- 1Department of Zoology, College of Science, King Saud University, Riyadh, Saudi Arabia
- 2Department of Biology, College of Science, Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia
- 3School of Pharmaceutical Science, Nanchang University, Nanchang, China
Objectives: Natural products hold significant potential in combating parasitic infections. Parasites belonging to the genus Eimeria are responsible for coccidiosis, which affects a wide variety of livestock worldwide. The emergence of drug resistance to coccidia has prompted renewed efforts to discover novel chemicals for alternative therapeutic techniques. This research was conducted to evaluate the effectiveness of Cinnamomum verum bark extract (CVBE) on behavior and the treatment of eimeriosis in mice.
Methods: The study involved the examination of five groups of mice, with E. papillate sporulation oocysts (1 × 103) being utilized to infect three groups by experimental means. The first group was the control, the second group was administered at a dosage of 100 mg/kg of CVBE only to test toxicity, and the third was the infected positive control. The fourth group was treated daily with 100 mg/kg of CVBE, while the fifth group received 50 mg/kg of amprolium via gavage. All mice were sedated using CO₂ and subsequently dissected for sample taking. Results FT-IR analysis, conducted using a Thermo Scientific optical spectrometer, revealed that CVBE contains 18 different phytochemical components. The administration of CVBE led to a significant decrease in the number of oocysts produced in the feces. Additionally, the parasite stages exhibited morphometric changes, with decreases in the measures compared with those of the infected but not treated mice. This improvement was accompanied by large increases in the number of goblet cells, which provided additional support for the effectiveness of CVBE as anticoccidial agents.
Conclusion: The findings indicate that Cinnamomum verum bark extract has effective effects in reducing the shedding of cysts, possesses anticoccidial, antioxidant, and anti-inflammatory activity against jejunum injury induced by the parasite Eimeria papillata.
1 Introduction
Coccidiosis is an intestinal disease that affects a wide variety of animals and affects the health and function of several vertebrates; it is caused by Eimeria spp. in birds and cattle or Isospora in felids and canids (1) The annual propagation rate of coccidiosis in domestic avians in Riyadh, Saudi Arabia, is 80% (2). These protozoan parasites present a threat to both animals and humans. They cause substantial harm to the economy of the cattle sector (3). Eimeria swiftly multiply within host cells, causing harm to their digestive systems (4). Disease symptoms include diarrhea, which varies from watery to hemorrhagic mucoid and is accompanied by feed malabsorption and reduced feed conversion efficiency, resulting in weight loss and, in severe instances, mortality (5). Mice infected with Eimeria papillata exhibit infections in their jejunum. This infection significantly affects the intestinal mucosa (6). Mice are the most commonly employed mammalian model in biomedical research. Thus, E. papillata serves as an exemplary model for the study of avian coccidiosis (7). Because parasites are becoming more resistant to antiparasitic drugs, treatment doses and residues in animal products have increased. This has made consumers want products without residues and led many researchers to suggest and develop other agents (8).
The growing interest in safe and effective options for coccidiosis management has resulted in the utilization of plant extracts, essential oils, and traditional medicinal preparations (9). Medicinal plants serve as the fundamental source for all alternative or natural medical systems and are considered therapeutic methods for acquiring potent chemical constituents (10). These medicines demonstrate organ-protective properties in hosts infected with Eimeria and specifically target parasites (11).
In recent decades, medicinal plant extracts have been shown to effectively safeguard animals from coccidia-induced harm, albeit without enhancing performance (12) The organic acid combinations suppressed both in vitro and in vivo pathogens, diminished inflammatory oxidative stress, and boosted the immune response by lowering reactive oxygen species, increasing manganese superoxide dismutase, and elevating short-chain fatty acid levels (13).
Cinnamomum verum, a natural product of the Lauraceae family, is abundant in several bioactive chemicals with diverse medicinal qualities. It comprises numerous secondary plant chemicals, including cinnamaldehyde, carvacrol, eugenol, camphor, and proanthocyanidins, as well as essential macro- and micronutrients (14). Owing to these bioactive components, botanicals, including essential oils, have demonstrated promising anticoccidial efficacy (15). These bioactive molecules exhibit antioxidant capabilities, hence mitigating the oxidative damage caused by Eimeria (16). C. verum, usually referred to as “cinnamon,” is a widely utilized spice recognized for its diverse therapeutic and pharmacological attributes, including antioxidant, anti-inflammatory, and antibacterial capabilities (17). Considering these characteristics, the present investigation was undertaken to evaluate the in vivo anticoccidial efficacy of C. verum essential against E. papillata infection and its impact on metabolic markers in mice.
2 Manuscript formatting
2.1 Extraction of Cinnamomum verum bark
The collection of C. verum bark came from spice markets in Riyadh. The Botany Department confirmed the taxonomic identity of the materials with number 24649. Once the bark was dried at 40 °C, it was chopped into small pieces. An electric blender was subsequently used to powder the bark. This powder was extracted by macerating it with 70% ethanol (18). We continuously mixed this mixture and then placed it in an incubator at 4 °C for 24 h. After centrifugation for 15 min at 5000 rpm, we carried out filtration. We concentrated the supernatant at 50 °C under reduced pressure via a rotary evaporator. To prepare dosages of CVBE, 100, 50, and 25 mg/kg, the powdered C. verum extract that was obtained was freeze-dried and stored at −80 °C.
2.2 Infrared spectroscopy of the CVBE
Upon extraction, a small fraction of the material was homogenized by blending it with an abundant amount of potassium bromide powder (1:99 wt%). The material subsequently underwent a coarse crushing procedure before being introduced into a pellet-forming die. The infrared spectrum was examined via a Thermo Scientific optical spectrometer [NICOLET 6700 Fourier transform infrared spectroscopy (FT-IR)]. This showed the forecasting of the most likely constituent classes. The maximum quantity of absorbed waves is represented by the term “wavenumber” (cm−1). Spectra were acquired at 25 °C, with a resolution of 4 cm−1, covering a spectral range from 4,000 cm−1 to 400 cm−1.
2.3 Design of the experiment
Eimeria papillata was obtained mainly from unpopulated oocysts by Prof. Mehlhorn at the University of Duesseldorf (Duesseldorf, Germany). Forty-five female BALB/C mice were obtained from an animal facility in Riyadh, Saudi Arabia. The mice were 9 ± 1-week-old, weighed 20 ± 2 g, and were maintained in settings that were free of any pathogens. Food and water were provided ad libitum, and the mice were kept on a 12-h light/dark cycle at a regulated temperature (26 ± 2 °C) and 65 ± 5% humidity. In addition, the feces of the mice were examined to guarantee that the animals were free of intestinal diseases, especially coccidia. A preliminary dose–response study was performed to determine the optimal dose of CVBE used 20 mice (injured 1 × 103 sporulated oocysts) distributed over four concentrations (12.5, 25, 50, and 100 mg/kg). In the final experiment, the animals were separated into five groups (5 mice/group), and an oral dose of 1 × 103 sporulated E. papillata oocysts was administered to all groups, except the first (negative control) and second groups (noninfected and treated with CVBE at a dose of 100 mg/kg). The positive control mice, which were infected but not treated, composed the third group. A dose of 100 mg/kg CVBE was administered to infected mice in the fourth group. In conclusion, the fifth group consisted of mice that had been infected and treated with amprolium at a dosage of 50 mg/kg. All mice were sedated using CO₂ and subsequently dissected for sample taking. The mice are placed in a sealed glass cage connected by a tube to a CO₂ cylinder, and a dose enough to anesthetize the mouse is given, proportionate to the mouse’s weight.
2.4 Evaluation of Anticoccidial activity
The anticoccidial index (ACI) was used to evaluate the preventive efficiency of CNBE against coccidia. Anticoccidial characteristics, including bloody diarrhea, oocyst value (OV), relative weight gain (RWG), lesion value (LV), and survival rate (SR), were assessed to compute the anticoccidial index (ACI) via the method of Habibi et al. (19).
ACI values less than 120 were deemed to indicate inactive anticoccidial action, although ACI values typically range from 0–200 (20) Consequently, ACI values ranging from 120–140, 140–160, 160–180, or over 180 were categorized as mild, moderate, marked, or outstanding. Bloody diarrhea in the mice was documented in the morning with daily veterinary surveillance and quantification of blood fragments in the feces during a period of 3 to 5 days post-infection. The feces were eliminated daily following each inspection to reveal the fresh bloody feces. The digits 0, 1, 2, 3, and 4 correspond to the respective values of the bloody fragments in the feces of each group (21).
Clinical symptoms and death were evaluated and documented daily following infection. Equation 5 was subsequently employed to determine the survival rate.
On the fifth day, the experiment ended at 5 DPI, during which all the mice (with a sample size of 1 mouse per duplicate, n = 5 mice per group) were subjected to 3 h of food deprivation, and their drinking water was provided ad libitum. The mice were subsequently euthanized and autopsied in compliance with the Animal Welfare Act. Following slaughter by severing the jugular vein, the mice were thoroughly bled, subsequently deskinned and eviscerated. The jejunum of each mouse was excised and analyzed. Lesion values were assessed based on the macroscopic lesions found during necropsy, utilizing a scale of 0–4, which was determined by the severity of the lesion and atrophy of the jejunum (including fluid buildup, epithelial coloration, and overall intestinal appearance). Zero indicates the absence of lesions, denoting a ‘normal condition’, whereas one signifies mild lesions characterized by small, scattered petechiae and normal jejunal wall thickness. Two findings indicate significant lesions: many petechiae and reduced jejunal wall thickness. Three indicates severe lesions characterized by significant hemorrhaging, distended jejunal walls, and morphological alterations of the jejunum; four denotes more severe lesions, including discoloration, blood clots, and atrophy of the jejunum, as well as pronounced hypertrophy of the jejunal walls, culminating in fatalities due to coccidiosis. Additionally, the jejunum lengths were documented as the mean ± SEM (in cm) to evaluate the degree of atrophy.
2.5 Oocyst number
On the fifth day post infection (p.i.), we isolated each mouse from the third, fourth, and fifth groups into individual cages (n = 5 mice) to obtain fresh fecal pellets. Recent fecal samples were obtained from the mice. The feces were suspended in a saturated saline solution containing 0.9% sodium chloride, with each sample meticulously blended and disseminated in 10 mL of water. The weight-to-volume ratio was 1:10. A calculation was conducted to determine the average quantity of oocysts excreted by both the treated and untreated groups via a glass microscope slide and sanitized square microscope coverslips measuring 18 × 18 mm via an Olympus optical microscope (Olympus Corporation, Tokyo, Japan) with a digital camera (22). The results were quantified as oocysts/g (OPG). The rates of reduction in oocyst generation and oocyst values were determined as specified by Smith et al. (23).
2.6 Histopathological examination and count of parasitic stages
The jejunum was subsequently separated with a phosphate-buffered saline solution (pH = 7.5) and promptly fixed in a 10% neutral buffered formalin solution at 24 °C for 1 day. The tissue samples were dehydrated via ethanol in increasing order, cleared with xylene, fixed in paraffin wax, and sectioned into 5 μm slices on glass slides. The samples were deparaffinized with xylene, and the tissue sections were stained with hematoxylin and eosin (H&E) and Masson’s trichrome (MTC). The slides were fixed and allowed to dry in anticipation of the examination. An Olympus compound microscope and a digital camera (Olympus 6.0, Tokyo, Japan) were used to capture images and analyze the samples (24).
