- 1Department of Anesthesia, An Nan Hospital, China Medical University, Tainan, Taiwan
- 2Graduate Institute of Medical Sciences, Chang Jung Christian University, Tainan, Taiwan
- 3Division of Cardiovascular Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan
- 4Institute of Imaging and Biomedical Photonics, National Chiao Tung University, Tainan, Taiwan
- 5Department of Physiology, National Cheng Kung University Medical College, Tainan, Taiwan
- 6Institute of Basic Medical Sciences, National Cheng Kung University Medical College, Tainan, Taiwan
Carfilzomib (CFZ, Kyprolis®) is widely recognized as an irreversible inhibitor of proteasome activity; however, its actions on ion currents in electrically excitable cells are largely unresolved. The possible actions of CFZ on ionic currents and membrane potential in pituitary GH3, A7r5 vascular smooth muscle, and heart-derived H9c2 cells were extensively investigated in this study. The presence of CFZ suppressed the amplitude of delayed-rectifier K+ current (IK(DR)) in a time-, state-, and concentration-dependent manner in pituitary GH3 cells. Based on minimal reaction scheme, the value of dissociation constant for CFZ-induced open-channel block of IK(DR) in these cells was 0.33 µM, which is similar to the IC50 value (0.32 µM) used for its efficacy on inhibition of IK(DR) amplitude. Recovery from IK(DR) block by CFZ (0.3 µM and 1 µM) could be well fitted by single exponential with 447 and 645 ms, respectively. The M-type K+ current, another type of K+ current elicited by low-threshold potential, was slightly suppressed by CFZ (1 µM). Under current-clamp condition, addition of CFZ depolarized GH3 cells, broadened the duration of action potentials as well as raised the firing frequency. In A7r5 vascular smooth muscle cells or H9c2 cardiac cells, the CFZ-induced inhibition of IK(DR) remained efficacious. Therefore, our study led us to reflect that CFZ or other structurally similar compounds should somehow act on the activity of membrane KV channels through which they influence the functional activities in different types of electrically excitable cells such as endocrine, neuroendocrine cells, smooth muscle cells, or heart cells, if similar in vivo findings occur.
Introduction
Carfilzomib (CFZ, Kyprolis®), an analog of epoxomicin, is a second-generation irreversible tetrapeptide epoxyketone class proteasome inhibitor which is recognized as an anti-cancer drug. CFZ was known to target the chymotrypsin-like β5 subunit of the constitutive 20S proteasome or the β5i subunit of the immunoproteasome 20Si, along with minimal cross reactivity to other proteases (Zhou et al., 2009; Dou and Zonder, 2014; Kaplan et al., 2017). Predominantly through the inhibition of proteasome-mediated proteolysis, the presence of CFZ can induce cell cycle arrest and apoptosis in different types of human cancer cell lines including multiple myeloma, lymphoma, and various solid tumors (Demo et al., 2007; Zhang and Sidhu, 2014; Kaplan et al., 2017). Moreover, there were case reports on CFZ associated neurotoxicity (Kaplan et al., 2017; Karademir et al., 2018; Luczkowska et al., 2018). However, how this agent and other structurally similar compounds exert any perturbations on membrane ionic currents is not thoroughly investigated, although CFZ might manipulate the energy and redox metabolism through a mechanism independent of its inhibition of proteasome activity (Soriano et al., 2016).
Recently, acute administration of CFZ was shown to produce short-term modifications on vascular and myocardial properties such as myocardial ischemia, arterial hypertension, or acute renal dysfunction (Gavazzoni et al., 2018; Heckmann et al., 2018; Efentakis et al., 2019). They reported that cardiotoxicity of CFZ could have been linked to its action through inhibition of AMPKα phosphorylation and autophagy-related proteins (Efentakis et al., 2019). Furthermore, previous studies have demonstrated that proteasome inhibitors (e.g., PSI [N-[(phenylmethoxy)carbonyl]-L-isoleucyl-L-α-glutamyl-tert-butyl ester-N-[(1S)-1-formyl-3-methylbutyl]-L-alaninamide]) could induce apoptotic changes in pituitary tumor (GH3) cells, suggesting that these agents were thought to be potential novel agents for treating pituitary tumors not amenable to other treatments (Yu et al., 2002).
Here, we investigated the effects of CFZ on ionic currents in pituitary tumor (GH3) cells, A7r5 vascular smooth muscle cells and heart-derived H9c2 cells. The possible efficacy of CFZ on spontaneous action potentials (APs) in GH3 cells was also examined. Findings from our study revealed that CFZ can interact directly with KV channels to alter the amplitude and gating (i.e., inactivation kinetics) of IK(DR) in response to membrane depolarization.
Materials and Methods
Chemicals, Drugs and Solutions
Carfilzomib (CFZ, Kyprolis®, (αS)-α-[[2-(4-morpholinyl)acetyl]amino]benzenebutanoyl-L-leucyl-N-[(1S)-3-methyl-1-[[(2R)-2-methyl-2-oxiranyl]carbonyl]butyl]-L-phenylalaninamide, C40H57N5O7) was obtained from Cayman Chemical (Ann Arbor, MI), and ML-213 and 9-phenanthrol were from Tocris (Bristol, UK), while 4-aminopyridine, diazoxide, tetraethyl ammonium chloride and tetrodotoxin were from Sigma-Aldrich (St. Louis, MO). For cell preparations, all culture media, fetal bovine serum, horse serum, L-glutamine, trypsin/EDTA, penicillin-streptomycin, fungizone, and trypsin were obtained from Invitrogen (Carlsbad, CA). All other chemicals, such as CdCl2, HEPES and aspartic acid, were commercially available and of reagent grade. The water was de-ionized using a Milli-Q water purification system (Millipore, Bedford, MA).
The composition of the extracellular solution (i.e., HEPES-buffered normal Tyrode’s solution) was 136.5 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 0.53 mM MgCl2, 5.5 mM glucose, and 5.5 mM HEPES-NaOH buffer, pH 7.4. To measure whole-cell M-type K+ current (IK(M)), high K+-bathing solution was composed of the following: 145 mM KCl, 0.53 mM MgCl2, and 5 mM HEPES-KOH buffer, pH 7.4. To record either whole-cell IK(DR) and IK(M) or membrane potential, we filled the recording pipettes by using a solution consisting of 130 mM K-aspartate, 20 mM KCl, 1 mM KH2PO4, 1 mM MgCl2, 3 mM Na2ATP, 0.1 mM Na2GTP, 0.1 mM EGTA, and 5 mM HEPES-KOH buffer, pH 7.2. The pipette solution used was filtered on the day of use with a syringe filter with a 0.22 mm pore size (Millipore).
Cell Preparations
GH3, a clonal cell line derived from a rat prolactin-secreting pituitary tumor, was obtained from the Bioresources Collection and Research Center (BCRC-60015; Hsinchu, Taiwan). Briefly, cells were cultured in Ham’s F-12 medium supplemented with 15% heat-inactivated horse serum (v/v), 2.5% fetal calf serum (v/v) and 2 mM L-glutamine in a humidified environment of 5% CO2/95% air (Wu et al., 2000; Wu et al., 2017). To promote cell differentiation, GH3 cells were transferred to a serum-free, Ca2+-free medium. Under our experimental conditions, cells remained 80–90% viable for at least two weeks.
The A7r5 cell line, derived from rat thoracic aortae, was obtained from American Type Culture Collection (ATCC; [CRL-1444], Manassas, VA), while the H9c2 cell line, derived from embryonic rat ventricles, also from ATCC ([CRL-1446]). A7r5 or H9c2 cells were maintained and subcultured in Dulbecco’s modified Eagle’s medium supplemented with heat-inactivated fetal bovine serum (10%), and 2 mM glutamine. They were grown in monolayer culture in 50 ml plastic culture flasks in a humidified environment of 5% CO2/95% air at 37 °C. The experiments were commonly performed after GH3, A7r5 or H9c2 cells reached confluence (usually 5–7 days).
Electrophysiological Measurements and Recordings
Shortly before the electrical recordings, cells (e.g., GH3, A7r5, or H9c2 cells) were harvested and transferred to a home-made recording chamber positioned on the stage of an inverted microscope. Cells were immersed at room temperature (22–25 °C) in normal Tyrode’s solution, the composition of which was described above. Patch-clamp recordings under the whole-cell mode were achieved with either an RK-400 (Bio-Logic, Claix, France) or an Axopatch-200B amplifier (Molecular Devices, Sunnyvale, CA) (Wu et al., 2000; Wu et al., 2017). Patch electrodes with tip resistances of 3–5 MΩ were made of Kimax-51 borosilicate capillaries (#34500; Kimble, Vineland, NJ) on either a PP-830 puller (Narishige, Tokyo, Japan) or a P-97 horizontal puller (Sutter, Novato, CA), and then fire-polished with an MF-83 microforge (Narishige). The signals, comprising voltage and current tracings, were stored online at 10 kHz in an ASUSPRO-BU401LG computer (ASUS, Taipei City, Taiwan) controlled by pCLAMP 10.7 software (Molecular Devices). Changes in membrane potential recorded from GH3 cells were measured under current-clamp configuration. In a separate set of whole-cell IK(DR) recordings with intracellular dialysis, the recording pipettes used were filled with the internal solution containing 0.3 µM CFZ.
Data Analyses
To evaluate concentration-dependent inhibition of CFZ on the amplitude of IK(DR), cells were immersed in Ca2+-free Tyrode’s solution. Each cell examined was maintained at −50 mV and 1−s depolarizing pulse from −50 to +50 mV was delivered. Current amplitudes at the level of +50 mV in response to depolarizing pulses were measured in the control and during cell exposure to different concentrations (0.01–10 µM) of CFZ. The concentration required to suppress 50% of current amplitude was determined by use of a Hill function:
where [C] represents the CFZ concentration; IC50 and nH are the concentration required for a 50% inhibition and the Hill coefficient, respectively; and maximal inhibition (i.e., 1−a) was also estimated from this equation. This equation converged reliably to produce the best-fit line and parameter estimates in Figure 2D.
