- 1Department of Respiratory Medicine and Critical Care Medicine, West China Hospital/West China School of Medicine, Sichuan University, Chengdu, China
- 2The Center of Gerontology and Geriatrics, West China Hospital/West China School of Medicine, Sichuan University, Chengdu, Sichuan, China
Background: The choice of empirical antibiotic treatment for patients with community-acquired pneumonia (CAP) who are admitted to non-intensive care unit (ICU) hospital wards is complicated by the limited availability of evidence. We systematically reviewed the efficacy and safety of strategies of empirical treatment with respiratory fluoroquinolone monotherapy and β-lactam with or without macrolide for non-ICU hospitalized CAP patients.
Methods: We searched databases including PubMed, the Cochrane Library (Issue11, 2018), EMbase, China National Knowledge Internet (CNKI), WanFang Data, VIP, and China Biology Medicine disc (CBMdisc) to identify randomized controlled trials (RCTs) involving the comparison of respiratory fluoroquinolone monotherapy and β-lactam with or without macrolide for the non-ICU hospitalized patients with CAP up to November 2018. Two reviewers independently screened literature according to the inclusion and exclusion criteria, extracted data, and assessed the risk of bias of the included studies. A meta-analysis was performed with the outcomes.
Results: A total of 22 studies involving 6,235 patients were included. The results of the meta-analysis showed a non-significant trend toward an advantage to the respiratory fluoroquinolone in overall mortality (RR 0.82, 95% CI 0.65–1.02). No significant difference was found between the two strategies in clinical success (the intention-to-treat population: RR 1.03, 95% CI 0.99–1.08; the clinically evaluable population: RR 1.03, 95% CI 0.999–1.055; the population in which it was unclear whether intention-to-treat or per-protocol analysis was used: RR 1.04, 95% CI 0.99–1.09), microbiological treatment success (RR 1.04, 95% CI 0.997–1.092), and length of stay (SMD −0.06, 95% CI −0.16 to 0.04). The advantage of respiratory fluoroquinolone was statistically significant on the drug-related adverse events (RR 0.87, 95% CI 0.77–0.97).
Conclusions: Current evidence shows that fluoroquinolone monotherapy has similar efficacy and favorable safety compared with β-lactam with or without macrolide for non-ICU hospitalized CAP patients. Since the limitation of region, quantity and quality of included studies, more RCTs with large scale and high quality are needed to verify the above conclusion.
Introduction
Long recognized as a major cause of death, community-acquired pneumonia (CAP) has been studied intensively since the late 1800s (Musher and Thorner, 2014). Despite the development of antimicrobial agents, pneumonia remains a major cause of hospitalization and death worldwide (Thomas et al., 2012; Welte et al., 2012).
Physicians must choose an optimal therapeutic regimen that eliminates the infection effectively, minimizes the risk of developing drug resistance and does not compromise the safety of the patient. Guidelines were written to develop a uniform set of recommendations that would provide appropriate antimicrobial therapy for the majority of patients with CAP. For patients with CAP who are admitted to a non-intensive-care-unit (ICU) ward, most guidelines recommend either respiratory fluoroquinolone monotherapy or β-lactam with or without macrolide for empirical treatment (Mandell et al., 2007; Lim et al., 2009; Woodhead et al., 2011; Cao et al., 2018). In America, guidelines recommend a respiratory fluoroquinolone monotherapy or a β-lactam plus a macrolide for the non-ICU inpatients (Mandell et al., 2007). In Britain, the British Thoracic Society suggests that amoxicillin is preferred for adults hospitalized with low severity CAP, while amoxicillin plus a macrolide is preferred for patients hospitalized with moderate severity CAP (levofloxacin, moxifloxacin, or doxycyline is alternative agent for those intolerant of penicillins or macrolides) (Lim et al., 2009). In Europe, guidelines recommend a respiratory fluoroquinolone monotherapy (levofloxacin or moxifloxacin), or a non-antipseudomonal cephalosporin, or a β-lactam (e.g., aminopenicillin) with or without a macrolide for non-ICU hospitalized patients (Woodhead et al., 2011). In China, a β-lactam (e.g., penicillins-β-lactamase-inhibitor combinations) with or without a macrolide, or respiratory fluoroquinolone monotherapy is suggested for the non-ICU inpatients (Cao et al., 2018). However, there is no consensus on which strategy is the best one. Level-one evidence for the comprehensive comparison of the two strategies is limited.
As main classes of antibiotics that have dominated the market for years, β-lactams, macrolides and fluoroquinolones are active against the major causative agents of CAP with different mechanisms (Walsh, 2003; Raja et al., 2004; Suda et al., 2018). β-lactam antibiotics work by inhibiting cell wall biosynthesis (inhibiting the β-lactam “binding protein” enzymes) in the bacterial organism (Fisher et al., 2005). They are effective against major causative bacteria of CAP (e.g., Streptococcus pneumonia) but not effective against Mycoplasma Pneumoniae (MP) or Chlamydia Pneumoniae (CP). Macrolides inhibit protein biosynthesis by binding to the P site on the 50S subunit of the bacterial ribosome and they are effective against Legionella Pneumophila, mycoplasma and chlamydia (Tenson et al., 2003). Physicians usually prescribe β-lactam plus macrolide for patients with CAP when infection with MP or CP is suspected. Fluoroquinolones eradicate bacteria by inhibiting the replication and transcription of bacterial DNA (preventing bacterial DNA from unwinding and duplicating) (Hooper, 2001; Aldred et al., 2014). Fluoroquinolones, especially respiratory fluoroquinolones (moxifloxacin, gemifloxacin, and levofloxacin) act against the major causative agents of CAP (including major causative bacteria, MP, CP and Legionella Pneumophila) and they are widely used as a monotherapy for patients with CAP.
Researchers from different countries and areas have performed randomized controlled trials (RCTs) to compare the efficacy of the two strategies. However, the results were not consistent. Finch et al. found that monotherapy with moxifloxacin was superior to that with a standard combination regimen of a β-lactam with or without a macrolide in the treatment of patients with CAP admitted to a hospital (Finch et al., 2002). Similarly, Huang G et al. reported that moxifloxacin was superior to cefuroxime with azithromycin in inpatients with low-moderate severity CAP (Huang et al., 2008). On the contrary, Erard et al. found that there were no significant differences between levofloxacin monotherapy and ceftriaxone with or without clarithromycin in non-ICU hospitalized CAP patients (Erard et al., 2004). Li BH et al. also reported that no significant differences were found between levofloxacin and cefuroxime with azithromycin in non-ICU hospitalized CAP patients (Li et al., 2009). Additionally, the small amount of patients enrolled in each trial limited the validity of the results.
Therefore, we conducted a systematic review and meta-analysis to conclusively and comprehensively compare the efficacy and safety of respiratory fluoroquinolone monotherapy vs. β-lactam with or without macrolide for empirical treatment for non-ICU hospitalized CAP patients.
Methods
Search Strategy
We searched databases including PubMed, the Cochrane Library (Issue11, 2018), EMbase, CNKI, WanFang Data, VIP and China Biology Medicine disc (CBMdisc) to identify RCTs up to November 2018. Search terms were “community-acquired pneumonia,” “fluoroquinolones” or “levofloxacin” or “moxifloxacin” or “gemifloxacin,” and “macrolides” or “β-lactams.” The search was restricted to RCTs. The language of the research papers was restricted to English and Chinese. All reference lists from relevant articles and reviews were hand-searched for additional eligible studies. We did not include abstracts from conferences because there is frequently considerable difference between data presented in conference abstracts and the subsequent peer-reviewed publications.
Study Selection
Two reviewers (SL and XT) independently carried out the literature search and examined relevant RCTs for further assessment. A checklist was used to assess whether studies met our inclusion criteria: (1) population: hospitalized patients diagnosed with CAP; (2) exposure: one of levofloxacin, moxifloxacin or gemifloxacin; (3) comparison group: β-lactams with or without macrolides; (4) outcome: at least include one of mortality, clinical treatment success, microbiological treatment success, length of hospital stay or adverse events; (5) study design: RCTs. Exclusion criteria eliminated duplicate reports and studies on patients aged < 18 years, outpatients, critically ill patients admitted to ICU, or patients identified as having some form of healthcare-associated pneumonia (HCAP).
