- 1Department of Radiation Oncology, The Sixth Affiliated Hospital of Sun Yat-sen University, Guangzhou, China
- 2Department of Radiation Oncology, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China
- 3Radiotherapy Department of Thorax and Abdomen Carcinoma, Cancer Center, The First People's Hospital of Foshan, Foshan, China
- 4Department of Colorectal Surgery, The Sixth Affiliated Hospital of Sun Yat-sen University, Guangzhou, China
Background: The addition of intensive preoperative chemotherapy and using of a longer waiting period between neoadjuvant radiotherapy and total mesorectal excision (TME) surgery lengthen the time interval from the initiation of neoadjuvant treatment to definitive surgery in patients with locally advanced rectal cancer (LARC). Here, we evaluated the prognostic value of different time intervals between the initiation of neoadjuvant treatment to TME surgery for LARC.
Methods: A total of 2,267 patients with LARC, who received neoadjuvant radiochemotherapy and TME surgery, between January 2010 through December 2018 were recruited. The entire cohort was divided into 4 subgroups based on total-time-to surgery, defined as the time interval between initiation of neoadjuvant treatment and TME surgery (TTS): <13 weeks (TTS-1), 13 to <15 weeks (TTS-2), 15 to <17 weeks (TTS-3), ≥17 weeks (TTS-4). Overall survival (OS), disease-free survival (DFS), distant metastasis-free survival (DMFS), and local recurrence-free survival (LRFS) rates in different TTS subgroup patients were compared, and hazard ratios (HR) for different demographic and clinicopathological variables, including TTS, were calculated to determine their prognostic significance.
Results: The median follow-up time was 42.0 (range, 5–162) months. The 3-year OS, DFS, DMFS, and LRFS rates were 87.0, 79.4, 80.9, and 93.8%, respectively. The varied OS, DFS, and DFMS rates were detected among these different TTS subgroups (P = 0.010, P < 0.001, and P < 0.001, respectively). Particularly, the lower survival outcome was mainly observed at patients in the shortest TTS group (TTS-1). Cox regression analysis confirmed that the only significant positive independent prognostic factor for 3-year DFS was a longer TTS (TTS 2–4 vs. TTS-1; HR 0.884, 95% CI 0.778–0.921, P < 0.001), while the significant negative independent prognosticfactors were moderate to poor tumor differentiation (vs. well-differentiated; HR 1.191, 95% CI 1.004–1.414, P = 0.045) and clinical N1-2 stage (vs. N0 stage; HR 1.190, 95% CI 1.052–1.347, P = 0.006).
Conclusion: For patients with LARC, an interval between the initiation of neoadjuvant treatment and TME surgery of longer than 13 weeks is associated with favorable disease-free survival.
Introduction
In the past few decades, the combination of neoadjuvant radiochemotherapy and total mesorectal excision (TME) surgery has markedly reduced the local recurrence rate, and serving as the standard therapeutic regimen for patients with locally advanced rectal cancer (LARC) (1, 2). However, the predominant cause of disease relapse in patients receiving this treatment is distant metastases, with the 5-year distant relapse rate estimated to be about 35% (3–5).
To attempt to address this issue, patients with LARC have been given additional adjuvant chemotherapy (ACT) after neoadjuvant radiochemotherapy and TME surgery. However, accumulated evidences showed that the effectiveness of ACT in LARC patients was limited (6–8). Considering that many patients declined or failed to complete ACT, efforts have been made to identify other more intensive systemic treatment approaches that can be completely administered in the neoadjuvant setting, with the aims of both downstaging locally advanced tumor and reducing the risks of metastatic disease prior to surgery. One selection was the administration of induction chemotherapy, followed by radiotherapy, and then subsequent consolidation chemotherapy, all prior to TME surgery. Another approach involved the use of total neoadjuvant therapy (TNT), in which all planned radiotherapy and intensive chemotherapy was delivered in the preoperative setting. Compared to ACT, these modified treatment strategies had a superior patient compliance rate, the long-term survival outcome results was however not yet to be published (9–12). Significantly, using of more intensive neoadjuvant chemotherapy (NCT) resulted in longer intervals between the beginning of neoadjuvant treatment and TME surgery. However, the appropriate period between the initiation of neoadjuvant therapy and TME surgery in patients with LARC remains unclear.
Likewise, there is debate about the optimal interval between the completion of neoadjuvant radiotherapy and TME surgery in patients with LARC. To achieve a high pathologic complete response (pCR) rate, which is considered a surrogate marker of favorable oncological outcomes (13), there has been a trend toward prolonging the interval between radiotherapy and TME surgery, especially when part of the aim is organ preservation. For the underlying reason, the amount of tumor regression after radiotherapy has been shown to be time-dependent (14). Taken Lyon R90-01 trial for example, a longer interval (6–8 weeks vs. up to 2 weeks) between radiotherapy and surgery was correlated with increased pCR rates, though there was no difference in long-term overall survival (OS) between two subgroups (15). Although the guideline of the National Comprehensive Cancer Network (NCCN) recommends that TME surgery should be done between 5 and 12 weeks after chemoradiotherapy (16), the GRECCAR-6 trial indicated that a longer interval of 11 weeks (vs. 7 weeks) between chemoradiotherapy and surgery resulted in increased postoperative morbidity and a poorer quality of TME surgery, without an increased pCR rate (17). Additionally, data collected from the United States National Cancer Database demonstrated that a delay in surgery of more than 8 weeks after neoadjuvant radiotherapy was associated with a higher rate of positive surgical margins as well as a decreased rate of survival in patients with LARC (18, 19). Given these somewhat contradictory results, the optimal period between neoadjuvant radiotherapy and TME surgery in LARC patients remains unclear.
In this study, we included a large size of LARC patients, who treated with intensive chemotherapy, radiotherapy, and TME surgery, with the aim of determining the impact of different time intervals from the initiation of neoadjuvant treatment to surgery, and of different waiting periods from the end of radiotherapy to surgery, on survival outcome and pCR rates.
Methods
Between January 2010 and December 2018, patients with newly diagnosed, biopsy-proven, non-metastatic LARC treated were included in this study. All patients were staged according to the 7th edition of the American Joint Commission on Cancer (AJCC) staging system (20). Patients with locally advanced rectal cancers were defined as stage II (T3-4N0) or stage III (T1-4N1-2) by magnetic resonance imaging (MRI), computed tomography (CT), and/or endorectal ultrasonography (EUS). All patients had adenocarcinomas with a distal border located <12 cm from the anal verge. Patients were excluded who had a previous history of any type of cancer, treatment duration from the beginning of neoadjuvant treatment to TME surgery of more than 9 months or waiting period from the end of radiotherapy to TME surgery of <4 weeks. The Institutional Review Board of the Sixth Affiliated Hospital at Sun Yat-sen University approved this retrospective study.
