- 1Department of Animal Medicine, Production and Health, University of Padova, Padova, Italy
- 2Department of Agricultural, Food, Environmental and Animal Sciences, University of Udine, Udine, Italy
- 3Independent Practitioner, Milan, Italy
- 4Department of Ecology and Emergence of Zoonotic Diseases, Helmholtz Institute for One Health, Greifswald, Germany
- 5Independent Practitioner, Turin, Italy
- 6Parco Faunistico Cappeller, Cartigliano, Italy
The fallow deer (Dama dama) is a widely distributed cervid in Europe, often managed in enclosed settings for conservation, education, or hunting purposes. Chemical immobilization is frequently required during routine handling; however, fallow deer remain understudied in the scientific literature with regard to standardized anesthetic protocols. This study aimed to evaluate the efficacy and safety of an anesthetic protocol combining detomidine (0.2 mg/kg), ketamine (2 mg/kg), and azaperone (0.3 mg/kg) in 22 captive fallow deer. Animals were immobilized for clinical procedures, including health checks, blood sampling, individual identification, and translocation. The quality of anesthesia and physiological parameters (heart rate, respiratory rate, oxygen saturation, end-tidal CO2, arterial blood pressure, temperature) were monitored throughout immobilization. Anesthetic depth was evaluated through the assessment of reflexes, muscle tone, and eye position. The quality of induction, immobilization at approach, and recovery were systematically evaluated using dedicated scoring systems. Induction was smooth in all animals, with lateral recumbency time, and time to first safe approach achieved in 10.7 ± 6.4 min, and 22.0 ± 5.1 min, respectively. Mean handling time was 49.8 ± 9.6 min. Physiological parameters remained within acceptable limits, with only transient hypoxemia. Recovery was uneventful in most individuals, with the majority exhibiting good to excellent recovery quality and standing within 20 min of atipamezole administration. Only one individual experienced a poor-quality and prolonged recovery. No complications were observed post-procedure. The detomidine-ketamine-azaperone combination proved to be an effective and safe protocol for immobilizing fallow deer, providing stable anesthesia and good recovery quality. Dedicated scoring systems enabled a standardized and repeatable evaluation of the quality of the used protocol and its suitability. This study is the first to describe the use of azaperone in combination with detomidine and ketamine in fallow deer, contributing new insights into non-opioid immobilization strategies for cervids.
1 Introduction
The fallow deer (Dama dama) is a widely distributed, naturalized species in Europe, commonly managed in fenced environments such as parks, zoos, and game reserves for conservation, educational, and hunting purposes (1, 2). Population management interventions, including translocations, clinical assessments or microchipping for individual identification, often require chemical immobilization to ensure the safety of both animals and personnel involved (3–5). However, fallow deer are highly sensitive to stress, making them particularly prone to capture-related complications such as respiratory depression, bloat, regurgitation, and potentially fatal capture myopathy (6–8).
Various drug protocols have been tested in cervids, including ultra-potent opioids like etorphine or thiafentanil, often combined with sedatives, to improve immobilization quality. Nonetheless, these combinations are associated with severe adverse effects, including dysrhythmias, hypoxemia, apnea, muscle rigidity, and prolonged recoveries (6, 9). Safer alternatives, such as α2-agonists (e.g., xylazine, detomidine, medetomidine) combined with dissociative agents like ketamine or tiletamine/zolazepam, have been explored (7, 10), but reports often lack standardized physiological monitoring or consistent dosing strategies.
Importantly, α2-agonists exert dose-dependent depressant effects on respiration (11), suggesting that dose optimization may mitigate risks while maintaining effective sedation. Although fallow deer are increasingly targeted for chemical immobilization, they remain underrepresented in the scientific literature. This highlights the need for further evaluation of non-opioid anesthesia protocols to establish safe and reliable immobilization strategies for this species.
The aim of this study was to evaluate the efficacy and safety of a non-opioid-based anesthetic protocol combining detomidine, ketamine, and azaperone in captive fallow deer. Specifically, we sought to:
• evaluate the clinical parameters during immobilization, including heart rate, respiratory rate, hemoglobin oxygen saturation, end-tidal CO2, rectal temperature, and arterial blood pressure;
• assess the quality of induction, immobilization, and recovery to determine the adequacy of sedation for conducting non-invasive procedures without supplemental dosing;
• identify any adverse effects or complications arising during immobilization, such as respiratory depression, hypoxemia, muscle rigidity, or prolonged recovery, and evaluate their clinical relevance.
It was hypothesized that the combination of detomidine, ketamine and azaperone would be an effective and safe protocol for the pharmacological immobilization of fallow deer.
2 Materials and methods
2.1 Animals
This study was approved by the Animal Welfare Body (OPBA) of the University of Padova (Protocol no. 80/2023), and all procedures complied with national ethical and professional standards. Chemical immobilizations of fallow deer were performed as part of routine management procedures, including health evaluations, blood sampling, individual identification via microchip and ear tag application, and translocation to new facilities. The study included captive fallow deer (Dama dama) of both sexes and various age groups, immobilized at three locations in Northern Italy. Immobilization sessions were conducted between February and April 2024 and in February 2025. The first location was the Antonio Servadei Farm of the University of Udine (46.12571° N, 13.17753° E, 169.00 m a.s.l.), where deer roamed within a ~20-hectare naturalistic enclosure. The second location was the Agricultural Institute Pastori, Brescia (45.52694° N, 10.261464° E, 132.00 m a.s.l.), which housed deer in a ~400 m2 outdoor enclosure with access to a covered shelter, trees, and a central pond. The third location was La Fodoma Farm, Chions (45.88861° N, 12.76589° E, 16,00 m a.s.l.), where a group of fallow deer lived on approximately 500 m2 of land. During the sessions, ambient temperatures ranged between 12 °C and 20 °C.
All deer lived in social groups and had ad libitum access to pasture grass, hay and water. Animals were selected for immobilization based on visible indicators of good physical condition, including normal posture, coat quality, and body condition. Individuals showing signs of poor health, abnormal behavior, or very young age were excluded from the study.
Animals were classified into age groups based on estimated age, body size, secondary sexual characteristics, dental wear, presence and size of antlers as follows: yearlings (approximately 1 year old), young adults (2–3 years), and adults (≥3 years).
2.2 Anesthesia protocol
Chemical immobilization was achieved using an intramuscular combination of detomidine (Sedaquick, 10 mg/mL, Fatro, Italy) 0.2 mg/kg, ketamine (Ketavet 100, 100 mg/mL, Intervet Productions Srl, Italy) 2 mg/kg, and azaperone (Suiwell, 40 mg/mL, ATI Azienda Terapeutica Veterinaria, Italy) 0.3 mg/kg. Animals were chemically immobilized without prior separation, remaining within their social group to minimize handling-related stress. The drugs were delivered remotely using a CO2-powered dart rifle (DAN-INJECT CO2 injection rifle, model J.M.SP. DAN-INJECT ApS, Børkop, Denmark) and 3-mL dart syringe with 2.0 x 30 mm collared needle with side ports (Dan-Inject, Børkop, Denmark). The distance to the animal, the CO2 pressure used for dart propulsion, and the anatomical site of dart impact were recorded. Drug dosages were calculated based on estimated body weight assessed by two experienced veterinarians (SP, WM).
A supplemental intramuscular dose was administered if adequate sedation was not achieved within 15 min due to incomplete drug effect or suspected dart failure.
