- 1Jiangsu Key Laboratory for Animal Genetic, Breeding and Molecular Design, Yangzhou University, Yangzhou, China
- 2Institute of Animal Husbandry and Veterinary Science, Zhejiang Academy of Agricultural Sciences, Hangzhou, China
Introduction: Green tea dust (GTD), a by-product of tea processing, exhibits promising potential as a functional feed additive owing to its rich protein profile and bioactive compounds.
Methods: This study evaluated the impact of GTD inclusion on biochemical parameters, oxidative stress markers, intestinal morphology, and cecal microbiota in Zhedong White geese. A cohort of 120 21-day-old male geese was randomly allocated to four dietary regimens: a basal diet (CTRL) and basal diets supplemented with 10% (LGTD), 15% (MGTD), or 20% ET (HGTD). The experimental period lasted seven weeks.
Results: Results indicated that GTD supplementation exerted no significant influence on plasma lipid metabolism. However, graded GTD doses markedly elevated systemic antioxidant activity, as evidenced by improved plasma antioxidant indices. Morphometric analysis revealed enhanced intestinal absorptive function, characterized by increased villus height (VH), reduced crypt depth (CD), and elevated VH/CD ratios in the duodenum. Furthermore, GTD supplementation modulated cecal microbial communities, promoting a favorable microbiota profile.
Conclusion: These findings underscore the utility of GTD as a dietary intervention to augment intestinal health and oxidative status in geese, providing empirical support for its broader adoption in poultry nutrition.
1 Introduction
Modern poultry production systems grapple with the dual challenges of sustaining growth efficiency and mitigating health risks under high-density rearing conditions. Of particular concern are oxidative stress and intestinal dysfunction, which compromise nutrient utilization, product quality, and sustainability (1). In response, the exploration of functional feed additives—particularly those derived from natural by—products—has gained traction as a strategy to enhance physiological resilience. Bioactive compounds, such as polyphenols and polysaccharides, are increasingly recognized for their dual capacity to modulate redox homeostasis and gastrointestinal health, offering a viable alternative to conventional growth promoters (2).
Green tea (Camellia sinensis) is a rich source of bioactive compounds, including polyphenols, polysaccharides, amino acids, and vitamins, which contribute to its well-documented pharmacological properties (3, 4). As a by-product of tea processing, green tea dust (GTD) retains many of these functional components but at a significantly reduced cost. Numerous studies have indicated the efficacy of green tea dust in anti-inflammation and antioxidant activity, due to its ingredients rich in tea polyphenols and tannins (5, 6). While studies in ruminants, such as sheep, have shown that GTD supplementation improves performance without compromising nutrient digestibility (7), its potential as a dietary supplement in poultry remains unexplored.
The intestinal tract is a critical site for nutrient assimilation, immune modulation, and host-microbiome interactions, with profound implications for overall animal health (8–11). Emerging evidence suggests that dietary interventions can modulate gut microbial composition and enhance systemic antioxidant responses (12–14). For instance, polysaccharide-based feed additives in poultry have been shown to promote microbial diversity, particularly enriching Firmicutes and Verrucomicrobiota, leading to improved growth performance, redox balance, and intestinal morphology (15). Similarly, supplementation with bioactive compounds such as tea tree oil has demonstrated efficacy in enhancing growth metrics and immune function through selective modulation of key microbial taxa (e.g., Clostridiaceae_1) (16).
The digestive physiology of geese, characterized by a highly developed cecal fermentation system, presents unique opportunities for utilizing fibrous feed ingredients to modulate microbial ecology and enhance oxidative resilience (3). As an economically significant indigenous breed in China, the Zhedong white goose serves as an ideal model to investigate the functional effects of unconventional dietary components. Thus, this study aimed to test the hypothesis that green tea dust supplementation can play role in antioxidant status and gut health as an additive in the diet of Zhedong White geese.
