- 1National R & D Center for Edible Fungus Processing Technology, Henan University, Kaifeng, China
- 2School of Biomedical Sciences, Huaqiao University, Xiamen, China
- 3Joint International Research Laboratory of Food & Medicine Resource Function, Henan Province, Henan University, Kaifeng, China
- 4Hebei Food Inspection and Research Institute, Shijiazhuang, China
- 5College of Forensic Medicine, Hebei Medical University, Shijiazhuang, China
Scheflera heptaphylla (L.)Frodin, a kind of Traditional Chinese Medicine, is commonly used in anti-inflammatory, analgesic, anti-viral, anti-tumor, and hemostasis. This study aimed to determine the anti-hepatoma components and its mechanism from the leaves of S. heptaphylla. The spectrum-effect relationships were analyzed by the method of Partial least squares, indicating that P1, P2, and P10 were positively correlated to inhibitory activity of Huh7 cells. Whereas others were negatively correlated. The technologies of component knock-out and UPLC-MS2 were used to determine compounds as 3,4-Dicaffeoylquinic acid (P6), 3,5-Dicaffeoylquinic acid (P7), 3α-Hydroxy-lup-20(29)-ene-23,28-dioic acid (P10, named Compound A). The results forecasted that Compound A had the best correlation with inhibitory activity. The effects of Compound A on the activities of human hepatoma cells (Huh7, SMMC-7721, HepG 2) and normal hepatocytes (L0-2, Chang liver) were evaluated. Cell apoptosis was observed with inverted microscope and flow cytometer. In addition, the proteins, related to apoptosis, were detected by Western blot. The results showed that Compound A (400 nM) could significantly inhibit the activity of three hepatoma cells (P < 0.001) with slight toxicity to normal hepatocytes, and the IC50 values were 285.3 and 315.1 nM, respectively, which were consistent with the prediction of spectrum-effect relationships. After treatment with Compound A, the number of hepatoma cells decreased significantly. And the apoptosis rate of Huh7 cells increased significantly (P < 0.001) in Compound A (200, 400 nM) groups, SMMC-7721 and HepG 2 were directly necrotic. Compound A groups could significantly improve the level of intracellular reactive oxygen species (ROS) (P < 0.05, P < 0.001) in Huh7 with no effect on normal hepatocytes. The content of apoptotic protein (Bax and Bim) in mitochondria was significantly increased in Compound A groups (P < 0.001). On the contrary, the content of anti-apoptotic protein (Bcl-xL and Mcl-1) decreased significantly (P < 0.001). These results demonstrated that Compound A was the main anti-hepatoma active component in the S. heptaphylla leaves. It achieved the effect of promoting apoptosis of Huh7 cells by regulating the levels of ROS and Bcl-2 family protein in mitochondrial apoptosis pathway.
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors in the world, with high morbidity and mortality (Ally et al., 2017; Fukuda et al., 2017; Yang et al., 2017; Yang et al., 2019; Wu et al., 2020). Primary liver cancer is the second leading cause of death in the world, of which 70% to 85% are HCC. The incidence of liver cancer in China ranks first worldwide (LV et al., 2015; Yang and Hou, 2016; Zhu and Liu, 2018).
According to statistics, the incidence of liver cancer is still growing rapidly, and China is a “Large country of liver cancer.” At present, the clinical treatment of liver cancer includes surgical treatment and non-operative treatment, and mainly choose the non-operative chemotherapy to improve the quality of life and prolong the life of patients (Julie et al., 2018). However, chemotherapeutic drugs could produce many side effects, such as myelosuppression, decreased immune function, organ damage, hair loss and so on, which significantly reduce the quality of life in patients. Ethnic drugs with wide efficacy and rich resources are widely used in the treatment of tumor, Such as Eucommia ulmoides Oliver, Poria cocos (Schw.) Wolf, and Schisandra chinensis (Turcz.) Baill (Li, 2011; Gui, 2012; Qian et al., 2019), which has the advantages of multi-target, multi-effect, little side effect, safety and effectiveness (Zhang et al., 2019). It could not only inhibit and kill tumor cells, but also has less toxicity to normal tissues and cells of the body. At the same time, it also can regulate the immune function of the body and improve clinical symptoms and signs (Wang and Hou, 2013). However, Traditional Chinses Medicine (TCM) is a whole concept in the process of treating diseases, it is difficult to determine the exact therapeutic components of TCM, which greatly restricts the application of TCM in clinical treatment of diseases.
Scheflera heptaphylla (L.)Frodin is commonly used as a hepatoprotective drug in Southeast Asia and has a significant anti-liver cancer effect. However, the anti-hepatoma effect of S. heptaphylla is not scientifically evaluated, and its active substances and mechanism are not clear. So, the anti-liver cancer active components of S. heptaphylla were investigated by the rapid method of “spectrum-effect relationship” (Wang et al., 2013), “knock-out technique,” and high-resolution mass spectrometry.
In this manuscript, the fingerprint of S. heptaphylla leaves was established by high performance liquid chromatography (HPLC) method, and the anti-hepatoma activity of 10 batches of extracts was evaluated in vitro. 10 quantitative chromatographic peaks were selected to analyze the correlation between the spectral efficacy and the efficacy data, and the spectral efficiency analysis model was established (Zeng et al., 2015). The peaks of anti-liver cancer active compounds were screened by spectrum-effect correlation, the target components were knocked out by knock-out technology (Gong et al., 2015), and the structures of the target components were identified by high resolution mass spectrometry. The effect and mechanism on anti-hepatoma of the target component was evaluated.
Results
Quantitative Determination of Chromatographic Peaks
Spectrum-effect relationship method was used to circumvent the weaknesses of the fingerprint technique and foster the strengths in TCM (Xu et al., 2014). The study of spectrum-effect relationship can reveal the material basis of the therapeutic values of TCM. This method has been widely applied in the researches of pharmacodynamic components, origins, harvest times, processing methods and batches of TCM (Hu et al., 2013; Zheng et al., 2014; Fang et al., 2016). Therefore, in order to accurately determine the material basis of their effects on anti-hepatoma activity, 10 batches of S. heptaphylla samples were selected to establish the fingerprint.
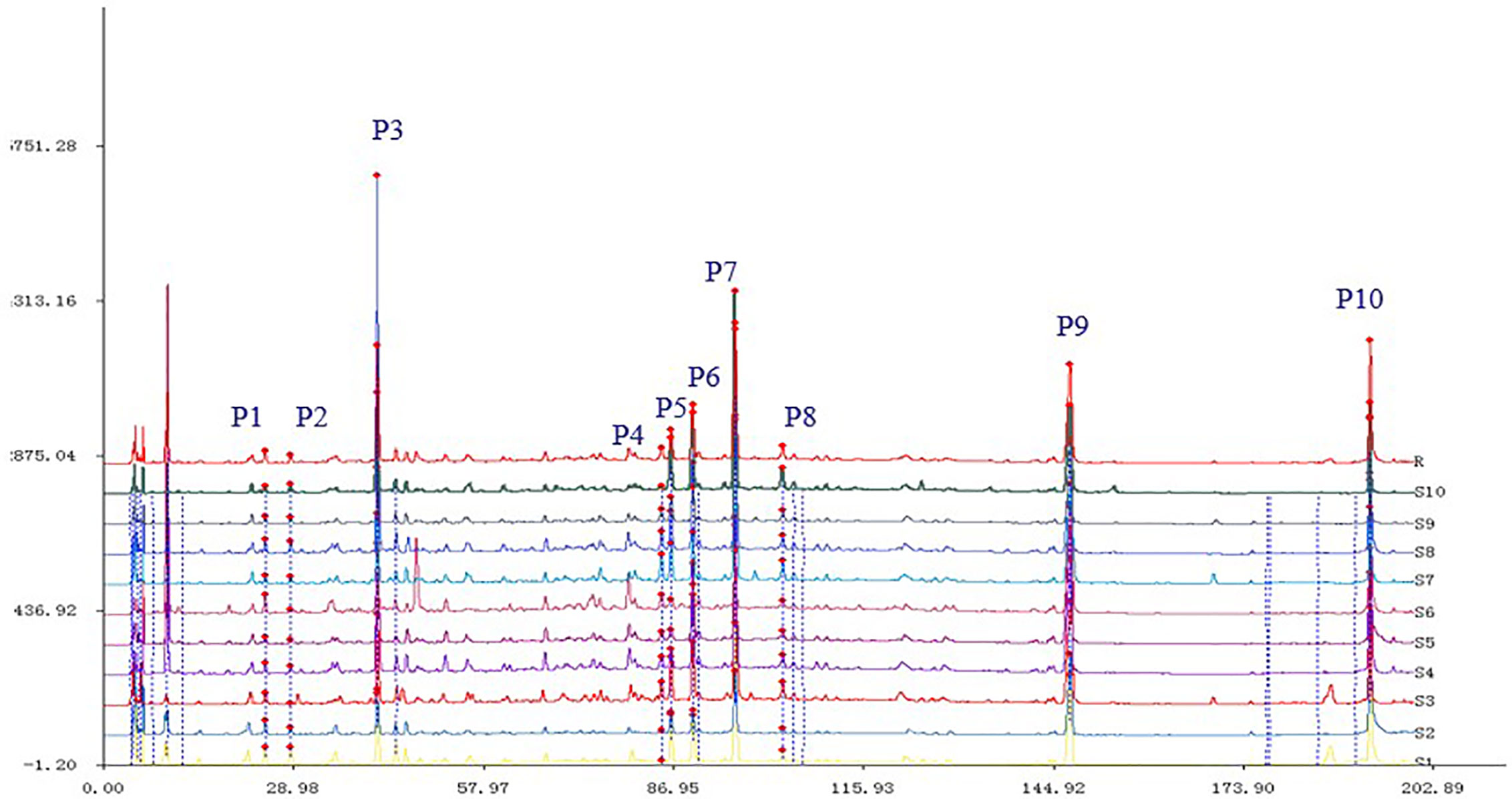
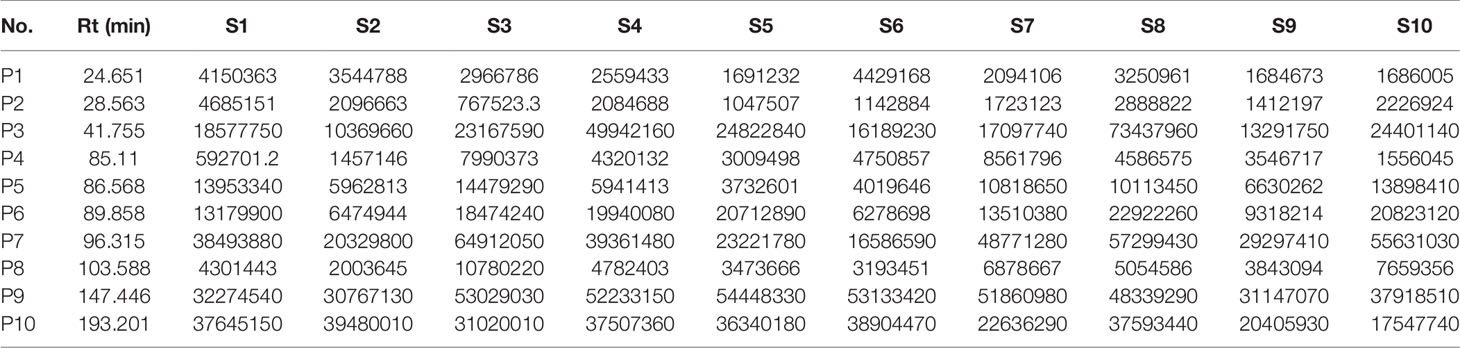
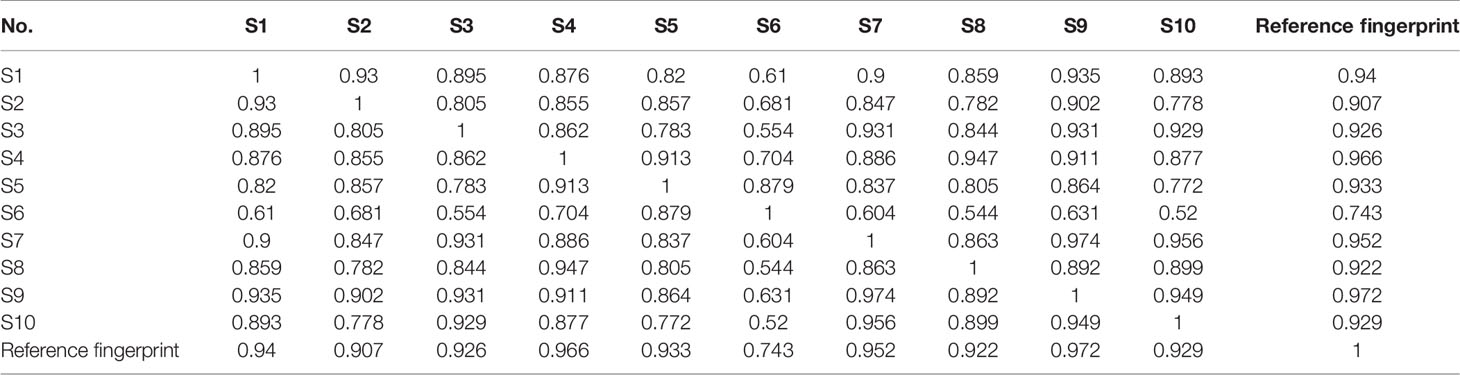
The chromatogram of S. heptaphylla ethanol extract was used as the reference spectrum, and the time window was set at 0.5. The chromatographic peaks with relatively high content and good separation in 10 batches of samples were selected for multi-point correction, and the average method was used to generate the control map by the software “Evaluation system of similarity of chromatographic fingerprint of traditional Chinese Medicine version 2004 1.0 A”. 10 common peaks out of the 10 feature maps were selected, as shown in Figure 1. The chromatographic peak matching data of S. heptaphylla leaves were shown in Table 1 and the similarity data were shown in Table 2.
