- 1Department of Public Health and Nursing, Faculty of Medicine and Health Science, Norwegian University of Science and Technology, Trondheim, Norway
- 2Department of Clinical and Molecular Medicine, Faculty of Medicine and Health Science, Norwegian University of Science and Technology, Trondheim, Norway
- 3Department of Thoracic Medicine, St. Olavs Hospital, Trondheim University Hospital, Trondheim, Norway
- 4K.G. Jebsen Centre for Genetic Epidemiology, Department of Public Health and Nursing, Norwegian University of Science and Technology, Trondheim, Norway
- 5MRC Integrative Epidemiology Unit, University of Bristol, Bristol, United Kingdom
- 6School of Epidemiology and Public Health, Faculty of Medicine, University of Ottawa, Ottawa, ON, Canada
- 7Clinic of Anesthesia and Intensive Care, St. Olavs Hospital, Trondheim University Hospital, Trondheim, Norway
Background: Prolonged sitting as a major sedentary behavior potentially contributes to illness, but its relation with lung cancer risk is unclear. Prolonged sitting can be presented in physically active or inactive individuals. Those who are extendedly seated and also physically inactive may represent the most sedentary people. We therefore aimed to prospectively examine if total sitting time daily itself or in combination with physical activity is associated with lung cancer incidence overall and histologic types.
Methods: We included 45,810 cancer-free adults who participated in the second survey of HUNT Study in Norway (1995–97), with a median follow-up of 18.3 years. Total sitting time daily and physical activity were self-reported at baseline. Lung cancer cases were ascertained from the Cancer Registry of Norway. Cox regression was used to estimate hazard ratios (HRs) with 95% confidence intervals (CIs).
Results: In total, 549 participants developed lung cancer during the follow-up. Total sitting time daily was not associated with the incidence of lung cancer overall and histologic subtypes. Compared with participants sitting < 8 h daily and being physically active, those sitting ≥8 h daily (prolonged sitting) and being physically inactive had an increased incidence of lung cancer (overall: adjusted HR = 1.44, 95% CI: 1.07–1.94; small cell lung cancer: adjusted HR = 2.58, 95% CI: 1.23–5.41). Prolonged sitting only or physical inactivity only was not associated with the incidence of lung cancer.
Conclusions: Our study suggested that prolonged sitting was not independently associated with lung cancer incidence. The combination of prolonged sitting and physical inactivity might increase the risk of lung cancer. However, residual confounding by smoking cannot be excluded completely even though smoking was adjusted for with detailed information.
Introduction
Lung cancer is one of the most common cancer types with a low survival rate (1). Small cell (SCLC) and non-small cell lung cancer (NSCLC) are the two major histologic types of lung cancer (2). Smoking is the most important risk factor for lung cancer, and less so for NSCLC than SCLC (3, 4). With a declining trend in smoking, other lifestyle factors may become more important for the incidence of lung cancer overall and histologic types. Physical activity or sedentary behavior has been suggested to be associated with the risk of cancer due to several plausible mechanisms including suppressed lipoprotein lipase activity (5, 6) and altered inflammatory pathways by lack of activities (7–9).
The relationship of physical activity with lung cancer risk has been extensively investigated. Recent meta-analysis studies have concluded that physical activity is associated with a reduced risk of lung cancer in smokers (10–12). Nonetheless, potential effects of physical activity and sedentary behavior might tangle in these meta-analysis studies since sedentary behavior was not properly taken into consideration in most of the individual studies.
Sedentary behavior describes a series of human behaviors requiring low energy expenditure in a sitting or reclining posture when awake (13). It is highly prevalent in western countries (14) and may be an independent risk factor for multiple health outcomes, including cancers (15, 16). Previous studies focused either on occupational sitting (17–19) or leisure-time TV watching (20, 21) in relation to lung cancer risk, with inconsistent results. Total sitting time daily is a better marker that reflects a sedentary lifestyle in the workplace, domestic environment and during leisure-time (22). However, there are limited studies on the relationship between total sitting time and lung cancer risk, and among them, one study found the association in a sub-population (23) and two studies did not adjust for physical activity properly due to lack of detailed information (21, 23). Physical inactivity and prolonged sitting are two distinct behaviors. Prolonged sitting can be present in physically active or inactive individuals. Those who are extendedly seated and also physically inactive may represent the most sedentary people. Thus, in the current study we aimed to prospectively examine the relationship between total sitting time daily and lung cancer risk (overall and major histologic types), taking smoking into consideration. We also investigated if different combinations of total sitting time and physical activity were associated with lung cancer incidence.
Materials and Methods
Study Design and Population
The HUNT study is a large population-based health study in Norway, which includes more than 97% Caucasian participants and well-represents the Norwegian population. It consists of three consecutive surveys; HUNT1 (1984–1986), HUNT2 (1995-1997), and HUNT3 (2006–2008) (24). At each survey, all adults 20 years or older living in the area of Nord-Trøndelag were invited to complete extensive health and lifestyle questionnaires and undergo a clinical examination (25).