Neoadjuvant and Adjuvant Treatment
All patients were treated with neoadjuvant intensity-modulated radiotherapy (IMRT). For each patient, the gross tumor volume (GTV) received 50 Gy in 25 fractions at 2.0 Gy per fraction, while the clinical target volume (CTV) received 45 Gy in 25 fractions at 1.8 Gy per fraction.
In addition to radiotherapy, all patients received neoadjuvant induction (before radiotherapy), concurrent (during radiotherapy), and/or consolidation (after radiotherapy) chemotherapy, based on the treatment guidelines for locally advanced rectal cancer. Neoadjuvant chemotherapy (NCT) consisted of a fluoropyrimidine-based regimen, determined at the discretion of the multi-disciplinary cancer team, involving one of the following: folinic acid, fluorouracil, and oxaliplatin (FOLFOX); capecitabine and oxaliplatin (CAPOX); capecitabine (Xeloda); or folinic acid, fluorouracil, oxaliplatin, and rinotecan (FOLFOXIRI).
A majority of the patients in this cohort also received a fluoropyrimidine-based ACT regimen, which was determined at the discretion of the multi-disciplinary cancer team. The same team also made the decision of the specific number of cycles of both NCT and ACT given to each patient. Total neoadjuvant therapy (TNT) in this study was defined as receiving 8 or more cycles of NCT and no ACT.
Total-Time-to-Surgery (TTS) and Waiting-Period-After-Radiotherapy (WPR)
Total-time-to-surgery (TTS) was defined as the time from the initiation of any neoadjuvant treatment to the date of TME surgery. The median TTS was 101 days (range, 58–265 days), and the interquartile TTS cut points were at 89, 101, and 117 days, which we approximated as 13, 15, and 17 weeks, respectively. Using these fixed cut points, patients were then divided into 4 groups: those who had TME surgery <13 weeks from the initiation of treatment (TTS-1), those who had TME surgery from 13 to <15 weeks from the initiation of treatment (TTS-2), those who had TME surgery from 15 to <17 weeks from the initiation of treatment (TTS-3), and those who had surgery 17 or more weeks from the initiation of treatment (TTS-4).
Waiting-period-after-radiotherapy (WPR) was defined as the time from the end of radiotherapy to the date of TME surgery. The median WPR was 56 days (range, 28–207 days). Patients were arbitrarily divided into 5 groups for every 2 weeks of WPR: those with waiting periods of 4 to <6 weeks (WPR-1), those with waiting periods of 6 to <8 weeks (WPR-2), those with waiting periods of 8 to <10 weeks (WPR-3), those with waiting periods of 10 to <12 weeks (WPR-4), and those with waiting period of 12 or more weeks (WPR-5).
Follow-Up
Follow-up duration was defined as the time from the first day of treatment to either the date of last examination or the date of death. Patients were routinely assessed at 3-month intervals during the first 3 years and at 6-month intervals thereafter or until death. Primary endpoints used in this study included the following: overall survival (OS), measured as the time from the initiation of treatment to death from any cause; disease-free survival (DFS), measured as the time from TME surgery to the first disease relapse at any site; distant metastasis-free survival (DMFS), measured as the time from TME surgery to the first distant relapse (recurrence outside the pelvis); and locoregional relapse-free survival (LRFS), measured as the time from TME surgery to the first locoregional relapse (recurrence within the pelvis).
Other clinicopathological characteristics evaluated in this study included the following: pathologic complete response (pCR), defined as the absence of viable adenocarcinoma cells in the TME surgical specimen (ypT0N0); pathologic stage after neoadjuvant therapy and TME surgery (ypTN stage); downstaging, defined as stage ypT0-2N0 after TME surgery; and surgical specimen pathology results (vascular invasion, neural invasion, and surgical margin status and measurement).
Statistical Methods
The χ2-test was used to compare the distributions of assorted demographic and clinicopathological characteristics in different TTS subgroups. Kaplan-Meier survival curves were used to compare patient outcomes (OS, DFS, DMFS, and LRFS) among different TTS and WPR subgroups. Statistical differences between curves were calculated using the log-rank test. The multivariate Cox proportional hazards model was utilized to estimate the hazard ratios (HR) and 95% confidence intervals (CI) for different demographic and clinicopathological characteristics, so that HRs for OS, DFS, and DMFS equated to the relative risks of death, disease relapse, and distant metastasis, respectively. All P-values were two-sided, and a P < 0.05 was considered statistically significant. Statistical analyses were performed with the Statistical Package for the Social Sciences (SPSS, version 24.0; SPSS, Inc, Chicago, IL).
Results
Totally, 2,267 patients with LARC were included in this study. Of whom, 1,540 (67.9%) were men and 727 (32.1%) were women, and the median age was 56.0 years (range, 15–87 years) (Table 1). Particularly, only 34 patients received TNT (3 in the TTS-3 group, 31 in the TTS-4 group). The clinicopathological factors of gender, as well as tumor differentiation and distance from the anal verge, and patients receiving TNT did not differ significantly among 4 TTS subgroups. Conversely, the age distributions of patients in the TTS groups was differed significantly, such that those with longer TTS (TTS-3 and TTS-4) were more frequently younger (56 years and younger) than older (57 years and older), whereas those in the shorter TTS-1 and TTS-2 subgroups were more frequently older than younger (P = 0.03). Additionally, cT stage, cN stage, and cTNM stage distributions of patients in the TTS groups were also differed significantly, that those with longer TTS (TTS-3 and TTS-4) were more frequently to have advanced cT stage (cT4 vs. cT1-3, P < 0.001), cN stage (cN+ vs. cN0, P = 0.005), and cTNM stage (III vs. II, P < 0.001).