Effect of Ethanol Extract From S. heptaphylla on the Viability of Huh7 Hepatoma Cells
In Figure 2A, the effects of 10 batches of S. heptaphylla ethanol extract on the viability of Huh7 showed when the concentrations of the extracts were 200, 400, 800 μg ·mL−1, the 10 batches of samples significantly inhibited the cells viability of Huh7 (P < 0.001, 0.001 < P < 0.01, P < 0.05) in a dose-dependent manner compared with the blank group in vitro.
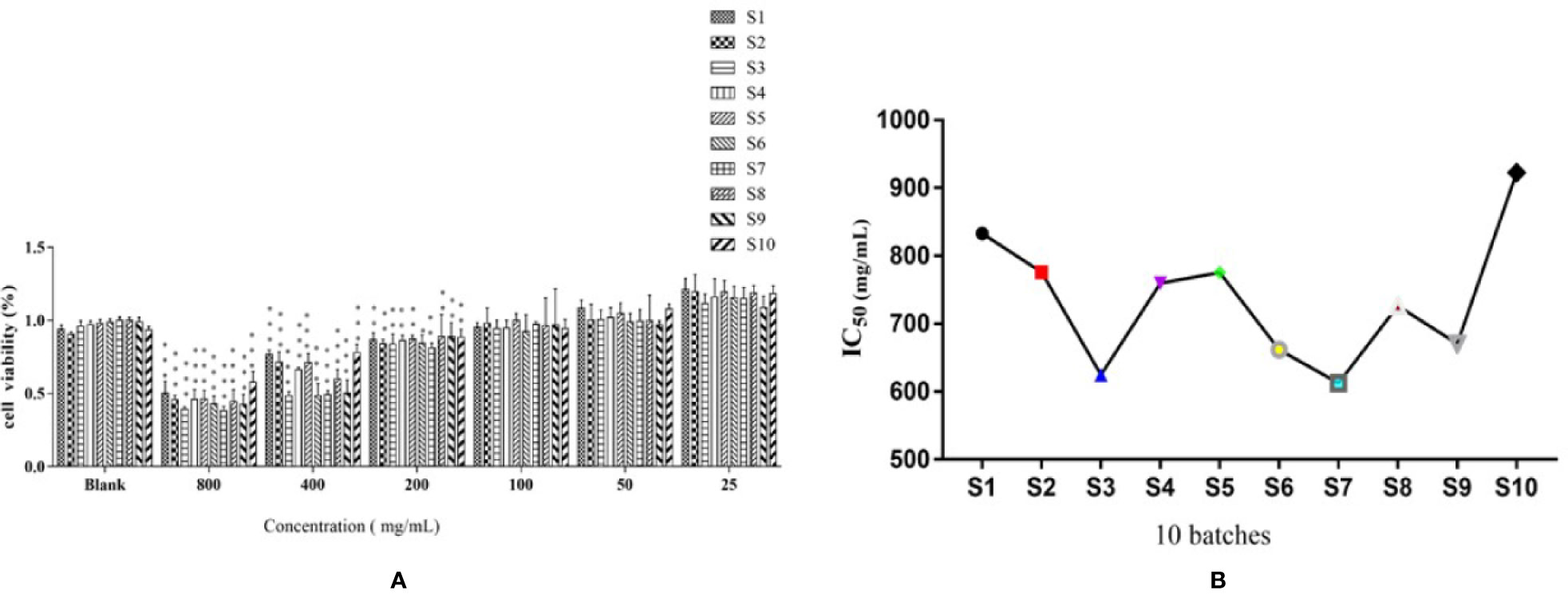
Figure 2 Effects of of S. heptaphylla ethanol extracts on the viability of Huh7 cells (A) and its IC50 values (B) in vitro. ***P < 0.001, 0.001 < **P < 0.01 vs. Blank group.
In Figure 2B, when the sample concentrations were at 1 g·mL−1 (equivalent to the original medicinal material), the IC50 values of 10 batches of S. heptaphylla on Huh7 cells showed a “W” shape, indicating that at this concentration, the anti-hepatoma effects of S. heptaphylla ethanol extract at different collection times were significantly different. The IC50 values were the lowest at S3 and S7, indicating that S. heptaphylla ethanol extracts had the best anti-liver cancer (Huh7) activity with no significant difference (P > 0.05).
Partial Least Square Regression Analysis
In recent years, spectrum-effect correlation analysis has been successfully applied to the analysis of active components of various traditional Chinese medicine. The main components of spectrum-effect relationship of traditional Chinese medicine include the establishment of fingerprint, pharmacodynamic evaluation and data processing of spectrum-effect correlation (Xu et al., 2014). The most common methods for establishing fingerprints are HPLC, UPLC, GC, and GC-MS (Zhou et al., 2008). Pharmacodynamics often uses experimental models in vitro or in vivo to obtain “efficacy” information (Zhang et al., 2012). The most common data processing methods are principal component analysis (PCA), canonical correlation analysis (CCA), partial least square analysis (PLSR) and grey correlation analysis (GRDA) (Zhao et al., 2004). Among them, the PLSR method is practical and stable, it can contain all the original fingerprint peaks, and the reaction information is more comprehensive (Alciaturi et al., 2014).
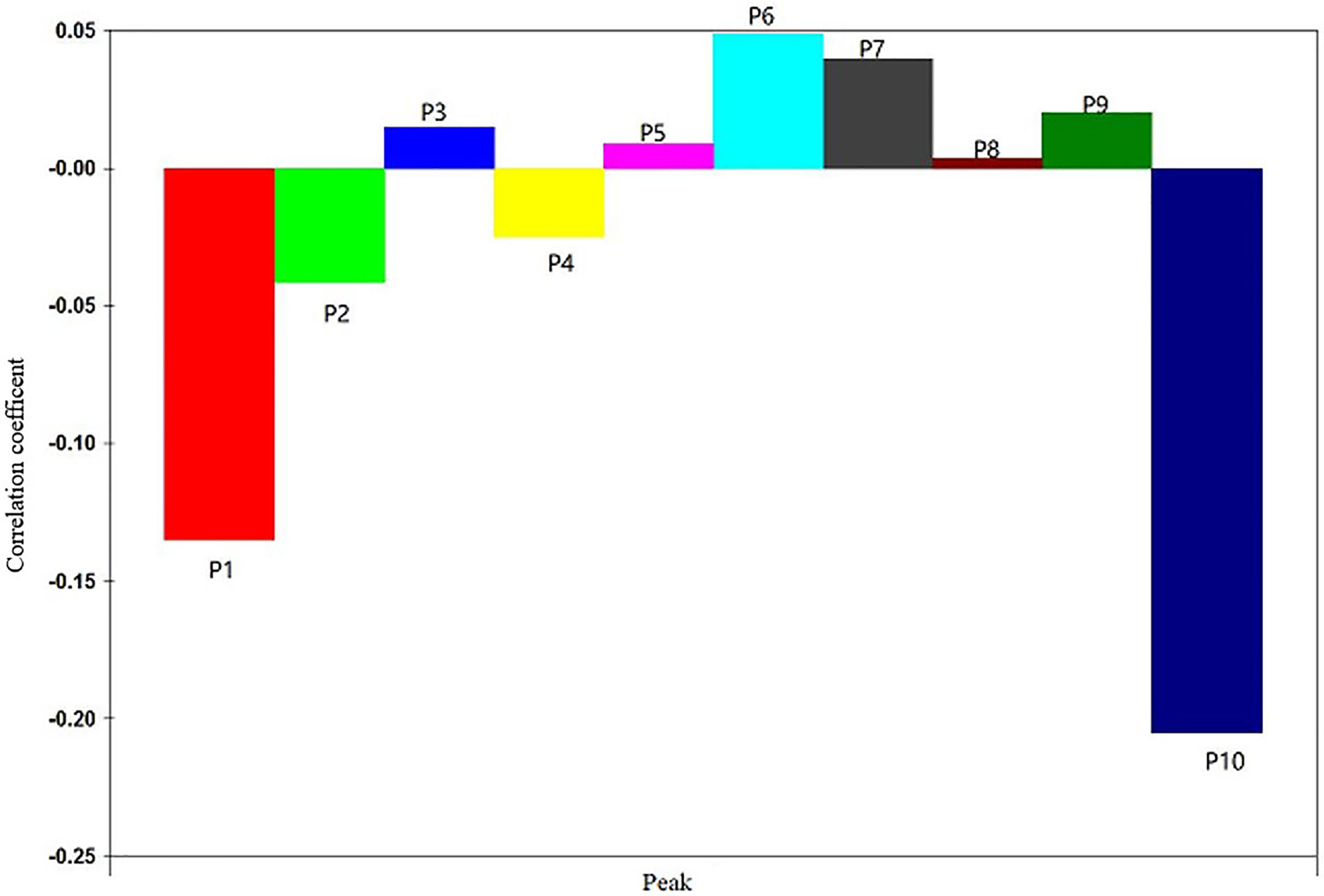
The IC50 values of anti-hepatoma activity of samples (equivalent to 1 g ·mL−1) were taken as dependent variables and 10 quantitative characteristic chromatographic peak areas as independent variables. The data were averaged by DPS 7.05 statistical software, and then partial least square regression analysis was carried out. After data standardization, the square sum of model error R2 increased with the increase of potential factor. When the potential factor reached 6, R2 reaches the maximum, and the variance of the explained independent variable is 89.62%.
