- Department of Oncology, Luohe Central Hospital, Luohe, China
Colorectal cancer (CRC) has emerged as a major public health concern in China during the last decade. In this study, we investigated the disease burden posed by CRC and analyzed temporal trends in CRC incidence and mortality rates in this country. We collected CRC incidence data from the Cancer Incidence in Five Continents, Volume XI dataset and the age-standardized incidence rate (ASIR) and age-standardized mortality rate (ASMR) of CRC by sex and age, from the 2016 Global Burden of Diseases Study. We used the average annual percentage change (AAPC) to quantify temporal trends in CRC incidence and mortality from 1990 to 2016 and found the ASIR of CRC increased from 14.25 per 100,000 in 1990 to 25.27 per 100,000 in 2016 (AAPC = 2.34, 95% confidence interval [CI] 2.29, 2.39). Cancer cases increased from 104.3 thousand to 392.8 thousand during the same period. The ASIR increased by 2.76% (95% CI 2.66%, 2.85%) and 1.70% (95% CI 1.64%, 1.76%) per year in males and females, respectively. The highest AAPC was found in people aged 15–49 years (2.76, 95% CI 2.59, 2.94). Cancer deaths increased from 81.1 thousand in 1990 to 167.1 thousand in 2016, while the ASMR remained stable (−0.04, 95% CI −0.13, 0.05), A mild increase (AAPC = 0.42, 95% CI 0.34, 0.51) was found among males and a significant decrease (AAPC = −0.75, 95% CI −0.90, −0.60) was found among females. Between 2016 and 2025, cancer cases and deaths are expected to increase from 392.8 and 167.1 thousand in 2016 to 642.3 (95% CI 498.4, 732.1) and 221.1 thousand (95% CI 122.5, 314.8) in 2025, respectively. Our study showed a steady increase in the CRC incidence in China over the past three decades and predicted a further increase in the near future. To combat this health concern, the prevention and management of known risk factors should be promoted through national polices. Greater priority should be given to CRC prevention in younger adults, and CRC screening should be widely adopted for this population.
Introduction
Colorectal cancer (CRC) is a common diagnosed malignant neoplasm, which ranks third among all cancers in terms of incidence and second in terms of mortality (1). In 2018, nearly 2.0 million newly diagnosed CRC cases and more than 0.8 million related deaths are expected to occur worldwide (1). CRC incidence rates vary substantially across the world, with the highest rates observed in parts of Europe (e.g., Slovakia, the Netherlands, Norway, and Hungary), in which the age-standardized incidence rates (ASIRs) have been as high as 60 per 100,000 for males and 35 per 100,000 for females (2), but CRC ASIRs in Asia have been two to three-fold lower than that in Europe (2, 3). The striking disparity in the global incidence of CRC reflects the strong impact of lifestyle factors on the occurrence of this cancer (4). Moreover, variations in genetic background between different populations, such as single nucleotide polymorphisms in certain genes, have been hypothesized to be associated with CRC genesis (5–7). CRC incidence and mortality rates have been stabilizing or decreasing in highly developed countries through the enormous efforts over the last decades (8). However, a rapid upward trend has been seen in many low-income and middle-income countries (4).
China has been the world's fastest-growing developing country for the past four decades. Urbanization, aging population, and shift to sedentary lifestyle and Westernized diet has led to a shift in the disease burden from infectious to non-communicable diseases (9). One of the major public health issues is the rapid rise in CRC incidence and the accompanying increase in disease burden (10, 11). According to cancer statistics for 2010 (12), ~274.8 thousand new CRC cases and 132.1 thousand CRC related deaths were estimated to occur in China, accounting for nearly one tenth of the global CRC burden. Knowing the epidemiological features of CRC, including its temporal trends and geographical patterns in China, therefore, is critical for both the prevention and management of cancer in this country.
This study used the CRC incidence and mortality data of China from the Global Burden of Diseases (GBD) 2016 Study and the data from the Cancer Incidence in Five Continents Volume XI of the International Agency for Research on Cancer. Both databases provide us with a unique opportunity to understand the CRC landscape in China. We described the contemporary geographical distribution of CRC incidence and assessed the temporal trends in its incidence and mortality from 1990 to 2016. Moreover, we conducted a forecasted CRC incidence and mortality up to 2025. The results yielded by our study should be useful for the assessment of current prevention strategies, to enhance our understanding of and planning to manage the disease burden, and to promote the evolution of China's Health System to respond to future challenges.
Materials and Methods
Study Data
We collected CRC incidence data from the Cancer Incidence in Five Continents Volume XI (CI5XI) dataset (13), which contains the average annual incidence of cancer (2008–2012) by sex, site, and age-group per 100,000 population from 36 cancer registries in China (Figure 1B, the table embedded in the Figure 1). The codes from 10th revision of the International Statistical Classification of Diseases and Related Health Problems (2010) corresponding to CRC (colon cancer: C18; rectal cancer: C19-20) were used to identify cancer cases. We combined colon cancer and rectal cancer to estimate the overall ASIR of CRC in different regions in China. We merged the data and provided a summary estimate for the provinces with several cancer registries. The ASIRs of CRC were plotted on a national map. We retrieved annual incident cases, ASIRs, mortality, age-standardized mortality rates (ASMRs), and Disability-Adjusted Life Years (DALYs), Years Lived with Disability (YLDs), and Years with Life Lost (YLLs) of CRC from 1990 to 2016, by sex and age group from the Global Health Data Exchange, a comprehensive online catalog of the GBD data (http://ghdx.healthdata.org/gbd-results-tool) (14). The cancer incidence was sought from individual cancer registries or aggregated databases of cancer registries, including Cancer Incidence in Five Continents (CI5) (15) and SEER (Surveillance, Epidemiology, and End Results) (16). Like data in CI5XI database, all cancer data stored in GBD were stratified by cancer site, country or region, age group, sex, and time. The general methods used in the 2016 GBD Study and the methods for estimating the disease burden of CRC have been detailed in previous studies (17). We matched all neoplasms as defined in the International Statistical Classification of Diseases and Related Health Problems to one of the 29 GBD cancer groups (18, 19). In the GBD Study, 95% uncertainty intervals (95% UI) were calculated to provide information on the variability of estimates resulting from errors related to the sampling process, and to non-sampling errors related to adjustments to data sources and modeling (18).