2 Materials and methods
2.1 Experimental design and sample collection
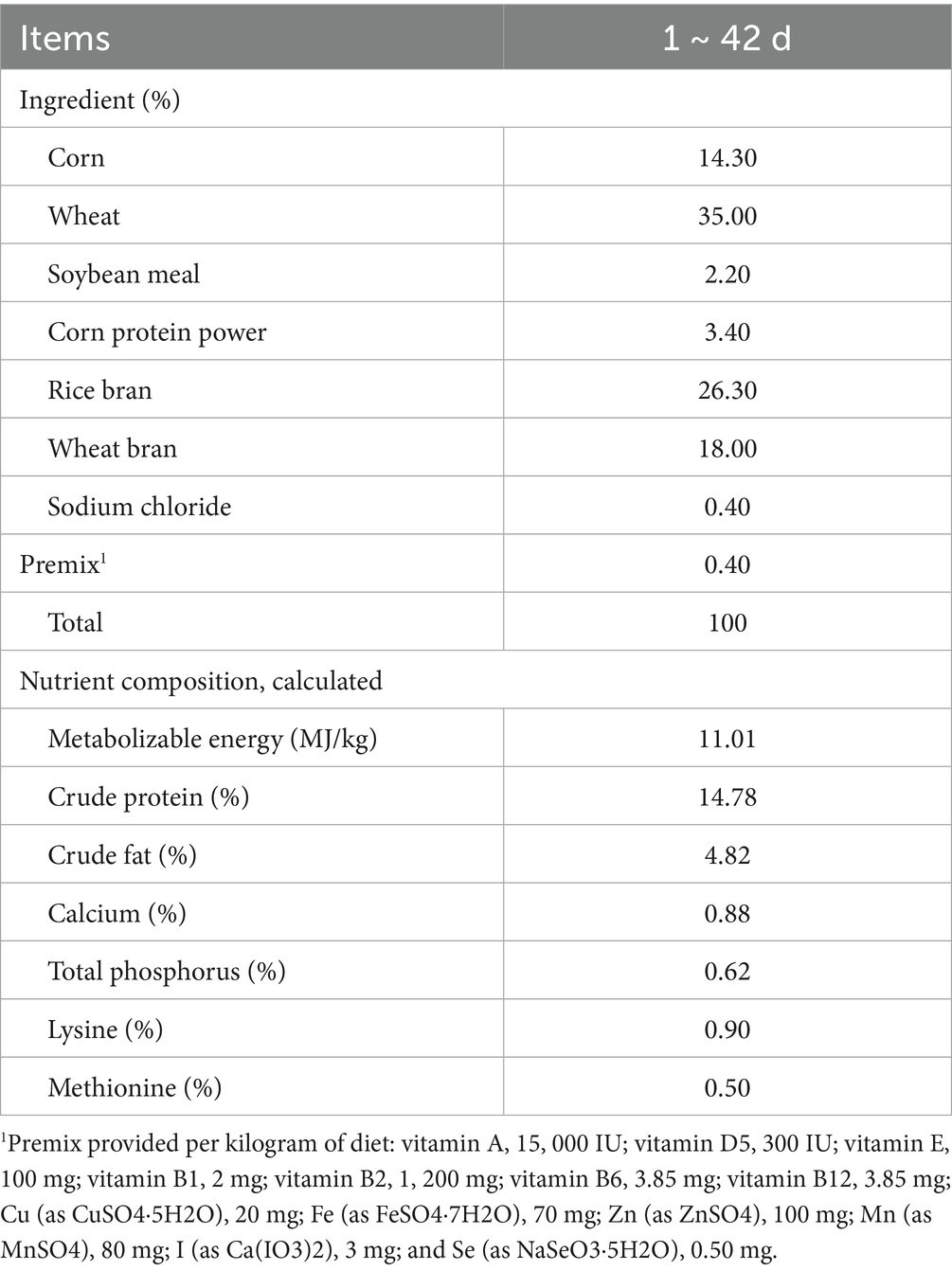
Male Zhedong White geese and green tea dust (a discarded waste material after industrial production of green tea beverages) were provided from the Zhejiang White Goose Research Institute and Tea Research Institute from Chinese Academy of Agricultural Sciences, respectively. One hundred and twenty 21-day-old healthy male Zhedong White geese were randomly divided into four groups: CTRL (the basal diet), LGTD (the basal diet with 10% tea dust), MGTD (the basal diet with 15% tea dust), and HGTD (the basal diet with 20% tea dust). The experimental period lasted seven weeks. At trial termination, six geese were randomly selected for sample collection and analysis. Dietary formulations and nutritional composition are detailed in Table 1.
Following the 7-week experimental period, blood was collected via subcutaneous venipuncture and processed by centrifugation (3,000 × g, 10 min, 4 °C) to isolate plasma, which was subsequently stored at −80 °C. Geese were humanely euthanized via intravenous sodium pentobarbital administration (200 mg/kg BW). Tissue specimens (liver, duodenum, and cecum content) were excised, flash-frozen in liquid nitrogen, and preserved at −80 °C for subsequent nutritional profiling and microbial genomic analysis.
2.2 Biochemical analysis
Liver total cholesterol (TC), triglycerides (TG), and non-esterified fatty acids (NEFA) were measured using commercially available kits (Jiancheng Biotechnology Inc., Nanjing, China) following the manufacturers’ instructions.
2.3 Oxidative stress biomarkers
Plasma oxidative status was assessed by measuring the total antioxidant capacity (T-AOC) and the activities of antioxidant enzymes (CAT, SOD, GSH-PX) using commercial assay kits (Solarbio, Beijing, China), with lipid peroxidation evaluated through malondialdehyde (MDA) quantification according the manufacturers’ instructions (Solarbio).
2.4 Histological observation
Duodenal samples were fixed in 4% paraformaldehyde and processed for histological analysis. Following dehydration in xylene (15–20 min) and paraffin embedding at 60 °C, 5-μm sections were prepared and stained with hematoxylin–eosin. Morphometric evaluation was performed by optical microscopy, with five serial sections analyzed per sample. From each section, six representative fields containing intact villi were selected for measurement. The sections were analyzed under an Olympus light microscope (Tokyo, Japan). Villus height was determined from the longest villus in each field, with corresponding crypt depth measured simultaneously. Mean values were calculated from all measurements for statistical analysis. All methods and detection were conducted by servicebio (Wuhan, China).