To evaluate the steady-state inactivation curve of IK(DR) during cell exposure to different CFZ concentrations, the relationships between the normalized amplitude of IK(DR) and the conditioning potentials achieved in the presence of 0.3 or 1 µM CFZ were appropriately fitted with a Boltzmann function of the following form:
where Imax is the maximal peak amplitude of IK(DR) taken at the level of +50 mV, V1/2 the voltage at which half-maximal inhibition occurs, q the apparent gating charge of the inactivation curve, F Faraday’s constant, R the universal gas constant, and T the absolute temperature.
Statistical Analyses
Linear or nonlinear curve-fitting (e.g., sigmoidal or exponential fitting) to data sets shown in this work was achieved with least-squares minimization procedure by use of different maneuvers including Microsoft Solver function embedded in Excel (Microsoft) and 64-bit OriginPro 2016 program (OriginLab). The averaged results are presented as the mean ± standard error of the mean (SEM) with sample sizes (n) indicating the cell numbers from which the results were collected. The paired or unpaired Student’s t-test and a one-way analysis of variance (ANOVA) followed by post-hoc Fisher’s least-significance difference method, were implemented for statistical evaluation of differences among means. Assuming that normality underlying ANOVA was violated, we used non-parametric Kruskal–Wallis test. Statistical analyses were performed using the SPSS 20 statistical software package (IBM Corp., Armonk, New York). Statistical significance was determined at a P-value of <0.05.
Results
Effect of CFZ on Delayed-Rectifier K+ Current (IK(DR)) in GH3 Cells
The whole-cell configuration of the patch-clamp technique was employed to investigate any modifications of CFZ on ionic currents inherently in GH3 cells. In an initial set of experiments, to measure IK(DR) and to avoid contamination of other types of ionic currents such as voltage-gated Na+ and Ca+ currents, and Ca2+-activated K+ currents, we bathed cells in Ca2+-free Tyrode’s solution containing tetrodotoxin (1 µM) and CdCl2 (0.5 mM), and, during electrophysiological recordings, we filled the recording pipette by using K+-containing solution. The compositions of these solutions used are detailed in Materials and Methods. As shown in Figures 1A, B, when the examined cell was maintained at −50 mV and a series of voltage pulses ranging from −60 to +50 mV in 10-mV increments were thereafter delivered, a family of K+ outward currents with slight inactivation was readily elicited. These outward K+ currents in response to membrane depolarization were previously proofed as the delayed-rectifier K+ current (IK(DR)) (Wu et al., 2000; Wang et al., 2008b). As cells were exposed to 1 mM tetraethyl ammonium chloride, a blocker of IK(DR), the amplitude of IK(DR) elicited by membrane depolarization from −50 to +50 mV was decreased to 86 ± 9 pA (n = 9, P < 0.05) from a control value of 282 ± 22 pA. However, the presence of 1 mM 4-aminopyridine, a blocker of transient outward K+ current, did not alter the amplitude of IK(DR) (282 ± 22 pA [in the absence of 4-aminopyridine] versus 281 ± 24 pA [in the presence of 4-aminopyridine; n = 11, P > 0.05]). Of particular interest, during exposure of cells to CFZ (0.3 µM for 1 min), the amplitude of IK(DR) measured at the end of 1−s depolarizing pulse was significantly reduced at the potentials ranging from 0 to +50 mV. For example, upon membrane depolarization from −50 to +50 mV, the presence of CFZ (0.3 µM) significantly decreased current density at the end of the voltage pulse from 10.1 ± 0.9 to 3.3 ± 0.6 pA/pF (n = 9, P < 0.05). This inhibitory effect was reversed to 7.6 ± 0.7 pA/pF (n = 7, P < 0.05) on washout of CFZ. Similarly, as cells were exposed to 0.3 µM CFZ, the whole-cell IK(DR) conductances (at the peak or steady-state level) measured at the voltages ranging between 0 and +50 mV were reduced (Figures 1C, D). However, as intracellular dialysis with 0.3 µM CFZ, the IK(DR) amplitude at the end of depolarizing pulse from −50 to +50 mV was not altered (356 ± 27 pA [in control] versus 354 ± 28 pA [in the presence of 0.3 µM CFZ]; n = 7, P > 0.05). Moreover, the activation kinetics of IK(DR) (i.e., maximal dI/dt value in the rising phase of the current by membrane depolarization from −50 to +50 mV) did not differ between the absence and presence of 0.3 µM CFZ (26.1 ± 3.2 pA/ms [in control] versus 26.3 ± 3.4 pA/ms [in the presence of 0.3 µM CFZ]; n = 9, P > 0.05).
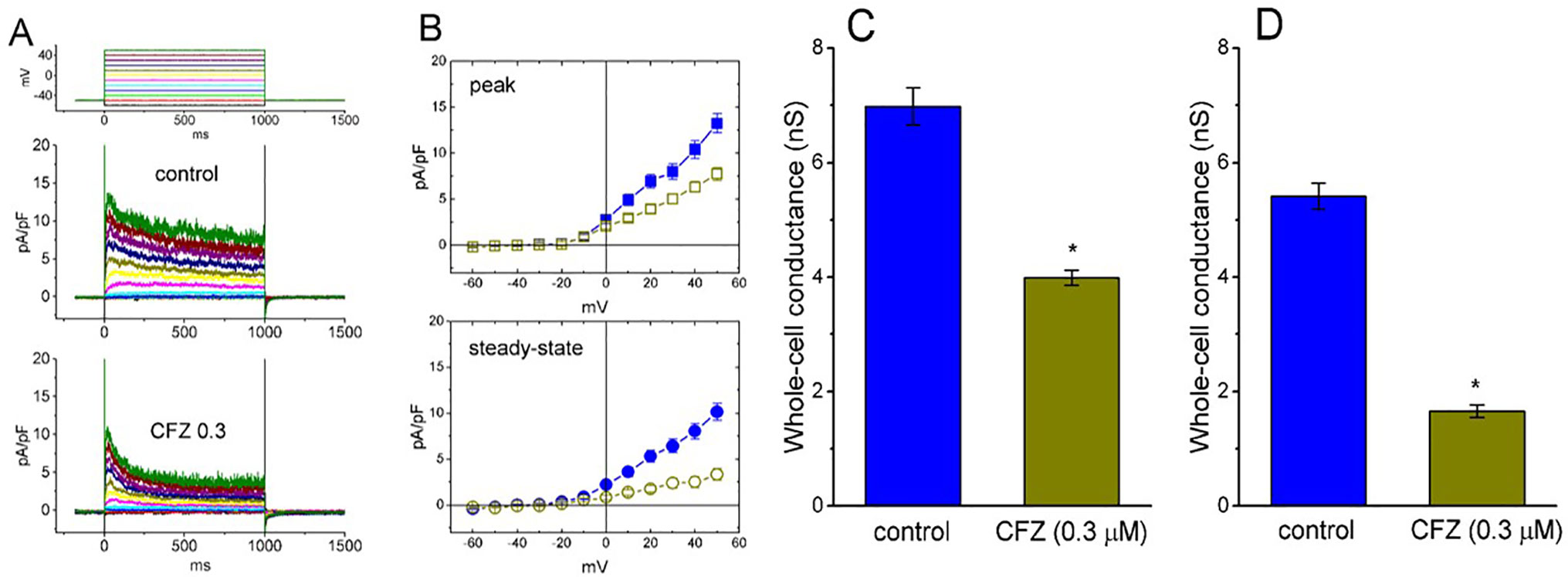
Figure 1 Effect of CFZ on IK(DR) density in GH3 cells. In these whole-cell current recordings, cells were immersed in Ca2+-free Tyrode’s solution and the recording pipette was filled with K+-containing solution. The examined cells were held at −50 mV and different voltage pulses ranging from −60 to +50 mV in 10-mV increments were applied. (A) Representative traces for IK(DR) density taken from the absence (upper; control) and presence (lower; CFZ 0.3) of 0.3 μM CFZ. The upper part in (A) indicates the voltage protocol applied. (B) Averaged current density versus voltage relationships of IK(DR) measured at the beginning (filled symbols; upper [peak]) and end (open symbols; lower [steady-state]) of each depolarizing pulse. The data points in the upper (peak) and lower (steady-state) panels were obtained from the absence (square symbols) and presence (circle symbols) of 0.3 μM CFZ, respectively. Each data point represents mean ± SEM (n = 9). Summary bar graphs in (C) and (D) respectively show the whole-cell conductances of IK(DR) at the beginning (i.e., peak conductance) and end (i.e., steady-state conductance) of depolarizing pulses ranging between 0 and +50 mV (mean ± SEM; n = 8 for each bar). *Significantly different from controls (P < 0.05).
Of note, an increase in the decaying time course of IK(DR) in response to sustained membrane depolarization was conceivably observed in the presence of CFZ (Figure 1A). These currents obtained after addition of CFZ activated a maximum and then rapidly decayed over time to a small fraction of their peak values seen at the voltages between 0 and +50 mV. However, in the presence of CFZ, there was no significant change in the activation kinetics (i.e., dI/dt) of these currents elicited by membrane depolarization. Averaged I–V relations for the amplitude of initial and steady-state components of IK(DR) in the control and during the exposure to CFZ (0.3 µM) are shown in Figure 1B, respectively, indicating that the presence of CFZ diminished the IK(DR) amplitude in a time-dependent manner, because of the increased relaxation in inactivation time course of IK(DR).
Kinetic Study of CFZ-Induced Block of IK(DR) in GH3 Cells
To provide quantitative estimate for CFZ-induced block of IK(DR), we further analyzed the time-dependent trajectories indicating relative block of IK(DR) (i.e., (Icontrol−ICFZ)/Icontrol) observed in these cells. We then fitted the time courses of relative block in the presence of different CFZ concentrations by a single-exponential function. The concentration dependence of IK(DR) decay elicited by long-lasting membrane depolarization was shown in Figures 2A, B. The results clearly showed that with the presence of CFZ, it produced a concentration-dependent raise in the rate (1/τ) of relative block. For example, as cells were depolarized from –50 to +50 mV with a duration of 1 s, the time constants (τ) of IK(DR) relative block obtained in the presence of 0.1, 0.3 and 1 µM CFZ were least-squares fitted by single exponential with the values of 173 ± 12, 88 ± 9, and 38 ± 7 ms (n = 9), respectively.