A total of 65,229 adults participated (70% of invited) in HUNT2. Every participant was followed-up from the date of participation in HUNT2 until the date of first diagnosis of lung cancer, the date of death or emigration from Norway or the end of follow-up on December 31, 2014, whichever came first. Diagnosis of lung cancer was obtained from the Cancer Registry of Norway. Information on vital status and emigration was obtained from the Central Population Registry.
Among the 65,229 participants, 59,070 self-reported no cancers at baseline. We included 45,810 cancer-free participants with complete information on total sitting time daily in the main cohort. Additionally, we investigated the combination of total sitting time and physical activity in relation to lung cancer risk in a sub-cohort of 33,793 participants who also provided complete information on physical activity.
The study was approved by the Regional Committee for Medical and Health Research Ethics of South-East Norway. All participants signed informed written consent on participation in HUNT, linkage to previous HUNT surveys and specific registries in accordance with the Declaration of Helsinki.
Ascertainment of Lung Cancer
By using the unique 11-digit personal identification number, participants' information from HUNT2 was linked to the Cancer Registry of Norway (26). Data from the Cancer Registry of Norway are reasonably accurate, complete (overall completeness 98.8% in 2001–05) and timely (27). The International Classification of Diseases version 10 (ICD-10) codes used for registration of lung cancer are C33-C34 (28). Histologic types were classified according to International Classification of Diseases of Oncology (ICD-O) (29).
Measurement of Exposures
Time spent sitting daily was measured by the question: “How many hours do you usually spend in the sitting position during a 24-h period? (work, meals, television, car etc.).” The participant reported the total number of hours as a positive integer. We categorized total sitting time daily into three categories (0–4, 5–7, and ≥8 h) based on similar cutoff criteria from former HUNT studies (30, 31) and two meta-analysis studies (32, 33). Occupational activity was used as an alternative marker for a sedentary lifestyle and measured by the question: “How would you describe your work?” Based on four response options, we categorized it into mostly sedentary work, much walking or lifting (two response options collapsed into one category), heavy physical work, and an “unknown” group with missing information.
Leisure-time physical activity was based on the question “How much of your leisure time have you been physically active per week during the last year?” Participants were asked to report average hours of light (no sweating or not being out of breath) and hard physical activity (sweating or out of breath) with the following response options for each intensity; none, <1 h, 1–2 h, and ≥3 h (reported as a positive integer). We classified participants' physical activity level as inactive (no any activity, or ≤2 h light activity only), low (≥3 h light activity only, or ≤2 h light activity and < 1 h hard activity), moderate (≥3 h light activity and < 1 h hard activity, or 1–2 h hard activity regardless of light activity), and high (≥3 h hard activity regardless of light activity). This classification has demonstrated a dose-response relationship with mortality (34). Based on information of total sitting time and physical activity, we defined four combined categories: (1) total sitting time < 8 h daily and physically active; (2) total sitting time < 8 h daily and physically inactive; (3) total sitting time ≥8 h daily and physically active; and (4) total sitting time ≥8 h daily and physically inactive. Physically active referred to physical activity level from low to high. Physically inactive referred to no any activity or ≤2 h light activity only.
Information on Other Important Baseline Variables
Age, sex, body mass index (BMI), active smoking (status and pack-years), and passive smoking status, alcohol consumption, education, economic difficulties, family history of cancer and chronic bronchitis were included a priori as potential confounders. Weight and height in HUNT2 were measured by health professionals at clinical examination. BMI was calculated as weight in kilograms divided by height squared in meter (kg/m2) and was grouped into three categories (< 25.0, 25.0–29.9, and ≥30.0 kg/m2) according to the recommendations of the World Health Organization (WHO) (35). Active smoking status was classified into never, former, current smokers and further classified based on pack-years (≤10.0, 10.1–20.0, and >20.1). Other variables were categorized as: passive smoking (never, only childhood, only adulthood, and both), alcohol consumption (never, 1–4, and ≥5 times/month), education (< 10, 10–12, and ≥13 years, reported as a positive integer), economic difficulty (During the last year, has it at any time been difficulty to meet the cost of food, transportation, housing and such? yes/no), family history of cancer (Is there any family member such as father, mother, siblings who reported cancer? yes/no), and chronic bronchitis (Have you had a cough with phlegm for periods of at least 3 months during each of the last 2 years? yes/no). All missing information on the aforementioned variables was taken into analysis as an “unknown” category.
Statistical Analysis
Baseline characteristics of participants were presented by the categories of exposure variables. We used Cox proportional hazard models to examine the associations between total sitting time, and its combinations with physical activty and lung cancer incidence overall and histologic types and presented crude and adjusted hazard ratios (HR) with 95% confidence intervals (CI). Age was used as the time scale, with entry and exit time defined as the subject's age at recruitment and age at lung cancer diagnosis or censoring, respectively. When potential linear association between total sitting time and lung cancer risk was evaluated by likelihood ratio test, non-linearity was suggested (p = 0.02 for comparison between total sitting time used as a categorical variable and it used as an ordinal variable). Thus, the three categories (0–4, 5–7, and ≥8 h) of total sitting time were applied in the main analysis. Total sitting time was also categorized into tertiles.
