- 1Department of Pharmacy, Faculty of Pharmacy, Mahidol University, Bangkok, Thailand
- 2Pharmacy Practice Division, Faculty of Pharmaceutical Sciences, Ubon Ratchathani University, Ubon Ratchathani, Thailand
- 3School of Pharmacy, Monash University Malaysia, Selangor, Malaysia
- 4Department of Medicine, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
- 5Section for Clinical Epidemiology and Biostatistics, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
- 6School of Epidemiology and Preventive Medicine, Monash University, Melbourne, VIC, Australia
- 7School of Public Health, Curtin University, Perth, WA, Australia
- 8Department of Internal Medicine, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand
- 9Center of Pharmaceutical Outcomes Research (CPOR), Naresuan University, Phitsanulok, Thailand
- 10School of Pharmacy, Monash University Malaysia, Selangor, Malaysia
- 11School of Pharmacy, University of Wisconsin, Madison, WI, United States
- 12Asian Centre for Evidence Synthesis in Population, Implementation and Clinical Outcomes (PICO), Health and Well-being Cluster, Global Asia in the 21st Century Platform, Monash University Malaysia, Bandar Sunway, Selangor, Malaysia
Background: Patients undergoing percutaneous coronary intervention (PCI) who require anticoagulant therapy are at increased risk of bleeding. The optimal regimen for these patients is uncertain. This study aimed to compare safety and efficacy of antithrombotic regimens used in patients undergoing PCI with concomitant anticoagulant therapy.
Methods: A systematic review and network meta-analysis was performed among studies comparing antithrombotic regimens for anticoagulated patients undergoing PCI. The primary outcome of interest was major bleeding. The secondary outcomes were coronary events. The reference intervention was classic triple therapy (aspirin plus clopidogrel plus VKA). Cluster rank incorporating risk (major bleeding) and benefit (all-cause death) was performed to identify the most appropriate regimen(s).
Results: There were 3 RCTs (6 interventions) and 29 non-RCTs (8 interventions) that met the inclusion criteria with 22,179 patients. Network meta-analysis of RCTs indicated that dual therapy (DT), either with vitamin K antagonist (VKA) or direct anticoagulant (DOAC) plus an antiplatelet, significantly reduced the risk of major bleeding compared to triple therapy (TT) [pooled RR of 0.51 (0.30–0.87) and 0.68 (0.49–0.94), respectively]. In addition, VKA-DT significantly reduced the risk of all-cause death compared to TT [pooled RR of 0.40 (0.17–0.93)]. Results from network meta-analysis of non-RCT paralleled that of RCTs. No significant differences of coronary events were found.
Conclusions: In conclusion, for anticoagulated patients undergoing PCI, dual therapy, either with warfarin or DOAC plus an antiplatelet, should be considered due to its optimal balance on efficacy and safety.
Introduction
Among patients who have an indication for anticoagulant therapy, approximately one third concurrently suffer from coronary artery disease where percutaneous coronary intervention (PCI) may be indicated (Dewilde et al., 2014). This situation therefore leads to a need for the concomitant use of both antiplatelet(s) and anticoagulant therapy which poses heighten risk of major bleeding (Rubboli et al., 2014a). A recent national registry suggested that the rates of fatal or nonfatal bleeding among atrial fibrillation patients admitted with myocardial infarction or for PCI significantly increased with increasing intensity of anti-thrombotic regimens. Patients receiving triple therapy [(TT): dual antiplatelet therapy (DAPT) plus an oral anticoagulant] experienced the highest bleeding rate at 14.2 events per 100 person-years with adjusted hazard ratio (HR) of 1.41 compared to dual therapy [(DT): vitamin K antagonist (VKA) plus single anti-platelet] (Lamberts et al., 2012). Major bleeding has been shown to increase 1-year mortality by several folds among PCI patients (Rao et al., 2005; Manoukian et al., 2007), most likely due to significant blood loss, hemodynamic compromise, or ischemic events secondary to the interruption or cessation of anti-thrombotic therapy.
The current situation is even more complex due to increasing usage of more potent P2Y12 inhibitors, namely prasugrel, and ticagrelor, and direct oral anticoagulants (DOAC), namely dabigatran, rivaroxaban, apixaban, and edoxaban. Unfortunately, direct head-to-head trials comparing efficacy and safety of these combinations are very limited despite the magnitude of the problem. Current practice guidelines are therefore based on limited data and expert consensus, recommending the use of a TT with aspirin, clopidogrel and an oral anticoagulant as the standard therapy. DT with clopidogrel and an oral anticoagulant is also recommended as an alternative for patients in whom the bleeding risk outweighs the ischemic risk (Kirchhof et al., 2016; Levine et al., 2016; Roffi et al., 2016; Valgimigli et al., 2018). However, recommendations regarding the use of newer antiplatelets and anticoagulants as a part of these regimens are still limited. We therefore performed a systematic review and network meta-analysis, where possible, to evaluate the relative efficacy and safety among various antithrombotic regimens.
Methods
Study Design
This study was conducted following the registered protocol with PROSPERO (CRD 42017052655) and was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) extension statement for network meta-analysis (Hutton et al., 2015). The study protocol was approved by the Institutional Review Board of Mahidol University (COE.No. MU-DT/PY-IRB 2017/022.2906).
Search Strategy and Study Selection
Relevant studies were identified from MEDLINE (via PubMed), Embase, Cochrane Central Register of Control Trials (CENTRAL) and ClinicalTrials.gov since inception to October 1, 2017 the following search terms were used: “PCI,” stent, “acute coronary syndrome,” “myocardial infarction,” revascularization, anticoagulant, antithrombotic, “dual antithrombotic,” “P2Y12 receptor antagonist*,” generic and trade names of antithrombotic agents (coumarins, warfarin, dabigatran, rivaroxaban, apixaban, edoxaban, aspirin, clopidogrel, prasugrel, ticagrelor), and synonymous words. Search strategies were described in Supplementary Appendix 1. Two investigators (W.B. and P.J.) independently performed the study selection. The reviewers independently screened titles and abstracts. Discrepancies were resolved by discussion. Reference lists of selected articles were also reviewed, and efforts to contact authors were made to obtain further study details. Both randomized controlled trials (RCTs) and non-RCTs were considered without language restrictions using the following criteria: (1) studied in patients who underwent PCI and received anticoagulants for prevention or treatment of thromboembolic complications (2) compared efficacy and safety among any pair of antithrombotic regimens (DAPT, DT (aspirin or a P2Y12 receptor antagonist plus an anticoagulant), and TT (aspirin plus a P2Y12 receptor antagonist plus an anticoagulant). Studies were excluded if the period of outcome measurement was < 1 month.
Data Extraction and Quality Assessment
Data were extracted including study design, baseline characteristics (e.g., age, underlying diseases, details of PCI procedure, and indications of anticoagulant therapy), antithrombotic regimens both in terms of composition and drug utilization, and outcomes of interest. Authors were contacted in case of incomplete or unclear data. Quality of studies was assessed depending on type of studies. For RCTs, the Cochrane Collaboration's tool for assessing risk of bias (ROB) was used (Higgins et al., 2016). This tool is comprised of 5 domains addressing biases in the randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. Meanwhile, the ROB in Non-randomized Studies tool (ROBINS-I) was used for non-RCTs. ROBINS-I is comprised of 7 domains addressing biases due to confounding, selection of participants, classification of interventions, deviations from intended interventions, missing data, measurement of outcomes and selection of the reported result (Sterne et al., 2016).
