell adhesion and protect the alimentary epithelia from shear-induced damage, while GJs are key in intracellular signaling (119). The cytoskeleton is a complex of proteins that supports the structure of all eukaryotic cells, and any damage to it can lead to an impairment of intestinal integrity (120).
The TJ facilitates the transportation of several materials and is controlled by signaling pathways (21). Claudin genes encode the protein “claudin” that forms strands at the TJ barrier, conferring cell–cell adhesion. Claudins are barrier-forming proteins responsible for regulating paracellular permeability through the pore pathway. Occludin modulates the paracellular leak pathway, and its phosphorylation results in the disruption of the TJ. Therefore, the concentration of claudin and occludin is vital for the regulation of TJ barrier functions (120). The TJ barrier is compromised in high-temperature environmental conditions, allowing luminal contents to enter the bloodstream (Figure 4). When this occurs, the gut becomes leaky and results in chronic systemic inflammation, which impairs the immune competence of birds (119).
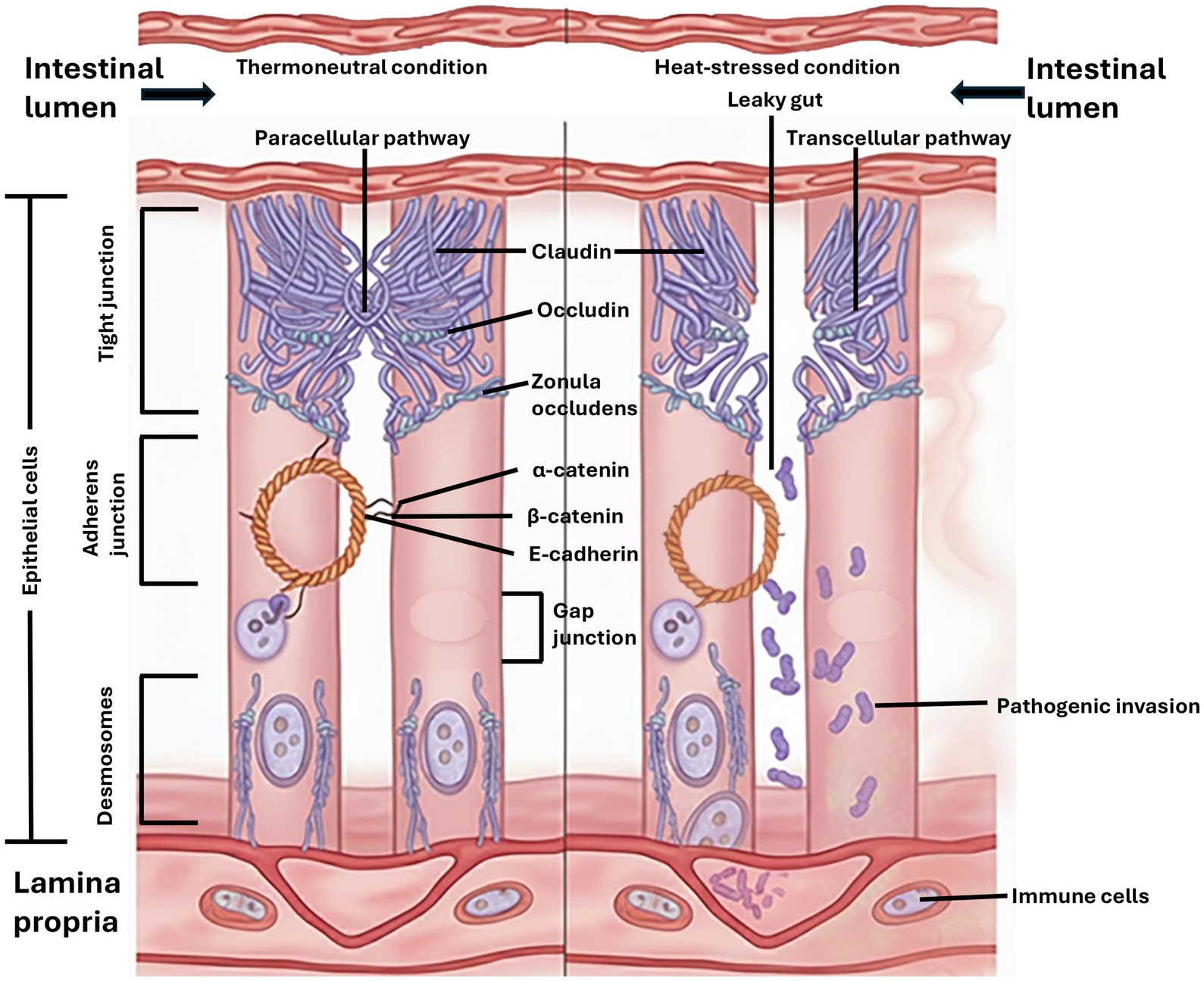
Figure 4. Mechanism of heat stress-induced intestinal permeability. Under thermoneutral conditions, the tight and adherens junctions form an intact barrier. Under heat stress, oxidative damage disrupts these junctions, widening the paracellular pathway and leading to the invasion of pathogenic bacteria and endotoxins into the lamina propria, triggering inflammation.
Several studies have indicated that HS negatively impacts nutrient digestion and absorption while enhancing the intestine’s sensitivity to pathogens (107, 108, 121–127). Heat stress alters intestinal morphology by causing abrasions in the duodenum, jejunum, and ileum (128). Due to increased reactive oxygen species levels, which heighten lipid peroxidation in the intestinal cell walls and pancreas, HS reduces the production of digestive enzymes (128). Additionally, there is a proliferation of pathogenic bacteria and a decline in beneficial microbiota, which compromises gut (121). This results in reduced meat and egg yields, compromised immune response, and hampered overall production performance.
3.5 Impact of heat stress on acid–base balance and electrolytes
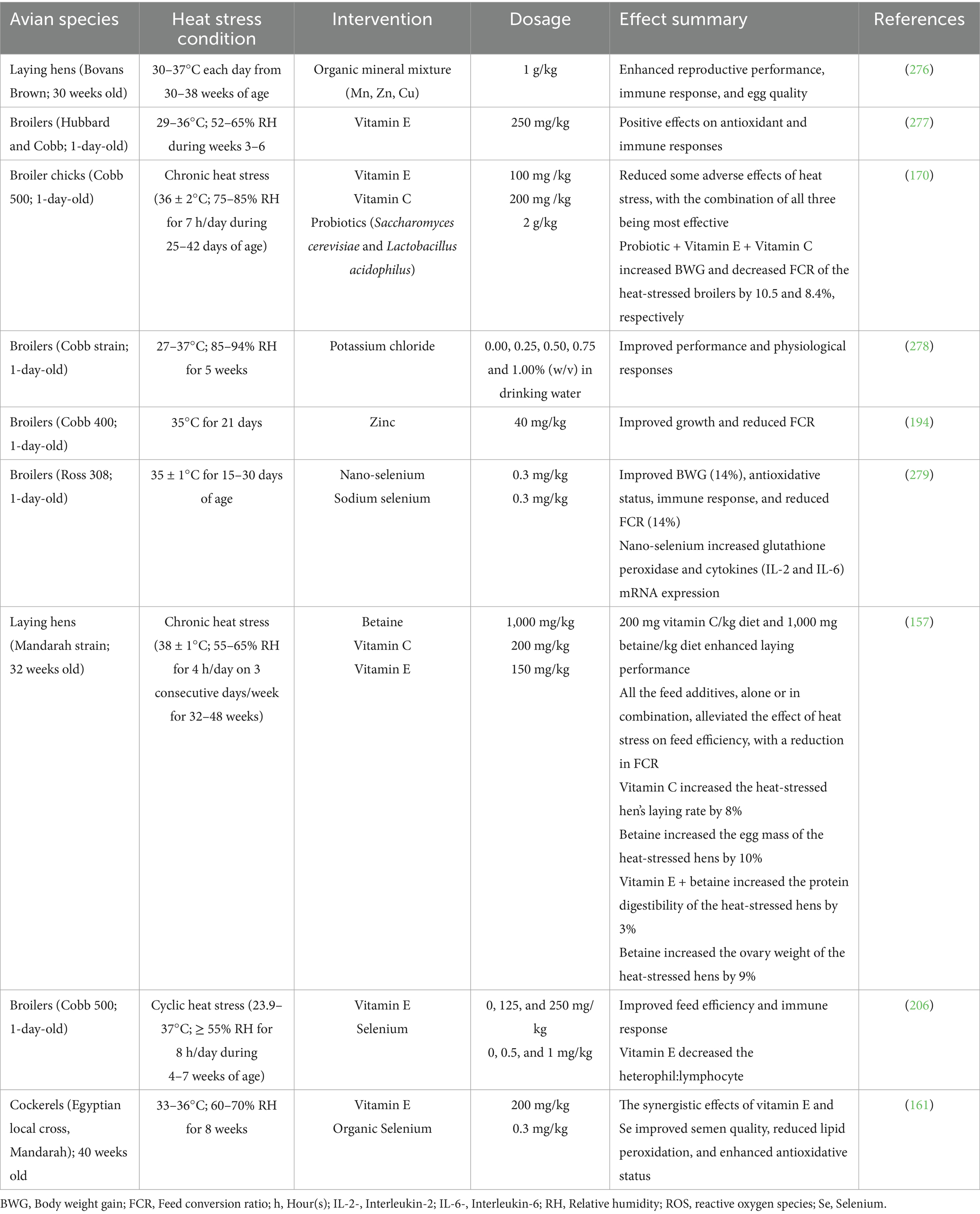
At normal temperatures, the kidneys and lungs function together to maintain acid–base equilibrium in the blood at a pH between 7.35–7.45 (Figure 5). Birds maintain pH via the bicarbonate buffer system and respiratory exchange of carbon dioxide (CO2). The hydration of CO2 generates hydrogen ion (H+) and bicarbonate (HCO3−), with hemoglobin and other buffers capturing H+ as CO2 is exhaled. This interaction of H+ with HCO3− to synthesize carbonic acid (H2CO3), thereby regulating the blood pH. Carbonic anhydrase converts H2CO3 into CO2 and H2O. The lungs expel the resulting CO2, while the kidneys excrete H+ ions along with HCO3− (27).
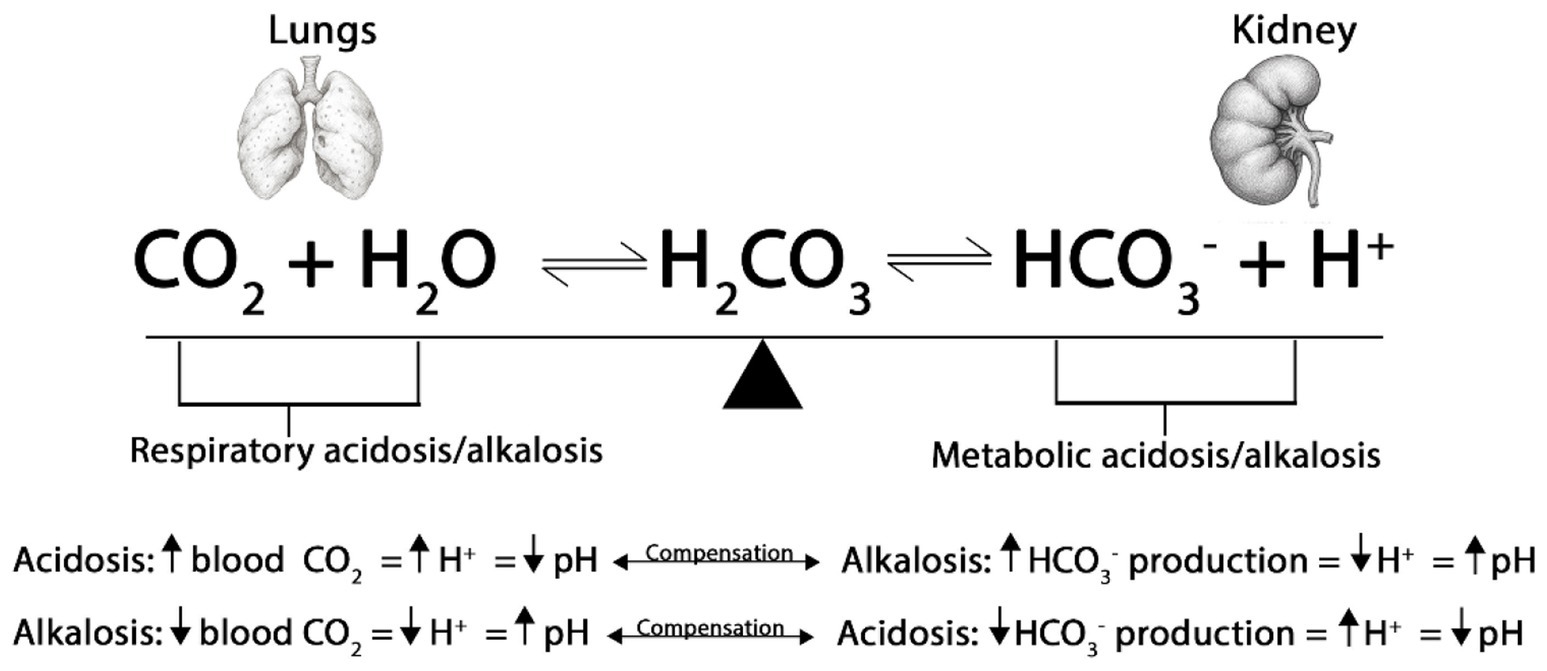
Figure 5. Respiratory and metabolic acidosis and alkalosis. The process of breathing leads to the production of CO2, which subsequently enters the bloodstream. Blood, primarily composed of water, facilitates a chemical reaction when carbon dioxide mixes with it, resulting in the formation of H2CO3. Carbonic acid is unstable and dissociates into HCO3− and H+. Thus, the lungs regulate blood pH by altering the PaCO2, while the kidneys regulate blood pH by altering the concentration of HCO3−. When the pH is too acidic, the kidneys absorb HCO3−, and when it is too alkaline, they excrete HCO3−.
During HS, a cascade of physiological changes occurs, starting with panting (respiratory rate increases from 25 breaths/min to 260 breaths/min), which increases CO2 exhalation relative to its cellular production, thereby altering the standard bicarbonate buffer system in the blood. The decreasing CO2 concentration in the blood results in a lower concentration of H2CO3 and H+. Conversely, the levels of HCO3− rise, leading to an increase in blood pH or respiratory alkalosis (28). Hyperventilation causes the dehydration of H2CO3 to CO2 and water (H2O), thereby depleting H+ and increasing pH (32). As a mechanism to normalize the blood pH, birds will begin to excrete higher levels of HCO3− and retain H+ in the kidney. Contrarily, the body may also experience a decrease in plasma HCO3− levels and a lower blood pH due to the increased production of lactic acid in the muscles, causing metabolic acidosis. This can occur when the body is not receiving enough O2 to meet the demands of the muscles during periods of high activity, such as panting. This altered H+ disturbs acid–base homeostasis, leading to respiratory alkalosis and metabolic acidosis, which are linked to reduced performance in poultry (129).
Respiratory acidosis involves a primary increase in the arterial partial pressure of carbon dioxide (PaCO2) and a consequent decrease in blood pH. Hypoventilation prevents adequate CO2 exhalation, leading to H2CO3 accumulation and an elevation of H+ (130). In acute respiratory acidosis, pH drops rapidly, and birds compensate for this by increasing HCO3− reabsorption and H+ excretion (renal compensation). In contrast, metabolic alkalosis results from elevated HCO3− levels, causing an increase in blood pH. This condition can occur due to a net loss of H+ or an accumulation of HCO3− (131). Respiratory compensation involves hypoventilation, which retains CO2, and the kidneys’ retention of H+. Metabolic alkalosis is often associated with hypokalemia because H+ exits cells in exchange for potassium ion (K+), resulting in an elevated blood pH, while the kidneys reabsorb Na+ alongside HCO3−.
