- 1Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina, Chapel Hill, NC, United States
- 2Division of Applied Regulatory Toxicology, Office of Applied Research and Safety Assessment, Center for Food Safety and Applied Nutrition, U.S. Food and Drug Administration, Laurel, MD, United States
- 3Department of Epidemiology and Biostatistics, University of Maryland School of Public Health, College Park, MD, United States
- 4Maryland Institute for Applied Environmental Health, University of Maryland School of Public Health, College Park, MD, United States
- 5B. P. Koirala Memorial Cancer Hospital, Bharatpur, Nepal
- 6Division of Public Health, University of Utah School of Medicine, Salt Lake City, UT, United States
Background: Of the 1.8 million global incident lung cancer cases estimated in 2012, approximately 60% occurred in less developed regions. Prior studies suggest sex differences in lung cancer risk and a potential role for reproductive and hormonal factors in lung cancer among women. However, the majority of these studies were conducted in developed regions. No prior study has assessed these relationships among Nepali women.
Methods: Using data from a hospital-based case-control study conducted in B. P. Koirala Memorial Cancer Hospital (Nepal, 2009–2012), relationships between reproductive and hormonal factors and lung cancer were examined among women aged 23–85 years. Lung cancer cases (n = 268) were frequency-matched to controls (n = 226) based on age (±5 years), ethnicity and residential area. The main exposures in this analysis included menopausal status, age at menarche, age at menopause, menstrual duration, gravidity, and age at first live-birth. Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated using multivariable logistic regression.
Results: Among postmenopausal women, those with a younger age at menopause (<45 years; 45–49 years) had an increased odds of lung cancer compared to those with an older (≥50 years) age at menopause [OR (95%CI): 2.14 (1.09, 4.17); OR (95% CI): 1.93 (1.07, 3.51)], after adjusting for age and cumulative active smoking years. No statistically significant associations were observed with the other reproductive and hormonal factors examined.
Conclusion: These results suggest that Nepali women with prolonged exposure to endogenous ovarian hormones, via later age at menopause, may have a lower odds of lung cancer.
Introduction
Lung cancer is the most commonly diagnosed cancer and the primary cause of cancer mortality worldwide (1–4). Moreover, approximately 60% of the global lung cancer incidence and mortality occurs in less developed regions (LDRs) (3), which include countries such as Nepal. Although lung cancer is one of the three most common cancers among Nepali women (5), epidemiologic data on the risk factors of lung cancer in LDRs remains scarce (6, 7).
While smoking is a well-established risk factor for lung cancer, several studies have suggested that reproductive and hormonal factors may also play a role in lung cancer development due to observed sex differences (8–12). These sex differences include increased susceptibility to the carcinogenic effects of tobacco (8–10) and a higher proportion of non-smoking lung cancer diagnosed (11) among women, as compared to men. Additionally, the distribution of histological subtypes differ by sex with adenocarcinoma being more common among women vs. squamous cell carcinoma in men (13). The presence of female sex-hormone receptors (estrogen-β and progesterone) on lung cancer cells (14) along with the patterns noted above support hypotheses related to reproductive and hormonal factors and lung cancer.
Prior epidemiological studies which investigated the relationship between age at menarche (15–27), age at first birth (15, 16, 18–25, 27, 28), and oral contraceptive use (17, 19–22, 24, 27, 29) in relation to lung cancer risk have reported no statistically significant associations, while only one study to date has examined the relationship between gravidity and lung cancer and observed no statistically significant association (16). More recent cohort studies conducted in the United States have observed an inverse association between lung cancer risk and age at menopause (20–23), and longer reproductive periods (22, 24) lending support to these potential associations. Despite these limited prior studies, reproductive and hormonal factors have not been examined in relation to lung cancer among Nepali women, an understudied population with differential patterns of these exposures.
Given the increased lung cancer global burden in LDRs (1, 2) such as Nepal, the suggested higher susceptibility among women (8–12), and the above-mentioned biological rationale, relations between reproductive and hormonal factors and lung cancer among Nepali women were examined. We hypothesized that the odds of lung cancer among Nepali women may differ by factors such as age at menarche, age at menopause, menstrual duration, number of pregnancies or gravidity, and age at first live-birth.
Methods
Study Population
This study utilizes data from a hospital-based case-control study, conducted between November 2009 and December 2012, at the B. P. Koirala Memorial Cancer Hospital (BPKMCH) in the city of Bharatpur, Chitwan district (30, 31). The primary study is described in previous publications (30, 31).
Briefly, 606 lung cancer cases and 606 frequency-matched controls, based on age (±5 years), sex, ethnicity, and residential area (district), were recruited, including 268 female lung cancer cases and 226 female frequency-matched hospital-based controls. One lung cancer case was excluded due to a previous breast cancer diagnosis. Thus, the final analytic population for this study included 493 women (267 cases and 226 controls).
Lung cancer was defined according the International Classification of Diseases for Oncology, 2nd Edition (ICD-O2) codes C33 (trachea) and C34 (bronchus and lung). Eligible cases included patients diagnosed with primary lung cancer at the BPKMCH. Eligible controls included visitors (family and friends) of non-lung cancer patients, subjects being screened for cancer and subjects accompanying the person being screened, at the BPKMCH during the study period. Although the primary study aimed to frequency-match cases and controls based on sex, hospital visitors were less likely to be women, and thus, fewer female controls were recruited. Lung cancer patients younger than 18 years of age and not residing in Nepal for at least 5 years were excluded.
All eligible participants were interviewed in-person by a trained nurse, who collected detailed information including tobacco and alcohol consumption, reproductive and hormonal factors, medical conditions, family history of cancer, anthropometrics and other factors using a standardized questionnaire. A target interval of 1 day and a maximal interval of 3 months between diagnosis and recruitment were used for data attainment in order to minimize selection bias. Informed written consent was obtained from the study participants prior to enrollment. Institutional Review Board approvals were obtained from the University of Utah, University of Maryland and the Nepal Health Research Council.
