- 1Department of Radiation Oncology, Affiliated Cancer Hospital and Institute of Guangzhou Medical University, Guangzhou, China
- 2State Key Laboratory of Respiratory Diseases, Guangzhou Institute of Respiratory Disease, Affiliated Cancer Hospital and Institute of Guangzhou Medical University, Guangzhou, China
- 3State Key Laboratory of Oncology in South China, Collaborative Innovation Center of Cancer Medicine, Sun Yat-sen University Cancer Center, Guangzhou, China
- 4Department of Radiation Oncology, Nanfang Hospital, Southern Medical University, Guangzhou, China
Zinc finger protein 671 (ZNF671) is a member of the largest transcription factor family in the human genome. However, the methylation status, expression, and prognostic role of ZNF671 in solid tumors remain unclear. The aim of this study was to explore the relationship between ZNF671 and the prognosis of patients with solid tumors. We performed a pan-cancer analysis of the methylation status and mRNA and protein expression of ZNF671 using The Cancer Genome Atlas (TCGA) database and the Human Protein Atlas. We further evaluated the prognostic value of ZNF671 expression among numerous cancer types using the “Kaplan–Meier plotter” (KM plotter) database. We found that downregulation of ZNF671 is associated with hypermethylation of its promoter. Survival analysis established that the downregulation of ZNF671 predicts poor prognosis in breast invasive carcinoma (BRCA), cervical squamous cell carcinoma and endocervical adenocarcinoma (CESC), head and neck squamous cell carcinoma (HNSC), kidney renal papillary cell carcinoma (KIRP), lung adenocarcinoma (LUAD), pancreatic adenocarcinoma (PAAD), and uterine corpus endometrial carcinoma (UCEC) solid tumors. CCK-8 and Transwell functional assays showed that ZNF671 could inhibit tumor cell proliferation, migration, and invasion. These results indicate that ZNF671 is an excellent predictive factor for BRCA, CESC, HNSC, KIRP, LUAD, PAAD, SARC, and UCEC solid tumors and may play crucial roles in the development and progression of these tumors.
Introduction
Epigenetic regulation is essential for the normal development and maintenance of gene expression in mammals. Many biological processes are involved in DNA methylation, histone modification, and nucleosome remodeling (1). Disruption of epigenetic processes can lead to activation of oncogenes and/or the inactivation of tumor suppressor pathways, and the accumulation of epigenetic changes in the molecular landscape is a hallmark of cancer (2). DNA methylation is an early event in tumorigenesis and plays a major role in tumor initiation and progression (3). Therefore, it is important to identify reliable prognostic and predictive biomarkers in solid tumors that may help in early diagnosis and treatment strategies.
Zinc finger (ZF) protein 671 (ZNF671) is a member of the KRAB-ZF (KRAB-ZFP) family of mammalian transcriptional repressors (4–6). Through recruitment of KRAB-associated protein-1 and other co-repressors, KRAB-ZFPs can regulate cell differentiation, proliferation, apoptosis, tumor suppression, and neoplastic transformation (7–11). The Illumina Human Methylation 450K Beadchip analysis and genome-wide methylation profiles identified ZNF671 as a gene with significantly higher methylation compared to other genes (12, 13). However, the methylation level and prognostic role of ZNF671 in other tumors are still not clear.
In this study, we systematically analyzed the methylation level and expression of ZNF671 and explored its correlation with solid tumors by analyzing The Cancer Genome Atlas (TCGA) database and the Human Protein Atlas (HPA). We evaluated the prognostic role of ZNF671 in 18 solid tumors using the Kaplan–Meier (KM) plotter database. Our results provide important insights into ZNF671 hypermethylation as a promising biomarker for eight solid tumors and thereby provide novel perspectives for the treatment of tumors.
Materials and Methods
TCGA Dataset Analysis
Gene methylation and expression data were downloaded from TCGA (http://cancergenome.nih.gov) and were interpreted, normalized, and log2 scaled using the online analysis tool. This study was approved by the Institutional Ethical Review Boards of Guangzhou Medical University Cancer Center.
Immunohistochemistry Analysis
The HPA database (https://www.proteinatlas.org/) was used to perform a pan-cancer analysis of ZNF671 protein expression (14). ZNF671 was entered in the database (https://www.proteinatlas.org/) to acquire ZNF671 expression for individual tumors of each cancer type (Scar bar = 200μm). All IHC images have been manually annotated by certified pathologists.
Prognostic Analysis
An online KM plotter database was used to assess the correlation of intra-individual ZNF671 mRNA expression with progression-free survival (15). ZNF671 was entered in the database (http://kmplot.com/analysis/) to acquire pan-cancer KM survival plots. Hazard ratio (HR), 95% confidence intervals, and log-rank P were determined and presented on the main plots.
