- 1Centre for Ethics, Philosophy and Public Affairs, University of St Andrews, St Andrews, Fife, United Kingdom
- 2Mace Animal Welfare, Dunfermline, Fife, United Kingdom
- 3School of Veterinary Medicine, College of Environmental and Life Sciences, Murdoch University, Murdoch, WA, Australia
- 4School of Environment and Science, Griffith University, Nathan, QLD, Australia
- 5Animal Welfare Research Group, Faculty of Health and Wellbeing, University of Winchester, Winchester, United Kingdom
Approximately 1.8 billion chicks are hatched worldwide in commercial hatcheries every month. A typical commercial hatchery is a high-speed and stressful environment. Not only is chick welfare impacted while at the hatchery, but also chickens’ early life experiences can have long-lasting impacts on their welfare once they leave the hatcheries. Additionally, chick embryos may have the capacity to experience stress and pain. This study systematically reviewed recent scientific studies exploring the starting point for the capacity to suffer in chicks and chick embryos. It found that the capacity to suffer (i.e., to experience pain, distress, or other prolonged negative welfare states) may commence by embryonic day 18—three days before hatching—and likely earlier. Based on this, serious and widespread welfare problems may exist for the 1.8 billion chicks hatched in hatcheries globally every month.
1 Introduction
Globally, an estimated 1.8 billion chicks are hatched every month primarily to serve the chicken meat and egg industries—but also to serve backyard chicken keepers, scientists, and other more fringe users of chickens (1). This amounts to roughly 900 million chicks per month in the USA alone (2) (p. 15). A typical hatchery is highly automated and processes chicks through the stages of hatching, conveying, sexing (for layers), maceration (especially for males from laying breeds which are unwanted), vaccination, and beak trimming (for layers)—up to roughly seven stages, as depicted in Figure 1 (3, 4). A hatchery conveyor belt can have an acceleration of up to 920 m/s2, and drops of up to 55 cm (5) (pp. 275–276), with ambient noise levels of up to 70 dB and both mechanical and manual handling (6) (p. 136, 138). The largest and most modern hatcheries can process up to 100,000 chicks per hour, amounting to 4 million per week [e.g., (see 7)]. Processing between 1 and 2 million chicks per week is not uncommon (3, 8).
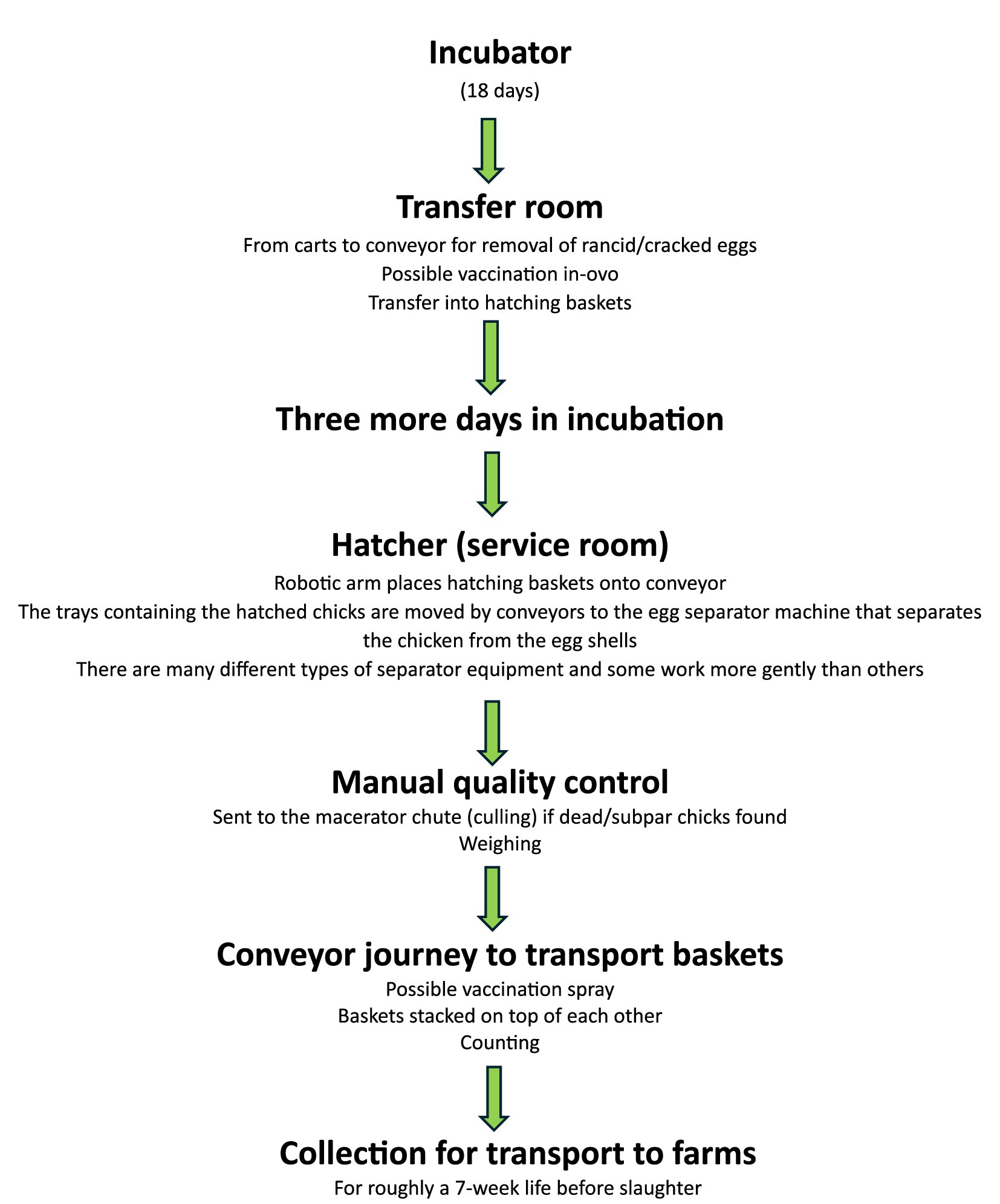
Figure 1. Representative stages in a commercial “broiler” chick hatchery, adapted from content in the USDA’s Poultry Industry Manual (4) (p. 16–18) and observations by Knowles et al. (3).
