- 1Department of Urology, Xianyang Central Hospital, Xianyang, China
- 2Center for Evidence-Based and Translational Medicine, Zhongnan Hospital of Wuhan University, Wuhan, China
- 3Department of Urology, Zhongnan Hospital of Wuhan University, Wuhan, China
- 4Department of Pharmacy, The Huaihua Second People's Hospital, Huaihua, China
- 5Center for Evidence-Based Medicine, Institute of Evidence-Based Medicine and Knowledge Translation, Henan University, Kaifeng, China
Objective: To systematically evaluate the quality of clinical practice guidelines (CPG) for medically treating benign prostatic hyperplasia (BPH), and to compare the context of recommendations in order to provide references for clinical application.
Methods: We searched databases of National Guideline Clearinghouse (NGC), Guidelines International Network (GIN), National Institute for Health and Clinical Excellence (NICE), Scottish Intercollegiate Guidelines Network (SIGN) and World Health Organization (WHO), PubMed, Embase, CNKI, VIP, WanFang Data, CBM, and Medlive from their establishment to October 13, 2019, to collect evidence-based guidelines and/or consensus on BPH. Method quality of included guidelines was assessed according to the Appraisal of Guidelines for Research and Evaluation II (AGREE II) instrument, and differences and similarities among recommendations were compared.
Results: A total of 22 guidelines were included, of which eight were updated versions. According to the AGREE II instrument, the median score of scope and purpose, stakeholder involvement, rigor of formulate, clarity of presentation, applicability, and editorial independence was 71.5%, 41%, 25%, 64%, 18%, and 28%, respectively. Based on recommendations for medical treatment, almost all guidelines recommended α1-blockers and 5α-reductase inhibitors, and most guidelines also recommended muscarinic receptor antagonists. In terms of drug combination therapy, most guidelines recommended “α1 blockers and 5α-reductase inhibitors”, and some guidelines also recommended “α1 blockers and muscarinic receptor antagonists”.
Conclusion: The recommendations from different guidelines were basically similar, only showing conflicts in some areas. The quality of included guidelines remains to be unified, and their context can provide valuable implications for development or improvement.
Introduction
A meta-analysis on studies from 25 countries showed that the lifetime prevalence of BPH was 26.2% [95% confidence interval (CI): 22.8–29.6%] and there were no regional or ethnic differences (Lee et al., 2017). In addition, in the United States alone, the annual spending on BPH treatment is estimated to be approximately $4 billion (Taub and Wei, 2006). With the advent of an aging society, BPH has become a serious burden to clinical work, society, and economy. The development and continuous updating of the BPH Clinical Practice Guide (CPG) (Wang, 2016) impose a positive impact on promoting the standardization of clinical medical work. In recent years, many countries, especially developed ones, have made great achievements in the development and application of BPH diagnosis and treatment guidelines in order to solve many problems faced in BPH clinical practice (Novara et al., 2006). Despite this progress, the quality of many CPG still appeared to fall below desirable standards. Therefore, this article studied and analyzed the basic content and development trend of global BPH clinical guidelines, used the AGREE II tool to scientifically evaluate the guidelines, compared the advantages and disadvantages of each guide from six domains. And focused on the content of drug treatment for BPH guidelines, hoping to provide help for frontline clinicians when referring to the guidelines, and also hoping to provide references for the specification of evidence-based guidelines for clinical treatment.
Methods
Inclusion and Exclusion Criteria
Inclusion globally published BPH-field clinical practice guidelines or consensus (the latest version) that meets the guidelines and is developed and issued by academic or national authorities. Guidelines must include recommendations for drug therapy. Exclude foreign direct translations or adapted foreign guides, guide interpretation documents, technical or operational instructions, lectures or expert writing, and knowledge manuals.
Search Strategy
Computer searched National Library of the United States (NGC), Guideline International Network (GIN), National Institute of Health and Clinical Demonstration (NICE), English Inter-Institutional Guide Network (SIGN), World Health Organization (WHO), PubMed, Embase, China National Knowledge Infrastructure (CNKI), Wanfang database, VIP database, China Biomedical Literature Data Road, and Medlive website from their inception to October 20, 2019, and a manual retrieval was also performed for relevant literature references. No language restrictions were applied to the search strategies. The search terms included BPH, benign prostate hyperplasia, enlarged prostate, BPH, prostatomegaly, prostatauxe, prostatic hypertrophy, benign prostatic enlargement, benign prostatic obstruction, lower urinary tract symptoms, LUTS, guideline, specification, etc.