Exposure Assessment
Reproductive and hormonal exposures assessed include menstrual duration (continuous in years), menopausal status (premenopausal, postmenopausal), age at menarche (<14, 14, ≥15 years), age at menopause (<45, 45–49, ≥50 years), number of pregnancies (≤2, 3–5, >5), age at first live-birth (<18, 18–20, ≥21 years) and birth control use (ever/never). Among postmenopausal women, menstrual duration was defined as the difference between the participants' reported age at menarche and age at menopause. Menopausal status was defined based on whether women reported still menstruating (premenopausal) or had stopped menstruating (postmenopausal) at the time of questionnaire completion. Menopausal status and age at menopause were combined into one variable for analyses (premenopausal, <45, 45–49, ≥50 years).
Covariate Assessment
Detailed information was collected on the type, duration and quantity of tobacco product smoked, including cigarettes with or without filter, bidi, choor or kankat, hooka or pipe, and hashish. Cumulative active smoking (CAS; continuous) was defined as the sum of the total years of use of the above tobacco products. Pack-years of smoking (continuous) was calculated by multiplying years of use by frequency of use (per day) divided by 20 for each tobacco product.
Statistical Analysis
Descriptive characteristics of the cases and controls were compared using t-tests and Chi-square tests. Odds ratios (ORs) and 95% confidence intervals (CIs) for associations between exposures noted above and lung cancer (outcome) were estimated using multivariable logistic regression. Continuous variable measurements were categorized based on distributions among the controls; missing values were categorized as “unknown.”
Based on prior literature, potential confounders were identified a priori, including age, smoking history, body mass index (BMI, kg/m2), residential history, ethnicity, marital status, education, family monthly income, first degree family history of cancer, history of medical condition (including tuberculosis, asthma and pneumonia), and alcohol consumption. As smoking is a well-established lung cancer risk factor, CAS was included in all models and the above-mentioned potential confounders were subsequently added to determine if estimated ORs changed by more than 10%. After adjusting for CAS, none of the above-mentioned potential confounders changed the estimated ORs by more than 10%. Thus, the final parsimonious models included adjustment for age and CAS, and are presented herein. Additionally, a final model that simultaneously adjusted for all reproductive and hormonal exposure variables of interest, in addition to adjustment for age and CAS, was performed using logistic regression. Model diagnostics were performed for the final models. One subject with missing cumulative active smoking history was excluded from all analyses.
Given the strong relationship between smoking and lung cancer, we also examined associations stratified by cumulative active smoking years. CAS categories (<15 years and ≥15 years), for the stratified analysis, were based on the mean cumulative active smoking years among the controls.
Tests for trend were performed by modeling each categorical exposure as an ordinal variable using logistic regression. The SAS statistical package, version 9.3, was used for all statistical analyses. All p-values were 2-sided.
Results
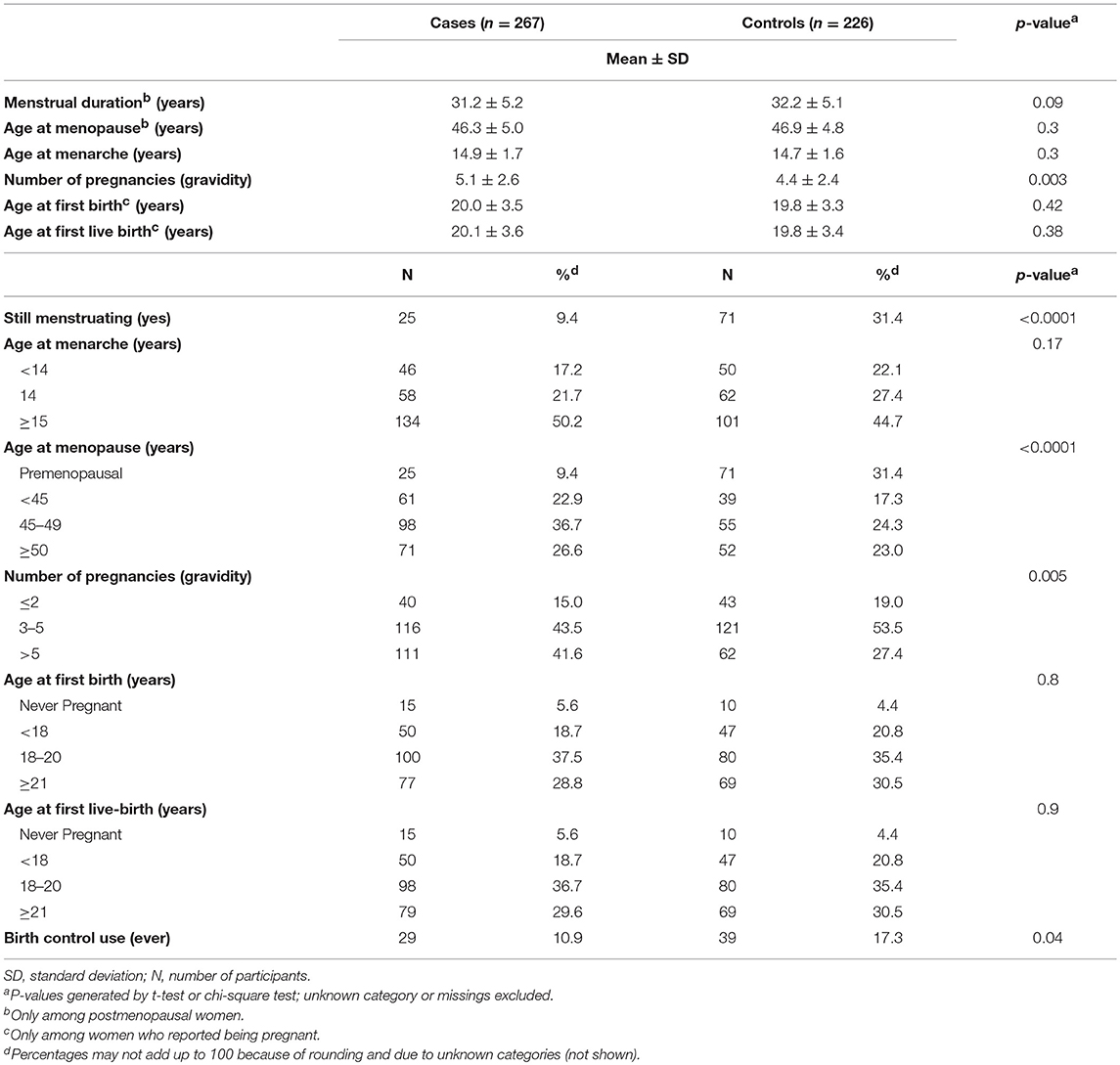
Descriptive characteristics of the study population are presented by case status (N = 267 cases, 226 controls) in Table 1A. Compared to controls, cases were slightly older (mean age (years) ± standard deviation (SD): 59.2 ± 11.6 vs. 53.2 ± 9.6, p < 0.0001), less educated (3.8% of cases completed high school vs. 12% of controls, p < 0.0001), and had lower family income (25.8% with a monthly family income ≤ 999 rupees compared to 12.8% of controls, p = 0.0002). Additionally, cases were more likely to be unmarried or widowed (30 vs. 15.5% of controls, p = 0.0002) and to reside in rural areas (86.9% compared to 79.2% of controls, p = 0.02). Cases and controls also varied in their ethnic background (p < 0.0001); controls had a higher proportion of Brahmins (29.2%) compared to cases (11.6%), and more than fifty percent of cases were included in the “other” ethnic groups.