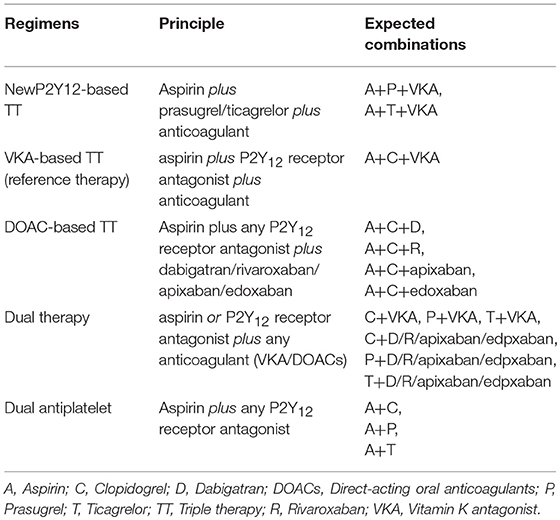
Type of Interventions and Reclassification of Regimens
Treatment regimens were combinations within/between drug classes including antiplatelet agents [aspirin (A), clopidogrel (C), ticagrelor (T), prasugrel (P)], and anticoagulants [VKA (e.g., warfarin, acenocoumarol, phenprocoumon), low molecular weight heparin (LMWH), dabigatran 150 mg BID (D), dabigatran 110 mg BID (d), rivaroxaban 15 mg OD (R), and rivaroxaban 2.5 mg BID (r)]. In addition, we reclassified these intervention into groups based on composition of regimens (Table 1). For RCT, we were able to classify regimens into 3 groups including TT (aspirin plus clopidogrel plus a VKA), VKA-DT (aspirin or a P2Y12 receptor antagonist plus a VKA) and DOAC-DT (aspirin or a P2Y12 receptor antagonist plus rivaroxaban or dabigatran). The reclassification is based on differences in the pharmacological profiles of anticoagulants (between VKAs vs. direct acting oral anticoagulants) and the intensity of antithrombotic therapy (TT vs. DT). We excluded two regimens of RCT from analysis. First was the regimen of aspirin plus clopidogrel plus 2.5 mg BID dose of rivaroxaban from PIONEER-AF PCI trial since this dose was an unapproved dose for stroke prevention (Gibson et al., 2016). Second, we extracted data from clopidogrel plus dabigatran 150 mg BID and its corresponding control arm but not from the 110 mg BID arms of the REDUAL-PCI trial. This was due to the fact that the control arm of each dabigatran dose was from the same pool of patient population with some adjustment in number and characteristics of the patients. If we included the data from both doses, it may create duplication of control arms (Cannon et al., 2017). We therefore chose to extract data from dabigatran 150 mg BID which is the most commonly approved dose worldwide. For non-RCT, based on the available data, we were able to reclassify interventions into 4 regimens including TT (aspirin plus clopidogrel plus an anticoagulant), newP2Y12TT (aspirin plus either prasugrel or ticagrelor plus an anticoagulant), VKA-DT (aspirin or a P2Y12 receptor antagonist plus a VKA), and DAPT (aspirin plus a P2Y12 receptor antagonist). With these reclassified regimens, we were able to evaluate the effects of newer antithrombotic therapy compared to the conventional regimens, which may potentially extend our knowledge beyond current clinical practice guidelines (Kirchhof et al., 2016; Levine et al., 2016; Roffi et al., 2016; Valgimigli et al., 2018).
Outcomes of Interest
The primary endpoint was major bleeding which was defined according to Bleeding Academic Research Consortium (BARC) type 3–5 (Mehran et al., 2011), and “compatible definition” if those could be standardized based on BARC type 3–5 criteria (see details of compatibility criteria in Supplementary Appendix 2). The secondary endpoints were stroke and/or systemic embolism, myocardial infarction, repeated revascularization, any stent thrombosis and all-cause death. In addition, we investigated the risk-benefit balance of various interventions by incorporating safety (major bleeding) and efficacy (all-cause death) using two-dimensional plots and clustering methods to rank these interventions. All-cause death was used as the efficacy outcome due to the lack of uniformity in the report of major cardiovascular events.
Data Synthesis and Statistical Methods
A pairwise meta-analysis and network meta-analysis were performed as follows. A pairwise meta-analysis, risk ratio (RR) along with 95% confidence interval (CI) was estimated and pooled using a random-effects model (DerSimonian and Laird, 1986). Heterogeneity was assessed using Cochrane Q test and I2 statistics (Maldonado et al., 2009). A network meta-analysis was performed to compare relative efficacy and safety among regimens. Relative treatment effects (RR) were estimated for each comparison vs. a common comparator of A+C+VKA or TT for re-classified. Subsequently, these RRs were pooled across studies using a meta-analysis with a consistency model (Jansen et al., 2011). We used the global inconsistency test to evaluate inconsistency in a network as a whole. If inconsistency was detected, we then used the loop-specific and node-splitting methods to identify which piece of evidence was responsible for inconsistency (Dias et al., 2010). Adjusted funnel plots were produced in order to determine small study effects (Mavridis and Salanti, 2014). The surface under the cumulative ranking curve (SUCRA) was performed to rank various antithrombotic regimens for each outcome. Finally, the cluster rank, a technique used to combine multidimensional aspects (usually risk and benefit) of an intervention, was performed to incorporate safety (major bleeding) and efficacy (all-cause death) simultaneously (Jinatongthai et al., 2017). The same approaches were used to compare 5 re-classified regimens including TT, newP2Y12TT, VKA-DT, DOAC-DT, and DAPT.
Pre-specified subgroup analyses were performed by patient characteristics (atrial fibrillation and atrial fibrillation predominant group, follow-up period (< 1, 1, >1 year), PCI-related predominant characteristics [i.e., ACS, elective PCI, bare-metal stent (BMS), drug-eluting stent (DES)], and study characteristics (study design and setting). Predominant groups were classified if characteristic prevalence was ≥50%. A pre-specified sensitivity analysis was performed on different major bleeding definitions and certain characteristics of studies (i.e., adjusted analysis, multicenter studies, omitting small sample size studies or serious-to-critical ROB). All analyses were stratified by non-RCTs and RCTs using STATA 14.0 (Stata Corp, College Station, TX). A p < 0.05 was considered statistically significant.
Results
Study Selection
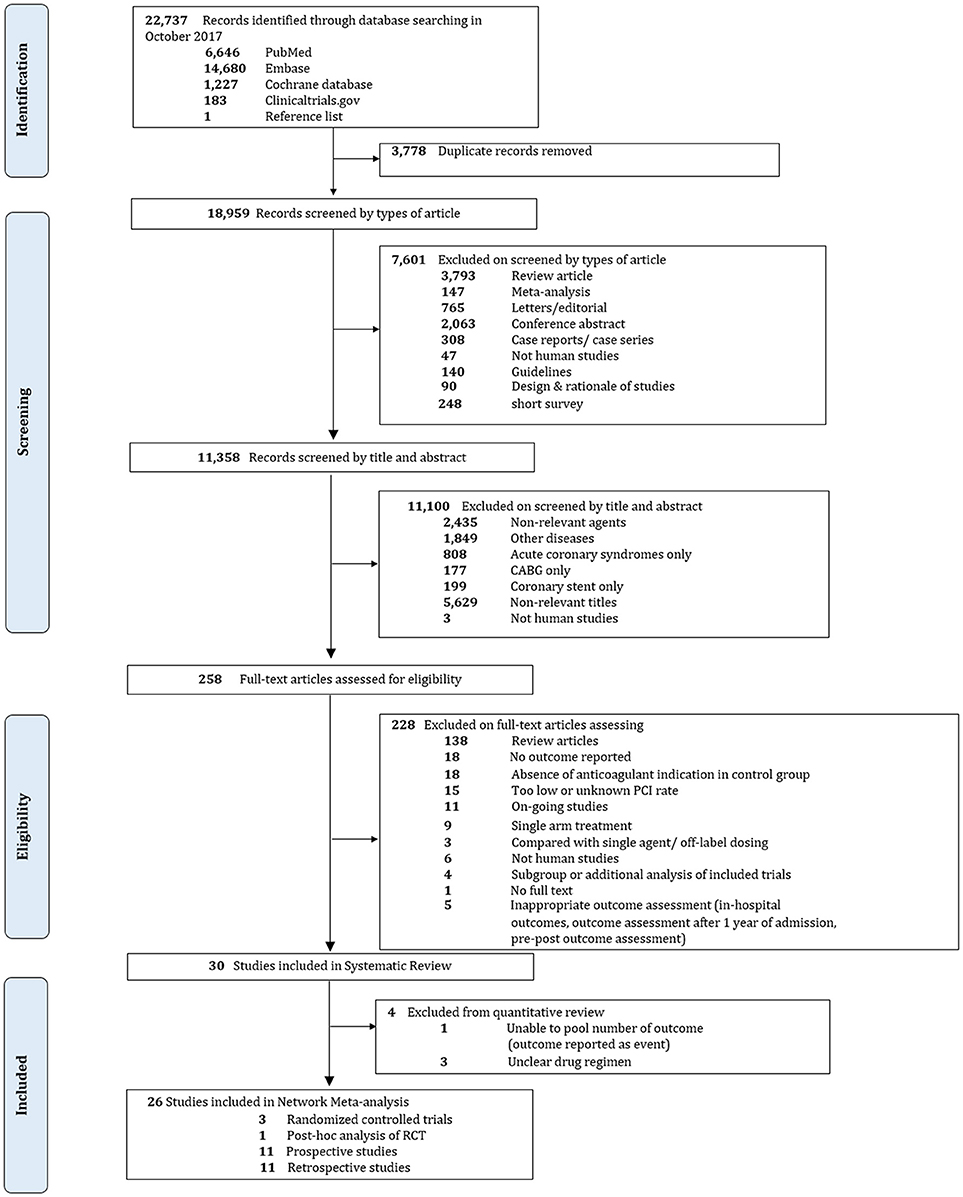
Overall, 22,737 records were identified, 258 potentially eligible articles were retrieved in full text. 200 and 28 articles were excluded, mostly due to absence of anticoagulant indication in the control group, contamination of patients without PCI or unknown rate of PCI, and no outcome of interest. Finally, 30 studies were included in our systematic review including 3 RCTs and 27 non-RCTs. Among 27 non-RCTs, only 23 studies were included in the quantitative analysis since 3 studies did not sufficiently specify composition of drug regimens while one study did not provide adequate outcome data. The PRISMA flow diagram is shown in Figure 1.
