- 1Department of Veterinary Medical Sciences, University of Bologna, Bologna, Italy
- 2Department of Veterinary Medicine, University of Teramo, Teramo, Italy
- 3Department of Translational Medicine and for Romagna, University of Ferrara, Ferrara, Italy
Background: Evidence suggests that the endocannabinoid system (ECS) is crucial for regulating inflammation, cell proliferation and pain. The ECS is composed of cannabinoid receptors such as type 1 (CBR1), type 2 (CBR2) and GPR55, endocannabinoids and enzymes. Proteins of ECS have previously been localized in the epidermal cells of the horse hooves. Given the physio-pathological role and cellular distribution of the ECS across species, the authors hypothesized that cannabinoid receptors are expressed within the inflammatory cells, fibroblasts and endothelial cells of the equine hoof laminae, going beyond the epidermal cells.
Objectives: To preliminary analyze the gene expression of Cn1r, Cn2r and GPR55 in the hoof laminae and test the specificity of the antibody against GPR55. To characterize the distribution and expression of CBRs in the inflammatory cells and fibroblasts of the laminar junction of equine healthy hooves and with laminitis.
Animals: Animals were divided into 3 groups: healthy, acute laminitis and chronic laminitis. A total of 18 samples were collected and processed from the front limb of animals slaughtered for consumption or euthanized (6 control animals, 4 acute laminitis, 8 chronic laminitis).
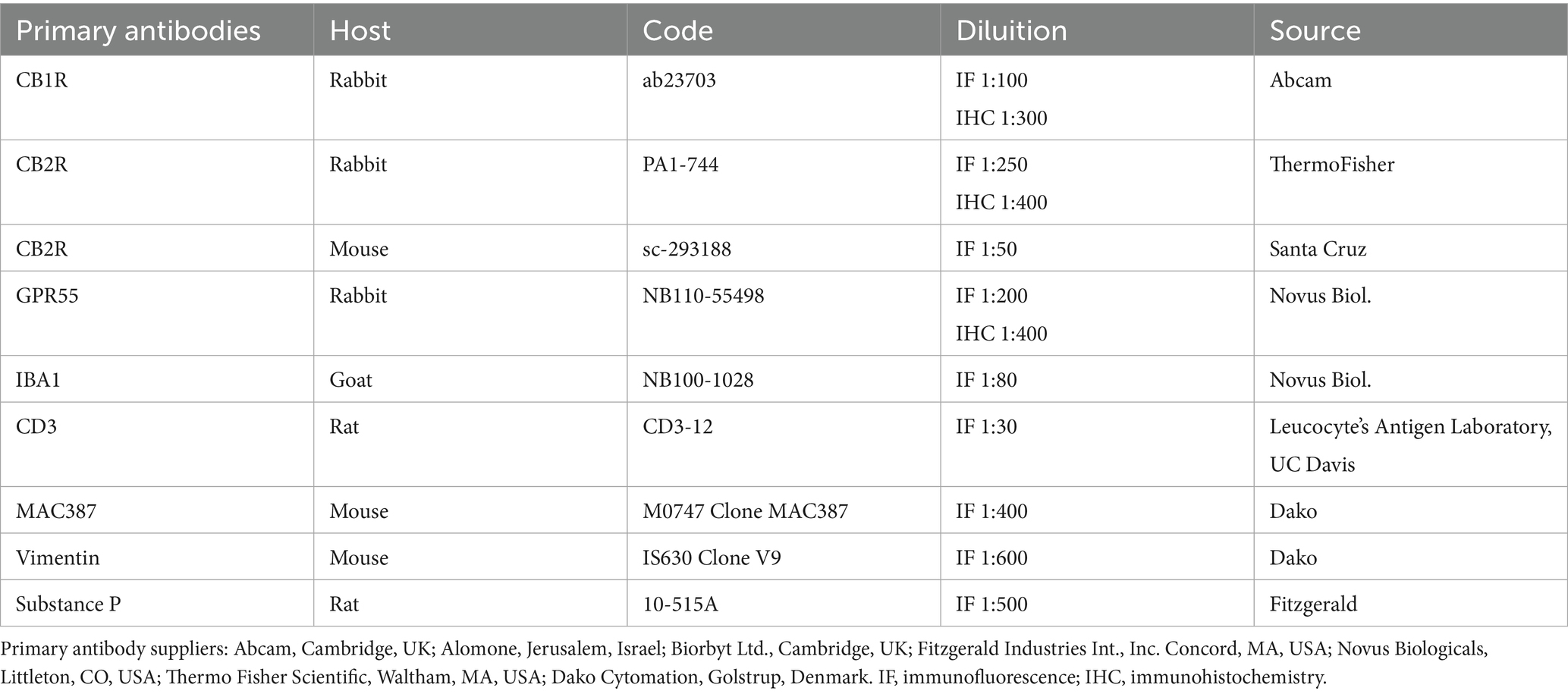
Methods: Analysis of CBR1, CBR2 and GPR55 protein expression was made by fluorescence microscopy with co-localization with antibodies against the macrophages marker IBA1, the T cell marker CD3, the neutrophils marker calprotectin (MAC387), the fibroblasts marker vimentin (Clove V9) and the nerve fibers marker Substance P. Preliminary analysis was performed to evaluate gene expression (Cnr1, Cnr2, and Gpr55) using real-time PCR and to verify the specificity of the primary antibody (Gpr55) with Western Blotting (WB).
Results: The resident pool of inflammatory cells in the normal laminae and the inflammatory infiltrate cells of the affected equine laminae showed protein expression of CB2R and GPR55; no CB1R staining was seen at the inflammatory cells. Equine dermal fibroblast and endothelial cells exhibited protein expressions of CB1R, CBR2 and GPR55. Substance P positive nerve fibers were positive for CB1R.
Conclusions and clinical importance: Cannabinoid receptors are expressed in different immune cell types of the hoof laminae, pointing to the role of the ECS in modulating inflammatory outburst, tissue degeneration and pain. Our results serve as a foundation for the development of new veterinary pharmacotherapies that target the ECS during laminitis.
1 Introduction
Laminitis is a complex and devastating condition affecting the hooves of horses; it is characterized by inflammation and failure of the laminae, namely the structures which secure the distal phalanx to the hoof wall (1). The pathogenesis of the disease involves multiple factors, including metabolic and endocrine disorders, gastrointestinal disturbances, and systemic inflammatory responses (2, 3). Horses affected with Pars Pituitary Intermedia Disfunction (PPID) or Insulin Dysregulation (ID) commonly present with lamellar damage (2, 4).
Inflammation is a central feature of laminitis, involving various cellular and molecular pathways. Upon the onset of laminitis, an inflammatory cascade is triggered, leading to the activation and recruitment of several inflammatory cell types to the laminae (5). Macrophages and dendritic cells (DCs) are pivotal in orchestrating the inflammatory response (6). Neutrophils are among the first responders to the site of inflammation; are essential for pathogen clearance but can also cause significant collateral damage to the laminar tissue (7). A significant increase in the number of neutrophils and T-cells is noted in horses with induced acute laminitis which points to the role of this cell type in the onset of the disease (7, 8).
In addition to the fluctuation of inflammatory cell populations during the disease process, recent studies have shown that the recruitment of inflammatory cells and cytokine production are also influenced by environmental factors (9). Neutrophil and lymphocyte concentrations in peripheral blood display opposing patterns during daylight hours, whereas during the night both cell populations tend to converge. Interleukin-6 (IL-6) levels peak around 8 a.m., while at 8 p.m. they reach their lowest concentrations (9). This rhythmic fluctuation over a 24-h period is known as a circadian cycle. Similarly, endocannabinoid molecules also exhibit circadian oscillations, being largely responsible for regulating stress responses, sleep, and neuronal oxidative balance (10–12). It is therefore plausible to hypothesize that the connection between the immune system and environmental cues is mediated, at least in part, by the expression of endocannabinoid system proteins within inflammatory cells.
Recent studies have identified cannabinoid receptors in equine tissue, suggesting a role for the endocannabinoid system (ECS) in mediating responses to pain and inflammation (13–16). The ECS proteins have being identified in the epidermal laminae of horses with and without laminitis (17), placing in evidence the fluctuation of gene expression and protein during this disease. The ECS is an intricate signaling network within the body, acting as a critical regulator of the various physiological processes, including pain, inflammatory response and endocrine pathways, responsible for maintaining homeostasis (18). This system includes endocannabinoids, receptors, and enzymes, all working in concert to modulate metabolic functions, neurotransmission and immune responses (19).
While the ECS is a conserved system across species, there are notable variations which reflect the unique physiological needs of different organisms. Understanding these variations is crucial for tailoring therapeutic interventions. In equine medicine, the ECS has garnered particular attention due to its potential implications in managing pain and stress (20–22). Studies have recently shown the therapeutic benefits of using cannabinoids as the drug of choice in treating mechanical allodynia and behavior disorders in horses (23, 24), and in reducing inflammatory response (22, 25). Given the physio-pathological role and cellular distribution of the ECS across species, the authors hypothesized that cannabinoid receptors are also expressed within the inflammatory cells, fibroblasts and endothelial cells of the equine hoof laminae, going beyond the epidermal cells. Thus, the aim of this study is to verify the protein expression of cannabinoid receptors at the inflammatory cells present at the hoof laminae during the course of laminitis.
2 Materials and methods
2.1 Type of study
Observational, non-blinded post-mortem analytical, cross-sectional study with a comparative design.
