- 1Jilin Agricultural Science and Technology College, Jilin, China
- 2College of Veterinary Medicine, Northeast Agricultural University, Harbin, China
- 3Heilongjiang Provincial Key Laboratory of Pathogenic Mechanism for Animal Disease and Comparative Medicine, Harbin, China
- 4Institute of Chinese Veterinary Medicine, Northeast Agricultural University, Harbin, China
Zearalenone (ZEA) is a non-steroidal estrogenic mycotoxin produced by Fusarium fungi, widely present in cereal feeds such as corn, barley, wheat, and sorghum. It not only impacts agricultural production and feed safety but also poses a serious threat to animal health. Extensive research demonstrates that natural products can effectively mitigate the toxic effects of zearalenone. This paper reviews zearalenone’s physicochemical properties and toxicological effects, with a focus on advances in the research on reducing zearalenone toxicity through plant, microbial, and mineral-derived natural products. The aim is to provide theoretical references for developing more efficient and safer zearalenone detoxification agents.
1 Introduction
Zearalenone (ZEA), also known as F-2 toxin, is a non-steroidal estrogenic mycotoxin produced by Fusarium graminearum fungi. It is one of the top three mycotoxins in animal feed globally (1). Grain feeds such as wheat and corn are susceptible to contamination by zearalenone and other toxins during production, processing, and transportation. Furthermore, ZEA exhibits chemical stability, produces numerous metabolites, and persists for extended periods. The Food and Agriculture Organization of the United Nations (FAO) estimates that approximately 25% of the world’s grain production is contaminated with mycotoxins (2). Based on ZEA monitoring data from China between 2004 and 2024, Wang et al. (3) Comprehensively analyzed ZEA contamination levels in Chinese feed and raw materials. International comparisons indicate that China’s zearalenone contamination levels exceed those in Europe.
The phenol-dihydroxy lactone structure of ZEA resembles that of estrogen. When animals consume ZEA-contaminated grain feed, it undergoes metabolic conversion within the body and is transported via the bloodstream throughout the system. There, it binds to estrogen receptors, triggering various pathological processes including estrogenic effects, oxidative stress, apoptosis, and inflammation. This leads to multi-organ damage affecting the uterus, testes, liver, kidneys, and spleen. Affected animals exhibit symptoms including prolonged estrus cycles, miscarriages, testicular atrophy, and poor sperm quality (4–7), causing substantial economic losses in livestock farming and feed processing industries. Currently, ZEA detoxification methods face limitations such as low efficacy, high costs, and significant toxicity (8–10).
In recent years, extensive research has focused on the antagonistic effects of natural plant extracts, microorganisms and their metabolites, and mineral materials against ZEA. Natural products, with their advantages of high safety, wide availability, and diverse mechanisms of action, have gradually become a hotspot in ZEA detoxification research. For example, a variety of bioactive substances found in natural plants, such as flavonoids, polysaccharides, and polyphenols, can successfully lessen the harm that ZEA does to organs, including the kidneys, liver, testes, and uterus. This is accomplished by scavenging reactive oxygen species, blocking inflammatory and apoptotic signaling pathways, and counteracting the estrogen-like actions of ZEA (11–14). This review systematically outlines the physicochemical properties, toxicity mechanisms, and mitigation methods of ZEA, with a focus on summarizing research progress in natural medicines for zearalenone mitigation. It aims to provide a theoretical foundation for the innovation of ZEA pollution prevention and control technologies and the industrial application of natural mitigants, thereby contributing to the improvement of food security and public health safeguards.
2 Zearalenone
2.1 Physicochemical properties of zearalenone
ZEA, chemically named 6-(10-hydroxy-6-oxo-undec-2-enyl)β-resorcylic acid lactone, has the molecular formula C₁₈H₂₂O₅. Its molecule contains two phenolic hydroxyl groups and one lactone ring, belonging to the β-resorcylic acid lactone class of compounds. Its chemical structure is shown in Figure 1 (15). ZEA exhibits stable chemical properties with a well-defined melting point. The pure compound melts at 164–165 °C and gradually decomposes when heated above 200 °C. Its toxicity is difficult to destroy through high-temperature treatment. At room temperature, its structure remains stable. Although hydrolysis occurs under alkaline conditions, its structure can be restored by lowering the pH. ZEA is readily soluble in polar organic solvents such as methanol, ethanol, acetone, and chloroform, but poorly soluble in water (Table 1).
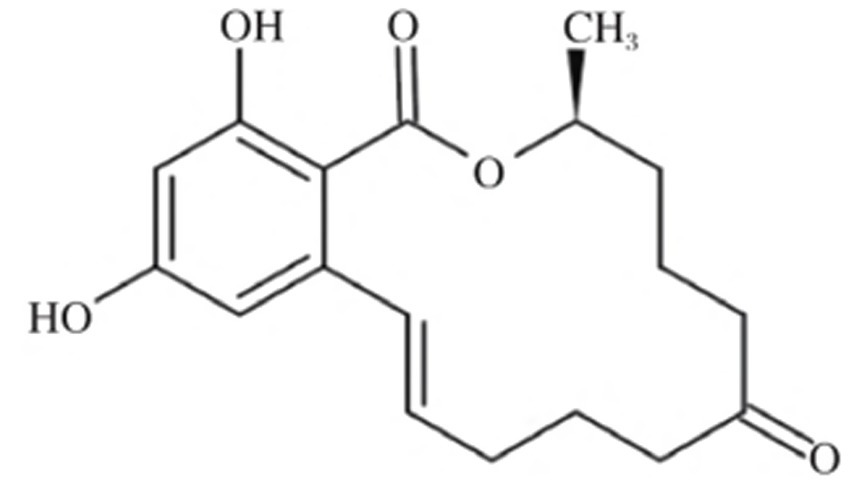
Figure 1. Chemical formula of zearalenone in corn. Reproduced with permission from Wu et al. (15).
2.2 Toxic effects of zearalenone
2.2.1 Reproductive toxicity
The reproductive organs are the primary target sites for ZEA toxicity. ZEA, structurally similar to estrogen, can competitively bind to estrogen receptors within the body, thereby disrupting the normal synthesis of steroid hormones such as estradiol, testosterone, and progesterone (16). When sows ingest ZEA-contaminated feed, it readily damages reproductive organs such as the uterus and ovaries, manifesting clinical symptoms including vulvar swelling, mammary gland redness and swelling, estrus cycle disruption, and abortion (17). Gilt sows are particularly sensitive to ZEA; estrogen poisoning can occur when feed contains ZEA levels exceeding 1 mg/kg (18). Research proved that ZEA can cause abnormal follicular development in laying hens, characterized by increased stromal cell numbers, vacuolation-induced edema, accompanied by congestion, hemorrhage, oocyte retraction, and separation of the granulosa and theca membranes (19, 20). At concentrations exceeding 5 mg/kg, ZEA leads to reduced egg production, heightened inflammation, impaired ovarian function, and disrupted sex hormone secretion (21). ZEA activates the phosphorylation of AMPK in endometrial epithelial cells. This process modulates TSC2 and Rheb, affecting mTOR phosphorylation levels and subsequently inducing autophagy. Concurrently, it upregulates the expression of proliferative genes PCNA and BCl2 while downregulating the apoptotic gene Bax. After promoting proliferation of endometrial epithelial cells, this ultimately leads to thickening of the endometrium and myometrium, increased density of uterine glands, and induces uterine hypertrophy (22) (Table 2; Figure 2).
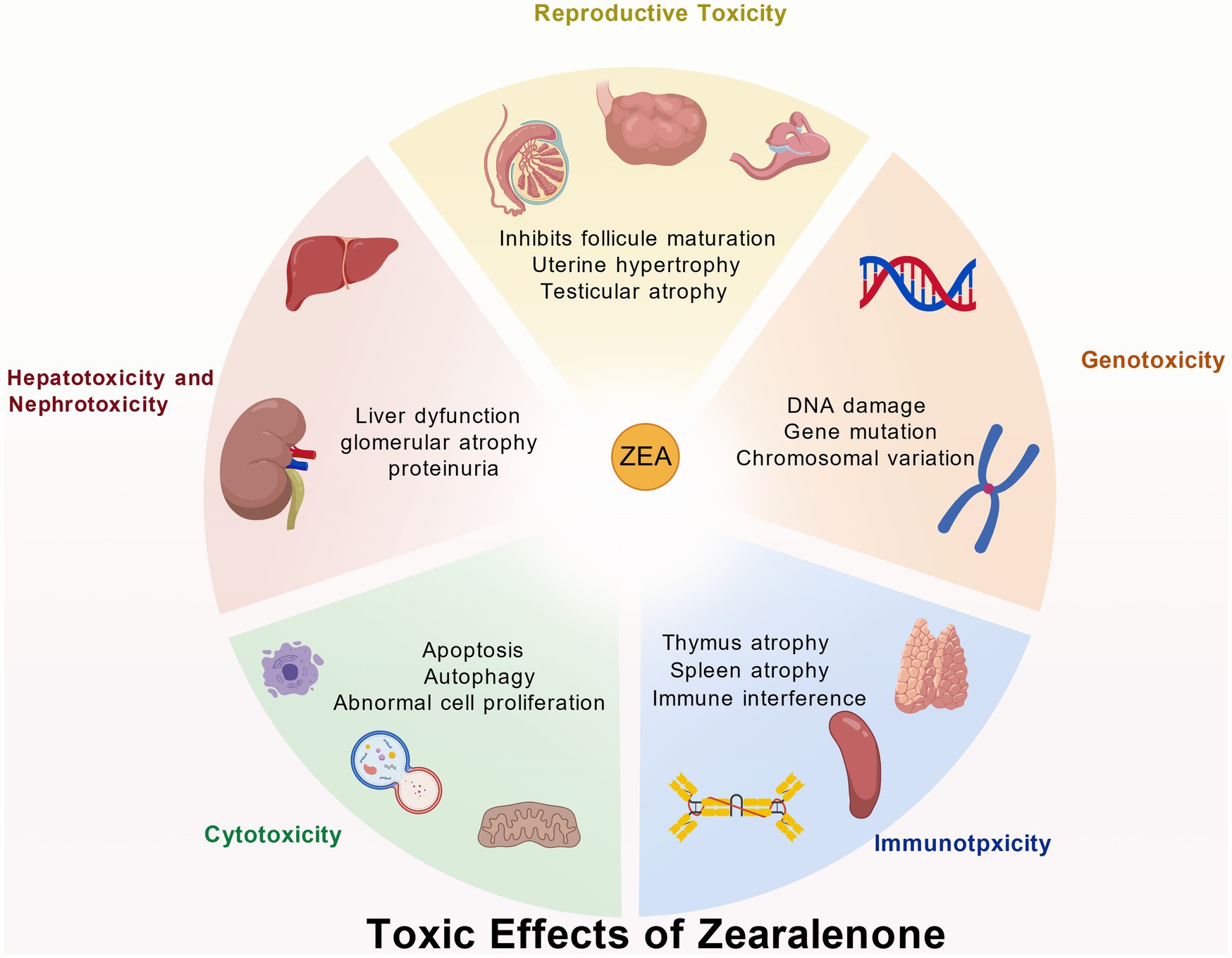
Figure 2. Toxic effects of zearalenone. Created with BioGDP.com.