Characteristics and Quality of Included Studies
A total of 12 antithrombotic regimens were identified including 1 DAPT (A+C), 6 DTs (A+VKA, C+VKA, T+VKA, C+R, C+D, C+d), and 5 TTs (A+C+VKA, A+P+VKA, A+T+VKA, A+C+r, A+C+LMWH). Detail of each regimen was summarized in Supplementary Appendix 3. Characteristics of all included studies are shown in Table 2.
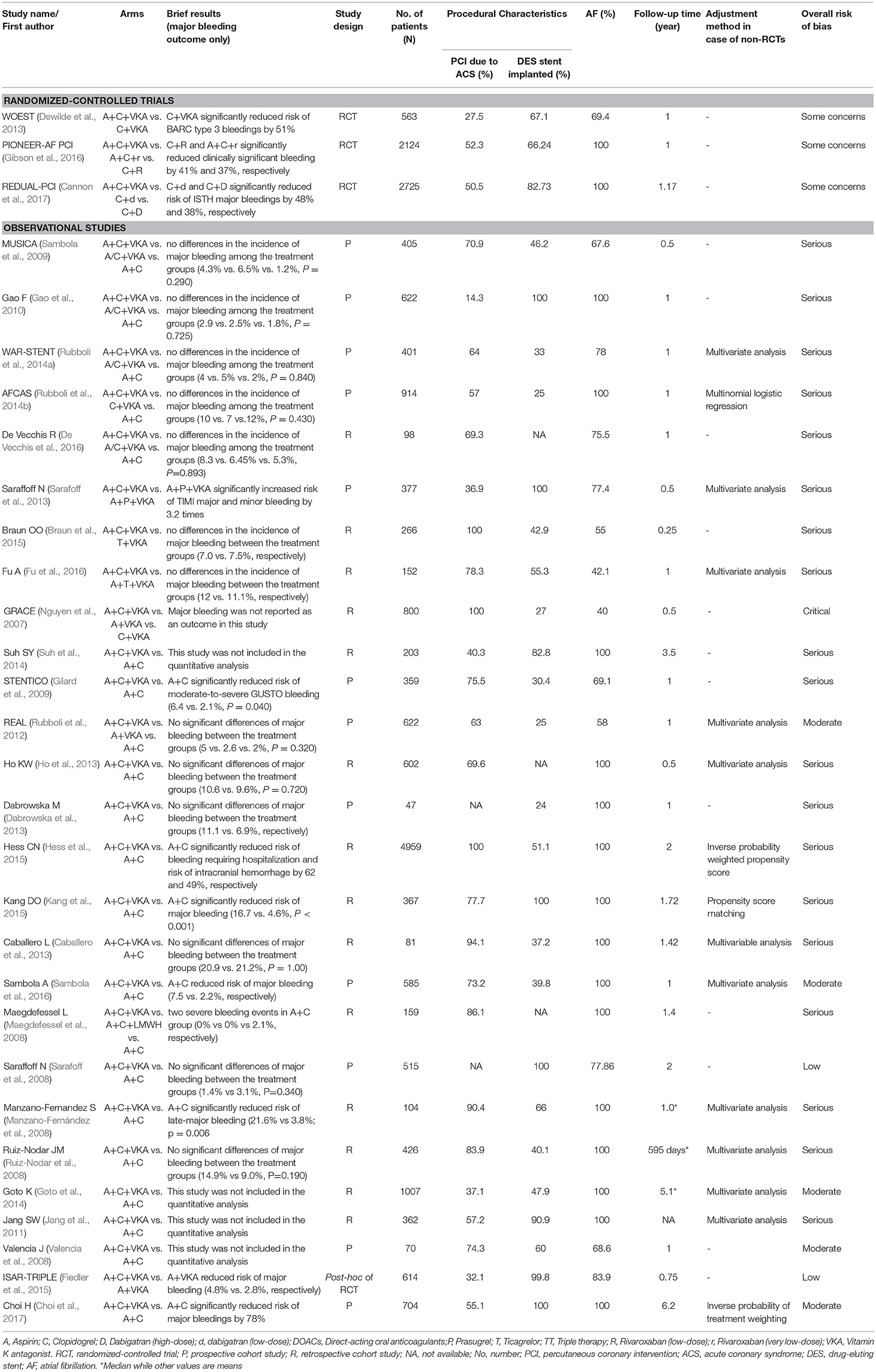
For 3 RCTs involving 5,412 patients, 1 trial was 2-arm RCT comparing A+C+VKA vs. C+VKA (Dewilde et al., 2013) while the others were 3-arm RCT comparing A+C+VKA vs. C+R vs. A+C+r (Gibson et al., 2016) and A+C+VKA vs. C+D vs. C+d (Cannon et al., 2017). Study settings, applied treatment regimens and patient baseline characteristics of these trials are summarized in Supplementary Appendix 4: eTables 4.1–4.5. Quality of included RCTs based on Cochrane ROB tool was assessed, which suggested some concerns with all trials. Cause of bias in these open-labeled trials was mainly due to lack of data about protocol deviations (Supplementary Appendix 4: eTable 4.6).
Among 27 non-RCTs, there was 1 post-hoc analysis of RCT (Fiedler et al., 2015), 12 prospective cohorts (Sarafoff et al., 2008, 2013; Valencia et al., 2008; Gilard et al., 2009; Sambola et al., 2009, 2016; Gao et al., 2010; Rubboli et al., 2012, 2014a,b; Dabrowska et al., 2013; Choi et al., 2017), and 14 retrospective cohorts (Nguyen et al., 2007; Maegdefessel et al., 2008; Manzano-Fernández et al., 2008; Ruiz-Nodar et al., 2008; Jang et al., 2011; Caballero et al., 2013; Ho et al., 2013; Goto et al., 2014; Suh et al., 2014; Braun et al., 2015; Hess et al., 2015; Kang et al., 2015; De Vecchis et al., 2016; Fu et al., 2016). Nineteen studies were 2-arm (Manzano-Fernández et al., 2008; Ruiz-Nodar et al., 2008; Sarafoff et al., 2008, 2013; Valencia et al., 2008; Gilard et al., 2009; Jang et al., 2011; Caballero et al., 2013; Dabrowska et al., 2013; Ho et al., 2013; Goto et al., 2014; Suh et al., 2014; Braun et al., 2015; Fiedler et al., 2015; Hess et al., 2015; Kang et al., 2015; Fu et al., 2016; Sambola et al., 2016; Choi et al., 2017) and 8 studies were 3-arm comparisons (Nguyen et al., 2007; Maegdefessel et al., 2008; Sambola et al., 2009; Gao et al., 2010; Rubboli et al., 2012, 2014a,b; De Vecchis et al., 2016). All studies used A+C+VKA as the reference. A total of 8 interventions were considered including A+C, A+VKA, C+VKA, T+VKA, A+C+VKA, A+P+VKA, A+T+VKA, and A+C+LMWH. Study settings and patient baseline characteristics of these studies are summarized in Supplementary Appendix 5: eTables 5.1–5.4. Various patterns of regimens used were found, especially duration of treatment (Supplementary Appendix 5: eTable 5.5). Among non-RCTs, 7, 19, 70, and 4% of studies were with low, moderate, serious, and critical risk, respectively (Supplementary Appendix 5: eTable 5.6).
Effect on the Primary and Secondary Outcomes
RCTs
Since there were only 3 RCTs including WOEST, PIONEER AF-PCI and REDUAL-PCI, meta-analysis on RCTs was not performed since there were too few trials. However, data from these trials were extracted and used to compare 3 re-classified regimens. Results of which are reported in the re-classified regimen section (Dewilde et al., 2013; Gibson et al., 2016; Cannon et al., 2017).
Non-RCTs
Results from Pairwise Meta-Analysis
For major bleeding, A+C significantly reduced risk of bleeding while A+P+VKA increased such risk compared to A+C+VKA with pooled RR 0.58 (0.40, 0.83) and pooled RR 5.00 (1.52, 16.67), respectively. For stroke, A+C increased risk of any stroke compared to A+C+VKA, with pooled RR 1.60 (1.04, 2.45). Overall, there was no statistically significant difference among these regimens in the risk of myocardial infarction, repeated revascularization, and stent thrombosis. For all-cause death, A+C+LMWH significantly increased the risk relative to A+C with pooled RR 4.17 (1.02, 16.67) (Supplementary Appendix 6).
Results from Network Meta-Analysis
The network of eligible comparisons for the primary outcome and secondary outcomes are shown in Figure 2. Global inconsistency was not found in each outcome (Supplementary Appendix 7). The pooled estimates of all outcomes were then based on consistency model.