2.2 Animals
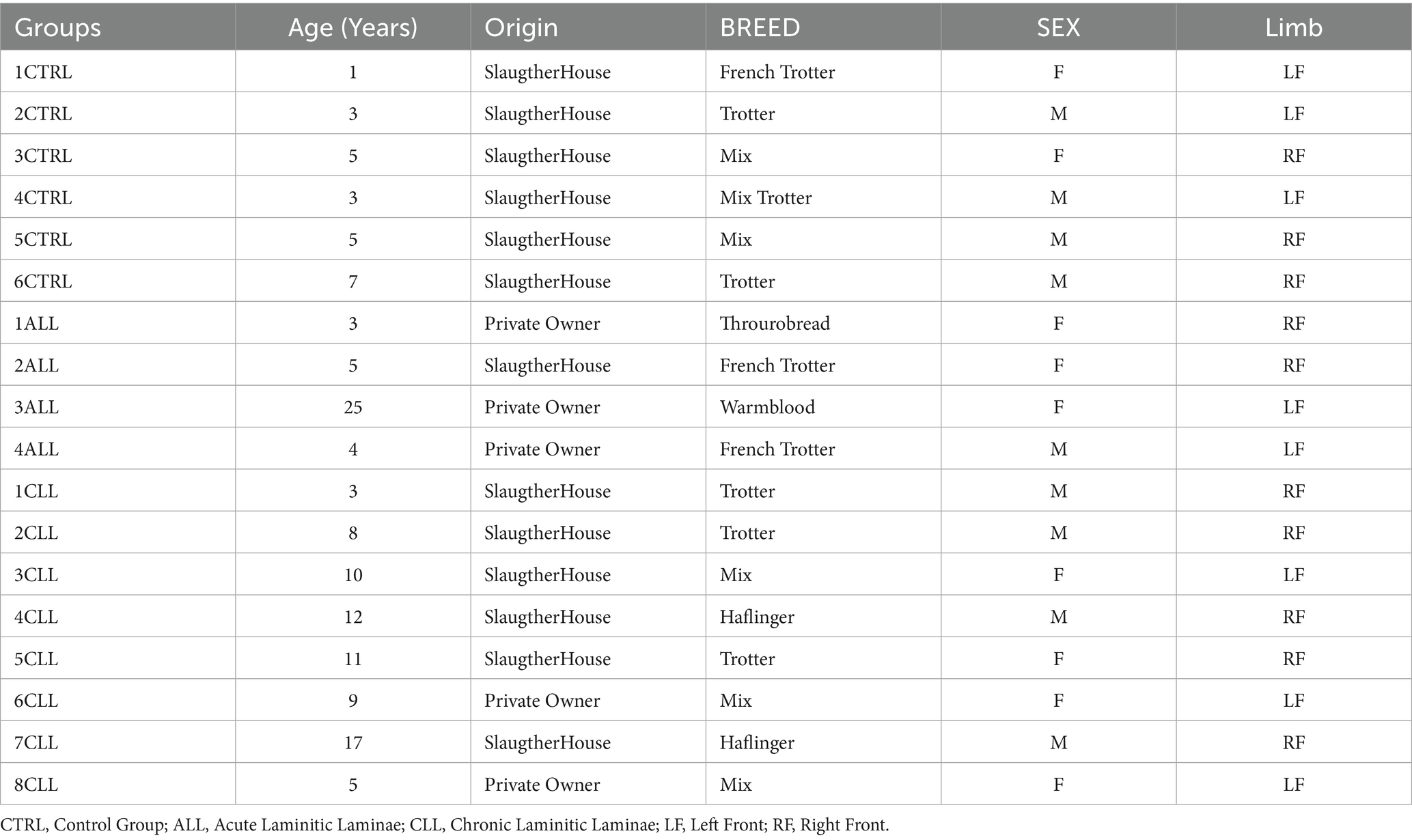
Laminar samples were collected post-mortem. Tissue collection occurred strictly from animals either euthanized for humane reasons (hospital patients – private owned) or slaughtered for human consumption (slaughterhouse). A total of 18 samples were collected from different animals. The characteristics of the horses are presented in Table 1.
Healthy laminae group (control) - The hooves were collected from the thoracic limbs of 6 healthy horses slaughtered for consumption, with ages ranging from 1 to 7 years. All the hooves collected were macroscopically intact.
Laminitic laminae group - The hooves were collected from the thoracic limbs of 12 laminitic horses, with ages ranging from 3 to 25 years. The horses were subdivided in two subgroups: the acute laminitic (ALL) horses (4/12), and the chronic laminitic (CLL) horses (8/12).
2.3 Ethical statement
According to Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 regarding the protection of animals used for scientific purposes, Italian legislation (D. Lgs. n. 26/2014) does not require any approval by competent authorities or ethics committees since this study did not influence any therapeutic decisions or induced any clinical outcomes. All samples were taken on demand after death. No disease was induced.
2.4 Group division
The samples were divided into three groups: Healthy Laminae (HL), Acute Laminitis (ALL), and Chronic Laminitis (CLL). Group division was based on a combination of clinical history, anamnesis, physical examination, and evidence of anatomical failure of the hoof (e.g., sinking of the third phalanx, rotation with severe angle, or excessive heel growth), as described (17).
Acute laminitis was defined as a clinical presentation occurring within a maximum of 72 h from the onset of clinical signs, with no prior history of laminitis and no evidence of structural failure (1, 26). Chronic laminitis was defined as a condition persisting for more than 72 h after the onset of primary clinical signs, with structural failure and/or a history of recurrent laminitic episodes (1, 27).
All cases were confirmed by post-mortem histopathological analysis carried out by a specialized pathologist, which included findings such as basement membrane separation, marked irregular hyperplasia of epidermal lamellae, distorted secondary lamellae, and inflammatory cell infiltration, as previously described (28). Detailed inclusion and exclusion criteria for each group are presented in Table 2.
2.5 Sample collection and tissue processing
The digits were disarticulated at the metacarpophalangeal joint. For each subject one limb was collected and the same limb was used for the experiments. Lamellar tissue, including epidermal and dermal lamellae was obtained by sectioning the hooves with a band saw according to the stablished protocol (Supplementary Figure 1) (17, 29, 30). All the tissues were collected a maximum of 4 h after death. For microscopic evaluation the tissue was then fixed and processed to obtain cryosection as described (17). The lamellar tissues were fixed for 48 h at 4 °C in 4% paraformaldehyde in phosphate buffer (0.1 M, pH 7.2). Tissues were subsequently rinsed overnight in phosphate-buffered saline (PBS; 0.15 M NaCl in 0.01 M sodium phosphate buffer, pH 7.2) and stored at 4 °C in PBS containing 30% sucrose and sodium azide (0.1%). The following day, the tissues were transferred to a mixture of PBS − 30% sucrose–azide and Optimal Cutting Temperature (OCT) compound (Sakura Finetek Europe, Alphen aan den Rijn, The Netherlands) at a ratio of 1:1 for an additional 24 h before being embedded in 100% OCT in Cryomold® (Sakura Finetek Europe). The sections were prepared by freezing the tissues in isopentane cooled in liquid nitrogen. Cryosections (14 μm thick) of lamellar tissues were cut on a cryostat and mounted on polilysinated slides, for each horse 5 non-consecutive slides (n = 5) were made for each co-localisation. Cryosections were oriented transverse to the major axis of the horse finger and included the primary (PEL) and secondary epidermal laminae (SEL), a portion of the innermost layer of the hoof capsule, and a large portion of the sub-lamellar dermal tissue.
In addition to the sampling for microscopic evaluation, aliquots of fixed and non-fixed tissue sample (frozen with liquid nitrogen) were harvested for gene expression (performed by RT-PCR) and western blotting data, respectively.
2.6 Analysis of gene expression by real-time polymerase chain reaction
For gene expression analysis to validate the receptor expression study, total RNA extraction was carried out using a RNeasy FFPE Kit (Qiagen Hilden, Germany) with a few modifications, as described by Rodrigo Zamith Cunha (17). Randomization was made with Artificial Intelligence (Chat GTP-4). After spectrophotometric quantification, the total RNA (1,000 ng) was reverse transcribed to cDNA using 5X iScript RT Supermix (Bio-Rad Laboratories Inc., Hercules, CA, United States) at a final volume of 20 μL. To evaluate the gene expression profiles, RT-PCR was carried out in a CFX96 thermal cycler (Bio-Rad Laboratories Inc.) using SYBR green detection to target the genes. RT-PCR was performed by using equine specific (15, 17) to evaluate the gene expression for Cannabinoid receptors 1 and 2 (Cn1r and Cn2r), and G protein-coupled receptor 55 (GPR55). Regarding the reference genes, GAPDH (Glyceraldehyde-3-phosphate dehydrogenase), HPRT (Hypoxanthine phosphoribosyltransferase 1) and β-Act (beta Actin) were based on horse sequences as previously reported (31). All the amplification reactions were carried out in 20 μL as previously described (17). The specificity of the amplified PCR products was confirmed by agarose gel electrophoresis and melting curve analysis. The relative expression of the genes of interest (IGs) was normalized based on the geometric mean of the three reference genes (RGs). The relative mRNA expression of the genes tested was evaluated using the ΔCt method with ΔCt = (Ct geometric mean ref. genes – Ct interested gene) which directly correlated with the expression level (ΔCt values very negative, lower expression; ΔCt values less negative higher expression) (Figure 1).
