- 1Chinese Academy of Medical Sciences and Peking Union Medical College, Institute of Hematology and Blood Diseases Hospital, Tianjin, China
- 2Gene Therapy Center, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States
- 3Department of Pharmacology, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States
- 4Department of Pediatrics, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States
A Corrigendum on
Efficient Capsid Antigen Presentation From Adeno-Associated Virus Empty Virions In Vivo
by Pei, X., Earley, L. F., He, Y., Chen, X., Hall, N. E., Samulski, R. J., et al. (2018). Front. Immunol. 9:844. doi: 10.3389/fimmu.2018.00844
In the original article, there was a mistake in the legend for Figure 5 as published. We used an incorrect description of AAV particles. The correct legend appears below.
“Figure 5. The capsid antigen presentation from AAV8OVA empty virions in mice. 2 × 1011 particles of AAV8OVA viruses (empty or full) were injected into C57BL/6 mice via retro-orbital vein, and at day 3 post AAV8OVA injection, 5 × 106 CFSE-labeled OT-1 T cells were transferred. Ten days posttransfer, proliferation of OT-1 cells was measured by flow cytometry. (A) Representative flow cytometric histograms. (B) Average T cell proliferation and SD for four mice. *p < 0.05 when compared to mice treated with AAV8OVA full particles.”
Additionally, there was a mistake in Figure 8 as published. In Figure 8B, the significant difference between empty particles and full particles should be shown and not PBS and empty particles. The corrected Figure 8 appears below.
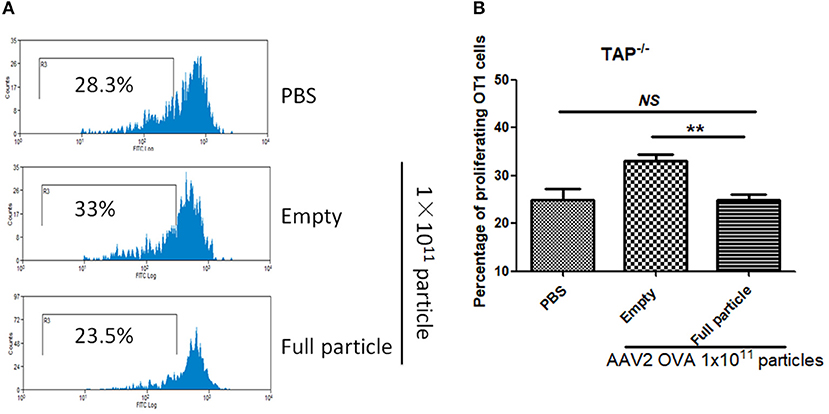
Figure 8. Blockage of proteasome pathway inhibits capsid antigen presentation from empty capsid. 1 × 1011 particles of AAV2OVA vectors (empty or full) were intravenously injected into TAP−/− mice via retro-orbital vein. At day 3, OT-1 cells were administered into the mice. Ten days later, spleen cells from mice were harvested for capsid antigen presentation analysis. (A) Representative flow cytometric histograms in TAP−/− mice treated with adeno-associated virus particles. (B) Average T cell proliferation and SD for five mice. **p < 0.01 when compared to mice treated with AAV2OVA full particles in TAP−/− mice.
Further, we erroneously indicated that “1.5 × 1011” instead of “1 × 1011” particles of AAV2OVA full particles were administered into TAP−/− mice and wild-type C57BL/6 mice.
A correction has been made to Results section, subsection Blockage of Proteasome Pathway Partially Inhibits Capsid Antigen Presentation From Empty Capsids, paragraph one:
“In our previous study, we demonstrated that capsid antigen presentation from AAV-transduced cells is proteasome dependent and requires endosomal escape (6). Utilization of proteasome inhibitors completely blocks capsid antigen presentation in 293/H-2Kb cells in a short incubation period. To study which antigen presentation pathway is involved in capsid antigen presentation in vivo after AAV transduction, TAP−/− mice were chosen since TAP deficiency blocks antigen presentation from proteasome-mediated degradation. 1 × 1011 particles of AAV2OVA full particles were administered into TAP−/− mice and wild-type C57BL/6 mice. At day 3 post AAV injection, OT-1 cells were transfused into the mice and 10 days later, spleen cells were collected for a capsid antigen presentation assay. Consistent to the finding in 293/H-2Kb cells, no capsid antigen presentation was documented in TAP−/− mice, although significantly higher capsid antigen presentation was observed in wild-type mice receiving AAV2OVA full particles when compared to control mice only receiving PBS injection (Figure 8). When 1 × 1011 particles of AAV2OVA virions were administered in TAP−/− mice, there was no significant difference in OT1 cell proliferation between mice injected with PBS or full particles; however, capsid antigen presentation from empty capsids was higher than that of AAV2OVA full particles. This result suggests that the capsid antigen presentation from AAV empty virions may use different mechanisms to process antigens, and this process is less dependent on TAP antigen presentation. It was noted that OT-1 cell proliferation in TAP−/− control mice was higher than that in wild-type C57BL/6 mice, the difference may result from the different immune background of mouse strains.”
The authors apologize for this error and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
Keywords: adeno-associated virus, capsid, antigen presentation, CD8+ T cells, empty virions
Citation: Pei X, Earley LF, He Y, Chen X, Hall NE, Samulski RJ and Li C (2020) Corrigendum: Efficient Capsid Antigen Presentation From Adeno-Associated Virus Empty Virions In Vivo. Front. Immunol. 10:3076. doi: 10.3389/fimmu.2019.03076
Received: 29 November 2019; Accepted: 17 December 2019;
Published: 04 February 2020.
Edited and reviewed by: Christoph T. Berger, University of Basel, Switzerland
Copyright © 2020 Pei, Earley, He, Chen, Hall, Samulski and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chengwen Li, Y2hlbmd3ZW5AbWVkLnVuYy5lZHU=
 Xiaolei Pei
Xiaolei Pei Lauriel Freya Earley2
Lauriel Freya Earley2 Xiaojing Chen
Xiaojing Chen Richard Jude Samulski
Richard Jude Samulski Chengwen Li
Chengwen Li