2.7 The enumeration of goblet cells
Alcian blue was used to stain 5 μm thick tissue sections for 5 min, followed by deparaffinization and processing, to quantify the number of goblet cells. The samples were repeatedly cleansed with distilled water, subsequently dehydrated with decreasing concentrations of water, and then deparaffinized with xylene and ethanol to remove moisture. The jejunum of each animal was examined for the quantity of goblet cells in a minimum of ten properly orientated villous–crypt units (VCUs). The findings are presented as the average count of goblet cells observed in each villus (25).
2.8 Oxidative stress in jejunal
The jejunum was prepared after weighing from each of the noninfected, nontreated, and infected groups of mice to assess alterations in the oxidation state. The sample was promptly homogenized in 50 mM phosphate-buffered saline (PBS) with a pH exceeding 7.4, followed by centrifugation at 5000 × g for 15 min at 4 °C. A 10% (wt/v) jejunum homogenate was obtained after the supernatant was collected, and 10% of the volume was used for biochemical investigation of the oxidative stress levels. The GSH (mg/mg), NO (μmol/mg), CAT (U/mg), and SOD (U/mg) levels were measured via ELISA kits according to the methods of Jollow et al. (25) and Buege and Aust (26). The optical density was assessed at 450 nm via a microplate reader.
2.9 Statistical analysis
The disparities were examined via one-way analysis of variance (ANOVA) in the SPSS Vision 21 statistical program. We employed the Duncan test to compare the mean values of the various substances. A probability of < 0.05 was deemed significant (p ≤ 0.05). We included the mean values alongside the standard deviation (mean±SD).
3 Result
Chemical analysis was performed via Fourier transform infrared spectroscopy (FTIR), and the results revealed that the ethanolic CNBV included 18 chemical compounds, with major absorption bands between 3932.03 cm − 1 and 672.40 cm − 1 (Figure 1). The analysis revealed the following active phytochemical components: O-H stretching, N-H stretching, O-H stretching, N-H stretching, N=N=N stretching, C=O stretching, N-O stretching, C-H bending, O-H bending, O-H bending, C-O stretching, C-O stretching, C-O stretching, C-O stretching, and C=C bending, with an absorbance of 400–4,000/cm − 1 (Table 1).
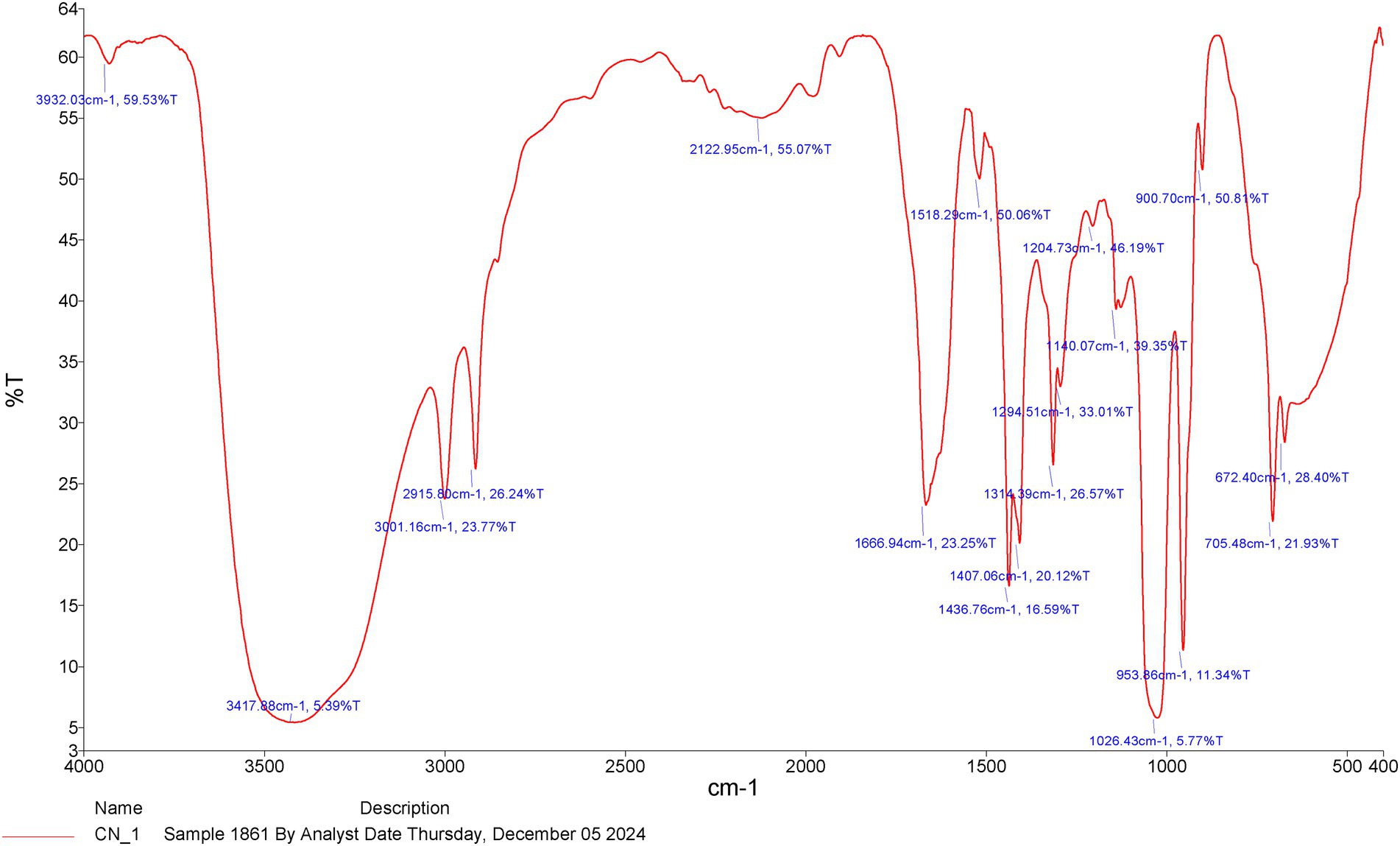
Figure 1. The functional characteristics of the active chemical compounds according to the FT-IR chromatograms of the Cinnamomum verum bark extract in methanol.
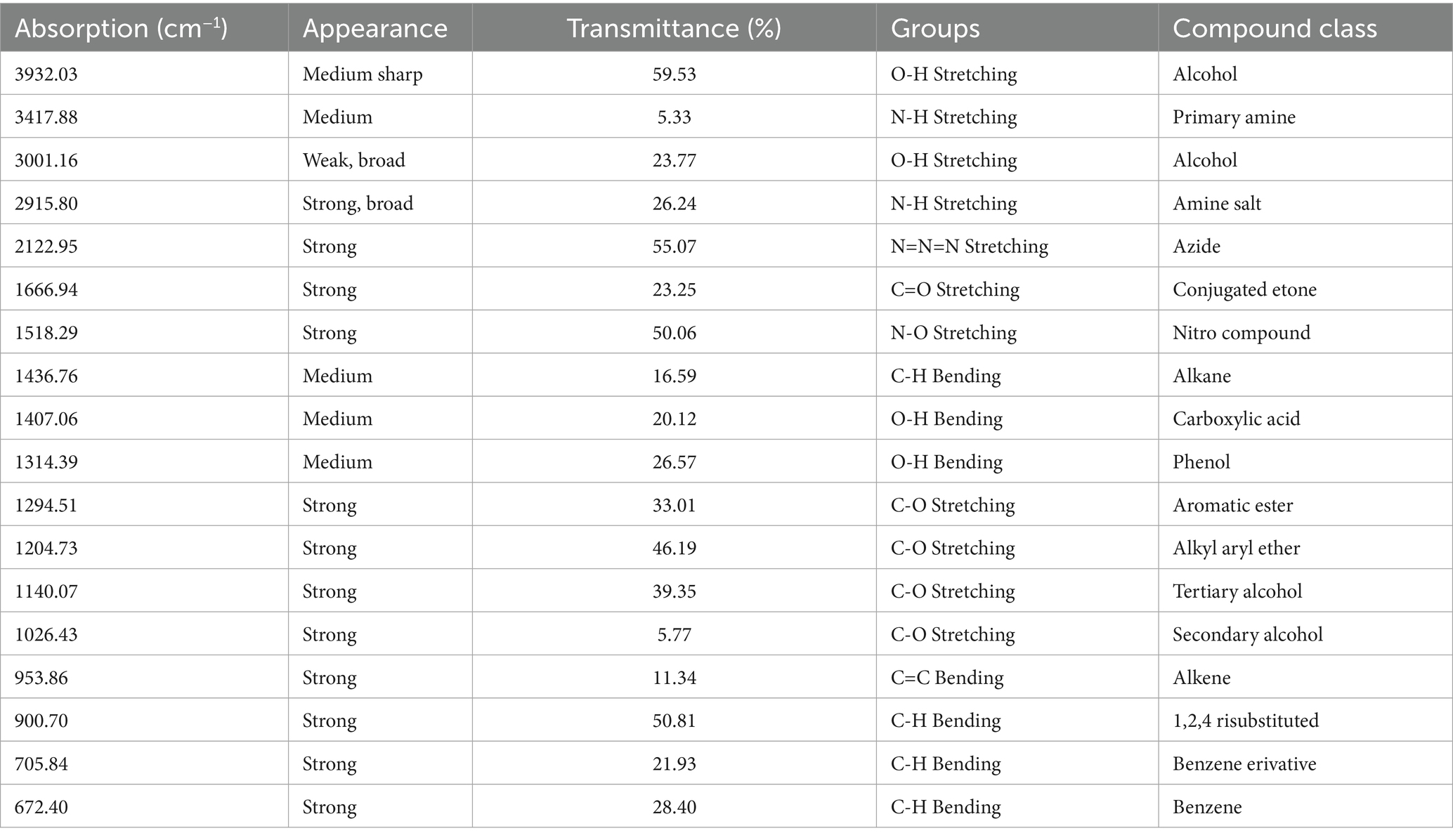
Table 1. Fourier transform infrared spectroscopy of Cinnamomum verum bark extracts in the frequency range.
Figure 2 illustrates the frequency of clinical symptoms observed in the mice. The clinical indications varied in intensity among the experimental mice. The most prevalent sign was diarrhea. Infected mice clearly displayed coccidiosis symptoms, such as crouching, inappetence, sadness, and ruffled skin, whereas uninfected or amprolium-treated mice did not.
