- Dept. of Neurology, St. Josef-Hospital, Ruhr-University Bochum, Bochum, Germany
Internuclear ophthalmoplegia (INO) is an eye movement disorder that occurs in approximately one-third of people with multiple sclerosis (MS). We used a novel, head-mounted, virtual reality (VR)-based oculography device (PRET™, machineMD AG, Switzerland) to objectively measure oculomotor symptoms reported by a patient with MS to aid in the clinical diagnosis confirmation of INO. The patient’s symptoms, primarily diplopia, were managed successfully with 4-aminopyridine. The VR-based eye-tracking tool documented the subjective improvement in oculomotor function tracking treatment effects.
1 Introduction
Internuclear ophthalmoplegia (INO) is caused by lesions in the medial longitudinal fasciculus (MLF) associated with inflammatory, vascular, infectious, or traumatic causes (Bolaños et al., 2004; Kleinsorge et al., 2021). INO is one of the most common oculomotor manifestations associated with multiple sclerosis (MS), affecting 15%–52% of individuals (Nij Bijvank et al., 2019; Jozefowicz-Korczynska et al., 2008; Hof et al., 2022).
Oculomotor dysfunction in INO is characterized by ipsilateral adduction palsy, often accompanied by dissociated horizontal jerk nystagmus of the contralateral eye during abduction (Omary et al., 2023). Regarding saccadic peak velocities, a specific pattern has been observed for INO, characterized by a reduction in the velocities of the adducting saccades of the affected eye, with abduction velocities falling within the normal range (Kirkham and Katsarkas, 1977). These eye movement abnormalities can be explained by Hering’s Law of Equal Innervation, which states that the muscles responsible for the coordination of eye movements receive equal and simultaneous innervation. A lesion in the MLF, however, disrupts signal transmission, the weaker adducting eye causing the other eye to exhibit greater movement, resulting in asymmetrical strabismus angles. This leads to the specific slowing of adduction saccades and dissociated nystagmus in the abducting eye (Nerrant and Tilikete, 2017; Aw et al., 2017). The most common resulting clinical symptoms of INO are diplopia and dizziness in lateral gaze (Nij Bijvank et al., 2019), with exotropia (Johkura et al., 2015) and loss of convergence (Wang et al., 2022). No gold-standard measurement technique exists for clinical INO diagnosis. Current clinical examinations focus on the identification of INO during horizontal gaze. However, mild INO can be easily overlooked in the case of slight deviations between adduction and abduction velocities between the eyes (Frohman et al., 2003). In such cases, relative slowing of the adduction saccades with full preservation of the adduction range may be the only manifestation of INO with the absence of abducting nystagmus (Serra et al., 2018). Early diagnosis of oculomotor symptoms of MS is vital for improving treatment outcomes and patients’ quality of life (Nij Bijvank et al., 2019).
To objectively measure and track INO, we used a VR-based oculography headset device (PRET™, machineMD, Switzerland) that utilizes a binocular infrared eye-tracking system operating at 200 Hz.
2 Case description
2.1 Patient history
A 51-year-old male patient experienced the first symptoms of MS, namely, diplopia and dizziness accompanied by nausea and vomiting, approximately 3 years prior to diagnosis, not resulting in a neurological work-up with the primary ophthalmological diagnosis of strabismus. He reported persisting oculomotor impairments involving double vision, i.e., diplopia, particularly during the right gaze, with vertigo and fatigue. Due to exophoria in the right eye, surgical intervention with bilateral retraction of the musculus rectus lateralis had been performed, resulting in short-term improvement. In an ophthalmological report after surgical intervention, a left internuclear ophthalmoplegia (INO) was mentioned for the first time as a potential differential diagnosis. The patient reported persistent impairment in everyday life activities due to diplopia, with only partial relief provided by prism glasses.
However, the diagnosis of MS was only made 3 years later due to the persistence of symptoms and the patient seeking a neurological consultation independently. This external neurological workup revealed positive findings for oligoclonal bands in the cerebrospinal fluid. There were no diagnostic findings suggestive of potential differential diagnoses. The MRI of the head and spine demonstrated supratentorial, brainstem, cerebellar, and spinal lesions. Dissemination in time and space was thus fulfilled according to the 2017 revision of the McDonald criteria. Following the diagnosis of MS, the patient was prescribed a disease-modifying treatment (teriflunomide, initiated January 2024) and referred to our center.