2.5 Microbial community analysis
Genomic DNA from cecal samples was isolated using a commercial extraction kit (Omega, Norcross, GA). The V3-V4 hypervariable regions of bacterial 16S rRNA genes were amplified using universal primers (341F: ACTCCTACGGGAGGCAGCA; 806R: GGACTACHVGGGTWTCTAAT). Amplicon libraries were prepared and sequenced on an Illumina MiSeq platform (2 × 300 bp) by Personalbio Technology (Shanghai). Sequence processing was conducted in QIIME 2, where amplicon sequence variants (ASVs) were generated through DADA2 denoising. Microbial diversity was assessed using α-diversity indices (Shannon, Chao1) and β-diversity metrics visualized via principal coordinate analysis (PCoA). Differential abundance of taxa between groups was determined using linear discriminant analysis effect size (LEfSe) with an LDA score threshold >2.0.
2.6 Statistical analysis
Data are presented as mean ± SD. Differences between groups were assessed by one-way ANOVA (SPSS 25.0), with statistical significance set at p < 0.05. Graphical representations were generated using GraphPad Prism 8.0.
3 Result
3.1 Lipid parameters
To determine whether dietary addition of tea dust could affect the fat deposition in body, the contents of TC, TG, and NEFA were analyzed. As shown in Table 2, dietary supplementation with different dose of tea dust did not alter the levels of plasma TC, TG, and NEFA (p > 0.05).
3.2 Antioxidant capacity
Plasma oxidative status parameters responded dose-dependently to tea dust supplementation (Figure 1). The tea dust supplementation groups exhibited significantly lower MDA levels than CTRL group (p < 0.05; Figure 1A) and the activities of antioxidant enzymes (SOD, CAT, T-AOC, GSH-PX) were significantly elevated compared to controls (p < 0.05; Figures 1B–D).
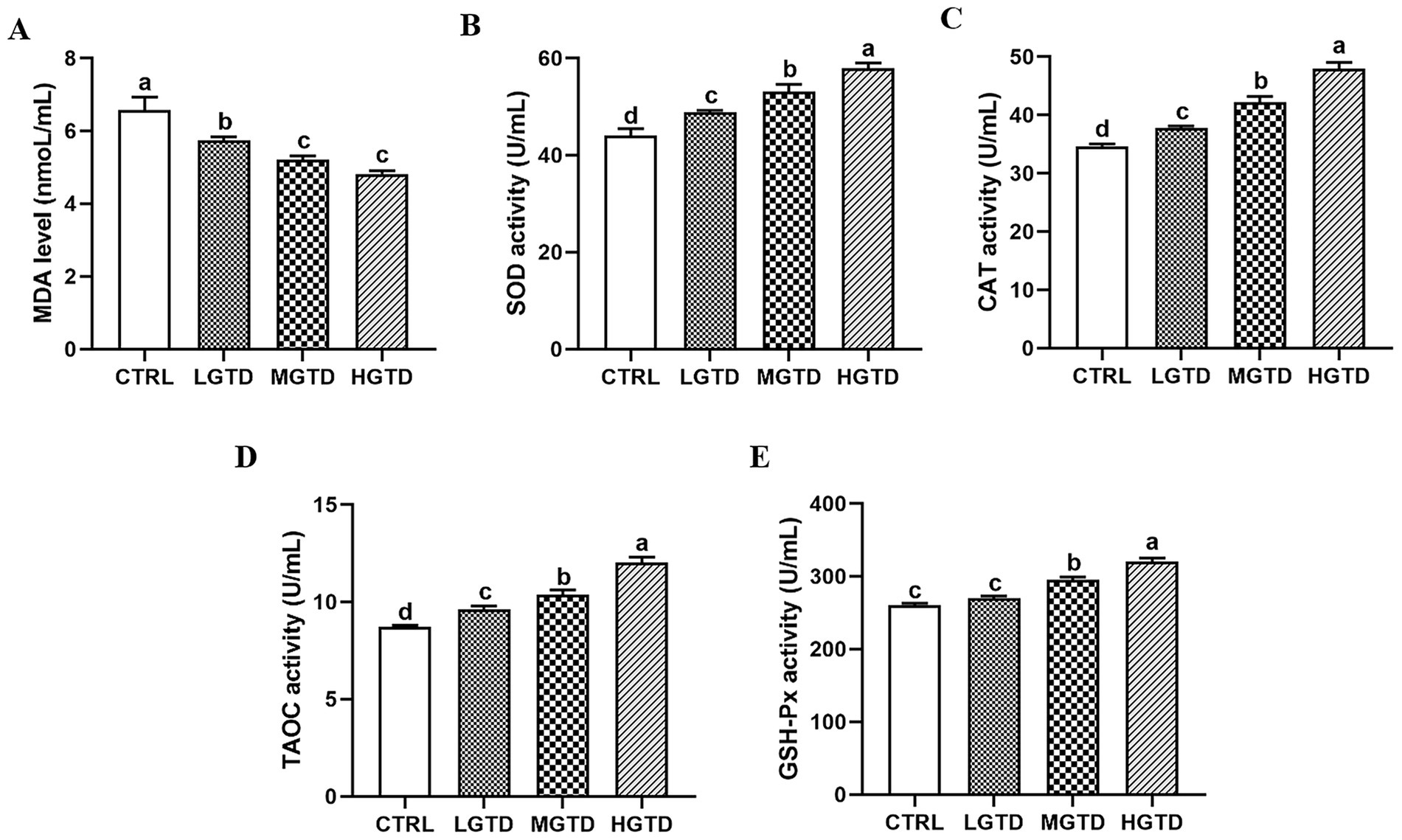
Figure 1. Effect of tea dust supplementation on plasma antioxidant capacity in Zhedong White geese. (A) MDA level. (B) SOD activity. (C) CAT activity. (D) TAOC activity. (E) GSH-PX activity. n = 6 for each group. a–dMeans with different letters are significantly different (p < 0.05).