Western Blot Analysis
After cells were transfected with the pSin-EF2-puro-ZNF671-HA or pSin-EF2-puro-vector plasmids (Land Hua Gene Biosciences, Guangzhou, China) for 48 h, Radio-Immunoprecipitation Assay (RIPA) lysis buffer (Beyotime, Shanghai, China) was used to isolate proteins. Proteins were separated by Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (SDS-PAGE, Beyotime), transferred onto polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA, USA), and incubated with primary anti-ZNF671 (1:500; Proteintech, Chicago, IL, USA) and anti-Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1:1,000, Proteintech, Chicago, IL, USA).
CCK-8 and Transwell Assays
For the CCK-8 assay, cells (1 × 103) were seeded into 96-well plates, incubated for 0–4 days, stained with CCK-8 (Dojindo, Tokyo, Japan), and absorbance values were determined at 450 nm using a spectrophotometer. Transwell migration and invasion assays were carried out using Transwell chambers (8 μm; Corning, Tewksbury, MA, USA) without or with Matrigel (BD Biosciences, San Jose, CA, USA). Cells were added to the upper chamber in serum-free medium, and the lower chamber contained culture medium with 20% FBS. The cells were incubated for 12 or 24 h at 37°C in 5% CO2 and were then fixed and stained. Cells on the undersides of the filters were observed and counted under i200 magnification.
Statistical Analysis
Statistical analysis was performed using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). Differences between two groups were analyzed using the two-tailed unpaired Student's t-test; P < 0.05 was considered statistically significant.
Results
ZNF671 Promoter Is Hypermethylated in Several Solid Tumor Types
To determine the methylation level of ZNF671 across cancer types, we analyzed human pan-cancer methylation data in the TCGA database. Among the 18 cancers examined, the promoter of ZNF671 was hypermethylated in bladder urothelial carcinoma (BLCA), breast invasive carcinoma (BRCA), cervical squamous cell carcinoma and endocervical adenocarcinoma (CESC), colon adenocarcinoma (COAD), head and neck squamous cell carcinoma (HNSC), kidney renal clear cell carcinoma (KIRC), kidney renal papillary cell carcinoma (KIRP), liver hepatocellular carcinoma (LIHC), lung adenocarcinoma (LUAD), lung squamous cell carcinoma (LUSC), pancreatic adenocarcinoma (PAAD), prostate adenocarcinoma (PRAD), rectum adenocarcinoma (READ), sarcoma (SARC), skin cutaneous melanoma (SKCM), stomach adenocarcinoma (STAD), and uterine corpus endometrial carcinoma (UCEC) (Figure 1, **P < 0.01). For thyroid carcinoma (THCA), the methylation level between tumor tissues and normal tissues did not differ.

Figure 1. ZNF671 is hypermethylated in solid tumors. ZNF671 methylation analysis from The Cancer Genome Atlas. BLCA, bladder urothelial carcinoma; BRCA, breast invasive carcinoma; CESC, cervical squamous cell carcinoma and endocervical adenocarcinoma; COAD, colon adenocarcinoma; HNSC, head and neck squamous cell carcinoma; KIRC, kidney renal clear cell carcinoma; KIRP, kidney renal papillary cell carcinoma; LIHC, liver hepatocellular carcinoma; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; PAAD, pancreatic adenocarcinoma; PRAD, prostate adenocarcinoma; READ, rectum adenocarcinoma; SARC, sarcoma; SKCM, skin cutaneous melanoma; STAD, stomach adenocarcinoma; THCA, thyroid carcinoma; UCEC, uterine corpus endometrial carcinoma.
ZNF671 mRNA Expression Is Downregulated in Several Solid Tumor Types
To determine the mRNA expression level of ZNF671 in different cancers, we further used the TCGA database to validate the mRNA expression of ZNF671 and found that ZNF671 was downregulated in BLCA, BRCA, CESC, COAD, HNSC, KIRP, LUAD, LUSC, PAAD, PRAD, READ, SARC, STAD, THCA, and UCEC, but the mRNA expression of ZNF671 was upregulated in KIRC, LIHC, and SKCM (Figure 2). Further correlation analysis found that the mRNA expression was negatively correlated with promoter methylation in the 18 cancers (Figure 3). These results indicate that the promoter hypermethylation of ZNF671 mediates its downregulation in BLCA, BRCA, CESC, COAD, HNSC, KIRP, LUAD, LUSC, PAAD, PRAD, READ, SARC, STAD, THCA, and UCEC cancers.