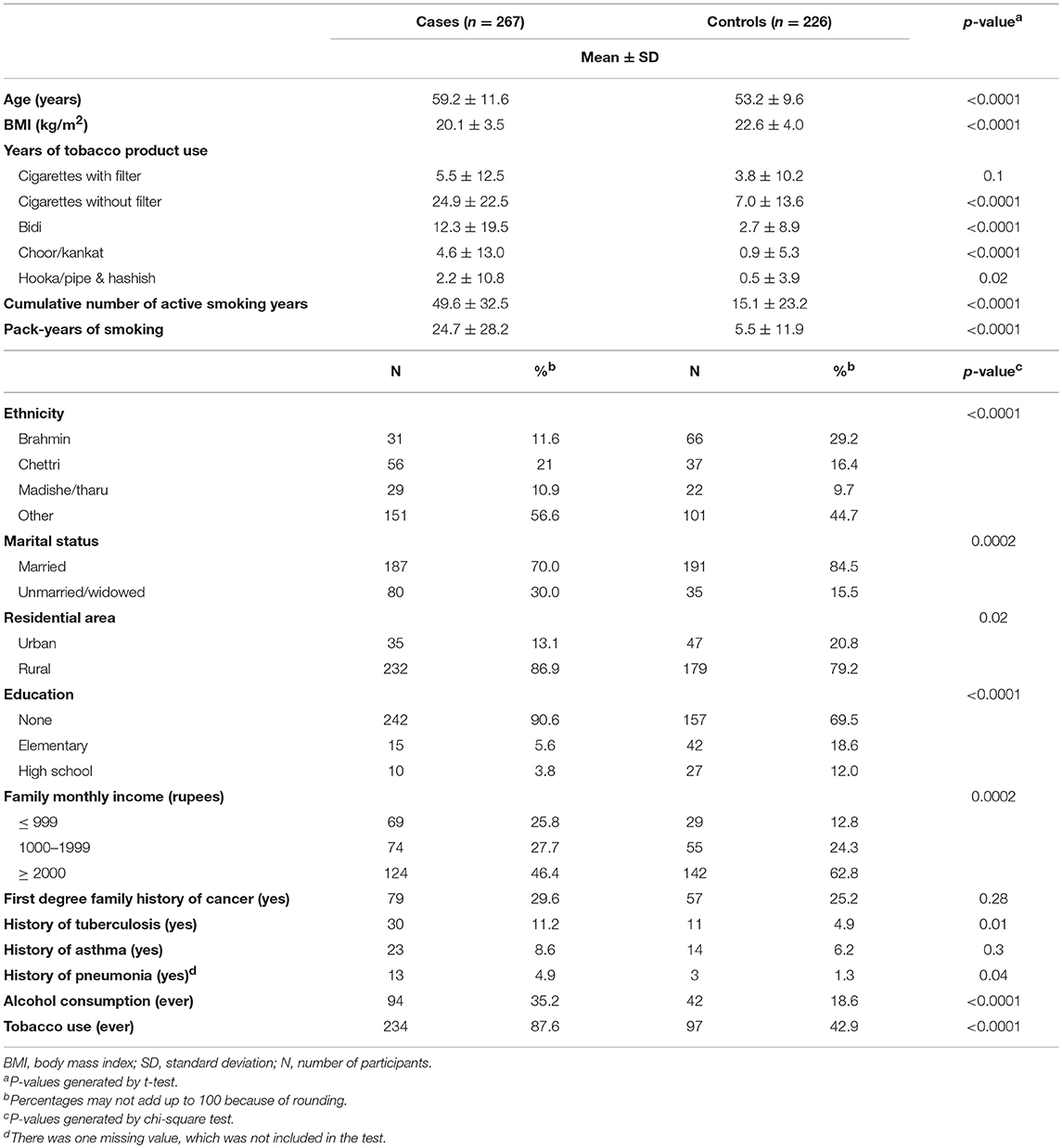
Table 1A. Characteristics of women enrolled in the hospital-based lung cancer case-control study in Bharatpur, Nepal, 2009–2012 (n = 493).