The culling of male chicks in hatcheries is gaining increasing attention and even being outlawed in some European countries such as Germany (9) (p. 30). However, the welfare concerns about chicks and potentially chick embryos in hatcheries extend beyond the culling of male chicks, as raised by Knowles et al. (3), RSPCA (10), and Animal Equality USA (46) in recent undercover footage. The concerns span avoidable injuries and deaths from the use of unsuitable equipment, ill-maintained machinery, and insufficient staff training or oversight. To assess the potential for suffering of chicks and chick embryos processed at typical hatcheries, this study systematically sourced and analyzed recent scientific evidence regarding the stage of life at which chicks begin to feel pain and distress (i.e., the capacity to suffer). This will also be of great importance for discussions surrounding the latest embryonic stage at which in-ovo sex identification should take place to enable the painless killing of male embryos.
For this study, widely accepted definitions of pain, suffering, distress, and welfare in nonhuman animals were used, as follows: “Pain is an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage” [International Association for the Study of Pain (IASP) (11); p. 2], and “Suffering is one or more bad feelings continuing for more than a short period” (12) (p. 60). To define distress, stress must first be defined: “Stress is the biological response elicited when an individual perceives a threat to its homeostasis” (13) (p. 1). Moberg and Mench (13) then define distress as occurring “when the stress response threatens an individual’s wellbeing” (p. 1). The welfare of an animal is a state which describes how well the animal is coping with his/her environment (12) (p. xiv). While pain and suffering are conceptually defined in the literature, their operationalization in studies involving chick embryos requires careful consideration. Given the absence of verbal communication and overt behavioral expression, researchers rely on indirect indicators such as the maturation of neural pathways (e.g., thalamocortical connections), electrophysiological responses to noxious stimuli, and the presence of coordinated motor reactions (14–16). These proxies are interpreted within a developmental framework, acknowledging that the capacity to suffer likely depends not only on nociceptive processing but also on integrative brain functions associated with affective experience (15). Therefore, in this review, we consider suffering as a multidimensional construct that is inferred from converging neurophysiological and behavioral evidence across embryonic stages.
2 Methodology
We conducted a systematic review of relevant scientific studies exploring the time point at which chicks and chick embryos start to have the capacity to suffer. We chose a systematic review in preference to other forms of evidence. The personal and potentially subjective opinions of experts are considered less reliable than more objective scientific literature analyses (17). Narrative literature reviews often focus on a subset of the literature, based on availability or author choice. These can create conscious or unconscious biases during the selection and inclusion of scientific evidence (18). In contrast, systematic literature reviews aim to minimize bias by identifying and analyzing all relevant studies on a specific topic, using robust and transparent criteria. These are considered to provide evidence of the greatest level of reliability when exploring scientific topics, and their use for such purposes is considered best practice (17–19). Systematic reviews require a transparent detailed search strategy and defined inclusion and exclusion criteria before starting the review. The identification process often utilizes bibliographic scientific literature databases, but can also be supplemented by checking reference lists or manually searching key journals to increase reliability and completeness.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines provide best practice guidelines for conducting systematic reviews (19). Accordingly, the PRISMA guidelines (2020 updated version) were adhered to in the present study. The following leading bibliographic scientific literature databases were used: Web of Science All Databases and Scopus. This concurs with current recommendations regarding the selection of databases for systematic reviews, namely, the use of at least three verified databases (20). Because the All Databases version of Web of Science comprises its Core Collection in addition to Medline and numerous supplementary databases, this fulfills and surpasses these criteria. Additionally, Web of Science Core Collection, Medline, and Scopus have recently been designated as “principal” databases that should be used for systematic reviews (21). They are also all either multidisciplinary or biomedically oriented, which is suitable for the field of enquiry at hand.
The following search string for all databases was devised after extensive piloting of different search strings, and following an initial review of key literature to guide keyword choices: (chick* OR galliform* OR “gallus gallus” OR “gallus domesticus” OR fowl OR bird OR avian OR poultry) AND (in-ovo OR embryo OR fetus OR foetus OR hatchling OR young OR neonatal OR newly-hatched OR day-old) AND (pain OR nocicep* OR suffer* OR distress OR discomfort) AND (stage OR neuron*) AND (development* OR incubation). This provided an appropriate balance of both sensitivity (ensuring key results were not missed) and specificity (ensuring irrelevant results were not included), as described by Bramer et al. (22). Our pilot review found that all “AND” components of our search terms were present within the abstracts of key studies we knew we needed to retrieve; thus, to ensure sufficient specificity, we required all of these components to be present by using “AND.” One digital skills librarian at the University of Winchester, UK, also confirmed the technical suitability of the search string, and recommended checking for other synonyms via EBSCOhost (a very large online research platform providing access to bibliographic databases and search features) and a thesaurus, which was done.