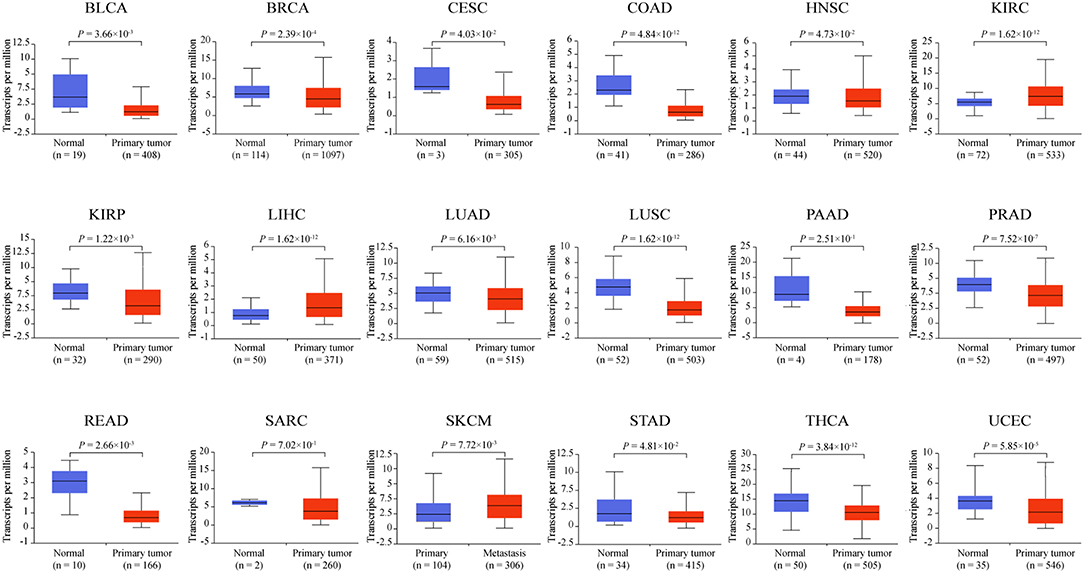
Figure 2. ZNF671 is downregulated in primary solid tumors. ZNF671 mRNA expression analysis from The Cancer Genome Atlas. BLCA, bladder urothelial carcinoma; BRCA, breast invasive carcinoma; CESC, cervical squamous cell carcinoma and endocervical adenocarcinoma; COAD, colon adenocarcinoma; HNSC, head and neck squamous cell carcinoma; KIRC, kidney renal clear cell carcinoma; KIRP, kidney renal papillary cell carcinoma; LIHC, liver hepatocellular carcinoma; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; PAAD, pancreatic adenocarcinoma; PRAD, prostate adenocarcinoma; READ, rectum adenocarcinoma; SARC, sarcoma; SKCM, skin cutaneous melanoma; STAD, stomach adenocarcinoma; THCA, thyroid carcinoma; UCEC, uterine corpus endometrial carcinoma.

Figure 3. Correlations between ZNF671 promoter methylation and mRNA expression in primary solid tumors. Analysis of data from The Cancer Genome Atlas. BLCA, bladder urothelial carcinoma; BRCA, breast invasive carcinoma; CESC, cervical squamous cell carcinoma and endocervical adenocarcinoma; COAD, colon adenocarcinoma; HNSC, head and neck squamous cell carcinoma; KIRC, kidney renal clear cell carcinoma; KIRP, kidney renal papillary cell carcinoma; LIHC, liver hepatocellular carcinoma; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; PAAD, pancreatic adenocarcinoma; PRAD, prostate adenocarcinoma; READ, rectum adenocarcinoma; SARC, sarcoma; SKCM, skin cutaneous melanoma; STAD, stomach adenocarcinoma; THCA, thyroid carcinoma; UCEC, uterine corpus endometrial carcinoma.
ZNF671 Protein Expression Is Downregulated in Several Solid Tumor Types
We performed a pan-cancer analysis of the protein expression of ZNF671 using the HPA, which presented the protein expression of ZNF671 in 17 different tumor types. As shown in Figure 4A, the protein expression level of ZNF671 was downregulated in most cancers. Statistical analysis indicated that ZNF671 expression was low and/or absent in urothelial (75%), breast (90.9%), cervical (90%), endometrial (83.3%), head and neck (100%), renal (100%), liver (77.8%), lung (100%), pancreatic (72.7%), prostate (100%), skin (100%), and thyroid (75%) cancers. ZNF671 protein expression was low and/or absent in only 41.7 and 45.4% of colorectal and stomach cancers, respectively (Figure 4B), and the protein expression of ZNF671 in sarcoma was unclear. These results indicate that ZNF671 protein expression is downregulated in urothelial, breast, cervical, endometrial, heck and neck, renal, liver, lung, pancreatic, prostate, skin, and thyroid cancers and that ZNF671 may play a tumor suppressor role in cancer progression.