2.2 Clinical findings
In addition to the standard clinical-neurological examination, a more detailed assessment of the patient’s horizontal eye movements was conducted. The patient was asked to fixate his gaze on an object moved by the physician and follow it with his gaze only. To detect INO, the examiner must observe both eyes of the patient while moving the object in one direction to detect the slowing or incompleteness of the adduction over the abduction and/or the jerk nystagmus. To detect strabismus, we performed the cover test and observed ocular alignment in binocular gaze and the saccadic correction of the respective uncovered eye. Only qualitative manual strabismus measurement was performed. Clinical examination at our center revealed the presence of a left INO, with decompensated exophoria of the right eye and saccadic gaze tracking. We defined strabismus as decompensated exophoria, as the patient was able to temporarily suppress the misalignment of the eyes, which is only visible in monocular gaze. We also found additional cerebellar and sensory signs, as evidenced by the Expanded Disability Status Scale (EDSS) score of 3.5 (functional scores: visual 1, brainstem 3, pyramidal 0, cerebellar 2, sensory 2, bowel/bladder 1, cerebral 2, and ambulation 0).
3 Diagnostic assessment
3.1 Diagnostic testing
The patient was examined as part of a prospective observational study (ethics approval 23-3974-BR, Medical Faculty, Ruhr-University Bochum, Germany) using the PRETTM device for standardized oculography measurement at baseline with a 5-month follow-up. This device utilizes a custom software platform and proprietary algorithms to conduct a neuro-ophthalmology screen divided into eight exam blocks (Coito et al., 2025). In our case, we specifically used the gaze direction and saccadic movement eye exams. The gaze direction exam measures gaze holding and ocular alignment during binocular fixation to monocular stimuli in all nine cardinal directions of gaze, with the stimuli presented for 3 s. For the same fixation points, the ocular alignment exam assesses the median deviation of phoria of each eye using an alternate cover test format (Coito et al., 2025), whereas the saccadic exam block assesses the horizontal (left and right) and vertical (up and down) saccadic eye movements and peak velocities by 10° (5 repetitions) and 20° (4 repetitions) to either side, respectively. This test also assesses how both eyes can individually track the same VR stimuli for two repetitions at two different velocities (slow and fast) of 15-degree amplitude and deceleration around the turning points. The resulting test report provides information about individual left (blue color) and right (red color) saccadic movement accuracy, speed, and peak movement velocity for each horizontal and vertical saccade sequence. This procedure allows for easy visual identification of INO by the relative slowing of the adducting compared to the abducting eye. The VR-based ocular alignment measurement is based on an alternate cover test. Its measurement principle has been evaluated against Harms (Vicini et al., 2025).
The ocular alignment plots at baseline revealed exophoria of both eyes based on the median eye position (Figure 1A) with reduction in horizontal saccadic peak velocities of the left adducting eye (blue color) representing a left INO (Figure 2A). The report also revealed decompensation of exophoria of the right eye during smooth pursuit (Figure 3A).