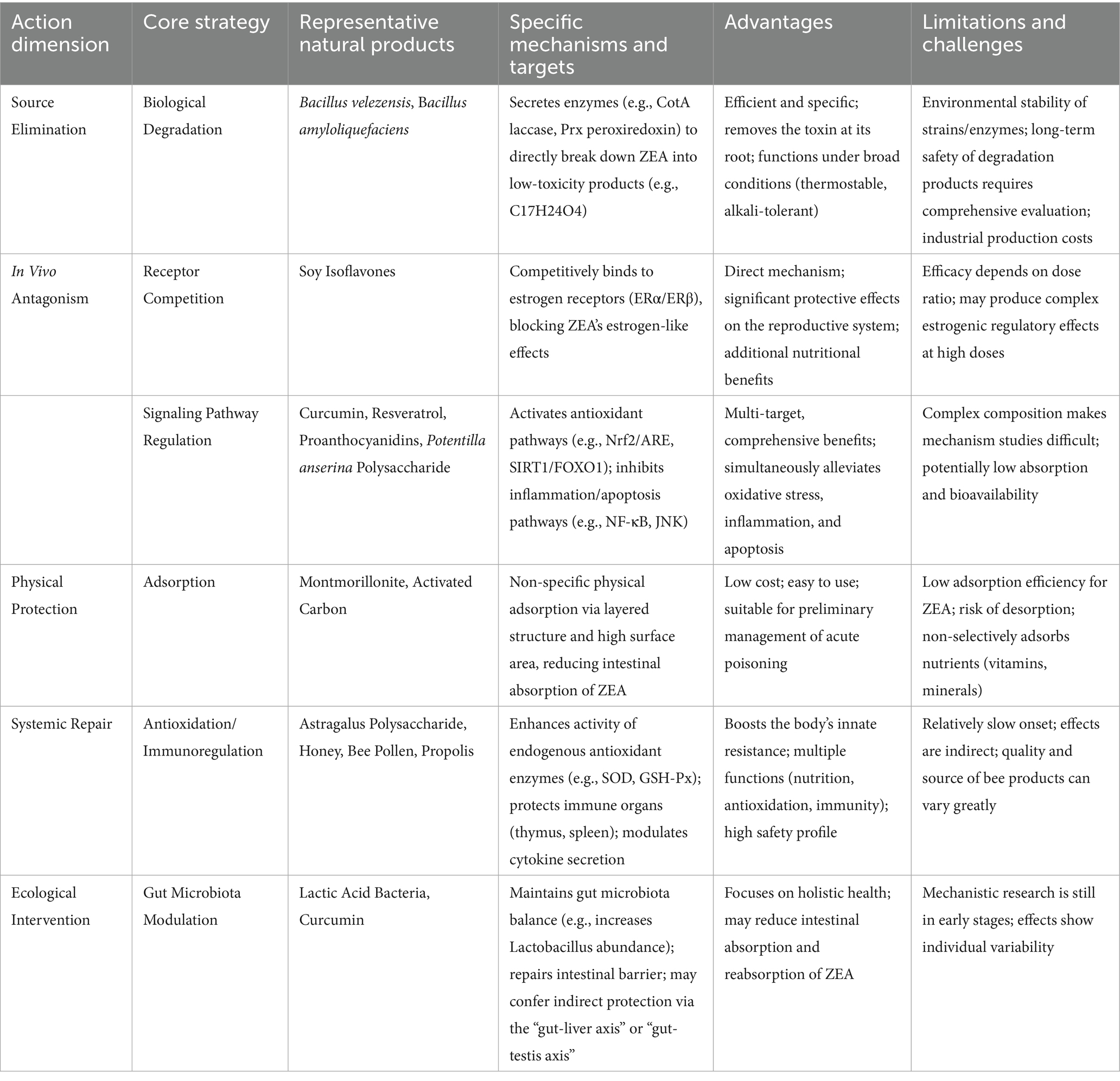
ZEA poisoning can also cause male animals to exhibit symptoms such as decreased sperm production and quality. Testicular interstitial cells are the primary site for testosterone synthesis in males. Studies indicate that ZEA significantly damages mitochondria in porcine testicular interstitial cells, inducing apoptosis through the PI3K-AKT signaling pathway mediated by mitochondria, which regulates the Bcl-2 protein family (23). ZEA induces mitochondrial morphological abnormalities and functional disorders, leading to mtDNA leakage into the cytoplasm. Free mtDNA acts as a damage-associated molecular pattern (DAMP), activating the endoplasmic reticulum transmembrane protein STING, which in turn triggers the NF-κB signaling pathway. This pathway not only upregulates inflammatory factor expression but also induces pyroptosis by regulating NLRP3 inflammasomes, resulting in damage to testicular and mouse testicular supporting cells (24). In both in vivo and in vitro studies of ZEA-induced male goat reproductive dysfunction, ZEA caused significant declines in sperm quality, disruption of seminiferous tubules, and impaired structure of goat testicular interstitial supporting cells along with blood-testis barrier function. Concurrently, supporting cells exhibited numerous vacuoles and excessive endoplasmic reticulum swelling (25) (Figure 3).
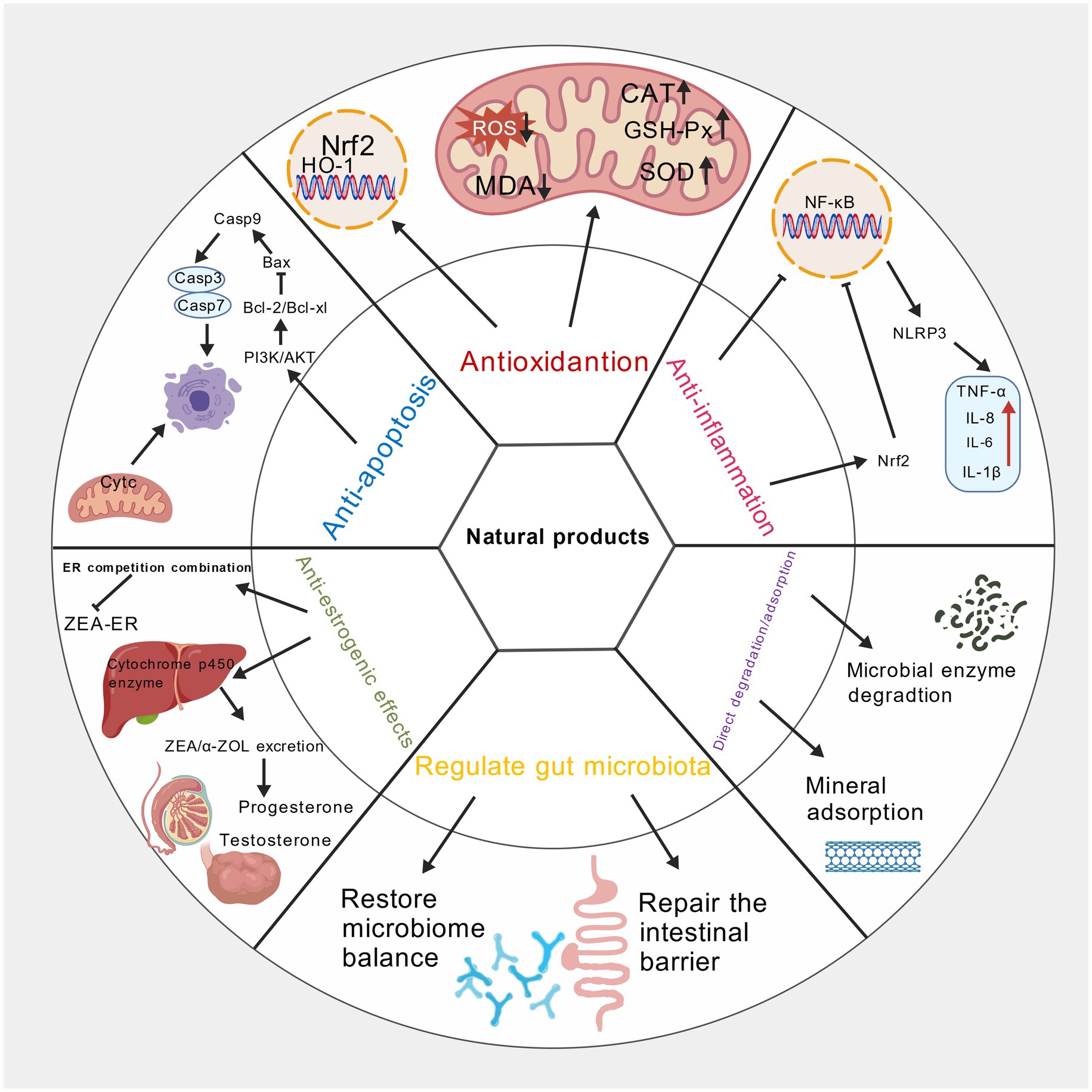
Figure 3. Mechanism diagram of natural products alleviating zearalenone toxicity. Created with BioGDP.com.
2.2.2 Genotoxicity
Extensive research indicates that ZEA exhibits genotoxicity, capable of inducing chromosomal aberrations, DNA damage, and gene mutations (26). In aquatic models, treating zebrafish embryos with 350–950 μg/L ZEA until 96 h post-fertilization (hpf) resulted in a highly significant increase in comet assay olive tail moments (OTM). Extensive apoptotic cells appeared in brain regions, accompanied by oxidative stress and DNA damage, with injury severity positively correlated with ZEA concentration and exposure duration (27). In rat primary testicular supporting cells, ZEA induced DNA damage and inhibited cell proliferation by activating the P-ATM → p53 → p21 signaling pathway, revealing its detrimental effects on male germ cell genetic material (28). Furthermore, in human breast cancer MCF7 cells, ZEA significantly elevated 5-methylcytosine levels and upregulated the expression of metabolism-related genes such as DNMT1, MGMT, IGF1, and HK2. However, it had no significant effect on DNA methylation or related gene expression in human normal breast epithelial MCF10F cells, suggesting that its genotoxicity exhibits cell specificity (29).
2.2.3 Immunotoxicity
The vast majority of immune cells express estrogen receptors on their surfaces. When ZEA binds to these receptors, it disrupts the normal functioning of the body’s immune system. The immune system comprises three major components: immune molecules, immune cells, and immune organs. In vitro experiments with chicken spleen lymphocytes showed that after 48 h of exposure to 25 μg/mL ZEA, IL-2 mRNA levels increased while IL-6 and IFN-γ levels decreased significantly, indicating that ZEA infection may interfere with cytokine secretion in chicken spleen lymphocytes (30). Literature reports indicate that in experiments where ZEA induces porcine spleen damage, ZEA upregulates the expression and synthesis of pro-inflammatory cytokines such as TNF-α and IL-8, activates the JNK pathway, and inhibits p38/MAPK and NF-κB, thereby disrupting immune homeostasis (31). Liang et al. (32) found that ZEA significantly inhibited mouse thymic epithelial cell proliferation in a dose- and time-dependent manner. Furthermore, literature reports indicate that ZEA and its metabolites can also affect intestinal and humoral immunity, such as altering intestinal mucosal IgA levels and inhibiting lymphocyte proliferation, with metabolites often exhibiting greater toxicity than ZEA itself (33).