The regression equation was obtained as follows:
The regression coefficient diagram of the partial least square regression equation was shown in Figure 3. P3 and P5, P6, P7, P8, and P9 were positively correlated with the IC50 of anti-liver cancer activity of S. heptaphylla ethanol extract. However, P1, P2, P4 and P10 were negatively correlated with the anti-hepatoma activity of S. heptaphylla ethanol extract. P10 had the strongest anti-liver cancer activity.
Composition Knock-Out Experiment
The Total Chromatogram of the Sample and the Chromatogram of the Knocked-Out Components
In Figure 4A, the full chromatogram of S. heptaphylla ethanol extract at 210 nm wavelength. Figures 4B–F are the chromatogram of the target components: P5, P6, P7, P9, and P10, respectively. According to the peak area normalization method, the purity of each target component was more than 85%, and the resolution was good (R > 1.5).
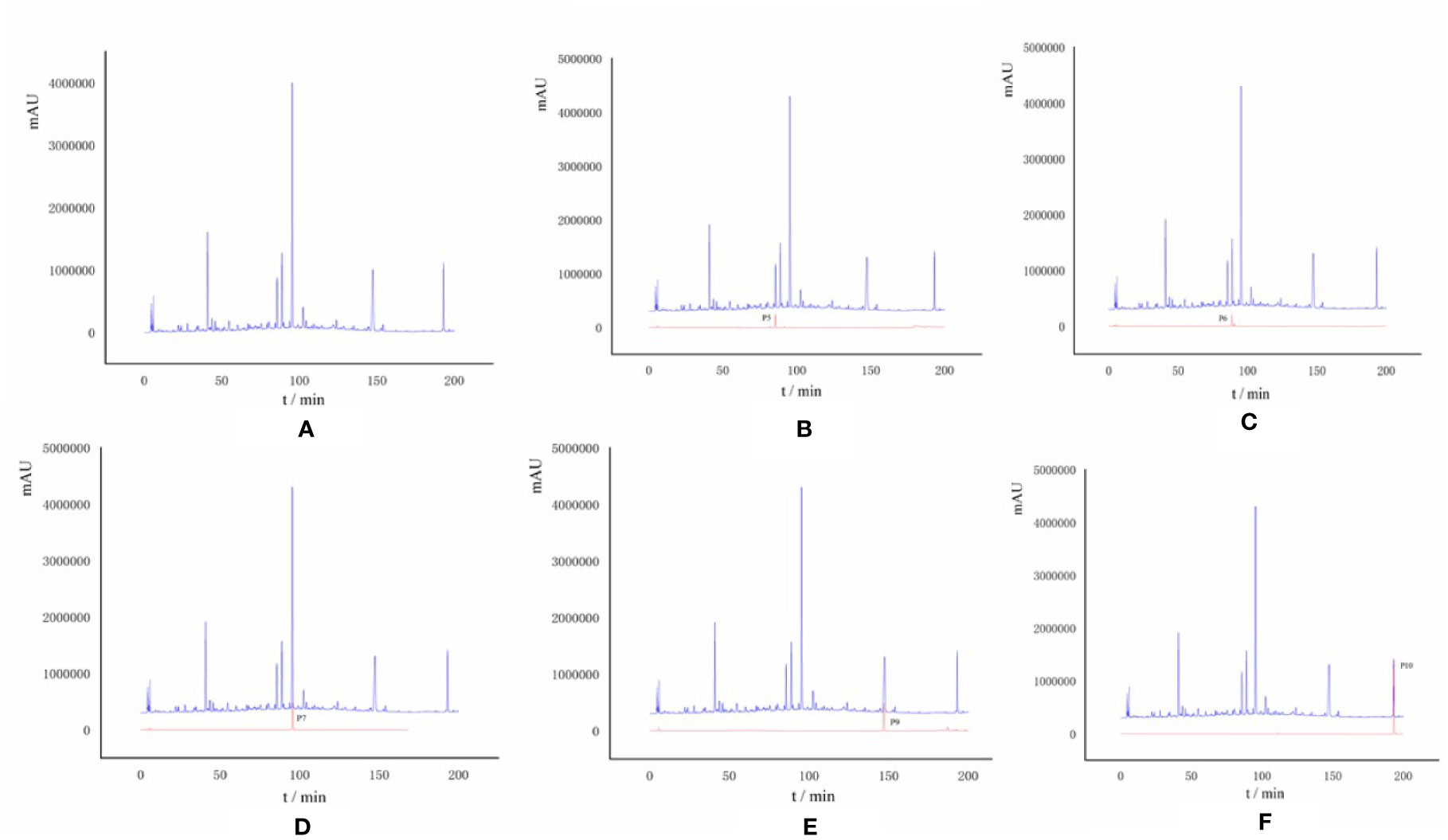
Figure 4 (A) HPLC chromatogram of S. heptaphylla ethanol extract; (B–F) HPLC chromatogram of Knock-Out components.
P6 (Rt = 89.858 min), the UPLC-MS2 results of the knock-out component was shown in Figure 5A, in which the molecular formula was C25H24O12. The results of secondary mass spectrometry showed that there were some ion fragments: 515.12/353.09/191.06/173.04/173.04/135.04. According to the reference (Yi, 2017), it was speculated to be 3,5-dicaffeoylquinic acid (Figure 5B). Moreover, the retention time of P6 was consistent with that of the reference substance.
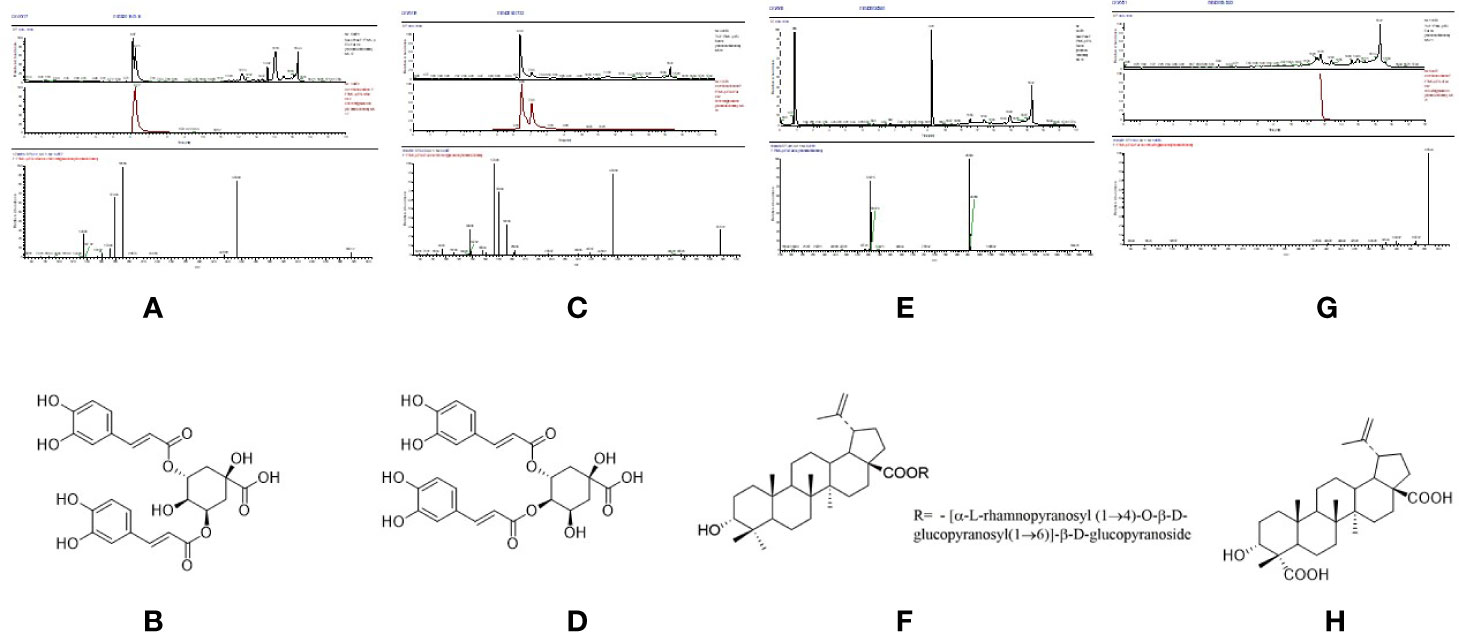
Figure 5 The high resolution mass spectra and chemical structures of Peak 6, 7, 9, 10 (A–H) knocked-out components.
P7 (Rt = 96.315 min), the UPLC-MS2 results of the knock-out component was shown in Figure 5C, in which the molecular formula was C25H24O12. The results of secondary mass spectrometry showed that there were some ion fragments: 515/353/191/179/173/135, 515/335.08/255.07/179.03/155.03/135.04, 515/335.1/317.07/299.06/173.04. According to the reference (Yi, 2017), it was speculated to be 3,4-dicaffeoylquinic acid (Figure 5D). In addition, the retention time was consistent with that of the reference substance.
P9 (Rt = 147.446 min), the UPLC-MS2 results of the knock-out component was shown in Figure 5E, in which the molecular formula was C48H76O19. According to the reference (Adam et al., 1982), it was speculated to be 3α-hydroxy-lup-20(29)- ene-23,28-dioic acid 28-O-[α-L-rhamnopyranosyl (1→4)-O-β-D-glucopyranosyl (1→ 6)]-β-D-glucopyrano-side (Figure 5F). In addition, the retention time of P9 was consistent with that of the reference substance.
P10 (Rt = 193.201 min), the UPLC-MS2 results of the knock-out component was shown in Figure 5G, in which the molecular formula was C30H46O5. The results of secondary mass spectrometry showed that there were some ion fragments: 485.33/467.32/439.32/423.33/, According to the reference (Adam et al., 1982), it was speculated to be 3α-hydroxy-lup-20(29)-ene-23,28-dioic acid (Figure 5H). Moreover, the retention time was consistent with that of the reference substance.