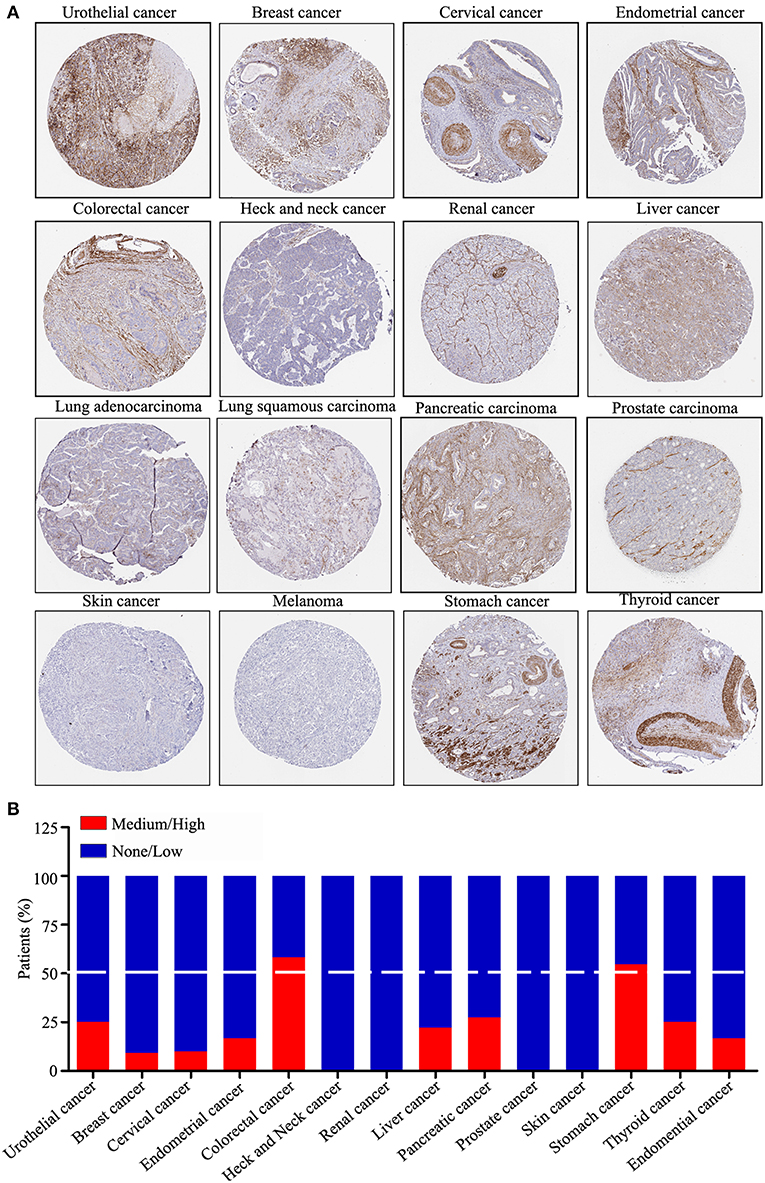
Figure 4. ZNF671 protein expression is downregulated in primary solid tumors. (A) Immunohistochemical stained tissues representing ZNF671 protein expression as shown in the Human Pathology Atlas database. (B) Protein expression levels across 14 cancer types analyzed by IHC in the Human Pathology Atlas database.
ZNF671 Is a Prognostic Biomarker in Several Solid Tumor Types
To further assess the prognostic role of ZNF671 in different cancers, we explored the relationship between mRNA expression of ZNF671 and patient survival using an online tool (http://kmplot.com). High ZNF671 mRNA expression was found to be significantly correlated with increased probability of survival for BRCA (HR = 0.56; P = 0.00045), CESC (HR = 0.5; P = 0.0049), HNSC (HR = 0.67; P = 0.0043), KIRC (HR = 0.55; P = 0.00018), KIRP (HR = 0.3; P = 2.4e-5), LUAD (HR = 0.58; P = 0.00022), PAAD (HR = 0.46; P = 5e-4), SARC (HR = 0.63; P = 0.025), and UCEC (HR = 0.55; P = 0.0062), but not in BLCA (HR = 0.89; P = 0.46), COAD (HR = 1.23; P = 0.3), LIHC (HR = 1.34; P = 0.11), LUSC (HR = 1.3; P = 0.072), PRAD (HR = 1.84; P = 0.38), READ (HR = 0.59; P = 0.18), SKCM (HR = 1.08; P = 0.025), STAD (HR = 1.27; P = 0.17), and THCA (HR = 0.62; P = 0.33; Figure 5).