Data Extraction
Two reviewers (SL and XT) independently extracted data from the trials included in the meta-analysis using a predesigned review form. In case of any disagreement between the two reviewers, a third reviewer extracted the data and the results were attained by consensus. The authors of trials were contacted for missing data when necessary. Data on first author, publication details, study design, included population, drug tested, endpoint data and adverse events during the treatment were extracted.
Assessment of Risk of Bias
Two reviewers (SL and XT) independently assess the risk of bias of the RCTs included in the meta-analysis. We use the domain-based method as recommended in The Cochrane Hand-book (Higgins and Altman, 2011a) according to: sequence generation, allocation concealment, blinding, incomplete outcome data addressed, free of selective reporting, and free of other bias. A third review author was responsible for resolving disagreements.
Outcomes
The primary outcome was all-cause mortality during the study period (treatment and follow-up period). Secondary outcomes included: clinical treatment success (“cure” was defined as resolution of all symptoms and signs of infections; “improvement” was defined as resolution of two or more of the baseline symptoms or signs of infections) (Frank et al., 2002; Writing Group of Guidance for Clinical Trials of Anti-bacterial Drugs, 2014) assessed at the test-of-cure (TOC) visit in the intention-to-treat population and clinically evaluable population; microbiological treatment success (defined as the eradication of baseline pathogens, or as presumed eradication based on the clinical outcomes when post-treatment cultures were not performed) (Frank et al., 2002; Writing Group of Guidance for Clinical Trials of Anti-bacterial Drugs, 2014); length of hospital stay; and adverse events probably related to the study regimens. Data was extracted preferentially by intention to treat.
Data Analysis and Statistical Methods
Heterogeneity was examined using the χ2 test (P ≤ 0.1) and the I2 test (I2 > 50% defining significant inconsistency). Publication bias was assessed using the funnel plot method and Egger's test. Risk ratios (RRs) were calculated for individual trials, with 95% confidence intervals (CIs). Meta-analysis was conducted using the Mantel–Haenszel fixed-effects model. We compared the fixed-effect model to a random-effects model when we observed significant heterogeneity between the trials (P ≤ 0.10). The results from the fixed-effects model are presented only when there was no significant heterogeneity between trials (P > 0.1); otherwise, the results from the random-effects model are presented. Analyses were conducted using Stata 11.0. For studies with multiple treatment groups, we assessed intervention groups for relevance for our review. If more than two groups were relevant, we combined groups to create a single pair-wise comparison.
Results
Study Selection Process
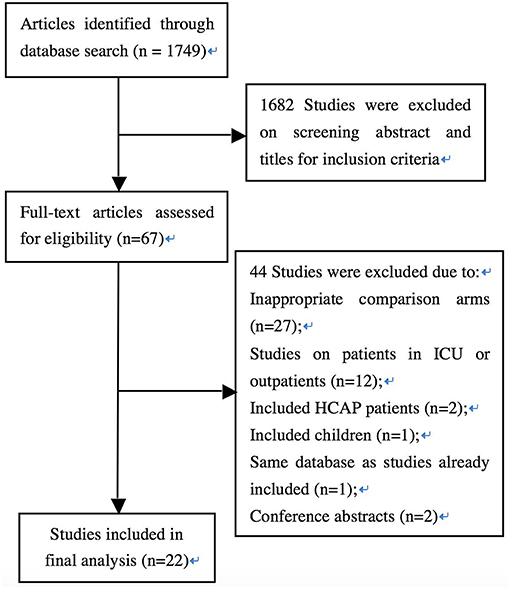
The flow diagram in Figure 1 shows the detailed screening and selection process applied before including trials in the meta-analysis. We identified a total of 1,749 citations from biomedical databases. After screening all titles and/or abstracts, 67 studies were identified for full text review. Forty-four studies were subsequently excluded for the following reasons: inappropriate comparison arms (n = 27); studies on patients in ICU or outpatients (n = 12); including HCAP patients (n = 2); including children (n = 1); same database as studies already included (n = 1); conference abstracts (n = 2). Twenty-two full-text publications involving 6,235 patients were ultimately identified (Finch et al., 2002; Frank et al., 2002; Lode et al., 2002; Erard et al., 2004; Leophonte et al., 2004; Zervos et al., 2004; Portier et al., 2005; Welte et al., 2005; Chang et al., 2006; Xu et al., 2006; Zhang et al., 2006; Lin et al., 2007; Zhao and Chen, 2007; Huang et al., 2008; Shao et al., 2008; Gao et al., 2009; Li et al., 2009; Yang and Zhang, 2009; Han et al., 2010; Lee et al., 2012; Liu et al., 2012; Postma et al., 2015).
Study Characteristics
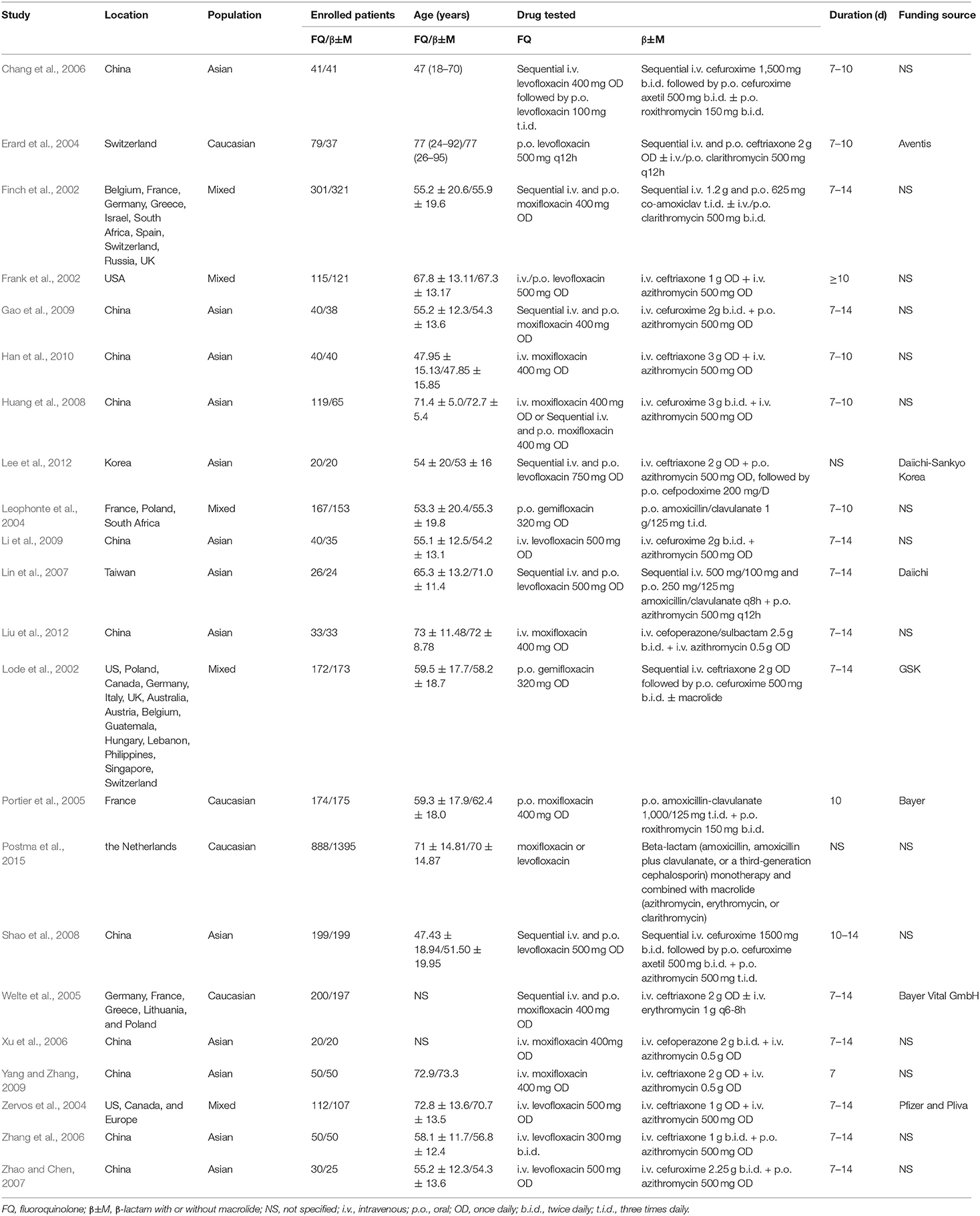
