- 1Department of Radiology, The Second Xiangya Hospital, Central South University, Changsha, China
- 2Department of Radiology, The First Hospital of Hunan University of Chinese Medicine, Changsha, China
- 3Department of Psychiatry, The Fourth People's Hospital of Wuhu, Wuhu, China
- 4Hunan Judicial Police Academy, Changsha, China
Background: A large portion of previous studies that have demonstrated brain gray matter reduction in individuals who use methamphetamine (MA) have focused on short-term abstinence, but few studies have focused on the effects of long-term abstinence of methamphetamine on brain structures.
Materials and Methods: Our study includes 40 healthy controls and 44 abstinent methamphetamine-dependent (AMD) subjects who have abstained for at least 14 months. For every AMD subject, the age when they first used MA, the total time of MA use, the frequency of MA use in the last month before abstinence, the duration of abstinence and the craving score were recorded. Here we used magnetic resonance imaging (MRI) to measure the gray matter volume (GMV) of each subject with voxel-based morphometry method. Two-sample t-test (AlphaSim corrected) was performed to obtain brain regions with different gray matter volume (GMV) between groups. In addition, partial correlation coefficients adjusted for age, years of education, smoking, and drinking were calculated in the AMD group to assess associations between the mean GMV values in significant clusters and variables of MA use and abstinence.
Results: Compared with the healthy control group, AMD group showed increased gray matter volumes in the bilateral cerebellum and decreased volumes in the right calcarine and right cuneus. Moreover, GMV of left cerebellum are positively correlated with the duration of abstinence in the AMD group (p = 0.040, r = 0.626).
Conclusions: The present study showed that the gray matter volume in some brain regions is abnormal in the AMD subjects with long-term abstinence. Changes in gray matter volume of visual and cognitive function regions suggested that these areas play important roles in the progress of MA addiction and abstinence. In addition, positive correlation between GMV of the left cerebellum crus and duration of abstinence suggested that prolonged abstinence is beneficial to cognitive function recovery.
Introduction
Methamphetamine is a highly addictive psychostimulant drug that principally affects the monoamine neurotransmitter systems of the brain and results in feelings of alertness, increased energy and euphoria (1–3). This drug has become a global public health problem due to its ease of production and has more rapid onset and serious neurotoxic effects compared with other traditional drugs (4, 5). World Drug Report 2018 (6) described that up to 2016, about 31 million drug users have shown to have problematic use of drugs. In China, it is reported that there are 2.5 million people that have problematic use of illicit drugs. Synthetic drugs remain the major source of abuse. Among the various drugs seized, methamphetamine and related products account for 31.6%, which is much higher than 10.6% for heroin and 8.1% for ketamine. As a consequence, methamphetamine abuse and related substances have become a serious health crisis (7). Methamphetamine abuse can cause various physical illnesses and psychotic disorders (8–11). Worse of all, even after undergoing substance abuse treatment, patients often relapse when they encounter stress and other high risk environments that may trigger drug use (12–14).
Researches have shown that methamphetamine abuse causes comprehensive changes to brain structures and functions (15–17). After a period of abstinence, brain structure and metabolism can be restored and improved to a certain extent (18–20). Among various methods to investigate brain changes, voxel-based morphometry (VBM) is an automated and efficient tool for whole-brain analysis to detect structural differences. This method is sensitive to subtle brain alterations in gray matter. VBM was developed to detect group differences in the relative concentration of gray matter tissues across the whole brain in a voxel-wise manner. Comparing with traditional morphometric approaches which rely on measuring brain volumes manually, it provides more rapid results and is used for various brain regions (21). Hence, it has been widely used in studies on psychiatric disorders including chemical substance addiction (22, 23). For example, Hanlon and Canterberry (24) indicated that the duration of abstinence was associated with increased gray matter volume (GMV) in the dorsolateral prefrontal cortex, posterior cingulate cortex, and superior parietal lobe by studying about 40 male cocaine abusers. A study on alcohol abstinent patients has shown that abstinence therapy is beneficial for the recovery of GMV in the frontal lobe (25). However, these studies using the VBM method mainly focused on short-term abstinence and results from various studies are inconsistent (26–28).