3.3 Intestinal morphology analysis
To determine the effects of tea dust on the intestinal absorption capacity, villi height (VH), crypt depth (CD) and VH/CD radio of the duodenum were assessed. As shown in Figure 2, duodenal histopathological analysis showed that the VH was significantly longer in the LGTD and HGTD group than that in the CTRL and HET group (p < 0.05). The CD was significantly smaller in the LGTD, MGTD and HGTD group than that in the CTRL group (p < 0.05). The VH/CD, which indicated the wall thickness of duodenum, was significantly greater in the LGTD and HGTD group than that in the CTRL and HGTD (p < 0.05). These findings indicated that tea dust could affect the morphology to improve the absorption capacity of the intestine.

Figure 2. Effect of tea dust supplementation on the duodenal morphology analysis in Zhedong White geese. (A) HE staining. (B) Villi height (VH). (C) Crypt depth (CD). (D) VH/CD radio. Magnification of 200X was used (Bar = 100 μm). n = 6 for each group. a–cMeans with different letters are significantly different (p < 0.05).
3.4 Gut microbiota compositions
To assess gut microbiota modulation by tea dust supplementation, we analyzed cecal microbiomes via 16S rRNA sequencing. The α diversity (represented by the Chao1, Simpson, Goods coverage, and Shannon indices) of the intestinal flora remained unchanged across groups (p > 0.05, Figure 3A). β-diversity analysis revealed overlapping microbial communities between CTRL and supplemented groups (Figure 3B). A Venn diagram showed that 886 amplicon sequence variants (ASVs) of the microbiota were obtained among the CTRL, LGTD, MGTD and HGTD groups (47, 19, 45, and 64 unique ASVs, respectively) (Figure 3C). As shown in Figure 3D, Bacteroidetes, Firmicutes and Proteobacteria were the most abundant phyla. Compared to the CTRL group, the relative abundance of Verrucomicrobia was significantly increased in HGTD group and the relative abundance of Actinobacteria was significantly decreased in MGTD group (Figures 3E,F). Furthermore, Bacteroidaceae, Ruminococcaceae, and Prevotellaceae were the dominant family (Figure 3G). LEfSe analysis showed that the genera Anaerobiospirillum, Fusobacterium, and Mucispirillum in the CTRL group, Weissella, Rothia, and Ruminococcus torques_group in the LGTD group, Sutterella, and Oscillospira in the MGTD group, and Lachnospira, Agathobacter, Solobacterium, and Megasphaera in the HGTD group were the predominant bacterial strains (Figure 4).

Figure 3. Effects of tea dust supplementation on the gut microbiota compositions in geese. (A) Variations in α diversity. (B) PCoA analysis. (C) Venn diagram. (D) Microbiota composition analysis at the phylum level. (E,F) The relative abundance of Verrucomicrobia and Actinobacteria. (G) Microbiota composition analysis at the family level. n = 6 for each group.

Figure 4. Analysis of the diversity of microbial communities with LEfSe analysis. (A) Cladogram. (B) LDA score.
4 Discussion
Green tea (Camellia sinensis), a non-fermented tea variety, has been extensively studied for its notable health-promoting properties (17, 18). Compared to other tea types, green tea exhibits particularly significant bioactive effects, with reported benefits in managing obesity (19, 20), modulating gut microbiota (21), reducing cancer risk (22, 23), improving cardiovascular health (24, 25), alleviating osteoarthritis symptoms (26), and mitigating metabolic disorders such as hypercholesterolemia and hyperglycemia (27). Our previous study demonstrated that green tea dust supplementation improved growth and slaughter performance in geese (28). In this study, we further confirmed green tea dust could enhance antioxidant capacity, intestinal microbiota composition in geese, while not altered lipid metabolism.
Several serum lipid measurements, such as TC, TG, and NEFA, are indicative of lipid metabolism status in general (29). TG converts to glycerol and free fatty acids in liver (30). Non-esterified fatty acids (NEFAs), primarily derived from adipose tissue lipolysis, serve as a key contributor to the hepatic triglyceride (TG) pool. Elevated circulating NEFA levels are associated with the development of insulin resistance in skeletal muscle and liver, as well as the onset of dyslipidemia (31). In this study, TG, TC and NEFA concentration in serum of geese showed no significance with dietary tea dust supplementation, suggesting that tea dust has little effect in lipid metabolism.