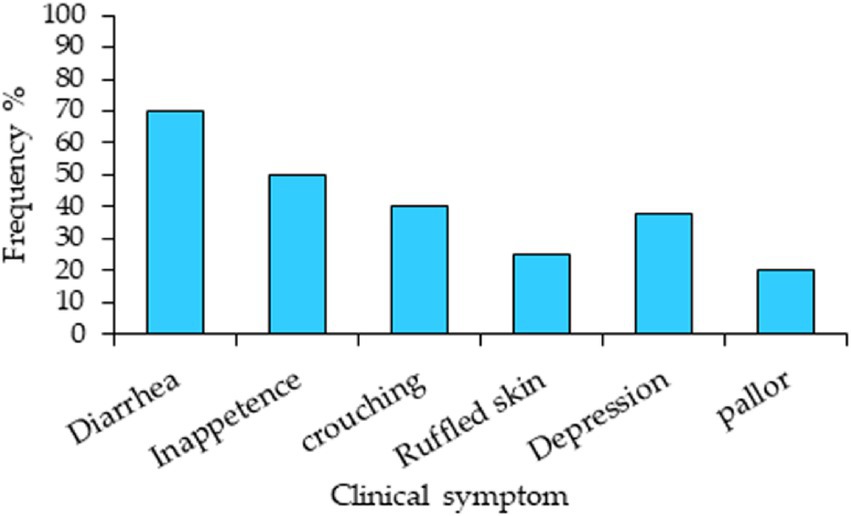
Figure 2. Clinical symptoms and their frequency in mice experimentally infected with Eimeria papillata without medication.
Table 2 shows the effects of CNB on the inhibition rate, incidence of bloody diarrhea, lesion score, and jejunal length for each therapy at 5 days postinjury. Pathological lesions in groups categorized from normal to severe. The scores of the infected group are shown in Table 2. It presents the length of the jejunum and the scores of the caeca, including jejunal atrophy, wall thickening, erosion, and black blood clots, which exhibited significant pathological abnormalities. Compared with those in the infected group, the jejunal morphology and length were considerably (p < 0.05) greater in the drug-treated group. When all the pathological parameters were aggregated, the lesion scores indicated that the herb-treated groups presented a significant (p < 0.05) reduction in unfavorable jejunal lesions induced by coccidiosis with increasing quantities of CNB extract (Table 2).
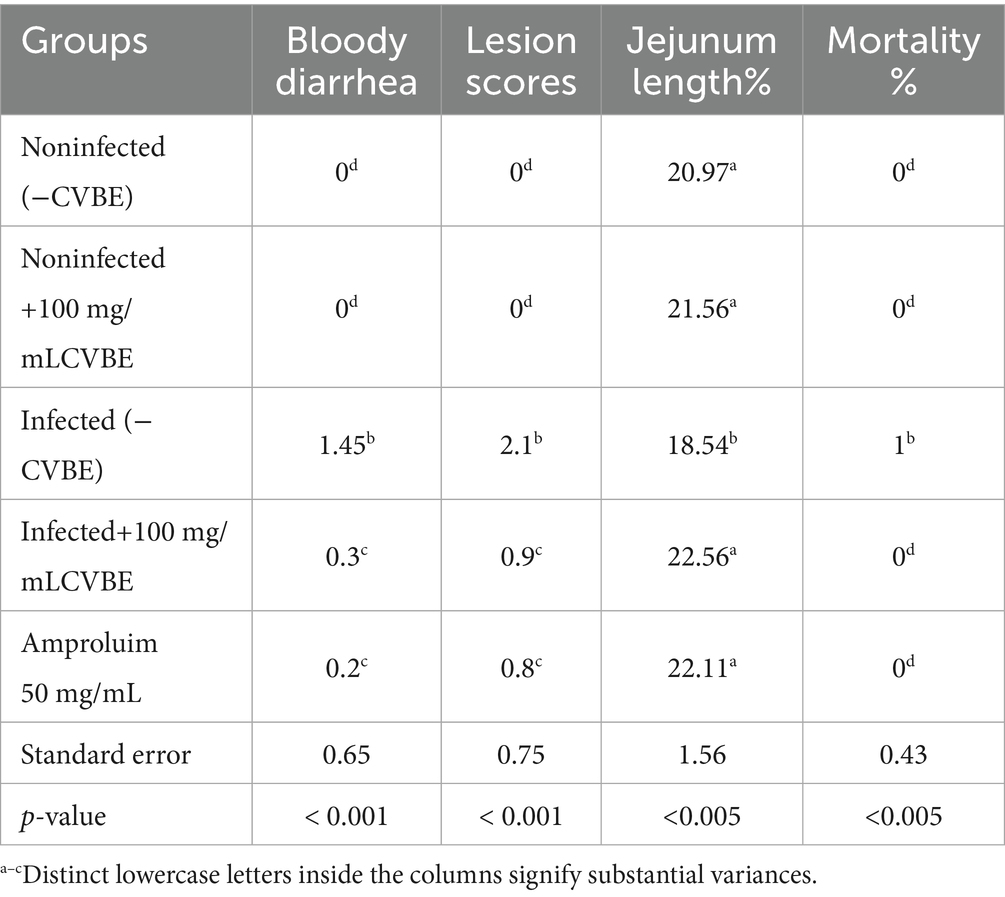
Table 2. Effects of the experimental groups on the incidence of diarrhea, lesion score, and jejunum length on the 5th day postinjury (5th DPI).
Herb exhibited the fewest pathological characteristics, including the lowest lesion scores, with increasing dosage. Compared with the infected group, all the groups that were administered CNB had significantly lower lesion ratings (p < 0.05). No mortality occurred in any of the experimental groups, except for one mouse in the infected group. Consequently, the death rate was 4% across all the experimental groups. Neither the amprolium-treated nor the uninfected groups experienced diarrhea throughout the study. No bloody diarrhea was present in the feces during the initial two days post infection. All infected groups, except the amprolium-treated group, experienced bloody diarrhea between days 3 and 5 following infection with E. papillate.
We have clarified that the lesion score in Table 2 represents the total lesion severity, which was calculated by summing the scores of specific jejunal lesions (atrophy, wall thickening, mucosal erosion, and hemorrhage) graded according to a standardized 0–4 scale. The total lesion score presented in Table 2 represents the sum of all individual lesion grades for each bird, with higher values indicating greater lesion severity.
Figure 3 shows the impact of the experimental groups on the anticoccidian index (ACI) of each group five days post dosage. We administered 100 mg/kg CNB and 50 mg/kg amprolium to both the infected groups. ACI scores ranging from 120–140, 140–160, 160–180, or over 180 were categorized as mild, moderate, marked, or outstanding, respectively.
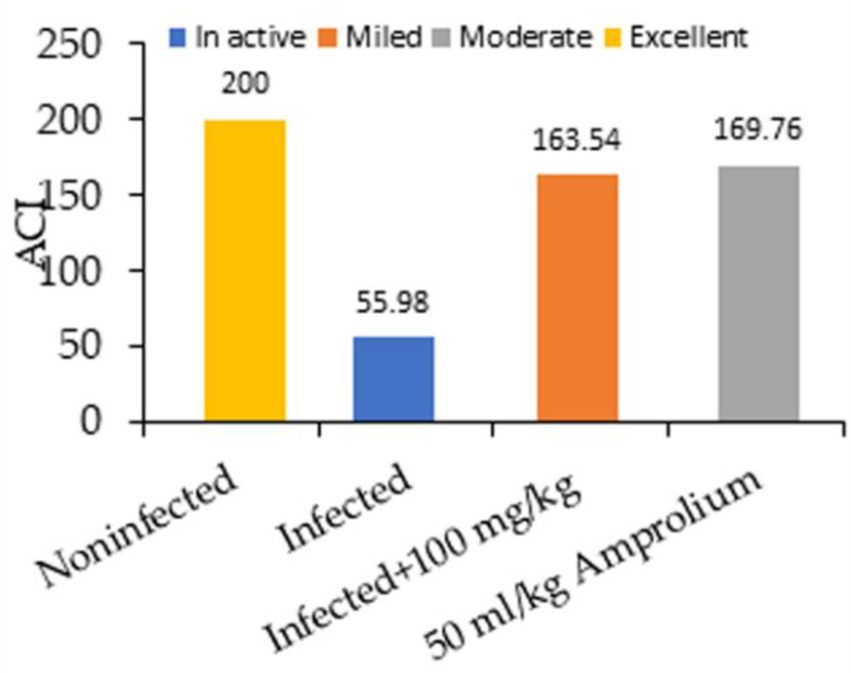
Figure 3. Impact of experimental groups and coccidiosis on the anticoccidial index (ACI) of each group five days post inoculation.
In general, the ratio of oocyst excretion was significantly (p ≤ 5) affected by the experimental groups, which decreased with concentrations of 100 mg/kg of extract and 50 mg/kg amprolium. High oocyst excretion ratios were detected at 12.5, 25, and 50 g/L (Figure 4). Infection of the mice with E. papillata resulted in oocyst production peaking at approximately 88% per gram of feces in the infected cohort. In the groups administered C. verum bark extracts, oocyst generation decreased by 34, 49, 64, and 66% at dosages of 12.5, 25, 50, and 100 mg/kg extract, respectively, alongside 50 mL/kg reference medication (Figure 5), indicating a considerable reduction. The 100 mg/kg dosage had the greatest capacity to suppress fecal oocyst excretion. Therefore, it was exclusively used in subsequent studies.
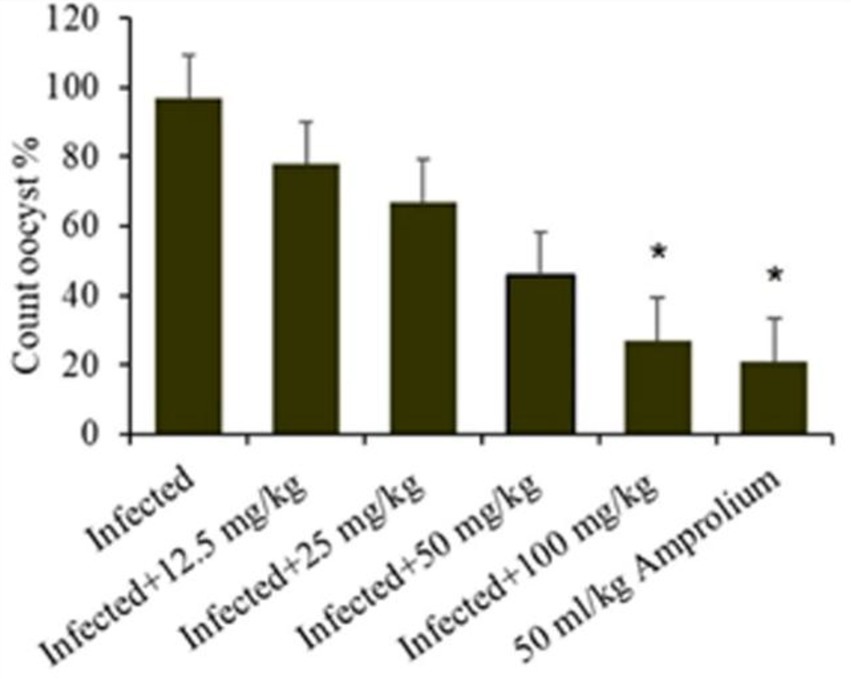
Figure 4. Shows the effect ratio of experimental Cinnamomum verum bark extracts on oocyst production on the fifth day following Eimeria papillata oocyst infection. Significance is relative to the infected cohort (p < 0.05).