Literature Screening and Data Extraction
The two evaluators independently completed literature screening and cross-checking according to the inclusion and exclusion criteria. If there were objections, the third evaluator would participate in the discussion and resolve the differences. Data were extracted according to a pre-designed data extraction table, and the extracted contents included the names of guideline, releasing country and organization, the earliest release or updating time, research area, drug treatment opinions, formulation methods, and references.
Quality Assessment
Pre-scoring was performed three times before formal scoring, and consistency was tested using intra-group correlation coefficient (ICC). ICC is one of the reliability coefficient indicators for measuring and evaluating the reliability between observers and retest reliability. Then, methodological quality was evaluated by two reviewers using the AGREE II (Wang, 2016) (Supplementary Table 1). The AGREE II consists of 23 key items organized within six domains followed by two global rating items (“overall assessment”). The six domains are “scope and purpose”, “stakeholder Involvement”, “rigor of development”, “clarity of presentation”, “applicability”, “editorial independence”. The two assessors received education regarding to the guideline development process and evidence-based nursing and were trained on the use of AGREE II. After the evaluation, answers from them were compared, and the score difference for each item greater than two points was defined as a large difference. Then, the two reviewers would give a new score after discussion. If differences still existed, a professor with extensive experience in using AGREE II would help. Three reviewers would combine all supporting materials and opinions to arrive a final score. We analyze the quality of included guidelines according to the following scheme: (1) score 23 key items within six domains; (2) each of the AGREE II items are rated on a seven-point scale (1–strongly disagree to 7–strongly agree); (3) the consistency of evaluations between the two reviewers was judged through calculating ICC value; score: domain score = (actual score-minimum possible score)/(maximum possible score-minimum possible score) × 100%. The higher the domain standardization score, the more complete the method, and the reporting when guidelines for the domain were developed.
Statistical Analysis
