- 1Department of Hematology, The Third Clinical Institute Affiliated to Wenzhou Medical University, People's Hospital of Wenzhou, Wenzhou, China
- 2Department of Urology, Affiliated Hospital of Yangzhou University, Yangzhou, China
- 3Department of Anorectal Surgery, Sixth Affiliated Hospital of Wenzhou Medical University (Lishui People's Hospital), Lishui, China
- 4Department of Urology, The Third Clinical Institute Affiliated to Wenzhou Medical University, People's Hospital of Wenzhou, Wenzhou, China
- 5Department of Urology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China
- 6Department of Respiratory, Rui'an People's Hospital, The Third Affiliated Hospital of the Wenzhou Medical University, Wenzhou, China
- 7Department of Urology, Changhai Hospital, Second Military Medical University, Shanghai, China
This study aimed to evaluate the preoperative prognostic value of systemic inflammation response index and platelet-to-lymphocyte ratio (SIRI-PLR) in patients with upper tract urothelial carcinoma (UTUC). The prognostic ability of SIRI-PLR was evaluated in a training cohort comprising 259 patients with UTUC who underwent radical nephroureterectomy and was further validated in an independent cohort comprising of 274 patients. Multivariate Cox regression models showed that SIRI was significantly associated with overall-survival (OS), cancer-specific survival (CSS), and metastatic-free survival (MFS), and PLR significantly affected OS and CSS (all P < 0.05). In particular, a simultaneously high SIRI-PLR value was considered an independent risk factor even after adjusting for confounding factors and was superior to SIRI alone in predicting survival among patients with UTUC. The analyses of concordance-index and receiver operating characteristic curve showed that incorporation of SIRI-PLR vs. without its incorporation into newly developed nomograms or currently available clinical parameters, such as pathologic T stage, N stage, or tumor grade, had higher accuracy in predicting urologic outcomes of patients with UTUC. These results were observed in the training cohort and were confirmed in the validation cohort. In conclusion, patients with a simultaneously high SIRI-PLR value had significantly poor prognosis. Incorporating SIRI-PLR into currently available clinical parameters can help in patient management.
Introduction
Upper tract urothelial carcinomas (UTUCs) are relatively rare types of urologic cancer, and they account for ~5–10% of all urothelial carcinomas (1, 2). Because of their aggressive clinical and biological nature, ~60% of UTUCs are already invasive and 7% have metastasized at the time of diagnosis (3); hence, the prognosis is usually poor (4). Radical nephroureterectomy (RNU) with or without cisplatin-based combination chemotherapy remains the gold standard treatment for non-metastatic UTUC (5). However, almost 50% of patients who undergo RNU treatment experience recurrence (6). Moreover, the 5-year cancer-specific survival (CSS) rates are <50% for pathologic T2 and T3 (pT2/pT3) disease and <10% for pathologic T4 (pT4) disease (7–9). Thus, the evaluation of risk factors is important to identify patients who are more likely to experience disease recurrence after RNU.
Accumulating evidence has revealed the significant role of inflammatory markers in the development and progression of tumors. Previous studies have shown that inflammation biomarkers, including platelet-to-lymphocyte ratio (PLR), neutrophil-to-lymphocyte ratio (NLR), monocyte-to-lymphocyte ratio (MLR), and systemic inflammation response index (SIRI), are associated with worse urologic outcomes (10–13). However, the correlation between NLR and the prognosis of UTUC remains controversial (10). Several studies have shown that SIRI, which is based on neutrophil, monocyte, and lymphocyte count, is a significant risk factor for several cancers, including pancreatic adenocarcinoma (14), esophageal squamous cell carcinoma (15), gastric adenocarcinoma (16), renal cell carcinoma (11), thyroid carcinoma (17), and nasopharyngeal carcinoma (18). However, no study has reported the prognostic value of SIRI in postoperative recurrence and survival among patients with UTUC. Therefore, this study aimed to evaluate the prognostic value of SIRI, NLR, PLR, MLR, and combined use of inflammatory markers in patients who presented with UTUC after RNU.
Patients and Methods
Patients and Data Collection
Consecutive patients with UTUC (pathological T1-4N0-1M0) who underwent RNU at the First Affiliated Hospital of Wenzhou Medical University, China from March 2005 to August 2015 were retrospectively enrolled and included in the training cohort. Moreover, we retrospectively included an independent-validation cohort of consecutive patients who presented with UTUC (pathological T1-4N0-1M0) after RNU at The Third Clinical Institute Affiliated to Wenzhou Medical University, People's Hospital of Wenzhou, China from July 2003 to December 2016. The present study was approved by the ethics committees of The First Affiliated Hospital of Wenzhou Medical University and The Third Clinical Institute Affiliated to Wenzhou Medical University, People's Hospital of Wenzhou and was conducted according to the Declaration of Helsinki. All patients provided written informed consent.
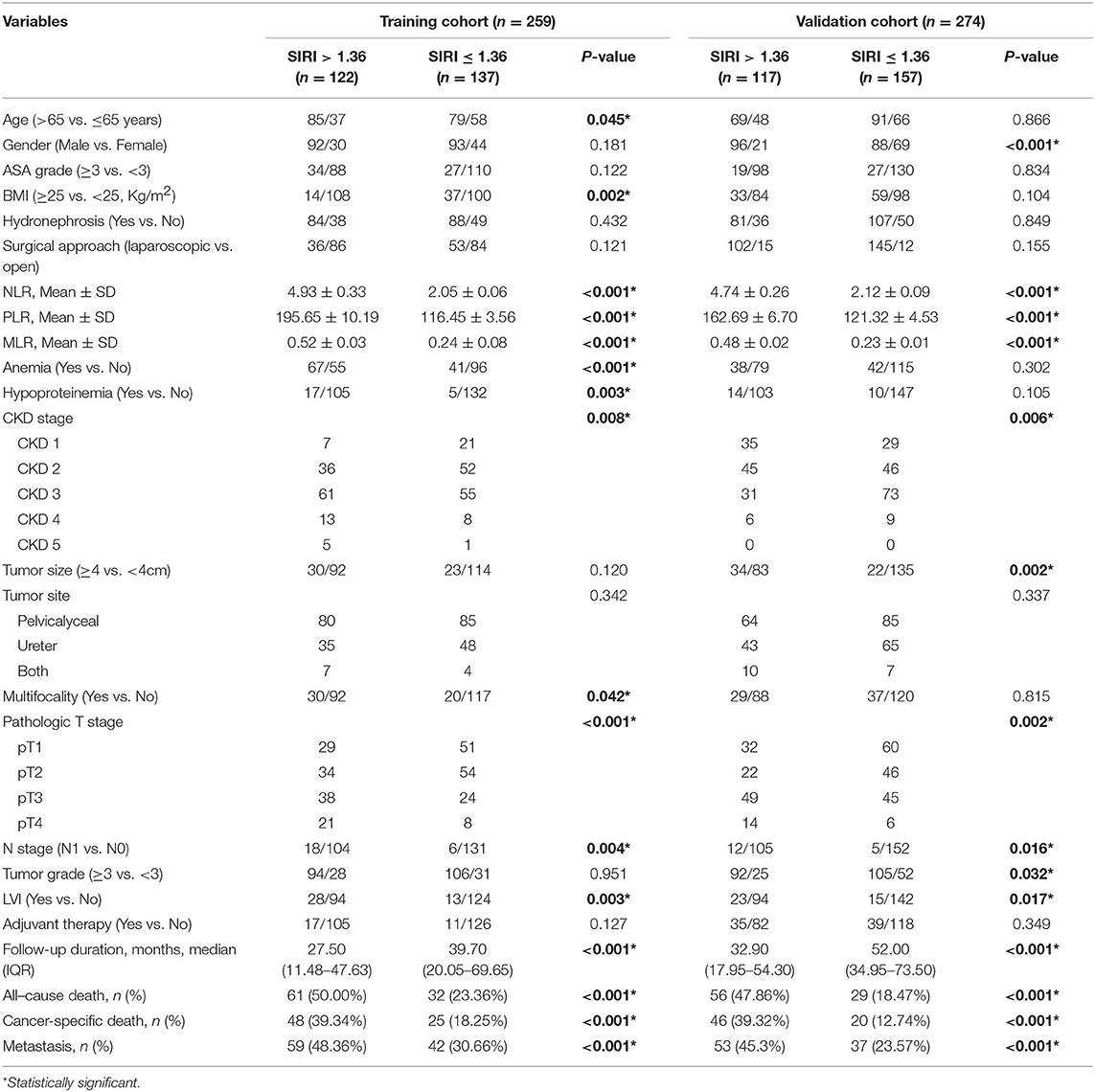
The exclusion criteria were as follows: patients who underwent palliative surgery instead of RNU, those who underwent kidney transplantation before surgery, those with evidence of metastatic disease at the time of surgery, those with incomplete preoperative medical information on SIRI, NLR, PLR, MLR, and other clinical parameters, and those with relevant comorbidity affecting systemic inflammatory response markers (i.e., chronic liver disease, immunosuppression, cytotoxic medications, leukemia, lymphoma, autoimmune diseases, and chronic inflammatory diseases). None of the patients included in the study received preoperative adjuvant chemotherapy, radiotherapy, or other anti-tumor therapies. The selection participants are summarized in Figure 1A.