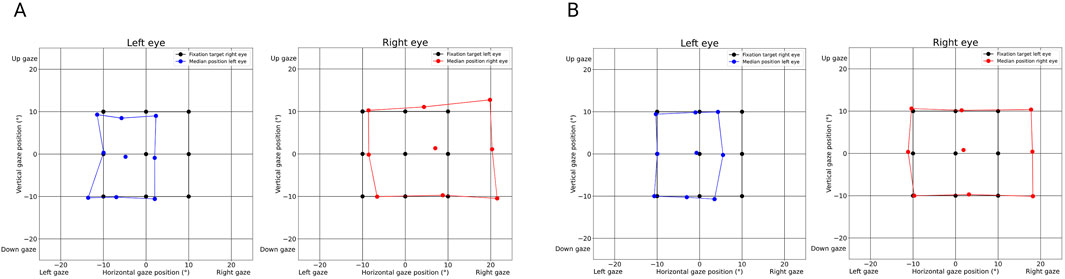
Figure 1. Ocular alignment graph derived from the PRET™ report illustrating strabismus in a patient with internuclear ophthalmoplegia (INO) affecting the left medial longitudinal fasciculus (MLF). (A) (baseline examination): Divergent strabismus (exotropia) of the left eye in the primary position (blue dots), while the right eye is fixating (black dots). During an alternate cover test, the median position of the covered eye is measured and marked by blue dots (left eye) or red dots (right eye). The position of the respective fixating eye is demonstrated by black dots. Physiologically, the position of the non-fixating left eye matches the position of the fixating right eye so that the black dots are covered by blue dots. The more the gaze moves to the right, the more the positions of the eyes differ from each other, representing the angle of exotropia that increases in right gaze and decreases in left gaze. The deviation of the right eye (red dots) shows the same deviation, with larger angles illustrated by the divergence between the position of the red dots compared to the black dots, especially in the right gaze. This larger “secondary angle” is typically observed when patients fixate with the eye with limited motility due to Hering’s law of equal innervation. The difference in the strabismus angle size between the left and right eyes indicates the magnitude of the limitation of motility. Again, the largest deviation is in the right gaze. (B) (follow-up after 5 months): Good alignment in primary gaze demonstrated by the central blue and red dots over the black dot in 0° vertical and 0° horizontal gaze position and residual exotropia in right gaze shown by the displacement of the median position of the right eye (red dots) enlarging in the horizontal right gaze.
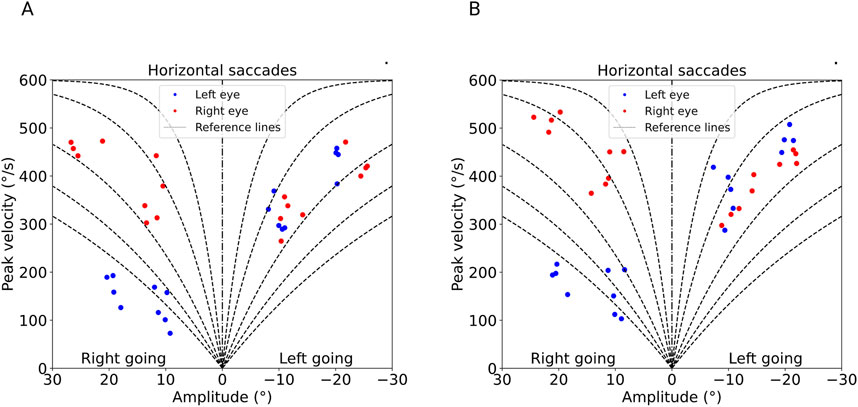
Figure 2. Saccadic peak velocities in horizontal gaze derived from the PRET™ report. (A) (baseline examination): Reduced saccadic peak velocity of the adducting left eye with right-going saccades (blue dots), with similar peak velocities of both eyes during left-going saccades demonstrating a typical INO pattern. The horizontal saccades are split into the two directions (left and right), demonstrated by amplitudes from +30 to −30°. The right eye (red dots) reaches velocities of almost 500° per second in both adduction (left-going saccades) and abduction (right-going saccades), whereas the left eye (blue dots) reaches velocities of around 200° per second in adduction (right going) and approximately 500° per second in abduction. This is where the adduction palsy becomes apparent. (B) (follow-up after 5 months): unchanged left INO pattern.