The production of calcium carbonate in the shell gland is essential for eggshell formation and depends on adequate HCO3− level in laying hens (129, 132). Alkalosis reduces the blood ionized calcium concentration, which impairs eggshell mineralization in laying hens and contributes to poor growth in broilers (133). Consequently, acute HS can jeopardize calcium-dependent processes, such as eggshell formation, along with overall homeostasis. Chronic HS worsens these problems since initial rapid, shallow panting can evolve into deeper panting, known as “thermal hyperpnea,” as birds attempt to cope with prolonged heat (134). Frank and Burger (135) and Balnave and Muheereza (136) reported that lower bicarbonate levels in the shell gland lumen negatively influence eggshell quality. Likewise, Miller and Sunde (137) stated that an abrupt increase in air temperature negatively affects eggshell quality within just one oviposition cycle, highlighting that respiratory alkalosis in hens sharply reduces blood ionized calcium levels (133).
Besides altering the acid–base balance, HS also alters electrolyte concentrations, particularly Na+, K+, and Cl−, which are crucial for intracellular and extracellular fluid homeostasis (138). Osmoregulation is preserved through tight control of intracellular K+ and extracellular Na+ and Cl−. In broiler chickens, the recommended dietary electrolyte balance (DEB; total of Na+ + K+ − Cl−, mEq/kg) is around 250 mEq/kg feed to support acid–base equilibrium (139), although this may vary with ambient temperature. To maintain electrolyte homeostasis in body fluids, the body increases the excretion of K+ and Na+ in feces and urine, while the Cl− concentration in the blood increases. According to Borges et al. (140), HS reduced plasma Na+, K+, and PaCO2 concentration, likely due to hemodilution from elevated water level consumption. Similarly, in heat-stressed birds, elevated temperatures decreased plasma Ca, Na, inorganic P, and Mg (141).
4 Nutritional supplements used in poultry for alleviating heat stress
Heat stress adversely impacts poultry growth, nutrient digestibility, immunity, and overall welfare, leading to poor performance, increased mortality rates, immune dysfunction, and economic losses (142, 143). Several strategies and management practices, including housing, physical cooling measures (134), ventilation, thermal manipulation, in ovo feeding, and breeding for heat-resistant related genes (144) have been implemented to alleviate HS in poultry. Yet, the effect of HS persists in the poultry industry. This has prompted the search for alternative strategies and nutritional manipulations to address this challenge (145, 146). The potential of several nutritional supplements, including vitamins, minerals, fat, and amino acids, has been investigated.
4.1 Vitamins
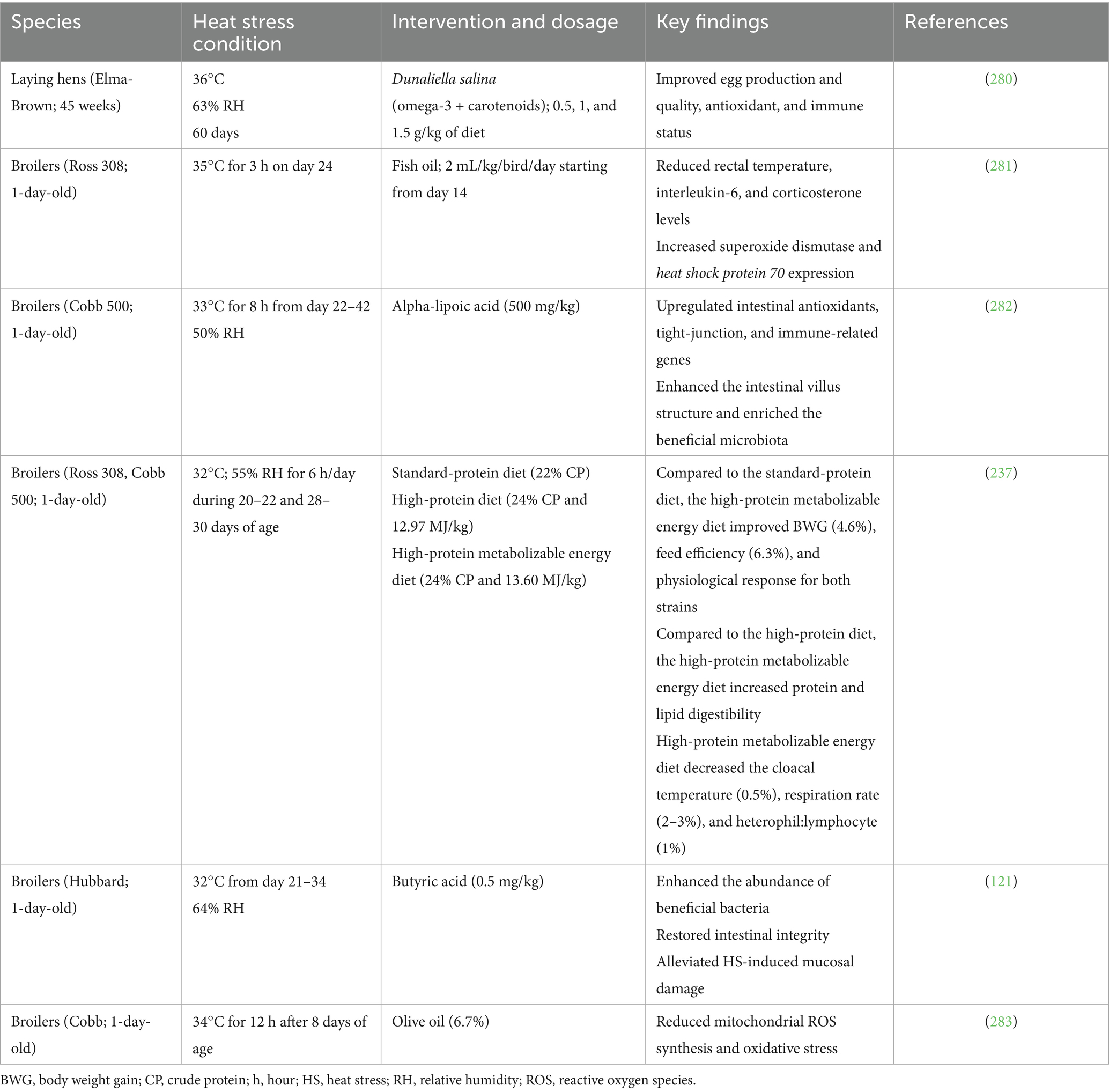
Researchers have extensively studied the role of vitamins in reducing the negative impacts of HS (147, 148), with vitamins C and E identified as having the most significant influence (34). Vitamins C and E are incorporated into poultry diets because of their anti-stress and antioxidant effects, and their production decreases during HS. Supplementing birds’ diet with vitamins A, C, and E improved egg production, hatchability, and fertility while also reducing egg breakages and mortality in laying hens in hot environments (149). Chung et al. (150) and Abd El-Gawad et al. (147) reported that supplementing vitamins C and E into the diet of heat-stressed broilers and quails improved eggshell quality, increased FI, and boosted BW.
4.1.1 Vitamin C
Vitamin C, or ascorbic acid, is a water-soluble antioxidant that protects cells from oxidative damage and is vital for stress tolerance, including HS in poultry (104). High temperature increases the birds’ demand for vitamin C. During hot weather, the tissues actively absorb and utilize vitamin C, leading to insufficient availability of plasma ascorbic acid and increased demand for vitamin C (34). Chickens naturally produce ascorbic acid in the kidney from glucose (151), which meets their normal physiological needs under typical conditions (152). During HS, the demand for ascorbic acid significantly increases because the endogenous synthesis of vitamin C fails to meet the birds’ physiological requirements. Ascorbic acid is an essential nutrient that is critical in maintaining efficient homeostasis. Chickens need vitamin C for the effective metabolism of amino acids and minerals, along with synthesizing hormones that help manage stress. It promotes leukocyte activity and contributes to antibody synthesis (104). As environmental temperatures rise, blood ascorbic acid levels decline; thus, supplemental ascorbic acid could help lessen the impact of HS in poultry.
Layers are susceptible to HS due to the higher metabolic heat produced during egg formation, ovulation, and oviposition (153). Ascorbic acid is crucial to several essential functions, like the release of adrenaline and corticosterone, the biosynthesis of 1,25-dihydroxy vitamin D and collagen, and the enhancement of calcium metabolism (154). Supplementing vitamin C enhances heat-stressed birds’ performance by boosting feed consumption and nutrient uptake. Asensio et al. (155) demonstrated that the inclusion of ascorbic acid in broiler diets significantly improved carcass weight and protein content, alongside a notable reduction in carcass fat. Supplementing diets with 50 and 100 mg/kg of vitamin C increases the fertility and hen-day egg production of broiler breeders (156). According to Attia et al. (157), supplementing 200 mg/kg vitamin C in the diets of heat-stressed laying hens improved FI, egg production, and egg quality. Furthermore, including 200–400 mg of ascorbic acid/kg of laying hens’ diet improved the number of produced eggs, survival rate, and FI (158). Also, Ahmed et al. (159) found that incorporating vitamin C into laying hens’ diets at 1,000 and 1,200 ppm/L of water optimized their egg production rate, egg weight, egg mass, livability, and FCR during HS.
4.1.2 Vitamin E
Vitamin E consists of various fat-soluble compounds known for their antioxidant benefits, including tocopherols and tocotrienols. Supplementing vitamin E in poultry diets is essential in poultry nutrition because birds do not have the capability to synthesize it (157). Including vitamin E in the diet during stressful periods enhances the immune response in poultry. During stressful conditions, vitamin E is a crucial natural antioxidant that reduces physiological stress induced by corticosterone and catecholamines. During HS, the hormonal levels of catecholamine and corticosterone increase, leading to the commencement of peroxidation of lipid in cell membranes (160). Vitamin E can serve as the primary defense against lipid damage and peroxidation of cells and tissues and effectively neutralizes free radicals (157, 161).
Heat stress induces oxidative stress, which impairs immune functions, but antioxidants can avert this impairment. Immune cells are rich in polyunsaturated fatty acids, making them highly susceptible to oxidative damage (162). Due to their lipophilic nature, antioxidants like vitamin E and carotenoids are efficacious in neutralizing damaging reactive species in lipid-rich membrane environments (163). According to Shakeri et al. (164), vitamin E is important in immune responsiveness by protecting plasma cells, lymphocytes, and macrophages from oxidative stress and likewise improves their viability and proliferation function. The exposure of Japanese quails to 34°C for 8 h/day, followed by 22°C for 32 days, led to a 29% decrease in blood tocopherol levels compared to the control group under normal temperature (165). This finding suggests that prolonged HS results in a reduction of antioxidant components.
Supplementing broiler chickens’ diet with vitamin E at 250 mg/kg effectively mitigates the severity of HS, potentially leading to improved bird performance and superior meat quality (166). Laying hens had improved feed utilization, egg production, and immunological response when vitamin E was supplemented into their diets at 125–250 mg/kg (164). Heat stress elevated malondialdehyde (MDA) levels in the blood and liver, whereas vitamin E supplementation reduced MDA formation, preventing lipid peroxidation and cell damage (167), to improve chicken performance. In high ambient temperature conditions, dietary vitamin E supplementation at 250 mg/kg increased the activity of glutathione peroxidase (GSH-Px) and reduced the levels of HSP60 in broilers (168). Likewise, supplementing 200 mg vitamin E/kg diet enhances the antioxidant defenses in chicken semen subjected to HS. This enhancement is due to a reduction in lipid peroxidation, improvement in GSH-Px activity, and an increase in total antioxidant capacity as measured by levels of MDA (161). Additionally, it enhances the fatty acid profile, feed consumption and efficiency, egg yield and quality, and oxidative stability of thigh muscle in broilers and quail raised under HS (148, 149, 169).
4.1.2.1 Effects of vitamins on growth and nutrient digestibility/utilization
Vitamins play significant roles in improving growth and nutrient utilization in heat-stressed birds (Table 3). Under cyclic HS (36 ± 2°C), broilers fed diets supplemented with vitamin E (100 mg/kg), vitamin C (200 mg/kg), and probiotics enhanced BWG and decreased the FCR of broiler chickens (170). Also, McKee et al. (171) reported that vitamin C (150 mg/kg in diet plus 400 mg/L in water) mitigated the reductions in cockerels’ weight gain, FI, and gain:feed ratio caused by heat exposure. Similarly, dietary inclusion of 100 mg vitamin E/kg enhanced feed utilization in broilers under heat stress, as evidenced by a significant decrease in FCR (172). These observed benefits could be associated with the antioxidant properties of the vitamins. By mitigating oxidative damage and gut permeability induced by HS, they stabilize the cell membranes and improve immune response, improving nutrient absorption and allowing birds to partition more energy toward growth.
In Japanese quails, Sahin and Kucuk (169, 173) demonstrated that dietary vitamin C (100–200 mg/kg) and vitamin E (125–500 mg/kg) increase final BW and feed efficiency. Also, the digestibility coefficients for dry matter, organic matter, and crude protein were improved. Combining vitamin C with folic acid increases mineral retention in Japanese quails. When vitamin E was combined with selenium (Se), the digestibility parameters, especially dry matter, were improved (173). In several studies, the combined use of vitamins with or without Se yielded synergistic effects on performance and nutrient utilization. Sahin and Kucuk (173) reported a significant interaction for final BW and feed efficiency between vitamin E and Se supplementation in Japanese quails exposed to HS (34°C). This suggests that strategic combinations of vitamins in the presence or absence of Se may be more effective in supporting growth performance and nutrient utilization in heat-stressed poultry than single vitamin supplementation.
4.1.2.2 Effect of vitamins on skeletal health
Heat stress induces oxidative stress that directly compromises the gut barrier function, leading to a massive reduction in the absorption of bone minerals like calcium and phosphorus (174). Vitamin supplementation could enhance the skeletal health of heat-stressed poultry by mitigating gut damage and maintaining efficient mineral absorption. Calik et al. (175) demonstrated that supplementing vitamin E and Se into the diets of broilers exposed to cyclic HS (35°C for 4 h/day) resulted in an enhanced bone mineral content (BMC) and bone mineral density (BMD). Due to their antioxidant properties, these additives protected the intestinal epithelium from this oxidative damage, maintaining the gut integrity. This facilitates the transport of calcium and phosphorus across the mucosa for utilization, thereby enhancing BMC and BMD. Similarly, Marques et al. (176) investigated the efficacy of 25-hydroxycholecalciferol (25-OH-D3) as an alternative to conventional vitamin D3 in broilers exposed to HS (31.1–32.9°C). Their findings indicated that 25-OH-D3 facilitated improved calcium and phosphorus deposition in bones, ensuring skeletal integrity without compromising overall performance, even when dietary Ca levels were reduced.
Contrarily, the observations of Mosleh et al. (177) regarding ascorbic acid supplementation indicated a limited impact on bone mineralization. While vitamin C alleviated oxidative stress in broilers exposed to chronic HS (39°C for 8 h/day over several durations), it did not significantly enhance BMD or other bone characteristics. This suggests that vitamin C alone may be insufficient to prevent heat-induced skeletal deterioration. However, Sahin et al. (178) found that combining 25-OH-D3 with soy isoflavones in quails exposed to 34°C for 8 h/day led to marked improvements in BMD, tibia ash content, and serum calcium and phosphorus concentrations. This suggests that a synergistic approach involving vitamin D metabolites and phytoestrogens may be particularly beneficial for maintaining bone health under HS conditions.
4.2 Minerals
Heat stress triggers several responses, including physiological and behavioral reactions in poultry. In response to excessive heat, birds reduce FI, which leads to insufficient mineral intake to meet their need. Therefore, heat-stressed birds are deficient in minerals, making the supplementation of certain minerals a priority. Minerals provide support for several biological and cellular functions, facilitating growth, enhancing nutrient utilization, strengthening immune responses, mitigating oxidative stress, and supporting the overall productivity of birds subjected to HS (179, 180). Heat stress in poultry causes increased mineral excretion, resulting in acid–base imbalances and respiratory alkalosis. This issue could be addressed with appropriate mineral supplementation at various production stages (181). This review focuses on zinc (Zn), Se, and chromium (Cr) supplementation due to their antioxidant properties, gut barrier integrity maintenance, proper immune functions, and glucose metabolism and energy utilization, which are negatively affected by HS.
4.2.1 Zinc
Zinc is an indispensable trace element that is important for homeostasis and acts as a cofactor for several enzymes involved in many biological and life processes, like skeletal development, antioxidant defense system, and immune response (106, 182). Zinc supplementation is vital because the body cannot store it. Furthermore, zinc preserves the integrity of the DNA, so its deficiency negatively impacts the DNA by reducing the effectiveness of zinc-dependent proteins (183). During HS, Zn protects the cells by enhancing antioxidant status, scavenging ROS, and attenuating heat shock responses. Zinc is vital for metallothionein synthesis, a free radical scavenger (182).