Redox homeostasis is essential for maintaining normal metabolic and physiological functions. Disruption of this equilibrium can lead to metabolic dysregulation and uncontrolled free radical reactions, contributing to oxidative stress (32, 33). Endogenous antioxidant enzymes, including superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px), constitute a primary defense system against oxidative damage (34, 35). SOD serves as the initial scavenger of reactive oxygen species (ROS), catalyzing the dismutation of superoxide anions (O₂−) into hydrogen peroxide (H₂O₂) and oxygen (O₂) (36). Subsequently, CAT facilitates the decomposition of H₂O₂ into water and molecular oxygen (37), while GSH-Px further eliminates H₂O₂ and detoxifies lipid peroxides, thereby protecting cellular integrity (38). In the present study, dietary supplementation with tea dust significantly enhanced plasma antioxidant capacity, as evidenced by elevated SOD, CAT, GSH-Px, and total antioxidant capacity (T-AOC) activities, alongside a marked reduction in malondialdehyde (MDA) levels. These findings align with previous reports demonstrating that fermented tea residue feed boosts SOD and GSH-Px activities while lowering serum MDA in laying hens (39). Similarly, dietary tea polyphenols have been shown to upregulate GSH-Px and T-AOC while suppressing MDA accumulation in poultry, further supporting the antioxidative potential of tea-derived compounds (40). This indicates that dietary with tea dust can reduce oxidative stress in geese.
The intestine is a key organ that helps digest and absorb nutrients. It also acts as a barrier to protect the body and is involved in cell communication (41, 42). Two important measurements of intestinal health are villus height (VH) and crypt depth (CD) (43). In the present study, dietary inclusion of 20% tea dust significantly reduced CD while increasing VH and the villus height-to-crypt depth ratio (V/C) compared to the control group. These morphological improvements suggest that tea dust supplementation enhances intestinal villus development, potentially optimizing nutrient assimilation and gut health (44).
The avian gut microbiota predominantly colonizes the cecal region, playing pivotal roles in host metabolism, immune modulation, and nutrient utilization (45, 46). Our findings revealed that while dietary tea dust supplementation did not significantly alter the relative abundance of dominant phyla Bacteroidetes and Firmicutes, it notably increased Verrucomicrobia and reduced Actinobacteria populations in the geese cecum. These microbial shifts suggest tea dust may selectively modulate specific bacterial taxa without disrupting the overall phylum-level composition. A previous study demonstrated that Verrucomicrobia usually represents a minor population of intestinal microbiota in response to dietary shifts in mice (47). Furthermore, Actinobacteria play a significant role in fiber degradation, particularly in metabolizing plant-based carbohydrates such as starch, inulin, and arabinoxylan (48). Notably, studies have shown that dietary CR or FCR supplementation leads to elevated Actinobacteria populations specifically in the ileal region (49). LEfSe analysis further validated the selective modulation of cecal microbiota by tea dust, suggesting that tea dust supplementation may enhance intestinal health through targeted microbial community restructuring. The above data indicated that the antioxidant capacity conferred by green tea dust supplementation is associated with the modulation of gut microbiota. Supporting this, tea polyphenol-induced amelioration of gut microbiota showed a strong correlation in enhancing antioxidant activity in mice (50). This evidence underpins the further exploration of the prebiotic potential inherent to green tea dust.
5 Conclusion
Collectively, our findings demonstrate that 20% tea dust inclusion in the diet enhances antioxidant capacity, intestinal function, and microbial ecology in Zhedong White geese. These physiological improvements support the practical application of tea dust supplementation as a nutritional strategy to optimize goose production performance.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://ngdc.cncb.ac.cn/gsa/index.jsp, CRA032681.