Descriptive analysis and presentation of results were completed using Excel 2007 software, and ICC values were calculated using SPSS 19.0 software. ICC value ranged between 0 and 1, and consistency would be poor when ICC value was less than 0.4. As for ICC locating between 0.4 and 0.75, it meant that the consistency was average. When ICC ≥ 0.75, the consistency was fine. ICC value should be above 0.7.
Results
Basic Features of Literature Search Results and Guidelines
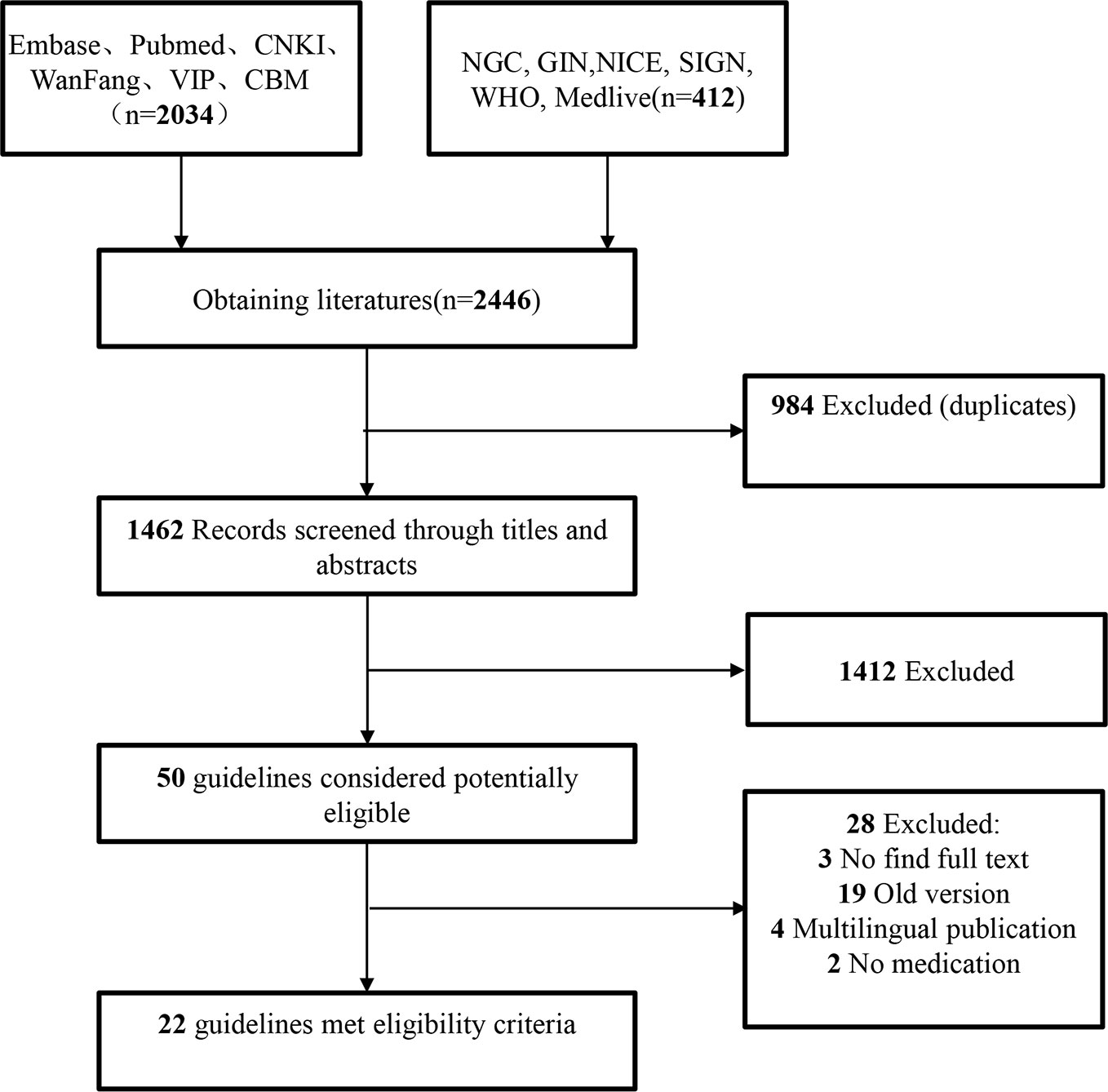
A total of 2,562 articles were obtained in the preliminary searching. After layer-by-layer screening, 22 guidelines were finally included (Cockett et al., 1991; Chang, 1998; Bereczky et al., 2006; Cavalcanti et al., 2006; Hofner, 2007; Zhang et al., 2007; Mcvary et al., 2011; Zhu et al., 2011; Spatafora et al., 2012; Tammela et al., 2012; Chapple, 2015; Gratzke et al., 2015; Wang, 2015; Yeo et al., 2016; Zhang et al., 2016; Homma et al., 2017; Sun et al., 2017; Yu and Gao, 2017; Zhang et al., 2017; Geng, 2018; Gravas et al., 2019; Nickel et al., 2018). The document screening process and results are shown in Figure 1. Of the 22 included guidelines, 2 were from European urology association (Gratzke et al., 2015; Gravas et al., 2019), eight from China (Zhang et al., 2007; Zhu et al., 2011; Wang, 2015; Zhang et al., 2016; Sun et al., 2017; Yu and Gao, 2017; Zhang et al., 2017; Geng, 2018), while only one from Japan (Homma et al., 2017), Brazil (Cavalcanti et al., 2006), Finland (Tammela et al., 2012), Germany (Hofner, 2007), the United Kingdom (Chapple, 2015), WHO (Cockett et al., 1991), Italy (Spatafora et al., 2012), Malaysia (Chang, 1998), Canada (Nickel et al., 2018), the United States (Mcvary et al., 2011), South Africa (Bereczky et al., 2006), and Korea (Yeo et al., 2016), respectively. The basic characteristics of the included guidelines are shown in Table 1.
AGREE II Evaluation Results
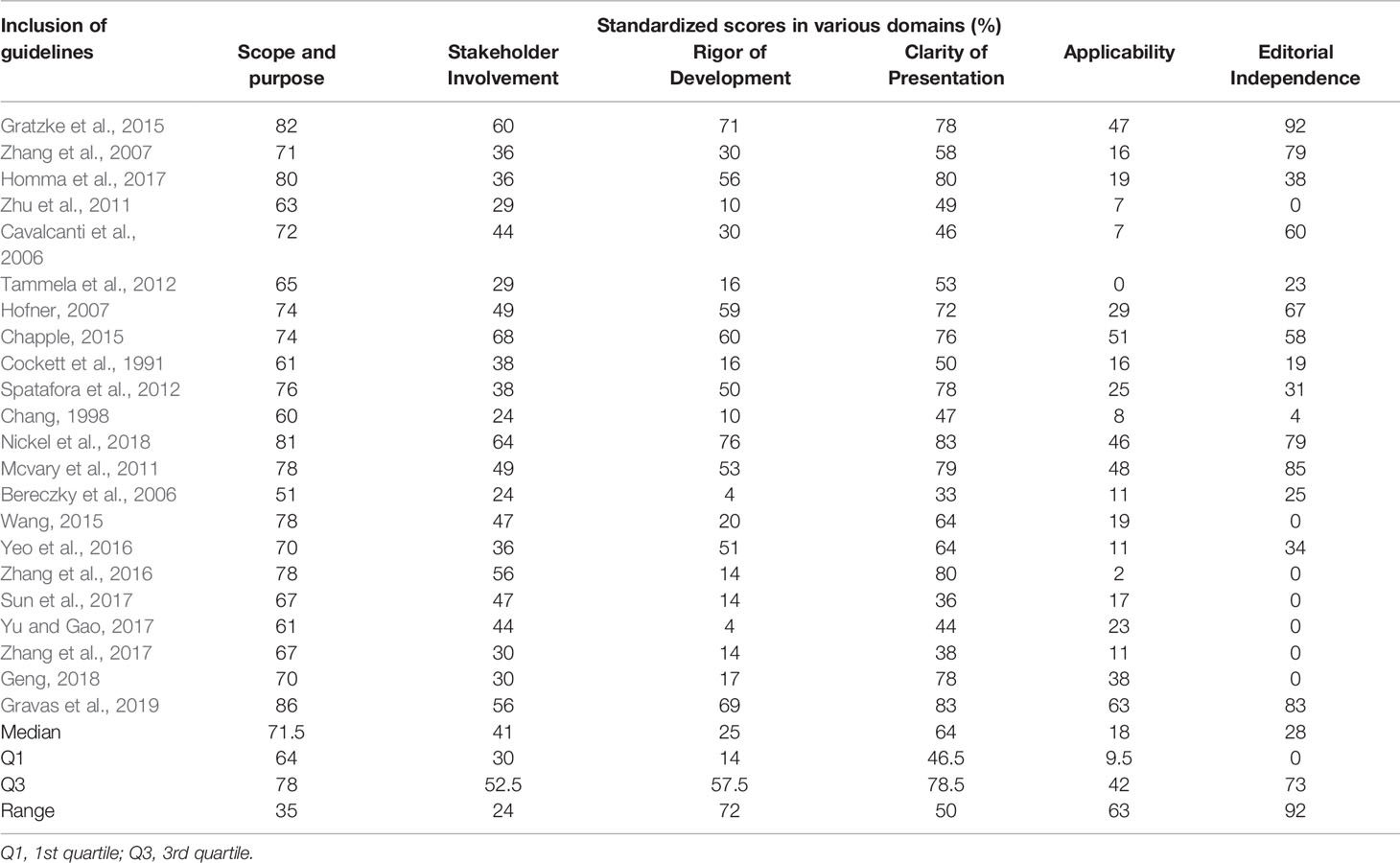
The results of consistency test showed that the ICC values of all guidelines were > 0.735 (0.735 to 0.994), indicating that their consistency was fine. The results of standardized scores in the six domains are shown in Table 2.
Scope and Purpose
The median (Q1, Q3) and full range in the domain were 71.5% (64%,78%) and 35%. The median score was highest in all areas. Almost all guides were well in this area, and no guideline score was below 50%.
Stakeholder Involvement
The median (Q1, Q3) and full range in this area were 41% (30%, 52.5%) and 24%. The median score ranked the third position across all areas, and the full range score was higher than the median score. The minimum score in this area was 22%, and only four guides possessed scores greater than 50%.
Rigor of Development
