- College of Bioengineering, Jiuquan Vocational and Technical University, Jiuquan, China
Bovine herpesvirus type 1 (BoHV-1) constitutes a major etiological agent associated with the onset of bovine respiratory disease in cattle. This virus is widely prevalent across global cattle populations, leading to significant economic losses. The advancement of early diagnostic methodologies and the formulation of effective vaccines are crucial for the prevention, control, and potential eradication of BoHV-1. This review offers a comprehensive overview of current diagnostic methodologies for BoHV-1, encompassing serological diagnostic approaches, molecular detection methods, and emerging novel techniques. Furthermore, recent development in vaccine research targeting BoHV-1 infection are discussed. Ultimately, this review aims to provide critical insights into the management of BoHV-1 infection, and guide future research efforts in the enhancement of diagnostic tools and vaccine strategies.
1 Introduction
Infectious bovine rhinotracheitis (IBR), attributed to bovine herpesvirus type 1 (BoHV-1), represents a significant infectious disease that results in substantial economic losses within the bovine industry (1). The disease caused by BoHV-1 infection has been listed a notifiable animal disease by the World Organization for Animal Health (WOAH). In addition to IBR, the clinical symptoms of this disease are characterized by reproductive disorders and the reduction in milk production in cows and central neural disease in calves (2). Moreover, BoHV-1 infection can also cause immunosuppression in cattle, thus the co-infection with BoHV-1 and other pathogens have been frequently monitored in clinical samples (3).
In the early 1940s, the initial documented case of BoHV-1 infection, referred to as bullous vaginitis, was identified in cattle within the United States. Following this, specific antibodies targeting BoHV-1 were detected in cattle in 1941 and the etiological agent (BoHV-1) was successfully isolated from clinical specimens in 1955 (4). Currently, BoHV-1 exhibits a high prevalence across various regions, including Europe (notably Ireland (5), Serbia (6), Iraq (7), and Irish (8)), Asia (specially Turkey (9) and Iran (10)), as well as the United States (11). In addition to cattle, this pathogen is capable of infecting a variety of mammals, including goats (12, 13) and deer (14, 15), thereby drawing significant attention to its potential threat to other animal industry.
Considering the persistent prevalence of BoHV-1 in cattle herds, the advancement of timely and accurate diagnostic approaches, as well as effective vaccines, is imperative for effective disease management and eradication. This review comprehensively synthesizes current advancements in diagnostic techniques and vaccine development targeting BoHV-1 infection, with the objective of establishing a foundational framework to support the prevention, control, and prospective elimination of this disease.
2 Genomic characteristics
BoHV-1 is an enveloped, double-stranded linear DNA virus that belongs to the members of Alpha-herpesvirinae subfamily (16). The DNA genome of BoHV-1 contains many open reading frames, which encode various structural proteins and regulatory proteins (17). The genome of BoHV-1 encodes multiple glycoproteins, including gL, gM, gB, gC, gD, and gE, etc., Among which, gB, gC, and gD play critical roles in viral entry into host cells and elicit the production of virus-neutralizing antibodies (18–20). The gE, gG, and the thymidine kinase (TK) enzyme are closely associated with virus virulence, deletion of these genes significantly attenuates viral pathogenicity without compromising viral replication or immunogenicity. Consequently, these genes are frequently targeted for the development of gene-deleted vaccines and differential diagnostic assays (21, 22).
According to the genetic characteristics of BoHV-1 strains, which are classified into three subtypes: BoHV-1.1, BoHV-1.2 (BoHV-1.2a and BoHV-1.2b), and BoHV-1.3 (also designated as BoHV-5) (23, 24). The subtypes BoHV-1.1 and BoHV-1.2a are typically associated with IBR and reproductive disorders, such as abortion, in infected animals. While infection with BoHV-1.2b strains usually leads to sexually transmitted disease that primarily affects the genital tract of cattle (24).
Due to the widespread used of the BoHV-1 modified-live virus (MLV) vaccines, recombinant strains derived from both field strains and MLV vaccines have been reported globally. For instance, Offay et al. isolated a novel recombinant BoHV-1 strain from an aborted fetus (25). The majority of its genome exhibited 100% sequence similarity to the field strain, while other regions shared higher homology to the MLV strain (25). Furthermore, the co-prevalence with BoHV-1 and BoHV-5 has increased the risk of genetic recombination between these viruses (26–28). For example, Maidana et al. conducted a phylogenetic analysis based on the gB and gD gene sequences of three isolates (26). The gB gene sequences of three recombinant strains were clustered into the same branch with that of those of BoHV-1 strains, whereas their gD genes showed higher sequence similarity to those of BoHV-5 strains (26).
3 Current diagnostic techniques for BoHV-1 detection
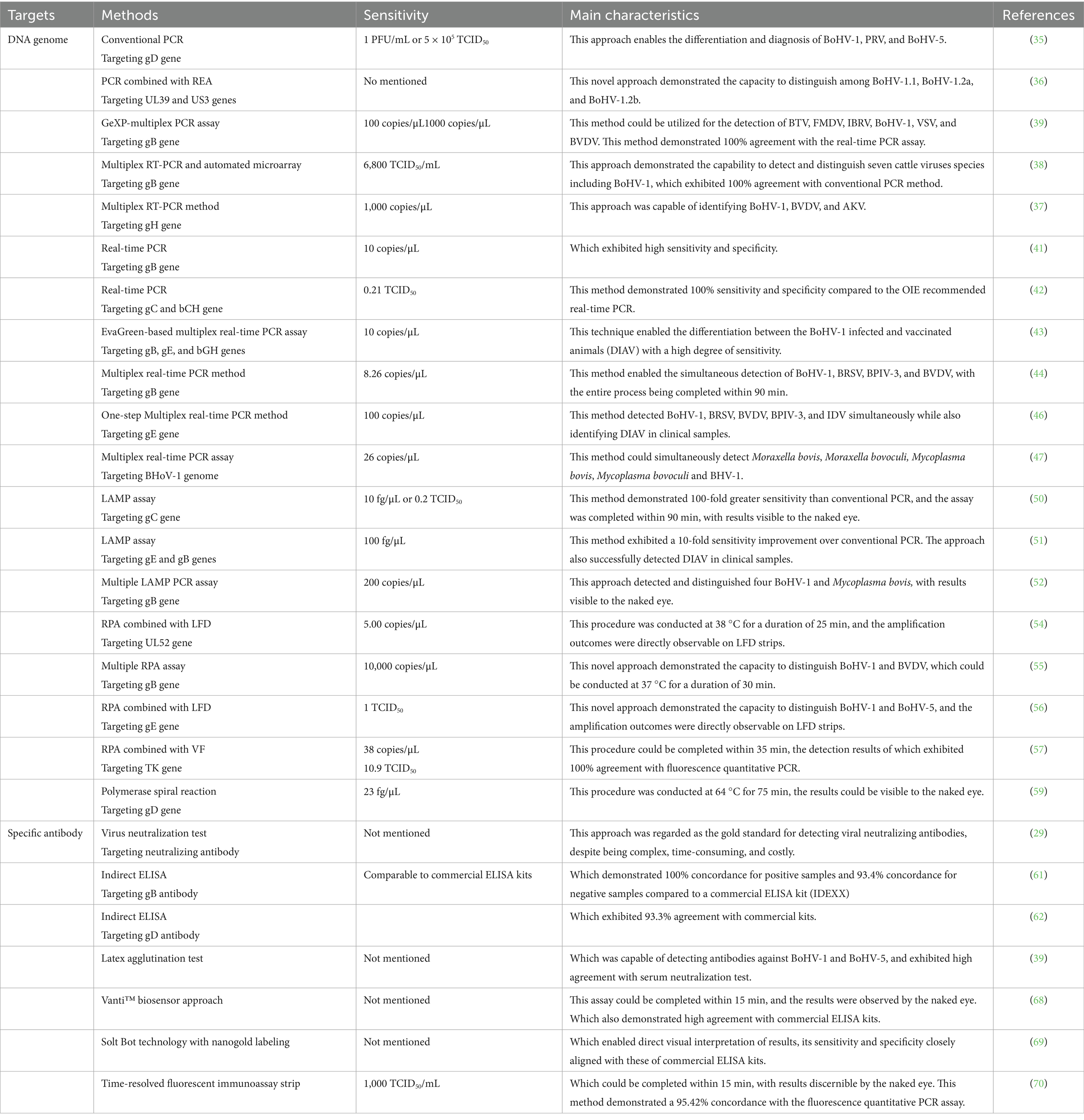
At present, multiple detection methodologies have been developed for the identification of BoHV-1, which can be broadly categorized into three main groups: (1) virus isolation and transmission electron microscopy (TM) techniques aimed at the direct visualization of viral particles; (2) molecular diagnostic approaches targeting viral nucleic acids, including conventional polymerase chain reaction (PCR), real-time PCR, loop-mediated isothermal amplification (LAMP), and recombinase polymerase amplification (RPA); (3) serological assays designed to detect specific antibodies against BoHV-1, such as enzyme-linked immunosorbent assay (ELISA). These representative detection methods are summarized in Table 1.
3.1 Virus isolation and transmission electron microscopy
3.1.1 Virus isolation
Virus isolation is widely considered the “gold standard” for pathogen identification and serves as a critical foundation for subsequent research endeavors, including investigations into viral infection mechanisms, antiviral drug screening, and vaccine development. A critical procedure for BoHV-1 isolation involves the collection of fresh clinical samples exhibiting a high viral load, particularly from the upper respiratory tract (e.g., nasal swab) and reproductive tract tissues of cattle infected with BoHV-1. Bovine testicular cells and Madin-Darby bovine kidney (MDBK) cells demonstrate susceptibility to BoHV-1 infection, rendering these cell lines frequently employed for the purposes of virus isolation, purification, and identification.
In brief, the supernatants derived from clinical samples were incubated with a monolayer of susceptible cells, and typical cytopathic effects will be observed within 48 to 72 h post-infection. Following this incubation period, both the cells and supernatants are collected for freeze–thaw cycles and centrifugation (29). After purification, further experiments are required to identify the isolated virus, these identification methods encompass immunofluorescence assays (IFA), molecular and serological testing, as well as transmission electron microscopy.