To investigate brain structure with long-term abstinence could further illuminate the nature of drug relapse, thus conducive to improve long-term abstinence treatment efficacy and rehabilitation programs. Yang et al. (29) utilized a non-human primate model of addiction and showed that neurochemical changes associated with long-term drug use do not persist after prolonged abstinence, suggesting therapeutic effects of long-term abstinence. Wang et al. (30) found that thalamic metabolism was recovered and was associated with improved performance in motor and verbal memory tasks in five long-term abstinent MA abusers. However, these studies which focused on long-term abstinence are either based on small samples or on non-human primate.
In order to overcome shortcomings of the related studies mentioned above, in this study, relatively larger samples (44 AMD subjects and 40 healthy controls) with a long abstinence duration (14–25 month) were collected to investigate the volume changes on gray matter of AMD patients compared with healthy controls (HC) using the VBM method.
Materials and Methods
Subjects
Our study includes 44 AMD subjects and 40 healthy control subjects. All AMD subjects are from Pingtang Mandatory Detoxification in Changsha City, Hunan Province. They all were diagnosed using the Diagnostic and Statistical Manual on Mental Disorders (DSM-V) and after that had received a long-term (14–25 month) compulsory abstinence. During abstinence, the participants were treated with medicine, education, and physical exercise and didn't show any significant abstinence symptoms. Inclusion criteria for all subjects included males, ranging in age from 18 to 45 years old, graduated from at least elementary school, normal visual acuity with or without lens correction, normal hearing, and right handed. Exclusion criteria included diseases that affect cognitive function such as head trauma history, cerebrovascular disease, epilepsy, severe mental illness, severe heart, liver, kidney diseases, drug use in the past 6 months, other substance use or dependence except nicotine in the past 5 years, contraindications to MR examination such as claustrophobia. In addition, for every AMD subject, the age when they first used MA, the total time of MA use, the frequency of MA use in the last month before abstinence, the abstinence duration, and the craving score were recorded.
The research protocol was approved by the Ethics Committee of the Second Xiangya Hospital, Central South University. All subjects volunteered to participate in this study and signed the informed consent form. Confidentiality of personal information and freedom to withdraw from the study were guaranteed.
MR Imaging Acquisition
All MRI data were acquired on a 3T Siemens Skyra MRI scanner (Magnetom Skyra, Siemens, Germany) equipped with a 32-channel head coil. The MRI scanning included T1-weight imaging (T1WI), T2-weight imaging (T2WI), and fluid attenuated inversion recovery (FLAIR) sequences. Each scan included a high-resolution T1-weighted anatomical magnetically prepared rapid acquisition gradient echo (3D MPRAGE) sequence with the following parameters: TR = 2,000 ms, TE = 2.6 ms, TI = 900 ms, flip angle = 8°, 176 slices, slice thickness = 1 mm, slice spacing = 1 mm, FOV = 256 × 256 mm2, acquisition matrix = 256 × 256, voxel size = 1 × 1 × 1 mm3. Subjects were placed in a supine position with foam padding between their head and the head coil to minimize head motions.
Voxel-Based Morphometry (VBM)
The quality of the T1-weighted images was visually checked for artifacts, structural abnormalities, and apparent head motion and no subject was excluded. Images were processed with Voxel-based Morphometry 8 (VBM8) toolbox (http://dbm.neuro.uni-jena.de/vbm/). Brain images were bias corrected, segmented and spatially normalized to the standard Montreal Neurological Institute (MNI) space. To preserve the actual gray matter values locally, segmented gray matter images were then modulated by a procedure in which the intensity value of each voxel was multiplied by the local value of the Jacobian determinants. Finally, the modulated gray matter volume were smoothed with an 8 mm full-width at half maximum (FWHM) Gaussian kernel.