The median (Q1, Q3) and full range in the domain was 25% (14%, 57.5%) and 72%. The median score ranked the third position among the six areas, and the full-range score was much larger than the median score. Five guides in the domain exhibited a median score greater than 50% and two guides showed a minimum score of 4%.
Clarity of Presentation
The median (Q1, Q3) and full range score in this area was 64% (46.5%, 78.5%) and 50%. The median score ranked the second position in all areas. Most guides were well in this area, and only six guides displayed scores below 50%.
Applicability
The median score in this domain was lowest across all domains (18%). Only one guideline scored above 50%, while one scored 0.
Editorial Independence
The median (Q1, Q3) and full range (full range) score for this domain were 28% (0,73%) and 92%. In this area, five guides scored over 70%, and 7 scored 0.
Medication Recommendations
Guidelines for drug treatment recommendations were shown in Figure 2. Four of the included guidelines purely involved traditional Chinese medicine, including no recommendations for other treatments. Almost all of the guidelines recommended α1-blockers and 5α-reductase inhibitors, and most of the guidelines recommended muscarinic receptor antagonists. Meanwhile, eight guidelines recommended the use of phosphodiesterase 5 inhibitor, while one guideline from China considered that currently, phosphodiesterase 5 inhibitor in our country had no indications for BPH/LUTS treatment, so such drug was not recommended for the time being. There were 10 guidelines recommending phytotherapy, but there were also two guidelines not recommending such approach for BPH. Although phytotherapy is popular in many parts of the world, both European and South African guidelines believed that currently, there was no objective evidence confirming its efficacy, mode of action, or biological effect. Besides, four guidelines recommended arginine vasopressin which was mainly adopted for treating polyuria at night. Three guidelines addressed recommendations for beta-3 agonist. Only seven guidelines from China and Japan recommended Chinese medicine treatment. Japanese guidelines recommended anti-androgen therapy alone. Guidelines for combined medication had a high degree of uniformity in recommending “α1-blockers and 5α-reductase inhibitors”, and 11 guidelines recommended “α1-blockers and muscarinic receptor antagonists”. A total of three guidelines from Japan, Germany, and China, respectively, recommended a combination of “α1-blockers and phosphodiesterase 5 inhibitors”.