In terms of lifestyle factors, cases were significantly more likely to use tobacco products (p < 0.0001), consume alcohol (p < 0.0001), and to have a slightly lower BMI (p < 0.0001). When smoking behaviors were analyzed in more detail, results indicated that cases reported greater cumulative active smoking years (mean ± SD: 49.6 ± 32.5 vs. 15.1 ± 23.2, p < 0.0001) and pack-years of smoking (mean ± SD: 24.7 ± 28.2 versus 5.5 ± 11.9, p < 0.0001). The difference between cases and controls was not statistically significant for years of filtered cigarette use (p = 0.1). Nevertheless, these groups were significantly different with respect to years of use of unfiltered cigarettes (p < 0.0001), bidi (p < 0.0001), choor or kankat (p < 0.0001), and hooka or hashish (p = 0.02). Cases were also more likely to report a prior history of tuberculosis (p = 0.01) and pneumonia diagnosis (p = 0.04).
Distributions of reproductive and hormonal characteristics were examined among cases and controls (Table 1B), with a similar mean age at menarche (p = 0.17), and age at first live-birth (p = 0.9) observed between both groups. However, a greater proportion of controls reported they were still menstruating (p < 0.0001), using birth control (p = 0.04), and on average, had fewer pregnancies (p = 0.005).
Among postmenopausal women, those with a younger age at menopause (<45 years and 45–49 years) had an increased odds of lung cancer compared to women who were ≥50 years at menopause [OR (95% CI): 2.14 (1.09, 4.17) and 1.93 (1.07, 3.51), respectively], after adjusting for age and CAS years (Table 2). The linear trend for age at menopause, with respect to lung cancer, was also statistically significant (p-trend: 0.02) suggesting a dose-response relationship. Furthermore, among postmenopausal women, women with a longer menstrual duration had a slightly decreased odds of lung cancer compared to those with a shorter menstrual duration [age and CAS adjusted OR (95% CI): 0.93 (0.88, 0.98)]. Lastly, women who were still menstruating had a decreased odds of lung cancer compared to those who had stopped menstruating [OR (95% CI): 0.41 (0.20, 0.85)]. All other reproductive and hormonal factors examined were not associated with lung cancer, after adjusting for age and CAS.
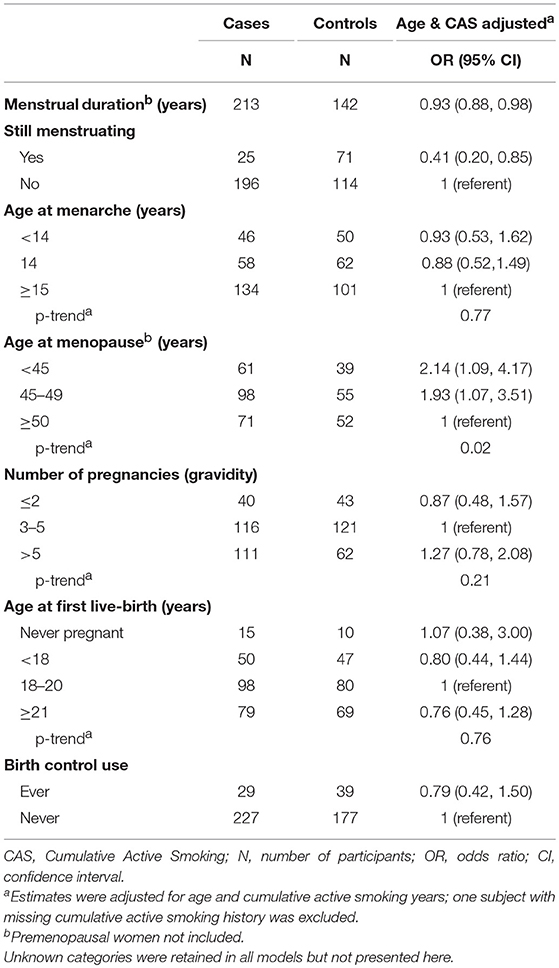
Table 2. Multivariable odds ratios and 95% confidence intervals for the relationship between reproductive and hormonal factors and lung cancer in the hospital-based lung cancer case-control study, 2009–2012.
While the magnitude of the odds of lung cancer among postmenopausal women with a younger age at menopause (<45 years and 45–49 years) remained elevated [OR (95% CI): 1.7 (0.90, 3.4) and OR (95% CI): 1.7 (0.90, 3.2), respectively], the estimates were no longer statistically significant after simultaneous adjustment for all reproductive and hormonal factors (Table 3). No other significant relationships with lung cancer were observed at α = 0.05.
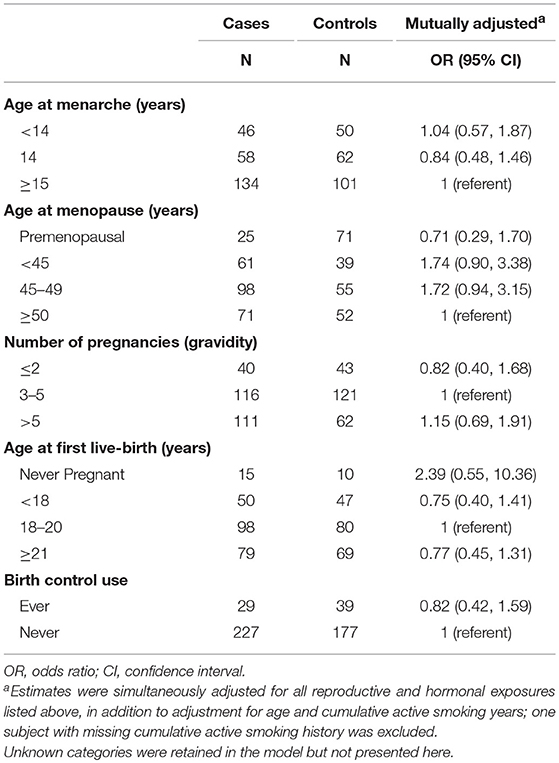
Table 3. Mutually adjusted odds ratios and 95% confidence intervals of reproductive and hormonal factors in relation to lung cancer in the hospital-based lung cancer case-control study.