The induction phase was systematically monitored by evaluating behavioral and postural indicators of sedation, with particular attention to the time required to attain stable lateral recumbency. Following drug administration, fallow deer were left undisturbed and continuously monitored throughout the induction period. Key timepoints were recorded from the moment of drug administration, including:
• ataxia time (onset of incoordination);
• sternal recumbency time (animal assuming a sternal position);
• lateral recumbency time (animal lying on its side);
• approach time.
Once the animal was in lateral recumbency and unresponsive to external stimuli, two experienced veterinarians proceeded with the approach. At this stage, the animal was blindfolded to minimize external stimuli, and transferred outside the enclosure to a more accessible, shaded area protected from radiant heat, where the scheduled procedures were carried out.
During the maintenance phase, the animal was continuously monitored. All animals underwent a complete physical and dental examination to assess their clinical condition. The body condition score (BCS) was also assessed using a 5-point scale (12). A blood sample was collected from each animal during immobilization for complete hematological and biochemical analysis. An abdominal ultrasound examination was also performed, primarily to evaluate the pregnancy status of the animals.
A jugular intravenous (IV) catheter 14G (Introcan Safety® 14G, 50 mm, B. Braun, Germany) was aseptically inserted. During immobilization, deer were maintained in sternal or in lateral recumbency with the neck elevated above the level of the rumen and the muzzle directed toward the ground to promote airway patency and minimize the risk of regurgitation or aspiration. Artificial tear drops were administered every 15 min to maintain ocular lubrication throughout the procedure. Propofol (Proposure 1%, Boehringer Ingelheim Animal Health Italia S.p.A., Italy) was available as rescue anesthesia at a dose of 1 mg/kg IV in cases where the depth of anesthesia was deemed insufficient, to ensure safety throughout the procedure.
At the end of the procedure, each animal was weighed using a digital dynamometer (Sauter FH 100, Sauter GmbH, Balingen, Germany) and subjects scheduled for translocation were placed in individual transport crates.
Eventually, recovery began with the intramuscular administration of atipamezole (Antisedan, 5 mg/mL, Vetoquinol Italia Srl, Italy) at a dose of 0.2 mg/kg, calculated based on the initial estimated body weight and administered at the end of the procedures. The following recovery times were recorded:
• time to first movements;
• time to sternal recumbency;
• time to standing position.
Handling time was defined as the time from the approach to the administration of atipamezole.
Actual body weight was recorded to retrospectively calculate the exact drug doses administered.
2.3 Clinical monitoring
Throughout the immobilization period, animals were continuously monitored, and their physiological parameters were recorded at 5-min intervals. Time zero (T0) corresponded to approach to the animal, followed by T5, T10, and subsequent time points.
Assessed clinical parameters included:
• heart rate (HR) via cardiac auscultation and cross-checked with a pulse oximeter (VE-H100B, Edan Instruments Inc., Germany) placed on the tongue;
• respiratory rate (RR) by visual observation of thoracic movements;
• hemoglobin oxygen saturation (SpO2) using the same pulse oximeter;
• end-tidal CO2 (ETCO2) via a portable capnograph (Emma™ Capnograph, Masimo, Switzerland) positioned at one nostril;
• arterial blood pressure (systolic SAP, diastolic DAP, mean MAP) using an oscillometric device (PetTrust, BioCare, Taiwan), with a cuff placed at the tail base, selected according to tail circumference;
• rectal temperature (Temp) using a digital thermometer.
The depth of anesthesia was assessed by evaluating palpebral and anal reflexes, muscle tone, and eye position, with corresponding scores assigned to these parameters (Table 1).
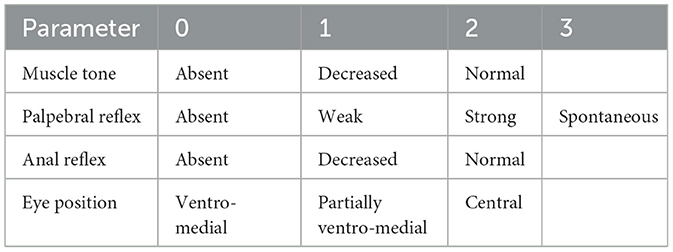
Table 1. Scoring system used to assess the depth of anesthesia during the chemical immobilization of fallow deer.
Additionally, fallow deer were monitored for signs of rumen distention and bloat visually. Measurement of blood lactate and glucose levels took place once in each animal during immobilization using handheld analyzers (Accu-Chek Guide Glucometer, Roche Diabetes Care GmbH, Germany; Lactate Scout 4, EKF Diagnostics, United Kingdom).
2.4 Anesthesia quality assessment
Dedicated scoring systems were developed specifically to evaluate the quality of induction, immobilization at approach, and recovery.
Induction quality was rated on a scale from 0 to 3 (Table 2), where 0 indicated poor and 3 excellent induction. The score was evaluated from the time of drug administration up to just before the approach to the animal.
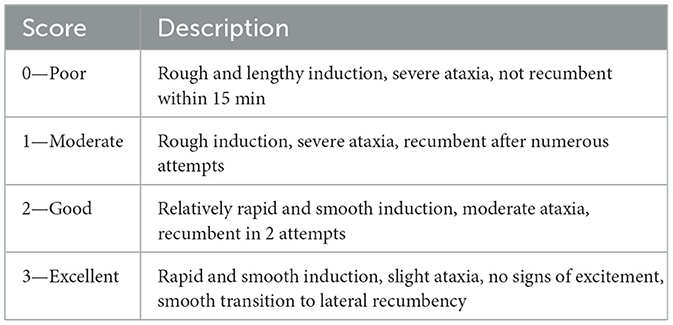
Table 2. Scoring system used to assess the quality of induction during the chemical immobilization of fallow deer.
Immobilization at approach was scored from 0 to 6 (Table 3), with 0 representing no visible effect and 6 indicating excessive immobilization.
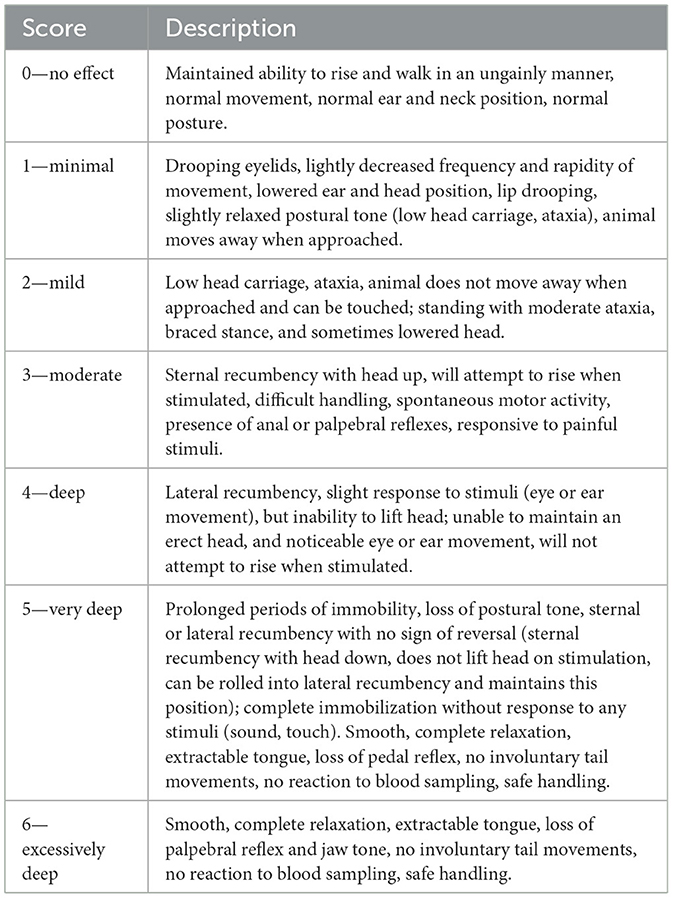
Table 3. Immobilization score at approach after administration of a combination of detomidine-ketamine-azaperone in fallow deer.