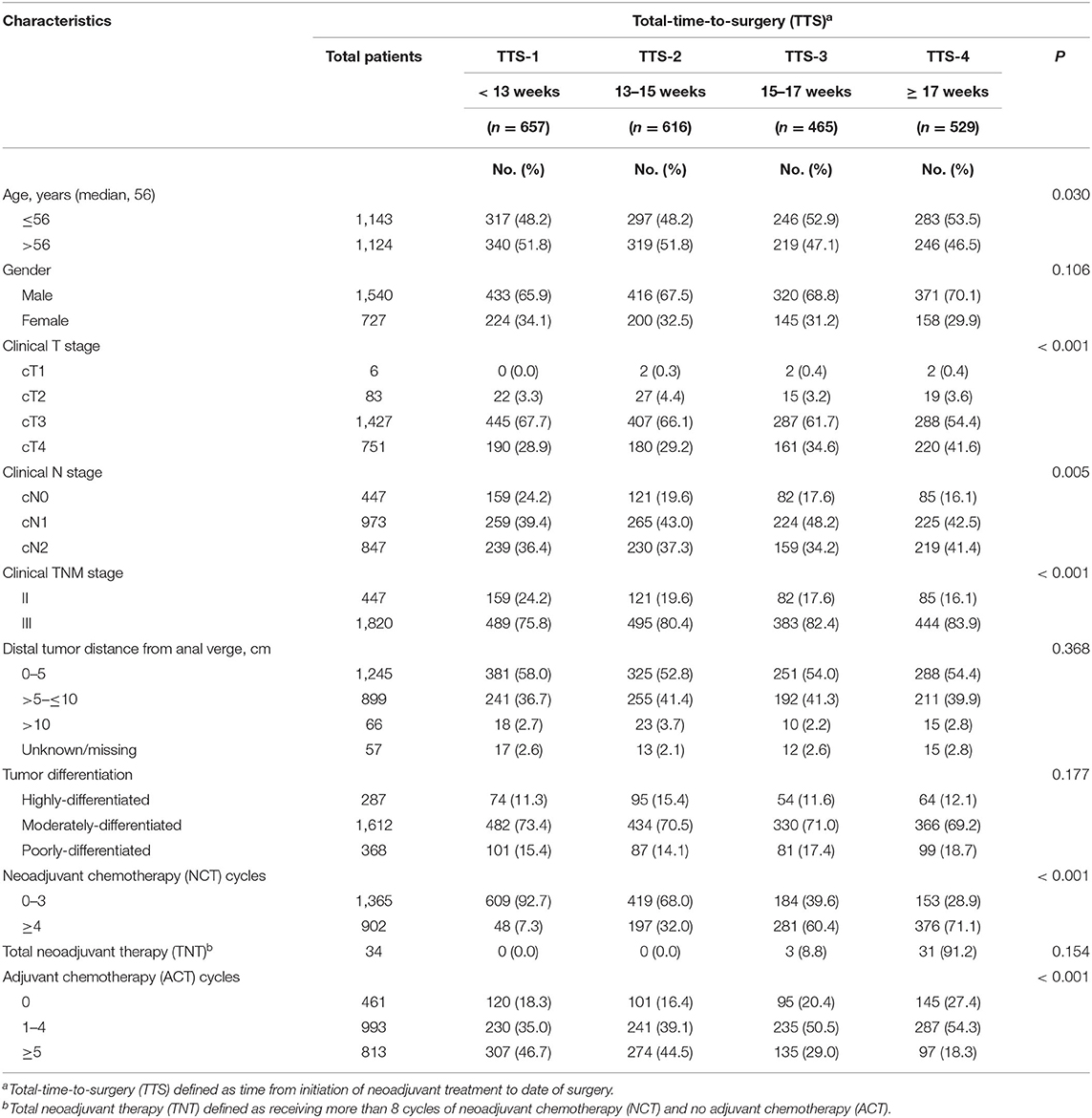
Table 1. Demographic and clinicopathological characteristics, by total-time-to-surgery (TTS)a, of 2,267 patients with locally advanced rectal cancer, January 2010 through December 2018.
Significantly, the distribution of patients among these 4 TTS subgroups, according to whether they received above or below the median number of NCT and ACT cycles, were differed greatly, shown as that those with longer TTS (TTS-3 and TTS-4) was prone to receive more NCT (4 or more cycles vs. 0–3 cycles, P < 0.001) and less ACT (0–4 cycles vs. 5 or more cycles, P < 0.001) (Table 1). Nevertheless, the total cycles of all chemotherapy (combining NCT and ACT) given to patients were similar across all TTS subgroups (range, 6.5 ± 2.8 to 7.3 ± 2.9, all P > 0.05) (Table 2).
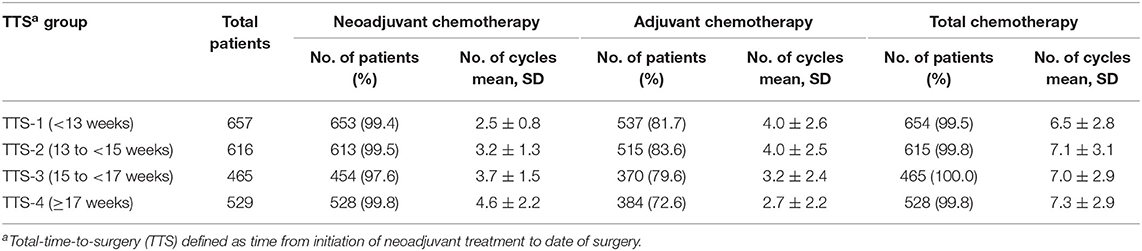
Table 2. Neoadjuvant, adjuvant, and total chemotherapy cycles, by total-time-to-surgery (TTS)a, in 2,267 patients with locally advanced rectal cancer, January 2010 through December 2018.
Of these 2,267 patients, 555 (24.5%) cases achieved a pCR (Table 3). Patients in the subgroups with longer TTS had significantly higher pCR rates: 18.3% in TTS-1, 24.2% in TTS-2, 26.0% in TTS-3, and 31.2% in TTS-4 (P < 0.001). Similarly, patients in the subgroups with progressively longer TTS also had significantly higher rates of downstaging (to ypT0-2N0): 23.1% in TTS-1, 29.2% in TTS-2, 31.4% in TTS-3, and 35.3% in TTS-4 (P < 0.001). The rates of positive surgical margins in all TTS subgroups were low (0.3% in TTS-1, 0.6% in TTS-2, 0% in TTS-3 and TTS-4) and did not differ significantly among the TTS subgroups (P = 0.121).