As an important constituent of antioxidant enzymes, like glutathione (GSH), superoxide dismutase (SOD), and glutathione S-transferase, Zn helps to mitigate free radical formation. Additionally, Zn is an element of carbonic anhydrase, which is responsible for catalyzing carbonate synthesis, which is crucial for eggshell mineralization (184). By negatively regulating the nuclear factor kappa-light-chain enhancer of activated B cells (NF-κB) signaling pathway, it helps to suppress inflammation responses. The import of Zn ions into cells via the Zn transporter protein family 8 (ZIP8) may inhibit the Ikappa-B kinase complex to downregulate NF-κB during inflammatory responses (185).
Zinc is often included in the diets of breeding birds to enhance reproductive function. Zinc deficiency reduces semen quality (about 10% decrease in sperm motility), lowers egg production, and leads to abnormal embryonic development and poorly performing offspring (186). Zhu et al. (187) found that maternal HS at 32°C during the age of 33 to 42 weeks negatively impacted both hatching performance and embryonic development in chickens; however, these negative effects could be mitigated by administering 110 mg Zn/kg of diets for 9 weeks. In heat-stressed broiler breeders, Zn supplementation at 110 mg/kg enhanced egg production and quality, improved antioxidant markers, and upregulated pancreatic and hepatic HSP expression (188). Turkey breeders that received supplemental Zn during the hot summer had increased egg production along with enhanced natural behaviors like feather cleaning and dustbathing (189). Also, corticosterone levels in plasma serve as a stress biomarker induction. Zinc supplementation lowered plasma corticosterone levels and enhanced egg production and BW in turkey breeders during the summer (190).
According to Long et al. (191), the supplementation of the organic form of zinc, zinc lactate, at varying doses of 40, 60, and 80 mg/kg in the diet enhanced broilers’ growth performance, intestinal morphology, antioxidant enzyme activity, immune response, and hepatic metallothionein. Organic Zn compounds might be more effective than inorganic Zn sources in reducing the adverse effects of HS in poultry. Zhang et al. (192) observed that the embryos from broiler breeders fed 80 mg Zn-Gly/kg of diet exhibited superior protection against HS-induced oxidative stress compared to those from breeders offered 80 mg ZnSO4/kg of diet. Additionally, Zn-lysine chelate significantly reduced HSP70 and HSP90 mRNA expression in heat-stressed broiler hepatocyte cultures compared to Zn oxide (193). In summer, broilers that received organic zinc supplementation at 40 mg/kg of feed had higher BWG, decreased lipid peroxide levels, and increased SOD enzyme activity (194).
Zinc performs several functions, including promoting metallothionein synthesis, regulating transition elements, and its connection with antioxidant vitamins like vitamins A and E. These functions might be responsible for its role in decreasing lipid peroxidation in birds receiving Zn supplements (195). Serving as a cofactor of the antioxidative enzyme, CuZn-SOD, Zn suppresses free radicals, impedes NADPH-dependent lipid peroxidation (196), and prevents lipid peroxidation by inhibiting the depletion of glutathione (197). Zinc can exert a direct antioxidant effect by competing and displacing transition metals (Fe, Cu) from binding sites, binding to the cell membrane, and decreasing free radical production (196, 198).
4.2.2 Selenium
Selenium is a trace element essential for at least 25 selenoproteins, including antioxidant enzymes like thioredoxin reductases (TrxR) and GSH-Px (199, 200), which protect cells from oxidative damage and maintain redox homeostasis. In poultry nutrition, two forms of Se are used as feed additives: inorganic forms, like sodium selenite and selenite, and organic forms, like selenomethionine and Se yeast, with organic forms generally showing higher bioavailability (201). For proper antioxidative function, chickens require an adequate Se dosage of about 0.1–0.3 mg/kg diet (202). Due to its high toxicity, there is a threshold for the inclusion of Se in poultry diets (203). Therefore, both deficiency and excess of Se can impair poultry performance. Incorporating a high level of Se (3 mg/kg) into the chickens’ diet induced oxidative stress characterized by low SOD and catalase activities (204). Likewise, Se deficiency (0.03 mg/kg for 40 days) exacerbates the inflammatory status of heat-stressed broilers by increasing the ratio of M1-type inflammatory macrophages relative to M2-type anti-inflammatory cytokine-producing macrophages (205).
Selenium has an antioxidant potential and is used to alleviate HS in poultry (206, 207). In heat-stressed Japanese quail (34°C for 8 h/day), the supplementation of 0.3 mg of sodium selenite or selenomethionine per kilogram of diet enhanced egg production, egg quality, and the antioxidant profile of the birds (208). The most pronounced effects were observed in the quails that received selenomethionine supplementation. Also, supplementing broiler feed with Se (0.3 mg/kg) enhanced bird weight and reduced FCR during HS (209). The supplementation of inorganic sodium and sodium selenite, at 0.1 mg/kg and 0.2 mg/kg of feed, enhanced carcass quality and overall performance of heat-stressed quails (173). Selenium enhances the productivity and reproductive ability of laying hens (210). During thermal stress, incorporating selenized yeast into laying hens’ diets significantly enhanced various response variables, including egg weight, egg production rates, haugh unit, and eggshell strength (211). During periods of HS, the addition of Se (0.15 and 0.30 mg/kg of feed sodium selenite or selenomethionine) to the diets of laying quails led to an increase in FI, BW, egg production, and improved feed efficiency (208). Also, the study showed that supplementing both forms of Se increased the haugh units and eggshell weights.
In chickens, Se supplementation reduces the expression of the HSP70 gene (204), whereas Se deficiency increases its expression in the spleen, thymus (212), and erythrocytes (213). Mahmoud and Edens (214) observed that the addition of Se yeast (0.2 mg/kg of diet) significantly modulates the HSP70 response, enhances antioxidant status, and mitigates the incidence of enteric bacterial infections in heat-stressed animals. Kumbhar et al. (168) reported that supplementing 0.2 mg/kg of Se and 250 mg/kg of vitamin E reduced the expression of HSP60, HSP70, and HSP90 in the breast muscle of heat-stressed chickens.
4.2.3 Chromium
Chromium is an important trace element that is crucial in nutrient metabolism, including carbohydrates, proteins, and fats (215). It is an essential element of chromodulin and is required for insulin function (216). Heat stress leads to greater mobilization of Cr from body tissues, thereby raising nutritional needs (217). Supplementing poultry diets with Cr enhances feed efficiency (173), improves nutrient digestibility and transporter functions (218), and affects orexin and glucose transporters, as well as various biochemical parameters (219). In laying hens, incorporating 0.4–2.0 mg of Cr/kg of feed as chromium picolinate (CrPic) enhanced immunity, improved egg quality, and increased the haugh unit (220). Additionally, this supplementation reduced the level of blood glucose, cholesterol, and triglycerides in the hens (221). Furthermore, evidence supports that Cr supplementation helps alleviate HS-induced egg quality deterioration and metabolic imbalances in laying hens (222).
Research has also highlighted the effectiveness of organic Cr, such as CrPic and Cr-histidinate, in improving nutrient digestibility and metabolic stability in heat-stressed birds. Orhan et al. (218) found that laying hens subjected to 34°C for 8 h/d for 12 weeks, exhibited improved glucose, protein, and intestinal fatty acid transporters when supplemented with 200 μg CrPic or Cr-histidinate per kilogram of diet. Similarly, in a study involving 360 Japanese quails, dietary Cr-Pic supplementation (500 or 1,000 μg Cr/kg diet) improved growth performance, BWG, and biochemical regulation under HS conditions (223). Organic Cr exhibits superior efficacy in mitigating oxidative stress linked to physiological damage in animals subjected to HS when compared to inorganic Cr (224). Chromium methionine, an organic Cr, improved broilers’ cellular and humoral immune responses during HS (225). This enhanced effectiveness is likely attributable to the greater biodistribution, bioavailability, and absorptive characteristics of organic Cr (226).
Chromium has been extensively studied for its role in lessening the impact of HS in birds, primarily due to its antioxidant, anti-inflammatory, and metabolic regulatory functions. A primary mechanism through which chromium mitigates oxidative stress involves decreasing lipid peroxidation while enhancing total antioxidant capacity and GSH levels, which are essential for cellular defense against oxidative damage (227). Additionally, Cr exerts anti-inflammatory effects by inhibiting pro-inflammatory cytokines such as TNF-α and interleukin 6 (IL-6) which are often elevated under HS conditions (228). According to Sahin et al. (229), CrPic was more effective at reducing TNF-α, IL-6, and C-reactive protein (CRP) levels than CrCl3 in heat-stressed quails. Chromium anti-inflammatory role is likely associated with its capacity to increase the expression of Nrf2 in heat-stressed birds (230). A study by Hamidi et al. (231) demonstrated that supplementing Cr nanoparticles or Cr-Pic (500, 1,000, or 1,500 mg/kg of diet) into broiler diets from 21 to 42 days of age enhanced immune responses by downregulating IFN-γ, which is crucial for ROS synthesis and inflammation under HS. Also, supplementing Cr-histidinate to heat-stressed quail diets (34°C for 8 h/day) can elevate hepatic inhibitor of NF-κB alpha (IκBα) levels while lowering NF-κB and influencing the hepatic IκB/NF-κB pathway (232). Furthermore, supplementation of Cr-histidinate in quails subjected to HS has been demonstrated to inhibit HSPs, specifically HSP60, HSP70, and HSP90 (233).
Beyond its antioxidant and anti-inflammatory roles, Cr is important for glucose metabolism and nutrient absorption, which are critical for maintaining bird performance under HS conditions. Chromium enhances insulin function by modulating chromodulin, GLUT-4, and uncoupling protein-3, leading to increased glucose and amino acid uptake and improved skeletal muscle mass (234). This metabolic regulation is crucial in birds subjected to HS, where energy balance and nutrient utilization are often compromised.
4.3 Dietary fat and fatty acids
The addition of fat to poultry diets increases the energy value of feed constituents and mitigates the effects of HS in birds. Heat stress accelerates gastrointestinal passage rates, but dietary fat addition slows the rate of food passage and enhances nutrient utilization (235). Additionally, higher-energy diets are effective in partially counteracting the harmful effects of HS, as fat metabolism generates lower heat increments compared to proteins and carbohydrates (236). The reduced thermogenic effect of fat, relative to carbohydrates and proteins, is attributable to differences in how these macronutrients are metabolized. Unlike carbohydrates, which undergo multiple metabolic transformations before being converted into body fat, fatty acids can be directly utilized in body fat synthesis without modification. This principle has led to the widespread practice of supplementing poultry diets with fat in hot climates, where the addition of fat helps increase dietary energy levels and reduce the detrimental impact of HS on bird performance.
Adding 5% fat to heat-stressed laying hens’ diets increased FI by 17% (158), while broilers fed a similar diet exhibited significant improvements in performance, nutrient digestibility, and carcass traits (237). Similarly, Attia et al. (238) stated that adding oil to high-protein diets helped alleviate the harmful effects of chronic HS on broiler performance, meat lipid composition, and physiological and immunological traits. Ghahremani et al. (239) showed that partially replacing dietary energy with soybean oil and increasing dietary energy concentration enhanced intestinal function and overall productivity in heat-stressed broilers. However, one notable outcome of fat supplementation is increased abdominal fat deposition in heat-stressed broilers (428).
Fatty acids, particularly short-chain and medium-chain fatty acids, are critical in stabilizing gut microbiota composition, regulating epithelial cell function, and alleviating oxidative stress caused by HS (Table 4). Short-chain fatty acids, with fewer than six carbon atoms, serve as key energy sources for gut microbiota and help maintain a healthy gut environment by lowering intestinal pH and inhibiting harmful bacterial growth (240). The improvement in gut epithelial structure helps protect against pathogenic bacterial invasion, thereby preventing intestinal permeability and enhancing overall growth performance in broilers.
4.4 Amino acids
Protein metabolism generates higher heat than fat and carbohydrates, thereby further increasing the adverse effect of HS in birds (241). While higher protein intake boosts heat production, reducing dietary protein can reduce BW. However, the adverse effects of supplying birds with low dietary protein can be countered through supplementation with crystalline limiting essential amino acids. Heat stress detrimentally impacts amino acids’ availability, transport, intestinal uptake, absorption, and utilization (145, 242). Therefore, the addition of balanced amino acids to low-protein diets can improve birds thermotolerance by lowering the energy cost for N excretion (243). In hot periods, diets lower in protein but enhanced with limiting amino acids yield better outcomes than high-protein diets.
Besides, maintaining amino acid balance and adequate amounts of limiting amino acids like lysine and arginine greatly helps in minimizing the effects of HS (166). Also, sulfur-containing amino acids, like methionine and cysteine, are essential for poultry nutrition. The supplementation of methionine reduced muscle oxidation and enhanced tissue antioxidant levels in broilers under HS (244). In another study, the supplementation of sulfur amino acids reduced chronic HS in broiler chickens by boosting antioxidant synthesis and protecting the permeability of the intestinal barrier (245, 246). According to Wu et al. (247), glutamine promotes growth performance, enhances gut development, and improves barrier function, including TJ protein expression. Additionally, Dai et al. (248) found that adding glutamine (0.5 and 1.0%) to the diets of broilers exposed to HS (28°C) mitigated losses in growth performance, carcass traits, meat quality, and meat color stability. Moreover, glutamine contributes significantly to stabilizing the intestinal microflora by enhancing the presence of Lactobacillus and Bifidobacterium in the cecum while decreasing the levels of Clostridium perfringens and E. coli in broiler chickens exposed to HS.
Glycine, a conditionally essential amino acid in poultry, is also crucial for improving production performance and reducing oxidative stress and intestinal dysfunction in heat-stressed birds (242, 249). Some non-essential amino acids and their derivatives, like betaine, taurine, L-citrulline, and L-theanine, can help reduce HS in poultry (242). These compounds exhibit strong biological effects, acting as anti-stress agents, antioxidants, anti-inflammatory agents, immune system boosters, and gut stimulants when administered to poultry experiencing HS.
4.5 Electrolytes
Under high temperatures, birds pant, causing a disruption in acid–base homeostasis and resulting in respiratory alkalosis. Electrolyte salts such as sodium chloride, sodium bicarbonate, ammonium chloride, potassium chloride, and potassium sulfate can be used for the restoration of the acid–base concentration in the blood (250, 251) as they dissociate to release electrolytes (ions). For every 1°C rise in ambient temperature, there is a corresponding 1.4% decrease in average daily FI and a 2.1% decline in average daily weight gain (252), while the demand for water intake is increased. At 38°C, chickens consume four times more water than at 21°C (253). Drinking more water aids in cooling the body and enhances heat dissipation, helping to alleviate the symptoms of heat exhaustion, especially when electrolytes like Na+, K+, and Cl− salts are supplemented in the water (254). Electrolyte supplementation in poultry drinking water restores essential nutrients that balance blood pH levels (32).
Electrolytes are vital for poultry health, especially during HS, and it is advisable to maintain adequate DEB levels under such conditions (255). The effect of electrolyte supplementation is contingent upon the DEB. Research indicates that moderate DEB values, ranging from 120 to 240 mEq, is beneficial to the physiological responses of heat-stressed broiler chickens (140). Heat stress causes hyperventilation, which disrupts the blood’s acid–base equilibrium, leading to respiratory alkalosis that hinders broiler chickens’ growth and laying hens’ egg production and eggshell quality. Farfán et al. (256) found that broilers under HS maintained a balanced electrolyte level of 240 mEq when provided with mineral-enriched water. This supplementation of minerals is associated with a decrease in metabolic heat hyperventilation. Bryden et al. (257) revealed that laying hens under HS showed improvements in both egg production and quality when given 0.5% hydrochloric acid in their drinking water.
Sodium bicarbonate is commonly utilized at high temperatures (258), and adding this salt to the diets of heat-stressed broiler chickens enhanced their performance (259). Also, providing NaHCO3 to laying hens can enhance eggshell quality, provided the hens have access to feed during the eggshell formation phase, facilitated by continuous light exposure (136). Water intake during HS is crucial, and incorporating electrolytes into poultry diet or drinking water helps to enhance water consumption (144), which improves performance. Supplementing 1% NH4Cl or 0.5% NaHCO3 (260) and 1.5 to 2.0% K in the form of KCl (261) can partially alleviate growth suppression in broilers. Feeding chickens 1.5–2.0% K from KCl reduced FCR during chronic HS. Additionally, supplementing drinking water with 0.2% NH4Cl, 0.150% KCl, and carbonated water further boosted chicken performance (144).