Effect of Compound A on the Viability of Normal Hepatocytes and Hepatocellular Carcinoma Cells
Compound A had the strongest anti-hepatoma activity, the effects of different concentrations of Compound A on the cell viability of normal hepatocyte lines (L0-2, Chang liver) and two hepatoma cell lines (Huh 7, HepG 2) after 24, 48, 72 h were detected by CCK-8 method. In Figure 6A, when the concentration of Compound A was higher than 125 nM, and 48 h treatment, Compound A had a slight inhibitory effect on human normal hepatocytes (L0-2), while the positive drugs showed significant cytotoxicity to normal hepatocytes in a time-dependent manner. In Figure 6B, after 24 h treatment, when the concentration of Compound A was higher than 500 nM, Compound A had significant cytotoxicity to Chang liver cells, compared with the blank group. After 48 h treatment, when the concentration of Compound A was higher than 62.5 nM, Compound A had significant cytotoxicity. The positive drug had significant cytotoxicity after 48 h. Compared with positive drugs, Compound A had less effect on the viability of normal hepatocytes. In Figure 6C, for Huh 7 hepatoma cells, when the concentration of Compound A was higher than 25 nM, it showed significant inhibitory effect on Huh 7 cells in a dose-dependent manner. The values of IC50 at 24, 48, and 72 h were 249.9, 285.3, and 199.5 nM, respectively. In Figure 6D, Compound A had a significant inhibitory effect on HepG 2 cells when the concentration of Compound A was higher than 25 nM. The values of IC50 at 24, 48, and 72 h were 657.7, 315.1, and 186.1 nM, respectively. The results showed that Compound A could significantly inhibit the viability of hepatoma cells, which was consistent with the results predicted by partial least square method. Then, the mechanism of Compound A against hepatoma cells was investigated.
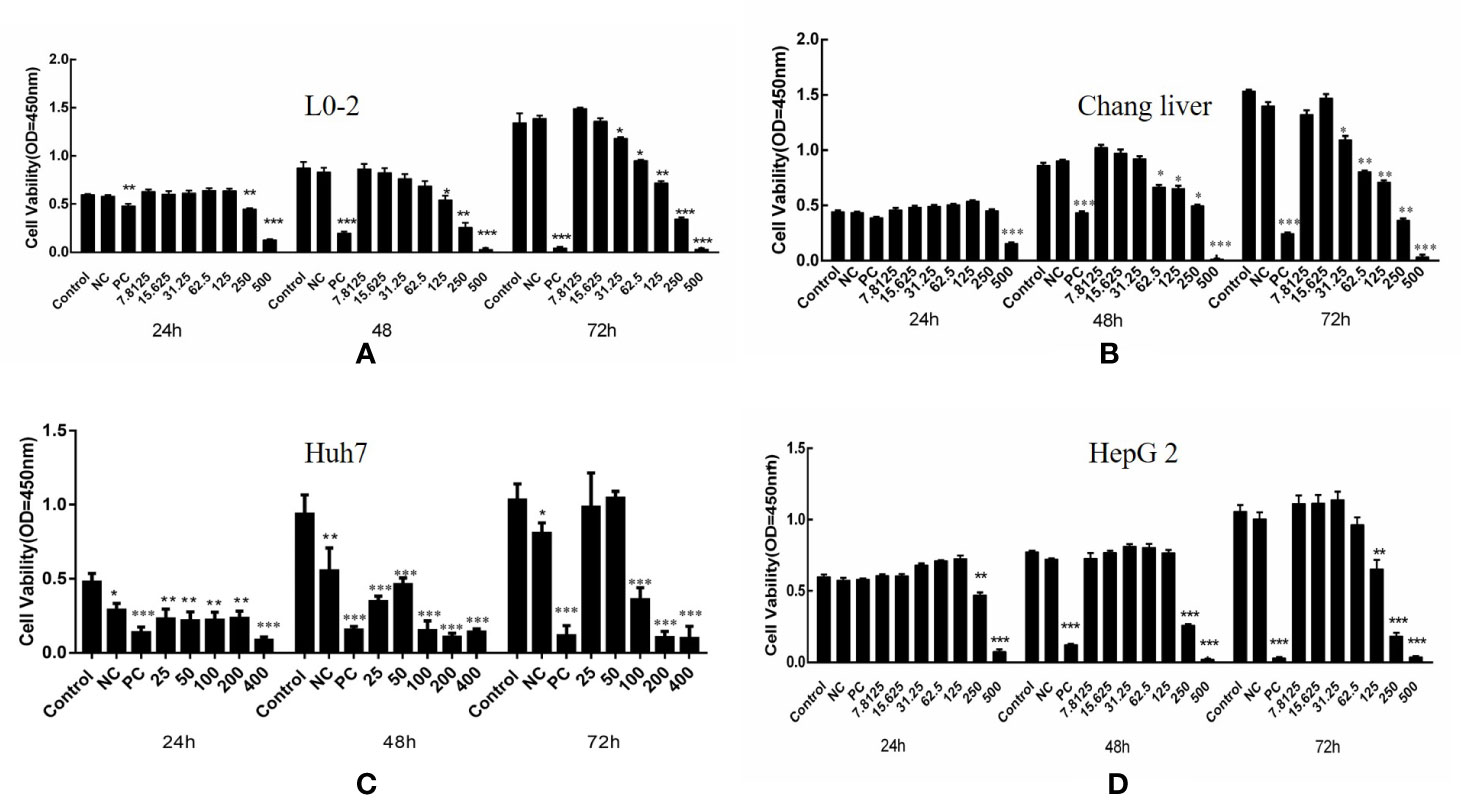
Figure 6 Effects of compound A on the activity of normal hepatocytes and hepatoma cells: L0-2 (A); Chang liver (B); Huh7 (C); HepG2 (D). ***P < 0.001, 0.001 < **P < 0.01, *P < 0.05 vs. control group.
Effects of Compound A on the Morphology of Two Kinds of Hepatoma Cells
The morphological changes of cells are closely related to the physiological phenomena such as cell proliferation, apoptosis and necrosis. Three concentrations of 100, 200, 400 nM were selected by CCK-8 experiment. HepG 2 and Huh7 hepatoma cells were treated with blank solvent, positive control drug (5-FU) and Compound A (100, 200, 400 nM) for 48 h, and then the morphological changes of HepG 2 and Huh7 hepatoma cells were observed by inverted microscope. Results were shown in Figure 7, the cells in the blank group were evenly spread over the bottom of the culture bottle, and the cells were regular, refractive and closely arranged, indicating that the cells were in good condition. While the number of cells in the positive control group and each concentration group of Compound A significantly decreased in a dose-dependent manner, and the cells showed morphological changes such as pseudopodia, deformation and floating.
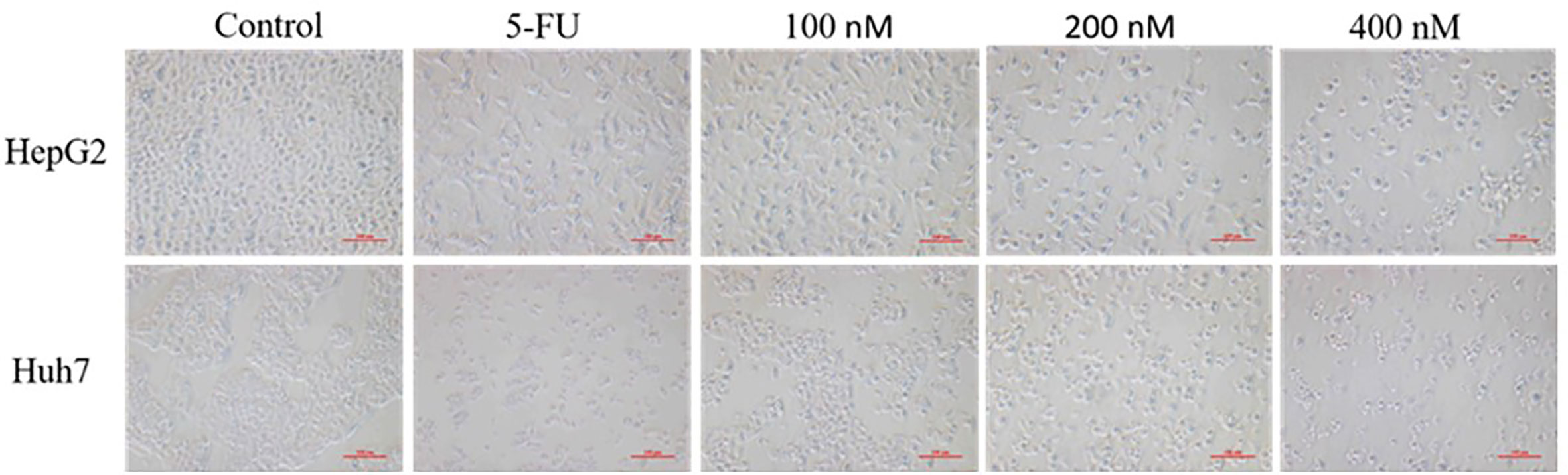
Figure 7 Effects of compound A at different concentrations on the morphology of HepG 2 and Huh 7 cells.
Thus, the question is whether the inhibitory effects of Compound A on hepatoma cells were achieved by directly causing cell necrosis or by promoting apoptosis? It needs to be further verified.
Effects of Compound A on Apoptosis of Normal Hepatocytes and Hepatoma Cells
In order to explore the mechanism of Compound A on inhibiting the proliferation of hepatoma cells, the effects of Compound A on apoptosis of normal hepatocytes (Chang liver) and hepatoma cells (Huh 7, HepG 2 and SMMC-7721) were analyzed by Annexin V/PI double staining and flow cytometry. Normal hepatocytes (Chang liver) and hepatoma cells (Huh 7, HepG 2 and SMMC-7721) were treated with blank solvents, positive drug and different concentrations of Compound A for 48 h. In Figure 8A, Compound A (200, 400 nM) and positive control group (5-FU) could significantly promote the apoptosis of Huh7 cells (P < 0.001), and the effect of Compound A (400 nM) was significantly better than that of positive control (5-FU) (P < 0.001). Therefore, we speculated that the inhibitory effect of Compound A on Huh 7 cells might be through promoting apoptosis. In Figures 8B, C, Compound A had no significant effects on the apoptosis of other hepatoma cells (HepG 2 and SMMC-7721), but could inhibit the viability of hepatoma cells by directly causing cell necrosis. However, did Compound A also have effects on normal hepatocytes? In Figure 8D, Compound A has no significant effect on the apoptosis and necrosis of normal hepatocytes, which further indicated that Compound A not only could inhibit hepatoma cells, but also has less toxic and side effects on normal hepatocytes. Then, we further studied the mechanism of Compound A in promoting apoptosis of Huh 7 cells.
