- 1Division of Child and Adolescent Psychiatry, University of Cape Town, Cape Town, South Africa
- 2Department of Pediatrics and Neurology, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, United States
- 3Tor Vergata University Hospital, Rome, Italy
- 4Hospital Necker-Enfants Malades, Paris Descartes University, Paris, France
- 5Novartis Pharmaceuticals Corporation, East Hanover, NJ, United States
- 6Novartis Pharma AG, Basel, Switzerland
- 7Adelphi Values, Bollington, United Kingdom
- 8Tuberous Sclerosis Multidisciplinary Management Clinic, Sydney Children’s Hospital, Randwick, NSW, Australia
Tuberous sclerosis complex (TSC) is a rare genetic disease associated with significant disease burden and considerable impact on health-related quality of life (HRQL). Currently no disease-specific clinical outcome assessments evaluate HRQL in individuals with TSC. A multi-center phase III study EXIST-3 (NCT01713946) assessed the efficacy and safety of two trough exposure ranges (Low exposure, LE: 3–7 ng/mL and high exposure, HE: 9–15 ng/mL) of adjunctive everolimus in patients aged 2–65 years with TSC and refractory partial-onset seizures (N = 366). Three age-specific HRQL measures were included as secondary endpoints including: quality of life in childhood epilepsy (QOLCE; caregiver-report for aged 2- < 11), the Quality of Life in Epilepsy Inventory for Adolescents-48 (QOLIE-AD-48; self-report, aged ≥ 11- < 18), and the Quality of Life in Epilepsy Inventory-31-Problems (QOLIE-31-P; self-report, aged ≥ 18). Intellectual ability was evaluated using the Wechsler Non-Verbal (WNV) Scale of Ability. Post hoc analyses were performed on the core phase primary data from EXIST-3 to evaluate the psychometric properties of the HRQL measures and calculate meaningful change estimates. Results showed that a significant subset of the trial sample (4–21 year olds) scored in the intellectual disability range, as assessed by the WNV. Psychometric analyses of the three epilepsy measures (including reliability, validity, and ability to detect change) supported the appropriateness for use in TSC. Distribution-based meaningful change estimates were generated for each HRQL measure, with estimates for the QOLIE-31-P total score largely consistent with the published literature. To our knowledge, this is the first evaluation using clinical trial data to establish the psychometric properties of the QOLCE, QOLIE-AD-48, and QOLIE-31-P for use in individuals with TSC. These findings increase confidence in the measures as valid and reliable for use in clinical trials and future research in patients with TSC.
Introduction
Tuberous sclerosis complex (TSC) is a rare genetic disease which causes benign tumors in many different organs (Henske et al., 2016). Neurological and TSC-Associated Neuropsychiatric Disorders (TAND) are associated with the greatest burden of disease (Curatolo et al., 2015; de Vries et al., 2015; Leclezio and de Vries, 2016). Reports from the patient perspective, identified through TSC patient and caregiver forums, underline the impact of TAND, in particular, on health-related quality of life (HRQL) with reports of time off work/school, emotional impacts such as depression and feelings of isolation, and cognitive impacts such as delays in speech and slow processing skills (Tuberous Sclerosis Alliance, 2017).
Although the disease burden associated with TSC is well-documented (Curatolo et al., 2015; de Vries et al., 2015; Leclezio and de Vries, 2016), there are currently no disease-specific clinical outcome assessments (COAs) that assess HRQL in individuals with TSC. This is most likely due to the relative rarity of the condition with a birth incidence around 1 in 6,000 live births (Osborne et al., 1991). Selecting the optimal COA measure to assess the individual experience of a rare condition can be challenging. In line with recommendations from the International Society For Pharmacoeconomics and Outcomes Research (ISPOR) Good Practices Task Force and European Organization for Rare Disorders (EURORDIS), existing item banks or COA measures on the target population or similar populations are considered a “practical solution given the obstacles associated with the development of a de novo COA for use in Rare Disease populations” (European Organisation for Rare Disorders, 2011; Benjamin et al., 2017).
The international, multi-center phase III study EXIST-3 (NCT01713946) assessed the efficacy and safety of two trough exposure ranges (Low exposure, LE: 3–7 ng/mL and high exposure, HE: 9–15 ng/mL) of adjunctive everolimus in patients aged 2–65 years with TSC and refractory partial-onset seizures (N = 366) (French et al., 2016). The trial included three age-specific patient-reported/observer-reported outcome (PRO/ObsRO) measures as secondary endpoints: the Quality of Life in Childhood Epilepsy (QOLCE) for individuals aged < 11 years (Sabaz et al., 2003), the Quality of Life in Epilepsy Inventory for Adolescents-48 (QOLIE-AD-48) for individuals aged 11 to < 18 years (Cramer et al., 1999), and the Quality of Life in Epilepsy Inventory-31-Problems (QOLIE-31-P) for individuals aged ≥ 18 years (Cramer et al., 1998). These measures were developed to assess HRQL in individuals with epilepsy. Even though these instruments were developed for individuals with a broad range of epilepsy syndromes rather than for TSC-specific epilepsy, comparison of the qualitative epilepsy literature (McEwan et al., 2004; Elliott et al., 2005) and TSC user/carer forums (Tuberous Sclerosis Alliance, 2017) confirms a clear conceptual overlap in the impacts reported by both groups and the items captured in these COAs. The measures have established psychometric properties including internal consistency, test–retest reliability, and construct validity in the general epilepsy population. However, the psychometric properties of these instruments have not been evaluated in relation to individuals with TSC with or without refractory seizures (Cramer et al., 1998, 1999; Sabaz et al., 2000).
Generating evidence of the psychometric properties of a COA measure in the relevant patient population is critical for its credibility and acceptance by stakeholders. In particular, regulatory agencies such as the European Medicines Agency (EMA) (European Medicines Agency, 2005) and the US Food and Drug Administration (FDA) (Food and Drug Administration, 2009), and clinicians who wish to use these measures in research or clinical practice. It is also important that HRQL data can be interpreted in the context of what level of change in scores over time is clinically meaningful (improvement or worsening). Currently, there are no clinically meaningful change estimates available for the QOLCE or QOLIE-AD-48 in any epilepsy-related population. Whilst there are clinically meaningful change estimates in the literature for the QOLIE-31-P (all assessed change over time within a group), these estimates were derived in individuals with refractory epilepsy, rather than in a TSC population (Wiebe et al., 2002; Cramer et al., 2004; Borghs et al., 2012).
Here, we used data from EXIST-3 to evaluate the psychometric properties of the QOLCE, QOLIE-AD-48, and QOLIE-31-P in a TSC population and to calculate meaningful change estimates for these three HRQL measures.
Materials and Methods
Clinical Trial Design
Full details of the clinical trial design (NCT01713946) are described elsewhere (French et al., 2016). In brief, EXIST-3 was a three-arm, randomized, multi-center (99 centers across 25 countries), double-blind, placebo-controlled phase III study. The study consisted of three phases: 8 week baseline phase, 18 week core phase and a ≥48 week extension phase (extension data not included here). The primary efficacy endpoint was change from baseline in seizure frequency for each of the two everolimus exposure ranges compared with placebo during the last 12 weeks of the core phase, defined as response rate by the EMA (reduction in seizure frequency) and median percentage reduction in seizure frequency by the FDA. HRQL and intellectual ability were analyzed as secondary endpoints, using the QOLCE, QOLIE-AD-48, or QOLIE-31-P and Wechsler Non-Verbal (WNV) Scale of Ability, respectively. Individuals with TSC or their caregivers, were required to complete the relevant age-specific HRQL measure (QOLCE; QOLIE-AD-48; QOLIE-31-P) and WNV were performed with individuals with TSC at baseline (week 0 – reflecting on 4 weeks prior to randomization) and at end of core phase (week 18 – reflecting on the last 4 weeks of core phase). The study also assessed safety, according to the National Cancer Institute Common Toxicity Criteria for Adverse Events version 4.03.
Measures Used
In line with recommendations from the FDA PRO guidance (Food and Drug Administration, 2009), the EMA reflection paper (European Medicines Agency, 2005) and the ISPOR Good Research Practices for the Assessment of Children and Adolescence Task Force (Matza et al., 2013), age-specific HRQL measures were administered to the relevant age groups within the clinical trial.