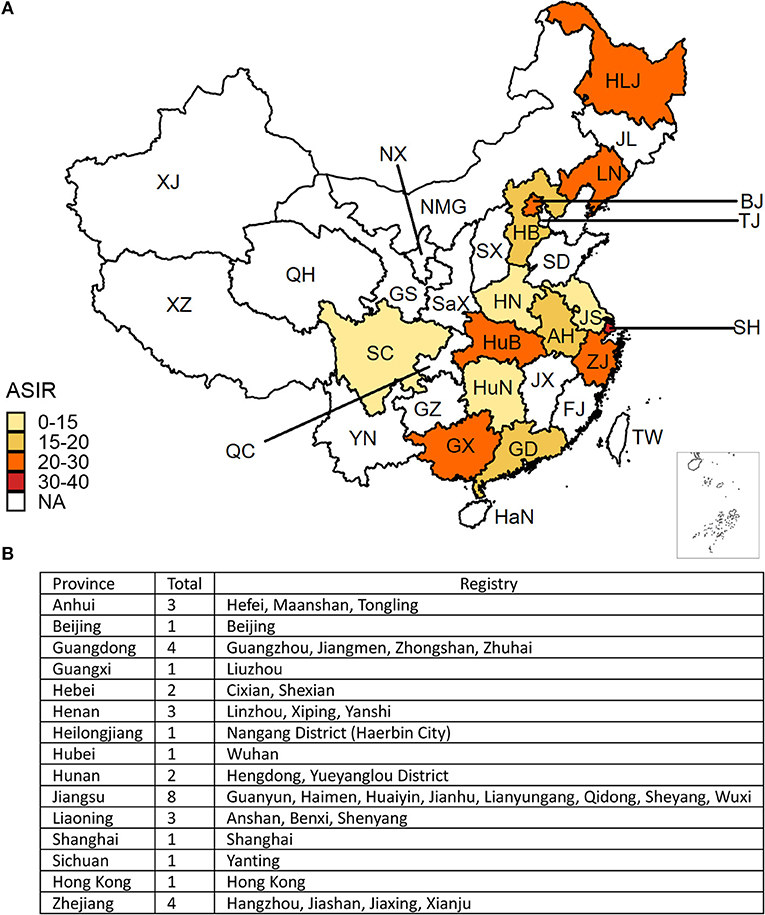
Figure 1. (A) The geographical distribution of the age-standardized incidence rate (ASIR) per 100,000 colorectal cancer cases in China. The deeper the color, the higher the rate. The white areas indicate no data were mapped. (B) The cancer registries included in this analysis. (BJ, Beijing; TJ, Tianjin; HB, Hebei; NMG, Inner Mongolia; SX, Shanxi; HLJ, Heilongjiang; JL, Jilin; LN, Liaoning; SD, Shandong; JS, Jiangsu; SH, Shanghai; ZJ, Zhejiang; AH, Anhui; FJ, Fujian; JX, Jiangxi; GD, Guangdong; GX, Guangxi; HaN, Hainan; YN, Yunnan; GZ, Guizhou; XZ, Tibet; SC, Sichuan; QC, Chongqing; HuN, Hunan; HuB, Hubei; HN, Henan; SaX, Shaanxi; GS, Gansu; NX, Ningxia; QH, Qinghai; XJ, Xinjiang).
Statistical Analysis
The Standard World Population 2000 was used to estimate ASIRs of CRC per 100,000 person years for all regions. We used the average annual percentage change (AAPC) to quantify temporal trends in CRC incidence and mortality from 1990 to 2016 (20). A regression line was fitted to the natural logarithm of the rates, i.e., y = α + βx + ε, where y = ln(ASIR or ASMR), and x = calendar year. The AAPC was calculated as 100 × (exp(β)-1). The 95% confidence interval (CI) of the AAPC was also calculated in the regression model. The ASIR (ASMR) showed an upward trend when the AAPC estimation and the lower boundary of its 95% CI were both > 0. In contrast, the ASIR (ASMR) showed a downward trend when the AAPC estimation and the upper boundary of its 95% CI were both < 0. Otherwise, the ASIR (ASMR) was deemed to be stable over time (21). For a clearer depiction of the temporal trend of the ASIR, we divided the study period into two discrete timeframes (1990–2010 and 2011–2016) and assessed the ASIR trend for each timeframe. The study period for the ASMR was also separated into two discrete periods (1990–2002 and 2003–2016), and the AAPC for each timeframe was also assessed separately. Furthermore, we predicted the numbers of cases and deaths from CRC between 2017 and 2025 by conducting a Bayesian age-period-cohort analysis using integrated nested Laplace approximations, which has been well-documented and validated in other studies (22, 23). All statistical analyses were performed using the R program (Version 3.4.1, R core team). The Bayesian age-period-cohort model was analyzed using the BAPC (Bayesian age-period-cohort) and INLA (integrated nested Laplace approximation) packages in R. A P < 0.05 was considered statistically significant.