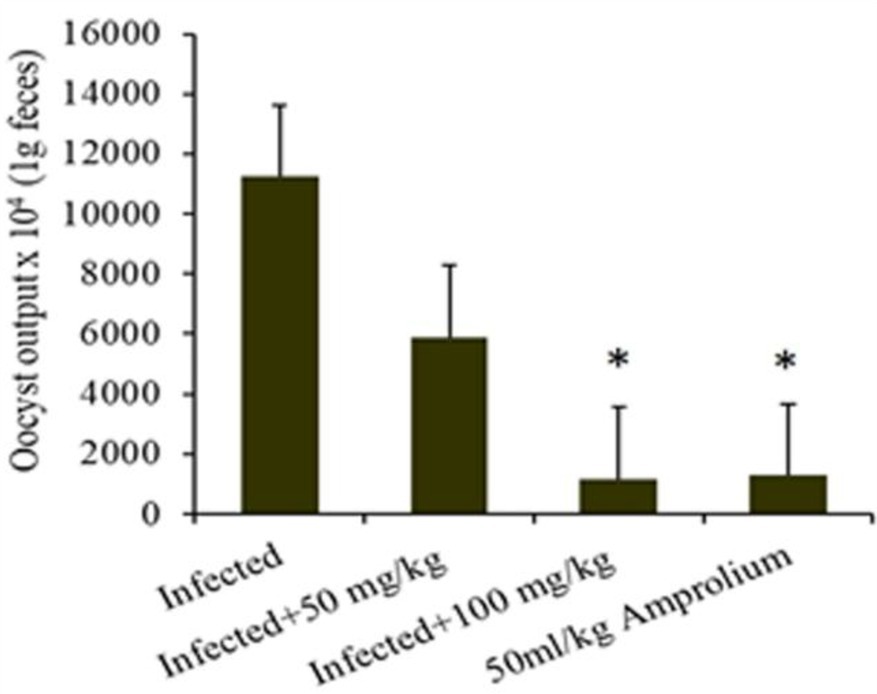
Figure 5. Illustrates the effect ratio of the experimental Cinnamomum verum bark extract sporulation oocyst count in 1 g of feces from infected, treatment (CVBE), and amprolium-treated mice. * The difference between the infected treated groups was significant at p ≤ 0.05.
E. papillate coccidian infection in mice led to oocyst excretion in fecal pellets, peaking at 228.75 × 103 ± 54.66 oocysts/g of feces on day 5 post infection in the infected cohort. The oocyst ejection rate following treatment with 100 mg/kg C. verum bark extract was 32.17 × 103 ± 5.25 oocysts/g feces, whereas it was 21.476 × 103 ± 5.42 oocysts/g feces in the control group.
Masson’s trichrome staining was performed on a segment of the murine jejunum to investigate the pathological alterations induced by infection of the jejunum. Histological examination via light microscopy revealed significant structural alterations in the gut wall. Prior to the administration of the extract, significant pathological abnormalities were observed in the jejunum of the infected group. The alterations included hemorrhaging in the intestinal villi, desquamation at the tips of the intestinal villi, and exfoliation of mucosal epithelial cells, alongside the emergence of parasite stages. Compared with the uninfected control mice, the infected group presented a marked reduction in villus height and crypt depth (Figure 6).
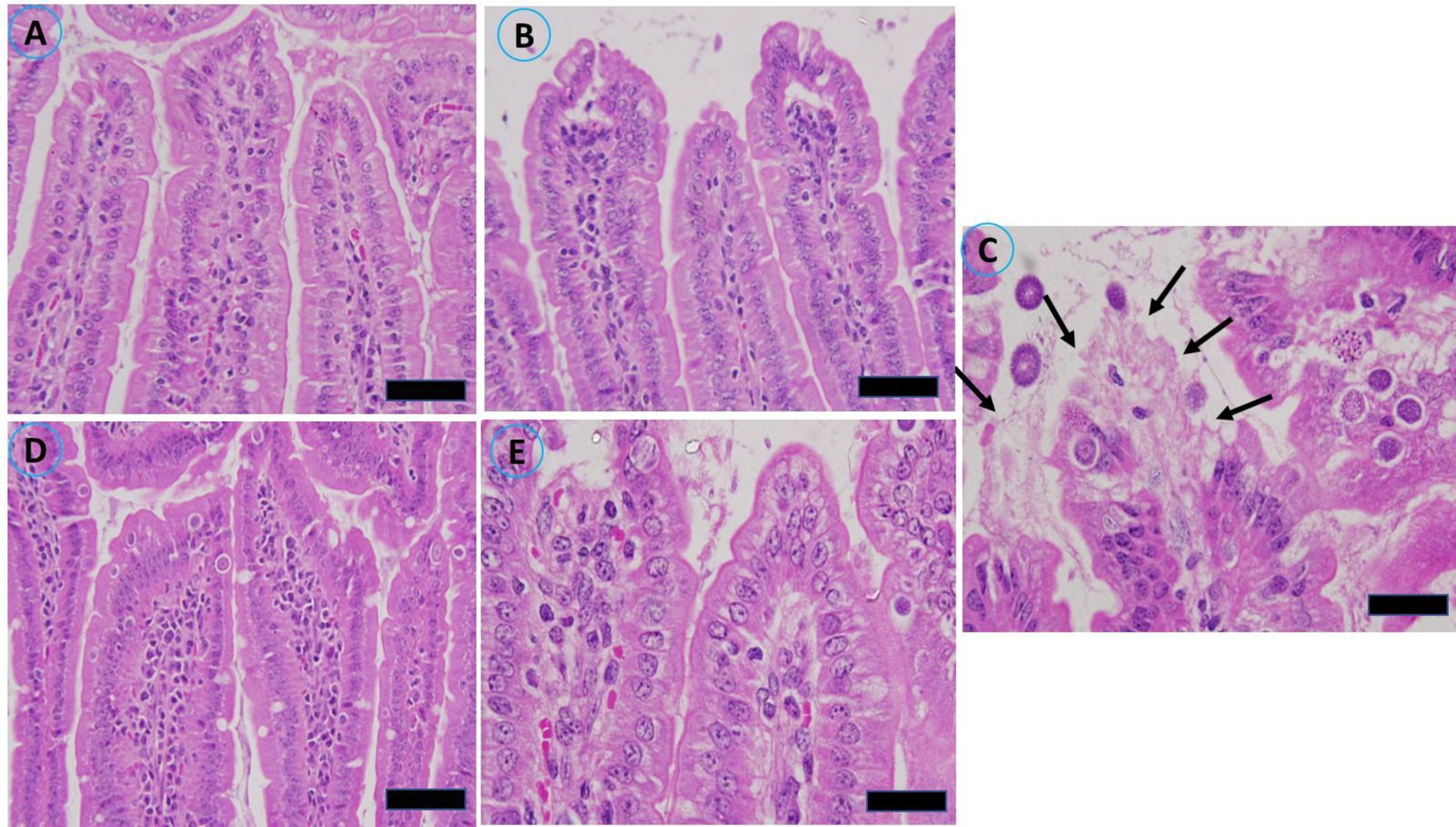
Figure 6. Histopathological alterations in the jejunal sections of mice resulting from infection with sporulated oocysts of Eimeria papillata, stained with Masson’s trichrome on day 5 post infection. (A) Noninfected (−CVBE); (B) Noninfected (+CVBE); (C) infected (−CVBE) denoted by arrows; (D) infected+100 mg/mL CVBE; (E) infected + amproluim 50 mg/mL. Scale Bar 20 μm.
Figure 7 shows that jejunal villous height (VH) was significantly lower in the infected group than in the noninfected group, crypt depth (CD) significantly affect. Anticoccidial treatments with 100 mg/mL of CVBE and 50 mg/mL Amprolium enhanced jejunal architecture in challenged mice; the villus height was greater in these groups than in the infected group, whereas the crypt depth was lower, and the VH-to-crypt depth ratio was greater in the CVBE and amprolium groups than in the other groups (Figure 7).
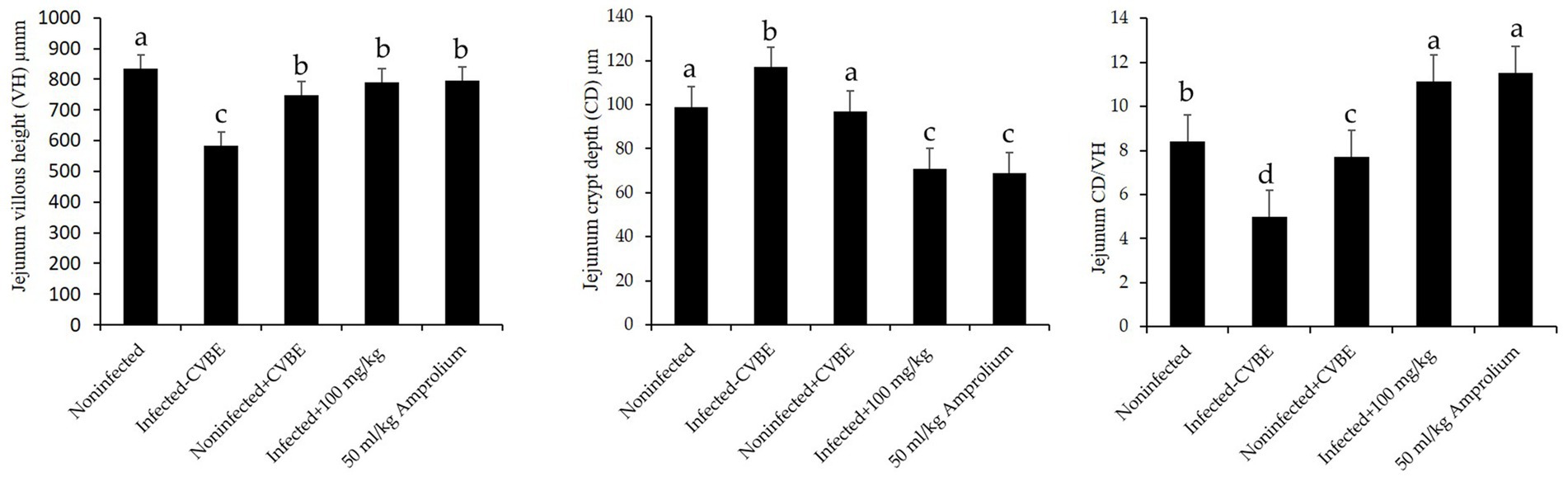
Figure 7. Impact of anticoccidial agents on jejunal histomorphometry in mice infected with Eimeria papillata. Noninfected group; infected group; noninfected group (+CVBE); infected with 100 mg/mL CVBE; infected with 50 mg/mL Amprolium. Data are presented as the mean values accompanied by their standard errors. Values denoted by distinct superscript letters are significantly different (p < 0.05).