The main characteristics of the included trials are shown in Table 1. The trials were carried out between 1997 and 2013 in more than 25 countries. With a mean or median age between 47 and 77 years, the patients enrolled were mainly Caucasian and Asian and mostly from European counties, China, and the United States (US). Data on the comparison of respiratory fluoroquinolone monotherapy with β-lactam monotherapy was available in two trials (Leophonte et al., 2004; Postma et al., 2015), β-lactam–macrolide combination therapy in 16 trials (Frank et al., 2002; Zervos et al., 2004; Portier et al., 2005; Xu et al., 2006; Zhang et al., 2006; Lin et al., 2007; Zhao and Chen, 2007; Huang et al., 2008; Shao et al., 2008; Gao et al., 2009; Li et al., 2009; Yang and Zhang, 2009; Han et al., 2010; Lee et al., 2012; Liu et al., 2012), and β-lactam with or without macrolide (β-lactam ± macrolide) in five trials (Finch et al., 2002; Lode et al., 2002; Erard et al., 2004; Welte et al., 2005; Chang et al., 2006). Patients received sequential intravenous to oral or intravenous antibiotics in 20 trials (Finch et al., 2002; Frank et al., 2002; Lode et al., 2002; Erard et al., 2004; Zervos et al., 2004; Welte et al., 2005; Chang et al., 2006; Xu et al., 2006; Zhang et al., 2006; Lin et al., 2007; Zhao and Chen, 2007; Shao et al., 2008; Gao et al., 2009; Li et al., 2009; Yang and Zhang, 2009; Han et al., 2010; Lee et al., 2012; Liu et al., 2012; Postma et al., 2015). Treatment was given orally initially in two trials (Leophonte et al., 2004; Portier et al., 2005). We did not find publication bias in the performed analyses.
Sequence generation (specified rule for allocating interventions to participants based on some random process) (Higgins and Altman, 2011a) was adequate in 6 studies (Frank et al., 2002; Welte et al., 2005; Lin et al., 2007; Li et al., 2009; Lee et al., 2012; Postma et al., 2015) and no information was available for other studies. With numbered sachets, only Léophonte's study (Leophonte et al., 2004) reported adequate allocation concealment (steps taken to secure strict implementation of random assignments by preventing foreknowledge of the forthcoming allocations) (Higgins and Altman, 2011a). Insufficient information was available for the other studies. One trial (Leophonte et al., 2004) was double-blinded and the remaining were open label. Details of the incomplete data for each outcome will be discussed in the following sections. We did not find any specific concerns over selective reporting. For other potential source of bias, we found that seven studies (Lode et al., 2002; Erard et al., 2004; Zervos et al., 2004; Portier et al., 2005; Welte et al., 2005; Lin et al., 2007; Lee et al., 2012) were sponsored by pharmaceutical companies, which might generate bias in the assessment of outcomes. Besides, one study (Postma et al., 2015) was a cluster-randomized, crossover trial comparing treatment strategies assigned to hospitals in defined study periods as the unit of randomization. Analyses in this study took into account cluster-period effects and center effects.
Mortality
Nine trials provided mortality outcomes (Finch et al., 2002; Frank et al., 2002; Lode et al., 2002; Erard et al., 2004; Leophonte et al., 2004; Zervos et al., 2004; Portier et al., 2005; Welte et al., 2005; Postma et al., 2015). In total, 114 (5.2%) of the 2,198 patients in the respiratory fluoroquinolone group and 191 (7.2%) of the 2,670 patients in the comparator group died during the course of the studies. A non-significant trend toward an advantage to the respiratory fluoroquinolone group was observed (RR 0.82, 95% CI 0.65–1.02) (Figure 2). No heterogeneity was observed (I2 = 0%).
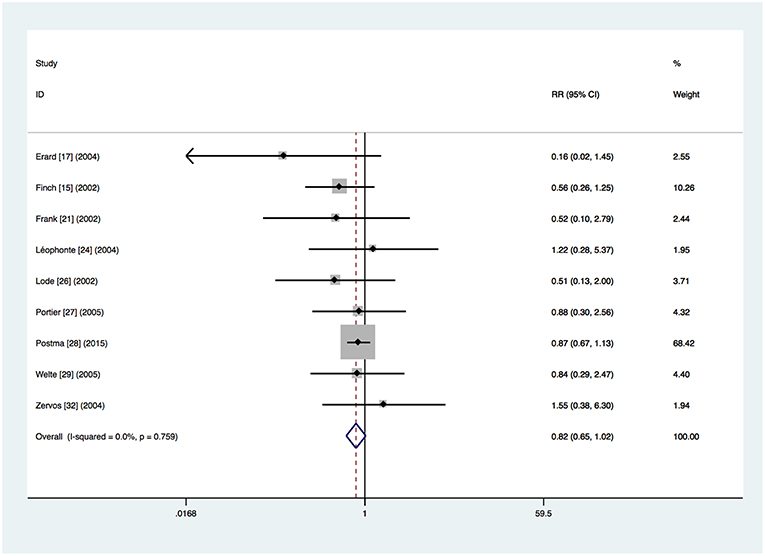
Figure 2. Mortality for respiratory fluoroquinolone monotherapy vs. β-lactam with or without macrolide. A fixed-effect Mantel–Haenszel (M–H) meta-analysis is shown with results presented as risk ratios with 95% confidence intervals (CIs).
Data about mortality of patients with β-lactam monotherapy was available for 2 trials (Leophonte et al., 2004; Postma et al., 2015) and no significant difference was found (RR 0.99, 95% CI 0.72–1.35). The non-significant advantage of the respiratory fluoroquinolone group was seen in the patients with β-lactam–macrolide combination therapy from 4 trials (RR 0.81, 95% CI 0.62–1.06) (Frank et al., 2002; Zervos et al., 2004; Portier et al., 2005; Postma et al., 2015). However, mortality rate was significantly lower in the respiratory fluoroquinolone group among patients with β-lactam ± macrolide regimen from 4 trials (RR 0.56, 95% CI 0.33–0.98) (Finch et al., 2002; Lode et al., 2002; Erard et al., 2004; Welte et al., 2005).
The same non-significant advantage of the respiratory fluoroquinolone group was seen when we excluded the cluster-randomized cross-over trial (RR = 0.70, 95% CI 0.46–1.07) (Postma et al., 2015).