Recovery quality was graded on a scale from 0 (very poor) to 5 (excellent) (Table 4).

Table 4. Scoring system used to assess the quality of recovery after administration of atipamezole in fallow deer.
At the end of each immobilization procedure, the attending veterinarians collectively discussed the scores to ensure consistency in evaluation.
2.5 Adverse effects and complications
Throughout the immobilization period, animals were continuously monitored for the occurrence of adverse effects or complications. Respiratory depression was defined as a respiratory rate below a predefined threshold (10 breaths/min), while hypoxemia was identified as a peripheral oxygen saturation (SpO2) below 90%. Potential re-sedation was monitored for 6 h post-immobilization. All behavioral and physiological responses potentially indicative of distress or drug-related side effects were documented.
Emergency equipment, including an oxygen source, endotracheal intubation devices, emergency drugs and fluids, was immediately available. Oxygen was ready to be administered in cases of hypoxemia and respiratory depression. For abdominal bloating, a rumen tube and trocar were available for decompression if abdominal massage failed to induce belching.
2.6 Statistical analysis
The Shapiro-Wilk test was used to assess the normality of continuous variables. Depending on data distribution, continuous variables were presented as mean ± standard deviation (SD) or median with interquartile range (IQR). Categorical variables were summarized as absolute counts and percentages.
Data analysis was performed using linear mixed models to investigate the effect of time on various clinical variables in the animals. The parameters analyzed for each individual included: HR, RR, SpO2, ETCO2, SAP, MAP, DAP, and Temp, all recorded at 5-min intervals starting from the approach (T0) to 40 min post-approach (T40).
For each clinical variable, a linear mixed-effects model was applied, with time as the fixed effect and individual animal as a random effect. This approach allowed for the assessment of temporal trends in each parameter while accounting for inter-individual variability.
All statistical analyses were conducted using R Studio (RStudio, PBC, Boston, MA, US) as interface for R (The R Foundation for Statistical Computing, Austria). A p-value < 0.05 was considered statistically significant.
3 Results
3.1 Animals
A total of 22 European fallow deer (Dama dama), comprising 12 males and 10 females, were included in the study. Two males were immobilized at the Antonio Servadei Farm, 15 fallow deer were housed at the Agricultural Institute, and 5 animals at La Fodoma Farm.
The study included 3 yearling males, 3 young adult males, 6 adult males, 3 yearling females, and 7 adult females. The BCS ranged from 3 to 4 in the animals. The median estimated weight was 60 (50–60) kg, and the mean actual weight was 52.2 ± 14.5 kg. All animals were weighed, except for one (D10), in which technical issues prevented completion of body weight measurement. Among the 20 deer with complete data, the estimated body weight was underestimated in 8 animals and overestimated in 12 animals. The mean difference was +5.8 kg for underestimated and −6.3 kg for overestimated animals, with an overall range from −28.8 to +15.0 kg. Based on clinical examination and hematobiochemical analyses, all animals were deemed healthy at the time of immobilization. Five females were confirmed to be pregnant.
3.2 Anesthesia protocol
All animals that entered the study met the inclusion criteria, completed the study without complications, and were included in the final analyses. The animals were never chased before being darted. In some cases, multiple animals were captured simultaneously. The drug combination was administered intramuscularly at different injection sites: 18 animals received the injection in the thigh, and the remaining in the shoulder. Animal D7 unintentionally received two full doses, the second of which intended for another animal, due to unexpected movement during darting. Two yearling deer (D8 and D11) were given doses intended for adults. Deer D5 and D18 were darted twice, with the first dart failing to discharge the drug after bouncing off. The mean shooting distance was 12.9 ± 2.5 meters, and the mean injection pressure was 4.9 ± 0.8 bar.
The immobilization protocol consisted of detomidine (0.2 mg/kg), ketamine (2 mg/kg), and azaperone (0.3 mg/kg), with doses based on visually estimated body weight. The drug doses were adjusted retrospectively based on actual body weight, with mean administered doses of detomidine 0.22 ± 0.07 mg/kg, ketamine 2.24 ± 0.69 mg/kg, and azaperone 0.34 ± 0.10 mg/kg.
A jugular venous catheter 14G was placed in all animals. No propofol or fluid administration was required in any case.
Not all timepoints during induction and recovery could be recorded for every animal, as some individuals were not fully visible to the observers, and in certain cases, time events overlapped. However, the time of intramuscular injection of detomidine, ketamine, and azaperone, as well as the times of sternal recumbency, approach, and atipamezole administration were consistently recorded for all subjects.
During induction, ataxia and lateral recumbency times were recorded for 13 and 10 animals, respectively. Ataxia, sternal recumbency, lateral recumbency and approach times were 4.0 ± 2.2 min, 5.2 ± 1.9 min, 10.7 ± 6.4 min, and 22.0 ± 5.1 min, respectively.
Handling time, from approach to atipamezole administration, was 49.8 ± 9.6 min.
Atipamezole was administered intramuscularly in the thigh to all animals. Times to first voluntary movement, sternal recumbency, and standing position were recorded in 2, 9, and 8 animals, respectively. Median times (interquartile ranges) were 3.5 min (2.3–4.8), 3.0 min (3.0–8.0), and 7.5 min (5.8–9.8), respectively.
At the time of antagonist administration, seven animals exhibited signs of awakening, whereas two required active physical restraint for placement into transport crates before receiving atipamezole. Consequently, the effective body weight of these two animals was not recorded.
All animals were able to stand and maintain a stable posture within 20 min from atipamezole injection, except for one animal that stood up after 30 min.
3.3 Clinical monitoring
Clinical monitoring began at the time of animal approach (T0), was conducted continuously, and recorded every 5 min for 38 ± 4.3 min.
Almost all parameters were consistently measured at all timepoints for all animals, except for subject D3, in which, due to instrumentation issues, it was possible to only record HR and RR every 5 min up to T40.
The parameters HR, RR, SpO2, ETCO2, SAP, MAP, DAP, and Temp are presented in Figure 1. Among these variables, only HR and Temp showed a significant change over time, while the others remained stable. Values at T0 were considered “baseline” values.