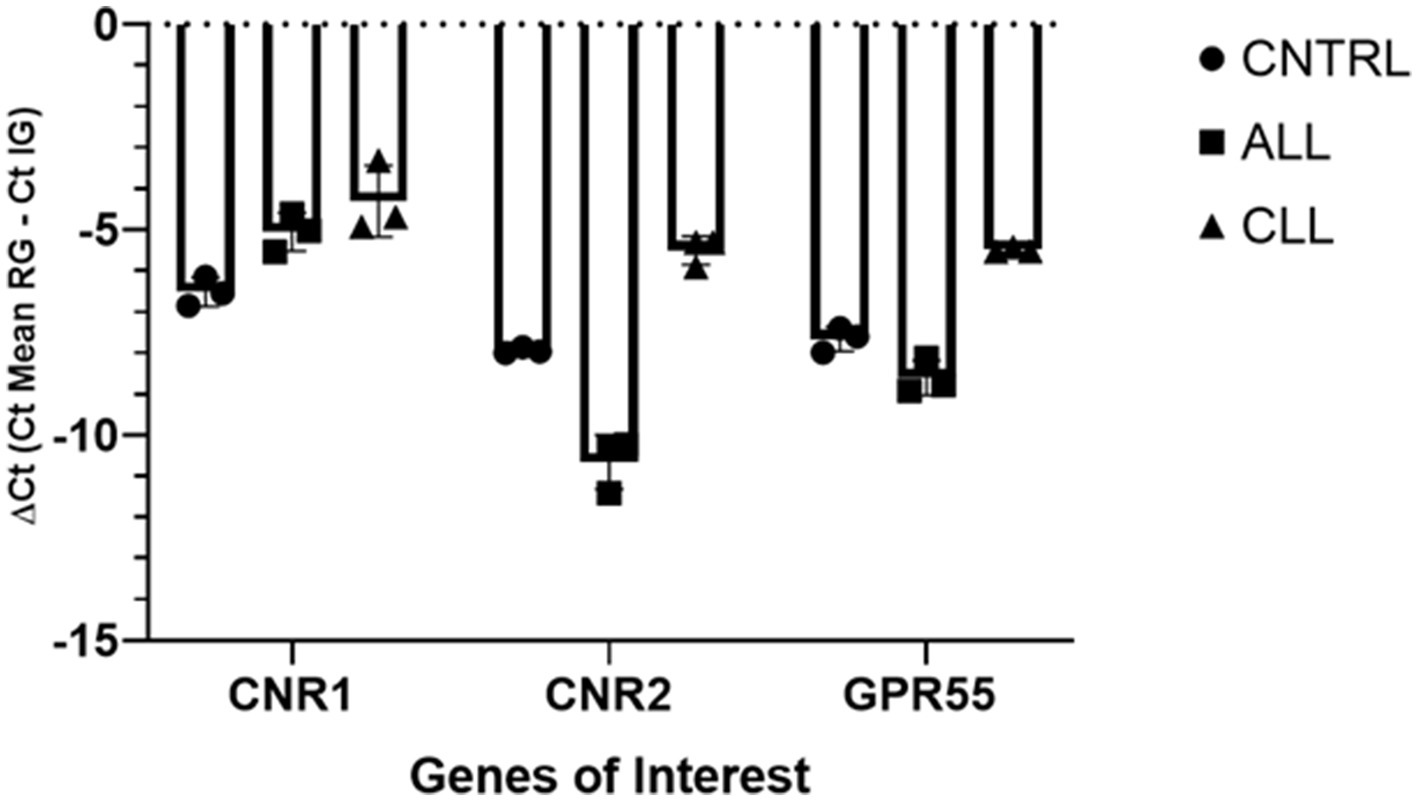
Figure 1. Gene expression of Cnr1, Cnr2, GPR55, in equine laminitic laminae in Healthy Laminae (CNTRL, n = 1), Acute Laminitis (ALL, n = 1), and Chronic Laminitis (CLL, n = 1). Each analysis was done in three independent experiments. The results are presented as ΔCt = (Ct Mean RG – Ct IG). For each gene, mean ± SD are indicated by horizontal bars.
2.7 Western blotting
Tissue collection and WB procedure were conducted following Galiazzo et al. (32) and Chiocchetti et al. (33). Briefly: lamellar sample was collected, frozen in liquid nitrogen and stored at −80 °C until sample processing. Following, 100 mg of tissue was homogenized in 1 mL of SDS buffer (Tris–HCl, 62.5 mM; pH 6.8; SDS, 2%; and glycerol, 20%) and supplemented with a protease inhibitor cocktail (Sigma-Aldrich, Co, St. Louis, MO, USA). Total protein content was determined by Peterson’s Modification of Lowry Method using a Protein Assay Kit. An amount of 20 μg of total proteins were separated with NuPage4–12% bis-Tris Gel (Life Technologies Ltd., Paisley, United Kingdom) for 30 min at 200 V and then using electrophoresis transferred onto a nitrocellulose membrane with a semi-dry system (Trans Turbo Blot Bio -Rad). Non-specific binding on nitrocellulose membranes was blocked with 5% milk powder in PBS-T20 (Phosphate Buffer Saline-0.1% Tween-20) for 1 h at room temperature. After blocking treatment, the membrane was incubated overnight at 4 °C first with the primary antibodies (GPR55) 1:500 diluted in PBS with 1% of milk, and then with an appropriate dilution of biotinylated secondary antibody. Immunoreactive bands were visualized using chemiluminescent substrate (Clarity Western ECL Substrate Bio Rad), according to the manufacturer’s instructions. The intensity of the luminescent signal was acquired by Chemidoc Instrument (Bio Rad) and the apparent molecular weight of the resultant bands was analyzed by Quantity One Software (Bio-Rad).
Western blot analysis of GPR55 revealed a stronger band of ~ 40 kDa (Figure 2).

Figure 2. Representative image of Western Blot analysis showing the specificity of anti GPR55 antibody. Lane 1, 20 ug of CNTRL laminae, lane 2, 20 ug of CNTRL laminae, lane 3, Molecular weight markers (kDa).
The primary antibody anti-CB1R (ab23703) utilized in current study had been validated and tested on horse tissue using WB analysis (13).
The rabbit anti-CB2R antibody (PA1-744) utilized in the current study had already been tested using WB analysis on horse tissue (34).
2.8 Immunohistochemistry on cryosections
Cryosections were hydrated in PBS. Endogenous peroxidase was blocked by immersion in 3% H2O2 in methanol for 30 min at room temperature (RT) (22–25 °C) and were then rehydrated. The blocking of non-specific antigenic sites was achieved by incubating the slides in Blocking solution (PBS with 3%of bovine serum albumin (Sigma Aldrich, Milan, Italy, Europe) and 0.25% Tween20 (Sigma Aldrich, Milan, Italy, Europe)) for 30 min at RT and then incubated overnight in a humid chamber at 4 °C with the primary antibodies diluted in blocking solution at the dilutions as described in Table 3. The slides were rinsed in TRIS buffer and subsequently incubated with a secondary biotinylated antibody (Vector Laboratories, Burlingame, CA, United States) diluted 1:200 in blocking solution. Following two additional washes in TRIS buffer, immunoreactivity was revealed using the avidin-biotin immunoperoxidase method (Vectastain Elite ABC Kit, Vector Laboratories, Burlingame, CA, United States) and visualized with the chromogen 3,3′-diaminobenzidine (0.05% w/v, cat# ACB999, Histo-Line Laboratories, Pantigliate, MI, Italy). The slides were then counterstained with Harris hematoxylin (cat# 01HEMH2; Histo-Line Laboratories) and permanently mounted using DPX medium (Fluka, Riedel-de Haen, Germany). Image acquisition was performed with an optical microscope (Eclipse E600; Nikon, Shinjuku, Japan) equipped with a USB 3.0 camera from the Imaging Source “33” Series (cat# DFK 33UX264; Bremen, Germany).
2.9 Immunofluorescence on cryosections
The cryosections from the 18 horses were hydrated in PBS and processed for immunostaining. To block non-specific bindings, the sections were incubated in a solution containing 20% normal donkey serum (Colorado Serum Co., Denver, CO, USA), 0.5% Triton X- 100 (Sigma Aldrich, Milan, Italy, and Europe) and bovine serum albumin (1%) in PBS for 1 h at room temperature (RT) (22–25 °C). The cryosections were incubated in a humid chamber overnight at RT with the antibodies directed against CB1R, CB2R and GPR55 (single immunostaining) or with a cocktail of primary antibodies (double immunostaining) (Table 3) diluted in 1.8% NaCl in 0.01 M PBS containing 0.1% sodium azide. After washing in PBS (3 × 10 min), the sections were incubated for 1 h at RT in a humid chamber with the secondary antibodies (Table 4) diluted in PBS. Laboratory procedures followed as described (16, 35). To identify macrophages, the anti-ionized calcium binding adapter molecule 1 (IBA1) antibody was employed. In addition, the antibody anti-MAC387 (calprotectin) identified neutrophils (36–38) To identify the fibroblasts, the antibody anti-vimentin (Clone V9) was used (39). To identify the lymphocytes T, the antibody anti-CD3 was used. To identify afferent peptidergic nervous fibbers, the antibody anti-substance P (SP) was used.
The preparations were examined using a Nikon Eclipse Ni microscope equipped with the appropriate filter cubes, and the images were recorded using a DS-Qi1Nc digital camera and NIS Elements software BR 4.20.01 (Nikon Instruments Europe BV, Amsterdam, The Netherlands, Europe). Slight brightness and contrast adjustments were made using Corel Photo Paint, whereas the figure panels were prepared using (Corel Draw Mountain View, Ottawa, ON, Canada).
2.10 Specificity of the primary antibodies
The specificity of the primary antibody anti-CB1R (ab23703) utilized in current study had been validated and tested on horse tissue using WB analysis (13).
The rabbit anti-CB2R antibody (PA1-744) utilized in the current study had already been tested using WB analysis on horse tissue (34). In this study, another anti-CB2R antibody, raised in mouse serum (sc-293188), was also used, whose specificity has not been tested on horse tissue; however, both the mouse and rabbit anti-CB2R antibodies were tested in a double-staining protocol and were co-localized in horse tissue (Supplementary Figure 2).
The rabbit anti-GPR55 antibody (NB110-55498) utilized in the current study has already recently been used on horse tissue (15). The immunogen used to obtain the anti-GPR55 antibody was a synthetic 20 amino acid peptide from the third cytoplasmic domain of Human GPR55 in amino acids 200–250. The homology between the full amino acid sequences of the horse and human GPR55 was 80%, and the correspondence with the specific sequence of the immunogen was 78%.1 The specificity of this antibody was tested in the present study.
The mouse anti-calprotectin (CAL) (clone MAC387), a complex of the mammalian proteins S100A8 and S100A9 (S100A8/A9), was recently shown to only label neutrophils on canine skin (40). The observation of Dapi-labeled multilobed nuclei confirmed that the CAL immunoreactive cells in the lamellar dermis of the horse were neutrophils.
The goat anti-IBA1 antibody, recently used on horse tissue (14, 15) was directed against a peptide having the sequence C-TGPPAKKAISELP, from the C Terminus of the porcine IBA1 sequence. Horse and porcine IBA1 molecules share 92.3% identity (see text footnote 1, respectively). It has been already used to identify macrophage and macrophage-like cells in horse tissue (15).
The rat anti-CD3 antibody was used to label the T lymphocytes in horses. CD3-12 is a rat-derived monoclonal antibody that recognizes the human CD3ε protein, a component of the T-cell receptor (TCR) complex essential for T-cell activation and development. Specific staining of equine T cells in paraffin-embedded sections has been demonstrated (41). The UniProt entry for the CD3 epsilon (CD3ε) chain, the target of the CD3-12 antibody, is P07766; the target for equine gene is A0A9L0RAV5_HORSE. Equine and Human CDε molecules shares 64.7% similarity (see text footnote 1, respectively). Staining with an antibody against the same protein has been demonstrated in horses (42).