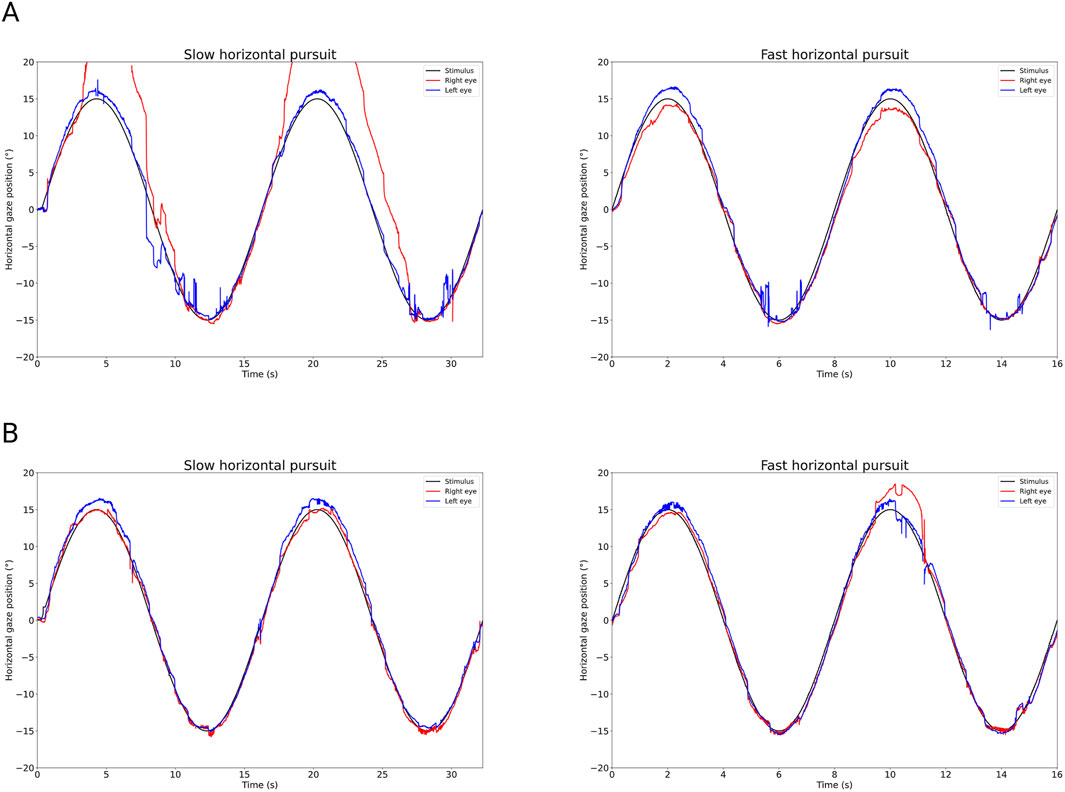
Figure 3. Horizontal smooth pursuit graph derived from the PRET™ report. (A) (baseline examination): The horizontal pursuit is demonstrated by the relation of the horizontal gaze position over time, positive degrees in position, meaning right gaze. The stimulus is represented by the black line, with blue indicating the gaze position of the left eye and red indicating the gaze position of the right eye. The deviation from the target of the right eye (red line) during right-going pursuit indicates persistent strabismus. Gain of pursuit is mildly reduced for left-going slow eye movements, resulting in catch-up saccades and jerky pursuit. The fast horizontal pursuit seems less impaired, also showing catch-up saccades and jerky pursuit. (B) (follow-up after 5 months): The impaired pursuit is barely detectable. Especially slow pursuit shows the improved alignment of eye positions and the position of the stimulus.
Upon reevaluation of the patient’s cerebral magnetic resonance imaging (MRI), we found a midbrain lesion of the MLF that had not previously been described (Figures 4A–D).
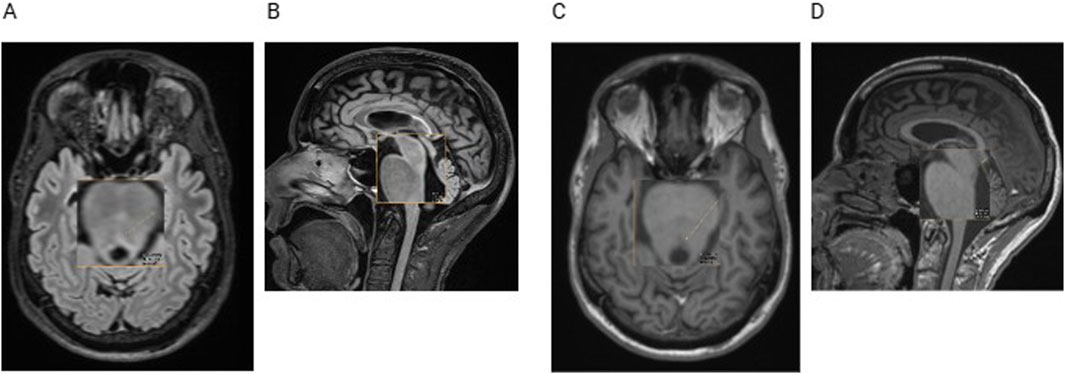
Figure 4. Cerebral MRI with transversal (A) and sagittal (B) fluid-attenuated inversion recovery (FLAIR) sequences and with transversal (C) and sagittal (D) T1-weighted sequences. The orange arrow indicates a small demyelinating lesion, not described in the radiological reports before.