Both databases were searched on 24th September, 2024. A flow diagram summarizing the systematic review stages is provided in Figure 2. No further refinements or exclusions were applied in the searches. Common reasons for excluding items were that studies only focused on adult chickens, on a different species, or on genetics or another insufficiently relevant topic. If it was unclear whether a title was relevant or not, it was retained for a review of the abstract or full paper at subsequent analysis stages; for instance, papers about sexing of chick embryos were retained in case they included information about neurological development relevant to pain perception. The reference lists of the final shortlist of records were also reviewed in case any additional items of importance were present. There were two shortlisted papers written in German, but we collectively possessed advanced German skills, so these were retained.
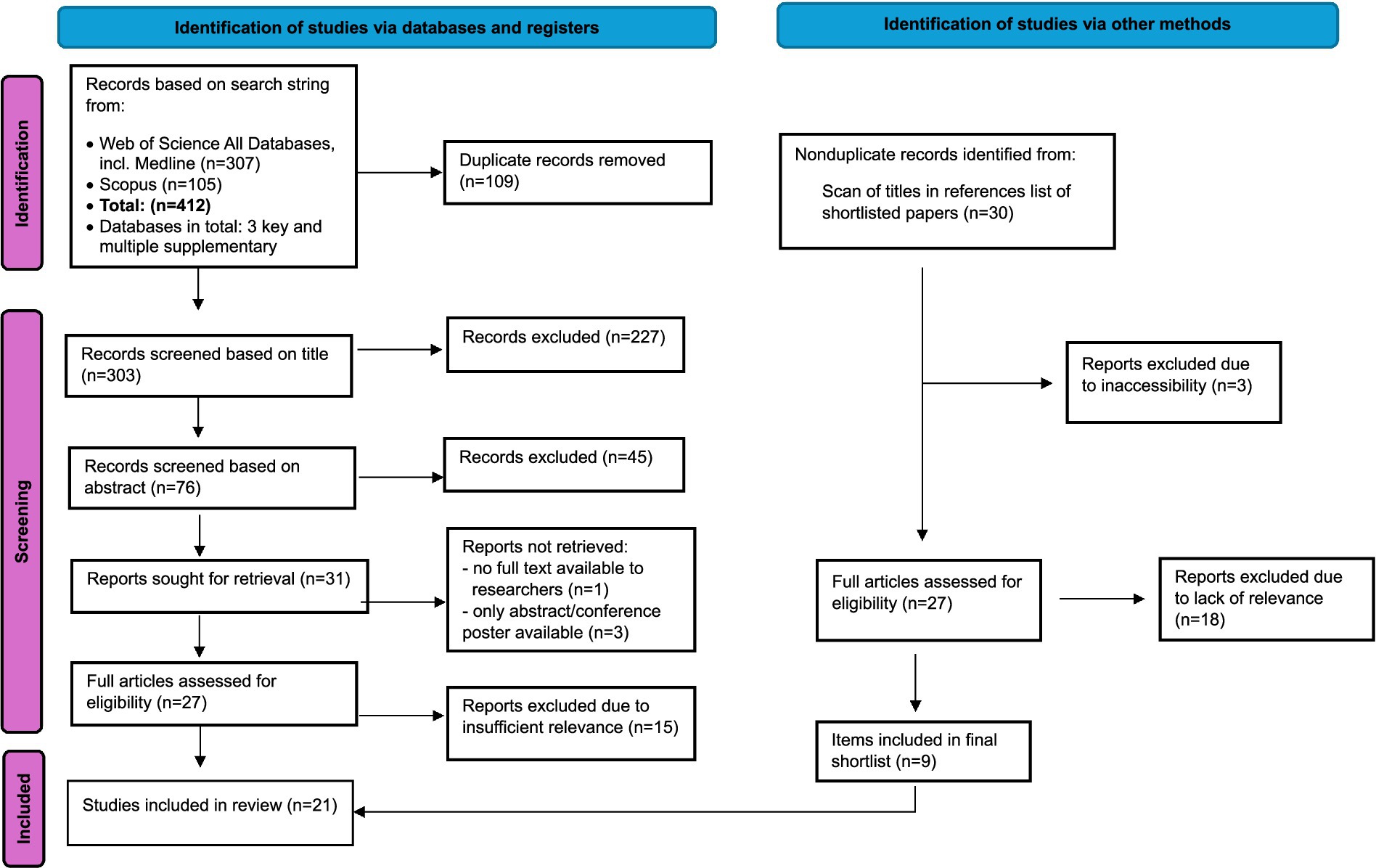
Figure 2. PRISMA flow diagram, adapted from Page et al. (19), used under CC BY 4.0 license.
The shortlisted items were then summarized into Table 1. Essential characteristics and findings were then tallied and analyzed. Consistent with systematic review best practice, the reliability and relevance of the shortlisted items were then assessed and indicated using red/amber/green colors to convey low/medium/high levels, respectively. However, a reliability analysis for each shortlisted study is not an essential component of the PRISMA (19) checklist. Thus, it should be noted, that due to resource limitations, the reliability analyses were based on the subjective assessment of the first author, primarily considering methodological factors (e.g., sample size, starting day of embryonic analysis, and any missing details), but also clarity of the write-up. Green was awarded if no problems or weaknesses were detected, amber if one to three weaknesses were detected, and red if more than three weaknesses were detected (or two major ones). The relevance of the items was likewise based on assessment by the first author. This centered on the relevance of the research questions of each shortlisted item. The scientific information collected was then analyzed to answer the research question: At what stage of development does the chick embryo begin to have the capacity to suffer?