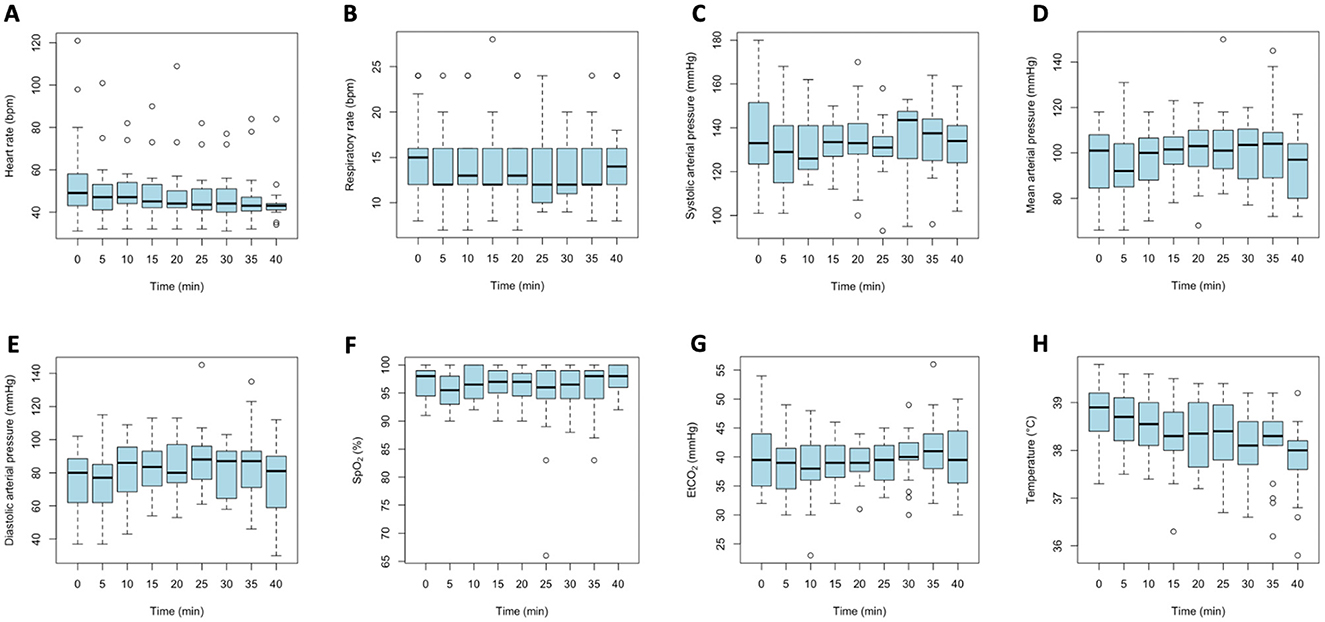
Figure 1. Time-course plots of physiological parameters recorded every 5 min during immobilization in fallow deer, starting from the moment of safe approach. Each panel represents a different parameter: (A) Heart rate; (B) Respiratory rate; (C) Systolic arterial pressure; (D) Mean arterial pressure; (E) Diastolic arterial pressure; (F) Peripheral oxygen saturation (SpO2); (G) End-tidal carbon dioxide (ETCO2); (H) Body temperature.
Specifically, for each time unit (every 5 min), there was a significant decrease in HR of approximately 0.17 bpm (p < 0.001). Considerable variability in HR was observed among animals' baseline values, with a standard deviation of 12.65 bpm.
Respiratory rate exhibited a slight negative trend over time (slope = −0.019), but this was not statistically significant (t = −1.33), indicating no significant temporal change in RR within the sample. Substantial variability was present between baseline values (standard deviation = 3.41 bpm).
Systolic arterial pressure showed a negligible and non-significant effect of time (t = −0.055), suggesting no systematic or relevant change during the observation period. Mild variability existed among animals' baseline values, with a baseline standard deviation of 6.65 mmHg.
Mean arterial pressure demonstrated a modest, non-significant positive trend over time (t = 1.359), indicating that MAP remained relatively stable throughout the observation. Mild inter-animal variability was observed (baseline standard deviation = 8.24 mmHg), with MAP consistently above 65 mmHg in all animals at all timepoints.
Diastolic arterial pressure also showed a modest and non-significant change over time (t = 1.733), with moderate variability between animals (baseline standard deviation = 9.73 mmHg), indicating no meaningful systematic change.
The SpO2 remained stable over time, with a negligible and non-significant effect of time (t = 0.052). Variability between animals was low (baseline standard deviation = 2.48%).
The ETCO2 showed a modest, non-significant temporal effect (t = 1.172), indicating no relevant systematic change over time. Moderate variability was seen between animals (baseline standard deviation = 2.26 units). Only two animals had single values above 50 mmHg (54 and 56 mmHg).
Body temperature demonstrated a significant and pronounced negative effect over time (t = −9.42), indicating a systematic decrease during the observation period. Moderate baseline variability was present among animals (standard deviation = 0.64 °C), while residual variability was lower (0.32 °C), suggesting the model effectively captured individual differences and temporal trends. Forty-seven measurements were below 38 °C, 89 ranged between 38 and 39 °C, and 43 were above 39 °C. Temperatures above 39.5 °C were recorded only at the first four time points (T0, T5, T10, or T15) in 4 animals.
Anesthesia depth was scored from 0 to 1 (Table 1) in all animals, based on palpebral and anal reflexes, muscle tone, and eye position, showing mostly absent reflexes and reduced muscle tone, indicating effective immobilization.
Lactate concentrations were measured approximately 30 min post-capture in 14 animals, with a mean value of 3.7 ± 0.7 mmol/L, while blood glucose was measured in 18 animals at about 40 min post-capture, with a mean of 159.3 ± 24.6 mg/dL.
3.4 Anesthesia quality assessment
Quality scores were assigned to all animals according to the criteria in Tables 2–4 and are presented in Table 5. Induction quality proved uniformly high, with 73 % of subjects rated as “excellent” and the remaining 27 % as “good”; no animal fell into the “poor” or “moderate” categories.
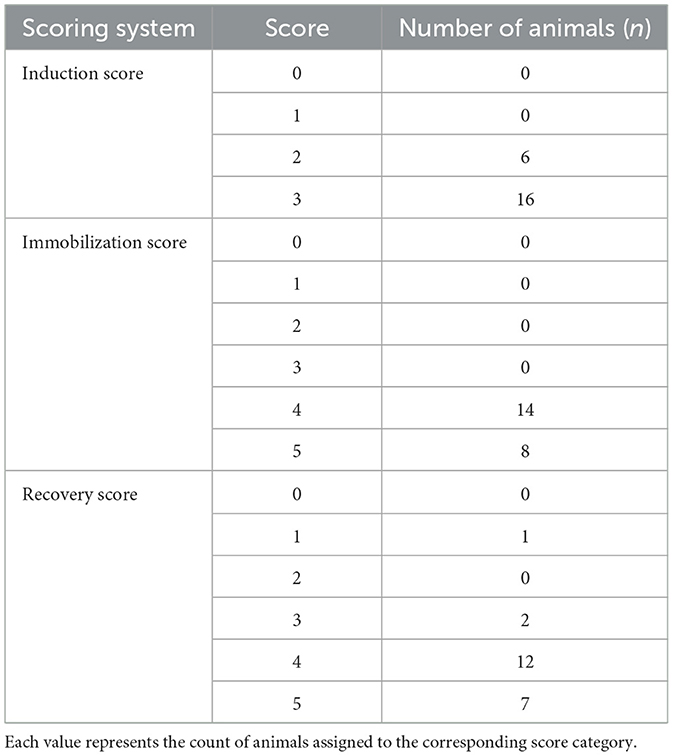
Table 5. Number of animals per score category for each scoring system: induction score (scores 0–3, see Table 2), immobilization score (scores 0–6, see Table 3), and recovery quality score (scores 0–5, see Table 4).
During immobilization, 64 % of animals exhibited a “deep” immobilization (score 4), while the other 36 % achieved “very deep” immobilization (score 5), and none showed either a more superficial or an excessively deep level.
Recovery quality was “moderate” in 54 % of cases and “excellent” in 32 %, with only two animals (D9, D14) receiving a “fair” score and a single animal (D21) experiencing a “poor” recovery.
3.5 Adverse effects and complications
During immobilization, rumen distention occurred in four animals (D1, D2, D5, and D13). In all cases, this was resolved through repositioning the animal in sternal recumbency and/or performing gentle ruminal massage.