Results
The CRC incidence data retrieved from the 36 cancer registries in 15 provinces were included in the study (Figure 1). The combined CRC ASIRs from 2008 to 2012 showed significant variation across the country, with the highest ASIR observed in Hong Kong (39.97 per 100,000), followed by Shanghai (32.30 per 100,000), Liaoning (29.62 per 100,000), and Zhejiang (24.30 per 100,000). At the national level, the ASIR of CRC increased from 14.25 (95% UI 13.75, 14.93) per 100,000 in 1990 to 25.27 (95% UI 24.02, 26.47) per 100,000 in 2016, with an overall AAPC of 2.34 (95% CI 2.29, 2.39). Cancer cases increased nearly three-fold from 104.3 thousand (95% UI 100.6, 109.4) to 392.8 thousand (95% UI 373.0, 412.4) during the same period (Table 1). CRC cases and ASIRs were significantly higher in males than females. Among males, the ASIR increased by 2.76% (95% CI 2.66, 2.85) per year from 1990 to 2016, and the cancer cases increased more than three times compared to 1990. Among females, the ASIR of CRC increased from 12.43 per 100,000 to 18.83 per 100,000, with an AAPC of 1.70 (95% CI 1.64, 1.76). Cancer cases among females increased from 46.9 thousand to 148.9 thousand during the study period (Table 1). The specific timeframes of the AAPCs in the ASIRs are presented in Figure 2A. In general, the CRC ASIRs of both males and females rapidly increased from 1990 to 2010; thereafter, the rising trends slowed. We also examined the CRC ASIRs among the three age groups. The highest ASIR was observed among individuals aged 50–69 years in terms of number of cancer cases and in those aged 70+ years in terms of the ASIR. However, the highest AAPC was found in people aged 15–49 years (2.76, 95% CI 2.59, 2.94) (Table 1).
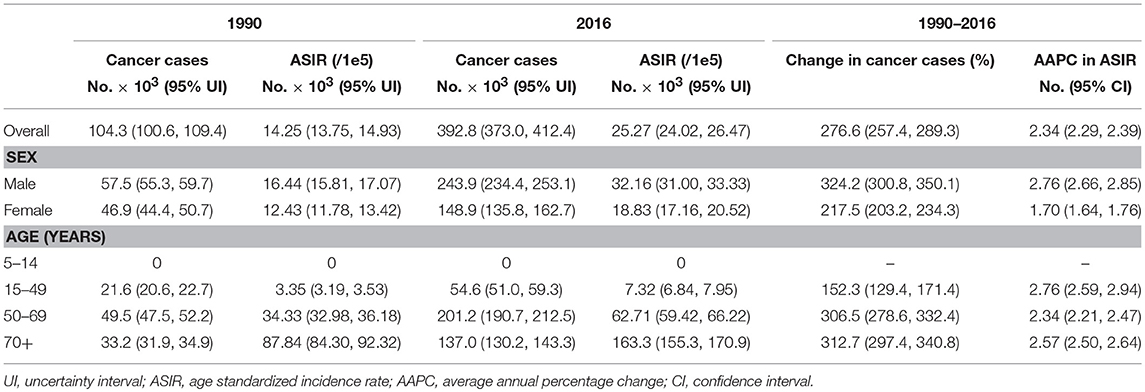
Table 1. The cancer cases and age standardized incidence rate of colorectal cancer in China in 1990 and 2016.
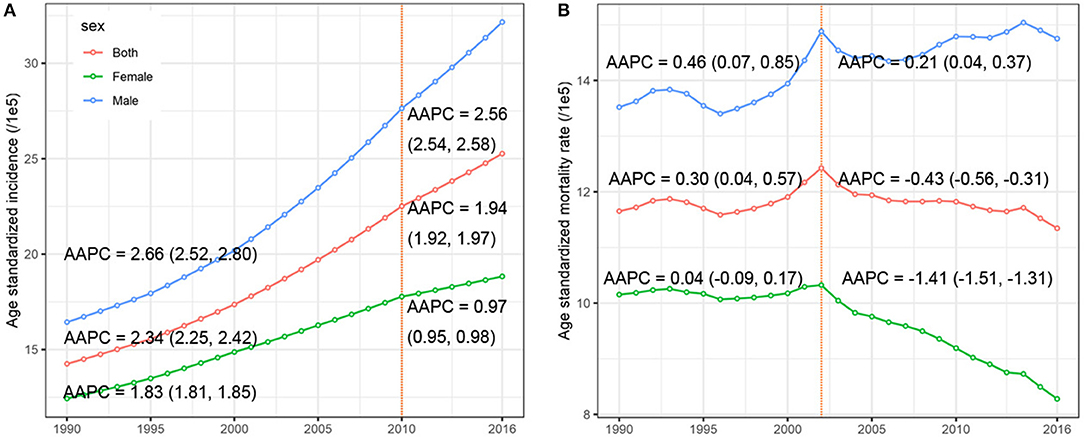
Figure 2. The temporal trends of the (A) age-standardized incidence rate, (B) age-standardized mortality rate, by sex, from 1990 to 2016. The AAPCs presented in (A,B) were derived from a period-specific data: (A) 1990–2010 and 2011–2016; (B) 1990–2002 and 2003–2016.