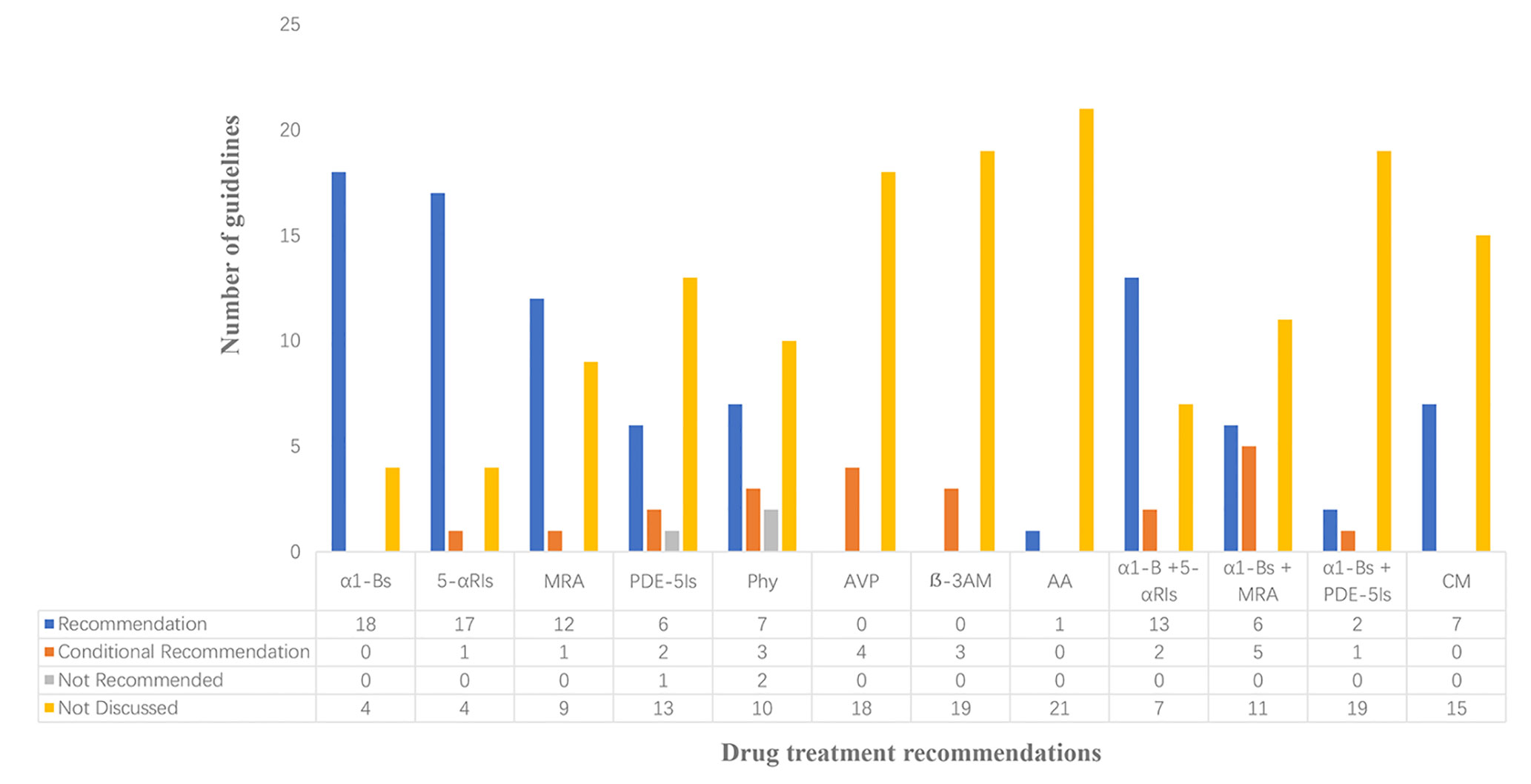
Figure 2 Analysis of drug treatment opinions α1-Bs, α1-Blockers; 5-αRIs,5α-Reductase inhibitors; MRA, Muscarinic receptor antagonist; PDE-5Is, Phosphodiesterase 5 inhibitor; Phy, Phytotherapies; AVP, Arginine vasopressin; β-3AM, Beta-3 agonist medications; AA, Anti-androgen; α1-B+5-αRIs, α1-Blockers+5α-Reductase inhibitor; α1-B+MRA, α1-Blockers + Muscarinic receptor antagonist; α1-B+PDE-5Is, α1-Blockers + phosphodiesterase 5 inhibitor; CM, Chinese medicine.