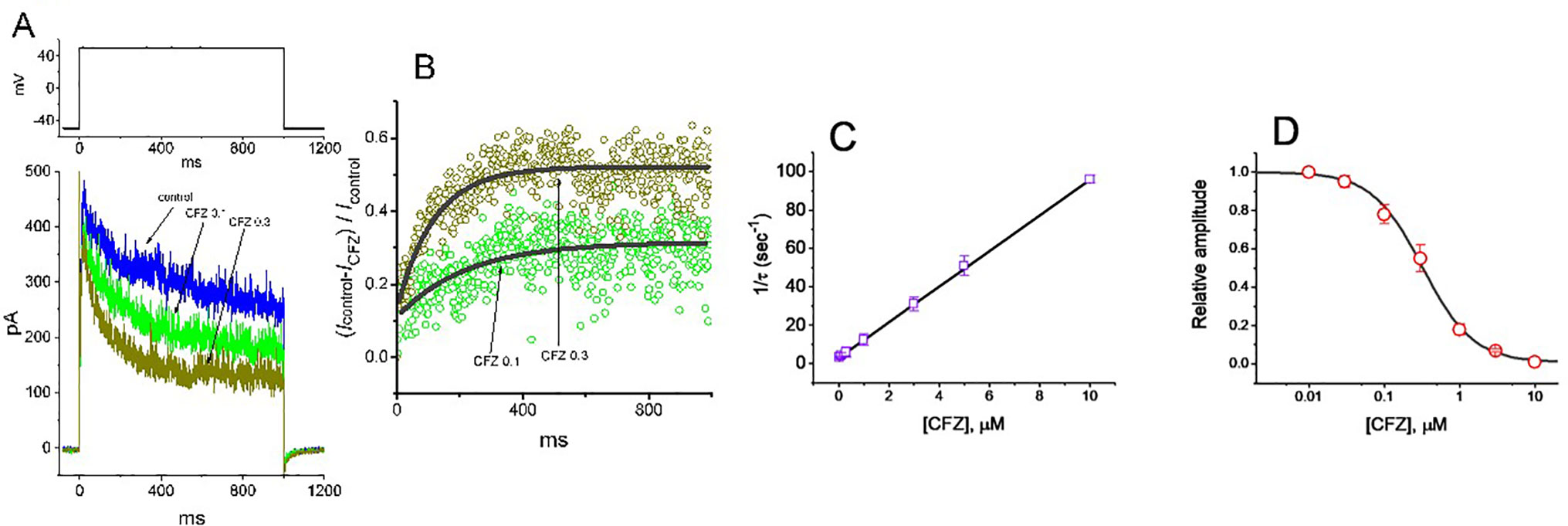
Figure 2 Evaluation of the kinetics of CFZ-induced block of IK(DR) elicited by membrane depolarization in GH3 cells. The IK(DR) established by depolarizing pulse from −50 to +50 mV with a duration of 1 s (indicated in the upper part of (A)) was measured as cells were exposed to different CFZ concentrations. In (A), representative IK(DR) traces was obtained in the absence (control) and presence of 0.1 μM CFZ (CFZ 0.1) or 0.3 μM CFZ (CFZ 0.3). In (B), the time courses of relative block of IK(DR) by 0.1 μM CFZ (CFZ 0.1) and 0.3 μM CFZ (CFZ 0.3) were well fitted by single exponential with a value of 181 and 91 ms (indicated by the smooth curves). The relative block (i.e., (Icontrol−ICFZ)/Icontrol) was evaluated by dividing the CFZ-sensitive current by the current taken from the control (i.e., in the absence of CFZ). In (C), the reciprocal of time constant (i.e., 1/τ) of relative block versus the CFZ concentration was plotted. Data points appearing in open squares were fitted by a linear regression, indicating that there is a molecularity of one. Mean ± SEM (n = 9 for each point). (D) Concentration-dependent inhibition of IK(DR) by CFZ (mean ± SEM; n = 9 for each point). Each cell was maintained at −50 mV and the depolarizing pulses from −50 to +50 mV with a duration of 1 s were applied. Current amplitudes measured at the end of depolarizing pulses in the presence of different CFZ concentrations were compared with the control value. The smooth curve was appropriately fitted by the Hill function detailed in Materials and Methods. The IC50 value obtained in the presence of CFZ was calculated to be 0.32 µM.
The inhibitory action of CFZ on IK(DR) measured from GH3 cells is explained by state-dependent block that preferentially binds to the open state of the channel. A minimal kinetic scheme was derived as the following:
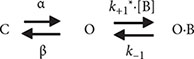
where [B] is the CFZ concentration; α and β are the voltage-gated rate constants for the opening and closing of KV channels, respectively; k+1* and k−1 those for blocking and unblocking produced by the presence of CFZ, respectively; and C, O, and OˑB shown in the scheme represent the closed, open, and open-blocked states, respectively.
The blocking (i.e., on) and unblocking (i.e., off) rate constants, k+1* and k−1, were determined from time constants (τ) of depolarization-elicited relative block (i.e., (Icontrol−ICFZ)/Icontrol) of IK(DR) (Caballero et al., 2004) obtained in different concentrations (0.01–5 µM) of CFZ. These rate constants (1/τ) were then allowed to be computed using the relation (Figure 2C):
where k+1* and k−1 are respectively acquired from the slope and from the y-axis intercept at [B] = 0 of the linear regression in which the reciprocal time constants (1/τ) versus the CFZ concentrations were interpolated, and [B] indicates the CFZ concentration.
Specifically, on the basis of the first-order reaction scheme, the relationship between 1/τ and [B] became linear with a correlation coefficient of 0.97 (Figure 2C). The blocking and unblocking rate constants were calculated to be 9.278 s−1µM−1 and 3.018 s−1, respectively; consequently, the results gave the value of dissociation constant (KD = k1/k+1*) of 0.33 µM.
Effect of CFZ on the Steady-State Inactivation Curve of IK(DR) in GH3 Cells
The steady-state inactivation of IK(DR) during cell exposure to CFZ was further studied. In this set of experiments, cells were bathed in Ca2+-free Tyrode’s solution and the electrode was filled with K+-containing solution. As whole-cell configuration was firmly established, we applied a two-pulse protocol to the examined cells which were exposed to different CFZ concentrations. The inactivation parameters of IK(DR) were then measured in the absence and presence of CFZ (1 or 3 µM). As illustrated in Figure 3, the normalized amplitudes of IK(DR) (i.e., I/Imax) was constructed against the conditioning potential and the smooth curve was fitted by a Boltzmann function described under Materials and Methods. In control (i.e., in the absence of CFZ), V1/2 = −46.3 ± 2.3 mV and q = 2.4 ± 0.3 e (n = 8). In the presence of 0.3 µM CFZ, V1/2 = −30.1 ± 2.2 mV and q = 3.6 ± 0.2 e (n = 8), while in the presence of 1 µM CFZ, V1/2 = −41.2 ± 2.1 mV and q = 3.6 ± 0.2 e (n = 8). Results from the above experiments showed that during the exposure to different CFZ concentrations, the inactivation curve of IK(DR) measured from GH3 cells could be altered.
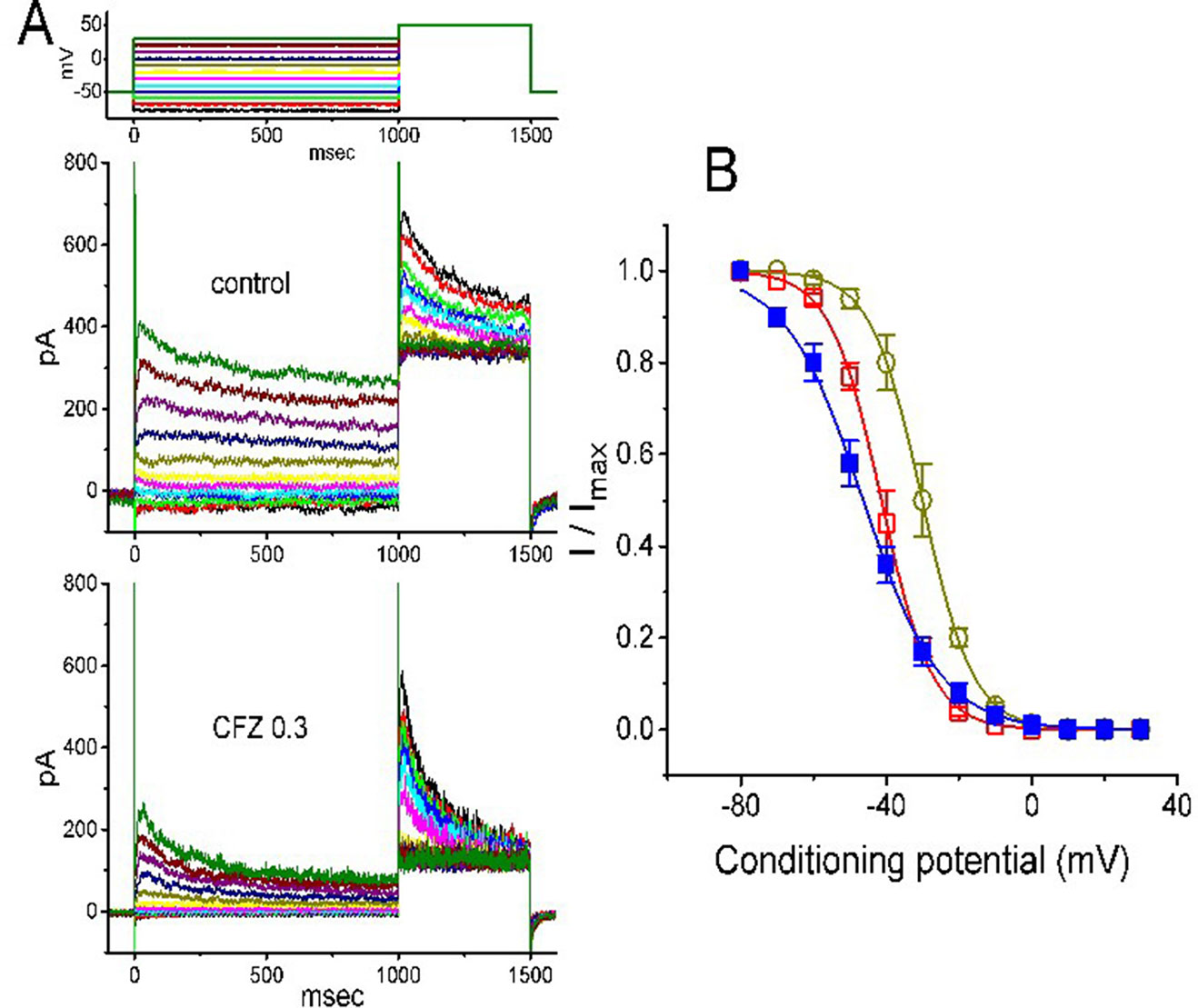
Figure 3 Effect of CFZ on the steady-state inactivation of IK(DR) in GH3 cells. (A) Representative IK(DR) traces obtained in the absence (upper) and presence (lower) of 0.3 µM CFZ. As the examined cell was held at −50 mV, the conditioning voltage pulses with a duration of 1 s to various membrane potentials ranging from −80 to +30 mV were delivered. Following each condition pulse applied, a test pulse to +50 mV with a duration of 500 ms was then applied to elicit IK(DR). The upper part indicates the voltage protocol used. (B) Steady-state inactivation curve of IK(DR) in the absence (filled squares) and presence of 0.3 µM (open circles) and 1 µM (open squares) CFZ (mean ± SEM; n = 8 for each point). The smooth curves were least-squares fitted by a Boltzmann function (detailed under Materials and Methods).