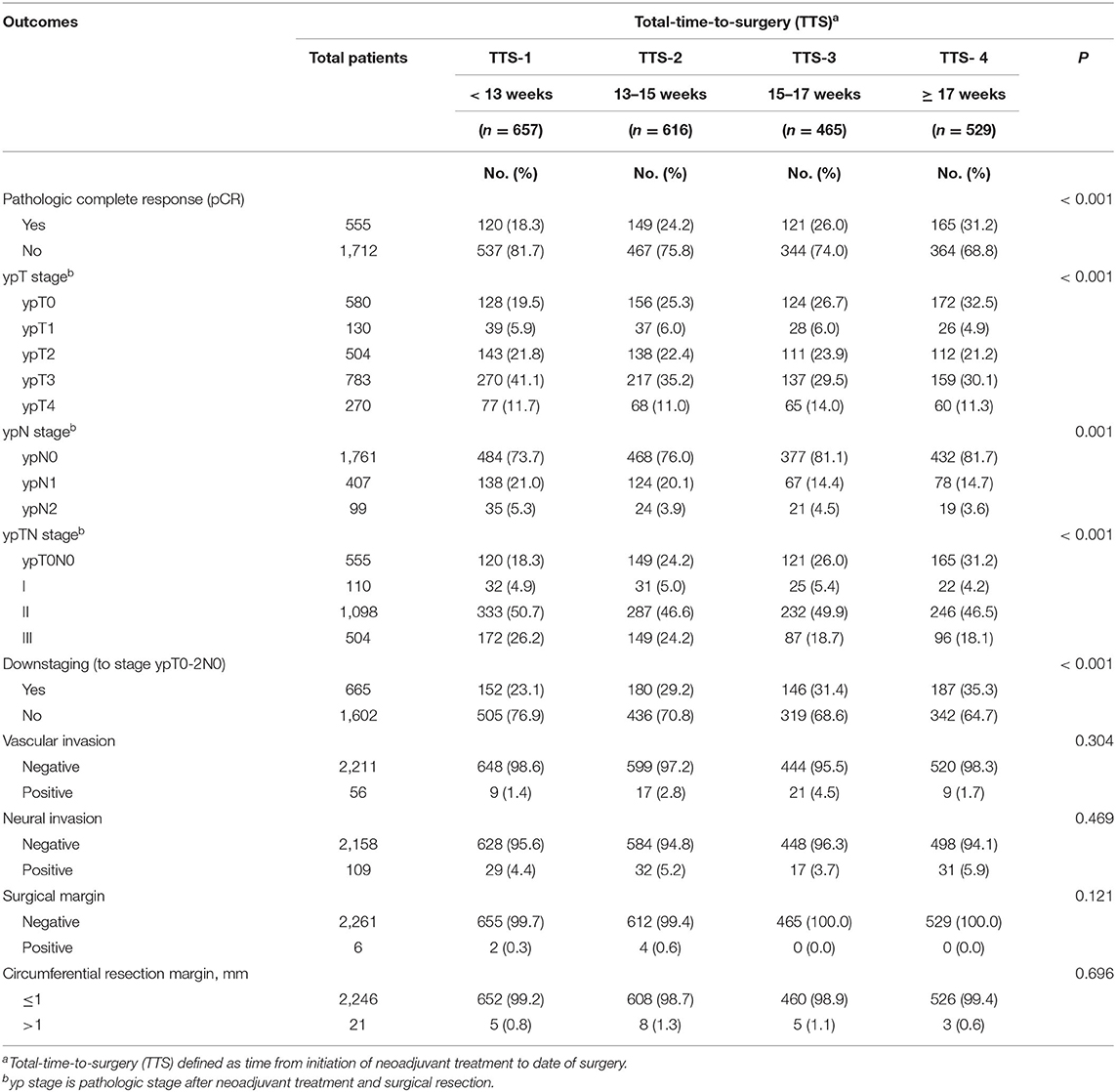
Table 3. Clinicopathological outcomes, by total-time-to-surgery (TTS)a, of 2,267 patients with locally advanced rectal cancer, January 2010 through December 2018.
The median follow-up duration for the entire cohort was 42.0 months (range, 5–162 months). The overall 3-year OS, DFS, DMFS, and LRFS rates for all patients were 87.0, 79.4, 80.9, and 93.8%, respectively. Univariate analysis displayed that tumor differentiation, number of NCT cycles, and TTS was the prognostic factors of OS (P < 0.001, P = 0.001, and P = 0.011, respectively); and clinical N stage, tumor distance from anal verge, tumor differentiation, and TTS was correlated with DFS (P = 0.030, P = 0.032, P = 0.001, and P < 0.001, respectively) and DMFS (P = 0.030, P = 0.039, P = 0.009, and P < 0.001, respectively). However, age, gender, clinical T stage, clinical TNM stage, and WPR did not correlate significantly with OS, DFS, or DMFS (Supplementary Table 1).
On initial multivariate analysis, only tumor differentiation and TTS were correlated with OS (P < 0.001 and P < 0.001, respectively), while cN stage, tumor differentiation, and TTS were correlated significantly with DFS (P = 0.023, P = 0.001, and P < 0.001, respectively) and DMFS (P = 0.021, P = 0.008, and P < 0.001, respectively) (Supplementary Table 1). Furthermore, compared to that of shorter interval TTS-1 subgroup, patients with longer interval time (TTS-2, TTS-3, and TTS-4 subgroups) had lower HR for 3-year relapse: 0.798 (95% CI 0.636–1.002, P = 0.052), 0.547 (95% CI 0.413–0.724, P < 0.001), and 0.680 (95% CI 0.525–0.880, P = 0.003), respectively.
The Kaplan-Meier survival curve analysis confirmed that OS, DFS, and DFMS rates differed significantly between different TTS subgroups (P = 0.010, P < 0.001, and P < 0.001, respectively). Moreover, the evident survival differences were mainly observed between TTS-1 subgroup and TTS-2/TTS-3/TTS-4 subgroups (Figure 1). As shown, the 3-year DFS rates were 74.7% for patients in the TTS-1 group, while was 79.8% for those in the TTS-2 group, 84.4% for those in the TTS-3 group, and 80.5% for those in the TTS-4 group.