3.1.2 Transmission electron microscopy
Transmission electron microscopy (TM) has been extensively employed to directly examine the morphological characteristics of viral particles (30, 31). In this procedure, cells infected with BoHV-1 or supernatants containing BoHV-1 are stained overnight with 2% phosphotungstic acid, and subsequently analyzed using EM. The viral particles are characterized by a roughly spherical shape, with diameters approximately between 150 and 200 nm. Moreover, the BoHV-1 particle comprises an icosahedral capsid core enveloped by a lipid membrane (30).
In addition to BoHV-1, other herpesviruses can infect cattle, including other subtypes of BoHV and pseudorabies virus (32, 33). These herpesviruses exhibit similar morphological characteristics and therefore cannot be directly identified by TM. Consequently, additional techniques such as PCR and IFA should be used in combination for accurate identification.
3.2 Molecular diagnostic techniques
3.2.1 Conventional PCR
In the past few years, conventional PCR technique has been widely employed for the molecular identification of pathogens. In general, PCR methods usually target the conserved regions of BoHV-1 genome, such as gD, gB, gE, and gC genes. Specifically, these positive PCR products can be sequenced for further analyzing its genotype and genetic variations (23, 34). Wang et al. developed a PCR method targeting the gD gene of herpesviruses, enabling the simultaneous differentiation and diagnosis of the gD genes of BoHV-1 (372 bp), BoHV-5 (206 bp and 440 bp), and PRV (303 bp) (35). The sensitivity of this assay for detecting BoHV-1 nucleic acid was determined to be 1 PFU/mL or 5 × 105 TCID50. These findings demonstrate that this PCR method possesses high specificity and sensitivity, allowing for the effective discrimination of BoHV-1, BoHV-5, and PRV in clinical samples (35).
BoHV-1 is classified into three sub-genotypes: BoHV-1.1, BoHV-1.2a, and BoHV-1.2b (36). A novel methodology has been established to enable rapid subtyping of BoHV-1 strains (36). In brief, gene fragments from UL39 (439 bp) and US3 (700 bp) are amplified by a standard multiplex PCR method. The resulting PCR products are then subjected to digestion with the HindIII restriction enzyme, to produce distinct fragment patterns (36). These fragment profiles allow for differentiation among the BoHV-1.1, BoHV-1.2a, and BoHV-1.2b sub-genotypes based on the number and size of the PCR fragments generated (36).
In recent years, a variety of multiplex RT-PCR based techniques have been developed to simultaneously detect BoHV-1 alongside other pathogens (37–40). Zhang et al. developed a multiplex RT-PCR method capable of identifying BoHV-1, BVDV, and Akabane virus (AKV), achieving a detection limit of 1,000 copies/μL for BoHV-1 (37). Similarly, Lung and his colleagues designed an RT-PCR assay integrated with a novel automated microarray system to concurrently detect BoHV-1 and seven additional pathogens, while the sensitivity of this approach for BoHV-1 was notably lower than conventional PCR method (38). Fan et al. successfully developed a GeXP-multiplex PCR assay for the simultaneous identification of six bovine viruses, including BoHV-1. Specifically, this innovative technology demonstrated a detection limit of 10 copies/μL for BoHV-1 (39).
3.2.2 Real-time PCR
Real-time PCR constitutes an advanced detection technology evolved from traditional PCR methodologies, enabling a transition from qualitative to quantitative analysis of viral nucleic acids, thereby providing enhanced sensitivity (40). This technique is extensively used for the early detection of pathogens, contributing significantly to the prevention and control of infectious diseases (41–44). Wang and colleagues established a TaqMan real-time fluorescence quantitative PCR assay targeting the gB gene of BoHV-1, which achieved a detection sensitivity of 10 copies/μL and demonstrated no cross-reactivity with other viruses. Application of this method to 183 clinical samples resulted in the identification of six BoHV-1 positive cases (41). In a comparable study, Pawar et al. developed an EvaGreen-based multiplex real-time PCR assay for simultaneously detecting the viral genes gB and gE, along with an internal positive control gene, which was capable of differentiating between wild-type and gE-deleted strains of BoHV-1 (43).
In clinical settings, BoHV-1 often co-infects alongside other pathogens, presenting with non-specific clinical manifestations that complicate differential diagnosis. Consequently, the development of multiplex fluorescent PCR assays are capable of simultaneously detecting BoHV-1 and other pathogens, which greatly reduce diagnostic costs and enhance detection efficiency (44–48). For example, Jiang et al. established a multiplex real-time PCR assay designed for the simultaneous identification of BoHV-1, BRSV, BPIV-3, and BVDV (44). This assay demonstrated detection limits of 8.26 copies/μL for BoHV-1, BVDV, BPIV-3, and 82.68 copes/μL for BRSV. Furthermore, this method exhibited high reproducibility and specificity, thereby providing robust technical support for the rapid detection of multiple pathogens (44).
3.2.3 Loop-medical isothermal amplification
Loop-medical isothermal amplification (LAMP) technology, developed by Notomi et al. enables nucleic acid amplification at constant temperatures. Unlike conventional PCR, this method eliminates the need for thermal cycling and reduces equipment complexity (49). LAMP system employs two or three pairs of primers targeting viral conserved regions, amplifying DNA fragments within nearly 1 h under isothermal conditions (60 ~ 65 °C). The amplification products generate magnesium pyrophosphate precipitates, enabling direct visual detection without specialized instrumentation (49).
Pawar et al. designed three primer pairs targeting the conserved region of the gC gene using PrimerExplorer V4 software to develop a LAMP assay for BoHV-1 nucleic acid detection (50). The assay achieved amplification within 90 min at 63 °C and detected as few as 10 viral genome copies, demonstrating 100-fold greater sensitivity than conventional PCR, while matching real-time PCR performance (50). Since most commercial attenuated and inactivated vaccines lack the gE gene, Pawar et al. also developed a LAMP assay targeting the gB and gE genes, which could be used to distinguish wild-type infections from animals vaccinated with gE-deleted vaccines (51). This assay exhibited a detection limit of 100 fg, representing a tenfold improvement over conventional PCR method, and its products could be visually assessed without requiring gel electrophoresis equipment (51).
Similar to multiplex real-time PCR, researchers designed two primers targeting the BoHV-1 gB gene and the Mycoplasma bovis uvrC gene. The gB gene internal primers were conjugated with FAM fluorescent probes, while the uvrC gene primers were labeled with CY5 probes (52). This assay detected the recombinant plasmid containing both target genes at 200 copies/μL, with amplification products showing distinct green fluorescence for BoHV-1 and red fluorescence for M. bovis (52). Clinical validation against WHO-recommended PCR methods revealed 95% ~ 96.6% sensitivity and 100% specificity, confirming its diagnostic utility (52).
3.2.4 Recombinase polymerase amplification
RPA is an isothermal nucleic acid detection approach that utilizes the recombinases, single-stranded binding proteins, and DNA polymerases to amplify target sequences at constant temperatures (53). This method could be accomplished in approximately 25 min and achieve higher efficiency than loop-mediated isothermal amplification (LAMP) (53). Hou et al. established an RPA assay targeting the conserved UL52 gene region of BoHV-1, combining it with lateral flow dipsticks (LFD) to create an RPA-LFD detection system (54). After incubating nucleic acid samples in the RPA system at 38 °C for 25 min, the amplification products subsequently were directly visualized on LFD strips (54). The system detected as few as 5 copies/μL and exhibited no cross-reactivity with related herpesviruses or bovine pathogens. Clinical evaluations demonstrated perfect agreement with real-time PCR results, confirming its high specificity, sensitivity, and practicality (54). Jiang et al. developed a multiple RPA assay using primers targeting the gB gene of BoHV-1 and the 5’UTR gene of BVDV, enabling simultaneous detection of both viruses (55). This multiplex assay produces results within 30 min at 37 °C, with the amplification products analyzed by agarose gel electrophoresis, achieving detection limits of 10 copies/μL for BVDV and 10,000 copies/μL for BoHV-1 (55).
In addition, Wu et al. developed an RPA assay for the simultaneous detection of BoHV-1 and BoHV-5, integrating the RPA amplification products with LFD test strips to create an RPA-LFD technology (56). This approach achieved visual results within 30 min, demonstrating a detection limit of 1 TCID50 and sensitivity equivalent to conventional real-time PCR (56). Separately, researchers in Jilin University from China designed an RPA-VF system combining RPA with a closed vertical flow visualization device (VF) targeting the BoHV-1 TK gene (57). The complete procedure spent about 35 min, including DNA genome extraction (5 min), RPA amplification at 42 °C (25 min), and visualization (5 min). The assay detected as few as 38 copies/μL of recombinant plasmid and 10.9 TCID50 of infectious viruses (57). In addition, this detection method did not show cross reactivity with other pathogenic nucleic acids, and the detection results of clinical samples were completely consistent with those of fluorescence quantitative PCR (100.0%) (57).
3.2.5 Polymerase spiral reaction
Polymerase spiral reaction (PSR) is an innovative isothermal nucleic acid amplification technique that utilizes DNA polymerase with chain displacement activity to efficiently amplify DNA fragments under constant temperature conditions. This characteristic makes it suitable for the rapid detection of pathogenic microorganisms (58). Malla et al. designed one pair of primers targeting the gD gene of BoHV-1 to establish a PSR method (59). This optimized reaction protocol involved an initial denaturation step at 95 °C for 5 min, followed by amplification at a constant temperature of 64 °C for 75 min (59). The positive PSR products appeared green after adding an appropriate amount of SYBR Green 1 dye, while the negative product were orange visually; in addition, the PSR amplification results could also be analyzed by agarose gel electrophoresis (59). Overall, the minimum detection limit of this method for target DNA was 23 fg/μL, which was consistent with the sensitivity of fluorescence quantitative PCR but 100 times higher than of traditional PCR; and this detection method exhibited no cross reactivity with other pathogens, therefore it had high specificity (59).
3.3 BoHV-1 antigen or antibody detection technologies
3.3.1 Serum neutralization test
Serum neutralization test (SNT) remains the gold-standard method for detecting virus-specific neutralizing antibodies, which play a pivotal role in the assessment of vaccine efficacy and serological studies (60). As for BoHV-1, infections of susceptible cell lines, such as MDBK cells, results in distinctive cytopathic effects, including cellular rounding, vacuolization, and subsequent plaque formation (29). When viral particles are pre-incubated with serum samples containing BoHV-1 specific neutralizing antibodies, viral infection would be efficiently suppressed, preventing or attenuating the CPE development.