2.2.4 Cytotoxicity
ZEA exerts cytotoxic effects on multiple cell types. It induces oxidative stress and activates apoptotic signaling pathways, leading to oxidative damage, apoptosis, or abnormal proliferation in various cells. In germ cells, ZEA upregulates ERS marker proteins such as GRP78, CHOP, and PERK in porcine endometrial epithelial cells (PECs), activates the JNK pathway, promotes β-catenin nuclear translocation, blocks G1 phase progression, and inhibits Bax and Caspase3, thereby inducing abnormal cell proliferation (34). In porcine trophoblast cells (pTr), ZEA activates the PERK-eIF2α-ATF4-CHOP and MAPK pathways, inhibits PI3K/AKT, elevates ROS and Ca2+, disrupts mitochondrial membrane potential, and induces autophagic apoptosis (35). In porcine endometrial stromal cells (ESCs), ZEA activates the ASK1-JNK pathway via the endoplasmic reticulum stress (ERS), elevates nuclear p-JNK, upregulates Bax and Caspase3/9 while downregulating Bcl-2, thereby intensifying apoptosis (36). In immune cells, 1–25 μg/mL ZEA inhibited LPS-activated mouse splenic lymphocyte proliferation with inhibition rates ranging from 5.62 to 88.17% over 24–72 h. It concurrently induced DNA fragmentation, with apoptosis rates increasing to 33.15% at higher concentrations (37).
2.2.5 Hepatotoxicity and nephrotoxicity
As the primary organs for ZEA metabolism and excretion in the body, the liver and kidneys are key targets for its toxicity. Regarding hepatotoxicity, studies have demonstrated that ZEA treatment of primary support cells for 24 h promotes intracellular ROS production, reduces cell viability and antioxidant enzyme activity, and causes a decrease in mitochondrial membrane potential. Concurrently, it induces activation of the Caspase-dependent apoptosis pathway and enhances autophagy activity (38), indicating that its hepatotoxic mechanism is closely related to oxidative stress (39, 40). The kidney serves as a secondary metabolic organ and is one of the primary target organs for zearalenone. After multiple enterohepatic circulations in vivo, zearalenone is filtered by the kidneys and ultimately excreted in urine. Specific effects include inducing glomerular atrophy, tubular epithelial cell degeneration, and proteinuria. In renal toxicity studies, female Wistar rats administered 40 mg/kg ZEA via gavage for one week exhibited significantly reduced kidney weight, markedly elevated serum blood urea nitrogen (BUN) and uric acid (UA) levels, significantly decreased creatinine (CRE), along with increased malondialdehyde (MDA) in kidney tissue. Along with marked decreases in antioxidant enzyme activities such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-px). Pathological lesions observed included renal vascular congestion, tubular dilatation, interstitial congestion, scattered cellular degeneration and necrosis, and glomerular cavity dilatation (41). Further mechanistic studies revealed that in porcine kidney epithelial cells (PK-15), ZEA induces apoptosis by activating ROS-mediated mitochondrial apoptosis pathways, upregulating pro-apoptotic proteins such as Bax and Caspase-3, and downregulating the anti-apoptotic protein Bcl-2 (42). Moreover, after ZEA enters the animal body, the liver can metabolize this toxin into two isomers: α-zearalenol (α-ZOL) and β-zearalenol (β-ZOL). The metabolite α-Zearalenol (α-ZOL) exhibits even greater cellular toxicity than ZEA itself, suggesting its metabolic activation process plays a critical role in liver and kidney toxicity (43).
2.3 Species-specific characteristics of ZEA toxicity
ZEA is a common mycotoxin that exerts toxic effects on various animal species. Pigs, chickens, cattle, and other animals are exposed to this toxin through consumption of ZEA-contaminated feed. Significant differences exist in ZEA sensitivity across species, primarily manifested in exposure routes, toxic doses, and typical clinical symptoms. Pigs are the most susceptible species to ZEA. Even trace amounts of ZEA in feed—as low as 0.1–0.15 mg/kg—can induce reproductive tract inflammation in sows. The maximum allowable levels of ZEA in feed for piglets and growing-finishing pigs are 0.1 mg/kg and 0.5 mg/kg, respectively. Typical clinical symptoms in pigs are concentrated in the reproductive system. For example, weaned gilts may exhibit vulvar hypertrophy and ovarian atrophy, while adult sows may experience infertility and pseudopregnancy. Boars may show testicular atrophy, sperm abnormalities, and reduced libido (4). In contrast, poultry and ruminants demonstrate higher tolerance. High ZEA doses (≥5 mg/kg) impair laying hen production performance and disrupt reproductive hormone secretion. At ZEA levels exceeding 5 mg/kg, laying hens exhibit significantly reduced average egg weight, markedly decreased blood luteinizing hormone (LH) levels, and markedly elevated progesterone levels, with these changes exhibiting a dose-dependent effect (44). Cattle, however, possess rumen microorganisms that partially degrade ZEA, resulting in a higher toxic threshold and atypical clinical symptoms. The primary potential impact is a slight reduction in reproductive performance. Case reports indicate that when corn zearalenone levels in feed reach 200 mg/kg, growing cattle exhibit toxic symptoms such as restlessness, reddened vaginal mucosa, and swollen labia (45).
These differences primarily stem from interspecies variations in metabolic pathways: pigs convert ZEA into the more toxic α-zearalenol, whereas poultry and ruminants produce less toxic metabolites. Therefore, subsequent research on natural product detoxification strategies for ZEA must account for species specificity: For swine, focus should be placed on developing formulations that block ZEA’s estrogen-like activity and promote excretion. For poultry and cattle, emphasis should shift toward additives that protect intestinal health and enhance overall production performance, enabling precise and effective detoxification interventions.
3 Research on natural products for ZEA attenuation
3.1 Plant-derived natural products
3.1.1 Flavonoids
Upon entering the animal body, ZEA can cause structural damage and functional impairment in multiple organs and tissues through various pathways, including oxidative stress, apoptosis, inflammatory responses, and endocrine disruption. In recent years, flavonoid natural medicines have garnered significant attention in ZEA poisoning research due to their wide availability, low toxicity, and multifaceted biological activities. Their detoxifying effects primarily achieve ZEA poisoning mitigation through multi-pathway, multi-target interventions.
Regarding the alleviation of oxidative damage, ZEA induces the production of large amounts of reactive oxygen species (ROS) upon entering the body. When ROS generation exceeds clearance, it triggers lipid peroxidation reactions, producing harmful substances such as malondialdehyde (MDA). Concurrently, it inhibits the activity of antioxidant enzymes like superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px), disrupting the body’s oxidative stress balance and causing oxidative damage to tissues like the uterus, liver, and spleen. Flavonoid-based natural medicines such as hyperoside, quercetin, rutin, and silymarin effectively address this issue. On one hand, they directly react with ROS to eliminate them, reducing ROS attacks on cells. On the other hand, they activate the body’s own antioxidant enzyme system, enhancing the activity and expression levels of antioxidants like SOD and GSH-Px, thereby strengthening the body’s ROS clearance capacity. Through these dual mechanisms, flavonoids significantly reduce the production of lipid peroxidation byproducts like malondialdehyde (MDA), maintain oxidative stress equilibrium, and mitigate ZEA-induced oxidative damage across multiple tissues and organs. These effects have been validated in various animal models and cellular experiments (46–51).
In inhibiting apoptosis and inflammatory responses, ZEA causes harm to the body by activating the apoptosis pathway. ZEA promotes the activation of Caspase family proteins while regulating the expression of apoptosis-related proteins, leading to the upregulation of pro-apoptotic protein Bax and the downregulation of anti-apoptotic protein Bcl-2. This disrupts the balance of apoptosis, resulting in massive cell death in tissues. Additionally, ZEA induces the release of inflammatory mediators such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), activating inflammatory signaling pathways like nuclear factor-κB (NF-κB) to trigger inflammatory damage. Flavonoid compounds like rutin and baicalin exhibit significant inhibitory effects against these mechanisms. Studies indicate that these compounds restore apoptotic balance by downregulating apoptosis-related proteins like Bax and Caspase-3 while upregulating Bcl-2 expression, thereby inhibiting excessive cell death (52, 53). For example, in a chick liver injury model established with 2.5 mg/kg ZEA, chicks were simultaneously administered 20 mg/kg, 40 mg/kg, or 80 mg/kg baicalin via oral gavage for one week. Immunohistochemical analysis revealed that baicalin dose-dependently reduced ZEA-induced hepatocyte apoptosis in chicks. Specifically, the 80 mg/kg baicalin group exhibited no significant difference in hepatocyte apoptosis compared to the blank control group, with virtually no discernible hepatocyte apoptosis observed (Xu Jingnan, 2022). In a ZEA-induced porcine endometrial stromal cell injury model, rutin was demonstrated to further enhance apoptosis inhibition by activating the nuclear factor E2-related factor 2 (Nrf2) signaling pathway (54). Treatment of porcine ESCs with 52.03 μM ZEA significantly increased the apoptosis rate. The addition of 25 μM rutin significantly reduced ZEA-induced apoptosis in porcine ESCs (p < 0.01). When Nrf2 was pre-silenced (si-Nrf2), ZEA-induced apoptosis further increased, and the addition of 25 μM rutin significantly suppressed this enhanced apoptotic effect (Z + N + R group vs. Z + N group, p < 0.01) (Chen et al., 2025). Concurrently, these flavonoids inhibit the activation of inflammatory signaling pathways such as NF-κB, reducing the secretion of inflammatory mediators like TNF-α and IL-6. This effectively alleviates inflammatory states in tissues like the liver and mitigates ZEA-induced inflammatory damage (55). In a model inducing porcine renal epithelial cell damage, quercetin targets CaSR to inhibit the CaSR/CaMKII pathway, regulating calcium homeostasis and maintaining mitochondrial dynamics stability, thereby preventing ZEA-induced apoptosis (56).
Regarding antagonizing endocrine-disrupting effects, ZEA exhibits estrogen-like activity by competitively binding to estrogen receptor alpha (ERα) and estrogen receptor beta (ERβ) in animals. This interferes with normal hormonal signaling pathways, leading to reproductive endocrine disorders and subsequent reproductive system abnormalities in animals, such as abnormal follicle development in sows and reduced sperm quality in boars. Flavonoid compounds like soy isoflavones demonstrate unique advantages in this context. They compete with ZEA for estrogen receptor binding sites. Due to their stronger binding affinity, they reduce ZEA’s receptor occupancy, thereby diminishing its estrogenic effects. Simultaneously, soy isoflavones regulate estrogen receptor expression levels, restoring normal hormone signaling pathways and maintaining reproductive endocrine balance (57). Furthermore, Practical application studies reveal that supplementing sow diets with soy isoflavones not only antagonizes ZEA’s estrogenic effects but may also accelerate the biotransformation and degradation of ZEA and its metabolites within the body by regulating the activity of relevant metabolic enzymes in tissues such as the liver. This approach reduces ZEA residues in the liver and muscle tissues of gilts during puberty and post-puberty, thereby mitigating the persistent harm caused by ZEA to animal organisms (58).