4.6 In ovo feeding
Recently, manipulation during embryogenesis, including thermal conditioning and in ovo feeding has been explored to combat HS. In ovo feeding (IOF) entails injecting nutrients or bioactive compounds into fertilized eggs during mid or late embryogenesis (around day 12–18) to support the embryo and early chick development and improve physiological performance (262). The delivery route for the nutrients varies and is dependent on time (262). During the early incubation phase, the air sac is chosen as the injection site (263). In contrast, at later stages, the amnion becomes the preferred route for injection because developing chicks can ingest amniotic fluid by that time (264).
In ovo injection of sulfur amino acids, cysteine (3.4 mg) and methionine (5.9 mg), during a high-temperature incubation (39.6°C for 6 h daily) from embryonic day (ED) 10 to 18 significantly downregulated stress markers (HSP90 and corticosterone) and elevated antioxidant defenses (total antioxidant capacity, glutathione, and glutathione:oxidized glutathione) in tissues (265). In the same study, IOF increased the intestinal villus area in the broiler embryos (265). Similarly, IOF of vitamin C (3 mg/egg) into the yolk at ED 11 reduced embryonic mortality and improved hatchability under an acute HS 40°C challenge (266). Mechanistically, IOF primes the chick’s antioxidant and immune systems and stimulates gut development before hatch.
5 Future direction
While significant progress has been made in understanding the effects of HS on poultry gut health and performance, and the potential of nutritional supplements to alleviate these effects, several knowledge gaps remain. The optimal dosages and combinations of vitamins, minerals, amino acids, and electrolytes are yet to be proven. Further research is needed to determine the precise quantities required to achieve maximum thermotolerance and to explore the interactions between different supplements and their synergistic effects. Additionally, most studies focused on short-term outcomes, so the long-term effects of continuous supplementation on poultry health and productivity are poorly understood. Future research should address the sustainability of these interventions, particularly in large-scale commercial production systems, to assess their cost-effectiveness and long-term feasibility.
Another area for exploration is the role of gut microbiota in HS responses. While some studies have shown that HS negatively impacts gut integrity, more research is needed to understand the mechanism behind specific nutritional supplements influencing the gut microbiota to mitigate these effects. Addressing these gaps will improve poultry resilience to HS and enhance global poultry production and food security in the face of climate change.
6 Conclusion
Heat stress remains a critical challenge in poultry production, with significant implications for bird health, welfare, and productivity. As climate change intensifies, the poultry industry faces growing pressure to mitigate the adverse impacts of rising temperatures on poultry. The physiological responses to HS, including altered metabolism, reduced FI, impaired gut integrity, and oxidative stress, necessitate effective mitigation strategies. Nutritional supplementation has proven to be an efficient approach to enhancing poultry resilience against HS. Research demonstrates that vitamins, minerals, fat, amino acids, and electrolytes can mitigate some of the adverse effects of HS by improving growth, enhancing antioxidant defense, modulating immune responses, and maintaining gut health. Vitamins A and C, along with minerals such as selenium, zinc, and chromium, are particularly effective in reducing oxidative stress, supporting immune responses, and stabilizing metabolic functions. These supplements have proven to improve feed intake, weight gain, and egg production, while also mitigating the adverse effects on gut integrity under HS conditions. Fat and amino acid supplementation, especially in combination with other dietary interventions, has been identified as beneficial for improving nutrient digestibility and enhancing poultry thermotolerance. Furthermore, electrolyte supplementation supplies minerals and plays a critical role in restoring acid–base balance and supporting hydration during heat exposure.
While current studies provide valuable insights, further research is needed to optimize supplement combinations, determine optimal dosages, and assess long-term effects in commercial poultry production. Integrating targeted nutritional strategies with improved management practices can significantly enhance poultry performance, gut health, and adaptability to rising global temperatures, ensuring sustainable production and food security.
Author contributions
SO: Writing – review & editing, Conceptualization, Writing – original draft, Data curation. SA: Conceptualization, Supervision, Writing – review & editing, Funding acquisition.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that Gen AI was used in the creation of this manuscript. Generative AI was used to restructure some sentences and generate ideas for images.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Murphy, S, and Allen, L. Nutritional importance of animal source foods. J Nutr. (2003) 133:3932S-5S. doi: 10.1093/jn/133.11.3932S
2. Sulaiman, U, Vaughan, RS, Siegel, P, Liu, D, Gilbert, ER, and Cline, MA. Embryonic heat conditioning increases Lipolytic gene expression in broiler chicks at day 4 post-hatch. Front Physiol. (2024) 15:1445569. doi: 10.3389/fphys.2024.1445569
3. Abo-Al-Ela, HG, El-Kassas, S, El-Naggar, K, Abdo, SE, Jahejo, AR, and Al Wakeel, RA. Stress and immunity in poultry: light management and nanotechnology as effective immune enhancers to fight stress. Cell Stress Chaperones. (2021) 26:457–72. doi: 10.1007/s12192-021-01204-6
5. USDA USDoA, Economic Research Service). (2025). Poultry & Eggs - Sector at a Glance. Available online at: https://www.ers.usda.gov/topics/animal-products/poultry-eggs/sector-at-a-glance?utm_source=chatgpt.com (accessed October 5, 2025).
7. Mottet, A, and Tempio, G. Global poultry production: current state and future outlook and challenges. Worlds Poult Sci J. (2017) 73:245–56. doi: 10.1017/s0043933917000071
9. Birhanu, MY, Osei-Amponsah, R, Yeboah Obese, F, and Dessie, T. Smallholder poultry production in the context of increasing global food prices: roles in poverty reduction and food security. Anim Front. (2023) 13:17–25. doi: 10.1093/af/vfac069
11. Paswan, C, Bhattacharya, TK, Nagaraj, CS, Chatterjee, RN, and Guru Vishnu, P. Role and present status of biotechnology in augmenting poultry productivity in India. Proc Nat Acad Sci India Biol Sci. (2014) 84:855–63. doi: 10.1007/s40011-014-0306-y
12. Tetteh, H, Bala, A, Fullana-i-Palmer, P, Balcells, M, Margallo, M, Aldaco, R, et al. Carbon footprint: the case of four chicken meat products sold on the Spanish market. Foods. (2022) 11:3712. doi: 10.3390/foods11223712
13. Selye, H. Forty years of stress research: principal remaining problems and misconceptions. Can Med Assoc J. (1976) 114:53–6.
14. Virden, WS, and Kidd, MT. Physiological stress in broilers: ramifications on nutrient digestibility and responses. J Appl Poult Res. (2009) 18:338–47. doi: 10.3382/japr.2007-00093
15. Bureau, C, Hennequet-Antier, C, Couty, M, and Guemene, D. Gene Array analysis of adrenal glands in broiler chickens following Acth treatment. BMC Genomics. (2009) 10:430. doi: 10.1186/1471-2164-10-430
16. Surai, PF. Selenium in poultry nutrition and health. Leiden, The Netherlands: Wageningen Academic. (2023). doi: 10.3920/978-90-8686-865-0
17. Soliman, A, and Safwat, AM. Climate change impact on immune status and productivity of poultry as well as the quality of meat and egg products. Climate Change Impacts Agric Food Security Egypt. (2020) 22:481–98. doi: 10.1007/978-3-030-41629-4_20
18. Rimoldi, S, Lasagna, E, Sarti, FM, Marelli, SP, Cozzi, MC, Bernardini, G, et al. Expression profile of six stress-related genes and productive performances of fast and slow growing broiler strains reared under heat stress conditions. Meta Gene. (2015) 6:17–25. doi: 10.1016/j.mgene.2015.08.003
19. Sejian, V, Bhatta, R, Gaughan, JB, Dunshea, FR, and Lacetera, N. Review: adaptation of animals to heat stress. Animal. (2018) 12:s431–44. doi: 10.1017/S1751731118001945
20. Shini, S, Kaiser, P, Shini, A, and Bryden, WL. Biological response of chickens (Gallus gallus domesticus) induced by corticosterone and a bacterial endotoxin. Comp Biochem Physiol B Biochem Mol Biol. (2008) 149:324–33. doi: 10.1016/j.cbpb.2007.10.003
21. Nawab, A, Ibtisham, F, Li, G, Kieser, B, Wu, J, Liu, W, et al. Heat stress in poultry production: mitigation strategies to overcome the future challenges facing the global poultry industry. J Therm Biol. (2018) 78:131–9. doi: 10.1016/j.jtherbio.2018.08.010
22. Katarzyna, O, and Sembratowicz, I. Stress as a factor modifying the metabolism in poultry. A review. Ann UMCS Zootech. (2012) 30:104. doi: 10.2478/v10083-012-0010-4
23. Smith, SM, and Vale, WW. The role of the hypothalamic-pituitary-adrenal Axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. (2006) 8:383–95. doi: 10.31887/DCNS.2006.8.4/ssmith
24. Dickens, MJ, Delehanty, DJ, and Michael Romero, L. Stress: an inevitable component of animal translocation. Biol Conserv. (2010) 143:1329–41. doi: 10.1016/j.biocon.2010.02.032
25. Mormede, P, Andanson, S, Auperin, B, Beerda, B, Guemene, D, Malmkvist, J, et al. Exploration of the hypothalamic-pituitary-adrenal function as a tool to evaluate animal welfare. Physiol Behav. (2007) 92:317–39. doi: 10.1016/j.physbeh.2006.12.003
26. Romero, LM, Platts, SH, Schoech, SJ, Wada, H, Crespi, E, Martin, LB, et al. Understanding stress in the healthy animal - potential paths for Progress. Stress. (2015) 18:491–7. doi: 10.3109/10253890.2015.1073255
27. Burden, YO. The role of feed additives in mitigating the effect of stressors on growth, digestibility, Intestinal morphology, permeability, and immune response in poultry. Lexington: University of Kentucky (2020).
28. Lara, LJ, and Rostagno, MH. Impact of heat stress on poultry production. Animals. (2013) 3:356–69. doi: 10.3390/ani3020356
29. Wang, WC, Yan, FF, Hu, JY, Amen, OA, and Cheng, HW. Supplementation of Bacillus Subtilis-based probiotic reduces heat stress-related behaviors and inflammatory response in broiler chickens. J Anim Sci. (2018) 96:1654–66. doi: 10.1093/jas/sky092
30. Baumgard, LH, and Rhoads, RP Jr. Effects of heat stress on Postabsorptive metabolism and energetics. Annu Rev Anim Biosci. (2013) 1:311–37. doi: 10.1146/annurev-animal-031412-103644
32. Wasti, S, Sah, N, and Mishra, B. Impact of heat stress on poultry health and performances, and potential mitigation strategies. Animals. (2020) 10:266. doi: 10.3390/ani10081266
33. Brugaletta, G, Teyssier, JR, Rochell, SJ, Dridi, S, and Sirri, F. A review of heat stress in chickens. Part I: insights into physiology and gut health. Front Physiol. (2022) 13:934381. doi: 10.3389/fphys.2022.934381
34. Goel, A. Heat stress Management in Poultry. J Anim Physiol Anim Nutr. (2021) 105:1136–45. doi: 10.1111/jpn.13496
35. Vaughan, R, Sulaiman, U, Flynn, A, Biase, F, Meiri, N, Liu, D, et al. Embryonic thermal conditioning and post-hatch heat challenge Alter hypothalamic expression of genes related to appetite, thermoregulation, and stress modulation in broiler chicks. Front Physiol. (2025) 16:1583958. doi: 10.3389/fphys.2025.1583958
36. Xu, Y, Lai, X, Li, Z, Zhang, X, and Luo, Q. Effect of chronic heat stress on some physiological and immunological parameters in different breed of broilers. Poult Sci. (2018) 97:4073–82. doi: 10.3382/ps/pey256
37. Gogoi, S, Kolluri, G, Tyagi, JS, Marappan, G, Manickam, K, and Narayan, R. Impact of heat stress on broilers with varying body weights: elucidating their interactive role through physiological signatures. J Therm Biol. (2021) 97:102840. doi: 10.1016/j.jtherbio.2021.102840
38. Lotvedt, P, Fallahshahroudi, A, Bektic, L, Altimiras, J, and Jensen, P. Chicken domestication changes expression of stress-related genes in brain, pituitary and adrenals. Neurobiol Stress. (2017) 7:113–21. doi: 10.1016/j.ynstr.2017.08.002
39. Farag, MR, and Alagawany, M. Physiological alterations of poultry to the high environmental temperature. J Therm Biol. (2018) 76:101–6. doi: 10.1016/j.jtherbio.2018.07.012
40. Binsiya, TK, Veerasamy, S, Bagath, M, Krishnan, G, Iqbal, H, Manimaran, A, et al. Significance of hypothalamic-pituitary-adrenal Axis to adapt to climate change in livestock. Int Res J Agric Food Sci. (2017) 2:1–20.
41. Mack, LA, Felver-Gant, JN, Dennis, RL, and Cheng, HW. Genetic variations Alter production and behavioral responses following heat stress in 2 strains of laying hens. Poult Sci. (2013) 92:285–94. doi: 10.3382/ps.2012-02589
42. Elnagar, SA, Scheideler, SE, and Beck, MM. Reproductive hormones, hepatic deiodinase messenger ribonucleic acid, and vasoactive intestinal polypeptide-Immunoreactive cells in hypothalamus in the heat stress-induced or chemically induced hypothyroid laying hen. Poult Sci. (2010) 89:2001–9. doi: 10.3382/ps.2010-00728
43. Beckford, RC, Ellestad, LE, Proszkowiec-Weglarz, M, Farley, L, Brady, K, Angel, R, et al. Effects of heat stress on performance, blood chemistry, and hypothalamic and pituitary Mrna expression in broiler chickens. Poult Sci. (2020) 99:6317–25. doi: 10.1016/j.psj.2020.09.052
44. Rajaei-Sharifabadi, H, Greene, E, Piekarski, A, Falcon, D, Ellestad, L, Donoghue, A, et al. Surface wetting strategy prevents acute heat exposure-induced alterations of hypothalamic stress- and metabolic-related genes in broiler chickens. J Anim Sci. (2017) 95:1132–43. doi: 10.2527/jas.2016.1290
45. Bobek, S, Sechman, A, Wieczorek, E, Wronska-Fortuna, D, Koziec, K, and Niezgoda, J. Reverse 3,3′,5′-triiodothyronine (Rt3) enhances hyperglycemic and lipemic effects of heat-stress in chickens. Horm Metab Res. (1997) 29:252–4. doi: 10.1055/s-2007-979031
46. Gonzalez-Rivas, PA, Chauhan, SS, Ha, M, Fegan, N, Dunshea, FR, and Warner, RD. Effects of heat stress on animal physiology, metabolism, and meat quality: a review. Meat Sci. (2020) 162:108025. doi: 10.1016/j.meatsci.2019.108025
48. Geraert, PA, Padilha, JC, and Guillaumin, S. Metabolic and endocrine changes induced by chronic heat exposure in broiler chickens: biological and Endocrinological variables. Br J Nutr. (1996) 75:205–16. doi: 10.1079/bjn19960125
49. de Andrade, AN, Rogler, JC, Featherston, WR, and Alliston, CW. Interrelationships between diet and elevated temperatures (cyclic and constant) on egg production and shell quality1. Poult Sci. (1977) 56:1178–88. doi: 10.3382/ps.0561178
50. Sohail, MU, Hume, ME, Byrd, JA, Nisbet, DJ, Ijaz, A, Sohail, A, et al. Effect of supplementation of prebiotic Mannan-oligosaccharides and probiotic mixture on growth performance of broilers subjected to chronic heat stress. Poult Sci. (2012) 91:2235–40. doi: 10.3382/ps.2012-02182
51. Harsini, SG, Habibiyan, M, Moeini, MM, and Abdolmohammadi, AR. Effects of dietary selenium, vitamin E, and their combination on growth, serum metabolites, and antioxidant defense system in skeletal muscle of broilers under heat stress. Biol Trace Elem Res. (2012) 148:322–30. doi: 10.1007/s12011-012-9374-0
52. Deng, W, Dong, XF, Tong, JM, and Zhang, Q. The probiotic Bacillus Licheniformis ameliorates heat stress-induced impairment of egg production, gut morphology, and intestinal mucosal immunity in laying hens. Poult Sci. (2012) 91:575–82. doi: 10.3382/ps.2010-01293
53. Adefioye, RA. The impacts of gut-supporting feed additives on the growth performance, gut health, blood chemistry, and immune status of broiler chickens exposed to different stressors. Lexington: University of Kentucky (2024).