Ethics statement
The animal study was approved by all animal experiments were approved by the Institutional Animal Care and Use Committee of Yangzhou University (Approval Date: December 2020). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
ZG: Conceptualization, Investigation, Methodology, Visualization, Writing – original draft. WX: Software, Writing – original draft. YT: Formal analysis, Writing – original draft. LL: Project administration, Writing – review & editing. GC: Funding acquisition, Software, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Zhejiang Province Major Agricultural Collaborative Promotion Project (2023ZDXT15), China Agriculture Research System of MOF and MARA (CARS-42-6).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Estévez, M. Oxidative damage to poultry: from farm to fork. Poult Sci. (2015) 94:1368–78. doi: 10.3382/ps/pev094
2. Jachimowicz, K, Winiarska-Mieczan, A, and Tomaszewska, E. The impact of herbal additives for poultry feed on the fatty acid profile of meat. Animals. (2022) 12:1054–1075. doi: 10.3390/ani12091054
3. Crespy, V, and Williamson, G. A review of the health effects of green tea catechins in in vivo animal models. J Nutr. (2004) 134:3431s–40s. doi: 10.1093/jn/134.12.3431S
4. Hwang, Y, Chang, B, Kim, T, and Kim, S. Ameliorative effects of green tea extract from tannase digests on house dust mite antigen-induced atopic dermatitis-like lesions in NC/Nga mice. Arch Dermatol Res. (2019) 311:109–20. doi: 10.1007/s00403-018-01886-6
5. Gaggìa, F, Baffoni, L, Galiano, M, Nielsen, DS, Jakobsen, RR, Castro-Mejía, JL, et al. Kombucha beverage from green, black and rooibos teas: a comparative study looking at microbiology, chemistry and antioxidant activity. Nutrients. (2018) 11:1–22. doi: 10.3390/nu11010001
6. Ouyang, Z, Zhu, W, Xie, Y, Yang, W, Liu, J, Pang, Q, et al. Green tea diet can effectively antagonize the toxicity induced by environmental-related concentrations of BPA: An implication from in vivo and in silico studies. J Agric Food Chem. (2024) 72:20633–45. doi: 10.1021/acs.jafc.4c05627
7. Ramdani, D, Budinuryanto, DC, and Mayasari, N. The effect of paddy straw and concentrate containing green tea dust on performance and nutrient digestibility in feedlot lambs. Turkish J Vet Anim Sci. (2020) 44:1–10. doi: 10.3906/vet-1909-10
8. van Kuijk, SJA, Han, Y, Garcia-Ruiz, AI, and Rodiles, A. Hydroxychloride trace minerals have a positive effect on growth performance, carcass quality and impact ileal and cecal microbiota in broiler chickens. J Anim Sci Biotechnol. (2021) 12:38. doi: 10.1186/s40104-021-00553-7
9. Xu, X, Xu, P, Ma, C, Tang, J, and Zhang, X. Gut microbiota, host health, and polysaccharides. Biotechnol Adv. (2013) 31:318–37. doi: 10.1016/j.biotechadv.2012.12.009
10. Richards, JD and Lange, JGFMDJCJoAS. The gastrointestinal microbiota and its role in monogastric nutrition and health with an emphasis on pigs: current understanding, possible modulations, and new technologies for ecological studies. (2005) 85: 421–435. doi: 10.4141/A05-049
11. Cui, J, Hao, Z, Zhou, Q, Qiu, M, Liu, Y, Liu, Y, et al. Chlorpyrifos induced autophagy and mitophagy in common carp livers through AMPK pathway activated by energy metabolism disorder. Ecotoxicol Environ Saf. (2023) 258:114983. doi: 10.1016/j.ecoenv.2023.114983
12. Muiños-Bühl, A, González-Recio, O, Muñoz, M, Óvilo, C, García-Casco, J, and Fernández, AI. Evaluating protocols for porcine Faecal microbiome recollection, storage and DNA extraction: from the farm to the lab. Curr Microbiol. (2018) 75:651–7. doi: 10.1007/s00284-017-1429-1
13. Dong, Z, Zhang, D, Wu, X, Yin, Y, and Wan, D. Ferrous Bisglycinate supplementation modulates intestinal antioxidant capacity via the AMPK/FOXO pathway and reconstitutes gut microbiota and bile acid profiles in pigs. J Agric Food Chem. (2022) 70:4942–51. doi: 10.1021/acs.jafc.2c00138
14. Cui, Y, Leng, X, Zhao, Y, Zhao, Y, and Wang, Q. Effects of dietary Artemisia annua supplementation on growth performance, antioxidant capacity, immune function, and gut microbiota of geese. Poult Sci. (2024) 103:103594. doi: 10.1016/j.psj.2024.103594
15. Yang, B, Li, X, Mesalam, NM, Elsadek, MF, and Abdel-Moneim, AE. The impact of dietary supplementation of polysaccharide derived from Polygonatum sibiricum on growth, antioxidant capacity, meat quality, digestive physiology, and gut microbiota in broiler chickens. Poult Sci. (2024) 103:103675. doi: 10.1016/j.psj.2024.103675