Table 2 presents the ASMR and cancer deaths from CRC in 1990 and 2016. Overall, cancer deaths increased from 81.1 thousand in 1990 to 167.1 thousand in 2016, whereas the ASMR remained stable (AAPC = −0.04, 95% CI −0.13, 0.05) during the same period. For males, a mild increase (AAPC = 0.42, 95% CI 0.34, 0.51) was observed in the ASMR during 1990-2016. In contrast, a significant decrease (AAPC = −0.75, 95% CI −0.90, −0.60) was found in females. The AAPC in the CRC ASMR was time dependent. As shown in Figure 2B, an increase in the overall ASMR was observed from 1990 to 2002, whereas the ASMR decreased thereafter (AAPC = −0.43, 95% CI −0.56, −0.31). The ASMR increased among males by 0.46 per year from 1990 to 2002, and the rising trend slowed from 2003 to 2016. Among females, a stable trend in the ASMR was found between 1990 and 2002. However, the ASMR showed a downward trend after 2002. The significant increase was detected only among people aged 70+ years (AAPC = 0.51, 95% CI 0.38, 0.63) (Table 2). We also retrieved the data on risk factors (including tobacco use, metabolic risks, high body-mass index [BMI], diet high in red meat, and diet high in processed meat) for CRC mortality during the study period. The temporal trends in the risk factors contribution for CRC mortality were estimated (Figure 3). Among the five well-established risk factors, the most significant contribution was tobacco use, followed by metabolic risks, high BMI, diet high in red meat, and diet high in processed meat. In contrast, the most pronounced increases in CRC death rate was found in diet high in red meat and diet high in processed meat.
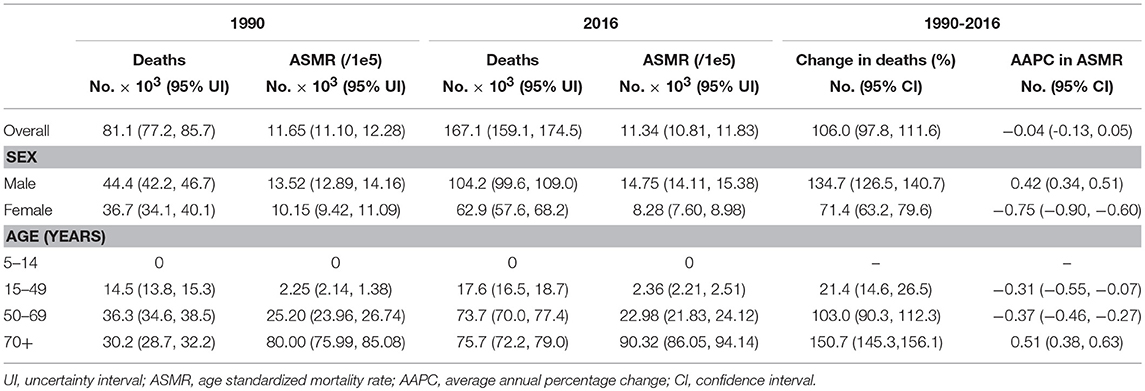
Table 2. The deaths and age standardized mortality rate of colorectal cancer in China in 1990 and 2016.
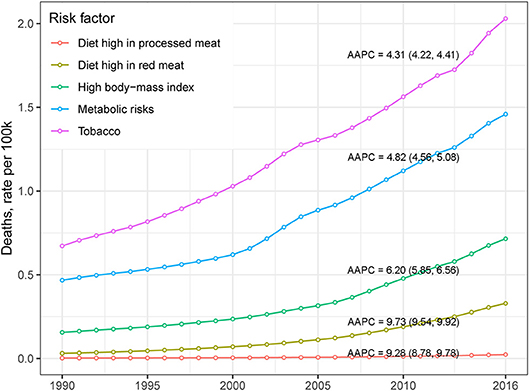
Figure 3. The temporal trends of CRC death rate, stratified by risk factor, in China from 1990 to 2016.
The annual Disability-Adjusted Life Years (DALYs), Years Lived with Disability (YLDs), and Years with Life Lost (YLLs) of CRC stratified by sex are presented in Figure 4. Overall, the disease burden posed by CRC was substantially increased in China during the study period. Specifically, the DALYs, YLDs, and YLLs increased 79.4, 354.4, and 64.5, respectively, between 1990 and 2016.
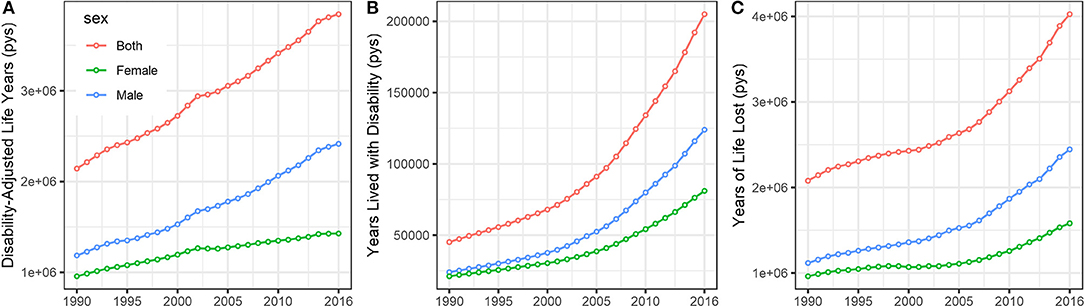
Figure 4. The temporal trends of the (A) Disability-Adjusted Life Years (DALYs), (B) Years Lived with Disability (YLDs), and (C) Years with Life Lost (YLLs) of CRC stratified by sex in China from 1990 to 2016. (pys, person years).