The specificity of the rat anti-SP antibody was previously tested on horse tissue (43).
The mouse anti-vimentin (Clone V9) antibody has already been used to label fibroblasts in the horse skin (16, 44).
2.11 Specificity of the secondary antibody
The specificity of the secondary antibodies was tested by applying them to the sections after the omission of the primary antibodies (17). No stained cells were detected after omitting the primary antibodies.
2.12 Immunofluorescence and immunohistochemistry qualitative and semi-quantitative analysis
The immunoreactivity of the antibodies was evaluated, and their cellular localization (cytoplasmic, membranous, and nuclear) was reported. Five different slides were evaluated for each horse on which at least three random areas were evaluated. The intensity of expression was assessed semi-quantitatively in images acquired with consistent exposure times, categorized as faint, moderate, or bright. The proportions of cells immunoreactive for markers of macrophages/DCs (IBA1), T-cells (CD3), and neutrophils (calprotectin/MAC387), which were also immunoreactive for one of the cannabinoid receptors (CB1R, CB2R, GPR55), were determined by examining fluorescently labeled, double-stained preparations. A total of thirty (n = 30) cells from each animal were counted for each inflammatory cell marker. The percentage of immunoreactive cells for each specific marker (IBA1, CD3, CAL), and immunoreactive for a cannabinoid receptor (CB1R, CB2R, GPR55) was calculated and expressed as mean ± standard deviation. The data analysis was carried out in duplicate by two different observers.
2.13 Statistical analysis
The number of inflammatory cells immunoreactive for each specific marker (IBA1, CD3, CAL), and immunoreactive for a cannabinoid receptor (CB1R, CB2R, GPR55) was calculated and expressed as a percentage. Data are presented as mean ± standard deviation (SD) for normally distributed variables and as median with interquartile range (IQR) for non-normally distributed variables. The normality of the data was assessed using both the Shapiro–Wilk and the Kolmogorov–Smirnov tests. The normal data were analyzed using an ordinary one-way analysis of variance (ANOVA) followed by the Tukey’s Multiple Comparison Test; The non-normal data were analyzed using the non-parametric Kruskal-Wallis’s test followed by the Dunn’s Multiple Comparison Test, with a 95% CI and a significance level of p < 0.05. Data analysis was carried out in duplicate by two different researchers. The statistical analyses were carried out using GraphPad Prism software.
3 Results
3.1 Immunohistochemistry on cryosections
Cannabinoid Receptor 1 - Moderate CB1R immunoreactivity (CB1R-IR) was observed in the cytoplasm of the endothelial cells of the blood capillaries in health and in laminitic primary dermal lamellae (PDL) and secondary dermal lamellae (SDL). Vascular smooth muscle cells also showed occasional faint positivity for CB1R (Figure 3). Inflammatory cells were negative for CB1R.
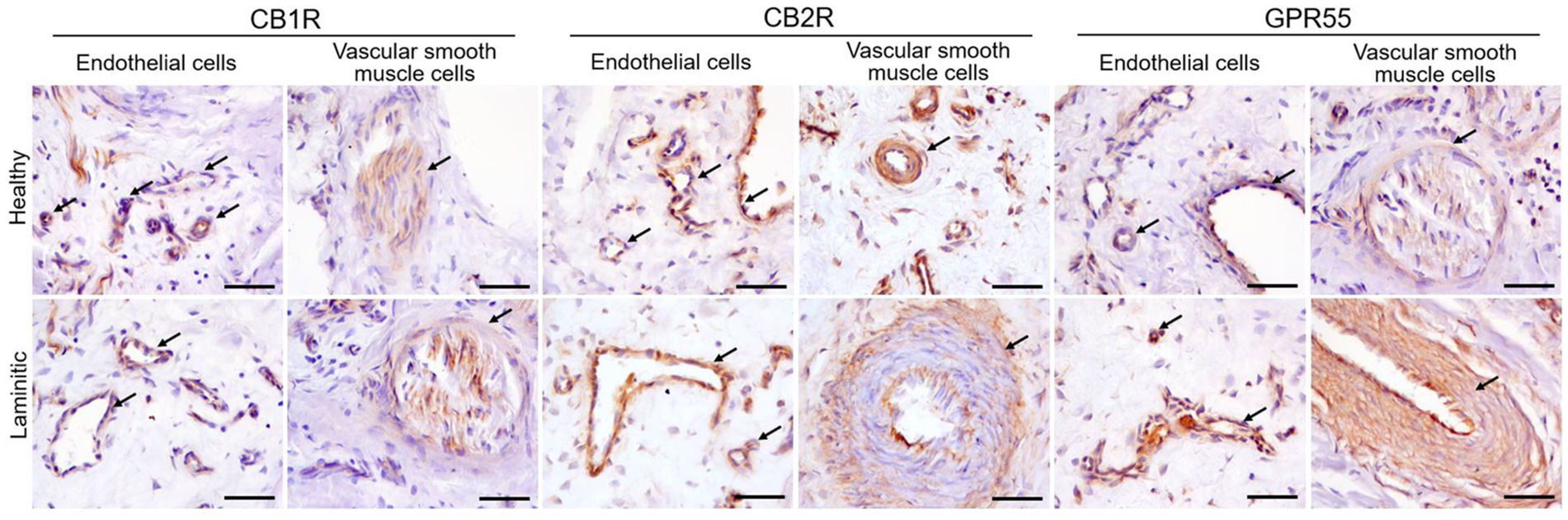
Figure 3. Representative immunohistochemical images on cryosections of HL and LL for anti-CB1R, -CB2R and -GPR55 antibodies. The arrows indicate endothelial cells and vascular smooth muscle cells positive for each respective cannabinoid receptor. Scale bar 50 μm.
Cannabinoid Receptor 2 - In the HL group, the cytoplasm of the endothelial cells of the PDL and the SDL blood vessels showed moderate expression of CB2R-IR; in the LL it showed bright expression. The cytoplasm of the vascular muscle cells showed moderate expression of CB2R (Figure 3). Cannabinoid receptor 2 was strongly expressed in the inflammatory cells present in the LL, whereas its expression was moderate in the inflammatory cells of the HL group. (Figure 4).

Figure 4. Representative immunohistochemical images of cryosections from HL and LL, stained with anti-CB1R, -CB2R and -GPR55 antibodies. The arrows highlight the inflammatory cells which exhibit the expression of CB2R and GPR55, but not of CB1R. Scale bar 50 μm.
G-Protein Coupled Receptor 55 - A moderate GPR55-IR was observed in the cytoplasm of the blood capillary endothelial and vascular smooth muscle cells in the healthy PDL and the SDL. In contrast, in the same cells of laminitic PDL and SDL, the expression of GPR55 was brighter (Figure 3); GPR55 was strongly expressed in the inflammatory cells of both the HL and LL groups (Figure 4).
3.2 Immunofluorescence on cryosections
3.2.1 Cannabinoid receptor 1
HL group - There was no CB1R immunolabeling on the inflammatory cells, fibroblasts or endothelial cells in the HL group. Nerve fibers of the dermis co-expressed CB1R- and SP-IR in all the horses (100%) (16/16) (Figure 5).

Figure 5. Photomicrographs of the laminar junction showing cannabinoid receptor 1 (CB1R) and Substance P (SP) immunoreactivity in the nerve fibers of the equine hoof. (a–d) the open arrows indicate the co-localization between CB1R- and SP-IR at the nerve fibers of the neurovascular plexus of the laminar junction. Scale bar: 50 μm.
LL group – The endothelial cells and fibroblasts of 25% (1/4) of the horses of the ALL group and 38% (3/8) of the CLL group expressed faint to moderate CB1R-IR.
3.2.2 Cannabinoid receptor 2
HL group - In the HL group, 100% of the horses (4/4) showed fibroblasts, vimentin-positive cells and vascular endothelial cells expressing moderate-to-bright cytoplasmatic CB2R-IR (Figure 6). Moderate CB2R-IR was also expressed by the cytoplasm of the macrophages/DCs and T-cells (Figure 7) present inside the blood vessels and around the neurovascular plexus within the deep dermis. The percentage of CD3 and IBA1 cells positive for CB2R were 52 ± 14.7% and 63 ± 7.3%, respectively (Figure 8).

Figure 6. Photomicrographs of the laminar junction of the equine hoof showing the co-localization between the anti-cannabinoid receptor type 2 (CB2R) antibody and the anti-vimentin antibody which is a marker for fibroblasts (a–h). (a–d) The epithelial cells of the secondary epidermal lamellae (SEL) showed bright CB2R immunoreactivity; the white arrows indicate the fibroblasts in the secondary dermal lamellae (SDL) showing bright vimentin immunoreactivity. The fibroblasts were also abundant in the primary dermal laminae (PDL). (e–h) The white arrows indicate vascular endothelial cells positive for CB2R; the open arrows indicate fibroblasts co-expressing vimentin and CB2R immunoreactivity. Bar: 50 μm.

Figure 7. Photomicrographs of the laminar junction of the equine hoof showing the co-localization between the anti-cannabinoid receptor type 2 (CB2R) antibody and the anti-IBA1 antibodies (marker for macrophages/dendritic cells) (a–d) and anti-CD3 (marker for T cells) (e-h). The open arrows indicate the Dapi-labeled nuclei of CB2R immunoreactive cells which were also IBA1 positive (a–d) and CD3 positive (e–h). Bar: 50 μm.

Figure 8. The graph illustrates the expression of cannabinoid receptors CB2 and GPR55 in different inflammatory cell markers (CD3, IBA1 and MAC387) across three groups: healthy laminae (CTRL), acute laminitis (ACUTE) and chronic laminitis (CHRONIC). The y-axis represents the percentage of cells expressing the respective cannabinoid receptors. Error bars indicate the standard error deviation and mean.