After stratifying by cumulative active smoking (CAS) years (Table 4), younger age at menopause (specifically <45 years) remained significantly associated with a higher odds of lung cancer among postmenopausal women with a cumulative active smoking history of at least 15 years [OR (95% CI): 2.27 (1.02, 5.04)]. Similarly, among postmenopausal women, the inverse relationship between menstrual duration and lung cancer only remained significant among those who reported smoking for ≥15 cumulative years over their lifetime. Women who reported to be still menstruating had decreased odds of lung cancer within both strata (<15 and ≥15 years) of CAS. Lastly, other factors examined (i.e., age at menarche, number of pregnancies, age at first birth, age at first live-birth and birth control use) were not associated with lung cancer within both strata of cumulative active smoking, similar to the overall analysis.

Table 4. Multivariable odds ratios and 95% confidence intervals of the relationship between reproductive and hormonal and lung cancer by smoking status.
Discussion
Findings from this first analysis of reproductive and hormonal factors in relation to lung cancer among Nepali women suggest a potential role of menstrual factors in lung cancer. Specifically, later age at menopause was significantly associated with a reduced odds of lung cancer, after adjusting for age and cumulative active smoking. These findings add to the growing body of literature that point to a complex pattern of association between reproductive hormones and lung cancer and contribute new information regarding these exposures among Nepali women, an understudied population.
Our findings with regard to age at menopause is consistent with the few large prospective cohort studies conducted among mainly Caucasian populations in the United States (US) (20, 21, 23), Canada (16) and Italy (22) that observed an inverse association between lung cancer and later age at menopause. However, mixed results have been observed among studies conducted among Chinese populations. More specifically, two cohort studies among non-smoking women (24, 26) and a case-control study (15), found an inverse association, whereas, a cohort study among female textile workers in Shanghai (25) found no association between age at menopause and lung cancer risk. Different smoking patterns and other life-style factors may play a role in the inconsistent results observed among these populations. Nepali women have been reported to have an average age at menopause between 46.8 and 49.9 years (32–36), which is similar to the average age at menopause observed in the present analysis (cases: 46.3 vs. controls: 46.9 years). The median age at menopause among white women in industrialized countries is reported to be between 50 and 52 years (37). Additional studies may be helpful in clarifying the relationships between these factors and lung cancer among the Nepali women population, with its unique distribution of sociodemographic, lifestyle, smoking and health-related behaviors.
Menstrual duration reflects a woman's reproductive period during which she is exposed to hormones produced and secreted by her ovaries, such as estrogen (38). We observed an inverse association between menstrual duration and lung cancer which is in-line with findings from previous studies that have also examined this relationship (22, 24). More specifically, a cohort study among lifetime non-smokers in China (24) and a case-control study among Italian women (22) also observed a reduced risk of lung cancer among women with a longer reproductive period. Potential mechanisms for this association are unclear, however, reproductive hormones, estrogen and progesterone, are suggested in the development of lung cancer, in part, due to sex differences in the observed incidence of lung cancer by subtype (13, 39). Additionally, evidence supports the presence of receptors for Estrogen-β and progesterone in lung cancer cells (14, 40). Prior studies have also reported that women may be more susceptible to the carcinogenic effects of tobacco (8–10), suggesting hormones may play a role. However, the specific mechanisms whereby duration of endogenous estrogen exposure, as measured by duration of menstruation and age at menopause in our study, influences lung cancer requires further mechanistic studies. Complex combinations of hormonal and environmental exposures, such as tobacco consumption, are likely to impact development of distinct histological subtypes of lung cancer.
Interestingly, a case-control analysis conducted by Koushik et al. among 422 women with lung cancer and 577 controls in Canada found an increased risk of lung cancer among women who had a non-natural menopause (predominantly including women who had a bilateral oophorectomy) compared to women who had a natural menopause (16). Koushik et al. also observed an inverse association between age at menopause and lung cancer risk (16). We were unable to conduct stratified analysis by type of menopause or lung cancer subtypes, or conduct a restricted analysis among non-smoking women only, which could provide further insights to our finding among the Nepali population.
Prior studies that examined age at menarche and lung cancer risk have been inconsistent. We found a null relationship between age at menarche and lung cancer which is consistent with various case-control (16–19) and prospective cohort studies (20, 24–27) conducted among Chinese (18, 24–26), Canadian (16, 27), American (19, 20), and German (17) women populations. However, other studies have also reported an inverse association between age at menarche and lung cancer among Chinese (15) and American (21) women. Based on prior studies, the mean age at menarche among Nepali girls ranges from 12 to 14.8 years of age (41–43). However, despite the older age at menarche among Nepali women in our study (mean ± SD: 14.7 ± 1.6) vs. US women (20) (mean ± SD: 12.5 ± 1.4), we also observed a null association. Our observations among Nepali women add important and much needed information to the existing body of literature in this area.
With regard to gravidity, age at first birth, and birth control use in relation to lung cancer, our null findings are also consistent with the majority of the epidemiologic studies that have examined these associations. Only one prior study has examined the relationship between gravidity and lung cancer (16). Similar to our findings, this population-based case-control study observed no statistically significant association between gravidity and lung cancer (16). Results from the current analysis of age at live-birth and age at first birth are similar to several studies that observed a null relationship between age at first birth and lung cancer (15, 16, 18–20, 22, 24, 25, 28). Conversely, Brinton et al. (21) and Kabat et al. (27) reported an inverse association between age at first birth and lung cancer in the NIH-AARP Diet and Health Study and the Canadian National Breast Screening Study cohorts. Differences in the study design as well as the prevalence of exposures may, in part, explain disparate results. The median age at first birth among Nepali women aged 25–49 years was reported to be 20.2 years based on national data (44), which is similar to the mean age at first birth within our study population (cases: 20.0 ± 3.5 years vs. controls: 19.8 ± 3.3 years). Based on the National Vital Statistics Reports (NVSR), the mean age at first birth for women in the US was 26.8 years in 2017 (45). As no prior study has examined the relationship between age at first birth and lung cancer among Nepali women, our results contribute to the understanding of reproductive risk factors in this unique population with a different exposure pattern. Lastly, the null association observed between birth control use and lung cancer risk in the current study, is consistent with the majority of prior studies (19–22, 24, 27, 29). Although the patterns of gravidity, age at first birth and birth control use among our Nepali population differ from those in the predominantly Caucasian or Chinese populations of the previous studies, the consistency of findings reported across multiple populations lend support to these associations.