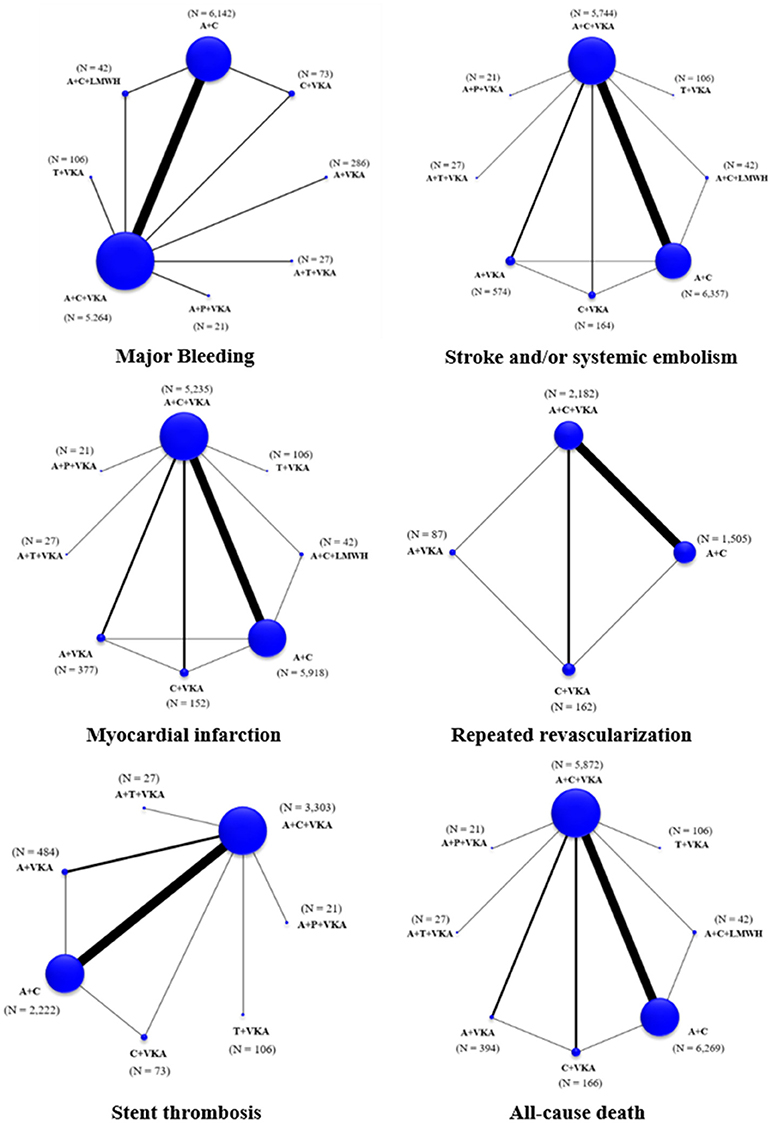
Figure 2. Network maps of treatment options for all outcomes. A+C, aspirin+clopidogrel; A+C+LMWH, aspirin+clopidogrel+low-molecular weight heparin; A+C+VKA, aspirin+clopidogrel+vitamin K antagonist; A+P+VKA, aspirin+prasugrel+vitamin K antagonist; A+T+VKA, aspirin+ticagrelor+vitamin K antagonist; A+VKA, aspirin+vitamin K antagonist; C+VKA, clopidogrel+vitamin K antagonist; T+VKA, ticagrelor+vitamin K antagonist.
A total of 17 studies (n = 11,961) consisting of 8 interventions reported major bleeding as BARC type 3–5 or compatible definitions (Supplementary Appendix 2). Results from network meta-analysis showed that A+C significantly reduced risk of major bleeding with a pooled RR of 0.57 (0.39–0.84) while A+P+VKA significantly increased such risk with a pooled RR of 5.09 (1.10–23.44) when compared to A+C+VKA. For stroke, network meta-analyses indicated that A+C significantly increased stroke risk compared to A+C+VKA with the pooled RR of 1.69 (1.06–2.68), respectively. For myocardial infarction, repeated revascularization, and stent thrombosis, there was no statistically significant difference among all regimens in these outcomes. For all-cause death, network meta-analysis showed that A+C+LMWH significantly increased risk of all-cause death compared to C+VKA, pooled RR of 4.55 (1.08,20). The forest plot for all outcomes compared to the reference therapy (A+C+VKA) is shown in Figure 3. Further information and ranking can be found in Supplementary Appendix 8–9. For a combination of risk-benefit outcomes, the cluster rank incorporating major bleeding and all-cause death showed that A+T+VKA and C+VKA were the best regimens. A+T+VKA had the lowest mortality risk while C+VKA had the lowest risk of major bleeding. A+C+LMWH and A+P+VKA were the worst regimens since A+C+LMWH was with the highest mortality risk while A+P+VKA was with the highest risk of major bleeding (Figure 4).
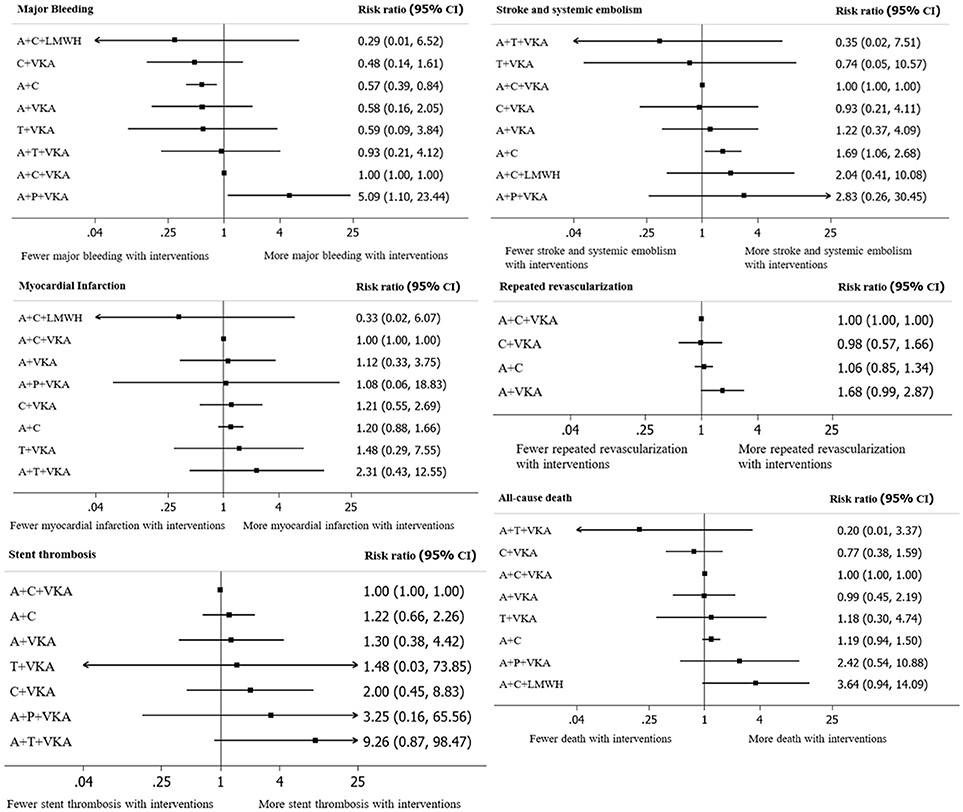
Figure 3. A forest plot of network meta-analysis of interventions compared with classic triple therapy (A+C+VKA). A+C, aspirin+clopidogrel; A+C+LMWH, aspirin+clopidogrel+low-molecular weight heparin; A+C+VKA, aspirin+clopidogrel+vitamin K antagonist; A+P+VKA, aspirin+prasugrel+vitamin K antagonist.
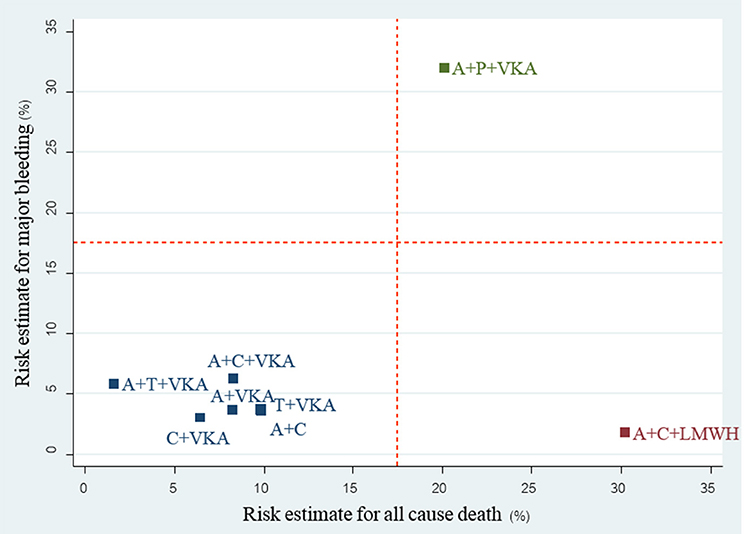
Figure 4. cluster rank incorporating risk estimate of major bleeding vs. all-cause death outcomes: main analysis (non-RCTs). A+C, aspirin + clopidogrel; A+C+LMWH, aspirin + clopidogrel + low-molecular weight heparin; A+C+VKA, aspirin + clopidogrel + vitamin K antagonist; A+P+VKA, aspirin + prasugrel + vitamin K antagonist; A+T+VKA, aspirin + ticagrelor + vitamin K antagonist; A+VKA, aspirin + vitamin K antagonist; C+VKA, clopidogrel + vitamin K antagonist; T+VKA, ticagrelor + vitamin K antagonist.