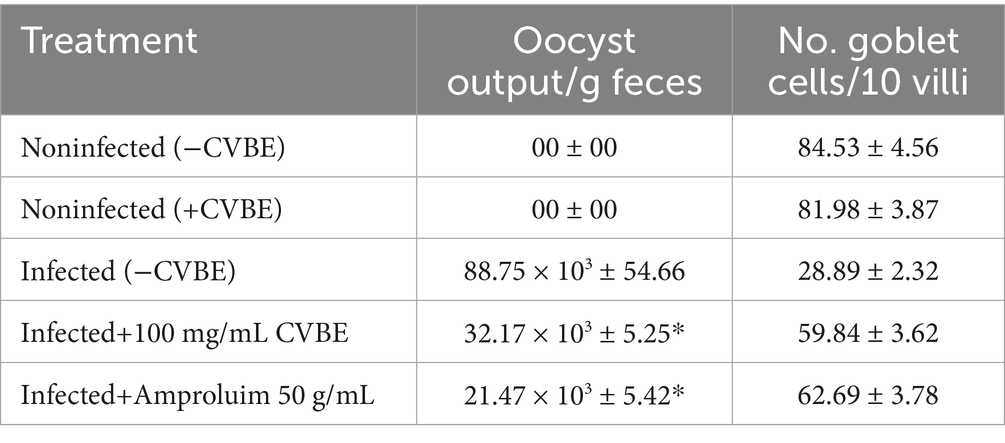
In Table 3, the results are presented as the average (SE), which represents the influence of C. verum bark extracts on the outcome of E. papillata infections. There was no fecal output of oocysts during the first-days post infection. On the fourth day the oocyst began to fall out, and in five-day post infection the highest oocyst output was 88.75 × 103 ± 54.66 oocysts/g of feces in infected mice. When the mice were infected with E. papillata, the number of goblet cells in their jejunum decreased from 84.53 ± 4.56 to 59.84 ± 3.62 after treatment with C. verum bark extract (Table 3).
Microscopic investigation of Alcian blue-stained jejunal sections revealed that E. papillata infection significantly reduced the number of goblet cells in the jejunum (p < 0.05) compared with that in the noninfected group. Conversely, compared with those in the infected group, the number of goblet cells in the jejunum of the mice that were administered CVBE significantly increased (Figures 8, 9).
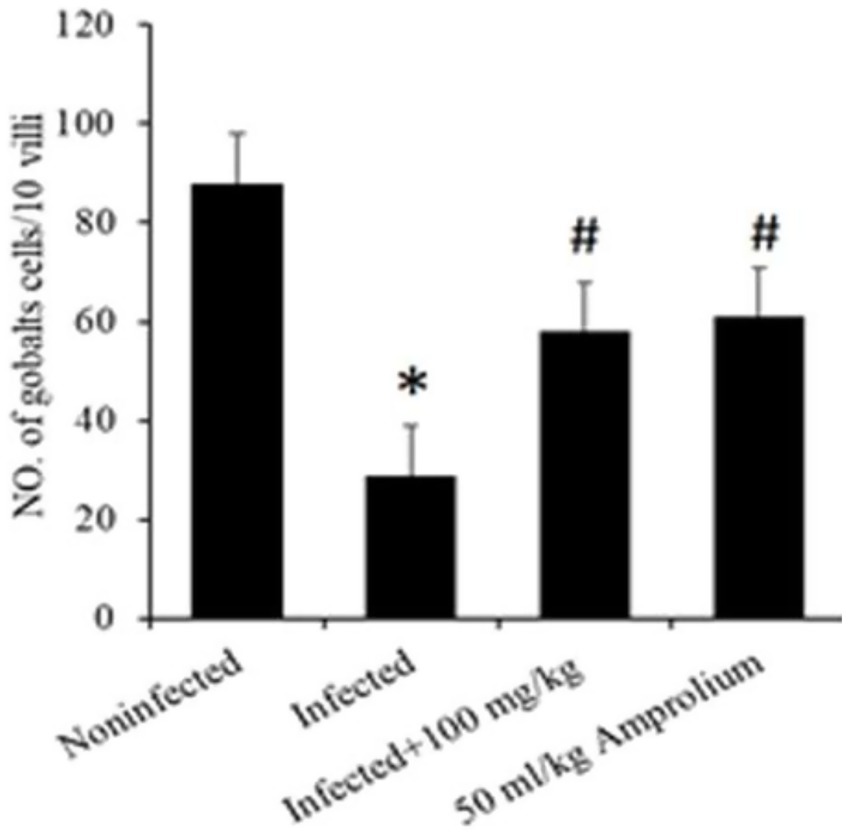
Figure 8. Impact of Cinnamomum verum bark extracts on the decrease in the goblet cell population in the jejunum of Eimeria papillata-infected mice. All values are shown as the means ± standard errors. Significant divergence from the control group (p ≤ 0.05) is indicated by *, #.
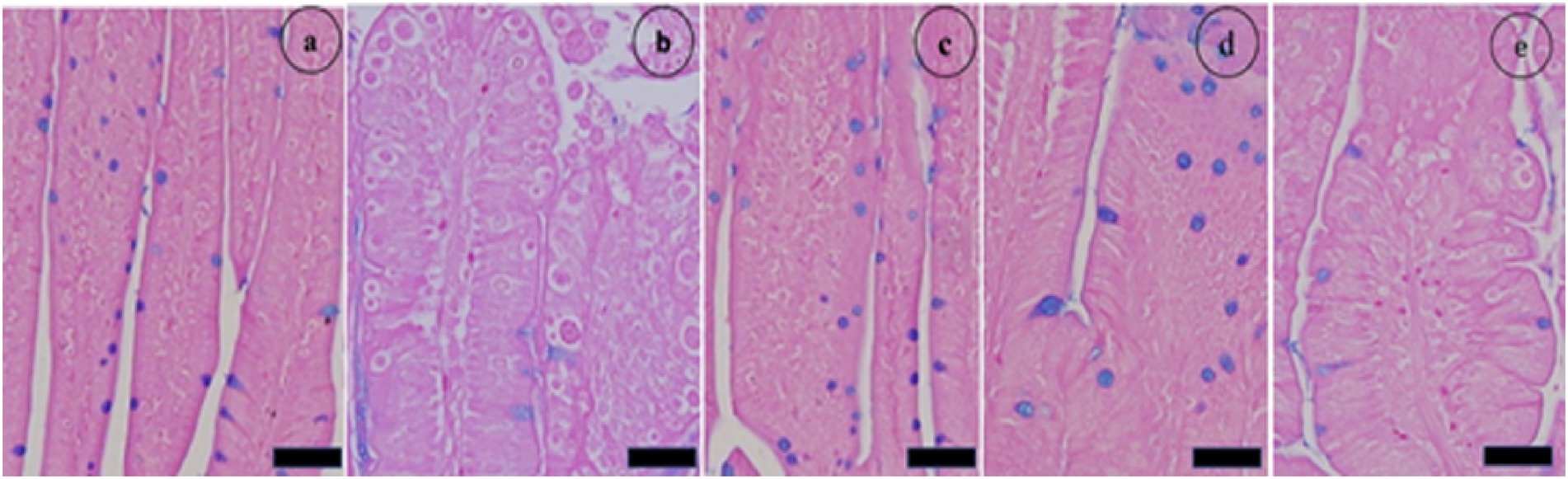
Figure 9. The impact of Cinnamomum verum bark extracts on the number of goblet cells in the jejunum of mice infected with Eimeria papillata. (a) denotes the noninfected group; (b) signifies the nontreated group exhibiting developmental stages; (c) indicates the noninfected group receiving Cinnamomum verum bark extracts only; (d) represents the treated group administered 100 mg/kg Cinnamomum verum bark extracts; and (e) refers to the infected group that received amprolium therapy. Alcian blue was used to stain the pieces. The scale bar represents 20 μm.
Figure 10 shows sections of jejunal villi stained with Masson’s trichrome. The administration of different dosages of C. verum bark extracts markedly reduced the total number of intracellular E. papillate stages, including the meronts, gamonts, and developing oocysts, especially at a dosage of 100 mg/kg C. verum bark extracts and reference drugs. This phenomenon was particularly evident in the mice that were administered extracts to address their disease. The epithelial cells in the jejunum of the mice infected with E. papillate presented different stages of parasite development. Compared with infection, treatment with C. verum bark extracts led to a substantial reduction of 69% in the number of parasite stages per ten villous–crypt units (p < 0.05).
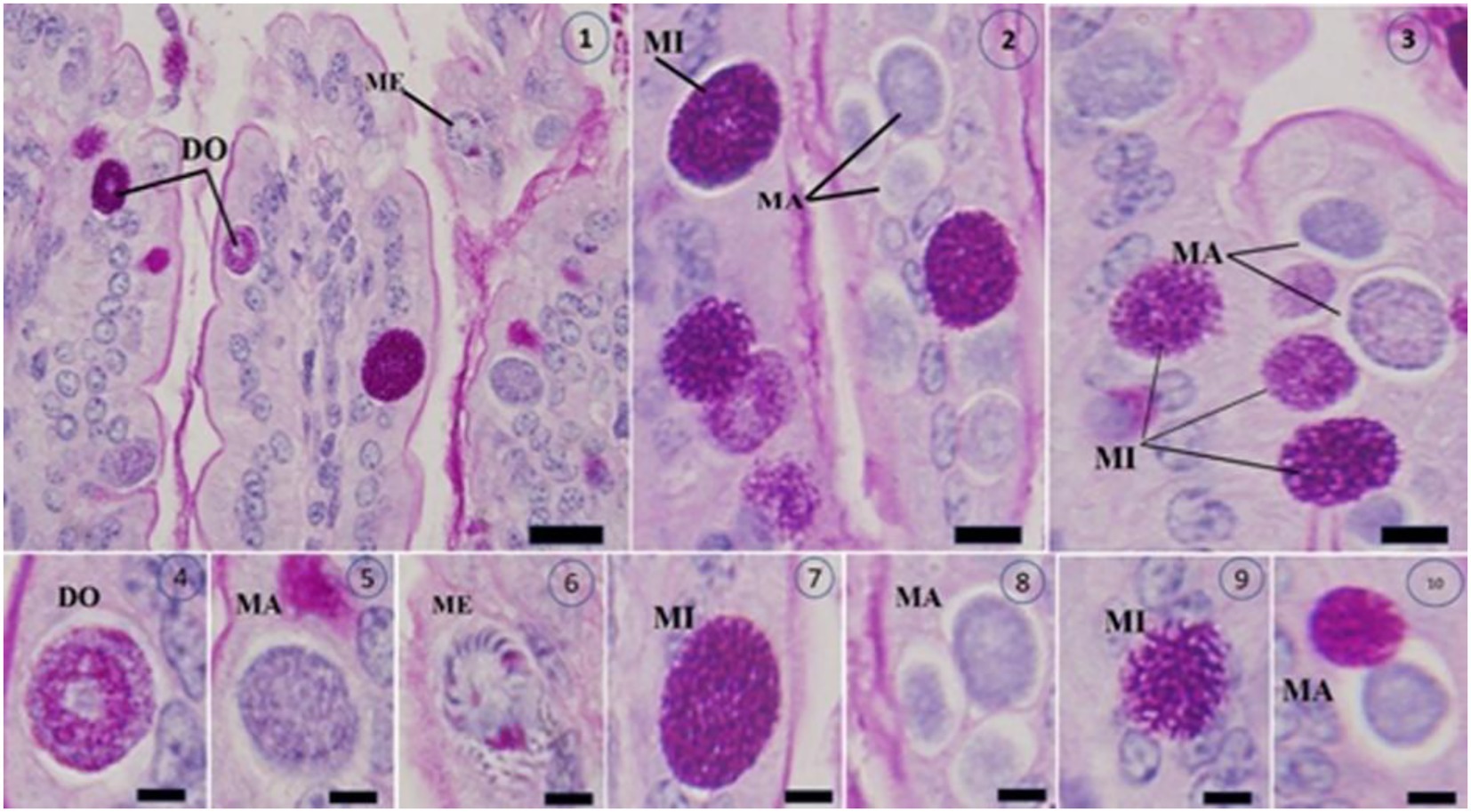
Figure 10. Shows Masson’s trichrome-stained slices of the jejunum infected with Eimeria papillata on day 5 after infection, revealing several developmental stages (1–10). The image displays a Meront (ME), macrogamont (MA), microgamont (MI), and developing oocyst (DO). Scale bar = 20 μm.