The procedure can be summarized as follows: BoHV-1 susceptible cells, such as MDBK cells, are seeded in 96-well culture plates, serial dilutions of the tested serum samples are subsequently prepared and combined with a predetermined titer of BoHV-1 virus before being added into the wells. The plates are incubated under controlled temperature conditions in a CO2 incubator, with daily monitoring for CPEs. The absence of BHoV-1 specific CPEs throughout the observation period indicates the presence of neutralizing antibodies against BoHV-1 in the serum sample, whereas their presence denotes a negative result. It is important to acknowledge that the virus neutralization test has several limitations, including its labor-intensive nature, extended assay duration, and the necessity for specialized laboratory equipment. Consequently, this method is less frequently employed in clinical practice for routine serological antibody detection.
3.3.2 ELISA
ELISA exploits the specific interaction between antigens and antibodies to detect the target molecules in serum, with broad applications including the identification of antibodies and antigens. The immunogenic glycoproteins gD, gC, gB, gE, and gG of BoHV-1 are commonly employed as targets in the developments of ELISA assays (61–63). Wu et al. expressed the BoHV-1 gB protein in a prokaryotic expression system to establish an indirect ELISA, which demonstrated 100% concordance for positive samples and 93.4% concordance for negative samples when compared with a commercial antibody detection kit (IDEXX), thereby confirming its diagnostic reliability (61). Similarly, Liu et al. developed an indirect ELISA based on the gD glycoprotein, which exhibited 93.3% agreement with commercial kits, as well as high sensitivity, specificity, and reproducibility (62).
ELISAs targeting the gE glycoprotein have proven effective in differentiating wild-type BoHV-1 infections from responses induced by gE-deleted vaccines, rendering them particularly useful for seroepidemiological investigations (64, 65). In recent year, Chinese researchers developed a gE-blocking ELISA characterized by intra- and inter-assay variability below 10%, and a 97.7% concordance rate with serum neutralization tests during clinical validation (66). Similarly, Chowdhury established a blocking ELISA technique targeting the cytoplasmic tail of BoHV-1 gE, which proved effective for distinguishing between animals infected with field strains and those infected with or vaccinated against gE-deleted viruses (67).
3.3.3 Other serological methods
In addition to ELISA, various serological detection techniques have been developed for identifying antibodies specific to BoHV-1. Fan et al. introduced a latex agglutination test capable of concurrently detecting antibodies against BoHV-1 and BoHV-5, When benchmarked against the serum neutralization test, this assay demonstrated a sensitivity of 94.68% and a specificity of 97.47%, suggesting its utility as a convenient diagnostic tools for BoHV-1 and BoHV-5 antibodies, albeit without the capacity to differentiate between these two strains (39). Crok et al. developed the Vanti™ biosensor method for the detection of BoHV-1 antibody, which exhibited specificity and sensitivity rates of 98.1 and 95.6%, respectively, relative to commercial ELISA kits (68). Notably, this approach offers reduced detection costs and a rapid turnaround time of 15 min, with results observable by the naked eye, obviating the need for costly instrumentation the thereby enhancing its practicality (68).
Japolla et al. devised an immunoassay that integrated Solt Blot technology with nanogold labeling for BoHV-1 antibody detection (69). This method afforded considerable convenience, enabling direct visual interpretation of result reliance on sophisticated equipment. Its sensitivity and specificity closely aligned with those of ELISA, indicating promising applicability in contexts lacking ELISA infrastructure (69). Additionally, Liu et al. developed a time-resolved fluorescent immunoassay strip for the detection of BoHV-1 antibody, capable of completing the assay within 15 min and achieving a sensitivity threshold of 1,000 TCID50/mL, with results discernible by the naked eye (70). This method demonstrated a 95.42% concordance with the fluorescence quantitative PCR assay endorsed by the WOAH, underscoring its suitability for monitoring BoHV-1 infections within dairy cattle populations (70).
4 Advances in vaccine development against BoHV-1
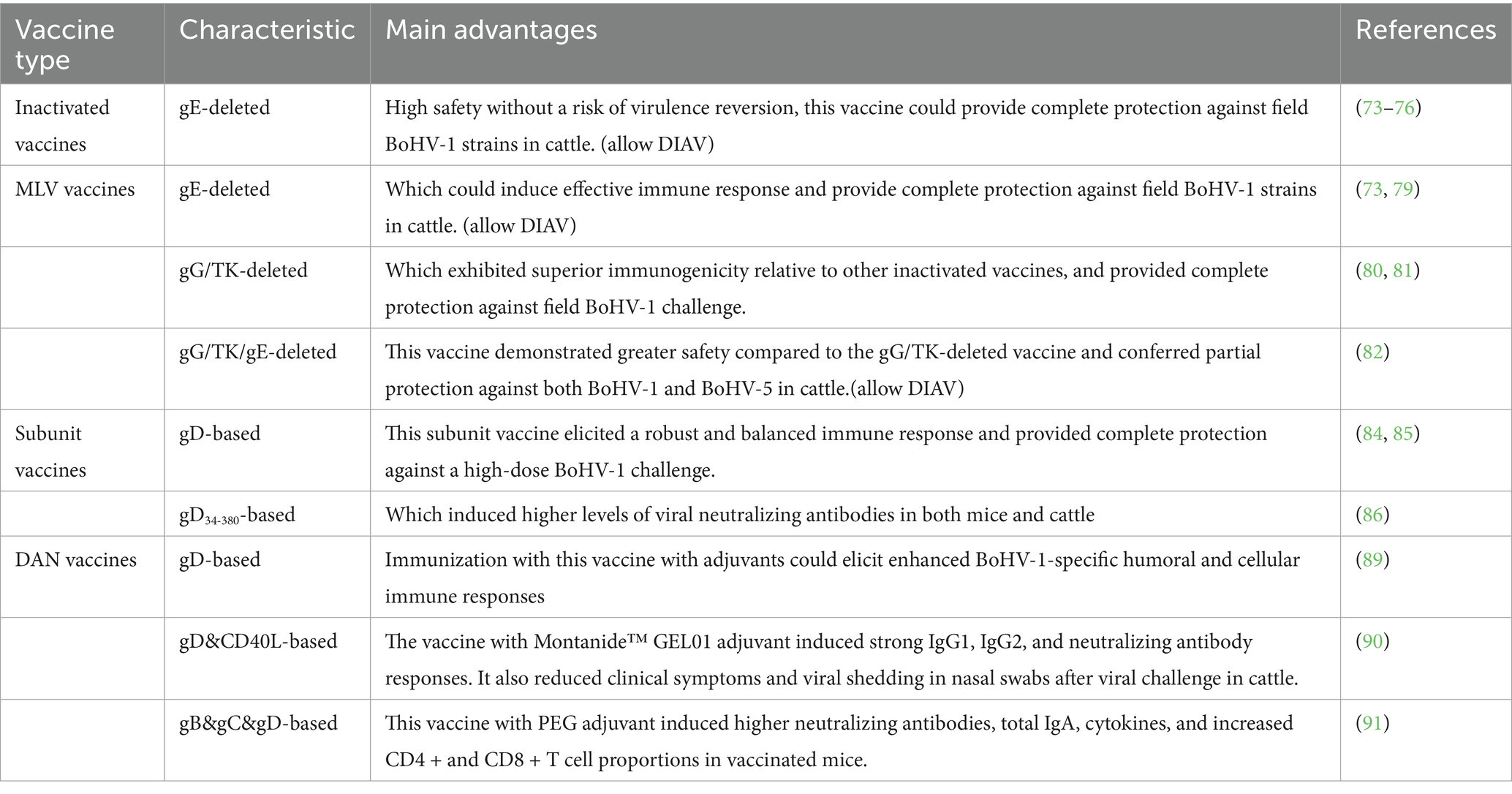
Vaccination represents the most effective strategy to combat infectious diseases such as BoHV-1. In recent years, much efforts have focused on developing various vaccines against BoHV-1, including inactivated, MLV, subunit, DNA, as well as vectored vaccines (as summarized in Tables 2, 3). The following section summarizes research progress pertaining to these vaccine classes.
4.1 Inactivated and modified-live virus vaccines
Alphaherpesvirinae gE protein is critically involved in viral infection progresses, immune evasion, and host cell interaction (71, 72), thus which has become a significant target antigen in the development of gene-modified vaccines, including inactivated and MLV vaccines. Notably, inactivated vaccines demonstrate excellent safety without potential risks of reversion to virulence, and which could provided efficient protein against BoHV-1 in water buffalo and cattle (73–76). Thus inactivated vaccines are widely used for the eradication of BoHV-1 in some Europe countries (65).
Currently, several MLV vaccines are commercially available and have substantially contribute to mitigating the economic impact associated with BoHV-1 infection (77, 78). Moreover, numerous MLV vaccine candidates have been enveloped, demonstrating effective protection against BoHV-1 infection (73, 79–82). For instance, Weiss et al. engineered a gE-deleted BoHV-1 strain expressing green fluorescent protein, and intramuscular administration of this genetically modified strain significantly enhanced the production of virus-neutralizing antibodies (80). Similarly, Zhang et al. developed a BoHV-1 strain with deletions in the gG and TK genes, immunization with this novel vaccine candidate elicited high titers of specific gB antibodies and neutralizing antibodies (80, 81). Furthermore, vaccination with this gG-/TK- gene-deleted vaccine strain conferred robust protection against high challenge with field BoHV-1 strain, and exhibited superior immunogenicity relative to other inactivated vaccines (80, 81). Subsequently, they constructed a recombinant BoHV-1 gG-/TK−/gE- triple deleted strain, which exhibited lower replication efficiency compared to the wild-type strain (82). Moreover, this vaccine candidate was safer to calves compared to BoHV-1 gG&TK gene-deleted strain, while which exhibited lower protection efficiency against wild-type BoHV-1 and BoHV-5 strains (82).
4.2 Subunit vaccines
Subunit vaccine constitutes a form of immunization developed through the isolation of distinct immunogenic antigens, including proteins, polysaccharides, or lipids, derived from pathogenic organisms (83). In contrast to the inactivated vaccines or MLV vaccines, subunit vaccines exclude the complete pathogen and instead employ specific structural components to elicit a target immune response and induce protective immunity (84).