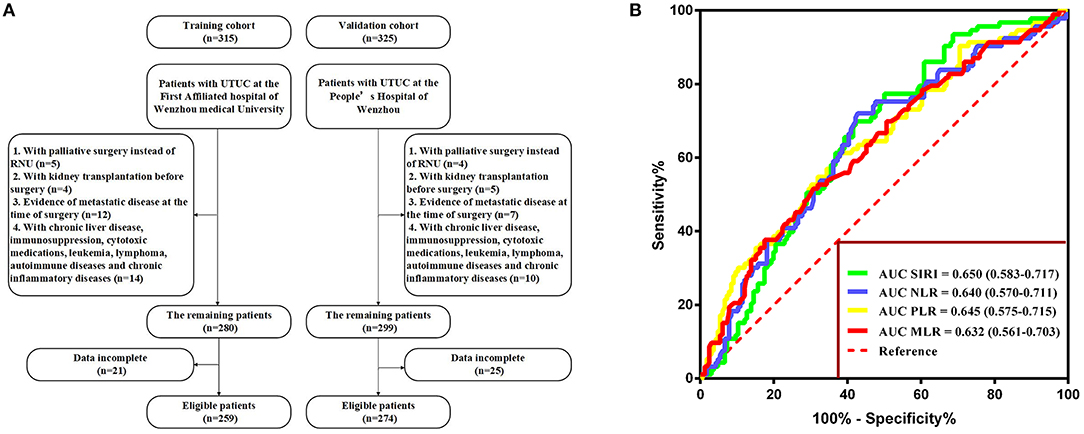
Figure 1. (A) Flow diagram of the study design. (B) Determination of the optimal cutoff value for SIRI, NLR, PLR, and MLR based on the ROC analysis.
The following patient-specific information was obtained from our database for analysis: age, gender, grade according to the American Society of Anesthesiologists (ASA) physical status classification system, body mass index (BMI), hydronephrosis, surgical approach, NLR, PLR, MLR, SIRI, anemia, hypoproteinemia, chronic kidney disease (CKD) stage, tumor size, tumor site, multifocality, pathologic T stage, N stage, tumor grade, lymphovascular invasion (LVI), adjuvant therapy, and postoperative outcomes, including overall survival (OS), CSS, and metastatic-free survival (MFS).
Tumors were staged according to the American Joint Committee on Cancer TNM Classification, 7th edition, and tumor grading was assessed based on the World Health Organization (WHO) 2004 grading system. Tumor size was defined as the largest diameter of the tumor according to a pathological report. SIRI was defined as follows: SIRI = (neutrophil × monocyte)/lymphocyte. NLR was calculated as neutrophil count divided by lymphocyte count, PLR as platelet count divided by lymphocyte count, and MLR as monocyte count divided by lymphocyte count. Follow-up care comprised blood and urine tests, and chest and abdominal computed tomography scan or magnetic resonance imaging were performed every 3 months within the first year, then every 6 months from the second year to the fifth year, and annually thereafter. OS, CSS, and MFS were determined from the date of surgery to the date of death from any cause, date of cancer-specific death, and date of the last follow-up of recurrence of radiologically or histologically confirmed distant metastasis, respectively.
Statistical Analysis
The cutoff values of SIRI, NLR, PLR, and MLR were determined using the Youden index by performing an analysis of the receiver operating characteristic (ROC) curves with OS as the endpoint. The comparisons between the clinicopathological characteristics of the patients and SIRI were performed using Student's t-test (normally distributed continuous variables), Mann-Whitney U test (non-normally distributed data), Pearson's chi-square test, or Fisher's exact test (categorical variables). The trends of SIRI for different PLR were analyzed using the Cochran-Armitage test. Survival patterns were identified using the means of the Kaplan-Meier curves. Univariate and multivariate Cox proportional hazards regression analyses were performed to evaluate the prognostic significance of each variable with respect to OS, CSS, and MFS. All P-values were two-tailed, and a P < 0.05 was considered statistically significant. Data were analyzed using SPSS (version, 25.0; IBM, Armonk, NY). Nomograms for the probability of OS, CSS, and MFS were established based on the results of the multivariate analysis using the R software (version 3.6.0) with rms, Hmisc, and ggplots packages. Calibration plot and concordance index (c-index) were used to evaluate the performance of the nomograms. A larger c-index represented a more accurate prognostic ability of the nomogram (low discriminative ability: 0.5–0.70, moderate discriminative ability: 0.71–0.90, and high discriminative ability: 0.90–1).
Results
Characteristics of the Participants
The clinicopathological characteristics of the two cohorts are summarized in Table 1. In the training cohort, 185 (71.4%) patients were men and 74 (28.6%) women, with a mean age of 67.5 ± 10.4 years. The median follow-up duration was 33.3 (interquartile range [IQR]: 15.5–64.2) months. During follow-up, 93 (35.9%) patients died, of whom 73 (28.2%) died of cancer-specific causes. Furthermore, 101 (39.0%) patients experienced distant metastasis. In the validation cohort, 184 (67.2%) patients were men and 90 (32.8%) women, with mean age of 65.9 ± 10.3 years. The median follow-up duration was 44.9 (IQR: 26.93–65.8) months. During follow-up, 85 (31.0%) patients died, whom 66 (24.1%) died of cancer-specific causes. In addition, 90 (32.8%) patients experienced distant metastasis.
Association Between SIRI and Clinicopathological Variables
With respect to OS, the optimal cutoff values were identified by ROC analysis and were as follows: SIRI, 1.36; NLR, 4.93; PLR, 195.65; and MLR, 0.52. The area under the curve (AUC) values of SIRI, NLR, PLR, and MLR were 0.650 (0.583–0.717), 0.640 (0.570–0.711), 0.645 (0.575–0.715), and 0.632 (0.561–0.703), respectively, with SIRI having the highest AUC (Figure 1B). The result indicated that SIRI was superior to the other three variables in terms of predicting survival.