Recovery From IK(DR) Block Induced by CFZ
In attempts to evaluate CFZ-induced block of IK(DR) in GH3 cells, recovery from block was again investigated. In this set of experiments, a stimulation protocol consisted of a first (conditioning) depolarizing pulses which are sufficiently long to allow block to reach a steady state was used. While cells were exposed to CFZ (0.3 or 1 µM), the membrane potential was stepped to +50 mV from −50 mV for a variable time, after then a second depolarizing pulse (test pulse) was applied at the same potential as the conditioning pulse (Figure 4A). Then, the ratios of the peak IK(DR) in response to the second (test) and first (conditioning) pulse were taken as a measure of recovery from block, and plotted versus interpulse interval. As shown in Figure 4A, the recovery of IK(DR) was occurring so fast that the protocol used was not adequate to measure it. Hence, the time course for the recovery of IK(DR) block was unable to be well illustrated. However, in our experimental protocol, IK(DR) recovery during the exposure to 0.3 µM CFZ was generally complete, and its time course described by a single exponential with time constant of 447 ± 22 ms (n = 7). Moreover, the time constant of such recovery was significantly raised to 645 ± 25 ms (n = 7, P < 0.05) during cell exposure to 1 µM CFZ (Figure 4B), strongly suggesting that the slowing of IK(DR) recovery produced by the presence of CFZ may result from open channel block.
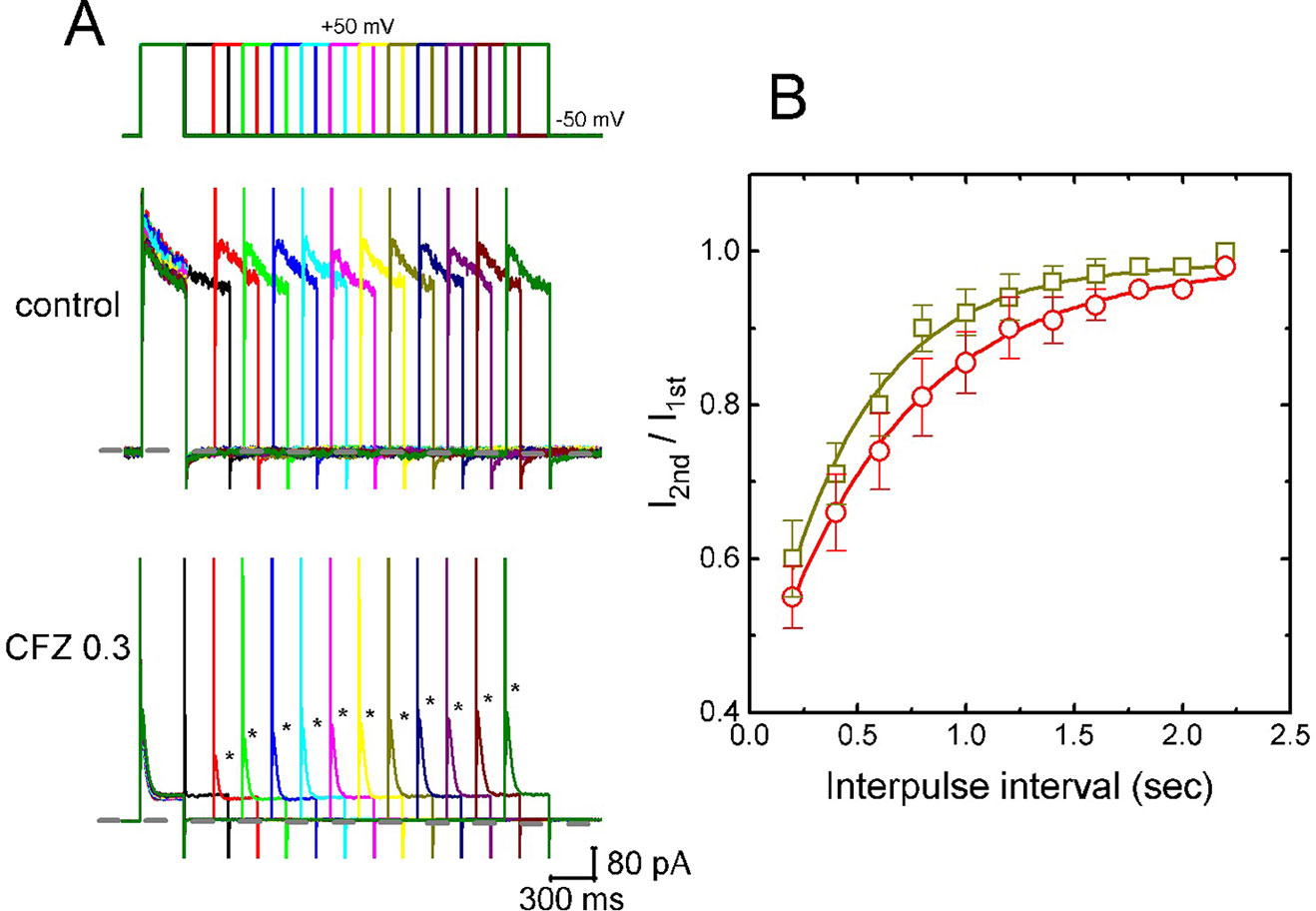
Figure 4 Time course of the recovery of IK(DR) in the presence of 0.3 and 1 µM CFZ. In these experiments, GH3 cells, bathed in Ca2+-free Tyrode’s solution, were depolarized from −50 to +50 mV with a duration of 300 ms and different interpulse durations were applied. (A) Representative IK(DR) traces in the absence (upper) and presence (lower) of 0.3 µM CFZ elicited by a two-pulse protocol with different interpulse durations (indicated in the upper part). Dashed line indicates the zero current level. (B) Time courses of recovery from IK(DR) inactivation caused by 0.3 µM CFZ (open squares) and 1 µM CFZ (open circles), respectively. Smooth curves in the presence of different CFZ concentrations were fitted by single exponential. Each point represents the mean ± SEM (n = 8). Asterisks indicate the peak IK(DR) elicited by different interpulse intervals.
Effect of CFZ on M-Type K+ Current (IK(M)) in GH3 Cells
Here, we further examined if addition of CFZ exerts any perturbations on other type of K+ current (i.e., IK(M)) inherently in GH3 cells. In this set of experiments, to amplify the magnitude of IK(M) and preclude contamination of Ca2+-activated K+ currents, we bathed cells in high-K+, Ca2+-free solution and the recording pipettes used were filled with K+-containing solution (Chen et al., 2018; Hsiao et al., 2019; Liu et al., 2019; Lu et al., 2019). As cells were exposed to ML-213 (10 µM), an activator of IK(M) (Yu et al., 2011), the amplitude of IK(M) in response to membrane depolarization from -50 to −10 mV increased from 34 ± 11 to 57 ± 14 pA (n = 9, P < 0.05). As shown in Figure 5, cell exposure to 0.3 µM CFZ had minimal effect on IK(M) elicited by membrane depolarization from −50 to −20 mV with a duration of 1 s. However, addition of 1 µM CFZ significantly diminished IK(M) amplitude, as evidenced by a significant reduction of current density from 1312 ± 172 to 884 ± 153 fA/pF (n = 9, P < 0.05). Alternatively, in continued presence of 1 µM CFZ, further application of ML-213 (10 µM) significantly reversed IK(M) density to 1324 ± 175 fA/pF (n = 8, P < 0.05). ML-213 was previously reported to stimulate IK(M) (Yu et al., 2011). The results thus suggest that the IK(M) in GH3 cells is relatively resistant to suppression by CFZ.