The majority of Nepali women, particularly the lung cancer cases, in our analytic population reported smoking (only 12.4% of the cases and 57.1% of the controls never smoked), limiting our ability to assess relationships between reproductive and hormonal exposures and lung cancer among non-smokers. Although we stratified our analysis by cumulative active smoking (<15, ≥15 years), few cases within our analytic population reported smoking <15 CAS years. A recent cross-sectional study among Nepali women 15–49 years of age reported the prevalence of tobacco consumption to be 43.6% (46). However, in the United States (and countries similar), the prevalence of smoking is much lower; 13.5% as reported by the Centers for Disease Control and Prevention (47). Given the long-standing relationship between tobacco smoking and lung cancer, it is not surprising that in our population, lung cancer cases smoked tobacco significantly longer compared to controls (mean (year): 49.6 vs. 15.1; p < 0.0001). Furthermore, our data reflect the high prevalence of smoking in this population. Nonetheless, due to the limited sample size within the CAS strata, findings from the smoking stratified analyses should be interpreted with caution and corroborated in a larger sample of non-smoking Nepali women. Additionally, the prevalence of EGFR mutations, ALK rearrangement and ROS1 within our study population is unknown as we have not measured these genetic markers. Associations also could not be analyzed by strata of menopausal status, due to the limited number of premenopausal women in our study population. Data on the type of menopause (natural, surgical, or other) was also not available. This information may help in elucidating relationships between age at menopause and lung cancer. Finally, information on lung cancer histology and specific types of birth control use may have provided additional clarification.
Despite these limitations, this is one of the few epidemiological studies examining potential lung cancer risk factors in Nepal (30, 31, 48–50), a country with limited cancer research resources and is the first study to examine the relationship between reproductive and hormonal factors and lung cancer among Nepali women. Additionally, studies that have examined the prevalence of reproductive and hormonal factors among Nepali women are scarce, and this study contributes much needed prevalence data on these exposures among Nepali women. The use of trained nurses via in-person interviews, standardized questionnaires, and detailed information on type, duration and quantity of tobacco products used are strengths of this study. In addition to contributing to the epidemiological body of evidence surrounding potential associations between reproductive and hormonal factors and lung cancer, this study contributes much needed information on the distribution of these risk factors among Nepali women.
Lung cancer is one of the three most common cancers diagnosed among Nepali women, and thus, it is crucial to understand and characterize the prevalence of risk factors in this population. However, research within the Nepali women population is lacking. Findings from this analysis contribute to our understanding of lung cancer occurring among Nepali women with a different prevalence of reproductive and hormonal factors and a high rate of lung cancer incidence. Further research is need to disentangle the relationship between smoking, reproductive and hormonal factors and lung cancer within this population and to assess the clinical applications of these associations.
Author Contributions
AS and MH designed and implemented the primary study. CD and SV conceptualized the ancillary study concept. AS, MH, CP, BT, and BS carried out the acquisition and quality control of data. SV performed statistical analysis of data. SV, CD and M-LL interpreted the results. SV wrote and drafted the manuscript. SV, CD, MH, and PW critically revised the manuscript for important intellectual content. All authors agreed to be accountable for the content of the work.
Funding
The study was financially supported by the International Agency for Research on Cancer (Lyon, France), the Huntsman Cancer Institute (Salt Lake City, Utah, United States) and the University of Maryland (College Park, Maryland, United States). AS was supported by US Fulbright Scholar Program to Nepal.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank the participants and the B. P. Koirala Memorial Cancer Hospital for their involvement in this study.
References
1. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. (2010) 127:2893–917. doi: 10.1002/ijc.25516
2. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. (2011) 61:69–90. doi: 10.3322/caac.20107
3. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. (2015) 136:E359–86. doi: 10.1002/ijc.29210
4. Bray F, Ren JS, Masuyer E, Ferlay J. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer. (2013) 132:1133–45. doi: 10.1002/ijc.27711
5. Pradhananga KK, Baral M, Shrestha BM. Multi-institution hospital-based cancer incidence data for Nepal: an initial report. Asian Pac J Cancer Prev. (2009) 10:259–62.
6. Poudel KK, Huang Z, Neupane PR, Steel R. Prediction of the cancer incidence in Nepal. Asian Pac J Cancer Prev. (2017) 18:165–8. doi: 10.22034/APJCP.2017.18.1.165
7. Piya MK, Acharya SC. Oncology in Nepal. South Asian J Cancer. (2012) 1:5–8. doi: 10.4103/2278-330X.96490
8. International Early Lung Cancer Action Program I, Henschke CI, Yip R, Miettinen OS. Women's susceptibility to tobacco carcinogens and survival after diagnosis of lung cancer. JAMA. (2006) 296:180–4. doi: 10.1001/jama.296.2.180
9. Risch HA, Howe GR, Jain M, Burch JD, Holowaty EJ, Miller AB. Are female smokers at higher risk for lung cancer than male smokers? A case-control analysis by histologic type. Am J Epidemiol. (1993) 138:281–93. doi: 10.1093/oxfordjournals.aje.a116857
10. Harris RE, Zang EA, Anderson JI, Wynder EL. Race and sex differences in lung cancer risk associated with cigarette smoking. Int J Epidemiol. (1993) 22:592–9. doi: 10.1093/ije/22.4.592
11. Wakelee HA, Chang ET, Gomez SL, Keegan TH, Feskanich D, Clarke CA, et al. Lung cancer incidence in never smokers. J Clin Oncol. (2007) 25:472–8. doi: 10.1200/JCO.2006.07.2983
12. Kiyohara C, Ohno Y. Sex differences in lung cancer susceptibility: a review. Gend Med. (2010) 7:381–401. doi: 10.1016/j.genm.2010.10.002
13. Siegfried JM, Hershberger PA, Stabile LP. Estrogen receptor signaling in lung cancer. Semin Oncol. (2009) 36:524–31. doi: 10.1053/j.seminoncol.2009.10.004
14. Cagle PT, Mody DR, Schwartz MR. Estrogen and progesterone receptors in bronchogenic carcinoma. Cancer Res. (1990) 50:6632–5.