In the sensitivity analysis, we first used occupational inactivity (mostly sedentary work) to test the robustness of our results on the assciation between total sitting time daily and lung cancer risk. Secondly, we performed analysis on the association of the combination groups of total sitting time and physcial activity with lung cancer risk after excluding the first three years of follow-up (n = 33,322) to reduce possible reverse causality by existing but undiagnosed lung cancer. In addition, we redefined the combination groups by including low activity into the physical inactivity level and repeated the analysis. Physical inactivity was thus redefined as no activity, any light activity only, or light activity ≤2 h and hard activity < 1 h weekly. In this way, we could test if the original category of the combined sitting time ≥8 h and physical inactivity captured the most sedentary individuals. We further conducted complete case analysis among individuals with complete information on smoking (n = 31,907) to minimize residual confounding from smoking. All statistical analyses were performed with STATA/SE 14.2 (College Station, TX, USA).
Results
In total, 549 participants developed lung cancer during a median follow-up time of 18.3 years among the 45,810 subjects. Tables 1, 2 describe the distribution of baseline characteristics of participants according to total sitting time and its combination with physical activity levels. Compared to participants sitting < 4 h or between 5 and 7 h daily, participants sitting ≥8 h were more likely to be males, frequent drinkers, have higher education and sedentary work (Table 1). Compared to participants who were sitting < 8 h daily and physically active, people who were sitting ≥8 h daily and physically inactive were older, lower educated, more frequent smokers and sedentary workers. Supplementary Table 1 shows that as compared to the original cancer-free population, participants in the main cohort had a higher percentage of family history of cancer and participants in the sub-cohort were relatively younger.
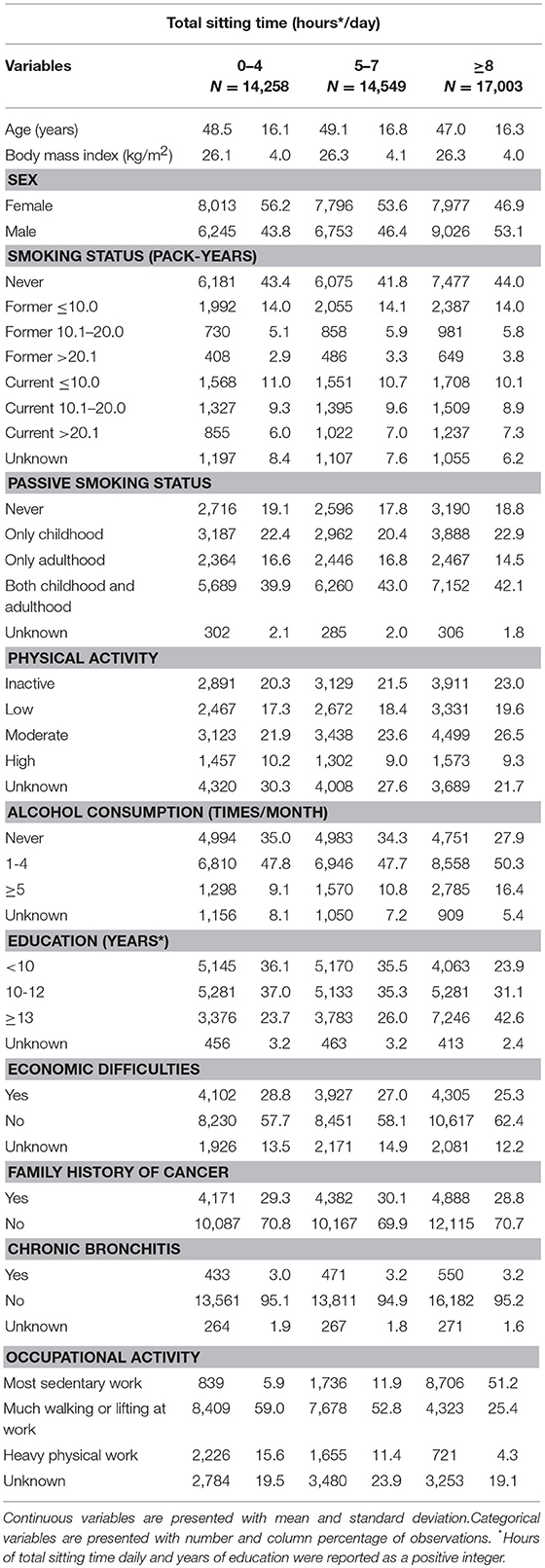
Table 1. Baseline characteristics of participants according to total sitting time, the HUNT Study, 1995–97 (N = 45,810).