The clinicopathological characteristics of the training and validation cohorts according to the cutoff value of SIRI are described in Table 1. In the training cohort, patients with higher SIRI were older than those with lower SIRI (P < 0.05). Lower BMI level, higher NLR, PLR, and MLR values, higher CKD stage, higher pathologic T stage, anemia, hypoproteinemia, multifocality, positive N status, and LVI were more commonly observed in patients with higher SIRI than in those with lower SIRI (all P < 0.05). In addition, patients with higher SIRI had a shorter follow-up duration, higher all-cause death, cancer-specific death, and were more likely to experience metastasis than those with lower SIRI (all P < 0.001). In the validation cohort, female sex, positive N status, LVI, higher NLR, PLR, and MLR values, higher CKD stage, larger tumor size, higher pathologic T stage, and higher tumor grade were more commonly observed in patients with higher SIRI than in those with lower SIRI (all P < 0.05). In addition, patients with higher SIRI had a shorter follow-up duration, higher all-cause death, cancer-specific death, and were more likely to experience metastasis than those with lower SIRI (all P values < 0.001).
Prognostic Significance of SIRI, NLR, PLR, and MLR
The Kaplan-Meier survival curves showed lower OS, CSS, and MFS in patients with higher SIRI, NLR, PLR, and MLR values both in the training and validation cohorts (all P < 0.05) (Figure 2 and Figure S1). The multivariate Cox regression model showed that SIRI was an independent risk factor of OS (hazard ratio [HR] = 2.005, 95%CI, 1.103–3.644, P = 0.022), CSS (HR = 2.271, 95%CI, 1.146–4.500, P = 0.019), and MFS (HR = 2.257, 95%CI, 1.277–3.987, P = 0.005) in the training cohort. Similar findings were observed in the validation cohort (OS: HR = 2.797, 95%CI, 1.249–6.262, P = 0.012; CSS: HR = 3.096, 95%CI, 1.262–7.599, P = 0.014; MFS: HR = 1.906, 95%CI, 0.931–3.905, P = 0.048). PLR was considered a significant risk factor for OS in the training cohort (HR = 1.786, 95%CI, 1.020–3.128, P = 0.043). However, in the validation cohort, PLR was a significant risk factor for both OS (HR = 1.839, 95%CI, 1.040–3.253, P = 0.036) and CSS (HR = 1.951, 95%CI, 1.047–3.634, P = 0.035). Other risk predictors included age, hydronephrosis, tumor size, pathologic T stage, N stage, LVI, adjuvant therapy, and tumor site (all P < 0.05) (Tables 2, 3 and Tables S1, S2).
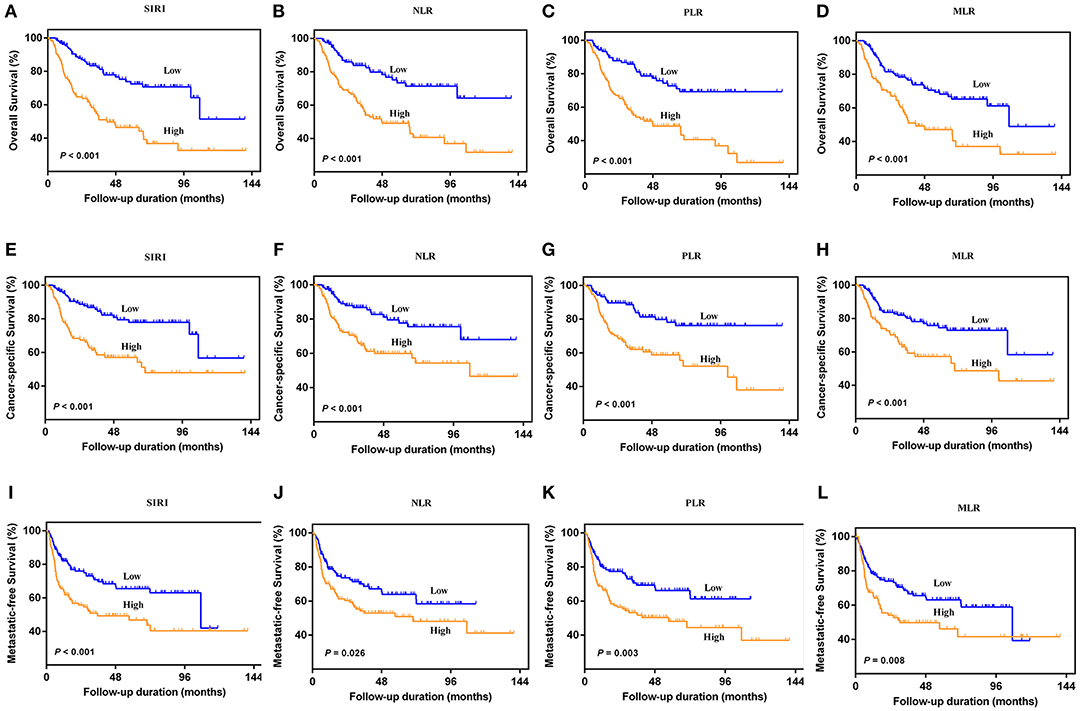
Figure 2. Kaplan-Meier curves for OS (A–D), CSS (E–H), and MFS (I–L) in UTUC patients stratified by SIRI, NLR, PLR, and MLR in the training cohort.
Table 2. Univariate analysis of variables for the prediction of survival outcomes in training cohort.
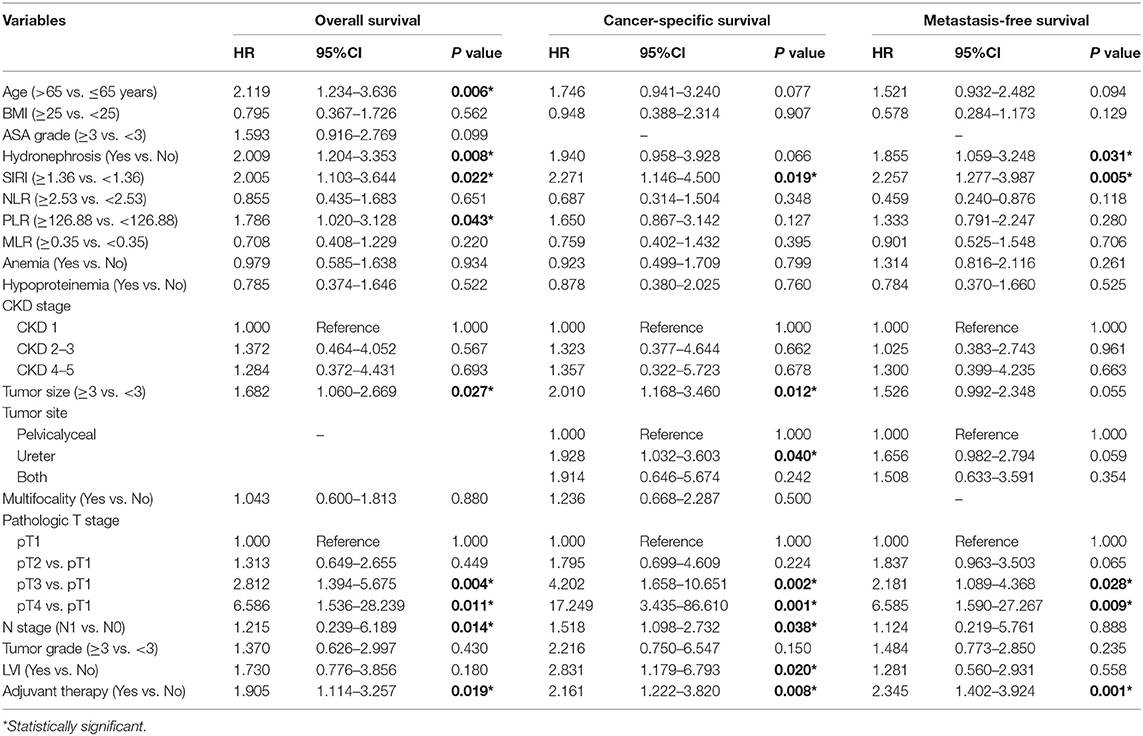
Table 3. Multivariate analysis of variables for the prediction of survival outcomes in training cohort.