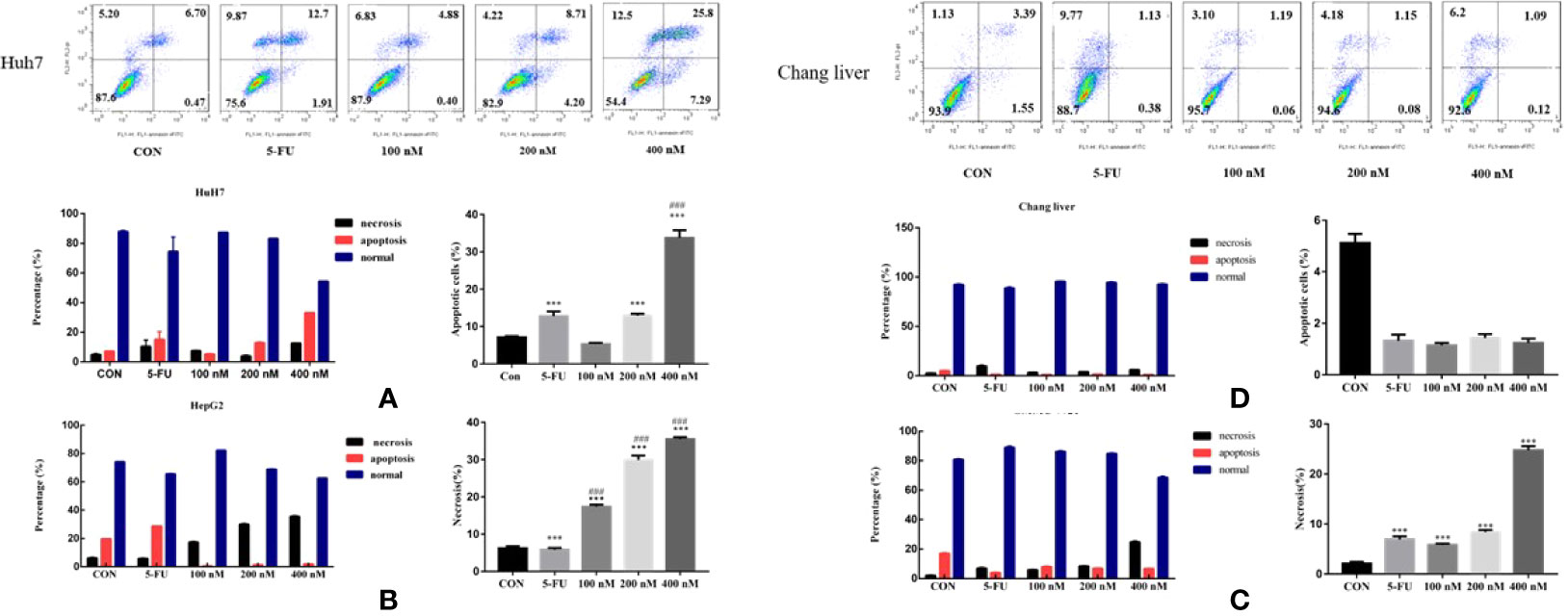
Figure 8 Effects of Compound A on apoptosis of hepatoma cells [(A): Huh 7; (B): HepG 2; (C): SMMC-7721] and normal hepatocytes [(D): Chang liver]. ***P < 0.001 vs. control group; ###P < 0.001 vs. 5-FU group.
Mechanism of Compound A on Promoting Apoptosis of Huh 7 Cells
Compound A Induces Apoptosis of Huh 7 Cells Through ROS
Cancer cells have a variety of apoptotic pathways, and ROS can affect mitochondrial metabolism and induce apoptosis (Masahiko and Masaya, 2013). Therefore, we studied whether Compound A induced apoptosis in Huh 7 cells by changing the level of reactive oxygen species (ROS). In Figures 9A, B, flow cytometry analysis showed that both the positive group (5-FU) and Compound A (100, 200, 400 nM) could significantly increase the level of intracellular ROS (P < 0.05, P < 0.001) compared with the blank group, in addition, the effects of Compound A (200, 400 nM) were significantly stronger than that of the positive control group (5-FU). For the normal hepatocytes (Chang liver), it was found that the positive control group (5-FU) could significantly increase the level of ROS (P < 0.001), compared with the blank group, while Compound A had no significant effect on the level of ROS in normal hepatocytes (P > 0.001), indicating that Compound A had less cytotoxicity to normal hepatocytes (Chang liver), and significantly lower than positive drugs (5-FU). It has been reported that the increase of intracellular ROS could induce the activation of mitochondrial apoptosis pathway. Then, the next study focused on the changes in the content of mitochondrial apoptotic proteins.
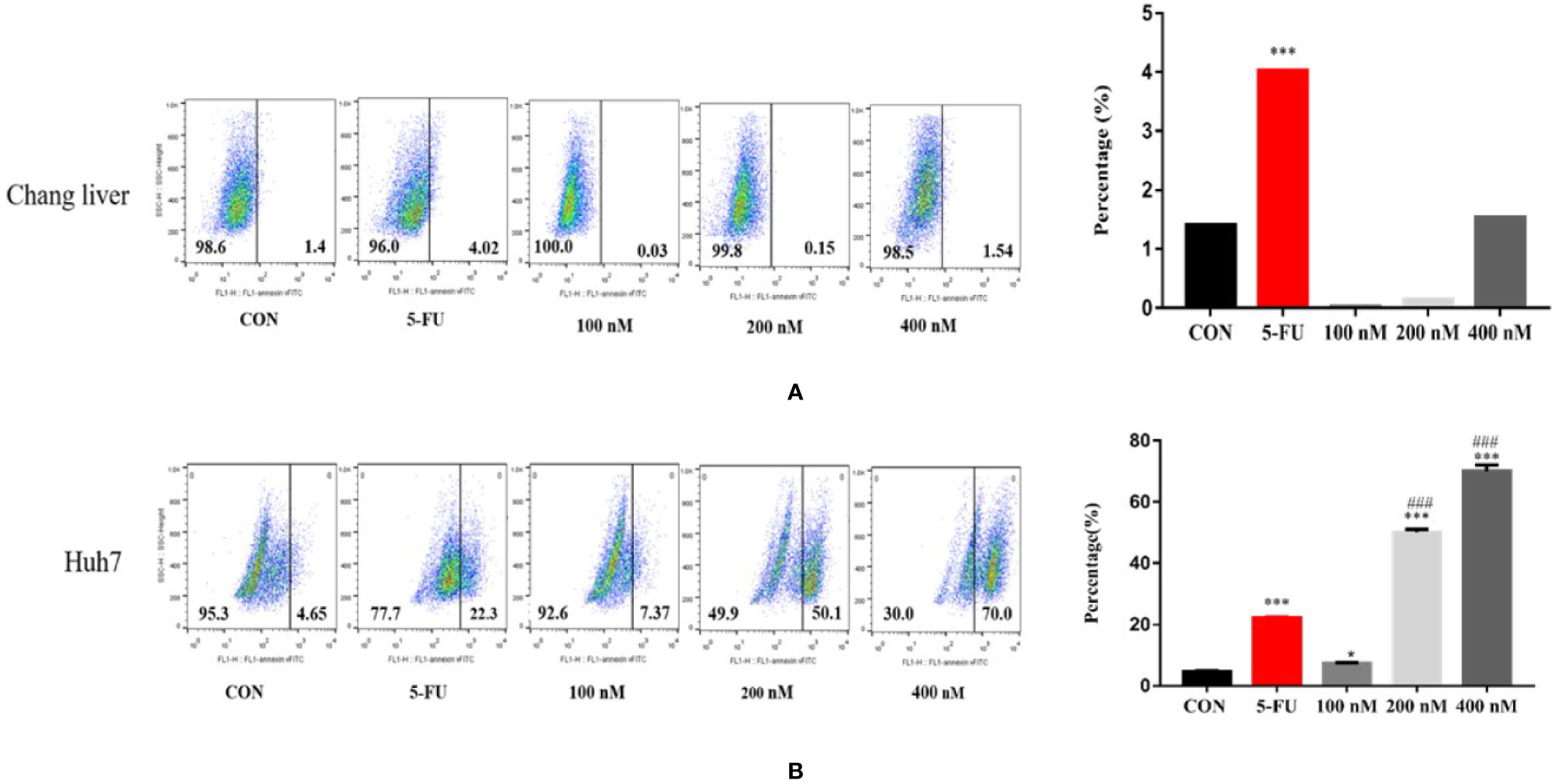
Figure 9 Effects of Compound A at different concentrations on ROS levels in Chang liver (A) and Huh 7 (B) cells. ***P < 0.001, *P < 0.05 vs. control group; ###P < 0.001 vs. 5-FU group.
Regulatory Effect of Compound A on the Expression of Apoptosis-Related Proteins in Huh 7 Cells
The increase of intracellular ROS level can induce the activation of mitochondrial apoptosis pathway. In order to further explore the mechanism of Compound A-induced apoptosis in Huh 7 cells, the expressions of Bcl-2 family proteins were detected. The change in the content of pro-apoptotic proteins was shown in Figure 10A, Compound A (100, 200, 400 nM) could significantly increase the content of mitochondrial pro-apoptotic proteins Bax and Bim (P < 0.001) compared with the blank group, and the effects were better than that of positive drug (5-FU). As for the anti-apoptotic protein, in Figure 10B, Compound A (100, 200, 400 nM) could significantly decrease the contents of Bcl-xL and Mcl-1 (P < 0.001), with no significant effect on the content of Bcl-2 (P > 0.001) compared with the blank group. Because the ratio of Bcl-2 to Bax determines the permeability of mitochondrial membrane, when the ratio is low, the permeability of mitochondrial membrane increases and apoptotic factors are transferred from mitochondria to the nucleus, which in turn induces cell apoptosis. On the contrary, it is difficult for apoptotic factors to transfer to the nucleus. In Figure 10C, Compound A (100, 200, 400 nM) and the positive control group (5-FU) significantly decreased the proportion of Bcl-2/Bax protein compared with the blank group (P < 0.001), and the effects of Compound A (100, 200, 400 nM) were significantly better than that of the positive control group (5-FU). It was inferred that Compound A might induce apoptosis of Huh 7 cells by regulating the expression of mitochondrial apoptotic proteins.