Clinical Treatment Success
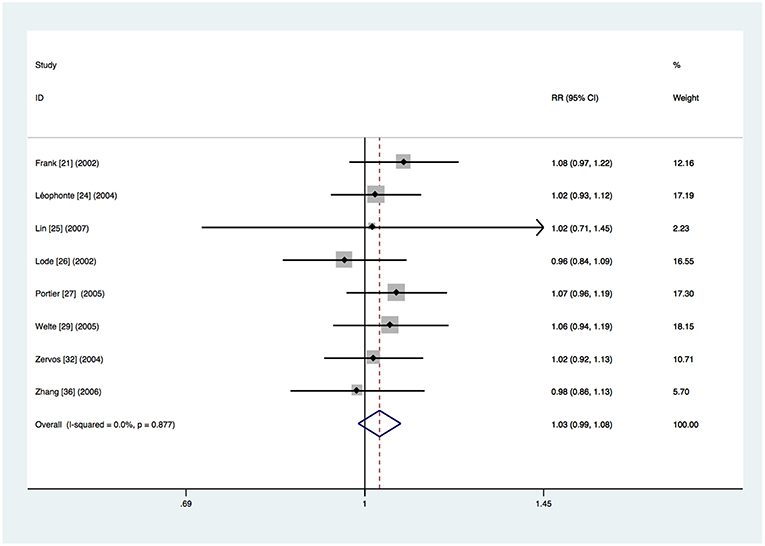
Data about clinical treatment success in the intention-to-treat population were available for 8 trials (Frank et al., 2002; Lode et al., 2002; Leophonte et al., 2004; Zervos et al., 2004; Portier et al., 2005; Welte et al., 2005; Zhang et al., 2006; Lin et al., 2007). Overall, treatment with respiratory fluoroquinolone was successful for 804 (80.9%) of the 994 patients. Treatment with comparator antibiotics was successful for 775 (78.4%) of the 988 patients. Meta-analysis showed that there was no significant difference (RR 1.03, 95% CI 0.99–1.08) (Figure 3). No heterogeneity was observed (I2 = 0%). The same conclusion was drawn from separate analyses of the studies on β-lactam–macrolide combination therapy (RR = 1.05, 95% CI 0.99–1.11) (Frank et al., 2002; Zervos et al., 2004; Portier et al., 2005; Zhang et al., 2006; Lin et al., 2007) and β-lactam ± macrolide regimen (RR 1.01, 95% CI 0.92–1.10) (Lode et al., 2002; Welte et al., 2005). Only one study (Leophonte et al., 2004) used β-lactam monotherapy and thus a combined analysis could not be performed. No significant difference was found in studies where treatment was given orally (RR 1.05, 95% CI 0.98–1.12) (Leophonte et al., 2004; Portier et al., 2005) or initially intravenously (RR 1.02, 95% CI 0.97–1.08) (Frank et al., 2002; Lode et al., 2002; Zervos et al., 2004; Welte et al., 2005; Zhang et al., 2006; Lin et al., 2007). No significant difference was found in the trials funded by pharmaceutical companies (RR 1.03, 95% CI 0.97–1.09) (Lode et al., 2002; Zervos et al., 2004; Portier et al., 2005; Welte et al., 2005; Lin et al., 2007) or not (RR 1.04, 95% CI 0.97–1.11) (Frank et al., 2002; Leophonte et al., 2004; Zhang et al., 2006).
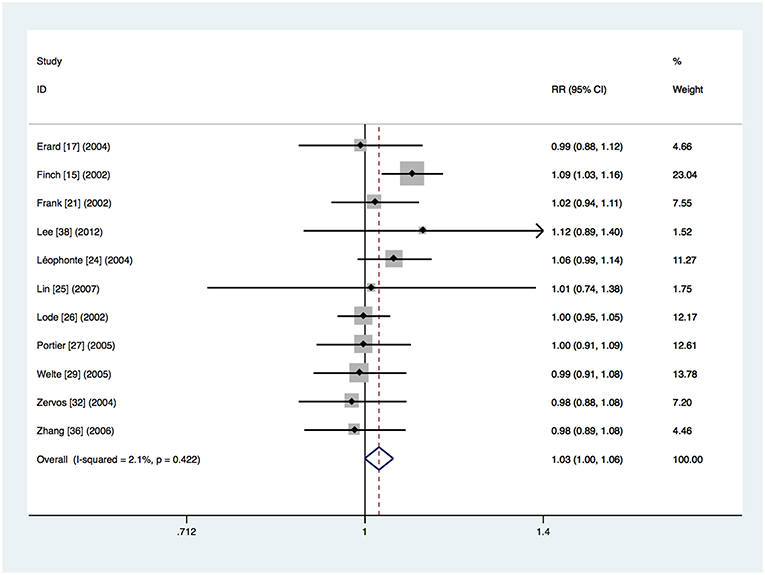
Eleven trials provided data about clinical treatment success in the clinically evaluable population (Finch et al., 2002; Frank et al., 2002; Lode et al., 2002; Erard et al., 2004; Leophonte et al., 2004; Zervos et al., 2004; Portier et al., 2005; Welte et al., 2005; Zhang et al., 2006; Lin et al., 2007; Lee et al., 2012). The clinical treatment success was 91.3% (1,048 of the 1,148 patients) in the respiratory fluoroquinolone group and 88.9% (984 of the 1,107 patients) in the comparator antibiotics group. Meta-analysis showed that there was no significant difference (RR 1.03, 95% CI 0.999–1.055) (Figure 4). No significant heterogeneity was observed (I2 = 2.1%). The same conclusion was drawn from separate analyses of the studies on β-lactam–macrolide combination therapy (RR 1.00, 95% CI 0.96–1.05) (Frank et al., 2002; Zervos et al., 2004; Portier et al., 2005; Zhang et al., 2006; Lin et al., 2007; Lee et al., 2012) and β-lactam ± macrolide regimen (RR 1.02, 95% CI 0.97–1.08) (Finch et al., 2002; Lode et al., 2002; Erard et al., 2004; Welte et al., 2005). Only one study used β-lactam monotherapy (Leophonte et al., 2004). No significant difference was found in studies where treatment was given orally (RR 1.03, 95% CI 0.97–1.09) (Leophonte et al., 2004; Portier et al., 2005) or initially intravenously (RR 1.03, 95% CI 0.996–1.059) (Finch et al., 2002; Frank et al., 2002; Lode et al., 2002; Erard et al., 2004; Zervos et al., 2004; Welte et al., 2005; Zhang et al., 2006; Lin et al., 2007; Lee et al., 2012). No significant difference was found in the trials funded by pharmaceutical companies (RR 1.00, 95% CI 0.96–1.04) (Lode et al., 2002; Erard et al., 2004; Zervos et al., 2004; Portier et al., 2005; Welte et al., 2005; Lin et al., 2007; Lee et al., 2012). However, the advantage of respiratory fluoroquinolone was statistically significant in the studies not funded by pharmaceutical companies (RR 1.06, 95% CI 1.02–1.10) (Finch et al., 2002; Frank et al., 2002; Leophonte et al., 2004; Zhang et al., 2006).
It was unclear whether intention-to-treat or per-protocol analysis was used in ten studies, which did not refer to dropouts or reported the total number of dropouts but did not give the numbers per study arm (Chang et al., 2006; Xu et al., 2006; Zhao and Chen, 2007; Huang et al., 2008; Shao et al., 2008; Gao et al., 2009; Li et al., 2009; Yang and Zhang, 2009; Han et al., 2010; Liu et al., 2012). The clinical treatment success was 93.7% (565 of the 603 patients) in the respiratory fluoroquinolone group and 89.5% (479 of the 535 patients) in the comparator antibiotics group. Heterogeneity was detected (I2 = 38.7%, P = 0.10) and meta-analysis done by the random-effects model showed no significant difference (RR 1.04, 95% CI 0.99–1.09) (Figure 5). The advantage of respiratory fluoroquinolone was statistically significant when compared with β-lactam–macrolide combination therapy (RR 1.05, 95% CI 1.01–1.09) (Xu et al., 2006; Zhao and Chen, 2007; Huang et al., 2008; Shao et al., 2008; Gao et al., 2009; Li et al., 2009; Yang and Zhang, 2009; Han et al., 2010; Liu et al., 2012) and the heterogeneity was reduced in this analysis (I2 = 25.8%, P = 0.21). Only one study used β-lactam ± macrolide regimen (Chang et al., 2006) and no trials used β-lactam monotherapy. Treatment was given initially intravenously in all trials. Not any study was funded by pharmaceutical companies.