Statistical Analysis
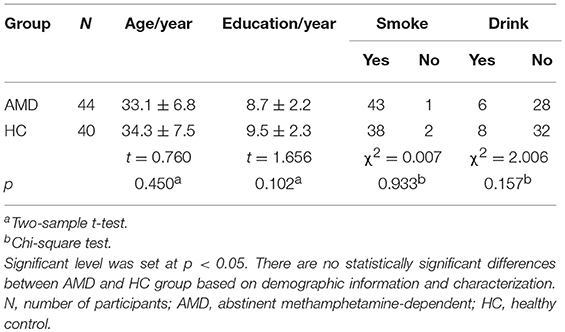
Demographics were compared between the groups with SPSS 21.0. Patients with AMD and healthy control groups were compared on age and the years of education using two-sample t-test; smoking and drinking using chi-square test, and the significance level was set to p < 0.05.
Voxel-wise GMV differences between two groups were computed using two-sample t-test with individual's total GMV, age, gender, and education as covariates. The group GMV difference was corrected for multiple comparisons to a significant level of p < 0.05 by combining the individual voxel p < 0.01 and cluster size >603 voxels. This correction was confined within a whole brain mask and was determined by Monte Carlo simulations using the DPABI AlphaSim program. On regions showed significantly different GMV between AMD group and healthy group, partial correlation coefficients adjusted for age, years of education, smoking, and drinking were calculated in AMD group to assess association between the mean GMV difference and their age when they first used MA, the total time of MA use, the frequency of MA use in the last month before abstinence, the abstinence duration and the craving score. Significance level was set to p < 0.05.
Results
Demographics and Clinical Characteristics of the Participants
Forty-four AMD subjects and forty healthy subjects are included in this study. There is no significant difference between AMD group and HC group in age (mean ± SD) (33.1 ± 6.8 for AMD group; 34.3 ± 7.5 for HC group; t = 0.760, p = 0.450), the years of education (8.7 ± 2.2 for AMD group; 9.5 ± 2.3 for HC group; t = 1.656, p = 0.102), smoking (43 of 44 AMD subjects smoke; 38 of 40 HC subjects smoke; χ2 = 0.007, p = 0.933) and drinking (16 of 44 AMD subjects drink; 8 of 40 HC subjects drink; χ2 = 2.006, p = 0.157) as showed in Table 1.
VBM Results
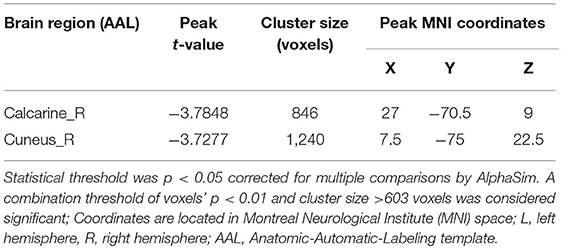
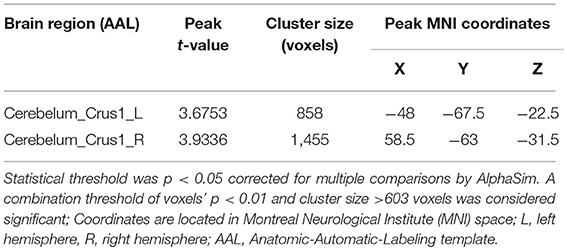
Group differences are shown in Tables 2, 3 and Figure 1. In comparison with HC group, the significant GMV reductions in AMD group were around right calcarine and right cuneus. In contrast, the significant GMV increases in AMD group are around the left cerebellum and right cerebellum.