16. Zhang, G, Zhao, J, Dong, W, Song, X, Zang, J, Ni, S, et al. Effects of tea tree oil supplementation on growth performance, antioxidant capacity, immune status and microbial community in weaned pigs. Arch Anim Nutr. (2021) 75:121–36. doi: 10.1080/1745039x.2021.1877074
17. Gordon, NC, and Wareham, DW. Antimicrobial activity of the green tea polyphenol (−)-epigallocatechin-3-gallate (EGCG) against clinical isolates of Stenotrophomonas maltophilia. Int J Antimicrob Agents. (2010) 36:129–31. doi: 10.1016/j.ijantimicag.2010.03.025
18. Wang, X, Tian, J, Jiang, J, Li, L, Ying, X, Tian, H, et al. Effects of green tea or green tea extract on insulin sensitivity and glycaemic control in populations at risk of type 2 diabetes mellitus: a systematic review and meta-analysis of randomised controlled trials. J Hum Nutr Diet. (2014) 27:501–12. doi: 10.1111/jhn.12181
19. Chen, IJ, Liu, CY, Chiu, JP, and Hsu, CH. Therapeutic effect of high-dose green tea extract on weight reduction: a randomized, double-blind, placebo-controlled clinical trial. Clin Nutr. (2016) 35:592–9. doi: 10.1016/j.clnu.2015.05.003
20. Janssens, PL, Hursel, R, and Westerterp-Plantenga, MS. Nutraceuticals for body-weight management: the role of green tea catechins. Physiol Behav. (2016) 162:83–7. doi: 10.1016/j.physbeh.2016.01.044
21. Cheng, M, Zhang, X, Zhu, J, Cheng, L, Cao, J, Wu, Z, et al. A metagenomics approach to the intestinal microbiome structure and function in high fat diet-induced obesity mice fed with oolong tea polyphenols. Food Funct. (2018) 9:1079–87. doi: 10.1039/c7fo01570d
22. Miyata, Y, Shida, Y, Hakariya, T, and Sakai, H. Anti-Cancer effects of green tea polyphenols against prostate Cancer. Molecules. (2019) 24:193–211. doi: 10.3390/molecules24010193
23. Tofolean, IT, Ganea, C, Ionescu, D, Filippi, A, Garaiman, A, Goicea, A, et al. Cellular determinants involving mitochondrial dysfunction, oxidative stress and apoptosis correlate with the synergic cytotoxicity of epigallocatechin-3-gallate and menadione in human leukemia Jurkat T cells. Pharmacol Res. (2016) 103:300–17. doi: 10.1016/j.phrs.2015.12.013
24. Li, G, Zhang, Y, Mbuagbaw, L, Holbrook, A, Levine, MA, and Thabane, L. Effect of green tea supplementation on blood pressure among overweight and obese adults: a protocol for a systematic review. BMJ Open. (2014) 4:e004971. doi: 10.1136/bmjopen-2014-004971
25. Li, G, Zhang, Y, Thabane, L, Mbuagbaw, L, Liu, A, Levine, MA, et al. Effect of green tea supplementation on blood pressure among overweight and obese adults: a systematic review and meta-analysis. J Hypertens. (2015) 33:243–54. doi: 10.1097/hjh.0000000000000426
26. Hashempur, MH, Sadrneshin, S, Mosavat, SH, and Ashraf, A. Green tea (Camellia sinensis) for patients with knee osteoarthritis: a randomized open-label active-controlled clinical trial. Clin Nutr. (2018) 37:85–90. doi: 10.1016/j.clnu.2016.12.004
27. Ahmad, RS, Butt, MS, Sultan, MT, Mushtaq, Z, Ahmad, S, Dewanjee, S, et al. Preventive role of green tea catechins from obesity and related disorders especially hypercholesterolemia and hyperglycemia. J Transl Med. (2015) 13:79. doi: 10.1186/s12967-015-0436-x
28. Li, R, Yu, A, Wu, L, Tian, Y, Li, H, Lu, L, et al. The effect of green tea dust supplementation on the growth performance, slaughter performance, and serum biochemical indicators in Zhedong white geese. Chin J Anim Sci. (2023) 59:232–5. doi: 10.19556/j.0258-7033.20220106-01
29. Mersmann, HJ, and MacNeil, MD. Relationship of plasma lipid concentrations to fat deposition in pigs. J Anim Sci. (1985) 61:122–8. doi: 10.2527/jas1985.611122x
30. Griffin, H, Grant, G, and Perry, M. Hydrolysis of plasma triacylglycerol-rich lipoproteins from immature and laying hens (Gallus domesticus) by lipoprotein lipase in vitro. Biochem J. (1982) 206:647–54. doi: 10.1042/bj2060647
31. Koutsari, C, and Jensen, MD. Thematic review series: patient-oriented research. Free fatty acid metabolism in human obesity. J Lipid Res. (2006) 47:1643–50. doi: 10.1194/jlr.R600011-JLR200
32. Babior, BM, Takeuchi, C, Ruedi, J, Gutierrez, A, and Wentworth, P Jr. Investigating antibody-catalyzed ozone generation by human neutrophils. Proc Natl Acad Sci USA. (2003) 100:3031–4. doi: 10.1073/pnas.0530251100
33. Djordjević, VB. Free radicals in cell biology. Int Rev Cytol. (2004) 237:57–89. doi: 10.1016/s0074-7696(04)37002-6
34. Sadi, G, Şahin, G, and Bostancı, A. Modulation of renal insulin signaling pathway and antioxidant enzymes with streptozotocin-induced diabetes: effects of resveratrol. Medicina. (2018) 55:3–14. doi: 10.3390/medicina55010003
35. Sezer Tuncsoy, B, Tuncsoy, M, Gomes, T, Sousa, V, Teixeira, MR, Bebianno, MJ, et al. Effects of copper oxide nanoparticles on tissue accumulation and antioxidant enzymes of galleria mellonella L. Bull Environ Contam Toxicol. (2019) 102:341–6. doi: 10.1007/s00128-018-2529-8