Episodes of respiratory depression, defined as a RR below 10 bpm, were observed in six animals. In D6 and D20, only a single timepoint measurement fell below this threshold, with values of 9 and 8 bpm, respectively. In contrast, animals D12, D18, D19, and D22 exhibited RR below 10 at two or more timepoints, with values ranging between 7 and 9 bpm. Despite these reductions in respiratory rate, SpO2 remained consistently above 90% in all affected animals, and ETCO2 levels were below 44 mmHg.
A total of 179 SpO2 measurements were recorded. Seven of the measurements of SpO2 were below 90% (ranging from 66 to 89%) in 4 animals, however mucous membranes remained pink, and subsequent measurements in those animals were above 90%. Forty-two measurements were between 90% and 95%.
All animals recovered without observable drug-related complications or behavioral signs of distress.
No evidence of re-sedation was observed in any individual during the subsequent 6 h, and no adverse effects were noted in the following 5 days. All of the female deer successfully carried their pregnancies to term.
4 Discussion
The detomidine-ketamine-azaperone combination provided effective and safe immobilization in fallow deer, characterized by smooth induction, adequate anesthetic depth, and good-quality recovery in the majority of animals. This study yielded valuable clinical insights into the effects and quality of immobilization afforded by this combination, thanks to comprehensive physiological monitoring and the use of detailed scoring systems for standardized assessment. Notably, this specific drug combination has not been previously described in the scientific literature for fallow deer.
The rationale for combining an α2-agonist with a dissociative anesthetic such as ketamine is supported by previous studies. The use of α2-agonists in association with ketamine or tiletamine/zolazepam has been described in fallow deer (7, 10, 13), generally providing acceptable immobilization. Among the α2-agonists, detomidine offers superior receptor selectivity compared to xylazine (11), but its use in fallow deer remains poorly documented. In the present study, detomidine was administered at 0.2 mg/kg, consistent with the dose reported by Galka et al. (7) in fallow deer. The ketamine dose (2 mg/kg) was slightly higher than in the protocol described by Avni-Magen et al. (13), where the mean immobilization time was only approximately 19 ± 3 min. However, it was lower than doses reported in other studies (7, 14). Despite this relatively low ketamine dose, anesthetic depth was adequate, likely enhanced by the synergistic effect of azaperone. Unlike α2-agonists and dissociative anesthetics, azaperone has rarely been studied in fallow deer and has previously been reported only in combination with fentanyl or thiafentanil (9, 15). Notably, Lapid et al. (9) evaluated azaperone exclusively in Persian fallow deer (Dama dama mesopotamica), a subspecies distinct from the European fallow deer. Azaperone has also been infrequently reported in deer (16, 17).
This butyrophenone drug, widely used for tranquilization in swine, has also been used in wild and farmed ungulates when combined with α2-agonists and opioids (5). In pigs, it has a reported duration of action of approximately 6 h, though its duration in wildlife remains unclear. In this study, the azaperone dose (0.3 mg/kg) was higher than that used by Lapid et al. (9), who administered 0.190 ± 0.030 mg/kg with thiafentanil, achieving immobilization for only 21.8 ± 5.2 min. Notably, Lapid et al. (9) also reported five perianesthetic morbidity and mortality events, including four cases of respiratory arrest approximately 10 min after drug administration. These adverse effects may have been related to the use of thiafentanil, a potent opioid known for its rapid onset and potential for respiratory depression. Lapid et al. (9) also reported a more uniform immobilization score with azaperone-thiafentanil than with etorphine-acepromazine, suggesting azaperone may provide more predictable immobilization quality.
In this study, the inclusion of azaperone may have contributed to the observed smooth induction, stable anesthesia, and satisfactory recovery, supporting its potential role in cervid anesthesia protocols. Moreover, its vasodilatory properties may have helped mitigate α2-agonist-induced hypertension (18, 19), further improving hemodynamic stability. Importantly, five pregnant females were successfully anesthetized and carried their pregnancies to term. This suggests that, despite the theoretical risk of α2-agonists affecting uterine perfusion (20, 21), the protocol was safe in these cases. Notably, detomidine is known not to affect reproductive organ perfusion while exerting typical cardiovascular effects (22). This supported its use in this study.
The results of this study also highlight the robustness and wide safety margin of the detomidine-ketamine-azaperone protocol. No complications were observed in this study, although some animals received drug doses that exceeded the calculated target amount, with a few receiving up to twice the intended dose. In chemical immobilization of wildlife, several factors may commonly affect dosing accuracy, including estimated body weight, dart volume limitations, and challenges associated with drug delivery under field conditions. For this reason, it is essential that pharmacological protocols offer a wide safety margin.
Another positive aspect of the protocol used in this study is the excellent quality of induction observed in the vast majority of animals (73%), and good in the remainder. Induction time is a critical factor in wildlife immobilization, as it helps to minimize stress and reduce the risk of trauma (23). In this study, mean times to ataxia (4.0 ± 2.2 min), sternal recumbency (5.2 ± 1.9 min), and lateral recumbency (10.7 ± 6.4 min) were recorded, with an average approach time of 22.0 ± 5.1 min.
Comparing the approach time used in this study to the induction times reported in other studies, these values are comparable to, or slightly longer than, those described in protocols for other cervids (7, 10, 13).
However, it is important to note that the approach time in this study reflects the actual physical approach of the operators, which was intentionally delayed to reduce stress in confined group settings. This differs from induction time reported in the literature, which usually refers to the interval from drug administration to loss of posture or recumbency. Previous research recommends waiting at least 15 min before approaching sedated wildlife, to allow full drug effect (24), as drug absorption after intramuscular injection is influenced by muscle perfusion and cardiac output. The onset of drug action varies with the agent used: approximately 15 min for α2-agonists, 1–2 min for ketamine, and around 10 min for azaperone (11). Loss of standing posture is considered a reliable indicator of deepening sedation, especially when associated with signs like head lowering, decreased respiratory rate, and reduced responsiveness. However, even when recumbent, animals may not yet be fully sedated, and early stimulation may trigger arousal or escape attempts. This reaction is commonly associated with α2-agonists and may be due to sudden stress-induced catecholamine release that counteracts sedation (24). Therefore, delaying approach after recumbency is generally advised. Despite these variations, no adverse events related to induction occurred in this study. All animals lost standing posture rapidly after darting, which effectively minimized the risk of injury or escape, and highlights the reliability and safety of the detomidine-ketamine-azaperone protocol under field conditions.
Immobilization quality was also consistently high, ranging between deep and very deep. Adequate anesthetic depth was confirmed by the absence of muscle tone, preserved relaxation, and a lack of nociceptive responses during painful procedures. The occasional presence of weak palpebral reflexes did not compromise the safety or adequacy of immobilization. Notably, no reactions were observed during venipuncture, microchip implantation, or ear tagging, indicating that the drug combination provided effective analgesia for minor procedures. This is consistent with the known, albeit short-lasting, analgesic effects of α2-agonists (11).
Continuous monitoring of vital signs during chemical immobilization revealed good cardiopulmonary stability throughout. Heart rates remained within expected ranges for α2-agonist/dissociative protocols (7, 10, 13) and were generally lower than those reported with opioid-based combinations (9, 25). A statistically significant decrease in heart rate was observed during anesthesia, consistent with other studies involving α2-agonists in other animal species (26, 27). However, the reduction (approximately 0.17 bpm every 5 min) was very minimal and not clinically relevant. Importantly, this decrease in heart rate was not associated with changes in blood pressure, which remained stable and within physiological limits for cervids sedated with similar protocols (9, 13). The inclusion of azaperone likely contributed to cardiovascular stability by attenuating detomidine-induced hypertension through peripheral vasodilation mediated by dopaminergic and α1-adrenergic antagonism (18). This balancing effect has also been reported in other cervid species when α2-agonists are combined with azaperone (19, 28), supporting its use in improving cardiovascular safety during immobilization. Mild variability in baseline heart rate and blood pressure values was noted among individual animals at the start of monitoring, likely reflecting differences in individual responses, stress levels, or temperament.