Figure 5. The prognostic effect of ZNF671 expression in solid tumors from KM plotter. Survival curves are plotted for all patients. BLCA, bladder urothelial carcinoma; BRCA, breast invasive carcinoma; CESC, cervical squamous cell carcinoma and endocervical adenocarcinoma; COAD, colon adenocarcinoma; HNSC, head and neck squamous cell carcinoma; KIRC, kidney renal clear cell carcinoma; KIRP, kidney renal papillary cell carcinoma; LIHC, liver hepatocellular carcinoma; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; PAAD, pancreatic adenocarcinoma; PRAD, prostate adenocarcinoma; READ, rectum adenocarcinoma; SARC, sarcoma; SKCM, skin cutaneous melanoma; STAD, stomach adenocarcinoma; THCA, thyroid carcinoma; UCEC, uterine corpus endometrial carcinoma.
The relationship between ZNF671 methylation, mRNA and protein expression, and prognosis among different cancer types is summarized in Table 1. Overall, the results indicate that epigenetic-mediated downregulation of ZNF671 predicts poor survival in BRCA, CESC, HNSC, KIRP, LUAD, PAAD, UCEC, and SARC and that ZNF671 may play a tumor suppressor role in tumor progression, which is consistent with previous studies in HNSC, UCEC, and KIRP (11, 16, 17).
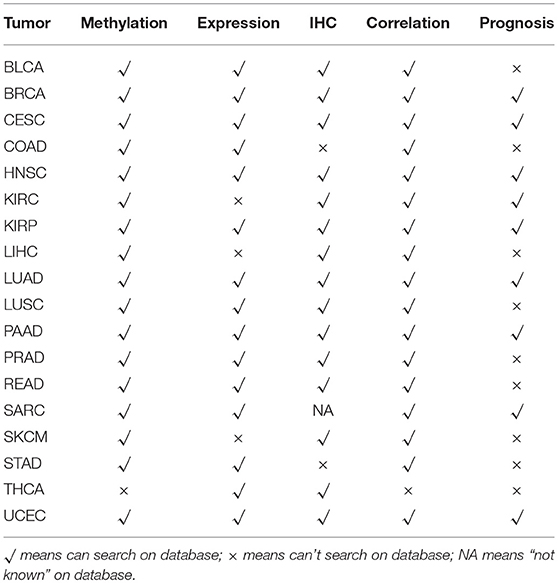
Table 1. Methylation status, gene expression, protein expression (IHC), correlation analysis and prognostic predictive value of ZNF671 in 18 solid tumors.
ZNF671 Inhibits Tumor Cell Proliferation, Migration, and Invasion
To assess the effects of ZNF671 in BRCA, CESC, LUAD, PAAD, and SARC, we performed CCK-8 and Transwell assays using MCF7, Hela, PC9, PANC1, and HOS cells transfected with ZNF671 or vector. As shown in Figure 6A, Western blot analysis validated that ZNF671 protein level was obviously elevated after transfection of ZNF671 plasmid in MCF7, Hela, PC9, PANC1, and HOS cells. Overexpression of ZNF671 suppressed the proliferation (Figure 6B) and migratory and invasive (Figures 6C–E) abilities of the tumor cells as determined by Transwell migration and invasion assays. These findings indicate that ZNF671 inhibits the proliferation and migratory and invasive abilities of BRCA, CESC, LUAD, PAAD, and SARC cells in vitro.
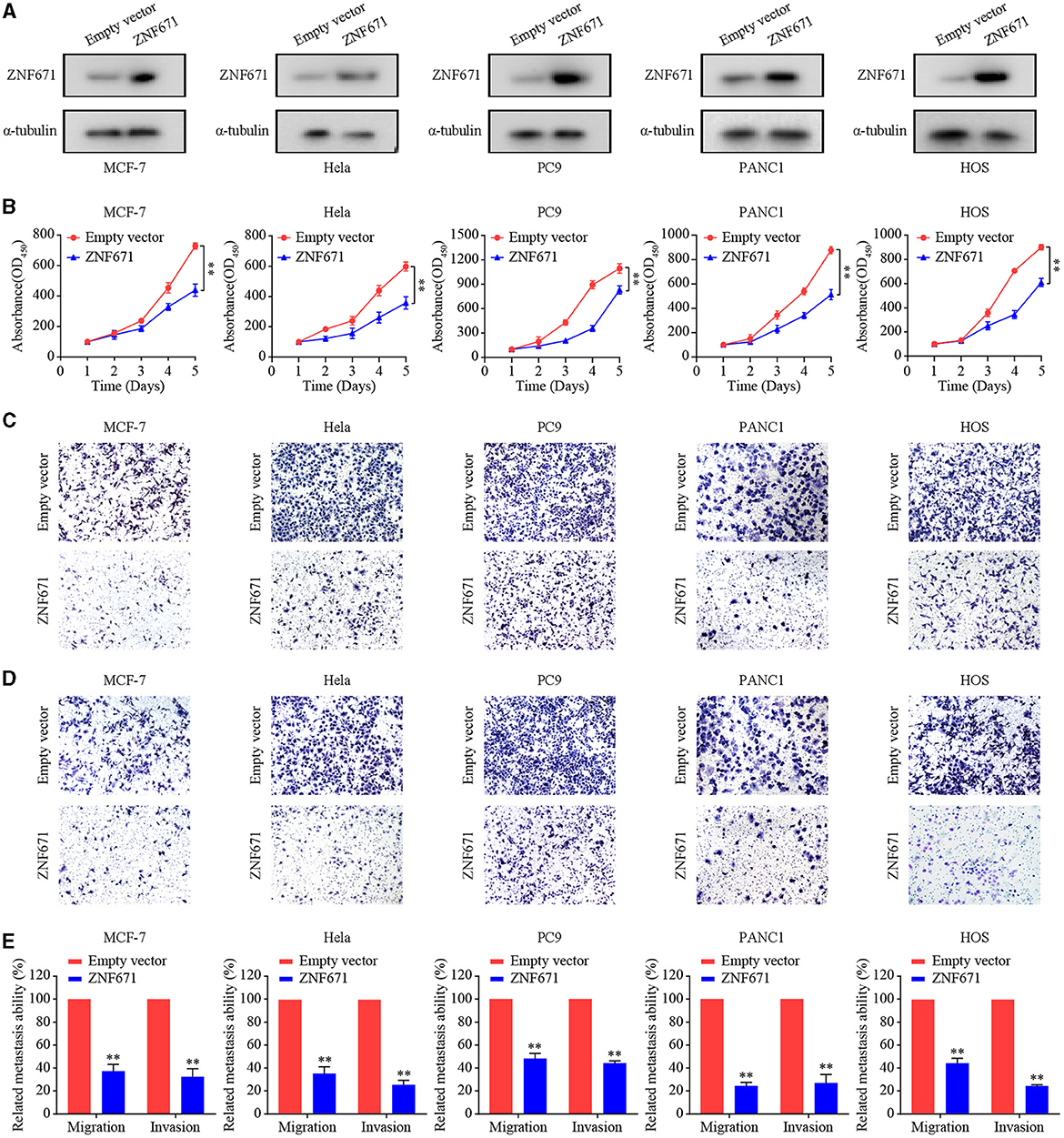