The QOLCE is an ObsRO measure (parent/caregiver reports the signs/symptoms and functional impacts observed in their child), designed to assess HRQL in individuals aged 4 to 18 years old with epilepsy (Sabaz et al., 2000, 2003). For the purpose of the trial the QOLCE was completed by parents/caregivers of individuals with TSC aged < 11 years old at randomization. The measure contains 92 items across five age-relevant domains namely physical function (12 items), emotional well-being (19 items), cognitive function (23 items), social function (12 items) and behavior (23 items). The QOLCE includes two general health items and one overall QoL item. In line with the literature, the cognitive subscales were completed only for individuals aged ≥ 6 to < 11 years old in the trial (Sabaz et al., 2003).
The QOLIE-AD-48 is a self-report PRO measure designed to assess HRQL in individuals aged 11–18 years old with epilepsy (Cramer et al., 1999). For the purpose of the trial the QOLIE-AD-48 was administered to individuals aged 11 to < 18 years old. The measure contains 48 items across eight domains namely epilepsy impact (12 items), memory/concentration (10 items), attitudes toward epilepsy (4 items), physical functioning (5 items), stigma (6 items), social support (4 items), school behavior (4 items), and health perceptions (3 items).
The QOLIE-31-P is a self-report PRO measure designed to assess HRQL in adults aged > 18 years old with epilepsy (Cramer et al., 1998). For the purpose of the trial the QOLIE-31-P was therefore administered to individuals aged ≥ 18 years old. The measure contains 39 items, of which 30 are used to make up seven domains namely seizure worry (5 items), overall QoL (2 items), emotional well-being (5 items), energy/fatigue (4 items), cognitive (6 items), medication effects (3 items), and social function (5 items). The remaining nine items (a distress item for each domain, an overall health item and an item which ranks the importance of each domain), did not contribute to the total score.
For each of the three HRQL measures scores can range from 0 to 100, with higher scores indicating a greater level of functioning and HRQL.
The WNV is a non-verbal assessment of general ability, designed for individuals aged 4–21 years old from diverse cultural and linguistic groups, those with limited language skills and those with language disorders (Wechsler et al., 2006). The single ability score derived for the full four subtests can range from 30 to 170 and the individual subtest scores can range from 10 to 90. Children aged 4 to 7 years 11 months completed the matrices and recognition subtests of the WNV, and individuals aged 8 to 21 years 11 months old completed the matrices and spatial span subtests, to derive prorated intellectual ability scores.
Psychometric Analyses
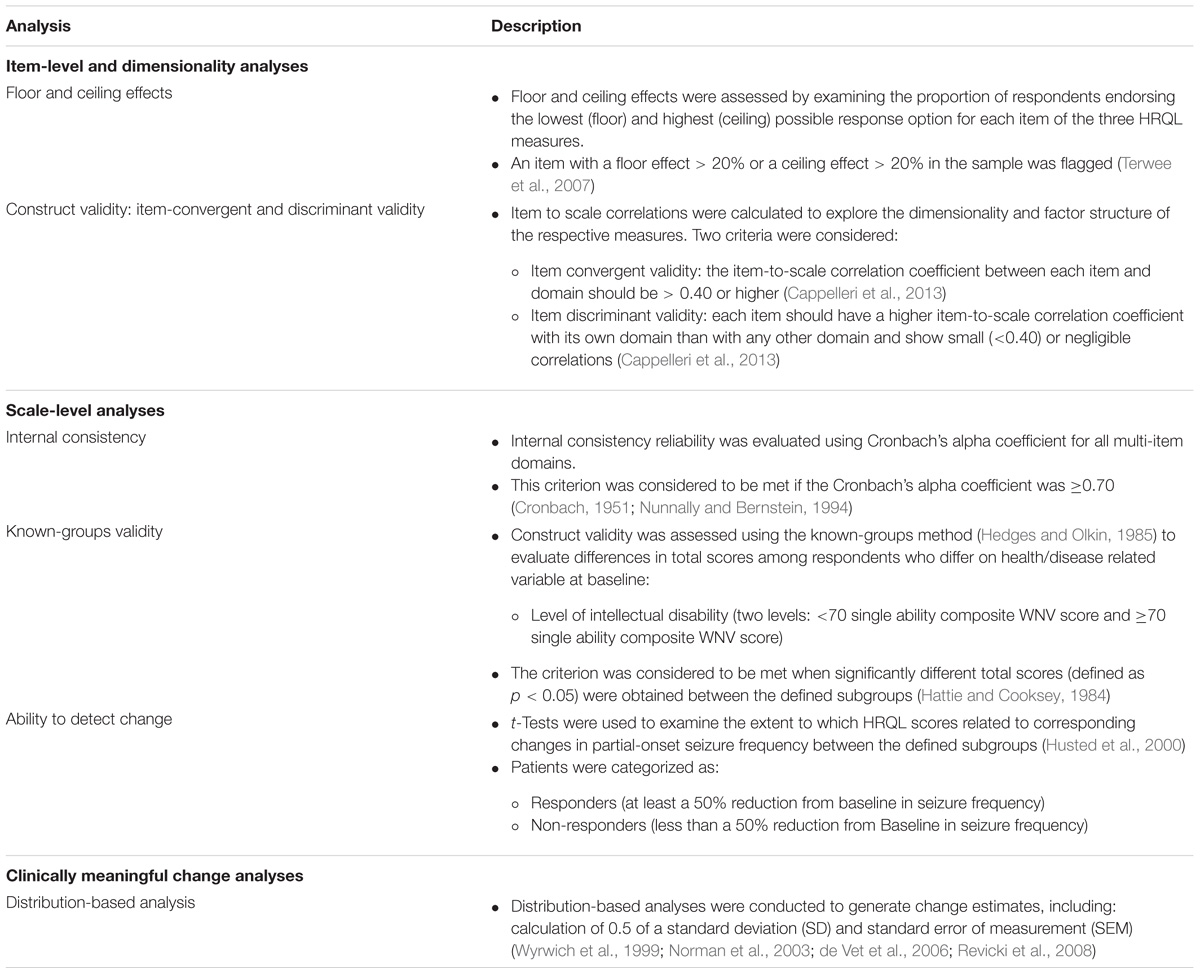
A range of psychometric analyses were performed post hoc on the core phase primary data (cutoff date October, 2015) from EXIST-3 to assess the measurement properties and generate meaningful change estimates for each of the three HRQL measures in patients with TSC and refractory partial-onset seizures. In line with best practice for evaluation of PROs for use in clinical trials of pharmaceutical products (Food and Drug Administration, 2009), three main domains of psychometric evaluation were selected – item-level and dimensionality analysis, scale-level analysis, and clinically meaningful change analysis. Table 1 outlines the range of psychometric analyses performed in each of these domains. Psychometric analyses were performed for each HRQL measure using the full analysis set (FAS; i.e., patients aged < 11, 11 to < 18, and ≥ 18 years at randomization) who completed the specific HRQL measure at baseline (week 0) and at end of core phase (week 18). Where there were violations in normalcy the appropriate non-parametric equivalent test was conducted.
Results
Completion Rates
At the end of core phase (week 18), the overall completion rate for the QOLCE (completed by caregivers) was high across treatment arms (84%, 166/197), compared to the lower completion rates for the patient self-report QOLIE-AD-48 (36%, 37/102) and QOLIE-31-P (49%, 33/67) (Table 2).
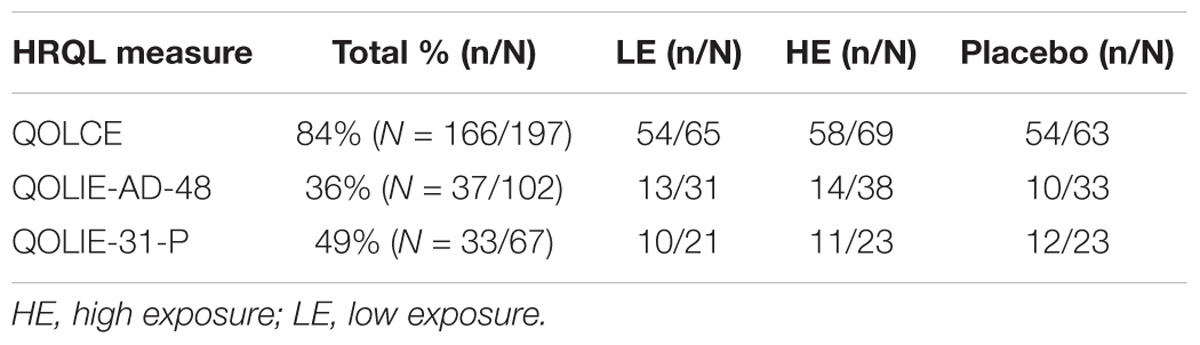
TABLE 2. Overall completion rates for each HRQL measure at end of core phase (week 18) across treatment arms.