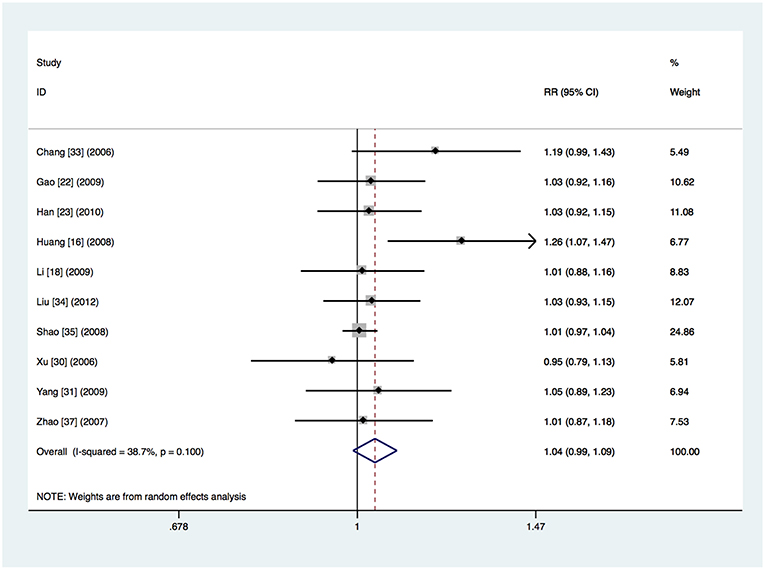
Figure 5. Clinical treatment success analysis for the studies in which it was unclear whether intention-to-treat or per-protocol analysis was used.
Microbiological Treatment Success
Eighteen studies reported microbiological treatment success outcomes (Finch et al., 2002; Frank et al., 2002; Lode et al., 2002; Leophonte et al., 2004; Zervos et al., 2004; Portier et al., 2005; Chang et al., 2006; Xu et al., 2006; Zhang et al., 2006; Lin et al., 2007; Zhao and Chen, 2007; Huang et al., 2008; Shao et al., 2008; Gao et al., 2009; Li et al., 2009; Yang and Zhang, 2009; Han et al., 2010; Lee et al., 2012). In the total microbiologically evaluable population, 513 (88.8%) of the 578 patients/isolates in the respiratory fluoroquinolone group and 462 (85.2%) of the 542 patients/isolates in the comparator group achieved eradication or presumed eradication of the baseline pathogens. The most common pathogens were S. pneumoniae, H. influenza, and M. pneumoniae. Details about drug resistance were reported in 9 trials (Finch et al., 2002; Frank et al., 2002; Lode et al., 2002; Erard et al., 2004; Leophonte et al., 2004; Zervos et al., 2004; Portier et al., 2005; Zhang et al., 2006; Postma et al., 2015). For respiratory fluoroquinolone, only one S. aureus isolate resistant to levofloxacin was found. S. pneumoniae strains resistant to the comparator antibiotics were more commonly found. Resistance was more prominent among macrolides than among β-lactams.
There was no significant difference in the overall microbiological treatment success rates between the two groups (RR 1.04, 95% CI 0.997–1.092). No significant heterogeneity was observed (I2 = 10.3%). The same conclusion was drawn from separate analyses of the studies on β-lactam–macrolide combination therapy (RR 1.05, 95% CI 0.98–1.12) (Frank et al., 2002; Zervos et al., 2004; Portier et al., 2005; Xu et al., 2006; Zhang et al., 2006; Lin et al., 2007; Zhao and Chen, 2007; Huang et al., 2008; Shao et al., 2008; Gao et al., 2009; Li et al., 2009; Yang and Zhang, 2009; Han et al., 2010; Lee et al., 2012) and β-lactam ± macrolide regimen (RR 1.07, 95% CI 0.99–1.15) (Finch et al., 2002; Lode et al., 2002; Chang et al., 2006). Only one study used β-lactam monotherapy (Leophonte et al., 2004). No significant difference was found in studies where treatment was given orally (RR 0.98, 95% CI 0.88–1.10) (Leophonte et al., 2004; Portier et al., 2005). In studies where treatment was given initially intravenously, the advantage of respiratory fluoroquinolone was statistically significant (RR 1.05, 95% CI 1.003–1.108) (Finch et al., 2002; Frank et al., 2002; Lode et al., 2002; Zervos et al., 2004; Chang et al., 2006; Xu et al., 2006; Zhang et al., 2006; Lin et al., 2007; Zhao and Chen, 2007; Huang et al., 2008; Shao et al., 2008; Gao et al., 2009; Li et al., 2009; Yang and Zhang, 2009; Han et al., 2010; Lee et al., 2012).
In addition, there was no significant difference between the respiratory fluoroquinolone group and the comparator group for the microbiological treatment success rates of S. pneumoniae (343 isolates, RR 0.99, 95% CI 0.85–1.17) (Finch et al., 2002; Frank et al., 2002; Lode et al., 2002; Leophonte et al., 2004; Zervos et al., 2004; Portier et al., 2005; Xu et al., 2006; Zhang et al., 2006; Lin et al., 2007; Han et al., 2010; Lee et al., 2012), H. influenzae (113 isolates, RR 1.04, 95% CI 0.87–1.25) (Finch et al., 2002; Frank et al., 2002; Lode et al., 2002; Leophonte et al., 2004; Zervos et al., 2004; Xu et al., 2006; Zhang et al., 2006; Lin et al., 2007; Han et al., 2010), M. pneumoniae (77 isolates, RR 1.08, 95% CI 0.96–1.23) (Finch et al., 2002; Lode et al., 2002; Han et al., 2010; Lee et al., 2012), C. pneumoniae (41 isolates, RR 1.03, 95% CI 0.83–1.27) (Finch et al., 2002; Lode et al., 2002; Han et al., 2010) and Legionella species (21 isolates, RR 0.99, 95% CI 0.60–1.63) (Finch et al., 2002; Lode et al., 2002; Leophonte et al., 2004).
Length of Hospital Stay
Data about the length of stay in hospital were available in 9 trials (Finch et al., 2002; Lode et al., 2002; Erard et al., 2004; Zervos et al., 2004; Welte et al., 2005; Lin et al., 2007; Shao et al., 2008; Li et al., 2009; Postma et al., 2015). Four trials provided the median duration of hospital stay and 0–2 days less duration was found in the respiratory fluoroquinolone group (Lode et al., 2002; Erard et al., 2004; Welte et al., 2005; Postma et al., 2015). Six trials provided the mean duration of hospital stay and no significant difference was found (SMD −0.06, 95% CI −0.22 to 0.11) (Finch et al., 2002; Zervos et al., 2004; Welte et al., 2005; Lin et al., 2007; Shao et al., 2008; Li et al., 2009). Among these studies, one trial provided both the median and the mean duration (Welte et al., 2005). Using the statistic methods recommended in the Cochrane Hand-book (Higgins and Altman, 2011b), we calculated the mean duration for all trials and performed an overall meta-analysis. No significant difference was found (SMD −0.06, 95% CI −0.16 to 0.04). Heterogeneity was moderate (I2 = 45.6%). However, the advantage of respiratory fluoroquinolone was statistically significant when compared with β-lactam ± macrolide regimen (SMD −0.18, 95% CI −0.28 to −0.07) (Finch et al., 2002; Lode et al., 2002; Erard et al., 2004; Welte et al., 2005) and the heterogeneity was reduced in this analysis (I2 = 9.7%). No significant difference was found when respiratory fluoroquinolone was compared with β-lactam–macrolide combination therapy (SMD 0.03, 95% CI −0.06 to 0.11) (Zervos et al., 2004; Lin et al., 2007; Shao et al., 2008; Li et al., 2009; Postma et al., 2015). Data of patients with β-lactam monotherapy was only available in one trial (Postma et al., 2015).