In summary, flavonoid-based natural medicines significantly mitigate ZEA toxicity through multiple mechanisms, including alleviating oxidative damage, inhibiting apoptosis and inflammatory responses, and antagonizing endocrine disruption. As research continues to advance, the application prospects of flavonoid-based natural medicines in alleviating ZEA poisoning will become increasingly broad, providing important research directions and potential solutions for addressing livestock production and food safety issues caused by ZEA contamination.
3.1.2 Polysaccharides
Polysaccharide-based natural medicines, characterized by their wide availability, high biocompatibility, minimal toxicity, and multifaceted biological activities, including antioxidant, immunomodulatory, and anti-inflammatory effects, have emerged as a significant research focus for alleviating ZEA poisoning. These substances exert protective effects through multidimensional and multitranslational pathways by precisely targeting key pathological stages of ZEA poisoning, offering novel strategies for its prevention and control.
Polysaccharides from diverse sources demonstrate unique advantages in mitigating ZEA-induced oxidative stress and apoptosis. Pteridium aquilinum polysaccharide (PAP-1b) exhibited significant protective effects in studies targeting testicular supporting cells of Changbai pigs (59). As critical cells maintaining spermatogenesis, testicular supporting cells are highly susceptible to ZEA toxicity. PAP-1b enhances cellular ROS scavenging capacity by boosting the activity of antioxidant enzymes such as superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px). Concurrently, it reduces levels of the lipid peroxidation product malondialdehyde (MDA) and the cellular damage marker lactate dehydrogenase (LDH), effectively mitigating ZEA-induced oxidative damage. Further studies revealed that PAP-1b also modulates apoptosis-related molecules to restore the Bax/Bcl-2 ratio and mitochondrial membrane potential, thereby inhibiting apoptosis. Its protective effects are closely associated with the PI3K-Akt signaling pathway and glutathione metabolism pathway. Key molecules in these pathways, GPX1 and SELENOK, play central roles in mediating PAP-1b’s detoxification effects. For example, a porcine testicular supporting cell damage model was established using 100 μM ZEA. After 4 h of pretreatment with 150 μg/mL Pteridium polysaccharide (PAP-1b), cells were cultured continuously for 48 h. flow cytometry analysis revealed significantly elevated apoptosis rates in the ZEA-treated group alone, whereas the PAP-1b-treated group exhibited significantly reduced apoptosis rates compared to the ZEA group (p < 0.05), with apoptosis levels approaching those of the blank control group (Shi et al., 2025).
Regarding the repair of ZEA-induced immune suppression, the thymus, as a central immune organ, is a critical site for T lymphocyte differentiation and maturation. ZEA can impair immune function by inducing thymocyte apoptosis. Astragalus polysaccharides (APS) significantly protect chicken thymocytes by downregulating the expression of endoplasmic reticulum stress-related genes such as ATF4, ATF6, and CRP78, as well as pro-apoptotic genes like Bax and Caspase-3, while simultaneously upregulating the expression of the anti-apoptotic gene Bcl-2, thereby inhibiting ZEA-induced thymocyte apoptosis. At a concentration of 200 μg/mL, APS exhibited optimal protective effects on thymocytes, effectively restoring thymic tissue morphology, enhancing immune cell activity, repairing ZEA-induced immune suppression, and strengthening the body’s antitoxic capacity (60).
Beyond the aforementioned polysaccharides, substances such as Poria cocos polysaccharide (PCP), Lycium barbarum polysaccharide (LBP), and selenium-chitosan also demonstrate promising potential in alleviating ZEA toxicity (61–63). PCP alleviates ZEA-induced oxidative damage across multiple tissues by elevating antioxidant enzyme activity (SOD, GPx) in mouse liver and kidney tissues, reducing MDA levels, and maintaining normal organ physiological functions. LBP specifically protects against ZEA-induced renal injury by inhibiting ZEA-induced mitochondrial apoptosis and autophagy in mouse kidneys. It reduces pathological damage and fibrosis in renal tissue by downregulating pro-apoptotic and autophagy-related molecules, thereby preserving renal filtration and metabolic functions. Selenium-chitosan, a conjugate of selenium and chitosan, demonstrated superior protective effects in a porcine endometrial epithelial cell injury model. It significantly downregulates ZEA-induced expression of genes, including JNK, ASK1, c-Jun, MKK4, and p53 by reducing intracellular ROS levels. By modulating the JNK/SAPK signaling pathway, it effectively mitigates ZEA-induced cell cycle arrest, mitochondrial damage, and apoptosis, offering a novel strategy for protecting endometrial function and reducing reproductive disorders in livestock and poultry.
In summary, polysaccharide-based natural medicines exert detoxification effects through multiple pathways, including enhancing antioxidant capacity, precisely regulating signaling pathways, and inhibiting apoptosis. Their mechanisms of action cover several key pathological stages of ZEA poisoning. These findings not only enrich the theoretical framework for ZEA poisoning prevention and control but also provide crucial experimental evidence for developing safe and effective natural anti-ZEA toxins. This research holds significant practical and applied value for promoting the healthy development of livestock and poultry farming and ensuring food safety.
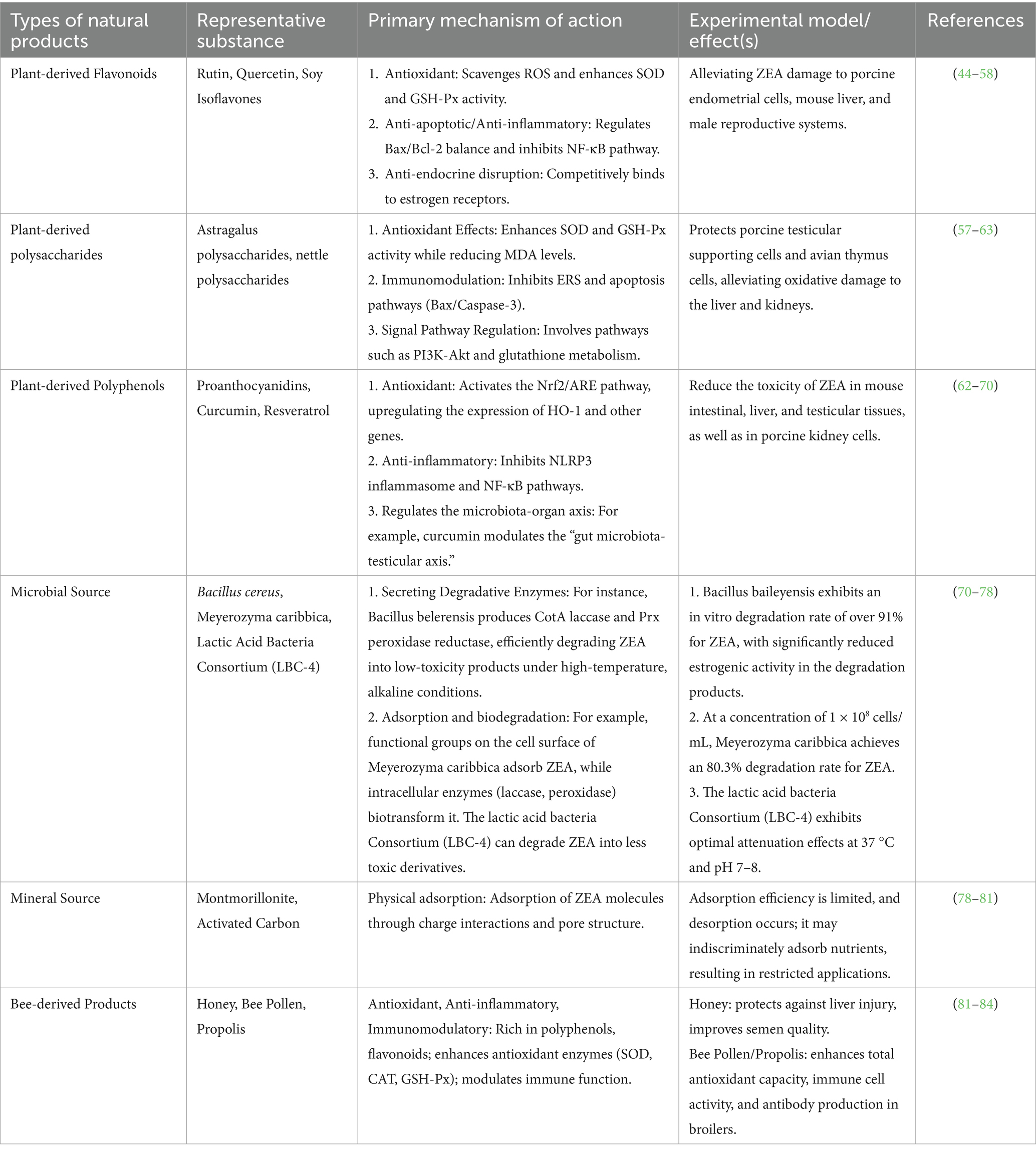
3.1.3 Polyphenols
ZEA is a common mycotoxin that damages the liver, reproductive system, and intestines. Polyphenolic natural medicines possess antioxidant, anti-inflammatory, and cell signaling pathway-modulating properties, enabling detoxification through multiple pathways. Proanthocyanidins (PCs) are internationally recognized as potent natural antioxidants and are widely used in ZEA detoxification research. Their protective effects primarily arise from regulating pathways associated with oxidative stress and apoptosis. In the mouse intestinal epithelial cell (MODE-K) model, ZEA activates the endoplasmic reticulum stress (ERS)-apoptosis pathway by upregulating ERS-related mRNA and protein expression, including CHOP, GRP78, JNK, and Caspase-12. Simultaneously reducing anti-apoptotic protein Bcl-2 levels and increasing pro-apoptotic protein Bax levels to induce apoptosis. It also inhibits superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) activity, decreases glutathione (GSH) content, and increases malondialdehyde (MDA) production, leading to oxidative damage. Conversely, PCs at concentrations of 5–15 μg/mL significantly reversed these changes by reducing apoptosis rates, restoring antioxidant enzyme activity and GSH content, inhibiting MDA elevation, and downregulating the expression of molecules associated with the ERS apoptosis pathway. This mechanism involves suppressing ERS-induced apoptosis pathways and alleviating oxidative stress (64). In mouse testicular supporting cells (TM4 cells), ZEA disrupts the Nrf2/ARE signaling pathway, downregulating mRNA and protein expression of Nrf2 and its downstream target genes HO-1, NQO1, GSH-Px, and γ-GCS, thereby weakening cellular antioxidant capacity. PCs (2.5–10 μg/mL) can activate the Nrf2/ARE pathway, upregulate the expression of the aforementioned antioxidant genes and proteins, enhance SOD and GSH-Px activity, reduce MDA accumulation, and decrease lactate dehydrogenase (LDH) release, thereby mitigating ZEA-induced cellular oxidative damage and apoptosis. The protective effect is optimal at a concentration of 5 μg/mL (65).