Throughout the immobilization, respiratory parameters also remained within acceptable clinical limits, indicating good physiological stability under the detomidine-ketamine-azaperone protocol. The SpO2 values showed a stable and non-significant trend over time. Similarly, ETCO2 exhibited a modest, non-significant upward trend, suggesting an absence of progressive hypoventilation or accumulation of CO2. Although two isolated values exceeded 50 mmHg (54 and 56 mmHg), these variations were not associated with any clinical signs of concern. Respiratory depression, defined as a respiratory rate below 10 breaths per minute, was observed in six animals. In two individuals, this occurred only at a single time point, while four animals displayed RR values between 7 and 9 bpm at multiple time points. Despite these reductions, SpO2 remained above 90% in all affected animals, and ETCO2 did not exceed 44 mmHg, suggesting that ventilation was still adequate and that hypoventilation did not progress to clinically significant hypoxia or hypercapnia. These findings are encouraging, particularly given that α2-agonists are known to cause reductions in respiratory rate in ruminants (29, 30). A total of seven SpO2 measurements fell below 90%, ranging from 66% to 89%, in four individuals. However, mucous membranes remained pink, and SpO2 values normalized in subsequent readings. Moreover, 42 measurements (across different animals and time points) were within the 90–95% range. Supplemental oxygen was intended to be administered via flow-by in cases where SpO2 dropped below 90% and RR was < 10 bpm. As no species-specific thresholds are available for fallow deer and the animals were breathing spontaneously, with no possibility to measure tidal volume or arterial blood gases, conservative criteria were applied to define respiratory depression in order to ensure animal safety.
Much lower SpO2 and RR values were frequently reported in similar species under different immobilization protocols (9, 13). Notably, the accuracy of pulse oximetry in fallow deer remains uncertain. In addition, it has been shown that SpO2 may underestimate arterial oxygen saturation (SaO2), due to poor agreement between these parameters in various wild species (31). This limitation may be further exacerbated by the use of α2-agonists, which can impair peripheral perfusion and pulse oximeter signal quality in animals (31, 32). Moreover, it is well known that the ability of pulse oximeters to accurately read SpO2 is influenced by the site of probe placement (32, 33). However, in the absence of direct blood gas analysis, which was not feasible in this study due to logistical and economic constraints, pulse oximetry provided a useful, albeit indirect, tool for monitoring respiratory adequacy. Regardless of the results of specific monitoring, supplemental oxygen is strongly recommended during field immobilization of cervids (34). Indeed, α2-agonist-induced reductions in arterial oxygen tension have been documented in sheep, highlighting the importance of oxygen support during sedation in ruminants (35). In this study, however, supplemental oxygen was not routinely administered, but rather, it was available if clinically necessary. This approach was adopted to better evaluate the actual impact of the anesthetic protocol on respiratory function in the field, where access to oxygen sources is not always guaranteed.
The protocol did not cause hyperthermia in any animal. In the study by Avni-Magen et al. (13) involving captive Persian fallow deer immobilized with medetomidine-ketamine or medetomidine-midazolam, body temperatures remained below 40.5 °C. In contrast, Lapid et al. (9) reported severe hyperthermia in 3 out of 20 Persian fallow deer immobilized with thiafentanil-azaperone, possibly due to muscle rigidity, a condition not observed in our study. On the contrary, we recorded a decrease in temperature over time, which is a common physiological response during anesthesia. Anesthetic agents, including sedatives, and α2-agonists, are known to widen the interthreshold range for thermoregulation, reducing the animal's ability to mount compensatory responses such as shivering (36).
Metabolic evaluation was limited but informative as only single-point lactate and glucose measurements could be collected due to the nature of the procedures. No cases of hypoglycemia or significant hyperglycemia were observed, despite the known hyperglycemic effects of α2-agonists (37), suggesting that the animals were not experiencing severe stress or excessive sympathetic stimulation. Lactate levels exceeded 2.5 mmol/L in several animals but remained below values reported in previous studies involving fallow deer immobilization (9), further supporting the notion of stable cardiovascular and metabolic status under the protocol used. Elevated lactate is commonly associated with increased muscular activity during capture and handling, which can also lead to hyperthermia, muscle enzyme leakage, and lactic acidosis. If uncompensated, these changes may progress to a clinical syndrome characterized by shock, myopathy, renal failure, muscle rupture, or even sudden death (24). The lactate values observed, together with the absence of significant increases in body temperature, suggest that the capture method and anesthetic protocol employed did not promote the pathophysiological cascade typically associated with capture myopathy. Future studies should evaluate these values at a minimum of two time points, immediately after the initial approach and prior to release, to provide a more accurate understanding of the effects of the protocol and handling on these parameters.
Overall, the cardiovascular, respiratory, and metabolic findings in this study support the conclusion that the detomidine-ketamine-azaperone combination provides a high degree of cardiopulmonary safety, with only minimal and clinically irrelevant respiratory depression and no evidence of significant metabolic derangement. Additionally, careful observation and timely interventions, such as repositioning animals and performing gentle ruminal massage, proved effective in managing ruminal distention when it occurred. This condition was observed in only four animals, despite being a well-documented side effect of chemical immobilization in polygastric herbivores with α2-agonists (30, 38, 39). The reduction in gastro-intestinal motility associated with α2-agonists is believed to result from the suppression of gastrointestinal regulatory centers within the medulla oblongata (40, 41). The low incidence and successful management of this complication further underscore the practical safety and field applicability of the protocol.
In this study, atipamezole was administered at a 1:1 ratio with detomidine. Recommended atipamezole doses vary by species and α2-agonists. Furthermore, the efficacy of antagonism depends on the sedative dose, time, and route of administration (11). Moreover, there are no specific studies evaluating the effective dose of atipamezole required to antagonize detomidine in cervids based on the time elapsed since drug administration. Notably, previous studies have successfully used a 1:1 ratio with antelopes and sheep (42, 43).
In the present study, no fallow deer exhibited clinical signs of re-sedation during the 6-h post-atipamezole monitoring period. This observation period was selected as a precautionary measure, since the pharmacokinetics of α2-agonists and their antagonism by atipamezole are not well established in fallow deer. Considering that atipamezole has a shorter half-life (approximately 1.5–2 h) than most α2-agonists (11), extended monitoring was deemed necessary to detect potential re-sedation or delayed adverse effects and to ensure animal welfare. Moreover, as field conditions allowed observation only under natural daylight, a 6-h period was deemed appropriate to ensure reliable monitoring and safeguard animal welfare. Based on our findings, it is reasonable to assume that no re-sedation occurred beyond this timeframe.
The safety of the protocol was further supported by the quality and rapidity of recovery observed in this study. Most animals exhibited moderate to excellent recovery quality, with only one individual classified as poor. Nonetheless, all animals were able to regain a standing position within 20 min post-procedure. These findings indicate that the detomidine-ketamine-azaperone combination allows for smooth and predictable recoveries, minimizing the risk of post-anesthetic complications and facilitating handling and safe release in field conditions. Similar recovery times were also reported by Avni-Magen et al. (13). In contrast, Lapid et al. (9) observed shorter recovery times; however, the quality of immobilization was inferior and several complications were noted.