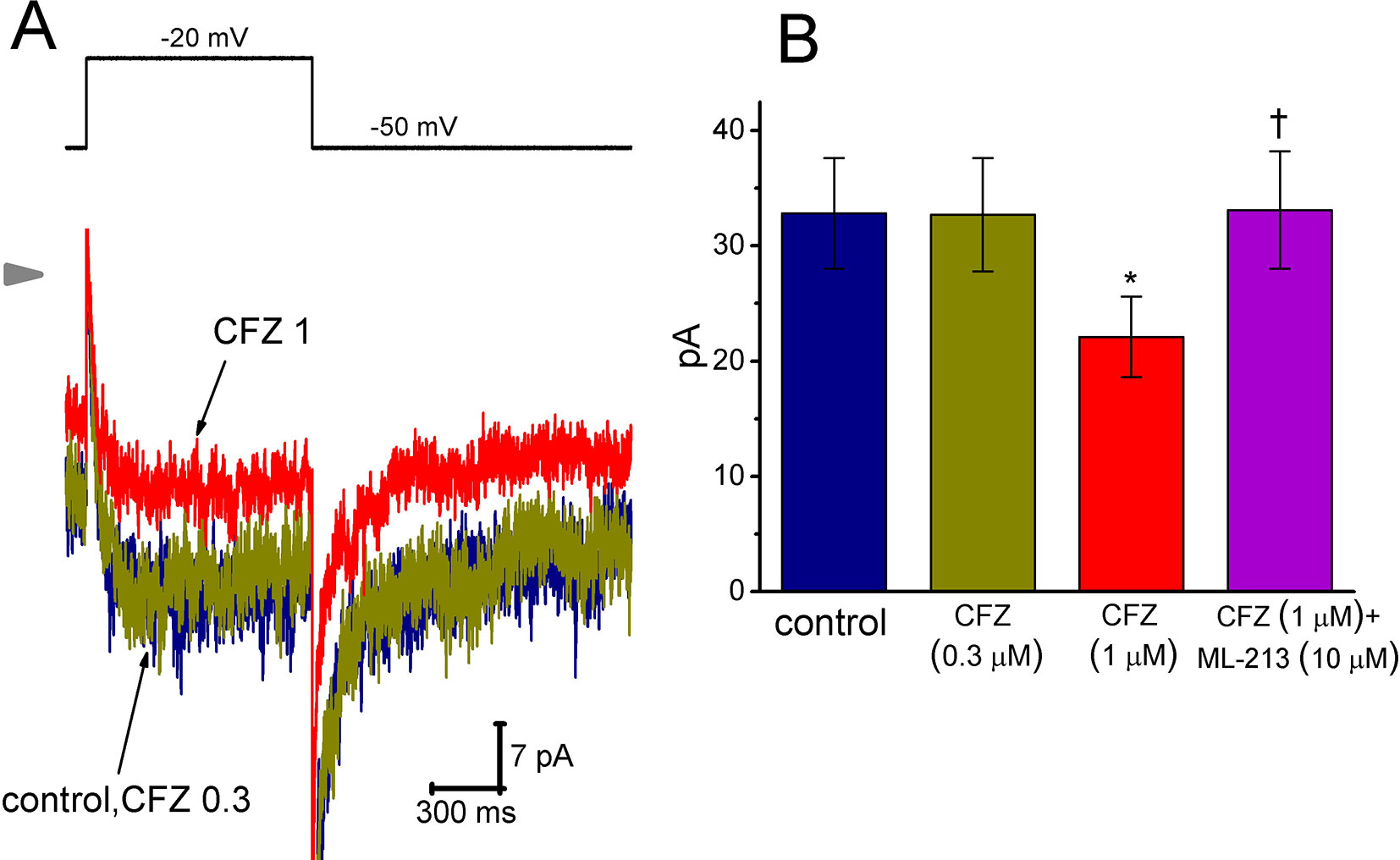
Figure 5 Effect of CFZ on IK(M) in GH3 cells. To record IK(M), we bathed cells in high-K+, Ca2+-free solution and filled the recording pipette by using K+-containing solution. (A) Representative IK(M) traces in response to membrane depolarization (indicated in the upper part). CFZ 0.3:0.3 µM CFZ; CFZ 1:1 µM CFZ. Arrowhead shows the zero current level. (B) Bar graph showing the effects of CFZ and CFZ plus ML-213 on IK(M) amplitude taken from GH3 cells (mean ± SEM; n = 8–9 for each bar). Current amplitude was measured at the end of depolarizing pulse from −50 to −20 mV. *Significantly different from control (P < 0.05) and †significantly different from 1 µM CFZ alone group (P < 0.05).
Effect of CFZ on the Firing of Spontaneous Action Potentials (APs) in GH3 Cells
In another set of experiments, we switch the mode to current-clamp condition for measurement of any changes in membrane potential in these cells. During these potential recordings, cells were bathed in normal Tyrode’s solution containing 1.8 mM CaCl2 and the pipette was filled with K+-containing solution. As shown in Figure 6, the presence of CFZ produced a progressive increase in the firing frequency of APs. For example, CFZ at a concentration of 0.3 and 1 µM significantly raised AP frequency to 0.67 ± 0.07 and 1.07 ± 0.08 Hz (n = 9, P < 0.05), respectively, from a control value of 0.42 ± 0.06 Hz (n = 9). Concomitant with these, during the exposure to 0.3 and 1 µM CFZ, the cells was significantly depolarized to −61 ± 2 and −48 ± 2 mV (n = 9, P < 0.05), respectively, from a control value of −66 ± 3 mV (n = 9). The duration of APs also became widened in the presence of 1 µM CFZ, as evidenced by a significant prolongation of AP duration from 45 ± 9 to 155 ± 14 ms (n = 9, P < 0.05). Therefore, it is conceivable from these results that CFZ-induced changes in membrane potential tend to be closely connected to its modification of IK(DR) amplitude and gating in these cells.
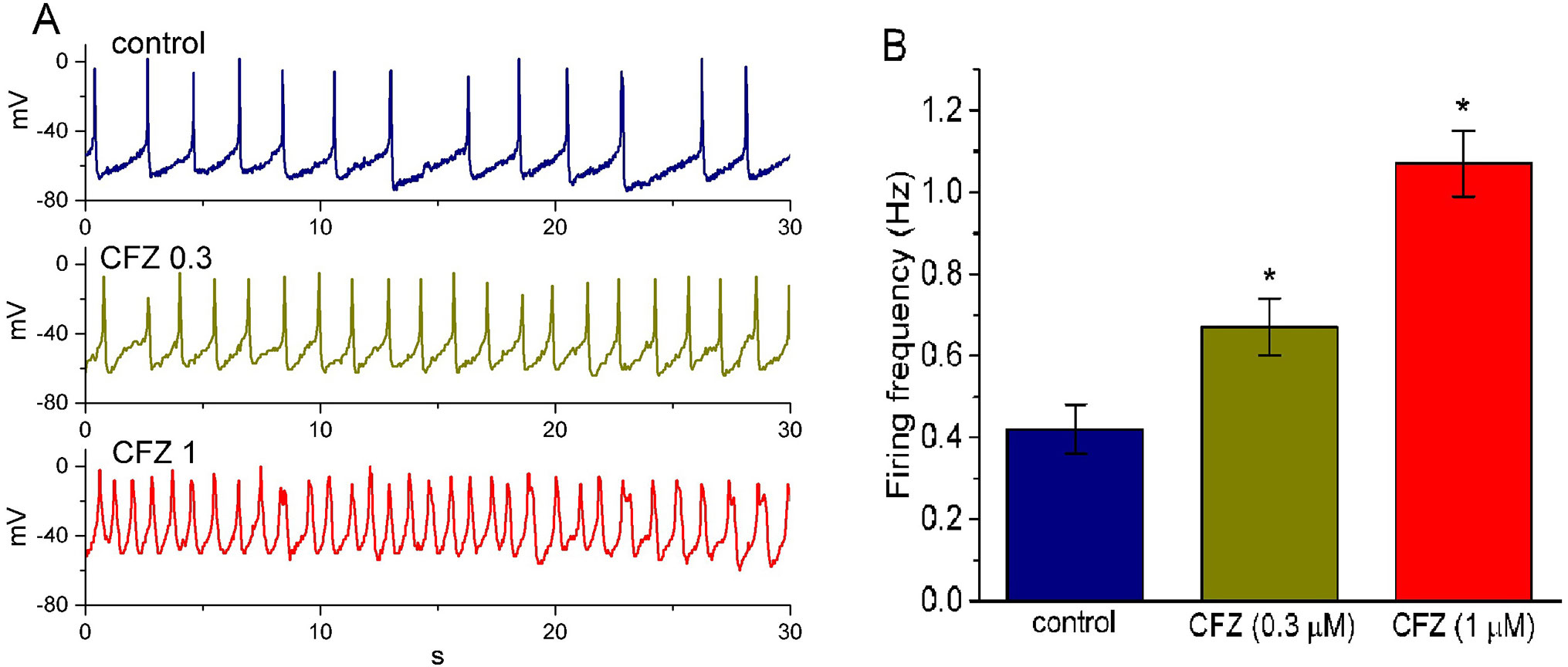
Figure 6 Effect of CFZ on spontaneous action potentials (APs) in GH3 cells. The experiments were conducted in cells bathed in normal Tyrode’s solution containing 1.8 mM CaCl2. As whole-cell configuration was achieved, the recording model was rapidly switched to current-clamp condition for measuring any changes in membrane potential. (A) Representative potential changes obtained in the absence (control) of CFZ and the presence of 0.3 µM CFZ (CFZ 0.3) and 1 µM CFZ (CFZ 1). (B) Bar graph depicting efficacy of CFZ to elevate the firing frequency of spontaneous APs in GH3 cells (mean ± SEM; n = 9 for each bar). *Significantly different from control (P < 0.05).