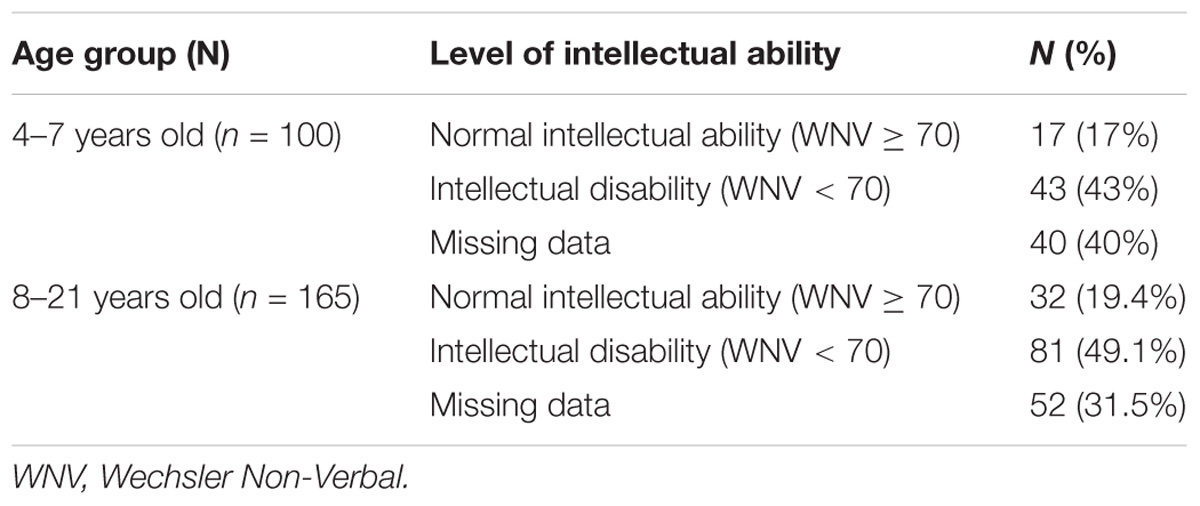
Intellectual Disability Before Starting Everolimus
At baseline (week 0) 43% of children aged 4 to 7 years old scored in the intellectual disability range, 17% of children scored in the normal intellectual ability range and 40% had missing data (see Table 3). At baseline (week 0) 49.1% of individuals aged 8 to 21 years old scored in the intellectual disability range, 19.4% scored in the normal intellectual ability range and 31.5% had missing data.
Item-Level and Dimensionality Analyses
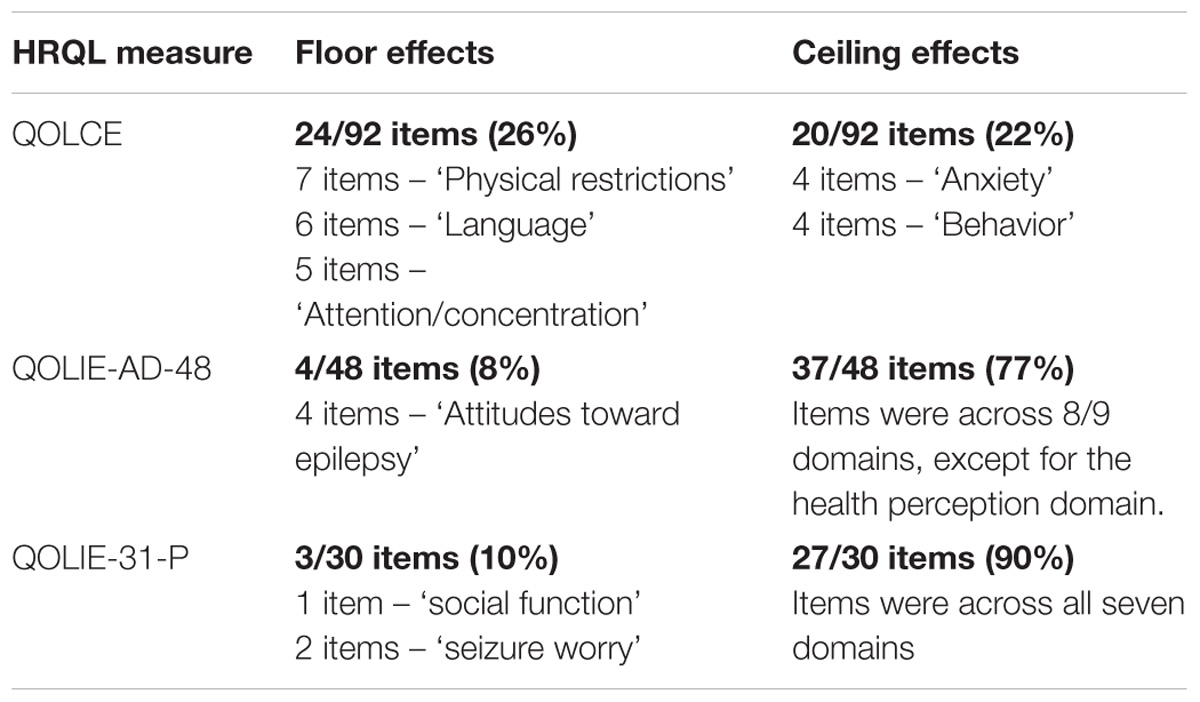
Floor and Ceiling Effects
Approximately 26% of QOLCE items across five subscales (physical restrictions, language, other cognitive, behavior, and attention/concentration subscales), demonstrated floor effects at baseline and at the end of the core phase (Table 4). Around 22% of QOLCE items across eight subscales (physical restrictions, depression, control/helplessness, anxiety, self-esteem, social interactions, stigma item, and behavior subscales) showed ceiling effects at baseline and end of core phase.
Around 8% of QOLIE-AD-48 items, in the attitudes toward epilepsy domain, showed floor effects at baseline and end of core phase. Conversely, 77% of items, across eight of the nine domains (except for the health perception domain) showed ceiling effects at baseline and end of core phase.
Around 10% of QOLIE-31-P items across two domains (social function and seizure worry) demonstrated floor effects at baseline and at the end of the core phase. Conversely, 90% of items, across all seven domains, showed ceiling effects at baseline and end of core phase.
Construct Validity: Item-Convergent and Discriminant Validity
Multi-trait analyses (see Table 1 for definition) at baseline and end of core phase demonstrated that for each of the HRQL measures, the majority of items were appropriately placed in the correct subscales, as they met the threshold for item convergent validity (r ≥ 0.40) and item discriminant validity criterion (r < 0.40) (Cappelleri et al., 2013).
Table 5 shows the items that did not meet the criteria for item convergent validity. Item discriminant validity was met in all cases.
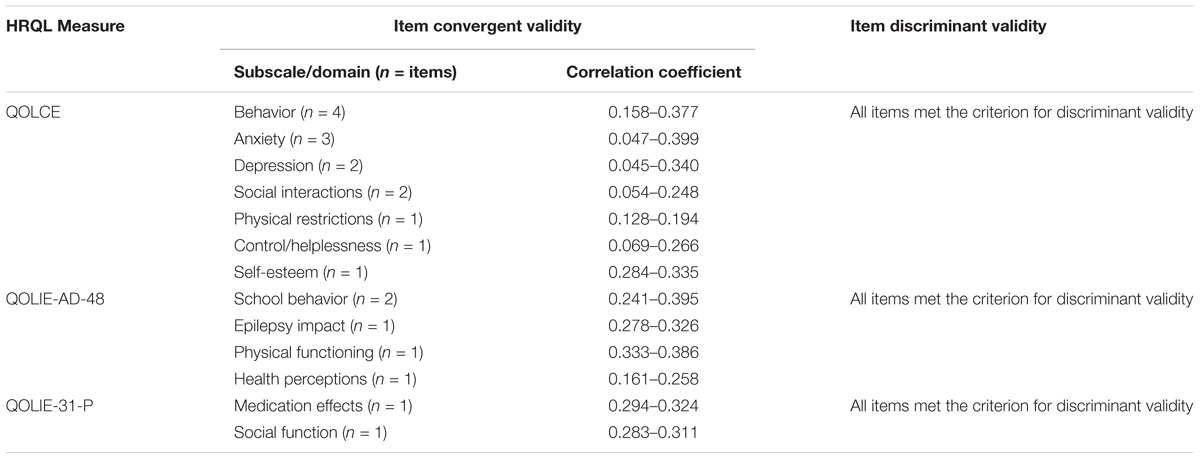
TABLE 5. Items with below threshold item-convergent validity at baseline (week 0) and end of core phase (week 18) for each HRQL measure.