Adverse Events
All but two trials reported on drug-related adverse outcomes. One trial did not refer to adverse events (Lin et al., 2007). One trial reported on complications while data on drug-related adverse outcomes was unavailable (Postma et al., 2015). The majority of the adverse events were mild to moderate. The most commonly studied adverse effects were gastrointestinal events (including nausea, diarrhea and vomiting) and liver function abnormalities. However, the definition of gastrointestinal events differed, some including all the three symptoms (nausea, diarrhea and vomiting) and some nausea alone, thereby excluding an accurate comparison for each symptom alone. QTc prolongation was reported in one trial with one patient in the co-amoxiclav ± clarithromycin group.
The advantage of respiratory fluoroquinolone was statistically significant on the adverse events (RR 0.87, 95% CI 0.77–0.97). No significant heterogeneity was observed (I2 = 25.9%). The same conclusion was drawn from analysis of the studies on serious adverse events (RR 0.67, 95% CI 0.51–0.88) (Finch et al., 2002; Frank et al., 2002; Leophonte et al., 2004; Zervos et al., 2004; Portier et al., 2005; Welte et al., 2005). The percentage of patients who were withdrawn from the trials because of adverse events was not significantly different between the two groups (RR 0.87, 95% CI 0.59–1.30) (Finch et al., 2002; Frank et al., 2002; Lode et al., 2002; Erard et al., 2004; Zervos et al., 2004; Welte et al., 2005; Chang et al., 2006; Liu et al., 2012). Respiratory fluoroquinolone was associated with significantly fewer adverse events compared with β-lactam–macrolide combination therapy (RR 0.74, 95% CI 0.61–0.90) (Frank et al., 2002; Zervos et al., 2004; Portier et al., 2005; Xu et al., 2006; Zhang et al., 2006; Zhao and Chen, 2007; Huang et al., 2008; Shao et al., 2008; Gao et al., 2009; Li et al., 2009; Yang and Zhang, 2009; Han et al., 2010; Lee et al., 2012; Liu et al., 2012). No significant difference was found when respiratory fluoroquinolone was compared with β-lactam ± macrolide regimen (RR 0.99, 95% CI 0.74–1.34) (Finch et al., 2002; Lode et al., 2002; Erard et al., 2004; Welte et al., 2005; Chang et al., 2006). Only one study used β-lactam monotherapy (Leophonte et al., 2004).
Gastrointestinal events were reported in 16 studies and were significantly less common in the respiratory fluoroquinolone group (RR 0.63, 95% CI 0.43 to 0.94) (Finch et al., 2002; Frank et al., 2002; Lode et al., 2002; Erard et al., 2004; Leophonte et al., 2004; Portier et al., 2005; Welte et al., 2005; Chang et al., 2006; Xu et al., 2006; Zhang et al., 2006; Huang et al., 2008; Shao et al., 2008; Gao et al., 2009; Han et al., 2010; Lee et al., 2012; Liu et al., 2012). Non-significant advantage of respiratory fluoroquinolone was found with regard to liver function abnormalities (RR 0.73, 95% CI 0.52 to 1.03) (Finch et al., 2002; Lode et al., 2002; Leophonte et al., 2004; Welte et al., 2005; Zhang et al., 2006; Zhao and Chen, 2007; Shao et al., 2008; Gao et al., 2009; Li et al., 2009; Yang and Zhang, 2009; Lee et al., 2012).
Discussion
This systematic review with meta-analysis compared the efficacy and safety of respiratory fluoroquinolone monotherapy and β-lactam with or without macrolide for non-ICU hospitalized CAP patients. A non-significant trend toward an advantage to respiratory fluoroquinolone was observed on overall mortality. No significant difference was found between the two strategies in clinical success, microbiological treatment success, and length of stay. The advantage of respiratory fluoroquinolone was statistically significant in the drug-related adverse events. The advantage of respiratory fluoroquinolone in clinical treatment success was statistically significant in the studies not funded by pharmaceutical companies based on the clinically evaluable population (RR 1.06, 95% CI 1.02–1.10) and the advantage in microbiological treatment success was statistically significant in the studies where treatment was given initially intravenously (RR 1.05, 95% CI 1.003–1.108). The results were consistent with those of the primary analysis for the subgroup of β-lactam–macrolide combination therapy except for the clinical success based on the data that it was unclear whether intention-to-treat or per-protocol analysis was used (RR 1.05, 95% CI 1.01–1.09). Analysis was available only in mortality for the subgroup of β-lactam monotherapy and no significant difference was found. For the subgroup of β-lactam ± macrolide regimen, respiratory fluoroquinolone was associated with significantly lower mortality and less length of stay, while no significant difference was found in clinical treatment success, microbiological treatment success and adverse events.
An earlier meta-analysis performed by Vardakas et al. (2008) investigated whether respiratory quinolone monotherapy was superior to other recommended antimicrobial regimens, including combination therapy consisting of a macrolide and β-lactam as well as monotherapy (macrolide, ketolide, or β-lactam alone), for the treatment of adults with CAP. While no significant difference was found in mortality, clinical success rates were significantly higher and adverse events were significantly fewer with fluoroquinolone monotherapy. However, we found no significant difference in the overall clinical treatment success. In our meta-analysis, we focused on direct comparison of respiratory fluoroquinolone monotherapy and β-lactam with or without macrolide for non-ICU hospitalized CAP patients, precluding the interference from outpatients or patients in ICU and the interference from other drugs. Furthermore, we included new trials performed in recent years, providing greater statistical confidence for our meta-analysis.
The moderate total mortality rates in the two groups of our meta-analysis (5.2% and 7.2%) supports the opinion that the patients admitted to non-ICU hospital wards are associate with moderate risk of death (Mandell et al., 2007; Lim et al., 2009). A non-significant trend toward an advantage to the respiratory fluoroquinolone group was observed and more RCTs are needed to further verify the result.
Overall, no significant difference was found in clinical treatment success. The advantage of respiratory fluoroquinolone was statistically significant in some subgroup analyses. However, we noticed that the advantage was not obvious (RR = 1.06 and RR = 1.05). Therefore, we considered that the advantages of respiratory fluoroquinolone in these subgroup analyses were limited in clinical significance.
Drug resistance was found more prominent in the comparator antibiotics and most commonly among macrolides, which was in correspondence with previous surveillance (Mandell et al., 2007; Ho et al., 2009; Lim et al., 2009). There was no significant difference in the microbiological treatment success. However, the amount of patients enrolled in the analysis was limited (578/542 patients/isolates) and the patients included in the analysis for atypical pathogens were mainly from European countries. Previous surveillance results showed that the resistance of M. pneumoniae to macrolides in Asian countries was significantly higher than in the European or North American countries (Mandell et al., 2007; Lim et al., 2009; Mikasa et al., 2016; Cao et al., 2018). Since the drug resistance pattern differs greatly in different areas and countries, more RCTs with large scale in different areas are needed to verify the above conclusion.
When respiratory fluoroquinolone was compared with β-lactam–macrolide combination therapy, no significant difference was found in mortality, clinical treatment success, microbiological treatment success and length of stay. Respiratory fluoroquinolone was associated with fewer adverse events. When respiratory fluoroquinolone was compared with β-lactam monotherapy, no significant difference was found in mortality. Because of the lack of studies in this subgroup, analyses for other outcomes were not available. This may be because researchers used β-lactam monotherapy mainly in outpatients with low severity and usually added macrolides for hospitalized patients with moderate to severe pneumonia. More studies or detailed data comparing respiratory fluoroquinolone with β-lactam monotherapy in hospitalized CAP patients under supervision are needed. In the studies with β-lactam ± macrolide regimen as control group, respiratory fluoroquinolone was associated with significantly lower mortality rate and less length of stay. No significant difference was found in clinical treatment success, microbiological treatment success and adverse events. As the comparator regimens in these studies were not exactly the same, the results of this subgroup analysis might introduce more bias and thus provided relatively less statistical confidence.