Association Between Survival and SIRI-PLR
Previous studies have shown that UTUC patients with elevated preoperative PLR had significantly worse survival outcomes (13, 19, 20). In addition, SIRI consists of neutrophil, monocyte, and lymphocyte count, but not platelet count. Therefore, we further evaluated the prognostic value of SIRI-PLR.
In the two independent cohorts, PLR was positively correlated to SIRI (Figure S2) (training cohort: r = 0.455, P < 0.001; validation cohort: r = 0.320, P < 0.001). Subsequently, patients were categorized into four groups: patients with low SIRI and low PLR, patients with high SIRI and low PLR, patients with low SIRI and high PLR, and patients with high SIRI and high PLR. The Kaplan-Meier curves showed that patients with high SIRI and high PLR had the lowest OS, CSS, and MFS (all P < 0.001) (Figure 3 and Figure S3). The multivariate analysis revealed that the combination of high SIRI and high PLR was a significant risk predictor for OS, CSS, and MFS in both the training cohort (OS: HR = 2.405, 95%CI, 1.288–4.490, P = 0.006; CSS: HR = 2.351, 95%CI, 1.141–4.844, P = 0.021; MFS: HR = 2.352, 95%CI, 1.283–4.333, P = 0.027) and the validation cohort (OS: HR = 3.829, 95%CI, 1.999–7.335, P < 0.001; CSS: HR = 4.322, 95%CI, 2.038-9.164, P < 0.001; and MFS: HR = 2.565, 95%CI, 1.388–4.739, P = 0.003) (Table 4 and Table S3). Figure S4 shows that SIRI-PLR was superior to SIRI alone in predicting survival. Therefore, high SIRI-PLR was considered an independent risk predictor of OS, CSS, and MFS in patients with UTUC.
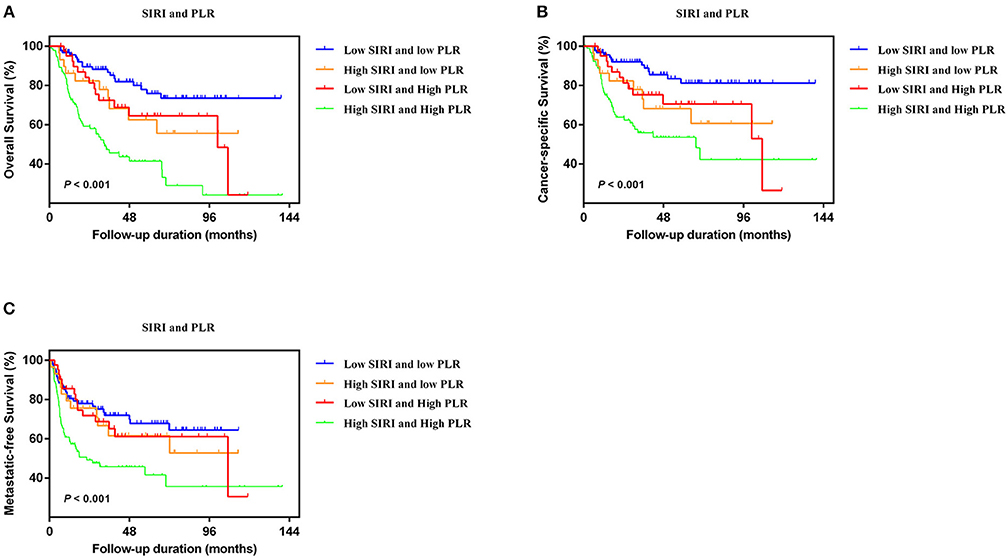
Figure 3. Kaplan-Meier analysis for OS (A), CSS (B), and MFS (C) in patients with UTUC who was divided into four groups based on SIRI-PLR in the training cohort.
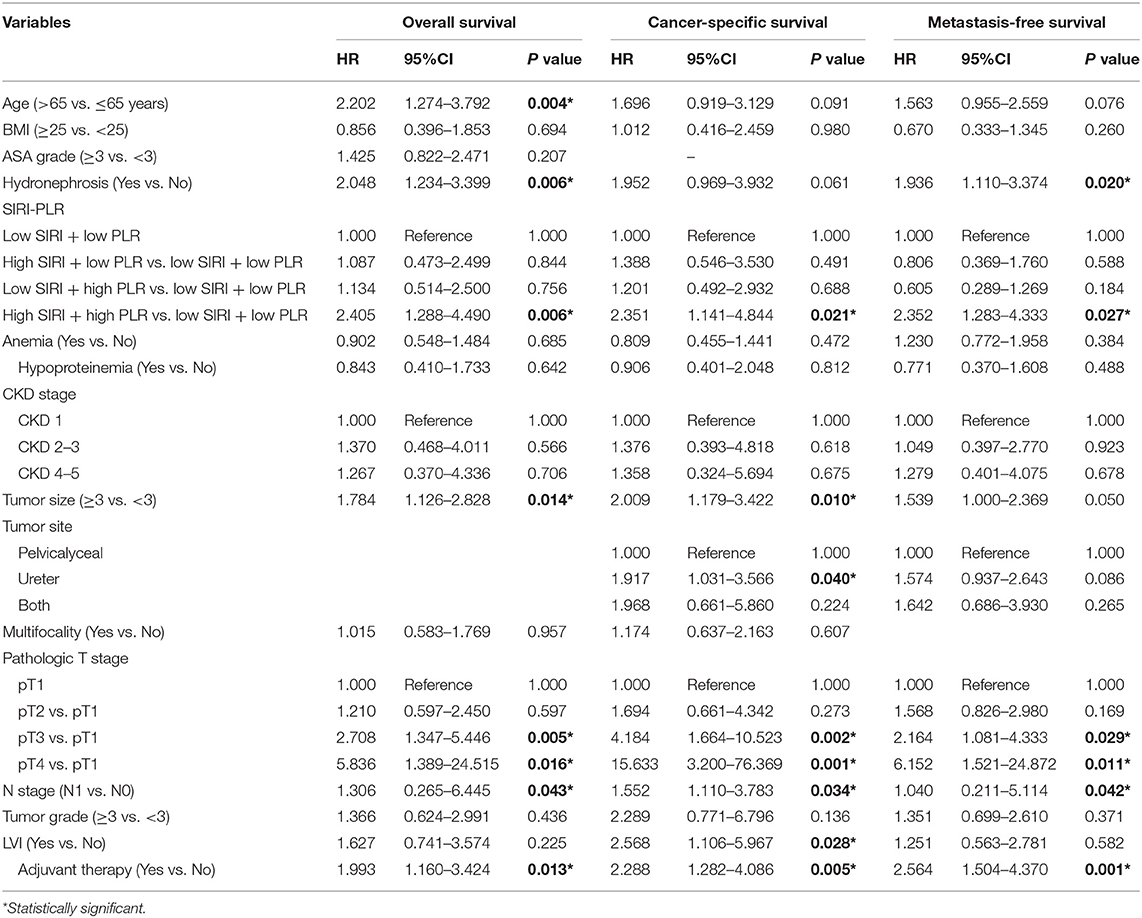
Table 4. Multivariate analysis of variables for the prediction of survival outcomes in training cohort when interrelated SIRI and PLR are combined.