Figure 6. Effects of ZNF671 overexpression on cell proliferation, migration, and invasion in vitro. (A) Representative Western blot analysis of ZNF671 overexpression in MCF7, Hela, PC9, PANC1, and HOS cell lines. (B) The CCK-8 assay showed overexpression of ZNF671 reduced the viability of MCF7, Hela, PC9, PANC1, and HOS cells. (C,D) Representative images of the effects of ZNF671 overexpression on migratory and invasive abilities of cells as determined by Transwell migration (C) and invasion (D) assays. (E) Quantification of the effects of ZNF671 overexpression on migratory and invasive abilities. All of the experiments were performed at least three times. Data presented are the mean ± SD; **P < 0.01 compared with control using Student’s t-test.
Discussion
ZNF671 belongs to the KRAB-ZF family of transcription factors, which contain C2H2-type ZFs and a Krüppel-associated box (KRAB) domain. KRAB-ZFPs are involved in regulating cell differentiation, proliferation, apoptosis, tumor suppression, and immune control (7, 9, 11, 18, 19). Our previous studies demonstrated that ZNF671 is a tumor suppressor that is epigenetically silenced by DNA methylation in nasopharyngeal carcinoma, but there is limited information regarding the role of ZNF671 methylation among different solid cancer types. The present study found that downregulation of ZNF671 was associated with its promoter hypermethylation among many different cancer types. Functional assays and prognostic analysis found that high ZNF671 expression suppressed tumor cell proliferation, migration, and invasion, and downregulation of ZNF671 was associated with poor clinical prognosis in BRCA, CESC, HNSC, KIRP, LUAD, PAAD, and UCEC. Thus, ZNF671 represents a novel predictive marker for the prognosis of patients with BRCA, CESC, HNSC, KIRP, LUAD, PAAD, SARC, and UCEC.
In this study, we found that the ZNF671 promoter is significantly methylated in 17 solid tumors, based on the TCGA datasets, which is consistent with studies in urothelial carcinoma, clear cell renal cell carcinomas, and cervical cancer (16, 17, 20). Next, we compared and analyzed the mRNA and protein expression levels of ZNF671 in these solid tumors. Statistical analysis revealed that the expression of ZNF671 was negatively correlated with its promoter hypermethylation in BLCA, BRCA, CESC, HNSC, KIRC, KIRP, LIHC, LUAD, LUSC, PAAD, READ, SARC, SKCM, and UCEC, suggesting a crucial role for ZNF671 in the progression of these cancer types.