Re-classified Regimen Analysis
For the 3 available RCTs including WOEST, PIONEER AF-PCI and REDUAL-PCI (Dewilde et al., 2013; Gibson et al., 2016; Cannon et al., 2017), we were able to compare 3 re-classified regimens including TT (A+C+VKA) vs. VKA-DT (C+VKA) vs. DOAC-DT (C+R, C+D). Results of network meta-analysis indicated that both VKA-DT and DOAC-DT significantly reduced the risk of major bleeding compared to TT [pooled RR of 0.51 (0.30–0.87); p = 0.014 and 0.68 (0.49–0.94); p = 0.02, respectively]. For stroke, myocardial infarction and stent thrombosis, there were no significant differences among these 3 regimens. However, VKA-DT significantly reduced the risk of all-cause death compared to TT [pooled RR of 0.40 (0.17–0.93); p = 0.034] (Supplementary Appendix 10). The cluster rank incorporating major bleeding and all-cause death showed that VKA-DT was the best regimens (Figure 5).
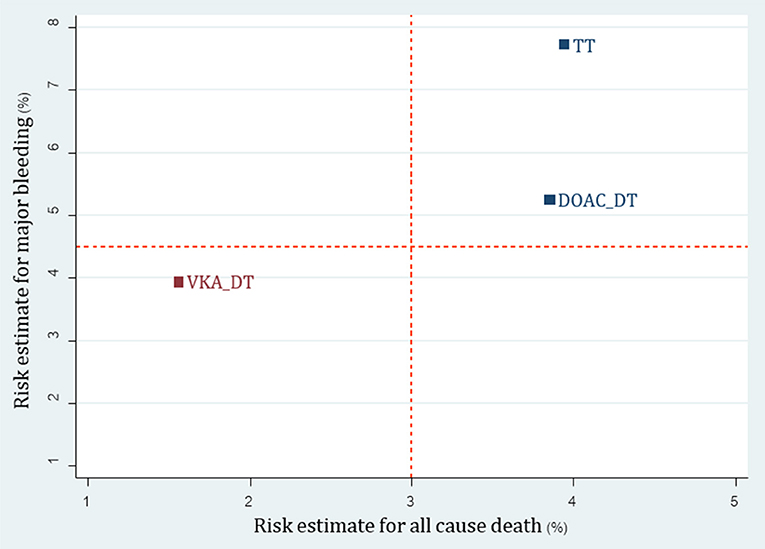
Figure 5. Cluster rank incorporating risk estimate of major bleeding vs. all-cause death outcomes: analysis of reclassified regimens among RCTs. DOAC, Direct-acting oral anticoagulant; DT, Dual therapy; TT, Triple therapy; VKA, Vitamin K antagonist.
For 23 non-RCTs, we reclassified 8 interventions into 4 groups including TT (A+C+VKA, A+C+LMWH), newP2Y12-based TT (A+P+VKA, A+T+VKA), VKA-DT (A+VKA, C+VKA, T+VKA), and DAPT (A+C). For major bleeding, SUCRA ranking showed that DAPT was the best regimen followed by DT, TT, and newP2Y12-based TT (Supplementary Appendix 11: eTable 11.1 and eFigure 11.1). Among efficacy outcomes, no statistical differences were found except increased risk of stroke from DAPT compared to TT with pooled RR 1.65 (1.08, 2.51) (Supplementary Appendix 11: eTable 11.2, eFigure 11.2). Based on the cluster rank of risk-benefit outcome, the best regimen was still VKA-DT in non-RCTs group (Figure 6).
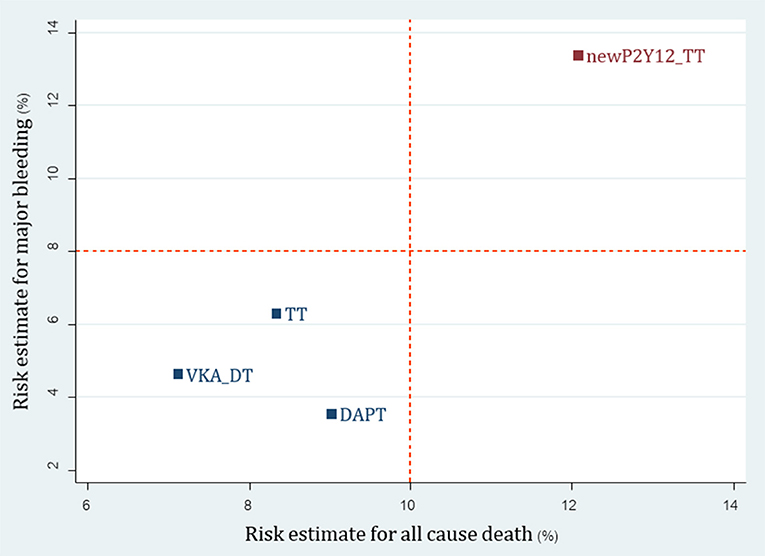
Figure 6. Cluster rank incorporating risk estimate of major bleeding vs. all-cause death outcomes: analysis of reclassified regimens among non-RCTs. DAPT, Dual antiplatelet; DT, Dual therapy; TT, Triple therapy; newP2Y12TT, New P2Y12 inhibitor-based triple therapy; VKA, Vitamin K antagonist.
Subgroup Analyses
For primary outcomes, all results from subgroup analyses (AF predominant group, ACS predominant group, stented with DES predominant group, and variety of period of follow-up) were consistent with the results in main analysis (Supplementary Appendix 12).
Sensitivity Analyses and Publication Bias
Sensitivity analyses with different types of major bleeding definitions were consistent with the main results (Supplementary Appendix 13: eTable 13.1). According to stroke subtypes, analysis showed that A+C reduced risk of hemorrhagic stroke compared to A+C+VKA and A+P+VKA with pooled RR 0.38 (0.16, 0.92) and pooled RR 0.02 (0.00, 0.48), respectively. We also performed sensitivity analysis among studies with adjusted RR, the results was consistent with main results. Further information from sensitivity analyses can be found in Supplementary Appendix 13. There was no clear evidence of small study effect, based on a lack of asymmetry shown in adjusted funnel plot analysis (Supplementary Appendix 14).
Discussion
This systematic review and network meta-analysis attempts to address one of the most controversial issues in the PCI era. Accessibility of PCI is increasing worldwide, however, appropriate drug combinations for patients whom anticoagulant therapy is indicated are still unknown. The current treatment guidelines recommend the TT of aspirin, clopidogrel and an oral anticoagulant as the standard therapy (Kirchhof et al., 2016; Levine et al., 2016; Roffi et al., 2016; Valgimigli et al., 2018). However, this regimen has been shown to increase the risk of major bleeding by 40%, and among those who suffered major bleeding, there were several fold increased in mortality (Lamberts et al., 2012). The magnitude of this problem is clearly evident since approximately one third of patients requiring anticoagulant therapy may require PCI (Dewilde et al., 2014). Attempts therefore have been made to find an alternative regimen that can prevent both stroke and coronary events while minimize the risk of bleeding. Based on several RCTs and observational studies, DT with clopidogrel and an oral anticoagulant has been shown to reduce bleeding. However, its ability to reduce both stroke and coronary events is less than certain since all trials did not have sufficient power to detect differences in stroke and coronary events. Despite such limitation, the current practice guidelines still recommend DT as a viable option in patients with high bleeding risk. In addition, data regarding the newer antiplatelets and anticoagulants were quite limited at the time when the guidelines were written. Therefore, a more comprehensive and updated analysis is needed to answer some of these issues.
To the best of our knowledge, our study is the first that compared regimens containing DOACs and new P2Y12 inhibitors using network meta-analysis. In addition, we tried to overcome the issue of varied bleeding definitions across studies by matching the definition of bleeding events reported in each trial with the BARC definition before including those studies into the primary outcome analysis. We also performed sensitivity analyses to assess the robustness of our conclusions based on different major bleeding definitions (Mehran et al., 2011). This is a key strength of this analysis compared to previous works.
Based on analysis of non-RCTs, A+P+VKA increased risk of major bleeding compared to most regimens. Furthermore, A+P+VKA tended to increase risk of hemorrhagic stroke in sensitivity analysis based on type of stroke. This finding paralleled the result of the TRITON-TIMI-38 (Wiviott et al., 2007). Although population and drug regimens in our analysis and TRITON-TIMI 38 are not identical, caution must be raised regarding the employment of prasugrel-based regimen.