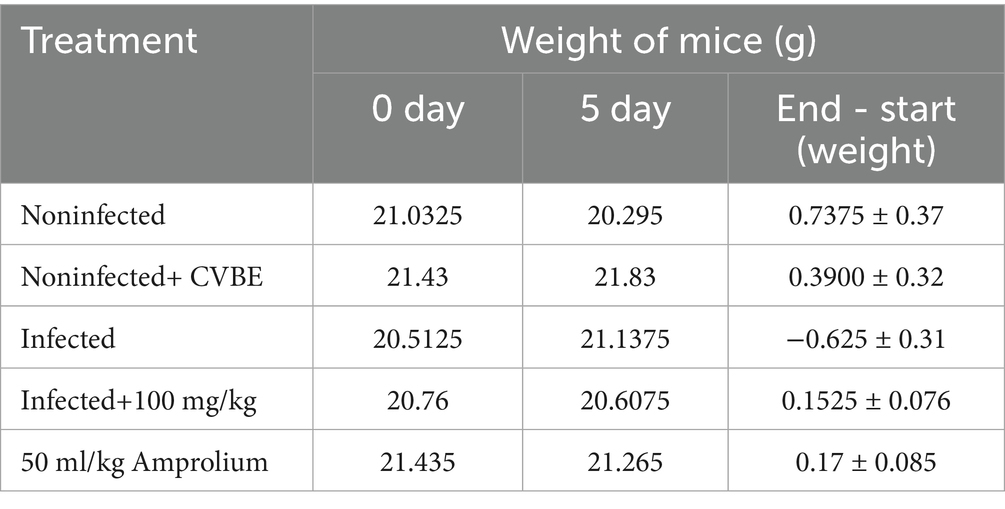
Compared with that in the control group, E. papilate parasite infection led to a decrease in weight in the infected group, whereas the weight in the treated groups showed minimal negative or positive effects (Table 4).
The infection disturbed the oxidative balance in the jejunum. A notable alteration occurred in the infected and treated mice. This was demonstrated by the evaluation of GSH levels, NO concentrations, and CAT activity.
E. papillata infection resulted in a substantial reduction (p < 0.01) in GSH levels in the jejunum of the mice. The reduction exceeded 40%; however, following the administration of C. verum bark extracts (100 mg/kg) to the sick mice, the GSH levels increased to nearly match those of the control groups. Compared with that of the reference medicine amprolium, the concentration of GSH was greater following treatment with the extracts (Figure 11).
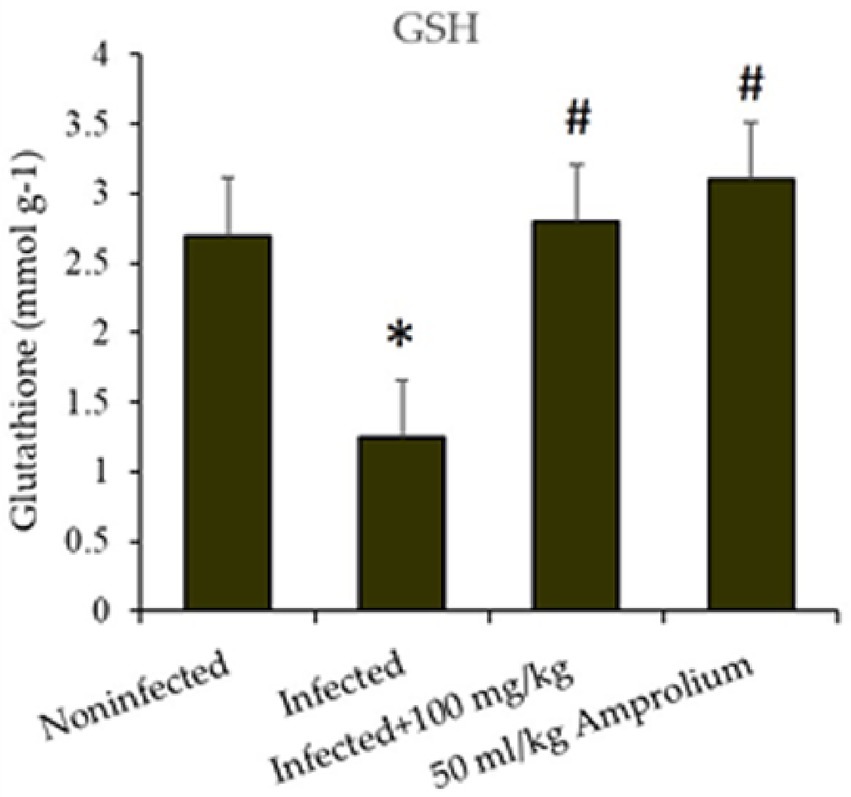
Figure 11. Impact of Cinnamomum verum bark extracts on jejunal glutathione levels in mice infected with Eimeria papillata. * and # indicate statistically significant differences between the control and infected groups, respectively, at p < 0.01.
As shown in Figure 12, E. papillata infection resulted in a substantial increase (p < 0.01) in nitric oxide levels in the jejunum. This increase was mitigated following the treatment of infected mice with extracts of C. verum bark, resulting in nitric oxide levels approaching those of the noninfected control groups. Compared with the reference medicine amprolium, the concentration of nitric oxide was greater following treatment with the C. verum bark extracts (Figure 12).
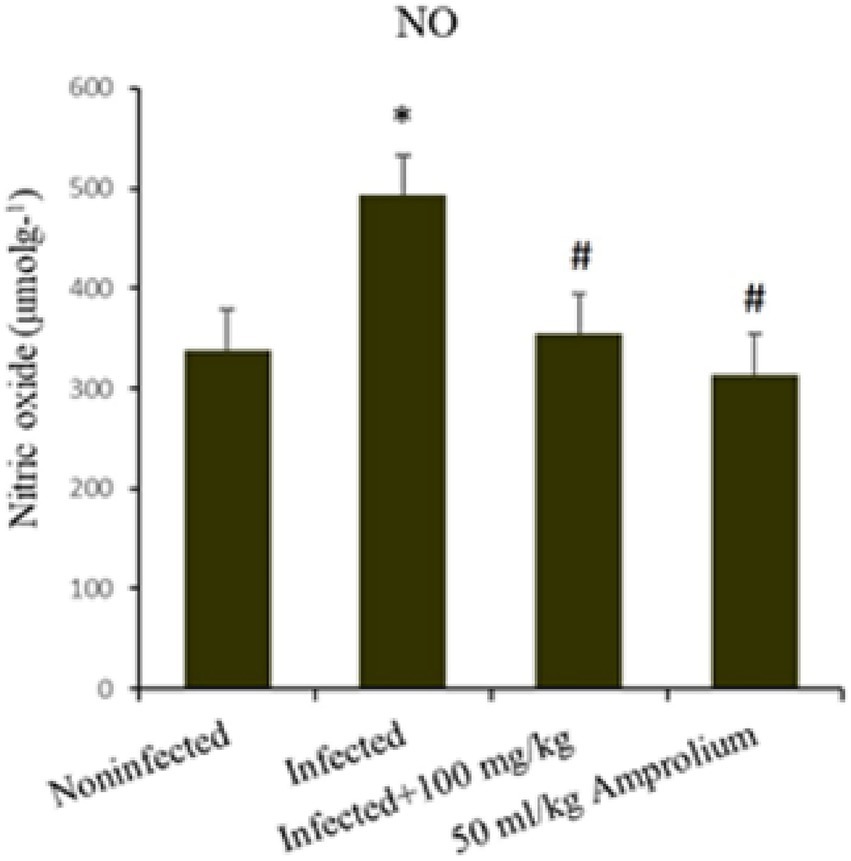
Figure 12. Impact of Cinnamomum verum bark extracts on jejunal nitric oxide levels in mice infected with Eimeria papillata. * and # indicate statistical significance in relation to the infected group at p < 0.01.
In the jejunum of infected mice, catalase enzyme activity was considerably diminished (p < 0.01) following infection with E. papillata. C. verum bark extracts increased catalase activity to match that of the control (Figure 13).
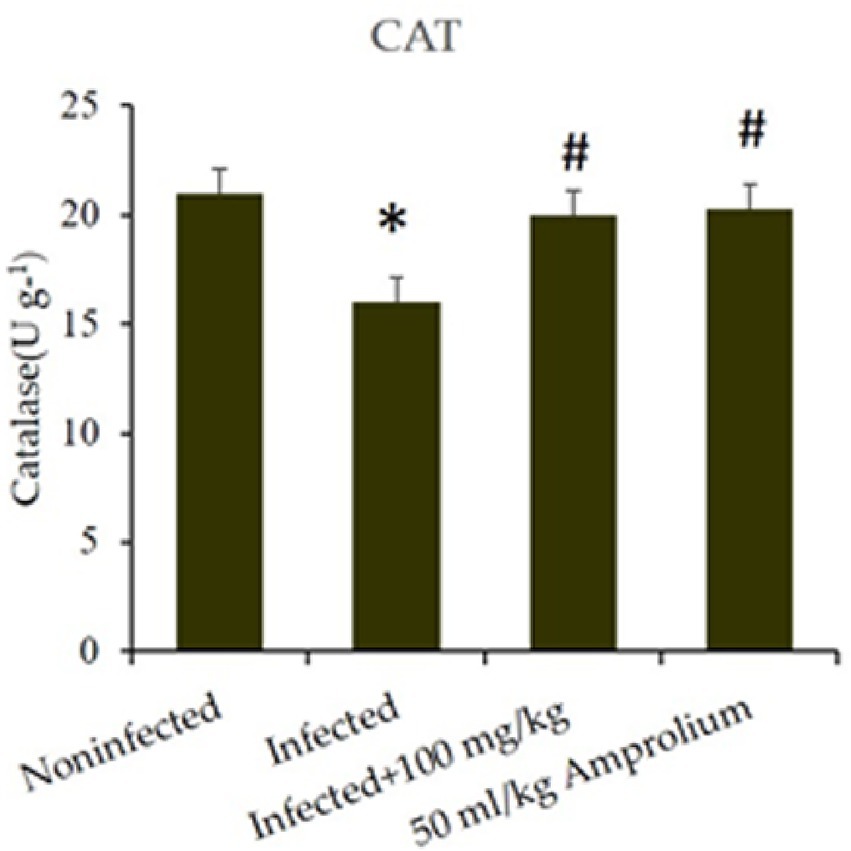
Figure 13. Impact of Cinnamomum verum bark extracts on jejunal catalase levels in mice infected with Eimeria papillata. * and # indicate statistical significance in relation to the infected group at p < 0.01.