Curcumin (CUR), an extract from the ginger family, exhibits antioxidant, anti-inflammatory, and gut microbiota-modulating effects, demonstrating protective actions against ZEA-induced damage to the liver, kidneys, and reproductive system. In a mouse liver injury model, ZEA (40 mg/kg) induced hepatocyte edema, mitochondrial vacuolation, elevated serum AST and ALT activity, increased ROS and MDA levels in liver tissue, decreased SOD, CAT, and GSH-Px activity, activated the NLRP3 inflammasome, and upregulated NLRP3, Caspase-1p20 protein, and IL-1β expression. Conversely, 150 mg/kg curcumin mitigated hepatic pathological damage through dual antioxidant and anti-inflammatory actions, restoring antioxidant enzyme activity, reducing ROS and MDA levels, inhibiting NLRP3 inflammasome activation and IL-1β release, and alleviating hepatic oxidative stress and inflammatory responses (66). In porcine kidney epithelial cells (PK-15 cells), ZEA (36.55 μg/mL) induces cellular oxidative stress, increases ROS and MDA production, and decreases SOD and CAT activity. Curcumin (6.25–25 μmol/L) activates the SIRT1/FOXO1 signaling pathway, upregulates SIRT1 protein expression, and reduces FOXO1 acetylation. This promotes mRNA and protein expression of downstream antioxidant enzymes Mn-SOD and CAT, scavenges ROS, reduces lipid peroxidation, and alleviates ZEA-induced oxidative damage in renal cells (42). Furthermore, in a male mouse model of reproductive damage, ZEA (40 mg/kg) disrupts gut microbiota balance by decreasing Lactobacillus abundance while increasing Prevotella and Bacteroides abundance. This activates the IL-17A-TNF-α signaling pathway in testes, reducing testosterone secretion, decreasing sperm survival rates, and increasing deformity rates. Curcumin at 200 mg/kg modulates gut microbiota structure, restores beneficial bacterial abundance, inhibits IL-17A pathway activation, elevates testosterone levels, and improves sperm quality. Its protective effects are associated with the regulation of the “gut microbiota-testicular axis” (67).
Resveratrol (RSV) is a natural polyphenolic antioxidant primarily found in plants such as grapes and Polygonum cuspidatum. RSV can reduce ZEA toxicity through multiple pathways. Regarding liver protection, in a ZEA-induced liver injury mouse model, RSV alleviated pathological damage, restored antioxidant enzyme activity, inhibited NF-κB nuclear translocation and inflammatory factor release, demonstrating optimal protective effects (68). In mouse testicular supporting cells, RSV activates the PI3K/Akt pathway, promotes Akt phosphorylation, thereby driving Nrf2 nuclear translocation and upregulating HO-1 expression. This enhances cellular antioxidant capacity, reduces ROS production, and inhibits apoptosis-related proteins (Caspase-3, PARP cleavage) while increasing the Bax/Bcl-2 ratio, thus preventing cell death (69). Regarding intestinal protection, ZEA disrupts the intestinal barrier in mice. A 100 mg/kg RSV dose restores intestinal structure and barrier function by activating the Nrf2 pathway and inhibiting the NF-κB pathway to alleviate damage (70).
In summary, polyphenolic natural compounds mitigate ZEA toxicity through multi-targeted, multi-pathway mechanisms, including regulating oxidative stress-related pathways, inhibiting inflammatory pathways, and improving gut microbiota dysbiosis. Different polyphenolic compounds exhibit distinct target profiles: proanthocyanidins and resveratrol primarily regulate intracellular antioxidant and apoptosis pathways, while curcumin combines antioxidant, anti-inflammatory, and gut microbiota-modulating effects. These findings provide theoretical foundations for ZEA poisoning prevention and treatment, laying the groundwork for polyphenolic applications in animal husbandry.
3.2 Microbial-derived natural products
3.2.1 Bacillus species
Bacillus species demonstrate exceptional performance in mitigating zearalenone toxicity due to their potent degradation capabilities and environmental adaptability. For instance, the Bacillus paeiliosus strain PA26-7 efficiently degrades zearalenone by secreting extracellular enzymes. This strain degrades zearalenone under a wide range of conditions: initial medium pH 4.0–8.0 and cultivation temperatures 25–60 °C. The degradation products exhibit lower cytotoxicity and estrogenic activity compared to zearalenone (71). Additionally, the Bacillus velezensis strain B.26 is particularly noteworthy. Studies indicate that this strain can degrade 91.64% of ZEA within 24 h at 70 °C and pH 10.0 (72). This high degradation efficiency is primarily attributed to its secreted CotA laccase and Prx peroxidoreductase. Through genomic mining and molecular cloning techniques, researchers successfully isolated and recombinantly expressed these two enzymes. CotA enzyme achieved over 91% degradation of ZEA within 6 h at 70 °C and pH 8.0, with its activity significantly enhanced by adding ions such as Na+ and Cu2+. Prx enzyme reached a degradation rate of 59.74% for ZEA within 6 h at 70 °C and pH 11.0. This indicates that both enzymes exhibit high degradation efficiency under both high-temperature and alkaline conditions. Their degradation products are low-toxicity compounds C17H24O4 and C12H16O4, without generating high-risk estrogen metabolites such as α-ZEL, thereby ensuring the safety of the degradation process.
Bacillus degradation enzymes show broad application prospects for mitigating zearalenone toxicity. These enzymes not only demonstrate high degradation efficiency under laboratory conditions but are also considered to possess significant potential for application in the food and feed industries due to their environmental friendliness and safety. With further technological optimization and commercialization, these degradative enzymes are expected to become powerful tools for degrading and removing mycotoxin contamination. Additionally, the Bacillus amyloliquefaciens strain XJ-140 was screened for its high-efficiency ZEA degradation capability, degrading 93.75% of ZEA (2 μg/mL) within 24 h of incubation. This degradation primarily occurs via extracellular enzymes, supplemented by cell wall adsorption (73).
3.2.2 Yeast
Yeast exhibits significant potential for degrading ZEA in corn. Recent studies have revealed unique mechanisms and high efficiency in ZEA degradation by various yeast strains. For instance, the Tibetan yeast strain Saccharomyces cerevisiae KAB68 demonstrates outstanding ZEA degradation capabilities. At a culture density of 1 × 108 cells/mL, this strain achieved an 80.3% degradation rate for ZEA. Studies indicate that this yeast strain removes ZEA through a dual mechanism involving adsorption and intracellular biodegradation (74). This dual-action mechanism not only enhances degradation efficiency but also ensures the environmental friendliness of the degradation process.
The mechanism of yeast-mediated ZEA degradation primarily involves adsorption and biotransformation. During adsorption, functional groups on the yeast cell surface—such as O–H, N–H, C═O, and C–O—bind to ZEA molecules, thereby reducing their free concentration in solution (75). During biotransformation, yeast secretes specific enzymes like laccase and peroxidase to degrade ZEA into less toxic metabolites. For instance, Pichia pastoris has been engineered to express zearalenone-degrading enzymes, which efficiently break down ZEA during fermentation (76). Additionally, components of the yeast cell wall participate in the adsorption of ZEA, further enhancing its degradation capacity.
These studies not only elucidate the molecular mechanisms by which yeast degrades ZEA but also provide a theoretical basis for developing yeast-based bio-detoxifiers. Future research should focus on further optimizing degradation conditions for yeast to enhance its efficiency and stability in practical applications, as well as identifying additional yeast strains with high degradation capacity.
3.2.3 Lactic acid bacteria
Lactic acid bacteria have garnered increasing attention in recent years for their role in degrading zearalenone (ZEA) due to their widespread application and safety profile in the food industry. Research indicates that lactic acid bacteria reduce ZEA toxicity through two primary mechanisms: adsorption and biotransformation. For instance, current research demonstrates that the novel lactic acid bacteria strain LBC-4 can degrade ZEA into less toxic derivatives, with optimal detoxification occurring at 37 °C and pH 7–8 (77). In animal studies, Lactobacillus significantly reduced ZEA’s toxic effects on rat blood, liver, kidneys, and uterus, while aiding in the restoration of normal physiological and biochemical parameters (78).
Although lactic acid bacteria show great potential for ZEA detoxification, further research is needed on their biodegradation mechanisms, the toxicity of degradation products, and microbial safety for animals.
3.3 Mineral-based natural products
Research on mineral-based natural products for reducing zearalenone toxicity has primarily focused on activated carbon and montmorillonite. As a natural mineral adsorbent, montmorillonite exhibits adsorption properties toward zearalenone. It can adsorb various mycotoxins, thereby reducing their levels in feed. First, most mineral adsorbents exhibit poor adsorption efficiency for ZEA. Experimental results indicate that the adsorption rate of sodium-based montmorillonite ZEA is only 4%, suggesting certain limitations in the physical adsorption method. This is attributed to ZEA’s weakly polar groups and low electrophilicity, making charge-based adsorption difficult (79). Second, when using montmorillonite as an adsorbent, desorption of zearalenone must be considered. Research indicates that montmorillonite exhibits good adsorption of zearalenone under acidic conditions at pH 3. However, desorption of adsorbed zearalenone occurs at both pH 3 and pH 5, with particularly pronounced desorption observed at pH 5 (80). This implies that in practical applications, relying solely on montmorillonite’s adsorption capacity may not fully eliminate zearalenone, necessitating comprehensive consideration of both adsorption and desorption phenomena. Additionally, activated carbon—a highly porous, insoluble powder with a large specific surface area—exhibits strong adsorption capacity but non-selectively binds feed nutrients, potentially causing adverse effects in animals. For instance, adding 2.5% or 5.0% montmorillonite to pig diets resulted in liver damage (81). Mineral-based natural products like montmorillonite and activated carbon show potential for reducing zearalenone toxicity, but limitations include restricted adsorption capacity, desorption issues, and indiscriminate nutrient binding. In production practice, the selection and optimization of adsorbent usage protocols should be based on factors such as feed pH, animal digestive physiology, and the type and concentration of zearalenone in the feed. This approach ensures more effective zearalenone removal, safeguarding feed safety and animal health.
3.4 Bee-derived natural products
ZEA primarily exhibits toxic effects including reproductive toxicity, immunosuppression, and liver damage. These effects not only disrupt normal physiological functions in animals but also lead to reduced livestock production efficiency and economic losses. Bee-derived natural products are substances formed through fermentation when bees collect raw materials such as plant pollen and nectar and combine them with their own secretions. Core categories include bee pollen, honey, and propolis, while other components with potential bioactivity encompass beeswax and royal jelly (82). Recent domestic and international studies confirm that bee-derived natural products are rich in bioactive substances such as polyphenols, flavonoids, polysaccharides, amino acids, vitamins, and minerals. They exhibit multiple pharmacological effects including antioxidant, anti-inflammatory, immunomodulatory, and hepatoprotective/nephroprotective properties (83, 84). This provides scientific rationale for their potential to antagonize ZEA toxicity and positions them as an emerging direction in ZEA detoxification research.