Our results for both pairwise and network meta-analyses indicated that A+C showed the lowest risk of major and any bleedings but it increased risk of stroke (RR = 1.69, 1.06–2.68). In ACTIVE-W trials, which investigated efficacy of this regimen vs. warfarin in patients with atrial fibrillation, A+C showed higher risk of stroke compared to warfarin (RR = 1.44, 1.18–1.76) (Connolly et al., 2006). Therefore, our result confirms the beneficial effect of anticoagulant therapy in patients with high thromboembolic risk.
Although we found no differences among all regimens in coronary outcomes, A+C+VKA was the most efficacious regimen in coronary outcomes based on SUCRA ranking. Risk-benefit outcome incorporating major bleeding and all-cause death showed that A+T+VKA and C+VKA were the most appropriate regimens. However, we caution readers to consider interpreting this finding carefully. The data of A+T+VKA was based entirely on a small observational study with only 27 patients using this intervention (Fu et al., 2016). In addition, beneficial effects of C+VKA among non-RCTs parallel the result of C+VKA in WOEST trial in terms of all-cause death reduction (Dewilde et al., 2013). As a result, C+VKA may be the best regimen based on our analysis.
With network meta-analysis and reclassification of antithrombotic regimens, we were able to perform analysis on the safety and efficacy of new P2Y12-based TT and DOACs-DT compared to conventional regimens. Based on RCTs, we were able to show that DOAC-DT significantly reduce major bleeding compared to TT and ranked favorably compared to TT when considering both major bleeding and all-cause death. Although VKA-DT was ranked best in risk-benefit outcome, we cautioned readers that this may be due to different patient characteristics along with types of antiplatelet used in WOEST compared to other trials. While all patients in both PIONEER AF-PCI and REDUAL-PCI were on anticoagulant therapy for at least 1 year, only 90% of patients in WOEST were on anticoagulant for 1 year. This may be due to the fact that WOEST trial included patients who were on anticoagulation for shorter term such as venous thromboembolism and apical thrombus. With shorter duration of treatment, bleeding rates may be lower compared to patients requiring life-long therapy in both PIONEER AF-PCI and REDUAL-PCI trials. In addition, newer and more potent antiplatelets were used in both PIONEER AF-PCI and REDUAL-PCI while clopidogrel was exclusively used in WOEST trial. Both issues may partly explain higher bleeding rates in PIONEER AF-PCI and REDUAL-PCI. For non-RCTs, cluster rank indicated that a new P2Y12-based TT may not be an appropriate option due to higher risk of both bleeding and all-cause death compared to all other regimens.
Prior to our study, there were a number of meta-analyses and one network meta-analysis evaluating the same issue (Bavishi et al., 2016; Briasoulis et al., 2016; Palla et al., 2016). Results from pairwise meta-analyses were with conflicting results. This is most likely due to difference of included studies and definition of major bleeding in each meta-analysis. For network meta-analysis, Liu et al. previously compared efficacy and safety of DAPT, A+VKA, C+VKA, and A+C+VKA (Liu et al., 2016). Our analysis was different in many aspects. First, we considered and included newer agents that have become increasingly used in clinical practice such as new P2Y12 inhibitors and DOACs. Second, we only included trials which all patients received PCI, while the previous study included trials which contained some populations who did not undergo PCI. Finally, previous analysis accepted major bleeding definition according to the original articles while we standardized bleeding based on the BARC definition.
Study Limitations
Our study has several key limitations. First, the majority of the data included in our analysis came from observational studies. Therefore, relative treatment effects were susceptible to the influence of confounding factors. Second, analysis of baseline characteristics and subgroup analyses were based on data of study level, not individual patient data level. Therefore, we could assess the data as “predominant characteristics,” which mean some contamination existed in some subgroup analyses. The reclassification of BARC bleeding was also done at a study level, not patient data level. Thirdly, due to a sparse number of studies of each combination, except A+C and A+C+VKA, our results depended mainly upon indirect comparisons from the network meta-analysis. Some findings were statistically significant with wide confidence intervals due to small sample size in each individual study. Therefore, results of our study are for hypothesis generation only. Lastly, we were unable to make any adjustment on the variation of treatment duration of each regimen. This may introduce heterogeneity on treatment duration of each regimen since regimen switching cannot be ruled out. Therefore, we could not avoid contaminating treatment effect at the point of outcome measurement in many studies. In addition, lack of information on time in therapeutic range for VKA therapy in each study may potentially affect the outcome. These limitations highlight the need for more high quality evidence for this controversial issue. Currently, there are several RCT being conducted to assess the efficacy and safety of DOACs and new P2Y12 inhibitor-based regimen including AUGUSTUS with apixaban, ENTRUST-AF PCI with edoxaban and MANJUSRI with ticagrelor (Lu et al., 2015; Vranckx et al., 2018). Results from these upcoming RCTs will add more information in the future. Until those high quality data become available, our systematic review may offer the most comprehensive data set and provide some guidance to tackle this issue.
Conclusion
In summary, our analysis shows that dual therapy, either with VKA or DOAC plus a single antiplatelet, may be an attractive option for patients with PCI whom anticoagulant are indicated. DT may offer an optimal balance on safety and efficacy by lowering risk of bleeding while maintaining antithrombotic effects both from stroke/systemic embolism and coronary events post PCI, compared to TT. However, more trials are warranted to clarify this issue.
Author Contributions
WB was responsible for concept and design, analysis, interpretation of data, critical writing, and final approval of the manuscript. PJ was responsible for concept and design, analysis, and final approval of the manuscript. PV was responsible for concept and design and final approval of the manuscript. AT was responsible for concept and design, revising the intellectual content, and final approval of the manuscript. CR was responsible for revising the intellectual content, and final approval of the manuscript. WW was responsible for concept and design, revising the intellectual content, and final approval of the manuscript. NC was responsible for concept and design, interpretation of data, revising the intellectual content, and final approval of the manuscript. SN was responsible for concept and design, interpretation of data, critical writing, revising the intellectual content, and final approval of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2018.01322/full#supplementary-material
Abbreviations
A+C, Aspirin + Clopidogrel; A+C+LMWH, Aspirin + Clopidogrel + Low-molecular weight heparin; A+C+r, Aspirin + Clopidogrel + Rivaroxaban 5 mg twice daily; A+C+VKA, Aspirin + Clopidogrel + Vitamin K antagonist; A+P+VKA, Aspirin + Prasugrel + Vitamin K antagonist; A+T+VKA, Aspirin + Ticagrelor + Vitamin K antagonist; A+VKA, Aspirin + Vitamin K antagonist; BARC, Bleeding Academic Research Consortium; C+D, Clopidogrel + Dabigatran 150 mg twice daily; C+d, Clopidogrel + Dabigatran 110 mg twice daily; C+R, Clopidogrel + Rivaroxaban 15 mg once daily; C+VKA, Clopidogrel + Vitamin K antagonist; DT, Dual therapy (single antiplatelet + single anticoagulant); SUCRA, The Surface Under the Cumulative Ranking curve; T+VKA, Ticagrelor + Viatamin K antagonist; TT, Triple therapy (dual antiplatelet + single anticoagulant).