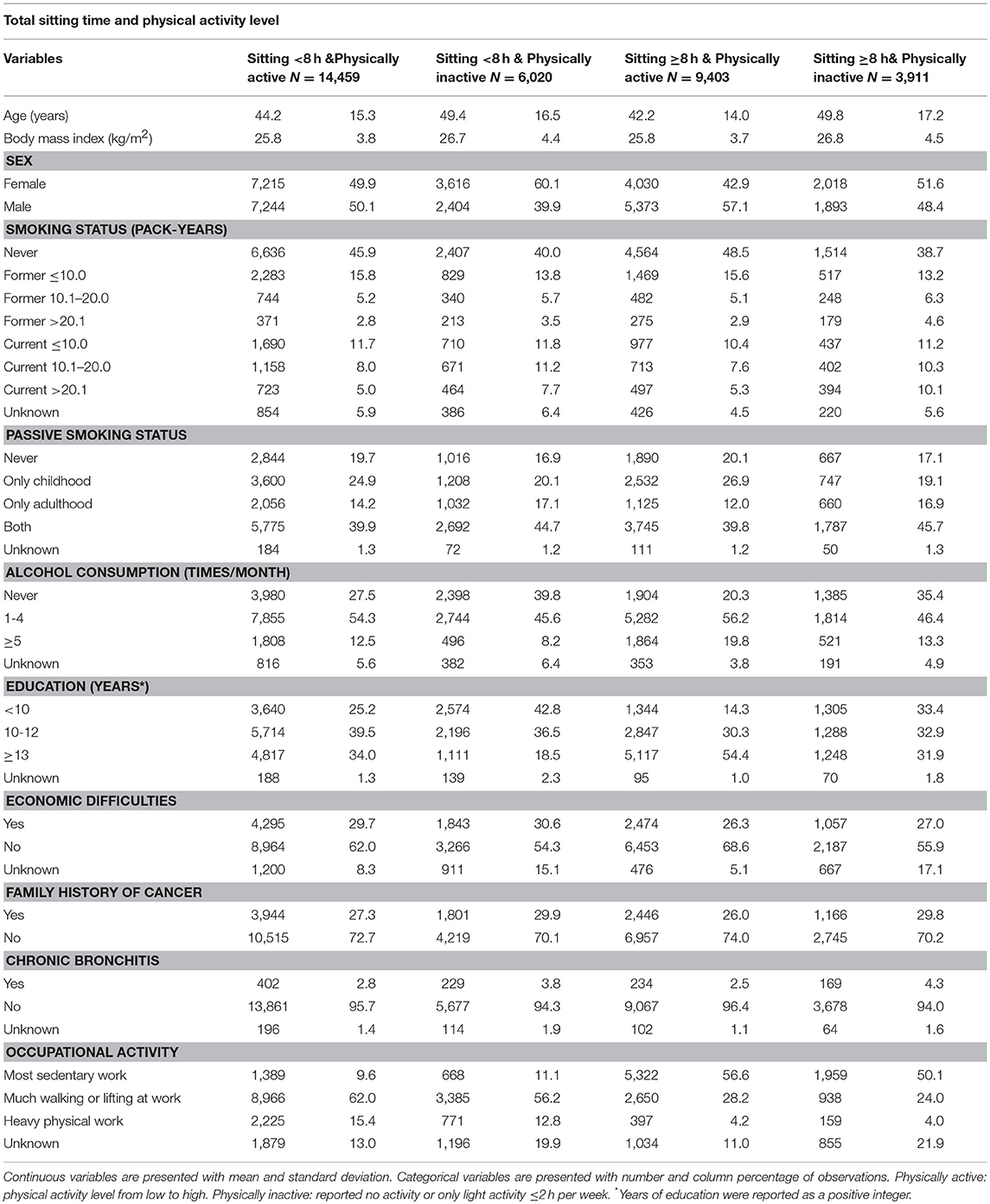
Table 2. Baseline characteristics of participants according to combined total sitting time and physical activity level, the HUNT Study, 1995–97 (N = 33,793).
In Table 3, categories of total sitting time daily were not associated with lung cancer risk overall, SCLC or NSCLC in the main cohort after adjustment for a number of potential confounding factors including smoking status and physical activity. Total sitting time classified by tertiles was not associated with lung cancer risk either (data not presented). Results in ever smokers were similar to the main cohort (Supplementary Table 2). We were not able to perform analysis in never smokers as there were only 26 cases of lung cancer overall, no cases of SCLC and 19 cases of NSCLC among the never smokers.
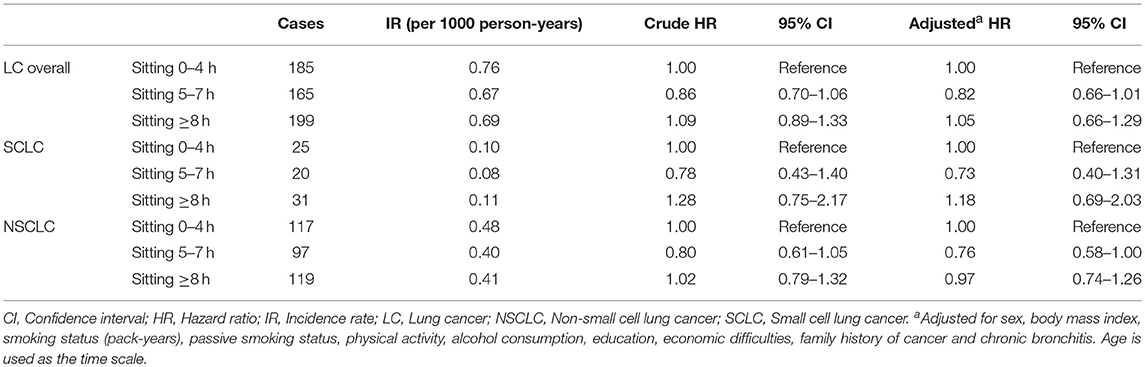
Table 3. The association of total sitting time with lung cancer risk overall and histologic types, the HUNT Study, 1995–97 to 2014 (N = 45,810).