From the perspective of the correlation between chemical composition and pharmacological effects, the detoxification potential of bee-derived natural products is closely related to their unique composition. Bee pollen, a granular substance formed when bees collect pollen grains from plant anthers and mix them with their own secretions, contains core active components such as flavonoids (e.g., rutin, quercetin), polyphenols, and polysaccharides. Among these, flavonoids can scavenge excess reactive oxygen species (ROS), activate the antioxidant pathway via nuclear factor E2-related factor 2 (Nrf2), enhance the activity of antioxidant enzymes such as superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px), and simultaneously inhibit the production of malondialdehyde (MDA), a lipid peroxidation product. This mechanism closely aligns with the previously described pathway by which plant-derived flavonoids (e.g., soy isoflavones) antagonize ZEA-induced oxidative damage. Regarding immune regulation, the polysaccharides in bee pollen promote T-lymphocyte and B-lymphocyte proliferation, elevate immunoglobulin (IgA, IgM) secretion levels, and enhance leukocyte activity, thereby repairing damage to immune organs. This is crucial for mitigating ZEA-induced immunosuppression. Research confirms that adding 2–3% bee pollen to broiler feed significantly alleviates thymus and spleen atrophy caused by ZEA (5 mg/kg) and reverses the decline in peripheral blood cytokine levels (e.g., IL-2, IFN-γ). This effect is mediated by activating the PI3K-Akt signaling pathway and suppressing excessive NF-κB inflammatory pathway activation, directly supporting the detoxification value of “bee pollen” highlighted in the keywords (84).
Honey, as the most extensively studied category among natural apicultural products, primarily consists of glucose and fructose (accounting for approximately 60–80%), alongside phenolic acids (such as caffeic acid and chlorogenic acid), flavonoids (including apigenin and kaempferol), and enzymes (like sucrase and catalase) (85, 86). These components confer honey with significant antioxidant and hepatoprotective activities: In a mouse model of alcoholic liver injury, 15 g/kg of red eucalyptus honey alleviated hepatic edema and inflammatory infiltration by upregulating mRNA and protein expression of CAT, SOD, and GSH-Px, while reducing serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activity (85). In ZEA-induced liver injury, phenolic acids in honey mitigated hepatocyte apoptosis by inhibiting NLRP3 inflammasome activation, reducing proinflammatory factor release (e.g., IL-1β), and enhancing mitochondrial membrane potential stability. This mechanism synergized with curcumin’s antagonism of ZEA hepatotoxicity. Furthermore, in studies on boar semen preservation, adding 10% buckwheat honey increased SOD and CAT activity in semen, reduced ROS accumulation, and improved sperm motility and survival rate (86). This finding suggests honey may mitigate ZEA-induced damage to male germ cells through antioxidant pathways, providing direction for future research.
Propolis is a resinous substance collected by bees from plant buds and tree bark, processed by mixing with their own secretions. Its primary active components include flavonoids (e.g., morin, pinobanksin), terpenoids, and phenolic esters (e.g., phenethyl caffeate). Modern pharmacological studies confirm propolis exhibits broad-spectrum antibacterial, anti-inflammatory, and immune-enhancing effects: On one hand, its flavonoid compounds competitively bind estrogen receptors (ERα/ERβ), reducing ZEA’s binding efficiency to these receptors and thereby attenuating its estrogen-like effects—a mechanism similar to how soy isoflavones antagonize ZEA’s reproductive toxicity. On the other hand, phenethyl caffeate in propolis can inhibit JNK signaling pathway activation, reduce the release of pro-inflammatory factors TNF-α and IL-6, and repair ZEA-damaged immune organs. In broiler chicken trials, supplementing feed with 0.1–0.2% propolis significantly enhanced total antioxidant capacity (T-AOC) in ZEA-exposed chickens, promoted spleen lymphocyte proliferation and antibody production, and synergistically improved ZEA-induced immunosuppression with bee pollen (84).
Overall, bee-derived natural products alleviate ZEA toxicity through multiple mechanisms—including antioxidant, anti-inflammatory, immunomodulatory, and endocrine-disrupting effects—due to their multi-component, multi-target characteristics. These effects complement the detoxification mechanisms of plant- and microbe-derived natural products. However, current research has limitations: First, the mechanisms of bee-derived natural products are primarily studied at the whole-animal or cellular level, with their active components (e.g., bee pollen flavonoids, propolis terpenoids) and direct interactions with ZEA (e.g., molecular docking, enzyme activity regulation) yet to be fully elucidated. Second, bee product quality is significantly influenced by plant sources and harvesting seasons, lacking standardized extraction and application protocols. Future research should integrate metabolomics and proteomics to decipher the key bioactive components and signaling pathways through which bee-derived natural products antagonize ZEA toxicity. Concurrently, establishing quality control standards for bee-derived detoxifiers will provide scientific support for their industrial application in preventing and controlling ZEA contamination within the livestock industry.
4 Summary and prospects
ZEA contamination is a complex global issue in agriculture and food safety, posing a multi-organ, multi-dimensional threat to animal health. This review demonstrates that relying on a single method is insufficient for effective mitigation. Natural products, due to their diverse mechanisms of action, offer an ideal solution for building a multi-layered, comprehensive defense system against toxicity. From microbial enzymes that degrade ZEA at the source, to plant active compounds that antagonize its effects in vivo, and to polysaccharides and bee products that systemically enhance the body’s antioxidant and immune capacity, various natural strategies have their own focus and work synergistically.
Current research has progressed from simple efficacy observation to the exploration of molecular mechanisms, revealing the central role of key signaling pathways such as Nrf2, NF-κB, and ERS in the detoxification process. However, challenges remain: most studies are still at the experimental stage, far from large-scale clinical application; the safety and stability of microbial agents, the bioavailability of plant extracts, the selectivity of mineral adsorbents, and the standardization of bee products urgently need to be resolved.
Future research should concentrate on the following directions: First, deepen the investigation of the molecular mechanisms underlying natural product detoxification. Utilize modern biotechnologies such as gene editing and proteomics to further elucidate their target sites and signaling pathways, providing more precise targets for developing novel detoxifiers. Second, optimize the extraction, purification, and formulation processes of natural products to enhance their stability and bioavailability, developing natural detoxifier products suitable for different animal species and farming environments. Third, strengthen ecotoxicological research on microbial degradation of ZEA to assess its long-term impacts in natural environments, ensuring the safety and sustainability of microbial degradation technologies. Fourth, conduct large-scale field trials to validate the efficacy and safety of natural detoxifiers in practical aquaculture settings, advancing their industrial application. Additionally, international cooperation and exchange should be enhanced to integrate global resources in jointly addressing ZEA pollution, thereby contributing to food security and public health.
Author contributions
NL: Writing – original draft. QZ: Writing – original draft. YP: Writing – review & editing. CS: Writing – review & editing. GS: Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by Jilin Province science and technology development Project (grant number 20240601087RC).
Acknowledgments
Image sources created with BioGDP.com.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gruber-Dorninger, C, Jenkins, T, and Schatzmayr, G. Global mycotoxin occurrence in feed: a ten-year survey. Toxins. (2019) 11:375. doi: 10.3390/toxins11070375
2. Marin, S, Ramos, AJ, Cano-Sancho, G, and Sanchis, V. Mycotoxins: occurrence, toxicology, and exposure assessment. Food Chem Toxicol. (2013) 60:218–37. doi: 10.1016/j.fct.2013.07.047
3. Wang, XY, Lou, X, Du, JH, Bai, YJ, Fan, ZH, and Liao, CS. Study on the contamination of zearalenone in feeds and feed raw materials in China. In proceedings of the 28th academic symposium of the veterinary pathology branch of the Chinese Association of Animal Science and Veterinary Medicine, the 27th academic symposium of the animal pathophysiology professional Committee of the Chinese Association of pathophysiology, the 7th academic symposium of the experimental pathology professional Committee of the Chinese Association for laboratory animal sciences, and the 6th academic symposium of the veterinary pathologist branch of the Chinese veterinary association. Henan: Henan University of Science and Technology (2025). doi: 10.26914/c.cnkihy.2025.035296
4. Xu, WJ, Wei, WJ, Chen, XG, Zhang, ZQ, Liu, YM, and Lü, QX. Research progress on the hazards of zearalenone to pigs and its prevention and control. Feed Res. (2024) 47:166–71. doi: 10.13557/j.cnki.issn1002-2813.2024.05.031
5. Jia, R, Ma, Q, Fan, Y, Ji, C, Zhang, J, Liu, T, et al. The toxic effects of combined aflatoxins and zearalenone in naturally contaminated diets on laying performance, egg quality, and mycotoxin residues in eggs of layers, and the protective effect of Bacillus subtilis biodegradation product. Food Chem Toxicol. (2016) 90:142–50. doi: 10.1016/j.fct.2016.02.010
6. Li, HM. Clinical symptoms, laboratory examination, prevention, and treatment of zearalenone poisoning in pigs. Modern Anim. Husbandry Sci. Technol. (2017) 10:98–137. doi: 10.19369/j.cnki.2095-9737.2017.10.090
7. Feng, YQ, Zhao, AH, Wang, JJ, Tian, Y, Yan, ZH, Dri, M, et al. Oxidative stress as a plausible mechanism for zearalenone to induce genome toxicity. Gene. (2022) 829:146511. doi: 10.1016/j.gene.2022.146511
8. Xue, LL, Zhang, PZ, Yang, XJ, Fan, SW, and Li, W. Research progress on contamination status and biological detoxification of zearalenone in corn and its by-products. Anim Husb Vet Med. (2024) 56:145–51.