54. Rostagno, MH. Effects of heat stress on the gut health of poultry. J Anim Sci. (2020) 98:90. doi: 10.1093/jas/skaa090
55. Babinszky, L, Halas, V, and Wa, M. Impacts of climate change on animal production and quality of animal food products. Climate Change - Socioeconomic Effects. Intech Open. (2011) 165–190. doi: 10.5772/23840
56. Dai, SF, Gao, F, Xu, XL, Zhang, WH, Song, SX, and Zhou, GH. Effects of dietary glutamine and gamma-aminobutyric acid on meat colour, Ph, composition, and water-holding characteristic in broilers under cyclic heat stress. Br Poult Sci. (2012) 53:471–81. doi: 10.1080/00071668.2012.719148
57. Sokołowicz, Z, Krawczyk, J, and Świątkiewicz, S. 4. Quality of poultry meat from native chicken breeds – a review. Ann Anim Sci. (2016) 16:347–68. doi: 10.1515/aoas-2016-0004
58. Kim, H-W, Cramer, T, Ogbeifun, OOE, Seo, J-K, Yan, F, Cheng, H-W, et al. Breast meat quality and protein functionality of broilers with different probiotic levels and cyclic heat challenge exposure. Meat Muscle Biol. (2017) 1:2. doi: 10.22175/mmb2017.01.0002
59. Lu, Q, Wen, J, and Zhang, H. Effect of chronic heat exposure on fat deposition and meat quality in two genetic types of chicken. Poult Sci. (2007) 86:1059–64. doi: 10.1093/ps/86.6.1059
60. Imik, H, Atasever, MA, Urcar, S, Ozlu, H, Gumus, R, and Atasever, M. Meat quality of heat stress exposed broilers and effect of protein and vitamin E. Br Poult Sci. (2012) 53:689–98. doi: 10.1080/00071668.2012.736609
61. Zhang, ZY, Jia, GQ, Zuo, JJ, Zhang, Y, Lei, J, Ren, L, et al. Effects of constant and cyclic heat stress on muscle metabolism and meat quality of broiler breast fillet and thigh meat. Poult Sci. (2012) 91:2931–7. doi: 10.3382/ps.2012-02255
62. Nawaz, AH, Amoah, K, Leng, QY, Zheng, JH, Zhang, WL, and Zhang, L. Poultry response to heat stress: its physiological, metabolic, and genetic implications on meat production and quality including strategies to improve broiler production in a warming world. Front Vet Sci. (2021) 8:699081. doi: 10.3389/fvets.2021.699081
63. Tang, S, Yu, J, Zhang, M, and Bao, E. Effects of different heat stress periods on various blood and meat quality parameters in young arbor Acer broiler chickens. Can J Anim Sci. (2013) 93:453–60. doi: 10.4141/cjas2013-041
64. Wang, RH, Liang, RR, Lin, H, Zhu, LX, Zhang, YM, Mao, YW, et al. Effect of acute heat stress and slaughter processing on poultry meat quality and postmortem carbohydrate metabolism. Poult Sci. (2017) 96:738–46. doi: 10.3382/ps/pew329
65. Geraert, PA, Padilha, JC, and Guillaumin, S. Metabolic and endocrine changes induced by chronic heat exposure in broiler chickens: growth performance, body composition and energy retention. Br J Nutr. (1996) 75:195–204. doi: 10.1079/bjn19960124
66. De Souza, LFA, Espinha, LP, Almeida, EA, Lunedo, R, Furlan, RL, and Macari, M. How heat stress (continuous or cyclical) interferes with nutrient digestibility, energy and nitrogen balances and performance in broilers. Livest Sci. (2016) 192:39–43. doi: 10.1016/j.livsci.2016.08.014
67. Kim, DH, Lee, YK, Lee, SD, Kim, SH, Lee, SR, Lee, HG, et al. Changes in production parameters, egg qualities, fecal volatile fatty acids, nutrient digestibility, and plasma parameters in laying hens exposed to ambient temperature. Front Vet Sci. (2020) 7:412. doi: 10.3389/fvets.2020.00412
68. Orhan, C, Kucuk, O, Sahin, N, Tuzcu, M, and Sahin, K. Effects of taurine supplementation on productive performance, nutrient digestibility and gene expression of nutrient transporters in quails reared under heat stress. J Therm Biol. (2020) 92:102668. doi: 10.1016/j.jtherbio.2020.102668
69. Wallis, IR, and Balnave, D. The influence of environmental temperature, age and sex on the digestibility of amino acids in growing broiler chickens. Br Poult Sci. (1984) 25:401–7. doi: 10.1080/00071668408454880
70. Song, ZH, Cheng, K, Zheng, XC, Ahmad, H, Zhang, LL, and Wang, T. Effects of dietary supplementation with enzymatically treated Artemisia Annua on growth performance, intestinal morphology, digestive enzyme activities, immunity, and antioxidant capacity of heat-stressed broilers. Poult Sci. (2018) 97:430–7. doi: 10.3382/ps/pex312
71. Al-Zghoul, MB, Alliftawi, ARS, Saleh, KMM, and Jaradat, ZW. Expression of digestive enzyme and intestinal transporter genes during chronic heat stress in the thermally manipulated broiler chicken. Poult Sci. (2019) 98:4113–22. doi: 10.3382/ps/pez249
72. Sun, X, Zhang, H, Sheikhahmadi, A, Wang, Y, Jiao, H, Lin, H, et al. Effects of heat stress on the gene expression of nutrient transporters in the jejunum of broiler chickens (Gallus Gallus Domesticus). Int J Biometeorol. (2015) 59:127–35. doi: 10.1007/s00484-014-0829-1
73. Habashy, WS, Milfort, MC, Fuller, AL, Attia, YA, Rekaya, R, and Aggrey, SE. Effect of heat stress on protein utilization and nutrient transporters in meat-type chickens. Int J Biometeorol. (2017) 61:2111–8. doi: 10.1007/s00484-017-1414-1
74. Abdelli, N, Ramser, A, Greene, ES, Beer, L, Tabler, TW, Orlowski, SK, et al. Effects of cyclic chronic heat stress on the expression of nutrient transporters in the jejunum of modern broilers and their ancestor wild jungle fowl. Front Physiol. (2021) 12:733134. doi: 10.3389/fphys.2021.733134
75. Calefi, AS, Quinteiro-Filho, WM, Ferreira, AJP, and Palermo-Neto, J. Neuroimmunomodulation and heat stress in poultry. Worlds Poult Sci J. (2017) 73:493–504. doi: 10.1017/S0043933917000472
76. Kumar, M, Ratwan, P, Dahiya, SP, and Nehra, AK. Climate change and heat stress: impact on production, reproduction and growth performance of poultry and its mitigation using genetic strategies. J Therm Biol. (2021) 97:102867. doi: 10.1016/j.jtherbio.2021.102867
77. Cornescu, GM, Panaite, TD, Untea, AE, Varzaru, I, Saracila, M, Dumitru, M, et al. Mitigation of heat stress effects on laying hens' performances, egg quality, and some blood parameters by adding dietary zinc-enriched yeasts, parsley, and their combination. Front Vet Sci. (2023) 10:1202058. doi: 10.3389/fvets.2023.1202058
78. Rozenboim, I, Tako, E, Gal-Garber, O, Proudman, JA, and Uni, Z. The effect of heat stress on ovarian function of laying hens. Poult Sci. (2007) 86:1760–5. doi: 10.1093/ps/86.8.1760
79. Buranawit, K, Imboonta, N, Tongsiri, S, Masuda, Y, and Phakdeedindan, P. Investigation of the effect of heat stress on egg production traits in Thai native chickens (Lueng hang Kao Kabin) as determined by the temperature-humidity index. Poult Sci. (2025) 104:105196. doi: 10.1016/j.psj.2025.105196
80. Li, G-M, Liu, L-P, Yin, B, Liu, Y-Y, Dong, W-W, Gong, S, et al. Heat stress decreases egg production of laying hens by inducing apoptosis of follicular cells via activating the Fasl/Fas and Tnf-Α systems. Poult Sci. (2020) 99:6084–93. doi: 10.1016/j.psj.2020.07.024
81. Abbas, IM, Schwaar, T, Bienwald, F, and Weller, MG. Predictable peptide conjugation ratios by activation of proteins with Succinimidyl Iodoacetate (Sia). Methods Protoc. (2017) 1:1002. doi: 10.3390/mps1010002
82. Vercese, F, Garcia, EA, Sartori, JR, Silva, ADP, Faitarone, ABG, Berto, DA, et al. Performance and egg quality of Japanese quail submitted to cyclic heat stress. Braz J Poult Sci. (2012) 14:37–41. doi: 10.1590/S1516-635X2012000100007
83. Kilic, I, and Simsek, E. The effects of heat stress on egg production and quality of laying hens. J Anim Vet Adv. (2013) 12:42–7. doi: 10.3923/javaa.2013.42.47
84. Tao, X, and Xin, H. Acute synergistic effects of air temperature, humidity, and velocity on homeostasis of market–size broilers. Trans ASAE. (2003) 46:491
85. Zulovich, J, and DeShazer, J. Estimating egg production declines at high environmental temperatures and Humidities. Paper No. 90-4021 (1990) 15.
86. Kim, H-R, Ryu, C, Lee, S-D, Cho, J-H, and Kang, H. Effects of heat stress on the laying performance, egg quality, and physiological response of laying hens. Animals. (2024) 14:1076. doi: 10.3390/ani14071076
87. Bonato, M, Malecki, IA, Rybnik-Trzaskowska, PK, Cornwallis, CK, and Cloete, SW. Predicting ejaculate quality and libido in male ostriches: effect of season and age. Anim Reprod Sci. (2014) 151:49–55. doi: 10.1016/j.anireprosci.2014.09.015
88. Riaz, A, Aleem, M, Ijaz, A, Saeed, MA, and Latif, A. Effect of collection frequency on the semen quality of broiler breeder. Br Poult Sci. (2004) 45:823–7. doi: 10.1080/00071660400012691
89. Banks, S, King, SA, Irvine, DS, and Saunders, PT. Impact of a mild scrotal heat stress on DNA integrity in murine spermatozoa. Reproduction. (2005) 129:505–14. doi: 10.1530/rep.1.00531
90. Igbokwe, NA. Effects of environmental heat stress on reproduction and its management in chickens. Niger Vet J. (2018) 39:101. doi: 10.4314/nvj.v39i2.2
91. Fouad, AM, Chen, W, Ruan, D, Wang, S, Xia, WG, and Zheng, CT. Impact of heat stress on meat, egg quality, immunity and fertility in poultry and nutritional factors that overcome these effects: a review. Int J Poult Sci. (2016) 15:81–95. doi: 10.3923/ijps.2016.81.95
92. Turk, G, Ceribasi, AO, Simsek, UG, Ceribasi, S, Guvenc, M, Ozer Kaya, S, et al. Dietary rosemary oil alleviates heat stress-induced structural and functional damage through lipid peroxidation in the testes of growing Japanese quail. Anim Reprod Sci. (2016) 164:133–43. doi: 10.1016/j.anireprosci.2015.11.021
93. Khan, RU, Rahman, ZU, Nikousefat, Z, Javdani, M, Tufarelli, V, Dario, C, et al. Immunomodulating effects of vitamin E in broilers. Worlds Poult Sci J. (2012) 68:31–40. doi: 10.1017/s0043933912000049
94. Akhigbe, RE, Oyedokun, PA, Akhigbe, TM, Hamed, MA, Fidelis, FB, Omole, AI, et al. The consequences of climate change and male reproductive health: a review of the possible impact and mechanisms. Biochem Biophys Rep. (2025) 41:101889. doi: 10.1016/j.bbrep.2024.101889
95. Ko, SH. Effects of heat stress-induced sex hormone dysregulation on reproduction and growth in male adolescents and beneficial foods. Nutrients. (2024) 16:3032. doi: 10.3390/nu16173032
96. Ameen, SA, Opayemi, OS, Ajayi, JA, and Adediwura, MA. Evaluation of semen quality of five different cockerel breed used in poultry industry in Nigeria. J Environ Issues Agric Dev Countries. (2014) 6:30–6.
97. McDaniel, CD, Bramwell, RK, and Howarth, B Jr. The male contribution to broiler breeder heat-induced infertility as determined by sperm-egg penetration and sperm storage within the hen's oviduct. Poult Sci. (1996) 75:1546–54. doi: 10.3382/ps.0751546
98. Obidi, JA, Onyeanusi, BI, Ayo, JO, Rekwot, PI, and Abdullahi, SJ. Effect of timing of artificial insemination on fertility and hatchability of Shikabrown breeder hens. Int J Poult Sci. (2008) 7:1224–6. doi: 10.3923/ijps.2008.1224.1226
99. Oguntunji, AO, and Alabi, OM. Influence of high environmental temperature on egg production and shell quality: a review. Worlds Poult Sci J. (2010) 66:739–50. doi: 10.1017/s004393391000070x
100. Ebeid, TA, Suzuki, T, and Sugiyama, T. High ambient temperature influences eggshell quality and Calbindin-D28k localization of eggshell gland and all intestinal segments of laying hens. Poult Sci. (2012) 91:2282–7. doi: 10.3382/ps.2011-01898
101. Ayo, JO, Obidi, JA, and Rekwot, PI. Effects of heat stress on the well-being, fertility, and hatchability of chickens in the northern Guinea Savannah zone of Nigeria: a review. ISRN Vet Sci. (2011) 2011:838606. doi: 10.5402/2011/838606
102. Lin, H, Jiao, HC, Buyse, J, and Decuypere, E. Strategies for preventing heat stress in poultry. Worlds Poult Sci J. (2019) 62:71–86. doi: 10.1079/wps200585
103. Yousaf, A, Jabbar, A, and Ditta, YA. Effect of pre-warming on broiler breeder eggs hatchability and post-hatch performance. J Anim Health Prod. (2017) 5:1–4. doi: 10.14737/journal.jahp/2017/5.1.1.4
104. Abidin, Z, and Khatoon, A. Heat stress in poultry and the beneficial effects of ascorbic acid (vitamin C) supplementation during periods of heat stress. Worlds Poult Sci J. (2013) 69:135–52. doi: 10.1017/s0043933913000123
105. Kala, M, Shaikh, MV, and Nivsarkar, M. Equilibrium between anti-oxidants and reactive oxygen species: a requisite for oocyte development and maturation. Reprod Med Biol. (2017) 16:28–35. doi: 10.1002/rmb2.12013
106. Sahin, K, Sahin, N, Kucuk, O, Hayirli, A, and Prasad, AS. Role of dietary zinc in heat-stressed poultry: a review. Poult Sci. (2009) 88:2176–83. doi: 10.3382/ps.2008-00560
107. Ma, X, Lin, Y, Zhang, H, Chen, W, Wang, S, Ruan, D, et al. Heat stress impairs the nutritional metabolism and reduces the productivity of egg-laying ducks. Anim Reprod Sci. (2014) 145:182–90. doi: 10.1016/j.anireprosci.2014.01.002
108. Cheng, CY, Tu, WL, Wang, SH, Tang, PC, Chen, CF, Chen, HH, et al. Annotation of differential gene expression in small yellow follicles of a broiler-type strain of Taiwan country chickens in response to acute heat stress. PLoS One. (2015) 10:e0143418. doi: 10.1371/journal.pone.0143418
109. Alagawany, M, Farag, MR, Abd El-Hack, ME, and Patra, A. Heat stress: effects on productive and reproductive performance of quail. Worlds Poult Sci J. (2017) 73:747–56. doi: 10.1017/s0043933917000782
110. Jahejo, AR, Sethar, A, Rao, M, Nisa, M, and Sethar, G. Effect of heat stress and ascorbic acid on gut morphology of broiler chicken. Sindh Univ Res J. (2016) 48:829–83.