Scale-Level Analyses
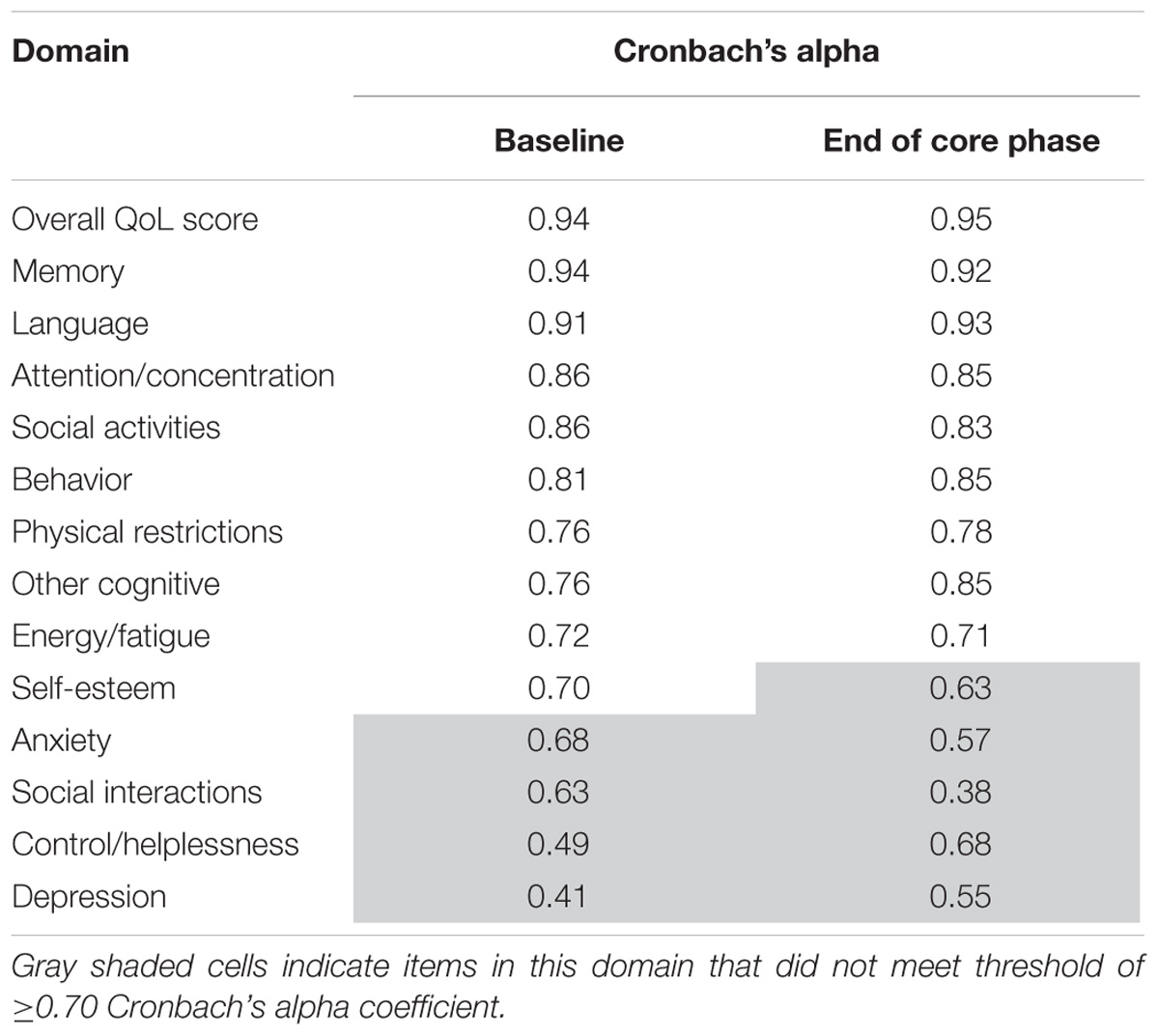
Internal Consistency Reliability
QOLCE
Table 6 shows the internal consistency reliability for the QOLCE overall QoL score and subscale scores. The total score and subscales were ≥0.70, apart from the five gray shaded subscales on the table (self-esteem, depression, anxiety, control/helplessness, and social interactions subscales).
QOLIE-AD-48
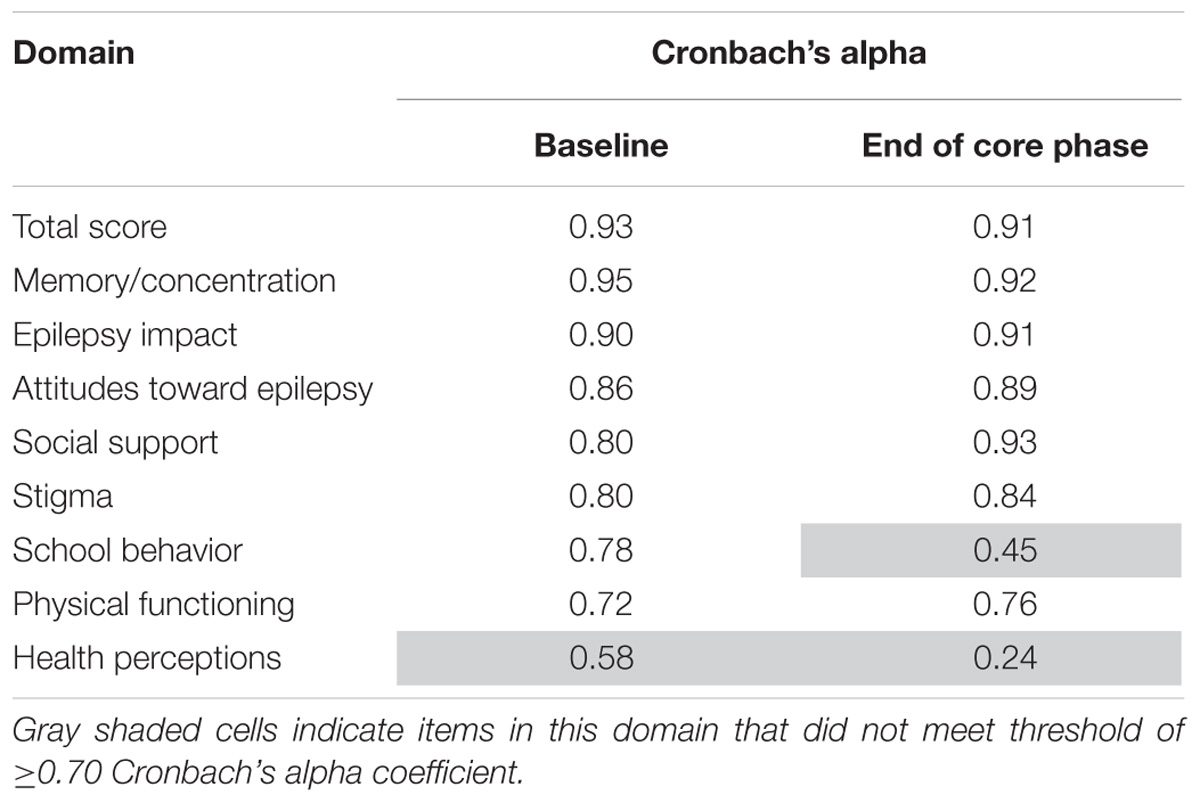
Table 7 shows the internal consistency reliability for the QOLIE-AD-48 total score and domain scores. The total score and all domains were ≥0.70, apart from the two gray shaded domains on the table (school behavior and health perceptions).