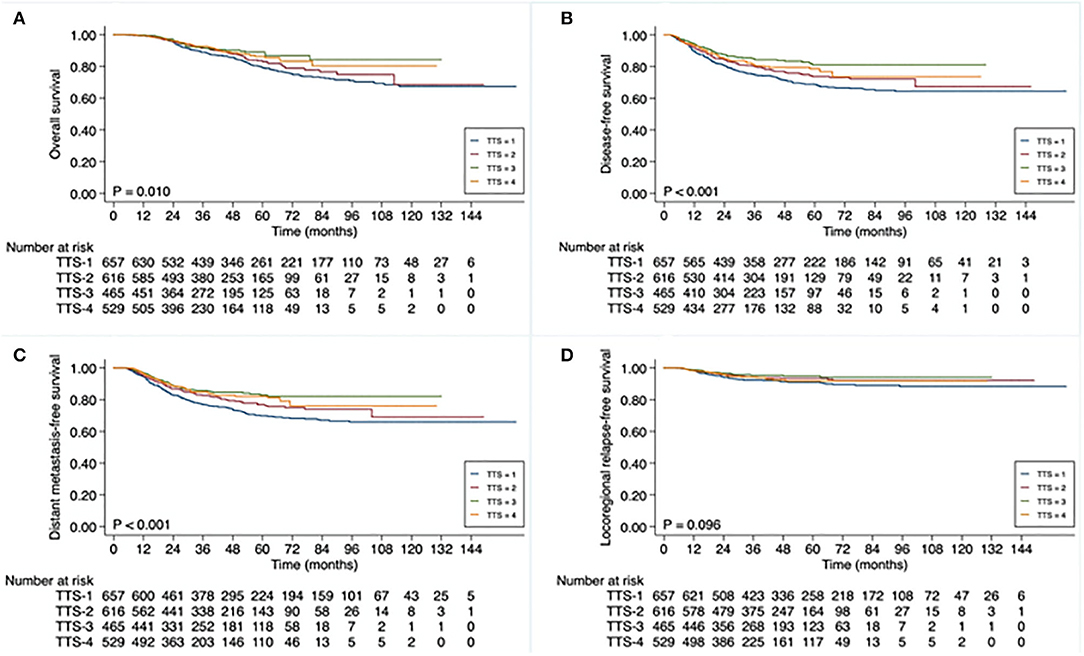
Figure 1. Kaplan-Meier curve analysis of (A) overall survival, (B) disease-free survival, (C) distant metastasis-free survival, and (D) locoregional relapse-free survival (D), based on total-time-to-surgery (TTS), of 2,267 patients with locally advanced rectal cancer. TTS defined as time from initiation of neoadjuvant treatment to date of surgery: <13 weeks (TTS-1), 13 to <15 weeks (TTS-2), 15 to <17 weeks (TTS-3), ≥17 weeks (TTS-4).
On the second univariate (Figure 2A) and multivariate analysis (Figure 2B), using some different referents and focusing only on 3-year DFS, the only significant clinically positive independent prognostic factor for 3-year DFS that remained was longer TTS (TTS 2–4 vs. TTS-1; HR 0.884, 95% CI 0.0.778–0.921, P < 0.001). The significant clinically negative independent prognostic factors were moderate to poor tumor differentiation (vs. well-differentiated; HR 1.191, 95% CI 1.004–1.414, P = 0.045) and cN1-2 stage (vs. cN0; HR 1.190, 95% CI 1.052–1.347, P = 0.006).
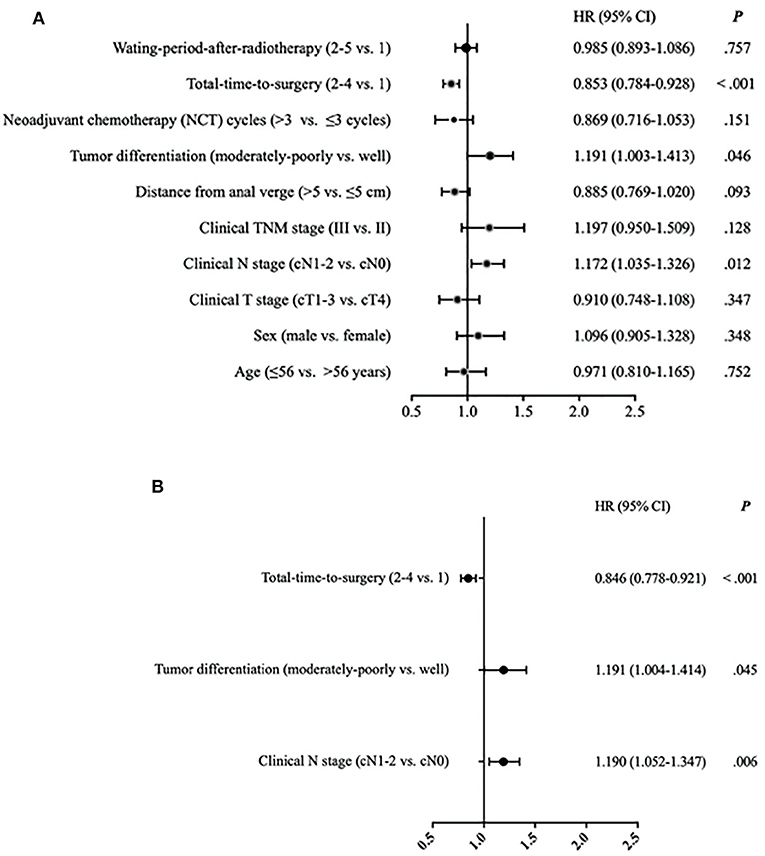
Figure 2. (A) Univariate analysis of risk of disease relapse (disease-free survival) for 2,267 patients with locally advanced rectal cancer. Hazard ratios (HR) with 95% confidence intervals (CI) on 3-year disease-free survival equate to relative risk of disease relapse. Waiting-period-after-radiotherapy (WPR) defined as time from end of radiotherapy to date of surgery: 4 to <6 weeks (WPR-1), 6 to <8 weeks (WPR-2), 8 to <10 weeks (WPR-3), 10 to <12 weeks (WPR-4), and ≥12 weeks (WPR-5). Total-time-to-surgery (TTS) defined as time from initiation of neoadjuvant treatment to date of surgery: <13 weeks (TTS-1), 13 to <15 weeks (TTS-2), 15 to <17 weeks (TTS-3), and ≥17 weeks (TTS-4). (B) Multivariate analysis of risk of disease relapse (disease-free survival) for 2,267 patients with locally advanced rectal cancer. Hazard ratios (HR) with 95% confidence intervals (CI) on 3-year disease-free survival equate to relative risk of disease relapse. Total-time-to-surgery (TTS) defined as time from initiation of neoadjuvant treatment to date of surgery: <13 weeks (TTS-1), 13 to <15 weeks (TTS-2), 15 to <17 weeks (TTS-3), and ≥17 weeks (TTS-4).