9. Qi, L, Li, Y, Luo, X, Wang, R, Zheng, R, Wang, L, et al. Detoxification of zearalenone and ochratoxin a by ozone and quality evaluation of ozonised corn. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. (2016) 33:1700–10. doi: 10.1080/19440049.2016.1232863
10. Feng, XL, Zhou, YH, Li, LA, and Jiao, XL. Research progress on the hazards of zearalenone and the detoxification effect of physical adsorbents. Modern Agric. Sci. Technol. (2021) 21:133–5. doi: 10.3969/j.issn.1007-5739.2021.21.048
11. Gao, G, Jiang, H, Lin, H, Yang, H, and Wang, K. Asiaticoside ameliorates uterine injury induced by zearalenone in mice by reversing endometrial barrier disruption, oxidative stress, and apoptosis. Rep. Biol. Endocrinol. (2024) 22:118. doi: 10.1186/s12958-024-01288-6
12. Sun, PX, Yin, LL, Meng, G, Sun, SX, Zhang, YY, Shi, YX, et al. Research progress on the elimination of zearalenone by plant extracts. Feed Res. (2023) 46:158–64. doi: 10.13557/j.cnki.issn1002-2813.2023.16.030
13. Jing, S, Liu, C, Zheng, J, Dong, Z, and Guo, N. Toxicity of zearalenone and its nutritional intervention by natural products. Food Funct. (2022) 13:10374–400. doi: 10.1039/D2FO01545E
14. Salem, IB, Boussabbeh, M, Neffati, F, Najjar, MF, Abid-Essefi, S, and Bacha, H. Zearalenone-induced changes in biochemical parameters, oxidative stress and apoptosis in cardiac tissue: protective role of crocin. Hum Exp Toxicol. (2016) 35:623–34. doi: 10.1177/0960327115597467
15. Wu, FY, Yang, XY, Li, JL, and Chen, BJ. Research progress on reproductive toxicity of zearalenone to sows. Acta Vet Zootech Sin. (2020) 51:227–33. doi: 10.11843/j.issn.0366-6964.2020.02.003
16. Kowalska, K, Habrowska-Górczyńska, DE, and Piastowska-Ciesielska, AW. Zearalenone as an endocrine disruptor in humans. Environ Toxicol Pharmacol. (2016) 48:141–9. doi: 10.1016/j.etap.2016.10.015
17. Wang, H. Epidemiological characteristics, clinical manifestations, prevention and treatment of zearalenone poisoning in pigs. Modern Animal Husb. Sci. Technol. (2019) 47:90–1. doi: 10.19369/j.cnki.2095-9737.2019.04.046
18. Xiao, ZJ. Zearalenone poisoning and reproductive disorders in pigs. China Anim Husb Vet Med. (2005) 32:45–6. doi: 10.3969/j.issn.1671-7236.2005.02.018
19. Yuan, T, Li, J, Wang, Y, Li, M, Yang, A, Ren, C, et al. Effects of zearalenone on production performance, egg quality, ovarian function and gut microbiota of laying hens. Toxins. (2022) 14:653. doi: 10.3390/toxins14100653
20. Zhang, WL, Li, QF, Luo, Y, Zhang, XD, Yang, H, and Deng, H. Effects of zearalenone on production performance, ovarian tissue structure and ERα expression in laying hens. China Poultry. (2021) 43:30–5. doi: 10.16372/j.issn.1004-6364.2021.08.006
21. Ling, AR, Guo, JL, Guo, WB, Yang, JH, and Zhao, ZH. Effects of zearalenone on production performance, blood indicators and reproductive hormone levels in laying hens. Acta Agric Shanghai. (2019) 35:100–6. doi: 10.15955/j.issn1000-3924.2019.04.18
22. Yang, L, Liao, W, Dong, J, Chen, X, Huang, L, Yang, W, et al. Zearalenone promotes uterine hypertrophy through AMPK/mTOR mediated autophagy. Toxins. (2024) 16:73. doi: 10.3390/toxins16020073
23. Lü, YM. Study on zearalenone-induced apoptosis of porcine Leydig cells via mitochondria-mediated PI3K-AKT signaling pathway [master's thesis]. Shenyang: Shenyang Agricultural University (2023).
24. Liu, P, Zheng, H, Gu, Y, Xu, Z, Zou, H, Gu, J, et al. Zearalenone toxin induces pyroptosis by activating mitochondrial DNA-STING-NFκB axis in testis and TM4 cell damage. Chem Biol Interact. (2025) 418:111618. doi: 10.1016/j.cbi.2025.111618
25. Liu, T, Liu, G, Xu, Y, Huang, Y, Zhang, Y, Wu, Y, et al. Zearalenone induces blood-testis barrier damage through endoplasmic reticulum stress-mediated paraptosis of Sertoli cells in goats. Int J Mol Sci. (2023) 25:553. doi: 10.3390/ijms25010553
26. Li, RF, Wu, J, Yuan, LY, Wu, Y, and Yuan, H. Research progress on genotoxicity of zearalenone. Shanghai J Anim Husband Vet Med. (2009) 6:26–7. doi: 10.3969/j.issn.10007725.2009.06.011
27. Muthulakshmi, S, Maharajan, K, Habibi, HR, Kadirvelu, K, and Venkataramana, M. Zearalenone induced embryo and neurotoxicity in zebrafish model (Danio rerio): role of oxidative stress revealed by a multi biomarker study. Chemosphere. (2018) 198:111–21. doi: 10.1016/j.chemosphere.2018.01.141
28. Cai, PR, Wang, BJ, Feng, NN, Wang, L, Li, Q, Zou, H, et al. Study on DNA damage of rat Sertoli cells induced by zearalenone. Chin Vet Sci. (2018) 48:1201–6. doi: 10.16656/j.issn.1673-4696.2018.0176
29. Karaman, EF, and Ozden, S. Alterations in global DNA methylation and metabolism-related genes caused by zearalenone in MCF7 and MCF10F cells. Mycotoxin Res. (2019) 35:309–20. doi: 10.1007/s12550-019-00358-8
30. Wang, YC, Deng, JL, Xu, SW, Peng, X, Zuo, ZC, Cui, HM, et al. Effects of zearalenone on IL-2, IL-6, and IFN-γ mRNA levels in the splenic lymphocytes of chickens. Sci World J. (2012) 2012:567327. doi: 10.1100/2012/567327
31. Pistol, GC, Braicu, C, Motiu, M, Gras, MA, Marin, DE, Stancu, M, et al. Zearalenone mycotoxin affects immune mediators, MAPK signalling molecules, nuclear receptors and genome-wide gene expression in pig spleen. PLoS One. (2015) 10:e0127503. doi: 10.1371/journal.pone.0127503
32. Liang, ZS, Xu, LN, Ma, YJ, Deng, XB, Li, Y, Fan, XL, et al. Toxic effect of zearalenone on mouse thymic epithelial cells. Chin J Vet Sci. (2009) 29:894–7. doi: 10.16303/j.cnki.1005-4545.2009.07.019
33. Bulgaru, CV, Marin, DE, Pistol, GC, and Taranu, I. Zearalenone and the immune response. Toxins. (2021) 13:248. doi: 10.3390/toxins13040248
34. Song, TT. Study on the mechanism of zearalenone-induced uterine hypertrophy in weaned piglets [master's thesis]. Tai’an: Shandong Agricultural University (2020).
35. Bai, J, Li, J, Liu, N, Jia, H, Si, X, Zhou, Y, et al. Zearalenone induces apoptosis and autophagy by regulating endoplasmic reticulum stress signalling in porcine trophectoderm cells. Anim Nutr. (2022) 12:186–99. doi: 10.1016/j.aninu.2022.08.016
36. Zhao, J, Hai, S, Chen, J, Ma, L, Rahman, SU, Zhao, C, et al. Zearalenone induces apoptosis in porcine endometrial stromal cells through JNK Signaling pathway based on endoplasmic reticulum stress. Toxins. (2022) 14:758. doi: 10.3390/toxins14110758
37. Ma, YJ, Xu, LN, Li, YG, Fan, XL, Liang, ZS, and Deng, XB. Effect of zearalenone on apoptosis of splenic lymphocytes in mice. J Domestic Anim Ecol. (2009) 30:52–6. doi: 10.3969/j.issn.1673-1182.2009.01.013
38. Liu, X, Xi, H, Han, S, Zhang, H, and Hu, J. Zearalenone induces oxidative stress and autophagy in goat Sertoli cells. Ecotoxicol Environ Saf. (2023) 252:114571. doi: 10.1016/j.ecoenv.2023.114571
39. Wu, J, Li, J, Wu, Y, Yang, M, Chen, Y, Wang, N, et al. Betulinic acid mitigates zearalenone-induced liver injury by ERS/MAPK/Nrf2 signaling pathways in mice. Food Chem Toxicol Int J Res Assoc. (2023) 177:113811. doi: 10.1016/j.fct.2023.113811
40. Han, JX, He, JB, Gao, F, Yang, SH, Liang, TT, Dong, S, et al. Protective effect of proanthocyanidins against zearalenone-induced oxidative damage to liver and kidney in mice. China Anim Husb Vet Med. (2016) 43:402–6. doi: 10.16431/j.cnki.1671-7236.2016.02.017
41. Ben Taheur, F, Mansour, C, Skhiri, SS, Chaaban, H, Jridi, M, Fakhfakh, N, et al. Kefir mitigates renal damage caused by zearalenone in female wistar rats by reducing oxidative stress. Toxicon Off J Int Soc Toxinol. (2024) 243:107743. doi: 10.1016/j.toxicon.2024.107743
42. Cui, HJ, Lu, CT, Pan, LQ, Hu, H, Zhong, PY, Zhu, JY, et al. Curcumin alleviates zearalenone-induced oxidative damage in porcine renal epithelial cells via the SIRT1/FOXO1 pathway. Sci Agric Sin. (2023) 56:1007–18. doi: 10.3864/j.issn.0578-1752.2023.05.015
43. Tatay, E, Espín, S, García-Fernández, AJ, and Ruiz, MJ. Oxidative damage and disturbance of antioxidant capacity by zearalenone and its metabolites in human cells. Toxicol Vitro Int J Pub Assoc BIBRA. (2017) 45:334–9. doi: 10.1016/j.tiv.2017.04.026
44. Ning, CM, An, JX, Zhao, Y, and Yang, Y. Toxic effects of zearalenone on animal reproductive performance and its mechanism. Chin J Anim Nutr. (2023) 35:2166–74. doi: 10.12418/CJAN2023.204
45. Lang, XZ. Case analysis of zearalenone mycotoxicosis in replacement heifers. Shandong J Anim Sci Vet Med. (2017) 38:96–7. doi: 10.16431/j.cnki.1671-7236.2024.01.044
46. Zhu, W, Ge, M, Li, X, Wang, J, Wang, P, Tai, T, et al. Hyperoside attenuates zearalenone-induced spleen injury by suppressing oxidative stress and inhibiting apoptosis in mice. Int Immunopharmacol. (2022) 102:108408. doi: 10.1016/j.intimp.2021.108408
47. Zhu, YD, Yang, Q, Wang, XF, Liu, XW, and Jiang, GJ. Mitigative effect of three traditional Chinese medicine components on zearalenone-induced liver injury in mice. China Anim Husb Vet Med. (2024) 51:434–42.
48. Chen, PD. Study on the mechanism of quercetin alleviating zearalenone-induced PNH/HL-059 cell injury [master's thesis]. Jilin: Jilin University (2025).
49. Wang, C, Chen, C, Wang, M, Rahman, SU, Wei, B, Ding, H, et al. Rutin attenuates zearalenone-induced ferroptosis of endometrial stromal cells in piglets through the p53 signaling pathway. Ecotoxicol Environ Saf. (2025) 290:117546. doi: 10.1016/j.ecoenv.2024.117546
50. Zhang, QQ, Wang, YN, Tang, Y, Su, X, Zhang, JY, Huang, SM, et al. Mitigative effect of rutin on zearalenone-induced reproductive organ injury in female mice. Chin J Anim Sci. (2023) 59:278–84. doi: 10.19556/j.0258-7033.20220414-03
51. Gao, X, Xiao, ZH, Liu, M, Zhang, NY, Khalil, MM, Gu, CQ, et al. Dietary silymarin supplementation alleviates zearalenone-induced hepatotoxicity and reproductive toxicity in rats. J Nutr. (2018) 148:1209–16. doi: 10.1093/jn/nxy114
52. Sayed, H, Zhang, Q, Tang, Y, Wang, Y, Guo, Y, Zhang, J, et al. Alleviative effect of rutin on zearalenone-induced reproductive toxicity in male mice by preventing spermatogenic cell apoptosis and modulating gene expression in the hypothalamic-pituitary-gonadal Axis. Toxins. (2024) 16:121. doi: 10.3390/toxins16030121
53. Xu, JN. Observation and study on the protective effect of baicalin against zearalenone-induced liver injury in chicks [master's thesis]. Foshan: Foshan University of Science and Technology (2022).