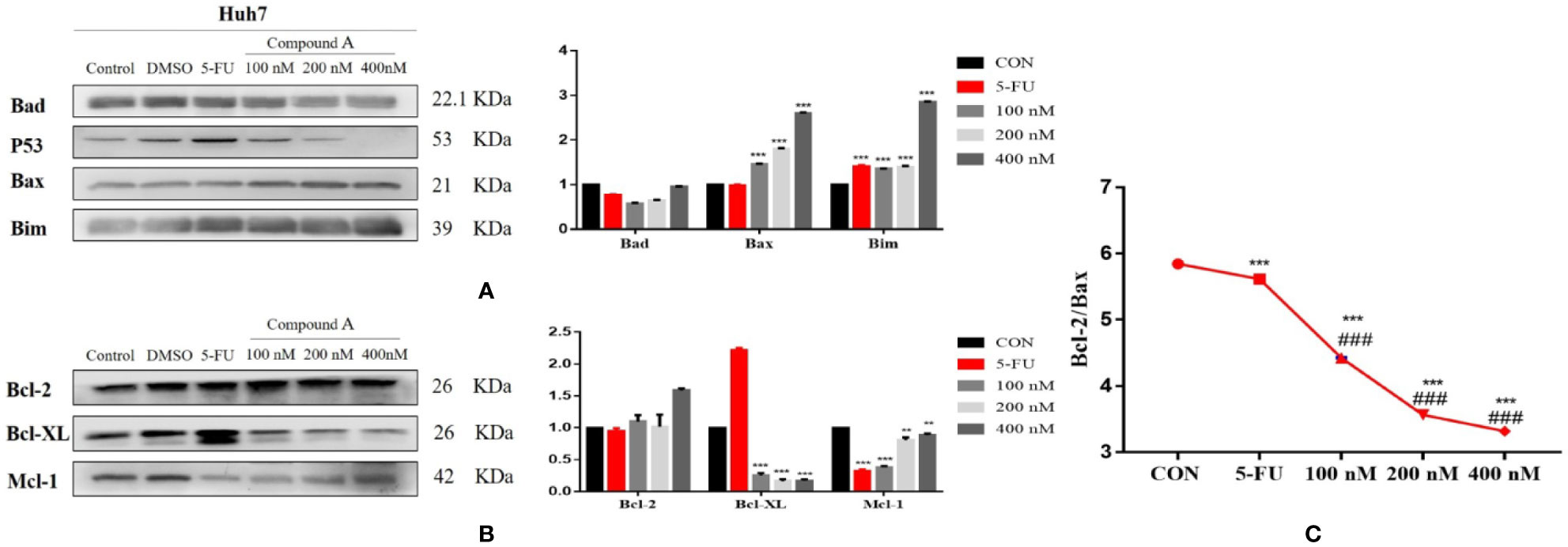
Figure 10 The regulatory effect of Compound A on the expression of apoptosis related proteins in Huh7 cells. (A) pro-apoptotic protein; (B) anti-apoptotic protein; (C) ratio of Bcl-2 to Bax. ***P < 0.001,**P < 0.01 vs. control group; ###P < 0.001 vs. 5-FU group.
Materials and Methods
General
Shimadzu High-Performance Liquid Chromatograph (Japan Shimadzu Co., Ltd). Thermo Ultimate 3000 UHPLC, Q Exactive (Thermo Scientific, US). chromatographic column Purospher® STAR LP RP-18 (Merck KGaA, Germany). MultiskanMK3 Full wavelength enzyme marker (Thermo Electron, US). GRP-9270 Constant temperature incubator (Sen xin Experimental instrument Co., Ltd, Shanghai). AB135-S Electronic balance (Mettler-Toledo Instruments Co., Ltd., Switzerland). Rotary evaporation meter (Tokyo physical and Chemical Instruments Co., Ltd.), Centrifuge precipitator (Shanghai Surgical Instruments Factory). Flow cytometry (Becton Dickinson, BD, US). Multicolor fluorescence, chemiluminescence and visible light imager (ProteinsiMpleFluorcheM Q). Electrophoretic apparatus (EPS600 Tanon); Inverted microscope (Nikon ECLiPSETS100).
Drugs and Reagents
Acetonitrile was purchased from Fisher (HPLC, US); pure water was obtained from Wahaha Group (Hangzhou); Dulbecco’s modified eagle medium (DMEM), Penicillin and streptomycin, Penicillin and streptomycin and PVDF membrane were purchased from Beijing Solarbio Science & Technology Co., Ltd. non-fat milk powder, Trypsin, EDTA, CCK-8 Kit, 6/96 well cell culture plate, Annexin V-FITC/Pl, Bax、Bcl-2、BID、Bcl-XL、Bak、Bim、β-actin and corresponding antibody were purchased from Becton Dickinson (BD, US); BCA protein quantitative Kit and Acrylamide were obtained from biyuntian biological Co., Ltd (Shanghai); fetal bovine serum (FBS) was purchased from Gibco (Grand Isoland, US); Thiazolyl blue(MTT) was purchased from Hualan Chemical Technology Co., Ltd (Shanghai).
Plant Material
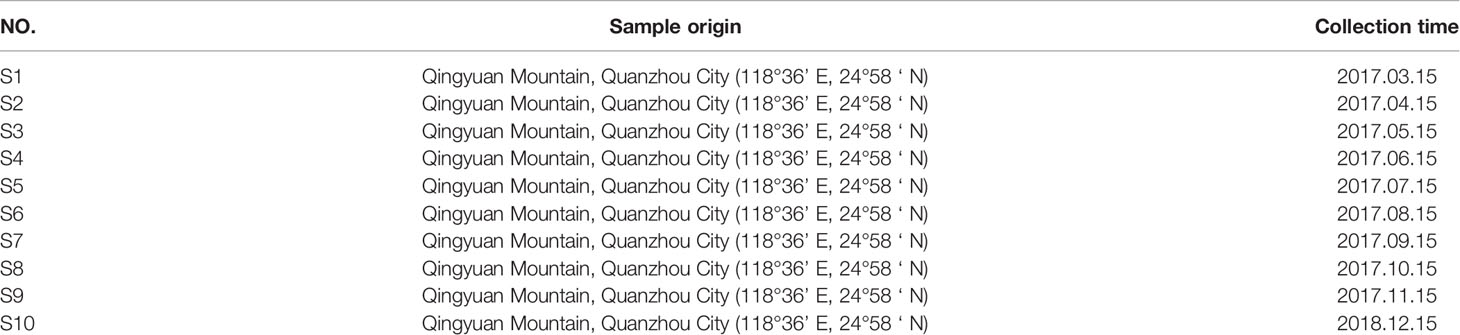
10 batches of S. heptaphylla leaves were collected from the foothills of Qingyuan Mountain in Quanzhou City, Fujian Province, as detailed in Table 3. All the samples were identified as the leaves of S. heptaphylla by Professor Changqin Li (Henan University). The samples now were stored in the Medical College of Huaqiao University and the National R & D Center for Edible Fungus Processing Technology, Henan University.
Experimental Methods
Sample Solution Preparation
10 batches of S. heptaphylla leaves were crushed (40 mesh), about 5.0 g, 10 times the amount of 70% ethanol, cold soaked for 3 times, each time for 3 days, combined with filtrate, decompressed and concentrated to obtain the total extract of S. heptaphylla leaves. The 200.00 mg extract was precisely weighed and dissolved in 10 mL 70% ethanol and filtered with a 0.22-μm micro porous membrane to prepare a solution, equivalent to 1 g· mL−1 of the original medicinal material.
Conditions for Fingerprint Analysis
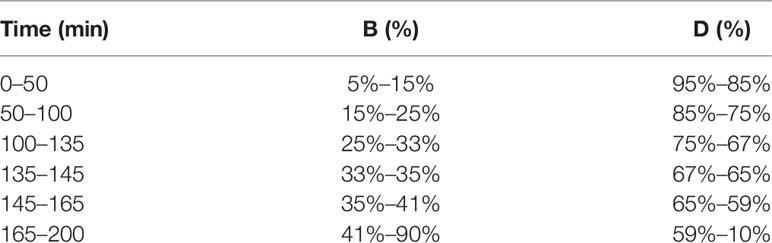
Chromatographic conditions: RP-18 endcapped column (4.6 mm × 250 mm, 5 μm); mobile phase was acetonitrile (B)-0.1% phosphate water (D). The elution procedure was shown in Table 4. The flow rate was set at 0.6 mL/min, and the column temperature was 25 °C. The detection wavelength was set at 210 nm with the injection volume of 5 μL.
The Method of Knocking Out the Target Components
Under the optimal conditions of high performance liquid chromatography (HPLC), the 70% ethanol extract (equivalent to 1 g· mL−1) was injected into 5 μL, and the chromatogram at 210 nm wavelength was recorded. According to the spectrum-effect relationship, the retention time of the chromatographic peaks related to the anti-hepatoma activity was analyzed, and the eluents of the corresponding peaks were collected and concentrated under reduced pressure to get the target compounds. Each target peak was prepared 10 times in liquid phase, and concentrated the eluate respectively. Dissolved with 0.3 mL 70% ethanol solution and passed through 0.22 μm microporous membrane to obtain the sample containing the target peak (marked as Px).
Mass Spectrometry Analysis
Chromatographic conditions: Watres BEHC-18 column (2.1 × 50 mm, 1.7 μm), mobile phase was acetonitrile (A)-0.1% formic acid aqueous solution (B), The elution procedure was follows: 0–5 min, (A)10%–(B) 90%; 5–30 min, (A) 95%–(B) 5%; 30 to 55 min, (A) 95% to (B) 5%; 55 to 56 min, (A) 10%–(B) 90%; 56–61 min, (A) 10%–(B) 90%. The flow rate was set at 0.3 mL/min, and injection 0.2 μL, determination.
Mass spectrometry conditions: sheath gas flow rate: 35 arb; auxiliary gas flow rate: 10 arb; spray voltage: 3.5 kV; capillary temperature: 320 °C, polarity: positive; full scan parameters: resolution 7000; AGC target: 3e6; Maximum IT: 100 ms; scanning range: 50-800 m/z. Second-level scanning parameters: resolution: 17500; AGC target: 1e5; Maximum IT: 50 ms; isolation window: 4.0 m/z; collision energy: 30.
Cell Culture
L0-2, Chang liver, HepG 2, SMMC-7721 and Huh 7 cells were cultured in the DMEM medium containing 10% FBS, 1% penicillin and streptomycin mixture and cultured in the incubator at 37 °C and 5% CO2. Take logarithmic growth phase cells for follow-up experiment.
Microscopic Observation of Cell Morphology
After the cells were treated with different concentrations of drugs for 48 h, the cells were collected and randomly taken 5 visual fields under an inverted microscope to take pictures (20 × objective lens).
Cell Viability Detection
MTT
The cells of logarithmic growth phase were inoculated in 96-well plates (1 × 104 cells/well), and incubated in incubator at 37 °C and 5% CO2 for 24 h. Thereafter, the supernatant was discarded by liquid transfer gun. The ethanol extracts of S. octophylla were diluted from 800 μg/mL to 800, 400, 200, 100, 50 and 25 μg/mL, respectively, and the blank control group was given DMEM medium with 6 compound holes in each concentration. Then incubated in 37 °C incubator for 24 h, 10 μL MTT solution was added to each well, then the supernatant was discarded after 4 h, and then DMSO solution was added to dissolve it. The absorbance was measured at 490 nm wavelength for 10 min.
CCK-8
Follow the operation steps of the CCK-8 test kit instructions. The logarithmic growth phase cells were inoculated into 96-well plates (2 × 103 cells/well). 12 h later, Compound A of different concentrations were added, and 6 compound holes were set up for each concentration. The cells were cultured at 37 °C for 24, 48 and 72 h, then the supernatant was discarded and washed with PBS twice. Then, 10% CCK-8 solution was added and cultured at 37 °C for 2 h. The absorbance was measured by enzyme labeling instrument at 450 nm wavelength.