LL group - In the ALL and CLL groups, the macrophages/DCs and T cells present in the inflammatory infiltrate and inside the blood capillaries of the dermis/PEL/SEL were brightly labeled for CB2R. In addition, the endothelial cells of the blood vessels and the smooth muscle cells of the arteries showed bright CB2R-IR. In the ALL horses, the dermal fibroblasts showed bright CB2R-IR, whereas, in the CLL group, 50% of the horses (4/8) showed moderate to bright CB2R-IR, and 50% of the horses (4/8) showed faint CB2R-IR. The mean percentage and standard deviation of CD3 and IBA1 positive cells for CB2R in the ALL group were 90 ± 8.1% and 89 ± 11.3%, respectively (Figure 8). For the CLL group, the mean percentage and standard deviation of CD3 and IBA1 cells positive for CB2R were 69 ± 19.5% and 83 ± 11.5%, respectively.
There was no immunolabeling of CB2R by neutrophils.
3.2.3 G-protein coupled receptor 55
HL group - In the HL group, dermal cells showed cytoplasmatic GPR55-IR (Figure 9). The cytoplasm of vimentin positive fibroblasts and endothelial cells of blood vessels showed bright GPR55R-IR. The cytoplasm of macrophages/DCs, T-cells, and non-identified immune cells present inside blood vessels and around the neurovascular plexus within the deep dermis showed moderate to bright GPR55-IR (Figure 10). The percentage of CD3 and IBA1 positive cells which co-expressed GPR55-IR were 75 ± 8.3% and 66 ± 5.6%, respectively (Figure 8). Rare MAC387 positive cells (neutrophils) were observed in the HL group; it was not possible to count 30 cells. In total there were seven (7) neutrophils, 22 ± 8.7% positive for GPR55, all located inside the blood vessels of the deep dermis.

Figure 9. Photomicrographs of the laminar junction of the equine hoof. (a–f) Immunolabeling of the anti-G-Protein Coupled Receptor 55 (GPR55) antibody. The white arrows indicate the inflammatory cells around the blood vessels (BVs), while the open arrows indicate the endothelial cells positive for GPR55-IR. Bar: 50 μm.

Figure 10. Photomicrographs of the laminar junction of the equine hoof. (a–d) Co-localization between the antibodies against the G-Protein Coupled Receptor 55 (GPR55) and macrophage marker IBA1 (white arrows) around a blood vessel (BV). (e–h) Co-localization between the cannabinoid-related receptor GPR55 and the antibody against T cells (CD3); the white arrow indicates the T cells positive for GPR55 around the blood vessels. Bar: 50 μm.
LL group - In the ALL group, the fibroblasts and the endothelial cells showed bright cytoplasmatic GPR55-IR. In the CLL group, the endothelial cells showed bright cytoplasmatic GPR55-IR; 63% (5/8) of the fibroblasts showed faint GPR55-IR, and 38% (3/8) showed moderate to bright GPR55-IR. In both groups, bright cytoplasmatic GPR55-IR was expressed by the macrophages/DCs, neutrophils, T-cells and non-identified immune cells of the inflammatory infiltrate and inside the blood capillaries of the dermis/PEL/ SEL. In the ALL group, the percentage of CD3, IBA1 and MAC387 positive cells which co-expressed GPR55-IR were 96 ± 6.3%, 89 ± 9.9%, and 98 ± 3.1%, respectively (Figure 8). In the CLL group, the mean percentage of CD3, IBA1, and MAC387 positive cells co-expressing GPR55 were 78 ± 18.6%, 82 ± 10.8% and 70 ± 17.3%, respectively (Figure 8).
3.3 Statistical analysis
Cannabinoid Receptor 1 – no analysis was carried out since none inflammatory cell was staining with CB1R antibody.
Cannabinoid Receptor 2 - The statistical analysis of the T cells positive for CB2R revealed a significant difference in T cell expression between the control and the acute laminitis groups (p = 0.0109). Comparisons between the control and the chronic groups (p = 0.4658), and between the acute and the chronic groups (p = 0.1579) were not statistically significant. Comparisons of the macrophages positive for CB2R revealed significant differences between the control and the acute laminitis groups (p = 0.0096), and between the control and the chronic laminitis groups (p = 0.0183).
G-Protein Coupled Receptor 55 - The statistical analysis of the T cells positive for GPR55 revealed no significant differences between the control and the acute laminitis groups (p = 0.1453), the control and the chronic laminitis groups (p = 0.9429) or the acute and the chronic laminitis groups (p = 0.1487). The statistical analysis of neutrophils positive for GPR55 revealed significant differences across all comparisons. There was a highly significant increase in GPR55 expression in the neutrophils between the control and the acute laminitis groups (p < 0.0001) as well as between the control and the chronic laminitis groups (p = 0.0002). In addition, a smaller but still significant difference was observed between the acute and the chronic laminitis groups (p = 0.0122). The statistical analysis of the macrophages positive for GPR55 revealed significant differences between the control and the acute laminitis groups (p = 0.0120), and between the control and the chronic laminitis groups (p = 0.0472); the difference between the acute and the chronic laminitis groups was not statistically significant (p = 0.4378).
4 Discussion
4.1 Laminae endocannabinoid system
The present protein expression confirmed by immunofluorescence and the presence of the gene for each protein evidenced by quantitative RT-PCR, provides confirmation of the distribution of novel pharmaceutical targets in the lamellar tissue. These findings are compatible with previous studies that showed cannabinoids receptors in the skin of horses (34), and in the epidermal cells of the hoof laminae (17). The G-protein coupled receptors of the ECS (type 1, type 2 and 55) are widespread in a compartmentalized way within the laminae. Its presence in different cell types and fluctuation across different disease stages suggests a pathophysiological role.
The Western Blot analysis of GPR55 revealed a major band of ~40 kDa, compatible with previous studies in other species (32, 45). Other faded bands were also noticed at ~20 kDa and ~55 kDa, this may be due to the existence of different heterodimers of this receptor. The G Proteins are made of 3 different sub-units: γ, α and β (46). The subunits α have a molecular weight between 40 to 45 kDa, while the subunits γ and β can have a molecular weight of ~37 to ~8 kDa (47). The receptor will change its structure depending on the signaling and binding. With the present result the antibody used is effective and specific for equine samples.
Accordingly, Kupczyk (34) found that for whole skin CBR1 has a molecular weight of ~50 kDa and CBR2 presented with two bands at ~70 kDa and ~40 kDa. After cell isolation, different bands were seeing. In keratinocytes CBR1 presented 3 bands, at ~50 kDa, ~55 kDa and ~33 kDa, while fibroblasts showed marked ~50 kDa and ~33 kDa bands; CBR2 presented with strong bands at ~70 kDa and ~40 kDa. These results holds in evidence the different subunits of the G-protein coupled receptors and align with the findings of the present study.
4.2 Endocannabinoid system role in inflammatory cells
Proteins of the ECS have being described in different species and its role on inflammatory regulation has been pointed (15, 16, 48, 49). Within the dermo-epidermal junction of the horse hoof, the macrophages, DCs, T cells, unidentified immune cells and blood capillaries strongly expressed CB2R and GPR55, while the neutrophils exclusively expressed GPR55. Previous studies have reported similar results in other species (36, 37).
The non-affected laminae analyzed in this study showed immunocytes inside the blood capillaries of the dermis (macrophages, lymphocytes and non-identified immune cells) strongly immunolabeled for CB2R and GPR55. Compatible with already described resident pool of lymphocytes and macrophages in the healthy laminae, which indicates that these leucocytes may play an important role in the early onset of laminar dysfunction and injury in laminitis (7, 50).
The macrophages present in the laminitic tissue in both the healthy and the laminitic hooves showed strong labeling for CB2R and GPR55, while the neutrophils present mostly in the laminitic horses were positive only for GPR55. Macrophages and fibroblasts produce IL-1β which is a pro-inflammatory cytokine and increases the expression of other inflammatory mediators including cicloossigenasi 2 (COX-2), IL-6, and matrix metalloproteinases, which are associated with the degradation and remodeling of tissue (51). In the present study, not only macrophages as also fibroblast were shown to be positive to CB2R and GPR55 at the normal and affected dermo-epidermal junction. A significantly increased migration of CD3 positive cells (T cells) into the lamellar junction was reported during the development and chronicity of laminitis (8), the present results showed strong CB2R and GPR55R immunolabeling in the same cells.
The significant differences observed in CB2R and GPR55 expressions in the neutrophils and macrophages during laminitis progression provide evidence that the ECS is actively involved in immune modulation. The higher number of neutrophils positive for GPR55 during the acute phase of the disease aligns with their well-established role (7, 52) in the early inflammatory response, reinforcing the fact that ECS components are engaged in immune cell recruitment and activation. As the disease progresses to a chronic stage, the reduction in neutrophil GPR55 expression and presence and the sustained increase in the macrophages showing CB2R- and GPR55-IR suggest a shift from an acute neutrophil-dominated response to macrophage-driven inflammatory regulation. Similarly, the significant increase in CB2R-positive T cells in acute laminitis suggests that T cells play an active role in modulating inflammation during the early phase of the disease. However, the lack of significant differences in GPR55 expressions in T cells across the different stages of disease indicates that its expressions do not depend on inflammatory stimuli. This dynamic receptor expression pattern indicates that the ECS plays a role in orchestrating immune cell behavior throughout different stages of laminitis. Furthermore, the persistent presence of CB2R- and GPR55-IR in both the resident immune cell pool and the inflammatory infiltrates highlights their role in immune regulation.
Supporting this hypothesis, a recent in-vitro study has shown that CBD at 4 μg/mL reduced the in vitro production of the inflammatory cytokines (TNF-α and IFN-γ) of the peripheral blood mononuclear cells from senior horses (53). Moreover, an in-vivo study involving senior horses pointed to a significant decrease in the inflammatory cytokine expression of IFN-γ for whole blood at day 60, and for IL-6 at days 60 and 90 for CBD-treated horses when compared to control horses (25), using an oil solution at a dose of 2 mg/kg orally for 90 days. Cannabinoids can actively suppress inflammation by downregulating the pro-inflammatory cytokines, such as IL-12, IL-6, IFN-γ, IL-8, IL-2, IL-1β, IL-15, and TNF-α (54), which are crucial pro-inflammatory molecules for the development of laminitis.