There were several limitations in our meta-analysis. First, our findings may be affected by the quality of trials included in the analysis. Sequence generation was adequate in 6 studies. Only one trial was double-blinded, and one trial reported adequate allocation concealment. A sensitivity analysis was performed including only trials that reported adequate sequence generation. The results were consistent with those of the primary analysis except for overall adverse events rate, which indicated non-significant advantage of the respiratory fluoroquinolone group. Second, the quantity of studies included in some subgroup analyses was small, resulting in limited statistical confidence. Third, we failed to perform a comprehensive analysis for β-lactam monotherapy because of the lack of studies comparing respiratory fluoroquinolone with it. Finally, seven studies were sponsored by pharmaceutical companies, which might generate bias in the assessment of outcomes. Sensitivity analyses limited to industry-funded and not industry-funded studies were performed. The results showed that for the clinical treatment success in the clinically evaluable population, the advantage of respiratory fluoroquinolone was statistically significant in the studies not funded by pharmaceutical companies but limited in clinical significance. For the overall adverse events, no significant difference was found in the studies not funded by pharmaceutical companies. Other analyses indicated similar findings with the primary analyses.
In conclusion, despite the limitations of our meta-analysis, we conclude that respiratory fluoroquinolone monotherapy has similar efficacy and favorable safety compared with β-lactam with or without macrolide for non-ICU hospitalized CAP patients. Since the limitation of region, quantity and quality of included studies, more RCTs with large scale and high quality are needed to verify the above conclusion.
Author Contributions
HF, SL, and XT conceived and designed the studies. SL and XT carried out the literature search, extracted data, assess the risk of bias of the RCTs. YM, DW, JH, LZ, MW, LW, and TL helped conduct the analyses. SL wrote the manuscript. All authors reviewed the manuscript.
Funding
This study was supported by National Key R&D Program of China (2017YFC1309703).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Aldred, K. J., Kerns, R. J., and Osheroff, N. (2014). Mechanism of quinolone action and resistance. Biochemistry 18, 1565–1574. doi: 10.1021/bi5000564
Cao, B., Huang, Y., She, D. Y., Cheng, Q. J., Fan, H., and Tian, X. L., et al. (2018). Diagnosis and treatment of community-acquired pneumonia in adults: 2016 clinical practice guidelines. Clin. Respir. J. 12, 1320–1360. doi: 10.1111/crj.12674
Chang, H. Q., Wang, J. X., and Xu, S. P. (2006). Observation of curative effect of levofloxacin on community-acquired pneumonia (Chinese). Beijing Med. J. 28:753. doi: 10.3969/j.issn.0253-9713.2006.12.021
Erard, V., Lamy, O., Bochud, P.-Y., Bille, J., Cometta, A., and Calandra, T. (2004). Full-course oral levofloxacin for treatment of hospitalized patients with community-acquired pneumonia. Eur. J. Clin. Microbiol. Infect. Dis. 23, 82–88. doi: 10.1007/s10096-003-1060-x
Finch, R., Schürmann, D., Collins, O., Kubin, R., McGivern, J., and Bobbaers, H., et al. (2002). Randomized controlled trial of sequential intravenous (iv) and oral moxifloxacin compared with sequential iv and oral co-amoxiclav with or without clarithromycin in patients with community-acquired pneumonia requiring initial parenteral treatment. Antimicrob. Agents Chemother. 46, 1746–1754. doi: 10.1128/AAC.46.6.1746-1754.2002
Fisher, J. F., Meroueh, S. O., and Mobashery, S. (2005). Bacterial resistance to beta-lactam antibiotics: compelling opportunism, compelling opportunity. Chem. Rev. 105, 395–424. doi: 10.1021/cr030102i
Frank, E., Liu, J., Kinasewitz, G., Moran, G. J., Oross, M. P., and Olson, W. H., et al. (2002). A multicenter, open-label, randomized comparison of levofloxacin and azithromycin plus ceftriaxone in hospitalized adults with moderate to severe community-acquired pneumonia. Clin. Ther. 24, 1292–1308. doi: 10.1016/S0149-2918(02)80034-0
Gao, W. H., Wu, K. S., Lin, Y. H., and Chen, Z. S. (2009). Comparison of moxifloxacin and cefuroxime combined with azithromycin for patients with community-acquired pneumonia (Chinese). Chin. J. Nosocomiol. 19, 3105–3107. doi: 10.3321/j.issn:1005-4529.2009.22.041
Han, B., Ning, X. C., and Chai, W. S. (2010). Clinical observation of moxifloxacin versus ceftriaxone combined with azithromycin in the treatment (Chinese). Chin. Pharm. 21, 3400–3401. Available online at: http://www.cnki.com.cn/Article/CJFDTotal-ZGYA201036025.htm
Higgins, J., and Altman, D. (2011a). Chapter 8:Assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration. Available online at: www.cochrane-handbook.org (accessed November 25, 2018).
Higgins, J., and Altman, D. E. (2011b). Chapter 7:Selecting studies and collecting data. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration. Available online at: www.cochrane-handbook.org
Ho, P. L., Cheng, V. C., and Chu, C. M. (2009). Antibiotic resistance in community-acquired pneumonia caused by Streptococcus pneumoniae, methicillin-resistant Staphylococcus aureus, and Acinetobacter baumannii. Chest 136, 1119–1127. doi: 10.1378/chest.09-0285
Hooper, D. C. (2001). Emerging mechanisms of fluoroquinolone resistance. Emerg. Infect. Dis. 7, 337–341. doi: 10.3201/eid0702.010239
Huang, G., Jiang, Y. Y., Zhou, Y. J., Ling, X. J., Qiu, T. F., and Zhou, Y., et al. (2008). Sequential moxifloxacin therapy in the treatment of elderly patients with community-acquired pnuemonia: clinical evaluation (Chinese). J. Nanjing Med. Nat. Sci. 28, 1457–1460. Available online at: http://jnmu.njmu.edu.cn/zr/ch/reader/view_abstract.aspx?file_no=aumn081123&flag=1
Lee, J., Kim, S., Kim, J., Ryu, Y., and Chang, J. (2012). High-dose levofloxacin in community-acquired pneumonia. Clin. Drug Invest. 32, 569–576. doi: 10.1007/BF03261911
Leophonte, P., File, T., and Feldman, C. (2004). Gemifloxacin once daily for 7 days compared to amoxicillin/clavulanic acid thrice daily for 10 days for the treatment of community-acquired pneumonia of suspected pneumococcal origin. Respir. Med. 98, 708–720. doi: 10.1016/j.rmed.2004.04.007
Li, B. H., Xue, J. M., and Song, J. M. (2009). A comparison between levofloxacin and cefuroxime with azithromycin in community-acquired pneumonia (Chinese). Chin. Gen. Pract. 12, 1532–1534. doi: 10.3969/j.issn.1007-9572.2009.16.027
Lim, W. S., Baudouin, S. V., George, R. C., Hill, A. T., Jamieson, C., and Le Jeune, I., et al. (2009). BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax. 64 (Suppl. 3), iii1–55. doi: 10.1136/thx.2009.121434
Lin, T., Lin, S., Chen, H., Wang, C., Wang, Y., and Chang, M., et al. (2007). An open-label, randomized comparison of levofloxacin and amoxicillin/clavulanate plus clarithromycin for the treatment of hospitalized patients with community-acquired pneumonia. Chang. Gung Med. J. 30:321.