However, certain limitations of this study must be acknowledged. The lack of a control or comparison group prevents direct evaluation against other commonly used protocols. The limited sample size, particularly across age classes, prevented subgroup analyses regarding dose effects or age-related sensitivity. Since the study was conducted on animals accustomed to human presence, the results may not be generalizable to free-ranging populations which could exhibit heightened stress responses. Additionally, all captures occurred under temperate conditions. Further studies are needed to evaluate the influence of environmental variables such as ambient temperature or seasonal changes on sedation and recovery quality. Testing the protocol under more challenging conditions, including in wild or free-ranging deer, would further assess its robustness.
5 Conclusions
The detomidine-ketamine-azaperone combination demonstrated excellent performance for the chemical immobilization of fallow deer, providing smooth induction, stable anesthesia, and good to excellent recovery quality in most animals. The protocol showed a favorable safety profile and represents a reliable, non-opioid option for routine clinical and management procedures in this species. While azaperone has previously been described in fallow deer in combination with thiafentanil or fentanyl, this is the first study to evaluate its use alongside detomidine and ketamine, highlighting its potential as a valuable adjunct in cervid anesthesia protocols. Further research is warranted to confirm these findings and optimize dosing strategies.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal studies were approved by Animal Welfare Body of the University of Padova. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
GD: Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. AB: Data curation, Investigation, Writing – original draft, Writing – review & editing. SP: Conceptualization, Investigation, Resources, Writing – review & editing. WM: Conceptualization, Investigation, Resources, Writing – review & editing. GM: Investigation, Writing – original draft, Writing – review & editing. AA: Investigation, Writing – review & editing. LB: Investigation, Writing – review & editing. FZ: Data curation, Formal analysis, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the Open Access funding provided by Università degli Studi di Padova | University of Padua, Open Science Committee.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Masseti M, Mertzanidou D. Dama Dama. The IUCN Red List of Threatened Species. e.T42188A10656554 (2008). doi: 10.2305/IUCN.UK.2008.RLTS.T42188A10656554.en
2. Bengsen AJ, Forsyth DM, Ramsey DSL, Amos M, Brennan M, Pople AR, et al. Estimating deer density and abundance using spatial mark-resight models with camera trap data. J Mammal. (2022) 103:711–22. doi: 10.1093/jmammal/gyac016
3. Pelliccioni ER, Riga F, Toso S. Linee Guida per la Gestione degli Ungulati: Cervidi e Bovidi. ISPRA, Manuali e Linee Guida. Rome: ISPRA (2013).
4. Caulkett NA, Arnemo JM. Cervids (deer). In: West G, Heard DJ, Caulkett N, editors. Zoo Animal and Wildlife Immobilization and Anesthesia. 2nd ed. Ames, IA: Wiley Blackwell (2014). p. 823–29. doi: 10.1002/9781118792919.ch59
5. Caulkett NA, Arnemo JM. Comparative immobilization and anesthesia – free-ranging terrestrial mammals. In:Lamont AL, Grimm KA, Robertson S, Love L, Schroeder C, , editors. Veterinary Anesthesia and Analgesia: The Sixth Edition of Lumb and Jones. Ames, IA: Wiley Blackwell. (2024). p. 1077–90. doi: 10.1002/9781119830306.ch55
6. Pearce PC, Kock RA. Physiological effects of etorphine, acepromazine and xylazine on the black fallow deer (Dama dama). Res Vet Sci. (1989) 46:380–6. doi: 10.1016/S0034-5288(18)31184-6
7. Galka ME, Aguilar JM, Quevedo MA, Santisteban JM, Gómez-Villamandos RJ. Alpha-2 agonist dissociative anesthetic combinations in fallow deer (Cervus dama). Zoo Wildl Med. (1999) 30:451–3.
8. Bengsen A, Hampton JO, Comte S, Freney S, Forsyth DM. Evaluation of helicopter net-gunning to capture wild fallow deer (Dama dama). Wild Res. (2021) 48:722–9. doi: 10.1071/WR21007
9. Lapid R, King R, Bdolah AT, Shilo-Benjamini Y. A retrospective comparison of chemical immobilization with thiafentanil, thiafentanil-azaperone, or etorphine-acepromazine in captive Persian fallow deer (Dama dama mesopotamica). J Zoo Wildl Med. (2017) 48:627–35. doi: 10.1638/2016-0280.1
10. Fernández-Morán J, Palomeque J, Peinado VI. Medetomidine/tiletamine/zolazepam and xylazine/tiletamine/zolazepam combinations for immobilization of fallow deer (Cervus dama). J Zoo Wildl Med. (2000) 31:62–4. doi: 10.1638/1042-7260(2000)031[0062:MTZAXT]2.0.CO;2
11. Creighton CM, Lamont LA. Sedatives and tranquilizers. In:Lamont AL, Grimm KA, Robertson S, Love L, Schroeder C, , editors. Veterinary Anesthesia and Analgesia: The Sixth Edition of Lumb and Jones. Ames, IA: Wiley Blackwell. (2024). p. 333–54. doi: 10.1002/9781119830306.ch22
12. Audige L, Wilson PR, Morris RS. A body condition score system and its use for farmed red deer hinds. New Zeal J Agr Res. (1998) 41:545–53. doi: 10.1080/00288233.1998.9513337
13. Avni-Magen N, Zafrir B, King R, Bdolah-Abram T, Shilo-Benjamini Y. Immobilization of captive Persian fallow deer (Dama dama mesopotamica) using medetomidine-ketamine or medetomidine-midazolam. Vet Anaesth Analg. (2019) 46:662–6. doi: 10.1016/j.vaa.2019.06.003
14. Stewart MC, English AW. The reversal of xylazine/ketamine immobilisation of fallow deer with yohimbine. Aust Vet J. (1990) 67:315–7. doi: 10.1111/j.1751-0813.1990.tb07812.x
15. Haigh JC. Fallow deer immobilization with fentanyl and a neuroleptic. Vet Rec. (1977) 100:386–7. doi: 10.1136/vr.100.18.386
16. Wilson PR, Biemans J, Stafford KJ, Veltman CJ, Spoorenberg J. Xylazine and xylaxine/fentanyl citrate/azaperone combination in farmed deer II: velvet antler removal and reversal combinations. N Zeal Vet J. (1996) 44:88–94. doi: 10.1080/00480169.1996.35942
17. Grunwald PJ, Ruder MG, Osborn DA, Muller LI, Goode KO, D'Angelo GJ. Comparison of butorphanol-azaperone-medetomidine and nalbuphine-medetomidine-azaperone for immobilization of white-tailed deer (Odocoileus virginianus). J Wildl Dis. (2025) 61:111–21. doi: 10.7589/JWD-D-23-00135
18. Lees P, Serrano L. Effects of azaperone on cardiovascular and respiratory functions in the horse. Br J Pharmacol. (1976) 56:263–9. doi: 10.1111/j.1476-5381.1976.tb07637.x
19. Pon K, Caulkett N, Woodbury M. Efficacy and safety of a medetomidine-azaperone-alfaxalone combination in captive white-tailed deer (Odocoileus virginianus). J Zoo Wildl Med. (2016) 47:29–37. doi: 10.1638/2015-0121.1
20. Carter JE. Anesthesia and sedation in the field. In:Cole C, Bentz B, Maxwell L, , editors. Equine Pharmacology. Ames, IA:Wiley Blackwell (2015). p. 44–62. doi: 10.1002/9781118845110.ch3
21. Dugdale A, Beaumont G, Bradbrook C, Gurney M. Sedation and premedication: small animals. In:Dugdale A, Beaumont G, Bradbrook C, Gurney M, , editors. Veterinary Anaesthesia: Principles to Practice. 2nd ed. Ames, IA: Wiley Blackwell (2020). p. 55–76.