BoHV-1 possesses a large DNA genome that encodes several envelope proteins. Among these, gD, gC, and gB have been identified as principle candidates for the development of subunit vaccines. Previous studies demonstrated that immunization with a truncated, secreted form of BoHV-1 gD combined with CpG oligodeoxynucleotides elicited a robust and balanced immune response in calves (84), which provided complete protection against a high-dose BoHV-1 challenge (85). Recently, Hoa et al. employed both baculovirus and Escherichia coli systems to produce the ectodomain of gD (gD34-380), designated as EgD and BgD, respectively (86). Immunization with BgD induced higher levels of viral neutralizing antibodies in mice compared to both the EgD and inactivated vaccine groups. Furthermore, the BgD subunit vaccine was able of eliciting robust neutralizing antibody responses against BoHV-1 in cattle (86). Similarly, recombinant BoHV-5 gD expressed in the Pichia pastoris system could generate elevated neutralizing antibody titers against both BoHV-5 and BoHV-1 (87).
4.3 DNA vaccines
DNA vaccines are developed by inserting the gene encoding a specific antigen into a plasmid vector, thereby enabling the expression of target antigen within eukaryotic cells (88). This vaccination strategy offers multiple advantages, such as straightforward design and production, cost efficiency, and favorable biosafety profiles (88). Langellotti et al. employed the plasmid pRSET-B to generate a recombinant plasmid encoding a truncated, secreted form of gD (PCIgD) (89). Immunization with pCIgD, combined with various adjuvants elicited enhanced BoHV-1-specific humoral and cellular immune responses compared to the pCIgD only group in murine models (89). Next, they constructed a recombinant plasmid that encoded both gD and the functional extracellular domain of bovine CD40 ligand (pCIgD-CD40L) using the pCIgD plasmid (89). Administration of pCIgD-CD40L alongside the adjuvant Montanide™ GEL01 resulted in increased levels of total IgG1, IgG2, and neutralizing antibodies against BoHV-1 in bovine serum (90). Furthermore, this immunization regimen significantly alleviated clinical symptoms and reduced viral shedding in nasal swabs following viral challenge in cattle (90).
In addition to gD, Liu et al. combined plasmids encoding gB, gC, and gD proteins, adsorbed onto PEI magnetic beads, and demonstrated that vaccination with this mixture and the adjuvant polyethylene glycol have induced the expression of viral proteins in the lung but not the spleen of mice (91). This approach also elicited elevated levels of neutralizing antibodies, total IgA, cellular cytokines and the increased proportions of CD4 + and CD8 + T cells in vaccinated mice (91).
4.4 Vectored vaccines developed using BoHV-1
Consistent with other members of the Alphaherpesvirinae subfamily, BoHV-1 possesses a large DNA genome that includes several non-essential genes, such as gE, TK, and gG. The deletion of these genes does not compromise viral replication efficiency (92). Consequently, the successful incorporation of exogenous genes into these non-essential genomic regions of BoHV-1, coupled with the efficient selection of recombinant viruses via homologous recombination techniques, indicating that BoHV-1 holds potential as a novel vaccine vector (92).
4.4.1 Recombinant vaccine candidate for management of BoHV-1 and PRV
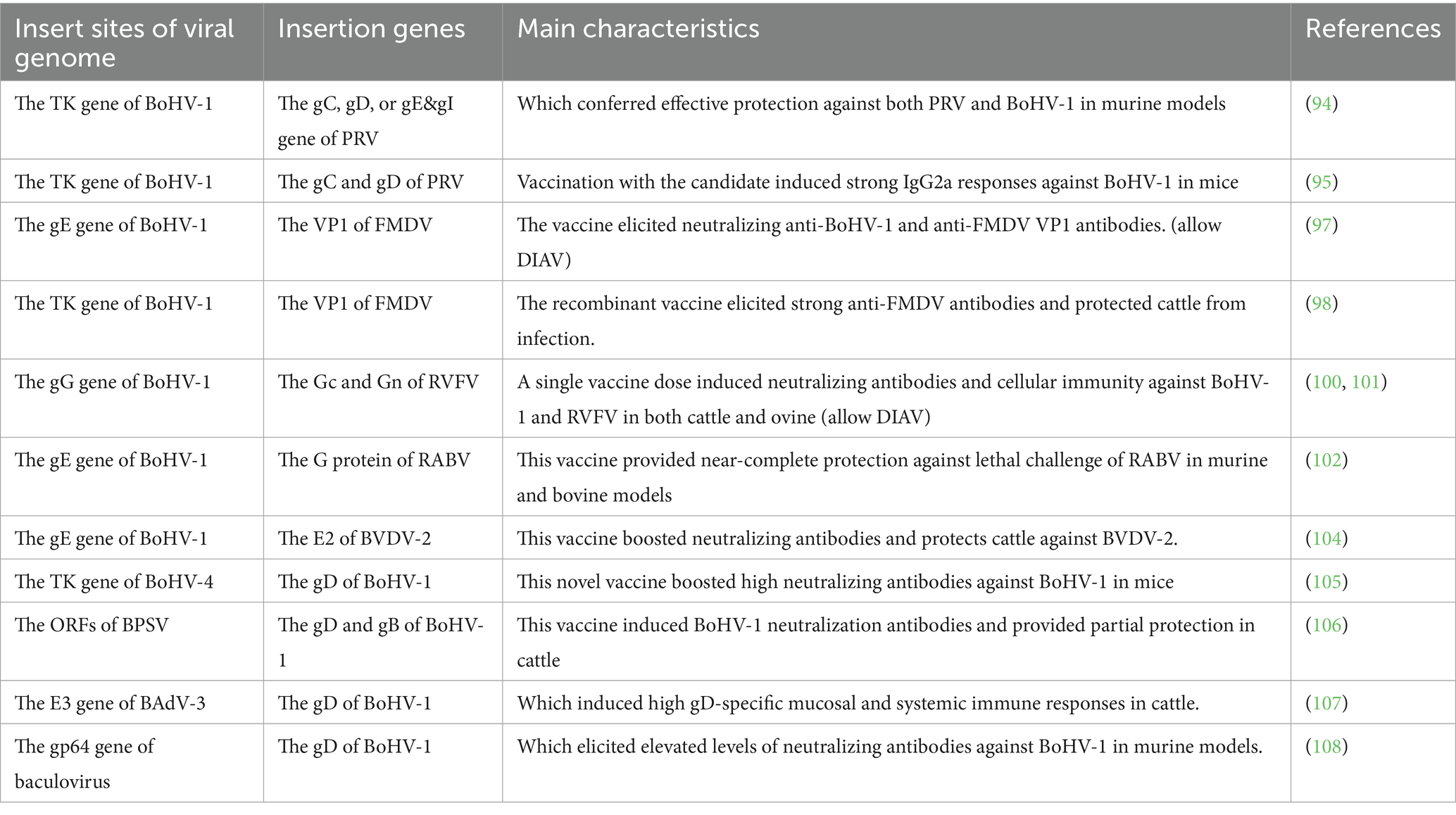
Pseudorabies virus (PRV) is considered a significant infectious agent posing a substantial threat to the development of the global swine industry (93). Furthermore, its ability to transmit across species, from pigs to other animals such as cattle and goats, has garnered considerable scientific interest (93). Otsuka et al. developed recombinant BoHV-1 strains that expressed the gB, gC, gD, or gE of PRV (94). Among these, recombinant strains expressing gC, gD, gE and gI but not gB, conferred effective protection against both PRV and BoHV-1 in murine models (94). Takashima et al. successfully incorporated the PRV gB and gC genes into the BoHV-1 genome, this recombinant strain demonstrated increased susceptibility to mouse tissue cell lines compared to the parental BoHV-1 strain (95). Moreover, immunization with this vaccine candidate induced robust IgG2a antibody responses against BoHV-1 in mice (95).
4.4.2 Recombinant vaccine candidate for management of BoHV-1 and FMDV
Foot-and-mouth disease virus (FMDV) represents a significant challenge to the global livestock industry, and its widespread prevalence also poses a potential threat to human populations (96). Ren et al. constructed a recombinant BoHV-1 strain engineered to express the VP1 protein of FMDV (97). Further experiments conducted in rabbits demonstrated that this vaccine candidate elicited robust viral neutralizing antibody response against BoHV-1, as well as generated specific antibodies targeting the FMDV VP1 protein (97). Kit et al. developed a recombinant BoHV-1 vector expressing the VP1 protein of FMDV, as verified by western blot analysis (98). Immunization with this recombinant vaccine induced high levels of IgG and neutralizing antibody responses against FMDV, conferring effective protection against FMDV infection in cattle (98).
4.4.3 Recombinant vaccine candidate for management of BoHV-1 and RVFV
Rift valley fever virus (RVFV) infection is associated with elevated mortality rates neonatal animals and induces abortion in pregnant livestock, such as cattle and sheep (99). Moreover, the prevalence of RVFV poses potential threats to human health (99). Pavulraj et al. developed a BoHV-1qmv-vectored vaccine expressing the amino-terminal glycoprotein (Gn) and carboxy-terminal glycoprotein (Gc) of RVFV (100). Notably, this recombinant strain demonstrated significant attenuation and safety in cattle (100, 101). A single vaccination dose of the vaccine elicited strong, and specific neutralizing antibody responses against both BoHV-1 and RVFV, in addition to inducing a cellular immune response (100). Similarly, this vaccine candidate has the potential to generate a strong humoral and cellular immune response against both BoHV-1 and RVFV in ovine subjects (101).
4.4.4 Recombinant vaccine candidate for management of BoHV-1 and RV
Rabies virus (RABV) has been endemic in over 150 countries globally, posing a significant risk to humans as well as various animal species, including livestock such as cattle and sheep (102). Zhao et al. constructed a recombinant BoHV-1 strain that expressed the RABV glycoprotein (G) while simultaneously deleting the gE (102). Importantly, the RABV G was located on the surface of this recombinant viral particles. A single administration of this novel vaccine candidate elicited robust activation of dendritic cells and B cells, and a specific neutralizing antibody response against RABV (102). This immune response conferred near-complete protection against a severe lethal challenge infection in both murine and bovine models (102).
4.4.5 Recombinant vaccine candidate for management of BoHV-1 and BVDV
Bovine viral diarrhea virus (BVDV) and BoHV-1 are significant pathogens contributing to the bovine respiratory disease complex on a global scale (103). Zhao et al. generated a recombinant BoHV-1 strain with quadruple gene mutations that concurrently expressed the E2 protein of BVDV-2 (104). Further analysis demonstrated that this novel vaccine candidate have elicited significantly elevated levels of neutralizing antibodies targeting BoHV-1 and BVDV-2, in comparison to the commercially available vaccines (104). Also, which could confer protection to the cattle against a virulent BVDV-2 challenge (104).