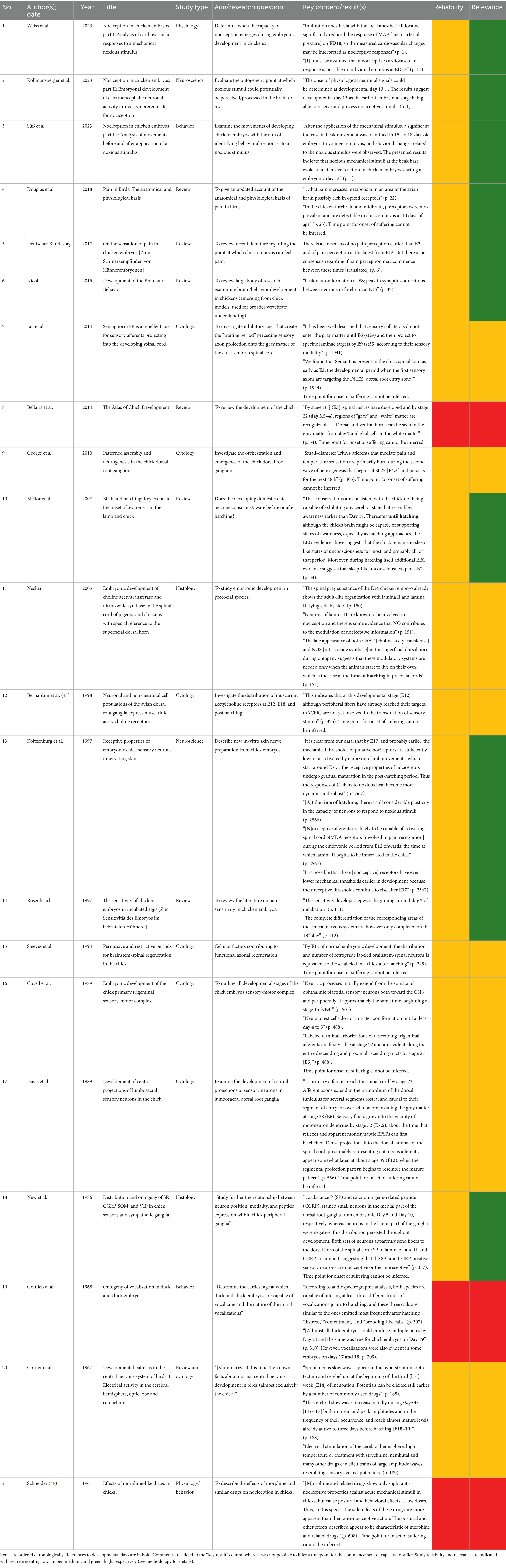
Table 1. Summary of systematic review results focusing on the question: at what stage of development do chick/chick embryos have the capacity to suffer?
3 Results and discussion
3.1 Essential characteristics of the shortlisted studies
Table 1 summarizes the results of the systematic review regarding the stage of life at which chicks begin to have the ability to suffer. Twenty-one relevant studies were located, comprising 6.5 reviews and 14.5 empirical studies. Most items (n = 18) were papers published in peer-reviewed journals, apart from one academic book chapter (23), one academic book (24), and one report from the science department of the German parliament (25). Over 50% of the shortlisted items were published after the year 2000. The 14.5 empirical studies comprised physiological (n = 3.5), behavioral (n = 2.5), and cytological/histological (n = 8.5) approaches, with the “0.5” number stemming from mixed-method studies. Of the 21 shortlisted items, only 10 had titles, aims, or results that were explicitly relevant to the key research question of the present report (i.e., those marked green in the Relevance column of Table 1). Nine of these 10 focused on pain/nociception rather than another aspect of the capacity to suffer, while one (26) focused on awareness, but without defining it. Five of these 10 most-relevant items explicitly defined pain using the IASP’s definition (see subsection 1, for details); another defined “nociceptor” briefly (27), while the remaining four gave no definition of pain or other terms related to suffering or welfare. This was also the case for the 11 less relevant items. See subsection 3.5 for a discussion of the reliability of the shortlisted items.
3.2 Key points of agreement
Among the shortlisted studies, there is consensus that the chicken species (Gallus gallus domesticus) is precocial. This means chicks reach an advanced stage of development before or at the time of hatching, as they are already relatively independent after hatching [(e.g., 28), p. 243; (29), p. 153; (23), p. 44]. There is consensus that avian neuroanatomy is comparable to that of mammals. For instance, avians, like mammals, have a lateralized brain, meaning it is split into different areas with each having a more specialized role. Weiss et al. (30) and Douglas et al. (31) also mention how both C-fibers and A-delta fibers have been found in numerous parts of an avian’s body. These are collectively responsible for sensing generalized, chronic, and low-level pain, as well as acute sharp pain. Again, similar to mammals, avians possess high-threshold nociceptors capable of receiving different types of sensory input, which are part of the peripheral nervous system (31). Transduction occurs to transmit messages to the brain via the spinal-thalamic tract (32) (p. 0.9); (31) (p. 20). The neurotransmitter substance P (which plays a key role in pain signal transmission) that is found in mammals, is also found in avians, as are laminae I and II, which are cellular layers of the gray matter of the spinal cord that are responsible for receiving and modulating sensory input, respectively (31, 33).
One key neuroanatomical difference between avians and mammals is the lack of a neocortex in the avian forebrain (31). Nevertheless, the avian and mammalian forebrains (cerebrums) are still thought to function similarly, with the avian hyperpallium, nidopallium, and mesopallium being largely analogous to the mammalian neocortex (32) (p. 9). These two points—being precocial and having similar functional neuroanatomy to mammals—point to the capacity to suffer being developed in day-old chicks at the very latest. However, there is actually a broad consensus for the capacity to suffer commencing prior to hatching during the late stages of the 21-day incubation period—by “embryonic day” (E) 18 at the latest.