3.2 Diagnosis
The diagnosis revealed MS-related INO in the left eye with diplopia and decompensated strabismus of the right eye due to a demyelinating MLF lesion.
3.3 Therapeutic intervention
We discussed treatment options with the patient and jointly agreed to use an off-label pharmacological approach for symptomatic management of INO. In addition to prism glasses, we initiated treatment with extended-release 4-aminopyridine at the standard dosage of 2 x 10 mg.
3.4 Follow-up and outcomes
Clinical follow-up at 2 months after 4-aminopyridine initiation revealed subjective reductions in double vision and improvement in activities of daily living. Exophoria, on clinical examination, was also found to be mostly compensated with improvement of gaze fixation. Double vision only occurred during extreme horizontal gaze to the right, where the left INO could still be detected. Side effects reported by the patient were vertigo, discomfort, and sleep disturbances that correspond to the known medication profile. To reduce these symptoms, we shifted the timing of intake (for both doses) forward for a few hours to increase the interval from the last dosing to going to sleep.
Five months after starting the treatment, the patient continued to experience a clear improvement. The VR-based oculography documented an improvement of oculomotor function with a reduced strabismus angle at central fixation (Figure 1B) and sustained compensation of right eye exophoria in smooth pursuit (Figure 3B) with unchanged left eye INO (Figure 2B).
The patient reported remission of the initial side effects with good tolerability.
4 Discussion
Our case highlights the importance of a neuro-ophthalmological and neurological clinical work-up of oculomotor impairments in a case of severely delayed MS diagnosis. MS-related neuro-ophthalmological findings are often missed during manual examination, which leads to delayed diagnosis and inadequate therapeutic management of MS (Frohman et al., 2003). Likewise, reappraisal of available MRI scans is useful, referring back to the clinical and VR-based examinations for lesion localization. This is particularly relevant for small demyelinating lesions frequently occurring in different brainstem regions (Lu et al., 2011). There is a significant discrepancy between clinical findings of INO and detection of lesions in the MLF on MRI (Nij Bijvank et al., 2019), creating a phenomenon of false-negative lesions because the specificity of MRI regarding lesions in the MLF is high (92.0%), but the sensitivity is low (46.2%) (Omary et al., 2023). VR systems have been shown to provide reliable measurements of horizontal and vertical deviations in patients with strabismus (Wenner et al., 2025) and demonstrate high specificity in their detection (Mori et al., 2025). There is sparse information but high potential for VR-based diagnosis of INO. Using quantitative saccade and video head impulse tests could be of assistance in identifying subtle INO (Manrique et al., 2022). The strengths of VR-based measurements, along with the differences between VR and traditional measurements, include the ability to provide immersive, controlled, and repeatable environments for the collection of objective data. VR-based devices can be rapidly and easily applied in routine settings, even without direct access to a neuro-ophthalmological assessment. However, the cost and accessibility of VR systems pose limitations, and more validation and standardization are required for their use in clinical practice.
Current treatment options for INO include surgical interventions to correct eye misalignment and conservative measures such as the use of prism glasses or occlusion therapy (Buckley and Elston, 1997). Patients with decompensated exophoria may not benefit from the use of prism glasses due to their reduced ability to adapt to prismatic corrections. This can cause eye strain and fatigue (Przekoracka-Krawczyk et al., 2019). Since surgical intervention and prism glasses only provided a transient effect in our MS patient, given the lack of new and/or active inflammatory lesions indicating MS relapse, we refrained from high-dose corticosteroid therapy (Pozzilli et al., 2004).