4.4.6 Vectored vaccines developed using other DNA viruses
In addition to BoHV-1, several large DNA viruses have been utilized as vectors to express the gD and/or gB proteins of BoHV-1, including BoHV-4, Bovine popular stomatitis virus (BPSV), bovine adenovirus-3 (BADV-3), and baculovirus (105–108). Notably, Delhon et al. constructed two recombinant BPSV strains expressing either the gD protein alone or both the gD and gB proteins of BoHV-1, respectively (107). Both of two vaccine candidates have induced robust neutralizing antibody responses against BoHV-1 in cattle, and conferred approximately 75% protection following challenge with high doses of BoHV-1 (107).
5 Perspective and conclusion remarks
BoHV-1 continues to be a primary infectious agent responsible for IBR, reproductive disorders and immunosuppression in cattle, resulting in significant economic losses worldwide. A meta-analysis conducted by Chen et al. evaluated the prevalence of BoHV-1 in China over the period from 1979 to 2018, revealing a pooled prevalence rate of 40.37% (17,535/43441) within cattle populations (109). González et al. reported a seroprevalence of BoHV-1 infection of 44.9% (325/725) in Colombia (11). Moreover, 21.43% (1,130/5273) of unvaccinated animals tested positive for BoHV-1 specific antibodies in samples collected from Ireland between 2018 and 2020 (110). Collectively, these findings indicate a substantial prevalence of BoHV-1 infection within cattle populations globally. In response to this challenge, numerous diagnostic techniques and vaccines have been developed, which are crucial for the effective management for this disease and the mitigation of its adverse impacts.
In recent years, a range of diagnostic methodologies has been established for the detection of specific antibodies, nucleic acids, or antigens of BoHV-1. These advancements have significantly enhanced researcher’s ability to elucidate the global epidemiological patterns of BoHV-1. However, this section presents several concerns. Firstly, despite the development of numerous detection methods, most were designed to target gene or antibodies other than the gE gene of BoHV-1. It is recommended that detection techniques specifically targeting the gE nucleic acid or antibodies be developed to enable differentiation between wild-type infections and animals vaccinated with gE-deleted vaccines. Secondly, considering the infrastructure constraints in many cattle farming regions, these practical, reliable, and cost-efficient DNA assays (such as specific LAMP or RPA) can be effectively deployed at the farm level by non-specialist personnel to facilitate regional eradication initiatives (53, 58).
In terms of the progress in vaccine development, the primary categories encompass inactivated vaccines, MLV vaccines, subunit vaccines, DNA vaccines, and live virus-vectored vaccines. Importantly, these vaccine types have demonstrated effective protection against BoHV-1 in animal models. However, several concerns are summarized in this section Firstly, virus-vectored vaccines have demonstrated significant potential in the prevention of various infectious diseases, including BoHV-1 infection. However, the strong immune response elicited by the host against the BoHV-1 vector itself may constrain the efficacy or durability of the immune response directed toward the foreign antigen following subsequent administrations (111). Secondly, similar with other animal herpesvirus, attenuated MLV vaccines incorporating deletions of one or multiple genes are considered safe and have the potential to confer effective immunity against BoHV-1 infection. However, the potential risk of genetic reversion to virulence or recombination with wild-type field strains remains a concern that cannot be overlooked (112). Thirdly, mucosal immunity is integral to the antiviral defense against BoHV-1 infection, particularly because BoHV-1 predominantly causes respiratory disease. Nevertheless, the capacity of these novel vaccines to elicit mucosal IgA antibody responses in cattle remains uninvestigated. Or the existing relationship between neutralizing antibody titers and effective mucosal protection has yet to be thoroughly examined.
In conclusion, BoHV-1 remains a significant pathogen affecting cattle globally, with high prevalence rates reported across different regions. Although various diagnostic tools and vaccines have been developed to combat the disease, challenges remain in differentiating infected from vaccinated animals and in ensuring accessibility in resource-limited settings. Targeting the gE gene in diagnostic assays could enhance this differentiation, while portable, rapid detection technologies such as RPA-LFD or biosensors offer practical solutions for field use. Vaccine development has progressed with promising candidates like live virus-vectored vaccines showing potential for multi-disease protection. However, issues related to cost, safety, and efficacy hinder their commercialization. Future research should focus on optimizing vaccine formulations, reducing production costs, and expanding vaccine applications to other susceptible species to better control BoHV-1 transmission and its economic impact.
Author contributions
JZ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Validation, Writing – original draft, Writing – review & editing. JC: Investigation, Methodology, Writing – original draft. LC: Investigation, Methodology, Writing – original draft. CZ: Conceptualization, Writing – original draft. LG: Conceptualization, Writing – original draft. XK: Conceptualization, Methodology, Writing – original draft. DL: Methodology, Writing – original draft. YZ: Conceptualization, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the High Efficiency Production Technology Innovation Team Project for Herbivores provided by Jiuquan Vocational and Technical University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Pastoret, PP, Thiry, E, Brochier, B, and Derboven, G. Bovid herpesvirus 1 infection of cattle: pathogenesis, latency, consequences of latency. Ann Rech Vet. (1982) 13:221–35.
2. Jones, C, and Chowdhury, S. A review of the biology of bovine herpesvirus type 1 (BHV-1), its role as a cofactor in the bovine respiratory disease complex and development of improved vaccines. Anim Health Res Rev. (2007) 8:187–205. doi: 10.1017/S146625230700134X
3. Sayers, RG. Associations between exposure to bovine herpesvirus 1 (BoHV-1) and milk production, reproductive performance, and mortality in Irish dairy herds. J Dairy Sci. (2017) 100:1340–52. doi: 10.3168/jds.2016-11113
4. Graham, DA. Bovine herpes virus-1 (BoHV-1) in cattle-a review with emphasis on reproductive impacts and the emergence of infection in Ireland and the United Kingdom. Ir Vet J. (2013) 66:15. doi: 10.1186/2046-0481-66-15
5. Graham, DA, Gallagher, C, Carden, RF, Lozano, JM, Moriarty, J, and O'Neill, R. A survey of free-ranging deer in Ireland for serological evidence of exposure to bovine viral diarrhoea virus, bovine herpes virus-1, bluetongue virus and Schmallenberg virus. Ir Vet J. (2017) 70:13. doi: 10.1186/s13620-017-0091-z
6. Milićević, V, Sapundžić, ZZ, Glišić, D, Kureljušić, B, Vasković, N, Đorđević, M, et al. Cross-sectional serosurvey of selected infectious diseases in wild ruminants in Serbia. Res Vet Sci. (2024) 170:105183. doi: 10.1016/j.rvsc.2024.10518
7. Abdulazeez, A, and Esmaeel, S. Molecular detection of bovine herpes virus-1 among cattle in Mosul city, Iraq. Bulg J Vet Med. (2024) 27:190–5. doi: 10.15547/bjvm.2022-0047
8. Barrett, D, Parr, M, Fagan, J, Johnson, A, Tratalos, J, Lively, F, et al. Prevalence of bovine viral Diarrhoea virus (BVDV), bovine herpes virus 1 (BHV1), leptospirosis and Neosporosis, and associated risk factors in 161 Irish beef herds. BMC Vet Res. (2018) 14:8. doi: 10.1186/s12917-017-1324-9
9. İnce, ÖB, and Şevik, M. Risk assessment and seroprevalence of bovine herpesvirus type 1 infection in dairy herds in the inner Aegean region of Turkey. Comp Immunol Microbiol Infect Dis. (2022) 80:101741. doi: 10.1016/j.cimid.2021.101741
10. Karimi, O, Bitaraf Sani, M, Bakhshesh, M, Zareh Harofteh, J, and Poormirzayee-Tafti, H. Prevalence of bovine herpesvirus 1 antibodies and risk factors in dairy cattle of Iran's central desert. Trop Anim Health Prod. (2022) 55:23. doi: 10.1007/s11250-022-03426-x
11. González, CB, Briñez, K, Tobón, JC, Corredor, DWS, Bauermann, FV, and Guzmán Barragán, BL. Cross-sectional seroprevalence study of bovine herpesvirus 1, bovine respiratory syncytial virus, and parainfluenza virus 3 in cattle from Villavicencio, Colombia. BMC Vet Res. (2025) 21:24. doi: 10.1186/s12917-024-04461-6