Table 4 presents the association of the combined groups of total sitting time and physical activity with lung cancer risk overall and different histologic types. Compared to participants sitting < 8 h and being physically active, participants sitting ≥8 h and being physically inactive had increased risks of lung cancer overall and SCLC in the main cohort (overall: adjusted HR = 1.44, 95% CI: 1.07–1.94; SCLC: adjusted HR = 2.58, 95% CI: 1.23–5.41). Neither of the group with prolonged sitting only or physical inactivity only was associated with lung cancer risk. Similar results were found among ever smokers (Supplementary Table 3).
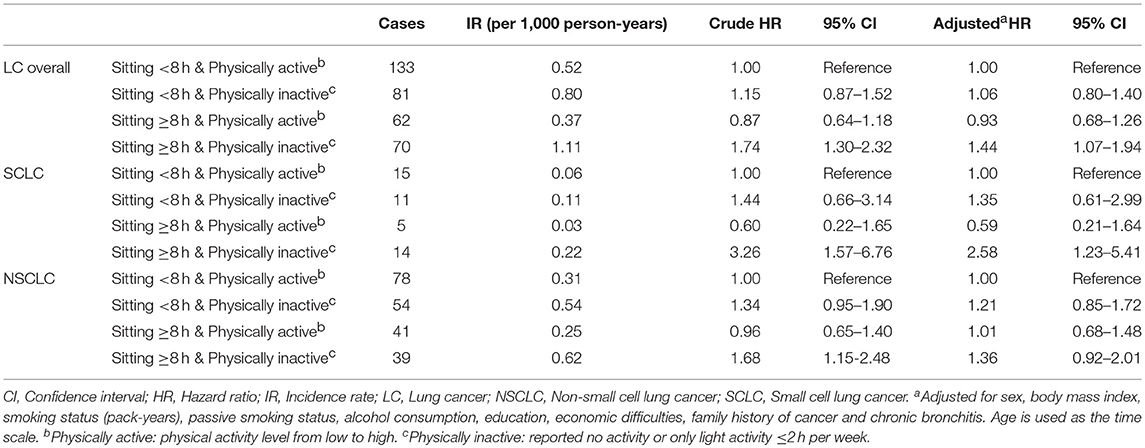
Table 4. The association of combined groups of total sitting time and physical activity with lung cancer risk overall and different histologic types, the HUNT Study, 1995–97 to 2014 (N = 33,793).
In the sensitivity analysis, we found no association between occupational inactivity (mostly sedentary work) and lung cancer risk (Supplementary Table 4). The association of combined sitting ≥8 h and physical inactivity with lung cancer risk was not altered after excluding the first 3 years of follow-up (Supplementary Table 5). When grouping low level physical activity into the physical inactivity group (Supplementary Table 6), the associations of the combined sitting time ≥8 h and physical inactivity with the risks of lung cancer overall and histologic types became weaker compared to the original results. Complete case analysis for information of smoking showed comparable results for lung cancer overall (Supplementary Table 7).
Discussion
Main Findings
In this prospective cohort study with 549 incident lung cancer cases, total sitting time daily was not associated with lung cancer. However, compared with participants sitting < 8 h daily and being physically active, participants sitting ≥8 h daily and being physically inactive appeared to have increased risks of lung cancer overall and SCLC.
Comparison With Previous Studies
Previous studies showed different results on the association between sedentary lifestyle and lung cancer risk (17–21, 23). Occupational sitting was shown to be either protective (17, 19) or not associated with lung cancer risk (18). Different adjustment for physical activity and education might explain the differences in the results. On the contrary, leisure-time TV watching was associated with an increased risk of lung cancer among Japanese men but not women (20). Residual confounding by smoking was likely to be the explanation. In addition, Japanese women seemed to be more active than men (4.5 h housework for women & 1 h for men daily), which might be another reason for the gender difference in lung cancer risk. However, no adjustment for physical activity was made in this study.
Total sitting time daily, as a better measure of sedentary lifestyle, was not associated with lung cancer risk in the current study. Our result was consistent with findings from the study by Wang et al. (23). We included both men and women and adjusted for levels of physical activity, whereas only menopausal women were included and no adjustment for physical activity was made in the referred study. In contrast, Lam et al. found a marginally increased risk of lung cancer associated with prolonged sitting among non-smokers (21). Although the cited study largely avoided confounding by smoking among non-smokers, the adjustment for physical activity only included vigorous activity. Leisure-time TV watching and occupational inactivity were also studied by Lam et al. but no associations with lung cancer risk were found. In our study, neither prolonged sitting nor occupational inactivity was independently associated with lung cancer incidence after adjustment for detailed categories of smoking and physical activity. However, we observed an increased risk of lung cancer among the most sedentary individuals who were both extendedly seated and physically inactive.