We instead opted for a non-invasive pharmacological approach using 4-aminopyridine, a reversible potassium channel blocker. This treatment is indicated for MS patients with gait impairment (Zhang et al., 2021) and has been proposed to induce symptom relief in patients with INO for off-label use (Kanhai et al., 2019). The most common adverse drug reactions for 4-aminopyridine include vertigo (Kanhai et al., 2019), insomnia, headaches, and nausea (Castelnovo et al., 2021), which are usually transient (Kanhai et al., 2019). The patient experienced vertigo, discomfort, and sleep disturbances shortly after starting 4-aminopyridine, which were not reported during the follow-up. Thus, we assume that these symptoms were due to the medication. 4-aminopyridine may show an increase in efficacy during the course of treatment (Filli et al., 2017). Therefore, we want to observe the patient over a longer period of time before further treatment approaches are established. As we only describe symptom improvement under 4-aminopyridine in a single case without placebo control, larger patient cohorts, or withdrawal–rechallenge settings, larger confirmation of the drug effect on eye movement is still needed for its general use in clinical routine.
4.1 Conclusion
We were able to measure strabismus, slowed adduction of the left eye, and impairment of stimulus pursuit as signs of clinically reported diplopia caused by INO using the VR-based oculography device, providing objective evidence for the presence of left INO and treatment response during follow-up. While pharmacological intervention using 4-aminopyridine led to a subjective improvement in the patient’s oculomotor symptoms, off-label treatment trials require thorough information and instruction of the affected person along with an exact documentation and understanding of the treatment response. Subjective perceptual disorders often evade standard clinical neurological examination. With the help of VR-based oculography, we were able to document the described disorder and reproducibly measure the response to treatment in the present case. This VR-based oculography approach enabled us to objectively monitor symptomatic treatment and support subjective findings.
Future studies will aim to further investigate the potential of VR-based oculography for treatment monitoring of disease-modifying drugs and early detection of disease progression, including progression independent of relapses (PIRA) with oculomotor function as an early surrogate marker (Müller et al., 2023). The efficacy of 4-aminopyridine must be further analyzed to ensure more frequent and rapid use in the future.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Ethics statement
The studies involving humans were approved by ethics approval 23-3974-BR; Medical Faculty, Ruhr-University Bochum, Germany. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
ER: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft. JL: Data curation, Writing – review and editing, Methodology. RS: Data curation, Investigation, Writing – review and editing, Formal Analysis, Validation. RK: Writing – review and editing, Data curation, Formal Analysis, Validation. JM: Conceptualization, Formal Analysis, Supervision, Validation, Writing – review and editing. RG: Formal Analysis, Supervision, Validation, Writing – review and editing. AS: Writing – original draft, Writing – review and editing, Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Supervision, Validation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The project underlying this case report (ethics approval 23-3974-BR) is funded by the regional association of North Rhine-Westphalia of the German MS Society (DMSG Landesverband NRW).