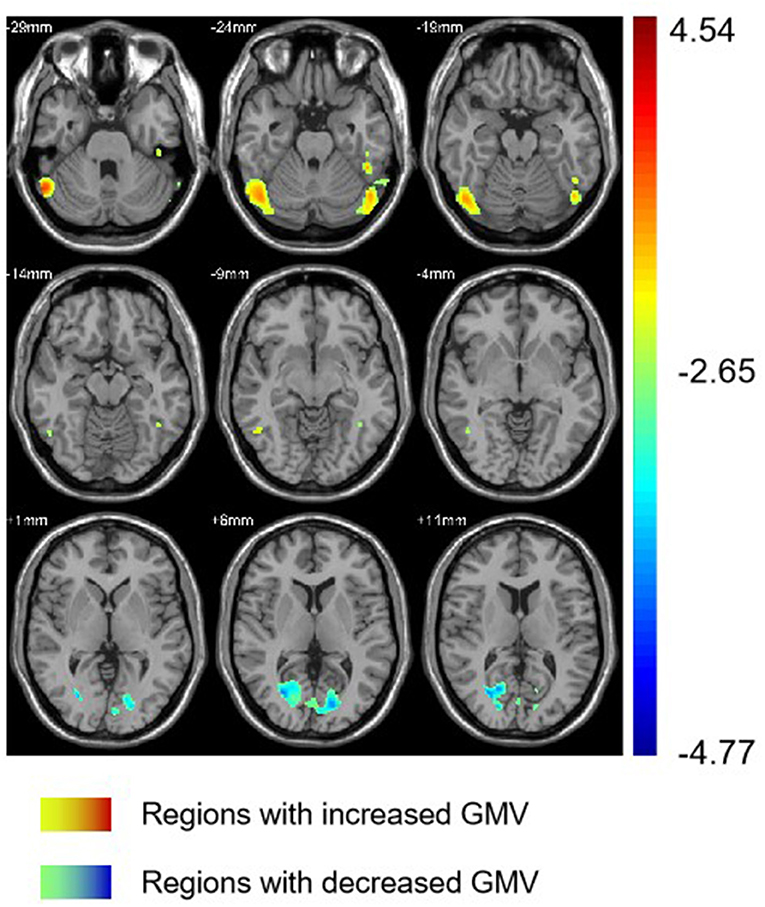
Figure 1. Regions with different GMV in AMD group compared with HC group. GMV, gray matter volume; AMD, abstinent methamphetamine-dependent; HC, healthy control.
Correlation Analyses
The length of abstinent duration of AMD subjects is positively correlated with GMV in left cerebellum crus as showed in Figure 2 (p = 0.040, r = 0.626). However, no other significant correlation was found in the AMD group.
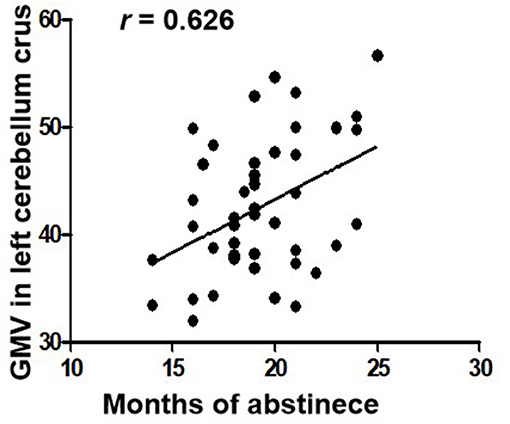
Figure 2. Correlations between duration of abstinence and GMV in the left cerebellum crus in AMD subjects. GMV, gray matter volume; AMD, abstinent methamphetamine-dependent.
Discussion
In our study, we compared GMV between abstinent methamphetamine-dependent group and healthy control group using VBM. We found that AMD group showed significant increased GMV in the bilateral cerebellum crus, and decreased GMV in the right calcarine and right cuneus. In addition, in the AMD group, duration of abstinence is positively correlated with GMV in the left cerebellum crus.
Numerous studies have confirmed drug abuse reduces volume of the cerebellum (31–33). Studies showed that the cerebellum is involved in the procedure of addiction, such as memory, predictive power, and executive control ability (34). Researches have shown that cerebellum crus is associated with cognitive and emotional function (35–37). In our study, the volume of bilateral cerebellar crus was increased in AMD group, which is consistent with previous studies. Kühn et al. (38) confirmed that cerebellar gray matter volume is negatively correlated with the degree of nicotine dependence. Schwartz et al. (39) found that patients with heroin dependence had an increased density of gray matter after 2 months of withdrawal, associated with cognitive, memory, and mood improvements, and with decreased levels of craving for drugs. A spectroscopy study on the abstinence of methamphetamine found that abstinence therapy contributes to the normalization of cerebellar neurometabolites (29). Therefore, we speculated that increased GMV of the cerebellum founded in our study may indicate partial recovery of cerebellar function in AMD patients. Importantly, we also found that the duration of abstinence was positively correlated with the left cerebellar volume, which may suggest that the long-term abstinence is beneficial for cerebellum recovery. Moreover, as the left cerebellar volume GMV increases over abstinence time, we inferred that this increase is probably associated with abstinence instead of addiction.