References
Bavishi, C., Koulova, A., Bangalore, S., Sawant, A., Chatterjee, S., Ather, S., et al. (2016). Evaluation of the efficacy and safety of dual antiplatelet therapy with or without warfarin in patients with a clinical indication for DAPT and chronic anticoagulation: a meta-analysis of observational studies. Catheter. Cardiovasc. Intervent. 88, E12–22. doi: 10.1002/ccd.26234
Braun, O. Ö., Bico, B., Chaudhry, U., Wagner, H., Koul, S., Tydén, P., et al. (2015). Concomitant use of warfarin and ticagrelor as an alternative to triple antithrombotic therapy after an acute coronary syndrome. Thrombosis Res. 135, 26–30. doi: 10.1016/j.thromres.2014.10.016
Briasoulis, A., Papageorgiou, N., Zacharia, E., Palla, M., El Abdallah, M. D., Androulakis, E., et al. (2016). Meta-analysis of oral anticoagulants with dual versus single antiplatelet therapy in patients after percutaneous coronary intervention. Am. J. Cardiovasc. Drugs 16, 103–110. doi: 10.1007/s40256-015-0154-z
Caballero, L., Ruiz-Nodar, J. M., Marin, F., Roldan, V., Hurtado, J. A., Valencia, J., et al. (2013). Oral anticoagulation improves the prognosis of octogenarian patients with atrial fibrillation undergoing percutaneous coronary intervention and stenting. Age Ageing. 42, 70–75. doi: 10.1093/ageing/afs121
Cannon, C. P., Bhatt, D. L., Oldgren, J., Lip, G. Y. H., Ellis, S. G., Kimura, T., et al. (2017). Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N. Engl. J. Med. 377, 1513–1524. doi: 10.1056/NEJMoa1708454
Choi, H. I., Ahn, J. M., Kang, S. H., Lee, P. H., Kang, S. J., Lee, S. W., et al. (2017). Prevalence, management, and long-term (6-Year) outcomes of atrial fibrillation among patients receiving drug-eluting coronary stents. JACC Cardiovasc. Intervent. 10, 1075–1085. doi: 10.1016/j.jcin.2017.02.028
Connolly, S., Pogue, J., Hart, R., Pfeffer, M., Hohnloser, S., Chrolavicius, S., et al. (2006). Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet (London, England) 367, 1903–1912. doi: 10.1016/S0140-6736(06)68845-4
Dabrowska, M., Ochała, A., Cybulski, W., and Tendera, M. (2013). Balancing between bleeding and thromboembolism after percutaneous coronary intervention in patients with atrial fibrillation. Could triple anticoagulant therapy be a solution? Postepy. Kardiol. Interwencyjnej. 9, 234–240. doi: 10.5114/pwki.2013.37501
De Vecchis, R., Cantatrione, C., and Mazzei, D. (2016). Clinical relevance of anticoagulation and dual antiplatelet therapy to the outcomes of patients with atrial fibrillation and recent percutaneous coronary intervention with stent. J. Clin. Med. Res. 8, 153–161. doi: 10.14740/jocmr2443w
DerSimonian, R., and Laird, N. (1986). Meta-analysis in clinical trials. Controlled Clin. Trials 7, 177–188. doi: 10.1016/0197-2456(86)90046-2
Dewilde, W. J., Janssen, P. W., Verheugt, F. W., Storey, R. F., Adriaenssens, T., Hansen, M. L., et al. (2014). Triple therapy for atrial fibrillation and percutaneous coronary intervention: a contemporary review. J. Am. College Cardiol. 64, 1270–1280. doi: 10.1016/j.jacc.2014.06.1193
Dewilde, W. J., Oirbans, T., Verheugt, F. W., Kelder, J. C., De Smet, B. J., Herrman, J. P., et al. (2013). Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet 381, 1107–1115. doi: 10.1016/S0140-6736(12)62177-1
Dias, S., Welton, N. J., Caldwell, D. M., and Ades, A. E. (2010). Checking consistency in mixed treatment comparison meta-analysis. Stat. Med. 29, 932–944. doi: 10.1002/sim.3767
Fiedler, K. A., Maeng, M., Mehilli, J., Schulz-Schüpke, S., Byrne, R. A., Sibbing, D., et al. (2015). Duration of triple therapy in patients requiring oral anticoagulation after drug-eluting stent implantation: the ISAR-TRIPLE trial. J. Am. Coll. Cardiol. 65, 1619–1629. doi: 10.1016/j.jacc.2015.02.050
Fu, A., Singh, K., Abunassar, J., Malhotra, N., Le May, M., Labinaz, M., et al. (2016). Ticagrelor in triple antithrombotic therapy: predictors of ischemic and bleeding complications. Clin. Cardiol. 39, 19–23. doi: 10.1002/clc.22486
Gao, F., Zhou, Y. J., Wang, Z. J., Shen, H., Liu, X. L., Nie, B., et al. (2010). Comparison of different antithrombotic regimens for patients with atrial fibrillation undergoing drug-eluting stent implantation. Circul. J. 74, 701–708. doi: 10.1253/circj.CJ-09-0880
Gibson, C. M., Mehran, R., Bode, C., Halperin, J., Verheugt, F. W., Wildgoose, P., et al. (2016). Prevention of bleeding in patients with atrial fibrillation undergoing, P. C. I. N. Engl. J. Med. 375, 2423–2434. doi: 10.1056/NEJMoa1611594
Gilard, M., Blanchard, D., Helft, G., Carrier, D., Eltchaninoff, H., Belle, L., et al. (2009). Antiplatelet therapy in patients with anticoagulants undergoing percutaneous coronary stenting (from STENTIng and oral antiCOagulants [STENTICO]). Am. J. Cardiol. 104, 338–342. doi: 10.1016/j.amjcard.2009.03.053
Goto, K., Nakai, K., Shizuta, S., Morimoto, T., Shiomi, H., Natsuaki, M., et al. (2014). Anticoagulant and antiplatelet therapy in patients with atrial fibrillation undergoing percutaneous coronary intervention. Am. J. Cardiol. 114, 70–78. doi: 10.1016/j.amjcard.2014.03.060
Hess, C. N., Peterson, E. D., Peng, S. A., de Lemos, J. A., Fosbol, E. L., Thomas, L., et al. (2015). Use and outcomes of triple therapy among older patients with acute myocardial infarction and atrial fibrillation. J. Am. Coll. Cardiol. 66, 616–627. doi: 10.1016/j.jacc.2015.05.062
Higgins, J. P. T. S. J., Savović, J., Page, M. J., Hróbjartsson, A., Boutron, I., Reeves, B., et al. (2016). “A revised tool for assessing risk of bias in randomized trials,” in Cochrane Methods, eds J. Chandler, J. McKenzie, I. Boutron, and V. Welch (Chichester: Cochrane Database of Systematic Reviews), Suppl. 1.
Ho, K. W., Ivanov, J., Freixa, X., Overgaard, C. B., Osten, M. D., Ing, D., et al. (2013). Antithrombotic therapy after coronary stenting in patients with nonvalvular atrial fibrillation. Can. J. Cardiol. 29, 213–218. doi: 10.1016/j.cjca.2012.08.008
Hutton, B., Salanti, G., Caldwell, D. M., Chaimani, A., Schmid, C. H., Cameron, C., et al. (2015). The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann. Inter. Med. 162, 777–784. doi: 10.7326/M14-2385
Jang, S. W., Rho, T. H., Kim, D. B., Cho, E. J., Kwon, B. J., Park, H. J., et al. (2011). Optimal antithrombotic strategy in patients with atrial fibrillation after coronary stent implantation. Korean Circul. J. 41, 578–582. doi: 10.4070/kcj.2011.41.10.578
Jansen, J. P., Fleurence, R., Devine, B., Itzler, R., Barrett, A., Hawkins, N., et al. (2011). Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 1. Value Health 14, 417–428. doi: 10.1016/j.jval.2011.04.002
Jinatongthai, P., Kongwatcharapong, J., Foo, C. Y., Phrommintikul, A., Nathisuwan, S., Thakkinstian, A., et al. (2017). Comparative efficacy and safety of reperfusion therapy with fibrinolytic agents in patients with ST-segment elevation myocardial infarction: a systematic review and network meta-analysis. Lancet 390, 747–759. doi: 10.1016/S0140-6736(17)31441-1
Kang, D. O., Yu, C. W., Kim, H. D., Cho, J. Y., Joo, H. J., Choi, R. K., et al. (2015). Triple antithrombotic therapy versus dual antiplatelet therapy in patients with atrial fibrillation undergoing drug-eluting stent implantation. Coronary Artery Dis. 26, 372–380. doi: 10.1097/MCA.0000000000000242
Kirchhof, P., Benussi, S., Kotecha, D., Ahlsson, A., Atar, D., Casadei, B., et al. (2016). 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. J. Cardiothorac. Surg. 50, e1–e88. doi: 10.1093/ejcts/ezw313
Lamberts, M., Olesen, J. B., Ruwald, M. H., Hansen, C. M., Karasoy, D., Kristensen, S. L., et al. (2012). Bleeding after initiation of multiple antithrombotic drugs, including triple therapy, in atrial fibrillation patients following myocardial infarction and coronary intervention: a nationwide cohort study. Circulation 126, 1185–1193. doi: 10.1161/CIRCULATIONAHA.112.114967
Levine, G. N., Bates, E. R., Bittl, J. A., Brindis, R. G., Fihn, S. D., Fleisher, L. A., et al. (2016). 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the american college of cardiology/american heart association task force on clinical practice guidelines: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention, 2011 ACCF/AHA guideline for coronary artery bypass graft surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease, 2013 ACCF/AHA guideline for the management of st-elevation myocardial infarction, 2014 AHA/ACC guideline for the management of patients with non-st-elevation acute coronary syndromes, and 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. Circulation 134, e123–55. doi: 10.1161/CIR.0000000000000404