4.3 Endocannabinoid system role in fibroblasts
Evidence showed that the ECS can modulate the functioning of fibroblasts (55). Connective tissue and keratinocytes are known to remodel and continually upgrade their spatial organization by the tightly controlled production of a specific class of zinc-dependent enzymes MMPs that, when activated, degrade the extracellular matrix and basal membrane components (56). The major functions of MMPs are the remodeling and degradation of the extra-cellular matrix and cell membranes during various biological processes, such as cell migration and keratinocyte proliferation as well as angiogenesis (57).
In healthy equine hoof wall laminae, metalloproteinase −2 (MMP-2) and metalloproteinase-9 (MMP-9) had already been isolated (56), and they were shown to play key roles in the degradation of the BM, which consists mainly of type IV collagen. In human fibroblasts, it is known that MMP-9 and MMP-2 secreted from fibroblasts could play important roles in the tissue metabolism, including cytokine-induced inflammation (58). The regulatory effect of CBD on the level and activity of MMPs is known (59). The present finding of CB2R and GPR55 expression in fibroblasts is compatible with previous studies (34, 40). CB1R was expressed in the diseased group, although in both groups’ gene presence was validated. The activation of the CB1R leads to fibrogenesis, while the enhancement of the CB2R inhibits fibrosis progression (60).
4.4 Endocannabinoid system role in blood vessels
Reduced lamellar blood flow is a key event in the pathogenesis of laminitis, with ischemia and subsequent reperfusion resulting in lamellar damage. Parallelly, the same pathologic mechanisms which underlie the cardiovascular disease associated with human metabolic syndrome, including changes in insulin signaling, inflammatory cytokines, and vascular/endothelial dysfunction, could contribute to laminitis (8, 61, 62).
By means of CB2R pathways, endocannabinoid molecules, such as N-Oleoylethanolamine and 2-arachidonoyglycerol, can modulate the human endothelial – leukocyte interaction by reducing the release and production of adhesion molecules (63) and selectins (64), and CB2R may play a role in the vasodilatory function (65). Furthermore, GPR55 was shown to play a role in the influx of calcium in the endothelial cells (66); it plays a role in mechanical hyperalgesia associated with inflammation and neuropathic pain (67), and with cardiovascular function and heart failure (68).
In addition, CB1R inhibition leads to decreased vascular angiotensin II type 1 receptor (AT1R) expression, NADPH oxidase activity and ROS production in vitro and in vivo; this antioxidative effect is associated with improved endothelial function in apolipoprotein E-deficient (ApoE−/−) mice, indicating the beneficial direct vascular effects of CB1R inhibition (69).
4.5 Endocannabinoid System Role in nerve fibers
The present findings demonstrated the presence of CB1R in nerve fibers at the dermo-epidermal junction of the equine hoof. This aligns with the existing literature, showing that CB1R is localized in peptidergic nerve fibers (Substance P positive) which are involved in pain and inflammatory responses (13, 70, 71). Pre-clinical studies have shown the effective management of pain in horses using CBD and enhanced quality of life, with a dose ranging from 1 to 3 mg/kg (22, 25, 72). To the day, there are no studies which deal with controlling pain in horses with laminitis using cannabinoids as the drug of choice.
Despite our findings, several limitations must be acknowledged. The study included a relatively small and uneven number of samples across groups, which may limit the statistical power and generalizability of the results. Additionally, the absence of complete clinical histories for all animals, particularly those from abattoirs, hindered a more robust correlation between the etiology and severity of laminitis and the observed patterns of protein expression. The study design was not blinded, which could introduce potential observer bias during immunohistochemical analysis. Moreover, gene expression quantitative analysis was not performed for this study, nor did was investigate receptor functionality, intracellular signaling, or dynamic changes in protein expression over time. The heterogeneity in the underlying causes of laminitis could influence receptor distribution, and this was not stratified or analyzed separately in the current design.
5 Conclusion
The present findings highlight the presence of cannabinoid receptors CB1, CB2, and GPR55 in the inflammatory cells, fibroblasts and endothelial cells of healthy and pathological hoof lamellar epithelial tissue. The modulation of CB1R, CB2R, and GPR55 signaling pathways could offer novel therapeutic approaches for managing hoof diseases. Future research should focus on elucidating the precise mechanisms by which these receptors function within the hoof tissue, using larger cohorts of animals and examining correlations between cause, severity, chronicity and protein expression.
Data availability statement
The datasets generated and analyzed during the current study are available in the AlmaDL Institutional Repository, managed by the University of Bologna. Data can be accessed through the re3data-registered repository at https://doi.org/10.17616/R3P19R (ISSN: 2038-7954).
Ethics statement
Ethical approval was not required for the studies involving animals in accordance with the local legislation and institutional requirements because According to Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 regarding the protection of animals used for scientific purposes, Italian legislation (D. Lgs. n. 26/2014) does not require any approval by competent authorities or ethics committees since this study did not influence any therapeutic decisions or induced any clinical outcomes. All samples were taken on demand after death. No disease was induced. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
RZ: Funding acquisition, Supervision, Writing – original draft, Software, Formal analysis, Writing – review & editing, Resources, Investigation, Methodology, Data curation, Visualization, Project administration, Conceptualization, Validation. FG: Methodology, Investigation, Data curation, Writing – review & editing, Writing – original draft, Formal analysis. MM: Writing – review & editing, Methodology, Supervision. GS: Writing – review & editing, Validation. AZ: Methodology, Writing – review & editing, Investigation, Formal analysis, Data curation. CB: Investigation, Writing – review & editing, Formal analysis, Data curation. AG: Funding acquisition, Writing – review & editing. RC: Resources, Writing – review & editing, Funding acquisition, Formal analysis, Supervision, Data curation, Methodology, Investigation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research received a grant from NBF LANES (Milan, Italy), Nº164/0403/2023.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2025.1723160/full#supplementary-material
Supplementary Figure 1 | Sampling procedure scheme. 1: disarticulation of the front limbs; 2: 1st transactional cut with a band saw; 3: transversal view of the two pieces of the hoof after 1st cut; 4: 2nd and 3rd cuts with band saw, transversally to the axis of anatomical piece. 5: Photograph of the middle section after 2nd and 3rd cuts, full thickening sample of the laminae is dissected using bistoury. 6: laminae sample pre fixation.
Supplementary Figure 2 | Co-localization between the rabbit anti-CB2R and mouse anti-CB2R antibodies.
Footnotes
1. ^https://www.uniprot.org/, accessed on 7 January 2025
References
1. Pollitt, CC. Equine laminitis. Clin Tech Equine Pract. (2004) 3:34–44. doi: 10.1053/j.ctep.2004.07.003
2. McGowan, C. The role of insulin in endocrinopathic laminitis. J Equine Vet Sci. (2008) 28:603–7. doi: 10.1016/j.jevs.2008.08.004
3. Fontaine, GL, Belknap, JK, Allen, D, Moore, JN, and Kroll, DL. Expression of interleukin-1β in the digital laminae of horses in the prodromal stage of experimentally induced laminitis. Am J Vet Res. (2001) 62:714–20. doi: 10.2460/ajvr.2001.62.714
4. Colmer, SF, Adams, AA, Adam, E, Miller, R, Stefanovski, D, Kulp, JC, et al. The effect of pre-dosing with metformin on the insulin response to oral sugar in insulin-dysregulated horses. Equine Vet J. (2024) 56:318–25. doi: 10.1111/evj.13979
5. Godman, JD, Burns, TA, Kelly, CS, Watts, MR, Leise, BS, Schroeder, EL, et al. The effect of hypothermia on influx of leukocytes in the digital lamellae of horses with oligofructose-induced laminitis. Vet Immunol Immunopathol. (2016) 178:22–8. doi: 10.1016/j.vetimm.2016.05.013
6. Lunn, DP, and Hurley, DJ. The role of leukocyte biology in laminitis. Vet Immunol Immunopathol. (2009) 129:158–60. doi: 10.1016/j.vetimm.2008.11.014
7. Leise, B. The role of neutrophils in equine laminitis. Cell Tissue Res. (2018) 371:541–50. doi: 10.1007/s00441-018-2788-z