As with patients in subgroups with longer TTS, patients in subgroups with progressively longer WPR also had significantly higher rates of pCR: 19.7% in WPR-1, 23.7% in WPR-2, 24.1% in WPR-3, 25.8% in WPR-4, and 32.7% in WPR-5 (P = 0.025). However, despite the increased pCR rates was associated with longer WPR, WPR did not significantly correlate with any of the survival endpoints (Supplementary Table 1; Figure 3).
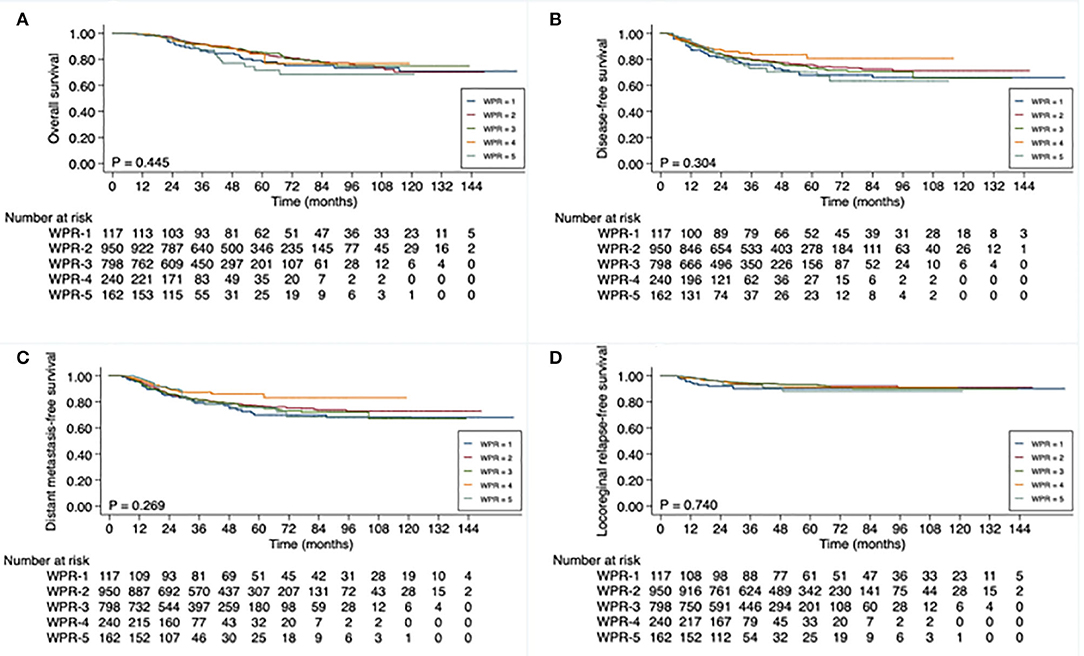
Figure 3. Kaplan-Meier curve analysis of (A) overall survival, (B) disease-free survival, (C) distant metastasis-free survival, and (D) locoregional relapse-free survival (D), based on waiting-period-after-radiotherapy (WPR), of 2,267 patients with locally advanced rectal cancer. WPR defined as time from end of neoadjuvant radiotherapy to date of surgery: 4 to <6 weeks (WPR-1), 6 to <8 weeks (WPR-2), 8 to <10 weeks (WPR-3), 10 to <12 weeks (WPR-4), and ≥12 weeks (WPR-5).
Discussion
In this study, we reported the prognostic value of total-time-to-surgery (TTS) in patients with locally advanced rectal cancer (LARC) who have received intensive neoadjuvant and/or adjuvant chemotherapy in addition to neoadjuvant radiotherapy and TME surgery, using multiple different survival rates as clinical endpoints. We identified TTS as an independent prognostic factor for both 3-year DFS and 3-year DMFS in patients with LARC. Specifically, our results showed that patients with LARC achieved better survival outcomes when they underwent TME surgery 13 or more weeks after the initiation of neoadjuvant treatment.
In the past, the standard approach for patients with LARC was concurrent neoadjuvant chemoradiotherapy, followed by TME surgery and then ACT. However, with this strategy distant metastases remained a common reason of failure (3–5). To address this, intensified chemotherapy has been administered, either by including more cytotoxic drugs in each chemotherapy cycle, or more total cycles of chemotherapy, prior to TME. This can be done by splitting the chemotherapy between NCT and ACT, or alternatively by giving all chemotherapy as NCT, which some have named as total neoadjuvant therapy (TNT). NCT certainly has some theoretical benefits over ACT, including potentially improved compliance with chemotherapy, earlier eradication of occult metastases, prompt identification of non-responders, and increased resectability of the primary tumor.
An example of adding cytotoxic drugs was the CAO/ARO/AIO-04 multicenter study, which involved patients with LARC who received neoadjuvant chemoradiotherapy, surgery, and ACT with or without oxaliplatin, and demonstrated 3-year DFS rates of 75.9 and 71.2%, respectively (21). TNT has been evaluated in a meta-analysis which involved 28 studies and demonstrated 3-year DFS and OS rates of 67 and 78.9%, respectively, in patients receiving TNT for LARC (22). Our FORWARC study, which also used an intensified NCT regimen in the preoperative setting, showed that 3-year DFS rates for fluorouracil plus radiotherapy, mFOLFOX6 plus radiotherapy, and mFOLFOX6 alone, were 72.9, 77.2, and 73.5%, respectively (23). Our present findings are similar to these reported studies, showing a 3-year DFS of 79.3% in all patients who received intensive NCT and/or ACT, in addition to radiotherapy and TME surgery.
While beneficial, the expanding use of NCT in the preoperative setting in recent years has resulted in an increase in the time from the initiation of neoadjuvant treatment to definitive surgery (24). For example, Garcia-Aguilar et al. (25) studied the impact of adding cycles of neoadjuvant chemotherapy on the time from the start of chemoradiation to surgery in patients with LARC (25). They explored the effect of adding 0, 2, 4, and 6 cycles of consolidation chemotherapy, after neoadjuvant chemoradiotherapy and before surgery, on pCR. They found that the mean total-time-to surgery for each of these 4 groups was 14.2, 17.1, 21.0, and 25.2 weeks, respectively. They also found 5-year DFS rates for each of these groups of 50, 81, 86, and 76%, respectively. Because of their study design, it was not possible to conclude that the survival benefits resulted solely from the longer intervals (26). However, their results were in line with our findings, that the 5-year DFS rate for patients with a TTS <13 weeks was significantly inferior to the rates for patients with longer TTS. These results provide evidence that a longer TTS may play an important role in the success of intensive treatment regimens in patients with LARC.