Acknowledgments
We thank our patient for his consent to publish this case. We additionally thank Avantika Naidu PT PhD (MachineMD Inc., US) and Dominik Brügger (machineMD, Switzerland) for proof-reading and feedback regarding technical information.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aw, S. T., Chen, L., Todd, M. J., Barnett, M. H., and Halmagyi, G. M. (2017). Vestibulo-ocular reflex deficits with medial longitudinal fasciculus lesions. J. neurology 264 (10), S. 2119–2129. doi:10.1007/s00415-017-8607-8
Bolaños, I., Lozano, D., and Cantú, C. (2004). Internuclear ophthalmoplegia: causes and long-term follow-up in 65 patients. Acta neurol. Scand. 110 (3), S. 161–165. doi:10.1111/j.1600-0404.2004.00278.x
Buckley, S. A., and Elston, J. S. (1997). “Surgical treatment of supranuclear and internuclear ocular motility disorders,”Eye Lond. Engl. 11. 377–380. doi:10.1038/eye.1997.79
Castelnovo, G., Gerlach, O., Freedman, M. S., Bergmann, A., Sinay, V., Castillo-Triviño, T., et al. (2021). Safety, patient-reported well-being, and physician-reported assessment of walking ability in patients with multiple sclerosis for prolonged-release fampridine treatment in routine clinical practice: results of the LIBERATE study. CNS drugs 35 (9), S. 1009–1022. doi:10.1007/s40263-021-00840-x
Coito, A., Naidu, A., Lehmann, J., Hauser, B., Brügger, D., and Abegg, M. (2025). Test-retest reliability of gaze precision of a novel virtual reality-based medical device. Front. Virtual Real 6. doi:10.3389/frvir.2025.1502679
Filli, L., Zörner, B., Kapitza, S., Reuter, K., Lörincz, L., Weller, D., et al. (2017). Monitoring long-term efficacy of fampridine in gait-impaired patients with multiple sclerosis. Neurology 88 (9), S. 832–841. doi:10.1212/WNL.0000000000003656
Frohman, T. C., Frohman, E. M., O'Suilleabhain, P., Salter, A., Dewey, R. B., Hogan, N., et al. (2003). “Accuracy of clinical detection of INO in MS: corroboration with quantitative infrared oculography,”Neurology 61. 848–850. doi:10.1212/01.wnl.0000085863.54218.72
Hof, S. N., Loonstra, F. C., Ruiter, L. R. J. de, van Rijn, L. J., Petzold, A., Uitdehaag, B. M. J., et al. (2022). The prevalence of internuclear ophthalmoparesis in a population-based cohort of individuals with multiple sclerosis. Multiple Scler. Relat. Disord. 63, 103824. doi:10.1016/j.msard.2022.103824
Johkura, K., Kudo, Y., Amano, Yu, Kikyo, H., Imazeki, R., Amari, K., et al. (2015). Gaze palsy and exotropia in internuclear ophthalmoplegia. J. neurological Sci. 353 (1-2), S. 158–160. doi:10.1016/j.jns.2015.04.017
Jozefowicz-Korczynska, M., Lukomski, M., and Pajor, A. (2008). Identification of internuclear ophthalmoplegia signs in multiple sclerosis patients. Saccade test analysis. J. neurology 255 (7), S. 1006–1011. doi:10.1007/s00415-008-0819-5
Kanhai, K. M. S., Nij, B., Wagenaar, Y. L., Klaassen, E. S., Lim, K. S., Bergheanu, S. C., et al. (2019). Treatment of internuclear ophthalmoparesis in multiple sclerosis with fampridine: a randomized double-blind, placebo-controlled cross-over trial. CNS Neurosci. and Ther. 25 (6), S. 697–703. doi:10.1111/cns.13096
Kirkham, T. H., and Katsarkas, A. (1977). An electrooculographic study of internuclear ophthalmoplegia. Ann. neurology 2 (5), 385–392. doi:10.1002/ana.410020507
Kleinsorge, M. T., Ebert, A., Förster, A., Weber, C. E., Roßmanith, C., Platten, M., et al. (2021). MRI topography of lesions related to internuclear ophthalmoplegia in patients with multiple sclerosis or ischemic stroke. J. neuroimaging official J. Am. Soc. Neuroimaging 31 (3), S. 471–474. doi:10.1111/jon.12847
Lu, Z., Zhang, B., Qiu, W., Kang, Z., Shen, L., Long, Y., et al. (2011). Comparative brain stem lesions on MRI of acute disseminated encephalomyelitis, neuromyelitis optica, and multiple sclerosis. PloS one 6 (8), e22766. doi:10.1371/journal.pone.0022766