Nomogram and Its Performance
We developed prognostic nomograms for OS, CSS, and MFS (Figure 4 and Figure S5) using independent predictors identified in the multivariate Cox regression models. A score was assigned to each predictor in the nomogram (top scale). The sum of these scores represented the probability of 3- and 5-year urological survival (bottom scale). The calibration plots of these nomograms were developed (Figure 5 and Figure S6), which showed that the nomograms were well-calibrated. In the training cohort, by incorporating the SIRI-PLR into the models, the c-indexes for the nomograms of OS, CSS, and MFS increased from 0.795 (0.748–0.842) to 0.808 (0.767–0.859), from 0.827 (0.785–0.859) to 0.844 (0.805–0.883), and from 0.733 (0.685–0.781) to 0.747 (0.699–0.795), respectively, indicating that this new biomarker can improve the prognostic accuracy in patients with UTUC. Similar results were observed in the validation cohort (Table 5 and Figure S7). In addition, the c-index value of SIRI-PLR combined with pT, N stage, LVI, or tumor grade for OS, CSS, or MFS in both cohorts was higher than that of SIRI-PLR or any indicator alone (Table 5). By incorporating SIRI-PLR into the models, the AUC and performance of the other indicators, which were associated with the prediction performance of nomograms for OS, CSS, and MFS, also improved (Table 6 and Figure 6). Therefore, combining SIRI-PLR and the currently available clinical parameters may help in patient risk stratification and clinical decision-making.
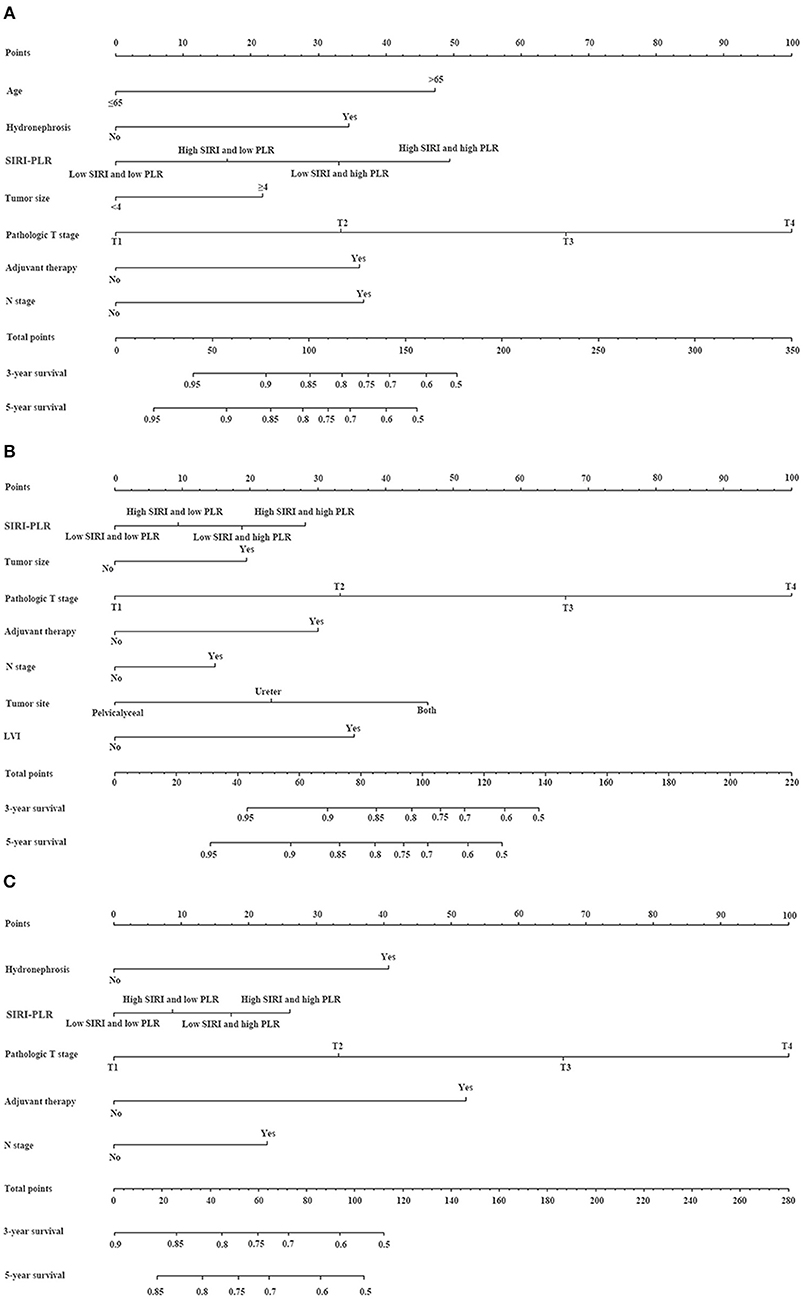
Figure 4. Establishment of nomograms for the prediction of OS (A), CSS (B), and MFS (C) in patients with UTUC after surgery. To use the nomogram, the value of individual patients with UTUC is shown on each variable axis, and a line is depicted upward to determine the number of points received for each variable value. Subsequently, the sum of these numbers is located on the total point axis, and a line is drawn downward to the survival axes to determine the likelihood of 3- and 5-year survival of OS, CSS, and MFS.
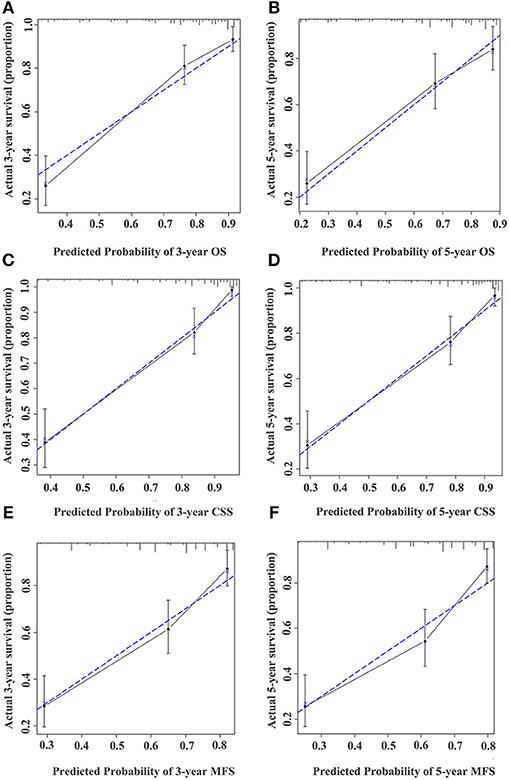
Figure 5. Calibration curve for predicting the 3- and 5-year survival of OS (A,B), CSS (C,D), and MFS (E,F) in UTUC patients in the training cohort. The actual OS, CSS, and MFS rates are plotted on the y-axis and nomogram-predicted OS, CSS, and MFS rates are plotted on the x-axis.