Discussion
This study aimed to evaluate the quality of CPG for BPH worldwide. We identified 22 CPGs related to BPH, which were published between 1991 and 2019. The median and range scores for the six AGREE II domains (scope and purpose, stakeholder involvement, rigor of development, clarity of presentation, applicability, and editorial independence) were 71.5%, 41%, 25%, 64%, 18%, and 28%. An increasing number of CPGs are being published. However, there are considerable potentials to elevate the quality of each domain. In the six major domains of the AGREE II tool scoring system, scores only in domains 1, “scope and purpose” and 4 “clarity of presentation” were >50%. Therefore, scores in the other four areas need to be improved. The median (range) of the scope and purpose scores was 71.5% (35%), indicating that these guidelines clearly described their ranges and purposes, and could help users to quickly determine whether they were what you needed. The stakeholder participation rate was 41% (24%), mainly because most guidelines did not take into account the views or wishes of target populations (patients, the public, etc.) of item 5. The rigor of development was 25% (72%), with a lower median and a larger range, indicating that a few criteria met the standards in the domain, and most guidelines did not report systematic retrieval or recommendation formation methods in the article. The clarity of the report was 64% (50%), indicating that the included guidelines met the criteria for most projects in the domain, but the recommendations of some guidelines were vague and difficult to identify quickly. The South African guidelines with the lowest scores (33%) recommended treatment but did not provide indicators such as duration and dose. The applicability was 18% (63%), mainly because the guidelines offered ambiguous descriptions on facilitators and obstacles during application, and only a small number of them provided different versions and supporting documents. Almost all guidelines did not mention potential resource inputs. The editorial independence was 28% (92%), the median was low, but the range was large, and only a few guidelines not only provided funding units but also clearly indicated whether they were affected by funding agency. Most guidelines either did not report funding agencies or reported funding agencies but did not state conflicts of interest.
In terms of drug treatment recommendations, they were basically the same. BPH is mainly featured by histological prostatic hyperplasia and glandular components, anatomical enlarged prostate (BPE), urodynamic bladder outlet obstruction (BOO), and low urinary tract symptoms (LUTS) (Wang, 2014). In treating BPH, pharmacological therapy may be not as effective as surgical therapy, but could sufficiently relieve symptoms for many patients, causing fewer adverse events (Kirby, 2000). Studies have shown that about 85% of patients receiving conservative treatment enjoy stable statuses during a follow-up of 1 year, and about 65% show no clinical progress within 5 years (Wang, 2014). Therefore, almost all guidelines reached a relatively uniform opinion on conservative treatment options. α1-Blockers, 5α-reductase inhibitors, and muscarinic receptor antagonists were recommended by most guidelines. Some research results suggested that the efficacy of plant preparations in BPH was equivalent to that of blockers and 5a proenzyme inhibitors, and that there were no obvious adverse reactions for plant preparations (Fourcade et al., 2008). Therefore, it has been recommended by Germany, China, Japan, Brazil, Finland, and Canada guidelines. Drugs such as phosphodiesterase 5 inhibitor, arginine vasopressin, and beta-3 agonist medications were also recommended by some guidelines, but such recommendation was not replicated by most guidelines. Regarding to the use of Chinese medicine in BPH, except those from China and Japan, guidelines from other countries did not discuss this aspect.
Our review had several strengths. First, we attempted to cover all published guidelines for our systematic review of qualities of CPGs on BPH. Our structured and explicit approach increased the validity of the findings. Second, we used the AGREE II instrument, which is a scientific and valid tool to assess the quality of CPGs. There were also some limitations in this study. First of all, the tool AGREE II, when targeting guidelines, only focused on evaluating their development methodology and the quality of their reporting. Consequently, the evaluation on their evidence quality and the authenticity of their recommendations was not enough. And score might fail to truly reflect quality. In addition, although evaluation scores are helpful in comparing the quality of clinical guidelines, they could do little in elevating the quality. Second, we did not limit expert consensus, and it differed from clinical practice guidelines in format and production methods. This was also a possible reason for lower AGREE II score in some areas. Finally, this study showed restriction in language, and clinical guidelines published in other databases might also be missed.