QOLIE-31-P
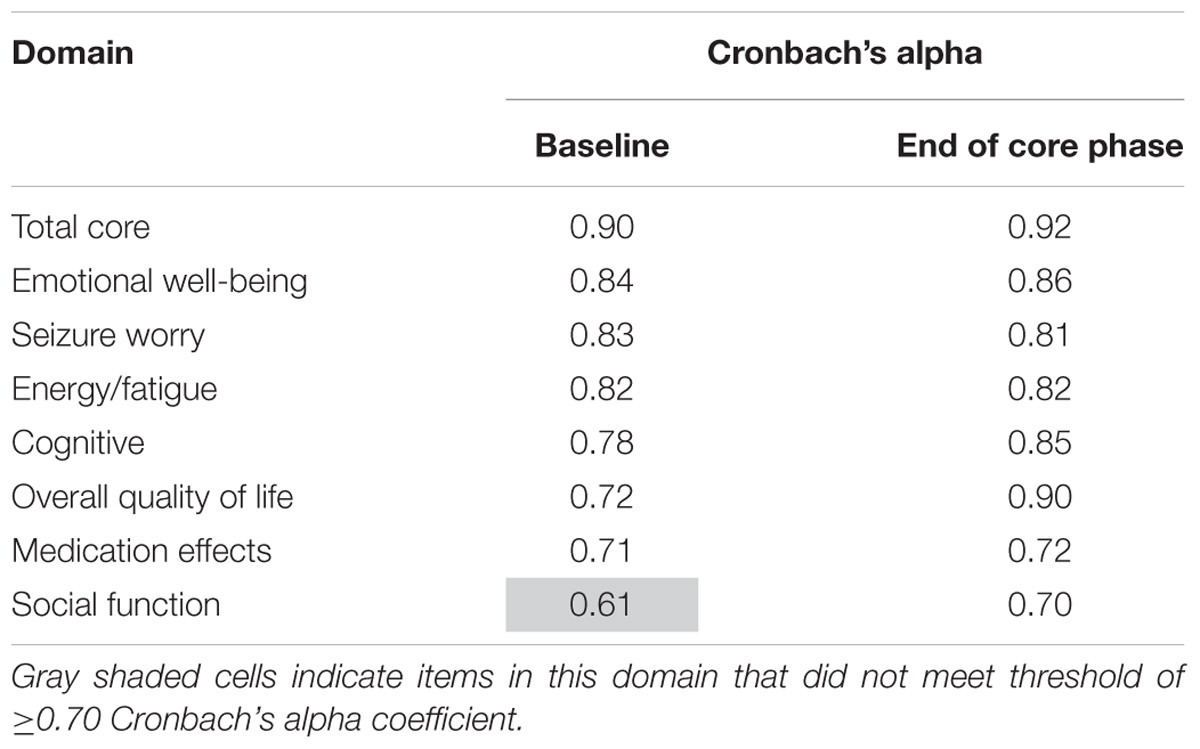
Table 8 shows the internal consistency reliability for the QOLIE-31-P total score and domain scores. The total score and all domains were ≥0.70, apart from the gray shaded domain on the table (social function).
Construct Validity: Known Groups Method
There was evidence of known groups validity in a TSC population using level of intellectual ability as a grouping variable (Table 9). Total scores on the QOLCE and QOLIE-AD-48 reported at baseline were able to discriminate between those individuals with or without intellectual disability, as categorized by single ability scores on the WNV. Individuals with normal intellectual ability demonstrated statistically significant higher overall HRQL scores at baseline, with a moderate effect size, on the QOLCE and QOLIE-AD-48 (non-significant trend). Construct validity for the QOLIE-31-P was not analyzed due to limitations in sample size.
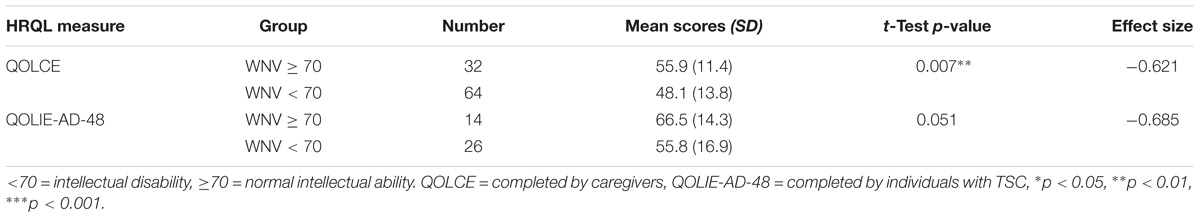
TABLE 9. Difference in QOLCE and QOLIE-AD-48 total scores with known groups categorized by intellectual ability status at baseline (week 0).
Ability to Detect Change
Tables 10–12 shows the extent to which the HRQL scores on each measure changed with a change (in either direction) in seizure response status. Mean change scores for the majority of domains across each of the measures were greatest for patients classified as ‘responders.’ Of note, 3/16 QOLCE subscales (depression, attention/concentration and social interactions) and 1/9 QOLIE-AD-48 domains (school behavior) did not follow this pattern.
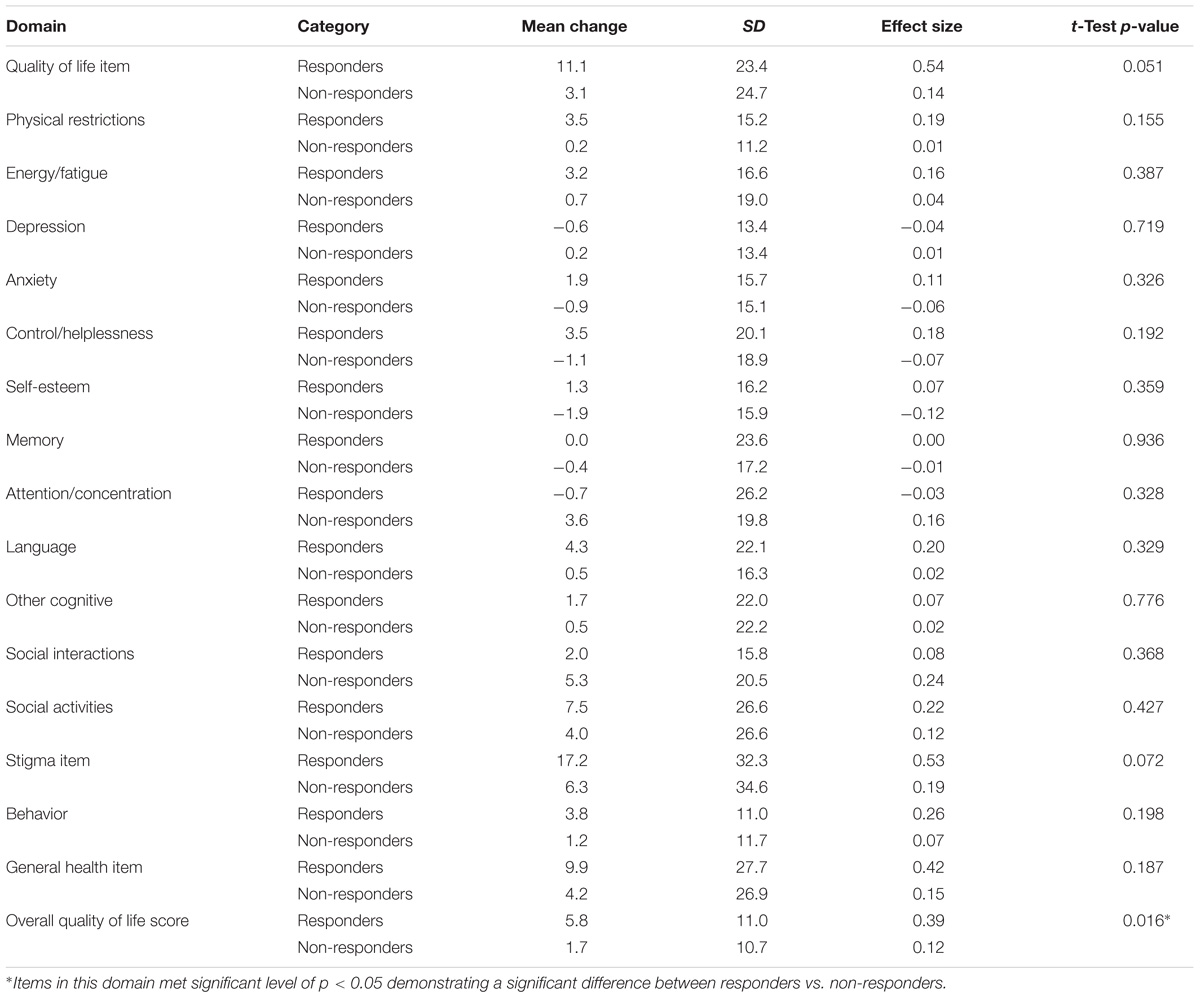
TABLE 10. Change scores and standardized effect sizes on the QOLCE based on the change from baseline in seizure frequency during the last 12 weeks of the core phase.