54. Chen, C, Wang, C, Jiang, H, Wang, M, Rahman, SU, Chen, C, et al. Rutin alleviates zearalenone-induced endoplasmic reticulum stress and mitochondrial pathway apoptosis in porcine endometrial stromal cells by promoting the expression of Nrf2. Toxins. (2024) 17:7. doi: 10.3390/toxins17010007
55. Wang, Y, Wang, Q, Wang, G, Zhang, Q, Guo, Y, Su, X, et al. Rutin, a natural flavonoid glycoside, ameliorates zearalenone induced liver inflammation via inhibiting lipopolysaccharide gut leakage and NF-κB signaling pathway in mice. Food Chem Toxicol Int J Pub Br Ind Biol Res Assoc. (2024) 191:114887. doi: 10.1016/j.fct.2024.114887
56. Chen, S, Xu, T, Xu, A, Chu, J, Luo, D, Shi, G, et al. Quercetin alleviates zearalenone-induced apoptosis and necroptosis of porcine renal epithelial cells by inhibiting CaSR/CaMKII signaling pathway. Food Chem Toxicol Int J Pub British Ind Biol Res Assoc. (2023) 182:114184. doi: 10.1016/j.fct.2023.114184
57. Wang, DF, Zhang, NY, Peng, YZ, and Qi, DS. Interaction of zearalenone and soybean isoflavone in diets on the growth performance, organ development and serum parameters in prepubertal gilts. J Anim Physiol Anim Nutr. (2012) 96:939–46. doi: 10.1111/j.1439-0396.2011.01212.x
58. Wang, DF, Zhou, HL, Hou, GY, Qi, DS, and Zhang, NY. Soybean isoflavone reduces the residue of zearalenone in the muscle and liver of prepubertal gilts. Animal. (2013) 7:699–703. doi: 10.1017/S1751731112002066
59. Shi, HX. Mechanism of potentilla anserina polysaccharide alleviating zearalenone-induced oxidative stress in porcine Sertoli cells [doctoral dissertation]. Lanzhou: Gansu Agricultural University (2025).
60. Hu, H, Xu, ZK, Zhang, KZ, Lu, CT, Zhou, JY, Cui, HJ, et al. Protective effect of astragalus polysaccharide against zearalenone-induced apoptosis of chicken thymus cells. Heilongjiang Anim Sci Vet Med. (2022) 10:109–14. doi: 10.13881/j.cnki.hljxmsy.2021.08.0383
61. Dai, D. J., Li, H., Zhang, Y. J., Zhu, S. X., Huang, J. S., and Kang, W. C. (2024). “Mitigative effect of poria cocos polysaccharide on zearalenone-induced toxicity in mice,” in Proceedings of the 11th National Toxicology Congress of Chinese Society of Toxicology (229–230). College of Animal Science and Technology, Guangxi University.
62. Chen, HM. Effect of lycium barbarum polysaccharide on zearalenone-induced renal mitochondrial apoptosis and autophagy in mice [master's thesis]. Guangzhou: South China Agricultural University (2021).
63. Wang, H, She, F, Chen, F, Li, K, and Qin, S. Selenium-chitosan protects porcine endometrial epithelial cells from zearalenone-induced apoptosis via the JNK/SAPK Signaling pathway. Biol Trace Elem Res. (2024) 202:2075–84. doi: 10.1007/s12011-023-03816-8
64. Long, M, Chen, X, Wang, N, Wang, M, Pan, J, Tong, J, et al. Proanthocyanidins protect epithelial cells from zearalenone-induced apoptosis via inhibition of endoplasmic reticulum stress-induced apoptosis pathways in mouse small intestines. Molecules. (2018) 23:1508. doi: 10.3390/molecules23071508
65. Shi, W. Protective effect of proanthocyanidins against zearalenone-induced oxidative damage in mouse testicular Sertoli cells [master's thesis]. Shenyang: Shenyang Agricultural University (2017).
66. Song, C, Fu, CQ, Huangfu, HP, Zhang, AG, Wang, YK, Shi, DM, et al. Effect of curcumin on zearalenone-induced hepatic oxidative stress and NLRP3 inflammasome activation in mice. Chin J Vet Sci. (2023) 43:1898–904. doi: 10.16303/j.cnki.1005-4545.2023.09.15
67. Peng, B, Guo, S, Niu, J, Guo, Y, Wang, Z, and Zhang, W. Curcumin attenuates zearalenone-induced reproductive damage in mice by modulating the gut microbe-testis Axis. Foods. (2025) 14:2703. doi: 10.3390/foods14152703
68. Zhu, GS. Protective effect of resveratrol against zearalenone-induced hepatic oxidative damage and inflammation in mice [master's thesis]. Yangzhou: Yangzhou University (2022).
69. She, JJ, Feng, NN, Zheng, H, Liu, SS, Zou, H, Gu, JH, et al. Protective effect of resveratrol against zearalenone-induced oxidative damage and apoptosis in TM4 cells. Chin Vet Sci. (2020) 50:1453–60. doi: 10.16656/j.issn.1673-4696.2020.0170
70. Xia, S, Yan, C, Gu, J, Yuan, Y, Zou, H, Liu, Z, et al. Resveratrol alleviates zearalenone-induced intestinal dysfunction in mice through the NF-κB/Nrf2/HO-1 signalling pathway. Foods. (2024) 13:1217. doi: 10.3390/foods13081217
71. Deng, FR, Chen, JH, Jia, SH, Yao, CY, Li, RJ, Deng, YQ, et al. Screening and performance evaluation of a zearalenone-degrading bacterial isolate PA26-7. Microbiol. China. (2023) 50:3404–16. doi: 10.13344/j.microbiol.china.221078
72. Wei, Z, Zhang, X, Shi, L, Jin, J, Yang, B, and Xing, F. Functional characterization and mechanism of two zearalenone-degrading enzymes from Bacillus velezensis B.26. J Agric Food Chem. (2025) 73:14629–40. doi: 10.1021/acs.jafc.5c03232
73. Xue, LL, Zhang, PZ, Yang, XJ, Fan, SW, and Li, W. Screening, identification, and degradation efficacy evaluation of zearalenone-degrading bacteria. J. Northwest A&F Univ. (2025) 53:13–22. doi: 10.13207/j.cnki.jnwafu.2025.01.002
74. Xu, ZL, Chen, YB, Meng, KL, Yang, CXY, Jiang, DD, Jiang, DS, et al. Research on the degradation efficacy and mechanism of zearalenone by Tibetan yeast Meyerozyma caribbica 68. Guangdong Agric Sci. (2025) 22:1–15.
75. Jing, S, Lan, X, Liu, Y, Sun, C, Ye, H, Wang, J, et al. Microbe-mediated removal of zearalenone using yeast strain Rhodotorula dairenensis isolated from the gut microbiome of zearalenone-treated mice. J Agric Food Chem. (2025) 73:9320–36. doi: 10.1021/acs.jafc.4c11881
76. Wang, Y, Wang, Y, Jiang, J, Zhao, Y, Xing, F, and Zhou, L. Sheng wu gong cheng xue bao. Chin J Biotechnol. (2020) 36:372–80. doi: 10.13345/j.cjb.190150
77. Murtaza, B, Wang, L, Li, X, Ali, A, Haq, SU, Ji-bin, L, et al. Novel lactobacillus consortium for effective zearalenone adsorption and biodegradation. Int Biodeterior Biodegrad. (2024) 194:105889. doi: 10.1016/J.IBIOD.2024.105889
78. Ali, SA, Ibrahim, HJ, Ali, MN, Ali, KM, Shahad, HH, and Rusul, NK. Bioremediation of zearalenone by using Lactobacillus acidophilus in albino rats bodies (in vivo). J Contemp Med Sci. (2015) 1:21–5. doi: 10.22317/jcms.v1i1.12
79. Zeng, L, Yan, CJ, Chen, G, and Yu, CC. Study on the adsorption properties of different silicate mineral materials for mycotoxins. Chin J Anim Sci. (2011) 47:64–6.
80. Liang, XW, Li, FD, Zhang, JM, Ma, YP, Zhao, QY, and Gao, HL. Study on the adsorption of montmorillonite and attapulgite to mycotoxins. Chin Anim Husb Vet Med. (2014) 41:133–8.
81. Elliott, CT, Connolly, L, and Kolawole, O. Potential adverse effects on animal health and performance caused by the addition of mineral adsorbents to feeds to reduce mycotoxin exposure. Mycotoxin Res. (2020) 36:115–26. doi: 10.1007/s12550-019-00375-7
82. Anjos, O, and Miguel, MDG. Unveiling the chemistry and bioactivity of bee products and their derivatives. Foods. (2025) 14:3058. doi: 10.3390/foods14173058
83. Wang, HZ, Wang, JZ, and Wang, CP. Research progress of bee products in promoting human health. Apic China. (2022) 73:52–5. doi: 10.3969/j.issn.0412-4367.2022.02.024
84. Al-Kahtani, SN, Alaqil, AA, and Abbas, AO. Modulation of antioxidant Defense, immune response, and growth performance by inclusion of Propolis and bee pollen into broiler diets. Anim MDPI. (2022) 12:1658. doi: 10.3390/ani12131658
85. Wang, JY, Lin, WK, and Tao, CL. Protective effect of jarrah honey against acute alcoholic liver injury in mice. Biol Chem Eng. (2024) 10:111–5. doi: 10.3969/j.issn.2096-0387.2024.02.022
Keywords: zearalenone, toxicity, natural products, detoxification methods, bee pollen
Citation: Liu N, Zhang Q, Piao Y, Sun C and Shi G (2025) Research progress on the prevention and treatment of zearalenone poisoning in animals using natural products. Front. Vet. Sci. 12:1710151. doi: 10.3389/fvets.2025.1710151
Edited by:
Baocheng Hao, Chinese Academy of Agricultural Sciences, ChinaReviewed by:
Hao Lu, Northwest A&F University, ChinaZhao-ying Liu, Hunan Agricultural University, China
Copyright © 2025 Liu, Zhang, Piao, Sun and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guangliang Shi, c2hpZ3VhbmdsaWFuZ0BuZWF1LmVkdS5jbg==
 Nannan Liu
Nannan Liu Qi Zhang
Qi Zhang Yulan Piao1
Yulan Piao1 Guangliang Shi
Guangliang Shi