The cortex around the calcarine fissure is the primary visual cortex, which accepts direct projection from the retina to recognize text, identify objects, determine the relationship between objects, distance difference, recent memory, and so on (40, 41). Neuroimaging studies often report that the activity in visual areas is significantly associated with drug cues exposure, treatment effect of drug abuse and prediction of relapse. It has shown that neural circuitry of addiction, consistently discriminates drug cues from neutral cues in substance dependence. A study by Helenna et al. based on VBM, found that visual associated cortices showed decreasing trends of cortical gray matter volumes on methamphetamine abusers, which may contribute to psychiatric symptoms (42). Our result that the volume of the visual cortex of AMD group decreased implies that drug cue-induced craving, which is one of the most robust factors to continued use and relapse across substances (43), is significant among methamphetamine abuser after long-term abstinence.
In our study, the AMD group also showed decreases in right cuneus volumes. The cuneus is involved in visual processing (44) and associated with cessation outcomes (43). A VBM study (45) on smokers showed that compared to relapsers, quitters had significantly smaller GMV in their right cuneus. Therefore, it is possible that the decreased GMV of cuneus has an impact on abstinence and relapse.
Strength and Limitations
The current study investigated AMD subjects' brain alterations after long-term abstinence. To the best of our knowledge, this is the first study to reveal brain gray matter alterations after an extended abstinence duration. Changes on visual and cognitive function regions suggest that these areas play important roles in the progress of MA addiction and abstinence.
There are several limitations in this study. (1) This is a cross-sectional study, the image data of AMD subjects before abstinence was not collected. As a result, the causal link of abnormal gray matter volume and the abstinence status was not determined. (2) Most of the subjects and controls have a history of smoking. The effect of smoking on the gray matter volume of the brain has been confirmed in the literature (46). Although smoking status is a covariate in this study, the influence of smoking on brain structure cannot be completely excluded. Further study with non-smoking subgroups would help to address this issue. (3) There are no female subjects in this study. The sex differences in the brain can influence the responses to drugs of abuse, progressive changes in the brain after exposure to drugs of abuse and whether addiction results from drug-taking experiences (47). For example, women exhibit more rapid escalation from casual drug taking to addiction, exhibit a greater withdrawal response with abstinence, and tend to exhibit greater vulnerability than men in terms of treatment outcome (48). Therefore, our results are only applicable in male subjects.
In summary, based on the VBM analysis, this study found gray matter changes in the bilateral cerebellar crus, the right calcarine and right cuneus in methamphetamine-dependent subjects with long-term abstinence. In addition, the volume of left cerebellum was positively correlated with abstinent duration, suggesting that prolonged abstinence may be beneficial to cognitive function recovery. This study provides an imaging basis for revealing the neural mechanism of long-term abstinence of methamphetamine.
Author Contributions
JL and JZ conceptualized and designed the research. PL, SH, LF, and YL performed the experiments. RY undertook the statistical analysis. ZZ, LH, and YJ wrote the first draft of the manuscript. ZZ and RY contributed to the final manuscript. All authors critically reviewed content and approved final version for publication.