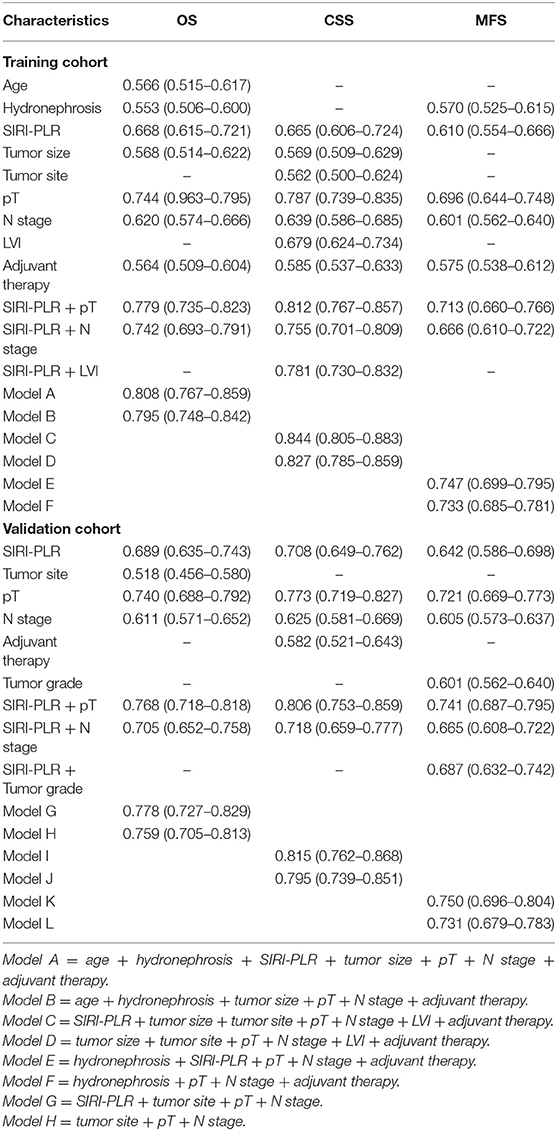
Table 5. C-index analysis of the prognostic accuracy of SIRI-PLR and other variables for OS, CSS, and MFS in training and validation cohorts.
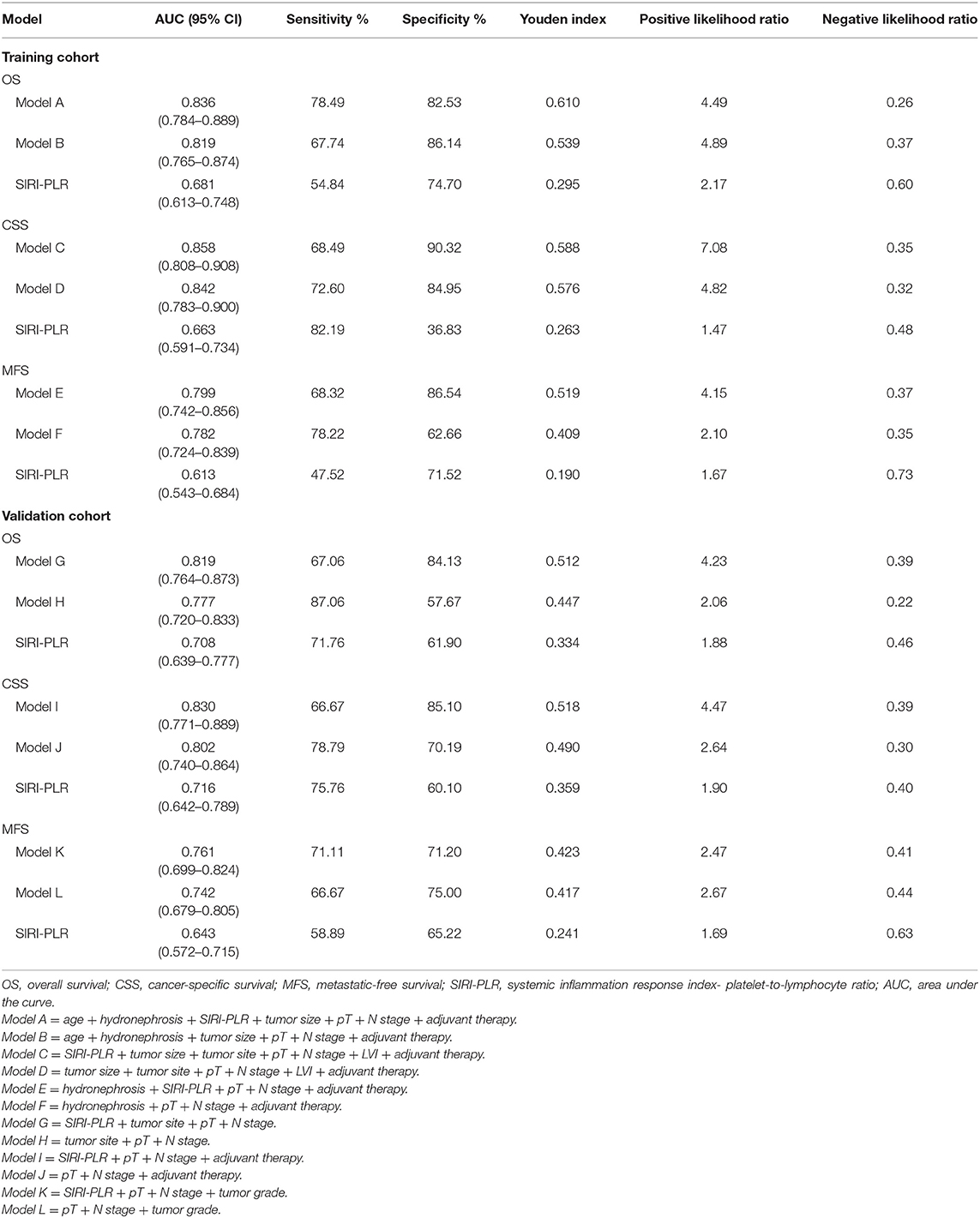
Table 6. ROC analysis of the prognostic accuracy of SIRI-PLR for OS, CSS, and MFS in training and validation cohorts.
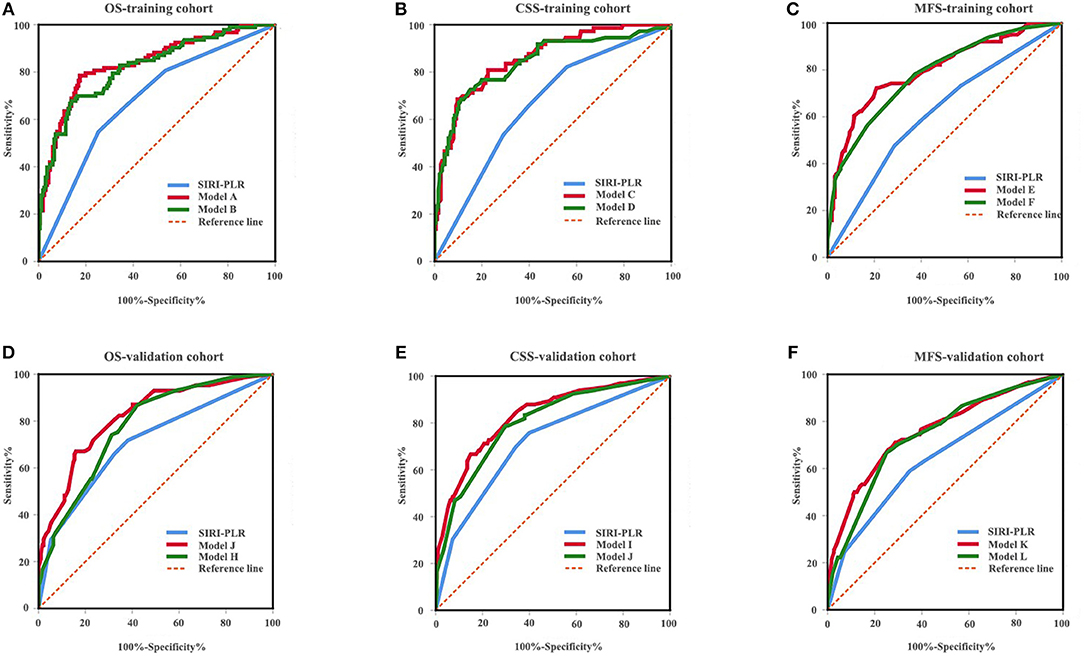
Figure 6. ROC analysis of the prognostic accuracy of SIRI-PLR for OS (A,D), CSS (B,E), and MFS (C,F) in training cohort and validation cohort.