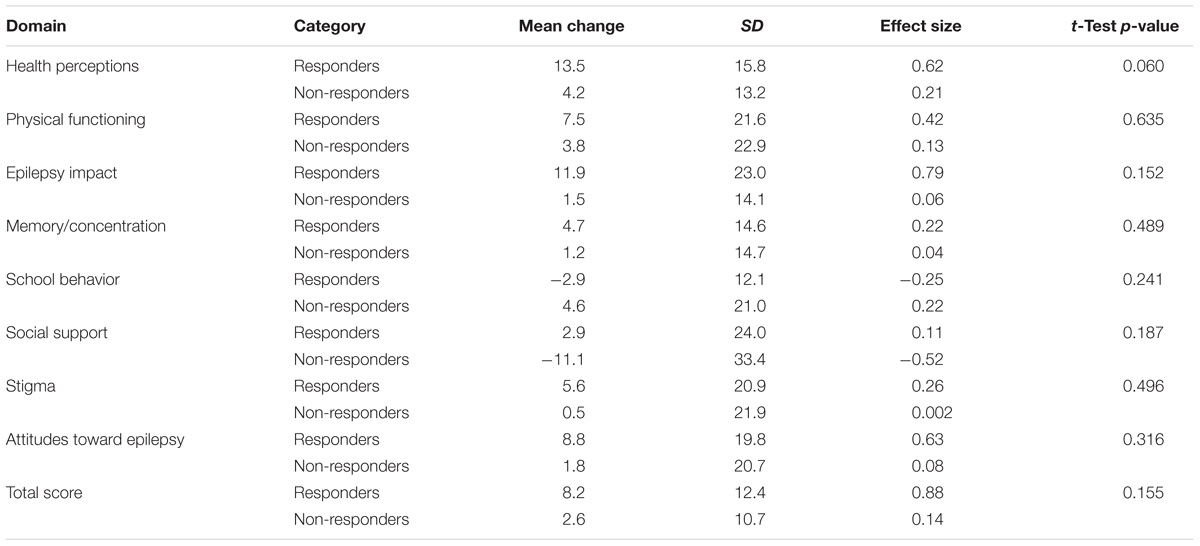
TABLE 11. Change scores and standardized effect sizes on QOLIE-AD-48 based on the change from baseline in seizure frequency during the last 12 weeks of the core phase.
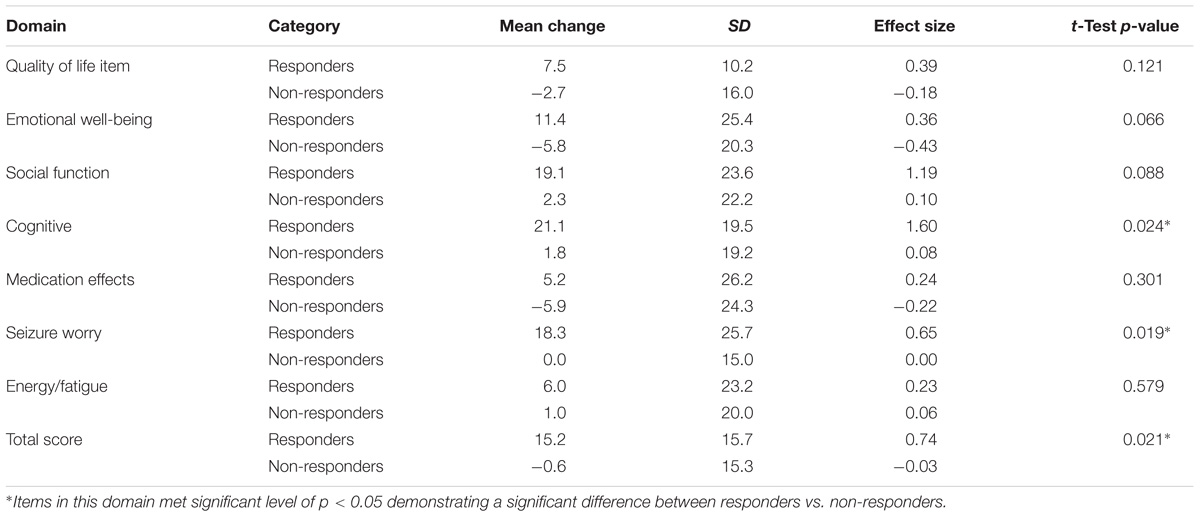
TABLE 12. Change scores and standardized effect sizes on QOLIE-31-P scores based on the change from baseline in seizure frequency during the last 12 weeks of the core phase.
Differences in mean changes observed among patients classified as ‘responders’ compared to those classified as ‘non-responders’ were statistically significant (p < 0.05) for the QOLCE overall QoL score and the QOLIE-31-P total score.
Clinically Meaningful Change Analysis
Table 13 shows the results of the distribution-based analyses for each of the HRQL measures. Using the 0.5 SD criterion (Norman et al., 2003; de Vet et al., 2006), estimates range from 6.0 for the QOLCE, 8.1 for the QOLIE-AD-48 and 11.0 for the QOLIE-31-P. Lower estimates were derived using the SEM (Wyrwich et al., 1999; de Vet et al., 2006), which range from 2.9 for the QOLCE, 4.2 for the QOLIE-AD-48, to 7.1 for the QOLIE-31-P.
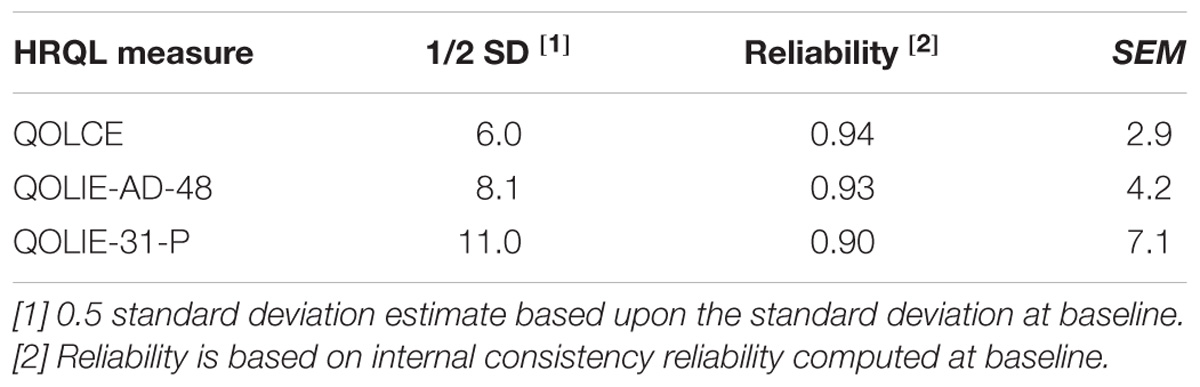
TABLE 13. Distribution-based methods: meaningful change estimates for QOLCE, QOLIE-AD-48, and QOLIE-31-P overall HRQL score.
Discussion
When selecting an optimal COA measure to assess the patient experience of a rare condition, it is often necessary, and acceptable, to use an existing COA measure developed for a similar population (European Organisation for Rare Disorders, 2011; Benjamin et al., 2017). However, generating evidence of the psychometric properties of a COA measure in the relevant patient population is critical for acceptance by stakeholders, and may identify unique psychometric characteristics of importance for clinical practice or research in the specific rare disease (European Medicines Agency, 2005). To our knowledge, this is the first evaluation using clinical trial data to establish the psychometric properties of the QOLCE, QOLIE-AD-48, and QOLIE-31-P for use in individuals with TSC.