The goals of giving NCT to patients with LARC in the preoperative setting include the eradication of occult micro-metastases and the downsizing of locoregional disease. Nevertheless, it is not possible in the majority of patients to eliminate all of the primary tumor without surgery. Indeed, we found that only 24.5% of patients in our study achieved pCR. However, the rate of pCR progressively increased with longer TTS, up to 31.2% in patients with a TTS of 17 weeks or more. Despite this, we noticed a slight decline in the 3-year DFS rates in patients in the TTS-4 subgroup (80.5%) compared to those in the TTS-3 subset (84.4%). Evidence exists that tumor cell repopulation might accelerate after chemotherapy or radiotherapy (27). Consequently, markedly prolonged time intervals between neoadjuvant treatment and definitive surgery may actually increase the risk of disease progression. Thus, there is still work to do to identify the optimal TTS, one that allows patients to receive the maximum therapeutically beneficial systemic neoadjuvant treatment while also avoiding an excessive delay that may result in disease progression.
It has been suggested that the waiting period between the completion of radiotherapy and the performance of surgery, which represents a component of TTS, may also play a role in determining survival rates in patients with LARC. Using pCR as a marker for oncological success, the Lyon R90-01 trial established a generally accepted waiting period after radiotherapy of 6–8 weeks (15), and the NCCN guideline recommended a range of 5–12 weeks as a proper interval (15, 16). However, neither of these addressed the impact of this waiting period on survival. In our study, the pCR rate increased when the WPR became longer, ranging from 19.7% up to 32.7% for a waiting period between radiotherapy and surgery of 12 weeks or longer. At the same time, we also found that WPR did not correlate with 3-year DFS. Therefore, the optimal waiting period between neoadjuvant radiotherapy and TME surgery remains to be clarified.
This study has several limitations. It included variations in patient baseline clinicopathological characteristics among the 4 different TTS subgroups. For example, the groups with longer TTS included a significantly larger proportion of patients who had advanced cTNM stages. Theoretically, this should correlate with inferior survival results in the groups with longer TTS, while the survival results were actually superior in these subgroups. Moreover, the subgroups with longer TTS included a significantly larger proportion of patients who had received more cycles of NCT and fewer cycles of ACT. These chemotherapy administration differences may have impact on the final oncological outcomes, it is noteworthy that the total number cycles of chemotherapy (combining NCT and ACT) received were similar among the 4 TTS subgroups. These variations were unavoidable given the retrospective nature of the present study. Finally, our study lacked data related to chemoradiotherapy and surgical complications, as well as to quality of life during and after treatment. This information may have been helpful in providing additional perspectives on different TTS and WPR.
Conclusions
In patients with LARC, the initiation of neoadjuvant treatment and surgery of longer than 13 weeks is associated with preferable disease-free survival outcomes. The appropriate interval between the initiation of treatment and surgery, including the ideal waiting period between neoadjuvant radiotherapy and surgery, warrants further study.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Ethics Statement
The study protocol was approved by the Central Ethics Committee of The Sixth Affiliated Hospital, Sun Yat-sen University (Guangzhou, China).
Author Contributions
X-BW and FH designed the study. FH performed the contouring, treatment planning, and statistical analysis. QZ, JZ, YL, and PL reviewed the data. All authors discussed the data. FH and MC drafted the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Natural Science Foundation of China (No. 81872188 to X-BW), Guangdong Science and Technology Project (No. 611231078086 to X-BW).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.01280/full#supplementary-material
Abbreviations
TME, total mesorectal excision; LARC, locally advanced rectal cancer; TTS, total-time-to-surgery; WPR, waiting-period-after-radiotherapy; TNT, total neoadjuvant therapy; NCT, neoadjuvant chemotherapy; ACT, adjuvant chemotherapy; DFS, disease-free survival; OS, overall survival; LRFS, local recurrence-free survival; DMFS, distant metastasis-free survival; pCR, pathologic complete response; AJCC, American Joint Commission on Cancer; CT, computed tomography; MRI, magnetic resonance imaging; EUS, endorectal ultrasonography; IMRT, intensity-modulated radiotherapy (IMRT); GTV, gross tumor volume; CTV, clinical target volume; CRM, circumferential resection margin; HR, hazard ratio; CI, confidence interval; NCCN, National Comprehensive Cancer Network.
References
1. Benson AB III, Bekaii-Saab T, Chan E, Chen YJ, Choti MA, Cooper HS, et al. Metastatic colon cancer, version 3.2013: featured updates to the NCCN Guidelines. J Natl Comprehens Cancer Netw. (2013) 11:141–52; quiz 52.