Liu, J., Fan, M., Zhao, J., Zhao, B., Zhang, C., Liu, C., et al. (2016). Efficacy and safety of antithrombotic regimens after coronary intervention in patients on oral anticoagulation: traditional and bayesian meta-analysis of clinical trials. Int. J. Cardiol. 205:89–96. doi: 10.1016/j.ijcard.2015.12.005
Lu, W., Chen, L., Wang, Y., Yao, Y., Fu, C., Zuo, P., et al. (2015). Rationale and design of MANJUSRI trial: a randomized, open-label, active-controlled multicenter study to evaluate the safety of combined therapy with ticagrelor and warfarin in AF subjects after PCI-eS. Contempor. Clin. Trials 40, 166–171. doi: 10.1016/j.cct.2014.12.002
Maegdefessel, L., Schlitt, A., Faerber, J., Bond, S. P., Messow, C. M., Buerke, M., et al. (2008). Anticoagulant and/or antiplatelet treatment in patients with atrial fibrillation after percutaneous coronary intervention. A single-center experience. Medizinische Klinik (Munich, Germany: 1983) 103, 628–632. doi: 10.1007/s00063-008-1101-4
Maldonado, J. R., Wysong, A., van der Starre, P. J., Block, T., Miller, C., and Reitz, B. A. (2009). Dexmedetomidine and the reduction of postoperative delirium after cardiac surgery. Psychosomatics 50, 206–217. doi: 10.1176/appi.psy.50.3.206
Manoukian, S. V., Feit, F., Mehran, R., Voeltz, M. D., Ebrahimi, R., Hamon, M., et al. (2007). Impact of major bleeding on 30-day mortality and clinical outcomes in patients with acute coronary syndromes: an analysis from the ACUITY Trial. J. Am. Coll. Cardiol. 49, 1362–1368. doi: 10.1016/j.jacc.2007.02.027
Manzano-Fernández, S., Pastor, F. J., Marín, F., Cambronero, F., Caro, C., Pascual-Figal, D. A., et al. (2008). Increased major bleeding complications related to triple antithrombotic therapy usage in patients with atrial fibrillation undergoing percutaneous coronary artery stenting. Chest 134, 559–567. doi: 10.1378/chest.08-0350
Mavridis, D., and Salanti, G. (2014). Exploring and accounting for publication bias in mental health: a brief overview of methods. Evid. Based Ment. Health 17, 11–15. doi: 10.1136/eb-2013-101700
Mehran, R., Rao, S. V., Bhatt, D. L., Gibson, C. M., Caixeta, A., Eikelboom, J., et al. (2011). Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation 123, 2736–2747. doi: 10.1161/CIRCULATIONAHA.110.009449
Nguyen, M. C., Lim, Y. L., Walton, A., Lefkovits, J., Agnelli, G., Goodman, S. G., et al. (2007). Combining warfarin and antiplatelet therapy after coronary stenting in the Global Registry of Acute Coronary Events: is it safe and effective to use just one antiplatelet agent? Eur. Heart J. 28, 1717–1722. doi: 10.1093/eurheartj/ehm186
Palla, M., Briasoulis, A., and Kondur, A. (2016). Oral anticoagulants with dual antiplatelet therapy versus clopidogrel in patients after percutaneous coronary intervention: a meta-analysis. Am. J. Therapeut. 1–8. doi: 10.1097/MJT.0000000000000466
Rao, S. V., O'Grady, K., Pieper, K. S., Granger, C. B., Newby, L. K., Van de Werf, F., et al. (2005). Impact of bleeding severity on clinical outcomes among patients with acute coronary syndromes. Am. J. Cardiol. 96, 1200–1206. doi: 10.1016/j.amjcard.2005.06.056
Roffi, M., Patrono, C., Collet, J. P., Mueller, C., Valgimigli, M., Andreotti, F., et al. (2016). 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the european society of cardiology (ESC). Eur. Heart J. 37, 267–315. doi: 10.1093/eurheartj/ehv320
Rubboli, A., Magnavacchi, P., Guastaroba, P., Saia, F., Vignali, L., Giacometti, P., et al. (2012). Antithrombotic management and 1-year outcome of patients on oral anticoagulation undergoing coronary stent implantation (from the Registro Regionale Angioplastiche Emilia-Romagna Registry). Am. J. Cardiol. 109, 1411–1417. doi: 10.1016/j.amjcard.2012.01.353
Rubboli, A., Saia, F., Sciahbasi, A., Bacchi-Reggiani, M. L., Steffanon, L., Briguori, C., et al. (2014a). Outcome of patients on oral anticoagulation undergoing coronary artery stenting: data from discharge to 12 months in the Warfarin and Coronary Stenting (WAR-STENT) Registry. J. Invasive Cardiol. 26, 563–569.
Rubboli, A., Schlitt, A., Kiviniemi, T., Biancari, F., Karjalainen, P. P., Valencia, J., et al. (2014b). One-year outcome of patients with atrial fibrillation undergoing coronary artery stenting: an analysis of the AFCAS registry. Clin. Cardiol. 37, 357–364. doi: 10.1002/clc.22254
Ruiz-Nodar, J. M., Marín, F., Hurtado, J. A., Valencia, J., Pinar, E., Pineda, J., et al. (2008). Anticoagulant and antiplatelet therapy use in 426 patients with atrial fibrillation undergoing percutaneous coronary intervention and stent implantation implications for bleeding risk and prognosis. J. Am. Coll. Cardiol. 51, 818–825. doi: 10.1016/j.jacc.2007.11.035
Sambola, A., Ferreira-González, I., Angel, J., Alfonso, F., Maristany, J., Rodríguez, O., et al. (2009). Therapeutic strategies after coronary stenting in chronically anticoagulated patients: the MUSICA study. Heart 95, 1483–1488. doi: 10.1136/hrt.2009.167064
Sambola, A., Mutuberria, M., Garcia Del Blanco, B., Alonso, A., Barrabes, J. A., Alfonso, F., et al. (2016). Effects of triple therapy in patients with non-valvular atrial fibrillation undergoing percutaneous coronary intervention regarding thromboembolic risk stratification. Circul. J. 80, 354–362. doi: 10.1253/circj.CJ-15-0923
Sarafoff, N., Martischnig, A., Wealer, J., Mayer, K., Mehilli, J., Sibbing, D., et al. (2013). Triple therapy with aspirin, prasugrel, and vitamin K antagonists in patients with drug-eluting stent implantation and an indication for oral anticoagulation. J. Am. Coll. Cardiol. 61, 2060–2066. doi: 10.1016/j.jacc.2013.02.036
Sarafoff, N., Ndrepepa, G., Mehilli, J., Dörrler, K., Schulz, S., Iijima, R., et al. (2008). Aspirin and clopidogrel with or without phenprocoumon after drug eluting coronary stent placement in patients on chronic oral anticoagulation. J. Inter. Med. 264, 472–480. doi: 10.1111/j.1365-2796.2008.01989.x
Sterne, J. A., Hernán, M. A., Reeves, B. C., Savovic, J., Berkman, N. D., Viswanathan, M., et al. (2016). ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355:i4919. doi: 10.1136/bmj.i4919
Suh, S. Y., Kang, W. C., Oh, P. C., Choi, H., Moon, C. I., Lee, K., et al. (2014). Efficacy and safety of aspirin, clopidogrel, and warfarin after coronary artery stenting in Korean patients with atrial fibrillation. Heart Vessels 29, 578–583. doi: 10.1007/s00380-013-0399-x
Valencia, J., Mainar, V., Bordes, P., Pineda, J., Gómez, S., and Sogorb, F. (2008). Observance of antiplatelet therapy after stent implantation in patients under chronic oral anticoagulant treatment. J. Intervent. Cardiol. 21, 218–224. doi: 10.1111/j.1540-8183.2008.00354.x
Valgimigli, M., Bueno, H., Byrne, R. A., Collet, J. P., Costa, F., Jeppsson, A., et al. (2018). 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the task force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 39, 213–260. doi: 10.1093/eurheartj/ehx419
Vranckx, P., Lewalter, T., Valgimigli, M., Tijssen, J. G., Reimitz, P.-E., Eckardt, L., et al. (2018). Evaluation of the safety and efficacy of an edoxaban-based antithrombotic regimen in patients with atrial fibrillation following successful percutaneous coronary intervention (PCI) with stent placement: rationale and design of the ENTRUST-AF PCI trial. Am. Heart J. 196, 105–112. doi: 10.1016/j.ahj.2017.10.009
Keywords: anticoagulants, antithrombosis, myocardial infarction, network meta-analysis, percutaneous coronary intervention
Citation: Bunmark W, Jinatongthai P, Vathesatogkit P, Thakkinstian A, Reid CM, Wongcharoen W, Chaiyakunapruk N and Nathisuwan S (2018) Antithrombotic Regimens in Patients With Percutaneous Coronary Intervention Whom an Anticoagulant Is Indicated: A Systematic Review and Network Meta-Analysis. Front. Pharmacol. 9:1322. doi: 10.3389/fphar.2018.01322
Received: 04 September 2018; Accepted: 29 October 2018;
Published: 19 November 2018.
Edited by:
Brian Godman, Karolinska Institutet (KI), SwedenReviewed by:
Tanja Mueller, University of Strathclyde, United KingdomJuhani Airaksinen, University of Turku, Finland
Copyright © 2018 Bunmark, Jinatongthai, Vathesatogkit, Thakkinstian, Reid, Wongcharoen, Chaiyakunapruk and Nathisuwan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Surakit Nathisuwan, c3VyYWtpdC5uYXRAbWFoaWRvbC5hYy50aA==
 Wipharak Bunmark
Wipharak Bunmark Peerawat Jinatongthai
Peerawat Jinatongthai Prin Vathesatogkit4
Prin Vathesatogkit4 Nathorn Chaiyakunapruk
Nathorn Chaiyakunapruk Surakit Nathisuwan
Surakit Nathisuwan