15. Brenner AV, Wang Z, Kleinerman RA, Lei S, Metayer C, Wang W, et al. Menstrual and reproductive factors and risk of lung cancer among Chinese women, Eastern Gansu Province, 1994–1998. J Epidemiol. (2003) 13:22–8. doi: 10.2188/jea.13.22
16. Koushik A, Parent ME, Siemiatycki J. Characteristics of menstruation and pregnancy and the risk of lung cancer in women. Int J Cancer. (2009) 125:2428–33. doi: 10.1002/ijc.24560
17. Kreuzer M, Gerken M, Heinrich J, Kreienbrock L, Wichmann HE. Hormonal factors and risk of lung cancer among women? Int J Epidemiol. (2003) 32:263–71. doi: 10.1093/ije/dyg064
18. Lim WY, Chen Y, Chuah KL, Eng P, Leong SS, Lim E, et al. Female reproductive factors, gene polymorphisms in the estrogen metabolism pathway, and risk of lung cancer in Chinese women. Am J Epidemiol. (2012) 175:492–503. doi: 10.1093/aje/kwr332
19. Meinhold CL, Berrington de Gonzalez A, Bowman ED, Brenner AV, Jones RT, Lacey JV Jr, et al. Reproductive and hormonal factors and the risk of nonsmall cell lung cancer. Int J Cancer. (2011) 128:1404–13. doi: 10.1002/ijc.25434
20. Baik CS, Strauss GM, Speizer FE, Feskanich D. Reproductive factors, hormone use, and risk for lung cancer in postmenopausal women, the Nurses' Health Study. Cancer Epidemiol Biomarkers Prev. (2010) 19:2525–33. doi: 10.1158/1055-9965.EPI-10-0450
21. Brinton LA, Gierach GL, Andaya A, Park Y, Schatzkin A, Hollenbeck AR, et al. Reproductive and hormonal factors and lung cancer risk in the NIH-AARP Diet and Health Study cohort. Cancer Epidemiol Biomarkers Prev. (2011) 20:900–11. doi: 10.1158/1055-9965.EPI-10-1325
22. Pesatori AC, Carugno M, Consonni D, Caporaso NE, Wacholder S, Tucker M, et al. Reproductive and hormonal factors and the risk of lung cancer: the EAGLE study. Int J Cancer. (2013) 132:2630–9. doi: 10.1002/ijc.27926
23. Schwartz AG, Ray RM, Cote ML, Abrams J, Sokol RJ, Hendrix SL, et al. Hormone use, reproductive history, and risk of lung cancer: the women's health initiative studies. J Thorac Oncol. (2015) 10:1004–13. doi: 10.1097/JTO.0000000000000558
24. Weiss JM, Lacey JV Jr, Shu XO, Ji BT, Hou L, Yang G, et al. Menstrual and reproductive factors in association with lung cancer in female lifetime nonsmokers. Am J Epidemiol. (2008) 168:1319–25. doi: 10.1093/aje/kwn257
25. Gallagher LG, Rosenblatt KA, Ray RM, Li W, Gao DL, Applebaum KM, et al. Reproductive factors and risk of lung cancer in female textile workers in Shanghai, China. Cancer Causes Control. (2013) 24:1305–14. doi: 10.1007/s10552-013-0208-y
26. Seow A, Koh WP, Wang R, Lee HP, Yu MC. Reproductive variables, soy intake, and lung cancer risk among nonsmoking women in the Singapore Chinese Health Study. Cancer Epidemiol Biomarkers Prev. (2009) 18:821–7. doi: 10.1158/1055-9965.EPI-08-0892
27. Kabat GC, Miller AB, Rohan TE. Reproductive and hormonal factors and risk of lung cancer in women: a prospective cohort study. Int J Cancer. (2007) 120:2214–20. doi: 10.1002/ijc.22543
28. Paulus JK, Asomaning K, Kraft P, Johnson BE, Lin X, Christiani DC. Parity and risk of lung cancer in women. Am J Epidemiol. (2010) 171:557–63. doi: 10.1093/aje/kwp441
29. Elliott AM, Hannaford PC. Use of exogenous hormones by women and lung cancer: evidence from the Royal College of General Practitioners' Oral Contraception Study. Contraception. (2006) 73:331–5. doi: 10.1016/j.contraception.2005.10.003
30. Hashibe M, Siwakoti B, Wei M, Thakur BK, Pun CB, Shrestha BM, et al. Socioeconomic status and lung cancer risk in Nepal. Asian Pac J Cancer Prev. (2011) 12:1083–8. Available online at: http://journal.waocp.org/article_25659_22288138f42055fa92d75893757e1cb0.pdf
31. Raspanti GA, Hashibe M, Siwakoti B, Wei M, Thakur BK, Pun CB, et al. Ethnic variation in consumption of traditional tobacco products and lung cancer risk in Nepal. Asian Pac J Cancer Prev. (2015) 16:5721–6. doi: 10.7314/APJCP.2015.16.14.5721
32. Rajbhandari S, Amatya A, Giri K. Relation of ethnicity and menopausal symptoms in Nepal. J South Asian Feder Menopause Soc. (2013) 1:50–5. doi: 10.5005/jp-journals-10032-1012
33. Acharya D, Gautam S, Neupane N, Kaphle HP, Singh JK. Health problems of women above forty years of age in rupandehi district of Nepal. Int J Health Sci Res. (2013) 3:29–36. Available online at: http://www.ijhsr.org/IJHSR_Vol.3_Issue.3_March2013/4.pdf