Funding
National Natural Science Foundation of China, Grant Number: 81671671. Natural Science Foundation of Hunan Province, China, Grant Number: 2015JJ4081. The National Key Research and Development Program of China (Grant number: 2016YFC0800908).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Kamei H, Nagai T, Nakano H, Togan Y, Takayanagi M, Takahashi K, et al. Repeated methamphetamine treatment impairs recognition memory through a failure of novelty-induced ERK1/2 activation in the prefrontal cortex of mice. Biol Psychiatry (2006) 59:75–84. doi: 10.1016/j.biopsych.2005.06.006
2. Gold MS, Kobeissy FH, Wang KK, Merlo LJ, Bruijnzeel AW, Krasnova IN, et al. Methamphetamine- and trauma-induced brain injuries: comparative cellular and molecular neurobiological substrates. Biol Psychiatry (2009) 66:118–27. doi: 10.1016/j.biopsych.2009.02.021
3. Panenka WJ, Procyshyn RM, Lecomte T, MacEwan GW, Flynn SW, Honer WG, et al. Methamphetamine use: a comprehensive review of molecular, preclinical and clinical findings. Drug Alcohol Depend. (2013) 129:167–79. doi: 10.1016/j.drugalcdep.2012.11.016
4. Var SR, Day TR, Vitomirov A, Smith DM, Soontornniyomkij V, Moore DJ, et al. Mitochondrial injury and cognitive function in HIV infection and methamphetamine use. AIDS (2016) 30:839–48. doi: 10.1097/QAD.0000000000001027
5. Darke S, Kaye S, Duflou J. Methamphetamine-related death is an under-addressed public health problem. Addiction (2017) 112:2204–5. doi: 10.1111/add.14035
8. Gonzalez R, Rippeth JD, Carey CL, Heaton RK, Moore DJ, Schweinsburg BC, et al. Neurocognitive performance of methamphetamine users discordant for history of marijuana exposure. Drug Alcohol Depend. (2004) 76:181–90. doi: 10.1016/j.drugalcdep.2004.04.014
9. Woods SP, Rippeth JD, Conover E, Gongvatana A, Gonzalez R, Carey CL, et al. Deficient strategic control of verbal encoding and retrieval in individuals with methamphetamine dependence. Neuropsychology (2005) 19:35–43. doi: 10.1037/0894-4105.19.1.35
10. Cruickshank CC, Dyer KR. A review of the clinical pharmacology of methamphetamine. Addiction (2009) 104:1085–99. doi: 10.1111/j.1360-0443.2009.02564.x
11. London ED, Kohno M, Morales AM, Ballard ME. Chronic methamphetamine abuse and corticostriatal deficits revealed by neuroimaging. Brain Res. (2015) 1628(Pt A):174–85. doi: 10.1016/j.brainres.2014.10.044
12. Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, et al. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. (2006) 26:6583–8. doi: 10.1523/JNEUROSCI.1544-06.2006
13. McKetin R, Najman JM, Baker AL, Lubman DI, Dawe S, Ali R, et al. Evaluating the impact of community-based treatment options on methamphetamine use: findings from the Methamphetamine Treatment Evaluation Study (MATES). Addiction (2012) 107:1998–2008. doi: 10.1111/j.1360-0443.2012.03933.x
14. Brecht ML, Herbeck D. Time to relapse following treatment for methamphetamine use: a long-term perspective on patterns and predictors. Drug Alcohol Depend. (2014) 139:18–25. doi: 10.1016/j.drugalcdep.2014.02.702
15. Chang L, Cloak C, Patterson K, Grob C, Miller EN, Ernst T. Enlarged striatum in abstinent methamphetamine abusers: a possible compensatory response. Biol Psychiatry (2005) 57:967–74. doi: 10.1016/j.biopsych.2005.01.039
16. Ernst T, Chang L. Adaptation of brain glutamate plus glutamine during abstinence from chronic methamphetamine use. J Neuroimmune Pharmacol. (2008) 3:165–72. doi: 10.1007/s11481-008-9108-4
17. Groman SM, Lee B, Seu E, James AS, Feiler K, Mandelkern MA, et al. Dysregulation of D(2)-mediated dopamine transmission in monkeys after chronic escalating methamphetamine exposure. J Neurosci. (2012) 32:5843–52. doi: 10.1523/JNEUROSCI.0029-12.2012