Inhibitory Effect of CFZ on IK(DR) Measured From A7r5 Vascular Smooth Muscle Cells and in Heart-Derived H9c2 Cells
Recent studies have recently demonstrated that CFZ might produce cardiovascular adverse events, although it evidently improves survival of patients suffering from multiple myeloma (Gavazzoni et al., 2018; Heckmann et al., 2018; Iannaccone et al., 2018; Mangla et al., 2018; Efentakis et al., 2019). For these reasons, we further evaluated whether addition of CFZ is able to exert any perturbations on IK(DR) inherently in A7r5 vascular smooth myocytes or H9c2 cardiac cells. In these experiments, A7r5 or H9c2 cells were bathed in Ca2+-free Tyrode’s solution and the IK(DR) was elicited when the examined cell was depolarized from −50 to +50 mV with a duration of 300 ms. As illustrated in Figure 7, the experimental results showed that CFZ at a concentration of 0.1 and 0.3 µM was effective at suppressing the IK(DR) in these cells. For example, in A7r5 cells, the presence of CFZ (0.3 µM) decreased IK(DR) density from 10.6 ± 2.1 to 4.4 ± 0.5 pA (n = 8, P < 0.05). However, distinguishable from its effect on GH3 or H9c2 cells, the presence of CFZ did not change the inactivation time course of IK(DR) observed in A7r5 cells, despite its ability to suppress current amplitude effectively. Moreover, CFZ-mediated inhibition of IK(DR) in A7r5 or H9c2 cells was unable to be attenuated by further addition of either diazoxide (30 µM), an activator of ATP-sensitive K+ currents, or 9-phenanthrol (3 µM), a stimulator of intermediate-conductance Ca2+-activated K+ channels (Garland et al., 2015; Chen et al., 2018). In A7r5 cells, the IK(DR) density obtained in the presence of 0.3 µM CFZ and 0.3 µM CFZ plus 30 µM diazoxide did not differ significantly (4.4 ± 0.5 pA [CFZ alone] versus 4.3 ± 0.6 pA [CFZ plus diazoxide]; n = 8, P > 0.05). Similarly, current density in the presence of 0.3 µM CFZ or 0.3 µM CFZ plus 3 µM 9-phenanthrol was not different (4.4 ± 0.5 pA [CFZ alone] versus 4.4 ± 0.6 pA [CFZ plus 9-phenanthrol]; n = 8, P > 0.05). Moreover, in H9c2 cells, the presence of CFZ decreased IK(DR) along with a significant reduction in inactivation time constant of the current (Figure 7C). The presence of CFZ at the concentration of 0.1 and 0.3 µM decreased the inactivation time constant of IK(DR) to 185 ± 11 (n = 9, P < 0.05) and 171 ± 9 ms (n = 9, P < 0.05), respectively, from a control value of 196 ± 13 ms (n = 9).
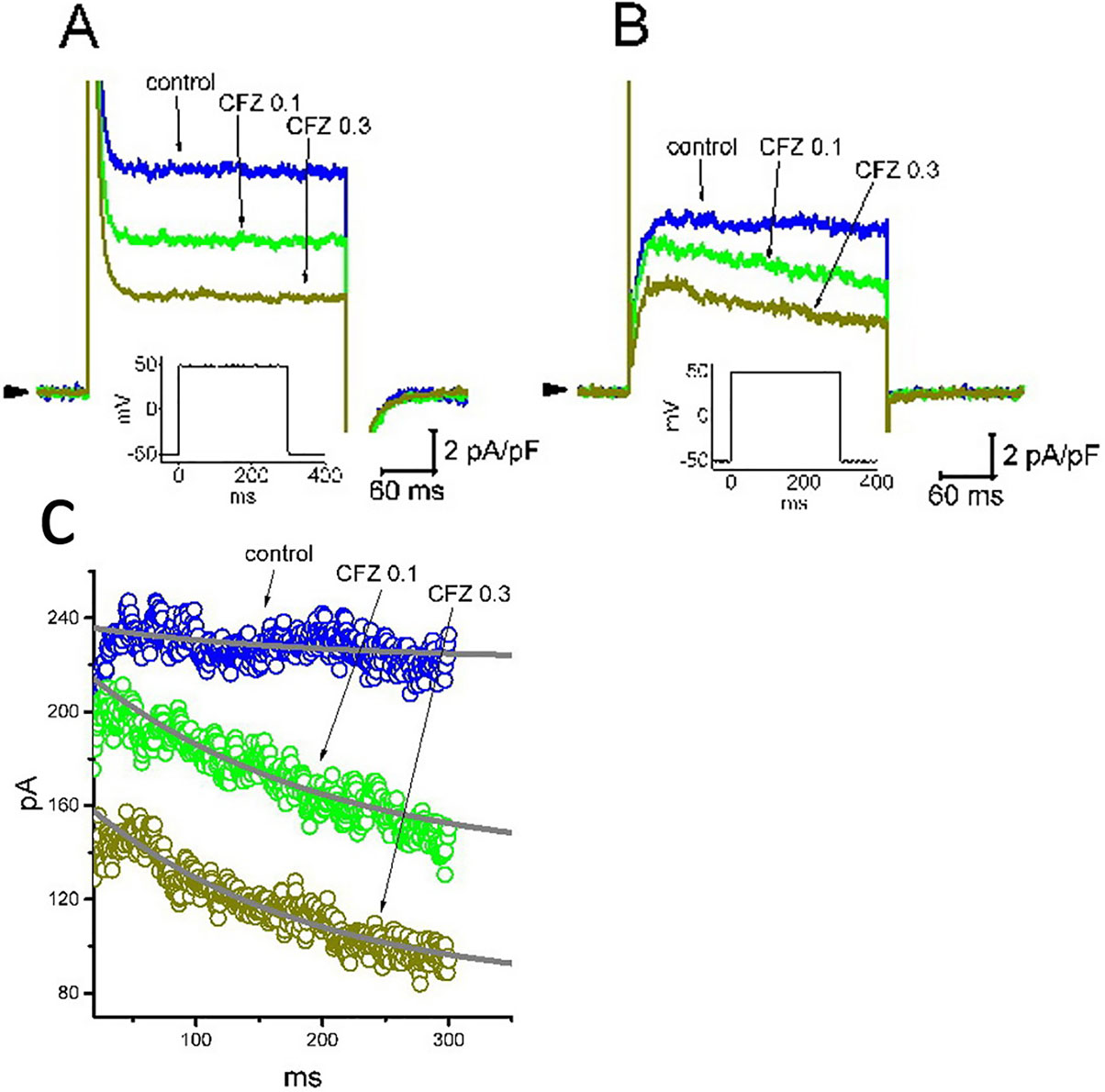
Figure 7 Inhibitory effect of CFZ on IK(DR) in A7r5 vascular smooth muscle cells (A) and in H9c2 cardiac cells (B). In these experiments, cells were bathed in Ca2+-free Tyrode’s solution and the pipette was filled with K+-containing solution. Superimposed IK(DR) traces recorded from either an A7r5 cell (A) or an H9c2 cell (B) were respectively obtained in the absence (control) and presence of 0.1 µM CFZ (CFZ 0.1) and 0.3 µ M CFZ (CFZ 0.3). Arrowhead indicates the zero current level, and inset shown in each panel denotes the voltage protocol applied. (C) Inactivation time course of IK(DR) in the absence and presence of 0.1 or 0.3 µM CFZ measured from H9c2 cells. Smooth curves labeled in control, CFZ 0.1 or CFZ 0.3 were least-squares fitted by single exponential.
Discussion
The present results demonstrated that in pituitary tumor (GH3) cells, CFZ produced an inhibitory efficacy on delayed rectifier K+ current (IK(DR)) in a concentration- and state-dependent fashion. The main action of CFZ on IK(DR) is thought to be principally through a state-dependent, open-channel block mechanism. Apart from its inhibitory effect on cytosolic proteasome or on changes in intracellular peptides (Dasgupta et al., 2014; Schäfer et al., 2017), block of CFZ of IK(DR) presented herein is of peculiar importance, since it may have characteristics making it significant from both pathophysiological, therapeutic, or toxicological point of view.
In this study, the mechanism of CFZ action on IK(DR) apparently differed from those on IK(M). In pituitary tumor (GH3) cells, the effect exerted by the presence of CFZ is a time-, and concentration-dependent increase of the inactivation process of IK(DR) elicited by long-lasting membrane depolarization, together with no change in the activation kinetics of the current. In other words, the inhibitory action of CFZ on IK(DR) tends to correlate over time with a considerable raise in the inactivation rate of the currents in response to membrane depolarization. Additionally, CFZ at higher concentrations could suppress the amplitude of IK(M) in GH3 cells. Both suppression of IK(DR) and IK(M) by CFZ might concertedly influence the electrical behavior of endocrine or neuroendocrine cells in vivo (Yu et al., 2002).
In light of steady-state inactivation curve of IK(DR) during the exposure to CFZ, the voltage for half-maximal inactivation was noted to be in the range of AP firing. Additionally, cell exposure to CFZ was also found to slow down the recovery of IK(DR) inactivation in GH3 cells. Therefore, it is conceivable that the CFZ molecule has a higher affinity toward the open-inactivated state of KV channels than toward the closed or resting state of the channels in these cells. In this way, the magnitude of CFZ-induced block on IK(DR) tends to be voltage-dependent, and the magnitude of its current inhibition on these currents can be synergistically influenced by different pre-existing level of resting potentials, the firing rate of APs, or the CFZ concentration applied, assuming that CFZ action in cells in vivo is the same as those on GH3 cells shown herein.
Earlier reports have shown the ability of CFZ to modify the energy and redox metabolism in neoplastic plasma cells or in heart cells (Al-Harbi, 2016; Soriano et al., 2016). However, in the present study, application of neither diazoxide nor 9-phenanthrol, in the presence of CFZ, attenuated its inhibitory efficacy on IK(DR) in A7r5 cells. Diazoxide is an activator of ATP-sensitive K+ current, while 9-phenanthrol can enhance the activity of intermediate-conductance Ca2+-activated K+ channels (Garland et al., 2015; Chen et al., 2018), though it could also suppress Ca2+-activated nonselective current (Simard et al., 2012). Therefore, the observed effect of CFZ on whole-cell IK(DR) enriched in A7r5 cells is not involved in the suppression of the activities derived from either ATP-sensitive or Ca2+-activated K+ channels. Moreover, in H9c2 cells, the presence of CFZ was able to inhibit IK(DR) together with a decrease in inactivation time constant of the current. The inhibitory effect on the amplitude and gating of IK(DR) caused by CFZ could be predominantly responsible for its interaction with macroscopic IK(DR).
Under current-clamp potential recordings, the addition of CFZ was found to depolarize the cells, to widen AP duration, and to raise the firing frequency of spontaneous APs recorded from GH3 cells in a concentration-dependent fashion. It is thus most likely that CFZ-induced increase of IK(DR) inactivation in endocrine or neuroendocrine cells may enhance a process of long-lasting facilitation or potentiation (Lin et al., 2008; Wang et al., 2008b; Wu et al., 2008; Kuo et al., 2018), which is conceivably associated with the toxicological actions of CFZ and other structurally similar compounds including variable forms of peripheral neuropathy (Cai et al., 2016; Kaplan et al., 2017; Karademir et al., 2018; Luczkowska et al., 2018).