4 Discussion
Amprolium functions as an anticoccidial ionophore that disrupts ion gradients across the membranes of Eimeria cells (27). The pursuit of synthetic anticoccidial alternatives is a vital research domain (22, 28, 29). We endeavored to replicate a synthetic anticoccidial by concentrating on identifying a conventional product exhibiting anticoccidial properties that are characterized by a distinctive, safe formulation devoid of tissue residues or drug resistance. Upon examining many plants, we concentrated on CNGE.
The many phytochemicals in specific herbs impart numerous bioactive characteristics to this shrub and spice (30). Initially, we concentrated on identifying a natural substance with anticoccidial qualities that may be further developed into a novel anticoccidial medication. Upon examining various herbs, we concentrated on the bark of C. verum. This spice contains a wealth of chemicals, such as cinnamaldehyde, cinnamate, cinnamic acid, alkaloids, flavonoids, various steroids, fatty acids, peptides, and several essential oils (31–33). It significantly lowers oocyst levels. Our data substantiate that the CNBE extract possesses considerable anticoccidial activity. The clinical manifestations of coccidiosis noted in the mice in this investigation included pallor, diarrhea, anorexia, depression, and ruffled fur, which aligns with other studies (34). In this trial, the incidence of bloody diarrhea in the infected treated groups was quantitatively lower than that in the infected groups. Consequently, diminished hemorrhagic diarrhea may safeguard diseased mice from secondary infections, inflammatory responses, and the consumption of hazardous agents (20). Cinnamon leaf extracts demonstrate a significant antidiarrheal effect in a castor oil-induced diarrhea model, confirming their efficacy in various diarrheal illnesses (29). Furthermore, cinnamon comprises active constituents, notably cinnamaldehyde, which enhances digestion and promotes the secretion of digestive enzymes (32, 35).
Consistent with other studies (21), the natural product significantly diminished the quantity of fecal oocysts in coccidia-infected mice. The herb-treated groups significantly diminished OPG production in the CNBE, which may be beneficial for managing extensive coccidiosis outbreaks on hen farms. The jejunum is one of the most essential digestive organs in mice. When Eimeria damages the epithelial cells of the jejunum, mice experience malabsorption and diarrhea, leading to reduced body weight gain (21). The extent of gross lesions and jejunal atrophy in the herb-treated groups varied from mild atrophy and dispersed petechiae to severe atrophy and extensive hemorrhaging in the infected group. Compared with those of the infected group, the data regarding jejunal lesions and oocyst reduction rates indicated that CNBE and amprolium effectively mitigated the problems associated with jejunal lesions (36).
These findings verify the presence of biologically active chemicals in CNGEs that impede or prolong the survival of E. papillate in vivo, leading to the suppression of lesions and oocyst excretion. Thus, cinnamon (37), which has direct antiparasitic effects on coccidian parasites, may significantly enhance the condition of afflicted mice due to its organ-protective attributes. Coccidiosis negatively impacted the affected group, which was mitigated by treatment with the natural product. The beneficial effects of cinnamon on anti-coccidiosis parameters may be attributed to the presence of substantial alkaloids, flavonoids, saponins, and tannins, which potentially exert synergistic effects on anti-parasitic, anti-inflammatory, antioxidant, and antidiarrheal activities in mice (30, 38). Eimeria infection in animals causes substantial weight loss (39), due to poor nutrition absorption and a reduced immunological response, resulting in intestinal damage (4). Our findings align with weight reduction in mice infected with E. papillate compared with their noninfected counterparts (6). This study corroborates the significant findings concerning the reduction in the number of oocysts and lesions in the jejunum; nonetheless, it has certain limitations. Although the 50 mg CNBE/kg dosage diminished the oocyst rate relative to that of the infected groups, it did not surpass that of the noninfected group. An overdose of coccidian oocysts or a subtherapeutic amount may be detrimental to the mice. The considerable potential effect of CNBE stems from the antioxidant and anti-inflammatory attributes of its constituent plant extract components (40, 41). Georgieva et al. (41), contend that coccidian infection disrupts the equilibrium between the body’s inherent antioxidant defenses and the generation of free radicals. These findings demonstrated that an E. papillata infection is associated with oxidative damage to the jejunum in mice, resulting in a decrease in antioxidant enzymes and GSH levels and CAT activity, while increasing NO concentrations. These oxidative parameters are crucial for safeguarding an animal’s body from free radical damage during Eimeria infection. Antioxidant enzymes employ the active sites of plant extracts to counteract the accumulation of reactive oxygen species that cause harmful changes in the jejunal epithelium (41). CNBE markedly inhibits the infection-induced depletion of these indicators and enhances their activity, which is generally diminished during oxidative damage caused by infection. This study utilized herbal extracts as anticoccidial agents to address E. papillate infection. This study revealed that CNBE therapy impedes the growth of this parasite, consequently resulting in an increase in the number of goblet cells due to damage. Murshed earlier, Vitis vinifera extract treatment exhibited significant efficacy and effectiveness against Eimeria (6). GSH functions as the initial line of defense against the harmful effects of reactive oxygen species (ROS), such as the toxicity associated with lipid peroxidation (42). This study indicates that the reduction in GSH observed in the jejunum of infected mice may signify increased consumption due to oxidative stress, suggesting that the intestinal lining is compromised by the Eimeria parasite. Nitric oxide synthases ultimately convert most of the nitric oxide produced in tissues into nitrate in bodily fluids (43). The increase in intestinal nitric oxide production is often linked to adverse consequences and is related to jejunal megaly (44). Catalase, an enzyme prevalent in living organisms, is essential for reducing oxidative stress by facilitating the breakdown of hydrogen peroxide (H2O2) into water and oxygen. This mechanism inhibits the buildup of reactive oxygen species (ROS) and subsequent oxidative damage to cells and tissues (45). This study evaluated catalase during E. papillate infection, as this enzyme directly controls certain free radicals, thereby diminishing them due to the reduced activity of the antioxidant defense system (46). CNBE demonstrates considerable anticoccidial efficiency. This is supported by studies demonstrating a reduction in oocyst excretion and sporulation, a decline in the stages of parasite proliferation in the jejuna, and a normalization of the goblet cell count.
5 Conclusion
Conclusions of in vivo studies have shown that these extracts markedly impede oocyst sporulation. Thorough pharmacological studies are essential for determining the new pharmacodynamic effects of bioactive components. Moreover, the essential mechanisms underlying these bioactivities require comprehensive examination. Clarifying the histological and molecular mechanisms behind sporulation suppression by CVBE and its protective effects against E. papillata-induced intestinal infection in vivo is crucial.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
Ethics approval and consent to participate: the research was conducted in accordance with the “Guide for the Care and Use of Laboratory Animals.” The study complied with the institutional restrictions on animal usage at King Saud University and met the standards set by the National Committee of Bio-Ethics (NCBE) in Saudi Arabia. The Royal Decree numbered M59 was issued on 14/9/1431H. The Research Ethics Committee of King Saud University (Approval No. KSU-Se-21-86) sanctioned all experimental techniques, guaranteeing animal rights, welfare, minimal stress, and the absence of injury or suffering. Regarding informed consent from all owners. Mice are bred in the department’s animal house in the university specifically for our experiments. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
MM: Conceptualization, Formal analysis, Methodology, Writing – original draft, Writing – review & editing, Project administration. HiA: Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. KI: Investigation, Resources, Software, Writing – original draft, Writing – review & editing. AA: Data curation, Investigation, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. HaA: Validation, Visualization, Writing – original draft, Writing – review & editing. SA-Q: Funding acquisition, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article.
Acknowledgments
Thanks for the Ongoing Research Funding Program No. ORF-2025-1081, at King Saud University. Also, Researchers Supporting Project No. (PNURSP2025R401), PrincessNourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Jones, K, and Garcia, G. Teaching of veterinary parasitology to university students in Trinidad, West Indies; are our local wildlife species neglected? Braz J Biol. (2021) 83:e248493. doi: 10.1590/1519-6984.248493
2. Al-Quraishy, S, Abdel-Baki, A, and Dkhil, ME. Eimeria tenella infection among broiler chicks Gallus domesticus in Riyadh city. J King Saud Univ Sci. (2009) 21:191–3. doi: 10.1016/j.jksus.2009.10.006
3. López-Osorio, S, Chaparro-Gutiérrez, JJ, and Gómez-Osorio, LM. Overview of poultry Eimeria life cycle and host-parasite interactions. Front Vet Sci. (2020) 7:384. doi: 10.3389/fvets.2020.00384
4. Adhikari, P, Kiess, A, Adhikari, R, and Jha, R. An approach to alternative strategies to control avian coccidiosis and necrotic enteritis. J Appl Poult Res. (2020) 29:515–34. doi: 10.1016/j.japr.2019.11.005
5. Alnassan, A, Kotsch, M, Shehata, A, Krüger, M, Daugschies, A, and Bangoura, B. Necrotic enteritis in chickens: development of a straightforward disease model system. Vet Rec. (2014) 174:555–5. doi: 10.1136/vr.102066
6. Murshed, M, Al-Tamimi, J, Aljawdah, HM, and Al-Quraishy, S. Pharmacological effects of grape leaf extract reduce eimeriosis-induced inflammation, oxidative status change, and goblet cell response in the jejunum of mice. Pharmaceuticals. (2023) 16:928. doi: 10.3390/ph16070928
7. Abdel-Latif, M, Abdel-Haleem, HM, and Abdel-Baki, A-AS. Anticoccidial activities of chitosan on Eimeria papillata-infected mice. Parasitol Res. (2016) 115:2845–52. doi: 10.1007/s00436-016-5035-0
8. Upadhaya, SD, Cho, SH, Chung, TK, and Kim, IH. Anti-coccidial effect of essential oil blends and vitamin D on broiler chickens vaccinated with purified mixture of coccidian oocyst from Eimeria tenella and Eimeria maxima. Poult Sci. (2019) 98:2919–26. doi: 10.3382/ps/pez040
9. Sidiropoulou, E, Skoufos, I, Marugan-Hernandez, V, Giannenas, I, Bonos, E, Aguiar-Martins, K, et al. In vitro anticoccidial study of oregano and garlic essential oils and effects on growth performance, fecal oocyst output, and intestinal microbiota in vivo. Front Vet Sci. (2020) 7:420. doi: 10.3389/fvets.2020.00420
10. Mosaddeghi, P, Eslami, M, Farahmandnejad, M, Akhavein, M, Ranjbarfarrokhi, R, Khorraminejad-Shirazi, M, et al. A systems pharmacology approach to identify the autophagy-inducing effects of traditional Persian medicinal plants. Sci Rep. (2021) 11:336. doi: 10.1038/s41598-020-79472-y
11. Wunderlich, F, Al-Quraishy, S, Steinbrenner, H, Sies, H, and Dkhil, MA. Toward identifying novel anti-Eimeria agents: trace elements, vitamins, and plant-based natural products. Parasitol Res. (2014) 113:3547–56. doi: 10.1007/s00436-014-4101-8
12. Ahmad, R, Yu-Hsiang, Y, Hua, K-F, Chen, W-J, Zaborski, D, Dybus, A, et al. Management and control of coccidiosis in poultry—a review. Animal bioscience. (2023) 37:1–15. doi: 10.5713/ab.23.0189
13. Balta, I, Marcu, A, Linton, M, Kelly, C, Stef, L, Pet, I, et al. The in vitro and in vivo anti-virulent effect of organic acid mixtures against Eimeria tenella and Eimeria bovis. Sci Rep. (2021) 11:16202. doi: 10.1038/s41598-021-95459-9
14. Ju, J, de Oliveira, MS, and Qiao, Y. Cinnamon: A medicinal plant and a functional food systems, vol. 31. Cham: Springer (2023).