12. Pourmahdi Borujeni, M, Abbasi, AH, Haji Hajikolaei, MR, and Seifi Abad Shapouri, MR. Seroepidemiology of bovine herpesvirus-1 in goats in South-Western Iran. Vet Med Sci. (2024) 10:e1574. doi: 10.1002/vms3.1574
13. Golender, N, Bumbarov, V, Kovtunenko, A, David, D, Guini-Rubinstein, M, Sol, A, et al. Identification and genetic characterization of viral pathogens in ruminant gestation abnormalities, Israel, 2015-2019. Viruses. (2021) 13:2136. doi: 10.3390/v13112136
14. Yatsentyuk, SP, Pchelnikov, AV, Safina, ER, and Krasnikova, MS. The first study on the occurrence of bovine herpesviruses in the wild fauna of the Moscow region, Russia. Vet World. (2022) 15:2052–8. doi: 10.14202/vetworld.2022.2052-2058
15. Rola, J, Larska, M, Socha, W, Rola, JG, Materniak, M, Urban-Chmiel, R, et al. Seroprevalence of bovine herpesvirus 1 related alphaherpesvirus infections in free-living and captive cervids in Poland. Vet Microbiol. (2017) 204:77–83. doi: 10.1016/j.vetmic.2017.04.006
16. Jones, C. Bovine herpesvirus 1 counteracts immune responses and immune-surveillance to enhance pathogenesis and virus transmission. Front Immunol. (2019) 10:1008. doi: 10.3389/fimmu.2019.01008
17. Pokhriyal, M, Ratta, B, Yadav, BS, Kumar, A, Saxena, M, Verma, OP, et al. Three newly identified immediate early genes of bovine herpesvirus 1 lack the characteristic octamer binding motif- 1. Sci Rep. (2018) 8:11441. doi: 10.1038/s41598-018-29490-8
18. Hou, LN, Wang, FX, Wang, YX, Guo, H, Liu, CY, Zhao, HZ, et al. Subunit vaccine based on glycoprotein B protects pattern animal guinea pigs from tissue damage caused by infectious bovine rhinotracheitis virus. Virus Res. (2022) 320:198899. doi: 10.1016/j.virusres.2022.198899
19. Liu, Y, Zhang, Q, Zou, M, Cui, J, Shi, X, Li, L, et al. Cell entry of bovine herpesvirus-1 through clathrin- and caveolin-mediated endocytosis requires activation of PI3K-Akt-NF-κB and Ras-p38 MAPK pathways as well as the interaction of BoHV-1 gD with cellular receptor nectin-1. Vet Microbiol. (2023) 279:109672. doi: 10.1016/j.vetmic.2023.109672
20. Li, Y, van Drunen Littel-van den Hurk, S, Babiuk, LA, and Liang, X. Characterization of cell-binding properties of bovine herpesvirus 1 glycoproteins B, C, and D: identification of a dual cell-binding function of gB. J Virol. (1995) 69:4758–68. doi: 10.1128/JVI.69.8.4758-4768.1995
21. Shaw, AM, Braun, L, Frew, T, Hurley, DJ, Rowland, RR, and Chase, CC. A role for bovine herpesvirus 1 (BHV-1) glycoprotein E (gE) tyrosine phosphorylation in replication of BHV-1 wild-type virus but not BHV-1 gE deletion mutant virus. Virology. (2000) 268:159–66. doi: 10.1006/viro.1999.0164
22. Miller, JM, Whetstone, CA, Bello, LJ, and Lawrence, WC. Determination of ability of a thymidine kinase-negative deletion mutant of bovine herpesvirus-1 to cause abortion in cattle. Am J Vet Res. (1991) 52:1038–43. doi: 10.2460/ajvr.1991.52.07.1038
23. Zhou, Y, Li, X, Ren, Y, Hou, X, Liu, Y, Wei, S, et al. Phylogenetic analysis and characterization of bovine herpesvirus-1 in cattle of China, 2016-2019. Infect Genet Evol. (2020) 85:104416. doi: 10.1016/j.meegid.2020.104416
24. Chothe, SK, Sebastian, A, Thomas, A, Nissly, RH, Wolfgang, D, Byukusenge, M, et al. Whole-genome sequence analysis reveals unique SNP profiles to distinguish vaccine and wild-type strains of bovine herpesvirus-1 (BoHV-1). Virology. (2018) 522:27–36. doi: 10.1016/j.virol.2018.06.015
25. d'Offay, JM, Fulton, RW, Eberle, R, Dubovi, EJ, and Chase, CCL. Complete genome sequence of bovine herpesvirus type 1.1 (BoHV-1.1) Los Angeles (LA) strain and its genotypic relationship to BoHV-1.1 Cooper and more recently isolated wild-type field strains. Arch Virol. (2019) 164:2843–8. doi: 10.1007/s00705-019-04398-4
26. Maidana, SS, Craig, PO, Craig, MI, Ludwig, L, Mauroy, A, Thiry, E, et al. Evidence of natural interspecific recombinant viruses between bovine alphaherpesviruses 1 and 5. Virus Res. (2017) 242:122–30. doi: 10.1016/j.virusres.2017.09.018
27. Romera, SA, Perez, R, Marandino, A, LuciaTau, R, Campos, F, Roehe, PM, et al. Whole-genome analysis of natural interspecific recombinant between bovine alphaherpesviruses 1 and 5. Virus Res. (2022) 309:198656. doi: 10.1016/j.virusres.2021.198656
28. Meurens, F, Keil, GM, Muylkens, B, Gogev, S, Schynts, F, Negro, S, et al. Interspecific recombination between two ruminant alphaherpesviruses, bovine herpesviruses 1 and 5. J Virol. (2004) 78:9828–36. doi: 10.1128/JVI.78.18.9828-9836.2004
29. Saha, T, Guha, C, Chakraborty, D, Pal, B, Biswas, U, Chatterjee, A, et al. Isolation and characterization of BoHV-1 from seropositive cows after inducing artificial stress in West Bengal, India. Pak J Biol Sci. (2013) 16:720–5. doi: 10.3923/pjbs.2013.720.725
30. Hoferer, M, Braun, A, and Sting, R. Creation of a bovine herpes virus 1 (BoHV-1) quantitative particle standard by transmission electron microscopy and comparison with established standards for use in real-time PCR. Biologicals. (2017) 48:121–5. doi: 10.1016/j.biologicals.2017.03.007
31. Kalthoff, D, Granzow, H, Trapp, S, and Beer, M. The UL49 gene product of BoHV-1: a major factor in efficient cell-to-cell spread. J Gen Virol. (2008) 89:2269–74. doi: 10.1099/vir.0.2008/000208-0
32. Khalid, A, Riaz, A, Yousaf, A, Khan, IH, Ur-Rehman, S, Moaeen-Ud-Din, M, et al. Epidemiological survey of bovine gammaherpesvirus 4 (BoHV-4) infection in cattle and buffalo from Pakistan. Vet Res Commun. (2023) 47:921–7. doi: 10.1007/s11259-022-10058-x
33. Tan, L, Zhu, P, Getu, Z, Yang, X, Zheng, S, Duan, Y, et al. Antiviral activity of nitazoxanide against pseudorabies virus infection in vitro. Front Vet Sci. (2025) 12:1623545. doi: 10.3389/fvets.2025.1623545
34. Dagalp, SB, Farzani, TA, Dogan, F, Alkan, F, and Ozkul, A. Molecular and antigenic characterization of bovine herpesvirus type 1 (BoHV-1) strains from cattle with diverse clinical cases in Turkey. Trop Anim Health Prod. (2020) 52:555–64. doi: 10.1007/s11250-019-02042-6
35. Wang, B, Zhang, M, Liu, Z, Chen, H, and Guo, A. Establishment of differential gD-PCR for detection of bovine herpesvirus infection. China Dairy Cattle. (2010) 12:3–6.
36. Maidana, SS, Miño, S, Apostolo, RM, De Stefano, GA, and Romera, SA. A new molecular method for the rapid subtyping of bovine herpesvirus 1 field isolates. J Vet Diagn Invest. (2020) 32:112–7. doi: 10.1177/1040638719898692
37. Zhang, FF, Wei, JZ, Li, JM, Tan, MF, Hu, LZ, and Zeng, YB. A multiplex RT-PCR assay for simultaneous detecting Akabane virus, bovine herpesvirus 1, and bovine viral diarrhea virus. Fujian J Agric Sci. (2024) 39:1242–7.
38. Lung, O, Furukawa-Stoffer, T, Burton Hughes, K, Pasick, J, King, DP, and Hodko, D. Multiplex RT-PCR and automated microarray for detection of eight bovine viruses. Transbound Emerg Dis. (2017) 64:1929–34. doi: 10.1111/tbed.12591
39. Fan, Q, Yao, L, Ding, M, Wang, DJ, Chen, HC, and Liu, ZF. Development of latex agglutination test for rapid detection of antibodies against bovine herpesvirus 1 and bovine herpesvirus 5 in cattle. J Vet Diagn Invest. (2012) 24:1162–5. doi: 10.1177/1040638712462376
40. Chen, Y, Luo, S, Tan, J, Zhang, L, Qiu, S, Hao, Z, et al. Establishment and application of multiplex real-time PCR for simultaneous detection of four viruses associated with porcine reproductive failure. Front Microbiol. (2023) 14:1092273. doi: 10.3389/fmicb.2023.1092273
41. Wang, J, Fu, R, Li, XB, Wang, SJ, Wang, SS, Li, W, et al. Establishment and application of real-time fluorescent quantitative PCR method for detection of bovine herpesvirus type 1. Lab Anim Sci. (2019) 36:35–42.
42. Pawar, SS, Meshram, CD, Singh, NK, Saini, M, Mishra, BP, and Gupta, PK. Development of a SYBR green I based duplex real-time PCR for detection of bovine herpesvirus-1 in semen. J Virol Methods. (2014) 208:6–10. doi: 10.1016/j.jviromet.2014.07.027
43. Pawar, SS, Meshram, CD, Singh, NK, Saini, M, Mishra, BP, and Gupta, PK. EvaGreen-based multiplex real-time PCR assay for rapid differentiation of wild-type and glycoprotein E-deleted bovine Herpesvirus-1 strains. Anim Biotechnol. (2017) 28:248–52. doi: 10.1080/10495398.2016.1268620
44. Sarangi, LN, Naveena, T, Rana, SK, Surendra, KSNL, Reddy, RVC, Bajibabu, P, et al. Evaluation of a specialized filter-paper matrix for transportation of extended bovine semen to screen for bovine herpesvirus-1 by real-time PCR. J Virol Methods. (2018) 257:1–6. doi: 10.1016/j.jviromet.2018.03.009
45. Jiang, LX, Wang, YH, He, SC, Hu, QY, Wang, WG, Lu, XH, et al. Establishment and application of quadruple fluorescence PCR assay for detection of BHV-1, BRSV, BPIV-3, and BVDV. Chin J Animal Health Inspect. (2021) 10:98–106.