36. Miao, LP, Zhou, MY, Zhang, XY, Yuan, C, Dong, XY, and Zou, XT. Effect of excess dietary fluoride on laying performance and antioxidant capacity of laying hens. Poult Sci. (2017) 96:2200–5. doi: 10.3382/ps/pex0002
37. Han, B, Yoon, SS, Su, J, Han, HR, and Zhong, DJAAJAS. Effects of selenium, copper and magnesium on antioxidant enzymes and lipid peroxidation in bovine fluorosis. Asian Australas J Anim Sci. (2004) 17:1695–9. doi: 10.5713/ajas.2004.1695
38. Reiter, RJ, Melchiorri, D, Sewerynek, E, Poeggeler, B, Barlow-Walden, L, Chuang, J, et al. A review of the evidence supporting melatonin's role as an antioxidant. J Pineal Res. (1995) 18:1–11. doi: 10.1111/j.1600-079x.1995.tb00133.x
39. Chen, X, Zhou, X, Li, S, Zhang, H, and Liu, Z. Effects of tea residues-fermented feed on production performance, egg quality, antioxidant capacity, caecal microbiota, and ammonia emissions of laying hens. Front Vet Sci. (2023) 10:1195074. doi: 10.3389/fvets.2023.1195074
40. Chen, X, Zeng, D, Zeng, X, and Zeng, Q. Effects of complex antioxidants added to chicken diet on growth performance, serum biochemical indices, meat quality, and antioxidant capacity. Animals. (2024) 14:360. doi: 10.3390/ani14030360
41. Fre, S, Huyghe, M, Mourikis, P, Robine, S, Louvard, D, and Artavanis-Tsakonas, S. Notch signals control the fate of immature progenitor cells in the intestine. Nature. (2005) 435:964–8. doi: 10.1038/nature03589
42. Turner, JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. (2009) 9:799–809. doi: 10.1038/nri2653
43. Zhang, C, Chen, KK, Zhao, XH, Wang, C, and Geng, ZY. Effect of l-theanine on the growth performance, immune function, and jejunum morphology and antioxidant status of ducks. Animal. (2019) 13:1145–53. doi: 10.1017/s1751731118002884
44. Xiao, Y, Gao, X, and Yuan, J. Substituting ethoxyquin with tea polyphenols and propyl gallate enhanced feed oxidative stability, broiler hepatic antioxidant capacity and gut health. Poult Sci. (2024) 103:104368. doi: 10.1016/j.psj.2024.104368
45. Zheng, L, Oh, ST, Jeon, JY, Moon, BH, Kwon, HS, Lim, SU, et al. The dietary effects of fermented Chlorella vulgaris (CBT(®)) on production performance, liver lipids and intestinal microflora in laying hens. Asian Australas J Anim Sci. (2012) 25:261–6. doi: 10.5713/ajas.2011.11273
46. Xiao, Y, Zou, H, Li, J, Song, T, Lv, W, Wang, W, et al. Impact of quorum sensing signaling molecules in gram-negative bacteria on host cells: current understanding and future perspectives. Gut Microbes. (2022) 14:2039048. doi: 10.1080/19490976.2022.2039048
47. Lu, J, Zhang, X, Liu, Y, Cao, H, Han, Q, Xie, B, et al. Effect of fermented corn-soybean meal on serum immunity, the expression of genes related to gut immunity, gut microbiota, and bacterial metabolites in grower-finisher pigs. Front Microbiol. (2019) 10:2620. doi: 10.3389/fmicb.2019.02620
48. Binda, C, Lopetuso, LR, Rizzatti, G, Gibiino, G, Cennamo, V, and Gasbarrini, A. Actinobacteria: a relevant minority for the maintenance of gut homeostasis. Dig Liver Dis. (2018) 50:421–8. doi: 10.1016/j.dld.2018.02.012
49. Azad, MAK, Jiang, H, Ni, H, Liu, Y, Huang, P, Fang, J, et al. Diets partially replaced with cassava residue modulate antioxidant capacity, lipid metabolism, and gut barrier function of Huanjiang Mini-pigs. Front Vet Sci. (2022) 9:902328. doi: 10.3389/fvets.2022.902328
50. Zhang, L, Gui, SQ, Wang, J, Chen, QR, Zeng, JL, Liu, AL, et al. Oral administration of green tea polyphenols (tp) improves ileal injury and intestinal flora disorder in mice with Salmonella typhimurium infection via resisting inflammation, enhancing antioxidant action and preserving tight junction. J Funct Foods. (2020) 64:103654. doi: 10.1016/j.jff.2019.103654
Keywords: tea dust, antioxidant capacity, intestinal absorption capacity, gut microbiota, goose
Citation: Gu Z, Xu W, Tian Y, Lu L and Chen G (2025) Effects of green tea dust on the biochemical parameters, antioxidant capacity, and intestinal microbiota composition in goose. Front. Vet. Sci. 12:1694350. doi: 10.3389/fvets.2025.1694350
Edited by:
Regiane R. Santos, Schothorst Feed Research, NetherlandsReviewed by:
Fernando Capela E Silva, University of Evora, PortugalMoyosore Joseph Adegbeye, University of Africa, Nigeria
Copyright © 2025 Gu, Xu, Tian, Lu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lizhi Lu, bHVsekB6YWFzLmFjLmNu; Guohong Chen, Z2hjaGVuMjAxOUB5enUuZWR1LmNu
 Zhuoya Gu1
Zhuoya Gu1 Wenwu Xu
Wenwu Xu Guohong Chen
Guohong Chen