111. Tuohy, KM, Probert, HM, Smejkal, CW, and Gibson, GR. Using probiotics and prebiotics to improve gut health. Drug Discov Today. (2003) 8:692–700. doi: 10.1016/s1359-6446(03)02746-6
112. Zoetendal, EG, Collier, CT, Koike, S, Mackie, RI, and Gaskins, HR. Molecular ecological analysis of the gastrointestinal microbiota: a review. J Nutr. (2004) 134:465–72. doi: 10.1093/jn/134.2.465
113. Kiela, PR, and Ghishan, FK. Physiology of intestinal absorption and secretion. Best Pract Res Clin Gastroenterol. (2016) 30:145–59. doi: 10.1016/j.bpg.2016.02.007
114. Goulet, O. Potential role of the intestinal microbiota in programming health and disease. Nutr Rev. (2015) 73:32–40. doi: 10.1093/nutrit/nuv039
115. Kamada, N, Seo, SU, Chen, GY, and Nunez, G. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol. (2013) 13:321–35. doi: 10.1038/nri3430
116. Chen, Y, Zhang, HS, Fong, GH, Xi, QL, Wu, GH, Bai, CG, et al. Phd3 stabilizes the tight junction protein Occludin and protects intestinal epithelial barrier function. J Biol Chem. (2015) 290:20580–9. doi: 10.1074/jbc.M115.653584
117. Hu, JY, Mohammed, AA, Murugesan, GR, and Cheng, HW. Effect of a Synbiotic supplement as an antibiotic alternative on broiler skeletal, physiological, and oxidative parameters under heat stress. Poult Sci. (2022) 101:101769. doi: 10.1016/j.psj.2022.101769
118. Salvo Romero, E, Alonso Cotoner, C, Pardo Camacho, C, Casado Bedmar, M, and Vicario, M. The intestinal barrier function and its involvement in digestive disease. Rev Esp Enferm Dig. (2015) 107:686–96. doi: 10.17235/reed.2015.3846/2015
119. Tellez, JG, Tellez-Isaias, G, and Dridi, S. Heat stress and gut health in broilers: role of tight junction proteins. Adv Food Technol Nutri Sci. (2017) 3:e1-e4. doi: 10.17140/AFTNSOJ-3-e010
120. Siddiqui, MR, Mayanil, CS, Kim, KS, and Tomita, T. Angiopoietin-1 regulates brain endothelial permeability through Ptpn-2 mediated tyrosine Dephosphorylation of Occludin. PLoS One. (2015) 10:e0130857. doi: 10.1371/journal.pone.0130857
121. Abdelqader, A, and Al-Fataftah, A-R. Effect of dietary butyric acid on performance, intestinal morphology, microflora composition and intestinal recovery of heat-stressed broilers. Livest Sci. (2016) 183:78–83. doi: 10.1016/j.livsci.2015.11.026
122. Burkholder, KM, Thompson, KL, Einstein, ME, Applegate, TJ, and Patterson, JA. Influence of stressors on Normal intestinal microbiota, intestinal morphology, and susceptibility to Salmonella Enteritidis colonization in broilers. Poult Sci. (2008) 87:1734–41. doi: 10.3382/ps.2008-00107
123. Cheng, HH, Kaiser, P, and Lamont, SJ. Integrated genomic approaches to enhance genetic resistance in chickens. Annu Rev Anim Biosci. (2013) 1:239–60. doi: 10.1146/annurev-animal-031412-103701
124. Gu, XH, Hao, Y, and Wang, XL. Overexpression of heat shock protein 70 and its relationship to intestine under acute heat stress in broilers: 2. Intestinal oxidative stress. Poult Sci. (2012) 91:790–9. doi: 10.3382/ps.2011-01628
125. Santos, RR, Awati, A, Roubos-van den Hil, PJ, Tersteeg-Zijderveld, MH, and Koolmees, PA. Fink-Gremmels J. Quantitative Histo-morphometric analysis of heat-stress-related damage in the small intestines of broiler chickens. Avian Pathol. (2015) 44:19–22. doi: 10.1080/03079457.2014.988122
126. Varasteh, S, Braber, S, Akbari, P, Garssen, J, and Fink-Gremmels, J. Differences in susceptibility to heat stress along the chicken intestine and the protective effects of galacto-oligosaccharides. PLoS One. (2015) 10:e0138975. doi: 10.1371/journal.pone.0138975
127. Liu, L, Fu, C, Yan, M, Xie, H, Li, S, Yu, Q, et al. Resveratrol modulates intestinal morphology and Hsp70/90, Nf-Kappab and Egf expression in the jejunal mucosa of black-boned chickens on exposure to circular heat stress. Food Funct. (2016) 7:1329–38. doi: 10.1039/c5fo01338k
128. Sharma, P. Repercussions and mitigation of heat stress in poultry: a review. J Vet Med Anim Sci. (2021) 4:1073
129. Borges, SA, Fischer Da Silva, AV, and Maiorka, A. Acid-Base balance in broilers. Worlds Poult Sci J. (2007):63. doi: 10.1079/WPS2005128
130. Patel, S, and Sharma, S. Respiratory acidosis. Treasure Island, FL: StatPearls Publishing (2023).
131. Tinawi, M. Pathophysiology, evaluation, and Management of Metabolic Alkalosis. Cureus. (2021) 13:e12841. doi: 10.7759/cureus.12841
132. Allahverdi, A, Feizi, A, Takhtfooladi, HA, and Nikpiran, H. Effects of heat stress on Acid-Base imbalance, plasma calcium concentration, egg production and egg quality in commercial layers. Global Veterinaria. (2013) 10:203–7. doi: 10.5829/idosi.gv.2013.10.2.7286
133. Odom, TW, Harrison, PC, and Bottje, WG. Effects of thermal-induced respiratory alkalosis on blood ionized calcium levels in the domestic hen. Poult Sci. (1986) 65:570–3. doi: 10.3382/ps.0650570
134. Renaudeau, D, Collin, A, Yahav, S, de Basilio, V, Gourdine, JL, and Collier, RJ. Adaptation to hot climate and strategies to alleviate heat stress in livestock production. Animal. (2012) 6:707–28. doi: 10.1017/S1751731111002448
135. Frank, FR, and Burger, RE. The effect of carbon dioxide inhalation and sodium bicarbonate ingestion on egg shell deposition. Poult Sci. (1965) 44:1604–6. doi: 10.3382/ps.0441604
136. Balnave, D, and Muheereza, SK. Improving eggshell quality at high temperatures with dietary sodium bicarbonate. Poult Sci. (1997) 76:588–93. doi: 10.1093/ps/76.4.588
137. Miller, PC, and Sunde, ML. The effects of precise constant and cyclic environments on shell quality and other lay performance factors with Leghorn pullets. Poult Sci. (1975) 54:36–46. doi: 10.3382/ps.0540036
138. Gezen, SS, Eren, M, and Deniz, G. The effect of different dietary electrolyte balances on eggshell quality in laying hens. Rev Med Vet. (2005) 156:491–7.
139. Mongin, P. Recent advances in dietary anion-cation balance: applications in poultry. Proc Nutr Soc. (1981) 40:285–94. doi: 10.1079/pns19810045
140. Borges, SA, Fischer da Silva, AV, Majorka, A, Hooge, DM, and Cummings, KR. Physiological responses of broiler chickens to heat stress and dietary electrolyte balance (sodium plus potassium minus chloride, Milliequivalents per kilogram). Poult Sci. (2004) 83:1551–8. doi: 10.1093/ps/83.9.1551
141. Koelkebeck, KW, and Odom, TW. Laying hen responses to acute heat stress and carbon dioxide supplementation: ii. Changes in plasma enzymes, metabolites and electrolytes. Comp Biochem Physiol. (1995) 112A:119–22.
142. Hosseini-Vashan, SJ, Golian, A, and Yaghobfar, A. Growth, immune, antioxidant, and bone responses of heat stress-exposed broilers fed diets supplemented with tomato pomace. Int J Biometeorol. (2015) 60:1183–92. doi: 10.1007/s00484-015-1112-9
143. Quinteiro-Filho, WM, Rodrigues, MV, Ribeiro, A, Ferraz-De-Paula, V, Pinheiro, ML, Sa, LR, et al. Acute heat stress impairs performance parameters and induces mild intestinal enteritis in broiler chickens: role of acute hypothalamic-pituitary-adrenal axis activation. J Anim Sci. (2012) 90:1986–94. doi: 10.2527/jas2011-3949
144. Lin, H, Decuypere, E, and Buyse, J. Acute heat stress induces oxidative stress in broiler chickens. Comp Biochem Physiol A Mol Integr Physiol. (2006) 144:11–7. doi: 10.1016/j.cbpa.2006.01.032
145. Mujahid, A. Nutritional Strategies to Maintain Efficiency and Production of Chickens under High Environmental Temperature. J Poult Sci. (2011) 48:145–54. doi: 10.2141/jpsa.010115
146. Rhoads, RP, Baumgard, LH, Suagee, JK, and Sanders, SR. Nutritional interventions to alleviate the negative consequences of heat stress. Adv Nutr. (2013) 4:267–76. doi: 10.3945/an.112.003376
147. Abd El-Gawad, AH, Hemid, AEA, and El-Wardany, I. Alleviating the effect of some environmental stress factors on productive performance in Japanese quail 1. Growth performance. World J Agric Sci. (2008) 4:605–11.
148. Vakili, R, and Rashidi, AA. The effects of dietary fat, vitamin E and zinc supplementation on fatty acid composition and oxidative stability of muscle thigh in broilers under heat stress. Afr J Agric Res. (2011) 6:2800–6. doi: 10.5897/AJAR11.118
149. Sahin, K, and Kucuk, O. Zinc supplementation alleviates heat stress in laying Japanese quail. J Nutr. (2003) 133:2808–11. doi: 10.1093/jn/133.9.2808
150. Chung, MK, Choi, JH, Chung, YK, and Chee, KM. Effects of dietary vitamins C and E on egg shell quality of broiler breeder hens exposed to heat stress. Asian Australas J Anim Sci. (2005) 18:545–51. doi: 10.5713/ajas.2005.545
151. Abdel-Moneim, AE, Shehata, AM, Khidr, RE, Paswan, VK, Ibrahim, NS, El-Ghoul, AA, et al. Nutritional manipulation to combat heat stress in poultry - a comprehensive review. J Therm Biol. (2021) 98:102915. doi: 10.1016/j.jtherbio.2021.102915
152. Pardue, SL, and Thaxton, JP. Ascorbic acid in poultry: a review. Worlds Poult Sci J. (1986) 42:107–23. doi: 10.1079/wps19860009
153. Tumova, E, and Gous, RM. Interaction of hen production type, age, and temperature on laying pattern and egg quality. Poult Sci. (2012) 91:1269–75. doi: 10.3382/ps.2011-01951
154. Panda, AK, Ramarao, SV, Raju, MV, and Chatterjee, RN. Effect of dietary supplementation with vitamins E and C on production performance, immune responses and antioxidant status of white leghorn layers under tropical summer conditions. Br Poult Sci. (2008) 49:592–9. doi: 10.1080/00071660802337233
155. Asensio, X, Abdelli, N, Piedrafita, J, Soler, MD, and Barroeta, AC. Effect of fibrous diet and vitamin C inclusion on uniformity, carcass traits, skeletal strength, and behavior of broiler breeder pullets. Poult Sci. (2020) 99:2633–44. doi: 10.1016/j.psj.2020.01.015
156. Skřivan, M, Marounek, M, Englmaierová, M, and Skřivanová, V. Influence of dietary vitamin C and selenium, alone and in combination, on the performance of laying hens and quality of eggs. Czeh J Anim Sci. (2013) 58:91–7. doi: 10.17221/6619-cjas
157. Attia, YA, Abd El-Hamid Ael, H, Abedalla, AA, Berika, MA, Al-Harthi, MA, Kucuk, O, et al. Laying performance, digestibility and plasma hormones in laying hens exposed to chronic heat stress as affected by betaine. Vitamin C Vitamin E Suppl. (2016) 5:1619. doi: 10.1186/s40064-016-3304-0
159. Ahmed, W, Ahmad, S, and Kamran, Z. Response of laying hens to vitamin C supplementation through drinking water under sub-tropical conditions. Avian Biol Res. (2008) 1:59–63. doi: 10.3184/175815508x360461
160. Abd El-Hack, M, Ashour, E, Elaraby, G, Osman, A, and Arif, M. Influences of dietary supplementation of Peanut skin powder (Arachis Hypogaea) on growth performance, carcass traits, blood chemistry, antioxidant activity and meat quality of broilers. Anim Prod Sci. (2018) 58:965–72. doi: 10.1071/AN16346
161. Ebeid, TA. Vitamin E and organic selenium enhances the Antioxidative status and quality of chicken semen under high ambient temperature. Br Poult Sci. (2012) 53:708–14. doi: 10.1080/00071668.2012.722192
162. Surai, PF. Natural antioxidants in avian nutrition and reproduction. Nottingham: Nottingham University Press (2002).
163. Pamplona, R, and Costantini, D. Molecular and structural antioxidant defenses against oxidative stress in animals. Am J Physiol Regul Integr Comp Physiol. (2011) 301:R843–63. doi: 10.1152/ajpregu.00034.2011
164. Shakeri, M, Oskoueian, E, Le, HH, and Shakeri, M. Strategies to combat heat stress in broiler chickens: unveiling the roles of selenium, vitamin E and vitamin C. Vet Sci. (2020) 7:71. doi: 10.3390/vetsci7020071
165. Sahin, K, Onderci, M, Sahin, N, Gursu, MF, Vijaya, J, and Kucuk, O. Effects of dietary combination of chromium and biotin on egg production, serum metabolites, and egg yolk mineral and cholesterol concentrations in heat-distressed laying quails. Biol Trace Elem Res. (2004) 101:181–92. doi: 10.1385/BTER:101:2:181
166. Saeed, M, Abbas, G, Alagawany, M, Kamboh, AA, Abd El-Hack, ME, Khafaga, AF, et al. Heat stress Management in Poultry Farms: a comprehensive overview. J Therm Biol. (2019) 84:414–25. doi: 10.1016/j.jtherbio.2019.07.025
167. Bergin, P, Leggett, A, Cardwell, CR, Woodside, JV, Thakkinstian, A, Maxwell, AP, et al. The effects of vitamin E supplementation on malondialdehyde as a biomarker of oxidative stress in Haemodialysis patients: a systematic review and Meta-analysis. BMC Nephrol. (2021) 22:126. doi: 10.1186/s12882-021-02328-8
168. Kumbhar, S, Khan, AZ, Parveen, F, Nizamani, ZA, Siyal, FA, El-Hack, MEA, et al. Impacts of selenium and vitamin E supplementation on Mrna of heat shock proteins, Selenoproteins and antioxidants in broilers exposed to high temperature. AMB Express. (2018) 8:112. doi: 10.1186/s13568-018-0641-0
169. Sahin, K, and Kucuk, O. Heat stress and dietary vitamin supplementation of poultry diets. CABI Rev. (2003) 73, 41–50. doi: 10.1079/cabireviews20033127283
170. Attia, YA, Al-Harthi, MA, El-Shafey, AS, Rehab, YA, and Kim, WK. Enhancing tolerance of broiler chickens to heat stress by supplementation with vitamin E, vitamin C and/or probiotics. Ann Anim Sci. (2017) 17:1155–69. doi: 10.1515/aoas-2017-0012
171. McKee, JS, Harrison, PC, and Riskowski, GL. Effects of supplemental ascorbic acid on the energy conversion of broiler chicks during heat stress and feed withdrawal. Poult Sci. (1997) 22:761278–86.