To further explore the potential prognostic value of ZNF671 in BLCA, BRCA, CESC, HNSC, KIRC, KIRP, LIHC, LUAD, LUSC, PAAD, READ, SARC, SKCM, and UCEC, we used KM plotter analysis to evaluate the correlation between ZNF671 expression and survival probability. KM plotter is used to analyze individual genes in different cancers (21). We found that low ZNF671 expression may predict poorer survival in BRCA, CESC, HNSC, KIRP, LUAD, PAAD, SARC, and UCEC. CCK-8 and Transwell migration and invasion assays showed that ZNF671 could inhibit tumor cell proliferation, migration, and invasion. However, additional studies are still needed to explore the molecular mechanism underlying the effects of ZNF671 expression in BRCA, CESC, HNSC, KIRP, LUAD, PAAD, SARC, and UCEC.
In conclusion, this is the first report to systematically evaluate the correlation between methylation of ZNF671 and prognosis among 18 solid tumors. The prognostic signature observed in our study was linked to hypermethylation of ZNF671. Our findings reveal that ZNF671 is hypermethylated in BRCA, CESC, HNSC, KIRP, LUAD, PAAD, SARC, and UCEC, and functional assays established that ZNF671 inhibits tumor cell proliferation, migration, and invasion. Further prognosis analysis indicated that high expression of ZNF671 was associated with longer overall survival (OS) and that ZNF671 expression is a favorable prognostic indicator in BRCA, CESC, HNSC, KIRP, LUAD, PAAD, SARC, and UCEC, but the mechanism remains unknown. Further studies are needed to clarify this issue. Our results confirm that ZNF671 can be used as a biomarker, with low expression predicting worse outcomes in patients with BRCA, CESC, HNSC, KIRP, LUAD, PAAD, SARC, and UCEC.
Author Contributions
JiZ and ZZ designed the research. JlZ, TX, YT, RL, BW, JL, and AX acquired and analyzed the data. JiZ, XH and YY wrote the manuscript.
Funding
This work was supported by grants from the Guangzhou Key Medical Discipline Construction Project Fund (B195002004042).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank professors Ying Sun, Jun Ma, and Na Liu (State Key Laboratory of Oncology in South China, Collaborative Innovation Center of Cancer Medicine; Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy; and Sun Yat-sen University Cancer Center, Guangzhou 510060, P.R. China) for supporting this work. We would like to thank the native English-speaking scientists of Elixigen Company (Huntington Beach, California) for editing our manuscript.
References
1. Dawson MA, Kouzarides T. Cancer epigenetics: from mechanism to therapy. Cell. (2012) 150:12–27. doi: 10.1016/j.cell.2012.06.013
2. Kanwal R, Gupta K, Gupta S. Cancer epigenetics: an introduction. Methods Mol Biol. (2015) 1238:3–25. doi: 10.1007/978-1-4939-1804-1_1
3. Suzuki H, Watkins DN, Jair KW, Schuebel KE, Markowitz SD, Chen WD, et al. Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nat Genet. (2004) 36:417–22. doi: 10.1038/ng1330
4. Witzgall R, O'Leary E, Leaf A, Onaldi D, Bonventre JV. The Kruppel-associated box-A (KRAB-A) domain of zinc finger proteins mediates transcriptional repression. Proc Natl Acad Sci USA. (1994) 91:4514–18.
5. Margolin JF, Friedman JR, Meyer WK, Vissing H, Thiesen HJ, Rauscher FJ III. Kruppel-associated boxes are potent transcriptional repression domains. Proc Natl Acad Sci USA. (1994) 91:4509–13.
6. Urrutia R. KRAB-containing zinc-finger repressor proteins. Genome Biol. (2003) 4:231. doi: 10.1186/gb-2003-4-10-231
7. Cheng Y, Geng H, Cheng SH, Liang P, Bai Y, Li J, et al. KRAB zinc finger protein ZNF382 is a proapoptotic tumor suppressor that represses multiple oncogenes and is commonly silenced in multiple carcinomas. Cancer Res. (2010) 70:6516–26. doi: 10.1158/0008-5472.CAN-09-4566
8. Friedman JR, Fredericks WJ, Jensen DE, Speicher DW, Huang XP, Neilson EG, et al. KAP-1, a novel corepressor for the highly conserved KRAB repression domain. Genes Dev. (1996) 10:2067–78.
9. Moosmann P, Georgiev O, Le Douarin B, Bourquin JP, Schaffner W. Transcriptional repression by RING finger protein TIF1 beta that interacts with the KRAB repressor domain of KOX1. Nucleic Acids Res. (1996) 24:4859–67.