22. Araujo RR, Ginther OJ. Vascular perfusion of reproductive organs in pony mares and heifers during sedation with detomidine or xylazine. Am J Vet Res. (2009) 70:141–8. doi: 10.2460/ajvr.70.1.141
23. Bergvall UA, Kjellander P, Ahlqvist P, Johansson Ö, Sköld K, Arnemo JM. Chemical immobilization of free-ranging fallow deer (Dama dama): effect of needle length on induction time. J Wildl Dis. (2015) 51:484–7. doi: 10.7589/2013-11-290
24. Caulkett N, Boysen S, Arnemo JM. Emergencies and complications. In:West G, Heard D, Caulkett N, , editors. Zoo Animal and Wildlife Immobilization and Anesthesia. 3rd ed. Ames, IA: Wiley Blackwell (2025). p. 103–20. doi: 10.1002/9781119539278.ch4
25. Shilo-Benjamini Y, Zafrir B, Avni-Magen N. Etorphine-medetomidine immobilization in 16 captive Persian fallow deer (Dama dama mesopotamica). Isr J Vet Med. (2021) 76:145–9.
26. Kasten JI, Messenger KM, Campbell NB. Sedative and cardiopulmonary effects of buccally administered detomidine gel and reversal with atipamezole in dogs. Am J Vet Res. (2018) 79:1253–60. doi: 10.2460/ajvr.79.12.1253
27. Tahmasbi T, Raisi A, Zakian A, Khaldari M. Comparing the effects of intravenous injection and intranasal atomisation of detomidine in sheep. Vet Med Sci. (2023) 9:353–62. doi: 10.1002/vms3.1023
28. Siegal-Willott J, Citino SB, Wade S, Elder L, Hayek LA, Lance WR. Butorphanol, azaperone, and medetomidine anesthesia in free-ranging white-tailed deer (Odocoileus virginianus) using radiotransmitter darts. J Wildl Dis. (2009) 45:468–80. doi: 10.7589/0090-3558-45.2.468
29. de Carvalho LL, Nishimura LT, Borges LP, Cerejo SA, Villela IO, Auckburally A, et al. Sedative and cardiopulmonary effects of xylazine alone or in combination with methadone, morphine or tramadol in sheep. Vet Anaesth Analg. (2016) 43:179–88. doi: 10.1111/vaa.12296
30. Nahvi A, Molaei MM, Samimi AS, Azari O, Mashayekhi H, Ebrahimzadeh F. Evaluation of the sedative and physiological effects of xylazine, detomidine, medetomidine and dexmedetomidine in goats. Vet Med Sci. (2022) 8:1205–10. doi: 10.1002/vms3.732
31. Morelli J, Rossi S, Fuchs B, Richard E, Barros DSB, Küker S, et al. Evaluation of three medetomidine-based anesthetic protocols in free-ranging wild boars (Sus scrofa). Front Vet Sci. (2021) 8:655345. doi: 10.3389/fvets.2021.655345
32. Dörfelt R, Diels J, Hartmann K. Evaluation of the performance of two new generation pulse oximeters in cats at different probe positions and under the influence of vasoconstriction. J Feline Med Surg. (2022) 24:1026–31. doi: 10.1177/1098612X211063768
33. De Benedictis GM, Contiero B, Bovo D, De Rosa V, Cardinali M, Zanusso F. Evaluation of the performance of three pulse oximeters at different probe positions in awake rabbits. PLoS One. (2025) 20:e0323044. doi: 10.1371/journal.pone.0323044
34. Evans AL, Lian M, Das Neves CG, Øs O, Andersen R, Aanes R, et al. Physiologic evaluation of medetomidine-ketamine anesthesia in free-ranging Svalbard (Rangifer tarandus platyrhynchus) and wild Norwegian reindeer (Rangifer tarandus tarandus). J Wildl Dis. (2013) 49:1037–41. doi: 10.7589/2013-03-049
35. Celly CS, McDonell WN, Young SS, Black WD. The comparative hypoxaemic effect of four alpha 2 adrenoceptor agonists (xylazine, romifidine, detomidine and medetomidine) in sheep. J Vet Pharmacol Ther. (1997) 20:464–71. doi: 10.1046/j.1365-2885.1997.00097.x
36. Grimm KA. Perioperative thermoregulation. In:Lamont AL, Grimm KA, Robertson S, Love L, Schroeder C, , editors. Veterinary Anesthesia and Analgesia: The Sixth Edition of Lumb and Jones. Ames, IA: Wiley Blackwell (2024). p. 246–53. doi: 10.1002/9781119830306.ch17
37. Sinclair MD. A review of the physiological effects of alpha2-agonists related to the clinical use of medetomidine in small animal practice. Can Vet J. (2003) 44:885–97.
38. Samimi AS, Sakhaee E, Iranmanesh F. Evaluation of sedative, analgesic, physiological, and laboratory effects of two doses of medetomidine and xylazine in dromedary calves. J Vet Pharmacol Ther. (2019) 42:411–9. doi: 10.1111/jvp.12779
39. Samimi AS, Molaei MM, Azari O, Ebrahimpour F. Comparative evaluation of sedative and clinical effects of dexmedetomidine and xylazine in dromedary calves (Camelus dromedarius). Vet Anaesth Analg. (2020) 47:224–8. doi: 10.1016/j.vaa.2019.11.004
40. Kästner SB. A2-agonists in sheep: a review. Vet Anaesth Analg. (2006) 33:79–96. doi: 10.1111/j.1467-2995.2005.00243.x
41. Garcia-Pereira FL, Greene SA, McEwen MM, Keegan R. Analgesia and anesthesia in camelids. Small Rumin Res. (2006) 61:227–33. doi: 10.1016/j.smallrumres.2005.07.013
42. Laricchiuta P, De Monte V, Campolo M, Grano F, Iarussi F, Crovace A, et al. Evaluation of a butorphanol, detomidine, and midazolam combination for immobilization of captive Nile lechwe antelopes (Kobus magaceros). J Wildl Dis. (2012) 48:739–46. doi: 10.7589/0090-3558-48.3.739
Keywords: fallow deer, chemical immobilization, detomidine, azaperone, ketamine, quality assessment
Citation: De Benedictis GM, Baggio A, Pesaro S, Magnone W, Miani G, Andolfatto A, Bono L and Zanusso F (2025) Evaluation of a detomidine–ketamine–azaperone combination for the chemical immobilization of fallow deer (Dama dama). Front. Vet. Sci. 12:1718243. doi: 10.3389/fvets.2025.1718243
Received: 03 October 2025; Accepted: 22 October 2025;
Published: 13 November 2025.
Edited by:
Apostolos D. Galatos, University of Thessaly, GreeceReviewed by:
Inhyung Lee, Seoul National University, Republic of KoreaSilke Pfitzer, Tshwane University of Technology Pretoria West Campus, South Africa
Copyright © 2025 De Benedictis, Baggio, Pesaro, Magnone, Miani, Andolfatto, Bono and Zanusso. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesca Zanusso, ZnJhbmNlc2NhLnphbnVzc29AdW5pcGQuaXQ=
 Giulia Maria De Benedictis
Giulia Maria De Benedictis Alice Baggio
Alice Baggio Stefano Pesaro
Stefano Pesaro William Magnone
William Magnone Giovanna Miani4
Giovanna Miani4 Alice Andolfatto
Alice Andolfatto Lucia Bono
Lucia Bono Francesca Zanusso
Francesca Zanusso