Discussion
In the present study, the prognostic value of SIRI was evaluated in a training cohort and was confirmed in a validation cohort. The ROC analysis showed that the cutoff value of SIRI was 1.36, and SIRI values higher than 1.36 were significantly associated with high pT stage, positive N status, positive LVI, high CKD stage, and other clinical parameters indicative of an aggressive phenotype. Multivariate Cox regression models showed that SIRI was an independent predictor of OS, CSS, and MFS, and PLR was also significantly associated with lower OS or CSS. Furthermore, a positive correlation was observed between PLR and SIRI. Moreover, SIRI-PLR was a significant risk factor of lower OS, CSS, and MFS and was superior than SIRI or PLR alone in predicting survival. When we further incorporated SIRI-PLR into the models or currently available clinical parameters, the prognostic accuracy of OS, CSS, and MFS improved. To the best of our knowledge, this report first showed that SIRI-PLR can be a significant indicator in predicting the prognosis of patients with UTUC; hence, it can be applied during risk stratification and clinical decision-making.
Increasing evidence has consistently shown that systemic inflammation could contribute to the growth, deterioration, and metastasis of cancer (21), thereby affecting impact survival patterns. Inflammatory processes involving cytokines, small inflammatory proteins, and immune cells are considered a hallmark of cancer (22). Inflammation-based factors and systemic inflammatory scores, including NLR, PLR, MLR, and systemic immune-inflammation index (SII), are considered independent markers of prognosis and can further improve the prognostic accuracy of the models established for multiple malignant tumors (10–12, 20, 23). SIRI, a novel inflammatory related marker, is significantly associated with postoperative recurrence and metastasis in patients with several types of carcinomas (11, 14–16, 18). Furthermore, numerous studies have shown that the predictive ability of SIRI is more powerful than that of other inflammatory factors, such as NLR, PLR, and MLR (11, 14, 15). Our findings are consistent with those of previous studies as only SIRI and PLR were considered as independent predictors.
There is uncertainty as to why high SIRI-PLR increases the risk of tumor recurrence and mortality, although this result might be explained by the functions of neutrophil, lymphocyte, monocyte, and platelet. Neutrophils may create an inflammatory microenvironment by producing anti-microbial and immunoregulatory mediators, resulting in tumor development, angiogenesis, progression, and metastasis and protecting tumor cells from immune surveillance (24, 25). Moreover, monocytes and monocyte-derived macrophages play an important role in tumor growth, invasion, and suppression of antitumor immunity and dissemination (26, 27). To some extent, monocyte count can represent a patient's tumor burden (28). Platelets facilitate tumor progression and metastasis (29, 30) and may also have other functions correlated to the generation of macrophages and neutrophils by recruiting and regulating monocytic and granulocytic cells (27). In contrast, lymphocytes enhance the anti-tumor efforts by secreting cytokines, such as interferon gamma (INF-γ) and tumor necrosis factor (TNF-α), thereby promoting cytotoxic cell death (25). The immune response to cancer mainly relies on the peripheral blood level of lymphocytes; however, they can be rapidly decreased due to systemic inflammation (11). For example, the activation of T cells can be impaired by increased circulating neutrophils attributed to the secretion of large amounts of nitric oxide, arginase, and reactive oxygen species (31). Accordingly, the crosstalk and cooperation between these inflammatory cells and related inflammatory mediators (i.e., chemokines and cytokines.) in the microenvironment of tumor inflammation may contribute to tumorigenesis and cancer progression. Therefore, our findings revealed that SIRI-PLR is an objective and reliable marker that reflects the tumor burden, and a simultaneously high circulating SIRI-PLR levels may be highly indicative of immune escape and increased circulating tumor cell levels, which may ultimately lead to poor urologic outcomes. These findings may be important for urologists in terms of clinical decision-making process and in particular identifying patients qualified for aggressive therapy.
Our study had several strengths. First, the predictive value of SIRI-PLR was confirmed in an independent cohort. Second, SIRI-PLR was first introduced in UTUC for evaluation, and this new biomarker was found to have a more powerful prognostic ability than NLR, PLR, MLR, and SIRI. Third, SIRI-PLR is advantageous as it is non-invasive, easy to assess, highly reproducible, affordable, and, more importantly, feasible, with a high accuracy in predicting the survival of patients who present with UTUC after RNU.
However, the current study also had some limitations. First, this study utilized data collected retrospectively, which may result in potential errors or misclassifications. However, all results were validated using data from another independent cohort. Moreover, although we adjusted multiple covariates in our multivariate Cox regression models, residual confounding cannot be ruled out. Hence, randomized controlled trials must be conducted to validate the prognostic ability of SIRI-PLR. Second, this study could not establish an optimal cutoff value for SIRI as the cutoff values for the two cohorts were inconsistent: 1.36 in the training cohort and 1.48 in the validation cohort. Thus, the lack of a consistent cutoff level might have influenced the prognostic ability of SIRI-PLR in different settings. Therefore, additional studies and meta-analysis should be further performed to establish the optimal cutoff values of SIRI. Third, we could not evaluate the effects of dynamic changes in SIRI-PLR on survival because of incomplete data. Thus, these effects must be further evaluated in future studies.
Conclusions
The combination of SIRI-PLR is a non-invasive, easily accessible, and highly accurate predictor of prognosis among patients who present with UTUC after RNU. Urologists should consider the assessment results of this novel inflammation-based biomarker during clinical decision-making.
Data Availability
All datasets generated for this study are included in the manuscript/Supplementary Files.
Ethics Statement
The studies involving human participants were reviewed and approved by the ethics committees of The First Affiliated Hospital of Wenzhou Medical University and The Third Clinical Institute Affiliated to Wenzhou Medical University, People's Hospital of Wenzhou. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
XG conceived and designed the study. YZ, YC, WC, and JC obtained the data. YP analyzed and interpreted the data. LB and XG drafted the manuscript. All authors contributed to data analysis, drafting and revising the article, and approved the final version of the manuscript to be published. Moreover, they are accountable for all aspects of the work.