2. Glimelius B, Tiret E, Cervantes A, Arnold D. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2013) 24(Suppl. 6):vi81–8. doi: 10.1093/annonc/mdt240
3. Gollins S, Sebag-Montefiore D. Neoadjuvant treatment strategies for locally advanced rectal cancer. Clin Oncol. (2016) 28:146–51. doi: 10.1016/j.clon.2015.11.003
4. van Gijn W, Marijnen CA, Nagtegaal ID, Kranenbarg EM, Putter H, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. (2011) 12:575–82. doi: 10.1016/S1470-2045(11)70097-3
5. Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. (2012) 30:1926–33. doi: 10.1200/JCO.2011.40.1836
6. Sainato A, Cernusco Luna Nunzia V, Valentini V, De Paoli A, Maurizi ER, Lupattelli M, et al. No benefit of adjuvant Fluorouracil Leucovorin chemotherapy after neoadjuvant chemoradiotherapy in locally advanced cancer of the rectum (LARC): Long term results of a randomized trial (I-CNR-RT). Radiother Oncol. (2014) 113:223–9. doi: 10.1016/j.radonc.2014.10.006
7. Bosset JF, Calais G, Mineur L, Maingon P, Stojanovic-Rundic S, Bensadoun RJ, et al. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long-term results of the EORTC 22921 randomised study. Lancet Oncol. (2014) 15:184–90. doi: 10.1016/S1470-2045(13)70599-0
8. Glynne-Jones R, Counsell N, Quirke P, Mortensen N, Maraveyas A, Meadows HM, et al. Chronicle: results of a randomised phase III trial in locally advanced rectal cancer after neoadjuvant chemoradiation randomising postoperative adjuvant capecitabine plus oxaliplatin (XELOX) versus control. Ann Oncol. (2014) 25:1356–62. doi: 10.1093/annonc/mdu147
9. Carvalho C, Glynne-Jones R. Challenges behind proving efficacy of adjuvant chemotherapy after preoperative chemoradiation for rectal cancer. Lancet Oncol. (2017) 18:e354–e63. doi: 10.1016/S1470-2045(17)30346-7
10. Ludmir EB, Palta M, Willett CG, Czito BG. Total neoadjuvant therapy for rectal cancer: an emerging option. Cancer. (2017) 123:1497–506. doi: 10.1002/cncr.30600
11. Franke AJ, Parekh H, Starr JS, Tan SA, Iqbal A, George TJ Jr. Total neoadjuvant therapy: a shifting paradigm in locally advanced rectal cancer management. Clin Colorectal Cancer. (2018) 17:1–12. doi: 10.1016/j.clcc.2017.06.008
12. Fokas E, Allgauer M, Polat B, Klautke G, Grabenbauer GG, Fietkau R, et al. Randomized Phase II trial of chemoradiotherapy plus induction or consolidation chemotherapy as total neoadjuvant therapy for locally advanced rectal cancer: CAO/ARO/AIO-12. J Clin Oncol. (2019) 37:3212–22. doi: 10.1200/JCO.19.00308
13. Maas M, Nelemans PJ, Valentini V, Das P, Rodel C, Kuo LJ, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. (2010) 11:835–44. doi: 10.1016/S1470-2045(10)70172-8
14. Cummings BJ, Rider WD, Harwood AR, Keane TJ, Thomas GM. Radical external beam radiation therapy for adenocarcinoma of the rectum. Dis Colon Rectum. (1983) 26:30–6. doi: 10.1007/BF02554676
15. Francois Y, Nemoz CJ, Baulieux J, Vignal J, Grandjean JP, Partensky C, et al. Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter-sparing surgery for rectal cancer: the Lyon R90-01 randomized trial. J Clin Oncol. (1999) 17:2396. doi: 10.1200/JCO.1999.17.8.2396
16. Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK, et al. Rectal cancer, version 2.2018, NCCN Clinical Practice Guidelines in oncology. J Natl Compr Canc Netw. (2018) 16:874–901. doi: 10.6004/jnccn.2018.0061
17. Lefevre JH, Mineur L, Kotti S, Rullier E, Rouanet P, de Chaisemartin C, et al. Effect of interval (7 or 11 weeks) between neoadjuvant radiochemotherapy and surgery on complete pathologic response in rectal cancer: a multicenter, randomized, controlled trial (GRECCAR-6). J Clin Oncol. (2016) 34:3773–80. doi: 10.1200/JCO.2016.67.6049
18. Huntington CR, Boselli D, Symanowski J, Hill JS, Crimaldi A, Salo JC. Optimal timing of surgical resection after radiation in locally advanced rectal adenocarcinoma: an analysis of the national cancer database. Ann Surg Oncol. (2016) 23:877–87. doi: 10.1245/s10434-015-4927-z
19. Sun Z, Adam MA, Kim J, Shenoi M, Migaly J, Mantyh CR. Optimal timing to surgery after neoadjuvant chemoradiotherapy for locally advanced rectal cancer. J Am Coll Surg. (2016) 222:367–74. doi: 10.1016/j.jamcollsurg.2015.12.017
20. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. (2010) 17:1471–4. doi: 10.1245/s10434-010-0985-4
21. Rodel C, Graeven U, Fietkau R, Hohenberger W, Hothorn T, Arnold D, et al. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. (2015) 16:979–89. doi: 10.1016/S1470-2045(15)00159-X
22. Petrelli F, Trevisan F, Cabiddu M, Sgroi G, Bruschieri L, Rausa E, et al. Total neoadjuvant therapy in rectal cancer: a systematic review and meta-analysis of treatment outcomes. Ann Surg. (2019) 271:440. doi: 10.1097/SLA.0000000000003471
23. Deng Y, Chi P, Lan P, Wang L, Chen W, Cui L, et al. Neoadjuvant modified FOLFOX6 with or without radiation versus fluorouracil plus radiation for locally advanced rectal cancer: final results of the Chinese FOWARC Trial. J Clin Oncol. (2019) 37:3223–33. doi: 10.1200/JCO.18.02309
24. Cercek A, Roxburgh CSD, Strombom P, Smith JJ, Temple LKF, Nash GM, et al. Adoption of total neoadjuvant therapy for locally advanced rectal cancer. JAMA Oncol. (2018) 4:e180071. doi: 10.1001/jamaoncol.2018.0071
25. Garcia-Aguilar J, Chow OS, Smith DD, Marcet JE, Cataldo PA, Varma MG, et al. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol. (2015) 16:957–66. doi: 10.1016/S1470-2045(15)00004-2
26. Marco MR, Zhou L, Patil S, Marcet JE, Varma MG, Oommen S, et al. Consolidation mFOLFOX6 chemotherapy after chemoradiotherapy improves survival in patients with locally advanced rectal cancer: final results of a multicenter phase II trial. Dis Colon Rectum. (2018) 61:1146–55. doi: 10.1097/DCR.0000000000001207
Keywords: locally advanced rectal cancer, neoadjuvant treatment, neoadjuvant treatment interval, prognostic value, surgery interval
Citation: Wan X-B, Zhang Q, Chen M, Liu Y, Zheng J, Lan P and He F (2020) Prognostic Value of Interval Between the Initiation of Neoadjuvant Treatment to Surgery for Patients With Locally Advanced Rectal Cancer Following Neoadjuvant Chemotherapy, Radiotherapy and Definitive Surgery. Front. Oncol. 10:1280. doi: 10.3389/fonc.2020.01280
Received: 07 February 2020; Accepted: 19 June 2020;
Published: 21 August 2020.
Edited by:
Jeremie Lefevre, Assistance Publique Hopitaux de Paris, FranceReviewed by:
Imerio Angriman, University of Padova, ItalyAlfonso Recordare, Ospedale dell'Angelo, Italy
Copyright © 2020 Wan, Zhang, Chen, Liu, Zheng, Lan and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiang-Bo Wan, d2FueGJvQG1haWwuc3lzdS5lZHUuY24=; Fang He, aGVmYW5nMjNAbWFpbC5zeXN1LmVkdS5jbg==
†These authors have contributed equally to this work
 Xiang-Bo Wan
Xiang-Bo Wan Qun Zhang
Qun Zhang Mo Chen3
Mo Chen3