The results of the forecast of CRC cases and deaths for 2017–2025 based on the data from the GBD Study are shown in Figure 5. New CRC cases and deaths will continue to rise in the near future. Cancer cases among males will increase from 243.9 thousand in 2016 to 410.9 thousand (95% CI 398.4, 422.4) in 2025, and the corresponding cancer-related deaths will increase from 104.2 thousand to 153.4 thousand (95% CI 142.2, 164.8) during this period. Among females, cancer cases will increase from 148.9 thousand in 2016 to 231.4 thousand (95% CI 213.2, 253.7) in 2025. Accordingly, the number of deaths caused by CRC will increase from 62.9 thousand to 67.7 thousand (95% CI 63.2, 73.7).
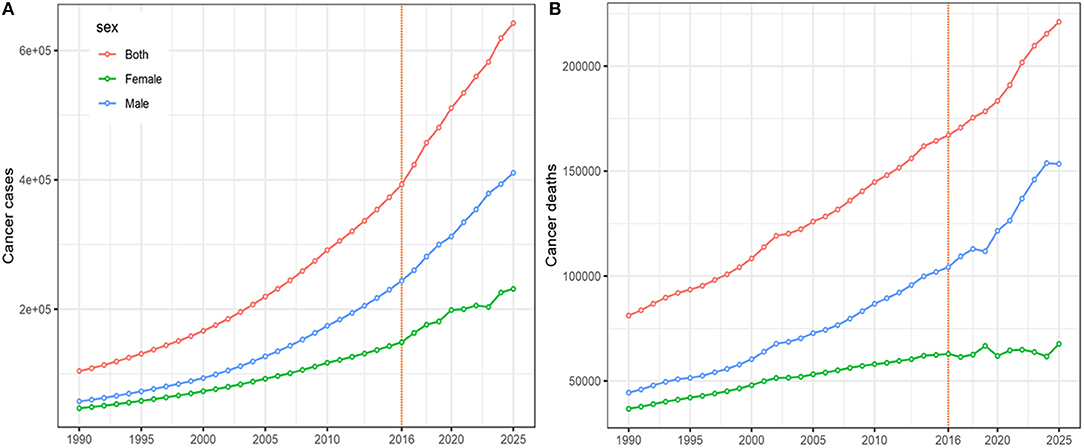
Figure 5. The projections of colorectal cancer cases (A) and deaths (B), by sex, from 2017 to 2025 in China.
Discussion
Colorectal cancer is one of the most common malignancies diagnosed worldwide (1). In this study, we analyzed the disease burden caused by CRC and temporal trends in CRC incidence and mortality in China. In general, the CRC incidence was relatively lower than that of developed countries (4, 24–26). However, this study showed an upward trend in its incidence, although there has been a downward trend in the mortality of CRC during the past three decades. The CRC incidence and mortality in males were almost twice as high as that of females. The most pronounced increase in the incidence of CRC was observed in younger and middle-aged adults.
Risk factors for CRC have been investigated extensively in the last decade. Previous epidemiological studies have suggested that alcohol consumption, diets high in meat intake, obesity, smoking, and physical inactivity are associated with an increased risk of CRC in various populations (27–31). Targeted prevention strategies for combating CRC have led to a significant decrease in its incidence in several developed countries (32, 33). However, an upward trend has been observed in most developing countries (4). Likewise, in this study, we found that CRC incidence has rapidly increased from 1990 to 2016 and will further increase in China over the next decade. This remarkable increase might be attributed mainly to the upward trend in Western dietary patterns (34), changes in occupational patterns (35), increases in high-risk behaviors (e.g., smoking and excessive calorie intake) (36), and an increase in the aging population (3). Moreover, changes in access to health care and early cancer screenings might contribute to variations in CRC incidence (37). In a report of a longitudinal analysis of dietary patterns of Chinese adults from 1991 to 2009, the authors expressed concern about the increasing popularity of the modern high-wheat dietary pattern, which consists of energy-dense foods, in the context of China's rapid economic changes (38). A Westernized diet, characterized by high intake in red meat and processed meat (39), might drive changes in cancer profiles, including an increase in CRC (34), which has been verified by epidemiological studies on immigrants (40, 41). For example, the CRC incidence among Asian migrants to the US has shifted to an intermediate level between their country of origin and their new country of residence (40, 41). The shift in diet pattern and the concurrently increase in CRC incidence rate in Chinese population emphasizes the importance of establishment of healthy diet for CRC prevention. In this regard, the general population might be benefitted more from a traditional Chinese diet or Mediterranean diet pattern (42, 43). Moreover, the prevalence of known risk factors, such as obesity, diabetes, and smoking, are still on the rise (44–47), although measures have been initiated to counter these risks. As a result, the incidence of CRC is expected to increase in the future as predicted in this study if effective interventions are not introduced.
Surprisingly, we found that the most pronounced increase in CRC incidence was observed in people aged 15–49 years. This finding is consistent with those of countries in which similar trends in younger populations have been reported over roughly the same period (48). For instance, in the US, the incidence rates of CRC among people below 50 years of age rose 1.61% per year in men and 1.46% per year in women from 1998 to 2009 (49). As more than 80% of young-onset CRC cases are diagnosed with symptomatic disease, which may be associated with a delay in diagnosis and poor prognosis (50), greater emphasis should be placed on young adults' risk reduction and awareness of CRC symptoms, and greater vigilance by healthcare providers is needed for the early detection of CRC in young patients.
Over the past two decades, the range of CRC screening modalities has expanded, and many population-based programs have been initiated (51). As expected, screening programs have been more frequently implemented in Western countries with higher rates of CRC and more available resources. Recommendations for CRC screening in the Asia Pacific region have been published and recently updated (52). Currently, those aged 40–74 years in China are screened using the fecal occult blood test, and followed up with a digital rectal exam and colonoscopy, which might influence the temporal trends in CRC, especially in the recent years. However, this program is far from complete and the national registry used to track clinical outcomes captures only 13% of the country's population, increasing the difficulty of planning healthcare services (53). The screening method also have impact on the identification of CRC subtypes. For example, more sigmoidoscopy screening would identify more rectal cases than colonoscopy does, though these screening methods have not widely adopted in China due to their cost and availability. In addition, screening methods with improved diagnostic ability or more sensitive biomarkers, such as methylated Septin9 (mSEPT9), have been proposed and developed (54), though these potential biomarkers might be expensive and also not recommended by the current global guidelines or Asian-pacific guidelines for CRC screening.