Conclusion
In summary, the overall quality of the included guidelines is uneven and needs to be unified. According to our analysis on the recommended uniformity of the acceptance guidelines, it could be concluded that in terms of drug treatment, α1-blockers and 5α-reductase inhibitors are more mature drugs for BPH treatment; therefore, it is recommended that in the future when CPG is formulated/revised Ability to use recognized standards wherever possible. Of course, in the light of actual conditions in countries and regions, based on recognized standards, it is also allowed to modify them to suit corresponding standards.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Author Contributions
X-FX, X-MQ, and Y-HJ designed this study. G-XL, CZ, and S-FY collected data. TD rechecked data. TD and X-FX performed analysis. X-FX wrote the manuscript. TD and Y-HJ reviewed the manuscript. All authors contributed to manuscript revision, and read and approved the submitted version.
Funding
This work was supported by the National Key Research and Development Plan of China (2016YFC0106300) and Technical Innovation Major Program of Hubei province (2016ACA152), without any financial interest or benefit. The Entrusted Project of National Center for Medical Service Administration, National Health and Family Planning Commission China (No. [2019]099).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Thanks to all authors for their contributions.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2020.00311/full#supplementary-material
References
Bereczky, Z., Bolus, M., Chetty, P., Du Toit, W., Enslin, J., Haffejee, M., et al. (2006). Management of benign prostatic hyperplasia - South African Urological Association guideline. Samj South Afr. Med. J. 96, 1275–1279.
Cavalcanti, A., Errico, G., Araújo, J. F. C. (2006). Hiperplasia prostática benigna (Projeto Diretrizes: Associação Médica Brasileira e Conselho Federal de Medicina).
Chang (1998). Consensus on Management of Benign Prostatic Hyperplasia. Malaysian Urol. Assoc. Malaysian Urological Association and Prostate Health Council of Malaysia.
Chapple, C. R. (2015). Lower urinary tract symptoms in men: management. Natl. Inst. Health Care Excellence.
Cockett, A. T., Aso, Y., Denis, L., Khoury, S., Barry, M., Carlton, C. E., et al. (1991). World Health Organization Consensus Committee recommendations concerning the diagnosis of BPH. Prog. Urol. 1, 957–972.
Fourcade, R. O., Theret, N., Taieb, C., Group, B. U. S. (2008). Profile and management of patients treated for the first time for lower urinary tract symptoms/benign prostatic hyperplasia in four European countries. BJU Int. 101, 1111–1118. doi: 10.1111/j.1464-410X.2008.07498.x
Geng, Q. (2018). Chinese Expert Consensus on Clinical Application of Huang E Capsules in Benign Prostatic Hyperplasia. Chin. J. Androl. 24, 85–88. doi: 10.13263/j.cnki.nja.2018.10.016
Gratzke, C., Bachmann, A., Descazeaud, A., Drake, M. J., Madersbacher, S., Mamoulakis, C., et al. (2015). EAU Guidelines on the Assessment of Non-neurogenic Male Lower Urinary Tract Symptoms including Benign Prostatic Obstruction. Eur. Urol. 67, 1099–1109. doi: 10.1016/j.eururo.2014.12.038
Gravas, S., Cornu, J. N., Drake, M. J. (2019). Management of non-neurogenic male LUTS (EAU Guidelines Edn presented at the EAU Annual Congress Copenhagen). https://uroweb.org/guideline/treatment- of-non-neurogenic-male-luts/
Hofner, K. (2007). Operative Therapie des benignen Prostatasyndroms. Med. Dtsch. Arztebl 104, 2424–2429.