The evidence for the capacity to suffer having developed by E18 first centers around cytological (cell-based) and histological (tissue-based) evidence (see Table 2). Second, this has been further confirmed through the detection of the ability to feel pain or distress at different embryonic timepoints. Through different studies, these tests have provided holistic confirmation. The studies have included physiological measures, such as cardiovascular (30) and EEG [electroencephalogram; (32)]. They have also included behavioral measures, such as vocalizations (34) and variations in bodily movement in response to both noxious stimuli and pain relief (35). As demonstrated in Table 1, a likely starting point for the capacity to suffer was referenced in over half (n = 11) of the shortlisted items, either explicitly or by inference. Of these, nine supported capacity for suffering by E18 at the latest. Timepoints listed ranged through E13 (n = 1), E15 (n = 4), E17 (n = 1), and E18 (n = 3).
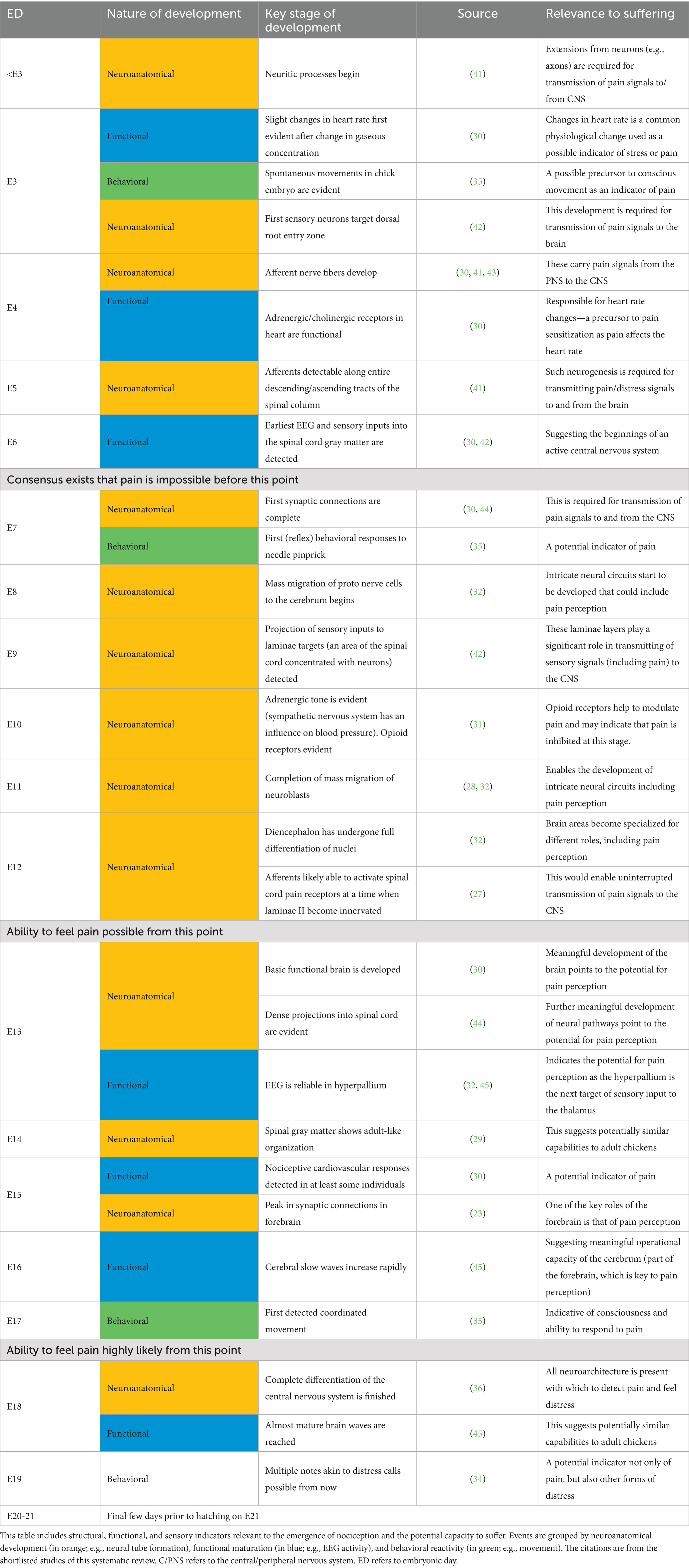
Table 2. Chronological overview of key neurodevelopmental milestones in chick embryos, by embryonic day (E).
Some of the most important findings summarized in Table 1 include significant increases in mean arterial pressure (MAP) and heart rate in response to a noxious stimulus, relative to a control (30). These responses were detected at E16 and E17 respectively, and in both cases, the responses reduced after administration of pain relief. This demonstrates the importance of using multiple measures, as some may have different sensitivities to detecting nociception. There is also some evidence to suggest that pain sensitivity may differ in degree at different stages of development. For instance, Weiss et al. (30) found significant differences in MAP rises in response to a noxious stimulus at E16, but the response was stronger at E18. Additionally, Weiss et al. (30) highlighted that different readings in heart rate and MAP for different individuals demonstrate the individuality of pain perception (pp. 10–11).
Another key finding is that of Süß et al. (35). At E15, these authors found significantly more beak movements and, at E18, more leg/foot movements after a “pinch,” versus “little pinch” or “touch.” The authors also suggest that the significant differences in beak movement between the three levels of touch may indicate pain perception specifically, rather than broader nociception. Tellingly, they also found reduced head movements at E18 after application of pain relief. Kollmansperger et al. (32) found an even earlier sign of brain processing of nociceptive information, with EEG recordings demonstrating activity from E13.