Although the incidence of CRC is on the rise, we have observed a mild decrease in the CRC mortality rate, especially in recent years. This decrease might involve at least two aspects. First, the implementation of CRC screening to detect cancer in the earlier stages has demonstrated its effectiveness in populations that have initiated early screening programs. Second, the clinical treatment and management of CRC patients has received substantial improvement. For example, the 5-year survival rate has increased from 49% in the 1960s to 77% in the 2000s among CRC patients (55). The unequilibrium between CRC incidence rate and mortality rate can be partly explained by these reasons. Other factors contributed to this unequilibrium warrant further investigations. Of note, cancer-related deaths have doubled between 1990 and 2016 and are expected to increase in the near future, which might be the results of population expansion and aging. CRC, therefore, is a major public health concern, and poses a heavy disease burden on China.
Our study has limitations. First, we used multiple data sources that represented a diverse population. The data were retrieved from different international agencies; therefore, incompatibilities between these data might exist. Second, changes in risk factors and population structures were not taken into account when we predicted the cancer cases and deaths in future years. Finally, since the diagnostic pattern of CRC depends strongly on the screening compliance in the population and the screening method might also have effect on the disease form, the results presented in our study might be interpreted with cautions.
In summary, our study presents evidence of a steady increase in CRC incidence in China over the past three decades. It also predicts further increases in the near future. The sample's diversity of age, gender, and geography may yield insights for the future development of national programs. For example, the prevention and management of known risk factors, e.g., obesity and smoking, should be improved through effective national polices. Efforts should be made to prioritize CRC prevention in younger adults, as the incidence of CRC has increased in this group, and it could be prevented through the adoption of screening programs for this population.
Author Contributions
TM: study design; LZ, FC, GZ, LS, SC, ZZ, and WZ: data collection; LZ and GZ: data analyses; all authors: results interpretations; LZ, FC, GZ, LS, SC, ZZ, and WZ: manuscript writing; TM and WZ: manuscript proofing.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
CRC, colorectal cancer; ASIR, age-standardized incidence rate; ASMR, age-standardized mortality rate; AAPC, average annual percentage change; GBD, global burden of diseases.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–24. doi: 10.3322/caac.21492
2. Douaiher J, Ravipati A, Grams B, Chowdhury S, Alatise O, Are C. Colorectal cancer-global burden, trends, and geographical variations. J Surg Oncol. (2017) 115:619–30. doi: 10.1002/jso.24578
3. Favoriti P, Carbone G, Greco M, Pirozzi F, Pirozzi RE, Corcione F. Worldwide burden of colorectal cancer: a review. Updates Surg. (2016) 68:7–11. doi: 10.1007/s13304-016-0359-y
4. Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut (2017) 66:683–91. doi: 10.1136/gutjnl-2015-310912
5. Takahashi Y, Sugimachi K, Yamamoto K, Niida A, Shimamura T, Sato T, et al. Japanese genome-wide association study identifies a significant colorectal cancer susceptibility locus at chromosome 10p14. Cancer Sci. (2017) 108:2239–47. doi: 10.1111/cas.13391
6. Tanikawa C, Kamatani Y, Takahashi A, Momozawa Y, Leveque K, Nagayama S, et al. GWAS identifies two novel colorectal cancer loci at 16q24.1 and 20q13.12. Carcinogenesis (2018) 39:652–60. doi: 10.1093/carcin/bgy026
7. Tanskanen T, van den Berg L, Valimaki N, Aavikko M, Ness-Jensen E, Hveem K, et al. Genome-wide association study and meta-analysis in Northern European populations replicate multiple colorectal cancer risk loci. Int J Cancer (2018) 142:540–6. doi: 10.1002/ijc.31076
8. Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. (2017) 67:177–93. doi: 10.3322/caac.21395
9. Yang G, Wang Y, Zeng Y, Gao GF, Liang X, Zhou M, et al. Rapid health transition in China, 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet (2013) 381:1987–2015. doi: 10.1016/s0140-6736(13)61097-1
10. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. (2016) 66:115–32. doi: 10.3322/caac.21338
11. Zheng R, Zeng H, Zhang S, Chen T, Chen W. National estimates of cancer prevalence in China, 2011. Cancer Lett. (2016) 370:33–8. doi: 10.1016/j.canlet.2015.10.003
12. Zheng ZX, Zheng RS, Zhang SW, Chen WQ. Colorectal cancer incidence and mortality in China, 2010. Asian Pac J Cancer Prev. (2014) 15:8455–60. doi: 10.7314/APJCP.2014.15.19.8455
13. Bray F, Colombet M, Mery L, Piñeros M, Znaor A, Zanetti R, Ferlay J. Cancer Incidence in Five Continents, Vol. XI (electronic version). Lyon: International Agency for Research on Cancer (2017). Available online at: http://ci5.iarc.fr (Accessed August, 30 2018).
14. Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2016 (GBD 2016) Results. Seattle: Institute for Health Metrics and Evaluation (IHME) (2017). Available Online at: http://ghdx.healthdata.org/gbd-results-tool
15. Bray F, Ferlay J, Laversanne M, Brewster DH, Gombe Mbalawa C, Kohler B, et al. Cancer incidence in five continents: inclusion criteria, highlights from Volume X and the global status of cancer registration. Int J Cancer (2015) 137:2060–71. doi: 10.1002/ijc.29670
16. Mertens AC, Yong J, Dietz AC, Kreiter E, Yasui Y, Bleyer A, et al. Conditional survival in pediatric malignancies: analysis of data from the childhood cancer survivor study and the surveillance, epidemiology, and end results program. Cancer (2015) 121:1108–17. doi: 10.1002/cncr.29170
17. Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. (2017) 3:524–48. doi: 10.1001/jamaoncol.2016.5688
18. GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet (2016) 388:1459–544. doi: 10.1016/s0140-6736(16)31012-1
19. Silva DAS, Tremblay MS, Souza MFM, Mooney M, Naghavi M, Malta DC. Mortality and years of life lost by colorectal cancer attributable to physical inactivity in Brazil (1990-2015): findings from the global burden of disease study. PLoS ONE (2018) 13:e0190943. doi: 10.1371/journal.pone.0190943
20. Clegg LX, Hankey BF, Tiwari R, Feuer EJ, Edwards BK. Estimating average annual per cent change in trend analysis. Stat Med. (2009) 28:3670–82. doi: 10.1002/sim.3733
21. Liu Z, Jiang Y, Yuan H, Fang Q, Cai N, Suo C, et al. The trends in incidence of primary liver cancer caused by specific etiologies: Results from the Global Burden of Disease Study 2016 and implications for liver cancer prevention. J Hepatol. (2018). doi: 10.1016/j.jhep.2018.12.001. [Epub ahead of print].
22. Pearson-Stuttard J, Guzman-Castillo M, Penalvo JL, Rehm CD, Afshin A, Danaei G, et al. Modeling future cardiovascular disease mortality in the united states: national trends and racial and ethnic disparities. Circulation (2016) 133:967–78. doi: 10.1161/circulationaha.115.019904
23. Riebler A, Held L. Projecting the future burden of cancer: Bayesian age-period-cohort analysis with integrated nested Laplace approximations. Biom J. (2017) 59:531–49. doi: 10.1002/bimj.201500263
24. Young JP, Win AK, Rosty C, Flight I, Roder D, Young GP, et al. Rising incidence of early-onset colorectal cancer in Australia over two decades: report and review. J Gastroenterol Hepatol. (2015) 30:6–13. doi: 10.1111/jgh.12792
25. Center MM, Jemal A, Smith RA, Ward E. Worldwide variations in colorectal cancer. CA Cancer J Clin. (2009) 59:366–78. doi: 10.3322/caac.20038
26. Brouwer NPM, Bos A, Lemmens V, Tanis PJ, Hugen N, Nagtegaal ID, et al. An overview of 25 years of incidence, treatment and outcome of colorectal cancer patients. Int J Cancer (2018) 143:2758–66. doi: 10.1002/ijc.31785
27. Dashti SG, Buchanan DD, Jayasekara H, Ait Ouakrim D, Clendenning M, Rosty C, et al. Alcohol consumption and the risk of colorectal cancer for mismatch repair gene mutation carriers. Cancer Epidemiol Biomarkers Prev. (2017) 26:366–75. doi: 10.1158/1055-9965.epi-16-0496
28. Feng YL, Shu L, Zheng PF, Zhang XY, Si CJ, Yu XL, et al. Dietary patterns and colorectal cancer risk: a meta-analysis. Eur J Cancer Prev. (2017) 26:201–11. doi: 10.1097/cej.0000000000000245
29. Bardou M, Barkun AN, Martel M. Obesity and colorectal cancer. Gut (2013) 62:933–47. doi: 10.1136/gutjnl-2013-304701
30. Liang PS, Chen TY, Giovannucci E. Cigarette smoking and colorectal cancer incidence and mortality: systematic review and meta-analysis. Int J Cancer (2009) 124:2406–15. doi: 10.1002/ijc.24191
31. Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet (2012) 380:219–29. doi: 10.1016/s0140-6736(12)61031-9
32. Siegel RL, Fedewa SA, Anderson WF, Miller KD, Ma J, Rosenberg PS, et al. Colorectal cancer incidence patterns in the United States, 1974-2013. J Natl Cancer Inst. (2017) 109. doi: 10.1093/jnci/djw322
33. Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer (2013) 49:1374–403. doi: 10.1016/j.ejca.2012.12.027
34. Mehta RS, Song M, Nishihara R, Drew DA, Wu K, Qian ZR, et al. Dietary patterns and risk of colorectal cancer: analysis by tumor location and molecular subtypes. Gastroenterology (2017) 152:1944–53.e1. doi: 10.1053/j.gastro.2017.02.015
35. Lo AC, Soliman AS, Khaled HM, Aboelyazid A, Greenson JK. Lifestyle, occupational, and reproductive factors and risk of colorectal cancer. Dis Colon Rectum. (2010) 53:830–7. doi: 10.1007/DCR.0b013e3181d320b1
36. Jayasekara H, English DR, Haydon A, Hodge AM, Lynch BM, Rosty C, et al. Associations of alcohol intake, smoking, physical activity and obesity with survival following colorectal cancer diagnosis by stage, anatomic site and tumor molecular subtype. Int J Cancer (2018) 142:238–50. doi: 10.1002/ijc.31049