46. Zhang, J, Wang, W, Yang, M, Lin, J, Xue, F, Zhu, Y, et al. Development of a one-step multiplex real-time PCR assay for the detection of viral pathogens associated with the bovine respiratory disease complex. Front Vet Sci. (2022) 9:825257. doi: 10.3389/fvets.2022.825257
47. Zheng, W, Porter, E, Noll, L, Stoy, C, Lu, N, Wang, Y, et al. A multiplex real-time PCR assay for the detection and differentiation of five bovine pinkeye pathogens. J Microbiol Methods. (2019) 160:87–92. doi: 10.1016/j.mimet.2019.03.024
48. Pansri, P, Katholm, J, Krogh, KM, Aagaard, AK, Schmidt, LMB, Kudirkiene, E, et al. Evaluation of novel multiplex qPCR assays for diagnosis of pathogens associated with the bovine respiratory disease complex. Vet J. (2020) 256:105425. doi: 10.1016/j.tvjl.2020.105425
49. Feng, Y, He, T, Zhang, B, Yuan, H, and Zhou, Y. Epidemiology and diagnosis technologies of human metapneumovirus in China: a mini review. Virol J. (2024) 21:59. doi: 10.1186/s12985-024-02327-9
50. Pawar, SS, Meshram, CD, Singh, NK, Sonwane, AA, Saini, M, Rautmare, SS, et al. Rapid detection of bovine herpesvirus 1 in bovine semen by loop-mediated isothermal amplification (LAMP) assay. Arch Virol. (2014) 159:641–8. doi: 10.1007/s00705-013-1869-2
51. Pawar, SS, Meshram, CD, Singh, NK, Saini, M, Mishra, BP, and Gupta, PK. Loop-mediated isothermal amplification for rapid detection and differentiation of wild-type bovine Herpesvirus-1 and glycoprotein E-deleted marker vaccine strain. Anim Biotechnol. (2015) 26:268–72. doi: 10.1080/10495398.2015.1015680
52. Fan, Q, Xie, Z, Xie, Z, Xie, L, Huang, J, Zhang, Y, et al. Development of duplex fluorescence-based loop-mediated isothermal amplification assay for detection of mycoplasma bovis and bovine herpes virus 1. J Virol Methods. (2018) 261:132–8. doi: 10.1016/j.jviromet.2018.08.014
53. Tan, M, Liao, C, Liang, L, Yi, X, Zhou, Z, and Wei, G. Recent advances in recombinase polymerase amplification: principle, advantages, disadvantages and applications. Front Cell Infect Microbiol. (2022) 12:1019071. doi: 10.3389/fcimb.2022.1019071
54. Hou, P, Wang, H, Zhao, G, He, C, and He, H. Rapid detection of infectious bovine Rhinotracheitis virus using recombinase polymerase amplification assays. BMC Vet Res. (2017) 13:386. doi: 10.1186/s12917-017-1284-0
55. Jiang, L, Zhang, G, Wang, P, Niu, X, Liu, Q, Zhang, S, et al. Simultaneous detection of bovine viral diarrhea virus (BVDV) and bovine herpesvirus 1 (BoHV-1) using recombinase polymerase amplification. Sci Rep. (2024) 14:10169. doi: 10.1038/s41598-024-56869-7
56. Wu, Y, Zhang, W, Yi, C, He, K, Hu, C, Ye, G, et al. Rapid, sensitive, and visible RPA-LFD assay for BoHV-1 and BoHV-5. Microbiol Spectr. (2025) 13:e0089524. doi: 10.1128/spectrum.00895-24
57. Wang, R, Huang, P, Huang, Z, Zhang, Y, Liu, M, Jin, K, et al. A rapid nucleic acid visualization assay for infectious bovine Rhinotracheitis virus that targets the TK gene. Microbiol Spectr. (2023) 11:e0185923. doi: 10.1128/spectrum.01859-23
58. Dixit, R, Kodali, NK, Biswal, M, Prakash, JAJ, Gopalan, N, Das, P, et al. Polymerase spiral reaction (PSR) as a point-of-care diagnostic assay: a systematic review. Expert Rev Mol Diagn. (2024) 24:79–88. doi: 10.1080/14737159.2024.2315286
59. Malla, JA, Chakravarti, S, Gupta, V, Chander, V, Sharma, GK, Qureshi, S, et al. Novel polymerase spiral reaction (PSR) for rapid visual detection of bovine herpesvirus 1 genomic DNA from aborted bovine fetus and semen. Gene. (2018) 644:107–12. doi: 10.1016/j.gene.2017.11.004
60. Mambetaliyev, M, Alieva, A, Abduraimov, Y, Rsaliyev, A, and Zhugunissov, K. Determination of the minimal level of neutralizing antibodies elicited following vaccination able to protect rabbits against virulent cowpox virus. Front Immunol. (2025) 16:1640056. doi: 10.3389/fimmu.2025.1640056
61. Wu, CT, Li, YM, and Tang, SG. Prokayotic expression of gB gene of bovine herpesvirus-1 and establishment of an indirect ELISA based on the recombinant fusion protein. Chin Vet Sci. (2010) 40:1259–64.
62. Liu, Q, Liang, R, Niu, X, Jiang, L, Zhang, G, Wang, P, et al. Develop an indirect ELISA utilizing gD protein to detect antibodies against bovine herpesvirus type 1. Front Cell Infect Microbiol. (2025) 15:1591304. doi: 10.3389/fcimb.2025.1591304
63. Zhang, Z, Gu, W, Fu, X, Lin, J, Ding, X, and Zhu, L. A B-cell epitope on the bovine herpesvirus 1 (BoAHV1) viral protein gC, referred to as PV116, is implicated in the generation of antibodies and the development of an ELISA kit for the detection of the virus antibody. BMC Microbiol. (2025) 25:70. doi: 10.1186/s12866-025-03782-2
64. Tignon, M, De Baere, M, Hanon, JB, Goolaerts, A, Houtain, JY, Delooz, L, et al. Characterization of three commercial ELISA kits for detection of BOHV-1 gE specific antibodies in serum and milk samples and applicability of bulk milk for determination of herd status. J Virol Methods. (2017) 245:66–72. doi: 10.1016/j.jviromet.2017.03.015
65. Alkan, F, Bilge-Dagalp, S, Karapınar, Z, Timurkan, MO, Coskun, N, and Burgu, I. Long-term study (2005-2010) on the vaccination with BoHV-1 glycoprotein E-deleted marker vaccine in selected two dairy herds in Turkey. Trop Anim Health Prod. (2018) 50:353–63. doi: 10.1007/s11250-017-1440-3
66. Li, JA. Development of bovine herpesvirus type 1 gE protein blocking ELISA antibody detection kit. China: Huazhong Agricultural University (2023).
67. Chowdhury, SI. Identification of an epitope within the bovine herpesvirus 1 glycoprotein E cytoplasmic tail and use of a monoclonal antibody directed against the epitope for the differentiation between vaccinated and infected animals. J Virol Methods. (2016) 233:97–104. doi: 10.1016/j.jviromet.2016.02.012
68. Cork, J, Jones, RM, and Sawyer, J. Low cost, disposable biosensors allow detection of antibodies with results equivalent to ELISA in 15 min. J Immunol Methods. (2013) 387:140–6. doi: 10.1016/j.jim.2012.10.007
69. Japolla, G, Cunha-Junior, JP, Pajuaba, ACAM, Taketomi, EA, Bührer-Sékula, S, Bataus, LAM, et al. Development of a nanogold slot blot inhibition assay for the detection of antibodies against bovine herpesvirus type 1. Arch Virol. (2018) 163:1549–57. doi: 10.1007/s00705-018-3763-4
70. Liu, W, Zhang, K, Cheng, J, Yu, S, Cheng, C, Jiang, B, et al. Development and evaluation of a time-resolved fluorescence labelled immunochromatographic strip assay for rapid and quantitative detection of bovine herpesvirus 1. Front Microbiol. (2024) 15:1371849. doi: 10.3389/fmicb.2024.1371849
71. Van Crombrugge, E, Ren, X, Glorieux, S, Zarak, I, Van den Broeck, W, Bachert, C, et al. The alphaherpesvirus gE/gI glycoprotein complex and proteases jointly orchestrate invasion across the host's upper respiratory epithelial barrier. MBio. (2024) 15:e0187324. doi: 10.1128/mbio.01873-24
72. Lamote, JAS, Kestens, M, Van Waesberghe, C, Delva, J, De Pelsmaeker, S, Devriendt, B, et al. The pseudorabies virus glycoprotein gE/gI complex suppresses type I interferon production by Plasmacytoid dendritic cells. J Virol. (2017) 91:e02276–16. doi: 10.1128/JVI.02276-16
73. Romera, SA, Puntel, M, Quattrocchi, V, Del Médico Zajac, P, Zamorano, P, Blanco Viera, J, et al. Protection induced by a glycoprotein E-deleted bovine herpesvirus type 1 marker strain used either as an inactivated or live attenuated vaccine in cattle. BMC Vet Res. (2014) 10:8. doi: 10.1186/1746-6148-10-8
74. Petrini, S, Martucciello, A, Grandoni, F, De Matteis, G, Cappelli, G, Giammarioli, M, et al. Evaluation of safety and efficacy of an inactivated marker vaccine against bovine alphaherpesvirus 1 (BoHV-1) in water Buffalo (Bubalus bubalis). Vaccines (Basel). (2021) 9:355. doi: 10.3390/vaccines9040355
75. Petrini, S, Righi, C, Iscaro, C, Viola, G, Gobbi, P, Scoccia, E, et al. Evaluation of passive immunity induced by immunisation using two inactivated gE-deleted marker vaccines against infectious bovine Rhinotracheitis (IBR) in calves. Vaccines (Basel). (2020) 8:14. doi: 10.3390/vaccines8010014
76. Ganguly, B, Tayshete, S, Melepat, DP, Awandkar, S, Karnati, S, Pattnaik, P, et al. An open-label, randomized field trial demonstrates safety and immunogenicity of inactivated gE-deleted marker vaccine against infectious bovine Rhinotracheitis in cattle. Vaccines (Basel). (2025) 13:579. doi: 10.3390/vaccines13060579
77. Kornuta, CA, Cheuquepán, F, Bidart, JE, Soria, I, Gammella, M, Quattrocchi, V, et al. TLR activation, immune response and viral protection elicited in cattle by a commercial vaccine against bovine Herpesvirus-1. Virology. (2022) 566:98–105. doi: 10.1016/j.virol.2021.11.014
78. Earley, B, Tiernan, K, Duffy, C, Dunn, A, Waters, S, Morrison, S, et al. Effect of suckler cow vaccination against glycoprotein E (gE)-negative bovine herpesvirus type 1 (BoHV-1) on passive immunity and physiological response to subsequent bovine respiratory disease vaccination of their progeny. Res Vet Sci. (2018) 118:43–51. doi: 10.1016/j.rvsc.2018.01.005