Manrique, L. G., Zhang, X., Kathryn, L., Marie, C., and Kattah, J. C. (2022). Mild bilateral internuclear ophthalmoplegia: the diagnostic role of the vertical posterior canal vestibulo-ocular reflex in acute brainstem demyelination, a clinical-radiologic correlation. J. neuro-ophthalmology official J. North Am. Neuro-Ophthalmology Soc. 42 (1), e281–e288. doi:10.1097/WNO.0000000000001262
Mori, D. M., Kuchhangi, A., Tame, J., Cooper, K., Hajkazemshirazi, L., Indaram, M., et al. (2025). Evaluation of a novel virtual reality simulated alternate cover test to assess strabismus: a prospective, masked study. Am. J. Ophthalmol. 269, 266–272. doi:10.1016/j.ajo.2024.08.042
Müller, J., Cagol, A., Lorscheider, J., Tsagkas, C., Benkert, P., Yaldizli, Ö., et al. (2023). Harmonizing definitions for progression independent of relapse activity in multiple sclerosis: a systematic review. JAMA neurol. 80 (11), S. 1232–1245. doi:10.1001/jamaneurol.2023.3331
Nerrant, E., and Tilikete, C. (2017). Ocular motor manifestations of multiple sclerosis. J. neuro-ophthalmology official J. North Am. Neuro-Ophthalmology Soc. 37 (3), S. 332–340. doi:10.1097/WNO.0000000000000507
Nij Bijvank, J. A., van Rijn, L. J., Balk, L. J., Tan, H. S., Uitdehaag, B. M. J., and Petzold, A. (2019). Diagnosing and quantifying a common deficit in multiple sclerosis: internuclear ophthalmoplegia. Neurology 92 (20), e2299–e2308. doi:10.1212/WNL.0000000000007499
Omary, R., Bockisch, C. J., Vere-Tyndall, A. de, Pazahr, S., Baráth, K., and Weber, K. P. (2023). Lesion follows function: video-oculography compared with MRI to diagnose internuclear ophthalmoplegia in patients with multiple sclerosis. J. neurology 270 (2), S. 917–924. doi:10.1007/s00415-022-11428-w
Pozzilli, C., Marinelli, F., Romano, S., and Bagnato, F. (2004). Corticosteroids treatment. J. neurological Sci. 223 (1), S. 47–51. doi:10.1016/j.jns.2004.04.019
Przekoracka-Krawczyk, A., Michalak, K. P., and Pyżalska, P. (2019). Deficient vergence prism adaptation in subjects with decompensated heterophoria. PloS one 14 (1), e0211039. doi:10.1371/journal.pone.0211039
Serra, A., Chisari, C. G., and Matta, M. (2018). Eye movement abnormalities in multiple sclerosis: pathogenesis, modeling, and treatment. Front. neurology 9, S 9, 31. doi:10.3389/fneur.2018.00031
Vicini, R., Brügger, D., Grabe, H., and Abegg, M. (2025). “Automated measurement of strabismus angle using a commercial virtual reality headset,”, Klin. Monatsblatter fur Augenheilkd. 242. 485–488. doi:10.1055/a-2466-0284
Wang, T., Cao, D., and Han, J. (2022). Case Report: a variant of wall-eyed bilateral internuclear ophthalmoplegia from unilateral pons infarction. Front. Neurosci. 16, 974645. doi:10.3389/fnins.2022.974645
Wenner, Y., Schneider, J., Drisser, T., Yang, Yu Yi, Demeter, T., Fronius, M., et al. (2025). “Virtual reality-based Harms tangent screen test for strabismus measurement,” in Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. doi:10.1007/s00417-024-06724-2
Keywords: internuclear ophthalmoplegia, multiple sclerosis, VR-based oculography, 4-aminopyridine, diplopia
Citation: Reuter E, Luerweg J, Schneider R, Klimas R, Motte J, Gold R and Salmen A (2025) Case Report: Improvement of diplopia due to severe internuclear ophthalmoplegia by 4-aminopyridine documented using a novel virtual reality-based oculography headset. Front. Virtual Real. 6:1595694. doi: 10.3389/frvir.2025.1595694
Received: 18 March 2025; Accepted: 12 June 2025;
Published: 03 July 2025.
Edited by:
Irene Fondon, Sevilla University, SpainReviewed by:
Sun-Uk Lee, Korea University Medical Center, Republic of KoreaDaniel B. Hier, Missouri University of Science and Technology, United States
Copyright © 2025 Reuter, Luerweg, Schneider, Klimas, Motte, Gold and Salmen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anke Salmen, YW5rZS5zYWxtZW5AcnViLmRl
 Emilie Reuter
Emilie Reuter Justus Luerweg
Justus Luerweg Jeremias Motte
Jeremias Motte Ralf Gold
Ralf Gold Anke Salmen
Anke Salmen