The values of KD (calculated from the first-order kinetic scheme) and IC50 (required for CFZ-mediated inhibition of IK(DR)) measured from GH3 cells were virtually indistinguishable (i.e., around 0.3 µM). These values are noted to be much lower than that used for PSI-mediated induction of apoptotic changes in GH3 cells (Yu et al., 2002). PSI, the structure of which is shared by CFZ, is another inhibitor of proteasome activity. The IC50 value for CFZ-induced suppression of cell viability in RPMI-8226 myeloma cells obtained during one-hour exposure was around 40 nM (Schäfer et al., 2017). Therefore, its effective concentration required for the inhibition of IK(DR) in different types of neoplastic cells probably overlaps with that for the suppression of proteasome activity (Yu et al., 2002; Schäfer et al., 2017). Because the maximal plasma concentration of CFZ reached in the blood in vivo was reported to be around 1000–2000 ng/ml (i.e., 1.4–2.8 µM) (Maruyama et al., 2018), such unanticipated modifications of IK(DR) amplitude and gating are expected to occur at a concentration achievable in humans.
Indeed, a previous study showed that berberine, an anti-diabetic herbal agent, could suppress IK(DR) in human myeloma (RPMI-8226) cells and that its inhibitory effects could be associated with inhibitory efficacy on cell proliferation or apoptosis (Wu et al., 1998; Hsu et al., 2012). It is thus tempting to speculate that, apart from its inhibition of proteasome, the direct inhibitory effect of CFZ on the amplitude and gating of IK(DR) contributes to its actions on myeloma cells. It remains to be further determined to what extent such blocking action is connected with CFZ-induced inhibition of proliferation in neoplastic cells such as myeloma, glioma or lung cancer cells (Demo et al., 2007; Baker et al., 2014; Booth et al., 2014; Zhang and Sidhu, 2014; Kaplan et al., 2017).
In this study, intracellular dialysis with CFZ (0.3 µM) had minimal changes in either the IK(DR) amplitude or the time course of IK(DR) inactivation. The results reflect that block by CFZ of IK(DR) seen in GH3, A7r5 or H9c2 cells tends to be rapid in onset once the channel is open. However, intracellular application of CFZ had minimal effects on IK(DR), thus ruling out an intracellular binding site for CFZ. Therefore, this binding site must be at the extracellular site in a location only accessible when the channel is in the open state. CFZ-mediated inhibition of IK(DR) or IK(M) presented here is direct and independent of its possible actions on the activity of cytosolic proteasomes or on any changes in intracellular peptide levels (Dasgupta et al., 2014; Schäfer et al., 2017). On the other hand, it would be interesting to explore whether the suppression by CFZ of proteasome activity would secondarily ascribes from its blocking of membranous IK(DR) to some extent in different types of neoplastic cells.
Recently, it has been demonstrated that administration of CFZ is able to affect adverse cardiovascular events (Gavazzoni et al., 2018; Heckmann et al., 2018; Iannaccone et al., 2018; Mangla et al., 2018; Efentakis et al., 2019). Protein quality control inside the cell, with which proteasome inhibitors mainly interfere, is thought to be an important part of the molecular machinery of cardiomyocytes, endothelial cells, and probably all other members of the cardiac environment (Heckmann et al., 2018). Previous studies also reported that inactivation of AMPKα and downregulation of autophagy-related proteins occurred rapidly after CFZ administration (Efentakis et al., 2019). However, in the present study, addition of CFZ effectively suppressed IK(DR) amplitude along with enhanced inactivation rate of the current in heart-derived H9c2 cells. In A7r5 vascular smooth muscle cells, the amplitude of IK(DR) was also suppressed by CFZ. Earlier studies have notably demonstrated the ability of aconitine, a diterpenoid cardiotoxin, to exert IK(DR) block in a concentration- and state-dependent fashion (Lin et al., 2008; Wu et al., 2008; Wang et al., 2008a; Wu et al., 2011). Aconitine was previously reported to induce apoptosis in H9c2 cardiac cells (Gao et al., 2018). Therefore, our findings lend the credence to the notion that the modifications by CFZ or aconitine of IK(DR) amplitude or gating inherently in vascular smooth muscle and in heart cells is intimately linked to its action on variable forms of cardiovascular catastrophes (Iannaccone et al., 2018; Mangla et al., 2018; Efentakis et al., 2019).
The effect of CFZ on the inactivation time course of IK(DR could be variable. Previous studies have demonstrated that the IK(DR) present in GH3 cells could be mediated by several different subtypes of KV channels, including KV1.2, KV1.4, KV1.5, KV2.1, KV2.2, KV3.2, KV4.1 and KV5.2 (Wulfsen et al., 2000). To what extent CFZ action on the inactivation kinetics is specific for a unique subset of KV channels remains to be further studied.
The present study provided evidence to show that interaction of CFZ with IK(DR) in different types of cells may not be fully explained only by its inhibition on proteasome activity. Regardless of the mode of CFZ action, the KV channels enriched in different types of electrically excitable cells are presumably an important target for the action of it or other structurally similar compounds. Findings from the present results also make a case for examining whether the mechanistic direction described herein for actions of CFZ or other structurally similar agents (e.g., bortezomib and ixazomib) (Zhou et al., 2009; Dou and Zonder, 2014) has pharmacological, toxicological and clinical relevance in humans.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Author Contributions
ES: manuscript preparation and data collection. P-YL: research idea and reference collection. C-CL: manuscript proof reading and preparation of figures. S-NW: performing the patchclamp and manuscript preparation.
Funding
This research was partly supported by a research fund (ANHRF107-13) from An Nan Hospital, China Medical University, granted to ES.
Conflict of Interest
The authors declared that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Al-Harbi, N. O. (2016). Carfilzomib-induced cardiotoxicity mitigated by dexrazoxane through inhibition of hypertrophic gene expression and oxidative stress in rats. Toxicol. Mech. Methods 26 (3), 189–195. doi: 10.3109/15376516.2016.1143071
Baker, A. F., Hanke, N. T., Sands, B. J., Carbajal, L., Anderl, J. L., Garland, L. L. (2014). Carfilzomib demonstrates broad anti-tumor activity in pre-clinical non-small cell and small cell lung cancer models. J. Exp Clin. Cancer Res. 33, 111. doi: 10.1186/s13046-014-0111-8
Booth, L., Cruickshanks, N., Tavallai, S., Roberts, J. L., Peery, M., Poklepovic, A., et al. (2014). Regulation of dimethyl-fumarate toxicity by proteasome inhibitors. Cancer Biol. Ther. 15 (12), 1646–1657. doi: 10.4161/15384047.2014.967992
Caballero, R., Gómez, R., Núñez, L., Moreno, I., Tamagrgo, J., Delpón, E. (2004). Diltiazem inhibits hKv1.5 and Kv4.3 currents at therapeutic concentrations. Cardiovasc. Res. 64 (3), 457–466. doi: 10.1016/j.cardiores.2004.07.022
Cai, X., Bhattacharyya, S., Plitt, A., Raibagkar, P., LaBuzetta, J. N., Schleicher, S. M., et al. (2016). Management of posterior reversible encephalopathy syndrome induced by carfilzomib in a patient with multiple myeloma. J. Clin. Oncol. 34 (2), e1–e5. doi: 10.1200/JCO.2013.49.6166
Chen, P. C., Ruan, J. S., Wu, S. N. (2018). Evidence of decreased activity in intermediate-conductance calcium-activated potassium channels during retinoic acid-induced differentiation in motor neuron-like NSC-34 cells. Cell Physiol. Biochem. 48 (6), 2374–2388. doi: 10.1159/000492653
Dasgupta, S., Castro, L. M., Dulman, R., Yang, C., Schmidt, M., Ferro, E. S., et al. (2014). Proteasome inhibitors alter levels of intracellular peptides in HEK293T and SH-SY5Y cells. PLoS One 9 (7), e103604. doi: 10.1371/journal.pone.0103604
Demo, S. D., Kirk, C. J., Aujay, M. A., Buchholz, T. J., Dajee, M., Ho, M. N., et al. (2007). Antitumor activity of PR-171, a novel irreversible inhibitor of the proteasome. Cancer Res. 67 (13), 6383–6391. doi: 10.1158/0008-5472.CAN-06-4086
Dou, Q. P., Zonder, J. A. (2014). Overview of proteasome inhibitor-based anti-cancer therapies: perspective on bortezomib and second generation proteasome inhibitors versus future generation inhibitors of ubiquitin-proteasome system. Curr. Cancer Drug Targets 14 (6), 517–536. doi: 10.2174/1568009614666140804154511
Efentakis, P., Kremastiotis, G., Varela, A., Nikolaou, P. E., Papanagnou, E. D., Davos, C. H., et al. (2019). Molecular mechanisms of carfilzomib-induced cardiotoxicity in mice and the emerging cardioprotective role of metformin. Blood 133 (7), 710–723. doi: 10.1182/blood-2018-06-858415