15. Bozkurt, M, Giannenas, I, Küçükyilmaz, K, Christaki, E, and Florou-Paneri, P. An update on approaches to controlling coccidia in poultry using botanical extracts. Br Poult Sci. (2013) 54:713–27. doi: 10.1080/00071668.2013.849795
16. El-Ghareeb, WR, Kishawy, AT, Anter, RG, Aboelabbas Gouda, A, Abdelaziz, WS, Alhawas, B, et al. Novel antioxidant insights of myricetin on the performance of broiler chickens and alleviating experimental infection with Eimeria spp.: crosstalk between oxidative stress and inflammation. Antioxidants. (2023) 12:1026. doi: 10.3390/antiox12051026
17. Maithani, M, Bansal, P, Gupta, V, Singh, R, Yadav, M, Joshi, G, et al. An updated literature-based mechanistic review: Phytochemistry, pharmacology and therapeutic promises of cinnamon (Cinnamomum verum) In: Science of spices and culinary herbs-latest laboratory, preclinical, and clinical studies, vol. 6 (Sharjah: Bentham Science) (2024). 76–92.
18. Dakrory, AI, Fahmy, SR, Soliman, AM, Mohamed, AS, and Amer, SA. Protective and curative effects of the sea cucumber Holothuria atra extract against DMBA-induced hepatorenal diseases in rats. Biomed Res Int. (2015):563652. doi: 10.1155/2015/563652
19. Habibi, H, Ghahtan, N, Tohidi, S, and Zarrinfar, A. Effect of composition of medicinal plants on growth performance, gut bacteria, hematological parameters, anticoccidial index, and optimum anticoccidial activity in domestic chicken. Comp Clin Path. (2022) 31:737–45. doi: 10.1007/s00580-022-03352-2
20. Shetshak, M, Suleiman, M, Jatau, I, Ameh, M, and Akefe, I. Anticoccidial efficacy of Garcinia kola (Heckel H.) against experimental Eimeria tenella infection in chicks. J Parasit Dis. (2021) 45:1034–48. doi: 10.1007/s12639-021-01389-8
21. Lan, L, Zuo, B, Ding, H, Huang, Y, Chen, X, and Du, A. Anticoccidial evaluation of a traditional Chinese medicine—Brucea javanica—in broilers. Poult Sci. (2016) 95:811–8. doi: 10.3382/ps/pev441
22. Al-Quraishy, S, Abdel-Maksoud, MA, Al-Shaebi, EM, and Dkhil, MAJSJBS. Botanical candidates from Saudi Arabian flora as potential therapeutics for plasmodium infection. Saudi J Biol Sci. (2021) 28:1374–9. doi: 10.1016/j.sjbs.2020.11.069
23. Smith, MK, Buhr, DL, Dhlakama, TA, Dupraw, D, Fitz-Coy, S, Francisco, A, et al. Automated enumeration of Eimeria oocysts in feces for rapid coccidiosis monitoring. Poult Sci. (2023) 102:102252. doi: 10.1016/j.psj.2022.102252
24. Abdel-Gaber, R, Hawsah, MA, Al-Otaibi, T, Alojayri, G, Al-Shaebi, EM, Mohammed, OB, et al. Biosynthesized selenium nanoparticles to rescue coccidiosis-mediated oxidative stress, apoptosis and inflammation in the jejunum of mice. Front Immunol. (2023) 14:1139899. doi: 10.3389/fimmu.2023.1139899
25. Jollow, D, Mitchell, J, Zampaglione, N, and Gillette, J. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. (1974) 11:151–69. doi: 10.1159/000136485
26. Buege, JA, and Aust, SD. Microsomal lipid peroxidation In: S Fleischer, L Packer, editors. Methods in enzymology : Elsevier (1978). 302–10.
27. Noack, S, Chapman, HD, and Selzer, PM. Anticoccidial drugs of the livestock industry. Parasitol Res. (2019) 118:2009–26. doi: 10.1007/s00436-019-06343-5
28. Al-Shaibani, I, Al-Khadher, A, and AlHibah, A. Anticoccidial activity of Allium sativum and Punica granatum against experimentally induced Eimeria tenella infection in broiler chickens. Asian J Res Anim Vet Sci. (2020) 5:20–9. doi: 10.9734/ajravs/2020/v3i2109
29. Qaid, MM, Mansour, L, Al-Garadi, MA, Alqhtani, AH, Al-Abdullatif, AA, Qasem, MA, et al. Evaluation of the anticoccidial effect of traditional medicinal plants, Cinnamomum verum bark and Rumex nervosus leaves in experimentally infected broiler chickens with Eimeria tenella. Ital J Anim Sci. (2022, 2022) 21:408–21. doi: 10.1080/1828051X.2022.2033139
30. Rao, PV, and Gan, SH. Cinnamon: a multifaceted medicinal plant. Evid Based Complement Alternat Med. (2014) 2014:642942. doi: 10.1155/2014/642942
31. Qaid, MM, Al-Mufarrej, SI, Azzam, MM, and Al-Garadi, MA. Anticoccidial effectivity of a traditional medicinal plant, Cinnamomum verum, in broiler chickens infected with Eimeria tenella. Poult Sci. (2021) 100:100902. doi: 10.1016/j.psj.2020.11.071
32. Murshed, M, Al-Tamimi, J, and Al Quraishy, S. In vitro comparative study of enzyme inhibitory properties of herbal plants and their phenolic, tannin, and flavonoid content as natural inhibitors in public health. Qual Assur Saf Crops Foods. (2025) 17:77–87. doi: 10.15586/qas.v17i4.1576
33. Förstermann, U, and Sessa, WC. Nitric oxide synthases: regulation and function. Eur Heart J. (2012) 33:829–37. doi: 10.1093/eurheartj/ehr304
34. Ali, A, Ponnampalam, EN, Pushpakumara, G, Cottrell, JJ, Suleria, HA, and Dunshea, FR. Cinnamon: a natural feed additive for poultry health and production- a review. Animals. (2021) 11:2026. doi: 10.3390/ani11072026
35. Yang, C, Kennes, YM, Lepp, D, Yin, X, Wang, Q, Yu, H, et al. Effects of encapsulated cinnamaldehyde and citral on the performance and cecal microbiota of broilers vaccinated or not vaccinated against coccidiosis. Poult Sci. (2020) 99:936–48. doi: 10.1016/j.psj.2019.10.036
36. Khater, HF, Ziam, H, Abbas, A, Abbas, RZ, Raza, MA, Hussain, K, et al. Avian coccidiosis: recent advances in alternative control strategies and vaccine development. Agrobiol Rec. (2020) 1:11–25. doi: 10.47278/journal.abr/2020.003
37. Bangoura, B, and Daugschies, A. Parasitological and clinical parameters of experimental Eimeria zuernii infection in calves and influence on weight gain and hemogram. Parasitol Res. (2007) 100:1331–40. doi: 10.1007/s00436-006-0415-5
38. Abdel-Moneim, A-ME, El-Saadony, MT, Shehata, AM, Saad, AM, Aldhumri, SA, Ouda, SM, et al. Antioxidant and antimicrobial activities of Spirulina platensis extracts and biogenic selenium nanoparticles against selected pathogenic bacteria and fungi. Saudi. J Biol Sci. (2022) 29:1197–209. doi: 10.1016/j.sjbs.2021.09.046
39. Georgieva, N, Koinarski, V, and Gadjeva, V. Antioxidant status during the course of Eimeria tenella infection in broiler chickens. Vet J. (2006) 172:488–92. doi: 10.1016/j.tvjl.2005.07.016
40. Surai, PF, Kochish, II, Fisinin, VI, and Kidd, MT. Antioxidant defense systems and oxidative stress in poultry biology: an update. Antioxidants. (2019) 8:235. doi: 10.3390/antiox8070235
41. Niu, B, Liao, K, Zhou, Y, Wen, T, Quan, G, Pan, X, et al. Application of glutathione depletion in cancer therapy: enhanced ROS-based therapy, ferroptosis, and chemotherapy. Biomaterials. (2021) 277:121110. doi: 10.1016/j.biomaterials.2021.121110
42. Mu, K, Yu, S, and Kitts, DD. The role of nitric oxide in regulating intestinal redox status and intestinal epithelial cell functionality. Int J Mol Sci. (2019) 20:1755. doi: 10.3390/ijms20071755
43. Rasheed, Z. Therapeutic potentials of catalase: mechanisms, applications, and future perspectives. Int J of Health Sci. (2024) 18:1.
44. Ighodaro, O, and Akinloye, O. First line defense antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defense grid. Alex J Med. (2018) 54:287–93. doi: 10.1016/j.ajme.2017.09.001
45. Nandi, A, Yan, LJ, Jana, CK, and Das, N. Role of catalase in oxidative stress‐and age‐associated degenerative diseases. Oxidative medicine and cellular longevity. (2019) 2019:9613090. doi: 10.1155/2019/9613090
Keywords: extraction, pharmaceutical industry, antioxidant, biological activity, plants, Eimeriosis, Cinnamomum verum bark, FTIR
Citation: Murshed M, Alzaylaee H, Ibrahim KE, Ammari A, Ahmed H and Al-Quraishy S (2025) Behavioral and pharmacological effects of phytochemicals in Cinnamomum verum bark extract for prevention of experimental murine coccidiosis. Front. Vet. Sci. 12:1715869. doi: 10.3389/fvets.2025.1715869
Edited by:
Vikrant Sudan, Guru Angad Dev Veterinary and Animal Sciences University, IndiaReviewed by:
Fatemeh Namazi, Shiraz University, IranBogning Zangueu Calvin, University of Douala, Cameroon
Copyright © 2025 Murshed, Alzaylaee, Ibrahim, Ammari, Ahmed and Al-Quraishy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mutee Murshed, bW11cnNoZWRAa3N1LmVkdS5zYQ==
 Mutee Murshed
Mutee Murshed Hind Alzaylaee2
Hind Alzaylaee2 Khalid Elfaki Ibrahim
Khalid Elfaki Ibrahim Aiman Ammari
Aiman Ammari