8. Black, SJ, Lunn, DP, Yin, C, Hwang, M, Lenz, SD, and Belknap, JK. Leukocyte emigration in the early stages of laminitis. Vet Immunol Immunopathol. (2006) 109:161–6. doi: 10.1016/j.vetimm.2005.08.017
9. Aragona, F, Rizzo, M, Giudice, E, Fazio, F, Costa, A, Di Bella, B, et al. Circadian oscillation of leukocyte subpopulations and inflammatory cytokines over a 24-H period in horses. Vet Sci. (2025) 12:386. doi: 10.3390/vetsci12040386
10. Kumar, D, Sharma, A, Taliyan, R, Urmera, MT, Herrera-Calderon, O, Heinbockel, T, et al. Orchestration of the circadian clock and its association with Alzheimer’s disease: role of endocannabinoid signaling. Ageing Res Rev. (2022) 73:101533. doi: 10.1016/j.arr.2021.101533
11. Murillo-Rodríguez, E, Budde, H, Veras, AB, Rocha, NB, Telles-Correia, D, Monteiro, D, et al. The endocannabinoid system may modulate sleep disorders in aging. Curr Neuropharmacol. (2020) 18:97–108. doi: 10.2174/1570159X17666190801155922
12. Sládek, M, Houdek, P, and Sumová, A. Circadian profiling reveals distinct regulation of endocannabinoid system in the rat plasma, liver and adrenal glands by light-dark and feeding cycles. Biochim Biophys Acta Mol Cell Biol Lipids. (2019) 1864:158533. doi: 10.1016/j.bbalip.2019.158533
13. Chiocchetti, R, Rinnovati, R, Tagliavia, C, Stanzani, A, Galiazzo, G, Giancola, F, et al. Localisation of cannabinoid and cannabinoid-related receptors in the equine dorsal root ganglia. Equine Vet J. (2021) 53:549–57. doi: 10.1111/evj.13305
14. Galiazzo, G, Tagliavia, C, Giancola, F, Rinnovati, R, Sadeghinezhad, J, Bombardi, C, et al. Localisation of cannabinoid and cannabinoid-related receptors in the horse ileum. J Equine Vet Sci. (2021) 104:103688. doi: 10.1016/j.jevs.2021.103688
15. Cunha, RZ, Zannoni, A, Salamanca, G, De Silva, M, Rinnovati, R, Gramenzi, A, et al. Expression of cannabinoid (CB1 and CB2) and cannabinoid-related receptors (TRPV1, GPR55, and PPARα) in the synovial membrane of the horse metacarpophalangeal joint. Front Vet Sci. (2023) 10:5030. doi: 10.3389/fvets.2023.1045030
16. Cunha, RZ, Semprini, A, Salamanca, G, Gobbo, F, Morini, M, Pickles, KJ, et al. Expression of cannabinoid receptors in the trigeminal ganglion of the horse. Int J Mol Sci. (2023) 24:949. doi: 10.3390/ijms242115949
17. Zamith Cunha, R, Gobbo, F, Morini, M, Zannoni, A, Mainardi, C, D’arpe, L, et al. Distribution of endocannabinoid system receptors in the equine hoof: dysregulation as a potential therapeutic target for laminitis. Histochem Cell Biol. (2025) 163:71. doi: 10.1007/s00418-025-02397-y
18. Di Salvo, A, Chiaradia, E, Sforna, M, and della Rocca, G. Endocannabinoid system and phytocannabinoids in the main species of veterinary interest: a comparative review. Vet Res Commun. (2024) 48:2915–41. doi: 10.1007/s11259-024-10509-7
19. Lowe, H, Toyang, N, Steele, B, Bryant, J, and Ngwa, W. The endocannabinoid system: a potential target for the treatment of various diseases. Int J Mol Sci. (2021) 22:9472. doi: 10.3390/ijms22179472
20. Cohen, L, Jones, T, Guay, K, Smith, WB, Nichols, J, and Elwonger, F. 62 evaluation of oral supplementation of cannabidiol (CBD) in horses. J Equine Vet Sci. (2021) 100:103525. doi: 10.1016/j.jevs.2021.103525
21. Eichler, F, Poźniak, B, Machnik, M, Schenk, I, Wingender, A, Baudisch, N, et al. Pharmacokinetic modelling of orally administered cannabidiol and implications for medication control in horses. Front Vet Sci. (2023) 10:551. doi: 10.3389/fvets.2023.1234551
22. Aragona, F, Tabbì, M, Gugliandolo, E, Giannetto, C, D’Angelo, F, Fazio, F, et al. Role of cannabidiolic acid or the combination of cannabigerol/cannabidiol in pain modulation and welfare improvement in horses with chronic osteoarthritis. Front Vet Sci. (2024) 11:473. doi: 10.3389/fvets.2024.1496473
23. Cunha, RZ, Felisardo, LL, Salamanca, G, Marchioni, GG, Neto, OI, and Chiocchetti, R. The use of cannabidiol as a novel treatment for oral stereotypic behaviour (crib-biting) in a horse. Vet Anim Sci. (2023) 19:100289. doi: 10.1016/j.vas.2023.100289
24. Ellis, KL, and Contino, EK. Treatment using cannabidiol in a horse with mechanical allodynia. Equine Vet Educ. (2021) 33:168. doi: 10.1111/eve.13168
25. Turner, S, Knych, HK, and Adams, AA. The effects of cannabidiol on immune function and health parameters in senior horses. Vet Immunol Immunopathol. (2023) 257:110549. doi: 10.1016/j.vetimm.2023.110549
26. Van Eps, AW. Acute laminitis: medical and supportive therapy. Vet Clin North Am Equine Pract. (2010) 26:103–14. doi: 10.1016/j.cveq.2009.12.011
27. Parks, A, and O’Grady, SE. Chronic laminitis: current treatment strategies. Vet Clin North Am Equine Pract. (2003) 19:393–416. doi: 10.1016/S0749-0739(03)00019-1
28. Kawasako, K, Higashi, T, Nakaji, Y, Komine, M, Hirayama, K, Matsuda, K, et al. Histologic evaluation of the diversity of epidermal laminae in hooves of horses without clinical signs of laminitis. Am J Vet Res. (2009) 70:186–93. doi: 10.2460/ajvr.70.2.186
29. Pollitt, CC. Basement membrane pathology: a feature of acute equine laminitis. Equine Vet J. (1996) 28:38–46. doi: 10.1111/j.2042-3306.1996.tb01588.x
30. Douglas, JE, and Thomason, JJ. Shape, orientation and spacing of the primary epidermal laminae in the hooves of neonatal and adult horses (Equus caballus). Cells Tissues Organs. (2000) 166:304–18. doi: 10.1159/000016744
31. Zannoni, A, Bombardi, C, Dondi, F, Morini, M, Forni, M, Chiocchetti, R, et al. Proteinase-activated receptor 2 expression in the intestinal tract of the horse. Res Vet Sci. (2014) 96:464–71. doi: 10.1016/j.rvsc.2014.03.006
32. Galiazzo, G, Giancola, F, Stanzani, A, Fracassi, F, Bernardini, C, Forni, M, et al. Localization of cannabinoid receptors CB1, CB2, GPR55, and PPARα in the canine gastrointestinal tract. Histochem Cell Biol. (2018) 150:187–205. doi: 10.1007/s00418-018-1684-7
33. Chiocchetti, R, Galiazzo, G, Tagliavia, C, Stanzani, A, Giancola, F, Menchetti, M, et al. Cellular distribution of canonical and putative cannabinoid receptors in canine cervical dorsal root ganglia. Front Vet Sci. (2019) 6:313. doi: 10.3389/fvets.2019.00313
34. Kupczyk, P, Rykala, M, Serek, P, Pawlak, A, Slowikowski, B, Holysz, M, et al. The cannabinoid receptors system in horses: tissue distribution and cellular identification in skin. J Vet Intern Med. (2022) 36:1508–24. doi: 10.1111/jvim.16467
35. Bombardi, C, Salamanca, G, Tagliavia, C, Grandis, A, Zamith Cunha, R, Gramenzi, A, et al. Cannabinoid receptors in the horse lateral nucleus of the amygdala: a potential target for ameliorating pain perception, stress and anxiety in horses. Int J Mol Sci. (2025) 26:7613. doi: 10.3390/ijms26157613
36. Chiocchetti, R, Salamanca, G, De Silva, M, Gobbo, F, Aspidi, F, Cunha, RZ, et al. Cannabinoid receptors in the inflammatory cells of canine atopic dermatitis. Front Vet Sci. (2022) 9:132. doi: 10.3389/fvets.2022.987132
37. Polidoro, G, Galiazzo, G, Giancola, F, Papadimitriou, S, Kouki, M, Sabattini, S, et al. Expression of cannabinoid and cannabinoid-related receptors in the oral mucosa of healthy cats and cats with chronic gingivostomatitis. J Feline Med Surg. (2021) 23:679–91. doi: 10.1177/1098612X20970510
38. Galiazzo, G, De Silva, M, Giancola, F, Rinnovati, R, Peli, A, and Chiocchetti, R. Cellular distribution of cannabinoid-related receptors TRPV1, PPAR-gamma, GPR55 and GPR3 in the equine cervical dorsal root ganglia. Equine Vet J. (2022) 54:788–98. doi: 10.1111/evj.13499
39. Zamith Cunha, R, Salamanca, G, Mille, F, Delprete, C, Franciosi, C, Piva, G, et al. Endocannabinoid system receptors at the hip and stifle joints of middle-aged dogs: a novel target for the therapeutic use of Cannabis sativa extract in canine Arthropathies. Animals. (2023) 13:833. doi: 10.3390/ani13182833
40. Chiocchetti, R, De Silva, M, Aspidi, F, Cunha, RZ, Gobbo, F, Tagliavia, C, et al. Distribution of cannabinoid receptors in keratinocytes of healthy dogs and dogs with atopic dermatitis. Front Vet Sci. (2022) 9:896. doi: 10.3389/fvets.2022.915896
41. Keresztes, G, Glávits, R, Krenács, L, Kurucz, É, and Andó, I. An anti-CD3ϵ serum detects T lymphocytes in paraffin-embedded pathological tissues in many animal species. Immunol Lett. (1996) 50:167–72. doi: 10.1016/0165-2478(96)02534-5
42. Deeg, CA, Reese, S, Gerhards, H, Wildner, G, and Kaspers, B. The Uveitogenic potential of retinal S-antigen in horses. Invest Opthalmol Vis Sci. (2004) 45:2286–92. doi: 10.1167/iovs.03-1226
43. Russo, D, Bombardi, C, Castellani, G, and Chiocchetti, R. Characterization of spinal ganglion neurons in horse (Equus caballus). A morphometric, neurochemical and tracing study. Neuroscience. (2011) 176:53–71. doi: 10.1016/j.neuroscience.2010.12.042
44. Cerrato, S, Ramió-Lluch, L, Brazís, P, Rabanal, RM, Fondevila, D, and Puigdemont, A. Development and characterization of an equine skin-equivalent model. Vet Dermatol. (2014) 25:e77:475. doi: 10.1111/vde.12134
45. Włodarczyk, M, Sobolewska-Włodarczyk, A, Cygankiewicz, AI, Jacenik, D, Krajewska, WM, Stec-Michalska, K, et al. G protein-coupled receptor 55 (GPR55) expresses differently in patients with Crohn’s disease and ulcerative colitis. Scand J Gastroenterol. (2017) 52:711–5. doi: 10.1080/00365521.2017.1298834