While the capacity to suffer could likely develop at an earlier timepoint than E18, there is a broad consensus that it is near impossible for the capacity to suffer to emerge before E7 [(e.g., 36), p. 112; (30), p. 2]. This is because, while the first afferent nerve fibers can be detected from E4, the earliest EEG readings are at E6.5 with the first synaptic connections being completed on E7. Exemplar stages in the developmental journey of a chick embryo are summarized in Table 2.
3.3 Key points of disagreement
Among the aforementioned 11 shortlisted items for which a starting point for the capacity to suffer is stated or can be inferred, there are two clear outliers. The authors of these outlier items point toward the capacity for suffering not beginning until the time of hatching or even later. They comprise a histological study by Necker (29) and a review by Mellor and Diesch (26). Necker (29) demonstrated that some pain modulating mechanisms are not fully developed until E20—just before hatching—suggesting that these systems are only required once the chick becomes independent. Mellor and Diesch (26) argued that, while chick embryos may have the neuronal capacity to experience awareness (and thus the capacity to suffer significantly) from E17, there are active neurosuppressors, such as adenosine, in play that maintain an unconscious state—including at the time of hatching and even immediately after. They state that the EEG readings support this too (p. 54). Another key concern Mellor and Diesch have is the reliance on nociception and extrapolation from mammals for inferring avian pain. Nociception comprises the physical/physiological aspects of pain, but not the emotional and subjective components (11)—i.e., it is a reaction to an aversive stimulus, rather than the perception of it. The authors deem this concern not only as a critique of individual papers/authors, but consider it a cultural/societal problem. Thus, there is concern about changing definitions of pain. This can be seen, for instance, in the paper by Süß et al. (35). These authors first introduce the definition by the IASP, but then proceed to state that, considering the problems of identifying pain without verbal report, pain could be defined as “a change in species-specific behavior as a possible consequence of a painful experience” (p. 2).
Some of these points are considered and countered by the other main cohort of authors. For instance, Kollmansperger et al. (32) contest that it is currently uncertain whether the embryonal electrical reading corresponds to a sleep-like state. Indeed, even Mellor and Diesch (26) themselves state that adenosine (neurosuppressor) levels are unknown in the chick (embryo). This could be an avenue of further investigation. Additionally, Koltzenburg and Lewin (27), among others, defend the use of nociception as one means of inferring pain in nonhuman animals. They contend that it is also necessary for inferring pain in nonverbal humans. Indeed, Douglas et al. (31) underscore that ascertaining pain in avians is particularly challenging because they are a prey species in which the flight response predominates. This means that overt signs of pain may be limited. Moreover, the same approaches (extrapolation from humans and use of nociception) are often used to assess pain in mammals (37), so it appears an inconsistency if this is permitted for mammals but not avians. Indeed, Weiss et al. (30) point out that MAP is a leading measure of nociception in mammals, as well as avians.
3.4 Implications of findings
National animal welfare legislation often excludes embryonic stages of life [e.g., England and Wales’ (38), s. 1.2]. This could be updated to reflect chick embryos’ potential to feel pain from embryonic day 13. This could affect hatchery practices such as the age at which relatively humane killing methods become important (e.g., urgent maceration versus discarding with other waste streams). It could also affect legislation in specialist areas. For instance, while the UK’s Animals (Scientific Procedures) Act (39) does currently cover chick embryos once they have reached the last third of incubation (s. 1.4.2), the time period covered could be extended to embryonic day 13 (roughly 2 days earlier). Emergent in-ovo sexing technologies are also increasingly being adopted to identify the sex of chick embryos prior to hatching (40). The intention of these technologies is to prevent the culling of newly hatched male chicks who are unwanted by the egg industry. Legislation should enforce their use by day 12 at the latest to specifically ensure that male chicks are destroyed prior to achieving sentience.
3.5 Limitations
Within any normal body of scientific evidence, various limitations are common. While no comprehensive reliability analysis of each included study was completed, the limitations of the items shortlisted within this systematic review mainly center around methodological choices and weaknesses in subsequent publications; hence, no item received a high (green) rating for reliability in Table 1. For instance, some studies only began examining embryo responses from E17 [(e.g., 27)], meaning signs present at earlier embryonal stages could be missed. Some studies had very small sample sizes [(e.g., 30)]. Some were exploratory studies only with no sophisticated power analyses [(e.g., 30); p. 3]. Others sometimes failed to include sufficient detail in the methodology such as whether the first day of incubation counts as E0 or E1 (32) (p. 9). Such limitations should be corrected in future research. Finally, more research that has the development of the capacity for suffering in chickens as its core focus could further strengthen the scientific evidence base. This could also involve examining potential differences between different breeds as Kollmansperger et al. (32) suggested. Notions of suffering could also be broadened away from a heavy focus on pain, to include distress and discomfort, for instance.
4 Conclusion
Limitations of scientific evidence such as those identified in this review are normal within scientific studies. These do not negate the ability to draw overall conclusions with reasonable certainty, given the collected weight of scientific evidence. From this systematic review of relevant scientific studies, it is clear that there is a general scientific consensus that the capacity to suffer in chicks is likely to commence from late stages of incubation, specifically, by E18 and potentially as early as E13. This is indicated by cell- and tissue-based developments, in addition to EEG readings, maturation of neuroarchitecture, and physiological and behavioral responses to both noxious stimuli and pain relief. This is significant for the welfare of the 1.8 billion chicks hatched globally each month. National legislation should be updated to protect embryonated chicks.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
JM: Formal analysis, Data curation, Writing – review & editing, Methodology, Writing – original draft, Investigation. AK: Resources, Project administration, Data curation, Supervision, Writing – review & editing, Methodology.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The authors declare that Animal Equality (USA) partially funded the research that this paper is based on. Representing Animals (UK) also funded its publication open access. However, these organizations were not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Acknowledgments
The authors would like to thank the peer reviewers for their feedback provided during the authorship of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any additional commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. McNeill, L. (2018). How many animals are born in the world every day? BBC. Available online at: https://www.bbc.co.uk/news/science-environment-44412495.