Intellectual Disability
Our analysis demonstrated that a significant subset of the trial sample (4–21 year olds) scored in the intellectual disability range, as assessed by the WNV, an observation consistent with the wide distribution of intellectual ability in individuals with TSC (Curatolo et al., 2015; de Vries et al., 2015; Leclezio and de Vries, 2016). This may have had implications on individuals’ ability to self-report on their HRQL and may, in part, explain the low completion rates for the two self-report PRO measures (QOLIE-AD-48 and QOLIE-31-P). Cognitive interviews to establish the appropriateness of self-report, and level of concordance between patient and informant-report, would support the transition of these measures from self-report to informant-report, which may result in more complete data (Matza et al., 2013).
Item Level Analysis
Investigation of item performance demonstrated ceiling effects for the majority of items on the QOLIE-AD-48 and QOLIE-31-P, indicating that a high proportion of patients were satisfied with their HRQL at baseline. As such, the amount of improvement possible is limited within domains in the QOLIE-AD-48 (8/9 domains, except for health perceptions) and the QOLIE-31-P (7/7 domains). These findings may suggest that in rare diseases such as TSC where a wide range of intellectual ability is observed, existing measures may not be sufficiently sensitive to capture change in HRQL at the ‘upper end’ of functioning. This may be a potential limitation and a potential avenue for future measure development.
Scale Level Analysis
The results of the scale-level analyses indicate good to excellent internal consistency (Cronbach’s alpha) for each of the HRQL measures in a TSC population, at the total score level and largely at the domain level. In line with the original development papers for the QOLCE (Sabaz et al., 2003), QOLIE-AD-48 (Cramer et al., 1999), and QOLIE-31-P (Cramer et al., 1998) (generated in epilepsy sample) the alpha coefficient surpassed 0.70 at the total score level. Interestingly, on the QOLCE 4 out of 16 subscales did not demonstrate acceptable levels of internal consistency reliability (i.e., <0.70). It should be noted that these fours subscales contain six or fewer items, and Cronbach’s alpha tends to be lower with lower number of items (Streiner et al., 2015). In addition, these results may suggest that the items contained within the subscales are not one-dimensional, for example the control/helplessness scale which includes items about feeling in control, as well as feeling excited or interested in something.
Construct validity was assessed using the known groups method. The QOLCE and QOLIE-AD-48 were able to distinguish between individuals with and without intellectual disability. Individuals with normal intellectual ability demonstrated higher overall HRQL scores at baseline, on the QOLCE (significant difference) and QOLIE-AD-48 (non-significant trend). The results may suggest that individuals who were able to complete the measures had higher overall ability and associated HRQL. However, as the WNV assesses individuals aged from 4–7 years old and 8–21 years old, the latter group encompasses individuals whose caregivers completed the QOLCE on their behalf, as well as individuals who completed the QOLIE-AD-48, meaning that it is hard to link the WNV data to the age-specific HRQL measures.
The QOLCE, QOLIE-AD-48, and QOLIE-31-P were shown to be responsive to improvements in seizure frequency. Across all three measures mean change scores were greatest for individuals who demonstrated a greater reduction in seizure frequency. Confirming the ability of an instrument to detect change strengthens the rationale to conduct meaningful change analysis, which focuses on establishing a threshold for meaningful change for each instrument.
Meaningful Change Analysis
As there were no appropriate external anchors (i.e., not sufficiently correlated to the HRQL scores), interpretation of change was only explored using distribution-based methods. Meaningful change estimates were generated for each HRQL measure, with estimates for the QOLIE-31-P total score largely in line with the literature, although domain estimates were higher than previously estimated (Wiebe et al., 2002; Cramer et al., 2004; Borghs et al., 2012). The variability in estimations of meaningful change may reflect differences in methodological approaches between the current study and previous work, i.e., distribution based vs. anchor approaches (previous work utilized a Patient Global Impression of Change item) and patient populations, i.e., epilepsy vs. TSC (Borghs et al., 2012). TSC is a very complex genetic disorder and the clinical disease spectrum is highly variable (Curatolo et al., 2015; de Vries et al., 2015; Leclezio and de Vries, 2016). Manifestations of the disorder can range from mild to profound intellectual disability (Curatolo et al., 2015; de Vries et al., 2015; Leclezio and de Vries, 2016), as evidenced by the WNV data collected in the current study. As such, there is also likely to be great heterogeneity in individual experiences of TSC. This variability could be reflected in the higher QOLIE-31-P domain meaningful change estimates compared to those previously generated in epilepsy populations (Wiebe et al., 2002; Cramer et al., 2004; Borghs et al., 2012).
Limitations
Low sample sizes, specifically for the QOLIE-AD-48 and QOLIE-31-P, may have impacted the level of statistical power required for the known-groups analysis and the ability to detect change analysis. Therefore, we recommend reassessment of these properties in future studies. More generally, the low completion rates for the QOLIE-AD-48 and QOLIE-31-P may limit the generalization of the current findings to the TSC population. To this end, the high levels of missing data for the WNV underlines the real-life challenge of identifying suitable standardized measures of intelligence such as IQ tests for individuals across the full range of neurodevelopmental ability. Individuals with severe-to-profound intellectual disability may struggle to participate in and understand many formal measurements; therefore the assessment of functional abilities, such as through quantification of adaptive behaviors, may be a more appropriate alternative, resulting potentially in more complete data.
While the distribution-based methods used in this study have provided insight regarding the level of change that may be important, the limitations of the approach should be recognized. Distribution-based methods do not connect back to the patient experience of the disease, they are more related to scale precision and can generate inflated estimates (de Vet et al., 2006). Future research should seek to supplement the present results by conducting anchor-based analyses, which may aid decision making surrounding the most appropriate meaningful change estimate for each HRQL measure (de Vet et al., 2006).
Conclusion
To our knowledge, this is the first evaluation using clinical trial data to establish the psychometric properties of the QOLCE, QOLIE-AD-48, and QOLIE-31-P for use in individuals with TSC, while there are some specific aspects across the measures that will require some further validation in future research. Importantly, we generated clinically meaningful change values specific to individuals with TSC. These findings should be useful for clinical practice and next steps in research.
Author Contributions
All authors contributed to the study concept, design, manuscript editing, and final reviewing.
Funding
This work was supported by Novartis Pharmaceuticals Corporation.
Conflict of Interest Statement
PdV reports grants and honoraria from Novartis. All fees have been donated to non-profit organizations. DF reports salary support from Cincinnati Childrens Hospital for consulting work, research grants, and speaker honoraria and travel reimbursement from Novartis. PC reports personal fees for advisory boards from Eisai, Shire, Novartis, and speaker honoraria and travel reimbursement from Novartis. RN reports grants from EU (FP7), Shire, Zogenix, GW Pharma, and Eisai Medical Research, and personal fees from Eisai, Zogenix, and Novartis. MN is an employee of Novartis Pharmaceuticals Corporation. FH is an employee of Novartis Pharma AG. KS, EB and BB are employees of Adelphi Values and were commissioned by Novartis Pharmaceuticals Corporation to conduct this work. JL reports personal fees and research grants from Novartis.
Acknowledgments
We thank the patients and their families, investigators, and the steering committee members of the EXIST-3 study.
References
Benjamin, K., Vernon, M. K., Patrick, D. L., Perfetto, E., Nestler-Parr, S., and Burke, L. (2017). Patient-reported outcome and observer-reported outcome assessment in rare disease clinical trials: an Ispor Coa emerging good practices task force report. Value Health 20, 838–855. doi: 10.1016/j.jval.2017.05.015
Borghs, S., De La Loge, C., and Cramer, J. A. (2012). Defining minimally important change in Qolie-31 scores: estimates from three placebo-controlled lacosamide trials in patients with partial-onset seizures. Epilepsy Behav. 23, 230–234. doi: 10.1016/j.yebeh.2011.12.023
Cappelleri, J. C., Zou, K. H., Bushmakin, A. G., Alvir, J. M. J., Alemayehu, D., and Symonds, T. (2013). Patient-Reported Outcomes: Measurement, Implementation and Interpretation. Boca Raton, FL: Crc Press.