Homma, Y., Gotoh, M., Kawauchi, A., Kojima, Y., Masumori, N., Nagai, A., et al. (2017). Clinical guidelines for male lower urinary tract symptoms and benign prostatic hyperplasia. Int. J. Urol. 24, 716–729. doi: 10.1111/iju.13401
Kirby, R. S. (2000). The natural history of benign prostatic hyperplasia: what have we learned in the last decade? Urology 56, 3–6. doi: 10.1016/S0090-4295(00)00747-0
Lee, S. W. H., Chan, E. M. C., Lai, Y. K. (2017). The global burden of lower urinary tract symptoms suggestive of benign prostatic hyperplasia: A systematic review and meta-analysis. Sci. Rep. 7, 7984. doi: 10.1038/s41598-017-06628-8
Mcvary, K. T., Roehrborn, C. G., Avins, A. L., Barry, M. J., Bruskewitz, R. C., Donnell, R. F., et al. (2011). Update on AUA guideline on the management of benign prostatic hyperplasia. J. Urol. 185, 1793–1803. doi: 10.1016/j.juro.2011.01.074
Nickel, J. C., Aaron, L., Barkin, J., Elterman, D., Nachabe, M., Zorn, K. C. (2018). Canadian Urological Association guideline on male lower urinary tract symptoms/benign prostatic hyperplasia (MLUTS/BPH): 2018 update. Can. Urol. Assoc. J. 12, 303–312. doi: 10.5489/cuaj.5616
Novara, G., Galfano, A., Gardi, M., Ficarra, V., Boccon-Gibod, L., Artibani, W. (2006). Critical review of guidelines for BPH diagnosis and treatment strategy. Eur. Urol. Suppl. 5, 418–429. doi: 10.1016/j.eursup.2006.02.005
Spatafora, S., Casarico, A., Fandella, A., Galetti, C., Hurle, R., Mazzini, E., et al. (2012). Evidence-based guidelines for the treatment of lower urinary tract symptoms related to uncomplicated benign prostatic hyperplasia in Italy: updated summary from AURO.it. Ther. Adv. Urol. 4, 279–301. doi: 10.1177/1756287212463112
Sun, Z. X., Song, C. S., Xing, J. P. (2017). Guidelines for the diagnosis and treatment of benign prostatic hyperplasia with integrated traditional Chinese and Western medicine (Trial version). Chin. J. Androl. 23, 280–285. doi: 10.13263/j.cnki.nja.2017.03.017
Tammela, T., Nurmi, M., Pétas, A. (2012). Eturauhasen hyvänlaatuinen liikakasvu: Käypä hoito–suosituksen päivitystiivistelmä. Duodecim 128, 1046–1047.
Taub, D. A., Wei, J. T. (2006). The economics of benign prostatic hyperplasia and lower urinary tract symptoms in the United States. Curr. Urol. Rep. 7, 272–281. doi: 10.1007/s11934-996-0006-0
Wang, X. H. (2014). Techniques for improving the safety of plasma transurethral resection of prostate. J. Mod. Urol. 19, 563–566. doi: 10.3969/j.issn.1009-8291.2014-09-01
Wang, J. Y. (2015). Consensus on drug treatment of benign prostatic hyperplasia / low urinary tract symptoms in the elderly, (2015). Chin. J. Geriatr. 34, 1380–1387. doi: 10.3760/cma.j.issn.0254-9026.2015.12.027
Wang, X. H. (2016). Development and evaluation of evidence-based clinical practice guidelines (Beijing: China Union Medical College Press).
Yeo, J. K., Choi, H., Bae, J. H., Kim, J. H., Yang, S. O., Oh, C. Y., et al. (2016). Korean clinical practice guideline for benign prostatic hyperplasia. Invest. Clin. Urol. 57, 30–44. doi: 10.4111/icu.2016.57.1.30
Yu, X. J., Gao, Q. H. (2017). Chinese Expert Consensus of Ning Bitai Capsule in Clinical Application of Lower Urinary Tract Symptoms. Chin. J. Androl. 23, 852–855. doi: 10.13263/j.cnki.nja.2017.09.016
Zhang, X. H., Wang, X. H., Wang, G. (2007). Guidelines for clinical diagnosis and treatment of benign prostatic hyperplasia. Chin. J. Surg. 45, 1704–1707. doi: 10.3760/j.issn:0529-5815.2007.24.018
Zhang, C. H., Li, Y. Q., Pei, X. H. (2016). Expert consensus on diagnosis and treatment of benign prostatic hyperplasia. Beijing J. Traditional Chin. Med. 77–81. doi: 10.16025/j.1674-1307.2016.11.026
Zhang, C. H., Li, Y. Q., Pei, X. H. (2017). Expert consensus on benign prostatic hyperplasia based on kidney deficiency and stasis theory. Chin. J. Androl. 31, 59–61. doi: 10.3969/j.issn.1008-0848.2017.01.015
Keywords: clinical practice guideline, benign prostatic hyperplasia, evidence-based evaluation, AGREE II instrument, medical treatment
Citation: Xu X-F, Liu G-X, Zhu C, Qiao X-M, Yu S-F, Deng T and Jin Y-H (2020) α1-Blockers and 5α-Reductase Inhibitors Are the Most Recommended Drugs in Treating Benign Prostatic Hyperplasia: An Evidence-Based Evaluation of Clinical Practice Guidelines. Front. Pharmacol. 11:311. doi: 10.3389/fphar.2020.00311
Received: 18 December 2019; Accepted: 02 March 2020;
Published: 25 March 2020.
Edited by:
Hailiang Hu, Duke University, United StatesCopyright © 2020 Xu, Liu, Zhu, Qiao, Yu, Deng and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tong Deng, ZGVuZ3RvbmdobkAxMjYuY29t; Ying-Hui Jin, amlueWluZ2h1aWVibUAxNjMuY29t
 Xiao-Feng Xu1
Xiao-Feng Xu1 Ying-Hui Jin
Ying-Hui Jin