Possible Mechanisms
Although the underlying mechanisms on how the most sedentary individuals might have an increased risk of lung cancer are unclear, animal studies showed that lack of activities might suppress lipoprotein lipase activity in skeletal muscles and reduce glucose uptake (5, 6). Both are related to metabolic disorder that have been shown to be risk factors for several malignancies (6, 36). In addition, some pre-clinical studies suggest that weight-bearing skeletal muscles are not highly engaged during inactivity (7–9). This may alter anti-cancer responses of myokines in the skeletal muscles and activate inflammatory pathways that are important for cancer development (7–9).
Strengths and Limitations
Scientific evidence regarding total sitting time daily in relation to lung cancer risk overall and histologic types is scarce. Our study is the first prospective cohort study to investigate lung cancer risk among people who were both extendedly seated and physically inactive. In addition, the sample size of our study is relatively large with homogeneous study population and the follow-up period is long to allow for study of rare disease outcome such as lung cancer. The Cancer Registry of Norway records information about lung cancer diagnosis and different histologic types 1 year after the first diagnosis, and the information is soundly complete and accurate (26). The distribution of key baseline characteristics in both the main and sub-cohorts are generally similar to the original cancer-free population, suggesting no substantial selection bias. We also had information on a panel of potential confounders at baseline. In addition, we excluded participants with cancer at baseline in the main analysis and excluded the first 3 years of follow-up in the sensitivity analysis. Thus, potential reverse causation due to preexisting but undiagnosed lung cancer may not be a major problem.
However, our study has several limitations. First, misclassification of total sitting time and physical activity was possible due to self-reporting, and weak correlations with accelerometer counts (r≈0.3) were reported in a previous study (31). Since all information on exposures was collected at baseline before the diagnosis of lung cancer, it was more likely to be non-differential misclassification. We further used occupational inactivity as an alternative marker of sedentary lifestyle to test the robustness of the association between total sitting time and lung cancer risk, and similar results were observed. Additionally, in our sensitivity analysis, the magnitude of association of sitting time ≥8 h in combination with physical inactivity with the lung cancer risk was reduced by grouping individuals who had low level physical activity into the inactive group. This suggested that the original combination of prolonged sitting and physical inactivity identified the most sedentary individuals and thereby the highest risk group for lung cancer.
Second, individuals who were extendedly seated and physically inactive were more likely to be smokers than those who were shortly seated and physically active. To minimize confounding by smoking, we used detailed information on smoking status together with pack-years to categorize this variable, but we were not able to perform analysis in never smokers among which there were only 26 lung cancer cases. We further conducted complete case analysis for information on smoking and similar results were obtained. In addition, we redefined smoking status by including cessation years for former daily smokers and categorized subjects into the groups of never smokers, ex-smokers with smoking cessation >10.1 years, ex-smokers with smoking cessation ≤10.0 years, current smokers with 0–20.0 pack-years, and current smokers with >20.1 pack-years. The results were similar to our original findings (data not presented). Nevertheless, residual confounding by smoking cannot be excluded entirely. Other unmeasured factors such as hazardous occupational exposures might also confound the association. Indoor radon exposure is suggested to be the second important risk factor for lung cancer after smoking (37), but the level of indoor radon at any of the seven municipalities in Nord-Trøndelag was shown to be in the safety range (< 200 Bq/m3) (38). At last, we could not look into specific histologic types of NSCLC such as adenocarcinoma and squamous cell carcinoma due to limited statistical power.
In conclusion, our study suggested that prolonged sitting was not independently associated with lung cancer risk, but prolonged sitting in combination with physical inactivity might increase the risk of lung cancer. However, our results should be interpreted with caution due to a possibility of residual confounding of smoking.
Ethics Statement
The study was carried out in accordance with the recommendation of the Regional Committee for Medical and Health Research Ethics of South-East Norway. All subjects gave written informed consent on participation in HUNT, linkage to previous HUNT surveys and specific registries in accordance with the Declaration of Helsinki. The protocol was approved by the Regional Committee for Medical and Health Research Ethics of South-East Norway.
Author Contributions
Y-QS and X-MM contributed to the study design and statistical analyses. AL and X-MM were responsible for data collection. LJ conducted statistical analyses, interpreted and wrote the initial draft of the manuscript. All authors participated in the data interpretation, contributed to the manuscript writing with important intellectual content and approved the final version of the manuscript.
Funding
LJ was supported by funding from the Extrastiftelsen through the Norwegian Cancer Society (Contract no. 77513001) and top-up funding from the collaboration partner between the Liaison Committee for Education, Research and Innovation in Central Norway and Central Norway Regional Health Authority. Y-QS was supported by the Norwegian Cancer Society (Project ID:5769155-2015).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The HUNT Study is collaboration between HUNT Research Center (Faculty of Medicine and Health Sciences, Norwegian University of Science and Technology—NTNU), Nord-Trøndelag County Council, Central Norway Regional Health Authority and the Norwegian Institute of Public Health.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2019.00101/full#supplementary-material
References
1. InternationalAgency for Research on Cancer. GLOBOCAN 2012, Estimated cancer incidence, mortality and prevalence worldwide in 2012. 2016. Available online at: http://globocan.iarc.fr/factsheet.asp