Cramer, J. A., Hammer, A. E., and Kustra, R. P. (2004). Quality of life improvement with conversion to lamotrigine monotherapy. Epilepsy Behav. 5, 224–230. doi: 10.1016/j.yebeh.2003.11.031
Cramer, J. A., Perrine, K., Devinsky, O., Bryant-Comstock, L., Meador, K., and Hermann, B. (1998). Development and cross-cultural translations of a 31-item quality of life in epilepsy inventory. Epilepsia 39, 81–88. doi: 10.1111/j.1528-1157.1998.tb01278.x
Cramer, J. A., Westbrook, L. E., Devinsky, O., Perrine, K., Glassman, M. B., and Camfield, C. (1999). Development of the quality of life in epilepsy inventory for adolescents: the Qolie-Ad-48. Epilepsia 40, 1114–1121. doi: 10.1111/j.1528-1157.1999.tb00828.x
Cronbach, L. J. (1951). Coefficient alpha and the internal structure of tests. Psychometrika 16, 297–334. doi: 10.1007/BF02310555
Curatolo, P., Moavero, R., and de Vries, P. J. (2015). Neurological and neuropsychiatric aspects of tuberous sclerosis complex. Lancet Neurol. 14, 733–745. doi: 10.1016/S1474-4422(15)00069-1
de Vet, H. C., Terwee, C. B., Ostelo, R. W., Beckerman, H., Knol, D. L., and Bouter, L. M. (2006). Minimal changes in health status questionnaires: distinction between minimally detectable change and minimally important change. Health Qual. Life Outcomes 4:54.
de Vries, P. J., Whittemore, V. H., Leclezio, L., Byars, A. W., Dunn, D., Ess, K. C., et al. (2015). Tuberous sclerosis associated neuropsychiatric disorders (Tand) and the Tand Checklist. Pediatr. Neurol. 52, 25–35. doi: 10.1016/j.pediatrneurol.2014.10.004
Elliott, I. M., Lach, L., and Smith, M. L. (2005). I just want to be normal: a qualitative study exploring how children and adolescents view the impact of intractable epilepsy on their quality of life. Epilepsy Behav. 7, 664–678. doi: 10.1016/j.yebeh.2005.07.004
European Medicines Agency (2005). Reflection Paper of the Regulatory Guidance for the use of Health-related Quality of Life (Hrql) Measures in the Evaluation of Medicinal Products. London: European Medicines Agency.
European Organisation for Rare Disorders (2011). Position Paper: Patients’ Priorities and Needs for Rd Research 2014–2020. Available at: http://www.eurordis.org/publication/research-priorities-rare-diseases
Food and Drug Administration (2009). Guidance for Industry: Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. Silver Spring, MD: Food and Drug Administration.
French, J. A., Lawson, J. A., Yapici, Z., Ikeda, H., Polster, T., Nabbout, R., et al. (2016). Adjunctive everolimus therapy for treatment-resistant focal-onset seizures associated with tuberous sclerosis (Exist-3): a phase 3, randomised, double-blind, placebo-controlled study. Lancet 388, 2153–2163. doi: 10.1016/S0140-6736(16)31419-2
Hattie, J., and Cooksey, R. W. (1984). Procedures for assessing the validities of tests using the” known-groups” method. Appl. Psychol. Meas. 8, 295–305. doi: 10.1177/014662168400800306
Hedges, L., and Olkin, I. (1985). Statistical Methods for Meta-Analysis. San Diego, CA: Academic Press.
Henske, E. P., Jozwiak, S., Kingswood, J. C., Sampson, J. R., and Thiele, E. A. (2016). Tuberous sclerosis complex. Nat. Rev. Dis. Primers 2:16035. doi: 10.1038/nrdp.2016.35
Husted, J. A., Cook, R. J., Farewell, V. T., and Gladman, D. D. (2000). Methods for assessing responsiveness: a critical review and recommendations. J. Clin. Epidemiol. 53, 459–468. doi: 10.1016/S0895-4356(99)00206-1
Leclezio, L., and de Vries, P. J. (2016). Towards an improved understanding of Tsc-associated neuropsychiatric disorders (TAND). Adv. Autism 2, 76–83. doi: 10.1108/AIA-12-2015-0025
Matza, L. S., Patrick, D. L., Riley, A. W., Alexander, J. J., Rajmil, L., Pleil, A. M., et al. (2013). Pediatric patient-reported outcome instruments for research to support medical product labeling: report of the Ispor Pro good research practices for the assessment of children and adolescents task force. Value Health 16, 461–479. doi: 10.1016/j.jval.2013.04.004
McEwan, M. J., Espie, C. A., Metcalfe, J., Brodie, M. J., and Wilson, M. T. (2004). Quality of life and psychosocial development in adolescents with epilepsy: a qualitative investigation using focus group methods. Seizure 13, 15–31. doi: 10.1016/S1059-1311(03)00080-3
Norman, G. R., Sloan, J. A., and Wyrwich, K. W. (2003). Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med. Care 41, 582–592. doi: 10.1097/01.MLR.0000062554.74615.4C
Nunnally, J. C., and Bernstein, I. H. (1994). Psychometric Theory, 3rd Edn, New York, NY: McGraw-Hill.
Osborne, J. P., Fryer, A., and Webb, D. (1991). Epidemiology of tuberous sclerosis. Ann. N. Y. Acad. Sci. 615, 125–127. doi: 10.1111/j.1749-6632.1991.tb37754.x
Revicki, D., Hays, R. D., Cella, D., and Sloan, J. (2008). Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J. Clin. Epidemiol. 61, 102–109. doi: 10.1016/j.jclinepi.2007.03.012
Sabaz, M., Cairns, D. R., Lawson, J. A., Nheu, N., Bleasel, A. F., and Bye, A. M. (2000). Validation of a new quality of life measure for children with epilepsy. Epilepsia 41, 765–774. doi: 10.1111/j.1528-1157.2000.tb00240.x
Sabaz, M., Lawson, J. A., Cairns, D. R., Duchowny, M. S., Resnick, T. J., Dean, P. M., et al. (2003). Validation of the quality of life in childhood epilepsy questionnaire in American epilepsy patients. Epilepsy Behav. 4, 680–691. doi: 10.1016/j.yebeh.2003.08.012
Streiner, D. L., Norman, G. R., and Cairney, J. (2015). Health Measurement Scales: A Practical Guide to their Development and Use. Oxford: Oxford University Press. doi: 10.1093/med/9780199685219.001.0001
Terwee, C. B., Bot, S. D., De Boer, M. R., Van Der Windt, D. A., Knol, D. L., Dekker, J., et al. (2007). Quality criteria were proposed for measurement properties of health status questionnaires. J. Clin. Epidemiol. 60, 34–42. doi: 10.1016/j.jclinepi.2006.03.012
Tuberous Sclerosis Alliance (2017). Tuberous Sclerosis Support Group and Discussion Community. Available: https://www.inspire.com/groups/tuberous-sclerosis-alliance/?section=topic&topic-2337=1 [Accessed December 13, 2017].
Wechsler, D., Naglieri, J. A., and Petermann, F. (2006). Wechsler Nonverbal Scale of Ability. San Antonio, TX: PsychCorp.
Wiebe, S., Matijevic, S., Eliasziw, M., and Derry, P. (2002). Clinically important change in quality of life in epilepsy. J. Neurol. Neurosurg. Psychiatry 73, 116–120. doi: 10.1136/jnnp.73.2.116
Keywords: tuberous sclerosis complex, refractory epilepsy, health related quality of life, psychometric properties, QOLCE, QOLIE-AD-48, QOLIE-31-P
Citation: de Vries PJ, Franz DN, Curatolo P, Nabbout R, Neary M, Herbst F, Sully K, Brohan E, Bennett B and Lawson JA (2018) Measuring Health-Related Quality of Life in Tuberous Sclerosis Complex – Psychometric Evaluation of Three Instruments in Individuals With Refractory Epilepsy. Front. Pharmacol. 9:964. doi: 10.3389/fphar.2018.00964
Received: 30 April 2018; Accepted: 03 August 2018;
Published: 30 August 2018.
Edited by:
Jean-Paul Deslypere, Besins Healthcare, ThailandReviewed by:
Kurt Neumann, Independent Researcher, Kerékteleki, HungaryFathi M. Sherif, University of Tripoli, Libya
Copyright © 2018 de Vries, Franz, Curatolo, Nabbout, Neary, Herbst, Sully, Brohan, Bennett and Lawson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Petrus J. de Vries, cGV0cnVzLmRldnJpZXNAdWN0LmFjLnph
 Petrus J. de Vries1*
Petrus J. de Vries1* Paolo Curatolo
Paolo Curatolo Kate Sully
Kate Sully Elaine Brohan
Elaine Brohan Bryan Bennett
Bryan Bennett