18. Brooks SJ, Burch KH, Maiorana SA, Cocolas E, Schioth HB, Nilsson EK, et al. Psychological intervention with working memory training increases basal ganglia volume: a VBM study of inpatient treatment for methamphetamine use. Neuroimage Clin. (2016) 12:478–91. doi: 10.1016/j.nicl.2016.08.019
19. Choi JK, Lim G, Chen YI, Jenkins BG. Abstinence to chronic methamphetamine switches connectivity between striatal, hippocampal and sensorimotor regions and increases cerebral blood volume response. Neuroimage (2018) 174:364–79. doi: 10.1016/j.neuroimage.2018.02.059
20. Stock AK, Radle M, Beste C. Methamphetamine-associated difficulties in cognitive control allocation may normalize after prolonged abstinence. Progr Neuro-Psychopharmacol Biol Psychiatry (2019) 88:41–52. doi: 10.1016/j.pnpbp.2018.06.015
21. Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neuroimage (2000) 11:805–21. doi: 10.1006/nimg.2000.0582
22. Morales AM, Lee B, Hellemann G, O'Neill J, London ED. Gray-matter volume in methamphetamine dependence: cigarette smoking and changes with abstinence from methamphetamine. Drug Alcohol Depend. (2012) 125:230–8. doi: 10.1016/j.drugalcdep.2012.02.017
23. Hartwell EE, Moallem NR, Courtney KE, Glasner-Edwards S, Ray LA. Sex differences in the association between internalizing symptoms and craving in methamphetamine users. J Addict Med. (2016) 10:395–401. doi: 10.1097/ADM.0000000000000250
24. Hanlon CA, Canterberry M. The use of brain imaging to elucidate neural circuit changes in cocaine addiction. Substance Abuse Rehabil. (2012) 3:115–28. doi: 10.2147/SAR.S35153
25. Durazzo TC, Mon A, Gazdzinski S, Yeh PH, Meyerhoff DJ. Serial longitudinal magnetic resonance imaging data indicate non-linear regional gray matter volume recovery in abstinent alcohol-dependent individuals. Addict Biol. (2015) 20:956–67. doi: 10.1111/adb.12180
26. Hser YI, Hoffman V, Grella CE, Anglin MD. A 33-year follow-up of narcotics addicts. Arch Gen Psychiatry (2001) 58:503–8. doi: 10.1001/archpsyc.58.5.503
27. Jan RK, Lin JC, Miles SW, Kydd RR, Russell BR. Striatal volume increases in active methamphetamine-dependent individuals and correlation with cognitive performance. Brain Sci. (2012) 2:553–72. doi: 10.3390/brainsci2040553
28. Li Q, Li W, Wang H, Wang Y, Zhang Y, Zhu J, et al. Predicting subsequent relapse by drug-related cue-induced brain activation in heroin addiction: an event-related functional magnetic resonance imaging study. Addict Biol. (2015) 20:968–78. doi: 10.1111/adb.12182
29. Yang S, Belcher AM, Chefer S, Vaupel DB, Schindler CW, Stein EA, et al. Withdrawal from long-term methamphetamine self-administration 'normalizes' neurometabolites in rhesus monkeys: a (1) H MR spectroscopy study. Addict Biol. (2015) 20:69–79. doi: 10.1111/adb.12078
30. Wang GJ, Volkow ND, Chang L, Miller E, Sedler M, Hitzemann R, et al. Partial recovery of brain metabolism in methamphetamine abusers after protracted abstinence. Am J Psychiatry (2004) 161:242–8. doi: 10.1176/appi.ajp.161.2.242
31. Nurmedov S, Metin B, Ekmen S, Noyan O, Yilmaz O, Darcin A, et al. Thalamic and cerebellar gray matter volume reduction in synthetic cannabinoids users. Eur Addict Res. (2015) 21:315–20. doi: 10.1159/000430437
32. Vnukova M, Ptacek R, Raboch J, Stefano GB. Decreased central nervous system Grey Matter Volume (GMV) in smokers affects cognitive abilities: a systematic review. Med Sci Monit. (2017) 23:1907–15. doi: 10.12659/MSM.901870
33. Zhou D, Rasmussen C, Pei J, Andrew G, Reynolds JN, Beaulieu C. Preserved cortical asymmetry despite thinner cortex in children and adolescents with prenatal alcohol exposure and associated conditions. Hum Brain Mapp. (2018) 39:72–88. doi: 10.1002/hbm.23818
34. Moulton EA, Elman I, Becerra LR, Goldstein RZ, Borsook D. The cerebellum and addiction: insights gained from neuroimaging research. Addict Biol. (2014) 19:317–31. doi: 10.1111/adb.12101
35. Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol. (2011) 106:2322–45. doi: 10.1152/jn.00339.2011
36. Leggio M, Olivito G. Topography of the cerebellum in relation to social brain regions and emotions. Handb Clin Neurol. (2018) 154:71–84. doi: 10.1016/B978-0-444-63956-1.00005-9
37. Schmahmann JD. The cerebellum and cognition. Neurosci Lett. (2018) 688:62–75. doi: 10.1016/j.neulet.2018.07.005
38. Kühn S, Romanowski A, Schilling C, Mobascher A, Warbrick T, Winterer G, et al. Brain grey matter deficits in smokers: focus on the cerebellum. Brain Struct Funct (2012) 217:517–22. doi: 10.1007/s00429-011-0346-5
39. Schwartz DL, Mitchell AD, Lahna DL, Luber HS, Huckans MS, Mitchell SH, et al. Global and local morphometric differences in recently abstinent methamphetamine-dependent individuals. Neuroimage (2010) 50:1392–401. doi: 10.1016/j.neuroimage.2010.01.056
40. Nordahl TE, Salo R, Natsuaki Y, Galloway GP, Waters C, Moore CD, et al. Methamphetamine users in sustained abstinence: a proton magnetic resonance spectroscopy study. Arch Gen Psychiatry (2005) 62:444–52. doi: 10.1001/archpsyc.62.4.444
41. Shmuel A, Leopold DA. Neuronal correlates of spontaneous fluctuations in fMRI signals in monkey visual cortex: implications for functional connectivity at rest. Hum Brain Mapp. (2008) 29:751–61. doi: 10.1002/hbm.20580
42. Nakama H, Chang L, Fein G, Shimotsu R, Jiang CS, Ernst T. Methamphetamine users show greater than normal age-related cortical gray matter loss. Addiction (2011) 106:1474–83. doi: 10.1111/j.1360-0443.2011.03433.x
43. Hanlon CA, Dowdle LT, Naselaris T, Canterberry M, Cortese BM. Visual cortex activation to drug cues: a meta-analysis of functional neuroimaging papers in addiction and substance abuse literature. Drug Alcohol Depend. (2014) 143:206–12. doi: 10.1016/j.drugalcdep.2014.07.028
44. Fink GR, Halligan PW, Marshall JC, Frith CD, Frackowiak RS, Dolan RJ. Where in the brain does visual attention select the forest and the trees? Nature (1996) 382:626–8. doi: 10.1038/382626a0
45. Froeliger B, Kozink RV, Rose JE, Behm FM, Salley AN, McClernon FJ. Hippocampal and striatal gray matter volume are associated with a smoking cessation treatment outcome: results of an exploratory voxel-based morphometric analysis. Psychopharmacology (2010) 210:577–83. doi: 10.1007/s00213-010-1862-3
46. Sharma A, Brody AL. In vivo brain imaging of human exposure to nicotine and tobacco. Handb Exp Pharmacol. (2009) 192:145–71. doi: 10.1007/978-3-540-69248-5_6
47. Becker JB, McClellan ML, Reed BG. Sex differences, gender and addiction. J Neurosci Res. (2017) 95:136–47. doi: 10.1002/jnr.23963
Keywords: addiction, methamphetamine, long-term abstinence, voxel-based morphometry, gray matter volume
Citation: Zhang Z, He L, Huang S, Fan L, Li Y, Li P, Zhang J, Liu J and Yang R (2018) Alteration of Brain Structure With Long-Term Abstinence of Methamphetamine by Voxel-Based Morphometry. Front. Psychiatry 9:722. doi: 10.3389/fpsyt.2018.00722
Received: 30 May 2018; Accepted: 07 December 2018;
Published: 20 December 2018.
Edited by:
Feng Liu, Tianjin Medical University General Hospital, ChinaReviewed by:
Quan Jiang, Henry Ford Health System, United StatesXiang Yang Zhang, University of Texas Health Science Center at Houston, United States
Liwei Zou, Second Hospital of Anhui Medical University, China
Liting Chen, First Affiliated Hospital of Nanchang University, China
Copyright © 2018 Zhang, He, Huang, Fan, Li, Li, Zhang, Liu and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Liu, anVubGl1MTIzQGNzdS5lZHUuY24=
Ru Yang, eXJfc211QDEyNi5jb20=
†These authors have contributed equally to this work
 Zhixue Zhang
Zhixue Zhang Lei He1†
Lei He1† Jun Liu
Jun Liu