2. Travis WD, Harris C. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. IARC Press (2004).
3. Khuder SA. Effect of cigarette smoking on major histological types of lung cancer: a meta-analysis. Lung Cancer (2001) 31:139–48. doi: 10.1016/S0169-5002(00)00181-1
4. Lee PN, Forey BA, Coombs KJ. Systematic review with meta-analysis of the epidemiological evidence in the 1900s relating smoking to lung cancer. BMC Cancer (2012) 12:385. doi: 10.1186/1471-2407-12-385
5. Bey L, Hamilton MT. Suppression of skeletal muscle lipoprotein lipase activity during physical inactivity: a molecular reason to maintain daily low-intensity activity. J Physiol. (2003) 551:673–82. doi: 10.1113/jphysiol.2003.045591
6. Hamilton MT, Hamilton DG, Zderic TW. Exercise physiology versus inactivity physiology: an essential concept for understanding lipoprotein lipase regulation. Exerc Sport Sci Rev. (2004) 32:161. doi: 10.1097/00003677-200410000-00007
7. Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. (2012) 8:457. doi: 10.1038/nrendo.2012.49.
8. Hojman P, Dethlefsen C, Brandt C, Hansen J, Pedersen L, Pedersen BK. Exercise-induced muscle-derived cytokines inhibit mammary cancer cell growth. Am J Physiol (2011) 301:E504–10. doi: 10.1152/ajpendo.00520.2010
9. Aoi W, Naito Y, Takagi T, Tanimura Y, Takanami Y, Kawai Y, et al. A novel myokine, secreted protein acidic and rich in cysteine (SPARC), suppresses colon tumorigenesis via regular exercise. Gut (2012) 62:882–9. doi: 10.1136/gutjnl-2011-300776
10. Schmid D, Ricci C, Behrens G, Leitzmann MF. Does smoking influence the physical activity and lung cancer relation? A systematic review and meta-analysis. Eur J Epidemiol. (2016) 31:1173–90. doi: 10.1007/s10654-016-0186-y
11. Brenner DR, Yannitsos DH, Farris MS, Johansson M, Friedenreich CM. Leisure-time physical activity and lung cancer risk: a systematic review and meta-analysis. Lung Cancer (2016) 95:17–27. doi: 10.1016/j.lungcan.2016.01.021
12. Zhong S, Ma T, Chen L, Chen W, Lv M, Zhang X, et al. Physical activity and risk of lung cancer: a meta-analysis. Clin J Sport Med. (2016) 26:173–81. doi: 10.1097/JSM.0000000000000219
13. Barnes J, Behrens TK, Benden ME, Biddle S, Bond D, Brassard P, et al. Letter to the Editor: Standardized use of the terms“ sedentary” and“ sedentary behaviours”. Appl Physiol Nutr Metab. (2012) 37:540–2. doi: 10.1139/h2012-024
14. Matthews CE, Chen KY, Freedson PS, Buchowski MS, Beech BM, Pate RR, et al. Amount of time spent in sedentary behaviors in the United States, 2003–2004. Am J Epidemiol. (2008) 167:875–81. doi: 10.1093/aje/kwm390
15. Thorp AA, Owen N, Neuhaus M, Dunstan DW. Sedentary behaviors and subsequent health outcomes in adults: a systematic review of longitudinal studies, 1996–2011. Am J Prevent Med. (2011) 41:207–15. doi: 10.1016/j.amepre.2011.05.004
16. Shen D, Mao W, Liu T, Lin Q, Lu X, Wang Q, et al. Sedentary behavior and incident cancer: a meta-analysis of prospective studies. PLoS ONE (2014) 9:e105709. doi: 10.1371/journal.pone.0105709
17. Steindorf K, Friedenreich C, Linseisen J, Rohrmann S, Rundle A, Veglia F, et al. Physical activity and lung cancer risk in the European prospective investigation into cancer and nutrition Cohort. Int J Cancer (2006) 119:2389–97. doi: 10.1002/ijc.22125
18. Thune I, Lund E. The influence of physical activity on lung-cancer risk: a prospective study of 81,516 men and women. Int J Cancer (1997) 70:57–62.