34. Marahatta RK. Study of menopausal symptoms among peri and postmenopausal women attending NMCTH. Nepal Med Coll J. (2012) 14:251–5. Available online at: https://pdfs.semanticscholar.org/fdf8/d0cf6993bb7aff38e0298a2cd8faf1326b66.pdf
35. Chuni N, Sreeramareddy CT. Frequency of symptoms, determinants of severe symptoms, validity of and cut-off score for menopause rating scale (MRS) as a screening tool: a cross-sectional survey among midlife Nepalese women. BMC Womens Health. (2011) 11:30. doi: 10.1186/1472-6874-11-30
36. Rajbhandari S, Subedi RK, Dangal G, Phuyal A, Vaidya A, Karki A, et al. Menopausal health status of Nepalese women. J Nepal Med Assoc. (2017) 56:107–11. doi: 10.31729/jnma.2907
37. Gold EB. The timing of the age at which natural menopause occurs. Obstet Gynecol Clin North Am. (2011) 38:425–40. doi: 10.1016/j.ogc.2011.05.002
38. Nelson LR, Bulun SE. Estrogen production and action. J Am Acad Dermatol. (2001) 45:S116–24. doi: 10.1067/mjd.2001.117432
39. Omoto Y, Kobayashi Y, Nishida K, Tsuchiya E, Eguchi H, Nakagawa K, et al. Expression, function, and clinical implications of the estrogen receptor beta in human lung cancers. Biochem Biophys Res Commun. (2001) 285:340–7. doi: 10.1006/bbrc.2001.5158
40. Stabile LP, Davis AL, Gubish CT, Hopkins TM, Luketich JD, Christie N, et al. Human non-small cell lung tumors and cells derived from normal lung express both estrogen receptor alpha and beta and show biological responses to estrogen. Cancer Res. (2002) 62:2141–50. Available online at: http://cancerres.aacrjournals.org/content/62/7/2141.full-text.pdf
41. Sharma M, Gupta S. Menstrual pattern and abnormalities in the high school girls of Dharan: a cross sectional study in two boarding schools. Nepal Med Coll J. (2003) 5:34–6.
42. Aryal TR. Age at menarche: differentials and determinants. J Nepal Med Assoc. (2004) 43:71–5. doi: 10.31729/jnma.562
43. Sunuwar L, Saha CG, KC A, Dhungel KU. Age at menarche of subpopulation of Nepalese girls. Nepal Med Coll J. (2010) 12:183–6.
44. Ministry of Health and Population, New ERA and ICF International, 2012. 2011 Nepal Demographic and Health Survey: Key Findings. Kathmandu and Calverton, MA: Ministry of Health and Population, New ERA and ICF International.
45. Martin JA, Hamilton BE, Osterman MJK, Curtin SC, Mathews TJ. Births: final data for 2012. In: National Vital Statistics Reports. Hyattsville, MD: National Center for Health Statistics (2013). Available online at: http://www.cdc.gov/nchs/data/nvsr/nvsr62/nvsr62_09.pdf
46. Khatri RB, Mishra SR, Khanal V. Tobacco use among rural Nepalese women: cross-sectional community based study. Indian J Cancer. (2015) 52:699–704. doi: 10.4103/0019-509X.178412
47. Jamal A, Phillips E, Gentzke AS, Homa DM, Babb SD, King BA, et al. Current cigarette smoking among adults—United States, 2016. Morbidity Mortality Weekly Rep. (2018) 67:53–9. doi: 10.15585/mmwr.mm6702a1
48. Chawla R, Sathian B, Mehra A, Kiyawat V, Garg A, Sharma K. Awareness and assessment of risk factors for lung cancer in residents of Pokhara Valley, Nepal. Asian Pac J Cancer Prev. (2010) 11:1789–93. Available online at: http://journal.waocp.org/article_25451_8e5d4324b5ad25d4d454dae355931bb9.pdf
49. Raspanti GA, Hashibe M, Siwakoti B, Wei M, Thakur BK, Pun CB, et al. Household air pollution and lung cancer risk among never-smokers in Nepal. Environ Res. (2016) 147:141–5. doi: 10.1016/j.envres.2016.02.008
Keywords: lung cancer, Nepal, women, reproductive factors, hormonal factors
Citation: Vohra SN, Sapkota A, Lee M-LT, Pun CB, Thakur B, Siwakoti B, Wiesenfeld PL, Hashibe M and Dallal CM (2019) Reproductive and Hormonal Factors in Relation to Lung Cancer Among Nepali Women. Front. Oncol. 9:311. doi: 10.3389/fonc.2019.00311
Received: 02 January 2019; Accepted: 05 April 2019;
Published: 07 May 2019.
Edited by:
Clement Adebamowo, University of Maryland School of Medicine, United StatesReviewed by:
Ahmad Ali, University of Mumbai, IndiaMelissa Ana Liriano Vyfhuis, University of Maryland School of Medicine, United States
Copyright © 2019 Vohra, Sapkota, Lee, Pun, Thakur, Siwakoti, Wiesenfeld, Hashibe and Dallal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amir Sapkota, YW1pcnNhcEB1bWQuZWR1
 Sanah N. Vohra
Sanah N. Vohra Amir Sapkota
Amir Sapkota Mei-Ling T. Lee3
Mei-Ling T. Lee3 Mia Hashibe
Mia Hashibe Cher M. Dallal
Cher M. Dallal