10. Zheng L, Pan H, Li S, Flesken-Nikitin A, Chen PL, Boyer TG, et al. Sequence-specific transcriptional corepressor function for BRCA1 through a novel zinc finger protein, ZBRK1. Mol Cell. (2000) 6:757–68.
11. Zhang J, Wen X, Liu N, Li YQ, Tang XR, Wang YQ, et al. Epigenetic mediated zinc finger protein 671 downregulation promotes cell proliferation and tumorigenicity in nasopharyngeal carcinoma by inhibiting cell cycle arrest. J Exp Clin Cancer Res. (2017) 36:147. doi: 10.1186/s13046-017-0621-2
12. Jiang W, Liu N, Chen XZ, Sun Y, Li B, Ren XY, et al. Genome-wide identification of a methylation gene panel as a prognostic biomarker in nasopharyngeal carcinoma. Mol Cancer Ther. (2015) 14:2864–73. doi: 10.1158/1535-7163.MCT-15-0260
13. Zhao W, Mo Y, Wang S, Midorikawa K, Ma N, Hiraku Y, et al. Quantitation of DNA methylation in Epstein-Barr virus-associated nasopharyngeal carcinoma by bisulfite amplicon sequencing. BMC Cancer. (2017) 17:489. doi: 10.1186/s12885-017-3482-3
14. Uhlen M, Zhang C, Lee S, Sjostedt E, Fagerberg L, Bidkhori G, et al. A pathology atlas of the human cancer transcriptome. Science. (2017) 357:eaan2507. doi: 10.1126/science.aan2507
15. Gyorffy B, Lanczky A, Szallasi Z. Implementing an online tool for genome-wide validation of survival-associated biomarkers in ovarian-cancer using microarray data from 1287 patients. Endocr Relat Cancer. (2012) 19:197–208. doi: 10.1530/ERC-11-0329
16. Yeh CM, Chen PC, Hsieh HY, Jou YC, Lin CT, Tsai MH, et al. Methylomics analysis identifies ZNF671 as an epigenetically repressed novel tumor suppressor and a potential non-invasive biomarker for the detection of urothelial carcinoma. Oncotarget. (2015) 6:29555–72. doi: 10.18632/oncotarget.4986
17. Tian Y, Arai E, Gotoh M, Komiyama M, Fujimoto H, Kanai Y. Prognostication of patients with clear cell renal cell carcinomas based on quantification of DNA methylation levels of CpG island methylator phenotype marker genes. BMC cancer. (2014) 14:772. doi: 10.1186/1471-2407-14-772
18. Xie W, Qiao X, Shang L, Dou J, Yang X, Qiao S, et al. Knockdown of ZNF233 suppresses hepatocellular carcinoma cell proliferation and tumorigenesis. Gene. (2018) 679:179–85. doi: 10.1016/j.gene.2018.08.070
19. Tie CH, Fernandes L, Conde L, Robbez-Masson L, Sumner RP, Peacock T, et al. KAP1 regulates endogenous retroviruses in adult human cells and contributes to innate immune control. EMBO Rep. (2018) 19:e45000. doi: 10.15252/embr.201745000
20. Schmitz M, Wunsch K, Hoyer H, Scheungraber C, Runnebaum IB, Hansel A, et al. Performance of a methylation specific real-time PCR assay as a triage test for HPV-positive women. Clin Epigenetics. (2017) 9:118. doi: 10.1186/s13148-017-0419-2
Keywords: epigenetic, ZNF671, prognosis, solid tumor, data mining
Citation: Zhang J, Zheng Z, Zheng J, Xie T, Tian Y, Li R, Wang B, Lin J, Xu A, Huang X and Yuan Y (2019) Epigenetic-Mediated Downregulation of Zinc Finger Protein 671 (ZNF671) Predicts Poor Prognosis in Multiple Solid Tumors. Front. Oncol. 9:342. doi: 10.3389/fonc.2019.00342
Received: 21 November 2018; Accepted: 15 April 2019;
Published: 07 May 2019.
Edited by:
Matiullah Khan, AIMST University, MalaysiaReviewed by:
Gayatri Sharma, Medical College of Wisconsin, United StatesDawn Sijin Nin, National University of Singapore, Singapore
Copyright © 2019 Zhang, Zheng, Zheng, Xie, Tian, Li, Wang, Lin, Xu, Huang and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yawei Yuan, eXVhbnlhd2VpQGd6aG11LmVkdS5jbg==
†These authors have contributed equally to this work
 Jian Zhang
Jian Zhang Ziqi Zheng
Ziqi Zheng Jieling Zheng4†
Jieling Zheng4† Yawei Yuan
Yawei Yuan