Flow Cytometry Analysis
The apoptosis of related cells was detected by FITC Annexin V apoptosis detection kit. The cells in logarithmic growth phase were inoculated in 6-well plates (1 × 106 cells/well) and cultured for 24 h. The original culture medium was abandoned and Compound A (100, 200, 400 nM) solution was added. The blank control group was DMSO, positive control group was 5-fluorouracil (5-FU). Each group was provided with 3 multiple holes and incubated in an incubator at 37 °C and 5% CO2 for 48 h. After discarding the original culture medium and washing with PBS for 3 times, the cells were digested with 0.25% trypsin, centrifuged for 5 min, washed twice with 1 mL precooled PBS, re-suspended in 200 μL binding buffer, then mixed with 10 μL Annexin V-FITC, 15 min, and 300 μL binding buffer were added away from light at room temperature, finally, 5 μL PI was added away from light, staining for 15 min to determine the apoptosis rate of each sample by flow cytometry. The left upper quadrant (Q1) was necrotic cells, the right upper quadrant (Q2) was late apoptotic cells, the right lower quadrant (Q3) was early apoptotic cells, and the left lower quadrant (Q4) was normal cells. Results the average percentage of apoptotic cells was analyzed by FlowJo software.
Detection of the Expression of Related Proteins in Apoptosis Signal Pathway by Western Blot
The cells in logarithmic growth phase were evenly inoculated in 6-well plates (1 × 106 cells/well), and then treated with Compound A (20, 50, 100 nM), blank control group (DMSO) and positive control group (5-FU) for 48 h in an incubator at 37 °C and 5% CO2. Then, cells were harvested and lysed on ice for 30 min in Radio Immunoprecipitation Assay (RIPA), and centrifuged at 4 °C, 12000 rpm for 20 min, to absorb the supernatant and transfer it to an EP tube. The protein concentration was measured by BCA protein concentration assay kit. Cell lysates were then loaded onto 10% SDS-PAGE for analysis of Bax、Bcl-2、BID、Bcl-XL、Bak、Bim, and β-actin. Proteins were transferred onto a polyvinylidene fluoride membranes (PVDF), which were blocked in 5% non-fat milk in Tris-buffered saline with 1% Tween 20 for 2 h. Then, the PVDF membrane was incubated with the primary antibody overnight at 4 °C. The membranes were washed thoroughly and incubated with horseradish peroxidase-conjugated secondary antibodies. After washing, protein bands were visualized using enhanced chemiluminescence (ECL) and use Imag J software to analyze the gray value of the protein.
Statistical Analysis
The partial least square regression analysis method was used to analyze the data of 10 quantitative characteristic maps and the anti-hepatoma activities of 10 batches S. heptaphylla extracts. The retention time of each peak of the characteristic spectrum of S. heptaphylla was corrected by using the software “similarity Evaluation system of chromatographic fingerprint of traditional Chinese Medicine version 1.0 A” recommended by Chinese Pharmacopoeia Committee, and the peak area was averaged. The data of 10 quantitative characteristic peaks were obtained. Using DPS 7.05 software to analyze the area of 10 quantitative characteristic peaks as independent variables, the IC50 values of (X), 10 batches of S. heptaphylla extract against liver cancer as dependent variables (Y), the partial least square regression equation was established, and the correlation between quantitative characteristic peaks and anti-hepatoma activity was screened.
The results were expressed by arithmetic mean and standard deviation, and the significant differences were compared by SPSS software single factor analysis of variance (One-Way ANOVA). All the pictures were drawn with Graphpad Prism 7.0.
Discussion
“Spectrum-Effect Relationship” and “Component Knock-Out” technology has been successfully applied in screening the active ingredients of natural products (Shi et al., 2018 and Li et al., 2019). In this paper, partial least square method was used to analyze the correlation between 10 quantitative characteristic chromatographic peaks of S. heptaphylla and the anti-hepatoma activity (IC50 value) of S. heptaphylla. It was inferred that P10 had the strongest anti-liver cancer activity by spectral correlation analysis. Bioactive components of natural products can be isolated quickly and efficiently by using “component knock-out” technology, which has advantages of accuracy and efficiency. In this manuscript, based on spectral correlation analysis, P10 with anti-hepatoma activity was prepared rapidly by component knock technique, and P10 was determined as 3α-hydroxy-lup-20(29)- ene-23,28-dioic acid (Compound A) by “Component knock-out” method and high resolution mass spectrometry. HPLC comparison showed that the results were consistent with reference standard.
In this manuscript, the results of CCK-8 test showed that Compound A could significantly inhibit the viability of hepatoma cells Huh 7, HepG 2 and SMMC-7721, which was consistent with the results speculated by spectral correlation analysis. Compound A could inhibit the viability of liver cancer cells by directly necrotizing tumor cells or indirectly inducing tumor cell apoptosis? The morphological changes of cells are often closely related to necrosis, apoptosis and so on. We found that Huh7 cells showed typical apoptotic morphology, which was further verified. The apoptosis of three kinds of hepatocellular carcinoma cells and normal hepatocytes were analyzed by flow cytometry. It was found that the apoptotic proportion of Huh7 cells significantly increased after Compound A treatment, while the necrotic proportion of HepG 2 and SMMC-7721 cells increased significantly, but there was no significant effect on normal hepatocytes.
The classical cytological theory points out that apoptosis is a spontaneous way of cell death, which mainly includes exogenous and endogenous apoptosis pathways (Daugas, 2001; Alciaturi et al., 2014). Including mitochondrial apoptosis pathway, death receptor apoptosis pathway and endoplasmic reticulum apoptosis pathway (Fulda and Debatin, 2006). Among them, mitochondrial apoptosis pathway is the most common one (Ashkenazi and Salvesen, 2014), which is regulated by Bcl-2 protein family, Caspase family and other key factors. Among them, Bcl-2 family regulates the permeability of mitochondrial inner and outer membrane, thus affecting the process of cell apoptosis (Martinou and Youle, 2011). The Bcl-2 protein family is mainly composed of anti-apoptotic proteins and pro-apoptotic proteins (Tsukahara et al., 2006). Anti-apoptotic proteins include Bcl-xl, Mcl-l and so on, in which Bcl-2 protein is the main sensor of apoptosis (Hockenbery et al., 1990; Boise et al., 1993). Anti-apoptotic proteins can stabilize the mitochondrial membrane, prevent the release of many kinds of mitochondrial apoptotic proteins such as Cyt-c, and prevent the further activation of Caspase (Kutuk and Basaga, 2006). Pro-apoptotic proteins, including Bax, Bak and Bad, could change the mitochondrial membrane potential and enhance the mitochondrial permeability. Cytochrome C (Cyt-c) is released from the mitochondria into the cytoplasm and further binds with apoptosis-related factors (Apaf-1) to form oligomers, which binds Pro-Caspase-9 to form apoptotic bodies to activate Caspase-9, and then activate Caspase-3, to lead cell apoptosis. In addition, the ratio of Bax to Bcl-2 protein may play a key role in the process of apoptosis (Liang and Cao, 2014). When the proportion is low, the permeability of the mitochondrial membrane increases, and the apoptotic factor is transferred from the mitochondria to the nucleus, which could induce the apoptosis. On the contrary, it is difficult to transfer the apoptotic factor to the nucleus. Intracellular ROS could affect mitochondrial metabolism (Masahiko and Masaya, 2013) and then activate the pathway of mitochondrial apoptosis.
It is reported that lupine pentacyclic triterpenes have strong and broad-spectrum anti-tumor activity, low toxicity and selectivity to tumor cells, and their mechanism is independent of p53 pathway, so they are expected to become excellent anti-tumor lead compounds (Wang et al., 2017). Betulinic acid can inhibit the proliferation of HepG 2 cells through Wnt pathway. Oleanolic acid inhibits proliferation and induces apoptosis of HepG 2 cells by regulating ROS and MMP (Miao et al., 2016). In addition, betulinic acid, 23-hydroxybetulinic acid, betulin and lupeol have significant anti-cancer activities through activating mitochondrial apoptosis pathway, and regulate the levels of Bcl-2 and ROS (Wang et al., 2017).
In order to further explore the mechanism of Compound A in promoting apoptosis of Huh7 cells. We detected the changes of apoptosis-related proteins of Bcl-2 family proteins and ROS in Huh7 cells. The results showed that Compound A (100, 200, 400 nM) could significantly increase the content of mitochondrial pro-apoptotic proteins Bax and Bim (P < 0.001), and its effect was better than that of positive drugs (5-FU). As for anti-apoptotic protein, Compound A (100, 200, 400 nM) could significantly reduce the content of Bcl-xL and Mcl-1 (P < 0.001), compared with the blank group, and Compound A (100, 200, 400 nM) and 5-FU could significantly reduce the proportion of Bcl-2/Bax protein (P < 0.001).
Conclusion
Spectrum Effect Relationship and Component Knock-Out were the rapid method to infer anti-hepatoma activity of compound in S. heptaphylla leaves and Compound A was determined to be one of the main anti-hepatoma active components. Compound A could significantly inhibit the proliferation of all kinds of hepatoma cells (Huh7, HepG2 and SMMC-7721), and had little toxicity to normal hepatocytes. It could promote the apoptosis of Huh7 cells by up-regulating the level of ROS in Huh7 cells, activating intracellular mitochondrial apoptosis pathway and regulating the level of Bcl-2 family proteins in mitochondrial apoptosis pathway.
Data Availability Statement
All datasets presented in this study are included in the article/supplementary material.
Author Contributions
WK and YZ conceived the research subject. XL, NJ, XX, and CL conducted the experiments, collected the plant specimens, separated and analysis compounds, analyzed and interpreted the data, as well as prepared the first draft. WK and XL critically read and revised the paper. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Key Project in Science and Technology of Henan Province (192102110112).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
ROS, reactive oxygen species; HPLC, high-performance liquid chromatography; CCK-8, cell counting kit-8; IC50, half-inhibitory concentration; DMSO, dimethyl sulfoxide; 5-FU, 5-fluorouracil; IRPA, radio immunoprecipitation assay; PVDF, polyvinylidene fluoride membranes; MTT, 3-(4,5)-dimethylthiahiazo (-z-y1)-3,5-di- phenytetrazolium bromide; DMEM, Dulbecco’s modified eagle medium; TCM, Traditional Chinese Medicine; ECL, enhanced chemiluminescence; Compound A, 3α-hydroxy-lup-20(29)-ene-23,28-dioic acid; HCC, hepatocellular carcinoma; FBS, fetal bovine serum.
References
Adam, G., Lischewski, M., Phiet, H. V. (1982). 3α-Hydroxy-lup-20(29)-ene-23, 28-dioic acid from Schefflera octophylla. Phytochemistry 21 (6), 1385. doi: 10.1016/0031-9422(82)80147-7
Alciaturi, C. E., Escobar, M. E., De La Cruz, C., Rincón, C. (2014). Partial least squares (PLS) regression and its application to coal analysis. Rev. Técnica La Facultad Ingeniería Universidad Del Zulia. 26 (3), 197–204.