Funding
This study was supported by the 2018 Zhejiang medical and health science and technology program (2018KY930), and the 2017 Lishui science and technology planning program (2017GYX14).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Editage for English language editing.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2019.00914/full#supplementary-material
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. (2016) 66:7–30. doi: 10.3322/caac.21332
2. Guyatt GH, Oxman AD, Kunz R, Falck-Ytter Y, Vist GE, Liberati A, et al. Going from evidence to recommendations. BMJ. (2008) 336:1049–51. doi: 10.1136/bmj.39493.646875.AE
3. Soria F, Shariat SF, Lerner SP, Fritsche HM, Rink M, Kassouf W, et al. Epidemiology, diagnosis, preoperative evaluation and prognostic assessment of upper-tract urothelial carcinoma (UTUC). World J Urol. (2017) 35:379–87. doi: 10.1007/s00345-016-1928-x
4. Soria F, Moschini M, Haitel A, Wirth GJ, Karam JA, Wood CG, et al. HER2 overexpression is associated with worse outcomes in patients with upper tract urothelial carcinoma (UTUC). World J Urol. (2017) 35:251–9. doi: 10.1007/s00345-016-1871-x
5. Roupret M, Babjuk M, Comperat E, Zigeuner R, Sylvester RJ, Burger M, et al. European association of urology guidelines on upper urinary tract urothelial cell carcinoma: 2015 update. Eur Urol. (2015) 68:868–79. doi: 10.1016/j.eururo.2015.06.044
6. Rink M, Sjoberg D, Comploj E, Margulis V, Xylinas E, Lee RK, et al. Risk of cancer-specific mortality following recurrence after radical nephroureterectomy. Ann Surg Oncol. (2012) 19:4337–44. doi: 10.1245/s10434-012-2499-8
7. Lughezzani G, Burger M, Margulis V, Matin SF, Novara G, Roupret M, et al. Prognostic factors in upper urinary tract urothelial carcinomas: a comprehensive review of the current literature. Eur Urol. (2012) 62:100–14. doi: 10.1016/j.eururo.2012.02.030
8. Jeldres C, Sun M, Isbarn H, Lughezzani G, Budaus L, Alasker A, et al. A population-based assessment of perioperative mortality after nephroureterectomy for upper-tract urothelial carcinoma. Urology. (2010) 75:315–20. doi: 10.1016/j.urology.2009.10.004
9. Abouassaly R, Alibhai SM, Shah N, Timilshina N, Fleshner N, Finelli A. Troubling outcomes from population-level analysis of surgery for upper tract urothelial carcinoma. Urology. (2010) 76:895–901. doi: 10.1016/j.urology.2010.04.020
10. Jan HC, Yang WH, Ou CH. Combination of the preoperative systemic immune-inflammation index and monocyte-lymphocyte ratio as a novel prognostic factor in patients with upper-tract urothelial carcinoma. Ann Surg Oncol. (2019) 26:669–84. doi: 10.1245/s10434-018-6942-3
11. Chen Z, Wang K, Lu H, Xue D, Fan M, Zhuang Q, et al. Systemic inflammation response index predicts prognosis in patients with clear cell renal cell carcinoma: a propensity score-matched analysis. Cancer Manag Res. (2019) 11:909–19. doi: 10.2147/cmar.s186976
12. Vartolomei MD, Kimura S, Ferro M, Vartolomei L, Foerster B, Abufaraj M, et al. Is neutrophil-to-lymphocytes ratio a clinical relevant preoperative biomarker in upper tract urothelial carcinoma? A meta-analysis of 4385 patients. World J Urol. (2018) 36:1019–29. doi: 10.1007/s00345-018-2235-5
13. Dalpiaz O, Krieger D, Ehrlich GC, Pohlmann K, Stojakovic T, Pummer K, et al. Validation of the preoperative platelet-to-lymphocyte ratio as a prognostic factor in a european cohort of patients with upper tract urothelial carcinoma. Urol Int. (2017) 98:320–7. doi: 10.1159/000452109
14. Qi Q, Zhuang L, Shen Y, Geng Y, Yu S, Chen H, et al. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer. (2016) 122:2158–67. doi: 10.1002/cncr.30057
15. Geng Y, Zhu D, Wu C, Wu J, Wang Q, Li R, et al. A novel systemic inflammation response index (SIRI) for predicting postoperative survival of patients with esophageal squamous cell carcinoma. Int Immunopharmacol. (2018) 65:503–10. doi: 10.1016/j.intimp.2018.10.002
16. Li S, Lan X, Gao H, Li Z, Chen L, Wang W, et al. Systemic Inflammation Response Index (SIRI), cancer stem cells and survival of localised gastric adenocarcinoma after curative resection. J Cancer Res Clin Oncol. (2017) 143:2455–68. doi: 10.1007/s00432-017-2506-3
17. Xie H, Wei B, Shen H, Gao Y, Wang L, Liu H. BRAF mutation in papillary thyroid carcinoma (PTC) and its association with clinicopathological features and systemic inflammation response index (SIRI). Am J Transl Res. (2018) 10:2726–36.
18. Chen Y, Jiang W, Xi D, Chen J, Xu G, Yin W, et al. Development and validation of nomogram based on SIRI for predicting the clinical outcome in patients with nasopharyngeal carcinomas. J Invest Med. (2019) 67:691–8. doi: 10.1136/jim-2018-000801
19. Son S, Hwang EC, Jung SI, Kwon DD, Choi SH, Kwon TG, et al. Prognostic value of preoperative systemic inflammation markers in localized upper tract urothelial cell carcinoma: a large, multicenter cohort analysis. Minerva Urol Nefrol. (2018) 70:300–9. doi: 10.23736/s0393-2249.18.02914-4
20. Huang J, Yuan Y, Wang Y, Zhang J, Kong W, Chen H, et al. Prognostic value of preoperative plasma fibrinogen level and platelet-to-lymphocyte ratio (F-PLR) in patients with localized upper tract urothelial carcinoma. Oncotarget. (2017) 8:36761–71. doi: 10.18632/oncotarget.13611
21. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. (2011) 144:646–74. doi: 10.1016/j.cell.2011.02.013
22. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. (2014) 15, e493–503. doi: 10.1016/s1470-2045(14)70263-3
23. Zhang H, Shang X, Ren P, Gong L, Ahmed A, Ma Z, et al. The predictive value of a preoperative systemic immune-inflammation index and prognostic nutritional index in patients with esophageal squamous cell carcinoma. J Cell Physiol. (2019) 234:1794–802. doi: 10.1002/jcp.27052
24. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. (2008) 454:436–44. doi: 10.1038/nature07205
25. Moses K, Brandau S. Human neutrophils: Their role in cancer and relation to myeloid-derived suppressor cells. Semin Immunol. (2016) 28:187–96. doi: 10.1016/j.smim.2016.03.018
26. Hamilton G, Rath B, Klameth L, Hochmair MJ. Small cell lung cancer: recruitment of macrophages by circulating tumor cells. Oncoimmunology. (2016) 5:e1093277. doi: 10.1080/2162402x.2015.1093277
27. Kim J, Bae JS. Tumor-associated macrophages and neutrophils in tumor microenvironment. Mediat Inflamm. (2016) 2016:6058147. doi: 10.1155/2016/6058147
28. Shibutani M, Maeda K, Nagahara H, Ohtani H, Sakurai K, Yamazoe S, et al. Prognostic significance of the lymphocyte-to-monocyte ratio in patients with metastatic colorectal cancer. World J gastroentero. (2015) 21:9966–73. doi: 10.3748/wjg.v21.i34.9966
29. Placke T, Kopp HG, Salih HR. The wolf in sheep's clothing: Platelet-derived “pseudo self” impairs cancer cell “missing self” recognition by NK cells. Oncoimmunology. (2012) 1:557–9. doi: 10.4161/onci.19367
30. Lavergne M, Janus-Bell E, Schaff M, Gachet C. Platelet integrins in tumor metastasis: do they represent a therapeutic target? Cancers. (2017) 9:E133. doi: 10.3390/cancers9100133
Keywords: upper tract urothelial carcinoma, systemic inflammation response index, platelet-to-lymphocyte ratio, prognosis, cancer
Citation: Zheng Y, Chen Y, Chen J, Chen W, Pan Y, Bao L and Gao X (2019) Combination of Systemic Inflammation Response Index and Platelet-to-Lymphocyte Ratio as a Novel Prognostic Marker of Upper Tract Urothelial Carcinoma After Radical Nephroureterectomy. Front. Oncol. 9:914. doi: 10.3389/fonc.2019.00914
Received: 06 July 2019; Accepted: 03 September 2019;
Published: 18 September 2019.
Edited by:
Walter J. Storkus, University of Pittsburgh, United StatesCopyright © 2019 Zheng, Chen, Chen, Chen, Pan, Bao and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yue Pan, cHh5XzYyOEAxNjMuY29t; Lianmin Bao, YmFvbGlhbm1pbjE5OTBAMTYzLmNvbQ==; Xiaomin Gao, ZG9jdG9yZ2FveGlhb21pbkBzbW11LmVkdS5jbg==
†These authors have contributed equally to this work
 Yangqin Zheng1†
Yangqin Zheng1† Xiaomin Gao
Xiaomin Gao