Gao, X., Zhang, X., Hu, J., Xu, X., Zuo, Y., Wang, Y., et al. (2018). Aconitine induces apoptosis in H9c2 cardiac cells via mitochondriamediated pathway. Mol. Med. Rep. 17 (1), 284–292. doi: 10.3892/mmr.2017.7894
Garland, C. J., Smirnov, S. V., Bagher, P., Lim, C. S., Huang, C. Y., Mitchell, R., et al. (2015). TRPM4 inhibitor 9-phenanthrol activates endothelial cell intermediate conductance calcium-activated potassium channels in rat isolated mesenteric artery. Br. J. Pharmacol. 172, 1114–1123. doi: 10.1111/bph.12985
Gavazzoni, M., Lombardi, C. M., Vizzardi, E., Gorga, E., Sciatti, E., Rossi, L., et al. (2018). Irreversible proteasome inhibition with carfilzomib as first line therapy in patients with newly diagnosed multiple myeloma: early in vivo cardiovascular effects. Eur. J. Pharmacol. 838, 85–90. doi: 10.1016/j.ejphar.2018.09.014
Heckmann, M. B., Doroudgar, S., Katus, H. A., Lehmann, L. H. (2018). Cardiovascular adverse events in multiple myeloma patients. J. Thorac. Dis. 10 (Suppl 35), S4296–S4305. doi: 10.21037/jtd.2018.09.87
Hsiao, H. T., Liu, Y. C., Liu, P. Y., Wu, S. N. (2019). Concerted suppression of Ih and activation of IK(M) by ivabradine, an HCN-channel inhibitor, in pituitary cells and hippocampal neurons. Brain Res. Bull. 149, 11–20. doi: 10.1016/j.brainresbull.2019.03.016
Hsu, Y. Y., Chen, C. S., Wu, S. N., Jong, Y. J., Lo, Y. C. (2012). Berberine activates Nrf2 nuclear translocation and protects against oxidative damage via a phosphatidylinositol 3-kinase/Akt-dependent mechanism in NSC34 motor neuron-like cells. Eur. J. Pharm Sci. 46 (5), 415–425. doi: 10.1016/j.ejps.2012.03.004
Iannaccone, A., Bruno, G., Ravera, A., Gay, F., Salvini, M., Bringhen, S., et al. (2018). Evaluation of cardiovascular toxicity associated with treatments containing proteasome inhibitors in multiple myeloma therapy. High Blood Press. Cardiovasc. Prev. 25 (2), 209–218. doi: 10.1007/s40292-018-0256-1
Kaplan, G. S., Torcun, C. C., Grune, T., Ozer, N. K., Karademir, B. (2017). Proteasome inhibitors in cancer therapy: treatment regimen and peripheral neuropathy as a side effect. Free Radic. Biol. Med. 103, 1–13. doi: 10.1016/j.freeradbiomed.2016.12.007
Karademir, B., Sari, G., Jannuzzi, A. T., Musunuri, S., Wicher, G., Grune, T., et al. (2018). Proteomic approach for understanding milder neurotoxicity of carfilzomib against bortezomib. Sci. Rep. 8 (1), 16318. doi: 10.1038/s41598-018-34507-3
Kuo, P. C., Yang, C. J., Lee, Y. C., Chen, P. C., Liu, Y. C., Wu, S. N. (2018). The comprehensive electrophysiological study of curcuminoids on delayed-rectifier K+ currents in insulin-secreting cells. Eur. J. Pharmacol. 819, 233–241. doi: 10.1016/j.ejphar.2017.12.004
Lin, M. W., Wang, Y. J., Liu, S. I., Lin, A. A., Lo, Y. C., Wu, S. N. (2008). Characterization of aconitine-induced block of delayed rectifier K+ current in differentiated NG108-15 neuronal cells. Neuropharmacology 54 (6), 912–923. doi: 10.1016/j.neuropharm.2008.01.009
Liu, Y. Y., Hsiao, H. T., Wang, J. C., Liu, Y. C., Wu, S. N. (2019). Parecoxib, a selective blocker of cyclooxygenase-2, directly inhibits neuronal delayed-rectifier K+ current, M-type K+ current and Na+ current. Eur. J. Pharmacol. 844, 95–101. doi: 10.1016/j.ejphar.2018.12.005
Lu, T. L., Chang, W. T., Chan, C. H., Wu, S. N. (2019). Evidence for effective multiple K+-current inhibitions by tolvaptan, a non-peptide antagonist of vasopressin V2 Receptor. Front. Pharmacol. 10, 76. doi: 10.3389/fphar.2019.00076
Luczkowska, K., Litwinska, Z., Paczkowska, E., Machalinski, B. (2018). Pathophysiology of drug-induce peripheral neuropathy in patients with multiple myeloma. J. Physiol. Pharmacol. 69 (2), 165–172. doi: 10.26402/jpp.2018.2.02
Mangla, A., Paydary, K., Liu, J., Mbachi, C., Yim, B., Lad, T. E. (2018). Carfilzomib-associated cardiovascular adverse events in a non-Caucasian cohort of patients with multiple myeloma: a real-world experience. Hematol. Oncol. 36 (4), 715–717. doi: 10.1002/hon.2535
Maruyama, D., Tobinai, K., Chou, T., Taniwaki, M., Shumiya, Y., Iida, S. (2018). Weekly carfilzomib and dexamethasone in Japanese patients with relapsed or refractory multiple myeloma: a phase 1 and PK/PD trial. Cancer Sci. 109 (10), 3245–3252. doi: 10.1111/cas.13753
Schäfer, J., Welti, L., Seckinger, A., Burhenne, J., Theile, D., Weiss, J. (2017). Cellular effect and efficacy of carfilzomib depends on cellular net concentration gradient. Cancer Chemother. Pharmacol. 80 (1), 71–79. doi: 10.1007/s00280-017-3335-4
Simard, C., Salle, L., Rouet, R., Guinamard, R. (2012). Transient receptor potential melastatin 4 inhibitor 9-phenanthrol abolishes arrhythmias induced by hypoxia and re-oxygenation in mouse ventricle. Br. J. Pharmacol. 165 (7), 2354–2364. doi: 10.1111/j.1476-5381.2011.01715.x
Soriano, G. P., Besse, L., Li, N., Kraus, M., Besse, A., Meeuwenoord, N., et al. (2016). Proteasome inhibitor-adapted myeloma cells are largely independent from proteasome activity and show complex proteomic changes, in particular in redox and energy metabolism. Leukemia 30 (11), 2198–2207. doi: 10.1038/leu.2016.102
Wang, Y. J., Chen, B. S., Lin, M. W., Lin, A. A., Peng, H., Sung, R. J., et al. (2008a). Time-dependent block of ultrarapid-delayed rectifier K+ currents by aconitine, a potent cardiotoxin, in heart-derived H9c2 myoblasts and in neonatal rat ventricular myocytes. Toxicol. Sci. 106 (2), 454–463. doi: 10.1093/toxsci/kfn189
Wang, Y. J., Lin, M. W., Lin, A. A., Peng, H., Wu, S. N. (2008b). Evidence for state-dependent block of DPI 201-106, a synthetic inhibitor of Na+ channel inactivation, on delayed-rectifier K+ current in pituitary tumor (GH3) cells. J. Physiol. Pharmacol. 59 (3), 409–423.
Wu, S. N., Chen, B. S., Lo, Y. C. (2011). Evidence for aconitine-induced inhibition of delayed rectifier K+ current in Jurkat T-lymphocytes. Toxicology 289 (1), 11–18. doi: 10.1016/j.tox.2011.07.003
Wu, S. N., Chen, B. S., Lin, M. W., Liu, Y. C. (2008). Contribution of slowly inactivating potassium current to delayed firing of action potentials in NG108-15 neuronal cells: experimental and theoretical studies. J. Theor. Biol. 252 (4), 711–721. doi: 10.1016/j.jtbi.2008.01.031
Wu, S. N., Yu, H. S., Jan, C. R., Li, H. F., Yu, C. L. (1998). Inhibitory effects of berberine on voltage- and calcium-activated potassium currents in human myeloma cells. Life Sci. 62 (25), 2283–2294. doi: 10.1016/S0024-3205(98)00209-4
Wu, S. N., Chern, J. H., Shen, S., Chen, H. H., Hsu, Y. T., Lee, C. C., et al. (2017). Stimulatory actions of a novel thiourea derivative on large-conductance, calcium-activated potassium channels. J. Cell Physiol. 232 (12), 3409–3421. doi: 10.1002/jcp.25788
Wu, S. N., Hwang, T., Teng, C. M., Li, H. F., Jan, C. R. (2000). The mechanism of actions of 3-(5’-(hydroxymethyl-2’-furyl)-1-benzyl indazole (YC-1) on Ca2+-activated K+ currents in GH3 lactotrophs. Neuropharmacology 39 (10), 1788–1799. doi: 10.1016/S0028-3908(00)00025-3
Wulfsen, I., Hauber, H. P., Schiemann, D., Bauer, C. K., Schwarz, J. R. (2000). Expression of mRNA for voltage-dependent and inward-rectifying K+ channels in GH3/B6 cells and rat pituitary. J. Neuroendocrinol. 12 (3), 263–272. doi: 10.1046/j.1365-2826.2000.00447.x
Yu, H., Wu, M., Townsend, S. D., Zou, B., Long, S., Daniels, J. S., et al. (2011). Discovery, synthesis, and structure activity relationship of a series of N-Aryl- bicyclo[2.2.1]heptane-2-carboxamides: characterization of ML213 as a novel KCNQ2 and KCNQ4 potassium channel opener. ACS Chem. Neurosci. 2 (10), 572–577. doi: 10.1021/cn200065b
Yu, R., Ren, S. G., Melmed, S. (2002). Proteasome inhibitors induce apoptosis in growth hormone- and prolactin-secreting rat pituitary tumor cells. J. Endocrinol. 174 (3), 379–386. doi: 10.1677/joe.0.1740379
Zhang, W., Sidhu, S. S. (2014). Development of inhibitors in the ubiquitination cascade. FEBS Lett. 588 (2), 356–367. doi: 10.1016/j.febslet.2013.11.003
Keywords: carfilzomib, delayed-rectifier K+ current, current inactivation, M-type K+ current, action potential, pituitary cell, vascular smooth muscle cell, heart cell
Citation: So EC, Liu P-Y, Lee C-C and Wu S-N (2019) High Effectiveness in Actions of Carfilzomib on Delayed-Rectifier K+ Current and on Spontaneous Action Potentials. Front. Pharmacol. 10:1163. doi: 10.3389/fphar.2019.01163
Received: 14 June 2019; Accepted: 10 September 2019;
Published: 07 October 2019.
Edited by:
Domenico Tricarico, University of Bari Aldo Moro, ItalyReviewed by:
Ricardo Gómez, University of La Laguna, SpainOsama F. Harraz, University of Vermont, United States
Copyright © 2019 So, Liu, Lee and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sheng-Nan Wu, c253dUBtYWlsLm5ja3UuZWR1LnR3
 Edmund Cheung So
Edmund Cheung So Ping-Yen Liu3
Ping-Yen Liu3 Sheng-Nan Wu
Sheng-Nan Wu