Ally, A., Balasundaram, M., Carlsen, R., Chuah, E., Clarke, A., Dhalla, N., et al. (2017). Comprehensive and Integrative Genomic Characterization of Hepatocellular Carcinoma. Cell 169 (7), 1327–1341. e23. doi: 10.1016/j.cell.2017.05.046
Ashkenazi, A., Salvesen, G. (2014). Regulated cell death: signaling and mechanisms. Annu. Rev. Cell Dev. Biol. 30, 337–356. doi: 10.1146/annurev-cellbio-100913-013226
Boise, L. H., González-García, M., Postema, C. E., Ding, L., Lindsten, T., Turka, L. A., et al. (1993). Bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell 74, 597–608. doi: 10.1016/0092-8674(93)90508-n
Daugas, E. (2001). Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature 410 (6828), 549. doi: 10.1038/35069004
Fang, C., Han, Z. H., Liu, X. H., Yang, Y. F., Lan, Z. H., Feng, S. L. (2016). Spectrum-effect relationship of active fraction from Hedysari Radix on improving immunity. Chin. Tradit. Herbal Drugs 47, 101–105. doi: 10.7501/j.issn.0253-2670.2016.01.015
Fukuda, K., Okumura, T., Abei, M., Fukumitsu, N., Ishige, K., Mizumoto, M., et al. (2017). Long-term outcomes of proton beam therapy in patients with previously untreated hepatocellular carcinoma. Cancer Sci. 108 (3), 497–503. doi: 10.1111/cas.13145
Fulda, S., Debatin, K. M. (2006). Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 25 (34), 4798. doi: 10.1038/sj.onc.1209608
Gong, A. G., Li, N., Lau, K. M., Lee, P. S., Yan, L., Xu, M. L., et al. (2015). Calycosin orchestrates the functions of DangguiBuxue Tang, a Chinese herbal decoction composing of Astragali Radix and Angelica Sinensis Radix: An evaluation by using calycosin-knock out herbal extract. J. Ethnopharmacol. 168, 150–157. doi: 10.1016/j.jep.2015.03.033
Gui, J. F. (2012). Study on antitumor activity of Poria cocos triterpene compositions and the quality analysis of Poria cocos medicinal materials [D] (Wuhan: Hubei University of Chinese Medicine).
Hockenbery, D., Nunez, G., Milliman, C., Schreiber, R. D., Korsmeyer, S. J. (1990). Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature 348, 334–336. doi: 10.1038/348334a0
Hu, X. Y., Liu, M. H., Qin, S., Zhang, S. J. (2013). Spectrum-effect relationship of antibacterial extracts from Isatidis Radix. Chin. Tradit. Herb. Drugs 44, 1615–1620. doi: 10.7501/j.issn.0253-2670.2013.12.018
Julie, K. H., Laura, M. K., Richard, S. F. (2018). AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 67 (1), 358–380. doi: 10.1002/hep.29086
Kutuk, O., Basaga, H. (2006). Bcl-2 protein family: Implications in vascular apoptosis and atherosclerosis. Apoptosis 11 (10), 1661–1675. doi: 10.1007/s10495-006-9402-7
Li, W. J., Zhang, Y., Shi, S. J., Yang, G., Liu, Z. H., Wang, J. M., et al. (2019). Spectrum-effect relationship of antioxidant and tyrosinase activity with Malus pumila flowers by UPLC-MS/MS and component knock-out method. Food Chem. Toxicol. 133, 110754. doi: 10.1016/j.fct.2019.110754
Li, b. (2011). Study on Separation and Purification Methods、Structural Identification and the Activity of Inhibiting Liver Cancer of Triterpenoid from Schisandra Chinensis (Turcz.) Baill [D] (Shenyang: Shenyang Agricultural University).
Liang, K., Cao, B. Z. (2014). Progress on cell apoptosis of mitochondrial regulation. BME &Clin Med. 18 (5), 501–505. doi: 10.13339/j.cnki.sglc.2014.05.049
LV, G. S., Chen, L., Wang, H. Y. (2015). Research progress and prospect of liver cancer in China. Chin. Bull. Life Sci. 27 (3), 237–248. doi: 10.13376/j.cbls/2015034
Martinou, J. C., Youle, R. J. (2011). Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev. Cell. 21, 92–101. doi: 10.1016/j.devcel.2011.06.017
Masahiko, T., Masaya, H. (2013). HTLV-1 Tax oncoprotein stimulates ROS production and apoptosis in T cells by interacting with USP10. Blood 122, 715–725. doi: 10.1182/blood-2013-03-493718
Miao, J. Q., Wang, X. R., Dong, X. S., He, S. H., Liang, T. G., Li, Q. S., et al. (2016). Apoptosis-inducing effect of oleanlic acid on human hepatoma cell line HepG 2 and its mechanism. Drugs Cli. 7 (31), 934–938.
Qian, W. D., Tan, A. J., Li, S. M., Chen, B. L., Du, A. D., Wang, S. Y., et al. (2019). Pentacyclic triterpenoids from Eucommia ulmoides and their antitumor activities. Chin. Tradit. Pat. Med. 41 (5), 1059–1065.
Shi, M. J., Zhang, Y., Song, M. M., et al. (2018). Screening the Marker Components in Psoralea corylifolia L. with the Aids of Spectrum-Effect Relationship and Component Knock-Out by UPLC-MS². Int. J. Mol. Sci. 19 (11), 3439. doi: 10.3390/ijms19113439
Tsukahara, S., Yamamoto, S., Shwe, T.-T.-W., Ahmed, S., Kunugita, N., Arashidani, K., et al. (2006). Inhalation of low-level formaldehyde increases the Bcl-2/Bax expression ratio in the hippocampus of immunologically sensitized mice. Neuroimmunomodulation 13 (2), 63–68. doi: 10.1159/000094829
Wang, B., Hou, W. (2013). A new target for TCM anti-tumor research. Cancer Res. Prev. Treat. 40 (12), 1200–1203.
Wang, J., Kong, H., Yuan, Z. M., Gao, P., Dai, W. D., Hu, C. X., et al. (2013). A novel strategy to evaluate the quality of traditional Chinese medicine based on the correlation analysis of chemical fingerprint and biological effect. J. Pharm. Biomed. Anal. 83, 57–64. doi: 10.1016/j.jpba.2013.04.035
Wang, Y. W., Xu, S. T., Xu, J. Y. (2017). Advances in Research on Lupane-Type-Pentacyclic Triterpenes and Their Anti-tumor Activities. Pharm. Clin. Res. 25 (4), 336–342. doi: 10.13664/j.cnki.pcr.2017.04.016
Wu, M., Fu, T., Chen, J. X., Lin, Y. Y., Yang, J. E., Zhuang, S. M. (2020). LncRNA GOLGA2P10 is induced by PERK/ATF4/CHOP signaling and protects tumor cells from ER stress-induced apoptosis by regulating Bcl-2 family members. Cell Death Dis. 11, 276. doi: 10.1038/s41419-020-2469-1
Xu, G. L., Xie, M., Yang, X. Y., Song, Y., Yan, C., Yang, Y., et al. (2014). Spectrum-Effect Relationships as a Systematic Approach to Traditional Chinese Medicine Research: Current Status and Future Perspectives. Molecules 19 (11), 17897–17925. doi: 10.3390/molecules191117897
Yang, W. T., Hou, E. C. (2016). The research progress of primary hepatic carcinoma treatment. Mod. Oncol. 24 (21), 3495–3499.
Yang, J. D., Larson, J. J., Watt, K. D., Allen, A. M., Wiesner, R. H., Gores, G. J., et al. (2017). Hepatocellular Carcinoma Is the Most Common Indication for Liver Transplantation and Placement on the Waitlist in the United States. Clin. Gastroenterol. Hepatol. 15 (5), 767–775. e3. doi: 10.1016/j.cgh.2016.11.034
Yang, J. D., Hainaut, P., Gores, G. J., Amadou, A., Plymoth, A., Roberts, L. R. (2019). A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 16, 589–604. doi: 10.1038/s41575-019-0186-y
Yi, Z. H. (2017). Quantum Chemistry Study on ESI-ITMSn Fragmentation of Caffeoylquinic Acids (Jiamusi: Jiamusi University).
Zeng, L. J., Lin, B., Song, H. T. (2015). Progress in study of spectrum-effect relationship of traditional Chinese medicine and discussions. China J. Chin. Mater. Med. 40 (8), 1425–1432. doi: 10.4268/cjcmm20150801
Zhang, L., Yan, J., Liu, X. M., Ye, Z. G., Yang, X. H., Meyboom, R., et al. (2012). Pharmacovigilance practice and risk control of Traditional Chinese Medicine drugs in China: current status and future perspective. J. Ethnopharmacol. 140 (3), 519. doi: 10.1016/j.jep.2012.01.058
Zhang, X. P., Shao, J. J., Ma, D. L., Liu, F., Liu, M. M. (2019). Research on antitumor active components and mechanisms of natural products. Acta Pharm. Sin. 54 (11), 1949–1957. doi: 10.16438/j.0513-4870.2019-0669
Zhao, X. P., Fan, X. H., Yu, J., Cheng, Y. Y. (2004). A method for predicting activity of traditional Chinese medicine based on quantitative composition-activity relationship of neural network model. China J. Chin. Mater. Med. 29 (11), 1082.
Zheng, Q., Zhao, Y., Wang, J., Liu, T., Zhang, B., Gong, M., et al. (2014). Spectrum-effect relationships between UPLC fingerprints and bioactivities of crude secondary roots of Aconitum carmichaelii Debeaux (Fuzi) and its three processed products on mitochondrial growth coupled with canonical correlation analysis. J. Ethnopharmacol. 153, 615–623. doi: 10.1016/j.jep.2014.03.011
Zhou, J., Qi, L., Li, P. (2008). Quality control of Chinese herbal medicines with chromatographic fingerprints. Se Pu. 26 (2), 153–159. doi: 10.1016/S1872-2059(08)60011-5
Keywords: S. octophylla, spectrum-effect relationship, active ingredient, Huh7, mechanism
Citation: Liu X, Jiang N, Xu X, Liu C, Liu Z, Zhang Y and Kang W (2020) Anti-Hepatoma Compound Determination by the Method of Spectrum Effect Relationship, Component Knock-Out, and UPLC-MS2 in Scheflera heptaphylla (L.)Frodin Harms and Its Mechanism. Front. Pharmacol. 11:1342. doi: 10.3389/fphar.2020.01342
Received: 30 June 2020; Accepted: 11 August 2020;
Published: 09 September 2020.
Edited by:
Yi Wang, Zhejiang University, ChinaReviewed by:
Liang Feng, China Pharmaceutical University, ChinaFatma Mohamady El-Demerdash, Alexandria University, Egypt
Copyright © 2020 Liu, Jiang, Xu, Liu, Liu, Zhang and Kang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhenhua Liu, bGl1emhlbmh1YTYyM0AxNjMuY29t; Yan Zhang, c25vd3dpbmdsdkAxMjYuY29t; Wenyi Kang, a2FuZ3dlbnlAaG90bWFpbC5jb20=
 Xuqiang Liu1,2
Xuqiang Liu1,2 Wenyi Kang
Wenyi Kang