19. Bak H, Christensen J, Thomsen BL, Tjønneland A, Overvad K, Loft S, et al. Physical activity and risk for lung cancer in a Danish cohort. Int J Cancer (2005) 116:439–44. doi: 10.1002/ijc.21085
20. Ukawa S, Tamakoshi A, Wakai K, Noda H, Ando M, Iso H. Prospective cohort study on television viewing time and incidence of lung cancer: findings from the Japan Collaborative Cohort Study. Cancer Causes Control (2013) 24:1547–53. doi: 10.1007/s10552-013-0231-z
21. Lam TK, Moore SC, Brinton LA, Smith L, Hollenbeck AR, Gierach GL, et al. Anthropometric measures and physical activity and the risk of lung cancer in never-smokers: a prospective cohort study. PLoS ONE (2013) 8:e70672. doi: 10.1371/journal.pone.0070672
22. Katzmarzyk PT, Church TS, Craig CL, Bouchard C. Sitting time and mortality from all causes, cardiovascular disease, and cancer. Med Sci Sports Exerc. (2009) 41:998–1005. doi: 10.1249/MSS.0b013e3181930355
23. Wang A, Qin F, Hedlin H, Desai M, Chlebowski R, Gomez S, et al. Physical activity and sedentary behavior in relation to lung cancer incidence and mortality in older women: The Women's Health Initiative. Int J Cancer. (2016) 139:2178–92. doi: 10.1002/ijc.30281
24. Holmen J, Midthjell K, Krüger Ø, Langhammer A, Holmen TL, Bratberg GH, et al. The Nord-Trøndelag Health Study 1995–97 (HUNT 2): objectives, contents, methods and participation. Norsk Epidemiologi. (2003) 13:19–32. doi: 10.5324/nje.v13i1.305
25. Krokstad S, Langhammer A, Hveem K, Holmen T, Midthjell K, Stene T, et al. Cohort profile: the HUNT study, Norway. Int J Epidemiol. (2013) 42:968–77. doi: 10.1093/ije/dys095
26. Cancer Registry of Norway. (2017). Available online: http://www.kreftregisteret.no/
27. Larsen IK, Småstuen M, Johannesen TB, Langmark F, Parkin DM, Bray F, et al. Data quality at the Cancer Registry of Norway: an overview of comparability, completeness, validity and timeliness. Eur J Cancer (2009) 45:1218–31. doi: 10.1016/j.ejca.2008.10.037
28. Cancer Registry of Norway. Cancer in Norway 2014-Cancer Incidence, Mortality, Survival and Prevalence in Norway. Oslo: Cancer Registry of Norway (2015).
29. ICD-O. International Classification of Diseases for Oncology (ICD-O). 3rd ed. World Health Organization (2013).
30. Åsvold BO, Midthjell K, Krokstad S, Rangul V, Bauman A. Prolonged sitting may increase diabetes risk in physically inactive individuals: an 11 year follow-up of the HUNT Study, Norway. Diabetologia (2017) 60:830–5. doi: 10.1007/s00125-016-4193-z
31. Chau JY, Grunseit A, Midthjell K, Holmen J, Holmen TL, Bauman AE, et al. Sedentary behaviour and risk of mortality from all-causes and cardiometabolic diseases in adults: evidence from the HUNT3 population cohort. Br J Sports Med. (2013) 49:737–42. doi: 10.1136/bjsports-2012-091974
32. Ekelund U, Steene-Johannessen J, Brown WJ, Fagerland MW, Owen N, Powell KE, et al. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. Lancet (2016) 388:1302–10. doi: 10.1016/S0140-6736(16)30370-1
33. Chau JY, Grunseit AC, Chey T, Stamatakis E, Brown WJ, Matthews CE, et al. Daily sitting time and all-cause mortality: a meta-analysis. PLoS ONE (2013) 8:e80000. doi: 10.1371/journal.pone.0080000
34. Moe B, Eilertsen E, Nilsen TI. The Combined Effect of Leisure-Time Physical Activity and Diabetes on Cardiovascular Mortality The Nord-Trøndelag Health (HUNT) cohort study, Norway. Diabetes Care (2013) 36:690–5. doi: 10.2337/dc11-2472
35. WHO. Preventing and Managing the Global Epidemic. Report of a WHO Consultation on Obesity. Geneva: World Health Organization (2004).
36. Wilmot EG, Edwardson CL, Achana FA, Davies MJ, Gorely T, Gray LJ, et al. Sedentary time in adults and the association with diabetes, cardiovascular disease and death: systematic review and meta-analysis. Diabetologia (2012) 55:2895–905. doi: 10.1007/s00125-012-2677-z
37. WHO. WHO Handbook on Indoor Radon: A Public Health Perspective. Geneva: World Health Organization (2009).
38. Kommunekartlegginger-radon (Municipal Surveys-Radon). (2013) Available online at: http://www.nrpa.no/temaartikler/90379/kart-og-data-radon (Accessed April 19, 2018).
Keywords: prolonged sitting, physical inactivity, lung cancer risk, prospective cohort, HUNT study
Citation: Jiang L, Sun Y-Q, Brumpton BM, Langhammer A, Chen Y, Nilsen TIL and Mai X-M (2019) Prolonged Sitting, Its Combination With Physical Inactivity and Incidence of Lung Cancer: Prospective Data From the HUNT Study. Front. Oncol. 9:101. doi: 10.3389/fonc.2019.00101
Received: 11 November 2018; Accepted: 04 February 2019;
Published: 25 February 2019.
Edited by:
Yawei Zhang, Yale University, United StatesReviewed by:
Manuela Marron, Leibniz Institute for Prevention Research and Epidemiology (LG), GermanyXiangqian Guo, Henan University, China
Copyright © 2019 Jiang, Sun, Brumpton, Langhammer, Chen, Nilsen and Mai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lin Jiang, bGluLmppYW5nQG50bnUubm8=
 Lin Jiang
Lin Jiang Yi-Qian Sun
Yi-Qian Sun Ben Michael Brumpton
Ben Michael Brumpton Arnulf Langhammer
Arnulf Langhammer Yue Chen6
Yue Chen6 Xiao-Mei Mai
Xiao-Mei Mai