172. Niu, ZY, Liu, FZ, Yan, QL, and Li, WC. Effects of different levels of vitamin E on growth performance and immune responses of broilers under heat stress. Poult Sci. (2009) 88:2101–7. doi: 10.3382/ps.2009-00220
173. Sahin, K, and Kucuk, O. Effects of vitamin E and selenium on performance, digestibility of nutrients, and carcass characteristics of Japanese quails reared under heat stress (34°C). J Anim Physiol Anim Nutr. (2001) 85:342–8. doi: 10.1046/j.1439-0396.2001.00340.x
174. Oluwagbenga, EM, and Fraley, GS. Heat stress and poultry production: a comprehensive review. Poult Sci. (2023) 102:103141. doi: 10.1016/j.psj.2023.103141
175. Calik, A, Emami, NK, White, MB, Walsh, MC, Romero, LF, and Dalloul, RA. Influence of dietary vitamin E and selenium supplementation on broilers subjected to heat stress, part I: growth performance, body composition and intestinal nutrient transporters. Poult Sci. (2022) 101:101857. doi: 10.1016/j.psj.2022.101857
176. Marques, MFG, Oliveira, RFM, Donzele, JL, Albino, LFT, Tizziani, T, Faria, LF, et al. 25-hydroxycholecalciferol as an alternative to vitamin D3 in diets with different levels of calcium for broilers reared under heat stress. Braz J Poult Sci. (2022) 24, 1–10. doi: 10.1590/1806-9061-2021-1559
177. Mosleh, N, Shomali, T, Nematollahi, F, Ghahramani, Z, Ahrari Khafi, MS, and Namazi, F. Effect of different periods of chronic heat stress with or without vitamin C supplementation on bone and selected serum parameters of broiler chickens. Avian Pathol. (2017) 47:197–205. doi: 10.1080/03079457.2017.1401212
178. Sahin, N, Balci, TA, Kucuk, O, Smith, MO, and Sahin, K. Effects of 25-hydroxycholecalciferol and soy Isoflavones supplementation on bone mineralisation of quail. Br Poult Sci. (2009) 50:709–15. doi: 10.1080/00071660903261944
179. Mir, SH, Pal, RP, Mani, V, Malik, TA, Ojha, L, and Yadav, S. Role of dietary minerals in heat-stressed poultry: a review. J Entomol Zool Stu. (2018) 6:820–6.
180. Adedokun, SA, and Olojede, OC. Optimizing gastrointestinal integrity in poultry: the role of nutrients and feed additives. Front Vet Sci. (2019) 5:348. doi: 10.3389/fvets.2018.00348
181. Onagbesan, OM, Uyanga, VA, Oso, O, Tona, K, and Oke, OE. Alleviating heat stress effects in poultry: updates on methods and mechanisms of actions. Front Vet Sci. (2023) 10:1255520. doi: 10.3389/fvets.2023.1255520
182. Prasad, AS, and Kucuk, O. Zinc in Cancer prevention. Cancer Metastasis Rev. (2002) 21:291–5. doi: 10.1023/A:1021215111729
183. Yan, M, Song, Y, Wong, CP, Hardin, K, and Ho, E. Zinc deficiency alters DNA damage response genes in Normal human prostate epithelial cells. J Nutr. (2008) 138:667–73. doi: 10.1093/jn/138.4.667
184. Stefanello, C, Santos, TC, Murakami, AE, Martins, EN, and Carneiro, TC. Productive performance, eggshell quality, and eggshell ultrastructure of laying hens fed diets supplemented with organic trace minerals. Poult Sci. (2014) 93:104–13. doi: 10.3382/ps.2013-03190
185. Jarosz, M, Olbert, M, Wyszogrodzka, G, Mlyniec, K, and Librowski, T. Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent Nf-kappab signaling. Inflammopharmacology. (2017) 25:11–24. doi: 10.1007/s10787-017-0309-4
186. Huang, L, Li, X, Wang, W, Yang, L, and Zhu, Y. The role of zinc in poultry breeder and hen nutrition: An update. Biol Trace Elem Res. (2019) 192:308–18. doi: 10.1007/s12011-019-1659-0
187. Zhu, Y, Liao, X, Lu, L, Li, W, Zhang, L, Ji, C, et al. Maternal dietary zinc supplementation enhances the epigenetic-activated antioxidant ability of Chick embryos from maternal Normal and high temperatures. Oncotarget. (2017) 8:19814–24. doi: 10.18632/oncotarget.15057
188. Liao, X, Li, W, Zhu, Y, Zhang, L, Lu, L, Lin, X, et al. Effects of environmental temperature and dietary zinc on egg production performance, egg quality and antioxidant status and expression of heat-shock proteins in tissues of broiler breeders. Br J Nutr. (2018) 120:3–12. doi: 10.1017/S0007114518001368
189. Bozakova, N. Influence of dietary zinc supplementation on Turkey welfare during the hot summer period. I. Behavioural aspects. Bulgarian J Ecol Sci. (2010) 9:20–6.
190. Bozakova, NA, Atanassov, AP, Sotirov, LK, Stoyanchev, KT, Yotova, IT, Uzunova, K, et al. Effect of dietary Zn supplement during the hot summer period on some productive traits of Turkey breeders. Bulgarian J Vet Med. (2011) 14:9–17.
191. Long, L, Zhao, X, Li, H, Yan, X, and Zhang, H. Effects of zinc lactate supplementation on growth performance, intestinal morphology, serum parameters, and hepatic Metallothionein of Chinese yellow-feathered broilers. Biol Trace Elem Res. (2022) 200:1835–43. doi: 10.1007/s12011-021-02785-0
192. Zhang, Y, Xie, L, Ding, X, Wang, Y, Xu, Y, Li, D, et al. Mechanisms underlying the protective effect of maternal zinc (Znso(4) or Zn-Gly) against heat stress-induced oxidative stress in chicken embryo. Antioxidants. (2022) 11:699. doi: 10.3390/antiox11091699
193. Li, T, He, W, Liao, X, Lin, X, Zhang, L, Lu, L, et al. Zinc alleviates the heat stress of primary cultured hepatocytes of broiler embryos via enhancing the antioxidant ability and attenuating the heat shock responses. Anim Nutr. (2021) 7:621–30. doi: 10.1016/j.aninu.2021.01.003
194. Rao, SVR, Prakash, B, Raju, M, Panda, AK, Kumari, RK, and Reddy, EPK. Effect of supplementing organic forms of zinc, selenium and chromium on performance, anti-oxidant and immune responses in broiler chicken reared in tropical summer. Biol Trace Elem Res. (2016) 172:511–20. doi: 10.1007/s12011-015-0587-x
195. Salgueiro, MJ, Zubillaga, M, Lysionek, A, Sarabia, MI, Caro, R, De Paoli, T, et al. Zinc as an essential micronutrient: a review. Nutr Res. (2000) 20:737–55. doi: 10.1016/S0271-5317(00)00163-9
196. Burke, JP, and Fenton, MR. Effect of a zinc-deficient diet on lipid peroxidation in liver and tumor subcellular membranes. Proc Soc Exp Biol Med. (1985) 179:187–91. doi: 10.3181/00379727-179-42083
197. Gibbs, PN, Gore, MG, and Jordan, PM. Investigation of the effect of metal ions on the reactivity of thiol groups in human 5-aminolaevulinate dehydratase. Biochem J. (1985) 225:573–80. doi: 10.1042/Bj2250573
198. Tate, DJ, Miceli, MV, and Newsome, DA. Zinc protects against oxidative damage in cultured human retinal pigment epithelial cells. Free Radic Biol Med. (1999) 26:704–13. doi: 10.1016/S0891-5849(98)00253-6
199. Naziroglu, M, Yildiz, K, Tamturk, B, Erturan, I, and Flores-Arce, M. Selenium and psoriasis. Biol Trace Elem Res. (2012) 150:3–9. doi: 10.1007/s12011-012-9479-5
200. Zhou, J, Huang, K, and Lei, XG. Selenium and diabetes--evidence from animal studies. Free Radic Biol Med. (2013) 65:1548–56. doi: 10.1016/j.freeradbiomed.2013.07.012
201. Yang, YR. Effect of organic and inorganic selenium supplementation on growth performance, meat quality and antioxidant property of broilers. Afr J Biotechnol. (2012) 11:3382. doi: 10.5897/ajb11.3382
202. Surai, PF, and Fisinin, VI. Selenium in poultry breeder nutrition: an update. Anim Feed Sci Technol. (2014) 191:1–15. doi: 10.1016/j.anifeedsci.2014.02.005
203. Kim, JH, and Kil, DY. Comparison of toxic effects of dietary organic or inorganic selenium and prediction of selenium intake and tissue selenium concentrations in broiler chickens using feather selenium concentrations. Poult Sci. (2020) 99:6462–73. doi: 10.1016/j.psj.2020.08.061
204. Xu, D, Li, W, Huang, Y, He, J, and Tian, Y. The effect of selenium and polysaccharide of Atractylodes Macrocephala Koidz. (Pamk) on immune response in chicken spleen under heat stress. Biol Trace Elem Res. (2014) 160:232–7. doi: 10.1007/s12011-014-0056-y
205. Yin, Y, Guo, J, Liu, Z, Xu, S, and Zheng, S. Selenium deficiency aggravates heat stress pneumonia in chickens by disrupting the M1/M2 balance. Biol Trace Elem Res. (2022) 200:3315–25. doi: 10.1007/s12011-021-02905-w
206. Habibian, M, Ghazi, S, Moeini, MM, and Abdolmohammadi, A. Effects of dietary selenium and vitamin E on immune response and biological blood parameters of broilers reared under Thermoneutral or heat stress conditions. Int J Biometeorol. (2014) 58:741–52. doi: 10.1007/s00484-013-0654-y
207. Habibian, M, Sadeghi, A, Ghazi Harsini, S, and Moeini, M. Selenium as a feed supplement for heat-stressed poultry: a review. Biol Trace Elem Res. (2015) 165:275. doi: 10.1007/s12011-015-0275-x
208. Sahin, N, Onderci, M, Sahin, K, and Kucuk, O. Supplementation with organic or inorganic selenium in heat-distressed quail. Biol Trace Elem Res. (2008) 122:229–37. doi: 10.1007/s12011-007-8075-6
209. Rahimi, S, Farhadi, D, and Valipouri, AR. Effect of organic and inorganic selenium sources and vitamin E on broiler performance and carcass characteristics in heat stress condition. Vet Res Biol Products. (2011) 24:25–35. doi: 10.22092/vj.2011.101097
210. Attia, YA, Abdalah, AA, Zeweil, HS, Bovera, F, Tag El-Din, AA, and Araft, MA. Effect of inorganic or organic selenium supplementation on productive performance, egg quality and some physiological traits of dual-purpose breeding hens. Czeh J Anim Sci. (2010) 55:505–19. doi: 10.17221/1715-cjas
211. Siske, V, Zeman, L, and Klecker, D. The egg Shell: A case study in improving quality by altering mineral metabolism – Naturally. Cham Springer (2000):327–346.
212. Khoso, PA, Liu, C, Liu, C, Khoso, MH, and Li, S. Selenium deficiency activates heat shock protein expression in chicken spleen and Thymus. Biol Trace Elem Res. (2016) 173:492–500. doi: 10.1007/s12011-016-0673-8
213. Zhao, J, Xing, H, Liu, C, Zhang, Z, and Xu, S. Effect of selenium deficiency on nitric oxide and heat shock proteins in chicken erythrocytes. Biol Trace Elem Res. (2016) 171:208–13. doi: 10.1007/s12011-015-0527-9
214. Mahmoud, KZ, and Edens, FW. Influence of organic selenium on Hsp70 response of heat-stressed and Enteropathogenic Escherichia Coli-challenged broiler chickens (Gallus Gallus). Comp Biochem Physiol C Toxicol Pharmacol. (2005) 141:69–75. doi: 10.1016/j.cca.2005.05.005
215. Khan, RU, Naz, S, Dhama, K, Saminathan, M, Tiwari, R, Jeon, GJ, et al. Modes of action and beneficial applications of chromium in poultry nutrition, production and health: a review. Int J Pharmacol. (2014) 10:357–67. doi: 10.3923/ijp.2014.357.367
217. Toghyani, M, Toghyani, M, Shivazad, M, Gheisari, A, and Bahadoran, R. Chromium supplementation can alleviate the negative effects of heat stress on growth performance, carcass traits, and meat lipid oxidation of broiler chicks without any adverse impacts on blood constituents. Biol Trace Elem Res. (2012) 146:171–80. doi: 10.1007/s12011-011-9234-3
218. Orhan, C, Tuzcu, M, Deeh, PBD, Sahin, N, Komorowski, JR, and Sahin, K. Organic chromium form alleviates the detrimental effects of heat stress on nutrient digestibility and nutrient transporters in laying hens. Biol Trace Elem Res. (2018) 189:529–37. doi: 10.1007/s12011-018-1485-9
219. Ozdemir, O, Tuzcu, M, Sahin, N, Orhan, C, Tuzcu, Z, and Sahin, K. Organic chromium modifies the expression of orexin and glucose transporters of ovarian in heat-stressed laying hens. Cell Mol Biol (Noisy-le-Grand). (2017) 63:93–8. doi: 10.14715/cmb/2017.63.10.15
220. Sahin, K, Ozbey, O, Onderci, M, Cikim, G, and Aysondu, MH. Chromium supplementation can alleviate negative effects of heat stress on egg production, egg quality and some serum metabolites of laying Japanese quail. J Nutr. (2002) 132:1265–8. doi: 10.1093/jn/132.6.1265
221. Torki, M, Zangeneh, S, and Habibian, M. Performance, egg quality traits, and serum metabolite concentrations of laying hens affected by dietary supplemental chromium picolinate and vitamin C under a heat-stress condition. Biol Trace Elem Res. (2014) 157:120–9. doi: 10.1007/s12011-013-9872-8
222. Sahin, N, Hayirli, A, Orhan, C, Tuzcu, M, Komorowski, JR, and Sahin, K. Effects of the supplemental chromium form on performance and metabolic profile in laying hens exposed to heat stress. Poult Sci. (2018) 97:1298–305. doi: 10.3382/ps/pex435
223. El-Kholy, MS, El-Hindawy, MM, Alagawany, M, Abd El-Hack, ME, and El-Sayed, S. Dietary supplementation of chromium can alleviate negative impacts of heat stress on performance, carcass yield, and some blood hematology and chemistry indices of growing Japanese quail. Biol Trace Elem Res. (2017) 179:148–57. doi: 10.1007/s12011-017-0936-z
224. Bin-Jumah, M, Abd El-Hack, ME, Abdelnour, SA, Hendy, YA, Ghanem, HA, Alsafy, SA, et al. Potential use of chromium to combat thermal stress in animals: a review. Sci Total Environ. (2020) 707:135996. doi: 10.1016/j.scitotenv.2019.135996
225. Jahanian, R, and Rasouli, E. Dietary chromium methionine supplementation could alleviate immunosuppressive effects of heat stress in broiler chicks. J Anim Sci. (2015) 93:3355–63. doi: 10.2527/jas2014-8807
226. Anderson, RA, Polansky, MM, and Bryden, NA. Stability and absorption of chromium and absorption of chromium Histidinate complexes by humans. Biol Trace Elem Res. (2004) 101:211–8. doi: 10.1385/BTER:101:3:211
227. Morvaridzadeh, M, Estêvão, MD, Morvaridi, M, Belančić, A, Mohammadi, S, Hassani, M, et al. The effect of conjugated linoleic acid intake on oxidative stress parameters and antioxidant enzymes: a systematic review and Meta-analysis of randomized clinical trials. Prostaglandins Other Lipid Mediat. (2022) 163:106666. doi: 10.1016/j.prostaglandins.2022.106666
228. Jain, SK, Rains, JL, and Croad, JL. Effect of chromium Niacinate and chromium Picolinate supplementation on lipid peroxidation, Tnf-alpha, Il-6, Crp, glycated hemoglobin, triglycerides, and cholesterol levels in blood of Streptozotocin-treated diabetic rats. Free Radic Biol Med. (2007) 43:1124–31. doi: 10.1016/j.freeradbiomed.2007.05.019
229. Sahin, K, Orhan, C, Tuzcu, M, Ali, S, Sahin, N, and Hayirli, A. Epigallocatechin-3-Gallate prevents lipid peroxidation and enhances antioxidant defense system via modulating hepatic nuclear transcription factors in heat-stressed quails. Poult Sci. (2010) 89:2251–8. doi: 10.3382/ps.2010-00749