37. Welch HG, Brawley OW. Scrutiny-dependent cancer and self-fulfilling risk factors. Ann Intern Med. (2018) 168:143–4. doi: 10.7326/m17-2792
38. Batis C, Sotres-Alvarez D, Gordon-Larsen P, Mendez MA, Adair L, Popkin B. Longitudinal analysis of dietary patterns in Chinese adults from 1991 to 2009. Br J Nutr. (2014) 111:1441–51. doi: 10.1017/s0007114513003917
39. Rai SK, Fung TT, Lu N, Keller SF, Curhan GC, Choi HK. The dietary approaches to stop hypertension (DASH) diet, western diet, and risk of gout in men: prospective cohort study. BMJ (2017) 357:j1794. doi: 10.1136/bmj.j1794
40. Flood DM, Weiss NS, Cook LS, Emerson JC, Schwartz SM, Potter JD. Colorectal cancer incidence in Asian migrants to the United States and their descendants. Cancer Causes Control (2000) 11:403–11. doi: 10.1023/A:1008955722425
41. Gomez SL, Le GM, Clarke CA, Glaser SL, France AM, West DW. Cancer incidence patterns in Koreans in the US and in Kangwha, South Korea. Cancer Causes Control (2003) 14:167–74. doi: 10.1023/A:1023046121214
42. Farinetti A, Zurlo V, Manenti A, Coppi F, Mattioli AV. Mediterranean diet and colorectal cancer: a systematic review. Nutrition (2017) 43–44:83–8. doi: 10.1016/j.nut.2017.06.008
43. Hang J, Cai B, Xue P, Wang L, Hu H, Zhou Y, et al. The joint effects of lifestyle factors and comorbidities on the risk of colorectal cancer: a large Chinese retrospective case-control study. PLoS ONE (2015) 10:e0143696. doi: 10.1371/journal.pone.0143696
44. Hu L, Huang X, You C, Li J, Hong K, Li P, et al. Prevalence of overweight, obesity, abdominal obesity and obesity-related risk factors in southern China. PLoS ONE (2017) 12:e0183934. doi: 10.1371/journal.pone.0183934
45. Zhang P, Wang R, Gao C, Jiang L, Lv X, Song Y, et al. Prevalence of central obesity among adults with normal BMI and its association with metabolic diseases in northeast China. PLoS ONE (2016) 11:e0160402. doi: 10.1371/journal.pone.0160402
46. Wang L, Gao P, Zhang M, Huang Z, Zhang D, Deng Q, et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. J Am Med Assoc. (2017) 317:2515–23. doi: 10.1001/jama.2017.7596
47. Wang M, Luo X, Xu S, Liu W, Ding F, Zhang X, et al. Trends in smoking prevalence and implication for chronic diseases in China: serial national cross-sectional surveys from 2003 to 2013. Lancet Res Med. (2018) 7:35–45. doi: 10.1016/S2213-2600(18)30432-6
48. Patel P, De P. Trends in colorectal cancer incidence and related lifestyle risk factors in 15-49-year-olds in Canada, 1969-2010. Cancer Epidemiol. (2016) 42:90–100. doi: 10.1016/j.canep.2016.03.009
49. Austin H, Henley SJ, King J, Richardson LC, Eheman C. Changes in colorectal cancer incidence rates in young and older adults in the United States: what does it tell us about screening. Cancer Causes Control (2014) 25:191–201. doi: 10.1007/s10552-013-0321-y
50. Quah HM, Joseph R, Schrag D, Shia J, Guillem JG, Paty PB, et al. Young age influences treatment but not outcome of colon cancer. Ann Surg Oncol. (2007) 14:2759–65. doi: 10.1245/s10434-007-9465-x
51. Schreuders EH, Ruco A, Rabeneck L, Schoen RE, Sung JJ, Young GP, et al. Colorectal cancer screening: a global overview of existing programmes. Gut (2015) 64:1637–49. doi: 10.1136/gutjnl-2014-309086
52. Sung JJ, Ng SC, Chan FK, Chiu HM, Kim HS, Matsuda T, et al. An updated Asia Pacific consensus recommendations on colorectal cancer screening. Gut (2015) 64:121–32. doi: 10.1136/gutjnl-2013-306503
53. Goss PE, Strasser-Weippl K, Lee-Bychkovsky BL, Fan L, Li J, Chavarri-Guerra Y, et al. Challenges to effective cancer control in China, India, and Russia. Lancet Oncol. (2014) 15:489–538. doi: 10.1016/s1470-2045(14)70029-4
54. Xie L, Jiang X, Li Q, Sun Z, Quan W, Duan Y, et al. Diagnostic value of methylated Septin9 for colorectal cancer detection. Front Oncol. (2018) 8:247. doi: 10.3389/fonc.2018.00247
Keywords: colorectal cancer, incidence, mortality, prediction, China
Citation: Zhang L, Cao F, Zhang G, Shi L, Chen S, Zhang Z, Zhi W and Ma T (2019) Trends in and Predictions of Colorectal Cancer Incidence and Mortality in China From 1990 to 2025. Front. Oncol. 9:98. doi: 10.3389/fonc.2019.00098
Received: 07 November 2018; Accepted: 04 February 2019;
Published: 21 February 2019.
Edited by:
Tianhui Chen, Zhejiang Academy of Medical Sciences, ChinaReviewed by:
Alireza Sadjadi, Tehran University of Medical Sciences, IranAga Syed Sameer, King Saud bin Abdulaziz University for Health Sciences, Saudi Arabia
Hamideh Salimzadeh, Tehran University of Medical Sciences, Iran
Copyright © 2019 Zhang, Cao, Zhang, Shi, Chen, Zhang, Zhi and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tianjiang Ma, bWVkcmVzZUAxNjMuY29t
†These authors have contributed equally to this work
 Lei Zhang†
Lei Zhang† Tianjiang Ma
Tianjiang Ma