2. USDA. (2024). Chickens and eggs. National Agricultural Statistics Service. Available online at: https://downloads.usda.library.cornell.edu/usda-esmis/files/fb494842n/np194x051/dv141d36t/ckeg0124.pdf.
3. Knowles, TG, Brown, SN, Warriss, PD, Butterworth, A, and Hewitt, L. Welfare aspects of chick handling in broiler and laying hen hatcheries. Anim Welf. (2004) 13:409–18. doi: 10.1017/s0962728600028669
4. USDA. (2013). Poultry industry manual. Available online at: https://www.aphis.usda.gov/sites/default/files/poultry_ind_manual.pdf.
5. de Haas, EN. Opportunities to improve the welfare of young chickens In: C Nicol, editor. Understanding the behaviour and improving the welfare of chickens. London: Burleigh Dodds Science (2020). 261–312.
6. Nielsen, SS, Alvarez, J, Bicout, DJ, Calistri, P, Canali, E, Drewe, JA, et al. Welfare of broilers on farm. EFSA J. (2023) 21:e07788. doi: 10.2903/j.efsa.2023.7788
7. Ceva Ecat-iD Campus. (2024). High speed solutions to maximize production. Available online at: https://www.ecat-id.com/en/products/high-speed-solutions.
8. Graber, R. (2019). Aviagen’s newest, largest hatchery to serve US and beyond. WATTPoultry. Available online at: https://www.wattagnet.com/broilers-turkeys/breeding-genetics/article/15529730/aviagens-newest-largest-hatchery-to-serve-us-and-beyond-wattagnet.
9. Niekerk, T. G. C. M., and Workamp, M. (2022). Scenarios for addressing the dilemma of the culling of day-old male chicks. Wageningen University & Research. Available online at: https://research.wur.nl/en/publications/scenarios-for-addressing-the-dilemma-of-the-culling-of-day-old-ma.
10. RSPCA. (2017). RSPCA welfare standards for hatcheries (chicks, poults and ducklings). Available online at: https://business.rspcaassured.org.uk/media/lykpuobe/hatcheries-standards-rspca-2017.pdf.
11. Raja, S, Carr, D, Cohen, M, Raja, SN, Carr, DB, Finnerup, NB, et al. The revised international association for the study of pain definition of pain: concepts, challenges, and compromises. Pain. (2020) 161:1976–82. doi: 10.1097/j.pain.0000000000001939
13. Moberg, GP, and Mench, JA. The biology of animal stress: Basic principles and implications for animal welfare. Wallingford: CABI (2000).
14. Mota-Rojas, D, Orihuela, A, Strappini, A, Ghezzi, M, Domínguez-Oliva, A, Napolitano, F, et al. Understanding animal pain: Behavioral foundations and implications for welfare optimization [Comprendiendo el dolor animal: Fundamentos asociados al comportamiento y optimización del bienestar animal]. Rural Soc Prod Environ. (2024) 24:133–58.
15. Sneddon, LU. Comparative physiology of nociception and pain. Physiology. (2018) 33:2–82. doi: 10.1152/physiol.00022.2017
16. Sneddon, LU. Evolution of nociception and pain: evidence from fish models. Philos Trans R Soc B. (2019) 374:20190290. doi: 10.1098/rstb.2019.0290
18. Uman, LS. Systematic reviews and meta-analyses. J Can Acad Child Adolesc Psychiatry. (2011) 20:57–9.
19. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n72. doi: 10.1136/bmj.n71
20. Mathew, MJ. Literature search in systematic reviews: how much is good enough? Clin Epidemiol Glob Health. (2024) 25:101485. doi: 10.1016/j.cegh.2023.101485
21. Gusenbauer, M, and Haddaway, NR. Which academic search systems are suitable for systematic reviews or meta-analyses? Evaluating retrieval qualities of Google scholar, PubMed, and 26 other resources. Res Synth Methods. (2020) 11:181–217. doi: 10.1002/jrsm.1378
22. Bramer, WM, de Jonge, GB, Rethlefsen, ML, Mast, F, and Kleijnen, J. A systematic approach to searching: an efficient and complete method to develop literature searches. J Med Libr Assoc. (2018) 106:531–41. doi: 10.5195/jmla.2018.283
23. Nicol, C. Development of the brain and behaviour In: The behavioural biology of chickens. Wallingford: CABI (2015). 35–57.
25. Deutscher Bundestag. (2020). Zum Schmerzempfinden von Hühnerembryonen Sachstand Wissenschaftliche Dienste. German parliament. Available online at: https://www.bundestag.de/resource/blob/805020/58284d172e611640db4dc17ec59d0865/WD-8-075-20-pdf-data.pdf.