79. Weiss, M, Brum, MC, Anziliero, D, Weiblen, R, and Flores, EF. A glycoprotein E gene-deleted bovine herpesvirus 1 as a candidate vaccine strain. Braz J Med Biol Res. (2015) 48:843–51. doi: 10.1590/1414-431X20154243
80. Zhang, S, Liu, G, Wang, C, Guo, A, and Chen, Y. Enhanced immunogenicity of a BoHV-1 gG−/tk- vaccine. Vaccine. (2025) 47:126704. doi: 10.1016/j.vaccine.2025.126704
81. Zhang, M, Fu, S, Deng, M, Xie, Q, Xu, H, Liu, Z, et al. Attenuation of bovine herpesvirus type 1 by deletion of its glycoprotein G and tk genes and protection against virulent viral challenge. Vaccine. (2011) 29:8943–50. doi: 10.1016/j.vaccine.2011.09.050
82. Marawan, MA, Deng, M, Wang, C, Chen, Y, Hu, C, Chen, J, et al. Characterization of BoHV-1 gG−/tk−/gE- mutant in differential protein expression, virulence, and immunity. Vet Sci. (2021) 8:253. doi: 10.3390/vetsci8110253
83. Ortiz-Prado, E, Kyriakidis, NC, López-Cortés, A, Vasconez-Gonzalez, J, Suarez, I, Pazmiño-Almeida, J, et al. Current and emerging Mpox vaccine strategies: a comprehensive review. Vaccine. (2025) 62:127598. doi: 10.1016/j.vaccine.2025.127598
84. Rankin, R, Pontarollo, R, Gomis, S, Karvonen, B, Willson, P, Loehr, BI, et al. CpG-containing oligodeoxynucleotides augment and switch the immune responses of cattle to bovine herpesvirus-1 glycoprotein D. Vaccine. (2002) 20:3014–22. doi: 10.1016/s0264-410x(02)00216-5
85. Van Drunen Littel-Van Den Hurk, S, Snider, M, Thompson, P, Latimer, L, and Babiuk, LA. Strategies for induction of protective immunity to bovine herpesvirus-1 in newborn calves with maternal antibodies. Vaccine. (2008) 26:3103–11. doi: 10.1016/j.vaccine.2008.02.018
86. Hoa, NT, Afzal, H, Gundegmaa, U, Raadan, O, Cheng, LT, Chu, CY, et al. Enhanced immune response with baculovirus-expressed BoHV-1 glycoprotein D in vaccine development. Vet J. (2024) 308:106228. doi: 10.1016/j.tvjl.2024.106228
87. Araujo, IL, Piraine, REA, Fischer, G, and Leite, FPL. Recombinant BoHV-5 glycoprotein (rgD5) elicits long-lasting protective immunity in cattle. Virology. (2023) 584:44–52. doi: 10.1016/j.virol.2023.04.004
88. Brisse, M, Vrba, SM, Kirk, N, Liang, Y, and Ly, H. Emerging concepts and Technologies in Vaccine Development. Front Immunol. (2020) 11:583077. doi: 10.3389/fimmu.2020.583077
89. Langellotti, CA, Gammella, M, Soria, I, Bellusci, C, Quattrocchi, V, Vermeulen, M, et al. An improved DNA vaccine against bovine Herpesvirus-1 using CD40L and a chemical adjuvant induces specific cytotoxicity in mice. Viral Immunol. (2021) 34:68–78. doi: 10.1089/vim.2020.0082
90. Kornuta, CA, Langellotti, CA, Bidart, JE, Soria, I, Quattrocchi, V, Gammella, M, et al. A plasmid encoding the extracellular domain of CD40 ligand and Montanide™ GEL01 as adjuvants enhance the immunogenicity and the protection induced by a DNA vaccine against BoHV-1. Vaccine. (2021) 39:1007–17. doi: 10.1016/j.vaccine.2020.11.071
91. Liu, XB, Yu, GW, Gao, XY, Huang, JL, Qin, LT, Ni, HB, et al. Intranasal delivery of plasmids expressing bovine herpesvirus 1 gB/gC/gD proteins by polyethyleneimine magnetic beads activates long-term immune responses in mice. Virol J. (2021) 18:60. doi: 10.1186/s12985-021-01536-w
92. Yu, W, Liu, J, Liu, Y, Forlenza, M, and Chen, H. Application of CRISPR/Cas9 for rapid genome editing of pseudorabies virus and bovine Herpesvirus-1. Viruses. (2024) 16:311. doi: 10.3390/v16020311
93. Tan, L, Yao, J, Yang, Y, Luo, W, Yuan, X, Yang, L, et al. Current status and challenge of pseudorabies virus infection in China. Virol Sin. (2021) 36:588–607. doi: 10.1007/s12250-020-00340-0
94. Otsuka, H, Xuan, X, Shibata, I, and Mori, M. Protective immunity of bovine herpesvirus-1 (BHV-1) recombinants which express pseudorabies virus (PRV) glycoproteins gB, gC, gD and gE. J Vet Med Sci. (1996) 58:819–24. doi: 10.1292/jvms.58.819
95. Takashima, Y, Nagane, N, Hushur, O, Matsumoto, Y, and Otsuka, H. Bovine herpesvirus-1 (BHV-1) recombinant expressing pseudorabies virus (PrV) glycoproteins B and C induces type 1 immune response in BALB/c mice. J Vet Med Sci. (2002) 64:589–96. doi: 10.1292/jvms.64.589
96. Stenfeldt, C, Eschbaumer, M, Humphreys, J, Medina, GN, and Arzt, J. The pathogenesis of foot-and-mouth disease virus: current understandings and knowledge gaps. Vet Res. (2025) 56:119. doi: 10.1186/s13567-025-01545-5
97. Ren, XG, Xue, F, Zhu, YM, Tong, GZ, Wang, YH, Feng, JK, et al. Construction of a recombinant BHV-1 expressing the VP1 gene of foot and mouth disease virus and its immunogenicity in a rabbit model. Biotechnol Lett. (2009) 31:1159–65. doi: 10.1007/s10529-009-9988-2
98. Kit, M, Kit, S, Little, SP, Di Marchi, RD, and Gale, C. Bovine herpesvirus-1 (infectious bovine rhinotracheitis virus)-based viral vector which expresses foot-and-mouth disease epitopes. Vaccine. (1991) 9:564–72. doi: 10.1016/0264-410x(91)90243-y
99. Nsengimana, I, Kelvin, D, Uwibambe, E, Rwagasore, E, Muvunyi, CM, Eastwood, G, et al. Inter-epidemic seroprevalence of Rift Valley fever virus and associated risk factors in humans in eastern Rwanda. PLoS Negl Trop Dis. (2025) 19:e0013405. doi: 10.1371/journal.pntd.0013405
100. Pavulraj, S, Stout, RW, Barras, ED, Paulsen, DB, and Chowdhury, SI. A novel quadruple gene-deleted BoHV-1-vectored RVFV subunit vaccine induces humoral and cell-mediated immune response against Rift Valley fever in calves. Viruses. (2023) 15:2183. doi: 10.3390/v15112183
101. Pavulraj, S, Stout, RW, Paulsen, DB, and Chowdhury, SI. A quadruple gene-deleted live BoHV-1 subunit RVFV vaccine vector reactivates from latency and replicates in the TG neurons of calves but is not transported to and shed from nasal mucosa. Viruses. (2024) 16:1497. doi: 10.3390/v16091497
102. Zhao, C, Gao, J, Wang, Y, Ji, L, Qin, H, Hu, W, et al. A novel rabies vaccine based on a recombinant bovine herpes virus type 1 expressing rabies virus glycoprotein. Front Microbiol. (2022) 13:931043. doi: 10.3389/fmicb.2022.931043
103. Zhou, Y, Shao, Z, Dai, G, Li, X, Xiang, Y, Jiang, S, et al. Pathogenic infection characteristics and risk factors for bovine respiratory disease complex based on the detection of lung pathogens in dead cattle in Northeast China. J Dairy Sci. (2023) 106:589–606. doi: 10.3168/jds.2022-21929
104. Chowdhury, SI, Pannhorst, K, Sangewar, N, Pavulraj, S, Wen, X, Stout, RW, et al. BoHV-1-vectored BVDV-2 subunit vaccine induces BVDV cross-reactive cellular immune responses and protects against BVDV-2 challenge. Vaccines (Basel). (2021) 9:46. doi: 10.3390/vaccines9010046
105. Bilge-Dagalp, S, Farzani, TA, Dogan, F, Akkutay Yoldar, Z, Ozkul, A, Alkan, F, et al. Development of a BoHV-4 viral vector expressing tgD of BoHV-1 and evaluation of its immunogenicity in mouse model. Braz J Microbiol. (2021) 52:1119–33. doi: 10.1007/s42770-021-00525-z
106. Delhon, G, Khatiwada, S, Doub, D, Harris, S, Chaulagain, S, El-Gaffary, M, et al. Bovine papular stomatitis virus as a vaccine vector for cattle. J Gen Virol. (2023) 104:001914. doi: 10.1099/jgv.0.001914
107. Kumar, P, Ayalew, LE, Godson, DL, Gaba, A, Babiuk, LA, and Tikoo, SK. Mucosal immunization of calves with recombinant bovine adenovirus-3 coexpressing truncated form of bovine herpesvirus-1 gD and bovine IL-6. Vaccine. (2014) 32:3300–6. doi: 10.1016/j.vaccine.2014.03.073
108. Peralta, A, Molinari, P, Conte-Grand, D, Calamante, G, and Taboga, O. A chimeric baculovirus displaying bovine herpesvirus-1 (BHV-1) glycoprotein D on its surface and their immunological properties. Appl Microbiol Biotechnol. (2007) 75:407–14. doi: 10.1007/s00253-006-0825-4
109. Chen, X, Wang, X, Qi, Y, Wen, X, Li, C, Liu, X, et al. Meta-analysis of prevalence of bovine herpes virus 1 in cattle in mainland China. Acta Trop. (2018) 187:37–43. doi: 10.1016/j.actatropica.2018.07.024
110. Barrett, D, Lane, E, Lozano, JM, O'Keeffe, K, and Byrne, AW. Bovine herpes virus type 1 (BoHV-1) seroprevalence, risk factor and bovine viral Diarrhoea (BVD) co-infection analysis from Ireland. Sci Rep. (2024) 9:867. doi: 10.1038/s41598-023-50433-5
111. Krishnagopal, A, and van Drunen Littel-van den Hurk, S. The biology and development of vaccines for bovine alphaherpesvirus 1. Vet J. (2024) 306:106152. doi: 10.1016/j.tvjl.2024.106152
Keywords: bovine herpesvirus type 1, cattle, diagnostic approaches, vaccine development, prevention and management
Citation: Zhang J, Cao J, Cao L, Zhang C, Gong L, Kang X, Li D and Zhang Y (2025) Research progress on the diagnostic techniques and vaccine development of bovine herpesvirus types 1. Front. Vet. Sci. 12:1703336. doi: 10.3389/fvets.2025.1703336
Edited by:
Dirk Werling, Royal Veterinary College (RVC), United KingdomReviewed by:
Nattawooti Sthitmatee, Chiang Mai University, ThailandSalam Abd Esmaeel, University of Mosul, Iraq
Copyright © 2025 Zhang, Cao, Cao, Zhang, Gong, Kang, Li and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianming Zhang, Z2FsbGFudHpoYW5nQDE2My5jb20=
 Jianming Zhang
Jianming Zhang Jian Cao
Jian Cao