230. Sahin, N, Hayirli, A, Orhan, C, Tuzcu, M, Akdemir, F, Komorowski, JR, et al. Effects of the supplemental chromium form on performance and oxidative stress in broilers exposed to heat stress. Poult Sci. (2017) 96:4317–24. doi: 10.3382/ps/pex249
231. Hamidi, O, Chamani, M, Ghahri, H, Sadeghi, A, Malekinejad, H, and Palangi, V. Effects of supplemental chromium nanoparticles on Ifn-Γ expression of heat stress broilers. Biol Trace Elem Res. (2022) 200:339–47. doi: 10.1007/s12011-021-02634-0
232. Orhan, C, Akdemir, F, Sahin, N, Tuzcu, M, Komorowski, JR, Hayirli, A, et al. Chromium Histidinate protects against heat stress by modulating the expression of hepatic nuclear transcription factors in quail. Br Poult Sci. (2012) 53:828–35. doi: 10.1080/00071668.2012.747084
233. Akdemir, F, Sahin, N, Orhan, C, Tuzcu, M, Sahin, K, and Hayirli, A. Chromium-Histidinate ameliorates productivity in heat-stressed Japanese quails through reducing oxidative stress and inhibiting heat-shock protein expression. Br Poult Sci. (2015) 56:247–54. doi: 10.1080/00071668.2015.1008992
234. Dalólio, FS, Albino, LFT, Silva, JN, Campos, PHRF, Lima, HJD, Moreira, J, et al. Dietary chromium supplementation for heat-stressed broilers. Worlds Poult Sci J. (2018) 74:101–16. doi: 10.1017/s0043933917001064
235. Mateos, GG, Sell, JL, and Eastwood, JA. Rate of food passage (transit time) as influenced by level of supplemental fat. Poult Sci. (1982) 61:94–100. doi: 10.3382/ps.0610094
236. Musharaf, NA, and Latshaw, JD. Heat increment as affected by protein and amino acid nutrition. Worlds Poult Sci J. (1999) 55:233–40. doi: 10.1079/WPS19990017
237. Ghazalah, AA, Elsamee, MO, and Ali, AM. Influence of dietary energy and poultry fat on the response of broiler chicks to heat therm. Int J Poult Sci. (2008) 7:359. doi: 10.3923/ijps.2008.355.359
238. Attia, YA, Al-Harthi, MA, and Sh Elnaggar, A. Productive, physiological and immunological responses of two broiler strains fed different dietary regimens and exposed to heat stress. Ital J Anim Sci. (2018) 17:686–97. doi: 10.1080/1828051x.2017.1416961
239. Ghahremani, A, Sadeghi, AA, Hesaraki, S, Chamani, M, and Shawrang, P. Effect of energy sources and levels on Caecal microbial population, Jejunal morphology, gene expression of Jejunal transporters (Sglt1, Fabp) and performance of broilers under heat stress. Kafkas Univ Vet Fak Derg. (2017) 23, 415–422. doi: 10.9775/kvfd.2016.16899
240. Pastell, H, Westermann, P, Meyer, AS, Tuomainen, P, and Tenkanen, M. In vitro fermentation of arabinoxylan-derived carbohydrates by Bifidobacteria and mixed fecal microbiota. J Agric Food Chem. (2009) 57:8598–606. doi: 10.1021/jf901397b
241. Torki, M, Nasiroleslami, M, and Ghasemi, HA. The effects of different protein levels in laying hens under hot summer conditions. Anim Prod Sci. (2017) 57:927. doi: 10.1071/an15463
242. Uyanga, VA, Oke, EO, Amevor, FK, Zhao, J, Wang, X, Jiao, H, et al. Functional roles of taurine, L-Theanine, L-Citrulline, and betaine during heat stress in poultry. J Anim Sci Biotechnol. (2022) 13:23. doi: 10.1186/s40104-022-00675-6
243. Zulkifli, I, Akmal, AF, Soleimani, AF, Hossain, MA, and Awad, EA. Effects of low-protein diets on acute phase proteins and heat shock protein 70 responses, and growth performance in broiler chickens under heat stress condition. Poult Sci. (2018) 97:1306–14. doi: 10.3382/ps/pex436
244. Zeitz, JO, Fleischmann, A, Ehbrecht, T, Most, E, Friedrichs, S, Whelan, R, et al. Effects of supplementation of dl-methionine on tissue and plasma antioxidant status during heat-induced oxidative stress in broilers. Poult Sci. (2020) 99:6837–47. doi: 10.1016/j.psj.2020.08.082
245. Ajayi, OI, Smith, OF, Oso, AO, and Oke, OE. Evaluation of in ovo feeding of low or high mixtures of cysteine and lysine on performance, intestinal morphology and physiological responses of thermal-challenged broiler embryos. Front Physiol. (2022) 13:972041. doi: 10.3389/fphys.2022.972041
246. Sarsour, AH, and Persia, ME. Effects of sulfur amino acid supplementation on broiler chickens exposed to acute and chronic cyclic heat stress. Poult Sci. (2022) 101:101952. doi: 10.1016/j.psj.2022.101952
247. Wu, QJ, Liu, N, Wu, XH, Wang, GY, and Lin, L. Glutamine alleviates heat stress-induced impairment of intestinal morphology, intestinal inflammatory response, and barrier integrity in broilers. Poult Sci. (2018) 97:2675–83. doi: 10.3382/ps/pey123
248. Dai, SF, Wang, LK, Wen, AY, Wang, LX, and Jin, GM. Dietary glutamine supplementation improves growth performance, meat quality and colour stability of broilers under heat stress. Br Poult Sci. (2009) 50:333–40. doi: 10.1080/00071660902806947
249. Deng, C, Zheng, J, Zhou, H, You, J, and Li, G. Dietary Glycine supplementation prevents heat stress-induced impairment of antioxidant status and intestinal barrier function in broilers. Poult Sci. (2023) 102:102408. doi: 10.1016/j.psj.2022.102408
250. Diarra, MS, and Malouin, F. Antibiotics in Canadian poultry productions and anticipated alternatives. Front Microbiol. (2014) 5:282. doi: 10.3389/fmicb.2014.00282
251. Pawar, SS, Basavaraj, S, Dhansing, LV, Pandurang, KN, Sahebrao, KA, Vitthal, NA, et al. Assessing and mitigating the impact of heat stress in poultry. Adv Anim Vet Sci. (2016) 4:332–41. doi: 10.14737/journal.aavs/2016/4.6.332.341
252. Ali Shah, SR, and Çeting, UL. Nutritional advances in production performance and product quality of poultry husbandry under heat stress. Online J Anim Feed Res. (2022) 12, 53–65. doi: 10.51227/ojafr.2022.8
253. Orakpoghenor, O, Ejum Ogbuagu, N, and Sa’Idu, N. Effect of environmental temperature on water intake in poultry. Adv Poultry Nutr Res. (2021) 1–7. doi: 10.5772/intechopen.95695
254. Gamba, JP, Rodrigues, MM, Garcia Neto, M, Perri, SHV, Faria Júnior, MJA, and Pinto, MF. The strategic application of electrolyte balance to minimize heat stress in broilers. Revista Brasileira de Ciência Avícola. (2015) 17:237–45. doi: 10.1590/1516-635x1702237-246
255. Mushtaq, T, Mirza, MA, Athar, M, Hooge, DM, Ahmad, T, Ahmad, G, et al. Dietary sodium and chloride for twenty-nine-to forty-two-day-old broiler chickens at constant electrolyte balance under subtropical summer conditions. J Appl Poult Res. (2007) 16:161–70. doi: 10.1093/japr/16.2.161
256. Farfán, C, Oliverosy, Y, and De Basilio, V. Effect of mineral supplying in water or feed on productive and physiological variables in broiler under heat stress conditions. Zootec Trop. (2010) 28:363–73.
257. Bryden, WL, Li, X, Ruhnke, I, Zhang, D, and Shini, S. Nutrition, feeding and laying hen welfare. Anim Prod Sci. (2021) 61:893–914. doi: 10.1071/an20396
258. Mushtaq, MMH, Pasha, TN, Mushtaq, T, and Parvin, R. Electrolytes, dietary electrolyte balance and salts in broilers: An updated review on growth performance, water intake and litter quality. Worlds Poult Sci J. (2019) 69:789–802. doi: 10.1017/s0043933913000810
259. Benton, CE, Balnave, D, Brake, J, Boyd, LJ, and Brown, MA. The use of dietary minerals during heat stress in broilers. Prof Anim Sci. (1998) 14:193–6. doi: 10.15232/s1080-7446(15)31828-3
260. Teeter, RG, Smith, MO, Owens, FN, Arp, SC, Sangiah, S, and Breazile, JE. Chronic heat stress and respiratory alkalosis: occurrence and treatment in broiler chicks. Poult Sci. (1985) 64:1060–4. doi: 10.3382/ps.0641060
261. Teeter, RG, and Smith, MO. High chronic ambient temperature stress effects on broiler Acid-Base balance and their response to supplemental ammonium chloride, potassium chloride, and potassium carbonate. Poult Sci. (1986) 65:1777–81. doi: 10.3382/ps.0651777
262. Das, R, Mishra, P, and Jha, R. In ovo feeding as a tool for improving performance and gut health of poultry: a review. Front Vet Sci. (2021) 8:754246. doi: 10.3389/fvets.2021.754246
263. Berrocoso, JD, Kida, R, Singh, AK, Kim, YS, and Jha, R. Effect of in Ovo injection of Raffinose on growth performance and gut health parameters of broiler chicken. Poult Sci. (2017) 96:1573–80. doi: 10.3382/ps/pew430
264. Ma, YB, Zhang, FD, Wang, J, Wu, SG, Qi, GH, and Zhang, HJ. Effect of in ovo feeding of Beta-Hydroxy-Beta-Methylbutyrate on hatchability, muscle growth and performance in prenatal and posthatch broilers. J Sci Food Agric. (2020) 100:755–63. doi: 10.1002/jsfa.10080
265. Elnesr, SS, Elwan, HAM, Xu, QQ, Xie, C, Dong, XY, and Zou, XT. Effects of in Ovo injection of sulfur-containing amino acids on heat shock protein 70, corticosterone hormone, antioxidant indices, and lipid profile of newly hatched broiler chicks exposed to heat stress during incubation. Poult Sci. (2019) 98:2290–8. doi: 10.3382/ps/pey609
266. Zhu, Y, Zhao, J, Wang, C, Zhang, F, Huang, X, Ren, Z, et al. Exploring the effectiveness of in Ovo feeding of vitamin C based on the embryonic vitamin C synthesis and absorption in broiler chickens. J Anim Sci Biotechnol. (2021) 12:86. doi: 10.1186/s40104-021-00607-w
267. Livingston, ML, Pokoo-Aikins, A, Frost, T, Laprade, L, Hoang, V, Nogal, B, et al. Effect of heat stress, dietary electrolytes, and vitamins E and C on growth performance and blood biochemistry of the broiler chicken. Front Anim Sci. (2022) 3:807267. doi: 10.3389/fanim.2022.807267
268. Alaqil, AA, Abd El-Atty, HK, and Abbas, AO. Intermittent lighting program relieves the deleterious effect of heat stress on growth, stress biomarkers, physiological status, and immune response of broiler chickens. Animals. (2022) 12:1834. doi: 10.3390/ani12141834
269. Greene, ES, Maynard, C, Owens, CM, Meullenet, JF, and Dridi, S. Effects of herbal Adaptogen feed-additive on growth performance, carcass parameters, and muscle amino acid profile in heat-stressed modern broilers. Front Physiol. (2021) 12:784952. doi: 10.3389/fphys.2021.784952
270. Slawinska, A, Zampiga, M, Sirri, F, Meluzzi, A, Bertocchi, M, Tavaniello, S, et al. Impact of Galactooligosaccharides delivered in Ovo on mitigating negative effects of heat stress on performance and welfare of broilers. Poult Sci. (2020) 99:407–15. doi: 10.3382/ps/pez512
271. Goo, D, Kim, JH, Park, GH, Delos Reyes, JB, and Kil, DY. Effect of heat stress and stocking density ongrowth performance, breast meat quality,and intestinal barrier function in broiler chickens. Animals. (2019) 9:107. doi: 10.3390/ani9030107
272. Imik, H, Ozlu, H, Gumus, R, Atasever, MA, Urcar, S, and Atasever, M. Effects of ascorbic acid and Α-lipoic acid on performance and meat quality of broilers subjected to heat stress. Br Poult Sci. (2012) 53:800–8. doi: 10.1080/00071668.2012.740615
273. Attia, YA, Hassan, RA, Tag El-Din, AE, and Abou-Shehema, BM. Effect of ascorbic acid or increasing metabolizable energy level with or without supplementation of some essential amino acids on productive and physiological traits of slow-growing chicks exposed to chronic heat stress. J Anim Physiol Anim Nutr. (2011) 95:744–55. doi: 10.1111/j.1439-0396.2010.01104.x
274. Crespo, R, and Grimes, J. Effect of brooding conditions on the blood chemistry and performance of Turkey poults. J Appl Poult Res. (2024) 33:100408. doi: 10.1016/j.japr.2024.100408
275. Albuquerque, K, Leighton, A, Mason, J, and Potter, L. The effects of environmental temperature, sex, and dietary energy levels on growth performance of large White turkeys. Poult Sci. (1978) 57:353–62. doi: 10.3382/ps.0570353
276. Saleh, AA, Eltantawy, MS, Gawish, EM, Younis, HH, Amber, KA, Abd El-Moneim, AEE, et al. Impact of dietary organic mineral supplementation on reproductive performance, egg quality characteristics, lipid oxidation, ovarian follicular development, and immune response in laying hens under high ambient temperature. Biol Trace Elem Res. (2020) 195:506–14. doi: 10.1007/s12011-019-01861-w
277. Rehman, ZU, Chand, N, and Khan, RU. The effect of vitamin E, L-carnitine, and ginger on production traits, immune response, and antioxidant status in two broiler strains exposed to chronic heat stress. Environ Sci Pollut Res Int. (2017) 24:26851–7. doi: 10.1007/s11356-017-0304-8
278. Yosi, F, Widjastuti, T, and Setiyatwan, H. Performance and physiological responses of broiler chickens supplemented with potassium chloride in drinking water under environmental heat stress. Asian J Poult Sci. (2016) 11:31–7. doi: 10.3923/ajpsaj.2017.31.37
279. El-Deep, MH, Ijiri, D, Ebeid, TA, and Ohtsuka, A. Effects of dietary Nano-selenium supplementation on growth performance, Antioxidative status, and immunity in broiler chickens under Thermoneutral and high ambient temperature conditions. J Poult Sci. (2016) 53:274–83. doi: 10.2141/jpsa.0150133
280. Madkour, M, Ali, SI, Alagawany, M, El-Kholy, MS, El-Baz, FK, Alqhtani, AH, et al. Dietary Dunaliella Salina microalgae enriches eggs with carotenoids and Long-chain Omega-3 fatty acids, enhancing the antioxidant and immune responses in heat-stressed laying hens. Front Vet Sci. (2025) 12:1545433. doi: 10.3389/fvets.2025.1545433
281. El-Tahan, HM, Lim, CI, Alhimaidi, AR, Ammari, AA, Cho, S, Kim, IH, et al. Fish oil a source of Omega-3 fatty acids affects hypothalamus heat resistance genes expressions and fatty acid composition in heat-stressed chicks. Domest Anim Endocrinol. (2025) 91:106915. doi: 10.1016/j.domaniend.2025.106915
282. Wasti, S, Sah, N, Lee, CN, Jha, R, and Mishra, B. Dietary supplementation of alpha-lipoic acid mitigates the negative effects of heat stress in broilers. PLoS One. (2021) 16:e0254936. doi: 10.1371/journal.pone.0254936
Keywords: gut health, heat stress, nutritional supplements, oxidative stress, performance, poultry
Citation: Olayiwola SF and Adedokun SA (2025) Heat stress in poultry: the role of nutritional supplements in alleviating heat stress and enhancing gut health in poultry. Front. Vet. Sci. 12:1691532. doi: 10.3389/fvets.2025.1691532
Edited by:
Arda Yıldırım, Gaziosmanpaşa University, TürkiyeReviewed by:
Sadid Al Amaz, University of Georgia, United StatesMuhammad Thohawi Elziyad Purnama, Airlangga University, Indonesia
Copyright © 2025 Olayiwola and Adedokun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sunday A. Adedokun, dGF5by5hZGVkb2t1bkB1a3kuZWR1
 Sammad F. Olayiwola
Sammad F. Olayiwola Sunday A. Adedokun
Sunday A. Adedokun