Liu, L. Y., Xiao, Y. H., Yan, P. K., Xu, J., and Guan, B. E. (2012). Efficacy of moxifloxacin vs. azithromycin plus cefoperazone/sulbactam for community acquired pneumonia (Chinese). Eval. Anal. Drug Use Hosp. China. 12, 543-545. Available online at: http://yypf.cbpt.cnki.net/WKD/WebPublication/paperDigest.aspx?paperID=18960cbc-afea-4ddd-b1c6-3e6848ee544b#
Lode, H., File, T. M., Mandell, L., Ball, P., Pypstra, R., and Thomas, M., et al. (2002). Oral gemifloxacin versus sequential therapy with intravenous ceftriaxone/oral cefuroxime with or without a macrolide in the treatment of patients hospitalized with community-acquired pneumonia: a randomized, open-label, multicenter study of clinical efficacy and tolerability. Clin. Ther. 24, 1915–1936. doi: 10.1016/S0149-2918(02)80088-1
Mandell, L. A., Wunderink, R. G., Anzueto, A., Bartlett, J. G., Campbell, G. D., and Dean, N. C., et al. (2007). Infectious diseases society of america/american thoracic society consensus guidelines on the management of community-acquired pneumonia in adults. Clin. Infect. Dis. 44 (Suppl. 2), S27–72. doi: 10.1086/511159
Mikasa, K., Aoki, N., Aoki, Y., Abe, S., Iwata, S., and Ouchi, K., et al. (2016). JAID/JSC guidelines for the treatment of respiratory infectious diseases: the japanese association for infectious Diseases/Japanese society of chemotherapy - The JAID/JSC guide to clinical management of infectious Disease/Guideline-preparing committee respiratory infectious disease WG. J. Infect. Chemother. 22(7 Suppl):S1–S65. doi: 10.1016/j.jiac.2015.12.019
Musher, D. M., and Thorner, A. R. (2014). Community-acquired pneumonia. N. Engl. J. Med. 371, 1619–1628. doi: 10.1056/NEJMra1312885
Portier, H., Brambilla, C., Garre, M., Paganin, F., Poubeau, P., and Zuck, P. (2005). Moxifloxacin monotherapy compared to amoxicillin-clavulanate plus roxithromycin for nonsevere community-acquired pneumonia in adults with risk factors. Eur. J. Clin. Microbiol. Infect. Dis. 24, 367–376. doi: 10.1007/s10096-005-1347-1
Postma, D. F., van Werkhoven, C. H., van Elden, L. J., Thijsen, S. F., Hoepelman, A. I., and Kluytmans, J. A., et al. (2015). Antibiotic treatment strategies for community-acquired pneumonia in adults. N. Engl. J. Med. 372, 1312–1323. doi: 10.1056/NEJMoa1406330
Raja, A., Lebbos, J., and Kirkpatrick, P. (2004). Telithromycin. Nat Rev Drug Discov. 3(9):733-734. doi: 10.1038/nrd1502
Shao, C. Z., He, L. X., Wang, G. F., Zhou, X., Shen, C., and Li, H. P., et al. (2008). A randomized controlled multicentre clinical trial of levofloxacin sequential therapy compared with combination therapy with cefuroxime and azithromycin in patients with community-acquired pneumonia (Chinese). Chin. J. Infect. Chemother. 8, 102–106. doi: 10.3321/j.issn:1009-7708.2008.02.005
Suda, K. J., Hicks, L. A., Roberts, R. M., Hunkler, R. J., Matusiak, L. M., and Schumock, G. T. (2018). Antibiotic expenditures by medication, class, and healthcare setting in the united states, 2010-2015. Clin Infect Dis. 6, 185–190. doi: 10.1093/cid/cix773
Tenson, T., Lovmar, M., and Ehrenberg, M. (2003). The mechanism of action of macrolides, lincosamides and streptogramin B reveals the nascent peptide exit path in the ribosome. J. Mol. Biol. 25, 1005–1014. doi: 10.1016/S0022-2836(03)00662-4
Thomas, C. P., Ryan, M., Chapman, J. D., Stason, W. B., Tompkins, C. P., and Suaya, J. A., et al. (2012). Incidence and cost of pneumonia in medicare beneficiaries. Chest. 142, 973–981. doi: 10.1378/chest.11-1160
Vardakas, K. Z., Siempos, I. I., Grammatikos, A., Athanassa, Z., Korbila, I. P., and Falagas, M. E. (2008). Respiratory fluoroquinolones for the treatment of community-acquired pneumonia: a meta-analysis of randomized controlled trials. CMAJ 179, 1269–1277. doi: 10.1503/cmaj.080358
Walsh, C. (2003). Where will new antibiotics come from? Nat. Rev. Microbiol. 1, 65-70. doi: 10.1038/nrmicro727
Welte, T., Petermann, W., Schürmann, D., Bauer, T. T., and Reimnitz, P. (2005). Treatment with sequential intravenous or oral moxifloxacin was associated with faster clinical improvement than was standard therapy for hospitalized patients with community-acquired pneumonia who received initial parenteral therapy. Clin. Infect. Dis. 41, 1697–1705. doi: 10.1086/498149
Welte, T., Torres, A., and Nathwani, D. (2012). Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax. 67, 71–79. doi: 10.1136/thx.2009.129502
Woodhead, M., Blasi, F., Ewig, S., Garau, J., Huchon, G., and Ieven, M., et al. (2011). Guidelines for the management of adult lower respiratory tract infections–full version. Clin. Microbiol. Infect. 17 (Suppl. 6), E1–E59. doi: 10.1111/j.1469-0691.2011.03672.x
Writing Group of Guidance for Clinical Trials of Anti-bacterial Drugs. (2014). Guidance for clinical trials of anti- bacterial drugs (Chinese). Chin. J. Clin. Pharmacol. 30, 844–856. doi: 10.13699/j.cnki.1001-6821.2014.09.030
Xu, S., Xiong, S., Xu, Y., Liu, J., Liu, H., and Zhao, J., et al. (2006). Efficacy and safety of intravenous moxifloxacin versus cefoperazone with azithromycin in the treatment of community acquired pneumonia. J. Huazhong Univ. Sci. Technol. Med. Sci. 26, 421–424. doi: 10.1007/s11596-006-0411-0
Yang, S. G., and Zhang, S. H. (2009). Efficacy and cost-effectiveness analysis of moxifloxacin hydrochloride and combination of ceftriaxone sodium with azithromycin in the treatment of moderate to severe community acquired pneumonia in elderly patients (Chinese). Chin. J. New. Drugs 28, 962–964. Available online at: http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgxyzz200910028
Zervos, M., Mandell, L. A., Vrooman, P. S., Andrews, C. P., McIvor, A., and Abdulla, R. H., et al. (2004). Comparative efficacies and tolerabilities of intravenous azithromycin plus ceftriaxone and intravenous levofloxacin with step-down oral therapy for hospitalized patients with moderate to severe community-acquired pneumonia. Treat. Respir. Med. 3, 329–336. doi: 10.2165/00151829-200403050-00006
Zhang, J. Y., Yin, C., Dong, B. R., Liu, Y. J., Chen, B., and Yang, R. X. (2006). Multicentre clinical study on levofloxacin intravenous injection for treatment of community-acquired pneumonia (Chinese). Int. J. Respir. 26, 245–247. doi: 10.3760/cma.j.issn.1673-436X.2006.04.002
Keywords: community-acquired pneumonia, fluoroquinolones, β-lactams, macrolides, systematic review, meta-analysis, randomized controlled trial
Citation: Liu S, Tong X, Ma Y, Wang D, Huang J, Zhang L, Wu M, Wang L, Liu T and Fan H (2019) Respiratory Fluoroquinolones Monotherapy vs. β-Lactams With or Without Macrolides for Hospitalized Community-Acquired Pneumonia Patients: A Meta-Analysis. Front. Pharmacol. 10:489. doi: 10.3389/fphar.2019.00489
Received: 23 December 2018; Accepted: 17 April 2019;
Published: 08 May 2019.
Edited by:
Tahir Mehmood Khan, University of Veterinary and Animal Sciences, PakistanReviewed by:
Lu Zhao, Zhejiang University, ChinaAmer Hayat Khan, University of Science, Malaysia, Malaysia
Copyright © 2019 Liu, Tong, Ma, Wang, Huang, Zhang, Wu, Wang, Liu and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Fan, ZmFuaG9uZ2ZhbkBxcS5jb20=
 Sitong Liu
Sitong Liu Xiang Tong
Xiang Tong Yao Ma
Yao Ma Dongguang Wang
Dongguang Wang Jizhen Huang
Jizhen Huang Li Zhang
Li Zhang Man Wu
Man Wu Lei Wang
Lei Wang Tao Liu
Tao Liu Hong Fan
Hong Fan