46. Bohm, A, Gaudet, R, and Sigler, PB. Structural aspects of heterotrimeric G-protein signaling. Curr Opin Biotechnol. (1997) 8:480–7. doi: 10.1016/S0958-1669(97)80072-9
47. Neer, EJ. Heterotrimeric C proteins: organizers of transmembrane signals. Cell. (1995) 80:249–57. doi: 10.1016/0092-8674(95)90407-7
48. Alcantara, KP, Malabanan, JWT, Nalinratana, N, Thitikornpong, W, Rojsitthisak, P, and Rojsitthisak, P. Cannabidiol-loaded solid lipid nanoparticles ameliorate the inhibition of Proinflammatory cytokines and free radicals in an in vitro inflammation-induced cell model. Int J Mol Sci. (2024) 25:4744. doi: 10.3390/ijms25094744
49. Yu, CHJ, and Rupasinghe, HPV. Cannabidiol-based natural health products for companion animals: recent advances in the management of anxiety, pain, and inflammation. Res Vet Sci. (2021) 140:38–46. doi: 10.1016/j.rvsc.2021.08.001
50. Faleiros, RR, Nuovo, GJ, Flechtner, AD, and Belknap, JK. Presence of mononuclear cells in normal and affected laminae from the black walnut extract model of laminitis. Equine Vet J. (2011) 43:45–53. doi: 10.1111/j.2042-3306.2010.00121.x
51. Yang, G, Im, H-J, and Wang, JH-C. Repetitive mechanical stretching modulates IL-1β induced COX-2, MMP-1 expression, and PGE2 production in human patellar tendon fibroblasts. Gene. (2005) 363:166–72. doi: 10.1016/j.gene.2005.08.006
52. Sheats, MK. A comparative review of equine SIRS, Sepsis, and neutrophils. Front Vet Sci. (2019) 6:69. doi: 10.3389/fvets.2019.00069
53. Turner, S, Barker, VD, and Adams, AA. Effects of Cannabidiol on the in vitro lymphocyte pro-inflammatory cytokine production of senior horses. J Equine Vet Sci. (2021) 103:103668. doi: 10.1016/j.jevs.2021.103668
54. Wang, B, Kovalchuk, A, Li, D, Rodriguez-Juarez, R, Ilnytskyy, Y, Kovalchuk, I, et al. In search of preventative strategies: novel high-CBD Cannabis sativa extracts modulate ACE2 expression in COVID-19 gateway tissues. Aging. (2020) 12:225. doi: 10.18632/aging.202225
55. Lowin, T, Apitz, M, Anders, S, and Straub, RH. Anti-inflammatory effects of N-acylethanolamines in rheumatoid arthritis synovial cells are mediated by TRPV1 and TRPA1 in a COX-2 dependent manner. Arthritis Res Ther. (2015) 17:321. doi: 10.1186/s13075-015-0845-5
56. Kyaw-Tanner, M, and Pollitt, CC. Equine laminitis: increased transcription of matrix metalloproteinase-2 (MMP-2) occurs during the developmental phase. Equine Vet J. (2004) 36:221–5. doi: 10.2746/0425164044877242
57. Gęgotek, A, Atalay, S, Rogowska-Wrzesińska, A, and Skrzydlewska, E. The effect of Cannabidiol on UV-induced changes in intracellular signaling of 3D-cultured skin keratinocytes. Int J Mol Sci. (2021) 22:1501. doi: 10.3390/ijms22031501
58. Kobayashi, T, Hattori, S, and Shinkai, H. Matrix Metalloproteinases-2 and -9 are secreted from human fibroblasts. Acta Derm Venereol. (2003) 83:105–7. doi: 10.1080/00015550310007436
59. Atalay, S, Gęgotek, A, and Skrzydlewska, E. Protective effects of Cannabidiol on the membrane proteome of UVB-irradiated keratinocytes. Antioxidants. (2021) 10:402. doi: 10.3390/antiox10030402
60. Mallat, A, Teixeira-Clerc, F, Deveaux, V, Manin, S, and Lotersztajn, S. The endocannabinoid system as a key mediator during liver diseases: new insights and therapeutic openings. Br J Pharmacol. (2011) 163:1432–40. doi: 10.1111/j.1476-5381.2011.01397.x
61. Hurley, DJ, Parks, RJ, Reber, AJ, Donovan, DC, Okinaga, T, Vandenplas, ML, et al. Dynamic changes in circulating leukocytes during the induction of equine laminitis with black walnut extract. Vet Immunol Immunopathol. (2006) 110:195–206. doi: 10.1016/j.vetimm.2005.09.015
62. Menzies-Gow, NJ, Wray, H, Bailey, SR, Harris, PA, and Elliott, J. The effect of tumour necrosis factor-α and insulin on equine digital blood vessel function in vitro. Inflamm Res. (2014) 63:637–47. doi: 10.1007/s00011-014-0736-2
63. Xu, X, Guo, H, Jing, Z, Yang, L, Chen, C, Peng, L, et al. N-Oleoylethanolamine reduces inflammatory cytokines and adhesion molecules in TNF-α-induced human umbilical vein endothelial cells by activating CB2 and PPAR-α. J Cardiovasc Pharmacol. (2016) 68:280–91. doi: 10.1097/FJC.0000000000000413
64. Gasperi, V, Evangelista, D, Chiurchiù, V, Florenzano, F, Savini, I, Oddi, S, et al. 2-Arachidonoylglycerol modulates human endothelial cell/leukocyte interactions by controlling selectin expression through CB1 and CB2 receptors. Int J Biochem Cell Biol. (2014) 51:79–88. doi: 10.1016/j.biocel.2014.03.028
65. Milman, G, Maor, Y, Abu-Lafi, S, Horowitz, M, Gallily, R, Batkai, S, et al. N -arachidonoyl l-serine, an endocannabinoid-like brain constituent with vasodilatory properties. Proc Natl Acad Sci U S A. (2006) 103:2428–33. doi: 10.1073/pnas.0510676103
66. Henstridge, CM, Balenga, NAB, Kargl, J, Andradas, C, Brown, AJ, Irving, A, et al. Minireview: recent developments in the physiology and pathology of the Lysophosphatidylinositol-sensitive receptor GPR55. Mol Endocrinol. (2011) 25:1835–48. doi: 10.1210/me.2011-1197
67. Staton, PC, Hatcher, JP, Walker, DJ, Morrison, AD, Shapland, EM, Hughes, JP, et al. The putative cannabinoid receptor GPR55 plays a role in mechanical hyperalgesia associated with inflammatory and neuropathic pain. Pain. (2008) 139:225–36. doi: 10.1016/j.pain.2008.04.006
68. Walsh, SK, Hector, EE, Andréasson, A-C, Jönsson-Rylander, A-C, and Wainwright, CL. GPR55 deletion in mice leads to age-related ventricular dysfunction and impaired Adrenoceptor-mediated inotropic responses. PLoS One. (2014) 9:e108999. doi: 10.1371/journal.pone.0108999
69. Tiyerili, V, Zimmer, S, Jung, S, Wassmann, K, Naehle, CP, Lütjohann, D, et al. CB1 receptor inhibition leads to decreased vascular AT1 receptor expression, inhibition of oxidative stress and improved endothelial function. Basic Res Cardiol. (2010) 105:465–77. doi: 10.1007/s00395-010-0090-7
70. Zurier, RB, and Burstein, SH. Cannabinoids, inflammation, and fibrosis. FASEB J. (2016) 30:3682–9. doi: 10.1096/fj.201600646R
71. Liu, X, Li, L, Jiang, J, Ge, W, Huang, Y, Jin, Z, et al. Role of type I cannabinoid receptor in sensory neurons in Psoriasiform skin inflammation and pruritus. J Invest Dermatol. (2023) 143:e3:812–21. doi: 10.1016/j.jid.2022.10.014
72. Interlandi, C, Tabbì, M, Di Pietro, S, D’Angelo, F, Costa, GL, Arfuso, F, et al. Improved quality of life and pain relief in mature horses with osteoarthritis after oral transmucosal cannabidiol oil administration as part of an analgesic regimen. Front Vet Sci. (2024) 11:1396. doi: 10.3389/fvets.2024.1341396
Glossary
ALL - Acute laminitic laminae
ANOVA - Analysis of variance
ApoE - apolipoprotein E
AT1R - Vascular angiotensin II type 1 receptor
BM - Basal membrane
BWE - Back-nut extract
CAL - Calprotectin
CBC - Complete blood count
CBD - Cannabidiol
CBGA - Cannabigerolic Acid
CB1R - Cannabinoid Receptor Type 1
CB2R - Cannabinoid Receptor Type 2
CLL - Chronic laminitic laminae
COX-2 - Cicloossigenasi 2
DAPI - 6-diamidino-2-phenylindole
DC - Dendritic cells
DRG - Dorsal Root Ganglia
ECS - Endocannabinoid System
GPR55 - G-Protein Coupled Receptor 55
HL - Healthy laminae
IBA1 - Ionized calcium binding adapter molecule 1
ID - Insulin Dysregulation
IFN-γ - Interferon gamma
IL - Interleukins
IR - Immunoreactivity
LL - Laminitic laminae
MMP - Metalloproteinases
NaCl - Sodium Chloride
OCT - Optimal Cutting Temperature
PBS - Phosphate-buffered saline
PDL - Primary Dermal Laminae
PEA - Palmitoylethanolamide
PEL - Primary Epidermal Laminae
PPID - Pars Pituitary Intermedia Disfunction
ROS - Reactive oxygen species
RT - Room temperature
SDL - Secondary Dermal Laminae
SEL - Secondary Epidermal Laminae
SIRS - Systemic inflammatory response syndrome
SP - Substance P
THCV - Tetrahydrocannabivarin Acid
TNF-α - Tumor necrosis factor-α
Wb - Western blot
α - Alfa
γ - Gamma
Keywords: endocannabinoid system, immune cells, macrophages, neutrophils, T-cells
Citation: Zamith Cunha R, Gobbo F, Morini M, Salamanca G, Zanoni A, Bernardini C, Gramenzi A and Chiocchetti R (2025) Cannabinoid and cannabinoid related receptors in fibroblasts, inflammatory and endothelial cells of the equine hoof with and without laminitis: novel pharmacological target. Front. Vet. Sci. 12:1723160. doi: 10.3389/fvets.2025.1723160
Edited by:
Robin Temmerman, European College of Veterinary Pharmacology and Toxicology (ECVPT), BelgiumReviewed by:
Claudia Interlandi, University of Messina, ItalyFrancesca Aragona, University of Messina, Italy
Copyright © 2025 Zamith Cunha, Gobbo, Morini, Salamanca, Zanoni, Bernardini, Gramenzi and Chiocchetti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rodrigo Zamith Cunha, cm9kcmlnb3phbWl0aGN1bmhhQGdtYWlsLmNvbQ==
 Rodrigo Zamith Cunha
Rodrigo Zamith Cunha Francesca Gobbo
Francesca Gobbo Maria Morini
Maria Morini Giulia Salamanca
Giulia Salamanca Augusta Zanoni
Augusta Zanoni Chiara Bernardini
Chiara Bernardini Alessandro Gramenzi
Alessandro Gramenzi Roberto Chiocchetti
Roberto Chiocchetti