26. Mellor, DJ, and Diesch, TJ. Birth and hatching: key events in the onset of awareness in the lamb and chick. N Z Vet J. (2007) 55:51–60. doi: 10.1080/00480169.2007.36742
27. Koltzenburg, M, and Lewin, GR. Receptive properties of embryonic chick sensory neurons innervating skin. J Neurophysiol. (1997) 78:2560–8. doi: 10.1152/jn.1997.78.5.2560
28. Steeves, JD, Keirstead, HS, Ethell, DW, Hasan, SJ, Muir, GD, Pataky, DM, et al. Permissive and restrictive periods for brainstem-spinal regeneration in the chick. Prog Brain Res. (1994) 103:243–62. doi: 10.1016/S0079-6123(08)61140-1
29. Necker, R. Embryonic development of choline acetyltransferase and nitric oxide synthase in the spinal cord of pigeons and chickens with special reference to the superficial dorsal horn. Anat Embryol. (2005) 210:145–54. doi: 10.1007/s00429-005-0018-4
30. Weiss, L, Saller, AM, Werner, J, Süß, SC, Reiser, J, Kollmansperger, S, et al. Nociception in chicken embryos, part I: analysis of cardiovascular responses to a mechanical noxious stimulus. Animals. (2023) 13:2710. doi: 10.3390/ani13172710
31. Douglas, JM, Sanchez-Migallon Guzman, D, and Paul-Murphy, JR. Pain in birds: the anatomical and physiological basis. Vet Clin North Am Exot Anim Practi. (2018) 21:17–31. doi: 10.1016/j.cvex.2017.08.008
32. Kollmansperger, S, Anders, M, Werner, J, Saller, AM, Weiss, L, Süß, SC, et al. Nociception in chicken embryos, part II: embryonal development of electroencephalic neuronal activity in ovo as a prerequisite for nociception. Animals. (2023) 13:2839. doi: 10.3390/ani13182839
33. New, HV, and Mudge, AW. Distribution and ontogeny of SP, CGRP, SOM, and VIP in chick sensory and sympathetic ganglia. Dev Biol. (1986) 116:337–46. doi: 10.1016/0012-1606(86)90137-5
34. Gottlieb, G, and Vandenbergh, JG. Ontogeny of vocalization in duck and chick embryos. J Exp Zool. (1968) 168:307–25. doi: 10.1002/jez.1401680303
35. Süß, SC, Werner, J, Saller, AM, Weiss, L, Reiser, J, Ondracek, JM, et al. Nociception in chicken embryos, part III: analysis of movements before and after application of a noxious stimulus. Animals. (2023) 13:2859. doi: 10.3390/ani13182859
37. Bateson, P. Assessment of pain in animals. Anim Behav. (1991) 42:827–39. doi: 10.1016/S0003-3472(05)80127-7
38. Animal Welfare Act (2006). Available online at: https://www.legislation.gov.uk/ukpga/2006/45/contents.
39. Animals (Scientific Procedures) Act (1986). Available online at: https://www.legislation.gov.uk/ukpga/1986/14/schedule/1.
40. Animal Welfare Committee. (2023). Opinion on alternatives to the culling of day-old male chicks. Department for Environment, Food & Rural Affairs. Available online at: https://assets.publishing.service.gov.uk/media/65eae6e062ff48ff7487b270/AWC_Opinion_on_chick_culling_alternatives.pdf.
41. Covell, DA, and Noden, DM. Embryonic development of the chick primary trigeminal sensory-motor complex. J Comp Neurol. (1989) 286:488–503. doi: 10.1002/cne.902860407
42. Liu, RQ, Wang, W, Legg, A, Abramyan, J, and O’Connor, TP. Semaphorin 5B is a repellent cue for sensory afferents projecting into the developing spinal cord. Development. (2014) 141:1940–9. doi: 10.1242/dev.103630
43. George, L, Kasemeier-Kulesa, J, Nelson, BR, Koyano-Nakagawa, N, and Lefcort, F. Patterned assembly and neurogenesis in the chick dorsal root ganglion. J Comp Neurol. (2010) 518:405–22. doi: 10.1002/cne.22248
44. Davis, BM, Frank, E, Johnson, FA, and Scott, SA. Development of central projections of lumbosacral sensory neurons in the chick. J Comp Neurol. (1989) 279:556–66. doi: 10.1002/cne.902790405
45. Corner, MA, SchadäEa, JP, Sedláček, J, Stoeckart, R, and Bot, APC. Developmental patterns in the central nervous system of birds. I. Electrical activity in the cerebral hemisphere, optic lobe and cerebellum. Prog Brain Res. (1967) 26(C:145–92. doi: 10.1016/S0079-6123(08)61422-3
46. Animal Equality. (2024). Undercover investigation inside a Foster farms hatchery. Available online at: https://animalequality.org/campaign/end-factory-farming/baby-chicks-factory-farms/.
47. Bernardini, N, De Stefano, ME, Tata, AM, Biagioni, S, and Augusti-Tocco, G. Neuronal and non-neuronal cell populations of the avian dorsal root ganglia express muscarinic acetylcholine receptors. Int J Dev Neurosci. (1998) 16:365–77. doi: 10.1016/S0736-5748(98)00038-0
Keywords: chicks, chick embryos, pain, capacity to suffer, hatcheries, chick welfare, chicken welfare
Citation: Mace JL and Knight A (2025) Development of the capacity to suffer in embryos and chicks: a systematic review of relevant studies. Front. Vet. Sci. 12:1698528. doi: 10.3389/fvets.2025.1698528
Edited by:
Daniel Mota-Rojas, Metropolitan Autonomous University, MexicoReviewed by:
Temple Grandin, Colorado State University, United StatesMarcelo Ghezzi, Universidad Nacional del Centro de Buenos Aires, Argentina
Copyright © 2025 Mace and Knight. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jenny L. Mace, am02MDlAc3QtYW5kcmV3cy5hYy51aw==
 Jenny L. Mace
Jenny L. Mace Andrew Knight
Andrew Knight